Figure 13-1 Routine views of the hand. A, Posteroanterior. B, Oblique. C, Lateral.
▪ Plain radiography is the standard imaging technique. It provides the vast majority of information needed in many hand and wrist disorders.
▪ Advanced imaging is necessary when disorders cannot be adequately assessed through routine measures.
▪ A bone scan is a minimally invasive technique that provides information about blood flow, soft tissue elements and bone pathology. Anatomic resolution is poor, yet bone scanning is an important adjunct to other imaging modalities. Bone scanning is useful especially when the diagnosis is enigmatic.
▪ Arteriography is a minimally invasive technique to accurately visualize arterial anatomy and integrity and may be necessary when considering vascular surgery or to view the vascularity of musculoskeletal lesions.
▪ Ultrasound is a noninvasive modality for viewing and differentiating masses within the musculoskeletal system, especially between solid and cystic masses. Ultrasound can provide a dynamic account of tendon excursion as well, and look for tendon injury.
▪ Computed Tomography (CT) is a noninvasive modality, but exposes patients to ionizing radiation. Benefits include the ability to observe occult fractures within the carpus, identify displacement of fracture fragments, and evaluate for bone healing and nonunion. CT is especially useful for looking at the distal radioulnar joint for subluxation or dislocation.
▪ Magnetic Resonance Imaging (MRI) is a noninvasive imaging technique that has become the gold standard in viewing and differentiating soft tissue lesions, identifying lesions of the carpal ligaments, triangular fibrocartilage, and occult fractures of the hand and wrist. Flexor tendon injuries and tenosynovitis is easily identified. MRI is contraindicated in patients with pacemakers and cochlear implants.
The diagnosis of upper extremity disorders relies on information obtained in the history, physical examination, and views of the region acquired through one or more imaging techniques. Plain radiography provides adequate visualization of most problems and, for that reason, is a standard part of the initial evaluation. Excellent depiction of fractures, fracture alignment, fracture healing site, dislocation, soft tissue calcification, foreign bodies, and bony detail can be obtained by this readily available, cost-effective, and noninvasive means. In certain cases, however, the problem cannot be properly assessed by routine measures; thus, other imaging modalities are needed to provide further diagnostic information. In this chapter, radiography and advanced imaging techniques are discussed in relation to the diagnosis of hand and wrist disorders.
Routine studies of the hand consist of posteroanterior (PA), lateral, and oblique views, which are evaluated for bone density, bony lesions, fractures and dislocations, integrity of the articular surfaces and joint spaces, and irregularities of the soft tissue (Fig. 13-1).1
Bone density, which is evaluated grossly, may be normal, less than normal (osteopenia), or greater than normal (osteosclerosis). Osteopenia is most often encountered in the elderly and is known as senile osteoporosis when associated with advanced age. Osteosclerosis occurs in conditions such as avascular necrosis (AVN) (Fig. 13-2), fracture healing, and metabolic bone disease.2 Plain films may reveal discrete or diffuse bone lesions, including primary or metastatic bone tumors, infection, and metabolic bone disease (Fig. 13-3). The cortical integrity is carefully inspected for evidence of acute fracture (Fig. 13-4) and the joint alignment evaluated for subluxation or dislocation.3 Abnormalities of the articular surface and cartilage joint space also are documented. Narrowing of the cartilage space may indicate arthritis, resulting from degeneration, inflammation, infection, or trauma (Figs. 13-5 and 13-6).4 Finally, the soft tissue shadows are evaluated for irregularities. Any evidence of calcification (Fig. 13-7), foreign bodies (Fig. 13-8, online),5 or soft tissue masses must be correlated with the clinical findings.
Figure 13-2 Avascular necrosis (AVN) of the lunate, or Kienböck’s disease. Coned-down posteroanterior views of the wrist demonstrate two manifestations of AVN: bony sclerosis, seen in the ulnar aspect of the lunate, and osteopenia, seen in the radial side of the lunate (arrow). The latter is due to increased blood flow as the bone attempts to heal.
Figure 13-3 An enchondroma is demonstrated in this posteroanterior view. Note the decreased bone density of the fourth metacarpal head and shaft, which also are increased in size. Within the area of decreased bone density are stippled calcifications—a classic finding in this type of lesion.
Figure 13-4 This posteroanterior view of the wrist shows a fracture of the proximal third of the scaphoid (arrow) with an associated cyst.
Figure 13-5 Posteroanterior views of the wrist demonstrating the hallmarks of rheumatoid arthritis: osteopenia, which may be the earliest sign of the disease (compare with normal bone density in Fig. 13-1, A), loss of articular cartilage, and erosions. In this patient, erosion is evident at the ulnar styloid (arrow).
Figure 13-6 Osteoarthritis. Oblique view of the wrist showing decreased height of the articular cartilage at the first metacarpal joint (arrow). The disease also has resulted in increased bone density on both sides of the joint.
Figure 13-7 Oblique view of the hand demonstrating extensive calcification in the soft tissues of the digits.
Figure 13-8 Posteroanterior (A) and lateral (B) views of the wrist and distal forearm showing multiple shotgun pellets. Note the air in the soft tissues (areas of lucency [arrow]) caused by the penetrating trauma.
If the history and physical examination indicate a localized problem, a coned-down view of the area can be obtained. This magnified spot film focuses on a specific area of the wrist, which is especially useful for isolated disease of the carpal bones because fractures and abnormalities in this region often cannot be seen on plain views (Fig. 13-9, online). Special positioning and maneuvers also are used to allow more complete visualization of a possible pathologic condition. For example, stress views may demonstrate joint malalignments that are not evident on routine films. Tears of the ulnar collateral ligament of the thumb metacarpophalangeal (MCP) joint are a case in point because these can become apparent by applying a force to this joint in radial deviation (Fig. 13-10).5 A special maneuver is often needed to demonstrate damage to the scapholunate ligament complex. This complex is important in stabilizing the scaphoid and preventing rotatory subluxation. When it is injured, the scapholunate interval may appear completely normal on a routine view, but it widens when a compression load is applied by clenching the fist in supination (Fig. 13-11). Recently, a modified clenched-fist view, called the “clenched-pencil” view, was described.6 This view allows the appropriate amount of pronation of the wrist to view the scapholunate interval most favorably. Special views, such as those of the trapezial ridge (Fig. 13-12) and hook of the hamate (Fig. 13-13), also are useful in demonstrating the bony anatomy of the carpal tunnel and certain fractures.
Figure 13-9 Coned-down posteroanterior views of the wrist provide greater bony detail and are useful when wrist pathology is suggested.
Figure 13-10 Thumb dislocation. A, This patient’s left thumb appears normal under radial stress. B, The right thumb, which had a disruption of the ulnar collateral ligament, shows subluxation of the first metacarpophalangeal joint when subjected to the same stress.
Figure 13-11 When this patient clenched the fist in supination, subluxation of the scaphoid (arrow) occurred.
When a diagnosis cannot be made on the basis of the routine clinical examination and plain radiographs, more advanced imaging techniques can be used to visualize the bone and soft tissue anatomy more completely. The decision to use another technique must consider the invasiveness and cost of the procedure (Table 13-1, online) as well as its specificity.
Table 13-1 Imaging Techniques Used in Diagnosing Hand Disorders: Invasiveness and Cost
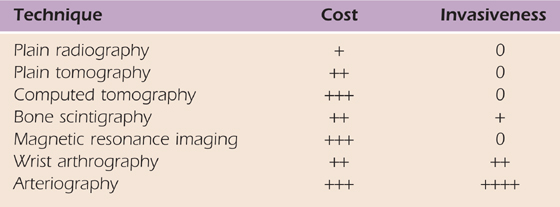
+, $50–300; ++, $400–900; +++, >$1000.
Plain tomography is capable of visualizing healing bone and fracture nonunion better than routine radiographs, especially in cases involving the scaphoid and hook of the hamate. This examination is noninvasive and relatively inexpensive. Computed tomography has largely replaced plain tomography in most centers. CT is more clinically useful than conventional tomography because it can obtain multiple contiguous sections with high resolution in a short time, multiplanar image reconstruction is possible, and additional information about adjacent soft tissues can be obtained. 3D reconstructions are now also available to help in planning surgery.7 CT remains the best modality for demonstrating bony architecture.8
CT is useful in evaluating complex fractures and bony lesions, providing superior detail of cortical invasion, marrow abnormalities, and matrix calcification.5,9,10 It is also an excellent means of depicting subluxations and dislocations of the distal radioulnar joint.11 In patients with metallic hardware, significant scatter often overlays the image of the bony segment. Image techniques can be manipulated to minimize this beam-hardening artifact to fine-tune visualization of the bony anatomy. The advantage of CT over conventional radiography lies in its ability to obtain transaxial images and provide excellent contrast resolution for evaluating soft tissues. This modality is also noninvasive, unless used in conjunction with contrast agents. CT exposes patients to potentially harmful ionizing radiation. With the advent of fast CT scanners, availability has led to increased radiation doses to patients.
