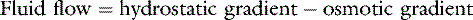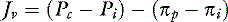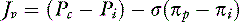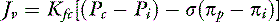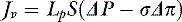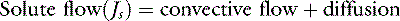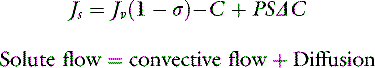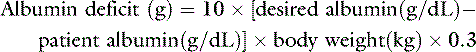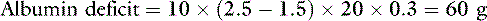Chapter 27 Fluid Therapy with Macromolecular Plasma Volume Expanders
“Those who fill our professional ranks are habitually conservative. This salutary mental attitude expresses itself peculiarly in our communal relations; namely, when a new idea appears which is more or less subversive to old notions and practices, he who originates the idea must strike sledge hammer blows in order to secure even a momentary attention. This must then be followed by a long, patient, propaganda and advertising until in the grand finale, the public, indifferent at first, is aroused, proceeds to discuss, and finally accepts the iconoclastic proposal as a long-accepted fact of its own invention and asks wonderingly, ‘Why such a bother? What after all is new about this? We knew it long ago!”
Howard A. Kelly, MD. Electrosurgery in gynaecology, Ann Surg 93:323, 1931.
In the late nineteenth century, Ernest Starling proposed the concept that the balance between hydrostatic and osmotic pressure gradients between the intravascular and interstitial fluid compartments governed transvascular fluid exchange.151 He postulated that a hydrostatic pressure gradient in excess of the osmotic gradient at the arterial end of the capillary bed results in a net transudation of fluid into the interstitium. At the venous end of the capillary bed, plasma proteins (which do not normally pass out of the blood vessels) exert an osmotic force in excess of the hydrostatic gradient, resulting in a net fluid flux into vessels. More than a century of research has confirmed that Starling’s hypothesis provides the foundation for microvascular fluid exchange but also has revealed that the anatomy and physiology of the microvasculature, interstitium, and lymphatic system are much more complex. Consequently, a much deeper understanding of transvascular fluid dynamics is necessary for a logical and rational approach to intravenous therapy with fluids containing macromolecules. This chapter assumes the reader is familiar with the information given in Chapter 1 explaining the fluid compartments of the body and the mechanisms of water and solute flow among compartments. Although this chapter discusses the anatomy, physiology, and biophysics of transvascular fluid dynamics in some depth, comprehensive reviews and texts are available on the subject for a more complete discussion of solute and solvent exchange among the microvasculature, interstitium, and lymphatics.5,127,154 The main aim of this chapter is to objectively address the complexities and controversies of colloid therapy while avoiding the tendency toward bias apparent in some articles dealing with the crystalloid-colloid controversy. A deeper appreciation of the relevant issues should ensure a more rational approach when deciding whether colloid therapy is appropriate. The present chapter is exhaustive in its dealing with some issues but not all-inclusive, and the reader also is referred to several reviews of colloid fluid therapy available in the veterinary31,54,98,135 and human medical literature.55,56,103,129,177
The microvascular barrier
In simple terms, the healthy microvascular barrier is a capillary wall that is relatively impermeable to protein. In addition to the endothelial cell and the capillary basement membrane, a luminal surface layer (the glycocalyx) and the interstitial matrix also contribute to the selective permeability of the microvascular barrier.5,127,180 The glycocalyx coats the luminal aspect of the endothelial cell and is composed of proteins, glycoproteins, and glycolipids that modify the permeability of the microvessel by occupying spaces within the wall or via electrostatic attraction or repulsion.93 Plasma proteins, especially albumin and orosomucoid, are thought to contribute significantly to maintaining the selective permeability of the endothelium.45-47,74,102
On a morphologic basis, capillary walls may be continuous, fenestrated, or discontinuous.122,158 Continuous capillaries, which are found in the majority of tissues and organs of the body, are so called because the wall is composed of a continuous endothelial cell and basement membrane. They are freely permeable to water and small solutes such as sodium but are relatively impermeable to macromolecules. The passage of smaller plasma proteins, such as albumin (molecular radius of 3.5 nm), is less restricted than the passage of larger plasma proteins. Fenestrated capillaries have a continuous basement membrane with regions that are only covered by thin endothelial diaphragms or are entirely devoid of endothelium. They are found in tissues characterized by large fluxes of water and small solutes such as the glomerulus and the intestine. Interestingly, the permeability of fenestrated capillaries to macromolecules is similar to that of continuous capillaries. This feature has been shown to be a result of a net negative charge of the basement membrane.12,146 Discontinuous capillaries are found in the liver, spleen, bone marrow, and some glands. They have gaps up to 1 μm between endothelial cells with no basement membrane and are therefore freely permeable to protein.
The permeability of the microvascular barrier has been explained by the presence of pores of differing sizes.111 These pore sizes often are extrapolated from experimental data regarding fluid and solute fluxes and do not always correlate with morphologic studies such as electron microscopy, implying that they represent functional rather than anatomic entities. The majority of experimental data suggest there are two effective pore sizes in the microvascular barrier in most tissues, with a high frequency of small pores that restrict efflux of macromolecules and a low frequency of large ones through which macromolecules can pass freely.127
Rather than being a free fluid space, the interstitium represents a dynamic environment that may contribute to the permeability characteristics of the microvascular barrier and modify the flow of fluid and macromolecules from the blood vessels to the lymphatics.5,10,11 The interstitium is composed of a collagen framework that contains a gel phase of glycosaminoglycans (of which hyaluronan is the most common), along with protein macromolecules and electrolytes in solution. The relative proportions of these constituents differ widely among organs and tissues, resulting in variations in the permeability and mechanical properties of the interstitium. Glycosaminoglycans are extremely long chains of repeating disaccharide subunits wound into random coils and entangled with each other and the collagen framework. They have molecular weights of the order of 107, and each molecule bears many thousand anionic moieties.5 This interstitial structure has been suggested to mechanically oppose distention (i.e., edema formation) and resists contraction during dehydration because of repulsion between the anionic moieties.71 The interstitial matrix itself is differentially permeable to macromolecules, and a colloid osmotic gradient also can exist from the perimicrovascular space across the interstitium to the lymphatics. Although the collagen network and many of the glycosaminoglycans are fixed in the interstitium, hyaluronan may be mobilized and removed via lymphatic drainage, thereby altering the permeability of the interstitium.5 Increased microvascular permeability may occur during inflammatory states thereby exacerbating macromolecule extravasation.
Transvascular fluid dynamics
Although not stated implicitly in his seminal article, Starling’s hypothesis was subsequently formalized to state simply that the hydrostatic pressure gradient between the capillary and the interstitium (Pc − Pi) is equal to the osmotic pressure gradient between the plasma and the interstitium (πp − π i). This expression can be expanded to describe fluid flux (Jv) across the microvascular barrier:
or
For a solute to exert its full osmotic pressure across a membrane, the membrane must be impermeable to the solute. If the membrane is partially permeable to the solute molecule, the equilibrium concentration gradient is lower, and the solute exerts only part of its potential osmotic pressure. The realization that the microvasculature was only partially impermeable to smaller macromolecules led to the inclusion of the reflection coefficient (σ) in the fluid flux equation.155
In descriptive terms, the reflection coefficient is the fraction of the total potential osmotic pressure exerted by the solute in question. Conceptually, one also can consider it as the proportion of the solute molecules reflected from the microvascular barrier. If a membrane is completely impermeable, no solute molecules pass through, the concentration gradient is maximal, and the solute exerts its full osmotic pressure (i.e., the reflection coefficient = 1). If the membrane is completely permeable to the solute in question, it passes through freely, no concentration difference exists, and no osmotic pressure can be exerted (i.e., the reflection coefficient = 0).
Further research showed that fluid flow from vessels differed among tissues depending on the surface area of the capillary beds in the organ and the hydraulic conductance (i.e., the ease of fluid flow) through the microvascular barrier. To account for this variability, the fluid flux equation is modified by the filtration coefficient (Kfc). This term simply implies that fluid flow is equal to a fraction of the effective hydrostatic and osmotic pressure gradients.
Each different constituent of plasma may differ in its rate of efflux from a vessel depending on such factors as its molecular radius, shape, and charge, and the permeability of the microvascular barrier to the constituent in question. The two major groups of molecules with respect to transvascular fluid flux are termed the solvent phase and the solute phase, and expressions were developed to predict the egress of both major groups of molecules from the microvasculature.83,92,110,115 The solvent phase includes water and those molecules that are not significantly impeded in their passage through the microvascular barrier, whereas the solute flux equation describes the passage of molecules that do not flow freely from the vasculature.
The solvent flow equation remains the same as the previous expression of fluid flow except that the filtration coefficient is subdivided into the hydraulic conductance (Lp) and the membrane surface area (S), and the hydrostatic and osmotic gradients are expressed as ΔP and Δπ, respectively:
The two major mechanisms of solute flow through the microvascular barrier are convection (i.e., carriage in a bulk flow of fluid) and diffusion (i.e., random motion resulting in net movement of molecules from an area of high concentration to an area of lower concentration).127 An analogy to illustrate the two mechanisms would be a wave breaking on a beach. Some of the sodium molecules in the wave will be moving away from the beach by diffusion; however, the forward convective flow of the wave carries them in the opposite direction.
The solute flow equation is the most relevant expression with respect to intravenous therapy with fluids containing macromolecules. It states that the rate of solute flux (Js) is equal to the sum of the convective flow and the diffusional movement.
