CHAPTER 115
Complex Regional Pain Syndrome: Types I and II
L. ANDREW KOMAN, MD, ZHONGYU LI, MD, PhD, BETH PATERSON SMITH, PhD, AND THOMAS L. SMITH, PhD
CRITICAL POINTS
▪ The primary difference between complex regional pain syndrome (CRPS) types I and II is that there is an identifiable peripheral nerve injury in type II.
▪ The diagnosis of CRPS is based on clinical findings, but a positive third-phase bone scan provides objective corroboration for the clinical diagnosis.
▪ Radiographs are used to determine whether osteopenia is present, and laser Doppler fluxmetry measurements are used to evaluate cutaneous perfusion.
▪ Decreased pain after stellate ganglion block is presumptive evidence that pain is sympathetically maintained, whereas no reduction in pain suggests that pain processing is sympathetically independent.
▪ Medical management and surgical management vary depending on the clinical presentation and the outcome of pharmacologic agents. However, hand therapy is considered an important aspect of nonsurgical management.
Reflex sympathetic dystrophy (RSD) is a generic term used to describe post-traumatic pain accompanied by inappropriate autonomic activity and impaired extremity function (Box 115-1). In Europe, this condition is called algodystrophy. However, the term complex regional pain syndrome (CRPS), as proposed by the International Association for the Study of Pain in 1993, provides descriptive terminology based on clinical features, location, and specifics of the injury, without implying mechanism, cause, or sympathetic maintenance.1 CRPS type I (traditional RSD or algodystrophy) occurs without an identifiable peripheral nerve injury; CRPS type II, causalgia, is clinical RSD with a peripheral nerve abnormality.2-5 Either form may be sympathetically maintained or sympathetically independent. The former is defined as pain occurring with relief or improvement of symptoms during or after sympatholytic blockade. CRPS is not synonymous with sympathetically maintained pain (SMP), in contradistinction to the consensus recommendation of the American Association for Hand Surgery in 1991.6 Physiologic manifestations of CRPS are normal responses to an initial noxious insult that are prolonged abnormally and persist in the absence of ongoing or impending cellular damage. Patients presenting with CRPS have incapacitating pain and functional compromise.7 In CRPS, a complex series of peripheral and central events affect peripheral autonomic control, modify central nervous system (CNS) activity, and produce pain, autonomic dysfunction, trophic changes, and functional impairment. Pain may be nociceptive or neuropathic. The former originates from a mechanical source in the absence of an identifiable nerve injury. Neuropathic pain emanates from an injury or dysfunction of a peripheral nerve combined with trophic changes, autonomic dysfunction, and functional impairment. Thus, a localized neuroma or neuroma-in-continuity, a cause of neuropathic pain, is not per se CRPS type II. The presence of a nociceptive focus (e.g., mechanical irritation of the wrist secondary to an unstable distal radioulnar joint or injury to a peripheral nerve) serves as a trigger to further exaggerate physiologic responses mediated through inappropriate α-adrenergic activity within the dorsal horn of the spinal cord.8,9 After a peripheral injury, excitation and sensitization of wide dynamic range neurons (internuncial connections in the dorsal horn of the spinal cord) increase the conscious appreciation of pain.4,10-12 SMP occurs when receptor disease [receptor disease refers to the concept that increased sensitivity of adrenergic receptors is responsible for CRPS] predominates and sympatholytic intervention (e.g., intravenous phentolamine or stellate ganglion block) decreases α-adrenergic tone with subsequent decrease in pain, normalization of autonomic function, and increased physical capability.8 Over time, physiologic and/or anatomic adaptation occurs or permanent structural damage may alter peripheral and CNS architectural structures. When this occurs, sympathetic blockade is no longer effective, and pain becomes sympathetically independent.
Box 115-1 Synonyms for Complex Regional Pain Syndrome
Acute atrophy of bone
Algodystrophy
Algoneurodystrophy
Causalgia state/syndrome
Chronic traumatic edema
Major causalgia
Major traumatic dystrophy
Mimocausalgia
Minor causalgia
Minor traumatic dystrophy
Neurodystrophy
Neurovascular dystrophy
Osteoneurodystrophy
Pain dysfunction syndrome
Painful post-traumatic osteoporosis
Peipheral trophoneurosis
Demographics
Although CRPS can develop in any patient, epidemiologically, white women who smoke cigarettes are affected most frequently. The estimated female preponderance of CRPS varies from 1 : 1.6 to 4.5 : 1.13-20 In the Netherlands, the incidence of CRPS was reported as 26.2 per 100,000 in a series of patients studied from 1996 through 2005.21
Age at onset is most frequently between 30 and 55 years (mean, 40 years); however, individuals of any age may be affected.22-26 An identifiable nociceptive injury is diagnosed in less than 50% of cases. CRPS is observed frequently (20%–40%) after fracture of the distal radius.22-25 In addition, traumatic or iatrogenic injuries to the following nerves have been documented to contribute to and to precipitate CRPS: the palmar cutaneous branch of the median nerve, the median nerve at any level, the dorsal branch of the ulnar nerve, the superficial radial nerve, the ulnar nerve at the elbow, and the posterior interosseous nerve.27 An association between CRPS after simultaneous median nerve decompression and palmar fasciectomy for Dupuytren’s disease has been reported.28,29 Familial or genetic factors have been implicated as risk factors for CRPS.30 Patients with CRPS do not report higher pain scores or lower levels of psychological distress than do patients with back pain or local neuropathy.31 Self-induced disorders such as factitious disorders may be confused with CRPS.32 See Chapter 136 for more information on self-inflicted disorders.
Diagnosis
General
The diagnosis of CRPS is based on clinical findings. In patients with CRPS, pain is initiated in the periphery by a noxious incident(s), is influenced by a post-traumatic event(s), is exacerbated by physiologic and/or anatomic variables, and is determined, in part, by congenital or genetic factors.27,33-35 There are no pathognomonic tests for CRPS; onset is frequently delayed and the average diagnosis is made 2 to 12 weeks after injury.
