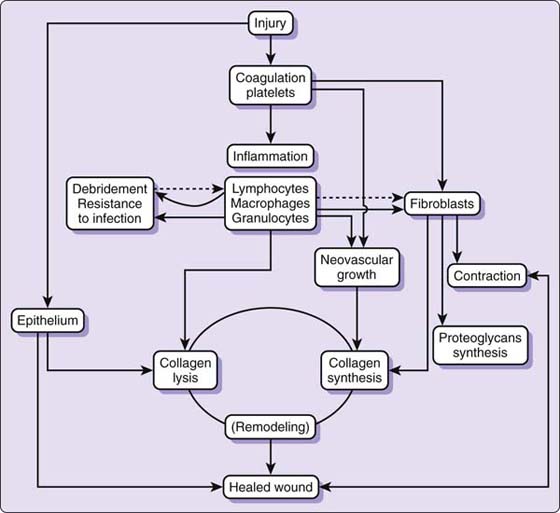
Figure 18-1 Schematic concept of wound healing. (From Hunt TK, Heppenstall RB, Pines E, Rovee D, eds. Soft and Hard Tissue Repair, New York, 1984, Praeger, an imprint of Greenwood Publishing Group, Westport, Connecticut.)
▪ Wound healing occurs in three stages and is characterized by changes in cellularity as different cell types migrate into and out of the wound bed.
▪ Recent advances in the bioregulation of normal wound repair include means for decreasing adhesion formation, the use of growth factors as a strategy for increasing early repair strength, and tissue engineering for the creation of replacement tissues.
▪ Wounds are evaluated in terms of risk factors for altered healing, the presence or absence of infection, physical location, size, appearance, and the stage of healing.
▪ The key issue in wound management is understanding the physiologic effect of such treatment steps as debridement, cleansing, disinfection, dressing, or the use of modalities of motion on the natural response of wound healing.
Comprehensive treatment of the patient with an upper extremity injury is often initiated within days following injury or surgery. Knowledge of the biology of the wound-healing process is an integral component of successful hand therapy practice. The purpose of this chapter is to review both seminal literature that has stood the test of time and offer current evidence as a means of advancing therapeutic intervention in the treatment of the healing wound.
Knowledge of wound biology and the physiology of tissue repair is the basis of clinical decision making in the treatment of the simple, complex, or multilayered wound.
Wound healing is a cellular event. Each phase of wound healing is characterized by changes in cellularity as different cell types, primarily neutrophils, monocytes, macrophages, fibroblasts, and endothelial cells, migrate into and out of the wound bed1-5 (Fig. 18-1). This cellular activity, initiated by tissue and platelet disruption, is regulated by a complex interaction of biochemical exchanges that orchestrate the events of phagocytosis, neovascularization, and biosynthesis of reparative collagen.6-11

Figure 18-1 Schematic concept of wound healing. (From Hunt TK, Heppenstall RB, Pines E, Rovee D, eds. Soft and Hard Tissue Repair, New York, 1984, Praeger, an imprint of Greenwood Publishing Group, Westport, Connecticut.)
The dramatic changes in wound-healing activity usually are divided into three overlapping stages.11-17 In the first stage of repair, the inflammatory or exudative stage, the neutrophil and macrophage are responsible for clearing the wound of debris to set the stage for subsequent repair.16,18,19 The macrophage is the most important regulatory cell in the inflammatory stage because it is critical to bactericidal control and is chemotactic to the fibroblast.11,20-23 Secretory products of the macrophage can enhance fibroblast proliferation and collagen synthesis.24 The macrophage also may be important in the normal process of angiogenesis—the formation of new blood vessels in granulation tissue.19,25,26 A nonmitogenic chemoattractant for endothelial cells, possibly derived from macrophages, has been isolated from wound fluid.27
The migration of epithelial cells, the process known as epithelialization, is initiated within hours of injury, sealing the cleanly incised and sutured wound within 6 to 48 hours.28 Epithelial cell movement is stimulated by an apparent loss of cellular contact that occurs with wounding and is stimulated by the process of contact guidance.28 This cellular migration is terminated when advancing cells meet similar advancing cells by the phenomenon known as contact inhibition.15 Epidermal cells migrate toward the area of cell deficit, following the predictable pattern of mobilization, migration, mitosis, and cellular differentiation.15 The cells maintain their numbers by mitosis, both in fixed basal cells away from the wound edges, and in migrating cells, with the net result being a resurfacing of the wound and thickening of the new epithelial layer.28,29 This reepithelialization process is influenced and possibly directed by a bath of cytokines arising from cells in the wound environment and in distant tissues.28
In the second stage of healing, the fibroblastic or reparative stage, the fibroblast begins the process of collagen synthesis.30,31 The fibroblast, signaled by the macrophage, growth factors, or other mononuclear cells, initially secretes the elements of ground substance, protein polysaccharides, and various glycoproteins, and at approximately the fourth to fifth day after wounding, collagen synthesis begins.10,21,29,32
The myofibroblast, a highly specialized form of fibroblast, is thought to be responsible for the phenomenon of wound contraction.33 This contractile fibroblast has the characteristics of both the smooth muscle cell and the fibroblast and is found in open granulating wounds, whereas fibroblasts are found in closed incised wounds.33 Researchers have suggested that the histologic existence of myofibroblasts is related to a transitional state of fibroblasts in granulation tissue, wherein they prepare to migrate from a healed wound.33
Endothelial cells form the new blood vessels in granulation tissue, which provide oxygen and nutrients to the wound site.34 These nutrients are necessary for the synthesis, deposition, and organization of the extracellular matrix.23 Angiogenesis is thought to be directed chemically by growth factors and macrophage-secreted angiogenic peptides.19,23,26,27,35-37
The third stage of wound healing is characterized by the maturation and remodeling of scar tissue or extracellular matrix manufactured during the second or reparative stage.11,15,38 This phase is typically observed after the 21st day, terminating months and perhaps years after the wound has occurred.39 As the healing tissues demonstrate decreased elasticity, a heightened awareness to stress at the wound site reduces the risk of skin breakdown during the reparative stage.39
Age is an important factor to consider in the analysis and management of the healing wound. Increased elasticity and strength of connective tissue facilitates both resilience and healing in the young patient.39 As aging occurs, a decrease in collagen elasticity and fat deposition hinder protective capabilities, resulting in skin that is more easily damaged.39 Chronic diseases, such as diabetes mellitus and renal failure, can confound the wound-healing process, leading to an increased risk for infection and amputation.40,41
Inasmuch as evidence of the cellular sequence of wound healing is abundant and consistent, histologic research has progressed to analyses of molecular events during the healing process. In the specialty of hand surgery, basic science research in the past two decades has focused on modulation of wound healing and adhesion formation, primarily in the healing flexor tendon.
Histologic research in wound healing has significantly evolved over the past three decades. Early studies manipulated the microenvironment of the wound and attempted to enhance cellular activity.42-51 Experimental modalities were pursued for the control or stimulation of wound healing21,37,51-58 and included peptides, cytokines, growth factors, and wound fluids.21,50,59-63 More recent advances in the bioregulation of normal wound repair include means for decreasing adhesion formation, the use of growth factors as a strategy for increasing early repair strength, and tissue engineering for the creation of replacement tissues.
Collagen is the most abundant protein in the human body, significantly contributing to the integrity of connective tissue structures. Despite the integral role of collagen synthesis in wound healing, excessive proliferation of collagen can limit excursion and, ultimately, the function of healing tissues in the upper extremity. Collagen synthesis has been associated with fibroblastic activity,10,21,29,32 and, as such, the chemical modulation of fibroblasts has been experimentally pursued.
Decreased synovial thickening and adhesion formation were observed following intraoperative application of 5-fluorouracil (5FU) in a chicken model.64 In a subsequent study, adhesion reduction did not result in significant differences in excursion, maximal load, or work of flexion compared with results in normal controls.65 5FU has been suggested to have a preferential effect on fibro-osseous fibroblasts,66 and these cells have been specifically targeted as highly responsible for adhesion formation.67 Other chemical inhibitors, including those targeting proteinase and prostaglandin, have been noted to decrease adhesions in animal models.68-71
Hyaluronic acid is a glycosaminoglycan found in the extracellular matrix of skin, cartilage, and synovial fluid. Noted to decrease scar formation and promote healing72-75 this carbohydrate has also been found in human amniotic fluid.76 Injection of human amniotic fluid coupled with tendon sheath repair resulted in fewer adhesions and a higher tensile strength in a rabbit model.76
Human amniotic fluid also contains growth factors, naturally occurring proteins that stimulate growth.76 Because research in adhesion modulation has offered limited clinically observable results, the tensile strength as afforded by growth factors has facilitated a transition toward the cellular repair processes that might accelerate tendon healing.
Vital to the process of wound healing, the differentiation of cell types in the intrasynovial flexor tendon has increased comprehension of both adhesions and tensile strength. Previously differentiated as extrinsic versus intrinsic, the analysis has expanded to consider the catalysts for collagen production at three distinct sites: the tendon sheath, epitenon, and endotenon.77 The delicate balance of scar tissue necessary for tendon integrity as opposed to adhesion formation has guided the careful analysis of growth factors and cell regulation.
The healing process of the intrasynovial flexor tendon has been studied using both in vitro and in vivo animal models. Using a rat model in vivo, Oshiro and colleagues78 demarcated the following sequence: superficial repair, initiation of collagen degradation at day 7, near completion of collagen degradation at day 21, synthesis of new collagen with remodeling, and neovascularization. In this study, preexisting endotenon fibroblasts were observed as the primary reparative cells when no gapping occurred.78 This concurred with seminal work by Gelberman and coworkers,79 who contrasted the role of epitenon fibroblasts in gapped tendons with endotenon fibroblasts in those tendons in which gapping had not occurred.79 In their study, Oshiro and colleagues78 also identified matrix metalloproteinases (MMPs) involved in collagen degradation and remodeling.
An in vitro study of normal rabbit tendons addressed the role of lactate in collagen production.77 Lactate was noted to stimulate both collagen and growth factors and to affect all cell types. Tendon sheath fibroblasts, however, exhibited the greatest proliferation and collagen production.77 Tendon sheath cells were also analyzed in a rat model to determine their presence in the healing flexor tendon.80 These cells were observed at 24 hours, increased in number through the fifth postoperative day, and decreased by day seven.80
The influence of vanadate added to drinking water was studied in two rat models. Following medial collateral ligament repair, vanadate yielded a significant increase in collagen fiber diameter, promoted collagen organization,81,82 and significantly increased biomechanical stiffness and ultimate force.82
Basic fibroblast growth factor (bFGF) has been detected in both normal and injured intrasynovial tendons83 with increased levels in the epitenon and tendon sheath cells during the first eight weeks of healing.84 This growth factor has been extensively studied, with implications toward increased proliferation of tenocytes for collagen production.85 Using a normal rabbit model in vivo, bFGF was observed to increase expression of nuclear factor κB (NF-κB), a cell proliferation regulator.85 NF-κB has been suggested as a possible signaling pathway among growth factors, cell proliferation, and collagen synthesis,85,86 yet clear cause and effect has not been established.87 The delivery of bFGF to the healing tendon has also been studied. Adeno-associated viral vectors (AAV2) significantly increased expression of bFGF in vitro,88 and bFGF-coated nylon suture increased strength and epitenon thickening in vivo.89
Transforming growth factor beta (TGF-β) has been noted to increase fibroblast recruitment and collagen production,90 producing significant increases in collagen observed in epitenon, endotenon, and tendon sheath cells in response to all isoforms.91 These proliferative characteristics spurred the research of TGF-β antibodies, found to decrease adhesions and increase range of motion after repair92 and generally reduce the profibrotic effects in all three types of cells.93
In vitro animal models have established the presence of vascular endothelial growth factor (VEGF) in the healing flexor tendon. This protein has been observed as more prevalent in intrinsic tenocytes than in epitenon cells,94 peaking between 7 and 10 days after repair and returning to baseline by day 14.95 An in vivo rat Achilles tendon repair study attributed increased tensile strength to VEGF.96 Platelet-derived growth factor (PDGF) has been differentially established as present in healing versus normal canine tendons.83 This protein is commercially available for use in chronic wound management, with improved rate of healing being documented in clinical trials.97,98
As understanding of the influence of growth factors has increased, comparative studies have helped to establish their individual roles. An in vitro rat tendon model comparing VEGF and PDGF identified the former as less favorable.99 VEGF was noted to significantly increase TGF-β, leading to adhesions, and the collagen produced was three times weaker than that stimulated by PDGF.99 PDGF and bFGF were also preferable to VEGF and bone morphogenetic protein 2 (BMP-2) in a normal canine model, with increased cell proliferation and collagen production comparatively.100
The concept of tissue engineering has been the focus of much study in the past decade. Woo and associates51 define tissue engineering as the manipulation of biochemical and cellular mediators to effect protein synthesis and to improve tissue remodeling. The new biologic therapies being developed from this basic science research include the application of growth factors to cutaneous wounds and the use of polymer scaffolds, cells, and growth factors to create replacement tissues.