Convective flow is equal to the product of fluid flow (Jv), the fractional permeability of the membrane (1 − σ ), and the mean intramembrane solute concentration,C. Diffusion is equal to the product of the solute permeability (P), the surface area of the microvascular barrier (S), and the solute concentration gradient across the membrane (ΔC). Therefore the expression representing macromolecular flux becomes:
At normal lymph flow rates, convection has been estimated to account for approximately 30% of the total flux of albumin into lymph.123 An important point that warrants further emphasis is that the rate of solute efflux is dependent on the rate of solvent efflux. Any condition that increases the rate of fluid flow across a membrane can increase the extravasation of macromolecules. Hence, intravenous fluid therapy with crystalloid or colloid can increase albumin loss into the interstitium.124
These mathematical expressions give the impression of a constant hydrostatic pressure gradient acting across a single membrane of static and uniform conductivity and permeability (homoporous), with filtration opposed by an osmotic pressure resulting from a single impermeant solute, the plasma “protein.” In fact, the hydrostatic pressure and osmotic pressure gradients vary among different tissues and at different levels of the capillary bed within the same tissue.121,156,159 In disease states, the differences among organs may be significant and the clinician must consider the possibility of individual organ edema (e.g., pulmonary, myocardial, or intestinal edema) even if there are no overt signs of a systemic edematous state. The total osmotic gradient is a summation of all the impermeant solutes present within plasma, which all have unique reflection coefficients and efflux rates.156 Furthermore, the surface area of the capillary bed may change depending on precapillary sphincter activity and the permeability of the microvascular barrier and interstitium may also vary physiologically and in disease states.8,71,113,180,181
Normal starling forces and the tissue safety factors
Plasma colloid osmotic pressure
Although in popular usage colloid is interpreted most often as referring to a macromolecule that cannot pass through a membrane, the strict definition refers to the dispersion in a gas, liquid, or solid medium of atoms or molecules that resist sedimentation, diffusion, and filtration. This definition is in contradistinction to crystalloids, which are freely diffusible. Oncotic pressure is defined as the osmotic pressure exerted by colloids in solution (hence it is redundant to use the phrase colloid oncotic pressure). Proteins in plasma are truly in solution, but they closely resemble a colloid solution and thus are referred to and treated as such. The osmotic pressure exerted by the naturally occurring colloids in plasma is higher than that calculated for an ideal solution in vitro. One of the main reasons for this discrepancy is that negatively charged proteins (such as albumin, which has a net negative charge of 17 at physiologic pH) retain cations within the intravascular space by electrostatic attraction (termed the Donnan effect).71 These cations contribute to the effective plasma protein osmotic pressure because osmotic pressure is proportional to the number of molecules present rather than their size. Therefore colloid osmotic pressure (COP) is the most correct term when referring to the osmotic pressure exerted by plasma proteins and their associated electrolyte molecules. For comparison, the oncotic pressure exerted by an albumin solution of 7 g/dL is 19.8 mm Hg, whereas the in vivo COP is actually 28 mm Hg, and the total osmotic pressure of all plasma solutes is 5400 mm Hg.71
By virtue of its relatively high concentration in the vascular space, albumin usually accounts for 60% to 70% of the plasma COP with globulins making up the remainder.108,168,176 Interestingly, the variation in COP in dogs may be because of differences in globulin concentration than in albumin concentration.65,108 Red blood cells and platelets do not contribute significantly to plasma COP.118 Serum albumin concentration is determined by the relative rates of synthesis, degradation, and loss from the body and its distribution between the extravascular and interstitial spaces. Albumin synthesis, which is unique to the liver, appears to be regulated, at least in part, by the hepatic plasma COP.53,117,130 Increases of plasma COP independent of albumin concentration, such as in hyperglobulinemia, are associated with decreased serum albumin concentration.18,131,132 The main site of albumin degradation is uncertain, but the reticuloendothelial system has been suggested. Equations have been derived to estimate plasma COP from plasma protein concentrations,108,160 but direct measurement with a colloid osmometer is more accurate.7,28,160,176
COPs measured in normal dogs and cats are given in Table 27-1.44,108,186
Table 27-1 Colloid Osmotic Pressure in Normal Cats and Dogs
| Species | Colloid Osmotic Pressure Mean ± SD (mm Hg) | Reference Number |
|---|---|---|
| Canine (plasma) | 20.8 ± 1.8 | 185 |
| Canine (plasma) | 17.5 ± 3.0 | 108 |
| Canine (whole blood) | 19.9 ± 2.1 | 44 |
| Feline (plasma) | 19.8 ± 2.4 | 185 |
| Feline (whole blood) | 24.7 ± 3.7 | 44 |
Interstitial colloid osmotic pressure
Capillaries are permeable to protein, despite the fact that the microvascular barrier greatly restricts macromolecular flux. Of the total quantity of albumin present in the body, 40% is intravascular and 60% is extravascular.133 Furthermore, all of the albumin present in plasma circulates through the interstitium every 24 hours.114 The interstitial COP varies from tissue to tissue depending on such factors as the permeability of the capillary wall to protein, the rate of transvascular solvent flow, the retention of protein in the interstitial matrix, and the rate of lymphatic clearance of protein. The microvascular barrier of skeletal muscle or subcutaneous tissue is relatively impermeable to protein, whereas the pulmonary capillary endothelium is more permeable with a reflection coefficient to albumin of approximately 0.5 to 0.64.113 Consequently, the normal protein concentration in lymph from skin or skeletal muscle is approximately 50% that of plasma compared with 65% in pulmonary lymph.113 Hyaluronan and its associated cations also may contribute to interstitial COP.5 Because of the volume occupied by the interstitial matrix, interstitial albumin is distributed in a volume that is less than the total interstitial volume. This phenomenon is called the volume exclusion effect, and the “excluded volume” with respect to albumin may be as high as one half to two thirds of the total interstitial volume.13,112,175 Consequently, in a normally hydrated interstitium, much less protein is required to exert a given osmotic pressure, and relatively smaller volumes of extravasated fluid result in greater decrements in interstitial COP. This effect maintains the intravascular-to-extravascular COP gradient in early edema formation. Conversely, when interstitial volume is overexpanded by fluid in edematous states, a dramatic increase occurs in the volume available for albumin sequestration.71 The increase in interstitial COP that occurs with dehydration acts to restrict mobilization of interstitial fluid.76
Intravascular hydrostatic pressure
Intravascular hydrostatic pressure is the main force that determines fluid egress from the vasculature. It may vary in different tissues and at different levels within each capillary bed. The normal hydrostatic pressure in the capillary bed is controlled by local myogenic, neurogenic, and humoral modulation of the arterial and venous resistances. Precapillary arteriolar constriction may reduce flow, and therefore hydrostatic pressure, through a capillary bed or shunt flow away from that bed, resulting in changes in the total surface area available for transvascular fluid movement. The hydrostatic pressure within a blood vessel at any particular site depends in part on where resistance to flow occurs, with hydrostatic pressures decreasing most across the areas of major resistance. In most tissues, the majority of resistance has been attributed to small arterioles, but experimental studies of the lung suggest that a significant pressure decrease may occur across the capillary bed itself.15,16,143
Interstitial hydrostatic pressure
As with all the other Starling forces, normal interstitial pressure also varies among tissues. Interestingly, in many tissues the resting pressure is slightly negative (subatmospheric), tending to favor rather than oppose fluid filtration from the microvasculature.179 This finding has been postulated to be the result of the molecular structure of the interstitial matrix, such that with normal hydration the biomechanical stresses on the molecules and the repulsion among like electrostatic charges act to expand the interstitium.5 In encapsulated organs, such as the kidney, normal interstitial pressures are positive. Interstitial pressures can also change depending on the functional state of the organ. For example, interstitial pressures in the nonabsorbing intestine are negative to slightly positive, whereas intestinal interstitial pressures are positive in the absorptive state.70 As mentioned before, the molecular structure of the interstitium mechanically opposes distention. Conventionally, it is said that one third of the total body water is found in the extracellular space and that the interstitium constitutes three fourths of the extracellular space. These figures are averages for the whole body, and the relative sizes of the intravascular and interstitial spaces vary among tissues. Tissues vary in their capacity to accommodate interstitial fluid depending on the size of the interstitial space relative to the total volume of the tissue and the nature of the interstitial matrix itself, especially its distensibility. The distensibility of an organ or tissue is termed its compliance, and depending on the nature of the tissue, the compliance of the interstitium may vary widely. Extreme examples would be tendon (which is relatively noncompliant) and loose subcutaneous connective tissue (which is relatively distensible). The accumulation of edema fluid in the peribronchovascular interstitium in canine lungs is likely the result of the higher compliance of this region of the pulmonary interstitium.
An extremely important concept related to the interstitial hydrostatic pressure is that of stress relaxation. In a normally hydrated animal, the interstitium in most tissues is relatively noncompliant. Small increases in volume caused by increased fluid extravasation result in large changes in interstitial hydrostatic pressure that act to oppose further extravasation of fluid and increase lymphatic drainage pressure—two of the tissue safety factors described later.72,157 As the interstitium becomes gradually more distended, it continues to oppose distention until a critical point is reached (suggested to correspond to the disordering of the interstitial matrix). Abruptly, the resistance to distention decreases (i.e., compliance increases), and fluid then can accumulate without a corresponding protective increase in interstitial pressure and lymph flow. At this point, the distended interstitium no longer opposes the movement of fluid and protein, resulting in increased extravasation and self-perpetuation of the edemagenic process. Furthermore, the greatly increased interstitial space provides a large volume for protein sequestration.
Tissue safety factors
From the previous discussion, it should be apparent that there are three main homeostatic mechanisms that prevent or limit accumulation of fluid in the interstitium. First, extravasation of fluid into a relatively nondistensible interstitium results in an increased interstitial hydrostatic pressure that opposes further extravasation. Second, after extravasation of low-protein fluid, interstitial COP decreases because of dilution and washout of protein, thereby maintaining or even enhancing the COP gradient between the intravascular space and interstitium. Third, the increased interstitial pressure results in an increased driving pressure for lymphatic drainage. These alterations in Starling forces that act to limit interstitial fluid accumulation have been termed the tissue safety factors.72,157 Their relative importance varies depending on the characteristics of the tissue.5,33 In a tissue that is relatively nondistensible (e.g., tendon), an increase in interstitial pressure may be the most important means by which to counteract filtration. In a tissue with moderate distensibility and with a relatively impermeable microvascular barrier (e.g., skin), the decrease in interstitial COP assumes more importance in protecting against interstitial fluid accumulation. In a distensible tissue that is quite permeable to protein (e.g., lungs), increased lymph flow appears to be the most important safeguard against interstitial edema.183
Pharmacokinetics and pharmacodynamics of macromolecular plasma volume expanders
Transvascular fluid dynamics are extremely complex. The balance of the hydrostatic and osmotic pressure gradients between the intravascular and interstitial fluid compartments forms the basis for microvascular fluid exchange. However, this simple concept is belied by the great heterogeneity in Starling forces and transvascular fluid dynamics that exists among and within tissues in both healthy and diseased states. The relative importance of the different tissue safety factors also varies among tissues, and the potential for self-regulation of transvascular fluid fluxes often is underestimated. When considering fluid therapy with macromolecular volume expanders, a great deal of emphasis has been placed on the manipulation of individual Starling forces (such as intravascular COP) in isolation rather than addressing the system in its entirety. Maintenance of intravascular volume depends on an intricate and dynamic interaction between the intravascular and interstitial Starling forces and the structure and function of the microvascular barrier, interstitium, and lymphatic system. Infusion of intravenous fluids can change all of the Starling forces, modify the permeability of the microvascular barrier, change the volume and composition of the interstitium, and increase lymphatic flow. Furthermore, the magnitude and relative significance of these changes vary among and within tissues. Consequently, it is a gross and potentially dangerous oversimplification to view the body as the homogenous sum of its individual parts when contemplating intravenous fluid therapy. From a clinical standpoint, the differences between the lungs and the systemic circulation are of the utmost importance. For example, in a dog with systemic inflammatory response syndrome and aspiration pneumonia causing pulmonary edema by means of increased microvascular permeability, colloid therapy may be effective in limiting subcutaneous edema at the expense of worsening pulmonary fluid extravasation.
