Placebo Analgesia
Introduction
For a patient experiencing pain, the perception that an effective treatment has been administered is often sufficient to produce significant analgesia. To the extent that the analgesia is due to the psychobiological effects of the treatment process, as opposed to an active property of the treatment, the person can be said to have experienced a placebo analgesic response. The actual treatment manipulation can take a variety of forms: a dummy tablet, nasal spray, surgical procedure, magnetic treatment, or topical cream.
Whatever the form, the most critical determinants of the analgesic efficacy of a placebo are (1) the presence of sensory cues that have been associated with effective treatment or pain relief in the past and (2) the expectation of pain relief.
The concept of placebo implies that there is a mismatch between what the patient expects and the treatment’s actual intrinsic efficacy. If the patient believes that the placebo treatment may be effective, positive expectations of analgesia are created, and these expectations are linked to pain relief. This, in turn, implies that the patient is deceived. However, it is also possible to create placebo effects through conditioning, the process of learning that sensory cues associated with the treatment context are linked with pain relief. In such cases, placebo effects may be independent of the patients’ conscious belief (Benedetti et al 2003), and it is thus possible in some cases to obtain placebo effects without explicit deception (Kaptchuk et al 2010).
It may also be possible to obtain expectancy (or treatment context–related benefits) in the context of active drug treatment, as is the case with patient-controlled analgesia (White 1988) and as demonstrated by studies of overt versus hidden drug treatment (Colloca et al 2004). In these cases, even though the active treatment cannot be called a placebo in the strict sense, the analgesic response that it elicits in the patient may be said to have a placebo component. Finally, the effects of expectations might, in some cases, interact with the active pharmacological mechanisms of drug treatments to produce synergistic effects (Kleijnen et al 1994). One striking example is a study of the drug proglumide, a cholecystokinin antagonist shown to relieve pain better than placebo alone when given overtly, with full patient awareness, but performs more poorly than placebo when given without patients’ awareness (Benedetti et al 1995).
In our view, placebo effects can thus arise in several situations—concomitant with conscious expectations about treatment (Kirsch 1985), following conditioning of pain relief with explicit sensory cues (with or without awareness of expectation; Benedetti et al 2003), and associated with the psychosocial context and ritual that surrounds treatment (Moerman and Jonas 2002, Barrett et al 2006). It is true that according to this definition the conceptual lines dividing placebo effects and effects of psychological therapies are blurred (Hrobjartsson 2002), but this definition respects the common origin of these effects in the brain of the patient. The brain mechanisms of the various psychological influences on pain, as well as whether they arise from common or distinct sources, are an empirical matter.
Although sham treatments can produce a powerful analgesic effect, in a typical clinical situation it is not usually obvious whether the improvement observed in a patient is due to a placebo response. As we describe later, this is often true even when the patient is known to have received a placebo treatment. Failure to appreciate this point has created confusion about what effects placebos do and do not have. Because of this, the first part of this chapter deals at length with definitions and with the phenomena that are most commonly confused with placebo analgesic responses. The second part of the chapter focuses on recent advances in understanding the neurobiology of the placebo response.
Terminology
The term placebo is most likely derived from the Latin stem placebit (“it will please”). Since the beginning of medicine, health professionals have knowingly deceived patients by giving them sham treatments—sometimes with well-meaning intentions and other times for self-serving purposes. However, the longevity of many treatments with probably no active effects (Shapiro 1959) and the success of physicians who prescribed them suggest that patients must have attributed some benefit to these ministrations. Furthermore, perhaps it is possible that the psychobiological effects can confer concrete physiological health benefits and even be an integral part of treatment. For example, Kong and colleagues (2009) cite the classic ancient Chinese text on acupuncture, the 1st-century BCE Yellow Emperor’s Inner Classic, as saying, “if a patient does not consent to therapy [acupuncture] with positive engagement, the physician should not proceed as the therapy will not succeed.” The study of placebo treatments, which have been selected to have no direct therapeutic benefit, is the study of the psychobiological effects of the treatment itself.
In this chapter we differentiate between the placebo, the placebo effect, and the placebo response. The placebo itself is a dummy treatment such as sham surgery or a sugar pill. The placebo effect is an observable difference between groups that is attributable to the efficacy of the placebo—for example, the difference in mean treatment effect between a group that has received a placebo treatment and one that received no treatment. The placebo analgesic response refers to the pain relief in an individual that results from the expectation of effectiveness of the therapeutic intervention.
The terminology surrounding placebo research can be confusing inasmuch as some authors use the term “placebo response” to mean any type of improvement in a placebo group in a clinical trial, even if that improvement is related to statistical artifacts such as sampling bias and regression to the mean or to the natural history of a clinical condition. Here, we reserve the term “response” for an active neurobiological process that occurs as a result of placebo treatment. Thus, from the standpoint of understanding mechanisms, it is the placebo response of the individual that is the most interesting and informative object of study.
Active Placebo Responses Versus Statistical Artifacts
The placebo response is widely misunderstood, in part because of modern clinical trial methodology and in part because of lack of understanding of the proximate mediating causes of clinical improvement. In clinical trials, the use of placebo treatment comparison groups is commonplace. The idea is to control for non-specific factors related to administration of the treatment and to the patient’s perception of the treatment. Frequently, in clinical trials of pain and a variety of other disorders, patients in the placebo group improve (Hróbjartsson and Gøtzsche 2001, 2004; Walsh et al 2002; Fournier et al 2010). The confusion begins with the assumption that the reason that such patients improve is because they received a placebo. This assumption is often unwarranted.
Patients in the placebo group might improve for several reasons. First, they might show improvement that would have happened with no treatment at all because of the natural history of the disease. Second, patients tend to enroll in trials or treatment when pain is at its worst, thereby resulting in apparent improvement with time as a consequence of regression to the mean. Third, patients may benefit from the positive psychosocial context of being enrolled in a study, which usually means increased medical attention, care, and assessment, as well as, increasingly, additional social support from other patients connected through Internet-based social media (the classic Hawthorne effect is a related phenomenon; Roethlisberger and Dickson 1939).
To illustrate the problem, consider the common condition of idiopathic headache. In most people the headaches that they experience will arise and subside completely without treatment. Thus any treatment given at the peak of headache severity (or no treatment) will tend to be followed by improvement. This is true whether the treatment is a starch pill or an active analgesic. To assess whether the placebo treatment had any actual psychobiological effects, it is necessary to compare improvement in a placebo treatment group with that in a no-treatment group (Fig. 27-1). This comparison can estimate the magnitude of the placebo effect (i.e., the benefit specifically attributable to taking the placebo), whereas improvement in the placebo group reflects a composite of many factors.
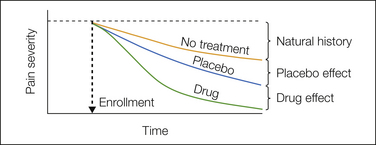
Figure 27-1 Estimating the placebo effect.
The graph shows the time course of pain severity after enrollment of three hypothetical groups in a clinical trial. The no-treatment group, shown in orange, may improve spontaneously because of a combination of the natural time course of the condition and regression to the mean. The vast majority of clinical trials do not contain such a group, but in those that do, the natural history effect is estimated as the change in pain severity relative to pain levels on enrollment. The placebo group, shown in blue, may show greater pain relief than the no-treatment group because of active psychological/brain processes. The difference between the placebo group and the no-treatment group thus defines the placebo effect. By contrast, the placebo response is the total change from enrollment levels in the placebo group (i.e., the placebo effect plus the natural history effect) and does not necessarily reflect any active psychological/brain changes induced by the placebo. The drug group, shown in green, demonstrates more rapid and complete pain reduction in trials of effective medications. The difference in pain severity between the drug and placebo groups after treatment defines the drug effect. The drug effect is the outcome of primary interest in nearly all clinical trials, and thus most clinical trials compare only the drug and placebo groups. Without a no-treatment group, however, it is impossible to isolate the active psychobiological effects of the placebo.
The various types of artifacts that can be mistaken for active placebo responses are described in more detail elsewhere (Atlas and Wager 2009, Atlas et al 2009), but it is worthwhile to elaborate briefly on regression to the mean, a pervasive problem illustrated in Figure 27-2. Imagine patients in a clinical trial for treatment of irritable bowel syndrome (IBS), a condition in which symptoms fluctuate over time but may be stable for a period of years (Agreus et al 2001). In this thought experiment, imagine that there is no change in the average symptom severity over time, only fluctuation around a stable value. Patients tend to enroll when symptoms are relatively severe, as marked by the arrows in Figure 27-2A. Because the symptoms fluctuate around a stable mean value, symptom severity on subsequent measurements will tend to be closer to the mean, and therefore the symptoms will appear to improve over time (Fig. 27-2B). Thus, even if there is no true improvement in the population over time, the time of study enrollment is not randomly sampled with respect to symptoms, and there is apparent improvement (Fig. 27-2C).
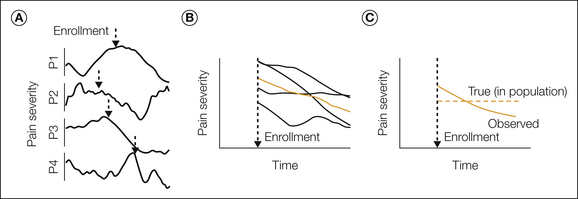
Figure 27-2 Natural history and regression to the mean.
