Hypocalcemia
Introduction
Hypocalcemia based on serum tCa is a relatively common laboratory abnormality and was observed in 13.5% of serum biochemical profiles of dogs in one clinical study.115 Based on serum iCa measurement in 1633 sick dogs, the prevalence of hypocalcemia was 31%,519 and in 434 sick cats, the prevalence was 27%.204 On the basis of serum tCa concentration, hypocalcemia is usually defined as a concentration less than 8.0 mg/dL in dogs and less than 7.0 mg/dL in cats. When serum iCa concentration is used, hypocalcemia is generally defined as a concentration less than 5.0 mg/dL (1.25 mmol/L) in dogs and less than 4.5 mg/dL (1.1 mmol/L) in cats. The most likely reason for submission of samples to measure calcium regulatory hormones in animals with hypocalcemia is for those with persistent hypocalcemia that is moderate to severe in magnitude and for which a known cause cannot be identified; most will be submitted with suspicion for a diagnosis of primary hypoparathyroidism.
In human patients, large and unexplained differences between ionized and tCa concentrations have been found in hypocalcemic conditions.320 This discordance is also seen in dogs and cats and is not predictable. Based on serum tCa measurement in 1633 sick dogs, 27% were classified hypocalcemic, but when iCa was measured, 31% were hypocalcemic.519 Using serum tCa measurement in 434 sick cats, 49% were classified hypocalcemic, but when iCa was measured, only 27% were actually hypocalcemic. Thus, in dogs, tCa measurement underestimated ionized hypocalcemia, and in cats, hypocalcemia was overestimated when using serum tCa concentration to predict iCa status.
Consequences of hypocalcemia and clinical signs
Clinical signs related to hypocalcemia are identical regardless of the underlying cause (Box 6-5). Low serum iCa increases excitability of neuromuscular tissue, which accounts for many of the clinical signs of hypocalcemia. Animals with mild decreases in iCa concentration may display no obvious clinical signs. The duration and magnitude of ionized hypocalcemia and the rate of decline in iCa concentration interact to determine the severity of clinical signs. Clinical signs in dogs often are not obvious until serum tCa concentration is less than 6.5 mg/dL, and some dogs show surprisingly few signs despite severe hypocalcemia (serum tCa concentration, <5.0 mg/dL), especially if the underlying disease has been chronic and there has been sufficient time for physiologic adaptation. Acute development of hypocalcemia is usually associated with severe clinical signs. In its most severe forms, hypocalcemia can cause death as a result of circulatory effects (e.g., hypotension and decreased myocardial contractility) and respiratory arrest from paralysis of respiratory muscles. Serum tCa concentration less than 4.0 mg/dL can cause left-sided myocardial failure154 and death,179 especially if the decline in serum calcium concentration was rapid.
Box 6-5 Clinical Signs Associated with Hypocalcemia
Other electrolyte and acid-base abnormalities can either magnify or diminish the signs of hypocalcemia. Correction of hypokalemia in cats with concurrent hypocalcemia may precipitate the onset of clinical signs of hypocalcemia.145,408 Patients with chronic hypocalcemia often display intermittent clinical signs despite seemingly stable serum tCa concentrations. Although unpredictable, clinical signs often follow periods of exercise or excitement that may be associated with respiratory alkalosis and subsequent decreases in iCa concentration. Rapid infusion of alkali to correct metabolic acidosis can cause seizures in animals with marginal or previously compensated hypocalcemia through further reduction in iCa concentration.
Clinical signs in dogs with chronic hypocalcemia (primary hypoparathyroidism) include seizures, muscle tremors or fasciculations, muscle cramping, stiff gait, and behavioral changes (e.g., restlessness, excitation, aggression, hypersensitivity to stimuli, and disorientation).87,115,145,530 Seizures often begin as focal muscle tremors that become more widespread. Most dogs in one series had a seizure during the initial 24 to 48 hours of hospitalization, a much higher frequency than that encountered with idiopathic epilepsy.179 Seizure activity associated with hypocalcemia may not be similar to that in idiopathic epilepsy because affected dogs may remain partially conscious and retain urinary continence during the seizure.179,446 Seizures are often preceded by apprehension or nervousness. The seizures may be as short as 60 seconds or as long as 30 minutes in some dogs. Most seizures resolve without treatment but often recur despite treatment with anticonvulsants. Growling attributable to pain or behavior change occurred in approximately 40% of dogs, and intense rubbing of the face with the paws or on the ground was observed in more than 50% of dogs. These signs were attributed to either paresthesia or pain from facial muscle spasms.87,179
Pyrexia may be caused by increased muscular activity with or without seizures. Lethargy and weakness are seen in approximately 33%, and polyuria and polydipsia occur in about 25% of cases as a result of psychogenic mechanisms or renal injury (nephrocalcinosis) from hypercalciuria associated with PTH deficiency in animals with hypoparathyroidism.504,530 Anterior and posterior lenticular cataracts occurred in more than 33% of affected dogs87,308 and also in cats.179,444 Tachycardia and electrocardiographic abnormalities (increased QT–interval) may also be encountered. Both hypertension and hypotension have been reported during hypocalcemia in humans.97,154
Neuromuscular signs in cats with chronic hypocalcemia associated with primary hypoparathyroidism are similar to those in dogs (e.g., muscle tremors, weakness, and generalized seizures).444 Anorexia and lethargy appear to be more common in cats than in dogs with primary hypoparathyroidism, but seizures have not been reported to be induced by excitement, as occurs in dogs. Prolapse of the third eyelid is occasionally observed in cats with acute hypocalcemia but is not a prominent finding during chronic hypocalcemia.
Clinical signs associated with acute postoperative hypocalcemia are similar in dogs and cats and are related to neuromuscular excitability. Focal twitching of facial muscles and vibrissae may be noticed before more generalized muscle tremors or seizures develop. Tetany or facial twitching has not been observed in cats after thyroidectomy until serum tCa concentration is less than 6.9 mg/dL.179,444,446 Severe hypocalcemia (<6.5 mg/dL) is often associated with muscular twitching, tetany, or seizures. Anorexia and lethargy are not often considered primary signs of hypocalcemia, but both signs decrease in cats during calcium infusion after thyroidectomy, suggesting a relationship between hypocalcemia and these signs.
Approach to hypocalcemia
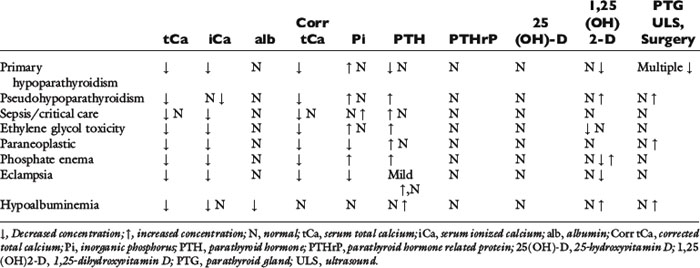
Hypocalcemia develops when bone mobilization of calcium is reduced, skeletal calcium accretion is enhanced, urinary losses of calcium are increased, gastrointestinal absorption of calcium is reduced, calcium is translocated intracellularly, or as a result of a combination of these mechanisms. Much like the initial approach to hypercalcemia, it is helpful to make the initial distinction as to whether hypocalcemia is parathyroid dependent or parathyroid independent. Ionized calcium concentration must be evaluated in conjunction with PTH concentration to determine whether PTH production is appropriate. Patients with low iCa and low PTH concentrations have absolute hypoparathyroidism (parathyroid dependent). A normal reference range PTH when iCa is low is inappropriate, because normal parathyroid glands should respond with increased PTH. Hypocalcemic patients with increased PTH are classified as having parathyroid-independent hypocalcemia. Normograms to determine the adequacy of the increased response of PTH to low iCa have not been established for dogs or cats. In cases of parathyroid-independent hypocalcemia, hypocalcemia exists from redistribution of calcium into other body spaces, excess phosphorus effects, or from deficiencies of vitamin D or dietary calcium. Patients with persistent moderate to severe hypocalcemia based on serum tCa should be evaluated for iCa and PTH concentrations; measurement of 25-hydroxyvitamin D and serum phosphorus is also helpful, and in rare circumstances, measurement of calcitriol may help provide a definitive diagnosis. The conditions associated with hypocalcemia in dogs and cats are listed in Box 6-6 according to their relative frequency, regardless of clinical signs or severity of decreased serum calcium concentration. The anticipated changes in calcium hormones and serum biochemistry in disorders causing hypocalcemia are noted in Table 6-4.
Box 6-6 Conditions Associated with Hypocalcemia
Uncommon
Improper sample anticoagulant (EDTA)
Infarction of parathyroid gland adenoma
Rapid intravenous infusion of phosphates
Acute calcium-free intravenous infusion (dilutional)
Intestinal malabsorption or severe starvation
Blood transfusion (citrated anticoagulant)
Differential diagnosis and mechanisms of hypocalcemia
Hypoalbuminemia
Hypoalbuminemia is the most common associated condition but perhaps the least important for clinical consequences, and it occurs in nearly one half of the dogs with hypocalcemia.115 Hypocalcemia associated with hypoalbuminemia is usually mild (serum tCa concentration, 7.5 to 9.0 mg/dL in dogs), and no signs referable to the functional effects of low serum calcium concentration are observed. Application of calcium correction formulas to serum tCa concentrations in dogs or cats with hypoproteinemia or hypoalbuminemia has been advocated in the past. However, these correction formulas do not improve the prediction of actual iCa concentration and in many cases increase the level of diagnostic discordance.519 Use of correction formulas to adjust serum tCa concentration to serum total protein or albumin concentration is not recommended.
Renal Failure
Renal failure is the second most common disorder associated with hypocalcemia in dogs.115 Decreased calcitriol synthesis by diseased kidneys and, to a lesser extent, mass law interactions of calcium with markedly increased serum phosphorus concentration are probable causes of the hypocalcemia observed in dogs and cats with CRF. To decrease iCa concentration by 0.1 mg/dL, serum phosphorus concentration must increase by 3.7 mg/dL.7 Calcitriol deficits are more important because hypocalcemia results from reduced intestinal calcium absorption and increased skeletal resistance to PTH.404 Animals with CRF and decreased serum tCa concentration are most often asymptomatic, possibly because of an increase in iCa concentration that accompanies metabolic acidosis.
Serum tCa concentration was 8.0 mg/dL or less in 10% of 268 dogs with clinical CRF, whereas low serum iCa concentrations were detected in 40% of affected dogs.118 In 23 dogs with CRF, iCa represented 40% of tCa as compared with 51% of tCa in normal dogs.303 Serum iCa was low in 56%, normal in 26%, and high in 17% of the dogs with CRF. Thus, iCa concentration was low in the majority of dogs despite the presence of metabolic acidosis in 83% of dogs, which would be expected to increase iCa.303 Hypocalcemia was diagnosed more frequently in a study of 489 dogs with CRF when determined by iCa measurement. Based on serum tCa measurement, hypocalcemia was noted in only 19% of dogs with CRF; when iCa concentration was measured, hypocalcemia was observed in 29% of dogs with CRF.519
In 74 cats with clinical CRF, 15% were hypocalcemic based on serum tCa.147 In cats with CRF, hypocalcemia was found more commonly with higher magnitudes of azotemia.27 In 73 cats with CRF, none had hypocalcemia based on tCa, but 3% of cats with moderate CRF and 23% of cats with advanced CRF did have hypocalcemia. In 47 cats with CRF, 14% with moderate CRF and 56% with advanced CRF had ionized hypocalcemia. Mean iCa for cats with advanced CRF was significantly lower than values from normal cats or cats with mild and moderate CRF. Hypocalcemia was underappreciated when based on results of tCa measurement, especially with advancing azotemia.
AIRF and postrenal failure can result in hypocalcemia that is more likely to be symptomatic because the degree of hyperphosphatemia is often greater than that observed in CRF. Dogs with AIRF had a mean serum tCa concentration of 9.8 ± 1.7 mg/dL, but iCa was not reported.589
Secondary hyperparathyroidism is common in association with renal failure, and is characterized by low or normal ionized calcium with elevated circulating concentration of serum PTH. In a study of 54 dogs with chronic kidney disease, secondary hyperparathyroidism was present in 76% of all dogs.130 In nonazotemic kidney disease (IRIS stage 1), 36% of dogs already had developed secondary hyperparathyroidism. With mild azotemia (IRIS stage 2), 50% of dogs had developed secondary hyperparathyroidism, and with moderate azotemia (IRIS state 3), 96% had secondary hyperparathyroidism. By the time severe azotemia was present (IRIS state 4), 100% of dogs had developed secondary hyperparathyroidism.
Emergency and Critical Care
Ionized hypocalcemia is common in critically ill humans in the intensive care setting and is more common in septic patients.106,333,644 The magnitude of hypocalcemia is correlated to severity of illness. Hypocalcemia with critical illness also occurs in veterinary patients.145,257 The causes of hypocalcemia in critical illness appear to be multifactorial because sepsis, systemic inflammatory response syndrome, hypomagnesemia, blood transfusions, and AIRF have been associated with hypocalcemia.145,332,636,644 In humans, hypocalcemia associated with critical illness involves decreased PTH secretion, hypercalcitonism, and altered calcium binding to proteins.465 The cause of the hypocalcemia is not related to enhanced urinary calcium excretion, decreased bone mobilization, or blunted secretion of PTH in septic patients.333 The presence of proinflammatory cytokines during sepsis is related to the development of hypocalcemia in septic patients.333 PTH is commonly elevated in this population even when normocalcemia exists.106,333 In a study of 141dogs admitted to an intensive care unit, 16% showed hypocalcemia based on iCa concentration.257 Hypocalcemia was associated with longer intensive care unit and hospital stays, but was not associated with decreased survival. Dogs with sepsis were more likely to exhibit hypocalcemia.
Up to 88% of hospitalized human patients had decreased iCa that correlated to severity of illness but not any specific diagnosis.644 The impact of hypocalcemia on patient survival has not yet been determined, although in one study, hypocalcemia and higher levels of PTH were more frequently associated with fatality.106
Ionized calcium concentration decreased in experimental dogs with hemorrhage-caused hypotension and continued to decline during replacement of blood volume with citrated whole blood.56 Hemorrhage also decreases iCa concentration in clinical dogs. Massive transfusions in 10 dogs resulted in significant ionized hypocalcemia.282
Cardiopulmonary resuscitation (CPR) may result in hypocalcemia. Dogs developed ionized hypocalcemia within 5 minutes of starting CPR in dogs with prolonged cardiac arrest and continued to decrease after 20 minutes.93,415 Serum tCa was not concordant with changes in iCa because mean serum tCa did not change, and iCa concentrations were negatively associated with lactate concentrations. Decreased iCa was most likely related to formation of complexes with lactate.
In horses with enterocolitis, decreased iCa was identified in nearly 80% of patients.575 Ionized hypocalcemia was associated with decreased iMg, increased serum phosphorus, decreased fractional urinary excretion of calcium, and increased PTH in 71% of cases. Hypocalcemia in 29% of these horses was a result of inadequate secretion of PTH, although impaired mobilization of calcium from bone and loss or sequestration of calcium within the gastrointestinal tract could not be excluded.
Acute pancreatitis may be associated with hypocalcemia. In 46 cats with acute pancreatitis, iCa concentration was low in 61% of cats.295 Suggested mechanisms that may account for low iCa in acute pancreatitis include sequestration of calcium into peripancreatic fat (saponification), increased free fatty acids, increased calcitonin secondary to hyperglucagonemia, and PTH resistance or deficit resulting from the effects of hypomagnesemia.45,145,277,505
In dogs with diabetes mellitus, 47% had ionized hypocalcemia.244 Normal iCa concentrations were noted in 49.4% of dogs from this study, and 3.5% had ionized hypercalcemia. Acute pancreatitis was diagnosed in 13% of these dogs, which could be the mechanism in some but not all of those with hypocalcemia. In a study of 127 dogs with diabetic ketoacidosis, 52% exhibited ionized hypocalcemia.286 Dogs that did not survive had lower iCa concentrations than those that did survive.
Puerperal tetany (eclampsia) typically occurs between 1 and 3 weeks postpartum in females of small dog breeds and is attributed to loss of calcium into milk during lactation, although parathyroid gland dysfunction has not been conclusively excluded.21,179 Proposed mechanisms for hypocalcemia include a poor dietary source of calcium, major loss of calcium during lactation, fetal skeletal ossification, and abnormal parathyroid gland function, including parathyroid gland atrophy. Hypophosphatemia may accompany the hypocalcemia, and clinical signs rarely occur before whelping.103 In 31 dogs with periparturient hypocalcemia, iCa was less than the reference range, and small breed dogs with large litters were typical.152 Median time from whelping to detection of clinical signs was 14 days, but variation was wide. Clinical signs most often included seizures, trembling, twitching, shaking, and stiffness. Nontypical signs included panting, behavioral changes, collapse, and whining; vomiting, diarrhea, and choking were rare. Rectal temperature was elevated, attributable to increased muscle activity. After treatment with intravenous calcium gluconate (mean dose, 115 mg/kg), iCa concentration normalized within 25 minutes in 90% of dogs. Most dogs received more than one injection of calcium gluconate, but the total calcium dose given did not correlate to initial iCa concentration. In one lactating bitch, severe hypocalcemia and hypomagnesemia occurred in association with acute onset of gastric and bladder atony, congestive heart failure, weakness, and paresis without muscle fasciculation or seizures.16
Puerperal tetany is rare in cats.602 Eclampsia was described in four cats in which hypocalcemia developed 3 to 17 days before parturition.174 Signs of depression, weakness, tachypnea, and mild muscle tremors were most common; vomiting and anorexia were less common, and prolapse of the third eyelid occurred in some cats. Hypothermia, instead of hyperthermia as seen in dogs, was observed. All cats responded to parenteral calcium gluconate initially and to oral calcium supplementation throughout gestation and lactation.
Ionized hypocalcemia is common in cats with urethral obstruction and is likely to develop in cats that also have hyperkalemia and metabolic acidosis. Cats with severe ionized hypocalcemia can exhibit compromised vital functions, although most survive with relief of urethral obstruction. In 199 cats with urethral obstruction, iCa was below the reference range in 34%, normal in 47%, and above the reference range in 19%.324 Of those with low iCa, 14% had moderate and 6% had severe hypocalcemia. In an earlier study, 75% of cats with urethral obstruction exhibited low iCa.153 Most of these cats had elevated tMg, probably from reduced renal function at the time of obstruction. Hypomagnesemia is not likely to account for the development of hypocalcemia in these cats, but iMg was not measured. Calcium regulatory hormones were not evaluated in either of these studies. Alkalinizing infusions designed to correct metabolic acidosis or for translocation of potassium into cells are often considered for treatment of cats with urethral obstruction, but these can decrease tCa and iCa concentrations.114
Rhabdomyolysis is sometimes associated with hypocalcemia, but clinical signs of hypocalcemia are rare. Mild hypocalcemia in dogs and cats with severe vehicular muscle trauma is occasionally observed (Chew, personal observations). Hypocalcemia likely occurs as a consequence of translocation of calcium into the damaged muscles. Symptomatic hypocalcemia resulting in death of three dystrophin-deficient cats occurred following anesthesia or mild exertion during restraint and subsequent acute rhabdomyolysis.208 Hypocalcemia was documented along with hyperphosphatemia, increased liver transaminases, and massive increases in creatine kinase. Hypocalcemia has been described in some dogs with fatal Vipera xanthina palestinae envenomation.17 The origin of the hypocalcemia may be multifactorial, including muscle necrosis. Renal transplantation in 14 cats resulted in decreased iCa in the 5-day postoperative period.624 All cats also had decreased serum iMg but normal tMg.
Small Intestinal Diseases
Hypocalcemia may occur in association with gastrointestinal disease. Ionized calcium concentration was below the reference range (mean, 0.99 ± 0.19 mmol/L; reference range, 1.13 to 1.33 mmol/L) in 12 dogs with intestinal lymphangiectasia.317 Ten of 13 dogs had hypoalbuminemia with a mean of 2.12 ± 0.70 g/dL, and “corrected” serum tCa was discordant with iCa measurement. Mechanisms for hypocalcemia could include calcium/fatty acid complexes in the intestinal lumen that could decrease intestinal calcium absorption. Hypovitaminosis D from malabsorption or hypomagnesemia may have contributed to hypocalcemia but was not evaluated. No dogs had clinical signs associated with hypocalcemia.
In five Yorkshire terriers and a shih tzu with protein-losing enteropathy, iCa and tMg concentrations were moderately to severely low.92,294 Concentration of PTH was increased (secondary hyperparathyroidism), and 25-hydroxyvitamin D concentration was below the reference range. It is not clear whether the apparent elevation in PTH was increased to an appropriate level in the face of low iCa, or whether maximum production was suppressed because of the effects of hypomagnesemia. Intravenous supplementation with fluids containing magnesium salts resulted in increases in PTH and iCa; 25-hydroxyvitamin D remained below the reference range.92 Following 8 weeks of treatment for inflammatory bowel disease, calcium homeostasis was normal based on normal iCa, PTH, tMg, and 25-hydroxyvitamin D concentrations. Magnesium repletion apparently resulted in resolution of hypocalcemia largely because of increased PTH secretion, whereas 25-hydroxyvitamin D concentration was still low. Resolution of weakness may have been the result of correction of hypocalcemia, hypomagnesemia, or both. Low iCa concentration with elevated PTH and low concentrations of both 25-hydroxyvitamin D and calcitriol were also noted in two dogs with protein-losing enteropathy.371 One dog was diagnosed with lymphangiectasia, and the other was diagnosed with chronic lymphocytic/plasmacytic enteritis.
Alkali Administration
The administration of alkaline agents may result in the development of hypocalcemia. Symptomatic hypocalcemia was documented in a cat treated for salicylate intoxication with sodium bicarbonate.2 Muscle fasciculation increased during treatment with sodium bicarbonate, and serum tCa was low. A single dose of intravenous sodium bicarbonate at 4 mEq/L to cats resulted in a maximal decrease of iCa 10 minutes following infusion; iCa remained below baseline for 3 hours.114 Part of the decrease in iCa was attributed to dilution and part to increased pH of serum, but most of the decrease was the result of unidentified factors. Similar findings were noted in dogs receiving sodium bicarbonate infusion.380 Twitching has been observed on rare occasion during or shortly after infusion of sodium bicarbonate solutions in cats with urethral obstruction and in dogs or cats with renal failure (Chew, personal observations) presumably because of decreases in serum iCa.
