Multiple Cartilaginous Exostosis
Multiple cartilaginous exostosis (MCE) is considered a developmental condition of growing dogs. There is evidence that the etiology of this condition may have a heritable component.332,358 The actual incidence of MCE is difficult to determine because affected dogs may show no signs and the diagnosis is often incidental. Lesions occur by the process of endochondral ossification when new bone is formed from a cartilage cap analogous to a physis. Lesions are located on bones, which form from endochondral ossification, and lesions stop growing at skeletal maturity. Malignant transformation of MCE lesions has been reported, but generally, they remain as unchanged, mature, bony projections from the surface of the bone from which they arose.333
Dogs typically present because of a nonpainful or moderately painful palpable mass on the surface of a bone or bones. The pain and lameness is thought to be due to mechanical interference of the mass with the overlying soft tissue structures. In the case of MCE of vertebral bodies, animals can present with clinical signs associated with spinal cord impingement. Radiographically, there is a bony mass on the surface of the affected bone, which has a benign appearance with fine trabecular pattern in the body of the mass (Figure 24-14). To obtain a histologic diagnosis, biopsy material must be collected so sections can include the cartilaginous cap and the underlying stalk of bone. Histologically, this cartilaginous cap gives rise to an orderly array of maturing bone according to the sequence of endochondral ossification. The cortical bone surfaces of the mass and the adjacent bone are confluent.331 A strong presumptive diagnosis is made by evaluation of the physical findings, history, and radiographic findings.
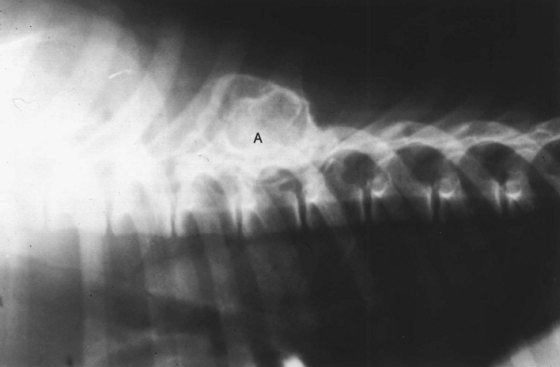
Figure 24-14 Lateral radiograph of a multiple cartilaginous exostosis lesion of the dorsal spinous process in a dog (A).
Treatment involves conservative surgical excision, but this is only necessary if signs do not abate after the dog is skeletally mature. Because of the likelihood of a heritable etiology, affected dogs should not be bred. Owners should also be advised of the possibility of late malignant transformation. Dogs with a previous history of MCE should be carefully evaluated for bone malignancy if signs return later in life.
Bone Cysts
Cysts are rare, benign lesions of bone. The majority of the veterinary literature pertaining to bone cysts centers on several small series of cases or single case reports.359-363 Affected animals are often young and present because of mild or moderate lameness; however, pathologic fracture can occur through cystic areas of long bones, leading to severe lameness. There appears to be a familial tendency in Doberman pinschers and Old English sheepdogs. The nomenclature in various reviews of canine bone cysts is confusing. By definition, a cyst is a fluid-filled sac lined by epithelium. The only true cyst of primary intraosseous origin is a simple bone cyst (SBC, or unicameral bone cyst). These lesions are usually in metaphyseal regions of long bones, and they can adjoin an open growth plate. Sometimes, however, unicameral bone cysts can be diaphyseal or epiphyseal. Neither the etiology nor the pathogenesis is known, but it is speculated that the lesions may be the result of trauma to the growth plate interfering with proper endochondral ossification. Others have theorized that with the rapid resorption and deposition of bone occurring in the metaphysis of a young animal, a cyst might develop if resorption is so rapid that a focus of loose fibrous tissue forms. The focus of fibrous tissue may then obstruct the thin-walled sinusoids, causing interstitial fluid to build up and form a cyst. The theory that appears to be partially substantiated is the synovial “rest” thesis. It is suggested that during fetal development a “rest” of synovial or perisynovial tissue becomes misplaced or incorporated into the adjacent osseous tissue. If this tissue remains or becomes functional, subsequent synovial secretion results in a cyst developing in the bone. Cysts have been described in bone just below articular cartilage (subchondral bone cysts or juxtacortical bone cysts).361,362,364 In these, it has often been possible to demonstrate direct communication with the articular synovial membrane. Radiographically, SBCs are single or, more commonly, multilocular, sharply defined, centrally located, radiolucent defects in the medullary canal of long bones. Variable degrees of thinning of the cortex with symmetric bone “expansion” are often a feature of the radiographs (Figure 24-15). The diagnosis cannot be reliably made from interpretation of radiographs alone. Lytic OS can be misdiagnosed as SBC. Diagnosis of an SBC relies on the histologic finding of a thin, fibrous wall lined by flat to slightly plump layers of mesothelial or endothelial cells. Treatment consists of meticulous curettage and packing the space with autogenous bone graft.
