Chapter 28 Peritoneal Dialysis
Dialysis is the process by which water and solutes move between two compartments that are separated by a semipermeable membrane. In peritoneal dialysis (PD), the two compartments consist of blood in the peritoneal capillaries and fluid (dialysate) instilled into the peritoneal cavity; the peritoneum serves as the semipermeable membrane. The primary indication for PD in animals is for renal failure to correct the resulting water, solute, and acid-base abnormalities and to remove uremic toxins.
Biology of the peritoneal membrane
The peritoneum is the serosal membrane that lines the abdominal cavity. The portion that covers the viscera and other intraabdominal structures is known as the visceral peritoneum, and that which lines the abdominal cavity is known as the parietal peritoneum. In humans, the surface area of the peritoneum is approximately the same as the body surface area (1 to 2 m2), and the visceral peritoneum accounts for approximately 80% of the total.10 Peritoneal surface area is proportionately larger in comparison to body surface area in infants and children,11 suggesting that this difference would also be true for dogs and cats.
Anatomically, the peritoneum consists of the mesothelium and underlying interstitial tissue (Figure 28-1). The mesothelium consists of a simple squamous epithelial-like monolayer supported by a basement membrane. The mesothelial cells have many apical microvilli that increase the functional surface area of the membrane. In humans, the basement membrane contains type IV collagen, proteoglycans, and glycoproteins. The interstitium is a layer of connective tissue below the basement membrane. Found within the connective tissue are extracellular matrix molecules, including collagen, fibronectin, and elastin. This layer has a gel-like character because of the presence of various proteoglycans. The peritoneal microvasculature is composed of true capillaries and postcapillary venules, which are supported by a negatively charged glycocalyx.61 These vessels are located at various distances from the mesothelial surface and can be found throughout the connective tissue layer. Lymphatics also are found in this layer, most commonly in the subdiaphragmatic peritoneum. These lymphatics drain primarily via stomata in the diaphragmatic peritoneum.10,48 The role of lymphatics in fluid and solute exchange from the peritoneum is poorly understood because of the difficulty in directly measuring lymph flow. Lymph flow is affected more by gravity than is blood flow through vessels, and therefore the upright posture of humans versus the quadruped stance of animals may mean that the role of peritoneal lymphatics differs between species.
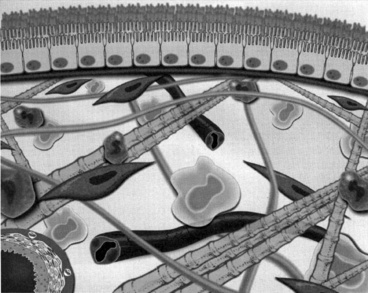
Figure 28-1 Diagrammatic representation of the peritoneal membrane.
(From Nagy JA, Jackman RW. Dialysis and transplantation: a companion to Brenner & Rector’s the kidney. Philadelphia: WB Saunders, 2000: 110.)
The most important function of the peritoneal membrane is to provide a protective, lubricating surface for the abdominal organs. Mesothelial cells secrete glycosaminoglycans including hyaluronic acid, proteoglycans such as decorin and biglycan, and phosphatidylcholine-containing lamellar bodies that allow free movement of the visceral organs during respiration and gastrointestinal peristalsis.48,61 Mesothelial cells play a role in a number of other processes, including antigen presentation, control of inflammation, tissue repair, coagulation, and fibrinolysis.13,35 It is generally believed, however, that the mesothelium does not represent a significant barrier to water transport.61 The anatomic structures that appear to play the most important role in fluid and solute transport are the walls of the capillaries and the extracellular matrix located in the submesothelial cell connective tissue.27,28,63 Peritoneal capillaries are composed primarily of nonfenestrated endothelial cells supported by a basement membrane. Endothelial cells contain aquaporins, which are 20-kDa cellular membrane proteins that are responsible for water transport. Intercellular clefts between endothelial cells also play a role in solute transport.36
Although the anatomic surface area of the peritoneum is large, the effective surface area—that area involved in fluid and solute movement—is considerably smaller. This discordance is because transport of water and solutes is primarily dependent on the surface area of peritoneal capillaries, rather than the mesothelium.3,10,36
Fluid and solute transport
The mechanisms by which fluids and solutes are transported across the peritoneal membrane involve several physical processes, including diffusion, convection, and ultrafiltration. Diffusion can be defined as the tendency for solutes to disperse within the available space.11 Solutes move by osmosis from a space with a higher concentration of that solute to one with a lower concentration. When this movement occurs across a semipermeable membrane, the rate of diffusion is governed by the permeability of the membrane, the available surface area for diffusion, and the concentration of solute on either side of the membrane. Diffusion is most rapid when the two solutions have markedly different solute concentrations, and the rate of movement of solute slows as the concentrations become more equal. Diffusion continues until the solutions on either side of the semipermeable membrane are of equal solute concentration.
Ultrafiltration is the removal of fluid (water) during PD. The rate of ultrafiltration is dependent on the osmotic or oncotic gradient between peritoneal capillary plasma and dialysate, as well as the effective peritoneal surface area and capillary blood flow.61,68 In PD, ultrafiltration is accomplished by instilling fluid into the peritoneal cavity that is of higher osmolality than that of plasma. Ultrafiltration is frequently desired when performing PD because animals are often overhydrated as the result of fluid administration.
Convection occurs when solutes are carried along with the bulk flow of water during ultrafiltration. This movement can occur even when the concentrations of solute on either side of the semipermeable membrane would not promote diffusion of the solute. This effect does not play an important role in PD; it is more important in hemodialysis, where this process can be mechanically manipulated (see Chapter 29).
Transport of water and solutes across the peritoneal membrane is best explained by the three pore theory.* Large pores, 100 to 200 Å in diameter, correspond to clefts in the endothelium and allow the transport of macromolecules such as albumin. They are present in very small numbers, accounting for less than 0.01% of the total pore surface area. Small pores, 20 to 25 Å in diameter, also correspond to clefts in the endothelium. They present in large numbers, representing more than 90% of the pore surface area, and allow the passage of low molecular weight substances such as urea. creatinine, and glucose. Ultrasmall pores, 4 to 6 Å in diameter, are aquaporin I channels found within peritoneal capillary and mesothelial cells, and transport only water.21,40,50-52,59,61 Aquaporins are a family of transmembrane polypeptides that permit water transport across the cellular membrane in response to an osmotic gradient.21,51,59,71 Aquaporin expression in mesothelial cells can be induced by exposure of the cells to hyperosmotic solutions.50
It has also been postulated that the location and the number of peritoneal capillaries affect the rate of transport of fluid and solutes.10,41,56 Those capillaries that are located farther from the mesothelium would participate less in the transport process.
In PD, diffusion is responsible for the transfer of urea, creatinine, and other small solutes from the compartment in which they are present in high concentration (plasma in peritoneal capillaries) to that in which they have low or no concentration (dialysate). The other factor affecting diffusion is the ability of a membrane to transport the specific solute that is directly proportional to the effective peritoneal surface area, and inversely proportional to the overall resistance. The mass transfer area coefficient (MTAC) is the theoretical clearance rate that would occur if the concentration gradient for a solute is infinitely high. The osmotic gradient, MTAC, and rate of diffusion are highest at the beginning of a dwell cycle, when the concentration gradient is highest.61,68 The rate of removal of a substance by diffusion is not only related to osmotic gradients but also to the size of the molecule and to the area available for diffusion. Urea has a relatively low molecular weight of 60 and diffuses more rapidly than creatinine, which has a molecular weight of 113. Larger molecules such as albumin (MW 69,000) are dependent on diffusion through larger pores, and the rate is comparably slower (Figure 28-2).