A, The time course of pain severity in four hypothetical patients (P1–P4) who experience fluctuating levels of pain over time but no change in their long-term average pain. The dashed arrow marks a probable point of enrollment in treatment for each person, which often occurs when pain is higher than average. In this hypothetical example, we assume a normal distribution of pain values that fluctuates slowly around a constant average for each patient. B, The time course of pain following enrollment for each person (black lines) and the group average (colored line). In this case the trial would show a substantial natural history effect. If the individuals shown had received a placebo treatment, the trial would appear to show a substantial placebo response. Because there is no true, long-term improvement in any patient, this effect is due to regression to the mean. C, The true population average across time (dashed line) versus the apparent natural history effect. The dashed line is flat because there is no true average change across time in this hypothetical example, only symptom fluctuation. The solid line shows pain reduction over time because of regression to the mean (i.e., patients tend to enroll when their pain is extreme, which thus tends to be less extreme on repeated measurements).
The clinical significance of regression to the mean in chronic pain is illustrated by the work of Whitney and Von Korff (1992). They conducted a population- and clinic-based study of people with temporomandibular disorders in which 147 patients who had been referred for treatment of “facial ache or pain in the jaw muscles, the joint in front of the ear or inside the ear (excluding infection)” were compared with 95 community cases identified in a random sample telephone survey of individuals who reported the same complaints but did not seek treatment. All subjects rated their pain severity at study entry and 1 year later. Pain severity at 1 year was much less than at entry for both the treated and untreated groups. The greatest improvement occurred in those with the highest level of pain at study entry, and when the subjects were matched for initial pain severity, no difference in pain levels at the 1-year follow-up was noted between the treated and untreated groups. However, at 4–6 weeks, many patients with temporomandibular disorders in the clinic group reported improvement and attributed their improvement to the treatment received.
Thus, improvement in placebo-treated groups in clinical trials is confounded by both natural healing processes and statistical artifacts such as regression to the mean. In a typical randomized placebo-controlled clinical trial of headache treatment, large numbers of patients in the placebo control group report improvement (e.g., de Craen et al 2000). Based on such improvement, it is frequently stated that a certain percentage of subjects or patients in a treatment trial are placebo responders. Indeed, Beecher’s oft quoted survey of clinical analgesic trials, from which he concluded that an average of 30% of patients respond to placebo treatments (Beecher 1955), is based on just such an estimate. In fact, assessing the benefit from taking a placebo requires comparison with a no-placebo group, which controls for natural history, regression to the mean, and other effects of enrolling in the study or manipulation of the type of placebo intervention (e.g., de Craen et al 2000, Kaptchuk et al 2008).
Evidence for Placebo Analgesia
Placebo Effects in Experimental and Clinical Studies
Consistent placebo analgesic effects have been demonstrated for dental postoperative pain, post-thoracotomy pain, low back pain, IBS pain, chronic neuropathic pain, and experimental somatic pain caused by noxious heat, laser, electric shock, intramuscular saline injections, rectal distention, esophageal stimulation, and exercise under ischemic conditions. Many well-controlled, experimental studies have demonstrated such effects (Enck et al 2008, Price et al 2008, Atlas et al 2009, Benedetti 2009, Vase et al 2009, Zubieta and Stohler 2009, Finniss et al 2010). Because placebo effects in experimental studies are well established and widely accepted, we devote space here to discussing the more contentious issue of whether placebo effects exist in clinical pain states.
Several meta-analyses have identified clinical trials with no-treatment control groups and have used these groups to estimate the magnitude of placebo analgesia (Hróbjartsson and Gøtzsche 2001, 2004; Vase et al 2002). These analyses show significant but modest placebo analgesia, with effect sizes estimated at d = 0.25 (Hróbjartsson and Gotzsche 2004) and d = 0.15 (Vase et al 2002) (d is the mean effect divided by its standard deviation). Effect sizes also varied significantly across trials. By contrast, studies of clinical pain with placebo treatments designed to elicit placebo analgesia have reported larger effects (e.g., Levine et al 1978, Gracely et al 1979, Vase et al 2005, Kaptchuk et al 2008). Experimental studies of placebo analgesia have reported even larger placebo effects (d = 0.95 and d = 1.00; Vase et al 2002, 2009). The larger effects in placebo analgesia studies and the substantial heterogeneity in placebo effects across clinical trials are probably related to the context and the instructions given to participants; those in placebo analgesia studies are typically told that the treatment will or may powerfully reduce pain, thereby leading to stronger expectations of analgesia. In addition, a direct comparison between placebo effects in experimental and clinical low back pain showed larger placebo effects for clinical pain (Charron et al 2006). This finding fits with the results of a meta-analysis showing that placebo effects are larger with more sustained pain and in the presence of hyperalgesia (Vase et al 2009).
In sum, placebo treatments can have a positive impact on clinical pain, with the most convincing evidence to date seen in patients with chronic low back pain and IBS. The presence of hyperalgesia and the psychological context in which placebo treatments are given also appear to matter considerably. In the clinical situation, the enthusiasm and belief of the physician and what is verbally communicated to the patient are critical, as are conditioning effects arising from previous exposure to an active (or inactive) analgesic drug. Other factors that probably influence the placebo effect include the physical properties of the placebo and how it is administered (Kaptchuk et al 2006).
Cognitive Bias as a Source of Placebo Effects
One limitation of the studies just discussed is that they all use patient-reported pain as an outcome. However, the judgment process that influences reports of pain and other phenomena can be biased in a number of ways. For example, judgments of simple facts such as “how far away is the moon?” are biased by suggested reference points (Tversky and Kahneman 1981), and judgments of economic value and basic perceptual similarity are biased by a number of cognitive variables, including the order in which the options are presented and the presentation of reference values that serve as anchors (Tversky 1977, Tversky and Kahneman 1981, Cheng and Holyoak 1985). Thus, under placebo treatment, patients may (1) establish a lower cognitive anchor point for pain and fail to sufficiently override their previous beliefs when making reporting decisions; (2) overweigh moments with lower pain experience when judging overall pain because of increased cognitive availability of experiences that match expectations; (3) desire to report what they believe the experimenter expects, in part because they believe that this conforms to “correct” or normative behavior; (4) desire to be consistent with previous behavior, which could include decreased reports of pain during prior treatment; and (5) bias their reports toward what they would like to happen (Metcalfe 1998). All these factors could create a placebo effect on reported pain that does not depend on changes in nociceptive sensory processing.
Consistent with these ideas, several studies have used sensory decision theory analysis to separate placebo effects on sensory discriminability—the ability to accurately detect which of two stimuli is more intense—from effects on pain report. These studies found that although placebo treatment decreases reported pain, it does not affect sensory discriminability (Clark 1969, Feather et al 1972). These studies provide some support for cognitive bias as a source of placebo effects (Allan and Siegel 2002), though there are limitations in the interpretation of signal detection measures (Gracely 2005).
Placebo Effects on Brain Correlates of Pain
The use of physiological markers of pain has become increasingly important as a way to gain leverage on whether placebo treatments produce meaningful changes in afferent nociceptive processing in the brain. The question of whether placebo responses reflect altered transmission in pain pathways has been addressed by using event-related potentials (ERPs), magnetoencephalography (MEG), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI). These studies show (1) reductions in pain-related activity in most brain correlates of pain experience, (2) activation with placebo treatment of areas important for modulation of pain-related regions and engagement of pain-modulating circuits, and (3) activation of the endogenous opioid and dopamine systems with placebo treatment. We review point 1 in this section and cover the others in the following section on mechanisms of placebo analgesia.
Placebo Effects on fMRI Responses to Noxious Stimuli
Several fMRI studies (summarized in Table 27-1 and Fig. 27-3) have shown reduced processing of noxious somatic stimuli with placebo treatment as compared with a no-placebo control. These studies have typically used intensity-matched stimuli to test the same individuals at two different times, once with placebo treatment and once with control treatment. The placebo treatments used have included placebo cream, intravenous administration of saline, and sham acupuncture, and the control treatments have consisted of matched cream application or injection with different instructions (e.g., “This cream will have a powerful pain-reducing effect” in the placebo condition versus “This cream will have no effect” in the control condition). Although we include sham acupuncture effects, we note that sham acupuncture may act as a counter-irritant, thus complicating its interpretation as a pure placebo treatment.
