Miscellaneous Tumors
 Section A
Section A
Hemangiosarcoma
Incidence and Risk Factors
Hemangiosarcoma (HSA), also known as malignant hemangioendothelioma or angiosarcoma, is a malignant neoplasm of vascular endothelial origin. HSA occurs more frequently in dogs than in any other species.1,2 It represents about 5% of all noncutaneous primary malignant neoplasms and 12% to 21% of all mesenchymal neoplasms in the dog.3-5 HSA accounts for 2.3% to 3.6% of skin tumors in dogs1,6 and 45% to 51% of splenic malignancies.2,7,8 It is less common in the cat, occurring in approximately 0.5% of cats examined at necropsy in one study and accounting for 2% of all neoplasms in another.9,10
HSA is seen mostly in middle-aged to older animals, although there are reports in dogs less than 3 years of age.7,10-14 German shepherds, golden retrievers, and Labrador retrievers are overrepresented in many case series.7,11,14-16 There may be a slight male predisposition in dogs.11,12,14,15,17
Although the etiology is unknown, reports in humans have been related to exposure to thorium dioxide, arsenicals, vinyl chloride, and androgens.18 It is reported as a rare late sequela to breast-conserving irradiation in humans with breast cancer.19 The human vascular neoplasm Kaposi’s sarcoma is causally associated with human herpesvirus 8, and although some studies have demonstrated herpesviral elements in human angiosarcoma as well, the majority have not.20-23 There is a documented increase in HSA development in dogs exposed to ionizing radiation prenatally or postnatally.24 Cutaneous HSAs are found more frequently in dogs with minimal pigmentation and thin hair coats25 and have been associated with ultraviolet light exposure in laboratory dogs.26
There is increasing evidence that dysregulation of molecular pathways governing angiogenesis may be important in the pathogenesis of HSA. Studies in humans and dogs have demonstrated abundant expression of angiogenic growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and angiopoietins-1 and -2 (Ang-1 and -2) in HSA cells and tissues and concomitant expression of the cellular receptors for VEGF, bFGF, and Ang-1.27-36 This suggests the potential for autocrine stimulation of one or more of these receptors leading to dysregulated proliferation and survival. Indeed, enforced overexpression of VEGF is sufficient to transform immortalized murine endothelial cells into HSA,37 and in vivo overexpression of VEGF has led to vascular tumor formation in mice.38
Mutations in tumor suppressor genes such as p53,39,40 Ras,41-43 and Tsc244 have likewise been implicated in the pathogenesis of HSA, based on murine or human studies. Recent studies suggest that p53 and Ras mutations are infrequent in canine HSA45-47; however, PTEN inactivation was demonstrated in greater than 50% of evaluated canine HSA samples in one study.48 Key growth- and apoptosis-regulating proteins such as pRB, cyclin D1, BCL2, and survivin appear overexpressed in HSA when compared with hemangiomas or normal tissues.46,49 Gene expression profiling studies indicate an enrichment for genes involved in inflammation and angiogenesis.47
Pathology and Natural Behavior
In the dog, the most common primary site for HSA is the spleen.1,11,12,14,15 Other frequent sites include the right atrium, skin and subcutis, and liver.11,14,25,50-53 Occurrence has also been reported in the lung, kidney, oral cavity, muscle, bone, urinary bladder, left ventricle, uterus, tongue, digit, and retroperitoneum.12,14,54-60 In the cat, cutaneous and visceral (e.g., spleen, liver, intestine) locations are evenly distributed.10,61 Other reported sites in the cat include the heart, thoracic cavity, eyelid, and nasal cavity.10,53,61-64 HSA is the most common splenic neoplasm encountered but is by no means the only differential for splenomegaly or splenic masses in dogs. The “double two-thirds rule” has been applied to canine splenic masses: approximately two-thirds of dogs with splenic masses will have a malignant tumor, and approximately two-thirds of those malignancies will be HSA2,8,17,65; however, recent studies have found that 63% to 70% of dogs with splenic masses presenting with nontraumatic hemoabdomen were HSA.66-68 Several splenic mass lesions (e.g., HSA, hematoma, hemangioma) can have a similar gross and ultrasonographic appearance, and large masses do not necessarily denote malignancy.69 Histopathology is necessary to establish a definitive diagnosis in these cases.
HSA may be solitary, multifocal within an organ, or widely disseminated at presentation. Grossly, they are of variable size, pale gray to dark red or purple, and soft or gelatinous, often containing blood-filled or necrotic areas on cut surface (Figure 33-1). They are poorly circumscribed, nonencapsulated, and often adhered to adjacent organs. They are extremely friable, and complications associated with rupture and hemorrhage are frequent presenting complaints. Histologically, HSA consists of immature, pleomorphic endothelial cells forming vascular spaces containing variable amounts of blood and/or thrombi.70,71 When such features are minimal but HSA is suspected, immunohistochemistry for von Willebrand’s factor (factor VIII–related antigen) or CD31/platelet endothelial cell-adhesion molecule (PECAM) can be used to demonstrate endothelial derivation and support the diagnosis of HSA.71-74 Recently, claudin-5 and CD117 (KIT) have also been identified as potentially useful immunohistochemical markers for distinguishing canine tumors of vascular endothelial origin.75,76
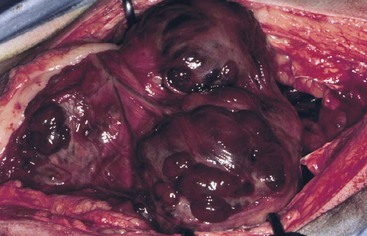
Figure 33-1 Intraoperative image of multifocal hemangiosarcoma (HAS) within the spleen of a dog. (Courtesy M.G. O’Brien, Madison, Wisconsin.)
Perhaps owing to its intimate association with the vasculature, facilitating extravasation and angiogenesis of metastatic clones, canine HSA is typified by very aggressive biologic behavior, with rapid and wide metastasis occurring frequently. An exception to this rule is pure cutaneous or dermal HSA, without any clinical or histologic evidence of subdermal infiltration.51 Metastasis is typically hematogenous or through transabdominal implantation following rupture. The most frequent metastatic sites are the liver, omentum, mesentery, and lungs.14,77 One study suggested that approximately, 25% of dogs with splenic HSA will also have right atrial involvement.77 However, the experience of the author suggests that this percentage at initial presentation is significantly lower (5% or less). Other reported sites of metastasis are the kidney, muscle, peritoneum, lymph nodes, adrenal gland, brain, and diaphragm. In dogs, HSA is considered the most common metastatic tumor to the brain.78,79
HSA in cats is considered to be a less aggressive disease. Cutaneous or subcutaneous HSA often behaves in a fashion similar to other soft tissue sarcomas, and local recurrence is the major concern.72,80,81 Reports do exist of more aggressive biologic behavior in a subset of cats with cutaneous HSA, however.80-82 Visceral HSAs have a higher metastatic rate, similar to that in dogs, and the common sites include the liver, omentum, diaphragm, pancreas, and lung.10,61,81,83,84
History and Clinical Signs
Presenting complaints vary, depending on the location of the primary tumor, and can range from vague, nonspecific signs of illness, to asymptomatic abdominal swelling, to acute collapse and death secondary to hemorrhagic/hypotensive shock. Common presenting complaints for visceral HSA are acute weakness or collapse. This may have been preceded by similar transient episodes occurring over a period of weeks or days, which resolved spontaneously in 12 to 36 hours. In such cases, it is theorized that HSA rupture leads to hemoperitoneum, followed by subsequent reabsorption (i.e., autotransfusion) of red blood cells. For those dogs that present asymptomatically or with vague signs (lethargy, inappetence, weight loss, abdominal distension), an abdominal mass may be palpated during examination. Dogs with cardiac HSA typically present with signs related to pericardial tamponade and associated right-sided heart failure, such as exercise intolerance, dyspnea, and ascites.
Common physical examination findings for visceral HSA include pale mucous membranes with delayed capillary refill, tachycardia with poor pulse quality, and a palpable fluid wave in the abdomen, with or without a palpable abdominal mass. Dogs with cardiac HSA may display ascites, muffled heart sounds, and/or pulsus paradoxus (variation in pulse quality associated with respiration).
In the cat, signs will depend on location and extent of tumor. Cats with visceral tumors usually have a history of lethargy, anorexia, vomiting, sudden collapse, dyspnea, or a distended abdomen.61,81,83 On physical examination, pallor, pleural or peritoneal fluid, and a palpable abdominal mass may be detected.
Diagnostic Techniques and Work-Up
Complete staging for an HSA suspect typically includes hematology and serum biochemistry, coagulation testing, thoracoabdominal imaging, ±abdominocentesis, and/or echocardiography.
In both dogs and cats, anemia is often seen, usually characterized by the presence of schistocytes (associated with microangiopathic hemolysis) and acanthocytes in the peripheral blood.* Anemia may be regenerative or nonregenerative, depending on duration. Blood typing and/or crossmatching may be indicated if surgery is planned in a severely anemic patient. In addition, a neutrophilic leukocytosis may be seen.61,66 Thrombocytopenia is observed in 75% to 97% of cases, ranging from mild to severe.66,67,86,87 Serum biochemistry changes are typically nonspecific and can include hypoalbuminemia, hypoglobulinemia, and mild elevations in liver enzymes.66,83
A coagulogram is very useful in animals with suspected HSA. Perturbations in some aspect of the coagulation cascade (prothrombin time [PT], activated partial thromboplastin time [APTT], platelet count, activated clotting time, fibrinogen concentration, fibrin degradation products) are seen in the majority of patients (Table 33-1)66,86-91 and approximately 50% have coagulation abnormalities that meet the criteria for disseminated intravascular coagulation (DIC).86,88 Tumor vasculature, especially in the case of HSA, differs significantly from normal vasculature, commonly containing blind-ended or irregular tortuous vessels, incomplete endothelial lining, arteriovenous shunts, exposed subendothelial collagen, and platelet-tumor aggregates.92 HSA-derived endothelial cells may also have an “activated” phenotype, further promoting initiation of the coagulation cascade.
Table 33-1
Coagulation Abnormalities in Dogs with Hemangiosarcoma

PT, Prothrombin time; APTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation.
Data from Pintar J, Breitschwerdt EB, Hardie EM, et al: Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987-2001), J Am Anim Hosp Assoc 39:518–522, 2003; Hammer AS, Couto CG, Swardson C, et al: Hemostatic abnormalities in dogs with hemangiosarcoma, J Vet Intern Med 5:11–14, 1991; Hargis AM, Feldman BF: Evaluation of hemostatic defects secondary to vascular tumors in dogs: 11 cases, J Am Vet Med Assoc 198:891–894, 1991; Maruyama H, Miura T, Sakai M, et al: The incidence of disseminated intravascular coagulation in dogs with malignant tumor, J Vet Med Sci 66:573–575, 2004; Mischke R, Wohlsein P, Schoon HA: Detection of fibrin generation alterations in dogs with haemangiosarcoma using resonance thrombography, Thromb Res 115:229–238, 2005; Rishniw M, Lewis DC: Localized consumptive coagulopathy associated with cutaneous hemangiosarcoma in a dog, J Am Anim Hosp Assoc 30:261–264, 1994; Maruyama H, Watari T, Miura T, et al: Plasma thrombin-antithrombin complex concentrations in dogs with malignant tumours, Vet Rec 156:839–840, 2005.
HSA effusions are serosanguineous (or frank blood) and usually do not clot. Cytology of effusions is rarely diagnostic; although tumor cells are likely present, they are heavily diluted with peripheral blood.