Acute Reversal of Chronic Hypercalcemia
The sudden correction of chronic hypercalcemia can result in hypocalcemia as a result of parathyroid gland atrophy and inadequate ability to synthesize and secrete PTH. This happens frequently in dogs with primary hyperparathyroidism caused by parathyroid gland adenoma following surgical excision of the affected parathyroid gland(s). The degree of parathyroid gland atrophy depends on the magnitude of hypercalcemia and its duration before correction. Two dogs with spontaneous infarction of a parathyroid gland adenoma have been reported with the development of hypocalcemia and clinical signs.485 Rapid correction of hypercalcemia following chemotherapy for lymphosarcoma or surgical excision of anal sac adenocarcinoma often results in mild hypocalcemia that is usually not associated with clinical signs, but clinical signs of hypocalcemia may occur.259 Persistent hypocalcemia has been observed in dogs following parathyroidectomy in association with hypomagnesemia. In three dogs, hypocalcemia resolved following supplementation with magnesium salts, but calcium regulatory hormones were not measured (Chew, unpublished observations).
Tumor Lysis Syndrome
Tumor lysis syndrome occurs when there is rapid destruction of sensitive tumor cells (usually lymphoid or bone marrow tumors) following chemotherapy.440 Release of intracellular products can result in hyperkalemia, hyperphosphatemia, and hyperuricemia. Hypocalcemia can develop as calcium-phosphate salts are deposited into soft tissues by mass-law effects from markedly increased serum phosphorus95,426,453 and may be associated with the development of AIRF. Tumor lysis syndrome is a rarely reported cause of symptomatic hypocalcemia in dogs,426,453 although it may be more common than previously reported because the Ohio State University oncology service has documented seven cases (Couto, personal communication, 2004).
Nutritional Secondary Hyperparathyroidism
Vitamin D deficiency and nutritional secondary hyperparathyroidism associated with low calcium and/or high phosphorus concentrations in the diet result in low serum iCa and phosphorus concentrations, with an increase in PTH secretion. Nutritional secondary hyperparathyroidism may also occur when severe gastrointestinal disease is present, limiting the absorption of calcium and vitamin D. Increased PTH secretion tends to return serum iCa concentration to normal, but decreases serum phosphorus concentration.623 The occurrence of nutritional secondary hyperparathyroidism has decreased dramatically since the advent of feeding commercially available, nutritionally complete and balanced pet food.286 Nutritional secondary hyperparathyroidism was induced in adult beagles by feeding a diet high in phosphorus and low in calcium, with a calcium to phosphorus ratio of 1:10.128 A significant increase in PTH production was seen at 10 weeks of feeding, and cancellous bone volume was reduced by 20% to 30%. Under experimental conditions, puppies fed a low-calcium, normal phosphorus content diet exhibited increased concentrations of PTH and calcitriol, with a decrease in 24,25-dihydroxyvitamin D concentration.239 In five German shepherd dog puppies fed a diet consisting of 80% steamed rice and 20% raw meat, nutritional secondary hyperparathyroidism was observed.289 This diet apparently had an adequate calcium concentration but contained an excess of phosphorus. All puppies showed moderate to marked fibrous osteodystrophy.
Serum iCa and phosphorus concentrations were below the reference range in six young cats with nutritional secondary hyperparathyroidism.573 Clinical signs referable to hypocalcemia (excitation, muscle twitching, seizures) and spontaneous fractures of bones were present in most cats. Renal secondary hyperparathyroidism preferentially affects the bones of the face (fibrous osteodystrophy), whereas nutritional secondary hyperparathyroidism tends to cause osteopenia of the long bones and vertebrae. Calcitriol concentration was mildly increased in three of four cats in which it was measured, whereas 25-hydroxyvitamin D was mildly decreased in three of three cats. PTH concentrations were increased in all cats and ranged from a minimal increase in one cat to a marked increase of 4 to 9.7 times the upper range in the remaining five cats. Cats had been fed meat only (three cats), meat combined with vegetables (two cats), or vegetables only (one cat). Dietary calcium intake was less than one tenth of the minimal nutritional requirement; dietary intake of phosphorus was mildly below the minimal requirements in five of six cats. An unfavorable calcium to phosphorus ratio existed for all diets.
A case of type 2 vitamin D-dependent rickets was described in a 5-month-old Pomeranian dog with intermittent lameness, and forelimb bowing and thickening.326 Vitamin D-dependent rickets type 2 is characterized by end-organ resistance to calcitriol. This puppy was fed a commercial puppy food and had persistent hypocalcemia with elevated PTH concentration. Despite high-dose calcitriol therapy, hypocalcemia persisted. A case of type 2 vitamin D-dependent rickets was described in a 4-month-old cat examined because of vomiting, diarrhea, muscle tremors, and mydriasis of acute onset.522 Serum tCa and tMg concentrations were decreased, and serum phosphorus, calcitriol, and PTH concentrations were increased, excluding hypoparathyroidism as the cause of hypocalcemia. Calcitriol and calcium salt supplementation resulted in the return to normocalcemia. Another case of vitamin D-dependent rickets type 2 was described in a 4-month-old kitten.556 In this kitten, serum iCa concentration was low, with elevations of both PTH and calcitriol. Serum concentration of 25-hydroxyvitamin D was within the reference range. The kitten was smaller than its littermates, had bilateral forelimb swelling, and a hunched appearance. Even with calcitriol therapy, the kitten failed to grow, had continual hypocalcemia, and died of unknown causes 7 months after diagnosis.
Vitamin D-dependent rickets type 1 has been described in a 5-month-old kitten.210 Vitamin D-dependent rickets type 1 is characterized by a deficiency of 1α-hydroxylase, which converts 25-hydroxyvitamin D to calcitriol. The kitten was examined because of generalized pain and reluctance to move. This kitten exhibited hypocalcemia, elevated serum PTH concentration, with low serum calcitriol concentration. The serum concentration of 25-hydroxyvitamin D was within the reference range. The kitten responded to calcitriol therapy.
Exotic animals may be at increased risk for the development of nutritional secondary hyperparathyroidism because nutritional requirements are not always known. Nutritional secondary hyperparathyroidism was documented in a 3-month-old tiger cub that was fed only beef with no calcium or vitamin supplementation.622 This tiger cub was reluctant to walk, exhibited osteodystrophy of the lumbosacral vertebrae, and had an elevated serum PTH concentration. Clinical signs improved after administration of vitamin D and calcium.
With the feeding of BARF (biologically appropriate raw food, or bones and raw food) and other homemade diets, the occurrence of nutritional secondary hyperparathyroidism is more likely. In a recent report, 6-week-old, large-breed puppies from two litters were fed a BARF diet on weaning.138 Puppies were weak, exhibited pain, and had abnormal-appearing joints, and some were unable to stand. In puppies that were radiographed, osteopenia was noted, with pathologic fractures apparent in multiple long bones. In euthanized puppies, the long bones were pliable, and cortices were thin. Parathyroid glands were prominent, and histologically, fibrous osteodystrophy was present in bones. Nutritional secondary hyperparathyroidism was attributable to a diet low in calcium and an inappropriate calcium to phosphorus ratio. Diffuse osteopenia and myelopathy occurred in a puppy fed a raw ground beef diet.557 This 8-month old puppy had been fed a commercially available organic premix mixed with ground beef for the previous 4 months. Vitamin D-dependent rickets Type I developed following the feeding of this nutritionally incomplete diet. While young animals may be more susceptible to nutritional secondary hyperparathyroidism, clinical signs can occur in adult dogs fed incomplete diets. Osteopenia occurred in a 6-year-old dog that had been fed a homemade diet for the previous year.136 This homemade diet did not include any vitamin or mineral supplements, and was deficient in both calcium and vitamin D. Plasma PTH concentration was elevated, and circulating 25-hydroxyvitamin D concentration was low. Severe osteopenia of the skull bones was present, with facial enlargement.
Secondary Hyperparathyroidism Associated with Hyperadrenocorticism
Hyperadrenocorticism has been associated with elevations in serum PTH concentration.461 In 68 dogs with hyperadrenocorticism, 92% had concentrations of serum PTH above the reference range. Ionized calcium was measured in 28 of these dogs, and was within or below the reference range in 20 dogs. However, the mean iCa concentration in dogs with hyperadrenocorticism was not significantly different from a group of 20 other hospital patients that did not have hyperadrenocorticism. Serum PTH concentration was significantly positively correlated to basal and post-ACTH cortisol concentrations, and to serum alkaline phosphatase concentration. The mechanism for the development of secondary hyperparathyroidism is unknown in these dogs, though glucocorticoids can decrease intestinal absorption of calcium and increase urinary calcium excretion. These effects may be sufficient to create a negative calcium balance, resulting in increased secretion of PTH.
The effect of trilostane treatment for hyperadrenocorticism on serum PTH and calcium concentrations was studied in 22 dogs.559 With treatment, serum PTH concentrations were significantly lower, and were not different from a control population that did not have hyperadrenocorticism. Serum calcium concentration increased significantly with trilostane therapy, even though there was no significant difference in calcium concentration between the pretreatment hyperadrenocorticism group and the control group. Serum phosphate concentrations also significantly decreased with trilostane therapy, but there was still a significant difference post-treatment between the dogs with hyperadrenocorticism and the control group.
Effects of drugs
Drug administration may cause a decrease in iCa. A significant decrease in iCa was observed in dogs administered enrofloxacin at 5 mg/kg intramuscularly once daily for 14 days.138 Mean iCa decreased to its nadir on day 3, remained below normal at day 10, and normalized by day 14 despite continued administration of enrofloxacin.
The administration of mithramycin or bisphosphonates can cause mild hypocalcemia as a side effect in humans, but symptomatic hypocalcemia is rare.570 The potential for development of hypocalcemia exists in dogs following mithramycin administration because normal dogs and those with malignancy-associated hypercalcemia undergo significant decreases in serum iCa and tCa.486,487 Use of mithramycin is reserved for emergency management of hypercalcemia refractory to other treatments because of severe toxicity in some dogs.
Phosphate enema administration can result in hypocalcemia after rapid absorption of phosphate, hyperphosphatemia, and subsequent mass law interaction with serum calcium. This is particularly a problem in cats and small dogs in which death can occur.18,281,512,574 Serum tCa decreased within 45 minutes of administration of a hypertonic phosphate enema to cats and persisted for 4 hours.18 Mean serum phosphorus was more than 14 mg/dL within 15 minutes, and increases persisted for 4 hours. Serum tCa concentrations were negatively correlated to serum phosphorus. Mild hypernatremia, severe hyperphosphatemia (mean, 37.6 mg/dL), and hypocalcemia were noted in five cats. Phosphate enemas should not be used in small dogs, cats, or in debilitated patients of any size.
Hypoparathyroidism
Hypoparathyroidism is an absolute or relative deficiency of PTH secretion that can be permanent or transient. Hypocalcemia and clinical signs referable to low iCa concentration are the hallmarks of advanced hypoparathyroidism. Hypoparathyroidism in dogs is most commonly idiopathic, whereas surgical removal of or injury to the parathyroid gland during thyroidectomy to correct hyperthyroidism is the most common cause in cats.
Idiopathic chronic inflammation of parathyroid tissue occurs sporadically in both dogs and cats but more commonly in dogs. It is presumed that parathyroiditis has an immune-mediated mechanism. Histopathologic study of affected parathyroid glands reveals inflammatory cell infiltration (lymphocytes, plasma cells, and neutrophils), fibrosis, and loss of secretory cells.87,179,444,446,530 Clinical signs occurred 1 to 26 weeks (mean, 7 weeks) before diagnosis of primary hypoparathyroidism in cats444 and 1 day to 25 weeks (mean, 3 weeks) before diagnosis in dogs.87 Primary hypoparathyroidism and parathyroiditis occur in dogs and cats of any age but more frequently in female dogs and male cats. In 735 dogs with primary hypoparathyroidism, 62% were female and 38% were male.466 Mean age was 7.0 ± 3.9 years, with 71% of diagnoses occurring in purebred dogs. The highest odds ratios for hypoparathyroidism correcting for breed popularity occurred in the standard schnauzer, Scottish terrier, miniature schnauzer, West Highland white terrier, and dachshund. Reduced risk was identified for the German shepherd dog, shih tzu, and Labrador retriever. In another study, 357 dogs were diagnosed with primary hypoparathyroidism over a 2-year period.521 Mixed-breed dogs accounted for 25% of the cases, with 13% schnauzers, 7% Labrador retrievers, 5% dachshunds, 4% Yorkshire terriers, 4% poodles, 3% golden retrievers, and 3% Scottish terriers without correction for breed popularity. There were 59 other dog breeds represented with an incidence of less than 3% each. In a study of 17 dogs, mixed-breed dogs, German Shepherd dogs, Saint Bernards, and terrier breeds were most commonly affected.503
Serum tCa concentration is usually less than 6.5 mg/dL (often 4.0 to 4.9 mg/dL) in dogs with primary hypoparathyroidism. Dogs that have episodes of tetany or seizures often have serum tCa concentrations less than 6.0 mg/dL. Serum phosphorus concentration is greater than serum calcium concentration in nearly all affected dogs and cats, and most have hyperphosphatemia. Parathyroid gland biopsy may confirm the diagnosis of lymphocytic parathyroiditis as the cause of primary hypoparathyroidism, but the parathyroid glands can be difficult or impossible to locate during surgical exploration because of atrophy and fibrosis. Parathyroid gland biopsy is not recommended to confirm hypoparathyroidism since the advent of validated PTH assays for use in the dog and cat.
Diagnosis of Hypoparathyroidism
Inappropriately low concentrations of PTH result in hypocalcemia, hyperphosphatemia, and decreased concentrations of 1,25-dihydroxyvitamin D (calcitriol). Hypocalcemia results from increased urinary loss of calcium (hypercalciuria), reduced bone resorption, and decreased intestinal absorption of calcium. Hyperphosphatemia results from decreased urinary loss of phosphorus (hypophosphaturia) that overrides the effects of decreased bone resorption and decreased intestinal absorption of phosphorus (secondary to calcitriol deficit) on serum phosphorus concentration. PTH is a potent stimulator and phosphorus is a potent inhibitor of the 25-hydroxyvitamin D-1α-hydroxylase enzyme system in renal tubules. Consequently, the absence of PTH and the presence of hyperphosphatemia act together to decrease renal synthesis of calcitriol. Decreased concentrations of calcitriol contribute to hypocalcemia via decreased intestinal calcium absorption. Hypocalcemia unrelated to low PTH concentrations may arise from increased uptake of calcium by bone after rapid correction of long-standing hyperparathyroidism or hyperthyroidism, both of which are associated with loss of bone calcium before treatment (“hungry bone” syndrome).548,567,617
Definitive diagnosis of primary hypoparathyroidism is based on the combination of clinical signs (see Box 6-5), low iCa concentration, and PTH concentration inappropriately low to the magnitude of ionized hypocalcemia. Hypoparathyroidism is the only possible diagnosis when low serum calcium concentration, high serum phosphorus concentration, normal renal function, and low PTH concentration are present in combination. Low serum calcium and high serum phosphorus concentrations can be encountered during nutritional and renal secondary hyperparathyroidism, after phosphate-containing enemas, and during tumor lysis syndrome, but PTH is increased in all of these conditions.
PTH should be measured in patients with chronic hypocalcemia of undetermined cause. Primary hypoparathyroidism requires lifelong treatment, and confirmation of the diagnosis with PTH measurement is recommended. It is not necessary to measure PTH routinely in patients with postsurgical hypocalcemia because this effect is usually transient and the cause obvious. PTH concentrations should be determined for patients in which hypocalcemia does not resolve. Absolute hypoparathyroidism is present if a PTH concentration below the reference range is detected simultaneously with hypocalcemia. Relative hypoparathyroidism is present if PTH concentration is inappropriately low but remains within the normal reference range. Increased serum phosphorus and decreased calcitriol concentrations provide further support for a diagnosis of hypoparathyroidism.232
Causes of Hypoparathyroidism
The causes of hypoparathyroidism can be divided into three categories: (1) suppressed secretion of PTH without parathyroid gland destruction,115,145 (2) sudden correction of chronic hypercalcemia, and (3) absence or destruction of the parathyroid glands. The most common category of hypoparathyroidism in dogs and cats is absence or destruction of the parathyroid glands.
Postoperative hypocalcemia developed 1 to 3 days after thyroidectomy in approximately 20% to 30% of cats.51,187,190,223,609 Some cats developed hypocalcemia as late as 1 to 2 weeks after surgery. The surgical technique used for thyroidectomy influences the chances that hypocalcemia will develop, and hypocalcemia occurred in more than 80% of cats when original extracapsular technique was used.187 When a modified intracapsular dissection technique was used, transient hypocalcemia developed in about 6% of cats postthyroidectomy.399 Hypocalcemia resolved in all cats within 6 days with therapy. Bilateral thyroidectomy results in loss of the two internal parathyroid glands, and hypoparathyroidism is permanent in patients in which the external parathyroid glands are completely removed during bilateral thyroidectomy. Hypocalcemia and hypoparathyroidism do not develop if the two external parathyroid glands are not excised or damaged during thyroidectomy. Normocalcemia can be maintained with one completely functional parathyroid gland.
Hypoparathyroidism is usually transient when the external parathyroid glands are retained but have their blood supply disrupted (parathyroid gland ischemia after physical trauma, vessel stretching, suture, cautery, or transection) during surgery. Permanent hypoparathyroidism is rare, but it may take as long as 3 months to be certain whether remaining parathyroid tissue can recover by hyperplasia.51,446,504 Similar injury to parathyroid glands can occur during any extensive surgery of the neck in dogs241,301 or cats or after exploration of the neck for unilateral parathyroid gland removal. Restored vascular supply to damaged parathyroid tissue seems unlikely as the mechanism for recovery from hypocalcemia. It is more likely that hyperplasia and hypertrophy of parathyroid gland remnants left behind during surgery or ectopic parathyroid tissue achieve sufficient mass to synthesize adequate amounts of PTH. Experimental cats subjected to parathyroidectomy predictably developed hypocalcemia and low serum PTH concentration, but, interestingly, the hypocalcemia resolved, although the PTH concentrations remained low.188 Autotransplantation of parathyroid tissue after bilateral thyroparathyroidectomy was associated with reduced morbidity and rapid return of serum calcium concentrations to normal in experimental cats.425
Long-standing ionized hypercalcemia causes normal parathyroid tissue to atrophy. If hypercalcemia is nonparathyroid in origin, PTH concentrations will already be low. Rapid correction of hypercalcemia results in hypocalcemia because the atrophic parathyroid glands cannot respond immediately to the need for increased PTH secretion. Surgical removal of a single parathyroid gland tumor (usually an adenoma) commonly causes postoperative hypocalcemia in this manner. Hypocalcemia severe enough to require treatment is likely to develop within 24 to 48 hours. Nearly 50% of dogs with primary hyperparathyroidism can be expected to develop clinical signs of hypocalcemia 3 to 6 days after surgical removal of a parathyroid gland tumor. Hypocalcemia is more likely to develop in dogs with higher presurgical iCa concentrations. More than one half of hyperparathyroid dogs exhibit a rapid decrease in serum iCa concentration that normalizes within 24 hours of surgery. Serum iCa concentrations in the remaining dogs usually normalize by 2 or 3 days after surgery, but some require as long as 5 days. Hypoparathyroidism resolves for most affected dogs in 8 to 12 weeks. Cats develop hypocalcemia less frequently than dogs after surgical correction of primary hyperparathyroidism.141,285
Hypoparathyroidism following spontaneous infarction of a parathyroid gland tumor previously causing hypercalcemia is a rare condition that can result in acute hypocalcemia in dogs.485 The rapid correction of cancer-associated hypercalcemia (e.g., with tumor excision and chemotherapy) can be associated with hypocalcemia and low PTH concentration, but hypocalcemia is usually minor and transient.
Both acute hypermagnesemia and severe magnesium depletion may suppress PTH secretion.63,465,572 As with hypocalcemia, mild acute hypomagnesemia stimulates PTH secretion, but severe magnesium depletion decreases PTH secretion, increases end-organ resistance to PTH, and may impair calcitriol synthesis. The end-organ resistance to PTH that develops during magnesium depletion may persist for days after magnesium repletion and resumption of normal PTH concentrations in humans. Until recently, hypomagnesemia has been reported rarely in dogs and cats with hypoparathyroidism. Normal serum tMg does not guarantee a normal iMg concentration because there is substantial discordance between these two measurements.
Magnesium depletion can cause functional hypoparathyroidism, and measurement of serum iMg concentration is recommended to exclude or identify this form of hypoparathyroidism. Serum tMg concentrations when measured in dogs and cats with primary hypoparathyroidism usually have been normal.87,179 In 357 dogs with primary hypoparathyroidism, mean iCa and mean PTH concentrations were below the reference range.521 The iMg concentration was below the reference range in 39%, within the reference range in 55%, and above the reference range in 6% of dogs with hypoparathyroidism. Of the 55% of dogs with iMg within the reference range, 69% had an iMg concentration within the lower half of the reference range, and only 31% had an iMg concentration within the upper half of the reference range.