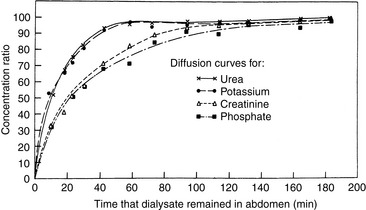
Figure 28-2 Equilibrium for urea, potassium, creatinine, and phosphate during peritoneal dialysis in dogs. Urea and potassium diffused rapidly and reached 85% of equilibrium in 40 minutes, whereas creatinine and phosphate were only 65% equilibrated. The flattening shapes of diffusion curves indicate that equilibration periods (dwell times) of 40 minutes or less were most efficient.
(From Kirk RW. Current veterinary therapy VII. Philadelphia: WB Saunders, 1980: 1107.)
Ultrafiltration is the removal of fluid (water) during PD. The rate of ultrafiltration is dependent on the osmotic or oncotic gradient between peritoneal capillary plasma and dialysate, as well as the effective peritoneal surface area and capillary blood flow.61,68
Convection (solvent drag) is the movement of solutes accompanying the flow of water from peritoneal capillaries into the peritoneal cavity. For most solutes, movement by convection does not occur in direct proportion to their concentration in blood. This effect is termed sieving and occurs because there is a greater barrier to solute than water movement across the peritoneum. Sieving coefficients vary depending upon the charge and molecular weight.10,15,36,61,68 As a result of sieving, the rate of decrease in solute concentration gradient gradually slows with longer dwell times. In people, physical properties of the peritoneal membrane vary, resulting in different coefficients. People treated with chronic PD undergo testing to determine the rate of ultrafiltration and solute clearance. One such test measures the rate at which creatinine appears in the dialysate compared with its concentration in plasma. The reason for performing such tests is that humans who are treated with chronic peritoneal dialysis have or develop changes in the peritoneal membrane that affect the rate at which solutes are transported. In low solute transporters, the osmotic gradient between plasma and dialysate remains high for a longer period, and therefore there is a high rate of ultrafiltration of water into dialysate. In high transporters, there is more efficient removal of urea, creatinine, and other uremic substances, but ultrafiltration is less efficient. In average transporters, the rates of solute and water movement are intermediate between those of low and high transporters.11,36,49 There is no such corresponding information available for clinical use in dogs and cats. Although such information would be useful in formulating an accurate dialysis prescription, its benefit in the treatment of acute disease is likely less important than for chronic disease.
Changes in the peritoneal membrane in chronic dialysis
Peritoneal dialysis is one of the methods of chronic renal replacement therapy in humans. Repeated, long-term instillation of dialysate fluid into the peritoneal cavity (3 to 4 years) results in a number of pathophysiologic changes that result in decreased diffusion and convection across the peritoneal membrane. Glucose-degradation products (GDPs) in PD fluids are most commonly implicated in membrane failure.61 Because virtually all of the PD done in veterinary patients is short-term, these changes are not likely to affect clinical management.
Indications for peritoneal dialysis
The primary indication for PD in animals is for the treatment of acute kidney injury. This includes oliguric or anuric renal failure, acute polyuric renal failure with severe uremia that is unresponsive to fluid therapy, and postrenal uremia resulting from ureteral obstruction or a rupture in the urinary collecting system. Although PD is less efficient than hemodialysis in correcting uremia, and water and solute abnormalities, it still has a number of therapeutic advantages (Box 28-1). The decreased efficacy may be beneficial in treating cats and small dogs, in which rapid water and electrolyte shifts can result in serious clinical consequences. The equipment and supplies used for PD are easily obtained, and the technique for performing PD, although labor intensive, is not difficult. This makes PD a useful therapeutic modality for private practices, especially those located in areas distant from hemodialysis facilities.
Although acute kidney injury is the most common indication for PD, it is not the only one. PD can be used for treatment of toxicities in which the offending toxin can be removed by diffusion across the peritoneal membrane. Such toxins include ethylene glycol, ethanol, and barbiturates. Severe metabolic disturbances, such as hypercalcemia, hyperkalemia, hepatic encephalopathy, and resistant metabolic acidosis, also can be corrected with PD. PD with hypertonic dialysate can be used to remove excess body water in animals with life-threatening fluid overload, such as may occur with heart failure. There are other disorders in which peritoneal lavage, using solutions and techniques very similar to those for PD, may be beneficial. These include hypothermia, hyperthermia resulting from heat stroke, and pancreatitis (Box 28-2).23
Published reports of PD in dogs and cats have noted varying outcomes.* Although most described improvement in renal function during dialysis, overall survival remained poor. More recent publications reported improved survival, perhaps indicating an improvement in technique or overall management of critically ill animals. In one study of 27 dogs and cats, 24% improved and were discharged from the hospital.20 However, 11 of 21 of the animals with acute renal failure suffered from ethylene glycol intoxication, which is known to result in a mortality rate approaching 100%.30,69 In another study, 45% (10 of 22) of cats treated with PD were discharged from the hospital.16 One study of PD for the treatment of leptospirosis in five dogs reported a survival rate of 80%.9 Complications identified in these dogs were hypokalemia (60%), hypoalbuminemia (40%), hypomagnesemia (20%), pelvic limb edema (40%), central nervous system signs (40%), dialysate retention (20%), and leakage from the catheter site (20%). A report of six cats treated with PD for acute renal failure of varying causes reported survival in five of the six cats, all of which eventually had normal renal parameters. All cats had complications, including subcutaneous edema (83%), hyperglycemia (67%), dialysate retention (50%), and hypoalbuminemia (50%).22
The success rate of PD must be compared with the overall survival rate of animals with acute renal failure treated with other means because animals undergoing dialysis traditionally have been those with the most severe renal failure. The survival rate of dogs with leptospirosis treated with fluids and antibiotics has been reported to be 59% to 85%.1,33,54 In a study of 99 dogs with acute renal failure, 43% were discharged from the hospital. Of these, 24% were left with residual renal dysfunction, and only 19% had return to normal renal function.69 In another report of 80 dogs with acute renal failure, only 20% survived to discharge; 44% of the dogs had ethylene glycol intoxication.30 It may be that the survival of animals with acute kidney injury treated with PD is more dependent on the underlying cause of disease than the technique.
PD could theoretically be used for the long-term management of animals with chronic renal failure. However, technical problems with catheter flow and complications such as infection make chronic PD difficult. Few such cases have been reported.12,20,58,62,67
Contraindications to peritoneal dialysis
There are few situations in which PD is absolutely contraindicated. In humans, these include peritoneal adhesions that prevent fluid distribution throughout the abdominal cavity and pleuroperitoneal leaks that would result in pleural effusion and respiratory compromise. Although adhesions are common in humans, especially those that have had abdominal surgery,13 they are not often seen in dogs and cats. Diaphragmatic or pericardiodiaphragmatic hernias are seen in animals and could result in respiratory or cardiac dysfunction. That said, pleural dialysis has been described in two dogs with acute renal failure.60 Relative contraindications for PD include recent thoracic or abdominal surgery, inguinal or abdominal hernias, and severe hypercatabolic states, such as those seen with cutaneous burns or skin denudation. Animals with recent abdominal surgery, especially gastrointestinal surgery, are at risk for dehiscence and infection during PD because of the increased abdominal pressure and potential fluid leakage through the incision site. Similarly, progressive herniation may occur as the result of increased intraabdominal pressure. Concomitant catabolic diseases contribute to the hypoalbuminemia that can occur during PD.
