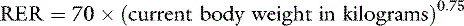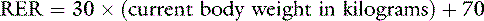Chapter 25 Parenteral Nutrition
Historical view of parenteral nutrition
Parenteral nutrition has been used routinely in human patients since the late 1960s. However, the use of parenteral nutrition goes as far back as 1656 when Sir Christopher Wren infused wine and beer into dogs using a goose quill and pig bladder.71 Although there were sporadic reports of the use of intravenous nutrition, such as the intravenous infusion of saline and milk into human cholera patients in 1832, it was not until the mid-1900s that physicians began conducting more organized experiments in providing nutrients via the intravenous route.71 Elman began administering protein hydrolysates and glucose via peripheral veins in the late 1930s and in 1947 published a book on parenteral alimentation in surgery.38 Meng and Early began using lipid emulsions in dogs in the 1940s and published an article on parenteral nutrition in dogs in 1949.38 However, it was in 1968 that physicians from the University of Pennsylvania published the seminal article on parenteral nutrition in dogs.25 These authors fed six male beagle puppies beginning at 12 weeks of age for a total of 72 to 256 days.25 The puppies were compared with their littermates that were fed orally during this same time period. The parenterally fed puppies grew at a faster rate and were larger at the end of the study compared with their littermates.25 In the same publication, Dudrick et al25 also reported on the results of feeding 30 human patients via total parenteral nutrition (TPN) for 10 to 200 days.
The Dudrick article became the start of a new era in nutritional support for hospitalized patients. After the recognition that people (and dogs) could be fed successfully for relatively long periods via the intravenous route, physicians and researchers proceeded to develop better ways to accomplish this goal. Two issues that were addressed early were developing better methods of central venous access and the formulation of parenteral nutrient admixtures. Once these techniques were further developed, a great deal of attention began to be focused on malnutrition (i.e., its prevalence, its detrimental effects, and methods for preventing and treating it). In 1976, Butterworth was the first to report an association between the mortality and morbidity associated with malnutrition in hospitalized patients.11 Soon it became widely recognized that hospital malnutrition was a major problem, which led to the concept of nutritional assessment and its role in overall patient management. In addition, clinical trials were conducted testing the benefits, complications, and timing of parenteral nutrition. In many studies, it has not been shown that routine use of perioperative TPN is justified, and often it is associated with more complications compared with enteral nutrition or no nutrition at all.8,24,29,32,70 However, certain patient populations, such as the malnourished, do appear to benefit from parenteral nutrition.8,32,70 More recently in human medicine, meta-analyses on parenteral nutrition have been conducted to determine specific patient populations in which parenteral nutrition would be most beneficial.8,29,32,64 Currently, research in parenteral nutrition in human medicine is focusing on optimizing the selection of patients who will benefit from parenteral nutrition and improved formulations, such as modified lipid and amino acid solutions.
Although dogs often have been used as models for the study of parenteral nutrition, the clinical use of parenteral nutrition in companion animal species is a relatively new modality of therapy. The first clinical report of the use of parenteral nutrition in companion animals was in 1977.12 In 1989, Lippert et al41 reported the use of TPN in seven normal cats for 14 days. In this study, cats were fed at maintenance energy requirements (MERs; calculated in that study as 1.4 × resting energy requirements [RERs]).41 However, in one group, the calories provided by protein were included in the calculations, whereas in the other group, protein calories were not included.41 Thus the latter group actually received calories in excess of MER.41 The cats that were fed more than MER developed vomiting, oral ulcerations, and hyperglycemia.41 However, all cats developed anemia, thrombocytopenia, hypertriglyceridemia, villous atrophy, and hepatocellular changes.41
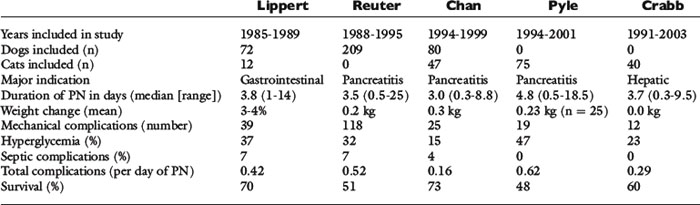
In 1993, Lippert et al43 published the first retrospective study on the use of TPN in clinical patients. This study reported the results of the use of TPN in 72 dogs and 12 cats.43 The median duration of TPN was 3.8 days, with a range of 1 to 14 days.43 Thirty-nine mechanical complications occurred, and metabolic complications were common, including hyperglycemia (37%), electrolyte abnormalities (30%), and hyperlipidemia (23%).43 Seven percent of animals developed sepsis (i.e., clinical signs of sepsis in combination with either a positive catheter tip culture or positive blood culture).43 In this study, both total calories and protein were administered at a higher level than is currently done at most institutions.43
The next major retrospective study was published in 1998 and reported the results of the use of TPN in 209 dogs.62 Similar to the Lippert study, the median duration of administration was 3.5 days (range, 0.5 to 25 days).62 One hundred-eighteen mechanical complications occurred, and 37% of the dogs had at least one mechanical complication.62 Hyperglycemia was the most common metabolic complication, with 32% of dogs evaluated developing this abnormality, but 329 individual metabolic complications occurred in this population of dogs.62 Seven percent of the animals developed sepsis.62 The overall complication rate was 0.52 complications per day of TPN.62
Two retrospective studies on the use of TPN specifically in cats also have been published.21,58 The median duration of TPN administration in these studies was 4.8 and 3.7 days, respectively.21,58 Although the number of mechanical and septic complications was similar between the two studies, 18% of cats in the Crabb et al study became hyperglycemic, compared with 47% in the larger Pyle et al study.21,58 The overall complication rate was 0.62 per day of TPN in the Pyle study and 0.29 in the Crabb study.21,58 There also were two interesting findings regarding hyperglycemia from these two studies.21,58 First, the study from Pyle et al showed that hyperglycemia was significantly associated with an increased mortality rate 24 hours after starting TPN.58 In this study, cats’ energy requirements were calculated by multiplying the resting energy requirements (RER) by an illness factor. In the Crabb et al study, cats in which the RER was multiplied by an illness factor were more likely to develop hyperglycemia than those in which energy requirements were provided at or below RER.21
Two studies have been published on partial parenteral nutrition (PPN) in clinical veterinary patients. One used a commercial three-in-one solution containing dextrose amino acids and lipid that provided 1.26 kcal/kg/hr for between 10 to 24 hr/day (n = 9) for a median of 36 hours.20 In this study, venous thrombus or thrombophlebitis was the most common cause of catheter failure.20 In a retrospective study of three-in-one PPN in 80 dogs and 47 cats, the median duration of PPN administration was 3.0 days.14 As in the previous studies, hyperglycemia was the main metabolic complication (15% of animals overall).14 Twenty-four other metabolic complications also occurred, and four septic cases were documented (3%).14 The complication rate was 0.18 and 0.15 complications per day of PPN for dogs and cats, respectively.14 One notable feature of this study is that animals that received some enteral nutrition in combination with PPN administration were more likely to survive compared with animals not receiving any enteral nutrition.14
To date, these are the most comprehensive published studies on results of parenteral nutrition use in clinical patients (see Table 25-1 for a summary of these studies). However, there are several other studies that have been helpful in enhancing the use of parenteral nutrition in dogs. The first is a study by Chandler et al19 in which an amino acid solution, a dextrose solution, and an electrolyte solution were administered to compare their effects on nitrogen balance. These solutions were individually administered to three healthy dogs via a peripheral vein for 10 hr/day for 4 days.19 Only the amino acid solution resulted in a positive nitrogen balance, suggesting that in healthy dogs it provided adequate amino acids to prevent breakdown of lean body mass.19 In 2001, Mauldin et al49 reported a study evaluating parenteral nutrition in healthy dogs. In this study, dogs received intravenous infusions of either nonlactated Ringer’s solution or isocaloric solutions containing 0, 1.36, or 2.04 g of amino acids per kilogram of body weight per day, with the remaining calories (to meet MER) provided by dextrose and lipid solutions for 12 hr/day for 7 days.49 On Ringer’s and 0 g/kg amino acids, dogs had negative nitrogen balance, and a regression analysis suggested that intravenous administration of 2.32 g/kg/day of amino acids would result in zero nitrogen balance in a healthy dog of beagle size (i.e., the minimum amount required to prevent catabolism of lean body mass by supplying basal amino acid requirements).49 Zentek et al published a study of parenteral nutrition in healthy laboratory dogs using a three-in-one solution with the majority of calories either from glucose or lipid.73 In this study, PPN was administered cyclically (over 10 hr/day) to meet MER.73 Depending on the formula (high glucose or high lipid), dogs had significant increases in blood glucose and triglycerides, respectively, but there were no significant differences in hormonal concentrations (e.g., insulin-like growth factor-1, insulin, glucagon, T3, T4).73 An in vitro study also was recently published assessing physical effects on the stability of lipid-based parenteral nutrition solutions.66 This is important information as factors such as temperature and handling can affect the nutrition and physical stability of parenteral nutrition solutions. This increasing number of studies has been helpful in better understanding the metabolism of parenteral amino acids in companion animals and will serve as a foundation on which to base future research into the specific requirements of ill and injured animals.
