Local Anesthetic Blocks and Epidurals
Introduction
Neuraxial and peripheral nerve blocks have been cornerstones in the management of acute, cancer, and chronic pain. Anesthesiologists became involved in pain medicine because of their skills in performing nerve blocks. In the United Kingdom they formed “The Intractable Pain Society,” an appropriate name at the time when neurolytic blocks with ethanol or phenol were the mainstay in the treatment of severe cancer-related pain. The largest group of individual members of the International Association for the Study of Pain (IASP) are pain-interested anesthesiologists. This is an indication that most anesthesiologists realize that although nerve blocks are important, collaboration with pain clinicians from other disciplines and professions in the IASP is necessary to help relieve patients with complex pain disorders. Nerve blocks can be helpful in diagnosing the location of the cause of a pain condition and can help define a sympathetically maintained pain component in complex pain conditions.
Repeated nerve blocks can relieve pain for periods far outlasting the effects of local anesthetics. Staffan Arnér’s classic and much-cited study documented this concept more than 20 years ago (Arnér et al 1990); however, we still do not fully understand the mechanisms of these prolonged effects. Breaking vicious cycles of “pain causing more pain” may possibly be involved in some cases, for example, pain-producing reflex muscle contractions and myogenic pain or release of noradrenaline from sympathetic efferent nerves producing more pain fiber firing via noradrenaline-hypersensitive afferents. Other possible mechanisms suggested by animal studies are reduced sprouting of damaged nerves in the spinal ganglia, decreased ephaptic transmission, and a reduction in the hyperexcitability of nociceptive nerves or neurons in the spinal cord dorsal horn. These effects are more pronounced if a glucocorticoid is added to a local anesthetic (Devor et al 1985). In addition to any local effects on the nerves being blocked, there are also systemic effects on the pain-modulating mechanisms in the central nervous system (CNS) from systemically absorbed local anesthetic drugs.
With optimal co-administration of a local anesthetic, an opioid, and adrenaline, neuraxial blocks using catheter infusions with external or internalized pump systems are powerful tools in managing intense acute as well as severe chronic pain.
This chapter does not provide a “how to do it” description of these techniques. Several excellent books describe in detail aspects of selecting and preparing patients, finding landmarks, step-by-step descriptions of how to perform the blocks, the aids needed, outcome measures, and how to prevent and handle immediate and delayed complications (Hill 2008, Cousins et al 2009).
Peripheral and Sympathetic Blocks
Types of Peripheral Nerve Blocks
Peripheral nerve blocks are effective in managing pain because they can interrupt the flow of impulses from the painful part of the body to the CNS. Traditionally, these have been divided into diagnostic nerve blocks, prognostic nerve blocks, and therapeutic nerve blocks (Curatolo and Bogduk 2010, Kvarstein 2010); see Box 37-1. For important aspects of peripheral nerve blocks see Box 37-2.
Diagnostic Nerve Blocks
These blocks are useful in searching for the location of the cause of the pain; infiltration of a local anesthetic into a neuroma, a painful joint, or a trigger point may indicate the source of the pain.
However, it may be difficult to categorically determine whether the pain has only a peripheral source or whether the pain is also or mainly due to CNS pathology. Reducing input from the periphery may dampen hyperexcitable neurons in the dorsal horn of the spinal cord. Failure to appreciate this has caused many peripheral nerves to be cut, burned, or frozen, which produces only short-lasting effects and leaves numbness and additional abnormal pain in the denervated area.
Specific sympathetic nerve blocks may help determine any sympathetically maintained pain component in more complex chronic pain states; see Box 37-3.
For a diagnostic block to be useful, detailed anatomical knowledge, technical skills, and experience are mandatory. The physician must have thorough knowledge of the pain syndromes being diagnosed and their overall evaluation and management. It is crucial that the block be conducted with finesse and gentleness so that nervous tissue is not damaged and the patient is not disturbed by a traumatic and painful experience.
The physician must be fully aware of potential side effects and complications of nerve blocks and must be prepared and skilled at dealing with any that do arise.
Nerves must be accurately targeted with a nerve stimulator, ultrasound, or fluoroscopy. Initially, a short-acting local anesthetic such as lidocaine or chloroprocaine should be used. A small volume (0.5–3 mL, depending on the site) must be used so that nearby nociceptors and nerve fibers do not become contaminated. If a short-acting local anesthetic block relieves the pain for about an hour, the block should be repeated with a longer-acting agent such as bupivacaine, which should give pain relief for at least 2 hours, depending on the concentration and volume injected.
If a diagnostic block is performed to localize a nerve to be treated with a neurolytic agent, cryoneurolysis, or radiofrequency denervation, repeated blocks (preferably with a placebo injection) should be performed (Crul et al 2008, Kvarstein and Högström 2008, Curatolo and Bogduk 2010, Kvarstein 2010).
Pain relief can follow the injection of saline alone. This can be a true placebo response but might also be caused by a counter-irritation effect, or peripheral input that dampens a central component of a chronic pain condition. Pain relief from a saline “block” certainly does not by itself mean that the pain is “psychogenic” or that the patient is malingering.
Prognostic Blocks
These blocks are meant to indicate whether destruction of the peripheral nerve will give long-lasting pain relief. However, this is unreliable, in part because the pain may be maintained by central dysfunction in the spinal cord or at higher CNS sites.
Preventive Nerve Blocks (Not “Pre-emptive” Blocks)
This concept received a lot of attention after the hypothesis was launched 2 decades ago that postoperative pain could be prevented by a nerve block established before surgery. This is not the case. However, a prophylactic block established before surgery and continued during and after surgery for as long as the patient has severe movement-triggered pain constitutes optimal postoperative pain management and may have prolonged beneficial effects, such as facilitating active rehabilitation after surgery and possibly reducing risk for the development of chronic pain after surgery (Breivik et al 1996).
Therapeutic Local Anesthetic Blocks
For acute pain after surgery or trauma, an appropriate nerve block can relieve the pain completely for the duration of the local anesthetic effect. The duration of pain relief can be extended by administering a dilute local anesthetic by continuous infusion or by patient-controlled bolus injections into a catheter placed near a nerve or a nerve plexus.
For chronic pain the efficacy of nerve blocks is less impressive. However, experienced pain clinicians are convinced that in some patients local anesthetic blocks may give pain relief that far outlasts the specific local anesthetic block of the peripheral nerve (Arnér et al 1990). This may be due to effects on hypersensitive nociceptors and peripheral C and Aδ fibers and/or to effects in the CNS from systemically absorbed local anesthetic drugs.
Regional blocks may facilitate a mobilization regime for patients with complex regional pain syndromes (CRPS).
Finally, there is a strong, non-specific, positive “context-sensitive therapeutic effect” when the physician demonstrates to the patient that the pain can be taken away completely, though transiently. It also helps in explaining pain and pain mechanisms to patients, reducing anxiety, and improving coping. A successful block may reinforce the effects of other measures taken to help the patient.
Neurolytic, Neurodestructive Nerve Blocks
Phenol in aqueous solution (up to 6.7%), phenol (8%) in glycerol (requiring a large-bore needle), or ethanol (up to 96%) is used to interrupt conduction of impulses in peripheral nerve fibers. Initially a burning and tender inflammatory reaction occurs, followed by a numbing pain relief that peaks after a few days. Unfortunately, the duration of nerve impulse block with these neurolytic agents is often disappointingly brief. Even worse, they induce a deafferentation–neuropathic type of pain after a few weeks to months in up to one-third of cases. Except for classic trigeminal neuralgia, they are not indicated for patients with chronic pain and a normal life expectancy. In patients with localized pain from advanced cancer disease, the duration of effect of such neurolytic blocks may be sufficient (Campbell 2008).
Cryoneurolysis (“cryoanalgesia”) causes its effect by freezing nerve segments to −70°C for 2–4 minutes; it is repeated two to three times with thawing in between. This is probably the most benign of the neurolytic procedures, with a low incidence of neuritis, although the risk for development of neuropathic pain is not zero (Kvarstein and Högström 2008).
