Molecular Biology of Sensory Transduction
Introduction
Pain, a sensory and emotional experience, is in the brain. As discussed elsewhere in this textbook, there are clearly cases, such as stroke, where pain can originate from within the central nervous system. However, the vast majority of the pain that we experience, including chronic pain associated with peripheral nerve injury and inflammatory disorders, arises from activity in primary afferent neurons. Moreover, the vast majority of this activity is due to the impact of thermal, chemical, and/or mechanical stimuli. Afferent activity may arise spontaneously under pathological conditions as a result of changes in the relative balance of ionic currents in the membrane (Liu et al 2000, 2002; Amir et al 2002), although even “spontaneous” activity may ultimately depend on membrane depolarization driven by a mechanical, thermal, or chemical stimulus impinging on the afferent, even though the source of the stimulus may not be readily apparent (Gold 2000a). The focus of this chapter is on the mechanisms that enable thermal, mechanical, and chemical stimuli to initiate neural activity.
By definition, sensory transduction is conversion of the energy of a stimulus into an electrical signal. For the special senses (vision, audition, olfaction, taste), transduction occurs in specialized organs via cellular events specific to the stimuli associated with these senses. Sensory information arising from the body, referred to as somatosensation, may also involve specialized sense organs. For example, Golgi tendon organs and Meissner’s corpuscles are involved in the transduction of tension on tendons and low-threshold mechanical stimuli on glabrous skin, respectively. The primary afferents or sensory neurons innervating these structures tend to have rapidly conducting myelinated axons and anatomically distinct, specialized endings that often incorporate non-neuronal cells (Caterina et al 2005). In contrast, afferents referred to as nociceptors respond to noxious or potentially tissue-damaging stimuli that are normally perceived as painful. Axons of these neurons tend to have slowly conducting unmyelinated (C fibers) or thinly myelinated (Aδ fibers) axons with peripheral terminals that are not associated with specific structures or cell types (Caterina et al 2005). Thus, nociceptors are said to have free nerve endings. Although recent data, discussed below, have forced investigators to rethink the contribution of other cell types to sensory transduction, an important implication of the free nerve ending is that the molecular machinery necessary for transduction of noxious stimuli must be intrinsic to the nociceptive afferents. The subsequent demonstration that subpopulations of isolated sensory neurons are responsive to thermal (both hot and cold) (Cesare and McNaughton 1996; Reichling and Levine 1997; Reid and Flonta 2001a, 2001b; Viana et al 2002; Thut et al 2003), mechanical (McCarter et al 1999, Drew et al 2004), and a variety of algogenic chemical stimuli (Gold and Gebhart 2010) is consistent with the idea that transduction is an intrinsic property of nociceptive afferents.
The available evidence indicates that the resting membrane potential of nociceptive afferents is negative to −40 mV, with values at the cell body ranging between −50 and −75 mV (Baccaglini and Hogan 1983, Gold et al 1996). Evidence from study of the putative nociceptive afferent somata in vitro suggests that the action potential threshold is relatively high at greater than −35 mV (Gold et al 1996; Petruska et al 2000, 2002; Flake et al 2005; Harriott et al 2006; Harriott and Gold 2009b). Thus, because action potential generation is necessary for propagation of sensory information to the central nervous system, transduction of nociceptive stimuli must ultimately result in membrane depolarization. The membrane depolarization resulting from a transduction event is called a generator potential.
A generator potential can be initiated in three primary ways. The first and most direct way involves the opening of an ion channel with an ion permeability ratio such that the equilibrium potential for the net charge movement through the channel is depolarized to the action potential threshold. In this case, sufficient activation of this ion channel will drive the membrane potential above threshold and thereby result in an action potential that can be propagated toward the central nervous system. Activation of the transient receptor potential vanilloid type 1 (TRPV1) channel is an example of such a transduction mechanism. As discussed below, the TRPV1 channel is activated or opened by thermal (heat) stimuli (Caterina et al 1997), as well as by a variety of chemical stimuli (Caterina et al 2005). It is a non-selective cation channel that is permeable to Ca2+, Na+, and K+ such that the equilibrium potential, or the potential at which there is no net flux of charge, is approximately 0 mV for this channel. Because this equilibrium potential is above the action potential threshold, activation of enough TRPV1 channels can ultimately result in action potential generation (Fig. 2-1).
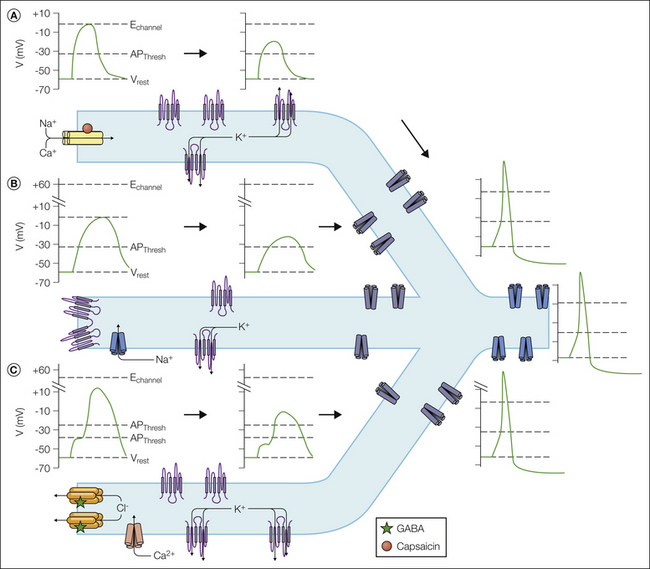
Figure 2-1 At least three mechanisms underlie the initiation of a generator potential.
A, One involves activation of an ion channel such as transient receptor potential vanilloid 1 (TRPV1) with a permeability ratio such that the equilibrium potential for ions flowing through the channel (Echannel) is above the action potential threshold (APThresh). Sufficient activation of such a channel will drive the membrane above the AP threshold. This generator potential is passively propagated to an action potential initiation site with a high density of voltage-gated Na+ channels. Decay of the generator potential is determined by the passive electrophysiological properties of the nociceptor terminal, which is dynamically influenced by the density, distribution, and activity of leak K+ channels such as the two-pore K+ channels TREK-1, TASK, and others. If the generator potential is above the AP threshold at the spike initiation site, an AP will be generated that is propagated toward the central nervous system. B, Another mechanism involves closing of a K+ channel such as a two-pore K+ channel. In this scenario there must be at least one other channel open, such as a persistent Na+ channel with an E channel above the AP threshold. Closing the K+ channels results in an increase in the relative permeability of the membrane for the depolarizing current, which may be sufficient to drive the membrane potential above the AP threshold. As in A, this generator potential must also be passively propagated toward the spike initiation zone. C, A third mechanism involves activation of a channel that has an equilibrium potential threshold. This appears to be the case for GABAA receptors in some nociceptive afferents. If this channel is close to a low-threshold voltage-activated channel, such as a low-voltage-activated Ca2+ channel, the depolarization driven by activation of the transducer may be sufficient to activate the voltage-gated channel and push the membrane potential above the AP threshold. As in A, this generator potential must also be passively propagated toward a spike initiation site. GABA, γ-aminobutyric acid.  leak K+ channels;
leak K+ channels;  voltage-gated K+ channels;
voltage-gated K+ channels;  voltage-gated Na2+ channels.
voltage-gated Na2+ channels.
A second mechanism underlying the generator potential involves the closing of a channel responsible for a hyperpolarizing current. K+ channels are the only channels capable of contributing such a current in nociceptive afferents. This is because of the distribution of ions inside and outside nociceptive afferents. That is, interstitial fluid has a relatively high concentration of Na+, Ca2+, and Cl− and a low concentration of K+. In contrast, in nociceptive afferents, the intracellular concentration of K+ is high, that of Cl− is relatively high (Rocha-Gonzalez et al 2008), and that of Na+ and Ca2+ is low. Closing of a K+ current is clearly an indirect mechanism of sensory transduction since it will result in a generator potential only if a resting depolarizing current is simultaneously active with the hyperpolarizing K+ current. Relatively high K+ conductance in the face of relatively low Na+ conductance will still enable the neuron to maintain a resting membrane potential in the expected range. If the decrease in K+ channels is sufficient in such a neuron, the result will be a generator potential capable of driving the membrane potential above the action potential threshold (see Fig. 2-1).
The third mechanism underlying a generator potential is also indirect, but in contrast to the second mechanism, it is dependent on a relatively close association between an ion channel capable of driving membrane depolarization and a low-threshold voltage-gated ion channel capable of pushing the membrane potential above the action potential threshold. That is not to say that the localization of ion channels is not critical for the ultimate success (i.e., generation of action potentials) of transduction via all three mechanisms, but this is particularly true for Cl− because of the unique regulation of Cl− in sensory neurons. There is evidence in some neurons that the concentration of intracellular Cl− may be high enough that the Cl− equilibrium potential is above the action potential threshold. Consequently, activation of a Cl− channel in these neurons, such as bradykinin-induced activation of the Ca2+-dependent Cl− channel TMEM16, may result in a generator potential sufficient for generation of an action potential (Liu et al 2010). Though depolarized relative to the resting membrane potential in almost all sensory neurons, the Cl− equilibrium potential is still below the action potential threshold in many sensory neurons. However, if there are low-threshold voltage-activated channels such as the T-type Ca2+ channel Cav3.2 in close association with Cl− channels, activation of a Cl− channel may still be sufficient for action potential generation even if the Cl− equilibrium potential is below the action potential threshold (see Fig. 2-1).
Many chemical stimuli act on the G protein–coupled receptors (GPCRs) expressed on nociceptors. In these cases, as discussed below, subsequent intracellular signaling cascades are needed to modify ion channel activity and drive initiation of the generator potential.
Chemo-, Thermo-, and Mechanotransducers
Transducers are often categorized according to the stimuli to which they are responsive. This is a useful way to think about transducers, particularly in the context of a particular type of pain or altered sensitivity such as cold allodynia or heat hyperalgesia, because it is reasonable to assume that these “types” of pain are due to afferent activity evoked with a specific stimulus. However, many, if not most of the putative thermo- and mechanotransducers respond to more than one stimulus modality and are therefore said to be polymodal. Given evidence that a variety of transducers are present and functional in non-neural tissue, it is also reasonable to categorize transducers according to whether they are intrinsic or extrinsic to the primary afferent. Furthermore, given evidence that at least one putative transducer (TRPV1) may be present and functional on subcellular organelles (Castro et al 2009), it is at least worth considering transducer localization despite evidence that the vast majority of transducers are membrane bound. Nociceptive afferents and consequently transducers are present throughout the body. Although a pinprick and noxious stretch are both mechanical stimuli, visceral afferents such as those innervating the colon are far more sensitive to stretch (i.e., colon distention) than other forms of mechanical stimuli (Ness and Gebhart 1990), whereas pinprick is a highly effective stimulus for activating nociceptive afferents innervating the skin (Caterina et al 2005). Consequently, it is important to consider the nature of the stimulus and the tissue being affected. Finally, even though a number of chemotransducers are activated by noxious chemicals in the environment, the majority, if not all, are responsive to endogenous chemicals. Therefore, it is also important to consider the source of the stimulus.
Chemotransducers
Of the three primary modalities of somatosensory stimuli, the process of chemotransduction is the most well understood. Specificity for one chemical over another is achieved through binding sites in the transducer that are unique, or at least relatively so, for a particular chemical. In the most direct form of chemotransduction, the transducer has a binding site, or receptor, for the chemical stimulus and is also an ion channel. Binding of the chemical to the receptor drives a conformational change in the transducer protein that opens the ion channel (e.g., see Mayer 2011). Thus, these transducers are also referred to as ionotropic receptors (Fig. 2-2). This is the most rapid form of chemical transduction, with signaling possible on the microsecond time scale. There is also an indirect form of chemotransduction whereby the conformational change in the transducer driven by chemical binding results in the activation of an intracellular signaling cascade. These transducers are referred to as metabotropic receptors (see Fig. 2-2). This form of chemical transduction is slower and occurs on a time scale of milliseconds to minutes. Guanine nucleotide–binding proteins, or GPCR receptors, are by far the most common type of metabotropic receptor, with the type of G protein being responsible for both initiation of the cellular signaling cascade and the type of cascade initiated (Bunnett and Cottrell 2010). Additional metabotropic receptors found in sensory neurons include receptors bearing intrinsic protein tyrosine kinase domains (i.e., Trk receptors), receptors that associate with cytosolic tyrosine kinases (i.e., non–tyrosine kinase receptors such as cytokine receptors, integrins), and protein serine/threonine kinases (i.e., transforming growth factor-α [TGF-α] receptors) (see Gold 2005 for review). This second form of signaling is very widespread and responsible for changes in the regulation of a variety of cellular processes, including ion channel properties (Fitzgerald et al 1999), cellular properties such as the regulation of intracellular Ca2+ (Werth et al 1996) and neurite extension (Yasuda et al 1990, Jones et al 2003), and gene expression (Huang and Reichardt 2003). Second-messenger signaling is complex with multiple points of convergence and interaction (Gold and Gebhart 2010). However, to keep this chapter tractable, I will consider only metabotropic receptor–mediated transduction events that are coupled to an ion channel that may initiate a generator potential.
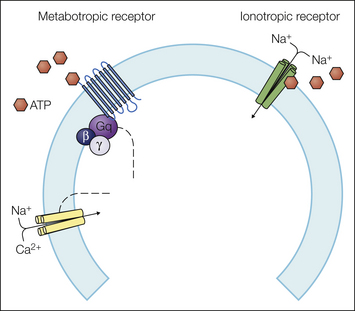
Figure 2-2 Ionotropic receptors are directly coupled to ion channels.
The purinergic P2X3 receptor is composed of three subunits. Binding of adenosine triphosphate (ATP) drives a conformational change in the subunit assembly that leads to opening of an Na+ channel. The result is rapid membrane depolarization. Metabotropic receptors initiate a second-messenger cascade. The purinergic P2Y2 receptor is a G protein–coupled receptor able to drive an action potential. The ion channel coupled to P2Y2 receptors has yet to be identified, but as a Gq-coupled G protein–coupled receptor, it could drive the activation of phospholipase C, which has been shown to activate transient receptor potential vanilloid 1 (TRPV1), a non-selective cation channel, secondary to cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2).