Despite this great heterogeneity, the concept that net fluid extravasation depends on the balance between intravascular COP and capillary hydrostatic pressure forms the basis for intravenous colloid therapy.64,73,90,174 By virtue of their larger molecular size, and in the absence of an increase in microvascular permeability, colloid molecules are retained within the vasculature to a greater degree than are crystalloids. Consequently, smaller volumes of colloid result in greater plasma volume expansion compared with crystalloid,51,144,145 and crystalloid is expected to leak into the interstitium to a greater degree than colloid and cause more interstitial expansion or edema.27 This may be beneficial if the animal has an interstitial fluid deficit or deleterious if there is interstitial edema. One hour after infusion of a crystalloid solution, as little as 10% of the infused volume may remain in the intravascular space.145 Some evidence indicates that tissue perfusion is better after volume expansion with colloids than with crystalloids, even when resuscitation is titrated to physiologic endpoints.63 Unfortunately larger colloids may reduce tissue perfusion by increasing plasma viscosity.22 Many factors influence the volume and duration of intravascular expansion associated with artificial colloids, including the species of animal, dose, specific colloid formulation, preinfusion intravascular volume status, and the microvascular permeability. These factors likely explain the great variability in intravascular persistence and volume expansion in published studies.
Artificial colloids are polydisperse; that is, they contain molecules of different molecular weight. In contrast, in a monodisperse colloid such as albumin, molecules are all the same size. The artificial colloids have extremely complex pharmacokinetics in part because of this large range of molecular sizes.84 The smaller molecules pass rapidly into the urine and interstitium, whereas the larger molecules remain in circulation and gradually are hydrolyzed by amylase or removed by the monocyte phagocytic system.161 This initial rapid excretion of small, osmotically active molecules followed by gradual elimination of large molecules results in an exponential decline in intravascular expansion. Manufacturer data sheets can be misleading because they may imply that a major proportion of the volume expansion lasts for 24 to 36 hours. Estimates of the degree of initial plasma volume expansion for hetastarch and dextran 70 vary from 70% to 170% of the infused volume.67,77,87,91,124 This decreases to approximately 50% of the infused volume after 6 hours. Volume expansion with hydroxyethyl starch then declines gradually from 60% to 40% of the infused volume during the next 12 to 18 hours, whereas with dextran 70 it decreases gradually from 40% to 20% of the infused volume.161 In experimental dogs, blood volume was increased by approximately 25% both immediately and 4 hours following infusion of 20mL/kg of both dextran 70 and hetastarch.147 In dogs with hypoalbuminemia of various causes receiving hydroxyethyl starch, COP was not significantly different from baseline 12 hours after infusion.106 In the authors’ experience, the duration of volume expansion with artificial colloids can be even shorter, especially with capillary leak syndromes. This relatively short duration of action and the high cost of artificial colloids have led some authors to question the cost-effectiveness of colloid infusions in veterinary patients.173
The duration of action of colloids may be expressed in terms of plasma colloid concentrations, plasma COP measurements, or degree of volume expansion. The initial volume of intravascular expansion is the result of the COP of the infused colloid, which is determined by the number of molecules, not their size. This concept is extremely important because the distribution of molecular weights is narrowed after intravenous infusion.57,58 The smaller molecules that are responsible for a large part of the COP and intravascular volume expansion are extravasated or excreted within hours. The intravascular colloid concentration (i.e., mass per unit volume) is still high due to the large molecules, but the COP is relatively low. COP and degree of volume expansion tend to decrease faster than does the plasma concentration of colloid. Data from an experimental study of euvolemic human volunteers given twice the usual dose of a high molecular weight form of hydroxyethyl starch may therefore have little bearing on the effects of commercially available hydroxyethylstarch in a dog with systemic inflammatory response syndrome in hypodynamic, septic shock.
To reiterate, the osmotic effect of macromolecules is because of their number rather than their size. Consequently, if more than 50% of the molecules leak into the interstitium, a net reduction in intravascular volume is likely as water leaves the intravascular space by osmosis along with the colloid. Therefore the difficulty is how to determine the magnitude of increase in permeability (i.e., the size of the “gaps” in the microvascular barrier). Although experimental techniques exist to detect an increase in microvascular permeability,14,25 they are not currently applicable in a clinical setting. A growing body of evidence suggests that hydroxyethyl starches can mitigate increases of microvascular permeability in several capillary leak states.34,97,109,184 The optimal molecular weight for this effect seems to be between 100 and 300 kDa.185 Unfortunately, relatively few products with molecules in this size range are available in the United States. Only 35% of the molecules in one preparation of hetastarch fall within this optimal size range.184 European formulations of hydroxyethyl starch (e.g., Haes-steril, Fresenius Kabi, Bad Homburg, Germany) contain more molecules in the optimal molecular size range.
Colloid preparations
The artificial colloids used most commonly worldwide fall into three major groups: the hydroxyethyl starch derivatives, the dextrans, and the gelatins. Availability varies among countries. The hydroxyethyl starches are synthesized by partial hydrolysis of amylopectin (the branched form of plant starch), the dextrans from a macromolecular polysaccharide produced from bacterial fermentation of sucrose, and the gelatins from hydrolysis of bovine collagen followed either by succinylation or linkage to urea. The preparations used most commonly in the United States are hydroxyethyl starch preparations and dextran 70, both of which are available as 6% (6 g/dL) solutions in 0.9% saline. Several gelatin-based products are available in Europe and Australia (Haemaccel, Intervet/Schering Plough Animal Health, Milton Keynes, UK; Gelofusine, Dechra Veterinary Products, Shrewsbury, UK).
Hydroxyethyl starches are manufactured by a complex process and are described using standardized pharmacologic terminology. An understanding of this terminology gives the clinician information about their molecular structure and allows estimation of their likely pharmacokinetics and pharmacodynamics. Amylopectin is a polysaccharide, which along with amylose, forms the plant structural polysaccharide, starch. Amylopectin is very similar in structure to glycogen and contains short chains of a-1,4-linked glucose units linked to other chains by α-1,6-links. Solutions of native starch would be unstable if injected as they are rapidly hydrolyzed by plasma amylases. Chemical modification is required to resist degradation and thereby increase intravascular persistence. This is achieved by substitution of the hydroxyl (-OH) groups on the glucose units with hydroxyethyl (-OCH2CH2OH) groups.
The terms “hetastarch,” “pentastarch,” and “tetrastarch” are nonspecific terms used to describe different preparations of hydroxyethyl starches. The term “hetastarch” and the abbreviation “HES” are sometimes used interchangeably, but this should be avoided; hetastarch is just one of the hydroxyethyl starches. The abbreviation, HES, may be correctly used as an umbrella term for all hydroxyethyl starches, which are then subclassified on the basis of their molecular structure. The HES family is most precisely described by reference to their molecular weight and their degree of substitution (e.g., HES 450/0.7 or HES 130/0.4). These characteristics are described more fully later. The C2/C6 hydroxyethylation ratio is another important pharmacologic characteristic that may be used as a descriptor but it is not routinely included in product descriptions at this time.177
Molecular Weight (MW)
In general, the molecules in HES preparations show great polydispersity. The molecules can range in size from a few thousand to a few million Daltons and in any one solution will generally follow a bell-shaped distribution. Hydroxyethyl starches have been arbitrarily divided into high molecular weight (>400 kDa), medium MW (200 to 400 kDa) and low MW (<200 kDa) preparations. The quoted MW represents the weight average molecular weight (e.g., 480 kDa for Hespan, 130 kDa for Voluven) but the actual range of sizes is wide. For example, the package insert for Hextend (Hospira, Lake Forest, Ill.) states that 80% of molecules fall between 2 and 2500 kDa, which means that 20% fall outside of this range. An independent analysis found that 85% of Hespan (Teva, Irvine, Calif.) consisted of molecules smaller than 300 kDa, 50% consisted of molecules smaller than 100 kDa, and molecular masses ranged up to 5000 kDa.184 It should also be remembered that the quoted MW only applies to the solution in vitro; as soon as the product is administered to a patient, the average MW will change as the product is subjected to excretion and hydrolysis. Measurement and interpretation of MW is further complicated as the quoted weight average molecular weight is only one way of calculating the “average” weight of a polydisperse polymer, the other method being the number average molecular weight. The number average molecular weight simply represents the total weight of polymer in solution divided by the number of molecules, whereas the weight average takes into account that the polymers are of different sizes and is exaggerated by larger particles in the mixture. As the detrimental effects of the colloids have been considered to be related to the presence of the higher molecular weight polymers, the weight average may be the more appropriate measure. The weight average MW is calculated from light scattering of the solution. The polydispersity index can be calculated from the ratio of the weight average to the number average for that solution. Hydroxyethyl starch with a weight average molecular mass of 100 to 300 kDa seems to provide the best compromise between colloid osmotic volume expansion and duration of action.40 Furthermore, this size distribution has less effect on coagulation164 and is the best size for reducing the increases in permeability present in patients with vascular leaks.184
Degree of Substitution and Molar Substitution Ratio
The amylopectin may be hydroxyethylated at carbons 2, 3, or 6 of the constituent glucose molecules. The degree of substitution is determined by measuring the number of substituted glucose molecules and dividing this by the total number of glucose molecules present. In contradistinction, the term substitution ratio or more correctly “molar substitution ratio” may also be used and refers to the total number of hydroxyethyl groups present divided by the quantity of glucose molecules. Although not identical, the terms are often used interchangeably with the degree of substitution being used most commonly in product descriptions. A higher degree of substitution prolongs intravascular persistence by slowing down the rate of hydrolysis (i.e., those products with a high degree of substitution will have longer persistence in the body). “Hetastarches” have the most hydroxyethylation (between 0.6 and 0.7), “pentastarches” a degree of substitution of 0.5, and “tetrastarches” a degree of substitution of 0.4.
C2/C6 Ratio
The hydroxyethylation can occur at carbons in position 2, 3, or 6 of the glucose unit. Individual glucose molecules can have from zero to three hydroxyethyl groups. Hydrolysis by amylase is slowed more by substitution at C2 as opposed to C6,166 thus those products with a high C2/C6 ratio will be metabolized more slowly and intravascular volume expansion will be expected to be longer. Hydroxyethylation at the C2 position confers greater resistance to degradation than the C6 position.
Hydroxyethyl starches are thus generally characterized by their weight average molecular weight, substitution ratio, and C2/C6 hydroxyethylation ratio.163 Hespan, for example, has an average molecular weight of 450 kDa and a substitution ratio of 0.7 and therefore is referred to as HES 450/0.7. Two forms of high molecular weight hydroxyethyl starch are available in the United States, HES 450/0.7 (Hespan, Teva, Irvine, Calif.) and Hextend (Hospira, Lake Forest, Ill.), which have a weight average molecular weight of 670 kDa, a molar substitution of 0.75, and a high C2/C6 ratio. In many European countries and Australia, the high molecular weight starches are no longer available. Several hydroxyethyl starch products are available in these countries with smaller average molecular weights, including pentastarch (HES 200/0.5) and HES 130/0.4 (Voluven, Fresenius Kabi). These lower molecular weight products have been developed to maximize volume expansion effects while minimizing the risk of adverse effects on the hemostatic system by reducing the number of large molecules. The FDA recently approved Voluven for use in the United States.