Symptoms and Signs
Pain, a prerequisite of CRPS, often is described as burning, throbbing, tearing, cutting, searing, shooting, and aching. Characteristic types of pain in CRPS include hyperalgesia, allodynia, and hyperpathia. Hyperalgesia, pain that is greater than expected for a given painful stimulus, is considered primary when it affects the immediate area surrounding the injury. Hyperalgesia is termed secondary when it causes distant discomfort in nontraumatized skin either proximal or distal to the initial injury area.36 Pain secondary to normally nonpainful stimuli is called allodynia. Hyperpathia is delayed pain that typically outlasts the initiating stimulus and spreads beyond normal dermatomal borders. Cold sensitivity (a painful response to cold exposure) is also commonly experienced by CRPS patients.13
Physiologic changes include trophic disease and vascular events. Trophic changes associated with CRPS include stiffness, edema, and atrophy of the hair, nails, and skin. Hyperkeratosis of the skin may occur. Symptoms of vasomotor and/or autonomic nervous system dysfunction occur in 80% of patients.37 Various testing methods can be used to demonstrate abnormal autonomic function in most patients.38-40
The subjective symptoms of patients with CRPS may be quantified by the use of validated instruments that evaluate pain, cold sensitivity, and numbness. These instruments include variations of the McGill Pain Questionnaire,41,42 the Carpal Tunnel Instrument,43 and the McCabe Cold Sensitivity Severity Scale.44 Extremity function may be analyzed using the Carpal Tunnel Instrument Function Scale43 and the Disabilities of the Arm, Shoulder, and Hand (DASH) form from the American Academy of Orthopaedic Surgery.45 The RAND SF-36 Health Survey (http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html) may be used to measure the health-related quality of life of CRPS patients. This instrument assesses physical, social, and emotional functioning; perceived health; overall life satisfaction; perceived pain; and work performance.46-48
Physical Examination
The physical examination must include an assessment of the whole patient, with an emphasis on the cervical spine, thoracic spine, shoulder girdle, involved extremity, contralateral limb, and both lower extremities. A careful neurologic assessment is required to determine the presence or absence of discogenic or degenerative cervical disease, peripheral neuropathy, arthritis, and/or arthrofibrosis. Restrictive shoulder range of motion (ROM), or adhesive capsulitis, is also a common finding. This condition, often called shoulder–hand syndrome, negatively affects health-related quality of life and requires specific treatment modalities. Shoulder–hand problems often are overlooked without a careful examination of the shoulder. The involved extremity must be assessed for sensibility, hyperpathia, allodynia, discoloration, swelling, atrophy, vasomotor and autonomic tone, neurologic function, vascular status, grip, and pinch. In addition, any nociceptive foci should be noted. Reevaluation after or during sympatholytic treatment may reveal additional findings, may facilitate the identification of trigger areas or delineate underlying inflammatory processes, and may clarify any structural injury. If these conditions are correctable, their treatment may have a positive impact on outcome.
Diagnostic Testing
There is no pathognomonic marker for CRPS; therefore, tests augment and/or quantify historical data and clinical findings. The clinical evaluation may be aided by information from validated instruments evaluating health-related quality of life, symptoms, and function. Combined with a standardized examination, this information provides reproducible and quantifiable data. Specific testing modalities provide an analysis of anatomic integrity, physiologic performance, and functional capacity. Categorization of the CRPS patient using reproducible physiologic assessments guides treatment decisions and documents disease status.27,49 Standardized tests are used to evaluate bone density and osteopenia, sudomotor performance, vasomotor and thermoregulatory control, components of blood flow, and endurance.
Radiographs may be used to assess the amount of regional osteopenia, a finding observed in 70% to 80% of CRPS patients.22,50 The classic radiographic finding associated with CRPS is periarticular osteopenia; however, CRPS may affect both cortical and cancellous bone.24,51,52 Genant and colleagues50 described five patterns of resorption that occur in CRPS: patchy, irregular trabecular bone and subperiosteal, intracortical, endosteal, and subchondral and juxtachondral surface erosions.50 However, radiographic changes occur relatively late in the progression of CRPS (Fig. 115-1).
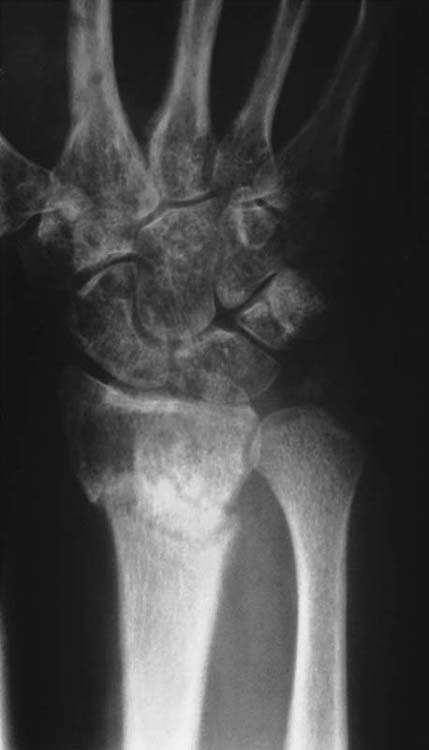
Figure 115-1 Plain radiograph of a patient with type I complex regional pain syndrome after fracture of the distal ends of the radius and ulna. The fracture line is visible. There is diffuse osteopenia in addition to juxtacortical demineralization and subchondral erosions and cysts. (From Koman LA. Department of Orthopaedic Surgery, Wake Forest University, Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1998.)
