Chapter 6 Disorders of Calcium
Hypercalcemia and Hypocalcemia
Calcium is required in the body for many vital intracellular and extracellular functions, as well as for skeletal support. Ionized calcium (iCa or Ca2+) is required for enzymatic reactions, membrane transport and stability, blood coagulation, nerve conduction, neuromuscular transmission, muscle contraction, vascular smooth muscle tone, hormone secretion, bone formation and resorption, control of hepatic glycogen metabolism, and cell growth and division.491 Intracellular calcium ions are one of the primary regulators of the cellular response to many agonists and serve as “an almost universal ionic messenger,” conveying signals received at the cell surface to the inside of the cell.463 In addition to serving as an intracellular messenger, the iCa concentration in the extracellular fluid (ECF) regulates cell function in many organs, including the parathyroid gland, kidneys, and thyroid C cells by binding to a newly identified cell membrane-bound calcium-sensing receptor.80 Normal homeostatic control mechanisms usually maintain the serum calcium concentration within a narrow range and guarantee an adequate supply of calcium for intracellular function. These mechanisms must be disrupted for hypercalcemia or hypocalcemia to develop. Abnormal serum calcium concentrations are of diagnostic value and contribute to the development of lesions and clinical signs. Technological advances in the measurement of serum iCa concentration, parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), and vitamin D metabolites have provided tools that allow greater diagnostic accuracy in the investigation of calcium disorders.
Veterinarians must frequently interpret abnormal serum calcium concentrations. Large deviations of serum calcium concentration from normal occur infrequently, but small deviations may be equally important because they also provide diagnostic clues to an underlying disease. The magnitude of altered serum calcium concentration often does not suggest a specific diagnosis or the extent of disease. Furthermore, a normal serum calcium concentration does not eliminate a disorder of calcium homeostasis.
Normal physiology
Overview of calcium homeostasis
Regulation of serum calcium concentration is complex and requires the integrated actions of PTH, vitamin D metabolites, and calcitonin (Fig. 6-1). PTH and calcitriol (1,25-dihydroxyvitamin D3) are the main regulators of calcium homeostasis and have major regulatory effects on each other.478 PTH is largely responsible for the minute-to-minute control of serum iCa concentration, whereas calcitriol maintains day-to-day control. In the fetus, the parathyroid glands and placenta produce PTHrP, which binds to PTH receptors and regulates calcium balance.582 After birth, the parathyroid glands modify their pattern of hormone secretion and produce predominantly PTH. Other hormones, including adrenal corticosteroids, estrogens, thyroxine, growth hormone, glucagon, and prolactin, have less influence on calcium homeostasis but may play a role during growth, lactation, or certain disease states.
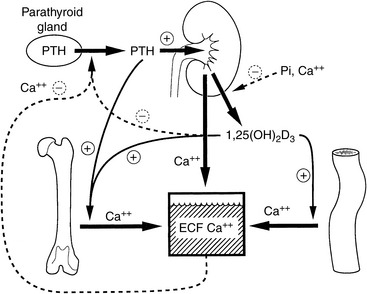
Figure 6-1 Regulation of extracellular fluid (ECF) calcium concentration by the effects of parathyroid hormone (PTH) and calcitriol (1,25-dihydroxyvitamin D3) on the gut, kidneys, bone, and parathyroid gland. The principal effect of PTH is to increase the ECF calcium concentration by mobilizing calcium from bone, increasing tubular calcium reabsorption, and, indirectly on the gut, by increasing calcitriol synthesis. The principal effect of calcitriol is to increase intestinal absorption of calcium, but it also exerts negative regulatory control of PTH synthesis and further calcitriol synthesis.
(Modified from Habner JF, Rosenblatt M, Pott JT. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev 1984;64:1000.)
The intestine, kidneys, and bone are the major target organs affected by calcium regulatory hormones. These interactions allow conservation of calcium in the ECF by renal tubular reabsorption, increased intestinal transport of calcium from the diet, and internal redistribution of calcium from bone (Fig. 6-2). The intestine and kidneys are the major regulators of calcium balance in health.176 Normally, dietary calcium intake equals the amount of calcium lost in urine and feces. The enteric absorption of calcium depends on the physiologic status of the intestines (e.g., acidity, presence of other dietary components, integrity of the villi or presence of small intestinal disease, and degree of enterocyte stimulation by calcitriol). Non–protein-bound calcium is filtered by the glomerulus and undergoes extensive renal reabsorption. This process results in reclamation of more than 98% of the filtered calcium in health.146,482
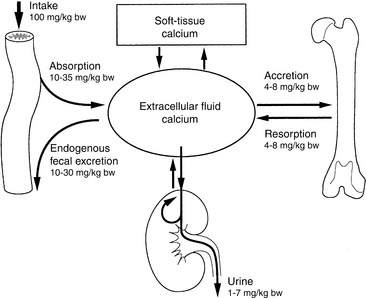
Figure 6-2 Normal calcium balance showing the major organs that supply or remove calcium from extracellular fluid: bone, gut, and kidneys. Total calcium input into extracellular fluid equals total calcium leaving the extracellular space.
(Modified from Hazewinkel HAW. Dietary influences on calcium homeostasis and the skeleton. In: Purina International Nutrition Symposium. Orlando, Fla: Ralston Purina, 1991: 52.)
The skeleton provides a major supply of calcium and phosphorus when intestinal absorption and renal reabsorption inadequately maintain normal serum calcium concentrations. Bone calcium mobilization is important in the acute regulation of blood calcium.433 Calcium and phosphorus can be mobilized from calcium phosphate in the bone ECF compartment, but these stores are rapidly depleted. The osteoblast is critical in limiting the distribution of calcium and phosphate between bone and ECF, and exchangeable bone water is separated from ECF water by the combined membranes of osteoblasts lining bone surfaces. For greater or prolonged release of calcium from bone, osteoclastic bone resorption must be activated. Osteoclasts secrete acid and proteases that result in dissolution of the mineralized matrix of bone and mobilize calcium and phosphorus.
Extracellular iCa concentration is the actively regulated fraction of total calcium (tCa).81,115 When blood iCa concentration decreases, PTH secretion is stimulated. PTH exerts direct effects on bone and the kidneys and indirect effects on the intestine through calcitriol. PTH increases synthesis of calcitriol by activating renal mitochondrial 1α-hydroxylation of 25-hydroxycholecalciferol. Calcitriol increases calcium absorption from the intestine and acts with PTH to stimulate osteoclastic bone resorption.104 Calcitriol is necessary for differentiation of osteoclasts from precursor mononuclear cells. PTH increases osteoclast number and stimulates osteoclast function to increase bone resorption and the release of calcium from bone to blood. Calcitriol also induces renal transport mechanisms activated by PTH that increase tubular reabsorption of calcium from the glomerular filtrate, thus preventing calcium loss in urine.404
Calcium distribution within the body
Approximately 99% of body calcium resides in the skeleton and is stored as hydroxyapatite, Ca10(PO4)6(OH)2. Most skeletal calcium is poorly exchangeable, and less than 1% is considered readily available. The small amount of rapidly exchangeable bone calcium arises from the ECF in bone that is present between osteoblasts and osteocytes and the bone matrix. Almost all of the nonskeletal calcium resides in the extracellular space, although small and biologically important quantities are found intracellularly.491
Extracellular Calcium
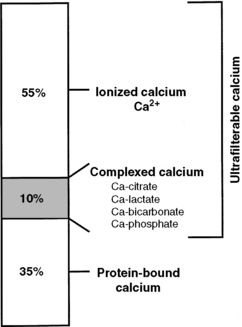
Calcium in plasma or serum exists in three fractions: ionized (iCa), complexed (bound to phosphate, bicarbonate, sulfate, citrate, and lactate), and protein bound (Fig. 6-3). In clinically normal dogs, protein-bound, complexed, and iCa account for approximately 34%, 10%, and 56% of serum tCa concentration, respectively.516 Ionized calcium is the most important biologically active fraction in serum, although an active biologic role for complexed calcium has been suggested.571 No biologic role for protein-bound calcium has been identified other than as a storage pool or buffering system for iCa.
Intracellular Calcium
Intracellular iCa is an important secondary messenger in the response to biochemical signals (such as hormones) transduced through the cell membrane.462,491 Therefore, intracellular iCa concentrations are maintained at a very low level (approximately 100 nM), 10,000-fold less than the serum concentration. This permits rapid diffusion into the cytoplasm from the ECF or endoplasmic reticulum. Intracellular calcium is rapidly buffered by cytosolic proteins and is transported into organelles or to the outside of the cell after an increase in intracellular iCa. If intracellular iCa is not maintained at a low concentration, it leads to toxicity and eventual cell death.
Most intracellular calcium is sequestered in organelles or bound to cellular membranes or proteins.276 Sequestration of iCa in mitochondria blunts an increase in cytosolic iCa, whereas endoplasmic reticulum serves as a reservoir to increase cytosolic iCa when necessary. Binding of calcium to specific cytosolic or membrane proteins is an efficient method for regulation of intracellular iCa concentration. Protein binding provides intracellular iCa buffering and also may act as a messenger system when protein configuration and activity are altered. Calbindin, calmodulin, and troponin C are important intracellular calcium-binding proteins.57
Cell Membrane Calcium Ion Sensing Receptor
In 1993, a novel iCa-sensing receptor was cloned and sequenced.78 The iCa receptor plays an integral role in iCa balance by regulating parathyroid chief cells, C cells, and renal epithelial cells.77,251 In parathyroid chief cells and C cells, the iCa receptor directly regulates intracellular iCa concentration, which controls PTH and calcitonin secretion. Ionized magnesium (iMg) is also an agonist of the iCa receptor. Stimulation of the iCa receptor caused by increased extracellular iCa concentration in the kidneys decreases NaCl, iCa, and iMg reabsorption in the proximal convoluted tubule and decreases water reabsorption in collecting ducts. This results in greater excretion of iCa and iMg in a more dilute urine.
Genetic diseases have been described related to both inactivating and activating mutations of the calcium receptor gene.23 Inactivating mutations lead to severe neonatal hypercalcemia when homozygous and to familial hypocalciuric hypercalcemia when heterozygous.562 Activating mutations of the calcium receptor produce hypoparathyroidism and hypocalcemia.564 Autoantibodies produced against the calcium receptor may either disable it, producing hyperparathyroidism with hypercalcemia,427,472 or activate it, producing hypoparathyroidism.219,293 Drugs that bind the Ca2+-sensing receptor may be useful in treating disorders of the parathyroid gland.
Parathyroid hormone
Structure
PTH is an 84-amino acid single-chain polypeptide that is synthesized and secreted by chief cells of the parathyroid glands.478 The amino acid sequences of PTH are known for the dog, cow, pig, rat, chicken, and human,313,488 and most mammals appear to have very similar amino-terminal portions of the molecule.404 Whereas the conserved amino end of PTH is vital for binding to cell membrane receptors, the role of the carboxyl terminus is to serve as a guide for PTH through the cellular secretory pathway.329
Synthesis and secretion
Synthesis, secretion, and degradation of PTH by chief cells are closely related. Little PTH is stored within the parathyroid glands,231 and synthesis of new specific messenger RNA (mRNA) and translation to PTH are required to maintain secretion.535 After secretion, PTH has a short half-life (3 to 5 minutes) in serum; thus, a steady rate of secretion is necessary to maintain serum PTH concentrations. Circulating PTH has many forms, not all of which have bioactivity,71,413 leading to potential confusion in assay interpretations.508,560,625
The amount of PTH available for secretion is a function of the balance of synthesis and degradation within chief cells (Fig. 6-4). Calcitriol, via the vitamin D receptor (VDR), and extracellular iCa concentration, via effects on the plasmalemmal calcium receptor,108,109,470 control these parathyroid cell processes. Because calcitriol regulates expression of the calcium receptor gene,99 calcitriol can be considered to exert overall control over PTH synthesis and secretion by the parathyroid cells. In general, the parathyroid gland has evolved most of its regulatory strategies to protect against hypocalcemia, with sensitive control of PTH synthesis and secretion being the dominant sites for regulation.82,536 However, high serum iCa concentrations increase the rate of degradation of PTH within the gland to protect against hypercalcemia.313
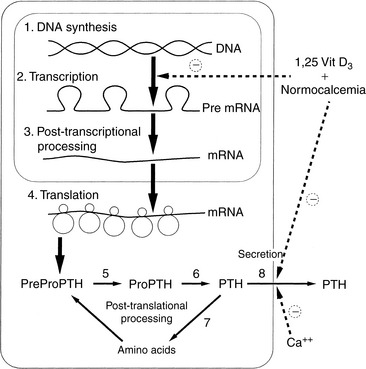
Figure 6-4 Synthesis and secretion of parathyroid hormone. Note sites of regulation of PTH biosynthesis by extracellular ionized calcium or calcitriol (1,25-[OH]2-vitamin D3) interaction.
(Modified from Habner JF, Rosenblatt M, Potts JT. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev 1984;64:1004.)
Except for minor diurnal variation, PTH secretion is relatively constant but may have a mild pulsatile pattern in response to minor fluctuations in the concentration of serum iCa.81 A relatively low rate of PTH secretion is needed normally to maintain serum iCa concentration. The basal secretory rate of PTH is approximately 25% of the maximal rate, and PTH is constantly secreted during normocalcemia. Complete inhibition of PTH secretion is not achieved even in the presence of severe hypercalcemia.313
Hypocalcemia is the principal stimulus for PTH secretion, but epinephrine, isoproterenol, dopamine, secretin, prostaglandin E2, and stimulation of nerve endings within the parathyroid gland may have minor effects.231 High concentrations of serum and intracellular iCa inhibit PTH secretion via increased arachidonic acid62,101 and possibly subsequent eicosanoid production.101 The control at PTH mRNA synthesis is also critically important.535
Calcitriol also plays an important role in the regulation of PTH synthesis and secretion.538 Calcitriol inhibits PTH mRNA synthesis537 and stimulates synthesis of the calcium receptor.99 These relationships explain the requirement for adequate blood concentrations of calcitriol to maintain the ability of the parathyroid gland to respond to changes in extracellular calcium concentrations.349,405 Increased intracellular iCa may also cooperate with calcitriol to reduce PTH synthesis in chief cells by inhibiting the expression of calreticulin (a blocker of VDR action).526,600 Animals with uremia and reduced serum calcitriol concentrations have poorly regulated chief cell function that results in renal secondary hyperparathyroidism,217,401 but a significant part of the hyperparathyroid response in uremic patients is the result of a glandular hyperplasia caused by the changes of both calcitriol and serum phosphorus.9 Serum phosphorus concentrations are generally considered to regulate PTH secretion principally by indirect means. Renal calcitriol synthesis is reduced early in uremia by modest hyperphosphatemia, and the plasma iCa concentration may decrease because of reduced effects of calcitriol on the intestine, bone, and kidneys. Markedly increased serum phosphorus concentrations (as seen in advanced renal failure) can lower the serum iCa concentration (mass law effect), resulting in an increase in PTH secretion because of the lowered calcium, but these effects do not occur early in renal failure when serum phosphorus is only moderately increased.401
Serum magnesium concentration has little role in the control of PTH secretion under normal conditions, but PTH secretion can be inhibited by very high concentrations of serum iMg.478 Paradoxically, hypomagnesemia or magnesium depletion also results in an inability to secrete PTH, but the cellular mechanism of this effect is unclear. This effect may be partially caused by reduced sensitivity of cell membrane receptors to iCa in the presence of low serum iMg concentrations.231,382
Set-Point for PTH Secretion
The set-point for PTH secretion is defined as the ECF iCa concentration that occurs at the serum PTH concentration that is midway between maximal and minimal values of PTH obtained experimentally.81 Normal serum iCa concentration is maintained slightly higher than the set-point; thus, PTH release normally is less than half-maximal (Fig. 6-5).
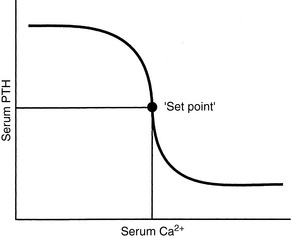
Figure 6-5 Relationship between secretion rate of parathyroid hormone and plasma calcium concentration. Small changes in plasma calcium concentration cause large changes in parathyroid hormone secretion, but secretion is not completely suppressed by high plasma calcium concentrations.
The rate of PTH secretion is inversely proportional to the concentration of extracellular calcium, but this proportional secretion of PTH occurs only over a narrow range corresponding to a serum tCa concentration of 7.5 to 11.0 mg/dL.231 An inverse sigmoidal curve with a steep slope results when the relationship between serum iCa concentration and PTH secretion is plotted over a larger range of calcium concentrations (see Fig. 6-5).81 This ensures large changes in PTH secretion for relatively small changes in iCa concentration in the physiologic range and precise control of serum iCa concentration. An approximate 10% decrease in serum iCa concentration elicits a nearly maximal PTH secretory response. The rate of decrease of serum iCa concentration is also important, and rapid decreases in serum iCa result in larger increases in PTH secretion. A 2% to 3% decrease in iCa concentration, if rapid in onset, may result in a 400% increase in PTH secretion.81
The cell membrane calcium receptor is responsible for establishing the relationship of the set-point for PTH secretion and extracellular iCa concentration.598 The calcium receptor regulates PTH secretion indirectly by controlling the intracellular iCa concentration by means of (1) release of iCa from intracellular stores, and (2) cell membrane calcium channels. Calcium channels span the parathyroid chief cell membrane and are important in allowing extracellular iCa access to the interior of the cell.186 The calcium channels are controlled by intracellular iCa concentration82 and membrane regulatory G proteins, which interact with the cell membrane calcium receptor.24
Calcitriol plays an important role in controlling the parathyroid gland set-point by regulating (1) synthesis of the cell membrane calcium receptor,76,99 (2) synthesis of cell membrane G proteins, and (3) function of cell membrane calcium channels.404 Therefore, adequate calcitriol is necessary to maintain the set-point for PTH secretion. The regulation of calcium receptor expression by calcitriol explains the observed “calcium set point” aberrations in control of PTH secretion in those with uremia.356 These patients have deficits in calcitriol production,116,617 as well as resistance in uremic parathyroids to calcitriol160,435; thus, they are less able of inducing synthesis of adequate numbers of calcium receptors.
Although regulations at each parathyroid cell may fail, thus producing abnormally increased PTH,214,460 changes may also be seen in the maximal secretory capacity dependent mostly on parathyroid cell numbers.506 It is likely that increased PTH secretion in patients with renal secondary hyperparathyroidism is primarily caused by parathyroid gland hyperplasia.157 One important role of calcitriol therapy in these patients is to prevent or reverse the parathyroid cellular hyperplasia.100,156,403
Inhibition of PTH Synthesis and Secretion
This topic has become important with the understanding of the toxicity of PTH in animals and humans with chronic renal failure (CRF) and accompanying secondary hyperparathyroidism.11,357,401,438 Recently, increased awareness of PTH toxicity stems from established relations to cardiovascular disease135 and mortality.546 PTH secretion is inhibited by increased serum iCa concentration,535,537 and the initial effect to decrease PTH secretion is rapid (occurring within 2 to 3 minutes), mediated by the calcium receptor with a cascade of resulting intracellular events67,137,251 and involving mediation by arachidonate.8 Slower effects are caused by inhibition of synthesis of PTH mRNA and its translation to hormone (Fig. 6-6).535
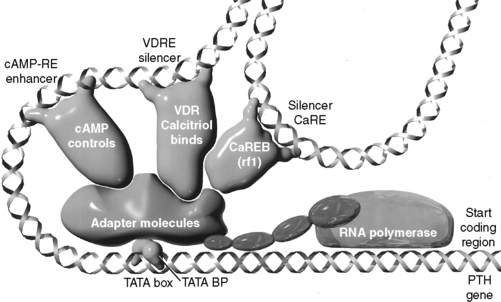
Figure 6-6 Simplified depiction of events regulating transcription of the parathyroid hormone (PTH) gene by RNA polymerase. Only the three transcription factors best understood to interact in this regulation are shown. Cyclic AMP (cAMP) stimulates phosphorylation of a transcription factor that binds to a cAMP response element (cAMP-RE) on the gene and enhances transcription. In contrast, the vitamin D receptor (VDR)-calcitriol complex and calcium response element-binding protein (CaREB, rf1) bind to their respective vitamin D (VDRE) and calcium (CaRE) response elements of the PTH gene, which function as “silencers” or negative regulators of gene transcription. Note that for calcium to exert its negative effect by means of the CaREB transcription factor, calcitriol and the vitamin D receptor must also be present. The adapter molecules (shown as a single structure) diagrammatically represent about 30 proteins termed accessory transcription factors. The TATA box is part of the gene promoter to which the TATA box binding proteins (BPs) bind.
(From Nagode LA, Chew DJ, Podell M. Benefits of calcitriol therapy and serum phosphorus control in dogs and cats with chronic renal failure. Vet Clin North Am Small Anim Pract 1996;26:1293–1330.)
