CHAPTER 135
Focal Hand Dystonia
NANCY N. BYL, MPH, PhD, PT, FAPTA
CRITICAL POINTS
▪ The etiology of focal hand dystonia is still considered idiopathic, but there is agreement that the condition is multifactorial.
▪ Focal hand dystonia includes involuntary end-range twisting postures of the fingers and wrist due to co-contractions of agonists and antagonists. The onset of the problem may initially manifest as an abnormality in the quality of sound produced by a musical instrument, increasing errors in task performance, unusual fatigue or sense of weakness, or involuntary and/or involuntary excessive movement of one or more digits when performing a certain task.
▪ The objective of retraining patients with focal dystonia must be to restore normal differentiation of cortical topography of the hand, enhance the quality and accuracy of sensorimotor feedback, and enable efficient and effective performance of motor pathways for the hand.
▪ Treatment of focal hand dystonia should be interdisciplinary and comprehensive to include education, stress management, fitness, good biomechanics, appropriate ergonomics, medications (e.g., botulinum toxin), counseling, brain retraining, and sometimes brain stimulation.
Repetitive strain injuries, or cumulative trauma disorders, are commonly reported in individuals who perform jobs demanding repetitive fine-motor movements such as typing, playing a musical instrument, and writing. Some of these individuals resolve the problem by taking time off work, decreasing the time and intensity at the task, and modifying the ergonomics of the worksite and the task. When ergonomic issues cannot be resolved, chronic pain or unusual, disabling, painless involuntary movements of the hand may develop, referred to as focal hand dystonia (occupational hand cramps, musician’s cramp, keyboarder’s cramp, writer’s cramp, task-specific dystonia). Usually these symptoms develop as a result of an accumulation of intrinsic (e.g., genetics, neurophysiology, anatomic structure) and extrinsic (e.g., environment, situation, personality) factors. This chapter reviews the history, etiology, diagnosis, and treatment strategies of patients with focal hand dystonia. The aims of this chapter are to (l) increase the awareness of hand dystonia in the health care community; (2) encourage clinicians to participate in educational programs to prevent hand dystonia and other repetitive strain injuries; (3) enable clinicians to make an early diagnosis of hand dystonia; (4) prepare multidisciplinary clinicians to provide patient support and encouragement for recovery; (5) encourage physicians and therapists to develop creative, innovative, insightful, and effective learning-based retraining programs to maximize normal neural adaptation, quality of life, and independent function; and (6) challenge clinicians to participate in multidisciplinary clinical and translational research studies to better understand the etiology and improve the effectiveness of treatment for hand dystonia.
History
An ad hoc committee of the scientific advisory board of the Dystonia Medical Research Foundation agreed on the following definition of dystonia: Dystonia is a syndrome including sustained and repetitive muscle contractions that cause twisting, end-range abnormal postures.1 A dystonia that involves a particular body part (e.g., hand, neck, foot) is called a focal limb dystonia. When the limb dystonia occurs only during the performance of a target task, it is called task-specific or action dystonia.2 Fahn and colleagues1 added that action dystonia involves an involuntary posturing (dystonia) superimposed on a voluntary movement, with task-specific limb dystonias being a subset of the action dystonias. The majority of task-specific dystonias involve the upper limb. Invariably, the tasks affected usually require (1) highly repetitive movements; (2) extreme motor precision; (3) interplay between conscious or at least “feedback-related” modulation; and/or (4) a repetitively executed motor plan.3 Although not a painful disorder, task-specific focal hand dystonia can bring an abrupt halt to a promising occupational, professional, or performance career.4 Figure 135-1 summarizes typical patterns of dystonia in musicians.

Figure 135-1 Typical patterns of dystonic posture in a pianist, a violinist, a flutist, and a trombone player. (From Altenmuller E, Jabusch HC. Focal hand dystonia in musicians: phenomenology, etiology and psychological trigger factors. J Hand Ther 2009;22:145.)
Limb dystonia can be divided into three categories: simple cramp (movements abnormal in relation to a single task), dystonic cramp (movements abnormal in relation to more than one task), and progressive cramp (abnormal movement that begins in relation to a single task and later becomes abnormal in other tasks). The limb and action dystonias can be further classified by occupation (e.g., musician’s dystonia, golfer’s “yip”) or by the task performed (e.g., writer’s cramp, keyboarder’s cramp).5-8
In 1833, Bell9 was the first to refer to hand dystonia as scrivener’s palsy. In 1861, Duchenne10 described a similar disability in French workers. As we moved into the 20th century, hand dystonia became a topic of growing interest among employers, employees, neurologists, educators, physical therapists, hand therapists, and behavioral scientists. It is estimated that some type of repetitive motion disorder of the upper limb may develop in 30% of musicians and aggressive computer users/data entry/programmers,11 but it is not clear how many of these individuals will develop a focal hand dystonia. Focal dystonia appears to be most common in males.12 Initially, writer’s cramp13,14 was considered the most common type of dystonia; however, quality epidemiologic studies are needed to clarify the incidence and prevalence as well as the etiologic intrinsic and extrinsic risk factors for the development of focal hand dystonia.15-17
Etiology of Focal Hand Dystonia
In the past 10 years, basic and clinical research as well as the integration of sophisticated genetic, neuroimaging, electrophysiologic, and psychophysical analyses have provided increased insight into the etiology of focal dystonia.18 Although the etiology of hand dystonia is still considered idiopathic, there is some consensus that the problem is multifactorial.15,16,19-22 It appears that intrinsic factors interact with extrinsic factors to produce the clinical phenotype of focal hand dystonia. For some individuals, intrinsic factors may be the strongest contributor (e.g., genetics, neurophysiologic dysfunction, anatomic restrictions, sensory deficits, aberrant homeostatic plasticity), whereas for others, extrinsic factors such as excessive repetition, trauma, or behavioral characteristics may drive the development of the phenotype.
Endophenotypic Factors
Genetics
One common intrinsic factor in dystonia is genetics. Some are known familial genes commonly characterizing primary general dystonia,23-26 but new genes for focal dystonia are being discovered.27 Patients with a genetic etiology of dystonia reportedly have a receptor abnormality for dopamine binding in the putamen. This genetic anomaly could decrease the dopamine normally secreted in rewarded, repetitive, associative, and nonassociative learning behaviors.28 Interestingly, despite a unilateral presentation of hand dystonia, abnormal neurophysiologic measures are reported in both cerebral hemispheres, reinforcing the possibility of a genetic etiology.29 In addition, approximately 30% of individuals with focal hand dystonia have a distant relative with dystonia.23 To date, no gene has been identified specifically for focal hand dystonia.5,30
In Germany, a gene was identified in a family in whom many members had a cervical dystonia.31 All those with the cervical dystonia had the gene, but cervical dystonia did not develop in all family members with the gene. None of the family members reported a focal hand dystonia.25 This type of gene is classified as one with low penetrance. Another gene for general dystonia, the DYTI gene in Ashkenazi Jewish families, has also been studied.32 Although those with generalized dystonia have the gene, in some family members with the gene, dystonia does not develop, and only a hand dystonia may develop in others.
Basal Ganglia–Thalamocortical Dysfunction
Some researchers report specific dysfunction in the basal ganglia–thalamocortical motor circuit in patients with dystonia.33 Within the basal ganglia, the striatum receives major input from the motor cortex with the output modems including the globus pallidus (internal segment), substantia nigra, and pars reticulata. Direct and indirect pathways connect to the input and output nuclei. Except for one excitatory projection, all pathways interconnecting the basal ganglia nuclei are inhibitory. Dystonia could result from either excessive activity in the direct striatum–globus pallidus (internal segment) pathway or from reduced activity in the indirect striatum–globus pallidus (external segment) pathway.34,35 Focal dystonia is sometimes considered a prototype of a hyperkinetic disorder resulting from an imbalance of excitation and inhibition in the globus pallidus.30
Hypoactivity of inhibitory pathways could also result from reduced basal ganglia output and/or reduced activity in the motor thalamus.36,37 Interestingly, the putamen and the pallidum show evidence of hypermetabolism in patients with general dystonia as well as some patients with hand dystonia.37,38 This may explain why a pallidotomy can reduce the severity of symptoms in patients with generalized dystonia.39-41
Inadequate Inhibition
Lack of inhibition at multiple levels of the nervous system seems to be a fundamental problem in the genesis of dystonia. The nervous system requires a balance between excitation and inhibition of neural circuits to facilitate smooth, coordinated motor control. A variety of forms of inhibition are used to control the precision and smoothness of movement, particularly in the hand, where individual finger movements require selective and specific activation of muscles.42
Reciprocal inhibition allows for control of muscles around a single joint. Lack of reciprocal inhibition at the spinal and peripheral levels leads to co-contractions of antagonistic muscles, which characterize patients with hand dystonia.43-46 Moving the intended fingers is associated with the firing of unnecessary adjacent fingers. Using electromyographic (EMG) technology, muscle overactivity has been documented with inappropriate co-contractions, prolongation of EMG bursts into muscle groups outside the intended movement, abnormal H reflexes, and abnormal long latency reflexes.45,47-49 These signs could also be viewed as a reflection of a loss of reflex inhibition,50 which is consistent with the deterioration of poor, fine, graduated movements observed in patients with hand dystonia.26
Using transcranial magnetic stimulation (TMS), abnormal cortical inhibition has also been demonstrated bilaterally even in patients with unilateral focal hand dystonia.34,51,52 Both inhibitory interneuronal activity and surround inhibition have been reported abnormal in patients with focal hand dystonia.53 Surround inhibition allows selective control of individual muscles by simultaneous inhibition of surrounding muscles. The indirect pathway of the globus pallidus also plays a major role in surround inhibition. Dysfunction of inhibitory interneurons that use γ-aminobutyric acid as a neurotransmitter could further mediate the abnormal surround inhibition at the cortical level.54-56
Anatomic Musculoskeletal Limitations
Some people have tight joints, muscles, and fascia and restrictions in the retinaculum in the hand. Restrictions in end-range finger spread, forearm rotation, and shoulder rotation have been documented in patients with focal hand dystonia.57-61 It is possible that anatomic restrictions put a patient at risk for the development of a hand dystonia, particularly under conditions of stressful, highly repetitious practice.
Leijinse and Hallett59 proposed a musculoskeletal etiologic model of focal hand dystonia in which defects in the musculoskeletal system combined with environmental factors such as overuse may accumulate in a focal hand dystonia.57-59 In this model, the assumption is that the acquisition of instrumental techniques requires high levels of physiologic movements under high performance demands with ergonomic limitations of the instrument and the individual. This model predicts that the development of focal dystonia is preceded by gradual changes in playing technique over a long period of time.
Based on this musculoskeletal hypothesis, ineffective or physiologically infeasible playing movements must be modified by voluntary (teaching) or involuntary (systemic) feedback. If the movement modification process does not converge to using muscle synergies that satisfy all constraints, movement modifications will continue until overcompensated muscle synergies are produced. Antagonists of the intended movements are recruited, and dystonic symptoms develop. Based on this model, musculoskeletal limitations should be addressed in the individual hand to resolve peripheral conflicts between constraints and tasks. These musculoskeletal limitations should be addressed before neurologic retraining.59
In a post-training anatomic study of nonhuman primates with behaviorally induced focal hand dystonia,62 one monkey had an anatomic defect of the profundus tendon, with adhesions on the middle and distal segments of the fourth digit on the trained side and of the third digit on the untrained side. On the side trained at a highly repetitive, attended, stereotypical task, movement dysfunction developed in 5 weeks, significantly earlier than in the other monkeys. The somatosensory representation of the hand was degraded, particularly the receptive fields for D4. However, on the untrained side, there were no signs of involuntary motor control or somatosensory degradation of the third digit.62 If biomechanical demands on the hands are not stressful, movement dysfunction might not develop, even when there are anatomic restrictions. These findings are consistent with a multifactorial theory of origin for focal hand dystonia.
Abnormal Motor Preparation
A striking characteristic of focal hand dystonia is its task specificity. Initially, symptoms are manifested only when patients are performing a specific task (e.g., writing, playing an instrument, using the keyboard). This specificity can extend to certain passages and not all aspects of playing an instrument. This interesting task specificity was initially thought to be psychiatric in origin.
Some researchers report a deficiency in the preparation and/or organization of established motor programs in patients with hand dystonia, not simply a deficiency during movement initiation.63,64 Using neuroimaging techniques, underactivity of motor areas have been reported during writing in patients with writer’s cramp.65 In further support for this hypothesis of a defective motor program, Stinear and Byblow181 observed defective intracortical inhibition only during voluntary movements, never at rest. In patients with dystonia, there are also some differences in set shifting compared with controls.66 Further, some researchers have reported abnormal sequential learning in patients with DYTI dystonia.42 We know even less about neurophysiologic processing differences between healthy controls and patients with hand dystonia during the acquisition of movement control.
Sensory Abnormalities
Although focal hand dystonia is characterized as a movement disorder, there is significant evidence of dysfunction in sensory processing in patients with focal hand dystonia. Cortical somatosensory receptive fields are abnormally enlarged and disorganized in patients with hand dystonia.67,68 In patients with unilateral focal hand dystonia, bilateral difficulty with discriminating sensory stimuli has been measured in both the spatial and temporal domains.69-71 These perceptual abnormalities have also been reported in the hands of patients with blepharospasm and those with cervical dystonia.
Sensory processing also may play a modulatory role in dystonia. For example, a sensory trick such as touching, holding, or taping can quiet the dystonic symptoms in an involved digit. In addition, tonic vibration can lead to a worsening of dystonia, whereas anesthetic blocks can relieve symptoms.70 In terms of intervention, sensory retraining in the form of tactile discrimination practice including reading Braille, stereotactic matching tasks, and interpreting information drawn on the skin71-76 can help ameliorate motor symptoms.
There is also evidence of abnormal sensorimotor integration in patients with focal hand dystonia. The modulation of sensory processing in response to movement (referred to as sensory gating) is reported as abnormal in patients with focal hand dystonia.77 This could contribute to problems of motor control as seen in dystonia. However, Nowak and colleagues78 evaluated the potential generalized impairment of sensorimotor integration in patients with writer’s cramp or musician’s cramp. These researchers measured grip force behavior. In this study, the researchers looked at adapting grip force when lifting a new object or adjusting grip force in anticipating or reacting to a change in load force when catching a weight. Interestingly, patients with focal dystonia and normal controls showed similar predictive grip force adjustments to expected changes in object load.
Patients with dystonia produced a grip force overshoot during the initial lifts. Patients with dystonia also had a shorter latency of grip force response than controls after an unexpected load increase. These researchers suggested those with dystonia had a greater level of preparatory motor activity or a disinhibited spinal reflex response. The researchers suggested increased grip force was likely to be a prelearned phenomenon rather than a primary disorder of sensorimotor integration.78
Unfortunately, there are no studies that document neurosensory processing competencies or abnormalities in patients before the onset of hand dystonia. Thus, it is unclear whether somatosensory and sensorimotor dysfunction predisposes a patient to the development of dystonia under conditions of highly stressful repetitive hand use or whether repetitive, nearly simultaneous overuse of the hands can degrade the somatosensory hand representation and lead to involuntary dystonic movements. In primate and rat animal studies, repetitive overuse can be associated with peripheral and central inflammatory responses as well as changes in somatosensory, sensorimotor, and motor cortices bilaterally, even when the training involves only one side and the dystonia is documented only on one side.79
Sensory abnormalities may drive dystonic movements. However, directed sensory training can relieve the dystonia. It is not clear how the sensory abnormalities alone can directly lead to the motor manifestations of dystonia. Although this discussion is still under debate at this time, more and more evidence suggests that there are both sensory and motor abnormalities in patients with hand dystonia.58,80
Maladaptive Homeostatic Plasticity
The central nervous system is plastic. New motor skills are acquired throughout one’s lifetime. With sensory, motor, and mental learning, the plasticity of the nervous system changes circuitry to accommodate new skill development. These changes in synaptic connections and circuitry occur with maturation, but can also be purposely driven by learning-based training activities in a dynamic environment. However, plasticity is not infinite. Further, although plasticity is usually controlled by homeostatic mechanisms, it seems that plasticity can potentially be excessive, leading to loss of control and destabilization. In patients with focal dystonia, there is some evidence that these homeostatic mechanisms may be abnormal.81-83
It was the hypothesis of Quartarone and colleagues81-83 that patients with dystonia had an impairment in the ability to keep cortical excitability within a normal physiologic range. Usually, anodal stimulation enhances the inhibitory effect of transmagnetic stimulation (TMS) in terms of corticospinal excitability and cathodal stimulation reverses the aftereffects of TMS, producing an increase in corticospinal excitability. In patients with writer’s cramp, after preconditioning with transcranial direct current stimulation (tDCS), there were no consistent changes in corticospinal excitability after TMS stimulation. Quartarone and colleagues81-83 interpreted these findings to mean that the homeostatic mechanisms stabilizing excitability levels are impaired in patients with writer’s cramp. Thus, there is excessive corticospinal excitability.
Another interpretation of the aberrant plasticity findings is that patients with focal dystonia have an exceptionally adaptive nervous system. Neural changes can exceed the neural operating limits. This abnormally enhanced plasticity could explain the abnormal organization of the sensory, sensorimotor, and motor maps and loss of motor control of the hand in patients with focal hand dystonia after repetitive hand use.84-88
Aberrant plasticity may also be generated by an environmental trigger. For example, practice and repetition usually lead to improved performance. Positive feedback and behavioral rewards increase acetylcholine, dopamine, and other modulatory neurotransmitters to generate continual positive neural adaptation.89 However, because of competition between neuronal pathways, neural adaptation is not infinite. When something changes in the practice cycle (e.g., modification of the equipment or technique, task complexity, time on task), there may be a marked turn in behavior. Now, increased investment of time leads to deterioration rather than to improvement in performance. As the operative movements become faster, the temporal inputs become nearly simultaneous, losing their individual differentiation. At some point, stereotypic repetition can lead to a degradation in the response. The more the practice, the more the fatigue, and ultimately the patient begins to sense some emergent incoordination. Finally, the fingers seem to develop a life of their own, uncontrollably curling when performing the target task.90
Cortical networks engage both excitatory and inhibitory neurons by strong input perturbation. Within a given cortical area, cortical pyramidal cells cannot be effectively re-excited by another perturbation for tens to hundreds of milliseconds. These integration times are dictated primarily by the time for recovery from inhibition, which dominates poststimulus excitability. The cortex continues to define its representation of the temporal aspects of behaviorally important inputs by generating more synchronous representations of sequenced input perturbations or events. These time constants both govern and limit the ability to “chunk” (i.e., to separately represent by distributed coordinated discharge) successive events within its processing channels.
Researchers such as Bara-Jimenez and colleagues67-69 propose that the abnormal reorganization of the cortex in patients with focal hand dystonia may not be based on rigidly learned highly repetitive movements. Rather, a congenital or remote acquired abnormal cortical deficit may explain the abnormal reorganization. Experience-based reshaping of cortical representation can be mediated by dynamic plastic operations of the brain, with subject–environment interactions affecting the organization of the somatosensory cortex.
Exophenotypic Factors
Repetitive Use
The common association between highly skilled manual performance and the development of focal hand dystonia is suggestive of an environmental contribution to focal dystonia, such as repetitive use. The theory was that while practice and repetition should normally have a positive effect on learning,91 it is possible that the repetition could be excessive or even associated with some peripheral damage and have a negative effect on the nervous system.92,93 A primate85,86,94 and a rat model84,87 of repetition suggest that excessive repetition can lead to focal hand dystonia. In one case, monkeys were trained to repetitively perform a complex manual task, and symptoms similar to those of dystonia developed in some. This led to the proposed learning-based sensorimotor learning hypothesis.85 Somatosensory cortical mapping of the primate hand representation was disorganized, similar to findings in patients with focal hand dystonia. The hypothesis of aberrant sensorimotor learning as one etiologic factor for focal hand dystonia has been confirmed with electrical and magnetic neuroimaging techniques.85,86
Goal-directed, repetitive movements are known to drive measurable change in structure, neuroenzymes, myelination, and function. Selective spatial and spectral cell assemblies have sharp segregation and result in more complex, efficient behaviors. These event-by-event complex signal representations are highly plastic.94-106 This positive adaptive learning can also be measured as an increase in the size of the cortical representation; smaller receptive fields; increased efficiency, amplitude, and density of evoked responses; and distinct, orderly representation. (See Box 135-1 for a summary of the principles of neuroplasticity.) Because fine-motor control of complex and simple finger movements requires accurate feedforward and feedback information from the primary sensory cortex and related pathways,107,108 the focal hand dystonia that develops in individuals involved in high levels of repetitive fine-motor work could represent a type of negative integrative neural adaptation.
Box 135-1 Neurophysiologic Principles of Neural Adaptivity (Neuroplasticity)
I. With learning, the distributed cortical representations of inputs and brain actions specialize in their representations of behaviorally important inputs and actions for skill learning.
A. There are important behavioral conditions that must be met in the learning phase of plasticity:
1. When behaviorally important stimuli repeatedly excite cortical neuron populations, the neurons will progressively grow in number.