Rationale for nutritional support in hospitalized animals
Ill and injured animals undergo metabolic changes that put them at high risk for malnutrition and its subsequent complications. In a healthy animal that receives insufficient calories to meet its needs, the body compensates for this calorie deficit in the short term by first using hepatic glycogen and then by mobilizing amino acids from muscle. Glycogen stores are rapidly depleted, particularly in carnivores such as cats. Although these processes can provide needed energy, they are inefficient energy sources; therefore after several days, the healthy animal adapts by decreasing protein turnover and preferentially using fat. By this process, a healthy animal can survive for a long period without food, provided that adequate water is available. In the ill or injured animal, however, this normal adaptive response to a calorie deficit does not occur, primarily as a result of alterations in the cytokine and hormonal milieu that are associated with the catabolic response. Thus these animals continue to mobilize protein, perpetuating the loss of lean body mass.
The problem with this continued loss of lean body mass is that all of the body’s protein is functional tissue, as compared with fat and carbohydrate, both of which have storage depots. In addition, loss of lean body mass negatively impacts wound healing, immune function, strength (both skeletal and respiratory muscle), and ultimately prognosis. Although it has not been demonstrated in companion animals, hospitalized people with weight loss have a worse outcome than those without. A loss of lean body mass in an ill or injured animal will occur, to a certain degree, even if the animal is provided with adequate calories. However, appropriate nutritional support can minimize the amount of lean body mass lost and the sequela of this loss. Therefore the goal of nutritional support in the hospitalized animal should be not only treatment of those that are already malnourished but also minimizing the development of malnutrition in animals at risk.
Patient selection
Any route of nutritional support carries some risk of complications, and parenteral nutrition is not an exception. Studies in people have shown that parenteral nutrition in some patient populations actually increases the risk of complications and worsens the outcome.* Therefore careful patient selection is particularly important in the case of parenteral nutrition. Ideally, one would select only those patients that would benefit from parenteral nutrition, but the appropriate selection criteria are not yet known in people or in companion animals. Most companion animals receive parenteral nutrition for relatively short periods (median, 3 to 4 days), and one must determine whether short-term provision of parenteral nutrition is likely to be beneficial. Occasionally, parenteral nutrition is administered for more prolonged periods, and as always the risk/benefit ratio must be considered. In a previously healthy dog that has been anorectic for 2 to 3 days and in which oral or enteral intake is likely to resume quickly, parenteral nutrition may not be beneficial. However, in a vomiting cat that has not eaten for 1 week at home and is not expected to be eating again soon, parenteral nutrition would be indicated.
The indications for parenteral nutritional support are situations in which an animal cannot voluntarily consume adequate calories and cannot tolerate enteral nutrition. The specific indications for parenteral nutrition are shown in Box 25-1. Proper patient selection is an important aspect of nutritional assessment because administration of parenteral nutritional support to patients unlikely to benefit from this form of nutrition only subjects them to risk of complications.
Nutritional assessment
In critically ill animals, nutrition often is not considered to be a priority during the early phases of resuscitation, stabilization, and diagnostic testing. However, this population is at high risk for developing malnutrition, and identification of animals that are already malnourished or those that are at high risk for becoming malnourished should be of high importance. Being aware of an animal’s nutritional status at admission and of changes that occur during hospitalization will optimize patient care.
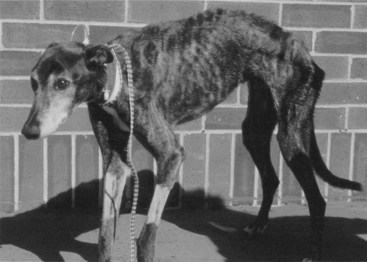
It is easy to recognize the classical picture of the starved patient as being malnourished (Figure 25-1). However, many of our patients have more subtle signs of malnutrition or develop malnutrition while hospitalized because the risk for malnutrition was not recognized early enough to prevent it (Figure 25-2, A). Even obese animals are at risk for malnutrition (Figure 25-2, B) because if they lose weight, they will lose lean body mass rather than fat. Assessment of nutritional status should be incorporated into the daily examination of each patient. Nutritional assessment identifies malnourished patients that require nutritional support and also identifies patients at risk for malnutrition in which nutritional support will help prevent malnutrition.15,53
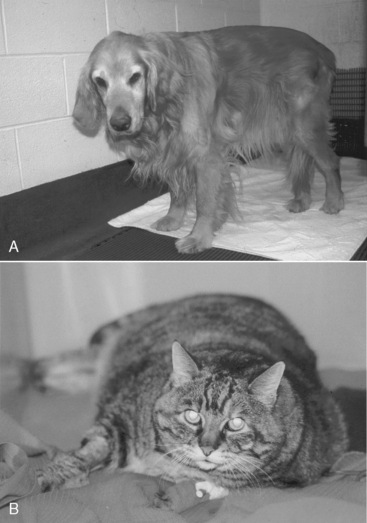
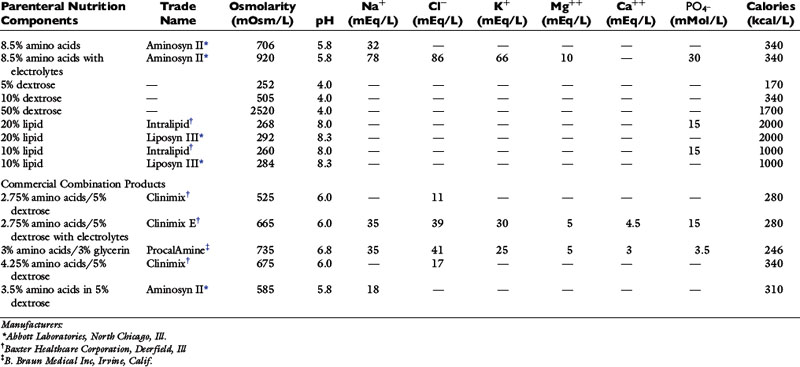
Figure 25-2 A, Malnutrition can be subtle in the early stages. This dog has been eating reduced amounts of food for 1 week as a result of chronic renal failure. It is not obviously thin but is already exhibiting muscle loss. Appropriate nutritional support can help to minimize further losses. B, Even an obese animal can become quickly malnourished in the hospital when ill or injured. If insufficient calories are supplied, the cat will lose weight but it will be functional lean body mass, rather than fat, that is lost.
For many years, investigators have attempted to develop a single measurement or group of measurements that will identify malnutrition in humans. Unfortunately, few of these have worked well on a clinical basis. Therefore, most nutritionists in human and veterinary medicine use a subjective global clinical assessment to identify patients in need of nutritional support (Box 25-2). This assessment includes historical information (e.g., duration of clinical signs, history of anorexia or weight loss), clinical parameters (e.g., underlying disease, degree of weight and/or muscle loss, severity of illness, clinical signs, anticipated course of recovery), and laboratory results. Any clinical or laboratory findings that would specifically alter the nutritional plan should be carefully considered. Examples include the presence of congestive heart failure (which would necessitate careful attention to fluid volume), electrolyte abnormalities, hyperglycemia, hypertriglyceridemia, or hepatic encephalopathy. These factors then are incorporated into an overall assessment of the degree of malnutrition or the animal’s risk for developing malnutrition. Prevention (or correction) of nutritional deficiencies and imbalances then can be accomplished by providing adequate energy substrates, protein, and micronutrients.
BOX 25-2 Indicators of Malnutrition in Dogs and Cats
* These laboratory abnormalities are not specific to malnutrition and generally are not present early in the process of developing malnutrition.