Growth factors have been marketed primarily via products that interact with biologic tissues, often through impregnation in biologic dressings. These dressings are often referred to as skin substitutes, dermal matrix products, or scaffolds, and function as a vascularized dermis for subsequent split-thickness skin grafting.101
Current clinical expectations for these dressings include the following: the dressings must be safe, not cause an immunogenic response, not transmit disease, not be cytotoxic, and not cause excess inflammation.102 Additional properties of interest include biodegradability, ample stability to support tissue reconstruction, sufficiently long shelf life, availability, and ease in handling.102 Integrated sheets, carriers, and sprays have been implemented successfully as dermal substitutes.101-103
Integra (Integra LifeSciences, Plainsboro, NJ) is a well-known dermal substitute that has been specifically reviewed following use in the hand.103 This collagen-based wound repair biomaterial was initially approved as a defect filler for the treatment of severe burns, allowing coverage of large full-thickness wounds to allow delayed split-thickness skin grafting.103 In a case study offered by Carothers and coworkers,103 Integra was sutured in a wound bed following tumor excision in the proximal palm. It is of note that both the median nerve and flexor tendons were exposed following tumor removal.103 The patient was discharged home 1 day postoperatively and encouraged to complete digital motion as a means of decreasing adherence of the exposed structures on the skin substitute.103 The patient underwent subsequent layering of Integra and split-thickness skin grafting 7 weeks after the initial coverage, resulting in full functional use without tendon adhesions.103
Four major challenges have been identified in the study of dermal replacement: safety, substitution for split-thickness skin grafting, improvement of angiogenesis in replacement tissue following graft, and increased ease of use.101 One identified benefit is that tissue-engineered skin could optimally decrease the use of animals in pharmaceutical testing.101
Growth factors have also been studied for direct application to healing bone and ligament. The use of BMP-2 has yielded successful results in human studies of spinal fusion.104-106
The interdisciplinary field of regenerative medicine includes the sciences of biology and engineering. Procedures in this field are enabled by the use of scaffolds, cells, and growth factors. Polymer scaffolds are the structural base on which tissues are grown.107 The ideal scaffold as described by Chong and associates107 is a biocompatible mechanism that demonstrates the integrity and ability to house cells until new tissue regenerates.107 Currently, the size of scaffolds has proved problematic in the maintenance of living cells,107 and technology for injectable systems is under study.108 Bioreactors have been employed for cyclic loading, a procedure that mimics stress and establishes desired physical and biochemical properties.44,109,110
The use of stem cells for tissue engineering has created notable public and scientific controversy. The ethical considerations associated with acquisition of embryonic stem cells and the possibilities of cloning are sources of heated debate. The plasticity of multipotent adult stem cells has been suggested as comparable to embryonic stem cells and is certainly less controversial.109 Adult mesenchymal stem cells can be harvested from bone marrow and fat,110 and these cells are capable of all types of differentiation, including osteogenesis, myogenesis, neurogenesis, and angiogenesis.109 In a rabbit model, epitenon tenocytes, tendon sheath cells, bone marrow–derived stem cells, and adipoderived stem cells all contributed to the successful engineering of flexor tendons; however, use of stem cells hastened proliferation.111
Growth factors are applied to facilitate collagen synthesis and subsequent accrual of strength in the engineered tissues. Despite continued research of growth factors, questions remain regarding necessary concentrations and optimal transfer techniques.110 A complete understanding of cell differentiation and signaling pathways for such differentiation has not been established.110
The successful engineering of a flexor tendon was reported by Cao and colleagues in 2002,112 and Wang and coworkers113 have quite recently engineered an extensor tendon complex using human fetal extensor tenocytes in an ex vivo rat model. Bone marrow–derived stem cells implanted via hydrogel scaffold have also contributed to cartilage formation in the subcutaneous tissue of mice.108
Continued experimental advances in wound healing and tissue engineering will predictably alter clinical management of repaired tendon, nerve, and the complex wound. The integration of biotechnology and the biochemical aspects of wound research may have tremendous relevance to our specialty because many of these new treatments will serve to regulate cellular activity. The application of a more scientific approach to wound healing may alter scar deposition and speed healing, decreasing the associated factors of morbidity: delayed healing, pain, excess fibrosis, longer treatment time, and increased expense. Treatment that may be helpful but not critical for the uncomplicated wound may be obligatory for the complex wound.
This new technology will most likely alter future treatment techniques,51,53,54,114 but these new techniques do not have much clinical application for the hand clinician as of this writing. For the most part, management of the upper extremity cutaneous wound by the hand surgeon or hand therapist is not an issue. The cleanly incised and sutured wound epithelializes within 6 to 48 hours,28 and the noninfected wound allowed to heal by secondary intention is expected to contract at a predictable pace.11,33 The normal phases of wound healing usually proceed without difficulty when the wound is managed with careful debridement of nonviable tissue, physiologic repair, and routine wound care with cleansing and dressing.115 The hand clinician, in most cases, focuses attention on the schedules for healing, immobilization, and mobilization for the deeper injured and repaired tissues. However, with complications of infection or dehiscence, and healing altered by malnutrition, irradiation, medication, immunosuppression, or a poor local blood supply, wound management becomes more of an issue and the significance of scientific clinical management becomes more apparent.29,115-123 Depressed healing associated with vasculitis, venostasis, diabetes, immunosuppression, and burn care has inspired much of the work produced by multidisciplinary specialties that has produced the new clinical treatments with biologic dressings, oxygen therapy, and growth factors.14,124,125 Cancer research has had a significant effect on the body of wound-healing knowledge, providing the early analysis of peptide growth factors.10,36
The next section addresses clinical decision making in wound evaluation and the effects of therapeutic management techniques on the cellular events in the different stages of wound healing.
Traditional wound assessment is well described in the literature.30,35,63,126-133 Wounds are evaluated in terms of risk factors for altered healing, the presence or absence of infection, physical location, size, appearance, and the stage of healing. Wound edema, presence of hematoma, vascular perfusion, and the status of the deeper tissues are noted. The rate of healing in relation to the date of injury or surgery and the duration of previous treatment or chronicity of the wound are important factors in treatment planning.
Assessment of infection includes a review of risk factors for the individual case, visual inspection, and tissue cultures.134-136 Before surgery or medical management, the surgeon will have established the factors that are predictive of susceptibility to infection or an altered rate of healing. This information determines timing of technique for wound closure or surgical management. Risk factors are determined based on an accurate history, including information concerning the mechanism of injury, the environment in which the injury occurred, the patient’s medical and immunosuppressive state, systemic or local nutritional status, and previous medical treatment with medication or radiation.18,115-118,121-123,134,137-139 These risk factors should be known to the hand therapist and the surgeon because the therapist in most cases is monitoring the wound more intensively than the surgeon. Patients at high risk for infection may need to be seen more often than those who will predictably experience benign wound healing.
Visual inspection helps determine whether a wound is healing with a normal inflammatory response or if, in fact, it has become infected. The cardinal signs of inflammation, redness, swelling, pain, and heat, accompany the biochemical and fluid aspects of the early inflammatory stage of wound healing and are not to be confused with infection.11,16 The therapist should understand that purulence does not always represent the presence of infection.29,140 If the inflammatory response is exaggerated or if the drainage is purulent, then bacterial counts must be obtained to determine the level of wound contamination.141
Clinical measurements of wound sepsis are determined by wound culture. The U.S. Institute of Surgical Research defines wound sepsis caused by bacterial overgrowth as bacterial counts exceeding 105 organisms per gram of tissue.57,142 Traumatic wounds with multiple layers of injury to skin, muscle, and bone are difficult to evaluate because the colony count may vary at each level.143
One must understand that wound healing in the clinical situation occurs in the presence of bacteria; it is the quantity of and not the mere presence of bacteria that alters the reparative process.29 Acceptable levels of endogenous, nonpathogenic microflora, as opposed to frank infection, determine the rate of wound healing and may actually stimulate tissue repair.137 Favorable microflora in the wound bed may stimulate epidermal cell migration and healing.144,145 Wound fluid monocyte and macrophage counts have been found to be markedly elevated and collagen deposition increased in wounds inoculated with 102 organisms.145 Lower bacterial counts or well-controlled infection have been found to enhance chemotactic and bactericidal activity.29
However, in the presence of significant infection (greater than 105 organisms per gram of tissue), impaired leukocyte function, decreased chemotaxis, impaired cellular migration, epithelialization, and intracellular killing are noted.29,146 Superficial infection may damage new epithelium through the release of neutrophil proteases,147 and bacterial counts greater than 105 may retard wound contraction.29 Infected wounds are affected adversely by the formation of thicker connective tissue and excessive angiogenesis, which is associated with prolific scar formation.29,30,36,119,145 Robson and colleagues,29 in a review of studies on the effect of bacterial count on fibroplasia, found the results to be inconsistent, but noted that collagen and hydroxyproline contents were consistently higher in infected wounds. Thus, infection control is important not only to the rate of healing but also to the management of scarring, which ultimately can interfere with tissue gliding and excursion for tendon, nerve, ligament, joint, and skin.
The wound healing by primary intention is usually simple to evaluate and treat. Attention in these cases is usually directed to protection of the deeper structures, the status of suture or staples, tension at the suture line, quality or quantity of drainage, and viability of the tissue. These wounds are described in terms of periwound edema, inflammation, infection, wound tension, viability, and rate of epithelialization.
If the wound closed by primary intention develops complications and dehisces, it becomes a wound healing by secondary intention.134 Wounds left to heal by secondary intention, or the chronic or infected wound, pose more complex questions and require more clinical problem-solving and decision-making skills of the health-care practitioner. The following section attempts to simplify decision making and treatment planning for the hand therapist who may be confused by the many issues surrounding the management of the complex wound.
A universal classification system introduced by Marion Laboratories in the late 1980s continues to be the standard with which open wounds are characterized. Their approach uses a “three-color concept” to describe wound status. Wounds are described as red, yellow, black, or a combination of two or three colors. The clinical application of this classification system is described by Cuzzel148-150 in several articles. The following color descriptions for evaluation and treatment are summaries of her articles. Clinical decision making as it relates to therapeutic management by debridement, cleansing, disinfecting, and dressing is reviewed in a brief synopsis as it relates to wound color1,6,149,151 (Table 18-1).
Table 18-1 Clinical Decision Making for Open Wounds
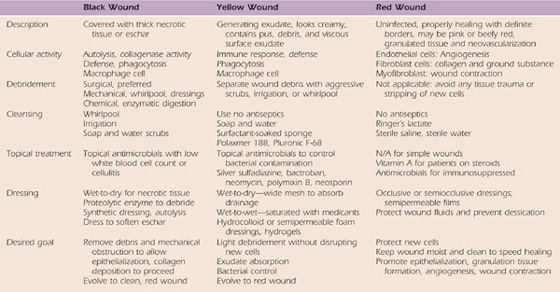
From Evans RB. An update on wound care. Hand Clin. 1991;7:418.
The red wound is uninfected, healing according to a predictable schedule, and characterized by definite borders, granulation tissue, and apparent revascularization (Fig. 18-2). The fibroblast, myofibroblast, endothelial, and epithelial cells are active in this wound, orchestrating the events of epithelialization, angiogenesis, and collagen synthesis. Skin-donor sites or surgical wounds healing by secondary intention, as in the case of an open Dupuytren’s release, are examples of red wounds often seen in the hand clinic. Superficial wounds and acute partial or second-degree burns are classified as red wounds if they are uniformly pink in appearance.
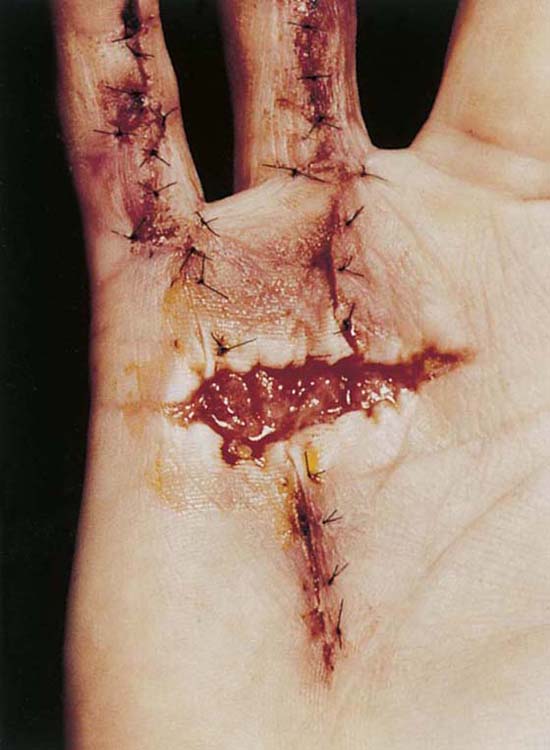
Figure 18-2 A red wound in a Dupuytren’s fasciectomy 3 days after surgery. The wound in the palm is beefy red, without infection, with epithelial, endothelial myofibroblast, and fibroblast cellular activity taking place. Note that the digital wounds are already epithelized.