Despite the relative paucity of published reports from cats, hypoparathyroidism was diagnosed in 27 cats during a 2-year period.521 Of cats with hypoparathyroidism, 59% were domestic shorthairs, 22% were an unspecified breed, and 15% were Siamese. Mean serum iCa concentration was below the reference range, and mean PTH concentration was in the lower half of the reference range. The iMg concentration was below the reference range in 37%, within the reference range in 59%, and above the reference range in 4%. Of the 59% of cats with iMg within the reference range, 88% had an iMg concentration within the lower half of the reference range, and only 12% had an iMg concentration within the upper half of the reference range. These results suggest that a large number of dogs and cats with hypoparathyroidism also exhibit subnormal or marginal iMg concentrations. The impact of magnesium supplementation in the treatment of hypoparathyroidism should be investigated. Although primary hypoparathyroidism is usually diagnosed in older cats, it has been reported in a 6-month-old kitten initially evaluated for lethargy, inappetence, muscle tremors, and seizures.34
Most causes of primary hypoparathyroidism have been attributed to immune destruction of parathyroid tissue. Early reports of hypoparathyroidism in dogs and cats did not consistently evaluate magnesium status and used tMg when it was reported. Based on discordance of magnesium status using iMg versus tMg, hypomagnesemia based on tMg assessments may have underestimated a role for hypomagnesemia in the genesis of hypoparathyroidism in animals. Hypomagnesemia may decrease cell membrane receptor sensitivity to iCa and PTH, as well as decrease PTH synthesis.325 Serum iMg concentration should be measured when iCa and PTH concentrations are determined.
The potential role of magnesium depletion in the development of post-thyroidectomy hypocalcemia in cats has not been explored. Magnesium depletion could play a role in the development of postoperative hypocalcemia in cats with hyperthyroidism, because hyperthyroidism can be associated with magnesium depletion.179
Canine distemper virus (CDV)-induced parathyroid hypofunction may contribute to development of hypocalcemia. Dogs infected with CDV had reduced serum tCa concentrations associated with ultrastructural evidence of parathyroid gland inactivity, degeneration, and viral inclusions.608
Miscellaneous Causes of Hypocalcemia
Metabolites of ethylene glycol can chelate calcium and become deposited in soft tissues, resulting in hypocalcemia. Both dogs and cats exhibit hypocalcemia after ethylene glycol ingestion.569 Seizures have been observed in dogs within hours of ingestion; renal function was normal at this time (Chew, personal observations). Hypocalcemia often develops later when renal function is severely reduced from acute renal failure and when hyperphosphatemia is severe.
Pansteatitis has been associated with severe hypocalcemia in a cat.641 A 13-year-old cat had a history of anorexia, lethargy, and abdominal enlargement; decreased serum iCa and tCa concentrations were observed with an elevation in PTH concentration (secondary hyperparathyroidism). No clinical signs of hypocalcemia were noted. Pansteatitis was confirmed on histopathology. The exact mechanism of hypocalcemia was not determined, although it was probably due to the formation of calcium soaps.
Acute decreases in iCa concentrations are most commonly caused by acute respiratory alkalosis in humans.465 It is likely that this phenomenon also occurs in dogs and cats subjected to the stresses of hypocalcemia and a visit to a veterinary clinic. This could explain the phenomenon of mild stress-induced seizures or tetany in dogs that have hypocalcemia, as the alkalosis shifts some calcium to the protein-bound state, causing more severe ionized hypocalcemia.
Treatment of hypocalcemia
Puerperal tetany is the condition most likely to require correction of hypocalcemia acutely, but chronic treatment is not needed. Hypoparathyroidism is the only condition requiring acute and chronic treatment to alleviate clinical signs of hypocalcemia. Other conditions associated with hypocalcemia are transient or result in minimal decreases in serum calcium concentration, do not cause obvious clinical signs, and only occasionally necessitate calcium replacement therapy. No treatment is indicated for hypocalcemia attributable entirely to hypoalbuminemia or hypoproteinemia, assuming that the iCa fraction is normal.
Treatment is individualized based on severity of clinical signs, magnitude of hypocalcemia, rapidity of decline in serum calcium concentration, and trend of serial serum calcium measurements (i.e., further decrease or stability). Aggressive treatment is prescribed for patients with severe clinical signs of hypocalcemia, patients with severe ionized hypocalcemia with or without signs, and patients in which serum calcium concentration is steadily or rapidly declining. Acute, subacute, and chronic rescue treatment regimens are available using supplementation with calcium salts and vitamin D metabolites. The goal of therapy is to increase serum calcium concentration to a level that alleviates the signs of hypocalcemia, minimizes the likelihood of the development of hypercalcemia, and reduces the magnitude of hypercalciuria (especially in patients with hypoparathyroidism). It is usually not necessary or desirable to return serum calcium concentration completely to normal because many clinical signs improve dramatically with slight increases in serum calcium concentration, and the consequences of overcorrection can be serious. For suspected temporary postsurgical hypoparathyroidism, it is desirable to keep the serum calcium concentration relatively low to maximize compensatory hypertrophy of remaining parathyroid glands.
In patients with hypoparathyroidism, no treatment regimen completely compensates for the full range of physiologic actions of the absent PTH. Vitamin D metabolite treatment corrects the low intestinal absorption of calcium but does not completely protect the kidneys from hypercalciuria as would occur in the presence of PTH. Similarly, vitamin D metabolites do not exert as powerful an effect on bone in the absence of PTH. Replacement therapy with once-daily subcutaneous injections of human PTH (1-34) in human subjects was highly effective in providing good 24-hour control of serum calcium concentration.616 Use of synthetic human amino-terminal PTH for the treatment of veterinary patients is possible because the amino-terminal portions of PTH are highly conserved, function in vivo in animals, and would be unlikely to elicit an immune response.
Hypocalcemia severe enough to cause clinical signs should be anticipated in dogs undergoing parathyroidectomy as treatment for hypercalcemia related to a parathyroid gland adenoma. Animals with very high concentrations of serum calcium, PTH, and serum ALP may be at greater risk of developing postoperative hypocalcemia. Postoperative hypocalcemia in this instance is the consequence of acute hypoparathyroidism resulting from chronic suppression of remaining parathyroid glands and calcium uptake into “hungry” bones. Hypocalcemia should be anticipated in cats that undergo bilateral thyroidectomy, because up to 30% of cats can be expected to have transiently lowered serum calcium concentrations.
Therapy should be instituted before the development of tetany. Preemptive therapy to increase serum calcium concentration may be a good choice for animals with marked hypocalcemia with no apparent clinical signs or for those in which serum calcium concentration is steadily or rapidly declining. Prophylactic therapy to prevent hypocalcemia in dogs undergoing surgery for hyperparathyroidism should be considered, especially in dogs with severe hypercalcemia. Active vitamin D metabolites should be started before surgery in these instances because there is a lag time until maximal effect is achieved. Vitamin D metabolites given at the time of surgery or just after surgery fail to prevent development of hypocalcemia.
Autotransplantation of normal parathyroid glands is a treatment option used to minimize postoperative hypocalcemia when it is obvious that damage has been done to the parathyroid glands during surgery (bilateral extracapsular thyroidectomy). Autotransplantation of normal parathyroid glands was studied in experimental cats following bilateral extracapsular thyroparathyroidectomy.425 External parathyroid glands were harvested and dissected from thyroid tissue, and small pieces of parathyroid tissue were embedded into the sternohyoideus muscle. Cats showed an average decrease of 44% in serum tCa with the nadir occurring 1.9 days following surgery. Hypocalcemia was present for a median of 14 days in cats having parathyroidectomy and autotransplantation in this study compared with a median of 71 days in cats of a previous report involving parathyroidectomy without autotransplantation.188 Seven of eight cats with autotransplantation of parathyroid glands regained normocalcemia within 20 days without oral calcium salt supplementation.425
Acute Management of Hypocalcemia Causing Tetany or Seizures
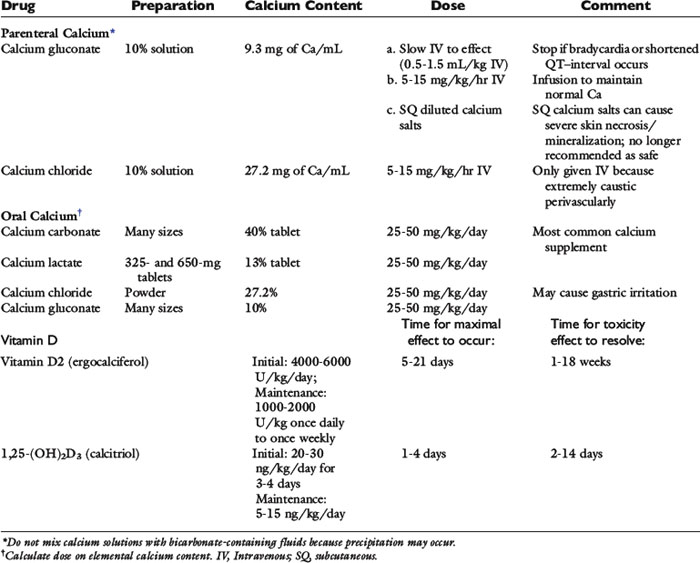
Tetany or seizures caused by hypocalcemia require treatment with intravenously administered calcium salts. Calcium is administered to effect, at a dosage of 5 to 15 mg/kg of elemental calcium (0.5 to 1.5 mL/kg of 10% calcium gluconate) over a 10- to 20-minute period.115,179,446,447 The calcium content of different calcium salts varies considerably (Table 6-5). There is no difference in effectiveness of calcium salts administered intravenously to correct hypocalcemia when the dose is based on elemental calcium content. Calcium gluconate is often the calcium salt of choice because it is nonirritating if the solution is inadvertently injected perivascularly. In contrast, calcium chloride is extremely irritating to tissues but provides more elemental calcium in each milliliter of solution (see Table 6-5).
The heart rate and electrocardiogram should be monitored during acute infusions of calcium salts. Bradycardia may signal the onset of cardiotoxicity arising from excessively rapid infusion of calcium. Sudden elevation of the ST segment or shortening of the QT–interval also may indicate cardiotoxicity resulting from the calcium infusion. Not all clinical signs abate immediately after acute correction of hypocalcemia; some may persist for 30 to 60 minutes. Nervousness, panting, and behavioral changes may persist despite return of normocalcemia during this period, perhaps reflecting a lag in equilibration between cerebrospinal fluid and ECF calcium concentrations.179,297,504 Hyperthermia that resulted from increased muscle activity or seizures may also take time to dissipate.
Subacute Management of Hypocalcemia
The initial bolus injection of elemental calcium can be expected to decrease signs of hypocalcemia for as little as 1 hour to as long as 12 hours if the underlying cause of hypocalcemia has not been corrected. Vitamin D metabolites should be administered as soon as possible because some of them require a few days before intestinal calcium transport is maximized. Calcitriol exerts initial effects on the intestine within 3 to 4 hours.601 Additional parenteral calcium salt administration is necessary until therapy with vitamin D metabolites is effective at maintaining serum calcium concentration at an acceptable level.
Multiple intermittent intravenous injections of calcium salts can be administered to control clinical signs, but this method is not recommended because wide fluctuations in serum calcium concentration are observed. Instead, continuous intravenous infusion of calcium is recommended at 60 to 90 mg/kg/day elemental calcium (2.5 to 3.75 mg/kg/hr) until oral medications provide control of serum calcium concentration.87,179,446,447 Initial doses in the higher range are administered to patients with more severe hypocalcemia, and the dose decreased according to the serum calcium concentration achieved. The intravenous dose of calcium is further reduced as oral calcium salts and vitamin D metabolites become more effective.
Ten milliliters of 10% calcium gluconate provides 93 mg of elemental calcium. A convenient method for infusing calcium is available when intravenous fluids are given at a maintenance volume of 60 mL/kg/day (2.5 mL/kg/hr). Approximately 1, 2, or 3 mg/kg/hr elemental calcium is provided by adding 10, 20, or 30 mL of 10% calcium gluconate, respectively, to each 250-mL bag of fluids. Calcium salts should not be added to fluids that contain lactate, acetate, bicarbonate, or phosphates because calcium salt precipitates can occur. Alkalinizing fluids that contain or generate bicarbonate should be avoided because they can decrease iCa and may unmask the clinical signs of hypocalcemia in animals with borderline hypocalcemia.
Subcutaneous administration of calcium gluconate has been regarded as safe for use in dogs with hypocalcemia when diluted to a ratio of at least 1:1 by volume. The use of calcium chloride is too caustic to ever be given subcutaneously. However, a recent report raises concerns about the safety of calcium gluconate administration subcutaneously. A 6-month-old border collie with hypoparathyroidism was initially treated with intravenous calcium gluconate, followed by oral calcitriol and calcium carbonate.513 This dog then received subcutaneous calcium gluconate three times daily for 2 days, and calcium gluconate was diluted as previously recommended. Fever and pain, swelling, and erythema of the ventral abdomen were obvious after 2 days of subcutaneous calcium gluconate treatments. Initial skin biopsy revealed calcinosis cutis with pyogranulomatous dermatitis and dermoepidermal separation. The dog’s condition worsened; ulceration involving about 80% of the skin developed over the trunk; and the dog was euthanized. A second skin biopsy revealed severe pyogranulomatous panniculitis with mineralization of adipocytes.
Reports of this reaction to the subcutaneous administration of calcium gluconate had not previously been reported in dogs despite its extensive use by some institutions (Feldman, personal communication, 2005). Unfortunately, we are aware of at least three other dogs with similar severe reactions to the subcutaneous administration of properly diluted calcium gluconate as treatment for primary hypoparathyroidism, resulting in euthanasia for most (Chew, personal communications, 2003, 2004). Differences in an individual animal’s susceptibility to the effects of calcium salts on subcutaneous tissues could account for severe reactions in some dogs. All dogs with this severe tissue reaction were also receiving calcitriol, which may potentiate more local dramatic effects in the subcutaneous tissues as compared with less active vitamin D metabolites (cholecalciferol, ergocalciferol, and dihydrotachysterol) commonly used in the past.
There are only two reports of cats with primary hypoparathyroidism that were treated with subcutaneous administration of calcium gluconate. No adverse effects were noted in one report.195 Iatrogenic calcinosis cutis, skin necrosis, and scarring occurred at sites of diluted calcium gluconate injection and sites where injected fluids pooled in one cat.501 This cat survived. Because of the severity of adverse reactions that have recently been observed in dogs and a cat, the administration of subcutaneous fluids containing calcium gluconate is no longer recommended as a safe and predictable treatment.
Subacute and Chronic Maintenance
Supplemental elemental calcium is administered orally (see Table 6-5) to guarantee adequate calcium for intestinal absorption after treatment with vitamin D metabolites. Oral calcium administered by pill or slurry is most important during initial treatment, especially if the animal is not eating. Active intestinal transport of calcium is under the control of calcitriol when calcium intake is low, but vitamin D-independent (passive) intestinal absorption of calcium occurs when calcium intake is high. The passive mechanisms for intestinal calcium transport can be used therapeutically before the actions of vitamin D take effect in the intestine. In most patients, normal dietary intake of calcium is sufficient to maintain adequate serum calcium concentrations in the presence of vitamin D metabolite treatment. Consequently, oral calcium salt supplementation can be tapered and discontinued in many instances as vitamin D compounds reach maximal effect.
Calcium carbonate is the most widely used oral preparation of the calcium salts because it contains the greatest percentage of elemental calcium. This approach allows fewer pills to be administered. The degree of calcium ionization from its salt and its bioavailability for absorption vary for each calcium salt and with conditions in the intestine. Consequently, it is not a simple matter to determine the bioavailable elemental calcium content of a specific oral calcium salt. Oral calcium is usually administered at 25 to 50 mg/kg/day elemental calcium in divided doses. Oral calcium carbonate serves as an intestinal phosphate binder in addition to providing calcium for intestinal absorption. It is advisable to continue oral calcium carbonate therapy for its intestinal phosphate-binding effects if serum phosphorus concentration remains increased. Lower serum phosphorus concentrations may allow increased endogenous synthesis of calcitriol because phosphate inhibits renal synthesis of calcitriol.
Vitamin D preparations (see Table 6-5) include ergocalciferol, cholecalciferol, 25-hydroxycholecalciferol (calcidiol), 1α-hydroxycholecalciferol, and calcitriol. Ergocalciferol and calcitriol are the preparations most commonly used in veterinary medicine. Lifelong treatment with some form of vitamin D metabolite is necessary for patients with primary hypoparathyroidism or postoperative hypocalcemia that fails to resolve spontaneously.
Ergocalciferol is favored by some because of its low cost,465 but it has several features that make it the least attractive agent for the treatment of hypocalcemia. Ergocalciferol and its immediate metabolite, 25-hydroxyergocalciferol, have low VDR avidity; thus, high doses are necessary. Ergocalciferol is highly lipid soluble, and several weeks are required to saturate body stores and achieve a maximal effect. It also has a long half-life. Consequently, prolonged periods of hypercalcemia occur after overdose with ergocalciferol. In addition, there is extreme individual variation in the dose of ergocalciferol required to achieve a target serum calcium concentration. Use of loading doses reduces the time required to achieve a maximal effect (see Table 6-5).
Calcitriol is the vitamin D metabolite of choice to provide calcemic actions because it has the most rapid onset of maximal action and the shortest biologic half-life. Calcitriol is approximately 1000 times as effective as parent vitamin D and 500 times as effective as its precursor, calcidiol (25-hydroxyvitamin D), in binding to the VDR. The dose of calcitriol can be adjusted frequently because of its short half-life and rapid effects on serum calcium concentration. If hypercalcemia occurs, it abates quickly after dose reduction. The half-life of calcitriol in blood is 4 to 6 hours, whereas its biologic half-life is 2 to 4 days. Loading protocols for use of calcitriol in animals have not been reported, but it is logical to use a loading protocol when more rapid correction of serum calcium concentration is desirable. A calcitriol dosage of 30 to 60 ng/kg/day has been recommended.87,179 This dosage may be satisfactory as a loading dose, but in our experience it is too high for chronic maintenance therapy. Calcitriol dosages for chronic maintenance therapy in humans range from 10 to 40 ng/kg/day, and doses are divided and given twice daily.232,465,616 We have used loading dosages of 20 to 30 ng/kg/day for 3 to 4 days and maintenance dosages of 10 to 20 ng/kg/day in most patients. The dose of calcitriol is divided and given twice daily to ensure sustained priming effects on intestinal epithelium for calcium transport. Calcitriol is commercially available in 0.25- and 0.50-μg capsules (250 and 500 ng per capsule, respectively). It is likely that reformulation of calcitriol in doses suitable for a variety of animal sizes will be necessary. It may be useful to prescribe calcitriol in liquid formulation so that small adjustments in dosage can be made accurately. A number of specialty pharmacies reformulate human drugs for veterinary use and can create any calcitriol dose needed.
Clinical follow-up and potential complications
Periods of hypocalcemia and hypercalcemia occur sporadically in patients during initial efforts to manage serum calcium concentration. Daily measurement of serum tCa concentration during stabilization is necessary. Weekly serum calcium measurements should suffice during maintenance therapy until target serum calcium concentration has been achieved and maintained. Measurement of serum tCa concentration is recommended every 3 months thereafter in animals with permanent hypoparathyroidism. Serum calcium concentration should be adjusted to just below the reference range. This not only lessens the likelihood that hypercalcemia will develop but also reduces the magnitude of hypercalciuria that occurs in patients with PTH deficiency. Maintaining a mildly decreased serum calcium concentration also ensures a continued stimulus for hypertrophy of the remaining parathyroid tissue in patients with postoperative hypoparathyroidism.
A change in dosage of vitamin D metabolites should only occur after maximal effect has occurred and should be altered gradually. The time lag for maximal effect varies with the different vitamin D metabolites (see Table 6-5). Dosage increases of 10% to 25% are recommended when serum calcium concentration is still below the target level.446,447 Vitamin D metabolite and calcium salt supplementation should be discontinued temporarily in patients that develop hypercalcemia.
Hypercalcemia is a serious adverse effect of treatment that can result in death or renal damage causing acute or CRF.111,115,314 Early signs of hypercalcemia should be explained to owners, who should be instructed to seek veterinary attention immediately if clinical signs suggest hypercalcemia. Clinical signs of hypercalcemia that clients are likely to recognize include polydipsia, polyuria, anorexia, vomiting, and lethargy. Animals with severe hypercalcemia require hospitalization. Fluids, furosemide, corticosteroids, bisphosphonates, calcitonin, or some combination may be required. All patients with symptomatic, vitamin D metabolite-induced hypercalcemia should be given a calcium-restricted diet because increased intestinal absorption of calcium contributes substantially to the development of hypercalcemia in hypervitaminosis D.
Patients that maintain serum iCa concentrations in the target zone are often managed successfully for years. Twenty-four of 25 dogs with primary hypoparathyroidism were managed successfully for more than 5 years,179 and long-term management was successful in a small number of cats.444 Patients that develop episodic or prolonged hypercalcemia during treatment have a poor prognosis. Management with calcitriol is easier and more successful in inducing and maintaining serum iCa concentrations in the target zone than are older therapeutic approaches.
Hypercalciuria, nephrocalcinosis, urolithiasis, and reduced renal function have occurred in humans treated for chronic hypoparathyroidism.232,572,616 As many as 80% of human patients treated for 2 years or longer have decreased creatinine clearance.616 These abnormalities can be attributed to episodes of hypercalcemia and hyperphosphatemia and to hypercalciuria that occurs in the absence of the actions of PTH on the renal tubules. In the absence of PTH, hypercalciuria occurs more readily at all serum iCa concentrations and is especially severe as iCa concentrations approach the normal range, which increases the filtered load of calcium. Nephrocalcinosis, reduced renal function, and CRF have also been suspected in veterinary patients receiving long-term treatment for hypoparathyroidism, but the risk for these disorders has not been critically evaluated.446
Vitamin D metabolite treatment is gradually tapered and then discontinued in patients with postsurgical hypoparathyroidism because hypocalcemia is usually transient. Most cats are able to maintain normal serum iCa concentrations 2 weeks after thyroidectomy, although some may take as long as 3 months. Dogs with hypocalcemia usually require 6 to 12 weeks of treatment after removal of a parathyroid gland adenoma. A reduction in dose of vitamin D metabolites is usually begun 1 month after initiation of therapy. If serum iCa concentration declines substantially, the previous dose is resumed, and reduction is attempted again 1 or 2 months later. Permanent hypoparathyroidism is likely if failure to maintain acceptable serum iCa concentration occurs after reduction of the vitamin D metabolite dose at 3 months.
1 Abou-Samra A.B., Juppner H., Force T., et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol triphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992;89:2732-2736.
2 Abrams K.L. Hypocalcemia associated with administration of sodium bicarbonate for salicylate intoxication in a cat. J Am Vet Med Assoc. 1987;191:235-236.