The authors categorize hospitalized animals into three groups: (1) those that are already malnourished (see Figure 25-1); (2) those that are not malnourished but are at high risk for developing malnutrition (Figure 25-3); and (3) those that are not malnourished and are at low risk for developing malnutrition (Figure 25-4). Animals in the first group require prompt nutritional support. Animals in the second group require nutritional support in the first 2 to 3 days of hospitalization, or at the time of anesthetizing them for diagnostic or therapeutic procedures, a feeding tube should be placed. Factors that put an animal at high risk for malnutrition include anorexia lasting longer than 3 days (be sure to include the time the animal has been anorectic at home before admission to the hospital), serious underlying disease (e.g., trauma, sepsis, peritonitis, pancreatitis, extensive gastrointestinal surgery), and large protein losses (e.g., protracted vomiting or diarrhea, open abdomen, or large draining wounds). Animals in the third group do not require immediate nutritional support and can be monitored to ensure adequate food intake. However, if the underlying disease does not resolve quickly or the animal continues to be anorectic, nutritional support may be required. Indicators of malnutrition are listed in Box 25-2.
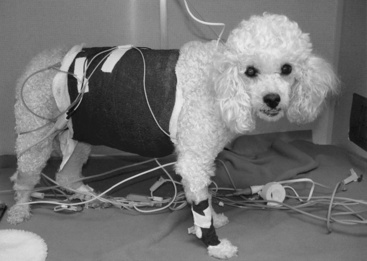
Figure 25-3 This dog is anorectic and is being treated with an open abdomen for septic peritonitis. Although not yet malnourished, it is at high risk for becoming so because of the lack of nutrient intake and the large protein losses via the abdomen.

Figure 25-4 A cat with asthma that is not malnourished and is at low risk for becoming so. This cat does not require immediate nutritional support and can be monitored to ensure adequate food intake. However, if the underlying disease does not resolve quickly or the animal continues to be anorectic, nutritional support may be required.
Route of nutritional support
Most clinicians have heard the adage, “If the gut works, use it.” This approach still holds true, and parenteral nutrition should not be the first consideration in an ill or injured animal. The suitability of the enteral route should always be addressed first because it is the safest, most convenient, most physiologically sound, and least expensive method of nutritional support (see Chapter 26.26 If only parts of the gastrointestinal tract are functional, consideration should be given to using those functional segments. For example, a dog or cat with severe esophagitis should be considered a candidate for a jejunostomy feeding tube. However, when patients are unable to tolerate any enteral feeding, parenteral nutrition should be considered. Before parenteral nutrition is instituted, however, it is critical that fluid, electrolyte, and acid-base abnormalities are corrected.
Parenteral nutrition
Parenteral nutrition can be delivered via a central vein (TPN) or a peripheral vein (PPN). TPN, as defined in this chapter, is the provision of all of the animal’s calorie and protein requirements (and ideally, all of the micronutrient requirements as well; see Other Nutrient Requirements section). PPN only supplies part of the animal’s energy, protein, and other nutrient requirements.74 In this chapter, we use the abbreviation PPN to refer to partial parenteral nutrition, which can be supplied through either a peripheral or central vein.
Because TPN will supply all of the animal’s calorie and protein requirements, it is usually the modality of choice for an animal requiring parenteral nutrition. The disadvantages are that it requires a jugular venous catheter and it may be associated with more metabolic complications. PPN may be an alternative to TPN in selected cases (Box 25-3), but it is important to be aware that it will not provide all of the animal’s requirements. Both TPN and PPN are typically a combination of dextrose, an amino acid solution, and a lipid solution. However, the concentration of some components (e.g., dextrose) varies depending on whether TPN or PPN is chosen. TPN or PPN can also be formulated with amino acids and dextrose alone, without any lipid.9Table 25-2 compares a PPN and a TPN admixture for a 20-kg dog.
BOX 25-3 Indications for Partial Parenteral Nutrition (PPN; i.e., providing less than the animal’s total calorie, protein, and micronutrient requirements)
• To maintain nutritional status, rather than replete the malnourished patient. Debilitated patients should get total parenteral nutrition (TPN) or a combination of PPN and enteral nutrition.
• Animals with average nutritional requirements. Those with high requirements (e.g., open abdomen, large draining wound, severe vomiting or diarrhea) should receive TPN.
• When only short-term nutritional support in a nondebilitated patient is anticipated (<5 days).
• To supplement oral or enteral nutrition.*
* Whenever possible, enteral nutrition should be used to supplement parenteral nutrition (even if provided at a very low rate) to prevent atrophy of the intestinal tract.
Table 25-2 Parenteral Nutrition (PPN) and Total Parenteral Nutrition (TPN) Formulations for 20-kg Dog with Acute Pancreatitis
| PPN | TPN | |
|---|---|---|
| 5% dextrose (mL) | 900 | — |
| 50% dextrose (mL) | — | 164 |
| 8.5% amino acids (mL) | 450 | 312 |
| 20% lipid (mL) | 77 | 139 |
| Total mL/day | 1427 | 615 |
| PPN mL/hr | 60 | 26 |
| Maintenance fluid rate (mL/hr) | 55 | 55 |
Formulation of parenteral nutrition requirements
Calorie Requirements
When formulating parenteral nutrition, the first step is to determine the animal’s calorie requirements. The patient’s RER is the number of calories required for maintaining homeostasis while the animal rests quietly. The RER is calculated using the formula:
This exponential equation will more accurately estimate the animal’s true requirements across all body weights. However, for animals weighing between 3 and 25 kg, the following linear formula gives a reasonable approximation of energy needs:
One should avoid using the linear equation for animals smaller than 3 kg or larger than 25 kg because the linear equation will underestimate or overestimate these animals’ energy requirements, respectively (Figure 25-5).
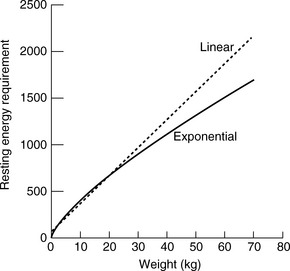
Figure 25-5 Comparison of resting energy requirements (RERs) as calculated using a linear equation [(30 × body weight) + 70] versus an exponential equation [70(body weight)0.75]. Note that the equations yield similar results for animals weighing between 3 and 25 kg. For animals that weigh more than 25 kg, the linear equation overestimates the animal’s RER.
For animals that are underweight, the authors recommend using the animal’s current weight for the RER calculation. The goal of parenteral nutrition should not be weight gain, which can be achieved after the animal’s underlying disease has been treated and the animal is able to tolerate enteral or oral feedings. Overfeeding for the animal’s current weight also increases the risk for metabolic complications (see Complications section). For animals that are overweight, one should feed an appropriate number of calories to prevent weight loss because seriously ill or injured animals lose lean body mass rather than fat. There are a number of ways to calculate parenteral nutrition requirements in markedly overweight animals (i.e., >25% above ideal body weight). One is to use the animal’s current body weight for the RER calculation while carefully monitoring body weight to ensure that the animal does not lose or gain weight. Another option is to use the assumption that 25% of excess weight is lean tissue and the remaining 75% is metabolically inactive fat (i.e., if a dog’s ideal weight is 20 kg and it weighs 30 kg, it has 10 kg of excess weight, 2.5 kg of which is lean tissue and 7.5 kg of which is fat). Therefore one can take the ideal weight plus 25% of the excess weight (to account for the extra lean body mass) as the weight to use for calculation of RER. Using the 30-kg dog and ideal weight of 20 kg from the example above, the adjusted body weight to use for calculation of RER would be 20 kg + (25% × 10 kg) or 20 kg + 2.5 kg = 22.5 kg. Thus the RER for this overweight dog would be 723 kcal/day.
In the past, the RER was multiplied by an illness factor between 1.0 and 2.0 to account for increases in metabolism associated with different conditions and injuries.1,10,42 Recently, there has been less emphasis on these subjective illness factors, and current recommendations are to use more conservative energy estimates to avoid overfeeding.15,28 Overfeeding can result in metabolic and gastrointestinal complications, hepatic dysfunction, and increased carbon dioxide production.5,6 We have shown in cats receiving TPN that those in which the RER was multiplied by an illness factor were more likely to develop hyperglycemia than those in which energy requirements were provided at or below RER.21 Critically ill cats, in particular, are at high risk for hyperglycemia due to a variety of hormonal alterations that are similar to those identified in critically ill people.17 Avoiding the development of hyperglycemia has been shown to be beneficial in certain populations of critically ill people.27,69 Newer recommendations for nutritional support in critically ill people emphasize the need to avoid overfeeding and reduce the risk of hyperglyceamia.40,47,60 In cats16,69 and in dogs,67 hyperglycemia is associated with reduced survival and longer hospitalization times. To reduce the risk of hyperglycemia and other complications, the authors use RER as an initial estimate of a critically ill patient’s energy requirements. Further adjustments are made based on the animal’s response to feeding, body weight, and changes in the underlying condition. Indirect calorimetry can accurately assess the caloric needs of individual patients, but it is rather cumbersome to use in a clinical setting. This technique may be more commonly used in the future, particularly for patients that are difficult to manage on nutritional support. Studies using indirect calorimetry support the hypothesis that the application of illness factors in calculating energy expenditure in clinical patients overestimates energy needs.57,72 At the current time, the key to successful nutritional support is vigilant monitoring after therapy has been initiated to ensure that provision of calories is adjusted as necessary.
