The Pathology of Neoplasia
Veterinary pathologists play a critical role in the management of neoplasia of companion animals by providing accurate diagnostic information to clinicians so that a prognosis can be determined and adequate treatment provided. The clinician needs to have knowledge of the pathology of neoplasia to understand neoplastic conditions and the limitations of histopathologic assessment in the diagnosis of neoplasia. Both the pathologist and the clinician must work together to determine optimal treatment for the patient because the diagnosis and treatment of neoplasia in veterinary medicine have become more complex. No longer is it adequate to simply determine if the tumor is benign or malignant. The tumor type needs to be identified as accurately as possible, and tumor subtypes should be identified if prognostically significant. Grading of tumors is increasingly important because the behavior of some tumors can be predicted by the grade of the tumor. In addition, the assessment of margins for completeness of surgical removal is very important. In some cases, the histologic assessment of tissue treated preoperatively is important in predicting treatment outcome. Special procedures such as immunohistochemistry (IHC), electron microscopy (EM), flow cytometry, or polymerase chain reaction (PCR) may be advantageous in some cases to correctly identify tumor type or subtype or to predict clinical behavior of certain tumors. Classification of neoplasia in veterinary medicine is becoming more commonly applied, and systems have become more advanced with application of molecular markers for more accurate classification and prognostic information. For example, a recently upgraded histopathologic classification and grading system for canine mammary tumors has been reported to better prognostically evaluate tumors and standardize studies for cross-comparison.1 As more is learned about the diagnosis and treatment of neoplasia in veterinary medicine, newer predictive/prognostic classifications and grading schemes will continue to be developed.
Sample Handling
The biopsy sample should be visually inspected by the clinician to help determine that the appropriate tissue was obtained. If the biopsy was a needle core or incisional specimen, the sample should be of sufficient size and consistency so that it remains intact in formalin and does not become lost in processing. Samples less than 1 mm3 are usually inadequate, although a needle core sample 1 mm wide but at least 5 mm long can be sufficient. If the biopsy samples are needle core samples, more than one core of tissue should be obtained, if possible.
Very small samples can easily be lost during shipping or in processing because sample shrinkage during fixation and processing is imminent. Given these precautions, some techniques can be utilized to maximize the chances that the sample will make it through the process. Samples less than 3 mm in size can be placed on paper (surgical glove paper is appropriate) before fixation. These samples will be tacky and adhere to the paper. Very small or pale samples can be circled with pencil to draw attention to the samples at the histology laboratory. The paper can then be folded around the sample, and the entire package can be placed in formalin for fixation and shipping. Alternatively, commercially available screened tissue cassettes can be used to house the sample during fixation and shipment. The sample is placed in the screened cassette at the time of surgery, and the cassette with the sample is placed directly into formalin for fixation. These techniques decrease the chance for small samples to be lost in larger formalin containers. Extremely small samples can also be dyed with India ink or other commercially available dyes to assist in the identification of the sample. Samples containing excessive blood, mucus, or necrotic material may not be diagnostic, and the biopsy procedure may need to be repeated. If the specimen was an excisional sample, the entire sample should be submitted if feasible, and margins of concern should be identified with suture or ink. For very large samples such as large splenic tumors, representative sections such as peripheral versus central or different-colored or textured areas can be submitted. Visualization by an experienced person is extremely important when selecting the sample so that viable tissue is selected. Usually it is best to submit at least three to five sections of large lesions in case some portions of the tumor have excessive distortion, necrosis, or inflammation. When taking representative samples, the tumor and normal tissue interface should be included so assessment of tumor invasiveness into normal tissue can be determined. Tissue samples should be handled gently because compression during biopsy sampling and excessive use of electrocautery, cryosurgery, or laser surgery can cause specimen artifact, which can prevent a definitive diagnosis from being made.2
The sample needs to be preserved in fixative. The most widely used fixative is 10% neutral buffered formalin, which is readily available and frequently supplied in individual specimen containers by most laboratories. During excessive cold conditions, samples can freeze during shipment and cause significant destructive tissue artifact. Addition of 20% ethylene glycol or ethanol to the formalin can prevent freezing and maintain tissue integrity. Prior to immersion in fixative, larger samples may need to be sliced, facilitating adequate fixation; however, one side such as the deep edge should be left intact rather than slicing all the way through the sample so that orientation and margin assessment are not lost. Slices less than 1 cm thick should be avoided because curling and distortion of the tissue during fixation can result. The volume of tissue to fixative should approximate 1 : 10. In cases in which this volume ratio is not feasible because of large tumor size, multiple representative sections can be obtained. It is advisable to save the remnants of the sample, if possible, in case these first sections are not adequate for a diagnosis. When mailing large samples, use of smaller volumes of fixative is acceptable if the specimen has been in the recommended initial volume for at least 12 hours.3
Sample containers must be properly labeled (on the container, not just the lid) prior to submission to the laboratory. During transportation by mail, courier, or unpacking at the laboratory, paperwork can be inadvertently separated from the sample container, and unless the container is properly labeled, samples could become mixed. Most important, adequate history, including signalment, pertinent clinical findings, radiographic findings, and pertinent treatment, should be provided to the pathologist. A drawing of the sample indicating the position of the tissue on the animal is helpful in some situations, especially when margin determination is needed. If margins or areas of special clinical interest are marked (labeled) on the sample, a clear description of these labels, margins, or areas should be present in the submission form. A proper history is crucial for accurate diagnosis; without this the pathologist can be severely handicapped. The end result might be an inaccurate diagnosis, culminating in an inaccurate prognosis and improper treatment.2,4
After arrival at the laboratory, the specimens are catalogued and assigned an identification number, visually examined, trimmed to fit into processing cassettes, processed into paraffin blocks, sectioned, and stained. These procedures are performed by trained technicians and automated laboratory equipment. In most laboratories, tissues are trimmed into cassettes on the day of arrival, processed overnight, and completed slides are ready for examination by the pathologist 24 hours after receipt. Hard tissue such as bone needs to be decalcified prior to sections and thus will take longer to process. Larger samples incompletely fixed, extremely bloody samples (e.g., spleen), or samples with abundant fatty tissue (e.g., mammary gland) may require additional time for fixation. This additional time is vital to assure fatty tissue at the margin of the surgically excised sample remains intact during trimming, processing, and sectioning. In the process of trimming in or sectioning on the microtome, margins could become distorted and orientation may be lost, in which case reexamination of the gross specimen by the pathologist may be necessary. Most laboratories will hold remnant wet tissues in formalin for 7 to 60 days in the event further examination or sectioning is needed. Most laboratories file paraffin blocks and glass slides indefinitely, permitting review of previously submitted tissue on a given patient or retrospective studies on a series of cases.
Although infrequent, frozen sections can be made during operative procedures to provide the surgeon with a more rapid diagnosis. Samples are quick-frozen, sectioned on a cryostat, fixed, stained, and examined within 20 to 35 minutes. This technique is often conducted during surgery to assist with intraoperative decisions and thus requires a diagnostic laboratory and facility on site. However, these tissue sections are inferior to those processed routinely into paraffin, and as a result diagnostic accuracy is not as high and is proportional to the experience of the pathologist interpreting frozen sections. Furthermore, only a few veterinary institutions provide this service. This procedure may be helpful in establishing the identity of the tissue, adequacy of surgical margins, or adequacy of the tissue for more routine processing. Sometimes a provisional diagnosis can be made or at least a distinction between benign and malignant processes can be determined. The frozen-section diagnosis is always confirmed by routine histopathologic assessment of a paraffin-embedded section, often using the same tissue sample.4
Molecular techniques are now routinely applied as diagnostic tests in veterinary medicine. Some of these such as PCR and IHC have been developed and characterized for use on formalin-fixed, paraffin-embedded samples. Once processed, these tissues are often viable indefinitely for these tests, although the time prior to fixation, the time in fixation, and the storage time can negatively affect test sensitivity. These factors need to be determined for individual tests, and their effect needs to be considered in interpretation of results. IHC on formalin-fixed tissues requires only unstained, routine sections on slides appropriate for IHC. Exposure to sunlight or extremes of temperature should be avoided. Requirements particular to individual testing laboratories should be determined prior to submitting samples for these tests. PCR requires thick sections (10 to 20 µm) to assure adequate DNA or RNA amounts. These are typically allowed to roll up during microtome sectioning and can be sent to the testing laboratory at room temperature in an air-tight container. Acquiring specifics of the test material needed and shipping requirements prior to collecting the sample or requesting the test is often beneficial and avoids frustration for the owner, submitter, and testing laboratory.
Terminology
Numerous terms associated with tumors or suspected tumors are encountered in the description of the features of a tissue sample. A clear understanding of this terminology is imperative to understand the implications of the histopathologic findings. A clinician’s responsibility extends beyond collection of the tumor and treating the patient. A clinician has a responsibility to interpret the histopathology report, to identify details that might help subclassify a tumor beyond the base of tumor identification, and to assist in determining a tailored treatment protocol for individuals. A firm grasp on terminology is required for this interpretation and to discuss details of the tumor with the pathologist.
A tumor is any tissue mass or swelling and may be neoplastic or not, although this term today more typically is a generic term used to describe any neoplasm. Neoplasia is the abnormal growth of a tissue into a mass that is not responsive to normal control mechanisms and may be benign or malignant. Growth of this mass is not affected when the inciting stimulus is removed. Cancer refers to a malignant neoplasm.5 All neoplasms arise in normal tissue and thus are composed of parenchymal and stromal cells—some can also incite secondary inflammation. Their differentiation state can be assessed with histopathology and is based on the appearance of the tumor cells, their organization, and their association with the supporting stroma. Differentiation is controlled at the molecular level by gene expression. Normal reversible processes of hyperplasia (a nonneoplastic increase in the number of cells present) and atrophy (a decrease in the number of cells present) are also composed of parenchymal cells and stroma. Their retention of near-normal architecture, just as well-differentiated neoplasms retain normal architecture, can make differentiation of the two processes difficult. At times, only removal of the inciting stimulus and time can distinguish between hyperplasia and well-differentiated cancer. One definition of cancer is a proliferation of a clonal population of cells that is no longer responsive to tissue homeostatic mechanisms. Molecular techniques to determine a clonal expansion of cells by their similar DNA sequence structure could help separate these conditions or identify occult cancer prior to tissue distortion at a microscopic level. These tests are few, but more are currently being developed, specifically for use in canine and feline lymphomas.6 Metaplasia is the abnormal transformation of a differentiated tissue of one kind into a differentiated tissue of another kind and not a neoplastic condition. Metaplasia should reverse or not progress with cessation of the chronic inciting stimulus. An example is squamous metaplasia in the prostate gland, where normally columnar epithelium becomes squamous under the influence of estrogen. Metaplastic cells can be targets for carcinogenesis if continued carcinogenic promotional events occur (e.g., bronchial squamous metaplasia in human smokers). In such cases, metaplasia often progresses and acquires dysplastic changes. Dysplasia is abnormal tissue development and can be a feature of neoplasia, but it is not necessarily a neoplastic condition. Dysplasia such as epithelial dysplasia in the oral cavity can be a preneoplastic condition. Anaplasia is a loss of differentiation or atypical differentiation and is a feature of many, but not all, malignancies.
