CHAPTER 90
Therapist’s Management of the Frozen Shoulder
MARTIN J. KELLEY, PT, DPT, OCS
CRITICAL POINTS
▪ The key clinical examination finding for frozen shoulder (FS) is the loss of at least 50% of passive external rotation (ER) at the side.
▪ Successful treatment is evidenced by significant symptom reduction, improved patient satisfaction, and restoration of functional motion.
Rehabilitating patients with frozen shoulder (FS) can be a challenging but rewarding endeavor. The therapist must understand the natural history of this unique pathology, and the patient must have patience. FS, or adhesive capsulitis, results in painful and limited active and passive shoulder range of motion (ROM). FS is reported to affect 2% to 5% of the general population1-4 increasing up to 10% and 38% of patients with thyroid disease and diabetes mellitus, respectively.1-6 Individuals with primary FS are commonly between 40 and 65 years old,7-9 and the incidence appears higher in females than males.1,3,6,10-12 The occurrence of FS in one shoulder increases the risk of contralateral shoulder involvement by 5% to 34%, and simultaneous bilateral shoulder involvement occurs as often as 14% of the time.1,13-15
Classification
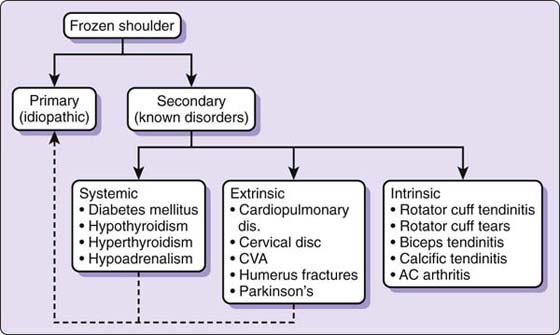
Zuckerman16 proposed a classification schema defined by primary and secondary FS (Fig. 90-1). Primary FS and idiopathic adhesive capsulitis are considered identical and not associated with a systemic condition or history of injury.16 Secondary FS was defined by three subcategories: systemic (e.g., diabetes, thyroid disease), extrinsic (e.g., myocardial infarction, cervical disk disease), and intrinsic (e.g., rotator cuff/biceps tendinopathy (see Fig. 90-1).16
Figure 90-1 Classification system. AC, acromioclavicular; CVA, cerebrovascular accident. (Reprinted with permission from Cuomo F. Diagnosis, classification and management of the stiff shoulder. In: Iannotti JP, Williams GR, eds. Disorders of the Shoulder: Diagnosis and Management. Philadelphia: Lippincott-Williams & Wilkins; 1999.)
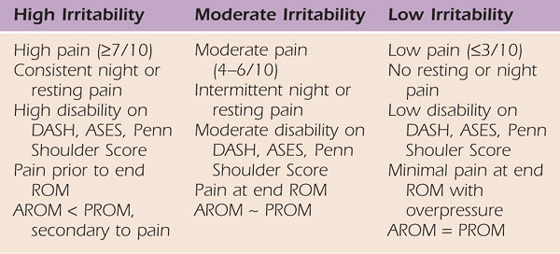
Another proposed classification system is based on the patient’s irritability level (low, moderate, and high) since most often the intervention is chosen based on tissue irritability (Table 90-1). Irritability is determined based on pain, ROM, and extent of disability. Patients with low irritability have lower disability, less pain, and capsular end-feels with little or no pain with over pressure; therefore, active and passive motion may be equal. These patients typically report stiffness rather than pain as a chief complaint. Patients with high irritability have significant pain, resulting in limited passive motion (due to muscle guarding) and greater disability. These patients typically report pain rather than stiffness as a chief complaint. Although these criteria are not time-based, most commonly patients with early-stage FS have a high level of irritability, and those with later stages have low irritability.
Table 90-1 Irritability Classification
Pathology
Hannafin and Chiaia12 described four stages incorporating the arthroscopic stages described by Neviaser and Neviaser,8 the clinical examination, and the histologic findings. Stage 1, the preadhesive stage, demonstrates mild erythematous synovitis. Patients present with mild end-range pain and are often misdiagnosed as having rotator cuff impingement. Stage 2, the acute adhesive, or “freezing,” stage, is characterized by a thickened red synovitis or angiogenesis (vascular proliferation). Patients frequently have a high level of discomfort and a high level of pain near end-range of movement. Even though this phase is represented by pain, examination under anesthesia reveals connective tissue changes resulting in loss of motion. Stage 3, the fibrotic, or “frozen,” stage, is characterized by less synovitis but more mature adhesions. Patients note significant stiffness with less pain. Based on examination under anesthesia, these patients have motion limited by established contracture as opposed to pain, as evidenced by equal passive motion to that when awake. Severe capsular restriction without apparent synovitis defines stage 4, or the “thawing,” phase. Patients present with painless stiffness and motion that typically improves by remodelling.