Denervation by heating the nerve to 70–80°C for brief periods with a radiofrequency probe that has a smaller dimension than the cryoprobe may cause more profound destruction in a localized area (Crul et al 2008).
Some Useful Peripheral Nerve Blocks
Local Infiltration
Chronic Pain Conditions
When such conditions are localized, they can be relieved temporarily with local anesthetic blocks, sometimes for prolonged periods. Spontaneous ectopic discharges in a “trigger point,” in a painful scar after surgery or trauma, or in an amputation stump can be suppressed by infiltration with a local anesthetic with a depot glucocorticoid added (Devor et al 1985).
“Trigger points” of myofascial syndromes are treated by infiltration of dilute local anesthetic solutions, with or without glucocorticoid. Depending on what is done after the infiltration (stretching, cold, massage, appropriate exercises), the beneficial effects may persist for longer periods. Patients with uncomplicated myofascial pain syndromes will benefit; patients with more complex chronic pain conditions will need a comprehensive approach, including appropriate cognitive therapy (Turk 2003).
Arthritic pain can be relieved effectively by intra-articular injection of dilute local anesthetic with glucocorticoid added (Shipley and Morris 2008). All major limb joints, as well as facet joints of the spine, can be injected (Cooper 2008). The duration of pain relief depends on the degree and duration of the arthritic changes.
Topical application of local anesthetic drugs is also a form of local infiltration. Jellies or creams are useful for mucous membrane pain from the urethra, urinary bladder, and rectum. Ointments and concentrated solutions can temporarily relieve the often excruciating pain from oral mucositis in cancer patients and bone marrow transplant patients. Eutectic mixtures, ointments, and patches are used on the allodynic and painful skin of patients with, for instance, post-herpetic neuralgia (Garnock-Jones and Keating 2009). Relief is obtained locally, but the local anesthetic is absorbed and the systemic effects may dampen the CNS hyperexcitability of this complex peripheral and central neuropathic pain (Hans et al 2009). Systemic toxic concentrations can occur if patches are kept on the skin for more than 12 hours.
Peripheral Nerves and Regional Nerve Plexuses
With good knowledge of anatomy and a neuromuscular stimulator and/or an ultrasound device, most nerves can be blocked specifically (Hill 2008). Catheters can be placed near peripheral nerves and nerve plexuses for continuous infusion of local anesthetic drugs. Excellent relief of acute pain is obtained. With simple patient-controlled devices, these techniques can be used safely at home (Chelly and Williams 2004).
Nerve Blocks of the Head and Neck
Nerve Blocks of the Upper Limb
Nerve Blocks of the Thorax and Abdomen
Nerve Blocks of the Lower Limb
Sympathetic Nerve Blocks
Selective sympathetic blocks are possible because anatomically the autonomic nervous system is (in part) separated from the somatic nervous system in the pre- and paravertebral regions. These blocks will interrupt both afferent and efferent sympathetic nerves. For thorough reviews, see Breivik (2008a) and Breivik and Cousins (2009).
The sympathetic efferent nerves arise from neurons in the intermediolateral column of the spinal cord, pass in the ventral roots from T1–L2, and then, via the white rami communicantes, join the sympathetic chain of paravertebral ganglia on each side of the vertebral bodies. In the cervical and lumbar regions, the sympathetic efferents are well separated from the somatic fibers; in the thoracic region they are closer.
The efferent sympathetic nerves then pass a variable distance up or down the sympathetic chain of ganglia, where they may synapse (cholinergic) with the post-ganglionic (adrenergic) neurons or pass on to the prevertebral ganglia (e.g., celiac plexus, superior hypogastric plexus) or on to post-ganglionic neurons in terminal ganglia close to the visceral organs. Then they synapse with the adrenergic post-ganglionic neurons (cholinergic to the sweat glands and some smooth muscles of vessels). The paravertebral sympathetic ganglia consist of 3 cervical ganglia, the stellate ganglion, 11 thoracic ganglia, 5 lumbar ganglia, 4 sacral ganglia, and 1 coccygeal ganglion (the “ganglion impar”).
From the post-ganglionic neurons, unmyelinated efferent fibers pass on to the somatic spinal nerves via the gray rami communicantes and follow these or vessels to effector cells (smooth muscles of vessels), sudomotor cells (cholinergic), and peripheral nociceptors, which may develop abnormal sensitivity to noradrenaline in some chronic pain states.
Afferent fibers (unmyelinated) carrying nociceptive impulses from viscera of the entire body pass through the sympathetic ganglia to the dorsal roots of the spinal nerves.
Diagnostic blocks are useful for evaluating sympathetically maintained pain in complex pain conditions. Therapeutic blocks of efferent sympathetic nerves or functional interruption of noradrenergic nerve endings of the peripheral sympathetic system (with guanethidine regional blocks) may reduce pain that is being maintained by excessive sympathetic outflow or by abnormal sensitivity to noradrenaline in nociceptors and nociceptive nerve fibers. Selective blocks of pre- or paravertebral ganglia may relieve visceral pain.
Indications for Sympathetic Blocks (See Box 37-3)
An example is pain in an ischemic limb (frostbite, embolus, or after accidental intra-arterial injection of a drug that is locally irritating). Although specific sympathetic blocks will relieve visceral pain, there are also somatic nociceptive pain components, and more complete pain relief is obtained with peripheral or regional blocks, such as a perivascular brachial plexus or epidural block. An epidural block will interrupt sympathetic afferent pain impulses transmitting the excruciating pain of acute pancreatitis, the pain associated with renal colic, uterine pain during childbirth, and cardiac ischemic pain. For these conditions the more specific sympathetic blocks are hardly indicated because a mixed sympathetic and somatic block is usually needed.
Acute pain of herpes zoster in the trigeminal area is relieved by stellate ganglion blocks (Box 37-4); such blocks may also reduce the risk for post-herpetic neuralgia (Tenicela et al 1985). The acute pain of herpes zoster in other locations is better treated with paravertebral or epidural blocks, which relieve the somatic as well as the visceral pain.
Chronic Visceral Pain and Sympathetically Maintained Pain (See Box 37-4)
Visceral pain from gastric and pancreatic cancer can be relieved by a celiac plexus block with ethanol (or phenol). This block relieves the deep aching pain that is difficult to relieve with opioids because of the often accompanying nausea. A celiac plexus block relieves the pain as well as the nausea and markedly or completely reduces the amount of opioids needed. Its duration lasts from weeks to months, and the block can be repeated if the pain recurs (Campbell 2008).
Visceral pain from cancer of the pelvic organs (descending and sigmoid colon, rectum, uterus and ovaries, vaginal fundus, prostate, bladder, testes and seminal vesicles) may be relieved by neurolytic block of the superior hypogastric plexus (Campbell 2008).
Visceral pain from the distal end of the rectum, perineum, vulva, and distal third of the vagina may be relieved by blocking the coccygeal sympathetic ganglion impar (Campbell 2008).
However, when abdominal and pelvic tumors invade the abdominal wall, perineum, and nerve plexuses, the pain is no longer only visceral and these selective sympathetic blocks will at best remove only part of the pain.
The pain of recurrent and chronic pancreatitis can be relieved completely for a few hours with a celiac plexus block with local anesthetic drugs, the duration often being prolonged for days to weeks if a depot steroid is added. Unfortunately, the duration of pain relief from neurolytic blocks of the celiac plexus decreases rapidly with repeated blocks, although occasionally the effect may last longer (Campbell 2008).
Lumbar sympathetic ganglion blocks, especially when pain is present at rest in the legs, may reduce pain from obliterative arterial disease. Neurolytic blocks with phenol or radiofrequency denervation of the lumbar sympathetic chain may facilitate exercise and result in lasting improvement.
Pain from chronic vasospastic disease (Raynaud’s disease, Raynaud’s phenomenon) and chronic pain after cold injuries are indications for a series of sympathetic ganglion blocks: stellate ganglion and first to second thoracic sympathetic ganglia for the upper limb and second to third (fourth) lumbar sympathetic ganglia for the lower limb.
A component of sympathetically maintained pain may be present in about one-third of cases with CRPS types 1 and 2. Diagnostic and subsequent therapeutic blocks may aid in the management and rehabilitation of patients with CRPS (Raja and Grabow 2002).