Protocol for peritoneal dialysis
Catheter types and placement
The key to successful PD is the catheter and its placement. An ideal catheter allows efficient inflow and outflow, is biocompatible, resists infection of both the peritoneum and subcutaneous tunnel, and retards leakage at the peritoneal exit site.18 One of the most common causes of catheter failure in small animals is catheter obstruction by omentum, resulting in failure to drain dialysate from the abdomen. There are many catheter designs available (Box 28-3), and most are modifications of a fenestrated silicone tube with Dacron (Invista, Wichita, Kan.) cuffs applied to promote fibrous attachments at the peritoneal and cutaneous exit sites (Figure 28-3). Simple tube catheters with stylets can be placed percutaneously in conscious animals using local anesthetics in an emergency situation.23,24,53 A percutaneous cystotomy tube catheter (Stamey percutaneous suprapubic catheter set, Cook, Spencer, Ind.) also has been used successfully for acute short-term PD (Figure 28-4). The Tenckhoff catheter, developed in 1968, is a straight soft Silastic tube fenestrated at the distal end and furnished with two Dacron velour cuffs.65 Because of the high rate of omental entrapment, surgical omentectomies are advocated when using this catheter in animals in which more than 3 days of dialysis is anticipated.12,23,24 PD for acute renal failure should be performed for a minimum of 48 to 72 hours, but the animal’s condition often necessitates a longer period of treatment.
Box 28-3 Suppliers of Peritoneal Dialysis Catheters
Ash Advantage Peritoneal Catheter
Coiled Adult Standard and Large
Kendall Healthcare/Kendall Vascular
A variety of peritoneal dialysis catheters
Straight and curled, one and two cuff peritoneal dialysis catheters
Acute and chronic peritoneal dialysis catheters, Tenckhoff and Spiral
Global Veterinary Products, Inc.
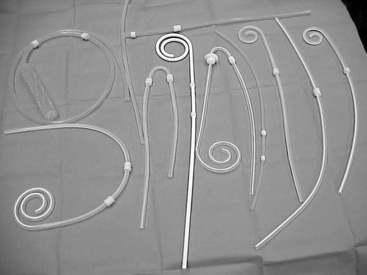
Figure 28-3 A collection of straight (Tenckhoff) and spiral fenestrated silicone peritoneal dialysis catheters.
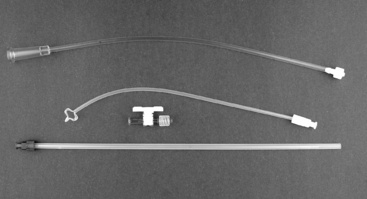
Figure 28-4 A Stamey percutaneous suprapubic catheter. May be used as a short-term peritoneal dialysis catheter (Cook Catheters, Spencer, Ind.).
An alternative catheter style called the fluted-T (Ash Advantage peritoneal dialysis catheter, Medigroup, Aurora, Ill.) has produced good results in dogs (Figure 28-5). In one study, 17 dogs had fluted-T catheters placed by peritoneoscope and were dialyzed daily. Only four catheters had outflow failure that occurred 7 to 18 days after placement. The remaining catheters functioned for the entire study period of 60 days.6,64 In the same study, Tenckhoff catheters in the dogs were obstructed by omentum 2 to 4 days after placement.
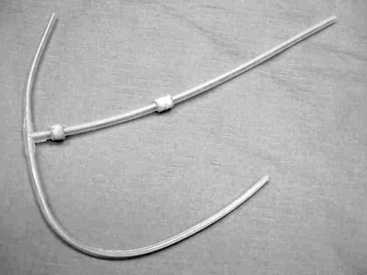
Figure 28-5 T-style fluted peritoneal dialysis catheter with folding crosspiece. Fluted design prevents omental obstruction (Ash Advantage Peritoneal Catheter, Medigroup, Inc, Aurora, Ill.).
The flutes are designed to offer minimal resistance to influx and efflux of fluid while preventing omental adhesion. The design of this catheter prevents outward migration. There is diminished hydraulic resistance during inflow and outflow because most of the dialysate flow pathways are around the catheter rather than within it. There is more complete drainage of the peritoneum through the limbs of the catheter, which are directed both cranially and caudally in the abdomen. Any residual omentum appears to have more difficulty attaching to the slots in the catheter than to the small holes in more traditional catheters. This silicone catheter has two Dacron cuffs that when implanted in the rectus muscle and subcutaneous layers become anchored by ingrowth of fibroblasts.6,64 The fluted aspect of the catheter is placed against the parietal peritoneum and oriented in a cranial to caudal plane. The catheter is flexible and designed to temporarily fold at the crosspiece to facilitate insertion. The fluted aspect of the catheter is 30 cm in length but can be cut to a shorter length for small patients. In humans, it is placed in a paramedian location, with the long aspect directed toward the inguinal ring. A subcutaneous tunnel is directed laterally for placement of the superficial cuff and the exit site of the catheter.7 A similar approach is used in both dogs and cats. Alternatives to the fluted-T catheter are the Swan Neck straight or curled Missouri catheter (Kendall Healthcare, Mansfield, Mass.) (Figure 28-6), commercial peritoneal dialysis catheters (15 Fr, 35 cm Cook Spiral Acute Peritoneal Catheter (Cook Veterinary Products, Spencer, Ind.), and the 10-cm PD catheter, coaxial design (Global Veterinary Products, New Buffalo, Mich.) (Figure 28-7). Other drainage tubes that may be used for PD in an emergency situation include the 15-Fr Blake surgical drain2 (Johnson and Johnson, Arlington, Tex.), the Jackson-Pratt fenestrated drains (Cardinal Health, Dublin, Ohio), 10 F wound drainage device (Hemovac, Zimmer, Dover, Ohio), Mila Chest tubes (Mila, Erlanger, Ky.), or appropriate-sized red rubber feeding tube and urethral catheter modified with multiple fenestrations (Tyco Health Care, Mansfield, Mass.). Although not specifically designed for PD, the Blake drain functions in a manner similar to the fluted-T catheter and has been used for PD in human infants.24
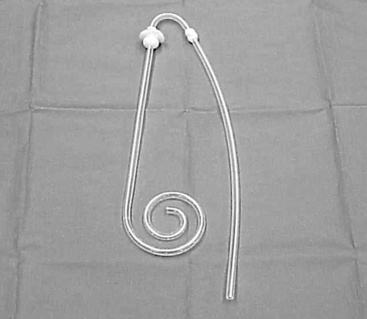
Figure 28-6 A surgically implantable catheter design. An omentectomy is required for optimal use (Curl Cath Missouri Peritoneal Dialysis Catheter, Kendall Healthcare, Mansfield, Mass.).
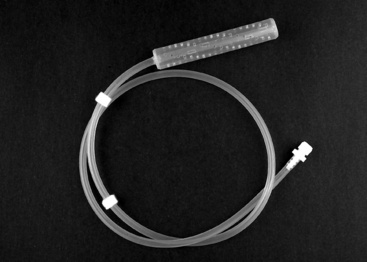
Figure 28-7 A surgically implantable catheter design. An omentectomy is required for optimal use.
(The coaxial design by Global Veterinary Products, Spencer, Ind.)
The fluted-T catheters are often cost prohibitive. The Swan Neck curled Missouri catheter is one the authors have found to be especially effective for long-term PD therapy and that is also more economical.
