Obstetric Pain
Introduction
Unrelenting dedication and hard work by clinicians, scientists, and professional organizations in the past 2 centuries have significantly increased our knowledge and improved the safety of obstetric anesthesia to make childbirth a more pleasant and memorable experience. However, the advance in obstetric analgesia and anesthesia is not without controversy and has been influenced significantly by social values and popular demands, as well as by scientific evidence.
The first use of an anesthetic for delivery can be credited to James Young Simpson, who administered diethyl ether to anesthetize a woman with a deformed pelvis for delivery on January 19, 1847 (Simpson 1871, Caton 1999). In search of a safer anesthetic than the dangerously flammable ether, Simpson pioneered the technique of chloroform anesthesia, which John Snow later refined. At that time the majority of physicians supported the view of Charles D. Meigs, who argued that labor pain was inseparable from contraction and should not be abolished (Levinson 1928). In addition, the documented deaths of 123 non-obstetric patients that “could be positively assigned to the inhalation of chloroform” in a report from the Royal Medical and Chirurgical Society provided further support for non-intervention in labor and delivery (Committee Appointed by the Royal Medical and Chirurgical Society 1864). Even when John Snow administered anesthesia to Queen Victoria for delivery of Prince Leopold in 1953, strong public criticism against obstetric anesthesia prevailed, including Thomas Wakely, the founding editor of Lancet. It was not until after the birth of the Queen’s first grandchild in 1860 when she remarked, “What a blessing she [Victoria, her oldest daughter] had chloroform,” that obstetric anesthesia became a more acceptable medical practice (Sykes 1982).
By the early 1900s, obstetric anesthesia had evolved to the use of morphine in combination with scopolamine, which was first introduced as Dammerschlaff or “twilight sleep” for labor analgesia by the Austrian physician Richard von Steinbuchel of Graz (1902) and later popularized by Gauss of Freiberg (1906). With the side effects of amnesia and disorientation and even a report of the death of a prominent advocate of this technique during childbirth, the initial resistance by many physicians to twilight sleep resembled earlier objections to etherization but differed in that a more critical scientific examination of drug effects on the fetus and the mother was conducted.
With the introduction of regional cocaine anesthesia for eye surgery by Carl Koller (1884) and the classic publication by Henry Head (1893) on the innervation of abdominal viscera, various publications on obstetric applications of regional techniques such as spinal, lumbar epidural, caudal, paravertebral, parasacral, and pudendal nerve blocks started to emerge between 1900 and 1930 (Kreis 1900, Schlimpert 1913, Bonar and Meeker 1923). However, inhalational and opioid forms of analgesia were still the preferred choices, probably because of limited availability of drugs and equipment for regional anesthesia and lack of knowledge on the advantages of regional over general anesthesia. Later, in 1933, John Cleland undertook a meticulous analysis of the nerve pathways mediating labor pain. A publication from Hingson and Edwards in 1941 described continuous caudal anesthesia with a malleable needle secured in place. Subsequently, with small flexible plastic catheters replacing malleable needles, continuous epidural anesthesia was made possible and resulted in continuous lumbar epidural analgesia and anesthesia, which remains the main effective technique for obstetric analgesia and anesthesia today.
Coincidentally, as regional analgesia and anesthesia started emerging, “natural childbirth,” originated by Dick-Read (1970), was gaining popularity. Dick-Read attributed labor pain purely to fear and suggested that sympathetic nervous system activity results in uterine ischemia and therefore pain. Contemporary childbirth preparation does not erroneously attribute pain purely to fear but rather helps patients manage pain through education (Lamaze and Vellay 1952, Atlee 1956, Lamaze 1956). The goals of natural childbirth and obstetric anesthesia have converged many aspects: to have an awake, participating mother during delivery without maternal, fetal, or neonatal side effects or a negative impact on the course of delivery. Different professional organizations from various countries have been formed to promote and accelerate the understanding and advancement of safe obstetric anesthesia not only for research but also to provide better clinical guidelines and education. The Society of Obstetric Anesthesia and Perinatology and the Obstetric Anaesthetists’ Association are two examples with worldwide stature that continue to flourish and grow. The availability of in-house obstetric anesthesiologists has also gained much appreciation from colleagues not just for pain relief but also for the multidisciplinary approach to overall improvement in patient care from resuscitation to management of hemorrhage and other maternal and fetal co-morbid conditions. Strong clinical evidence supports the notion that properly administered analgesia/anesthesia for labor and delivery has a very low overall risk and improves maternal and perinatal mortality and morbidity (Ruppen et al 2006, Davies et al 2009, Hawkins et al 2011). More than a century ago, Simpson (1871), after administering his first obstetric anesthetic, profoundly and insightfully stated, “It will be necessary to ascertain anesthesia’s precise effect, both upon the action of the uterus and on the assistant abdominal muscles; its influence, if any, upon the child; whether it has a tendency to hemorrhage or other complications.” With this, he identified the essential issues and provided a road map to guide many in the quest for better obstetric care. The surge of interest in obstetric pain relief in the past 50 years is attested to by many publications on the subject. Much of the research has been focused only on refinement of techniques or drugs to provide better analgesia with improvement in neonatal and maternal safety. Despite the many advances in understanding the neurobiology of pain, relatively little has emerged specifically regarding labor pain. Laboring patients mostly receive a local anesthetic with an opioid adjuvant aimed at blocking the spinal transmission of pain-related signals. Various clinical reports have suggested a relatively high incidence of inadequate or failed labor epidural analgesia that has resulted in patient dissatisfaction (Eappen et 1998, Pan et al 2004). Many consider intrapartum pain relief an unmet medical need. Better understanding of the biological basis of labor pain will allow the development of target-directed therapy that may provide alternatives to achieve a better quality of intrapartum pain relief. In addition, recent research involving better modeling of labor pain, obstetric pharmacogenetics, prediction of the severity of acute and chronic obstetric pain, and its associated long-term public health concerns is likely to result in further improvement in the relief of labor pain.
Measurement and Severity of Obstetric Pain
Many early descriptions of labor pain were inaccurate and confusing but influenced perceptions of the magnitude of pain endured by laboring parturients. Although most modern child-birth preparations, such as Lamaze’s psychoprophylaxis, acknowledge the existence of pain during labor, some may still consider labor pain to be minor or unimportant (Lamaze and Vellay 1952, Lamaze 1956). The early work of Bonica from personal observation and interviews indicated that 65% of laboring parturients had moderate to severe pain (Scott-Palmer and Skevington 1981). Melzack subsequently confirmed Bonica’s findings through systematic use of the McGill Pain Questionnaire and showed that women experiencing their first childbirth without training rated their labor pain to be as painful as digit amputation. More than 65% of women of mixed parity rated labor pain as being severe or very severe (Melzack 1984) (Fig. 55-1). Nulliparous women tend to have a higher total mean pain rating than parous women do. Twenty-five percent of primiparas and 11% of multiparas rated labor pain as “horrible.” However, there is considerable interindividual variability in rating the intensity of labor pain. Javert and Hardy reported that some laboring women equated labor pain to the “ceiling pain” generated by a second-degree burn from a radiant heat source applied to the skin (Hardy and Javert 1949, Javert and Hardy 1951). Linear regression of the same data suggested that cervical dilation resulted in severe pain. Others have reported the intensity of labor pain to be associated with the magnitude of uterine contraction pressure (Algom and Lubel 1994), menstrual pain intensity, age, parity (Melzack et al 1981, Melzack 1984), maternal fatigue (Melzack 1984), and even a history of a previous pain experience before childbirth (Niven and Gijsbers 1989). It is possible that interindividual variation in labor pain may be partially due to genetic differences, including genetic polymorphisms regulating drug responses, cytokine production, functions, and responses (Reid et al 2001; also see Chapter 10). In evaluating and studying labor pain and treatment, most previous reports tended to compare labor pain by using one of two discrete pain scores. However, because labor itself and its associated pain are both dynamic processes that are affected by many factors and thus result in significant interindividual differences, better identification of the covariates affecting labor progress and pain is needed.
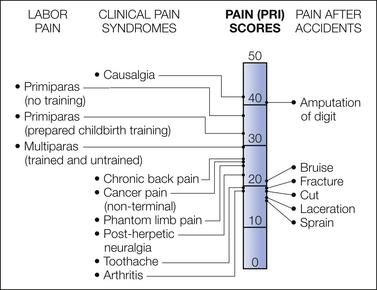
Figure 55-1 Comparison of pain scores with the McGill Pain Questionnaire obtained from women during labor and from patients in a general hospital pain clinic and an emergency department.
PRI, Pain Rating Index. (From Melzack R 1984 The myth of painless childbirth (the John J. Bonica Lecture). Pain 19:321–337, Fig. 2.)
Recently, Conell-Price and co-authors (2008) reported the development and validation of a dynamic model to account for labor progress in assessment of labor pain. Subsequently, Debiec and colleagues (2009), at the same institution, combined a bi-exponential model describing labor progress with the sigmoidal labor pain model to test the influence of patient covariates on labor pain. Both studies used retrospective patient data to develop and test their models. The prediction error for the pain score in the former study was large, but the purpose of the model was to remove the variability associated with labor progress so that other factors, such as genetic polymorphisms, can be quantitatively studied. In their study population, cervical dilation accounted for only 16–20% of the variability in reported pain (Conell-Price et al 2008). In the latter study, covariates such as ethnicity were found to have statistically significant but clinically trivial effects on labor progress (Debiec et al 2009). The technique suggested by these authors may provide a useful quantitative tool for future studies to identify and assess effect or lack of effect of patient or environmental covariates on labor progress, labor pain, and therapeutic responses. Better understanding of the underlying causes of interindividual variability in labor progress, pain, and response to therapy is likely to lead to better tailored therapy (Fisher and Eisenach 2009).
Meaning, Significance, and Impact of Obstetric Pain
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damages” by the International Association for the Study of Pain (Merskey 1979). Fear, anxiety, and apprehension stimulate the sympathetic nervous system and also affect pain perception and behavior during labor. Unplanned pregnancy results in higher reported labor pain scores (Reading and Cox 1985), whereas the presence of a partner in the labor room leads to lower pain scores (Melzack 1984). Other emotional factors, such as motivation, cultural influences, and cognitive intervention with preparatory classes, can affect modulation of sensory transmission and increase confidence in handling labor, which in turn is a strong predictor of a less painful labor experience (Lowe 1989). Even when the pain level is the same, the meaning of labor pain may be different from that of non-labor pain. Bajaj and co-workers (2002) compared pain descriptors in laboring women and those with dysmenorrhea or spontaneous abortion. Those with non-labor pain used words implying suffering such as punishing and wretched, whereas laboring women generally did not. Some have associated labor pain with a sense of euphoria, as in pain derived from mountain climbing (Lowe 2002) or a process making them more mature and resulting in a stronger personality (Lundgren and Dahlberg 1998), but others find no deeper meaning in labor pain and expect complete pain relief. Some parturients planning natural childbirth but ending up receiving analgesia when the pain becomes intolerable may experience guilt, anger, and failure (Melzack et al 1981). By contrast, unrelieved severe labor pain can also result in long-term emotional and even physical consequences that negatively affect the parturient’s emotion and bonding with her newborn, relationship with her partner, and postpartum depression (Melzack et al 1981, Melzack 1984).
Many women undergo delivery without negative sequelae, but some may experience significant persistent postpartum pain and even depression. Recent investigations of acute and chronic postpartum pain have shown a 7% incidence of perineal pain 8 weeks after vaginal delivery (Thompson et al 2002, Macarthur and Macarthur 2004), a 48% incidence of punctate hyperalgesia at 48 hours, and a 23% incidence of residual pain 6 months after cesarean delivery (Lavand’homme et al 2007). Eisenach and colleagues (2008), in a multicenter, prospective, longitudinal cohort study of 1288 parturients delivering by either cesarean or vaginal delivery, tested whether the mode of delivery had an independent role in persistent pain and depression at 8 weeks postpartum. The impact of mode of delivery on acute postpartum pain, persistent pain, depressive symptoms, and their interrelationships was assessed by regression analysis and propensity adjustment. They reported a 10.9% prevalence of severe acute pain within 36 hours postpartum and a 9.8 and 11.2% prevalence of persistent pain and depression at 8 weeks postpartum, respectively. The severity of acute postpartum pain, but not the mode of delivery, was independently related to the risk for persistent pain and depression, both of which also resulted in negative effects on activities of daily living and on sleep (Eisenach et al 2008). Those with severe acute postpartum pain had a 2.5- and 3.0-fold increased risk for persistent pain and depression, respectively, when compared with those with mild acute postpartum pain (Eisenach et al 2008). These findings suggest that this morbidity may not be related to the degree of trauma to physical tissues but rather to an individual’s pain response to that injury. Furthermore, long-term follow-up of the postpartum patients showed that incidence of chronic pain at 6 months and 1 year later was remarkably low at 0.3% and 0.1%, respectively, as compared to surgery with similar tissue injury. Whether pregnancy, the process of labor and bonding with the newborn have a protective effect against chronic pain, or whether the severity of pain during labor and delivery would show the same correlation to postpartum morbidity as acute postpartum pain does has not been well studied. Studies in animals suggest that acute intervention at the time of tissue injury reduces the likelihood of chronic pain developing (Hefferan et al 2003). It is likely that the severity of the acute pain is not just a marker of chronic pain but rather an active participatory component in the pathophysiology of transitioning from acute to chronic pain (Eisenach et al 2008). More careful attention to pain treatment and follow-up in the days following childbirth may potentially reduce long-term morbidity and improve overall outcomes. Further research is needed to define predictive factors to identify those at risk for severe acute and chronic postpartum pain.
Mechanism and Pathways of Pain of Childbirth
Pain experienced during the first stage of labor predominantly originates from afferents with peripheral terminals in the lower uterine segment and the cervix rather than the uterine body. In the absence of inflammation, uterine body afferents may be much less important in labor pain during uterine distention, as shown in laboratory animals (Bradshaw et al 1999), whereas manual cervical distention reproduces labor pain in humans (Javert and Hardy 1951). Furthermore, Bonica and Chadwick (1989) showed that patients undergoing cesarean section under a local anesthetic block did not experience pain from uterine distention but did from cervical distention, which resembled labor pain. Afferents innervating the lower uterine segment and endocervix have cell bodies in the thoracolumbar dorsal root ganglia and are different from those innervating the vaginal surface of the cervix and the vagina, which have cell bodies in the sacral dorsal root ganglia (Berkley et al 1993). Dilation of the endocervix in rats, resembling first-stage labor, leads to activation of afferents from the endocervix and lower uterine segment, thus suggesting significant roles for these afferents in first-stage labor pain (Papka et al 2002) (Fig. 55-2). However, afferents from the vaginal surface of the cervix and vagina are activated only during delivery, not during labor, and stimulation may result in antinociception or mating behavior in rats (Sandner-Kiesling et al 2002). From Cleland (1933), Berkley and colleagues (1993), and other investigators’ work we now know that first-stage labor pain is transmitted via visceral afferents with peripheral terminals in the lower uterine segment and cervix. They pass through the paracervical ganglion and the hypogastric nerve and plexus together with the lumbar sympathetic chain and enter the spinal cord in the T10–L1 region (Fig. 55-3). From the spinal cord, second-order cells send axons to supraspinal sites as discussed in Chapter 51. Visceral stimulation activates similar areas in the brain as somatic stimulation does (Strigo et al 2003). Interestingly, visceral stimulation is perceived to be more unpleasant than somatic stimulation of similar intensity. The diffuse localization of visceral pain in first-stage labor versus the precise location of somatic pain in second-stage labor helps clinicians determine the appropriate type of regional analgesia needed at these times (Pan and Eisenach 2010).