Tissue oxygenation determines the color of the wound. A chronic red wound has pale pink to beefy red granulation tissue and usually is in the late stages of repair. Red wounds closing by secondary intention fill with granulation tissue from the edge of the wound to the center, closing by contraction and epithelialization, or they may be closed by grafting at the appropriate time.
Cellular activity in the clean red wound must be protected and facilitated by the appropriate therapy. Therapeutic goals are to protect the local wound environment, maintain humidity, protect the wound fluids from desiccation, and protect the newly forming granulation tissue and epithelial cells. These wounds should be cleansed with lactated Ringer’s solution or for home care with a nondetergent, mild pump soap such as Ivory or Dove. The soap should be applied to the periwound area only and rinsed with running water. Antiseptics should not be used on the red wound. Topical treatment may include an antibiotic ointment if the patient is at high risk for developing infection. The newly forming cells should be protected from noxious mechanical forces (tapes, dry dressings, wet-to-dry dressings, whirlpool agitation, and wound scrubbing). Occlusive or semiocclusive dressings, which are described in a later section, may be used to protect the local wound environment and wound humidity.
The yellow wound may range in color from a creamy ivory to a canary yellow. Colonization with Pseudomonas gives the wound a yellow-green appearance and a distinctive odor. The yellow wound is draining, purulent, and characterized by slough that is liquid or semiliquid in texture; it contains pus, yellow fibrous debris, or viscous surface exudate (Fig. 18-3). The exudate may promote bacterial growth. Cellular activity is dominated by the macrophage, which is stimulated by the presence of bacteria and inflammation. The macrophage functions to clear the tissue of debris and to remove pathogenic organisms; thus, it is critical to bactericidal control and phagocytosis.23
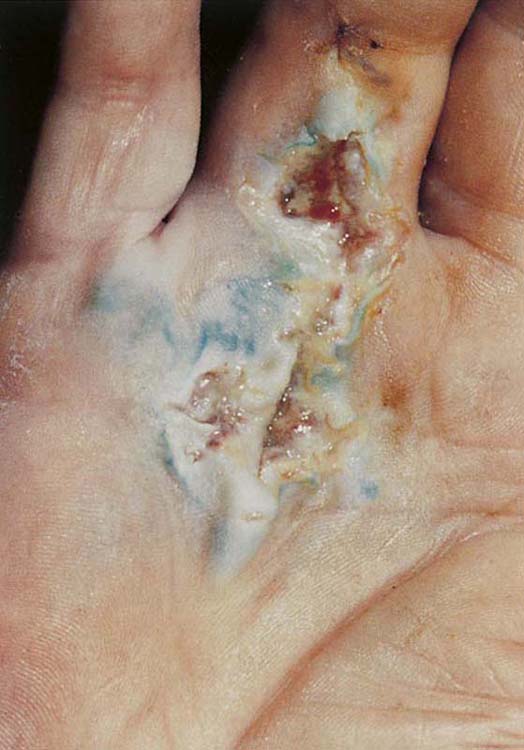
Figure 18-3 A yellow wound infected with Pseudomonas in a postsurgical Dupuytren’s fasciectomy closed by primary intention with subsequent dehiscence. Although some of the green color is from skin pencil, the wound is yellow-green with a distinctive odor. Cellular activity is dominated by the macrophage, which is stimulated by bacteria and inflammation. (From Evans RB: An update on wound management. Hand Clin. 1991;7:419-432.)
Epithelialization and wound contraction, activity controlled by the epithelial cells and myofibroblasts, may be occurring at the pink wound margins but, in general, are delayed until infection or excessive inflammation are under control.
The goal of treatment in the case of the yellow wound is to facilitate cellular activity so that it can evolve into a red wound. Continual cleansing, removal of nonviable tissue, and absorption of excess drainage are important to decrease the workload of the macrophage.17 These wounds may be aggressively washed with soap and water, irrigated with a water pick152 or syringe, or treated with sterile whirlpools to separate surface debris and necrotic tissue. Topical antiseptics are cytotoxic and depress leukocyte function, thus depleting the body’s natural defense mechanism and are thus to be avoided.138,139,142,153,154 If bacterial proliferation requires control, an antibiotic such as Silvadene or Bactroban, and not a topical antiseptic, should be used in the wound.135,155 Wet-to-dry dressings should be placed over only the wound because their application to the periwound area may cause skin maceration. Wet-to-dry dressings should be used with care because their removal may disturb new cells that are forming at the edge of the wound in addition to necrotic tissue. Dressings that absorb excess exudate while maintaining a moist environment, such as semipermeable foams, hydrocolloids, or hydrogels, may be used in the noninfected wound.142
The black wound ranges in color from dark brown to gray-black; it is covered with eschar or thick necrotic tissue (Fig. 18-4). Cellular activity represents several stages of wound repair that may be occurring simultaneously. The macrophage is working to clear the area of bacteria and debris and to signal fibroblasts to the area. The fibroblast and endothelial cells are beginning to synthesize collagen and new vessels as the debris is removed. This cellular activity is facilitated by the removal of the eschar surgically, mechanically, or enzymatically, in an effort to decrease the workload of the macrophage and to allow for unimpeded cellular migration. Eschar impedes cellular migration and proliferation by acting as a mechanical block and provides a medium in which bacteria can proliferate.
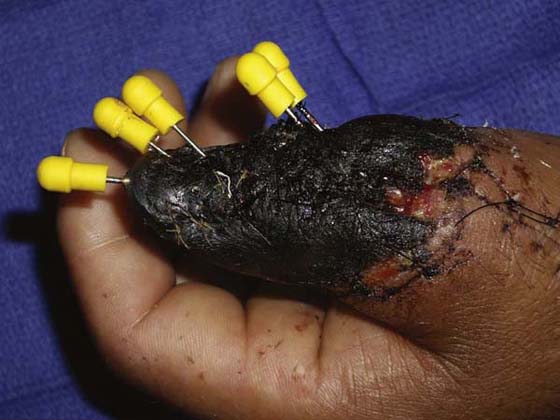
Figure 18-4 A black wound with evident necrosis of the distal thumb following replantation. (Photo courtesy of Dr. Miguel Pirela-Cruz, El Paso, Texas.)
Debridement is the therapeutic goal for the black wound. Meticulous and timely debridement decreases the risk of infection and hastens healing by facilitating normal cellular response. These wounds may be debrided surgically, mechanically, or with proteolytic enzymes135,142,148 such as Travase or Elase. Before mechanical debridement, the tissue may be softened as it is cleansed with scrubs or whirlpool to loosen dead tissue from the viable wound bed.
Topical antibiotics may soften eschar and decrease bacterial count. These wounds should be dressed to protect the wound environment, soften eschar, and facilitate autolysis. Synthetic dressings may facilitate autolysis by protecting wound fluids that contain the white cells responsible for phagocytosis.
Understanding the normal cellular activity of wound repair and regeneration is critical to accurate wound assessment, which in turn determines successful wound treatment. The key issue in wound management is understanding the physiologic effect of such treatment steps as debridement, cleansing, disinfection, dressing, or the use of modalities or motion on the natural response of wound healing. These treatments all contribute, either positively or negatively, to that cellular response.
The therapist can contribute to the wound-healing process by using management techniques that protect wound fluids, help prevent or control infection, minimize adverse mechanical influences, and control the collagen maturation process. Physical agents may facilitate cellular movement associated with increased blood flow, epithelialization, and macrophage or fibroblast activity and may have a role in the stimulation of growth factors. The deleterious effects of desiccation, mechanical trauma, and some topical treatments have been studied in terms of their inhibition of normal cellular function, and the results should alter some currently popular, but unscientific, wound management techniques.147,151,156-160
The positive role of humidity in wound resurfacing, first reported more than four decades ago,161-163 has been recognized as one of the most important factors in wound healing by several researchers.17,56,114,126-130,133,164-168 The maintenance of a moist wound environment in the noninfected wound facilitates both biochemical and cellular activity.4,23,37,132,133,139,142,169-173 Wound fluids contain certain growth factors that interact with the host tissue, promote cellular activity, and contribute to wound metabolism.4,10,16,21,23,27,131,170,174,175 The tissue fluids that accumulate in a wound create a favorable environment for angiogenesis and granulation tissue formation on which epithelialization can occur.3,9,16,22,142,163,170,176
An accelerated rate of healing in moist wounds is supported by histologic evaluation of full-thickness wounds in porcine skin.3 Neutrophils and macrophages decreased in number more rapidly under moist conditions, and the proliferative phase cells (fibroblasts and endothelial cells) increased more rapidly. More rapid progression to the remodeling phase and advanced angiogenesis were noted in moist compared with dry wounds.3
In an unprotected wound, evaporation occurs within hours of tissue disruption, allowing wound fluids to escape the wound bed.142 An open wound exposed to air for 2 to 3 hours becomes necrotic to a depth of 0.2 to 0.3 mm.163 The desiccated dermis or scab impedes epithelial cell migration and acts as a mechanical barrier, creating a dell or depression in the wound as the epidermal cells are required to migrate from the wound margins deep beneath the dried tissue.162 This process is minimized in a wound that is occluded and not allowed to dessicate.128
Occlusion refers to the ability of a wound dressing to allow the transfer of water vapor and gases from a wound surface to the atmosphere.17 The concept of sequestering wound fluids in the noninfected open wound for the purpose of enhancing cellular activity or facilitating autolytic debridement with occlusive or semiocclusive dressings has led to the development of many environmental dressings.56,129,132,142,164-166,168,169,176-179
These microenvironmental dressings may be categorized as films, foams, hydrocolloids, hydrogels, and calcium alginates.17,142,165,166,169,172,173,177,180-184 Although there are substantial differences in these dressings, they are similar in that they maintain wound humidity (are impermeable to water but not always water vapor), may permit exchange of gases, reduce pain, reduce mechanical trauma associated with dressing removal, and absorb exudate in some cases.142 The properties and indications for the dressings are summarized in Table 18-2), and several excellent review articles are recommended for more detailed study.17,58,114,133,142,169,176,182,184,185
Table 18-2 Properties, Indications, and Precautions for Microenvironmental Dressings*
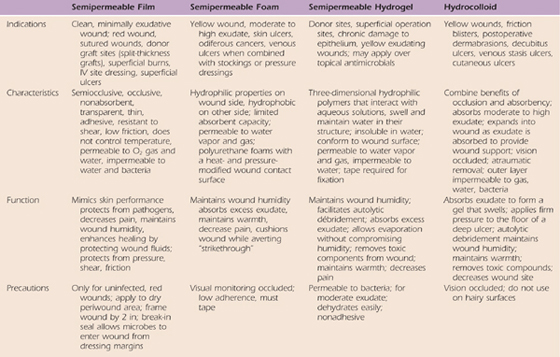
* Disclaimer/contraindications: All environmental dressings must be used in accordance with product information, which provides guidelines for indications, application, and contraindications. Some contraindications are wounds ulcerated into the muscle, tendon, bone; third-degree burns edge-to-edge eschar; wounds associated with osteomyelitis and active vasculitis, ischemic ulcers, and infected wounds. These products are all-inclusive and are not necessarily endorsed by the author or publisher but are provided as a source for further study.
Human skin has measurable transcutaneous electrical potential differences that are decreased with wounding.176,186,187 Dehydration of wound tissue may decrease the lateral electrical gradient thought to control epidermal cell migration.142,188,189 Exposed wounds tend to be more inflamed and necrotic than occluded wounds.17 In later stages, the dermis of exposed wounds is more fibroblastic, fibrotic, and scarred.17
The therapist can contribute to infection control by using the appropriate therapeutic techniques to maintain a clean wound bed free of necrotic tissue or excess drainage, by protecting the wound from its external environment with the proper dressings, and by instructing the patient concerning home wound care.
