Wrist Reconstruction: Salvage Procedures
▪ Wrist salvage procedures provide options for patients with a history of long-standing wrist instability, degeneration, and/or pain that severely limit function. They are performed to decrease pain and to restore stability and strength to the wrist to allow for optimal hand function, not to maximize range of motion (ROM).
▪ When considering a total wrist arthrodesis, a period of immobilization in a removable orthosis is recommended before surgery to determine the best individual wrist position and to allow the patient to become accustomed to an immobile wrist.
▪ The postoperative therapy goal is pain-free functional wrist motion. A stable wrist that has a pain-free arc of limited ROM is more functional than an unstable, painful wrist that has full ROM.
▪ A wrist salvage procedure will likely result in restricted wrist ROM, but these restrictions are not necessarily detrimental to daily function. Palmer and colleagues concluded that functional range of wrist motion was 5 degrees of flexion, 30 degrees of extension, 10 degrees of radial deviation, and 15 degrees of ulnar deviation.
▪ Protective Phase. Postoperative care after a wrist salvage procedure will vary depending on the procedure performed. The average time needed for healing to occur will vary with each method of fixation and will determine the length of the protective phase.
▪ ROM Phase. When radiographs indicate acceptable healing, the cast is removed, a removable orthosis is fabricated, and active ROM is initiated (unless a wrist fusion was performed).
▪ Strengthening Phase. This phase begins when the fusion is able to tolerate the increased forces associated with resistive activity. The patient may now be weaned from the orthosis. Gentle active assisted or passive ROM may be initiated, if needed, to increase ROM. Forceful manipulations and joint mobilizations are not appropriate. Treatment may be progressed to include isometric exercise, followed by gentle strengthening exercises such as progressive resistive exercise and graded grip strengthening. Heavier resistance, lifting simulations, and more strenuous activity are avoided until bony healing is confirmed on radiographs, which may vary from 8 to 16 weeks.
▪ Return to work for heavy laborers may take as long as 6 months, depending on the procedure performed.
The wrist is the link between the hand and the upper extremity. It must be mobile to allow positioning of the hand to perform activities of daily living (ADLs), work, and recreation. It must be stable to allow for force transfer for lifting, gripping, and carrying. This mobile yet stable joint is dependent on intact ligaments and proper bony alignment of articular surfaces to function properly. There are no muscle bellies crossing the wrist joint that can be strengthened to compensate for ligament insufficiencies.
The wrist joint, by its anatomic position, is subject to frequent use, high stress, and injury. Undiagnosed and untreated carpal fracture and/or ligament injury produces carpal malalignment. This malalignment, while allowing continued function, places abnormal stress on the articular surfaces of the wrist causing degeneration. Progressive degeneration of the wrist will result in pain, weakness, loss of wrist motion, and inability to use the hand for ADLs. Many patients first present for medical treatment when this degenerative arthritis has progressed to the point of functional limitation.
This chapter reviews salvage procedures for the degenerated wrist due to fracture, ligamentous instability, avascular necrosis (AVN), and arthritis. Intercarpal arthrodesis, replacement arthroplasty, proximal-row carpectomy (PRC), and total wrist arthrodesis procedures are also reviewed, discussing surgical indications, expected clinical results, postoperative therapeutic protocols, and outcomes data.
A “normal” wrist has a greater ROM than that required for most ADLs. A wrist salvage procedure will likely result in restricted wrist ROM. Motion can be expended to achieve stability without compromising function. The amount of motion required to perform ADLs has been evaluated by several authors. Palmer and colleagues1 measured wrist motion in 10 normal subjects using a triaxial electrogoniometer. They evaluated 52 standardized tasks. The normal functional range of wrist motion in this study was 5 degrees of flexion, 30 degrees of extension, 10 degrees of radial deviation, and 15 degrees of ulnar deviation. Ryu and associates.2 studied 40 subjects using a biaxial wrist electrogoniometer. They evaluated 31 activities that reflected major functional wrist requirements, categorizing them as palm placement, personal care and hygiene, diet and food preparation, and work activities. The entire group of activities could be performed with a minimum of 60 degrees of extension, 54 degrees of flexion, 17 degrees of radial deviation, and 40 degrees of ulnar deviation. Most of the activities (except rising from a chair and perineal care) could be performed with 40 degrees of flexion and extension, 10 degrees of radial deviation, and 30 degrees of ulnar deviation. The authors concluded that this is the minimal ROM needed to perform a majority of ADLs. They also concluded that wrist ulnar deviation and extension are the most important positions for wrist activities. This position must be taken into consideration with orthotic fabrication and reconstructing the wrist.
De Smet3 studied 205 patients who had undergone a variety of reconstructive wrist procedures. He correlated wrist ROM with disability of the upper limb using the Disability of the Arm, Shoulder, and Hand (DASH) score, finding a significant but weak correlation. When wrist arthrodesis was included, a stronger correlation was found, illustrating the impact of complete loss of motion versus restricted motion. Reconstructive salvage procedures should strive to create a stable wrist and maintain functional ROM.
These studies provide a measure of functional wrist motion. Wrist extension and ulnar deviation are the most important positions for wrist activities. When a functional ROM cannot be maintained as a result of a salvage procedure, extension and ulnar deviation must be maintained with arthrodesis.
Wrist salvage procedures provide options for patients with a history of long-standing wrist instability, degeneration, and/or pain that severely limit function. They are performed to decrease pain and to restore stability and strength to the wrist to allow optimal hand function, not to maximize ROM. For this reason, the postoperative therapy goal is pain-free functional wrist motion. A stable wrist that has a pain-free arc of limited ROM is more functional than an unstable, painful wrist that has full ROM. Functional and pain-free wrist motion is a sum of all the movements of this complicated articular complex and dependent on equilibrium of the dynamic system. These complicated surgical procedures require skilled hand therapy to achieve optimal function, maximize surgical efforts and goals, and avoid unnecessary complications.