Bone scintigraphy involves the intravenous (IV) injection of 99mTc-labeled monodiphosphonate (MDP), which is preferentially taken up by bone. Flow images are generated after 60 seconds, and a nuclear medicine angiogram is obtained. Delayed images are generated after 3 hours. Areas of abnormal blood flow and bone turnover can be detected on the scan, making it useful in the evaluation of infection, AVN of the lunate, tumors, and reflex sympathetic dystrophy. It also is used to screen patients who have unexplained wrist pain (Fig. 13-14).12
Figure 13-14 This bone scan demonstrates increased uptake in the right lunate, the right pisiform/triquetral area, and the first carpal metacarpal joint (arrow). Such findings are nonspecific. Avascular necrosis of the lunate was demonstrated on other imaging studies.
The examination is minimally invasive and moderately expensive. The poor anatomic resolution of the scan is its primary drawback. The test also is nonspecific, making it impossible to distinguish various processes that can cause increased uptake.13 The results must be carefully correlated with the clinical findings to be properly interpreted. Today, MRI has largely replaced scintigraphy in most centers in musculoskeletal imaging.14
Arteriography is the most specific means of evaluating vascular anatomy and pathology; it is used to evaluate aneurysms, arteriovenous malformations, tumors of vascular origin, traumatic vessel injuries, and solid tumor vascularity (Fig. 13-15).8,15 The examination is performed by threading a catheter into the brachial artery using a femoral approach, injecting contrast material, and then taking multiple radiographs. The procedure is often painful, even with the use of new nonionic contrast material. Complications include bleeding hematoma, pseudoaneurysm formation, dissection, and thrombosis. The contrast medium may cause arterial spasm, although this can be controlled with the use of vasodilators. Anaphylaxis is another potential complication when iodinated contrast material is used, but severe episodes are rare, occurring in only 1 of 40,000 cases.
Figure 13-15 Contrast arteriogram of the wrist showing a hemangioma (arrow) in the ulnar aspect of the palm.
In the diagnosis of bone and soft tissue tumors, conventional angiography has largely been replaced with MRI and magnetic resonance angiography (MRA) to avoid potential complications.8
Arthrography is used to evaluate the integrity of the carpal ligaments and triangular fibrocartilage (TFC) (Fig. 13-16, online). The examination involves injecting dye into the radiocarpal, radioulnar, and midcarpal compartments, so it must be performed carefully.1 The injection of contrast material into the joint can highlight intra-articular soft tissue structures such as ligaments and fibrocartilaginous structures.16 Communication of dye between the compartments of the wrist can be caused by a clinically unimportant perforation and does not necessarily indicate pathology.17 Thus, the test results must be correlated with the clinical examination and interpreted with a great deal of caution. The examination is invasive but can be administered with minimal discomfort to the patient.
Figure 13-16 Arthrography of the wrist. A, This normal arthrogram was obtained after injecting dye into the radiocarpal joint. No leakage into the midcarpal joint or radioulnar joint is seen. B, Leakage into the distal radioulnar joint (arrow) is evident in this patient. Clinical correlation is needed to accurately interpret this finding.
The role of arthrography had declined because of the excellent visualization of intra-articular structures by MRI.18,19 Arthrography has also been found to be only 60% accurate in detecting tears in the triangular fibrocartilage complex (TFCC), scapholunate ligament, or lunotriquetral ligament.20 Arthrography is currently used as a complement to MRI and CT to enhance the diagnostic accuracy.21
Continuous radiographic imaging has many applications such as arthrography, tenography, bursography, arteriography, and percutaneous bone and soft tissue biopsy.21 Evaluation of the wrist in motion is a useful method for detecting the rigidity of a freshly fixed fracture or for dynamic carpal instability. This noninvasive and inexpensive technique is the diagnostic imaging procedure of choice for determining and documenting the presence of dynamic rotatory subluxation of the scaphoid.22 The role of MRI in this capacity is evolving but remains problematic. Dynamic MRI is a newer technique that may yield more comprehensive clinically relevant information with regard to wrist kinematics than conventional MRI.23,24
MRI visualizes tissue by applying a strong magnetic field with radiofrequency pulses to record differences in tissue signal intensity. A variety of magnet strengths are available from 0.2 to 3 T, with higher strengths providing greater contrast resolution.13,25 MRI provides high resolution and high-contrast tissue segmented information about the integrity of joint and soft tissue structures. It also is completely noninvasive, involves no ionizing radiation, and is able to obtain images through cast and fiberglass materials that limit the resolution of conventional radiography and CT.8 Among its minor drawbacks are its contraindications in patients with pacemakers, cochlear implants, or ferromagnetic aneurysm clips. Implanted metallic objects create “holes” and distort the images of tissues hoped to be visualized.8,21 In addition, the examination is performed with the patient lying in a narrow cylinder within the bore of a magnet, a closed space that may cause some individuals to become claustrophobic. In the event of such anxiety, IV benzodiazepam may be administered to the patient, provided that the patient can be monitored properly during sedation. Open MRIs have evolved and are advantageous in this setting. The diagnostic accuracy of low-field open scanners has been found to be comparable to that of high-field scanners.26 Evaluating a suggested pathology with MRI involves the use of multiple sequences, including T1, T2, short tau inversion recovery (STIR), and spin echo; for this reason, close communication between the orthopedist and radiologist is essential.
MRI is of great value in defining soft tissue abnormalities (Figs. 13-17 to 13-19, all online). In the evaluation of tumors, it cannot provide a specific diagnosis, but it can define the size of the lesion and the extent of involvement of marrow and neurovascular structures (Fig. 13-20, online).27 Other soft tissue abnormalities diagnosed more easily by MRI include ganglions, ligament tears, and cartilage abnormalities (Fig. 13-21).28,29 Dorsal wrist pain can be attributed to hypertrophy of the dorsal capsule as well as ganglions that may be occult and not palpable. Patients with dorsal wrist pain of unknown origin are therefore candidates for MRI evaluation. MRI is especially helpful in diagnosing tears of the scapholunate and lunotriquetral ligaments, particularly when dissociation of the scapholunate is not evident on plain films.30 Excellent depiction of the TFC can be achieved with MRI, but the image must be interpreted carefully; thinning of the disk occurs in many patients, but a tear of this structure is not diagnosed unless an avulsion from the ulnar or radial insertion can be observed.31-33
Figure 13-17 Synovial sarcoma. A, Sagittal magnetic resonance image showing a soft tissue mass (arrow). The white object (curved arrow) is a marker (raw almond) placed over the palpable mass (straight arrow). B, Axial magnetic resonance image obtained after contrast enhancement demonstrates the mass again (arrows) and shows that the neurovascular bundle is encased by tumor.
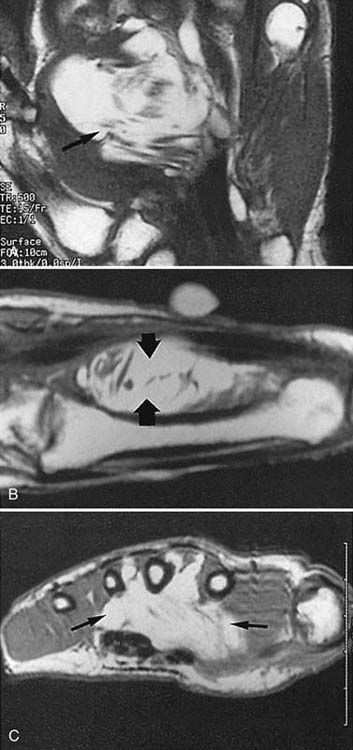
Figure 13-18 Infiltrating lipoma of the palm. Magnetic resonance images of the sagittal (A), coronal (B), and axial (C) planes show the extent of the lesion (arrows).
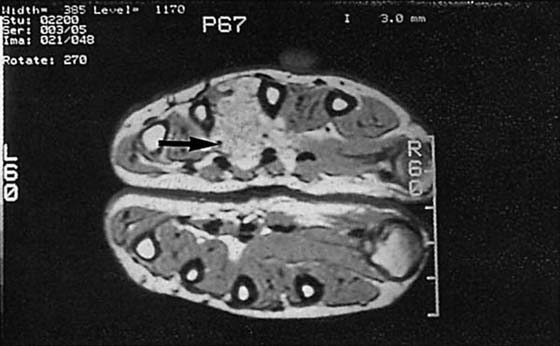
Figure 13-19 Hemangioma. Axial magnetic resonance images through the palm show a mass between the third and fourth metacarpals (arrow). MRI is capable of depicting the extent of a soft tissue mass but cannot provide a specific pathologic diagnosis. A contrast arteriogram of this hand is shown in Figure 13-15.
