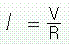Electrical Stimulation
Description
People have been passing electrical currents through their bodies for healing purposes for thousands of years. In just the last century a systematic attempt has been made to describe the therapeutic effects of electricity and to organize them in such a way to make them useful. Much like the case with cryotherapy, we have collectively learned that there are a number of different forms and therapeutic uses of electricity, and their popularity in athletic health care facilities is growing. The recent growth in the use of electrotherapy is probably related to recent manufacturing improvements in electrotherapy devices that make them easier than ever to use and provide more treatment options than ever before. Although this has certainly bolstered the clinical use of electrotherapy, it has also created a strong tendency to use “cookbook“ electrotherapy protocols rather than protocols based on specific goals for the patient. In fact, many clinicians now learn to use only preset protocols that are factory programmed into the machine, and they have great difficulty in creating a custom protocol to accomplish their goals. Worse yet, many of the factory preset protocols for electrotherapy devices have little or no basis in basic research or outcomes data and may not be effective at all. Similarly, because of a great deal of inconsistency in manufacturers’ terminology, practitioners must first translate the instruction manuals into a common set of terms before they can understand them. For these reasons, it is important that practitioners have a good understanding of the basics of electrotherapy and the ability to apply these basics to produce the outcomes desired. This section provides a basic framework and description of electrotherapy, but it can not substitute for a comprehensive course in therapeutic modalities.
How We Think It Works
Electricity fundamentals and terminology
A basic familiarity with electricity is assumed for this discussion, but a brief review of a few essential concepts that influence the clinical use of electrotherapy is provided here.58 First, electricity is the flow of electrons from an area of high concentration to an area of lower concentration. Because electrons carry a negative charge, the area of high electron concentration has a negative charge or negative polarity, and the area of low concentration has a positive charge or positive polarity. Therefore, electricity flows from a negatively charged area, called the negative pole or cathode, to a positively charged area, called the positive pole or anode. An electrically conductive pathway connecting the negative pole to the positive pole is called a circuit. Electrotherapy treatments work by making the targeted tissues a part of this circuit. The flow of electricity along a circuit is known as current. Electrical currents can be either continuous, like water constantly running through a garden hose, or interrupted, which is like turning the spigot for our garden hose on and off quickly and repeatedly so that separate spurts of water travel through the hose. The amount of electricity flowing along the circuit is measured in amperes and is analogous to the volume of water in the garden hose. The force that moves the electrons along the circuit is referred to as voltage, and it is analogous to water pressure in our garden hose. The relationship between force and flow (voltage and current) is describe by Ohm’s law (Box 8-10).
For most of the things that clinical electrotherapy is used for, the direction of the current flow (i.e., which end of the circuit is positive or negative) is seldom as important as ensuring that current actually flows through the target tissue in a sufficient amount to cause the physiologic response being sought. The flow of current is always unidirectional, from the negative pole to the positive pole. If the two poles at the ends of the circuit never change polarity while the current is on, the direction of current flow is constant and is called direct current (DC). If the two poles at the ends of the circuit switch polarity, the direction of current flow also switches and is called alternating current (AC). AC is the type of current available from electrical wall outlets, and it switches direction at a constant rate of 60 cycles per second (60 Hz) in North America. DC is the type of current available from a battery.
By connecting the electrical circuit to an oscilloscope, we can visualize the shape, or waveform, of the current (Fig. 8-5). The waveform for DC would be entirely on one side of the horizontal baseline and would continue along indefinitely until the current is turned off (A). Because the current is always moving in one direction, the charge would always have the same polarity and would remain on one side of the baseline. DC can therefore be said to have a single phase and is often called monophasic current. With AC, the waveform would initially be on one side of the baseline and then switch to the other side when the direction of the current and therefore the polarity alternated (B). The graph would repeat this switching as long as the current is flowing. Thus, AC can be said to have two phases (one positive and one negative) and is therefore often called biphasic current. A third type of current, polyphasic, actually has three or more phases and is typically produced by simultaneously overlaying an interrupted current over a continuous biphasic current called a carrier frequency. Common examples of polyphasic waveform devices are interferential stimulators and Russian stimulators.155
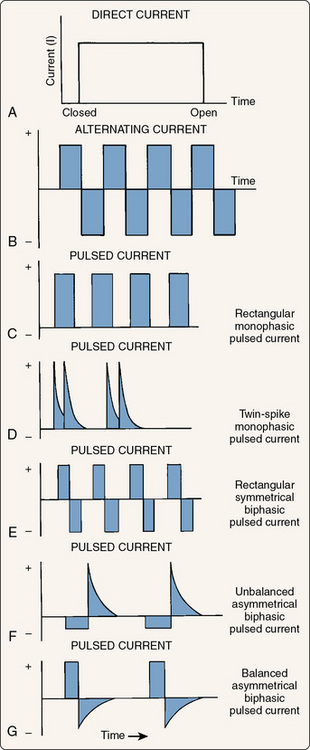
Figure 8-5 Graphic representation of the three types of electrical current. A, Direct current. B, Alternating current. C through G, Pulsed currents.
(Modified from Robinson, A.J. [1989]: Basic concepts and terminology in electricity. In: Snyder-Mackler, L., and Robinson, A.J. [eds.]. Clinical Electrophysiology. Baltimore, Williams & Wilkins, pp. 9, 11, 13.)
