TEST INSTRUMENT CONSIDERATIONS
PATHOMECHANICS AND DEGREES OF INJURY
NERVE CONDUCTION VELOCITY: A COMPANION ASSESSMENT
TOUCH–PRESSURE THRESHOLD TESTING (SEMMES–WEINSTEIN-STYLE MONOFILAMENTS)
▪ Accurate sensibility tests are useful for early recognition of peripheral nerve problems and allow early intervention and monitoring.
▪ Screening of touch threshold perception according to the zones and areas of peripheral nerve innervations and illustrating the results using a color-coded hand map is possible using Semmes–Weinstein-style monofilaments.
▪ Other sensibility tests, such as those for two-point discrimination, may add to the overall assessment of patient status.
▪ Assessments of sensory and motor nerve conduction velocity along with sensibility tests form a crucial basis for treatment decisions in patients with peripheral nerve problems.
Neurophysiologists are interested in “normal” sensory function.1-6 Clinicians are interested in accurately assessing abnormal sensibility and detection thresholds.7-12 The examiner of sensibility must determine how a client’s performance on the spectrum of sensibility tests compares with a normal baseline and, if abnormal, be able to quantify the degree of change in measurable increments. In this chapter, abnormal results for sensibility measured by clinicians via various tests are referenced against normal values so that degrees of loss can be accurately assessed and monitored.
Sensibility measurement instruments must have instrument integrity before test results can be considered valid in clinical studies.8,13-16 Instrument accuracy can only be as good as the quality of stimulus input the instrument provides. For example, a sensibility test instrument that is not repeatable in force of application lacks the needed accuracy, does little to clarify, and can actually be misleading in results.
In any given skin area of 10 mm2 there are more than 3000 sensory end organs.3 Sensibility tests are intended to measure the physiologic function of the peripheral nerves by assessing response of their respective end-organ mechanoreceptors in the skin. All force and pressure tests used to excite sensory mechanoreceptor nerve endings in the skin (which respond to stretch or deformation) should be in repeatable force or pressure units defined by the National Institute of Standards and Technology (NIST).
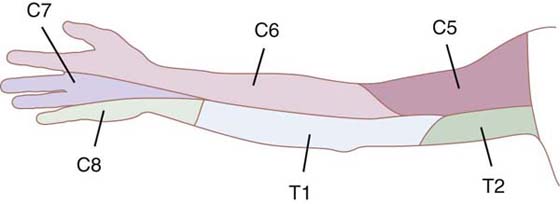
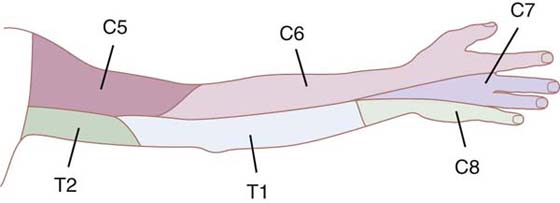
The examiner of sensibility should be aware of the normal patterns of sensory nerve innervation in the hand and upper extremity as well as the typical sensory signs and symptoms that result from different levels of lesions along nerve pathways17-20 (Figs. 11-1 and 11-2). The prognosis for recovery of function of the sensory nerves depends on which nerve structures are involved, and their degree of damage (axons, endoneurium, perineurium, or epineurium). Modes of nerve injury are mechanical, thermal, chemical, or ischemic. Swelling and inflammation can exacerbate internal and external nerve compression and compromise vital nutrition to nerve axons, particularly where the nerves must pass through tight structures. Direct injury can result in a nerve lesion in continuity (axonal conduction disruption) or in complete transection.21,22
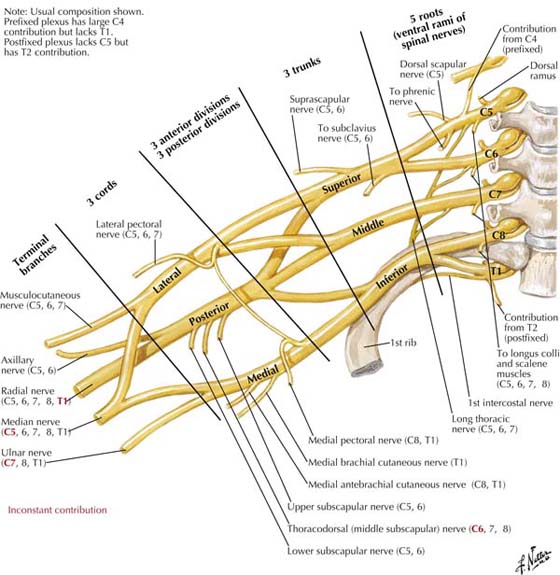
Figure 11-2 Nerves of the brachial plexus. (Netter illustration from www.netterimages.com. © Elsevier Inc. All rights reserved.)
Observation and interview before testing may help to identify apprehensive patients, those with exaggerated symptoms, and those whose intention is secondary gain from an injury. Often, patients provide clues as to their condition by how they hold their arms, sign papers, manipulate objects, and present themselves. If patients have a history of symptoms suggestive of peripheral nerve involvement, but test within normal limits, the examiner may use certain provocative positions, or otherwise stress the nerve in question in order to provoke symptoms that can then be measured. Stress testing can be static (e.g., sustained wrist flexion or extension at end range for 1 minute), or dynamic (e.g., putty squeezed for 5 minutes, or provocative work and activity), with sensibility measurement before and after stress.15,21,23-25
The objective of nerve conduction velocity (NCV) assessment is to determine the speed of neural conduction and, if slowed or abnormal, to determine whether more than one nerve or site is involved.25-27 NCV does not determine if and how much a patient can and cannot actually “feel.” In order to determine a subject’s touch threshold detection and discrimination, tests of sensibility need to be done along with NCV.28,29 It is important for clinicians to understand how NCV test results fit in with sensibility test results to enable an overall interpretation of peripheral nerve status.
A common peripheral nerve condition in the upper extremity is median nerve compression at the level of the wrist, thus the median nerve is frequently initially targeted for NCV testing when numbness or tingling occur in a patient’s fingers. But the astute clinician is aware that other peripheral nerves can be involved and more than one level of involvement, referred to as a “double-crush” syndrome. Furthermore, there can be, and frequently is, bilateral involvement. Patients can have nerve lesions in continuity at all segments of upper extremity nerves, including brachial plexus and thoracic outlet.30-32 Sensory involvement usually precedes motor. Hunter and others maintain that high-level traction neuropathies commonly result from high-speed vehicular trauma, falls on the outstretched arm, repetitive assembly work (lateral abduction or overhead lifting), overuse (when heavy work exceeds physical capacity), and poor posture.18,33,34 NCV testing from the neck to the fingers bilaterally is recommended when the initial history and physical do not readily suggest the level, type, and degree of involvement. Although valuable, NCV testing is known to vary according to the time of day, temperature of the extremity, size of the electrodes, placement of the electrodes, and instrument. In skilled hands using correctly calibrated machines, NCV testing can be accurate and repeatable.26,33 Results of NCV and sensibility tests do loosely correlate but do not always directly correlate as evidence of peripheral nerve involvement.14,27 “Slowing” of sensory NCV, or abnormal amplitude, along with abnormal sensibility testing, help confirm abnormal nerve function.
When NCV examination results show a “slowed” nerve conduction response, and light touch threshold tests such as the Semmes–Weinstein monofilament test demonstrate results that are “within normal limits,” the clinician can assume that there is not yet a detectable change in sensory threshold detection. NCV can be reported as “absent,” while the heaviest monofilaments, or a pinprick, can still be detected in some instances, signaling the nerve does have residual viable function that could potentially improve if treated. These differences in the test results do not mean that one is more sensitive or objective, but that they are different tests and measurements of neural physiologic function.7,35 See Chapter 15 for a detailed discussion of nerve conduction studies.
Five hierarchal levels of sensibility testing have been described by Fess15 and LaMotte.2 They include the following: autonomic/sympathetic response, detection of touch, touch discrimination, quantification, and identification.
Callahan15 divides sensibility tests into four categories: threshold tests, functional tests, objective tests and provocative or stress tests. Selected tests from these categories are discussed in the following sections. For another perspective and more details, the reader is referred to Callahan’s archived chapter on sensibility assessment on the companion Web site of this text.
Touch–pressure threshold testing involves the use of nylon monofilaments of standard length and increasing diameters that provide controlled gradients of force to the mechanoreceptors in the skin to determine light-touch to deep-pressure detection thresholds (Fig. 11-3). The advantage of monofilament testing is that it provides clear, quantified, and repeatable information about the patient’s detection of touch. The pattern of sensibility loss reflected by the monofilament testing helps to identify pathology.

Figure 11-3 Semmes–Weinstein-style monofilaments: Hand/body screen monofilament set (5 of 20 available in full set). Critical monofilaments for monitoring in the reduced set needed for most evaluations—marking numbers 2.83, 3.61, 4.31, 4.56, and 6.65.
It is important to note any changes in touch–pressure detection thresholds with repeated testing, but this requires the clinician’s interpretation to guide the course of further treatment. Improvement in light-touch to deep-pressure thresholds may be seen even in chronic conditions, episodes of worsening may occur, and the course of recovery following nerve release or suture can be predictable or complicated by scar and fibrosis. All of these occurrences can be reflected with repeat testing of touch–pressure thresholds.
The monofilament form of testing is used in patients with neuropathies, entrapment or compression syndromes, lacerations, and other abnormalities, including patients with diabetes.36-38 The test can reveal sensibility losses in patients with Hansen’s disease (HD), in which early changes are often superficial, and with worsening of the disease process, changes can mimic other peripheral nerve lesions or compression. Early testing in this situation can lead to prevention, reversal, or improvement of neural damage with timely use of steroids and other anti-inflammatory medications.39-41
Monofilament testing is easy to perform yet profound in the information it provides. Results are understood by the examiner, physician, insurance provider, health care gatekeeper, the patient, and others involved in treatment and employment. The versatile monofilament form of testing can be used in a 10-minute screen or for a full map showing clearly and completely in detail the area and degree of abnormality. Screening examinations are used to evaluate the autonomous areas of cutaneous innervation of the median, ulnar, or radial nerves or other sites of potential or suggested involvement.
Monofilament test results are mapped to identify the area and degree of abnormality, and repeat testing reflects changes over time. For normative studies, it is critical to include a lighter, above-threshold monofilament from the set of 20 so the study includes above-threshold sensitivities. Szabo7,42 and others use all of the lighter monofilaments in the within-normal-limits functional level, along with the hand screen monofilament sizes when testing patients with possible compression or entrapment, considering it important to use the most sensitive possible. This is an accepted variation of the test when time allows, which adds additional data to the hand screen. See studies by Szabo,43 Gelberman,44 and Lundborg45 for their protocols.