Table 27-1
Neuroimaging Studies of Placebo Analgesia
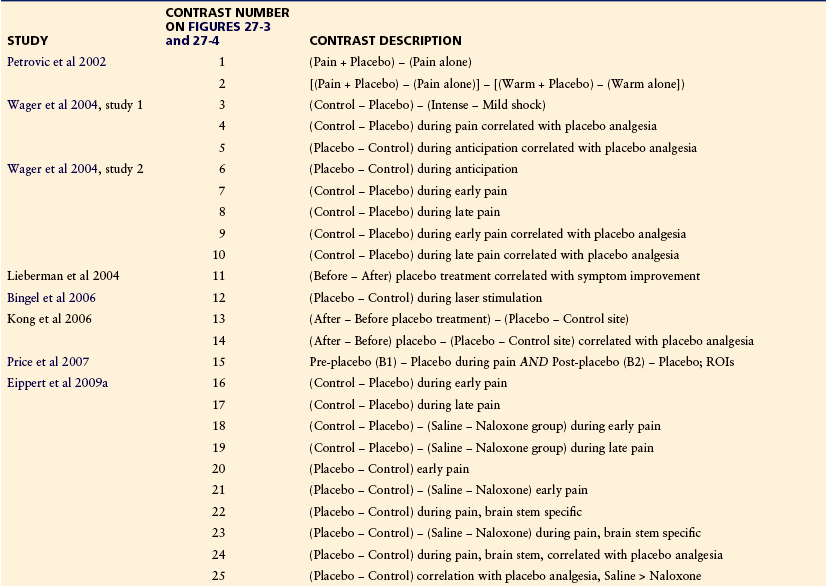
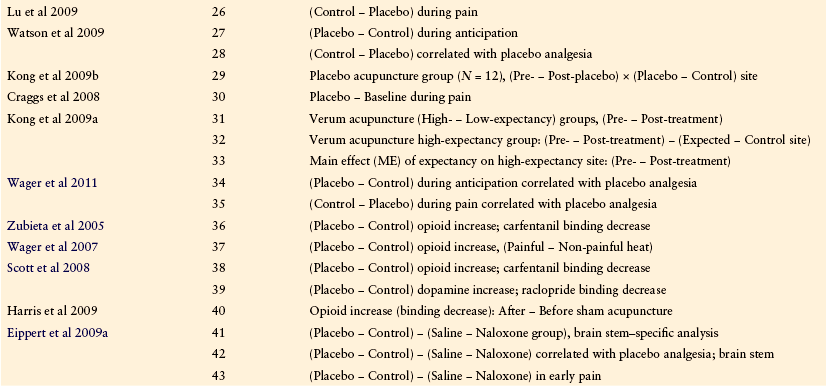
Note: Results from each of these studies are coded by the specific comparisons between conditions used (contrasts) and plotted in Figures 27-3 and 27-4.
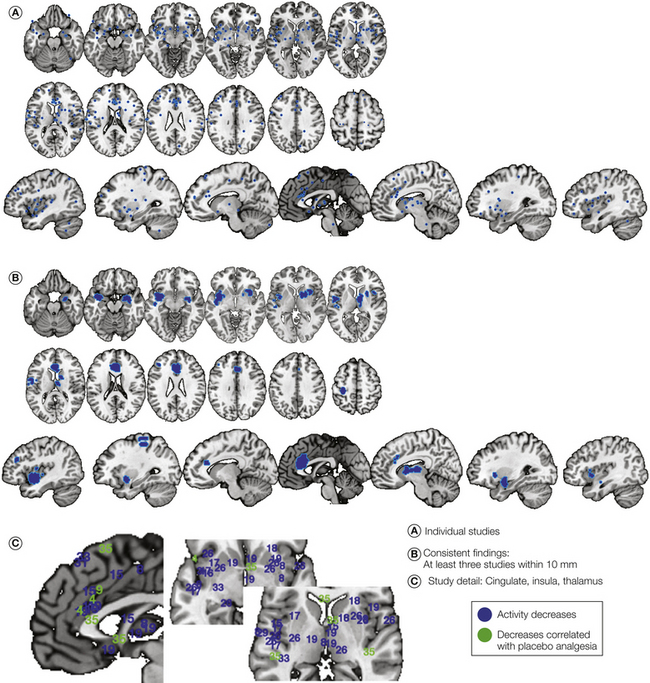
Figure 27-3 Modulation of pain processing–related brain activity by placebo treatment.
A, Reported stereotactic coordinates reflecting placebo-induced decreases in functional magnetic resonance imaging activity during painful stimulation. Coordinates from statistical contrasts showing reduced activity with placebo (e.g., [Placebo < Control]) in group analyses are presented in blue, and coordinates showing correlations between reduced brain activity and placebo analgesia in subjective reports are presented in green. Contrasts are numbered according to the specific comparisons listed in Table 27-1. In this and all figures, coordinates from the same contrast within 12 mm were averaged together before plotting. B, Consensus regions showing effects within 10 mm in at least three separate contrast maps. The map shows decreased activity during pain in (from bottom to top in the brain) the amygdala and basal forebrain, anterior insula and operculum, medial thalamus, striatum, anterior cingulate, and parts of the prefrontal cortex. C, Detail showing individual study findings (numbered as in Table 27-1) around the insula, thalamus, and striatum.
Wager and colleagues (2004) studied decreases in fMRI responses to noxious stimuli in two experiments. In study 1, a sample of 24 community volunteers was exposed to noxious shock delivered to the right arm under placebo and control conditions. All participants served as their own controls in a within-subjects design. In study 2, to increase the magnitude of the placebo effect, a conditioning procedure (surreptitious lowering of stimulus intensity when inert cream was applied; Voudouris et al 1985, 1989) was used to associate the application of a placebo cream with effective pain relief. In this case, noxious heat was delivered to the left arm. The fMRI results were similar in studies 1 and 2. Placebo-induced reductions were found in the contralateral anterior insula, medial thalamus, and rostral dorsal cingulate (corresponding to Vogt’s anterior mid-cingulate region). As with all studies discussed here, the locations of placebo-relevant brain effects are plotted with numbers coding for each unique study contrast in Figure 27-3. The precise contrasts tested (i.e., the comparison of conditions that produced the statistical map of brain placebo effects) are listed by number in Table 27-1. This study, for example, corresponds to contrasts 3–10 in Table 27-1 and Figure 27-3. In addition, importantly, this and all other studies demonstrating decreases in pain processing showed significant placebo analgesia effects on reported pain, thus replicating other, purely behavioral findings (e.g., Voudouris et al 1985, 1989; Montgomery and Kirsch 1997; Price et al 1999; Benedetti et al 1998, 1999, 2005; Benedetti 2008; Morton 2010).
Since then, other studies have replicated these reductions. Price and colleagues (2007; contrast 4 in Table 27.1 and Fig. 27-3) found decreases in the same regions (anterior insula [aINS], rostral dorsal anterior cingulate cortex [rdACC], and medial thalamus) accompanying large behavioral placebo effects in IBS patients. They reported decreases in the secondary somatosensory cortex (SII) and several other pain-responsive regions as well, although it was unclear whether these changes were due to placebo or habituation over time. Lu and co-workers (2010; contrast 26) found substantial placebo-induced reductions in these regions, as well as the sensorimotor regions around SI/SII. Eippert and associates (2009; contrasts 16–19) replicated the decreases in each region. Furthermore, decreases in each region (and in peri-SI/SII) were blocked by the opiate antagonist naloxone, thus implicating the opioid system. High verbally induced expectations were associated with reductions in some overlapping areas, including the rdACC, nearby dorsomedial prefrontal cortex, and other areas, including the amygdala and ventral striatum. Watson and co-authors (2009; contrast 28) also reported placebo-induced reductions that correlated with the magnitude of placebo analgesia in the rdACC and peri-SI. In addition, in a re-analysis of the combined individual difference data across both studies by Wager and colleagues (2011), correlations were found between placebo-induced reductions in signal magnitude in a number of pain-processing regions and reported placebo analgesia; these regions included the anterior cingulate and thalamus. Even larger correlations were found between reduced pain and reduced activity in the ventral striatum, thereby potentially implicating valuation and learning systems distinct from those that typically encode the intensity of noxious stimulation.
Findings from all these studies are summarized in Figure 27-3A, with numbers corresponding to the contrasts listed in Table 27-1 for each study. Figure 27-3B shows regions in which placebo-induced decreases were replicated in at least three separate study maps. A close-up view of individual study results in the key regions around the insula, cingulate, thalamus, and striatum is shown in Figure 27-3C. In summary, although the results vary, there is clear consensus on decreased processing of noxious stimuli in typical “pain-processing regions,” including the rdACC, aINS, and medial thalamus, as well as reductions in pain-responsive regions of the ventral striatum, amygdala, and sensorimotor cortex.
Placebo Effects on Event-Related Potential Responses to Noxious Stimuli
In addition to their role in processing nociceptive input, both the rostral cingulate and insula are increasingly being viewed as cortical “hubs” that contribute to a variety of processes, including perceiving others in pain, non–pain-related social or economic loss, conflict detection, and behavioral decision making. Thus, nociception-related patterns of activity in these regions must be interpreted with caution, and their role in placebo analgesia may not be straightforward. Second, the time resolution of fMRI is poor relative to that of the underlying neural activity. For example, the imaging studies reviewed earlier did not have the ability to resolve events at a lower time resolution than several seconds, which leaves ample time for evaluative processes beyond nociception to influence the observed signals. In this respect, ERP studies provide an important complement to fMRI studies because of their excellent time resolution.