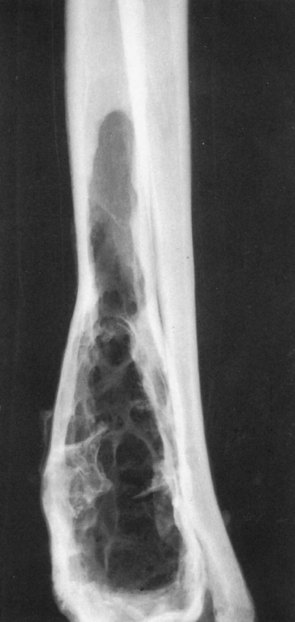
Figure 24-15 Lateral radiograph of a distal radial bone lesion histologically confirmed as a simple bone cyst (SBC). Typical radiographic features include a multilocular, sharply defined, centrally located radiolucency, without evidence of cortical destruction.
Aneurysmal bone “cysts” (ABCs) are spongy, multiloculated masses filled with free-flowing blood. The walls of an ABC are rarely lined by epithelium, and the lesion is possibly an arteriovenous malformation. A proposed pathogenesis of ABCs is that a primary event such as trauma or a benign bone tumor occurs within the bone or periosteum. This event disrupts the vasculature, resulting in a rapidly enlarging lesion with anomalous blood flow, which damages the bone mesenchyme. The bone reacts by proliferating. As the vascular anomaly becomes stabilized, the reactive bone becomes more consolidated and matures. It is important to differentiate these lesions from OS or other malignant lesions of bone. The age of affected dogs ranges from 2 to 14 years, but it has been reported in a 6-month-old dog.360 Treatment can be achieved by en bloc resection and reconstruction, but extensive curettage with packing of the defect with autogenous bone graft can be effective.
Primary Bone Tumors of Cats
Primary tumors involving the bones of cats are rare. An estimate of the incidence of all bone tumors in cats is 4.9 per 100,000.365 Between 67% and 90% of bone tumors in cats are histologically malignant. OSs are the most common primary bone tumor in cats accounting for 70% to 80% of all primary malignant cancer of cats. Feline OS occurs in appendicular or axial skeletal sites and extraskeletal sites. OS occurs in appendicular long bones approximately twice as often as in axial skeleton sites.366 However, in one study, 50 of 90 skeletal OS cases were appendicular and 40 of 90 were axial.367 Axial OS originates most commonly in the skull (especially oral cavity) and pelvis but is also reported in the ribs and vertebrae. The disease in cats differs from that in dogs in that the primary lesions occur more often in hind limbs in cats (distal femur and proximal tibia) and the disease is reported to be far less metastatic than in dogs.368 In a large feline OS case study, 56 of 146 cases were extraskeletal in origin, with the most commonly affected sites being those commonly used for vaccination: interscapular, dorsal lumbar, or thigh areas. Other locations included ocular/orbital, oral, intestinal, and mammary sites. No mention is made of any differences in the pathology of these tumors in comparison to skeletal sites.367 There are case reports of feline extraskeletal OSs in the flank, liver, and duodenum.369-371
OS generally affects older cats (reported mean ages of 8.5,366 10,368 10.2,368 and 10.7372 years), but the age range of reported cases is large (1 to 20 years), so bone lesions in younger cats cannot be ruled out as OSs based on age criteria alone. The age at presentation for axial OS is greater than appendicular OS.367 Conflicting reports on gender predisposition exist with either no difference between sexes or a slight male predisposition.366-368,372 OS was reported to arise after a limb fracture was repaired with an intramedullary pin in one cat and following RT in another.38
MCE is a disease that occurs after skeletal maturity in cats. This is in contrast to dogs in which exostoses develop before closure of growth plates. Also, in contrast to dogs, the lesions seldom affect long bones, are rarely symmetric, and are probably of viral rather than familial origin. There does not appear to be any breed or sex predisposition, although early reports of this condition were in the Siamese.373 Affected cats range in age from 1.3 to 8 years (mean 3.2 years).374 Virtually all cats with MCEs will test positive for feline leukemia virus (FeLV) antigenemia. This disease has an aggressive natural behavior.