Bone Scintigraphy
Three-phase bone scans “should not be used as a major criterion in diagnosing reflex sympathetic dystrophy.”53 A third-phase scan with positive findings is not a prerequisite for the diagnosis of CRPS or SMP. The diagnostic importance of abnormal phases of bone scans is a subject of debate, and abnormal findings on scans in any phase assist in the corroboration of the diagnosis of RSD.54,55 Although it has been stated that only a third-phase scan correlates with RSD,56 abnormal findings in any phase of a three-phase scan document abnormal physiology of blood flow, bone turnover, or both. Unfortunately, bone scan findings do not correlate with the traditional staging criteria for RSD, do not predict recovery, do not determine the potential for response to treatment, and do not necessarily revert to normal after successful treatment. However, the presence of positive third-phase bone scan findings provides objective corroboration for the clinical diagnosis of CRPS (Figs. 115-2 and 115-3).
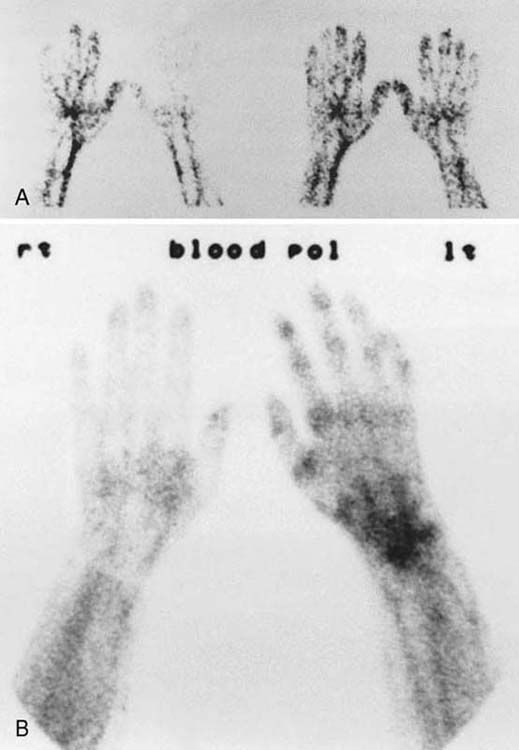
Figure 115-2 Three-phase bone scans. A, Phase I, a dynamic phase, evaluates vascular perfusion by visual or quantitative analysis or radiotracer uptake after an intravenous injection. Each image represents a 3- to 5-second interval and allows an assessment of flow dynamics. B, Phase II, blood pool image, documents total tissue uptake of tracer during the first 3 to 5 minutes after injection. Phase III is a conventional bone scan (see Fig. 115-3). (From Koman LA. Department of Orthopaedic Surgery, Wake Forest University, Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1998.)
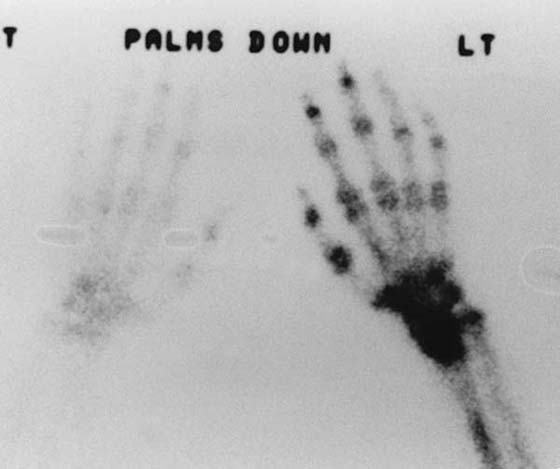
Figure 115-3 An abnormal (phase III) bone scan demonstrating increased periarticular uptake throughout the hand. This is a scan of the patient whose radiograph is shown in Figure 115-1. (From Koman LA. Department of Orthopaedic Surgery, Wake Forest University, Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1998.)
Sudomotor performance may be evaluated using resting sweat output,15,57 galvanic skin response, peripheral autonomic surface potentials,58 sympathetic skin response,59-61 and quantitative sudomotor axon reflex test.57 These tests provide an objective measure of sweat function by indirect or direct means.
Vasomotor and thermoregulatory control can be assessed by monitoring digital pulp temperature(s), laser Doppler fluxmetry measurements of cutaneous perfusion (Fig. 115-4), or laser Doppler perfusion imaging of cutaneous perfusion profiles. These studies indirectly evaluate cutaneous perfusion and provide reproducible profiles of physiologic capacity if combined with a physiologic stressor.38-40,62-64
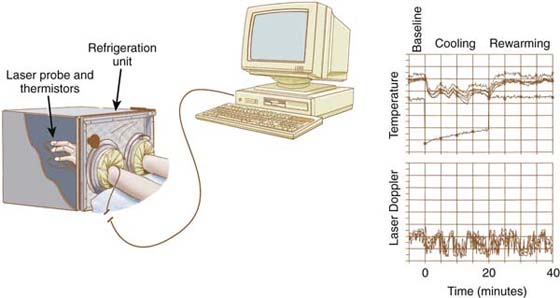
Figure 115-4 Digital microvascular physiology can be evaluated by using an isolated cold stress test combining digital temperature and laser Doppler fluxmetry measurements. Digital temperatures are monitored with thermistors attached to each digit of both extremities. Microvascular cutaneous perfusion is assessed with a laser Doppler probe attached to one digit of each extremity. Digital temperature and laser Doppler fluxmetry measurements are sampled by using custom computer software, and the results of the test are plotted for analysis. (From Koman LA. Department of Orthopaedic Surgery, Wake Forest University, Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1998.)
Under normal conditions, 80% to 90% of total finger blood flow is involved in thermoregulation. However, in patients with CRPS, the percentage of blood flow involved in thermoregulatory activity may vary. Thermoregulatory flow is calculated by subtracting the nutritional flow from the total flow. Nutritional flow may be measured by using vital capillaroscopy.64,65 This technique measures nutritional blood flow of the nail fold capillaries by direct evaluation of red blood cell flow through a single capillary loop by using an epi-illumination microscope and computerized analyses of flow (Fig. 115-5). Patients with CRPS have reduced nutritional flow and are unable to modulate flow compared with normal patients.27,66 Decreased nutritional flow occurs in patients with longstanding CRPS and may be secondary, in part, to irreversible changes in arteriovenous shunt mechanisms and/or arteriovenous shunt control.