2. Repetitive, behaviorally important stimuli processed in skill learning lead to progressively greater specificity in spectral (spatial) and temporal dimensions.
B. A growing number of selectively responding neurons discharge with progressively stronger temporal coordination (distributed synchronicity).
C. With learning, the selection of behaviorally important inputs is a product of strengthening input-coincidence-based connections (synapses).
II. Plasticity is constrained by the following:
A. Anatomic sources and convergent-divergent spreads of inputs
B. The time constants governing coincident input co-selection
C. The time structures and potentially achievable coherences of extrinsic and intrinsic cortical input sources
D. Top-down influences
1. Cortical field-specific differences in input sources, distributions, and time-structured inputs create different representational structures.
2. Temporal dimensions of behaviorally important inputs influence representational specialization.
III. The integration time (processing time) in the cortex is itself subject to powerful learning-based plasticity.
IV. Plasticity processes are competitive.
V. Learning is modulated as a function of behavioral state.
VI. The scale of plasticity in progressive skill learning is massive.
A. Enduring cortical plasticity changes appear to be accounted for by local changes in neural anatomy.
B. Cortical plasticity processes in child development represent progressive, multiple-stage skill learning.
Although the observed problem is a disorder of movement, the initial electrophysiologic and magnetic resonance imaging studies highlighted the changes in the somatosensory cortex. Byl and colleagues85,86,109,110 reported significant dedifferentiation of the sensory representation of the hand in nonhuman primates in which motor dysfunction resulted from highly repetitive, stereotypical digital opening and closing movements of the hand. In these primates, the receptive fields increased in size, with the evoked neural response engaging a broadened neuronal network across adjacent digits and across dorsal and glabrous surfaces. This degradation in representation disrupted the accuracy of the feedback control and ultimately interfered with accurate and specific motor control (sensorimotor learning hypothesis) (Figs. 135-2 and 135-3). More recently, in human studies, using magnetoencephalography, these same researchers not only confirmed the degradation in topography, but also documented problems in timing and spatial processing in both the somatosensory (S1 and S2), premotor, and motor cortices in both the ipsilateral and contralateral hemispheres of the affected and unaffected digits in patients with dystonia.
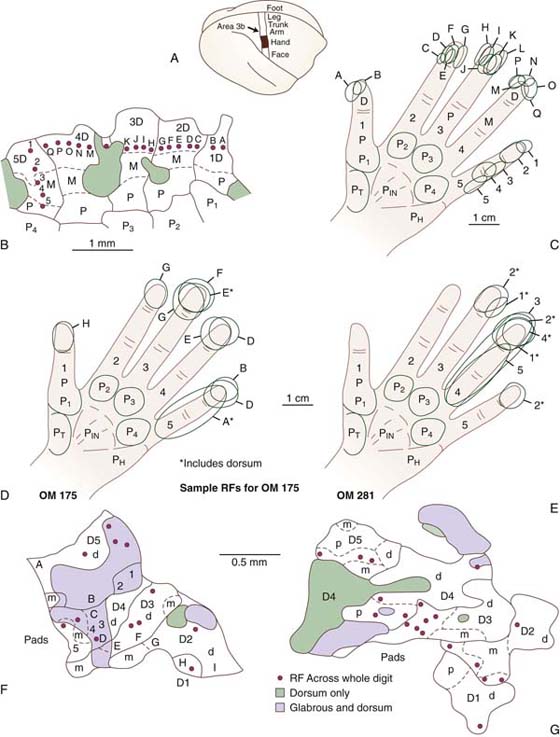
Figure 135-2 A, Hand zone in cortical area 3b. B, Typical normal receptive fields on the hand representing the surface topography. Penetration sites 1 to 5 and A to Q are noted. C, Receptive fields drawn on the hand representing the area of the skin associated with each marked cortical penetration. The gray areas represent the dorsum. D and E, Abnormally large receptive fields drawn on the fingers representing the electrode penetrations. Many of the electrode penetrations had more than one receptive field, some overlapping adjacent digits, and some overlapping of the glabrous and dorsal surfaces. F and G, Representation of the abnormal surface topography for two animals trained at a repetitive hand task. OM, owl monkey; RF, receptive field. (From Byl NN, Merzenich M, Jenkins W. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced de-differentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology 1996;47:508.)
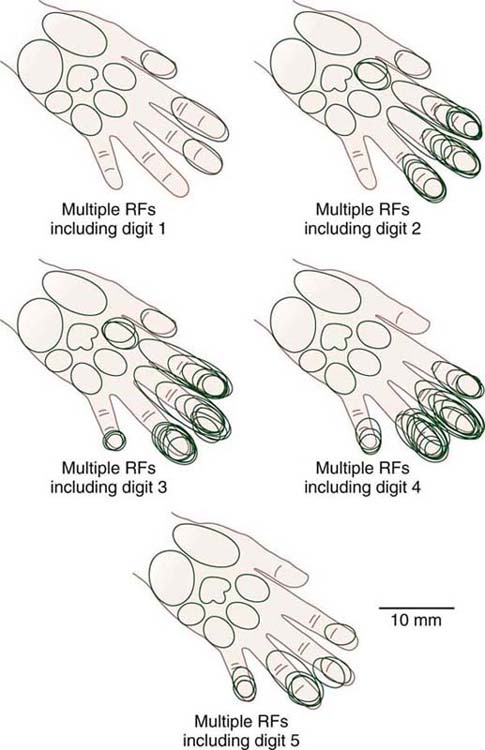
Figure 135-3 All digital receptive fields from owl monkeys (OM 281) recorded in area 3b zone sorted by individual digits. Compare with Figure 135-2C. In a normal condition, each electrode penetration would be associated with a single receptive field on one finger only. However, in this trained primate, many of the electrode penetrations were associated with multiple receptive fields extending across multiple digits. RF, receptive field. (From Byl NN, Merzenich M, Jenkins W. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced de-differentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology 1996;47:508.)
In comprehensive animal models of dystonia research, there is evidence of both peripheral and central changes occurring after repetitive overuse of the hand. For example, using a learning-based111 rat model to study peripheral inflammation associated with repetition under different force conditions,84,87,112 Barbe and colleagues,84 Elliot and colleagues,112 and Coq and colleagues87 documented a progressive model of dysfunction post-repetition. This dysfunction began by a cascade of peripheral inflammation that crossed over midline and then centralized. In a series of experiments, local inflammation was initially observed in the heavily used extremity. Then the inflammatory signs spread to the opposite side and then spread centrally. When repetitive movements continued, a noticeable, inefficient movement dysfunction in feeding was observed in the reaching hand. Clinically, the rat lost dexterity and a scooping strategy was noted.
Under blinded conditions, aberrant cortical changes were measured in the somatosensory, sensorimotor, and motor cortices in the rats that developed the inefficient hand strategy of scooping for food. Other researchers have also shown a change in somatosensory cortical topography after repetitive motor movements.113 As in the primate studies, the somatosensory and sensorimotor cortices lost accurate differentiation of the digits, and the motor cortex became excessively sensitive. A low current excessively excited the neurons, and now firing of agonists and antagonists was seen across the wrist and the digits.87
Sanger and Merzenich114 translated the learning-based sensorimotor hypothesis into a computational model to explain the changes in topography and the abnormal motor output in individuals in whom focal hand dystonia develops (Fig. 135-4). This model explains several features of focal dystonia: (1) symptoms develop in otherwise healthy individuals in response to highly attended repetitive movements, (2) evolution of symptoms is variable in terms of time, (3) symptoms appear only during the performance of a target-specific task, (4) dystonic movements persist despite stopping the task, (5) symptoms can be decreased but not remediated with dopamine-depleting drugs or botulinum toxin, and (6) evidence of abnormalities in motor and sensory cortical representations of the dystonic limb.
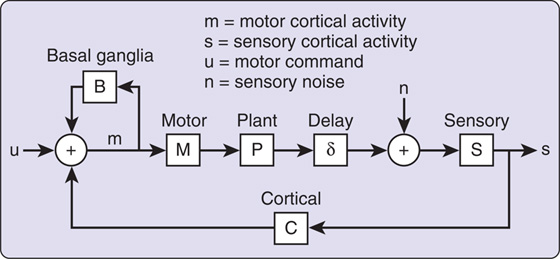
Figure 135-4 Diagram of the computational model of sensory feedback in focal dystonia developed by Sanger and Merzenich.114 Here, disorganized representations in somatosensory cortex (S) contribute to excessive gain in a mapping from sensory to motor cortex (C), leading to saturation in motor cortical activity (m) when combined with normal inputs from feedback through the basal ganglia-corticostriatal circuit (B) and the initial motor command (u). (Reprinted with permission from Sanger TD, Merzenich MM. A computational model of the role of sensory representations in focal dystonia. J Neurophysiol. 2000;84:2458-2464.)
In this model, gain can be increased by expanding or even shrinking the sensory cortical representation of a limb as a result of adaptation to repetitive use or specific increased, simultaneous firing; coupling of multiple sensory signals; and voluntary coactivation of muscles. The loop through the deep nuclei, including the cortex, basal ganglia, and thalamus, combined with the sensorimotor loop gain, contributes to instability.114 If only certain mechanical models of the sensorimotor loop are unstable, a focal dystonia, rather than a generalized dystonia, can develop.
Based on this computational model of focal hand dystonia, treatment must decrease the imbalance in the loop gain. A permanent solution requires a redifferentiation of cortical and subcortical representations to release excessive gain. Retraining may not be possible in the context of severe dystonia without temporarily breaking the cycle. This might include botulinum toxin injections to stop the abnormal muscle firing at the periphery. Another approach would be to increase the variability of practiced movements so that there are many uncorrelated movement components, each with only a few relevant sensory neurons. This is comparable with behaviors uncoupling the pathologically coupled modes.
Trauma
Focal dystonia can develop after an insult such as trauma, disease, vascular insufficiency, or anatomic restriction. For example, dystonic movements have been reported in patients after a fall on an outstretched arm,115 a cervical neck injury or a degenerative condition with radiculopathy,115 a neurovascular entrapment in the thoracic outlet,116 or an entrapped ulnar nerve at the elbow.117,118 In some of these cases, surgical intervention has resolved the dystonia.118 Unusual hand movements that sometimes are referred to as dystonic in character have also been described in patients with severe chronic pain (e.g., complex regional pain syndrome with and without sympathetic signs). In addition, dystonic hand movements can emerge after a central nervous system insult such as a head injury or a stroke.15
Psychological Risk Factors
For years, psychological factors were believed to be the underlying cause of focal dystonia.119 These occupational neuroses were emphasized until around 1982. At that time, Sheehy and colleagues13,14 suggested that the problem of dystonia had a neurologic pathomechanism.
Recently, Altenmuller and Jabusch2,4,120,121 compared the personality characteristics of musicians with and without dystonia with musicians with chronic pain. These researchers studied a variety of personality inventories and questionnaires regarding competence and control orientation, anxiety disorders (stage fright, panic attacks, free-floating anxiety), phobias (agoraphobia, social phobia, and specific phobias such as acrophobia, claustrophobia), life satisfaction, perfectionism, and social orientation. In addition, they asked patients about their anxieties before and after the onset of dystonia or pain. They also asked patients whether the specific anxieties had been present or absent before the onset of pain or dystonia and whether their attitudes concerning perfectionism were the same or different before and after onset of dystonic symptoms.
Anxieties were significantly more common in patients with dystonia and chronic pain than in normal healthy musician controls. Those with dystonia admitted the anxieties had been present before the onset of the disorder. Musicians with dystonia had more problems with social phobias than healthy musicians, while musicians with chronic pain did not have social phobias. Musicians with focal hand dystonia had significantly greater perfectionism than controls or those with chronic pain. The patients also noted they had problems with perfectionism even before the onset of dystonia. This suggests the psychological features contributed to the development of the dystonia rather than representing a psychoreactive response to the disorder. However, dystonia does not necessarily develop in all patients with these psychological disorders.2,4,120,121
In conclusion, the etiology of focal hand dystonia is still considered idiopathic. There is agreement the problem is multifactorial. It is not yet clear whether endophenotypic factors cause focal dystonia or simply increase the risk of its development under stressful exophenotypic conditions such as high levels of repetitive hand use. To determine whether an imbalance in neuroanatomic and neurophysiologic excitation and inhibition, aberrant homeostatic plasticity, anatomic restrictions, degraded cortical topography, and/or abnormal sensorimotor processing put patients at risk for the development of focal hand dystonia under environmental stress (e.g., repetition, trauma, behavioral characteristics), preplanned longitudinal studies are needed across a heterogeneous population of patients who work in highly repetitive jobs. This research will take years to conduct.
In the meantime, researchers need to clarify some important factors that could assist in developing more effective remediation strategies. For example, basic, clinical, and translational research is needed to determine whether topographic degradation occurs in the somatosensory, sensorimotor, and the motor and premotor domains, whether cortical topography interferes with both spatial and temporal processing of sensory input and motor output, whether there are problems in motor preparation for a learned task as well as problems in processing during skill acquisition, and whether variations in clinical patient presentations are uniquely correlated with severity or with different types of aberrant neurophysiologic processing. This information could help guide the next iteration of research regarding the effectiveness of intervention strategies.
Clinical Presentation: Focal Hand Dystonia
The onset of the signs and symptoms of focal hand dystonia is variable. The problem may initially manifest as an abnormality in the quality of sound produced by a musical instrument (e.g., a deterioration of vibration in a violinist),122 increasing errors in task performance, unusual fatigue or sense of weakness, or involuntary and/or excessive movement of a single digit or multiple digits). Initially, the symptoms are subtle and virtually indistinguishable from the normal variations that may be seen in the execution experienced by all musicians studying technically demanding music or a software engineer who is spending excessive hours at the computer. Clinicians must always be alert to the possibility that a musician, a keyboard user, a professional dentist or dental hygienist performing repetitive fine-motor techniques on the teeth, or a worker performing repetitive fine-motor assembly tasks who presents with minimal pain but vague motor control problems and/or somatosensory dysfunction may be manifesting early signs of focal dystonia.123
Although co-contractions of flexors and extensors can be observed while an individual with hand dystonia performs the target task, at rest and during the performance of nontarget tasks, the hand appears to function normally. Some patients demonstrate a variety of subtle abnormalities such as a reduced arm swing; loss of smooth, controlled grasping; a physiologic tremor; hypermobility of the interphalangeal joints; decreased range of motion in some upper limb joints (e.g., shoulder abduction, external rotation, finger abduction, forearm pronation); neurovascular entrapment; compression neuropathy; and/or poor posture.58,60,61,124-126 Patients with hand dystonia also usually describe a sensory trick to minimize the dystonia, but do not necessarily describe specific awareness of dysfunction in sensory discrimination.
The initial presentation often varies by the type of hand dystonia. A good example is a recent review of the clinical features of nearly 1000 musicians with focal, target-specific musician’s dystonia.126 The patients had a mean age of 35.7 years (standard deviation, 10.6) at onset with a male predominance (80%). Although both hands were affected in 4% of the patients, more commonly the problem was unilateral with the right hand affected in 64% of cases and the left in 32%. When the dystonia primarily affected one finger, the third finger was the most common, followed by the index and ring fingers. In cases with multiple affected fingers, there were four frequent patterns: (1) combination 4, 5 (32%); (2) combination 3, 4 (17%); (3) combination 3, 4, 5 (17%); and (4) combination 2, 3 (10%). Flexion of one or more fingers was the most common phenotype (54%). Isolated extension was less commonly reported (13%), and a combination of flexion and extension was seen in 17%. Side of involvement varied by type of musician. For example, although the right hand was predominantly affected in keyboard players (77%) and plucked string players (guitar, lute, 78%), the left hand was predominantly affected in bowed string players (68%) and flutists (81%). Either hand was affected in woodwind, percussion, and brass players.
Frucht126 also observed that task-specific hand dystonia seemed to begin after motor skills had been acquired rather than during skill acquisition. Thus, focal hand dystonia in a musician is probably not a disorder of motor learning, but rather a corruption of acquired, complex, motor programs. The data also suggested that peripheral environmental influences seemed to play an important role in molding the dystonic phenotype. For example, the hand performing the more complex musical tasks (e.g., right hand in pianists and guitarists, left hand in violinists) seemed to be more predisposed to the development of dystonia. In addition, the dystonia usually began in one finger and spread to adjacent fingers, rarely skipping a finger. Further, the ulnar side of the hand (fingers 4 and 5) was disproportionately affected, potentially because of the challenging ergonomics of the musical instrument as well as technical burdens required for this part of the hand in terms of gripping and activation of individual finger movements.25
There also seemed to be a correlation between physiologic patterns, dystonic phenotype, and the musical instrument. For example, flexion of the right fourth and fifth fingers was common in pianists, but flexion of the left fourth and fifth fingers was common in violinists. Flexion of the right third through fifth fingers was commonly reported in guitarists, whereas isolated extension of digit 3 was common in woodwind players. Frucht125 also noted that once a phenotype was established in a given patient, the pattern rarely varied, even if the patient took an extended break from playing his or her instrument. Thus, patterns seem to result from the technical requirements of the instrument, coupled with the technical burden of the hand. However, the observations also reinforce the possibility that with continued repetition of an abnormal, dystonic sensorimotor network, the network actually becomes entrained and learned.
In other studies, different patterns of dystonic presentations have been documented for patients with different types of hand dystonia. In a study by McKenzie and colleagues,11 there were significant differences in multifactorial measures of musculoskeletal, sensory, and motor performance for patients with writer’s cramp compared with patients with musician’s cramp. Patients with musician’s cramp had a higher level (P < 0.05) of functional independence and better range of motion, but more neural tension and less strength in the affected upper limb than patients with writer’s cramp. Compared with subjects with writer’s cramp, on the affected side, subjects with musician’s cramp demonstrated greater (P < 0.05) accuracy on graphesthesia, kinesthesia, and localization, but less accuracy and speed in stereognosis. It also seems that patients with writer’s cramp have a reduced response to vibration or oscillation.54,127 No between-group differences were noted in motor performance.
Rosenkranz and colleagues128,129 also reported differences in pathophysiologic factors including sensorimotor reorganization in patients with musician’s dystonia compared with patients with writer’s cramp. Applying a vibration stimulus to measure effects on cortical excitability and short latency intracortical inhibition, these researchers noted that vibration increased the amplitude of excitability and decreased the short latency intracortical inhibition in healthy nonmusicians. In patients with writer’s cramp, vibration had no measurable effect on either excitability or inhibition. In patients with musician’s cramp, vibration strongly reduced the short latency intracortical inhibition. It is possible that repetition in the musician leads to significant reorganization in the motor cortex, which ultimately interferes with rather than assists controlled fine-motor movements. The lack of response to sensory vibration in patients with writer’s cramp may suggest that sensory processing plays less of a role in provoking pathologic, dystonic changes than in patients with musician’s cramp. In summary, despite common trends in clinical presentation, patients with focal hand dystonia not only demonstrate variability in the affected limb and fingers, but may also have different problems in the neuromusculoskeletal system.
Diagnosis
Signs and symptoms of focal dystonia usually begin slowly, with the hand initially feeling thick and then uncoordinated in a few specific movements (e.g., alternating movements) while retaining the ability to do other movements.30,130 Often, the problem develops in musicians at the time when they are attempting to play dynamically restrained passages.90 The diagnosis may be readily apparent from the patient history; however, it may be difficult to make a specific diagnosis until the patient literally cannot complete a target task without disruption from the writhing type of involuntary movements.1,131 The signs and symptoms occasionally appear more acutely, for example, after an acute trauma (e.g., after a motor vehicle accident or after a fall on an outstretched arm). There is significant subjectivity in the early symptoms, and the movements associated with a specific instrument are idiosyncratic.60 Wilson and colleagues60 and Wagner132 proposed that occupational hand cramp or focal dystonia be defined as sustained and functionally significant loss of a previously attained manual skill, satisfying all of the following conditions.60
1. The affected skill is impaired by movements in which there are errors in timing, force, or trajectory associated with stereotypical tonic postures and/or cramping sensations that are absent at rest.
2. Abnormal movements are initiated by the attempt to exercise a specific motor skill within a characteristic context and may fail to develop except under those conditions.
3. Skill loss at the outset cannot be explained by diminished practiced efforts.
4. Degraded movements cause the individual to function at a reduced level of skill despite any masking strategies adopted to disguise or circumvent the problem.
5. The movement abnormality persists despite resolution of any and all antecedent inflammatory, toxic, traumatic, myopathic, and neuropathic abnormalities.
As part of the history, it is important to inquire about a family history of dystonia, previous injuries, chronic pain, or other medical problems such as a cardiovascular accident and head injury. As with many conditions in which the pathophysiology and the treatment are not well understood, it is assumed that the problems of repetitive strain injury originate as a result of biomechanical, anatomic, and psychological stress.119,133 Psychological stress is part of a high motivational drive and literally a basis of survival for most musicians and other successful professionals.
In addition, there seems to be an integral connection between sensory and motor function, performance anxiety, and the limbic system. When an individual begins to expect movement dysfunction when performing the target task, then the expectation could reinforce the outcome of poor motor control.134 Continued negative expectations could potentially contribute to abnormal learning. Thus, psychosocial issues and expectations should be thoughtfully reviewed as part of the patient history.