At least three additional factors have an impact on the efficacy of chemoreceptor signaling. First, the spatial distribution of transducers relative to other ion channels in the membrane, particularly those responsible for action potential initiation, is a critical determinant of whether a generator potential will result in an action potential. To add to the complexity of metabotropic receptor signaling, there is evidence that second-messenger coupling is dynamic and changes in response to a number of different conditions, including hormonal status (Dina et al 2001) and history of prior stimulation (Parada et al 2005). Furthermore, it is dependent not only on the appropriate localization of transducers and targeted ion channel but also on the appropriate cellular signaling machinery. Consequently, metabotropic receptor activation may not always result in a generator potential. Second, allosteric modulation of chemotransducers, where a second chemical binding site is located at a site different from that of the chemical that activates the receptor, is common and can result in profound changes in chemotransducer activity. An extreme example is the N-methyl-D-aspartate (NMDA) type of ionotropic glutamate receptor: for glutamate to activate the receptor, it must also be bound to glycine (Ren and Dubner 1999, Dubner 2004, Salter 2005). Third, a number of chemotransducers may be activated by several distinct chemicals. In the case of TRPV1, a transducer activated by protons and capsaicin (the pungent component of chili peppers), the binding sites or receptors for these compounds are on distinct parts of the protein (Gavva et al 2005).
Ionotropic Receptor Families
Acid-Sensing Ion Channels
Ionotropic receptors are generally classified according to their structure and genetic homology. The acid-sensing ion channels (ASICs) are, as their name implies, activated by extracellular protons, although this is true for only three of the four genes encoding ASIC channel subunits (ASIC1, 3, and 4) since ASIC2 does not appear to be activated by protons (Lingueglia 2007). All four subunits are detected in sensory neurons (Alvarez de la Rosa et al 2002, Hughes et al 2007, Bohlen et al 2010), including the two splice variants of ASIC1 (1a and 1b) and ASIC2 (2a and 2b). The ASICs are trimeric proteins with homology to the epithelial Na+ channel (ENaC)/degenerin family that can form homomeric or heteromeric channels (Qadri et al 2012). ASIC3 was originally thought to be specific to dorsal root and trigeminal ganglion neurons (Chen et al 1998), where it is enriched in nociceptive afferents, but it has subsequently been shown to be more widely expressed (Sanchez-Freire et al 2011, Sole-Magdalena et al 2011). It is the most sensitive to protons—activated by a decrease of less than 0.2 pH units—and this sensitivity is dramatically enhanced with lactate (Immke and McCleskey 2001). Intense muscle use produces lactic acidas, a metabolic produce that is thought to contribute to exercise and ischemic muscle pain. Thererfore, ASIC3 is one of several transducers present in muscle afferents that may also be referred to as metaboreceptors (Molliver et al 2005). ASIC3 is enriched in specific subpopulations of afferents, including those innervating the heart (Benson et al 1999) and dura (Yan et al 2011), where it has been suggested to contribute to the pain associated with coronary ischemia and migraine, respectively. Evidence from null mutant mice suggests that ASIC3 contributes to hyperalgesia of an inflamed muscle (primary hyperalgesia) whereas ASIC1 contributes to the hyperalgesia observed at sites distant from the inflamed muscle (secondary hyperalgesia) (Walder et al 2010), although the details underlying the basis for this distinct pattern have yet to be fully clarified. Recent data indicating that venom from the western coral snake can directly activate ASIC1 (Bohlen et al 2010) further support a role for this subunit in the response to pain-producing stimuli and suggest that these channels are responsive to a wider variety of stimuli than originally thought. The observation that the pH sensitivity of ASIC2a was dramatically increased by coral snake venom also led to the suggestion that this subunit may function as a coincidence detector for as yet to be identified compounds. Along these same lines, recent evidence suggests that ASIC3 and P2X5 may act as coincidence detectors since binding of adenosine 5′-triphosphate (ATP) to P2X5 dramatically increases the sensitivity of ASIC3 to protons (Birdsong et al 2010).
Cys-Loop Family of Ligand-Gated Ion Channels
ACh/5-HT3/GABAA: One of the larger families of ligand-gated ion channels is the Cys-loop family, so named because of the characteristic loop in the extracellular N-terminal domain of the α subunit formed by a disulfide bond between two cysteine (Cys) residues (Tsetlin et al 2011). Members of three of the four major subfamilies of Cys-loop receptors are present in sensory neurons. These include nicotinic acetylcholine receptors (nAChRs), serotonin type 3 (5-HT3) receptors, and type A γ-aminobutyric acid (GABA) receptors (GABAA). All Cys-loop receptors contain five subunits, which may be homomeric or heteromeric, depending on the receptor subtype and subunit composition.
Both homomeric (α7; Shelukhina et al 2009) and heteromeric (α3–6, β2–4; Xiao et al 2002, Rau et al 2005, Spies et al 2006) nAChRs composed of two α and three β subunits are present in sensory neurons, with evidence that both forms contribute to nociceptive processing (Carstens et al 1998, Schmelz et al 2003). Although there are several potential non-neural sources of acetylcholine (ACh) in the periphery, it is important to note that ACh levels are increased in inflamed tissue (Wessler et al 2003, Gahring et al 2010). Furthermore, peripheral administration of ACh- or nAChR-selective agonists results in burning pain (although recent evidence suggests that at least some of the pain associated with nicotine may be due to activation of TRPA1 (Talavera et al 2009). Interestingly, recent evidence also suggests that the relative contribution of nAChR subtypes to peripheral pain may depend on the target of innervation. That is, α7 nAChRs appear to suppress activity in colonic dorsal root ganglion (DRG) neurons (Abdrakhmanova et al 2010), whereas the burning pain associated with the application of nAChR agonists to the skin is probably mediated by heteromeric receptors (Carstens et al 1998, Schmelz et al 2003).
The 5-HT3 receptor is the only ionotropic serotonin receptor. It may exist as a homomeric receptor composed of five 5-HT3A subunits or as a heteromeric receptor composed of a 5-HT3A subunit with one of the other four 5-HT3 subunits (5-HT3B–3E) (Thompson and Lummis 2007). It was originally thought to have a relatively limited role in nociception because of its expression pattern in afferents with a medium to large cell body diameter (Tecott et al 1993). Subsequent analysis, however, suggested that this receptor is critical for both serotonin-induced pain and, more importantly, full expression of the second phase of pain behavior in the formalin test (Zeitz et al 2002), a behavior thought to reflect spontaneous inflammatory pain. Given that the gastrointestinal (GI) tract is the largest source of serotonin in the body, it should not be surprising that this receptor plays an even more important role in visceral pain. In fact, several 5-HT3 receptor antagonists have been approved for use in treating the pain associated with irritable bowel and other visceral pain syndromes (Fayyaz and Lackner 2008).
Unlike the nAChRs and 5-HT3 receptors, which have ion channels permeable to Na+, K+, and to varying degrees, Ca2+, GABAA receptors have an ion pore selective for anions (primarily Cl− and to a less degree HCO3−) (Michels and Moss 2007, Picton and Fisher 2007). Like nAChRs and 5-HT3 receptors, GABAA receptors may exist as homomeric or heteromeric proteins. The homomeric proteins are composed of one of the three ρ subunits (originally called GABAC receptors) and have been most studied extensively in the retina (Qian 1995–2005). Recent evidence suggests that ρ subunit–containing receptors are present in sensory neurons. However, heteromeric GABAA receptors are far more common throughout the nervous system, including sensory neurons. Although 19 subunits of the GABAA receptor have been identified, the number of possible receptors is constrained by a subunit stoichiometry that consists of two α subunits, two β subunits, and one of the remaining seven non-ρ subunits. GABAA receptor subunit composition determines the pharmacological and biophysical properties of the receptor, as well as its cellular distribution (Michels and Moss 2007).
Given that GABAA receptors underlie the vast majority of fast inhibitory synaptic transmission in the adult central nervous system, it may be surprising to find these receptors among a list of chemotransducers. However, because, as noted above, the concentration of intracellular Cl− is maintained at levels considerably higher in nociceptive afferents than in neurons in the central nervous system (Rocha-Gonzalez et al 2008), GABAA receptor activation results in membrane depolarization in nociceptive afferents (Price et al 2009), which may still be inhibitory in the absence of tissue injury. However, following tissue injury, GABAA receptor activation may result in action potential generation in nociceptive afferents (Willis 1999). This can occur at the central terminals of nociceptive afferents, a process referred to as the dorsal root reflex. More importantly in the context of the present discussion, GABAA receptor activation in the periphery can excite nociceptive afferents (Carlton et al 1999, Carr et al 2010). It should be noted that although there do not appear to be sources of peripheral GABA necessary to achieve the concentrations needed for activation of low-affinity synaptic receptors, recent evidence suggests that high-affinity receptors, which may be activated by GABA concentrations close to the resting levels observed in extracellular fluid, are present in sensory neurons (Lee et al 2012). Thus, even though there is evidence that a shift in GABAA signaling in the spinal cord contributes to both the initiation and maintenance of inflammatory hypersensitivity, high-affinity GABAA receptors in the periphery may also contribute to ongoing afferent drive in the presence of inflammation.
Glutamate Receptors
Ionotropic glutamate receptors play a critical role in fast excitatory synaptic transmission in the central nervous system and are therefore generally studied in the context of synaptic transmission. These receptors have historically been classified according to their response to three selective agonists: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, and NMDA (Mayer 2011). Subsequent analysis has indicated that these receptors are composed of heteromeric combinations of four subunits, with AMPA receptors being composed of GluR1–4, kainate receptors being composed of GluR5–7 (GluK1–3) and/or KA1–2 (GluK4–5), and NMDA receptors being composed of a combination of NR1, NR2A–D, and/or NR3A–B. All three types of receptors are present and functional in primary afferent neurons (Miller et al 2011). Interestingly, even though these receptors are present on the central terminals of primary afferents, where they appear to contribute to synaptic transmission in the spinal cord, they are also present in the periphery, where they can drive action potential generation (Cairns et al 1998, 2001b; Lam et al 2009a, 2009b) and pain (Cairns et al 2001a). There are a number of potential sources of peripheral glutamate, including the afferent itself, where peripherally released glutamate could serve as a form of feedback excitation to amplify injury-induced activation of nociceptive afferents.
HCN Channels
Hyperpolarization-activated, cyclic nucleotide–gated (HCN) ion channels are not typical chemotransducers since they are not directly activated by extracellular ligand binding (Wickenden et al 2009). Furthermore, because these non-selective cation channels are activated by membrane hyperpolarization and close with membrane depolarization, their biophysical properties argue against a role in the initiation of a generator potential capable of driving the membrane above the action potential threshold. Under resting conditions, these channels are activated only during the increase in membrane potential associated with the after-hyperpolarization that follows an action potential, where they provide a depolarizing drive for subsequent spike initiation (Ingram and Williams 1994). There are four HCN family members (HCN1–4), which consistent with their homology to Kv family members, form homomeric tetramers (Wickenden et al 2009). mRNA for all four HCN channels is detectable in sensory neurons, with HCN1 being differentially distributed in large- and small-diameter neurons and HCN3 enriched in small-diameter neurons (Chaplan et al 2003, Kouranova et al 2008).
These channels have been included in the list of chemotransducers for several reasons. The voltage dependence of channel activation is regulated by intracellular cyclic adenosine monophosphate (cAMP) such that an increase in cAMP drives a depolarizing shift in the voltage dependence of channel activation. This shift can be sufficiently large that HCN channels contribute to the depolarization of resting membrane potential and, more relevantly, to a depolarizing drive that facilitates action potential generation (Emery et al 2011). The channel is also a putative target for a number of metabotropic receptors coupled to second-messenger pathways that result in an increase in cAMP. There is an increase in HCN current density in large-diameter neurons following traumatic peripheral nerve injury, where the increase appears to be responsible for ectopic activity (Chaplan et al 2003). Recent evidence from null mutant mice suggests that HCN2 in Nav1.8-expressing neurons plays a particularly important role in the generation of inflammatory thermal hyperalgesia, as well as peripheral nerve injury–induced thermal and mechanical hypersensitivity (Emery et al 2011).
Purinergic Receptors—P2X
Ionotropic purinergic, or P2X, receptors are activated by ATP. The functional receptor is a trimer. Seven subunits have been identified, P2X1–7; all but P2X6 can form functional homomers, and each appears to be able to form a heteromeric protein with at least one other subunit (Burnstock 2006, Jarvis and Khakh 2009). At least six, if not all seven, subunits are present in sensory neurons, with all but P2X4 being differentially distributed among subpopulations of DRG neurons (Kobayashi et al 2005). P2X3, which forms a heteromer with P2X2, has been the most extensively studied member of this family with respect to nociceptor activation (Jarvis 2003). This subunit was originally shown to be enriched in a subpopulation of neurons with a small cell body diameter that did not express the neuropeptides substance P or calcitonin gene–related peptide (CGRP) (Bradbury et al 1998), the so-called non-peptidergic afferents. Although the distribution of P2X3 receptors among specific subpopulations of sensory neurons was subsequently shown to depend on the target of innervation (e.g., see Ambalavanar et al 2005), pharmacological and molecular biological analysis confirmed that this subunit plays a dominant role in mediating the nociceptive response to the application of ATP, as well as the response to a variety of noxious stimuli applied to various tissues (Jarvis 2003). Interestingly, this subunit appears to mediate the activation of nociceptive afferents observed following damage to neighboring cells (Cook and McCleskey 2002), thus making this transducer a critical player in the initial pain observed in response to tissue injury. As noted above, more recent work has highlighted a potential role for P2X5 in the sensitization of ASIC currents in sensory neurons and consequently the pain associated with muscle ischemia (Birdsong et al 2010).
Transient Receptor Potential Channels
The TRP channels involved in chemotransduction come from a large family of ion channels that encompass eight subfamilies ranging from TRPA (for ankyrin) to TRPV (for vanilloid), with TRPC (for canonical), TRPM (for melastatin), TRPML (for mucolipins), TRPP (for polycystins), and TRPN (for NO-mechanopotential C) in between (Nilius and Owsianik 2011). Unifying features of this family include a channel protein formed from four subunits, each with a structure analogous to that for voltage-gated K+ ion channel subunits with a six-transmembrane segment and a pore loop between transmembrane segments 5 and 6. Specific to TRP channels are ankyrin domains on the intracellular N-terminus and proline-rich domains on the intracellular C-terminus. All TRP family members form Ca2+-permeable cation channels. Many of the family subunits have a voltage sensor in segment 4 and consequently exhibit voltage-sensitive properties. As discussed below, several of the channels are activated by different stimulus modalities, with the biophysical properties of the channel activity appearing to depend on how the channel is activated. For example, capsaicin-induced activation of TRPV1 desensitizes in the presence of extracellular Ca2+, whereas heat-evoked activation of TRPV1 does not (Caterina et al 1997). The channels also appear to be used differentially across phyla. For example, TRPA1 functions as a cold receptor among other modalities of transduction in mammals (Story et al 2003, Karashima et al 2009) but underlies infrared detection in snakes (Gracheva et al 2010). The relative contribution of various TRP channels to nociceptive processing is an active area of investigation.