The goal of intercarpal arthrodesis is to provide the patient with a painless wrist that remains durable under prolonged stress, maintains functional mobility, and is not subject to overload and subsequent degenerative joint disease elsewhere in the wrist.4 The primary pathology and its secondary pattern of degeneration will determine which carpal bones should be fused. Common diagnoses that can be treated by intercarpal arthrodesis include chronic scapholunate instability, lunate AVN, and degenerative and post-traumatic arthritis.
The following is a general postoperative therapy guideline. Treatment is based on the procedure performed, fixation used, and bone healing.
A cast is applied and worn for 4 weeks after surgery. Delayed union requires a longer period of immobilization. The home exercise program should include edema control and active ROM (AROM) of the joints proximal and distal to those immobilized in the cast.
At week 4, a custom thermoplastic wrist orthosis is fabricated. If there is scaphoid involvement, a forearm-based thumb spica orthosis is required. The thumb is placed in a position opposing the index finger. The orthosis is worn full time except for hygiene. Edema control and AROM of the uninvolved joints continue. Scar management techniques may be initiated.
Gentle wrist AROM can be initiated at 6 weeks. Generally, by 8 weeks, one can expect to see a 60- to 80-degree flexion–extension arc of motion.5 As ROM improves and symptoms resolve, gentle isometric exercises for grip, wrist, and forearm may be performed with care to avoid increasing painful symptoms. Treatment may be progressed to include strengthening exercises and passive range of motion (PROM) when radiographs confirm bony healing.
Adaptive equipment, ergonomic adjustments, and task modifications should be addressed. Wrist wraps and gloves may be used as needed for comfort and pain relief. Heavy activity should be avoided for 3 months postoperatively. Expect the arthrodesis to be secure by 10 to 12 weeks; full symptom resolution and strength recovery may ultimately require 6 to 12 months.5
Minami and colleagues6 reported on 12 of 15 wrists (4 radiocarpal/11 intercarpal) that showed an average of 32 degrees of flexion and 33 degrees of extension at both 22 and 89 months postoperatively. Grip strength was nearly identical at both visits. These results suggest that the clinical and radiographic results were maintained at the final follow-up visit and that the effect of limited wrist fusion does not deteriorate.6
Fusion of the scaphotrapezial trapezoidal (STT) joint is indicated in the treatment of degenerative arthritis of the STT joint (Fig. 76-1). STT fusion offers good pain relief in patients with STT arthrosis.7 It maintains grip and pinch strength without sacrificing functional motion. STT arthrodesis is also indicated in the treatment of AVN of the lunate.8 The STT fusion has been shown to be effective in unloading the lunate at the expense of increased radioscaphoid contact. This load transfer may eventually lead to degenerative arthritis of the radioscaphoid joint.9,10 STT fusion has also been advocated for scapholunate instability.11 Long-term studies have demonstrated significant problems with the procedure, with a 50% complication rate.12 For this reason, it is important that postoperative therapy include education regarding long-term joint protection techniques.
The surgical technique involves fusion of the distal pole of the scaphoid to the trapezium and trapezoid via a dorsal approach. Internal fixation is used to maintain the scaphoid in an extended position to maintain proper carpal alignment (Fig. 76-2). Distal radial bone grafting is used. The procedure prevents scaphoid flexion and extension and will alter carpal motion.
Immediately after STT arthrodesis, a short-arm thumb spica cast is applied. During this time, AROM may be performed proximally at the shoulder and elbow as well as at those remaining joints of the hand not included in the cast.
At 4 weeks, the cast is usually removed. The duration of this protective phase may vary depending on the type of fixation used and the status of healing. Smokers may require a longer period of immobilization.
At about 4 weeks, a full wrist-thumb spica orthosis is fabricated. During fabrication, care must be taken to ensure that the thumb is positioned in palmar abduction. In some cases, the surgeon may choose to place the patient in a long-arm thumb spica or Munster (Fig. 76-3, online) cast for optimum control of forearm rotation, thereby ensuring immobilization at the fusion site. Generally, gentle AROM of the wrist may begin at 4 weeks. Edema control and scar management are performed as indicated.
A review of the literature reveals that after STT fusion, one can expect to see an average arc of wrist flexion–extension ranging from 67 to 115 degrees and an average arc of radioulnar deviation ranging from 31 to 56 degrees.7-9,13,14
At 6 weeks, gentle assisted AROM (AAROM) and later PROM may be initiated in wrists that are stiff and have limited motion. Forceful, painful manipulations are to be avoided. Isometric exercises may be initiated at 6 weeks, and gentle strengthening exercises may begin at 8 weeks when approved by the surgeon based on healing of the fusion. This may include graded grip strengthening, isotonic exercises, and light work simulations. A review of the literature indicates that after STT arthrodesis, one can expect to achieve grip strength ranging from 65% to 83% of that of the uninvolved extremity.7,8,10,13-17
When there is evidence of solid healing, heavier resistance and job simulations may be added to the program, usually by week 12. Watson and colleagues,13 in a series of 800 STT fusions, reported that 88% of the patients and 80% of the heavy laborers returned to preoperative employment. Similar findings are reported by Sauerbier and colleagues18 and Kleinman and colleagues,11 with 80% and 92%, respectively, of their patients returning to their original occupations.
Several authors reported the use of the DASH as an outcome measure after STT fusion. This questionnaire, which has been found to be a valid, reliable, and responsive tool, evaluates symptoms and physical function.19 A higher score reflects greater disability. Following STT fusion, DASH scores ranging from 24.8 to 29 have been reported, indicating less pain and disability.9,16,18,20
Watson and Ballet21 described the scapholunate advanced collapse (SLAC) deformity arising from chronic scapholunate instability. The scaphoid rotates, the capitate pushes in between the scaphoid and lunate, and degeneration occurs first at the scaphoid radial styloid joint (Fig. 76-4A) and then at the capitate lunate joint (see Fig. 76-4B). The radiolunate joint is spared and maintains good articular cartilage in most patients. Pain occurs from degenerative arthritis at the radial scaphoid and capitate lunate joints. For surgical treatment to succeed, this pathology must be addressed. The four-bone arthrodesis procedure removes the scaphoid to eliminate this focus of degeneration and fuses the lunate, capitate, hamate, and triquetrum to stabilize the wrist (Fig. 76-5B, online). This choice of intercarpal fusion prevents further capitate migration. It eliminates the midcarpal degeneration between the capitate and lunate and maintains carpal height. This fusion is also indicated in patients with radiocarpal arthritis from scaphoid nonunion and scaphoid AVN.
