Soft Tissue Sarcomas
Incidence and Risk Factors
Soft tissue sarcomas are a heterogeneous population of mesenchymal tumors that comprise 15% and 7% of all skin and subcutaneous tumors in the dog and cat, respectively.1 The annual incidence of soft tissue sarcomas in companion animals is about 35 per 100,000 dogs at risk and 17 per 100,000 cats at risk.2 In dogs, sarcomas have been associated with radiation, trauma, foreign bodies, orthopedic implants, and the parasite Spirocerca lupi.3-9
Most soft tissue sarcomas are solitary tumors in middle-aged to older dogs and cats. There is no specific breed or sex predilection for soft tissue sarcomas with the possible exception of synovial cell sarcomas in dogs. Earlier reports indicate a slight male predilection10,11; however, in a recent study, males and females were equally represented.12 Another exception is the occurrence of rhabdomyosarcoma in young dogs.13 Soft tissue sarcomas tend to be overrepresented in large breed dogs.
Pathology and Natural History*
Soft tissue sarcomas are a heterogeneous group of tumors whose classification is based on similar pathologic appearance and clinical behavior. Sarcomas arise from mesenchymal tissues and have features similar to the cell type of origin (Table 21-1). These tumors originate in connective tissues, including muscle, adipose, neurovascular, fascial, and fibrous tissue, and can give rise to benign and malignant entities. Only malignant soft tissue sarcomas will be covered in this chapter. Malignant neoplasms in this category include fibrosarcoma, peripheral nerve sheath tumor (PNST; also known as malignant schwannoma, neurofibrosarcoma, or hemangiopericytoma), myxosarcoma, undifferentiated sarcoma, liposarcoma, malignant fibrous histiocytoma, and rhabdomyosarcoma (Table 21-2).14,15 The term soft tissue sarcoma generally excludes those tumors of hematopoietic or lymphoid origin. Hemangiosarcoma (covered briefly here), mast cell sarcoma, oral sarcoma, osteosarcoma, and chondrosarcoma are covered separately in other chapters. Feline sarcomas and vaccine-associated sarcomas are covered in a separate section at the end of this chapter.
Table 21-1
Histiogenic Classification and Metastatic Potential of Canine Soft Tissue Sarcomas
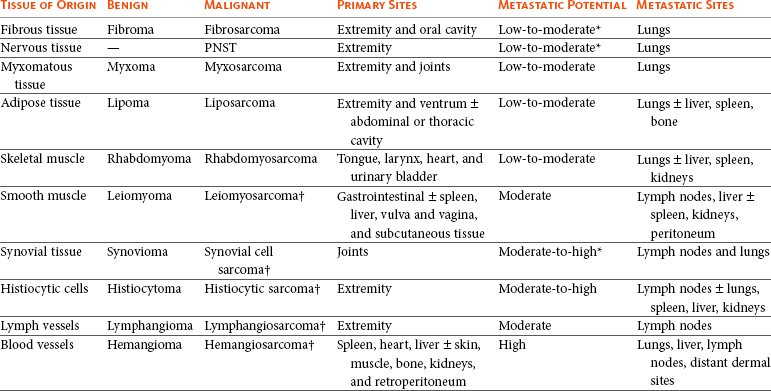
PNST, Peripheral nerve sheath tumor.
*Dependent on histologic grade.
†Atypical soft tissue sarcoma with higher metastatic rate of metastasis to regional lymph node.
Table 21-2
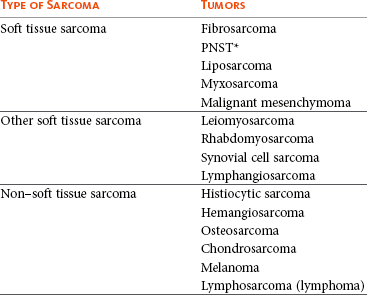
Tumors listed under “Soft tissue sarcoma” have similar biologic behavior characterized by local aggression and low-to-moderate metastatic potential. Tumors listed under “Other soft tissue sarcoma” are atypical soft tissue sarcomas because of different location (e.g., leiomyosarcoma and synovial cell sarcoma) or higher metastatic rate (leiomyosarcoma, rhabdomyosarcoma, lymphangiosarcoma ± synovial cell sarcoma). Some sarcomas not considered as soft tissue sarcomas are discussed in this chapter because of similarities in location to other soft tissue sarcomas (e.g., histiocytic sarcoma and dermal hemangiosarcoma).133
PNST, Peripheral nerve sheath tumor.
*PNSTs include tumors previously classified as hemangiopericytoma, malignant schwannoma, and neurofibrosarcoma.
Soft tissue sarcomas may arise from any anatomic site in the body, although skin and subcutaneous sites are most common. Soft tissue sarcomas have the following important common features with regard to their biologic behavior:
• They tend to appear as pseudoencapsulated soft-to-firm tumors but have poorly defined histologic margins or infiltrate through and along fascial planes, and they are locally invasive.
• Local recurrence after conservative surgical excision is common.
• Sarcomas tend to metastasize hematogenously in up to 20% of cases.
• Regional lymph node metastasis is unusual (except for synovial cell sarcoma).10
• Histopathologic grade is predictive of metastasis, and resected tumor margins predict local recurrence.16
• Measurable or bulky (>5 cm in diameter) tumors generally have a poor response to chemotherapy and radiation therapy (RT).
Soft tissue sarcomas present a diagnostic challenge. Many of these tumors have histologic patterns with overlapping features not only among themselves but also with a variety of other neoplasms with different histogenesis. The development of immunocytochemical procedures, the availability of monoclonal antibodies and polyclonal antibodies to various tissue markers, and tissue microarray technology have improved diagnosis of soft tissue sarcomas in human pathology and, to a limited degree, in veterinary pathology.12,17-22
The histologic nomenclature for some sarcomas may vary from pathologist to pathologist. Before the initiation of the appropriate therapy for the treatment of soft tissue sarcomas, it is necessary to know the histologic type, size, site, and grade and the stage of disease. Histologic grading (e.g., low, intermediate, or high or I, II, III) is assigned after histologic characterization from adequate biopsy specimens (Table 21-3).
Table 21-3
Soft Tissue Sarcoma Grading System

Grade I: Cumulative score of ≤4 for the three categories.
Grade II: Cumulative score of 5-6.
Grade III: Cumulative score of ≥7.
*Mitosis is calculated as the number of mitotic figures/10 HPF.
Specific Tumor Types
Soft tissue sarcoma is a collective term used to describe a number of different types of tumors with similar histologic features and biologic behavior. The histogenesis of soft tissue sarcomas is controversial and may be difficult to differentiate on the basis of routine histologic and immunohistochemical analysis. Some pathologists have recommended the use of a more generic term such as soft tissue sarcoma or spindle cell tumor of canine soft tissue because of the difficulty in differentiating tumors such as fibrosarcoma, PNST, and hemangiopericytoma.23 Moreover, histologic distinction of tumor type is not clinically important because most soft tissue sarcomas have a similar biologic behavior (i.e., locally aggressive with a low-to-moderate distant metastatic rate). Some types of soft tissue sarcomas covered briefly in this chapter are hemangiosarcomas, lymphangiosarcomas, and synovial cell sarcomas, which are atypical because their biologic behavior is different with a higher rate and different distribution of metastasis.
Tumors of Fibrous Origin
Nodular Fasciitis (Fibromatosis, Pseudosarcomatous Fibromatosis)
Nodular fasciitis is a benign nonneoplastic lesion arising from the subcutaneous fascia or superficial portions of the deep fascia in dogs. These lesions are usually nodular, poorly circumscribed, and very invasive.24 Histologically, nodular fasciitis is characterized by large plump or spindle-shaped fibroblasts in a stromal network of variable amounts of collagen and reticular fibers with scattered lymphocytes, plasma cells, and macrophages.24 The morphologic and pathologic characteristics of nodular fasciitis can result in these lesions being misdiagnosed as fibrosarcoma. Infantile desmoid-type fibromatosis is a variant of nodular fasciitis and is characterized by fibroblast proliferation with a dense reticular fiber network and mucoid material.25 Wide excision of both nodular fasciitis and infantile desmoid-type fibromatosis lesions is usually curative.26 Local recurrence is possible with incomplete resection. These tumors do not metastasize.24
Fibrosarcoma
Most fibrosarcomas arise from the skin, subcutaneous tissue, or oral cavity and represent malignant fibroblasts. Tumors can be well differentiated, exhibiting spindle-shaped tumor cells with scant cytoplasm. The more anaplastic tumor is very cellular with closely packed spindle-shaped fibroblasts showing many mitotic figures and marked cellular pleomorphism.27 Tumors tend to occur in older dogs and cats with no breed or sex predilection; however, one reference states a higher predilection in golden retrievers and Doberman Pinschers.28 A unique form, histologically low-grade, yet biologically high-grade fibrosarcoma, is seen in the oral cavity and has a tendency to grow quite large and invade deeper structures, including bone. Metastasis can be seen in up to 20% of the cases.19 Metastasis is rare, but these tumors are infiltrative, with microscopic tumor cells invading along fascial planes, and often recur after surgical excision.
Tumors of the Peripheral Nerves
Peripheral Nerve Sheath Tumor (Neurofibrosarcoma, Malignant Schwannoma, Hemangiopericytoma)
The PNSTs are malignant tumors of nerve sheath origin and have been referred to as neurofibrosarcoma, malignant schwannoma, and hemangiopericytoma. The confusion regarding hemangiopericytoma is the fact that blood vessel pericyte origin has yet to be proven in dogs. Most hemangiopericytomas will have features of nerve sheath tumors histologically.27 Malignant PNSTs will stain positive with S-100 and vimentin, indicating peripheral nerve origin.29
Regardless of the nomenclature, these tumors can occur anywhere in the body. A PNST may involve nerves away from the brain or spinal cord (peripheral group), or they may involve nerves immediately adjacent to the brain or spinal cord (root group) or the brachial or lumbosacral plexus (plexus group).30 The true peripheral form is much more treatable than the plexus or root form. Despite appearing encapsulated at surgery, these tumors are similar to fibrosarcomas and occur as poorly defined tumors without histologic encapsulation. Most of the tumors are adherent to deeper tissues and may infiltrate underlying fascia, muscle, and skin. Although these tumors are considered malignant, they have a modest metastatic rate. As with fibrosarcomas, local recurrence for PNSTs is common following conservative surgery. They tend to grow slowly and can range in size from 0.5 cm to over 10 to 12 cm in diameter. In some cases, they can easily be confused with lipomas on initial clinical examination.27
PNSTs located in the proximal axial region may result in the compression of nerves. The vast majority of cases will show signs of unilateral lameness, muscle atrophy, paralysis, and pain.30 They can invade the spinal cord, and about 50% will invade the cord if a grade III tumor is diagnosed.30 However, local disease usually limits survival before metastasis occurs.
Histiocytic Disorders
The reader is referred to Chapter 33, Section F, for a complete discussion of histiocytic disorders because the biology, nomenclature, and management of this collection of benign and malignant conditions continue to evolve. Malignant fibrous histiocytoma as a histopathologic diagnosis, however, is considered to be more consistent with soft tissue sarcomas in general and is managed with guidelines discussed later in this chapter.
Tumors of Adipose Tissue
Lipomas are benign tumors of adipose tissue. Variants of lipomas have been reported and include angiolipoma and angiofibrolipoma.31 Lipomas are relatively common in older dogs, especially in subcutaneous locations, and are rarely symptomatic. Lipomas can also occur in the thoracic cavity, abdominal cavity, spinal canal, and vulva and vagina of dogs and can cause clinical abnormalities secondary to either compression or strangulation.32-40 Parosteal and infiltrative lipomas have been rarely reported and these tumors can have a more aggressive behavior despite their benign histologic appearance.41-46 Marginal resection is recommended for lipomas that interfere with normal function; however, the majority are asymptomatic and do not require surgical intervention. Lipomas can be differentiated from liposarcomas based on morphologic and histologic appearance. Histologically, lipomas have indistinct nuclei and cytoplasm resembling normal fat, whereas liposarcomas are characterized by increased cellularity, distinct nuclei, and abundant cytoplasm with one or more droplets of fat.38 Surgical resection is usually curative, but local recurrence has been reported.39
Intermuscular Lipoma
Intermuscular lipomas are a variant of the subcutaneous lipoma and are located in the intermuscular region of the caudal thigh of dogs, particularly between the semitendinosus and semimembranosus muscles (Figure 21-1).47 Clinically, intermuscular lipomas appear as a slow-growing, firm, and fixed mass in the caudal thigh region and may occasionally cause lameness.47 Cytologic analysis of fine-needle aspirates is usually diagnostic. The recommended treatment is surgical resection, involving blunt dissection and digital extrusion, and placement of either a Penrose or negative-suction drain. Seromas are a common complication in dogs in which a drain is not used. The prognosis is excellent with no recurrence reported in 11 dogs following surgical removal.47
Infiltrative Lipoma
Infiltrative lipomas are uncommon tumors composed of well-differentiated adipose cells without evidence of anaplasia. These tumors cannot be readily distinguished from the more common simple lipoma by cytology or small biopsy specimens. They are considered “benign” and do not metastasize. However, infiltrative lipomas are locally aggressive and commonly invade adjacent muscle, fascia, nerve, myocardium, joint capsule, and even bone.42,48,49 Computed tomography (CT) is used to better delineate these tumors; however, they do not contrast enhance and differentiating infiltrative lipomas from normal fat is problematic.47 One retrospective analysis of 16 cases reported a 4 : 1 female-to-male ratio.44 Aggressive treatment, including amputation, may be necessary for local control. RT can be considered either alone or in combination with surgical excision.46
Liposarcoma
Liposarcomas are uncommon malignant tumors originating from lipoblasts in older dogs.50 Liposarcomas apparently do not arise from malignant transformation of lipomas. Specific causes are not known, but foreign body–associated liposarcoma has been reported in one dog.5 There is no breed or sex predilection.50 They are commonly reported in subcutaneous locations, especially along the ventrum and extremities, but can also occur in other primary sites such as bone and the abdominal cavity. Liposarcomas are differentiated from lipomas based on morphologic appearance and cytologic characteristics. Liposarcomas are usually firm and poorly circumscribed. They are locally invasive with a low metastatic potential. Metastatic sites include the lungs, liver, spleen, and bone.27,50
The prognosis for liposarcoma is good with appropriate surgical management. The median survival time (MST) following wide surgical excision is 1188 days; this is significantly better than either marginal excision or incisional biopsy, which have MSTs of 649 days and 183 days, respectively (Figure 21-2).50 Liposarcoma is histologically classified as well-differentiated, myxoid, round cell (or poorly differentiated), pleomorphic, or dedifferentiated. This classification scheme has clinical and prognostic importance in humans because pleomorphic liposarcomas have a high metastatic rate, myxoid liposarcomas are more likely to metastasize to extrapulmonary soft tissue structures, and well-differentiated liposarcomas are unlikely to metastasize.51-53 In a retrospective study in dogs, histologic subtype was not prognostic, but metastatic disease was more common in dogs with pleomorphic liposarcomas.50

Figure 21-2 Kaplan-Meier survival curve of 56 dogs with liposarcoma treated with either incisional biopsy, marginal resection, or wide excision. The median survival time (MST) is significantly longer, at 1188 days, following wide surgical resection than less aggressive techniques. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Baez JL, Hendrick MJ, Shofer FS, et al: Liposarcomas in dogs: 56 cases (1989-2000), J Am Vet Med Assoc 224:887, 2004.) J Am Vet Med Assoc
Tumors of Smooth Muscle
Leiomyomas and leiomyosarcomas are tumors arising from smooth muscle cells. The gastrointestinal (GI) tract is most commonly affected, but other primary sites include the spleen, liver, genitourinary tract, retroperitoneal space, vessel wall, and subcutaneous tissue.54-57 Paraneoplastic syndromes associated with smooth muscle tumors, particularly GI leiomyomas and leiomyosarcomas, include hypoglycemia, nephrogenic diabetes insipidus, and secondary erythrocytosis.55,58-61
Leiomyomas are benign and usually small, localized, and well encapsulated. Leiomyomas of the vagina and vulva are often pedunculated, protrude from the vulva, and are hormonally dependent. Ovariohysterectomy is recommended in the management of dogs with vulval or vaginal leiomyomas.