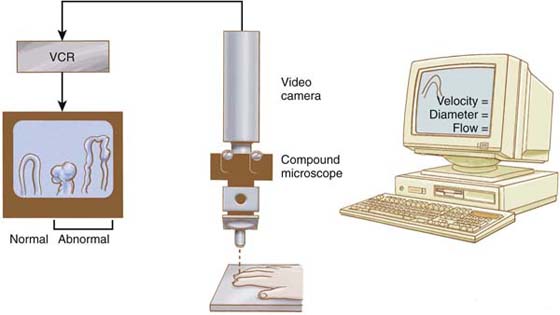
Figure 115-5 Nutritional capillaries may be visualized directly through a compound microscope, which provides epi-illumination from the microscope. Magnification within the microscope and use of a video camera allow direct visualization of cell motion within the capillaries and permit the identification of normal and/or abnormal capillary morphology. Videotape analysis facilitates quantitation of the diameter of the capillaries and velocity of red blood cell flow within the ascending and descending capillary loop. Abnormal morphology diagnostic of collagen vascular disease also can be observed. (From Koman LA. Department of Orthopaedic Surgery, Wake Forest University, Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1998.)
Endurance testing may be performed by using a variety of computerized diagnostic techniques that quantify muscle energy, strength, and endurance. Subtle abnormalities in function may be assessed only by careful analysis of these test results.
Diagnostic blockade may be used to determine whether CRPS is sympathetically maintained or sympathetically independent. The most common diagnostic blockade procedures performed are stellate ganglion block, a phentolamine test, epidural injection, and controlled trials of oral sympatholytic medications. Diagnostic blockade is performed classically with stellate ganglion block or phentolamine67; however, phentolamine currently is not available in the United States.
Stellate ganglion block is performed by injecting the cervical sympathetic trunk with a short- to medium-acting stabilizing agent (e.g., lidocaine) (Fig. 115-6). Decreased pain after injection is presumptive evidence that pain is sympathetically maintained.8,67,68 The inability to obtain pain reduction after or during the test suggests either an alternative diagnosis or that irreversible peripheral changes have occurred that have changed the previously SMP to sympathetically independent pain. A brief response to oral sympatholytic medications is presumptive evidence of complex regional pain that is sympathetically maintained.
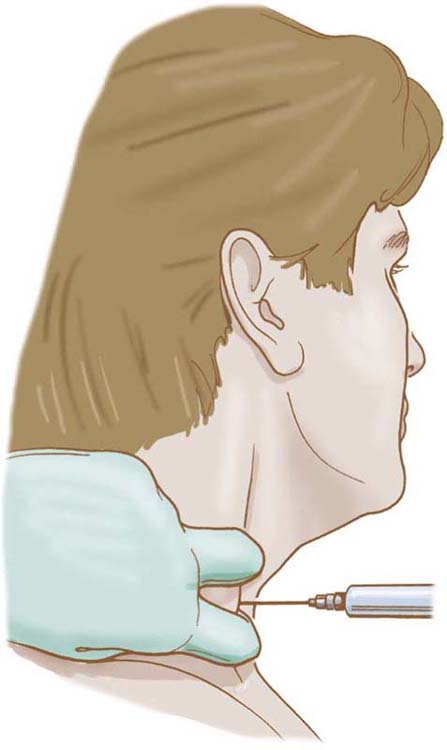
Figure 115-6 Schematic of technique of needle placement for stellate block.
Treatment
General Principles
Of CRPS patients treated within the first year of injury, 80% will show significant improvement; only 50% of those treated after 1 year will improve. However, patients with CRPS after distal radius fractures have a poorer prognosis; stiffness and decreased finger function at 3 months correlate with residual impairment and morbidity at 10 years.69 Although early intervention is important, it is not always possible, and many patients are treated inadvertently before diagnosis. Thus, the natural history may be improved by medical intervention despite an incorrect or incomplete diagnosis.
Effective treatment of CRPS requires recognition and prompt intervention.7 Before initiating treatment, it is appropriate to stage the CRPS patient by determining whether pain is sympathetically maintained or sympathetically independent, total blood flow is high or low, evidence of abnormal arteriovenous shunting and/or nutritional deprivation exists, and permanent structural damage has occurred. The degree of soft tissue contracture should be assessed because patients with significant arthrofibrosis and atrophy often have sustained irreversible trophic changes that indicate a less favorable prognosis. Nociceptive foci should be identified and treated. Effective sympatholytic treatment often requires the use of multiple modalities.70
Hand Therapy
Hand therapy is an important aspect of management of CRPS affecting the upper extremity. The active involvement of a hand therapist in the management of a CRPS patient provides an independent assessment of progress, continuity of care, and a vital feedback loop among the patient, physician, insurance carriers, and rehabilitation personnel. Pain modulation techniques used in hand therapy include contrast baths, desensitization, Fluidotherapy (Chattanooga Medical, Chattanooga, TN), static orthotic positioning, electrical stimulation, and ultrasound. Edema is controlled by using manual lymphatic drainage techniques or light compression garments. ROM activity can be aided by ultrasound and paraffin baths along with dynamic orthotic positioning and continuous passive motion equipment as long as the patient’s pain does not increase.27,71
Pharmacologic Interventions
The use of oral, topical, and parenteral pharmacologic intervention is well documented in the management of CRPS. The most commonly used drugs include antidepressants, anticonvulsants, membrane-stabilizing agents, adrenergic agents, and steroids. The use of these medications is largely empirical; however, theoretical mechanisms support their use in chronic pain. Oral medications (e.g., anticonvulsants, local anesthetics) may stabilize hypersensitive membranes, may provide competitive inhibition of neurotransmitters (e.g., bretylium), may block or influence neurotransmitter activity and/or receptor affinity or both, or may increase nutritional flow and/or decrease inappropriate arteriovenous shunting.72
The use of almost all the agents discussed are unlabeled indications according to the U.S. Food and Drug Administration. Therefore, the individual practitioner must be familiar with the pharmacology, physiologic actions, side effects, potential complications, indications, potential drug interactions, and contraindications of any medications prescribed.