TRPA1: TRPA1 was originally identified through a combined bioinformatic and expression strategy designed to identify additional TRP family members (Story et al 2003). It is the only member of its subfamily defined by the exceptional number (14) of N-terminal ankyrin repeats. Although TRPA1 was first thought to function primarily as a cold transducer (see below), subsequent analysis has indicated that TRPA1 is responsive to a variety of noxious compounds. This list first included allyl isothiocyanates (i.e., mustard oil) and cannabinoids (Jordt et al 2004) and was rapidly expanded to include the pungent ingredients of garlic (Bautista et al 2005), cinnamon, wintergreen oil, clove oil, and ginger (Bandell et al 2004), as well as a variety of environmental irritants such as acrolein, CO2, and formalin (McNamara et al 2007). TRPA1 is also activated by a number of endogenous mediators, including products of oxidative stress (Andersson et al 2008) and cyclooxygenase-dependent fatty acid metabolites (Materazzi et al 2008, Taylor-Clark et al 2008). TRPA1 appears to be expressed in a subset of TRPV1-expressing neurons and, like TRPV1, appears to be a target of endogenous algogenic compounds such as bradykinin (Bandell et al 2004). There is also evidence that TRPA1 may interact with TRPV1 at the level of a complex (Akopian 2011). As a result, activation of one channel influences the response resulting from activation of the other. Interestingly, despite its role in mediating the acute painful response to a variety of chemicals, a gain-of-function mutation in TRPA1 is not associated with ongoing pain. Rather, individuals with the TRPA1 mutation experience episodic upper body pain triggered by fasting or physical stress (Kremeyer et al 2010).
TRPM2: TRPM2 is widely expressed throughout the body but is particularly enriched in immune cells such as macrophages (Harteneck 2005, Jiang et al 2011). It was originally suspected that TRPM2 would have enzymatic properties in addition to its putative function as a Ca2+ channel based on the presence of a Nudix box on its C-terminus, a motif common to enzymes, particularly those that degrade nucleoside diphosphates (Perraud et al 2001). It is likely that this motif serves as a binding site for adenosine diphosphate (ADP)-ribose, which was subsequently shown to activate the channel. It was soon realized, however, that TRPM2 plays a significant role in mediating the cellular response to stress since it is activated by reactive oxygen species such as hydrogen peroxide (Takahashi et al 2011). The channel has recently been shown to be present in sensory neurons, where it enables sensitivity to hydrogen peroxide (Naziroglu et al 2011). Recent data indicate that the channel plays a prominent role in facilitating the inflammatory response to infection (Yamamoto et al 2008) and is likely to contribute to inflammatory hypersensitivity. However, though present in sensory neurons (Naziroglu et al 2011, Özgül and Naziroglu 2012), the pro-nociceptive role of this channel is likely to be indirect via facilitation of the release of chemokines such as CXCL2 from immune cells and microglia.
TRPM3: TRPM3 is another member of the melastatin subfamily of TRP receptors implicated in nociception. This TRP channel is expressed in a variety of different tissues, including a relatively broad population of sensory neurons (Vriens et al 2011). The most potent known agonist for TRMP3 is pregnenolone sulfate. Although this compound acts at a number of receptors and ion channels, it has been shown to induce nociceptive behavior when administered to mice, behavior that is eliminated in TRPM3 null mutant mice (Vriens et al 2011).
TRPM8: The discovery of TRPM8 was reported almost simultaneously by two different groups that used complementary strategies of bioinformatics (Peier et al 2002a) and expression cloning (McKemy et al 2002) to identify novel TRP channels. This channel was shown to be responsive to both cooling and menthol. The channel is present in a small subpopulation of non-peptidergic afferents that does not overlap with TRPV1/TRPA1-expressing neurons (McKemy et al 2002, Peier et al 2002a). Because the sensation of menthol is not usually described as painful, this transduction mechanism would generally be grouped with those associated with the transduction of non-noxious stimuli. It is included here for the sake of completeness and, as noted below, because of evidence that the channel contributes to the perception of cold pain (Knowlton et al 2011). Interestingly, recent genome-wide association studies have linked TRPM8 to migraine without aura (ref PMID:21666692 and 22683712), although it remains to be determined how a polymorphism in this channel contributes to the increased risk for the presence of migraine.
TRPV1: The field of sensory transduction broke open in 1997 with the discovery of TRPV1, a TRP channel activated by capsaicin, the pungent compound in chili peppers (Caterina et al 1997). This receptor had long been sought because of an extensive body of both preclinical and clinical data supporting a link between the actions of capsaicin and pain (Holzer 1991). Interestingly, although the only sensation associated with capsaicin in the short term is pain (Schmelz et al 2000), in the long term, treated tissue can become desensitized to subsequent noxious stimuli (Nolano et al 1999). Probably because of species differences in the distribution of TRPV1 among subpopulations of nociceptive afferents, the modality specificity of this desensitization appears to depend on the species being studied; it appears to be specific to noxious heat in the mouse (Cavanaugh et al 2009), but it encompasses heat, mechanical, and possibly chemical stimuli in the rat, dog, and primate (Nolano et al 1999). Various preparations of TRPV1 agonist and administration routes are still being explored for the treatment of chronic pain (Wong and Gavva 2009, Anand and Bley 2011).
A number of interesting features of TRPV1 have been revealed since its original discovery. As discussed below, it is not just a receptor for capsaicin but is activated by noxious heat and plays a critical role in the manifestation of thermal hyperalgesia (Caterina et al 2000). In addition to capsaicin, TRPV1 is responsive to a number of different pungent compounds found in plants, including resiniferatoxin, piperine, and camphor (Tominaga et al 1998), as well as endogenous mediators such as protons (though with considerably lower potency than ASIC3) and lipids (e.g., oxidized linoleic acid metabolites; Patwardhan et al 2009, 2010). There is evidence that phosphatidylinositol 4,5-bisphosphate (PIP2) can both activate and inhibit TRPV1 (Vriens et al 2009). In a model in which PIP2 is inhibitory, it was proposed that algogenic compounds such as bradykinin are able to activate TRPV1 subsequent to the activation of phospholipase C, which frees TRPV1 from inhibition following PIP2 hydrolysis (Prescott and Julius 2003). The channel is a target for a number of different protein kinases, thereby resulting in dynamic regulation of channel properties and membrane distribution (Gold and Gebhart 2010). Channel translation and expression are also dynamically regulated and enable the channel to contribute to both the initiation and maintenance of persistent pain. Prolonged activation of the receptor results in a process referred to as pore dilation, where the size of the channel increases to the point that it becomes permeable to large macromolecules (Chung et al 2008). A comparable process has recently been described in TRPA1 (Chen et al 2009). Although the physiological consequences of this process have yet to be fully elucidated, it may be possible to exploit this property for therapeutic purposes and deliver molecules such as membrane-impermeable local anesthetics to provide selective blockade of nociceptive afferents (Binshtok et al 2007).
TRPV4: TRPV4 was first thought to function as an osmosensor responsive to cell swelling (see below) (Strotmann et al 2000). Subsequent analysis has indicated that the channel is also a thermosensor (see below) responsive to warming (Guler et al 2002). Data from knockout or knockdown experiments suggest that the channel contributes to inflammatory pain and sensitivity (Alessandri-Haber et al 2005, 2006), particularly of visceral structures such as the bladder (Everaerts et al 2010), although this appears to be due to its function as a mechanotransducer (see below). Nevertheless, like other TRP channel family members, TRPV4 is responsive to a plant derivative, as well as to artificial ligands such as 4-α-PDD (Vriens et al 2007), thus raising the possibility that there are endogenous ligands that have yet to be identified.
Two-Pore Potassium Channels
Two-pore potassium (K2P) channels are a large family of resting, or background, K+ channels that when active, inhibit cell excitability (Honore 2007). The channels are widely distributed throughout the body. Family members present in sensory neurons include TREK-1–2, TRAAK, TASK1–3, and TRESK, although recent data suggest that additional family members are detectable in mRNA extracted from whole ganglia (Marsh et al 2012). The name for TREK-1 comes from TWIK-related K+ channel, where TWIK stands for a tandem of P domains in a weak inward rectifier K+ channel (Patel and Honore 2001). TREK-1 was cloned in 1996 based on homology to TWIK and was first shown to be activated by arachidonic acid. TREK-2 and TRAAK (TWIK-related arachidonic acid–activated K+ channel) are also activated by arachidonic acid. TWIK-related acid-sensitive K+ channels (TASK) are present in putative nociceptive afferents (Rau et al 2006) and are inhibited by protons and serotonin (Hopwood and Trapp 2005). The S-type K2P TRESK activity appears to be regulated primarily via second-messenger signaling cascades, with activity being increased by calcineurin and inhibited by kinase activity, including protein kinase A, and by 14–3–3 adaptor protein docking (Czirjak and Enyedi 2010). That inhibition of K2P activity is sufficient to drive afferent activation is suggested by the observation that K2P channel blockers such as sanchool can drive activity in isolated DRG neurons (Bautista et al 2008). Moreover, DRG neurons from TRESK null mutant mice are hyperexcitable (Dobler et al 2007) and hyper-responsive to noxious stimuli. TRESK is also down-regulated in primary afferents following peripheral nerve injury, and this down-regulation is associated with an increase in afferent excitability (Tulleuda et al 2011). Most recently, a dominant negative mutation in TRESK was linked to a form of familial migraine with aura (Lafreniere et al 2010). Finally, there is evidence that TREK-1 and -2 are down-regulated in colonic afferents in a mouse model of colitis (La and Gebhart 2011, La et al 2011).
Metabotropic Receptors
Seemingly endless lists of metabotropic receptors have been identified in sensory neurons, including a long list of GPCRs and neurotrophin receptors and a growing list of receptors for cytokines and chemokines. As discussed above, there is evidence that the activity of a number of chemotransducers and ion channels may be regulated directly by activation of metabotropic receptors. For example, TRPV1 can be activated by metabotropic receptors coupled to phospholipase C (Chuang et al 2001). More commonly, however, metabotropic receptor activation results in “sensitization” of a transducer or ion channel such that the ion channel is more readily activated by natural stimuli such as a change in membrane potential (as for HCN channels) or temperature (as for TRPV1 channels). Thus, under the appropriate conditions, all metabotropic receptors are theoretically capable of acting as a chemotransducer. Not surprisingly, although there are a number of cases in which the ion channels underlying a metabotropic receptor–mediated generator potential have been identified, such as the Ca2+-dependent Cl− channel TMEM16A (or ONO1) underlying the actions of bradykinin in a subpopulation of DRG neurons (Liu et al 2010), in many cases the ion channels responsible for the generator potential are unknown.
Despite the large number of metabotropic receptors expressed in sensory neurons, the number that normally activates nociceptive afferents is relatively small. These include the B1 and B2 receptors for bradykinin (Mense 1982), the H1 receptor for histamine (Fu et al 1997, Jafri et al 1997, Schmelz et al 2003), P2Y2 receptors for ATP (Molliver et al 2002, Stucky et al 2004), the endothelin A receptor for endothelin-1 (Gokin et al 2001, Namer et al 2008), the protease-activated receptor 2 (PAR-2) for extracellular proteases (Patwardhan et al 2006), and IP receptors for prostacyclin (Birrell et al 1991). Understanding signaling via metabotropic receptors in sensory neurons is complicated by two factors. First, there are metabotropic receptor homologues for many of the ionotropic receptors activated by endogenous ligands. For example, afferents express both ionotropic and metabotropic receptors for ACh (Spies et al 2006, Nandigama et al 2010), ATP (Molliver et al 2002), glutamate (Willcockson and Valtschanoff 2008, Carlton et al 2009), and serotonin (Pierce et al 1996, Zeitz et al 2002). The result may be a unique pattern of activity as has been observed for ATP, with the initial burst of activity being mediated by the ionotropic receptor and a more slowly developing, longer-lasting burst being driven by the metabotropic receptor (Molliver et al 2002) (Fig. 2-3). Second, the metabotropic receptors may be coupled to inhibitory second-messenger pathways, which can result in yet another pattern of activity. A variety of GPCRs are discussed further in Chapter 3 of this volume.
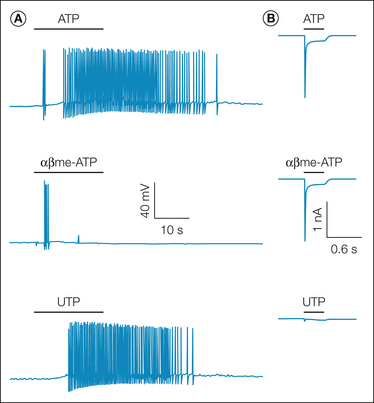
Figure 2-3 Action potentials (A) and currents (B) evoked in a sensory neuron by adenosine triphosphate (ATP) and its analogues.
A single application of ATP evoked two series of action potentials: a brief train occurred immediately after the application of ATP, and then a much longer train occurred after a delay and then persisted for many seconds after removal of ATP. Application of alpha-beta methylene ATP (αβme-ATP), which selectively stimulates sensory neuron ATP-gated ion channels (P2X receptors), evoked the initial train of action potentials but not the prolonged one. Application of uridine triphosphate (UTP), which activates G protein–coupled ATP receptors (P2Y), evoked the delayed, persistent train but not the rapid, brief one. ATP and αβme-ATP both evoked the large currents typical of P2X3 channels, but UTP evoked only a very small inward current; KCNQ has been shown to underlie much of this current. (Reproduced from Molliver DC, Cook SP, Carlsten JA, et al 2002 ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. European Journal of Neuroscience 16:1850–1860. Copyright 2002 with permission from Blackwell Publishing Ltd.)
Chemotransducer Ligands
A common feature of the chemotransducers in sensory neurons is their ability to respond to both exogenous and endogenous compounds. Given the array of noxious chemicals and pathogens in the environment, it makes intuitive sense that the body would adapt ways of detecting the presence of these potential sources of threat or injury. Some classes of receptors for structurally conserved components of bacteria, fungi, viruses, and other organisms, such as the toll-like receptors, are specialized to detect ligands derived from exogenous sources. Many of these receptors, including 7 of the 13 known toll-like receptors, are present in sensory neurons (Ochoa-Cortes et al 2010), and although the second-messenger pathways activated by these receptors could result in a generator potential, these receptors appear to be responsible primarily for afferent sensitization rather than activation (e.g., see Diogenes et al 2011). On the other hand, a number of chemotransducers are clearly specialized to detect endogenous signaling molecules, an extreme example being the glutamate receptors specialized to subserve fast synaptic transmission. The TRP channels are prime examples of receptors with the capacity to respond to both exogenous and endogenous compounds. It should therefore not be surprising that identification of endogenous and exogenous ligands for chemotransducers in sensory neurons is an active area of investigation. Notable in this regard are the creative strategies used. In one compelling example, researchers reasoned that the intense and prolonged pain associated with a burn injury may be due to mediators released in response to the heating of tissue. This led to the discovery of oxidized linoleic acid metabolites, which turned out to be potent endogenous agonists for TRPV1 (Patwardhan et al 2009, 2010). A second compelling example is the use of microdialysis probes in patients with painful oral cancers (Hardt et al 2011). In this case, the researchers again reasoned that oral cancers are particularly painful because of the compounds released within the cancer. Although this research team has only just begun to identify the endogenous source of this form of cancer pain, it appears to involve an increase in proteolytic activity that drives the activation of PARs (Lam and Schmidt 2010). A third example involves a screen of toxins associated with intense pain in humans. This led to the discovery of a toxin in western coral snake venom that acts on ASIC channels as described above (Bohlen et al 2010). This first example highlights the possibility that transduction of any stimulus may involve a multiple-step process whereby the initial stimulus, such as heat or a bacterial cell wall, results in the activation of another cell type, such as a resident immune cell in the case of the bacteria, that releases the chemical ultimately responsible for the generator potential in sensory neurons.