Leiomyosarcomas are malignant tumors with a moderate metastatic potential, depending on the primary site.55,62 The metastatic rate for dogs with hepatic leiomyosarcoma is apparently 100% but is approximately 50% for other primary intraabdominal sites and 0% for dermal smooth muscle tumors.55,61,63,64 Regional lymph nodes, mesentery, and liver are the most common metastatic sites for GI leiomyosarcoma, although other sites include the spleen, kidneys, and peritoneum.55,61-64
Leiomyosarcoma is the second most common GI tumor in dogs and has a predilection for the jejunum and cecum, but any region of the GI tract can be affected from the esophagus to the rectum.55,61-63,65 An immunohistochemical review of previously diagnosed GI leiomyomas and leiomyosarcomas has resulted in the vast majority of these tumors being reclassified as GI stromal tumors (GISTs) or GI stromal-like tumors.66,67 Leiomyosarcomas have strong immunoreactivity to actin and desmin and rarely stain positively with c-kit, CD34, or S-100 protein. In contrast, true GISTs are consistently associated with mutations of the tyrosine kinase receptor gene c-kit and will have strong immunoreactivity to c-kit and CD34 proteins with variable reactivity to actin, desmin, and S-100 protein.62,65-67 The GI stromal-like tumors have an identical immunohistochemical profile to GISTs, except they do not express c-kit.66
Older dogs are more commonly affected and there is no sex or breed predisposition.55,61-63 In contrast, there is a male predisposition for GI leiomyomas and these tumors have a predilection for the stomach rather than the jejunum and cecum.62,68 Presenting signs can include inappetence, weight loss, vomiting, diarrhea, polyuria, polydipsia, anemia, and hypoglycemia.55,61-63
Surgical resection is the recommended treatment for dogs with leiomyosarcomas. Intestinal perforation with localized to diffuse peritonitis is relatively common in dogs with GI leiomyosarcoma and is reported in up to 50% of cases.61 Local tumor control is good following complete resection, but recurrence has been reported following incomplete resection of a gastric leiomyoma and cutaneous leiomyosarcomas.64,66,68
Prolonged survival and possibly cure has been reported following surgery in dogs with gastrointestinal leiomyosarcoma and GIST tumors.54,55,59,67 Prognostic factors in humans with GIST include tumor size, metastasis, and histologic criteria such as tumor necrosis, number of mitotic figures, and proliferating cell nuclear antigen (PCNA) index69; however, prognostic factors have not been investigated in dogs with leiomyosarcoma. The MST for dogs with GI leiomyosarcoma and GIST surviving the immediate postoperative period is up to 37.4 months, with 1-, 2-, and 3-year survival rates of 75% to 83%, 62% to 67%, and 60%, respectively.55,61,63,66,67 In addition, metastasis did not have a negative impact on survival time in one report with a 21.7 month MST for dogs with documented metastasis at the time of surgery.61 However, other investigators have found metastasis significantly decreases survival time.63 The MST is 8 months for dogs with splenic leiomyosarcoma, and all dogs with hepatic leiomyosarcoma in one series had evidence of metastasis at initial surgery and were euthanized.55
Tumors of Skeletal Muscle
Rhabdomyosarcomas are rare malignant tumors originating from myoblasts or primitive mesenchymal cells capable of differentiating into striated muscle cells.70 In dogs, rhabdomyosarcomas are most frequently reported to arise from skeletal muscle of the tongue, larynx, myocardium, and urinary bladder. They are locally invasive with a low-to-moderate metastatic potential. Metastatic sites include the lungs, liver, spleen, kidneys, and adrenal glands.70
Rhabdomyosarcomas are histologically classified as embryonal, botryoid, alveolar, and pleomorphic. The histologic diagnosis of rhabdomyosarcoma is difficult and immunohistochemical staining for vimentin, skeletal muscle actin, myoglobin, myogenin, and myogenic differentiation (MyoD) may be required for definitive diagnosis.71 Embryonal rhabdomyosarcomas have a predilection for the head and neck region of older dogs, such as the tongue, oral cavity, and larynx. In contrast, botryoid rhabdomyosarcoma commonly arises in the urinary bladder of young, female large breed dogs with Saint Bernard dogs possibly being overrepresented in one data set.70 Botryoid tumors are characterized by their grapelike appearance. The histologic classification scheme for rhabdomyosarcoma has prognostic significance in humans, but this has not been investigated in dogs. In humans, botryoid rhabdomyosarcoma has a good prognosis, embryonal rhabdomyosarcoma has an intermediate prognosis, and alveolar rhabdomyosarcoma has a poor prognosis.70,72
Rhabdomyosarcomas, particularly those involving the extremities, have a high rate of metastasis in humans; major metastatic sites include the lungs, lymph nodes, and bone marrow. The metastatic potential and prognosis in dogs with rhabdomyosarcoma have not been determined because it is rarely diagnosed and even more rarely treated with curative intent. However, disease-free and overall survival times have been encouraging in the few dogs treated with surgical resection with or without RT and chemotherapy.73-77 In humans, in contrast to many other types of soft tissue sarcomas, multimodality treatment with surgery, RT, and chemotherapy has significantly improved survival rates, particularly in children with embryonal and botryoid rhabdomyosarcoma.51,53,72
Tumors of Vascular and Lymphatic Tissue
Lymphangiosarcomas are rare tumors seen in young dogs and cats that arise from lymphatic endothelial cells.27,78,79 They are usually soft, cystic-like, and edematous, usually occurring in the subcutis (Figure 21-3).27 In most cases, clinical signs are associated with extensive edema and drainage of lymph through the skin or a cystic mass. Aspiration may reveal a fluid-filled mass. Histopathologically, these tumors resemble normal endothelial cells and may be confused with hemangiosarcomas because of the vascular channels; however, red blood cells are not seen.27 Lymphangiosarcomas tend to have a moderate-to-high metastatic potential.80 In a single case report, a dog treated with surgery that had local recurrence had a complete response following doxorubicin chemotherapy with no evidence of recurrence or metastasis 9 months after remission.81
Hemangioma
Hemangiomas and hemangiosarcomas (HSAs) are tumors of vascular endothelial origin. Hemangioma is a benign tumor that can occur in a variety of sites, including skin, liver, spleen, kidneys, bone, tongue, and heart.82-84 Dermal hemangiomas may be induced by ultraviolet (UV) light in short-haired dogs with poorly pigmented skin.27 Despite their benign biologic behavior, hemangiomas can cause severe anemia secondary to tumor-associated blood loss.82,84 In humans, hemangiomas can spontaneously regress or be responsive to intralesional corticosteroids, but this has not been observed in dogs.27 Complete surgical resection is usually curative.
Hemangiosarcoma
HSAs are highly malignant tumors. The most common primary sites in cats and dogs involve visceral organs, especially the spleen, right atrial appendage, and liver.24 Other primary sites include the skin, pericardium, lung, kidneys, oral cavity, muscle, bone, genitourinary tract, and peritoneum and retroperitoneum.24,85 A thorough review of HSAs is presented in Chapter 33, Section A. The discussion here is limited to dermal and subcutaneous HSAs. Primary dermal HSAs have a predilection for light-haired or poorly pigmented skin on the ventral abdomen and preputial region of dogs, particularly those confined to the dermis, and solar radiation has been proposed as a cause of dermal HSAs in dogs.86
Canine cutaneous HSAs are clinically staged according to depth of involvement with stage I tumors confined to the dermis, stage II tumors extending into subcutaneous tissue, and stage III HSAs involving the underlying muscle (Figure 21-4).86 Stage II and III dermal HSAs are typically large and poorly circumscribed and have a bruised appearance that can be mistaken for a traumatic hematoma. The recommended treatment is wide surgical resection and adjuvant doxorubicin.87 Doxorubicin-based chemotherapy protocols have been investigated in dogs with nonresectable stage II and III cutaneous HSAs, with approximately 40% of dogs showing a measurable response with a median response duration of 53 days.88 Treatment with doxorubicin-based chemotherapy protocols may downstage dogs with large subcutaneous and intramuscular HSAs and thus decrease the size of the tumor, allowing for complete surgical excision.88
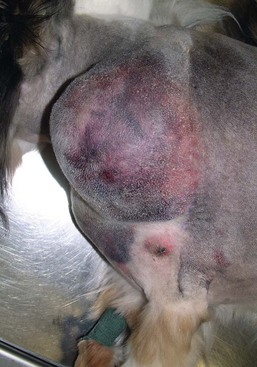
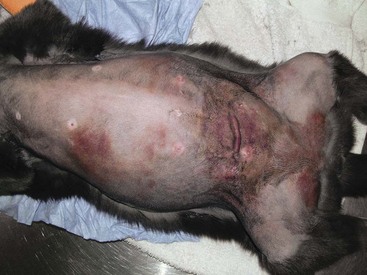
Figure 21-4 A dog with a subcutaneous hemangiosarcoma invading into the underlying musculature of the thoracic limb (stage III) with a typical bruised appearance. Local tumor control is more difficult in these cases and the prognosis is worse for dogs with higher grade dermal hemangiosarcoma if logoregional tumor control is not achieved.
Clinical staging provides prognostic information on the success of local treatment, metastatic potential, and survival time. Dogs with stage I dermal HSAs have a complete surgical resection rate of 78%, 30% metastatic rate with all metastases occurring in distant dermal sites, and a MST of 780 days.86 In contrast, dogs with stage II and III hypodermal HSAs have a complete surgical resection rate of only 23%, principally because these tumors are larger and less well circumscribed; are 60% metastatic to lungs, regional lymph nodes, and distant dermal sites; and have a significantly lower MST of 172 to 307 days (Figure 21-5).86 For dogs with aggressive local control (using adequate surgery with or without RT) of subcutaneous or intramuscular HSA, the use of adjuvant doxorubicin results in encouraging survival times. The median disease-free interval (DFI) and survival time for dogs with subcutaneous and intramuscular HSAs treated with surgery and doxorubicin are 1553 and 1189 days and 266 and 273 days, respectively.87
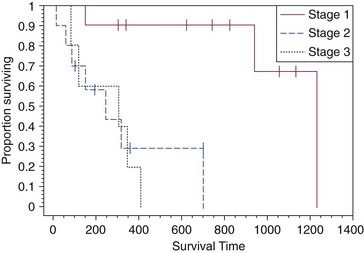
Figure 21-5 Kaplan-Meier survival curve for dogs with stage I (cutaneous), stage II (subcutaneous), and stage III (muscle) hemangiosarcoma. (Reprinted with permission from Ward H, Fox LE, Calderwood-Mays MB, et al: Cutaneous hemangiosarcoma in 25 dogs: A retrospective study, J Vet Intern Med 8:345, 1994.)
Feline cutaneous HSAs are usually solitary tumors in older cats, with a mean age of 11.5 to 12.5 years and males possibly being overrepresented.89,90 Unlike cutaneous hemangiomas, which have no site predilection, cutaneous HSAs occur primarily in poorly pigmented skin, particularly the skin of the pinna, head, and ventral abdomen and subcutaneous tissue of the inguinal region.89-91 Local tumor control is poor following surgical resection, with local recurrence reported in 50% to 80% of cases at a median of 420 days postoperatively.89-91 Metastasis has been reported but occurs less frequently than in dogs with dermal and hypodermal HSAs.89-91 The MST of greater than 1460 days for cats treated with wide surgical resection is significantly better than the MST of 60 days reported for untreated cats with cutaneous HSAs.90
Tumors of Synovial Tissue
Synovial cell sarcomas are malignant tumors arising from synoviocytes of the joint capsule and tendon sheath. The two types of synoviocytes are type A synoviocytes, which are phagocytic and resemble macrophages, and type B synoviocytes, which are fibroblastic.92 Synovial cell sarcomas have been classified as monophasic or biphasic, depending on the proportion of malignant epithelial (synovioblastic) and mesenchymal (fibroblastic) cells within the tumor. However, this classification scheme was adopted from human medicine and is probably not applicable in small animals because of the rarity of epithelial cells in canine synovial cell sarcomas.92 To add further confusion to the nomenclature, synovial cell sarcomas and histiocytic sarcomas are often considered to be different types of joint tumors, but both may originate from synovial cells, with synovial cell sarcomas arising from type B synoviocytes and histiocytic sarcomas arising from type A synoviocytes. Expression of CD18 does not differentiate between the macrophages (or type A synoviocytes) and dendritic antigen-presenting cell (APC) lineage of histiocytic cells (P.F. Moore, personal communication). Recent immunohistochemical evidence, however, indicates that periarticular histiocytic sarcomas do arise from subsynovial dendritic APCs and not type A synoviocytes (P.F. Moore, unpublished data). Rare canine synovial myxomas have been reported. They were most common in large breed middle-aged dogs93 and were reported most often at the stifle or a digit and may be confused with the more common synovial cell sarcoma.
The diagnosis of synovial cell sarcoma is controversial and immunohistochemistry (IHC) is recommended to differentiate synovial cell sarcomas from other types of joint tumors.12,94,95 The immunohistochemical panel should include vimentin and cytokeratin for synovial cell sarcoma, CD18 for histiocytic sarcoma, and actin for malignant fibrous histiocytoma.94 However, other investigators have questioned the value of IHC in the diagnosis of synovial cell sarcoma.95 Synovial cell sarcomas are histologically graded from I to III based on criteria such as nuclear pleomorphism, mitotic figures, and necrosis, and this grading scheme provides prognostic information.12
Synovial cell sarcomas are locally aggressive with a moderate-to-high metastatic potential, depending on histologic grade.12 Up to 32% of dogs with synovial cell sarcomas have evidence of metastatic disease at diagnosis and 54% by the time of euthanasia.10,12 Regional lymph nodes and lungs are the most common metastatic sites.12 Synovial cell sarcomas have a greater metastatic potential and a higher incidence of lymph node metastasis compared to typical soft tissue sarcomas. Synovial cell sarcomas are rare in cats, but feline and canine synovial cell sarcomas are similar in terms of histologic appearance, biologic behavior, and distribution of metastatic lesions.96
In dogs, synovial cell sarcomas usually occur in large breeds, with a predisposition for flat-coated and golden retrievers. Middle-aged dogs are most commonly affected, and there is no sex predilection. Synovial cell sarcomas usually involve the larger joints, particularly the stifle, elbow, and shoulder, but any joint can be affected. Lameness is the most common presenting complaint and can be confused with other orthopedic conditions.12 Radiographic features of synovial cell sarcomas in dogs include periarticular soft tissue swelling and bone invasion, ranging from an ill-defined periosteal reaction to multifocal punctate osteolytic lesions, involving bones on either side of the joint.12,92 Bone involvement is rare in cats.96 Limb amputation is recommended for treatment of the local tumor because local tumor recurrence is significantly lower compared to marginal or wide resection.12,95 Local tumor recurrence following limb amputation, known as stump recurrence, occurs at a relatively higher rate than other types of tumors for which limb amputation is commonly performed.12 Thus the level of amputation should be as proximal as possible to minimize the risk of local tumor recurrence, such as forequarter amputation for synovial cell sarcomas of the thoracic limb and coxofemoral disarticulation or hemipelvectomy for synovial cell sarcomas of the pelvic limb. The role of adjuvant therapy is unknown, but synovial cell sarcomas in humans are more responsive to chemotherapy agents such as anthracyclines and ifosfamide than many other types of soft tissue sarcomas.51 Doxorubicin-based chemotherapy protocols may be warranted in dogs with nonmetastatic grade III synovial cell sarcomas.12,97
Prognostic factors in dogs with synovial cell sarcoma include clinical stage, histologic grade, and extent of surgical treatment. Dogs with evidence of lymph node or lung metastasis at diagnosis have a MST of less than 6 months compared to 36 months or greater if there is no evidence of metastasis.12 The MST for dogs treated with limb amputation is 850 days, which is significantly better than the 455 days reported following marginal resection.95 Finally, the MST for dogs with grade III synovial cell sarcomas is 7 months and significantly worse than either grade I or II synovial cell sarcomas, with MSTs greater than 48 months and 36 months, respectively.12 In one study comparing canine joint tumors, the metastatic rate and MST for dogs with synovial cell sarcoma was 25% and 31.8 months, 0% and 30.7 months for dogs with synovial myxoma, 91% and 5.3 months for dogs with histiocytic sarcoma, and 100% and 3.5 months for dogs with other types of periarticular sarcomas.94
Tumors of Uncertain Histogenesis
Myxosarcomas are neoplasms of fibroblast origin with an abundant myxoid matrix composed of mucopolysaccharides. These rare tumors occur in middle-aged or older dogs and cats. The majority are subcutaneous tumors of the trunk or limbs,27 but there are reports of myxosarcomas arising from the heart, eye, and brain.98-100 These tumors tend to be infiltrative growths with ill-defined margins.27
Malignant Mesenchymoma
Malignant mesenchymomas are rare soft tissue sarcomas comprising a fibrous component with two or more different varieties of other types of sarcoma.24 Malignant mesenchymomas have been reported in the lungs, thoracic wall, liver, spleen, kidney, digits, and soft tissue.101-106 They have a slow rate of growth and can grow very large. Metastasis has been reported.105,106 The outcome for dogs with splenic mesenchymomas is better than other types of splenic sarcomas, with a MST of 12 months and a 1-year survival rate of 50%.101
History and Clinical Signs
Soft tissue sarcomas generally present as a slow-growing mass anywhere in the body. Rapid tumor growth, intratumoral hemorrhage, or necrosis can be seen in some cases. Symptoms are directly related to site of involvement and tumor invasiveness. However, one exception is tumor hypoglycemia, which has been reported in dogs with smooth muscle tumors.59,107 There is marked variability in the physical features of soft tissue sarcoma, but they are generally firm and adherent (fixed) to skin, muscle, or bone. Often, soft tissue sarcomas can be soft and lobulated, mimicking lipomas.