Examiners sometime include additional monofilaments in the heavy range with the hand screen monofilaments. The 5.07, 10-g level monofilament is most frequently added, specifically for testing of the diabetic foot for measuring gross protective sensation of the plantar surface.46,47 Some prefer 4.56, 4-g level monofilament (already included in the hand screen kit) for screening protective sensation of the foot.7,13
It is important to consider that the patient’s activities immediately prior to testing can influence the testing results. For example, if the patient has had a relatively stress-free morning, after sleeping late, having a good breakfast, and a more quietly paced and lighter-duty work schedule than is normal routine, the results of testing may be better than after heavy-duty work of a few hours’ duration. Hunter terms this condition transient stress neuropathy and recommends monofilament testing after activities and positions that reproduce their symptoms.33
Monofilament testing can detect abnormal sensory threshold responses all over the body, even on the face, where sensitivities exceed that of the hand, and on the plantar contact area of the foot, where slightly heavier detection thresholds allow for callus.7,48,49 The force range of the monofilaments in available diameter sizes is 4.5 mg, for the lightest, to over 300 g for the heaviest. Semmes and Weinstein50 and Weinstein51,52 found that within normal subjects, differences can be found between men and women, the left- and right-handed, and among age groups. For most clinical testing, however, it is not as important to use the very lightest above-threshold monofilaments as it is to determine if the patient is normal or not. The 2.83 marking number (50-mg level monofilament) is then the most important of the monofilaments available. It is the last of the lightest monofilaments falling within normal limits for screening of men and women and right and left hands.50-53
Nylon monofilament force of application was examined by Bell-Krotoski and Buford at the Paul W. Brand Research Laboratory.7,13,54-56 Nylon material was obtained, tested, and used for sets researched and produced at the former Gillis W. Long Hansen’s Disease Center (GWLHDC), Carville, Louisiana, in 1989. All sets were made to Weinstein’s original specifications for diameter size and length of 38 mm. Bell-Krotoski provided these original specifications for sets first produced by North Coast Medical (NCM). Both the GWLHDC sets and NCM sets were then used in a normative study by six examiners who tested 131 subjects (262 hands, 520 tests; 182 feet, total 364 tests).48 In this study, which used the standard protocol detailed in this chapter and included all of the lighter monofilaments, the 2.83 (marking number) 50-mg level monofilament was confirmed as the optimal size for within-normal-limits screening for males and females, right and left hands, and all over the body, except the plantar contact area of the foot where the 3.61 (marking number) 200-mg level was found to be a better predictor of normal57 (Fig. 11-4, online).
A normal person is not expected to detect the 2.83, 50-mg level monofilament 100% of the time, but a normal person can detect this level of monofilament force of application most of the times it is applied. The correlation of monofilament sizes with functional levels of detection was developed by von Prince and Butler, Werner and Omer, and as used today by Bell (Bell Krotoski).7,58,59 Those who detect a 3.61-size monofilament (but not lighter) also have difficulty in discerning textures and symbols drawn on the fingertips (“diminished light touch”) (Table 11-1).
Table 11-1 Interpretation Scale for Monofilaments
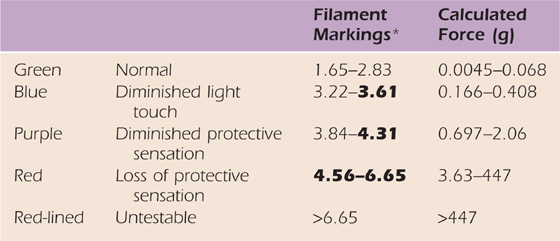
*Minikit monofilaments are in bold. Descriptive levels based on other scales of interpretation and collapse of data from 200 patient tests.
Force data, used with permission, from Semmes J, Weinstein S: Somatosensory Changes After Penetrating Brain Wounds in Man, Cambridge, Mass.: Harvard University Press, 1960.
In repeated measurements over time using standard specifications for this size monofilament (7 mil diameter, and 38-mm length) the 3.61 monofilament size was found to measure a more sensitive 200-mg level, not actually reaching the 400-mg level shown in Weinstein’s original calculated forces13,57,60-62 (Table 11-2). Both 200- and 400-mg forces fall in the diminished-light-touch functional level of detection. It is important that manufacturers of the monofilaments appreciate the fact that if the length or diameter of a monofilament is changed from that used in the normative and clinical studies according to Weinstein’s original specifications, these studies no longer apply for interpretation of test results with the changed monofilaments for accuracy and reliability. It is recommended that the force of application for each monofilament should remain that of the original design with the standard 38-mm length and specified diameters.13,63
Table 11-2 Monofilament Marking Numbers, Force Comparisons, and Diameters
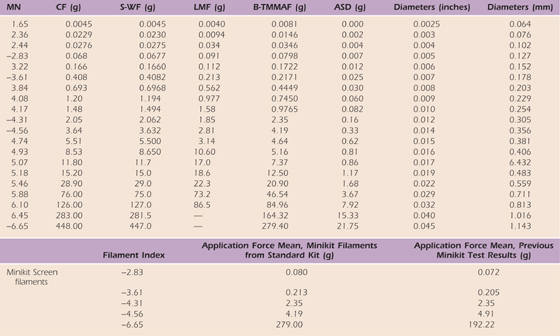
One tester, seven complete testing kits.
The range of forces for all filaments can be read as the mean plus or minus the SD value. Note in offset the comparison of means of filaments in the minikits and minikit filaments tested in the long kits.
ASD, average standard deviation; B-TMMAF, Bell-Tomancik measured mean application force; CF, calculated force based on buckling equation; LMF, Levin Pearsall, and Ruderman measured force; MN, marking number derived from log scale; S-WF, Semmes–Weinstein force.
Reprinted, with permission, from Table V, Bell JA, Tomancik E. Repeatability of testing with Semmes–Weinstein monofilaments. J Hand Surg. 1987;12A:155.
Touch–pressure detection thresholds increase to gram levels with more severe degrees of nerve loss.63-65 Hand screening determines magnitude of response in established functional sensibility levels, beginning with within-normal limits and progressing to diminished light touch, “diminished protective sensation,” “loss of protective sensation,” “deep pressure sensation,” or unresponsive.
The monofilaments clearly have been shown to be accurate and their results repeatable if the instrument is calibrated correctly.65 The monofilaments bend when the predetermined threshold for that size is applied to the patient and cannot go beyond if applied correctly.13 The elastic properties of the nylon monofilament material provides the instrument with the unique ability to dampen the vibration of the examiner’s hand that occurs with hand-applied devices that do not control for this vibration.63 In extensive materials testing, characteristics of the nylon used to manufacture the monofilaments was found to be important.35 Additives during manufacture can change nylon’s physical properties, that is, the force of application. Nylon fishing line is beginning to be used by some manufacturers of the filaments, but it is extruded on rolls instead of in straight lengths. Any rolled nylon when extruded does not hold repeatable calibration, even if artificially heat-straightened and, thus, cannot be recommended. Clinicians should recognize and replace any nylon monofilament that is bent and stays bent.
Straight-length, extruded nylon holds calibration, even if curved slightly, until damaged, because nylon has an indefinite shelf life.57 The elasticity of straight-length nylon monofilament and its bending and recovery at a specific force means the force it can apply is limited and controlled, thus the monofilament form of testing with pure straight nylon is force-controlled.
Some of the monofilaments in the full set of 20 have been found to be so close in force of application that they occasionally overlap and represent the same force62 (see Table 11-2). Those examiners concerned with losing test sensitivity because they are not using all 20 monofilaments need to consider that sensitivity is better using the hand screen set where the forces never overlap, rather than the full set, where some overlap is possible.
Sidney Weinstein and his physicist son Curt Weinstein developed the Weinstein Enhanced Sensory Test (WEST) following review and discussion with Bell Krotoski and Fess regarding abnormal functional levels, and the Hand Screen set.57,66 The Weinsteins improved on Bell Krotoski’s “pocket filament” prototype of the Hand Screen monofilaments in one handle, by rounding monofilament tips, and designing a less fragile handle35,57,66 (Fig. 11-5). The Weinsteins certify the WEST monofilament force of application, and do slightly adjust the lengths toward Weinstein’s original calculated forces. The WEST monofilament set is recommended for research (available through Connecticut Bioinstruments, Connecticut) but the difference in stimulus needs to be considered when attempting to compare the WEST with threshold and functional scales of interpretation developed from Weinstein’s standard style monofilaments. The tip geometry has changed, and force of application differs slightly from standard sets used. Although the instruments are very close in stimuli, additional clinical testing is needed to determine how the WEST compares with the original standard monofilament design in test stimuli and if the interpretation scales are applicable for the WEST.
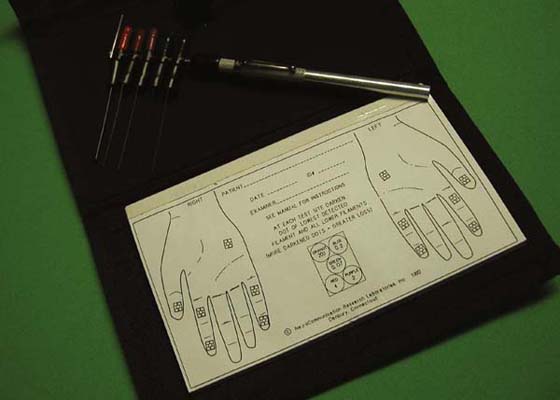
Figure 11-5 Weinstein Enhanced Sensory Test (WEST). Hand screen/body screening monofilaments in one handle, and rounded application tips.
Instrument handle variations may not affect the specified monofilament force of application if the nylon is maintained at a 90-degree angle to the handle, and force of the monofilament applied to the skin is correct and at a distance from the examiner’s hand. These two criticisms of Semmes–Weinstein-style monofilaments have been addressed in a new handle design: the examiner’s difficulty in seeing the tip of the lightest monofilaments on application, and the monofilaments’ breaking at the point it leaves the handle. The new handle design extends over the monofilament where it exits from the handle and includes a light to illuminate the skin being tested, while keeping the 90-degree orientation of the monofilament to the rod handle. This new handle design prevents the monofilament from being sheared off by a neighboring monofilament, from being laid down upon itself, and from damage when inadvertently dropped55 (available at timelyneuropathytesting.com) (Fig. 11-6).

Figure 11-6 BK CLEAR Lighted Monofilaments. Bell-Krotoski JA. Device for evaluating cutaneous sensory detection, notice of publication of application, United States Patent and Trademark Office, US-2009-0105606-A1, 2009.
If made of pure nylon, and the diameter size and length are correct, the standard Semmes–Weinstein-style monofilament instrument stimulus has been found to be repeatable within a small specified standard deviation.13,65 The monofilament length can be checked to be 38 mm with a millimeter ruler. Diameters can be checked with a micrometer. Because the monofilaments are not always perfectly round, diameters are taken three times and averaged. (See diameters in Table 11-2).