3 Adamantos S., Boag A. Total and ionised calcium concentrations in dogs with hypoadrenocorticism. Vet Rec. 2008;163(1):25-26.
4 Adami S., Zamberlan N. Adverse effects of bisphosphonates; a comparative review. Drug Saf. 1996;14:158-170.
5 Adams J.S., Sharma O.P., Diz M.M., et al. Ketoconazole decreases the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J Clin Endocrinol Metab. 1990;70:1090-1095.
6 Adin D.B., Taylor A.W., Hill R.C., et al. Intermittent bolus injection versus continuous infusion of furosemide in normal adult greyhound dogs. J Vet Intern Med. 2003;17:632-636.
7 Adler A.J., Ferran N., Berlyne G.M. Effect of inorganic phosphate on serum ionized calcium concentration in vitro: a reassessment of the “trade-off hypothesis.”. Kidney Int. 1985;28:932-935.
8 Almaden Y., Canalejo A., Ballesteros E., et al. Regulation of arachidonic acid production by intracellular calcium in parathyroid cells: effect of extracellular phosphate. J Am Soc Nephrol. 2002;13:693-698.
9 Almaden Y., Felsenfeld A.J., Rodriguez M., et al. Proliferation in hyperplastic human and normal rat parathyroid glands: role of phosphate, calcitriol, and gender. Kidney Int. 2003;64:2311-2317.
10 Almirall J., Lopez T., Vallve M., et al. Safety and efficacy of sevelamer in the treatment of uncontrolled hyperphosphataemia of haemodialysis patients. Nephron Clin Pract. 2004;97:c17-22.
11 Amin M., Fawzy A., Hamid M.A., et al. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124:2093-2097.
12 Anderson T.E., Legendre A.M., McEntee M.M. Probable hypercalcemia of malignancy in a cat with bronchogenic adenocarcinoma. J Am Anim Hosp Assoc. 2000;36:52-55.
13 Andress D.L. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kid Int. 2006;69:33-43.
14 Anthony L.B., May M.E., Oates J.A. Case report: lanreotide in the management of hypercalcemia of malignancy. Am J Med Sci. 1995;309:312-314.
15 Arceneaux K.A., Taboada J., Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc. 1998;213:658-664.
16 Aroch I., Ohad D.G., Baneth G. Paresis and unusual electrocardiographic signs in a severely hypomagnesaemic, hypocalcaemic lactating bitch. J Small Anim Pract. 1998;39:299-302.
17 Aroch I., Segev G., Klement E., et al. Fatal Vipera xanthina palestinae envenomation in 16 dogs. Vet Hum Toxicol. 2004;46:268-272.
18 Atkins C.E., Tyler R., Greenlee P. Clinical, biochemical, acid-base, and electrolyte abnormalities in cats after hypertonic sodium phosphate enema administration. Am J Vet Res. 1985;46:980-988.
19 Aubin J.E., Heersche J.N. Vitamin D and osteoblasts. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:313-328.
20 Aucella F., Scalzulli R.P., Gatta G., et al. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure; a synergistic effect with r-HuEpo. Nephron Clin Pract. 2003;95:c121-7.
21 Austad R., Bjerkas E. Eclampsia in the bitch. J Small Anim Pract. 1976;17:793-798.
22 Bae B.K., Kim C.W., Choi U.S., Choi E.W., Jee H., Kim D.Y., et al. Hypercalcemia and high parathyroid hormone-related peptide concentration in a dog with a complex mammary carcinoma. Vet Clin Pathol. 2007;36(4):376-378.
23 Bai M., Quinn S., Trivedi S., et al. Expression and characterization of inactivating and activating mutations in the human Ca2+ o-sensing receptor. J Biol Chem. 1996;271:19537-19545.
24 Bai M. Structure-function relationship of the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:197-207.
25 Banerjee D., Asif A., Striker L., et al. Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J Kidney Dis. 2003;41:E18.
26 Barber P.J., Elliott J., Torrance A.G. Measurement of feline intact parathyroid hormone: assay validation and sample handling studies. J Small Anim Pract. 1993;34:614-620.
27 Barber P.J., Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:108-116.
28 Barber P.J., Elliott J. Study of calcium homeostasis in feline hyperthyroidism. J Small Anim Pract. 1996;37:575-582.
29 Barber P.J., Rawlings J.M., Markwell P.J., et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40:62-70.
30 Barber P.J., Torrance A.G., Elliott J. Carboxyl fragment interference in assay of feline parathyroid hormone. J Vet Intern Med. 1994;8:168.
31 Barr F.J., Patterson M.W., Lucke V.M., et al. Hypercalcemic nephropathy in three dogs: sonographic appearance. Vet Radiol. 1989;30:169-173.
32 Barrett S., Sheafor S.E., Hillier A., et al. Challenging cases in internal medicine “What’s your diagnosis?”. Vet Med. 1998;93:35-44.
33 Basoglu A., Sevinc M., Sen I., et al. The blocking effect of verapamil in hypercalcemic dogs. Turkish J Vet Anim Sci. 1997;21:331-333.
34 Bassett J.R. Hypocalcemia and hyperphosphatemia due to primary hypoparathyroidism in a six-month-old kitten. J Am Anim Hosp Assoc. 1998;34:503-507.
35 Behrend E.N., Kemppainen R. Adrenocortical disease. In: August J., editor. Consultations in feline internal medicine. 4th ed. Philadelphia: WB Saunders; 1980:159-168.
36 Bennett P.F., DeNicola D.B., Bonney P., et al. Canine anal sac adenocarcinomas: clinical presentation and response to therapy. J Vet Intern Med. 2002;16:100-104.
37 Bereket A., Erdogan T. Oral bisphosphonate therapy for vitamin D intoxication of the infant. Pediatrics. 2003;111:899-901.
38 Berenson J., Hirschberg R. Safety and convenience of a 15-minute infusion of zoledronic acid. Oncologist. 2004;9:319-329.
39 Berenson J.R., Rosen L., Vescio R., et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37:285-290.
40 Berger B., Feldman E.C. Primary hyperparathyroidism in dogs: 21 cases (1976-1986). J Am Vet Med Assoc. 1987;191:350-356.
41 Bergman S.M., O’Mailia J., Krane N.K., et al. Vitamin-A-induced hypercalcemia: response to corticosteroids. Nephron. 1988;50:362-364.
42 Bernardi R.J., Johnson C.S., Modzelewski R.A., et al. Antiproliferative effects of 1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology. 2002;143:2508-2514.
43 Berndt T., Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol. 2007;69:341-359.
44 Bhang D.H., Choi U.S., Kim M.K., Choi E.H., Kang M.S., Hwang C.Y., et al. Epitheliotropic cutaneous lymphoma (mycosis fungoides) in a dog. J Vet Sci. 2006;7(1):97-99.
45 Bhattacharya S.K., Luther R.W., Pate J.W., et al. Soft tissue calcium and magnesium content in acute pancreatitis in the dog: calcium accumulation, a mechanism for hypocalcemia in acute pancreatitis. J Lab Clin Med. 1985;105:422-427.
46 Bienzle D., Jacobs R.M., Lumsden J.H. Relationship of serum total calcium to serum albumin in dogs, cats, horses and cattle. Can Vet J. 1993;34:360-364.
47 Bienzle D., Silverstein D.C., Chaffin K. Multiple myeloma in cats: variable presentation with different immunoglobulin isotypes in two cats. Vet Pathol. 2000;37:364-369.
48 Bilezikian J.P., Singer F.R. Acute management of hypercalcemia due to parathyroid hormone and parathyroid hormone-related protein. In: Bilezikian J.P., Levine M.A., Marcus R., editors. The parathyroids. New York: Raven Press; 1994:359-372.
49 Bilezikian J.P. Clinical utility of assays for parathyroid hormone-related protein. Clin Chem. 1992;38:179-181.
50 Biller D.S., Bradley G.A., Partington B.P. Renal medullary rim sign: ultrasonographic evidence of renal disease. Vet Radiol Ultrasound. 1992;33:286-290.
51 Birchard S.J., Peterson M.E., Jacobson A. Surgical treatment of feline hyperthyroidism: results of 85 cases. J Am Anim Hosp Assoc. 1984;20:705-709.
52 Biswas P.N., Wilton L.V., Shakir S.A. Pharmacovigilance study of alendronate in England. Osteoporos Int. 2003;14:507-514.
53 Black K.S., Mundy G.R. Other causes of hypercalcemia: local and ectopic secretion syndromes. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. New York: Raven Press; 1994:341-358.
54 Blackwood L., Sullivan M., Lawson H. Radiographic abnormalities in canine multicentric lymphoma: a review of 84 cases. J Small Anim Pract. 1997;38:62-69.
55 Block G.A., Martin K.J., de Francisco A.L., et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516-1525.
56 Blumenthal S.R., Williams T.C., Barbee R.W., et al. Effects of citrated whole blood transfusion in response to hemorrhage. Lab Anim Sci. 1999;49:411-417.
57 Boden S.D., Kaplan F.S. Calcium homeostasis. Orthop Clin North Am. 1990;21:31-42.
58 Body J.J., Coleman R.E., Piccart M. Use of bisphosphonates in cancer patients. Cancer Treat Rev. 1996;22:265-287.
59 Body J.J. Clinical research update: zoledronate. Cancer. 1997;80(Suppl.):1699-1701.
60 Body J.J. Hypercalcemia of malignancy. Semin Nephrol. 2004;24:48-54.
61 Bolliger A.P., Graham P.A., Richard V., et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol. 2002;31:3-8.
62 Bourdeau A., Souberbielle J.C., Bonnet P., et al. Phospholipase-A2 action and arachidonic acid metabolism in calcium-mediated parathyroid hormone secretion. Endocrinology. 1992;130:1339-1344.
63 Bourke E., Delaney V. Assessment of hypocalcemia and hypercalcemia. Clin Lab Med. 1993;13:157-181.
64 Bowers G.N.Jr, Brassard C., Sena S.F. Measurement of ionized calcium in serum with ion-selective electrodes: a mature technology that can meet the daily service needs. Clin Chem. 1986;32:1437-1447.
65 Bowman A.R., Epstein S. Drug and hormone effects on vitamin D metabolism. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:797-829.
66 Bregazzi V.S., Fettman M.J., Twedt D.C. Hypergastrinemia associated with hypercalcemia in the dog. J Vet Intern Med. 2000;14:389.
67 Breitwieser G.E., Miedlich S.U., Zhang M. Calcium sensing receptors as integrators of multiple metabolic signals. Cell Calcium. 2004;35:209-216.
68 Brennan S.F., O’Donovan J., Mooney C.T. Changes in canine ionised calcium under three storage conditions. J Small Anim Pract. 2006;47:383-386.
69 Brenza H.L., DeLuca H.F. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381:143-152.
70 Breslau N.A. Normal and abnormal regulation of 1,25-(OH)2D synthesis. Am J Med Sci. 1988;296:417-425.
71 Bringhurst F.R. Circulating forms of parathyroid hormone: peeling back the onion. Clin Chem. 2003;49:1973-1975.
72 Bronner F. Mechanisms and functional aspects of intestinal calcium absorption. J Exp Zoolog A Comp Exp Biol. 2003;300:47-52.
73 Bronner F. Mechanisms of intestinal calcium absorption. J Cell Biochem. 2003;88:387-393.
74 Brossard J.H., Cloutier M., Roy L., et al. Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923-3929.
75 Brown A.J., Dusso A., Lopez-Hilker S., et al. 1,25-(OH)2D receptors are decreased in parathyroid glands from chronically uremic dogs. Kidney Int. 1989;35:19-23.
76 Brown A.J., Zhong M., Finch J., et al. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270:F454-F460.
77 Brown E.M., Conigrave A., Chattopadhyay N. Receptors and signaling for calcium ions. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:127-142.
78 Brown E.M., Gamba G., Riccardi D., et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575-580.
79 Brown E.M., Hebert S.C. Calcium-receptor-regulated parathyroid and renal function. Bone. 1997;20:303-309.
80 Brown E.M., Pollak M., Seidman C.E., et al. Calcium-ion-sensing cell-surface receptors. N Engl J Med. 1995;333:234-240.
81 Brown E.M. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371-411.
82 Brown E.M. Homeostatic mechanisms regulating extracellular and intracellular calcium metabolism. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. New York: Academic Press; 1994:15-54.
83 Brown J.E., Neville-Webbe H., Coleman R.E. The role of bisphosphonates in breast and prostate cancers. Endocr Relat Cancer. 2004;11:207-224.
84 Brown M.A., Haughton M.A., Grant S.F., et al. Genetic control of bone density and turnover: role of the collagen 1alpha1, estrogen receptor, and vitamin D receptor genes. J Bone Miner Res. 2001;16:758-764.
85 Brownie C.F. Confusion over jasmine and jessamine. J Am Vet Med Assoc. 1987;191:613-614.
86 Brunette M.G., Vary J., Carriere S. Hyposthenuria in hypercalcemia: a possible role of intrarenal blood-flow (IRBF) redistribution. Pflugers Arch. 1974;350:9-23.
87 Bruyette D.S., Feldman E.C. Primary hypoparathyroidism in the dog: report of 15 cases and review of 13 previously reported cases. J Vet Intern Med. 1988;2:7-14.
88 Burritt M.F., Pierides A.M., Offord K.P. Comparative studies of total and ionized serum calcium values in normal subjects and patients with renal disorders. Mayo Clin Proc. 1980;55:606-613.
89 Burtis W.J., Brady T.G., Orloff J.J., et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990;322:1106-1112.
90 Burtis W.J., Dann P., Gaich G.A., et al. A high abundance midregion species of parathyroid hormone-related protein: immunological and chromatographic characterization in plasma. J Clin Endocrinol Metab. 1994;78:317-322.
91 Burtis W.J. Parathyroid hormone-related protein: structure, function, and measurement. Clin Chem. 1992;38:2171-2183.
92 Bush W.W., Kimmel S.E., Wosar M.A., et al. Secondary hypoparathyroidism attributed to hypomagnesemia in a dog with protein-losing enteropathy. J Am Vet Med Assoc. 2001;219:1732-1734. 1708
93 Cairns C.B., Niemann J.T., Pelikan P.C., et al. Ionized hypocalcemia during prolonged cardiac arrest and closed-chest CPR in a canine model. Ann Emerg Med. 1991;20:1178-1182.
94 Caldin M., Tommaso F., Lubas G., et al. Incidence of persistent hypercalcemia in dogs and its diagnostic approach. European Society of Veterinary Internal Medicine Congress. 2001. Dublin
95 Calia C.M., Hohenhaus A.E., Fox P.R., et al. Acute tumor lysis syndrome in a cat with lymphoma. J Vet Intern Med. 1996;10:409-411.
96 Campbell A. Calcipotriol poisoning in dogs. Vet Rec. 1997;141:27-28.
97 Campese V.M. Calcium, parathyroid hormone, and blood pressure. Am J Hypertens. 1989;2:34S-44S.
98 Camus C., Charasse C., Jouannic-Montier I., et al. Calcium free hemodialysis: experience in the treatment of 33 patients with severe hypercalcemia. Intensive Care Med. 1996;22:116-121.
99 Canaff L., Hendy G.N. Human calcium-sensing receptor gene: vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337-30350.
100 Canalejo A., Almaden Y., Torregrosa V., et al. The in vitro effect of calcitriol on parathyroid cell proliferation and apoptosis. J Am Soc Nephrol. 2000;11:1865-1872.
101 Canalejo A., Canadillas S., Ballesteros E., et al. Importance of arachidonic acid as a mediator of parathyroid gland response. Kidney Int Suppl. 2003;85:S10-S13.
102 Canalis E., Hock J.M., Raisz L.G. Anabolic and catabolic effects of parathyroid hormone on bone and interactions with growth factors. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. New York: Raven Press; 1994:65-82.
103 Capen C.C., Martin S.L. Calcium metabolism and disorders of parathyroid glands. Vet Clin North Am. 1977;7:513-548.
104 Capen C.C., Rosol T.J. Hormonal control of mineral metabolism. In: Bojrab M.J., editor. Disease mechanisms in small animal surgery. Philadelphia: Lea & Febiger; 1993:841-857.
105 Care A.D. The placental transfer of calcium. J Dev Physiol. 1991;15:253-257.
106 Carlstedt F., Lind L., Rastad J., et al. Parathyroid hormone and ionized calcium levels are related to the severity of illness and survival in critically ill patients. Eur J Clin Invest. 1998;28:898-903.
107 Carothers M., Chew D.J., Nagode L.A. 25-OH-cholecalciferol intoxication in dogs. Proceedings Am Coll Vet Intern Med Forum. 1994.
108 Chang W., Shoback D. Extracellular Ca2+-sensing receptors-an overview. Cell Calcium. 2004;35:183-196.
109 Chen R.A., Goodman W.G. Role of the calcium-sensing receptor in parathyroid gland physiology. Am J Physiol Renal Physiol. 2004;286:F1005-F1011.
110 Cheteri M.B., Stanford J.L., Friedrichsen D.M., et al. Vitamin D receptor gene polymorphisms and prostate cancer risk. Prostate. 2004;59:409-418.
111 Chew D.J., Capen C.C. Hypercalcemic nephropathy and associated disorders. In: Kirk R.W., editor. Current veterinary therapy VII. Philadelphia: WB Saunders; 1980:1067-1072.
112 Chew D.J., Carothers M., Nagode L.A., et al. 25-OH-cholecalciferol intoxication in 12 dogs (14 episodes). Proceedings of the 39th Annual Congress of World Small Animal Veterinary Association. Berlin, 1993.
113 Chew D.J., Carothers M. Hypercalcemia. Vet Clin North Am Small Anim Pract. 1989;19:265-287.
114 Chew D.J., Leonard M., Muir W.3rd. Effect of sodium bicarbonate infusions on ionized calcium and total calcium concentrations in serum of clinically normal cats. Am J Vet Res. 1989;50:145-150.
115 Chew D.J., Meuten D.J. Disorders of calcium and phosphorus metabolism. Vet Clin North Am Small Anim Pract. 1982;12:411-438.
116 Chew D.J., Meuten D.J. Primary hyperparathyroidism. In: Kirk R.W., editor. Current veterinary therapy VIII. Philadelphia: WB Saunders; 1983:880-884.
117 Chew D.J., Nagode L., Rosol T.J., et al. Utility of diagnostic assays in the evaluation of hypercalcemia and hypocalcemia: parathyroid hormone, vitamin D metabolites, parathyroid hormone-related protein, and ionized calcium. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII: small animal practice. Philadelphia: WB Saunders; 1995:378-383.
118 Chew D.J., Nagode L.A. Renal secondary hyperparathyroidism. Proc Soc Comp Endocrinol. 1990.
119 Chew D.J., Schaer M., Liu S.K., et al. Pseudohyperparathyroidism in a cat. J Natl Cancer Inst. 1975;11:46-52.
120 Chew D.J., Schenck P.A. Advances in the treatment of hypercalcemia: the veterinary perspective. Proceedings from the American College of Veterinary Internal Medicine Meeting. Anaheim, Calif. 2010.
121 Ching S.V., Fettman M.J., Hamar D.W., et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J Nutr. 1989;119:902-915.
122 Ching S.V., Norrdin R.W. Histomorphometric comparison of measurements of trabecular bone remodeling in iliac crest biopsy sites and lumbar vertebrae in cats. Am J Vet Res. 1990;51:447-450.
123 Chisholm M.A., Mulloy A.L., Taylor A.T. Acute management of cancer-related hypercalcemia. Ann Pharmacother. 1996;30:507-513.
124 Chorev M., Alexander J.M., Rosenblatt M. Interactions of parathyroid hormone and parathyroid hormone-related protein with their receptors. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:53-92.
125 Christakos S., Beck J.D., Hyliner S.J. Calbindin-D 28K. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:209-221.
126 Coburn J.W., Maung H.M. Use of active vitamin D sterols in patients with chronic kidney disease, stages 3 and 4. Kidney Int Suppl. 2003;85:S49-S53.
127 Coburn J.W. An update on vitamin D as related to nephrology practice: 2003. Kidney Int Suppl. 2003;85:S125-0000130.
128 Cook S.D., Skinner H.B., Haddad R.J. A quantitative histologic study of osteoporosis produced by nutritional secondary hyperparathyroidism in dogs. Clin Orthop Relat Res. May 1983:105-120.
129 Cooke N.E., Haddad J.G. Vitamin D binding protein. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:87-101.
130 Cortadellas O., Fernandez del Palacio M.J., Talavera J., Bayon A. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J Vet Intern Med. 2010;24:73-79.
131 Coyne D.W. Cinacalcet should not be used to treat secondary hyperparathyroidism in stage 3-4 chronic kidney disease. Nature Clin Pract Nephrol. 2008;4:364-365.
132 Crews L.J., Sharkey L.C., Feeney D.A., Jessen C.R., Ruska T. Evaluation of total and ionized calcium status in dogs with blastomycosis: 38 cases (1997-2006). J Am Vet Med Assoc. 2007;231(10):1545-1549.
133 D’Amour P., Brossard J.H., Rousseau L., et al. Amino-terminal form of parathyroid hormone (PTH) with immunologic similarities to hPTH(1-84) is overproduced in primary and secondary hyperparathyroidism. Clin Chem. 2003;49:2037-2044.
134 Davainis G.M., Chew D.J., Nagode L.A., et al. Calcium regulation in the cat with chronic renal failure. European Society of Veterinary Internal Medicine/European Society of Veterinary Endocrinology Annual Meeting. Dublin. 2001.
135 De Boer I.H., Gorodetskaya I., Young B., et al. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol. 2002;13:2762-2769.
136 de Fornel-Thibaud P., Blanchard G., Escoffier-Chateau L., Segond S., Guetta F., Begon D., et al. Unusual case of osteopenia associated with nutritional calcium and vitamin D deficiency in an adult dog. J Am Anim Hosp Assoc. 2007;43(1):52-60.
137 De Luisi A., Hofer A.M. Evidence that Ca(2+) cycling by the plasma membrane Ca(2+)-ATPase increases the “excitability” of the extracellular Ca(2+)-sensing receptor. J Cell Sci. 2003;116:1527-1538.