In fact, ERP studies have found encouragingly replicable results on early nociception-related components. Wager and colleagues (2006), using a brief conditioning manipulation, tested placebo effects on noxious laser evoked potentials (LEPs), specifically the N2/P2 complex, a midline potential sensitive to noxious stimulus intensity that occurs at around 180 msec (N2) and 250–300 msec (P2) and whose probable neural generator is the anterior cingulate (Garcia-Larrea et al 2003). They found evidence of placebo-induced reduction of P2 that decreased over time as P2 habituated. Watson and co-workers (2007) replicated these findings in a pre– versus post–placebo treatment design and found reduced N2 and P2 potentials after placebo treatment but not after an inactive control treatment. They found no evidence of habituation in the control group, thus arguing against a habituation-related explanation for the placebo effects. Colloca and colleagues (2008b) also used a between-groups design to test for placebo-induced reduction in the N1 and N2/P2 complex with suggestion alone (group 1) or with conditioning to reduced stimulus intensity (group 2) in comparison to a no-placebo control (group 3). They found N2/P2 reductions that were particularly large in group 2 and correlated with placebo analgesia (r = 0.54) in group 1. Morton and associates (2010) tested placebo effects on LEPs in two separate sessions at least 2 weeks apart. Reductions in LEPs before and after placebo treatment were correlated with placebo analgesia (r = 0.040 and 0.41 in each session). Also of note was that both placebo analgesia and LEP reductions were reliable across the two sessions (r = 0.75 and r = 0.41, respectively). Finally, Aslaksen and co-workers (2011) also found reductions in N2/P2 potentials and accompanying reductions in self-reported stress, but only in males (the experimenters were female). These studies are complemented by other ERP studies of learned expectations not strictly classified as “placebo” because the expectations are not about treatment. For example, Lorenz and colleagues (2005) found expectancy-based modulation of MEG activity around 165 msec after noxious laser stimulation localized to SII. Overall, the evidence indicates that placebo treatments can modulate responses to early nociceptive processes.
Placebo Effects on Spinal Nociceptive Processes
The neurophysiological effects described above provide evidence that pain-related processing is reduced with placebo analgesia and that these reductions are correlated in some cases with reductions in the pain experience. However, these studies do not address the question of whether and to what degree these brain changes reflect decreased aversion, reactivity, or attention to pain at the supraspinal level and to what degree they activate descending antinociceptive mechanisms that can reduce pain at the spinal level (see Heinricher and Fields, Chapter 8 of this volume).
To date, there is limited direct evidence for spinal inhibition. In one study, Matre and colleagues (2006) assessed placebo effects on an area of secondary mechanical hyperalgesia created by sustained painful heat. The heat created a hypersensitive area on each participant’s arm, and a subsequent test for pain induced by light touch was performed without participants looking at the arm. Placebo treatment reduced the size of the hyperalgesic area, which the authors argued implies reductions in central sensitization of pain at the spinal level. Goffaux and associates (2007) assessed the effects of instructions on diffuse noxious inhibitory control (DNIC, created here by a cold water bath) suppression of RIII reflex responses to sural nerve stimulation. They found significant biasing of reflex amplitude with placebo treatment, but this was primarily driven by “nocebo” conditions in which subjects were instructed that the cold-water bath would amplify their pain. Finally, Eippert and colleagues (2009b) used fMRI to directly image the cervical spinal cord during painful heat with and without placebo. They found that placebo treatment significantly reduced spinal fMRI activity in response to heat.
Although these results are promising, questions remain. If placebo treatment reduces nociception at the spinal level, one might expect it to reduce pain-related activity in all relevant areas of the cerebrum. However, studies have not yet unequivocally demonstrated reductions in pain-related processing in the sensory thalamus, SII, and dorsal posterior insula. Importantly, these are the areas activated most specifically by noxious somatic stimulation (e.g., Hua et al 2005, Kross et al 2011). It is possible that the widespread effects of spinal inhibition are masked by increases in placebo-related activity driven by cortical sources (some of which could reflect the metabolic demands of, for example, activation of inhibitory interneurons) or paradoxically reduced thresholds in specific nociceptive pathways.
Ingredients of Placebo Analgesia: What Makes a Placebo Responder?
A number of processes contribute to the creation of placebo analgesia at both the psychological and neural level, and different factors may influence the magnitude of the placebo response in different situations. Relationships between placebo effects and personality measures have proved inconsistent (Liberman 1964, Shapiro et al 1979), and placebo responses are not highly correlated across types of pain and variations in situational context. For example, Liberman (1964) assessed placebo response magnitude in the same group of women in three kinds of pain and found that placebo responses were uncorrelated across the types of pain. More recently, Whalley and colleagues (2008) tested for correlations in placebo responses in the same pain modality, but with different brand names for the placebo. Responses were uncorrelated.
However, more recent studies suggest a number of promising psychological correlates of placebo response magnitude, including suggestibility (De Pascalis et al 2002, Morton et al 2010), optimism (Morton et al 2009), expectation (Vase et al 2003, Zubieta et al 2005, Atlas et al 2010, Morton et al 2010), behavioral activation (Schweinhardt et al 2009), desire for relief (Vase et al 2003), reductions in anticipatory anxiety with placebo treatment (Lyby et al 2010), and sensitivity to opiate drugs (Amanzio and Benedetti 1999). These factors may underlie some of the brain correlates described elsewhere in this chapter; that is, increased optimism and positive expectations may be linked to greater anticipatory frontostriatal activity and reduced anticipatory anxiety, thereby potentiating the release of endogenous opioids through prefrontal–brain stem pathways and reducing noxious stimulus–induced activity in pain-processing regions and in the learning and motivation-related ventral striatal circuits.
How can these apparently conflicting findings be reconciled? Placebo effects are influenced by both stable individual differences such as optimism and past experiences with pain, treatments, and treatment contexts and cues. These two kinds of predisposing factors contribute to the psychological and brain processes that shape the emotional, sensory, and evaluative processing of pain. Thus, placebo responses are likely to be elicited in individuals who are receptive to the particular treatment context offered. For example, person A might be responsive to placebo injections in part because of positive past experiences with injected analgesics. Person B might be more responsive to a placebo cream. Person A might be quite optimistic and show strong placebo responses to laboratory pain but have anxiety about the pain of childbirth that blocks placebo responses in that context, whereas person B might have different predispositions toward childbirth that permit placebo responses to develop. This notion is consistent with a fundamental idea in psychology that personality traits alone are insufficient to describe how a person will respond, and predisposition × situation interactions must be considered (Mischel 2004). Furthermore, predisposing factors might combine to elicit stronger or weaker expectations about pain in the moment, which may be proximal mediators of how strong the placebo effect will be for a given person in a given situation (e.g., Wager et al 2011). However, we might not expect a person to respond similarly to different situations and different types of pain (Liberman 1964).
Much attention has been given to how previous experiences with drug and context cues influence placebo effects. The process of learning that drug cues signal pain relief and/or drug-induced changes in the brain’s neurochemistry is known as conditioning. Conditioned cues can have strong influences on pain in basic and clinical contexts (Wickramasekera 1980; Voudouris et al 1985, 1990; Amanzio and Benedetti 1999; Atlas et al 2010). The clearest evidence for conditioning effects in the clinical situation is derived from placebo-controlled crossover trials of analgesic medications. In a study of acute pain in hospitalized patients, Kantor and colleagues (1966) and Laska and Sunshine (1973) compared placebo and several different doses of an active analgesic. What they found was a clear conditioning effect. When placebo was given as a second treatment 24 hours after administration of an active analgesic, the magnitude of placebo analgesia was positively correlated with the dose of the previously administered active medication. These results indicate a conditioning effect of pairing the treatment context (the hospital, physician, nurse, and capsule) with the analgesic effect of the drug through its direct action on the central nervous system. This is similar to the classic conditioning of drug effects as described by Pavlov (Pavlov and Anrep 1927). One could thus consider such a placebo manipulation to be a conditioned response. In this case, the contextual cues (white coat, pill, or needle) are the conditional stimuli, the direct drug effect on the brain is the unconditioned stimulus, and the analgesic effect of the drug is the unconditional response.
Pharmacological conditioning is well documented in humans (Amanzio and Benedetti 1999, Colloca and Benedetti 2006) and animals (e.g., Herrnstein 1962, Guo et al 2010). However, conditioning trials with analgesic drugs are not required to produce subsequent placebo analgesic responses. Voudouris and co-workers (1990) produced conditioned analgesia by simulating an analgesic effect. They first applied a noxious stimulus to the skin to determine the subject’s pain threshold. They then applied an inert cream to the skin and reapplied the stimulus but surreptitiously reduced its intensity to suggest to the subject that the cream had an analgesic effect. After this simulated analgesia, the placebo “analgesic” cream was applied and the original noxious stimulus was delivered to the same area of skin. When compared with a group given the cream with no conditioning, the conditioned group showed significant pain reduction by the placebo cream. This same basic conditioning paradigm can influence both pain and pain-related physiology (Table 27-1, Fig. 27-3). Furthermore, larger numbers of conditioning trials create larger placebo effects that are more resistant to extinction (Colloca et al 2010). Thus, cues associated with the experience of reduced pain per se can have a substantial analgesic effect when presented during later pain. However, critical, unanswered questions remain. Little is known about the precise mechanisms of pharmacological conditioning versus conditioning to reduced pain (see Wager et al 2007 for discussion). In addition, it is unknown why cues that signal reduced pain elicit opioid-mediated analgesia whereas in other studies, cues that signal increased pain produce opioid-mediated analgesia (Fanselow 1986). Both are likely to be at least in part conditioned responses, but they have opposite effects on pain.