Albumin, obtained from purified human plasma, has been used to provide colloid support in human medicine for many years. Albumin most commonly is given to small animal patients as stored or fresh frozen plasma, stored whole blood, or fresh whole blood. Human serum albumin has been used in small animal patients and a canine serum albumin preparation has recently become available. Albumin has a molecular weight of approximately 69 kDa and a molecular radius of 3.5 nm. It is a monodisperse colloid (i.e., all albumin molecules are the same size). In addition to its role in maintaining plasma COP, it carries a wide range of substances such as bilirubin, fatty acids, metals and other ions, hormones, and drugs.134 Albumin equilibrates with the interstitial space more rapidly and to a greater extent than artificial colloids, and relatively large volumes must be given to achieve a sustained increase in plasma COP. In human medicine, a large trial comparing fluid resuscitation of critical patients with either saline or albumin found no significant differences between the groups in a number of variables, including 28-day outcome.60 The authors of the study concluded that decisions about which fluid to use should be based on clinician preference, possible adverse effects, and cost. Although administration of human albumin solutions to critically ill dogs has been associated with effective increases in serum albumin concentration, refractometric total solids, and COP with relatively few adverse effects,32,167 administration to healthy animals has been associated with serious adverse outcomes.36,61
When considering chronic albumin supplementation, as opposed to acute volume expansion, the amount of albumin required can be estimated using an equation that corrects for the expected volume of distribution across the intravascular and interstitial spaces75:
To increase the serum albumin concentration from 1.5 g/dL to 2.5 g/dL in a 20-kg dog:
This amount is equivalent to 2 L of plasma or 4 L of fresh whole blood and does not take into account ongoing losses. Hence, administration of albumin in plasma is an inefficient means of providing colloid support.
Clinical uses
Support Of Intravascular Volume
The principle use of colloids is for intravascular volume expansion in patients with hypovolemic or distributive shock. Colloids are generally considered to be more effective for this purpose on a mL/kg basis than isotonic crystalloids. However, given the many factors involved in the efficacy and persistence of colloid therapy and the heterogeneous nature of the patient population in which they are used, it is crucial to carefully assess the need for colloidal therapy and the clinical response of the patient. Colloid therapy is not a panacea; rather it represents one more group of drugs with specific indications, contraindications, benefits, and risks. The treatment of critically ill human patients with colloid solutions recently has been questioned in several meta-analyses of randomized clinical trials in human patients. 17,35,116,139,171 Despite the limitations of randomized clinical trials and meta-analyses,128 all showed a trend toward increased mortality when colloids were used to resuscitate human trauma patients. Subdivision of the patients in one study171 demonstrated that in trauma patients there was a 12.3% difference in mortality rate in favor of crystalloid therapy, and when data from studies that used nontrauma patients were pooled, there was a 7.8% difference in mortality rate in favor of colloid treatment. The authors concluded:
“in patients with trauma who are septic and in whom the capillary leak syndrome leads to adult respiratory distress syndrome, it may be assumed that colloid resuscitation would be no better than crystalloid resuscitation. In this study the meta-analysis of published data showed that this form of treatment is deleterious. In patients who are nonseptic or having elective surgery, however, the basement membrane is intact, and meta-analysis of data in this setting showed that treatment with colloids would be efficacious.”
The likely explanation of these results is that if vascular leak is sufficiently severe to allow significant colloid extravasation then colloids may worsen outcome compared with crystalloids. A meta-analysis that was designed a priori to investigate resuscitation after trauma showed a lower mortality rate associated with the use of crystalloid fluids.35
Similar large scale studies do not yet exist within the veterinary field. In experimental dogs given standard boluses of isotonic saline (80 mL/kg) or hetastarch (20 mL/kg), volume expansion was initially significantly greater with isotonic saline, was not significantly different 30 minutes following administration, and was greater, although not significantly so, in the hetastarch group after 4 hours.147 Hetastarch was more effective than crystalloid in reversing isoflurane-induced hypotension in healthy experimental beagles.1 Two small clinical studies comparing dextran/hypertonic saline with isotonic saline for fluid resuscitation of dogs suffering from gastric dilatation-volvulus syndrome137 and trauma138 did not reveal a consistent benefit with either approach.
Recommended Dose
The recommended dosage for the high molecular weight hydroxyethyl starches, the gelatins, and the dextrans is 20 mL/kg/day. Dependent on the patient’s status, this may be administered as one or more boluses with the speed of bolus administration being dependent on the clinical status of the patient. Although higher dosages have been used without apparent adverse effects,106,150 deleterious effects on coagulation (see later discussion) occur more commonly at and above this dosage. The newer low molecular weight hydroxyethyl starches available in Europe may be used at higher doses of up to 50 mL/kg/day.89 A dosage of 20 mL/kg represents one quarter of a dog’s blood volume, and if repeated doses are required to maintain perfusion, the underlying reason should be pursued aggressively. Cats seem to be much more likely to develop volume overload than dogs, at least in part due to their smaller blood volume as a percentage of body weight. The suggested dose in cats is 5 mL/kg. Lastly, colloid solutions may not contain a bacteriostat and such formulations are therefore intended for single-dose usage.
Colloid therapy in pulmonary disease
Many pulmonary diseases result in accumulation of excess fluid in the interstitium alone or in the interstitium and alveoli. This increase in so-called extravascular lung water is synonymous with pulmonary edema. The lung is relatively resistant to the edemagenic effects of hypoproteinemia,182 and the two most important mechanisms by which pulmonary edema occurs are an increase in pulmonary hydrostatic pressure and an increase in pulmonary microvascular permeability.153 High-pressure edema may occur secondary to left-sided heart failure or volume overload, whereas increased permeability edema may be caused by conditions such as pneumonia, toxic lung injury, and systemic inflammatory response syndrome. In some clinical settings, the pathogenesis of pulmonary edema may be unclear or include both components (e.g., neurogenic and reexpansion edema).
The pulmonary endothelium is relatively permeable to protein compared with other tissues, and albumin170 and HES86 equilibrate more rapidly with the interstitial space even in a normal lung. Consequently, the effective COP gradient that can be generated between the intravascular space and the pulmonary interstitium is lower than in other tissues. Therefore the lung must rely more on increased lymph flow than interstitial COP dilution to protect against pulmonary edema.153 Certain types of lung injury, such as pneumonia or chemical injury, further increase the permeability of the capillary endothelium to protein. When one considers the Starling equation, it becomes obvious that the capillary hydrostatic pressure becomes the major determinant of edema formation. Smaller increases in capillary hydrostatic pressure result in much greater fluid extravasation than occurs when the endothelium remains intact. This finding clearly explains clinical and experimental studies that show that colloid therapy significantly worsens pulmonary edema caused by increased microvascular permeability.78 If the alveolar epithelium is also damaged, interstitial edema can rapidly progress to alveolar flooding because there is, in effect, a direct conduit from the vasculature to the alveolar space.
Absorption of water, solutes, and protein occurs via different mechanisms and at vastly different rates. Resorption of sodium-containing alveolar fluid occurs mainly via active transport by the alveolar epithelium, most likely via a sodium-potassium pump with glucose cotransport, which β-adrenergic agonists stimulate.100,136 Fluid absorption occurs against the colloid osmotic gradient, which increases as fluid is reabsorbed and protein remains behind. Protein is cleared from the alveoli at a very slow rate,99 which is one of the reasons for the protracted resolution often seen with edema caused by increased permeability.
Colloid therapy may worsen pulmonary edema if the increase in endothelial permeability is such that the majority of colloid molecules can pass through the pulmonary capillary endothelium.79 This is particularly true if a significant increase in pulmonary capillary pressure occurs simultaneously, as is more likely with colloid infusion. Considering the extremely slow clearance of macromolecules from the alveolar space, this increase in edema may be life threatening. Conversely, if the increase in permeability is insufficient to allow loss of colloid into the interstitium, prudent colloid therapy can reduce extravascular lung water. Therefore it is important to critically evaluate the patient’s response to a test infusion of colloid. An increase in COP should be titrated to prevent an increase in respiratory rate and effort, or, at worst, a decrease in arterial oxygen concentration. In more advanced critical care settings, pulmonary capillary wedge pressure or even measurement of extravascular lung water may be used to guide fluid therapy. When using colloids in the patient with a systemic vascular leak state, and in the absence of hemorrhage, failure to retain colloid in the intravascular space for an appropriate time period suggests that extravascular leakage of colloid could be worsening, not helping, hypovolemia and edema. If arterial oxygenation worsens after colloid therapy in an animal with pulmonary edema caused by altered permeability, one must consider the possibility that the colloid is contributing to the pulmonary edema.
The use of colloids in patients with high-pressure pulmonary edema is controversial because of their greater propensity for volume overload and because existing therapies for heart failure are so effective. Therefore colloid therapy should be used with extreme caution to prevent increases in pulmonary capillary hydrostatic pressure. Colloid support in the patient with left-sided heart failure should only be used in a critical care environment with invasive monitoring capabilities. Increased left atrial pressure secondary to left-sided heart failure results in increased pulmonary capillary pressure and increased fluid extravasation into the pulmonary interstitium.73 Lymph flow in the lung increases to protect against interstitial fluid accumulation,182 but as extravasation increases, fluid begins to accumulate in the interstitium. In the alveoli, where gas exchange occurs, the capillary endothelial cell is closely apposed to the alveolar epithelial cell, and the perimicrovascular interstitium is relatively noncompliant. In contrast, the peribronchovascular interstitial tissue is more compliant, and fluid tends to accumulate as peribronchovascular edema cuffs, thereby protecting gas exchange.38,39 Eventually, edema fluid distends all parts of the pulmonary interstitium and ultimately fills the airspaces of the lung. Current theory suggests that because the alveolar membrane is so impermeable to solutes, alveolar filling does not occur by fluid flow through the epithelium, but rather fluid spills into the airspaces at the junction of the alveolar and airway epithelia.152 In the absence of increases in permeability, maintenance of intravascular COP via colloid administration can be protective against cardiogenic pulmonary edema.174 Furosemide also increases COP, and, contrary to popular belief, it does not appear to reduce plasma volume.48,142 Because of the opposing effects of intravascular hydrostatic pressure and COP, monitoring the gradient between pulmonary artery occlusion pressure and COP has been suggested in the management of pulmonary edema.48,119,120
Chronic hypoproteinemia
The effective COP acting to retain fluid within the intravascular space is the net difference between the intravascular COP and interstitial COP. As intravascular COP decreases, fluid with a lower COP passes from the vasculature and dilutes the interstitial protein concentration such that interstitial COP also decreases. Consequently, the gradient between intravascular and interstitial COP is preserved. In addition, increased lymphatic drainage will also protect against edema formation. Hence, a low plasma COP per se does not necessitate colloid therapy in the absence of clinical signs such as hypovolemia or edema. Indeed, people with an hereditary form of complete albumin deficiency have plasma COP that still is half of normal because of increased globulin concentrations, and affected individuals exhibit minimal peripheral edema.9,49 There also appear to be no serious clinical signs in an autosomal recessive hereditary albumin deficiency in rats.107 Interestingly, affected rats have marked hypercholesterolemia.