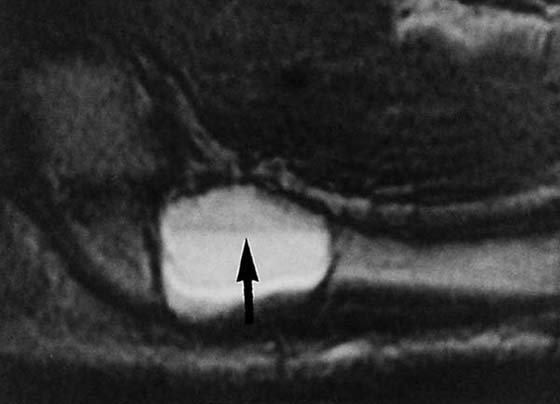
Figure 13-20 Aneurysmal bone cyst. This magnetic resonance image reveals an abnormality at the base of the second metacarpal. The fluid-filled level (arrow) is a typical finding in such cases.
Figure 13-21 Ganglion. A, This axial magnetic resonance image demonstrates a dorsal mass (arrow) that was not palpable on clinical examination. B, Coronal magnetic resonance image in another patient showing a mass in the abductor canal (arrow).
MRA is another tool that can easily demonstrate vascular abnormalities. MRA provides detailed information about the anatomy of the small vessels that constitute the carpal arches.23 MRA is noninvasive because it does not require the use of IV contrast material (Figs. 13-22 and 13-23, both online). Rather, it relies on the property of blood flow, and special pulsed sequences are used to enhance the fluid within the vessels.21
Figure 13-22 Magnetic resonance angiogram of a normal radial (curved arrow) and ulnar (straight arrow) artery.
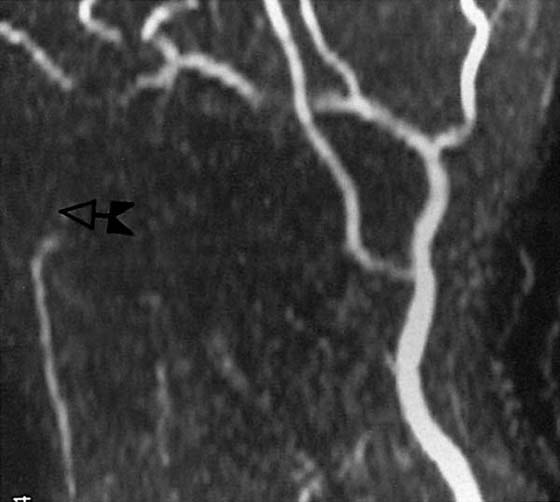
Figure 13-23 Magnetic resonance (MR) angiogram demonstrating ulnar artery thrombosis (left). (Compare with normal MR angiogram in Figure 13-22.)
Conventional radiography is still considered the first-line means of examining the wrist. More recently, CT has been used, especially for examining fractures. However, MRI has revolutionized the manner in which the orthopedic surgeon approaches diagnosis and decision making,23 although the gold standard for assessment of the wrist for pathology is arthroscopy.
Ultrasound (US) uses high-frequency sound waves to produce images. The images produced are a reflection of the echogenicity of a structure, which determines its brightness on the ultrasound. Cysts and tendons have low echogenicity, whereas solid masses have high echogenicity and appear white on ultrasound. Doppler US can image motion and has been used routinely for imaging blood vessels.13,25
US is noninvasive, does not emit ionizing radiation, is readily available, and has high patient acceptance.18 The two major indications for US are muscle injuries and tendon lesions. Joint effusions, ganglia, and ruptures of larger ligaments (not interosseous carpal ligaments) can be observed. Dynamic US can be used to view tendon movements. Nonorthogonal scanning, however, can produce hyperechoic zones mimicking partial tendon tears or tendinitis.7
The use of US has been limited mainly because of the inability to penetrate bone and view osseous pathology. US is also operator-dependent, involves long examination times, and produces static images that may be incomprehensible when viewed at a later time.18,25 The accuracy and reliability of US for detecting ganglions, solid lesions, tenosynovitis, and tendon lesions are limited.34,35
The extensor tendons are contained in six dorsal compartments numbered from the radial (I) to ulnar (VI) sides of the wrist. Lister’s tubercle separates compartments II and III. Extensor disorders outside of compartments I or VI are rare; thus, fluid in multiple compartments usually represents synovitis and is often a marker for an inflammatory arthritis such as rheumatoid arthritis.
More commonly, the tendons of compartment I (abductor pollicis longus and extensor pollicis brevis tendons) are affected with stenosing tenosynovitis. This disorder is termed de Quervain’s disease and is related to overuse. It is more common in women and also may occur as a complication of pregnancy. Patients typically present with pain radiating from the radial styloid to the thumb and proximally into the forearm. Pain is increased with passive movement of the thumb and swelling or tenderness over the first dorsal compartment of the wrist. This disorder usually is diagnosed clinically. On MRI, fluid within the tendon sheath is increased, and the tendons themselves, which may show increased intrasubstance signal, are enlarged. On coronal images, there is focal obliteration of the adjacent subcutaneous fat and intense synovial enhancement following gadolinium administration. This stenosing tenosynovitis is analogous to adhesive capsulitis in the shoulder.
Compartment VI contains the extensor carpi ulnaris (ECU) tendon, which is another common site for disorders such as synovitis or tenosynovitis. ECU subluxation may occur, which can lead to synovitis or intrasubstance tears; thus, dynamic imaging may be performed to confirm the diagnosis. It should be recognized that artifactual increased signal within the ECU is commonly seen, which may represent subclinical degeneration or normal fascicular anatomy.
The ligamentous anatomy of the wrist is complex because of the stabilization required for the numerous carpal bones and extensive range and axes of motion in this joint. The intrinsic carpal ligaments connect the carpal bones to each other, and the extrinsic ligaments connect the carpus to both the radius and ulna and to the metacarpals. The extrinsic ligaments are more important for overall carpal stability and have a complex nomenclature. In general, tears of the extrinsic ligaments are less common and more difficult to diagnose than intrinsic ligament tears. Ligaments can be injured by trauma or by inflammatory processes such as rheumatoid arthritis (RA).
The two main intrinsic ligaments of the wrist are the scapholunate (SL) and lunatotriquetral (LT) ligaments. SL ligament tears may be spontaneous, secondary to a fall on an outstretched hand, or associated with the subtypes of carpal instability. LT ligament tears also can be spontaneous or posttraumatic. LT tears are not uncommonly associated with instability patterns of the wrist and also have a high association with TFC tears.
Mechanically, both the SL and LT ligaments are divided into three portions: dorsal, volar, and central. The central or membranous portion is thin, whereas the dorsal and volar portions are thicker with individual fascicles usually visible. Membranous tears can cause pain but are biomechanically unimportant. The dorsal and volar aspects of these ligaments are more important to the mechanical stability of the carpus. The dorsal SL ligament is the more significant portion that helps prevent dorsal intercalated segment instability (DISI), whereas the volar portion of the LT ligament is vital to prevent volar intercalated segment instability (VISI).
The carpal ligaments are best visualized on thin-section 3D gradient recalled echo (GRE) MR sequences in the coronal and axial planes. They appear as signal voids bridging the carpal bones. Ligament strains can be diagnosed if the signal is abnormal or the structure is attenuated. Discontinuity, complete absence, and increased intercarpal distances are findings compatible with ligament tears. The best finding for an intrinsic tear is fluid violating the space extending from the radiocarpal to the midcarpal joints. Normally, the ends of the lunate, scaphoid, and triquetrum should appear smoothly rounded. The presence of osteophytes is abnormal and often is caused by biomechanically incompetent ligaments, usually resulting from partial dorsal or volar aspect tears. The presence of marrow edema, subchondral sclerosis, or other signs of focal articular disease should suggest ligament dysfunction. Focal offset of two adjacent carpal bones and the lack of the normal articular parallelism also are signs suggestive of ligament dysfunction. Remember that perforation of the SL or LT ligament occurs with aging; thus, in a patient older than 35 to 40 years, a tear should be diagnosed only if the ligament is morphologically abnormal.