Screening for metastasis is mandatory prior to definitive surgery for HSA. Dogs with gross evidence of metastasis have a grave prognosis, and surgery is purely palliative. Thoracic radiographs should be obtained in all cases. One study reported a 78% sensitivity and 74% negative predictive value for detecting pulmonary manifestations of HSA, and obtaining three views significantly decreased the false-negative rate.93 Dogs with hemopericardium secondary to cardiac HSA will typically have a globoid cardiac silhouette, with or without distension of the caudal vena cava.94 Abdominal radiographs may reveal a cranial abdominal mass in many cases; however, abdominal ultrasound is a superior modality for imaging the abdomen. Many animals with visceral HSA will have ascites, which diminishes abdominal detail with radiographs but not ultrasound. Ultrasound also allows for the more thorough evaluation of the rest of the abdomen for evidence of metastatic disease. HSA typically has a heteroechoic appearance, ranging from anechoic/cavitated to hyperechoic, or a targetoid appearance (Figure 33-2).95,96
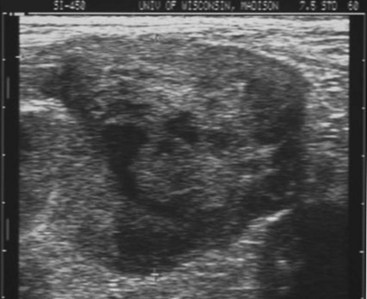
Figure 33-2 Ultrasound image of canine splenic hemangiosarcoma (HSA). (Courtesy R.T. O’Brien, University of Illinois, College of Veterinary Medicine.)
Typical electrocardiographic signs consistent with pericardial effusion (decreased amplitude QRS complex, electrical alternans) can be seen in dogs with cardiac HSA and hemopericardium. Echocardiography can be useful in dogs with suspected pericardial effusion, and a right atrial mass may be visible in 65% to 90% of cases (Figure 33-3)97-100; however, some dogs may develop blood clots within the pericardium that can have a mass effect. Thus the absence of a mass does not rule out HSA, and the presence of a mass is not pathognomonic. Despite this, detection of a mass on echocardiography is strongly associated with a worse prognosis in dogs with pericardial effusion.94,99 Other negative prognostic factors include history of collapse and presence of ascites.94 Routine evaluation of the heart for metastatic disease in a dog with subcutaneous or visceral HSA without evidence of other metastasis is rarely rewarding.
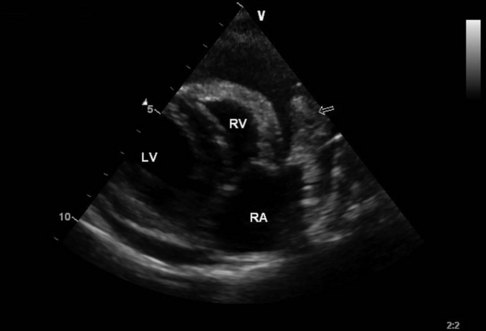
Figure 33-3 Echocardiographic image of right atrial hemangiosarcoma (HSA) with pericardial effusion. LV, Left ventricle; RV, right ventricle; RA, right atrium. Arrow indicates mass in region of right atrial appendage. (Courtesy J Boon, Colorado State University, Veterinary Teaching Hospital.)
A clinical staging system for HSA is given in Table 33-2.
Ultimately, a definitive diagnosis of HSA usually requires a surgical biopsy. Needle aspiration cytology of suspicious masses, although simple and cost-effective, is of low diagnostic utility due to the hemodilution that usually accompanies sampling.101 Needle core biopsies can be obtained of cutaneous or subcutaneous lesions but should not be performed on suspect visceral lesions due to the risk of induction of hemorrhage and subsequent seeding of the peritoneum. If only small samples of suspected HSA are submitted, histopathology may only reveal blood clots or other nonspecific findings. Submission of large samples of suspected HSA is preferred to maximize the likelihood of capturing a true tumor if present. This includes submission of entire spleens whenever possible or, at the very least, numerous parts of the mass.
Several investigators are evaluating additional tests, which may allow more accurate presurgical diagnosis. Pericardial fluid pH was suggested to correlate with presence or absence of neoplasia in one study102; however, subsequent studies have shown pericardial fluid analysis to be of little diagnostic benefit.103,104 Recent studies have found a significant difference in concentrations of troponin I, an indicator of myocardial damage, between dogs with cardiac HSA and dogs with idiopathic pericardial effusions.105,106 Plasma concentrations of VEGF and urine concentrations of bFGF are elevated in dogs with HSA versus normal controls27,28; however, neither was found to correlate with remission status, disease stage, or outcome. Advanced imaging techniques such as contrast-enhanced ultrasound,107-113 contrast-enhanced computed tomography (CT),114 and magnetic resonance imaging (MRI)115 have been useful in discriminating malignant versus benign lesions in preliminary studies. Additional blood-based biomarkers showing early promise include measurement of serum alpha-1 acid glycoprotein,116 lipocalin region of collagen XXVII alpha 1,117 and thymidine kinase activity,118,119 as well as multiparameter flow cytometry of peripheral blood utilizing a variety of endothelial and primitive hematopoietic cell markers.120
Treatment
Surgery remains the primary method of treatment for almost all dogs and cats with HSA. Prior to surgery, appropriate treatment for shock (e.g., crystalloids, colloids) and correction of severe hematologic or coagulation abnormalities should be addressed with blood products as necessary. Surgery should be as aggressive as possible to remove all locally affected tissue.
For cutaneous or subcutaneous HSA, surgical considerations are similar to those for other soft-tissue sarcomas (see Chapter 21). Dermal HSA is usually discrete, and surgical margins of 1 to 2 cm are often adequate. Intramuscular HSA will often present with a large blood clot admixed with actual tumor. Wide margins can be difficult to achieve, short of limb amputation when possible. Debulking may provide short-term relief.
For splenic HSA, splenectomy is required and can be performed with sutures, staples, or electrothermal vessel sealant devices (e.g., LigaSure Force Triad, Covidien, Mansfield, MA).121 The spleen should be carefully delivered from the abdomen to avoid iatrogenic rupture. Attached omentum should be removed en bloc. Intraoperative autotransfusion of blood has been described but requires specialized equipment and personnel and still remains controversial.122 At the time of splenectomy, the abdomen should be thoroughly explored and any suspicious lesions in the liver or omentum, especially if actively bleeding, should be excised and submitted for histopathology. Biopsy of grossly normal livers is probably not useful. The abdomen should be thoroughly lavaged and instruments changed prior to closure to minimize the risk of abdominal/wound bed or suture line seeding. Dogs undergoing splenectomy are prone to develop ventricular arrhythmias following surgery. In one study of 59 dogs, 24% developed arrhythmias.123 Poor myocardial perfusion secondary to hypoxia, hypovolemia, or anemia or a neurohormonal response associated with manipulation of the spleen are all potential causes. An electrocardiogram should be monitored intraoperatively and in the postoperative period, and arrhythmias treated as they arise. Arrhythmias usually resolve within 24 to 48 hours.
Surgery can be performed for primary cardiac HSA. An open or thoracoscopic pericardiectomy can be used as a palliative procedure, allowing effusion to escape into the thorax rather than accumulating in the pericardium, where a small volume can readily restrict function. Right atrial appendage masses can be resected with a stapling device or hand stitching,124,125 and reconstructive procedures have been described in cases where extensive resection is required.126,127 A recent retrospective study suggests that dogs surviving the perioperative period have a prognosis similar to dogs with HSA of other visceral sites.125
Chemotherapy
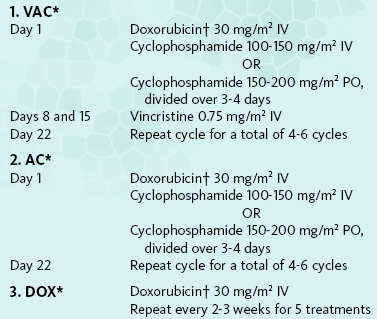
Given the very high metastatic rate of most canine HSA and the poor outcome associated with surgery alone (see later), adjuvant chemotherapy is indicated in all cases, with the exception of pure cutaneous tumors. Single-agent and combination doxorubicin (DOX)-based chemotherapy protocols are most frequently used (Box 33-1).128-134 Other combinations such as vincristine, cyclophosphamide, and methotrexate (VCM) have yielded only modest improvements in survival time.14 Recently, ifosfamide was shown to also have some modest antitumor activity in canine HSA.134,135 Both epirubicin and liposome-encapsulated DOX appear to have roughly equivalent activity to conventional DOX.136,137 In cats, similar DOX-based protocols are employed, although reports of systematic evaluations are lacking. For cases in which surgery is declined or impossible due to size/location or presence of metastasis, DOX-based chemotherapy induces meaningful tumor regression in a large minority of animals138-140; however, remissions are typically incomplete and brief in duration. Isolated reports of antitumor activity have been reported with carboplatin as well.141
Immunotherapy
Very few studies have been conducted to evaluate biologic therapy for HSA (see Chapter 13). One study used a mixed killed bacterial vaccine following surgery and showed some improvement in survival time in dogs with splenic HSA.14 More recently, a surgical adjuvant study was conducted in dogs with splenic HSA to compare chemotherapy (DOX and cyclophosphamide) to the same chemotherapy combined with immunotherapy using liposome-encapsulated muramyl tripeptide-phosphatidylethanolamine (L-MTP-PE). The median survival time (MST) for those dogs treated with chemotherapy alone was 5.7 months versus 9.1 months (p = 0.03) for those treated with L-MTP-PE and chemotherapy, with 40% of dogs in the L-MTP-PE group experiencing long-term survival.133 L-MTP-PE is currently unavailable in the United States but has been granted orphan drug status for the treatment of pediatric osteosarcoma in the European Union. Investigations of allogeneic tumor cell vaccine approaches in combination with chemotherapy have shown initial promise in small numbers of dogs.142
Radiation Therapy
Radiation therapy (RT) is rarely utilized for HSA due to the anatomic sites involved and high metastatic rate. Coarsely fractionated (palliative) RT for peripheral masses (e.g., cutaneous and subcutaneous lesions) may dramatically decrease local disease but may not significantly impact overall survival.143-145 It is conceivable that a combination of palliative RT and chemotherapy may provide better control, but this awaits further investigation. Full-course (definitive) RT may be reasonable for dogs with incompletely resected, solitary dermal HSA or feline nonvisceral HSA due to their reduced potential for metastasis.
Novel Therapies
Given the endothelial derivation of HSA, therapy directed against angiogenesis is a logical avenue of exploration, and murine HSA has been used as a model for the evaluation of novel antiangiogenic strategies. Antitumor activity has been seen in a murine HSA model to a variety of antiangiogenic treatments, such as VEGF receptor kinase inhibitors,146 TNP-470,147 and several others,148-150 and objective responses have been observed with several angiogenic receptor tyrosine kinase inhibitors in human reports.151,152 Interleukin-12 (IL-12) has been shown to retard tumor growth in a canine HSA xenograft as well.33
A small pilot study recently reported the outcome following treatment of dogs with splenic HSA with a combination of splenectomy, a nonsteroidal antiinflammatory drug (NSAID), and low-dose continuous (metronomic) chemotherapy with alternating courses of cyclophosphamide and etoposide. Outcome was similar to what has been reported following DOX-based injectable chemotherapy.153
Current studies are focusing on whether combinations of conventional DOX-based chemotherapy followed by “maintenance” therapy with low-dose continuous chemotherapy or VEGF receptor kinase inhibitors such as toceranib may improve postsurgical outcome in patients with HSA; the results of these studies are eagerly anticipated.