Examination
In addition to taking a comprehensive history, the following three questions help to determine the current stage of the patient’s FS. First, “Can you sleep through the night?” The ability to sleep well indicates low irritability. Secondly, “Do you have more pain or stiffness?” Greater pain reflects active synovitis, whereas stiffness indicates resolving synovitis and stage progression from stage 2 to 3. The third question, “Is it better or worse in the last 3 weeks?,” can help determine if the patient is moving into or out of stage 2.
A full upper quarter examination is performed to rule out cervical spine and neurologic pathologies. The examination typically reveals significant global limitations of both active and passive ROM with elevation usually less than 120 degrees,17-20 but motion limitations are stage-dependent. Scapular substitution frequently accompanies active shoulder motion.21,22
Loss of motion of greater than 25% in at least two planes, a 50% loss of passive ER, or less than 30 degrees of ER are indicative of a patient with FS.14,15,17,19,20,23-31 Passive motions should be assessed supine to appreciate the quality of the resistance to motion at the end of passive movement—that is, the end-feel. Frequently, passive glenohumeral motions are restricted due to pain at or before end-range, and muscle guarding can often be appreciated at end-range. I believe muscle guarding can masquerade as a capsular end-feel. I examined six patients prior to manipulation both before and after administration of anesthesia. The examinations revealed an increase in passive motion of 10 to 30 degrees during anesthetization in five of six patients. A capsular end-feel was appreciated in all patients before anesthesia; however, this was a false interpretation. Partial improvement in motion related to diminished pain and muscle guarding has been reported after local or regional anesthetic.11
Patients with FS are considered to have normal strength and painless resisted motions.27 However, markedly reduced shoulder isometric maximal voluntary force has been reported in patients with FS.32-34 Objective shoulder strength measurements using a hand-held dynamometer revealed significant weakness of the shoulder internal rotators33,34 and elevators32,33 in patients with FS.
Special tests such as impingement signs and Jobe’s test are not helpful in differentiating FS from rotator cuff tendinopathy since they require painful end-range positioning.
Interventions
Multiple interventions have been studied; however, definitive treatment remains unclear.24,30,35-37 Unfortunately, comparison between studies is difficult due to varied inclusion criteria, different protocols, and varied outcome assessment tools. One of the major difficulties in assessing efficacy is defining success criteria because the majority of patients with FS significantly improve in approximately 1 year regardless of how they were treated. Successful treatment should not be defined by the return of “normal” motion, but rather as significant symptom reduction, improved patient satisfaction, and improved functional motion. Therefore the initial goal is pain reduction by influencing synovial tissue. Typically, as pain lessens, the patient moves well within the passive limits and “feels” greatly improved. The capsuloligamentous complex (CLC) tissue length is restored, and motion returns as dense fibrotic collagen remodeling occurs over time.
Modalities
Little data support the use of frequently employed modalities such as heat, ice, ultrasound, or electric stimulation. Modalities may influence pain and muscle relaxation, and therefore they might enhance the effect of exercises and manual techniques. Hot packs can be applied before or during ROM exercises. Application of moist heat in conjunction with stretching has been shown to improve muscle extensibility.38 This may occur by a reduction of muscle viscosity and neuromuscular-mediated relaxation.29,39 Gursel and colleagues40 demonstrated the lack of efficacy of ultrasound compared with sham ultrasound in treating shoulder soft tissue disorders. Transcutaneous electrical nerve stimulation (TENS) together with a prolonged low-load stretch resulted in less pain and improved motion in patients with FS.41
Stretching
Outcomes have been reported in patients with FS treated primarily with stretching exercises in therapy. Stretching appears to influence pain and improve motion over time. Studies have shown that exercise results in improved ROM and outcomes; however, aggressive stretching may be detrimental.14,42 An impressive finding among multiple studies is symptom improvement and minimal or no difference in outcomes (at 3–6 months) in patients treated with a therapist-directed home exercise program compared with other interventions.24,30,31,43
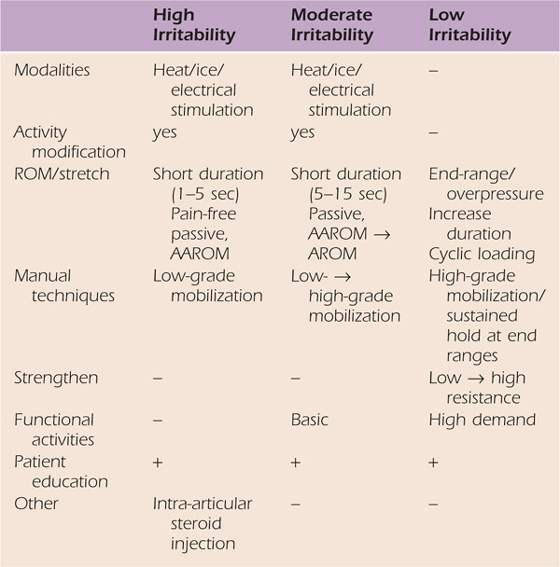
Applying the correct “dose” of stretching is important and based on the stage of FS and the patient’s irritability classification (Table 90-2). In patients with high irritability, low intensity and short-duration ROM exercises are performed to simply alter the joint receptors input, reduce pain, decrease muscle guarding, and increase motion.44 Three factors should be considered when calculating “dose” or total amount of stress delivered to a tissue: intensity, frequency, and duration. The total end-range time (TERT)45,46 is the total amount of time the joint is held at or near end-range position. TERT is calculated by multiplying the frequency and duration of the time spent at end-range daily and is a useful way of measuring the dose of tissue stress.45,46 Intensity remains an important factor in tensile stress dose but is typically limited by pain. Traditional ROM exercises are considered lower forms of tensile stress, whereas the highest tensile stress doses are achieved by low-load prolonged stretching (LLPS) because TERT is maximized. Therefore, the goal with each patient is to determine the therapeutic level of tensile stress required based on the patient’s irritability level and response to treatment.