Clinicians either need to request actual monofilament calibration measurements on test sets they use or measure their sets to confirm calibration. Monofilaments are applied 10 times and averaged for application force measurement. Nevertheless, it is most accurate to use the same set for retesting.57 It is not enough just to state in studies that a calibrated instrument was used, or say sets used are made to specifications based on a published calculated table of application force.50
A known problem in measurement of monofilament application force is the use of top-loading balance scales in an attempt to measure monofilament force of application. For most who attempt monofilament calibration, these scales are inaccurate in measuring the monofilament “dynamic” force of application because the instruments are intended for measuring static weight, and depend on an internal spring mechanism that works against the elasticity of the nylon.13
Bell and Buford specifically engineered an instrument measurement system to be sensitive enough to measure dynamic force application and range of the monofilaments, in addition to any other hand-held sensory testing instruments (Fig. 11-7). The signature of a monofilament repeatedly applied was accurately displayed in real time on an oscilloscope, measured, and examined for spikes in force, vibration of the tester’s hand, or subthreshold application.13,65 The lightest monofilaments were applied to a calibrated strain gauge accurate to less than 1 g. If applied too quickly (less than 1.5 seconds) and “bounced” against the skin, the monofilament force of application will spike, overshoot, and exceed intended force of application, thus technique of application is important.65 A spectrum analyzer (not shown) was used in the measurement system to detect the force frequency of application. Frequency signal outputs from the lightest to the heaviest monofilament were detected throughout the available frequency spectrum, negating claims that the monofilaments only test low- or high-frequency (slowly or quickly adapting) end-organ response.13,63
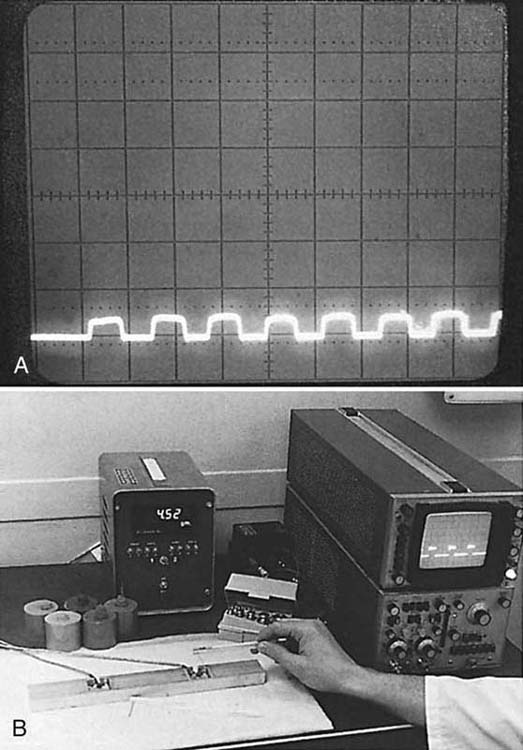
Figure 11-7 A, Oscilloscope screen showing force on repeated application of the 2.83 (marking number) within normal limits Semmes–Weinstein monofilament (250 mg/division). The instrument application force is highly repeatable within a very small range if the lengths and diameters of the monofilaments are correct. B, Sensory Instrument Measurement System used to measure any hand-held instrument “dynamic” force of application.
In recent years, mechanical engineer researchers James Foto and Dave Giurintano have reproduced the earlier strain-gauge transducer design and engineered the force output to be read directly on a computer. This system, like the original, can measure any hand-held sensibility testing device, but allows former analog measurements to be digital in real time. Computer calculations of force of application and standard deviation help eliminate potential examiner error in calculations of average force (developed in Labview scientific program, National Instruments, Austin, Texas). A still more recent development adds a motorized attachment to apply the monofilament to the measurement system (Fig. 11-8). All variations in hand-held application are thus eliminated for measurement. Foto and Giurintano initiated this automation in a project between Louisiana State University and the Paul W. Brand Research Laboratory, National Hansen’s Disease, Programs (NHDP) now in Baton Rouge, Louisiana.
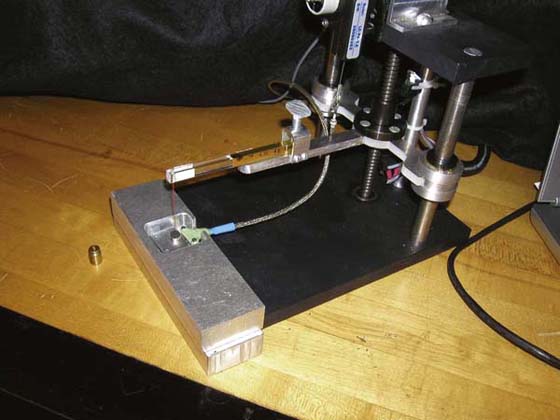
Figure 11-8 Automated application to Sensory Instrument Measurement System for elimination of any hand-held variable in force measurement.
Three applications of the lightest monofilaments are used in clinical testing even though the patient usually responds to the first application. It was found in instrument testing that one touch of these extremely light monofilaments may not reach the required threshold, but one out of three always reaches intended threshold.13,66,68 Clinical studies and papers that require two out of three, three out of five, or one out of five, and so forth for a correct response are incorrect, as this is not the test protocol used in normative studies and clinical studies for functional levels (which requires one correct response out of three trials).50,52,57,61
When calibrated and applied correctly, the monofilaments are a valid test for determining sensibility detection thresholds. Studies have clearly demonstrated their ability to accurately detect intended clinical conditions.15,21,27,44,69 Used in standard consistent protocols, the monofilament test is able to compare patient data in individual and multicenter studies and is providing information regarding peripheral nerve changes with treatment not previously available with less sensitive and uncontrolled instruments.9,40,41,70 When calibrated correctly, it is one of the few, if not the only, sensibility measurement instrument that approaches requirements for an objective test.
Weinstein found that the normal detection threshold for touch pressure does not vary widely over the entire body.51 The relative consistency is what makes light-touch/deep-pressure threshold mapping of the cutaneous innervation of the peripheral nervous system possible. This is in contrast to point localization and two-point discrimination thresholds.
Depending on the question, one may need more than one test of sensibility to obtain an adequate picture of neural abnormality. The monofilaments do not measure end-organ innervation density. Once monofilament threshold is screened or mapped to establish areas of abnormality, other tests such as two-point discrimination and point localization can be focused in abnormal areas to help further qualify and quantify abnormal sensibility as to innervation density and localization of touch.
It is known that monofilament testing can sometimes reveal peripheral nerve compression before conventional two-point discrimination tests and reveal return of innervation long before two-point discrimination is measurable at the fingertips.57 Authors and clinicians have traditionally championed one or more methods of sensibility testing, and clinicians should understand that to determine the relative control and validity of another instrument versus the monofilaments, a comparison study requires a valid protocol with direct comparison of instrument stimulus and results, not just opinion.
The first sign of nerve return after laceration and repair is not the heaviest monofilament but a positive Tinel’s test distal to the site of repair.71 A positive Tinel’s test—in which there is perception of shocking and shooting electrical sensations—is a valuable, albeit subjective, indicator of returning nerve physiologic response after laceration concurrent with or before the heaviest touch–pressure threshold can be measured. Since peripheral nerve return occurs from proximal to distal, Tinel’s test, like the monofilament test, shows improvement proximally to distally over time.
Von Frey72 was the inventor of the monofilament form of testing using horsehairs only capable of producing light thresholds. Weinstein first invented the 20 nylon monofilaments, added heavier levels of detection, and did normative studies.50 The range of forces of the monofilaments occur simply from available diameter sizes of nylon. In studies, they cannot be treated as occurring at equal mathematical increments as some researchers have done. Today the monofilament log numbers are primarily used as marking numbers for ordering and specifying diameter size35,63-65 (see Table 11-1).
Von Prince58 was greatly influenced by Moberg’s emphasis on sensibility and hand function.73 She began investigating the residual function of patients who had sustained a variety of peripheral nerve injuries from war wounds. She observed that of two patients who could not tell a difference in testing between one and two points, one could feel a match that would burn his finger, and the other could not. She thus described the all important “level of protective sensation” that was not being measured by the Weber74 two-point discrimination test frequently used in practice. She also noticed that of two patients who responded to a pinprick, one would have the ability to discriminate textures and one would not. Thus she first described a “level of light touch sensation” that could be equated with the patient’s ability to discriminate textures. As she searched for tests that would be able to discern these differences in patients, she found the answer in Weinstein’s monofilament test. Von Prince published her findings but was transferred overseas before fully completing her investigation. Omer realized the value of the monofilament test and insisted it be continued by Werner.59
James M. Hunter, originator of the Hand Rehabilitation Center, Ltd., in Philadelphia, realized the value of monofilament testing in producing information on patient neural status that was not forthcoming from other examinations. He insisted on this form of testing for his patients, many of whom came to him with previously longstanding unresolved peripheral nerve problems. But the test originally took 2 hours when included with other sensory tests and was confounded by inconsistent coding and the inclusion of other tests for interpretation.
Working with Hunter in 1976, Bell (later Bell-Krotoski) made changes to the test to make consistent peripheral nerve mappings and eliminate variables. Changes included (1) a constant scale of interpretation for the entire upper extremity rather than the interpretation scale changed for thumb, fingers, and palm, (2) eliminating two-point discrimination as a requirement for light touch, (3) eliminating point localization as a requirement for a “yes” response,” and (4) adding consistent colors from cool to warm for quick recognition of increase in force required for detection of touch–pressure. Results of mappings serially compared for changes in neural status could then be easily recognized in seconds numerically and visually for extent and severity of peripheral nerve abnormality. The mappings were found to predict the rate of neural return or diminution, as well as of the quality of neural return or severity of diminution (Figs. 11-9 to 11-11).
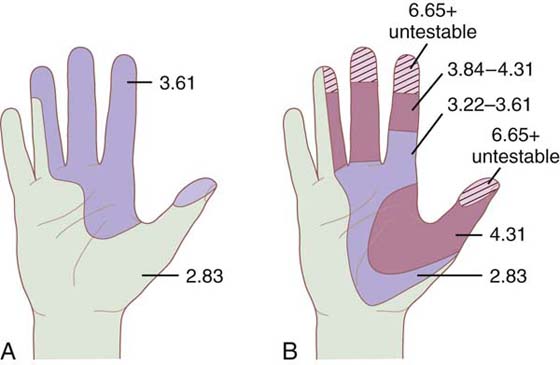
Figure 11-9 A, Monofilament mapping showing a median nerve compression as measured in a woman with a history of numbness for 2 years and no corrective intervention. B, Same patient as measured 4 months later. Touch–pressure recognition has become worse from diminished light touch to untestable with monofilaments in fingertips.
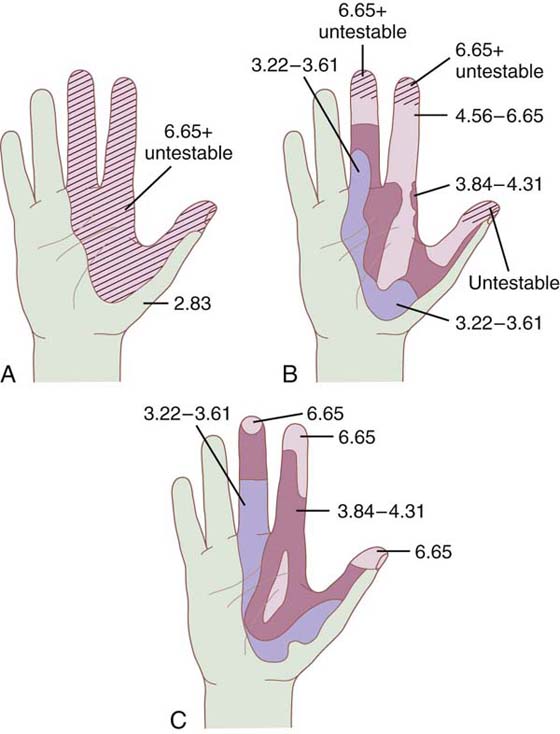
Figure 11-10 A, Monofilament mapping showing a median nerve laceration before surgery. Two-point untestable. B, Same patient 3 months after surgery. Two-point untestable. C, Same patient 7 months after surgery. Two-point untestable, fingertips now testable with monofilaments.