In our clinical experience and in experimental studies,182 animals with severe hypoproteinemia (COP, <11 mm Hg) may exhibit peripheral edema but rarely develop pulmonary edema. In dogs with hypoalbuminemia, hydroxyethyl starch has been shown to result in clinical improvement of peripheral edema or ascites.150 The role of macromolecules in maintaining the selective permeability of the microvascular barrier46,102 provides a rationale for the prophylactic use of albumin or artificial colloid. It is, however, most important to diagnose and treat the underlying cause of the hypoproteinemia rather than administer palliative colloid therapy. Furthermore, if large ongoing losses are present, as can be the case with protein losing nephropathies and enteropathies, colloid support may not be effective.106
Treatment complications and adverse effects
Effects on hemostasis
The debate about whether artificial colloids cause abnormalities in coagulation is largely redundant because all of the older, higher molecular weight artificial colloids can cause abnormalities of primary and secondary hemostasis. The more important question is whether these coagulopathies are clinically relevant. Despite many studies supporting a lack of clinically relevant bleeding, there also is a large amount of clinical and experimental evidence documenting serious, potentially life-threatening bleeding after administration of hydroxyethyl starches and dextran.6,21,42,162,172 This apparently conflicting evidence implies that coagulation abnormalities are clinically relevant only in some cases. The effects on coagulation appear to be directly related to the intravascular concentration of artificial colloid.172 Higher plasma concentrations of colloid may occur after larger doses, repeated administration, or reduced intravascular degradation. However, although high molecular weight has been considered to be one of the key factors in determining coagulation effects of HES products,164 in general a reduction in molecular weight has also been associated with a reduction in degree of substitution. Recent intriguing work evaluating the coagulation effects of products with differing MW but the same low degree of substitution (HES 130/0.42, HES 500/0.42, and HES 900/0.42) that demonstrated similar effects on coagulation for all three preparations suggests that molecular weight has less effect than the degree of substitution.95 A further study evaluating HES 700 with varying degrees of substitution and C2/C6 ratios suggests that effects on coagulation are minimized when there is a low degree of substitution and a low C2/C6 ratio.169 This opens up the possibility for development of HES products with higher MW (and thus potentially better intravascular persistence) but minimal effects on coagulation. With repeated administration, the small colloid molecules are constantly excreted, and the relative concentration of larger molecules increases. This fact explains why many studies reporting clinically relevant bleeding refer to patients who received repeated doses of colloid over a period of days.
The exact mechanism of action by which coagulation is affected still is not fully understood; however, great progress has been made over recent years. Older studies reported reductions in factor VIII and von Willebrand’s factor (greater than those expected by dilution) and weakened clot formation.2-4,68,69,82 Colloid molecules may impair the action of endothelial adhesion molecules, thereby reducing endothelial release of von Willebrand’s factor.37 Decreases in vWF and factor VIII may also occur due to binding with HES molecules and accelerated elimination of the complex.88 In essence, colloids can cause an acquired type 1 form of Von Willebrand disease (VWD). Dogs that already have mild to moderate VWD may experience severe reductions in VWF and factor VIII following colloid infusion. Colloids should be avoided in known cases of VWD. Platelet dysfunction independent of von Willebrand factor is also present; its mechanism has not been fully elucidated but is at least in part due to the ability of HES molecules to coat the surface of platelets and interfere with ligand binding.52
The reductions in factor VIII (FVIII), which is stabilized by vWF in circulation, accounts for the mildly prolonged activated partial thromboplastin times that have been observed in people after HES administration.81 Hydroxyethylstarches decrease agonist-induced expression and activation of platelet integrin αIIbß3 (formerly known as GPIIb/IIIa).62 Integrin αIIbß3 on the surface of the platelet binds fibrinogen, and therefore plays a vital role in platelet aggregation and formation of a platelet plug. It has also been shown that HES molecules coat the surface of the platelet, limiting binding of ligands to cell surface receptors, which may decrease function of platelets independent of the integrin αIIbß3 blockade.52
The clinical relevance of platelet dysfunction after HES administration has been manifest in people as increased postoperative blood loss or increased transfusion requirements in some patient populations.20 Nevertheless, some studies in surgical patients have also shown no significant increase in blood loss.80,94 Trials in postoperative patients that have used rapidly degradable HES solutions found no difference in rates of blood loss and transfusion requirements when compared to albumin or gelatin.88 A recent pooled analysis of studies in major surgery comparing HES 130/0.4 (Voluven) and HES 200/0.5 (Starquin) found that estimated blood loss and transfusion requirements were significantly reduced in the group receiving HES 130/0.4.89
In veterinary medicine, administration of hetastarch 670/0.75 as compared with 0.9%NaCl has been shown to affect canine platelet function both in vitro178 and in vivo148 in clinically healthy dogs. A single dose of HES 670/0.75 at 20 mL/kg IV causes platelet dysfunction in dogs for at least 5 hours after injection. The clinical impact of platelet dysfunction induced by HES solutions in veterinary medicine remains to be established but it is reasonable to assume that it could be of clinical significance. Assessing platelet function by performing a buccal mucosal bleeding time (or other platelet function analysis if available) following colloid infusion would seem prudent in select at-risk patients.
It seems prudent to supplement clotting factors in animals at risk by use of fresh frozen plasma. In addition, desmopressin has been shown to increase factor VIII:C activity after hydroxyethyl starch infusion and should be considered as adjunctive therapy along with fresh frozen plasma administration.41
The observation that colloids impair the action of endothelial adhesion molecules also raises the possibility that colloids may reduce neutrophil adhesion in sepsis37 and explain the higher neutrophil counts observed after dextran 70 infusion in endotoxic shock.105
Intravascular volume overload
Because colloids are retained within the vascular system to a greater extent than are crystalloids, there is a greater likelihood of volume overload with injudicious administration of colloids. Most clinicians are more familiar with crystalloid than with colloid infusion rates, and a helpful method to ensure a safe colloid infusion rate is to estimate the equivalent crystalloid infusion rate. Approximately 20% to 25% of crystalloid remains within the intravascular space 1 hour after infusion compared with 100% of the volume of infused colloid. Therefore multiplying the colloid infusion rate by four allows one to conceptualize the volume expansion effects of the colloid in terms of an equivalent crystalloid volume: 20 mL/kg/hr of colloid is equivalent to 80 mL/kg/hr of crystalloid. Animals with heart, lung, or brain disease or oliguria/anuria should be closely monitored during colloid administration, ideally by direct monitoring of central venous pressure. Cats are more likely to develop volume overload than dogs. This is due in part to their smaller blood volume as a percentage of body weight, but also to inadvertently administering the canine dose to a cat.
Effects on the kidney
The low molecular weight dextrans such as dextran 40 have been reported to cause acute renal failure.59,96 Renal dysfunction has also been associated with HES solutions although recent reports suggest that concerns regarding renal function related to the older high MW HES should not be extrapolated to the new low MW products.19,177 In people, there is growing evidence that there is a dose-related association between the use of slowly degradable hydroxyethyl starch solutions and acute kidney injury in certain subsets of patients, such as in sepsis.29,141 Glomerular filtration of a high concentration of small colloid molecules is postulated to cause obstruction of the renal tubules or osmotic nephrosis.59,96 Another concern in patients with oliguric or anuric renal failure is that the kidneys are the major route of excretion for all artificial colloids; a situation analogous to the pharmacokinetics of mannitol. There is no other rapid excretion route for colloids, so animals with renal failure and reduced glomerular filtration rate will be at much greater risk of volume overload. Although published evidence is lacking in veterinary medicine, it is prudent to limit or avoid artificial colloid therapy in patients with documented renal failure and in those at high risk of renal tubular injury/renal failure. If use of colloids is deemed necessary in these patients, urine output and renal function should be monitored closely and the dose of colloid (both total cumulative and duration) should be minimized.
Anaphylaxis
Anaphylactic or anaphylactoid reactions have been reported in people following the administration of dextrans, hydroxyethyl starches, and gelatins,125 but the incidence of serious complications is extremely low.126 Hydroxyethyl starch was associated with pruritus in up to 33% of patients treated with long-term infusions.66 Deposits of hydroxyethyl starch in cutaneous nerves101 and histiocytic skin infiltrates43 were thought to be responsible. Interestingly, pruritus also has been reported after infusion of lactated Ringers solution.24 Several studies have raised concerns about the potential effects of plasma substitutes on reticuloendothelial function.140 Decreased concentrations of the opsonic plasma factor, fibronectin, have been reported with use of hydroxyethyl starch165 and gelatins.26
Laboratory tests and interpretation, clinical evaluation, and monitoring
Refractometry does not accurately reflect the concentration or the osmotic effect of synthetic colloids.30 The older forms of hydroxyethyl starch and dextran 70 available in the United States both yield refractometric total solids (RTS) readings of 4.5 g/dL. As plasma volume is replaced by artificial colloid, the measured RTS should approach that of the artificial colloid. Theoretically, administering artificial colloid to an animal with an initial RTS concentration greater than 4.5 g/dL will reduce the measured RTS, whereas administering artificial colloids to an animal with an initial RTS concentration less than 4.5 g/dL should increase the measured RTS toward 4.5 g/dL. However, in vitro addition of either of these colloid preparations (in an amount corresponding to a 22-mL/kg dose in a patient) to a 2.5% solution of human serum albumin (initial RTS concentration, <2.5 g/dL) led to minimal increases in the RTS concentration despite an increase in measured COP.30 As more artificial colloid was added to the albumin solution, the RTS concentration did increase, but the amount of colloid necessary to cause this change was greater than the volume likely to be used in clinical patients.
The in vivo situation is more complicated because of other effects such as extravasation, excretion of colloid, and osmotic fluid shifts into the vascular space after administration. In the authors’ experience, most patients with preinfusion RTS concentration of 5 g/dL have a decrease in RTS concentration after colloid administration. Conversely, increases in RTS after colloid administration seem to be uncommon, regardless of the initial RTS. The clinician should anticipate the dilutional effect caused by intravascular volume expansion that occurs with colloid infusion.
Hematologic and biochemical parameters may decrease due to simple dilution. Objective measures of hemodilution (e.g., serum albumin concentration and PCV) almost invariably decrease after colloid infusion. Platelet count and serum potassium concentration also seem to be reliably decreased. Failure to recognize the dilutional decrease in RTS or albumin concentrations could cause the clinician to misinterpret the decrease as an indication for more colloid and increase the likelihood of volume overload. In contrast, administration of HES may result in increased serum amylase activity (200% to 250% of normal) because of its binding to HES and decreased excretion.23,85,104
Unfortunately, assays for the quantitative determination of serum colloid concentrations are not readily available. Therapy with artificial colloids would ideally be monitored by measurement of plasma COP using a colloid osmometer but this is rarely possible. In most clinical practice, the response to colloids is assessed indirectly by monitoring the cardiovascular response to infusion. Because artificial colloids are excreted primarily by the kidneys, the presence of colloid molecules in the urine will typically lead to an increase in urine specific gravity (USG) measured after administration.149 Urine specific gravities in excess of 1.080 may occur. Changes are somewhat variable and depend upon the excretion rate of colloid and the volume of urine being produced. The increase in USG due to colloids means that it can no longer be used as an indicator of renal concentrating ability following colloid administration.