Pathology and Natural Behavior
The histologic characteristics of feline OS are similar to canine OS. OS of cats is composed of mesenchymal cells embedded in malignant osteoid. There may be a considerable amount of cartilage present, and osteoid may be scant. A feature of some feline OS cases is the presence of multinucleate giant cells, which may be numerous. Reactive host bone and remnants of host bone are often present in specimens. Tumors are seen to be invasive; however, some surrounding soft tissue may be compressed rather than infiltrated. There is often variation of the histologic appearance within the tumor, with some portions having a more fibrosarcomatous appearance and others more cartilaginous. Some authors have described subtypes that resemble those seen in dogs: chondroblastic, fibroblastic, and telangiectatic, as well as the giant cell variant. These histologic subtypes, however, do not appear to confer any prognostic predictive value.375,376 OSs in cats are locally aggressive but have a low metastatic rate compared to canine OS. Feline OS can be of the juxtacortical type.
Osteochondroma may occur as a solitary lesion in cats, but there is a form that is multicentric (osteochondromatosis). The lesions are composed of hard, irregular exostoses with a fibrous and cartilaginous cap.377 Endochondral ossification occurs from the cartilage cap, which extends to a variable thickness. This cap tends to blend with adjacent tissue, making its surgical removal difficult. Cats usually develop multiple sites of disease, and there is a potential for malignant transformation and metastasis. The presence of FeLV antigenemia is also a foreboding prognostic finding for these cats.
History and Clinical Signs
The most common signs of OS are lameness, swelling, and deformity, depending on the location of the lesion. The lesions may appear radiographically similar to the OS in dogs with mixed osteoblastic and osteolytic aggressive bone lesion with an ill-defined zone of transition between normal and tumor-affected bone; however, some cats have lesions arising from the periosteal surface (juxtacortical OS).366 Tumors can reach a large size without evidence of severe clinical signs. It is rare for cats to have metastatic OS at presentation.
Cats with viral-associated MCE have rapidly progressing, conspicuous, hard swellings over affected sites causing pain and loss of function. Common sites for lesion development are the scapula, vertebrae, and mandible; however, any bone can become affected. Radiographically, the lesions are either sessile or pedunculated protuberances from bone surfaces with indistinct borders with the normal bone. There may be a loss of smooth contour with evidence of lysis, particularly if there is malignant transformation.
Diagnostic Work-Up
Both OS and MCE may be suspected, based on the radiographic appearance of the lesions and the FeLV status of the cat. Definitive diagnosis is made by histopathologic evaluation of properly collected biopsy tissue. Metastatic rates for cats with primary bone tumors are low compared to dogs (5% to 10% versus >90%), and three-view thoracic radiographs are recommended as part of the staging process. Presurgical evaluation with a CBC, chemistry profile, and urinalysis is recommended to rule out concurrent disease.
Therapy and Prognosis
Amputation is the recommended treatment for feline appendicular OS in which there are no clinically detectable metastatic lesions. Complete surgical excision of the primary tumor is prognostic for increased survival time, DFI, and recurrence-free interval.372 Due to the low metastatic rate and prolonged MSTs of 24 to 44 months with amputation alone,366,368 adjuvant chemotherapy is not indicated or recommended in cats, which is in contrast to the situation in dogs. No adjuvant therapy is known to be efficacious in extending survival time in cats. The MST for feline OS in axial sites (6.7 months) is lower than that of appendicular and extraskeletal OS sites.367 This likely reflects the difficulty of achieving complete resection and local tumor control in these anatomic sites rather than a difference in biologic behavior. A combination of surgical resection and RT may be appropriate in these cases. SRT has been used in several cats for local tumor control in appendicular and axial sites.