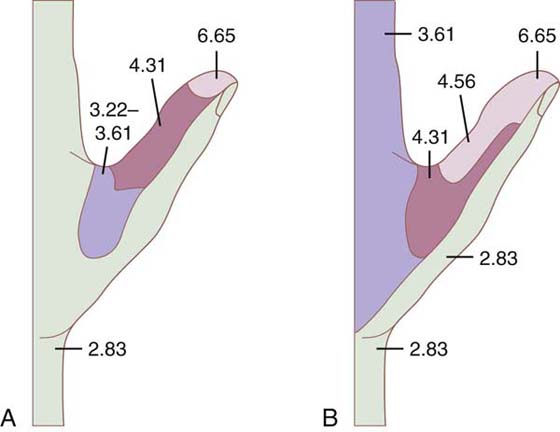
Figure 11-11 A, Two years after incomplete amputation of right thumb. Digital nerves were not resutured. Small centimeter pedicle of dorsal skin was intact. B, Same patient after injection of lidocaine around median nerve to determine whether innervation was radial nerve or median. Notice that although the volar thumb sensation was downgraded, it and the palmar area of the median nerve did not become asensory. This finding brings into question the blocking of the contralateral nerve in testing. Such blocks may be incomplete and lead to false conclusions.
After 2 years of testing with the revised test in a battery of other sensibility tests, it was found that not all of the monofilaments were needed to obtain results in functional levels of sensibility7 (Fig. 11-12). This work was based on over 200 tests of patients with nerve compressions and lacerations at the Hand Rehabilitation Center, Ltd, Philadelphia, PA. The interpretation scale and test protocol were later used in still other studies, becoming the standard in monofilament testing.7
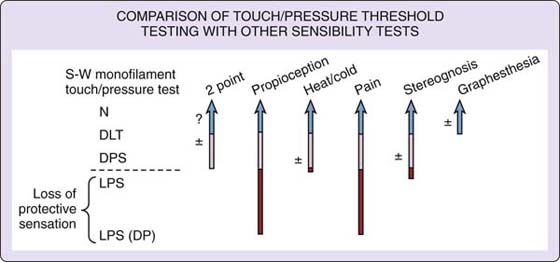
Figure 11-12 Comparison of monofilament touch–pressure detection testing with other sensibility tests.
At the time this work was published in the first edition of this text, the scale of interpretation was found to largely agree with threshold studies of von Prince and colleagues, and that of Omer and Werner in levels of “functional discrimination and recognition.” These independent works are significant in their similar agreement corroborating the relative relationship of monofilament threshold detection with functional discrimination. The hand screen monofilaments evolved from the standard scale of interpretation by selecting the heaviest monofilament falling within normal limits, and one monofilament corresponding with each functional discrimination level.7
All sensibility testing is best performed in a quiet room that has good light and normal room temperature. A quiet testing area is mandatory. The hand/extremity being tested is extended by the patient and comfortably relaxed resting on a rolled towel. A folder or screen is used to block the patient’s line of vision from the site tested. (Theraputty as an anchor and blindfolds are not recommended.)
A thorough patient history helps focus testing where most needed. Unless a higher lesion is suggested, it is often necessary to examine only the hands with the monofilaments, although it is possible to test the entire body when indicated.
Using the 2.83 monofilament it is important to establish a reference area that is within normal limits that the patient always responds to. This can be done while demonstrating the monofilaments and reassuring the patient that they do not hurt. An area more proximal on the extremity or alternate extremity usually suffices, but the back, or face, can also be used. This reference area helps the clinician to eliminate guessing and ensure attention. If the patient does not respond in a test area, then the reference area is revisited in order to ensure there is still a response in the reference area used as a control. Then the test area can be rechecked to confirm that what the patient does not detect in the test area can be detected in the reference area.
On the hand map, colors from cool to warm are used to document the monofilament level detected for each area tested. Each color corresponds with force of application detected and its corresponding functional sensibility level.
Monofilament testing begins with filaments in the normal threshold level and progresses to filaments of increasing force/pressure until the patient can identify touch. The filaments 1.65 to 4.08 are applied three times to the same test spot, with one response out of three considered an affirmative response.
All the filaments are applied in a perpendicular fashion to the skin surface in 1 to 1.5 seconds, continued in pressure in 1 to 1.5 seconds, and lifted in 1 to 1.5 seconds. The filaments 1.65 to 6.45 should bend to exert the specific pressure. The 6.65 filament is relatively stiff and found most repeatable if applied just to bending.
On initial patient testing, the patient’s baseline detection level is established. On subsequent testing, the previous test serves as a comparison to establish the direction of change and improvement or worsening, if any. It is most accurate for the same examiner to repeat successive evaluation, if possible. But if instrument specification, protocol, and technique are kept standard, testing can be repeatable among examiners, whether in Japan or California. Testing by other examiners is often used in a double-blind design for studies.
A hand screen examination facilitates more frequent testing and monitoring of patients over time with treatment. The hand screen monofilament sizes include normal, 2.83 (marking number) 50-mg level; diminished light touch, 3.61 (marking number) 200-mg level; diminished protective sensation 4.31 (marking number) 2-g level; and loss of protective sensation. Two filaments are included for the loss of protective sensation level, the lightest of these is 4.56 (marking number) 4-g level, important to define loss of protective sensation versus lighter diminished protective sensation, and the heaviest 6.65 (marking number) over 300-g level, needed for determining residual sensation or returning nerve function.
Test sites specific to the median nerve are the tip of the thumb, index, and proximal index. (The radial base of the palm is avoided to eliminate innervation from the recurrent branch of the median nerve.) Test sites specific to the ulnar nerve are the distal little finger, proximal phalanx, and ulnar base of the palm. The test site specific for the radial nerve is the dorsal aspect of the thumb webspace. These represent the minimum critical consistent data points for monitoring the peripheral nerves (Fig. 11-13A, B).
The test protocol for the hand screen (can also screen any area of the body) is the same as for mapping, except that fewer test sites are used. Usually monofilaments always used are the five most critical, although sets of six monofilaments are becoming available [including the 5.07 (marking number) 10-g level]. Predetermined, consistent sites are recorded for monofilament response for that site. Because there are limited test sites in a screen test versus a mapping, an examiner revisits a nonresponsive test site at least three times to ensure a monofilament is not detected at that site.
Note and draw on a recording form any unusual appearances on the hands, including sweat patterns, blisters, dry or shiny skin, calluses, cuts, blanching of the skin, and so on.
1. Draw a probe across the area to be tested in a radial-to-ulnar and proximal-to-distal manner. Ask the patient to describe where and if his or her feeling changes. Do not ask for numbness because the patient’s interpretation of numbness varies. Draw the area described as “different” with an ink pen (Fig. 11-14). The examination is easier if the patient can identify the gross area of involvement as a reference; if the patient cannot, proceed the same way on testing but allow more testing time.
2. Establish an area of normal sensibility as a reference. Familiarize the patient with the filament to be used and demonstrate it in a proximal control reference area. Then, with the patient’s eyes occluded, apply the filament in the reference area until the patient can easily identify the 2.83 (marking number) 50-mg level monofilament. Test the involved hand (volar surface) by applying the same filament (2.83) to the fingertips first and working proximally. Dot the spots correctly identified with a green felt-tip pen. (Explain to the patient that one touch is a marking of the pen.) In general, the patient is tested distally to proximally, but a consistent pattern is not used to avoid patient anticipation of the area to be touched. When all the area on the volar surface of the hand that can be identified as within normal limits is marked in green, proceed to the dorsum of the hand and test in the same fashion. Because the sensibility on the dorsum of the hand is not always as well defined as the volar surface, it is easier to establish areas of decreased sensibility on the volar surface first. Now the gross areas of normal and decreased sensibility have been defined.
3. Return to the volar surface of the hand. Proceed to the filaments within the level of diminished light touch (see Tables 11-1 and 11-2, and color maps Figs. 11-9 to 11-11), but change the color of the marking pen for this level to blue. Test as discussed earlier in the unidentified areas remaining, working again first on the volar surface and then on the dorsum.
4. If areas remain unidentified, proceed to the filaments in the diminished protective sensation level (purple) and then loss of protective sensation level (red) and continue testing until all the areas have been identified.
5. Record the colors and filament numbers on the report form to produce a sensory mapping. (Color and mark hands on the form.) Note any variations and unusual responses, especially delayed responses. Delayed responses (more than 3 seconds) are considered abnormal and should be noted. Note the presence and direction of referred touch with arrows.
Within normal limits is the level of normal recognition of light touch and therefore deep pressure.64
Diminished light touch is diminished recognition of light touch. If a patient has diminished light touch, provided that the patient’s motor status and cognitive abilities are intact, he or she has fair use of the hand; graphesthesia and stereognosis are both close to normal and adaptable; he or she has good temperature appreciation and definitely has good protective sensation; he or she most often has fair to good two-point discrimination; and the patient may not even realize he or she has had a sensory loss.
Diminished protective sensation is diminished use of the hands, difficulty manipulating some objects, and a tendency to drop some objects; in addition, the patient may complain of weakness of the hand, but still have an appreciation of the pain and temperature that should help keep him or her from injury, and the patient has some manipulative skill. Sensory reeducation can begin at this level. It is possible for a patient to have a gross appreciation of two-point discrimination at this level (7–10 mm).
Loss of protective sensation is compromised use of the hand; a diminished, if not absent, temperature appreciation; an inability to manipulate objects outside line of vision; a tendency to be injured easily; and potential danger for the patient with sharp objects and around machinery. Instructions on protective care are needed to prevent injury.
Deep-pressure sensation is a rudimentary deep pressure detected with the heaviest monofilament. Patients describe this as a sensation of heavy weight, but without any other tactile discrimination. The patient still has deep pressure recognition, which does not make the affected area totally asensory. Instructions on protective care are critical to prevent injury.
Untestable is no response to monofilament threshold testing. A patient may or may not feel a pinprick but has no other discrimination of levels of feeling. If a patient feels a pinprick in an area otherwise untestable, it is important to note that some potential nerve response is still present. Instructions on protective care are critical to prevent the normally occurring problems associated with the asensory hand.
Further interpretation of the effect that a decrease or loss of sensibility has on patient function depends on the area and extent of loss and whether musculature is diminished. Light-touch/deep-pressure threshold measurements can be used to consider the need for treatment, changes in treatment, and the success of treatment.
Maintenance of protective sensation is a major goal for preventing injury from loss of sensory feedback. Patients with loss of protective sensation are injured easily and experience repetitive injuries, which can lead to lifelong psychological stress, as well as deformity and disability48,56,68,75,76 Certainly, when it comes to sensory abnormality and functional discrimination or recognition, whether or not protective sensation is present is a defining factor in patient treatment because it determines if the patient is still relatively safe with sharp or hot objects, or conversely, in danger of wounds and amputations from complications of burns and injuries that could be incurred through use of everyday objects.