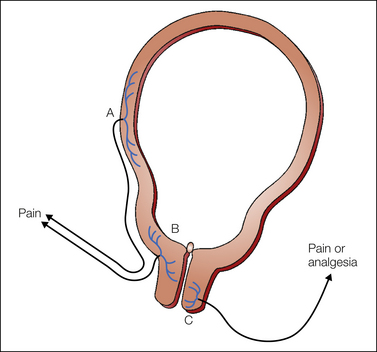
Figure 55-2 Uterocervical afferents activated during the first stage of labor.
Uterine body afferents (A) partially regress during pregnancy and may contribute to the pain of the first stage of labor. However, the major input is from afferents in the lower uterine segment and endocervix (B). By contrast, at least in animals, activation of afferents that innervate the vaginal surface of the cervix (C) results in analgesia, not pain, and they enter the spinal cord in sacral areas rather than at the site of referred pain in labor. (From Pan PH, Eisenach JC 2010 The pain of childbirth and its effects on mother and fetus. In: Chestnut DH, Polley LS, Tsen LC, et al (eds) Chestnut’s obstetric anesthesia principles and practice, 4th ed. Philadelphia, Mosby-Elsevier, pp 387–403, Fig. 20-4.)
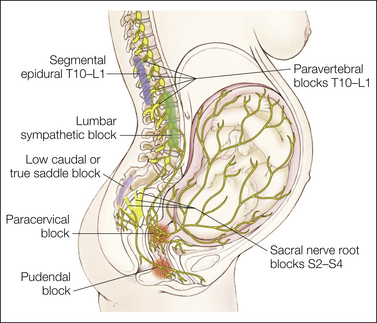
Figure 55-3 Transmission of labor pain.
Labor pain has a visceral component and a somatic component. Uterine contractions may result in myometrial ischemia, which causes the release of potassium, bradykinin, histamine, and serotonin. In addition, stretching and distention of the lower segments of the uterus and the cervix stimulate mechanoreceptors. These noxious impulses follow the sensory nerve fibers accompanying sympathetic nerve endings, travel through the paracervical region and the pelvic and hypogastric plexus, and enter the lumbar sympathetic chain. Through the white rami communicantes of the T10, T11, T12, and L1 spinal nerves, they enter the dorsal horn of the spinal cord. These pathways could be mapped successfully by demonstration that blockade at different levels along this path (sacral nerve root blocks of S2–4, pudendal block, paracervical block, low caudal or true saddle block, lumbar sympathetic block, segmental epidural blocks of T10–L1, and paravertebral blocks T10–L1) can alleviate the visceral component of labor pain. (From Eltzschig HK, Lieberman ES, Camann WR 2003 Regional anesthesia and analgesia for labor and delivery. New England Journal of Medicine 348:319–322, Fig. 1.)
Second Stage of Labor
Second-stage labor begins with completed cervical dilation, at which time the pain tends to be sharp and well localized to the vagina and perineum as a result of distention, ischemia, and tissue injury. Second-stage labor pain includes pain transmitted by afferents as in the first stage of labor but also with additional somatic afferents innervating the vaginal surface of the cervix, vagina, and perineum. These afferents travel via the pudendal nerves to dorsal root ganglia located at the S2–4 levels and terminate in the superficial laminae of the dorsal horn with limited rostrocaudal extension. Of note, studies in animal and non-pregnant women have demonstrated an antinociceptive or minor analgesic effect of stimulation of the vaginal surface of the cervix. Its role in reduction of second-stage labor pain is unclear but suggests possible activation of endogenous analgesia during labor in the presence of noxious stimuli. In the late first stage and during the second stage of labor, aching, burning, and cramping discomfort may develop in the thighs, legs, and back in some parturients. This is probably due to painful stimulation from stretching and tension in the pelvic cavity, bladder, urethra, and rectum and from pressure on roots of the lumbosacral plexus, as in the case of an abnormal occiput posterior position of the fetus. Understanding the anatomic basis of pain during the various stages of labor allows an appropriate choice of different regional anesthesia techniques to relieve the labor pain. The visceral pain in first-stage labor can be relieved with a bilateral paracervical plexus or lumbar sympathetic block and second-stage somatic pain with a bilateral pudendal nerve block, whereas an epidural or intrathecal block and their variations can provide analgesia during both stages of labor with appropriate extension of the block (see Fig. 55-3).
Neurobiological Basis of Labor Pain
Sensitization of peripheral afferents may play a significant role in labor pain and its relief. Peripheral inflammation is common with acute and chronic pain, but peripheral synthesis and release of the inflammatory products (such as prostaglandin E2 [PGE2], cytokines, and growth factors) associated with labor and cervical ripening may play an important role in labor pain besides its role in preterm labor. Animal studies have demonstrated that PGE2 induces peripheral sensitization by activation of protein kinase A (Aley and Levine 1999) and nitric oxide synthase (Aley et al 1998). These sensitizing substances can amplify the perception and severity of pain (Pan and Eisenach 2010). Furthermore, estrogen receptor signaling may modulate the response to pain by sensitizing mechanosensitive afferents, whereas long-term estrogen exposure increases the proportion of hypogastric afferents innervating the cervix that express transient receptor potential vanilloid 1 (TRPV1). TRPV1 receptor antagonists have been shown to reduce hypogastric afferent responses to cervical distention (Tong et al 2006, Yan et al 2007). Peripheral sensitization during labor may be responsible for the increase in labor pain associated with labor progression, as well as provide potential new targets specific for the relief of labor pain.
Role of Inhibitory Receptors
Endogenous inhibitory receptors modulating pain responses are expressed in the peripheral afferent terminals, spinal cord, and supraspinal central nervous system to provide analgesia. Opioid receptor agonists, in particular, mu (μ) and kappa (κ) receptors, have been more commonly studied and used for analgesia. μ-Opioid receptor agonists produce antinociception in response to uterine cervical distention (UCD) through actions in the supraspinal central nervous system and the spinal cord but not in the peripheral afferent terminals (Sandner-Kiesling and Eisenach 2002) (Fig. 55-4). With somatic stimulation, tonic estrogen exposure reduces the supraspinal but not the spinal (intrathecal) analgesic effect of μ-opioid receptor agonists (Cicero et al 2002). κ-Opioid receptor agonists, which are unaffected by tonic estrogen exposure and have greater analgesic efficacy in women than in men, produce their antinociceptive effect through actions on visceral peripheral afferents and the supraspinal central nervous system (Cicero et al 2002) (see Fig. 55-4).
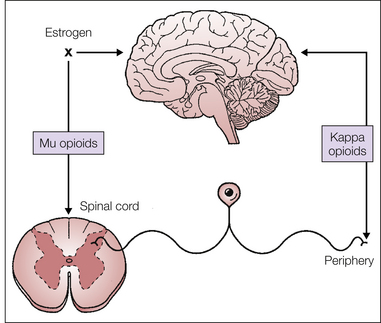
Figure 55-4 Supraspinal, spinal, and peripheral effects of opioid receptor agonists during the first stage of labor.
κ-Opioid receptor agonists act primarily at visceral afferent terminals in the periphery and in the supraspinal central nervous system to provide analgesia during the first stage of labor, whereas μ-opioid receptor agonists act in the spinal cord and the supraspinal central nervous system. Estrogens block the effect of μ-opioid receptor agonists at supraspinal sites. (From Pan PH, Eisenach JC 2010 The pain of childbirth and its effects on mother and fetus. In: Chestnut DH, Polley LS, Tsen LC, et al (eds) Chestnut’s obstetric anesthesia principles and practice, 4th ed., Philadelphia, Mosby-Elsevier, pp 387–403, Fig. 20-9.)
Role of the Spinal Cord
As a result of nociceptive stimuli, action potentials entering the spinal cord cause voltage-gated calcium channels to open and hence increased intracellular calcium concentrations, which in turn through a multistep process leads to the release of multiple excitatory neurotransmitters (amino acids such as glutamate and aspartate or peptides such as substance P, calcitonin gene–related peptide [CGRP], and neurokinin A) that interact with different receptors on spinal cord neurons (Ludwig et al 2002). Release of neurotransmitters at sensory afferent terminals is controlled by presynaptic receptors that mainly control the flux of intracellular calcium as action potentials arrive. Animal studies show that inhibition of calcium channels with gabapentin or related compounds provides antinociception in response to visceral stimulation by preventing the multistep process leading to the release of neurotransmitters (Feng et al 2003).
Sensitization and amplification of nociception can occur at the spinal cord level following repetitive nociceptor activation. Intensive noxious stimuli causing sustained release of the excitatory amino acid glutamate activates N-methyl-D-aspartate (NMDA) receptors, which leads to sustained depolarization and enhanced excitability of projection neurons (Headley and Grillner 1990). In 1933, Cleland had already reported skin hypersensitivity on dermatomes T11–12 in laboring women. This hypersensitivity, which is ablated by a paravertebral local anesthetic injection, is most likely due to enhanced sensitization of spinal cord neurons receiving ongoing nociceptive visceral input from the cervix and input from skin at these dermatomes. More recently, studies in rats have shown that UCD significantly increases spinal cord c-Fos immunoreactivity from T12–L2, which is a spinal cord neuronal activation pattern similar to that occurring with other noxious visceral stimuli. Furthermore, inhibition of c-Fos expression by prior lidocaine injection of the cervix or intrathecal ketorolac (cyclooxygenase inhibitor) suggests a role of spinal cyclooxygenase in UCD-evoked nociception and probably labor pain (Tong et al 2003). (For a summary of pain transmission in the spinal cord, see Chapter 51.)
Physiological Effects of Labor Pain
The stress and pain of labor stimulate the sympathetic system, which results in an increase in plasma concentrations of epinephrine by 300–600%, norepinephrine by 200–400%, cortisol by 200–300%, and cardiac output, all of which reach peak values at or around delivery (Lederman et al 1977, Ohno et al 1986). These increases together with the reduced carbon dioxide tension associated with hyperventilation during labor result in a net reduction in uteroplacental perfusion in animal models (Bonica 1973, Shnider et al 1979). The β-adrenergic effects of epinephrine on the myometrium may result in dysfunctional labor, which may then become normal when labor analgesia is achieved.
Ferguson’s reflex involves neural input and results in enhanced release of oxytocin. However, even in women with disruption of this circuit, spontaneous labor and delivery are not abolished (Hingson and Hellman 1956). Papka and Shew (1993) suggested that afferent terminals in the lower uterine segment and cervix release stimulatory substances (substance P, glutamate, vasoactive intestine peptide) or inhibitory substances (CGRP, nitric oxide) that act on myometrial contraction and cervical dilatation when released locally into the cervix and lower uterine segment during afferent terminal depolarization as a result of the contraction-associated tissue distortion. Better understanding of how these released stimulatory and inhibitory substances affect the labor process and labor pain may provide opportunities for better management of labor and labor pain.
Cardiac, Respiratory, and Gastrointestinal Effects
Labor and labor pain exert stress on the cardiovascular and respiratory systems by increasing plasma catecholamines, which in turn elevate maternal cardiac output, blood pressure, and peripheral vascular resistance and reduce uteroplacental blood flow (Shnider et al 1979). Ueland and Hansen (1969) showed that maternal cardiac output increased throughout pregnancy when measured in the lateral position. The increase in cardiac output during pregnancy was due to an increased stroke volume and heart rate with the accompanying increase in blood volume. With the onset of labor, cardiac output further increased above the prelabor level by 15, 30, 45, and 65–80% during the early first stage, late first stage, second stage, and immediate after delivery, respectively (Hendricks and Quilligan 1956, Ueland and Hansen 1969) (Fig. 55-5). Return of the 250–300 mL of blood extruded from the uterus during contraction to the maternal venous circulation accounts for about half the increase in labor-associated maternal cardiac output, whereas the other half is due to sympathetic stimulation. Furthermore, maternal systolic and diastolic blood pressure can increase by 20–30 mm Hg with uterine contraction during labor without analgesia. The labor pain–associated changes in maternal hemodynamics and catecholamine concentrations, which may be deleterious to parturients with cardiovascular co-morbid conditions or uteroplacental insufficiency, can be lessened by half with complete analgesia, such as epidural or intrathecal analgesia (Shnider et al 1983, Walls and Melzack 1999).
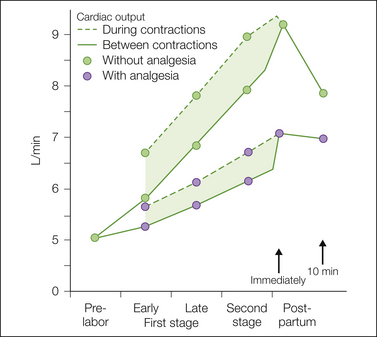
Figure 55-5 Cardiac output during the various phases of labor between contractions and during contractions.
In a group of patients in labor without analgesia, the progressive increase between contractions and the further increase during each contraction were much greater than the changes in the group of patients who received continuous epidural analgesia. (Developed from data from Hendricks CH, Quilligan EJ 1956 Cardiac output during labor. American Journal of Obstetrics and Gynecology 71:953–972; and Ueland K, Hansen JM 1969 Maternal cardiovascular dynamics. II. Posture and uterine contractions. American Journal of Obstetrics and Gynecology 103:1–7.)
Changes in lung volume during normal pregnancy are shown in Figure 55-6. The increased ventilation during pregnancy is due in part to an increase in CO2 production (metabolism) and the hormonal effect of sensitizing the respiratory center to CO2 as a direct respiratory stimulant (Pernoll et al 1975, Skatrud et al 1978). Labor pain is a powerful respiratory stimulus that results in a further increase in tidal volume, minute ventilation, and alveolar ventilation above the already increased values during pregnancy. During labor, maternal PaCO2 can be reduced from 32 to as low as 16–20 mm Hg, which in combination with the elevated plasma catecholamine concentrations may result in decreases in cerebral and uterine blood flow. The persistent repetitive nature of labor pain also results in periods of hyperventilation during uterine contractions, followed by compensatory periods of hypoventilation between contractions, which in turn may lead to transient episodes of maternal and even fetal hypoxemia. Relief of labor pain, such as with neuraxial analgesia, reduces the increases in minute ventilation, oxygen consumption, catecholamine release, and lipolytic metabolism during the first stage of labor to levels comparable to pre-labor values. However, the expulsive effort of second-stage labor can result in a further increase in ventilation and metabolism even with neuraxial analgesia (Hägerdal 1983).