Phantom limb pain and central pain after stroke may sometimes have a sympathetically maintained component that will be revealed only by specific sympathetic blocks.
Aspects of Performing Sympathetic Blocks
When sympathetic efferent blockade is needed but does not have to be specific, an appropriate neuraxial or peripheral nerve block (e.g., epidural block) will suffice. When a specific sympathetic blockade is needed for diagnosis, sympathetic ganglion blocks at the appropriate levels are required. These blocks can be achieved by local bolus injection of an anesthetic, repeated as necessary, or by catheter infusion for more prolonged effect. Neurolytic or radiofrequency denervation of sympathetic ganglia may give more lasting effects, but unfortunately, there is a risk for denervation hyperpathia, which will complicate the pain condition even further.
An intravenous regional sympathetic block with guanethidine depletes the noradrenergic efferent nerve endings of their transmitter substance and causes an efferent sympathetic block that lasts for days to weeks. This is a low-risk, easily performed sympathetic block that is effective only if the patient has sympathetically maintained pain (Breivik and Cousins 2009). Unfortunately, “controlled” studies have compared intravenous regional lidocaine blocks with or without guanethidine in patients with various regional neuropathic-type pain conditions. These negative outcome studies have at least two fatal flaws (Breivik 1997):
Unfortunately, such studies with false-negative outcomes have resulted in guanethidine becoming unavailable in many countries. An intravenous regional guanethidine block, as described by Hannington-Kiff (1974), is carried out as a traditional Bier block with injection of 10–30 mg of guanethidine dissolved in 25–40 mL of saline into a distal intravenous catheter after inflating a blood pressure cuff above systolic pressure. The limb is kept isolated from the circulation for at least 20 minutes thereafter. Pain may increase as noradrenaline is released from sympathetic nerve endings, but such pain decreases gradually over the next few hours. It is often necessary to give an intravenous bolus dose of a rapid, short-acting opioid (e.g., alfentanil) during the procedure. Lidocaine should not be used instead of saline to reduce the procedural pain because lidocaine will ruin the effect of the guanethidine (Joyce et al 2002). There is a tendency for transient orthostatic hypotension and a stuffy nose from the systemic effects of guanethidine after release of the tourniquet, and patients need to be warned of these effects. Very rarely, transient cardiac dysrhythmias have occurred in elderly patients.
Epidural and Intrathecal Analgesia
Epidural analgesia is still the “gold standard” for the management of severe pain aggravated by deep breathing, coughing, and movement after surgery (or trauma) on the thorax and upper part of the abdomen (Box 37-5). It is also the gold standard for relieving severe labor pain during vaginal delivery. A thoracic paravertebral block has been proposed as a safer alternative. However, paravertebral blocks are neither more effective nor safer than epidural analgesia, as discussed below (and see Norum and Breivik 2010).
Epidural analgesia is not a standardized procedure; it is practiced in many different ways, and not all are effective and not all are safe (Breivik 2008b). The practice of epidural analgesia requires good anatomical and pharmacological knowledge, technical skills, and experience. It is not without risk to the patient and requires a robust system for monitoring its effects and possible adverse effects (Box 37-6). Constant effort is required to maintain vigilance for discovering early symptoms of infrequent but potentially catastrophic complications from bleeding or infection in the spinal canal. Early detection and high preparedness for treating complications are mandatory to prevent permanent neurological sequelae. Therefore, the practice of effective and safe epidural analgesia is labor-intensive.
The following is a brief description of how I use this extremely elegant and useful tool after more than 3 decades of continuously improving efficacy and safety of epidural analgesia (Breivik et al 1995, Niemi and Breivik 2002, Breivik 2008b).
Why Use Epidural Analgesia?
The intense pain that is provoked by deep breathing, coughing, or moving a body part after major surgery or trauma involving the abdominal or thoracic cavities and thoracic wall causes immobility, ineffective cough, and lack of deep breathing and markedly increases the risk for the development of complications in the lungs and cardiocirculatory organs. Such “incident” pain can best be relieved and the accompanying risk for complications reduced by continuous thoracic epidural analgesia. With an ongoing, optimally conducted epidural analgesic regimen started before induction of general anesthesia, patients can be awake and extubated early and immediately be able to breathe deeply and cough effectively. This will prevent retention of secretions and the development of atelectasis, pneumonia, and sepsis. Patients can get out of bed, move about without pain or orthostatic hypotension, pass urine, and empty their bowels early. Rehabilitation of normal function will be greatly facilitated and the risk for thromboembolic complications reduced (Breivik et al 1995, Ballantyne et al 1998, Ballantyne 2004, Caputo et al 2011, Royse 2011).
This is in such striking contrast to patients waking up from general anesthesia without an epidural that no “blinded, randomized controlled” study is needed to document the differences.
However, it is also true that a poorly managed epidural is even worse than no epidural. The patient is bound to have poor pain relief when the epidural is activated only after the end of surgery, when the epidural catheter is situated too low (Fig. 37-1 for correct positions of the thoracic epidural catheter) or is on one side, and when excessively high doses of local anesthetic and opioid are administered to compensate for a malpositioned epidural catheter. The patient will also most likely be hypotensive and have weak legs, which makes mobilization difficult or impossible. In addition, the patient is likely to have urinary retention, nausea, and severe pruritus.
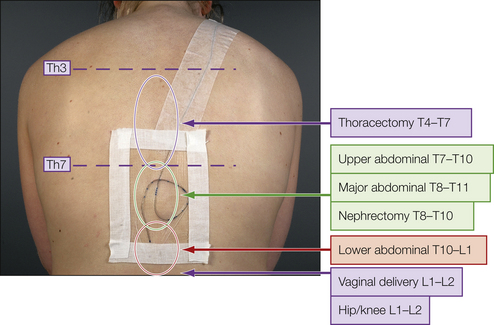
Figure 37-1 The three components of the epidural solution, bupivacaine, fentanyl, and adrenaline, cause additive (and potentially synergistic) analgesia when reaching the dorsal horn of the spinal cord at the appropriate segmental level relative to the surgery or trauma. Correct segmental placement of the epidural catheter is therefore of utmost importance for obtaining optimal epidural analgesia with only small amounts of drugs and reducing dose-related side effects to a minimum. (Adapted from Breivik H, Curatolo M, Niemi G, et al 2007 How to implement an acute postoperative pain service: an update. In: Breivik H, Shipley M (eds) Pain best practice and research compendium. Elsevier, London, p xiii, Plate I.)
Some studies comparing “epidural” with other forms of perioperative pain relief have had poor control of the quality of the epidural procedures and regimens. It does not help to have hundreds of patients randomized to the two groups if half the epidural catheters are poorly situated or come out early accidentally (Rigg et al 2002). Equally important is what is administered into the epidural catheter—it is still common to see too little, too much, or far from the best combinations of synergistically acting analgesic drugs being used (Figs. 37-2 and 37-3).
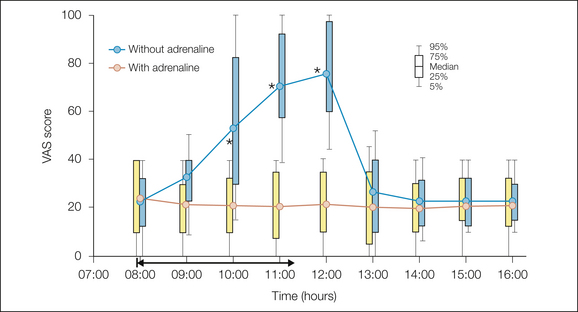
Figure 37-2 Pain intensity on a 0–100 visual analog scale (VAS) during deep breathing and coughing after thoracoabdominal surgery.
The patients had an ongoing epidural infusion of a triple analgesic mixture (bupivacaine, 1 mg/mL; fentanyl, 2 μg/mL; adrenaline, 2 μg/mL) from surgery the day before. At 8:00 AM they received in a double-blind, randomized manner the triple mixture or only bupivacaine plus fentanyl without the adrenaline. When pain became unbearable with the mixture without adrenaline, the triple-component epidural infusion was resumed and the pain then returned to mild intensity. (Modified from Niemi G, Breivik H 1998 Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery. Acta Anaesthesiologica Scandinavica 42:897–909.)