All patients need a complete neurologic examination. Some parts of the neurologic examination should be elaborated, such as the sensory and motor control components. Both hands should be evaluated carefully. Hochberg and colleagues135 suggested that it was important to carefully define the abnormal movement and which fingers are most involved. For example, one or more terminal phalanges may go into flexor spasm or the extensors of the fingers may contract simultaneously with the flexors. In other cases, the wrist turns inward with ulnar deviation or there may be irregular hyperpronation of the forearm with lifting at the elbow.135 It is important to assess whether contraction of the flexors drives the contraction of the extensors or vice versa. Biofeedback can be used to determine this. In addition, all patients should be videotaped while performing the target task.136,137 It is important to analyze which normal movements have been preserved while performing the target task. It is also important to apply some type of rating scale to score the severity of the movement dysfunction to serve as a baseline to measure change. Suggestions for grading these dystonic movements are summarized in Figures 135-5 through 135-8 (online).
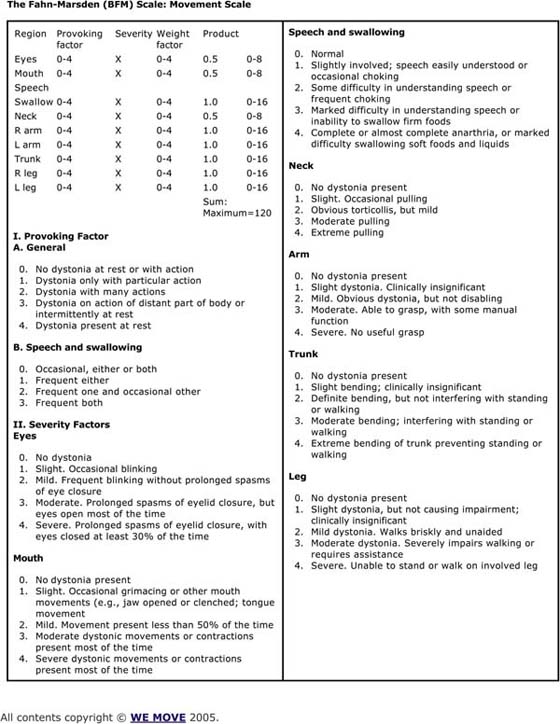
Figure 135-5 The Fahn-Marsden Scale: movement scale.
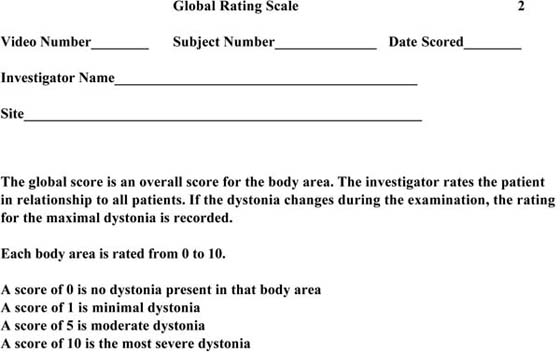
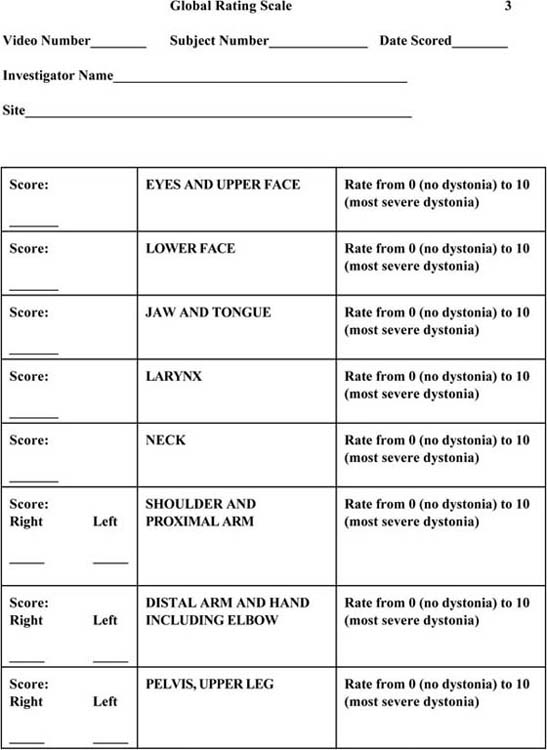
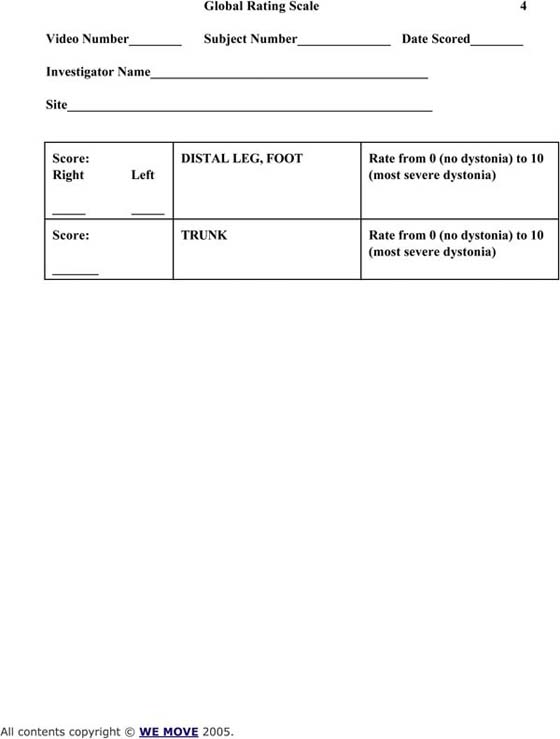
Figure 135-6 Global Rating Scale.


Figure 135-7 Toronto Western Spasmodic Torticollis Rating Scale.

Figure 135-8 Unified Dystonia Rating Scale Revised.
It is important to carefully differentiate between the signs and symptoms of hand dystonia, peripheral neuropathy, and other central movement disorders. Some patients with hand dystonia have a peripheral neuropathy. This neuropathy may be a risk factor for hand dystonia, but usually it is not a sufficient cause. Dystonia also needs to be distinguished from other central, hypertonic movement disorders such as spasticity and rigidity.138
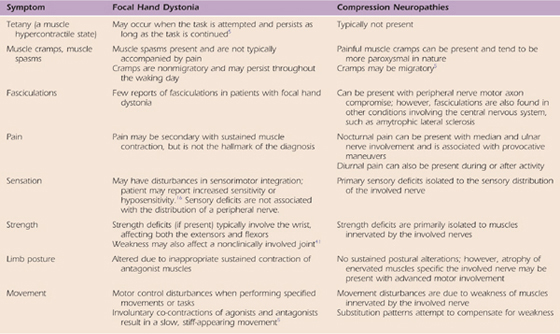
Goldman and colleagues139 provide a thorough comparison of the signs and symptoms of a peripheral nerve compression in the upper limb and focal hand dystonia (Table 135-1). They outlined and differentiated hand dystonia and compression neuropathy in terms of tetany (muscle hypercontractility), muscle spasm (cramps), fasciculations, pain, sensation, strength, limb posture, and movement disturbance. Compared with patients with a peripheral neuropathy, patients with focal hand dystonia usually do not have pain. However, if the muscle co-contractions are maintained over a long period of time, vasoconstriction may occur and the cramping is painful. In addition, patients with hand dystonia are likely to demonstrate tetany, disturbances in sensorimotor integration, strength deficits in wrist flexors and extensors, inappropriate sustained co-contractions of agonists and antagonists, and motor control problems at a target-specific task. On the other hand, compared with a patient with hand dystonia, the patient with a peripheral neuropathy is more likely to have painful migratory muscle cramps and fasciculations, nocturnal pain, sensory deficits in the distribution of the sensory nerve, and weakness in muscles innervated by the involved nerves without sustained postural alterations, with movement disturbance primarily due to weakness of the muscles. Muscle spasm is generally secondary to pain, trauma, nerve compression, a degenerative joint condition, or a neural degenerative condition of the dorsal horn or dorsal root. The muscles involved are often two-joint muscles, with the spasm more likely to occur when the muscles are too short or too long.
Table 135-1 Comparing Neuromuscular Findings of Focal Hand Dystonia and Compression Neuropathies
Based on muscular resistance, the speed of passive movement, the presence of a fixed posture, the pattern of muscle activation, and the impact of the emotional state, one can usually distinguish between the common central movement disorders of spasticity, rigidity, and dystonia (Table 135-2). Although spasticity can be characterized by a velocity-dependent resistance to passive motion, rigidity appears to be independent of the speed of passive movement or position, and dystonia tends to involve a sustained or intermittent co-contraction of agonists and antagonists when performing a target task.139
Table 135-2 Comparing Clinical Neuromuscular Findings of Dystonia, Spasticity, and Rigidity
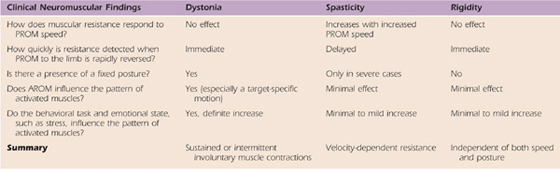
Spasticity represents an exaggerated stretch reflex usually secondary to a central condition involving cortical spinal pathways. Traditionally, spasticity is evaluated by moving the limb quickly through the range of motion and noting whether this is smooth and easy or whether there is resistance, clonus, or a sudden catch (stop to the motion) followed with a release after stopping. Usually the postural righting muscles are most frequently involved (e.g., in the upper limb: shoulder abductors, shoulder internal rotators, forearm pronators, and elbow, wrist, and finger flexors; in the lower limb: hip flexion, knee extension, ankle inversion, and plantar flexion). The stretch reflex is exaggerated. Slow positioning can inhibit the tone.
Rigidity involves a co-contraction of the flexors and extensors. The co-contraction of the muscles blocks voluntary movement. This co-contraction may not be equal, limiting movements like dorsiflexion and eversion of the ankle as well as wrist extension and finger spread. Usually patients have difficulty initiating movements, but once started, they can proceed, particularly if the movement is rhythmical. The co-contractions restrict not only movement range but also movement variability. There is some evidence that there is excessive inhibition in patients with rigidity. Positioning does not seem to help relieve the rigidity. The stretch reflex may be decreased.
Dystonia is also based on a co-contraction of the flexors and extensors, with either the flexors or the extensors dominating the movement, leading to end-range twisting motions. The observation that patients can use a sensory trick to inhibit the dystonic movements and that the dystonia is often target specific is unique to dystonia. In this case, there is inadequate inhibition of the antagonist muscle. The stretch reflex is generally normal.
A good sensory examination is important. Although two-point discrimination and differentiation of sharp and dull stimuli appear intact, cortical sensory problems are commonly reported (e.g., abnormal graphesthesia, stereognosis, kinesthesia). Poor temporal and spatial processing140,141 and poor somesthetic temporal discrimination142 have also been reported. These findings are consistent with reports that patients with idiopathic focal dystonia demonstrate increased abnormal dystonic posturing after the vibration of the tendon or muscle belly, abnormal perception of arm movements with tonic vibration, a reduction in dystonic posturing after cutaneous and proprioceptive stimuli, and anesthesia of the muscle spindle.6,13,70,143 Some report that patients with focal hand dystonia have decreased motor accuracy and decreased efficiency of fine-motor control of the hand.144-146 It is not clear whether this dysfunction is related to the sensory problems or directly related to the dystonia. All the measurements from the neurologic and physical examinations should be objectively recorded: (1) range of motion and strength,58,60,61 (2) neurovascular entrapment,147 (3) peripheral nerve entrapments,8,15,118,148,149 (4) somatosensory function,71,114,118,150 (5) sympathetic signs, and (6) pain.151 For the sensory examination, sharp/dull discrimination, detection of light touch threshold with the Semmes–Weinstein monofilament,140 and two-point discrimination152 should be performed. However, more time should be spent on cortical sensory discrimination testing such as localization, graphesthesia, stereognosis, and kinesthesia.153 Unfortunately, many of these latter tests are standardized for children, and therapists need to be certified to administer the Sensory Integration Praxis Tests. Box 135-2 provides some suggestions for clinical sensory discrimination testing. Because occupational hand cramps can develop in both hands, the examination and the measurements should be recorded bilaterally.17,132
Box 135-2 Suggestions for Sensory Discrimination Assessment Localization
Draw an outline of a hand. Place points on this hand pattern that will serve as the sites for you to deliver stimuli to your patient. Put target points on each segment of each finger as well as on the palm of the hand. Make a few similar points on the dorsum. Give the patient a fine-tip marker pen. Ask the patient to hold the pen close to the tip. The examiner also needs a fine-tip marker pen that is a different color from the one used by the patient. The examiner explains the task to the patient: “I will ask you to close your eyes, and then I will be placing a mark on your hand. Please take the pen I gave you and mark where you felt me touch you. Once you place the pen on the skin, please do not move it around. Keep it at the location until I have time to measure it. Now, close your eyes and we will begin.” Using a marker pen, the examiner lightly touches on one of the points (do not depress the skin more than 1 mm). After the patient places a pen mark on the place that he or she felt the stimulus, the examiner must take the measurement between where the stimulus was delivered and where the patient thought it was delivered. Although there is some variability in performance, adult patients will be 0 to 1.5 cm from the point of contact.15,16 This test is designed after the localization test of the Sensory Integrative Praxis Test.4
KINESTHESIA
Take a piece of 11 × 14-inch paper and make lines of different lengths going in different directions. Have a clear starting place and a clear ending point. Mark each line from 1 to 10 and indicate right or left (five trials per hand). Place the piece of paper in front of the patient while you sit across from him or her. Ask the patient to close his or her eyes. Take the third finger of the patient’s right hand and place it on the start position (beginning of the line, right-1). Now say, “With your eyes closed, I am going to move your finger to another position, and then I will return your finger to where we started. Then, I want you to take your finger back to the place I took it. Please keep your finger there until I can take the measurement.” Normal adult subjects usually will be less than 1.5 cm off target.15,16 This recommendation for testing is designed after the kinesthesia test of the Sensory Integrative Praxis Test.4
GRAPHESTHESIA
For testing graphesthesia, make a chart with 4 columns and 10 rows. Label one column Affected and another column Unaffected. Draw different simple designs approximately 0.5 inch in size on each row in the first of two columns for the affected and unaffected digits. Make the answer sheet large enough so that you have room to copy the drawing that the patient produces for you after the stimulus is delivered. Now explain to the patient, “I will ask you to close your eyes. I am going to draw a design on each of your finger pads. After I draw the design, I want you to try to draw it exactly like I drew it on your finger. Make the design similar in size and orientation to the one I drew.” After the subject draws the design, grade the design as follows: 2, completely correct; 1, partially correct; or 0, incorrect. Each hand will have a total of 10 tests, with a maximum score of 20. Determine the percentage of the designs that are correct for each side. This recommendation for testing is designed after the graphesthesia test from the Sensory Integration Praxis Test.4
STEREOGNOSIS
Collect 12 keys that have the same base but different cuts. Make a photocopy of each key. Then put six of the keys on each of two separate key rings. Create an answer sheet that includes a photocopy of each key. Show the patient a photocopy of one key. Hand the patient a ring of keys that includes the photocopied key. Instruct the patient as follows: “Put the keys under the table where you cannot see them. Feel each key until you find the one that matches the one you see. I will note the time that it requires for you to find the key and your accuracy.” Using a stopwatch, record how long it takes the subject to match the key on the ring with the photocopy of the same key. Alternate between sides (i.e., have a ring of keys for the right and the left sides). Have an answer sheet where you can record both the time required and whether the match was correct. This test has been used in our practice setting for research purposes. The reliability is high on test–retest (>90%). It takes between 30 seconds and 2 minutes for the subject to evaluate each key. Thus, the test takes from 10 to 20 minutes to administer. We have been using a six-key set; however, we are in the process of designing a new Byl-Cheney-Bocsai Discriminator that can be used as an alternative to the key test. It will require less time to administer than the key test.
SENSORIMOTOR ACCURACY
On an 11 × 14-inch piece of paper, start at the middle of the bottom of the paper and draw a wavy oval shape with a medium felt-tip pen. The oval should come back to the starting place at the middle, bottom of the sheet. Measure the entire length of the oval that you drew. Use this as the master from which to make copies. Give the patient a red pen to draw with using the right hand and a blue pen for the left hand. Instruct the patient as follows: “Start here and try to follow this line as carefully and as quickly as you can. I will be timing you, but I want you to try to be as accurate as possible.” A stopwatch must be used to time how long the subject requires to copy the line. Have the subject begin with the unaffected side. Then repeat the same test with the affected side. Again, time how long is required to complete the test. After the subject has completed the task, grade the test by measuring and then summing up the length of the areas where the subject was off the line. Subtract the total length of the line where the subject was off the line from the total line length. Divide this calculated length by the total length of the line to create a percentage. Normal subjects should be able to stay on the line approximately 80% to 90% of the time and complete the line tracing in approximately 30 seconds. This test is designed after the motor accuracy test of the Sensory Integration Praxis Test.4
Dynamic EMG testing can be included as part of the diagnostic workup. It is not uncommon to detect a peripheral neuropathy of the ulnar, median, or musculocutaneous nerve in a patient with hand dystonia. EMG changes in conduction may be noted even before weakness or sensory changes are detected. In addition, the electromyogram can document classic co-contractions of antagonists and agonists as well as confirm that muscles are recruited with excessive force. In addition, the electromyogram can show that once the muscles are contracted, not only is it difficult to release the contraction,47,154 but it is difficult to hold a maximum contraction given the interference of the contractions of the antagonists.45 This inability to hold a consistent firing pattern can be used to determine whether a muscle should be injected with botulinum toxin.
Even though pain is not usually an issue with patients with focal hand dystonia, it is still important to evaluate pain as part of the initial workup. The most common self-rated measurement instrument is the visual analog scale. This scale includes either rating pain on an ordinal scale of 0 to 10 or drawing the severity on a 10-cm line.151
Severity of Dystonia
There are a number of standard scales that can be used to quantify the severity of the dystonia. The common scales include Fahn-Marsden Scale (see Fig. 135-5, online); the Global Rating Scale (see Fig. 135-6, online); the Toronto Western Spasmodic Torticollis Rating Scale (see Fig. 135-7, online); and the Unified Dystonia Rating Scale (see Fig. 135-8, online). These are ordinal scales and may include an evaluation of the hand as part of the scoring, but do not uniquely target the hand.
In the case of task-specific hand dystonia, the general scales are not particularly detailed to measure objective changes in hand function. Further calculations of statistical significance are questionable based on small sample sizes and ordinal scales. The severity of dystonia in patients with hand/arm dystonia can be rated using the Arm Dystonia Disability Scale (ADDS).131 The ordinal scale for the ADDS is 0 = normal, 1 = mild difficulty in playing/writing, 2 = moderate difficulty in playing/writing, 3 = marked difficulty in playing/writing. It is difficult to objectively determine mild from moderate to marked difficulty. Technical performance is also rated on an ordinal scale according to the Tubiana and Chamagne Scale155,156 with the following ordinal assignment: 0 = unable to play, 1 = plays several notes but stops because of blockage or lack of facility, 2 = plays short sequences without rapidity and with unsteady fingering, 3 = plays easy pieces but avoids difficult passages for fear of motor problems, 5 = returns to concert performances. The Tubiana and Chamagne Scale more objectively defines the severity; however, with this scale, good performance is a high score and poor performance is a low score (just the opposite of the other scales). Patients may also be subjectively asked to assess the effects of treatment based on a percentage scale or a percentage scale can be created by subtracting their pretreatment motor performance from the post-treatment performance. If patients have writer’s cramp, a video should be made during writing, a force sensor placed on a pen, and an ordinal score used to grade the force of the grip, the force of the pressure down on the paper, the legibility of writing, and the amplitude of the letters.157
Despite the shortcomings of the scales, dystonia severity ratings have been correlated with movement kinematics. Mean stroke frequency is significantly reduced in patients with dystonia. In patients with focal hand dystonia, drawing movements showed a greater decrease in stroke frequency than handwriting movements. During circle drawing, mean vertical peak velocity was more variable in patients compared with controls. This may indicate an impaired ability to maintain the reproduction of the same kinematic pattern over time. An increase in vertical writing pressure was only observed during handwriting, but not during circle drawing. It is possible that this increase in vertical writing pressure reflected a compensatory effort to stabilize the pencil. Kinematic measures and individual scores on the ADDS and Toronto Western Spasmodic Torticollis Rating Scale were not correlated. The lack of correlation should not be surprising given ADDS, the Toronto torticollis scale, and kinematic analyses probe different aspects of motor impairments. The ADDS characterizes how dystonia affects a set of fine manual tasks, whereas the torticollis scale scores the manifestation of dystonia during handwriting. Therefore, the clinical scores and kinematic analysis of handwriting provide complementary insights into the motor impairments of patients with hand dystonia. Future studies need to address which combinations of clinical scores and kinematic measures are needed to determine the most appropriate method to quantify impairments in patients with writer’s and musician’s cramp.158
Psychosocial Assessment
Some studies report pathologic behaviors in patients with writer’s cramp.159 It has been reported that patients with focal dystonia have difficulty with shifting mindsets, increased perseveration, and obsessive compulsivity that reflect complex neurophysiologic dysfunction (dorsolateral, orbitofrontal, and motor frontostriatal).66,160
In musicians, studies confirm the presence of anxiety, perfectionism, and phobias as risk factors for the development of hand dystonia. Thus, it may be helpful to administer a personality inventory, a questionnaire on anxiety, and perhaps some standard questioning on perfectionism. This may highlight some of the personality risk factors that could contribute to the development of dystonia but may also help guide comprehensive intervention to remediate the dystonia.