138 DeLay J., Laing J. Nutritional osteodystrophy in puppies fed a BARF diet. AHL Newsletter. 2002;6:23.
139 DeLuca H.F., Krisinger J., Darwish H. The vitamin D system: 1990. Kidney Int Suppl. 1990;29:S2-S8.
140 Demay M. Muscle: a nontraditional 1,25-dihydroxyvitamin D target tissue exhibiting classic hormone-dependent vitamin D receptor actions. Endocrinology. 2003;144:5135-5137.
141 den Hertog E., Goossens M.M., van der Linde-Sipman J.S., et al. Primary hyperparathyroidism in two cats. Vet Q. 1997;19:81-84.
142 Deniz A., Mischke R. Ionized calcium and total calcium in the cat. Berl Munch Tierarztl Wochenschr. 1995;108:105-108.
143 DeVries S.E., Feldman E.C., Nelson R.W., et al. Primary parathyroid gland hyperplasia in dogs: six cases (1982-1991). J Am Vet Med Assoc. 1993;202:1132-1136.
144 Dhaliwal R.S., Tang K.N. Parathyroid hormone-related peptide and hypercalcaemia in a dog with functional keratinizing ameloblastoma. Vet Comp Oncol. 2005;3(2):98-100.
145 Dhupa N., Proulx J. Hypocalcemia and hypomagnesemia. Vet Clin North Am Small Anim Pract. 1998;28:587-608.
146 DiBartola S.P., Chew D.J., Jacobs G. Quantitative urinalysis including 24-hour protein excretion in the dog. J Am Anim Hosp Assoc. 1980;16:537-546.
147 DiBartola S.P., Rutgers H.C., Zack P.M., et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973-1984). J Am Vet Med Assoc. 1987;190:1196-1202.
148 Divieti P., John M.R., Juppner H., et al. Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology. 2002;143:171-176.
149 Dougherty S.A., Center S.A., Dzanis D.A. Salmon calcitonin as adjunct treatment for vitamin D toxicosis in a dog. J Am Vet Med Assoc. 1990;196:1269-1272.
150 Dow S.W., Legendre A.M., Stiff M., et al. Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc. 1986;188:706-709.
151 Drazner F.H. Hypercalcemia in the dog and cat. J Am Vet Med Assoc. 1981;178:1252-1256.
152 Drobatz K.J., Casey K.K. Eclampsia in dogs: 31 cases (1995-1998). J Am Vet Med Assoc. 2000;217:216-219.
153 Drobatz K.J., Hughes D. Concentration of ionized calcium in plasma from cats with urethral obstruction. J Am Vet Med Assoc. 1997;211:1392-1395.
154 Drop L.J. Ionized calcium, the heart, and hemodynamic function. Anesth Analg. 1985;64:432-451.
155 Drueke T.B., McCarron D.A. Paricalcitol as compared with calcitriol in patients undergoing hemodialysis. N Engl J Med. 2003;349:496-499.
156 Drueke T.B. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11:1141-1152.
157 Drueke T.B. Parathyroid gland hyperplasia in uremia. Kidney Int. 2001;59:1182-1183.
158 Duncan A.R. The use of subcutaneous pamidronate. J Pain Symptom Manage. 2003;26:592-593.
159 Dusso A.S., Finch J., Brown A., et al. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72:157-164.
160 Dusso A.S. Vitamin D receptor: mechanisms for vitamin D resistance in renal failure. Kidney Int Suppl. 2003;85:S6-S9.
161 Dust A., Norris A.M., Valli V.E.O. Cutaneous lymphosarcoma with IgG monoclonal gammopathy, serum hyperviscosity and hypercalcemia in a cat. Can Vet J. 1982;23:235-239.
162 Dzanis D.A., Kallfelz F.A. Recent knowledge of vitamin D toxicity in dogs. Proceedings Am Coll Vet Intern Med Forum. 1988;6.
163 Earm J.H., Christensen B.M., Frokiaer J., et al. Decreased aquaporin-2 expression and apical plasma membrane delivery in kidney collecting ducts of polyuric hypercalcemic rats. J Am Soc Nephrol. 1998;9:2181-2193.
164 Eelen G., Verlinden L., van Camp M., et al. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133-146.
165 Eiam-Ong S., Punsin P., Sitprija V., et al. Acute hypercalcemia-induced hypertension: the roles of calcium channel and alpha-1 adrenergic receptor. J Med Assoc Thai. 2004;87:410-418.
166 Elliott J., Dobson J., Dunn J., et al. Hypercalcemia in the dog: a study of 40 cases. J Small Anim Pract. 1991;32:564-571.
167 Engelman R.W., Tyler R.D., Good R.A., et al. Hypercalcemia in cats with feline-leukemia-virus-associated leukemia-lymphoma. Cancer. 1985;56:777-781.
168 Estepa J.C., Lopez I., Felsenfeld A.J., et al. Dynamics of secretion and metabolism of PTH during hypo- and hypercalcaemia in the dog as determined by the “intact” and “whole” PTH assays. Nephrol Dial Transplant. 2003;18:1101-1107.
169 Eubig P.A., Brady M.S., Gwaltney-Brant S.M., Khan S.A., Mazzaferro E.M., Morrow C.M. Acute renal failure in dogs after the ingestion of grapes or raisins: a retrospective evaluation of 43 dogs (1992-2002). J Vet Intern Med. 2005;19(5):663-674.
170 Falchetti A. Calcium agonists in hyperparathyroidism. Expert Opin Investig Drugs. 2004;13:229-244.
171 Fan T.M., de Lorimier L.P., Charney S.C., et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74-80.
172 Fan T.M., Simpson K.W., Trasti S., et al. Calcipotriol toxicity in a dog. J Small Anim Pract. 1998;39:581-586.
173 Farese G., Mager M., Blatt W.F. A membrane ultrafiltration procedure for determining diffusible calcium in serum. Clin Chem. 1970;16:226-228.
174 Fascetti A.J., Hickman M.A. Preparturient hypocalcemia in four cats. J Am Vet Med Assoc. 1999;215:1127-1129.
175 Favus M.J., Langman C.B. Evidence for calcium-dependent control of 1,25-dihydroxyvitamin D3 production by rat kidney proximal tubules. J Biol Chem. 1986;261:11224-11229.
176 Favus M.J. Intestinal absorption of calcium, magnesium, and phosphorus. In: Coe F.L., Favus M.J., editors. Disorders of bone and mineral metabolism. New York: Raven Press; 1992:57-81.
177 Feldman E.C., Hoar B., Pollard R., Nelson R.W. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987-2004). J Am Vet Med Assoc. 2005;227(5):756-761.
178 Feldman E.C., Nelson R.W. Hypercalcemia and primary hyperparathyroidism. In: Feldman E.C., editor. Canine and feline endocrinology and reproduction. Philadelphia: WB Saunders; 2004:660-715.
179 Feldman E.C., Nelson R.W. Hypocalcemia and primary hypoparathyroidism. In: Feldman E.C., editor. Canine and feline endocrinology and reproduction. Philadelphia: WB Saunders, 2004.
180 Feldman E.C., Wisner E.R., Nelson R.W., et al. Comparison of results of hormonal analysis of samples obtained from selected venous sites versus cervical ultrasonography for localizing parathyroid masses in dogs. J Am Vet Med Assoc. 1997;211:54-56.
181 Fenton A.J., Kemp B.E., Hammonds R.G.Jr, et al. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of parathyroid hormone-related protein; PTHrP[107-111]. Endocrinology. 1991;129:3424-3426.
182 Fenton A.J., Kemp B.E., Kent G.N., et al. A carboxyl-terminal peptide from the parathyroid hormone-related protein inhibits bone resorption by osteoclasts. Endocrinology. 1991;129:1762-1768.
183 Finco D.R., Rowland G.N. Hypercalcemia secondary to chronic renal failure in the dog: a report of four cases. J Am Vet Med Assoc. 1978;173:990-994.
184 Finco D.R. Interpretations of serum calcium concentration in the dog. Comp Contin Educ. 1983;5:778-787.
185 Fingeroth J.M., Smeak D.D. Intravenous methylene blue infusion for intraoperative identification of parathyroid gland tumors in dogs. Part III: Clinical trials and results in three dogs. J Am Anim Hosp Assoc. 1988;24:673-678.
186 Fitzpatrick L.A., Brandi M.L., Aurbach G.D. Control of PTH secretion is mediated through calcium channels and is blocked by pertussis toxin treatment of parathyroid cells. Biochem Biophys Res Commun. 1986;138:960-965.
187 Flanders J.A., Harvey H.J., Erb H.N. Feline thyroidectomy: a comparison of postoperative hypocalcemia associated with three different surgical techniques. Vet Surg. 1987;16:362-366.
188 Flanders J.A., Neth S., Erb H.N., et al. Functional analysis of ectopic parathyroid activity in cats. Am J Vet Res. 1991;52:1336-1340.
189 Flanders J.A., Scarlett J.M., Blue J.T., et al. Adjustment of total serum calcium concentration for binding to albumin and protein in cats: 291 cases (1986-1987). J Am Vet Med Assoc. 1989;194:1609-1611.
190 Flanders J.A. Surgical therapy of the thyroid. Vet Clin North Am Small Anim Pract. 1994;24:607-621.
191 Fleisch H. Bisphosphonates: pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs. 1991;42:919-944.
192 Fleisch H. Mechanisms of action of the bisphosphonates. Medicina (B Aires). 1997;57(Suppl. 1):65-75.
193 Foley R.N. Phosphorus comes of age as a cardiovascular risk factor. Arch Intern Med. 2007;167:873-874.
194 Fooshee S.K., Forrester S.D. Hypercalcemia secondary to cholecalciferol rodenticide toxicosis in two dogs. J Am Vet Med Assoc. 1990;196:1265-1268.
195 Forbes S., Nelson R.W., Guptill L. Primary hypoparathyroidism in a cat. J Am Vet Med Assoc. 1990;196:1285-1287.
196 Fournel-Fleury C., Ponce F., Felman P., et al. Canine T-cell lymphomas: a morphological, immunological, and clinical study of 46 new cases. Vet Pathol. 2002;39:92-109.
197 Fradkin J.M., Braniecki A.M., Craig T.M., et al. Elevated parathyroid hormone-related protein and hypercalcemia in two dogs with schistosomiasis. J Am Anim Hosp Assoc. 2001;37:349-355.
198 Franceschini N., Joy M.S., Kshirsagar A. Cinacalcet HCl: a calcimimetic agent for the management of primary and secondary hyperparathyroidism. Expert Opin Investig Drugs. 2003;12:1413-1421.
199 Fraser D., Jones G., Kooh S.W., et al. Calcium and phosphate metabolism. Philadelphia: WB Saunders. 1986:1317-1372.
200 Fraser D., Jones G., Kooh S.W. Calcium and phosphate metabolism. In: Tietz N.W., editor. Fundamentals of clinical chemistry. Philadelphia: WB Saunders; 1987:705-728.
201 Frolik C.A., Black E.C., Cain R.L., et al. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372-379.
202 Fukagawa M., Kitaoka M., Kurokawa K. Renal failure and hyperparathyroidism. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:1227-1239.
203 Galitzer H., Ben-Dov I., Lavi-Moshayoff V., et al. Fibroblast growth factor 23 acts on the parathyroid to decrease parathyroid hormone secretion. Curr Op Nephrol Hyper. 2008;17:363-367.
204 Gao P., Scheibel S., D’Amour P., et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605-614.
205 Garcion E., Sindji L., Nataf S., et al. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol (Berl). 2003;105:438-448.
206 Garlock S.M., Matz M.E., Shell L.G. Vitamin D3 rodenticide toxicity in a dog. J Am Anim Hosp Assoc. 1991;27:356-360.
207 Garrett I.R. Bone destruction in cancer. Semin Oncol. 1993;20(Suppl. 2):4-9.
208 Gaschen F., Gaschen L., Seiler G., et al. Lethal peracute rhabdomyolysis associated with stress and general anesthesia in three dystrophin-deficient cats. Vet Pathol. 1998;35:117-123.
209 Gascon-Barre M. The vitamin D 25-hydroxylase. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:41-56.
210 Geisen V., Weber K., Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med. 2009;23(1):196-199.
211 Gertz B.J., Holland S.D., Kline W.F., et al. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther. 1995;58:288-298.
212 Ghazarian J.G. The renal mitochondrial hydroxylases of the vitamin D3 endocrine complex: how are they regulated at the molecular level? J Bone Miner Res. 1990;5:897-903.
213 Goldstein R.E., Long C., Swift N.C., et al. Percutaneous ethanol injection for treatment of unilateral hyperplastic thyroid nodules in cats. J Am Vet Med Assoc. 2001;218:1298-1302.
214 Goodman W.G., Belin T., Gales B., et al. Calcium-regulated parathyroid hormone release in patients with mild or advanced secondary hyperparathyroidism. Kidney Int. 1995;48:1553-1558.
215 Goodman W.G., Veldhuis J.D., Belin T.R., et al. Calcium-sensing by parathyroid glands in secondary hyperparathyroidism. J Clin Endocrinol Metab. 1998;83:2765-2772.
216 Goodman W.G. New assays for parathyroid hormone (PTH) and the relevance of PTH fragments in renal failure. Kidney Int Suppl. 2003;85:S120-124.
217 Goodman W.G. Recent developments in the management of secondary hyperparathyroidism. Kidney Int. 2001;59:1187-1201.
218 Gosling P. Analytical reviews in clinical biochemistry: calcium measurement. Ann Clin Biochem. 1986;23:t146-t156.
219 Goswami R., Brown E.M., Kochupillai N., et al. Prevalence of calcium sensing receptor autoantibodies in patients with sporadic idiopathic hypoparathyroidism. Eur J Endocrinol. 2004;150:9-18.
220 Gouget B., Gourmelin Y., Blanchet F., et al. Ca2+ measurement with ion selective electrodes: the French coordinated evaluation of seven analyzers, for a better clinical relevance and acceptance. Ann Biol Clin (Paris). 1988;46:419-434.
221 Gow A.G., Gow D.J., Bell R., Simpson J.W., Chandler M.L., Evans H., et al. Calcium metabolism in eight dogs with hypoadrenocorticism. J Small Anim Pract. 2009;50(8):426-430.
222 Grant W.B., Garland C.F. Evidence supporting the role of vitamin D in reducing the risk of cancer. J Intern Med. 2002;252:178-179. author reply 179–180
223 Graves T.K. Complications of treatment and concurrent illness associated with hyperthyroidism in cats. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII: small animal practice. Philadelphia: WB Saunders; 1995:369-372.
224 Green M.D. Oral bisphosphonates and malignancy. Med J Aust. 1997;167:211-212.
225 Greenlee P.G., Filippa D.A., Quimby F.W., et al. Lymphomas in dogs: a morphologic, immunologic, and clinical study. Cancer. 1990;66:480-490.
226 Grone A., McCauley L.K., Capen C.C., et al. Cloning and sequencing of the 3′-region of the canine parathyroid hormone-related protein gene and analysis of alternate mRNA splicing in two canine carcinomas. Domest Anim Endocrinol. 2002;22:169-177.
227 Grosenbaugh D.A., Gadawski J.E., Muir W.W. Evaluation of a portable clinical analyzer in a veterinary hospital setting. J Am Vet Med Assoc. 1998;213:691-694.
228 Gunther R., Felice L.J., Nelson R.K., et al. Toxicity of a vitamin D3 rodenticide to dogs. J Am Vet Med Assoc. 1988;193:211-214.
229 Gutierrez O., Isakova T., Rhee E., et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accelerates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205-2215.
230 Gwaltney-Brant S., Holding J.K., Donaldson C.W., et al. Renal failure associated with ingestion of grapes or raisins in dogs. J Am Vet Med Assoc. 2001;218:1555-1556.
231 Habener J.F., Rosenblatt M., Potts J.T.Jr. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984;64:985-1053.
232 Halabe A., Arie R., Mimran D., et al. Hypoparathyroidism: a long-term follow-up experience with 1 alpha-vitamin D3 therapy. Clin Endocrinol (Oxf). 1994;40:303-307.
233 Ham K., Greenfield C.L., Barger A., Schaeffer D., Ehrhart E.J., Pinkerton M., et al. Validation of a rapid parathyroid hormone assay and intraoperative measurement of parathyroid hormone in dogs with benign naturally occurring primary hyperparathyroidism. Vet Surg. 2009;38(1):122-132.
234 Hare W.R., Dobbs C.E., Slayman K.A., et al. Calcipotriene poisoning in dogs. Vet Med. 2000;95:770-778.
235 Harrison H.E., Harrison H.C. Transfer of Ca45 across intestinal wall in vitro in relation to action of vitamin D and cortisol. Am J Physiol. 1960;199:265-271.
236 Haruna A., Kawai K., Takab T., et al. Dietary calcinosis in the cat. J Anim Clin Res Round. 1992;1:9-16.
237 Haussler M.R., Haussler C.A., Jurutka P.W., et al. The nuclear vitamin D receptor: from clinical radioreceptor assay of the vitamin D hormone to genomics, proteomics and a novel ligand. J Clin Ligand Assay. 2002;25:221-228.
238 Hayes C.E., Nashold F.E., Spach K.M., et al. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand). 2003;49:277-300.
239 Hazewinkel H.A., Tryfonidou M.A. Vitamin D3 metabolism in dogs. Mol Cell Endocrinol. 2002;197:23-33.
240 Hazewinkel H.A.W. Dietary influences on calcium homeostasis and the skeleton. In: Purina International Nutrition Symposium. Orlando, Fla: Ralston Purina; 1991:52.
241 Henderson R.A., Powers R.D., Perry L. Development of hypoparathyroidism after excision of laryngeal rhabdomyosarcoma in a dog. J Am Vet Med Assoc. 1991;198:639-643.
242 Henry D.A., Goodman W.G., Nudelman R.K., et al. Parenteral aluminum administration in the dog: I. Plasma kinetics, tissue levels, calcium metabolism, and parathyroid hormone. Kidney Int. 1984;25:362-369.
243 Henry H. The 25-hydroxyvitamin D 1-alpha-hydroxylase. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:57-68.
244 Hess R.S., Saunders H.M., Van Winkle T.J., et al. Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993-1998). J Am Vet Med Assoc. 2000;217:1166-1173.
245 Hickford F.H., Stokol T., van Gessel Y.A., et al. Monoclonal immunoglobulin G cryoglobulinemia and multiple myeloma in a domestic shorthair cat. J Am Vet Med Assoc. 2000;217:1029-1033.
246 Hilbe M., Sydler T., Fischer L., et al. Metastatic calcification in a dog attributable to ingestion of a tacalcitol ointment. Vet Pathol. 2000;37:490-492.
247 Hill R.C., Van Winkle T.J. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. A retrospective study of 40 cases (1976-1989). J Vet Intern Med. 1993;7:25-33.
248 Hirt R.A., Kneissl S., Teinfalt M. Severe hypercalcemia in a dog with a retained fetus and endometritis. J Am Vet Med Assoc. 2000;216:1423-1425.
249 Hoare S.R., Usdin T.B. Molecular mechanisms of ligand recognition by parathyroid hormone 1 (PTH1) and PTH2 receptors. Curr Pharm Des. 2001;7:689-713.
250 Hodges R.D., Legendre A.M., Adams L.G., et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med. 1994;8:409-413.
251 Hofer A.M., Brown E.M. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530-538.
252 Holick M.F. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone. 1995;17(Suppl.):107S-111S.
253 Holick M.F. Photobiology of vitamin D. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:33-40.
254 Holick M.F. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362-371.
255 Holley D.C., Evans J.W. Determination of total and ultrafilterable calcium and magnesium in normal equine serum. Am J Vet Res. 1977;38:259-262.
256 Hollis B.W., Kamerud J.Q., Kurkowski A., et al. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42:586-592.
257 Holowaychuk M.K., Hansen B.D., DeFrancesco T.C., Marks S.L. Ionized hypocalcemia in critically ill dogs. J Vet Intern Med. 2009;23(3):509-513.
258 Hori Y., Uechi M., Kanakubo K., Sano T., Oyamada T. Canine ovarian serous papillary adenocarcinoma with neoplastic hypercalcemia. J Vet Med Sci. 2006;68(9):979-982.
259 Horn B., Irwin P.J. Transient hypoparathyroidism following successful treatment of hypercalcaemia of malignancy in a dog. Aust Vet J. 2000;78:690-692.
260 Horst R.L., Reinhardt T.A., Hollis B.W. Improved methodology for the analysis of plasma vitamin D metabolites. Kidney Int Suppl. 1990;29:S28-S35.
261 Horst R.L., Reinhardt T.A. Vitamin D metabolism. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:13-31.
262 Hostutler R.A., Chew D.J., Jaeger J.Q., et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med. 2005;19:29-33.
263 Hostutler R.A., DiBartola S.P., Chew D.J., et al. Comparison of the effects of daily and intermittent-dose calcitriol on serum parathyroid hormone and ionized calcium concentrations in normal cats and cats with chronic renal failure. J Vet Intern Med. 2006;20:1307-1313.
264 How K.L., Hazewinkel H.A., Mol J.A. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol. 1994;96:12-18.
265 Hristova E.N., Cecco S., Niemela J.E., et al. Analyzer-dependent differences in results for ionized calcium, ionized magnesium, sodium, and pH. Clin Chem. 1995;41:1649-1653.
266 Hsu C.H., Patel S.R. Altered vitamin D metabolism and receptor interaction with the target genes in renal failure: calcitriol receptor interaction with its target gene in renal failure. Curr Opin Nephrol Hypertens. 1995;4:302-306.
267 Hulter H.N., Halloran B.P., Toto R.D., et al. Long-term control of plasma calcitriol concentration in dogs and humans: dominant role of plasma calcium concentration in experimental hyperparathyroidism. J Clin Invest. 1985;76:695-702.
268 Hume D.Z., Drobatz K.J., Hess R.S. Outcome of dogs with diabetic ketoacidosis: 127 dogs (1993-2003). J Vet Intern Med. 2006;20(3):547-555.
269 Hung S.H., Tsai W.Y., Tsao P.N., et al. Oral clodronate therapy for hypercalcemia related to extensive subcutaneous fat necrosis in a newborn. J Formos Med Assoc. 2003;102:801-804.
