Cancer Pain Assessment and Syndromes
Approach to Cancer Pain Assessment
Assessment is an ongoing and dynamic process that includes evaluation of the patient’s problems, elucidation of pain syndromes and pathophysiology, and formulation of a comprehensive plan for continuing care. The objectives of cancer pain assessment include (1) accurate characterization of the pain, including the pain syndrome and inferred pathophysiology, and (2) evaluation of the impact of the pain and the role that it plays in the overall suffering of the patient.
Such assessment is predicated on establishment of a trusting relationship with the patient in which the clinician emphasizes relief of pain and suffering as being central to the goal of therapy and encourages open communication about symptoms. Clinicians should not be cavalier about the potential for under-reporting of symptoms; they are frequently described as complaints, and there is a common perception that a “good patient” refrains from complaining (Oldenmenger et al 2009). The prevalence of pain is so great that an open-ended question about the presence of pain should be included at each patient visit in routine oncological practice. If the patient is either unable or unwilling to describe the pain, a family member may need to be questioned to assess the distress or disability of the patient.
Pain Syndromes
Cancer pain syndromes are defined by the association of particular pain characteristics and physical signs with specific consequences of the underlying disease or its treatment. Syndromes are associated with distinct causes and pathophysiologies and have important prognostic and therapeutic implications. Pain syndromes associated with cancer can be either acute or chronic. Whereas the acute pains experienced by cancer patients are usually related to diagnostic and therapeutic interventions, chronic pains are most commonly caused by direct tumor infiltration. Adverse consequences of cancer therapy, including surgery, chemotherapy, and radiation therapy, account for 15–25% of chronic cancer pain problems, and a small proportion of the chronic pain experienced by cancer patients is caused by pathology unrelated to either the cancer or therapy for the cancer.
Pain Characteristics
Evaluation of the characteristics of the pain provides some of the data essential for identification of the syndrome. These characteristics include intensity, quality, distribution, and temporal relationships.
Intensity
Evaluation of pain intensity is pivotal to therapeutic decision making. It indicates the urgency with which relief is needed and influences the selection of analgesic drugs, route of administration, and rate of dose titration (Breivik et al 2008). Furthermore, assessment of pain intensity may help characterize the pain mechanism and underlying syndrome. For example, the pain associated with radiation-induced nerve injury is rarely severe; the occurrence of severe pain in a previously irradiated region therefore suggests the existence of recurrent neoplasm or a radiation-induced second primary neoplasm.
Quality
The quality of the pain often suggests its pathophysiology. Somatic nociceptive pain is usually well localized and described as sharp, aching, throbbing, or pressure-like. Visceral nociceptive pain is generally diffuse and may be gnawing or crampy when caused by obstruction of a hollow viscus or may be aching, sharp, or throbbing when caused by involvement of organ capsules or mesentery. Neuropathic pain may be described as burning, tingling, or shock-like (lancinating).
Distribution
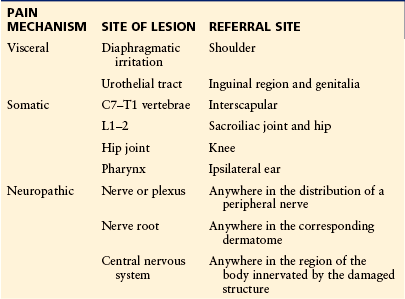
Patients with cancer pain commonly experience pain at more than one site. Distinction between focal, multifocal, and generalized pain may be important in the selection of therapy, such as nerve blocks, radiotherapy, or surgical approaches. The term “focal” pain, which is used to denote a single site, has also been used to depict pain that is experienced in the region of the underlying lesion. Focal pain can be distinguished from pain referred to a site remote from the lesion. Familiarity with pain referral patterns is essential to target appropriate diagnostic and therapeutic maneuvers (Table 73-1). For example, a patient in whom progressive shoulder pain develops without evidence of focal pathology needs to undergo evaluation of the regions above and below the diaphragm to exclude the possibility of referred pain from diaphragmatic irritation.
Temporal Relationships
Cancer-related pain may be acute or chronic. Acute pain is defined by recent onset and a natural history characterized by transience. The pain is often associated with overt pain behavior (such as moaning, grimacing, and splinting), anxiety, or signs of generalized sympathetic hyperactivity, including diaphoresis, hypertension, and tachycardia. Chronic pain has been defined by persistence for 3 or more months beyond the usual course of an acute illness or injury, by a pattern of recurrence at intervals over months or years, or by association with a chronic pathological process. Chronic tumor-related pain is usually insidious in onset, often increases progressively with tumor growth, and may regress with tumor shrinkage. Overt pain behavior and sympathetic hyperactivity are often absent, and the pain may be associated with affective disturbances (anxiety and/or depression) and vegetative symptoms, such as asthenia, anorexia, and sleep disturbance.
Transitory exacerbations of severe pain over a baseline of moderate pain or less may be described as “breakthrough pain” (Portenoy and Hagen 1990). Breakthrough pain is common in both acute and chronic pain states. These exacerbations may be precipitated by volitional actions of the patient (so-called incident pains), such as movement, micturition, coughing, or defecation, or by non-volitional events, such as bowel distention. Spontaneous fluctuations in pain intensity can also occur without an identifiable precipitant.
Inferred Pain Mechanisms
It is increasingly becoming clear that the physiology of neuropathic and nociceptive pain may share common features of peripheral and central sensitization. Nonetheless, clinical inference about the predominant mechanisms that may be responsible for the pain continues to be helpful in evaluation of the pain syndrome and management of cancer pain. The assessment process usually provides the clinical data necessary to infer a predominant pathophysiology. Nociceptive pain, neuropathic pain, and idiopathic pain may be recognized. The basis of these types of pain is described in earlier chapters in this book.
Stepwise Approach to the Evaluation of Cancer Pain
A practical approach to assessment of cancer pain incorporates a stepwise approach that begins with data collection and ends with a clinically relevant formulation.
Data Collection
Careful review of the patient’s past medical history and chronology of the cancer is important to place the pain complaint in context. The pain-related history must elucidate the relevant pain characteristics, as well as the responses of the patient to previous disease-modifying and analgesic therapies. The presence of multiple pain problems is common, and if more than one is reported, each must be assessed independently. Validated pain assessment instruments can provide a format for communication between the patient and health care professionals and can also be used to monitor the adequacy of therapy (see below).
The clinician should assess the consequences of the pain, including impairment in activities of daily living; psychological, familial, and professional dysfunction; disturbed sleep, appetite, and vitality; and financial concerns. The patient’s psychological status, including current level of anxiety or depression, suicidal ideation, and the perceived meaning of the pain, is similarly relevant. Pervasive dysfunctional attitudes, such as pessimism, idiosyncratic interpretation of pain, self-blame, catastrophizing, and perceived loss of personal control, can usually be detected through careful questioning. It is important to assess the patient–family interaction and to note both the type and frequency of pain behavior and the nature of the family response.
Most patients with cancer pain have multiple other symptoms, and the clinician should evaluate the severity and distress caused by each of these symptoms. Symptom checklists and quality-of-life measures may contribute to this comprehensive evaluation (Bruera et al 1991, Portenoy et al 1994b).
Examination
Physical examination, including neurological evaluation, is a necessary part of the initial pain assessment. The need for a thorough neurological assessment is justified by the high prevalence of painful neurological conditions in this population (Gonzales et al 1991, Clouston et al 1992). The physical examination should attempt to identify the underlying etiology of the pain problem, clarify the extent of the underlying disease, and discern the relationship of the pain complaint to the disease.
Provisional Assessment
The information derived from these investigations provides the basis for a provisional pain diagnosis, an understanding of the patient’s disease status, and identification of other concurrent concerns. This provisional diagnosis includes inferences about the pathophysiology of the pain and an assessment of the pain syndrome.
Additional investigations are often required to clarify areas of uncertainty in the provisional assessment. The extent of diagnostic investigation must be appropriate to the patient’s general status and the overall goals of care. For some patients, comprehensive evaluation may require numerous investigations, some targeted at the specific pain problem and others needed to clarify the extent of disease or concurrent symptoms.
The lack of a definitive finding on an investigation should not be used to override a compelling clinical diagnosis. In the assessment of bone pain, for example, plain radiographs provide only a crude assessment of bony lesions, and further investigation with bone scintigraphy, computed tomography (CT), or magnetic resonance imaging (MRI) may be indicated. To minimize the risk for error, the physician ordering the diagnostic procedures should personally review them with the radiologist to correlate the pathological changes detected with the clinical findings.
Pain should be managed during the diagnostic evaluation. Comfort will improve compliance and reduce the distress associated with procedures. No patient should be inadequately evaluated because of poorly controlled pain.
Comprehensive assessment may also require additional evaluation of other physical or psychosocial problems identified during the initial assessment. Expert assistance from other physicians, nurses, social workers, or others may be essential.
Formulation and Therapeutic Planning
The evaluation should enable the clinician to appreciate the nature of the pain, its impact, and concurrent concerns that further undermine quality of life. The findings of this evaluation should be reviewed with the patient and appropriate others. Through candid discussion, current problems can be prioritized to reflect their importance to the patient.
This evaluation may also identify potential outcomes that would benefit from contingency planning. Examples include evaluation of resources for home care, pre-bereavement interventions with the family, and provision of assistive devices in anticipation of compromised ambulation.
Measurement of Pain and Its Impact on Patient Well-being
Pain measurement has an important role in the routine monitoring of cancer patients in treatment settings (Au et al 1994, Rhodes et al 2001). Since observer ratings of symptom severity correlate poorly with patient ratings and are generally an inadequate substitute for patient reporting (Grossman et al 1991), patient self-report is the primary source of information for the measurement of pain.
Pain Measures in Routine Clinical Management
Guidelines from the World Health Organization (1996, 2007), National Comprehensive Cancer Network (2010), Agency for Health Care Policy and Research (1994), and the American Pain Society (2005) recommend the regular use of rating scales to assess pain severity and relief in all patients who commence or change treatments. These recommendations also suggest that clinicians teach patients and families to use assessment tools in the home to promote continuity of pain management in all settings.
The two most commonly used scales for adults are a verbal descriptor scale (i.e., “Which word best describes your pain: none, mild, moderate, severe, or excruciating?”) or a numerical scale (i.e., “On a scale from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain you can imagine, how would you rate your pain?”) (Agency for Health Care Policy and Research 1994, National Comprehensive Cancer Network 2010).
Au and colleagues (1994) demonstrated that use of a simple verbal pain assessment tool improved the caregiver’s understanding of pain status in hospitalized patients. Routinely measuring pain intensity as a fifth vital sign by using a pain scale incorporated into the bedside chart can help make pain a visible parameter that is monitored dynamically (Coyle et al 1993).
Acute Pain Syndromes
Cancer-related acute pain syndromes are most commonly due to diagnostic or therapeutic interventions (Jain and Chatterjee 2010) and generally pose little diagnostic difficulty. Although some tumor-related pain has an acute onset (such as pain from a pathological fracture), most such pain will persist unless effective treatment of the underlying lesion is provided.
Acute Pain Associated with Diagnostic and Therapeutic Interventions
Many investigations and treatments are associated with predictable, transient pain. In patients with a pre-existing pain syndrome, otherwise innocuous manipulations can also precipitate incident pain.
Acute Pain Associated With Diagnostic Interventions
Lumbar Puncture Headache
Lumbar puncture (LP)-related headache is the best characterized acute pain syndrome associated with a diagnostic intervention. This syndrome is typified by the delayed development of a positional headache that is precipitated or markedly exacerbated by an upright posture. Less commonly, dural puncture may also cause back pain, arm pain, thoracic pain, and bowel and bladder dysfunction. The pain is believed to be related to a reduction in cerebrospinal fluid (CSF) volume as a result of ongoing leakage through the defect in the dural sheath and compensatory expansion of the pain-sensitive intracerebral veins (Levine and Rapalino 2001). The incidence of headache is related to the caliber of the LP needle. Risk for LP headache can be reduced by several strategies: when using a regular beveled needle, longitudinal insertion of the needle bevel, which causes less trauma on the longitudinal elastic fibers in the dura, may reduce the incidence of headache (Kempen and Mocek 1997).