Radiographs and Neuroimaging
Neither a radiograph of the hand nor a magnetic resonance image (MRI) of the hand is generally ordered by the physician unless there is a history of an injury, a structural biomechanical problem, or signs and symptoms of some type of soft tissue lesion (e.g., ganglion, cyst, ligamentous tear, fibrosis of the retinaculum, trigger finger). However, a physician may request brain magnetic resonance imaging or a computed tomography scan to rule out a stroke, brain tumor, or structural anomaly that could explain the movement dysfunction. However, these expensive techniques may not necessarily rule in the diagnosis of focal hand dystonia.161,162 Thus, at this time, health insurance will usually not pay for expensive imaging tests unless the neurologist suspects pathology consistent with a brain tumor or a vascular event.
On the other hand, advances in noninvasive imaging have expanded the opportunities to understand the etiology of dystonia. Imaging is useful for clarifying what areas of the brain may be contributing to the motor deficits. Detailed brain anatomy and unusual deviations in volume, density, morphology, and temporal and spatial processing in cortical and subcortical structures may also be examined with MRI and functional MRI (fMRI). For example, with basic MRI and fMRI paired with TMS, several cortical abnormalities have been noted in patients with focal hand dystonia: (1) a reduction in cortical blood flow and abnormal transient asymmetry in movement-related cortical potentials,163-167 (2) asymmetrical muscle response to double-pulsed magnetic stimulation,50,122 and (3) asymmetry in the tonic vibration reflex after intramuscular lidocaine.70
Diffusion tensor imaging can measure water diffusion across myelinated central nervous system structures to reconstruct the white matter tracts (e.g., the expansion of gray matter volume has been noted in the putamen of patients with dystonia).28 In highly active brain regions, positron emission tomography and fMRI provide indirect measures of neural activity through the consumption of oxygen. For example, compared with controls, patients with writer’s cramp demonstrate an increase in the amplitude of the blood oxygen level–dependent signal in the fMRI in the basal ganglia.168,169 On the other hand, using positron emission tomography, hypermetabolism has been noted in the frontal cortex and the basal ganglia in patients with dystonia.37 Spectroscopy (a special advanced application of MRI) can identify biochemical markers such as neurotransmitters (e.g., γ-aminobutyric acid and glutamate). Although proton magnetic resonance spectroscopy provides evidence of mitochondrial dysfunction as the pathophysiology of primary dystonia, in one study of 14 patients with primary focal hand dystonia, no statistically significant differences were found in any of the measured brain metabolites.49
Functional imaging methods like electroencephalography (EEG) and magnetoencephalography (MEG) can record brain activity through changes in the electrical current or changes in the magnetic fields across the scalp. MEG studies are noninvasive and can be safely used to look at cortical organization in humans.170,171 MEG studies confirm sensory, sensorimotor, and motor degradation in patients with focal hand dystonia.19,80,82,85,172 For example, in patients with focal hand dystonia, the size of the sensory receptive fields is enlarged, the separation of the digit representations is reduced, and the digital receptive fields are altered in their sequential order. Further, MEG cutaneous stimuli delivered to the digits produce somatosensory evoked fields with decreased amplitude and longer duration in the contralateral hemisphere of the affected hand for both the early (S1) and late (S2) responses.172,173
However, in S2, both earlier and higher amplitude responses have been measured in the ipsilateral and contralateral hemispheres of patients with hand dystonia.
An example of the variations in the somatosensory evoked responses can be seen in Figures 135-9 through 135-12. After sensory stimuli were delivered to the digits, the somatosensory evoked potentials in patients with dystonia were disorganized, with a reduced amplitude, excessive oscillations, and early peak responses.67,174-176 In addition, although the poststimulus somatosensory responses for digits 1-5 (D1-D5) were progressively ordered medial to lateral and inferior to superior, there was significant overlap across dorsal and lateral surfaces, between digits, as well as across segments within digits.67,174,175 Sanger and colleagues141 also quantified the overlap in the somatosensory evoked responses after simultaneous stimulation of adjacent digits in normal subjects and in subjects with hand dystonia. The magnitude of response when stimulating D2 and D3 in normal subjects was equivalent to a serial addition of the response of the individual digits alone. In patients with focal hand dystonia, the response of simultaneous stimulation was less than the addition of each digit stimulated individually, suggesting overlap and redundancy between adjacent digits. This is similar to the overlap recorded electrophysiologically in a behavioral monkey model, supporting the sensorimotor learning hypothesis.85,86
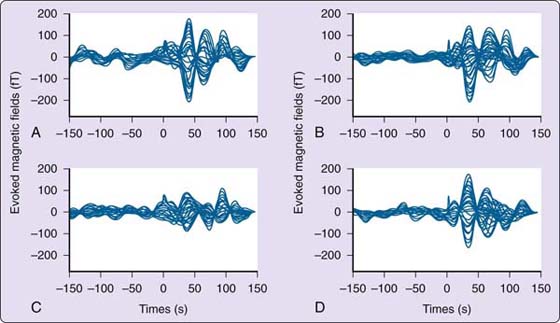
Figure 135-9 Somatosensory evoked magnetic fields for a healthy musician. A, Right D1. B, Left D1. C, Right D2. D, Left D2. The pattern is organized with the peak response at approximately 50 msec with approximately two half oscillations poststimulus. (From Byl NN, McKenzie A, Nagarajan SS, et al. Phys Ther Case Rep. 2000;3:93-113.)
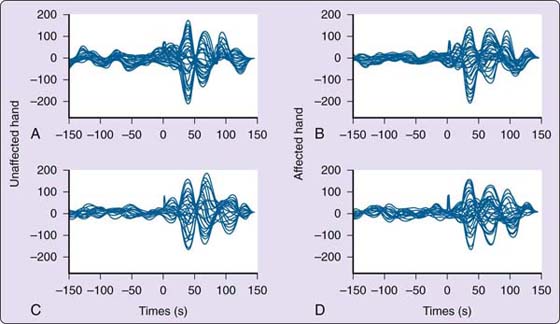
Figure 135-10 Somatosensory evoked magnetic fields for a musician with focal hand dystonia. A, Unaffected side with matched uninvolved finger before training. B, Affected side with uninvolved finger before training. C, Unaffected side with matched uninvolved finger after training. D, Affected side with matched uninvolved finger after training. For the involved digit (D2), there is little change in the pattern and organization of the somatosensory evoked response before and after training. (From Byl NN, McKenzie A, Nagarajan SS, et al. Phys Ther Case Rep. 2000;3:93-113.)
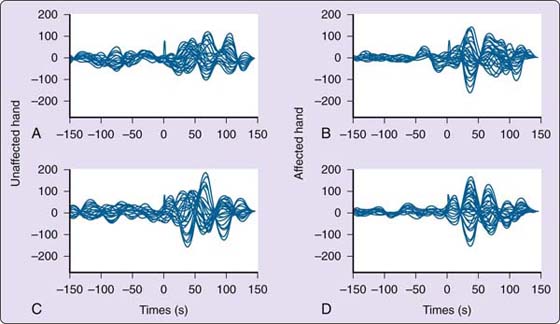
Figure 135-11 Somatosensory evoked response for a musician with focal hand dystonia. Uninvolved digit 4 (A) and involved digit 4 (B) before sensory training. The peak amplitude occurs closer to 40 msec, and the pattern appears disorganized on the unaffected and affected sides. At 150 msec, the neuronal activity still has not quieted down. C, Somatosensory evoked potential of uninvolved digit 4. D, Somatosensory evoked potential of involved digit 4 after sensory training. After sensory training, the pattern of the response is more organized, especially on the trained side, and the peak response is no longer the first response. The quieting of the neural response is more consistent after the stimulus. (From Byl NN, McKenzie A, Nagarajan SS, et al. Phys Ther Case Rep. 2000;3:93-113.)
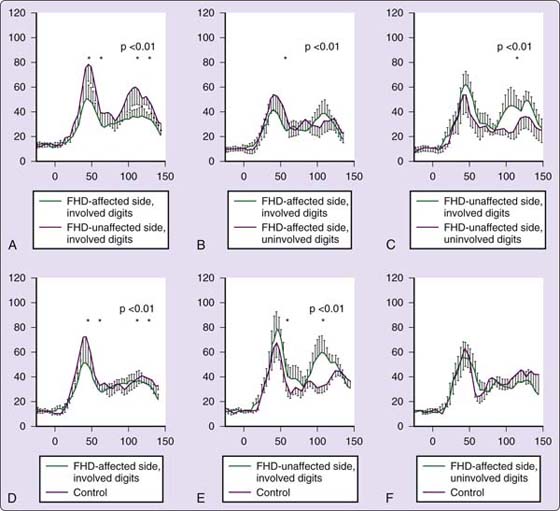
Figure 135-12 Integration of the amplitude of the somatosensory evoked response over time. A–C, Compare the differences of the patients with focal dystonia on the affected and unaffected sides. The amplitude is reduced significantly on the involved digits on the affected side. On the unaffected side, with the digits matched for those that are involved versus uninvolved, there is a significant increase in amplitude at the late response for the involved digits. D–F, Compared with controls, there is a significant reduction in the amplitude for the subjects with dystonia on the affected side’s involved digits. On the unaffected side, matching the involved digits, the amplitude is higher for the subjects with focal hand dystonia at the early and late peak. There was no difference in the amplitude over time on the affected side’s uninvolved digits for the controls and for those with dystonia. FHD, focal hand dystonia.
Change in cortical activity has also been noted in the M1 when doing a motor task.177 However, cortical activity in regions such as the premotor cortex (PMC) and supplementary motor area is reduced when a patient with dystonia performs a manual task or receives magnetic stimulation of the affected digits.65 In other studies, excessive plasticity in the motor cortex seems to generalize beyond the specific task in patients with dystonia, suggesting a endophenotypic disorder.82
High-speed three-dimensional video images and force and acceleration data have also been used to characterize dystonia. In addition, MIDI technology and kinematic motion analysis have also been gaining acceptance in motor research laboratories to help document timing and force information.17,172 Clinical tests provide important information to help the clinician understand the neurophysiologic and neuromusculoskeletal dynamics of dystonia, but it is still critical for the physician and the therapist to observe and quantify the clinical manifestations of the movement disorder while the patient is performing the target task.
At this time, there is no one clinical test that can be administered to rule in or rule out the diagnosis of focal hand dystonia. A considerable amount of research is still needed to correlate the diagnosis of a focal, especially a task-specific, hand dystonia with a specific gene, consistent anatomic restrictions, and generalized imbalances of neurophysiologic mechanisms. Do endophenotypic factors cause a primary hand dystonia or are the endophenotypic neurophysiologic imbalances a result of the hand dystonia? Is it conceivable that exophenotypic factors cause dystonia when the endophenotypic risk factors are present. This has been documented in animals with no known natural cause of focal hand dystonia in which repetitive behaviors dedifferentiate multiple cortical areas not only in the contralateral hemisphere of the involved limb but also in the ipsilateral hemisphere of the trained limb.84-86,178,179
Patients who participate in imaging studies after they already have focal hand dystonia demonstrate bilateral degradation of topographic organization as well as spatial and temporal processing problems bilaterally. In addition, when using imagery to perform a task, excitation is seen in uninvolved areas. Further, patients with dystonia have different cortical responses compared with healthy controls. If the neurophysiologic degradation is generalized and relates to endophenotypic risk factors,67,68,80,82,83,147 then why do the patients have a primary focal hand dystonia and not a generalized dystonia? Clarifying the cause versus the effect of neurophysiologic differences may be critical to both the prevention of hand dystonia as well as more effective treatment.127,180
It is also difficult to explain why some neuroimaging studies report increased activity in M1 while others report a decrease.113,181 Perhaps these differences represent some interaction between different brain regions complicated by the body part or the task affected. It is possible that these differences may become disentangled through continued MEG and fMRI studies combined with MRI and diffusion tension imaging studies of anatomic connectivity.182-184 Ultimately, it may be possible to measure the effects of retraining by looking at clinical changes and neuroimaging changes. Documentation of improvement in neurophysiologic processing could strengthen our understanding of etiology and potentially the receptivity to remediation.
Treatment for Focal Hand Dystonia
The treatment for focal hand dystonia varies by provider, interpretation of the etiology of hand dystonia, and the type of dystonia presented. Given the multifactorial origin of hand dystonia, the treatment must be not only comprehensive, but also specific to the individual patient. The whole person is affected by the problem, both physically and mentally.
In particular, the treatment for musician’s dystonia differs from the treatment for writer’s cramp, blepharospasm, and torticollis. Musicians are extraordinarily motivated to pursue treatment. These individuals are completely dependent on their hands for performance. When the signs of dystonia initially appear, most musicians respond by practicing harder. When practice does not remediate the dysfunction, the musician usually seeks help from teachers, coaches, physicians, and physical therapists. All these mentors try to help make musicians more aware of their biomechanics and their body movements. Commonly, the musician engages in slow practice to consciously control individual finger and hand movements. Oral medications such as anticholinergics may improve the foundation for retraining.4 Although a 75% improvement may be beneficial for a patient with blepharospasm or torticollis or even writer’s cramp, a 75% recovery is usually insufficient for a concert artist.
The treatment for focal hand dystonia must establish a solid foundation of positive expectations; wellness; postural alignment and reactions; cardiopulmonary, musculoskeletal, and neuromuscular fitness; trunk and extremity sensibility; stability; flexibility and strength within the context of good environmental and job ergonomics; task biomechanics; and psychological factors.185 The intervention should also maximize what we know about neural plasticity and retraining the brain.
It is essential to initiate intervention with an ergonomic analysis of the workstation and tool design and to analyze the unique demands imposed by unusual mechanics of different musical instruments or keyboards.186,187 Strategies used in job and musical performance must be analyzed carefully to avoid excessive force and unnecessary overuse. In some cases, special ergonomic equipment may be incorporated into the job. For musicians, there are special changes that can be made to the instrument to reduce stress, and special ergonomic-type instruments are available. It is important to try these new instruments before purchasing them. Some musicians suggest the sound quality is compromised with the instrument modification.
When initiating treatment for patients with focal hand dystonia, each individual should be encouraged to do the following: (1) establish a regular cardiopulmonary exercise program; (2) eat a healthy diet; (3) maintain hydration (e.g., to maximize oxygen delivery to heavily used tissues); (4) adapt strategies to reduce the effects of stress (e.g., diaphragmatic breathing, relaxation techniques, resolution of personal and job-related issues); (5) reduce unnecessary biomechanical trauma to the hands by using stress-free hand techniques in activities of daily living (e.g., let the surface of an object provide the sensory stimulus to open the hand and to grade the force needed when using the hand); (6) learn how to balance posture and to maintain flexibility of muscle, fascia, and neural tissue; (7) perform self-massage and soft tissue mobilization for muscles and fascia that tighten with stress; and (8) enhance the strength of the intrinsic muscles of the hand to enable maintenance of the functional, structural position of the hand. Based on this healthy foundation, patients will be able to focus on more effective strategies to remediate the dystonia.
Most patients come to see a health professional for treatment of their hand dystonia with a long list of previous, usually ineffective therapies. Intervention strategies can include muscle strengthening, modification of the musician’s instruments or keyboards, education, drugs to deplete dopamine or injections of botulinum toxin, special diets, physical therapy, hypnosis, acupuncture, biofeedback, reading Braille, Rolfing, movement training (Feldenkrais, Alexander techniques, Pilates, yoga), behavioral therapies, imagery, immobilization, constraint-induced movement therapy, TMS, and surgically implanted deep brain stimulators. Hochberg and colleagues135 evaluated a series of these treatment approaches and concluded that different approaches still remain empirical and may temporarily reduce the severity of the dystonia, but no single treatment modality has yet proven to be 100% effective. Thus, the objectives of the intervention are to manage the signs and symptoms; decrease progression of symptoms; reduce impairments, handicaps, and disability; and maximize community participation, including a return to work. Based on recent research, the multifactorial nature of the etiology, and the abnormal findings in the ipsilateral and contralateral hemispheres of the affected and unaffected hand, retraining strategies should be bilateral.
Medications: Botulinum Toxin
The treatment of focal dystonia was revolutionized with the approval of botulinum toxin in the late 1980s. Botulinum toxin is most commonly used for cervical dystonia, blepharospasm, and spasmodic dysphonia. However, botulinum toxin is also used with patients with hand cramps. The toxin should be injected by experienced experts and should be performed under EMG guidance.188-197 The botulinum toxin is injected into the overactive muscles to peripherally block the neurotransmitter acetylcholine at the neuromuscular junction.124,190-194 The biological activity of the toxin extends to 2.5 to 4.5 cm, restricting its specificity and potentially intruding into areas that are not desirable.192 Botulinum toxin is not a specific therapy to remediate the cause of the dystonia. The effects of the injection usually last for a couple of months. Most patients experience mild weakness for a couple of weeks after the injection and then enjoy greater benefit once the weakness dissipates and the clinical benefit remains. There is some evidence that botulinum toxin exerts effects peripherally and centrally.195-197 However, the primary benefits of the injection are based on subjective and/or clinical ratings. Permanent, significant changes in neurophysiology or function have been measured in the typical pathologic EMG patterns, including co-contractions, tremor, prolongation of EMG bursts, and lack of selectivity.47,193 Some researchers have measured a decrease in long latency reflexes after botulinum toxin. Most patients require the injections to be repeated every 3 to 4 months as the effects of the toxin wear off. However, the ability to use the hands in a coordinated way to play an instrument is usually not restored.189,198 Many musicians discontinue the injections after a year or two due to lack of a functional improvement.
TMS
Current neuroimaging studies find a reduction in the activation of the primary motor cortex and hyperactivity of areas of the frontal nonprimary motor areas in patients with writer’s cramp. Intervention strategies that could decrease the excitation of the nonprimary motor areas (e.g., PMC and supplementary motor area) might decrease dystonic symptoms. Subthreshold, low-frequency (0.2 Hz) repetitive TMS (rTMS) can exert an inhibitory action on the cortex. Pascual-Leone and colleagues199 also demonstrated that TMS could modulate muscle responses during acquisition of a new fine-motor task.
Given that deficient cortical inhibition has been reported in patients with hand dystonia, it is possible rTMS applied to the supplementary motor area and the PMC could increase inhibition and improve motor control. Murase and colleagues77 demonstrated that stimulation of the PMC significantly improved the quality and efficiency of handwriting in patients with writer’s cramp. This stimulation was also associated with a decrease in pen pressure and a prolonged silent period in patients with writer’s cramp. Stimulation of other sites or using a sham coil in the patient group revealed no physiologic or clinical changes. However, the increased susceptibility of the PMC in patients with hand dystonia suggests the decreased excitability of the motor cortex may be secondary to the hyperactivity of the PMC neurons.200,201
Desensitization
In France, Tubiana developed a four-stage treatment program for musicians. This program is based on desensitization or deprogramming of the acquired “bad habits.”155,156,167,185,202 In this program, there is an attempt to restructure the body image, selectively improve muscle differentiation and relaxation, individually retrain the muscles, and then provide technical retraining on the instrument. Usually, postural and shoulder imbalances are found along with the specific hand dystonia. These imbalances must also be addressed in treatment. Tubiana156,167 and Chamagne185 regard the rehabilitation as a long-term, cooperative, supportive relationship if success is to be achieved. Having seen a large population of subjects, they estimate that it takes a year for treatment. Of 438 patients, 95 returned to concert performance, 57 did not improve, and the remainder achieved partial improvement.
Negative Reinforcement
Liversedge and Sylvester203 developed a negative reinforcement protocol for treating typists with writer’s cramp. They focused on breaking down the primary manifestations of the motor dysfunction to identify the sensorimotor aspects that needed to be treated. The patient held a metal stylus and inserted it in holes of decreasing size, traced a flat zigzag on a metal plate, or wrote with a pen that reacted to excessive pressure from the thumb. When a “mistake” was made, an electric shock was delivered to the palm. An apparatus was rigged for a typist so that a shock was received when the fingers curled into the palm. Six patients were treated, and all improved significantly, with improvement maintained for varying periods (weeks to months of follow-up).