Histologic grade, using a grading scheme that evaluates tumor vascular invasion, pleomorphism, mitotic index, and tumor matrix and cell necrosis, is prognostic for survival.372 Specifically, tumors with high number of mitoses have a higher hazard ratio for decreased recurrence-free intervals. The metastatic potential of OS in cats (5% to 10%) is much less than for the same disease in dogs or humans. Reported anatomic sites for metastases are lung, kidney, liver, brain, and spleen. Cats with MCE have a guarded prognosis. Lesions may be removed surgically for palliation; however, local recurrences are common, or new, painful, debilitating lesions may occur. No reliably effective treatment is known for this condition in cats.
Fibrosarcoma, Chondrosarcoma, and Hemangiosarcoma
Non-OS primary bone tumors in cats are rare. Fibrosarcoma is the second most common primary bone tumor of cats.375 Chondrosarcoma is reported to be next in terms of frequency, and hemangiosarcomas rarely involve bones of cats.368 Little is known about the biologic behavior of these rare lesions. Aggressive surgical resection is the preferred treatment for these tumors. The metastatic rate is low; however, metastases have been seen in cats with chondrosarcoma and hemangiosarcoma.368,376
References
1. Brodey, RS, Mc, GJ, Reynolds, H. A clinical and radiological study of canine bone neoplasms. I. J Am Vet Med Assoc. 1959;134:53–71.
2. Brodey, RS, Riser, WH. Canine osteosarcoma: a clinicopathologic study of 194 cases. Clin Orthop Relat Res. 1969;62:54–64.
3. Brodey, RS, Sauer, RM, Medway, W. Canine bone neoplasms. J Am Vet Med Assoc. 1963;143:471–495.
4. Dorfman, SK, Hurvitz, AI, Patnaik, AK. Primary and secondary bone tumours in the dog. J Small Anim Pract. 1977;18:313–326.
5. Ling, GV, Morgan, JP, Pool, RR. Primary bone tumors in the dog: a combined clinical, radiographic, and histologic approach to early diagnosis. J Am Vet Med Assoc. 1974;165:55–67.
6. Priester, WA, McKay, FW. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980:1–210.
7. Withrow, SJ, Powers, BE, Straw, RC, et al. Comparative aspects of osteosarcoma: dog versus man. Clin Orthop Relat Res. 1991;Sept(270):159–168.
8. Alexander, JW, Patton, CS. Primary tumors of the skeletal system. Vet Clin North Am Small Anim Pract. 1983;13:181–195.
9. Brodey, RS, Abt, DA. Results of surgical treatment in 65 dogs with osteosarcoma. J Am Vet Med Assoc. 1976;168:1032–1035.
10. Jongeward, SJ. Primary bone tumors. Vet Clin North Am Small Anim Pract. 1985;15:609–641.
11. Knecht, CD, Priester, WA. Musculoskeletal tumors in dogs. J Am Vet Med Assoc. 1978;172:72–74.
12. Misdorp, W. Skeletal osteosarcoma: animal model: canine osteosarcoma. Am J Pathol. 1980;98:285–288.
13. Misdorp, W, Hart, AA. Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst. 1979;62:537–545.
14. Nielsen, SW, Schroder, JD, Smith, DL. The pathology of osteogenic sarcoma in dogs. J Am Vet Med Assoc. 1954;124:28–35.
15. Spodnick, GJ, Berg, J, Rand, WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988). J Am Vet Med Assoc. 1992;200:995–999.
16. Straw, RC, Withrow, SJ, Richter, SL, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med. 1991;5:205–210.
17. Tjalma, RA. Canine bone sarcoma: estimation of relative risk as a function of body size. J Natl Cancer Inst. 1966;36:1137–1150.
18. Wolke, RE, Nielsen, SW. Site incidence of canine osteosarcoma. J Small Anim Pract. 1966;7:489–492.
19. Smith, RL, Sr. Osteosarcoma in dogs in the Brisbane area. Aust Vet Pract. 1988;18:97–100.
20. Phillips, L, Hager, D, Parker, R, et al. Osteosarcoma with a pathologic fracture in a six-month-old dog. Vet Radiol. 1986;27:18–19.
21. Feeney, DA, Johnston, GR, Grindem, CB, et al. Malignant neoplasia of canine ribs: clinical, radiographic, and pathologic findings. J Am Vet Med Assoc. 1982;180:927–933.