The diagnosis of many patterns of carpal instability can be established by routine radiographic findings. The lateral projection is useful in evaluating both DISI and VISI. In DISI, the lunate is tilted dorsally, with the scaphoid displaced vertically. The angle of intersection between the main longitudinal axis (along the radius, lunate, capitate, and third metacarpal) and the long axis of the scaphoid is greater than 60 degrees where it should normally be 30 to 60 degrees. The radioscapholunate ligament should be evaluated for tears. This injury is typically degenerative in origin and is thought to be the sequela of trauma to the outstretched hand in a young adult. In VISI, the lunate is flexed toward the palm, and the angle between the two longitudinal axes is less than 30 degrees. Note that this finding may be normal if bilateral and in a young female patient. VISI is less common than DISI overall. Most cases are thought to be degenerative in origin, although there is an association with RA. These patterns must also be carefully interpreted on sagittal MRI as the lunate may appear more dorsally tilted, mimicking a DISI pattern in otherwise normal wrists.36
The evaluation of these ligaments for abnormalities has been challenging. In the past, evaluation was limited to conventional arthrography. Proponents state that wrist arthrography has the potential to provide an equivalent diagnostic evaluation to arthroscopy, provided the technique is meticulously followed and the operator possesses fundamental knowledge regarding anatomy and age-related changes of the wrist.16 With advanced imaging techniques, such as MRA, the extrinsic and intrinsic ligaments can easily be seen.37
Advanced cases of carpal instability are obvious on routine radiographic studies. Subtle instability may require advanced imaging. Yet there has been a discrepancy in the literature about the sensitivity and specificity of MRI in detecting intrinsic ligament tears.19,38,39 Recently, CT arthrography was found to have high sensitivity and specificity in detecting interosseous ligament injuries.40,41 Schmid and colleagues41 compared CT arthrography with MRI and found the sensitivity, specificity, and accuracy of MRI to detect palmar and central tears of the SL and LT ligaments to be less than 80%. The volar portion of the ligament had a sensitivity and specificity of 0 and 100, respectively. CT arthrography was better at detecting these ligament injuries. Accurate visualization of the interosseous ligaments has also been established with MR arthrography.37,42 Haims and coworkers43 discovered improved sensitivity in detecting SL ligament tears with indirect MR arthrography over unenhanced MRI.
Carpal fusion or coalition is clinically important because it places the patient at a somewhat higher risk for carpal instability and secondary SL ligament tears. There are three types of carpal fusion:
1. Congenital: Isolated fusions involve bones of the same carpal row, whereas syndrome-related fusions may affect bones in different rows (both proximal and distal). LT and capitate–hamate are the most common types of isolated fusion, although fusion may occur in almost any combination. LT fusion may be asymptomatic, but widening of the scapholunate interosseous space may be seen radiographically. Partial fusions may be associated with pain and cystic changes in the adjacent bones. Massive carpal fusion, which affects both carpal rows, is associated with congenital syndromes and chromosomal anomalies (e.g., Turner’s, Holt–Oram, Ellis–van Creveld).
2. Surgical or post-traumatic.
3. Inflammatory: Pericapitate fusions are seen in adult Still’s disease.
The most common internal derangement of the wrist involves the TFCC, which consists of the triangular fibrocartilage, the meniscal homologue, the ulnolunate and ulnotriquetral (volar ulnocarpal) ligaments, and the extensor carpi ulnaris tendon. The TFC is a biconcave fibrocartilage band that normally appears dark on all MRI sequences and is surrounded by higher-signal synovial fluid or hyaline cartilage. Its function is similar to that of the menisci in the knee. The TFC arises from the ulnar aspect of the distal radius and extends to the junction between the ulnar head and styloid process, adjacent to the meniscal homologue. The meniscal homologue at the ulnar edge of the TFC is made up of fibrofatty tissue that appears as high signal on T1-weighted and proton-density images in contrast to the low-signal TFC. Many authorities currently believe that the homologue is not a fibrofatty structure, but rather fat interposed between the extrinsic ligaments at the ulnar aspect of the wrist. The meniscal homologue should never appear similar to fluid on T2-weighted images.
The TFC is best demonstrated on coronal images, especially on thin-section 3D-GRE sequences. A suggested tear of the TFC is a common indication for MRI examination of the wrist. These tears are divided into degenerative and traumatic types.44
Degenerative tears are much more common and are often termed central tears. These occur in the thinnest portion of the TFC, which is central but slightly eccentric toward the radial aspect of this structure. Traumatic tears occur after a discrete injury and usually are located at the ulnar or radial side of the TFC. Traumatic tears tend to be perpendicular to the long axis of the TFC and are associated with fluid in the distal radioulnar joint (DRUJ) and, to a lesser degree, with excessive fluid in the radiocarpal joint. TFCC tears can be partial or full thickness. Ulnar-sided tears may be in the region of the meniscal homologue. It is these traumatic TFC tears that are most easily missed on MRI. High-signal intensity in this region on T2-weighted sequences is the most reliable finding (Fig. 13-24, online). Any fluid at the periphery of the TFCC on T2-weighted images that is not in the prestyloid recess or ECU tendon sheath should be described as a peripheral tear. TFCC tears are typically associated with LT, and less frequently, SL ligament tears.
Figure 13-24 Coronal magnetic resonance image showing an avulsion of the triangular fibrocartilage complex from its attachment to the ulna (arrow).
Although the TFC is usually low in signal on all pulse sequences, increased internal signal may sometimes be seen on GRE or T1-weighted images. This represents degeneration of the TFC and not a true tear. This degenerative signal is globular, is hypointense to cartilage, and does not extend to both sides of the TFC. Another diagnostic pitfall is the line of high-signal hyaline cartilage seen at the insertion of the TFC onto the radius, which can be mistaken for a tear. True radial-sided tears are rare. They usually are caused by acute trauma and consist of a radial avulsion of the TFC with increased signal intensity on T2 images. Furthermore, TFC slit-like communicating lesions can be found in asymptomatic wrists.45 Tears should be seen on at least two pulse sequences.
Multiple reports have emerged on the accuracy of current modalities in imaging the TFCC. MRI has been found in one report to have limited utility in imaging the peripheral attachment of the TFCC, with sensitivity and specificity of 17% and 79%, respectively.43 A high signal intensity at the ulnar insertion was found to have low sensitivity and specificity as a marker for a peripheral tear. Blazar and associates46 discussed the fact that MRIs coming in to most hand surgeons’ practices are heterogeneous and not obtained in a standardized fashion, having been done at various locations. Less experienced radiologists also were found to have lower sensitivity, specificity, and accuracy in predicting a tear and its location. Potter and colleagues47 found higher accuracy rates, but images were obtained using a standardized protocol. MR arthrography has also been evaluated as a potential modality to increase the prediction of TFCC injury. Haimes and coworkers48 compared indirect MR arthrography with unenhanced MRI and found no improvement in the ability to evaluate central tears of the TFCC. Direct MR arthrography, on the other hand, found improved sensitivities, specificities, and accuracy over unenhanced MRI and conventional arthrography, and the highest rates when combining MR arthrography with conventional arthrography.49 In another study, CT arthrography was highly accurate in detecting central TFC tears but not peripheral tears.40
There are four types of problems affecting the DRUJ: incongruity, instability, impaction, and isolated tears of the TFC.50 The presence or absence of each can be confirmed by using the various imaging techniques in a logical sequence.
Joint incongruity is evaluated on plain PA radiographs, which can demonstrate irregularity, sclerosis, spurs, and erosions of the articular surface of the sigmoid notch of the radius and ulnar head (Fig. 13-25). Instability is caused by a deficiency of the fibrocartilaginous support structure of the DRUJ, which can be determined on the basis of the clinical examination. Plain radiographs taken with the patient properly positioned will confirm the diagnosis if subluxation or dislocation of the ulnar head within the sigmoid notch of the radius is shown (Fig. 13-26). CT or MRI also can confirm the subluxation or dislocation by demonstrating the orientation of the DRUJ in the axial plane.11,51
Figure 13-25 Incongruity of the distal radioulnar joint. Narrowing of the normal cartilage space (arrow) demonstrates loss of the articular cartilage.
Ulnar variance refers to the relationship between the distal ulna and radius, excluding their respective styloid processes. Normally, the articular surfaces of these bones are aligned. Changes in the length of the ulna relative to that of the radius alter the compressive forces across the wrist. In positive ulnar variance, the ulna is longer than the adjacent articular aspect of the radius, and with negative variance the ulna is shorter. Any variance greater than 2.5 mm is biomechanically significant. Ulnar variance can be viewed with conventional radiography using both neutral rotation and pronated grip PA radiographs.52 Ulnar variance may be difficult to accurately assess on MRI, other than to describe the relationship between the ulna and the sigmoid sulcus of the radius. Negative ulnar variance is weakly associated with Kienböck’s disease. Positive ulnar variance is associated with degenerative type TFC tears due to mechanical erosion.