If the current is turned on and off repeatedly, individual phases with periods of no current flow (no charge) between them (C) would occur, and this is often called pulsed or interrupted current. The majority of electrotherapy devices use interrupted current, although a few exceptions are discussed later in the chapter. Electrotherapy devices allow the practitioner to control the number of these individual pulses per second (pulse rate). With low pulse rates (below 30), pulsing muscle contractions can be induced. By increasing the pulse rate to somewhere between 30 and 50 pulses per second, the muscle contraction appears smooth and sustained. A muscle that is contracting in a smooth and sustained fashion is said to be in tetany. Even higher pulse rates are often used and also cause tetanic contractions. It should be noted (D to G) that by using different combinations of polarity, voltage, and phase duration, the shape of each phase can be controlled. Common phase shapes are rectangular or square (E), spiked or twin spiked (D), asymmetric (F and G) in which the positive phase and negative phase have different shapes, and sinusoidal (Fig. 8-6). If the positive phase and negative phase have the same voltage (height), the waveform is said to be balanced (G).
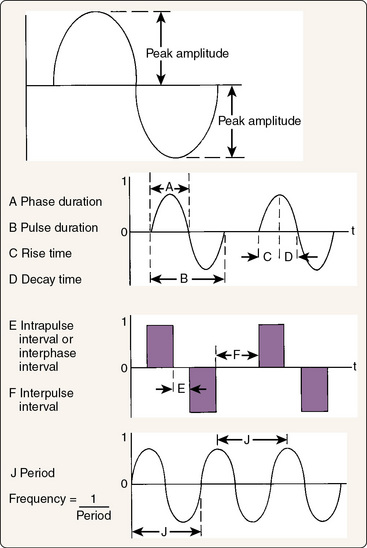
Figure 8-6 Characteristics of electricity.
(Modified from Robinson, A.J. [1989]: Basic concepts and terminology in electricity. In: Snyder-Mackler, L., and Robinson, A.J. [eds.]. Clinical Electrophysiology. Baltimore, Lippincott, Williams & Wilkins, p. 15.)
In examining a waveform, a specific set of terms is used to describe the phases and pulses (see Fig. 8-6). A phase is a single positively or negatively charged bolus of current, whereas a pulse is several consecutive phases that are continuous. All currents have at least one phase, but pulses are seen only with interrupted current and are separated from each other by brief intervals when no phase is present. The height, or amplitude, of each phase represents the voltage (the driving force for the current). The width of each phase represents the phase duration or phase width, usually in milliseconds. A related concept is pulse width, or the combined duration of all of the phases within a single pulse of interrupted current. Related to phase and pulse widths are phase and pulse intervals, or the duration between phases when there is no current (phase interval) or between pulses where there is no current (pulse interval). Phase interval and pulse interval are terms that are sometimes used interchangeably.
The lack of consistency in terminology across manufactures and textbooks is an ongoing problem in electrotherapy, which leads us to a fourth and sometimes confusing waveform concept called frequency. The frequency of a waveform can mean two very different things. Technically, it represents the number of cycles per second for the current. A cycle, also called a period, is one complete waveform including all of its phases. In clinical usage, the term frequency more commonly means the number of pulses per second and is also called the pulse rate. Remember that a pulse consists of several consecutive cycles of a current in an interrupted current. For example, if you use the electricity coming from your wall outlet, it is a continuous sinusoidal biphasic current with a frequency of 60 Hz. Now, if you are using an outlet connected to a switch and you manually turn the current on and off 10 times per second, the resulting current would be an interrupted sinusoidal biphasic current with a frequency of 60 Hz and a pulse rate of 10 Hz. Obviously, this terminology can be somewhat confusing, and most clinicians and manufacturers generally use “frequency“ to mean “pulse rate“ and the actual frequency of the current being interrupted is ignored.
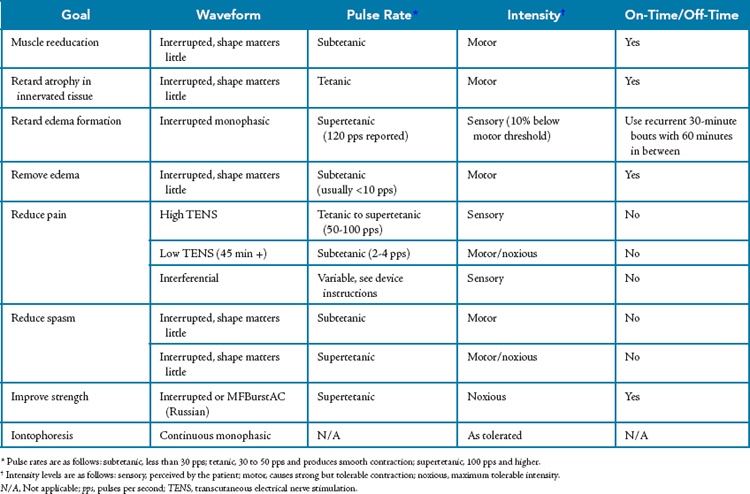
Although scientific study of electrotherapy is progressing, not as much is known about the physiology of this modality as is known about cryotherapy, superficial thermotherapy, or ultrasound, and the precise mechanisms by which electrotherapy is effective have not yet been described. As is the case for these better-studied modalities, electrotherapy is also characterized by a conspicuous lack of outcomes research. Even though the literature is still sparse, electrotherapy has been suggested to be effective in achieving a number of rehabilitative goals (Table 8-10), most of which are related to the ability of electricity to depolarize nerves. In addition, some the effects proposed are related to the electrical charge fields around the electrodes and do not rely on nerve depolarization.