In humans with various vascular tumors, antitumor activity has been observed following the local administration of IL-2,154,155 and the local or systemic administration of interferon-α (IFNα) in combination with traditional cytotoxics.156-159 Additionally, paclitaxel, docetaxel, and gemcitabine appear to have activity against human HSA.157,160-162
Prognosis
A summary of the results of several reports on the treatment of splenic HSA is presented in Table 33-3. Overall, the prognosis for dogs with splenic HSA treated by surgery alone is extremely poor. MSTs for splenic HSA range from 19 to 86 days, and less than 10% survive to 12 months.2,8,14,163,164 Primary hepatic HSA is thought to carry an equally poor prognosis. In contrast, one study suggests that dogs with primary renal HSA may have a more favorable outcome than those with HSA of other visceral sites.60
Table 33-3
Comparison of Survival Times in Dogs with Splenic Hemangiosarcoma
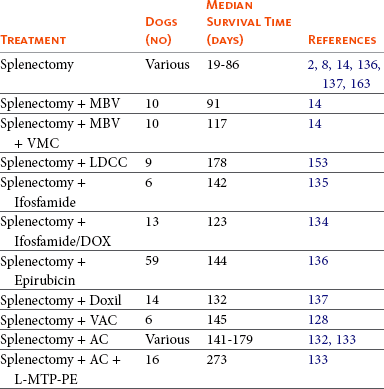
MBV, Mixed bacterial vaccine; VMC, vincristine, methotrexate, cyclophosphamide; LDCC, Low-dose, continuous chemotherapy; DOX, doxorubicin; VAC, vincristine, doxorubicin, cyclophosphamide; AC, doxorubicin, cyclophosphamide; L-MTP-PE, liposome muramyl tripeptide phosphatidylethanolamine.
Surgery plus anthracycline-based chemotherapy following surgery will increase median survival times to 141 to 179 days.* Even with the addition of chemotherapy, the 12-month survival percentage is 10% or less. In one report, the addition of immunotherapy (L-MTP-PE) to standard chemotherapy increased median survival time to 273 days.133 In splenic HSA, stage I (nonruptured) tumors may have a more favorable outcome than stage II (ruptured) tumors when postoperative chemotherapy is used.14,130,133 One study employed a histologic grading scheme, and demonstrated that dogs with low-grade tumors had a better prognosis than dogs with intermediate- or high-grade tumors.129
In a study of surgically treated cutaneous HSA, tumors involving the dermis (without subdermal invasion) had a MST of 780 days (n = 10).51 Tumors with invasion into the subcutaneous tissues or muscle generally have worse outcomes than those confined to the dermis.11,51,165,166 Therefore adjuvant medical therapy should be offered for subcutaneous or intramuscular HSA, although reports regarding efficacy are divergent.165,166
Overall, the prognosis is poor for cardiac HSA. In dogs undergoing surgery for right atrial HSA, the average survival time ranges from 1 to 4 months.99,124 A recent study reported an outcome following surgery and chemotherapy roughly equivalent to that reported for HSA of the spleen.125
Feline
In cats, the prognosis for visceral HSA is poor. Most cats die from recurrence of the primary tumor or metastasis, and MSTs are generally short, owing to metastasis.61,84 HSAs located in cutaneous and subcutaneous sites have recurrence rates of 60% to 80%,61,72 although one recent case series reported a favorable outcome following aggressive surgery, and complete excision is associated with improvement in outcome.80,81 Metastasis can develop following surgical resection in some cases, although the frequency is unknown.82
In summary, surgery still offers the best approach to diagnose and treat HSA even though it is generally only palliative. Novel avenues for adjuvant chemotherapy need evaluation. New approaches to treatment such as immunotherapy or antiangiogenic therapy may provide alternatives, and additional investigation into the biology of canine HSA, hopefully leading to clinical trials, is desperately needed.
Comparative Aspects
In humans, a spectrum of endothelial tumors, including hemangioma, hemangioblastoma, Kaposi’s sarcoma, hemangioendothelioma, and angiosarcoma, is seen. Angiosarcoma is extremely rare in humans and can be a late sequela to RT in women treated for breast cancer.167 With this exception, it has a lesion distribution and behavior similar to canine HSA. As in dogs, metastasis is frequent and adjuvant chemotherapy provides minimal benefit.
References
1. Priester, W, McKay, F. The occurrence of tumors in domestic animals. Washington, DC: National Cancer Institute Monograph; 1980.
2. Spangler, WL, Culbertson, MR. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985-1989). J Am Vet Med Assoc. 1992;200:829–834.
3. Bastianello, SS. A survey on neoplasia in domestic species over a 40-year period from 1935 to 1974 in the republic of South Africa. VI. Tumors occurring in dogs. Onderspoort J Vet Res. 1983;50:199–220.
4. Dorn, CR, Taylor, DON, Schneider, R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318.
5. MacVean, DW, Monlux, AW, Anderson, PS, et al. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978;145:700–715.
6. Rostami, M, Tateyama, S, Uchida, K, et al. Tumor in domestic animals examined during a ten-year period (1980 to 1989) at Miyazaki University. J Vet Med Sci. 1991;56:403–405.
7. Day, MJ, Lucke, VM, Pearson, H. A review of pathological diagnoses made from 87 canine splenic biopsies. J Small Anim Pract. 1995;36:426–433.
8. Spangler, WL, Kass, PH. Pathologic factors affecting postsplenectomy survival in dogs. J Vet Intern Med. 1997;11:166–171.
9. Carpenter, JL, Andrews, LK, Holzworth, J. Tumors and tumor-like lesions. In: Holzworth J, ed. Diseases of the cat: medicine and surgery. Philadelphia: WB Saunders, 1987.
10. Patnaik, AK, Liu, SK. Angiosarcoma in cats. J Small Anim Pract. 1977;18:191–198.
11. Schultheiss, PC. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J Vet Diagn Invest. 2004;16:522–526.
12. Oksanen, A. Haemangiosarcoma in dogs. J Comp Pathol. 1978;88:585–595.
13. Arp, LH, Grier, RL. Disseminated cutaneous hemangiosarcoma in a young dog. J Am Vet Med Assoc. 1984;185:671–673.
14. Brown, NO, Patnaik, AK, MacEwen, EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985;186:56–58.
15. Srebernik, N, Appleby, EC. Breed prevalence and sites of haemangioma and haemangiosarcoma in dogs. Vet Rec. 1991;129:408–409.
16. Moe, L, Gamlem, H, Dahl, K, et al, Canine neoplasia–population-based incidence of vascular tumours. APMIS Suppl 2008:63–68.
17. Gamlem, H, Nordstoga, K, Arnesen, K. Canine vascular neoplasia–a population-based clinicopathologic study of 439 tumours and tumour-like lesions in 420 dogs. APMIS. 2008;Suppl:41–54.
18. Falk, H, Herbert, J, Crowley, S, et al. Epidemiology of hepatic angiosarcoma in the United States: 1964-1974. Environ Health Perspect. 1981;41:107–113.
19. Weaver, J, Billings, SD. Postradiation cutaneous vascular tumors of the breast: a review. Semin Diagn Pathol. 2009;26:141–149.
20. Gessi, M, Cattani, P, Maggiano, N, et al. Demonstration of human herpesvirus 8 in a case of primary vaginal epithelioid angiosarcoma by in situ hybridization, electron microscopy, and polymerase chain reaction. Diagn Mol Pathol. 2002;11:146–151.
21. Remick, SC, Patnaik, M, Ziran, NM, et al. Human herpesvirus-8-associated disseminated angiosarcoma in an HIV-seronegative woman: report of a case and limited case-control virologic study in vascular tumors. Am J Med. 2000;108:660–664.
22. Fink-Puches, R, Zochling, N, Wolf, P, et al. No detection of human herpesvirus 8 in different types of cutaneous angiosarcoma. Arch Dermatol. 2002;138:131–132.
23. Palacios, I, Umbert, I, Celada, A. Absence of human herpesvirus-8 DNA in angiosarcoma. Br J Dermatol. 1999;140:170–171.
24. Benjamin, SA, Lee, AC, Angleton, GM, et al. Mortality in beagles irradiated during prenatal and postnatal development. II. Contribution of benign and malignant neoplasia. Radiat Res. 1998;150:330–348.
25. Hargis, AM, Ihrke, PJ, Spangler, WL, et al. A retrospective clinicopathologic study of 212 dogs with cutaneous hemangiomas and hemangiosarcomas. Vet Pathol. 1992;29:316–328.
26. Nikula, KJ, Benjamin, SA, Angleton, GM, et al. Ultraviolet radiation, solar dermatosis, and cutaneous neoplasia in beagle dogs. Radiat Res. 1992;129:11–18.
27. Clifford, CA, Hughes, D, Beal, MW, et al. Plasma vascular endothelial growth factor concentrations in healthy dogs and dogs with hemangiosarcoma. J Vet Intern Med. 2001;15:131–135.
28. Duda, LE, Sorenmo, KU, Urine basic fibroblast growth factor in canine hemangiosarcoma. 73, Chicago. Proceedings of the Veterinary Cancer Society Annual Conference 1997 .
29. Amo, Y, Masuzawa, M, Hamada, Y, et al. Observations on angiopoietin 2 in patients with angiosarcoma. Br J Dermatol. 2004;150:1028–1029.
30. Brown, LF, Tognazzi, K, Dvorak, HF, et al. Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 1996;148:1065–1074.
31. Hashimoto, M, Oshawa, N, Ohnishi, A, et al. Expression of vascular endothelial growth factor and its receptor mRNA in angiosarcoma. Lab Invest. 1995;73:859–863.
32. Yamamoto, T, Umeda, T, Yokozeki, H, et al. Expression of basic fibroblast growth factor and its receptor in angiosarcoma. J Am Acad Dermatol. 1999;41:127–129.
33. Akhtar, N, Padilla, ML, Dickerson, EB, et al. Interleukin-12 inhibits tumor growth in a novel angiogenesis canine hemangiosarcoma xenograft model. Neoplasia. 2004;6:106–116.
34. Fosmire, SP, Dickerson, EB, Scott, AM, et al. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Invest. 2004;84:562–572.
35. Yonemaru, K, Sakai, H, Murakami, M, et al. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and their receptors (flt-1, flk-1, and flg-1) in canine vascular tumors. Vet Pathol. 2006;43:971–980.
36. Kodama, A, Sakai, H, Matsuura, S, et al. Establishment of canine hemangiosarcoma xenograft models expressing endothelial growth factors, their receptors, and angiogenesis-associated homeobox genes. BMC Cancer. 2009;9:363.
37. Arbiser, JL, Larsson, H, Claesson-Welsh, L, et al. Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo. Am J Pathol. 2000;156:1469–1476.
38. Lee, RJ, Springer, ML, Blanco-Bose, WE, et al. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901.
39. Donehower, LA, Harvey, M, Slagle, BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221.
40. Naka, N, Tomita, Y, Nakanashi, H, et al. Mutations of p53 tumor-suppressor gene in angiosarcoma. Int J Cancer. 1997;71:952–955.
41. Arbiser, JL, Moses, MA, Fernandez, CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866.
42. DeVivo, I, Marion, MJ, Smith, SJ, et al. Mutant c-Ki-ras p21 protein in chemical carcinogenesis in humans exposed to vinyl chloride. Cancer Causes Control. 1994;5:273–278.
43. Garcia, JM, Gonzalez, R, Silva, JM, et al. Mutational status of K-ras and TP53 genes in primary sarcomas of the heart. Br J Cancer. 2000;82:1183–1185.
44. Onda, H, Lueck, A, Marks, PW, et al. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695.
45. Mayr, B, Zwetkoff, S, Schaffner, G, et al. Tumour suppressor gene p53 mutation in a case of haemangiosarcoma of a dog. Acta Vet Hung. 2002;50:157–160.
46. Yonemaru, K, Sakai, H, Murakami, M, et al. The significance of p53 and retinoblastoma pathways in canine hemangiosarcoma. J Vet Med Sci. 2007;69:271–278.
47. Tamburini, BA, Phang, TL, Fosmire, SP, et al. Gene expression profiling identifies inflammation and angiogenesis as distinguishing features of canine hemangiosarcoma. BMC Cancer. 2010;10:619.
48. Dickerson, EB, Thomas, R, Fosmire, SP, et al. Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet Pathol. 2005;42:618–632.
49. Murakami, M, Sakai, H, Kodama, A, et al. Expression of the anti-apoptotic factors Bcl-2 and survivin in canine vascular tumours. J Comp Pathol. 2008;139:1–7.