Table 90-2 Treatment Strategies Based on Irritability Level
Joint Mobilization
Several studies have examined the effect of joint mobilization in patients with FS, and although there is evidence that it may be beneficial there is little evidence that it is more efficacious than other forms of treatment.24,25,47-49 One study treated one group with high-grade (III and IV) and another with low-grade (I and II) mobilizations without any other intervention for three sessions a week for 12 weeks. Outcome measures for both groups improved, and although the high-grade mobilization group did better, only a minority of comparisons reached statistical significance and the overall differences between the two interventions was small.25 In other words, just performing low-grade mobilizations (for pain) brought about significant improvement in motion and function.
Clearly some patients appear to react very favorably to joint mobilization, demonstrated by their response to treatment. Further research is required to determine if certain patient characteristics respond to specific interventions such as joint mobilization.
Corticosteroids
Corticosteroids can be administered by injection to dampen the inflammatory response in patients with FS. Significant evidence demonstrates that intra-articular corticosteroid injections provide significant improvement of symptoms in the first 4 to 6 weeks of the intervention.24,30,35,36,43 Although no long-term differences can be attributed to corticosteroids, the belief is that the patient can be made more comfortable at rest and with functional movement and the synovitis stage possibly shortened, thereby hastening the time needed to achieve end-range stretching of fibrotic tissue.
Proposed Intervention Algorithm
An algorithmic approach offering conservative and surgical interventions can effectively address the FS continuum (Fig. 90-2). The patient is seen by the shoulder service surgeon, physician, or therapist and diagnosed with FS. The patient is given one of four treatment options based on the known pathology, and evidence-based literature. The patient is given the option to receive an intra-articular corticosteroid injection. Whether he or she receives the injection or not, the patient is referred to physical therapy for either a home exercise program (HEP) or supervised therapy (ST). There is not clear evidence to determine which patients may need formal ST rather than simply a HEP. Therefore the decision is made based on the physician’s and patient’s preference with input from the therapist after initial evaluation. Factors that may favor use of ST may be increased irritability, greater disability, more comorbidities, lower social support, lower educational level, or high fear and anxiety.
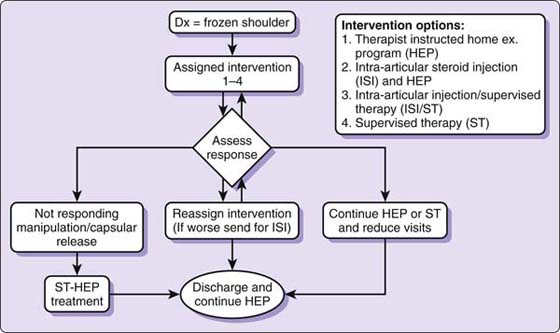
Figure 90-2 Frozen shoulder intervention algorithm.
Regardless of the intervention, the patient is asked to return to the physician in 6 to 8 weeks. The algorithm is followed until the patient responds or is discharged. If the patient elects to receive an intra-articular injection, no more than three are given and usually only one to two are required.50 Most patients respond to the interventions, but some improve very slowly and continue to have impairments (pain and functional deficits) but are willing to continue with the HEP after the other conservative options are attempted. If the symptoms and motion are unresponsive to the various levels of treatment over time (typically 6 months) and quality of life is compromised, a manipulation under anesthesia or surgical capsular release should be considered. If the patient is unwilling to have a manipulation or surgery, he or she is discharged but encouraged to continue with a daily stretching program.
Therapy Intervention
When seen in therapy, the patient is given a complete examination. A key principle of the examination is to establish the patient’s irritability level. After a thorough evaluation, the therapist educates the patient about the pathology and the natural course of FS. The patient is instructed to use heat before exercise and, if needed, ice following. All patients are instructed in a HEP based on irritability level regardless of whether they are returning for ST or treated with a HEP. In patients classified as having severe or moderate irritability the stretching time intervals are kept short (1–5 sec). Although there is no evidence for exercise frequency, the patient is asked to perform each exercise 20 times two or three times a day. The core exercises include the pendulum exercise; passive supine forward elevation; passive ER with the arm in approximately 40 degrees in the plane of the scapula; and AAROM in extension, internal rotation (IR), and horizontal adduction (Fig. 90-3). These exercises primarily influence different regions of the synovial and CLC and have been used in supervised therapy programs and HEPs in patients with FS.14,30,31,42,43 Any exercise found to be too painful to perform is removed from the program. Patients with moderate irritability may be instructed in pulley use for elevation. Patients classified with low irritability may be instructed in the same exercises and pulley but hold at end-range for up to 30 sec. The pulley exercise is performed with the elbow close to full extension and shoulder in forward flexion. This allows normal obligatory, humeral IR to occur. If the arm is raised with the elbow bent, the initial “forced” ER takes up all the CLC slack, thereby limiting elevation (Fig. 90-4).