The osteocyte-derived phosphatonin43 fibroblast growth factor-23 (FGF-23) is both protectively phosphaturic229 and inhibitory of PTH secretion.203 FGF-23 is induced by calcitriol304 and in a feedback loop, FGF-23 inhibits calcitriol synthesis.229 The calcimimetic cinacalcet (Sensipar), previously used as an alternative to calcitriol’s PTH suppression13 during kidney disease,543,630 has recently been shown to be contraindicated131 due primarily to hyperphosphatemic consequences.284 High phosphorus is increasingly recognized193,291,359 as the major driver of cardiovascular calcification,280,367 which is the major cause of mortality in human patients with chronic renal disease.387 Calcitriol, in part due to its induction of FGF-23304 with its phosphaturic effects,229 can protect against vascular calcification,358,603,627 which is likely instrumental in the now widely recognized improved survival associated with use of calcitriol621 and other active vitamin D metabolites.620 Cinacalcet, although it suppresses PTH,630 fails to affect FGF-23, so with PTH suppressed, there is no protection against hyperphosphatemia caused by failure of renal excretion. Oral calcitriol improves survival in human renal failure patients before dialysis,584 a group that corresponds to the dogs and cats with chronic renal failure for which veterinary use of calcitriol has been recommended.401 A large body of work now demonstrates in humans the life protection conferred by calcitriol,617,643 and in placebo-controlled studies in the dog.456
Calcitriol is an important inhibitor of PTH synthesis, and it completes a negative feedback loop from the kidneys because PTH stimulates renal calcitriol synthesis. Short and long negative feedback loops complement each other to control normal secretion of PTH.313 The long negative feedback loop is completed when an increased serum iCa concentration results from PTH stimulation of renal calcitriol production and subsequent enhanced gastrointestinal absorption of calcium. This effect takes hours to develop because calcium-binding proteins associated with calcium absorption must be induced in enterocytes.72,601 The short negative feedback loop is mediated by the binding of calcitriol to VDRs in parathyroid cells, with inhibition of transcription of the PTH gene.535 The calcitriol receptor (VDR) is expressed in parathyroid chief cells at concentrations equal to those in intestinal epithelial cells that regulate calcium absorption in the gastrointestinal tract. The VDR was found to be depleted in the parathyroid glands of dogs and humans with uremia because of a lack of renal production of calcitriol.75 After the VDR binds calcitriol, the VDR-calcitriol complex acts in the nucleus of the parathyroid chief cells by binding to specific regions of the PTH gene called vitamin D response elements (VDREs) and inhibiting transcription of the PTH gene (see Fig. 6-6).313,401 For calcitriol to suppress synthesis of PTH, a normal concentration of iCa must be present because it would be inappropriate to suppress PTH synthesis in a hypocalcemic patient.
Clearance and metabolism of parathyroid hormone
The intact PTH molecule (84 amino acids) circulates in the bloodstream with a half-life of 3 to 5 minutes and is removed by fixed macrophages.313,478 A significant amount of cleavage is close to the amino terminus of the PTH molecule. Regardless of where the endopeptidase cleavage occurs, the amino-terminal portion of PTH is completely degraded within the phagocytes.
The kidneys and bone also participate in destruction of intact PTH. Fragments of PTH are filtered by the glomeruli. This mechanism of excretion is most important for the excretion of the carboxyl-terminal PTH fragments because carboxyl-terminal PTH (released from either the parathyroid gland or Kupffer cells) is cleared only by glomerular filtration (Fig. 6-7). The carboxyl-terminal fragments of PTH are not important for calcium metabolism. The circulating half-life of carboxyl-terminal PTH is much longer than that of intact PTH, and serum concentrations of carboxyl-terminal PTH can be very high during primary or secondary hyperparathyroidism and can be nonspecifically increased during renal failure.
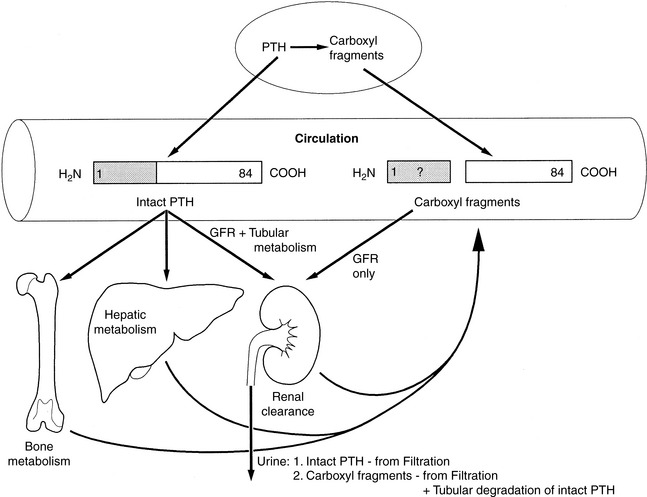
Figure 6-7 Degradation and clearance of parathyroid hormone (PTH). PTH (1-84) is secreted intact from the parathyroid gland into the circulation. Biologically inactive carboxy-terminal (COOH) fragments of PTH are also secreted by the parathyroid gland, but amino-terminal PTH is not secreted and does not circulate in biologically relevant concentrations. Peripheral metabolism of intact PTH to carboxy-terminal PTH fragments occurs mostly in the liver but may also occur in the kidneys and bone. Both intact PTH and carboxy-terminal PTH are cleared by glomerular filtration, but only intact PTH is metabolized in the liver, kidneys, and bone. The half-life of intact PTH in vivo is short compared with that of the carboxy-terminal fragments of PTH.
(Modified from Endres DB, Villaneuva R, Sharp CF, et al. Measurement of parathyroid hormone. Endocrinol Metab Clin North Am 1989;18:614.)
Actions of parathyroid hormone
PTH is the principal hormone involved in the minute-to-minute fine regulation of blood calcium concentration. It exerts its biologic actions directly by influencing the function of target cells primarily in bone and the kidneys and indirectly in the intestine to maintain plasma calcium at a concentration sufficient to ensure the optimal functioning of a wide variety of body cells.
In general, the most important biologic effects of PTH on calcium are to (1) increase the blood calcium concentration; (2) increase tubular reabsorption of calcium, resulting in decreased calcium loss in the urine; (3) increase bone resorption and the numbers of osteoclasts on bone surfaces; and (4) accelerate the formation of the principal active vitamin D metabolite (1,25-dihydroxyvitamin D, or calcitriol) by the kidneys through a trophic effect to both induce synthesis of and activate the 1α-hydroxylase in mitochondria of renal epithelial cells in the proximal convoluted tubules.
An important action of PTH on bone is to mobilize calcium from skeletal reserves into ECF.102 The increase in blood calcium concentration results from an interaction of PTH with receptors on osteoblasts that stimulate increased calcium release from bone and direct an increase in osteoclastic bone resorption.393
The response of bone to PTH is biphasic. The immediate effects are the result of increasing the activity of existing bone cells. This rapid effect of PTH depends on the continuous presence of hormone and results in an increased flow of calcium from deep in bone to bone surfaces through the action of an osteocyte-osteoblast “pump” in order to make fine adjustments in the blood calcium concentration.433 The later effects of PTH on bone are potentially of greater magnitude and are not dependent on the continuous presence of hormone. Osteoclasts are primarily responsible for the long-term action of PTH on increasing bone resorption and overall bone remodeling.102,393
PTH also has the potential to serve as an anabolic agent in bone and stimulate osteoblastic bone formation.201,552 Intermittent administration of exogenous 1-34 PTH has been reported to increase bone mass in humans and animals.554
The ability of PTH to enhance the renal reabsorption of calcium is of considerable importance. This effect of PTH on tubular reabsorption of calcium is caused by, in part, a direct action on the distal convoluted tubule.631 PTH may also increase calcium reabsorption in the ascending thick limb of Henle’s loop indirectly by increasing the net positive charge in the nephron lumen and creating a stimulus for diffusion out of the lumen. PTH also regulates the conversion of 25-hydroxycholecalciferol to calcitriol and other metabolites of vitamin D.
Parathyroid Hormone C-Terminal 7-84 as PTH Antagonist
It was originally thought that PTH 35-84 and other fragments cleaved between residues 24 and 43 dominated the carboxyl-terminal fragments of PTH secreted by chief cells. The C-terminal fragments can be measured using C-terminal-specific immunoassays. The function of PTH 35-84 and its receptor is unknown, but it may regulate bone cell function. The larger C-terminal fragment, PTH 7-84,279 may be significantly increased in renal secondary hyperparathyroidism386 and can antagonize the effects of PTH 1-84 in vivo.321 The antagonistic action of PTH 7-84 is likely attributable to binding to an alternate PTH receptor and not to the PTH1 receptor that is used by PTH 1-34 and PTH 1-84.148,414
Parathyroid Hormone Receptor
The receptor for N-terminal PTH (amino acids 1 to 34), the region important in calcium regulation, has been cloned and sequenced in humans, dogs, and other species.1,416,542 It is a seven-transmembrane domain receptor that is expressed in renal epithelial cells, osteoblasts, and some other cells. The N-terminal regions of PTH and PTHrP bind this receptor with equal affinity. The PTH receptor is also located on many cell types, such as dermal fibroblasts, that are not associated with the action of PTH. It is assumed that the receptor functions as the binding protein for PTHrP in these tissues. The currently used terminology for this receptor is the PTH1 receptor, but it is often described as the PTH/PTHrP receptor. The PTH2 receptor is present in the brain and binds to both PTH and tuberoinfundibular peptide but not to PTHrP.249
Parathyroid hormone-related protein: a polyhormone
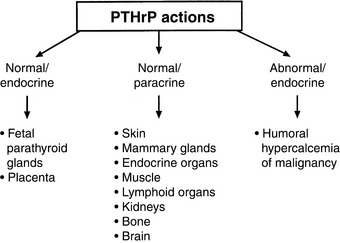
PTHrP is not strictly a calcium-regulating hormone, but it was identified in 1982 as an important PTH-like factor that plays a central role in the pathogenesis of humoral hypercalcemia of malignancy (HHM).480 PTHrP is produced widely in the body and has numerous actions in the developing fetus and adult animal independent of its role in cancer-associated hypercalcemia.451 This is in contrast to PTH, which is produced by the parathyroid glands and functions principally in regulation of calcium balance. PTHrP has multiple actions that are specific to the N-terminal, midregion, and C-terminal regions of the protein, making PTHrP a true polyhormone.
Some of the actions of PTHrP involve normal regulation of calcium metabolism.491 For example, PTHrP functions as a calcium-regulating hormone in the fetus and is produced by the fetal placenta.343 In the adult, PTHrP circulates in the blood in low concentrations (<1 pM) but is produced by many different tissues and functions principally as an autocrine, paracrine, or intracrine cellular regulator. PTHrP is produced by the lactating mammary gland and is secreted into milk. Mammary gland production of PTHrP likely facilitates mobilization of calcium from maternal bones and may play a role in the transport of calcium into milk during lactation.628,629 PTHrP acts as an abnormal systemic calcium-regulating hormone and mimics the actions of PTH in patients with HHM. PTHrP not only plays a major role in most forms of HHM but also has been demonstrated in many normal tissues, including epithelial cells of the skin and other organs; endocrine glands; smooth, skeletal, and cardiac muscle; lactating mammary glands; placenta; fetal parathyroid glands; bone; brain; and lymphocytes.451,478 Therefore, PTHrP functions as (1) a hormone in an endocrine manner in the fetus and lactating dams,582 (2) a paracrine factor in many fetal and adult tissues, and (3) an abnormal hormone in an endocrine manner in adults with HHM (Fig. 6-8). PTHrP is necessary for normal endochondral bone formation in the fetus and neonate. Knockout of the PTHrP gene results in short-limb dwarfism and death at birth as a result of a failure of cartilage proliferation at the growth plates and premature ossification.287
PTHrP is a 139- to 173-amino acid peptide originally isolated from human and animal tumors associated with HHM.480 PTHrP shares 70% sequence homology with PTH in its first 13 amino acids. The N-terminal region of PTHrP (amino acids 1 to 34) binds and stimulates PTH receptors in bone and kidney cells with affinity equal to that of PTH, so that PTHrP functions similarly to PTH in patients with HHM.124,423 The midregion of PTHrP is responsible for stimulating iCa uptake by the fetal placenta,343and the C-terminal region can inhibit osteoclastic bone resorption.181
The complementary DNA (cDNA) for canine and feline PTHrP has been cloned and sequenced.492,555 The sequence of canine PTHrP cDNA and gene indicated that the dog PTHrP gene is more closely related to the human PTHrP gene than are the PTHrP genes in rats, mice, and chickens.226 The deduced amino acid sequence of the N-terminal region (amino acids 1 to 36) is identical in five mammalian species (dog, cat, human, rat, and mouse), and there is a high degree of homology of the midregion of PTHrP in these species.350,492,551,555,633 The high degree of interspecies homology indicates the importance of the N terminus and midregion in the function of PTHrP.
There is less homology of the C-terminal region of canine PTHrP with that from other species. The function of the C-terminal region is unknown. PTHrP (107 to 111) and PTHrP (107 to 139) may inhibit osteoclastic bone resorption.182,548 Increased urine concentrations of C-terminal PTHrP have been demonstrated in humans and mice with cancer-associated hypercalcemia275,288 and in patients with renal failure.89 Increased C-terminal PTH is also seen in the sera of patients with renal failure and indicates that the kidneys are an important site of excretion of C-terminal PTHrP. C-terminal PTHrP may have a longer serum half-life than N-terminal or midregion PTHrP.
Parathyroid hormone-related protein in the fetus
Fetuses maintain higher concentrations of serum iCa than their dams. Fetal parathyroid glands produce low levels of PTH,105 and PTHrP functions to maintain iCa balance in the fetus.342,343 PTHrP is secreted by fetal parathyroid chief cells, and PTHrP is produced by the placenta, which is necessary for iCa uptake by the fetus.628 The midregion of PTHrP is the most active portion that stimulates iCa and iMg transport by the placenta. The placenta expresses the iCa-sensing receptor, which may contribute to the regulation of placental calcium transport.309 PTHrP is also produced by the uterus, where it is important in permitting relaxation of the smooth muscle of the muscularis as the fetus grows.565
Vitamin D
Vitamin D (calciferol) is classified as a secosteroid hormone.261 In tetrapods, the role of vitamin D via the calcitriol-activated VDR has evolved into one dominated by calcium regulatory mechanisms, but the roles in primitive species, including regulation of detoxification enzymes, have commonly been retained in more evolved life forms.596,613 These pleiotropic actions of vitamin D330 include, among others, important roles as antiproliferative and prodifferentiative mediators25 working in part via control of DNA replication164 and roles as immunomodulators,238 including effects on glomerulonephritis431 and encephalitis.205 A role of calcitriol to regulate expression of the insulin receptor has been described,345 as has a role in muscle.140 Of particular interest in uremic patients is the calcitriol increase of erythroid proliferation via burst-forming units.20 These pleiotropic effects of calcitriol can be related to important clinical applications in patients with renal or other metabolic disease.252 They may explain the clinical improvements noticed in dog and cat uremic patients treated preventively with low doses of calcitriol401 that were accomplished when calcitriol was used before any PTH elevation had occurred.
Vitamin D metabolism
The cholecalciferol (parent vitamin D3 of animal origin) metabolites 25-hydroxyvitamin D3 (calcidiol), 1,25-dihydroxyvitamin D3 (calcitriol), and 24,25-dihydroxyvitamin D3 are the most important of at least 30 metabolites. In domestic mammals, the same three metabolites derived from vitamin D2 (ergocalciferol of plant origin) are equally bioactive; thus, generic use of the terms 1,25-dihydroxyvitamin D and calcitriol is assumed to include metabolites of vitamin D3 or D2 derived from animal or plant origin, respectively. The 25-hydroxyvitamin D that is produced in liver is the major circulating form of vitamin D209 and serves as a pool for further activation by 1α-hydroxylation or catabolism by 24-hydroxylation.243,421 Only 25-hydroxylation and 1α-hydroxylation are important in the function of vitamin D.139
Synthesis
In humans, the requirement for vitamin D can be met by consumption of vitamin D2 or D3 or by synthesis of vitamin D3 (cholecalciferol) in the skin. Cholecalciferol is synthesized in the skin from 7-dehydrocholesterol after exposure to ultraviolet light. 7-Dehydrocholesterol forms previtamin D3 in the presence of ultraviolet B light at 288 nm, followed by further thermal conversion from pre vitamin D3 to vitamin D3.253 Dogs and cats inefficiently photosynthesize vitamin D in their skin and consequently are dependent on vitamin D in their diet.264 Vitamin D ingested in the diet is absorbed intact from the intestine.
Vitamin D-binding protein transports vitamin D to the liver and other target sites (Fig. 6-9).129 Hydroxylation of vitamin D occurs in the liver to produce 25-hydroxyvitamin D (calcidiol). The 25-hydroxylase activity is not influenced by calcium or phosphorus.209 Calcidiol does not have any known action in normal animals,139 but during vitamin D intoxication, high levels of calcidiol are produced by the liver and can induce hypercalcemia.
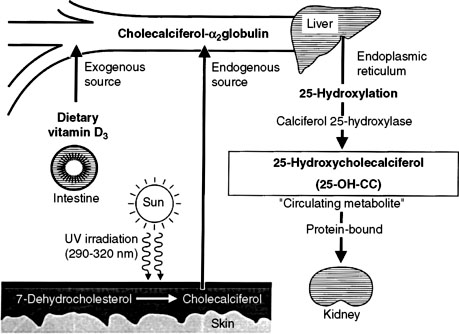
Figure 6-9 Metabolism of vitamin D. The initial step of metabolic activation of vitamin D3 from endogenous (photoactivation) and dietary sources is in the liver to form 25-hydroxycholecalciferol (25-hydroxyvitamin D3). Photoactivation is poor in dogs and cats; consequently, they depend on dietary sources of vitamin D3.
The most important step in bioactivation of vitamin D occurs as 25-hydroxyvitamin D is further hydroxylated to calcitriol in the proximal tubules of the kidneys.243 This reaction is tightly regulated by ionic and hormonal control mechanisms that modulate the activity of the hydroxylase enzyme systems (Fig. 6-10). The two principal enzyme systems involved are 25-hydroxyvitamin D-1α-hydroxylase (resulting in active calcitriol formation) and 25-hydroxyvitamin D-24R-hydroxylase (the first step of catabolism to inactive vitamin D metabolites). The activities of these enzymes are reciprocally regulated.421
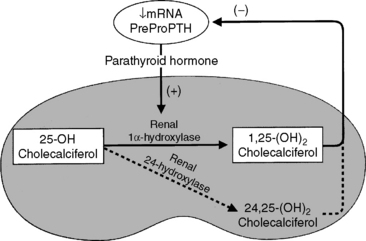
Figure 6-10 Parathyroid hormone increases renal synthesis of 1,25-dihydroxycholecalciferol (calcitriol) by stimulating the 1α-hydroxylase activity in renal epithelial cells that converts 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. Negative feedback is exerted by 1,25-dihydroxycholecalciferol (calcitriol) on parathyroid chief cells to decrease the rate of PTH synthesis and secretion, which in turn decreases the rate of formation of 1,25-dihydroxycholecalciferol. Calcitriol also directly suppresses synthesis of the renal 1α-hydroxylase enzyme.
The 1α-hydroxylase enzyme activity is localized within mitochondria of the convoluted tubules and portions of the straight proximal tubules of the kidneys. Little extrarenal 1α-hydroxylation of 25-hydroxyvitamin D occurs in other tissues except in human and rat placenta and skin and in some lymphoproliferative disorders.5,159 The 24-hydroxylation can also metabolize calcitriol, generating 1,24,25-trihydroxyvitamin D as the first step in the major catabolic pathway of calcitriol to biologically inactive calcitroic acid.261 Inactive vitamin D catabolites are excreted through the bile into feces, which is the only important excretory route; less than 4% is excreted into urine.139
Stimulation of Calcitriol Synthesis
Serum PTH, calcitriol, phosphorus, and calcium concentrations are the principal regulators for renal calcitriol synthesis.243 Chronic changes in serum calcium concentration regulate the synthesis of calcitriol, and these calcium changes can override signals from serum phosphorus and PTH concentrations.267 Deficiencies of phosphorus, calcium, and calcitriol lead to increased calcitriol formation.402 Low calcium or calcitriol concentrations lead to increased serum PTH concentrations. In the kidneys, PTH mediates dephosphorylation of renal ferredoxin (renoredoxin) and results in increased synthesis of calcitriol.212,533 Renoredoxin is the regulatory constituent of the 1α-hydroxylase enzyme system and is inhibited by phosphorylation in the presence of high concentrations of phosphorus or calcium in the renal tubule.243 PTH not only activates the renal 1α-hydroxylase but also induces synthesis of the enzyme from the renal gene encoding it.158,159
Several drugs and hormones have effects on vitamin D metabolism, some of which are stimulatory.65 Hypocalcemia and calcitonin directly stimulate 1α-hydroxylation independent of PTH.70 Estrogens increase calcitriol synthesis after up-regulation of PTH receptors in the kidneys,70 and testosterone may also increase calcitriol synthesis.640 Reduced dietary calcium intake can lead to stimulation of renal 1α-hydroxylase in the absence of detectable hypocalcemia.640
Inhibition of Calcitriol Synthesis
Calcitriol synthesis is inhibited by calcitriol, hypercalcemia, FGF-23, and phosphate loading.70,203,243 Calcium directly and indirectly inhibits calcitriol synthesis.175 The indirect action is caused by inhibition of PTH synthesis and secretion, thus removing the stimulus provided by PTH. The inhibitory effects of chronic hypercalcemia can override the stimulatory effects of increased PTH concentrations in calcitriol production, as may occur in primary hyperparathyroidism.267 The inhibitory effects of high concentrations of phosphorus on calcitriol synthesis are important and affect the activity of existing enzyme molecules.401,402
Actions of Calcitriol
Calcitriol is the only natural form of vitamin D with significant biologic activity.139,467 It is approximately 1000 times as effective as parent vitamin D and 500 times as effective as its precursor calcidiol (25-hydroxyvitamin D) in binding to the natural calcitriol receptor (VDR) in target cells.404 Calcitriol increases serum calcium and phosphorus concentrations, and its major target organ for these effects is the intestine.72 However, there is also an important contribution from bone,549 and calcitriol stimulates the kidneys to reabsorb both calcium and phosphorus from the glomerular filtrate. Calcitriol has multiple indirect effects on calcium balance, including up-regulation of calcitriol receptors in patients with uremia, regulation of PTH synthesis and secretion by the parathyroid gland,635 and prevention or reversal of parathyroid gland hyperplasia in the uremic patient.202,401
The calcitriol receptor
The VDR for calcitriol is present in many tissues in addition to bone, kidneys, intestine, and parathyroid gland.237 The importance of calcitriol in tissue is proportional to the abundance of the VDR in the cells, and this is highly regulated.311 Intestinal epithelial cells and parathyroid gland chief cells have equal and high concentrations of VDR. VDR genetic polymorphisms are thought to generate variation of efficiency of the VDR.84,110 Calcitriol initially dissociates from its serum binding protein, diffuses across the cell membrane, and binds with its receptor.