Restoration of Physiologic Posture and a Balanced Hand
Chamagne185 outlined a four-part program of rehabilitation that has been successful for his own rehabilitation as well as for others with focal hand dystonia. Part 1 focuses on reestablishing a balanced, stable posture with gravity. Part 2 involves the integration and function of the upper limb with a balanced posture. This part focuses on balancing the scapula and the head on the trunk as well as on breathing. Part 3 focuses on strengthening the muscles of the scapular girdle, forearm, and hand (wrist, finger extensors, thumb opposition, hand intrinsics). Part 4 includes retraining on the instrument to restore control of the pressure of the digits to actuate the desired sound. This may be the longest part of the rehabilitation process. This part may require dynamic and resting orthotic positioning to improve biomechanical control.185
Constrained-Use Paradigms
Constrained-use paradigms have been used in stroke rehabilitation programs.204-207 When the unaffected side is constrained, the patient is forced to use the affected side. In this forced-use situation, patients must engage the involved hand in meaningful ways to facilitate adaptive, functional use. For focal hand dystonia, effectiveness studies have been reported in case studies and small pilot studies.204-206,208-210
In a paradigm designed by Candia and colleagues,208-210 patients with focal dystonia followed the principles of constraint-induced movement therapy. The therapy is based on the principles of neuroplasticity. The specific guiding principles are to (1) determine the most dystonic finger; (2) avoid constraining the most dystonic finger; (3) identify the fingers that constrain the independent use of the dystonic finger; (4) constrain the fingers in a position that is similar to the normal resting angle used in performing the target task; (5) perform selected exercises with the dystonic finger; (6) progressively increase the speed at which the dystonic finger is required to move in concert with the other fingers, and then progressively decrease, expecting more exacting requirements (shaping); (7) generalize the daily practice needed to sustain patient motivation to the target task; (8) practice intensively (i.e., massed practice), but not to the point that it creates excessive fatigue or increases the dystonic movement postures; and (9) outline a home practice program.
In the Candia paradigm,178,208,209 the movement of the affected extremity was restricted for 2 weeks. The most affected extremity received intensive training for 6 hours per day for the 10 weekdays. The treatment involved identifying one finger as being the main focal dystonic digit and one or two other digits as being involved in performing the target-specific task. An orthosis was then created that left free the digit exhibiting the main dystonic symptoms. The orthosis had the flexibility of releasing other digits to participate with the dystonic finger as well. One or several of the other digits were immobilized, and extensive practice was directed toward performing individual movements of the focal dystonic finger in coordination with movements of the other fingers. Once it was confirmed that a given finger was the most dystonic, other digits were immobilized and the subject was required to perform sequences of movements with the finger. The immobilization of adjacent fingers was simply a means to enable independent movement of the most involved digit(s).
During fixation, the finger was held in the rest position that was used to accomplish the target task. The involved finger should begin to perform alternating individual finger movements with all possible permutations of the other fingers of the dystonic hand. This should include sequential movements of one finger and then of two or three digits, including the focal dystonic digit. The patient does this for 10 minutes in an ascending and then descending order, with continuous repetitions. Then, a 2-minute rest was instituted after the sequence of movements of two or three fingers. Five such blocks were carried out in an hour.
Performance was paced by a metronome, starting at a medium tempo (60 beats per minute), then speeded up, and gradually decreased in rate. The speed sequencing was then reinitiated with the goal of having the subject generate faster and faster, then slower and slower, alternating movements in successive sequences (shaping). This was fatiguing. After completion of the first five blocks, the orthosis was removed, and the subjects were given a 10-minute rest. Then they received four more 10-minute blocks of exercises with 2 minutes of rest between blocks. Various permutations of possible finger movement were used.
After the specific motor training, the subjects were encouraged to play their instruments without the orthosis. They played approximately 10 bars from a self-selected musical piece (15–30 seconds). If they could not do this, they were encouraged to try again. After two successful repetitions, they were asked to play a different 10-bar segment and then asked to play portions of musical pieces for longer and longer duration, until they had played for a period of 15 minutes (excluding rest intervals). Subjects were fearful of having the dystonic movement return, but they were encouraged to continue. The complexity and duration of the practice within the performance period without the orthosis were based on the therapist’s judgment. Success encouraged more success and continued performance. After a rest of 5 minutes, if the subject was not too fatigued, the orthosis was replaced and a second series of alternating digital maneuvers was performed for half the time taken by the first series. This regimen was continued for 8 consecutive days.
On the last treatment day, the subjects were given the orthosis constructed for them and asked to practice for 1 hour daily over a period of 1 year post-therapy. The subjects were also instructed to do the repertoire for 10% of the usual and customary practice time without the orthosis. This period was increased by 10% in each succeeding month if there was no deterioration in the level of motor control.
Some of the subjects have been followed for 24 months. This rehabilitation routine has improved the function of the affected limb in a series of 80 patients. However, a specific controlled trial of treatment was reported on only 5 subjects. The therapist monitored a dexterity/displacement device that continuously recorded digital displacement during metronome-paced movements of two fingers and a spectral analysis of the record reporting on smoothness of the movements. A dystonic evaluation scale, in which patients rated how well they were performing movement sequences and passages from their repertoire without the orthosis, was administered at the beginning of each treatment day. A one-way analysis of variance revealed that the scores at pre-treatment, post-treatment, and 1-month follow-up were significantly different from one another. Post hoc testing indicated that the pretreatment scores were significantly lower than post-treatment scores up to 1-month follow-up. One patient did not do the home program and relapsed. The others did the home program and were stable for up to 14 months.
Learning-Based Sensorimotor Training
With the growing evidence supporting the presence of abnormal differentiation of the hand in the somatosensory, sensorimotor, motor, and premotor cortices in patients with focal hand dystonia, the challenge is to determine the best way to remediate the underlying dedifferentiation. The challenge is to coordinate somatosensory and sensorimotor training to improve motor control. If the cortex is adaptive, and goal-attended repetitive behaviors can enhance its representation,95,187,211-216 it is conceivable that highly attended, stereotypical, excessively repetitious movements of the digits could degrade the representation of the hand at multiple levels. However, it is also possible that progressive, attended, rewarded, repetitive but progressively challenged sensory and motor activities could restore the normal sensory and motor representation of the hand, facilitating the recovery of normal motor control.217 Thus, intervention must follow the principles of neuroplasticity (see Box 135-1) and retraining the brain (Box 135-3).
Box 135-3 Principles of Plasticity for Retraining
Although basic science research confirms that it is possible to modify our nervous system, the exact retraining protocol to follow to integrate science into practice and maximize learning is less well understood. The principles that should be followed to guide rehabilitation are as follows:
1. Use it: Stay active and keep challenging learning; failure to regularly engage specific and general brain functions in learning something new can lead to serious degradation.
2. Try to improve performance: Engage in training behaviors that drive efficiency or new effectiveness of old and new specific brain functions.
3. Be specific in your training: The training experience must match the desired outcomes; the new connections driven by neural plasticity are dictated by the nature of the training.
4. Repetition, repetition, repetition: Learning requires repetition; learning must be reinforced, progressed in difficulty, and spaced over time.
5. Training must be intense: Plasticity changes require sufficient intensity of training to ensure that new connections and pathways are durable.
6. Match training to outcomes: To facilitate neural adaptation, the training must be salient and consistent with the outcome behavior desired.
7. Age is important: Training-induced plasticity occurs most readily in a young brain, but neural adaptation continues across the life span after learning-based training.
8. Transfer learning: Neural adaptation in response to one training experience can also enhance acquisition of similar behaviors and adaptation in other experiences and other parts of the body.
9. There can be interference: Plastic changes after one training experience may interfere with the acquisition of changes in similar systems.
10. Expect improvement: Patient expectation can facilitate the outcomes of training; patients who expect to get better can enhance their learning.
11. Feedback is necessary: Feedback and reinforcement allow modification of training behaviors, correcting errors, and improving accuracy of learning.
A foundation of good posture, healthy blood flow to the soft tissues, a balance of intrinsic and extrinsic muscle strength, a positive mental attitude, appropriate stress management, good performance ergonomics and biomechanics, and adequate cardiopulmonary fitness all serve as a healthy foundation with which to begin sensorimotor retraining. Ideally, the patient should take a break from the tasks that create the dystonia to improve one’s focus on getting better. Abnormal movements must be minimized or stopped to prevent repetition and continued negative learning. Patients must expect to get better and should begin by imaging what it was like to perform the target task normally and the joy of performing the task. Boxes 135-4 and 135-5 provide suggestions for learning-based training and sensorimotor guidelines.
Box 135-4 Specific Practice Training Suggestions to Improve the Effectiveness of Learning-Based Training: Therapists, Patients, and Family
A. Each individual must set his or her own unique goals and objectives for retraining.
1. Each individual should encourage the family to be involved in the retraining activities.
2. Create activities to achieve goals and objectives that require attention and are repetitive, progressed in difficulty, increased in variety and depth, spaced over time, rewarded, and complimented with feedback on accuracy.
B. Each individual must set his or her own retraining schedule.
1. It is important to determine how to integrate work and retraining.
2. Ideally, individuals should take time off work if work increases the dystonia.
C. The training paradigms will be somewhat unique for each individual but share common paradigms.
1. Link activities temporally (in time) and spatially but sequence the training and minimize excessive simultaneity (except in the case of training dual tasking).
2. Make the stimulus strength adequate for detection and appropriate to avoid abnormal overstimulation.
3. Integrate stimulus-induced behaviors into meaningful functional activities.
4. Try to make training activities age appropriate and fun.
5. Integrate training activities across multiple sensory modalities.
6. Perform training activities in different postural orientations.
7. Make sensory input relevant to desired outputs.
8. Match training behaviors with progression of healing and recovery as well as development.
9. Strengthen positive responses with multisensory feedback modalities.
10. Consider making it difficult for the unaffected side to be used (e.g., wearing a glove).
11. Begin sensory training stimulation by using the most mature or accurate sensory receptors.
12. Create training situations in which desired behaviors are performed in different environmental contexts.
13. Do the training in the positions that stimulated the best performance.
14. Avoid activities that stimulate repetition of abnormal movements.
15. Maintain high levels of attention and cognitive function to all activities.
a. Learn something new each day.
b. Make a schedule of activities to complete each day.
c. It is not necessary to do all activities every day.
d. Maintain a positive perspective on keeping a balance of daily activities.
D. Retrain the key components of the sensory, motor, and cognitive impairments.
1. Stop the activities that stimulate the abnormal movements.
2. Focus on elements of control that can drive positive change.
3. Think positively about recovery.
a. Improve self-esteem.
b. Imagine and practice remapping the cortical somatosensory (touch, pain), sensorimotor (proprioception, vibration, length of muscle spindles) audition, vision, and motor representations.
4. Learn to turn muscles on and off on command.
a. Retrain inhibition (stop unwanted movements); you may need to use electrical, auditory, and tactile feedback.
b. Stop the co-contractions of agonists and antagonists (the flexors and extensors).
c. Decrease neural sensitivity and irritability (decrease overexcitation by grading the activation of the muscles and quieting the nervous system).
d. Set reasonable schedules of practice with attention but minimize excessive plasticity associated with heavy schedules of repetition.
Box 135-5 Guide for Patients: Stages of Learning-Based Sensorimotor Training
I. Think positively about recovery; expect to get better.
II. Commit to a fitness program including stress management, good nutrition, hydration, and exercise.
III. Curtail, if not stop, the abnormal movements.
A. Stop performing the task(s) that leads to the abnormal movements.
B. Watch others performing the target task with normal movements.
1. Be sensitive to even the slightest abnormal muscle contractions.
2. Imagine that it is you performing the task.
C. Practice looking at your instrument and do not think about the dystonia.
IV. Imagine normal movements at the target task.
A. Imagine performing the target tasks normally.
1. Imagine the success of completing the target task without abnormal, involuntary movements.
2. Imagine the satisfaction and joy involved in performing the target task.
B. Go back in time as far as is necessary to remember when you performed with your instrument and it was easy, comfortable, and enjoyable; try to transition the memories to the current day.
V. Improve sensory discrimination skills.
A. Improve the ability of your touch receptors to actively discriminate fine differences.
B. Think sensory rather than motor; let the sensation of the object determine how the hand should be opened.
C. Do sensory discrimination activities in a variety of venues.
1. Put rough or sticky surfaces on objects that you regularly use (e.g., pen, pencil, toothbrush, hairbrush).
2. When a surface is coarse, the brain will automatically reduce the force that you need to exert to hold the object.
3. Put specific objects that can be matched into a bowl filled with beans, rice, and other stimuli; then try to match the objects.
4. Put objects in your pocket (coins, small toys, paperclips of different sizes) that you can match while walking around, sitting, or driving.
5. Ask friends to help you improve your discrimination skills by:
a. Touching different fingers when your eyes are closed
b. Drawing designs, letters, and words on your fingers that you try to reproduce
6. Learn to read Braille.
VI. Develop strategies to provide feedback to help you turn on and off desired muscles to create fluid movement.
A. Tape the involved fingers.
1. Put on tape (paper tape or other tape) to give you information about where your fingers are in space.
2. Tape the fingers, but keep the pads of the fingers free.
B. Get a biofeedback machine to provide auditory and visual feedback about when a muscle is firing.
1. Put the electrode over a muscle that you would like to quiet (e.g., the extensors) and then try to bend the fingers, wrist, or elbow while keeping the extensors quiet.
2. Put the electrode over the muscle that you want to turn on; make the muscle contract so there is auditory firing.
C. Use a mirror.
1. Put your good hand in front of the mirror; the mirror image looks like the other hand.
2. Put your affected hand behind the mirror.
3. Pick up objects, stack objects, do simple movements (e.g., tap the finger), turn palm up and down, write, or put fingers on a keyboard on either side of the mirror and watch the mirror image; make your affected hand look just like the mirror image.
4. Occasionally look at the affected hand to see whether it actually is moving like the mirror image.
VII. Practice placing the hand on the target instrument.
A. Learn to place the hand on the target instrument without feeling tension.
1. You may have to do this just for a few seconds (to keep the muscles from excessively firing).
2. If it is not possible to touch the instrument without increasing the firing of the muscles, determine whether there is an alternative position that you can assume to be able to place your hand comfortably on the instrument.
B. Handle the instrument (pick it up and put it down).
C. Pick up the instrument and put it into the playing position but do not play it.
VIII. Practice graded movements.
A. Controlling the amount of force is critical in fine-motor movements.
B. Practice doing graded movements.
1. Lightly put the hand on a record without altering the sound.
2. Lightly put the hand on the belt of a treadmill and let the belt move under the fingers.
3. Put the hand on a scale.
a. Maintain a fixed amount of pressure for several seconds.
b. Place different amounts of force on the scale.
c. Simulate the amount of force that you need to depress a key on a keyboard or a flute.
IX. Practice nontarget tasks.
A. Identify the tasks that are similar to the tasks that lead to the dystonic movements.
B. Practice performing nontarget tasks with controlled, smooth movements.
C. Improve speed and quality of task performance.
1. Put pegs in holes of different sizes.
2. Practice painting instead of writing.
3. Screw things together.
4. Shuffle and deal cards.
5. Take objects in and out of small places.
X. Evaluate your performance posture at the target task.
A. Determine whether you can get into a position that allows you to do the target task without the dystonic movements.
1. Lie on your back or stomach, upside down, or on your side.
2. Stand instead of sit.
3. Once you can perform the target task in an unusual posture, begin to perform the target task in the usual position.
B. Pay attention to good posture: head lined up over shoulders, hips, and ankles.
XI. Practice individual fine-motor movements.
A. Teach yourself to recruit selective, desired muscles.
1. Avoid recruiting the antagonist muscles (move the fingers in the opposite direction).
2. Move the fingers at the base joint using the intrinsic muscles of the hand (inside the hand).
a. With palm up, move each finger one at a time, up toward the ceiling.
b. Keep the other joints of the fingers in the same position (slightly bent).
3. Place the hand on a surface, palm down, and press finger down on surface moving from the base joint.
a. Try to press one finger down at a time.
b. Release the pressure down; do not lift the finger up.
c. Alternate the fingers and then sequentially move the fingers.
d. Move the fingers in pairs.
B. Integrate practice of individual fine-motor movements into the target task.
XII. Practice the target task.
A. When you can place your hand quietly on the instrument without dystonic movements, you may be ready to begin to perform the target task; you may need to put more sensory cues on the instrument.
B. Think about all the fingers resting down, even when you only want to press one down.
C. Try to rotate the forearm as the strategy to place a finger down or lift the fingers off the surface.
D. Initially avoid rapid alternating, stereotypical movement patterns.
E. If you are a musician,
1. Begin with new music, not an established repertoire.
2. Begin practice for only 5 minutes at a time.
3. Consider working with a teacher to modify techniques.
F. If you are not a musician, consider working with a physical therapist to initiate retraining.
G. If you heavily use a computer keyboard,
1. Consider an ergonomic evaluation of your workplace to adjust furniture and equipment.
2. Consider using voice-activated software.
3. Take regular breaks (initially every 5 minutes), and then you can extend practice up to 30 minutes and then take a break.
4. Stop using touch-typing techniques; move from the shoulder and elbow.
All behaviors must be goal directed and highly attended. The behaviors must be adequately repetitive, with progression of difficulty to enable learning. The patient must be able to accomplish the sensory and motor tasks correctly (e.g., 80%–90% accuracy). Appropriate performance and accuracy must be rewarded. When performance is accurate, progression of training complexity is in order.
The first objective is to improve the accuracy of discriminating sensory information for all somatosensory modalities (superficial cutaneous [rapid and slowly adaptive], deep touch, muscle afferents and Golgi tendon organs, kinesthesia, vibration). Sensory discrimination training can also be reinforced with auditory discrimination training.212 Stimuli must be accessed actively (searching and discriminating objects) as well as discriminating stimuli passively delivered to the skin (e.g., graphesthesia). Ideally, the stimulation should be static and dynamic (i.e., still as well as moving). The stimuli should be delivered in both the temporal and spatial domains. When the patient can accurately interpret the information from one stimulus, therapists should consider providing two stimuli proximate in time. The two stimuli could be delivered together on opposite limbs or delivered on the same limb with spatial or slight temporal separation. Initially, the therapist should make the stimuli the same and then make the two stimuli different. The stimuli should be brought closer and closer together in time and then in space. The tasks must be progressed in difficulty.
The sensory tasks must be performed without increasing muscle tension. If muscle co-contractions are triggered, the sensory tasks should be done first with the unaffected side until there is some familiarity with the sensory task. All the sensory tasks should be performed with the patient blindfolded or with the eyes closed to minimize visual input. It might also be helpful to begin sensory discriminative training in an atypical position (e.g., lying on the back rather than sitting) to decrease tension. Ultimately, it is important to train in the position in which the task is usually performed.
Ideally, the most dystonic finger is identified. Sensory retraining should be started with the most involved digits. However, if a lot of tension is created by doing sensory training with the most involved finger, it may be necessary to begin with the least involved digit(s) or compensatory digits. When multiple digits are involved, the sensory retraining should progress from the tips of the fingers to the pads to the lateral and medial surfaces between the adjacent digits. The training paradigms with animals demonstrated that it was possible to degrade the somatosensory, sensorimotor, and motor cortices with training once daily for 1 to 1.5 hours or twice daily in 30-minute sessions for 8 weeks. Thus, to restore the differentiation of the digits, a similar training schedule may be needed. If abnormal dystonic movements are continued, then retraining may take more repetitions and more time to counter the repetition of the abnormal movements. However, the training sessions may be spread in smaller time segments throughout the day.
Patients should carry games, coins, puzzles, shapes, and other objects in pockets to constantly challenge sensory exploration. The palpation of objects must be purposeful and not just random. For example, objects should be manipulated and functionally matched or assembled. When doing any task, the patient should think sensory (what it feels like rather than focusing on the motor control). Eventually sensory training needs to be performed on the surface of the target instrument.
Once sensory processing accuracy is improved, the patient should begin to explore the sensory interface of the surface of the target instrument. It is critical for the patient to be able to touch and handle the instrument without creating abnormal movement or tension. The sensory training should be done with a variety of surfaces, with the patient assuming a variety of positions to access multiple sensory maps. For example, a guitarist may be able to hold the guitar and touch the strings without involuntary muscle contractions when he is lying on his back but not while sitting. A person may be able to write on a pad on the chair while lying on his or her stomach, but not when writing a check at the store. If Velcro (rough surface) is placed on the keys of a computer keyboard, a patient with dystonia may be able to depress the key without involuntary finger curling.
Next, the sensory processing needs to be integrated into performing the target task. At this point, retraining should focus on sensorimotor feedback and fine-motor control. First, the therapist and the patient should identify a position in which the patient can perform the target task normally. If this position can be found, the patient can begin to at least handle the instrument in that position. When the patient can place his or her hand on the instrument and move it around without feeling the dystonic movements, he or she is ready to begin to practice the target task in that position. Initial practice of the target task should begin with mental imagery, with the hands resting on the instrument but not playing. The imagery can strengthen the pathways for learning the task.184,218 Physical practice should begin only after the patient is able to imagine performing the task normally. Before beginning to practice the whole task, the task must be broken down into small components that are simple and can be performed normally. The patient and the therapist may need to do this together and write down these tasks.