Non-traumatic, conically tipped needles with a lateral opening are associated with substantially lower risk for post-LP headaches than regular cannulas are (Corbey et al 1997, Toyka et al 2002). The evidence that recumbency after LP reduces the incidence of this syndrome is controversial (Gonzalez 2000, Ebinger et al 2004).
LP headache, which usually develops hours to several days after the procedure, is typically described as a dull occipital discomfort that may radiate to the frontal region or to the shoulders. The pain is commonly associated with nausea and dizziness. The duration of the headache is usually 1–7 days, and routine management relies on rest, hydration, and analgesics. Persistent headache may necessitate the application of an epidural blood patch. Although a controlled study suggested that prophylactic administration of a blood patch may reduce this complication (Martin et al 1994), the incidence and severity of the syndrome do not warrant such treatment. Severe headache has also been reported to respond to treatment with intravenous or oral caffeine (Morewood 1993).
Transthoracic Needle Biopsy
Transthoracic fine-needle aspiration of an intrathoracic mass is generally a non-noxious procedure. Severe pain has, however, been associated with this procedure when the underlying diagnosis was a neurogenic tumor (Jones et al 1993).
Transrectal Prostatic Biopsy
Transrectal ultrasound-guided prostate biopsy is an essential procedure in the diagnosis and management of prostate cancer. In a prospective study, 16% of patients reported pain of moderate or greater severity and 19% would not agree to undergo the procedure again without anesthesia (Irani et al 1997). Subsequent studies have demonstrated a low rate of severe pain (Sheikh et al 2005). When present, pain may persist up to 4 weeks after the biopsy (Naughton et al 2000). Periprostatic lidocaine infiltration (Gurbuz et al 2010), intrarectal introduction of 2% lidocaine cream (Skriapas et al 2009), and a unilateral pudendal nerve block (Bhomi et al 2007) substantially reduce the pain associated with this procedure.
Mammography Pain
The breast compression associated with mammography can cause moderate and, rarely, severe pain (Sapir et al 2003). The duration of the pain is generally short (Sapir et al 2003). Unless patients are adequately counseled and treated, occasional patients will refuse repeat mammograms because of pain (Leaney and Martin 1992). Pain may be reduced by using a lower level of compression, and in a systematic review the only intervention found to significantly reduce the pain was patient-controlled compression (Miller et al 2002).
Acute Pain Associated with Therapeutic Interventions
Postoperative Pain
Acute postoperative pain is universal unless adequately treated. Unfortunately, undertreatment is endemic despite the availability of adequate analgesic and anesthetic techniques (“Postoperative pain undertreated in the United States” 2008). Guidelines for management have been reviewed (American Society of Anesthesiologists Task Force on Acute Pain Management 2004, NHS Quality Improvement Scotland 2004). Postoperative pain that exceeds the normal duration or severity should prompt careful evaluation for the possibility of infection or other complications.
Radiofrequency Tumor Ablation
Radiofrequency tumor ablation is commonly used for the management of liver metastases. It is also increasingly being used in other settings, including adrenal metastases and renal tumors, as well as for lung, breast, and bone tumors. Percutaneous ablation of liver tumors may be associated with severe right upper quadrant abdominal pain or pain radiating to the right shoulder (Buscarini and Buscarini 2004) in 5–10% of patients. Pain has also been reported after radiofrequency ablation of renal lesions (Baker et al 2007), and it is presumably possible with the use of this approach in other sites.
Cryosurgery
Cryotherapy is commonly used for the management of skin, cervical, and prostatic tumors. Cutaneous cryotherapy typically causes a local painful reaction that decreases in severity over a period of 2–7 days (Thai et al 2004). Cryosurgery of the cervix for the treatment of an intraepithelial neoplasm frequently produces an acute cramping pain syndrome. The severity of the pain is related to the duration of the freeze period and is not diminished with the administration of prophylactic non-steroidal anti-inflammatory drugs (NSAIDs) (Harper 1994).
Other Interventions
Invasive interventions other than surgery are commonly used in cancer therapy and may also result in predictable acute pain syndromes. Examples include the pain associated with tumor embolization techniques (Ryu et al 2003), radio-embolization of liver tumors (Sato et al 2008), and chemical pleurodesis (Shaw and Agarwal 2004).
Acute Pain Associated with Analgesic Techniques
Local Anesthetic Infiltration Pain
Intradermal and subcutaneous infiltration of lidocaine produces a transient burning sensation before the onset of analgesia. This can be modified with the use of buffered solutions (Palmon et al 1998). Other maneuvers, including warming the solution (Martin et al 1996) and slowing the rate of injection, (Scarfone et al 1998) do not diminish injection pain.
Opioid Injection Pain
Intramuscular and subcutaneous injections are painful. When repetitive dosing is required, the intramuscular route of administration is not recommended (Agency for Health Care Policy and Research: Acute Pain Management Panel 1992, Agency for Health Care Policy and Research 1994). The pain associated with subcutaneous injection is influenced by the volume injected and the chemical characteristics of the injectant.
Opioid Headache
Rarely, a reproducible generalized headache develops after opioid administration. Although its cause is not known, speculation suggests that it may be due to opioid-induced release of histamine.
Spinal Opioid Hyperalgesia Syndrome
Intrathecal and epidural injection of high opioid doses is occasionally complicated by pain (typically perineal, buttock, or leg pain), hyperalgesia, and associated manifestations, including segmental myoclonus, piloerection, and priapism. This is an uncommon phenomenon that remits after discontinuation of the infusion (Cartwright et al 1993).
Spinal Injection Pain
Back, pelvic, or leg pain may be precipitated by an epidural injection or infusion. The incidence of this problem has been estimated to be approximately 20% (Naumannet al 1999, Willis and Doleys 1999). It is speculated that it may be caused by compression of an adjacent nerve root by the injected fluid (Buchser and Chedel 1992). Similar problems have been described with intrathecal injections associated with pericatheter fibrosis (Gaertner et al 2003).
Acute Pain Associated with Anticancer Therapies
Acute Pain Related to Chemotherapy Infusion Techniques
Intravenous Infusion Pain: Pain at the site of infusion of a cytotoxic substance is a common problem. Four pain syndromes related to the intravenous infusion of chemotherapeutic agents are recognized: venous spasm, chemical phlebitis, vesicant extravasation, and anthracycline-associated flare. Venous spasm causes pain that is not associated with inflammation or phlebitis and may be modified by the application of a warm compress or reduction of the rate of infusion. Chemical phlebitis can be caused by cytotoxic medications, including amsacrine, dacarbazine, carmustine, and vinorelbine, as well as the infusion of potassium chloride and hyperosmolar solutions (Pucino et al 1988). The pain and linear erythema associated with chemical phlebitis must be distinguished from the more serious complication of vesicant cytotoxic extravasation (Langer 2010). Extravasation of vesicant may produce intense pain followed by desquamation and ulceration. Finally, a brief venous flare reaction is often associated with intravenous administration of the anthracycline doxorubicin. The flare is typically accompanied by local urticaria, and occasional patients report pain or stinging (Vogelzang 1979, Curran et al 1990).
Hepatic Artery Infusion Pain: Cytotoxic infusions into the hepatic artery (for patients with hepatic metastases) are frequently associated with the development of diffuse abdominal pain (Kemeny 1991, Barnett and Malafa 2001). Continuous infusions can lead to persistent pain. In some patients the pain is due to the development of gastric ulceration or erosions (Shike et al 1986) or cholangitis (Batts 1998). If the latter complications do not occur, the pain usually resolves with discontinuation of the infusion. A dose relationship is suggested by the observation that some patients will comfortably tolerate re-initiation of the infusion at a lower dose (Kemeny 1992).
Intraperitoneal Chemotherapy Pain: Transient mild abdominal pain associated with sensations of fullness or bloating is reported by approximately 25% of patients after intraperitoneal chemotherapy (“ACOG Committee Opinion No. 396” 2008). A further 25% of patients reports moderate or severe pain necessitating opioid analgesia or discontinuation of therapy (Almadrones and Yerys 1990). Moderate or severe pain is generally caused by chemical serositis or infection (Jaaback and Johnson 2006). Chemical serositis is a common complication of intraperitoneal administration of the anthracycline agents mitoxantrone and doxorubicin and with paclitaxel (Taxol), but it is relatively infrequent with 5-fluorouracil (5-FU) or cisplatin. Pain may indicate suboptimal drug distribution within the abdominal cavity. Some patients experience discomfort related to sensing abdominal distention or from intercostal nerve irritation. Abdominal pain associated with fever and leukocytosis in blood and peritoneal fluid is suggestive of infectious peritonitis.
Intravesical Chemotherapy or Immunotherapy: Intravesical bacille Calmette-Guérin (BCG) therapy for transitional cell carcinoma of the urinary bladder usually causes a transient bladder irritability syndrome characterized by frequency and/or micturition pain (Shelley et al 2003, 2004). Similarly, intravesical doxorubicin (Matsumura et al 1992), mitomycin C (Shelley et al 2004), and thiotepa (Choe et al 1995) can also cause a painful chemical cystitis. Rarely, intravesical BCG treatment may trigger a painful polyarthritis, sometimes associated will full-blown Reiter’s syndrome (Okamoto et al 2010) or localized regional or systemic infections with abscess formation (Alvarez-Mugica et al 2009).
Acute Pain Associated with Chemotherapy Toxicity
Mucositis: Severe mucositis is an almost invariable consequence of the myeloablative chemotherapy and radiotherapy that precede bone marrow transplantation, but it is less common with standard-intensity therapy (Peterson and Lalla 2010). The cytotoxic agents most commonly associated with mucositis are cytarabine, doxorubicin, etoposide, 5-FU, and methotrexate. Pretreatment oral pathology and poor dental hygiene increase the risk for chemotherapy-induced mucositis. Younger patients have a relatively greater risk for chemotherapy-induced stomatitis, perhaps related to a higher epithelial mitotic rate. Damaged mucosal surfaces may become superinfected with microorganisms such as Candida albicans and herpes simplex virus (Peterson and Lalla 2010). The latter complication is most likely to occur in neutropenic patients, who are also predisposed to systemic sepsis arising from local invasion by aerobic and anaerobic oral flora.
Corticosteroid-Induced Perineal Discomfort: A transient burning sensation in the perineum is described by some patients following the rapid infusion of large doses (20–100 mg) of dexamethasone (Perron et al 2003). Clinical severity is variable and the burning may be severe. Experience suggests that this syndrome can be prevented by slow infusion.
Steroid Withdrawal Pseudorheumatism: Withdrawal of corticosteroids may produce a pain syndrome manifested as diffuse myalgias, arthralgias, and tenderness of muscles and joints. These symptoms occur with rapid or slow withdrawal and may develop in patients taking these drugs for a long or short period. Treatment consists of reinstituting the steroids at a higher dose and withdrawing them more slowly (Weissman et al 1991).
Painful Peripheral Neuropathy: Chemotherapy-induced painful peripheral neuropathy, which is usually associated with vinca alkaloids, cisplatin, oxaliplatin, and paclitaxel, can have an acute course. The vinca alkaloids (particularly vincristine) are also associated with other, presumably neuropathic acute pain syndromes, including pain in the jaw, legs, arms, or abdomen that may last from hours to days (Rosenthal and Kaufman 1974). Vincristine-induced orofacial pain in the distribution of the trigeminal and glossopharyngeal nerves occurs in approximately 50% of patients at the onset of vincristine treatment (McCarthy and Skillings 1992). The pain, which is severe in about half of those affected, generally begins 2–3 days after vincristine administration and lasts for 1–3 days. It is usually self-limited, and if recurrence occurs, it is generally mild (McCarthy and Skillings 1992). Vinorelbine is associated with mild paresthesias in about 20% of patients, but severe neuropathy is rare (Scalone et al 2004). The neuropathy associated with paclitaxel is dose related and generally subacute in onset with resolution after completion of therapy (Postma et al 1995); however, in a proportion of patients it can be severe and persistent (Argyriou et al 2006a).