Effects of Calcitriol on the Intestine
Calcitriol enhances the transport of calcium and phosphate from the intestinal lumen to plasma across the enterocyte.73,601 Energy in the form of adenosine triphosphate (ATP) is required to transport calcium from the enterocytes into the blood and to absorb phosphate from the intestinal lumen. Calcitriol induces synthesis of the plasma membrane calcium pump (ATPase) that removes calcium from the enterocytes432 and the Na+-phosphate cotransport protein that transports phosphorus into the enterocyte. In addition, calcitriol increases the brush border permeability to calcium and induces the synthesis of calbindin-D 9k.125,567 Calbindins serve as buffers to protect enterocytes from toxic concentrations of calcium ion while ferrying calcium across the cell.601 Calcitriol also directly stimulates rapid calcium transport (transcaltachia) across the enterocyte.418 Normal dogs have a progressive decrease in the number of calcitriol receptors and calbindin concentrations that regulate the efficiency of calcium absorption in enterocytes from the duodenum to the ileum.307 Longer transit times in certain portions of the intestinal tract (e.g., ileum) can still lead to significant calcium absorption despite low transport efficiency.601
Effects of Calcitriol on Bone
Calcitriol is necessary for bone formation and mineralization because it ensures an adequate source of calcium and phosphorus from the intestinal tract. Deficiencies in vitamin D lead to impaired bone growth, such as rickets in growing animals and osteomalacia in adults.478 Calcitriol is necessary for normal bone development and growth because it regulates the production of multiple bone proteins produced by osteoblasts, including alkaline phosphatase (ALP), collagen type I, osteocalcin, and osteopontin.19,544 Calcitriol is also necessary for normal bone resorption because it promotes differentiation of monocytic hematopoietic precursors in the bone marrow into osteoclasts.549 This relationship between calcitriol and osteoclasts explains the dependence of PTH on calcitriol for optimal bone resorption.403
Effects of Calcitriol on the Kidneys
An important effect of calcitriol in the kidneys is direct inhibition of 25-hydroxyvitamin D-1α-hydroxylase in the renal tubule, preventing overproduction of calcitriol.467 In addition, calcitriol facilitates calcium and phosphorus reabsorption from the glomerular filtrate.318 Calcitriol is necessary to work with PTH to reabsorb urinary calcium into blood. Glomerular podocytes contain the VDR for calcitriol and respond to low doses of calcitriol with decreased injury and loss of podocytes.316 In glomerulonephritis, low doses of calcitriol decreased mesangial proliferative nephritis, which involved calcitriol abrogation of inflammatory mediators interleukin (IL)-1α, tumor necrosis factor-α (TNF-α), and IL-6 in the mesangium.430 Although calcitriol has generally been thought to protect the kidneys during CRF by preventing the damage from excess PTH,403,635 it is becoming clear that calcitriol has direct beneficial effects on the diseased kidney as well.
Effects of Calcitriol on the Parathyroid Gland
Calcitriol inhibits the production of PTH in the parathyroid gland by direct and indirect means.531,537 Binding of calcitriol to its receptor in parathyroid chief cells directly inhibits PTH synthesis. Second, calcitriol stimulates intestinal calcium absorption, which indirectly reduces PTH secretion by increasing serum iCa concentration. Calcitriol suppression of PTH synthesis is dose dependent and occurs before serum iCa concentration is increased by the delayed effects of calcitriol on intestinal calcium transport.540 Calcitriol may be considered the primary controlling factor for transcription of the PTH gene and subsequent synthesis of PTH because suppression of PTH synthesis cannot occur in the absence of calcitriol even in the presence of hypercalcemia (see Fig. 6-6).402,537 PTH secretion decreases 12 to 24 hours after exposure to calcitriol. Whereas PTH stimulates renal calcitriol synthesis, calcitriol is a negative regulator of PTH. Long-standing calcitriol deficiency results in chief cell hypertrophy and hyperplasia, demonstrating that calcitriol is important in limiting cellular proliferation in the parathyroid gland.537 Calcitriol treatment of uremia in dogs and humans has resulted in regression of parathyroid gland hyperplasia.202,404 Calcitriol can be used to prevent development of hyperparathyroidism in dogs and cats with early stages of CRF.401 This has proved to be highly successful and is consistent with developing thinking in the human medical profession.642
Calcitriol in cancer therapy?
Many studies focus on the benefits of calcitriol therapy in cancer.222,254 Part of the great interest stems from the antiproliferative role of calcitriol,25 with specific effects on DNA replication genes164 and with a potentially important effect on proliferation of blood vessel endothelial cells.42 Studies are focused on human prostate cancer312 and also on breast and colon cancers.254 Although a discussion is beyond the scope of this chapter, its dynamic character indicates it will be important for many years to come.
Calcitonin
Calcitonin is a 32-amino acid polypeptide hormone that is synthesized by C cells in the thyroid gland.389,478 An important role of calcitonin is to limit the degree of postprandial hypercalcemia. This effect, in concert with PTH, acts to maintain serum iCa concentration within a narrow range. Calcitonin is secreted in response to hypercalcemia and also to a calcium-rich meal. Calcitonin secretion increases during hypercalcemia, but the effects of calcitonin on normal calcium homeostasis are considered to be minor. The major target site for calcitonin is bone, where it inhibits osteoclastic bone resorption. The effects of calcitonin in bone are transitory, which has limited the usefulness of calcitonin as a treatment for hypercalcemia. At high doses, calcitonin may promote urinary calcium excretion.81
Normal homeostatic response to hypocalcemia
Hypocalcemia elicits corrective responses that are mediated by PTH and calcitriol.478 Acute effects occur in seconds to minutes; subacute effects occur over several hours; and chronic effects occur over days to weeks. A marked increase in PTH secretion occurs in response to mild hypocalcemia, and this response occurs in seconds. Acute secretion of preformed PTH can maintain PTH concentrations for 1 to 1.5 hours during hypocalcemia. Hypocalcemia decreases the proportion of PTH that is degraded in the parathyroid chief cells, making more PTH available for secretion. This effect is relatively rapid (approximately 40 minutes). During increased PTH secretion, renal calcium reabsorption and phosphorus excretion are increased within minutes, whereas bone mobilization of calcium and phosphate occurs within 1 to 2 hours.
After several hours of hypocalcemia, increased PTH secretion stimulates the synthesis and secretion of calcitriol. Increased intestinal transport of calcium and phosphorus into blood follows, providing an external source of calcium in addition to the internal mobilization from bone. Hypocalcemia increases transcription of the PTH gene and synthesis of PTH mRNA, enhancing the ability of the chief cells to produce PTH. This effect also occurs within hours of hypocalcemia. Over days or weeks of hypocalcemia, further increases in PTH secretion are achieved largely by hypertrophy and hyperplasia of chief cells in the parathyroid gland.497 In addition, the proportion of chief cells actively synthesizing PTH is increased.
Normal homeostatic response to hypercalcemia
Most of the effects that occur during hypercalcemia are the opposite of those described earlier for hypocalcemia.478 Hypercalcemia results in decreased PTH secretion, increased intracellular degradation of PTH in chief cells, and decreased PTH synthesis. Increased calcitonin secretion is stimulated in an attempt to minimize the magnitude of hypercalcemia. In addition, hyperplasia of C cells in the thyroid gland results if the hypercalcemic stimulus is sustained, but this mechanism is ineffective for controlling hypercalcemia because of the transitory effect of calcitonin on osteoclastic bone resorption.420,484 Calcitriol synthesis is decreased both through direct inhibition by iCa and as a result of decreased stimulation because of decreased PTH concentration.
Diagnostics
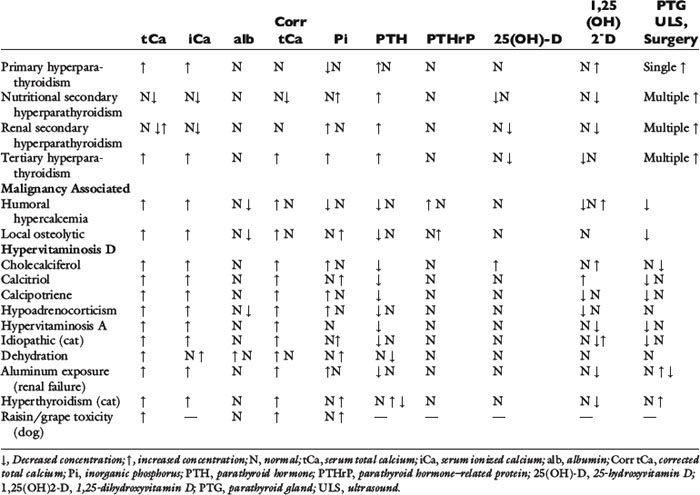
Table 6-1 lists the normal values for serum tCa,115 iCa,114 PTH,404,577 PTHrP,489 and vitamin D metabolites that are useful in the diagnostic workup of patients with calcium disorders.478
Table 6-1 Normal Serum Concentrations
| Dog | Cat | |
|---|---|---|
| Total Calcium | ||
| mg/dL | 9.0-11.5 | 8.0-10.5 |
| mmol/L | 2.2-3.8 | 2.0-2.6 |
| Ionized Calcium | ||
| mg/dL | 5.0-6.0 | 4.5-5.5 |
| mmol/L | 1.2-1.5 | 1.1-1.4 |
| Parathyroid Hormone (PTH) | ||
| Intact (pmol/L) | 2-13* | 0-4* |
| N-terminal (pg/mL) | 5-55 | 8-28 |
| Parathyroid Hormone Related protein (PTHrP) (pmol/L) (intact or N-terminal) | <1.0* | <1.0* |
| 25-Hydroxyvitamin D | 60-215* | 65-170* |
| (calcidiol) (nmol/L) | ||
| 1,25-Dihyroxyvitamin D | ||
| (calcitriol) (pg/mL) | ||
| Adults | 20-50 | 20-40 |
| 10-12-week old | 60-120 | 20-80 |
* Data from Endocrine Diagnostic Section, Diagnostic Center for Population and Animal Health, Lansing, Michigan.
Total calcium
Despite the fact that only the iCa fraction is physiologically active, the calcium status of animals is usually initially based on evaluation of the serum tCa concentration. Measurement of tCa concentration is more readily available than iCa measurement, but it does not always accurately reflect the iCa concentration of the patient. The serum tCa concentration has been assumed to be directly proportional to iCa, but in many clinical conditions, this may lead to erroneous interpretation of laboratory data. In humans with disorders of calcium balance, measurement of serum tCa concentrations failed to predict serum iCa concentrations in 31% of all patients566 and in 26% of patients with renal disease.88 In 1633 canine samples, diagnostic disagreement between serum iCa and tCa was 27%, and in dogs with CRF, this disagreement was 36%.519 In cats, serum iCa concentrations were only moderately correlated with serum tCa concentrations,142 and a 40% diagnostic disagreement between serum iCa and tCa measurement was noted in 434 cats.518 In dogs, tCa measurement overestimated normocalcemia and underestimated hypocalcemia,519 and in cats, hypercalcemia and normocalcemia were underestimated, and hypocalcemia was overestimated when using serum tCa concentration to predict iCa status.518
Analytical Methods
Fasting serum or heparinized plasma samples should be submitted for analysis. Oxalate, citrate, and ethylenediaminetetraacetic acid (EDTA) anticoagulants should not be used because calcium is bound to these chemicals and becomes unavailable for analysis.623
Serum tCa concentrations vary with the method used. Isotope dilution with subsequent mass spectrometry constitutes the definitive method for calcium measurement but is not readily available.200 For clinical determination of serum tCa concentration, simple colorimetric reactions and spectrophotometry are usually employed using automated or manual methods. Ortho-cresolphthalein complexone is a metal dye that is commonly used to form a color complex with calcium. This method is accurate and reproducible.200 Hemolysis can result in formation of an interfering hemoglobin-chromogen complex that falsely increases measured calcium concentration. High concentrations of bilirubin falsely decrease, and acetaminophen and hydralazine falsely increase serum tCa concentration. Lipemia can result in spuriously high calcium concentrations,380 with values exceeding 20 mg/dL in some instances of severe lipemia.
Caution should be exercised in the interpretation of tCa measurements performed on small serum or plasma volumes. When submitted volume is inadequate, dilution with water or saline is often performed. In an in-house commercial laboratory study, when samples were diluted 1:3, serum tCa concentrations were nearly 3 mg/dL lower than when analyzed in undiluted samples (Antech newsletter May 1999).
Normal Values
The range for serum tCa concentration in normal dogs and cats is wide and varies among laboratories (see Table 6-1). Each laboratory should establish normal values. Variability may result from differences in age, diet, duration of fasting before sampling, and time of sampling, in addition to differences in analytical methods.
Normal serum tCa concentrations in mature dogs and cats are approximately 10.0 and 9.0 mg/dL, respectively. No difference in serum tCa concentration has been ascribed to breed or sex in normal dogs and cats, but an effect of aging has been observed in the dog.115,240 Dogs younger than 3 months of age have slightly higher mean serum calcium concentrations (approximately 11.0 mg/dL) than those of dogs older than 1 year (approximately 10.0 mg/dL), probably because of normal bone growth. In a small percentage of normal young dogs, serum tCa concentrations may be greater than 12.0 mg/dL and as high as 15.0 mg/dL.417 Dietary calcium, phosphorus, and vitamin D supplementation should be evaluated in dogs with serum tCa concentrations greater than 12.0 mg/dL.
Adjusted Total Calcium
It has been reported that serum tCa concentrations should be “corrected” or “adjusted” relative to the total serum protein or albumin concentration to improve diagnostic interpretation.184,376 Such correction seemed logical because binding of serum calcium to protein is substantial, and 80% to 90% of the calcium bound to proteins is bound to albumin. The correlation between serum tCa and serum albumin or total protein concentrations was moderate, and adjustment formulas were developed for use in dogs older than 1 year. These adjustment formulas were not recommended for use in cats because there was no linear relationship between serum tCa and serum albumin and total protein concentrations in this species.189
It has been assumed that serum tCa concentrations that correct into the normal range are associated with normal serum iCa concentration. Likewise, samples with values that fail to correct into the normal range are presumed to have abnormal serum iCa concentrations. However, these formulas were developed without verification by serum iCa measurements. Correction of serum tCa concentration for albumin did not improve the correlation between serum tCa and iCa concentrations.385 In 1633 canine serum samples, the use of an adjustment formula to predict iCa status showed a higher diagnostic disagreement than did serum tCa measurement alone.519 Diagnostic disagreement was 37% between tCa adjusted to total protein and iCa measurement and 38% between tCa adjusted to albumin and iCa measurement. In 490 dogs with CRF, diagnostic disagreement between adjusted tCa and iCa measurement increased to 53%, indicating the poor performance of the adjustment formulas in the prediction of iCa status. In all dogs, hypercalcemia and normocalcemia were overestimated, and hypocalcemia was underestimated when either adjustment formula was used. In dogs with CRF, however, hypercalcemia was overestimated, and normocalcemia and hypocalcemia were underestimated. Because of the high degree of diagnostic disagreement between adjusted tCa and iCa measurement, the use of adjustment formulas to predict iCa status cannot be recommended.
Ionized calcium
Ionized calcium is the biologically active form of calcium, and its homeostasis is important for many physiologic functions.478 Calcium ion regulates its own homeostasis directly by binding to cell membrane receptors specific for iCa.79 The cell membrane calcium receptors are present in parathyroid chief cells and C cells of the thyroid gland, in which iCa regulates PTH and calcitonin secretion, respectively. Calcium receptors are also present on renal tubular cells, and iCa directly regulates its own tubular reabsorption rate. Therefore, serum iCa concentration is controlled by interacting feedback loops that involve iCa, phosphate, PTH, calcitriol, and calcitonin. These mechanisms help maintain serum iCa concentration in a narrow range.
For accurate assessment of calcium status, iCa must be measured directly. Ionized calcium measurement has been shown to be superior to serum tCa measurements in many conditions, especially in hyperparathyroidism, renal disease, hypoproteinemia and hyperproteinemia, acid-base disturbances, and critical illnesses.218,519,638 Changes in the magnitude of serum protein concentration, individual protein binding capacity and affinity, serum pH, and complexed calcium all interact to determine the iCa concentration, independent of the tCa concentration. Fasting serum samples collected at the same time in the morning are advised.
Analytical Methods
Use of automated equipment with a calcium ion-selective electrode allows easy and accurate measurement of iCa in blood, plasma, or serum.64 Newly developed electrodes minimize interference by other ions (e.g., magnesium, lithium, and potassium), protein, or hemolysis.220 Nevertheless, differences among analyzers exist, and it is recommended that reference ranges be established for each analyzer.265
Recently, portable clinical analyzers have been developed for cage-side analysis of iCa concentration. These analyzers use a disposable cartridge containing an impregnated biosensor for iCa and other analytes. Heparinized whole blood is used for analysis, but caution should be exercised when interpreting these results. Ionized calcium concentrations in dogs are typically 0.05 to 0.26 mmol/L lower, and 0.05 to 0.14 mmol/L lower in cats, when heparinized whole blood is compared with serum iCa measurement.227 The greatest underestimation of iCa concentration occurred when serum iCa concentrations were greater than 1.3 mmol/L. When iCa concentration in heparinized whole blood was measured using both ion-selective electrode methodology and portable clinical analyzer methods, the correlation (r) was only 0.71.398 The portable clinical analyzer method resulted in an iCa concentration that was approximately 2.6% lower than that measured with an ion-selective electrode.334 However, in a study of dogs and horses, there were no differences in iCa concentrations using heparinized whole blood measured with an ion-selective electrode and portable clinical analyzer.337 Because the quantity and type of heparin used and volume of blood collected also have an effect on iCa measurement, it is best to establish a rigid protocol for blood collection when using a portable clinical analyzer. Reference ranges should also be established for the analyzer using this standard protocol.
Sample Handling Techniques
Concentration of iCa can be determined in samples handled under both anaerobic and aerobic conditions. The most precise determination of iCa concentration and physiologic pH requires that samples be collected and processed anaerobically to ensure that no increase in pH occurs because of loss of CO2. The pH of blood or serum has a significant effect on serum iCa concentration. Acidic pH favors dissociation of calcium from protein and increases the amount of iCa in the sample. Alkaline pH occurs with loss of CO2 and favors calcium binding to protein, thus decreasing the amount of iCa. Mixing serum with air results in increased pH and decreased measured iCa concentration because of loss of CO2 from the sample.515 Exposure to air in partially filled serum tubes also can affect iCa concentration; tubes that were only 25% or 50% filled had 0.07 or 0.04 mmol/L lower concentrations of iCa when compared with measurements from tubes that were 100% filled.587
Ionized calcium can be measured in whole blood or heparinized plasma, but measurement is problematic. Heparinized canine blood provided stable iCa measurements when stored up to 9 hours at 4° C, but pH was significantly increased after 3 hours.553 In practice, it may be impossible to analyze the sample within this period. The amount and type of heparin used for whole blood or plasma samples also affect the measurement of iCa. When zinc heparin is used as an anticoagulant, iCa concentration is overestimated most likely because of a decrease in pH, which displaces calcium from proteins.338,340 Lithium heparin causes an underestimation in iCa concentration,338 and an electrolyte-balanced heparin may underestimate or overestimate iCa concentration depending on whether hypocalcemia, normocalcemia, or hypercalcemia is present. The amount of heparin used is critical in the measurement of iCa in blood. Using syringes containing a premeasured quantity of lithium heparin or electrolyte-balanced heparin, iCa measurement was underestimated when a less than recommended quantity of blood was collected for analysis.338,339 When using heparinized whole blood for measurement of iCa concentration, it is imperative to collect the same volume of blood for each sample to avoid the dilutional effects of heparin. Syringes containing a premeasured amount of dry heparin are preferable to coating a syringe manually with an unknown and variable quantity of liquid heparin.