In recent studies, vitamin C, at a dose of 500 mg/day, has been observed to decrease the incidence of CRPS after distal radius fractures and may provide a prophylactic advantage.73 A tricyclic antidepressant often is used in conjunction with a serotonin reuptake inhibitor. For example, amitriptyline and paroxetine may be used simultaneously (Tables 115-1 and 115-2). Anticonvulsants are commonly used because of their theoretical effect on membrane stabilization (Table 115-3). The use of oral membrane-stabilizing agents such as mexiletine and tocainide is limited because of the numerous side effects associated with these agents. Steroids are used frequently and are advocated by many as a primary oral medication54,72,74,75 (Table 115-4).
Table 115-1 Antidepressants
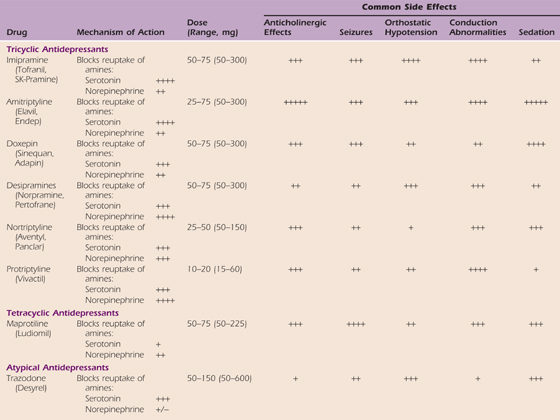
Table 115-2 Selective Serotonin Reuptake Inhibitors
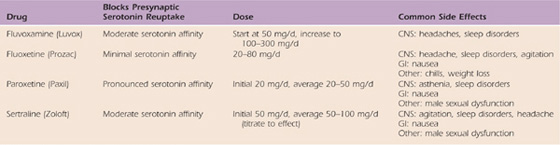
Table 115-3 Anticonvulsants
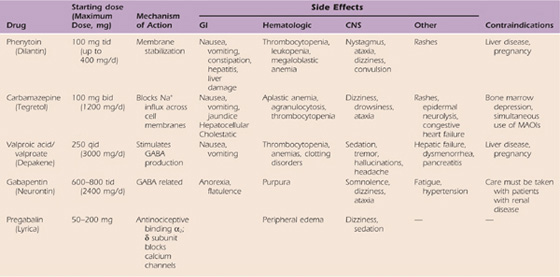
Table 115-4 Comparative Characteristics of Intravenous and Oral Local Anesthetic Agents Used for Chronic Pain: Membrane-Stabilizing Agents
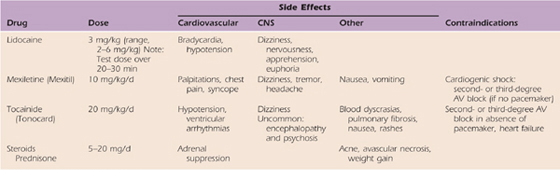
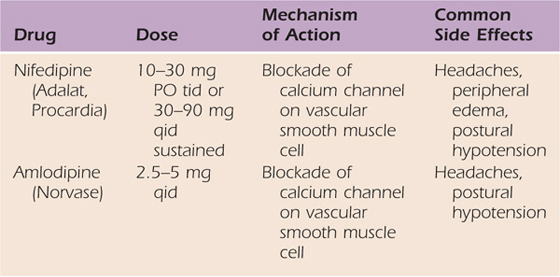
Adrenergic agents have direct effects on presynaptic and postsynaptic hypersensitivity, and, therefore, theoretically, they should benefit many patients with CRPS (Table 115-5). Because calcium channel blockers increase nutritional perfusion, their use is valuable in patients with increased blood flow and inappropriate arteriovenous shunting or in patients with nutritionally compromised extremities as a result of reduced blood flow (Table 115-6).76
Table 115-5 Adrenergic Agents
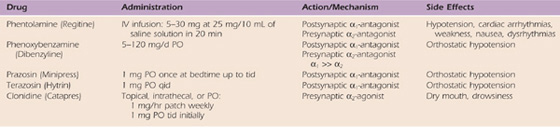
Table 115-6 Calcium Channel Blockers
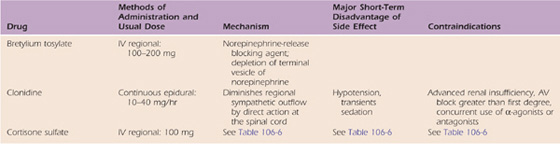
In the past, parenteral agents (guanethidine, steroids, lidocaine, and bretylium tosylate) were used to manage CRPS. However, the efficacy of these drugs is not supported by controlled studies.77-81 Therefore, these agents are no longer commonly used. Continuous epidural infusions with clonidine have been demonstrated to be effective for patients with advanced refractory cases of CRPS (Table 115-7).82,83 Drugs that have been used recently to manage CRPS include calcitonin, bisphosphonates, N-methyl-D-aspartate receptor antagonists, and dimethylsulfoxide.84
Table 115-7 Parenteral Medications
Surgical and Ablative Therapies
Sympathectomy may be achieved by cervicothoracic ablation, periarterial stripping, or neurolytic blockade. Cervicothoracic ablation provides relatively short-term efficacy that is maintained for 6 to 12 weeks. This period is followed by increased peripheral hypersensitivity secondary to receptor up-regulation. Periarterial sympathectomy that is performed in the periphery does not result in receptor up-regulation or increased sensitivity of a receptor to a transmitter; however, it is not documented to be effective in any controlled studies involving CRPS. The use of percutaneous cervicothoracic sympathectomy using phenol and alcohol provides ease of administration with reversibility and eliminates the potential problems of receptor up-regulation. Alternative therapies include thorascopic transection of the superior cervical ganglia85 and radiofrequency ablation.86,87 Implantable stimulators in the spinal cord and neurosurgical instrumentation in the thalamus and gray matter, as well as cingulotomy (i.e., transection of a portion of the brain) have been performed.88-94
Surgical Management
Surgical management of a nociceptive focus is important in the management of patients with CRPS types I and II. A mechanical irritant exaggerates or incites abnormal peripheral fibers with resultant secondary CNS adaptations. Sympatholytic medications or treatment may be required to reduce pain and hypersensitivity sufficiently to delineate underlying nociceptive foci. If possible, these mechanical or neural areas of irritation then should be surgically repaired under the protection of continuous autonomic blockade. In CRPS type II, common nociceptive foci include neuroma, neuroma-in-continuity, compression neuropathy, irritation of the superficial radial nerve (often by a fixation pin), irritation of the median nerve, and irritation of the dorsal branch of the ulnar nerve. In CRPS type I, stenosing tenosynovitis of the first, second, or third dorsal compartment; internal derangements of the wrist; and osteochondral fractures often are precipitating and exacerbating events.27