Positive ulna variance can eventually lead to impaction of the lunate by the ulna with or without a degenerative TFC tear (Fig. 13-27). This is known as ulnalunato abutment syndrome, or ulnar impaction syndrome. This also may affect the triquetral bone of the carpus. On MRI, abutment is manifested by cartilage loss of the lunate with resultant marrow edema, subchondral cysts and eventual subchondral sclerosis. Cartilage defects are best seen on high-resolution images, whereas the sclerosis is seen as low signal on T1- and T2-weighted sequences. GRE sequences often accentuate this dark signal. Characteristic focal signal changes are seen at the proximal ulnar aspect of the lunate, and often the triquetrum.53
Figure 13-27 Positive ulnar variance of the distal radioulnar joint seen in this posteroanterior view has resulted in impaction of the ulna into the lunate. The sclerosis indicates a chronic bone reaction to this trauma. Loss of the articular cartilage also is evident.
AVN is common in the wrist. It may be the result of overuse, occur spontaneously, or be secondary to local trauma or systemic disease. Examples of systemic processes that can lead to AVN include steroid usage and systemic lupus erythematosus (SLE).
An example of AVN related to overuse occurs in the lunate and is termed Kienböck’s disease. Repetitive microtrauma is thought to be the major cause, although it may occur after a single major traumatic event. The dominant extremity is usually affected, and young males are more commonly affected than females. The blood supply to the lunate is tenuous because most of this bone is covered with hyaline cartilage. The proximal aspect of the lunate is most vulnerable to injury, and if AVN involves only one part of the bone, it affects the radial side. Kienböck’s disease also is associated with negative ulnar variance. The MR staging of Kienböck’s disease (modified from plain film staging) is as follows:
Stage 1: Normal contour of the lunate, with a radiolucent or radiodense line representing the compression fracture; fracture is seen as a zone of decreased T1 signal
Stage 2: Resorption of bone along the fracture line
Stage 3: All of the changes of stages 1 and 2, plus sclerosis of the bone; necrotic bone has variably decreased T1 signal
Stage 4: Fragmentation or flattening of the lunate
Stage 5: Secondary osteoarthritis of the radiocarpal and intercarpal joints; fragmentation and decreased T1 signal of the lunate combined with osteophyte formation, subchondral sclerosis, or edema involving the adjacent articular surfaces; often an associated joint effusion
Because of its anatomic specificity, MRI is capable of demonstrating marrow abnormalities caused by AVN, and it can reveal these changes earlier than is possible with bone scintigraphy (Fig. 13-28).29,54-57 Bone islands have a similar appearance on MR images, so the results must be correlated with plain films. The imaging findings of Kienböck disease show marrow replacement, best assessed on the T1-weighted images as decreased signal.29,54-57 The T2 appearance varies somewhat but usually shows increased signal. As with all cases of AVN in the wrist, the classic “double-line” sign is rarely seen. The surrounding soft tissues are usually normal, and a joint effusion is variably present.
Figure 13-28 Magnetic resonance image demonstrating a scaphoid fracture (disruption in the black cortical line at the thin arrow). The marrow of the scaphoid is relatively darker (thick arrow), indicating edema from the fracture.
Post-traumatic AVN may be seen in any carpal bone; however, it is most common in the scaphoid. The proximal pole of the scaphoid is affected by AVN secondary to a disruption in the blood supply. The proximal pole is fed from the terminal branches of the artery entering the distal pole and passing through the middle segment of the scaphoid. A scaphoid waist fracture may disrupt this supply, and AVN may result in up to 60% of cases. The risk of AVN increases as the fracture becomes more proximal. Fractures that have a visible step-off also have a higher risk of AVN. On MRI, the proximal pole of the fractured scaphoid may show decreased bone marrow signal on T1 images. The contour of the cortex may become indistinct and irregular subsequent to collapse. MRI also can show reactive changes in the distal fracture fragment, with AVN rarely occurring here. Again, the double-line sign on T2 images is rarely seen. An acutely “negative” MR for scaphoid AVN is unreliable because false-negative results can occur up to 1 to 2 months. Gadolinium-enhanced MRI may better assess the proximal pole vascularity and is currently considered the best technique.58 The proximal pole can appear hypointense on a T1-weighted image and hyperintense on a proton-density–weighted fat-suppressed image, resembling a necrotic proximal pole; but a gadolinium-enhanced T1-weighted fat-suppressed MRI can show some vascularized tissue within the fragment. This could represent spontaneous revascularization, or may be some unspecific reactive inflammatory tissue originating from the nonunion site. Also, if the fatty content of the proximal pole appears normal, then the absence of enhancement with gadolinium does not indicate AVN.
Spontaneous or idiopathic AVN occurring in the scaphoid is termed Preiser disease. Preiser disease demonstrates complete marrow replacement on T1-weighted images, with typical edema on T2-weighted images. Associated ligamentous injury is uncommon, as is collapse. The capitate bone is the third most common carpal bone to undergo AVN, but this occurs rarely, and when it does, is usually spontaneous in origin.
The carpal tunnel is bounded by the concave volar surface of the carpus in continuity with the flexor retinaculum, which extends from the trapezium to the hook of the hamate. It consists of the eight flexor digitorum tendons [flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP)], the flexor pollicis longus tendon, and the median nerve.
Carpal tunnel syndrome (CTS) is a common syndrome characterized by pain, paresthesias, and weakness in the distribution of the median nerve. It is bilateral in 50% of cases and may be associated with repetitive mechanical activity, such as typing. Other causes of CTS include tenosynovitis, RA, amyloidosis, infection, mass (intrinsic or extrinsic), pregnancy, or developmental anomalies. Diagnosis is usually made clinically and confirmed with electromyography (EMG) examinations.
MRI plays a limited role in the examination of patients with possible carpal tunnel disease. By and large, the diagnosis of this problem should be reached clinically, but MRI may be used occasionally if the symptoms derive from a soft tissue mass or an infection within the carpal tunnel.59,60 Indications for wrist MRI include atypical symptoms, a lack of EMG findings, high clinical suspicion for a mass, young patient age (possible congenital anomalies), and recurrent symptoms in postoperative patients.
On MRI, the flexor retinaculum is normally taut to minimally convex, the median nerve is of uniform size (4 × 2 mm) and ovoid in shape with signal isointense to muscle, and the flexor tendons are not distinguishable from one another on T2-weighted sequences. MRI findings of CTS include volar bowing of the flexor retinaculum, synovitis of the tendon sheaths within the carpal tunnel, focal enlargement of the median nerve at the level of the pisiform, and increased signal of the nerve itself. The median nerve may alternatively appear flattened. Note that these findings are nonspecific in the absence of symptoms. High signal within the median nerve is the least reliable sign and is often seen in asymptomatic individuals. Mild bowing of the flexor retinaculum may be physiologic; thus, CTS should not be suggested unless this finding is marked. Masses rarely cause CTS, but the search for masses must be diligent. Ganglion cysts, lipomas extending from the thenar or hypothenar eminences, or focal amyloid deposition may be causative lesions. A dedicated extremity MRI may be a cost-effective way to make this diagnosis.
In postoperative patients, it is important to check for incomplete lysis of the flexor retinaculum. Although the retinaculum may have been completely incised at surgery, it may regrow and appear intact. Assessment for recurrent or residual masses or recurrent synovitis also should be performed. Postoperatively, the flexor tendons migrate volarly, and the median nerve usually regains normal size and signal.
Compression of the ulnar nerve also may occur at the wrist, resulting in tingling and pain along the hypothenar region and ulnar side of the fourth and fifth fingers. The ulnar nerve, along with the ulnar artery and vein, travels within Guyon’s canal. This space lies superficial to the retinaculum of the carpal tunnel, adjacent to the hook of the hamate. Processes that affect or extend into this space may cause the characteristic symptoms.
Guyon’s canal is formed on the medial side by the pisiform and flexor carpi ulnaris muscle and on the lateral side by the hook of the hamate. The splitting of the flexor retinaculum forms the roof of Guyon’s canal. Within the canal lies the ulnar nerve, ulnar artery, and vena comitans.61 Pain in the ulnar aspect of the palm may be caused by a pathologic condition in the canal and may involve the hook of the hamate, pisiform, ulnar artery or vein, or ulnar nerve. A tumor such as a lipoma or ganglion that extends into or arises within the canal also may cause symptoms by compressing any of these structures.
Fractures involving the hamate or pisiform may be difficult to visualize on plain films and are best evaluated on a carpal tunnel view or CT scan. An anteroposterior oblique view also should be obtained to assess the possibility of pisotriquetral arthritis. Pisotriquetral arthritis may be idiopathic or the result of a fracture. MRI is the best option for examining vascular and other soft tissue structures. Routine MRI and MRA can definitively establish vessel patency noninvasively.
As with CTS, MRI may reveal masses, most commonly ganglion cysts or inflammatory changes within Guyon’s canal. Compression of the ulnar nerve most commonly results from fibrous bands, which may not be visualized on MRI. Beware of looped ulnar vessels, which may mimic ganglia in this region.
Several types of arthritis affect the wrist, including osteoarthritis (OA), RA, crystal deposition diseases (most notably gout and calcium pyrophosphate deposition disease), and hemochromatosis. The presence of cysts and the specific distribution of disease can be helpful in differentiating the various types of arthritis.