Thermotransduction
The Molecular Thermometer (TRPA1–TRPV2)
The cloning of TRPV1 was not just a watershed moment for the understanding of chemotransduction in nociceptive afferents; it also facilitated the understanding of thermotransduction as well: TRPV1 was not only the capsaicin receptor but was additionally a thermotransducer activated by noxious heat (Caterina et al 1997). Within 6 years of the cloning of TRPV1 six different TRP channels had been identified that together had the biophysical properties necessary to build a molecular thermometer that spanned the full range of human thermosensory perception (Fig. 2-4). TRPA1 was found to be a transducer for noxious cold that encodes drops in temperature from approximately 18 to 4°C (Story et al 2003). TRPM8 had the properties of a cool receptor that was responsive to small decreases in temperature lower than 30°C (McKemy et al 2002). TRPV3, though present in keratinocytes rather than sensory neurons, was responsive to innocuous increases in temperature above 37°C (Peier et al 2002b, Xu et al 2002). TRPV4 was also activated by innocuous warming and was clearly expressed in sensory neurons (Guler et al 2002). TRPV1 was responsive to noxious heat with a threshold for activation roughly comparable to that associated with a heat pain threshold (Caterina et al 1997). Finally, TRPV2 was responsive to heat with a threshold for activation (>48°C) close to that associated with heat pain tolerance in psychophysical studies (Caterina et al 1999). The observation that TRPM8, TRPV4, TRPV1, and TRPV2 are present in different populations of neurons was consistent with in vivo single-unit electrophysiological and psychophysical data (Bessou and Perl 1969, Iggo 1969, Hensel and Iggo 1971, Darian-Smith et al 1973, Dubner et al 1975, LaMotte and Campbell 1978, Campbell and LaMotte 1983, Treede et al 1995) and was used to provide compelling support for the argument that a population code (i.e., activity in different populations of afferents) is used for thermosensation and that there was a “labeled line,” at least from the periphery, for heat pain. Furthermore, the overlap between TRPV1 and TRPA1 (Story et al 2003) was used to explain the psychophysical observation that noxious cold is perceived as burning pain. This idea has proved overly simplistic, however, as discussed below.
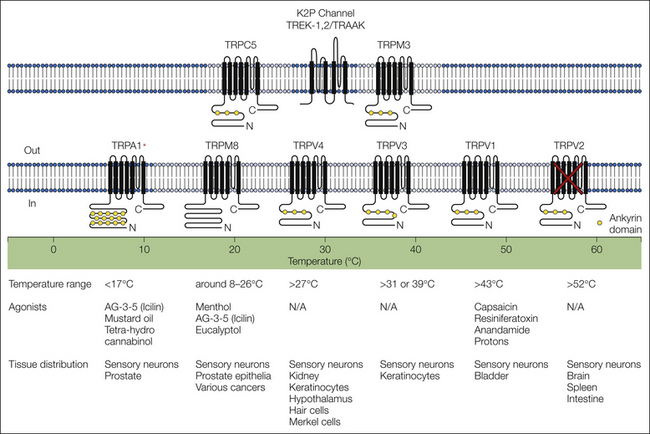
Figure 2-4 Thermosensitive channels respond to a wide range of temperatures.
This diagram depicts mammalian channels that have been demonstrated or proposed to underlie the neural response to thermal stimuli, arranged according to their temperature response profiles when examined in heterologous expression systems. Listed below each channel are their reported thermal thresholds and the range of temperatures to which they respond. Recent data suggest that there may be additional channels that contribute to thermosensation. Most problematic is evidence in support of a role for TRPV2 in thermosensation since null mutant mice have no detectable thermal phenotype. Also problematic is TPRA1, which clearly functions as a thermoreceptor for noxious cold in heterologous systems and in isolated sensory neurons but appears to contribute to the response to noxious cold only in the presence of tissue injury. Additional channels have also been identified that appear to contribute to thermosensation. These include the two-pore K+ channels, TREK-1, TREK-2, and TRAAK, which are activated at between approximately 28 and 42°C. This property enables these channels to contribute to the response to both warming (via inhibition of afferent activity) and cooling (via a decrease in inhibition). TRPM3 has a relatively low threshold for activation (≈30°C) but appears to contribute to the response to noxious heat. Finally, TRPC5 is activated with decreases in temperature from 37 to 25°C and may contribute to the response to cooling. (Modified from Jordt SE, McKenny DD, Julius D 2003 Lessons from peppers and peppermint: the molecular logic of thermosensation. Current Opinion in Neurobiology 13:487–492. Copyright 2003 Elsevier Ltd.)
TRPA1: Concerns over the molecular thermometer model were raised almost immediately, with some of the most contentious disagreements being focused on TRPA1. In heterologous expression systems, cold-evoked TRPA1 responses were transient, which differed from the sustained responses to noxious cold observed in isolated sensory neurons (Thut et al 2003), single-unit electrophysiology (Bessou and Perl 1969, Iggo 1969, Hensel and Iggo 1971), and psychophysical studies (Kenshalo and Scott 1966, Johnson et al 1973). Data from some of the initial studies of cold transduction in isolated sensory neurons implicated the closing of K+ channels as a contributing (Viana et al 2002) if not primary mechanism (Reid and Flonta 2001a). Others failed to detect a response to noxious cold with TRPA1, even in comparable heterologous expression systems (Jordt et al 2004). Subsequent data with TRPA1 null mutant mice suggested that the contribution of TRPA1 to the response to acute noxious cold stimuli was minimal, if detectable at all (Bautista et al 2006, Kwan et al 2006). Data derived from TRPA1-selective antagonists confirmed the negative behavioral data obtained in TRPA1 null mutant mice, thus suggesting that the channel has no detectable influence on cold sensitivity in naïve animals (Chen et al 2011). With some distance from this particular debate and time for more detailed analysis, it is clear that TRPA1 is gated by noxious cold (Karashima et al 2009). It is also clear that the channel contributes to injury-induced cold hypersensitivity (del Camino et al 2010). Finally, as yet unidentified mechanisms must contribute to the response to acute noxious cold stimuli.
TRPM8: Data from a number of different lines of investigation support the notion that TRPM8 contributes to the response to innocuous cooling. This includes single-cell polymerase chain reaction of isolated sensory neurons (Nealen et al 2003), single-unit electrophysiology, and behavioral data from null mutant mice (Bautista et al 2007, Knowlton et al 2011). The only point of contention over TRPM8 is whether this channel also contributes to nociceptive behavior. In the absence of tissue injury, the answer to this question appears to be no because the channel does not code well into the noxious range, it is present in a subpopulation of neurons that have properties of non-nociceptive afferents (Nealen et al 2003, Bautista et al 2007), and there is little change in behavior in response to noxious cold stimuli in TRPM8 null mutant mice (Bautista et al 2007, Babes et al 2011). However, high-dose menthol applied topically in humans is used to generate hyperalgesia. Moreover, in the presence of tissue injury, cold hypersensitivity is attenuated in TRPM8 null mutant mice (Xing et al 2007) and with a TRPM8 antagonist (Knowlton et al 2011). Nonetheless, several additional lines of evidence argue against a role for TRPM8 in the cold sensitivity associated with nerve injury (Katsura et al 2006, Caspani et al 2009). Whether the putative role for TRPM8 in nociception is due to de novo expression of TRPM8 in nociceptive afferents (Djouhri et al 2004; but see Caspani et al 2009) and/or changes in the central nervous system such that input via cool-responsive neurons is able to engage a nociceptive circuit remains to be determined.
TRPV3 and TRPV4: Unfortunately, because warm fibers are generally absent in rodents, progress in our understanding of the relative contribution of TRPV3 and TRPV4 to the response to warmth has been slow. Whether these channels contribute to the response to innocuous warming in other species remains to be determined. However, results from null mutant mice and the use of non-selective TRP channel blockers (St Pierre et al 2009) indicate that these channels have little role in the afferent or behavioral response to innocuous warm or noxious heat. Because rodents appear to have the ability to discriminate temperatures in the innocuous warm range, these observations suggest that other channels must contribute to warmth transduction.
TRPV1: There has also been little dispute over whether TRPV1 is activated by noxious heat. The response to heating in some isolated sensory neurons is blocked by TRPV1 antagonists and absent in TRPV1 null mutant mice (Caterina et al 2000). The single-unit response to noxious heat ramps may be attenuated but is not lost in null mutant mice (Caterina et al 2000), and the behavioral response to noxious heat is also not lost with TRPV1 antagonists (Wong and Gavva 2009) and in null mutant mice (Caterina et al 2000). The most pronounced phenotype, at least with respect to thermosensation, in null mutant mice is the absence of inflammatory heat hyperalgesia (Caterina et al 2000). Together, these observations have several important implications. The complete loss of a heat response in isolated sensory neurons from TRPV1 null mutant mice in the face of heat sensitivity that persists in vivo suggests that (1) there are other thermotransducers and (2) TRPV1 may not be present in primary afferents that contribute to thermosensation. The possibility that these other transducers act in concert with TRPV1 to generate a “normal” response to heat is suggested by the decrease in the slope of the single-unit stimulus–response function (Caterina et al 2000). Subsequent data indicating the presence of a subpopulation of afferents deficient in TRPV1 immunoreactivity that were responsive to noxious heating and still present in TPRV1 null mutant mice confirmed that there were additional heat transducers (Woodbury et al 2004). Finally, the prominent role of TRPV1 in inflammatory heat hyperalgesia supported the conclusion that activity in specific subpopulations of afferents mediates specific types of pain.
TRPV2: The thermal threshold for TRPV2, as well as its presence in a subpopulation of DRG neurons with a “medium-diameter” cell body, was an excellent fit with the available single-unit and psychophysical data (Treede et al 1995). Previous data from the primate had revealed two types of nociceptive afferents with axons conducting in the Aδ range. Type II AMHs (A fibers responsive to mechanical and heat stimuli) a higher threshold for activation than did heat-responsive C fibers but a very short utilization time (i.e., were activated very rapidly). These fibers were thought to be primarily responsible for the behavioral responses to acute noxious stimuli. Data from rodents indicated that there was a rough correlation between cell body diameter and axon conduction velocity and suggested that neurons giving rise to axons conducting in the Aδ range should be medium diameter (Lawson 2002). Thus, TRPV2 had the right biophysical properties and was in the right population of afferents. Closer inspection of these correlational studies, however, reveals that there is no correlation between cell body size and axon conduction velocity for neurons with a medium-diameter cell body (Lawson 2002). More problematic is that TRPV2 null mutant mice appear to have no deficits in heat sensitivity, even with very intense stimuli (Park et al 2010). Therefore, if there is another heat transducer in this population of afferents, it has yet to be identified.
Other Thermotransducers
K2P Channels: Consistent with the evidence described above that there must be other thermotransducers contributing to temperature sensation, at least four other channels have been identified that are gated by temperature. The first of these were the K2P channels TREK-1 and -2 and TRAAK (Noel et al 2009). These channels are activated by increases in temperature over a range from approximately 28 to 42°C. Because they are active at rest, the converse is also true, with channel activity being decreased over the same range. TASK has comparable temperature sensitivity but far less dramatic changes in activity associated with warming (Noel et al 2009). There is also evidence that a 4-aminopyridine–sensitive K+ channel contributes to cold transduction (Viana et al 2002), but this channel has yet to be identified. As noted above, all four thermosensitive K2P channels are present in sensory neurons. The involvement of a leak K+ conductance in mediating the response to cooling is consistent with the underlying mechanism predicted in one of the first characterizations of cooling-evoked responses in isolated sensory neurons (Reid et al 1999). Although the increase in K2P channel activity associated with warming should result in a decrease in afferent activity potentially, thereby explaining the therapeutic value of heat for the treatment of inflammatory pain, one would predict that these channels do not contribute the warming-induced increase in activity in warm fibers. Consistent with this prediction, double-null mutant mice deficient in TRAAK and TREK-1 have an increased response to heating and an inflammatory heat hyperalgesia that is fully intact (Noel et al 2009). The cooling phenotype in these mice is a little harder to interpret because the animals show an increase in cold sensitivity following injury. This is in contrast to the prediction that the loss of a channel that normally closes as a means to enable a response to cooling should be associated with an increase in the response to cooling in the absence of injury.
Recent evidence indicates that TRPM3 is also a heat-sensitive channel (Vriens et al 2011). It has a threshold for activation of about 30°C, which can be sensitized by the co-application of pregnenolone sulfate. Like TRPA1 and V1, the thermal sensitivity of TRPM3 appears to reflect a temperature-dependent shift in the voltage dependence of channel gating. Interestingly, in contrast to TRPM2, 4, and 5, which also have thermal sensitivity in a temperature range comparable to TRPM3, TRPM3 null mutant mice exhibit a deficit in noxious heat sensitivity (Vriens et al 2011). Although the majority of TRPM3-expressing neurons also express TRPV1, the observation that TRPM3 is present in a subpopulation of TRPV1-negative neurons suggests that this transducer may contribute to the heat sensitivity observed in afferents from TRPV1 null mutant mice. Nevertheless, the observation that heat sensitivity is still detectable in neurons from TRPM3 null mutant mice in the presence of TRPV1 blockers suggests that at least one more heat transducer has yet to be identified.