One possibility is that conditioned analgesia is mediated by changes in brain connectivity in nociceptive or affective circuits that reduce pain in a relatively unconscious, automatic way. Conditioned placebo effects that are insensitive to verbal instructions revealing that the treatment is a sham have been demonstrated in other domains (Benedetti et al 2003), and recently, Kaptchuk and colleagues (2010) found substantial placebo effects on IBS pain even when the patients were told that they were being given placebo. On the other hand, other studies have demonstrated that suggestion alone is enough to produce some degree of analgesia (Amanzio and Benedetti 1999, Wager et al 2004) and possibly opioid release (Zubieta et al 2005). Conscious expectations of relief are correlated with reduced anticipatory responses in brain regions linked with anticipatory anxiety and reduced placebo analgesia (Wager et al 2011), with prefrontal activity that mediates cue-evoked changes in pain (Atlas et al 2010), and with placebo-induced opioid release in the limbic and paralimbic regions (Zubieta et al 2006). These studies suggest that conditioning can work in at least two different ways—by eliciting conscious expectations of drug relief and by brain mechanisms independent of conscious expectations (Stewart-Williams and Podd 2004).
Just as conditioning can produce positive expectations and brain changes that reduce pain, it can also produce negative expectations and brain changes that increase pain. Such phenomena have often been referred to as “nocebo” effects, and reduction of anxiety-related hyperalgesia is one potential mechanism of action for placebo treatments (Vase et al 2005, Aslaksen and Flaten 2008), although more complex relationships between placebo analgesia and anxiety are also possible (Benedetti and Amanzio 1997, Staats et al 2001).
One clear example of nocebo expectations is provided by a study of Dworkin and colleagues (1983) on the effect of nitrous oxide on pain elicited by tooth pulp stimulation. Using verbal instruction, these investigators were able to turn the effect of nitrous oxide from analgesia to hyperalgesia. Several recent experimental studies have yielded similar results. Goffaux and associates (2007) found that the largest effects of instructions on spinal reflexes were nocebo effects: instructions that a normally analgesic counterstimulation treatment would create hyperalgesia offset or reversed the effects of the treatment. Bingel and colleagues (2011) found that nocebo instructions offset or reversed the normally analgesic effects of remifentanil on pain reports and fMRI responses associated with pain. Finally, nocebo instructions appear to have larger and longer-lasting effects than placebo instructions do (Colloca et al 2008a, 2010) and produce stronger physiological responses, such as on cortisol (Johansen 2003, Benedetti et al 2006).
Mechanisms of Placebo Analgesia
Engagement of the Evaluative and Visceromotor Brain Systems
Thus far we have reviewed evidence on whether placebos can produce reductions in biological markers of nociceptive processes and some of the probable ingredients of placebo analgesia. Brain-based studies of placebo can also elucidate the proximal mechanisms by which placebo treatments work, including changes in brain activity, brain connectivity, and neurochemistry. Understanding these mechanisms is essential for understanding what brain–body processes placebos can affect, how placebo responses can be triggered in patient care, and how placebo-related interventions can be combined with standard medical treatments. Important insights into the mechanisms of placebo analgesia have come from studies that use PET and fMRI to measure brain activity and neurochemistry, pharmacological manipulations to manipulate neurochemistry, and transcranial magnetic stimulation to manipulate brain electrical activity.
The brain circuits important for creating and maintaining expectations, re-evaluating the significance of noxious stimuli, and activating endogenous antinociceptive systems are likely to show increased metabolic activity when anticipating and experiencing pain under placebo conditions because increased activity is usually tightly correlated with increased processing load in the brain. Figure 27-4A summarizes consistent findings on increases in fMRI and PET responses in placebo versus control conditions. Consistent increases in placebo conditions are found in the bilateral posterior dorsolateral prefrontal cortex (DLPFC), anterior prefrontal cortex, and orbitofrontal cortex (OFC), the pre-genual anterior cingulate cortex (pgACC), and the midbrain periaqueductal gray (PAG; red in Fig. 27-4; see Table 27-1 for details of numbered effects on the figure). In each of these regions, increases in activity in at least one study—and typically more—were correlated with the magnitude of placebo analgesia in reported pain (yellow in Fig. 27-4). These regions constitute a probable control circuit that generates expectations of pain relief and altered appraisals of imminent and ongoing pain in the placebo context. Involvement of the PAG points to possible activation of descending control systems and altered affective–motivational states.
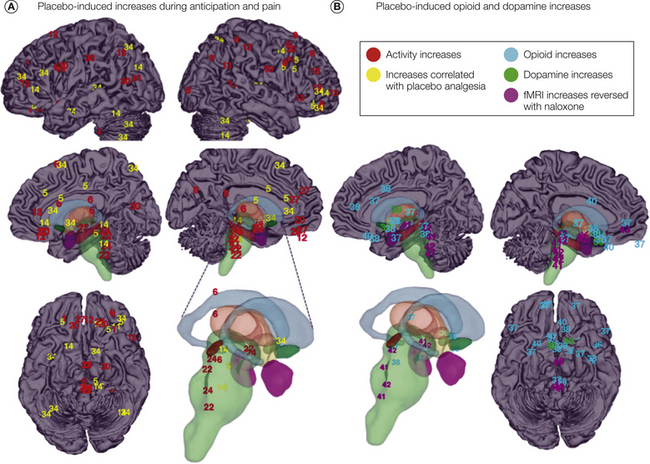
Figure 27.4 Mechanisms of placebo analgesia revealed through neuroimaging.
A, Coordinates from individual studies numbered according to Table 27-1 and associated with placebo-induced increases in activity. Overall increases in activity in group analyses (e.g., [Placebo > Control] during anticipation or the experience of pain) are shown in red, whereas increases in activity correlated with the magnitude of placebo analgesia are shown in yellow. Subcortical structures are colored for visibility and include the caudate (blue), thalamus (brown), brain stem (green), nucleus accumbens (darker green), hypothalamus (yellow), and amygdala (purple). Consistent changes in at least three separate contrasts were found in the dorsal pons, periaqueductal gray, bilateral midlateral orbitofrontal cortex, anterior insula, and bilateral posterior lateral prefrontal cortex. B, Coordinates associated with placebo-induced increases in endogenous neurotransmitter/neuropeptide activity, including opioid increases (light blue) and dopamine increases (green). Also shown are coordinates associated with placebo-induced increases in activity that were blocked by naloxone (purple). Consistent changes in at least three separate contrasts were found in the dorsal pons, subgenual anterior cingulate, nucleus accumbens, hypothalamus, and periaqueductal gray. fMRI, functional magnetic resonance imaging.
Further evidence on the relationship of brain activity to analgesia comes from correlations between individual differences in the magnitude of changes in brain activity with placebo analgesia. The most extensive treatment of predictors of individual differences in placebo analgesia to date was done by Wager and colleagues (2011; contrast 34 in Fig. 27-4A), who used multivariate patterns of brain activation to determine which regions have activity that most accurately predicts subjects’ reports of placebo analgesia. The strongest links during anticipation of pain were found in the anterior prefrontal cortex and superior parietal cortex, thus confirming the importance of anticipatory evaluative processes. Both placebo analgesia and pre-scan expectations of analgesia were also associated with reduced anticipatory activity in the pgACC, a region linked with anticipatory value, anxiety, and cardiovascular responses in other studies. These patterns explained up to 44% of the variance in individual placebo analgesia, which suggests that these brain changes might be reliable enough to be clinically useful.
This pattern of placebo-related influences is consonant with current models of affect regulation in other domains. The DLPFC, operating in conjunction with the parietal cortex, has been linked to maintenance of context information in short-term memory in numerous other studies and may play a large role in establishing the cognitive set that generates placebo analgesia. Recently, Krummenacher and co-authors (2010) reported that transcranial magnetic stimulation of the left DLPFC, which is thought to disrupt or inhibit ongoing processing in the stimulated region, blocked placebo analgesia without affecting baseline pain, thus corroborating the fMRI findings. Stimulation of the lateral prefrontal cortex in the rat also produces analgesia blocked by naloxone infusion in the PAG (Zhang et al 1997a, 1997b, 1998). The midlateral OFC has also been implicated in the generation and updating of reward value and hedonic processes, with neurons that encode both appetitive and aversive qualities of reinforcers. Recently, Petrovic and colleagues (2010) noted that this area was more reliably activated by placebo than by verum opiate treatment, which raises the possibility that placebos can engage evaluative mechanisms that could complement active treatment.
The heaviest anatomical connections with the PAG and other parts of the descending nociception control systems, however, are in the pgACC and other areas within the ventromedial prefrontal cortex (VMPFC), which has been termed the “visceromotor” cortex by virtue of its influences on the brain stem neuroendocrine and autonomic systems (Price 2005). This area is heavily connected with the lateral OFC and also with the amygdala, nucleus accumbens, ventral pallidum and striatum, PAG, hypothalamus, and other brain stem nuclei involved in pain regulation such as the parabrachial complex and rostral ventral medulla. Reliable metabolic increases here were first noted by Petrovic and colleagues (2002) in the first neuroimaging study of placebo analgesia and were co-localized with areas showing opiate-induced increases. Activity in more ventral parts of this region reliably track anticipated hedonic value and the desirability of economic outcomes, whereas activity more dorsal in the anterior cingulate responds to a variety of manipulations that increase anticipatory anxiety and stressor-evoked physiological changes (see Wager et al 2009). Several neuroimaging studies report increased functional coupling between the VMPFC or nearby dorsal cingulate cortex and the PAG or pontine areas under placebo conditions (Petrovic et al 2002, Bingel et al 2006, Wager et al 2007), thus providing additional support for placebo engagement of cortical–brain stem pain regulatory circuits.