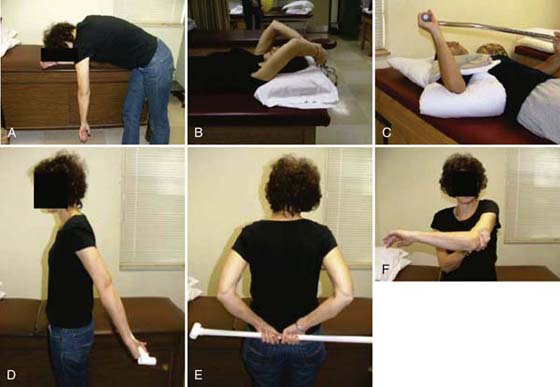
Figure 90-3 Core stretching exercises. A, Pendulum. B, Forward elevation. C, External rotation (stretch performed with heat application). D, Extension. E, Internal rotation. F, Horizontal adduction.
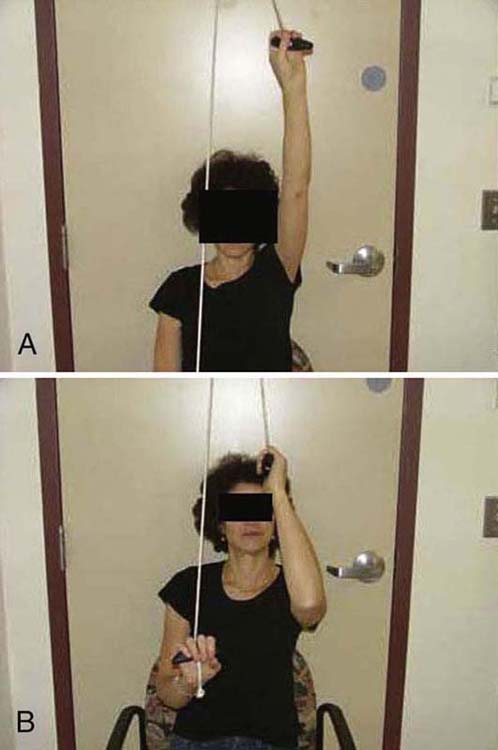
Figure 90-4 Pulley exercise. A, Performed correctly. B, Incorrectly.
Patients performing only a HEP are instructed to call if pain increases or they feel they are not performing the exercises correctly. Patients who have difficulty performing the exercises independently are asked to return within the week to be sure they are doing them correctly.
If followed in ST, the patient is asked to perform the exercises for 1 week and return. The patient’s response to the week of HEP determines prognosis and treatment frequency. At the 1-week visit, the patient is asked to report any change in symptoms, especially night pain. A reassessment is performed assessing “cold” measurements (pretreatment) and compared with the initial examination. End-range “irritability” is determined with passive ER and elevation.
Treatment begins with moist heat on the affected side with the patient supine and the arm placed in approximately 40 degrees of abduction in the plane of the scapula. After 5 minutes with heat, the patient begins to perform short-interval (5-sec) intermittent passive ER stretching (with the heat still applied) for 20 repetitions (see Fig. 90-3C). Following this, passive ER motion is reassessed. Patients often gain 10 to 15 degrees of motion following heat and stretching, indicating muscle guarding and pain as the initial motion barrier. The patient with low irritability may gain nothing or minimal motion (5 degrees) following heat and stretch. This may indicate that the “fibroblastic wall,” and not pain, is the motion barrier. The patient performs passive supine elevation, undergoes joint mobilizations or gentle manual stretching, and performs the rest of the prescribed ROM and pulley exercises. Mobilizations are initially performed in the loose pack position anteriorly, inferiorly, and posteriorly. Traction and rotational oscillations are applied between translational glides.
Determining Treatment Frequency
Those who are able to sleep through the night or have a significant overall reduction in irritability (decreased pain or tolerable end-range overpressure with less pain) after the first week of HEP have an excellent prognosis and are seen once a week or every other week. If minimal response is noted relative to pain and motion, the patient presents with high to moderate irritability and demonstrates a significant “in treatment response” (>15 degree motion gain in ER and or elevation), he or she is seen twice a week. If the patient’s condition has significantly worsened, he or she may be referred back to the physician for a glenohumeral intra-articular corticosteroid injection.