Summary
Although the pathophysiology of complex regional pain remains ill defined, the use of sequential treatment modalities that decrease pain and restore function is vital for a successful outcome. Early recognition, diagnosis, and intervention in patients with complex regional pain are important; however, most patients receive partial treatment before definitive diagnosis. CRPS is common after fractures of the distal radius and ulna and may complicate relatively “trivial” trauma with exaggerated pain responses. After surgery, CRPS can create a stormy postoperative course and should be suspected if pain seems excessive. Often, patients with an expected postsurgical course have mild CRPS that complicates recovery; these patients will respond dramatically to sympatholytic drugs, not narcotics. Treatment should be multifactorial and should be based on physiologic criteria. Treatment paradigms should decrease inappropriate autonomic function, restore appropriate arteriovenous shunting, and identify and correct underlying nociceptive foci.
REFERENCES
1. Stanton-Hicks M, Jänig W, Hassenbusch S, et al. Reflex sympathetic dystrophy, changing concepts and taxonomy. Pain. 1995;63:127–133.
2. Boas RA. Complex regional pain syndromes: symptoms, signs, and differential diagnosis. In: Janig W, Stanton-Hicks M, eds. Reflex Sympathetic Dystrophy: A Reappraisal—Progress in Pain Research and Management.vol 6. Seattle: IASP Press; 1996.
3. Campbell JN, Meyer RA, Raja SN. Is nociceptor activation by alpha-1 adrenoreceptors the culprit in sympathetically maintained pain? Am Pain Soc J. 1992;1:3.
4. Campbell JN., et al. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89.
5. Stanton-Hicks M, Baron R, Boas R, et al. Consensus report: complex regional pain syndromes—guidelines for therapy. Clin J Pain. 1998;14:155.
6. Amadio PC, Mackinnon SE, Merritt WH, et al. Reflex sympathetic dystrophy syndrome: consensus report of an ad hoc committee of the American Association for Hand Surgery on the definition of reflex sympathetic dystrophy syndrome. Plast Reconstr Surg. 1991;87:371–375.
7. Pappagallo M, Rosenberg AD. Epidemiology, pathophysiology, and management of complex regional pain syndrome. Pain Pract. 2001;1(1):11.
8. Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698.
9. Raja SN, Turnquist JL, Meleka S, Campbell JN. Monitoring adequacy of α-adrenoceptor blockade following systemic phentolamine administration. Pain. 1996;64:197–204.
10. Campbell JN., et al. Diagnosis and management of sympathetically maintained pain. Fields HL, Liebeskind JC, eds. Progress in Pain Research and Management.vol 1. Seattle: IASP Press; 1994.
11. Dotson RM. Causalgia: reflex sympathetic dystrophy—sympathetically maintained pain: myth and reality. Muscle Nerve. 1993;16:1049.
12. Ochoa JL, Verdugo RJ. The mythology of reflex sympathetic dystrophy and sympathetically maintained pains. Phys Med Rehab Clin North Am. 1993;4:151.
13. Allen G, Galar BS, Schwartz L. Epidemiology of CRPS: a retrospective chart review of 134 patients. Pain. 1999;80:539.
14. An HS, Hawthorne KB, Jackson WT. Reflex sympathetic dystrophy and cigarette smoking. J Hand Surg. 1988;13A:458.
15. Low PA, Wilson PR, Sandroni P, et al. Clinical characteristics of patients with reflex sympathetic dystrophy (sympathetically maintained pain) in the USA. Janig W, Stanton-Hicks M, eds. Reflex Sympathetic Dystrophy: A Reappraisal—Progress in Pain Research and Management.vol 6. Seattle: ISAP Press; 1996:49–66.
16. Mailis A, Wade J. Profile of Caucasian women with possible genetic predisposition to reflex sympathetic dystrophy: a pilot study. Clin J Pain. 1994;10:210.
17. Ochoa JL, Verdugo RJ, Campero M. Pathophysiological spectrum of organic and psychogenic disorders in neuropathic pain patients fitting the description of causalgia or reflex sympathetic dystrophy. Gebhart GF, Hammond DL, Jensen TJ, eds. Progress in Pain Research and Management.vol 2. Seattle: IASP Press; 1994. Proceedings of the 7th World Congress on Pain
18. Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57.
19. Subbarao J, Stillwell GK. Reflex sympathetic dystrophy syndrome of the upper extremity: analysis of total outcome of management of 125 cases. Arch Phys Med Rehabil. 1988;62:549.