Terms associated with cellular or growth features are frequently encountered in descriptions of neoplasia. Pleomorphism is the occurrence of multiple forms, shapes, and sizes of cells and nuclei (cellular and nuclear pleomorphism respectively). Anisocytosis and anisokaryosis are greater than normal variations in cell and nuclear size, respectively. Round or polygonal cell shapes are usually associated with epithelial or hematologic tumors, whereas spindle cell shapes are usually associated with mesenchymal tumors. A scirrhous or desmoplastic response is an abundant fibroblastic proliferation with collagen formation that occurs in some malignant invasive cancers. In situ refers to a malignancy, usually limited to lesions of epithelial origin, that has not yet become invasive or invaded beyond the natural confines of its basement membrane.2,4,5
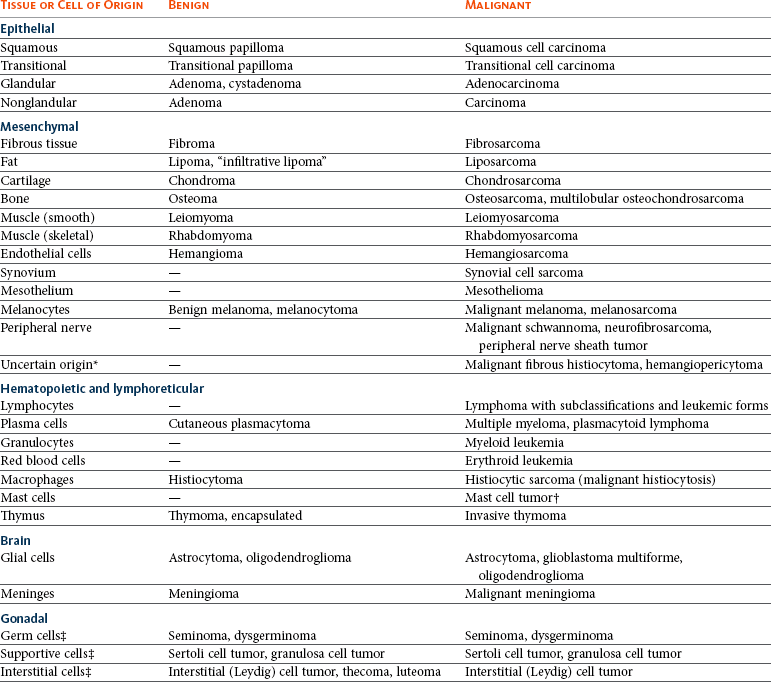
For each type of tumor, specific terminology is used to denote the origin of the tumor and whether the tumor is benign or malignant (Table 3-1). In basic terms, although benign tumors can cause tissue distortion, they typically do not have a high mortality. In contrast, malignant tumors (cancer) are more destructive of tissues and will often ultimately lead to death if the patient is left untreated. There are exceptions to these rules. Tumors can develop from any normal tissue type; therefore there are a considerable number of different tumor types. As more is learned about certain tumors, names and subclassifications may change, creating some confusion. More recent advances in molecular techniques applied to tumors have allowed additional subclassification of tumors beyond the histologic realm. More focused techniques such as IHC, targeted PCR for genetic alterations and mutations, and flow cytometry for cell surface markers can be applied once specific alterations in tumor types have been identified and determined to be markers in a specific tumor or tumor subtype. Broad encompassing techniques for large genomic, proteomic, and more recently metabolomic fingerprinting of tumors are used as discovery tools to scan for alterations that define subclasses of tumor types previously unidentified by more traditional methods. The identification of genetic mutations, varied cell surface receptor expression, altered signaling pathways, or altered cellular metabolic response to these genetic modifications may identify unique fingerprints for a tumor, which, combined with traditional histologic methods, might allow subclassification that is more accurate at identifying a tumor’s behavior and its response to tailored cancer treatment (see Chapter 8).
Table 3-1
Nomenclature of Common Tumor Types in Veterinary Medicine
*Pathologists disagree about the origin of these tumors; some feel they are a class of peripheral nerve sheath tumors or perivascular wall tumors.
†Theoretically, all mast cell tumors are potentially malignant, but grade 1 mast cell tumors are clinically benign.
‡Unfortunately, the terminology of these tumors does not distinguish between benign and malignant forms.
Benign tumors of epithelial origin are termed adenoma, papilloma, or epithelioma. Benign tumors of mesenchymal origin are designated by the suffix -oma after the tissue type (e.g., fibroma, osteoma). Malignant tumors of epithelial origin are termed carcinoma or adenocarcinoma if forming glands and ducts, whereas malignant tumors of mesenchymal origin are termed sarcoma. In some cases, the -oma suffix is used when the tumor is malignant, as in malignant melanoma and lymphoma. Leukemia, a malignant neoplasia of blood cells (occasionally referred to as “liquid tumors”) in hematopoietic tissues and usually in the blood, has no benign counterpart, although a leukemoid reaction is a nonmalignant condition that mimics leukemia.2,4,7 Although nomenclature for human cancer can often be applied to animal cancer, all nomenclature cannot be directly applied across species due to differences in tumor types and tumor behavior. Similarities and differences must be taken into account when considering whether nomenclature can have cross-species application. For example, direct comparison of canine mammary gland tumors to those of the human mammary classification system have identified differences that require an independent classification system.8
Histologic Features of Neoplasia
Despite recent advances in a number of areas of pathology, including molecular techniques, evaluation of tissue by light microscopy remains the standard technique for tumor diagnosis.3 Neoplasia has certain histologic features that distinguish it from hyperplasia or inflammation, and there are features that distinguish benign from malignant neoplasia. In some cases, these features can be difficult to observe. Definitive diagnosis of malignant versus benign versus inflammation or hyperplasia may not always be possible. In these cases, a repeat biopsy, either immediately or after a period of clinical observation, may facilitate a definitive diagnosis.
When inflammation is present, the cellular features of reactive fibroblasts and reactive endothelial cells can be misleading.2,9 However, in reactive tissue with inflammation, the fibroblasts and endothelial cells usually are oriented perpendicular to one another (reactive granulation tissue) and usually a substantial amount of inflammation relative to reactive tissue is present. When granulomatous inflammation occurs, large reactive and epithelioid macrophages can be mistaken for tumor cells, but the pattern of tissue involvement and presence of other inflammatory cells helps rule out neoplasia. In some tumors, especially those with surface ulceration or necrosis (e.g., some synovial cell and soft tissue sarcomas), an extensive amount of inflammation can obscure neoplasia but is considered a secondary process.
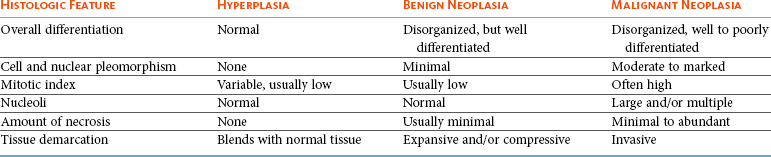
Benign tumors may be most difficult to distinguish from hyperplasia (Table 3-2) because both have a proliferation of well-differentiated cells that are easy to identify. There is a distortion or loss of normal tissue architecture in benign neoplasia, and usually the tumor grows in an expansive manner, causing compression rather than invasion of adjacent tissue. These tumors are often defined by a fibrous tumor capsule. Hyperplasia tends to retain normal tissue orientation and does not compress adjacent tissue. It often lacks a fibrous capsule. In general, if allowed to grow, benign neoplasia will attain a larger size than a hyperplastic lesion. In some instances, such as thyroid gland adenoma versus adenomatous hyperplasia of the thyroid gland in cats or sebaceous gland adenoma versus sebaceous gland hyperplasia the distinction between benign tumor and hyperplasia is not clinically important.
Features that distinguish malignant from benign neoplasia include more dramatic loss of tissue organization, increased anisocytosis and anisokaryosis, increased nuclear and cellular pleomorphism, increased and variable nuclear : cytoplasmic ratio, abnormal nuclear chromatin, increased mitotic figures, increased and abnormal mitotic figures, abnormal large and/or multiple nucleoli, increased necrosis, amount and character of the supporting stroma, and invasiveness of malignant tumors (see Table 3-2). With invasion, individual cells or groups of tumor cells infiltrate extensively into surrounding tissue, may invade into vascular or lymphatic spaces, and may invoke a scirrhous or desmoplastic response characterized by an excessive fibrous reaction. A further feature of malignancy is destruction of the normal tissue or obliteration of normal tissue architecture. Evidence of lymph node or more widespread metastasis obviously distinguishes malignant from benign tumors.2,4,10 However, in certain tumors, histologic features do not correlate with behavior (e.g., canine histiocytoma and benign plasmacytoma). Both have histologic features of malignancy but are clinically benign. Histologically low-grade, yet biologically high-grade, fibrosarcomas of the canine head have histologic features of a benign condition but are clinically malignant.11 Similarly, bronchial carcinomas in cats will retain organized epithelial structures composed of well-differentiated ciliated pseudostratified columnar epithelium even at distant metastatic sites, including the digit, eye, heart, and kidneys.12 In these instances, knowledge of clinical history and tumor behavior is needed to distinguish benign from malignant neoplasia.
Generally, a pathologist makes the diagnosis of tumor versus reactive tissue and sometimes tumor type at relatively low magnification after evaluating the overall tissue pattern and behavior with respect to adjacent normal tissue. Higher magnification is then used to confirm the low magnification impression, to classify tumor type if not already done, and to assess nuclear features and mitotic index. Immediate use of high magnification is a mistake because very reactive tissue and inflammation, especially when macrophages are present, may be mistaken for neoplasia. For this reason, a definitive diagnosis may be difficult to establish with small samples or with samples that lack some normal tissue. There are numerous instances such as with osteosarcoma, mast cell tumor, transitional cell carcinoma, some soft tissue sarcomas, and squamous cell carcinoma in which histologic features are sufficiently distinct to make a definitive diagnosis on a small sample if the tumor was sampled correctly. In other cases such as with lymphoma or granulomatous inflammation, small samples may be inadequate to establish a final diagnosis because the overall cellular pattern and interaction with normal tissue is an important diagnostic feature.