270 Hutson C.A., Willauer C.C., Walder E.J., et al. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987-1989). J Am Vet Med Assoc. 1992;201:777-781.
271 Ihle S.L., Nelson R.W., Cook J.R.Jr. Seizures as a manifestation of primary hyperparathyroidism in a dog. J Am Vet Med Assoc. 1988;192:71-72.
272 Imaizumi T., Tsuruta M., Kitagaki T., et al. Single dose toxicity studies of calcipotriol (MC903) in rats and dogs. J Toxicol Sci. 1996;21(Suppl. 2):277-285.
273 Imaizumi T., Tsuruta M., Koike Y., et al. A 26-week repeated percutaneous dose toxicity study of calcipotriol (MC903) in dogs. J Toxicol Sci. 1996;21(Suppl. 2):365-387.
274 Imaizumi T., Tsuruta M., Koike Y., et al. A 4-week repeated percutaneous dose toxicity study of calcipotriol (MC903) followed by a 4-week recovery test in dogs. J Toxicol Sci. 1996;21(Suppl. 2):309-323.
275 Imamura H., Sato K., Shizume K., et al. Urinary excretion of parathyroid hormone-related protein fragments in patients with humoral hypercalcemia of malignancy and hypercalcemic tumor-bearing nude mice. J Bone Miner Res. 1991;6:77-84.
276 Irvine R.F. Calcium transients: mobilization of intracellular Ca2+. Br Med Bull. 1986;42:369-374.
277 Izquierdo R., Bermes E.Jr, Sandberg L., et al. Serum calcium metabolism in acute experimental pancreatitis. Surgery. 1985;98:1031-1037.
278 Jackson I.T., Saleh J., van Heerden J.A. Gigantic mammary hyperplasia in pregnancy associated with pseudohyperparathyroidism. Plast Reconstr Surg. 1989;84:806-810.
279 John M.R., Goodman W.G., Gao P., et al. A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: implications for PTH measurements in renal failure. J Clin Endocrinol Metab. 1999;84:4287-4290.
280 Jono S., McKee M.D., Murry C.E., et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:e10-e17.
281 Jorgensen L.S., Center S.A., Randolph J.F., et al. Electrolyte abnormalities induced by hypertonic phosphate enemas in two cats. J Am Vet Med Assoc. 1985;187:1367-1368.
282 Jutkowitz L.A., Rozanski E.A., Moreau J.A., et al. Massive transfusion in dogs: 15 cases (1997-2001). J Am Vet Med Assoc. 2002;220:1664-1669.
283 Kadar E., Rush J.E., Wetmore L., et al. Electrolyte disturbances and cardiac arrhythmias in a dog following pamidronate, calcitonin, and furosemide administration for hypercalcemia of malignancy. J Am Anim Hosp Assoc. 2004;40:75-81.
284 Kalantar-Zadeh K., Kovesdy C.P. Is it worth correcting hyperparathyroidism if hyperphosphatemia and hypocalcemia worsen? A Cinacalcet story. Am J Kidney Dis. 2009;53:183-188.
285 Kallet A.J., Richter K.P., Feldman E.C., et al. Primary hyperparathyroidism in cats: seven cases (1984-1989). J Am Vet Med Assoc. 1991;199:1767-1771.
286 Kallfelz F.A. Nutritional supplements in small animal practice: boon or bane?. Proceedings of the 8th Am Coll Vet Intern Med Forum. 1990. Washington, DC
287 Karaplis A.C., Luz A., Glowacki J., et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277-289.
288 Kasahara H., Tsuchiya M., Adachi R., et al. Development of a C-terminal-region-specific radioimmunoassay of parathyroid hormone-related protein. Biomed Res. 1992;13:155-161.
289 Kawaguchi K., Braga I.S.3rd, Takahashi A., et al. Nutritional secondary hyperparathyroidism occurring in a strain of German shepherd puppies. Jpn J Vet Res. 1993;41:89-96.
290 Kawasaki T. Creatinine unreliable indicator of renal failure in ferrets. J Small Anim Exotic Med. 1991;1:28-29.
291 Kestenbaum B., Sampson J.N., Rudser K.D., et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-528.
292 Khosla S., van Heerden J.A., Gharib H., et al. Parathyroid hormone-related protein and hypercalcemia secondary to massive mammary hyperplasia. N Engl J Med. 1990;322:1157.
293 Kifor O., McElduff A., LeBoff M.S., et al. Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. J Clin Endocrinol Metab. 2004;89:548-556.
294 Kimmel S.E., Waddell L.S., Michel K.E. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire terriers: five cases (1992-1998). J Am Vet Med Assoc. 2000;217:703-706.
295 Kimmel S.E., Washabau R.J., Drobatz K.J. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996-1998). J Am Vet Med Assoc. 2001;219:1105-1109.
296 Kirby R., Iverson W., Schaer M. Hypercalcemic nephropathy in a young dog resembling human milk alkali syndrome. J Am Anim Hosp Assoc. 1992;28:119-123.
297 Kirk G.R., Breazile J.E., Kenny A.D. Pathogenesis of hypocalcemic tetany in the thyroparathyroidectomized dog. Am J Vet Res. 1974;35:407-408.
298 Kiupel M., Teske E., Bostock D. Prognostic factors for treated canine malignant lymphoma. Vet Pathol. 1999;36:292-300.
299 Klausner J.S., Bell F.W., Hayden D.W., et al. Hypercalcemia in two cats with squamous cell carcinomas. J Am Vet Med Assoc. 1990;196:103-105.
300 Klausner J.S., Fernandez F.R., O’Leary T.P., et al. Canine primary hyperparathyroidism and its association with urolithiasis. Vet Clin North Am Small Anim Pract. 1986;16:227-239.
301 Klein M.K., Powers B.E., Withrow S.J., et al. Treatment of thyroid carcinoma in dogs by surgical resection alone: 20 cases (1981-1989). J Am Vet Med Assoc. 1995;206:1007-1009.
302 Knecht T.P., Behling C.A., Burton D.W., et al. The humoral hypercalcemia of benignancy: a newly appreciated syndrome. Am J Clin Pathol. 1996;105:487-492.
303 Kogika M.M., Lustoza M.D., Notomi M.K., et al. Serum ionized calcium in dogs with chronic renal failure and metabolic acidosis. Vet Clin Pathol. 2006;35(4):441-445.
304 Kolek O., Hines E.R., Jones M.D., et al. 1alpha, 25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036-G1042.
305 Koller H., Zitt E., Staudacher G., et al. Variable parathyroid hormone(1-84)/carboxylterminal PTH ratios detected by 4 novel parathyroid hormone assays. Clin Nephrol. 2004;61:337-343.
306 Koo W.S., Jeon D.S., Ahn S.J., et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72:424-428.
307 Korkor A.B., Kuchibotla J., Arrieh M., et al. The effects of chronic prednisone administration on intestinal receptors for 1,25-dihydroxyvitamin D3 in the dog. Endocrinology. 1985;117:2267-2273.
308 Kornegay J.N. Hypocalcemia in dogs. Compend Contin Educ. 1982;4:1785-1792.
309 Kovacs C.S., Ho-Pao C.L., Hunzelman J.L., et al. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. J Clin Invest. 1998;101:2812-2820.
310 Kremer R., Goltzman D. Assays for parathyroid hormone-related protein. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. New York: Raven Press; 1994:321-340.
311 Krishnan A.V., Feldman D. Regulation of vitamin D receptor abundance. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:179-200.
312 Krishnan A.V., Shinghal R., Raghavachari N., et al. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59:243-251.
313 Kronenberg H.M., Bringhurst F.R., Segre G.V., et al. Parathyroid hormone biosynthesis and metabolism. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:17-30.
314 Kruger J.M., Osborne C.A., Nachreiner R.F., et al. Hypercalcemia and renal failure. Etiology, pathophysiology, diagnosis, and treatment. Vet Clin North Am Small Anim Pract. 1996;26:1417-1445.
315 Kruger J.M., Osborne C.A., Polzin D.J. Treatment of hypercalcemia. In: Kirk R.W., editor. Current veterinary therapy IX. Philadelphia: WB Saunders; 1986:75-90.
316 Kuhlmann A., Haas C.S., Gross M.L., et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004;286:F526-F533.
317 Kull P.A., Hess R.S., Craig L.E., et al. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996-1998). J Am Vet Med Assoc. 2001;219:197-202.
318 Kumar R. Vitamin D and the kidney. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:275-292.
319 Kyles A.E., Stone E.A., Gookin J., et al. Diagnosis and surgical management of obstructive ureteral calculi in cats: 11 cases (1993-1996). J Am Vet Med Assoc. 1998;213:1150-1156.
320 Ladenson J.H., Lewis J.W., McDonald J.M., et al. Relationship of free and total calcium in hypercalcemic conditions. J Clin Endocrinol Metab. 1979;48:393-397.
321 Langub M.C., Monier-Faugere M.C., Wang G., et al. Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135-1138.
322 Larsson L., Ohman S. Effect of silicone-separator tubes and storage time on ionized calcium in serum. Clin Chem. 1985;31:169-170.
323 LeBlanc C.J., Echandi R.L., Moore R.R., Souza C., Grooters A.M. Hypercalcemia associated with gastric pythiosis in a dog. Vet Clin Pathol. 2008;37(1):115-120.
324 Lee J.A., Drobatz K.J. Characterization of the clinical characteristics, electrolytes, acid-base, and renal parameters in male cats with urethral obstruction. J Vet Emerg Crit Care. 2003;13:227-233.
325 Levi J., Massry S.G., Coburn J.W., et al. Hypocalcemia in magnesium-depleted dogs: evidence for reduced responsiveness to parathyroid hormone and relative failure of parathyroid gland function. Metabolism. 1974;23:323-335.
326 LeVine D.N., Zhou Y., Ghiloni R.J., Fields E.L., Birkenheuer A.J., Gookin J.L., et al. Hereditary 1,25-dihydroxyvitamin D-resistant rickets in a Pomeranian dog caused by a novel mutation in the vitamin D receptor gene. J Vet Intern Med. 2009;23(6):1278-1283.
327 Leyland-Jones B. Treating cancer-related hypercalcemia with gallium nitrate. J Support Oncol. 2004;2:509-516. discussion 516–520
328 Lifton S.J., King L.G., Zerbe C.A. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986-1995). J Am Vet Med Assoc. 1996;209:2076-2081.
329 Lim S.K., Gardella T.J., Baba H., et al. The carboxy-terminus of parathyroid hormone is essential for hormone processing and secretion. Endocrinology. 1992;131:2325-2330.
330 Lin R., White J.H. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21-28.
331 Lincoln S.D., Lane V.M. Serum ionized calcium concentration in clinically normal dairy cattle, and changes associated with calcium abnormalities. J Am Vet Med Assoc. 1990;197:1471-1474.
332 Lind L., Bucht E., Ljunghall S. Pronounced elevation in circulating calcitonin in critical care patients is related to the severity of illness and survival. Intensive Care Med. 1995;21:63-66.
333 Lind L., Carlstedt F., Rastad J., et al. Hypocalcemia and parathyroid hormone secretion in critically ill patients. Crit Care Med. 2000;28:93-99.
334 Lindemans J., Hoefkens P., van Kessel A.L., et al. Portable blood gas and electrolyte analyzer evaluated in a multiinstitutional study. Clin Chem. 1999;45:111-117.
335 Lins L.E. Renal function in hypercalcemic dogs during hydropenia and during saline infusion. Acta Physiol Scand. 1979;106:177-186.
336 Long C.D., Goldstein R.E., Hornof W.J., et al. Percutaneous ultrasound-guided chemical parathyroid ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc. 1999;215:217-221.
337 Looney A.L., Ludders J., Erb H.N., et al. Use of a handheld device for analysis of blood electrolyte concentrations and blood gas partial pressures in dogs and horses. J Am Vet Med Assoc. 1998;213:526-530.
338 Lyon M.E., Bremner D., Laha T., et al. Specific heparin preparations interfere with the simultaneous measurement of ionized magnesium and ionized calcium. Clin Biochem. 1995;28:79-84.
339 Lyon M.E., Guajardo M., Laha T., et al. Electrolyte balanced heparin may produce a bias in the measurement of ionized calcium concentration in specimens with abnormally low protein concentration. Clin Chim Acta. 1995;233:105-113.
340 Lyon M.E., Guajardo M., Laha T., et al. Zinc heparin introduces a preanalytical error in the measurement of ionized calcium concentration. Scand J Clin Lab Invest. 1995;55:61-65.
341 Machado C.E., Flombaum C.D. Safety of pamidronate in patients with renal failure and hypercalcemia. Clin Nephrol. 1996;45:175-179.
342 MacIsaac R.J., Caple I.W., Danks J.A., et al. Ontogeny of parathyroid hormone-related protein in the ovine parathyroid gland. Endocrinology. 1991;129:757-764.
343 MacIsaac R.J., Heath J.A., Rodda C.P., et al. Role of the fetal parathyroid glands and parathyroid hormone-related protein in the regulation of placental transport of calcium, magnesium and inorganic phosphate. Reprod Fertil Dev. 1991;3:447-457.
344 MacKenzie C.P. Poisoning in four dogs by a compound containing warfarin and calciferol. J Small Anim Pract. 1987;28:433-445.
345 Maestro B., Davila N., Carranza M.C., et al. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223-230.
346 Mahgoub A., Hirsch P.F., Munson P.L. Calcium-lowering action of glucocorticoids in adrenalectomized-parathyroidectomized rats: specificity and relative potency of natural and synthetic glucocorticoids. Endocrine. 1997;6:279-283.
347 Major P., Lortholary A., Hon J., et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558-567.
348 Major P. The use of zoledronic acid, a novel, highly potent bisphosphonate, for the treatment of hypercalcemia of malignancy. Oncologist. 2002;7:481-491.
349 Malberti F., Surian M., Cosci P. Improvement of secondary hyperparathyroidism and reduction of the set point of calcium after intravenous calcitriol. Kidney Int Suppl. 1993;41:S125-130.
350 Mangin M., Ikeda K., Broadus A.E. Structure of the mouse gene encoding parathyroid hormone-related peptide. Gene. 1990;95:195-202.
351 Marchetti V., Lubas G., Baneth G., Modenato M., Mancianti F. Hepatozoonosis in a dog with skeletal involvement and meningoencephalomyelitis. Vet Clin Pathol. 2009;38(1):121-125.
352 Markowitz G.S., Fine P.L., Stack J.I., et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64:281-289.
353 Marquez G.A., Klausner J.S., Osborne C.A. Calcium oxalate urolithiasis in a cat with a functional parathyroid adenocarcinoma. J Am Vet Med Assoc. 1995;206:817-819.
354 Martin L.G. Hypercalcemia and hypermagnesemia. Vet Clin North Am Small Anim Pract. 1998;28:565-585.
355 Martin T.J., Grill V. Hypercalcemia in cancer. J Steroid Biochem Mol Biol. 1992;43:123-129.
356 Martin-Salvago M., Villar-Rodriguez J.L., Palma-Alvarez A., et al. Decreased expression of calcium receptor in parathyroid tissue in patients with hyperparathyroidism secondary to chronic renal failure. Endocr Pathol. 2003;14:61-70.
357 Massry S.G. Pathogenesis of uremic toxicity, Part 1. Parathyroid hormone as a uremic toxin. In: Massry S.G., Glassock R.J., editors. Textbook of nephrology. Baltimore: Williams & Wilkins; 1989:1126-1144.
358 Mathew S., Lund R.J., Chaudhary L.R., et al. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509-1519.
359 Mathew S., Tustison K.S., Sugatani T., et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092-1105.
360 Matus R.E., Leifer C.E., MacEwen E.G., et al. Prognostic factors for multiple myeloma in the dog. J Am Vet Med Assoc. 1986;188:1288-1292.
361 Matwichuk C.L., Taylor S.M., Daniel G.B., et al. Double-phase parathyroid scintigraphy in dogs using technetium-99m-sestamibi. Vet Radiol Ultrasound. 2000;41:461-469.
362 Matwichuk C.L., Taylor S.M., Wilkinson A.A., et al. Use of technetium Tc 99m sestamibi for detection of a parathyroid adenoma in a dog with primary hyperparathyroidism. J Am Vet Med Assoc. 1996;209:1733-1736.
363 Mazzaferro S., Barberi S., Scarda A., et al. Ionised and total serum magnesium in renal transplant patients. J Nephrol. 2002;15:275-280.
364 McCauley L.K., Rosol T.J., Stromberg P.C., et al. Effects of interleukin-1 alpha and cyclosporin A in vivo and in vitro on bone and lymphoid tissues in mice. Toxicol Pathol. 1991;19:1-10.
365 McClain H.M., Barsanti J.A., Bartges J.W. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc. 1999;35:297-301.
366 McElwain M.C., Modzelewski R.A., Yu W.D., et al. Vitamin D: an antiproliferative agent with potential for therapy of squamous cell carcinoma. Am J Otolaryngol. 1997;18:293-298.
367 McIntyre C.W. The functional cardiovascular consequences of vascular calcification. Semin Dial. 2007;20:122-128.
368 Mealey K.L., Willard M.D., Nagode L.A., et al. Hypercalcemia associated with granulomatous disease in a cat. J Am Vet Med Assoc. 1999;215:959-962.
369 Mellanby R.J., Craig R., Evans H., Herrtage M.E. Plasma concentrations of parathyroid hormone-related protein in dogs with potential disorders of calcium metabolism. Vet Rec. 2006;159(25):833-838.
370 Mellanby R.J., Mee A.P., Berry J.L., Herrtage M.E. Hypercalcaemia in two dogs caused by excessive dietary supplementation of vitamin D. J Small Anim Pract. 2005;46(7):334-338.
371 Mellanby R.J., Mellor P.J., Roulois A., Baines E.A., Mee A.P., Berry J.L., et al. Hypocalcaemia associated with low serum vitamin D metabolite concentrations in two dogs with protein-losing enteropathies. J Small Anim Pract. 2005;46(7):345-351.
372 Mellanby R.J., Mellor P., Villiers E.J., Herrtage M.E., Halsall D., O’Rahilly S., et al. Hypercalcemia associated with granulomatous lymphadenitis and elevated 1,25 dihydroxyvitamin D concentration in a dog. J Small Anim Pract. 2006;47(4):207-212.
373 Meller Y., Kestenbaum R.S., Yagil R., et al. The influence of age and sex on blood levels of calcium-regulating hormones in dogs. Clin Orthop Relat Res. 1984;187:296-299.
374 Merryman J.I., Rosol T.J., Brooks C.L., et al. Separation of parathyroid hormone-like activity from transforming growth factor-alpha and -beta in the canine adenocarcinoma (CAC-8) model of humoral hypercalcemia of malignancy. Endocrinology. 1989;124:2456-2463.
375 Messinger J.S., Windham W.R., Ward C.R. Ionized hypercalcemia in dogs: a retrospective study of 109 cases (1998-2003). J Vet Intern Med. 2009;23(3):514-519.
376 Meuten D.J., Chew D.J., Capen C.C., et al. Relationship of serum total calcium to albumin and total protein in dogs. J Am Vet Med Assoc. 1982;180:63-67.
377 Meuten D.J., Cooper B.J., Capen C.C., et al. Hypercalcemia associated with an adenocarcinoma derived from the apocrine glands of the anal sac. Vet Pathol. 1981;18:454-471.
378 Meuten D.J., Kociba G.J., Capen C.C., et al. Hypercalcemia in dogs with lymphosarcoma: biochemical, ultrastructural, and histomorphometric investigations. Lab Invest. 1983;49:553-562.
379 Meuten D.J., Segre G.V., Capen C.C., et al. Hypercalcemia in dogs with adenocarcinoma derived from apocrine glands of the anal sac: biochemical and histomorphometric investigations. Lab Invest. 1983;48:428-435.
380 Meuten D.J. Hypercalcemia. Vet Clin North Am. 1984;14:891-910.
381 Midkiff A.M., Chew D.J., Randolph J.F., et al. Idiopathic hypercalcemia in cats. J Vet Intern Med. 2000;14:619-626.
382 Miki H., Maercklein P.B., Fitzpatrick L.A. Effect of magnesium on parathyroid cells: evidence for two sensing receptors or two intracellular pathways? Am J Physiol. 1997;272:E1-E6.
383 Miller D., Edmonds M.W. Hypercalcemia due to hyperparathyroidism treated with a somatostatin analogue. CMAJ. 1991;145:227-228.
384 Milner R.J., Farese J., Henry C.J., et al. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597-604.
385 Mischke R., Hanies R., Lange K., et al. The effect of the albumin concentration on the relation between the concentration of ionized calcium and total calcium in the blood of dogs. Dtsch Tierarztl Wochenschr. 1996;103:199-204.
386 Miwa N., Nitta K., Kimata N., et al. An evaluation of 1-84 PTH measurement in relation to bone alkaline phosphatase and bone Gla protein in hemodialysis patients. Nephron Clin Pract. 2003;94:c29-c32.
387 Mizobuchi M., Towler D., Slatopolosky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453-1464.
388 Mohn K.L., Jacks T.M., Schleim K.D., Harvey C.E., Miller B., Halley B., et al. Alendronate binds to tooth root surfaces and inhibits progression of feline tooth resorption: a pilot proof-of-concept study. J Vet Dent. 2009;26(2):74-81.
389 Mol J.A., Kwant M.M., Arnold I.C., et al. Elucidation of the sequence of canine (pro)-calcitonin: a molecular biological and protein chemical approach. Regul Pept. 1991;35:189-195.
390 Moore F.M., Kudisch M., Richter K., et al. Hypercalcemia associated with rodenticide poisoning in three cats. J Am Vet Med Assoc. 1988;193:1099-1100.
391 Morita T., Awakura T., Shimada A., et al. Vitamin D toxicosis in cats: natural outbreak and experimental study. J Vet Med Sci. 1995;57:831-837.
392 Morris J.G. Vitamin D synthesis by kittens. Vet Clin Nutr. 1996;3:88-92.
393 Morris S.A., Bilezikian J.P. Signal transduction in bone physiology: messenger systems for parathyroid hormone. In: Bilezikian J.P., Raisz L.G., Rodan G.A., editors. Principles of bone biology. New York: Academic Press; 1996:1203-1215.