50. Priester, WA. Hepatic angiosarcomas in dogs: an excessive frequency as compared with man. J Natl Cancer Inst. 1976;57:451–454.
51. Ward, H, Fox, LE, Calderwood-Mays, MB, et al. Cutaneous hemangiosarcoma in 25 dogs: a retrospective study. J Vet Intern Med. 1994;8:345–348.
52. Ware, WA, Hopper, DL. Cardiac tumors in dogs: 1982-1995. J Vet Intern Med. 1999;13:95–103.
53. Aupperle, H, Marz, I, Ellenberger, C, et al. Primary and secondary heart tumours in dogs and cats. J Comp Pathol. 2007;136:18–26.
54. Erdem, V, Pead, J. Haemangiosarcoma of the scapula in three dogs. J Small Anim Pract. 2000;41:461–464.
55. Liptak, JM, Dernell, WS, Ehrhart, EJ, et al. Retroperitoneal sarcomas in dogs: 14 cases (1992-2002). J Am Vet Med Assoc. 2004;224:1471–1477.
56. Liptak, JM, Dernell, WS, Withrow, SJ. Haemangiosarcoma of the urinary bladder in a dog. Aust Vet J. 2004;82:215–217.
57. Pirkey-Ehrhart, N, Withrow, SJ, Straw, RC, et al. Primary rib tumors in 54 dogs. J Am Anim Hosp Assoc. 1995;31:65–69.
58. Wobeser, BK, Kidney, BA, Powers, BE, et al. Diagnoses and clinical outcomes associated with surgically amputated feline digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol. 2007;44:362–365.
59. Dennis, MM, Ehrhart, N, Duncan, CG, et al. Frequency of and risk factors associated with lingual lesions in dogs: 1,196 cases (1995-2004). J Am Vet Med Assoc. 2006;228:1533–1537.
60. Locke, JE, Barber, LG. Comparative aspects and clinical outcomes of canine renal hemangiosarcoma. J Vet Intern Med. 2006;20:962–967.
61. Scavelli, TD, Patnaik, AK, Mehlhaff, CJ, et al. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J Am Vet Med Assoc. 1985;187:817–819.
62. Merlo, M, Bo, S, Ratto, A. Primary right atrium haemangiosarcoma in a cat. J Feline Med Surg. 2002;4:61–64.
63. Pirie, CG, Dubielzig, RR. Feline conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of eight cases (1993-2004). Vet Ophthalmol. 2006;9:227–231.
64. Newkirk, KM, Rohrbach, BW. A retrospective study of eyelid tumors from 43 cats. Vet Pathol. 2009;46:916–927.
65. Johnson, KA, Powers, BE, Withrow, SJ, et al. Splenomegaly in dogs: predictors of neoplasia and survival after splenectomy. J Vet Intern Med. 1989;3:160–166.
66. Pintar, J, Breitschwerdt, EB, Hardie, EM, et al. Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987-2001). J Am Anim Hosp Assoc. 2003;39:518–522.
67. Hammond, TN, Pesillo-Crosby, SA. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003-2005). J Am Vet Med Assoc. 2008;232:553–558.
68. Aronsohn, MG, Dubiel, B, Roberts, B, et al. Prognosis for acute nontraumatic hemoperitoneum in the dog: a retrospective analysis of 60 cases (2003-2006). J Am Anim Hosp Assoc. 2009;45:72–77.
69. Mallinckrodt, MJ, Gottfried, SD. Mass-to-splenic volume ratio and splenic weight as a percentage of body weight in dogs with malignant and benign splenic masses: 65 cases (2007-2008). J Am Vet Med Assoc. 2011;239:1325–1327.
70. Pulley, LT, Stannard, AA. Tumors of the skin and subcutaneous tissues. In Moulton JE, ed.: Tumors in domestic animals, ed 3, Berkeley: University of California Press, 1990.
71. Gamlem, H, Nordstoga, K. Canine vascular neoplasia–histologic classification and inmunohistochemical analysis of 221 tumours and tumour-like lesions. APMIS. 2008;Suppl:19–40.
72. Miller, MA, Ramos, JA, Kreeger, JM. Cutaneous vascular neoplasia in 15 cats: clinical, morphologic, and immunohistochemical studies. Vet Pathol. 1992;29:329–336.
73. von Beust, BR, Suter, MM, Summers, BA. Factor VIII-related antigen in canine endothelial neoplasms: An immunohistochemical study. Vet Pathol. 1988;25:251–255.
74. Ferrer, L, Fondevila, D, Rabanal, RM, et al. Immunohistochemical detection of CD31 antigen in normal and neoplastic canine endothelial cells. J Comp Pathol. 1995;112:319–326.
75. Jakab, C, Halasz, J, Kiss, A, et al. Claudin-5 protein is a new differential marker for histopathological differential diagnosis of canine hemangiosarcoma. Histol Histopathol. 2009;24:801–813.
76. Sabattini, S, Bettini, G. An immunohistochemical analysis of canine haemangioma and haemangiosarcoma. J Comp Pathol. 2009;140:158–168.
77. Waters, D, Caywood, D, Hayden, D, et al. Metastatic pattern in dogs with splenic hemangiosarcoma. J Small Anim Pract. 1988;29:805–814.
78. Waters, D, Hayden, D, Walter, P. Intracranial lesions in dogs with hemangiosarcoma. J Vet Intern Med. 1989;3:222–230.
79. Snyder, JM, Lipitz, L, Skorupski, KA, et al. Secondary intracranial neoplasia in the dog: 177 cases (1986-2003). J Vet Intern Med. 2008;22:172–177.
80. McAbee, KP, Ludwig, LL, Bergman, PJ, et al. Feline cutaneous hemangiosarcoma: a retrospective study of 18 cases (1998-2003). J Am Anim Hosp Assoc. 2005;41:110–116.
81. Johannes, CM, Henry, CJ, Turnquist, SE, et al. Hemangiosarcoma in cats: 53 cases (1992-2002). J Am Vet Med Assoc. 2007;231:1851–1856.
82. Kraje, AC, Mears, EA, Hahn, KA, et al. Unusual metastatic behavior and clinicopathologic findings in eight cats with cutaneous or visceral hemangiosarcoma. J Am Vet Med Assoc. 1999;214:670–672.
83. Culp, WT, Drobatz, KJ, Glassman, MM, et al. Feline visceral hemangiosarcoma. J Vet Intern Med. 2008;22:148–152.
84. Gordon, SS, McClaran, JK, Bergman, PJ, et al. Outcome following splenectomy in cats. J Feline Med Surg. 2010;12:256–261.
85. Hirsch, V, Jacobsen, J, Mills, J. A retrospective study of canine hemangiosarcoma and its association with acanthocytosis. Can Vet J. 1981;22:152–155.
86. Hammer, AS, Couto, CG, Swardson, C, et al. Hemostatic abnormalities in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:11–14.
87. Hargis, AM, Feldman, BF. Evaluation of hemostatic defects secondary to vascular tumors in dogs: 11 cases. J Am Vet Med Assoc. 1991;198:891–894.
88. Maruyama, H, Miura, T, Sakai, M, et al. The incidence of disseminated intravascular coagulation in dogs with malignant tumor. J Vet Med Sci. 2004;66:573–575.
89. Mischke, R, Wohlsein, P, Schoon, HA. Detection of fibrin generation alterations in dogs with haemangiosarcoma using resonance thrombography. Thromb Res. 2005;115:229–238.
90. Rishniw, M, Lewis, DC. Localized consumptive coagulopathy associated with cutaneous hemangiosarcoma in a dog. J Am Anim Hosp Assoc. 1994;30:261–264.
91. Maruyama, H, Watari, T, Miura, T, et al. Plasma thrombin-antithrombin complex concentrations in dogs with malignant tumours. Vet Rec. 2005;156:839–840.
92. Thamm, DH, Helfand, SC. Acquired coagulopathy III: neoplasia. In Feldman BF, Zinkl JG, Jain NC, eds.: Schalm’s veterinary hematology, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2000.
93. Holt, D, Van Winkle, T, Schelling, C, et al. Correlation between thoracic radiographs and postmortem findings in dogs with hemangiosarcoma: 77 cases (1984-1989). J Am Vet Med Assoc. 1992;200:1535–1539.
94. Stafford Johnson, M, Martin, M, Binns, S, et al. A retrospective study of clinical findings, treatment and outcome in 143 dogs with pericardial effusion. J Small Anim Pract. 2004;45:546–552.
95. Cuccovillo, A, Lamb, CR. Cellular features of sonographic target lesions of the liver and spleen in 21 dogs and a cat. Vet Radiol Ultrasound. 2002;43:275–278.
96. Wrigley, RH, Park, RD, Konde, LJ, et al. Ultrasonographic features of splenic hemangiosarcoma in dogs: 18 cases (1980-1986). J Am Vet Med Assoc. 1988;192:1113–1117.
97. Thomas, W, Sisson, D, Bauer, T, et al. Detection of cardiac masses in two-dimensional echocardiography. Vet Radiol. 1984;25:65–71.
98. Berg, RJ, Wingfield, W. Pericardial effusion in the dog: a review of 42 cases. J Am Anim Hosp Assoc. 1984;20:721–730.
99. Dunning, D, Monnet, E, Orton, EC, et al. Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985-1996). J Am Vet Med Assoc. 1998;212:1276–1280.
100. Fruchter, A, Miller, C, O’Grady, M. Echocardiographic results and clinical considerations in dogs with right atrial/auricular masses. Can Vet J. 1992;33:171–174.
101. Bertazzolo, W, Dell’Orco, M, Bonfanti, U, et al. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet Clin Pathol. 2005;34:28–34.
102. Edwards, NJ. The diagnostic value of pericardial fluid pH determination. J Am Anim Hosp Assoc. 1996;32:63–67.
103. Fine, DM, Tobias, AH, Jacob, KA. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17:525–529.
104. Sisson, D, Thomas, WP, Ruehl, WW, et al. Diagnostic value of pericardial fluid analysis in the dog. J Am Vet Med Assoc. 1984;184:51–55.
105. Shaw, SP, Rozanski, EA, Rush, JE. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med. 2004;18:322–324.
106. Chun, R, Kellihan, HB, Henik, RA, et al. Comparison of plasma cardiac troponin I concentrations among dogs with cardiac hemangiosarcoma, noncardiac hemangiosarcoma, other neoplasms, and pericardial effusion of nonhemangiosarcoma origin. J Am Vet Med Assoc. 2010;237:806–811.
107. O’Brien, RT, Iani, M, Matheson, J, et al. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs. Vet Radiol Ultrasound. 2004;45:547–553.
108. Kutara, K, Asano, K, Kito, A, et al. Contrast harmonic imaging of canine hepatic tumors. J Vet Med Sci. 2006;68:433–438.
109. O’Brien, RT. Improved detection of metastatic hepatic hemangiosarcoma nodules with contrast ultrasound in three dogs. Vet Radiol Ultrasound. 2007;48:146–148.
110. Ohlerth, S, Dennler, M, Ruefli, E, et al. Contrast harmonic imaging characterization of canine splenic lesions. J Vet Intern Med. 2008;22:1095–1102.
111. Webster, N, Holloway, A. Use of contrast ultrasonography in the diagnosis of metastatic feline visceral haemangiosarcoma. J Feline Med Surg. 2008;10:388–394.
112. Ivancic, M, Long, F, Seiler, GS. Contrast harmonic ultrasonography of splenic masses and associated liver nodules in dogs. J Am Vet Med Assoc. 2009;234:88–94.
113. Nakamura, K, Takagi, S, Sasaki, N, et al. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet Radiol Ultrasound. 2010;51:79–85.
114. Fife, WD, Samii, VF, Drost, WT, et al. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography. Vet Radiol Ultrasound. 2004;45:289–297.
115. Clifford, CA, Pretorius, ES, Weisse, C, et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004;18:330–338.
116. Yuki, M, Machida, N, Sawano, T, et al. Investigation of serum concentrations and immunohistochemical localization of alpha1-acid glycoprotein in tumor dogs. Vet Res Commun. 2010;35:1–11.