22. Heyman, SJ, Diefenderfer, DL, Goldschmidt, MH, et al. Canine axial skeletal osteosarcoma. A retrospective study of 116 cases (1986 to 1989). Vet Surg. 1992;21:304–310.
23. Ru, G, Terracini, B, Glickman, LT. Host related risk factors for canine osteosarcoma. Vet J. 1998;156:31–39.
24. Cooley, DM, Beranek, BC, Schlittler, DL, et al. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1434–1440.
25. Liptak, JM, Dernell, WS, Straw, RC, et al. Proximal radial and distal humeral osteosarcoma in 12 dogs. J Am Anim Hosp Assoc. 2004;40:461–467.
26. Gamblin, RM, Straw, RC, Powers, BE, et al. Primary osteosarcoma distal to the antebrachiocarpal and tarsocrural joints in nine dogs (1980-1992). J Am Anim Hosp Assoc. 1995;31:86–91.
27. Bleier, T, Lewitschek, HP, Reinacher, M. Canine osteosarcoma of the penile bone. J Vet Med A Physiol Pathol Clin Med. 2003;50:397–398.
28. Lucroy, MD, Peck, JN, Berry, CR. Osteosarcoma of the patella with pulmonary metastases in a dog. Vet Radiol Ultrasound. 2001;42:218–220.
29. LaRue, SM, Withrow, SJ, Wrigley, RH. Radiographic bone surveys in the evaluation of primary bone tumors in dogs. J Am Vet Med Assoc. 1986;188:514–516.
30. Bech-Nielsen, S, Haskins, ME, Reif, JS, et al. Frequency of osteosarcoma among first-degree relatives of St. Bernard dogs. J Natl Cancer Inst. 1978;60:349–353.
31. Kuntz, CA, Dernell, WS, Powers, BE, et al. Extraskeletal osteosarcomas in dogs: 14 cases. J Am Anim Hosp Assoc. 1998;34:26–30.
32. Langenbach, A, Anderson, MA, Dambach, DM, et al. Extraskeletal osteosarcomas in dogs: a retrospective study of 169 cases (1986-1996). J Am Anim Hosp Assoc. 1998;34:113–120.
33. Patnaik, AK. Canine extraskeletal osteosarcoma and chondrosarcoma: a clinicopathologic study of 14 cases. Vet Pathol. 1990;27:46–55.
34. Ringenberg, MA, Neitzel, LE, Zachary, JF. Meningeal osteosarcoma in a dog. Vet Pathol. 2000;37:653–655.
35. Thamm, DH, Mauldin, EA, Edinger, DT, et al. Primary osteosarcoma of the synovium in a dog. J Am Anim Hosp Assoc. 2000;36:326–331.
36. Owen, LN. Transplantation of canine osteosarcoma. Eur J Cancer. 1969;5:615–620.
37. Gellasch, KL, Kalscheur, VL, Clayton, MK, et al. Fatigue microdamage in the radial predilection site for osteosarcoma in dogs. Am J Vet Res. 2002;63:896–899.
38. Bennett, D, Campbell, JR, Brown, P. Osteosarcoma associated with healed fractures. J Small Anim Pract. 1979;20:13–18.
39. Sinibaldi, K, Rosen, H, Liu, SK, et al. Tumors associated with metallic implants in animals. Clin Orthop Relat Res. 1976:257–266.
40. Stevenson, S, Hohn, RB, Pohler, OE, et al. Fracture-associated sarcoma in the dog. J Am Vet Med Assoc. 1982;180:1189–1196.
41. Rosin, A, Rowland, GN. Undifferentiated sarcoma in a dog following chronic irritation by a metallic foreign body and concurrent infection. J Am Anim Hosp Assoc. 1981;17:593–598.
42. Knecht, CD, Priester, WA. Osteosarcoma in dogs: a study of previous trauma, fracture, and fracture fixation. J Am Anim Hosp Assoc. 1978;14:82–84.
43. Vasseur, PB, Stevenson, S. Osteosarcoma at the site of a cortical bone allograft in a dog. Vet Surg. 1987;16:70–74.
44. Gillette, SM, Gillette, EL, Powers, BE, et al. Radiation-induced osteosarcoma in dogs after external beam or intraoperative radiation therapy. Cancer Res. 1990;50:54–57.