20. Veldman PHJM, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–1016.
21. de Mos M, de Bruijn AG, Huygen FJ, et al. The incidence of complex reginal pain syndrome: a population-based study. Pain. 2007;129:12–20.
22. Atkins RM, Duckworth T, Kanis JA. Algodystrophy following Colles’ fracture. J Hand Surg. 1989;14B:161.
23. Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after Colles’ fracture. J Bone Joint Surg. 1990;72B:105.
24. Bickerstaff DR, Charlesworth D, Kanis JA. Changes in cortical and trabecular bone in algodystrophy. Br J Rheumatol. 1993;32:46.
25. Laulan J, Bismuth J-P, Sicre G, Garaud P. The different types of algodystrophy after fracture of the distal radius: predictive criteria of outcome after 1 year. J Hand Surg. 1997;22B:441–447.
26. Wilder RT, Berde CB, Wolohan M, et al. Reflex sympathetic dystrophy in children: clinical characteristics and follow-up of seventy patients. J Bone Joint Surg. 1992;74A:910–919.
27. Koman LA, Poehling GG, Smith TL. Complex regional pain syndrome: reflex sympathetic dystrophy and causalgia. In: Green DP, ed. Green’s Operative Hand Surgery. New York: Churchill Livingstone; 1999.
28. Gonzalez F, Watson HK. Simultaneous carpal tunnel release and Dupuytren’s fasciectomy. J Hand Surg. 1991;16B:175.
29. Nissenbaum M, Kleinert HE. Treatment considerations in carpal tunnel syndrome with coexistent Dupuytren’s disease. J Hand Surg. 1980;5:544.
30. Griepp ME, Thomas AF. Familial occurrences of reflex sympathetic dystrophy. Clin J Pain. 1991;7:48.
31. Ciccone DS, Bandilla EB, Wu W. Psychological dysfunction in patients with reflex sympathetic dystrophy. Pain. 1997;71:323.
32. Mailis-Gagnon A, Nicholson K, Blumberger D, Zurowski M. Characteristics and period prevalence of self-induced disorder in patients referred to a pain clinic with the diagnosis of complex regional pain syndrome. Clin J Pain. 2008;24(2):176–185.
33. de Takats G. Sympathetic reflex dystrophy. Med Clin North Am. 1965;49:117.
34. de Takats G, Miller DS. Post-traumatic dystrophy of the extremities: a chronic vasodilator mechanism. Arch Surg. 1943;46:469.
35. Koman LA., et al. Reflex sympathetic and other dystrophies. In: Peimer C, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill; 1996.
36. Sieweke N, Birklein F, Riedl B, et al. Patterns of hyperalgesia in complex regional pain syndrome. Pain. 1999;80:171–177.
37. Baron R, Blumberg H, Janig W. Clinical characteristics of patients with complex regional pain syndrome in Germany with special emphasis on vasomotor function. Janig W, Stanton-Hicks M, eds. Reflex Sympathetic Dystrophy: A Reappraisal—Progress in Pain Research and Management.vol 6. Seattle: IASP Press; 1996.
38. Pollock FE Jr, Koman LA, Smith BP, et al. Measurement of hand microvascular blood flow with isolated cold stress testing and laser Doppler fluxmetry. J Hand Surg. 1993;18A:143–150.
39. Pollock FE, Koman LA, Smith BP, Poehling GG. Patterns of microvascular response associated with reflex sympathetic dystrophy of the hand and wrist. J Hand Surg. 1993;18A:847–852.
40. Schürmann M, Gradl G, Andress HJ, et al. Assessment of peripheral sympathetic nervous function for diagnosing early post-traumatic complex regional pain syndrome type I. Pain. 1999;80:149–159.
41. Davidoff G, Morey K, Amann M, Stamps J. Pain measurement in reflex sympathetic dystrophy syndrome. Pain. 1988;32:27–34.
42. Huskisson EC. Measurement of pain. Lancet. 1974;2:1127.
43. Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg. 1993;75A:1585–1592.
44. McCabe SJ, Mizgala C, Glickman L. The measurement of cold sensitivity of the hand. J Hand Surg. 1991;16A:1037.
45. American Academy of Orthopaedic Surgeons, Council of Musculoskeletal Specialty Societies, Institute for Work and Health. Disabilities of the Arm, Shoulder, and Hand: outcomes data collection package, version 1.1, March 1996.
46. RAND Health Sciences Program. RAND 36-Item Health Survey 1.0. Santa Monica, Calif: RAND Corp; 1992.
47. Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. JAMA. 1989;262:907–913.
48. Stewart AL, Ware JE, Brook RH. Advances in the measurements of functional status: construction of aggregate indexes. Med Care. 1981;XIX:473–488.
49. Sandroni P, Low PA, Ferrer T, et al. Complex regional pain syndrome I (CRPSI): prospective study and laboratory evaluation. Clin J Pain. 1998;14:282–289.
50. Genant HK, Kozin E Bekerman C, et al. The reflex sympathetic dystrophy syndrome. Radiology. 1975;117:21–32.
51. Atkins RM, Tindale W, Bickerstaff D, Kanis JA. Quantitative bone scintigraphy in reflex sympathetic dystrophy. Br J Rheumatol. 1993;32:41–45.
52. Otake T, Ieshima H, Ishida H, et al. Bone atrophy in complex regional pain syndrome patients measured by microdensitometry. Can J Anaesth. 1998;45:831–838.
53. Lee GW, Weeks PM. The role of bone scintigraphy in diagnosing reflex sympathetic dystrophy. J Hand Surg. 1995;20A:458.
54. Kozin F, Ryan LM, Carerra GF, et al. The reflex sympathetic dystrophy syndrome (RSDS). III. Scintigraphic studies, further evidence for the therapeutic efficacy of systemic corticosteroids, and proposed diagnostic criteria. Am J Med. 1981;70:23.