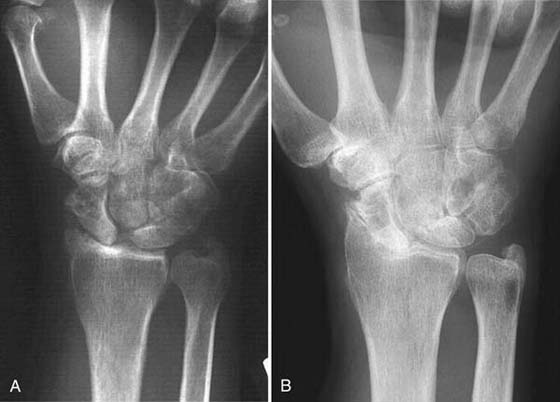
Figure 76-4 A, Scapholunate advanced collapse (SLAC) wrist. Note arthritis at scaphoid radial styloid joint. B, Severe SLAC wrist. Lunate radius joint is spared from arthritic involvement.
Figure 76-5 B, Four bone fusion lateral X-ray. Note correction of lunate extension to align the central axis of capitate and lunate.
Four-bone intercarpal fusion maintains 50% to 60% of normal wrist motion and 80% of the grip strength on the contralateral side. Long-term studies demonstrate no secondary radiolunate degeneration.6,22
The most common complication after this procedure is dorsal radiocarpal impingement in wrist extension.22,23 This occurs when the lunate is fused in an extended position. The capitate impinges on the dorsal rim of the radius with wrist extension (Fig. 76-6, online). Cohen and Kozin23 proposed fusing the lunate in slight flexion relative to the capitate to avoid this complication and, as a result, provide greater wrist extension.
Figure 76-6 Four-bone fusion: lunate fused in an extended position causes capitate impingement on dorsal radius, limiting wrist extension.
Postoperative care will vary depending on the type of fixation used at the time of surgery. A variety of methods used to secure the arthrodesis are described in the literature, such as K-wires,23,24 Spider plates,25 and staples and screws.24 The average time needed to achieve fusion will vary with each method of fixation. El-Mowafi and colleagues24 estimate that for K-wires (which provide minimal stability and no compression26), approximately 8 to 10 weeks is needed to achieve fusion. Krakauer and colleagues27 indicate that this period of immobilization may be shortened if fusion was accomplished with compression screws (which provide increased stability and allow for early mobilization).26 Therefore, the chosen method of fixation will determine the postoperative course of therapy. During this phase of treatment, while the patient is in a protective cast, therapy focuses on edema control and AROM of the uninvolved joints.
When radiographs indicate sufficient healing, a thermoplastic thumb spica orthosis is fabricated (Fig. 76-7, online). In some cases, the surgeon may choose to place the patient in a long-arm thumb spica or Munster orthosis for optimum control of forearm rotation, thereby ensuring immobilization at the fusion site. It is worn fulltime with removal for hygiene and exercise for the next 4 weeks. Gentle AROM may now be initiated at the wrist. During this phase, undue stress at the repair site such as grip-strengthening exercises and testing that significantly load the carpus should be avoided.27 Edema control continues as indicated. The patient is instructed in scar management. Modalities such as moist heat may be initiated to enhance ROM and tendon-gliding exercises.
A review of the literature reveals that after four-bone fusion one can expect to see an average flexion–extension arc of approximately 72 to 80 degrees and an average ulnoradial deviation arc ranging from 37 to 53 degrees22,23 (Fig. 76-8, online). Krakauer and colleagues27 report significantly less motion with a 54-degree flexion–extension arc, whereas El Mowafi and colleagues24 report significantly more motion, achieving 75% to 79% of normal contralateral wrist motion.
Figure 76-8 A, B, Case study. A 45-year-old right hand dominant (RHD) security officer treated for a scaphoid fracture 10 years earlier presents with a painful wrist. Evaluation demonstrates a scaphoid nonunion advanced collapse deformity. A scaphoid excision with four-corner fusion was performed (see Fig. 76-5). At 4 weeks postoperatively, a thumb spica orthosis was fabricated and gentle wrist active range of motion (AROM) was initiated. At 8 weeks postoperatively, the patient was pain free with normal digital ROM. Wrist AROM was 40 degrees of extension (Fig. 76-8A) and 35 degrees of flexion (Fig. 76-8B), 20/15 degrees of radial/ulnar deviation, and 75/75 degrees of pronation/supination. Grip strength at level III on a Jamar dynamometer was 53 pounds on the right and 70 pounds on the left.
This phase begins when the surgeon indicates that the fusion is able to tolerate the increased forces associated with resistive activity. The orthosis may now be weaned. Gentle AAROM may be initiated, if needed, to increase ROM. Forceful manipulations and joint mobilizations are not appropriate. Treatment may be progressed to include isometric exercise followed by gentle strengthening. Strenuous activity is avoided until bony fusion is confirmed on radiographs (which may vary from 8 to 16 weeks).28 A review of the literature reveals that after this procedure one can expect to achieve about 80% of normal contralateral grip strength.22-24
High patient satisfaction and good pain relief are documented in the literature.22-24 Cohen and Kozin23 reported the use of the SF-36 Health Survey and the Patient-Related Wrist Evaluation (PRWE) in their series of 19 patients. The PRWE is a valid and reliable wrist-specific outcome measurement tool.29 The score is based on a scale of 100. Cohen and Kozin23 reported an average score of 27. Lower scores are associated with less pain and disability and a more successful outcome. Using the DASH, De Smet3 reported a slightly higher average score of 38 in his series of 18 patients who underwent four-corner fusion.