Headache: Intrathecal methotrexate for the treatment of leukemia or leptomeningeal metastases produces an acute meningitis syndrome in 5–50% of patients (Weiss et al 1974). Headache is the prominent symptom but may be accompanied by vomiting, nuchal rigidity, fever, irritability, and lethargy. Rarely, it may be manifested as a polyradiculopathy (Pascual et al 2008). Symptoms usually begin hours after intrathecal treatment and persist for several days. CSF examination reveals a pleocytosis that may mimic bacterial meningitis. Patients at increased risk for the development of this syndrome include those who have received multiple intrathecal injections and patients undergoing treatment for proven leptomeningeal metastases (Weiss et al 1974). The syndrome tends to not recur with subsequent injections.
Systemic administration of L-asparaginase for the treatment of acute lymphoblastic leukemia produces thrombosis of the cerebral veins or dural sinuses in 1–2% of patients (Kieslich et al 2003). This complication typically occurs after a few weeks of therapy, but its onset may be delayed until after the completion of treatment. Headache is the most common initial symptom, but seizures, hemiparesis, delirium, vomiting, or cranial nerve palsies may also occur. The diagnosis is established by MRI (Kieslich et al 2003).
Trans-retinoic acid therapy, which may be used for the treatment of acute promyelocytic leukemia (APML), can cause a transient severe headache (Avvisati and Tallman 2003). The mechanism may be related to pseudotumor cerebri induced by hypervitaminosis A.
Diffuse Bone Pain: Trans-retinoic acid therapy in patients with APML often produces a syndrome of diffuse bone pain (Pilatrino et al 2005). The pain is generalized, of variable intensity, and closely associated with transient neutrophilia. The latter observation suggests that the pain may be due to marrow expansion.
Taxol-Induced Arthralgia and Myalgia: Administration of paclitaxel generates a syndrome of diffuse arthralgia and myalgia in 10–20% of patients (Loprinzi et al 2007). These symptoms are related to individual doses; associations with the cumulative dose and duration of infusion are less clear. Diffuse joint and muscle pain generally appears 1–2 days after the infusion and lasts for a median of 4–5 days. Pain is commonly located in the back, hips, shoulders, thighs, legs, and feet. The pain is often exacerbated by weight bearing, walking, or tactile contact. Steroids may reduce the tendency for the development of myalgia and arthralgia (Markman et al 1999).
5-Fluorouracil–Induced Anginal Chest Pain: Ischemic chest pain may develop in patients receiving 5-FU (Saif et al 2009). Although the overall risk is generally considered to be low (1–2%), this is debated and some evidence suggests that the risk is as high as 20% (Saif et al 2009). Overall, the risk is higher in patients treated with a continuous infusion than in those receiving bolus therapy and higher in patients with pre-existing ischemic heart disease (Labianca et al 1982). It is widely speculated that coronary vasospasm may be the underlying mechanism (Saif et al 2009). Similar ischemic cardiotoxicity may occur in patients receiving the 5-FU prodrug capecitabine (Monsuez et al 2010).
Palmar–Plantar Erythrodysesthesia Syndrome: Also called acral erythema, hand–foot syndrome, toxic erythema of the palms and soles, and Burgdorf’s syndrome, this painful rash is seen in association with continuously infused 5-FU, capecitabine (Gressett et al 2006), and liposomal doxorubicin (Alberts and Garcia 1997). It has also been reported with paclitaxel (Vukelja et al 1993) and the tyrosine kinase inhibitors sorafenib and sunitinib (Lipworth et al 2009). It is characterized by the development of a tingling or burning sensation in the palms and soles followed by the development of an erythematous rash. Its pathogenesis is unknown. Management often requires discontinuation of therapy, and symptoms may be more manageable with lower doses of therapy. Symptomatic measures are frequently required (Bellmunt et al 1988), and treatment with pyridoxine has been reported to induce resolution of the lesions (Fabian et al 1990).
Post-chemotherapy Gynecomastia: Painful gynecomastia can occur as a delayed complication of chemotherapy. Testis cancer is the most common underlying disorder (Uygur and Ozen 2003), but it has been reported after therapy for other types of cancers as well (Glass and Berenberg 1979, Trump et al 1982). Gynecomastia typically develops after a latency of 2–9 months and resolves spontaneously within a few months. Persistent gynecomastia is occasionally observed (Trump et al 1982).
Chemotherapy-Induced Acute Digital Ischemia: Raynaud’s phenomenon, or transient ischemia of the toes, is a common complication of cis-platinum, vinblastine, and bleomycin (PVB) treatment of testicular cancer (Aass et al 1990). Rarely, irreversible digital ischemia leading to gangrene has been reported after bleomycin (Elomaa et al 1984). Capecitabine and vincristine have been implicated in case reports (Gottschling et al 2004, Coward et al 2005).
Chemotherapy-Induced Tumor Pain: Pain at the site of tumor is reported to occur in some patients (7%) after treatment with vinorelbine. Typically, the pain begins within a few minutes of the vinorelbine infusion, is moderate to severe in intensity, and requires analgesic therapy. Premedication with ketorolac may prevent recurrence in some cases (De Marco et al 1999).
Acute Pain Associated with Hormonal Therapy
Luteinizing Hormone–Releasing Factor Tumor Flare in Prostate Cancer: Initiation of luteinizing hormone–releasing factor (LHRF) hormonal therapy for prostate cancer produces a transient symptom flare in 5–25% of patients (Chrisp and Goa 1991). The flare is presumably caused by an initial stimulation of release of luteinizing hormone before suppression is achieved. The syndrome is typically manifested as an exacerbation of bone pain or urinary retention; spinal cord compression and sudden death have been reported (Thompson et al 1990). Symptom flare is usually observed within the first week of therapy and lasts 1–3 weeks in the absence of androgen antagonist therapy. Co-administration of an androgen antagonist during the initiation of LHRF agonist therapy can prevent this phenomenon (Labrie et al 1987).
Hormone-Induced Pain Flare in Breast Cancer: Any hormonal therapy for metastatic breast cancer can be complicated by the sudden onset of diffuse musculoskeletal pain commencing within hours to weeks of initiation of therapy (Plotkin et al 1978). Other manifestations of this syndrome include erythema around cutaneous metastases, changes in liver function study results, and hypercalcemia. It may be associated with increased metabolic activity at tumor sites, which can be detected with positron emission tomography (PET) (Mortimer et al 2001). The underlying mechanism is not understood. and the flare reaction is often predictive of later tumor response to the hormonal therapy.
Aromatase Inhibitor–Induced Arthralgia: The aromatase inhibitor medications used in hormonal therapy for breast cancer may cause a multifocal arthralgia syndrome in 10–20% of patients (Chlebowski 2009). It is most commonly manifested as early morning stiffness and hand/wrist pain. Pain intensity is variable and in some patients it precludes or interferes in daily activities. Occasionally, it is reason to discontinue treatment because of severe symptoms (Chlebowski 2009). The possible mechanisms are unclear, but an association with the development of osteoporosis has been noted (Muslimani et al 2009). Treatment options for arthralgia (primarily NSAIDs) are often inadequate, and areas of active research include high-dose vitamin D and new targeted therapies to inhibit bone loss.
Acute Pain Associated with Immunotherapy
Interferon-Induced Acute Pain: Virtually all patients treated with interferon experience an acute syndrome consisting of fever, chills, myalgias, arthralgias, and headache (Quesada et al 1986). The syndrome usually begins shortly after initial dosing and frequently improves with continued administration of the drug (Quesada et al 1986). Doses of 1–9 million units of interferon alfa are generally tolerated, but doses of 18 million units or higher usually produce moderate to severe toxicity (Quesada et al 1986). Acetaminophen pretreatment is often useful in ameliorating these symptoms.
Acute Pain Associated with Bisphosphonates
Bisphosphate-Induced Bone Pain: Bisphosphonates are widely used in the care of patients with bony metastases. Infusion of bisphosphonates is commonly associated with the development of multifocal bone pain and/or myalgia. Typically, pain occurs within 24 hours of infusion and may last up to 3 days. The intensity of the pain is variable and it may be severe. The condition is self-limited but may require analgesic therapy (Lipton 2007).
Acute Pain Associated with Growth Factors
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) commonly produce mild to moderate bone pain and constitutional symptoms such as fever, headache, and myalgias during the period of administration (Veldhuis et al 1995). In studies of G-CSF in dose-dense chemotherapy, the prevalence of pain was 20–30% (Venturini et al 2005). Pain intensity is variable and may be severe. Co-administration of dexamethasone may reduce the prevalence and severity of bone pain (Heuft et al 2004).
Subcutaneous administration of recombinant human epoetin alfa (r-HuEPO alfa) is associated with pain at the injection site in about 40% of cases (Frenken et al 1991). Subcutaneous injection of r-HuEPO alfa is more painful than r-HuEPO beta (Morris et al 1994). Epoetin alfa injection pain can be reduced by dilution of the vehicle with benzyl alcohol saline, reduction of the volume of vehicle to 1.0–0.1 mL (Frenken et al 1994), or the addition of lidocaine (Alon et al 1994).
Acute Pain Associated with Radiotherapy
Incident pain can be precipitated by transport and positioning of the patient for radiotherapy. Other pain can be caused by acute radiation toxicity, which is most commonly associated with inflammation and ulceration of skin or mucous membranes within the radiation port. The syndrome produced is dependent on the involved field: head and neck irradiation can cause stomatitis or pharyngitis, treatment of the chest and esophagus can cause esophagitis, and pelvic therapy can cause proctitis, cystitis–urethritis, vaginal ulceration, or radiation dermatitis.
Oropharyngeal Mucositis: Radiotherapy-induced mucositis is invariable with doses higher than 1000 cGy, and ulceration is common at doses above 4000 cGy. Although the severity of the associated pain is variable, it is often severe enough to interfere with oral alimentation. Painful mucositis can persist for several weeks after completion of the treatment (Scully et al 2004).
Acute Radiation Enteritis and Proctocolitis: Acute radiation enteritis occurs in as many as 50% of patients undergoing abdominal or pelvic radiotherapy. Involvement of the small intestine can give rise to cramping abdominal pain associated with nausea and diarrhea (Andreyev et al 2005). Pelvic radiotherapy can cause painful proctocolitis, with tenesmic pain being associated with diarrhea, mucous discharge, and bleeding (Babb 1996, Andreyev et al 2005). These complications typically resolve shortly after completion of therapy but may have a slow resolution over a period of 2–6 months (Andreyev et al 2005). Acute enteritis is predictive of increased risk for late-onset radiation enteritis (see below).
Early-Onset Brachial Plexopathy: Transient brachial plexopathy has been described in breast cancer patients immediately following radiotherapy involving the chest wall and adjacent nodal areas (Salner et al 1981, Pierce et al 1992) and after mantle radiotherapy for Hodgkin’s disease (Churn et al 2000). In retrospective studies the incidence of this phenomenon has been variably estimated to be 1.4–20% (Salner et al 1981, Pierce et al 1992); clinical experience suggests that lower estimates are more accurate. Median latency until the development of symptoms was 4.5 months (range, 3–14 months) in one survey (Salner et al 1981). Paresthesias are the most common initial symptoms, with pain and weakness occurring less frequently. The syndrome is self-limited and is not predictive of the subsequent development of delayed-onset, progressive plexopathy.
Subacute Radiation Myelopathy: Subacute radiation myelopathy is an uncommon phenomenon that may occur following radiotherapy for extraspinal tumors (Ang and Stephens 1994, Schultheiss 1994). It is most frequently found to involve the cervical cord after radiation treatment of head and neck cancers and Hodgkin’s disease. In the latter case, shock-like pains develop in the neck and are precipitated by neck flexion (Lhermitte’s sign); such pain may radiate down the spine and into one or more extremities. The syndrome usually begins weeks to months after completion of radiotherapy and typically resolves over a period of 3–6 months (Ang and Stephens 1994).