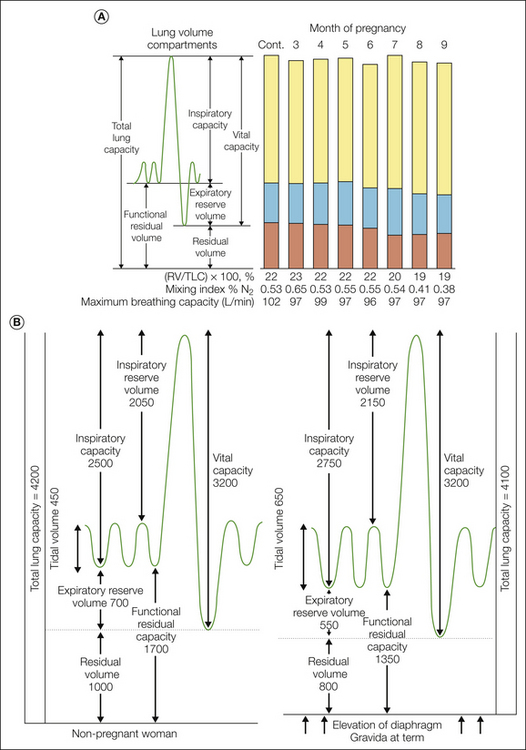
Figure 55-6 A, Serial measurements of lung volume compartments, pulmonary mixing index, and maximum breathing capacity during normal pregnancy. Control values were obtained from the same women 4–9 months postpartum. Mean values were obtained from nine women by Cugell and colleagues (1953). Note that changes begin after the fifth month. B, Pulmonary volumes and capacities in milliliters in the non-pregnant state and in the term gravida. (From Prowse CM, Gaensler EA 1965 Respiratory and acid-base changes during pregnancy. Anesthesiology 26:381–392. Copyright 1965 Lippincott Williams & Wilkins.)
Labor pain, anxiety, and emotional stress increase gastrin release and inhibit the segmental and suprasegmental reflex of gastrointestinal and urinary motility (Marx and Greene 1964, Buchan 1980), which results in an increase in gastric acidity and volume and a delay in bladder emptying. These changes are further aggravated by recumbent positions, opioids, and depressant medications. Consequently, laboring parturients are at risk for pulmonary aspiration, especially during emergency induction of general anesthesia.
Methods to Relieve Labor Pain
It is important to remember that labor pain is contextually unique with different meanings and significance to different parturients, depending on their culture, psychological state, experiences, expectations, support, and education. It is interesting to note that pain relief or the extent of pain relief in labor does not equate to overall satisfaction, which is correlated more with overall personal control, rapport, and participation in decision making. Despite the justifiable popularity and enthusiasm of neuraxial labor analgesia (now reaching 50–90% in developed countries), the majority of parturients around the world, especially in developing countries, may not have access to or the resources for neuraxial labor analgesia. Furthermore, some parturients prefer and treasure the experience of feeling the movement, rotations, and delivery of their baby through the birth canal. Various non-pharmacological methods, such as childbirth education, emotional and physical therapy support, psychoprophylaxis, hypnosis, hydrotherapy, transcutaneous electrical nerve stimulation (TENS), acupuncture, and intradermal water injection, are available to help parturients cope with labor pain. However, most of these non-pharmacological techniques have not been subjected to rigorous scientific testing and validation. Consequently, strong conclusive evidence of their efficacy in relieving labor pain is mostly not available.
Childbirth education may help the parturient understand, prepare for, and cope with the labor process and should include participation of the support person. The psychoprophylactic method of analgesia (modification of the original Dick-Read method of “natural birth”) emphasizes patient control over the labor process and the belief that childbirth pain is a result of uterine tension secondary to fear and that childbirth itself is not inherently painful. The psychoprophylactic method was initially (in the 1950s) popularized in Russia and then modified by Lamaze (1956), who successfully introduced it into the United States about the same time as regional anesthesia was reintroduced. The technique incorporates various controlled muscle relaxation and breathing exercises, which are claimed to have a salutary effect on the pain experience. This technique demands close communication and coordination among the teacher, the patient, support persons, and the health care team to foster confidence in the parturient for pleasant fulfillment of the childbirth experience. The presence of a partner or friend can often provide emotional support to the parturient. Systematic reviews also suggest that laboring parturients who receive continuous labor support (defined here as non-medical support of the parturient by trained personnel such as a doula) have slightly shorter labor, fewer operative deliveries and analgesic interventions, and greater satisfaction without a significant influence on neonatal outcomes (Simkin and O’hara 2002, Hodnett et al 2007). However, results from North American studies of continuous labor support are less positive than those from Europe or Asia (Simkin and Bolding 2004).
The use of water at delivery and immersion in warm water to cover the lower half of the body up to the abdomen during labor (hydrotherapy) has been stated by some to provide relaxation to parturients and reportedly to have the potential to reduce labor pain and analgesic requirements according to systematic reviews (Simkin and O’Hara 2002, Cluett et al 2004). In general, parturients tend to be able to use coping skills from psychoprophylactic techniques during early labor, but as labor progresses, success becomes progressively lower, with less than a third of parturients being able to use the technique by the onset of second-stage labor. Furthermore, Melzack (1981) showed that more than 90% of nulliparas and 75% of multiparas who had prepared childbirth training still rated their labor pain as moderate to severe. It is important to note that childbirth education classes may sometimes create false expectations and even cause a sense of failure and inferiority and subsequent negative psychological reactions if the parturient does not enjoy the “natural” delivery or requires other forms of labor analgesia. The hypnotic technique, another form of non-pharmacological analgesia that has been used for labor analgesia, demands complete cooperation between the parturient and the hypnotizer and requires considerable time, concentration, and dedication to the technique, as well as belief in the patient’s own inner self and strength. In Bonica’s evaluation of the hypnotic method for labor analgesia, he found that only 5–10% experienced little or no pain, 15–20% had their pain and analgesic requirement decreased, and the rest had no decrease in pain but had less fear and anxiety (Bonica 1980).
Acupuncture, or stimulation at meridians, when applied to parturients according to the different stages of labor in several randomized controlled trials resulted in lower pain scores and less use of neuraxial or systemic analgesia than in women receiving placebo (Hantoushzadeh et al 2007, Borup et al 2009). Although acupuncture may hold some promise for labor analgesia, the need for trained personnel to perform the time-consuming procedure alone may limit its widespread use or attempts at intrapartum analgesia. TENS is the application of patient-controlled low-intensity, high-frequency electrical impulses via surface electrodes placed on paravertebral positions in the lower part of the back at the T10–L1 and S2–4 levels bilaterally. Theoretically, electrical stimulation produces antinociception as a result of activation of large afferent myelinated mechanosensory neurons, central release of endogenous endorphin, or an effect of distraction. Most studies of TENS have reported no, minimal, or inconsistent analgesic effect when used for intrapartum pain relief (Simkin and Bolding 2004, Dowswell et al 2009). However, Chao and co-authors (2007) reported that the application of TENS at specific acupuncture points resulted in decreased pain perception. On the other hand, intradermal or intracutaneous injection of water (0.1 mL sterile water) into the lower back region at four points defined by the sacral borders (so-called water block) is claimed to reduce severe lower back pain intensity for up to an average of 60–80 minutes during labor, but the requirement for other analgesics is not reduced (Simkin and Bolding 2004). Since the injection itself is quite painful and works only for a limited time, its use may be not appealing to most parturients.
Systemic Analgesia: Parenteral and Inhalational Analgesia
In early labor or with Braxton Hicks–type contractions, sedatives and hypnotics can be administered mostly to provide sedation, relieve anxiety, and improve disruptive sleep patterns before the onset of active labor. Some of the agents used commonly and their relative therapeutic effects are listed in Table 55-1. As labor progresses and pain worsens, two common forms of systemic analgesia are available: parenteral opioids and inhalational analgesia.
Table 55-1
Sedatives, Hypnotics, and Ataractics
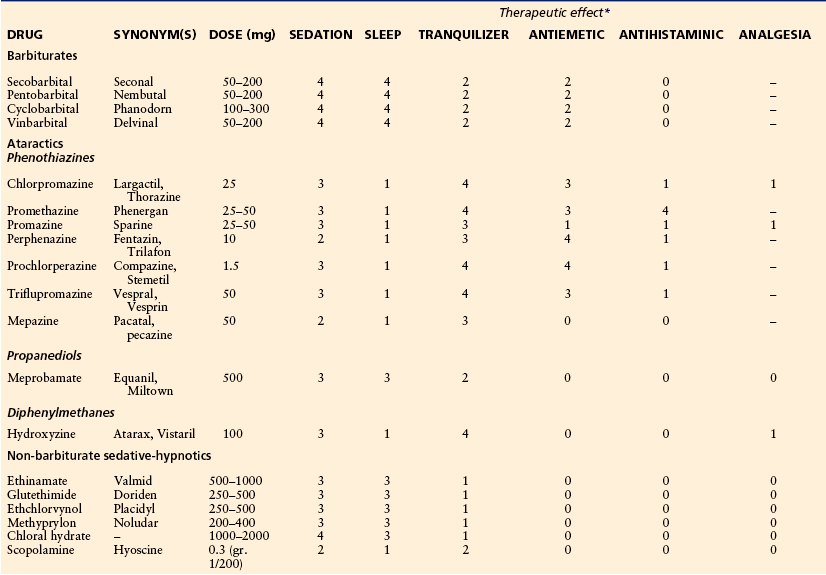
*0, no effect; 1, minimum effect; 2, moderate effect; 3, good effect; 4, maximum effect; –, analgesic effect.
Parenteral Agents
The use of parenteral analgesia or no analgesia for labor has been decreasing in the United States. However, parenteral labor analgesia with or without neuraxial analgesia is still being used in more than 34% of laboring parturients in a survey of U.S. hospitals (Bucklin et al 2005). Opioids (including agonists and agonist–antagonists) are the primary agents used parenterally for labor analgesia, with meperidine being one of the most commonly used. The effectiveness of opioid labor analgesia is at best incomplete and is much less than that of neuraxial analgesia, but it may be needed for parturients with contraindications to neuraxial analgesia. All parenteral opioids cross the placenta and result in dose-dependent maternal, fetal, and neonatal side effects. Common side effects include emesis, delayed gastric emptying, and respiratory depression. In addition, fetal bradycardia and a decrease in beat-to beat variability can occur, as well as neonatal respiratory depression (Caldwell et al 1978, Hill et al 2003). Meperidine (pethidine, Demerol) is a synthetic opioid with an intermediate half-life of about 3 hours, but its long-acting active metabolite normeperidine is a potent respiratory depressant and has a potential half-life longer than 48 hours in neonates (Caldwell et al 1978). Table 55-2 summarizes the pharmacokinetics and pharmacodynamics of the opioids commonly used for labor analgesia. Agonist–antagonists, such as butorphanol and nalbuphine, are also frequently used for labor analgesia and have the potential advantages of less nausea and vomiting and a “ceiling effect” on respiratory depression when compared with pure opioid agonists. However, their use is limited by incomplete labor analgesia, as with other opioids. It has been reported that placental transfer of the drug may produce a false sinusoidal fetal heart rate pattern complicating the interpretation of a true ominous sinusoid fetal heart rate pattern (Feinstein et al 1986), as well as the potential precipitation of withdrawal syndrome in opioid-dependent parturients and newborns (Weintraub and Naulty 1985).
Table 55-2
Opioid Analgesics Used for Moderate to Severe Pain: Pharmacokinetic and Pharmacodynamic Data
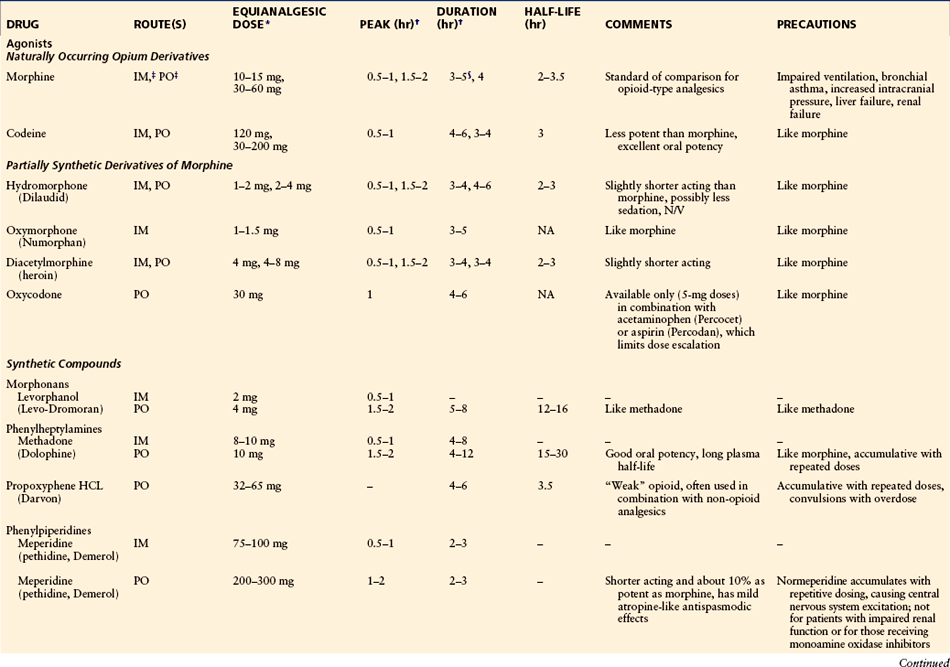
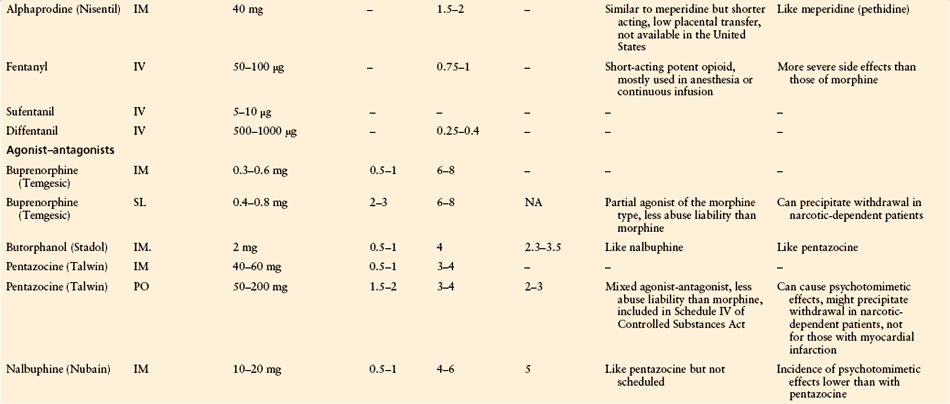
IM, intramuscular; IV, intravenous; NA, not available; PO, oral; SL, sublingual.
*Recommended starting doses from which the optimal dose for each patient is determined by titration and the maximal dose is limited by adverse effects.
†Peak time and duration of analgesia are based on mean values and refer to the stated equianalgesic doses.
‡For a single oral dose, the ratio of IM to PO is 1:6; for repeated doses the ratio is closer to 1:3.
§Plasma half-life, at least for morphine, is age dependent: it increases with age.
After Bonica JJ, McDonald JS 1995 Principles and practice of obstetric analgesia and anesthesia, 2nd ed. Baltimore, Williams & Wilkins. Copyright 1995 Lippincott Williams & Wilkins.
Scientific data are inadequate to show that any one opioid is better than another for labor analgesia, but patient-controlled intravenous analgesia (PCIA) using short-acting opioids with short latency may offer potential theoretical advantages over intermittent boluses administered by health care providers. Remifentanil, a short-acting opioid with an estimated half-life of 1.3 minutes and rapid metabolism by both maternal and neonatal plasma esterases, may have the theoretical advantage of minimal accumulation from the prolonged administration needed during labor. Use of remifentanil PCIA with a bolus dose ranging from 0.2–1.0 μg/kg (median effective bolus dose of 0.4 μg/kg) and a lockout interval of 1–5 minutes with or without infusion rates of 0.025–0.1 μg/kg/min has been reported (Volmanen et al 2002, Blair et al 2005, Balki et al 2007). Because of limited data, it is premature to advocate the safety, efficacy, and superiority of remifentanil over other opioids for labor analgesia. It is important to note that with labor PCIA, the total dose of opioids required for labor analgesia is often amazingly high. Some studies have reported that patients needed in excess of 300 μg/hr of fentanyl for labor analgesia, with 16% of neonates requiring naloxone, though without adverse neonatal outcomes (Morley-Forster et al 2000, Halpern et al 2004). Avoiding the use of a basal infusion rate with PCIA may be important to provide an additional safety margin. The implication of the residual effects of a large cumulative amount of opioids received during labor is unclear, especially in the immediate postpartum period when noxious stimulation is absent. All these concerns highlight the need for an appropriately established monitoring protocol and the presence or immediate availability of appropriate health care providers to ensure patient safety when labor PCIA is used. A PCIA protocol used at Forsyth Medical Center, Winston-Salem, North Carolina, for parturients in whom neuraxial labor analgesia is contraindicated or who are unable to receive it is shown in Figure 55-7.