Treatment Progression
As pain and irritability lessen, the grade of mobilization and intensity of stretching increases. Mobilization positions are moved to end-range to influence connective tissue length (Fig. 90-5). The hold–relax technique is used near end-range to maximize relaxation by reducing muscle guarding (antagonistic inhibition). Mobilization with movement is used to recruit agonistic and inhibit antagonistic musculature. Specific mobilizations are performed to target the rotator cuff interval (RCI) since contracture of this structure is often found with FS (Fig. 90-6).26,51-53 The RCI limits ER and provides stability against inferior translation with the arm adducted. ER motion can be improved not only with anterior but also posterior glides (Fig. 90-7).47
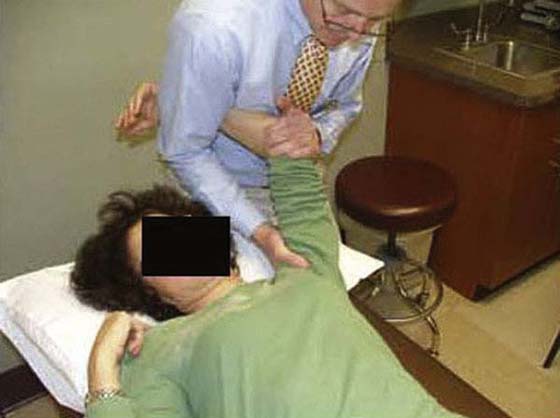
Figure 90-5 Inferior glide at end-range.
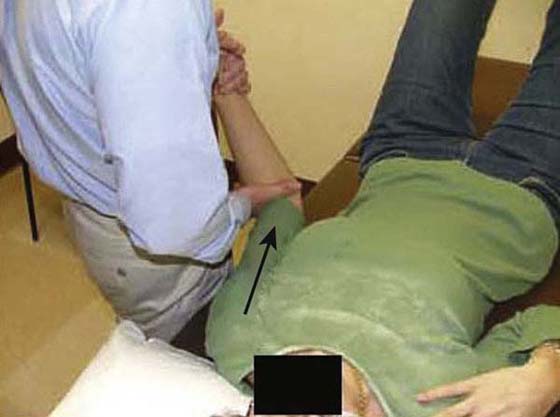
Figure 90-6 Inferior glide in adduction and external rotation to target the rotator cuff interval.
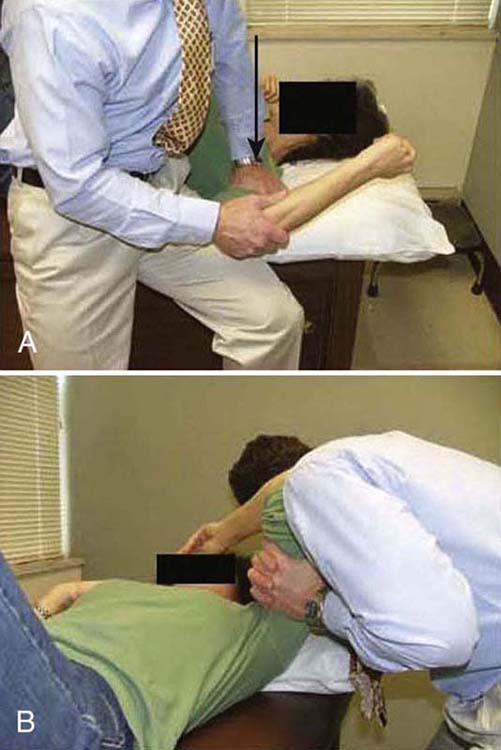
Figure 90-7 A, Posterior glides to help regain external rotation motion. B, Posterolateral glide.
The rehabilitation program is progressed to a quadrant stretch with the patient supine by bringing the patient’s locked hands to the top of the head and letting the elbow fall to the surface (Fig. 90-8). Pectoral activity controls this stretch, and as the patient relaxes the shoulder moves into a slightly painful range. Patients are asked to bring their elbow forward after approximately 5 to 10 sec and repeat 10 to 20 times. The intensity and duration of the stretching is based on irritability level, but painful aggressive stretching is avoided. A sign of appropriate stretch intensity is elimination of pain once the limb is removed from end-range. Patients with limited functional IR are thought to have tightness of superior capsular structures in addition to posterior structures. Pouliart and coworkers54 found a posterior superior ligamentous structure that limits IR with the arm at the side; therefore an inferior glide in adduction or extension and IR can be performed to influence this structure (Fig. 90-9). To stretch the superior CLC structures the patient is placed in a sidelying position with the involved hand on hip and the elbow is gently pushed down (arm adducted) (Fig. 90-10). When irritability allows, the exercise can be progressed to a “sleeper stretch” (Fig. 90-11). Care must be taken since these stretches can be very painful.
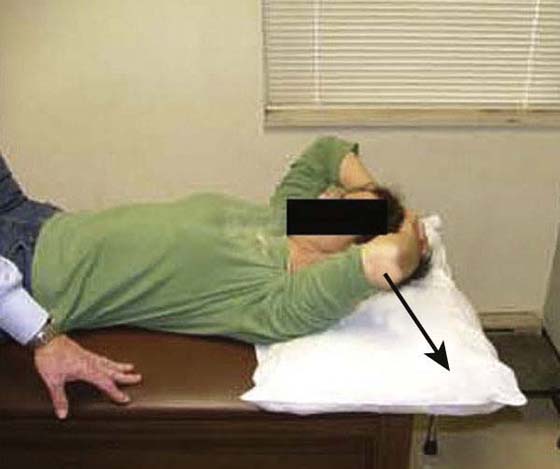
Figure 90-8 Quadrant stretch.