Cleanly incised and sutured wounds may be washed with a mild soap and running water as early as 24 hours after surgery.190 The red wound may be rinsed with lactated Ringer’s solution, which is more biologically compatible with the wound environment than saline. Some wound therapists currently believe that the pH of saline is too acidic for wound care. Saline, however, continues to serve as a common choice for wound cleansing as it does not cause harm to normal tissue and adequately cleanses most wounds.191
The red wound should not be scrubbed because this mechanical trauma could disrupt newly forming epithelium and vessels.192 The yellow and black wound may be scrubbed with a mild soap and water. Dove or Ivory soap are recommended for home care,193 or Pluronic F-68 or Poloxamer 188, nontoxic surfactants, can be used when more vigorous cleansing is needed.139,151 A high-porosity sponge (90 ppi) may be used for mechanical scrubs because it is minimally abrasive and thus inflicts less tissue damage.139 The object of wound cleansing is to separate soil, particle, and debris from the wound but not to create cellular destruction. Hydraulic irrigation with a water pick or whirlpool are indicated only for yellow and black wounds to loosen debris from the wound bed.155,172 Pressures between 4 and 15 psi are recommended for wound cleansing.191
Cleansing solutions such as Hibiclens, hexachlorophene, and povidone-iodine (Betadine) may be used on intact skin before surgery on the periwound area, but if applied to the wound itself, they are cytotoxic and invite infection by destroying macrophages.118,138,151,158,194
Several authors have studied the adverse effects of povidone-iodine.118,139,151,194 Aronoff and coworkers195 has demonstrated that long-term povidone-iodine topical application may result in systemic absorption with resulting negative effects. Wound epithelialization and early tensile strength are affected negatively by 1% povidone-iodine solution. Researchers have reported that this solution must be diluted to 0.001% concentration to be nontoxic to human fibroblasts.196 At this strength, the solution is still bactericidal to Staphylococcus aureus. However, Rodeheaver151 has demonstrated that cleansing with povidone-iodine offers no advantage over cleansing with saline solution. He found the same level of viable bacteria in wounds contaminated with S. aureus when treated with either saline or povidone-iodine. Both hexachlorophene and povidone-iodine scrubs have been found to instantaneously lyse white blood cells that are critical to wound defense,151 and povidone-iodine damages red blood cells, resulting in significant hemolysis.197 Feedar and Kloth142 urge that povidone-iodine solution in whirlpools and on gauze dressings be reconsidered, and other authors139,151,198 recommend cessation of this practice altogether.
Although we all have observed wound healing in the presence of these cleansing agents, it may be that the wounds we have treated could have responded more quickly, decreasing time, discomfort, and expense, had we more carefully protected the wound fluids and cellular environment.
Many wound specialists have condemned the practice of decontaminating a wound after cleansing with topical antiseptics. The often-quoted adage that “the only solution that should be placed in a wound is one that can safely be poured in the physician’s eye” is supported by most wound therapists.156 Rodeheaver and colleagues155 has demonstrated that all antiseptic agents are cytotoxic, and their only mechanism of action is to destroy cell walls. Almost four decades ago he reviewed commonly used antiseptics and found iodine, chlorhexidine, peroxide, boric acid, alcohols, hexachlorophene, formaldehyde, hypochlorite, acetic acid, silver nitrate, merthiolate, gentian violet, permanganate, and aluminum salts to be cytotoxic.155
Hydrogen peroxide (H2O2), which has little bactericidal action, is perhaps misused as often as povidone-iodine. Hydrogen peroxide is appropriately used on a crusted wound, or to cleanse periwound skin, but should not be used after crust separation, on new granulation tissue, or on closed wounds.142
Researchers have suggested that topical antibiotics are the only antimicrobial agents that are nontoxic and beneficial to wound cellular activity.155 Mupiricin (Bactroban) is a broad-spectrum antimicrobial recommended for its bactericidal capacity, which is greater than that of other topical antimicrobials. Neosporin ointment has a wide spectrum of bactericidal activity, including against most gram-positive and gram-negative bacteria found in both human and porcine skin.142 Zinc bacitracin, which is one of the three antibacterial components of Neosporin, was found to increase epidermal healing by 25% compared with controls.142 Contaminated blister wounds treated with the triple antibiotic in Neosporin (neomycin, polymyxin B, and bacitracin) ointment demonstrated lower bacterial counts and faster healing than with similar wounds treated with only protection or antiseptics.199 One percent silver sulfadiazine (Silvadene) cream acts on a wide range of gram-negative and gram-positive bacteria as well as fungi. It has been used to prevent infection in burn wounds200 and to salvage some or all parts of questionable flaps.135 Silvadene treatment has been reported to reduce bacterial counts in wounds contaminated with less than 105 bacteria in 100% of the cases tested.151 Silvadene also has been found to speed epithelialization in experimental animal studies.151
With each dressing change, the wound should be cleansed thoroughly of these ointments, and surface coagulum should be gently removed so that the fresh application of the topical antibiotic can be in contact with the wound bed.138,201 By using only antibiotic ointments and avoiding the use of cytotoxic antiseptics, bacterial count is controlled and macrophage function, so critical to wound defense, is protected. These ointments may speed epithelialization by keeping the wound moist, thereby preventing crust formation and desiccation, which serve as mechanical barriers to cell migration.
Necrotic tissue promotes bacterial growth and, by mechanical impedance, interferes with epithelial cell migration.139 Removal of this necrotic tissue by meticulous debridement may be the most critical aspect of care to prevent infection in the acute wound and in the management of the contaminated or chronic wound.139,202
Debridement can be accomplished mechanically, enzymatically, or biologically through the normal phagocytic activity of white blood cells (autolysis).142 Mechanical debridement by the surgeon is a critical component of both primary and chronic care. The therapist can remove small areas of black or gray eschar or debris from combination yellow and black wounds with fine forceps and sharp scissors. The necrotic debris should be separated from the wound edges, working toward the center, to facilitate the process of wound contraction. The yellow wound can be gently debrided with a small bone curette, but care must be taken not to fracture new capillaries at the wound edges. Before mechanical debridement, the wound may be cleansed and softened in a clear-water whirlpool. A scab may serve as a biologic dressing and left in place on a superficial wound, but if drainage occurs from beneath the scab, it must be debrided.135
Enzymatic debridement with topical fibrinolysin enzymes such as Travase or Elase may be used to hasten separation of eschars, scabs, or fibrinous coagulum.135,142 Collagenase debridement products Biozyme C and Santyl may hydrolyze undenatured collagen or facilitate debridement of difficult necrotic tissue.142 Feedar and Kloth142 categorize these topical enzymes as selective, that is, working on only necrotic tissue, and their claim is supported by others who have demonstrated that these enzymes spare viable tissue.203,204 Although these proteolytic enzymes may depress leukocyte phagocytosis, they do not significantly interfere with wound healing.154 Hydrocolloid, alginate, or hydrogel dressings can be used to achieve natural autolytic cleansing.184
Autolytic, or biologic, debridement is considered the most selective because it relies on the body’s natural defense system.142 The noted importance of this natural phagocytic activity has led to the concept of sequestering wound fluids to facilitate macrophage debridement and has led to the development of synthetic dressings that enhance autolytic debridement.142,184
Selective debridement by careful mechanical, proteolytic, or autolytic means facilitates positive cellular response and is indicated for the yellow or black wound. Nonselective debridement, or that which indiscriminately removes both viable and nonviable tissue from the wound, may disturb new epithelial cells and granulation tissue and should be used with discretion. Nonselective methods includes wet-to-dry, wet-to-wet, and dry-to-dry dressings, whirlpool therapy, vigorous scrubs, Dakin’s solution, or hydrogen peroxide solutions.142
The act of covering a wound is an attempt to reproduce the barrier function of epithelium.17 The primary dressing, or that which is placed in direct contact with the wound, provides a barrier to the external environment and functions to prevent infection. Nonadherent, nonabsorbent contact layers, such as Adaptic, Xeroform, Aquaphor, or Transite, may help prevent desiccation and adhesion of the secondary dressing to the wound. These nonadherent contact layers are used postsurgically before the wound is sealed or epithelialized or can be used on a clean, red wound. The red wound can be protected from its environment with nonabsorbent film dressings such as Tegaderm, Opsite, or Bioclusive (see Table 18-1). These dressings are impermeable to water and provide all the benefits of moist wound healing previously described. Tegaderm has been used successfully to protect the humidity of exposed tendon in the digit. This film allows complete motion, adheres with an airtight seal to the periwound area, and prevents tendon desiccation until the wound is closed by secondary intention or surgical means (Fig. 18-5). These dressings provide a physiologic solution to a difficult problem, but they are not appropriate for infected wounds.
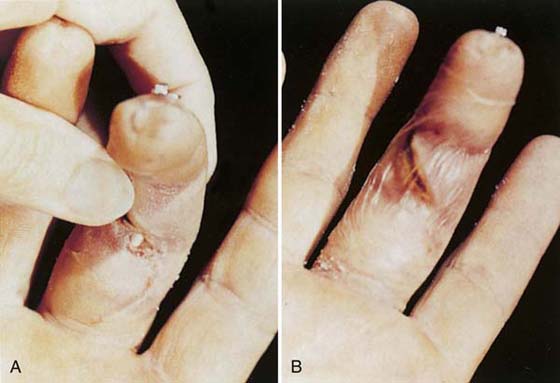
Figure 18-5 A, Exposed repaired flexor tendon 3 weeks after surgery. B, A Tegaderm dressing sequestered wound fluids and protected the exposed tendon until the wound was revised.
When excess drainage or infection is present, dressing changes should be performed as often as demanded by the accumulation of debris and fluid and the overload of absorbent materials.17 The absorption of exudate into a dressing reduces the work requirement of the macrophage for phagocytosis and autolytic debridement and also removes a potential substrate for microbial growth.17 The accumulation of wound fluid to the point of flooding causes maceration and bacterial overgrowth. Therefore, an absorbent dressing should imbibe exudate, but not allow fluid accumulation to the level of the most superficial dressing layer (“strikethrough”).17 If “strikethrough” does occur, a channel is created that will allow microorganisms to enter the wound from the external environment.17 The absorbent dressing should match the requirement of the wound. The acute, noninfected wound exudes maximally at 24 hours after surgery and may have as its secondary layer sterile absorbent woven or nonwoven gauze products that usually are made of cotton or rayon.17
Yellow, noninfected, exudating wounds may be dressed with some of the newer microenvironmental dressings that function to absorb fluid without “strikethrough,” encourage autolytic debridement, and maintain body temperature17,116,166,176 (see Table 18-2). Hydrocolloid and hydrogel dressings are those that combine the benefits of occlusion and absorption and are most often used for chronic yellow wounds.17,173,177,183 Alginates absorb exudate, facilitate moist wound healing, and can be used to obtain hemostasis in an oozing wound.166,172,181 Excess exudate also may be absorbed with hydrophilic products.79 Yellow wounds should be irrigated of the yellow-brown gelatinous mass that remains on the wound after dressing removal when hydrocolloids are used.17 The yellow wound also may be dressed with a wide-meshed 4 × 4 dressing changed every 4 to 6 hours to absorb purulent drainage, or with a wet dressing saturated with saline or topical medicants. If topical medicants are used, they should not be allowed to dry out because drying increases the concentration of the medication, possibly rendering it cytotoxic.135
Open wounds with dead space should be lightly packed with a fine-mesh strip gauze (e.g., Nu Gauze) to keep the superficial portions of the wound open while the deeper layers contract. Tight packing should be avoided because it could retard drainage and create tissue ischemia. A fine-mesh (44/36) gauze prevents epithelial growth into the weave.135 Despite the negative consequences to healing tissue, the use of wet-to-dry dressings perseveres due to its simplicity and long tradition.191
In a systematic review comparing dressings used for surgical wounds healing by secondary intention, insufficient data limited a correlation between dressing choice and wound healing.205 Limited evidence was found, however, to support the use of foam rather than gauze in terms of pain, patient satisfaction, and decreased nursing time.205
Mechanical influences that affect healing include wound edema, hematoma, tension at the wound site or incision line, foreign bodies, crust or necrotic tissue, iatrogenic manipulation, and overly aggressive debridement.
Swelling is a normal occurrence in the inflammatory stage of wound healing, but its control decreases the inflammatory response, which in turn decreases fibrosis.16 The edematous wound environment contributes to sustaining and perpetuating a chronic inflammatory state associated with excessive scarring.142 Gross edema in the periwound area decreases vascularization by altering hydrostatic capillary pressure; decreased oxygen and nutrient supply subsequently decrease the proliferation of granulation tissue.36,124,125 Therapeutic management of edema includes elevation techniques, controlled motion when possible, and application of a bulky dressing during the inflammatory stage. Single self-adherent Coban wraps control edema in digital wounds. The application of stress to injured tissues with manual exercise should be applied judiciously in all phases of wound healing. Exercise that is painful or increases swelling should be avoided.
Hematoma formation compromises the repair process. The space-occupying blood coagulum decreases perfusion capability, may cause graft separation or wound dehiscence, and increases the workload of the phagocytic cells.206 The increased inflammatory response associated with hematoma increases fibrosis and scar. Hematoma also serves as a perfect culture medium for bacteria and increases the risk for infection.