As mentioned previously (see Treatment Complications and Adverse Effects section), tests of primary and secondary hemostasis may be affected by colloid administration. For primary hemostasis, platelet function defects have been documented in dogs178 so it is likely that buccal mucosal bleeding times may also be prolonged. Increases in partial thromboplastin time may also be seen, presumably due to colloid-induced reductions in factor VIII and direct interference in clot formation. Finally, colloids have been reported to increase plasma viscosity,22 and hydroxyethyl starch can produce predictable but potentially misleading results in blood typing and crossmatching.50
Acknowledgments
The authors gratefully acknowledge Dr. Lisa Smart BVSc, DACVECC for her assistance with the sections on treatment complications and adverse effects and laboratory tests and interpretation, clinical evaluation, and monitoring.
1 Aarnes T.K., Bednarski R.M., Lerche P., et al. Effect of intravenous administration of lactated Ringer’s solution or hetastarch for the treatment of isoflurane-induced hypotension in dogs. Am J Vet Res. 2009;70:1345.
2 Aberg M., Arfors K.E., Bergentz S.E. Effect of dextran on factor VIII and thrombus stability in humans. Significance of varying infusion rates. Acta Chir Scand. 1977;143:417.
3 Aberg M., Hedner U., Bergentz S.E. Effect of dextran 70 on factor VIII and platelet function in von Willebrand’s disease. Thromb Res. 1978;12:629.
4 Aberg M., Hedner U., Bergentz S.E. Effect of dextran on factor VIII (antihemophilic factor) and platelet function. Ann Surg. 1979;189:243.
5 Aukland K., Reed R.K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1.
6 Baldassarre S., Vincent J.L. Coagulopathy induced by hydroxyethyl starch. Anesth Analg. 1997;84:451.
7 Barclay S.A., Bennett E.D. The direct measurement of colloid osmotic pressure is superior to colloid osmotic pressure derived from albumin or total protein. Intensive Care Med. 1987;13:114.
8 Bates D.O., Curry F.E. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol. 1996;271:H2520.
9 Bennhold H. Volume regulation and renal function in analbuminemia. Lancet. 1960;2:1169.
10 Bent-Hansen L. Initial plasma disappearance and tissue uptake of 131I-albumin in normal rabbits. Microvasc Res. 1991;41:345.
11 Bent-Hansen L. Whole body capillary exchange of albumin. Acta Physiol Scand Suppl. 1991;603:5.
12 Bent-Hansen L., Feldt-Rasmussen B., Kverneland A., et al. Plasma disappearance of glycated and non-glycated albumin in type 1 (insulin-dependent) diabetes mellitus: evidence for charge dependent alterations of the plasma to lymph pathway. Diabetologia. 1993;36:361.
13 Bert J.L., Mathieson J.M., Pearce R.H. The exclusion of human serum albumin by human dermal collagenous fibres and within human dermis. Biochem J. 1982;201:395.
14 Berthezene Y., Vexler V., Jerome H., et al. Differentiation of capillary leak and hydrostatic pulmonary edema with a macromolecular MR imaging contrast agent. Radiology. 1991;181:773.
15 Bhattacharya J., Nanjo S., Staub N.C. Factors affecting lung microvascular pressure. Ann N Y Acad Sci. 1982;384:107.
16 Bhattacharya S., Glucksberg M.R., Bhattacharya J. Measurement of lung microvascular pressure in the intact anesthetized rabbit by the micropuncture technique. Circ Res. 1989;64:167.
17 Bisonni R.S., Holtgrave D.R., Lawler F., et al. Colloids versus crystalloids in fluid resuscitation: an analysis of randomized controlled trials. J Fam Pract. 1991;32:387.
18 Bjorneboe M., Schwartz M. Investigations concerning the changes in serum proteins during immunization: the cause of hypoalbuminemia with high gamma globulin levels. J Exp Med. 1959;110:259.
19 Boldt J., Brosch C., Ducke M., et al. Influence of volume therapy with a modern hydroxyethyl starch preparation on kidney function in cardiac surgery patients with compromised renal function: a comparison with human albumin. Crit Care Med. 2007;35:2740.
20 Boldt J., Haisch G., Suttner S., et al. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend) on measures of coagulation. Br J Anaes. 2002;89:722.
21 Boldt J., Knothe C., Zickmann B., et al. Influence of different intravascular volume therapies on platelet function in patients undergoing cardiopulmonary bypass. Anesth Analg. 1993;76:1185.
22 Boldt J., Zickmann B., Rapin J., et al. Influence of volume replacement with different HES-solutions on microcirculatory blood flow in cardiac surgery. Acta Anaesthesiol Scand. 1994;38(5):432-438.
23 Boon J.C., Jesch F., Ring J., et al. Intravascular persistence of hydroxyethyl starch in man. Eur Surg Res. 1976;8:497.
24 Bothner U., Georgieff M., Vogt N.H. Assessment of the safety and tolerance of 6% hydroxyethyl starch (200/0.5) solution: a randomized, controlled epidemiology study. Anesth Analg. 1998;86:850.
25 Brigham K.L., Harris T.R., Owen P.J. [14C]urea and [14C]sucrose as permeability indicators in histamine pulmonary edema. J Appl Physiol. 1977;43:99.
26 Brodin B., Hesselvik F., von Schenck H. Decrease of plasma fibronectin concentration following infusion of a gelatin-based plasma substitute in man. Scand J Clin Lab Invest. 1984;44:529.
27 Brown R.H., Zerhouni E.A., Mitzner W. Visualization of airway obstruction in vivo during pulmonary vascular engorgement and edema. J Appl Physiol. 1995;78:1070.
28 Brown S.A., Dusza K., Boehmer J. Comparison of measured and calculated values for colloid osmotic pressure in hospitalized animals. Am J Vet Res. 1994;55:910.
29 Brunkhorst F.M., Engel C., Bloos F., et al. Competence Network Sepsis (SepNet) (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125.
30 Bumpus S.E., Haskins S.C., Kass P.H. Effect of synthetic colloids on refractometric readings of total solids. J Vet Emerg Crit Care. 1998;8:21.
31 Chan DL Colloids. Current recommendations. Vet Clin N Amer Sm Anim Pract. 2008;38:587.
32 Chan D.L., Rozanski E.A., Freeman L.M., et al. Retrospective evaluation of human albumin use in critically ill dogs. J Vet Emerg Crit Care. 2004;14:S8.
33 Chen H.I., Granger H.J., Taylor A.E. Interaction of capillary, interstitial, and lymphatic forces in the canine hindpaw. Circ Res. 1976;39:245.
34 Chi O.Z., Lu X., Wei H.M., et al. Hydroxyethyl starch solution attenuates blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Anesth Analg. 1996;83:336.
35 Choi P.T., Yip G., Quinonez M.D., et al. Crystalloids vs colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200.
36 Cohn L.A., Kerl M.E., Lenox C.E., et al. Response of healthy dogs to infusion of human serum albumin. Am J Vet Res. 2007;68:657.
37 Collis R.E., Collins P.W., Gutteridge C.N., et al. The effect of hydroxyethyl starch and other plasma volume substitutes on endothelial cell activation; an in vitro study. Intensive Care Med. 1994;20:37.
38 Conhaim R.L., Lai-Fook S.J., Eaton A. Sequence of interstitial liquid accumulation in liquid-inflated sheep lung lobes. J Appl Physiol. 1989;66:2659.
39 Conhaim R.L., Lai-Fook S.J., Staub N.C. Sequence of perivascular liquid accumulation in liquid-inflated dog lung lobes. J Appl Physiol. 1986;60:513.
40 Conhaim R.L., Rosenfeld D.J., Schreiber M.A., et al. Effects of intravenous pentafraction on lung and soft tissue liquid exchange in hypoproteinemic sheep. Am J Physiol. 1993;265:H1536.
41 Conroy J.M., Fishman R.L., Reeves S.T., et al. The effects of desmopressin and 6% hydroxyethyl starch on factor VIII:C. Anesth Analg. 1996;83:804.
42 Cope J.T., Banks D., Mauney M.C., et al. Intraoperative hetastarch infusion impairs hemostasis after cardiac operations. Ann Thorac Surg. 1997;63:78.
43 Cox N.H., Popple A.W. Persistent erythema and pruritus, with a confluent histiocytic skin infiltrate, following the use of a hydroxyethylstarch plasma expander. Br J Dermatol. 1996;134:353.
44 Culp A.M., Clay M.E., Baylor I.A., et al. Colloid osmotic pressure (COP) and total solids (TS) measurement in normal dogs and cats (abstract). Fourth International Emergency and Critical Care Symposium. 1994:705. San Antonio, Tex
45 Curry F.E. Effect of albumin on the structure of the molecular filter at the capillary wall. Fed Proc. 1985;44:2610.
46 Curry F.E., Michel C.C., Phillips M.E. Effect of albumin on the osmotic pressure exerted by myoglobin across capillary walls in frog mesentery. J Physiol. 1987;387:69.
47 Curry F.E., Rutledge J.C., Lenz J.F. Modulation of microvessel wall charge by plasma glycoprotein orosomucoid. Am J Physiol. 1989;257:H1354.
48 da Luz P., Shubin H., Weil M.H., et al. Pulmonary edema related to changes in colloid osmotic and pulmonary artery wedge pressure in patients after acute myocardial infarction. Circulation. 1975;51:350.
49 Dammacco F., Miglietta A., D’Addabbo A., et al. Analbuminemia: report of a case and review of the literature. Vox Sang. 1980;39:153.
50 Daniels M.J., Strauss R.G., Smith-Floss A.M. Effects of hydroxyethyl starch on erythrocyte typing and blood crossmatching. Transfusion. 1982;22:226.
51 Dawidson I.J., Willms C., Sandor Z.F., et al. Lactated Ringer’s solution versus 3% albumin for resuscitation of a lethal intestinal ischemic shock in rats. Crit Care Med. 1990;18:60.
52 Deusch E., Gamsjager T., Kress H.G., et al. Binding of hydroxyethyl starch molecules to the platelet surface. Anesth Analg. 2003;97:680.
53 Dich J., Hansen S.E., Thieden H.I.D. Effect of albumin concentration and colloid osmotic pressure on albumin synthesis in the perfused rat liver. Acta Physiol Scand. 1973;89:352.
54 Driessen B., Brainard B. Fluid therapy for the traumatized patient. J Vet Emerg Crit Care. 2006;16:276.
55 Ertmer C., Rehberg S., Van Aken H., Westphal M. Relevance of non-albumin colloids in intensive care medicine. Best Pract Res Clin Anaesthesiol. 2009;23:193.
56 Falk J.L., Rackow E.C., Weil M.H. Colloid and crystalloid fluid resuscitation. In: Shoemaker W.C., Ayres S., editors. Textbook of critical care. Philadelphia: WB Saunders, 1989.