Because the wrist is a non-weight-bearing joint, OA is usually secondary to other processes, such as prior trauma, inflammatory arthridites (RA), or infection. The radial distribution of OA is well recognized, with changes usually confined to the first carpometacarpal joint and the trapezioscaphoid space of the midcarpal joint. Clinical symptoms include pain, restricted movement, and instability. Increasing radial subluxation of the metacarpal base, narrowing of the interosseous space, sclerosis and cystic change of the subchondral bone, and osteophytosis is seen radiographically. Changes of OA on MRI are characterized by joint space loss, subchondral cyst or geode formation, and bony production manifested by subchondral sclerosis and osteophyte formation.
Focal arthritis also may occur secondary to a type II lunate or in the scapholunate advanced collapse (SLAC) wrist. OA at the base of the first metacarpal often demonstrates marked proliferative change, with large osteophyte production and synovial cysts that can sometimes mimic masses on MRI. This form of OA also can mimic a subluxation because the osteophytes may appear to displace the base of the first metacarpal radially. OA about the scaphoid is common and is not usually related to instability; however, this type of OA can be the sequela of a ligament tear or instability, particularly if the arthritis is focal. Usually, this focal type of arthritis occurs as the result of an SL or LT ligament tear.
The SLAC wrist may occur as a complication in patients with long-standing SL ligament tears and secondary widening of the SL interval. Proximal migration of the capitate is present, leading to focal arthritis at the capitoscaphoid and capitolunate joints. On MRI, the SLAC wrist demonstrates cartilage loss and subtle marrow edema at the distal central SL joint and the proximal capitate. The SLAC wrist may lead to CTS because of the shortening of the carpal tunnel with an infolding of the median nerve.
The last subtype of OA occurs in patients with a type II lunate. In this variant, an extra facet of the lunate articulates with the hamate. This is a common finding, occurring in approximately half of affected individuals. Accelerated cartilage loss with underlying marrow edema may be seen as the sequelae of the type II lunate. Frank OA may appear at the lunatohamate junction. The edema from the cartilage loss in this entity may mimic AVN or stress fractures of the hamate.
The next most common type of arthritis to affect the wrist is RA. RA has certain characteristic sites of involvement in the wrist, including the ulnar styloid (secondary to extensor carpi ulnaris synovitis) and the first and second MP joints. The cartilage loss in RA presents as diffuse, symmetrical joint space loss in contrast to the focal changes seen in OA. Joint effusions are common, with marked synovial proliferation (pannus) often seen. This pannus mimics fluid on T2-weighted images but may also demonstrate low signal secondary to ferritin deposition. The number and size of erosions, as well as the volume of pannus, are good markers for rheumatoid activity. These findings may be used to assess the effectiveness of medical intervention in this disease.
Calcium pyrophosphate deposition disease (CPPD) often affects the wrist. Usually, calcification is seen in the TFC, but it also may affect the SL and LT ligaments. Capsular calcification also can occur, but this type of calcification is usually senile or related to hyperparathyroidism caused by renal failure. On MRI, it may be difficult to see the calcifications of CPPD. This is because the TFC is normally low in signal intensity; therefore the signal void of calcification does not show up against it. Occasionally, the calcifications may appear bright, particularly on intermediate-weighted images. Calcifications may mimic a TFC, SL, or LT ligament tear because of the artifactual junction between the low signal calcification and the higher signal of a degenerated, but not torn, ligament.
The urate crystal deposition of gout tends to be low in signal on T1- and T2-weighted images. Sharply marginated erosions usually are seen, and pancarpal involvement often occurs, with variably sized lesions of high signal on T2-weighted images. Diseases with similar signal characteristics include amyloidosis, synovial chondromatosis, and pigmented villonodular synovitis (PVNS). All of these entities also show variable, but often significant, susceptibility artifact. Of note, some types of productive OA can occasionally have a peculiar appearance, which may mimic gout or PVNS. Last, the arthritis resulting from silicone implants also can have a similar appearance to this group of disorders.
Most displaced wrist fractures can be adequately assessed by plain film. Frontal and lateral views are routine projections, with radiographs obtained during radial and ulnar deviation helpful in the evaluation of the carpal bones. In addition to evaluation for carpal fracture, the orientation of the carpal rows and the intercarpal relationships are important in the assessment of carpal instability. Fractures of the scaphoid are most common, with classification and prognosis for healing or complications based on the location of the fracture line and displacement of the fracture. Isolated fractures of the triquetrum (dorsal surface), lunate, and hamate are less common but also are seen.
The most commonly suggested occult fracture of the wrist involves the scaphoid. In the clinical setting, advanced imaging is often required because scaphoid fractures are difficult to visualize on routine radiographs. Proper radiographic evaluation of the scaphoid has been proposed as requiring four views: a PA in ulnar deviation with fist position, a lateral view, and oblique views with 60 degrees of pronation and supination.62 In the past, scintigraphy, routine tomography, and CT had been used to examine occult fractures, but even in cases with negative CT or tomographs, patients often were treated as if a true fracture were present, if the clinical suspicion was high enough. Treatment was withdrawn if follow-up radiographs remained negative.
More recently, MRI has become the test of choice to assess for the presence of a scaphoid fracture, having been found to have sensitivity and specificity of 95% to 100%, with 100% interobserver agreement.63 In patients in whom scaphoid fractures are clinically suggested, MRI can clearly demonstrate the abnormality, which appears as a dark, linear fracture line on T1-weighted images. It also has the advantage of showing other associated occult fractures, usually involving the triquetrum or distal radius. The MRI protocol should include coronal T1 and STIR images. If the STIR images are negative, a fracture can be confidently excluded. Many patients with suggested scaphoid fractures have only soft tissue injuries, including collateral ligament tears, peripheral TFCC tears, radial TFCC avulsions, and SL or LT ligament tears.
Other imaging modalities used to detect scaphoid fractures, such as scintigraphy and CT, are also used and may be necessary in patients who are unable to have an MRI. CT and conventional radiography have been compared in detecting displacement of scaphoid fractures greater than 1 mm.64 Radiography had low sensitivity (52%) because of its inability to detect volar/dorsal displacement. Likewise, CT had difficulty detecting radial/ulnar displacement (sensitivity 49%). Scintigraphy was found to detect bony abnormalities more frequently than CT, although patients preferred CT since there is less of a time requirement.65 The sensitivities and specificities of MRI compared with scintigraphy in detecting occult scaphoid fractures at 19 days from injury have been found to be 100% and 100% for MRI, respectively, and 83% and 95%, respectively, for scintigraphy.66 Finally, when MRI is compared with CT, MRI is better able to detect trabecular involvement, whereas CT more reliably detected cortical involvement.67
Occult fractures may occur elsewhere in the wrist, but they are much less common and have infrequent complications. Triquetral fractures are the second most common carpal fracture, but they rarely require advanced imaging for diagnosis. The third most common carpal bone fracture involves the hamate. Hamate fractures usually occur either dorsally or involve the hook, with both areas often difficult to visualize radiographically. Thin-section axial CT images through this area may be required to diagnose a fracture. On MRI, hook of the hamate fractures are best evaluated on axial images, and they are clinically important because of the proximity to Guyon’s canal and the possibility of ulnar nerve impingement. Even though lunate fractures are rare, AVN is a common complication (similar to the scaphoid). Some cases of Kienböck’s disease likely result from this post-traumatic scenario. Occasionally, cysts in the lunate may mimic fractures.
Bone bruises are intimately related to occult fractures, and differentiating the two by MRI alone can be difficult. Bone bruises should be diffuse, have little articular or cortical extension, and show no obvious fracture line. In the wrist, however, occult fractures may be present with these findings alone. Bone bruises, although they may be symptomatic, heal spontaneously in 8 to 10 weeks without treatment and have no known sequelae. Occult fractures, on the other hand, are true fractures and may go on to nonunion and the other typical complications. It is our philosophy that in the hand and wrist, abnormalities seen on both T1 and STIR (or fat-suppressed T2 images) represent true fractures. Those injuries with abnormalities on STIR sequences only should be termed bone bruises.
Lunate fractures, proximal capitate fractures, and hook of hamate fractures may occur because of stress injuries; however, stress fractures are much less common in the upper extremity than the lower extremity. The differentiation of an acute traumatic fracture from a stress fracture is made on the basis of time. An acute traumatic fracture occurs at one specific episode of time, but a stress fracture has no discrete episode leading to it. In general, stress fractures are more common in the lower extremities, but they can occur in the wrist, especially in active sports participants. Before a stress fracture occurs, there is the normal physiologic response of the bone to attempt to remodel (Wolff’s law). This remodeling might be visible on MRI as subtle areas of marrow edema. The stress response may be painful and can show uptake of moderate intensity on bone scintigraphy. The stress response can be thought of as a bone bruise, which can be a self-limited condition that heals spontaneously once the causative activity is stopped. If this activity continues, a fatigue-type stress fracture may result. This occurs in normal bones undergoing abnormal stresses. In older individuals, insufficiency-type fractures may occur when injury is due to normal stresses applied to abnormal underlying bone (usually as a result of osteoporosis).