Ionized calcium and pH are more stable in serum than in whole or heparinized blood. The analysis of serum eliminates the potential interference of heparin and allows a longer storage period before analysis. Silicone separator tubes should not be used; the iCa concentration was increased in serum separated by use of silicone separator tubes because of release of calcium from the silicone gel.322 Measured iCa in canine and equine sera was stable after storage for 72 hours at 23° C or 4° C and for 7 days at 4° C.514,515 Use of serum collected anaerobically and stored at 4° C allows sufficient time for shipment to a reference laboratory for anaerobic measurement of iCa and pH.
Ionized calcium may also be accurately measured in samples handled aerobically. Mathematical formulas have been developed to correct the iCa concentration in samples exposed to air (with increased pH) to the actual pH of the patient or to a pH of 7.4.331,400 In a study of serum samples from 61 dogs and 21 cats, there was good correlation between iCa measured anaerobically and again aerobically after shipment to a diagnostic laboratory (Schenck and Chew, unpublished observations). These pH correction formulas are species specific, and formulas developed in humans should not be used. A mathematical correction formula should be derived for each species in each laboratory setting. Although not as precise as anaerobic measurement, aerobic measurement under proper laboratory conditions offers a diagnostically accurate methodology for iCa determination with simplified shipping and handling requirements.
Some iCa analyzers will automatically mathematically manipulate the iCa concentration and actual pH value of the sample and yield an adjusted value for iCa concentration that theoretically would occur at a pH of 7.4. These correction formulas were developed for use in humans and should not be used in animals. When using anaerobically collected samples, corrected iCa concentrations have not been advocated for use in humans because insight into the pathophysiology of the patient is gained by evaluation of the in vivo iCa concentration and pH.199 This may be especially true for patients with renal disease.498 If anaerobic sampling is possible (typically in an in-house setting), there is no necessity or benefit in correcting the iCa concentration to a pH of 7.4. Only when samples are handled aerobically is there a need for correction to a standard pH. In one study, iCa measurement was stable in aerobically handled serum for up to 48 hours at 4° C; however, in this study samples were undisturbed for this period so they had minimal mixing with air.68 During shipment to a laboratory, serum mixes considerably with air, causing a significant decrease in iCa due to an increase in pH. Thus, it is not recommended to measure iCa in shipped aerobically handled samples without adjustment to a standard pH of 7.4.
Normal Values
The range for serum iCa concentration in normal dogs and cats varies among laboratories but is approximately 5.0 to 5.8 mg/dL (1.25 to 1.45 mmol/L) in adult dogs516 and 4.6 to 5.4 mg/dL (1.15 to 1.35 mmol/L) in adult cats.142 An effect of aging has been observed in both the dog and cat. Young dogs and cats (up to 2 years of age) have serum iCa concentrations that are 0.1 to 0.4 mg/dL higher than those reported in older animals.142,385 Normal values should be established for each laboratory based on the age of the animal, type of sample, and analyzer used.
Fractionation of Serum Calcium
In addition to measuring the ionized concentration in the serum, the protein-bound and complexed fractions of calcium can be quantified using fractionation techniques. Ionized calcium and complexed calcium are diffusible, and together are referred to as ultrafilterable calcium. To separate protein-bound from ultrafilterable serum calcium, a micropartition system based on the filtration method has been used.173,516 The micropartition system contains a filter through which ultrafilterable calcium (complexed and ionized) passes. It is important that serum be collected anaerobically before ultrafiltration to allow accurate measurement of the calcium fractions and to prevent changes in serum pH.
Protein-bound, ionized, and complexed calcium fractions in serum were 34%, 56%, and 10% in normal dogs516 and 40%, 52%, and 8% in normal cats, respectively (Schenck, unpublished observations). Ultrafilterable calcium (ionized and complexed fractions) in dogs,516 horses,255 and cats (Schenck, unpublished observations) accounted for 66%, 63%, and 60% of serum tCa, respectively. The iCa fraction has the smallest variation, with larger variations occurring in the protein-bound and complexed fractions. This observation supports the concept that the iCa fraction is tightly regulated and represents the biologically active fraction of serum calcium.
Complexed and protein-bound calcium fractions have not been assessed in metabolic disorders associated with abnormal calcium concentrations. Measurement of the protein-bound and complexed calcium fractions in addition to the iCa fraction may facilitate detection of disease processes that affect calcium metabolism. In dogs with CRF, two subgroups have been identified based on calcium fractionation. Dogs with normal to elevated serum tCa concentrations had a significantly higher concentration of circulating complexed calcium as compared with those dogs with low concentrations of tCa, even though there was no difference in iCa or protein-bound calcium between groups.517 Further studies are needed to determine whether prognosis or effectiveness of therapy differs between these groups.
Parathyroid hormone
PTH circulates predominantly as intact PTH (1-84) and carboxyl-terminal fragments. Only intact PTH is biologically active, and it is best to measure this form in serum or plasma. Samples should be stored and shipped frozen to prevent degradation of intact PTH. Stability is best in plasma collected with EDTA, but serum is adequate if stored frozen after separation from blood. Because of the sequence homology of human and animal PTH, commercial assays developed for humans have been used successfully for some veterinary species.117 An amino-terminal-specific radioimmunoassay (RIA) was used for more than 50 mammalian species but is no longer commercially available.402 A two-site immunoradiometric assay (IRMA) for intact human PTH has been validated in the dog and cat.26,577 Normal values for serum PTH concentration using this assay were 2 to 13, 0 to 4, and 0 to 2 pmol/L in the dog, cat, and horse, respectively (Endocrine Diagnostic Section, Diagnostic Center for Population and Animal Health, Lansing, MI). Unfortunately, this assay has been discontinued. The two-site assays have not proved useful for measurement of PTH in reptiles. The expected responses of PTH in various conditions will be discussed later (see Hypercalcemia and Hypocalcemia sections).
The two-site IRMA measures both the intact PTH-(1-84) and the PTH-(7-84) fragment, because the amino-terminal antibodies react near the tenth amino acid.74,133,413 A new third-generation IRMA “whole” PTH assay has been developed for use in humans that measures only PTH-(1-84).204 This new assay could offer a better measure of whole PTH, especially in patients with secondary hyperparathyroidism because the PTH-(7-84) fragment is increased in these patients.386 High concentrations of carboxyl-terminal PTH fragments, which occur in cats with CRF, may interfere with intact PTH immunoassays.30 Using ratios of “whole” PTH versus “intact” PTH to clarify low bone turnover, renal osteodystrophy406 or the dynamics of PTH secretion507 have been attempted.216,305 The “whole” PTH assay may also be of better diagnostic value in dogs than the “intact” PTH assay because PTH-(7-84) fragments may be increased in dogs as compared with humans.168 Whole PTH (1-84) and intact PTH (1-84 and 7-84) have been measured in dogs, and it was observed that the whole PTH/intact PTH ratio in dogs (about 36%) was less than in humans, and the ratio did not change during acute hypocalcemia.168 In preliminary studies in cats, a third-generation PTH-(1-84) assay resulted in higher PTH values than a second generation assay that also measures the PTH-(7-84) fragment.134 Although this is opposite of what is found in humans, it is not unexpected because cat and other mammalian PTH is more similar to human PTH in the first few amino acids than in the region of the tenth amino acid.
Parathyroid hormone-related protein
Two-site IRMA and N-terminal RIA are available for the measurement of human PTHrP.49,310 These assays are useful for measuring biologically active PTHrP in the dog (see Cancer-Associated Hypercalcemia section)117,489 because of the high degree of sequence homology of PTHrP between species, especially in the N-terminal 111 amino acids.91 An N-terminal RIA for human PTHrP did not prove useful for measuring circulating PTHrP in a small number of horses.490 PTHrP is susceptible to degradation by serum proteases, and PTHrP concentrations must be measured in fresh or frozen plasma using EDTA as an anticoagulant. EDTA complexes with plasma calcium, which is required for function of many proteases. The addition of protease inhibitors, such as aprotinin and leupeptin, may provide further inhibition of proteolysis in plasma.429
The circulating forms of PTHrP are not completely understood because PTHrP rapidly undergoes proteolysis intracellularly and extracellularly after secretion into the blood.429 The forms of PTHrP that are present in vivo include intact PTHrP, an N-terminal peptide, a combined N-terminal and midregion peptide, a midregion peptide, and a C-terminal peptide.90,632 Fragments that have PTH-like biologic activity in vivo include N-terminal PTHrP (1-36), PTHrP (1-86), and intact PTHrP (1-141). The two-site immunologic assays measure intact PTHrP (1-141) and PTHrP (1-86) because antibodies bind to the N terminus and midregion. The N-terminal RIAs measure intact PTHrP (1-141), PTHrP (1-86), and N-terminal PTHrP (1-36). The C-terminal PTHrP accumulates in the sera of human patients with renal failure, which suggests that C-terminal PTHrP peptides are excreted by the kidneys, as occurs with PTH.89
Vitamin D metabolites
Measurement of vitamin D metabolites is occasionally helpful in diagnosing disorders of calcium homeostasis (see Table 6-1). 25-Hydroxyvitamin D (calcidiol) and calcitriol are the metabolites of clinical interest for detection of hypovitaminosis D, hypervitaminosis D, and abnormalities of the renal hydroxylase system (e.g., renal failure). The metabolites are stable during refrigeration and freezing, but samples should not be exposed to light for long periods.
The metabolites of vitamin D are chemically identical in all species, thus receptor-binding assays or RIAs developed for use in humans are satisfactory for the measurement of the same metabolites in animals.256,260 Young growing dogs have higher calcitriol concentrations than mature dogs, and most mammals appear to share this attribute during rapid growth.373
Concentrations of 25-hydroxyvitamin D are a good indicator of vitamin D ingestion or production in vivo and can be used to diagnose hypovitaminosis D or hypervitaminosis D.107 Calcitriol assays can be used to detect genetic errors of vitamin D metabolism, low concentrations of calcitriol in patients with renal failure, or high concentrations of calcitriol in some patients with cancer-associated hypercalcemia.478
Bone biopsy and bone marrow aspiration
Bone marrow aspiration or core biopsy is frequently part of the diagnostic evaluation of animals without an obvious cause of hypercalcemia. Its greatest utility is in the discovery of lymphoma, myeloproliferative disease, or multiple myeloma. Biopsy of the iliac crest is recommended for standardization, particularly when histomorphometric analysis is available for the quantitative evaluation of bone formation and bone resorption. A procedure for iliac crest bone biopsy has been described.122,486 Direct biopsy of focal bone lesions may be diagnostic, particularly when such lesions are caused by lymphoma, multiple myeloma, or a metastatic bone tumor.
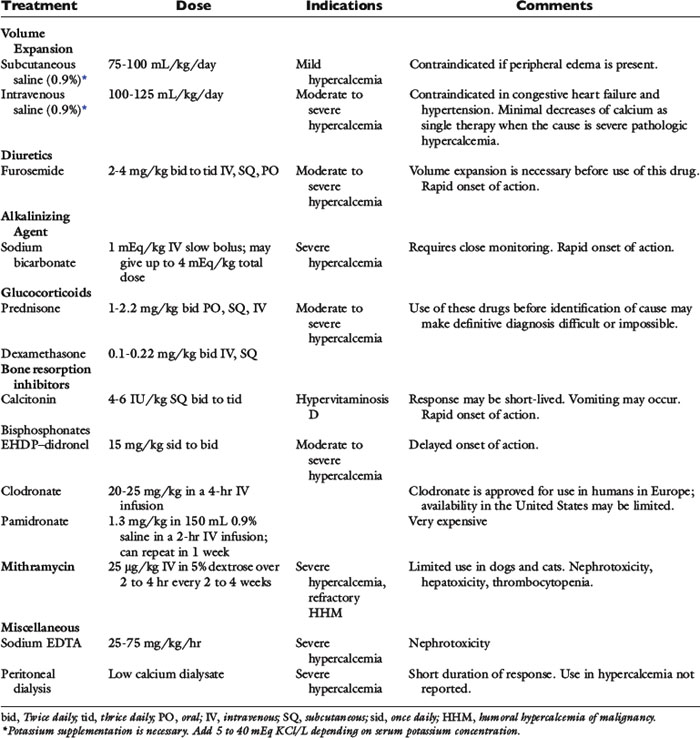
Hypercalcemia
Hypercalcemia is an uncommon but important electrolyte disturbance of dogs and cats. The frequency of finding hypercalcemia based on evaluation of serum tCa in more than 10,000 canine serum samples analyzed during a 6-month period at one private veterinary diagnostic laboratory was 1.5%.94 Of these, 28% were found to be from young growing dogs, 62% were found to be transient, and 18% were persistent and associated with pathology.
Hypercalcemia can serve as a marker of disease or can create disease. Increases in serum iCa concentration above normal often have adverse pathophysiologic consequences. Hypercalcemia represents a clinically relevant increase above an individual animal’s own normal serum calcium concentration, usually defined as a fasting serum tCa concentration greater than 12.0 mg/dL in dogs or greater than 11.0 mg/dL in cats. Ionized calcium measurements can provide greater sensitivity and specificity for the diagnosis of some hypercalcemic disorders. A serum iCa concentration greater than 6.0 mg/dL (1.5 mmol/L) in dogs and greater than 5.7 mg/dL (1.4 mmol/L) in cats constitutes ionized hypercalcemia.
Toxicity of hypercalcemia and clinical signs
Excessive calcium ions are toxic to cells,462 and increased serum iCa concentration decreases cellular function by causing alterations in cell membrane permeability and cell membrane calcium pump activity. Increased intracellular iCa content can ultimately result in cell death caused by deranged cellular function and reduced energy production. Although all tissues may be subject to the dangerous effects of hypercalcemia, effects on the central nervous system, gastrointestinal tract, heart, and kidneys are of most importance clinically.
Polydipsia, polyuria, anorexia, lethargy, and weakness are the most common clinical signs in dogs with hypercalcemia,113,178 but individual animals often display remarkable differences in clinical signs despite similar magnitudes of hypercalcemia. The severity of clinical signs and development of lesions of hypercalcemia depend not only on the magnitude of hypercalcemia but also on its rate of development and duration. Simultaneous disturbances in other electrolyte concentrations and in acid-base balance, as well as organ dysfunction secondary to hypercalcemia, all contribute to clinical signs, laboratory abnormalities, and lesions. Box 6-1 lists the signs and conditions associated with hypercalcemia.
Box 6-1 Clinical Signs and Conditions Associated with Hypercalcemia
| Common | Uncommon |
|---|---|
| Polydipsia and Polyuria | Constipation |
| Anorexia | Cardiac arrhythmia |
| Dehydration | Seizures or twitching |
| Lethargy | Death |
| Weakness | Acute intrinsic renal failure |
| Vomiting | Calcium urolithiasis |
| Prerenal azotemia | |
| Chronic renal failure |
Clinical signs are most severe when hypercalcemia develops rapidly, as can occur with vitamin D intoxication or during rapid infusion of calcium-containing fluids. Dogs with similar magnitudes of hypercalcemia may display minimal clinical signs when hypercalcemia has developed gradually. Regardless of the rate of increase in serum calcium concentration, clinical signs become more severe as the magnitude of hypercalcemia increases. Serum tCa concentrations of 12.0 to 14.0 mg/dL may not be associated with severe clinical signs, but most animals with concentrations greater than 15.0 mg/dL show systemic signs. Dogs with serum calcium concentrations greater than 18 mg/dL are often severely ill, and concentrations greater than 20 mg/dL may constitute a life-threatening crisis. Exceptions do occur, however, and some dogs are severely affected by mild hypercalcemia, whereas others are relatively unaffected by severe hypercalcemia. Clinical signs and histopathologic changes are more likely to develop the longer hypercalcemia has been present, regardless of its magnitude. Progressive hypercalcemia may also contribute to the severity of clinical signs, as occurs in animals with malignant neoplasia or hypervitaminosis D related to rat bait ingestion.
Changes in serum sodium and potassium concentrations can magnify the clinical signs of hypercalcemia by their effects on cell membrane excitability, particularly in nerve and muscle (see Chapter 5). Acidosis increases the proportion of serum calcium that is ionized, worsening clinical signs, whereas alkalosis lessens toxicity and clinical signs by decreasing the proportion of calcium that is ionized.
Mineralization of soft tissues (especially the heart and kidneys) is an important complication of hypercalcemia. The serum phosphorus concentration at the time hypercalcemia develops is important in determining the extent of soft tissue mineralization. Soft tissue mineralization is most severe when the product of calcium (mg/dL) times phosphorus (mg/dL) is greater than 60.115 Soft tissue mineralization occurs regardless of the serum phosphorus concentration in severe hypercalcemia.
Renal Effects of Hypercalcemia
Abnormal renal function frequently accompanies hypercalcemia, and rapid deterioration in renal function occasionally occurs. The functional effects of hypercalcemia on the kidneys are readily reversible, but structural changes may not be reversible if renal lesions are advanced. Azotemia occurred commonly in 34 dogs with hypercalcemia related to malignancy, hypoadrenocorticism, CRF, and hypervitaminosis D.314 The frequency of azotemia was higher in dogs with malignancy (71%) than in those with hypercalcemia related to primary hyperparathyroidism (11%). Azotemia caused by hypercalcemia can result from any combination of the following mechanisms: prerenal reduction in ECF volume (anorexia, hypodipsia, vomiting, and polyuria); renal vasoconstriction from ionized hypercalcemia; decreased permeability coefficient of the glomerulus (Kf); acute tubular necrosis from the ischemic and toxic effects of hypercalcemia; and CRF caused by nephron loss, nephrocalcinosis, tubulointerstitial inflammation, and interstitial fibrosis.
Decreased urinary concentrating ability and polyuria are early functional effects of hypercalcemia in dogs. The concentrating defect is often out of proportion to the observed reduction in glomerular filtration rate (GFR) and increase in serum creatinine or blood urea nitrogen (BUN) concentration. Urine specific gravity is consistently less than 1.030 in dogs and was less than 1.020 in more than 90% of hypercalcemic dogs in one study.314 Urinary concentration may be well preserved in some cats with hypercalcemia that do not have CRF. Defective urinary concentrating ability results from a combination of reduced tubular reabsorption of sodium and impaired action of antidiuretic hormone on tubular cells of the collecting duct. This results in a form of nephrogenic diabetes insipidus characterized by hyposthenuria if the diluting segment of the nephron (medullary thick ascending limb of Henle’s loop) is unaffected. These effects are caused by intrinsic responses of the kidneys to hypercalcemia. Some of these effects are mediated by calcium-sensing receptors on the renal epithelial cells,79 whereas others may be related to effects of hypercalcemia on aquaporin expression, cell trafficking, and delivery to apical membranes of the collecting tubules.163,459,597 Additional direct effects of hypercalcemia on the kidneys include reduced tubular calcium reabsorption and antagonism of the actions of PTH. These responses by the kidneys facilitate calcium excretion and help to ameliorate the clinical effects of hypercalcemia. Renal medullary blood flow is increased in dogs with experimental hypercalcemia86 and can result in medullary washout as another mechanism contributing to hyposthenuria. Isosthenuria develops if the diluting segments have been structurally altered by long-standing hypercalcemia. Polydipsia occurs as compensation for obligatory polyuria, but there is evidence that polydipsia can be caused by direct stimulation of the thirst center by hypercalcemia.115 Mineralization of renal tubules, basement membranes, or the interstitium; tubular degeneration; and interstitial fibrosis are structural changes that may occur in the kidneys secondary to hypercalcemia and can contribute to impaired urinary concentrating ability.
Dehydration is common owing to increased fluid losses from vomiting and polyuria. Substantial contraction of the ECF volume results in a reduction in GFR severe enough to increase BUN and serum creatinine concentrations and to cause prerenal azotemia. The clinical axiom that dilute urine in association with azotemia is caused by intrinsic renal lesions may not be true in animals with hypercalcemia because the urinary concentrating defect can occur without structural renal lesions. This condition is commonly misdiagnosed as primary renal failure when it is actually prerenal failure caused by dehydration and a renal concentrating defect early in the course of hypercalcemia.
Intrarenal causes of azotemia during hypercalcemia can be functional or structural. Hypercalcemia can induce renal vasoconstriction, resulting in decreased renal blood flow (RBF) and GFR. In an acute model of hypercalcemia, reduced RBF and GFR were observed consistently in conscious dogs when serum tCa concentration exceeded 20 mg/dL, but only one half of the dogs had significant reductions in GFR and RBF when serum calcium concentration was 15 to 20 mg/dL. Little effect on RBF and GFR was observed when serum calcium concentration was less than 15 mg/dL. These findings are in contrast to those in studies of anesthetized dogs, which demonstrated much more severe functional changes during hypercalcemia.335 Impaired renal autoregulation related to the effects of hypercalcemia may result in azotemia at early stages of dehydration because GFR would otherwise be maintained by afferent arteriolar vasodilatation.