First described by Stamm in 1944,30 PRC attempts to convert a complex link articulation in the wrist to a simple hinge joint. Imbriglia and colleagues31 demonstrated that the motion is not that of a hinge joint; rather, the motion between the capitate and radius is rotational and translational with a moving center of rotation.
The procedure is indicated for disease affecting the proximal carpal row, including scaphoid nonunion, radioscaphoid arthritis, scapholunate instability, and AVN of the lunate or scaphoid. It is contraindicated if degeneration is present and involves the capitate or lunate facet of the radius. The theoretical advantage of the PRC over arthrodesis includes increased motion, decreased period of immobilization, elimination of internal fixation, bone grafting, and potential nonunion.
The surgical approach is dorsal, with removal of the scaphoid, lunate, and triquetrum (Fig. 76-9). Care is taken to preserve the radioscaphocapitate ligament to prevent instability. The dorsal capsule is imbricated and reattached to the dorsal radius.
Several authors23,31-33 demonstrated good short- and long-term results with PRC. The average wrist flexion–extension arc was 70 to 80 degrees with 8 degrees of radial deviation and 19 degrees of ulnar deviation. The grip strength averaged 71% to 79% of that of the opposite wrist. Cohen and Kozin23 found no difference in the results of a PRC compared with a four-bone fusion.
Loss of motion and strength32 is the result of relative shortening of the wrist and lengthening of the extrinsic muscles and tendons. The postoperative course of therapy must take into account and make adjustments for this dynamic change.
Immediately after PRC, the wrist is casted in 0 to 10 degrees of extension.31,33-36 At this time, full active digital and thumb ROM are encouraged. Digital flexion is often limited because of the altered length-tension relationship of the flexor tendons. Gentle forearm ROM may also be performed in addition to AROM at the elbow and shoulder. Edema control is performed as indicated.
Wrist position sets the critical tension of the extrinsic muscle-tendon units.31 By immobilizing the wrist, relative shortening of the lengthened extrinsic muscles and tendons is achieved.37 Performing AROM of the fingers and thumb in the orthosis provides isometric muscle contractions that will later contribute to grip strength recovery.
At 4 weeks, a thermoplastic wrist orthosis is fabricated with the wrist in neutral and is worn full time with removal for exercise and hygiene. Gentle isolated AROM may now be initiated at the wrist. Composite wrist/digit extension/flexion should be avoided to prevent stretching of the extrinsic muscles.
Treatment now includes modalities in preparation for functional activity, scar management, desensitization if needed, and light functional activity. The program is progressed slowly. Care is taken to avoid tendon inflammation and to respect pain. It is also important to educate the patient regarding appropriate expectations for wrist motion after PRC.
A review of the literature reveals that after PRC one can expect to see an average flexion–extension arc of approximately 52% to 84% of that of the contralateral wrist.23,27,30-44 It has also been documented that radial deviation is most limited by this procedure with several researchers reporting as little as 5 to 9 degrees of radial deviation.23,31-33,35,37 Ulnar deviation is reasonably preserved, with several authors reporting 23 and 24 degrees.23,31,32 Although AROM is restricted after PRC, the average ROM as reported previously falls within the range of functional motion as reported by Palmer and colleagues.1
As inflammation and pain decrease and the patient gains motion and strength, the orthosis may be gradually weaned. For patients who require additional light support, a step-down orthosis such as a neoprene wrist wrap may be used. Treatment may be progressed at 6 weeks to include gentle AAROM and isometric exercise. Forceful manipulations and joint mobilizations are not appropriate. Gentle strengthening exercises such as graded grip strengthening, isotonic exercise, and progressive resistive exercises may begin at 8 weeks, followed by job simulations.
Grip strength deficits may be more marked initially due to the altered length-tension relationship of the tendons and the potential for active insufficiency.28,40 A review of the literature reveals that PRC results in weakness of grip with strength averaging 50% to 83% of that of the contralateral side.23,27,31-33,38-45 Several studies indicate that as much as 91% to 100% of contralateral grip strength may be regained, but it may take up to 1 year to reach this plateau.34-36
Return to work is an important measure of any procedure. Stamm,30 in his original description of PRC, stated that the final result cannot be expected in less than 4 months. Patients having sedentary-type jobs may return to work as early as 3 months. However, return to heavy labor usually requires up to 6 months. Imbriglia and colleagues31 have shown that the capacity for heavy, repetitive tasks after PRC is equal to that after partial wrist fusion and superior to that after full fusion. In their series, 84% of the patients who performed vigorous manual labor returned to their previous occupations within 4.5 months. Tomaino and colleagues33 reported similar findings with 69% of the heavy laborers returning to work. However, Culp and colleagues32 cautioned that PRC should be used selectively in laborers because they found that only 20% of the laborers in their series returned to work. The therapist must consider precautions related to the progressive degenerative changes in the wrist that may occur with heavy labor. This typically involves job and task analysis and modifications such as the use of shock-absorbing tools, wrist orthoses for work, and other job-specific ergonomic adjustments.
Several authors reported the use of the DASH as an outcome measure after PRC. They reported an average DASH score ranging from 9 to 28 points,3,34,36-40,42,44 indicating successful outcomes with regard to disability and pain. The PRWE was used in two studies. Cohen and Kozin23 reported a score of 18 in their series of 19 patients after PRC. De Smet40 reported a mean score of 25 in his series of 51 patients. Both reported scores are low, also indicating less pain and disability and a more successful outcome.
Replacement arthroplasty of the wrist has been largely restricted to patients with rheumatoid arthritis and a low-demand wrist. The indications are being extended to include patients with post-traumatic arthritis. The design of the prosthesis continues to evolve as the wrist kinematics are better understood and applied to the prosthetic design. This has the advantage of greater motion, less loosening, and greater implant longevity.
The objective of total wrist arthroplasty is to maintain wrist motion while relieving pain and correcting deformity.46,47 Expectations and limitations of this procedure should be discussed among the patient, surgeon, and therapist. Permanent restrictions are placed on the patient, including lifting no more than 10 pounds, limited repetitive activity, no weight-bearing activities, and no impact sports.48 In a patient with bilateral involvement, it may be desirable to perform a wrist arthroplasty for limited ROM and dexterity and a wrist arthrodesis on the other extremity for strength.