Intraabdominal tumors often compress the GI tract and patients may present with vomiting, diarrhea, and melena. A mass may be palpable, and weight loss and anorexia is common. Leiomyosarcomas are seen most commonly in the GI tract, spleen, and urogenital tract. GI leiomyosarcomas generally result in intestinal obstruction and may cause intestinal perforation leading to peritonitis.61 Rhabdomyosarcomas, seen in young dogs and involving the bladder, present with signs of hematuria, dysuria, and rarely, hypertrophic osteopathy.108,109
PNSTs, involving the brachial or lumbosacral plexus, usually result in pain, lameness, muscle atrophy, and eventual paralysis. These tumors generally are located axially and are difficult to palpate, making early diagnosis difficult because patients do not present with clinical signs until the tumors are quite large and there is significant lameness and muscle atrophy.
Diagnostic Techniques and Work-Up
Fine-needle aspiration (FNA) is recommended to exclude other differentials (e.g., abscesses, cysts, or mast cell tumors [MCTs]). However, cytologic evaluation of FNA may not be sufficient for definitive diagnosis because false-negative results can occur because of nonrepresentative samples subject to variable degrees of necrosis and poor exfoliation of cells in comparison to epithelial and round cell tumors.24 In one study in which FNA was performed on soft tissue sarcomas from 40 dogs, 15% of dogs were incorrectly diagnosed and a further 23% were nondiagnostic.110 Biopsy methods for definitive preoperative diagnosis of soft tissue sarcomas include needle-core, punch, incisional, or excisional biopsies. The biopsy should be planned and positioned so that the biopsy tract can be included in the curative-intent treatment, whether it be surgery with or without RT, without increasing the surgical dose or size of the radiation field. Excisional biopsies are not recommended because they are rarely curative and the subsequent surgery required to achieve complete histologic margins is often more aggressive than surgery following core or incisional biopsies, resulting in additional morbidity and treatment costs. Furthermore, multiple attempts at resection, including excisional biopsy, prior to definitive therapy have a negative impact on survival time in dogs with soft tissue sarcomas.111
Diagnostic tests performed for work-up and clinical staging depend on the type of soft tissue sarcoma, especially with atypical forms (e.g., HSAs, histiocytic sarcomas, lymphangiosarcomas, and synovial cell sarcomas), but usually involve routine hematologic and serum biochemical blood tests, three-view thoracic radiographs, and regional imaging of the soft tissue sarcoma. Blood tests are usually within the normal reference range for most dogs with soft tissue sarcomas; however, there are some notable exceptions. Hematologic abnormalities such as anemia and thrombocytopenia are relatively common in dogs with disseminated histiocytic sarcomas and HSAs.24,112,113 Hypoglycemia has been reported in dogs with intraabdominal leiomyomas and leiomyosarcomas.55,58,59,107 Excessive production of insulin-like growth factor II has been implicated as the cause of hypoglycemia in humans with mesenchymal tumors and this has also been demonstrated in one dog with a gastric leiomyoma.114-116
Imaging studies of the local tumor may be required for planning of the surgical approach or RT if the tumor is fixed to underlying structures or located in an area that may make definitive treatment difficult, such as the pelvic region. Three-dimensional (3D) imaging techniques such as CT and magnetic resonance imaging (MRI) are particularly useful for staging local disease.117 Other imaging modalities for staging of the local tumor include survey radiographs, ultrasonography, angiography, and nuclear scintigraphy.
Diagnostic tests for staging of metastatic disease include thoracic radiographs, abdominal ultrasonography or advanced imaging, and FNA or biopsy of the regional lymph nodes. Three-view thoracic radiographs should be performed prior to definitive treatment because the lungs are the most common metastatic site for typical soft tissue sarcomas.16 Lymph node metastasis is uncommon. FNA or biopsy of regional lymph nodes should be performed in dogs with clinically abnormal lymph nodes, high-grade (III) soft tissue sarcomas, or atypical soft tissue sarcomas having a high rate of metastasis to regional lymph nodes, such as HSAs, histiocytic sarcomas, lymphangiosarcomas, synovial cell sarcomas, leiomyosarcomas, and possibly rhabdomyosarcomas.* Abdominal imaging is recommended for the assessment of metastasis to intraabdominal organs, especially the lymph nodes, spleen, and liver, in animals with suspected intraabdominal neoplasia (e.g., GI leiomyosarcoma or splenic sarcoma) or advanced stage and high-grade pelvic limb soft tissue sarcoma.
Clinical Staging
A modified staging system has been described for soft tissue sarcomas in dogs.24 The American Joint Committee on Cancer (AJCC) staging system currently used in humans with soft tissue sarcomas has been substantially modified from the original staging system, on which the modified animal staging system is based. The most important change to the AJCC staging system is categorization of local disease, with less emphasis on tumor size, which is an arbitrary assignment, and greater emphasis on depth of invasion.51,120 A superficial tumor is defined as a soft tissue sarcoma located above the superficial fascia and that does not invade the fascia, whereas a deep tumor is located deep to the superficial fascia, invades the fascia, or both.108 Based on the current AJCC staging system, an updated modified staging system for animals is summarized in Box 21-1. The stage grouping takes into account both clinical staging criteria (TNM staging system) and histologic grade. The original and updated staging systems for animals with soft tissue sarcomas have not been investigated and the prognostic significance of either of these systems is unknown.
Treatment
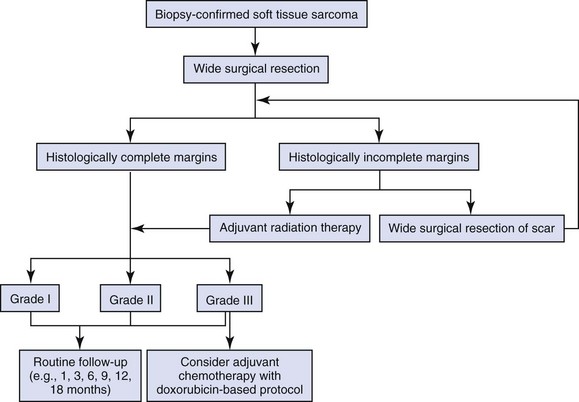
There are over 20 different histologic subtypes of soft tissue sarcomas described in dogs, but the vast majority of these subtypes have a very similar biologic behavior characterized by locally aggressive invasiveness and a low-to-moderate metastatic potential depending on histologic grade.16 Local tumor control is the most important consideration in the management of soft tissue sarcomas because of their locally aggressive behavior. As such, surgical resection is the principal treatment for dogs with soft tissue sarcomas. RT also plays an important role in local tumor control, especially for incompletely resected and unresectable soft tissue sarcomas. However, definitive treatment options depend on histologic subtype (especially for atypical soft tissue sarcomas such as HSAs, histiocytic sarcomas, and lymphangiosarcomas), tumor location, clinical stage, histologic grade, and completeness of surgical margins.24,121 A strategy for managing dogs with typical soft tissue sarcomas is presented in Figure 21-6.
Surgery
Soft tissue sarcomas are locally aggressive tumors that grow along paths of least resistance and invade surrounding tissue, resulting in the formation of a pseudocapsule of compressed viable tumor cells.24 The pseudocapsule gives the false impression of a well-encapsulated tumor. However, surgical removal of the encapsulated mass without adequate margins will result in incomplete resection and a high risk of local tumor recurrence.16 The minimum recommended margins for surgical resection are 3 cm lateral to the tumor and one fascial layer deep to the tumor (Figure 21-7).121,122 Biopsy tracts and any areas of fixation, including bone and fascia, should be resected en bloc with the tumor using the recommended surgical margins. Radical surgery such as limb amputation or hemipelvectomy may be required to achieve adequate surgical margins and local tumor control. Alternatively, planned multimodal therapy with marginal surgical excision and postoperative RT may be limb sparing and reduce patient morbidity.
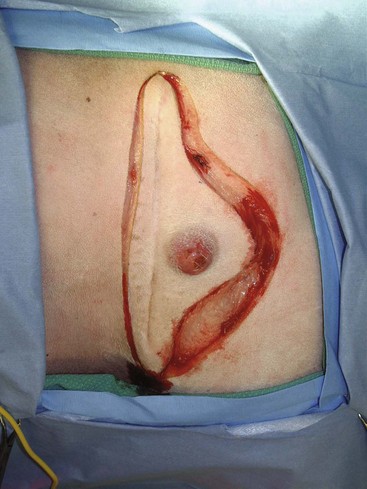
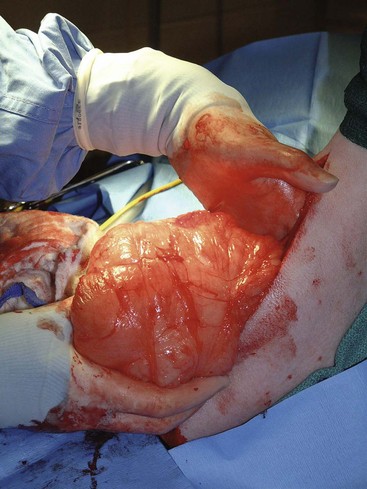
Figure 21-7 Intraoperative image of a dog with a recurrent soft tissue sarcoma. The soft tissue sarcoma is being resected with 3-cm margins around the recurrent tumor and previous scar because both are considered contaminated. Deep margins are one fascial layer.
The resected tumor should be pinned out to the original dimensions to prevent shrinkage during formalin fixation (Figure 21-8)123; the lateral and deep margins should be inked to aid in histologic identification of surgical margins; and any areas of concern should be tagged with suture material, inked in a different color, or submitted separately for specific histologic assessment. Surgical margins and tumor grade are important in determining the need and type of further treatment. For instance, tumor grade is important in deciding whether a soft tissue sarcoma resected with complete but close surgical margins (i.e., 1 to 3 mm) will require further local treatment because surgical margins may be adequate with a low-grade soft tissue sarcoma but not with a high-grade soft tissue sarcoma.121,122
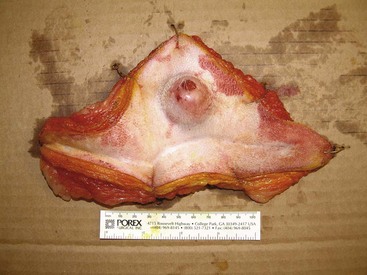
Figure 21-8 Following surgical resection, the lateral and deep margins should be inked (yellow ink was used in this case) to assist in identification of the margins histologically and pinned out on cardboard to prevent sample shrinkage during formalin fixation.
The first surgery provides the best opportunity for local tumor control as the management of incompletely resected tumors increases patient morbidity and treatment costs, increases the risk of further local tumor recurrences, and decreases survival time.111,121,124-128 Traditionally, if a soft tissue sarcoma had been incompletely resected, then the surgical scar was managed with either a second aggressive surgery or RT because the entire surgical wound was considered contaminated and neoplastic.121 However, recent evidence suggests that this may not necessarily be true and a more conservative approach may be employed, particularly for low-grade soft tissue sarcomas. In one study, histologic evidence of residual tumor was identified in only 22% of 39 dogs with incompletely excised tumors.129 Following excision of the surgical scar in these dogs with 0.5- to 3.5-cm margins, the local recurrence rate was 15%.129 In a study of 104 canine soft tissue sarcomas managed with surgery alone in nonreferral practices, fewer than 10% were excised with 3-cm margins and local tumor recurrence was reported in 28% of dogs.130 In a similar pathologic study, 75% of 139 canine subcutaneous soft tissue sarcomas were incompletely excised and the local tumor recurrence rate was significantly associated with histologic grade, with local tumor recurrence reported in only 7% of dogs with grade I soft tissue sarcomas compared to 34% and 75% of dogs with grade II and III tumors, respectively.131 This was further supported by another study in which 11% of 35 dogs with low-grade soft tissue sarcomas of the extremities (below the elbow and stifle) had local tumor recurrence following marginal excision.132 Thus, based on this evidence, dogs with incompletely excised soft tissue sarcomas, especially grade I and perhaps grade II tumors, can be managed with either active surveillance (i.e., frequent observation for local tumor recurrence and appropriate treatment if the tumor recurs) or a staging surgery. Staging surgery is a decision-making surgery. The surgical scar is excised with minimal margins (0.5 to 1.0 cm) with the aim being to determine if there is histologic evidence of residual tumor cells.129 If there is no evidence of tumor cells, then no further treatment is required and these dogs should be monitored regularly for local tumor recurrence. If there is evidence of residual tumor cells, then wide resection of the surgical scar should be performed with the same margins recommended for primary soft tissue sarcomas, 3 cm lateral to the tumor and one fascial layer deep to the tumor,117,121,126,127 or the entire surgical scar should be irradiated.126,127 Surgery is preferred to RT for management of incompletely resected soft tissue sarcomas in humans because local tumor control is better with repeat surgical resection than adjunctive RT alone.126,127 Another option for the management of dogs with incompletely excised soft tissue sarcomas may include metronomic (i.e., low-dose continuous) chemotherapy. The administration of piroxicam and low-dose cyclophosphamide in 30 dogs with incompletely excised soft tissue sarcomas resulted in a significantly prolonged DFI when compared to a nonrandomized control group of 85 dogs with incompletely excised soft tissue sarcomas and no metronomic chemotherapy.133 Additional studies are needed to confirm this treatment alternative.
Surgery and Radiation Therapy
RT can be used as an adjunct to surgery following either planned marginal resection or unplanned incomplete resection. Marginal surgical resection combined with full-course postoperative RT is an attractive alternative to limb amputation for extremity soft tissue sarcoma (Figure 21-9). This multimodality approach requires additional planning and expense but preserves the limb and limb function. Surgery involves completely removing all grossly visible tumor and then marking the lateral, proximal, and distal extents of the surgical field with radiopaque clips to assist in planning of RT. Migration of the radiopaque clips has been reported but does not significantly influence the planned radiation field.134
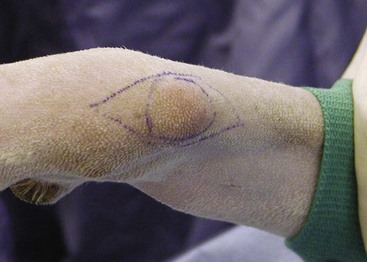
Figure 21-9 Planned marginal resection of a soft tissue sarcoma in a dog. Marginal resection followed by full-course postoperative radiation therapy (RT) provides excellent local tumor control and preserves both the limb and limb function. RT should not involve the limb circumferentially to preserve both lymphatic and venous drainage of the distal extremity. If close but clean margins were obtained for a grade I soft tissue sarcoma, observation alone may be an acceptable alternative.
RT should be started a minimum of 7 days postoperatively to minimize the risk of radiation-induced complications with the surgical wound, such as delayed healing and dehiscence.135 Full-course fractionated protocols are recommended with reported schedules including 3.0- to 4.2-Gy fractions on a Monday-to-Friday or Monday-Wednesday-Friday schedule for a total dose of 42 to 63 Gy.136,137 The optimal fractionation and total dose schemes for canine soft tissue sarcoma have not been determined, but cumulative doses greater than 50 Gy are recommended, and local tumor control is better with higher cumulative doses.136 Acute side effects of RT such as moist desquamation are relatively mild and transient.137
Local tumor control and survival time are excellent when incompletely resected soft tissue sarcomas are treated with postoperative RT. The median time to local recurrence is 700 days to more than 798 days, with local tumor recurrence reported in 5% to 30% of dogs by 1 year and 16% to 60% of dogs in the long term (Figure 21-10).111,136-140 The overall MST for incompletely resected nonoral soft tissue sarcomas treated with postoperative RT is 2270 days with 2-, 4-, and 5-year survival rates of 87%, 81%, and 76%, respectively (Figure 21-11).136,137
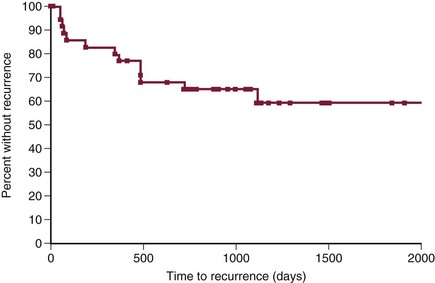
Figure 21-10 Kaplan-Meier curve for time to local tumor recurrence in 35 dogs treated with surgery (incomplete resection) and postoperative radiation therapy (RT). (Reprinted with permission from Forrest LJ, Chun R, Adams WM, et al: Postoperative radiotherapy for canine soft tissue sarcoma, J Vet Intern Med 14:578, 2000.)