A computer coding method was developed in 1984, for monitoring large numbers of HD patients in the United States and for overseas projects.61 This relatively simple method is available for coding patient peripheral nerve status. Data can be digitized for computer analysis by giving each filament a weighted score similar to muscle testing, where 5 = normal, 4 = fair, 3 = good, 2 = poor, and 1 = trace. In sensibility coding, green, 2.83 (marking number) = 5; blue, 3.61 (marking number) = 4; purple, 4.31 (marking number) = 3; red, 4.56 (marking number) = 2; red-orange, 6.65 (marking number) = 1; no response equals zero. Scores can be totaled for each nerve and overall. A normal hand then would have a score of 15 for the median nerve, 15 for the radial nerve, and 5 for the radial, for a total of 35 points.39 Computer entry and grading can be done in Microsoft Excel or Access programs to record and analyze hand screen site response.
The optimally designed computerized instrument automatically applies and controls the stimulus with a built-in limit on how much force is applied.9,15,69,77,78 Computerized instruments reported to be accurate and sold for clinical testing of patients—just as hand-held instruments—need to have their forces of application measured and these measurements made available along with other instrument specifications.63
If the instrument still depends on a hand-held application of the stimulus, it is still subject to the same limitations of any hand-held instrument.13,79-81 Computer averaging of force stimuli can hide peaks of higher force and examiner hand vibration79,80,82 (Fig. 11-15).
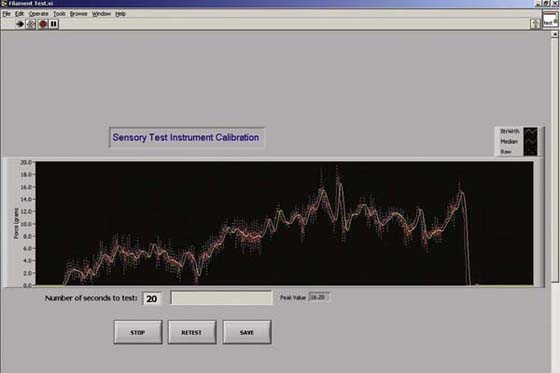
Figure 11-15 Variable force versus time application—significantly variable force which results from holding a probe from any hand-held instrument without force control.
Two-point discrimination is a classic test of sensibility used by hand surgeons over several decades.83-85 The test is believed by many to be a test of innervation density. Some think two-point discrimination a good predictor of patient function and manipulation. It does follow that once normal two-point discrimination has returned to the fingertips after nerve repair, the quality of sensibility is good. The test overall has yet to be related to the presence or absence of protective sensation.
At one time it was popular to use a paper clip to test two-point discrimination, but this is not recommended. The probe tips should be blunt, of the same geometry, and not so sharp that pain is elicited. Commonly available hand-held two-point discrimination test instruments range from the relatively light Disk-Criminator (P.O. Box 16392, Baltimore, Maryland),14 to a heavy Boley Gauge (Boley Gauge, Research Designs, Inc., Houston, Texas) (Fig. 11-16).
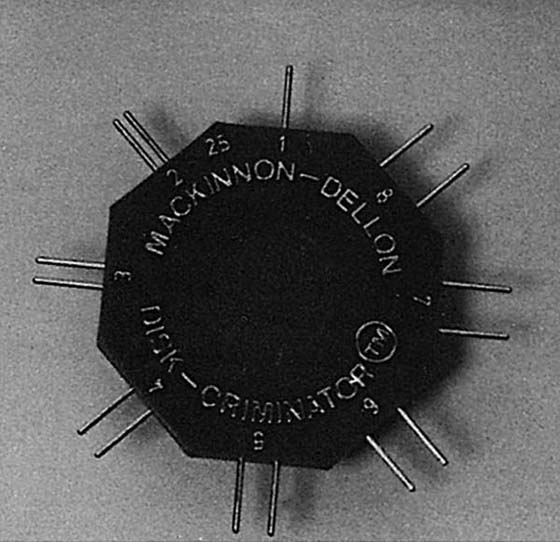
Figure 11-16 Disk-Criminator. The weight of the instrument improves control on force of application but does not totally eliminate variable force from hand-held application.
In addition to touch–pressure thresholds, Weinstein published a table for two-point discrimination thresholds all over the body (Fig. 11-17). The widely variable pattern for two-point discrimination does not correlate with touch detection threshold (0.17), but does correlate and is almost identical to that for localization of touch (0.92).51 With common scoring methods, two-point discrimination testing is most accurate at the fingertips. The clinician should know that studies have found subjects with entrapments and compressions in which two-point discrimination is normal, but monofilament and nerve conduction testing are abnormal.27,70
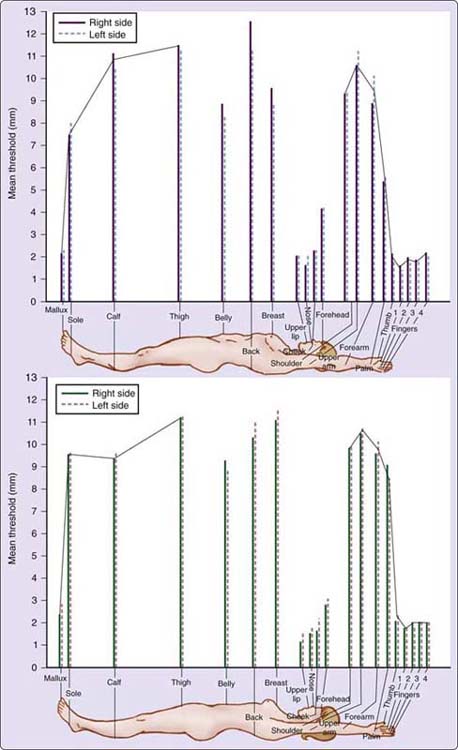
Figure 11-17 Weinstein’s two-point discrimination body thresholds. (Reprinted, with permission, from Bell-Krotoski JA. Advances in sensibility evaluation. Hand Clin. 1991;7:534, Figure 11-3; and Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: Kenshalo DR, ed. The Skin Senses. Springfield, Ill, Charles C Thomas. 1968; pp 195–222.)
The known uncontrolled variable in two-point discrimination testing is the unspecified force at which all of the hand-held two-point discrimination instruments are applied. Unless the instruments are force-controlled for hand-held application, they are relatively incapable of producing repeatable application force. Different instruments vary in weight and configuration, and the test probes vary. For this reason the examiner should always use the same instrument for repeated testing.
Many think the two-point discrimination test is objective and calibrated because it can be numerically adjusted to repeatable distances at its probe tips for measurement.63 Two-point discrimination may be a very good test if force-controlled, but most hand-held instruments do not provide the opportunity to produce consistent repeatable data13,63 (Fig. 11-18). Specifying the stimulus in pressure (pressure being force/unit area) is no more accurate than force calculation. The “moving” two-point discrimination test is more uncontrolled in force of application (or pressure) because of the varying topography of the finger as the test probes are moved across the joints and fingertip. Both “static” and moving forms of testing need limits on the force applied, or at a minimum to show that clinical results from testing are not different when applied with various force/pressures and instruments.
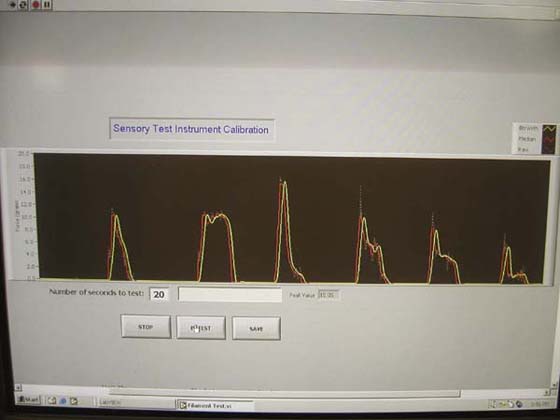
Figure 11-18 Variable force from conventional two-point discrimination instruments. Six applications to Sensory Instrument Measurement System.
A prototype controlled two-point discrimination instrument has been designed using heavier monofilaments that hold their distance when on a sliding scale (available at timelyneuropathytesting.org; patent pending) (Fig. 11-19). This is a force-controlled two-point discrimination design which can apply 5 g, 10 g, or 30 g of force.
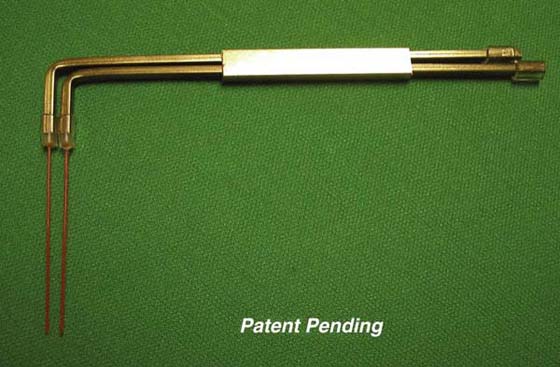
Figure 11-19 Design for controlled monofilament two-point discrimination instrument, showing that force control design for two-point discrimination is possible and could potentially improve the test. (Bell-Krotoski JA: Device for evaluating cutaneous sensory detection, notice of publication of application, United States Patent and Trademark Office, US-2009-0105606-A1, 2009.)
Two-point discrimination testing has been clinically useful despite the lack of control of the application force. The potential for greater usefulness depends on the development of instruments that are designed with the force of application controlled.71,86
Weber invented the two-point discrimination test.74 Moberg and others have advocated that the test is closely related to “tactile gnosis” and functional ability of the patient to use the hand for fine motor and skilled tasks.73,87 Dellon introduced the moving two-point discrimination test.83 His objective and rationale is that fingertip sensibility is highly dependent on motion.
Moberg agreed with the need for force control of two-point discrimination instruments after hearing a critique of the force-control weaknesses in two-point discrimination instruments, suggesting a design for a prototype instrument that would use 5- and 10-g weights.71,86
Usually only the fingertips are assessed in static two-point discrimination testing, as norms vary widely farther up the extremity. The patient’s hand should be fully supported on a towel. The examiner should take care not to touch the patient’s hand with anything except the instrument, as touch by the examiner adds extraneous touch stimuli and may confuse the patient. A very light application of two versus one point of the instrument is used. Vision is occluded—usually by obscuring the line of vision with a folder rather than a blindfold. Results can be recorded on a hand screen form or hand drawing.
Testing begins with 5 mm of distance between the two points in a random sequence with one point applied in a longitudinal orientation to avoid overlapping the innervation zones of the digital nerves. The point of blanching has been suggested as a control for force of application, but this is problematic as blanching has been found to occur at different forces on different fingers and tissue areas, somewhat dependent on condition of the skin.13 Seven of 10 responses is necessary to be considered accurate. If there is no response or an inaccurate response, the distance between the ends is increased by increments of 1 mm, until 7 of 10 responses are accurate. Testing is stopped at 15 mm.