TRPC5
TRPC5 is the most recent addition to the thermosensitive family of ion channels (Zimmermann et al 2011). It is proposed to function as a cool receptor with channel activity that is steeply temperature sensitive between 37 and 25°C, where interestingly, only the homomeric channel is cold sensitive whereas the TRPC5/TRPC1 heteromeric channel is not. The channel is detectable in approximately 32% of DRG neurons in a proportion that mirrors the size distribution of the entire population of DRG neurons. The protein appears to be targeted to peripheral terminals, many of which terminate in the superficial layers of the skin. The channel does not contribute to cold-evoked currents in isolated sensory neurons from TRPC5 null mutant mice, possibly because of preferential targeting in the periphery (Zimmermann et al 2011). There are several compensatory changes in the afferent properties of the TRPC5 null mutant, which was used to explain the paradoxical increase in cooling-evoked activity in mechanosensitive C fibers. Thus, absence of the expected cooling phenotype in these animals (i.e., the loss of a cooling response) may be due to compensatory changes. Alternatively, it was suggested that TRPC5 could play a role in responses to cooling that are not tied to behavior (i.e., regulation of peripheral blood flow). Additional data will be needed to further define the role of this putative cold transducer.
Nav1.8
In contrast to other transduction modalities, transduction of noxious cold stimuli is entirely dependent on the biophysical properties of the voltage-gated Na+ channels that underlie action potential initiation (Zimmermann et al 2007). As already touched on above, the ultimate fate of a generator potential depends on a number of factors, not the least of which are the density, relative distribution, and biophysical properties of the voltage-gated Na+ channels that ultimately transform the passive depolarization initiated by the transducer into an action potential. In nociceptive afferents, the voltage-gated Na+ channel Nav1.8 plays a particularly important role in this process. This channel has a number of unique features, including relatively slow kinetics of activation and inactivation but recovery from inactivation that is exceptionally fast (Elliott and Elliott 1993, Flake et al 2004), a high threshold for activation, and a voltage dependence of inactivation curve that is relatively depolarized (Akopian et al 1996). These properties alone can account for many of the unique properties of nociceptive afferents, such as a high threshold for activation and the ability to continue to fire action potentials in the presence of sustained membrane depolarization (Gold 2000b). An additional feature of Nav1.8 is that the channel is not inactivated by noxious cold temperatures (Zimmermann et al 2007). This is in contrast to other voltage-gated Na+ channels responsible for action potential initiation in low-threshold afferents. This difference accounts for why cold tissue feels both “numb” and “on fire” at the same time.
Mechanotransduction
Despite the fact that mechanosensation is the dominant modality of somatosensation and that mechanical hypersensitivity is far more common than thermal or even chemical hypersensitivity (e.g., see Backonja and Stacey 2004), mechanotransduction remains the most poorly understood of the stimulus modalities that activate the somatosensory system. This lack of understanding is not due to a dearth of putative mechanotransducers since a number of ion channels have been shown to be gated by mechanical stimuli. Rather, the problem appears to be due to the fact that data from parallel lines of evidence are not internally consistent. Not only are there differences between the results obtained with isolated neurons, isolated organ preparation, and behavioral assays, but there are also differences between behavioral assays engaging different body regions. Nevertheless, at least two conclusions can be drawn from this data set at present. First, the process of mechanotransduction appears to involve several different mechanotransducers, and second, the underlying mechanisms are likely to vary as a function of both the type of stimulus (stretch versus pressure) and the tissue stimulated.
ASIC Channels
Although ASICs have yet to demonstrate intrinsic mechanosensitivity when expressed in heterologous expression systems, several lines of evidence suggest that these channels may play a role in mechanotransduction. First, the channels are members of a larger family of ion channels, degenerins, identified in Caenorhabditis elegans in genetic screens for mechanosensory defects (Goodman et al 2002, O’Hagan et al 2005). The closest mammalian homologue, ENaCs, have been shown to possess intrinsic mechanosensitivity in lipid bilayers (Ismailov et al 1997) and in heterologous expression systems (Kizer et al 1997). Second, like degenerins, ASICs are also sensitive to amiloride and related compounds (Kizer et al 1997). Third, the channels are present in peripheral terminals (Price et al 2000, 2001; Garcia-Anoveros et al 2001). Unfortunately, there is considerably more conflicting evidence or evidence against a role for ASICs in mechanotransduction than for it. Mechanically evoked currents in isolated sensory neurons from ASIC2/3 double-null mutant mice are fully intact (Drew et al 2004). The mechanical stimulus–response properties of afferents from ASIC1 null mutant mice were comparable to those in wild-type mice when studied in a skin nerve preparation (Page et al 2004). A reduction in the slope of the stimulus–response function with no change in threshold was observed in one study of rapidly adapting low-threshold mechanosensitive afferents from an ASIC2 null mutant (Price et al 2000); however, comparable changes were not observed in a subsequent study (Roza et al 2004). Even more confusing was the observation that in ASIC3 null mutant mice there was a decrease in the mechanosensitivity of Aδ mechanosensitive afferents but an increase in the mechanosensitivity of low-threshold, rapidly adapting afferents. An increase in inflammatory mechanical hypersensitivity was also observed in these mice (Price et al 2001). In contrast to the role of ASICs in the skin, ASIC1, 2, and 3 appear to contribute to the mechanosensitivity of visceral afferents. ASIC1 appears to inhibit mechanosensitivity such that afferents of the GI tract are even more excitable in ASIC1 null mutant mice (Page et al 2004, 2005). The response properties of some subpopulations of GI afferents were increased whereas others were decreased in ASIC2 null mutant mice (Page et al 2005). Most striking, however, was the suppression of mechanosensitivity observed in all but one subpopulation of GI afferents defined by the response properties to various mechanical stimuli (Jones et al 2005, Page et al 2005).
Piezo1 and 2
The most recent additions to the list of channels with intrinsic mechanosensitivity are Piezo1 and 2 (Coste et al 2010). These channels were identified through a heroic expression cloning approach in a heterologous expression system in which “hits” were identified by the cell’s response to a “poke” with a small pipette (Fig. 2-5). Both channels are widely expressed across phyla, as well as in a number of tissues in rodents. Piezo2 appears to play a particularly important role in the rapidly activating and rapidly inactivating “poke”-evoked currents in isolated sensory neurons. The contribution of these channels to mechanosensation in vivo has yet to be determined, but Piezo does appear to be a bona fide mechanotransducer. That is, when expressed it is sufficient to carry an inward current when the cell is mechanically stimulated. There are probably many ancillary proteins that modulate the mechanically induced currents, several discussed here, but this is not a sufficient basis to consider them mechanotransducers.
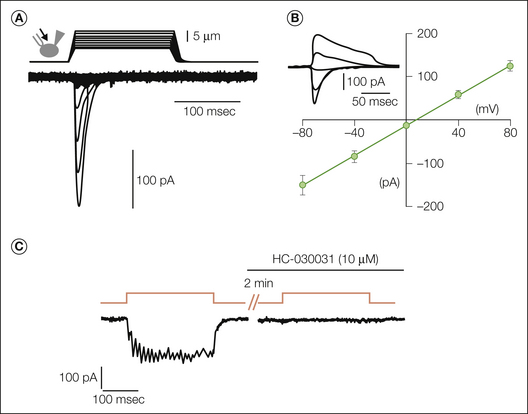
Figure 2-5 Piezo2 underlies rapidly activating and rapidly inactivating mechanically activated (MA) currents in Neuro2A (N2A) cells and sensory neurons.
A, Representative traces of MA inward currents expressed in N2A cells. Cells were subjected to a series of mechanical steps consisting of 1-μm movements with a stimulation pipette (inset drawing, arrow) in the whole-cell patch configuration at a holding potential of −80 mV. B, Average current–voltage relationships of MA currents in N2A (n = 11) cells. The inset shows representative MA currents evoked at holding potentials ranging from −80 to +40 mV (applied 0.7 second before the mechanical step). A comparable current is detectable in sensory neurons that is selectively reduced with small interfering RNA against Piezo2. Sustained MA currents are also detected in sensory neurons (C). However, the sustained current is completely blocked by the TRPA1-selective antagonist HC-030031. Results from these studies indicate that there are several mechanotransducers in sensory neurons. (A and B, From Coste B, Mathur J, Schmidt M, et al 2010 Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60; C, from Vilceanu D, Stucky CL 2010 TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One 5:e12177.)
TRPA1
TRP Channels
One of the most controversial putative mechanotransducers is TRPA1. The first suggestion that the channel might function as a mechanotransducer came from observations that the channel is present in hair cells, where it is localized in the hair tips, and that protein knockdown results in the inhibition of receptor cell function (Corey et al 2004). Subsequent data from TRPA1 null mutant mice ruled out a role for TRPA1 as the mechanotransducer in cochlea hair cells (Kwan et al 2006). In isolated cells, there is evidence both for and against a role for TRPA1 in mechanically activated (MA) currents. In ND-C cells, a cell line derived from neonatal rat DRGs, MA currents are cationic, rapidly activating, and slowly inactivating. TRPA1 is not present in these cells normally, thus suggesting that other channels underlie the MA evoked currents (Rugiero and Wood 2009). Furthermore, transfecting ND-2 cells with TRPA1 failed to alter the MA currents in these cells. In contrast, slowly inactivating MA currents are completely absent in neurons from TRPA1 null mutant mice and completely blocked with the TRPA1 antagonist HC-030031 (Vilceanu and Stucky 2010) (see Fig. 2-5). Interestingly and at least partially consistent with results obtained in ND-C cells, heterologous expression of TRPA1 in HEK293 cells failed to alter the MA currents in these cells, which led the authors to suggest that TRPA1 alone was not sufficient to mediate MA evoked currents.
In isolated skin nerve preparations from TRPA1 null mutant mice, the entire mechanical stimulus–response function in C fibers and only the top of the stimulus–response function in Aδ fibers were suppressed (Kwan et al 2009), with no change in threshold in either population. Surprisingly, the stimulus–response function for slowly adapting Aβ fibers is also suppressed in TRPA1 null mutant mice, whereas that for rapidly adapting Aβ fibers and D hairs is increased. Evidence of TRPA1 in keratinocytes was offered as one potential explanation for the widespread impact of the loss of TRPA1. However, in contrast to the original descriptions of TRPA1 in which it was indicated that channel expression was restricted to a subpopulation of small-diameter TRPV1-expressing sensory neurons (Story et al 2003), evidence of TRPA1 expression was detected in all sizes of DRG neurons, as well as in the terminals of low-threshold afferents. These results suggested that changes in firing properties were due to the actions, or lack thereof, of TRPA1 in all populations of sensory neurons. The subsequent observation that a TRPA1 antagonist produced a suppression of activity in C fibers confirmed a role for TRPA1 in the response to mechanical stimulation in these neurons (Kerstein et al 2009). However, failure of the TRPA1 antagonist to influence the mechanical response properties of Aδ fibers or the response of wide–dynamic range neurons to low-intensity mechanical stimuli (McGaraughty et al 2010) raises the possibility that at least some of the results obtained with the null mutant mouse are due to compensatory changes rather than the loss of TRPA1 itself. Of note, the observation that TRPA1 expression in primary afferents may be more generalized than originally thought has yet to be repeated. Interestingly, TRPA1 appears to contribute to the response of all types of visceral afferents, including vagal afferents, to punctate mechanical stimuli but not to stretch (Brierley et al 2009), again highlighting the importance of both the type and site of stimulation on the relative contribution of a putative transducer.
The behavioral data are consistent with a role for TRPA1 in the response to noxious mechanical stimuli. The response to punctate mechanical stimuli (i.e., the von Frey test) is attenuated in TRPA1 null mutant mice, as is the response to noxious paw pressure in rats following administration of the TRPA1 antagonist HC-030031 (Wei et al 2009). However, one would have predicted that the response to colonic distention would be unaffected by the loss of TRPA1 given the selective deficit in the response to punctate mechanical stimuli in TRPA1 null mutants. Nevertheless, this was observed in only one of two studies in which TPRA1 null mutant mice were used (Cattaruzza et al 2010). In a second study, the response to distention was attenuated in the null mutant. The response to distention was also attenuated following antisense knockdown of TRPA1 (Kondo et al 2009). Unfortunately, interpretation of the behavioral data has been complicated by evidence that TRPA1 acts at the central terminal of nociceptive afferents, where it facilitates nociceptive signaling. Consequently, spinal block of TRPA1 is antinociceptive (McGaraughty et al 2010, Wei et al 2010). Although TRPA1 remains a target of active investigation, if conclusions can be drawn from the data available on TRPA1 and its potential role as a mechanotransducer, they are the following: First, the role of TRPA1 in mechanotransduction is limited to modulation of evoked activity. This implies that other channels or transducers play a dominant role in the process. Second, consistent with this modulatory role, TRPA1 plays a significant role in the injury-induced increase in mechanosensitivity, as has been demonstrated in models of both somatic and visceral inflammation, as well as in several models of peripheral nerve injury (Petrus et al 2007, Eid et al 2008, Wei et al 2009, da Costa et al 2010, McGaraughty et al 2010).
TRPV4
TRPV4 was known to function as an osmolality-gated ion channel (Strotmann et al 2000) before the realization that the channel also functions as a thermoreceptor. The channel is activated by hypertonic solutions, presumably because of the mechanical stress associated with cell shrinkage. The channel is widely distributed in a number of different cell types, including primary afferents, probably reflecting the fact that the ability to respond to changes in tonicity is essential to most cell types, particularly epithelial cells. However, in sensory neurons the channel is essential for the pain behavioral response associated with hypertonic solutions (Alessandri-Haber et al 2005). Interestingly, although TRPV4 is responsive to swelling, chemical, and thermal stimuli, each modality activates the channel via distinct pathways (Vriens et al 2004). Like other TRP channels, TRPV4 may play a more important role in mechanosensation in the presence of injury, as suggested by the observation that mechanical hypersensitivity is attenuated in TRPV4 null mutant mice (Alessandri-Haber et al 2004, Chen et al 2007, Cenac et al 2008, Zhang et al 2008).
K2P
There are at least three K2P channels present in sensory neurons that have been shown to have mechanosensitivity: TREK-1, TREK-2, and TRAAK (Maingret et al 1999a, 1999b; Bang et al 2000). TREK-1 was first described as a chemotransducer activated by arachidonic acid (Fink et al 1998) and inhibited by cAMP (Fink et al 1996). It was subsequently shown to be activated by osmotic swelling, stretch, and membrane crenators (Maingret et al 1999b). TREK-2 and TRAAK have the same mechanosensitive properties as TREK-1. Despite evidence that the channels are differentially regulated in the presence of tissue injury in a manner consistent with a role in the injury-induced hypersensitivity model (Marsh et al 2012), the relative contribution of K2P channels to mechanosensitivity remains to be determined.