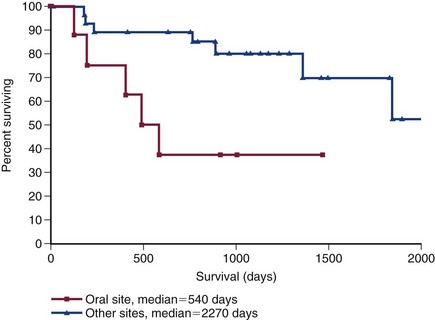
Figure 21-11 Kaplan-Meier survival curve of 35 dogs treated with surgery (incomplete resection) and postoperative RT after soft tissue sarcomas of oral sites were separated from other locations. (Reprinted with permission from Forrest LJ, Chun R, Adams WM, et al: Postoperative radiotherapy for canine soft tissue sarcoma, J Vet Intern Med 14:578, 2000.)
Radiation Therapy
RT can be employed along with surgery in the treatment of soft tissue sarcomas with a curative intent, either preoperatively or postoperatively, or as a sole treatment for pain palliation. Radiotherapy alone as a single modality treatment using doses of 50 Gy resulted in 1-year tumor control rates of 50% that dropped to 33% at 2 years.141 Measurable and palpable (i.e., macroscopic) soft tissue sarcomas are resistant to long-term control with conventional doses of irradiation alone (40 to 48 Gy).142,143 Although one study reported a 30% complete response rate with RT alone,144 these tumors do not rapidly regress after radiation, or if there is significant tumor shrinkage, it is not a durable response. As a single modality, RT is generally considered palliative with control defined as a slowly regressing or stable-in-size tumor mass. Palliative radiation, with 4 fractions of 8 Gy for a total dose of 32 Gy, has recently been investigated with similar results to full-course radiation with a 50% response rate in 16 dogs with measurable soft tissue sarcomas, median time to progression of 155 days, and a MST of 309 days.145
Inadequate durable tumor control using RT as a single modality resulted in the development of combination therapies using RT with hyperthermia and surgery, yielding superior control rates.* Current results of therapeutic clinical studies in dogs demonstrate that RT is the best treatment for incompletely resected soft tissue sarcomas.15,136,137,149
Although higher doses of irradiation will have higher control rates, the chances of normal tissue complications will also increase. In some studies, hyperthermia combined with irradiation showed promise for improved control versus irradiation alone and may also decrease the time to recurrence.144,146,147 The median duration of local control with RT plus hyperthermia is 750 days and significantly greater than the 350 days with RT alone. Difficulty in homogeneous heating of large tumors limits the routine use of hyperthermia for treating soft tissue sarcoma in conjunction with radiation. Addition of whole-body hyperthermia does not improve response rate when compared to RT and local hyperthermia and may have increased the risk for metastasis.148 The response rate and survival time in dogs treated with irradiation and hyperthermia may be predicted with dynamic contrast-enhanced MRI of soft tissue sarcomas before and after the first hyperthermia.150
As mentioned previously, the combination of radiation and surgery provides long-term local control. Postoperative irradiation may be utilized if surgical removal is incomplete and further surgery is not feasible. Irradiation of microscopic tumors after excision is generally superior to radiation of measurable tumors. Control rates for adjuvant radiation after histologically incomplete resection of soft tissue sarcomas are 80% to 95% at 1 year and 72% to 91% at 2 years, with an expected 3-year and 5-year survival rate of 68% to 76%, respectively.136,137,151 Dogs with soft tissue sarcomas with a mitotic rate greater than 9/10 HPF were more likely to have local tumor recurrence and shorter survival times in one study of 39 dogs treated with orthovoltage and doxorubicin as a radiation sensitizer.151 Although not statistically significant, dogs with hemangiopericytoma had a 3-year survival rate of 92% in one retrospective study.136
Preoperative RT is becoming commonplace in veterinary oncology. The rationale and advantage of administering RT prior to surgery are that (1) the radiation field is smaller because, after surgery, the entire surgical site must be included in the field and this may contribute to local toxicity; (2) a large number of peripheral tumor cells are inactivated (with reduced contamination of the surgical site); and (3) tumor reduction may make surgical resection less difficult.15,152-154 In a study of 112 human patients with soft tissue sarcomas, it was noted that there was no significant difference between preoperative or postoperative radiotherapy in terms of relapse-free survival, local control, or overall survival. However, wound complications were significantly more frequent in preoperative radiotherapy patients.154 A subsequent review supported these findings.155 Lower doses of preoperative radiotherapy (less than 50 Gy) are used to reduce the risk of surgical complications. The therapeutic goal of preoperative radiotherapy is to eradicate the microscopic tumor cells at the peripheral margins. Generally, preoperative radiotherapy is reserved for initially inoperable tumors as an alternative to radical surgery.
Chemotherapy
The role of chemotherapy in the management of dogs with soft tissue sarcoma is not yet known. The metastatic rate for cutaneous soft tissue sarcoma is grade dependent and varies from less than 15% for grade I and II soft tissue sarcoma to 41% for grade III soft tissue sarcoma.16 Metastasis often occurs late in the course of disease, with a median time to metastasis of 365 days,16 and this may minimize the beneficial effects of postoperative chemotherapy on the development of metastatic disease. However, there are clinical situations in which postoperative chemotherapy should be considered, including dogs with grade III soft tissue sarcoma, metastatic disease, intraabdominal soft tissue sarcoma (e.g., leiomyosarcoma and splenic sarcoma), and histologic subtypes with a higher rate of metastasis, such as histiocytic sarcomas, hypodermal HSAs (stage II or III), synovial cell sarcomas, rhabdomyosarcomas, and lymphangiosarcomas.24,88,156-159
Doxorubicin-based protocols, either alone or in combination with cyclophosphamide, have shown the most promise in dogs with soft tissue sarcoma with an overall response rate of 23%.160 The need to combine cyclophosphamide with doxorubicin is debatable because single-agent doxorubicin has been shown to be equally as effective in the management of dogs with HSAs as doxorubicin combined with either cyclophosphamide or cyclophosphamide and vincristine.161 Mitoxantrone, a chemotherapeutic drug related to doxorubicin, has a variable effect against soft tissue sarcomas in dogs, with the response rate varying from 0% in 6 dogs to 33% in 12 dogs.162,163 Ifosfamide has also shown some potential with a complete response rate of 15% (2 dogs) in 13 dogs with solid sarcomas of the skin, bladder, and spleen.164 Doxorubicin and ifosfamide are the most effective single agents in the management of soft tissue sarcoma in humans, but response rates are less than 30% and metaanalyses show single- and multiple-agent chemotherapy protocols do not significantly increase overall survival times compared to surgery alone.165,166 A similar finding of no improvement in survival times has been reported in dogs with grade III soft tissue sarcomas treated with surgery alone compared to those treated with surgery and adjuvant doxorubicin.167
Postoperative systemic chemotherapy has also been shown to significantly improve disease-free survival times, but not overall survival time, in humans with soft tissue sarcomas, regardless of histologic grade.165,166 Adjuvant chemotherapy has not shown the same effect on local tumor control in dogs with soft tissue sarcomas,167 but metronomic and local chemotherapy protocols may be effective in decreasing the rate of local tumor recurrence and improving DFIs in dogs with soft tissue sarcomas. Metronomic chemotherapy with piroxicam and low-dose cyclophosphamide significantly prolongs DFI in a small group of dogs with incompletely excised soft tissue sarcomas.133 Local release of cisplatin from a biodegradable polymer implanted into the surgical bed of incompletely resected soft tissue sarcomas may also decrease the risk of local tumor recurrence, with a 31% rate of local tumor recurrence in 32 dogs at a median of greater than 640 days postoperatively.168
Prognosis
The prognosis for dogs with soft tissue sarcoma is good. Local tumor control is often the most challenging aspect of managing soft tissue sarcomas.16,110 Local tumor recurrence rates following either surgery alone or surgery and RT varies from 7% to 32%.110,111,138,167,169 Poor prognostic factors for local tumor control include large tumor size, incomplete surgical margins, and high histologic tumor grade.16 In one study of 75 dogs, the local tumor recurrence rate following incomplete resection was 28% and 11 times more likely than soft tissue sarcomas resected with complete margins (Figure 21-12).16 Tumor size has been reported to have a negative effect on local tumor control16 but probably influences the ability to achieve complete resection rather than having a direct effect on local tumor recurrence. Furthermore, tumor size has not been identified as a prognostic factor in other studies110,138,169 and does not influence local tumor control in dogs treated with surgery and adjuvant RT or RT alone.141,146,169,170 Management of recurrent soft tissue sarcomas is usually more difficult than management of primary tumors, which emphasizes the need for an aggressive approach initially. Curative-intent treatment of recurrent soft tissue sarcomas often requires a more aggressive approach, resulting in increased treatment-related morbidity, whereas the DFI is shorter, the metastatic rate higher, and survival times are decreased in comparison to dogs with naïve tumors.110,121,124-128 Local tumor recurrence is still possible after either complete resection or incomplete resection combined with adjunctive RT.16,136,137 Consequently, examination of the treatment site is recommended at regular intervals, such as monthly for the first 3 months, then every 3 months for the first 12 months, and then every 6 months thereafter.121 The median time to local tumor recurrence was 368 days in one report of 75 dogs with soft tissue sarcomas, which emphasizes the need for long-term follow-up in these cases.16

Figure 21-12 Kaplan-Meier curve for disease-free interval (DFI) in 39 dogs with complete surgical removal of soft tissue sarcomas and 36 dogs with incomplete surgical margins. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Kuntz CA, Dernell WS, Powers BE, et al: Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996), J Am Vet Med Assoc 211:1147, 1997.) J Am Vet Med Assoc
The overall metastatic rate in dogs with soft tissue sarcoma varies from 8% to 17% with a median time to metastasis of 365 days.16,136,137 Factors that increase the risk of metastatic disease include histologic grade, number of mitotic figures, percentage of tumor necrosis, and local tumor recurrence. As mentioned previously, the metastatic rate for dogs with grade I or II soft tissue sarcomas is less than 15% compared to 41% to 44% for grade III soft tissue sarcomas.16,167 Metastasis is 5 times more likely when tumors have a mitotic rate of 20 or more mitotic figures/10 HPF compared to fewer than 20 mitotic figures/10 HPF.16
The MST for dogs with soft tissue sarcoma ranges from 1416 days following surgery alone to 2270 days with surgery and adjunctive RT.16,136,137 Overall, up to 33% of dogs eventually die of tumor-related causes.16 Tumor-related deaths in dogs with soft tissue sarcoma is 2.8 times more likely with greater than 10% tumor necrosis and 2.6 times more likely with a mitotic rate of 20 or more mitotic figures/10 HPF.16 The MSTs for dogs with 10 or fewer, 10 to 19, and 20 or more mitotic figures/10 HPF is 1444 days, 532 days, and 236 days, respectively (Figure 21-13).16 The prognosis for specific subtypes of soft tissue sarcoma are discussed separately earlier in this chapter in the section on specific tumor types.

Figure 21-13 Kaplan-Meier survival curve for 75 dogs with soft tissue sarcomas based on mitotic rates. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Kuntz CA, Dernell WS, Powers BE, et al: Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996), J Am Vet Med Assoc 211:1147, 1997.) J Am Vet Med Assoc
Feline Sarcomas and Vaccine-Associated Sarcomas
The following events are linked to the development of postvaccinal sarcomas in the cat. The prevalence of feline rabies led to the enactment of a law requiring rabies vaccinations for cats in Pennsylvania in 1987.171 In addition, two changes in vaccines occurred in the mid-1980s: development of a killed rabies vaccine licensed for subcutaneous administration and a killed vaccine for feline leukemia virus (FeLV). Two pathologists, M. J. Hendrick and M. H. Goldschmidt, and their colleagues at the University of Pennsylvania School of Veterinary Medicine were the first to recognize the increased incidence of reactions and formation of sarcomas at sites of rabies vaccinations.172-174 Epidemiologic studies have shown a strong association between the administration of inactivated feline vaccines, such as FeLV and rabies, and subsequent development of soft tissue sarcoma at vaccine sites.172-176 Additionally, some authors report reaction to vaccines was additive and this increased the likelihood of sarcoma development with multiple vaccines given at the same site simultaneously.175 The development of soft tissue sarcoma at sites of vaccine administration of FeLV or rabies is believed by some to be as high as 1/1000,177,178 whereas others report a prevalence between 1 and 3.6/10,000 cases.176,179,180
The time to tumor development postvaccination has been reported to be 4 weeks to 10 years and is associated with a robust inflammatory reaction around the tumor.176 Adjuvant-containing vaccines have been proposed to be more likely to cause a vaccine site reaction and/or develop into a soft tissue sarcoma.176 However, two large epidemiologic studies did not provide evidence that aluminum-containing vaccines pose a greater risk.165,182 Thus it remains unclear whether nonadjuvant vaccines are safer. A multicenter study of cats in the United States and Canada found that no single vaccine manufacturer or vaccine type had a higher or lower association with the development of soft tissue sarcoma.182 Additionally, vaccine practices such as needle gauge, syringe reuse, use and shaking of multidose vials, mixing vaccines in a single syringe, and syringe type had no role in the development of tumors.182
The hypothesis that postvaccination inflammatory reactions lead to uncontrolled fibroblast and myofibroblast proliferation and eventual tumor formation either alone or along with immunologic factors is a popular theory.183-188 The thought that inflammation precedes tumor development is supported by histologic identification of transition zones from inflammation to sarcoma and microscopic foci of sarcoma located in areas of granulomatous inflammation. A similar phenomenon of intraocular sarcoma development exists in cats after trauma or chronic uveitis.189-191
Growth factors regulate the cellular events involved in granulation tissue formation and wound healing. When these factors are added to fibroblast cultures, the cells develop a neoplastic phenotype. Immunohistochemical identification and localization of growth factors and their receptors are being investigated in vaccine-associated sarcomas. Through immunohistochemical study of growth factors and their receptors, Hendrick has found that vaccine-associated sarcomas are immunoreactive for platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and its receptors, and transforming growth factor-β (TGF-β). Conversely, non–injection-site fibrosarcomas are negative or faintly positive.192,193 Hendrick also found that lymphocytes in vaccine-associated sarcomas are positive for PDGF, but lymphocytes in non–injection-site fibrosarcomas and normal lymph nodes are negative.192 Regional macrophages also stain positively for PDGF receptor (PDGFR). Neoplastic cells that are closest to lymphocytes in these tumors have the strongest staining for PDGFR, which has led to a hypothesis that lymphocytes in vaccine-associated sarcomas may secrete PDGF, recruit macrophages, and lead to fibroblast proliferation.192,193 The expression of C-jun, a proto-oncogene coding for the transcriptional protein AP-1, has also been examined in vaccine-associated sarcomas. C-jun was found to be strongly positive in vaccine-associated sarcomas and not expressed in non–injection-site fibrosarcomas.192,193 It appears that FeLV and the feline sarcoma virus are not involved in the pathogenesis of feline vaccine-associated sarcomas. Using immunohistochemical analysis and polymerase chain reaction (PCR) techniques, FeLV was not detected in vaccine-associated sarcomas.194
p53 mutations have been evaluated in feline vaccine-associated sarcomas.195-198 p53 is a tumor suppressor gene that encodes a nuclear protein critical in the regulation of the cell cycle. Normal or wild-type p53 will increase in response to DNA damage resulting in cell arrest at the G1 interphase, allowing for DNA repair before replication or apoptosis if damage is irreparable. Cells lacking normal p53 proceed through the cell cycle unregulated, leading to aberrant clones and possible malignancy.186 Anti-p53 antibodies have immunoreactivity in feline sarcomas and may play a role in predicting clinical outcome.198
Recent papers continue to link growth factors with development of vaccine-associated sarcomas in cats. Continued immunohistochemical probing of feline vaccine-associated sarcomas document expression of growth-regulating proteins: p53 protein, basic fibroblast growth factor β (bFGF-β), and TGF-α.198 Researchers recently concluded that PDGF and its receptor play an important role in the in vitro growth of vaccine-associated sarcoma cell lines, both alone and in the presence of chemotherapeutic agents. Furthermore, they found that a signal transduction inhibitor, imatinib mesylate, inhibits the PDGF-dependent cell growth in a dose-dependent manner.199
The term vaccine-associated sarcoma has been used interchangeably with injection-site sarcoma because sarcomas arising at sites of injections other than vaccines, such as lufenuron and microchips, have been reported.200-202 Vaccine-associated sarcoma has been used in this chapter because of the overwhelming prevalence of vaccines as a cause of these injection-site sarcomas.