Other Nutrient Requirements
After calorie requirements are determined, one must also address protein requirements. Animals require a nitrogen source and essential amino acids. These are typically provided parenterally by an amino acid solution. Essential fatty acids (linoleic acid in the dog, linoleic and arachidonic acids in the cat) are also required. These are provided by a lipid emulsion; however, fat is not required on a daily basis. Some nutritionists formulate parenteral nutrition without lipids or provide an intermittent infusion of a lipid emulsion when animals remain on parenteral nutrition for prolonged periods over which essential fatty acid supplementation would be required.9
Electrolytes, vitamins, and trace elements also may be added to the parenteral nutrition formulation. Depending on the hospital and the patient, electrolytes can be added individually to the admixture, added as an electrolyte mixture, included as part of the amino acid solution, or left out altogether and managed separately in the animal’s crystalloid fluid prescription. The most appropriate method will depend upon the individual case, on the facilities, and on clinician preference. Because most animals receive parenteral nutrition for only a short duration, fat-soluble vitamins usually are not limiting, and supplementation with TPN vitamin preparations designed for humans usually is not indicated. The exception is obviously malnourished animals in which supplementation may be desirable. Conversely, because B vitamins are water soluble, they are more likely to become depleted, particularly in anorectic animals. Therefore B vitamins should be routinely supplemented in the parenteral nutrient admixture. Trace elements serve as cofactors in a variety of enzyme systems and can become depleted in malnourished animals or during long-term parenteral nutrition. In people receiving parenteral nutrition, zinc, copper, manganese, and chromium are routinely included in the parenteral nutrient admixture. These are sometimes added to parenteral nutrition preparations for malnourished animals, but in the authors’ practice, they are not routinely included.
Other nutritional requirements will depend on the patient’s underlying disease, clinical signs, and laboratory test results. Adjustments to the nutritional plan may include sodium restriction for cardiac patients, protein restriction for encephalopathic and end-stage renal failure patients, and fat restriction for patients with hypertriglyceridemia. Finally, there may be certain nutrients that may have benefits when added in amounts above their nutrient requirements. This concept is often called nutritional pharmacology. The addition of immune-modulating substances such as arginine, glutamine, antioxidants, and n-3 fatty acids to parenteral nutrition preparations may offer added benefits, but few studies have been conducted in companion animals.13,18,52
Parenteral nutrition components
Typically, the desired parenteral nutrition formula is calculated, and the components are compounded into a parenteral nutrient admixture. This solution is most often composed of amino acids, dextrose, and lipid along with vitamins and minerals. Information on the various parenteral nutrition components is presented in Table 25-3.
Amino Acids
Amino acid solutions provide a nitrogen source and essential amino acids. Amino acid solutions are available in varying concentrations from 3.5% to 10%. However, the worksheets in this chapter use amounts specific for an 8.5% amino acid solution. Other concentrations could be used, but the amounts would need to be adjusted accordingly. Most amino acid solutions are available in two formulations: one with electrolytes and one without (see Table 25-3). The majority of animals receive parenteral nutrition formulated using amino acids with electrolytes. However, amino acid solutions without electrolytes can be used for patients with marked electrolyte disturbances, and these electrolyte imbalances are corrected separately via other administered intravenous fluids.
The standard amount of protein included in the parenteral formulation is 4 to 5 g/100 kcal for dogs and 6 to 8 g/100 kcal for cats (although the optimal concentration for ill and injured dogs and cats has not been determined).15 This concentration can be reduced for animals with protein intolerance (e.g., those with hepatic encephalopathy or severe renal failure) or increased for those with higher needs (e.g., animals with large draining wounds or hypoalbuminemia).
It is important to note that the essential amino acids provided in these solutions are intended to meet the essential amino acid requirements in people. Currently, no amino acid solutions are made specifically for dogs or cats, and therefore these solutions do not meet all of these species’ needs or provide amino acids in the optimal proportions. However, when used for short-term nutritional support, this situation is unlikely to result in clinically relevant deficiencies. This may not be the case for certain amino acids such as taurine, which could become limiting if parenteral nutrition were to be used long term.
Other amino acid products are commercially available for people, including those intended for patients with renal failure, hepatic failure (e.g., high concentrations of branched chain amino acids), and for neonates. Some of these products may meet companion animal needs, but their additional cost usually does not justify their hypothetical benefits.
Dextrose
Dextrose is a component of nearly all parenteral nutrition formulations. In the formulations provided in this chapter, a 5% dextrose solution is used in PPN, whereas a 50% dextrose solution is used in TPN. Different concentrations can be used, but the worksheets would need to be adjusted accordingly. A 50% dextrose solution provides 1.7 kcal/mL, whereas the 5% solution provides 0.17 kcal/mL. Typically, in TPN, half of the nonprotein calories are provided by dextrose, but the ratio between dextrose and lipid can be adjusted depending on the individual circumstances (e.g., a greater proportion of lipid compared with dextrose would be given to a hyperglycemic animal). In people, the maximal amount of dextrose that can be oxidized is 5 mg/kg/min. In fact, dextrose infusion rates exceeding 4 mg/kg/min have been associated with the development of hyperglycemia in nondiabetic human patients.63 In light of these findings, the authors recommend limiting the amount of dextrose infused during parenteral nutrition to less than 4 mg/kg/min. When formulating parenteral nutrition for diabetic patients, a greater proportion of calories should be provided from amino acids and lipids. Despite adjustments to the formulation, diabetic patients often require adjustment of insulin therapy during parenteral nutritional support.
Lipid
Lipid emulsions are used in parenteral nutrition as an energy source (a 20% solution provides 2 kcal/mL) and as a source of essential fatty acids. Commercial lipid emulsions in the United States are usually based on soybean oil or soybean and safflower oil. They also include egg yolk phospholipids, glycerin, and water. The presence of soybean and safflower oil means that these solutions are composed primarily of n-6 fatty acids. High doses of lipid can cause immunosuppression via granulocyte and reticuloendothelial cell dysfunction.31,34,37 In addition to immunologic effects, lipids can have hemodynamic and inflammatory effects, the latter mediated by the more inflammatory eicosanoids produced from n-6 fatty acids.30 In other countries, different types of lipid emulsions are available that may be preferable to the standard soybean-based emulsions (e.g., n-3 fatty acids, n-9 fatty acids, medium-chain triglycerides, structured lipids), but these are not commercially available in the United States. The authors try to limit the lipid dosage in dogs and cats to 2.0 g/kg/day to prevent the potential for immunosuppression. Animals with hypertriglyceridemia also require lower doses of lipid and may require a TPN formulation without any lipid. Although some dogs with pancreatitis have hypertriglyceridemia and require reduction (or elimination) of the lipid dose, dogs with pancreatitis without hypertriglyceridemia do not need any reduction in the amount of lipid provided from the standard calculation.
Recently, the use of intravenous lipids for the treatment of moxidectin toxicosis in a dogs was reported.22 A proposed mechanism as to how intravenous lipids can be used to treat certain toxicities relies on the lipid solubility of the drug and the creation of a “lipid sink” by infusion of lipids. The protocol used involved administering a 1.5 mL/kg bolus of a 20% intralipid solution followed by a 0.25 mL/kg/min infusion for 60 minutes. The amount of lipids used in this protocol is well within the guidelines of administered 2 g/kg/day of intralipids.
Minerals
As previously mentioned, parenteral nutrition can be formulated without any electrolytes or electrolytes can be included, either as a component of an amino acid solution, added individually, or added as a combination of TPN electrolytes (most commonly, a combination of sodium, potassium, calcium, magnesium, chloride, and acetate). The most effective method will depend on the individual hospital and, in some cases, the individual patient. In certain situations, additional potassium or magnesium may be added directly to parenteral nutrition. However, the disadvantage of adding directly to the parenteral nutrient admixture is that if the animal’s requirements change during the day (or over a few days if more than 1 day of parenteral nutrition is compounded at one time) and the electrolyte is already in the admixture, the parenteral admixture must be reformulated or the animal will receive a less than optimal electrolyte composition. Adjusting electrolytes separately from the parenteral nutrition allows greater flexibility.