57 Farrow S.P., Hall M., Ricketts C.R. Changes in the molecular composition of circulating hydroxyethyl starch. Br J Pharmacol. 1970;38:725.
58 Ferber H.P., Nitsch E., Forster H. Studies on hydroxyethyl starch. Part II: Changes of the molecular weight distribution for hydroxyethyl starch types 450/0.7, 450/0.5, 450/0.3, 300/0.4, 200/0.7, 200/0.5, and 200/0.1 after infusion in serum and urine of volunteers. Arzneimittelforschung. 1985;35:615.
59 Ferraboli R., Malheiro P.S., Abdulkader R.C., et al. Anuric acute renal failure caused by dextran 40 administration. Ren Fail. 1997;19:303.
60 Finfer S., Bellomo R., Boyce N., et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247.
61 Francis A.H., Martin L.G., Haldorson G.J., et al. Adverse reactions suggestive of Type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc. 2007;230:873.
62 Franz A., Bräunlich P., Gamsjäger T., Felfernig M., Gustorff B., Kozek-Langenecker S.A. The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg. 2001;92:1402-1407.
63 Funk W., Baldinger V. Microcirculatory perfusion during volume therapy. A comparative study using crystalloid or colloid in awake animals. Anesthesiology. 1995;82:975.
64 Gaar K.A.J., Taylor A.E., Owens L.J., et al. Effect of capillary pressure and plasma protein on development of pulmonary edema. Am J Physiol. 1967;213:79.
65 Gabel J.C., Scott R.L., Adair T.H., et al. Errors in calculated oncotic pressure of dog plasma. Am J Physiol. 1980;239:H810-H812.
66 Gall H., Schultz K.D., Boehncke W.H., et al. Clinical and pathophysiological aspects of hydroxyethyl starch-induced pruritus: evaluation of 96 cases. Dermatology. 1996;192:222.
67 Gollub S., Kangwalklai K., Schaefer C. Treatment of experimental hemorrhage with colloid-crystalloid mixtures. J Surg Res. 1969;9:311.
68 Gollub S., Schaefer C. Structural alteration in canine fibrin produced by colloid plasma expanders. Surg Gynecol Obstet. 1968;127:783.
69 Gollub S., Schaefer C., Squitieri A. The bleeding tendency associated with plasma expanders. Surg Gynecol Obstet. 1967;124:1203-1211.
70 Granger D.N., Barrowman J.A. Gastrointestinal and liver edema. In: Staub N.C., Taylor A.E., editors. Edema. New York: Raven Press, 1984.
71 Granger H.J., Laine G.A., Barnes G.E., et al. Dynamics and control of transmicrovascular fluid exchange. In: Staub N.C., Taylor A.E., editors. Edema. New York: Raven Press, 1984.
72 Guyton A.C., Granger H.J., Taylor A.E. Interstitial fluid pressure. Physiol Rev. 1971;51:527.
73 Guyton A.C., Lindsay N.W. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959;7:649.
74 Haraldsson B., Rippe B. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol Scand. 1987;129:127.
75 Hardin T.C., Page C.P., Schwesinger W.H. Rapid replacement of serum albumin in patients receiving total parenteral nutrition. Surg Gynecol Obstet. 1986;163:359.
76 Heir S., Wiig H. Subcutaneous interstitial fluid colloid osmotic pressure in dehydrated rats. Acta Physiol Scand. 1988;133:365.
77 Hempel V., Metzger G., Unseld H., et al. The influence of hydroxyethyl starch solutions on circulation and on kidney function in hypovolaemic patients. Anaesthesist. 1975;24:198.
78 Holcroft J.W., Trunkey D.D., Carpenter M.A. Extravasation of albumin in tissues of normal and septic baboons and sheep. J Surg Res. 1979;26:341.
79 Holcroft J.W., Trunkey D.D., Carpenter M.A. Sepsis in the baboon: factors affecting resuscitation and pulmonary edema in animals resuscitated with Ringer’s lactate versus Plasmanate. J Trauma. 1977;17:600.
80 Hüttner I., Boldt J., Haisch G., et al. Influence of different colloids on molecular markers of haemostasis and platelet function in patients undergoing major abdominal surgery. Br J Anaes. 2000;85:417.
81 Jamnicki M., Bombeli T., Seifert B., et al. Low- and medium-molecular-weight hydroxyethyl starches - comparison of their effect on blood coagulation. Anesthesiology. 2000;93:1231.
82 Jones P.A., Tomasic M., Gentry P.A. Oncotic, hemodilutional, and hemostatic effects of isotonic saline and hydroxyethyl starch solutions in clinically normal ponies. Am J Vet Res. 1997;58:541.
83 Kedem O., Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958;27:229.
84 Klotz U., Kroemer H. Clinical pharmacokinetic considerations in the use of plasma expanders. Clin Pharmacokinet. 1987;12:123.
85 Kohler H., Kirch W., Horstmann H.J. Hydroxyethyl starch-induced macroamylasemia. Int J Clin Pharmacol Biopharm. 1977;15:428.
86 Korent V.A., Conhaim R.L., McGrath A.M., et al. Molecular distribution of hetastarch in plasma and lung lymph of unanesthetized sheep. Am J Respir Crit Care Med. 1997;155:1302.
87 Korttila K., Grohn P., Gordin A., et al. Effect of hydroxyethyl starch and dextran on plasma volume and blood hemostasis and coagulation. J Clin Pharmacol. 1984;24:273.
88 Kozek-Langenecker S.A. Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology. 2005;103:654.
89 Kozek-Langenecker S.A., Jungheinrich C., Sauermann W., et al. The effects of hydroxyethyl starch (130/0.4) on blood loss and use of blood products in major surgery: a pooled analysis of randomised clinical trials. Anesth Analg. 2008;107:382.
90 Kramer G.C., Harms B.A., Bodai B.I., et al. Effects of hypoproteinemia and increased vascular pressure on lung fluid balance in sheep. J Appl Physiol. 1983;55:1514.
91 Lamke L.O., Liljedahl S.O. Plasma volume changes after infusion of various plasma expanders. Resuscitation. 1976;5:93.
92 Landis E.M., Pappenheimer J.R. Exchange of substances through the capillary walls. Hamilton W.F., Dow P., editors. Handbook of physiology, vol 2. Baltimore: Williams and Wilkins, 1963.
93 Luft J.H. The structure and properties of the cell surface coat. Int Rev Cytol. 1976;45:291.
94 Macintyre E., Mackie I.J., Ho D., et al. The haemostatic effects of hydroxyethyl starch (HES) used as a volume expander. Intensive Care Med. 1985;11:300.
95 Madjdpour C., Dettori N., Frascarolo, et al. Molecular weight of hydroxyethyl starch: is there an effect on blood coagulation and pharmacokinetics? Br J Anaesth. 2005;94:569.
96 Mailloux L., Swartz C.D., Capizzi R., et al. Acute renal failure after administration of low-molecular weight dextran. N Engl J Med. 1967;277:1113.
97 Marx G., Pedder S., Smith L., et al. Attenuation of capillary leakage by hydroxyethyl starch (130/0.42) in a porcine model of septic shock. Crit Care Med. 2006;34:3005.
98 Mathews K.A. The various types of parenteral fluids and their indications. Vet Clin North Am Small Anim Pract. 1998;28:483.
99 Matthay M.A., Berthiaume Y., Staub N.C. Long-term clearance of liquid and protein from the lungs of unanesthetized sheep. J Appl Physiol. 1985;59:928.
100 McAuley D.F., Frank J.A., Fang X., et al. Clinically relevant concentrations of ß2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid induced lung injury. Crit Care Med. 2004;32(7):1470-1476.
101 Metze D., Reimann S., Szepfalusi Z., et al. Persistent pruritus after hydroxyethyl starch infusion therapy: a result of long-term storage in cutaneous nerves. Br J Dermatol. 1997;136:553.
102 Michel C.C., Phillips M.E., Turner M.R. The effects of native and modified bovine serum albumin on the permeability of frog mesenteric capillaries. J Physiol. 1985;360:333.
103 Mishler J.M. Synthetic plasma volume expanders: their pharmacology, safety, and clinical efficacy. Clin Haematol. 1984;13:75.
104 Mishler J.M., Durr H.K. Macroamylasaemia following the infusion of low molecular weight-hydroxyethyl starch in man. Eur Surg Res. 1979;11:217.
105 Modig J. Beneficial effects of dextran 70 versus Ringer’s acetate on pulmonary function, hemodynamics and survival in a porcine endotoxin shock model. Resuscitation. 1988;16:1.
106 Moore L.E., Garvey M.S. The effect of hetastarch on serum colloid oncotic pressure in hypoalbuminemic dogs. J Vet Intern Med. 1996;10:300.
107 Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979;205:590.
108 Navar P.D., Narar L.G. Relationship between colloid osmotic pressure and plasma protein concentration in the dog. Am J Physiol. 1977;233:H295-H298.
109 Oz M.C., FitzPatrick M.F., Zikria B.A., et al. Attenuation of microvascular permeability dysfunction in postischemic striated muscle by hydroxyethyl starch. Microvasc Res. 1995;50:71.
110 Pappenheimer J.R. Passage of molecules through capillary walls. Physiol Rev. 1953;33:387.
111 Pappenheimer J.R., Renkin E.M., Borrero L.M. Filtration, diffusion and molecular sieving through peripheral capillary membranes. A contribution to the pore theory of capillary permeability. Am J Physiol. 1951;167:13.
112 Parker J.C., Falgout H.J., Grimbert F.A., et al. The effect of increased vascular pressure on albumin-excluded volume and lymph flow in the dog lung. Circ Res. 1980;47:866.
113 Parker J.C., Perry M.A., Taylor A.E. Permeability of the microvascular barrier. In: Staub N.C., Taylor A.E., editors. Edema. New York: Raven Press, 1984.
114 Parving H.H., Rasmussen S.M. Transcapillary escape rate of albumin and plasma volume in short- and long-term juvenile diabetics. Scand J Clin Lab Invest. 1973;32:81.
115 Patlak C.S., Goldstein D.A., Hoffman J.F. The flow of solute and solvent across a two membrane system. J Theor Biol. 1963;5:426.
116 Perel P., Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients (Cochrane review). Cochrane Database Syst Rev. 4, 2007.
117 Pietrangelo A., Panduro A., Chowdhury J.R., et al. Albumin gene expression is down-regulated by albumin or macromolecule infusion in the rat. J Clin Invest. 1992;89:1755.
118 Prather J.W., Gaar K.A., Guyton A.C. Direct continuous recording of plasma colloid osmotic pressure of whole blood. J Appl Physiol. 1968;24:602.
119 Rackow E.C., Fein I.A., Leppo J. Colloid osmotic pressure as a prognostic indicator of pulmonary edema and mortality in the critically ill. Chest. 1977;72:709.