Table 8-10 Common Therapeutic Goals for Electrotherapy
| Goal | Rationale |
|---|---|
| Muscle reeducation | Retrain firing patterns or overcome neuromuscular inhibition in intact muscles following injury or pathology. The mechanism is thought to be related to increasing the quantity of motor units recruited or decreasing the inhibition of the motor nerves that is preventing normal function |
| Retard atrophy | Cause the muscle to contract in an effort to reduce the effects of immobilization or paralysis on atrophy. |
| Retard edema formation | Limit the formation of edema during acute inflammation by inhibiting the increase in vascular permeability with sensory-level stimulation. |
| Remove edema | Remove edema that is already present through a muscle pump mechanism with motor-level stimulation. |
| Reduce pain | Interfere with the transmission or perception of pain through a variety of different electrotherapy approaches. |
| Reduce spasm | Reduce acute spasms by either decreasing the muscle’s contraction frequency or by fatiguing the muscle until it fails (very uncomfortable). Electrotherapy can also be used to manage the spasticity associated with neuromuscular diseases or spinal cord trauma. |
| Increase strength | Increase muscle force output in nonpathologic tissue by causing hypertrophy of the muscle. Although muscular strength can be improved with motor-level stimulation, the protocols required are very uncomfortable and are not nearly as effective as resistance exercise. This is not generally an appropriate goal for electrotherapy. |
| Increase range of motion | A commonly cited but misleading goal. Electrotherapy can be effective in reducing muscle spasticity or edema and thereby countering loss of ROM associated with these conditions, but electrotherapy is not an effective means of improving ROM by itself. |
| Transport medications | Iontophoresis: deliver medications by driving electrically charged ions through the skin. |
| Tissue healing | Microcurrent: some evidence has shown that microcurrent may augment tissue repair in individuals with fractures or slow-healing skin ulcerations. The mechanism has yet to be described. |
ROM, Range of motion.
Muscle reeducation
Neuromuscular function is altered through inhibition following injury as discussed previously with regard to rehabilitative cryotherapy. In fact, the great majority of early muscular strength and power loss following an injury is thought to be the result of neurologic inhibition rather than more morphologic causes, such as loss of muscle mass. Loss of muscular strength and power is seen immediately following injury before loss of muscle mass has even occurred. In fact, loss of muscle mass as an explanation for the loss of strength becomes valid only after several weeks have passed. The goal of muscle reeducation is to counteract postinjury inhibition in an attempt to allow more normal use of the surrounding musculature. By reducing the degree of inhibition seen in these muscles in the early period following injury, earlier and more functional exercise can begin, and it is this exercise that is the most important tool in returning the athlete to competition. This is sometimes thought of as helping a muscle “remember“ how to contract. Similarly, reeducation can be used to help reestablish neuromuscular pathways after periods of immobilization or even to help correct pathologic neuromuscular patterns such as seen when a patient compensates for gait or postural abnormalities. An additional means by which electrotherapy can help in reeducation is by overcoming the inhibition associated with the injury.90
The primary means by which electrotherapy is thought to be an effective tool for muscle reeducation is through the recruitment of motor units that are not otherwise being recruited. The muscle tissue is not directly induced to contract, however. Instead, the electrical current stimulates motor neurons to depolarize and thereby causes contraction of their respective muscle fibers. By artificially recruiting these motor units through stimulation of the motor nerves it is thought that we may somehow overcome the inhibitory stimuli that are interfering with their voluntary recruitment at some location up the neurologic tree.94 Despite common anecdotal agreement about the efficacy of muscle reeducation, very little research has directly examined its effects.
Retard atrophy
As is the case with muscle reeducation, the use of electrotherapy to retard atrophy is based on the ability of electrical current to induce muscle contraction.156,157 During disuse, immobilization, or paralysis, the relative inactivity of the muscles leads them to atrophy. Likewise, lack of muscle contraction–induced stress on the bones can also lead them to atrophy, eventually to the point where they can become fragile if the disuse is of sufficient duration. Electrotherapy is frequently used in cases in which prolonged immobilization is anticipated, such as with casting for fractures or with paralysis. The premise is that electrotherapy can be used to induce low-intensity isometric contractions of the muscle that can retard the progression of atrophy without compromising the immobilization. Note that the word retard was used rather than prevent. Prevention implies that we can completely counteract the atrophy that is occurring (Box 8-11). Instead, we are more likely to slow its progression.
Box 8-11 Three Reasons Why Electrotherapy Cannot Completely Prevent Atrophy
Edema management
Management of edema with electrotherapy can take two different forms. The first is retarding the formation of edema. The second is removing edema that is already present. The technique is different for each strategy and should be used only in the correct situation. For example, electrotherapy should be used to retard the formation of edema only in the period while edema is forming immediately following the injury. Use of this approach after a large amount of edema is already present may actually inhibit removal of the edema.
Sensory-level, high-voltage pulsed electrical stimulation applied directly to the area of the injury has been shown to limit the volume of edema following uniform injuries in an animal model.88,90-93 Two mechanisms have been proposed for this retarding of edema through the application of electrotherapy. The first is to combat the increasing permeability of the capillaries during the initial acute inflammatory response and thereby reduce the efflux of fluid from the circulatory system into the injured tissues. As explained in Chapter 2, one of the major events of the acute inflammatory response is a marked increase in capillary permeability that results from the release of numerous chemical mediators. The increase in permeability occurs when adjacent endothelial cells in capillaries do not adhere to each other as tightly as they normally do. This makes the capillaries “leaky“ and allows the transcapillary Starling forces to exert an even greater influence. The second—and less accepted—of the proposed mechanisms is that pulsed monophasic current causes vascular spasm that limits delivery of fluid to the injured area. Regardless of the suggested physiologic explanations for the effectiveness of the technique, the protocol used is very specific, requires a specific waveform, and is outlined in the later section on technique and dosage. One of the key elements of the protocol is the timing in relation to injury. This approach is effective only when it is begun before meaningful edema has developed. Therefore, the time window for initiating this modality is quite literally the first few minutes following the injury.