Normal two-point discrimination is considered less than 6 mm, fair is 6 to 10 mm, and poor is 11 to 15 mm (see guidelines of the American Society for Surgery of the Hand,7 and International Federation of Hand Surgery Societies).75,88
In the moving two-point discrimination test, testing is begun with an 8-mm distance between the two instrument tip points.83 The instrument is moved parallel to the long axis of the finger (testing ends side by side). Testing begins proximal to distal toward the fingertip. For a correct response, the patient has to respond accurately to 7 of 10 stimuli of one or two points, before the distance is narrowed for testing with a smaller distance. Testing is stopped at 2 mm, which is considered normal.
The moving two-point stimulus is more easily detected than the static two-point. Several authors have reported correlation between moving two-point discrimination and object recognition.11,83
Dellon recommends the pressure-specified sensory device (PSSD) as optimal for two-point and moving two-point discrimination testing (PSSD, Post Office Box 16392, Baltimore, MD).79,80,89 For the specific testing technique for the PSSD, the reader is referred to literature available with the instrument.80,89 Dellon, based on two-point discrimination research with the PSSD, reports finding that clinical results can be quite different at various thresholds of pressure (force) of application.80,89 Other computerized force and pressure-controlled devices have been tried experimentally.69,90 Used for research, these may help determine optimum target thresholds for two-point discrimination testing.13,81,91-93
The objective of using a point localization test after nerve injury or repair is to determine the accuracy of localization of a touch stimulus and when inaccurate to determine and record the direction and distance in centimeters to another point, area, or finger to which the touch is referred. The regenerating peripheral nerve after laceration or suture does not always find and innervate the same mechanoreceptor end organs in the skin, and until a certain density of endings has returned, insufficient data is available for a patient to accurately determine localization. Localization generally improves over time as nerve healing and regeneration progresses, but localization also requires an integrated level of perception and cortical interpretation by the patient (Fig. 11-20).
Weinstein tested normal subjects and published a table for localization of touch sensitivities all over the body. The variable pattern for localization in normal subjects is quite different from that found for touch detection threshold, but very similar to that found for two-point discrimination. The ability to localize in 48 normal adults was found to have a high correlation with two-point discrimination thresholds (0.92), but did not have high correlation with light-touch threshold (0.28).51
Types of test instruments used have varied from dowels, used by Werner and Omer, to monofilaments. The monofilaments do provide a repeatable force of application and therefore are recommended for a stimulus probe. Whatever instrument is used, it should be reported and used consistently for the same and subsequent patients in order to be the most meaningful.
Localization early after nerve repair is poor and generally improves with time and use of the affected part. Poor localization after nerve repair can seriously limit function. Localization may vary with the cognitive ability of a patient to adapt to new sensory pathways more than as a result of the actual level of return of the nerve and its response to touch stimuli.
Shortly after a lacerated nerve begins to show a Tinel’s94 sign distal to the suture line, a touch with a probe or heaviest monofilament may be detected by the patient (pressure recognition), but is not usually correctly localized. When asked, a patient may refer to another finger or area. Some patients never regain normal point localization, but most regain area localization.59 Few would contest that improvement in point localization is faster and better if the affected area is used for grasp and manipulation, and in the reverse, is slower, and of poor quality if not frequently used after repair.
Errors in localization can frequently be reduced in one treatment session with reeducation, indicating that the change is in part relearning, rather than a physiologic change in the nerve. The research of Rosen and Lundborg regarding the plasticity of the brain supports this concept.95
Localization can easily be tested and recorded on a hand screen form after other sensibility tests such as monofilament testing to help further determine the quality of sensibility for patients who have had nerve lacerations.96 Localization should be tested separately and not used as a requirement with other tests of sensibility. This was once the procedure with monofilament testing but it confounded the results of testing and their interpretation.7,16
As the repair matures, that skin area initially reinnervated subsequently tends to improve toward lighter touch threshold detection. As touch–pressure recognition improves, the distance the touch is referred tends to lessen and can be recorded in millimeters.
Werner and Omer described differences in both area and point localization, with area localization being the first to return. In area localization, after being touched, the patient responds by indicating the area that was touched. In point localization, the patient responds by covering the point touched with a wooden dowel within a centimeter.
In a recommended test protocol by Callahan, the lightest Semmes–Weinstein monofilament perceived is used for testing localization over the involved area.15 A grid divided into zones is used for recording the results of this test on standard hand or arm recording forms.16,97 With the patient’s vision occluded, the monofilament is applied. Patients are instructed to open their eyes and point to the exact spot touched. If inaccurate, the distance from the correct spot is measured in millimeters. Arrows are drawn on the recording form from the correct stimulus point to the incorrect point indicated by the patient. If the stimulus is correctly localized, a dot is marked on the recording form. Nakada96 provides a method to more objectively document and score errors by using a 4.17 (marking number) Semmes–Weinstein monofilament and measuring errors in localization with a vernier caliper.
The objective of a test for vibration is to determine frequency response of mechanoreceptor end organs. Some neurologists believe that vibration as a separate sense does not exist, but is rather the perception of variable changes in stimuli.82 Dellon has advocated 30- and 256-Hz tuning forks for measuring patient response to vibration.63 However, the vibratory stimulus with the tuning forks is not controlled and the stimulus varies with the examiner’s technique and force of application.
Szabo,98 and Gelberman,27 have investigated vibratory sensory testing with computerized instruments, but these are not sufficiently controlled in force of application and vibration to be recommend for clinical use. Horch developed a computerized instrument with control of applied force of its probe that has been used by Hardy and coworkers, and Lundborg has reported results with a force-controlled computerized instrument, but these are not yet commercially available9,69,90,99
Monofilament threshold detection levels can predict patient function and thus are a first-order functional assessment. Results of testing can help focus reeducation on what is realistic relative to the degree of physiologic nerve return.7,54,55,58,59,100 For example, light touch is necessary for the epicritic quality of sensibility. In addition, protective sensation, at a minimum, is especially important to prevent injury to hands during use. Instruction in protective techniques to prevent injury to the skin is needed until protective sensation has returned to the fingertips in the affected area. But touch–pressure threshold testing does not include measurement of secondary skill and adaptation.12,70 Human brains adapt very quickly after injury, and even without nerve return patients exhibit varying adaptations and use of extremities.
The classic Moberg pickup test87 is an observational object recognition test requiring motor participation and is most appropriate for median or combined median and ulnar nerve lesions. The Functional Performance Sensory Test (FPST) is standardized, but is not specific for nerve change.56 A still relatively untapped frontier for clinicians is the development of standardized functional skill assessments.
Functional skill tests are particularly indicated when the objective of testing is to determine an injured individuals’ potential for return to life activity and work.101,102 These can indicate the need for sensory reeducation and training or retraining (as long as the test used for reeducation is not also used for the primary assessment in the same patient).83,92,93,103
Disagreement among clinical investigators about which instrument best tests patient function frequently results from the fact that they are considering both patient physiologic function (which needs to be measured without cortical reasoning and accommodation) and patient adaptation (which includes coping and learned skill). Whereas a patient’s level of physiologic function can often predict tactile gnostic function, which correlates in general with patient functional performance, his or her ability to cope with change and the degree of adaptation cannot be used as direct measurement of neural status.56 Both physiologic function and adaptive function are important, but they need to be clearly defined and considered separately.
Moberg highlighted the need for the hand to be used after injury, referring to the hand without median and ulnar nerve sensibility as “blind.”84,87,104 He considered a loss in sensibility to be greatly underestimated and argued to rate the total loss of sensibility in the palm of the hand as 100% disability. He spoke of sensibility as a tactile “gnosis,” with the hand being the “eye” for the body and touch.
The now classic Moberg pickup test uses an assortment of common office objects placed on a table (paper clip, piece of cotton, etc.). The patient is instructed to quickly pick up the objects and place them into a box, first with the involved hand, then with the uninvolved, while the examiner times and observes. This process is repeated with the patient’s eyes closed. With eyes closed, the patient tends not to use the fingers or other sensory surfaces with poor sensibility. Ng and colleagues105 and others have proposed a standard protocol using standard objects for administering the test. Dellon86 modified the pickup test by standardizing the items used and requiring object identification (Fig. 11-21). No more than 30 seconds is permitted to identify the object, which is presented twice. King correlated the results of Semmes–Weinstein-style monofilament testing in carpal tunnel syndrome patients with their response times for texture and object recognition and found a significant association between level of touch perceived and time required to identify the test items.106
Waylett designed a standard tactile discrimination test.107 The Flinn Functional Performance Sensory Test (FPST) is a standard and objective test of functional performance that is sensitive but not specific for sensory abnormality.56,75,101
Onne108 and Richards109 describe vasomotor and nutritional aspects of peripheral nerve function. Sudomotor function is important for hydrating and lubricating the skin and maintaining its normal pliability. When examined, the skin in an area with abnormal sensibility also often has changes in sweat and vascularity. Affected skin that does not receive specific care is dry to the touch and can crack easily, leading to infection. Soaking of the skin and applying an oil before drying is an effective way of maintaining suppleness and skin condition.68
The objective of using Moberg’s ninhydrin sweat test is to help document sweat and indicate change in sensibility in early injury.50,110,111 Moberg scored ninhydrin test results on a scale of 0 to 3, with 0 representing absent sweating and 3 representing normal sweating.21 Perry112 and Phelps97 describe a commercially available ninhydrin developer and fixer. The patient’s hand is cleaned, rinsed, and wiped with ether, alcohol, or acetone with a minimum of a 5-minute waiting period during which nothing contacts the fingers. Then the fingertips are placed against the bond paper for 15 seconds and traced with a pencil. The paper is sprayed with ninhydrin spray reagent (N-0507) (Sigma Chemical Company, St. Louis, Missouri) and dried for 24 hours or heated in an oven for 5 to 10 minutes at 200°F (93°C). After development, the prints are sprayed with the ninhydrin fixer reagent (N-0757). In a normal print, dots representing sweat glands can be clearly seen, and the lack of dots indicates a lack of sweating.
The objective of O’Riain’s wrinkle test is to demonstrate a lack of wrinkling.113 It was observed that a denervated hand placed in warm water (40°C; 104°F) for 30 minutes does not wrinkle. This test is rated using a 0 to 3 scoring system. It is most useful for determining areas of denervation from lacerations in adults who cannot be responsive and in young children. Phelps noted that wrinkling can return without return of sensibility, so this should be used early after injury.97
The objective of thermal testing is to demonstrate temperature recognition. Temperature sensibility, like pain, is difficult to quantify precisely.114 Normal discrimination between hot and cold temperatures has been reported to be within 5°C, but hot and cold test tubes as used by clinicians greatly exceed this range. Diminished temperature recognition can be shown to occur when touch–pressure detection thresholds are at the diminished and loss of protective sensation levels.115 Most forms of temperature testing are relatively gross, however, and not capable of revealing small changes, which may better correlate with lighter levels of touch–pressure change. Controlled and sensitive laboratory instruments that can measure temperature and pain are available to neurophysiologists and other researchers, but these are expensive and primarily used experimentally. Instrument reliability in these would need to be improved before clinical use, as some have been found to change in stimulus with various electrical voltages or change with the charge of their battery.66
Early detection of developing nerve problems offers the best opportunity for improvement.48,58,116,117 At a minimum, Semmes–Weinstein-style monofilament testing using hand screen size monofilaments (for hand and body) is recommended. The following battery is recommended after obtaining a thorough patient history. The tests should be administered in a manner designed to minimize variables, and they should be knowledgeably interpreted. Optimally, testing is done both before and after treatment intervention, including surgery, in order to clearly show the direction of change, whether improvement, the same, or worsening. Testing is repeated using a standard protocol at intervals specific to the individual case.