The patients must have functioning wrist extensor tendons for the procedure to succeed. The procedure is indicated in rheumatoid patients with bilateral wrist involvement when motion in one wrist is desirable for function. The most common complication is distal component loosening and/or cut out from the metacarpal.
Cobb and Beckenbaugh49 reported on 64 biaxial implant arthroplasties with a 5-year follow-up. Their results were pain relief in 75%. The average ROM was 36 degrees of extension, 29 degrees of flexion, 10 degrees of radial deviation, and 20 degrees of ulnar deviation; 8 of 11 failures occurred as a result of distal implant loosening.
Total wrist implant failure is salvaged by total wrist fusion if component revision is not feasible.
During the patient’s first postoperative visit with the surgeon, he or she is referred for a custom-made thermoplastic orthosis. A volar wrist orthosis allows full digit/thumb ROM and is worn full time for 6 weeks with removal for hygiene.
The patient should be instructed in digit, elbow, forearm, and shoulder AROM. Edema reduction techniques such as elevation and compression garments are begun as indicated. The patient may or may not attend formal therapy at this time.
Scar management should begin at 2 weeks, after suture removal, to ensure decreased adherence of the scar over the extensor tendons. If required, desensitization techniques should be incorporated into the patient’s program both at home and in the clinic. Edema control continues as indicated. Compression garments such as therapeutic gloves (Isotoner gloves) or self-adherent elastic wraps (Coban wraps) may also be used to combat persistent edema.
The initiation of gentle AROM of the wrist varies from 2 to 6 weeks after surgery depending on prosthetic fit and soft tissue integrity.50 The patient should continue with AROM of the uninvolved joints to avoid the consequences associated with immobilization.
Over the next 4 weeks, the patient may wean off the wrist orthosis and wear a soft support for comfort. The patient should continue to use the thermoplastic wrist orthosis if ambulation with a cane, walker, or crutches is required. Regular use of the upper extremities for support during ambulation or transfers is a contraindication after wrist arthroplasty.47
The patient should achieve full digital motion and a 60-degree wrist flexion–extension arc of motion. If wrist stiffness persists, then gentle passive motion, dynamic orthosis, or static progressive night orthosis may be incorporated into the program with the physician’s approval.46 When fabricating the orthosis, the therapist must also remember the primary precautions: (1) maintain stability, (2) avoid overstress, and (3) respect new motion axis.51
Isometric strengthening exercises for the hand, wrist, and forearm should be initiated as tolerated at 6 weeks with a progression to isotonic strengthening. At this time, the patient may have a better idea of what tasks can be accomplished relatively easily and those that may require adaptive equipment. Jar openers, ergonomic knives, and door openers are relatively inexpensive ways to promote independence.
The goal after arthroplasty is a symptom-free wrist that can perform basic ADLs such as dressing, writing, typing, and perineal care. Education regarding adaptive devices and techniques, joint protection, and energy conservation should be incorporated into the therapy program. The therapist should take into account job duties and daily tasks that may require ergonomic adjustments and modifications. If flare-ups occur, the patient may be required to resume wearing the wrist orthosis.
Formal therapy may be discontinued at this time. The patient should continue to perform the home exercise program and maintain the precautions stated earlier. The expected wrist flexion–extension arc of motion is 60 degrees with 10 degrees of radial deviation and 25 degrees of ulnar deviation. Digital AROM should be full.47 Generally, maximum motion is not expected until 6 months postoperatively.46,47
Divelbiss and associates,52 in their prospective study of the Universal total wrist prosthesis, looked at 22 prostheses implanted in 19 patients. They noted significant improvement for all wrist and forearm motions. Wrist extension, radial deviation, and supination, which were most limited before surgery, improved the most postoperatively. DASH scores improved 14 points at 1 year and 24 points at 2 years.
Menon45 reported his results using the Universal Total Wrist Implant. He achieved results similar to those with other prostheses. He found consistently good pain relief (90%) and a functional ROM. Average motion was 36 degrees of extension, 41 degrees of flexion, 7 degrees of radial deviation, and 13 degrees of ulnar deviation.
The final salvage procedure for the wrist is a total arthrodesis. All motion, flexion, extension, and radioulnar deviation is sacrificed for stability and pain relief. Supination and pronation are preserved, improving function and quality of life.53 The indications for total wrist arthrodesis include post-traumatic arthritis involving the midcarpal and radiocarpal joints, failed intercarpal fusion, and rheumatoid arthritis.
Many techniques for performing wrist fusion have been described. Most authors now agree that stable internal fixation will significantly increase the success of union.5 The following joints are always included in the fusion mass: radioscaphoid, scapholunate, scaphocapitate, lunocapitate, and capitate long finger metacarpal. If the primary pathology includes the ulnar side of the wrist, the lunate hamate and triquetrum are included in the fusion. The index carpometacarpal joint does not need to be included in the fusion.
The results of fusion by the AO method with plate fixation (Fig. 76-10) and local bone graft are consistently good, with minimal nonunion and good pain relief.5,54,55 Complications include plate tenderness in 19%, extensor tendon adhesions requiring tenolysis in 3.5%, carpal tunnel syndrome in 4% to 10%, and distal radioulnar joint pain in up to 3.5%.
The functional outcome of total wrist fusion has been studied in 23 patients.55 Fifteen returned to their original jobs. Most tasks were performed with modification because of loss of functional wrist ROM. The most difficult daily tasks for a patient with a fused wrist were perineal care and manipulation of the hand in a tight space.