Figure 55-7 A patient-controlled intravenous analgesia (PCIA) protocol for relief of labor pain at the Forsyth Medical Center for parturients in whom neuraxial analgesia is contraindicated or they are unable to receive it.
(BP, blood pressure; DBP, diastolic BP; HR, heart rate; OB, obstetrics; SBP, systolic BP.)
Inhalational Agents
Labor inhalational analgesia is the administration of a subanesthetic concentration of inhaled anesthetic for intrapartum pain relief. Intrapartum pain relief with inhalational analgesia is much less common in the United States than in the United Kingdom and other countries. Nitrous oxide, in a premixed blend of 50% nitrous oxide and 50% oxygen (Entonox), can be self-administered intermittently during contractions or continuously and is the more common form of labor inhalational analgesia used. Special scavenging equipment is needed (as with all inhalational anesthetic agents) to prevent environmental contamination. The parturient is taught to inhale the correct mixture at about 15 seconds before the peak uterine contraction pain to obtain maximum analgesic benefit. Such intermittent application seems to be safe for both the mother and fetus without significant adverse reports, but its efficacy for labor analgesia remains controversial because of inconsistent results from different studies. Careful monitoring of patients is required because maternal hypoxemia can occur, especially with the concomitant use of systemic opioids (Carstoniu et al 1994, Lucas et al 2000, Rooks 2007).
The labor analgesia provided by volatile halogenated anesthetic agents is partial but better than that with nitrous oxide/oxygen and can result in significant dose-dependent maternal sedation and uterine relaxation. Although newer agents with lower blood–gas solubility coefficients, such as desflurane and sevoflurane, may allow more rapid onset and recovery from effects of the drug, its safety and effect on labor progress remain to be determined. Yeo and colleagues (2007a, 2007b) determined that 0.8% sevoflurane was the optimal concentration for labor analgesia and reported better median pain relief scores with sevoflurane than with Entonox. Intermittent inhalation of a volatile halogenated anesthetic agent may be useful for labor analgesia in selected patients with contraindications to neuraxial analgesia. Investigations are needed for refinement and to prove the safety of the techniques. Besides safety concerns, the need for specialized equipment, additional monitoring, and added workload with dedicated anesthesia providers is a factor limiting its more common use.
Regional Analgesia
A variety of regional analgesia techniques can be used individually or in combination to provide optimal and effective labor analgesia with fewer drug-induced maternal and fetal side effects than seen with systemic analgesics. Among the various regional techniques, neuraxial labor analgesia is the most commonly used and is the most effective and complete labor analgesia, whereas lumbar sympathetic block is performed much less frequently and paracervical, pudendal, and local perineal infiltration techniques are performed occasionally by obstetricians, sometimes as a supplement to inadequate neuraxial analgesia or sometimes as sole labor analgesia. The techniques for pudendal and paracervical block are illustrated in Figures 55-8 and 55-9. About one-third of parturients in the United Kingdom chose neuraxial labor analgesia according to the U.K. National Health Service Maternity Statistics of 2005–2006 (National Health Service 2005). In the last major obstetric workforce survey performed in U.S. hospitals, about 77% of parturients in hospitals with 1500 or more deliveries received some form of neuraxial labor analgesia, and many hospitals with a larger number of deliveries may have a neuraxial labor analgesia rate as high as 80–90% (Bucklin et al 2005). Among the types of neuraxial labor analgesia, the continuous lumbar epidural technique is most commonly used, but the combined spinal epidural (CSE) technique has been gaining popularity over recent decades. Single-shot techniques and caudal analgesia remain uncommon as a result of limited duration and the requirement for a large amount of local anesthetic, respectively. Continuous spinal analgesia is not commonly used because of the lack of appropriate and approved equipment such as small-gauge spinal introducer needles and intrathecal catheters. Occasionally and despite the risk for post–dural puncture headache (PDPH), an intrathecal catheter is inserted either intentionally or unintentionally with the epidural needle puncturing the dura. Not only does continuous (epidural or intrathecal) catheter-based neuraxial analgesia provide excellent labor analgesia, but such catheters also allow rapid conversion of neuraxial analgesia to anesthesia for cesarean delivery and minimize the need for general anesthesia and manipulation of the airway. Recent findings of potential apoptotic neurodegeneration in developing brains and long-term behavioral consequences in juvenile animals exposed to drugs involving NMDA and/or γ-aminobutyric acid (GABA) receptors (which include almost all sedative–hypnotics and inhalational anesthetics) have prompted in a joint statement from the American Society of Anesthesiologists, Society of Pediatric Anesthesia, American Academy of Pediatrics, and Food and Drug Administration in 2011 encouraging research to more fully evaluate these potential neurological changes. Even though prospective human data are lacking and the applicability of animal data to clinical practice is uncertain, these current findings remain a theoretical reason to consider regional analgesia/anesthesia over systemic analgesia for relief of labor pain or for general anesthesia for cesarean delivery or maternal surgery during pregnancy (Loepke and Soriano 2008).
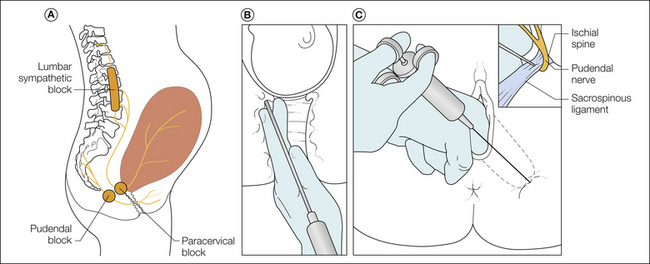
Figure 55-8 A, Sites of three regional techniques for obstetric analgesia. Lumbar sympathetic blockade is rarely used, but it is highly effective in relieving pain of the first stage and may be preferable to a paracervical block, especially with high-risk pregnancies. B, Coronal section of the vagina and lower part of the uterus containing the baby’s head in which the technique for a paracervical block are shown. The 22-gauge needle is within a guide, with its point protruding only 5–7 mm beyond the surface of the mucosa. After negative aspiration, injection of 8–10 mL of 0.25% bupivacaine at the 4- and 8-o’clock positions on the cervical fornix will produce relief of uterine pain for several hours. C, Transvaginal technique of blocking the pudendal nerve. The two fingers of the left hand are inserted into the vagina to guide the needle point into the sacrospinous ligament. As long as the bevel of the needle is in the ligament, there is some resistance to injection of local anaesthetic, but as soon as the bevel passes through the ligament, a sudden lack of resistance is felt and indicates that the needle point is next to the nerve. (From Bonica JJ 1967 Principles and practice of obstetric analgesia and anesthesia, vol 1. Philadelphia, FA Davis.)
Neuraxial Analgesia
Effect of Neuraxial Analgesia on Obstetric Outcomes
Although observational studies often conclude that parturients receiving neuraxial labor analgesia have a higher incidence of cesarean and instrument delivery and a longer duration of labor, a cause-and-effect relationship has not been proved. Furthermore, parturients with early onset of severe labor pain may be having signs of an abnormal labor pattern leading to an increased risk for operative delivery; at the same time these patients may be more likely to request neuraxial analgesia. Findings from a number of randomized controlled studies in recent years have helped clarify and alleviate some of the concerns of the influence of neuraxial analgesia on obstetric outcomes.
Influence on Cesarean Delivery
The impact of neuraxial analgesia on cesarean delivery rates has been examined from several aspects: (1) change in the neuraxial labor analgesia rate, (2) concentrations of local anesthetic and regimens used for neuraxial analgesia, and (3) timing of neuraxial analgesia administration. Yancey and associates (1999), in a large impact study, showed that the cesarean rate remained unchanged (19% versus 19.4%) when their epidural analgesia rate dramatically changed from 1–80% in 1 year at the Tripler U.S. Army Hospital in Hawaii. A meta-analysis that included nine impact studies with more than 37,000 parturients further confirmed no association between the use of neuraxial labor analgesia and cesarean delivery (Segal et al 2000) (Fig. 55-10). Furthermore, both a Cochrane review including 20 randomized clinical trials and a meta-analysis of 17 studies that included more than 6700 women showed no difference in the cesarean delivery rate between laboring parturients receiving systemic or epidural analgesia (Anim-Somuah et al 2005, Halpern and Leighton 2005). In one randomized trial with 1300 parturients at Parkland Hospital, Dallas, Texas, the authors reported a cesarean rate of 9% in parturients who received epidural analgesia versus 3.9% in those who received systemic meperidine (Ramin et al 1995). However, this study did not use an intent-to-treat analysis and had a high crossover rate such that about one-third of women did not receive their assigned treatment. Subsequently, two studies from the same group comparing systemic PCIA with meperidine versus epidural analgesia and systemic meperidine on request versus CSE analgesia found that the use of neuraxial labor analgesia did not increase the cesarean delivery rate (Sharma et al 2002, 2004). The impact of the timing of neuraxial analgesia on obstetric outcomes was evaluated earlier by Chestnut (1994a, 1994b) and more recently by Wong and colleagues (2005, 2009), Ohel and colleagues (2006), and Wang and co-workers (2009). Chestnut and colleagues (1994a) randomized nulliparous women in early spontaneous labor (median cervical dilation of 3.5 cm) to receive early epidural analgesia (with an intermittent epidural bolus of 0.25% bupivacaine) or early intravenous nalbuphine followed by the late administration of the same epidural analgesia when the cervix dilated to 5 cm or more. The authors performed a similar study in women under oxytocin induction (Chestnut et al 1994b). Both studies showed that early administration of epidural analgesia did not prolong labor or increase the frequency of operative deliveries. Wong and associates (2005, 2009) randomized nulliparous women in early spontaneous labor (median cervical dilation of 2 cm) or under oxytocin induction, respectively, to early CSE analgesia (intrathecal fentanyl, followed by initiation of patient-controlled epidural analgesia [PCEA] at request) or to early systemic hydromorphone followed by late PCEA initiated when the cervix dilated to 4 cm or greater (Wong et al 2005, 2009). The authors found no difference in the cesarean delivery rate between the early neuraxial and the systemic analgesia groups in both studies. These results were further confirmed by findings from a 2007 meta-analysis that included 3320 subjects (Marucci et al 2007) and a randomized trial in 2009 involving more than 12,000 nulliparous women over a 5-year period (Wang et al 2009), both showing no increase in the cesarean delivery rate with neuraxial analgesia administered early (latent phase) in labor as compared with late (active phase) in labor. Other studies investigating epidural dose effects (traditional high dose of 0.25% bupivacaine versus a low dose of 0.1% or a lower concentration of bupivacaine with fentanyl) and different epidural regimens (CSE, epidural intermittent bolus or continuous infusion) also showed that the cesarean delivery rate was not affected by different doses or regimens of neuraxial labor analgesia (Nageotte et al 1997, Comparative Obstetric Mobile Epidural Trial Study Group UK 2001).
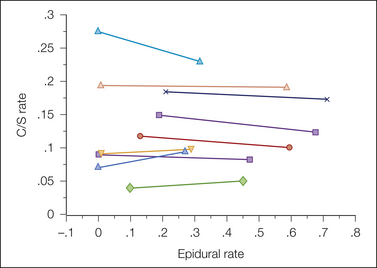
Figure 55-10 Meta-analyses of impact studies involving the influence of neuraxial labor analgesia on the rate of cesarean section (C/S) delivery. (From Segal S, Su M, Gilbert P 2000 The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. American Journal of Obstetrics and Gynecology 183:974–978, Fig. 1.)
Influence on Instrument Delivery
Besides the risk for cesarean delivery, instrumental vaginal delivery is another concerning obstetric outcome related to neuraxial analgesia. Several meta-analyses, including ones by Halpern and Leighton (2005), Liu and Sia (2004), and Sharma and colleagues (2004), compared the effects of labor epidural versus systemic analgesia on the risk for instrumental vaginal delivery and reported a statistically significantly higher odds ratio ranging from 1.6–1.9 for instrumental delivery with epidural analgesia versus systemic analgesia. Chestnut and co-workers in a series of three studies (1987a, 1987b, 1990), as well as a study by Vertommen and colleagues (1991), reported that effective second-stage analgesia with a relatively higher concentration and amount of local anesthetic administered neuraxially may result in an increased incidence of instrumental delivery when compared with neuraxial administration of a lower dose of local anesthetic or systemic analgesia. In a number of other studies (Nageotte et al 1997, Comparative Obstetric Mobile Epidural Trial Study Group UK 2001) using much lower concentrations and amounts of local anesthetic with epidural or CSE techniques, the incidence of instrumental vaginal delivery was shown to be lower than when a traditional epidural with a higher concentration and amount of local anesthetic was used. It is important to note that not all epidural techniques are the same and their impact on some obstetric outcomes may be different, depending on the concentration, amount, and types of drugs used; the mode of drug delivery, such as continuous infusion, intermittent boluses, or PCEA; and the technique used, such as CSE or conventional epidural administration. However, completely effective second-stage analgesia often requires a denser blockade with a higher concentration or larger amount of local anesthetic. As a result, current evidence seems to support a potentially increased risk for instrumental vaginal delivery with completely effective second-stage neuraxial analgesia when a higher concentration and/or amount of local anesthetic is used.
Influence on Duration of the First and Second Stages of Labor
Evidence of the impact of neuraxial analgesia on the duration of the first stage of labor is obtained mostly as a secondary outcome in studies evaluating its effects on cesarean and/or instrumental vaginal delivery. The evidence suggests variable effects as a result of about 30 minutes’ prolongation in some parturients (Sharma et al 2004) to shortening of the first stage of labor in others (Wong et al 2005, 2009; Ohel et al 2006) and even speeding up of the rate of cervical dilatation in some (Tsen et al 1999). The definition of the onset of first-stage labor often varies among studies, and assessment of when the first stage begins and ends in many studies may be inaccurate because of the imprecise timing and long interval between cervical assessment. Although the definition of the duration of second-stage labor is usually consistent, the accuracy and frequency of assessment and the application of early or delayed pushing can greatly influence results when measuring the duration of the second stage of labor. Evidence from meta-analyses suggests that parturients receiving neuraxial analgesia (especially with effective second-stage analgesia) (Sharma et al 2004, Halpern and Leighton 2005) have a longer second stage of labor by about 15 minutes than do those receiving opioid systemically. As Chestnut points out in his series of three studies (Chestnut 1987a, 1987b, 1990), lower concentrations of local anesthetic, even with opioid, may provide second-stage labor analgesia that is only marginally better than that with saline placebo.