Radiopharmaceutical-Induced Pain Flare: Strontium 89, rhenium 186, hydroxyethylidene diphosphonate, and samarium 153 are systemically administered β-emitting calcium analogues that are taken up by bone in areas of osteoblastic activity and may help relieve the pain caused by blastic bony metastases (McEwan 1997). A “flare” response, characterized by transient worsening of pain 1–2 days after administration, occurs in 15–20% of patients (Robinson et al 1995). This flare usually resolves after 3–5 days, and a good analgesic response subsequently develops in most affected patients (Robinson et al 1995).
Acute Pain Associated with Infection
A significantly increased incidence of acute herpetic neuralgia occurs in cancer patients, especially those with hematological or lymphoproliferative malignancies and those receiving immunosuppressive therapies (Portenoy et al 1986, Rusthoven et al 1988, Insinga et al 2005). The pain, which may be continuous or lancinating, usually resolves within 2 months (Galer and Portenoy 1991). Pain persisting beyond this interval is referred to as post-herpetic neuralgia (PHN) (see below). Patients with active tumor are more likely to have a disseminated infection (Rusthoven et al 1988). In those predisposed by chemotherapy, the infection usually develops less than 1 month after the completion of treatment. The dermatomal location of the infection is often associated with the site of the malignancy (Rusthoven et al 1988). The infection also occurs twice as frequently in previously irradiated dermatomes as in non-radiated areas (Dunst et al 2000).
Acute Pain Associated with Vascular Events
Thrombosis is the most frequent complication and the second most common cause of death in patients with overt malignant disease (Battinelli and Ansell 2005). Thrombotic episodes may precede the diagnosis of cancer by months or years and represent a potential marker for occult malignancy (Prandoni and Piccioli 2006). Postoperative deep vein thrombosis (DVT) is more frequent in patients who undergo surgery for malignant diseases than for other disorders, and both chemotherapy and hormone therapy are associated with increased risk for thrombosis (Battinelli and Ansell 2005).
Possible prothrombic factors in cancer include the capacity of tumor cells and their products to interact with platelets, clotting and fibrinolytic systems, endothelial cells, and tumor-associated macrophages. Cytokine release, acute phase reaction, and neovascularization may contribute to vivo clotting activation (Sood 2009). Data from a very large cohort of 66,329 cancer patients demonstrated that bone, ovarian, brain, and pancreatic cancers are associated with the highest incidence of thrombosis and that distant metastases, chemotherapy, and hormonal therapy all lead to 1.5–2.5 times greater risk (Blom et al 2006).
Lower Extremity Deep Venous Thrombosis
Pain and swelling are the most common initial features of lower extremity DVT (Criado and Burnham 1997). The pain is variable in severity and often mild. It is commonly described as a dull cramp or diffuse heaviness. The pain most frequently affects the calf but may involve the sole of the foot, the heel, the thigh, the groin, or the pelvis. Pain usually increases on standing and walking. On examination, suggestive features include swelling, warmth, dilatation of superficial veins, tenderness along venous tracts, and pain induced by stretching (Criado and Burnham 1997).
Rarely, tissue ischemia or frank gangrene may develop, even without arterial or capillary occlusion; this syndrome is called phlegmasia cerulea dolens. It is most commonly seen in patients with underlying neoplasm (Lorimer et al 1994) and is characterized by severe pain, extensive edema, and cyanosis of the legs. Gangrene can occur unless the venous obstruction is relieved. Intravenous thrombolytic therapy (Tardy et al 2006) may be more effective than the traditional treatment of anticoagulation and thrombectomy (Perkins et al 1996). Until recently, the mortality rate in patients with ischemic venous thrombosis was about 30–40%, the cause of death usually being the underlying disease or pulmonary emboli (Vysetti et al 2009).
Upper Extremity Deep Venous Thrombosis
The three major clinical features of upper extremity venous thrombosis are edema, dilated collateral circulation, and pain (Flinterman et al 2008). Approximately two-thirds of patients have arm pain. Among patients with cancer the most common causes are central venous catheterization and extrinsic compression by tumor (Flinterman et al 2008). Although thrombosis secondary to intrinsic damage usually responds well to anticoagulation alone and rarely causes persistent symptoms, when extrinsic obstruction is the cause, persistent arm swelling and pain are commonplace (Flinterman et al 2008).
Superior Vena Cava Obstruction
Superior vena cava (SVC) obstruction is most frequently due to extrinsic compression by enlarged mediastinal lymph nodes (Wan and Bezjak 2009). In contemporary series, lung cancer and lymphomas are the most commonly associated conditions. Increasingly, thrombosis of the SVC is being caused by intravascular devices (Rice et al 2006), particularly with left-sided ports and when the catheter tip lies in the upper part of the vena (Puel et al 1993). Patients usually have facial swelling and dilated neck and chest wall veins. Chest pain, headache, and mastalgia are less common manifestations.
Acute Mesenteric Vein Thrombosis
Acute mesenteric vein thrombosis is most commonly seen in patients with hypercoagulability states. Rarely, it has been associated with extrinsic venous compression by malignant lymphadenopathy (Traill and Nolan 1997) or with extension of venous thrombosis (Vigo et al 1980), occurs as a result of an iatrogenic hypercoagulable state (Sahdev et al 1985), or is an adverse effect of pancreatic resection (Zyromski and Howard 2008).
Superficial Thrombophlebitis
Superficial thrombophlebitis is more common in patients with cancer and may be an initial symptom of cancer (van Doormaal et al 2010). It is characterized by the development of a palpable tender cord in the course of a superficial vein, often associated with erythema of the overlying skin. Duplex ultrasound should be considered to rule out occult DVT, particularly when the greater or lesser saphenous veins are involved (Blumenberg et al 1998).
Trousseau’s syndrome, or migratory thrombophlebitis, is a rare condition characterized by a recurrent and migratory pattern and involvement of superficial veins, frequently in unusual sites such as the arm or chest (Varki 2007).
Chronic Pain Syndromes
Most chronic cancer-related pain is caused directly by the tumor. Data from the largest prospective survey of cancer pain syndromes revealed that almost one-quarter of patients experienced two or more pains. Over 90% of patients had one or more tumor-related pains and 21% had one or more pains caused by cancer therapies. Somatic pain (71%) was more common than neuropathic (39%) or visceral (34%) pain (Caraceni and Portenoy 1999). Bone pain and compression of neural structures are the two most common causes (Daut and Cleeland 1982, Foley 1987, Banning et al 1991, Grond et al 1996, Twycross et al 1996).
Bone Pain
Bone metastases are the most common cause of chronic pain in cancer patients. Cancers of the lung, breast, and prostate most often metastasize to bone, but any tumor type may be complicated by painful bony lesions. Although bone pain is usually associated with direct tumor invasion of bony structures, more than 25% of patients with bony metastases are pain free (Wagner 1984), and patients with multiple bony metastases typically report pain in only a few sites. The pathophysiology of bone pain is reviewed in Chapter 69.
Differential Diagnosis
Bone pain secondary to metastatic tumor needs to be differentiated from less common causes. Non-neoplastic causes in this population include osteoporotic fractures, including those associated with multiple myeloma; focal osteonecrosis, which may be idiopathic or related to chemotherapy, corticosteroids, or radiotherapy (see below); and osteomalacia (Shane et al 1997). Rarely, paraneoplastic osteomalacia, which is associated with elevated levels of fibroblast growth factor 23, can mimic multiple metastases (Jan de Beur 2005).
Multifocal or Generalized Bone Pain
Bone pain may be focal, multifocal, or generalized. Multifocal bone pain is most commonly experienced by patients with multiple bony metastases. A generalized pain syndrome is occasionally produced by replacement of bone marrow (Hesselmann et al 2002, Lin et al 2002). This bone marrow replacement syndrome has been observed in patients with hematogenous malignancies (Beckers et al 2002) and, less commonly, with solid tumors (Cohen et al 1982, Wong et al 1993) and brain tumors (Rajagopalan et al 2005). This syndrome can occur in the absence of abnormalities on bone scintigraphy or radiography, thus increasing the difficulty of diagnosis. It is best demonstrated on MRI (Ollivier et al 2006).
Vertebral Syndromes
The vertebrae are the most common sites of bony metastases. More than two-thirds of vertebral metastases are located in the thoracic spine; lumbosacral and cervical metastases account for approximately 20% and 10%, respectively. Multiple-level involvement is common and occurs in more than 85% of patients (Constans et al 1983). Early recognition of pain syndromes caused by tumor invasion of vertebral bodies is essential since pain usually precedes compression of adjacent neural structures and prompt treatment of the lesion may prevent the subsequent development of neurological deficits. Several factors often confound accurate diagnosis: referral of pain is common, and the associated symptoms and signs can mimic a variety of other disorders, both malignant (e.g., paraspinal masses) and non-malignant.
Atlantoaxial Destruction and Odontoid Fracture
Nuchal or occipital pain is the typical result of destruction of the atlas or fracture of the odontoid process. Pain often radiates over the posterior aspect of the skull to the vertex and is exacerbated by movement of the neck, particularly flexion (Lakemeier et al 2009). Pathological fracture may result in secondary subluxation with compression of the spinal cord at the cervicomedullary junction. This complication is usually insidious and may begin with symptoms or signs in one or more extremity. Typically, there is early involvement of the upper extremities and the occasional appearance of so-called pseudo-levels suggestive of more caudal spinal lesions; these deficits can slowly progress to involve sensory, motor, and autonomic function in the extremities (Sundaresan et al 1981).
C7–T1 Syndrome
Invasion of the C7 or T1 vertebra can result in pain referred to the interscapular region. These lesions may be missed if radiographic evaluation is mistakenly targeted to the painful area caudal to the site of damage. Additionally, visualization of the appropriate region on routine radiographs may be inadequate because of obscuration by overlying bone and mediastinal shadows. Patients with interscapular pain should therefore undergo radiography of both the cervical and the thoracic spine.
T12–L1 (Thoracolumbar Junction) Syndrome
A T12 or L1 vertebral lesion can refer pain to the ipsilateral iliac crest or the sacroiliac joint. Imaging procedures directed at pelvic bones can miss the source of the pain.
Sacral Syndrome
Severe focal pain radiating to buttocks, perineum, or posterior of the thighs may accompany destruction of the sacrum (Nader et al 2004). The pain is often exacerbated by sitting or lying and is relieved by standing or walking (Payer 2003). The neoplasm can spread laterally to involve muscles that rotate the hip (e.g., the pyriformis muscle). This may produce severe incident pain induced by motion of the hip or a malignant “pyriformis syndrome” characterized by buttock or posterior leg pain that is exacerbated by internal rotation of the hip. Local extension of the tumor mass may also involve the sacral plexus (see below).
Back Pain and Epidural Compression
Epidural compression (EC) of the spinal cord or cauda equina is the second most common neurological complication of cancer and occurs in up to 10% of patients (Posner 1987). In a large retrospective series, 0.23% of cancer patients were found to have EC at diagnosis of their disease, and 2.5% of patients dying of cancer had at least one admission for cord compression in the 5 years preceding death (Loblaw et al 2003). Breast, lung, and prostate cancer each accounts for 20–25% of events (Loblaw et al 2003, 2005). Most EC is caused by posterior extension of vertebral body metastasis to the epidural space. Occasionally, EC is due to tumor extension from the posterior arch of the vertebra or infiltration of a paravertebral tumor through the intervertebral foramen.
Untreated, EC leads inevitably to neurological damage. Effective treatment can potentially prevent these complications. The most important determinant of the efficacy of treatment is the degree of neurological impairment at the time that therapy is initiated. Seventy-five percent of patients who begin treatment while ambulatory remain so; the efficacy of treatment declines to 30–50% in those who begin treatment while markedly paretic and is 10–20% in those who are plegic (Prasad and Schiff 2005). Nonetheless, delays in diagnosis are commonplace (Levack et al 2002).