Grading and Staging of Neoplasia
In certain tumors, grading the degree of malignancy is predictive of biologic behavior,2,4,10 and, in the future, quite likely the behavior of more tumors will be shown to be related to histologic grade. Grading of tumors is somewhat subjective, and reproducibility between pathologists can be variable.13 Despite this limitation, in one study of 440,000 cases of cancer in humans, interobserver variation did not have a sufficient impact to alter the relationship between grade and outcome.14 A recent international study applying the World Health Organization (WHO) system of lymphoma classification to canine lymphomas demonstrated a reproducibility of 83% to 87% if the classification entities were well described and illustrated for reference by those applying the classification system. With preparative training of those using the system and careful application to well-described criteria, a high level of accuracy can be achieved in diagnosing canine lymphoma or any other disease entity.15
Another difficulty is that tumors are heterogeneous, and patterns as well as features of increased malignancy may vary from area to area. If heterogeneity is present, the most malignant areas are usually assessed for grading purposes. Sampling variation with small biopsy samples can dramatically affect the representation of different components in a heterogeneous tumor; therefore, if the sample is small, accurate grading is not possible and grades should be interpreted with caution.
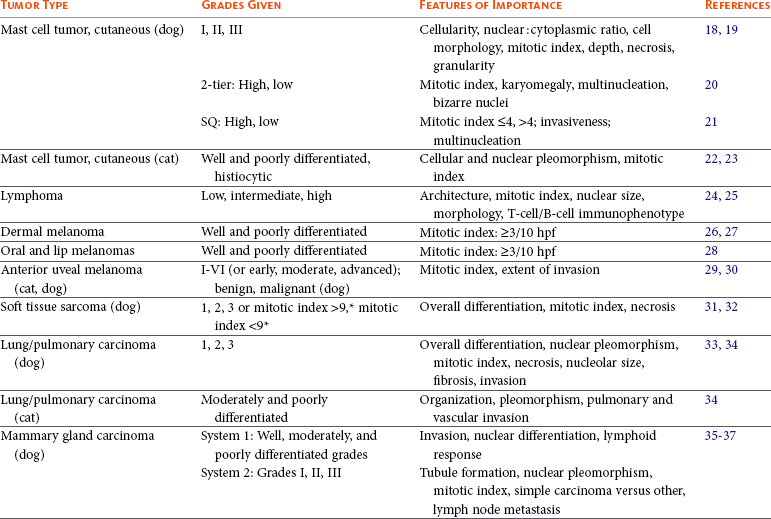
Features of tumors that are often evaluated to assess grade include (1) degree of differentiation, (2) mitotic index (number of mitotic figures per 10 high-power 400× fields), (3) degree of cellular or nuclear pleomorphism, (4) amount of necrosis, (5) invasiveness, (6) stromal reaction, (7) nucleolar size and number, (8) overall cellularity, and (9) lymphoid response (Table 3-3). Of these features, mitotic index, amount of necrosis, and nucleolar features are the only objective, quantifiable features that can be quantified with manual counting, computerized morphometry, or chemical quantification.2,4,10 Often, in determining a grade, individual features are scored, and then each score is added to obtain a total tumor score. The tumor scores are then separated into ranges that are associated with a tumor grade. Current grading scales are efficient, cost-effective, and involve no new technology. As the identification of molecular markers for tumor subtypes and prognostic or predictive parameters progresses and as the techniques become more time and cost efficient, yielding quicker turnaround times and easier application, they will become more routine and, likely, a valuable component of updated grading systems.
Table 3-3
Molecular Features Underlying Grading Criteria
| Grading Criteria | Underlying Molecular Mechanisms |
| Mitotic index | Cyclins, cyclin-dependent kinases (CDKs), proliferating cell nuclear antigen (PCNA), Ki67, bromodeoxyuridine (BrdUrd), labeling index (LI)/growth fraction (GF) |
| Percent necrosis | Inflammatory mediators, including eicosanoids (prostaglandins), cytokines (interleukin [IL], tumor necrosis factor alpha [TNF-α]), microvessel density (MVD) |
| Invasiveness | Matrix metalloproteinases (MMPs), plasminogen activators (PA), integrin expression, CAM (cell adhesion molecules) |
| Stromal reaction | Transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), MVD mediators |
| Nucleolar size | RNA transcriptional activity, silver staining nucleolar organizing regions (AgNORs) |
| Overall cellularity | Growth fraction, apoptosis factors (i.e., FasL, caspases), tumor doubling time |
| Inflammatory (lymphoid) response | TNF-α, interferon gamma (IFN-γ), IL-2, increased MHCII, intercellular adhesion molecule (ICAM) |
The quantifiable criteria previously mentioned for tumor grading have more recently been assessed with image analysis (computerized analytical morphometry). The use of image analysis allows for a more objective, repeatable measure decreasing interobserver variation and bias. Examples of this methodology demonstrate nuclear features such as nuclear area, mean diameter, and perimeter that correlate with mast cell tumor histologic grade, which is predictive of tumor biologic aggressiveness.16,17 Currently, the routine application of computerized morphometry is not practical for routine use in diagnostic pathology due to time and effort restrictions, but it is only a matter of time before automation overcomes these limitations.
The rationale behind the effectiveness of tumor grade is the indirect assessment of molecular features. Many of the histologic criteria used in grading scales probably reflect the underlying molecular mechanisms (Table 3-4). One example would be the correlation between tumor necrosis evaluated in many tumor grades and the underlying mechanism of tumor microvessel density (MVD) and factors that affect the density of these vessels. Lack of adequate tumor vessel density will result in tumor hypoxia and thus tumor necrosis. An underlying mechanism of tumor vessel density includes the vascular endothelial growth factor (VEGF) signaling pathway. Studies have demonstrated the strong association of MVD and VEGF expression with tumor grade and biologic tumor aggressiveness.52,53 Another example involves the induction of hypoxia-inducible factor-1α (HIF-1α) by hypoxic tumor cells. The HIF-1 gene product is the alpha subunit of transcription factor HIF-1 (http://www.ncbi.nlm.nih.gov/gene/3091). HIF-1α is a regulator of the cellular response to hypoxia by activating transcription of many genes involved in energy metabolism, angiogenesis, apoptosis, and other genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia. HIF-1α thus plays an essential role in angiogenesis and pathophysiology in the hypoxic environment present in many rapidly growing tumors. HIF-1α overexpression in brain, breast, cervical, esophageal, oropharyngeal, and ovarian cancers is correlated with treatment failure and mortality, as well as tumor progression.54
Table 3-4
Neoplasia with Grades or Histologic Features Having Prognostic Significance
*Sum of mitoses in ten 400× fields.
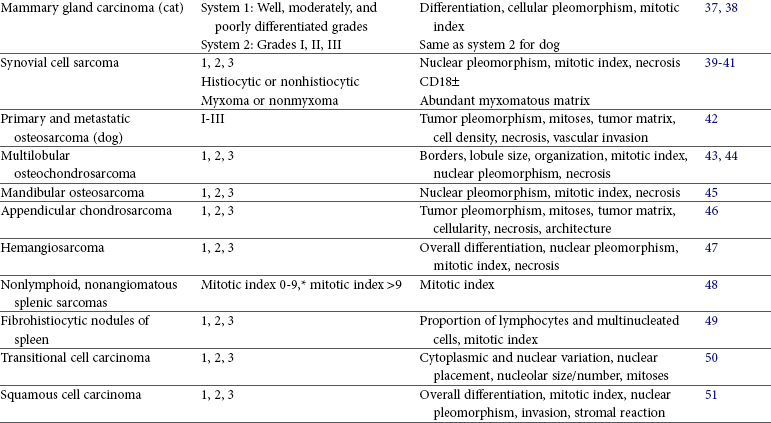
Tumor grade may correlate with survival, metastatic rate, disease-free interval, or with frequency and/or speed of local recurrence. Tumors in which grade or histologic features have been determined to be prognostic for biologic behavior in dogs include mast cell tumor18-21; lymphoma24,25; dermal, oral, and ocular melanoma26,29,54,55; mammary gland carcinoma1,35; synovial cell sarcoma39,40; multilobular osteochondrosarcoma43,44; hemangiosarcoma47; nonhematogenous sarcoma and fibrohistiocytic nodules of the spleen48,49; transitional cell carcinoma of the urinary bladder50; squamous cell carcinoma of the tongue51; lung carcinoma33,34; appendicular osteosarcoma42,56; mandibular osteosarcoma45; chondrosarcoma46; and soft tissue sarcoma31,32,57 (see Table 3-3). In humans and dogs with soft tissue sarcoma, the histologic grade is more important than the tumor type.2,32,58 Tumors whose grade or histologic features are predictive of biologic behavior in cats include lung carcinoma34 and mammary gland carcinoma,38 with conflicting reports regarding feline mast cell tumor and fibrosarcoma22,23,59,60 (see Table 3-4).
Grading systems have not been well established for some malignant tumors, yet the pathologist can make an assessment of presumed biologic behavior based on the overall degree of tumor differentiation. In these cases, the terms well differentiated, moderately differentiated or poorly differentiated may be suggestive of a low-grade, medium-grade, and high-grade malignancy, respectively.61 This type of assessment is most commonly done for squamous cell carcinomas, some sarcomas, and carcinomas of the mammary gland, salivary gland, gastrointestinal tract, liver, exocrine pancreas, and perianal gland. Tumor grading probably will become even more widespread and important in the future, especially as novel analytical techniques and molecular tumor markers are included. Not only can prognosis be determined based on tumor grade and differentiation, but also treatment may be modified to apply more aggressive therapies to tumors of higher grade.
The pathologist also may assist in staging of cancer by assessing tumor size, depth of tumor invasion, the presence of tumor in regional lymph nodes, and identification of tumor in distant sites. This information is needed to stage tumors into the T (tumor size and/or invasion), N (nodal involvement), and M (distant metastasis) system.10 Cytologic assessment of draining lymph nodes has been shown as a sensitive alternative to histopathology for lymph node metastasis needed for staging.62 For some tumors such as bladder cancer in humans, tumor staging is based largely on depth of tumor invasion into the bladder wall.2,4 This may prove to be useful in cases of bladder cancer in pets and has been shown to correlate with tumor grade in dogs.50 In both processes of tumor grading or tumor staging, these procedures are useful only if they have been shown to correlate with clinical behavior.
Assessment of Tumor Margins
Tumor margin assessment is an essential part of the pathology report whenever curative-intent surgical excision is attempted.3 It may be the best determinant of adequate surgical treatment and may serve as a predictor of treatment outcome.63 Grossly, the surgical margin can be defined as the margin beyond which tissue remains in the surgical bed or any region of the biopsy specimen adjacent to or contiguous with tissue that remains in vivo.63 Microscopically, the surgical margin is the region between the neoplastic process and the surgical edge of the biopsy specimen. Margin assessment should be performed for both benign and malignant lesions, although detailed characterization of the margin (e.g., objective measurement, tissue constituents, viability) is typically more critical for malignancies. Obtaining accurate surgical margin information on the pathology report is critically dependent on (1) specimen handling and information submitted by the clinician, (2) method of tissue trimming at the diagnostic laboratory, and (3) observations reported by the pathologist.