Normal movement should be reinforced with visual, mirror, and motor imagery even though the patient is actually beginning to initiate training on the target instrument.219,220 Movement practice should start with simple movement sequences. A musician should take time off from required instrumental play. When ready to return to play, new music should be pursued. Patients should not perform well-learned difficult repertoires. Patients need to start with simple movements on the instrument and then practice simple sequences of movement. Partial tasks and other tasks similar to the target task should be performed accurately, smoothly, and at different speeds before proceeding to complete the target task.
Initial practice might include just repeatedly dropping the digit on a key or a string. It might include rotating the hand back and forth (pronation and supination) to drop a finger on a key or lift a finger off a key. The individual could progress to dropping down alternating fingers. As part of this training, the patient should also work on restoring graded movements such as lightly holding the hand/digits on a moving record, lightly holding the fingers near the blades of a small portable plastic fan without stopping the movement of the blades, or moving the hand smoothly in different directions over a moving surface (e.g., the moving belt of a treadmill).
The patient should then physically practice the aspects of the target task that can be performed normally. If the patient cannot perform the task without triggering the abnormal movements, the patient should try to do the task with the unaffected side or with the foot. Well-learned, nearly automatic rhythmic tasks (e.g., walking, skipping, writing, singing) and well-learned complex voluntary tasks (e.g., playing a musical instrument, typing) are mapped on the cortex by both function and geographic location.220 Thus, when retraining, it may help to drive cortical changes by activating the functional map, even if the task is performed by a different extremity.
Biofeedback should be used to increase awareness of the presence of co-contractions. For example, if the electrodes are placed on the extensors of the wrist and fingers while a patient practices bending the finger, the goal would be to stop the co-contractions of the antagonists (extensors). The goal might also be to turn the agonist on or off (e.g., quickly extend the wrist and release it). This might also include retraining the muscles to contract with minimal versus maximal force. The therapist must carefully evaluate whether the agonist drives the firing of the antagonist or the contraction of the antagonist drives the firing of the agonist. It is not uncommon to note a patient trying to flex the finger and involuntarily firing the extensors.
The biofeedback can be sensory (e.g., using tape, pulling the hair, getting into strange positions to provide a sensory trick). It can also be electronic (sensing electrical charges from muscle contractions). Ideally, the patient should be able to take the biofeedback unit home. At home, the patient can become more aware of how the muscles are sequenced in all activities of daily living as well as in the target task. A metronome can be used to progressively increase the speed of the movements. Adjacent fingers may need to be resting down on a surface to keep them still while focusing on the movement of an individual digit.
If a patient continues to have difficulty controlling involuntary movements, mirror training can be used. When the unaffected hand is in front of a mirror, it looks like the affected limb. With the unaffected hand in front of the mirror and the affected hand behind the mirror, the patient looks at the mirror image of the unaffected hand (which looks like the affected hand) and copies this image. The patient can practice picking up objects and putting them down. The patient can hold a pen in both hands and practice writing. A keyboard can be placed on a surface with the mirror placed on the keyboard, and practice takes place on the instrument with mirror feedback (Fig. 135-13).
Figure 135-13 Pictures of mirror imagery. A, The right, unaffected hand is placed in front of the mirror. The mirror image of the right hand looks like the left affected hand. B, The affected and unaffected hands perform the same task simultaneously. The patient watches the mirror images and receives positive mirror feedback that the hand is moving normally.
Trunk and hand posture continues to be important as the patient returns to practicing the target task. Learning to maintain the alignment of the trunk with gravity and keeping the functional position of the hand are critical when retraining on the instrument. This means maintaining the carpal, oblique, and longitudinal arches of the hand; using forearm rotation to decrease the demands for individual finger movements and lifting of the digits; and allowing the instrument interface to open the hand. Pressure on the instrument and stabilization of the hand with the recruitment of the intrinsic muscles are essential. Avoiding adduction of the thumb, excessive ulnar deviation of the wrist, and hyperextension of interphalangeal joints is requisite to set the stage for safe hand use.
To date, three case reports and three intervention studies have been completed to evaluate the effectiveness of this learning-based approach to intervention. These studies provide initial evidence that learning-based sensorimotor re-education can effectively improve task-specific motor control and facilitate the return to work.11,72,144,221-223
In the most recent study of learning-based sensorimotor training,223 13 subjects (16 hands) with hand dystonia (musician’s cramp, writer’s cramp, keyboarder’s cramp) participated in a combination of learning-based practice that included sensorimotor and memory training. Subjects were randomly assigned to a home program of training (with written instructions) or supervised practice before beginning the home program. The subjects who came in for supervised training (1–2 weeks before starting the home program) were more compliant with the home program. Those who were compliant with the training made the greatest gains. All the subjects improved 85% to 90% in their performance at the target task and were able to return to their previous jobs. Some of the musicians had to change their repertoire for successful performance, and one had to remove an instrument from his performance repertoire. One musician continued Braille training up to a year after the study to maintain her musical performance skills. When followed 6 months later, the subjects continued to do well.
The response to learning-based sensorimotor treatment also varies by type of hand dystonia: musician’s cramp versus writer’s cramp. In the study by McKenzie and colleagues,11 there were 14 patients with musician’s cramp and 13 with writer’s cramp. Outcomes relative to physical parameters, somatosensory parameters, and fine-motor parameters were measured. As in other studies, the participants were asked to develop healthful habits (e.g., regular aerobic exercise, good nutrition and hydration, adequate sleep and relaxation) during the retraining. In addition, they were asked to avoid the activities that specifically triggered the dystonia. Each participant worked under supervision of the primary investigator for a month, reinforced with home-based training for 2 hours per day. In the second month, the participants were seen weekly and continued to progress in the home program.
When all the subcategories were combined into the physical, sensory, and motor factors, there were no between-group differences in composite scores at baseline or at 6-month follow-up. However, there were within-factor differences by group. With training, both groups improved significantly in posture, but the improvement was greater for those with musician’s cramp. Both groups improved significantly on functional independence, scoring within the average range for healthy adults. In terms of range of motion, only the musicians significantly improved range of motion. In terms of sensory parameters, both groups improved in kinesthesia, localization, and graphesthesia after treatment. However, the gains in accuracy were greater in kinesthesia for patients with writer’s cramp and greater in terms of localization and graphesthesia for patients with musician’s cramp. Relative to motor control at the target task, both groups improved to 80% of normal. This study provides additional evidence of the effectiveness of learning-based sensorimotor training. However, it reinforces the need to evaluate each individual patient carefully and not only match the treatment but also progress the treatment based on the problems noted.
The major constraint in implementing this paradigm of sensorimotor retraining has been patient compliance, both in terms of taking a break from performing the target task and in performing the goal-directed, specific, repetitive retraining activities. In addition, treatment has been provided within the constraints of the reimbursement of the health care system, limiting the frequency and duration of treatment. A large randomized, controlled clinical trial is essential to objectively validate this approach to treatment.
Braille as a Sensorimotor Retraining Strategy
In patients with writer’s cramp, Zeuner and colleagues74,75,158,224,225 studied the isolated benefits of Braille training in terms of improving writing ability. In these studies, patients were not instructed in general exercise, posture, physical strengthening, or broad-based sensory retraining. Patients trained and practiced Braille reading for 8 weeks, 30 to 60 minutes daily. This was done under supervision. Patients made significant gains and continued to improve for up to 1 year if the patient continued to practice Braille reading as a sensory task.
In another study, Zeuner and colleagues76 used target-specific motor training instead of Braille reading (e.g., writing practice). Focusing on trying to decrease abnormal overflow of movement to fingers not involved in a task, a motor training program was developed for individualized finger movement. Ten patients with writer’s cramp participated in the motor training program. Evaluation was based on the Fahn-Marsden dystonia scale, patient self-report of improvement, kinematic analysis of handwriting, and response to TMS and EEG. Clinical improvement of dystonia was significant (Fahn dystonia scale), and 6 patients reported an improvement in writing. The handwriting analysis showed a trend for improvement in simple exercises after training. There were no changes in cortical excitability measured by TMS or EEG. Thus, Braille training for 4 weeks led to mild subjective improvement and some objective improvements in handwriting, but it was not sufficient to reverse motor cortex abnormalities measured by TMS and EEG.
Zeuner and colleagues224 compared the benefits of general fine-motor retraining and those of task-specific handwriting for patients with writer’s cramp. Subjects were randomly assigned to two types of retraining: one group of patients trained with drawing and writing movements using a pen attached to the bottom of a finger orthosis and the second group used therapeutic putty to train fine-motor finger sagittal and horizontal movements without doing drawing and writing movements. Training lasted for 8 weeks, 30 to 60 minutes daily. Before the retraining started, the affected hand and forearm were immobilized for 4 weeks to facilitate the responsiveness to retraining. Dystonia was assessed during handwriting using the Writer’s Cramp Rating Scale. Although no clinical improvement was observed immediately after immobilization, 8 weeks of retraining was associated with a reduction in task-specific dystonia relative to baseline (P < 0.005). Both training modalities were equally effective. The more severely affected patients benefited most. There were no correlations between disease duration and the individual treatment response. Retraining also improved hand function, as indexed by the Arm Dystonia Disability Scale (ADDS) (P < 0.008). Kinematic handwriting analysis showed that retraining lowered vertical force level and enhanced the fluency of handwriting. They concluded that retraining does not need to specifically focus on the task affected by dystonia to be clinically effective.
Zeuner and colleagues74,75 hypothesized that more than 8 weeks of Braille reading would be needed to restore writing skills. Ten patients with writer’s cramp who had practiced Braille reading for 8 weeks 30 to 60 minutes daily were encouraged to continue Braille practice after the 8 weeks. After 8 weeks, the 10 subjects significantly improved spatial acuity (grating orientation discrimination task) and reduced dystonia severity (Fahn scale). Interestingly, three patients (self-selected) continued with the Braille training for up to 1 year. These 3 subjects showed even further improvements in the grating orientation discrimination task, writing a standard paragraph, and self-rating dystonia scales. Although there was no random assignment and the subjects who decided to continue practice were completely self-selected, it is possible that ongoing sensory Braille training may be a good management strategy for patients with writer’s cramp.
Transcutaneous Electrical Nerve Stimulation
Based on the hypothesis of the presence of sensory dysfunction in patients with focal hand dystonia, transcutaneous electrical nerve stimulation (TENS) is part of the sensory retraining. It is conceivable that TENS could remodel the balance of excitatory and inhibitory relationships in the central nervous system. If activation of large-diameter muscle afferents could restore the balance between agonist and antagonist muscles, then voluntary fine-motor movements at the target task could be enabled. In a randomized, placebo-controlled study including 10 patients with simple writer’s cramp, Tinazzi and colleagues226 documented improvement of motor control in patients with hand dystonia after a 2-week period of TENS treatment. Unfortunately, there is some question about the lasting qualities of this type of sensory retraining (e.g., may be limited to about 3 weeks).
Intensive Practice
It seems that the basic mechanisms of motor learning may be intact in patients with hand dystonia. These individuals achieve exceptional performance with intensive practice.76,158,209 The problem of dystonia may develop as a corruption in learned motor programming. The resolution of the training-induced dysfunction depends on intensive retraining (practice) to correct the imbalance. This type of practice could be general fine-motor practice or task-specific training. In either case, the individual must begin at the level of the impairment and progress to restore function.
Different strategies may fit into more than one classification of retraining. Sensorimotor retuning, for example, is one form of intensive practice. This is also a variant of constraint-induced movement therapy. Individual fingers are trained to perform controlled movements while the other fingers are positioned orthotically to prevent movement.210 Task performance (exciting desired muscle contraction and inhibiting undesired muscle contractions) is practiced until controlled movements are restored. Based on this paradigm, in one research paradigm, pianists experienced significant improvement in dystonic features and cortical redifferentiation.209,210 Similarly, with intensive target-specific and nonspecific target practice (30–40 min/day), Zeuner and colleagues76 reported a significant decrease in the severity of hand dystonia. Pesenti and colleagues227 also explored the benefit of intensive fatigue training. They had patients with hand dystonia repetitively make a strong grip until the patient was completely fatigued. They documented a temporary decrease in the dystonia and improved function. They did not study the effectiveness of repeating this type of training over time.
Handwriting Retraining
In 1994, Mai and Marquardt137,228 developed a handwriting program that focused specifically on retraining handwriting movements. Practice of integrated handwriting motor exercises has been used to facilitate efficient writing with appropriate conditions such as the use of an altered pen, a change in the grip of the pen, and the use of a writing pad.136,137,225,228 This strategy was also applied by Schenk and colleagues.229 These researchers reported that this approach led to permanent improvements in the kinematic handwriting measures.
Later, Baur and colleagues230 studied the direct effect of a modified pen grip (e.g., pen held between the proximal phalanges of the index and middle fingers). This modified pen grip was associated with a significant reduction in writing pressure and grip force but no change in writing performance. Continued practice with alternative pen grips continued to reduce pressure and grip force.69 Unfortunately, these methods did not document a complete reversal of the writing disorder.
Immobilization
Inactivity-dependent neuroplasticity is an alternative approach to remediate the effects of maladaptive plasticity. Inactivity of a limb (no motor movement) can induce effects at multiple levels of the motor system. First, prolonged immobilization can be associated with changes in skeletal muscle properties (e.g., muscle atrophy, strength reduction) as well as bone demineralization and change in ratios of type I and II muscle fibers.
In a group of healthy controls, Hortobagyi and colleagues231 reported a significant reduction of type I and II fibers with an increase in the dysregulation in myosin gene expression after immobilization. It has also been suggested that prolonged reduction in muscular activity could induce a restriction of motor neuron firing to lower firing rates and reduce afferent input. In addition to peripheral and spinal changes, immobilization may reduce the representation of the immobilized part on the motor cortex.
Applying TMS after ankle immobilization, Liepert and colleagues146 found a reduction in the size of the motor cortex representational map of the ankle/foot. Facchini and colleagues232 reported reduced motor evoked potentials (MEPs) as measured by TMS after finger immobilization. Kaneko and colleagues233 also reported reduced MEP amplitudes during motor imagery after arm immobilization. More recently, Huber and colleagues234 reported that only 12 hours of arm immobilization in normal subjects could induce plastic changes in the sensorimotor cortex, including a depression of somatosensory evoked potentials and MEPs. The hypothesis that plastic cortical changes related to immobilization account for the improvement of dystonic phenomena also receives support from the transient improvement measured in patients with focal dystonia after motor fatigue.227 Fatigue transiently reduced and reshaped motor cortical areas, including a reduction in motor output.
To test the hypothesis that immobilization could normalize an abnormally enlarged cortical representation of dystonic muscles and secondarily reduce excessive motor output, Pesenti and colleagues213 and Priori and colleagues235 measured the effects of 4 weeks of immobilization of the hand using a plastic orthosis in eight patients with early-onset idiopathic occupational focal dystonia. After 4 weeks, when the orthosis was removed, a significant objective improvement was measured in the arm dystonia. This reduction in dystonia lasted up to 6 months in seven of the eight patients. After 6 months, three of the patients showed additional moderate improvement (20%–33% from baseline). From baseline performance measures, four patients had markedly improved by 70%.
In a larger sample of patients with focal occupational dystonia (19 patients; 4 affected by writer’s cramp and 15 by musician’s cramp), Pesenti and colleagues213 also found a positive effect of immobilization. In this study, subjects underwent 4 to 6 weeks of orthotic positioning. After 3 months, the patients were instructed to progressively resume activities. In most of the patients (79%), immobilization induced some improvement. A third of the patients noted marked improvement (>75% as quantified by the self-rating scale). None of the patients worsened or had severe side effects. The improvement seemed to peak at 24-week visits after orthosis removal. The patients with a good response to immobilization had more severe dystonia at onset and evidence of overuse, were young, and had significantly shorter disease duration (<5 years). Limb immobilization may be a simple, inexpensive, and safe treatment for early-onset severe focal occupational dystonia of the hand and forearm in selected patients. Although there have been other reports of improvement in motor control in patients with focal dystonia after immobilization,198,236-238 the findings are not consistent and further studies are needed to define the most effective type and duration of orthotic use as well as the type of rehabilitation needed after orthotic positioning. Immobilization could be useful as a preparatory phase for other rehabilitative strategies. Zeuner and colleagues224 immobilized patients before beginning motor retraining using Braille and other fine-motor strategies. They hypothesized that the patient would be more stable before beginning the retraining.
Biofeedback
Biofeedback has generically been used to retrain general and neurophysiologic processing (e.g., decrease muscle spasm in areas of pain, increase muscle contractions in areas of weakness, lower blood pressure and heart rate). In focal dystonia, the goal of biofeedback is to provide information to the patient about the status of muscle contractions. The biofeedback system provides a mechanism to give feedback to a patient about the status of a motor or sensory event. Biofeedback can be used to decrease overexcitation (quiet an agonist muscle) or to quiet an antagonist muscle. Usually the skin-applied electrodes monitor a group of muscle activity. If there are two channels, the aim is to simultaneously train a patient to turn “on” a specific set of muscles or to turn “off” a specific set of muscles. Ideally, the biofeedback system can be set to be more or less sensitive to the underlying firing of the muscles. This type of intervention is usually adjunctive to other retraining strategies.
In focal dystonia, biofeedback may be used to enhance voluntary task performance, smooth fine-motor movements, or change grip force and downward surface pressure in writing. Although there are no randomized, controlled studies on the effectiveness of biofeedback for the restoration of voluntary motor control in patients with focal hand dystonia, there are some case studies supporting the benefit of biofeedback as a supplemental training technique.
Grip Force Biofeedback Training for Patients With Writer’s Cramp
Most patients are unaware of the force that they exert during writing. Sensory dysfunction might contribute to this unawareness. Thus, direct grip force feedback might be very helpful for patients to try to decrease the pressure through voluntary effort. Based on the principles of handwriting retraining by Mai and Marquardt,137 auditory grip force feedback was provided during writing exercises for patients with writer’s cramp.239
In a study by Baur and colleagues,239 seven right-handed subjects with isolated writer’s cramp of the right hand (54.3 ± 18.0 years of age with a mean duration of writer’s cramp of 12.6 ± 16.9 years) with normal touch sensibility (two-point discrimination) participated in force pressure and grip handwriting training based on the principles of neuroplasticity outlined by Mai and Marquardt.136 The goal was to reduce inappropriate writing strategies (decrease pressure on the pen and the desk and overflexion or overextension of the finger joints or wrist). Each individual was evaluated and matched to the appropriate motor exercise. Subjects were instructed to softly bend and stretch the fingers before or interspersed with writing to improve the mobility of finger joints. Subjects were asked to reduce the force of the pen grip. Each subject was also instructed to draw lines quickly from left to right to enhance arm transport. External writing conditions were also changed as necessary to decrease the dystonia (e.g., writing on the lap instead of on the desk or writing with eyes closed). Patients started by writing letters and progressed to words and sentences. Patients participated in 7 hours of training. Five of them trained for 2 weeks and two patients trained for 7 weeks. A conventional pen was used for 50 minutes and a writing stylus was used the last 10 minutes. The writing stylus had sensors to measure grip force and provided auditory grip force feedback. Average grip force during handwriting in healthy controls varies from 5 to 20 N.240 Tone increased with increasing grip force levels. The goal was to hear a pleasant low-frequency tone (Fig. 135-14). The outcomes measured included the Fahn dystonia scale, a scale for subjective writing performance and pain.

Figure 135-14 Grip-force-measuring writing device. (From Baur B, Furholzer W, Marquardt C, Hermsdorfer J. Auditory grip force feedback in the treatment of writer’s cramp. J Hand Ther. 2009;2:163–171.)
Within one session, all patients reduced grip force to less than 20 N (within normal range), and the subjects could do the exercises using a normal grip force level most of the time. The script generally improved in legibility. Writing frequency and fluency did not change significantly after treatment. The mean writing pressure decreased in all the patients with the post-treatment values more than three times smaller than the pretreatment values. The grip force exerted on the pen diminished in all but one patient after training, but the amount of change was not statistically significant. Pain decreased in six of seven subjects, and subjective writing performance improved in all patients. The Fahn scores did not improve significantly during treatment. There was a high negative correlation between the Fahn score and pain before and after training (0.193) and a high positive correlation between subjective performance and the Fahn score after training (0.85).
Based on this small study, the combination of handwriting retraining and biofeedback appears to be a promising strategy. However, further research with a larger sample size, long-term follow-up, and a more detailed analysis of handwriting speed and quality is needed. This retraining could potentially be simulated by putting pressure-sensitive pads on a pen where the force is generated to a computer screen to provide visual feedback.
Deep Brain Stimulation
A few studies have reported on the usefulness of stereotactic thalamotomy for medication-refractory writer’s cramp. Taira and Hori241 obtained satisfactory results in relieving uncontrollable cramping in 12 patients with writer’s cramp using the same procedure. Fukaya and colleagues36 successfully treated five patients with writer’s cramp by thalamic deep brain stimulation (DBS). However, due to the risks of surgical complications and the costs associated with lesional and DBS surgery, these deep brain neurosurgical approaches are limited to select patients with severe focal hand dystonia refractory to other treatment interventions.