In addition to retarding the formation of edema during acute inflammation, electrotherapy can also be a valuable adjunct in removal of the edema that is already present. The proposed mechanism by which existing edema is removed is somewhat different from that for retarding the formation of edema, however. Although retarding the formation of edema is based on limiting the permeability of the vasculature, such a strategy may actually hinder the ability to remove edema. When present, edema is removed by the lymphatic system rather than the circulatory system. Removal requires that the edematous fluid be absorbed into lymph capillaries, where it flows to larger collecting vessels and eventually to one of the lymphatic ducts, and the fluid is then returned to the circulatory system. If the permeability of the lymphatic capillaries is limited, a potential outcome of the edema retardation electrotherapy protocol, movement of fluid into the lymphatic system may actually be hindered and thus reduce the effectiveness of edema removal.
Instead of using protocols aimed at altering permeability,t edema removal protocols focus on moving fluid into the lymphatic system and then moving it along the lymphatic vessels and away from the injury site.158-163 This is accomplished by motor-level electrical stimulation in which the muscles are induced to contract in a pulsing fashion with interrupted current. Each muscle contraction exerts external pressure on the lymphatic vessels. Squeezing of the lymphatic vessels causes the fluid in them to move. This strategy for removal of edema is sometime called the “muscle pump“ strategy. When fluid reaches the collecting vessels of the lymphatic system, its flow essentially becomes unidirectional because of the presence of one-way valves within the vessels. Much like the valves located in veins, pressure exerted on the lymphatic vessel from muscular contractions causes the upstream valve to close and the downstream valve to open, thereby allowing the fluid to flow back only toward the circulatory system. Because fluid moves with each muscular contraction, electrotherapy pulse rates that are below the level needed for tetany are used. Tetanic contractions would produce only a single pressure pulse and would not be expected to move as much fluid as would repetitive contractions.
Pain management
Management of pain with electrotherapy is among the most common, best documented, and most successful uses of this modality.164-174 Much of the pain reduction literature deals with a subform of electrotherapy called transcutaneous electrical nerve stimulation (TENS). TENS typically involves the use of pulsed, sensory-level stimulation to interfere with the transmission of pain signals in the spinal cord through a mechanism known as gait control. Gait control uses sensory information on A-β afferent nerves to interfere with the transmission of pain on A-δ and C afferent fibers. To be effective, adequate stimulation of A-β fibers must occur. The literature suggests that this is most likely to occur at rates between 60 and 150 pulses per second, and rates of 100 to 150 pulses per second are most common.1,2,5 The high pulse rates have led this protocol to be called high-frequency TENS. High-frequency TENS units generally use a short pulse duration (20 to 60 μsec) combined with a 50- to 100-Hz frequency of stimulation. Additionally, the combination of sensory-level TENS and cryotherapy has been shown to provide greater pain relief than with either of these modalities used individually.
Although the gait control strategy is certainly the most common, it is not the only strategy for controlling pain with electrotherapy. Motor-level stimulation with a high-voltage (> 150 V) stimulator can be used to stimulate the release of endogenous opiate-like substances from nerve fibers. Sometimes this protocol is referred to as “low-frequency TENS“ because of its low pulse rate (2 to 4 pulses per second). It is frequently uncomfortable during application but results in relief of pain following the treatment. This is somewhat different for conventional high-frequency TENS, in which pain relief is usually experienced during the treatment and for a short time thereafter. The release-of-opiates strategy is not as common as the gate control strategy; however, it may provide longer-lived pain relief.
Another pain relief technique with electrotherapy involves the use of a sensory-level polyphasic current known as interferential current.168,170,171 Interferential current is actually the combination of two different biphasic currents that are out of phase with each other. The two currents each have different frequencies and are carried on two different circuits (or channels) that are applied more or less perpendicular to each other. The differing frequencies of the currents and the fact that they are out of phase with each other produce a constantly changing waveform in the region where the currents cross. The key feature of interferential electrotherapy is this ever-changing waveform. Because the waveform is constantly changing, the body has a very difficult time accommodating to it. Accommodation is the process by which the sensory system learns to “ignore“ sensory stimuli that are unchanging. For example, anyone married for more than a few months no longer senses the wedding ring against their finger. The body has a reasonably good ability to accommodate to electrotherapy, particularly sensory-level electrotherapy. This accommodation reduces the efficacy of the treatments. With interferential electrotherapy, the constantly changing waveform reduces the accommodation and allows the treatment be more effective than fixed-waveform treatments. The mechanism of pain relief is thought to be the same as for high-frequency TENS. In fact, many TENS units now use modulated waveforms that change throughout the treatment in an attempt to overcome accommodation.
Spasticity management
Management of spasticity is among the more commonly cited uses of electrotherapy, although it is generally less applicable to athletic injuries than to other conditions, such as neurologic lesions. Management of the spasticity associated with neurologic lesions frequently involves stimulating the antagonist muscles and relying on reciprocal relaxation. In fact, the vast majority of the literature on the use of electrotherapy for spasticity focuses on the type of spasticity resulting from spinal cord trauma, cerebral vascular accidents, brain trauma, and disease.175 Because these lesions are not common with athletic injuries, they will not be discussed further in this chapter. On the other hand, management of muscle spasm associated with athletic injuries is a common use of electrotherapy and needs to be addressed, although the literature describing such use is very sparse.