Appropriate assessment of the surgical margin is at the onset critically dependent on information provided by the submitting clinician and moreover by appropriate tissue demarcation (e.g., inking) prior to submission. Inking is superior to other methods of denoting surgical margins (e.g., suture placement) because surgical ink is visible at both the gross and microscopic levels.4,64 At the gross specimen level, the surgical ink impacts the regions of the specimen that are obtained by laboratory personnel during trimming for microscopic examination. At the microscopic level, assuming appropriate corresponding information was provided on the submission form by the clinician (e.g., yellow ink = deep margins, black ink = lateral margin), the ink allows the pathologist to appropriately assess and report the margins relative to a specific region. Surgical ink should only be placed on regions of the specimen that are true surgical margins or of specific concern to the clinician. The ink should also be allowed to dry (typically 5 to 10 minutes) prior to placement into a fixative (e.g., 10% neutral buffered formalin) to prevent the ink from washing off or unintentionally coating insignificant areas.63
Once at the laboratory, the specimen will be trimmed routinely with guidance from the information provided on the submission form and based on any tissue markings. If desired and if it enhances communication, annotated sketches or images of the specimen may also be submitted. The most common method of trimming for routine specimens is known as the cross-sectioning method. The mass is bisected along its short axis, after which each remaining half is bisected along its long axis, creating quarter sections. Ultimately, this method is perpendicular sectioning and allows for margin evaluation at four lateral and four deep regions of the specimen.65 Additional techniques exist that can increase the region of marginal tissue evaluated although increased costs may also be incurred. These techniques include “parallel” sectioning, “modified” sectioning (a combination of parallel and cross-sectioning), and “tangential” sectioning.63 An additional method for evaluating excisional completeness is to evaluate the “tumor bed,” which is the in vivo tissue that was adjacent to or contiguous with the excised specimen. Small regions/scrapings from the tumor bed may be submitted in addition to the excised mass but should be submitted in a separate container and clearly specified that it is “tumor bed” tissue. If neoplastic cells exist within the tumor bed tissue, this indicates presence of residual disease in the patient.
Specimens come in all shapes and sizes, thus no one blanket trimming method can be recommended. Additionally, each method has advantages and disadvantages. The most important fundamental aspects of trimming to recognize are that the regions selected for microscopic examination (1) adequately represent the mass lesion for diagnosis and (2) are the most appropriate for margin evaluation for that specific specimen. It is essential for clinicians to understand the various trimming methods that exist and the methods by which their own specimens are being trimmed, to have an understanding of the percentage of marginal tissue that is evaluated relative to the entire surgical margin, and to recognize that they can greatly influence the region of the tissue examined microscopically through inking and information provided on the submission form.
Assuming tissue demarcation, specimen submission, and tissue trimming have all been appropriately performed, microscopic reporting of the surgical margins should be clear, concise, and thorough, furnishing the clinician with essential information such that they can make informed decisions and recommendations regarding further management of the cancer patient. Thorough microscopic evaluation of the tissue margin should include (1) a description of the neoplastic cells closest to the margin (e.g., individual cells, nests of cells, cells at the periphery of the mass itself); (2) an objective measurement (via stage or ocular micrometer) from the tissue edge to the closest neoplastic cell (this parameter is precluded for tangentially trimmed sections); and (3) a description of the tissue constituents (e.g., adipose tissue, dense connective tissue, skeletal muscle) and quality of these constituents (e.g., normal, necrotic, inflamed) composing the margin because different tissue types provide variable barriers against invasion and infiltration of neoplastic cells.63 Objective measurements may be provided in any appropriate metric (e.g., micrometers, millimeters), but the metric used should remain consistent for all margins reported for a given specimen. Vague and ambiguous terminology such as clean, dirty, close, or narrow, should be avoided, since these are subjective and introduce interpretative variability.
Microscopic evaluation of surgical margins to assess excisional completeness is not a perfect science but is approached to provide the best possible assessment. If surgical resection is determined to be complete by microscopic evaluation, the chance of local recurrence is reduced, but by no means is local tumor control guaranteed. Recurrence of soft tissue sarcomas in humans has been shown to occur in about 10% of cases in which margins were deemed complete66; a similar situation likely exists in veterinary medicine. Additionally, “complete excision” of local disease does not address the potential for systemic or metastatic disease and thus in no way can confirm a disease-free state.
Many tumor-specific (especially canine soft tissue sarcoma and mast cell tumor) studies have been performed with the goal of correlating surgical margins to clinical outcome.32,67-73 To this end, it is important to note that tissue shrinkage subsequent to formalin fixation does occur and the degree of shrinkage varies relative to tissue type.74-77 For cutaneous biopsies, shrinkage can occur up to 30%.75,77 Additionally, tissue shrinkage is impacted by inherent postexcisional tissue retraction, as well as dehydration steps during processing. These changes may result in reported surgical margins that appear to be significantly less than the clinician believed were obtained at surgery.
Assessment of Treatment Response
Sometimes histologic assessment of preoperative treatment response may help predict outcome and could even alter subsequent therapeutic options. Assessment of preoperative therapy is most common with osteosarcoma and soft tissue sarcoma. Percent tumor necrosis is the most commonly used parameter to quantify the impact of presurgical chemotherapy or radiation therapy. In dogs with osteosarcoma, percent tumor necrosis is a good predictor for local recurrence following limb-sparing surgery subsequent to neoadjuvant radiation therapy and/or chemotherapy. Tumor necrosis rates of 90% or more, between 80% and 90%, and below 80% are associated with 91%, 78%, and 28% local control rates, respectively.78,79 In humans, the percent of tumor necrosis in osteosarcoma following preoperative chemotherapy is also predictive for survival, and poor responders may be treated with more aggressive alternative chemotherapeutic regimes.80 In soft tissue sarcomas, percent tumor necrosis has been used to assess presurgical therapy in humans81 and could be done in companion animals.
The effect of previous treatment also may be evaluated histologically when there is progressive growth of tissue in an area treated previously with radiation, surgery, chemotherapy, or photodynamic therapy. In these cases, distinguishing between reactive tissue and neoplasia is important but also may be extremely difficult. Inflammation, fibrovascular proliferation (granulation tissue), or epithelial hyperplasia (if applicable) is usually present in the area. Furthermore, especially after radiation therapy, the area may contain some bizarre reactive cells, including fibroblasts (called radiation atypical fibroblasts) with many features of malignancy, although these cells are not neoplastic.82 If tumor cells are identified, the clinician may wish to know if these cells are viable, dead, or rendered viable but sterilized (reproductively dead) by radiation or chemotherapy. Distinction between a viable and dead cell is often possible, but determining if a cell is viable but sterilized, or nonclonogenic, is not possible with routine microscopy. However, the presence of numerous mitotic figures in a viable-appearing tumor is suggestive of active regrowth.
Molecular techniques hold promise in identifying the adequacy of treatment and/or treatment response. Use of PCR to identify residual circulating lymphoma cells in dogs during clinical remission holds promise as an early indicator of tumor reoccurrence prior to clinical and histologic/cytologic identifiable disease. New techniques that identify large portions of the tumor’s genomic expression (gene expression microarrays) and molecular phenotype (tissue microarrays, protein microarrays, and mass spectrometry) can identify tumor gene and phenotypic profiles that are predictive of a tumor’s biologic behavior, a tumor’s response to treatment, or early tumor recurrence.
Special Procedures
Approximately 90% of oncologic cases in humans can be diagnosed by light microscopy using hematoxylin and eosin (H&E) stains.3 This likely approximates the situation in veterinary medicine as well. In the remaining 10% of cases, special stains or special procedures such as IHC or EM may help. IHC, flow cytometry, or other molecular techniques may also be useful in predicting tumor behavior or may help in distinguishing benign from malignant tumors (see Chapter 8).
Special Histochemical Stains
Histochemical stains consist of chemical substances that, when applied to tissue sections, result in a direct chemical reaction with tissue constituents. For all intents and purposes, routine H&E is a histochemical stain; however, many additional special histochemical stains exist. In veterinary oncologic pathology, these stains are most commonly used to assist in the diagnosis of certain poorly differentiated tumors.10 Toluidine blue and Giemsa are two of the more common histochemical stains that aid in the identification of mast cell granules in poorly differentiated mast cell tumors. Periodic acid–Schiff (PAS), another histochemical stain, is often used in feline and ferret mast cell tumors because the granules in these species are often better visualized with PAS.
Silver stains such as Pascual’s, Grimelius, or Sevier-Munger can aid in the identification of neuroendocrine tumors. Sudan black and Oil Red O stains are specific for lipid and thus may aid in the diagnosis of poorly differentiated liposarcomas or lipid-rich variants of some carcinomas and other tumor types.83-86 It should be noted that these stains must be performed on nonprocessed tissue because exposure to xylene during processing will dissolve lipid components. A melanin bleach or iron stain (Prussian blue) may help distinguish between melanin and hemosiderin, respectively, in suspected cases of melanoma. Masson’s trichrome or other trichrome stains may be used to identify collagen fibrils; this can help differentiate certain mesenchymal tumors, such as those derived from muscle (leiomyomas, leiomyosarcomas, rhabdomyoma, rhabdomyosarcomas), and those that produce collagen matrix (fibromas, fibrosarcomas). It is important to keep in mind, however, that muscle-derived tumors may have a small amount of collagen inherent in the surrounding microenvironment, and poorly differentiated fibrosarcomas may produce only minimal, if any, collagen. Phosphotungstic acid hematoxylin (PTAH) can aid in differentiating a rhabdomyosarcoma from leiomyosarcoma or other tumor as it enhances visibility of the cytoplasmic cross-striations present in skeletal muscle. Alcian blue stain may help identify ground substance glycosaminoglycans that may be seen in some neurofibrosarcomas or myxosarcomas. Mucicarmine stain or PAS is useful in mucosal tissues for identifying poorly differentiated carcinomas, whereas PAS is also helpful in the diagnosis of granular cell tumor as it reacts with the intracytoplasmic lysosomes.