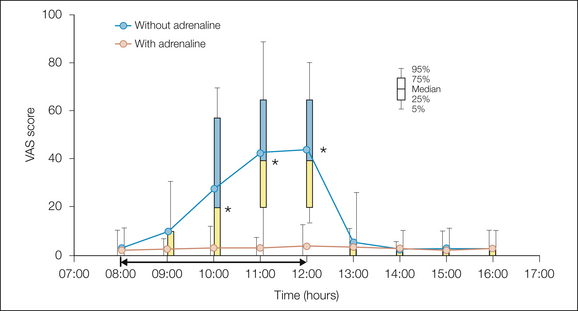
Figure 37-3 Pain intensity on a 0–100 visual analog scale (VAS) at rest after thoracoabdominal surgery.
The patients had an ongoing epidural infusion of a triple analgesic mixture (bupivacaine, 1 mg/mL; fentanyl, 2 μg/mL; adrenaline, 2 μg/mL) from surgery the day before. At 8:00 AM they received in a double-blind, randomized manner the triple mixture or only bupivacaine plus fentanyl without the adrenaline. When the pain became unbearable with the mixture without adrenaline, the triple-component epidural infusion was resumed and the pain then returned to almost zero intensity. (Modified from Niemi G, Breivik H 1998 Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery. Acta Anaesthesiologica Scandinavica 42:897–909.)
Recently, resurgent interest in paravertebral blocks has created the claim that paravertebral blocks are more effective and safer than epidural analgesia. This may be true if one compares a poorly conducted epidural (see above) with paravertebral blocks using large volumes of concentrated local anesthetics. However, this is an “easy” way of avoiding proper performance of epidurals. Paravertebral blocks can have serious complications (Norum and Breivik 2010).
Although an individually tailored, optimally conducted thoracic epidural, in the hands of a dedicated acute pain team on surgical wards, provides the best possible pain relief, it is only one of many aspects of good rehabilitation after major surgery on the thorax and abdomen. Risks for postoperative complications and morbidity are reduced, the speed and quality of recovery are improved (Kehlet 1997, Basse et al 2002, Ballantyne 2004, Caputo et al 2011, Royse 2011), and postoperative mortality is clearly reduced in studies with large numbers (Wijeysundera et al 2008). It seems important enough to save one life for about every 500 who had an epidural (Wijeysundera et al 2008), in addition to the improved quality of life during early rehabilitation after major surgery (Royse 2011).
Thoracic, Not Lumbar, Epidural Catheter
Postoperative myocardial infarction, respiratory and renal failure, stroke, and in some studies even mortality were reduced with a perioperative thoracic epidural (see Fig. 37-1), but not with lumbar epidural analgesia (Meissner et al 1997, Rodgers et al 2000, Beattie et al 2001, Waurick and Van Aken 2005). During a thoracic epidural infusion, the analgesic drugs reach the dorsal horn of the spinal cord; a lumbar epidural infusion causes mainly local anesthetic nerve block of the cauda equina, with only a small part reaching the spinal cord.
For the important contrasting effects of thoracic and lumbar epidural analgesia, see Boxes 37-7 and 37-8 (Meissner et al 1997, Waurick and Van Aken 2005). A lumbar epidural may even increase cardiac risk, and it does not improve pulmonary function or gastrointestinal motility. Lack of awareness of these important differences between thoracic and lumbar epidural analgesia is one reason for the confusion and conflicting opinions on the effects of epidural analgesia on outcome after surgery.
Risks to the Patient from Epidural Analgesia
Side Effects That Can Be Minimized by Adrenaline
With adrenaline added to the epidural drugs, doses of local anesthetic and opioid can be minimized; they are only slowly absorbed into the systemic circulation and produce no systemic side effects.
If adrenaline is not added, higher doses are required for adequate epidural analgesia with local anesthetics and opioids; they are rapidly absorbed into the systemic circulation and give rise to systemic effects and side effects (Niemi and Breivik 1998, 2002, 2003; Niemi 2004).
If an excessive dose (in comparison to what is needed when adrenaline is co-administered) of a local anesthetic is administered epidurally, orthostatic hypotension, motor blockade (with low thoracolumbar and lumbar catheters), and urinary retention (with lumbar catheters) may develop.
If an excessive dose of an opioid is administered epidurally, the following may occur:
Serious Complications
Bleeding and infection in the epidural space are rare but potentially catastrophic complications (Moen et al 2004). Leg weakness and new back pain are early warning symptoms of hematoma or abscess formation in the spinal canal. It is essential to have a robust monitoring regimen to be able to detect these early symptoms and act swiftly to treat and prevent permanent damage (see Box 37-6). Excessive doses of local anesthetics in the lumbar or low thoracolumbar epidural space will cause motor blockade and thereby conceal early signs of spinal cord compression and ischemia (Breivik 1998, Breivik et al 2010).
Perioperative Epidural Analgesia
The epidural catheter should be placed in an appropriate segmental level before surgery and tested for bilateral effects before induction of general anesthesia so that the intense nociceptive impulse inflow to the spinal cord and efferent sympathetic outflow to the myocardium can already be dampened during surgery. In this way, only light general anesthesia is needed for surgery and the patient can be completely awake immediately after the end of surgery without any hangover and nausea from lingering general anesthetic drugs. The patient can then immediately benefit from the postoperative epidural analgesic infusion.
Starting epidural analgesia only after the patient wakes up from a deep general anesthetic is one common reason for the difficulty in obtaining a good epidural analgesic state and stable cardiorespiratory function after thoracic and upper abdominal surgery.
On the postoperative ward and on the surgical wards, nurses, with the assistance of a pain nurse from the acute pain team when appropriate, adjust the infusion rate of the epidural analgesic mixture. Patients, when awake and cooperative and have stable cardiorespiratory functions, are allowed to use the dose administration button (Figs. 37-4 and 37-5) of the patient-controlled infusion pump to give themselves boluses when they feel that stronger analgesia is needed. The segmental distribution of the epidural analgesia is determined by the position of the epidural catheter and by the infusion rate. A bolus injection increases the quality of analgesia (Niemi 2004).
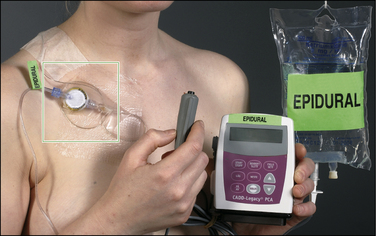
Figure 37-4 Illustration of the hygienic, low–infection risk, closed system for continuous epidural infusion with the possibility of a patient-controlled bolus epidural injection (see also Fig. 37-5 for details).
The bag contains 550 mL of a three-component epidural analgesic solution (bupivacaine, fentanyl, and adrenaline), and the electronic, tamper-proof pump delivers the solution from the bag via a microfilter to the epidural catheter. The patient may obtain a rescue dose by pushing the dose button. (From Breivik H, Curatolo M, Niemi G, et al 2007 How to implement an acute postoperative pain service: an update. In: Breivik H, Shipley M (eds) Pain best practice and research compendium. Elsevier, London, p xiv, Plate III.)
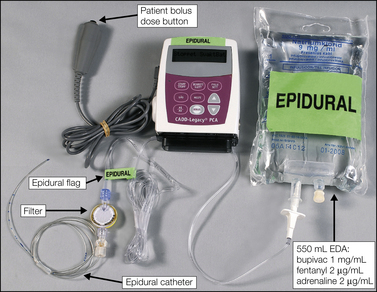
Figure 37-5 Details of the hygienic, low–infection risk, closed system for continuous epidural infusion with the possibility of patient-controlled epidural analgesia.
The bag contains 550 mL of a three-component epidural analgesic solution (EDA = bupivacaine, 1 mg/mL; fentanyl, 2 μg/mL; adrenaline 2 μg/mL), and the electronic, tamper-proof pump delivers the solution via a microfilter to the epidural catheter. (From Breivik H, Curatolo M, Niemi G, et al 2007 How to implement an acute postoperative pain service: an update. In: Breivik H, Shipley M (eds) Pain best practice and research compendium. Elsevier, London, p xiii, Plate II.)