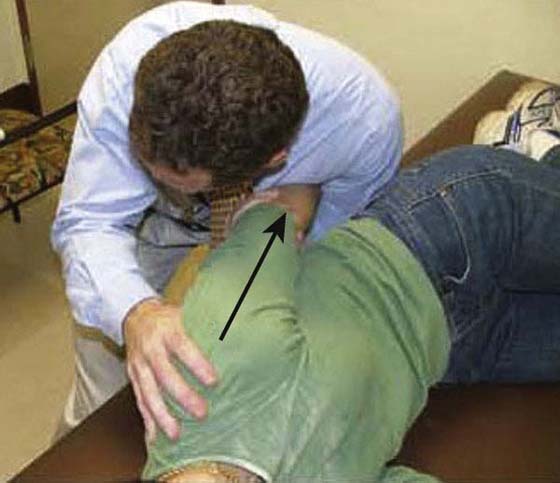
Figure 90-9 Inferior glide in adduction and extension to target the posterosuperior capsuloligamentous complex.
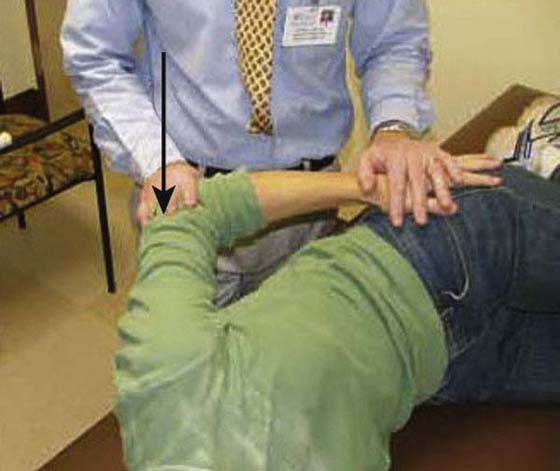
Figure 90-10 Superior structures stretch.
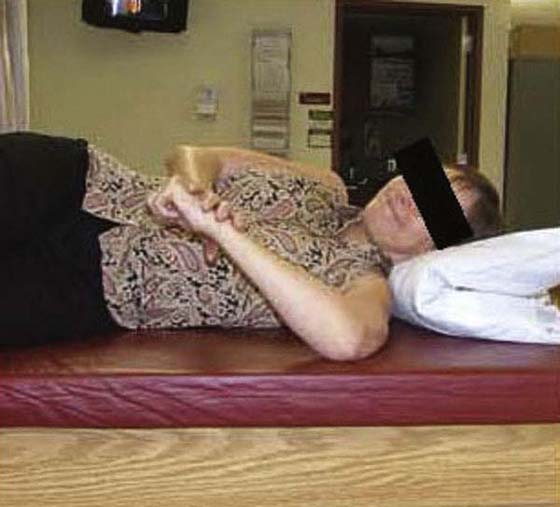
Figure 90-11 “Sleeper’s” stretch to target the posterior capsuloligamentous complex.
Before each subsequent session begins, ROM and end-range reactivity are assessed. At the end of the session, the therapist reassesses ROM with particular attention to ER and elevation. If the patient’s symptoms remain or worsen and severe irritability persists during that initial period (usually within 3 to 4 visits), the patient is sent back to the referring physician for a glenohumeral intra-articular corticosteroid injection. After receiving a corticosteroid injection the patient is asked to perform only pendulum exercise several times a day to allow maximal medication effect without tissue provocation. After approximately 4 days, the patient can return to supervised physical therapy or the HEP.
Constant reassessment of symptoms and modifying the “dosage” of tensile load is continued for 4 to 6 weeks. If the patient’s symptoms continue to lessen, the TERT concept is used so that LLPS is performed (Fig. 90-12). The time between visits increases to a visit every 1 to 3 weeks. Discharge is considered based on these criteria: low irritability, minimal or no end-range pain, 10 degrees or less within treatment session gain, and stagnant motion gain between visits. These criteria represent the presence of fibroblastic contracture at that time, and continued stretching will improve tissue remodeling. Some patients making slow progress may meet the discharge criteria and are discharged but are asked to return in 8 to 12 weeks for program progression.

Figure 90-12 Prolonged stretch into external rotation and elevation.
Patients return to the referring surgeon or physician every 6 to 8 weeks. As per the algorithm, if the symptoms are unresponsive to the various levels of treatment over time and quality of life is compromised, a manipulation or capsular release is offered. Approximately 7% to 10% of conservative treatment approaches fail, and manipulation or capsular release is then required.14,55 If the patient is unwilling to have a manipulation or surgery, he or she is discharged but encouraged to continue with a daily stretching program.