Acute intrinsic renal failure (AIRF) occasionally develops as a consequence of hypercalcemia, but chronic intrinsic renal failure is more common. Sustained renal vasoconstriction related to hypercalcemia may result in ischemic tubular injury, promoting the development of both AIRF and chronic intrinsic renal failure and potentiating the direct toxic effects of calcium on tubular cells. The toxic effects of ionized hypercalcemia are enhanced by high concentrations of PTH in animals with CRF because excess PTH increases calcium entry into cells.403 The ascending limb of Henle’s loop and distal convoluted tubule show the earliest structural lesions, but lesions in the collecting system are ultimately the most pronounced. Thickening and mineralization of tubular basement membranes are most apparent in the proximal tubule. Tubular atrophy, mononuclear cell infiltration, and interstitial fibrosis occur in the chronic stages. Degenerative and necrotic tubules also are observed. Granular and tubular cell casts contribute to intrarenal obstruction and azotemia.314
Calcium-oxalate urolithiasis occasionally occurs in animals with long-standing hypercalcemia and has been described in dogs and cats with primary hyperparathyroidism. Nephrocalcinosis and linear mineralization along the renal diverticula are nonspecific findings discovered by radiography or ultrasonography in some dogs with long-standing hypercalcemia. Increased renal echogenicity and the medullary rim sign have been described during renal ultrasonography in dogs with hypercalcemia.28,53 These changes can occur in other normocalcemic conditions and in forms of dystrophic mineralization.
Effects of Hypercalcemia on Other Organs
Anorexia, vomiting, and constipation can result from hypercalcemia by reduction of the excitability of gastrointestinal smooth muscle and from direct effects on the central nervous system. Gastric hyperacidity and subsequent gastric ulceration caused by increased secretion of gastrin and direct stimulation of hydrogen ion secretion from parietal cells by hypercalcemia may account for some of the vomiting. Gastrin concentration was increased in four of six dogs with hypercalcemia in one preliminary report.66 Increased gastrin concentration occurs secondary to reduced renal clearance as a consequence of the hypercalcemia. Decreased excitability of skeletal muscle contributes to generalized weakness. Lethargy is commonly observed in severe hypercalcemia because of direct effects on the central nervous system and rarely can progress to stupor and coma. Seizures and muscle twitching are unusual neuromuscular manifestations of hypercalcemia.271
Clinically important cardiac effects of hypercalcemia are not commonly detected in dogs and cats, but PR–interval prolongation and QT–interval shortening can be observed on the electrocardiogram. Serious arrhythmias (including ventricular fibrillation) can be caused by the direct effects of severe hypercalcemia or may be a consequence of mineralization of cardiac tissue. Hypertension has been demonstrated in humans and rats during both acute and chronic hypercalcemia. The increase in blood pressure is proportional to the increase in serum calcium concentration in acute studies.97 In a study of acute hypercalcemia, hypertension was attributed to a direct effect of calcium on vascular smooth muscle and to an indirect effect of calcium to increase secretion of catecholamine with activation of adrenergic receptors.165 Whether hypertension is a clinically relevant complication in dogs and cats with hypercalcemia is unknown.
Mechanisms and differential diagnosis of hypercalcemia
Increased entry of calcium into ECF, decreased egress of calcium from ECF, reduced plasma volume, or a combination of these factors must occur for hypercalcemia to develop (Fig. 6-11). Increased calcium input can arise from increased intestinal absorption, increased bone resorption, or increased renal tubular reabsorption of calcium. Decreased glomerular filtration and decreased bone accretion result in decreased egress of calcium from ECF. Volume contraction is common in the presence of hypercalcemia because of the effects of anorexia, vomiting, and obligatory polyuria. The mechanisms of hypercalcemia vary with the specific causes, but much attention has been focused on the importance of increased bone resorption.
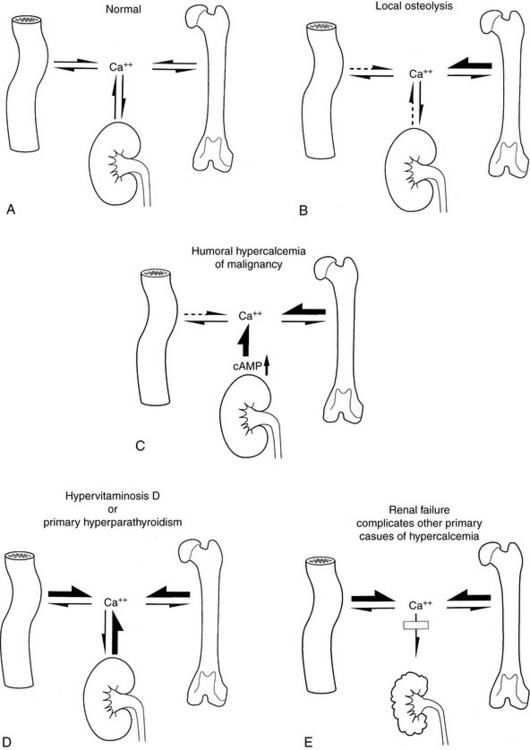
Figure 6-11 Patterns of calcium transport between extracellular fluid and gut, kidneys, and bone in various states of hypercalcemia. A, Normal. B, Osteolysis. C, Humoral hypercalcemia of malignancy. D, Hypervitaminosis D or primary hyperparathyroidism. E, Hypercalcemia complicated by renal failure. Size of arrows is proportional to the degree of calcium influx or efflux. Dashed arrows indicate possible response of decreased PTH secretion to hypercalcemia of nonparathyroid origin.
(Modified from Mundy GR. Malignancy and hypercalcemia-humoral hypercalcemia of malignancy, hypercalcemia associated with osteolytic metastases. In: Mundy GR, editor. Calcium homeostasis: hypercalcemia and hypocalcemia. London: Martin Dunitz, 1989: 65.)
Box 6-2 provides a list of possibilities in the differential diagnosis of hypercalcemia. Characterization of the hypercalcemia as transient or persistent, pathologic or nonpathologic, mild or severe, progressive or static, and acute or chronic is helpful in determining its cause. Persistent, pathologic hypercalcemia occurs most often in association with malignancy. Most studies in dogs attribute hypercalcemia to malignancy in more than 50% of the cases,46,166,586 although in one series malignancy accounted for only one third of the cases.314
Box 6-2 Conditions Associated with Hypercalcemia
Pathologic or Consequential—Persistent
Hypoadrenocorticism, renal failure, primary hyperparathyroidism, hypervitaminosis D, and inflammatory disorders sporadically account for hypercalcemia in dogs. In a study of 109 dogs with ionized hypercalcemia, 58% had underlying neoplasia, 17% had renal failure, 13% were diagnosed with hyperparathyroidism, 5% had hypoadrenocorticism, and 3% had vitamin D toxicity.375 It is often difficult to determine the cause of hypercalcemia in animals with mild or transient hypercalcemia. No definitive diagnosis could be made for 2% to 9% of hypercalcemic dogs in two reports.166,586 No definitive diagnosis was reported in 13% of cats with hypercalcemia in one report, but the actual percentage is much higher based on sample submissions to veterinary endocrinology laboratories.511
In serum samples from 332 hypercalcemic cats, 80% had parathyroid-independent hypercalcemia, 10% had parathyroid-dependent hypercalcemia, and 10% were equivocal.61 Approximately 10% of these hypercalcemic cats had PTHrP levels above the reference range, suggesting malignancy as the cause. Hypercalcemic cats have parathyroid-independent hypercalcemia more commonly than dogs. Samples from 5722 hypercalcemic dogs from the same laboratory categorized the hypercalcemia as parathyroid dependent in about 40%, parathyroid independent in 50%, and equivocal in 10%.466
General approach to diagnostic workup of patients with hypercalcemia
It is important to ensure that the hypercalcemia initially detected is repeatable, especially if the magnitude of hypercalcemia is modest. The likely cause of the hypercalcemia will be obvious in some patients from findings in the history (hypervitaminosis D) or from physical examination (masses and effusions). When the cause is not immediately apparent, body cavity imaging with chest radiographs, abdominal radiographs, and abdominal ultrasound is recommended to determine whether organomegaly or infiltrative processes are present that could account for the hypercalcemia. Fine needle aspiration, needle biopsy, or wedge biopsy of abnormal tissues will often yield the cause of the hypercalcemia. Patients with cytopenia (neutropenia, anemia, and thrombocytopenia) should undergo bone marrow evaluation if the diagnosis has not already been established by other means. Bone marrow evaluation in the absence of cytopenia does not often result in a diagnosis. Radiographs of painful bones may reveal lesions associated with hypercalcemia. Aspiration of focal bone lesions may reveal the cause of the hypercalcemia. Bone survey of all bones is sometimes useful in finding lesions even in those without demonstrable bone pain (multiple myeloma). Bone scintigraphy may be considered when a diagnosis is lacking despite exhaustive diagnostic testing.
High frequency ultrasonography of the cervical region can be performed to help determine whether the hypercalcemia is parathyroid dependent (large parathyroid glands) or parathyroid independent. In parathyroid-independent hypercalcemia, parathyroid glands are not enlarged or may not be identified; some may be atrophic if ionized hypercalcemia of malignancy or hypervitaminosis D has been long standing.
If the increase in serum tCa is minimal, measurement of serum iCa is important to determine whether the increase is clinically significant. Measurement of iCa in patients with renal failure is essential because renal failure can be associated with nonionized or ionized hypercalcemia. Serum iCa should be measured in association with PTH determination to assess the appropriateness of PTH response to serum iCa concentration.
If the cause of hypercalcemia is not apparent following history, physical examination, hematology, routine serum biochemistry, and body cavity imaging, then measurement of calcium-regulating hormones is needed to establish or suggest a definitive cause. The first step is to determine whether the hypercalcemia is parathyroid dependent (disease of the parathyroid glands is causing the hypercalcemia) or parathyroid independent (normal parathyroid glands suppress PTH secretion in response to hypercalcemia). Measurement of PTHrP is helpful if malignancy is suspected, but PTHrP concentrations are not always increased in malignancy. If extensive imaging methodologies are not available, measurement of serum iCa, PTH, and PTHrP may be performed before extensive body cavity imaging or bone marrow evaluation. Measurement of 25-hydroxyvitamin D is useful in cases of potential cholecalciferol or ergocalciferol ingestion. Measurement of 1,25-dihydroxyvitamin D (calcitriol) is occasionally useful if excess calcitriol is the cause of hypercalcemia. The anticipated changes in calcium hormones and serum biochemistry in disorders causing hypercalcemia are noted in Table 6-2.
Nonpathologic hypercalcemia
Serum calcium concentrations in animals may be mildly increased after feeding; consequently, a 12-hour fast is recommended before blood sampling. Laboratory error or detergent contamination of the serum or sample tube may result in artifactual hypercalcemia.380 Lipemia frequently causes erroneously high serum tCa concentrations because of colorimetric interference. Normal young growing dogs may have mildly higher serum calcium concentrations than older dogs.385
Transient or Inconsequential Hypercalcemia
Inconsequential hypercalcemia does not cause injury, resolves rapidly, or is only mild. Dehydration can result in mild hypercalcemia attributed to hemoconcentration. Furthermore, dehydration and volume contraction stimulate increased sodium and calcium reabsorption in the kidneys. An increased serum concentration of protein, especially albumin, can result in an increased serum tCa concentration as more calcium binds to protein. Dehydration in dogs is occasionally associated with serum tCa concentrations of 12.0 to 13.5 mg/dL that rapidly return to normal after dehydration is corrected. Increased serum tCa and decreased iCa concentrations can occur transiently after plasma transfusion because of excess citrate-calcium ion complexes.385
Hypoadrenocorticism
Hypoadrenocorticism is the second most common cause of hypercalcemia in dogs (after malignancy), accounting for 11% to 45% of cases in five studies,115,166,314,586,614 but no cases were reported in one study.46 Hypercalcemia was reported in 28% to 31% of dogs with glucocorticoid- and mineralocorticoid-deficient hypoadrenocorticism,442,445 in some dogs with glucocorticoid-deficient hypoadrenocorticism,328 and in 1 of 10 cats.443 In a study of 36 dogs with hypoadrenocorticism, 42% had an increase in tCa, but only 22% had increases in both iCa and tCa.3 The ionized hypercalcemia was typically mild, but a few dogs exhibited moderate to severe hypercalcemia. In eight dogs with hypoadrenocorticism, tCa was elevated in all eight, but iCa was elevated in only five of seven dogs in which iCa was measured.221 PTH was within or below the reference range in all dogs, 25-hydroxyvitamin D was within or below the reference range in all dogs, and calcitriol was within the reference range in seven of eight dogs. Hypoadrenocorticism is rarely recognized in cats, and hypercalcemia is present in only 8% of cases.35 Hypercalcemia was present in one cat with iatrogenic secondary hypoadrenocorticism and diabetes mellitus.541 Magnitude of hypercalcemia was greatest in the most severely affected dogs, but the mechanism is unknown. A correlation between the degree of hyperkalemia and hypercalcemia was detected when the serum potassium concentration was greater than 6.0 to 6.5 mEq/L, and serum tCa concentration was often 11.4 to 13.5 mg/dL.178 Increases in serum iCa may or may not develop in hypoadrenocorticism.595 Serum tCa concentration rapidly returns to normal after 1 to 2 days of corticosteroid replacement therapy in dogs,442 and IV volume expansion can return serum calcium concentration to normal within a few hours. Hypoadrenocorticism should always be included in the differential diagnosis of hypercalcemia, because the clinical signs of hypoadrenocorticism and hypercalcemia are similar.
Chronic Renal Failure
The findings of hypercalcemia and primary renal azotemia pose a special diagnostic problem because hypercalcemia can cause renal failure or develop as a consequence of CRF. Serum PTH concentration is often increased in patients with hypercalcemia related to renal failure, and these animals must be differentiated from those with primary hyperparathyroidism. Serum iCa concentration is increased in primary hyperparathyroidism but is usually normal or low in patients with CRF.118,314
Deleterious effects of hypercalcemia occur in patients with renal failure only if it is associated with increases in serum iCa concentration. Consequently, clinical signs of hypercalcemia are uncommon in CRF patients, and measurement of serum iCa concentration to assess calcium status in CRF patients is critical. In CRF patients, the serum tCa measurement incorrectly assessed iCa status in 36% of dogs and 32% of cats.518,519 The use of the “adjusted tCa” value incorrectly assessed iCa status in approximately 53% of dogs with CRF. In dogs, serum tCa measurement or adjusted tCa measurement overestimated hypercalcemia and underestimated hypocalcemia. In cats with CRF, serum tCa measurement overestimated normocalcemia and underestimated hypercalcemia. Thus, to accurately assess calcium status in patients with CRF, iCa concentration must be directly measured.
Fewer than 10% of all dogs with CRF have increased serum iCa concentrations. In one study, approximately 6% exhibited ionized hypercalcemia.118 In a recent study of 490 dogs with CRF, 9% exhibited hypercalcemia, 55% were normocalcemic, and 36% were hypocalcemic based on serum iCa concentrations.519 In another recent study of dogs with hypercalcemia, 17% were diagnosed with renal failure and were hypercalcemic based on their iCa concentration.375 Of these dogs, 89% had chronic renal failure and 11% had acute renal failure. Cats with CRF appear to have a higher incidence of ionized hypercalcemia as compared with dogs. In 102 cats with CRF, 29% were hypercalcemic, 61% were normocalcemic, and 10% were hypocalcemic based on iCa concentration.518
Many dogs and cats with CRF have normal serum tCa concentrations.147,183,380 Hypercalcemia based on measurement of serum tCa concentration occurs sporadically in dogs and cats with CRF and is usually listed as second or third in frequency of causes of hypercalcemia in dogs. Elevated tCa occurs in up to 14% of dogs with CRF, with a range of 12.1 to 15.2 mg/dL.118,183,314,405 In 71 hypercalcemic cats, CRF was noted in 38%.511 In cats with CRF, the reported incidence of serum total hypercalcemia ranged from 11.5%147 to 58%.27
The incidence of elevated tCa increases with severity of azotemia. In 73 cats with CRF, serum tCa was increased in 8%, 18%, and 32% of those with mild, moderate, or severe azotemia, respectively.27 However, increases in serum iCa do not show a strong association with the degree of azotemia.134 In 47 of the previous 73 cats with CRF, iCa was increased in 0%, 9%, and 6% of those with mild, moderate, or severe azotemia, respectively.27 Hypercalcemia was also not correlated with serum phosphorus concentration in dogs with experimental renal failure.419,583
The parathyroid glands must be present for hypercalcemia to develop,583 and partial parathyroidectomy ameliorates hypercalcemia in some dogs with CRF.183 Treatment of dogs with CRF and hypercalcemia with low-dose calcitriol to reduce PTH synthesis and secretion can result in decreased iCa concentration. Low-dose calcitriol therapy does not appreciably increase intestinal calcium absorption.401,402 In patients with CRF, increased serum PTH concentration (renal secondary hyperparathyroidism) contributes to the progression of renal disease.402 Oral administration of low doses of calcitriol reduces toxic concentrations of PTH, improves quality of life, reduces progression of renal disease, and leads to prolongation of life.403,524
Some cases of ionized hypercalcemia and CRF may be associated with the use of calcium carbonate intestinal phosphate binders. In these cases, serum iCa concentration rapidly returns to normal after discontinuation of treatment. In humans with CRF, therapeutic use of calcitriol is limited by development of hypercalcemia in patients also being treated with calcium-based dietary phosphorus binders.126,403 In veterinary medicine, use of aluminum-based phosphorus binders or sevelamer largely precludes this problem.10 “Noncalcemic analogues” of calcitriol have been developed for use in humans,539 such as paricalcitol, 22-oxacalcitriol (OCT), and doxercalciferol.155 These analogues have a very short half-life (several minutes), and this short half-life is responsible for their weak stimulation of intestinal calcium absorption. Doses of noncalcemic analogues needed to suppress PTH synthesis are approximately eightfold higher than that of calcitriol539 and are up to 12 times the cost. If hypercalcemia develops with calcitriol therapy, a twice-weekly dosing strategy of calcitriol is used. This dosing regimen will suppress PTH but be much less effective at stimulating intestinal calcium absorption. Noncalcemic analogues are not needed and are financially impractical in veterinary medicine.
Ionized hypercalcemia occurs in patients with CRF who receive excessive doses of calcitriol. Hypercalcemia is very uncommon in animals treated with the lower dosages of calcitriol (2.5 to 4.0 ng/kg daily). If hypercalcemia is caused by excessive calcitriol, the serum tCa concentration decreases during the week after its discontinuation. Most CRF patients who develop an elevated tCa during low-dose calcitriol treatment have normal or low serum iCa concentrations. Serum tCa concentration may not decrease when calcitriol is discontinued if the increased serum tCa concentration is caused by increased complexed calcium.
The mechanisms of increased serum tCa concentration in CRF have not been well characterized.183,314,478,583 In dogs with CRF, serum total hypercalcemia, and normal iCa concentrations, the increase in serum tCa is caused by an increase in the complexed calcium fraction.517 In CRF, organic anions such as citrates, phosphates, lactates, bicarbonates, and oxalates are capable of complexing with calcium. Complexed calcium accounted for 24% of serum tCa in those dogs with CRF and elevated serum tCa as compared with 11% in those dogs with CRF and low serum tCa. Increased PTH-mediated bone resorption as a consequence of CRF could increase serum tCa concentration. If elevated iCa is also present, then the reduced GFR caused by loss of renal mass could cause increased iCa concentration as the filtered load of calcium declines. Hyperplasia of the parathyroid gland chief cells could account for increased PTH secretion and serum calcium concentration because chief cells secrete small amounts of PTH that are nonsuppressible regardless of serum iCa concentration.214
Tertiary hyperparathyroidism refers to the condition of a subset of patients with CRF who develop ionized hypercalcemia and excessive PTH secretion that is not inhibited by high serum iCa concentration. It is likely that such patients had high PTH concentrations in association with normal or low serum iCa concentration (renal secondary hyperparathyroidism) earlier in the clinical course of CRF. Autonomous secretion of PTH from the parathyroid gland is unlikely, but the set-point for PTH secretion may be altered in CRF such that higher concentrations of iCa are necessary to inhibit PTH secretion.215 Decreased serum calcitriol concentrations, decreased numbers of calcitriol receptors in the parathyroid gland, and decreased calcitriol-VDR interactions with chief cell DNA caused by uremic toxins may contribute to this increase in set-point,75,266,435 as may decreased levels of the calcium receptor, which both establish the set-point and depend on calcitriol functionality for synthesis of its mRNA from the parathyroid cells’ DNA.99 Ten dogs with CRF and increased serum tCa concentration were compared with those with normal serum tCa concentration (Fig. 6-12). Serum amino-terminal PTH concentration was markedly increased in both groups of uremic dogs, but those with increased tCa had higher PTH concentrations. Calcitriol concentration was decreased to a similar extent in both groups. It was proposed that the hypercalcemic and more markedly hyperparathyroid uremic dogs might have had greater calcitriol receptor (VDR) deficits in their parathyroid cells, which would lead to poorly controlled PTH synthesis and chief cell hyperplasia.405 Deficient calcitriol functionality caused by VDR deficits would also lead to calcium receptor deficits and the “set-point” elevations involved in the observed hypercalcemia.99
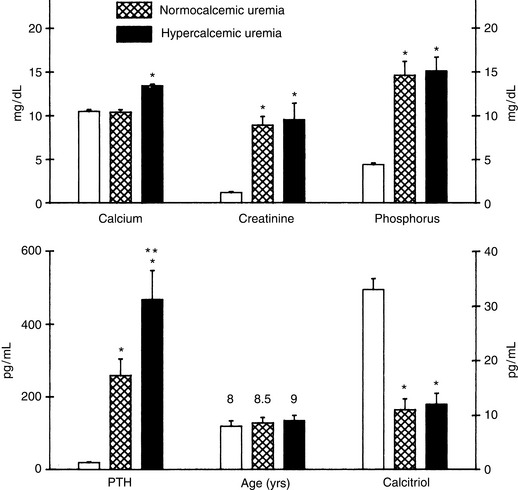
Figure 6-12 Comparison of biochemical data for dogs with renal failure and hypercalcemia or normocalcemia. Dogs with renal failure were normalized for age and had similar concentrations of serum creatinine, phosphorus, and calcitriol. Serum concentrations of PTH were greater in the hypercalcemic dogs than in the normocalcemic dogs. Data are mean ± SEM. For normal and hypercalcemic uremic dogs, n = 10; for normocalcemic uremic dogs, n = 20. Significant differences were *P <.0001 (from normal) and **P <.02 (from normocalcemic uremia PTH) by Student t test.