Epidural Analgesia for Labor Pain
When the uterine cervix is dilated during the late stage I of vaginal delivery, sensory nerve impulses pass from the uterus to the sympathetic ganglia and enter the spinal cord dorsal horn at T10–L1 (Breivik 2008b). Infusion of a low-concentration triple-component epidural analgesic mixture (Box 37-9; also see Box 37-6) through an epidural catheter placed in the low thoracic or thoracolumbar region will relieve pain effectively during this most painful and protracted part of vaginal delivery. There will be no or minimal motor blockade and vascular effects, the parturient can move around and use her abdominal muscles during delivery, blood pressure will be stable, and nausea and pruritus are only minor problems. Importantly, the baby is not affected by fentanyl because the adrenaline reduces systemic absorption of fentanyl from the epidural space (Niemi and Breivik 1998). This epidural labor analgesia can be reinforced or be initiated with a minimal dose of bupivacaine and fentanyl (or sufentanil) given intrathecally if the pain is already severe—a combined epidural-spinal technique.
Again, an important aspect of “optimal” epidural analgesia is placement of the epidural catheter in the correct segmental level to obtain the most concentrated spinal cord analgesia there (see Fig. 37-1). Lumbar placement of an epidural catheter for labor analgesia causes leg weakness, urinary retention, and need for higher doses, which will cause more side effects (Breivik 2008b). The use of neuraxial analgesia for childbirth is discussed further in Chapter 55.
Optimizing the Efficacy and Safety of Epidural Analgesia
The efficacy and safety of epidural analgesia are optimized by exploiting the principle of synergy in combining two or more drugs with different mechanisms of analgesia and different side effect profiles (Breivik 2000). For 2 decades (Breivik 1993, Breivik et al 2007) we have managed around 50,000 patients who underwent major surgery or labor analgesia with a combination of low concentrations of the following:
These three drugs cause spinal cord analgesia by at least three separate mechanisms acting on the pain-processing mechanisms in the dorsal horn of the spinal cord. Fentanyl and adrenaline—like clonidine—act on pre- and post-synaptic opioid receptors and α2 receptors, respectively (Collins et al 1984). They inhibit transmission of pain impulses from the primary afferent nociceptive neurons to the transmission neurons in the dorsal horn of the spinal cord. Subanesthetic doses of bupivacaine and other local anesthetic drugs inhibit excitatory synaptic mechanisms in the same area of the spinal cord. Exploiting their synergistic antinociceptive effects by concurrent administration of these three pain-inhibiting drugs allows a reduction in the dose of each drug (Breivik et al 1995; Niemi and Breivik 1998, 2001, 2002, 2003).
The three drugs have different dose-related side effects, and therefore the overall risk for adverse effects is reduced when the dose of each drug is decreased. This is true for respiratory depression, nausea, itching, decreased gastrointestinal motility, sedation, hypotension, urinary retention, motor blockade, and leg weakness (Niemi 2004).
The upper and lower border of analgesia is determined by where the epidural catheter is placed (see Fig. 37-1) and by the infusion rate. The quality of analgesia can be improved by a bolus injection, when needed (Niemi 2004).
Adrenaline Markedly Increases the Effectiveness of the Epidural Analgesic Infusion
In randomized, double-blind, crossover studies we documented the powerful effects of adrenaline in an epidural mixture with bupivacaine (or ropivacaine) and fentanyl. Pain intensity with this triple mixture was practically zero during rest and only mild during coughing after major abdominal or thoracic surgery. When adrenaline was removed from the mixture, pain increased despite more patient-administered epidural bolus doses and intravenous morphine rescue. When our standard mixture with adrenaline was reintroduced, pain relief again became optimal; see Figures 37-2 and 37-3 (Niemi and Breivik 1998, 2002).
In a dose-finding study, we documented that adrenaline (epinephrine), 0.5 and 1.0 μg/mL, had less effect on the efficacy of the analgesic mixture than did 1.5 μg/mL, which had almost the same effect as 2.0 μg/mL (Niemi and Breivik 2003). Adrenaline is rapidly oxidized to inactive adrenochrome unless the mixture contains an antioxidant (Kjønniksen et al 2000); with disodium edentate adrenaline in this triple solution is stable for several months without the addition of bisulphite (Brustugun J et al personal communication).
These beneficial effects of adrenaline are true only for thoracic epidurals because with lumbar epidural infusions the adrenaline will reinforce the local anesthetic motor nerve blocking effects and cause weak legs and urinary retention.
Adrenaline Increases the Safety of Bupivacaine and Fentanyl Epidural Analgesia
Adrenaline is important for the safety of prolonged epidural infusion of the analgesic mixture because absorption of fentanyl and bupivacaine into the systemic circulation is reduced. With adrenaline, 2 μg/mL, fentanyl was almost undetectable in serum; when adrenaline was removed from the epidural infusion, the serum fentanyl concentration increased and patients experienced adverse effects from systemic absorption—sedation, nausea, and itching (Niemi and Breivik 1998). Adrenaline, unlike the more specific α2 receptor agonist clonidine, does not cause sedation or hypotension (Paech et al 1997). Adrenaline, unlike clonidine, causes epidural vasoconstriction and less systemic absorption of concurrently administered drugs.
A human error in programming the epidural infusion pump (80 mL/hr instead of 8 mL/hr) illustrated the important safety benefit of having adrenaline in the epidural mixture: when the error was discovered after about 3 hours, the patient had profound analgesia and weak legs, but no spinal or systemic adverse effects occurred (Breivik et al 1995).
Adrenaline increases the stickiness of platelets and may reduce the risk for bleeding in the epidural space (Breivik et al 2010).
Epidural Adrenaline, 2 μg/mL at 5–15 mL/hr, Does Not Decrease Spinal Cord Blood Flow
The well-known vasoconstrictive action of adrenaline on vessels outside the CNS has led to unfounded concern about the blood supply to the spinal cord when administering adrenaline-containing epidural infusions. Vessels in the CNS are not constricted by adrenaline. There are no human data documenting spinal cord ischemia even with very large doses of adrenaline, up to 1000-μg boluses directly into cerebrospinal fluid (CSF) (Bromage 1997). Up to 200-μg boluses of adrenaline directly into the subarachnoid CSF did not decrease spinal cord blood flow in cats or dogs (Porter et al 1985).
Adrenaline administered epidurally reduces epidural blood flow markedly (Kozody et al 1984). This causes positive pharmacokinetic interactions with epidural fentanyl and local anesthetic drugs (see Box 37-9). Epidural infusion of adrenaline, 2 μg/mL at 5–15 mL/hr, results in a very low concentration of adrenaline in CSF in comparison to what has commonly been used for spinal anesthesia, usually 200 μg adrenaline with tetracaine or lidocaine into CSF.
We have had extensive clinical experience with adrenaline in our standard triple-component epidural analgesic mixture for more than 2 decades. We are therefore convinced that the minute dose of adrenaline that we use (about 20 μg/hr) not only is advantageous for the analgesic effect and overall safety of epidural analgesia but also carries no risk for spinal cord ischemia.
Alternative Epidural Analgesic Mixtures
There are many alternative recipes for epidural analgesia. Ropivacaine is an alternative to bupivacaine; it is less cardiotoxic but more expensive (Niemi and Breivik 2002). When co-administering bupivacaine with fentanyl and adrenaline for epidural infusion analgesia, very low doses are required so that cardiotoxicity is not an issue.
Sufentanil is more potent than fentanyl; it is well documented and approved for epidural administration in several countries. With adrenaline and bupivacaine, sufentanil appears to function as well as fentanyl (Cohen et al 1993). Our hospital pharmacy is unable to obtain sufentanil (or ropivacaine) dry substance for our triple-component epidural mixture, so we stick to the cheapest and simplest: bupivacaine, fentanyl, and adrenaline, the latter requiring disodium edentate as an antioxidant (Kjønniksen et al 2000, Brustugun et al, personal communication).