Correct surgical management with proper hemostasis before closure, adequate approximation of tissue defects, and drains where deemed necessary may prevent hematoma. The postoperative bulky dressing should be used for 24 to 48 hours with most surgical wounds about the hand or wrist and continued with each dressing change for as long as the first 5 to 7 days after surgery for surgeries in the vascular forearm, such as tumor excision or release of the median or radial nerves at this level.
Large-mesh grafts, such as those that provide coverage for the dorsum of the hand, can be dressed with 4 × 4 wide-mesh gauze dressings, wet with saline, to provide a continuous wicking action for exudate and to reduce fluid viscosity. These dressings serve to protect the graft from hematoma and excess accumulation of exudate, which should cause graft separation. These dressings must be kept wet, because allowing them to dry out causes adherence and disturbs the graft tissue during removal. This regimen is carried out in a hospital environment with 24-hour elevation.207
Dressings that function to maintain the configuration of a wound or to ensure graft contact to the wound, such as a bolus, should be made of cotton instead of synthetic materials, which compress and lose their shape.208
Wound site tension may reduce the rate of repair, compromise tensile strength, and increase the final width of the scar.15,209,210 Excessive tension at the wound site may cause necrosis by jeopardizing local blood supply.176,211 Sutures that are tied too tightly may need to be released; tension on an incision line may be relieved with wound tapes, pressure dressings, or orthoses that limit motion and stress.155,156,212
Negative-pressure wound therapy is a technique for preventing hematoma formation or serum collection postoperatively as an alternative to protecting skin grafting with bolstered dressings.213,214 The technique, termed vacuum-assisted closure (VAC), uses negative pressure to eliminate fluid collections, to increase oxygen tension, and to decrease contamination. Negative-pressure wound therapy is postulated to increase perfusion, nutrient delivery, and rate of granulation and decrease bacterial levels in wound tissue.215 It was approved by the FDA in 1995 and has gained popularity due to cost savings in dressings, decreased length of stay, and decreased need for nursing care.216
The treatment consists of insertion of sterile sponge into the wound bed connected to the negative-pressure device by a suction hose. Pump settings are adjustable based on specific wound characteristics.216 For acute wounds, the device is operated at a negative pressure of 125 mm Hg with a 5-minute on, 2-minute off cycle.213,216 Following mesh skin graft, a continuous pressure setting of 50 to 75 mm Hg is recommended.216 A nonadhesive layer is placed between the graft and the sponge, with VAC treatment commencing after 5 days.216 Negative-pressure wound therapy has been used successfully in multitraumatic upper extremity injuries requiring extensive reconstruction and skin-grafting procedures (Fig. 18-6).
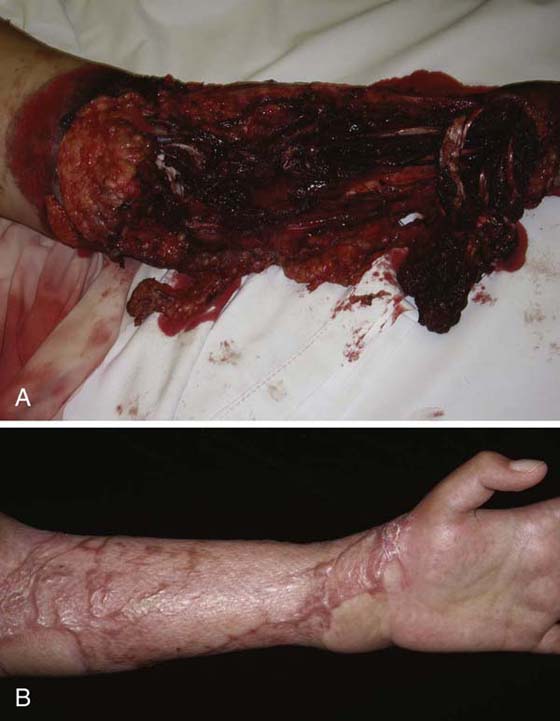
Figure 18-6 A, The distal left, nondominant upper extremity of a 16 year-old female after motor vehicle accident involving an 18-wheeler. B, Final outcome following negative-pressure wound therapy. (Photos courtesy of Dr. Miguel Pirela-Cruz, El Paso, Texas.)
Complications with VAC use are reported as primarily technical and include pressure from evacuation tubing on surrounding tissues, maceration of skin caused by occlusive dressing, growth of granulation tissue into the foam dressing, and pain.216 Precautions for use include active bleeding, the use of anticoagulants, and difficult hemostasis of the wound.216 It is contraindicated for patients with untreated osteomyelitis and for wounds that are malignant, necrotic, or demonstrate eschar formation.216 In the postoperative phase, the wound site, VAC parameters, and fluid collection are all closely monitored.216
VAC has been used in the treatment of degloving injuries,217 for the coverage of radial forearm free-flap donor-site complications,206 and for closure of chronic wounds.218 Recently, three systematic reviews have been completed to assess recommendations and outcomes for clinical use of VAC. The first review, completed by the Agency for Healthcare Research and Quality, found the literature to be insufficient to support conclusions for effectiveness in completeness of healing, time for healing, or readiness for surgical closure.219 A second systematic review of VAC for partial-thickness burns reported significant differences in burns at day 3 and 5 that decreased by day 14.220 No complications were found in the studies under review; however, absence of clinically relevant outcomes such as wound-healing parameters of rate, total time, area, and proportion were identified.220 The third review included articles that compared topical negative pressure with moistened gauze and other wound care products, such as hydrocolloids, gels, and alginates.221 The authors found no valid or reliable evidence that topical negative pressure facilitates chronic wound healing, and no difference in effect was found in comparison to alternative dressing choices.221
Physical agents offer the therapist additional options in wound management. The use of physical agents and their effect on cellular function is yet another expanding frontier in the area of wound manipulation, but a detailed discussion is beyond the scope of this chapter. Several sources are suggested for further study on the application of exogenously applied electrical stimulation,8,25,53,56,184,188,222-224 the effects of ultrasound (US) on the various connective tissues,56,225-232 and the use of heat and cold as an adjunct to wound treatment.233-235
Definitive research is lacking for clinical application of these modalities.8 A number of review articles offer perspective to the clinician.53,56,224,229,233
A few important studies are mentioned to emphasize the point that with all applied modalities, as with other wound management techniques, the therapist should understand the physiologic response of the tissues to the treatment applied.
Therapeutic doses of electric current have been shown to augment healing in chronic wounds in human subjects and induced wounds in animal models.188 Studies of cell cultures have shown that electric fields can influence migrating, proliferative, and functional capacity of cells involved in the healing process, and that growth factors may be stimulated by electric current.188 One clinical study has demonstrated the beneficial effect of pulsed electric stimulation on the healing of stage II, III, and IV chronic dermal ulcers, with treatment times that do not exceed 60 minutes per day, 5 to 7 days per week,188 a period substantially less than the 20 to 42 hours of stimulation per week recommended in earlier studies.236 In vitro electric stimulation has been shown to stimulate local growth factor activity, affecting human dermal fibroblasts and leading to greater collagen synthesis.222
Isolated epidermal cells, cell clusters, and cell sheets have demonstrated galvanotaxis (electrotaxis) in migrating toward the cathode in in vitro studies.186,187 Macrophages have been shown to migrate toward the anode,237 whereas neutrophils have been observed to migrate toward both the anode and cathode.238
Researchers have demonstrated that dermal fibroblasts in culture, stimulated with pulsed current at 100 pulses per second (pps) and 100 V, increased the expression of receptors for transforming growth factor-β that were six times greater than those of control fibroblasts.222 A negative effect has been demonstrated on tendon healing. The effect of pulsed electromagnetic fields (PEMF) stimulation on early flexor tendon healing in a chicken model (using a similar stimulus to that used clinically) caused a decrease in tensile strength and an increase in peritendinous adhesions.239
An in vivo rat model was employed to study the effects of pulsed magnetic field (PMF) on repaired Achilles tendons.240 Following surgery, two 30-minute sessions of PMF were applied at differing levels.240 At 3 weeks, a 69% increase in tensile strength was noted compared with controls. The researchers in this study identified PMF as contributory to the speed and efficiency of the cellular response.240
The effect of low-intensity US on the healing strength of 24 repaired rabbit Achilles tendons was studied.241 The tendons were excised after nine treatments and compared with non-US-treated tendons. The US group demonstrated a significant increase in tensile strength, tensile stress, and energy absorption capacity. These findings suggest that high-intensity US is not necessary to augment the healing strength of tendons, but that low-intensity US may enhance the healing process of surgically repaired Achilles tendons.241 Rat models have also been used to study the response of Achilles tendons to ultrasound with comparable results. Increased tensile and mechanical breaking strength242 and increased density and orientation of collagen fibrils243 have been noted in response to therapeutic US.
A study that describes the influence of US administered at different postoperative intervals on several aspects of healing in the surgically repaired zone II flexor tendon in 76 white leghorn chickens has indicated that use during the early stages of wound healing increases range of motion, decreases scar formation, and shows no adverse effect on strength.244
The effect of US on the healing human tendon has not yet been established, and US is not yet ready for clinical application. However, experimental studies suggest that positive effects are found with sonication limited to the very earliest stages of healing, and negative results when sonication is continued for several weeks.241 Clinical guidelines in regard to timing, duration, and intensity of application have not yet been established, but these parameters evidently must be matched to specific cellular activity to maximize the effectiveness of this modality.
The effect of US on bone repair has been investigated recently. It has been demonstrated that low-intensity US can accelerate the healing of fresh fractures.227,232 Some preliminary evidence suggests its usefulness in treatment of delayed healing and nonunions as well.227,228 A single case study credits low-intensity US with union of an ununited hook of the hamate fracture.226
Nussbaum229 provides an interesting review of studies on US of clinical interest to the hand clinician.
Scar management should be addressed from the first wounding day. Judicious planning of surgical procedures, full-thickness skin grafting where necessary, and infection control are immediate concerns.33 Control of the variables that could lead to infection, an exaggerated inflammatory state, or a dehydrated wound, help minimize fibrosis and hypertrophic scarring.17
Adhesion control in the case of the deeper tissues is discussed in other chapters on rehabilitation of the tendon, ligament, and joint. The negative biochemical and biomechanical changes in immobilized connective tissue studied both experimentally and clinically have been defined in many articles and are not reiterated here, except to point out that some controlled motion for these deeper tissues should be applied where possible with respect to the tensile strength of the repair to maintain tissue homeostasis and to promote a more organized deposition of collagen at the wound site.245 Motion in the inflammatory stage biochemically stimulates cellular response.42,54,245 Early passive motion has been correlated to an increased fibronectin (FN) concentration in the adult canine tendon; by 7 days after repair, controlled motion flexor tendons had FN concentrations two times that of immobilized tendons.42 Fibroblast chemotaxis and adherence to the substrate in the days after injury and repair appear to be directly related to FN concentration.47 Motion during the reparative stages biomechanically affects tissue glide for tendon and ligament and nutrient transport for cartilage. Tissue engineering promises to alter the management of healing connective tissue in the future,51 but at this writing, none of these new experimental techniques have been as effective as the application of controlled load to healing tendon and ligament.54
Techniques for the management of cutaneous scars—pressure garments, elastomeres, silicone gel sheeting, and the use of paper tape—are well known to the hand therapist.34,123,167,171,210,212,246,247
Hypertrophic scars are found most commonly in areas of high tension and movement as with the flexor surfaces of the extremities.210 Applying tension to suture lines with aggressive orthotic positioning and exercise can contribute to hypertrophic scarring on these surfaces and functional limitation. An example of commonly misapplied force by the hand therapist occurs with postoperative Dupuytren’s contractures. These cases, along with others, fare better by eliminating tension via orthotic fabrication and careful exercise technique.