Back pain is the initial symptom in almost all patients with EC (Posner 1987, Ruckdeschel 2005), and in 10% it is the only symptom at the time of diagnosis (Greenberg et al 1980). Since pain usually precedes neurological signs by a prolonged period, it should be viewed as a potential indicator of EC, which can lead to treatment at a time when a favorable response is most likely. Back pain, however, is a non-specific symptom that can result from bony or paraspinal metastases without epidural encroachment, from retroperitoneal or leptomeningeal tumor, from epidural lipomatosis secondary to steroid administration, or from a large variety of other benign conditions.
Clinical Features of Epidural Extension
Some pain characteristics are particularly suggestive of epidural extension (Helweg-Larsen and Sorensen 1994). Rapid progression of back pain in a crescendo pattern is an ominous occurrence (Rosenthal et al 1992). Radicular pain, which can be constant or lancinating, has similar implications (Helweg-Larsen and Sorensen 1994). It is generally unilateral in the cervical and lumbosacral regions and bilateral in the thorax, where it is often experienced as a tight, belt-like band across the chest or abdomen (Helweg-Larsen and Sorensen 1994). The likelihood of EC is also greater when back or radicular pain is exacerbated by recumbency, coughing, sneezing, or straining (Ruff and Lanska 1989). Other types of referred pain are also suggestive, including Lhermitte’s sign (Ventafridda et al 1991) and central pain from spinal cord compression, which is usually perceived some distance below the site of the compression and is typically a poorly localized, non-dermatomal dysesthesia (Posner 1987).
Weakness, sensory loss, autonomic dysfunction, and reflex abnormalities generally occur after a period of progressive pain (Helweg-Larsen and Sorensen 1994). Weakness may begin segmentally if related to nerve root damage or in a multisegmental or pyramidal distribution if the cauda equina or spinal cord, respectively, is injured.
The rate of progression of weakness is variable; in the absence of treatment, paralysis will develop within 7 days of the onset of weakness in one-third of patients (Barron et al 1959). Without effective treatment, sensory abnormalities, which may also begin segmentally, may ultimately evolve to a sensory level, with complete loss of all sensory modalities below the site of injury. The upper level of sensory findings may correspond to the location of the epidural tumor or be below it by many segments (Helweg-Larsen and Sorensen 1994). Ataxia without pain is the initial manifestation of EC in 1% of patients; this finding is presumably due to early involvement of the spinocerebellar tracts (Gilbert et al 1978). Bladder and bowel dysfunction occurs late, except in patients with a conus medullaris lesion, who may have acute urinary retention and constipation without preceding motor or sensory symptoms (Helweg-Larsen and Sorensen 1994).
Other features that may be evident on examination of patients with EC include scoliosis, asymmetrical wasting of the paravertebral musculature, and gibbus (palpable step in the spinous processes). Spinal tenderness on percussion, which may be severe, often accompanies the pain.
Imaging Modalities
Definitive imaging of the epidural space confirms the existence of EC (and thereby indicates the necessity and urgency of treatment), defines the appropriate radiation portals, and determines the extent of epidural encroachment (which influences the prognosis and may alter the therapeutic approach). Options for definitive imaging include MRI, myelography and CT-myelography, or spiral CT without myelographic contrast enhancement.
MRI offers accurate imaging of the vertebrae and intraspinal and paravertebral structures. When available, it is generally the preferred mode of evaluation (Loblaw et al 2005). Whenever possible, total spine imaging should be performed since multiple-level involvement is common and other sites may be clinically occult. In a study of 65 patients with cord compression, 32 (49%) had involvement of multiple levels, and of these, 28 (66%) were clinically occult (Heldmann et al 1997). MRI has multiple advantages: metastases can be distinguished from other pathological processes involving the axial skeleton, epidural and intradural spaces, and spinal cord. This is particularly true for bacterial abscesses, leptomeningeal carcinomatosis, intradural extramedullary or, rarely, intramedullary metastases or primary tumors, and infectious or inflammatory myelitis.
Post-myelographic CT is a useful tool that provides additional information about the vertebral and paravertebral structures. It can usually define the extent of the cord compression (Boesen et al 1991) and may help in distinguishing between cord compression caused by displaced bony fragments and soft tissue extension and in identifying paraspinal tumors with extension through the intervertebral foramina (Helweg-Larsen et al 1992). Besides immediate patient discomfort, myelography is often complicated by post-procedure side effects that include back pain, headache, vomiting, seizures, and adverse neurobehavioral reactions. Risk for adverse effects is related to the gauge and type of needle used (Wilkinson and Sellar 1991), the contrast medium (Killebrew et al 1983), and the anatomy of the EC.
Similar to MRI, CT is non-invasive and provides excellent visualization of the vertebrae, vertebral structural integrity, paravertebral soft tissues, and vertebral foramina. The improved resolution observed with contemporary spiral techniques facilitates very clear imaging of the spinal canal contents.
Pain Syndromes of the Bony Pelvis and Hip
The pelvis and hip are common sites of metastatic involvement. Lesions may involve any of the three anatomical regions of the pelvis (ischiopubic, iliosacral, or periacetabular), the hip joint itself, or the proximal end of the femur (Papagelopoulos et al 2007). The weight-bearing function of these structures, essential for normal ambulation, contributes to the propensity of disease at these sites to cause incident pain with walking and weight bearing.
Hip Joint Syndrome
Tumor involvement of the acetabulum or head of the femur typically produces localized hip pain that is aggravated by weight bearing and movement of the hip (Singh et al 2006). The pain may radiate to the knee or medial aspect of the thigh, and occasionally, pain is limited to these structures (Sim 1992). Medial extension of an acetabular tumor can involve the lumbosacral plexus as it traverses the pelvic sidewall. Evaluation of this region is best accomplished with CT or MRI, both of which can demonstrate the extent of bony destruction and adjacent soft tissue involvement more sensitively than possible with other imaging techniques. Important differential diagnoses include avascular necrosis, radicular pain (usually L1), or occasionally, occult infections.
Acrometastasis
Acrometastasis, or metastasis in the hands and feet, is rare and often misdiagnosed or overlooked. In the feet, the larger bones containing higher amounts of red marrow, such as the os calcis or talus, are usually involved (Kouvaris et al 2005, Bahk et al 2006). Symptoms may be vague and can mimic other conditions such as osteomyelitis, gouty rheumatoid arthritis, Reiter’s syndrome, Paget’s disease, osteochondral lesions, and ligamentous sprains.
Arthritis
Hypertrophic Pulmonary Osteoarthropathy
Hypertrophic pulmonary osteoarthropathy (HPOA) is a paraneoplastic syndrome that incorporates clubbing of the fingers, periostitis of long bones, and occasionally, a rheumatoid-like polyarthritis (Martinez-Lavin 1997). Periosteitis and arthritis can produce pain, tenderness, and swelling in the knees, wrists, and ankles. The onset of symptoms is usually subacute, and it may precede discovery of the underlying neoplasm by several months. It is most commonly associated with non–small cell lung cancer with an incidence in this population of 1–5% (Ito et al 2010, Izumi et al 2010). Less commonly it may be associated with benign mesothelioma, pulmonary metastases from other sites, smooth muscle tumors of the esophagus, breast cancer, and metastatic nasopharyngeal cancer. HPOA is diagnosed on the basis of physical findings, radiological appearance, and radionuclide bone scan (Martinez-Lavin 1997). Therapeutic approaches have recently been subjected to a systematic review (Nguyen and Hojjati 2011). Effective antitumor therapy is sometimes associated with regression of symptoms (Hung et al 2000, Kishi et al 2002); bisphosphonate therapy may help relieve the symptoms (Suzuma et al 2001, Amital et al 2004), and there are case reports of resolution after vagotomy (Treasure 2006, Ooi et al 2007).
Other Polyarthritides
Rarely, rheumatoid arthritis, systemic lupus erythematosus, and an asymmetrical polyarthritis may occur as paraneoplastic phenomena that resolve with effective treatment of the underlying disease (Racanelli et al 2008). A syndrome of palmar–plantar fasciitis and polyarthritis characterized by palmar and digital polyarticular painful capsular contractions has been associated with ovarian (Giannakopoulos et al 2005), breast (Saxman and Seitz 1997), and gastric (Enomoto et al 2000) cancer.
Muscle Pain
Persistent muscle cramps in cancer patients are usually caused by an identifiable neural, muscular, or biochemical abnormality (Siegal 1991). In one series of 50 patients, 22 had peripheral neuropathy, 17 had root or plexus pathology (including 6 with leptomeningeal metastases), 2 had polymyositis, and 1 had hypomagnesemia. In this series, muscle cramps were the initial symptom of recognizable and previously unsuspected neurological dysfunction in 64% (27 of 42) of the identified causes (Steiner and Siegal 1989). Cramps have been reported as an adverse effect of imatinib (Breccia et al 2005), goserelin (Ernst et al 2004), and vincristine (Haim et al 1994).
Skeletal Muscle Tumors
Soft tissue sarcomas arising from fat, fibrous tissue, or skeletal muscle are the most common tumors involving the skeletal muscles. Skeletal muscle is one of the most unusual sites of metastasis from any malignancy (Cekinmez et al 2009). They occur disproportionally at sites of previous muscle trauma (Magee and Rosenthal 2002). Lesions are usually painless but may be accompanied by persistent ache.
Headache and Facial Pain
Headache in cancer patients results from traction, inflammation, or infiltration of pain-sensitive structures in the head or neck. Early evaluation with appropriate imaging techniques may identify the lesion and allow prompt treatment, which may reduce pain and prevent the development of neurological deficits (Vecht et al 1992).
Intracerebral Tumor
The prevalence of headache in patients with brain metastases or primary brain tumors is 60–90% (Kirby and Purdy 2007), and headache is frequently the first symptom of patients with brain tumors or metastases (Kirby and Purdy 2007) and is often associated with dizziness (Hird et al 2009). Among 183 patients with new-onset chronic headache as an isolated symptom, investigation revealed underlying tumor in 15 (Vazquez-Barquero et al 1994). The headache is presumably produced by traction on pain-sensitive vascular and dural tissue. Patients with multiple metastases and those with posterior fossa metastases are more likely to report this symptom (Kirby and Purdy 2007).
The pain may be focal, overlying the site of the lesion, or generalized. Headache has lateralizing value, especially in patients with supratentorial lesions (Suwanwela et al 1994, Argyriou et al 2006b, Kirby and Purdy 2007). Posterior fossa lesions often cause a bifrontal headache. The quality of the headache is usually throbbing or steady, and the intensity is generally mild to moderate (Suwanwela et al 1994, Argyriou et al 2006b).
In children headache is the most common initial symptom of brain tumors (Wilne et al 2006). Clinical features predictive of underlying tumor include sleep-related headache, headache in the absence of a family history of migraine, vomiting, absence of visual symptoms, headache of less than 6 months’ duration, confusion, and abnormal findings on neurological examination (Wilne et al 2006). The headache is frequently worse in the morning and is exacerbated by stooping, sudden head movement, or Valsalva maneuvers (cough, sneeze, or strain) (Suwanwela et al 1994).
Leptomeningeal Metastases
Leptomeningeal metastases, which are characterized by diffuse or multifocal involvement of the subarachnoid space by metastatic tumor, occur in 1–8% of patients with systemic cancer (Grossman and Krabak 1999, Taillibert et al 2005). Non-Hodgkin’s lymphoma and acute lymphocytic leukemia both demonstrate a predilection for meningeal metastases (Nolan and Abrey 2005); the incidence is lower for solid tumors alone (Bruno and Raizer 2005). Of solid tumors, adenocarcinomas of the breast and small cell lung cancer predominate (Bruno and Raizer 2005).
Leptomeningeal metastases are associated with focal or multifocal neurological symptoms or signs that may involve any level of the neuraxis. The most common initial symptoms are headache, cranial nerve palsies (Yamanaka et al 2011), and radicular pain in the low back region and buttocks (van Oostenbrugge and Twijnstra 1999). More than one-third of patients have evidence of cranial nerve damage, including double vision, hearing loss, facial numbness, and decreased vision (Wasserstrom et al 1982, Yamanaka et al 2011). Less common features include seizures, papilledema, hemiparesis, ataxic gait, and confusion (Balm and Hammack 1996).