Morphine or diamorphine with bupivacaine, with or without adrenaline, may be a bit less sensitive to optimal segmental location of the epidural catheter, but nausea and pruritus often reduce the global quality of analgesia (Wheatley et al 2001).
Conduct and Monitoring of Epidural Analgesia: The Importance of a Dedicated Acute Pain Team
The need for safe and effective practice of epidural analgesia on surgical wards was one of the main reasons that acute pain services were established in many hospitals some 20 years ago (Breivik 1993). Extensive clinical experience has confirmed that these services are essential and that they have made prolonged epidural analgesia more readily available to patients who really need this effective way of controlling the most severe dynamic pain after major surgery.
Intravenous opioids and other forms of patient-controlled analgesia became available because of the early efforts of acute pain teams. Basic pharmacological treatment of acute postoperative pain has been upgraded and optimized thanks to the ongoing educational programs, quality assurance, and research activities of acute pain teams in university hospitals (Breivik et al 2007, Counsell et al 2008).
Alternatives for Epidural Analgesia
When the patient has congenital or drug-induced hemostatic disturbances and high risk for intraspinal bleeding provoked by the epidural needle or catheter analgesia, alternative techniques for pain relief must be found (Breivik et al 2010). When there is an evidence base for reduced morbidity and even more so when an epidural may reduce postoperative mortality, it is possible to accept some increased risk for bleeding. However, when such is not the case, even a moderately increased risk for bleeding is a relatively strong contraindication to epidural (and subarachnoid spinal) analgesia.
Alternatives for Thoracic Epidural Anesthesia in a Patient with Increased Risk for Bleeding
There is no safe alternative nerve block technique:
Alternatives for Lumbar Epidural Analgesia in a Patient with Increased Risk for Bleeding
Long-Term Epidural Infusions for Chronic Pain
The use of external or internal (implanted) pumps may be indicated for cancer-related pain. Experience shows that it is technically difficult to maintain an epidural infusion for prolonged periods because of epidural fibrosis and adhesions.
However, the often prolonged epidural infusion is maintained as long as it functions well. In patients with advanced cancer and severe pain and nausea, an epidural infusion using the standard triple epidural infusion (described above) is performed. This is the most straightforward, inexpensive, and uncomplicated approach. When the epidural infusion fails, an intrathecal infusion directly into CSF can then be started (Raphael and Grady 2008).
Intrathecal Infusion
Indications for continuous intrathecal administration of an opioid and a local anesthetic (with or without clonidine or adrenaline) are severe cancer pain resistant to traditional treatment, including high doses of oral or parenteral opioids, or when intolerable side effects prevent dose escalation (Raphael and Grady 2008) (Box 37-10).
Refractory non-malignant pain may also be an indication for intrathecal treatment in pain management centers where this technique is well established and only when more traditional treatments have not achieved sufficient pain relief or have resulted in intolerable side effects. Ziconotide, an antagonist at N-type voltage-sensitive calcium channels of presynaptic terminals in the dorsal horn of the spinal cord, appears to have a specific effect in chronic pain conditions. It is difficult to titrate but, in selected cases, clearly produces good analgesia without respiratory depression (Raphael and Grady 2008).
Epidural Steroids for Spinal Radicular Pain
Injection of local anesthetics into the caudal epidural space for sciatica had been used for some 50 years before hydrocortisone was added in the early 1950s. This was attempted because the spinal nerve roots appeared inflamed and swollen during operations for prolapsed intervertebral discs. Early randomized controlled trials (RCTs) confirmed that epidural steroids did relieve irradiating sciatic pain better than saline or local anesthetic alone did (Dilke et al 1973, Breivik et al 1976).
In one RCT, most patients had radiologically verified radiculitis (arachnoiditis) or a herniated disc and were randomized to receive bupivacaine epidurally (caudal injection) with depot methylprednisolone (80 mg) added or saline (up to 100 mL) administered after the bupivacaine injection, up to three times at weekly intervals. The patients and the neurologist observer were blinded. Beneficial effects on pain and function were documented in two-thirds of the patients who had bupivacaine and steroid injections as opposed to one-third of those receiving bupivacaine and saline injections. Relief was limited to the irradiating, radicular pain and did not include any low back pain (Breivik et al 1976).
In a subsequent study on patients with sciatic pain of 3 months’ duration from a radiologically verified herniated lumbar disc, we tested the local epidural effect versus the systemic effect from the intramuscular injection of depot methylprednisolone. These patients had a lumbar epidural injection of bupivacaine plus methylprednisolone (80 mg) and a placebo (saline) intramuscular injection, or they were randomized to receive lumbar epidural bupivacaine alone and the depot methylprednisolone given intramuscularly. The patients and the neurologist observer were blinded. Epidural application of steroids produced a significant relieving effect on the irradiating pain with improved function in two-thirds of the patients as opposed to only one-third of those who received depot steroid intramuscularly (Hesla and Breivik 1979).
A number of studies and systematic reviews with conflicting conclusions have since been published. It appears that there are at least three causes of this confusion:
“Low back pain” comprises many different musculoskeletal causes of back pain. For these, it is not reasonable to expect effects from steroids injected into the epidural space.
Lack of documentation of correct placement of the injection is significant because our study from 1979 (Hesla and Breivik 1979) indicated that it is important to have the steroid placed locally near the affected nerve root or roots (Box 37-11). Systemically administered steroids must be given in higher doses to have effects similar to those of locally applied steroids (Green 1975). The addition of a local anesthetic will provide reasonable verification that the affected segmental nerve root pathology is reached when the patient experiences relief of pain as long as the local anesthetic acts. X-ray documentation cannot prove that the steroid solution reached the structures causing the pain.
One early negative study, otherwise well designed and receiving high scores for quality in subsequent systematic reviews, clearly had no documentation of correct placement of the epidural injection (Snoek et al 1977). Additionally, a volume of only 2 mL was used, which is not enough to reach the anterior portions of the epidural space where the nerve root pathology is most often located. Such flaws are not always appreciated in meta-analyses.
Some more recent RCTs support the experience that radicular pain can be relieved by epidural steroid injections into the cervical as well as the lumbar segments—but there are also studies that found only transient effects (Samanta and Samanta 2004). The duration of pain relief varies after epidural steroid injections. It is reasonable to expect a longer effect in patients without recurrent mechanical irritation of the nerve root. If the anti-swelling and anti-inflammatory effects of the steroids on the nerve root and surrounding tissues create sufficient space and mobility of the nerve root, the pain may be gone for this episode. However, when the disc reherniates or there is bony impingement on the nerve root in the intervertebral foramen, pain relief from epidural steroids is likely to be transient.
Neither low back pain nor spinal stenosis is an indication for epidural steroids (see Box 37-11). Additionally, epidural “fibrosis” has not been documented as being relieved by epidural steroids. Epiduroscopy with mechanical intervention for fibrotic changes and direct application of local anesthetic and steroid onto inflamed areas in the epidural space appear to have some merit (Igarashi et al 2004, Richardson 2004).
Epidural steroids have less effect in patients after “failed back surgery” or for pain caused by injury at work with an ongoing compensation or litigation process. The success rate is higher in those with radicular pain of less than 3 months’ duration than in those who have a much longer history of sciatic pain.
Transforaminal, periradicular injection of steroid (Box 37-12) directly onto the ventral aspect of the lumbar nerve root sleeve and the dorsal aspect of the disc herniation is performed through the intervertebral foramen or via a posterior trans-midline approach from the opposite side. Theoretically, this approach should be more effective than the traditional epidural application of the steroid, but again, results vary from only short-term relief (Karppinen et al 2001) to highly significant and long-lasting relief (Vad et al 2002). There have been serious complications from the spinal cord—possibly from injection into or damaging of a spinal cord radicular artery when performing transforaminal steroid injections (Glaser and Falco 2005).
The references for this chapter can be found at www.expertconsult.com.
References
Arnér S. Intravenous phentolamine test: diagnostic and prognostic use in reflex sympathetic dystrophy. Pain. 1991;46:17–22.
Arnér S., Lindblom U., Meyerson B.A., et al. Prolonged relief of neuralgia after regional anesthetic blocks. A call for further experimental and systematic clinical studies. Pain. 1990;43:287–297.