45. Lloyd, RD, Taylor, GN, Angus, W, et al. Distribution of skeletal malignancies in beagles injected with 239Pu citrate. Health Phys. 1994;66:407–413.
46. McEntee, MC, Page, RL, Theon, A, et al. Malignant tumor formation in dogs previously irradiated for acanthomatous epulis. Vet Radiol Ultrasound. 2004;45:357–361.
47. Miller, SC, Lloyd, RD, Bruenger, FW, et al. Comparisons of the skeletal locations of putative plutonium-induced osteosarcomas in humans with those in beagle dogs and with naturally occurring tumors in both species. Radiat Res. 2003;160:517–523.
48. Powers, BE, Gillette, EL, McChesney, SL, et al. Bone necrosis and tumor induction following experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1989;17:559–567.
49. Robinson, E, Neugut, AI, Wylie, P. Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst. 1988;80:233–240.
50. Tillotson, C, Rosenberg, A, Gebhardt, M, et al. Postradiation multicentric osteosarcoma. Cancer. 1988;62:67–71.
51. White, RG, Raabe, OG, Culbertson, MR, et al. Bone sarcoma characteristics and distribution in beagles fed strontium-90. Radiat Res. 1993;136:178–189.
52. White, RG, Raabe, OG, Culbertson, MR, et al. Bone sarcoma characteristics and distribution in beagles injected with radium-226. Radiat Res. 1994;137:361–370.
53. Dahlin, DC, Coventry, MB. Osteogenic sarcoma: a study of six hundred cases. J Bone Joint Surg Am. 1967;49:101–110.
54. McKenna, RJ, Schwinn, CP, Higginbotham, NL. Osteogenic sarcoma in children. CA Cancer J Clin. 1966;16:26–28.
55. Dubielzig, RR, Biery, DN, Brodey, RS. Bone sarcomas associated with multifocal medullary bone infarction in dogs. J Am Vet Med Assoc. 1981;179:64–68.
56. Riser, WH, Brodey, RS, Biery, DN. Bone infarctions associated with malignant bone tumors in dogs. J Am Vet Med Assoc. 1972;160:414–421.
57. Prior, C, Watrous, BJ, Penfold, D. Radial diaphyseal osteosarcoma with associated bone infarction in a dog. J Am Anim Hosp Assoc. 1986;22:43–48.
58. Ansari, MM. Bone infarcts associated with malignant sarcomas. Comp Cont Ed Pract Vet. 1991;3:367–370.
59. Boulay, J, Wallace, L, Lipowitz, A. Pathologic fracture of long bones in the dog. J Am Anim Hosp Assoc. 1987;23:297–303.
60. Marcellin-Little, DJ, DeYoung, DJ, Thrall, DE, et al. Osteosarcoma at the site of bone infarction associated with total hip arthroplasty in a dog. Vet Surg. 1999;28:54–60.
61. Holmberg, BJ, Farese, JP, Taylor, D, et al. Osteosarcoma of the humeral head associated with osteochondritis dissecans in a dog. J Am Anim Hosp Assoc. 2004;40:246–249.
62. Barnhart, MD. Malignant transformation of an aneurysmal bone cyst in a dog. Vet Surg. 2002;31:519–524.
63. Johnson, AS, Couto, CG, Weghorst, CM. Mutation of the p53 tumor suppressor gene in spontaneously occurring osteosarcomas of the dog. Carcinogenesis. 1998;19:213–217.
64. Kirpensteijn, J, Kik, M, Teske, E, et al. TP53 gene mutations in canine osteosarcoma. Vet Surg. 2008;37:454–460.
65. Levine, RA, Fleischli, MA. Inactivation of p53 and retinoblastoma family pathways in canine osteosarcoma cell lines. Vet Pathol. 2000;37:54–61.
66. Loukopoulos, P, Thornton, JR, Robinson, WF. Clinical and pathologic relevance of p53 index in canine osseous tumors. Vet Pathol. 2003;40:237–248.
67. Mendoza, S, Konishi, T, Dernell, WS, et al. Status of the p53, Rb and MDM2 genes in canine osteosarcoma. Anticancer Res. 1998;18:4449–4453.