With the advancement of IHC (discussed later), many histochemical stains have lost popularity but are still available and useful in the appropriate setting. Silver staining of nucleolar organizer regions (AgNOR) is an additional histochemical stain that has been shown to be prognostically relevant in canine malignant lymphoma87 and mast cell tumors.88
Immunohistochemistry
Immunohistochemistry (IHC) can aid the classification of several tumors in veterinary medicine and is a widely used diagnostic technique. IHC is a staining procedure that employs commercial antibodies to identify specific cellular and extracellular molecules ex vivo, such as cytoplasmic intermediate filaments, secretory substances, and cell surface markers. IHC can be performed on frozen sections or specimens routinely fixed in formalin and processed into paraffin blocks. The tissue sections are incubated with primary antibodies to specific cell proteins (the antigens). These sections with bound primary antibody are then exposed to secondary antibodies directed against the primary antibody. The secondary antibodies are linked to peroxidase or avidin-biotin peroxidase complexes. The peroxidase catalyzes a reaction in the presence of dye that precipitates at the site of the complex and is visible with light microscopy.3,89 As an alternative, alkaline phosphatase enzyme systems are also available. Commonly used immunohistochemical stains include those for intermediate filaments, such as vimentin for mesenchymal cells, cytokeratin for epithelial cells, and desmin or actin for muscle cells (myocytes).3,90-92 A list of common diagnostic IHC markers used in veterinary oncology and the respective tumor types in which their use is indicated is provided in Table 3-5.
Table 3-5
Common Diagnostic Immunohistochemical Markers/Panels and Respective Tumor Types in Cats and Dogs
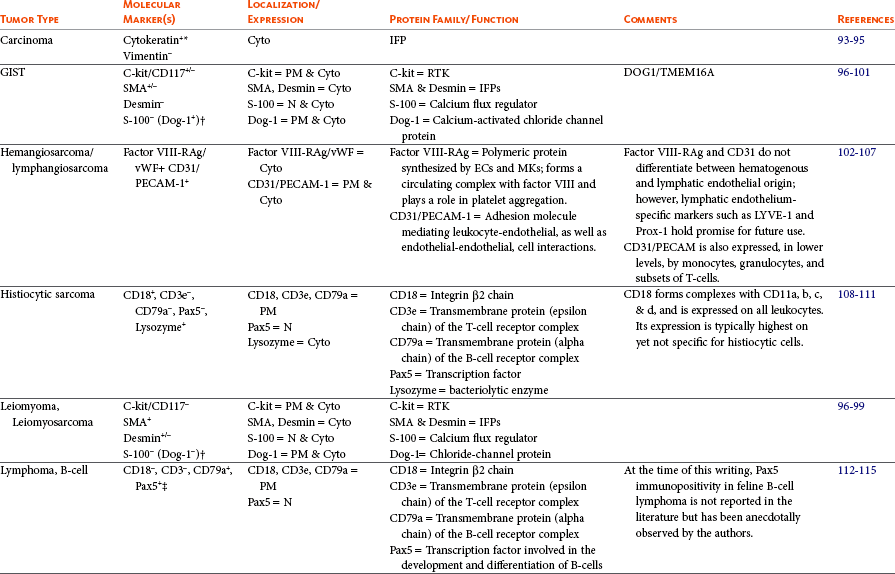
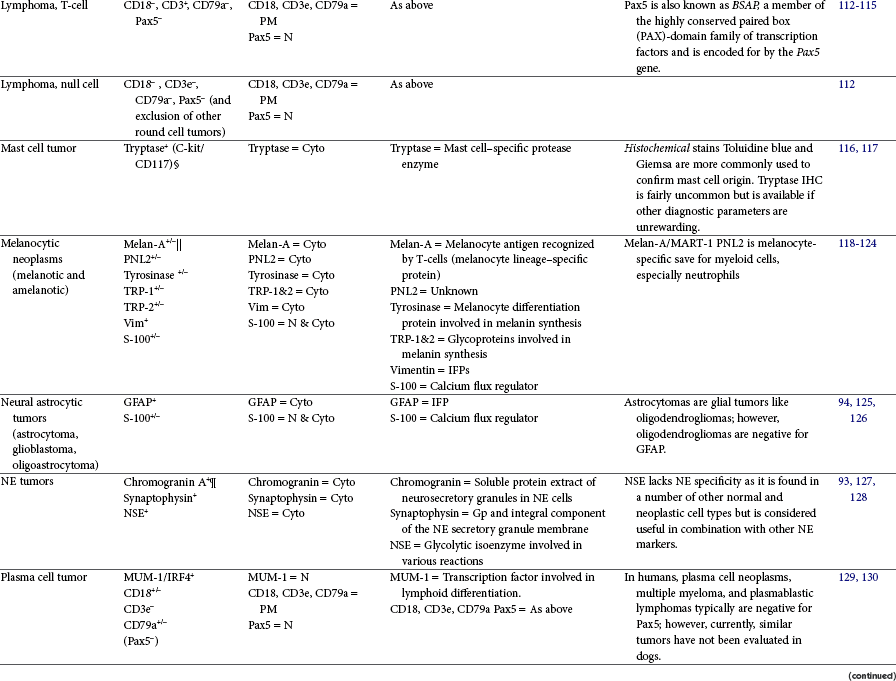
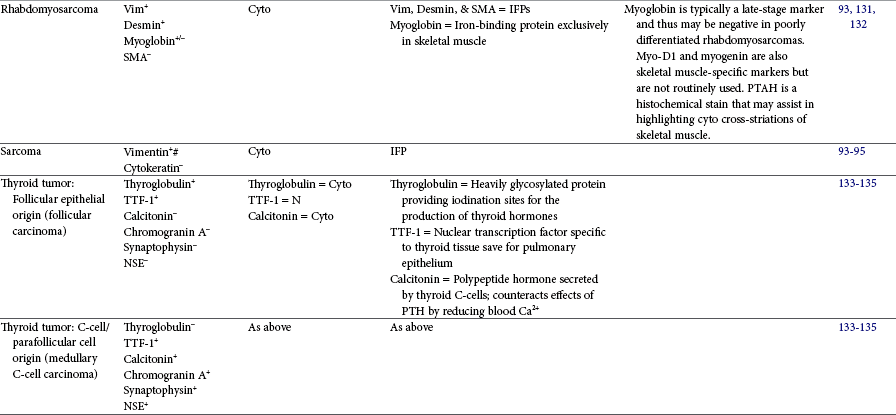
This table provides common diagnostic immunohistochemical markers utilized in veterinary oncologic pathology. For a complete list of cell-specific markers available for use in veterinary samples, visit www.ihc.sdstate.org.
Immunohistochemical stains should always be interpreted in conjunction with routine (hematoxylin & eosin [H&E]) histopathologic evaluation and in the presence of appropriately stained positive and negative control tissues.
BSAP, B-cell–specific activator protein; Cyto, cytoplasmic; DOG1, discovered on gastrointestinal tumor 1; EC, endothelial cell; Factor VIII-RAg/vWF, factor VIII–related antigen/von Willebrand factor; GFAP, glial fibrillary acidic protein; GIST, gastrointestinal stromal tumor; Gp, glycoprotein; IFP, intermediate filament protein; IHC, immunohistochemistry; MKs, megakaryocytes; MUM-1/IRF4, multiple myeloma 1/interferon regulatory factor 4; N, nuclear; NE, neuroendocrine; NSE, neuron-specific enolase; PECAM, platelet endothelial cell adhesion molecule; PM, plasma membrane; PTH, parathyroid hormone; PTAH, phosphotungstic acid hematoxylin; RTK, tyrosine kinase receptor; SMA, smooth muscle actin; TRP, tyrosinase-related protein; TTF-1, thyroid transcription factor-1; Vim, vimentin.
*Generic marker for tumors of epithelial origin.
†A well characterized diagnostic marker for GISTs in humans and, at the time of this writing, under investigation for veterinary application by one of the chapter authors (BEP).
‡At the time of this writing, reported only in the dog; however, anecdotally observed in feline lymphoma by the authors.
§c-kit/CD117 is not diagnostic for mast cell tumors because it is not mast-cell specific, but it has been shown to carry prognostic relevance in canine cutaneous mast cell tumor based on its cellular localization/expression pattern determined via immunohistochemistry.116
¶Rare positivity in amelanotic melanomas.
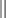 Generic markers for tumors of neuroendocrine (NE) origin. A panel of all three markers is strongly recommended because NE tumors may be positive for only one of the three.
Generic markers for tumors of neuroendocrine (NE) origin. A panel of all three markers is strongly recommended because NE tumors may be positive for only one of the three.
#Generic marker for tumors of mesenchymal cell origin.
IHC can also be useful in determining cellular proliferation or the tumor growth fraction that may carry prognostic relevance.116 This can be done using Ki-67 and PCNA staining, markers of multidrug resistance (e.g., P-glycoprotein),136 or altered proto-oncogenes such as p53, CD117/c-Kit, p21, Rb, and PTEN.137-139 Other markers that have been explored as potential prognostic markers via IHC in a variety of veterinary tumor types include VEGF, cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), human epidermal growth factor receptor-2 (HER2), urokinase plasminogen activator (UPA), and heat shock proteins (HSPs), among others. As the realm of IHC in veterinary medicine continues to advance, so too will the discovery of tumor-specific diagnostic markers, as well as markers for prognostic (biologic aggressiveness) and predictive (response to therapy) utility.
Although IHC can be a valuable tool, some complicating factors exist. A negative stain does not exclude a certain cell type as technical difficulties or tumor cell dedifferentiation, causing loss of expression of expected proteins/markers, may result in a negative stain. One of the more common technical problems that can cause negative staining is prolonged formalin fixation that results in excessive cross-linking of the antigenic components or loss of soluble proteins into the fixative. Antibody-specific antigens (epitopes) that have been masked by protein cross-linking can often be “unmasked” by pretreating sections with trypsin or pepsin or by using heat-induced epitope retrieval (HIER) techniques.89 Decalcification of tissue may also result in alteration of target proteins so that they are no longer recognized by the respective antibody; however, the type and duration of decalcifying solution may mitigate these deleterious effects.89 Areas of tissue necrosis, autolysis, hemorrhage, section drying, and sometimes collagenous matrix components can cause excessive nonspecific background staining. A skilled pathologist who is familiar with the IHC stain should be asked to differentiate background stain from tumor-specific stain and to navigate technical difficulties. Additionally, IHC does not distinguish between neoplastic and nonneoplastic tissue, normal, or hyperplastic. For example, normal bladder mucosal epithelium (urothelium), urothelial hyperplasia, and urothelial carcinoma (transitional cell carcinoma) would all be immunopositive for cytokeratin. The differentiation between neoplastic and nonneoplastic is made via routine H&E light microscopy based on hallmark features of neoplasia (e.g., loss of organization, cellular atypica, invasion). IHC is an ancillary diagnostic tool that aids in determining histogenesis for poorly differentiated tumors. Considerable cross-reactivity of staining in different tumor types may occur because some markers lack specificity and can be found in a variety of cells or tumors (e.g., S-100 in melanomas, cartilage, and certain epithelial cells).3,140 Because most tumor markers have limitations, the best and most reliable results may be obtained by using a panel of IHC stains wherein both marker-specific immunopositive and immunonegative results may be anticipated (e.g., rhabdomyosarcoma should be immunopositive for vimentin and desmin but immunonegative for smooth muscle actin) rather than relying on a single stain. Additionally, IHC stains can only be appropriately interpreted in the presence of appropriate species-specific controls. For example, if one seeks to support the diagnosis of or immunophenotype an intestinal lymphoma in a cat, the appropriate positive control tissue that must be run simultaneously would be a section of normal feline lymphoid tissue (e.g., lymph node, spleen, tonsil, or other). It must be of feline origin and contain normal lymphoid tissue in order for the pathologist to confirm that the IHC stain was successfully performed and to appropriately interpret the immunoreaction of the test tissue. Similarly, a negative control that consists of the test tissue treated either with nonspecific antibody or omission of the primary antibody must also be run to assist in ruling out background/nonspecific staining. Finally, IHC can be a powerful tool providing information that could not otherwise be determined on routine microscopy alone (e.g., confirmation of tumor histogenesis); however, an IHC stain should never be interpreted in and of itself but rather should always be evaluated in conjunction with routine light microscopic findings and knowledge of relevant clinical information.