Ballantyne J.C. Does epidural analgesia improve surgical outcome? British Journal of Anaesthesia. 2004;92:4–6.
Ballantyne J.C., Carr D.B., DeFerranti S., et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesthesia and Analgesia. 1998;89:598–612.
Basse L., Raskov H.H., Jakobsen D., et al. Accelerated postoperative recovery program after colonic resection improves physical performance, pulmonary function and body composition. British Journal of Surgery. 2002;89:446–453.
Beattie W., Badaner N., Choi P. Epidural analgesia reduced postoperative myocardial infarction: a meta-analysis. Anesthesia and Analgesia. 2001;93:853–858.
Breivik H. Recommendations for foundation of a hospital-wide postoperative pain service—a European view. Pain Digest. 1993;3:27–30.
Breivik H. Chronic pain and the sympathetic nervous system. Acta Anaesthesiologica Scandinavica. 1994;41:131–134.
Breivik H. Neurological complications in association with spinal and epidural analgesia—again. Acta Anaesthesiologica Scandinavica. 1998;42:609–613.
Breivik H. High-tech versus low-tech approaches to postoperative pain management. Progress in Pain Research and Management. 2000;16:787–807.
Breivik H. Sympathetic blocks. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:322–336.
Breivik H. Epidural analgesia for acute pain after surgery and during labor, including patient-controlled epidural analgesia. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:311–321.
Breivik H., Bang U., Jalonen J., et al. Nordic guidelines for neuraxial blocks in disturbed haemostasis from the Scandinavian Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiologica Scandinavica. 2010;54:16–41.
Breivik H., Breivik E.K., Stubhaug A. Clinical aspects of pre-emptive analgesia: prevention of post-operative pain by pretreatment and continued optimal treatment. Pain Reviews. 1996;3:63–78.
Breivik H., Cousins M.J. Sympathetic neural blockade of upper and lower extremity. In: Cousins M.J., Carr D.B., Horlocker T.T., et al, eds. Cousins & Bridenbaugh`s neural blockade in clinical anesthesia and pain medicine. 4th ed. Philadelphia: Wolters-Kluwer/Lippincott, Williams & Wilkins; 2009:848–885.
Breivik H., Curatolo M., Niemi G., et al. How to implement an acute postoperative pain service: an update. In: Breivik H., Shipley M., eds. Pain best practice and research compendium. London: Elsevier; 2007:255–270.
Breivik H., Hesla P.E., Molnar I., et al. Treatment of chronic low back pain and sciatica: comparison of caudal epidural injections of bupivacaine followed by saline. In: Bonica J.J., Albe-Fessard D., eds. Advances in pain research and therapy. New York: Raven Press; 1976:927–932.
Breivik H., Niemi G., Haugtomt H., et al. Optimal epidural analgesia: importance of drug combinations and correct segmental site of injection. Best Practice & Research. Clinical Anaesthesiology. 1995;9:493–512.
Bromage P.R. Neurological complications of subarachnoid and epidural anaesthesia. Acta Anaesthesiologica Scandinavica. 1997;41:439–444.
Campbell W.I. Neurolytic blocks. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:337–348.
Caputo M., Alwair H., Rogers C.A., et al. Thoracic epidural anesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2011;114:380–390.
Chelly J.E., Williams B.A. Continuous perineural infusion at home: narrowing the focus. Regional Anesthesia and Pain Medicine. 2004;29:1–3.
Cohen S., Armar D., Pantuck C.B., et al. Postcesarean delivery epidural patient-controlled analgesia. Fentanyl or sufentanil? Anesthesiology. 1993;78:486–491.
Collins J.G., Kitahata L.M., Suzukawa M. Spinally administered epinephrine suppresses noxiously evoked activity of WDR neurons in the dorsal horn of the spinal cord. Anesthesiology. 1984;60:269–275.
Cooper R. Facet (zygapophyseal) joint injections and medial branch block. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:361–369.
Counsell D., Macintyre P.E., Breivik H. Organization and role of acute pain services. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:579–603.
Cousins M.J., Carr D.B., Horlocker T.T., et al, eds. Cousins & Bridenbaugh’s neural blockade in clinical anesthesia and pain medicine, 4th ed, Philadelphia: Wolters-Kluwer/Lippincott, Williams & Wilkins, 2009.
Crul B.J.P., van Zundert J.H.M., van Kleef M. Radiofrequency lesioning and treatment of chronic pain. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:389–403.
Curatolo M., Bogduk N. Diagnostic blocks for chronic pain. Scandinavian Journal of Pain. 2010;1:186–192.
Devor M., Govrin-Lippman R., Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22:127–137.
Dilke T.F.W., Burry H.C., Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. British Medical Journal. 1973;2:635–637.
Garnock-Jones K.P., Keating G.M. Lidocaine 5% medicated plaster: a review of its use in postherpetic neuralgia. Drugs. 2009;69:2149–2165.
Glaser S.E., Falco F. Paraplegia following thoracolumbar transforaminal epidural steroid injection. Pain Physician. 2005;8:1533.
Green L.N. Dexamethasone in the management of symptoms due to herniated lumbar disc. Journal of Neurosurgery and Psychiatry. 1975;38:1211–1217.
Hannington-Kiff J.G. Pain relief. London: Heinemann; 1974. p 68
Hans G., Joukes E., Verhulst J., et al. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Current Medical Research and Opinion. 2009;25:2737–2743.
Hesla P.E., Breivik H. Epidural analgesia and epidural steroid injections for treatment of chronic low back pain and sciatica [in Norwegian]. Tidsskrif for den Norske Laegeforening. 1979;99:936–939.
Hill D. Peripheral nerve blocks: practical aspects. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:255–292.
Igarashi T., Hirabayashi Y., Seo N., et al. Lysis of adhesions and epidural injection of steroid/local anaesthetic during epiduroscopy potentially alleviate low back and leg pain in elderly patients with lumbar spinal stenosis. British Journal of Anaesthesia. 2004;93:181–187.
Joyce P.I., Rizzi D., Caló G., et al. The effect of guanethidine and local anesthetics on the electrically stimulated mouse vas deferens. Anesthesia and Analgesia. 2002;95:1339–1343.
Karppinen J., Malmivaara A., Mauno K., et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine. 2001;26:1059–1067.
Kehlet H. A multi-modal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia. 1997;78:606–617.
Kjønniksen I., Brustugun J., Niemi G., et al. Stability of an epidural analgesic solution containing adrenaline, bupivacaine and fentanyl. Acta Anaesthesiologica Scandinavica. 2000;44:864–867.
Kozody R., Palahniuk R.J., Wade J.G., et al. The effect of subarachnoid epinephrine and phenylephrine on spinal cord blood flow. Canadian Anaesthesia Society Journal. 1984;31:503–508.
Kvarstein G. Nerve block—a reliable diagnostic tool? Scandinavian Journal of Pain. 2010;1:184–185.
Kvarstein G., Högström H. Cryoanalgesia. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:378–388.
Meissner A., Norbert Rolf N., Van Aken H. Thoracic epidural anesthesia and the patient with heart disease: benefits, risks, and controversies. Anesthesia and Analgesia. 1997;85:517–528.
Moen V., Dahlgren N., Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–99. Anesthesiology. 2004;101:950–959.
Niemi G. Optimizing postoperative epidural analgesia. Thesis for the Degree of Doctor of Medical Sciences. University of Oslo: Faculty of Medicine; 2004.
Niemi G., Breivik H. Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery. Acta Anaesthesiologica Scandinavica. 1998;42:897–909.
Niemi G., Breivik H. Epidural fentanyl markedly improves thoracic epidural analgesia in a low-dose infusion of bupivacaine, adrenaline and fentanyl. A randomized, double-blind crossover study with and without fentanyl. Acta Anaesthesiologica Scandinavica. 2001;45:221–232.
Niemi G., Breivik H. Epinephrine markedly improves thoracic epidural analgesia produced by a small-dose infusion of ropivacaine, fentanyl and epinephrine after major thoracic or abdominal surgery: a randomized, double blind cross-over study with and without epinephrine. Anesthesia and Analgesia. 2002;94:1598–1605.