120 Rackow E.C., Fein I.A., Siegel J. The relationship of the colloid osmotic-pulmonary artery wedge pressure gradient to pulmonary edema and mortality in critically ill patients. Chest. 1982;82:433.
121 Renkin E.M. B.W. Zweifach Award lecture: regulation of the microcirculation. Microvasc Res. 1985;30:251.
122 Renkin E.M. Multiple pathways of capillary permeability. Circ Res. 1977;41:735.
123 Renkin E.M., Joyner W.L., Sloop C.H., et al. Influence of venous pressure on plasma-lymph transport in the dog’s paw: convective and dissipative mechanisms. Microvasc Res. 1977;14:191.
124 Rieger A. Blood volume and plasma protein. 3. Changes in blood volume and plasma proteins after bleeding and immediate substitution with Macrodex, Rheomacrodex and Physiogel in the splenectomized dog. Acta Chir Scand Suppl. 1967;379:22.
125 Ring J. Anaphylactoid reactions to plasma substitutes. Int Anesthesiol Clin. 1985;23:67.
126 Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466.
127 Rippe B., Haraldsson B. Transport of macromolecules across microvascular walls: the two pore theory. Physiol Rev. 1994;74:163.
128 Rizoli S.B. Crystalloids and colloids in trauma resuscitation: a brief overview of the current debate. J Trauma. 2003;54:S82.
129 Roberts J.S., Bratton S.L. Colloid volume expanders. Problems, pitfalls and possibilities. Drugs. 1998;55:621.
130 Rothschild M.A., Oratz M., Evans C., et al. Alterations in albumin metabolism following serum and albumin infusions. J Clin Invest. 1964;43:1874.
131 Rothschild M.A., Oratz M., Franklin E.C., et al. The effect of hypergammaglobulinemia on albumin metabolism in hyperimmunized rabbits studied with albumin I131. J Clin Invest. 1962;41:1564.
132 Rothschild M.A., Oratz M., Mongelli J., et al. Albumin metabolism in rabbits during gamma globulin infusions. J Lab Clin Med. 1965;66:733.
133 Rothschild M.A., Oratz M., Schreiber S.S. Extravascular albumin. N Engl J Med. 1979;301:497.
134 Rothschild M.A., Oratz M., Schreiber S.S. Serum albumin. Hepatology. 1988;8:385-401.
135 Rudloff E., Kirby R. Colloid and crystalloid resuscitation. Vet Clin North Am Small Anim Pract. 2001;31:1207.
136 Sakuma T., Okaniwa G., Nakada T., et al. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305.
137 Schertel E.R., Allen D.A., Muir W.W., et al. Evaluation of a hypertonic-dextran solution for treatment of dogs with shock induced by gastric dilatation volvulus. J Am Vet Med Assoc. 1997;210:226.
138 Schertel E.R., Allen DAMuir W.W., et al. Evaluation of a hypertonic sodium chloride/dextran solution for treatment of traumatic shock in dogs. J Am Vet Med Assoc. 1996;208:366.
139 Schierhout G., Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961.
140 Schildt B., Bouveng R., Sollenberg M. Plasma substitute induced impairment of the reticuloendothelial system function. Acta Chir Scand. 1975;141:7.
141 Schortgen F., Lacherade J.C., Bruneel F., et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicenter randomised study. Lancet. 2001;357:911.
142 Schuster C.J., Weil M.H., Besso J., et al. Blood volume following diuresis induced by furosemide. Am J Med. 1984;76:585.
143 Shepard J.M., Gropper M.A., Nicolaysen G., et al. Lung microvascular pressure profile measured by micropuncture in anesthetized dogs. J Appl Physiol. 1988;64:874.
144 Shoemaker W.C. Comparison of the relative effectiveness of whole blood transfusions and various types of fluid therapy in resuscitation. Crit Care Med. 1976;4:71.
145 Shoemaker W.C., Schluchter M., Hopkins J.A., et al. Comparison of the relative effectiveness of colloids and crystalloids in emergency resuscitation. Am J Surg. 1981;142:73.
146 Simionescu M., Simionescu N., Palade G.E. Preferential distribution of anionic sites on the basement membrane and the abluminal aspect of the endothelium in fenestrated capillaries. J Cell Biol. 1982;95:425.
147 Silverstein D.C., Aldrich J., Haskins S.C., et al. Assessment of changes in blood volume in response to resuscitative fluid administration in dogs. J Vet Emerg Crit Care. 2005;15:185.
148 Smart L., Jandrey K.E., Hass P.H., et al. The effect of hetastarch (670/0.75) in vivo on platelet closure time in the dog. J Vet Emerg Crit Care. 2009;19:444.
149 Smart L., Hopper K., Aldrich J., et al. The effect of hetastarch (670/0.75) on urine specific gravity and osmolality in the dog. J Vet Intern Med. 2009;23:388.
150 Smiley L.E., Garvey M.S. The use of hetastarch as adjunct therapy in 26 dogs with hypoalbuminemia: a phase two clinical trial. J Vet Intern Med. 1994;8:195.
151 Starling E.H. On the absorption of fluid from the connective tissue spaces. J Physiol (Lond). 1896;19:312.
152 Staub N.C. Alveolar flooding and clearance. Am Rev Respir Dis. 1983;127:S44.
153 Staub N.C. Pulmonary edema. In: Staub N.C., Taylor A.E., editors. Edema. New York: Raven Press, 1984.
154 Staub N.C., Taylor A.E. Edema. New York: Raven Press, 1984.
155 Staverman A.J. Non-equilibrium thermodynamics of membrane processes. Trans Faraday Soc. 1952;48:176.
156 Taylor A.E. Capillary fluid filtration: Starling forces and lymph flow. Circ Res. 1981;49:557.
157 Taylor A.E. The lymphatic edema safety factor: the role of edema dependent lymphatic factors (EDLF). Lymphology. 1990;23:111.
158 Taylor A.E., Granger D.N. Exchange of macromolecules across the microcirculation. In: Renkin E.M., Michel C.C., editors. Handbook of physiology: microcirculation. Bethesda, Md: American Physiological Society, 1985.
159 Taylor A.E., Moore T., Khimenko P. Microcirculatory exchange of fluid and protein and development of the third space. In: Zikria B.A., Oz M.O., Carlson R.W., editors. Reperfusion injuries and clinical capillary leak syndrome. Armonk, New York: Futura Publishing Company, 1994.
160 Thomas L.A., Brown S.A. Relationship between colloid osmotic pressure and plasma protein concentration in cattle, horses, dogs, and cats. Am J Vet Res. 1992;53:2241.
161 Thompson W.L., Fukushima T., Rutherford R.B., et al. Intravascular persistence, tissue storage, and excretion of hydroxyethyl starch. Surg Gynecol Obstet. 1970;131:965.
162 Treib J., Haass A., Pindur G. Coagulation disorders caused by hydroxyethyl starch. Thromb Haemost. 1997;78:974.
163 Treib J., Haass A., Pindur G., et al. A more differentiated classification of hydroxyethyl starches is necessary. Intensive Care Med. 1997;23:709.
164 Treib J., Haass A., Pindur G., et al. Avoiding an impairment of factor VIII:C by using hydroxyethyl starch with a low in vivo molecular weight. Anesth Analg. 1997;84:1391.
165 Treib J., Haass A., Pindur G., et al. Decrease of fibronectin following repeated infusion of highly substituted hydroxyethyl starch. Infusionsther Transfusionsmed. 1996;23:71.
166 Treib J., Haass A., Pindur G., et al. HES 200/0.5 is not HES 200/0.5. Influence of the C2/C6 hydroxyethylation ratio of hydroxyethyl starch (HES) on hemorheology, coagulation and elimination kinetics. Thromb Haemost. 1995;74:1452.
167 Trow A.V., Rozanski E.A., Delaforcade A.M., Chan D.L. Evaluation of use of human albumin in critically ill dogs: 73 cases (2003-2006). J Am Vet Med Assoc. 2008;233:607.
168 Tullis J.L. Albumin. 1. Background and use. JAMA. 1977;237:355-360.
169 Von Roten I., Madjdpour C., Frascarolo P., et al. Molar substitution and C2/C6 ratio of hydroxyethyl starch: influence on blood coagulation. Br J Anaesth. 2006;96:455.
170 Vaughan T.R.J., Erdmann A.J., Brigham K.L., et al. Equilibration of intravascular albumin with lung lymph in unanesthetized sheep. Lymphology. 1979;12:217.
171 Velanovich V. Crystalloid versus colloid fluid resuscitation: a meta-analysis of mortality. Surgery. 1989;105:65.
172 Villarino M.E., Gordon S.M., Valdon C., et al. A cluster of severe postoperative bleeding following open heart surgery. Infect Control Hosp Epidemiol. 1992;13:282.
173 Wall P.L., Nelson L.M., Guthmiller L.A. Cost effectiveness of use of a solution of 6% dextran 70 in young calves with severe diarrhea. J Am Vet Med Assoc. 1996;209:1715.
174 Wareing T.H., Gruber M.A., Brigham K.L., et al. Increased plasma oncotic pressure inhibits pulmonary fluid transport when pulmonary pressures are elevated. J Surg Res. 1989;46:29.
175 Weiderhelm C.A., Black L.L. Osmotic interaction of plasma proteins with interstitial macromolecules. Am J Physiol. 1976;231:638.
176 Weisberg H.F. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978;8:155-164.
177 Westphal M., James M.F.M., Kozek-Langenecker S., et al. Hydroxyethyl starches. Different products - different effects. Anesthesiology. 2009;111:187.
178 Wieringa J.R., Jandrey K.E., Haskins S.C., et al. In vitro comparison on the effects of two forms of hydroxyethyl starch solutions on platelet function in dogs. Am J Vet Res. 2007;68:605.
179 Wiig H., Reed R.K. Volume-pressure relationship (compliance) of interstitium in dog skin and muscle. Am J Physiol. 1987;253:H291.
180 Wissig S.L., Charonis A.S. Capillary ultrastructure. In: Staub N.C., Taylor A.E., editors. Edema. New York: Raven Press, 1984.
181 Yuan Y., Granger H.J., Zawieja D.C., et al. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641-H646.
182 Zarins C.K., Rice C.L., Peters R.M., et al. Lymph and pulmonary response to isobaric reduction in plasma oncotic pressure in baboons. Circ Res. 1978;43:925.
183 Zarins C.K., Rice C.L., Smith D.E., et al. Role of lymphatics in preventing hypooncotic pulmonary edema. Surg Forum. 1976;27:257.
184 Zikria B.A. A biophysical approach: sealing of capillary leak by intravenous biodegradable macromolecules. In: Zikria B.A., Oz M.O., Carlson R.W., editors. Reperfusion injuries and clinical capillary leak syndrome. Armonk, NY: Futura Publishing Company, 1994.
185 Zikria B.A., King T.C., Stanford J., et al. A biophysical approach to capillary permeability. Surgery. 1989;105:625.
186 Zweifach B.W., Intaglietta M. Measurement of blood plasma colloid osmotic pressure. II. Comparative study of different species. Microvasc Res. 1971;3:83.