Lumbar Epidural Analgesia
Lumbar epidural analgesia is a safe and effective method of providing a T10–L1 sensory block for analgesia during first-stage labor and has the flexibility of extending the block for sacral (S2–4) analgesia during second-stage labor or converting to epidural anesthesia for operative delivery, if needed. Over the past 2 decades, modification of techniques and new drugs and adjuvants have provided effective analgesia with minimal motor block and minimal maternal and fetal/neonatal side effects. Typically, labor lumbar epidural analgesia is administered sterilely with a 17- or 18-gauge Tuohy epidural needle, through which a 19- or 20-gauge flexible single- or multi-orifice catheter is inserted epidurally for continuous or intermittent bolus administration of epidural drugs. After an appropriate test dose or doses to exclude inadvertent intrathecal or intravenous catheter insertion, an initial loading dose of epidural drug is administered to initiate analgesia and confirm that the epidural catheter is functioning appropriately to provide a bilateral sensory block as intended. Continual epidural analgesia is then maintained, often with continuous epidural infusion (CEI) with or without PCEA boluses or scheduled/programmed intermittent epidural boluses (PIEBs) and, less commonly, clinician-administered intermittent boluses (CAIEBs) at patient demand (without PCEA or CEI). If breakthrough pain occurs and is not relieved with PCEA boluses, top-up boluses can be administered commonly by anesthesia providers (in some institutions by trained labor nurses). When comparing CEI and CAIEB on demand (without PCEA or CEI), CEI resulted in higher total local anesthetic consumption, fewer clinician-administered boluses, and higher patient satisfaction but greater motor block and potentially increased risk for instrumental vaginal delivery (Bogod et al 1987). These results suggest that some form of continual delivery of epidural drugs, whether by CEI, PCEA, PIEB, or their combinations, would be appropriate to reduce the frequency of clinician top-ups and therefore workload. Other studies have explored which mode of delivery would be best to provide continual delivery of epidural drugs for optimal analgesia with the least need for clinician intervention, least motor block, and lowest total drug consumption. A meta-analysis by van der Vyver and co-workers (2002) showed that PCEA (without background basal CEI) seemed to be more favorable and resulted in less clinician intervention (top-ups), less total local anesthetic consumption, and less motor blockade than occurred with CEI without PCEA. The next logical questions seem to be (1) whether background infusion should be used with PCEA and, (2) if so, should the background be administered as CEI or PIEB. Various studies exploring the first question of whether background CEI is needed for PCEA seemed to show inconsistent results. Boseli and colleagues (2004) compared basal CEI rates of 0, 3, 6, and 9 mL/hr, all with PCEA, and found that patient satisfaction, clinician intervention, and analgesia were all similar with or without any of the basal CEI rates with PCEA but that total drug consumption was higher only when a basal CEI rate of 6 or 9 mL/hr was used. Bremerich and associates (2005) compared PCEA with or without background CEI and reported that periods with visual analog scale scores higher than 40 mm were significantly (P < 0.001) more frequent with PCEA without background CEI (22.4%) than with PCEA with background CEI (7.5%) in treating labor pain without increasing total drug consumption. Halpern and Carvalho (2009) recently reviewed studies comparing PCEA with or without basal CEI and concluded that when using a dilute concentration of local anesthetic for maintenance of epidural analgesia, the use of basal CEI with PCEA improved analgesia and decreased clinician interventions (especially with a long duration of labor) and that a larger bolus (>5 mL) seemed to provide better analgesia than smaller boluses with PCEA. If some form of background delivery for PCEA is favorable, should the background infusion be delivered by CEI or PIEB? Fettes and colleagues (2006), in comparing 10-mL/hr CEI versus PIEB of 10 mL every hour, both without PCEA, in 40 parturients after receiving epidural (not CSE) analgesia in a randomized, controlled trial, showed fewer supplemental injections, lower total drug consumption, and longer time to the first rescue bolus with PIEB than with CEI. Similarly, other authors have compared PIEB and CEI with or without PCEA for maintenance of epidural analgesia after initiation with CSE intrathecal analgesia in randomized clinical trials (Lim et al 2005, Wong et al 2006). These studies showed mostly similar results. That is, PIEB provided lower total local anesthetic consumption per hour, a smaller percentage of patients required rescue boluses (top-ups) by a clinician, and/or patient satisfaction was higher (Lim et al 2005, Wong et al 2006, 2011), as well as less motor block (Capogna et al 2011). However, one study using 6 mL of dilute local anesthetic with PIEB every 30 minutes versus CEI each after initial CSE analgesia and with PCEA indicated that among parturients with a duration of labor (from analgesia to delivery) shorter than about 3 hours (median of the groups), the differences (number of rescue boluses required and hourly local anesthetic consumption) observed between groups diminished to insignificant (Wong et al 2006). Another study reported that PIEB, 2.5 mL every 15 minutes, was not different from CEI, 10 mL/hr (Lim et al 2010). Collectively, these studies suggest that PIEB may be more favorable (less drug, less rescue, higher patient satisfaction), especially for longer labor, than CEI, each with or without PCEA, but the optimal volume and frequency of the boluses administered via PIEB remained to be determined.
The traditional technique of continuous epidural analgesia using CEI and the extent and intensity of the analgesia during the first and second stages of labor and for delivery are summarized in Figure 55-11. Commonly used epidural regimens (local anesthetic and adjuvants) for initiation, continuous infusion, and PCEA are listed in Table 55-3. Opioid (fentanyl or sufentanil), with its local anesthetic-sparing effect, is commonly added to the epidural local anesthetic solution to provide synergistic analgesia, to reduce the concentration and total amount of local anesthetic needed for analgesia, and to decrease the amount of motor block. Other less commonly used adjuvants, such as neostigmine, clonidine, and epinephrine, have been attempted. Varying degrees of success and associated side effects limit some of these adjuvants from more popular use. During the second stage of labor, forceps delivery or episiotomy and supplement epidural doses with a higher concentration of local anesthetic (e.g., 5–10 mL of 1–2% lidocaine or 2–3% 2-chloroprocaine) may be required in some patients for sacral analgesia to block the pain from distention and pressure on the perineum, vagina, and pelvic floor.
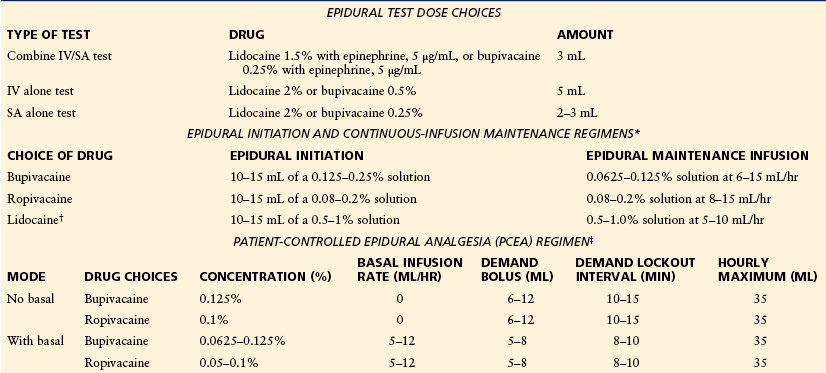
Table 55-3
Suggested Epidural Regimens: Test Doses, Initiation, Maintenance, Patient Controlled with or without Basal Infusion
IV, intravenous; SA, subarachnoid.
*Opioid, such as fentanyl, 2 μg/mL, or sufentanil, 0.2–0.33 μg/mL, is commonly added to each of the solutions for epidural maintenance infusion, as well as epidural initiation, to reduce motor block and the total amount of local anesthetic needed. Epinephrine (1.25–5 μg/mL) is often also added to the lower-concentration maintenance solution. The lower volume/rate within the range is for the higher-concentration solution, whereas the upper volume/rate is for the lower-concentration solution.
†Lidocaine is not commonly used for maintenance because of its short duration of action.
‡Opioid, such as fentanyl, 1.5–3 μg/mL, or sufentanil, 0.2–0.33 μg/mL, is commonly added to each of the solutions to reduce motor block and the total amount of local anesthetic needed.
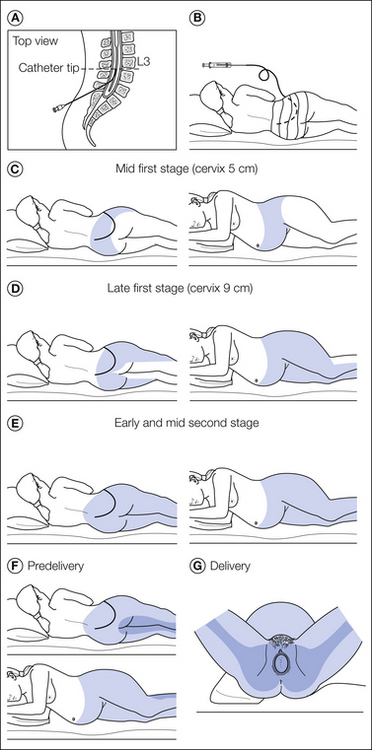
Figure 55-11 Technique of continuous epidural analgesia and the extent and intensity of analgesia during the first and second stages of labor and for delivery.
A, Epidural needle and catheter in place. After removing the needle, the catheter is taped to the patient’s back and a test dose of local anesthetic is given. B, If after 5 minutes there is no sign of accidental intravenous or subarachnoid injection, a bolus of 5 mL of local anesthetic is injected while the patient is in the lateral position. C, After signs of epidural analgesia of T9–10 to L1–2 are noted, the catheter is connected to the continuous infusion system and the solution is administered at a rate of 10–12 mL/hr with the patient in a 15–20-degree head-up position and lying on her side. D, Extent of analgesia after 1.5–2 hours of infusion. E, Extent of analgesia in the early and mid second stage. F, After internal rotation has occurred, injection of a bolus of 10 mL of local anesthetic solution, such as 1% lidocaine (lignocaine), produces an increase in the intensity of analgesia as indicated by the more heavily shaded area involving the lower sacral segments. G, The patient is ready for delivery. Note the wedge under the right hip and lower region to help displace the uterus to the left. (See text for details.) (From Wall PD, Melzack R 1999 Textbook of pain. Edinburgh, Churchill Livingstone, 1999, with the permission of Elsevier.)
Combined Spinal and Epidural Labor Analgesia
The first crude CSE approach was described in the 1930s, but it was not until a couple of decades ago that CSE re-emerged to gradually gain popularity for neuraxial labor analgesia. With CSE, a small amount of opioid with or without a small amount of local anesthetic is administered intrathecally via a 25- or 27-gauge pencil-point spinal needle inserted 12–15 mm through the tip of a standard 18- or 17-gauge epidural needle after the epidural space is identified. After removal of the spinal needle, the epidural catheter is inserted and used in the standard manner. Advantages of CSE labor analgesia are a faster onset (2–5 minutes with CSE versus 5–20 minutes with an epidural) of an excellent quality of analgesia that lasts about 90–180 minutes, less motor and sympathetic blockade, and even potentially faster speed of cervical dilatation (Tsen et al 1999) than with the traditional epidural technique (Simmons et al 2007). Local anesthetic (usually bupivacaine) is commonly added to the intrathecal opioid (usually fentanyl or sufentanil) to prolong the duration of analgesia with intrathecal opioid alone and also to provide an earlier onset of more complete sacral analgesia than can be achieved with an epidural technique alone, especially during advanced stages of labor. Therefore, CSE labor analgesia is often administered during advanced stages of labor and in multiparous women expecting fast labor. However, it is reasonable to consider its use even in nulliparous women not in an advanced stage of labor if the patient is already in severe labor pain and/or prefers more control of motor function or ambulation. After analgesia established by the intrathecal drug or drugs in the CSE technique, an epidural test dose is commonly administered via the epidural catheter to exclude intrathecal and intravenous catheter placement, and then maintenance of continual epidural analgesia is usually initiated immediately afterward with either CEI or PIEB, each with or without PCEA, in the same manner as with the epidural technique alone.
Commonly cited disadvantages of CSE are dural puncture, dose-dependent opioid-induced pruritus (Simmons et al 2007), potentially increased risk for fetal bradycardia (Clarke et al 1994), and untested epidural catheter function. The overall incidence of PDPH resulting from CSE is not different from that with the epidural technique alone. Insertion of the spinal needle during the CSE procedure may guide or prevent inadvertent dural puncture by the Tuohy needle, especially in cases with poorly defined loss of resistance to the suspected epidural space, thus balancing the small risk for PDPH associated with dural puncture by the small-gauge spinal needle. Therefore, the overall risk for PDPH after CSE does not seem to be increased over that with the standard epidural technique alone. Pruritus occurs in a dose-dependent manner much more frequently with intrathecal opioid (almost 100%) than with epidural opioid and is probably mediated via a central μ-opioid receptor, central inhibitory neurotransmitter, central 5-hydroxytryptamine (5-HT3) receptor, or their combination (Scott and Fischer 1982, Ganesh and Maxwell 2007). The symptoms are usually worse during the initial 30 minutes, but the need for treatment is relatively low and the symptoms can be treated effectively with nalbuphine (2.5–5 mg) or naloxone (40–120 μg) if needed. Fetal bradycardia occurring shortly after CSE is thought to be related to the precipitous decrease in maternal plasma epinephrine in relation to norepinephrine, which can lead to uterine hypertonicity, hypoperfusion, and ultimately, if persistent, fetal bradycardia (Clark et al 1994). Fetal bradycardia and uterine hypertonicity can occur 15–45 minutes after the initiation of either epidural or CSE analgesia (Shnider et al 1983). A systematic review conducted by Mardirosoff and colleagues (2002) showed that the use of intrathecal opioid analgesia may result in an increased risk for fetal bradycardia when compared with non-opioid intrathecal analgesia. Van de Velde and co-authors (2004) reported a dose-dependent increase in the incidence of fetal bradycardia with intrathecal sufentanil, but no difference in operative delivery. However, Nielsen and colleagues (1996) did not report any difference in fetal heart rate abnormalities (including bradycardia) when comparing parturients receiving intrathecal sufentanil versus epidural bupivacaine. Fortunately, these episodes of fetal bradycardia are usually transient and self-limited and do not alter overall obstetric and neonate outcomes, such as the cesarean delivery rate. Treatment consists of exclusion of other causes and supportive treatment, such as discontinuing any exogenous uterotonic agents, maintaining normal hemodynamics, uterine displacement, administration of supplemental oxygen, and administration of a short-acting uterine muscle relaxant such as nitroglycerin or terbutaline if the uterine hypertonus and fetal bradycardia persist.
The other disadvantage of CSE is the untested catheter since the function of the epidural catheter is not completely known or tested until the spinal analgesia of CSE wears off. Therefore, it is common for clinicians to not use CSE analgesia in patients with increased risk or anticipated airway difficulty for operative delivery, for which a functioning epidural catheter that allows rapid institution of epidural anesthesia is critical to avoid the risks associated with general anesthesia. However, even with the conventional epidural technique, the epidural catheter may still have the potential to fail at any time during labor or operative delivery. Various retrospective reviews and quality assurance data suggest that the incidence of epidural catheter failure requiring replacement during the course of labor ranges from 5–13% (Eappen et al 1998, Eisenach 2002). In a retrospective review of more than 12,000 neuraxial labor analgesia procedures, Pan and co-authors (2004) reported a 6.8% catheter replacement rate during the course of labor even after the initial analgesia provided from the epidural catheter is adequate. However, the use of CSE was not associated with an increase in epidural catheter failure during labor or when needed for operative delivery over that with the traditional epidural technique.