Pathology
There are many similarities between the histologic subtypes and biologic behavior of soft tissue sarcomas in cats and dogs. The three principal exceptions in cats are vaccine-associated sarcomas, virally induced multicentric fibrosarcoma, and the relative rarity of PNST, synovial cell sarcoma, and histiocytic sarcoma.188,203 There are significant differences between vaccine-associated sarcoma and non–vaccine-associated sarcoma. Tumors that develop after vaccination are typically mesenchymal in origin and include fibrosarcomas, rhabdomyosarcomas, malignant fibrous histiocytomas, undifferentiated sarcomas, and extraskeletal osteosarcomas and chondrosarcomas.192,204,205 Vaccine-associated sarcomas have histologic features consistent with a more aggressive biologic behavior than non–vaccine-associated sarcomas, such as marked nuclear and cellular pleomorphism, increased tumor necrosis, high mitotic activity, and the presence of a peripheral inflammatory cell infiltrate consisting of lymphocytes and macrophages.187,192,206 In a series of 91 cats with histologically confirmed and graded vaccine-associated sarcomas, the prevalence of high-grade lesions was substantially higher than reported in dogs,16 with 59% of cats diagnosed with grade III tumors and only 5% with grade I tumors.207 Microscopically, areas of transition between inflammation and tumor development are frequently observed in cats with vaccine-associated sarcoma.161,192,208 The macrophages in these peripheral inflammatory cell infiltrates often contain a bluish-gray foreign material that has been identified as aluminum and oxygen by electron probe x-ray microanalysis.188 Aluminum hydroxide is one of several adjuvants used in currently available feline vaccines.188 Injection-site sarcomas are histologically similar to mesenchymal tumors arising in the traumatized eyes of cats, which suggests a common pathogenesis of inflammation and the development of soft tissue sarcomas in these cats.190-193 The presence of inflammatory cells, fibroblasts, and myofibroblasts in and adjacent to vaccine-associated sarcomas supports this hypothesis.24,209,210
Diagnosis and Work-Up
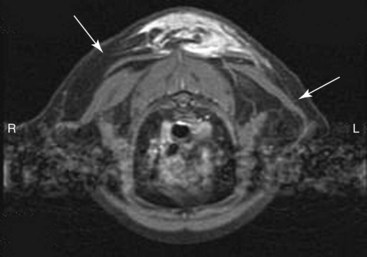
The diagnostic techniques and clinical staging tests recommended for cats with suspected vaccine-associated sarcoma are similar to those described in dogs earlier in this chapter. Advanced imaging such as contrast-enhanced CT or MRI is recommended for local staging of the tumor because these 3D-imaging modalities provide essential information for proper planning of surgery and/or RT (Figure 21-14).188 The volume of tumor based on contrast-enhanced CT is approximately twice the volume measured using calipers during physical examination.185 Accurate pretreatment knowledge of the extent of disease is important because vaccine-associated sarcomas are very invasive, are frequently located in areas in which regional anatomy can complicate an aggressive surgical approach (e.g., interscapular area, body wall, and proximal pelvic limb), and have a high rate of local tumor recurrence, especially if incompletely resected. Excisional biopsy of a suspected vaccine-associated sarcoma is not recommended because the risk of local tumor recurrence is increased, and DFI and survival time are significantly decreased.211,212
Treatment
The Vaccine-Associated Feline Sarcoma Task Force has recommended that masses at vaccination sites be treated if the mass is still evident 3 or more months after vaccination, is larger than 2 cm in diameter, or is increasing in size more than 4 weeks after vaccine administration.188 Vaccine-associated sarcomas are very invasive tumors, and aggressive treatment is required, with both wide surgical resection and full-course RT, to achieve adequate local control.
Surgery
Vaccine-associated sarcomas are poorly encapsulated tumors with extension and infiltration along fascial planes.213,214 The Vaccine-Associated Feline Sarcoma Task Force has recommended surgical resection with a minimum of 2-cm margins both lateral and deep to the tumor.188 Marginal resection or excisional biopsy should not be attempted. The median DFI and survival time are significantly decreased with marginal resection, with an increased number of surgical interventions, and if surgery is performed by nonreferral surgeons (Figure 21-15).205,211,212 The median time to first recurrence following marginal resection is 79 days compared to 325 to 419 days for wide resection or radical surgery.212 In addition, the median time to first recurrence is only 66 days when the first surgery is performed at a nonreferral institution compared to 274 days at referral institutions.211 Inadequate biopsy planning, preoperative staging, and/or attempts at first surgery will result in an increase in tumor margins and may make further surgical treatment impossible. The first attempt at surgical management of cats with vaccine-associated sarcomas should be performed by a referral surgeon with experience in aggressive resection, especially in the interscapular and pelvic regions, to increase the chance of a successful outcome.185

Figure 21-15 Kaplan-Meier curve for time to first local tumor recurrence in 47 cats with vaccine-associated sarcomas treated by referring veterinarians (squares) compared to 14 cats treated at referral institutions (triangles). Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Hershey AE, Sorenmo KU, Hendrick MJ, et al: Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986-1996), J Am Vet Med Assoc 216:58, 2000.) J Am Vet Med Assoc
Similar to dogs with soft tissue sarcomas, biopsy tracts and any areas of fixation, including bone and fascia, should be resected en bloc with the tumor. In cats with vaccine-associated sarcomas, wide surgical resection of tumors located in the interscapular region will often involve excision of dorsal spinous processes and perhaps the dorsal aspect of the scapula (Figure 21-16), whereas thoracic or body wall resection is often required for truncal tumors.211,212 Limb amputation or hemipelvectomy is usually required to achieve adequate surgical margins and local tumor control for vaccine-associated sarcomas located on the extremity.
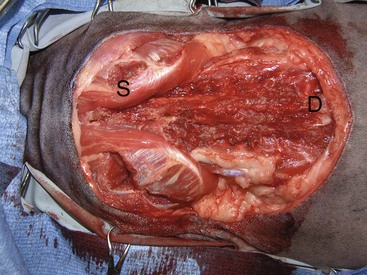
Figure 21-16 Aggressive resection of vaccine-associated sarcomas is required for prolonged local tumor control. In this cat, the vaccine-associated sarcoma was resected with 5-cm margins, including the dorsal spinous processes (D). The scapulae (S) were spared because the tumor could be resected with two fascial planes without involving the scapula.
Local tumor control is still disappointing with curative-intent surgery. Despite attempting aggressive surgery with 2- to 3-cm lateral margins and one fascial layer for deep margins, complete resection is achieved in less than 50% of cats.211,212 Furthermore, overall 1- and 2-year disease-free rates are only 35% and 9%, respectively.211 Median DFI and survival time are both greater than 16 months following complete histologic resection and significantly better than incompletely resected tumors. Local tumor control is improved for extremity vaccine-associated sarcomas, presumably because the lateral and deep surgical margins achieved with limb amputation are superior to other locations.211 However, aggressive surgery is possible in other locations with good results. Chest wall and body resection, using a minimum of 3-cm margins, was well tolerated in six cats and local tumor recurrence was not reported in any of these cats at a minimum of 12 months postoperatively.215
A more aggressive surgical approach is recommended because of the low rate of complete resection and subsequent poor local tumor control, with surgical excision using 2 to 3 cm. This recommendation is based on a study of 91 cats with vaccine-associated sarcoma in various locations, including interscapular, trunk, and extremity, treated with surgery alone using 5-cm lateral margins and two fascial planes for deep margins.207 Major surgical complications were reported in 11% of cats. Wound dehiscence following excision of interscapular tumors was the most common complication.207 Complete histologic resection was achieved in 97% of cats and local tumor recurrence was reported in only 14% of cats.207 These findings are supported by an earlier study of 57 cats treated with 4- to 5-cm lateral margins and one fascial layer for deep margins in which complete histologic excision was achieved in 95% of cats.216 These results suggest that extrapolation from the surgical management of canine soft tissue sarcomas and the recommendations of the Vaccine-Associated Feline Sarcoma Task Force are inadequate for the surgical treatment of feline vaccine-associated sarcomas and better rates of complete surgical resection and local tumor control can be achieved using a more aggressive surgical approach.
Surgery and Radiation Therapy
As a result of the high rate of local tumor recurrence following wide surgical resection with 3-cm margins, full-course RT is highly recommended in the management of cats with vaccine-associated sarcoma.205,217-220 The timing of surgery and RT is controversial. The advantages of preoperative radiation include greater antitumor effect because of a smaller population of resistant hypoxic cells as blood supply to the tumor is not disturbed; a smaller radiation field because, after surgery, the entire surgical site must be included in the field; reduction in tumor size, which facilitates surgical resection; and decreased risk of disseminating tumor cells during surgery.153 The principal disadvantage of preoperative RT is the increased risk of surgical complications, such as wound dehiscence.153 Postoperative RT may provide better tumor control because RT is more effective against microscopic disease than gross tumor and does not delay definitive surgery.153 However, surgery increases the size of the radiation field and the population of radioresistant hypoxic tumor cells by altering the local blood supply, and there is a risk of tumor cells repopulating in the interval between surgery and the start of RT.153,205
In two studies investigating preoperative RT, local tumor recurrence was reported in 40% to 45% of cats at a median of 398 to 584 days postoperatively.217,219 In both studies, complete resection significantly improved the time to local recurrence, with a 700- to 986-day median DFI for completely excised tumors and 112- to 292-day median DFI for tumors resected with incomplete margins. However, despite the prolonged interval to local tumor recurrence, complete resection following preoperative RT does not appear to improve local control rates because local tumor recurrence was reported in 42% of 59 cats with complete margins and 32% of 33 cats with incomplete margins.219
The outcome following postoperative RT is similar to preoperative RT. In one study, local tumor recurrence was reported in 41% of 76 cats at a median of 405 days postoperatively.217 In another study investigating the effects of chemotherapy in cats treated with surgery and postoperative RT, local tumor recurrence occurred in 28% of 25 cats with a median time to first recurrence not reached in cats treated with surgery and RT alone and 661 days in cats also treated with doxorubicin.218 In a recent study of 46 cats treated with surgery and curative-intent postoperative RT, the median progression-free interval (PFI) was 37 months with 1- and 2-year progression-free rates of 63% and 60%, respectively.219 In comparison, 27 cats treated with postoperative palliative coarse fractionation protocols had a median PFI of 10 months and a MST of 24 months.219 Importantly, RT should start 10 to 14 days postoperatively as DFI and survival time decreases as the interval between surgery and starting RT increases.205 Local tumor recurrence does not influence survival time and, regardless of the timing of RT relative to surgery, survival data are encouraging with MSTs of 600 to 1300 days and 1-, 2-, and 3-year survival rates of 86%, 44% to 71%, and 28% to 68%, respectively.205,217,218,220
Local tumor control is still disappointing with 28% to 45% of tumors recurring after multimodality treatment with surgery and RT.205,217-220 The radiation field used in these studies typically included a minimum of 3-cm margins around the tumor or surgical scar. The majority of tumors recur within the radiation field, although tumors have been reported to recur outside the radiation field.218 Similar to surgery alone, a more aggressive approach may be warranted to improve local tumor control, such as higher radiation doses, larger radiation fields, and more aggressive surgical resections.
Radiation Therapy
Although RT alone is rarely effective for the management of cats and dogs with measurable soft tissue sarcomas, RT may have a role in the palliative setting for cats with large and unresectable vaccine-associated sarcomas. In a pilot study of 10 cats with vaccine-associated sarcomas, 7 cats achieved partial responses and 2 cats had complete responses following treatment with liposomal doxorubicin as a radiation sensitizer and irradiation with a median of 5 fractions of 4 Gy for a total dose of 20 Gy; however, PFIs were not durable (median of 117 days).221
Chemotherapy
The role of chemotherapy in the management of cats with vaccine-associated sarcoma remains undefined. Metastasis has been reported in 0% to 26% of cats with vaccine-associated sarcomas, despite the aggressive histologic appearance and prevalence of high-grade lesions in these tumors, with a median time to metastasis of 265 to 309 days.* Injection-site sarcoma cell lines have shown in vitro sensitivity at clinically relevant doses to doxorubicin, mitoxantrone, vincristine, and paclitaxel.222,223 Clinically, partial and complete responses to doxorubicin, either alone or in combination with cyclophosphamide, have been reported in 39% to 50% of cats with gross tumors, but these responses are often short-lived with a median duration of 84 to 125 days.224,225 However, MSTs are significantly prolonged in cats that respond to chemotherapy with 242 days for responders and 83 days for nonresponders.224 Furthermore, MSTs are significantly increased for cats with gross residual disease following surgical excision and treated with postoperative RT and chemotherapy with a MST of 29 months compared to 5 months for cats treated with surgery and postoperative RT.220
Postoperative chemotherapy has minimal impact on survival in cats treated with curative-intent surgery and RT.207,216-218,225 Chemotherapy may, however, have beneficial effects on local tumor control and time to local tumor recurrence. Doxorubicin and liposome-encapsulated doxorubicin significantly prolong the DFI following surgery, with a median DFI of 393 days for cats receiving chemotherapy and 93 days for those in which chemotherapy was not administered (Figure 21-17).225 The completeness of surgical margins may be a confounding factor in this analysis because the median DFI was greater than 449 days in cats with complete surgical margins compared to 281 days following incomplete resection.225 Carboplatin was associated with an insignificant but numerically superior median DFI of greater than 986 days in cats treated with preoperative RT and surgery.219 Other studies have shown no effect of adjunctive chemotherapy on either local tumor control or survival time.217,218
Figure 21-17 Kaplan-Meier curve for disease-free interval (DFI) in 75 cats with vaccine-associated sarcomas treated with surgery and postoperative chemotherapy (liposome-encapsulated doxorubicin or doxorubicin) or surgery alone (historic control). (Reprinted with permission from Poirier VJ, Thamm DH, Kurzman ID, et al: Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of vaccine-associated sarcomas in cats, J Vet Intern Med 16:726, 2002.)
Novel treatments such as electrochemotherapy and immunotherapy are also being investigated with some encouraging results. In one study, cats with high-grade soft tissue sarcomas were treated either intraoperatively or postoperatively with intratumoral bleomycin followed by 8 biphasic pulses of up to 1300 V/cm.226 The median time to local tumor recurrence in the control (surgery only) group was 4 months compared to 12 months for cats treated intraoperatively and 19 months for cats treated postoperatively. Of further interest, the metastatic rate was only 1.7%.226
Immunotherapy, using recombinant viruses expressing interleukin-2 (IL-2), has shown some promise in improving local tumor control rates in cats with vaccine-associated sarcomas. Following surgical resection and iridium-based RT, the 1-year local tumor recurrence rate was 61% in cats receiving no adjunctive treatment, 39% in cats administered human IL-2 using a vaccinia virus vector, and 28% with feline IL-2 using a canary pox virus vector.227 In a phase I trial, two doses of intratumoral feline IL-2, interferon γ (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by magnetofection prior to surgical resection resulted in a 1-year local recurrence rate of 24%.228 In another phase I trial by the same research group, 2 doses of intratumoral GM-CSF followed by magnetofection prior to surgical resection resulted in a 1-year local recurrence rate of 50% and minimal treatment-related toxicities.229 Other immunotherapeutic agents such as IL-12 and acemannan have been investigated with less favorable results.230-232
Prognosis
It has become clear that the prognosis for cats with vaccine-associated sarcomas treated with surgical excision alone, using traditional recommendations, can be poor with respect to tumor control, especially when the first surgery is marginal and multiple surgeries are employed as the method of treatment. However, long-term survival, even in the face of multiple recurrences, is the norm with this disease. The median time to first recurrence was 2 months for cats treated by conservative surgery (referring veterinarian) and 9 months for those cats treated by more aggressive surgery (referral institution); the overall survival time was 19 months.212 Another study of single modality surgical excision reported an overall DFI of 10 months, which increased to longer than 16 months when excision was complete.211 The prognosis with surgery alone is improved with aggressive surgical excision using 5-cm lateral margins and two fascial layers for deep margins.207 A recent study looked at 3D histologic margins in 48 cats with vaccine-associated sarcomas and evaluated the predictive value of histologic margins and tumor grading on local recurrence.233 Tumor margins were noninfiltrated in 32 cats and the remaining 16 cases had infiltrated margins. Overall, 6/32 (19%) tumors with noninfiltrated margins and 11/16 (69%) with infiltrated margins had tumor recurrence. Tumors with infiltrated margins recurred about 10 times more frequently compared to tumors with noninfiltrated margins.233 The degree of infiltration of tumor margins may provide an indication for the need for adjuvant RT following surgical excision.
Outcomes are improved when cats with vaccine-associated sarcomas are treated with multimodality therapy, especially following surgical excision with less aggressive intent. Optimal outcome occurs when cats are treated aggressively initially and not after multiple failed resections.220 The combination of radiotherapy either preoperatively or postoperatively has improved the DFI and overall survival time in cats with vaccine-associated sarcomas. DFI and MSTs range from 13 to 37 months and 23 to 43 months, respectively.205,215-218 Injection-site sarcomas are locally invasive; however, approximately 15% to 24% will metastasize to the lungs or other sites.207,208,212,216,217 Continued research into the treatment of vaccine-associated sarcomas is needed because there is room for improvement in outcomes.