Summary
FS is a commonly treated musculoskeletal problem, yet the cause remains elusive. Patients present with a recognized history, physical examination results, and natural course of recovery. Multiple interventions have been investigated. I feel the FS algorithm provides efficacious intervention options. Corticosteroid intra-articular injections are favored in patients with high irritability or those who have not responded to ST or a HEP. Assessing the response to treatment determines the frequency of treatment and alternative interventions. The discharge criteria satisfy the initially stated short-term success goals (significant pain relief, return of functional movement, patient satisfaction) and are based on the belief that once pain is ameliorated, CLC remodeling occurs over a prolonged period. The patient with a recalcitrant FS has the option of manipulation or capsular release (or both) if conservative treatment fails.
REFERENCES
1. Lundberg BJ. The frozen shoulder: clinical and radiographic observation: the effect of manipulation under general anesthesia: structure and glycosaminoglycan content in the joint capsule. Acta Orthop Scand. 1969;119:1–59.
2. Pal B, Anderson J, Griffiths I. Limitation of joint mobility and shoulder capsulitis in insulin and non-insulin dependent diabetes. Br J Rheumatol. 1986;25:147–151.
3. Aydeniz A, Gursoy S, Guney E. Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J Int Med Res. 2008;36(3):505–511.
4. Bridgman J. Periarthritis of the shoulder and diabetes mellitus. Ann Rheum Dis. 1972;31:69.
5. Balci N, Balci MK, Tuzuner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complications. 1999;13(3):135–140.
6. Milgrom C, Novack V, Weil Y, et al. Risk factors for idiopathic frozen shoulder. Isr Med Assoc J. 2008;10(5):361–364.
7. Neviaser RJ. Painful conditions affecting the shoulder. Clin Ortho Rel Res. 1983 Mar.(173):63–69.
8. Neviaser RJ, Neviaser TJ. The frozen shoulder. Diagnosis and management. Clin Ortho Rel Res. 1987.(223):59–64.
9. Neviaser JS. Adhesive capsulitis and the stiff and painful shoulder. Orthop Clin N Am. 1980;11(2):327–331.
10. Binder A, Bulgen D, Hazleman B. Frozen shoulder: an arthrographic and radionuclear scan assessment. Ann Rheum Dis. 1984;43:365.
11. Sheridan MA, Hannafin JA. Upper extremity: emphasis on frozen shoulder. Orthop Clin N Am. 2006;37:531–539.
12. Hannafin JA, Chiaia T. Adhesive capsulitis. Clin Ortho Rel Res. 2000;372:95–109.
13. Bulgen DY, Binder A, Hazleman BL, Park JR. Immunological studies in frozen shoulder. J Rheumatol. 1982;9(6):893–898.
14. Griggs S, Ahn A, Green A. Idiopathic adhesive capsulitis: a prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am. 2000;82(10):1398–1417.
15. Shaffer B, Tibone JE, Kerlan RK. Frozen shoulder. A long-term follow-up. J Bone Joint Surg Am. 1992;74(5):738–746.
16. Zuckerman JD. Definition and classification of frozen shoulder: a consensus approach. J Shoulder Elbow Surg. 1994;3(1):S73.
17. Bunker T, Anthony P. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg [Br]. 1995;77:677–683.
18. Quin C. “Frozen shoulder”: evaluation of treatment with hydrocortisone injections and exercises. Ann Phys Med. 1965;8:22.
19. Neviaser JS. Adhesive capsulitis of the shoulder. A study of the pathological findings in periarthritis of the shoulder. J Bone Joint Surg Am. 1945;27(2):211–222.
20. Rizk TE, Pinals RS. Frozen shoulder. Semin Arthr Rheum. 1982;11(4):440–452.
21. Vermeulen HM, Stokdijk M, Eilers PH, et al. Measurement of three dimensional shoulder movement patterns with an electromagnetic tracking device in patients with a frozen shoulder. Ann Rheum Dis. 2002;61(2):115–120.
22. Rundquist PJ, Anderson DD, Guanche CA, Ludewig PM. Shoulder kinematics in subjects with frozen shoulder. Arch Phys Med Rehabil. 2003;84(10):1473–1479.
23. Binder AI, Bulgen DY, Hazleman BL, Roberts S. Frozen shoulder: a long-term prospective study. Ann Rheum Dis. 1984;43(3):361–364.
24. Bulgen DY, Binder A, Hazleman BL, et al. Frozen shoulder: prospective clinical study with an evaluation of three treatment regimens. Ann Rheum Dis. 1984;43:353.
25. Vermeulen HM, Rozing PM, Obermann WR, et al. Comparison of high-grade and low-grade mobilization techniques in the management of adhesive capsulitis of the shoulder: randomized controlled trial. Phys Ther. 2006;86(3):355–368.
26. Neer CS, Satterlee CC, Dalsey RM, Flatow EL. The anatomy and potential effects of contracture of the coracohumeral ligament. Clin Orthop. 1992.(280):182–185.
27. Cyriax J. Diagnosis of Soft Tissue Lesions. Baltimore: Williams & Wilkins; 1970.
28. Reeves B. The natural history of the frozen shoulder syndrome. J Rheumatol. 1975;4:193.
29. Wadsworth CT. Frozen shoulder. Phys Ther. 1986;66(12):1878–1883.
30. Carette S, Moffet H, Tardif J, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo-controlled trial. Arthritis Rheum. 2003;48(3):829–838.