Field and colleagues56 suggest a period of immobilization before surgery in a removable orthosis to determine the best individual wrist position and also to allow the patient to become accustomed to an immobile wrist. The patient’s AROM of the ipsilateral upper extremity should be evaluated before wrist fusion. Compensatory movements of the ipsilateral shoulder and elbow are necessary to do more normal tasks when the wrist is fused.57 The wrist is usually fused in 10 to 20 degrees of extension and slight ulnar deviation. This position increases hand function.2
Generally after a total wrist fusion, a postoperative wrist cast or orthosis is fabricated and worn full time until radiographs confirm bony healing. This may range from 2 to 6 weeks. If healing is delayed or nonunion occurs, the period of immobilization is increased. The therapist should monitor for complications such as infection, hematoma, excessive edema, and partial dehiscence of the incision site. The patient should be instructed in AROM of the uninvolved joints and edema management. Blocking exercises and gentle PROM for the digits are initiated, if needed. Emphasis should be placed on extensor digitorum communis gliding exercises to avoid scar adherence over the extensor tendons. If an extensor lag of the metacarpophalangeal (MCP) joints is noted, a thermoplastic MCP joint extension support can be attached to the wrist orthosis for nighttime use. If decreased MCP joint flexion is noted, an exercise orthosis can be fabricated to promote MCP joint flexion (Fig. 76-11).
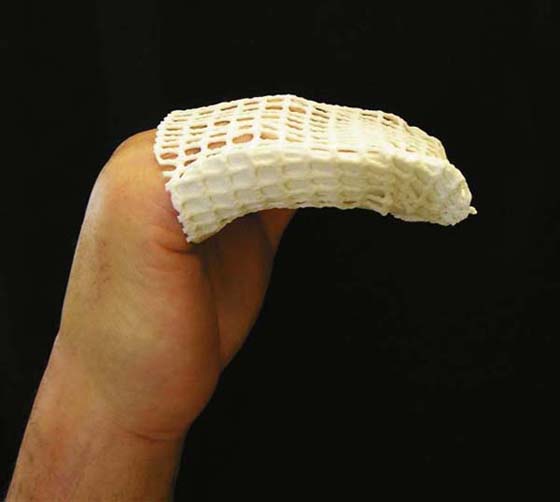
Figure 76-11 This blocking orthosis may be fabricated for use during active exercises to promote metacarpophalangeal joint flexion.
Edema control and digital ROM are continued as tolerated. The patient should be instructed in scar management techniques and wear nightly silicon gel scar pads or molds if required. Desensitization is initiated if hypersensitivity is present. Fine-motor and manipulation tasks are added to the patient’s therapy program to increase dexterity.
At approximately 6 weeks, depending on bony healing, the patient is able to stop using a wrist orthosis except for precarious situations. Therapy should continue to address any AROM limitations, persistent edema, dysfunction in ADLs, or persistent pain/hypersensitivity.
Patients are given a 5-pound weight limit for the first 8 weeks.5 Isometric exercises for grip strength are initiated with surgeon approval because it is dependent on the fixation. Difficulties in ADLs may still persist for perineal hygiene, fine-motor manipulations, and horizontal use of a screwdriver.55 Adaptive equipment, ergonomic adjustments, and task modifications should be addressed per specific patient limitations. According to Hastings,5 patients should expect a 6-month learning/adaptation period following fusion; 92% of tasks will be performed in a normal manner without undue delay, and grip strength will not plateau until 1 year postoperatively. Maximum improvement in function after arthrodesis requires an average of 6 to 14 months.55,57
Barbier and colleagues57 looked at 18 patients at 7 years after arthrodesis and found that all patients were pleased with the procedure and had satisfactory pain relief. Of the 23 patients studied by Weiss and colleagues,55 15 returned to their original jobs. All patients were able to perform the vast majority of tasks with modifications; however, perineal care and manipulating the hand in tight spaces were noted to be most difficult. Gohritz and colleagues58 noted that total arthrodesis after failed partial arthrodesis reduced pain in 18 of 20 reexamined wrists by 67% at rest and 46% when stressed. Complete pain relief at rest was noted in 7 of the 20 patients, and 2 patients reported complete pain relief under stress conditions. Grip strength of the involved hand averaged 53% of that of the uninvolved hand.
Lengthy periods of wrist immobilization may lead to stiffness, pain, and weakness. Skirven developed an alternative orthosis to allow for limited postoperative wrist motion. This flexible wrist orthosis was described in 1989 by Bora and colleagues59 for use after scaphoid fracture (Fig. 76-12). They proposed the following benefits over complete immobilization: (1) control and preservation of short-arc wrist motion, (2) decreased bone osteopenia, (3) maintenance of articular cartilage health, (4) stimulation of healing, and (5) increased independence in the performance of ADLs. The orthosis allows approximately 30 degrees of a wrist flexion–extension arc of motion, limits ulnar and radial deviation, and at rest provides support and stability. This orthosis may be used after intercarpal fusions, after an appropriate time period of postoperative casting, when there is radiographic evidence of healing, and with a reliable patient. It is worn full time and removed only for hygiene.
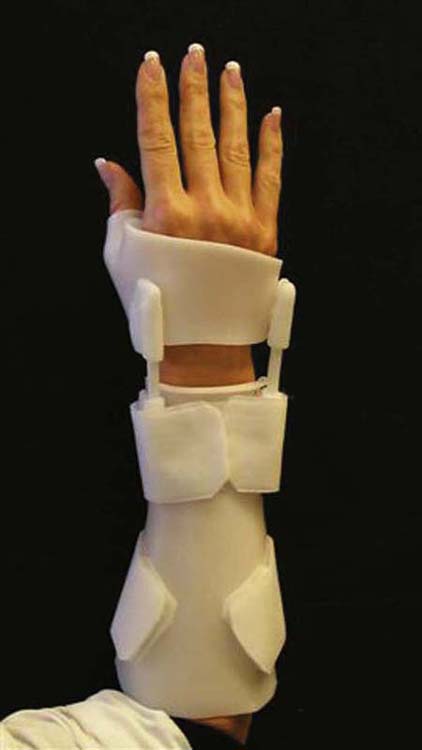
Figure 76-12 A flexible wrist orthosis may be used postoperatively for limited and controlled wrist motion.