Electron Microscopy
EM involves preserving very small representative tumor samples (1 × 1 mm) in special fixatives such as glutaraldehyde, processing tissue into epoxy-based plastic blocks, and sectioning at 1 µm for thick sections to determine the adequacy of the sample and inclusion of appropriate tumor cells. Subsequently, sectioning is done at about 600Å, stained with heavy-metal-based stains, and examined with the aid of the electron microscope. Samples fixed in formalin can be used, although the quality of the subsequent sections is less than ideal. EM may help identify certain specific features, such as intercellular junctions or basal lamina in epithelial cells, melanosomes in melanocytic cells, mast cell granules in mast cells, neurosecretory granules in neuroendocrine cells, or mucin droplets in certain epithelial cells. These features are useful in distinguishing carcinomas from lymphomas and identifying melanomas, mast cell tumors, and neuroendocrine tumors. Unless a specific feature is sought, however, EM will be no more useful than a higher magnification of a tumor that could not be diagnosed with the light microscope. Furthermore, EM is not useful for distinguishing benign from malignant cells in many cases because the magnification is too high and the tumor pattern in the tissue is not evident.4,10 Not all veterinary diagnostic laboratories have the technical support and equipment needed for EM.
Flow Cytometry and Polymerase Chain Reaction
Flow cytometry is an analytic procedure that can be used to evaluate cell suspensions obtained from suspected neoplastic masses or fluids. In human medicine, this procedure is frequently used to diagnose and occasionally to monitor for the recurrence of various tumors, such as bladder carcinoma. Use of flow cytometry for humans is especially useful for detecting neoplastic cells in the urine of bladder cancer patients and in the evaluation of cell suspensions of suspected leukemia and lymphoma. In solid tumors, the cells must first be disassociated to create a single cell suspension. Cell suspensions are stained with specific fluorochromes, passed through the flow cytometer chamber, and analyzed and sorted by use of a focused laser beam. The most routine analysis is to determine DNA content or ploidy of the cells. Malignant cells may be diploid (normal DNA content) or aneuploid (nondiploid), but normal tissue, benign tumors, and reactive tissues are usually diploid. Occasionally, however, benign tumors and reactive tissue can be aneuploid. In some instances, aneuploidy may be prognostically significant and can be predictive of survival time. Flow cytometry also can be used to evaluate S-phase distribution or cell cycle time if the tumor is sampled at appropriate times after injecting the patient with bromodeoxyuridine (BrdUrd).141
Flow cytometry has been used to evaluate tumors in dogs and is becoming a more routine diagnostic procedure combined with other tests, including histopathology and cytopathology, immunocytochemistry, and PCR. In the earliest report, various canine tumors were characterized for DNA ploidy.142 In subsequent studies, tumor cell heterogeneity, comparisons of primary and metastatic tumors, and the positive predictive value of kinetic parameters in canine osteosarcomas were evaluated.143,144 Other studies have indicated the value of flow cytometry in predicting the behavior of various canine tumors, including lymphomas,145,146 myeloproliferative disease,147-149 mammary gland tumors,150 melanomas,151 osteosarcomas,143 and plasmacytomas.152 Flow cytometry can also be useful for analyzing abnormal populations of white blood cells in blood or fluid, helping to distinguish lymphoma from reactive processes. As samples to be evaluated by flow cytometry are often cell suspensions derived from tumor masses, a correlate sample from the same site or same specimen should always be taken for histopathologic assessment. Histologic correlation is necessary because flow cytometry cannot distinguish a benign from a malignant diploid tumor, nor can it always identify tumor type.3 Currently the use of flow cytometry in diagnostic medicine to identify tumor subtypes, occult disease with a leukemic component, or cell surface prognostic markers is crossing over from investigational to practical diagnostic veterinary medicine.
PCR is a technique now commonly used to distinguish lymphoma from reactive processes by evaluating for the presence of antigen-receptor rearrangements (PARR) to determine clonality. The PCR is used to amplify DNA encoding the antigen-binding region of lymphocytes, and a clonal or single-size product indicates malignancy. In an early study, 91% of lymphomas were identified by this technique.6 This technique also accurately identified B- or T-cell immunophenotype. Currently, air-dried aspirates, fresh, or formalin-fixed tissue can be used for this technique. Mutations resulting in internal tandem duplication in exon 11 of the c-Kit gene, CD117 or stem cell growth factor receptor, have been identified as a mutation constitutively activating this tyrosine kinase receptor, resulting in a more aggressive biologic behavior in canine mast cell tumors.153,154 PCR is currently used to identify this mutation in a diagnostic setting as a biologic marker of prognosis.
Clinical-Pathologic Correlation and Second Opinions
Sometimes the pathologist cannot make an accurate diagnosis without clinical correlations.2,4 This is especially true for some primary bone tumors or secondary tumors involving bone. Diagnosis of a surface or juxtacortical osteosarcoma is based on both radiographic and histologic features. An osteoma may be difficult to distinguish from reactive bone without a corroborative radiograph. A synovial cell sarcoma may be difficult to distinguish from other sarcomas or even inflammatory or immune-mediated joint disease unless there is radiographic or gross evidence of joint involvement and bone invasion. An acanthomatous epulis may be difficult to distinguish from a fibrous epulis unless there is bone invasion in the former that cannot be identified without appropriately deep biopsy samples that include underlying bone. The best example of the need for clinical and pathologic correlation is with histologically low-grade yet biologically high-grade fibrosarcomas of the canine head in which the histologic appearance is of benign fibrous tissue, but the clinical presentation is an aggressive invasive mass, often recurrent after conservative surgery, causing bone destruction.11 These examples demonstrate the necessity for an accurate history with pertinent clinical results being provided to the pathologist along with the biopsy sample. In some cases, photographs of the tumor site or inclusion of radiographs or radiographic findings are most helpful.
Before any major treatment is undertaken or if a pathology diagnosis is not consistent with clinical presentation, a second opinion should be requested from the pathologist. In human medicine, a review of mandatory second-opinion surgical pathology at major hospitals revealed 1.4% to 5.8% major changes in diagnosis that resulted in change of therapy or prognoses. It was concluded that despite the extra cost, mandatory second opinions should be obtained whenever a major therapeutic endeavor is considered or if treatment decisions are based primarily on the pathologic diagnosis.155-157
The two major categories of errors that may occur at the pathology laboratory are technical errors and errors in interpretation of the tissue.61 Technical errors may occur if the histotechnologist improperly labels specimens, tissue blocks, or slides or fails to process all the critical tissue submitted by the clinician. If tissue is improperly processed because of equipment malfunction or because it is poorly sectioned, artifacts can occur that make the tissue specimen impossible to interpret. Errors in interpretation by the pathologist may occur in difficult cases. If a pathology service staffed by physicians is used, certain tumors such as histiocytoma, mast cell tumor, transmissible venereal tumor, or perianal gland adenoma may be misdiagnosed, as these do not have a human counterpart. Many pathologists will obtain opinions from other pathologists when confronted with difficult cases, just as clinicians will seek second opinions on difficult radiographs or clinical problems. The clinician should never hesitate to ask for a second opinion nor should the pathologist be offended by the request. Each pathologist approaches a section differently and in some cases, one approach might prove more accurate than another. A second or even third pathologist can offer a different perspective on a difficult case, offer an alternative diagnosis, confirm the primary pathologist’s diagnosis, or confirm that an accurate diagnosis is not possible. Since the patient’s treatment options or decisions regarding euthanasia are often based on the final pathology diagnosis, it is not at all unreasonable for the clinician to request a second opinion. A misdiagnosis can result in costly, ineffective, and untimely treatments that can cause undo discomfort for patients. They can result in unnecessary surgery, unnecessary chemotherapy or radiation therapy, insufficient treatment resulting in cancer progression, and, worst of all, unwarranted euthanasia. Considering these possible scenarios, second opinions are not only prudent but highly recommended.
The clinician needs to have knowledge of the pathology of neoplasia to understand neoplastic conditions and understand the limitations of histopathologic assessment in the diagnosis of neoplasia. In the case of tumor diagnosis, histopathologic assessment of a thin slice of tissue may not always be an exact science. The pathologist and the clinician must work together to establish the most appropriate diagnosis so that proper treatment can be initiated.
References
1. Goldschmidt, M, et al. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48(1):117–131.
2. Bonfiglio, TA, Stoler, MH. The pathology of cancer. In Rubin P, ed.: Clinical Oncology, ed 7, Philadelphia: WB Saunders, 1993.
3. Pfeifer, J, Wick, M. The pathologic evaluation of neoplastic diseases. In: Murphy G, Lawrence W, Lenhard R, eds. Clinical oncology. Washington, DC: Pan American Health Organization, 1991.
4. Pfeifer, J, Wick, M. The pathologic evaluation of neoplastic diseases. In: Murphy G, Lawrence W, Lenhard R, eds. Clinical oncology. Washington, DC: Pan American Health Organization, 1995.
5. Stedman, T. Stedman’s medical dictionary, ed 23. Baltimore: Lippincott Williams & Wilkins; 1976.
6. Burnett, RC, et al. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40(1):32–41.
7. Jacobs, R, Messick, H, Valli, V. Tumors of the hemolympatic system. In Meuten D, ed.: Tumors in domestic animals, ed 4, Ames, Iowa: Iowa State Press, 2002.
8. Sorenmo, KU, et al. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet Pathol. 2011;48(1):85–97.
9. Misdorp, W. General considerations. In Meuten J, ed.: Tumors of domestic animals, ed 3, Berkeley, Calif: University of California Press, 1990.