Nonunion or delayed union occurs when a fracture does not heal within the first 6 months following the injury. Delayed union refers to complete healing occurring after a significant time interval, but healing never occurs in nonunion. Systemic diseases such as alcoholism, pancreatitis, cirrhosis, and malnutrition all contribute to delayed union. Nonunion is usually related to the type and location of the fracture and whether it was immobilized adequately or not. In particular, scaphoid fractures have a relatively high incidence of nonunion. Scaphoid nonunion may be fibrous, cartilaginous, or synovial (pseudoarthrosis).
Untreated occult fractures of the scaphoid that become nonunions can develop cystlike defects seen on conventional radiographs, often misreported as plain cysts.68 Advanced imaging is appropriate for patients with persistent wrist pain and negative plain radiographs in whom an occult scaphoid fracture is suggested. On MRI, a fibrous nonunion demonstrates low signal on T1- and T2-weighted images. Because this appearance is similar to the MRI characteristics of some united fractures, only the presence of motion on physical examination can definitely define nonunion. Evaluation with thin-section CT may be a helpful adjunct in evaluating for subtle bony bridging. Motion at a site of nonunion is indirectly visible on MRI as high T2 signal about the fracture. This perifractural edema is either a sign of healing or abnormal motion, and distinguishing between the two can be difficult. In small bones, this edema is more likely secondary to pathologic motion. Cartilaginous nonunion shows intermediate T1- and T2-weighted signal and also can demonstrate motion or edema around the fracture line. The presence of synovial fluid in the fracture line after 6 months indicates a pseudoarthrosis or synovial nonunion. To diagnose a pseudoarthrosis on MRI, the fluid at the fracture site should be differentiated from the perifractural edema of healing by using heavily T2-weighted images. The granulation tissue of normal fracture healing fades on these long TE (time to echo) images.
The most common mass in the wrist is a ganglion. Occasionally, these can extend intraosseously, typically into the lunate. Ganglia are often signs of an internal derangement and may be a cause of pain if occult. The second most common wrist mass is a lipoma, characteristically located in the thenar or hypothenar eminence. Within the hand, tendon sheath fibromas and giant-cell tumors of the tendon sheath may occur. Distally, masses may occur in the tuft of the digits. These include glomus tumors, foreign body granulomas, paronychia, or felons. MRI is the preferred modality for imaging soft tissue masses in the extremities.69
Dorsal wrist pain may be caused by pathology in the extensor tendon compartment, dorsal wrist capsule, or bony structures.
Evaluation of the bony structures begins with routine plain views. If the bone density is increased, advanced Kienböck’s disease is a possibility; otherwise, early AVN of the lunate must be ruled out. Bone scintigraphy is then performed and, in Kienböck’s disease, may show decreased uptake within the first 48 hours and then increased uptake as the bone repairs. Bone scintigraphy is nonspecific, however, and demonstrates increased uptake wherever bone turnover is increased, such as in joints with synovitis. It also provides poor anatomic resolution and does not effectively distinguish between the joint space and the bone. MRI provides far greater resolution than bone scintigraphy in the evaluation of AVN. It also can reveal pathology of the bone vascularity earlier than any other imaging techniques (Fig. 13-29).
Figure 13-29 Avascular necrosis of the lunate (arrow). Coronal magnetic resonance image shows a dark area in the marrow. The corresponding nuclear medicine scan is seen in Figure 13-14.
Hypertrophy of the synovium of the dorsal capsule may be clinically indistinguishable from other causes of dorsal wrist pain, but MRI can provide a definitive diagnosis because of its excellent resolution of soft tissue structures (Fig. 13-30, online).
Figure 13-30 This patient’s dorsal wrist pain was caused by hypertrophy of the dorsal capsule (arrow), seen in this sagittal magnetic resonance imaging (MRI) scan. MRI is useful in distinguishing this condition from avascular necrosis of the lunate.
Disorders of the hand and wrist can be complex. Plain radiography is a standard imaging technique for many disorders, yet advanced imaging with bone scan, computed tomography, and magnetic resonance imaging may be necessary for evaluation. Advanced imaging provides the ability to identify painful conditions such as occult fractures and avascular necrosis in a more timely fashion. Accurate assessment of fracture alignment and healing is possible. Visualization of important soft tissue structures can also be detected. Cost–benefit aspects should be considered as well to prevent unnecessary use of more costly imaging modalities. Finally, imaging modalities provide information that allow health care workers to have knowledgeable discussions with patients and help provide direction for appropriate treatment options.
1. Linn MR, Mann FA, Gilula LA. Imaging the symptomatic wrist. Orthop Clin. 1990;21:515–543.
2. Mann FA, Wilson AJ, Gilula LA. Radiographic evaluation of the wrist: what does the hand surgeon need to know. Radiology. 1992;184:15–24.
3. Manaster BJ. Cortical efficacy of triple injection arthrography. Radiology. 1991;178:267–270.
4. Buckwalter KA, Swan JS, Braunstein EM. Evaluation of joint disease in the adult hand and wrist. Hand Clin. 1991;7:135–151.
5. Reinus WR, Conway WF, Totty WG, et al. Carpal avascular necrosis MR imaging. Radiology. 1986;160:689–693.
6. Lawand A, Foulkes GD. The clenched pencil view: a modified clenched fist scapholunate stress view. J Hand Surg. 2003;28A:414–418.
7. Heuck A, Bonél H, Stäbler A, Schmitt R. Imaging in sports medicine: hand and wrist. Eur J Radiol. 1997;26:2–15.
8. Peterson JJ, Bancroft LW, Kransdorf MJ. Principles of bone and soft tissue imaging. Hand Clin. 2004;20:147–166.
9. Biondetti PR, Vannier MW, Gilula LA, Knapp R. Wrist: coronal and transaxial CT scanning. Radiology. 1987;163:149–151.
10. Sowa DT, Holder LE, Patt PG, Weiland AJ. Application of magnetic resonance imaging to ischemic necrosis of the lunate. J Hand Surg. 1989;14A:1008–1016.
11. Metz VM, Schratter M, Dock WI. Age associated changes of the triangular fibrocartilage: evaluation of the diagnostic performance of MR imaging. Radiology. 1992;184:217–220.
12. Perlik PC, Guilford WB. Magnetic resonance imaging to assess vascularity of scaphoid nonunion. J Hand Surg. 1991;16A:479–484.
13. Greenspan A. Upper limb III: distal forearm, wrist, hand. In: Orthopaedic Imaging: A Practical Approach. ed 4 Philadelphia: Lippincott Williams & Wilkins; 2004:165–214.
14. Bohndorf K, Imhof H, Pope TL. Trauma. In: Musculoskeletal Imaging: A Concise Multimodal Approach. New York: George Thieme Verlag; 2001.
15. Swan JS, Heiner JP, Rao VK, Weber DM. Preoperative evaluation of giant cell tumors of the radius with MR angiography. J Hand Surg. 1993;18A:499–503.
16. Linkous MD, Gilula LA. Wrist arthrography today. Radiol Clin North Am. 1998;36:651–672.
17. Vogelzang RL. Arteriography of the hand and wrist. Hand Clin. 1991;7:63–86.
18. Bohndorf K, Kilcoyne RF. Traumatic injuries: imaging of peripheral musculoskeletal injuries. Eur Radiol. 2002;12:1605–1616.
19. Oneson SR, Scales LM, Timins ME, et al. MR imaging interpretation of the Palmer classification of triangular fibrocartilage complex lesions. Radiographics. 1996;16:97–106.
20. Weiss A-PC, Akelman E, Lambiase R. Comparison of the findings of triple-injection cinearthrography of the wrist with those of arthroscopy. J Bone Joint Surg. 1996;78A:348–356.
21. Greenspan A. Imaging techniques in orthopaedics. In: Orthopaedic Imaging: A Practical Approach. ed 4 Philadelphia: Lippincott Williams and Wilkins; 2004:17–37.
22. Pin PEG, Semenkovich JW, Young VL. Role of radionuclide imaging in the evaluation of wrist pain. J Hand Surg. 1988;13A:810–814.
23. Miller RJ. Information that orthopedists still need to know and what is missing from the MR images of the wrist. Semin Musculoskelet Radiol. 2001;5:211–216.