Failure of the spinal analgesia portion of CSE has reportedly ranged from 0.5–15% (Rigler et al 1991, Grondin et al 2009). A commonly cited reason for failure is deviation of needle placement from the midline resulting in no return of spinal fluid when the spinal needle is inserted through the epidural needle after the epidural space is identified. Figure 55-12 shows some of the common reasons for failure of the CSE procedure. Grondin and co-authors (2009) also reported that if there was no return of spinal fluid through the spinal needle during the CSE procedure, subsequent insertion of the epidural catheter was associated with a significantly higher catheter replacement rate (28.6%) because of subsequent inadequate epidural analgesia during the course of labor than in those with initial fluid return (4.1%; P < 0.03).
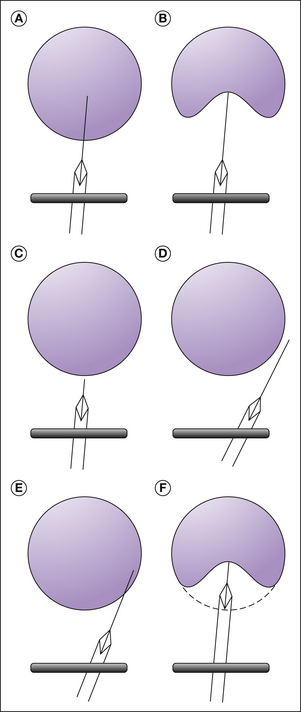
Figure 55-12 Successful continuous spinal epidural analgesia (A) and possible reasons for failure (B–F).
A, Successful dural puncture. B, The dura is “tented” rather than punctured. C, A short spinal needle fails to reach the dura. D, Lateral deviation of the epidural needle causing the spinal needle to miss the dural sac. E, Similar to D except that the dura is punctured laterally, which may result in an inability to aspirate cerebrospinal fluid. F, “Tenting” of the dura with “too short” a spinal needle. Further advancement of the epidural needle may result in the dura rebounding over both the spinal and epidural needle tips and cause an accidental “wet tap” with the epidural needle. (From D’Angelo R, Thomas JA 2002 Regional analgesia in obstetrics. In: Palmer CM, D’Angelo R, Paech MJ (eds) Handbook of obstetric anesthesia, Oxford, BIOS Scientific Publishers, pp 41–67, Fig. 5-4.)
Continuous Spinal Labor Analgesia
Continuous spinal analgesia requires the insertion of a catheter into the low lumbar subarachnoid space, through which spinal medication can be administered intermittently or continuously. In the early 1990s, a series of cases of cauda equina syndrome in non-pregnant patients was reported in association with the administration of continuous spinal anesthesia via 32-gauge spinal microcatheters (Rigler et al 1991). Subsequently, the spinal microcatheter was removed from clinical use by the U.S. Food and Drug Administration. The reason for neurological injury is not fully understood but is thought to be due to subarachnoid maldistribution of a relatively high concentration of agents (local anesthetic and/or dextrose) (Rigler and Drasner 1991). More recently, a randomized controlled trial comparing conventional continuous epidural labor analgesia with continuous spinal labor analgesia via a 28-gauge spinal catheter showed no statistically significant difference in neurological complications or incidence of PDPH between groups (spinal, 9%; epidural, 4%) (Arkoosh et al 2008). However, the authors reported a higher incidence of technical difficulty and catheter failure in the continuous spinal group and also indicated that safe use of the spinal catheter needed to be confirmed with larger studies. Subsequently, the manufacturer did not market the spinal catheter. Because of the lack of an appropriate small spinal introducer needle and spinal catheter, continuous spinal labor analgesia is usually performed by puncturing the dura with the standard epidural needle and inserting the standard epidural catheter as a spinal catheter. Besides the risk for PDPH with a large spinal catheter and its large introducer needle, the risk for neurological injury and complications (high spinal or total spinal block, accidental administration of the epidural dose through the spinal catheter, which is actually the usual epidural catheter) is probably increased. When these complications develop in the relatively uncontrolled setting of the labor and delivery suite, there is a further increase in risk for lethal catastrophic outcomes. Therefore, continuous spinal labor analgesia is uncommonly performed and should be reserved only for selected cases with extra special precautions. Examples of such cases are intentional or unintentional dural puncture with the epidural needle in patients in whom epidural catheter placement has proved difficult or not possible (e.g., scoliosis, morbid obesity). For initiation of spinal analgesia, a typical CSE dose, such as 1.5–2.5 mg bupivacaine with 10–20 μg fentanyl, can be used. Maintenance of continuous spinal analgesia can be provided by the continuous infusion or intermittent bolus method (Table 55-4). If operative delivery is needed, analgesia can be converted to surgical anesthesia quickly. The maintenance medication can be the standard epidural solution, such as 0.1% bupivacaine with 2 μg/mL fentanyl running at 1–2 mL/hr and then titrated to effect. A standard PCEA pump may be used to deliver the spinal medication either by continuous infusion or by provider-administered intermittent boluses without risking contamination from repeated opening of the spinal catheter connector for each bolus administration. However, special labeling of the catheter (and the PCEA pump if used) and clear communication to make all providers aware of the spinal catheter (not the epidural catheter), meticulous sterile technique, and careful frequent assessment of the patient are essential for the safe use of continuous spinal labor analgesia.
Side Effects and Complications of Neuraxial Labor Analgesia
The details of each side effect and complication are beyond the scope of this chapter. Commonly cited side effects from neuraxial analgesia are hypotension, emesis, fever, urinary retention, pruritus, and reactivation of herpes simplex virus (HSV). Overall, the incidence of hypotension after neuraxial analgesia is about 10% and not different between CSE and the contemporary epidural technique using a low-dose/low-concentration local anesthetic solution with opioid (Simmons et al 2007). Nausea and vomiting are common with labor and delivery itself but can often be a result of hypotension from the sympathetic block of neuraxial analgesia or from aortocaval compression. For hypotension, vasopressor treatment, such as with ephedrine and phenylephrine, should be administered promptly together with left lateral tilt positioning to relieve aortocaval compression. Hydration has a slow onset and is probably ineffective by itself. Standard antiemetics and a 5-HT3 antagonist (ondansetron or dolasetron), as well as naloxone and even a small dose of propofol, have all been administered to treat persisting emetic symptoms after hypotension is excluded or corrected.
Most current evidence suggests an association between epidural analgesia and maternal fever during labor (Camann et al 1991, Lieberman et al 1997). Some studies suggest that an inflammatory mechanism is manifested as fever. The significance of a low-grade maternal fever on the mother or the fetus is not clear, but it may lead to an increase in evaluation for neonatal sepsis even though the actual neonatal sepsis rate does not appear to be increased (Lieberman et al 1997). Interestingly, several reports suggest that intrathecal morphine, commonly administered for post-cesarean analgesia, can occasionally be associated with mild hypothermia (as low as 34°C) lasting about 6 hours without treatment (Hess et al 2005, Hui et al 2006). The mechanism is also unclear and usually only a small percentage of these patients are symptomatic with diaphoresis and feeling hot (instead of feeling cold and shivering). Active warming seems to be ineffective, whereas lorazepam has been used successfully to treat the hypothermia and associated symptoms, with rapid cessation of symptoms and restoration of temperature (Hess et al 2005).
Intrapartum and postpartum urinary retention in patients receiving neuraxial analgesia is observed to be increased over those with systemic or no labor analgesia (Weiniger et al 2006). Neuraxial local anesthetics block the sacral nerve roots S2–4 controlling the detrusor muscle and sphincter function of the bladder. Likewise, intrathecal opioids can cause a dose-dependent suppression of detrusor muscle contractility and a decrease in the urge to micturate (Kuipers et al 2004). However, a study of 771 women without neuraxial analgesia reported the rate of postpartum urinary retention to be 9.7% and 15.8% after normal and instrumental vaginal delivery, respectively (Kekre et al 2011), whereas protracted (defined as >3 days postpartum) urinary retention was uncommon (0.18%), as reported in a study of more than 30,000 postpartum women (Groutz et al 2011). Therefore, parturients should be monitored in the intrapartum and postpartum period to prevent urinary retention and bladder distention and, if needed, be treated with catheterization or naloxone if the problem is related to the neuraxial opioid.
The side effect of pruritus has already been discussed in the CSE section. However, some have postulated that facial scratching as a result of neuraxial morphine–induced pruritus may be responsible in part for reactivation of HSV. Some evidence suggests that reactivation of HSV by neuraxial morphine may be due to a molecular imbalance between viral transcription and the central nervous system host immunity that normally maintains HSV latency (Boyle 1995). Overall, the evidence suggests a higher incidence of postpartum oral HSV reactivation in parturients receiving neuraxial morphine than in those receiving systemic morphine for post–cesarean delivery analgesia. However, the majority (72–86%) of parturients with a history of herpes labialis did not have a recurrence after receiving neuraxial morphine (Bauchat 2010). Currently, there is no strong evidence or randomized clinical trial data to indicate that the risk for reactivation of HSV is increased with the use of other neuraxial opioids (other than morphine) for labor analgesia or other postoperative analgesia. Therefore, most clinicians do not withhold neuraxial opioids for labor analgesia or even neuraxial morphine for post-cesarean analgesia since the benefits most likely outweigh the risks.
Serious complications with neuraxial analgesia and anesthesia are fortunately rare, but they are potentially devastating, especially when occurring in obstetric patients. In a prospective audit of 145,550 obstetric epidural procedures in the United Kingdom, the incidence of complications was 1 in 5000 for intravascular injection, 1 in 2900 for intrathecal injection, 1 in 4200 for subdural injection, and 1 in 16,200 for high or total spinal block (Jenkins 2005). The Royal College of Anaesthetists conducted a national audit of practice in the United Kingdom for 1 year between 2006 and 2007, and the results suggested an optimistic estimate of 1 per 320,000 to a pessimistic estimate of 1 per 80,000 for complications with permanent injury from obstetric neuraxial anesthesia (Cook et al 2009). The Serious Complication Repository (SCORE) project conducted under the Society for Obstetric Anesthesia and Perinatology prospectively collected information on complications from 307,495 deliveries with 256,795 total anesthetics (251,463 were regional anesthetics) during October 2004 to June 2009. The reported incidence of complications was similar to that in other studies, with the estimated incidence of epidural hematoma being 1 per 251,463, that of epidural abscess and meningitis being 1 per 62,866, overall neurological injury requiring neurological consultation or imaging occurring in 1 per 35,923, and a relatively high incidence of high spinal block requiring intubation and resuscitation in 1 per 4336 (personal communications). Removal of the 0.75% epidural bupivacaine solution from clinical use, aspiration of the catheter before each dose, and the use of test doses and especially small fractionated dosing have significantly reduced catastrophic results from the rare complication of systemic toxic reaction and some of the total spinal block from the administration of epidural drug through a misplaced epidural catheter. There were no obstetric anesthesia malpractice claims from 1990–2003 in the Closed Claims Project involving inadvertent intravenous injection of epidural local anesthetic. However, there were still 10 cases of high spinal block resulting from intrathecally placed epidural catheters that were not identified with aspiration or a test dose (Davies et al 2009). This further highlights the importance of small fractionated dosing for every dose followed by assessment of effects from the dose.
Other serious complications from obstetric neuraxial analgesia/anesthesia remain rare. A robust risk estimate of neurological injury is difficult to obtain because of its low incidence. Ruppen and co-authors (2006) reported the risk for neurological injury from a total of 1.37 million obstetric epidural analgesia/anesthesia procedures obtained from 27 articles. Among articles published after 1990 and with a study sample size larger than 100,000, the estimated risks were as follows: epidural hematoma, 1 per 168,000; deep epidural infection, 1 per 145,000; persistent neurological injury, 1 per 240,000; and transient neurological injury, 1 per 6700. These findings are quite similar to those reported in the SCORE project discussed earlier. Earlier studies before 1990 with a smaller sample size tended to report higher but still very low risk estimates. In a Swedish survey of about 1.2 million spinal anesthetics and 450,000 epidural anesthetics during 1990–1999, Moen and co-workers (2004) reported only 1 case of epidural abscess and no cases of meningitis after 200,000 obstetric epidural anesthetics and no cases of meningitis after 55,000 obstetric spinal anesthetics. The incidence of epidural abscess and meningitis in the obstetric population was reported to be much lower than that in the non-obstetric population. This is probably due to the generally healthier state and better immune system in the obstetric population than in the non-obstetric population receiving neuraxial analgesia/anesthesia. It is reassuring to see that the risk for bleeding and infectious neurological injury is low after obstetric neuraxial analgesia/anesthesia, but it is important to continue to be vigilant and use sterile technique (Hebl 2006) (such as including the use of an alcohol-based chlorhexidine preparation solution, gloves, mask, and hat), together with careful evaluation and selection of patients appropriate for neuraxial labor analgesia and anesthesia.
Anesthesia-Related Maternal Mortality
Anesthesia-related maternal mortality is extremely low in developed countries and has significantly decreased from an estimated 4.3 per million live births in the triennium 1979–1981 to 1.0 per million live births in 2003–2005 in the United States, which represents less than 2% of pregnancy-related maternal mortality (Chang et al 2003, Hawkins et al 2011). Improvement of similar magnitude has likewise been observed in the United Kingdom. Hawkins and co-authors (2011) also reported a 59% reduction in anesthesia-related maternal mortality between the periods 1979–1990 and 1991–2002. They estimated the case fatality rate for general anesthesia to be 16.8 per million in 1991–1996 versus 6.5 per million in 1997–2002; for neuraxial anesthesia the figures were 2.5 and 3.8 per million for the same two periods, respectively. The risk ratios between general and neuraxial anesthesia decreased significantly from 6.7 to 1.7 for the two periods, respectively (Hawkins et al 2011). These results suggest that the safety of obstetric general anesthesia has improved significantly and continually from 1980–2002, most likely the result of improved vigilance of the airway and management, the availability of newer effective airway equipment and monitors, and use of the difficult airway algorithm during that period. Even though obstetric neuraxial analgesia/anesthesia is very safe, the level of safety has not improved much in the past 2 decades, possibly partly because of the increased use of neuraxial anesthesia for higher-risk and sicker patients now more than 2 decades ago. Furthermore, the top leading causes of anesthesia-related maternal death in 1991–2002 were failed intubation or induction issues (about 67%) in those who received general anesthesia and high neuraxial block (26%), respiratory failure (19%), and drug reaction (19%) in those who received neuraxial anesthesia. A review of anesthesia-related maternal mortality in Michigan from 1985–2003 shed some light on the emerging problems that we may be encountering (Mhyre et al 2007). Of the eight anesthesia-related maternal deaths in the Michigan report, all were due to airway mishaps during emergence and/or recovery but not during induction of general anesthesia. Furthermore, obesity and African American race were risk factors, with 75% of the deaths occurring in obese patients and 75% in African Americans. It was found that lapses in postoperative monitoring and inadequate supervision by the anesthesiologist seemed to be responsible for 50% of the deaths. Finally, the American Society of Anesthesiologists Closed Claims Project may reveal useful insight and lessons on trends and mechanisms of injuries, even though accurate denominators to calculate event rates are not available (Ruppen et al 2006). Obstetric anesthesia claims still account for about 12% of all perioperative claims since pre-1990–2003. Although newborn death or brain damage claims have decreased and maternal nerve injury claims have increased over this period, newborn death or brain injury, maternal nerve injury, and maternal death remain the most common injuries resulting in claims in the post-1990 period. Unfortunately, substandard anesthesia care, anesthetic delay, and poor communication are three main factors associated with almost all the newborn death and brain injury cases. Among claims for maternal death or brain injury in patients who received neuraxial anesthesia, high neuraxial block was the most common cause and accounted for 37% of these claims, whereas maternal hemorrhage and failed intubation were the first and second most common causes (29% and 25% of the claims, respectively) in patients who received general anesthesia. Of the claims from high neuraxial block, two-thirds were the result of administration of epidural drugs into an unrecognized intrathecal catheter intended for the epidural space even after negative aspiration and uneventful test doses. Of note, cardiac arrest is likely to follow after a high or total spinal. Lee and colleagues (American Society of Anesthesiologists Task Force on Obstetric Anesthesia 2007) reported that neuraxial block–associated cardiac arrest was the most common cause of regional anesthesia–related maternal death or brain injury during the period 1980–1999. Maternal nerve injury claims are nearly all associated with regional anesthesia and also most commonly associated with vaginal deliveries. Although 80% of the nerve injuries were temporary and non-disabling, 11% of the claims were for serious spinal cord injury resulting in paraplegia. Some of the known causes of spinal cord injury were epidural hematoma in four, epidural abscesses in four, direct cord injection in two, and anterior spinal artery syndrome in one.