Wrist salvage procedures are performed to decrease pain and to restore stability and strength to the wrist to allow optimal hand function, not to maximize ROM. Regaining a mobile wrist must be balanced with preserving stability. When a wrist is stiff after a salvage procedure because of inflammation and scar formation (in addition to a surgical fusion limiting motion), the therapist and the physician must determine this balance. Orthoses may be used to apply low load-prolonged stress to increase motion when cleared by the surgeon, when radiographs confirm complete bony consolidation (which, for an intercarpal fusion, may vary from 8 to 16 weeks),28 when the fusion has sufficient tensile strength and no longer requires protective orthotic use, and when the wrist motion achieved is less than the expected motion (given the procedure performed) and it is not functional for the patient. Orthotic fabrication for the stiff, salvaged wrist requires appropriate goal setting and logical guidelines. Studies on functional motion of the wrist1,2 as well as outcome studies identifying average ROM achieved after various procedures provide baseline goals. The therapist must expect an altered arc of motion after a salvage procedure. If the therapist and/or the patient attempt to exceed the ROM limits, failure of the fusion may occur. When wearing an orthosis to achieve greater wrist ROM after a salvage procedure, the therapist must make sure to maintain stability, observe altered kinematics, avoid overstress of the fusion, and respect new motion.51 Shultz-Johnson51 emphasized the use of the objective evaluation of tissue response to the stress applied in therapy to guide the course of treatment. Pain, heat, redness, edema, and loss of ROM or strength in response to therapy are all indicators of inappropriate levels of stress.
The goal of a wrist salvage procedure is to provide a pain-free stable wrist to improve the function of the hand and upper extremity. When possible, the surgeon and therapist will strive to achieve a functional ROM postoperatively, unless a total wrist fusion has been performed. The surgical option used is determined by the wrist pathology and the carpal bones involved. The postoperative rehabilitation is procedure specific, determined by the procedure performed and the expected ROM. The patient, surgeon, and therapist must work together to achieve optimal, pain-free function. Communication between the surgeon and therapist is essential to maximize the patient’s ultimate ROM and functional result.
1. Palmer AK, Werner FW, Murphy D, Glisson R. Functional wrist motion: a biomechanical study. J Hand Surg Am. 1985;10:39.
2. Ryu J, Cooney WP 3rd, Askew LJ, et al. Functional ranges of motion of the wrist. J Hand Surg Am. 1991;16:409.
3. De Smet L. Does restricted wrist motion influence the disability of the upper limb? Acta Orthop Belg. 2007;73:446.
4. Watson HK, Dhillon HS. Intercarpal arthrodesis. 4th edGreen DP, ed. Operative Hand Surgery.Vol 1. New York: Churchill Livingstone; 1999:108–130.
5. Hastings H II. Arthrodesis (partial and complete). In: Green DP, Hotchkiss RN, Peterson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed New York: Churchill Livingstone; 2005:489–534.
6. Minami A, Kato H, Iwasaki N, Minami M. Limited wrist fusions: comparison of results 22 and 89 months after surgery. J Hand Surg Am. 1999;24:133.
7. Srinivasa VB, Matthews JP. Results of scaphotrapeziotrapezoid fusion for idiopathic arthritis. J Hand Surg Br. 1996;21:378.
8. Watson HK, Monacelli DM, Milford RS, Ashmead D IV. Treatment of Kienbock’s disease with scaphotrapezio-trapezoid arthrodesis. J Hand Surg Am. 1996;21(1):9–15.
9. Meier R, van Griensven M, Krimmer H. Scaphotrapeziotrapezoid (STT)-arthrodesis in Keinbock’s disease. J Hand Surg Br. 2004;29(6):580–584.
10. Watson HK, Fink JA, Monacelli DM. Use of triscaphe fusion in the treatment of Keinbock’s disease. Hand Clin. 1993;9:493–499.
11. Kleinman WB, Steichen JB, Strickland JW. Management of chronic rotary subluxation of the scaphoid by scapho-trapezio-trapezoid arthrodesis. J Hand Surg Am. 1982;7:125.
12. Kleinman WB, Carroll C IV. Scapho-trapezio-trapezoid arthrodesis for treatment of chronic static and dynamic scapho-lunate instability: a 10 year perspective on pitfalls and complications. J Hand Surg Am. 1990;15:408.
13. Watson HK, Wollstein R, Joseph E, et al. Scaphotrapeziotrapezoid arthrodesis: a follow-up study. J Hand Surg Am. 2003;28(3):397.
14. Yasuda M, Masada K, Takeuchi E, Ando Y. Scaphotrapeziotrapezoid arthrodesis for the treatment of Lichtman stage 3B Kienbock disease. Scand J Plast Reconstr Surg Hand Surg. 2005;39(4):242.
15. Ishida O, Tsai TM. Complications and results of scapho-trapezio-trapezoid arthrodesis. Clin Orthop Relat Res. 1993;287:125.
16. Meier R, Prommersberger KJ, Krimmer H. Scapho-trapezio-trapezoid arthrodesis (triscaphe arthrodesis). Handchir Mikrochir Plast Chir. 2003;35(5):323–327.
17. Wollstein R, Watson HK. Scaphotrapeziotrapezoid arthrodesis for arthritis. Hand Clin. 2005;21(4):539.
18. Sauerbier M, Tränkle M, Erdmann D, et al. Functional outcome with scaphotrapeziotrapezoid arthrodesis in the treatment of Kienbock’s disease stage III. Ann Plast Surg. 2000;44(6):618.
19. Beaton DE, Katz JN, Fossel AH, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the disabilities of the arm, shoulder and hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2).
20. Kalb K, Fuchs V, Bartelmann U, et al. Experiences with the STT (scapho-trapezio-trapezoid) arthrodesis: a retrospective evaluation. Handchir Mikrochir Plast Chir. 2001;33(3):181.
21. Watson HK, Ballet FL. The SLAC wrist: scapholunate advanced collapse pattern of degenerative arthritis. J Hand Surg Am. 1984;9:358–365.
22. Ashmead D 4th, Watson HK, Damon C, et al. Scapholunate advanced collapse wrist salvage. J Hand Surg Am. 1994;19:741.
23. Cohen MS, Kozin SH. Degenerative arthritis of the wrist: proximal row carpectomy versus scaphoid excision and four corner arthrodesis. J Hand Surg Am. 2001;26:94.