T-Type Ca2+ Channel (Cav3.2)
Transient or T-type Ca2+ channels are members of the voltage-activated Ca2+ channel family that have a low threshold for activation (Catterall et al 2005). They are therefore also referred to as low-threshold voltage-activated channels. The α subunit of these channels is a large molecule with four homologous domains, each of which has six transmembrane segments with a pore loop between segments 5 and 6 and a voltage sensor in segment 4. Thus, the α subunit has all the components necessary for a functional channel. Three α subunits for the low-threshold channel have been identified and designated Cav3.1–3.3. Of these, Cav3.2 is enriched in a subpopulation of sensory neurons that appear to innervate D hairs (Shin et al 2003). More importantly, the response to mechanical stimulation of the receptive field of D-hair units is selectively attenuated with the T-type channel blocker mibefradil. There is evidence that T-type currents are also enriched in a subpopulation of nociceptive afferents and that sensitization of these channels results in a decrease in the mechanical threshold (Todorovic and Jevtovic-Todorovic 2006). However, data from a Cav3.2 null mutant mouse suggest that the mechanical response properties of nociceptive afferents are minimally altered, thus indicating a minor role for this channel in these afferents. This is in contrast to the response of D-hair fibers in this knockout line, which is reduced by more than 50%, largely as a result of an increase in the mechanical threshold and utilization time (Shin et al 2003). Although data in support of a role for T-type channels in the mechanical response properties of D hairs are compelling, the channel has received the most attention for its role in mediating the hypersensitivity observed in response to a number of pain-producing manipulations, including hydrogen sulfide injection, diabetic and post-traumatic neuropathy (including a compressed DRG model; Wen et al 2006), and a model of irritable bowel syndrome (Marger et al 2011). Consistent with these observations is that a number of small-molecule inhibitors of T-type channels have antinociceptive efficacy in a variety of animal models of persistent pain (Zamponi et al 2009).
Polymodality
It is clear from the preceding discussion that in primary afferents, transducer specificity is rare. Many transducers are activated by several stimulus modalities. Even though the functional implications of this polymodality are still being worked out, the chemosensitivity of many of the thermo- and mechanotransducers makes interpretation of results from intact preparations difficult, at least with respect to the contribution of a specific transducer to the response to a specific stimulus. For example, it will be difficult to distinguish the relative contribution of the mechanosensitive properties of the transducer from its chemosensitive properties if it is possible that chemicals that activate the transducer are released from other cells in response to mechanical stimuli. Evidence abounds that chemicals are released from thermally (Patwardhan et al 2010) and mechanically (Burnstock 2009) stimulated tissue, thus making this a serious technical hurdle.
Indirect Signaling Pathways
The focus up until now has been on transduction in sensory neurons. However, it is becoming increasingly clear that many putative transducers are not only present but also functional in other cell types. The bladder epithelium, or urothelial cells, are probably the best characterized in this regard. A wide variety of chemo-, thermo-, and mechanotransducers are reportedly present on these cells, including nAChRs, bradykinin receptors (B1 and B2), and TRP channels (TRPA1, TRPM8, and TRPV1–2, 4) (see Birder 2011 for review). The only transducer present in urothelial cells minimally represented in sensory neurons (Hermanstyne et al 2008) is the ENaC, which is related to ASIC channels (see above). Urothelial cells are activated by thermal, mechanical, and chemical stimuli, and they release a variety of mediators that are able to activate and/or sensitize afferents. These mediators include ACh, ATP, reactive oxygen species (nitric oxide), peptides, neurotrophins, cyclooxygenase metabolites, and cytokines (Birder 2011). Of these, ATP has received the most attention because it was the first mediator shown to be released in response to bladder stretch. This observation provided a mechanism for mechanical transduction via ATP binding P2X receptors on primary afferents that terminate in close contact to urothelial cells. Consistent with this model, there is an increase in release of ATP from the urothelium of both animals (Birder et al 2003) and humans (Sun and Chai 2002) with interstitial cystitis, a painful inflammation of the bladder. TRPV1 is another channel that has received a lot of attention both because it appears to contribute to normal bladder function (i.e., bladder afferents from TRPV1 null mutant mice have a lower response to bladder distention) (Birder et al 2002) and because TRPV1 agonists can be used to produce afferent desensitization and provide some relief for patients with pain associated with bladder hypersensitivity disorders (Cruz 1998).
The bladder urothelium is proving to be far from unique with respect to its potential role in sensory transduction inasmuch as similar roles have been implicated for epithelial cells lining the GI tract (Wynn et al 2004) and airway (Button et al 2007). Epithelial cell signaling appears to be even more complex in the skin, where keratinocytes have been shown to express not only a wide variety of transducers but also channels that could serve to facilitate signaling, such as voltage-gated Na+ channels (Zhao et al 2008, Dussor et al 2009, Hou et al 2011). Like the bladder, there is considerable heterogeneity among keratinocytes with regard to the expression of various transducers and ion channels. Consistent with the fact that skin consists of stratified epithelium, there is also heterogeneity in the distribution of channels between layers. Interestingly, this pattern appears to be disrupted in the presence of tissue injury and under pathological conditions (Zhao et al 2008), thus raising the possibility that these changes contribute to the associated alterations in sensation. Much remains to be determined, however, with respect to the role of these cells in signal transduction, not the least of which is the answer to how modality specificity is achieved in the nervous system if all modalities of stimuli result in the release of common mediators such as ATP.
Lessons Learned from Injury-Induced Changes
Our understanding of the molecular mechanisms of transduction is complicated by the apparent paradox between naïve and injured tissue with regard to the relative contribution of putative transducers to sensation since the contribution often appears to be greater in the presence of tissue injury. This may be due to the fact that in some tissues (e.g., skin), the relative contribution of C-fiber activity is minimal under normal conditions and becomes apparent only in the presence of injury-induced hypersensitivity (Khasar and Levine 1996). This differential contribution of fiber types may also explain why putative transducers appear to contribute more significantly to the response of naïve visceral tissue, given the relative dearth of myelinated fibers that innervate visceral structures. Of course, the implication of such a suggestion is that we have yet to identify transducers underlying the response of the more rapidly conducting Aβ and Aδ fibers thought to dominate the response to acute noxious stimulation of naïve cutaneous tissue. The paradox may also reflect the fact that many of the known transducers are dramatically up-regulated in the presence of tissue injury. Injury-induced changes in TRPV1 are an excellent example of transducer up-regulation. The channel is sensitized via an array of second-messenger pathways, including those involving protein kinase A (PKA), protein kinase C (PKC), phosphatidylinositol-3′-kinase (PI3K), calcium-calmodulin–dependent kinase II protein (CaMKII), and p38/mitogen-activated protein kinase (MAPK), which results in an increase in channel activity, a decrease in desensitization, and/or an increase in receptor density as a result of translocation to the membrane (Gold and Gebhart 2010). On a slower time scale, there is evidence that TRPV1 protein is increased via post-transcriptional mechanisms (Ji et al 2002) and that the distribution of the channel is increased in DRG neurons, thus suggesting alterations in transcriptional machinery as well (Breese et al 2005). Following nerve injury there is evidence that the channel is even expressed in A fibers (Rashid et al 2003). These observations highlight the importance of both the time course of changes after injury and injury-induced changes in the relative contribution of afferent subpopulations to pain after injury.
Changes in the properties of ion channels regulating the passive and active electrophysiological properties of the afferent are also likely to contribute to the increase in the relative impact of putative transducers on the generation of afferent activity in the presence of tissue injury. That is, the fate of the generator potential depends on both the passive and active electrophysiological properties of the afferent terminal, with passive properties that include resting membrane potential and input resistance influencing the magnitude of the generator potential and the distance over which it is passively spread. The resting membrane potential will also influence the availability of many of the ion channels underlying active electrophysiological properties. Channels that contribute to passive properties in primary afferents include the P2K channels, which are dynamically regulated by a variety of mediators (see above), as well as over the long term by changes in expression (Marsh et al 2012). Voltage- and Ca2+-modulated ion channels underlie active electrophysiological properties, and these will determine the action potential threshold, the amplitude and duration of the action potential, the amplitude and duration of the afterpotential, and more stimulus–response properties such as the interspike interval and burst duration (Harriott and Gold 2009a).
Because the generator potential provides the underlying drive for activation of these other ion channels, the interaction between the amplitude and duration of the generator potential with the biophysical properties of the channels underlying the active electrophysiological properties can have a profound influence on the output of the neuron. For example, very slow depolarization in a neuron in which initiation of an action potential is dependent on a voltage-gated Na+ channel subject to steady-state inactivation may drive the inactivation of Na+ channels before initiation of the action potential. Similarly, a large and rapid depolarization associated with activation of TRPV1 may drive the membrane potential to 0 mV, the reversal potential for TRPV1, and enable the generation of few if any action potentials before a depolarization-induced inactivation of voltage-gated Na+ channels. Another critical point of interaction between the transducer and ion channels underlying the active electrophysiological properties is at the level of the permeant ions. That is, TRPV1 is highly permeable to Ca2+, whereas ASIC3 is far more selective for Na+. The presence of a high density of Ca2+-dependent ion channels that contribute to determination of the action potential threshold and/or burst duration should respond very differently to stimuli engaging TRPV1 than to those engaging ASIC3. Conversely, Ca2+ influx via voltage-gated Ca2+ channels is not only important for peripheral transmitter release and therefore the efferent function of afferents but can also facilitate the desensitization of channels such as TRPV1 (Vyklicky et al 2008).
The literature is now full of descriptions of injury-induced changes in an array of ion channels that underlie active electrophysiological properties. This includes changes in a variety of voltage-gated K+ channels, Na+ channels, Ca2+ channels, and Ca2+-dependent K+ and Cl− channels in a manner consistent with an increase in afferent excitability (Harriott and Gold 2009a). Changes in all channel types are associated with both the acute actions of inflammatory mediators and longer-term changes in channel distribution and gene expression. Importantly, as noted above, the nature and timing of the changes depend on a number of factors, including the type of injury, the site of injury, the previous history of the injured tissue, age, and sex.
Finally, data from nerve injury models have highlighted the importance of transducer distribution on the emergence of ectopic activity. There is evidence that transducers may be inserted into the axon membrane following nerve injury and thereby result in the emergence of mechanical, thermal, and presumably chemical sensitivity at sites along the axon (Michaelis et al 2000, Grossmann et al 2009, Janig et al 2009). This process appears to occur much more readily in muscle afferents. The process is also likely to occur within ganglia and contribute to the emergence of ectopic activity arising from within the ganglia following traumatic nerve injury (Devor 1999). The emergence of sources of activity at locations remote from the site of injury or even the painful tissue can add to the difficulty in treating neuropathic pain with peripherally targeted interventions.
Conclusion
The past 15 years have yielded an explosion of information regarding the molecular mechanisms of sensory transduction. An array of putative transducers have been identified, as have details regarding mechanisms underlying their activation. As is true of many aspect of science, the more we learn about something, the more complicated it becomes. This has been particularly true of our understanding of thermal transduction, which seemed so clear 5 years ago but is considerably less so now. This complexity has clearly proved to be a barrier to the development of novel therapeutic approaches for the treatment of pain. With multiple channels working in parallel and/or differentially contributing to the response in one fiber type or after a particular type of injury, it should not be surprising that an effective blocker of a particular channel has not emerged as the next silver bullet for the treatment of pain. Nevertheless, although it is clear that there is still much to learn about sensory transduction, novel approaches are on the horizon that should provide relief for many in need.
The references for this chapter can be found at www.expertconsult.com.
References
Abdrakhmanova G.R., AlSharari S., Kang M., et al. α7-nAChR–mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;299:G761–G768.
Akopian A.N. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Current Pharmaceutical Biotechnology. 2011;12:89–94.
Akopian A.N., Sivilotti L., Wood J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262.
Alessandri-Haber N., Dina O.A., Joseph E.K., et al. A transient receptor potential vanilloid 4–dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. Journal of Neuroscience. 2006;26:3864–3874.
Alessandri-Haber N., Dina O.A., Yeh J.J., et al. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. Journal of Neuroscience. 2004;24:4444–4452.
Alessandri-Haber N., Joseph E., Dina O.A., et al. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79.
Alvarez de la Rosa D., Zhang P., Shao D., et al. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2326–2331.
Ambalavanar R., Moritani M., Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–291.
Amir R., Liu C.N., Kocsis J.D., et al. Oscillatory mechanism in primary sensory neurones. Brain. 2002;125:421–435.
Anand P., Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. British Journal of Anaesthesia. 2011;107:490–502.
Andersson D.A., Gentry C., Moss S., et al. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. Journal of Neuroscience. 2008;28:2485–2494.
Babes A., Ciobanu A.C., Neacsu C., et al. TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Current Pharmaceutical Biotechnology. 2011;12:78–88.
Baccaglini P.I., Hogan P.G. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:594–598.
Backonja M.M., Stacey B. Neuropathic pain symptoms relative to overall pain rating. Journal of Pain. 2004;5:491–497.
Bandell M., Story G.M., Hwang S.W., et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857.
Bang H., Kim Y., Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. Journal of Biological Chemistry. 2000;275:17412–17419.
Bautista D.M., Jordt S.E., Nikai T., et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282.
Bautista D.M., Movahed P., Hinman A., et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12248–12252.
Bautista D.M., Siemens J., Glazer J.M., et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208.
Bautista D.M., Sigal Y.M., Milstein A.D., et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nature Neuroscience. 2008;11:772–779.
Benson C.J., Eckert S.P., McCleskey E.W. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circulation Research. 1999;84:921–928.
Bessou P., Perl E.R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. Journal of Neurophysiology. 1969;32:1025–1043.
Binshtok A.M., Bean B.P., Woolf C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610.
Birder L.A. Urothelial signaling. Handbook of Experimental Pharmacology. 2011;202:207–231.
Birder L.A., Barrick S.R., Roppolo J.R., et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. American Journal of Physiology. Renal Physiology. 2003;285:F423–F429.
Birder L.A., Nakamura Y., Kiss S., et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature Neuroscience. 2002;5:856–860.
Birdsong W.T., Fierro L., Williams F.G., et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749.
Birrell G.J., McQueen D.S., Iggo A., et al. PGI2-induced activation and sensitization of articular mechanonociceptors. Neuroscience Letters. 1991;124:5–8.
Bohlen C.J., Chesler A.T., Sharif-Naeini R., et al. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2010;479:410–414.
Bradbury E.J., Burnstock G., McMahon S.B. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Molecular and Cellular Neurosciences. 1998;12:256–268.
Breese N.M., George A.C., Pauers L.E., et al. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49.
Brierley S.M., Hughes P.A., Page A.J., et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 137, 2009. 2084–2095, e3
Bunnett N.W., Cottrell G.S. Trafficking and signaling of G protein–coupled receptors in the nervous system: implications for disease and therapy. CNS & Neurological Disorders Drug Targets. 2010;9:539–556.
Burnstock G. Purinergic signalling—an overview. Novartis Foundation Symposium. 2006;276:26–48. discussion 48–57, 275–281
Burnstock G. Purinergic mechanosensory transduction and visceral pain. Molecular Pain. 2009;5:69.
Button B., Picher M., Boucher R.C. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. Journal of Physiology. 2007;580:577–592.