Topical silicone gel sheeting (SGS) is used to prevent, control, and reduce hypertrophic scar formation.210 Clinically, it decreases scar redness and elevation of the scar area, and patients seem to have fewer complaints about itching and painful sensations.248
The mechanism of action of the SGS is unknown.210,248 Pressure exerted by the SGS applied to the scar with paper tape is negligible (<3 mm Hg).249 Temperature and differences in oxygen transmission have been excluded. The gel is occlusive, with a water vapor loss rate lower than that of skin (4.5 vs. 8.5 g/m2/hr).123 However, other polyurethane films do not have the same effect on hypertrophic scar. Researchers postulate that because the scar surface does not become wet or macerated with prolonged wear, the SGS may promote hydration of the scar, but changes in scar water content have not been directly measured. Researchers also postulate that the reduction in water vapor loss may decrease capillary activity, thereby reducing collagen deposition and scar hypertrophy.250 There is no histologic or scanning electron microscopic evidence of silicone absorption, but a chemical effect has not been excluded. Several clinical studies have demonstrated the clinical benefits of SGS in minimizing hypertrophic scarring in surgical scars and keloid scars with at least 12 hours of treatment daily over periods of up to 6 months.251
SGS also has been found to prevent development of these scars and is effective as a wound dressing. In a controlled analysis of fresh surgical incisions, SGS was found to significantly inhibit the formation of hypertrophic scar when used at least 12 hours daily for 2 months.248 They have been used with postoperative punch grafting to prevent cobblestoning and graft dislocation, to provide sterile atmosphere for the grafts,252 and as a method of treating painful fingertip injuries in children.246
Paper tape applied longitudinally along an incision line as soon as epithelialization takes place prevents wound site tension and clinically appears to minimize scarring. This technique has been studied,167,171,212 with one researcher finding that it is even more effective than SGS.167 Reiffel applied paper tape longitudinally along susceptible wounds and found that hypertrophic scarring was prevented.212 He hypothesized that the tape worked by preventing longitudinal stretching of the incision line. He also proposed that SGS was effective in scar management because it prevents tension at the incision line, not because of compression forces.212
The wound-healing process is subject to manipulation and facilitation by the clinician. The importance of a physiologic approach to the management of both the simple and complex wound may prove to speed the processes of epithelialization and contraction and help control collagen deposition. The importance of careful attention to this aspect of our discipline cannot be overemphasized.
1. Cohen IK, Diegelmann RE, Lindblad WJ. Wound Healing. Biomechanical and Clinical Aspects. Philadelphia: WB Saunders; 1992.
2. Davidson JM. Wound repair. J Hand Ther. 1998;11:80–94.
3. Dyson M, Young S, Pendel CL, et al. Comparison of the effects of moist and dry conditions on dermal repair. J Invest Dermatol. 1988;91:434–439.
4. Falcone PA, Caldwell MP. Wound metabolism. Clin Plast Surg. 1990;17:443–456.
5. Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25:321–340.
6. Evans RB. An update on wound management. Hand Clin. 1991;7:409–432.
7. Folkman J, Klagsbrun M. Angiogenesis factors. Science. 1987;235:442–447.
8. Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen. 1999;7:495–503.
9. Hunt TK, Hussain Z. Wound microenvironment. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:274–281.
10. McGrath MH. Peptide growth factors and wound healing. Clin Plast Surg. 1990;17:421–432.
11. Peacock EE. Wound Repair. 3rd ed Philadelphia: WB Saunders; 1984.
12. Clark RAF, Folkvord JM, Hart CE, et al. Platelet isoforms of platelet derived growth factor stimulate fibroblasts to contract collagen lattices. J Clin Invest. 1989;84:1036–1040.
13. Hunt TK, Knighton DR, Thakral KK, et al. Cellular control of repair. In: Hunt TK, Heppenstall RB, Pines E, Rovee D, eds. Soft and Hard Tissue Repair: Biological and Clinical Aspects. New York: Praeger; 1984:3–19.
14. Kloth LC, McCulloch JM, Feedar JA. Wound Healing: Alternatives in Management. Philadelphia: FA Davis; 1990.
15. Rohrich RJ. The biology of wound healing. Techniques of wound closure, abnormal scars, envenomation. Select Readings Plast Surg. 1988;5:1.
16. Wahl SM, Allen JB. T-lymphocyte dependent mechanisms of fibrosis. In: Barbul A, Pines E, Caldwell M, Hunt TK, eds. Growth Factors and Other Aspects of Wound Healing: Biological and Clinical Implications. New York: Alan R Liss; 1988:147–160.
17. Wiseman DM, Rovee DT, Alvarez OM. Wound dressings: design and use. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Biochemical and Clinical Aspects of Wound Healing. Philadelphia: WB Saunders; 1992:562–580.
18. Barbul A. Immune aspects of wound repair. Clin Plast Surg. 1990;17:433–442.
19. Thakral KK, Goodson WH, Hunt TK. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res. 1979;26:430–436.
20. Browder W, Williams D, Lucore P, et al. Effect of enhanced macrophage function on early wound healing. Surgery. 1988;104:224–230.
21. Grotendorst GR. Chemoattractants and growth factors. In: Cohen IK, Diegelman RF, Linblad WJ, eds. Wound Healing. Philadelphia: WB Saunders; 1992:237–246.
22. Knighton DR, Ciresi KF, Fiegel VD, et al. Classification and treatment of chronic non-healing wounds. Ann Surg. 1986;204:322–330.
23. Ten Dijke P, Iwata KK. Growth factors for wound healing. Bio/Technology. 1989;7:793–798.
24. Schmidt JA, Mizel SB, Cohen D, Green I. Interleukin-1, a potential regulator of fibroblast proliferation. J Immunol. 1982;128:2177–2182.
25. Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther. 1988;68:503–508.
26. Whalen GF, Zetter BR. Angiogenesis. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:77–95.
27. Banda MJ, Knighton DR, Hunt TK, Web Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci USA. 1982;79:7773–7777.
28. Stenn KS, Malhotra R. Epithelialization. In: Cohen IK, Diegelman RE, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:115–129.
29. Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg. 1990;17:485–492.
30. Dieglemann RR, Lindblad WJ. Cellular sources of fibrotic collagen. Fundam Appl Toxicol. 1985;5:219–227.
31. Morgan CJ, Pledger J. Fibroblast proliferation. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:63–76.
32. Madden JW, Peacock EE. Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery. 1968;64:288–294.
33. Rudolph R, VandeBerg J, Ehrlich HP. Wound contracture. In: Cohen IK, Diegelman RE, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:96–114.
34. Forrester JC, Zederfeldt BH, Hayes TL, Hunt TK. Wolff’s law in relation to the healing skin wound. J Trauma. 1970;10:770–779.
35. Brown PW. Open injuries of the hand. In: Green DP, ed. Operative Hand Surgery. 3rd ed New York: Churchill Livingstone; 1993:1619–1653.
36. Hunt TK, LaVan FB. Enhancement of wound healing by growth factors. N Engl J Med. 1989;321:111–112.
37. Knighton DR, Fiegel VD, Doucette NM, et al. The use of topically applied platelet growth factors in chronic non-healing wounds: a review. Wounds. 1989.71–78.
38. Mignatti P, Welgus HG, Rifkin DB. Role of degradative enzymes in wound healing. In: Clark RAF, Henson PM, eds. The Molecular and Cell Biology of Wound Repair. New York: Plenum Press; 1988:497–523.
39. Harvey C. Wound healing. Orthop Nurs. 2005;24:143–157.
40. Gonzalez MH, Bochar S, Novotny J, et al. Upper extremity infections in patients with diabetes mellitus. J Hand Surg. 1999;24A:682–686.
41. Jain AJ, Witbreuk M, Ball C, et al. Influence of methotrexate on wound complications after elective rheumatoid hand and wrist surgery. J Hand Surg. 2002;27A:449–455.
42. Amiel D, Gelberman R, Harwood F, Siegel D. Fibronectin in healing flexor tendons subjected to immobilization and early controlled passive motion. Matrix. 1991;II:184–189.
43. Clark RAF. Cutaneous tissue repair: basic biologic considerations I. J Am Acad Dermatol. 1985;13:701–725.
44. Cordeiro PG, Seckel BR, Lipton SA, et al. Acidic fibroblast growth factor enhances peripheral nerve regeneration in vivo. Plast Reconstr Surg. 1989;83:1013–1019.
45. Fernandez E, Pallini R, Mercanti D. Effects of topically administered nerve growth factor on axonal regeneration in peripheral nerve autographs implanted in the spinal cord of rats. Neurosurgery. 1990;26:37–42.
46. Frykman GK. The quest for better recovery from peripheral nerve injury: current status of nerve regeneration research. J Hand Ther. 1993;6:83–88.
47. Gelberman RH, Steinberg D, Amiel D, Akeson W. Fibroblast chemotaxis after repair. J Hand Surg. 1991;16A:686–693.
48. Morris J, et al. Platelet derived growth factor enhances healing in a rabbit deep flexor tendon model, unpublished research, 1992.
49. Pierce CF, Mustoe TA, Altrock BW, et al. Role of platelet derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326.
50. Rich KM, Alexander TD, Pryor JC, Hollowell JP. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105:162–170.
51. Woo SL-Y, Hildebrand K, Watanabe N, et al. Tissue engineering of ligament and tendon healing. Clin Orthop. 1999;367(Suppl):S312–S323.
52. Bello YM, Phillips TJ. Recent advances in wound healing. JAMA. 2000;283:716–718.
53. Braddock M, Campbell CJ, Zuder D. Current therapies for wound healing: electrical stimulation, biological therapeutics, and the potential for gene therapy. Int J Dermatol. 1999;38:808–817.
54. Buckwalter JA, Grodzinsky AJ. Loading of healing bones, fibrous tissue, and muscle: implications for orthopaedic practice. J Am Acad Orthop Surg. 1999;7:291–299.
55. Gibson T, Kenedi RM. Biomechanical properties of skin. Surg Clin North Am. 1967;47:279–294.
56. Houghton PE, Campbell KE. Choosing an adjunctive therapy for the treatment of chronic wounds. Ostomy Wound Manage. 1999;45:43–52.
57. Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg. 1998;176(2A suppl):80S–82S.
58. Sefton MV, Woodhouse KA. Tissue engineering. J Cutan Med Surg. 1998.(Suppl 1):18–23.
59. Deuel TF, Senior KM, Chang D. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci USA. 1981;78:4584–4587.
60. Deuel TF, Senior RM, Huang JS, Griffin GL. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982;69:1046–1049.
61. Eisinger M, Sadan S, Soehnochen R, Silver IA. Wound healing by epidermal derived factors: experimental and preliminary clinical studies. In: Barbul A, ed., et al. Growth Factors and Other Aspects of Wound Healing, Biological and Clinical Implications. New York: Alan R Liss; 1987:291–302.
62. Pessa ME, Bland KL, Copeland EM III. Growth factors and determinants of wound repair. J Surg Res. 1987;42:207–217.
63. Wahl LM, Wahl SM. Inflammation. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:40–62.
64. Alkali A, Khan U, Khaw PT, et al. Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Recon Surg. 1999;103:151–158.
65. Moran SL, Ryan CK, Orlando GS, et al. Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg. 2000;25A:242–251.
66. Khan U, Occleston NL, Khaw PT, et al. Single exposures to 5-fluorouracil: a possible mode of targeted therapy to reduce contractile scarring in the injured tendon. Plast Reconstr Surg. 1997;99:465–471.
67. Khan U, Occleston NL, Khaw PT, et al. Differences in proliferative rate and collagen lattice contraction between endotenon and synovial fibroblasts. J Hand Surg. 1998;23A:223–232.
68. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: An experimental study in the rabbit. J Hand Surg. 1993;18A:68–75.
69. Komurcu M, Akkus O, Basbozkurt M, et al. Reduction of restrictive adhesions by local aprotinin application and primary sheath repair in surgically traumatized flexor tendons of the rabbit. J Hand Surg. 1997;22A:826–832.
70. Miller JA, Ferguson RL, Powers DL, et al. Efficacy of hyaluronic acid/non-steroidal anti-inflammatory drug systems in preventing post-surgical tendon adhesions. J Biomed Mater Res. 1997;38:25–33.
71. Szabo RM, Younger E. Effects of indomethacin on adhesion formation after repair of zone II tendon lacerations in the rabbit. J Hand Surg. 1990;15A:480–483.
72. Hagberg L, Gerdin B. Sodium hyaluronate as an adjunct in adhesion prevention after flexor tendon surgery in rabbits. J Hand Surg. 1992;17A:935–941.
73. Hagberg L, Tenblad A, Gerdin B. Hyaluronic acid in flexor tendon sheath fluid after sheath reconstructions in rabbits: A comparison between tendon sheath transplantation and conventional two stage procedures. Scand J Plast Reconstr Hand Surg. 1991;25:103–107.
74. Isik S, Öztürk S, Gürses S, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg. 1999;52:373–379.
75. Thomas SC, Jones LC, Hungerford DS. Hyaluronic acid and its effect on postoperative adhesions in the rabbit flexor tendon: a preliminary look. Clin Orthop. 1986;206:281–289.
76. Özgenel GY, Samli B, Özcan M, et al. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J Hand Surg. 2001;26A:332–339.
77. Klein MB, Pham H, Yalamanchi N, et al. Flexor tendon wound healing in vitro: the effect of lactate on tendon cell proliferation and collagen production. J Hand Surg. 2001;26A:847–854.
78. Oshiro W, Lou J, Xing X, et al. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg. 2003;28A:814–823.
79. Gelberman RH, Vandeberg JS, Manske PR, et al. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg. 1985;10A:776–784.
80. Harrison RK, Mudera V, Grobbelaar AO, et al. Synovial sheath cell migratory response to flexor tendon injury: an experimental study in rats. J Hand Surg. 2003;28A:987–993.