117. Kirby, GM, Mackay, A, Grant, A, et al. Concentration of lipocalin region of collagen XXVII alpha 1 in the serum of dogs with hemangiosarcoma. J Vet Intern Med. 2011;25:497–503.
118. von Euler, HP, Rivera, P, Aronsson, AC, et al. Monitoring therapy in canine malignant lymphoma and leukemia with serum thymidine kinase 1 activity—evaluation of a new, fully automated non-radiometric assay. Int J Oncol. 2008;34:505–510.
119. Thamm, DH, Kamstock, DS, Sharp, CR, et al. Elevated serum thymidine kinase activity in canine splenic hemangiosarcoma. Vet Comp Oncol. 2011. [Epub ahead of print].
120. Lamerato-Kozicki, AR, Helm, KM, Jubala, CM, et al. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006;34:870–878.
121. Rivier, P, Monnet, E. Use of a vessel sealant device for splenectomy in dogs. Vet Surg. 2011;40:102–105.
122. Bower, MR, Ellis, SF, Scoggins, CR, et al. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol. 2011;18:166–173.
123. Keyes, M, Rush, J. Ventricular arrhythmias in dogs with splenic masses. Vet Emerg Crit Care. 1994;3:33–38.
124. Aronsohn, M. Cardiac hemangiosarcoma in the dog: a review of 38 cases. J Am Vet Med Assoc. 1985;187:922–926.
125. Weisse, C, Soares, N, Beal, MW, et al. Survival times in dogs with right atrial hemangiosarcoma treated by means of surgical resection with or without adjuvant chemotherapy: 23 cases (1986-2000). J Am Vet Med Assoc. 2005;226:575–579.
126. Brisson, BA, Holmberg, DL. Use of pericardial patch graft reconstruction of the right atrium for treatment of hemangiosarcoma in a dog. J Am Vet Med Assoc. 2001;218:723–725.
127. Morges, M, Worley, DR, Withrow, SJ, et al. Pericardial free patch grafting as a rescue technique in surgical management of right atrial HSA. J Am Anim Hosp Assoc. 2011;47:224–228.
128. Hammer, AS, Couto, CG, Filppi, J, et al. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:160–166.
129. Ogilvie, GK, Powers, BE, Mallinckrodt, CH, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;10:379–384.
130. Sorenmo, K, Duda, L, Barber, L, et al. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J Vet Intern Med. 2000;14:395–398.
131. Sorenmo, KU, Baez, JL, Clifford, CA, et al. Efficacy and toxicity of a dose-intensified doxorubicin protocol in canine hemangiosarcoma. J Vet Intern Med. 2004;18:209–213.
132. Sorenmo, KU, Jeglum, KA, Helfand, SC. Chemotherapy of canine splenic hemangiosarcoma with doxorubicin and cyclophosphamide. J Vet Intern Med. 1993;7:370–376.
133. Vail, DM, MacEwen, EG, Kurzman, ID, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: A randomized multi-institutional clinical trial. Clin Cancer Res. 1995;1:1165–1170.
134. Payne, SE, Rassnick, KM, Northrup, NC, et al. Treatment of vascular and soft-tissue sarcomas in dogs using an alternating protocol of ifosfamide and doxorubicin. Vet Comp Oncol. 2003;1:171–179.
135. Rassnick, KM, Frimberger, AE, Wood, CA, et al. Evaluation of ifosfamide for treatment of various canine neoplasms. J Vet Intern Med. 2000;14:271–276.
136. Kim, SE, Liptak, JM, Gall, TT, et al. Epirubicin in the adjuvant treatment of splenic hemangiosarcoma in dogs: 59 cases (1997-2004). J Am Vet Med Assoc. 2007;231:1550–1557.
137. Sorenmo, K, Samluk, M, Clifford, C, et al. Clinical and pharmacokinetic characteristics of intracavitary administration of pegylated liposomal encapsulated doxorubicin in dogs with splenic hemangiosarcoma. J Vet Intern Med. 2007;21:1347–1354.
138. Ogilvie, GK, Reynolds, HA, Richardson, RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195:1580–1583.
139. Wiley, JL, Rook, KA, Clifford, CA, et al. Efficacy of doxorubicin-based chemotherapy for non-resectable canine subcutaneous haemangiosarcoma. Vet Comp Oncol. 2010;8:221–233.
140. Dervisis, NG, Dominguez, PA, Newman, RG, et al. Treatment with DAV for advanced-stage hemangiosarcoma in dogs. J Am Anim Hosp Assoc. 2011;47:170–178.
141. Kisseberth, WC, Vail, DM, Yaissle, J, et al. Phase I clinical evaluation of carboplatin in tumor-bearing cats: a Veterinary Cooperative Oncology Group study. J Vet Intern Med. 2008;22:83–88.
142. U’Ren, LW, Biller, BJ, Elmslie, RE, et al. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. 2007;21:113–120.
143. Blake, MK, LaRue, S, Withrow, SJ, Palliative radiation therapy of solid soft tissue malignancies. In Proceedings of the Veterinary Cancer Society, Estes Park, CO, 20 1988.
144. Lawrence, JA, Thamm, DH, Adams, WM, et al, Soft tissue sarcomas: a retrospective analysis of 16 dogs treated with palliative radiotherapy (1996-2001). In Proceedings of the Veterinary Cancer Society, Kansas City, MO, 19 2004.
145. Hillers, KR, Lana, SE, Fuller, CR, et al. Effects of palliative radiation therapy on nonsplenic hemangiosarcoma in dogs. J Am Anim Hosp Assoc. 2007;43:187–192.
146. Gingrich, DE, Reddy, DR, Iqbal, MA, et al. A new class of potent vascular endothelial growth factor receptor tyrosine kinase inhibitors: structure-activity relationships for a series of 9-alkoxymethyl-12-(3-hydroxypropyl)indeno[2, 1-a]pyrrolo[3,4-c]carbazole-5-ones and the identification of CEP-5214 and its dimethylglycine ester prodrug clinical candidate CEP-7055. J Med Chem. 2003;46:5375–5388.
147. Ma, G, Masuzawa, M, Hamada, Y, et al. Treatment of murine angiosarcoma with etoposide, TNP-470 and prednisolone. J Dermatol Sci. 2000;24:126–133.
148. Liekens, S, Verbeken, E, De Clercq, E, et al. Potent inhibition of hemangiosarcoma development in mice by cidofovir. Int J Cancer. 2001;92:161–167.
149. Bai, X, Cerimele, F, Ushio-Fukai, M, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507.
150. Ruggeri, BA, Robinson, C, Angeles, T, et al. The chemopreventive agent oltipraz possesses potent antiangiogenic activity in vitro, ex vivo, and in vivo and inhibits tumor xenograft growth. Clin Cancer Res. 2002;8:267–274.
151. Maki, RG, D’Adamo, DR, Keohan, ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140.
152. Kiesel, H, Muller, AM, Schmitt-Graeff, A, et al. Dramatic and durable efficacy of imatinib in an advanced angiosarcoma without detectable KIT and PDGFRA mutations. Cancer Biol Ther. 2009;8:319–321.
153. Lana, S, U’Ren, L, Plaza, S, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007;21:764–769.
154. Masuzawa, M, Ohkawa, T, Inamura, K, et al. Effects of intralesional injective recombinant interleukin-2 immunotherapy on malignant hemangioendothelioma. Jpn J Dermatol. 1989;99:1459–1466.
155. Takano, M, Suzuki, Y, Asai, T, et al. A dramatic effect of continuous intra-arterial injective recombinant interleukin-2 immunotherapy on malignant hemangioendothelioma. Jpn J Dermatol. 1991;101:719–725.
156. Abdullah, JM, Mutum, SS, Nasuha, NA, et al. Intramedullary spindle cell hemangioendothelioma of the thoracic spinal cord–case report. Neurol Med Chir (Tokyo). 2002;42:259–263.
157. Jackel, A, Deichmann, M, Waldmann, V, et al. Regression of metastatic angiosarcoma of the skin after systemic treatment with liposome-encapsulated doxorubicin and interferon-alpha. Br J Dermatol. 1999;140:1187–1188.
158. Spieth, K, Gille, J, Kaufmann, R. Therapeutic efficacy of interferon alfa-2a and 13-cis-retinoic acid in recurrent angiosarcoma of the head. Arch Dermatol. 1999;135:1035–1037.
159. Durie, BG, Clouse, L, Braich, T, et al. Interferon alfa-2b-cyclophosphamide combination studies: in vitro and phase I-II clinical results. Semin Oncol. 1986;13:84–88.
160. Fata, F, O’Reilly, E, Ilson, D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034–2037.
161. Isogai, R, Kawada, A, Aragane, Y, et al. Successful treatment of pulmonary metastasis and local recurrence of angiosarcoma with docetaxel. J Dermatol. 2004;31:335–341.
162. Stacchiotti, S, Palassini, E, Sanfilippo, R, et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian Rare Cancer Network. Ann Oncol. 2011;23(2):501–508.
163. Prymak, C, McKee, LJ, Goldschmidt, MH, et al. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). J Am Vet Med Assoc. 1988;193:706–712.
164. Wood, CA, Moore, AS, Gliatto, JM, et al. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991-1993). J Am Anim Hosp Assoc. 1998;34:417–421.
165. Bulakowski, EJ, Philibert, JC, Siegel, S, et al. Evaluation of outcome associated with subcutaneous and intramuscular hemangiosarcoma treated with adjuvant doxorubicin in dogs: 21 cases (2001-2006). J Am Vet Med Assoc. 2008;233:122–128.
166. Shiu, KB, Flory, AB, Anderson, CL, et al. Predictors of outcome in dogs with subcutaneous or intramuscular hemangiosarcoma. J Am Vet Med Assoc. 2011;238:472–479.
167. Simonart, T, Heenen, M. Radiation-induced angiosarcomas. Dermatology. 2004;209:175–176.
 Section B
Section B
Thymoma
Carlos Henrique de Mello Souza
Incidence and Risk Factors
Thymomas are one of the most common tumors of the cranial mediastinum, second only to lymphoma, yet they are uncommon in both dogs and cats. Thymomas can occur at any age but usually affect older patients. The reported peak age for presentation is 9 and 10 years in dogs and cats, respectively.1,2 Breed predisposition has not been reported, and most studies do not show a sex predisposition.1,2 Risk factors predisposing animals to thymoma have not been identified.