The headache is variable and may be associated with changes in mental status (e.g., lethargy, confusion, or loss of memory), nausea, vomiting, tinnitus, or nuchal rigidity. Pain that resembles cluster headache (DeAngelis and Payne 1987) or glossopharyngeal neuralgia with syncope (Sozzi et al 1987) has also been reported.
Increasingly, gadolinium-enhanced MRI of the neuraxis is the investigation of choice when leptomeningeal metastases are suspected. When compared with CSF cytological examination, MRI is probably more sensitive; however, it is less specific because false-positive cytological findings are rare (Straathof et al 1999). If gadolinium-enhanced MRI is non-diagnostic and if the pain distribution indicates spinal involvement, sensitivity is enhanced by performing an examination of the whole spine. Spinal MRI may be positive in almost 50% of patients without clinical findings related to the spinal region and in 60% of patients with negative CSF cytology (Gomori et al 1998). Additionally, findings of contrast enhancement of the basilar cisterns, parenchymal metastases, hydrocephalus without a mass lesion, or spinal subarachnoid masses or enhancement may all have therapeutic implications (Grossman and Krabak 1999).
The diagnosis of leptomeningeal metastases may be confirmed through analysis of CSF, which may reveal elevated pressure, increased protein, depressed glucose, and/or lymphocytic pleocytosis. Ninety percent of patients ultimately show positive cytology, but multiple evaluations may be required. After a single LP, the false-negative rate may be as high as 55%; this falls to only 10% after three LPs (Olson et al 1974, Wasserstrom et al 1982, Kaplan et al 1990). Despite these measures, CSF cytology is persistently negative in as many as 20% of patients with clinically or radiographically unequivocal leptomeningeal involvement, presumably because the malignant cells adhere to the leptomeninges rather than float in CSF. The sensitivity and specificity of CSF cytology may be enhanced by the use of fluorescence in situ hybridization (FISH) (van Oostenbrugge et al 1998, 2000) or immunocytochemical techniques (Thomas et al 2000). Tumor markers such as lactate dehydrogenase isoenzymes (Wasserstrom et al 1982), carcinoembryonic antigen (Liu et al 2009), β2-microglobulin (Twijnstra et al 1987), and tissue polypeptide antigen (Soletormos and Bach 2001) may help delineate the diagnosis. Flow cytometry for detection of abnormal DNA content may be a useful adjunct to cytological examination (Schinstine et al 2006).
Untreated, leptomeningeal metastases cause progressive neurological dysfunction at multiple sites, followed by death in 4–6 weeks. Current treatment strategies, which include radiation therapy delivered to the area of symptomatic involvement, corticosteroids, and intraventricular or intrathecal chemotherapy or systemic chemotherapy, are of limited efficacy, and in general patient outlook remains poor (Clarke et al 2010).
Base of Skull Metastases
Base of skull metastases are associated with well-described clinical syndromes (Greenberg et al 1981, Laigle-Donadey et al 2005) that are named according to the site of metastatic involvement: orbital, parasellar, middle fossa, jugular foramen, occipital condyle, clivus, and sphenoid sinus. Cancers of the breast, lung, and prostate are most commonly associated with this complication (Greenberg et al 1981, Laigle-Donadey et al 2005), but any tumor type that metastasizes to bone may be responsible. When base of skull metastases are suspected, axial imaging with CT (including bone window settings) is the usual initial procedure (Laigle-Donadey et al 2005). MRI is more sensitive in assessing soft tissue extension, and CSF analysis may be needed to exclude leptomeningeal metastases.
Orbital Syndrome
Orbital metastases usually cause progressive pain in the retro- and supraorbital area of the affected eye (Ahmad and Esmaeli 2007). Blurred vision and diplopia may be associated complaints. Signs may include proptosis, chemosis of the involved eye, external ophthalmoparesis, ipsilateral papilledema, and decreased sensation in the ophthalmic division of the trigeminal nerve.
Parasellar Syndrome
Parasellar syndrome is typically manifested as unilateral supraorbital and frontal headache, which may be associated with diplopia (Yi et al 2000). Ophthalmoparesis (Besada et al 2007) or papilledema may be present, and formal visual field testing may demonstrate hemianopia or quadrantanopia.
Middle Cranial Fossa Syndrome
Middle cranial fossa syndrome is characterized by facial numbness, paresthesias, or pain, which is usually referred to the cheek or jaw (in the distribution of the second or third divisions of the trigeminal nerve) (Lossos and Siegal 1992). The pain is typically described as a dull continual ache, but it may also be paroxysmal or lancinating. On examination, patients may exhibit hypoesthesia in the trigeminal nerve distribution and signs of weakness in the ipsilateral muscles of mastication. Occasional patients have other neurological signs, such as abducens palsy (Greenberg et al 1981).
Jugular Foramen Syndrome
Jugular foramen syndrome is usually associated with hoarseness or dysphagia. Pain is generally referred to the ipsilateral ear or mastoid region and may occasionally be manifested as glossopharyngeal neuralgia, with or without syncope (Greenberg et al 1981, Laigle-Donadey et al 2005). Pain may also be referred to the ipsilateral neck or shoulder. Neurological signs include ipsilateral Horner’s syndrome and paresis of the palate, vocal cord, sternocleidomastoid, or trapezius. Ipsilateral paresis of the tongue may also occur if the tumor extends to the region of the hypoglossal canal.
Occipital Condyle Syndrome
Occipital condyle syndrome is typified by unilateral occipital pain that is worsened with neck flexion (Martinez Salamanca et al 2006). The patient may complain of neck stiffness. Pain intensity is variable but can be severe. Examination may reveal head tilt, limited movement of the neck, and tenderness on palpation over the occipitonuchal junction. Neurological findings may include ipsilateral hypoglossal nerve paralysis and sternocleidomastoid weakness (Capobianco et al 2002).
Clivus Syndrome
Clivus syndrome is characterized by vertex headache, which is often exacerbated by neck flexion. Lower cranial nerve (VI–XII) dysfunction may develop (Ulubas et al 2005, Malloy 2007) and may become bilateral (Fink et al 1987).
Sphenoid Sinus Syndrome
Sphenoid sinus metastasis often causes bifrontal and/or retro-orbital pain, which may radiate to the temporal regions (Lawson and Reino 1997). Associated features of nasal congestion and diplopia may be present (Mickel and Zimmerman 1990). Physical examination is often unremarkable, although unilateral or bilateral sixth nerve paresis may be observed.
Painful Cranial Neuralgias
As noted, specific cranial neuralgias can result from metastases in the base of the skull or leptomeninges. They are most commonly observed in patients with prostate and lung cancer (Gupta et al 1990, McDermott et al 2004). Invasion of the soft tissues of the head or neck or involvement of sinuses can also eventuate in such lesions. Each of these syndromes has characteristic findings. Early diagnosis may allow effective treatment of the underlying lesion before progressive neurological injury occurs.
Glossopharyngeal Neuralgia
Glossopharyngeal neuralgia has been reported in patients with leptomeningeal metastases (Sozzi et al 1987), jugular foramen syndrome (Greenberg et al 1981), or head and neck malignancies (Ribeiro et al 2007). This syndrome is associated with severe pain in the throat or neck, which may radiate to the ear or mastoid region. Pain may be induced by swallowing. In some patients, pain is associated with sudden orthostasis and syncope (Ribeiro et al 2007).
Trigeminal Neuralgia
Trigeminal pain may be continual, paroxysmal, or lancinating. Pain that mimics classic trigeminal neuralgia can be induced by tumors in the middle or posterior fossa (Benoliel et al 2007) or leptomeningeal metastases (DeAngelis and Payne 1987). Sometimes, pain may be caused by perineural spread without evidence of a discrete mass (Boerman et al 1999). Continual pain in a trigeminal distribution may be an early sign of acoustic neuroma (Payten 1972). All cancer patients in whom trigeminal neuralgia develops should be evaluated for the existence of an underlying neoplasm.
Ear and Eye Pain Syndromes
Otalgia
Otalgia is the sensation of pain in the ear, whereas referred otalgia is pain felt in the ear but originating from a non-otological source. The rich sensory innervation of the ear is derived from four cranial nerves and two cervical nerves, which also supply other areas in the head, neck, thorax, and abdomen. Pain referred to the ear may originate in areas far removed from the ear itself. Otalgia may be caused by acoustic neuroma (Morrison and Sterkers 1996) and metastases to the temporal bone or infratemporal fossa (Hill and Kohut 1976, Shapshay et al 1976, Leonetti et al 1998). Referred otalgia is reported in patients with tumors involving the oropharynx or hypopharynx (Scarbrough et al 2003).
Eye Pain
Blurring of vision and eye pain are the two most common symptoms of choroidal metastases (De Potter 1998). More commonly, chronic eye pain is related to metastases to the bony orbit (Shih et al 2007), intraorbital structures such as the rectus muscles (Weiss et al 1984, Friedman et al 1990), the optic nerve (Laitt et al 1996), or the cavernous sinus (Rodriguez et al 2007).
Uncommon Causes of Headache and Facial Pain
Headache and facial pain in cancer patients may have many other causes. Unilateral facial pain can be the initial symptom of an ipsilateral lung tumor (Sarlani et al 2003, Evans 2007, Navarro et al 2009). Presumably, this referred pain is mediated by vagal afferents. Facial squamous cell carcinoma of the skin may cause facial pain as a result of extensive perineural invasion (Schroeder et al 1998). Patients with Hodgkin’s disease may have transient episodes of neurological dysfunction that has been likened to migraine (Dulli et al 1987). In some cases it may be a reversible posterior leukoencephalopathy syndrome, which is characterized by headache, disturbances in consciousness, seizures, and cortical visual loss with the neuroimaging finding of edema in posterior regions of the brain (Miyazaki et al 2004).
Headache may occur with cerebral infarction or hemorrhage, which may be due to non-bacterial thrombotic endocarditis or disseminated intravascular coagulation. Headache is also the usual finding in patients with sagittal sinus occlusion, which may be due to tumor infiltration, a hypercoagulable state, or treatment with L-asparaginase therapy (Sigsbee et al 1979). Headache attributable to pseudotumor cerebri has also been reported to be the manifestation of SVC obstruction in a patient with lung cancer (Portenoy et al 1983). Tumors of the sinonasal tract may be accompanied by deep facial or nasal pain (Marshall and Mahanna 1997).
Neuropathic Pain Involving the Peripheral Nervous System
Neuropathic pain involving the peripheral nervous system is common. Syndromes include painful radiculopathy, plexopathy, mononeuropathy, and peripheral neuropathy.
Painful Radiculopathy
Radiculopathy or polyradiculopathy may be caused by any process that compresses, distorts, or inflames nerve roots. Painful radiculopathy is an important manifestation in patients with epidural tumor and leptomeningeal metastases (see above).
Post-herpetic Neuralgia
The precise clinical definition of PHN has been a matter of dispute. There is agreement that acute herpetic neuralgia refers to pain preceding or accompanying the eruption of a rash that persists up to 30 days from its onset. Some authorities refer to all persistent pain as PHN, whereas others describe a subacute herpetic neuralgia that persists beyond healing of the rash but resolves within 4 months of onset and reserve the term PHN for pain persisting beyond 4 months from the initial onset of the rash (Dworkin and Portenoy 1996). One study suggested that PHN is two to three times more frequent in the cancer population than in the general population (Rusthoven et al 1988).
Cervical Plexopathy
In cancer patients cervical plexus injury is frequently due to tumor infiltration or treatment (including surgery or radiotherapy ) of neoplasms in this region (Jaeckle 2004). Tumor invasion or compression of the cervical plexus can be caused by direct extension of a primary head and neck malignancy or neoplastic (metastatic or lymphomatous) involvement of the cervical lymph nodes (Jaeckle 2004). Pain may be experienced in the preauricular (greater auricular nerve) or postauricular (lesser and greater occipital nerves) regions or the anterior part of the neck (transverse cutaneous and supraclavicular nerves). Pain may be referred to the lateral aspect of the face or head or to the ipsilateral shoulder. The overlap in pain referral patterns from the face and neck may relate to the close anatomical relationship between the central connections of the cervical afferents and the afferents carried in cranial nerves V, VII, IX, and X in the upper cervical spinal cord.