Cairns B.E., Hu J.W., Arendt-Nielsen L., et al. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. Journal of Neurophysiology. 2001;86:782–791.
Cairns B.E., Sessle B.J., Hu J.W. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. Journal of Neuroscience. 1998;18:8056–8064.
Cairns B.E., Sessle B.J., Hu J.W. Characteristics of glutamate-evoked temporomandibular joint afferent activity in the rat. Journal of Neurophysiology. 2001;85:2446–2454.
Campbell J.N., LaMotte R.H. Latency to detection of first pain. Brain Research. 1983;266:203–208.
Carlton S.M., Du J., Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Research. 2009;1248:86–95.
Carlton S.M., Zhou S., Coggeshall R.E. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722.
Carr R.W., Sittl R., Fleckenstein J., et al. GABA increases electrical excitability in a subset of human unmyelinated peripheral axons. PLoS One. 2010;5:e8780.
Carstens E., Kuenzler N., Handwerker H.O. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. Journal of Neurophysiology. 1998;80:465–492.
Caspani O., Zurborg S., Labuz D., et al. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383.
Castro J., Aromataris E.C., Rychkov G.Y., et al. A small component of the endoplasmic reticulum is required for store-operated Ca2+ channel activation in liver cells: evidence from studies using TRPV1 and taurodeoxycholic acid. Biochemical Journal. 2009;418:553–566.
Caterina M.J., Gold M.S., Meyer R.A. Molecular biology of nociceptors. In: Hunt S., Koltzenburg M., eds. The neurobiology of pain. Oxford: Oxford University Press; 2005:1–33.
Caterina M.J., Leffler A., Malmberg A.B., et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313.
Caterina M.J., Rosen T.A., Tominaga M., et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441.
Caterina M.J., Schumacher M.A., Tominaga M., et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824.
Cattaruzza F., Spreadbury I., Miranda-Morales M., et al. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;298:G81–G91.
Catterall W.A., Perez-Reyes E., Snutch T.P., et al. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacological Reviews. 2005;57:411–425.
Cavanaugh D.J., Lee H., Lo L., et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9075–9080.
Cenac N., Altier C., Chapman K., et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. 946.e1–e2
Cesare P., McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15435–15439.
Chaplan S.R., Guo H.Q., Lee D.H., et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. Journal of Neuroscience. 2003;23:1169–1178.
Chen C.C., England S., Akopian A.N., et al. A sensory neuron–specific, proton-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10240–10245.
Chen J., Joshi S.K., DiDomenico S., et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172.
Chen J., Kim D., Bianchi B.R., et al. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Molecular Pain. 2009;5:3.
Chen X., Alessandri-Haber N., Levine J.D. Marked attenuation of inflammatory mediator–induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Molecular Pain. 2007;3:31.
Chuang H.H., Prescott E.D., Kong H., et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962.
Chung M.K., Guler A.D., Caterina M.J. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nature Neuroscience. 2008;11:555–564.
Cook S.P., McCleskey E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47.
Corey D.P., Garcia-Anoveros J., Holt J.R., et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730.
Coste B., Mathur J., Schmidt M., et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60.
Cruz F. Desensitization of bladder sensory fibers by intravesical capsaicin or capsaicin analogs. A new strategy for treatment of urge incontinence in patients with spinal detrusor hyperreflexia or bladder hypersensitivity disorders. International Urogynecology Journal and Pelvic Floor Dysfunction. 1998;9:214–220.
Czirjak G., Enyedi P. TRESK background K(+) channel is inhibited by phosphorylation via two distinct pathways. Journal of Biological Chemistry. 2010;285:14549–14557.
da Costa D.S., Meotti F.C., Andrade E.L., et al. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–437.
Darian-Smith I., Johnson K.O., Dykes R. “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. Journal of Neurophysiology. 1973;36:325–346.
del Camino D., Murphy S., Heiry M., et al. TRPA1 contributes to cold hypersensitivity. Journal of Neuroscience. 2010;30:15165–15174.
Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;6(Suppl):S27–S35.
Dina O.A., Aley K.O., Isenberg W., et al. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. European Journal of Neuroscience. 2001;13:2227–2233.
Diogenes A., Ferraz C.C., Akopian A.N., et al. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. Journal of Dental Research. 2011;90:759–764.
Djouhri L., Wrigley D., Thut P.D., et al. Spinal nerve injury increases the percentage of cold-responsive DRG neurons. Neuroreport. 2004;15:457–460.
Dobler T., Springauf A., Tovornik S., et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. Journal of Physiology. 2007;585:867–879.
Drew L.J., Rohrer D.K., Price M.P., et al. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. Journal of Physiology. 2004;556:691–710.
Dubner R. The neurobiology of persistent pain and its clinical implications. Supplements to Clinical Neurophysiology. 2004;57:3–7.
Dubner R., Sumino R., Wood W.I. A peripheral “cold” fiber population responsive to innocuous and noxious thermal stimuli applied to monkey’s face. Journal of Neurophysiology. 1975;38:1373–1389.
Dussor G., Koerber H.R., Oaklander A.L., et al. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Research Reviews. 2009;60:24–35.
Eid S.R., Crown E.D., Moore E.L., et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Molecular Pain. 2008;4:48.
Elliott A.A., Elliott J.R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. Journal of Physiology. 1993;463:39–56.
Emery E.C., Young G.T., Berrocoso E.M., et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466.
Everaerts W., Zhen X., Ghosh D., et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19084–19089.
Fayyaz M., Lackner J.M. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Therapeutics and Clinical Risk Management. 2008;4:41–48.
Fink M., Duprat F., Lesage F., et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862.
Fink M., Lesage F., Duprat F., et al. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308.
Fitzgerald E.M., Okuse K., Wood J.N., et al. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. Journal of Physiology. 1999;516:433–446.
Flake N.M., Bonebreak D.B., Gold M.S. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. Journal of Neurophysiology. 2005;93:1585–1597.
Flake N.M., Lancaster E., Weinreich D., et al. Absence of an association between axotomy-induced changes in sodium currents and excitability in DRG neurons from the adult rat. Pain. 2004;109:471–480.
Fu L.W., Pan H.L., Longhurst J.C. Endogenous histamine stimulates ischemically sensitive abdominal visceral afferents through H1 receptors. American Journal of Physiology. 1997;273:H2726–H2737.
Gahring L.C., Osborne A.V., Reed M., et al. Neuronal nicotinic alpha7 receptors modulate early neutrophil infiltration to sites of skin inflammation. Journal of Neuroinflammation. 2010;7:38.
Garcia-Anoveros J., Samad T.A., Zuvela-Jelaska L., et al. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. Journal of Neuroscience. 2001;21:2678–2686.
Gavva N.R., Tamir R., Klionsky L., et al. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Molecular Pharmacology. 2005;68:1524–1533.
Gokin A.P., Fareed M.U., Pan H.L., et al. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. Journal of Neuroscience. 2001;21:5358–5366.
Gold M.S. Spinal nerve ligation: what to blame for the pain and why. Pain. 2000;84:117–120.
Gold M.S. Sodium channels and pain therapy. Current Opinion in Anaesthesiology. 2000;13:565–572.
Gold M.S. Molecular basis of receptors. In: Merskey H., Loeser J.D., Dubner R., eds. The paths of pain 1975-2005. Seattle: IASP Press; 2005:49–67.
Gold M.S., Dastmalchi S., Levine J.D. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275.
Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nature Medicine. 2010;16:1248–1257.
Goodman M.B., Ernstrom G.G., Chelur D.S., et al. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042.
Gracheva E.O., Ingolia N.T., Kelly Y.M., et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011.
Grossmann L., Gorodetskaya N., Teliban A., et al. Cutaneous afferent C-fibers regenerating along the distal nerve stump after crush lesion show two types of cold sensitivity. European Journal of Pain. 2009;13:682–690.
Guler A.D., Lee H., Iida T., et al. Heat-evoked activation of the ion channel, TRPV4. Journal of Neuroscience. 2002;22:6408–6414.
Hardt M., Lam D.K., Dolan J.C., et al. Surveying proteolytic processes in human cancer microenvironments by microdialysis and activity-based mass spectrometry. Proteomics. Clinical Applications. 2011;5:636–643.
Harriott A.M., Dessem D., Gold M.S. Inflammation increases the excitability of masseter muscle afferents. Neuroscience. 2006;141:433–442.
Harriott A.M., Gold M.S. Contribution of primary afferent channels to neuropathic pain. Current Pain and Headache Reports. 2009;13:197–207.
Harriott A.M., Gold M.S. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. Journal of Neurophysiology. 2009;101:3126–3134.
Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2005;371:307–314.
Hensel H., Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflugers Archiv: European Journal of Physiology. 1971;329:1–8.
Hermanstyne T.O., Markowitz K., Fan L., et al. Mechanotransducers in rat pulpal afferents. Journal of Dental Research. 2008;87:834–838.
Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacology Reviews. 1991;43:143–201.
Honore E. The neuronal background K2P channels: focus on TREK1. Nature Reviews. Neuroscience. 2007;8:251–261.
Hopwood S.E., Trapp S. TASK-like K+ channels mediate effects of 5-HT and extracellular pH in rat dorsal vagal neurones in vitro. Journal of Physiology. 2005;568:145–154.
Hou Q., Barr T., Gee L., et al. Keratinocyte expression of calcitonin gene–related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–2051.
Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annual Review of Biochemistry. 2003;72:609–642.
Hughes P.A., Brierley S.M., Young R.L., et al. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. Journal of Comparative Neurology. 2007;500:863–875.
Iggo A. Cutaneous thermoreceptors in primates and sub-primates. Journal of Physiology. 1969;200:403–430.
Immke D.C., McCleskey E.W. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nature Neuroscience. 2001;4:869–870.
Ingram S.L., Williams J.T. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–186.
Ismailov I.I., Berdiev B.K., Shlyonsky V.G., et al. Mechanosensitivity of an epithelial Na+ channel in planar lipid bilayers: release from Ca2+ block. Biophysical Journal. 1997;72:1182–1192.
Jafri M.S., Moore K.A., Taylor G.E., et al. Histamine H1 receptor activation blocks two classes of potassium current, IK(rest) and IAHP, to excite ferret vagal afferents. Journal of Physiology. 1997;503:533–546.
Jänig W., Grossmann L., Gorodetskaya N. Mechano- and thermosensitivity of regenerating cutaneous afferent nerve fibers. Experimental Brain Research. 2009;196:101–114.
Jarvis M.F. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opinion on Therapeutic Targets. 2003;7:513–522.
Jarvis M.F., Khakh B.S. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215.
Ji R.R., Samad T.A., Jin S.X., et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68.
Jiang L.H., Gamper N., Beech D.J. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Current Drug Targets. 2011;12:724–736.
Johnson K.O., Darian-Smith I., LaMotte C. Peripheral neural determinants of temperature discrimination in man: a correlative study of responses to cooling skin. Journal of Neurophysiology. 1973;36:347–370.
Jones D.M., Tucker B.A., Rahimtula M., et al. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. Journal of Neurochemistry. 2003;86:1116–1128.
Jones R.C., 3rd., Xu L., Gebhart G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. Journal of Neuroscience. 2005;25:10981–10989.
Jordt S.E., Bautista D.M., Chuang H.H., et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265.
Jordt S.E., McKenny D.D., Julius D., et al. Lessons from peppers and peppermint: the molecular logic of thermosensation. Current Opinion in Neurobiology. 2003;13:487–492.
Karashima Y., Talavera K., Everaerts W., et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1273–1278.
Katsura H., Obata K., Mizushima T., et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Experimental Neurology. 2006;200:112–123.
Kenshalo D.R., Scott H.A., Jr. Temporal course of thermal adaptation. Science. 1966;151:1095–1096.
Kerstein P.C., del Camino D., Moran M.M., et al. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Molecular Pain. 2009;5:19.
Khasar S.G., Levine J.D. Neonatal capsaicin attenuates mechanical nociception in the rat. Neuroscience Letters. 1996;205:141–143.
Kizer N., Guo X.L., Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1013–1018.
Knowlton W.M., Daniels R.L., Palkar R., et al. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894.
Kobayashi K., Fukuoka T., Yamanaka H., et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. Journal of Comparative Neurology. 2005;481:377–390.
Kondo T., Obata K., Miyoshi K., et al. Transient receptor potential A1 mediates gastric distention–induced visceral pain in rats. Gut. 2009;58:1342–1352.
Kouranova E.V., Strassle B.W., Ring R.H., et al. Hyperpolarization-activated cyclic nucleotide–gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience. 2008;153:1008–1019.
Kremeyer B., Lopera F., Cox J.J., et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680.
Kwan K.Y., Allchorne A.J., Vollrath M.A., et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289.
Kwan K.Y., Glazer J.M., Corey D.P., et al. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. Journal of Neuroscience. 2009;29:4808–4819.
La J.H., Gebhart G.F. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2011;301:G165–G174.
La J.H., Schwartz E.S., Gebhart G.F. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187.
Lafreniere R.G., Cader M.Z., Poulin J.F., et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nature Medicine. 2010;16:1157–1160.
Lam D.K., Schmidt B.L. Serine proteases and protease-activated receptor 2–dependent allodynia: a novel cancer pain pathway. Pain. 2010;149:263–272.
Lam D.K., Sessle B.J., Hu J.W. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Research. 2009;1253:48–59.
Lam D.K., Sessle B.J., Hu J.W. Glutamate and capsaicin effects on trigeminal nociception I: activation and peripheral sensitization of deep craniofacial nociceptive afferents. Brain Research. 2009;1251:130–139.
LaMotte R.H., Campbell J.N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. Journal of Neurophysiology. 1978;41:509–528.
Lawson S.N. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Experimental Physiology. 2002;87:239–244.
Lee K.-Y., Charbonnet M., Gold M.S. Upregulation of high affinity GABAA receptors in cultured rat dorsal root ganglion neurons. Neuroscience. 2012;208:133–142.
Lingueglia E. Acid-sensing ion channels in sensory perception. Journal of Biological Chemistry. 2007;282:17325–17329.
Liu B., Linley J.E., Du X., et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. Journal of Clinical Investigation. 2010;120:1240–1252.
Liu C.N., Devor M., Waxman S.G., et al. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. Journal of Neurophysiology. 2002;87:2009–2017.
Liu C.N., Michaelis M., Amir R., et al. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. Journal of Neurophysiology. 2000;84:205–215.
Maingret F., Fosset M., Lesage F., et al. TRAAK is a mammalian neuronal mechano-gated K+ channel. Journal of Biological Chemistry. 1999;274:1381–1387.
Maingret F., Patel A.J., Lesage F., et al. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. Journal of Biological Chemistry. 1999;274:26691–26696.
Marger F., Gelot A., Alloui A., et al. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11268–11273.