Pathology and Natural Behavior
Thymomas are neoplasms of thymic epithelial cells.1 Different histologic types of thymoma have been described and include differentiated epithelial, lymphocyte-rich, and clear-cell type. The degree of lymphocyte infiltration within thymomas in dogs and cats has been shown to correlate positively with survival.1,3 In cats, cystic thymomas seem to be the most common form, although squamous cell carcinoma variants and a thymolipoma have been reported.1,4-8 The terms benign or malignant thymoma are commonly used and are based on clinical criteria of invasiveness and resectability rather than on histologic features of malignancy. Distant metastasis has been rarely reported in dogs.5,9-11 The same is probably true in cats, although one study revealed a 20% metastatic rate in cystic thymomas in the species.6 The differential diagnoses for mediastinal masses should include lymphoma, ectopic thyroid tumor, branchial cysts, rare sarcomas, and metastatic neoplasms. Tumors extending from the ribs or sternum into the mediastinum may sometimes resemble a mediastinal mass.12
History and Clinical Signs
Clinical signs are usually related to organ displacement due to the presence of a mediastinal mass and include lethargy, coughing, tachypnea, and dyspnea. Less commonly, cranial vena cava syndrome (edema of the head, neck, and front limbs) may occur and is related to obstruction of vessels draining the cranial part of the body.3-7,10-12 Paraneoplastic syndromes are common in dogs and cats and may occur in as many as 67% of dogs with thymoma.3,4 Reported paraneoplastic syndromes include myasthenia gravis (MG), exfoliative dermatitis, erythema multiforme, hypercalcemia, T-cell lymphocytosis, anemia, and polymyositis. Myasthenia gravis may occur in up to 40% of dogs with thymoma and has also been reported in cats.3,10,11 Concurrent megaesophagus and aspiration pneumonia has been reported in as many as 40% of canine patients.3 Paraneoplastic syndromes may occur at presentation, later in the course of the disease, or after tumor removal.*
Diagnostic Techniques and Work-Up
Physical examination findings may include edema of the head, cervical area, or front limbs, secondary to cranial vena cava syndrome. The jugular veins may be dilated and tortuous. Auscultation of the thoracic cavity may reveal decreased or absent lung sounds in the cranial mediastinum related to displacement by the mass or pleural effusion. Cardiac displacement may also occur, and the heart sounds may be heard either more dorsally, caudally, or both. In small dogs and cats, decreased compressibility of the anterior thorax may also be detected.3-6,10-11,22
Complete blood count (CBC) is usually normal, but anemia, thrombocytopenia (secondary to immune-mediated destruction), and lymphocytosis may occur. Hypercalcemia, although uncommon, has been reported in association with thymomas but is much more common in cases of mediastinal lymphoma.5,11,23 In both tumors, hypercalcemia is usually the result of excessive production of parathyroid hormone–related peptide (PTHrP). The presence of hypercalcemia in an animal with a mediastinal mass should not be used as the sole means to differentiate thymoma from lymphoma.22-26
Thoracic radiographs may reveal the presence of a cranial mediastinal mass, pleural effusion, and cardiac displacement (Figure 33-4). Dilatation of the esophagus in the presence of megaesophagus secondary to MG and an increase in alveolar or interstitial lung pattern suggestive of aspiration pneumonia may also be detected. When MG is suspected, demonstration of serum antibodies against acetylcholine receptor is indicated. The Tensilon test (edrophonium chloride, which is an ultrashort anticholinesterase agent) can be used in cases suspected of having MG, in which muscle weakness and fatigue are observed. A marked short-lived improvement in muscle strength is considered a positive response.18-21 In cases presenting with pleural effusion, fluid analysis after thoracocentesis usually reveals a modified transudate and numerous small mature lymphocytes or a mixed lymphocyte population.3-5,10,22 Thoracic ultrasonography can be used to determine a presumptive diagnosis of thoracic masses, including thymomas. In addition ultrasound can guide biopsy needles for sampling of the mass.27,28 More recently, endoscopic thoracic ultrasound has been described in dogs with the reported advantage of a decrease in artifacts caused by the lungs.29 Transthoracic ultrasound-guided fine-needle aspiration (FNA) of the mediastinum can be easily and safely performed after deep sedation and analgesia or general anesthesia. The diagnosis of thymoma is made when the presence of neoplastic epithelial cells interspersed between large numbers of small mature lymphocytes (and occasional mast cells) is observed.30,31 Unfortunately, nondiagnostic samples are common due to either a small percentage of epithelial cells, the presence of only small mature lymphocytes in the samples, or the presence of cysts within the mass. The diagnosis is further complicated due to the fact that both lymphoma and thymoma may be composed primarily of small lymphocytes. In three studies, a presumptive diagnosis of thymoma was achieved in approximately 20%, 40%, and 77% of mediastinal masses after FNA and cytology. In addition, Hassall’s corpuscles, cytoplasmic structures present in thymocytes, are not usually visualized in Wright-Giemsa preparations in comparison to hematoxylin-eosin (H&E) used for formalin-fixed samples.3,10,30,31
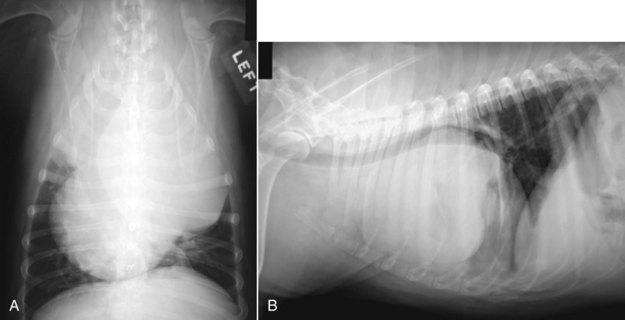
Figure 33-4 A, Ventrodorsal thoracic radiograph of a large left-sided cranial mediastinal mass that severely displaces the trachea and the heart. B, Lateral view of the same mass showing dorsal displacement of the trachea and caudal displacement of the heart. A small amount of pleural effusion can also be observed. (Courtesy Dr. Christine Warzee, College of Veterinary Medicine, Michigan State University.)
Flow cytometry has been recently reported to aid in the specific diagnosis of mediastinal tumors. Flow cytometry of the thymus is based on the fact that thymic lymphocytes can be differentiated from peripheral lymphocytes by simultaneous expression of CD4 and CD8. In that study, all cases of thymoma expressed 10% lymphocytes co-expressing CD4 and CD8, whereas six of seven lymphomas contained less than 2% of CD4+CD8+ lymphocytes. The additional case of lymphoma was readily differentiated from thymoma by flow cytometric scatter plot analysis.32
Advanced imaging modalities such as CT are now used to help the surgeon evaluate the possibility of resection. Three-dimensional (3D) CT reconstructions can estimate the size and volume of the mass, in addition to identifying neighboring structures and whether invasion of these structures has occurred. If the tumor is deemed inoperable, CT will still be helpful if radiotherapy is to be employed (Figure 33-5). Furthermore, CT-guided biopsies can be obtained during the process. Despite these advantages, nonangiographic CT has been shown to have limitations, which were outlined by a recent study. In that study, nonangiographic CT scanning significantly underestimated vascular invasion when compared to surgical exploration. These limitations can be potentially overcome by the use of CT angiography.33-36
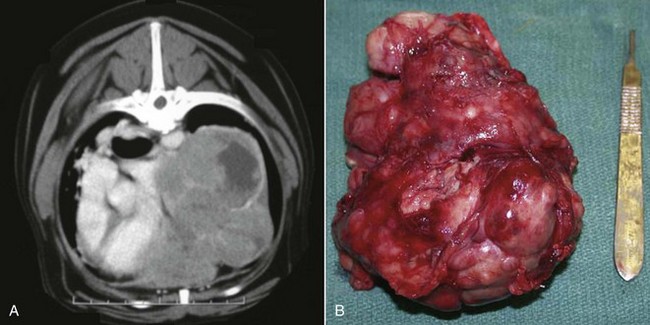
Figure 33-5 A, CT view of the mass. The mass occupies most of the lateral aspect of the thoracic cavity, and despite significant organ displacement, no evidence of vascular invasion is detected. B, Surgical specimen of the large mass after sternotomy and complete resection. (Courtesy Dr. Christine Warzee, College of Veterinary Medicine, Michigan State University.)
Therapy
A variety of different modalities have been described for the treatment of thymomas in dogs and cats, including surgery, RT, and chemotherapy and multimodality treatments. Unfortunately, there are no available studies comparing outcomes of animals treated with these different modalities. In addition, in many studies, animals were treated by a combination of different methods.* In a recent retrospective study that evaluated 11 dogs and 9 cats with invasive and noninvasive thymomas treated by surgery alone, the median survival was 790 days and 1825 days, respectively. The 1- and 3-year survival rates were 64% and 42% for dogs and 89% and 74% for cats, respectively.3 The successful resection of noninvasive thymomas in two dogs by video-assisted thoracoscopy has also been reported.38
In another retrospective study, 17 dogs and 7 cats with thymoma were treated with RT alone or as an adjunctive therapy. Twenty cases were available for follow-up, and a response rate of 75% (11 partial responses [PRs]; 4 complete responses [CRs]) was observed. MSTs for dogs and cats were 248 days and 720 days, respectively. In that study, the total radiation dose (15 to 54 Gy) and treatment interval (from daily to once weekly) varied markedly among animals. To further confound the effects of RT in thymomas, the same study reported that many animals received concurrent chemotherapy.37
The effects of chemotherapy for the treatment of thymomas have not been well evaluated in veterinary patients. Long-term remission and stable disease after treatment of thymomas in humans and dogs have been reported (single-case reports) with the use of high-dose prednisone. In one report a cat with thymoma achieved partial remission after treatment with DOX. Likely, the effects of chemotherapy and RT against thymomas reflect, at least in part, a greater reduction in the nonneoplastic lymphocyte population in the thymus, rather than in the primary carcinoma cells.3-5,10,39
Prognosis
Surgically resectable thymomas in dogs without megaesophagus and aspiration pneumonia are thought to carry a good prognosis.3,5,10 The definition of surgical resectability will depend on the experience and ability of the surgeon. Vascular invasion, which could potentially limit resection in the past, may be managed with a jugular vein autograft for reconstruction of the cranial vena cava in a dog with invasive thymoma.40
As mentioned previously, thymomas with a significant lymphocytic infiltrate demonstrate improved survival. Age of the dog or cat, invasiveness of the tumor, and mitotic index had no effect on prognosis.3
In cats, cystic thymomas are commonly reported and they have been associated with better prognosis, although no other possible prognostic factors such as surgical resectability were critically evaluated.3,41
In conclusion, long-term survival should be expected for dogs and cats with thymomas that can be completely resected. Vascular invasion may increase surgical complexity but not necessarily exclude surgery as an option.
Comparative Aspects
Thymic neoplasms in humans constitute 30% of anterior mediastinal masses in adults and less than 15% in children. The majority will occur in elderly patients, 60 years or older, and no sex or race predilection is thought to occur.42,43 A clinicopathologic classification has been adopted by the World Health Organization (WHO) and correlates well with behavior. In the WHO system, cells are classified as spindle (predominant in the medullary), oval and epithelioid (predominant in the cortex), or dendritic. The tumors are then further divided into medullary, mixed, predominantly cortical and cortical thymomas, and well-differentiated and high-grade thymic carcinoma. Medullary and mixed thymomas are considered benign tumors even in the face of capsular invasion. Predominantly cortical and cortical thymomas display intermediate aggressiveness and have a low risk of relapse independent of their invasiveness. Well-differentiated and high-grade thymomas are considered to be highly invasive and are associated with a high frequency of relapse and death. A staging system that employs both surgical and histologic signs of invasiveness to describe five different stages correlates well with prognosis.42,44
MG is the most common paraneoplastic syndrome associated with thymomas, occurring in 30% to 50% of patients. Red cell aplasia and hypogammaglobulinemia are reported to occur in 5% to 10% of patients.
Complete surgical resection is considered the best predictor for long-term survival for humans with thymomas and is the standard of care in patients with resectable tumors. RT is indicated most commonly for extensive or recurrent disease. A variety of chemotherapy drugs have been used to treat inoperable thymomas or in cases in which gross residual disease is present after surgery. Cisplatin, ifosfamide, and prednisone are considered the most effective agents. In addition neoadjuvant chemotherapy has been shown to influence long-term survival for thymomas of Masaoka stages III and IVa.45
References
1. Parker, GA, Casey, HW. Thymomas in domestic animals. Vet Pathol. 1976;13:353–364.
2. Aronsohn, M. Canine thymomas. Vet Clin North Am. 1985;15:755–767.
3. Zitz, JC, Birchard, SJ, Couto, GC, et al. Results of excision of thymomas in cats and dogs: 20 cases (1984-2005). J Am Vet Med Assoc. 2008;232(8):1186–1192.
4. Aronsohn, MG, Schunk, KL, Carpenter, JL, et al. Clinical and pathologic features of thymomas in 15 dogs. J Am Vet Med Assoc. 1984;184:1355–1362.
5. Atwater, SW, Powers, BE, Park, RD, et al. Canine thymomas: 23 cases (1980-1991). J Am Vet Med Assoc. 1994;205:1007–1013.
6. Patnaik, AK, Lieberman, PH, Erlandson, RA, et al. Feline Cystic thymomas: a clinicopathologic, immunohistologic, and electron microscopic study of 14 cases. J Feline Med Surg. 2003;5:27–35.