Other areas are likely to be involved in this circuit as well—such as the ventral striatum/nucleus accumbens, parahippocampal cortex, and brain stem areas, including the rostral ventral medulla. Placebo-related increases in activity in each of these areas has been reported (Fig. 24-4A). Though not yet replicated multiple times, these areas are heavily interconnected with the VMPFC (and pgACC) and insula, and the neuropharmacological results described below suggest their importance in placebo analgesia. In addition, other studies reinforce the importance of the ventral striatum/accumbens, which is heavily implicated in approach and avoidance motivation and value-driven learning, in placebo analgesia. The ventral striatum/accumbens is robustly activated by cues that predict monetary gain (Knutson et al 2001) and loss (Jensen et al 2007), perhaps in different local regions (Seymour et al 2007; compare with Yacubian et al 2006); that predict shock (whether it can be avoided; Jensen et al 2003); and that predict pain relief (Baliki et al 2010). In addition, it is specifically activated in response to better than expected outcomes (Hare et al 2008; Rutledge et al 2010) and loss avoidance (Pessiglione et al 2006), but also during pain itself (Becerra et al 2001). Together, these findings suggest a central role for this structure in regulating the response to sensory stimuli with intrinsic motivational salience. Since relief of pain is negatively reinforcing, the expectation of pain relief might be viewed as reward predictive, and placebo treatment would be expected to activate the ventral striatum. Additionally, if the ventral striatum is critical for learning the motivational value of pain relief–related cues, it might play an important role in the development of placebo effects.
Schweinhardt and colleagues (2009) found that gray matter density in the human ventral striatum/accumbens was positively correlated with both placebo analgesia and a composite personality measure essentially reflecting approach motivation and risk seeking. Paralleling these findings, Wager and co-authors (2011) reported that the reduced ventral striatal responses during pain were among the strongest correlations between activity during pain and placebo analgesia (perhaps indicating reduced aversive processing or reduced demand for pain avoidance learning in the ventral striatum). In another study, Atlas and co-workers (2010) manipulated expectations about pain intensity with predictive cues. They found robust effects of high-pain cues on pain and responses in classic “pain-processing” circuits, including the rdACC, medial thalamus, aINS, and SII. These effects were mediated by anticipatory increases in the VMPFC (near the pgACC) and ventral striatum (near the accumbens), thus tracing a pathway from anticipatory processes in these “value-related” regions and responses in the established pain circuitry. This study and other similar ones (e.g., Koyama et al 2005, Keltner et al 2006) were not included in Figure 27-3 because they do not manipulate expectations about a treatment per se, as in the classic placebo paradigm, but the pain expectancy–manipulation paradigm is nearly indistinguishable from other recent studies that used conditioning to similar cues to elicit placebo effects (e.g., Lui et al 2010).
Neurochemical Mechanisms of Placebo Analgesia
One of the first discoveries that implied a role for placebos in shaping nociceptive processing was the finding of Levine, Gordon, and Fields (1978) that placebo effects could be reversed by the opiate antagonist naloxone, thereby implicating the endogenous opioid system. Other studies have since replicated and extended this finding in humans and animals (Benedetti and Amanzio 1997, Amanzio and Benedetti 1999, Guo et al 2010), although placebo effects are not always sensitive to naloxone (Vase et al 2005), particularly when they are created via pharmacological conditioning with a non-opiate drug (Amanzio and Benedetti 1999).
More recently, PET studies have directly assessed activity at μ-opioid and dopamine D2 receptors by using radioactively labeled compounds that bind to these receptors. Results from these studies are shown in Figure 27-4B, and they implicate many of the same brain structures as the fMRI studies do. Zubieta and colleagues (2005; contrast 36 in Fig. 27-4B) compared binding of the μ-opioid receptor–specific agonist carfentanil during intramuscular pain induced by injecting saline into the masseter muscle in the jaw. Subjective pain levels were matched by using an adaptive procedure, and the higher saline infusion rate required to maintain pain provided evidence of a placebo effect on pain. The higher rate of infusion was accompanied by decreases in binding (evidence of increased opioid system activation) in the pgACC, nucleus accumbens, DLPFC, and other areas, many of which were found to be correlated with subjective pain (Zubieta et al 2006). Wager and co-workers (2007; contrast 37) imaged μ-opioid binding with carfentanil during matched levels of noxious thermal heat with and without placebo (the typical design used in the fMRI studies described earlier) and found opioid system increases in these areas and in the bilateral OFC, medial thalamus, and PAG. This latter finding was particularly important because the PAG is a major source of opioids in the brain. They also found evidence of increased correlation in carfentanil binding between the rostral anterior cingulate and PAG (as in Bingel et al 2006, Kong et al 2008) and between a number of other placebo-responsive regions, consistent with the idea that placebo treatment causes central opioid release.
More recently, Scott and colleagues (2007, 2008; contrasts 38 and 39) provided additional evidence implicating both the endogenous opioid and dopamine systems in placebo analgesia. In the first paper, they used raclopride PET to image dopamine binding and scanned the same subjects with fMRI in a monetary incentive delay task to assess responses of the nucleus accumbens to impending reward. They found correlations between dopamine activity and fMRI responses in the accumbens, which were also correlated with the magnitude of placebo analgesia in a separate test. Subsequently, they imaged subjects in a placebo paradigm with both carfentanil and raclopride PET in separate sessions. They replicated the finding of placebo-induced increases in the PAG and reported correlated responses of both the dopamine and opioid systems in the nucleus accumbens. In general, these results fit with other fMRI studies showing correlations between pain-related decreases in the ventral striatum/pallidum and placebo analgesia (Wager et al 2011), correlations with ventral striatal gray matter density measures and placebo analgesia (Schweinhardt et al 2009), and ventral striatal mediation of the effects of pain-predictive cues (which elicit expectations of higher versus lower pain) on pain processing and pain report (Atlas et al 2010). Because opioids typically exert a local inhibitory effect on neural transmission, increased opioid responses might be expected to be associated with reduced fMRI activity during pain; however, the relationships between tonic and phasic dopamine, opioid, and fMRI responses are likely to be complex and remain to be fully elucidated.
Finally, Eippert and associates (2009) compared fMRI responses to noxious heat under placebo and control (inert instruction) conditions, as in previous studies, but this time with two groups: one group was treated with naloxone before testing, and the other was treated with saline. In addition to reversing pain-related decreases in established pain-processing regions, as discussed earlier, naloxone reversed placebo-related increases in the pgACC, DLPFC, and several brain stem regions, including the PAG, pons, and rostral ventral medulla. These latter findings are important because they establish links with the descending antinociceptive systems (Fields 2004), and they were detected in a unique, brain stem–specific analysis, which is a promising approach for future studies.
The Placebo Response in Clinical Practice
Very little published information is available on the extent to which placebo effects contribute to outcome in clinical practice, but studies of clinical treatments suggest that their effects might be quite large (Haake et al 2007, Kaptchuk et al 2008). Deliberate enhancement of the placebo component of an active clinical treatment is usually accomplished when the health care professional makes positive suggestions about the treatment’s efficacy, thus leading to an increase in the patient’s expectation of pain relief, or when attention is paid to the context cues and route of administration associated with treatment, including the place, time, and look and feel of the treatment. The extent to which health professionals actually make positive statements (or for that matter, negative statements) probably varies considerably and may play a larger role in the effectiveness of care than is commonly appreciated. Thus, in contrast to the overall view and conclusions of Hróbjartsson and Gøtzsche (2001), it is likely that at least some physicians, psychologists, physical therapists, and nurses elicit strong placebo effects and that improved pain management, at least in the short term, could occur by teaching such professionals about the relevant factors.
Several obvious factors related to the doctor–patient relationship and patients’ expectations may have an important impact on treatment outcome. First, the more ineffective treatments that a patient receives, the more likely it is that future treatments will fail. This means that it is important to find the optimal therapy early in the course of treatment, and it is important that patients believe that they can improve. This can be a major challenge for patients whose pain has persisted through many different therapies. Second, it is important for the person who is providing the treatment to communicate to the patient why a particular therapeutic approach is being used. If the practitioner doubts the efficacy of the treatment and this doubt is communicated to the patient, it may have a negative impact on treatment. Third, explaining the effect of expectancy to patients may be helpful, particularly if there is reason to believe that expectancy may be contributing to the resistance of the patient to treatment. Fourth, the use of prognostic drug infusions may be helpful in demonstrating to the patient that relief is possible. It is also possible that a conditioning effect of such infusions could add to the efficacy of the same class of drug when given orally. Of course, if the infusions do not work, the conditioning will work in the wrong direction.
Once these and other factors that contribute to placebo analgesia have been identified and studied in the clinical setting, they could be optimized in clinical practice. Even though optimizing clinician–patient interactions is likely to be complex and involve an understanding of the various reactions that patients may have to the same information, we are optimistic that placebo-related psychological principles could be systematically used to patients’ benefit. In addition, these principles may be applied without deception by using conditioning procedures (Benedetti et al 1998, Amanzio and Benedetti 1999) and providing positive, supporting information (Vase et al 2003, Kaptchuk et al 2010). The potential utilization of these techniques in clinical practice is an area ripe for future investigation.