Depending on the catheter selected, there are three placement methods: laparoscopically (with a spiral catheter guide), blind (guidewire or trocar), and dissective (surgical).4,19 In veterinary medicine, in the emergency situation, a simple catheter that is placed percutaneously for short-term use often is chosen. After a catheter has been selected, the animal is placed in dorsal recumbency, and the abdomen is shaved and scrubbed for a surgical procedure. It is essential that the animal be draped and an aseptic technique maintained to prevent contamination of the peritoneal catheter system. The simple tube catheters are placed by trocar. Using aseptic technique, the catheter (over the trocar) is inserted through a stab incision 3 to 5 cm lateral to the umbilicus oriented toward the pelvis (Figure 28-8).24,53 The trocar is tunneled subcutaneously for several centimeters before being inserted through the abdominal muscles into the abdomen. The catheter then is threaded over the trocar until fully in the abdomen.24 The subcutaneous tunnel ideally should create a snug fit, but a purse-string suture should be placed to secure the catheter, and a tape butterfly can be added to secure the catheter to the skin on the lateral abdomen. Sutures have been reported to promote tunnel infections in human patients; therefore for long-term use it is recommended that a catheter with Dacron cuffs be used and no purse-string suture placed. If a cuffed catheter is to be used, the cuffs should be soaked in sterile saline before placement to remove air and facilitate fibroblast cuff invasion.5,24 The inner cuff is placed in the rectus muscle, and the other cuff is placed in the subcutaneous tunnel. A tight subcutaneous tunnel with fibrous ingrowth into the cuff decreases the incidence of dialysate leak.39,44
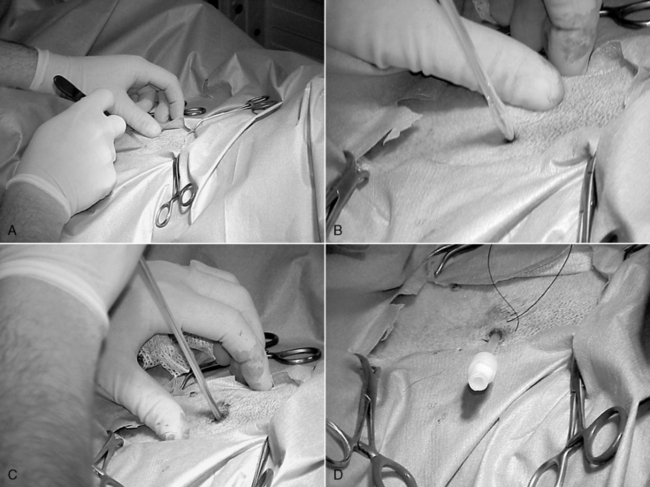
Figure 28-8 Placement of a percutaneous dialysis catheter. A, A stab incision made 3 cm lateral to the umbilicus. B, The catheter with trocar is inserted through the stab incision. C, Once inserted the catheter and trocar are directed toward the pelvis. D, Once the catheter is fully inserted the trocar is removed.
When it is believed PD will be performed for longer than 24 hours, a surgically placed catheter should be used. Although some catheters such as the fluted-T or the Quinton Swan Neck curled catheter have been designed to be placed either via laparoscope or blind trocarization in human medicine, it is preferable to place these catheters surgically in dogs and cats. Omentectomy is necessary to provide adequate exchanges for long durations. The curled tip catheter or the tip of the Missouri catheter should be positioned in the inguinal area. The subcutaneous tunnel should be such that there is a gentle bend in the catheter that does not kink and that exits caudally and off midline by 3 to 5 cm (Figure 28-9). The Swan Neck catheters are manufactured with a gentle bend and use of these catheters avoids the error of overbending or kinking of a straight catheter in the subcutaneous tunnel.
Initially, large volumes of dialysate should be avoided to minimize excess intraabdominal pressure, which can promote leaks and retard healing of exit sites.18,32,43 It is recommended that one quarter to one half of the calculated prescription volume for the first 24 hours be infused at the start of dialysis exchanges. The catheter should be attached to a sterile closed exchange system and carefully bandaged into position with dry sterile dressings. The use of topical antibiotic ointments is not recommended because of the potential to cause maceration of the exit site tissues and fibroblast inhibition. Minimizing catheter movement during the invasion of fibroblasts into the cuffs is crucial for minimizing exit site leaks and infections. After placement of the dialysis catheter, the tail of the catheter tubing is connected to a transfer tubing set, which previously has been attached to and primed with a prewarmed bag of dialysate. Strict sterile technique should be maintained throughout all manipulations. Connections should be protected with povidone-iodine connection shields or chlorhexidine-soaked sponges.
Dialysate solutions
The biocompatibility of a PD solution can be defined as the ability of a solution formulation to permit long-term dialysis without any clinically relevant changes in the functional characteristics of the peritoneum and is of paramount importance not only in maintaining the health of the membrane but also in permitting PD to be a successful long-term therapy. Solution components can affect leukocyte, mesothelial cell, endothelial cell, and fibroblast function, resulting in alterations in cytokine, chemokine, and growth factor networks, up-regulation of proinflammatory and profibrotic pathways, impaired peritoneal host defense, and the induction of carbonyl and oxidative stress.17 Such perturbations of normal physiology have been proposed as causative factors contributing to changes in peritoneal structure, such as peritoneal fibrosis, sclerosis, and vasculopathy, and changes in peritoneal function including increased solute permeability and ultrafiltration failure.17
The ideal solution for PD should not be unduly hypertonic, should not impair host defenses, and should not damage the peritoneal membrane. It should be bicarbonate-based with normal pH. It should be sterilized in a manner that does not promote generation of glucose degradation products (GDPs). Most existing glucose-based solutions are lactate-based, have low pH and high tonicity, contain GDPs, and glycosylate the peritoneal membrane.
Commercially prepared dialysate solutions containing various concentrations of dextrose are available. Dialysis for removal of solutes generally is performed using 1.5% dextrose. Dialysates containing 2.5% and 4.25% dextrose are used in moderate to severely overhydrated patients. Dialysate solutions are buffered, slightly hyperosmolar crystalloid solutions designed to pull fluid, potassium, urea, and phosphate from the plasma into the dialysate while providing diffusible buffer and other needed compounds such as magnesium and calcium.42
Hypertonic dextrose-containing dialysate solutions are effective for minimizing edema in overhydrated patients and for enhancing ultrafiltration (removal of water) in all patients. Hypertonic dextrose appears to favor capillary vasodilatation and promotes solute drag. A 1.5% dextrose dialysate is used in dehydrated or normovolemic patients. The 2.5% and 4.25% dialysates should be used in mildly to severely overhydrated patients. Intermittent use of a 4.25% dialysate solution may increase the efficiency of dialysis in all patients.42 Heparin (250 to 1000 U/L) should be added to the dialysate for the first few days after catheter placement to help prevent occlusion of the catheter by fibrin deposition. This heparin is minimally absorbed by the patient’s circulation and is unlikely to prolong clotting times.18,24,43 The recommended infusion volume for small animals is 5 to 20 mL/kg for the first 24 hours and then 30 to 40 mL/kg. The dialysate should be warmed to 38° C to improve permeability of the peritoneum. The dialysate line should be placed in a fluid warmer to help maintain this temperature.
Adding dextrose to lactated Ringer’s solution can make a suitable dialysate solution. Osmolality should closely approximate that of the patient, and the dextrose concentration should be at least 1.5%. Adding 30 mL of 50% glucose to 1 L of lactated Ringer’s solution will result in a 1.5% dextrose solution.