10. Cullen, J, Page, R, Misdorp, W. An overview of cancer pathogenesis, diagnosis and management. In Meuten D, ed.: Tumors in domestic animals, ed 4, Ames, Iowa: Iowa State Press, 2002.
11. Ciekot, PA, et al. Histologically low-grade, yet biologically high-grade, fibrosarcomas of the mandible and maxilla in dogs: 25 cases (1982-1991). J Am Vet Med Assoc. 1994;204(4):610–615.
12. Gottfried, SD, et al. Metastatic digital carcinoma in the cat: a retrospective study of 36 cats (1992-1998). J Am Anim Hosp Assoc. 2000;36(6):501–509.
13. Northrup, NC, et al. Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. J Vet Diagn Invest. 2005;17(3):245–248.
14. Carriaga, MT, Henson, DE. The histologic grading of cancer. Cancer. 1995;75(1 Suppl):406–421.
15. Valli, VE, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48(1):198–211.
16. Maiolino, P, et al. Nucleomorphometric analysis of canine cutaneous mast cell tumours. J Comp Pathol. 2005;133(2-3):209–211.
17. Strefezzi Rde, F, Xavier, JG, Catao-Dias, JL. Morphometry of canine cutaneous mast cell tumors. Vet Pathol. 2003;40(3):268–275.
18. Patnaik, AK, Ehler, WJ, MacEwen, EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21(5):469–474.
19. Bostock, DE. The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract. 1973;14(1):27–41.
20. Kiupel, M, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48(1):147–155.
21. Thompson, JJ, et al. Canine subcutaneous mast cell tumor: characterization and prognostic indices. Vet Pathol. 2011;48(1):156–168.
22. Wilcock, BP, Yager, JA, Zink, MC. The morphology and behavior of feline cutaneous mastocytomas. Vet Pathol. 1986;23(3):320–324.
23. Molander-McCrary, H, et al. Cutaneous mast cell tumors in cats: 32 cases (1991-1994). J Am Anim Hosp Assoc. 1998;34(4):281–284.
24. Carter, RF, Valli, VE, Lumsden, JH. The cytology, histology and prevalence of cell types in canine lymphoma classified according to the National Cancer Institute Working Formulation. Can J Vet Res. 1986;50(2):154–164.
25. Teske, E, et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205(12):1722–1728.
26. Bostock, DE. Prognosis after surgical excision of canine melanomas. Vet Pathol. 1979;16(1):32–40.
27. Laprie, C, et al. MIB-1 immunoreactivity correlates with biologic behaviour in canine cutaneous melanoma. Vet Dermatol. 2001;12(3):139–147.
28. Esplin, DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol. 2008;45(6):889–896.
29. Wilcock, BP, Peiffer, RL, Jr. Morphology and behavior of primary ocular melanomas in 91 dogs. Vet Pathol. 1986;23(4):418–424.
30. Kalishman, JB, et al. A matched observational study of survival in cats with enucleation due to diffuse iris melanoma. Vet Ophthalmol. 1998;1(1):25–29.
31. Bostock, DE, Dye, MT. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet Pathol. 1980;17(5):581–588.
32. Kuntz, CA, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996). J Am Vet Med Assoc. 1997;211(9):1147–1151.
33. McNiel, EA, et al. Evaluation of prognostic factors for dogs with primary lung tumors: 67 cases (1985-1992). J Am Vet Med Assoc. 1997;211(11):1422–1427.
34. Hahn, KA, McEntee, MF. Prognosis factors for survival in cats after removal of a primary lung tumor: 21 cases (1979-1994). Vet Surg. 1998;27(4):307–311.
35. Kurzman, ID, Gilbertson, SR. Prognostic factors in canine mammary tumors. Semin Vet Med Surg (Small Anim). 1986;1(1):25–32.
36. Karayannopoulou, M, et al. Histological grading and prognosis in dogs with mammary carcinomas: application of a human grading method. J Comp Pathol. 2005;133(4):246–252.
37. Misdorp, W. Tumors of the mammary gland. In: Meuten D, ed. Tumors in Domestic Animals. ed 4. Ames, Iowa: Iowa State Press; 2002:575–606. [764].
38. Weijer, K, et al. Feline malignant mammary tumors. I. Morphology and biology: some comparisons with human and canine mammary carcinomas. J Natl Cancer Inst. 1972;49(6):1697–1704.
39. Vail, DM, et al. Evaluation of prognostic factors for dogs with synovial sarcoma: 36 cases (1986-1991). J Am Vet Med Assoc. 1994;205(9):1300–1307.
40. Craig, LE, Julian, ME, Ferracone, JD. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol. 2002;39(1):66–73.
41. Craig, LE, Krimer, PM, Cooley, AJ. Canine synovial myxoma: 39 cases. Vet Pathol. 2010;47(5):931–936.
42. Kirpensteijn, J, et al. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol. 2002;39(2):240–246.
43. Straw, RC, et al. Multilobular osteochondrosarcoma of the canine skull: 16 cases (1978-1988). J Am Vet Med Assoc. 1989;195(12):1764–1769.
44. Dernell, WS, et al. Multilobular osteochondrosarcoma in 39 dogs: 1979-1993. J Am Anim Hosp Assoc. 1998;34(1):11–18.
45. Straw, RC, et al. Canine mandibular osteosarcoma: 51 cases (1980-1992). J Am Anim Hosp Assoc. 1996;32(3):257–262.
46. Farese, JP, et al. Biologic behavior and clinical outcome of 25 dogs with canine appendicular chondrosarcoma treated by amputation: a Veterinary Society of Surgical Oncology retrospective study. Vet Surg. 2009;38(8):914–919.
47. Ogilvie, GK, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;10(6):379–384.
48. Spangler, WL, Culbertson, MR, Kass, PH. Primary mesenchymal (nonangiomatous/nonlymphomatous) neoplasms occurring in the canine spleen: anatomic classification, immunohistochemistry, and mitotic activity correlated with patient survival. Vet Pathol. 1994;31(1):37–47.
49. Spangler, WL, Kass, PH. Pathologic and prognostic characteristics of splenomegaly in dogs due to fibrohistiocytic nodules: 98 cases. Vet Pathol. 1998;35(6):488–498.
50. Valli, VE, et al. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J Comp Pathol. 1995;113(2):113–130.
51. Carpenter, L, Withrow, S, Powers, B. Squamous cell carcinoma of the tongue in 10 dogs. J Am Anim Hosp Assoc. 1993;29:17–24.
52. Pakos, EE, et al. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005;25(5):3591–3596.
53. Yudoh, K, et al. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br J Cancer. 2001;84(12):1610–1615.
54. Hansen, AE, et al. Hypoxia-inducible factors–regulation, role and comparative aspects in tumourigenesis. Vet Comp Oncol. 2011;9(1):16–37.
55. Smedley, RC, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48(1):54–72.
56. Loukopoulos, P, Robinson, WF. Clinicopathological relevance of tumour grading in canine osteosarcoma. J Comp Pathol. 2007;136(1):65–73.
57. Dennis, MM, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol. 2011;48(1):73–84.
58. Coindre, JM, et al. Histopathologic grading in spindle cell soft tissue sarcomas. Cancer. 1988;61(11):2305–2309.
59. Bostock, DE, Dye, MT. Prognosis after surgical excision of fibrosarcomas in cats. J Am Vet Med Assoc. 1979;175(7):727–728.
60. Davidson, EB, Gregory, CR, Kass, PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg. 1997;26(4):265–269.
61. Bonfiglio, T, Terry, R, The pathology of cancer. Rubin P, ed. Clinical oncology ed 6, 1983.
62. Langenbach, A, et al. Sensitivity and specificity of methods of assessing the regional lymph nodes for evidence of metastasis in dogs and cats with solid tumors. J Am Vet Med Assoc. 2001;218(9):1424–1428.
63. Kamstock, DA, et al. Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. Vet Pathol. 2011;48(1):19–31.
64. Rochat, MC, et al. Identification of surgical biopsy borders by use of india ink. J Am Vet Med Assoc. 1992;201(6):873–878.
65. Abide, JM, Nahai, F, Bennett, RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73(3):492–497.
66. Rydholm, A. Surgical margins for soft tissue sarcoma. Acta Orthop Scand Suppl. 1997;273:81–85.
67. Baker-Gabb, M, Hunt, GB, France, MP. Soft tissue sarcomas and mast cell tumours in dogs; clinical behaviour and response to surgery. Aust Vet J. 2003;81(12):732–738.
68. Bacon, NJ, et al. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999-2004). J Am Vet Med Assoc. 2007;230(4):548–554.
69. Stefanello, D, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996-2006). Vet Surg. 2008;37(5):461–465.
70. McSporran, KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol. 2009;46(5):928–933.
71. Simpson, AM, et al. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2004;224(2):236–240.
72. Fulcher, RP, et al. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2006;228(2):210–215.
73. Schultheiss, PC, et al. Association of histologic tumor characteristics and size of surgical margins with clinical outcome after surgical removal of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2011;238(11):1464–1469.
74. Johnson, RE, et al. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19(4):281–286.
75. Reimer, SB, et al. Evaluation of the effect of routine histologic processing on the size of skin samples obtained from dogs. Am J Vet Res. 2005;66(3):500–505.
76. Wang, L, et al. [The extensibility and retractility of surgical margins in digestive tract cancer]. Zhonghua Wai Ke Za Zhi. 2002;40(4):271–273.
77. Kerns, MJ, et al. Shrinkage of cutaneous specimens: formalin or other factors involved? J Cutan Pathol. 2008;35(12):1093–1096.
78. Powers, BE, et al. Percent tumor necrosis as a predictor of treatment response in canine osteosarcoma. Cancer. 1991;67(1):126–134.
79. Withrow, SJ, et al. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991;270:159–168.
80. Rosen, G, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221–1230.
81. Willett, CG, et al. The histologic response of soft tissue sarcoma to radiation therapy. Cancer. 1987;60(7):1500–1504.
82. Fajardo, LF, Berthrong, M, Anderson, RE. Differential diagnosis of atypical cells in irradiated tissues. In: Berthrong M, Fajardo LF, Anderson RE, eds. Radiation pathology. New York: Oxford University Press, 2001.
83. Masserdotti, C, et al. Use of Oil Red O stain in the cytologic diagnosis of canine liposarcoma. Vet Clin Pathol. 2006;35(1):37–41.
84. Kwon, HJ, et al. Round cell variant of myxoid liposarcoma in a Japanese Macaque (Macaca fuscata). Vet Pathol. 2007;44(2):229–232.
85. Kamstock, DA, Fredrickson, R, Ehrhart, EJ. Lipid-rich carcinoma of the mammary gland in a cat. Vet Pathol. 2005;42(3):360–362.