Associated features can include ipsilateral Horner’s syndrome or hemidiaphragmatic paralysis. The diagnosis must be distinguished from EC of the cervical spinal cord and leptomeningeal metastases. MRI or CT of the neck and cervical spine is usually required to evaluate the etiology of the pain.
Brachial Plexopathy
The two most common causes of brachial plexopathy in cancer patients are tumor infiltration and radiation injury. Less common causes of painful brachial plexopathy include trauma during surgery or anesthesia, radiation-induced second neoplasms, acute brachial plexus ischemia, and paraneoplastic brachial neuritis.
Malignant Brachial Plexopathy
Plexus infiltration by tumor is the most prevalent cause of brachial plexopathy. Malignant brachial plexopathy is most common in patients with lymphoma, lung cancer, or breast cancer. The invading tumor usually arises from adjacent axillary, cervical, and supraclavicular lymph nodes (lymphoma and breast cancer) or from the lung (superior sulcus tumors or so-called Pancoast’s tumors) (Kori et al 1981, Kori 1995, Jaeckle 2004). Pain is nearly universal (occurring in 85% of patients) and often precedes neurological signs or symptoms by months (Kori 1995). Lower plexus involvement (C7, C8, T1 distribution) is typical and is reflected in the pain distribution, which usually involves the elbow, medial part of the forearm, and fourth and fifth fingers. Pain may sometimes localize to the posterior portion of the arm or elbow. Severe aching is generally reported, but patients may also experience constant or lancinating dysesthesias along the ulnar aspect of the forearm or hand.
Tumor infiltration of the upper plexus (C5–6 distribution) is less common. This lesion is characterized by pain in the shoulder girdle, lateral part of the arm, and hand. A pan-plexopathy subsequently develops in 75% of patients with upper plexopathy, and 25% of patients are initially seen with pan-plexopathy (Kori et al 1981).
Cross-sectional imaging is essential in all patients with symptoms or signs compatible with plexopathy. Although comparative data on the sensitivity and specificity of MRI and CT in evaluating lesions of the brachial plexus are not available, MRI is widely thought to be the best choice for evaluating the anatomy and pathology of the brachial plexus (van Es et al 2010). Limited experience has been reported for PET-CT imaging (Luthra et al 2006).
Electrodiagnostic studies may be helpful in patients with suspected plexopathy, particularly when findings on neurological examination and imaging studies are normal (Ferrante and Wilbourn 2002). Though not specific for tumor, abnormalities on electromyography or somatosensory evoked potentials may establish the diagnosis of plexopathy and thereby confirm the need for additional evaluation.
Patients with malignant brachial plexopathy are at high risk for epidural extension of the tumor (Portenoy et al 1989, Jaeckle 2004). Epidural encroachment can occur as the neoplasm grows medially and invades vertebrae or tracks along nerve roots through the intervertebral foramina. In the latter case, there may be no evidence of bony erosion on imaging studies. The development of Horner’s syndrome, evidence of pan-plexopathy, or the finding of paraspinal tumor or vertebral damage on CT or MRI is highly associated with epidural extension and should lead to definitive imaging of the epidural tumor (Portenoy et al 1989, Jaeckle 2004).
Radiation-Induced Brachial Plexopathy
Two distinct syndromes of radiation-induced brachial plexopathy have been described: (1) early-onset transient plexopathy (see above) and (2) delayed-onset progressive plexopathy. Delayed-onset progressive plexopathy can occur 6 months to 20 years after a course of radiotherapy that included the plexus in the radiation portal. In contrast to tumor infiltration, pain is a relatively uncommon initial symptom (18%) and, when present, is usually less severe (Kori et al 1981). After supraclavicular node radiotherapy there is a progressively increasing incidence over time that rises to 56% after 20 years (Bajrovic et al 2004). Weakness and sensory changes predominate in the distribution of the upper plexus (C5–6 distribution) (Schierle and Winograd 2004, Jaeckle 2010). Radiation-induced changes in the skin and lymphedema are commonly associated.
The typical appearance of radiation fibrosis of the plexus on CT studies is diffuse infiltration and loss of tissue planes without a mass lesion (Cascino et al 1983). There is often associated lymphedema in the arm, evident on CT, and occasionally, radiation necrosis of the clavicle, rib, or humeral head occurs at the adjacent level (Schulte et al 1989). Tumor infiltration of the plexus cannot be differentiated from radiation fibrosis by CT when diffuse infiltration is noted. On MRI the most common findings observed with radiation fibrosis are thickening and diffuse enhancement of the brachial plexus without a focal mass and/or soft tissue changes and low signal intensity on both T1- and T2-weighted images (Wittenberg and Adkins 2000).
Electrodiagnostic studies in patients with radiation fibrosis have been demonstrated to show signs of fibrillation and positive waves associated with denervation. Widespread myokymia is strongly suggestive of radiation-induced plexopathy (Lederman and Wilbourn 1984). Although a careful history combined with neurological findings and the results of tomographic and electrodiagnostic studies can strongly suggest the diagnosis of radiation-induced injury, repeated assessments over time may be needed to confirm the diagnosis. Rare patients require surgical exploration of the plexus to exclude neoplasm and establish the etiology. When caused by radiation, plexopathy is usually progressive (Killer and Hess 1990, Jaeckle 2004), although some patients plateau for a variable period.
Uncommon Causes of Brachial Plexopathy
A malignant peripheral nerve tumor or a second primary tumor in a previously irradiated site can account for pain recurring late in a patient’s course (Binder et al 2004). Pain has been reported to occur as a result of brachial plexus entrapment in a lymphedematous shoulder (Vecht 1990) and as a consequence of acute ischemia many years after axillary radiotherapy (Gerard et al 1989). Idiopathic brachial plexopathy has also been described in patients with Hodgkin’s disease (Lachance et al 1991). Paraneoplastic brachial plexopathy associated with anti-amphiphysin antibodies has been described in patients with small cell lung cancer (Coppens et al 2006).
Lumbosacral Plexopathy
In the cancer population, lumbosacral plexopathy is generally caused by neoplastic infiltration or compression. Radiation-induced plexopathy also occurs, and the lesion occasionally develops as a result of surgical trauma, infarction, cytotoxic damage, infection of the pelvis or psoas muscle, abdominal aneurysm, or idiopathic lumbosacral neuritis. Polyradiculopathy from leptomeningeal metastases or epidural metastases can mimic lumbosacral plexopathy.
Malignant Lumbosacral Plexopathy
The primary tumors most frequently associated with malignant lumbosacral plexopathy include colorectal, cervical, breast, sarcoma, and lymphoma (Jaeckle et al 1985, Jaeckle 2004). Most tumors involve the plexus by direct extension from an intrapelvic neoplasm; metastases account for only one-fourth of cases. Rarely, plexopathy can arise by perineural spread from prostate cancer along prostatic nerves into the lumbosacral plexus (Ladha et al 2006).
Pain is typically the first symptom and is experienced by almost all patients at some point, and it is the only symptom in nearly 20% of patients. Its quality is aching, pressure-like, or stabbing; dysesthesias are relatively uncommon. Numbness, paresthesias, or weakness develops weeks to months after the pain begins in most patients. Common signs include leg weakness that involves multiple myotomes, sensory loss that crosses dermatomes, reflex asymmetry, focal tenderness, leg edema, and positive direct or reverse straight leg raising signs.
A lower plexopathy is most common and accounts for just more than 50% of patients with malignant lumbosacral plexopathy (Jaeckle et al 1985). It is usually caused by direct extension from a pelvic tumor, most frequently rectal cancer, gynecological tumors, or pelvic sarcoma. Pain may be localized in the buttocks and perineum or referred to the posterolateral aspect of the thigh and leg. Associated symptoms and signs conform to an L4–S1 distribution. Examination may reveal weakness or sensory changes in the L5 and S1 dermatomes and a depressed ankle jerk. Other findings include leg edema, bladder or bowel dysfunction, sacral or sciatic notch tenderness, and a positive straight leg raising test. A pelvic mass may be palpable.
An upper plexopathy occurs in almost one-third of patients with lumbosacral plexopathy (Jaeckle et al 1985). Pain may be experienced in the back, lower part of the abdomen, flank or iliac crest, or the anterolateral portion of the thigh. Examination may reveal sensory, motor, and reflex changes in an L1–4 distribution.
A subgroup of these patients have a syndrome characterized by pain and paresthesias limited to the lower part of the abdomen or inguinal region, variable sensory loss, and no motor findings. CT may show tumor adjacent to the L1 vertebra (the L1 syndrome) (Jaeckle et al 1985) or along the pelvic sidewall, where it presumably damages the ilioinguinal, iliohypogastric, or genitofemoral nerves.
Another subgroup has neoplastic involvement of the psoas muscle and a syndrome characterized by upper lumbosacral plexopathy, painful flexion of the ipsilateral hip, and a positive psoas muscle stretch test; this has been termed malignant psoas syndrome (Stevens and Gonet 1990, Agar et al 2004). Similarly, pain in the distribution of the femoral nerve has been observed in the setting of recurrent retroperitoneal sarcoma (Zografos and Karakousis 1994), and tumor in the iliac crest can compress the lateral cutaneous nerve of the thigh and produce a pain that mimics meralgia paresthetica (Tharion and Bhattacharji 1997).
Sacral plexopathy may occur as a result of direct extension of a sacral lesion or a presacral mass (Payer 2003). It may be manifested predominantly as involvement of the lumbosacral trunk, which is characterized by numbness over the dorsal medial aspect of the foot and sole and weakness of knee flexion, ankle dorsiflexion, and inversion. Other patients demonstrate particular involvement of the coccygeal plexus, with prominent sphincter dysfunction and perineal sensory loss. The latter syndrome occurs with low pelvic tumors, such as those arising from the rectum or prostate.
A pan-plexopathy with involvement in an L1–S3 distribution occurs in almost one-fifth of patients with lumbosacral plexopathy (Jaeckle et al 1985). Local pain may occur in the lower part of the abdomen, back, buttocks, or perineum. Referred pain can be experienced anywhere in distribution of the plexus. Leg edema is extremely common. Neurological deficits may be confluent or patchy within the L1–S3 distribution, and a positive straight leg raising test is usually present.
Cross-sectional imaging with either CT or MRI is the preferred diagnostic procedure for evaluating lumbosacral plexopathy. Scanning should be done from the level of the L1 vertebral body through the sciatic notch. Limited data suggest superior sensitivity of MRI over CT (Taylor et al 1997). Definitive imaging of the epidural space adjacent to the plexus should be considered in patients who have features indicative of a relatively high risk for epidural extension, including bilateral symptoms or signs, unexplained incontinence, or a prominent paraspinal mass (Jaeckle et al 1985, Portenoy et al 1989).
Radiation-Induced Lumbosacral Plexopathy
Radiation fibrosis of the lumbosacral plexus is a rare complication that may occur 1 to more than 30 years following radiation treatment (Jaeckle 2004). The use of intracavitary radium implants for carcinoma of the cervix may be an additional risk factor (Stryker et al 1990, Gonzalez-Caballero et al 2000). Radiation-induced plexopathy is typically manifested as progressive weakness and leg swelling; pain is not usually a prominent feature (Thomas et al 1985, Stryker et al 1990). Weakness typically begins distally in the L5–S1 segments and is slowly progressive. The symptoms and signs may be bilateral (Thomas et al 1985). If CT demonstrates a lesion, it is generally a non-specific diffuse infiltration of the tissues. Electromyography may show myokymic discharges (Thomas et al 1985).