Trace elements are sometimes added to the parenteral nutrient admixture, but the authors only add them for animals that are malnourished or are receiving parenteral nutrition for 5 days or more. The most common trace elements supplemented are zinc, copper, manganese, and chromium, with copper considered the most limiting of these elements. The authors use a commercial trace element product containing (per 5 mL): 4 mg zinc, 1 mg copper, 0.8 mg manganese, and 10 μg chromium at a dosage of 0.2 to 0.3 mL/100 kcal (4 Trace Elements, Abbott Laboratories, North Chicago, Ill.).
Vitamins
For most hospitalized animals, including a B vitamin complex to the parenteral nutrient admixture is sufficient. Some B vitamins, particularly riboflavin, are light sensitive. Therefore sufficient B vitamin complex should be given such that the riboflavin dose is administered within the first 6 hours of the parenteral nutrition infusion. When using a commercial B vitamin complex containing thiamine, niacin, pyridoxine, pantothenic acid, riboflavin, and cyanocobalamin (B vitamin complex, Veterinary Laboratories, Lenexa, Kan.), a dosage of 0.2 mL/100 kcal should provide this amount of riboflavin.
For debilitated animals or those that receive parenteral nutrition for prolonged periods, a TPN vitamin complex can be included in the nutrient admixture. These products typically contain vitamins A, D, E, and C, in addition to the B vitamins.
Although certain medical conditions may result in vitamin K deficiency (e.g., biliary obstruction, hepatic disease), vitamin K is not typically administered intravenously and therefore is not added to the parenteral nutrition admixture. Interestingly, lipid solutions do contain vitamin K, which can be at sufficient concentrations to interfere with warfarin therapy in human patients.39,46 The amount of vitamin K found in lipid solutions has not been reported to cause anaphylactic reactions in companion animals. When deemed necessary, vitamin K supplementation is administered subcutaneously with dosages appropriate for the medical condition.
Commercial Combination Parenteral Nutrition Products
Although veterinarians have clinically used single nutrient solutions (e.g., amino acids or dextrose alone), these solutions do not provide balanced nutrition and are problematic when used alone (e.g., 50% dextrose is too hyperosmolar to be administered through a peripheral vein; 5% dextrose is too low in calories to be beneficial when administered alone). If 5% dextrose were administered at 66 mL/kg/day to an 11-kg dog, it would provide only 123 kcal/day (<30% of the dog’s calorie requirements and no protein). Administering lipid as a single solution can suppress immune function and, like dextrose, provides no protein. The osmolarity of amino acid solutions (1144 mOsm/L) makes them inappropriate for peripheral administration, and if amino acids are provided without sufficient calories, the amino acids will be used for calories rather than protein synthesis. Therefore, single nutrient solutions should be avoided.
However, there are a number of combination products commercially available that combine an amino acid source with a calorie source. These are listed in Table 25-3. Because dextrose cannot be sterilized in combination with amino acids, the approach to these products is to use dual-chamber bags in which the two compartments are separate until the seal between them is broken by squeezing the bag and the solutions are mixed (e.g., Clinimix, Baxter Healthcare Corporation). In another product (ProcalAmine, B. Braun Medical Inc., Irvine, Calif.), glycerin, which can be safely sterilized with amino acids, is used as a calorie source along with the amino acids. The advantages of these commercial combination products are their availability and the fact that they require no compounding. There are several different formulations of the dextrose/amino acid solutions. Lower concentration products (i.e., Clinimix 2.75 amino acid in 5% dextrose or ProcalAmine), which have an osmolarity that allows them to be administered via a long, nonthrombogenic catheter in a peripheral vein (see later discussion), provide all of a dog’s (and most of a cat’s) protein requirements but only 30% to 40% of their energy requirements when administered at maintenance fluid rates (cats, 50 mL/kg/day; dogs, 66 mL/kg/day) by continuous-rate infusion. Concentrations with higher concentrations (i.e., Clinimix 5% amino acid in 25% dextrose) can provide 100% of RER but must be administered via the jugular vein because of their high osmolarity. The electrolyte composition of these products varies, but they generally are high in potassium. Therefore they should be used with caution in critically ill patients. The authors typically use these products as a temporary measure for parenteral nutritional support (i.e., overnight or on weekends) or in combination with low-dose enteral nutrition. They should be used with care in animals with renal or hepatic failure because the ratio of protein to calories cannot be modified.
Compounding parenteral nutrition
The authors recommend using a parenteral nutrient admixture, which refers to the inclusion of the dextrose, amino acids, and lipids (with or without electrolytes, vitamins, trace elements) in a single bag. The calculations of calorie requirements, as well as the amino acid, lipid, and dextrose components for TPN and PPN, are shown in Boxes 25-4 and 25-5.
BOX 25-4 Worksheet for Calculating a Total Parenteral Nutrition Formulation
| 2. Protein requirements | Canine | Feline |
| >Standard | 4-5 g/100 kcal | 6 g/100 kcal |
| >Decreased requirements (hepatic/renal failure) | 2-3 g/100 kcal | 4-5 g/100 kcal |
| >Increased requirements (protein-losing conditions) | 5-6 g/100 kcal | 6-8 g/100 kcal |
3. Volumes of nutrient solutions required each day
>Be sure to adjust the patient’s other fluids accordingly.
>Note: Fluids can be added directly to TPN if desired (at the time of compounding only).
>The monitoring required will depend on the individual patient. However, at least the following should be measured daily:
*Glucose, total solids (check hematocrit tubes for lipemia)
*Electrolytes (especially potassium) should be monitored at least every other day
BOX 25-5 Worksheet for Calculating a Partial Parenteral Nutrition Formulation
1. Resting energy requirement (RER)
2. Partial energy requirement (PER)
Be sure to adjust the patient’s other intravenous fluids accordingly.
Notes:
* Fluids can be added directly to the PPN solution (at the time of compounding only).
* In some cases, the calculated PPN rate may be greater than maintenance fluid requirements or greater than what the animal can tolerate (e.g., cardiac disease).
* The monitoring required will depend on the individual patient. However, at least the following should be measured daily:
Other methods of calculating parenteral nutrition formulations have been described,42,48,61 but the methods listed in this chapter reflect the ones currently used by the authors. The TPN worksheet produces a parenteral nutrient admixture with an osmolarity greater than 1000 mOsm/L, and it must be administered via a jugular vein. The PPN worksheet produces an admixture with an osmolarity less than 700 mOsm/L, which can be administered via a peripheral vein, provided that a long, nonthrombogenic catheter is used. Calculating the actual osmolarity of the parenteral nutrition admixture can be done using the osmolarity of each component listed in Table 25-3 and the equation listed in Box 25-6. The PPN worksheet is designed for simplicity and not to provide optimal proportions of nutrients based on body weight categories. The rationale for the weight categories is that these calculations will provide PPN at approximately maintenance fluid rates. Animals with metabolic disturbances or those that require volume restriction may require adjustments in the PPN calculations or may require TPN, which allows more flexibility.
BOX 25-6 Calculation of Osmolarity of Parenteral Nutrition Admixture
[(mL of amino acids × osmolarity of amino acid solution) + (mL of dextrose × osmolarity of dextrose solution) + (mL of lipid × osmolarity of lipid solution) + (mL of additional fluids × osmolarity of fluids)] ÷ total volume of parenteral nutrition
Drug-nutrient compatibility is a very critical and very complex issue for parenteral nutrition.33,54,68 A number of deaths have been reported in people from parenteral nutrition because of precipitation of calcium phosphate in the admixture.33 At the time of compounding TPN or PPN, some additives can be included using aseptic technique, but others definitely are not compatible. Commonly used drugs that are compatible with parenteral nutrition admixtures include insulin, heparin, and metoclopramide. It is strongly recommended that a pharmacist experienced in parenteral nutrition compounding be consulted before considering adding anything to parenteral nutrition admixtures.