24. Miller RJ. Wrist MRI and carpal instability: what the surgeon needs to know, and the case for dynamic imaging. Semin Musculoskelet Radiol. 2001;5:235–240.
25. Lee JHE. Imaging modalities. In: Johnson TR, Steinbach LS, eds. Essentials of Musculoskeletal Imaging. Rosemont, Ill.: American Academy of Orthopaedic Surgeons; 2004:3–30.
26. Merl T. Results of a prospective multicenter study for evaluation of the diagnostic quality of an open whole-body low-field MRI unit: a comparison with high-field MRI measured by the applicable gold standard. Eur J Radiol. 1999;30:43–53.
27. Binkovitz LA, Berquist TH, McLeod RA. Masses of the hand and wrist: detection and characterization with MR imaging. AJR Am J Roentgenol. 1990;154:323–526.
28. Binkovitz LA, Ehman RL, Cahill DR, Berquist TH. Magnetic resonance imaging of the wrist: normal cross-sectional imaging and selected abnormal cases. Radiographics. 1988;8:1171–1202.
29. Zeiss J, Jakab E, Khimji T, Imbriglia J. Ulnar tunnel at the wrist (Guyon’s canal): normal MR anatomy and variants. AJR Am J Roentgenol. 1992;158:1081–1085.
30. Protas JM, Jackson WR. Evaluating carpal instabilities with fluoroscopy. AJR Am J Roentgenol. 1980;135:137–140.
31. Cerofolini E, Luchetti R, Pederzini L, et al. MR evaluation of triangular fibrocartilage complex tears in the wrist. J Comput Assist Tomogr. 1990 Nov-Dec;14:963–967.
32. Golimbu CN, Firooznia H, Melone CP Jr, et al. Tears of the TFCC of the wrist: MR imaging. Radiology. 1989;173:731–733.
33. Mesgarzadeh M, Schneck CD, Bonakdarpour A, et al. Carpal tunnel: MR imaging. Part II. Carpal tunnel syndrome. Radiology. 1989;171:749–754.
34. Teefey SA, Middleton WD, Patel V, et al. The accuracy of high-resolution ultrasound for evaluating focal lesions of the hand and wrist. J. Hand Surg. 2004 May;29A:393–399.
35. Naredo E, Moller I, Moragues C, et al. Interobserver reliability in musculoskeletal ultrasonography: results from a teach the teachers rheumatologist course. Ann Rheum Dis. 2006;65:14–19.
36. Zanetti M, Hodler J, Gilula LA. Assessment of dorsal or ventral intercalated segmental instability configurations of the wrist: reliability of sagittal MR images. Radiology. 1998;206:339–345.
37. Theumann NH, Pfirrmann CWA, Antonio GE, et al. Extrinsic carpal ligaments: normal MR arthrographic appearance in cadavers. Radiology. 2003;226:171–179.
38. Scheck RJ, Romagnolo A, Hierner R, et al. The carpal ligaments in MR arthrography of the wrist: correlation with standard MRI and wrist arthroscopy. J Magn Reson Imaging. 1999;9:468–474.
39. Manton GL, Schweitzer ME, Weishaupt D, et al. Partial interosseous ligament tears of the wrist: difficulty in utilizing either primary or secondary MRI signs. J Comput Assist Tomogr. 2001 Sep-Oct;25:671–676.
40. Bille B, Harley B, Cohen H. A comparison of CT arthrography of the wrist to findings during wrist arthroscopy. J Hand Surg. 2007;32A:834–841.
41. Schmid MR, Schertler T, Pfirrmann CW, et al. Interosseous ligament tears of the wrist: comparison of multi-detector row CT arthrography and MR imaging. Radiology. 2005;237:1008–1013.
42. Theumann NH, Etechami G, Duvoisin B, et al. Association between extrinsic and intrinsic carpal ligament injuries at MR arthrography and carpal instability at radiography: initial observations. Radiology. 2006;238:950–957.
43. Haims AH, Schweitzer ME, Morrison WB, et al. Limitations of MR imaging in the diagnosis of peripheral tears of the triangular fibrocartilage of the wrist. Am J Roentgenol. 2002;178:419–422.
44. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg. 1989;14A:594–606.
45. Zanetti M, Linkous MD, Gilula LA, Hodler J. Characteristics of triangular fibrocartilage defects in symptomatic and contralateral asymptomatic wrists. Radiology. 2000;216:840–845.
46. Blazar PE, Chan PSH, Kneeland JB, et al. The effect of observer experience on magnetic resonance imaging interpretation and localization of triangular fibrocartilage complex lesions. J Hand Surg. 2001;26A:742–748.
47. Potter HG, Weiland AJ, Schatz JA, et al. The utility of high-resolution magnetic resonance imaging in the evaluation of the triangular fibrocartilage complex of the wrist. J Bone Joint Surg. 1997;79A:1675–1684.
48. Haims AH, Schweitzer ME, Morrison WB, et al. Internal derangement of the wrist: indirect MR arthrography versus unenhanced MR imaging. Radiology. 2003;227:701–707.
49. Berná-Serna JD, Martínez F, Reus M, et al. Evaluation of the triangular fibrocartilage in cadaveric wrists by means of arthrography, magnetic resonance (MR) imaging, and MR arthrography. Acta Radiol. 2007;1:96–103.
50. Mino DE, Palmer AK, Levinsohn EM. Role of radiography and computerized tomography in the diagnosis of the subluxation and dislocation of the distal radioulnar joint. J Hand Surg. 1983;8:23.
51. Park MJ, Kim JP. Reliability and normal values of various computed tomography methods for quantifying distal radioulnar joint translation. J Bone Joint Surg. 2008;90A:145–153.
52. Tomaino MM. The importance of the pronated grip x-ray view in evaluating ulnar variance. J Hand Surg. 2000;25A:352–357.
53. Imaeda T, Nakamura R, Shionoya K, Makino N. Ulnar impaction syndrome: MR imaging findings. Radiology. 1996;201:495–500.
54. Nathan R, Schneider LH. Classification of distal radioulnar joint disorders. Hand Clin. 1991;7:239–247.
55. Reicher MA, Kellerhouse L. Carpal Instability in MRI of the Wrist and Hand. New York: Raven Press; 1990.
56. Russell RC, Williamson DA, Sullivan JW, et al. Detection of foreign bodies in the hand. J Hand Surg. 1991;16A:2–11.
57. Schweitzer ME, Brahme SK, Hodler J, et al. Chronic wrist pain: spin-echo and short tau inversion recovery MR imaging and conventional and MR arthrography. [published erratum appears in Radiology 1992;184:583] Radiology. 1992;182:205–211.
58. Zanetti M, Saupe N, Nagy L. Role of MR imaging in chronic wrist pain. Eur Radiol. 2007;17:927–938.
59. Meema HE. Radiologic study of endosteal, intracortical, and periosteal surfaces of the hand bones in metabolic bone disease. Hand Clin. 1991;7:37–51.
60. Mesgarzadeh M, Schneck CD, Bonakdarpour A, et al. Carpal tunnel: MR imaging. Part I. Normal anatomy. Radiology. 1989;171:743–748.
61. Wilson AJ, Gilula LA, Mann FA. Unidirectional joint communications in wrist arthrography: an evaluation of 250 cases. Am J Roentgenol. 1991;157:105–109.
62. Toth F, Sebestyen A, Balint L, et al. Positioning of the wrist for scaphoid radiography. Eur J Radiol. 2007 Oct;64:126–132.
63. Breitenseher MJ, Metz VM, Gilula LA, et al. Radiographically occult scaphoid fractures: value of MR imaging in detection. Radiology. 1997;203:245–250.
64. Temple CL, Ross DC, Bennett JD, et al. Comparison of sagittal computed tomography and plain film radiography in a scaphoid fracture model. J Hand Surg. 2005 May;30A:534–542.
65. Groves AM, Cheow H, Balan K, et al. 16-MDCT in the detection of occult wrist fractures: a comparison with skeletal scintigraphy. Am J Roentgenol. 2005;184:1470–1474.
66. Fowler C, Sullivan B, Williams LA, et al. A comparison of bone scintigraphy and MRI in the early diagnosis of occult scaphoid waist fracture. Skeletal Radiol. 1998;27:683–687.
67. Memarsadeghi M, Breitenseher MJ, Schaefer Prokop C, et al. Occult scaphoid fractures: comparison of multidetector CT and MR imaging—initial experience. Radiology. 2006;240:169–176.
68. Rennie WJ, Finlay DB. Posttraumatic cyst-like defects of the scaphoid: late sign of occult microfracture and useful indicator of delayed union. AJR Am J Roentgenol. 2003;180:655–658.
69. Peh WCG, Trouong NP, Totty WG, Gilula LA. Pictorial review: magnetic resonance imaging of benign soft tissue masses of the hand and wrist. Clin Radiol. 1995;50:519–525.