Uncommon Causes of Lumbosacral Plexopathy
Lumbosacral plexopathy may occur following intra-arterial cisplatin infusion (see below) and embolization techniques. This syndrome has been observed following attempted embolization of a bleeding rectal lesion. Raised intrapelvic pressure from a compression syndrome (Lin et al 2009) or, rarely, a massively distended bladder or rectum may sometimes cause a plexus compression syndrome with radicular pain. Benign conditions that may produce similar findings include hemorrhage or abscess in the iliopsoas muscle (Chad and Bradley, 1987), abdominal aortic aneurysms, diabetic radiculoplexopathy, vasculitis, and an idiopathic lumbosacral plexitis analogous to acute brachial neuritis (Chad and Bradley 1987).
Painful Mononeuropathy
Tumor-Related Mononeuropathy
Tumor-related mononeuropathy usually results from compression or infiltration of a nerve from tumor arising in an adjacent bony structure. The most common example of this phenomenon is intercostal nerve injury in a patient with rib metastases. Other examples include the cranial neuralgias previously described, sciatica associated with invasion of the sciatic notch by tumor, common peroneal nerve palsy associated with primary bone tumors of the proximal end of the fibula, and neuralgia involving the lateral cutaneous nerve of the thigh associated with iliac crest tumors.
Other Causes of Mononeuropathy
Mononeuropathy from many other causes also develops in cancer patients. Post-surgical syndromes are well described (see below), and radiation injury to a peripheral nerve occurs occasionally. Rarely, nerve entrapment syndromes (such as carpal tunnel syndrome) related to edema or direct compression by tumor (Desta et al 1994) can develop in cancer patients.
Painful Peripheral Neuropathies
Painful peripheral neuropathies have multiple causes, including nutritional deficiencies, other metabolic derangements (e.g., diabetes and renal dysfunction), neurotoxic effects of chemotherapy, and rarely, paraneoplastic syndromes.
Painful Paraneoplastic Peripheral Neuropathy
Painful paraneoplastic peripheral neuropathy can be related to injury to the dorsal root ganglion (also known as subacute sensory neuronopathy or ganglionopathy) or injury to peripheral nerves (Grisold and Drlicek 1999). These syndromes may be the initial manifestation of an underlying malignancy. Except for the neuropathy associated with myeloma (Kissel and Mendell 1996, Rotta and Bradley 1997), their course is usually independent of the primary tumor (Dalmau and Posner 1997, Grisold and Drlicek 1999).
Subacute sensory neuronopathy is characterized by pain (usually dysesthetic), paresthesias, sensory loss in the extremities, and severe sensory ataxia (Brady 1996). Some patients have predominantly sensory symptoms, whereas in others, ataxia is dominant (Oki et al 2007). Although it is usually associated with small cell carcinoma of the lung (van Oosterhout et al 1996), other tumor types, including breast cancer (Peterson et al 1994), Hodgkin’s disease (Plante-Bordeneuve et al 1994), and varied solid tumors, are rarely associated. The pain generally develops before the tumor is evident, and its course is typically independent. Co-existing autonomic, cerebellar, or cerebral abnormalities are common (Brady 1996). An antineuronal IgG antibody (“anti-Hu”) that recognizes a low-molecular-weight protein present in most small cell lung carcinomas has been associated with the condition (Dalmau and Posner 1997). Recently, a number of other paraneoplastic antibodies have been identified, including anti-CV2 (Antoine et al 2001) and anti-Ri (Fasolino et al 2004).
A sensorimotor peripheral neuropathy, which may be painful, has been observed in association with diverse neoplasms, particularly Hodgkin’s disease and paraproteinemias (Kelly and Karcher 2005). Clinically evident peripheral neuropathy occurs in approximately 15% of patients with multiple myeloma, and electrophysiological evidence of this lesion can be found in 40% (Kissel and Mendell 1996). The pathophysiology of the neuropathy is unknown.
Pain Syndromes of the Viscera and Miscellaneous Tumor-Related Syndromes
Pain may be caused by pathology involving the luminal organs of the gastrointestinal or genitourinary tract, the parenchymal organs, the peritoneum, or the retroperitoneal soft tissues. Obstruction of hollow viscera, including the intestine, biliary tract, and ureter, produces visceral nociceptive syndromes that are well described in the surgical literature (Silen 1983). Pain arising from retroperitoneal and pelvic lesions may involve mixed nociceptive and neuropathic mechanisms if both somatic structures and nerves are affected.
Hepatic Distention Syndrome
Pain-sensitive structures in the region of the liver include the liver capsule, blood vessels, and biliary tract (Coombs 1990). The nociceptive afferents that innervate these structures travel via the celiac plexus, the phrenic nerve, and the lower right intercostal nerves. Extensive intrahepatic metastases or gross hepatomegaly associated with cholestasis may produce discomfort in the right subcostal region and less commonly in the right midback region or flank (Harris et al 2003). Referred pain may be experienced in the right side of the neck or shoulder or in the region of the right scapula (Moghadam et al 2008).
In occasional patients who experience chronic pain as a result of hepatic distention, an acute intercurrent subcostal pain can develop that may be exacerbated by respiration. Physical examination may demonstrate a palpable or audible rub. These findings suggest the development of an overlying peritonitis, which can develop in response to some acute event, such as a hemorrhage into a metastasis (La Fianza et al 1999).
Midline Retroperitoneal Syndrome
Retroperitoneal pathology involving the upper part of the abdomen may produce pain by injury to deep somatic structures of the posterior abdominal wall, distortion of pain-sensitive connective tissue and vascular and ductal structures, local inflammation, and direct infiltration of the celiac plexus. The most common causes are pancreatic cancer (Kelsen et al 1997) and retroperitoneal lymphadenopathy (Sponseller 1996), particularly celiac lymphadenopathy (Schonenberg et al 1991). Reasons for the high frequency of perineural invasion and the presence of pain in pancreatic cancer may be related to locoregional secretion and activation of nerve growth factor and its high-affinity receptor TrkA. These factors are involved in stimulating epithelial cancer cell growth and perineural invasion (Zhu et al 1999). In contrast, tumors with overexpression of a low-affinity receptor were associated with less pain (Dang et al 2006).
In some instances of pancreatic cancer, obstruction of the main pancreatic duct with subsequent ductal hypertension generates pain that can be relieved by stenting of the pancreatic duct (Tham et al 2000).
The pain is experienced in the epigastrium, in the low thoracic region of the back, or in both locations. It is often diffuse and poorly localized. It is generally dull and boring in character, exacerbated with recumbency, and improved by sitting. The lesion can usually be demonstrated by CT, MRI, or ultrasound scanning of the upper part of the abdomen.
Intestinal Obstruction
Abdominal pain is an almost invariable manifestation of intestinal obstruction, which may occur in patients with abdominal or pelvic cancer (Ripamonti et al 2005). Factors that contribute to this pain include smooth muscle contractions, mesenteric tension, and mural ischemia. Obstructive symptoms may be due primarily to the tumor or, more likely, to a combination of mechanical obstruction and other processes, such as autonomic neuropathy and ileus from metabolic derangements or drugs. Both continuous and colicky pains occur and may be referred to the dermatomes represented by the spinal segments supplying the affected viscera. Vomiting, anorexia, and constipation are important associated symptoms.
Peritoneal Carcinomatosis
Peritoneal carcinomatosis occurs most often by transcoelomic spread of abdominal or pelvic tumor; except for breast cancer, hematogenous spread of an extra-abdominal neoplasm in this pattern is rare. Carcinomatosis can cause peritoneal inflammation, mesenteric tethering, malignant adhesions, and ascites, all of which can cause pain. Pain and abdominal distention are the most common initial symptoms. Adhesions can also cause obstruction of hollow viscera with consequent intermittent colicky pain (Averbach and Sugarbaker 1995). CT may demonstrate evidence of ascites, omental infiltration, and peritoneal nodules (Archer et al 1996).
Malignant Perineal Pain
Tumors of the colon or rectum, female reproductive tract, and distal genitourinary system are most commonly responsible for perineal pain (Stillman 1990, Boas et al 1993). Severe perineal pain following resection of pelvic tumors often precedes evidence of detectable disease and should be viewed as a potential harbinger of progressive or recurrent cancer (Stillman 1990, Boas et al 1993, Rigor 2000). Some evidence suggests that this phenomenon is due to microscopic perineural invasion by recurrent disease (Seefeld and Bargen 1943). The pain, which is typically described as constant and aching, is frequently aggravated by sitting or standing and may be associated with tenesmus or bladder spasms (Stillman 1990).
Tumor invasion of the musculature of the deep pelvis can also result in a syndrome that appears similar to the so-called tension myalgia of the pelvic floor (Sinaki et al 1977). The pain is typically described as a constant ache or heaviness that is exacerbated with an upright posture. When caused by tumor, the pain may be concurrent with other types of perineal pain. Digital examination of the pelvic floor may reveal local tenderness or a palpable tumor.
Adrenal Pain Syndrome
Large adrenal metastases, common in lung cancer, may produce unilateral flank pain and, less commonly, abdominal pain. The pain is of variable severity, and it can be severe (Berger et al 1995b). Adrenal metastases can be complicated by hemorrhage, which may cause severe abdominal pain (Karanikiotis et al 2004).
Ureteric Obstruction
Ureteric obstruction is most frequently caused by tumor compression or infiltration within the true pelvis (Harrington et al 1995, Russo 2000). Less commonly, obstruction can be more proximal and associated with retroperitoneal lymphadenopathy, an isolated retroperitoneal metastasis, mural metastases, or intraluminal metastases. Cancers of the cervix, ovary, prostate, and rectum are most commonly associated with this complication. Pain may or may not accompany ureteric obstruction. When present, it is typically a dull chronic discomfort in the flank with radiation to the inguinal region or genitalia (Little et al 2003). If pain does not occur, ureteric obstruction may be discovered when hydronephrosis is discerned on abdominal imaging procedures or renal failure develops. Ureteric obstruction can be complicated by pyelonephritis or pyonephrosis, which are often accompanied by features of sepsis, loin pain, and dysuria. Diagnosis of ureteric obstruction can usually be confirmed by demonstration of hydronephrosis on renal sonography. The level of obstruction can be identified by pyelography, and CT scanning techniques will usually demonstrate the cause (Russo 2000).
Ovarian Cancer Pain
Moderate to severe chronic abdominopelvic pain is the most common symptom of ovarian cancer; it is reported by almost two-thirds of patients in the 2 weeks before the onset or recurrence of the disease (Portenoy et al 1994a). Pain is experienced in the low back region or abdomen (Goff et al 2004, Webb et al 2004). In patients who have previously been treated, it is an important symptom of potential recurrence (Portenoy et al 1994).
Lung Cancer Pain
Even in the absence of involvement of the chest wall or parietal pleura, lung tumors can produce a visceral pain syndrome. In a large case series of lung cancer patients, pain was unilateral in 80% and bilateral in 20%. Among patients with hilar tumors, the pain was reported to refer to the sternum or the scapula. Upper and lower lobe tumors referred pain to the shoulder and the lower part of the chest, respectively (Marino et al 1986, Marangoni et al 1993). As mentioned previously, early lung cancers can cause ipsilateral facial pain (Sarlani et al 2003). It is postulated that this pain syndrome is generated via vagal afferent neurons.
Other Uncommon Visceral Pain Syndromes
Sudden-onset severe abdominal or loin pain may be caused by non-traumatic rupture of a visceral tumor. It has most frequently been reported with hepatocellular cancer (Miyamoto et al 1991) but also with other liver metastases (Marini et al 2002). Kidney rupture as a result of renal metastasis from adenocarcinoma of the colon (Wolff et al 1994), ovarian tumor rupture (Malhotra et al 2010), gastrointestinal stromal tumor rupture (Fiscon et al 2009), splenic rupture in acute leukemia (Rajagopal et al 2002), rupture of adrenocortical cancers (Stamoulis et al 2004), and metastasis-induced perforated appendicitis (Ende et al 1995) have been reported. Torsion of pedunculated visceral tumors can produce a cramping abdominal pain. Uncommonly, peripheral lung tumors can rupture and cause pleuritis (Iwata et al 2008).