Glucose itself is harmful to the peritoneum. The glucose concentration of dialysate solutions is high. The tissues of the peritoneal membrane are continuously exposed to glucose concentrations that are clearly in the diabetic range. These concentrations of glucose are toxic to the mesothelium. Glucose activates the polyol pathway and the secretion of transforming growth factor-β1 (TGF-β1), monocyte chemoattractant protein-1 (MCP-1), and fibronectin. in vitro data suggest that glucose is involved in the development of peritoneal fibrosis.70 Glucose is likely to be involved in the development of peritoneal neoangiogenesis. The clinical importance of this finding is that it leads to enlargement of the peritoneal vascular surface area, resulting in loss of the osmotic gradient, which ultimately impairs ultrafiltration.70 A third mechanism by which glucose can damage the peritoneal tissue is by inducing nonenzymatic glycosylation of tissue proteins, which leads to the formation of advanced glycosylation end products (AGEs). The deposition of AGEs in the vascular wall also leads to ultrafiltration failure.70
GDPs are formed during the heat sterilization process of dialysate solutions. GDPs consist of aldehydes such as formaldehyde and dicarbonyl products such as glyoxal and methylglyoxal. GDPs may affect the peritoneal membrane by three mechanisms. They are toxic to fibroblasts. Methylglyoxal enhances the production of vascular endothelial growth factor (VEGF). Finally, GDPs trigger the formation of AGEs at a much faster rate than glucose.70
Thus standard glucose-based PD solutions have long-term detrimental effects on the peritoneum because of the presence of high concentrations of lactate, glucose, GDPs, and low pH, which may result in diminished defense mechanisms and ultrafiltration failure.70 However, for short-term use in veterinary medicine, no adverse effects have been recognized.
Until recently, there have been few practical alternatives to glucose. Now polyglucose (icodextrin) (Extrarenal, Baxter Healthcare Corporation, Deerfield, Ill.) is available. Icodextrin (7.5% polyglucose) is a mixture of high molecular weight, water-soluble glucose polymers isolated by fractionation of hydrolyzed cornstarch. Icodextrin is a glucose polymer of MW 16,800 and osmolality of 285 mOsm/kg. No diffusion into the blood occurs, and the colloid osmotic gradient and ultrafiltration are maintained as the dwell proceeds. Ultrafiltration occurs by colloid osmosis via small pores. Minimal ultrafiltration occurs via ultrapores, through which glucose mainly acts, and consequently there is no sodium sieving. Icodextrin is absorbed via lymphatics and metabolized to maltose. No toxicity has been identified. However, a number of adverse effects have been reported with icodextrin use (e.g., sterile peritonitis, peritoneal mononucleosis, antibody formation). In humans undergoing chronic ambulatory PD, icodextrin is used during the long dwell periods.46,47,70 Icodextrin’s role in veterinary PD has yet to be investigated.
Bicarbonate-based solutions are being developed to increase solution biocompatibility and thus protect the peritoneal membrane. Their formulation also reduces infusion pain. These solutions require use of a double chamber bag to separate bicarbonate from calcium. A 1.1% amino acid solution now is available in many countries to supplement protein intake and treat or prevent malnutrition.25,70 One exchange of the 1.1% amino acid solution per day has been shown to improve nitrogen balance and biochemical markers of nutrition in malnourished continuous ambulatory PD patients.37
Delivery technique
Aseptic technique during delivery of dialysate is essential to minimize the risk of peritonitis (Box 28-4). Hands should be thoroughly washed and sterile gloves used while changing the dialysate bags or lines because the most common cause of peritonitis is contamination of the bag spike.12,24 Routine use of a face mask while doing bag exchanges and catheter maintenance has been shown to be unnecessary as long as proper hand care is maintained.29 Every line connection should be covered with a povidone-iodine connection shield or chlorhexidine-soaked dressings covered with sterile gauze. All injection ports should be scrubbed with chlorhexidine and alcohol before injections, and the use of multiple-dose vials (e.g., heparin or potassium chloride) for dialysate supplements should be avoided to decrease the risk of introducing microorganisms.
Box 28-4 Guidelines for Preventing Infection During Peritoneal Dialysis
1. Wash hands before beginning. Wear sterile gloves when changing bags or handling lines. Work in a clean environment.
2. Use povidone-iodine connection shields or chlorhexidine-soaked dressings covered with sterile gauze over all line connections.
3. Scrub injection ports for 2 minutes before injections, or allow chlorhexidine to sit on injection ports and medication bottles for 5 minutes before use.
4. Avoid multiple dose vials for dialysate additives.
5. Adjust dialysate prescription to prevent exit site leaks.
6. Minimize catheter movement at cutaneous exit site. Wash the area with chlorhexidine scrub, and dry with sterile gauze once daily. Dry sterile bandages are recommended at catheter exit site from body wall.
7. Examine dialysate for cloudiness before and after each exchange.
8. Provide adequate nutritional support to the patient by enteral or parenteral routes.
Although dialysis can be performed with a straight-line transfer set, use of a closed, flush system has been associated with lower infection rates.12,45 The closed “Y” system allows the lines to be flushed free of possible bacterial contamination before each dialysate infusion without opening the system to outside air.
The exchange procedure
For the first 24 to 48 hours after catheter placement, exchange volumes should be one quarter to one half the calculated ideal volume (postsurgical placement use one fourth the ideal volume, or 10 mL/kg) to assess the degree of abdominal distention, the effect on respiratory function, and the potential for dialysate leakage. After the first 48 hours, the dialysate is infused at a dosage of 30 to 40 mL/kg during a 10-minute period.12,14,39,57 The dialysate is allowed to remain in the peritoneal cavity for 30 to 40 minutes (dwell time) and then is drained into a collection bag by gravity during a 20- to 30-minute period. A 90% to 100% recovery of dialysate is expected. This process is repeated continually, and the dialysate formula and dwell times are adjusted every 12 to 24 hours according to the animal’s need.
A Y-set tubing with a fresh dialysate bag and a drainage container attached to either segment is connected to the catheter tubing or transfer set (Figure 28-10). First, a small amount of fresh dialysate is flushed into the drainage bag, and then the peritoneal cavity is drained, so that any contaminants introduced during the connection procedures are flushed into the drainage bag and not into the peritoneal cavity. After drainage, the fresh dialysate is infused. This “drain first-infuse later” principle has markedly decreased the incidence of peritonitis in humans on PD as compared with the “infuse first-drain later” principle used in the straight single-spiked system.5,39
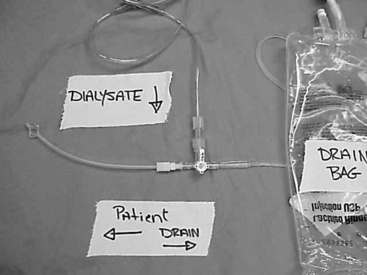
Figure 28-10 The “Y-set” system. Dialysate bag upper left and the collecting bag at the bottom right of the photo. The connecting tubing to the patient is to the left.
The exchange technique for severe uremia should follow the protocol described below:
1. The dialysate should remain in the abdomen for 30 to 40 minutes.
2. Dialysis cycles should be repeated every 1 to 2 hours until the animal is clinically improved and blood urea nitrogen (BUN) and serum creatinine concentrations have decreased.
3. This initial intensive dialysis typically continues for 24 to 48 hours. Do not attempt to bring BUN and serum creatinine concentrations into the normal range. A reasonable target is a BUN concentration of 60 to 100 mg/dL and a serum creatinine concentration of 4.0 to 6.0 mg/dL.
4. The animal then can be changed to the chronic dialysis cycle.
The chronic dialysis protocol includes the following:
1. Dialysate should remain in the abdomen for 3 to 6 hours.
2. Three to four exchanges per day are performed. The dialysate should remain in the abdomen during these extended exchange periods.
3. Rate of infusion can be rapid in most cases without problems. If the animal shows signs of discomfort during infusion, slow the rate of infusion and check that the solution temperature is not too hot or too cold.