TMS
Based on the etiology of maladaptive or aberrant plasticity of the brain creating abnormal sensorimotor integration, enlarged cortical motor representations and dedifferentiated somatosensory representations in patients with focal dystonia,26,67,85,86,195 behavioral interventions should be based on restoring normal sensory and motor function through plasticity processes. The challenge is what type of training strategies should be used (sensory, sensorimotor, motor, or a combination) and how the retraining should be designed to most efficiently and effectively minimize the abnormal movements and recruit and retrain neuronal adaptation.
TMS and tDCS are noninvasive methods to stimulate the brain and to modulate its function.242 TMS is based on the application of a magnetic field outside the scalp to induce an electric field able to excite neurons and to produce action potentials. tDCS consists of applying a weak constant electric current that modulates brain excitability, without inducing the generation of action potentials. rTMS or tDCS can be applied over selected cortical regions to modulate specific cortical–subcortical networks.
Several TMS studies have investigated the balance between inhibitory and excitatory systems within the motor cortex in patients with dystonia. This type of stimulation was based on the hypothesis that reduction in the inhibitory mechanism might ultimately result in cortical overactivity.130,242 Even though hyperexcitability of the motor cortex in focal hand dystonia may be interpreted as a phenomenon secondary to different pathophysiologic mechanisms (i.e., abnormal sensorimotor integration, maladaptive motor plasticity), its down-regulation should be considered a suitable target to improve dystonia.
In 1997, Chen and colleagues201,243 studied the effects of low-frequency TMS in terms of motor cortex excitability. Given documentation of impaired inhibition in patients with writer’s cramp during voluntary muscle activation, low-frequency rTMS over the lateral PMC induced lasting changes in regional activation and function.201,243 Repeated paired associative stimulation of the median nerve followed by 20 to 25 msec of TMS of the hand area of the motor cortex has been shown to lead to a prolonged increase in the size of MEPs in median nerve–innervated hand muscles. A similar finding was reported in patients with focal hand dystonia, even though there is less focal facilitation than in normal subjects.81 The effects of rTMS on cortical excitability depend on the frequency and intensity of the stimulation. Low-frequency (<1 Hz) rTMS decreases cortical excitability, whereas high-frequency (>5 Hz) rTMS increases cortical excitability.
With the aim of increasing inhibition in the motor areas of the cerebral cortex, the therapeutic trials performed with patients with focal hand dystonia used low-frequency rTMS over the PMC (M1) and premotor cortex (PM). Siebner and colleagues244 evaluated the effect of low-frequency (1 Hz) stimulation of the PMC in 16 patients with writer’s cramp. The intensity of stimulation was set at 10% below the resting motor threshold of the right first dorsal interosseous muscle. Low-frequency rTMS (30 minutes, 1800 pulses) restored normal intracortical inhibition. Moreover, after rTMS, six patients noted a marked improvement of handwriting lasting for more than 3 hours. In two of these patients, the improvement persisted for several days. Even though the PMC was the first rTMS target to be stimulated with the aim to down-regulate cortical excitability, it was recently reported that rTMS over the dorsal PMC could reduce intracortical inhibition more than rTMS over the motor cortex.245 In a group of nine patients with writer’s cramp, Murase and colleagues77 compared the clinical effect of 20 minutes of 0.2-Hz rTMS over the PMC, the supplementary motor cortex (SMC) and the PMC plus the SMC. These researchers reported that the combination of stimulation of the PMC and SMC was the best for obtaining a therapeutic effect, with 78% of patients reporting a significant improvement in their symptoms (compared with only 37% after PMC stimulation, 56% after supplementary motor cortex stimulation, and 11% after sham rTMS). Tyvaert and colleagues246 demonstrated significant improvement in handwriting up to 40 minutes after 30 minutes of 1-Hz rTMS over the PMC in eight patients with writer’s cramp.
None of these studies reported any adverse events related to magnetic stimulation. Nevertheless, the clinical use of rTMS for treatment of focal hand dystonia is limited. Performance improvements reported are moderate in magnitude and somewhat transient. Further research is needed to determine how to make the changes more permanent.
Another form of stimulation is transcutaneous deep cortical stimulation (tDCS). This type of stimulation is an alternative to rTMS to modulate cortical excitability in patients with focal hand dystonia. However, clinically, this type of stimulation has not been performed in patients specifically with dystonia. Rather, tDCS has elicited encouraging results in other patients with hyperkinetic movement disorders. Depending on the polarity, strength, and duration of stimulation, tDCS can up-regulate or down-regulate excitability of the underlying brain areas by synaptic and nonsynaptic mechanisms.247,248 tDCS may also have an important role to play to help prime the effects of rTMS.249
TMS and tDCS are noninvasive interventions that may be beneficial adjunctive therapy for patients with hand dystonia. Future controlled studies are needed to determine how rTMS and tDCS can modify cortical processing in patients with hand dystonia and what the appropriate rehabilitative treatment strategy needs to be for restoring task-specific function in patients with focal hand dystonia.
Thalamotomy
A more invasive approach to treating musicians with dystonia has been used in Japan, where botulinum toxin injection is not available. Thalamotomy of the ventralis oralis anterior thalamus contralateral to the affected hand has been performed in patients with musician’s cramp with surprisingly good results.241 In the United States, some patients with severe musician’s cramp have been treated neurosurgically (e.g., lesioning of the thalamus or DBS). As a general rule, these approaches are more commonly reserved for patients with severe generalized or cervical dystonia.
Summary
Focal hand dystonia can be a very disabling condition. At this time, there is no consensus regarding the etiology of focal hand dystonia. It seems to be a condition that develops as an accumulation of multifactorial risk factors ranging from endophenotypic to exophenotypic factors. There is increasing evidence that patients with focal hand dystonia have problems balancing excitation and inhibition as well as aberrant homeostatic plasticity. These inherent neurophysiologic processing problems involve the cortex, the basal ganglia, and the thalamus. Patients with focal hand dystonia have measurable dedifferentiation of the hand representation in the somatosensory, sensorimotor, and premotor cortices. The presence of innate underlying neurophysiologic dysfunction in homeostatic plasticity, sensory processing, and inhibition may put individuals at risk for the development of focal hand dystonia under conditions of stress, repetition, trauma, or perfection. The objective of intervention must be to control stress, restore fitness and wellness, and create a balance between excitation and inhibition and good ergonomics and biomechanics. The objective of retraining must be to restore normal differentiation of cortical topography, enhance the quality and accuracy of sensorimotor feedback, and enable efficient and effective performance of motor pathways. A combination of medications, education, counseling, brain retraining, and sometimes brain stimulation is currently used to manage this disorder. Research is still needed to more clearly understand the etiology of the disorder and clarify the best integrative training strategies.
REFERENCES
1. Fahn S, Marsden CD, Calne D. Classification and investigation of dystonia. Marsden CD, Fahn S, eds. Movement Disorders.Vol 2. London: Butterworth; 1987.
2. Jabusch HC, Altenmuller E. Anxiety as an aggravating factor during onset of focal dystonia in musicians. Med Probl Perform Art. 2004;192:75.
3. Utti R, Vingerhoets FJG, Tsui JKC. Limb dystonia. In: Tsui JKC, Calne DB, eds. Handbook of Dystonia. New York: Marcel Dekker; 1995.
4. Jabusch HC, Altenmuller E. Epidemiology, phenomenology and therapy of musician’s cramp. In: Altenmuller E, Kesselring J, Wiesendanger M, eds. Music, Motor Control and the Brain. Oxford: Oxford University Press; 2006:265–282.
5. Marsden CD, Sheehy MP. Writer’s cramp. Trends Neurosci. 1990;13:148. (review)
6. Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108:463–483.
7. McDaniel KD, Cummings JL, Shain S. The “yips”: a focal dystonia of golfers. Neurology. 1989;39:192–195.
8. Newmark J, Hochberg F. Isolated painless manual incoordination in musicians. J Neurol Neurosurg Psychiatry. 1987;50:291–295.
9. Bell C. The Hand: Its Mechanism and Vital Endowments as Evincing Design. London: William Pickering; 1883.
10. Duchenne de Bologne GB. Spasme fonctionel et paralysies musculaires fonctionelles. In: L’Electrisation Localisee. Paris: Balliere; 1861.
11. McKenzie AL, Goldman S, Barango C, et al. Differences in physical characteristics and response to rehabilitation for patients with hand dystonia: musician’s cramp compared to writers’ cramp. J Hand Ther. 2009;22:172–181.
12. Soland VL, Bhatia KP, Marsden CD. Sex prevalence of focal dystonia. J Neurol Neurosurg Psychiatry. 1996;60:204–205.
13. Sheehy M, Marsden CD. Writer’s cramp: a focal dystonia. Brain. 1982;105:461–480.
14. Sheehy MP, Rothwell JC, Marsden CD. Writer’s cramp. Adv Neurol. 1988;50:457–472.
15. Jankovic J, Fahn S. Dystonic disorders. In: Jankovic J, Tolosa E, eds. Parkinson’s Disease and Movement Disorders. Baltimore: Williams & Wilkins; 1993.
16. Jankovic J, Shale H. Dystonia in musicians. Semin Neurol. 1989;9:131–135.
17. Wilson F. Digitizing digital dexterity: a novel application for MIDI recordings of keyboard performance. Psychomusicology. 1992;11:79–95.
18. Lim VK, Altenmuller E, Bradshaw JL. Focal dystonia: current theories. Hum Mov Sci. 2001;20:875–914.
19. Lin PT, Shamim EA, Hallett M. Focal hand dystonia. Pract Neurol. 2006;6:278–287.
20. Lin PT, Hallett M. The pathophysiology of focal hand dystonia. J Hand Ther. 2009;22:109–113.
21. Rhoad R, Stern P. Writer’s cramp: a focal dystonia–etiology, diagnosis, and treatment. J Hand Surg Am. 1993;18:541–544.
22. Rothwell J, Obeso JA, Day BL, Marsden CD. Pathophysiology of dystonias. Adv Neurol. 1983;39:851–863.
23. Dhaenens CM, Krystkowiak P, Doury X, et al. Clinical and genetic characteristics in a French population presenting with primary focal dystonia. Mov Dis. 2005;20:822–825.
24. Illarioshkin N, Markova ED, Slominsky PA, et al. The GTP cyclohydrolase I gene in Russian families with dopa-responsive dystonia. Arch Neurol. 1998;55:789–792.
25. Levangie PK, Norkin CC. Joint Structure and Function: A Comprehensive Analysis. Chapter 9. 2nd ed Philadelphia: FA Davis; 1992.
26. Odergren T, Iwasaki N, Borg J, Forssberg H. Impaired sensory motor integration during grasping in writer’s cramp. Brain. 1996;119:569–583.
27. Ozelius LJ, Hewett JW, Page CE, et al. The early onset of torsion dystonia gene (dyt1) encodes an ATD-binding protein nature. Genetics. 1997;17:40.
28. Black KJ, Ongur D, Perlmutter JS. Putamen volume in idiopathic focal dystonia. Neurology. 1998;51:819–824.
29. Meunier S, Hallett M. Endophenotyping: a window to the pathophysiology of dystonia. Neurology. 2007;65:792–793.
30. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285.
31. Leube B, Rudnicki D, Ratzlaff T, et al. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1997;5:1673–1677.
32. Gasser T, Bove CM, Ozelius LJ, et al. Haplotype analysis at the DYT1 locus in Ashkenazi Jewish patients with occupational hand dystonia. Mov Disord. 1996;11:163–166.
33. Tempel L, Perlmutter JS. Abnormal cortical responses in patients with writer’s cramp. Neurology. 1993;43:2252–2257.
34. Hallett M. Physiology of dystonia. Adv Neurol. 1998;78:11.
35. Vitek JL, Zhang J, Evatt M, et al. GPi pallidotomy for dystonia: clinical outcome and neuronal activity. Adv Neurol. 1998;78:211–219.
36. Fukaya C, Katayama Y, Kano T, et al. Thalamic deep brain stimulation for writer’s cramp. J Neurosurg. 2007;104:977–982.
37. Karbe H, Holthoff VA, Rudolf J, et al. Positron emission tomography demonstrates frontal cortex and basal ganglia hypometabolism in dystonia. Neurology. 1992;42:1540–1544.
38. DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543.
39. Lozano A, Kumar R, Gross RE, et al. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord. 1997;12:865–870.
40. Perlmutter JS, Stambuk MK, Markham J, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843–850.
41. Wichmann T, Bergman H, Starr PA, et al. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409.
42. Ghilardi MF, Carbon M, Silvestri G, et al. Impaired sequence learning in carriers of the DYTI dystonia mutation. Ann Neurol. 2003;54:102–109.
43. Chen R, Tsai C, Lu C. Reciprocal inhibition in writer’s cramp. Mov Disord. 1995;10:556–561.
44. Crossman AR, Brotchie JM. Pathophysiology of dystonia. Adv Neurol. 1998;78:19–25.
45. Nakashima K, Rothwell JC, Day BL, et al. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain. 1969;112:681–697.
46. Panizza M, Hallett M, Nilsson J. Reciprocal inhibition in patients with hand cramps. Neurology. 1989;39:85–89.
47. Cohen L, Hallet M. Hand cramps: clinical features and electromyographic patterns in focal dystonia. Neurology. 1988;38:1005–1012.
48. Goetz C, Penn R, Tanner C. Efficacy of cervical cord stimulation in dystonia. Adv Neurol. 1988;50:645–649.
49. Naumann M, Becker G, Toyka KV, et al. Lenticular nucleus lesion in idiopathic dystonia detected by transcranial sonography. Neurology. 1996;47:1284–1290.
50. Ridding MC, Sheean G, Rothwell JC, et al. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–498.
51. Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–140.
52. Hallett M. Pathophysiology of writer’s cramp. Hum Mov Sci. 2006;225:454–463.
53. Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2001;56:529–537.
54. Cimatti A, Schwartz DP, Pourdain F, et al. Time-frequency analysis reveals decreased high frequency oscillations in writer’s cramp. Brain. 2007;130:198–205.
55. Eidelberg D, Moeller JR, Ishikawa T, et al. The metabolic topography of idiopathic torsion dystonia. Brain. 1995;118:1473–1484.
56. Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol. 2002;51:93–101.
57. Leijnse JN. Anatomical factors predisposing to focal dystonia in the musician’s hand: principles, theoretical examples, clinical significance. J Biomech. 1997;30:659–669.
58. Leijinse JM. Anatomical factors predisposing to focal dystonia in the musician’s hand—principles, theoretical examples, clinical significance. J Biomech. 1997;30:659–669.
59. Leijinse JW, Hallett M. Etiological musculo-skeletal factors in focal dystonia in a musician’s hand: a case study of the right hand of a guitarist. Mov Dis. 2007;22:1803–1808.
60. Wilson F, Wagner C, Homberg V. Biomechanical abnormalities in musicians with occupational cramp/focal dystonia. J Hand Ther. 1993;6:298–307.
61. Wilson F, Wagner C, Hömberg V, Noth J. Interaction of biomechanical and training factors in musicians with occupational cramps/focal dystonia. Neurology. 1991;4:291–292.
62. Topp KS, Byl NN. Movement dysfunction following repetitive hand opening and closing: anatomical analysis in owl monkeys. Mov Disord. 1999;14:295–306.
63. Hamano T, Kaji R, Katayama M, et al. Abnormal contingent native variations in writer’s cramp. Clin Neurophysiol. 1999;2230:508–515.
64. Toro C, Deuschl G, Hallett M. Movement related electroencephalographic desynchronization in patients with hand cramps: evidence for motor cortical involvement in focal dystonia. Ann Neurol. 2000;47:456–461.
65. Ibanex V, Sadata N, Karp B, et al. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53:96–105.
66. Bugalho P, Correa B, Guimaraes J, Xavier M. Set-shifting and behavioral dysfunction in primary focal dystonia. Mov Disord. 2008;23:200–206.
67. Bara-Jimenez W, Catalan M, Hallett M. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44:828–831.
68. Bara-Jimenez S, Shelton P, Sanger TD, Hallett J. Sensory discrimination capabilities in patients with focal hand dystonia. Ann Neurol. 2000;47:377–380.
69. Bara-Jimenez W, Shelton P, Hallett M. Spatial discrimination is abnormal in focal hand dystonia. Neurology. 2000;55:1869–1873.
70. Kaji R, Rothwell JC, Katayama M, et al. Tonic vibration reflex and muscle afferent block in writer’s cramp. Ann Neurol. 1995;38:155–162.
71. Byl NN, Wilson F, Merzenich M, et al. Sensory dysfunction associated with repetitive strain injuries of tendinitis and focal hand dystonia: a comparative study. J Orthop Sports Phys Ther. 1996;23:234–244.
72. Byl NN, Topp KS. Focal hand dystonia. Phys Ther Case Rep. 1998;1:39–52.
73. Byl NN, McKenzie A, Nagarajan SS. Differences in somatosensory hand organization in a healthy flutist and a flutist with focal hand dystonia: a case report. J Hand Ther. 2000;13:302–309.
74. Zeuner KE, Bara-Jimenez M, Naguchi PS, et al. Sensory training for patients with focal hand dystonia. Ann Neurol. 2002;51:593–598.
75. Zeuner KE, Hallett M. Sensory training as treatment for focal dystonia: a one year follow up. Mov Disord. 2003;18:1044.
76. Zeuner KE, Shill HA, Sohn YH, et al. Motor training as treatment in focal hand dystonia. Mov Disord. 2005;20:335.
77. Murase N, Rothwell JC, Kaji R, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain. 2005;128(1):104–115.
78. Nowak DA, Rosenkranz K, Topka H, Rothwell J. Disturbances of grip force behavior in focal hand dystonia: evidence for a generalized impairment of sensory-motor integration? J Neurol Neurosurg Psychiatry. 2005;76:953–959.
79. Molloy FM, Carr TD, Zeuner KE, et al. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182.
80. Tinazzi M, Rosso T, Fiaschi A. Role of the somatosensory system in primary dystonia. Mov Disord. 2003;18:605–622.
81. Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain. 2003;126:2586–2596.
82. Quartarone A, Rizzo V, Bagnato S, et al. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain. 2005;128:1943–1950.
83. Quartarone A, Morgante F, Sant’angelo A, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79(9):985–990.
84. Barbe MF, Barr AE, Gorzelang I, Arnin M, Gauglan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of RSI. J Ortho Res. 2003;21:167–176.
85. Byl NN, Merzenich M, Jenkins W. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced de-differentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Ann Neurol. 1996;47:508–520.
86. Byl NN, Merzenich MM, Cheung S, et al. A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther. 1997;77:269–284.
87. Coq JO, Barr AE, Strata F, et al. Peripheral and central changes combine to induce motor behavioral deficits in a moderate repetitive task. Exp Neurol. 2009 Dec;220(2):234–245.
88. Weise D, Schramm A, Stefan K, et al. The two sides of associative plasticity in writer’s cramp. Brain. 2006;129(10):2709–2721.
89. Jenkins W, Allard T, Nudo R. Cortical representational plasticity. In: Raskic P, Singer W, eds. Neurobiology of the Neocortex. New York: John Wiley & Sons; 1988.
90. Gould G. Unpublished diary, 1977, Glenn Gould Papers, Music Division, National Library of Canada (permission to quote from the estate).
91. Jenkins W, Merzenich M. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–266.
92. Juliano SL, Ma W, Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci USA. 1991;88:780–784.
93. Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356.
94. Byl N. Learning-based animal models: Task-specific focal hand dystonia. ILAR J. 2007;48:411–431.
95. Jenkins W, Merzenich MM, Ochs MT, et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104.
96. Merzenich MM. Development and maintenance of cortical somatosensory representations: functional “maps” and neuroanatomical repertoires. In: Barnard KE, Brazelton TB, eds. Touch: the Foundation of Experience. Madison, CT: International Universities Press; 1991.
97. Merzenich M. Cognitive neuroscience: seeing in the sound zone. Nature. 2000;404:820–821.
98. Merzenich MM, Allard T, Jenkins WM. Neural ontogeny of higher brain function: implications of some recent neurophysiological findings. In: Franzen O, Westman P, eds. Information Processing in the Somatosensory System. London: Macmillan Press; 1991.
99. Merzenich MM, DeCharms RC. Neural representations, experience and change. In: Llinas R, Churchland P, eds. The Mind-Brain Continuum. Boston: MIT Press; 1995.
100. Merzenich MM, Jenkins WM. Cortical representation of learned behaviors. In: Andersen P, ed. Memory Concepts. Amsterdam: Elsevier; 1993.
101. Merzenich MM, Jenkins WM. Cortical plasticity, learning and learning dysfunction. In: Jules B, Kovacs I, eds. Maturational Windows and Adult Cortical Plasticity. New York: Addison-Wesley; 1995.
102. Merzenich MM, Kaas JH, Wall JT, et al. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 2 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665.
103. Merzenich MM, Kaas JH, Wall J, et al. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55.
104. Merzenich MM, Nelson RJ, Stryker MP, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605.
105. Recanzone G, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol. 1992;67:1015–1030.
106. Recanzone GH, Merzenich MM, Jenkins WM, et al. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056.
107. Gerloff C, Corwell B, Chen R, et al. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998;121:1695–1709.
108. Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116.