394 Morrow C.M.K., Valli V.E., Volmer P.A., et al. Canine renal pathology associated with grape or raisin ingestion: 10 cases. J Vet Diagn Invest. 2005;17:223-231.
395 Mosdell K.W., Visconti J.A. Emerging indications for octreotide therapy, part 1. Am J Hosp Pharm. 1994;51:1184-1192.
396 Moseley J.M., Kubota M., Diefenbach-Jagger H., et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA. 1987;84:5048-5052.
397 Mundy G.R., Oyajobi B. Other local and ectopic hormone syndromes associated with hypercalcemia. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:691-706.
398 Murthy J.N., Hicks J.M., Soldin S.J. Evaluation of i-STAT portable clinical analyzer in a neonatal and pediatric intensive care unit. Clin Biochem. 1997;30:385-389.
399 Naan E.C., Kirpensteijn J., Kooistra H.S., Peeters M.E. Results of thyroidectomy in 101 cats with hyperthyroidism. Vet Surg. 2006;35(3):287-293.
400 Nachreiner R.F., Refsal K.R. The use of parathormone, ionized calcium and 25-hydroxyvitamin D assays to diagnose calcium disorders in dogs. Proc Am Coll Vet Intern Med Forum. 1990;8.
401 Nagode L.A., Chew D.J., Podell M. Benefits of calcitriol therapy and serum phosphorus control in dogs and cats with chronic renal failure: both are essential to prevent or suppress toxic hyperparathyroidism. Vet Clin North Am Small Anim Pract. 1996;26:1293-13330.
402 Nagode L.A., Chew D.J., Steinmeyer C.L. The use of low doses of calcitriol in the treatment of renal secondary hyperparathyroidism. Proc 15th Waltham Symposium (Endocrinology). Columbus, Ohio. 1992.
403 Nagode L.A., Chew D.J. Nephrocalcinosis caused by hyperparathyroidism in progression of renal failure: treatment with calcitriol. Semin Vet Med Surg (Small Anim). 1992;7:202-220.
404 Nagode L.A., Chew D.J. The use of calcitriol in treatment of renal disease of the dog and cat. Proc 1st Purina Int Nutr Symp. 1991.
405 Nagode L.A., Steinmeyer C.L., Chew D.J., et al. Hyper- and normo-calcemic dogs with chronic renal failure: relations of serum PTH and calcitriol to PTG Ca++ set-point. In: Norman A.W., Schaefer K., Grigoleit H.G., et al, editors. Vitamin D. Molecular, cellular and clinical endocrinology. Berlin: Walter de Gruyter; 1988:799-800.
406 Negri A.L., Alvarez Quiroga M., Bravo M., et al. Whole PTH and 1-84/84 PTH ratio for the non invasive determination of low bone turnover in renal osteodystrophy. Nefrologia. 2003;23:327-332.
407 Nemeth E.F., Heaton W.H., Miller M., et al. Pharmacodynamics of the type II calcimimetic compound Cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627-635.
408 Nemzek J.A., Kruger J.M., Walshaw R., et al. Acute onset of hypokalemia and muscular weakness in four hyperthyroid cats. J Am Vet Med Assoc. 1994;205:65-68.
409 Neuman N.B. Acute pancreatic hemorrhage associated with iatrogenic hypercalcemia in a dog. J Am Vet Med Assoc. 1975;166:381-383.
410 Neves M., Gano L., Pereira N., et al. Synthesis, characterization and biodistribution of bisphosphonates Sm-153 complexes: correlation with molecular modeling interaction studies. Nucl Med Biol. 2002;29:329-338.
411 Neville-Webbe H., Coleman R.E. The use of zoledronic acid in the management of metastatic bone disease and hypercalcaemia. Palliat Med. 2003;17:539-553.
412 Neville-Webbe H.L., Holen I., Coleman R.E. The anti-tumour activity of bisphosphonates. Cancer Treat Rev. 2002;28:305-319.
413 Nguyen-Yamamoto L., Rousseau L., Brossard J.H., et al. Origin of parathyroid hormone (PTH) fragments detected by intact-PTH assays. Eur J Endocrinol. 2002;147:123-131.
414 Nguyen-Yamamoto L., Rousseau L., Brossard J.H., et al. Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology. 2001;142:1386-1392.
415 Niemann J.T., Cairns C.B. Hyperkalemia and ionized hypocalcemia during cardiac arrest and resuscitation: possible culprits for postcountershock arrhythmias? Ann Emerg Med. 1999;34:1-7.
416 Nissenson R.A. Receptors for parathyroid hormone and parathyroid hormone-related protein: signaling and regulation. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:93-104.
417 Normal blood values for cats and normal blood values for dogs. St. Louis: Ralston-Purina; 1975.
418 Norman A.W. Rapid biological responses mediated by 1,25-dihydroxyvitamin D3: a case study of transcaltachia (rapid hormonal stimulation of intestinal calcium transport). In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:233-256.
419 Norrdin R.W., Miller C.W., LoPresti C.A., et al. Observations on calcium metabolism, 47Ca absorption, and duodenal calcium-binding activity in chronic renal failure: studies in beagles with radiation-induced nephropathy. Am J Vet Res. 1980;41:510-515.
420 Okada H., Merryman J.I., Rosol T.J., et al. Effects of humoral hypercalcemia of malignancy and gallium nitrate on thyroid C cells in nude mice: immunohistochemical and ultrastructural investigations. Vet Pathol. 1994;31:349-357.
421 Omdahl J.L., May B. The 25-hydroxyvitamin D 24-hydroxylase. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:69-86.
422 Ong S.C., Shalhoub R.J., Gallagher P., et al. Effect of furosemide on experimental hypercalcemia in dogs. Proc Soc Exp Biol Med. 1974;145:227-233.
423 Orloff J.J., Reddy D., de Papp A.E., et al. Parathyroid hormone-related protein as a prohormone: posttranslational processing and receptor interactions. Endocr Rev. 1994;15:40-60.
424 Osborne C.A., Lulich J.P., Thumchai R., et al. Feline urolithiasis: etiology and pathophysiology. Vet Clin North Am Small Anim Pract. 1996;26:217-232.
425 Padgett S.L., Tobias K.M., Leathers C.W., et al. Efficacy of parathyroid gland autotransplantation in maintaining serum calcium concentrations after bilateral thyroparathyroidectomy in cats. J Am Anim Hosp Assoc. 1998;34:219-224.
426 Page R.L. Acute tumor lysis syndrome. Semin Vet Med Surg (Small Anim). 1986;1:58-60.
427 Pallais J.C., Kifor O., Chen Y.B., et al. Acquired hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor. N Engl J Med. 2004;351:362-369.
428 Panciera D.L. Diagnostic approach to disorders of calcium homeostasis. In August J., editor: Consultations in feline internal medicine, 2nd ed, Philadelphia: WB Saunders, 1994.
429 Pandian M.R., Morgan C.H., Carlton E., et al. Modified immunoradiometric assay of parathyroid hormone-related protein: clinical application in the differential diagnosis of hypercalcemia. Clin Chem. 1992;38:282-288.
430 Panichi V., Migliori M., Taccola D., et al. Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int. 2001;60:87-95.
431 Panichi V., Migliori M., Taccola D., et al. Effects of calcitriol on the immune system: new possibilities in the treatment of glomerulonephritis. Clin Exp Pharmacol Physiol. 2003;30:807-811.
432 Pannabecker T.L., Chandler J.S., Wasserman R.H. Vitamin-D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem Biophys Res Commun. 1995;213:499-505.
433 Parfitt A.M. Bone and plasma calcium homeostasis. Bone. 1987;8(Suppl. 1):S1-S8.
434 Patel R.T., Caceres A., French A.F., McManus P.M. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol. 2005;34(4):341-352.
435 Patel S.R., Ke H.Q., Vanholder R., et al. Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J Clin Invest. 1995;96:50-59.
436 Pecherstorfer M., Brenner K., Zojer N. Current management strategies for hypercalcemia. Treat Endocrinol. 2003;2(4):273-292.
437 Penny D., Henderson S.M., Brown P.J. Raisin poisoning in a dog. Vet Rec. 2003;152:308.
438 Perkovic V., Hewitson T.D., Kelynack K.J., et al. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res. 2003;26:27-33.
439 Perry C.M., Figgitt D.P. Zoledronic acid: a review of its use in patients with advanced cancer. Drugs. 2004;64:1197-12211.
440 Persons D.A., Garst J., Vollmer R., et al. Tumor lysis syndrome and acute renal failure after treatment of non-small-cell lung carcinoma with combination irinotecan and cisplatin. Am J Clin Oncol. 1998;21:426-429.
441 Peterson E.N., Kirby R., Sommer M., et al. Cholecalciferol rodenticide intoxication in a cat. J Am Vet Med Assoc. 1991;199:904-906.
442 Peterson M.E., Feinman J.M. Hypercalcemia associated with hypoadrenocorticism in sixteen dogs. J Am Vet Med Assoc. 1982;181:802-804.
443 Peterson M.E., Greco D.S., Orth D.N. Primary hypoadrenocorticism in ten cats. J Vet Intern Med. 1989;3:55-58.
444 Peterson M.E., James K.M., Wallace M., et al. Idiopathic hypoparathyroidism in five cats. J Vet Intern Med. 1991;5:47-51.
445 Peterson M.E., Kintzer P.P., Kass P.H. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979-1993). J Am Vet Med Assoc. 1996;208:85-91.
446 Peterson M.E. Hypoparathyroidism. In: Kirk R.W., editor. Current veterinary therapy IX: small animal practice. Philadelphia: WB Saunders; 1986:1039-1045.
447 Peterson M.E. Treatment of canine and feline hypoparathyroidism. J Am Vet Med Assoc. 1982;181:1434-1436.
448 Petrie G. Management of hypercalcemia using dichloromethylene bisphosphonate (clodronate). Proc Cong Eur Soc Vet Intern Med. 1996;6.
449 Petzinger E., Ziegler K. Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther. 2000;23:91-98.
450 Pezzilli R., Billi P., Barakat B., et al. Octreotide for the treatment of hypercalcemia related to B cell lymphoma. Oncology. 1997;54:517-518.
451 Philbrick W.M., Wysolmerski J.J., Galbraith S., et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127-173.
452 Phillips D.E., Radlinsky M.G., Fischer J.R., et al. Cystic thyroid and parathyroid lesions in cats. J Am Anim Hosp Assoc. 2003;39:349-354.
453 Piek C.J., Teske E. Tumor lysis syndrome in a dog. Tijdschr Diergeneeskd. 1996;121:64-66.
454 Pollak M.R., Brown E.M., Chou Y.H., et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297-1303.
455 Pollard R.E., Long C.D., Nelson R.W., et al. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc. 2001;218:1106-1110.
456 Polzin D.J., Ross S., Osborne C.A. Calcitriol. In: Bonagura J., Twedt D.C., editors. Kirks current veterinary therapy XIV. St. Louis: WB Saunders; 2009:892-895.
457 Powell G.J., Southby J., Danks J.A., et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059-3061.
458 Pressler B.M., Rotstein D.S., Law J.M., et al. Hypercalcemia and high parathyroid hormone-related protein concentration associated with malignant melanoma in a dog. J Am Vet Med Assoc. 2002;221:263-265.
459 Procino G., Carmosino M., Tamma G., et al. Extracellular calcium antagonizes forskolin-induced aquaporin 2 trafficking in collecting duct cells. Kidney Int. 2004;66:2245-2255.
460 Ramirez J.A., Goodman W.G., Belin T.R., et al. Calcitriol therapy and calcium-regulated PTH secretion in patients with secondary hyperparathyroidism. Am J Physiol. 1994;267:E961-E967.
461 Ramsey I.K., Tebb A., Harris E., Evans H., Herrtage M.E. Hyperparathyroidism in dogs with hyperadrenocorticism. J Small Anim Pract. 2005;46(11):531-536.
462 Rasmussen H., Barrett P., Smallwood J., et al. Calcium ion as intracellular messenger and cellular toxin. Environ Health Perspect. 1990;84:17-25.
463 Rasmussen H. The cycling of calcium as an intracellular messenger. Sci Am. 1989;261:66-73.
464 Rasor L., Pollard R., Feldman E.C. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc. 2007;43(2):70-77.
465 Reber P.M., Heath H.3rd. Hypocalcemic emergencies. Med Clin North Am. 1995;79:93-106.
466 Refsal K.R., Provencher-Bolliger A.L., Graham P.A., et al. Update on the diagnosis and treatment of disorders of calcium regulation. Vet Clin North Am Small Anim Pract. 2001;31:1043-1062.
467 Reichel H., Koeffler H.P., Norman A.W. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980-991.
468 Renoe B.W., McDonald J.M., Ladenson J.H. Influence of posture on free calcium and related variables. Clin Chem. 1979;25:1766-1769.
469 Reusch C. Ultrasonography of the parathyroid glands in dogs-a review. Schweiz Arch Tierheilkd. 2001;143:55-62.
470 Riccardi D. The role of extracellular calcium in the regulation of intracellular calcium and cell function (II). Some answers and more questions. Cell Calcium. 2004;35:179-181.
471 Richard V., Lairmore M.D., Green P.L., et al. Humoral hypercalcemia of malignancy: severe combined immunodeficient/beige mouse model of adult T-cell lymphoma independent of human T-cell lymphotropic virus type-1 tax expression. Am J Pathol. 2001;158:2219-2228.
472 Rickels M.R., Mandel S.J. Hypocalciuric hypercalcemia and autoantibodies against the calcium-sensing receptor. N Engl J Med. 2004;351:2237-2238. author reply 2237–2238
473 Rijnberk A., Elsinghorst T.A., Koeman J.P., et al. Pseudohyperparathyroidism associated with perirectal adenocarcinomas in elderly female dogs. Tijdschr Diergeneeskd. 1978;103:1069-1075.
474 Rodan G.A., Balena R. Bisphosphonates in the treatment of metabolic bone diseases. Ann Med. 1993;25:373-378.
475 Roemer-Becuwe C., Vigano A., Romano F., et al. Safety of subcutaneous clodronate and efficacy in hypercalcemia of malignancy: a novel route of administration. J Pain Symptom Manage. 2003;26:843-848.
476 Rohrer C.R., Phillips L.A., Ford S.L., et al. Hypercalcemia in a dog: a challenging case. J Am Anim Hosp Assoc. 2000;36:20-25.
477 Rosenberg M.P., Matus R.E., Patnaik A.K. Prognostic factors in dogs with lymphoma and associated hypercalcemia. J Vet Intern Med. 1991;5:268-271.
478 Rosol T.J., Capen C.C. Calcium-regulating hormones and diseases of abnormal mineral (calcium, phosphorus, magnesium) metabolism. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical biochemistry of domestic animals. San Diego: Academic Press; 1997:619-702.
479 Rosol T.J., Capen C.C. Cancer-associated hypercalcemia. In: Feldman B.F., Zinkl J.G., Jain N.C., editors. Schalm’s veterinary hematology. Philadelphia: Lippincott Williams & Wilkins; 2000:660-666.
480 Rosol T.J., Capen C.C. Mechanisms of cancer-induced hypercalcemia. Lab Invest. 1992;67:680-702.
481 Rosol T.J., Capen C.C. Pathogenesis of humoral hypercalcemia of malignancy. Domest Anim Endocrinol. 1988;5:1-21.
482 Rosol T.J., Capen C.C. Pathophysiology of calcium, phosphorus, and magnesium metabolism in animals. Vet Clin North Am Small Anim Pract. 1996;26:1155-1184.
483 Rosol T.J., Capen C.C. The effect of low calcium diet, mithramycin, and dichlorodimethylene bisphosphonate on humoral hypercalcemia of malignancy in nude mice transplanted with the canine adenocarcinoma tumor line (CAC-8). J Bone Miner Res. 1987;2:395-405.
484 Rosol T.J., Capen C.C. Tumors of the parathyroid gland and circulating parathyroid hormone-related protein associated with persistent hypercalcemia. Toxicol Pathol. 1989;17:346-356.
485 Rosol T.J., Chew D.J., Capen C.C., et al. Acute hypocalcemia associated with infarction of parathyroid gland adenomas in two dogs. J Am Vet Med Assoc. 1988;192:212-214.
486 Rosol T.J., Chew D.J., Couto C.G., et al. Effects of mithramycin on calcium metabolism and bone in dogs. Vet Pathol. 1992;29:223-229.
487 Rosol T.J., Chew D.J., Hammer A.S., et al. Effect of mithramycin on hypercalcemia in dogs. J Am Anim Hosp Assoc. 1994;30:244-250.
488 Rosol T.J., McCauley L.K., Steinmeyer C.L., et al. Nucleotide sequence of canine preproparathyroid hormone. In: Dacke C., Danks J., Caple I., et al, editors. The comparative endocrinology of calcium regulation. Bristol, UK: Journal of Endocrinology Ltd; 1996:201-203.
489 Rosol T.J., Nagode L.A., Couto C.G., et al. Parathyroid hormone (PTH)-related protein, PTH, and 1,25-dihydroxyvitamin D in dogs with cancer-associated hypercalcemia. Endocrinology. 1992;131:1157-1164.
490 Rosol T.J., Nagode L.A., Robertson J.T., et al. Humoral hypercalcemia of malignancy associated with ameloblastoma in a horse. J Am Vet Med Assoc. 1994;204:1930-1933.
491 Rosol T.J., Chew D.J., Nagode L.A., et al. Pathophysiology of calcium metabolism. Vet Clin Pathol. 1995;24:49-63.
492 Rosol T.J., Steinmeyer C.L., McCauley L.K., et al. Sequences of the cDNAs encoding canine parathyroid hormone-related protein and parathyroid hormone. Gene. 1995;160:241-243.
493 Rosol T.J., Tannehill-Gregg S.H., Corn S., et al. Animal models of bone metastasis. Cancer Treat Res. 2004;118:47-81.
494 Rosol T.J., Tannehill-Gregg S.H., LeRoy B.E., et al. Animal models of bone metastasis. Cancer. 2003;97(Suppl.):748-757.
495 Rosol T.J. Pathogenesis of bone metastases: role of tumor-related proteins. J Bone Miner Res. 2000;15:844-850.
496 Ross J.T., Scavelli T.D., Matthiesen D.T., et al. Adenocarcinoma of the apocrine glands of the anal sac in dogs: a review of 32 cases. J Am Anim Hosp Assoc. 1991;27:349-355.
497 Roth S.I., Capen C.C. Ultrastructural and functional correlations of the parathyroid gland. Int Rev Exp Pathol. 1974;13:161-221.
498 Rudnicki M., Frolich A., Haaber A., et al. Actual ionized calcium (at actual pH) vs adjusted ionized calcium (at p. 7.4) in hemodialyzed patients. Clin Chem. 1992;38:1384.
499 Rumbeiha W.K., Fitzgerald S.D., Kruger J.M., et al. Use of pamidronate disodium to reduce cholecalciferol-induced toxicosis in dogs. Am J Vet Res. 2000;61:9-13.
500 Rumbeiha W.K., Kruger J.M., Fitzgerald S.F., et al. Use of pamidronate to reverse vitamin D3-induced toxicosis in dogs. Am J Vet Res. 1999;60:1092-1097.
501 Ruopp J.L. Primary hypoparathyroidism in a cat complicated by suspect iatrogenic calcinosis cutis. J Am Anim Hosp Assoc. 2001;37:370-373.
502 Ruslander D.A., Gebhard D.H., Tompkins M.B., et al. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo. 1997;11:169-172.
503 Russell N.J., Bond K.A., Robertson I.D., Parry B.W., Irwin P.J. Primary hypoparathyroidism in dogs: a retrospective study of 17 cases. Aust Vet J. 2006;84(8):285-290.
504 Russo E.A., Lees G.E. Treatment of hypocalcemia. In: Kirk R.W., editor. Current veterinary therapy IX: small animal practice. Philadelphia: WB Saunders; 1986:91-94.
505 Ryzen E., Rude R.K. Low intracellular magnesium in patients with acute pancreatitis and hypocalcemia. West J Med. 1990;152:145-148.
506 Salusky I.B., Goodman W.G. Parathyroid gland function in secondary hyperparathyroidism. Pediatr Nephrol. 1996;10:359-363.
507 Santamaria R., Almaden Y., Felsenfeld A., et al. Dynamics of PTH secretion in hemodialysis patients as determined by the intact and whole PTH assays. Kidney Int. 2003;64:1867-1873.
508 Santini S.A., Carrozza C., Vulpio C., et al. Assessment of parathyroid function in clinical practice: which parathyroid hormone assay is better? Clin Chem. 2004;50:1247-1250.
509 Sato R., Yamagishi H., Naito Y., et al. Feline vitamin D toxicosis caused by commercially available cat food. J Jpn Vet Med Assoc. 1993;46:577-581.
510 Saunders Y., Ross J.R., Broadley K.E., et al. Systematic review of bisphosphonates for hypercalcaemia of malignancy. Palliat Med. 2004;18:418-431.
511 Savary K.C., Price G.S., Vaden S.L. Hypercalcemia in cats: a retrospective study of 71 cases (1991-1997). J Vet Intern Med. 2000;14:184-189.
512 Schaer M., Cavanaugh P., Hause W., et al. Iatrogenic hyperphosphatemia, hypocalcemia, and hypernatremia in a cat. J Am Anim Hosp Assoc. 1977;13:39.
513 Schaer M., Ginn P.E., Fox L.E., et al. Severe calcinosis cutis associated with treatment of hypoparathyroidism in a dog. J Am Anim Hosp Assoc. 2001;37:364-369.
514 Schenck P.A., Chew D.J., Brooks C.L. Effects of storage on serum ionized calcium and pH from horses with normal and abnormal ionized calcium concentrations. Vet Clin Pathol. 1996;25:118-120.
515 Schenck P.A., Chew D.J., Brooks C.L. Effects of storage on serum ionized calcium and pH values in clinically normal dogs. Am J Vet Res. 1995;56:304-307.
516 Schenck P.A., Chew D.J., Brooks C.L. Fractionation of canine serum calcium, using a micropartition system. Am J Vet Res. 1996;57:268-271.