31. Kivimaki J, Pohjolainen T, Malmivaara A, et al. Manipulation under anesthesia with home exercise versus home exercises alone in the treatment of frozen shoulder: a randomized controlled trial with 125 patients. J Shoulder Elbow Surg. 2007;16:722–726.
32. Sokk J, Gapeyeva H, Ereline J, Kolts I, Paasuke M. Shoulder muscle strength and fatigability in patients with frozen shoulder syndrome: the effect of 4-week individualized rehabilitation. Electromyogr Clin Neurophysiol. 2007;47:205–213.
33. Leggin B, Kelley MJ, Pontillo M. Impairments and function in patients with frozen shoulder compared to patients with rotator cuff tendonopathy. Second International Congress of Shoulder Therapists. Bahia: Brazil; 2007.
34. Jurgel J, Rannama L, Gapeyeva H, Ereline J, Kolts I, Paasuke M. Shoulder function in patients with frozen shoulder before and after 4-week rehabilitation. Medicina (Kaunas). 2005;41(1):30–38.
35. Arslan S, Celiker R. Comparison of the efficacy of local corticosteroid injection and physical therapy for the treatment of adhesive capsulitis. Rheumatol Int. 2001;21(1):20–23.
36. van der Windt DA, Koes BW, Deville W, et al. Effectiveness of corticosteroid injections versus physiotherapy fro the treatment of painful stiff shoulder in primary care: randomized trial. Br Med J. 1998;317:1292–1296.
37. Hazleman BL. The painful stiff shoulder. Rheumatol Phys Med. 1972;11(8):413–421.
38. Jarvinen TA. Muscle injuries: biology and treatment [Review]. Am J Sports Med. 2005;33(5):745–764.
39. Safran M, Garrett W Jr, Seaber A, et al. The role of warmup in muscular injury prevention. Am J Sports Med. 1988;16(2):123–129.
40. Gursel YK, Ulus Y, Bilgic A, et al. Adding ultrasound in the management of soft tissue disorders of the shoulder: a randomized placebo-controlled trial. Phys Ther. 2004;84(4):336–343.
41. Rizk TE, Christopher RP, Pinals RS, et al. Adhesive capsulitis (frozen shoulder): a new approach to its management. Arch Phys Med Rehabil. 1983;64(1):29–33.
42. Diercks RL, Stevens M. Gentle thawing of the frozen shoulder: a prospective study of supervised neglect versus intensive physical therapy in seventy-seven patients with frozen shoulder syndrome followed up for two years. J Shoulder Elbow Surg. 2004;13(5):499–502.
43. Ryans I, Montgomery A, Galway R, et al. A randomized controlled trial of intra-articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology. 2005;44(4):529–535.
44. Wyke BD. The neurology of joints. Ann R Coll Surg. 1967;41:2550.
45. McClure PW, Blackburn LG, Dusold C. The use of splints in the treatment of joint stiffness: biologic rationale and an algorithm for making clinical decisions. Phys Ther. 1994;74(12):1101–1107.
46. Flowers KR, LaStayo P. Effect of total end range time on improving passive range of motion. J Hand Ther. 1994;7:150–157.
47. Johnson AJ, Godges JJ, Zimmerman G, Ounanian LL. The effect of anterior versus posterior glide joint mobilization on external rotation range of motion in patients with the shoulder adhesive capsulitis. J Orthop Sports Phys Ther. 2007;37(3):88–99.
48. Nicholson G. The effects of passive joint mobilization on pain and hypomobility associated with adhesive capsulitis of the shoulder. Orthop Sports Phy Ther. 1985;6:238–246.
49. Vermeulen HM, Obermann WR, Burger BJ, et al. End-range mobilization techniques in adhesive capsulitis of the shoulder joint: a multiple-subject case report. Phys Ther. 2000;80(12):1204–1213.
50. Shah N, Lewis M. Shoulder adhesive capsulitis: systematic review of randomised trials using multiple corticosteroid injections. Br J Gen Prac. 2007;57:662–667.
51. Omari A, Bunker T. Open surgical release for frozen shoulder: surgical findings and results of the release. J Shoulder Elbow Surg. 2001;10:353–357.
52. Ozaki J, Nakagawa Y, Sakurai G, Tamai S. Recalcitrant chronic adhesive capsulitis of the shoulder. Role of contracture of the coracohumeral ligament and rotator interval in pathogenesis and treatment. J Bone Joint Surg Am. 1989;71(10):1511–1515.
53. Wiley AM. Arthroscopic appearance of frozen shoulder. Arthroscopy. 1991;7(2):138–143.
54. Pouliart N, Somers K, Eid S, Gagey O. Variations in the superior capsuloligamentous complex and description of a new ligament. J Shoulder Elbow Surg. 2007;16(6):821–836.
55. Murnaghan JP. Adhesive capsulitis of the shoulder: current concepts and treatment. Orthopedics. 1988;11(1):153–158.