109. Byl NN. What can we learn from animal models of focal hand dystonia. Rev Neurol (Paris). 2003 Oct;159(10 Pt 1):857–873.
110. Byl NN. Focal hand dystonia may result from aberrant neuroplasticity. Ann Neurology. 2004;94:19–28.
111. Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurol. 1997;17:851–861.
112. Elliot MD, Barr AE, Clark BD, et al. High force reaching task induces widespread inflammation, increased spinal cord neurochemical and neuropathic pain. Neuroscience. 2009;158:922–931.
113. Braun C, Heinz U, Schweizer R, et al. Dynamic organization of the somatosensory cortex induced by motor activity. Brain. 2001;124:2259–2267.
114. Sanger TD, Merzenich MM. A computational model of the role of sensory representations in focal dystonia. J Neurophysiol. 2000;84:2458–2464.
115. Katz R, Williams C. Focal dystonia following soft tissue injury: three case reports with long-term outcomes. Arch Phys Med Rehabil. 1990;71:345–349.
116. Quartarone A, Girlanda P, Risitano G, et al. Focal hand dystonia in a patient with thoracic outlet syndrome. J Neurol Neurosurg Psychiatry. 1998;65:272–274.
117. Charness ME. Unique upper extremity disorders of musicians. In: Millender LH, Louis DS, Simmons BP, eds. Occupational Disorders of the Upper Extremity. New York: Churchill Livingstone; 1992.
118. Charness M. The relationship between peripheral nerve injury and focal dystonia in musicians. Am Acad Neurol. 1993;162:21–27.
119. Grafman J, Cohen L, Hallett M. Is focal hand dystonia associated with psychopathology? Mov Disord. 1991;6:29–35.
120. Altenmueller E, Jabusch H-C. Focal hand dystonia in musicians: phenomenology, etiology, and psychological trigger factors. J Hand Ther. 2009;22:144–154.
121. Jabusch HC, Muller SV, Altenmuller E. Anxiety in musicians with focal dystonia and those with chronic pain. Mov Disord. 2004;19:1169–1175.
122. Critchley M. Occupational palsies in musical performers. In: Critchley M, Henson RA, eds. Music and the Brain. London: Heinemann Medical Books; 1977.
123. Hays B. “Painless” hand problems of string pluckers. Med Probd Perform Art. 1987;2:39.
124. Altenmueller E. Causes and cures of focal limb dystonia in musicians. Proceedings of the 1997 York conference In: Scott R, Black J, eds. Health and the Musician. London: BAPA Publications; 1997.
125. Frucht SJ. Focal task-specific dystonia in musicians. Adv Neurol. 2004;94:225–230.
126. Frucht SJ. Focal task specific dystonia of the musicians’ hand—a practical approach for the clinician. J Hand Ther. 2009;22:136–142.
127. Butz M, Timmermann L, Gross J, et al. Oscillatory coupling in writing and writer’s cramp. J Physiol. 2006;99:14–20.
128. Rosenkranz K, Williamon A, Butler K, et al. Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain. 2005;128:918–931.
129. Rosenkranz K, Butler K, Williamon A, et al. Sensorimotor reorganization by proprioceptive training in musician’s dystonia and writer’s cramp. Neurology. 2008;70:304–315.
130. Rona S, Berardelli A, Vacca L, et al. Alterations of motor cortical inhibition in patients with dystonia. Mov Disord. 1998;13:118–124.
131. Fahn W. Assessment of the primary dystonias. In: Munsat TL, ed. Quantification of Neurologic Deficit. Boston: Butterworths; 1989:241–270.
132. Wagner C. Determination of finger flexibility. Eur J Appl Physiol. 1974;32:259–278.
133. Frommer J. Idiopathic writing cramp as a psychosomatic disease: a qualitative analysis of three case reports. Z Psychosom Med Psychoanal. 1992;38:49–62.
134. Umphrod D, Byl N, Rollor P, Lazaro L. Intervention for patients with neurological disease. In: Umphred DA, ed. Neurological Rehabilitation. 5th ed St. Louis, MO: Mosby Elsevier; 2007.
135. Hochberg F, Harris S, Blattert T. Occupational hand cramps: professional disorders of motor control. Hand Clin. 1990;6:417–428.
136. Mai N, Marquardt C. Treatment of writer’s cramp: kinematic measures as an assessment tool for planning and evaluating training procedures. In: Faure C, Keuss P, Lorette G, Vinter A, eds. Advances in Handwriting and Drawing: A Multidisciplinary Approach. Paris: Europia; 1994:445–461.
137. Mai N, Marquardt C. Schreibtraining in der neurologischen Rehabilitation. In: Mai N, Ziegler W, Kerkhoff G, Toppmann N, eds. EKN-Materialien fur die Rehabilitation, Dortmund. Germany: Borgmann; 1999.
138. Sanger TD, Delgado MR, Gaebler-Spira D, et al. Task Force on Childhood Motor Disorders, Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):89–97.
139. Goldman SB, Brininger TL, Antczak A. Clinical relevance of neuromuscular findings and abnormal movement patterns: A comparison between focal hand dystonia and upper extremity entrapment neuropathies. J Hand Ther. 2009;22:115–123.
140. Dellon A. Somatosensory Testing and Rehabilitation. Bethesda, MD: American Occupational Therapy Association; 1997.
141. Sanger TD, Tarsy D, Pascual-Leone A. Abnormalities of spatial and temporal sensory discrimination in writer’s cramp. Mov Dis. 2001;16:94–99.
142. Tinazzi M, Frasson E, Bertolasi L, et al. Temporal discrimination of somesthetic stimuli is impaired in dystonic patients. Neuroreport. 1999;10:1547–1550.
143. Grunewald F, Yoneda Y, Shipman JM, Sagar HJ. Idiopathic focal dystonia is a disorder of muscle afferent processing. Brain. 1997;120:2179–2185.
144. Byl NN, McKenzie A. Treatment effectiveness of patients with a history of repetitive hand use and focal hand dystonia: a planned, prospective follow-up study. J Hand Ther. 2000;13:289–301.
145. Ikeda A, Shibasaki H, Kaji R, et al. Abnormal sensorimotor integration in writer’s cramp: study of contingent negative variation. Mov Disord. 1999;17:683–690.
146. Liepert J, Tegenhoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386.
147. Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol. 1992;67:1057–1070.
148. Chen R, Hallett M. Focal dystonia and repetitive motion disorders. Clin Orthop. 1998;351:102–106.
149. Fry H. Overuse syndromes in musicians 100 years ago: an historical review. Med J Australia. 1986;146:620–625.
150. Byl NN, Hamati D, Wilson F, et al. The sensory consequences of repetitive strain injury in musicians: focal dystonia of the hand. J Back Musculoskel Rehabil. 1996;7:27–39.
151. Carlsson AM. Assessment of chronic pain: I–aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16:87–101.
152. Shimokata H, Kuzuya F. Two-point discrimination test of the skin as an index of sensory aging. Gerontology. 1995;42:267–272.
153. Ayres J. Sensory Integration Praxis Test. Los Angeles: Western Psychological Association; 1989.
154. Von Reis G. Electromyographical studies in writer’s cramp. Acta Med Scand. 1954;149:253–260.
155. Tubiana R, Chamagne P. Les affecions professionnelles du member superior chez les musiciaens. Bull Acad Natl Med. 1993;177:203–216.
156. Tubiana R. Incidence: classification of severity and results of therapy. In: Winspur I, Parry CBW, eds. The Musician’s Hand. London: Martin Dunitz; 1998.
157. Zeuner KE, Peller M, Knutzen A, et al. How to assess motor impairment in writer’s cramp. Mov Disord. 2007;22(8):1102.
158. Zeuner KE, Molloy FM. Abnormal reorganization in focal hand dystonia: sensory and motor training programs to retrain cortical function. NeuroRehabilitation. 2008;23:43–53.
159. Windgassen K, Ludoph A. Psychiatric aspects of writer’s cramp: Eur Arch Psychiatry. Clin Neurosci. 1991;241:170–176.
160. Harrington RC, Wieck A, Marks IM, Marsden CD. Writer’s cramp: not associated with anxiety. Mov Disorder. 1988;3:195.
161. Breakefield XO, Blood AJ, Li Y, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234.
162. Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;230:1183–1193.
163. Chase T, Tamminga C, Burrows H. Positron emission tomographic studies of regional cerebral glucose metabolism in idiopathic dystonia. Adv Neurol. 1988;50:237–241.
164. Rutledge JN, Hilal SK, Silver AJ, et al. Magnetic resonance imaging of dystonic states. Adv Neurol. 1988;50:265–275.
165. Deuschl G, Toro C, Matsumoto J, Hallett M. Movement-related cortical potentials in writer’s cramp. Ann Neurol. 1995;38:862–868.
166. Tecce JJ, Cattanach L. Contingent negative variation. In: Niedermeyer L, da Silva F, eds. Electroencephalography Related Fields. Baltimore: Williams & Wilkins; 1993.
167. Tubiana R, Amadio PC. Medical Problems of the Instrumentalist Musician. London: Martin Dunitz; 2000.
168. Blood AJ, Flaherty AW, Choi JK, et al. Basal ganglia activity remains elevated after movement in focal hand dystonia. Ann Neurol. 2004;55:744–748.
169. Oberman M, Yaldizli O, de Greiff A, et al. Increased basal-ganglia activation performing a non-dystonia related task in focal dystonia. Eur J Neurol. 2008;8:831–838.
170. Roberts TPL, Poeppel D, Rowley HA. Magnetoencephalography and magnetic source imaging. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:49–64.
171. Rowley HA, Roberts TPL. Functional localization by magnetoencephalography. Neuroimaging Clin N Am. 1995;5:695–710.
172. Hinkley LBN, Webster R, Byl NN, Nagarajan SS. Neuroimaging characterstics of patients with focal hand dystonia. J Hand Ther. 2009;22:125–134.
173. McKenzie A, Nagarajan S, Roberts TP, Merzenich MM, Byl NN. Somatosensory representation of the digits and clinical performance in patients with focal hand dystonia. Am J Phys Med Rehabil. 2003;82(10):737–749.
174. Elbert T, Pantev C, Wienbruch C, et al. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307.
175. Elbert T, Candia V, Altenmüller E, et al. Alternation of digital representation in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9:3571–3575.
176. McKenzie AL, Nagarajan SS, Byl N, et al. Somatosensory representation of the digits and performance: patients with focal hand dystonia. Abstract and podium presentation at the annual meeting of the California chapter of the American Physical Therapy Association, October 1999, Palm Springs, California.
177. Lerner A, Shill H, Hanakawa T, et al. Regional cerebral blood flow correlates of the severity of writer’s cramp symptoms. Neuroimage. 2004;32:904–913.
178. Blake DT, Cheung S, Bedenbaugh P, et al. Sensory representation abnormalities that parallel focal hand dystonia in a primate model. Somatosens Mot Res. 2002;19:347–357.
179. Blake D, Byl N, Merzenich M. The owl monkey model of focal dystonia. In: LeDoux M, ed. Animal Models of Movement Disorders. New York: Lippincott Publishers; 2005.
180. Oga T, Honda M, Toma K, et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer’s cramp: an fMRI study. Brain. 2002;125(4):895–903.
181. Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb Cortex. 2004;14:555–561.
182. Gilman S, Junck L, Young A, et al. Cerebral metabolic activity in idiopathic dystonia studied with positron emission tomography. Adv Neurol. 1988;50:231–236.
183. Godschalk M, Lemon RN, Nijs HG, Kuypers HG. Behaviour of neurons in monkey peri-arcuate and precentral cortex before and during visually guided arm and hand movements. Exp Brain Res. 1981;44:113–116.
184. Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501.
185. Chamagne P. Functional assessment and rehabilitation of musician’s focal dystonia. In: Tubiana R, Amadio PC, eds. Medical Problems of the Instrumentalist Musician. London: Martin Dunitz; 2000.
186. Markison RE. Treatment of musical hand: redesign of the interface. Hand Clin. 1990;6:525–544.
187. Meagher SW. Tool designing for prevention of hand and wrist injuries. J Hand Surg. 1987;12A:855–877.
188. Karp BI. Botulinum toxin treatment of occupational and focal hand dystonia. Mov Disord. 2004;19(S8):116.
189. Schuele S, Jabusch HC, Lederman RJ, Altenmuller E. Botulinum toxin injections in the treatment of musician’s dystonia. Neurology. 2005;64:341–343.
190. Cole R, Hallett M, Cohen L. Double-blind trial of botulinum toxin for treatment of focal hand dystonia. Mov Disord. 1995;10:466–471.
191. Pullman S, Greene P, Fahn S, Pedersen SF. Approach to the treatment of limb disorders with botulinum toxin A. Arch Neurol. 1996;53:617–624.
192. Ross MH, Charness ME, Sudarsky L, Logigian EL. Treatment of occupational cramp with botulinum toxin: diffusion of toxin to adjacent noninjected muscles. Muscle Nerve. 1997;20:593–598.
193. Tsui J, Bhatt M, Calne S, Calne DB. Botulinum toxin in the treatment of writer’s cramp: a double-blind study. Neurology. 1993;43:183–185.
194. Van den Bergh P, Francart J, Mourin S, et al. Five-year experience in the treatment of focal movement disorders with low-dose Dysport botulinum toxin. Muscle Nerve. 1995;18:720–729.
195. Byrnes ML, Thickbroom GW, Wilson SA, et al. The corticomotor representation of upper limb muscles in writer’s cramp and changes following botulinum toxin injection. Brain. 1998;121:977–988.
196. Ceballos-Baumann AO, Sheean G, Passingham RE, et al. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp: a PET study. Brain. 1997;120:571–582.
197. Gilo F, Curra A, Lorenzano C, Modugno, et al. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol. 2000;48:20–26.
198. Schuele S, Lederman RJ. Long term outcome of focal dystonia in string instrumentalists. Mov Disord. 2004;19:43.
199. Pascual-Leone A, Nguyen D, Cohen LG. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045.
200. Chen R, Wassermann E, Canos M, Hallett M. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059.
201. Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve. 2000;999:S26–S32.
202. Tubiana R, Chamagne P. Occupational “cramps” of the upper limb. Ann Chir Main. 1983;2:134–142.
203. Liversedge LA, Sylvester JD. Conditioning techniques in the treatment of writer’s cramp. Lancet. 1955;4:1147–1149.
204. Taub E. Somatosensory deafferentation research with monkeys: implications for rehabilitation medicine. In: Ince LP, ed. Behavioral Psychology in Rehabilitation Medicine: Clinical Applications. Baltimore: Williams & Wilkins; 1980.
205. Taub E, Crago JE, Uswatte G. Commentary: constraint-induced movement therapy—a new approach to treatment in physical rehabilitation. Rehabil Psychol. 1998;43:152–170.
206. Taub E, Miller NE, Novack TA, et al. Technique to improve motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354.
207. Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132.
208. Candia V, Elbert T, Altenmüller E, et al. Constraint-induced movement therapy for focal hand dystonia in musicians. Lancet. 1999;353:42.
209. Candia V, Schafer T, Taub E, et al. Sensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch Phys Med Rehabil. 2002;83:1342–1348.
210. Candia V, Rosset-Liobet J, Elbert T, Pascual-Leone A. Changing the brain through therapy for musicians’ hand dystonia. Ann N Y Acad Sci. 2005;1060:335–342.
211. Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digit syndactyly. J Neurophysiol. 1991;66:1048–1058.
212. Nagarajan SS, Blake DT, Wright BA, et al. Practice-related improvements in somatosensory integral discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci. 1999;18:1559–1570.
213. Pesenti A, Barbierri S, Priori A. Limb immobilization for occupational dystonia: a possible alternative treatment for selected patients. Adv Neurol. 2004;94:247–254.
214. Wang X, Merzenich MM, Sameshima K, et al. Afferent input integration and segregation in learning are input timing dependent. Neurosci Abstr. 1994;20:1427.
215. Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodeling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75.
216. Xerri C, Merzenich MM, Jenkins W, Santucci S. Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex. 1999;9:264–276.
217. Byl N, Priori R. The development of focal dystonia in musicians as a consequence of maladaptive plasticity: implications for intervention. In: Altenmuller E, Wiesendanger M, Kesselring J, eds. Music, Motor Control and the Brain. Hanover, Germany: Oxford University Press; 2006.
218. Abbrazese G, Trompetto C, Schieppati M. The excitability of the human motor cortex increases during execution and mental imagination of sequential but not repetitive finger movements. Exp Brain Res. 1996;111:476–482.
219. Porro CA, Francescato MP, Cettolo V, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698.
220. Rijintjes M, Dettmers C, Büchel C, et al. A blueprint for movement: functional and anatomical representation in the human motor system. J Neurosci. 1999;19:8043–8048.
221. Byl NN, Nagarajan SS, McKenzie A. Effectiveness of sensory retraining: three case studies of patients with focal hand dystonia. Presented at the annual meeting of the Society of Neuroscience, New Orleans, Louisiana, November 6, 2000 (abstract and poster).
222. Byl N, Barbe M, Barr A. Repetitive stress pathology: soft tissue. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. Canada: Saunders: Elsevier; 2008.
223. Byl N, Archer E, McKenzie A. Effectiveness of a home program of fitness and learning-based sensorimotor and motor training. J Hand Ther. 2009;22:183–198.
224. Zeuner KE, Peller M, Knutzen H, et al. Motor retraining does not need to be task specific to improve writer’s cramp. Mov Disord. 2008;23(16):2319–2327.
225. Zeuner KE, Baur B, Siebner H. Therapy of sensorimotor dysfunction of the hand: focal hand dystonia. In: Nowak DA, Hermsdoerfer J, eds. Sensorimotor Control of Grasping: Physiology and Pathophysiology. Cambridge, UK: Cambridge University Press; 2009.
226. Tinazzi M, Farina S, Bhatia K, et al. TENS for the treatment of writer’s cramp dystonia: a randomized, placeo-controlled study. Neurology. 2005;64:1946–1948.
227. Pesenti A, Priori A, Scarlato G, Barbieri S. Transient improvement induced by motor fatigue in focal occupational dystonia: the handgrip test. Mov Disord. 2001;16:1143–1147.
228. Marquardt C, Mai N. A computational procedure for movement analysis in handwriting. J Neurosci Methods. 1994;52:39–45.
229. Schenk T, Baur B, Steidle B, Marquardt C. Does training improve writer’s cramp? An evaluation of a behavioral treatment approach using kinematic analysis. J Hand Ther. 2004;17:349–363.
230. Baur B, Furholzer W, Jasper I, et al. Effects of modified pen grip and handwriting training on writer’s cramp. Arch Phys Med Rehabil. 2009;90(5):867–875.
231. Hortobagyi T, Dempsey L, Fraser D, et al. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol. 2000;524:293–304.
232. Facchini S, Romani M, Tinazzi M, Aglioti SM. Time-related changes of excitability of the human motor system contingent upon immobilisation of the ring and little fingers. Clin Neurophysiol. 2002;113:367–375.
233. Kaneko F, Murakami T, Onari K, et al. Decreased cortical excitability during motor imagery after disuse of an upper limb in humans. Clin Neurophysiol. 2003;114:2397–2403.
234. Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176.
235. Priori A, Pesenti A, Cappellari A, et al. Limb immobilization for the treatment of focal occupational dystonia. Neurology. 2001;57:405–409.
236. Badarny S, Meer J, Drori T, Zivziner S, Honigman J. Writer’s cramp treated with hand immobilization. Mov Disord. 2002;17:1020.
237. Lederman R. Drummer dystonia. Med Probl Perform Art. 2003;19:70.
238. Schuele S, Lederman RJ. Focal dystonia in woodwind instrumentalists: long term outcome. Med Probl Perform Art. 2003;18:15.
239. Baur B, Furholzer W, Marguardt C, Hermsdorfer J. Auditory grip force feedback in the treatment of writer’s cramp. J Hand Ther. 2009;22:163–171.
240. Hermsdoerfer J, Marquardt C, Schneider A, Fuerholzer W, Baur B. Pen grip force in writer’s cramp. Hum Move Sci. 2010; in press.
241. Taira T, Hori T. Stereotactic ventrooralis thalamotomy for task-specific focal hand dystonia (writer’s cramp). Stereotact Funct Neurosurg. 2003;30:88–91.
242. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation. Nat Rev Neurosci. 2007;8:559–567.
243. Chen R, Classen J, Gerloff C, Celnick P, Wassermann EM, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403.
244. Siebner H, Tormos J, Ceballos-Baumann A, et al. Low frequency repetitive transcranial magnetic stimulation of the motor cortex. Neurology. 1999;52:529–537.
245. Rizzo V, Siebner HR, Modugno N, et al. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495.
246. Tyvaert L, Cassim C, Devanne H, et al. Subthreshold low frequency rTMS over the premotor cortex and sensorimotor integration in patients with writer’s cramp. Neurology. 2006;66(A):179.
247. Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–663.
248. Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301.
249. Lang N, Siebner HR, Ernst D, et al. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639.



