Two strategies are predominantly used for managing athletic injury–related acute muscle spasm with electrotherapy, although only one of them is very tolerable to the patient. The more tolerable of the two strategies involves using motor-level stimulation of the spastic muscles in an attempt to alter their frequency of contraction. Recall that the rate of firing of the motor units determines whether a muscle’s contraction will be pulsing or smooth (tetanic). Smooth contractions occur with firing rates higher than 30 to 50 pulses per second. When a muscle is in spasm, it is generally contracting smoothly and continuously. By using a muscle stimulator with relatively high intensity and less than a tetanic pulse rate, it may be possible to slow the firing rate and cause the spasm to abate. The other and probably more effective strategy involves using the stimulator to fatigue the muscle to the point where the spasm ceases. This strategy has been shown to be effective, but it is not very comfortable and most patients may not tolerate it well. Generally, the technique involves using high–pulse rate, maximum tolerable intensity stimulation with a high-voltage stimulator to recruit as many motor units as possible. This will eventually lead to muscular fatigue, and the spasm will lessen or stop altogether. Although this technique is uncomfortable, it can be made more comfortable and more effective if it is combined with static stretching of the affected muscles. Cryotherapy is also a common adjunct with this technique.
Increasing strength
Even though electrotherapy can indeed be used to improve strength, this is among the most often misused forms of this modality. To understand this use of electrotherapy, a distinction must first be made between using electrotherapy for muscle reeducation versus electrotherapy for increasing strength. As discussed earlier, muscle reeducation involves using electrotherapy to overcome the neuromuscular inhibition that is interfering with normal muscle function. Said another way, muscle reeducation uses an electrical stimulator in an attempt to improve pathologic muscle function by reestablishing the normal and appropriate neurologic pathways for muscle contraction. This is different from using a stimulator to improve muscle strength.
When a stimulator is used to improve strength, the goal is not to overcome inhibition or other neuromuscular pathology. Instead, electrotherapy is used to strengthen tissue that has normal neuromuscular function but is not as strong as desired. Said another way, muscle strengthening with electrotherapy involves causing the contractile elements within muscle to overload in an attempt to induce them to adapt by becoming stronger. This is precisely the same goal as used during resistance training to improve muscle strength, but the catch is that resistance training is considerably more effective in achieving this goal than electrotherapy is.
To strengthen muscle tissue, regardless of the method, the muscle must be made to exert more force than it is accustomed to exerting. This principle is known as overload. Overload of a muscle can be accomplished in two ways: increasing the rate of firing of motor units and recruiting a larger number of motor units.60 During resistance training, the body’s strategy is to do both, but to predominantly favor recruiting more motor units. In fact, during exercise the body varies its motor unit recruitment pattern. At the beginning of an exercise, the smaller-diameter motor neurons of the fine control motor units are recruited. If more force is required, the larger-diameter motor neurons controlling the stronger but less finely controlled motor units are recruited. As the muscle begins to fatigue, the fine control motor units fail first and additional large motor units are recruited until they eventually fail. This is why some shaking and less coordination are observed as someone fatigues during resistance training. When a muscle stimulator is used, however, a different pattern of recruitment takes place.1,2,5
Use of a muscle stimulator to improve strength also depends on overload, and both a high rate of motor unit firing and measures aimed at recruiting more motor units are used. In strengthening with electrotherapy, a pulse rate at or near the maximum available for the stimulator is used in an effort to produce as high a rate of motor unit firing as possible. In addition, as high an intensity (voltage) as tolerable is used because higher intensities lead to better penetration of the nerve by the electrical current and therefore recruitment of an increased number of motor units. The unfortunate catch in this strategy, however, is that recruitment of motor units with electrotherapy appears to be the reverse of normal voluntary recruitment. With electrotherapy, motor units that are of larger diameter and closer to the surface of the nerve are preferentially recruited. These same motor units appear to be stimulated over and over rather than recruiting different motor units as some of them fatigue, as is the case with normal exercise. This repeated firing of the same motor units is the reason that muscular fatigue is experienced so quickly with electrical stimulation and not as quickly with exercise. The repeated recruitment of a limited set of motor units also limits the effectiveness of electrotherapy-based strengthening programs. Strengthening adaptations are essentially limited to the motor units that are being recruited, and electrotherapy recruits fewer motor units than active exercise does. Although some degree of strengthening of normal tissue can occur with electrotherapy, the strengthening is not as effective as with active exercise in a resistance training program. Therefore, the use of electrotherapy for muscle reeducation is recommended in athletic therapy, but electrotherapy as a tool to strengthen muscles that have normal function is not.
Increasing range of motion
Many modalities texts suggest that electrotherapy can be used to improve range of motion, and it most certainly can do so, but not in the way that we are usually seeking with athletic injuries. The use of electrotherapy to improve range of motion is appropriate and effective in only a few limited situations. The most common involves a patient with neurologic trauma or disease that results in spasticity. For example, it is not uncommon to see spasticity in the gastrocnemius and soleus of a spinal cord–injured patient. Such spasticity leads to lack of ankle dorsiflexion range of motion. Electrotherapy can be applied to the dorsiflexors to both stimulate them and inhibit the gastrocnemius and soleus. This would allow greater dorsiflexion range of motion because of the reduction in spasticity of the plantar flexors. Though useful in some cases for neurologic injuries and disease, this type of improvement in range of motion is of little value in the rehabilitation of common athletic injuries. Another limited situation that is of more use with athletic injuries would be improving range of motion that is limited by pain, edema, or both. As discussed earlier, electrotherapy can be used for the management of these conditions and may result in an indirect improvement in range of motion as well.