24. El-Mowafi H, El-Hadidi M, Boghdady GW, Hasanein EY. Functional outcome of four-corner arthrodesis for treatment of grade IV scaphoid non-union. Acta Orthop Belg. 2007;73(5):604–611.
25. Enna M, Hoepfner P, Weiss AP. Scaphoid excision with four-corner fusion. Hand Clin. 2005;21:531.
26. Brach P, Goitz R. An update on the management of carpal fractures. J Hand Ther. 2003;16(2):152.
27. Krakauer JD, Bishop AT, Cooney WP. Surgical treatment of scapholunate advanced collapsed. 19. 1994.
28. Wright TW, Michlovitz SL. Management of carpal instabilities. J Hand Ther. 1996;9(2):148–156.
29. MacDermid JC. Development of a scale for patient rating of wrist pain and disability. J Hand Ther. 1996;9(2):178.
30. Stamm TT. Excision of the proximal row of the carpus. Proc R Soc Med. 1944;38:74.
31. Imbriglia JE, Broudy AS, Hagberg WC, McKernan DL. Proximal row carpectomy: clinical evaluation. J Hand Surg Am. 1990;15:426.
32. Culp RW, McGuigan FX, Turner MA, et al. Proximal row carpectomy: a multicenter study. J Hand Surg Am. 1993;18:19.
33. Tomaino MM, Delsignore J, Burton RI. Long-term results following proximal row carpectomy. J Hand Surg Am. 1994;19:694.
34. DiDonna ML, Kiefhaber TR, Stern PJ. Proximal row carpectomy: study with a minimum of ten years of follow-up. J Bone Joint Surg Am. 2004;86:2359.
35. Neviaser RJ. Proximal row carpectomy for posttraumatic disorders of the carpus. J Hand Surg. 1983;8:301.
36. Stern PJ, Agabegi SS, Kiefhaber TR, Didonna ML. Proximal row carpectomy. J Bone Joint Surg Am. 2005;87(Suppl 1):166. Pt 2
37. Nagelvoort R, Kon M, Schuurman AH. Proximal row carpectomy: a worthwhile salvage procedure. Scand J Plast Reconstr Surg Hand Surg. 2002;36:289.
38. Baumeister S, Germann G, Dragu A, et al. Functional results after proximal row carpectomy (PRC) in patients with SNAC/SLAC wrist stage II. Handchir Mikrochir Plast Chir. 2005;37(2):106.
39. Dacho AK, Baumeister S, Germann G, Sauerbier M. Comparison of proximal row carpectomy and midcarpal arthodesis for the treatment of scaphoid nonunion advanced collapse (SNAC-wrist) and scapholunate advanced collapse (SLAC-wrist) in stage II. J Plast Reconstr Aesthet Surg. 2008;61:1210.
40. De Smet L. Outcome of proximal row carpectomy. Scand J Plast Reconstr Surg Hand Surg. 2006;40(5):302.
41. Jebson PJ, Hayes EP, Engber WD. Proximal row carpectomy: a minimum 10-year follow-up study. J Hand Surg Am. 2003;28(4):561–569.
42. Streich NA, Martini AK, Daecke W, et al. Proximal row carpectomy—an adequate procedure in carpal collapse. Int Orthop Nov. 2006.
43. Steenwerckx A, De Smet L, Zachee B, Fabry G. Proximal row carpectomy: an alternative to wrist fusion? Acta Orthop Belg. 1997;63:1.
44. Trankle M, Sauerbier M, Blum K, et al. Proximal row carpectomy: a motion-preserving procedure in the treatment of advanced carpal collapse. Unfallchirurg. 2003;106(12):1010.
45. Menon J. Universal total wrist implant: experience with a carpal component fixed with 3 screws. J Arthroplasty. 1998;13:515–523.
46. Adams B. Complications of wrist arthroplasty. Atlas Hand Clin. 2005;10:271.
47. Adams B. Total wrist arthroplasty. Atlas Hand Clin. 2005;10:263.
48. Kirkpatrick WH, Kozin S, Uhl R. Early motion after arthroplasty. Hand Clin. 1996;12(1):73.
49. Cobb TK, Beckenbaugh RD. Biaxial total-wrist arthroplasty. J Hand Surg Am. 1996;21:1101.
50. Kozin S. Arthroplasty of the hand and wrist: Surgeon’s perspective. J Hand Ther April-June. 1999;12(2):123–132.
51. Schultz-Johnson K. Splinting the wrist: mobilization and protection. J Hand Ther April-June. 1996;9(2):165–177.
52. Divelbiss BJ, Sollerman C, Adams B. Early results of the Universal total wrist arthroplasty in rheumatoid arthritis. J Hand Surg Am. 2002;27:195.
53. Toma CD, Machacek P, Bitzen P, et al. Fusion of the wrist in rheumatoid arthritis. J Bone Joint Surg Br. 2007;89:1620.
54. Weiss APC, Hastings H. Wrist arthrodesis for traumatic conditions: a study of plate and local bone graft application. J Hand Surg Am. 1995;20:50.
55. Weiss APC, Wiedman G Jr, Quenzer D, et al. Upper extremity function after wrist arthrodesis. J Hand Surg Am. 1995;20A:813.
56. Field J, Herbert TJ, Prosser R. Total wrist fusion. J Hand Surg Br. 1996;21(4):429.
57. Barbier O, Sales P, Rombouts JJ, Thonnard JL. Long term functional results or wrist arthrodesis in rheumatoid arthritis. J Hand Surg Br. 1999;24(1):27.
58. Gohritz A, Gohla T, Stutz N, et al. Special aspects of wrist arthritis management for SLAC and SNAC wrists using midcarpal arthrodesis. Bull Hosp Joint Dis. 2005;63(1&2):41.
59. Bora FW Jr, Culp RW, Osterman AL, Skirven T. A flexible wrist splint. J Hand Surg Am. 1989;14:574.