Prognostic factors were investigated in one study of 42 cats with surgically resected nonvisceral soft tissue sarcomas, including 8 cats with presumed vaccine-associated sarcomas.234 The overall MST was 608 days. The margins of excision lateral and deep to the tumor were not reported, but excision was incomplete in 74% of cats and local tumor recurrence was reported in 62% of cats. The median time to recurrence was 261 days and the MST in cats with local tumor recurrence was 576 days. Metastasis was suspected in 14% of cats and the MST in cats with suspected metastasis was 562 days. Prognostic factors included tumor size and histologic type. Cats with soft tissue sarcomas smaller than 2 cm in diameter had a MST of 643 days, which was significantly longer than the 558 days for cats with tumors 2 to 5 cm in diameter and 394 days for cats with soft tissue sarcomas larger than 5 cm in diameter. The MSTs for cats with fibrosarcoma (640 days) and PNST (645 days) were significantly better than for cats with malignant fibrous histiocytoma (290 days). Tumor location and injection-site versus non–injection-site sarcomas were not prognostic factors in this study.234 Although these results are encouraging, the rates of incomplete excision and local tumor recurrence suggest that a more aggressive surgical approach and multimodal therapy with adjuvant RT should also be considered for cats with non–vaccine-associated sarcomas.
In two studies, one with 57 cats with vaccine-associated sarcomas treated primarily with wide surgical excision (4- to 5-cm lateral margins and one fascial layer for deep margins)216 and the other with 91 cats treated with aggressive surgery alone (5-cm lateral margins and two fascial layers for deep margins),207 prognostic factors for survival included histologic grade, local tumor recurrence, and distant metastasis. Local tumor recurrence was reported in 39% of the cats and was not associated with tumor diameter, tumor location, or histologic grade.216 The MST for cats with local tumor recurrence was 365 to 499 days compared to 1098 to 1461 days for cats without local tumor recurrence.207,216 The MST for cats without distant metastasis was 929 to 1528 days compared to 165 to 388 days for the 20% to 21% of cats that developed distant metastasis.207,216 Furthermore, distant metastasis was more likely in cats with grade III sarcomas, suggesting that chemotherapy should be considered for cats with high-grade vaccine-associated sarcomas.216
Prevention
The recommendations on preventing vaccine-associated sarcomas in cats are controversial. These include changing the sites of vaccine administration, decreasing the use of polyvalent vaccines, using nonadjuvanted vaccines, avoiding the use of aluminum-based adjuvants, and increasing the interval between vaccinations.24,178,235,236
The Vaccine-Associated Feline Sarcoma Task Force has recommended that no vaccine be administered in the interscapular region, rabies vaccines be administered in the distal aspect of the right pelvic limb, FeLV vaccines be administered in the distal aspect of the left pelvic limb, and all other vaccines be administered in the right shoulder.24 The location of each injection, the type of vaccine, and the manufacturer and serial number of the vaccine should be documented in the patient records. These recommendations are intended to provide epidemiologic information rather than prevent vaccine-associated sarcomas. Vaccines should be administered into the distal, rather than mid to proximal, aspects of the limb to aid in earlier detection and increase the chance of achieving complete resection. Subcutaneous and intramuscular administration can both cause local inflammatory reactions and result in the development of vaccine-associated sarcomas.24 Subcutaneous administration is preferred to intramuscular injection because vaccine-associated sarcomas developing from subcutaneous sites are more readily palpable and diagnosed earlier in the course of disease. The Vaccine-Associated Feline Sarcoma Task Force has recommended that masses at vaccination sites be treated if the mass is evident 3 or more months after vaccination, is larger than 2 cm in diameter, or is increasing in size more than 4 weeks after vaccine administration.185 Unfortunately, feline vaccine-associated sarcomas are still occurring at sites not recommended by the Vaccine-Associated Feline Sarcoma Task Force and are often difficult to cure with surgery and RT.237
Traditionally, annual boosters have been recommended for most vaccines in cats. The U.S. Department of Agriculture–Animal Plant Health Inspection Service (USDA-APHIS) does not require duration of immunity studies for licensing of vaccines. However, if a duration of immunity study has not been performed, the USDA-APHIS requires that vaccine labels include a recommendation for annual revaccination.188 Recently, vaccine practices have been questioned by the profession and this has been supported by duration of immunity studies. The duration of immunity for a single commercially available inactivated, adjuvanted combination of feline panleukopenia, herpesvirus, and calicivirus is greater than 7 years with persistent antibodies against all three viruses for more than 3 years.238,239 Local and state requirements often mandate annual rabies boosters, despite a duration of immunity of at least 3 years, because of the significant public health concern of rabies infection.188 The Vaccine-Associated Feline Sarcoma Task Force has recommended that the administration of vaccines is a medicinal procedure and vaccination protocols should be customized for individual cats.188 A vaccine should not be administered until the medical importance and zoonotic potential of the infectious agent, risk of exposure, and legal requirements have been considered and balanced against the risk of vaccine-associated sarcomas and other adverse effects.188
Comparative Aspects
In general, soft tissue sarcomas have a similar pathologic appearance, clinical presentation, and behavior in humans and animals. However, a higher incidence is seen in young people as opposed to young companion animals, with the exception of rhabdomyosarcoma, which is seen in young dogs. The distribution of soft tissue sarcoma in humans is similar to animals. In humans, 43% are in the extremities, with two-thirds occurring in the lower limb, and 34% are intraperitoneal, with 19% visceral in origin and 15% retroperitoneal. Soft tissue sarcomas of the trunk occur in 10% of human patients, and the remaining 13% occur at other sites. Metastasis is generally hematogenous and appears to be more common in human soft tissue sarcoma than in dogs, which may partially be explained by the higher numbers of nerve sheath tumors (with lower metastatic rate) seen in the dog.
Most sarcomas recognized in humans are also diagnosed in animals, although the specific incidences may vary markedly. There are many more histologic subtypes recognized in humans, which are often site dependent. With the exception of benign smooth muscle tumors and subcutaneous lipomas, there is little evidence that these lesions arise from their mature (differentiated) tissue counterparts. One current theory is that switching on a set of genes that programs mesenchymal differentiation in any mesenchymal cell may give rise to any type of mesenchymal tumor. Common subtypes of soft tissue sarcomas seen in the extremities of humans are liposarcomas, malignant fibrous histiosarcomas, synovial cell sarcomas, and fibrosarcomas. In the retroperitoneal location, liposarcomas and leiomyosarcomas are the most common histotypes noted in humans. The most common subtype noted viscerally are GISTs. Overall, leiomyosarcoma is the most common genitourinary sarcoma. Ten percent to 15% of all sarcomas occur in children, and the subtypes most commonly represented are rhabdomyosarcomas, Ewing’s sarcoma, and primitive neuroectodermal tumors.
Prognostic variables in humans include clinical stage, histologic grade, necrosis, site, size, lymph node involvement, and aggressiveness of surgery or radiation. The histopathologic grading system used and shown to be predictive for metastasis in dogs is a grading system adopted from human pathology that is also predictive for survival.24 Additionally, it appears that histologic grade is the predominant predictor of early recurrence, whereas tumor size plays a more important role for late recurrence. It is unclear whether age plays a prognostic role in human soft tissue sarcoma.
Surgical treatment is the mainstay of therapy for soft tissue sarcoma in the control of local disease. However, aggressive surgical resection combined with RT and chemotherapy, used before or after resection, allows effective limb-sparing treatment in 90% of patients. Amputation is reserved for patients with unresectable tumors, no evidence of metastasis, and the potential for good long-term rehabilitation. Local recurrence is greater in those patients undergoing limb-sparing therapies as compared to amputation; however, there is no difference in disease-free survival between the two groups. Since size is a prognostic factor for both local recurrence and metastasis, the recommended treatment is different. In patients with tumors smaller than 5 cm, complete surgical resection with 1-cm margins is usually sufficient. Larger lesions are treated with a combination of therapies, including chemotherapy and RT. RT techniques include external-beam RT and/or brachytherapy. Because half the human patients develop distant metastasis, despite local control, adjuvant chemotherapy is often used (doxorubicin alone or in combination with other chemotherapeutics). Ifosfamide is currently the most active salvage agent for patients who have failed doxorubicin-based protocols. Metaanalysis of combination therapy for soft tissue sarcoma reports a disease-free survival of 52% and an overall survival of 57% with a median follow-up of 9.4 years. Permanent local control with the first treatment is related to long-term survival. High-risk soft tissue sarcoma patients are treated with combined chemoradiation prior to surgical resection. The chemotherapy protocol often used is doxorubicin, ifosfamide, mesna, and dacarbazine. Local and systemic toxicity included expected wound-healing complications. In human patients with metastatic disease, combination chemotherapy produces response rates of 20%, and most patients are candidates for investigational agents.
References
1. Theilen, GH, Madewell, BR. Tumors of the skin and subcutaneous tissues. In: Theilen GH, Madewell BR, eds. Veterinary cancer medicine. Philadelphia: Lea & Febiger, 1979.
2. Dorn, ER. Epidemiology of canine and feline tumors. J Am Anim Hosp Assoc. 1976;12:307–312.
3. Hardy, WD. The etiology of canine and feline tumors. J Am Anim Hosp Assoc. 1976;12:313–334.
4. Madewell, BR, Theilen, GH. Etiology of cancer in animals. In: Theilen GH, Madewell BR, eds. Veterinary cancer medicine. Philadelphia: Lea & Febiger; 1979:13–25.
5. McCarthy, PE, Hedlund, CS, Veazy, RS, et al. Liposarcoma associated with a glass foreign body in a dog. J Am Vet Med Assoc. 1996;209:612–614.
6. Thrall, DE, Goldschmidt, MH, Biery, DN. Malignant tumor formation at the site of previously irradiated acanthomatous epulides in four dogs. J Am Vet Med Assoc. 1981;178:127–132.
7. Johnstone, PA, Laskin, WB, DeLuca, AM, et al. Tumors in dogs exposed to experimental intraoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:853–857.
8. Barnes, M, Duray, P, DeLuca, A, et al. Tumor induction following intraoperative radiotherapy: late results of the National Cancer Institute canine trials. Int J Radiat Oncol Biol Phys. 1990;19:651–660.
9. Sinibaldi, K, Rosen, H, Liu, SK, et al. Tumors associated with metallic implants in animals. Clin Orthop. 1976;118:257–266.
10. McGlennon, NJ, Houlton, JEF, Gorman, NT. Synovial cell sarcoma-a review. J Small Anim Pract. 1988;29:139–152.
11. Murray, JA. Synovial sarcoma. Orthop Clin North Am. 1977;8:963–972.
12. Vail, DM, Powers, BE, Getzy, DM, et al. Evaluation of prognostic factors for dogs with synovial cell sarcoma: 36 cases (1986-1991). J Am Vet Med Assoc. 1994;205:1300–1307.
13. Kim, DY, Hodgin, EC, Cho, DY, et al. Juvenile rhabdomyosarcomas in two dogs. Vet Pathol. 1996;33:447–450.
14. Duda, RB. Review: Biology of mesenchymal tumors. Cancer J. 1994;7:52–62.
15. Thrall, DE, Gillette, EL. Soft-tissue sarcomas. Semin Vet Med Surg Small Anim. 1995;10:173–179.
16. Kuntz, CA, Dernell, WS, Powers, BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996). J Am Vet Med Assoc. 1997;21:1147–1151.
17. Ordonez, NG. Immunocytochemistry in the diagnosis of soft-tissue sarcomas. Cancer Bull. 1993;45:13–23.
18. Madewell, BR, Munn, RJ. The soft tissue sarcomas: Immunohistochemical and ultrastructural distinctions. ACVIM Proc. 1991;9:717–720.
19. Ciekot, P, Powers, B, Withrow, S, et al. Histologically low-grade, yet biologically high-grade, fibrosarcoma of the mandible and maxilla in dogs: 25 cases (1982–1991). J Am Vet Med Assoc. 1994;204:610–615.
20. Zhang, P, Brooks, JS. Modern pathological evaluation of soft tissue sarcoma specimens and its potential role in soft tissue sarcoma research. Curr Treat Options Oncol. 2004;5:441–450.
21. Hogendoorn, PC, Collin, F, Daugaard, S, et al. Pathology and Biology Subcommittee of the EORTC Soft Tissue and Bone Sarcoma Group. Changing concepts in the pathological basis of soft tissue and bone sarcoma treatment. Eur J Cancer. 2004;40:1644–1654.
22. Nilbert, M, Engellau, J. Experiences from tissue microarray in soft tissue sarcomas. Acta Orthop Scand Suppl. 2004;75:29–34.
23. Dennis, MM, McSporran, KD, Bacon, NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol. 2011;48:73–84.
24. MacEwen, EG, Powers, BE, Macy, D, et al. Soft tissue sarcomas. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. Philadelphia: Saunders, 2001.
25. Cook, JL, Turk, JR, Pope, ER, et al. Infantile desmoid-type fibromatosis in an Akita puppy. J Am Anim Hosp Assoc. 1998;34:291–294.
26. Gwin, RM, Gelatt, KN, Peiffer, RL. Ophthalmic nodular fasciitis in the dog. J Am Vet Med Assoc. 1977;170:611–614.
27. Goldschmidt, MH, Hendrick, MJ. Tumors of the skin and soft tissue. In: Meuten DJ, ed. Tumors in domestic animals. Ames, Iowa: Iowa State Press, 2002.
28. Goldschmidt, MH, Shofer, FS. Skin tumors of the dog and cat. Oxford: Butterworth Heinemann; 1998.
29. Chijiwa, K, Uchida, K, Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet Pathol. 2004;41:307–318.
30. Brehm, D, Steinberg, H, Haviland, J, et al. A retrospective evaluation of 51 cases of peripheral nerve sheath tumors in the dog. J Am Anim Hosp Assoc. 1995;31:349–359.
31. Liggett, AD, Frazier, KS, Styler, EL. Angiolipomatous tumors in dogs and a cat. Vet Pathol. 2002;39:286–289.
32. Brodey, RS, Rozel, JF. Neoplasms of the canine uterus, vagina, vulva: a clinicopathologic survey of 90 cases. J Am Vet Med Assoc. 1967;151:1294–1307.
33. Woolfson, JM, Dulisch, ML, Tams, TR. Intrathoracic lipoma in a dog. J Am Vet Med Assoc. 1984;185:1007–1009.
34. Anderson, SM, Lippincott, CL. Intrathoracic lipoma in a dog. Calif Vet. 1989;43:9.
35. Umphlet, RC, Vicini, DS, Godshalk, CP. Intradural-extramedullary lipoma in a dog. Compend Contin Educ Pract Vet. 1989;11:1192.
36. McLaughlin, R, Kuzma, AB. Intestinal strangulation caused by intra-abdominal lipomas in a dog. J Am Vet Med Assoc. 1991;199:1610–1611.
37. Plummer, SB, Bunch, SE, Khoo, LH, et al. Tethered spinal cord and an intradural lipoma associated with a meningocele in a Manx-type cat. J Am Vet Med Assoc. 1993;203:1159–1161.
38. Head, KW, Else, RW, Dubielzig, RR. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in domestic animals. Ames, Iowa: Iowa State Press, 2002.
39. Mayhew, PD, Brockman, DJ. Body cavity lipomas in six dogs. J Small Anim Pract. 2002;43:177–181.
40. Gibbons, SE, Straw, RC. Intra-abdominal lipoma in a dog. Aust Vet Pract. 2003;33:86.
41. Gleiser, CA, Jardine, JH, Raulston, GL, et al. Infiltrating lipomas in the dog. Vet Pathol. 1979;16:623–624.
42. McChesney, AE, Stephens, LC, Lebel, J, et al. Infiltrative lipoma in dogs. Vet Pathol. 1980;17:316–322.
43. Doige, CE, Farrow, CS, Presnell, KR. Parosteal lipoma in a dog. J Am Anim Hosp Assoc. 1980;16:87.
44. Bergman, PJ, Withrow, SJ, Straw, RC, et al. Infiltrative lipoma in dogs: 16 cases (1981-1992). J Am Vet Med Assoc. 1994;205:322–324.
45. McEntee, MC, Page, RL, Mauldin, GN, et al. Results of irradiation of infiltrative lipoma in 13 dogs. Vet Radiol Ultrasound. 2000;41:554–556.
46. McEntee, MC, Thrall, DE. Computed tomographic imaging of infiltrative lipoma in 22 dogs. Vet Radiol Ultrasound. 2001;42:221–225.
47. Thomson, MJ, Withrow, SJ, Dernell, WS, et al. Intermuscular lipomas of the thigh region in dogs: 11 cases. J Am Anim Hosp Assoc. 1999;35:165–167.