Iontophoresis of pharmaceuticals
Iontophoresis, or the use of an electrical current to drive medications through the skin, is another common form of electrotherapy. This technique has some real advantages in that it can be used to deliver medications locally without having to inject them. This can be particularly useful for patients with fear of needles or for pediatric patients. In fact, iontophoresis with local anesthetics is gaining popularity as a preinjection technique to lessen the discomfort of pediatric immunizations. Though potentially advantageous, there is also some controversy over iontophoresis in the literature.130,176-182 Reports conflict regarding whether iontophoresis delivers enough medication to a deep enough tissue depth to be effective for many conditions. Moreover, outcome data for iontophoresis are limited and have not yet adequately demonstrated that the technique is of much benefit to patients with musculoskeletal injuries.
Iontophoresis requires a very specific type of electrical current and is not possible with typical muscle stimulators. To use electrical current to move medications, the medications must dissociate into electrically charged ions in solution. When an electrical current is applied to the medication solution, the charge at each electrical pole repels the medication ions with like charges and attracts the ions with opposite charges. Therefore, it is critical to apply the medication to the electrode that has the same polarity as the ion of interest in the medication. Likewise, only direct (monophasic) current can be used because alternating (biphasic) current would both repel and retract the medication and produce no net transport through the skin. The current used also needs to be continuous. Interrupted currents do not repel the drug long enough for it to travel through the skin. For these reasons, iontophoresis stimulators are different from other electrotherapy devices and are designed expressly for use in iontophoresis. These devices are used with single-use electrodes and deliver low-intensity DC in a monopolar setup as described later. The dosage is typically the product of the duration of treatment and the amount of current used and is expressed in milliampere minutes (mA • min). The specific dosage depends on the medication being used, and treatments generally last between 10 and 20 minutes, although they can be longer if lower amounts of current must be used because the patient does not tolerate DC well. A new iontophoresis device, the IontoPatch, was introduced in April 2001 and is somewhat different. Instead of using the typical 10- to 20-minute treatment with low to moderate amounts of current, the IontoPatch uses a much longer duration (usually 24 hours) and an extremely low level of current delivered from a self-contained battery in the patch. The lower level of current means fewer complications in terms of skin burns, and anecdotal reports have been very favorable, although the device is new enough that research literature is lacking.
A relatively small number of medications, usually corticosteroids, are commonly used with iontophoresis in athletic medicine settings. However, from a technical standpoint, any medication that dissociates into ions in solution and produces the desired effects could be used. The most common medications are presented in Table 8-11.
Table 8-11 Nonsteroidal Ions and Radicals
| Ion or Radical (Charge) | Features* |
|---|---|
| Magnesium (+) | From magnesium sulfate (Epsom salts), 2% aqueous solution; excellent muscle relaxant, good vasodilator, mild analgesic |
| Mecholyl (+) | Familiar derivative of acetylcholine, 0.25% ointment; powerful vasodilator, good muscle relaxant and analgesic; used for discogenic low back radiculopathies and sympathetic reflex dystrophy |
| Iodine (−) | From Iodex ointment, 4.7%; bactericidal, fair vasodilator, excellent sclerolytic agent; used successfully for adhesive capsulitis (“frozen shoulder“), scars |
| Salicylate (−) | From Iodex with methyl salicylate, 4.8% ointment (if desired without the iodine, can be obtained from Myoflex ointment—trolamine salicylate, 10%—or from a 2% aqueous solution of sodium salicylate powder); a general decongestant, sclerolytic, and antiinflammatory agent; used successfully for frozen shoulders, scar tissue, warts, and other adhesive or edematous conditions |
| Calcium (+) | From calcium chloride, 2% aqueous solution; believed to stabilize the irritability threshold in either direction, as dictated by the physiologic needs of the tissues; effective for spasmodic conditions, tics, “snapping joints“ |
| Chlorine (−) | From sodium chloride, 2% aqueous solution; good sclerolytic agent; useful for scar tissue, keloids, burns |
| Zinc (+) | From zinc oxide ointment, 20%; trace element necessary for healing; especially effective for open lesions and ulcerations |
| Copper (+) | From 2% aqueous solution of copper sulfate crystals; fungicide, astringent, useful for intranasal conditions (e.g., allergic rhinitis—hay fever), sinusitis, and dermatophytosis (athlete’s foot) |
| Lidocaine (+) | From Xylocaine, 5% ointment; anesthetic and analgesic, especially for acute inflammatory conditions (e.g., bursitis, tendinitis, tic douloureux, and temporomandibular joint pain) |
| Lithium (−) | From lithium chloride or carbonate, 2% aqueous solution; effective as an exchange ion for gouty tophi and hyperuricemia† |
| Acetate (−) | From acetic acid, 2% aqueous solution; dramatically effective as a sclerolytic exchange ion for calcific deposits‡ |
| Hyaluronidase (+) | From Wydase crystals in aqueous solution, as directed; for localized edema |
| Tap water (+/−) | Usually administered with alternating polarity, sometimes with glycopyrronium bromide for hyperhidrosis |
| Ringer solution (+/−) | With alternating polarity; used for open decubitus lesions |
| Citrate (+) | From potassium citrate, 2% aqueous solution; reported effective for rheumatoid arthritis |
| Priscoline (+) | From benzazoline hydrochloride, 2% aqueous solution; reported effective for indolent ulcers |
| Antibiotics: gentamicin sulfate (+) | 8 mg/mL; for suppurative ear chondritis |
* All solutions are 2%; ointments are also low-percentage compounds. The literature and clinical reports agree that the lower the percentage, the more effective the ionic exchange and transfer. Whether this is purely a physical chemistry phenomenon or an example of the Arndt-Schultz law, which states that “the smaller the stimulant, the greater the physiologic response,“ remains to be proved.
† The lithium ion replaces the weaker sodium ion in the insoluble sodium urate tophus and converts it to soluble lithium urate.