Administration
Catheters
Administration of parenteral nutrition requires a catheter that is placed using aseptic technique (Figure 25-6). Adherence to aseptic technique is crucial because the skin has been identified as the most common source of catheter-related infections.2,7,50,56 Long catheters composed of silicone, polyurethane, or tetrafluoroethylene are recommended for use with parenteral nutrition to reduce the risk of thrombophlebitis. Short catheters and catheters composed of polyvinyl chloride or polyethylene should be avoided. The catheter should be “dedicated” (i.e., it should not be used for any other purpose [e.g., administering medications, collecting blood samples, measuring central venous pressure] than administration of parenteral nutrition). If a multilumen catheter is used, a single port should be dedicated to parenteral nutrition.56 Multilumen catheters are very useful for patients receiving parenteral nutrition because they can remain in place for longer periods compared with normal catheters and provide additional ports for blood sampling and administration of additional intravenous fluids and medications. All catheters should be well secured and wrapped, but the bandage should be changed daily so that the catheter site can be evaluated. This practice will help to identify swelling, erythema, or malpositioning of the catheter. All handling of the catheter and lines should be done using aseptic technique. Appropriate catheter care has been shown to be one of the most effective measures in reducing catheter-related complications.2,56

Figure 25-6 The catheter for parenteral nutrition should be placed with aseptic technique and should be a dedicated catheter.
There also has been some concern associated with parenteral nutrition admixtures leaching plasticizers (e.g., diethylhexylphthalate (DEHP) from polyvinylchloride infusion lines and bags causing hepatic injury.44,45 The risk if particularly high in infants when long-term infusions ( >1 month) are used. It is unknown if this is a problem in animals receiving parenteral nutrition for less than 5 days, which is the typical course of parenteral nutrition in animals. Recommendations for reducing the risk of DEHP toxicity involves using lines and bags free of DEHP and made with alternatives such as ethyl vinyl acetate.
Parenteral Nutrition Solutions
For logistical and economical reasons, more than 1 day’s supply of parenteral nutrition usually is compounded at one time. As such, the bag of parenteral nutrition for the current day should be set up for the animal, and the other bags should be stored in a refrigerator until the time of use. No more than a 5-day supply of parenteral nutrition should be compounded and stored at a time. However, some authors recommend that no more than a 2-day supply is compounded ahead of time, and this practice may be more appropriate for patients that are critically ill in which frequent adjustments to the parenteral nutrition admixture may become necessary (e.g., decreasing dextrose or lipid content).66 Parenteral nutrition admixtures should never be frozen, and any unused portions should be discarded (i.e., not saved for use at a later time or in another patient).
Initiating Parenteral Nutrition
The worksheets in this chapter provide an admixture that is intended to last 24 hours when administered at a constant-rate infusion. Bags of parenteral nutrition admixtures should not be at room temperature for more than 24 hours. The bag should be administered during the 24-hour period via a fluid infusion pump (Figure 25-7). During this time, the lines should not be disconnected from the bag or the patient (i.e., it should remain a closed system). When taking dogs outside, either the pump should accompany the dog or the bag can be removed from the pump (if this does not disconnect the lines from the bag or the patient) and carried along. In the latter situation, one must be careful to allow the parenteral nutrition to continue to drip slowly (i.e., avoid clamping it off completely but ensure that it is not administered at a faster than desired rate during this time) and keep the drip chamber upright. At the end of each 24-hour period, the infusion should be complete, and the empty bag, along with the lines, can be changed using an aseptic technique and a new bag and lines substituted. All parenteral nutrition should be administered through a 1.2-µm in-line filter (extension set with 1.2-µm downstream filter, Baxter Healthcare Corp.), but not all hospitals use these. The filter can help to prevent lipid globules or precipitates (particularly calcium phosphate) from being introduced to the patient.4,51 Because of the high osmolarity of the TPN solution, it should be administered through a central venous (jugular) catheter. However, some authors have downplayed the role of high osmolality in increasing risk of thrombophlebitis in people.36 It is unknown if this holds true in dogs and cats and further investigation is required. PPN (as formulated using the worksheet in this chapter) can be administered through a peripheral or jugular catheter, but because it is more dilute, it can only provide a portion of the patient’s energy requirements.
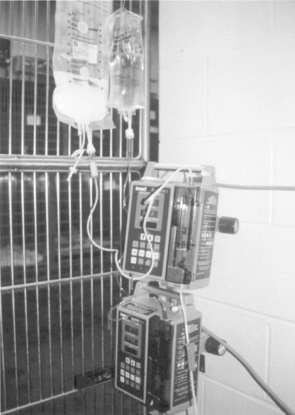
Figure 25-7 Parenteral nutrition should be infused over a 24-hour period by continuous-rate infusion via a fluid pump.
TPN should be instituted gradually over 48 to 72 hours. Most animals tolerate receiving 50% of total requirements on the first day and 100% on the second day. Animals that have been without food for long periods may require slower introduction (i.e., 33% on the first day, 66% on the second day, and 100% on the third day). PPN does not require gradual introduction and can be initiated at 100% on the first day. It is important to adjust the animal’s other intravenous fluids when initiating parenteral nutritional support to prevent fluid volume overload.
Potential Complications
A number of possible complications can be associated with parenteral nutrition, and these generally are grouped into one of three categories (Box 25-7). Metabolic complications are the most common, with hyperglycemia typically seen most frequently.14,43 Electrolyte disturbances can develop either after instituting nutritional support or may worsen in animals with preexisting abnormalities. Refeeding syndrome is uncommon in companion animals but can be difficult to manage when it occurs.3,35 Refeeding syndrome refers to a potentially fatal complication secondary to nutritional management of severely malnourished patients.23,55,63 It includes the development of hypophosphatemia with or without hypokalemia, hypomagnesemia, thiamine deficiency, and fluid shifts.23,65 It can develop when nutritional support, either parenteral or enteral, is initiated in a severely malnourished animal (particularly those that have not eaten for a prolonged period). The glucose provided stimulates insulin secretion that drives extracellular ions (e.g., phosphorus, potassium, magnesium) intracellularly and stimulates protein synthesis. The result may be clinically significant hypophosphatemia, hypokalemia, and hypomagnesemia. The shift to carbohydrate metabolism increases demand for important cofactors such as thiamine, which may already be depleted in malnourished patients, and neurologic manifestations of thiamine deficiency may occur.23,55,65 Congestive heart failure also can occur secondary to fluid shifts. It is important, particularly in animals with prolonged anorexia, to initiate parenteral nutrition slowly, to supplement vitamins (particularly thiamine), and to monitor serum electrolytes for the first 3 to 4 days after initiation.
BOX 25-7 Potential Complications of Parenteral Nutrition
Metabolic
Septic (Clinical Signs of Sepsis in Conjunction with a Positive Catheter Tip or Blood Culture)
How to Reduce the Risk
Catheter composed of materials of low thrombogenicity
Placing catheters, handling catheters and lines with aseptic technique
Using parenteral nutrition for the least amount of time deemed necessary
Monitor body temperature, catheter site, general attitude
If sepsis is suspected, parenteral nutrition solution and catheter tip should be cultured
Remove catheter as soon as possible once parenteral nutrition is discontinued
The most important factor in reducing the risk of mechanical and septic complications is prevention protocols. Careful attention to catheter placement and catheter and line care will reduce the risk of problems. Placement of catheters by experienced personnel has been shown to reduce mechanical and septic complications.43,56 Elizabethan collars should be used for any animal that shows a propensity to chew lines. Creative solutions may be needed for animals that circle in the cage or are otherwise fractious. Protocols for catheter placement, handling catheters and line with aseptic technique, and maintaining dedicated catheters also are beneficial in minimizing the incidence of sepsis. If clinical evidence of sepsis does develop, conventional recommendations include submission of the parenteral nutrition admixture and the catheter tip for bacteriologic cultures. Often, sepsis develops as a result of the underlying disease rather than being related to parenteral nutrition. However, another indirect link exists between sepsis and parenteral nutrition. An increased risk for bacterial translocation is present because villous atrophy occurs when an animal is fed parenterally (i.e., when the animal is not receiving any nutrients via the enteral route). This factor is another argument for reinstituting oral or enteral nutrition as soon as possible in animals receiving parenteral nutrition.