(From Nagode LA, Steinmeyer CL, Chew DJ, et al. Hyper- and normo-calcemic dogs with chronic renal failure: relations of serum PTH and calcitriol to parathyroid gland Ca++ set-point. In: Norman AW, Schaefer K, Grigoleit HG, et al, editors. Vitamin D 1988. Chemical, biochemical and clinical endocrinology. Berlin: Walter de Gruyter & Co, 1988: 799–800.)
Aluminum accumulation in the development of hypercalcemia in dogs or cats with renal disease being treated with aluminum-containing intestinal phosphate binders has not been investigated despite the fact that such treatment is common. Experimental dogs exposed to aluminum developed mild hypercalcemia within minutes of a single intravenous injection. During chronic daily exposure to aluminum during a period of weeks, serum calcium concentration progressively increased, and azotemia developed.242
Two of 15 cats with CRF developed hypercalcemia while eating a phosphate-restricted veterinary diet designed for treatment of renal failure. Hypercalcemia in these cats was associated with a decrease in serum phosphorus and low or undetectable PTH concentrations. Serum calcium returned to normal, and PTH and phosphorus increased with the feeding of a maintenance diet.29
Pathologic or consequential hypercalcemia
Cancer-Associated Hypercalcemia
The most common cause of hypercalcemia in dogs is cancer-associated hypercalcemia. Cancer is third in frequency of association with hypercalcemia in cats. There are three mechanisms (Fig. 6-13) of increased serum calcium concentration induced by neoplasms: (1) HHM, (2) hypercalcemia induced by metastases of solid tumors to bone (local osteolytic hypercalcemia [LOH]), and (3) hematologic malignancies growing in the bone marrow (LOH).479,480
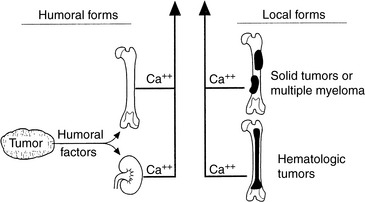
Figure 6-13 Pathogenesis of cancer-associated hypercalcemia. Humoral and local forms of cancer-associated hypercalcemia increase circulating concentrations of calcium by stimulation of osteoclastic bone resorption and increased renal tubular reabsorption of calcium.
Humoral Hypercalcemia of Malignancy
HHM is a syndrome associated with many tumors in people and animals.480 Characteristic clinical findings in patients with HHM include hypercalcemia, hypophosphatemia, hypercalciuria (often with decreased fractional calcium excretion), increased fractional excretion of phosphorus, increased nephrogenous cyclic adenosine monophosphate (cAMP), and increased osteoclastic bone resorption. Hypercalcemia is induced by humoral effects on bone, kidneys, and possibly the intestine (Fig. 6-14).481 Increased osteoclastic bone resorption is a consistent finding in HHM and increases calcium release from bone. The kidneys play a critical role in the pathogenesis of hypercalcemia because PTHrP stimulates calcium reabsorption, which binds and activates renal PTH-PTHrP receptors. The level of renal function in the patient may also contribute to the development of hypercalcemia. Animals with dehydration or impaired renal function are more susceptible to developing hypercalcemia or may have more severe hypercalcemia because of decreased renal excretion of calcium. In some forms of HHM, increased serum 1,25-dihydroxyvitamin D concentrations may increase calcium absorption from the intestine.489
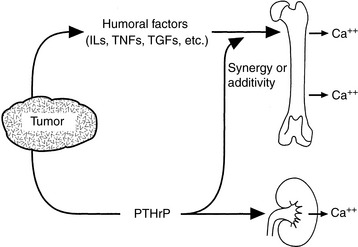
Figure 6-14 Humoral factors such as parathyroid hormone-related protein (PTHrP), interleukin-1 (IL-1), tumor necrosis factors (TNFs), or transforming growth factors (TGFs) produced by tumors induce humoral hypercalcemia of malignancy (HHM) by acting as systemic hormones and stimulating osteoclastic bone resorption or increasing tubular reabsorption of calcium.
Malignancies that are commonly associated with HHM in dogs include T-cell lymphoma and adenocarcinomas derived from the apocrine glands of the anal sac.22,36,479,607,615 In a retrospective study of 109 dogs with hypercalcemia, 58% had hypercalcemia due to underlying neoplasia. Lymphosarcoma was present in 78% of these patients, with 11% diagnosed with carcinoma and 6% diagnosed with anal sac adenocarcinoma.375 Dogs with cancer and HHM are expected to have shorter survival. In addition, sporadic cases of HHM occur in dogs with thymoma, myeloma, melanoma, or carcinomas originating in the lungs, pancreas, thyroid gland, skin, mammary gland, nasal cavity, and adrenal medulla.61,458,479-481 Tumors associated with hypercalcemia in cats include lymphosarcoma, multiple myeloma, squamous cell carcinoma, bronchogenic carcinoma/adenocarcinoma, osteosarcoma, fibrosarcoma, undifferentiated sarcoma, undifferentiated renal carcinoma, anaplastic carcinomas of the lungs and diaphragm, and thyroid carcinoma.* Lymphosarcoma and squamous cell carcinoma are the two most common causes of hypercalcemia in cats.511 Of 11 hypercalcemic cats with lymphosarcoma, two each had renal, generalized, gastrointestinal, or mediastinal involvement, and one each had laryngeal, nasal, or cutaneous disease.61,119,161,167,511 Squamous cell carcinoma has been found in mandibular, maxillary, pulmonary, and ear canal locations.61,270,299,511
Excessive secretion of biologically active PTHrP plays a central role in the pathogenesis of hypercalcemia in most forms of HHM, but cytokines such as IL-1, TNF-α, and transforming growth factor (TGF)-α and -ß or calcitriol may have synergistic or cooperative actions with PTHrP (see Fig. 6-14). Before PTHrP was identified, it was recognized that tumors associated with HHM induced a syndrome that mimicked primary hyperparathyroidism with secretion of a PTH-like factor that was antigenically unrelated to PTH.396,606 PTHrP binds to the N-terminal PTH-PTHrP receptor in bone and the kidneys but does not cross-react immunologically with native PTH (Fig. 6-15). PTHrP stimulates adenylyl cyclase and increases intracellular calcium in bone and the kidneys cells by binding to and activating the cell membrane PTH-PTHrP receptors. This binding results in stimulation of osteoclastic bone resorption, increased renal tubular calcium reabsorption, and decreased renal tubular phosphate reabsorption. IL-1 stimulates bone resorption in vivo and in vitro and is synergistic with PTHrP.364,480 TGF-α and -ß can stimulate bone resorption in vitro and have been identified in tumors associated with HHM, including adenocarcinomas derived from apocrine glands of the anal sac in dogs.374
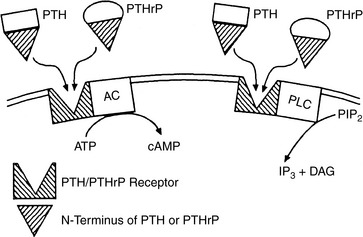
Figure 6-15 Parathyroid hormone-related protein (PTHrP) induces many of the effects of parathyroid hormone (PTH) by interacting with the PTH receptor in bone and kidneys and activating adenylyl cyclase (AC) to form cyclic AMP (cAMP) and phospholipase C (PLC) to form inositol triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol (PIP2). Stimulation of the PTH receptor results in increased osteoclastic bone resorption and renal tubular reabsorption of calcium, inhibition of renal tubular reabsorption of phosphorus, and stimulation of renal production of 1,25-dihydroxyvitamin D3 (calcitriol).
Lymphoma
Hypercalcemia is found in 20% to 40% of dogs with lymphoma (Fig. 6-16).321,342 Most dogs with lymphoma and hypercalcemia have HHM because increased osteoclastic resorption is present in bones without evidence of tumor metastasis. Lymphoma is an uncommon cause of mild HHM in ferrets.290 Lymphomas associated with HHM are usually of the T-cell type.607 T-cell lymphoma occurred in 22% of dogs with lymphoma, and hypercalcemia only occurred in dogs with CD4+ lymphoma in one study.502 The pathogenesis of hypercalcemia in dogs with lymphoma and HHM resembles that occurring in humans with lymphoma or leukemia induced by human T-cell lymphotropic virus type I (HTLV-I). Neoplastic cells from humans with HTLV-I-induced lymphoma have increased PTHrP production.471
Figure 6-16 Lateral (A) and ventrodorsal (B) thoracic radiographs of a 5-year-old boxer dog with hypercalcemia of malignancy caused by mediastinal lymphoma (arrows). Severe hypercalcemia (serum total calcium concentration, 20.6 mg/dL) was detected on initial presentation.
(From Chew DJ, Carothers M. Hypercalcemia. Vet Clin North Am Small Anim Pract 1989;19:265–287.)
Most dogs with lymphoma and hypercalcemia have T-cell lymphoma.561,607 Dogs with T-cell lymphoma were significantly more likely to have early relapse and death compared with those with B-cell lymphoma. Shorter remissions and survival times have been noted by others for T-cell lymphoma compared with B-cell lymphoma in dogs.225 In another study, 46 (32.8%) of 140 lymphomas were classified as T cell in origin, and 16 of these dogs (35%) were hypercalcemic.196 In 37 dogs with lymphoma and hypercalcemia, calcium concentration was not related to prognosis; mean remission was 10.4 months, and median remission was 6 months.477 The presence of a mediastinal mass had an adverse effect on remission in these hypercalcemic dogs. Serum tCa concentration may return to normal despite minimal reduction in tumor mass following chemotherapy, as happened in 5 of 12 dogs with lymphoma and initial hypercalcemia.610 The finding of hypercalcemia in dogs with lymphoma was not prognostic for survival or time to remission, but T-cell origin lymphoma did adversely affect prognosis.298,561,590
Most dogs with lymphoma and HHM have increased circulating PTHrP concentrations, but concentrations are lower than those in dogs with carcinomas and HHM, and PTHrP concentrations are not correlated with serum calcium concentration (Fig. 6-17).489 Elevated PTHrP concentrations were found in 12 of 14 dogs with underlying malignancy, but was within the reference range in 17 normocalcemic dogs with lymphoma.369 Epithelial T-cell cutaneous lymphoma (mycosis fungoides) has been associated with hypercalcemia in one dog, but histopathology failed to detect PTHrP in the neoplastic cells.44 These findings indicate that PTHrP is an important marker of HHM in dogs with lymphoma but is not the sole humoral factor responsible for stimulation of osteoclasts and development of hypercalcemia. It is likely that cytokines such as IL-1 or TNF function synergistically with PTHrP to induce HHM in dogs with lymphoma (see Fig. 6-14).479,480
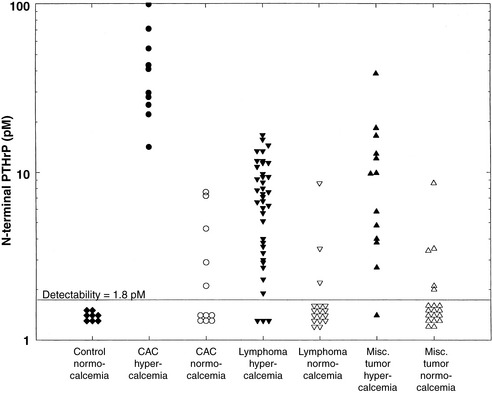
Figure 6-17 Circulating N-terminal parathyroid hormone-related protein (PTHrP) concentrations in normal dogs (CONTROL); dogs with hypercalcemia (>12 mg/dL) and anal sac adenocarcinomas (CAC), lymphoma, or miscellaneous tumors (MISC TUMOR); and dogs with normocalcemia (<12 mg/dL) and anal sac adenocarcinomas, lymphoma, or miscellaneous tumors.
(From Rosol TJ, Nagode LA, Couto CG, et al. Parathyroid hormone-related protein, parathyroid hormone, and 1,25-dihydroxyvitamin D in dogs with cancer-associated hypercalcemia. Endocrinology 1992;131:1157–1164.)
Some dogs and human patients with lymphoma and hypercalcemia have increased serum calcitriol concentrations, which may contribute to the induction of hypercalcemia.489,527 Some lymphocytes contain the 1α-hydroxylase (similar to that found in renal tubules) that converts 25-hydroxyvitamin D to the active metabolite 1,25-dihydroxyvitamin D (calcitriol). Therefore, lymphomas that retain this capability may synthesize excessive calcitriol, which could increase calcium absorption from the intestinal tract and facilitate development of hypercalcemia.
An early report indicated that a mediastinal mass was detected in most dogs with lymphoma and hypercalcemia.378 However, a recent report indicates that the presence of a cranial mediastinal mass was not required for development of hypercalcemia in dogs, and mediastinal masses were not disproportionately more common in those dogs with hypercalcemia.54
Canine Adenocarcinoma Derived from Apocrine Glands of the Anal Sac
The adenocarcinoma derived from apocrine glands of the anal sac of dogs consistently fulfills the criteria for HHM.377,379,473 This tumor appears primarily in middle-aged (mean, 10 years) dogs and rarely metastasizes to bone. Clinical signs are referable to hypercalcemia (polyuria, polydipsia, anorexia, and weakness), a mass in the perineum (tenesmus, ribbon-like stools, increased odor, and protruding mass), a mass in the sublumbar region, or more distant metastases. Apocrine adenocarcinomas often require rectal and anal sac palpation to confirm their presence because their sizes range from 7 mm to 6 × 8 cm (Fig. 6-18). Dogs with this tumor and HHM have hypercalcemia (tCa, 12 to 24 mg/dL); hypophosphatemia; decreased immunoreactive PTH concentration; increased urinary excretion of calcium, phosphorus, and cAMP; and increased osteoclastic bone resorption.36,379,615 This tumor should not be confused with the common perianal adenomas or the uncommon perianal adenocarcinomas that arise from the circumanal glands and have entirely different biologic behavior. Perianal adenomas and adenocarcinomas affect primarily male dogs and are not associated with hypercalcemia.591
Figure 6-18 Hypercalcemia of malignancy associated with apocrine gland adenocarcinoma of the anal sac in an elderly female dog. Transverse section of the anal sac and associated malignancy (arrows).
(From Chew DJ, Meuten DJ. Disorders of calcium and phosphorus metabolism. Vet Clin North Am Small Anim Pract 1982;12:411–438.)
Hypercalcemia was present at the time of diagnosis in 80% to 100% of affected dogs in early studies.357,360 Recent reports in dogs with earlier detection note the incidence of hypercalcemia to be lower, at 33%,496 27%,615 and 53% of cases.36 Early reports also noted a strong bias toward the occurrence of this tumor in female dogs, but equal sex distribution has been more recently noted.615 In some instances, the finding of hypercalcemia during routine serum biochemistry testing prompts rectal palpation and subsequent discovery of an apocrine gland adenocarcinoma. Surgical removal or radiation therapy of the adenocarcinoma results in rapid return to normal of serum calcium and phosphorus concentrations, increased serum PTH concentration, and decreased calcitriol concentration.489 Postsurgical survival of dogs with apocrine gland adenocarcinoma and hypercalcemia ranged from 2 to 21 months, with a mean of 8.8 months. Sublumbar metastases occur in a high percentage (72%) of affected dogs and are associated with recrudescence of the biochemical alterations in serum and urine.36 In one study, dogs with hypercalcemia and anal sac adenocarcinoma had shorter survival times compared with normocalcemic dogs with this tumor (356 vs. 584 days)615; in another study, survival was not influenced by the presence of hypercalcemia.36
Most dogs with HHM have increased concentrations of circulating PTHrP (see Fig. 6-17). Plasma concentrations of PTHrP are highest (10 to 100 pmol/L) in dogs with apocrine adenocarcinomas of the anal sac and sporadic carcinomas associated with HHM.489 Serum calcium concentrations in affected dogs correlate well with circulating PTHrP concentrations, which is consistent with the concept that PTHrP plays a primary role in the pathogenesis of HHM in these dogs. Dogs with apocrine adenocarcinomas and normocalcemia may have increased plasma PTHrP concentrations (2 to 15 pmol/L), but the concentrations are lower than those in dogs with hypercalcemia.
Some dogs with apocrine adenocarcinomas have inappropriate concentrations (normal or increased) of calcitriol for the degree of hypercalcemia.489 This finding suggests that the humoral factors produced by the neoplastic cells are capable of stimulating renal 1α-hydroxylase and increasing the formation of calcitriol even in the presence of increased serum calcium concentration. PTH concentrations were not increased in hypercalcemic dogs and were significantly lower than those observed in dogs with primary hyperparathyroidism. Parathyroid glands from dogs with apocrine adenocarcinoma were atrophic or inactive, and there was nodular hyperplasia of C cells in the thyroid glands because of prolonged hypercalcemia.379
Hematologic Malignancies
Some types of hematologic malignancies present in the bone marrow produce hypercalcemia by inducing bone resorption locally.479,480 This effect occurs most commonly in multiple myeloma and lymphoma. Hypercalcemia has been reported in 17% of dogs360 and 20% of cats with multiple myeloma.435 Hypercalcemia has also been noted in a case of erythrophagocytic multiple myeloma in a cat.605 In one dog with multiple myeloma, an increase in tCa was noted, but iCa concentration was within the reference range.580 In this case the increase in tCa concentration was presumptively due to an increase in the protein-bound serum calcium concentration. A number of paracrine factors or cytokines may be responsible for the stimulation of bone resorption in this setting. The cytokines most often implicated in the pathogenesis of local bone resorption are IL-1, TNF-α, and TNF-ß (lymphotoxin).355,397 Other cytokines or factors that may play a role include IL-6, TGF-α and TGF-ß, and PTHrP.53 Production of small amounts of PTHrP by a tumor in bone may stimulate local bone resorption without inducing a systemic response. Prostaglandins (especially prostaglandin E2) may also be responsible for local stimulation of bone resorption.
Some dogs with lymphoma and hypercalcemia have localized bone resorption associated with metastases to medullary cavities without evidence of increased bone resorption at sites distant from the tumor metastases.378 Hypercalcemic dogs with lymphoma and bone metastases had decreased PTH and calcitriol concentrations, increased excretion of hydroxyproline, calcium, phosphorus, and increased concentrations of the prostaglandin E2 metabolite 13,14-dihydro-15-ketoprostaglandin E2. Prostaglandin E2 may be an important local mediator of bone resorption in these dogs. Other potential mediators include IL-1 and TNFs.
Tumors Metastatic to Bone
Solid tumors that metastasize widely to bone can produce hypercalcemia by the induction of local bone resorption associated with tumor growth. This is not common in animals but is an important cause of cancer-associated hypercalcemia in humans.479,493,494 Tumors that often metastasize to bone and induce hypercalcemia in human patients include breast and lung carcinomas. Carcinomas of the mammary gland, prostate, liver, and lungs were most frequently reported to metastasize to bone in dogs, and the humerus, femur, and vertebrae were the most common sites of metastasis.380,495 Primary bone tumors are not often associated with hypercalcemia in dogs or cats.
The pathogenesis of enhanced bone resorption is not well understood, but two primary mechanisms are secretion of cytokines or factors that stimulate local bone resorption and indirect stimulation of bone resorption by tumor-induced cytokine secretion from local immune or bone cells.207 Cytokines or factors that may be secreted by tumor cells and stimulate local bone resorption include PTHrP,457 TGF-α and TGF-ß, and prostaglandins (especially prostaglandin E2). In some cases, bone-resorbing activity can be inhibited by indomethacin, which suggests that prostaglandins are either directly or indirectly associated with stimulation of bone resorption. The cytokines most often implicated in indirect stimulation of bone resorption by local immune cells include IL-1 and TNFs.