For Nerve Lesions in Continuity
• NCV to help define site of involvement and severity of slowed or absent conduction
• Semmes–Weinstein-style monofilament hand screen or mapping to determine the touch–pressure threshold in the involved areas
• Static and moving two-point discrimination (optional for comparison)
• For patients with intermittent symptoms, stress testing with provocative activity or positioning (in coordination with referring physician) followed with repeat NCV or Semmes–Weinstein-style touch–pressure threshold tests
• Functional and other tests as indicated by need
• Examination of the hand for evidence of sympathetic dysfunction
• Tinel’s test distal to the repair to determine distal progression of regenerating axons
• Semmes–Weinstein-style monofilament hand screen or mapping to assess level and area of touch–pressure return and to reveal changes over time
• Pinprick test if tested areas are unresponsive to the thickest diameter (6.65 marking number), 300-g+ level Semmes–Weinstein-style monofilament
• Static and moving two-point discrimination tests on the fingertips (if indicated)
• Touch localization testing distal to nerve repair
• Dellon modification of the Moberg pickup test for median or median and ulnar nerve dysfunction
• Functional tests and use assessment of the hand in activities of daily living (FPST where available) 101
Note: For a child younger than 4, the wrinkle test, possibly the ninhydrin sweat test, and the Moberg pickup test may provide the best information.
The clinician needs to be a peripheral nerve detective and begin an evaluation with screening of all of the peripheral nerves of the upper extremity, even when a referral has been made to test a specific nerve and level. Accurate testing and common understanding of the need for thorough evaluation among the surgeons, therapists, and others involved in the peripheral nerve assessment facilitates early diagnosis and accurate resolution of developing nerve problems. Data from consistent tests can be numerically quantified and compared among measurements, following treatment, and among patient groups. Semmes–Weinstein-style monofilament screening or mapping of detection thresholds enables the examiner to “see” what is otherwise invisible. Hand-held instruments without sufficient control on their application do not produce repeatable results and are therefore invalid in clinical testing. Many of our traditional hand-held tests need to have a means of control developed for their test stimulus. Computerized test instruments could help to eliminate the uncontrolled variables of hand-held tests. But computerized instruments must also meet sensitivity and repeatability requirements for objective testing. NCV and sensibility tests together hold the potential to improve patient outcome by enabling earlier recognition of developing problems and intervention at a point before nerve damage is irreversible.
1. Greenspan JD, LaMotte RH. Cutaneous mechanoreceptors of the hand: experimental studies and their implications for clinical testing of tactile sensation. J Hand Ther. 1993;6(2):75–82.
2. LaMotte RH. Assessment of Cutaneous Sensibility, Sensory Discrimination and Neural Correlation. Carville, LA: Symposium presented at National Hansen’s Disease Center; 1980.
3. LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate between vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychophysical measurements. J Neurophysiol. 1975;38:539–559.
4. LaMotte RH, Srinivasan MA. Tactile discrimination of shape: responses of quickly adapting mechanoreceptive afferents to a step stroked across the monkey fingerpad. J Neurosci. 1987;8:1672–1697.
5. Looft FJ, Williams WJ. On-line receptive field mapping of cutaneous receptors. Trans Biomed Eng BME. 1979;26:350–356.
6. Werner G, Mountcastle VB. Neural activity in mechanoreceptive cutaneous afferents: stimulus-response relations, Weber functions and information transmission. J Neurophysiol. 1965;28:359–397.
7. Bell JA. Sensibility evaluation. In: Hunter JM, ed. Rehabilitation of the Hand. St Louis: Mosby; 1978:273–291.
8. Brand PW. Rehabilitation of the hand with motor and sensory impairment. Orthop Clin North Am. 1973;4:1135–1138.
9. Jimenez S, Hardy MA, Horch K, Jabaley M. A study of sensory recovery following carpal tunnel release. J Hand Ther. 1993;6(2):124–129.
10. MacDermid JC, Kramer JF, Roth JH. Decision making in detecting abnormal Semmes-Weinstein monofilament thresholds in carpal tunnel syndrome. J Hand Ther. 1994;7:158–162.
11. Novak CB, Patterson JM, Mackinnon SE. Evaluation of hand sensibility with single and double latex gloves. Plast Reconstr Surg. 1999;103:128–131.
12. Rosén BL. Recovery of sensory and motor function after nerve repair: a rational for evaluation. J Hand Ther. 1996;9:315–327.
13. Bell-Krotoski J, Buford W Jr. The force/time relationship of clinically used sensory testing instruments. J Hand Ther. 1988;1:76.
14. Breger D. Correlating Semmes-Weinstein monofilament mappings with sensory nerve conduction parameters in Hansen’s disease patients: an update. J Hand Ther. 1987;Oct-Dec:33–37.
15. Callahan AD. Sensibility assessment for nerve lesions in continuity and nerve lacerations. In: Mackin EJ, Callahan AD, Skirven TM, eds., et al. Rehabilitation of the Hand and Upper Extremity. 5th ed St Louis: Mosby; 1995:214–239.
16. Fess E. The need for reliability and validity in hand assessment instruments. J Hand Surg. 1986;11A:621–623.
17. Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 3rd ed Philadelphia: WB Saunders; 1993.
18. Gilliat RW, Harrison MJG. Nerve compression and entrapment. In: Asbury AK, Gilliat RW, eds. Peripheral Nerve Disorders. London: Butterworths; 1984.
19. Haymaker W, Woodhall B. Peripheral Nerve Injuries: Principles of Diagnosis. 2nd ed Philadelphia: WB Saunders; 1953.
20. Voerman VF, van Egmond J, Crul BJ. Normal values for sensory thresholds in the cervical dermatomes: a critical note on the use of Semmes-Weinstein monofilaments. Am J Phys Med Rehabil. 1999;78:24–29.
21. Chochinov RH, Onyot LE, Moorehouse JA. Sensory perception thresholds in patients with juvenile diabetes and their close relatives. N Engl J Med. 1972;286(32):1233–1237.
22. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7:547–556.
23. Gillenson SP, Parets N, Bear-Lehman J, Stanton DB. The effect of wrist position on testing light touch sensation using the Semmes-Weinstein pressure aesthesiometer: a preliminary study. J Hand Ther. 1998;11:27–31.
24. Koris MR, Gelberman RH, Duncan K, et al. Carpal tunnel syndrome: evaluation of a quantitative provocational diagnostic test. Clin Orthop Relat Res. 1990;251:157–161.
25. Read R. Stress testing in nerve compression. Hand Clin. 1991;7:521–526.
26. Almquist E, Eeg-Olofsson O. Sensory nerve conduction velocity and two-point discrimination in sutured nerves. J Bone Joint Surg. 1970;52A:791–796.
27. Gelberman RH, Szabo RM, Williamson RV, Dimick MP. Sensibility testing in peripheral nerve compression syndromes: an experimental study in humans. J Bone Joint Surg. 1983;65A:632–638.
28. Omer GE Jr. Median nerve compression at the wrist. Hand Clin. 1992;8:317–324.
29. Omer GE Jr. Sensation and sensibility in the upper extremity. Clin Orthop Relat Res. 1974;104:30–36.
30. Luoma A, Nelems B. Thoracic outlet syndrome: thoracic surgery perspective. Neurosurg Clin N Am. 1991;2:187–226.
31. Roos DB. The thoracic outlet syndrome is underrated. Arch Neurol. 1990;47:327–328.
32. Wilbourn AJ. Thoracic outlet syndromes: a plea for conservatism. Neurosurg Clin N Am. 1991;2:235–245.
33. Hunter JM. Recurrent carpal tunnel syndrome, epineural fibrous fixation, and traction neuropathy. Hand Clin. 1991;7:491–504.
34. Hunter JM, Read RL, Gray R. Carpal tunnel neuropathy caused by injury: reconstruction of the transverse carpal ligament for the complex carpal tunnel syndromes. J Hand Ther. 1993;6(2):145–151.
35. Bell-Krotoski JA. “Pocket filaments” and specifications for the Semmes-Weinstein monofilaments. J Hand Ther. 1993;3(1):26–29.
36. Costigan DA, Wilbourn AJ. The elevated arm stress test: specificity in the diagnosis of thoracic outlet syndrome. Neurology. 1985;35 (Suppl):74. (poster abstract)
37. Kets CM, Van Leerdam ME, Van Brakel WH, et al. Reference values for touch sensibility thresholds in healthy Nepalese volunteers. Lepr Rev. 1996;67:28–38.
38. Lehman LF, Orsini MB, Nicholl AR. The development of the Semmes-Weinstein monofilaments in Brazil. J Hand Ther. 1993;6:290–297.
39. Bell-Krotoski JA. Correlating sensory morphology and tests of sensibility with function. In: Hunter JM, Schneider LH, Mackin EJ, eds. Tendon and Nerve Surgery in the Hand. St. Louis: Mosby; 1997:49–62.
40. Naafs B, Dagne T. Sensory testing: a sensitive method in the follow-up of nerve involvement. Int J Lepr Other Mycobact Dis. 1978;45:364–368.
41. Premkumar RE, Daniel E, Suneetha S, Yovan P. Quantitative assessment of facial sensation in leprosy. Int J Lepr Other Mycobact Dis. 1998;66:348–355.
42. Szabo R, Chidgey L. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg. 1989;14A:624–627.
43. Szabo RM, Gelberman RH, Dimick MP. Sensibility testing in patients with carpal tunnel syndrome. J Bone Joint Surg. 1984;66A:60–64.
44. Gelberman RH, Pfeffer GB, Galbraith RT, et al. Results of treatment of severe carpal tunnel syndrome without internal neurolysis of the median nerve. J Bone Joint Surg. 1987;69A:896–903.
45. Lundborg G, Gelberman RH, Minteer-Convery M, et al. Median nerve compression in the carpal tunnel: functional response to experimentally induced controlled pressure. J Hand Surg. 1982;7:252–259.
46. Jeng CJ, Michelson J, Mizel M. Sensory thresholds of normal human feet. Foot Ankle Int. 2000;21:501–504.
47. Kuipers M, Schreuders T. The predictive value of sensation testing in the development of neuropathic ulceration on the hands of leprosy patients. Lepr Rev. 1994;65:253–261.
48. Brand PW. Coping with a chronic disease: the role of the mind and spirit. Patient Education Counsel. 1995;26(1):107–112.
49. Trumble TE, Venderhooft E, Khan U. Sural nerve grafting for lower extremity nerve injuries. J Orthop Trauma. 1995;9:158–163.
50. Semmes J, Weinstein S, Ghent G, et al. Somatosensory Changes After Penetrating Brain Wounds in Man. Cambridge, Mass: Harvard University Press; 1960.
51. Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: Kenshalo DR, ed. The Skin Senses. Springfield, Ill: Charles C Thomas; 1968:195–218.