81. Chen J, Iosifidis M, Zhu J, et al. Vanadate ingestion enhances the organization and collagen fibril diameters of rat healing medial collateral ligaments. Knee Surg Sports Traumatol Arthrosc. 2006;14:750–755.
82. Jiang DP, Li ZZ, Jiang ZT. Systemic vanadate ingestion improves early medial collateral ligament repair. J Int Med Res. 2007;35:819–826.
83. Duffy FJ Jr, Seiler JG, Gelberman RH, et al. Growth factors and canine flexor tendon healing: initial studies in uninjured and repair models. J Hand Surg. 1995;20A:645–649.
84. Chang J, Most D, Thunder R, et al. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J Hand Surg. 1998;23A:1052–1058.
85. Tang JB, Xu Y, Ding F, et al. Tendon healing in vitro: Promotion of collagen gene expression by bFGF with NF-κB gene activation. J Hand Surg. 2003;28A:215–220.
86. Tang JB, Xu Y, Ding F, et al. Expression of genes for collagen production and NF-κB gene activation of in vivo healing flexor tendons. J Hand Surg. 2004;29A:564–570.
87. Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg. 2004;29A:551–563.
88. Wang XT, Liu PY, Xin K-Q, et al. Tendon healing in vitro: (FGF gene transfer to tenocytes by adeno-associated viral vectors promotes expression of collagen genes. J Hand Surg. 2005;30A:1255–1261.
89. Hamada Y, Katoh S, Hibino N, et al. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J Hand Surg. 2006;31A:530–540.
90. Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737.
91. Klein MB, Yalamanchi N, Pham H, et al. Flexor tendon healing in vitro: effects of TGF-ß on tendon cell collagen production. J Hand Surg. 2002;27A:615–620.
92. Chang J, Thunder R, Most D, et al. Studies in flexor tendon wound healing: Neutralizing antibody to TGF-β1 increases post-operative range of motion. Plast Reconstr Surg. 2000;105:148–155.
93. Zhang AY, Pham H, Ho F, et al. Inhibition of TGF-β-induced collagen production in rabbit flexor tendons. J Hand Surg. 2004;29A:230–235.
94. Bidder M, Towler DA, Gelberman RH, et al. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. J Orthop Res. 2000;18:247–252.
95. Boyer MI, Watson JT, Lou J, et al. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res. 2001;19:869–872.
96. Zhang F, Liu H, Stile F, et al. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112:1613–1619.
97. Embil JM, Papp K, Sibbald G, et al. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open label clinical evaluation of efficacy. Wound Repair Regen. 2000;8:162–168.
98. Kallianinen LK, Hirshberg J, Marchant B, et al. Role of platelet-derived growth factor as an adjunct to surgery in the management of pressure ulcers. Plast Reconstr Surg. 2000;106:1243–1248.
99. Wang XT, Liu PY, Tang JB. Tendon healing in vitro: Modifications of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor β but minimally affects expression of collagen genes. J Hand Surg. 2005;30A:222–229.
100. Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg. 2005;30A:441–447.
101. MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880.
102. Shevchenko RV, Sibbons PD, Sharpe JR, et al. Use of a novel porcine collagen paste as a dermal substitute in full-thickness wounds. Wound Rep Regen. 2008;16:198–207.
103. Carothers JT, Brigman BE, Lawson RD, et al. Stacking of a dermal regeneration template for reconstruction of a soft-tissue defect after tumor excision from the palm of the hand: a case report. J Hand Surg. 2005;30A:1322–1326.
104. Boden SD. Bioactive factors for bone tissue engineering. Clin Orthop. 1999;367(Suppl):S84–S94.
105. Boden SD, Kang J, Sandhu H, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans—A prospective, randomized clinical pilot trial—2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673.
106. Mulconrey DS, Bridwell KH, Flynn J, et al. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery. Spine. 2008;33:2153–2159.
107. Chong AKS, Chang J. Tissue engineering for the hand surgeon: a clinical perspective. J Hand Surg. 2006;31A:349–358.
108. Sharma B, Williams CG, Khan M, et al. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112–120.
109. Conrad C, Huss R. Adult stem cell lines in regenerative medicine and reconstructive surgery. J Surg Res. 2005;124:201–208.
110. Huang D, Balian G, Chhabra B. Tendon tissue engineering and gene transfer: the future of surgical treatment. J Hand Surg. 2006;31A:693–704.
111. Kryger GS, Chong AKS, Costa M, et al. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg. 2007;32A:597–605.
112. Cao Y, Liu Y, Liu W, et al. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg. 2002;110:1280–1289.
113. Wang B, Liu W, Zhang Y, et al. Engineering of extensor tendon complex by an ex vivo approach. Biomaterials. 2008;29:2954–2961.
114. Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1997;6:279–288.
115. Robson MC. Disturbances of wound healing. Ann Emerg Med. 1988;17:1274–1278.
116. Altemeier W. The significance of infection in trauma. AORNJ. 1972;15:92–97.
117. Barbul A. Role of the immune system. In: Cohen IK, Diegelman RF, Lindblad WJ, eds. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:282–291.
118. Becker GD. Identification and management of the patient at high risk for wound infection. Head Neck Surg. 1986;8:205–210.
119. Bucknall TE. The effect of local infection upon wound healing: an experimental study. Br J Surg. 1980;67:851–855.
120. Levenson SM, Demetriou AA. Metabolic factors. In: Cohen IK, Diegelman RF, Linblad WJ, eds. Wound Healing, Biochemical and Clinical Aspects. Philadelphia: WB Saunders; 1992:248–273.
121. Miller SH, Rudolph R. Healing in the irradiated wound. Clin Plast Surg. 1990;17:503–508.
122. Morain WD, Colen LB. Wound healing in diabetes mellitus. Clin Plast Surg. 1990;7:493–501.
123. Ohmori S. Effectiveness of silastic sheet coverage in the treatment of scar keloid. Aesthetic Plast Surg. 1988;12:95–99.
124. Knighton DR, Hunt TK, Scheuenstuhl H, et al. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285.
125. LaVan FB, Hunt TK. Oxygen and wound healing. Clin Plast Surg. 1990;17:463–472.
126. Alper JC, Tibbetts LL, Sarazen AA. The in vitro response of fibroblasts to the fluid which accumulates under a vapor permeable membrane. J Invest Dermatol. 1985;84:513–515.
127. Alper JC, Welch EA, Ginsberg M, et al. Moist wound healing under a vapor permeable membrane. J Am Acad Dermatol. 1983;8:347–353.
128. Alvarez O. Moist environment for healing: matching the dressing to the wound. Ostomy Wound Manage. 1988;21:64–83.
129. Alvarez OM, Hefton JM, Eaglstein WE. Healing wounds: occlusion or exposure. Infect Surg. 1984;3:171–181.
130. Alvarez OM, Mertz PA, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and reepithelialization in superficial wounds. J Surg Res. 1983;35:142–148.
131. Spencer EM, Skover G, Hunt TK. Somatomedins: do they play a pivotal role in wound healing. In: Barbul A, ed., et al. Growth Factors and Other Aspects of Wound Healing: Biological and Clinical Implications. New York: Alan R Liss; 1988.
132. Varghese MC, Balin AK, Carter DM, Caldwell D. Local environment of chronic wounds under synthetic dressings. Arch Dermatol. 1986;122:52–57.
133. Wheeland RG. The newer surgical dressings and wound healing. Dermatol Clin. 1987;5:393–407.
134. Burkhalter WE. Care of war injuries of the hand and upper extremity: report of the war injury committee. J Hand Surg. 1983;8:810–813.
135. Smith KL. Wound care for the hand patient. In: Hunter JM, ed., et al. Rehabilitation of the Hand. 3rd ed St Louis: Mosby; 1990:172–180.
136. Trott A. Mechanisms of surface soft tissue trauma. Ann Emerg Med. 1988;17:1279–1283.
137. Cruse PJE, Foord R. The epidemiology of wound infection. Surg Clin North Am. 1980;66:27–40.
138. Edlich RF, Rodeheaver GT, Thacker JG, Edgerton MT. Technical factors in wound management. In: Hunt TK, Dunphy JE, eds. Fundamentals of Wound Management. New York: Appleton-Century-Crofts; 1979:364–454.
139. Edlich RF, Rodeheaver GT, Morgan RF, et al. Principles of emergency wound management. Ann Emerg Med. 1988;17:1284–1302.
140. Robson MC, Heggars JP. Quantitative bacteriology and inflammatory mediators in soft tissue. In: Hunt TK, ed., et al. Soft and Hard Tissue Repair: Biological and Clinical Aspects. New York: Praeger; 1984:483–507.
141. Mann RJ. In: Infections of the Hand. Philadelphia: Lea & Febiger; 1988:63–71.
142. Feedar JA, Kloth LC. Conservative management of chronic wounds. In: Kloth LC, McCulloch JM, Feedar JA, eds. Wound Healing: Alternatives in Management. Philadelphia: FA Davis; 1990:135–172.
143. Burkhalter WE. personal communication, 1990.
144. Laato M, Lehtonen OP, Niinikoski J. Granulation tissue formation in experimental wounds inoculated with Staphylococcus aureus. Acta Chir Scand. 1985;151:313–318.
145. Laato M, Niinikoski J, Lundberg C, Gerdin B. Inflammatory reaction and blood flow in experimental wounds, inoculated with Staphylococcus aureus. Eur Surg Res. 1988;20:33–38.
146. McCall CE, Caves J, Cooper R, DeChatlet L. Functional characteristics of human toxic neutrophils. J Infect Dis. 1971;124:68–75.
147. Orgill D, Deming RH. Current concepts and approaches to wound healing. Crit Care Med. 1988;16:899–908.
148. Cuzzell J. The new red, yellow, black color code. Am J Nurs. 1988;10:1342–1346.
149. Cuzzell J. Tell it like it is: a realistic approach to wound documentation. Am J Nurs. 1986;86:600–601.
150. Stotts N, Cuzzell J. Toward a Universal Standard for Wound Assessment and Therapy. Seminar Proceedings from the American Association of Critical Care Nurses Teaching Institute. Kansas City, Mo: Marion Laboratories; 1988.
151. Rodeheaver G. Controversies in topical wound management. Ostomy Wound Manage. 1988;20:58–68.
152. Trelstad A, Osmundson D. Water pick: wound cleansing alternative. Plast Surg Nurs. 1989;9:117–119.
153. Custer J, Edlich RF, Prusak M, et al. Studies in the management of the contaminated wound. An assessment of the effectiveness of pHisoHex and Betadine surgical scrub solutions. Am J Surg. 1971;121:572–575.
154. Rodeheaver G. Side-effects of topical proteolytic enzyme treatment. Surg Gynecol Obstet. 1979;148:562–566.
155. Rodeheaver GT, Smith SL, Thacker JG, et al. Mechanical cleansing of contaminated wounds with a surfactant. Am J Surg. 1975;129:241–245.
156. Brånemark PI, Albrektsson B, Linström J, Lundborg G. Local tissue effects of wound disinfectants. Acta Chir Scand. 1966;357(suppl):166–176.
157. Faddis D, Daniel D, Boyer J. Tissue toxicity of antiseptic solutions. A study of rabbit articular and periarticular tissues. J Trauma. 1977;17:895–897.
158. Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;184:50–56.
159. Johnson AR, White AC, McAnalley. Comparison of common topical agents for wound treatment: cytotoxicity for human fibroblasts in culture. Wounds. 1989;1:186–192.
160. Oberg MS. Do not put hydrogen peroxide or povidone iodine into wounds!. Am J Dis Child. 1987;141:27–28.
161. Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–378.
162. Winter GD. The formation of the scab and the rate of epithelialization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294.
163. Winter GD, Scales JT. Effect of air drying and dressings on the surface of wound. Nature. 1963;197:91–92.
164. Eaglstein WH. Experiences with biosynthetic dressings. J Am Acad Dermatol. 1985;12:434–440.
165. Hom DB, Adams G, Koreis M, Maisel R. Choosing the optimal dressing for irradiated tissue wounds. Otolaryngol Head Neck Surg. 1999;121:591–598.
166. Ladin DA. Understanding dressings. Clin Plast Surg. 1998;25:433–441.
167. Niessen FB, Spauwen PH, Schalkwijk J, Kon M. The use of silicone sheeting (Sil-K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plast Reconstr Surg. 1998;102:1962–1972.
168. Pirone LA, Monte KA, Shannon RJ, Bolton LL. Wound healing under occlusion and non-occlusion in partial thickness wounds in swine. Wounds. 1990;2:74–78.
169. Auböck J. Synthetic dressings. Curr Probl Dermatol. 1999;27:26–48.
170. Barbul A, Pines E, Caldwell M, Hunt TK. Growth factors and other aspects of wound healing; biological and clinical implications. Barbul A, Pines E, Caldwell M, Hunt TK, eds. Progress in Clinical and Biological Research.Vol 266. New York: Alan R Liss; 1988.