Marsh B., Acosta C., Djouhri L., et al. Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Molecular and Cellular Neurosciences. 2012;49:375–386.
Materazzi S., Nassini R., Andre E., et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12045–12050.
Mayer M.L. Structure and mechanism of glutamate receptor ion channel assembly, activation and modulation. Current Opinion in Neurobiology. 2011;21:283–290.
McCarter G.C., Reichling D.B., Levine J.D. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neuroscience Letters. 1999;273:179–182.
McGaraughty S., Chu K.L., Perner R.J., et al. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Molecular Pain. 2010;6:14.
McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58.
McNamara C.R., Mandel-Brehm J., Bautista D.M., et al. TRPA1 mediates formalin-induced pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13525–13530.
Mense S. Reduction of the bradykinin-induced activation of feline group III and IV muscle receptors by acetylsalicylic acid. Journal of Physiology. 1982;326:269–283.
Michaelis M., Liu X., Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. Journal of Neuroscience. 2000;20:2742–2748.
Michels G., Moss S.J. GABAA receptors: properties and trafficking. Critical Reviews in Biochemistry and Molecular Biology. 2007;42:3–14.
Miller K.E., Hoffman E.M., Sutharshan M., et al. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacology & Therapeutics. 2011;130:283–309.
Molliver D.C., Cook S.P., Carlsten J.A., et al. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. European Journal of Neuroscience. 2002;16:1850–1860.
Molliver D.C., Immke D.C., Fierro L., et al. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular Pain. 2005;1:35.
Namer B., Hilliges M., Orstavik K., et al. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137:41–49.
Nandigama R., Bonitz M., Papadakis T., et al. Muscarinic acetylcholine receptor subtypes expressed by mouse bladder afferent neurons. Neuroscience. 2010;168:842–850.
Naziroglu M., Ozgul C., Cig B., et al. Glutathione modulates Ca(2+) influx and oxidative toxicity through TRPM2 channel in rat dorsal root ganglion neurons. Journal of Membrane Biology. 2011;242:109–118.
Nealen M.L., Gold M.S., Thut P.D., et al. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. Journal of Neurophysiology. 2003;90:515–520.
Ness T.J., Gebhart G.F. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234.
Nilius B., Owsianik G. The transient receptor potential family of ion channels. Genome Biology. 2011;12:218.
Noel J., Zimmermann K., Busserolles J., et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318.
Nolano M., Simone D.A., Wendelschafer-Crabb G., et al. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145.
Ochoa-Cortes F., Ramos-Lomas T., Miranda-Morales M., et al. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;299:G723–G732.
O’Hagan R., Chalfie M., Goodman M.B. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature Neuroscience. 2005;8:43–50.
Özgül C., Naziroglu M. TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca(2+) influx in rat dorsal root ganglion. Physiology & Behavior. 2012;106:122–128.
Page A.J., Brierley S.M., Martin C.M., et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747.
Page A.J., Brierley S.M., Martin C.M., et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415.
Parada C.A., Reichling D.B., Levine J.D. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190.
Park U., Vastani N., Guan Y., et al. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. Journal of Neuroscience. 2010;31:11425–11436.
Patel A.J., Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends in Neurosciences. 2001;24:339–346.
Patwardhan A.M., Akopian A.N., Ruparel N.B., et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. Journal of Clinical Investigation. 2010;120:1617–1626.
Patwardhan A.M., Diogenes A., Berg K.A., et al. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–124.
Patwardhan A.M., Scotland P.E., Akopian A.N., et al. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18820–18824.
Peier A.M., Moqrich A., Hergarden A.C., et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715.
Peier A.M., Reeve A.J., Andersson D.A., et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049.
Perraud A.L., Fleig A., Dunn C.A., et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599.
Petrus M., Peier A.M., Bandell M., et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Molecular Pain. 2007;3:40.
Petruska J.C., Napaporn J., Johnson R.D., et al. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. Journal of Neurophysiology. 2000;84:2365–2379.
Petruska J.C., Napaporn J., Johnson R.D., et al. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115:15–30.
Picton A.J., Fisher J.L. Effect of the alpha subunit subtype on the macroscopic kinetic properties of recombinant GABA(A) receptors. Brain Research. 2007;1165:40–49.
Pierce P.A., Xie G.X., Levine J.D., et al. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996;70:553–559.
Prescott E.D., Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288.
Price M.P., Lewin G.R., McIlwrath S.L., et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011.
Price M.P., McIlwrath S.L., Xie J., et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083.
Price T.J., Cervero F., Gold M.S., et al. Chloride regulation in the pain pathway. Brain Research Reviews. 2009;60:149–170.
Qadri Y.J., Rooj A.K., Fuller C.M. ENaCs and ASICs as therapeutic targets. American Journal of Physiology. Cell Physiology. 2012;302:C943–C965.
Qian H. GABAC receptors in the vertebrate retina. In: Kolb H., Fernandez E., Nelson R., eds. Webvision: the organization of the retina and visual system [Internet, updated June 1, 2007]. Salt Lake City: University of Utah Health Sciences Center, 1995–2005.
Rashid M.H., Inoue M., Kondo S., et al. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. Journal of Pharmacology and Experimental Therapeutics. 2003;304:940–948.
Rau K.K., Cooper B.Y., Johnson R.D. Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neuroscience. 2006;141:955–963.
Rau K.K., Johnson R.D., Cooper B.Y. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. Journal of Neurophysiology. 2005;93:1358–1371.
Reichling D.B., Levine J.D. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7006–7011.
Reid G., Flonta M. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neuroscience Letters. 2001;297:171–174.
Reid G., Flonta M.L. Physiology. Cold current in thermoreceptive neurons. Nature. 2001;413:480.
Reid G., Scholz A., Bostock H., et al. Human axons contain at least five types of voltage-dependent potassium channel. Journal of Physiology. 1999;18:681–696.
Ren K., Dubner R. Central nervous system plasticity and persistent pain. Journal of Orofacial Pain. 1999;13:155–163. discussion 164–171
Rocha-Gonzalez H.I., Mao S., Alvarez-Leefmans F.J. Na+, K+, 2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. Journal of Neurophysiology. 2008;100:169–184.
Roza C., Puel J.L., Kress M., et al. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. Journal of Physiology. 2004;558:659–669.
Rugiero F., Wood J.N. The mechanosensitive cell line ND-C does not express functional thermoTRP channels. Neuropharmacology. 2009;56:1138–1146.
Salter M.W. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Current Topics in Medicinal Chemistry. 2005;5:557–567.
Sanchez-Freire V., Blanchard M.G., Burkhard F.C., et al. Acid-sensing channels in human bladder: expression, function and alterations during bladder pain syndrome. Journal of Urology. 2011;186:1509–1516.
Schmelz M., Schmid R., Handwerker H.O., et al. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571.
Schmelz M., Schmidt R., Weidner C., et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. Journal of Neurophysiology. 2003;89:2441–2448.
Shelukhina I.V., Kryukova E.V., Lips K.S., et al. Presence of alpha7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective alpha-neurotoxins in histochemistry. Journal of Neurochemistry. 2009;109:1087–1095.
Shin J.B., Martinez-Salgado C., Heppenstall P.A., et al. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nature Neuroscience. 2003;6:724–730.
Sole-Magdalena A., Revuelta E.G., Menenez-Diaz I., et al. Human odontoblasts express transient receptor protein and acid-sensing ion channel mechanosensor proteins. Microscopy Research and Technique. 2011;74:457–463.
Spies M., Lips K.S., Kurzen H., et al. Nicotinic acetylcholine receptors containing subunits alpha3 and alpha5 in rat nociceptive dorsal root ganglion neurons. Journal of Molecular Neuroscience. 2006;30:55–56.
St Pierre M., Reeh P.W., Zimmermann K. Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro. Experimental Brain Research. 2009;196:31–44.
Story G.M., Peier A.M., Reeve A.J., et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829.
Strotmann R., Harteneck C., Nunnenmacher K., et al. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biology. 2000;2:695–702.
Stucky C.L., Medler K.A., Molliver D.C. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44.
Sun Y., Chai T.C. Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU International. 2002;90:381–385.
Takahashi N., Kozai D., Kobayashi R., et al. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–287.
Talavera K., Gees M., Karashima Y., et al. Nicotine activates the chemosensory cation channel TRPA1. Nature Neuroscience. 2009;12:1293–1299.
Taylor-Clark T.E., Undem B.J., Macglashan D.W., Jr., et al. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Molecular Pharmacology. 2008;73:274–281.
Tecott L.H., Maricq A.V., Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1430–1434.
Thompson A.J., Lummis S.C. The 5-HT3 receptor as a therapeutic target. Expert Opinion on Therapeutic Targets. 2007;11:527–540.
Thut P.D., Wrigley D., Gold M.S. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience. 2003;119:1071–1083.
Todorovic S.M., Jevtovic-Todorovic V. The role of T-type calcium channels in peripheral and central pain processing. CNS & Neurological Disorders Drug Targets. 2006;5:639–653.
Tominaga M., Caterina M.J., Malmberg A.B., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543.
Treede R.D., Meyer R.A., Raja S.N., et al. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. Journal of Physiology. 1995;483:747–758.
Tsetlin V., Kuzmin D., Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. Journal of Neurochemistry. 2011;116:734–741.
Tulleuda A., Cokic B., Callejo G., et al. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Molecular Pain. 2011;7:30.
Viana F., de la Pena E., Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nature Neuroscience. 2002;5:254–260.
Vilceanu D., Stucky C.L. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010;5:e12177.
Vriens J., Appendino G., Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Molecular Pharmacology. 2009;75:1262–1279.
Vriens J., Owsianik G., Hofmann T., et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494.
Vriens J., Owsianik G., Janssens A., et al. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. Journal of Biological Chemistry. 2007;282:12796–12803.
Vriens J., Watanabe H., Janssens A., et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:396–401.
Vyklicky L., Novakova-Tousova K., Benedikt J., et al. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiological Research/Academia Scientiarum Bohemoslovaca. 2008;57(Suppl 3):S59–S68.
Walder R.Y., Rasmussen L.A., Rainier J.D., et al. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. Journal of Pain. 2010;11:210–218.
Wei H., Chapman H., Saarnilehto M., et al. Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil–treated non-diabetic rat. Neuropharmacology. 2010;58:578–584.
Wei H., Hamalainen M.M., Saarnilehto M., et al. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154.
Wen X.J., Li Z.J., Chen Z.X., et al. Intrathecal administration of Cav3.2 and Cav3.3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta Pharmacologica Sinica. 2006;27:1547–1552.
Werth J.L., Usachev Y.M., Thayer S.A. Modulation of calcium efflux from cultured rat dorsal root ganglion neurons. Journal of Neuroscience. 1996;16:1008–10015.
Wessler I., Kilbinger H., Bittinger F., et al. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sciences. 2003;72:2055–2061.
Wickenden A.D., Maher M.P., Chaplan S.R. HCN pacemaker channels and pain: a drug discovery perspective. Current Pharmaceutical Design. 2009;15:2149–2168.
Willcockson H., Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell and Tissue Research. 2008;334:17–23.
Willis W.D., Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Experimental Brain Research. 1999;124:395–421.
Wong G.Y., Gavva N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Research Reviews. 2009;60:267–277.
Woodbury C.J., Zwick M., Wang S., et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. Journal of Neuroscience. 2004;24:6410–6415.
Wynn G., Ma B., Ruan H.Z., et al. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287:G647–G657.
Xiao H.S., Huang Q.H., Zhang F.X., et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8360–8365.
Xing H., Chen M., Ling J., et al. TRPM8 mechanism of cold allodynia after chronic nerve injury. Journal of Neuroscience. 2007;27:13680–13690.
Xu H., Ramsey I.S., Kotecha S.A., et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186.
Yamamoto S., Shimizu S., Kiyonaka S., et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nature Medicine. 2008;14:738–747.
Yan J., Edelmayer R.M., Wei X., et al. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113.
Yasuda T., Sobue G., Ito T., et al. Nerve growth factor enhances neurite arborization of adult sensory neurons; a study in single-cell culture. Brain Research. 1990;524:54–63.
Zamponi G.W., Lewis R.J., Todorovic S.M., et al. Role of voltage-gated calcium channels in ascending pain pathways. Brain Research Reviews. 2009;60:84–89.
Zeitz K.P., Guy N., Malmberg A.B., et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. Journal of Neuroscience. 2002;22:1010–1019.
Zhang Y., Wang Y.H., Ge H.Y., et al. A transient receptor potential vanilloid 4 contributes to mechanical allodynia following chronic compression of dorsal root ganglion in rats. Neuroscience Letters. 2008;432:222–227.
Zhao P., Barr T.P., Hou Q., et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008;139:90–105.
Zimmermann K., Leffler A., Babes A., et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858.
Zimmermann K., Lennerz J.K., Hein A., et al. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18114–18119.
Birder L.A. Urothelial signaling. Handbook of Experimental Pharmacology. 2011;202:207–231.
Birdsong W.T., Fierro L., Williams F.G., et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749.
Burnstock G. Purinergic mechanosensory transduction and visceral pain. Molecular Pain. 2009;5:69.
Cairns B.E., Hu J.W., Arendt-Nielsen L., et al. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. Journal of Neurophysiology. 2001;86:782–791.
Caterina M.J., Schumacher M.A., Tominaga M., et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824.
Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;6(Suppl):S27–S35.
Dussor G., Koerber H.R., Oaklander A.L., et al. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Research Reviews. 2009;60:24–35.
Emery E.C., Young G.T., Berrocoso E.M., et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466.
Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nature Medicine. 2010;16:1248–1257.
Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annual Review of Biochemistry. 2003;72:609–642.
Lawson S.N. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Experimental Physiology. 2002;87:239–244.
Michaelis M., Liu X., Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. Journal of Neuroscience. 2000;20:2742–2748.
Parada C.A., Reichling D.B., Levine J.D. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190.
Price T.J., Cervero F., Gold M.S., et al. Chloride regulation in the pain pathway. Brain Research Reviews. 2009;60:149–170.
Schmelz M., Schmid R., Handwerker H.O., et al. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571.
Schmelz M., Schmidt R., Weidner C., et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. Journal of Neurophysiology. 2003;89:2441–2448.
Tominaga M., Caterina M.J., Malmberg A.B., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543.
Vriens J., Appendino G., Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Molecular Pharmacology. 2009;75:1262–1279.
Wong G.Y., Gavva N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Research Reviews. 2009;60:267–277.
Woodbury C.J., Zwick M., Wang S., et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. Journal of Neuroscience. 2004;24:6410–6415.
Zamponi G.W., Lewis R.J., Todorovic S.M., et al. Role of voltage-gated calcium channels in ascending pain pathways. Brain Research Reviews. 2009;60:84–89.
Zimmermann K., Leffler A., Babes A., et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858.