The frequency of the dialysis exchanges and the duration of the dwell time are adjusted for each animal’s individual needs. The goal of PD for an animal with renal failure is to remove enough urea to maintain the BUN concentration at 70 mg/dL.24,57 The amount of solute transferred across the peritoneal membrane is determined by the concentration gradient for each solute. If there is a need to increase the removal of large molecules such as creatinine, the dwell time for each exchange is extended.
Dialysis should be continued until renal function has normalized or is adequate to maintain the patient without dialysis as determined by urine output, stabilization of laboratory values, and clinical signs. Gradual reduction of the number of exchanges and having “rest” periods are recommended. This intermittent PD should be done during a 3- to 4-day period, with continual reevaluation of the patient’s clinical state. If the animal receiving aggressive, well-managed continual PD has not improved according to biochemical parameters or uremic signs after several days, chronic PD, chronic hemodialysis, renal transplantation, or euthanasia should be considered.
Monitoring
Careful records of the dialysate volume infused and recovered during each exchange period should be maintained (Figure 28-11). Less fluid may be recovered from the abdomen than was delivered for the first few exchanges. As dialysis proceeds, outflow should approximate or exceed inflow if the patient is adequately hydrated.
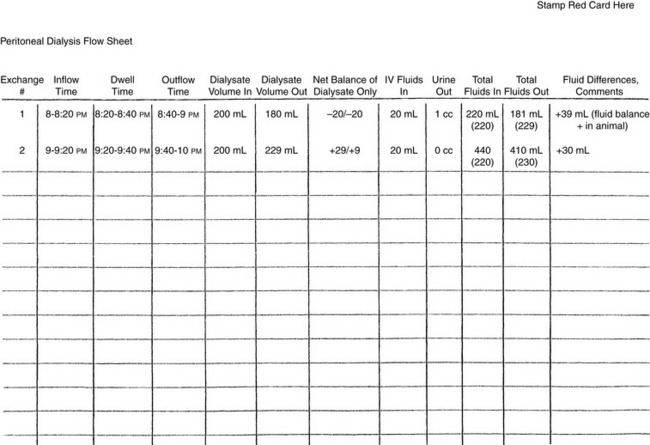
Figure 28-11 Flow chart used at the authors’ institution for monitoring dialysate and fluid volumes.
In the acute setting, body weight and hydration status should be monitored frequently, with body weight recorded consistently (i.e., either with or without dialysate in the abdomen). Measurement of central venous pressure (CVP) through a jugular catheter is a relatively sensitive method for detecting overhydration and should be performed every 4 hours. Determination of packed cell volume (PCV) and total protein should be performed at least twice daily (Box 28-5). Serum electrolyte concentrations and other blood chemistries including BUN, creatinine, albumin, and acid-base should be assessed initially every 8 to 12 hours and then daily (Figure 28-12).39
Box 28-5 Guidelines for Monitoring the Animal Undergoing Peritoneal Dialysis
1. Weigh the animal twice daily before dialysate infusion.
2. Check central venous pressure (CVP) every 4 to 6 hours.
3. Check systemic arterial blood pressure every 6 to 8 hours.
4. Check body temperature every 6 to 8 hours.
5. Record heart rate and respiratory rate every 2 hours. Note if there is respiratory difficulty with dialysate infusion.
6. Perform adequate peritoneal catheter exit site care, and evaluate for exit site infection daily.
7. Evaluate serum urea nitrogen (BUN), creatinine, electrolyte, albumin, and venous blood gas analysis once to twice daily depending on severity of azotemia. Evaluate serum magnesium every 3 days.
8. Record or weigh the amount of dialysate infused and recovered with each exchange.
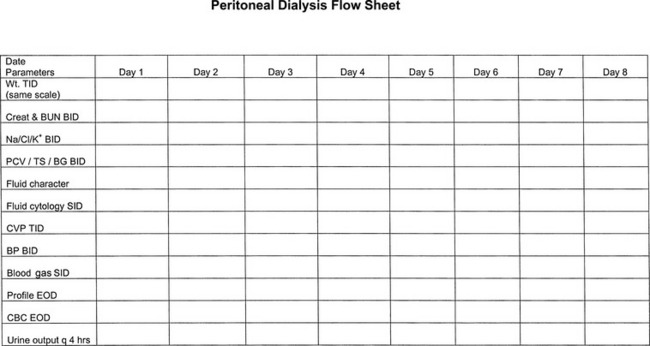
Figure 28-12 Flow chart used at the authors’ institution to monitor dialysis patient’s laboratory values.
A number of metabolic aberrations may occur in patients on PD, including alterations in serum sodium, potassium, magnesium, and glucose concentrations as well as changes in acid-base status. Frequent monitoring and adjustment in dialysate and supplemental parenteral fluid composition may be necessary.
In cases of acute kidney injury, the objectives of PD are to reduce azotemia, resolve the clinical signs of uremia, and to help correct fluid, electrolyte, and acid-base imbalances until the animal’s kidney function can recover sufficiently. Conversion of the anuric or oliguric state to a polyuric state and stabilization or improvement of azotemia are the primary indications for discontinuation of PD.
Complications
Complications are common with PD, but they are manageable if recognized early (Box 28-6). The most common complications include catheter flow problems, exit site leaks, hypoalbuminemia, peritonitis, pleural effusion, dyspnea resulting from increased abdominal pressure, changes in hydration status, and electrolyte abnormalities.
Catheter flow obstruction by fibrin and omentum leading to dialysate retention are common problems when catheters are placed percutaneously.14,39 In one study, 30% of dogs undergoing PD developed such obstructions.20 Careful catheter placement and management are important preventative steps. Heparinized saline flushes of the catheter for the first few days may decrease the occurrence of omentum wrapping around the catheter.24 If a clot in the catheter is suspected, a high-pressure saline flush or the addition of 15,000 U of urokinase to the catheter for 3 hours may dislodge clots.5 Decreasing volumes of dialysate during outflow or abdominal pain on dialysate inflow are evidence of omental entrapment. If omental entrapment occurs, catheters can be repositioned or replaced to correct this problem. For this reason, it is strongly recommended that catheters be surgically placed and an omentectomy performed if use of the PD catheter is anticipated for longer than 48 hours.
Protein losses can be clinically important with PD. Losses may increase dramatically (50% to 100%) when peritonitis is present. Hypoalbuminemia was the most common complication in a review of PD cases in dogs and cats, and 41% of the animals were affected.20 Hypoalbuminemia may be the result of low dietary protein intake, gastrointestinal or renal protein loss, loss in the dialysate itself, uremic catabolism, and concurrent diseases. Usually, the animal can maintain normal serum protein concentrations if nutritional intake is adequate. However, anorexia and vomiting are common in uremic patients, and adequate enteral nutrition may be difficult to maintain. Supportive measures to maintain positive nitrogen balance often must be used. Nutritional support includes feeding tubes, partial parenteral nutrition, total parenteral nutrition, and a technique of PD using 1.1% amino acid solutions.24,37 Gastrostomy and jejunostomy tubes are contraindicated during PD because of increased risk of infection and abdominal wall exit site dialysate leaks.