7. Carpenter, JL, Valentine, BA. Brief communications and case reports: squamous cell carcinoma arising in two feline thymomas. Vet Pathol. 1992;29:541–543.
8. Vilafranca, M, Font, A. Thymolipoma in a cat. J Feline Med Surg. 2005;7:125–127.
9. Robinson, WC, Cantwell, HD, Crawley, RR, et al. Invasive thymoma in a dog: a case report. J Am Anim Hosp Assoc. 1977;13:95–97.
10. Bella, JR, Stiff, ME, Russel, RG. Thymoma in the dog: two case reports and review of 20 additional cases. J Am Vet Med Assoc. 1983;183:306–311.
11. Day, MJ. Review of thymic pathology in 30 cats and 36 dogs. J Small Anim Pract. 1997;38:393–403.
12. Bell, FW. Neoplastic diseases of the thorax. Vet Clin North Am Small Anim Pract. 1987;17:387.
13. Darke, PG. Myasthenia gravis, thymoma, and myositis in a dog. Vet Rec. 1975;97:392–395.
14. Carpenter, JL, Holzworth, J. Thymoma in 11 cats. J Am Vet Med Assoc. 1982;181:248–251.
15. Turek, MM. Cutaneous paraneoplastic syndromes in dogs and cats: A review of the literature. Vet Dermatol. 2003;14:279–296.
16. Rottenberg, S, von Tscharner, C, Roosje, PJ. Thymoma-associated exfoliative dermatitis in cats. Vet Pathol. 2004;41:429–433.
17. Uchida, K, Awamura, Y, Nakamura, T, et al. Thymoma and multiple thymic cysts in a dog with acquired myasthenia gravis. J Vet Med Sci. 2002;64:637–640.
18. Stenner, VJ, Parry, BW, Holloway, SA. Acquired myasthenia gravis associated with a non-invasive thymic carcinoma in a dog. Aust Vet J. 2003;81:543–546.
19. Paciello, O, Maiolino, P, Navas, L, et al. Acquired canine myasthenia gravis associated with thymoma: histological features and immunolocalization of HLA type II and IgG. Vet Res Commun. 2003;27(Suppl 1):715–718.
20. Moffet, AC. Metastatic thymomas and acquired generalized myasthenia gravis in a beagle. Can Vet J. 2007;48:91–93.
21. Singh, A, Boston, SE, Poma, R. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51:757–760.
22. Theilen, GH, Madewell, BR. Tumors of the respiratory tract and thorax. In: Veterinary cancer medicine. Philadelphia: Lea & Febiger; 1979.
23. Foley, P, Shaw, D, Runyon, C. Serum parathyroid hormone-related protein concentration in a dog with thymomas and persistent hypercalcemia. Can Vet J. 2000;41:867–870.
24. Harris, CL, Klausner, JS, Caywood, DD, et al. Hypercalcemia in a dog with thymomas. J Am Anim Hosp Assoc. 1991;27:281–284.
25. Bolliger, AP, Graham, PA, Richard, V, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol. 2002;31:3–8.
26. Marconato, L, Stefanello, D, Valenti, P, et al. Predictors of long-term survival in dogs with high-grade multicentric lymphoma. J Am Vet Med Assoc. 2011;238:480–485.
27. Reickle, JK, Wisner, ER. Non-cardiac thoracic ultrasound in 75 feline and canine patients. Vet Radiol Ultrasound. 2000;41:154–162.
28. Larson, MM. Ultrasound of the thorax (non-cardiac). Vet Clin North Am. 2009;39:733–745.
29. Gashen, L, Kircher, P, Hoffman, G, et al. Endoscopic ultrasound for the diagnosis of intrathoracic lesions in two dogs. Vet Radiol Ultrasound. 2003;44:292–299.
30. Rae, CA, Jacobs, RM, Couto, CG. A comparison between the cytological and histological characteristics in thirteen canine and feline thymomas. Can Vet J. 1989;30:497–500.
31. Cowell, RL, Tyler, RD, Meinkoth, JH, The lung parenchyma. Diagnostic cytology of the dog and cat ed 2. St Louis: Mosby; 1999.
32. Lana, S, Plaza, S, Hampe, K, et al. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med. 2006;20:1161–1165.
33. Yoon, J, Feeney, DA, Cronk, DE, et al. Computed tomographic evaluation of canine and feline mediastinal masses in 14 patients. Vet Radiol Ultrasound. 2004;45:524–526.
34. Zekas, LJ, Crawford, JT, O’Brien, RT. Computed tomographic-guided biopsy of intrathoracic lesions in 50 dogs and cats. Vet Radiol Ultrasound. 2005;46:200–204.
35. Hylands, R. Veterinary diagnostic imaging. Thymoma. Can Vet J. 2006;47:593–596.
36. Scherrer, W, Kyles, A, Samii, V, et al. Computed tomographic assessment of vascular invasion and resectability of mediastinal masses in dogs and cats. NZ Vet J. 2008;56:330–333.
37. Smith, AN, Wright, JC, Brawner, WR, Jr., et al. Radiation therapy in the treatment of canine and feline thymomas: a retrospective study (1985-1999). J Am Anim Hosp Assoc. 2001;37:489–496.
38. Mayhew, PD, Friedberg, JS. Video assisted thoracoscopic resection of noninvasive thymomas using one-lung ventilation in two dogs. Vet Surg. 2008;37:756–762.
39. Moore, AS. Chemotherapy for intrathoracic cancer in dogs and cats. Prob Vet Med. 1992;4:351–364.
40. Holsworth, IG, Kyles, AE, Bailiff, NL. Use of a jugular vein autograft for reconstruction of the vena cava in a dog with invasive thymoma and cranial vena cava syndrome. J Am Vet Med Assoc. 2004;225:1205–1210.
41. Gores, BR, Berg, J, Carpenter, JL. Surgical treatment of thymomas in cats: 12 cases (1987-1992). J Am Vet Med Assoc. 1994;204:1782–1785.
42. Tomaszek, S, Wigle, DA, Keshavjee, S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg. 2009;87:1973–1980.
43. Masaoka, A, Monden, W, Nakahara, K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492.
44. Ried, M, Guth, H, Potzger, T, et al. Surgical resection of thymomas still represents the first choice of treatment. Thorac Cardiovasc Surg. 2011;60(2):145–149.
45. Cardillo, G, Carleo, F, Giunti, R, et al. Predictors of survival in patients with locally advanced thymomas and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg. 2010;37:819–823.
 Section C
Section C
Canine Transmissible Venereal Tumor
Incidence and Risk factors
Canine transmissible venereal tumor (TVT), also known as transmissible venereal sarcoma and Sticker’s sarcoma, is a naturally occurring, horizontally transmitted infectious histiocytic tumor of dogs usually spread by coitus, but it may also be spread by licking, biting, and sniffing tumor-affected areas.1-5 It has been observed occasionally in other canids, such as foxes, coyotes, and jackals.1,2 It has also been known as infectious sarcoma, venereal granuloma, canine condyloma, transmissible sarcoma, and transmissible lymphosarcoma.6,7
Although TVT has a worldwide distribution, its prevalence is highest in tropical and subtropical areas, particularly in the southern United States, Central and South America, southeast Europe, Ireland, Japan, China, the Far East, the Middle East, and parts of Africa.1,2 In enzootic areas, where breeding is poorly controlled and there are high numbers of free-roaming sexually active dogs, TVT is the most common canine tumor.1-3,8 In North America, prevalence of TVT is correlated with increased rainfall and mean annual temperature.9 Occasional cases occur in regions otherwise free of TVT following travel to endemic areas as a result of tourism.10 Pets travelling abroad can be exposed to TVT and carry it back home to nonendemic areas; therefore veterinarians may act as the first line of defense against the introduction of TVT as an emerging disease in nonendemic areas.
Because TVT is primarily spread by coitus, free-roaming, sexually intact mature dogs are at greatest risk.2 Dogs of any breed, age, or sex are susceptible.1,2,6 No heritable breed-related predisposition has been found.2,6 In endemic areas, although dogs over 1 year of age are at high risk, TVT is most common in dogs 2 to 5 years of age.2 The physical exertions associated with coitus in the dog with extensive abrasions and bleeding make both sexes susceptible to injury to the genital mucosa, which facilitates the exfoliation and implantation of tumor cells.2,6 Transmission can occur efficiently in either direction between the dog and the bitch. The most common sites of involvement are the external genitalia, but other sites that can be affected through licking or sniffing include the nasal and oral cavities, subcutaneous tissues, and the eyes.1-4,6,11-14 Spontaneous regressions occur and have been associated with immune responses against the tumor. Hence immunosuppression from any cause may be a risk factor for the development and maintenance of TVT and may predispose to widespread dissemination. When spontaneous regressions occurs, it usually starts within 3 months after implantation but rarely after 9 months of tumor.6
TVT is a transmissible allograft spread directly from dog to dog across major histocompatibility complex (MHC) barriers through transplantation of viable tumor cells on damaged mucosa through coitus or sniffing and licking.15 TVT and Tasmanian devil facial tumor disease (DFTD) are the only known naturally occurring clonally transmissible cancers that behave like an infectious parasitic neoplastic tissue graft.16 These cancers have overcome the limitations of existing within the single host, which gave rise to the tumors by gaining the ability to spread between individuals and thus survive long after the original hosts have died.
Pathology and Natural Behavior
TVT was initially recognized in 1876 and was utilized for the first successful experimental transmission of a tumor.6 A number of characteristics of TVT suggest that the tumor originated in inbred wolves or dogs about 10,000 to 15,000 years ago around the time that the dog was domesticated and subsequently the tumor has been spread worldwide.17 TVT has evolved into a transmissible parasite representing the oldest known colony of cloned somatic mammalian cells in continuous propagation.
Tumor growth generally appears on the external genitalia or nasal or oral mucosa within 2 to 6 months of mating and can either grow slowly and unpredictably for years or grow invasively and eventually become malignant and metastasize.1,2,6 Extragenital lesions can occur both alone (in isolation) and in association with genital lesions; however, it has been suggested that in most cases neoplastic foci can be detected on the genitalia.
TVT usually remains localized, but metastasis occurs in up to 5% to 17% of cases to draining regional lymph nodes (i.e., inguinal, iliac, tonsils), subcutaneous tissue, skin, eyes, oral mucosa, liver, spleen, peritoneum, hypophysis, brain, and bone marrow.2,11,18-20 Because TVT is also transmitted by licking, sniffing, and biting, many cases of reported metastases may instead actually be spread of the growth by mechanical extension or autotransplantation or heterotransplantation. Spontaneous regression can occur within 3 to 6 months of implantation, but the chance of self-regression is remote if the tumor is present for over 9 months.2
TVT is commonly described as a round (or discrete) cell tumor and suggested to be of histiocytic origin.6 This is supported by immunohistochemical (IHC) expression of vimentin, lysozyme, alpha-1-antitrypsin (AAT), and macrophage-specific ACM1, as well as negative IHC staining specific for other cell types.4,6,21,22 Immunohistochemistry has been helpful in confirming metastatic TVT in various anatomic locations.18-20 Furthermore, there have been reports describing TVT cells with intracellular Leishmania organisms also suggesting a histiocytic origin.14,23
Cytogenetic and genetic analyses have provided robust evidence of clonality. Whereas normal canine cell chromosomes consist of 76 acrocentric autosomes plus submetacentric X and Y sex chromosomes, TVT cells have a vastly rearranged karyotype consisting of 57 to 59 chromosomes, including 15 to 17 submetacentric chromosomes as a result of multiple centric fusions.4,17 However, the total number of chromosome arms in TVT is grossly comparable to the normal dog, so it appears the karyotypic rearrangement is not associated with significant change in DNA content.15,17 Although TVT cells are aneuploid, they exhibit remarkably stable and similar karyotypes in samples obtained from widely separate geographic regions (i.e., different continents).15 Likewise, molecular genetic studies of globally distributed TVT tumors provide evidence of a monophyletic origin, which has diverged into two subclades.15,24
In addition, TVT cells all share an insertion of a long interspersed nuclear element (LINE-1) upstream of the c-myc oncogene that is not found in normal dog genomes.25-29 This insertion has the potential to disrupt transcriptional regulation of downstream genes, possibly initiating oncogenic activity, and may have been causally involved in the origin of the tumor.30 This unique rearranged LINE-c-myc gene sequence has been used with polymerase chain reaction (PCR) as a diagnostic marker of TVT to confirm diagnosis.31,32 TVT cells have also demonstrated point mutations in the tumor suppressor gene p53, which is responsible for protecting the integrity of the DNA.29,33,34 Mutations of such a key regulator of the cell cycle, apoptosis, and senescence may be another factor in the oncogenesis of TVT.