The other critical aspect in reducing the risk of complications is vigilant monitoring. Checking the catheter site daily can identify malpositioning of the catheter and phlebitis or cellulitis early, before serious problems develop. Body weight should be monitored daily in animals receiving parenteral nutrition. Fluid shifts also can explain rapid changes in weight during hospitalization, emphasizing the need for continued nutritional assessment. Use of the RER as the patient’s caloric requirement is merely a starting point. The number of calories provided may need to be increased to prevent weight loss or to keep up with the patient’s changing needs. To prevent complications with parenteral nutrition, the patient should be monitored carefully and frequently. Body temperature, heart rate, and respiratory rate should be recorded several times a day. Metabolic complications can occur frequently in animals receiving parenteral nutrition, and monitoring is crucial to detect and address them early, if necessary. The clinical situation should dictate the frequency and spectrum of monitoring required because some patients will need more intensive monitoring. Each case is individual, and good clinical judgment is imperative. In animals receiving parenteral nutrition, the authors recommend that general attitude, body weight, temperature, blood glucose concentration, total solids (check the serum for gross lipemia or hemolysis), and serum electrolyte concentrations should be assessed daily or more frequently if indicated. Other variables that may require monitoring include ammonia (for animals that are at risk of developing hepatic encephalopathy), triglycerides (for those with gross lipemia), and bilirubin. The development of metabolic abnormalities usually does not require discontinuation of parenteral nutrition but may require reformulation (e.g., a reduction in the lipid content for animals that develop hypertriglyceridemia). Box 25-7 lists the methods that can be used to reduce the risk of the common complications. Other variables to monitor include gastrointestinal signs and appetite so that enteral nutrition or oral intake can be initiated as soon as possible. Finally, the overall nutritional plan should be reassessed on a regular basis so that it can be adjusted to meet the animal’s changing needs. For example, an animal receiving PPN for 3 days may need to be switched to TPN if its underlying disease has not resolved, or a small amount of enteral nutrition can be introduced in conjunction with PPN if tolerated.
Discontinuing Parenteral Nutrition
Transitioning to oral intake or enteral nutrition should be done as soon as possible to prevent the problem of gut atrophy that is associated with lack of oral intake. In veterinary medicine, parenteral nutrition typically is administered for less than 1 week. However, it is important to ensure that the patient is tolerating oral intake or enteral nutrition and is ingesting sufficient amounts (at least 50% of RER) before discontinuing parenteral nutrition. Once the patient is able to eat, it should be offered food regularly to assess its appetite, or a feeding tube should be placed if the animal is anorectic. When the animal is voluntarily consuming or enterally receiving at least 50% of RER, TPN can be gradually decreased over a period of 4 to 8 hours (while monitoring blood glucose concentration). To accomplish this withdrawal, TPN is administered at half the calculated rate for 4 to 8 hours and then discontinued completely. If TPN is discontinued abruptly, there is a small risk of rebound hypoglycemia. PPN can be discontinued abruptly without this gradual decrease.
How to Obtain Parenteral Nutrition
To compound the parenteral nutrient admixtures (dextrose, amino acid, and lipid) calculated using the TPN and PPN worksheets provided in this chapter, there are a number of different options. One option is an automated compounder, which provides quick and accurate mixing. However, these compounders are expensive and usually are not cost-effective unless parenteral nutrition is used frequently. A second option for compounding parenteral nutrition solutions manually is using a three-in-one bag (Empty three-in-one mixing container with attached 3-lead transfer set, Abbott Laboratories; All-in-One Container for gravity transfer, Baxter Healthcare Corp.). These bags have three attached leads that can be connected using aseptic technique to bags of dextrose, amino acids, and lipids, respectively. The components then are added to the recipient bag in a closed system by gravity. To make this system more accurate, the recipient bag should be weighed to ensure that an accurate amount of each solution is added, especially in very small animals. Like the automated compounder, these all-in-one bags require a knowledgeable person to perform the compounding, a very clean environment, and good aseptic technique. Many hospitals that do not use parenteral nutrition frequently do not find this method to be time- or cost-effective. Alternatives include making arrangements with a large veterinary referral hospital that compounds parenteral nutrition or with a human hospital in the community. Another solution that has worked very well for many veterinary hospitals is to make arrangements with a human home health care company. These companies compound parenteral nutrition for human patients who often are receiving it for many years in their homes. A formula can be provided to the company, and the solution can be delivered to the veterinary hospital within a short time period. In many cases, this approach works out to be more cost- and time-effective than compounding parenteral nutrition in an individual veterinary hospital.
Future goals
Parenteral nutrition can now be safely provided to hospitalized dogs and cats, and it is an important part of their optimal care. Future directions in parenteral nutrition research include developing species-specific amino acid solutions rather than being limited to preparations designed for humans, and determining the optimal proportions of nutrients for critically ill animals. Nutritional pharmacology, such as the use of glutamine, n-3 fatty acids, or antioxidants also may prove to be beneficial. One of the most exciting areas of research in human critical care medicine is stricter control of blood glucose concentrations. Critically ill cats respond similarly in terms of glucose regulation to critically ill people, and more careful control of blood glucose concentrations also may have similar benefits in companion animals.17 Finally, efficacy studies continue to be performed in human medicine to determine the patients most likely to benefit from parenteral nutrition and those most likely to have complications. Similar types of studies are needed in veterinary patients to most effectively use this exciting nutritional support modality and to most successfully care for our hospitalized patients.
1 Abood S.K., Mauterer J.V., et al. Nutritional support of hospitalized patients. In: Slatter D., editor. Textbook of small animal surgery. 2nd ed. Philadelphia: WB Saunders; 1993:63-83.
2 Adal K.A., Farr B.M. Central venous catheter-related infection: a review. Nutrition. 1996;12:208-213.
3 Armitage-Chan E.A., O’Toole T., Chan D.L. Management of prolonged food deprivation, hypothermia and refeeding syndrome in a cat. J Vet Emerg Crit Care. 2006;16(S1):S34-S41.
4 Ball P.A. Intravenous in-line filters: filtering the evidence. Curr Opin Clin Nutr Metab Care. 2003;6:319-325.
5 Barton R.G. Nutrition support in critical illness. Nutr Clin Pract. 1994;9:127-139.
6 Biffl W.L., Moore E.E., Haenel J.B., et al. Nutritional support of the trauma patient. Nutrition. 2002;18:960-965.
7 Bjornson H.S., Colley R., Bowen R.H., et al. Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery. 1982;92:720-727.
8 Braunschweig C.L., Levy P., Sheean P.M., et al. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534-542.
9 Buffington T., Holloway C., Abood A. Clinical dietetics. In: Buffington T., Holloway C., Abood S., editors. Manual of veterinary dietetics. St. Louis: WB Saunders; 2004:49-141.
10 Burkholder W.J. Metabolic rates and nutrient requirements of sick dogs and cats. J Am Vet Med Assoc. 1995;206:614-618.
11 Butterworth C.E. Skeleton in the hospital closet. Nutr Today. 1976;9:4.
12 Carter J.M., Freedman A.B. Total intravenous feeding in the dog. J Am Vet Med Assoc. 1977;171:71-76.
13 Chan D.L. The role of nutrients in modulating disease. J Small Anim Pract. 2008;49(6):266-271.
14 Chan D.L., Freeman L.M., Labato M.A., et al. Retrospective evaluation of partial parenteral nutrition in dogs and cats. J Vet Intern Med. 2002;16:440-445.
15 Chan D.L. Nutritional requirements of the critically ill patient. Clin Tech Small Anim Pract. 2004;19:1-5.
16 Chan D.L., Freeman L.M., Rozanski E.A., et al. Prevalence of hyperglycemia in cats presented to the emergency service. J Vet Emerg Crit Care. 2002;12(3):199.
17 Chan D.L., Freeman L.M., Rozanski E.A., et al. Alterations in carbohydrate metabolism in critically ill cats. J Vet Emerg Crit Care. 2006;16:S7-S13.
18 Chan D.L. Parenteral nutrition. In: Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine. 7th ed. Philadelphia: Saunders; 2010:701-707.
19 Chandler M.L., Guilford W.G., Maxwell A., et al. A pilot study of protein sparing in healthy dogs using peripheral parenteral nutrition. Res Vet Sci. 2000;69:47-52.
20 Chandler M.L., Payne-James J.J. Prospective evaluation of a peripherally administered three-in-one parenteral nutrition product in dogs. J Small Anim Pract. 2006;47:518-523.
21 Crabb S.E., Chan D.L., Freeman L.M. Retrospective evaluation of total parenteral nutrition in cats: 40 cases (1991–2003). J Vet Emerg Crit Care. 2006;16(S1):S21-S26.
22 Crandell D.E., Weinberg G.L. Moxidectin toxicosis in a puppy successfully treated with intravenous lipids. J Vet Emerg Crit Care. 2009;19(2):181-186.
23 Crook M.A., Hally V., Pantelli J.V. The importance of the refeeding syndrome. Nutrition. 2001;17:632-637.
24 Detsky A.S., Baker J.P., O’Rourke K., et al. Perioperative parenteral nutrition: a meta-analysis. Ann Intern Med. 1987;107:195-203.
25 Dudrick S.J., Wilmore D.W., Vars H.M., et al. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery. 1968;64:134-142.