‡ The acetate radical replaces the carbonate radical in the insoluble calcium carbonate calcific deposit and converts it to soluble calcium acetate.
From Kahn, J. (1987): Non-steroid iontophoresis. Clin. Manage., 7:15. Reprinted from Clinical Management with the permission of the American Physical Therapy Association.
Clinical Considerations
The specific waveform chosen for electrotherapy can be absolutely critical in some cases and can make very little difference in others. One of the hallmarks of a skilled practitioner is to understand the difference. To understand this difference, some familiarity with the electricity fundamentals and terminology discussed earlier is required, as is understanding of the following concepts. First, it has already been discussed that waveforms can be monophasic (also known as DC current), biphasic (also known as AC current), or polyphasic (a mixed waveform, such as interferential current). It has also been discussed that waveforms can be either continuous or interrupted. These features can be combined to produce some general classes of waveforms that have an impact on clinical treatments.
Continuous versus interrupted current
Continuous currents are found in only very few devices and are used in very specific situations. The first of these situations is for iontophoresis, where a continuous monophasic current is used. Continuous currents must be used in this case because interrupted currents do not have a sufficient duration of current flow to move ions across the skin. A second situation in which continuous current is used involves the stimulation of denervated muscle to prevent disuse atrophy. In this case, continuous current is used because the longer current duration makes it easier to induce depolarization of what little remains of the motor nerves or perhaps even the muscle itself. Aside from these two situations, continuous currents are normally found only as biphasic carrier frequencies in polyphasic currents. In these situations, they are normally used to help create a perpetually changing waveform to help overcome accommodation, as is the case with both interferential current and a waveform used for strengthening known as MFBurstAC, or Russian current.155
Polarity
Electrotherapy devices that offer monophasic waveforms can readily be distinguished from biphasic or polyphasic waveforms by nature of the ability to select a polarity for the treatment electrodes. Because biphasic and polyphasic waveforms have both positively and negatively charged phases that alternate, no specific polarity can be assigned to the electrodes. In reality, only in very few situations does the specific polarity of the electrodes make a clinical difference; however, it makes a very big difference in a few. For example, iontophoresis can be accomplished only with a continuous monophasic current and, even then, only when the drug is applied to the correct electrode. The other situation in which polarity clearly matters involves the stimulation of denervated muscle for preventing disuse atrophy; however, this application is less important in the rehabilitation of athletic injuries. Another situation in which polarity has been suggested to make a difference involves the use of electrotherapy to retard the formation of edema. In this case, cathodal (negative polarity) stimulation has been shown to be effective, as discussed later. There is little evidence to suggest that polarity is an important consideration in the management of pain or in the ability to produce contractions in innervated muscle tissue.
Unipolar versus bipolar
One of the easiest to understand yet most misunderstood application technique related to electrotherapy is unipolar versus bipolar electrode configuration. A unipolar electrode configuration means that the active effects of the stimulator are seen only in the electrodes attached to one of the two electrical poles. Bipolar configuration means that the active effects of the stimulator are seen in the electrodes attached to both poles. Recall that to have an electrical circuit, the electricity must have a pathway to flow from one pole (negative) to another pole (positive). In DC these poles maintain constant polarity, and in AC they switch polarity. For purposes of electrode configuration, the actual polarity of the electrode does not matter except in the cases discussed earlier. Whether an electrode displays “active effects“ of the current depends on the current density under the electrode, and this is determined by the combination of current intensity and size of the electrode. For a given amount of current, a bigger electrode will have the current spread over a larger area, and a smaller electrode will have the current spread over a smaller area. The amount of current per unit of area is the current density. To have an active effect, such as inducing sensory or motor stimulation, the current density must be adequate. The smaller the electrode, the greater the current density, and therefore greater stimulation effects will be seen. Conversely, the larger the electrode, the smaller the current density, and therefore little or no stimulation effect will occur.
In a unipolar configuration, current density is manipulated by using electrode size. One pole has a relatively small electrode (or several small electrodes), and the other pole has a relatively large electrode, usually called a dispersive electrode. Because a dispersive electrode has a large area, it has a small current density that does not produce active effects. Unipolar configurations are standard on monophasic high-voltage stimulators and are very useful in situations in which you want to move the active electrode, such as with trigger point stimulation. Bipolar configurations use similarly sized electrodes at both poles, so similar effects are seen at both electrodes. Bipolar configurations are more common on newer devices and biphasic stimulators. Bipolar configurations are useful for situations in which you do not plan to move the electrodes or when exposure of enough skin to use a dispersive electrode can compromise a patient’s modesty.
The names unipolar and bipolar can sometimes be confusing because they sound similar to polarity. In reality, unipolar and bipolar configurations have absolutely nothing to do with the polarity of the electrical current under the electrode. Although unipolar arrangements are typically the standard electrode setup on monophasic high-voltage stimulators, this is actually a matter of convention rather than necessity. In reality, any stimulator can be used in either a unipolar or bipolar configuration. The choice is really a matter of clinical convenience rather than association with a specific stimulator. To convert a unipolar configuration to a bipolar configuration, the large dispersive electrode would simply be replaced with an electrode similar in size to the other active electrode. Similarly, a greater effect can be produced with the stimulator by merely using smaller electrodes. To convert a bipolar configuration to a unipolar configuration, one of the active electrodes would simply be replaced with a larger dispersive electrode. One unique case of a unipolar setup involves using electrotherapy while immersed in water, such as a whirlpool or ice slush. In this case, a relatively small electrode is attached to a motor point of a body part that is immersed in water (e.g., the gastrocnemius). This small (active) electrode must be attached to a motor point that is out of the water. The other electrode lead wire is immersed in the water along with the body part. The water acts as a very large dispersive electrode, and the small electrode acts as an active electrode. This technique is generally used for edema retardation or removal protocols.