517 Schenck P.A., Chew D.J. Determination of calcium fractionation in dogs with chronic renal failure. Am J Vet Res. 2003;64:1181-1184.
518 Schenck P.A., Chew D.J. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res. 2010;74:209-213.
519 Schenck P.A., Chew D.J. Prediction of serum ionized calcium concentration by serum total calcium measurement in dogs. Am J Vet Res. 2005;66:1330-1336.
520 Schenck P.A., Chew D.J., Refsal K., et al. Calcium metabolic hormones in feline idiopathic hypercalcemia. J Vet Intern Med. 2004;18:442.
521 Schenck P.A. Serum ionized magnesium concentrations in dogs and cats with hypoparathyroidism. J Vet Intern Med. 2005;19(3):462.
522 Schreiner C.A., Nagode L.A. Vitamin D-dependent rickets type 2 in a four-month-old cat. J Am Vet Med Assoc. 2003;222:337-339. 315–316
523 Schultz R.M., Johnson E.G., Wisner E.R., Brown N.A., Byrne B.A., Sykes J.E. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J Vet Intern Med. 2008;22(4):851-859.
524 Schwarz U., Amann K., Orth S.R., et al. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696-1705.
525 Sekine M., Takami H. Combination of calcitonin and pamidronate for emergency treatment of malignant hypercalcemia. Oncol Rep. 1998;5:197-199.
526 Sela-Brown A., Russell J., Koszewski N.J., et al. Calreticulin inhibits vitamin D’s action on the PTH gene in vitro and may prevent vitamin D’s effect in vivo in hypocalcemic rats. Mol Endocrinol. 1998;12:1193-1200.
527 Seymour J.F., Gagel R.F. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;82:1383-1394.
528 Sharma O.P. Vitamin D, calcium, and sarcoidosis. Chest. 1996;109:535-539.
529 Sheafor S.E., Gamblin R.M., Couto C.G. Hypercalcemia in two cats with multiple myeloma. J Am Anim Hosp Assoc. 1996;32:503-508.
530 Sherding R.G., Meuten D.J., Chew D.J., et al. Primary hypoparathyroidism in the dog. J Am Vet Med Assoc. 1980;176:439-444.
531 Sherwood L.M., Cantley L., Russell J. Effects of calcium and 1,25-(OH)2D3 on the synthesis and secretion of parathyroid hormone. In: Cohn D.V., Martin T.J., Meunier P.J., editors. Calcium regulation and bone metabolism: basic and clinical aspects. Amsterdam: Elsevier Science; 1987:778-781.
532 Shoben A.B., Rudser K.D., de Boer I.H., et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613-1619.
533 Siegel N., Wongsurawat N., Armbrecht H.J. Parathyroid hormone stimulates dephosphorylation of the renoredoxin component of the 25-hydroxyvitamin D3-1 alpha-hydroxylase from rat renal cortex. J Biol Chem. 1986;261:16998-17003.
534 Sih T.R., Morris J.G., Hickman M.A. Chronic ingestion of high concentrations of cholecalciferol in cats. Am J Vet Res. 2001;62:1500-1506.
535 Silver J., Kilav R., Naveh-Many T. Mechanisms of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2002;283:F367-F376.
536 Silver J., Kronenberg H.M. Parathyroid hormone-molecular biology and regulation. In: Bilezikian J.P., Raisz L.G., Rodan G.A., editors. Principles of bone biology. San Diego: Academic Press; 1996:325-337.
537 Silver J., Naveh-Many T. Vitamin D and the parathyroid glands. In: Feldman D., editor. Vitamin D. San Diego: Academic Press; 1997:353-367.
538 Silver J., Yalcindag C., Sela-Brown A., et al. Regulation of the parathyroid hormone gene by vitamin D, calcium and phosphate. Kidney Int Suppl. 1999;73:S2-S7.
539 Slatopolsky E., Finch J., Brown A. New vitamin D analogs. Kidney Int Suppl. 2003;85:S83-17.
540 Slatopolsky E., Lopez-Hilker S., Delmez J., et al. The parathyroid-calcitriol axis in health and chronic renal failure. Kidney Int Suppl. 1990;29:S41-000047.
541 Smith S.A., Freeman L.C., Bagladi-Swanson M. Hypercalcemia due to iatrogenic secondary hypoadrenocorticism and diabetes mellitus in a cat. J Am Anim Hosp Assoc. 2002;38:41-44.
542 Smock S.L., Vogt G.A., Castleberry T.A., et al. Molecular cloning and functional characterization of the canine parathyroid hormone/parathyroid hormone related peptide receptor (PTH1). Mol Biol Rep. 2001;28:235-243.
543 Sprague S.M., Coyne D. Control of secondary hyperparathyroidism by Vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:512-518.
544 St. Arnaud R., Glorieux F.H. Vitamin D and bone development. In: Feldman D., editor. Vitamin D. San Diego: Academic Press; 1997:293-303.
545 Stern P.H. Vitamin D and bone. Kidney Int Suppl. 1990;29:S17-S21.
546 Stevens L.A., Djurdjev O., Cardew S., et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770-779.
547 Storms T.N., Clyde V.L., Munson L., et al. Blastomycosis in nondomestic felids. J Zoo Wildl Med. 2003;34:231-238.
548 Strewler G.J. Physiological actions of PTH and PTHrP: Skeletal actions. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:213-226.
549 Suda T., Takahashi N. Vitamin D and osteoclastogenesis. In: Feldman D., editor. Vitamin D. San Diego: Academic Press; 1997:329-340.
550 Sueda M.T., Stefanacci J.D. Ultrasound evaluation of the parathyroid glands in two hypercalcemic cats. Vet Radiol Ultrasound. 2000;41:448-451.
551 Suva L.J., Winslow G.A., Wettenhall R.E., et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893-896.
552 Swarthout J.T., D’Alonzo R.C., Selvamurugan N., et al. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1-17.
553 Szenci O., Brydl E., Bajcsy C.A. Effect of storage on measurement of ionized calcium and acid-base variables in equine, bovine, ovine, and canine venous blood. J Am Vet Med Assoc. 1991;199:1167-1169.
554 Takahashi H.E., Tanizawa T., Hori M., et al. Effect of intermittent administration of human parathyroid hormone (1-34) on experimental osteopenia of rats induced by ovariectomy. Cell Mater. 1991:113-117.
555 Tannehill-Gregg S., Kergosien E., Rosol T.J. Feline head and neck squamous cell carcinoma cell line: characterization, production of parathyroid hormone-related protein, and regulation by transforming growth factor-beta. In Vitro Cell Dev Biol Anim. 2001;37:676-683.
556 Tanner E., Langley-Hobbs S.J. Vitamin D-dependent rickets type 2 with characteristic radiographic changes in a 4-month-old kitten. J Feline Med Surg. 2005;7(5):307-311.
557 Taylor M.B., Geiger D.A., Saker K.E., Larson M.M. Diffuse osteopenia and myelopathy in a puppy fed a diet composed of an organic premix and raw ground beef. J Am Vet Med Assoc. 2009;234(8):1041-1048.
558 Teare J.A., Krook L., Kallfelz F.A., et al. Ascorbic acid deficiency and hypertrophic osteodystrophy in the dog: a rebuttal. Cornell Vet. 1979;69:384-401.
559 Tebb A.J., Arteaga A., Evans H., Ramsey I.K. Canine hyperadrenocorticism: effects of trilostane on parathyroid hormone, calcium and phosphate concentrations. J Small Anim Pract. 2005;46(11):537-542.
560 Terry A.H., Orrock J., Meikle A.W. Comparison of two third-generation parathyroid hormone assays. Clin Chem. 2003;49:336-337.
561 Teske E., van Heerde P., Rutteman G.R., et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205:1722-1728.
562 Tfelt-Hansen J., Chattopadhyay N., Yano S., et al. Calcium-sensing receptor induces proliferation through p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase but not extracellularly regulated kinase in a model of humoral hypercalcemia of malignancy. Endocrinology. 2004;145:1211-1217.
563 Thadhani R. Is calcitriol life-protective for patients with chronic kidney disease? J Am. Soc Nephrol. 2009;20:2285-2290.
564 Thakker R.V. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:275-282.
565 Thiede M.A., Daifotis A.G., Weir E.C., et al. Intrauterine occupancy controls expression of the parathyroid hormone-related peptide gene in preterm rat myometrium. Proc Natl Acad Sci USA. 1990;87:6969-6973.
566 Thode J., Juul-Jorgensen B., Bhatia H.M., et al. Comparison of serum total calcium, albumin-corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand J Clin Lab Invest. 1989;49:217-223.
567 Thomasset M. Calbindin-D 9K. In: Feldman D., editor. Vitamin D. New York: Academic Press; 1997:223-232.
568 Thompson K.G., Jones L.P., Smylie W.A., et al. Primary hyperparathyroidism in German shepherd dogs: a disorder of probable genetic origin. Vet Pathol. 1984;21:370-376.
569 Thrall M.A., Grauer G.F., Mero K.N. Clinicopathologic findings in dogs and cats with ethylene glycol intoxication. J Am Vet Med Assoc. 1984;184:37-41.
570 Thurlimann B., Waldburger R., Senn H.J., et al. Plicamycin and pamidronate in symptomatic tumor-related hypercalcemia: a prospective randomized crossover trial. Ann Oncol. 1992;3:619-623.
571 Toffaletti J. Ionized calcium measurement: analytical and clinical aspects. Lab Management. July 1983:31-35.
572 Tohme J.F., Bilezikian J.P. Hypocalcemic emergencies. Endocrinol Metab Clin North Am. 1993;22:363-375.
573 Tomsa K., Glaus T., Hauser B., et al. Nutritional secondary hyperparathyroidism in six cats. J Small Anim Pract. 1999;40:533-539.
574 Tomsa K., Steffen F., Glaus T. Life threatening metabolic disorders after application of a sodium phosphate containing enema in the dog and cat. Schweiz Arch Tierheilkd. 2001;143:257-261.
575 Toribio R.E., Kohn C.W., Chew D.J., et al. Comparison of serum parathyroid hormone and ionized calcium and magnesium concentrations and fractional urinary clearance of calcium and phosphorus in healthy horses and horses with enterocolitis. Am J Vet Res. 2001;62:938-947.
576 Torley D., Drummond A., Bilsland D.J. Calcipotriol toxicity in dogs. Br J Dermatol. 2002;147:1270.
577 Torrance A.G., Nachreiner R. Human-parathormone assay for use in dogs: validation, sample handling studies, and parathyroid function testing. Am J Vet Res. 1989;50:1123-1127.
578 Torrance A.G., Nachreiner R. Intact parathyroid hormone assay and total calcium concentration in the diagnosis of disorders of calcium metabolism in dogs. J Vet Intern Med. 1989;3:86-89.
579 Tras B., Maden M., Bas A.L., et al. Investigation of biochemical and haematological side-effects of enrofloxacin in dogs. J Vet Med A Physiol Pathol Clin Med. 2001;48:59-63.
580 Tripp C.D., Bryan J.N., Wills T.B. Presumptive increase in protein-bound serum calcium in a dog with multiple myeloma. Vet Clin Pathol. 2008;38(1):87-90.
581 Troy G.C., Forrester D., Cockburn C., et al. Heterobilharzia americana infection and hypercalcemia in a dog: a case report. J Am Anim Hosp Assoc. 1987;23:35-40.
582 Tucci J., Hammond V., Senior P.V., et al. The role of fetal parathyroid hormone-related protein in transplacental calcium transport. J Mol Endocrinol. 1996;17:159-164.
583 Tuma S.N., Mallette L.E. Hypercalcemia after nephrectomy in the dog: role of the kidneys and parathyroid glands. J Lab Clin Med. 1983;102:213-219.
584 Tweedy C.R., Rees G.M. Octreotide acetate in the treatment of hypercalcemia accompanying small cell carcinoma. South Med J. 1992;85:561.
585 Tyrrell C.J., Collinson M., Madsen E.L., et al. Intravenous pamidronate: infusion rate and safety. Ann Oncol. 1994;5(Suppl. 7):S27-29.
586 Uehlinger P., Glaus T., Hauser B., et al. Differential diagnosis of hypercalcemia-a retrospective study of 46 dogs. Schweiz Arch Tierheilkd. 1998;140:188-197.
587 Unterer S., Lutz H., Gerber B., et al. Evaluation of an electrolyte analyzer for measurement of ionized calcium and magnesium concentrations in blood, plasma, and serum of dogs. Am J Vet Res. 2004;65:183-187.
588 Urena P., Frazao J.M. Calcimimetic agents: review and perspectives. Kidney Int Suppl. 2003;85:S91-16.
589 Vaden S.L., Levine J., Breitschwerdt E.B. A retrospective case-control of acute renal failure in 99 dogs. J Vet Intern Med. 1997;11:58-64.
590 Vail D.M., Kisseberth W.C., Obradovich J.E., et al. Assessment of potential doubling time (Tpot), argyrophilic nucleolar organizer regions (AgNOR), and proliferating cell nuclear antigen (PCNA) as predictors of therapy response in canine non-Hodgkin’s lymphoma. Exp Hematol. 1996;24:807-815.
591 Vail D.M., Withrow S.J., Schwarz P.D., et al. Perianal adenocarcinoma in the canine male: a retrospective study of 41 cases. J Am Anim Hosp Assoc. 1990;26:329-334.
592 Walker M.C., Schaer M. Percutaneous ethanol treatment of hyperthyroidism in a cat. Feline Practice. 1998;28:10-12.
593 Walker P., Watanabe S., Lawlor P., et al. Subcutaneous clodronate. Lancet. 1996;348:345-346.
594 Walker P., Watanabe S., Lawlor P., et al. Subcutaneous clodronate: a study evaluating efficacy in hypercalcemia of malignancy and local toxicity. Ann Oncol. 1997;8:915-916.
595 Walser M., Robinson B.H.B., Duckett J.W.Jr. The hypercalcemia of adrenal insufficiency. J Clin Invest. 1963;42:456-465.
596 Walters M.R. Newly identified effects of the vitamin D endocrine system: update 1995. In: Bikle D.D., Negrovilar A., editors. Hormonal regulation of bone mineral metabolism. Bethesda: Endocrine Society; 1995:47-56.
597 Wang W., Li C., Kwon T.H., et al. AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol. 2002;283:F1313-F1325.
598 Ward D.T. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217-228.
599 Warrell R.P.Jr, Murphy W.K., Schulman P., et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9:1467-1475.
600 Waser M., Mesaeli N., Spencer C., et al. Regulation of calreticulin gene expression by calcium. J Cell Biol. 1997;138:547-557.
601 Wasserman R.H. Vitamin D and the intestinal absorption of calcium and phosphorus. In: Feldman B.F., editor. Vitamin D. New York: Academic Press; 1997:259-273.
602 Waters C.B., Scott-Moncrieff J.C. Hypocalcemia in cats. Compend Contin Educ. 1992;14:497-506.
603 Watson K.E., Abrolat M.L., Malone L.L., et al. Active serum Vitamin D levels are inversely correlated with coronary calcifications. Circulation. 1997;96:1755-1760.
604 Weaver M.E., Morrissey J., McConkey C.Jr, et al. WR-2721 inhibits parathyroid adenylate cyclase. Am J Physiol. 1987;252:E197-E201.
605 Webb J., Chary P., Northrup N., Almy F. Erythrophagocytic multiple myeloma in a cat. Vet Clin Pathol. 2008;37(3):302-307.
606 Weir E.C., Burtis W.J., Morris C.A., et al. Isolation of 16,000-dalton parathyroid hormone-like proteins from two animal tumors causing humoral hypercalcemia of malignancy. Endocrinology. 1988;123:2744-2751.
607 Weir E.C., Norrdin R.W., Matus R.E., et al. Humoral hypercalcemia of malignancy in canine lymphosarcoma. Endocrinology. 1988;122:602-608.
608 Weisbrode S.E., Krakowka S. Canine distemper virus-associated hypocalcemia. Am J Vet Res. 1979;40:147-149.
609 Welches C.D., Scavelli T.D., Matthiesen D.T., et al. Occurrence of problems after three techniques of bilateral thyroidectomy in cats. Vet Surg. 1989;18:392-396.
610 Weller R.E., Theilen G.H., Madewell B.R. Chemotherapeutic responses in dogs with lymphosarcoma and hypercalcemia. J Am Vet Med Assoc. 1982;181:891-893.
611 Wellington K., Goa K.L. Zoledronic acid: a review of its use in the management of bone metastases and hypercalcaemia of malignancy. Drugs. 2003;63:417-437.
612 Wells A.L., Long C.D., Hornof W.J., et al. Use of percutaneous ethanol injection for treatment of bilateral hyperplastic thyroid nodules in cats. J Am Vet Med Assoc. 2001;218:1293-1297.
613 Whitfield G.K., Dang H.T., Schluter S.F., et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144:2704-2716.
614 Willard M.D., Schall W.D., McCaw D.E., et al. Canine hypoadrenocorticism: report of 37 cases and review of 39 previously reported cases. J Am Vet Med Assoc. 1982;180:59-62.
615 Williams L.E., Gliatto J.M., Dodge R.K., et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985-1995). J Am Vet Med Assoc. 2003;223:825-831.
616 Winer K.K., Yanovski J.A., Cutler G.B.Jr. Synthetic human parathyroid hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA. 1996;276:631-636.
617 Winkelmayer W.C., Levin R., Avorn J. The nephrologist’s role in the management of calcium-phosphorus metabolism in patients with chronic kidney disease. Kidney Int. 2003;63:1836-1842.
618 Wisner E.R., Nyland T.G. Ultrasonography of the thyroid and parathyroid glands. Vet Clin North Am Small Anim Pract. 1998;28:973-991.
619 Wisner E.R., Penninck D., Biller D.S., et al. High-resolution parathyroid sonography. Vet Radiol Ultrasound. 1997;38:462-466.
620 Wolf M. Active Vitamin D and survival. J Am Soc Nephrol. 2008;19:1442-1443.
621 Wolf M., Thadhani R. Vitamin D in patients with renal failure: a summary of observational mortality studies and steps moving forward. J Steroid Biochem and Mol Biol. 2007;103:487-490.
622 Won D.S., Park C., In Y.J., et al. A case of nutritional secondary hyperparathyroidism in a Siberian tiger cub. J Vet Med Sci. 2004;66:551-553.
623 Woo J., Cannon D.C. Metabolic intermediates and inorganic ions, 17th ed. Philadelphia: WB Saunders. 1984:133-179.
624 Wooldridge J.D., Gregory C.R. Ionized and total serum magnesium concentrations in feline renal transplant recipients. Vet Surg. 1999;28:31-37.
625 Worth G.K., Vasikaran S.D., Retallack R.W., et al. Major method-specific differences in the measurement of intact parathyroid hormone: studies in patients with and without chronic renal failure. Ann Clin Biochem. 2004;41:149-154.
626 Wright K.N., Breitschwerdt E.B., Feldman J.M., et al. Diagnostic and therapeutic considerations in a hypercalcemic dog with multiple endocrine neoplasia. J Am Anim Hosp Assoc. 1995;31:156-162.
627 Wu-Wong J.R., Melnick J. Vascular calcification in chronic kidney failure: role of Vitamin D receptor. Curr Opin Investig Drugs. 2007;8:237-247.
628 Wysolmerski J.J., Stewart A.F., Martin J.T. Physiological actions of PTH and PTHrP: epidermal, mammary, reproductive, and pancreatic tissues. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The parathyroids. San Diego: Academic Press; 2001:275-292.
629 Wysolmerski J.J. The evolutionary origins of maternal calcium and bone metabolism during lactation. J Mammary Gland Biol Neoplasia. 2002;7:267-276.
630 Yajima A., Pasch A., Nitta K. Efficacy and safety of Cinacalcet in chronic kidney disease stage III and IV. Clin Med Thera. 2009;1:1661-1666.
631 Yanagawa N., Lee D.B.N. Renal handling of calcium and phosphorus. In: Coe F.L., Favus M.J., editors. Disorders of bone and mineral metabolism. New York: Raven Press; 1992:3-40.
632 Yang K.H., dePapp A.E., Soifer N.E., et al. Parathyroid hormone-related protein: evidence for isoform- and tissue-specific posttranslational processing. Biochemistry. 1994;33:7460-7469.
633 Yasuda T., Banville D., Rabbani S.A., et al. Rat parathyroid hormone-like peptide: comparison with the human homologue and expression in malignant and normal tissue. Mol Endocrinol. 1989;3:518-525.
634 Yu J., Papavasiliou V., Rhim J., et al. Vitamin D analogs: new therapeutic agents for the treatment of squamous cancer and its associated hypercalcemia. Anticancer Drugs. 1995;6:101-108.
635 Yudd M., Llach F. Current medical management of secondary hyperparathyroidism. Am J Med Sci. 2000;320:100-106.
636 Zaloga G.P., Chernow B. The multifactorial basis for hypocalcemia during sepsis: studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987;107:36-41.
637 Zaloga G.P., Malcolm D., Holaday J., et al. Verapamil reverses calcium cardiotoxicity. Ann Emerg Med. 1987;16:637-639.
638 Zaloga G.P., Willey S., Tomasic P., et al. Free fatty acids alter calcium binding: a cause for misinterpretation of serum calcium values and hypocalcemia in critical illness. J Clin Endocrinol Metab. 1987;64:1010-1014.
639 Zawada E.T.Jr, Saelens D.A., Lembke J.M. Influence of calcium infusion on plasma atrial natriuretic peptide in conscious dogs: intervention with calcium antagonist, verapamil. Miner Electrolyte Metab. 1990;16:369-377.
640 Zelikovic I., Chesney R.W. Vitamin D and mineral metabolism: the role of the kidney in health and disease. World Rev Nutr Diet. 1989;59:156-216.
641 Zini E., Hauser B., Ossent P., Dennler R., Glaus T.M. Pansteatitis and severe hypocalcaemia in a cat. J Feline Med Surg. 2006;9(2):168-171.
642 Zitterman A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552-572.
643 Zittermann A., Schleithoff S.S., Frisch S., et al. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55:1163-1170.
644 Zivin J.R., Gooley T., Zager R.A., et al. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis. 2001;37:689-698.