48. Kramek, BA, Spackman, CJA, Hayden, DW. Infiltrative lipoma in three dogs. J Am Vet Med Assoc. 1985;186:81–82.
49. Frazier, KS, Herron, AJ, Dee, JF, et al. Infiltrative lipoma in a stifle joint. J Am Anim Hosp. 1993;29:81–83.
50. Baez, JL, Hendrick, MJ, Shofer, FS, et al. Liposarcomas in dogs: 56 cases (1989-2000). J Am Vet Med Assoc. 2004;224:887–891.
51. Brennan, MF, Singer, S, Maki, RG, et al. Soft tissue sarcoma. In: DeVita RT, Hellman S, Rosenberg SA, eds. Cancer: Principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2008.
52. Chang, HR, Hajdu, SI, Collin, C, et al. The prognostic value of histologic subtypes in primary extremity liposarcoma. Cancer. 1989;64:1514–1520.
53. Choong, PFM, Pritchard, DJ. Common malignant soft-tissue tumors. In: Simon MA, Springfield D, eds. Surgery for bone and soft-tissue tumors. Philadelphia: Lippincott-Raven, 1998.
54. Bruecker, KA, Withrow, SJ. Intestinal leiomyosarcomas in six dogs. J Am Anim Hosp Assoc. 1988;24:281.
55. Kapatkin, AS, Mullen, HS, Matthiesen, DT, et al. Leiomyosarcoma in dogs: 44 cases (1983-1988). J Am Vet Med Assoc. 1992;201:1077–1079.
56. Callanan, JJ, McCarthy, GM, McAllister, H. Primary pulmonary artery leiomyosarcoma in an adult dog. Vet Pathol. 2000;37:663–664.
57. Miller, MA, Ramos-Vara, JA, Dickerson, MF, et al. Uterine neoplasia in 13 cats. J Vet Diagn Invest. 2003;15:515–522.
58. Leifer, CE, Peterson, ME, Matus, RE, et al. Hypoglycemia associated with nonislet cell tumor in 13 dogs. J Am Vet Med Assoc. 1985;186:53–55.
59. Bagley, RS, Levy, JK, Malarkey, DE. Hypoglycemia associated with intra-abdominal leiomyoma and leiomyosarcoma in six dogs. J Am Vet Med Assoc. 1996;208:69–71.
60. Sato, K, Hikasa, Y, Morita, T, et al. Secondary erythrocytosis associated with high plasma erythropoietin concentrations in a dog with cecal leiomyosarcoma. J Am Vet Med Assoc. 2002;220:486–490.
61. Cohen, M, Post, GS, Wright, JC. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107–110.
62. Frost, D, Lasota, J, Miettinen, M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42–54.
63. Crawshaw, J, Berg, J, Sardinas, JC, et al. Prognosis for dogs with nonlymphomatous, small intestinal tumors treated by surgical excision. J Am Anim Hosp Assoc. 1998;34:451–456.
64. Liu, SM, Mikaelian, I. Cutaneous smooth muscle tumors in the dog and cat. Vet Pathol. 2003;40:685–692.
65. Larock, RG, Ginn, PE. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol. 1997;34:303–311.
66. Maas, CP, ter Haar, G, van der Gaag, I, et al. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation. Vet Surg. 2007;36:302–313.
67. Russell, KN, Mehler, SJ, Skorupski, KA, et al. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990-2003). J Am Vet Med Assoc. 2007;230:1329–1333.
68. Kerpsack, SJ, Birchard, SJ. Removal of leiomyomas and other noninvasive masses from the cardiac region of the canine stomach. J Am Anim Hosp Assoc. 1994;30:500.
69. Kontogianni, K, Demonakou, M, Kavantzas, N, et al. Prognostic predictors of gastrointestinal stromal tumors: a multi-institutional analysis of 102 patients with definition of a prognostic index. Eur J Surg Oncol. 2003;29:548–556.
70. Cooper, BJ, Valentine, BA. Tumors of muscle. In: Meuten DJ, ed. Tumors in domestic animals. Ames, Iowa: Iowa State Press, 2002.
71. Kobayashi, M, Sakai, H, Hirata, A, et al. Expression of myogenic regulating factors, myogenin and MyoD, in two canine botryoid rhabdomyosarcomas. Vet Pathol. 2004;41:275–277.
72. Russell, HV, Pappo, AS, Nuchtern, JG, et al. Solid tumors of childhood. In: DeVita RT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2008.
73. Senior, DF, Lawrence, DT, Gunison, C, et al. Successful treatment of botryoid rhabdomyosarcoma in the bladder of a dog. J Am Anim Hosp Assoc. 1993;29:386.
74. Block, G, Clarke, K, Salisbury, SK, et al. Total laryngectomy and permanent tracheostomy for treatment of laryngeal rhabdomyosarcoma in a dog. J Am Anim Hosp Assoc. 1995;31:510–513.
75. Lascelles, BDX, McInnes, E, Dobson, JM, et al. Rhabdomyosarcoma of the tongue in a dog. J Small Anim Pract. 1998;39:587–591.
76. Ueno, H, Kadosawa, T, Isomura, H, et al. Perianal rhabdomyosarcoma in a dog. J Small Anim Pract. 2002;43:217–220.
77. Takiguchi, M, Watanabe, T, Okada, H, et al. Rhabdomyosarcoma (botryoid sarcoma) of the urinary bladder in a Maltese. J Small Anim Pract. 2002;43:269–271.
78. Kelly, WR, Wilkinson, GT, Allen, PW. Canine angiosarcoma (lymphangiosarcoma): A case report. Vet Pathol. 1981;18:224–227.
79. Walsh, K, Abbott, DP. Lymphangiosarcoma in two cats. J Comp Pathol. 1984;94:611–614.
80. Diessler, ME, Castellano, MC, Massone, AR, et al. Cutaneous lymphangiosarcoma in a young dog: clinical, anatomopathological and lectin histochemical description. J Vet Med A Physiol Pathol Clin Med. 2003;50:452–456.
81. Itoh, T, Mikawa, K, Mikawa, M, et al. Lymphangiosarcoma in a dog treated with surgery and chemotherapy. J Vet Med Sci. 2004;66:197–199.
82. Widmer, WR, Carlton, WW. Persistent hematuria in a dog with renal hemangioma. J Am Vet Med Assoc. 1990;197:237–239.
83. Mott, JCP, McAnulty, JF, Darien, DL, et al. Nephron sparing by partial median nephrectomy for treatment of renal hemangioma in a dog. J Am Vet Med Assoc. 1996;208:1274–1276.
84. Schoofs, SH. Lingual hemangioma in a puppy: a case report and literature review. J Am Anim Hosp Assoc. 1997;33:161–165.
85. Liptak, JM, Dernell, WS, Ehrhart, EJ, et al. Retroperitoneal sarcomas in dogs: 14 cases (1992-2002). J Am Vet Med Assoc. 2004;224:1471–1477.
86. Ward, H, Fox, LE, Calderwood-Mays, MB, et al. Cutaneous hemangiosarcoma in 25 dogs: a retrospective study. J Vet Intern Med. 1994;8:345–346.
87. Bulakowski, EJ, Philibert, JC, Siegel, S, et al. Evaluation of outcome associated with subcutaneous and intramuscular hemangiosarcoma treated with adjuvant doxorubicin in dogs: 21 cases (2001-2006). J Am Vet Med Assoc. 2008;233:122–128.
88. Wiley, JL, Rook, KA, Clifford, CA, et al. Efficacy of doxorubicin-based chemotherapy for non-resectable canine subcutaneous haemangiosarcoma. Vet Comp Oncol. 2010;8:221–233.
89. Miller, MA, Ramos, JA, Kreeger, JM. Cutaneous vascular neoplasia in 15 cats: clinical, morphologic, and immunohistochemical studies. Vet Pathol. 1992;29:329–336.
90. McAbee, KP, Ludwig, LL, Bergman, PJ, et al. Feline cutaneous hemangiosarcoma: a retrospective study of 18 cases (1998-2003). J Am Anim Hosp Assoc. 2005;41:110–116.
91. Scavelli, TD, Patnaik, AK, Mehlhall, CJ, et al. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J Am Vet Med Assoc. 1985;187:817–819.
92. Pool, RR, Thompson, KG. Tumors of joints. In: Meuten DJ, ed. Tumors in domestic animals. Ames, Iowa: Iowa State Press, 2002.
93. Craig, LE, Krimer, PM, Cooley, AJ. Canine synovial myxoma: 39 cases. Vet Pathol. 2010;47(5):931–936.
94. Craig, LE, Julian, ME, Ferracone, JD. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol. 2002;39:66–73.
95. Fox, DB, Cook, JL, Kreeger, JM, et al. Canine synovial cell sarcoma: a retrospective assessment of described prognostic criteria in 16 cases (1994-1999). J Am Anim Hosp Assoc. 2002;38:347–355.
96. Liptak, JM, Withrow, SJ, Macy, DW, et al. Metastatic synovial cell sarcoma in two cats. Vet Comp Oncol. 2004;2:164–170.
97. Tilmant, LL, Gorman, NT, Ackerman, N, et al. Chemotherapy of synovial cell sarcoma in a dog. J Am Vet Med Assoc. 1986;188:530–532.
98. Foale, RD, White, RA, Harley, R, et al. Left ventricular myxosarcoma in a dog. J Small Anim Pract. 2003;44:503–507.
99. Briggs, OM, Kirberger, RM, Goldberg, NB. Right atrial myxosarcoma in a dog. J S Afr Vet Assoc. 1997;68:144–146.
100. Richter, M, Stankeova, S, Hauser, B, et al. Myxosarcoma in the eye and brain in a dog. Vet Ophthalmol. 2003;6:183–189.
101. Spangler, WL, Culbertson, MR, Kass, PH. Primary mesenchymal (nonangiomatous/nonlymphomatous) neoplasms occurring in the canine spleen: anatomic classification, immunohistochemistry, and mitotic activity correlated with patient survival. Vet Pathol. 1994;31:37–47.
102. McDonald, RK, Helman, RG. Hepatic malignant mesenchymoma in a dog. J Am Vet Med Assoc. 1986;188:1052–1053.
103. Hahn, KA, Richardson, RC. Use of cisplatin for control of metastatic malignant mesenchymoma and hypertrophic osteopathy in a dog. J Am Vet Med Assoc. 1989;195:351–353.
104. Carpenter, JL, Dayal, Y, King, NW, et al. Distinctive unclassified mesenchymal tumor of the digit of dogs. Vet Pathol. 1991;28:396–402.
105. Watson, AD, Young, KM, Dubielzig, RR, et al. Primary mesenchymal or mixed-cell-origin lung tumors in four dogs. J Am Vet Med Assoc. 1993;202:968–970.
106. Robinson, TM, Dubielzig, RR, McAnulty, JF. Malignant mesenchymoma associated with an unusual vasoinvasive metastasis in a dog. J Am Anim Hosp Assoc. 1998;34:295–299.
107. Beaudry, D, Knapp, D, Montgomery, T, et al. Hypoglycemia in four dogs with smooth muscle tumors. J Vet Intern Med. 1995;9:415–418.
108. Pletcher, JM, Dalton, L. Botryoid rhabdomyosarcoma in the urinary bladder of a dog. Vet Pathol. 1981;18:695–697.
109. Kuwamura, M, Yoshida, H, Yamate, J, et al. Urinary bladder rhabdomyosarcoma (sarcoma botryoides) in a young Newfoundland dog. J Vet Med Sci. 1998;60:619–621.
110. Baker-Gabb, M, Hunt, GB, France, MP. Soft tissue sarcomas and mast cell tumours in dogs: clinical behaviour and response to surgery. Aust Vet J. 2003;81:732–738.
111. Postorino, NC, Berg, RJ, Powers, BE, et al. Prognostic variables for canine hemangiopericytoma: 50 cases (1979-1984). J Am Anim Hosp Assoc. 1988;24:501–509.
112. Spangler, WL, Kass, PH. Splenic myeloid metaplasia, histiocytosis, and hypersplenism in the dog (65 cases). Vet Pathol. 1999;36:583–593.
113. Skorupski, KA, Clifford, CA, Paoloni, MC, et al. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Intern Med. 2007;21:121–126.
114. LeRoith, D, Clemmons, D, Nissley, P, et al. Insulin-like growth factors in health and disease. Ann Intern Med. 1992;116:854–862.
115. Cryer, P. Glucose homeostasis and hypoglycemia. In: Wilson J, Foster D, eds. Williams textbook of endocrinology. Philadelphia: Saunders, 1992.
116. Boari, A, Venturoli, M, Minuto, F. Non-islet-cell tumor hypoglycemia in a dog associated with high levels of insulin-like growth factor II. World Small Animal Vet Assoc Cong Proc. 1992;17:678.
117. Sugiura, H, Takahashi, M, Katagiri, H, et al. Additional wide resection of malignant soft tissue tumors. Clin Orthop. 2002;394:201–210.
118. Affolter, VK, Moore, PF. Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol. 2000;22:40–48.
119. Affolter, VK, Moore, PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83.
120. Greene, FL, Page, DL, Fleming, ID, et al. AJCC cancer staging manual. New York: Springer; 2002.
121. Dernell, WS, Withrow, SJ, Kuntz, CA, et al. Principles of treatment for soft tissue sarcoma. Clin Tech Small Anim Pract. 1998;13:59–64.
122. Banks, TA, Straw, RC, Withrow, SJ, et al. Prospective study of canine soft tissue sarcoma treated by wide surgical excision: quantitative evaluation of surgical margins. Vet Cancer Soc Proc. 2003;23:21.
123. Reimer, SB, Séguin, B, DeCock, HE, et al. Evaluation of the effect of routine histologic processing on the size of skin samples obtained from dogs. Am J Vet Res. 2005;66:500–505.
124. Ramanathan, RC, A’Hern, R, Fisher, C, et al. Prognostic index for extremity soft tissue sarcomas with isolated local recurrence. Ann Surg Oncol. 2001;8:278–289.
125. Stojadinovic, A, Leung, DHY, Hoos, A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434.
126. Zagars, GK, Ballo, MT, Pisters, PWT, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservative surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–2543.
127. Zagars, GK, Ballo, MT, Pisters, PWT, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer. 2003;97:2544–2553.
128. Eilber, FC, Rosen, G, Nelson, SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226.
129. Bacon, NJ, Dernell, WS, Ehrhart, N, et al. Evaluation of primary re-excision after inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999-2004). J Am Vet Med Assoc. 2007;230:548–554.
130. Chase, D, Bray, J, Ide, A, et al. Outcome following removal of canine spindle cell tumours in first opinion practice: 104 cases. J Small Anim Pract. 2009;50:568–574.
131. McSporran, KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol. 2009;46:928–933.
132. Stefanello, D, Morello, E, Roccabianca, P, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996-2006). Vet Surg. 2008;37:461–465.
133. Elmslie, RE, Glawe, P, Dow, SW. Metronomic chemotherapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Vet Intern Med. 2008;22:1373–1379.
134. McEntee, MC, Samii, VF, Walsh, P, et al. Postoperative assessment of surgical clip position in 16 dogs with cancer: a pilot study. J Am Anim Hosp Assoc. 2004;40:300–308.
135. Henry, CJ, Stoll, MR, Higginbotham, ML, et al. Effect of timing of radiation initiation on post-surgical wound healing in dogs. Vet Cancer Soc Proc. 2003;23:52.
136. Forrest, LJ, Chun, R, Adams, WM, et al. Postoperative radiotherapy for canine soft tissue sarcoma. J Vet Intern Med. 2000;14:578–582.
137. McKnight, JA, Mauldin, GN, McEntee, MC, et al. Radiation treatment of incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc. 2000;217:205–210.
138. Graves, GM, Bjorling, DE, Mahaffey, E. Canine hemangiopericytoma: 23 cases (1967-1984). J Am Vet Med Assoc. 1988;192:99–102.
139. Atwater, SW, LaRue, SM, Powers, BE, et al. Adjuvant radiation therapy of soft-tissue sarcomas in dogs. Vet Cancer Soc Proc. 1992;12:41.
140. Mauldin, GN, Meleo, KA, Burk, RL. Radiation therapy for the treatment of incompletely resected soft tissue sarcomas in dogs: 21 cases. Vet Cancer Soc Proc. 1993;13:111.
141. McChesney, SL, Withrow, SJ, Gillette, EL, et al. Radiotherapy of soft tissue sarcomas in dogs. J Am Vet Med Assoc. 1989;194:60–63.
142. Hilmas, DE, Gillett, EL. Radiotherapy of spontaneous fibrous connective-tissue sarcomas in animals. J Natl Cancer Inst. 1976;56:365–368.
143. Banks, WC, Morris, E. Results of radiation treatment of naturally occurring animal tumors. J Am Vet Med Assoc. 1975;166:1063–1064.
144. Richardson, RC, Anderson, VL, Voorhees, WD, et al. Irradiation-hyperthermia in canine hemangiopericytomas: Large-animal model for therapeutic response. J Natl Cancer Inst. 1984;73:1187–1194.