The prevalence of peritonitis (22%) in veterinary patients on PD is higher than that reported for humans.20 The most common source of peritonitis is contamination of the bag spike or tubing by the handler, but intestinal, hematogenous, and exit site sources of infection do occur. To minimize exit site sources of infection, it is important to recognize pericatheter leaks.24 Peritonitis is diagnosed when two of the following three criteria are recognized: (1) cloudy dialysate effluent, (2) greater than 100 inflammatory cells per liter of effluent or positive culture results, and (3) clinical signs of peritonitis.24 Because Staphylococcus spp. is the most common organism, cephalosporins administered systemically and intraperitoneally are recommended empirically. In a study at the authors’ institution, peritonitis was not identified in any of the PD cases reviewed during a 4-year period.9
Acute pleural effusion is an uncommon complication and usually occurs early in the course of treatment. A common PD complication at the authors’ institution is overhydration of the patient. If the patient is gaining weight, the CVP is increasing, or the effluent recovered is not at least 90% of the dialysate infused, the prescription should be changed to ultrafiltration with more concentrated dextrose (2.5% or 4.25%) solutions. The most frequent complication at the authors’ institution is dialysate leakage into the subcutaneous tissue (Figure 28-13). This complication is managed by having the surgeon closely appose the abdominal incision (simple interrupted suture pattern only), starting the initial exchange volumes at one quarter of the calculated infusion amount, and if leakage does occur, intermittently wrapping the limbs to promote mobilization of the edema.
Dialysis dysequilibrium is a rare complication characterized by dementia, seizures, or death. Dysequilibrium may occur during early exchanges, especially in patients with extreme azotemia, acidosis, hypernatremia, or hyperglycemia. Rapid removal of urea and other small solutes apparently causes influx of water into brain cells and neurologic dysfunction.20,24 If evidence of dysequilibrium occurs, the dialysate prescription should be adjusted to remove urea and small solutes at a slower rate (i.e., fewer exchanges or longer dwell times).
Conclusion
PD is a realistic option for veterinary patients with acute nonresponsive renal failure or dialyzable toxin exposure. The protocol requires careful intraperitoneal catheter placement and care, aggressive exchange prescriptions, and careful monitoring for complications. Veterinarians should recognize that PD is an extremely effective tool in human medicine and should consider it as a treatment modality in an acute critical care setting.
The future role of PD in veterinary medicine may be as alternative management therapy for end-stage renal failure when hemodialysis and transplantation are not options. As advanced renal replacement therapy becomes a more common treatment modality, we may find chronic ambulatory PD is the next area to emerge. In some patients, chronic hemodialysis is not a viable option because of poor vascular access, other underlying diseases, the size of the animal, or unavailability of a hemodialysis center. Continuous ambulatory PD may serve as a treatment option for these patients. Cats that are not transplant candidates and are too small for hemodialysis are ideal candidates for chronic ambulatory PD. The active lifestyles of most dogs traditionally have made PD challenging for them. However, with a dedicated owner, chronic ambulatory PD may serve a role in renal replacement therapy. Typically, the patient is maintained in the hospital while the dialysis prescription is formulated, the incision heals, and the animal becomes accustomed to the dialysis process. Long-term care at home with outpatient visits is a goal for the future. Success will necessitate active cooperation among the owner, veterinarian, and technical staff. Long-term maintenance of low-profile gastrostomy tubes and low-profile cystostomy tubes has become commonplace. The challenge of maintaining a chronic ambulatory PD catheter will require developing and establishing excellent aseptic technique, daily bandage changes, and early recognition of any signs of infection. Investigational use of intradialytic amino acid solution and alternatives to traditional dialysate solutions will become areas of investigation in veterinary PD as more chronic dialysis is performed.
1 Adin C.A., Cowgill L.D. Treatment and outcome of dogs with leptospirosis: 36 cases (1990-1998). J Am Vet Med Assoc. 2000;216:371.
2 Alexander J.W., Aerni S.E. Comparison of fluted silicone and polyvinyl chloride drains for closed suction drainage following cholecystectomy. University of Cincinnati and Johnson and Johnson Products Inc; 1984.
3 Anglani F., Forino M., Del Prete D., et al. Molecular biology of the peritoneal membrane; in between morphology and function. Contrib Nephrol. 2001;131:61.
4 Ash S.R. Techniques of peritoneal access placement, short courses in the clinical practice of nephrology. Proceedings of the ASN annual meeting. Boston. 1993:27.
5 Ash S.R., Carr D.J., Diaz-Buxo J.A. Peritoneal access devices: hydraulic function and biocompatibility. In: Nissenson A., editor. Clinical dialysis. Stanford: Appleton and Long; 1995:212-236.
6 Ash S.R., Janle E.M. T-fluted peritoneal dialysis catheter. Adv Perit Dial. 1993;9:223.
7 Ash Advantage Peritoneal Catheter. Implantation instructions using a modified y-tec peritoneoscopic procedure. Aurora, Ill: Medigroup Inc; 1996.
8 Avellini G., Fruganti G., Morettini B., et al. Peritoneal dialysis in the treatment of canine leptospirosis. Atti della Societa Italiana Delle Scienze Veterinairie. 1973;27:377.
9 Beckel N., O’Toole T., Rozanski E., et al. Peritoneal dialysis in the management of acute renal failure in five dogs with leptospirosis. J Vet Emerg Crit Care. 2005;15(3):201-205.
10 Blake P.G., Daugirdas J.T. Physiology of peritoneal dialysis. In: Daugirdas J.T., Blake P.G., Ing T.S., editors. Handbook of dialysis. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2001:281-296.
11 Burkhart J.M. Peritoneal dialysis. In: Brenner B.M., editor. The kidney. 6th ed. Philadelphia: WB Saunders; 2000:2454-2517.
12 Carter L.J., Wingfield W.E., Allen T.E. Clinical experience with peritoneal dialysis in small animals. Compend Contin Educ Pract Vet. 1989;11:1335.
13 Chegini N. Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci. 2002;7:91.
14 Christie B.A., Bjorling D.E. Kidneys. In: Slatter D., editor. Textbook of small animal surgery. 2nd ed. Philadelphia: WB Saunders; 1993:1439-1440.
15 Coester A.M., Smit W., Struijk D.G., et al. Fluid transport with time on peritoneal dialysis: The contribution of free water transport and solute coupled water transport. Contrib Nephrol. 2009;163:22.
16 Cooper R.L., Labato M.A. Peritoneal Dialysis in cats with acute kidney injury: 22 cases 2001-2006. 2010. unpublished manuscript
17 Cooker L.A., Holmes C.J., Hoff C.M. Biocompatibility of icodextrin. Kidney Int. 2002;62:S34.
18 Cowgill L.D. Application of peritoneal dialysis and hemodialysis in the management of renal failure. In: Osborne C.A., editor. Canine and feline nephrology and urology. Baltimore: Lea and Febiger; 1995:573.
19 Crabtree J.H., Fishman A. Laparoscopic implantation of swan neck presternal peritoneal dialysis catheters. J Laparoendosc Adv Surg Tech A. 2003;13:131.
20 Crisp M.S., Chew D.J., DiBartola S.P., et al. Peritoneal dialysis in dogs and cats: 27 cases (1976-1987). J Am Vet Med Assoc. 1989;195:1262.
21 Devuyst O., Ni J., Verbavatz J.M. Aquaporin-1 in the peritoneal membrane: implications for peritoneal dialysis and endothelial cell function. Biol Cell. 2005;97:667.
22 Dorval P., Boysen S. Management of acute renal failure in cats using peritoneal dialysis: a retrospective study of six cases (2003-2007). J Feline Med Surg. 2009;11:107.
23 Dzyban L.A., Labato M.A., Ross L.A. CVT update: peritoneal dialysis. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
24 Dzyban L.A., Labato M.A., Ross L.A., et al. Peritoneal dialysis: a tool in veterinary critical care. J Vet Emerg Crit Care. 2000;10:91.
25 Feriani M., Passlich-Deetjen J., Jaeckle-Meyer I., et al. Individualized bicarbonate concentrations in the peritoneal dialysis fluid to optimize acid-base status in CAPD patients. Nephrol Dial Transplant. 2004;19:195.