Malignant neoplasms with osseous metastases may cause moderate to severe hypercalcemia and hypercalciuria, but serum ALP activity and phosphorus concentrations are usually normal or only moderately increased. It is believed these changes are caused by release of calcium and phosphorus into the blood from areas of bone destruction at rates greater than can be cleared by the kidneys and intestine. Bone involvement can be multifocal but is usually sharply demarcated and localized to the area of metastasis.
Primary Hyperparathyroidism
Primary hyperparathyroidism is an uncommon cause of hypercalcemia in dogs40,87 and is even less common in cats.141,285 In hypercalcemic cats, primary hyperparathyroidism was found in 4 of 71 cases.511 Excessive and inappropriate secretion of PTH by the parathyroid glands relative to the serum iCa concentration characterizes this condition. In a review of 210 dogs with primary hyperparathyroidism, all dogs had hypercalcemia, and 65% had hypophosphatemia. BUN and serum creatinine concentrations were within or below the reference range in 95% of cases. Serum PTH concentration was within the reference range in 73% of dogs. Primary hyperparathyroidism was caused by a solitary parathyroid gland adenoma in approximately 90% of dogs, whereas parathyroid gland carcinoma and parathyroid gland hyperplasia each accounted for 5% of cases in one large series.178 Adenomas occurred with nearly equal frequency in the external and internal parathyroid glands in one study,40 but external gland adenomas predominated in another report in dogs.619 Idiopathic parathyroid gland hyperplasia may affect one or more glands and has been reported in six older dogs.143 Although remnant parathyroid tissue may be found in the cranial mediastinum near the base of the heart, neoplastic transformation has not been reported at this site in dogs or cats. An ectopic parathyroid gland adenoma cranial to the thoracic inlet has been described in one dog.618 In cats, the underlying lesion is typically benign, owing to an adenoma, bilateral cystadenomas, or hyperplasia,141,172,511,550 but unilateral or bilateral carcinomas have also been diagnosed.178,285,353,452
Primary parathyroid gland hyperplasia has been reported in two German shepherd dog puppies.568 Diffuse hyperplasia was present in all four parathyroid glands. In retrospect, this family of German shepherd dogs probably had an inactivating mutation in the gene for the calcium-sensing receptor. Mutations in one or both of the calcium-sensing receptor genes in humans result in familial hypocalciuric hypercalcemia or neonatal severe hypercalcemia, respectively, because of an inadequate ability to sense extracellular calcium concentration and coordinate the appropriate cellular response.454 The affected puppies had a disease syndrome that mimicked neonatal severe hypercalcemia in humans. Neonatal severe hypercalcemia is lethal unless total parathyroidectomy is performed early in life to markedly reduce increased PTH concentrations.
Dogs with primary hyperparathyroidism are older: in one review of 210 dogs with primary hyperparathyroidism, the mean age of affected dogs was 11.2 years (range, 6 to 17 years);177 in another study, the mean age of affected dogs was 10.5 years (range, 5 to 15 years).178 The mean age in affected cats was 12.9 years (range, 8 to 15 years).285 No sex predisposition has been noted, but keeshonds constituted 36% of affected dogs, and five of eight cats were Siamese.380 Parathyroid gland masses usually cannot be palpated in dogs, but 50% of cats with primary hyperparathyroidism had a palpable cervical mass.141,285 Clinical signs related to hypercalcemia are either mild (e.g., lethargy, polydipsia, polyuria, and weakness) or absent in many affected dogs.40,178 In one study, most owners of affected dogs were not convinced that their dogs had a serious illness,40 but some owners retrospectively recognized subtle signs after hypercalcemia resolved.178 In 210 dogs with primary hyperparathyroidism, the most common clinical signs were related to urinary tract infections or urolithiasis.177 No abnormalities were noted in 71% of dogs. Urinary tract infection was present in 29% and urolithiasis was present in 31% of dogs in this study. Other studies have shown that calcium-containing uroliths (calcium phosphate, calcium oxalate, or mixtures) occurred in approximately 30% of dogs and in a cat with primary hyperparathyroidism.178,300,353 Urolithiasis is attributed to hypercalcemia and subsequent hypercalciuria. Interestingly, hypercalcemia arising from other causes has not been associated with urolithiasis except in cats with idiopathic hypercalcemia (IHC).520 More prominent clinical signs and serious consequences can occur when hyperparathyroidism and severe hypercalcemia are long standing and associated with renal failure.115,116
The diagnostic workup to confirm primary hyperparathyroidism often begins with the fortuitous finding of increased serum calcium concentration on routine clinical chemistry testing.178 The diagnosis of primary hyperparathyroidism is easy in dogs and cats that have increased serum tCa concentration, normal renal function, and increased concentration of immunoreactive PTH. The appropriateness of the PTH concentration must be interpreted in relation to the serum iCa concentration. Additional support for the diagnosis of primary hyperparathyroidism is provided by the finding of increased serum iCa concentration, increased serum ALP, low serum phosphorus concentration, increased or normal calcitriol concentration, undetectable PTHrP, and calcium-containing uroliths. The most consistent laboratory abnormality in dogs with primary hyperparathyroidism is increased serum calcium concentration.178
Hypercalcemia results from a combination of effects following PTH binding to receptors in kidneys and bone. PTH also acts indirectly to increase serum iCa concentration by enhancing renal conversion of 25-hydroxyvitamin D to calcitriol. Hypophosphatemia secondary to PTH-enhanced urinary excretion of phosphorus was observed in 5 of 21 dogs.40 Serum phosphorus concentration is typically low,178 and calcitriol concentrations were mildly increased or in the high-normal range in three of four dogs with primary hyperparathyroidism.489
The diagnosis of primary hyperparathyroidism is more challenging when PTH is within the reference range. A PTH concentration in the upper part of the reference range in association with hypercalcemia is inappropriate. Confirmed primary hyperparathyroidism has been noted in dogs and cats with hypercalcemia and reference range PTH concentrations.178,285 In a cat with persistent hypercalcemia related to primary hyperparathyroidism, PTH concentration was increased on two occasions but within the reference range on five other occasions.141 PTH concentrations measured in blood collected from either the left or right jugular vein did not differ, and sampling from a specific side was not valuable for localizing the site of an enlarged parathyroid gland.180 Circulating PTHrP concentrations were undetectable in six dogs with primary hyperparathyroidism.489
Ultrasonography of the neck is helpful in the diagnosis of primary hyperparathyroidism in dogs and cats, but it requires an ultrasound unit with a high-frequency (7.5- to 10-MHz) transducer to achieve the necessary level of resolution rather than the widely available 5- or 7.5-MHz units used for abdominal studies.178,618 With a 10-MHz linear transducer, the parathyroid glands of normal dogs can routinely be identified especially in larger dogs.469 Parathyroid gland masses greater than 5 mm can usually be identified, and some masses as small as 2 mm may be detected. Enlarged parathyroid glands are expected to be hypoechoic or anechoic, well marginated, and easily contrasted with thyroid tissue. False-positive results are rare, but false-negative findings may occur. Ultrasonography correctly identified the presence and location of a solitary parathyroid gland mass in 10 of 11 dogs in a prospective study in which the mass was confirmed at surgery.180 Sonography identifies the location of the parathyroid gland tumor and allows presurgical planning.
Double-phase scintigraphy of the parathyroid glands using 99mTc sestamibi was useful in the diagnosis of parathyroid gland adenoma in initial reports from two dogs.362,626 In a study of 15 dogs with hypercalcemia, scintigraphy correctly identified 3 of 3 dogs with hypercalcemia of malignancy as negative for hyperfunctioning parathyroid glands.361 Scintigraphy identified only one of six dogs with a parathyroid gland adenoma and only one of six dogs with parathyroid hyperplasia. Based on these results, parathyroid gland scintigraphy is not recommended to identify abnormal parathyroid glands because of very poor sensitivity and specificity.
Surgical exploration of the cervical region in patients with parathyroid gland adenoma or carcinoma usually reveals enlargement of one parathyroid gland, and the remaining three are small or impossible to identify because hypercalcemia results in atrophy of normal parathyroid tissue. Primary parathyroid gland hyperplasia may affect more than one gland, and clinical signs can recur if only the largest gland is removed surgically. Parathyroid gland tumors may be difficult to identify if the tumor is embedded in fat or if it arises from the internal parathyroid gland. Failure to visualize a parathyroid gland tumor is rarely attributed to the occurrence of a tumor in ectopic parathyroid tissue. Methylene blue infusion to enhance visualization of parathyroid glands should be reserved for patients in whom a tumor is strongly suspected but not readily identified during surgery because clinically relevant side effects of methylene blue administration include hemolytic anemia and acute renal failure.185 In 47 dogs with primary hyperparathyroidism treated with parathyroidectomy, 94% of surgeries resulted in control of hypercalcemia.464 Hypercalcemia resolved within 1 to 6 days, and remained within the reference range for a median of 561 days. The use of a rapid PTH assay for intraoperative measurement of PTH may be helpful to demonstrate a decrease in PTH concentration after excision of autonomously secreting parathyroid tissue.233
Ultrasound-guided chemical ablation was used safely and effectively as an alternative treatment to surgery in eight dogs with a solitary parathyroid gland mass and hypercalcemia.336 Serum tCa and iCa concentrations were within reference ranges 24 hours after treatment in seven dogs and within 5 days in one dog. Transient hypocalcemia developed in four dogs during the first 5 days after treatment; one dog required treatment for hypocalcemic tetany. Dysphonia was noted in two of eight dogs in this study, but Horner syndrome, laryngeal paralysis, and death were not encountered as has been described with ethanol injection of thyroid glands of hyperthyroid cats.213,592,612 It is likely that the low volume of ethanol injected into a single parathyroid mass provides less potential for leakage beyond the parathyroid mass. In a review of treatment of 110 dogs with primary hyperparathyroidism, 72% of ethanol ablation procedures resulted in a control of hypercalcemia.464 Hypercalcemia resolved in 1 to 4 days, and remained within normal limits for a median of 540 days.
Ultrasonographically guided radiofrequency heat ablation of parathyroid masses in dogs has become the preferred treatment at some referral hospitals. In one study, 11 dogs with either one or two masses on ultrasonography were treated by radiofrequency heat following anesthesia and insertion of a 20-gauge over-the-needle catheter into the mass.455 Hypocalcemia developed in five of the eight successfully treated dogs, all of which required treatment. The only other adverse effect was a transient voice change in one dog. In 49 dogs with primary hyperparathyroidism treated with heat ablation, 90% of procedures resulted in a control of hypercalcemia.464 Hypercalcemia resolved in 1 to 6 days, and remained within normal limits for a median of 581 days.
Hypervitaminosis D
Hypervitaminosis D refers to toxicity resulting from excess cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2). Metabolites of vitamin D can also exert toxicity, and the term hypervitaminosis D has been extended clinically to include toxicity from 25-hydroxyvitamin D, dihydrotachysterol, and 1,25-dihydroxyvitamin D (calcitriol), as well as newer analogues of calcitriol. Vitamin D toxicity is better referred to as 25-hydroxyvitamin D toxicity, because vitamin D is rapidly transformed into this metabolite in vivo.199 Vitamin D and its immediate metabolite, 25-hydroxyvitamin D, have little biologic activity at physiologic concentrations because they have low binding affinity for the VDR. Pharmacologic concentrations of 25-hydroxyvitamin D that occur during hypervitaminosis D exert hypercalcemic effects, because 25-hydroxyvitamin D competes with calcitriol for binding to the VDR in target tissues.162,404 Hypercalcemia results from increased intestinal absorption of calcium, but increased osteoclastic bone resorption and calcium reabsorption from renal distal tubules may also contribute.
Vitamin D intoxication and hypercalcemia may result from excessive dietary supplementation or may be caused iatrogenically during the treatment of hypoparathyroidism. Accurate dosing with cholecalciferol and ergocalciferol is difficult because they have a slow onset and prolonged duration of action.40,530 Hypercalcemia developed in 7 of 16 hypoparathyroid dogs during treatment with vitamin D and calcium salt supplementation.40 Ingestion of toxic plants that contain glycosides of calcitriol (e.g., Cestrum diurnum, Solanum malacoxylon, and Trisetum flavescens) is a potential cause of hypercalcemia in small animals.428 Vitamin D toxicity associated with ingestion of C. diurnum has been reported in a cat.151 C. diurnum, day-blooming jessamine, has achieved increasing popularity as a house plant and should not be confused with jasmine, which is an indoor climbing plant without active vitamin D metabolites.85
A diagnosis of hypervitaminosis D in dogs and cats increased with the introduction of cholecalciferol-containing rodenticides in 1985, but this source of intoxication is less common today. Cholecalciferol bait is delivered as pellets that are palatable to some animals and are very toxic when ingested. One manufacturer claimed a low hazard to dogs (oral median lethal dose, 88 mg/kg), but toxicity at a lower dosage (10 mg/kg) was demonstrated.162,228 High-risk groups include dogs weighing 12 kg or less and those younger than 9 months. Recovery from previous cholecalciferol toxicity can be a risk factor for subsequent occurrence because removal of the source from the premises may not be possible.112 Toxicity in four cats has also been reported.390,441 One reason for the few reports of vitamin D toxicity in cats is that they appear to be resistant to cholecalciferol toxicity when the diet is otherwise complete and balanced.534
Clinical signs are usually vague and include anorexia, lethargy, vomiting, tremors, constipation, and polyuria. These signs are usually attributed to the effects of hypercalcemia. Hypercalcemia is reversible with early and aggressive therapy by providing enough time for 25-hydroxyvitamin D to be eliminated from the body.107,149,162 Death occurred in approximately 45% of dogs after developing hypercalcemia from hypervitaminosis D in early reports,162,228,344,500 but the survival rate was higher in dogs of a later series.107
Hypercalcemia usually develops within 24 hours after ingestion,228 and hypercalcemia is often severe unless serum samples were obtained within 24 hours of ingestion. Mild hyperphosphatemia is often noted. Azotemia is initially absent but can develop subsequently. Serum creatinine concentration usually is less than 3 mg/dL unless treatment has been delayed, in which case azotemia may be marked. It may take as long as 72 hours for azotemia to develop as a result of renal lesions caused by hypercalcemia. Measurement of serum 25-hydroxyvitamin D concentration can provide conclusive evidence for hypervitaminosis D after exposure to cholecalciferol or ergocalciferol. Serum concentrations of 25-hydroxyvitamin D were increased to at least twice the upper limit of normal, with a mean concentration approximately 10 times the normal in dogs with hypervitaminosis D107 and were increased for weeks to months in some instances.149 In ten episodes of cholecalciferol intoxication, concentrations of cholecalciferol were increased above the normal range for 10 to 61 days.112 The half-life for cholecalciferol was 29 days in experimental dogs.500 Serum calcitriol concentrations were also increased early in the syndrome,107 but suppression of calcitriol synthesis occurs later.
Hypervitaminosis D with hypercalcemia, azotemia, high concentrations of 25-hydroxyvitamin D, and/or renal calcification has been described in cats from Japan fed fish-based commercial cat food.236,391,509 Cholecalciferol content of these diets exceeded the dietary requirements of vitamin D by more than 100 times. Renal disease and failure occurred within 4 to 14 months in a large number of cats fed a commercial cat food containing 30 times the vitamin D requirement.392 All commercial cat foods provide vitamin D in excess of the minimal requirements, and there is no regulated upper limit on the quantity of vitamin D that can be included. Other factors may modulate the toxicity of hypervitaminosis D, such as increased dietary calcium and phosphorus or dietary reduction in magnesium.534 Hypervitaminosis D with hypercalcemia, high concentrations of serum 25-hydroxyvitamin D and calcitriol concentrations have been described in two dogs that consumed a commercial diet containing more than 100 times the manufacturer-stated vitamin D concentration.370 Accidental oversupplementation of commercial dog and cat foods with excessive vitamin D resulted in a large recall of products in 2006.
Hypercalcemia attributed to the effects of increased calcitriol occasionally occurs during calcitriol treatment in animals with hypoparathyroidism and rarely during treatment of renal secondary hyperparathyroidism. When hypercalcemia is observed, it is usually in patients given doses more than 3.5 ng/kg daily. Discontinuation of calcitriol should result in normocalcemia within 1 week. Dosing with calcitriol at twice the daily dosage every other day up-regulates fewer intestinal epithelial cells for calcium absorption and decreases the chance for further development of hypercalcemia. No adverse effects were noted when using this intermittent dosing protocol in one study in 20 cats.263 Formulation errors have also been encountered in which the concentration of calcitriol in a compounded product was too high. There are no veterinary preparations of calcitriol; thus, the available preparations of calcitriol must be diluted in pharmaceutical oils for appropriate dosing. Hypercalcemia has also been encountered when dosing errors have been made (mg/kg amounts given as opposed to ng/kg amounts). High serum concentrations of calcitriol have been observed in some dogs with lymphoma and hypercalcemia,489 but it is not clear whether the excess calcitriol was synthesized by the tumor or by the kidneys under stimulation of PTHrP.
Topical ointments containing potent vitamin D analogues (calcipotriene) for treatment of human psoriasis can result in hypercalcemia when toxic quantities are ingested by dogs.* Minimal toxic dose is 10 μg/kg; minimal lethal dose is 65 μg/kg; and the oral LD50 is between 100 and 150 μg/kg in dogs.234 In 25 dogs with calcipotriene ingestion, 28% died and 50% experienced AIRF. Phosphorus, tCa, and iCa are elevated with calcipotriene toxicity.230,234 The affinity of calcipotriene for vitamin D-binding protein is much lower than that of calcitriol; thus, free calcipotriene is readily available for binding to VDRs. The rapid binding to VDRs accounts for the rapid onset of hypercalcemia and hyperphosphatemia and also for the rapid catabolism of calcipotriene. Hypercalcemia decreases after several days rather than being prolonged for weeks to months as seen in cholecalciferol toxicity. Exposure to calcipotriene has not yet been reported in cats, although there are several anecdotal reports of cats that developed hypercalcemia after licking calcipotriene from their owner’s skin. Telephone calls to animal poison control centers indicate that exposure to this ointment has been increasing in dogs.354 Whether calcipotriene cross-reacts with calcitriol in the measurement of vitamin D metabolites has not yet been determined, but it is not detected by methods to measure 25-hydroxyvitamin D.
Granulomatous Disease
Hypercalcemia can result from calcitriol synthesis by activated macrophages during granulomatous inflammation. Normal macrophages express 1α-hydroxylase activity (which converts 25-hydroxyvitamin D to calcitriol) when stimulated by interferon or lipopolysaccharide. Macrophages in granulomatous inflammation express such activity without stimulation.159 Blastomycosis is a granulomatous disease in dogs that is occasionally (5% to 14% of cases) associated with hypercalcemia. Hypercalcemia is usually mild but can be severe.15,150 In a study of 38 dogs with blastomycosis, 5% had elevated serum iCa but only 2.6% had elevated serum tCa.132 In this study, 61% of dogs with blastomycosis had low serum tCa, but no dogs were hypocalcemic based on serum iCa concentration, showing an overestimation of hypocalcemia based on serum tCa measurement. Reports of granulomatous diseases associated with hypercalcemia include a dog with gastric pythiosis,323 a dog with granulomatous lymphadenitis,372 two cats with disseminated histoplasmosis250 and dogs with coccidioidomycosis or schistosomiasis.178,581 In one dog with schistosomiasis, PTHrP levels were undetectable,476 but in two other dogs with schistosomiasis, PTHrP levels were increased with no malignancy found at necropsy.197 In cats, elevated calcitriol concentrations were documented in cases of Nocardia and atypical mycobacteria infection.368 Cats with blastomycosis, cryptococcosis, actinomyces, and injection site granulomas (Chew and Peterson, unpublished observations on injection site granuloma)368,511,547 have been noted with hypercalcemia possibly because of enhanced synthesis of calcitriol.150 Severe hypercalcemia was observed in association with noninfectious granulomatous dermatitis in two dogs in which excess synthesis of calcitriol was suspected (Kwochka and Chew, unpublished observations). PTH, PTHrP, and 25-hydroxyvitamin D concentrations were not increased. Hypercalcemia resolved as the inflammation subsided. In a dog with granulomatous lymphadenitis, serum PTH concentration was low but serum calcitriol concentration was elevated.372 Nodular panniculitis with hypercalcemia has been reported in dogs, and calcitriol concentrations were two to three times the normal in one instance.166,448
Idiopathic Hypercalcemia of Cats
Hypercalcemia may be less common in cats than in dogs, although the incidence of hypercalcemia from primary care practices is not reported. Within the past 10 years, IHC has been recognized in cats365,381 and is now the most common cause of ionized hypercalcemia in cats in the United States. Even though some suggest that IHC is a local geographic phenomenon,178 it is widespread across the United States and is being recognized in other parts of the world.