The importance of history is the lessons that we learn from it. Although anesthesia-related maternal morbidity and mortality are rare and neuraxial labor analgesia is very safe, it is important for anesthesia providers to remain vigilant with patients and meticulous with techniques and for anesthesia practice and obstetric anesthesia societies to establish protocols and guidelines.
The references for this chapter can be found at www.expertconsult.com.
References
Aley K.O., Levine J.D. Role of protein kinase A in the maintenance of inflammatory pain. Journal of Neuroscience. 1999;19:2181–2186.
Aley K.O., McCarter G., Levine J.D. Nitric oxide signaling in pain and nociceptor sensitization in the rat. Journal of Neuroscience. 1998;18:7008–7014.
Algom D., Lubel S. Psychophysics in the field: perception and memory for labor pain. Perception & Psychophysics. 1994;55:133–141.
American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology 106:843–863
Anim-Somuah M., Smyth R., Howell C. Epidural versus non-epidural or no analgesia in labour. Cochrane Database of Systematic Reviews. 2005;4:CD000331.
Arkoosh V.A., Palmer C.M., Yun E.M., et al. A randomized, double-masked, multicenter comparison of the safety of continuous intrathecal labor analgesia using a 28-gauge catheter versus continuous epidural labor analgesia. Anesthesiology. 2008;108:286–298.
Atlee H.B. Natural childbirth. Springfield, IL: Charles C Thomas; 1956.
Bajaj P., Drewes A.M., Gregersen H., et al. Controlled dilatation of the uterine cervix—an experimental visceral pain model. Pain. 2002;99:433–442.
Balki M., Kasodekar S., Dhumne S., et al. Remifentanil patient-controlled analgesia for labour: optimizing drug delivery regimens. Canadian Journal of Anaesthesia. 2007;54:626–633.
Bauchat J.R. Focused review: neuraxial morphine and oral herpes reactivation in the obstetric population. Anesthesia and Analgesia. 2010;111:1238–1241.
Berkley K.J., Robbins A., Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. Journal of Neurophysiology. 1993;69:533–544.
Blair J.M., Dobson G.T., Hill D.A., et al. Patient controlled analgesia for labour: a comparison of remifentanil with pethidine. Anaesthesia. 2005;60:22–27.
Bogod D.G., Rosen M., Rees G.A. Extradural infusion of 0.125% bupivacaine at 10 ml h−1 to women during labour. British Journal of Anaesthesia. 1987;59:325–330.
Bonar B.E., Meeker W.R. The value of sacral nerve block anesthesia in obstetrics. JAMA: Journal of the American Medical Association. 1923;81:1079–1083.
Bonica J.J. Maternal respiratory changes during pregnancy and parturition. In: Marx G.F., ed. Parturition and perinatology. Philadelphia: F A Davis, 1973.
Bonica J.J. Obstetric analgesia and analgesia, ed 2. Seattle: World Federation of Societies of Anaesthesiologists, Amsterdam, University of Washington Press; 1980.
Bonica J.J., Chadwick H.S., Labour pain. Wall P.D., Malzack R., eds. Textbook of pain, ed 2. New York: Churchill-Livingstone; 1989:482–499.
Borup L., Wurlitzer W., Hedegaard M., et al. Acupuncture as pain relief during delivery: a randomized controlled trial. Birth. 2009;36:5–12.
Boselli E., Debon R., Cimino Y., et al. Background infusion is not beneficial during labor patient-controlled analgesia with 0.1% ropivacaine plus 0.5 microg/ml sufentanil. Anesthesiology. 2004;100:968–972.
Boyle R.K. A review of anatomical and immunological links between epidural morphine and herpes simplex labialis in obstetric patients. Anaesthesia and Intensive Care. 1995;23:425–432.
Bradshaw H.B., Temple J.L., Wood E., et al. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197.
Bremerich D.H., Waibel H.J., Mierdl S., et al. Comparison of continuous background infusion plus demand dose and demand-only parturient-controlled epidural analgesia (PCEA) using ropivacaine combined with sufentanil for labor and delivery. International Journal of Obstetric Anesthesia. 2005;14:114–120.
Buchan P.C. Emotional stress in childbirth and its modification by variations in obstetric management. Epidural analgesia and stress in labor. Acta Obstetricia et Gynecologica Scandinavica. 1980;59:319–321.
Bucklin B.A., Hawkins J.L., Anderson J.R., et al. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
Caldwell J., Wakile L.A., Notarianni L.J., et al. Maternal and neonatal disposition of pethidine in childbirth—a study using quantitative gas chromatography—mass spectrometry. Life Sciences. 1978;22:589–596.
Camann W.R., Hortvet L.A., Hughes N., et al. Maternal temperature regulation during extradural analgesia for labour. British Journal of Anaesthesia. 1991;67:565–568.
Capogna G., Camorcia M., Stirparo S., Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesthesia and Analgesia. 2011;113:826–831.
Carstoniu J., Levytam S., Norman P., et al. Nitrous oxide in early labor. Safety and analgesic efficacy assessed by a double-blind, placebo-controlled study. Anesthesiology. 1994;80:30–35.
Caton D. What a blessing. She had chloroform: the medical and social response to the pain of childbirth from 1800 to the present. New Haven, CT: Yale University Press; 1999.
Chang J., Elam-Evans L.D., Berg C.J., et al. Pregnancy-related mortality surveillance—United States, 1991–1999. MMWR. Surveillance Summaries: Morbidity and Mortality Weekly Report. 2003;52(2):1–8.
Chao A.S., Chao A., Wang T.H., et al. Pain relief by applying transcutaneous electrical nerve stimulation (TENS) on acupuncture points during the first stage of labor: a randomized double-blind placebo-controlled trial. Pain. 2007;127:214–220.
Chestnut D.H., Bates J.N., Choi W.W. Continuous infusion epidural analgesia with lidocaine: efficacy and influence during the second stage of labor. Obstetrics and Gynecology. 1987;69:323–327.
Chestnut D.H., Laszewski L.J., Pollack K.L., et al. Continuous epidural infusion of 0.0625% bupivacaine–0.0002% fentanyl during the second stage of labor. Anesthesiology. 1990;72:613–618.
Chestnut D.H., McGrath J.M., Vincent R.D., Jr., et al. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80:1201–1208.
Chestnut D.H., Vandewalker G.E., Owen C.L., et al. The influence of continuous epidural bupivacaine analgesia on the second stage of labor and method of delivery in nulliparous women. Anesthesiology. 1987;66:774–780.
Chestnut D.H., Vincent R.D., Jr., McGrath J.M., et al. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin? Anesthesiology. 1994;80:1193–1200.
Cicero T.J., Nock B., O’Connor L., et al. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. Journal of Pharmacology and Experimental Therapeutics. 2002;300:695–701.
Clarke V.T., Smiley R.M., Finster M. Uterine hyperactivity after intrathecal injection of fentanyl for analgesia during labor: a cause of fetal bradycardia? Anesthesiology. 1994;81:1083.
Cleland J.C. Paravertebral anaesthesia in obstetrics. Surgery, Gynecology & Obstetrics. 1933;57:51–62.
Cluett E.R., Nikodem V.C., McCandlish R.E., et al. Immersion in water in pregnancy, labour and birth. Cochrane Database of Systematic Reviews. 2004;2:CD000111.
Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK. Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet. 2001;358:19–23.
Conell-Price J., Evans J.B., Hong D., et al. The development and validation of a dynamic model to account for the progress of labor in the assessment of pain. Anesthesia and Analgesia. 2008;106:1509–1515.
Cook T.M., Counsell D., Wildsmith J.A., et al. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. British Journal of Anaesthesia. 2009;102:179–190.
Davies J.M., Posner K.L., Lee L.A., et al. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology. 2009;110:131–139.
Debiec J., Conell-Price J., Evansmith J., et al. Mathematical modeling of the pain and progress of the first stage of nulliparous labor. Anesthesiology. 2009;111:1093–1110.
Dick-Read G. Childbirth without fear: the principles and practice of natural childbirth. New York: Harper & Row; 1970.
Dowswell T., Bedwell C., Lavender T., et al. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews. 2009;2:CD007214.
Eappen S., Blinn A., Segal S. Incidence of epidural catheter replacement in parturients: a retrospective chart review. International Journal of Obstetric Anesthesia. 1998;7:220–225.
Eisenach J.C. The treatment of pain: remaining challenges and future opportunities. Canadian Journal of Anaesthesia. 2002;49:R1–R3.
Eisenach J.C., Pan P.H., Smiley R., et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94.
Feinstein S.J., Lodeiro J.G., Vintzileos A.M., et al. Sinusoidal fetal heart rate pattern after administration of nalbuphine hydrochloride: a case report. American Journal of Obstetrics and Gynecology. 1986;154:159–160.
Feng Y., Cui M., Willis W.D. Gabapentin markedly reduces acetic acid–induced visceral nociception. Anesthesiology. 2003;98:729–733.
Fettes P.D., Moore C.S., Whiteside J.B., et al. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. British Journal of Anaesthesia. 2006;97:359–364.
Fisher D.M., Eisenach J.C. Science marches forward: a new tool to study the progress of labor. Anesthesiology. 2009;111:936–937.
Ganesh A., Maxwell L.G. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333.
Gauss C.J. Die Anwendung des Skopolamin-Morphium-Dammerschlafes in der Geburtshilfe. Medizinische Klinik. 1906;2:136–138.
Grondin L.S., Nelson K., Ross V., et al. Success of spinal and epidural labor analgesia: comparison of loss of resistance technique using air versus saline in combined spinal-epidural labor analgesia technique. Anesthesiology. 2009;111:165–172.
Groutz A., Levin I., Gold R., et al. Protracted postpartum urinary retention: the importance of early diagnosis and timely intervention. Neurourology and Urodynamics. 2011;30:83–86.
Hägerdal M., Morgan C.W., Sumner A.E., et al. Minute ventilation and oxygen consumption during labor with epidural analgesia. Anesthesiology. 1983;59:425–427.
Halpern S.H., Carvalho B. Patient-controlled epidural analgesia for labor. Anesthesia and Analgesia. 2009;108:921–928.
Halpern S.H., Leighton B.L. Epidural analgesia and the progress of labor. In: Halpern S.H., Douglas M.J., eds. Evidence-based obstetric anesthesia. Oxford: Blackwell; 2005:10–22.
Halpern S.H., Muir H., Breen T.W., et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesthesia and Analgesia. 2004;99:1532–1538.
Hantoushzadeh S., Alhusseini N., Lebaschi A.H. The effects of acupuncture during labour on nulliparous women: a randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2007;47:26–30.
Hardy J.D., Javert C.T. Measurements of pain intensity in childbirth. Journal of Clinical Investigation. 1949;28:153–162.
Hawkins J.L., Chang J., Palmer S.K., et al. Anesthesia-related maternal mortality in the United States: 1979–2002. Obstetrics and Gynecology. 2011;117:69–74.
Head H. On disturbances of sensation with especial reference to the pain of visceral disease. Brain. 1893;16:1–113.
Headley P.M., Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends in Pharmacological Sciences. 1990;11:205–211.
Hebl J.R. The importance and implications of aseptic techniques during regional anesthesia. Regional Anesthesia and Pain Medicine. 2006;31:311–323.
Hefferan M.P., O’Rielly D.D., Loomis C.W. Inhibition of spinal prostaglandin synthesis early after L5/L6 nerve ligation prevents the development of prostaglandin-dependent and prostaglandin-independent allodynia in the rat. Anesthesiology. 2003;99:1180–1188.
Hendricks C.H., Quilligan E.J. Cardiac output during labor. American Journal of Obstetrics and Gynecology. 1956;71:953–972.
Hess P.E., Snowman C.E., Wang J. Hypothermia after cesarean delivery and its reversal with lorazepam. International Journal of Obstetric Anesthesia. 2005;14:279–283.
Hill J.B., Alexander J.M., Sharma S.K., et al. A comparison of the effects of epidural and meperidine analgesia during labor on fetal heart rate. Obstetrics and Gynecology. 2003;102:333–337.
Hingson R.A., Edwards W.B. Continuous caudal analgesia: an analysis of the first ten thousand confinements thus managed with the report of the authors’ first thousand cases. JAMA: Journal of the American Medical Association. 1943;123:538–546.
Hingson RA, Hellman LM. Anesthesia for obstetrics, Lippincott, Philadelphia, p 74.
Hodnett E.D., Gates S., Hofmeyr G.J., et al. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews. 2007;3:CD003766.
Hui C.K., Huang C.H., Lin C.J., et al. A randomised double-blind controlled study evaluating the hypothermic effect of 150 microg morphine during spinal anaesthesia for caesarean section. Anaesthesia. 2006;61:29–31.
Javert C.T., Hardy J.D. Influence of analgesic on pain intensity during labor; with a note on natural childbirth. Anesthesiology. 1951;12:189–215.
Jenkins J.G. Some immediate serious complications of obstetric epidural analgesia and anaesthesia: a prospective study of 145,550 epidurals. International Journal of Obstetric Anesthesia. 2005;14:37–42.
Kekre A.N., Vijayanand S., Dasgupta R., et al. Postpartum urinary retention after vaginal delivery. International Journal of Gynaecology and Obstetrics. 2011;112:112–115.
Koller C. On the use of cocaine for producing anaesthesia on the eye. Lancet. 1884;124:990–992.
Kreis O. Über Medullarnarkose bei Gebärenden. Centralblatt Gynäkologie. 1900;28:724–727.
Kuipers P.W., Kamphuis E.T., van Venrooij G.E., et al. Intrathecal opioids and lower urinary tract function: a urodynamic evaluation. Anesthesiology. 2004;100:1497–1503.
Lamaze F. Qu’est-ce que l’accouchement sans douleur par la methode psychoprophylactique? Ses principles, sa realization, ses resultants. Paris: Savoir et Connaitre; 1956.
Lamaze F., Vellay P. L’accouchement sans douleur par la methode psycholophysique: premiers resultants portent sur 500 cas. Gazette Medicale de France. 1952;59:1445.
Lavand’homme P.M., Roelants F., Waterloos H., et al. Postoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean delivery. Anesthesiology. 2007;106:1220–1225.
Lederman R.P., McCann D.S., Work B., Jr., et al. Endogenous plasma epinephrine and norepinephrine in last-trimester pregnancy and labor. American Journal of Obstetrics and Gynecology. 1977;129:5–8.
Levinson A. The three Meigs and their contribution to pediatrics. Annals of Medical History. 1928;10:138–148.