Acknowledgment
Thanks to Mathieu Roy, Liane Schmidt, Jenna Reinen, and Yoni Ashar for helpful comments on the manuscript. This work was supported by grants R01MH076136 and R01DA027794 to T.D.W. The Matlab code implementing the plots shown in the figures is available at http://psych.colorado.edu/~tor.
The references for this chapter can be found at www.expertconsult.com.
References
Agreus L., Svardsudd K., Talley N.J., et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. American Journal of Gastroenterology. 2001;96:2905–2914.
Allan L., Siegel S. A signal detection theory analysis of the placebo effect. Evaluation & the Health Professions. 2002;25:410–420.
Amanzio M., Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. Journal of Neuroscience. 1999;19:484–494.
Aslaksen P.M., Bystad M., Vambheim S.M., et al. Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosomatic Medicine. 2011;73:193–199.
Aslaksen P.M., Flaten M.A. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosomatic Medicine. 2008;70:811–818.
Atlas L.Y., Bolger N., Lindquist M.A., et al. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30:12964–12977.
Atlas L.Y., Wager T.D. The placebo response. In: Banks W., ed. Encyclopedia of consciousness. New York: Elsevier; 2009:201–216.
Atlas L.Y., Wager T.D., Dahl K., et al. Placebo effects. In: Cacioppo J.T., Berntson G.G., eds. Handbook of neuroscience for behavioral psychologists. Hoboken, NJ: John Wiley & Sons; 2009:1236–1259.
Baliki M.N., Geha P.Y., Fields H.L., et al. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160.
Barrett B., Muller D., Rakel D., et al. Placebo, meaning, and health. Perspectives in Biology and Medicine. 2006;49:178–198.
Becerra L., Breiter H.C., Wise R., et al. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946.
Beecher H.K. The powerful placebo. Journal of the American Medical Association. 1955;159:1602–1606.
Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annual Review of Pharmacology and Toxicology. 2008;48:33–60.
Benedetti F. Placebo effects: understanding the mechanisms in health and disease. York, UK: New Oxford University Press; 2009.
Benedetti F., Amanzio M. The neurobiology of placebo analgesia: from endogenous opioids to cholecystokinin. Progress in Neurobiology. 1997;52:109–125.
Benedetti F., Amanzio M., Baldi S., et al. The specific effects of prior opioid exposure on placebo analgesia and placebo respiratory depression. Pain. 1998;75:313–319.
Benedetti F., Amanzio M., Maggi G. Potentiation of placebo analgesia by proglumide. Lancet. 1995;346:1231.
Benedetti F., Amanzio M., Vighetti S., et al. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. Journal of Neuroscience. 2006;26:12014–12022.
Benedetti F., Arduino C., Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. Journal of Neuroscience. 1999;19:3639–3648.
Benedetti F., Mayberg H.S., Wager T.D., et al. Neurobiological mechanisms of the placebo effect. Journal of Neuroscience. 2005;25:10390–10402.
Benedetti F., Pollo A., Lopiano L., et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. Journal of Neuroscience. 2003;23:4315–4323.
Bingel U., Lorenz J., Schoell E., et al. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15.
Bingel U., Wanigasekera V., Wiech K., et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science Translational Medicine. 3(70), 2011. 70ra14
Charron J., Rainville P., Marchand S. Direct comparison of placebo effects on clinical and experimental pain. Clinical Journal of Pain. 2006;22:204–211.
Cheng P.W., Holyoak K.J. Pragmatic reasoning schemas. Cognitive Psychology. 1985;17:391–416.
Clark W.C. Sensory-decision theory analysis of the placebo effect on the criterion for pain and thermal sensitivity. Journal of Abnormal Psychology. 1969;74:363–371.
Colloca L., Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133.
Colloca L., Lopiano L., Lanotte M., et al. Overt versus covert treatment for pain, anxiety, and Parkinson`s disease. Lancet Neurology. 2004;3:679–684.
Colloca L., Petrovic P., Wager T.D., et al. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439.
Colloca L., Sigaudo M., Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218.
Colloca L., Tinazzi M., Recchia S., et al. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2008;139:306–314.
Craggs J., Price D.D., Perlstein W.M., et al. The dynamic mechanisms of placebo induced analgesia: Evidence of sustained and transient regional involvement. Pain. 2008;139(3):660–669.
de Craen A.J., Tijssen J.G., de Gans J., et al. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. Journal of Neurology. 2000;247:183–188.
De Pascalis V., Chiaradia C., Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402.
Dworkin S.F., Chen A.C., LeResche L., et al. Cognitive reversal of expected nitrous oxide analgesia for acute pain. Anesthesia and Analgia. 1983;62:1073–1077.
Eippert F., Bingel U., Schoell E., et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543.
Eippert F., Finsterbusch J., Bingel U., et al. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404.
Enck P., Benedetti F., Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:95–206.
Fanselow M.S. Conditioned fear–induced opiate analgesia: a competing motivational state theory of stress analgesia. Annals of the New York Academy of Sciences. 1986;467:40–54.
Feather B., Chapman C.R., Fisher S. The effect of a placebo on the perception of painful radiant heat stimuli. Psychosomatic Medicine. 1972;34:290–294.
Fields H. State-dependent opioid control of pain. Nature Reviews. Neuroscience. 2004;5:565–575.
Finniss D.G., Kaptchuk T.J., Miller F., et al. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695.
Fournier J.C., DeRubeis R.J., Hollon S.D., et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA: Journal of the American Medical Association. 2010;303:47–53.
Garcia-Larrea L., Frot M., Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiologie Clinique. 2003;33:279–292.
Goffaux P., Redmond W.J., Rainville P., et al. Descending analgesia—when the spine echoes what the brain expects. Pain. 2007;130:137–143.
Gracely R.H. Evaluation of pain sensations. In: Merskey H., Loeser J.D., Dubner R., eds. The paths of pain 1975–2005. Seattle: IASP Press, 2005.
Gracely R.H., Dubner R., McGrath P.A. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203:1261–1263.
Guo J.Y., Wang J.Y., Luo F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. Journal of Psychopharmacology. 2010;24:1561–1567.
Haake M., Muller H., Schade-Brittinger C., et al. German Acupuncture Trials (GERAC) for chronic low back pain: randomized, multicenter, blinded, parallel-group trial with 3 groups. Archives of Internal Medicine. 167, 2007. 1892
Hare T., O’Doherty J., Camerer C., et al. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623.
Harris R.E., Zubieta J.K., Scott D.J., et al. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs. Neuroimage. 2009;47(3):1077–1085.
Herrnstein R.J. Placebo effect in the rat. Science. 1962;138:677–678.
Hróbjartsson A. What are the main methodological problems in the estimation of placebo effects? Journal of Clinical Epidemiology. 2002;55:430–435.
Hróbjartsson A., Gøtzsche P.C. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. New England Journal of Medicine. 2001;344:1594–1602.
Hróbjartsson A., Gotzsche P.C. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. Journal of Internal Medicine. 2004;256:91–100.
Hua L.H., Strigo I.A., Baxter L.C., et al. Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. American Journal of Physiology. Regulatory. Integrative and Comparative Physiology. 2005;289:R319–R325.
Jensen J., McIntosh A.R., Crawley A.P., et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257.
Jensen J., Smith A.J., Willeit M., et al. Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping. 2007;28:294–302.
Johansen O. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosomatic Medicine. 2003;65:786–790.
Kantor T.G., Sunshine A., Laska E., et al. Oral analgesic studies: pentazocine hydrochloride, codeine, aspirin, and placebo and their influence on response to placebo. Clinical Pharmacology and Therapeutics. 1966;7:447–454.
Kaptchuk T.J., Friedlander E., Kelley J.M., et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5:e15591.
Kaptchuk T.J., Kelley J.M., Conboy L.A., et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003.
Kaptchuk T.J., Stason W.B., Davis R.B., et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ. 2006;332:391–397.
Keltner J.R., Furst A., Fan C., et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience. 2006;26:4437–4443.
Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40:1189–1202.
Kleijnen J., de Crain A.J., van Everdingen J., et al. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet. 1994;344:1347–1349.
Knutson B., Adams C.M., Fong G.W., et al. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 21(16), 2001. RC159
Kong J., Gollub R.L., Rosman I.S., Webb J.M., et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388.
Kong J., Gollub R.L., Polich G., et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. Journal of Neuroscience. 2008;28:13354–13362.
Kong J., Kaptchuk T.J., Polich G., et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. NeuroImage. 2009;45:940–949.
Kong J., Kaptchuk T.J., Polich G., et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo nalgesia. Neuroimage. 2009;45(3):940–949.
Kong J., Kaptchuk T.J., Polich G., et al. An MRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage. 2009;47(3):1066–1076.
Koyama T., McHaffie J.G., Laurienti P.J., et al. The subjective experience of pain: where expectations become reality. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12950–12955.
Kross E., Berman M.G., Mischel W., et al. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6270–6275.
Krummenacher P., Candia V., Folkers G., et al. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374.
Laska E.M., Sunshine A. Anticipation of analgesia. A placebo effect. Headache. 1973;13:1–11.