Current modulation
In addition to the manipulations of electrical currents already discussed, a number of other current modulations are also common. Current modulation is simply an alteration in the current’s waveform. It includes applications already discussed, such as using interrupted current, as well as a few other alterations that will be discussed here. It is used for a variety of reasons, such as counteracting accommodation, increasing patient comfort, minimizing fatigue, or making contraction easier.
Among the most common modulations is varying the pulse rate of an interrupted current to minimize accommodation. Most contemporary stimulators have built-in presets to modulate the pulse rate. Typically, these presets vary the pulse rate up and down within a specific band of frequencies that corresponds to a desired effect. For example, among the more common presets is one that varies the pulse rate up and down between 1 and 10 pulses per second, obviously in the subtetanic range if used with muscle contraction. With sufficient intensity, this setting would cause the muscle to visibly twitch at a varying rate that would not become a tetanic contraction. Similar presets can be found in the pulse rate range just above the threshold for tetany and also at much higher frequencies such as those commonly used for pain management or muscle strengthening.
Another common modulation is ramping the intensity with an interrupted current. When using a ramp setting, the current is not at its maximum intensity when it first comes on. Instead, each successive pulse of current increases slightly in intensity until the desired maximum is reached. The ramp setting allows the user to specify the time that it takes for the maximum to be reached. Some devices also allow a ramp setting for when the current is ending. Ramp settings are used to increase patient comfort, with it generally being more comfortable to ramp up to the maximum rather than being hit with it all at once.
Another modulation related to patient comfort is controlling the pulse width. There is an indirect relationship between the pulse duration (width) and the pulse amplitude (the intensity) when inducing a muscle contraction. For patients who have a hard time tolerating high amplitudes, the amplitude can be reduced to tolerable levels and the pulse width increased to still induce muscle contraction. Increasing pulse width can also improve the ability to induce a contraction in other situations as well.
The use of “on-time” and “off-time” is another common modulation with electrotherapy. Recall that muscle stimulators induce recruitment of the same set of motor units over and over. Because this does not match the normal physiologic recruitment pattern for motor units, it often leads to rapid fatigue in the muscle. Although in some protocols fatigue is desirable, such as when trying to overcome spasticity, muscle fatigue is to be avoided with most electrotherapy protocols. For this reason, many stimulators provide the practitioner with the ability to have either “continuous” current flow or current flowing for a certain period followed by a rest period with no flow of current. This type of modulation is often called interrupted, but it should not be confused with interrupted current as discussed previously. Normally, when we speak of current interruption, we mean that discreet pulses of current alternate with brief (microseconds to milliseconds) intervals of no current. When on and off modulation is used, current flows during the on-time, and this current is almost pulsed at whatever pulse rate setting is chosen. Likewise, the continuous setting on most stimulators means that the flow of interrupted (pulsed) current is constant rather than meaning a noninterrupted current. Obviously, the terminology gets to be confusing and becomes worse when different manufacturers use their own terminology. There is little consensus on the correct durations for on-time or off-time or even the correct on-off ratio. Even though research is required to better explore this parameter, many clinicians anecdotally report using a 1:1 ratio or less of on-time to off-time.
Intensity
The intensity setting controls the amplitude (voltage) of the waveform and therefore controls the quantity of current flowing through the circuit. Recall that the relationship between force and flow (voltage and current) is describe by Ohm’s law (see Box 8-10). The greater the intensity, or driving force, the greater the flow of current. Although most contemporary stimulators provide the user with a readout of intensity or current levels, use of a numerical value can be misleading. Because of variability in electrode placement, electrode size, skin conductivity, moisture, and other factors, the voltage used for one treatment to induce muscle contraction would not necessarily induce the same degree of contraction with a different treatment for the same patient. For this reason, it is more useful to think of intensity levels in terms of their effects rather than their numerical value. Perhaps the easiest scheme involves classifying intensity progressively as subsensory, sensory, motor, and noxious (Table 8-12). Subsensory levels are obviously not perceived by the patient and are rarely used outside microcurrent electrotherapy. Sensory levels of intensity imply that the patient can feel the current but the current does not cause muscle contraction. Motor levels of intensity cause muscle contractions. As the name implies, noxious levels of intensity are rather uncomfortable and are rarely if ever used, but they provide the maximum tolerable level of muscle contraction.
Table 8-12 Classification of Intensity Level by Its Effects
| Subsensory level | The level is not perceived by the patient and is rarely used outside microcurrent electrotherapy. |
| Sensory level | The patient can feel the current but the current does not cause muscle contraction. |
| Motor level | Motor levels of intensity cause muscle contractions. As the name implies, noxious levels of intensity are rather uncomfortable and are rarely if ever used, but they provide the maximum tolerable level of muscle contraction. |
Techniques and Dosage
This chapter presents only a general framework for electrotherapy techniques. For more complete and detailed information, a full text on modalities should be consulted. To simplify this section, general strategies and parameters are summarized in Table 8-13. Very few data are available regarding appropriate treatment durations for most protocols. By convention, most are 15 to 30 minutes in duration unless otherwise noted.
Future Questions
Many questions are still unanswered about electrotherapy because very little research has actually has examined specific combinations of parameters to determine their efficacy. Similarly, very few quality outcomes studies are available. Currently, some very promising work is being conducted in the areas of retarding edema formation and in the management of pain. Similarly, iontophoresis also receives sporadic and conflicting examinations. As a general statement, electrotherapy as a whole remains a vast and little explored area with many areas for further study.