In IHC, serum calcium concentration may be increased for months to more than 1 year. In 427 cases of feline IHC, 46% had no clinical signs, 18% had mild weight loss with no other clinical signs, 6% had inflammatory bowel disease, 5% had chronic constipation, 4% were vomiting, and 1% were anorectic.520 Uroliths or renoliths were observed in 15%, and calcium oxalate stones were noted in 10% of cases. Cats ranged in age from 0.5 to 20 years, and long-haired cats accounted for 27% of the cases (compared with an overall submission rate of 14% from long-haired cats). There was no sex predilection. Serum iCa concentration was increased; PTH concentration was in the lower half of the reference range; and PTHrP was negative in all samples. Concentration of iMg was normal, and mean concentration of 25-hydroxyvitamin D was within the reference range. Calcitriol was measured in a small number of these cats and was suppressed. In another study, 1 of 7 cats exhibited an increased concentration of calcitriol, and 2 of 11 cats had increased PTHrP in the absence of underlying neoplasia following extensive diagnostic evaluation, survival for many months, and necropsy.381 It appears that excessive PTH, 25-hydroxyvitamin D, or calcitriol concentration is not the cause of IHC in most cats. However, normal concentrations of calcitriol could result in hypercalcemia if there are mutations of the VDR or an increase in the number of calcitriol receptors. Normal concentrations of iMg indicate that PTH secretion is not inhibited by decreased or excess iMg.520 Renal function, based on BUN and serum creatinine concentration, is usually normal initially, but some cats develop CRF secondary to long-standing IHC.381 Results of serology testing for feline leukemia virus and feline immunodeficiency virus have been negative, and serum thyroxine concentrations have been normal. Chronic acidosis could explain chronic elevations of iCa,121 but venous blood gas analysis has not revealed significant acid-base disturbances. Exploration of the cervical region has not identified primary hyperparathyroidism, and subtotal parathyroidectomy has not resolved hypercalcemia in cats in which this procedure was performed.381
As many as 35% of cats with calcium oxalate urinary stones have hypercalcemia. Even though the specifics of the underlying diagnoses were not detailed,424 it is likely that most had IHC. The occurrence of ureterolithiasis in cats was very uncommon before 1993. Eleven cases of calcium oxalate ureterolithiasis were recently described in cats, and four had mild to moderate hypercalcemia.319 It appears that the frequency of hypercalcemia in calcium oxalate stone-forming cats has decreased substantially (Lulich, personal communication, 2003).
Specific treatment for IHC is impossible because the pathogenesis remains unknown. Increased bone resorption, increased intestinal absorption, or decreased renal excretion of calcium or combinations of these mechanisms could be responsible for hypercalcemia. The feeding of increased dietary fiber decreased serum calcium in some cats365 but not in others.381 The beneficial effect of a higher fiber diet may be because of decreased intestinal absorption of dietary calcium. The effects of fiber on intestinal absorption are complex and depend on the types and amounts of fiber in the diet and other nutrients present.
The feeding of veterinary renal diets may result in normocalcemia in some cats with IHC. These diets are generally low in calcium and phosphorus and are considered alkalinizing or at least less acidifying than maintenance diets. Some cats that show an initial decrease in serum calcium concentration following any type of dietary change will have a return to hypercalcemia over time.
In those cats that do not respond to a change in diet, prednisone therapy may result in a long-term decrease in iCa. The effects of glucocorticosteroid treatment may last for months to years in some cats with doses of 5 to 20 mg prednisone/cat/day. There is an escape from the effects of glucocorticosteroid treatment in some cats and a return to hypercalcemia despite maximal doses of prednisone. When dietary modification and treatment with prednisolone have been unsuccessful in resolving IHC, intravenous pamidronate treatment can be considered.
Beneficial effects from the chronic administration of subcutaneous fluids or oral furosemide to cats with IHC have not been evaluated. Treatment with calcimimetics could be of benefit. Calcimimetics interact with the calcium receptor and are effective in decreasing calcium, phosphorus, and PTH in human patients.55
Uncommon Causes of Hypercalcemia
AIRF in dogs is occasionally associated with mild hypercalcemia. Hypercalcemia may occur more commonly after conversion of oliguria to polyuria, possibly as calcium salts that were deposited during oliguria are mobilized from soft tissues. Sudden improvement in renal function also may result in a rapid decrease of serum phosphorus concentration, changing mass law interactions between phosphorus and calcium and resulting in transient hypercalcemia. Mild hypercalcemia (11.5 to 12.5 mg/dL) is observed uncommonly in some dogs with severe oliguria and decreased GFR during intrinsic renal failure. Animals with severe hyperphosphatemia during AIRF usually have normal or low serum calcium concentrations.
Nonmalignant skeletal lesions are occasionally associated with hypercalcemia in dogs. Bacterial and fungal osteomyelitis can potentially result in hypercalcemia if the rate of osteolysis is sufficient.111 Hypercalcemia associated with hepatozoonosis and skeletal involvement has been reported.351 In 30 dogs with systemic aspergillosis, 27% had hypercalcemia.523 Neonatal septicemia has been associated with hypercalcemia on rare occasions in puppies after septic embolization of bone and subsequent osteolysis.111 Mild hypercalcemia occurs in some dogs with hypertrophic osteodystrophy, and the hypercalcemia may be aggravated by ascorbic acid supplementation.558 Hypothermia has caused hypercalcemia in one cat.429 One cat with pancreatitis and hypercalcemia has been described, even though hypocalcemia is more common in cases of pancreatitis.247 In one report, a dog receiving intermittent calcium therapy for hypocalcemia developed hypercalcemia and acute pancreatic hemorrhage that may have been related to excessive calcium therapy.409 Dehydration may cause mild and reversible hypercalcemia, especially with normal kidney function. Disuse osteoporosis after prolonged immobilization can rarely contribute to the development of mild hypercalcemia because weight bearing is necessary to maintain the balance between new bone formation and resorption of old bone. Serum total hypercalcemia has been noted in a small percentage of hyperthyroid cats,28,511 but iCa concentration is normal. In cats with untreated hyperthyroidism, mild ionized hypercalcemia that resolved following conversion to euthyroidism with treatment has been uncommonly noted (Chew, unpublished observations). Overuse of calcium-containing intestinal phosphate binders can occasionally cause hypercalcemia.111 An unusual case of hypercalcemia was attributed to the chronic ingestion of calcium carbonate in the form of limestone rocks.296 Malignant histiocytosis in dogs was reported in association with hypercalcemia in one dog.586
The ingestion of large amounts of grapes or raisins may result in hypercalcemia. Seven of 10 dogs with renal failure associated with grape or raisin ingestion had increased serum tCa concentrations (12.3 to 26 mg/dL) and increased serum phosphorus (6.4 to 22 mg/dL) 24 hours to several days following ingestion.230 In four dogs, ingestion was estimated to be from 0.41 to 1.1 ounces of grapes or raisins per kilogram of body weight. Oliguria or anuria was noted in 5 of 10 dogs, and 5 of 10 dogs survived. These cases were clustered from 1999 to 2001, and raisin/grape toxicity had not been previously reported.
Vomiting following ingestion of what appears to be a trivial quantity of raisins or grapes in some dogs leads to the development of AIRF usually within 48 hours. Not all dogs that consume grapes or raisins develop clinical signs or acute renal failure. Of 132 dogs reported with raisin or grape ingestion, 33 developed no clinical signs or azotemia, and 14 of 133 dogs developed clinical signs but no azotemia.169 Of 132 cases, 43 dogs developed clinical signs and AIRF. The pathogenesis of nephrotoxicity associated with raisins and grapes remains unknown, but it is speculated that ochratoxin may be a toxic component.449 Tubular degeneration and necrosis of varying severity are consistently described and most pronounced in proximal tubules.169,394
In some cases of grape or raisin ingestion with AIRF, mild to severe hypercalcemia develops, and in some dogs, serum tCa concentration can change dramatically from day to day during various treatments.363 With acute renal failure following ingestion of raisins or grapes, hypercalcemia was detected in 93% of affected dogs, and tCa ranged from 8 to 26 mg/dL.169,230 Of 40 dogs, 23 (57.5%) survived, and 17 (42.5%) failed to survive; 15 of 23 underwent complete resolution of azotemia. Initial and peak serum tCa concentrations and initial and peak calcium X phosphorus products were significantly higher in those that did not survive as compared with those that did survive. Hypercalcemia was documented in 1 of 3 dogs evaluated within 24 hours of ingestion, in 2 of 8 dogs within 24 to 48 hours, and in 12 of 13 dogs evaluated for the first time 48 to 72 hours after ingestion. Total calcium concentration returned to the normal range in a median of 11 days (range, 2 to 51 days). Unfortunately, iCa measurements have yet to be reported for any dogs with raisin toxicity, AIRF, and hypercalcemia based on serum tCa. Because many dogs with severe AIRF have hyperphosphatemia, some of the increased serum tCa may be because of complex formation with phosphate. The observation that serum tCa concentration can dramatically increase or decrease daily during treatment suggests that its origin is related to extracellular or intravascular fluid volume dynamics.
A favorable outcome is possible in about 50% of cases, but several weeks of hospitalization with intensive fluid treatment is often needed in those with AIRF, especially if oliguric. About 50% of affected dogs can be expected to develop oliguria or anuria.169,230,363 A case of AIRF with a fatal outcome occurred after ingestion of 450 grams of raisins in a vizsla dog despite intensive treatment, including peritoneal dialysis.437 Aggressive treatment has been recommended for any dogs suspected of having ingested large, or even small, quantities of grapes or raisins, including induction of emesis, gastric lavage, and administration of activated charcoal, followed by intravenous fluid therapy for a minimum of 48 hours.230 However, some dogs may consume relatively large quantities of grapes or raisins without development of ill effects.
Hypercalcemia was reported in a dog with a retained fetus and endometritis.248 Serum PTH was suppressed, and 25-hydroxyvitamin D concentration was within the normal range. Biopsy of the removed uterus documented neutrophilic inflammation but no granulomatous inflammation as a possible cause of the hypercalcemia. Serum iCa was normal 4 days after surgical removal of the uterus, and serum tCa was normal 6 weeks later.
Humoral hypercalcemia of benignancy is a phrase used to describe the association of humoral factors such as PTHrP and hypercalcemia in the absence of malignancy.197,302 One dog with massive mammary gland hyperplasia, severe ionized hypercalcemia, and increased PTHrP in the absence of malignancy at necropsy has been observed (Chew, unpublished observations). This phenomenon has rarely been described in humans.278,292
Treatment of hypercalcemia
Philosophy of Treatment
There is no absolute serum calcium concentration that can be used as a guideline for the decision to treat hypercalcemia aggressively.113,184 The magnitude of hypercalcemia, its rate of development, whether the serum calcium concentration is stable or progressively increasing, and the modifying effects of other electrolyte and acid-base disturbances must all be considered when deciding on a treatment plan. The clinical condition of the animal ultimately dictates how aggressive treatment should be, but a serum calcium concentration of 16 mg/dL or greater has been recommended as a basis for aggressive therapy.184 Animals with serum calcium concentrations approaching 20 mg/dL should be considered candidates for crisis management. Animals with serum calcium concentrations less than 16 mg/dL may also require aggressive treatment, depending on the degrees of neurologic, cardiac, and renal dysfunction induced by the hypercalcemia and concurrent deleterious factors. Acidosis can magnify the effects of hypercalcemia at all serum calcium concentrations by shifting more calcium to the ionized fraction. The serum phosphorus concentration at the time of hypercalcemia is also an important modulating factor in clinical decision making because soft tissue mineralization is potentiated by hyperphosphatemia. Animals with rapid and progressive development of hypercalcemia usually display serious clinical signs that require aggressive therapy.
Definitive Therapy
Removal of the underlying cause is the definitive treatment for hypercalcemia. Most animals with pathologic hypercalcemia have an associated malignancy that is quickly diagnosed but often not readily treated. Complete excision of isolated neoplasms (e.g., apocrine gland adenocarcinoma of the anal sac and parathyroid gland adenoma) abolishes hypercalcemia. In animals with disseminated metastases, multicentric neoplasia, or nonresectable primary malignancy, the tumor burden and hypercalcemia may be decreased by appropriate chemotherapy, radiation therapy, and immunotherapy. Chemotherapy may disrupt neoplastic cellular metabolism to such an extent that the tumor may no longer be able to synthesize enough humoral factors to sustain hypercalcemia. Decreased serum calcium concentrations can occur despite a lack of obvious reduction in tumor size in these instances.
Antifungal treatment with amphotericin B, ketoconazole, or itraconazole effectively lowers increased serum calcium concentrations in dogs with systemic mycoses as the infectious agent is eradicated and inflammation resolves. For animals with hypercalcemia associated with hypoadrenocorticism, replacement therapy with mineralocorticoids and glucocorticoids after fluid volume replacement definitively manages the condition. Discontinuing all vitamin D supplementation in animals with hypervitaminosis D and hypercalcemia removes the external cause of intoxication, but excessive body stores of vitamin D may continue to contribute to hypercalcemia for several weeks.
Supportive Therapy
Supportive therapy is often necessary to decrease serum calcium concentration to a less toxic level while waiting for a definitive diagnosis to be established, for definitive treatment to reduce serum calcium concentration permanently, or for chronic management of hypercalcemia when the underlying cause cannot be removed. Box 6-3 and Table 6-3 list the general and specific treatments for the management of hypercalcemia. Unfortunately, no single treatment protocol is consistently effective for all causes of hypercalcemia. Consequently, regimens must be tailored for the individual patient. Supportive treatments reduce the magnitude of hypercalcemia by increasing renal calcium excretion, inhibiting bone resorption, promoting soft tissue deposition of calcium, causing a shift of intravascular calcium to other body compartments, promoting extrarenal calcium loss, reducing calcium transport across the gut, or some combination of these effects.113,315,354
Initial Considerations for Treatment
Parenteral fluids, furosemide, sodium bicarbonate, glucocorticoids, or combinations of these treatments effectively reduce serum calcium concentrations in most animals. Repeatable serum hypercalcemia should be confirmed before prescribing aggressive treatments. It is not necessary to reduce serum calcium concentration to within normal limits, but substantial resolution of serious clinical signs may occur when serum tCa concentration decreases by as little as 1 to 3 mg/dL.
Fluid Therapy
Parenteral fluid therapy is an important first treatment for all animals with hypercalcemia. The first goal of fluid therapy is to correct dehydration because hemoconcentration contributes to increased serum calcium concentration. In addition, the kidneys respond during ECF volume contraction with more avid reabsorption of sodium and calcium from the glomerular ultrafiltrate. Correction of dehydration abrogates this effect and allows calciuresis and natriuresis to occur.
Dehydration should be corrected with intravenous fluids within 4 to 6 hours of presentation in animals with severe clinical signs attributable to hypercalcemia. Additional expansion of ECF volume with parenteral fluids is then indicated, but sufficient fluid for rehydration and volume expansion is often provided simultaneously. Fluid therapy alone may be sufficient in some animals to reduce the magnitude of hypercalcemia adequately when the initial serum calcium concentration is less than 14 mg/dL, but often other treatments must be added. Normocalcemia may be restored by fluid therapy alone if hypercalcemia was initially mild (12 to 13 mg/dL).
Physiologic saline (0.9% NaCl) is the solution of choice for correction of the intravascular volume deficit and for further slight volume expansion. Slight volume expansion with 0.9% NaCl promotes calcium loss in urine secondary to increased GFR and increased filtered load of calcium, and competition from the additional sodium ions results in reduced renal tubular calcium reabsorption and enhanced calciuresis. Eleven dogs with hypercalcemia (most with malignancy and one each with primary hyperparathyroidism and hypervitaminosis D) were treated for 24 hours with IV 0.9% NaCl at 60 mL/kg. A decrease in serum tCa was observed in 7 of 11 dogs (1.3 mg/dL mean; 0.5 to 2.8 mg/dL), no change in 1 of 11, and an increase in 3 out of 11 (0.6 mg/dL mean; 0.1 to 0.7 mg/dL). Overall there was a decrease in mean serum tCa of 0.7 mg/dL, but tCa concentration did not return to the reference range in any dog.487
ECF volume expansion with lactated Ringer’s solution (6 mg/dL calcium) in dogs results in decreased total protein, tCa, and iCa concentrations. Decreases in tCa concentration were greater (12.4%) than those observed for iCa concentration (3.5%).468 Thus, volume expansion with solutions that contain some calcium can be beneficial because the dilutional effect supersedes the effect of the additional calcium that is administered. However, physiologic saline (0.9% NaCl) is preferred because it is devoid of additional calcium and contains more sodium than that in lactated Ringer’s solution (154 versus 130 mEq/L). Consequently, 0.9% NaCl results in a more rapid reduction in serum calcium concentration. An initial fluid volume of two to three times maintenance needs (120 to 180 mL/kg/day) usually corrects dehydration, provides maintenance needs, and results in mild volume expansion. The use of sodium phosphate is not recommended because of the potential detrimental effects of soft tissue mineralization.184
Diuretics (Calciuretics)
Administration of furosemide follows rehydration and fluid volume expansion as second in importance for treatment of persistent hypercalcemia. Furosemide promotes enhanced urinary calcium loss, but calciuresis does not follow the use of all diuretics. In particular, thiazides should not be used because they may result in hypocalciuria and potentially may aggravate hypercalcemia. Pivotal work was performed in dogs that provide the basis for this therapy.422 An acute model of hypercalcemia was created in dogs following administration of high doses of cholecalciferol (vitamin D3) and calcium chloride added to the food until the target range for serum tCa of 13 to 15 mg/dL was achieved. Furosemide was given at 5 mg/kg IV as a bolus, followed by a constant rate infusion (CRI) of 5 mg/kg/hr for the next hour, and IV fluids were replaced based on measurement of “ins and outs.” Over baseline, mean urinary output increased by fortyfold, urinary sodium excretion by 200-fold, urinary calcium excretion by sevenfold, and the GFR by 1.4-fold. Mean serum tCa decreased by 2.7 mg/dL (14.3 mg/dL to 11.3 mg/dL; a 19.3% decrease), and serum total magnesium decreased from a mean of 1.56 mg/dL to 1.07 mg/dL. In a model of chronic treatment of hypercalcemia in dogs, hypercalcemia was created by twice daily SQ injections of PTH to achieve a target serum tCa of 13 to 15 mg/dL. Furosemide at 2.5 to 15 mg/kg was given IM twice daily for 4 to 7 days, and saline was added to the diet to match the volume of urinary loss. Diuresis, natriuresis, and calciuresis followed furosemide treatment, but there was no change in serum tCa. Calciuresis was eight times greater with IV furosemide in the acute treatment model compared with the chronic treatment model.
Furosemide IV was compared for its effect in normal adult Greyhounds by bolus or CRI.6 The same total dose was given with intermittent bolus furosemide IV at 3 mg/kg at 0 and 4 hours and at 0.66 mg/kg loading dose followed by 0.66 mg/kg/hr over 8 hours. Urine sodium and calcium losses were greater and urinary potassium loss less when furosemide was given by CRI compared with intermittent bolus. Cacliuresis, based on total milligrams of calcium excreted, was 1.6 times greater when furosemide was given by CRI compared with an intermittent bolus. Unfortunately, serum calcium was not measured because of technical errors during the study. CRI may be safer than intermittent bolus treatments with furosemide due to more continuous delivery of furosemide to nephrons with less variability in serum and tubular drug concentrations achieved. Restlessness was observed in Greyhounds receiving the CRI of furosemide but not for those with the intermittent boluses. This observation has not been made before in humans, dogs, or horses. Whether this is specific to Greyhounds or occurs in other breeds of dogs is not known.
It is important to maintain hydration at all times. Proper hydration ensures more delivery of furosemide to the proximal tubules where it must be secreted in order to exert its subsequent effect in the ascending limb of the loop of Henle. Large magnitude diuresis occurs with the bolus followed by high-dose CRI furosemide methods. It can be challenging to keep up with this magnitude of diuresis and adequately replace the volume loss with enough IV fluids. Ongoing dehydration results in worsening of the hypercalcemia from contraction of the ECFV and may pose a risk for ischemic renal injury. Lower dose regimens are most often employed by clinicians at 1 mg/kg followed by 1 mg/kg/hour CRI furosemide. Alternatively, furosemide at 2 to 4 mg/kg two to three times daily can be given IV or IM, but this is less effective in lowering serum calcium. Serum magnesium can decrease substantially during aggressive furosemide induced diuresis, so this should be measured and replacement salts given in IV fluids as needed.
Sodium Bicarbonate
Infusion of sodium bicarbonate has been advocated for acute or crisis management of hypercalcemia, but most often it is mentioned for use in the presence of metabolic acidosis.6,113,314 Serum iCa concentration is reduced as acidosis is corrected or mild alkalosis is created because more calcium becomes bound to serum proteins, and there is increased binding of calcium to bicarbonate.468 Decreases in ionized and tCa concentrations after bicarbonate infusions have been observed in dogs380 and cats.114 Based on studies in normal cats, a slow IV bolus of sodium bicarbonate from 0.5 to 4.0 mEq/kg114,314 results in a 5% to 12% decrease in ionized calcium (0.2 to 0.7 mg/dL). This effect is dose dependent and lasts 120 to 180 minutes. A small component of this effect is from decreases in serum albumin and serum proteins, as well as increases in serum or plasma pH, which change the number or affinity of calcium binding sites. Some of this effect may also be from increased binding to circulating complexes (HCO3).114 Too much alkalinity can promote tissue mineralization during the presence of hypercalcemia, so this treatment is given to effect for only a very short while. Reduction in serum calcium concentration is slight after administration of sodium bicarbonate alone, but the effect increases with larger doses. Sodium bicarbonate infusion is most likely to be helpful in combination with other treatments.