Niemi G., Breivik H. Minimally effective concentration of epinephrine in a low-concentration thoracic epidural analgesic infusion of bupivacaine, fentanyl, and epinephrine after major surgery. Acta Anaesthesiologica Scandinavica. 2003;47:1–12.
Norum H.M., Breivik H. A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy. Scandinavian Journal of Pain. 2010;1:12–23.
Paech M.J., Pavy T.J., Orlaikowski C.E., et al. Postoperative epidural infusion: a randomized, double-blind, dose-finding trial of clonidine in combination with bupivacaine and fentanyl. Anesthesia and Analgesia. 1997;84:1323–1328.
Petersen P.L., Mathiesen O., Torup H., et al. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiologica Scandinavica. 2010;54:529–535.
Porter S.S., Albin M.S., Watson W.A., et al. Spinal cord and cerebral blood flow responses to subarachnoid injection of local anesthetics with and without epinephrine. Acta Anaesthesiologica Scandinavica. 1985;29:330–338.
Rafael J., Grady K. Intrathecal drug delivery. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:370–378.
Raja S., Grabow T.S. Complex regional pain syndrome I (reflex sympathetic dystrophy). Anesthesiology. 2002;96:1254–1260.
Renck H. Wound infiltration with local anaesthetics. Acta Anaesthesiologica Scandinavica. 1994;38:2–6.
Richardson J. A (pain free) step in the right direction. British Journal of Anaesthesia. 2004;93:173–174.
Rigg J.R.A., Jamrozik K., Myles P.S., et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359:1276–1282.
Rodgers A., Walker W.S., McKee A., et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomized trials. British Medical Journal. 2000;321:1493–1497.
Romundstad L., Breivik H., Niemi G., et al. Methylprednisolone intravenously one day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiologica Scandinavica. 2004;48:1223–1231.
Royse C. Epidurals for cardiac surgery. Can we substantially reduce surgical morbidity or should we focus on quality of recovery? Anesthesiology. 2011;114:232–233.
Samanta A., Samanta J. Is epidural injection of steroids effective for low back pain? British Medical Journal. 2004;328:1509–1510.
Shipley M., Morris V. Intra-articular and soft-tissue injections. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:349–360.
Snoek W., Weber H., Jorgenson B. Double-blind evaluation of extradural methyl prednisolone for herniated lumbar discs. Acta Orthopaedica Scandinavica. 1977;48:635–641.
Takeda K., Sawanmura S., Sekiyama H., et al. Effect of methylprednisolone on neuropathic pain and spinal glial activation in rats. Anesthesiology. 2004;100:1249–1257.
Tenicela R., Lovasik D., Eaglestein W. Treatment of herpes zoster with sympathetic blocks. Clinical Journal of Pain. 1985;1:63–67.
Turk D.C. Cognitive–behavioral approach to the treatment of chronic pain patients. Regional Anesthesia and Pain Medicine. 2003;28:573–579.
Vad V., Bhat A.L., Lutz G.E., et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002;27:11–15.
Warncke T. Neurophysiological studies of peripheral and central mechanisms of primary and secondary hyperalgesia. Thesis for the degree of Doctor of Medical Sciences. University of Oslo: Faculty of Medicine; 2001. pp 24–27
Waurick R., Van Aken H. Update in thoracic epidural anaesthesia. Best Practice and Research. Clinical Anaesthesiology. 2005;19:201–213.
Wheatley R.G., Schug S.A., Watson D. Safety and efficacy of postoperative epidural analgesia. British Journal of Anaesthesia. 2001;87:47–61.
Wijeysundera D.N., Beattie W.S., Austin P.C. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet. 372, 2008. 562–469
Ballantyne J.C. Does epidural analgesia improve surgical outcome? British Journal of Anaesthesia. 2004;92:4–6.
Breivik H. High-tech versus low-tech approaches to postoperative pain management. Progress in Pain Research and Management. 2000;16:787–807.
Breivik H. Epidural analgesia for acute pain after surgery and during labor, including patient-controlled epidural analgesia. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:311–321.
Breivik H., Bang U., Jalonen J., et al. Nordic guidelines for neuraxial blocks in disturbed haemostasis from the Scandinavian Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiologica Scandinavica. 2010;54:16–41.
Breivik H., Breivik E.K., Stubhaug A. Clinical aspects of pre-emptive analgesia: prevention of post-operative pain by pretreatment and continued optimal treatment. Pain Reviews. 1996;3:63–78.
Breivik H., Cousins M.J. Sympathetic neural blockade of upper and lower extremity. In: Cousins M.J., Carr D.B., Horlocker T.T., et al, eds. Cousins & Bridenbaugh’s neural blockade in clinical anesthesia and pain medicine. ed 4. Philadelphia: Wolters-Kluwer/Lippincott, Williams & Wilkins; 2009:848–885.
Breivik H., Curatolo M., Niemi G., et al. How to implement an acute postoperative pain service: an update. In: Breivik H., Shipley M., eds. Pain best practice and research compendium. London: Elsevier; 2007:255–270.
Collins J.G., Kitahata L.M., Suzukawa M. Spinally administered epinephrine suppresses noxiously evoked activity of WDR neurons in the dorsal horn of the spinal cord. Anesthesiology. 1984;60:269–275.
Counsell D., Macintyre P.E., Breivik H. Organization and role of acute pain services. In: Breivik H., Campbell W.I., Nicholas M.K., eds. Clinical pain management: practice and procedures. London: Hodder-Arnold; 2008:579–603.
Curatolo M., Bogduk N. Diagnostic blocks for chronic pain. Scandinavian Journal of Pain. 2010;1:186–192.
Hans G., Joukes E., Verhulst J., et al. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Current Medical Research and Opinion. 2009;25:2737–2743.
Joyce P.I., Rizzi D., Caló G., et al. The effect of guanethidine and local anesthetics on the electrically stimulated mouse vas deferens. Anesthesia and Analgesia. 2002;95:1339–1343.
Kehlet H. A multi-modal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia. 1997;78:606–617.
Kjønniksen I., Brustugun J., Niemi G., et al. Stability of an epidural analgesic solution containing adrenaline, bupivacaine and fentanyl. Acta Anaesthesiologica Scandinavica. 2000;44:864–867.
Kvarstein G. Nerve block—a reliable diagnostic tool? Scandinavian Journal of Pain. 2010;1:184–185.
Niemi G. Optimizing postoperative epidural analgesia. Thesis for the Degree of Doctor of Medical Sciences. University of Oslo: Faculty of Medicine; 2004.
Niemi G., Breivik H. Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery. Acta Anaesthesiologica Scandinavica. 1998;42:897–909.
Niemi G., Breivik H. Epidural fentanyl markedly improves thoracic epidural analgesia in a low-dose infusion of bupivacaine, adrenaline and fentanyl. A randomized, double-blind crossover study with and without fentanyl. Acta Anaesthesiologica Scandinavica. 2001;45:221–232.
Niemi G., Breivik H. Epinephrine markedly improves thoracic epidural analgesia produced by a small-dose infusion of ropivacaine, fentanyl and epinephrine after major thoracic or abdominal surgery: a randomized, double blind cross-over study with and without epinephrine. Anesthesia and Analgesia. 2002;94:1598–1605.
Norum H.M., Breivik H. A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy. Scandinavian Journal of Pain. 2010;1:12–23.
Petersen P.L., Mathiesen O., Torup H., et al. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiologica Scandinavica. 2010;54:529–535.
Romundstad L., Breivik H., Niemi G., et al. Methylprednisolone intravenously one day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiologica Scandinavica. 2004;48:1223–1231.
Royse C. Epidurals for cardiac surgery. Can we substantially reduce surgical morbidity or should we focus on quality of recovery? Anesthesiology. 2011;114:232–233.
Waurick R., Van Aken H. Update in thoracic epidural anaesthesia. Best Practice and Research. Clinical Anaesthesiology. 2005;19:201–213.
Wijeysundera D.N., Beattie W.S., Austin P.C. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet. 2008;372:562–569.