TVT is an immunogenic tumor and the immunologic response of the host appears to play a critical role in determining the natural behavior of the disease. The course of disease is divided into a progressive phase (P) in which the tumor grows for 3 to 6 months, then a short stationary phase (S), which is followed by a regressive phase (R) in most dogs, unless the dog is elderly, in poor general condition, or immunologically compromised.6,8,35-44
Initially, in the P phase, the tumor downregulates its MHC class I β2-microglobulin and class II expression, which allows it to evade the host’s histocompatibility barrier, particularly T-cell cytotoxicity.45 Some cell-surface MHC class I expression remains, likely to prevent recognition and killing by natural killer (NK) cells. This immunoevasion is partly due to the high concentration of tumor-secreted transforming growth factor-β1 (TGF-β1), which inhibits tumor MHC antigen expression and NK cell activity.40 TVT also targets and damages dendritic cells (DCs).45 It has been suggested that TVT has evolved under survival pressures to escape host immunosurveillance.46
Tumor-infiltrating lymphocytes (TILs) produce IFNγ but fail to promote tumor MHC expression due to inhibition of IFNγ effects by tumor-derived TGF-β1, which also suppresses the cytotoxicity of the NK cells that migrate to the tumor site because of low tumor-antigen expression.36,39,40 However, late in the P phase, TILs produce high concentrations of the proinflammatory cytokine IL-6, which acts synergistically with host-derived IFNγ to antagonize the immunoinhibitory activity of TGF-β1 and results in MHC expression in up to 40% of tumor cells and restores NK cytotoxicity.37,47 A critical threshold level of IL-6 secreted by TILs has to be reached to trigger TVT into R phase.40,47 Therefore, after progressive growth for 3 to 4 months, the tumor spontaneously regresses with upregulation of MHC antigen expression possibly under epigenetic control.46
In addition to cell-mediated immunity, TVT also elicits a humoral immune response demonstrable by antibodies against TVT antigens.35,48 Experimentally, immunocompromised dogs do not demonstrate regression but rather continued progression of TVT and widespread metastatic disease.6 Conversely, dogs recovered from TVT have serum-transferable immunity to reinfection and puppies born to bitches exposed to TVT are less susceptible to the disease.49 In addition to the host immune response, during TVT regression stromal cells and extracellular matrix (ECM) react comparably to wound repair with collapse of the tumor parenchyma and replacement by fibrous stroma.43
History and Clinical Signs
The archetypical TVT patient is a sexually intact young adult dog either living in or having travelled to an area endemic for TVT, with a history of contact (coitus, sniffing, licking, or biting) with dogs of similar signalment.1,2,6,7 The primary lesions are usually on the external genitalia. In the male, the tumor is usually located on the caudal part of the penis, requiring caudal retraction of the penile sheath for visualization (Figure 33-6).2,6 Occasionally, it is on the prepuce. In the bitch, the tumor is usually in the posterior vagina or vestibule.2,6 Tumors appear initially as small 1 to 3 mm hyperemic papules that progress by fusing together into nodular, papillary, multilobulated cauliflower-like or pedunculated proliferations up to 10 to 15 cm in diameter. The mass is firm but friable, with an ulcerated inflamed surface. The tumor often oozes a serosanguineous or hemorrhagic fluid. Examples of extragenital sites are illustrated in Figure 33-7.
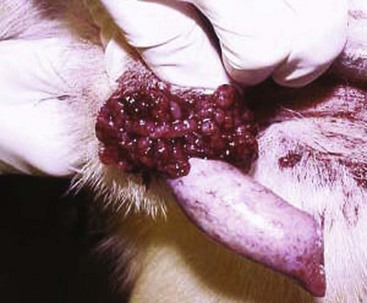
Figure 33-6 Typical appearance of a transmissible venereal tumor (TVT) in a male dog. The tumor is located at the base of the penis, has a cauliflower-like appearance, is very friable, and bleeds easily.
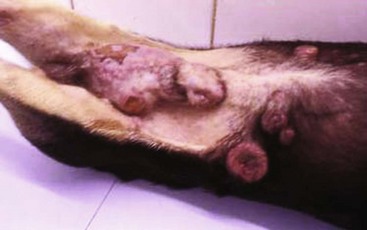
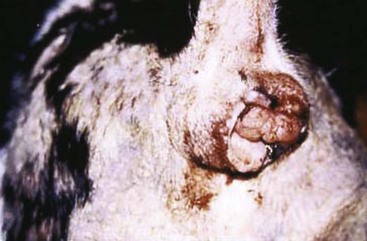
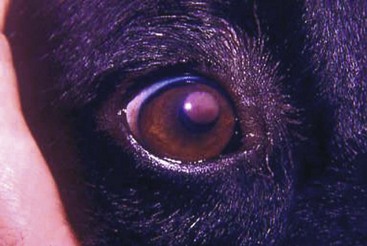
Figure 33-7 Other primary tumor sites. A, Male dog with cutaneous dissemination of transmissible venereal tumor (TVT) on the ventral abdomen. B, Mucocutaneous TVT of the anal area in a dog. C, Corneal involvement with TVT in a dog. (Courtesy Pr. Noeme S. Rocha.)
Clinical signs vary according to the location of the lesions. Genital lesions often manifest with chronic signs of discomfort or hemorrhagic discharge from the penile sheath or vulva for weeks to months prior to diagnosis, which can result in anemia.2,6,7 Lesions can predispose to ascending bacterial urinary tract infections but rarely interfere with micturition.50 Extragenital lesions cause a variety of signs, depending on anatomic location, such as sneezing, epistaxis, epiphora, halitosis, tooth loss, exophthalmos, skin masses, facial deformation, and regional lymph node enlargement.
Diagnostic Techniques and Work-Up
A presumptive diagnosis of TVT can be obtained based on history (including travel), signalment, clinical signs, and physical findings in dogs with the classic presentation. Definitive diagnosis is based on cytologic examination of cells obtained by swabs, FNAs, or imprints of the tumors or histologic examination of a biopsy from the mass. TVT is described as a discrete (or round) cell tumor. TVT has a characteristic morphologic appearance on cytopathology and is often diagnosed without need for histopathology (see Figure 7-37). Exfoliative cytology demonstrates uniform discrete round to polyhedral-shaped cells with moderately abundant pale blue cytoplasm and an eccentrically located nucleus, with occasional binucleation and mitotic figures.4,6 Single or multiple nucleoli are often observed, surrounded by clumped chromatin. The most characteristic feature is the presence of numerous discrete clear cytoplasmic vacuoles. In R phase, TVTs contain a higher number of infiltrating lymphocytes. Other round cell tumors, including lymphomas, mast cell tumors, plasma cell tumors, histiocytomas, and amelanotic melanomas, are important differential diagnoses but are generally not confused with TVT on cytopathology.
Histopathology of TVT reveals compact masses of round or polyhedral cells with slightly granular, vacuolated, eosinophilic cytoplasm.4,6 The neoplastic cells are arranged in a diffuse pattern and supported by a thin trabecula of fibrovascular tissue. Regressing tumors are infiltrated by lymphocytes, plasma cells, and macrophages.6 For atypical TVTs, if there is doubt about the diagnosis, specific molecular techniques can be utilized (e.g., in situ PCR of the rearranged LINE-c-myc gene sequence).31
The incidence of metastatic spread of TVT has been reported as less than 15%. However, in most cases of TVT, tumor staging is not performed. Therefore the metastatic rate might be higher. Regional lymph nodes should always be evaluated for metastasis by palpation and cytopathology. A thorough physical examination is essential to rule out other possible sites of involvement (i.e., skin, subcutis, nasal and oral cavities, the eye, the orbit). Diagnostic imaging is usually not required except with invasive TVT of the nasal cavity, orbit, or unusual locations. However, abdominal ultrasound may be used to image regional lymph nodes. CBC, serum biochemistry profile, and urinalysis do not reveal specific changes. Dogs bearing a large tumor burden of TVT have been associated with a paraneoplastic erythrocytosis that may require temporary symptomatic therapy.6
Therapy
TVTs will respond to many forms of therapy; however, chemotherapy is the most effective. Single-agent vincristine (0.5 to 0.7 mg/m2 intravenous [IV], once weekly for 3 to 6 treatments) obtains a complete and durable response in 90% to 95% of treated dogs.* Other single-agent and combination multiagent protocols employing cyclophosphamide, vinblastine, methotrexate, and prednisolone have not demonstrated superiority to vincristine alone.52,53,56 Resistant cases can be treated with DOX (25 to 30 mg/m2 IV, every 21 days for 3 treatments).1
RT has demonstrated efficacy against TVT. In a study using orthovoltage radiation at 1000 to 3000 cGy, all 18 dogs treated responded with a complete and durable response.57 Seven dogs were cured with a single coarse fraction of 1000 cGy. The other 11 dogs required 2 or 3 fractions to achieve complete response. Three of the 18 dogs were presented after recurrence following chemotherapy. Another study using megavoltage radiation from a Cobalt-60 unit reported all 15 dogs achieved complete and durable responses with 3 fractions administered over 1 week, for an average dose of 1500 cGy.11
Four of the 15 dogs had been resistant to vincristine. Therefore RT can be considered an effective treatment for TVT, particularly for lesions showing resistance to chemotherapy or located in sanctuary sites from chemotherapy (i.e., brain, testicle, eye).
Surgery can be an effective treatment for small localized TVT; however, surgery has an overall recurrence rate of 30% to 75%.58,59 Marginal surgical excision is not effective, and it can be difficult to obtain wide surgical margins in the areas in which TVT typically appears.58,59 In addition, tumor transplantation into the surgical wound by contamination from instruments or gloves may also cause postoperative tumor recurrence.
Other therapies described in spontaneous and experimentally induced cases include biologic-response modifiers, piroxicam, cryosurgery, radiofrequency ablation, laser ablation, and electrochemotherapy.60-64
Reduction of the incidence of TVT is possible by having dog owners and breeders carefully examine all males and females before mating to avoid breeding from affected animals.2 In addition, stray dogs can act as a TVT reservoir; therefore the mingling of breeding dogs with strays should be prevented. In some areas, the control of ownerless, free-roaming dogs can drastically reduce the incidence of TVT. TVT can enter the wild canid population through physical contact (licking, biting, or mating), and it is not known whether TVT could pose a threat to endangered wild canids.65
Prognosis
In most cases, TVT remains localized and rarely becomes disseminated in an immunocompetent host. In fact, a number of dogs will have their TVT spontaneously regress. For those dogs requiring treatment with vincristine or radiation, the prognosis for complete and durable clinical remission is excellent. Therefore the overall prognosis of canine TVT is generally very good to excellent. In stark contrast, the other naturally occurring transmissible tumor allograft, the Tasmanian DFTD, is highly virulent and kills most affected animals within 6 months by obstructing their ability to feed or breathe. It is speculated that the difference in virulence between the two allografts is due to the loss of MHC diversity in the Tasmanian devils, and perhaps one reason for MHC diversity in vertebrates is to ensure that cancer is not communicable between hosts like an infectious disease.15,17,46