Lieberman E., Lang J.M., Frigoletto F., Jr., et al. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics. 1997;99:415–419.
Lim Y., Chakravarty S., Ocampo C.E., et al. Comparison of automated intermittent low volume bolus with continuous infusion for labour epidural analgesia. Anaesthesia and Intensive Care. 2010;38:894–899.
Lim Y., Sia A.T., Ocampo C. Automated regular boluses for epidural analgesia: a comparison with continuous infusion. International Journal of Obstetric Anesthesia. 2005;14:305–309.
Liu E.H., Sia A.T. Rates of caesarean section and instrumental vaginal delivery in nulliparous women after low concentration epidural infusions or opioid analgesia: systematic review. British Medical Journal. 2004;328:1410.
Loepke A.W., Soriano S.G. An assessment of the effects of general anesthesia on developing brain structure and neurocognitive function. Anesthesia and Analgesia. 2008;106:1681–1707.
Lowe N.K. Explaining the pain of active labor: the importance of maternal confidence. Research in Nursing & Health. 1989;12:237–245.
Lowe N.K. The nature of labor pain. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl Nature):S16–S24.
Lucas D.N., Siemaszko O., Yentis S.M. Maternal hypoxaemia associated with the use of Entonox in labour. International Journal of Obstetric Anesthesia. 2000;9:270–272.
Ludwig M., Sabatier N., Bull P.M., et al. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89.
Lundgren I., Dahlberg K. Women’s experience of pain during childbirth. Midwifery. 1998;14:105–110.
Macarthur A.J., Macarthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. American Journal of Obstetrics and Gynecology. 2004;191:1199–1204.
Mardirosoff C., Dumont L., Boulvain M., et al. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG: An International Journal of Obstetrics and Gynaecology. 2002;109:274–281.
Marucci M., Cinnella G., Perchiazzi G., et al. Patient-requested neuraxial analgesia for labor: impact on rates of cesarean and instrumental vaginal delivery. Anesthesiology. 2007;106:1035–1045.
Marx G.F., Greene N.M. Maternal lactate, pyruvate, and excess lactate production during labor and delivery. American Journal of Obstetrics and Gynecology. 1964;90:786–793.
Melzack R. The myth of painless childbirth (the John J. Bonica lecture). Pain. 1984;19:321–337.
Melzack R., Taenzer P., Feldman P., et al. Labour is still painful after prepared childbirth training. Canadian Medical Association Journal. 1981;125:357–363.
Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979.
Merskey H. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249.
Mhyre J.M., Riesner M.N., Polley L.S., et al. A series of anesthesia-related maternal deaths in Michigan, 1985–2003. Anesthesiology. 2007;106:1096–1104.
Moen V., Dahlgren N., Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–1999. Anesthesiology. 2004;101:950–959.
Morley-Forster P.K., Reid D.W., Vandeberghe H. A comparison of patient-controlled analgesia fentanyl and alfentanil for labour analgesia. Canadian Journal of Anaesthesia. 2000;47:113–119.
Nageotte M.P., Larson D., Rumney P.J., et al. Epidural analgesia compared with combined spinal-epidural analgesia during labor in nulliparous women. New England Journal of Medicine. 1997;337:1715–1719.
, National Health Service Maternity Statistics, 2005. Available at, http://www.ic.nhs.uk/statistics-and-data-collections/hospital-care/maternity/nhs-maternity-statistics-2005-06. Accessed March 1, 2011
Nielsen P.E., Erickson J.R., Abouleish E.I., et al. Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labor analgesia: incidence and clinical significance. Anesthesia and Analgesia. 1996;83:742–746.
Niven C.A., Gijsbers K.J. Do low levels of labour pain reflect low sensitivity to noxious stimulation? Social Science & Medicine. 1989;29:585–588.
Ohel G., Gonen R., Vaida S., et al. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. American Journal of Obstetrics and Gynecology. 2006;194:600–605.
Ohno H., Yamashita K., Yahata T., et al. Maternal plasma concentrations of catecholamines and cyclic nucleotides during labor and following delivery. Research Communications in Molecular Pathology and Pharmacology. 1986;51:183–194.
Pan P.H., Bogard T.D., Owen M.D. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. International Journal of Obstetric Anesthesia. 2004;13:227–233.
Pan P.H., Eisenach J.C. The pain of childbirth and its effects on mother and fetus. In: Chestnut D.H., Polley L.S., Tsen L.C., et al, eds. Chestnut’s obstetric anesthesia principles and practice. ed 4. Philadelphia: Mosby-Elsevier; 2010:387–403.
Papka R.E., Hafemeister J., Puder B.A., et al. Estrogen receptor-alpha and neural circuits to the spinal cord during pregnancy. Journal of Neuroscience Research. 2002;70:808–816.
Papka R.E., Shew R.L. Neural input to the uterus and influence on uterine contractility. In: Garfield R.E., Tabb T.N., eds. Control of uterine contractility. London: CRC Press; 1993:375–399.
Pernoll M.L., Metcalfe J., Kovach P.A., et al. Ventilation during rest and exercise in pregnancy and postpartum. Respiration Physiology. 1975;25:295–310.
Ramin S.M., Gambling D.R., Lucas M.J., et al. Randomized trial of epidural versus intravenous analgesia during labor. Obstetrics and Gynecology. 1995;86:783–789.
Reading A.E., Cox D.N. Psychosocial predictors of labor pain. Pain. 1985;22:309–315.
Reid J.G., Simpson N.A., Walker R.G., et al. The carriage of pro-inflammatory cytokine gene polymorphisms in recurrent pregnancy loss. American Journal of Reproductive Immunology. 2001;45:35–40.
Report of the Committee Appointed by the Royal Medical and Chirurgical Society to inquire into the uses and the physiological, therapeutical, and toxical effects of chloroform, as well as into the best mode of its administration, Medico-Chirurgical Transactions 47:323–442
Rigler M.L., Drasner K. Distribution of catheter-injected local anesthetic in a model of the subarachnoid space. Anesthesiology. 1991;75:684–692.
Rigler M.L., Drasner K., Krejcie T.C., et al. Cauda equina syndrome after continuous spinal anesthesia. Anesthesia and Analgesia. 1991;72:275–281.
Rooks J.P. Nitrous oxide for pain in labor—why not in the United States? Birth. 2007;34:3–5.
Ruppen W., Derry S., McQuay H., et al. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399.
Sandner-Kiesling A., Eisenach J.C. Pharmacology of opioid inhibition to noxious uterine cervical distension. Anesthesiology. 2002;97:966–971.
Sandner-Kiesling A., Pan H.L., Chen S.R., et al. Effect of kappa opioid agonists on visceral nociception induced by uterine cervical distension in rats. Pain. 2002;96:13–22.
Schlimpert H. Concerning sacral anaesthesia. Surgery. Gynecology & Obstetrics. 1913;16:488–492.
Scott P.V., Fischer H.B. Spinal opiate analgesia and facial pruritus: a neural theory. Postgraduate Medical Journal. 1982;58:531–535.
Scott-Palmer J., Skevington S.M. Pain during childbirth and menstruation: a study of locus of control. Journal of Psychosomatic Research. 1981;25:151–155.
Segal S., Su M., Gilbert P. The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. American Journal of Obstetrics and Gynecology. 2000;183:974–978.
Sharma S.K., Alexander J.M., Messick G., et al. Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology. 2002;96:546–551.
Sharma S.K., McIntire D.D., Wiley J., et al. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142–148.
Shnider S.M., Abboud T.K., Artal R., et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. American Journal of Obstetrics and Gynecology. 1983;147:13–15.
Shnider S.M., Wright R.G., Levinson G., et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology. 1979;50:524–527.
Simkin P., Bolding A. Update on nonpharmacologic approaches to relieve labor pain and prevent suffering. Journal of Midwifery & Women’s Health. 2004;49:489–504.
Simkin P.P., O’hara M. Nonpharmacologic relief of pain during labor: systematic reviews of five methods. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl Nature):S131–S159.
Simmons S.W., Cyna A.M., Dennis A.T., et al. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database of Systematic Reviews. 2007;3:CD003401.
Simpson W.G., ed. The works of Sir JY Simpson, vol. II. Edinburgh: Anaesthesia. Adam and Charles Black; 1871:199–200.
Skatrud J.B., Dempsey J.A., Kaiser D.G. Ventilatory response to medroxyprogesterone acetate in normal subjects: time course and mechanism. Journal of Applied Physiology. 44, 1978. 393–344, 1978
Strigo I.A., Duncan G.H., Boivin M., et al. Differentiation of visceral and cutaneous pain in the human brain. Journal of Neurophysiology. 2003;89:3294–3303.
Sykes W.S., Essays on the first hundred years of anaesthesia, vol. I. Park Ridge, IL: Wood Library Museum of Anesthesiology; 1982.
Thompson J.F., Roberts C.L., Currie M., et al. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29:83–94.
Tong C., Conklin D., Clyne B.B., et al. Uterine cervical afferents in thoracolumbar dorsal root ganglia express transient receptor potential vanilloid type 1 channel and calcitonin gene–related peptide, but not P2X3 receptor and somatostatin. Anesthesiology. 2006;104:651–657.
Tong C., Ma W., Shin S.W., et al. Uterine cervical distension induces cFos expression in deep dorsal horn neurons of the rat spinal cord. Anesthesiology. 2003;99:205–211.
Tsen L.C., Thue B., Datta S., et al. Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology. 1999;91:920–925.
Ueland K., Hansen J.M. Maternal cardiovascular dynamics. II. Posture and uterine contractions. American Journal of Obstetrics and Gynecology. 1969;103:1–7.
van der Vyver M., Halpern S., Joseph G. Patient-controlled epidural analgesia versus continuous infusion for labour analgesia: a meta-analysis. British Journal of Anaesthesia. 2002;89:459–465.
Van de Velde M., Teunkens A., Hanssens M., et al. Intrathecal sufentanil and fetal heart rate abnormalities: a double-blind, double placebo-controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesthesia and Analgesia. 2004;98:1153–1159.
Vertommen J.D., Vandermeulen E., Van Aken H., et al. The effects of the addition of sufentanil to 0.125% bupivacaine on the quality of analgesia during labor and on the incidence of instrumental deliveries. Anesthesiology. 1991;74:809–814.
Volmanen P., Akural E.I., Raudaskoski T., et al. Remifentanil in obstetric analgesia: a dose-finding study. Anesthesia and Analgesia. 2002;94:913–917.
von Steinbuchel R. Vorlaufige Mittheilung uber die Anwendung von Skopolamin-Morphium-injektionen in der Geburtshilfe. Centralblatt Gynäkologie. 1902;30:1304–1346.
Wall P.D., Melzack R. 1999 Textbook of pain. Edinburgh: Churchill Livingstone; 1999.
Wang F., Shen X., Guo X., et al. Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a five-year randomized controlled trial. Anesthesiology. 2009;111:871–880.
Weiniger C.F., Wand S., Nadjari M., et al. Post-void residual volume in labor: a prospective study comparing parturients with and without epidural analgesia. Acta Anaesthesiologica Scandinavica. 2006;50:1297–1303.
Weintraub S.J., Naulty J.S. Acute abstinence syndrome after epidural injection of butorphanol. Anesthesia and Analgesia. 1985;64:452–453.
Wong C.A., McCarthy R.J., Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesthesia Analgesia. 2011;112:904–911.
Wong C.A., McCarthy R.J., Sullivan J.T., et al. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstetrics and Gynecology. 2009;113:1066–1074.
Wong C.A., Ratliff J.T., Sullivan J.T., et al. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesthesia and Analgesia. 2006;102:904–909.
Wong C.A., Scavone B.M., Peaceman A.M., et al. The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. New England Journal of Medicine. 2005;352:655–665.
Yan T., Liu B., Du D., et al. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesthesia and Analgesia. 2007;104:1246–1250.
Yancey M.K., Pierce B., Schweitzer D., et al. Observations on labor epidural analgesia and operative delivery rates. American Journal of Obstetrics and Gynecology. 1999;180:353–359.
Yeo S.T., Holdcroft A., Yentis S.M., et al. Analgesia with sevoflurane during labour: I. Determination of the optimum concentration. British Journal of Anaesthesia. 2007;98:105–109.
Yeo S.T., Holdcroft A., Yentis S.M., et al. Analgesia with sevoflurane during labour: II. Sevoflurane compared with Entonox for labour analgesia. British Journal of Anaesthesia. 2007;98:110–115.
Bucklin B.A., Hawkins J.L., Anderson J.R., et al. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
Caton D. What a blessing. She had chloroform: the medical and social response to the pain of childbirth from 1800 to the present. New Haven, CT: Yale University Press; 1999.
Chestnut D.H., Bates J.N., Choi W.W. Continuous infusion epidural analgesia with lidocaine: efficacy and influence during the second stage of labor. Obstetrics and Gynecology. 1987;69:323–327.
Chestnut D.H., Vandewalker G.E., Owen C.L., et al. The influence of continuous epidural bupivacaine analgesia on the second stage of labor and method of delivery in nulliparous women. Anesthesiology. 1987;66:774–780.
Conell-Price J., Evans J.B., Hong D., et al. The development and validation of a dynamic model to account for the progress of labor in the assessment of pain. Anesthesia and Analgesia. 2008;106:1509–1515.
Debiec J., Conell-Price J., Evansmith J., et al. Mathematical modeling of the pain and progress of the first stage of nulliparous labor. Anesthesiology. 2009;111:1093–1110.
Eisenach J.C., Pan P.H., Smiley R., et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94.
Halpern S.H., Carvalho B. Patient-controlled epidural analgesia for labor. Anesthesia and Analgesia. 2009;108:921–928.
Halpern S.H., Leighton B.L. Epidural analgesia and the progress of labor. In: Halpern S.H., Douglas M.J., eds. Evidence-based obstetric anesthesia. Oxford: Blackwell; 2005:10–22.
Hawkins J.L., Chang J., Palmer S.K., et al. Anesthesia-related maternal mortality in the United States: 1979–2002. Obstetrics and Gynecology. 2011;117:69–74.
Loepke A.W., Soriano S.G. An assessment of the effects of general anesthesia on developing brain structure and neurocognitive function. Anesthesia and Analgesia. 2008;106:1681–1707.
Melzack R. The myth of painless childbirth (the John J. Bonica lecture). Pain. 1984;19:321–337.
Mhyre J.M., Riesner M.N., Polley L.S., et al. A series of anesthesia-related maternal deaths in Michigan, 1985–2003. Anesthesiology. 2007;106:1096–1104.
Papka R.E., Shew R.L. Neural input to the uterus and influence on uterine contractility. In: Garfield R.E., Tabb T.N., eds. Control of uterine contractility. London: CRC Press; 1993:375–399.
Ruppen W., Derry S., McQuay H., et al. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399.
Simmons S.W., Cyna A.M., Dennis A.T., et al. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database of Systematic Reviews. 2007;3:CD003401.
Ueland K., Hansen J.M. Maternal cardiovascular dynamics. II. Posture and uterine contractions. American Journal of Obstetrics and Gynecology. 1969;103:1–7.
Wong C.A., Ratliff J.T., Sullivan J.T., et al. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesthesia and Analgesia. 2006;102:904–909.
Wong C.A., Scavone B.M., Peaceman A.M., et al. The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. New England Journal of Medicine. 2005;352:655–665.
Yeo S.T., Holdcroft A., Yentis S.M., et al. Analgesia with sevoflurane during labour: I. Determination of the optimum concentration. British Journal of Anaesthesia. 2007;98:105–109.