86. Avakian, A, et al. Lipid-rich pleural mesothelioma in a dog. J Vet Diagn Invest. 2008;20(5):665–667.
87. Kiupel, M, Teske, E, Bostock, D. Prognostic factors for treated canine malignant lymphoma. Vet Pathol. 1999;36(4):292–300.
88. Kravis, LD, et al. Frequency of argyrophilic nucleolar organizer regions in fine-needle aspirates and biopsy specimens from mast cell tumors in dogs. J Am Vet Med Assoc. 1996;209(8):1418–1420.
89. Ramos-Vara, JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42(4):405–426.
90. Andreasen, CB, Mahaffey, EA, Duncan, JR. Intermediate filament staining in the cytologic and histologic diagnosis of canine skin and soft tissue tumors. Vet Pathol. 1988;25(5):343–349.
91. Sandusky, GE, Carlton, WW, Wightman, KA. Diagnostic immunohistochemistry of canine round cell tumors. Vet Pathol. 1987;24(6):495–499.
92. Espinosa de los Monteros, A, et al. Coordinate expression of cytokeratins 7 and 20 in feline and canine carcinomas. Vet Pathol. 1999;36(3):179–190.
93. Dabbs, D. Diagnostic immunohistochemistry, ed 2. Philadelphia: Churchill Livingstone Elsevier; 2006.
94. Moore, AS, Madewell, BR, Lund, JK. Immunohistochemical evaluation of intermediate filament expression in canine and feline neoplasms. Am J Vet Res. 1989;50(1):88–92.
95. Desnoyers, MM, Haines, DM, Searcy, GP. Immunohistochemical detection of intermediate filament proteins in formalin fixed normal and neoplastic canine tissues. Can J Vet Res. 1990;54(3):360–365.
96. Frost, D, Lasota, J, Miettinen, M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40(1):42–54.
97. Russell, KN, et al. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990-2003). J Am Vet Med Assoc. 2007;230(9):1329–1333.
98. Fatima, N, Cohen, C, Siddiqui, MT. DOG1 utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. Cancer Cytopathol. 2011;119(3):202–208.
99. Espinosa, I, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32(2):210–218.
100. West, RB, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1):107–113.
101. Morini, M, et al. C-kit gene product (CD117) immunoreactivity in canine and feline paraffin sections. J Histochem Cytochem. 2004;52(5):705–708.
102. von Beust, BR, Suter, MM, Summers, BA. Factor VIII-related antigen in canine endothelial neoplasms: an immunohistochemical study. Vet Pathol. 1988;25(4):251–255.
103. Ferrer, L, et al. Immunohistochemical detection of CD31 antigen in normal and neoplastic canine endothelial cells. J Comp Pathol. 1995;112(4):319–326.
104. Wilting, J, et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16(10):1271–1273.
105. Sagartz, JE, et al. Lymphangiosarcoma in a young dog. Vet Pathol. 1996;33(3):353–356.
106. Galeotti, F, et al. Feline lymphangiosarcoma–definitive identification using a lymphatic vascular marker. Vet Dermatol. 2004;15(1):13–18.
107. Sugiyama, A, et al. Lymphangiosarcoma in a cat. J Comp Pathol. 2007;137(2-3):174–178.
108. Moore, PF. Characterization of cytoplasmic lysozyme immunoreactivity as a histiocytic marker in normal canine tissues. Vet Pathol. 1986;23(6):763–769.
109. Affolter, VK, Moore, PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39(1):74–83.
110. Thio, T, et al. Malignant histiocytosis of the brain in three dogs. J Comp Pathol. 2006;134(2-3):241–244.
111. Fulmer, AK, Mauldin, GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48(10):1041–1043. [1046–1050].
112. Willmann, M, et al. Pax5 immunostaining in paraffin-embedded sections of canine non-Hodgkin lymphoma: a novel canine pan pre-B- and B-cell marker. Vet Immunol Immunopathol. 2009;128(4):359–365.
113. Ferrer, L, et al. Immunohistochemical detection of CD3 antigen (pan T marker) in canine lymphomas. J Vet Diagn Invest. 1993;5(4):616–620.
114. Milner, RJ, et al. Immunophenotypic classification of canine malignant lymphoma on formalin-mixed paraffin wax-embedded tissue by means of CD3 and CD79a cell markers. Onderstepoort J Vet Res. 1996;63(4):309–313.
115. Caniatti, M, et al. Canine lymphoma: immunocytochemical analysis of fine-needle aspiration biopsy. Vet Pathol. 1996;33(2):204–212.
116. Webster, JD, et al. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004;41(4):371–377.
117. Walls, AF, et al. Immunohistochemical identification of mast cells in formaldehyde-fixed tissue using monoclonal antibodies specific for tryptase. J Pathol. 1990;162(2):119–126.
118. Smedley, RC, et al. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet Pathol. 2011;48(1):32–40.
119. Koenig, A, et al. Expression of S100a, vimentin, NSE, and melan A/MART-1 in seven canine melanoma cells lines and twenty-nine retrospective cases of canine melanoma. Vet Pathol. 2001;38(4):427–435.
120. Giudice, C, et al. Immunohistochemical investigation of PNL2 reactivity of canine melanocytic neoplasms and comparison with Melan A. J Vet Diagn Invest. 2010;22(3):389–394.
121. Ramos-Vara, JA, Miller, MA. Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Vet Pathol. 2011;48(2):443–450.
122. Ramos-Vara, JA, et al. Melan A and S100 protein immunohistochemistry in feline melanomas: 48 cases. Vet Pathol. 2002;39(1):127–132.
123. Kawakami, Y, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180(1):347–352.
124. Schneider, J, et al. Overlapping peptides of melanocyte differentiation antigen Melan-A/MART-1 recognized by autologous cytolytic T lymphocytes in association with HLA-B45.1 and HLA-A2.1. Int J Cancer. 1998;75(3):451–458.
125. Lipsitz, D, et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40(6):659–669.
126. Stoica, G, et al. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41(1):10–19.
127. Hawkins, KL, et al. Immunocytochemistry of normal pancreatic islets and spontaneous islet cell tumors in dogs. Vet Pathol. 1987;24(2):170–179.
128. Leblanc, B, et al. Immunocytochemistry of canine thyroid tumors. Vet Pathol. 1991;28(5):370–380.
129. Ramos-Vara, JA, Miller, MA, Valli, VE. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet Pathol. 2007;44(6):875–884.
130. Feldman, AL, Dogan, A. Diagnostic uses of Pax5 immunohistochemistry. Adv Anat Pathol. 2007;14(5):323–334.
131. Andreasen, CB, et al. Desmin as a marker for canine botryoid rhabdomyosarcomas. J Comp Pathol. 1988;98(1):23–29.
132. Murakami, M, et al. Cytologic, histologic, and immunohistochemical features of maxillofacial alveolar rhabdomyosarcoma in a juvenile dog. Vet Clin Pathol. 2010;39(1):113–118.
133. Liptak, JM, et al. Cranial mediastinal carcinomas in nine dogs. Vet Comp Oncol. 2008;6(1):19–30.
134. Ramos-Vara, JA, Miller, MA, Johnson, GC. Usefulness of thyroid transcription factor-1 immunohistochemical staining in the differential diagnosis of primary pulmonary tumors of dogs. Vet Pathol. 2005;42(3):315–320.
135. Ramos-Vara, JA., et al. Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet Pathol. 2002;39(4):480–487.
136. Bergman, PJ, Ogilvie, GK, Powers, BE. Monoclonal antibody C219 immunohistochemistry against P-glycoprotein: sequential analysis and predictive ability in dogs with lymphoma. J Vet Intern Med. 1996;10(6):354–359.
137. Sagartz, JE, et al. p53 tumor suppressor protein overexpression in osteogenic tumors of dogs. Vet Pathol. 1996;33(2):213–221.
138. London, CA, et al. Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. 1996;115(4):399–414.
139. Koenig, A, et al. Expression and significance of p53, rb, p21/waf-1, p16/ink-4a, and PTEN tumor suppressors in canine melanoma. Vet Pathol. 2002;39(4):458–472.
140. Lewis, RE, Johnson, WW, Cruse, JM. Pitfalls and caveats in the methodology for immunoperoxidase staining in surgical pathologic diagnosis. Surv Synth Pathol Res. 1983;1:134.
141. Nunez, R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3(3):67–70.
142. Johnson, TS, et al. Ploidy and DNA distribution analysis of spontaneous dog tumors by flow cytometry. Cancer Res. 1981;41(8):3005–3009.
143. LaRue, SM, et al. Impact of heterogeneity in the predictive value of kinetic parameters in canine osteosarcoma. Cancer Res. 1994;54(14):3916–3921.
144. Fox, MH, et al. Comparison of DNA aneuploidy of primary and metastatic spontaneous canine osteosarcomas. Cancer Res. 1990;50(19):6176–6178.
145. Gibson, D, et al. Flow cytometric immunophenotype of canine lymph node aspirates. J Vet Intern Med. 2004;18(5):710–717.
146. Gelain, ME, et al. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Vet Immunol Immunopathol. 2008;121(3-4):179–188.
147. Weir, EG, Borowitz, MJ. Flow cytometry in the diagnosis of acute leukemia. Semin Hematol. 2001;38(2):124–138.
148. Wilkerson, MJ, et al. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Vet Immunol Immunopathol. 2005;106(3-4):179–196.
149. Villiers, E, et al. Identification of acute myeloid leukemia in dogs using flow cytometry with myeloperoxidase, MAC387, and a canine neutrophil-specific antibody. Vet Clin Pathol. 2006;35(1):55–71.
150. Hellmen, E, et al. Prognostic factors in canine mammary tumors: a multivariate study of 202 consecutive cases. Vet Pathol. 1993;30(1):20–27.
151. Bolon, B, Calderwood Mays, MB, Hall, BJ. Characteristics of canine melanomas and comparison of histology and DNA ploidy to their biologic behavior. Vet Pathol. 1990;27(2):96–102.
152. Frazier, KS, et al. Analysis of DNA aneuploidy and c-myc oncoprotein content of canine plasma cell tumors using flow cytometry. Vet Pathol. 1993;30(6):505–511.
153. Letard, S, et al. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. 2008;6(7):1137–1145.
154. Webster, JD, et al. The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia. 2006;8(2):104–111.
155. Abt, AB, Abt, LG, Olt, GJ. The effect of interinstitution anatomic pathology consultation on patient care. Arch Pathol Lab Med. 1995;119(6):514–517.
156. Kronz, JD, Westra, WH, Epstein, JI. Mandatory second opinion surgical pathology at a large referral hospital. Cancer. 1999;86(11):2426–2435.
157. Kronz, JD, Westra, WH. The role of second opinion pathology in the management of lesions of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):81–84.