52. Weinstein S. Tactile sensitivity of the phalanges. Percept Motor Skills. 1962;14:351–354.
53. van Turnhout AA, Hage JJ. Lack of difference in sensibility between the dominant and non-dominant hands as tested with Semmes-Weinstein monofilaments. J Hand Surg [Br]. 1997;22:768–771.
54. Bell JA, Buford WL Jr. Assessment of levels of cutaneous sensibility. Presented at the Sixteenth Annual Meeting of the United States Public Health Professional Association, Houston. 1979.
55. Bell-Krotoski JA. Device for evaluating cutaneous sensory detection, notice of publication of application, United States Patent and Trademark Office, US-2009-0105606-A1. 2009.
56. Bell-Krotoski JA. Psychological Aspects of Sensibility Impairment: a thesis, Texas Woman’s University. 2009.
57. Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments: a comparative study. J Hand Ther. 1995;8:155–162.
58. von Prince K, Butler B. Measuring sensory function of the hand in peripheral nerve injuries. Am J Occup Ther. 1967;21:385–395.
59. Werner JL, Omer GE. Evaluating cutaneous pressure sensation of the hand. Am J Occup Ther. 1970;24(5):347–356.
60. Bell-Krotoski JA. Advances in sensibility evaluation. Hand Clin. 1991;7:527–546.
61. Bell-Krotoski JA. A study of peripheral nerve involvement underlying physical disability of the hand in Hansen’s disease. J Hand Ther. 1992;3:133–142.
62. Levin S, Pearsall G, Ruderman RJ. Von Frey’s method of measuring pressure sensibility in the hand: an engineering analysis of the Weinstein-Semmes pressure aesthesiometer. J Hand Surg. 1978;3:211–216.
63. Bell-Krotoski J, Weinstein S, Weinstein C. Testing sensibility, including touch-pressure, two-point discrimination, point localization, and vibration. J Hand Ther. 1993;6(2):114–123.
64. Bell JA, Buford WL Jr. Comparison of forces and interpretation scales as used with the von Frey Aesthesiometer. Paper presented at Hand Surgery Correlated with Hand Therapy Meeting, Philadelphia. 1978.
65. Bell JA, Tomancik E. Repeatability of testing with Semmes-Weinstein monofilaments. J Hand Surg. 1987;12A:155–161.
66. Weinstein S. Fifty years of somatosensory research from the Semmes-Weinstein monofilaments to the Weinstein enhanced sensory test. J Hand Ther. 1993;6(1):11–22.
67. van Vliet DC, Novak CB, Mackinnon SE. Duration of contact time alters cutaneous pressure threshold measurements. Ann Plast Surg. 1993;31:335–339.
68. Brand PW. Symposium, Assessment of Cutaneous Sensibility. Comments of Chairman. Carville, La: National Hansen’s Disease Center; 1980.
69. Hardy M, Jimenez S, Jabaley M, Horch K. Evaluation of nerve compression with the Automated Tactile Tester. J Hand Surg. 1992;17:838–842.
70. Rosen BL, Dahlin LB, Lundborg G. Assessment of functional outcome after nerve repair in a longitudinal cohort. Scand J Plast Reconst Surg Hand Surg. 2000;43:71–78.
71. Omer GE Jr. Bell-Krotoski JA. Sensibility testing. In: Omer GE, Spinner M, Van Beeks AL, eds. Management of Peripheral Nerve Problems. 2nd ed Philadelphia: WB Saunders; 1998:11–28.
72. Von Frey M. Zur Physiologie der Juckempfindung. Arch Neurol Physiol. 1922;7:142–145.
73. Moberg E. Nerve repair in hand surgery: an analysis. Surg Clin North Am. 1968;48:985–991.
74. Weber EH. Ueber den Tastsinn. Muller Archiv. 1935;61:152–160.
75. Bell Krotoski JA. Sensibility testing. In: Burke SL, Higgins JP, McClinton MA, eds., et al. Hand and Upper Extremity Rehabilitation. 3rd ed 2005:53–85.
76. Pham HD, Armstrong DG, Harvey C, et al. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606–611.
77. Dyck PJ, Zimmerman IR, O’Brien PC, et al. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol. 1978;4:502–510.
78. O’Brien PC, Dyck PJ, Kosanke JL. A Computer Evaluation of Quantitative Algorithms for Measuring Detection Thresholds of Cutaneous Sensation Clinical Applications. Boston: Butterworths; 1989.
79. Dellon AL, Keller K. Validation of cutaneous pressure threshold measurement in carpal and cubital tunnel syndrome. Ann Plast Surg. 1997;38:493–502.
80. Dellon ES, Mourey R, Dellon AL. Human pressure perception values for constant and moving one- and two-point discrimination. Plast Reconstr Surg. 1992;90(1):112–117.
81. Stranbi SK, Rossi B, De Michelle G, et al. Trans Biomed Eng. 1999;7:895–898.
82. Dyck PJ. Assessment of Cutaneous Sensibility. Carville, La: Symposium presented at National Hansen’s Disease Center; 1980.
83. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3:474–481.
84. Moberg E. Criticism and study of methods for examining sensibility in the hand. Neurology. 1962;12:8–19.
85. MacKinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg. 1985;10A:906–907.
86. Dellon AL. The sensational contributions of Erik Moberg. J Hand Surg [Br]. 1990;15:14–24.
87. Moberg E. Objective methods of determining the functional value of sensibility of the hand. J Bone Joint Surg. 1958;40B:454–476.
88. Bell-Krotoski JA. Sensibility testing: current concepts. In: Hunter JM, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. 4th ed St Louis: Mosby; 1995:129–152.
89. Dellon AL, Mackinnon SE, Brandt KE. The markings of Semmes-Weinstein nylon monofilaments. J Hand Surg. 1993;18A:756–757.
90. Horch K, Hardy M, Jimenez S, Jabaley M. An automated tactile tester for evaluation of cutaneous sensibility. J Hand Surg. 1992;17A:829–837.
91. Brunelli SG. Gnostic rings for assessment of tactile gnosis. Am Soc Surg Hand Newslett. 1981;53.
92. Buford WL, Bell JA. Dynamic properties of hand held tactile assessment stimuli. Proceedings of the Thirty-fourth Annual Conference of Engineering in Medicine and Biology. 1981;23:307.
93. Imai HT, Tajima T, Natsuma Y. Interpretation of cutaneous pressure thresholds (Semmes-Weinstein monofilament measurement) following median nerve repair and sensory reeducation in the adult. Microsurgery. 1989;10:142–144.
94. Tinel J. The “tingling” sign in peripheral nerve lesions (translated by Emanual B. Kaplan). In: Spinner M, ed. Injuries to the Major Branches of Peripheral Nerves of the Forearm. 2nd ed Philadelphia: WB Saunders; 1978:8–13.
95. Lundborg GB, Rosén B, Linström K, Lindberg S. Artificial sensibility based on the use of piezoresistive sensors. Preliminary observations. J Hand Surg [Br]. 1998;23:620–626.
96. Nakada M. Localization of a constant-touch and moving touch stimulus in the hand: a preliminary study. J Hand Ther. 1993;6:23–28.
97. Phelps P, Walker E. Comparison of the finger wrinkling test results to established sensory tests in peripheral nerve injury. Am J Occup Ther. 1977;31(9):565–572.
98. Szabo RM, Gelberman RH, Williamson RV, et al. Vibratory sensory testing in acute peripheral nerve compression. J Hand Surg. 1984;98A:104–109.
99. Lundborg G, Lie-Stenström AK, Sollerman C, et al. Digital Vibrogram: a new diagnostic tool for sensory testing in compression neuropathy. J Hand Surg. 1986;11A(5):693–699.
100. Vallbo AB, Johansson RS. The tactile sensory innervation of the glabrous skin of the human hand. In: Gordon G, ed. Active Touch. Oxford, England: Pergamon Press; 1978:29–54.
101. Flinn-Wagner S, Maddinicky A, Goodman G, et al. Characteristics of upper extremity injured individuals making a successful transition to work. J Hand Ther. 1990;2(2):51–55.
102. Rosén B, Lundborg G. A new tactile gnosis instrument in sensibility testing. J Hand Ther. 1998;11:251–257.
103. Millesi H, Renderer D. A method of training and testing sensibility of the fingertips, from the Department of Plastic and Reconstructive Surgery, Surgical University Clinic of Vienna and Ludwig-Boltzmann, Institute for Experimental Plastic Surgery, Vienna, Austria, 1978.
104. Moberg E. The unsolved problem—how to test the functional value of hand sensibility. J Hand Ther. 1991;4(3):105–110.
105. Ng CL, Ho DD, Chow SP. The Moberg Pickup Test: results of testing with a standard protocol. J Hand Ther. 1999;12:309–312.
106. King PM. Sensory function assessment: a pilot comparison study of touch pressure threshold with texture and tactile discrimination. J Hand Ther. 1997;10:24–28.
107. Waylett-Rendall J, Niemeyer LO. Exploratory analysis to identify factors impacting return-to-work outcomes in cases of cumulative trauma disorder. J Hand Ther. 2004;17(1):50–57.
108. Onne L. Recovery of sensibility and sudomotor activity in the hand after nerve suture. Acta Chir Scand. 1962;300 (Suppl):1.
109. Richards RL. Vasomotor and nutritional disturbances following injuries to peripheral nerves. In: Seddon HJ, ed. Peripheral Nerve Injuries. London: Her Majesty’s Stationery Office; 1954.
110. Flynn JE, Flynn WF. Median and ulnar nerve injuries: a long range study with evaluation of the Ninhydrin test, sensory and motor return. Ann Surg. 1962;156:1002–1009.
111. Stromberg WB, McFarlane RM, Bell JL, et al. Injury of the median and ulnar nerves: one hundred and fifty cases with an evaluation of Moberg’s Ninhydrin test. J Bone Joint Surg. 1961;43A:717.
112. Perry JF, Hamilton GF, Lachenbruch PA, Bevin AG. Protective sensation in the hand and its correlation to the Ninhydrin sweat test following nerve laceration. Am J Phys Med. 1974;53:113–118.
113. O’Riain S. New and simple test of nerve function in the hand. Br Med J. 1973;22:615–616.
114. Quintner JL. A study of upper limb pain and paraesthesias following neck injury in motor vehicle accidents: assessment of the brachial plexus tension test of Elvey. Br J Rheumatol. 1989;28:528–533.
115. McCabe SJ, Mizgala C, Glickman L. The measurement of cold sensitivity of the hand. J Hand Surg. 1991;16A:1037–1040.
116. Rosen BL, Lundborg G. The long term recovery curve in adults after median or ulnar nerve repair: A reference interval. Br J Hand Surg. 2001;26(3):196–200.
117. van Breckel WH, Brakel WH, Kets CM, Van Leerdam IB. Functional sensibility of the hand in leprosy patients. Lepr Rev. 1997;68:25.

* I gratefully acknowledge Bill Buford, bioengineer, University of Texas at Galveston, formerly at the Paul W. Brand Research Laboratory, Gillis W. Long Hansen’s Disease Center, Carville, Louisiana, for his help in reviewing the monofilament calculations, in developing instrument measurements, and collaborating on sensibility test design.