Levine J.D., Gordon N.C., Fields H.L. The mechanism of placebo analgesia. Lancet. 1978;2:654–657.
Liberman R. An experimental study of the placebo response under three different situations of pain. Journal of Psychiatric Research. 1964;33:233–246.
Lieberman M.D., Jarcho J.M., Berman S., et al. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22(1):447–455.
Lorenz J., Hauck M., Paur R.C., et al. Cortical correlates of false expectations during pain intensity judgments—a possible manifestation of placebo/nocebo cognitions. Brain, Behavior, and Immunity. 2005;19:283–295.
Lu H.-C., Hsieh J.-C., Lu C.-L., et al. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: A 3T-fMRI study. Pain. 2010;148:75–83.
Lui F., Colloca L., Duzzi D., et al. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824.
Lyby P.S., Aslaksen P.M., Flaten M.A. Is fear of pain related to placebo analgesia? Journal of Psychosomatic Research. 2010;68:369–377.
Matre D., Casey K.L., Knardahl S. Placebo-induced changes in spinal cord pain processing. Journal of Neuroscience. 2006;26:559–563.
Metcalfe J. Cognitive optimism: self-deception or memory-based processing heuristics? Personality & Social Psychology Review: Special Issue: Metacognition. 1998;2:100–110.
Mischel W. Toward an integrative science of the person. Annual Review of Psychology. 2004;55:1–22.
Moerman D.E., Jonas W.B. Deconstructing the placebo effect and finding the meaning response. Annals of Internal Medicine. 2002;136:471–476.
Montgomery G.H., Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113.
Morton D.L. Cognitive changes as a result of a single exposure to placebo. Neuropsychologia. 2010;48:1958–1964.
Morton D.L., El-Deredy W., Watson A., et al. Placebo analgesia as a case of a cognitive style driven by prior expectation. Brain Research. 2010;1359:137–141.
Morton D.L., Watson A., El-Deredy W., et al. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain. 2009;146:194–198.
Pavlov I.P., Anrep G.V. Conditioned reflexes; an investigation of the physiological activity of the cerebral cortex. Humphrey Milford, London: Oxford University Press; 1927.
Pessiglione M., Seymour B., Flandin G., et al. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045.
Petrovic P., Kalso E., Petersson K.M., et al. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–1740.
Petrovic P., Kalso E., Petersson K.M., et al. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150:59–65.
Price D.D., Craggs J., Verne G., et al. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72.
Price D.D., Finniss D.G., Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annual Review of Psychology. 2008;59:565–590.
Price D.D., Milling L.S., Kirsch I., et al. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156.
Price J.L. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. Journal of Comparative Neurology. 2005;493:132–139.
Roethlisberger F.J., Dickson W.J. Management and the Worker. Cambridge, MA: Harvard University Press; 1939.
Rutledge R.B., Dean M., Caplin A., et al. Testing the reward prediction error hypothesis with an axiomatic model. Journal of Neuroscience. 2010;30:13525–13536.
Schweinhardt P., Seminowicz D.A., Jaeger E., et al. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. Journal of Neuroscience. 2009;29:4882–4887.
Scott D., Stohler C., Egnatuk C., et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336.
Scott D., Stohler C., Egnatuk C., et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry. 2008;65:220–231.
Seymour B., Daw N.D., Dayan P., et al. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27:4826–4831.
Shapiro A.K. The placebo effect in the history of medical treatment: implications for psychiatry. Am J Psychiatry. 1959;116:298–304.
Shapiro A.K., Struening E.L., Shapiro E. The reliability and validity of a placebo test. Journal of Psychiatric Research. 1979;15:253–290.
Staats P.S., Staats A., Hekmat H. The additive impact of anxiety and a placebo on pain. Pain Medicine. 2001;2:267–279.
Stewart-Williams S., Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological Bulletin. 2004;130:324–340.
Tversky A. Features of similarity. Psychological Review. 1977;84:327–352.
Tversky A., Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458.
Vase L., Petersen G.L., Riley J.L., et al. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44.
Vase L., Riley J.L., Price D.D. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452.
Vase L., Robinson M., Verne G., et al. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25.
Vase L., Robinson M., Verne G., et al. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347.
Voudouris N.J., Peck C.L., Coleman G. Conditioned placebo responses. Journal of Personality and Social Psychology. 1985;48:47–53.
Voudouris N.J., Peck C.L., Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38:109–116.
Voudouris N.J., Peck C.L., Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128.
Wager T.D., Atlas L.Y., Leotti L.A., et al. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. Journal of Neuroscience. 2011;31:439–452.
Wager T.D., Matre D., Casey K.L. Placebo effects in laser-evoked pain potentials. Brain, Behavior, and Immunity. 2006;20:219–230.
Wager T.D., Rilling J.K., Smith E.E., et al. Placebo-induced changes in FMRI in the anticipation and experience of pain: supplementary material. Science. 2004;303:1162–1167.
Wager T.D., Scott D.J., Zubieta J.K. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–11061.
Wager T.D., van Ast V.A., Hughes B.L., et al. Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851.
Walsh B.T., Seidman S.N., Sysko R., et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA: Journal of the American Medical Association. 2002;287:1840–1847.
Watson A., El-Deredy W., Vogt B.A., et al. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport. 2007;18:771–775.
Watson A., El-Deredy W., Iannetti G.D., et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30.
Whalley B., Hyland M., Kirsch I. Consistency of the placebo effect. Journal of Psychosomatic Research. 2008;64:537–541.
White P.F. Use of patient-controlled analgesia for management of acute pain. JAMA: Journal of the American Medical Association. 1988;259:243–247.
Whitney C.W., Von Korff M. Regression to the mean in treated versus untreated chronic pain. Pain. 1992;50:281–285.
Wickramasekera I. A conditioned response model of the placebo effect. Biofeedback and Self-Regulation. 1980;5:5–18.
Yacubian J., Gläscher J., Schroeder K., et al. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. Journal of Neuroscience. 2006;26:9530–9537.
Zhang S., Tang J.S., Yuan B., et al. Involvement of the frontal ventrolateral orbital cortex in descending inhibition of nociception mediated by the periaqueductal gray in rats. Neuroscience Letters. 1997;224:142–146.
Zhang S., Tang J.S., Yuan B., et al. Inhibitory effects of electrical stimulation of ventrolateral orbital cortex on the rat jaw-opening reflex. Brain Research. 1998;813:359–366.
Zhang Y.Q., Tang J.S., Yuan B., et al. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 1997;72:127–135.
Zubieta J.K., Bueller J.A., Jackson L.R., et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. Journal of Neuroscience. 2005;25:7754–7762.
Zubieta J.K., Stohler C. Neurobiological mechanisms of placebo responses. Annals of the New York Academy of Sciences. 2009;1156:198–210.
Zubieta J.K., Yau W.Y., Scott D.J., et al. Belief or need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain, Behavior, and Immunity. 2006;20:15–26.
Atlas L.Y., Bolger N., Lindquist M.A., et al. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30:12964–12977.
Atlas L.Y., Wager T.D., Dahl K., et al. Placebo effects. In: Cacioppo J.T., Berntson G.G., eds. Handbook of neuroscience for behavioral psychologists. Hoboken, NJ: John Wiley & Sons; 2009:1236–1259.
Bingel U., Lorenz J., Schoell E., et al. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15.
Eippert F., Bingel U., Schoell E., et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543.
Eippert F., Finsterbusch J., Bingel U., et al. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404.
Enck P., Benedetti F., Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:195–206.
Fields H. State-dependent opioid control of pain. Nature Reviews. Neuroscience. 2004;5:565–575.
Finniss D.G., Kaptchuk T.J., Miller F., et al. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695.
Hrobjartsson A., Gotzsche P.C. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. Journal of Internal Medicine. 2004;256:91–100.
Keltner J.R., Furst A., Fan C., et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience. 2006;26:4437–4443.
Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40:1189–1202.
Kong J., Gollub R.L., Polich G., et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. Journal of Neuroscience. 2008;28:13354–13362.
Kong J., Kaptchuk T.J., Polich G., et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. NeuroImage. 2009;45:940–949.
Koyama T., McHaffie J.G., Laurienti P.J., et al. The subjective experience of pain: where expectations become reality. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12950–12955.
Kross E., Berman M.G., Mischel W., et al. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6270–6275.
Krummenacher P., Candia V., Folkers G., et al. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374.
Lui F., Colloca L., Duzzi D., et al. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824.
Petrovic P., Kalso E., Petersson K.M., et al. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–1740.
Price D.D., Craggs J., Verne G., et al. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72.
Schweinhardt P., Seminowicz D.A., Jaeger E., et al. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. Journal of Neuroscience. 2009;29:4882–4887.
Scott D., Stohler C., Egnatuk C., et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336.
Scott D., Stohler C., Egnatuk C., et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry. 2008;65:220–231.
Wager T.D., Atlas L.Y., Leotti L.A., et al. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. Journal of Neuroscience. 2011;31:439–452.
Wager T.D., Rilling J.K., Smith E.E., et al. Placebo-induced changes in FMRI in the anticipation and experience of pain: supplementary material. Science. 2004;303:1162–1167.
Wager T.D., Scott D.J., Zubieta J.K. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–11061.
Watson A., El-Deredy W., Iannetti G.D., et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30.