145. Lawrence, J, Forrest, L, Adams, W, et al. Four-fraction radiation therapy for macroscopic soft tissue sarcomas in 16 dogs. J Am Anim Hosp Assoc. 2008;44:100–108.
146. McChesney-Gillette, S, Dewhirst, MW, Gillette, EL, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: a randomized phase II study. Int J Hyperthermia. 1992;8:309–320.
147. Brewer, WG, Turrel, JM. Radiotherapy and hyperthermia in the treatment of fibrosarcomas in the dog. J Am Vet Med Assoc. 1982;181:146–150.
148. Thrall, D, Prescott, D, Samulski, T, et al. Radiation plus local hyperthermia versus radiation plus the combination of local and whole-body hyperthermia in canine sarcomas. Int J Radiation Oncol Biol Phys. 1996;34:1087–1096.
149. Mauldin, GN. Soft tissue sarcomas. Vet Clin North Am Small Anim Pract. 1997;27:139–148.
150. Viglianti, BL, Lora-Michiels, M, Poulson, JM, et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of clinical outcome in canine spontaneous soft tissue sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2009;15:4993–5001.
151. Simon, D, Ruslander, DM, Rassnick, KM, et al. Orthovoltage radiation and weekly low dose of doxorubicin for the treatment of incompletely excised soft-tissue sarcomas in 39 dogs. Vet Rec. 2007;160:321–326.
152. Kalnicki, S, Bloomer, W. Radiation therapy in the treatment of bone and soft tissue sarcomas. Philadelphia: Saunders; 1992.
153. MacLeod, DA, Thrall, DE. The combination of surgery and radiation in the treatment of cancer. A review. Vet Surg. 1989;18:1–6.
154. Cheng, EY, Dusenbery, KE, Winters, MR, et al. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99.
155. Strander, H, Turesson, I, Cavallin-Stahl, E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–531.
156. Rassnick, KM, Moore, AS, Russel, DS, et al. Phase II, open-label trial of single-agent CCNU in dogs with previously untreated histiocytic sarcoma. J Vet Intern Med. 2010;24:1528–1531.
157. Skorupski, KA, Rodriguez, CO, Krick, EL, et al. Long-term survival in dogs with localized histiocytic sarcoma treated with CCNU as an adjuvant to local therapy. Vet Comp Oncol. 2009;7:139–144.
158. Klahn, SL, Kitchell, BE, Dervisis, NG. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J Am Vet Med Assoc. 2011;239:90–96.
159. Rassnick, KM. Medical management of soft tissue sarcomas. Vet Clin North Am Small Anim Pract. 2003;33:517–531.
160. Ogilvie, GK, Reynolds, HA, Richardson, RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195:1580–1583.
161. Ogilvie, GK, Powers, BE, Mallinckrodt, CH, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;6:379–384.
162. Ogilvie, GK, Obradovich, JE, Elmslie, RE, et al. Efficacy of mitoxantrone against various neoplasms in dogs. J Am Vet Med Assoc. 1991;198:1618–1621.
163. Henry, CJ, Buss, MS, Potter, KA, et al. Mitoxantrone and cyclophosphamide combination chemotherapy for the treatment of various canine malignancies. J Am Anim Hosp Assoc. 1999;35:236–239.
164. Rassnick, KM, Frimberger, AE, Wood, CA, et al. Evaluation of ifosfamide for treatment of various canine neoplasms. J Vet Intern Med. 2000;14:271–276.
165. Sarcoma Meta-Analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647–1654.
166. Komdeur, R, Hoekstra, HJ, van den Berg, E, et al. Metastasis in soft tissue sarcomas: prognostic criteria and treatment perspectives. Cancer Metastasis Rev. 2002;21:167–183.
167. Selting, KA, Powers, BE, Thompson, LJ, et al. Outcome of dogs with high-grade soft tissue sarcomas treated with and without adjuvant doxorubicin chemotherapy: 39 cases (1996-2004). J Am Vet Med Assoc. 2005;227:1442–1448.
168. Dernell, WS, Withrow, SJ, Straw, RC, et al. Intracavitary treatment of soft tissue sarcomas in dogs using cisplatin in a biodegradable polymer. Anticancer Res. 1997;17:4499–4505.
169. Bostock, DE, Dye, MT. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet Pathol. 1980;17:581–588.
170. Evans, SM. Canine hemangiopericytoma: a retrospective analysis of response to surgery and orthovoltage radiation. Vet Radiol. 1987;28:13–16.
171. Hauck, M. Feline injection site sarcomas. Vet Clin North Am Small Anim Pract. 2003;33:553–557.
172. Hendrick, MJ, Goldschmidt, MH, Shofer, FS, et al. Postvaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992;52:5391–5394.
173. Hendrick, MJ, Goldschmidt, MH. Do injection site reactions induce fibrosarcomas in cats? J Am Vet Med Assoc. 1991;199:968.
174. Hendrick, MJ, Shofer, FS, Goldschmidt, MH, et al. Comparison of fibrosarcomas that developed at vaccination sites and at nonvaccination sites in cats: 239 cases (1991-1992). J Am Vet Med Assoc. 1994;205:1425–1429.
175. Kass, PH, Barnes, WG, Spangler, WL, et al. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc. 1993;203:396–405.
176. Macy, DW, Hendrick, MJ. The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin North Am Small Anim Pract. 1996;26:103–109.
177. Lester, S, Clemett, T, Burt, A. Vaccine site-associated sarcomas in cats: clinical experience and a laboratory review (1982-1993). J Am Anim Hosp Assoc. 1996;32:91–95.
178. Hendrick, MJ, Kass, PH, McGill, LD, et al. Postvaccinal sarcomas in cats. J Natl Cancer Inst. 1994;86:341–343.
179. Coyne, MJ, Reeves, NCP, Rosen, DK, et al. Estimated prevalence of injection sarcomas in cats during 1992. J Am Vet Med Assoc. 1997;210:249–251.
180. Gober, GM, Kass, PH. World Wide Web-based survey of vaccination practices, postvaccinal reactions, and vaccine site-associated sarcomas in cats. J Am Vet Med Assoc. 2002;220:1477–1482.
181. Reference deleted in pages.
182. Kass, PH, Spangler, WL, Hendrick, MJ, et al. Multicenter case-control study of risk factors associated with development of injection-site sarcomas in cats. J Am Vet Med Assoc. 2003;223:1283–1292.
183. Hendrick, MJ. Feline injection-site sarcomas. Cancer Invest. 1999;17:273–274.
184. Dubielzig, RR, Hawkins, KL, Miller, PE. Myofibroblastic sarcoma originating at the site of rabies vaccination in a cat. J Vet Diagn Invest. 1993;5:637–638.
185. McEntee, MC, Page, RL. Feline injection-site sarcomas. J Vet Intern Med. 2001;15:176–182.
186. McNiel, EA. Vaccine-associate sarcomas in cats: a unique cancer model. Clin Orthop. 2001;382:21–27.
187. Séguin, B. Injection site sarcomas in cats. Clin Tech Small Anim Pract. 2002;17:168–173.
188. Morrison, WB, Starr, RM. Injection-site Feline Sarcoma Task Force: Injection-site feline sarcomas. J Am Vet Med Assoc. 2001;218:697–702.
189. Peiffer, RL, Monticello, T, Bouldin, TW. Primary ocular sarcomas in the cat. J Small Anim Pract. 1988;29:105.
190. Dubielzig, RR, Everitt, J, Shadduck, JA, et al. Clinical and morphologic features of post-traumatic ocular sarcomas in cats. Vet Pathol. 1990;27:62–65.
191. Hakanson, N, Forrester, SD. Uveitis in the dog and cat. Vet Clin North Am Small Anim Pract. 1990;20:715–735.
192. Hendrick, MJ, Brooks, JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol. 1994;31:126–129.
193. Hendrick, MJ. Feline injection-site sarcomas: current studies on pathogenesis. J Am Vet Med Assoc. 1998;213:1425–1426.
194. Ellis, JA, Jackson, ML, Bartsch, RC, et al. Use of immunohistochemistry and polymerase chain reaction for detection of oncornaviruses in formalin-fixed, paraffin-embedded fibrosarcomas from cats. J Am Vet Med Assoc. 1996;209:767–771.
195. Mayr, B, Reifinger, M, Alton, K, et al. Novel p53 tumour suppressor mutations in cases of spindle cell sarcoma, pleomorphic sarcoma and fibrosarcoma in cats. Vet Res Comm. 1998;22:249–255.
196. Mayr, B, Blauesnsteiner, J, Edlinger, A, et al. Presence of p53 mutations in feline neoplasms. Res Vet Sci. 2000;68:63–70.
197. Hershey, AE, Dubielzig, RR, Padilla, ML, et al. Aberrant p53 expression in feline vaccine-associated sarcomas and correlation with prognosis. Vet Pathol. 2005;42:805–811.
198. Nieto, A, Sánchez, MA, Martínez, E, et al. Immunohistochemical expression of p53, fibroblast growth factor-b and transforming growth factor-α in feline injection-site sarcomas. Vet Pathol. 2003;40:651–658.
199. Katayama, R, Huelsmeyer, MK, Marr, AK, et al. Imatinib mesylate inhibits platelet-derived growth factor activity and increases chemosensitivity in feline injection-site sarcoma. Cancer Chemother Pharmacol. 2004;54:25–33.
200. Esplin, DG, Bigelow, M, McGill, LD, et al. Fibrosarcoma at the site of a lufenuron injection in a cat. Vet Cancer Soc Newsletter. 1999;23:8.
201. Daly, MK, Saba, CF, Crochik, SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg. 2008;10:202–205.
202. Carminato, A, Vascellari, M, Marchioro, W, et al. Microchip-associated fibrosarcoma in a cat. Vet Dermatol. 2011;22(6):565–569.
203. Moore, PF, Affolter, VK. Canine and feline histiocytic diseases. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. St Louis: Elsevier Saunders, 2005.
204. Heldmann, E, Anderson, MA, Wagner-Mann, C. Feline osteosarcoma: 145 cases (1990-1995). J Am Anim Hosp Assoc. 2000;36:518–521.
205. Cohen, M, Wright, JC, Brawner, WR, et al. Use of surgery and electron beam irradiation, with or without chemotherapy, for treatment of injection-site sarcomas in cats: 78 cases (1996-2000). J Am Vet Med Assoc. 2001;219:1582–1589.
206. Doddy, FD, Glickman, LT, Glickman, NW, et al. Feline fibrosarcomas at vaccination sites and nonvaccination sites. J Comp Pathol. 1996;114:165–174.
207. Phelps, HA, Kuntz, CA, Milner, RJ, et al. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998-2002). J Am Vet Med Assoc. 2011;239:97–106.
208. Esplin, DG, McGill, LD, Meininger, AC, et al. Postvaccination sarcomas in cats. J Am Vet Med Assoc. 1993;202:1245–1247.
209. Madewell, BR, Griffey, SM, McEntee, MC, et al. Feline injection-site fibrosarcoma: an ultrastructural study of 20 tumors (1996-1999). Vet Pathol. 2001;38:196–202.
210. Couto, SS, Griffey, SM, Duarte, PC, et al. Feline injection-site fibrosarcoma: morphologic distinctions. Vet Pathol. 2002;39:33–41.
211. Davidson, EB, Gregory, CR, Kass, PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg. 1997;26:265–269.
212. Hershey, AE, Sorenmo, KU, Hendrick, MJ, et al. Prognosis for presumed feline injection-site sarcoma after excision: 61 cases (1986-1996). J Am Vet Med Assoc. 2000;216:58–61.
213. Brown, NO, Patnaik, AK, Mooney, S, et al. Soft tissue sarcomas in the cat. J Am Vet Med Assoc. 1978;173:744–749.
214. Kahler, S. Collective effort needed to unlock factors related to feline injection site sarcomas. J Am Vet Med Assoc. 1993;202:1551–1554.
215. Lidbetter, DA, Williams, FA, Krahwinkel, DJ, et al. Radical lateral body-wall resection for fibrosarcoma with reconstruction using polypropylene mesh and a caudal superficial epigastric axial pattern flap: a prospective clinical study of the technique and results in 6 cats. Vet Surg. 2002;31:57–64.
216. Romanelli, G, Marconato, L, Olivero, D, et al. Analysis of prognostic factors associated with injection-site sarcomas in cats: 57 cases (2001-2007). J Am Vet Med Assoc. 2008;232:1193–1199.
217. Cronin, K, Page, RL, Spodnick, G, et al. Radiation therapy and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound. 1998;39:51–56.
218. Bregazzi, VS, LaRue, SM, McNiel, E, et al. Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with injection-site sarcomas: 25 cases (1995-2000). J Am Vet Med Assoc. 2001;218:547–550.
219. Kobayashi, T, Hauck, ML, Dodge, R, et al. Preoperative radiotherapy for injection-site sarcoma in 92 cats. Vet Radiol Ultrasound. 2002;43:473–479.
220. Eckstein, C, Guscetti, F, Roos, M, et al. A retrospective analysis of radiation therapy for treatment of feline vaccine-associated sarcoma. Vet Comp Oncol. 2009;7:54–68.
221. Kleiter, M, Tichy, A, Willmann, M, et al. Concomitant liposomal doxorubicin and daily palliative radiation therapy in advanced feline soft tissue sarcomas. Vet Radiol Ultrasound. 2010;51:349–355.
222. Williams, LE, Banerji, N, Klausner, JS, et al. Establishment of two injection-site feline sarcoma cell lines and determination of in vitro chemosensitivity to doxorubicin and mitoxantrone. Am J Vet Res. 2001;62:1354–1357.
223. Banerji, N, Li, X, Klausner, JS, et al. Evaluation of in vitro chemosensitivity of injection-site feline sarcoma cell lines to vincristine and paclitaxel. Am J Vet Res. 2002;63:728–732.
224. Barber, LG, Sorenmo, KU, Cronin, KL, et al. Combined doxorubicin and cyclophosphamide chemotherapy for nonresectable feline fibrosarcoma. J Am Anim Hosp Assoc. 2000;36:416–421.
225. Poirier, VJ, Thamm, DH, Kurzman, ID, et al. Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of injection-site sarcomas in cats. J Vet Intern Med. 2002;16:726–731.
226. Spugnini, EP, Baldi, A, Vincenzi, B, et al. Intraoperative versus postoperative electrochemotherapy in high grade soft tissue sarcomas: a preliminary study in a spontaneous feline model. Cancer Chemother Pharmacol. 2007;59:375–381.
227. Jourdier, TM, Moste, C, Bonnet, MC, et al. Local immunotherapy of spontaneous feline fibrosarcomas using recombinant poxviruses expressing interleukin 2 (IL2). Gene Ther. 2003;10:2126–2132.
228. Jahnke, A, Hirschberger, J, Fischer, C, et al. Intra-tumoral gene delivery of feIL-2, feIFN-gamma and feGM-CSF using magnetofection as a neoadjuvant treatment option for feline fibrosarcoma: a phase I trial. J Vet Med A Physiol Pathol Clin Med. 2007;54:599–606.
229. Hüttinger, C, Hirschberger, J, Jahnke, A, et al. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: a phase I trial. J Gene Med. 2008;10:655–667.
230. Kent, EM. Use of an immunostimulant as an aid in treatment and management of fibrosarcoma in three cats. Fel Pract. 1993;21:13.
231. King, GK, Yates, KM, Greenlace, PG, et al. The effect of acemannan immunostimulant in combination with surgery and radiation therapy on spontaneous canine and feline fibrosarcomas. J Am Anim Hosp Assoc. 1995;31:439–447.
232. Quintin-Colonna, F, Devauchelle, P, Fradelizi, D, et al. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleukin-2. Gene Ther. 1996;3:1104–1112.
233. Giudice, C, Stefanello, D, Sala, M, et al. Feline injection-site sarcoma: recurrence, tumour grading and surgical margin status evaluated using the three-dimensional histological technique. Vet J. 2010;186:84–88.
234. Dillon, CJ, Mauldin, GN, Baer, KE. Outcome following surgical removal of nonvisceral soft tissue sarcomas in cats: 42 cases (1992-2000). J Am Vet Med Assoc. 2005;227:1955–1957.
235. Macy, DW. Current understanding of vaccination site-associated sarcomas in the cat. J Feline Med Surg. 1999;1:15–21.
236. Martano, M, Morello, E, Buracco, P. Feline injection-site sarcoma: past, present and future perspectives. Vet J. 2011;1888:136–141.
237. Shaw, SC, Kent, MS, Gordon, IK, et al. Temporal changes in characteristics of injection-site sarcomas in cats: 392 cases (1990-2006). J Am Vet Med Assoc. 2009;234:376–380.
238. Scott, FW, Geissinger, CM. Duration of immunity in cats vaccinated with an inactivated feline panleukopenia, herpesvirus, and calicivirus vaccine. Fel Pract. 1997;25:12.
239. Scott, FW, Geissinger, CM. Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. Am J Vet Res. 1999;60:652–658.