55. Kozin F, Soin JS, Ryan LM, et al. Bone scintigraphy in the reflex sympathetic dystrophy syndrome. Radiology. 1981;138:437–443.
56. Mackinnon SE, Holder LE. The use of three-phase radionuclide bone scanning in the diagnosis of reflex sympathetic dystrophy. J Hand Surg. 1984;9A:556.
57. Low PA, Caskey PE, Tuck RR, et al. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580.
58. Knezevic W, Bajada S. Peripheral autonomic surface potential: a quantitative technique for recording autonomic neural function in man. Clin Exp Neurol. 1985;21:201.
59. Chu N. Recovery of sympathetic skin responses after digit-to-digit replantation and toe-to-digit transplantation in humans. Ann Neurol. 1996;40:67.
60. Fagius J, Karhuvaara S, Sundlöf G. The cold pressor test: effects of sympathetic nerve activity on human muscle and skin nerve fascicles. Acta Physiol Scand. 1989;137:325.
61. Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608.
62. Baron R, Maier C. Reflex sympathetic dystrophy: skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain. 1996;67:317.
63. Gulevich SJ, Conwell TD, Lane J, et al. Stress infrared telethermography is useful in the diagnosis of complex regional pain syndrome, type I (formerly reflex sympathetic dystrophy). Clin J Pain. 1997;13:50–59.
64. Koman LA, Smith BP, Smith TL. Stress testing in the evaluation of upper-extremity perfusion. Hand Clin. 1993;9:59.
65. Fagrell B. Microcirculation of the skin. Mortillaro NA, ed. The Physiology and Pharmacology of the Microcirculation.vol 2. Orlando: Academic Press; 1984.
66. Ostergren J, Fagrell B. Skin microvascular circulation in the sympathetic dystrophies evaluated by videophotometric capillaroscopy and laser Doppler fluxmetry. Intl J Microcirc. 1988;7:289.
67. Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;68:571.
68. Raja SN, Davis KD, Campbell JN. The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg. 1992;8:63.
69. Field J, Warwick D, Bannister GC. Features of algodystrophy ten years after Colles’ fracture. J Hand Surg [Br]. 1992;17(3):318.
70. Viel E, Ripart J, Pelissier J, et al. Management of reflex sympathetic dystrophy. Ann Med Intern. 1999;150:205–210.
71. Bengtson K. Physical modalities for complex regional pain syndrome. Hand Clin. 1997;13:443.
72. Czop C, Smith TL, Rauck R, Koman LA. The pharmacologic approach to the painful hand. Hand Clin. 1996;12:633–642.
73. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am. 2007;89(7):1424–1431.
74. Kozin F, McCarty DJ, Sims J, Genant H. The reflex sympathetic dystrophy syndrome. I. Clinical and histologic studies: evidence for bilaterally, response to corticosteroids and articular involvement. Am J Med. 1976;60:321–331.
75. Magni G. The use of antidepressants in the treatment of chronic pain: a review of current evidence. Drugs. 1991;42:730.
76. Muizelaar JP, Kleyer M, Hertogs IA, DeLange DC. Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia): management with the calcium channel blocker nifedipine and/or the α-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg. 1997;99:26–30.
77. Hord AH, Rooks MD, Stephens BO, et al. Intravenous regional bretylium and lidocaine for treatment of reflex sympathetic dystrophy: a randomized, double-blind study. Anesth Analg. 1992;74:818–821.
78. Jadad AR, Carroll D, Glynn CJ, McQuay HJ. Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double-blind crossover study. J Pain Symptom Manage. 1995;10:13–20.
79. Lake AP. Intravenous regional sympathetic bloc: past, present and future? Pain Res Manag. 2004;9(1):35.
80. Livingstone JA, Atkins RM. Intravenous regional guanethidine blockade in the treatment of post-traumatic complex regional pain syndrome type 1 (algodystrophy) of the hand. J Bone Joint Surg Br. 2002;84(3):380.
81. Ramamurthy S, Hoffman J. Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia: a randomized, double-blind study. Guanethidine Study Group. Anesth Analg. 1995;81(4):718.
82. Eisenach JC, Rauck RL, Buzzanell C, Lysak SZ. Epidural clonidine analgesia for intractable cancer pain: phase I. Anaesthesiology. 1989;71:647–652.
83. Rauck RL, Eisenach JC, Jackson K, et al. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology. 1993;79:1163–1169.
84. Eisenberg E, Geller R, Brill S. Pharmacotherapy options for complex regional pain syndrome. Expert Rev Neurother. 2007;7(5):521.
85. Sharony R, Saute M, Uretzky G. Video assisted thoracic surgery: our experience with 102 patients. J Cardiovasc Surg. 1994;35:173.
86. Wilkinson HA. Percutaneous radiofrequency upper thoracic sympathectomy: a new technique. Neurosurg. 1984;15:811.
87. Wilkinson HA. Radiofrequency percutaneous upper-thoracic sympathectomy: technique and review of indications. N Engl J Med. 1984;311:34.
88. Barolat G, Schwartzmann R, Woo R. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Appl Neurophysiol. 1987;50:442.
89. Calvillo O, Didie J, Smith K. Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg. 1998;64:58.
90. Hassenbusch SJ, Stanton-Hicks M, Schoppa D, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415–423.
91. Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans: report of 122 cases, 1970-1984. J Neurosurg. 1986;64:543.
92. Robaina FJ, Dominguez M, Díaz M, et al. Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery. 1989;24:63–67.
93. Santo JL, Arias LM, Barolat G, et al. Bilateral cingulumotomy in the treatment of reflex sympathetic dystrophy. Pain. 1990;41:55–59.
94. Walker AE, Nulson F. Electrical stimulation of the upper thoracic portion of the sympathetic chain in man. Arch Neurol Psychiatry. 1948;59:559.