26 Eirmann L., Michel K.E. Enteral Nutrition. In: Silverstein DC., Hopper K. editors. Small animal critical care medicine. St Louis: Saunders Elsevier; p. 53–62
27 Finney S.J., Zekveld C., Elia A., et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041-2047.
28 Freeman L.M., Chan D.L. Parenteral and enteral nutrition. Compend Stand Care Emerg Crit Care. 2001;3:1-7.
29 Gramlich L., Kichian K., Pinilla J., et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843-848.
30 Grimes J.B., Abel R.M. Intravenous fat emulsion in dogs. JPEN J Parenter Enter Nutr. 1979;3(2):40-44.
31 Hamawy K.J., Moldawer L.L., Geogieff M., et al. The effect of lipid emulsions on reticuloendothelial system function in the injured animal. JPEN J Parenter Enteral Nutr. 1985;9:559-565.
32 Heyland D.K., MacDonald S., Keefe L., et al. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA. 1998;280:2013-2019.
33 Hill S.E., Heldman L.S., Goo E.D.H., et al. Fatal microvascular pulmonary emboli from precipitation of a total nutrient admixture solution. JPEN J Parenter Enteral Nutr. 1996;20:81-87.
34 Jarstrand C., Berghem L., Lahnborg G. Human granulocyte and reticuloendothelial system function during intralipid infusion. JPEN J Parenter Enteral Nutr. 1978;2:663-670.
35 Justin R.B., Hohenhaus A.E. Hypophosphatemia associated with enteral alimentation in cats. J Vet Intern Med. 1995;9:228-233.
36 Kane K.F., Cologiovanti L., McKiernan J., et al. High osmolality feedings do not increase the incidence of thrombophlebitis during peripheral IV nutrition. JPEN J Parenter Enter Nutr. 1996;20(3):194-197.
37 Kang J.H., Yang M.D. Effect of a short-term infusion with soybean oil-based lipid emulsion on phagocytic responses of canine peripheral blood polymorphonuclear neutrophilic leukocytes. J Vet Intern Med. 2008;22(5):1166-1173.
38 Kinney J.M. History of parenteral nutrition, with notes on clinical biology. In: Rombeau J.L., Rolandell R.H., editors. Clinical nutrition: parenteral nutrition. Philadelphia: WB Saunders; 2001:1-20.
39 Lennon C., Davidson K.W., Sadowski J.A., et al. The vitamin K content of intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 1993;17:142-144.
40 Lin L.Y., Lin H.C., Lee P.C., et al. Hyperglycemia correlates with outcomes in patients receiving total parenteral nutrition. Am J Med Sci. 2007;333(5):261-265.
41 Lippert A.C., Faulkner J.E., Evans A.T., et al. Total parenteral nutrition in clinically normal cats. J Am Vet Med Assoc. 1989;194:669-676.
42 Lippert A.C. The metabolic response to injury: enteral and parenteral nutritional support. In: Murtaugh R.J., Kaplan P.M., editors. Veterinary emergency and critical care medicine. St. Louis: Mosby Yearbook; 1992:593-617.
43 Lippert A.C., Fulton R.B., Parr A.M. A retrospective study of the use of total parenteral nutrition in dogs and cats. J Vet Intern Med. 1993;7:52-64.
44 Loof P.D., Subotic U., Oulmi-Kagermann J., et al. Diethylhexylphthalate extracted by typical newborn lipid emulsions from polyvinylchloride infusion systems causes significant changes in histology of rabbit liver. JPEN J Parenter Enter Nutr. 2007;31(3):188-193.
45 Loff S., Hannmann T., Subotic U., et al. Extraction of diethylhexylphthalate by home total parenteral nutrition from polyvinyls chloride infusion lines commonly used in the home. J Pediatr Gastroenterol Nutr. 2008;47(1):81-86.
46 Lutomski D.M., Palascak J.E., Bower R.H. Warfarin resistance associated with intravenous lipid administration. JPEN J Parenter Enteral Nutr. 1987;11:316-318.
47 Marik P.E. Maximizing efficacy from parenteral nutrition in critical care: appropriate patient populations, supplemental parenteral nutrition, glucose control, parenteral glutamine, and alternate fat sources. Curr Opin Gastroenterol Rep. 2007;9(4):345-353.
48 Marks S.L. Enteral and parenteral nutritional support. In: Ettinger S.J., Feldman E.D., editors. Textbook of veterinary internal medicine. 5th ed. Philadelphia: WB Saunders; 2000:275-283.
49 Mauldin G.E., Reynolds A.J., Mauldin G.N., et al. Nitrogen balance in clinically normal dogs receiving parenteral nutrition solutions. Am J Vet Res. 2001;62:912-920.
50 McGee D.C., Gould M.K. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123-1133.
51 McKinnon B.T. FDA safety alert: hazards of precipitation associated with parenteral nutrition. Nutr Clin Pract. 1996;11:59-65.
52 Michel K.E. Interventional nutrition for the critical care patient: optimal diets. Clin Tech Small Animal Pract. 1998;13:204-210.
53 Michel K.E. Prognostic value of clinical nutritional assessment in canine patients. J Vet Emerg Crit Care. 1993;3:96-104.
54 Michel K.E., Higgins C. Nutrient-drug interactions in nutritional support. J Vet Emerg Crit Care. 2002;12:163-168.
55 Miller C.C., Bartges J.W. Refeeding syndrome. In: Bonagura J.D., editor. Current veterinary therapy XIII. Philadelphia: WB Saunders; 2000:87-89.
56 O’Grady N.P., Alexander M., Dellinger E.P., et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23:759-769.
57 O’Toole E., Miller C.W., Wilson B.A., et al. Comparison of the standard predictive equation for calculation of resting energy expenditure with indirect calorimetry in hospitalized and healthy dogs. J Am Vet Med Assoc. 2004;255:58-64.
58 Pyle S.C., Marks S.L., Kass P.H. Evaluation of complications and prognostic factors associated with administration of total parenteral nutrition in cats: 75 cases (1994–2001). J Am Vet Med Assoc. 2004;225:242-250.
59 Ray C.C., Callahan-Clark J., Beckel N.F., et al. The prevalence and significance of hyperglycemia in hospitalized cats. J Vet Emerg Crit Care. 2009;19(4):347-351.
60 Reeds D. Near-normal glycemia for critically ill patients receiving nutrition support: fact or folly. Curr Opin Gastroenterol. 2010;26(2):152-155.
61 Remillard R.L., Saker K.E. Parenteral-assisted feeding. In: Hand M.S., Thatcher C.D., Remillard R.L., et al, editors. Small animal clinical nutrition. 5th ed. Topeka, Kan: Mark Morris Institute; 2010:477-498.
62 Reuter J.D., Marks S.L., Rogers Q.R., et al. Use of total parenteral nutrition in dogs: 209 cases (1988–1995). J Vet Emerg Crit Care. 1998;8:201-213.
63 Rosmarin D.K., Wardlaw G.M., Mirtallo J. Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr Clin Pract. 1996;11:151-156.
64 Simpson F., Doig G.S. Parenteral vs. enteral nutrition in the critically ill patient: A meta-analysis of trials using the intention to treat principle. Intensive Care Med. 2005;31:12-23.
65 Solomon S.M., Kirby D.F. The refeeding syndrome: a review. JPEN J Parenter Enteral Nutr. 1990;14:90-97.
66 Thomovsky E.J., Backus R.C., Mann F.A., et al. Effects of temperature and handling conditions on lipid emulsion stability in veterinary parenteral nutrition admixtures during simulated intravenous administration. Am J Vet Res. 2008;69:652-658.
67 Torre D.M., deLaforcade A.M., Chan D.L. Incidence and clinical relevance of hyperglycemia in critically ill dogs. J Vet Intern Med. 2007;21:971-975.
68 Trissel L.A., Gilbert D.L., Martinez J.F., et al. Compatibility of medications with 3-in-1 parenteral nutrition admixtures. JPEN J Parenter Enteral Nutr. 1999;23:67-74.
69 van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;8:1359-1367.
70 Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525-532.
71 Vinnars E., Wilmore D. History of parenteral nutrition. JPEN J Parenter Enteral Nutr. 2003;27:225-231.
72 Walton R.S., Wingfield W.E., Ogilvie G.K. Energy expenditure in 104 postoperative and traumatically injured dogs with indirect calorimetry. J Vet Emerg Crit Care. 1998;6:71-79.
73 Zentek J., Stephan I., Kramer S., et al. Response of dogs to short-term infusions of carbohydrate- or lipid-based parenteral nutrition. J Vet Med. 2003;50:313-321.
74 Zsombor-Murray E., Freeman L.M. Peripheral parenteral nutrition. Compend Contin Educ Pract Vet. 1999;21:512-523.