171. Sawada Y, Urushidate S, Nibei Y. Hydration and occlusive treatment of a sutured wound. Ann Plast Surg. 1998;41:508–512.
172. Timmons J. Alginates and hydrofibre dressings. Prof Nurse. 1999;14:501.
173. Williams C. Hydrocoll: a “new breed” of hydrocolloid wound dressing. Br J Nurs. 1998;7:1337–1340.
174. Nemeth AJ, Hebda PA, Eaglstein WH. Stimulating effect of human wound fluid on epidermal outgrowth from porcine skin explant cultures, abstracted. J Invest Dermatol. 1986;86:497.
175. Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF (alpha) and other growth factors in vivo: analysis by an RNA phenotyping. Science. 1988;241:708–712.
176. Falanga V. Occlusive wound dressings: why, when, which? Arch Dermatol. 1988;124:872–877.
177. Draye JP, Delaney B, Van de Voorde A, et al. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19:1677–1687.
178. Kahanovich S, Aladjem M, Shafir R, et al. The best of wound cover methods. Qtr Med Rev. 1987;1:10–12.
179. Mertz PM, Eaglstein WH. The effect of a semiocclusive dressing on the microbial population in superficial wounds. Arch Surg. 1984;119:287–289.
180. Elton RC, Bouzard WC. Gunshot and fragment wounds of the metacarpus. South Med J. 1975;68:833–843.
181. Ichioka S, Harii K, Nakahara M, Sato Y. An experimental comparison of hydrocolloid and alginate dressings, and the effect of calcium ions on the behavior of alginate gel. Scand J Plast Reconstr Surg Hand Surg. 1998;32:311–316.
182. Lyall PW, Sinclair SW. Australian survey of split skin donor site dressings. Aust N Z J Surg. 2000;70:114–116.
183. Matzen S, Peschardt A, Alsbjorn B. A new amorphous hydrocolloid for the treatment of pressure sores: a randomized controlled study. Scand J Plast Reconstr Hand Surg. 1999;33:13–15.
184. Senet P, Meaume S. Decubitus sores in geriatric medicine. Local and general treatment of pressure sores in the aged. Presse Med. 1999;28:1840–1845.
185. Cross SE, Roberts MS. Defining a model to predict the distribution of topically applied growth factors and other solutes in excisional full-thickness wounds. J Invest Dermatol. 1999;112:36–41.
186. Cooper NS, Schliwa M. Electrical and ionic control of tissue cell locomotion in a DC electrical field. J Neurosci Res. 1985;13:223–244.
187. Erickson CA, Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electrical fields. J Cell Biol. 1984;98:296–307.
188. Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71:639–649.
189. Jaffe LF, Vanable JW. Electrical fields and wound healing. Clin Dermatol. 1984;2:34–44.
190. Noe JM, Keller M. Can stitches get wet? Plast Reconstr Surg. 1988;81:82–84.
191. Mendez-Eastman S. A glimpse at the specialty of wound care. Plast Surg Nurs. 1999;19:67–73.
192. Wheeler CB, Rodeheaver GT, Thacker JG, et al. Side effects of high pressure irrigation. Surg Gynecol Obstet. 1976;234:775–778.
193. Crossland M. personal communication, 1990.
194. Brennan SS, Leaper DJ. The effect of antiseptics on the healing wound: a study using the rabbit ear chamber. Br J Surg. 1985;72:780–782.
195. Aronoff GR, Friedman SJ, Doedens DJ, Lavelle KJ. Increased serum iodide concentration from iodide absorption through wounds treated topically with povidone-iodine. Am J Med Sci. 1980;297:173–176.
196. Lineaweaver W, Howard R, Soucy D, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120:267–270.
197. Bryant CA, Rodeheaver GT, Reem EM, et al. Search for a non-toxic surgical scrub solution for periorbital laceration. Ann Emerg Med. 1984;13:317–321.
198. Thomas C. Nursing alert—wound healing halted with the use of povidone-iodine. Ostomy Wound Manage. 1988;18:30–33.
199. Leyden JJ, Bartelt NM. Comparison of topical antibiotic ointments, a wound protectant, and antiseptics for the treatment of human blister wounds contaminated with Staphylococcus aureus. J Fam Pract. 1987;24:601–604.
200. Hermans RP. Topical treatment of serious infections with special reference to the use of a mixture of silver sulfadiazine and cerium nitrate: two clinical studies. Burns Incl Therm Inj. 1984;2:59–62.
201. Edlich RF, Madden JE, Prusak M, et al. Studies in the management of contaminated wounds: VI. The therapeutic value of gentle scrubbing in prolonging the limited period of effectiveness of antibiotics in contaminated wounds. Am J Surg. 1971;121:668–672.
202. Haury B, Rodeheaver G, Vensko J, et al. Debridement: an essential component of traumatic wound care. Am J Surg. 1978;135:238–242.
203. Harmel RP, Vane DP, King DR. Burn care in children: special considerations. Clin Plast Surg. 1986;13:95–105.
204. Zawacki BE. The effect of Travase on heat injured skin. Surgery. 1975;77:132–136.
205. Vermeulen H, Ubbink D, Goossens A, et al. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2004.(Issue 1):
206. Greer SE, Longaker MT, Margiotta M, et al. The use of subatmospheric pressure dressing for the coverage of radial forearm free flap donor-site exposed tendon complications. Ann Plast Surg. 1999;43:551–554.
207. Burkhalter WE. Mutilating injuries of the hand. Hand Clin. 1986;2:45–68.
208. Singer PI, Moore JH, Byron PM. Management of skin grafts and flaps. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand. 3rd ed St Louis: Mosby; 1990:253–263.
209. Brown GL, Nanney LB, Griffin J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321:76–79.
210. Su CW, Alizadeh K, Boddie A, Lee RC. The problem scar. Clin Plast Surg. 1998;25:451–465.
211. Adamson JE, Fleury AF. Incisions in the hand and wrist. In: Green DP, ed. Operative Hand Surgery. 2nd ed New York: Churchill Livingstone; 1988:1785–1804.
212. Reiffel RS. Prevention of hypertrophic scars by long term paper tape application. Plast Reconstr Surg. 1995;96:1715–1718.
213. Blackburn JH II, Boemi L, Hall WW, et al. Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg. 1998;40:453–457.
214. Kalailieff D. Vacuum-assisted closure: wound care technology for the new millennium. Perspectives. 1998;22:28–29.
215. Morykwas M, Argenta L, Shelton Brown E, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–661.
216. Mendez-Eastman S. Negative pressure wound therapy. Plast Surg Nurs. 1998;18:27–37.
217. Meara JG, Guo L, Smith JD, et al. Vacuum-assisted closure in the treatment of degloving injuries. Ann Plast Surg. 1999;42:589–594.
218. Hartnett JM. Use of vacuum-assisted wound closure in three chronic wounds. J Wound Ostomy Continence Nurs. 1998;25:281–290.
219. Samson D, Lefevre F, Aronson N. Wound healing technologies: low-level laser and vacuum-assisted closure. Summary, Evidence Report/Technology Assessment No. 111. (Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence-based Practice Center, under Contract No. 290-02-0026.) AHRQ Publication No. 05-E005-1. Rockville, MD: Agency for Healthcare Research and Quality; December 2004.
220. Wasiak J, Cleland H. Topical negative pressure (TNP) for partial thickness burns. Cochrane Database Syst Rev. 2007.(Issue 3):
221. Ubbink DT, Westerbos SJ, Evans D, et al. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008.(Issue 3):
222. Falanga V, Bourguignon GL, Bourguignon LYN. Electrical stimulation increases the expression of fibroblast receptors for transforming growth factor-beta, abstracted. J Invest Dermatol. 1987;88:488.
223. Kloth LC, Feedar JA. Electrical stimulation in tissue repair. In: Kloth LC, McCulloch JM, Feedar JA, eds. Wound Healing: Alternatives in Management. Philadelphia: FA Davis; 1990:222–256.
224. Lampe KE. Electrotherapy in tissue repair. J Hand Ther. 1998;11:131–139.
225. Dyson M. Role of ultrasound in wound healing. In: Kloth LC, McCulloch JM, Feedar JA, eds. Wound Healing: Alternatives in Management. Philadelphia: FA Davis; 1990:229–285.
226. Fujioka H, Tsunoda M, Noda M, et al. Treatment of ununited fracture of the hook of hamate by low-intensity pulsed ultrasound: a case report. J Hand Surg. 2000;25A:77–79.
227. Hadjiargyrou M, McLeod K, Ryaby JP, Rubin C. Enhancement of fracture healing by low intensity ultrasound. Clin Orthop Relat Res. 1998;Oct(Suppl):S216–S229.
228. Mayr E, Rankel V, Ruter A. Ultrasound: an alternative healing method for nonunions? Arch Orthop Trauma Surg. 2000;120:1–8.
229. Nussbaum E. The influence of ultrasound on healing tissues. J Hand Ther. 1998;11:140–147.
230. Sun JS, Tsuang YH, Lin FH, et al. Bone defect healing enhanced by ultrasound stimulation: an in vitro tissue culture model. J Biomed Mater Res. 1999;46:253–261.
231. Taskan I, Ozyazgan I, Tercan M, et al. A Comparative study of the effect of ultrasound and electrostimulation on wound healing in rats. Plast Reconstr Surg. 1997;100:966–972.
232. Warden SJ, Bennell KL, McMeeken JM, Wark JD. Acceleration of fresh fracture repair using the sonic accelerated fracture healing system (SAFHS): a review. Calcif Tissue Int. 2000;66:157–163.
233. Hardy M, Woodall W. Therapeutic effects of heat, cold, and stretch on connective tissue. J Hand Ther. 1998;11:148–156.
234. Lehmann JF, DeLateur BJ. Therapeutic heat. In: Lehmann JF, ed. Therapeutic Heat and Cold. 4th ed Baltimore: Williams & Wilkins; 1990:429–433.
235. Michlovitz SL. Thermal Agents in Rehabilitation. Philadelphia: FA Davis; 1990.
236. Carley P, Wainapel S. Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil. 1985;66:443–446.
237. Orida N, Feldman JD. Directional protrusive pseudopodia activity and motility in macrophage induced by extracellular electric fields. Cell Motil. 1982;2:243–255.
238. Monguio J. Über die polar wirkung des galvanischen stromes auf leukozyten. Z Biol. 1933;93:553–559.
239. Robotti E, Zimbler AG, Kenna D, Grossman JA. The effect of pulsed electromagnetic fields on flexor tendon healing in chickens. J Hand Surg Br. 1999;24B:56–58.
240. Strauch B, Patel MK, Rosen DJ, et al. Pulsed magnetic field therapy increases tensile strength in a rat Achilles’ tendon repair model. J Hand Surg. 2006;31A:1131–1135.
241. Enwemeka CS, Rodriguez O, Mendoza S. The biomechanical effects of low intensity ultrasound on healing. Ultrasound Med Biol. 1990;16:801–807.
242. Jackson BA, Schwane JA, Starcher BC. The effect of ultrasound therapy on the repair of Achilles tendon injuries in rats. Med Sci Sports Exerc. 1991;23:171–176.
243. Da Cunha A, Parizotto NA, Vidal B, et al. The effect of therapeutic ultrasound on repair of the Achilles tendon (tendo calcaneus) of the rat. Ultrasound Med Biol. 2001;27:1691–1696.
244. Gan BS, Huys S, Sherebrin MH, Scilley CG. The effects of ultrasound on flexor tendon healing in the chicken limb. J Hand Surg. 1995;20B:809–814.
245. Woo SL-Y, Buckwalter JA, eds. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, Ill: American Academy of Orthopaedic Surgeons; 1988.
246. O’Donovan DA, Mehdi SY, Eadie PA. The role of Mepitel silicone net dressings in the management of fingertip injuries in children. J Hand Surg Br. 1999;24B:727–730.
247. Zukin DD. New wound closure tapes. J Emerg Med. 1987;5:553.
248. Katz BE. Silastic gel sheeting is found to be effective in scar therapy. Cosm Derm. 1992;5:32–34.
249. Quinn KJ. Silicone gel in scar treatment. Burns Incl Therm Inj. 1987;13:S33–S40.
250. Davey RB, Wallis KA, Bowering K. Adhesive contact media: an update on graft fixation and burn scar management. Burns. 1991;17:313–319.
251. Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel for the prevention and treatment of hypertrophic scar. Arch Surg. 1991;126:499–504.
252. Agarwal US, Jain D, Gulati R, et al. Silicone gel sheet dressings for prevention of post-minigraft cobblestoning in vitiligo. Dermatol Surg. 1999;25:102–104.