26 Flessner J.F. The peritoneal dialysis system: importance of each component. Perit Dial Int. 1997;17:S91.
27 Flessner M.F. Peritoneal transport physiology: insights from basic research. J Am Soc Nephrol. 1991;2:122.
28 Flessner M., Henegar J., Bigler S., et al. Is the peritoneum a significant transport barrier in peritoneal dialysis? Perit Dial Int. 2003;23:542.
29 Figueiredo A.E., de Figueiredo C.E., d’Avila D.O. Peritonitis prevention in CAPD: to mask or not? Perit Dial Int. 2000;20:354.
30 Forrester S.D., McMillan N.S., Ward D.L. Retrospective evaluation of acute renal failure in dogs. J Vet Intern Med. 2002;16:354. (abstract)
31 Fox L.E., Grauer G.F., Dubielzig R.R., et al. Reversal of ethylene glycol-induced nephrotoxicosis in a dog. J Am Vet Med Assoc. 1987;191:1433.
32 Goldrach I., Mariano M. One-step peritoneal catheter replacement in children. Adv Perit Dial. 1993;9:325.
33 Harkin K.R., Gartrell C.L. Canine leptospirosis in New Jersey and Michigan: 17 cases (1990-1995). J Am Anim Hosp Assoc. 1996;32:495.
34 Jackson R.F. The use of peritoneal dialysis in the treatment of uremia in dogs. Vet Rec. 1964;76:1481.
35 Jörres A. PD: a biological membrane and a non-biological fluid. Contrib Nephrol. 2003;140:1.
36 Khanna R. Peritoneal transport: clinical implications. In: Dialysis and transplantation. Philadelphia: WB Saunders; 2000:129-143.
37 Kopple J.D., Bernard D., Messana J., et al. Treatment of malnourished CAPD patients with an amino acid based dialysate. Kidney Int. 1995;47:1148.
38 Kirk R.W. Peritoneal lavage in uremia in dogs. J Am Vet Med Assoc. 1957;131:101.
39 Labato M.A. Peritoneal dialysis in emergency and critical care medicine. Clin Tech Small Anim Pract. 2000;15:126.
40 Lai K.N., Li F.K., Lan H.Y., et al. Expression of aquaporin-1 in human peritoneal mesothelial cells and its upregulation by glucose in vitro. J Am Soc Nephrol. 2001;12:1036.
41 Lameire N., Mortier S., DeVriese A. Peritoneal microcirculation. Contrib Nephrol. 2003;140:56.
42 Lane I.F., Carter L.J. Peritoneal dialysis and hemodialysis. In: Wingfield W., editor. Veterinary emergency medicine secrets. Philadelphia: Hanley and Belfus; 1997:350.
43 Lane I.F., Carter L.J., Lappin M.R. Peritoneal dialysis: an update on methods and usefulness. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XI. Philadelphia: WB Saunders; 1992:865-870.
44 Langston C. Advanced renal therapies: options when standard treatments are not enough. Vet Med. 2003;98:999.
45 Maiorca R., Cancarini G. Experiences with the Y-system. Contemp Issue Nephrol Perit Dial. 1990;22:167-190.
46 Martin J., Sansone G., Cirugeda A., et al. Severe peritoneal mononucleosis associated with icodextrin use in continuous ambulatory peritoneal dialysis. Adv Perit Dial. 2003;19:191.
47 Moberly J.B., Mujais S., Gehr T., et al. Pharmacokinetics of icodextrin in peritoneal dialysis patients. Kidney Int. 2002;62:S23.
48 Nagy J.A., Jackman R.W. Peritoneal membrane biology. In: Dialysis and transplantation. Philadelphia: WB Saunders; 2000:109-128.
49 Oreopoulos D.G., Rao P.S. Assessing peritoneal ultrafiltration, solute transport, and volume status. In: Daugirdas J.T., Blake P.G., Ing T.S., editors. Handbook of dialysis. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2001:361-372.
50 Ota T., Kuwahara M., Fan S., et al. Expression of aquaporin-1 in the peritoneal tissues: localization and regulation by hyperosmolality. Perit Dial Int. 2002;22:307.
51 Pannekeet M.M., Krediet R.T. Water channels in the peritoneum. Perit Dial Int. 1996;16:255.
52 Pannekeet M.M., Mulder J.B., Weening J.J., et al. Demonstration of aquaporin-CHIP in peritoneal tissue of uremic and CAPD patients. Perit Dial Int. 1996;16:S54.
53 Parker H.R. Peritoneal dialysis and hemofiltration. In: Bovee K., editor. Canine nephrology. New York: Harwal; 1984:723-744.
54 Rentko V.T., Clark N., Ross L.A., et al. Canine leptospirosis: a retrospective study of 17 cases. J Vet Intern Med. 1992;6:235.
55 Rippe B. A three-pore model of peritoneal transport. Perit Dial Int. 1993;13(Suppl. 2):35.
56 Ronco C., Feriani M., Chiaramonte S. Peritoneal blood flow: does it matter? Perit Dial Int. 1996;16:S70.
57 Ross L.A. Peritoneal dialysis. In: Morgan R.V., editor. Handbook of small animal practice. New York: Churchill Livingstone; 1988:585-588.
58 Rubin J., Jones Q., Quillen E., et al. A model of long-term peritoneal dialysis in the dog. Nephron. 1983;35:259.
59 Schoenicke G., Diamant R., Donner A., et al. Histochemical distribution and expression of aquaporin 1 in the peritoneum of patients undergoing peritoneal dialysis: relation peritoneal transport. Am J Kidney Dis. 2004;44:146.
60 Shahar R., Holmberg D.L. Pleural dialysis in the management of acute renal failure in two dogs. J Am Vet Med Assoc. 1985;187:952.
61 Sharma A., Blake P.G. Peritoneal dialysis. In: Brenner B.M., editor. The Kidney. 8th ed. Philadelphia: Saunders Elsevier; 2008:2007-2036.
62 Simmons E.E., Lockard B.S., Moncrief J.W., et al. Experience with continuous ambulatory peritoneal dialysis and maintenance of a surgically anephric dog. Southwest Vet. 1980;33:129.
63 Stelin G., Rippe B. A phenomenological interpretation of the variation in dialysate volume with dwell time in CAPD. Kidney Int. 1989;35:1234.
64 Stone R.W. A protocol for peritoneal dialysis. J Vet Crit Care. 1985;8:2.
65 Thornhill J.A. Peritoneal dialysis: applications in acute and chronic end-stage kidney disease in the dog and cat. Proceedings of the 15th ACVIM Forum. Lake Buena Vista, Fla. 1997:31.
66 Thornhill J.A., Ash S.R., Dhein C.R., et al. Peritoneal dialysis with the Purdue column disc catheter. Minn Vet. 1980;20:27.
67 Thornhill J.A., Hartman J., Boon G.D., et al. Support of an anephric dog for 54 days with ambulatory peritoneal dialysis and a newly designed peritoneal catheter. Am J Vet Res. 1984;45:161.
68 Twardowski Z.J. Pathophysiology of peritoneal transport. Contrib Nephrol. 2006;150:13.
69 Vaden S.L., Levine J., Breitschwerdt E.B. A retrospective case control of acute renal failure in 99 dogs. J Vet Intern Med. 1997;11:58.
70 Vardham A., Zweers M.M., Gokal R., et al. A solutions portfolio approach to peritoneal dialysis. Kidney Int. 2003;64:S114.
71 Verkman A.S. Aquaporins in endothelia. Kidney Int. 2006;69:1120.