68. Sagartz, JE, Bodley, WL, Gamblin, RM, et al. p53 tumor suppressor protein overexpression in osteogenic tumors of dogs. Vet Pathol. 1996;33:213–221.
69. Setoguchi, A, Sakai, T, Okuda, M, et al. Aberrations of the p53 tumor suppressor gene in various tumors in dogs. Am J Vet Res. 2001;62:433–439.
70. van Leeuwen, IS, Cornelisse, CJ, Misdorp, W, et al. P53 gene mutations in osteosarcomas in the dog. Cancer Lett. 1997;111:173–178.
71. Thomas, R, Wang, HJ, Tsai, PC, et al. Influence of genetic background on tumor karyotypes: evidence for breed-associated cytogenetic aberrations in canine appendicular osteosarcoma. Chromosome Res. 2009;17:365–377.
72. Levine, AJ, Chang, A, Dittmer, D, et al. The p53 tumor suppressor gene. J Lab Clin Med. 1994;123:817–823.
73. Wadayama, B, Toguchida, J, Shimizu, T, et al. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994;54:3042–3048.
74. Levine, RA, Forest, T, Smith, C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet Pathol. 2002;39:372–378.
75. Angstadt, AY, Motsinger-Reif, A, Thomas, R, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosomes Cancer. 2011;50:859–874.
76. Phillips, JC, Lembcke, L, Chamberlin, T. A novel locus for canine osteosarcoma (OSA1) maps to CFA34, the canine orthologue of human 3q26. Genomics. 2010;96:220–227.
77. Phillips, JC, Stephenson, B, Hauck, M, et al. Heritability and segregation analysis of osteosarcoma in the Scottish deerhound. Genomics. 2007;90:354–363.
78. Urfer, SR, Gaillard, C, Steiger, A. Lifespan and disease predispositions in the Irish Wolfhound: a review. Vet Q. 2007;29:102–111.
79. Rosenberger, JA, Pablo, NV, Crawford, PC. Prevalence of and intrinsic risk factors for appendicular osteosarcoma in dogs: 179 cases (1996-2005). J Am Vet Med Assoc. 2007;231:1076–1080.
80. Fox, MH, Armstrong, LW, Withrow, SJ, et al. Comparison of DNA aneuploidy of primary and metastatic spontaneous canine osteosarcomas. Cancer Res. 1990;50:6176–6178.
81. Liao, AT, McCleese, J, Kamerling, S, et al. A novel small molecule Met inhibitor, PF2362376, exhibits biological activity against osteosarcoma. Vet Comp Oncol. 2007;5:177–196.
82. Liao, AT, McMahon, M, London, C. Characterization, expression and function of c-Met in canine spontaneous cancers. Vet Comp Oncol. 2005;3:61–72.
83. MacEwen, EG, Kutzke, J, Carew, J, et al. c-Met tyrosine kinase receptor expression and function in human and canine osteosarcoma cells. Clin Exp Metastasis. 2003;20:421–430.
84. Ferracini, R, Angelini, P, Cagliero, E, et al. MET oncogene aberrant expression in canine osteosarcoma. J Orthop Res. 2000;18:253–256.
85. Liao, AT, McMahon, M, London, CA. Identification of a novel germline MET mutation in dogs. Anim Genet. 2006;37:248–252.
86. Fieten, H, Spee, B, Ijzer, J, et al. Expression of hepatocyte growth factor and the proto-oncogenic receptor c-Met in canine osteosarcoma. Vet Pathol. 2009;46:869–877.
87. MacEwen, EG, Pastor, J, Kutzke, J, et al. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J Cell Biochem. 2004;92:77–91.
88. Khanna, C, Prehn, J, Hayden, D, et al. A randomized controlled trial of octreotide pamoate long-acting release and carboplatin versus carboplatin alone in dogs with naturally occurring osteosarcoma: evaluation of insulin-like growth factor suppression and chemotherapy. Clin Cancer Res. 2002;8:2406–2412.
89. Gorlick, R, Huvos, AG, Heller, G, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–2788.
90. Scotlandi, K, Manara, MC, Hattinger, CM, et al. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing’s sarcoma. Eur J Cancer. 2005;41:1349–1361.
91. Flint, AF, U’Ren, L, Legare, ME, et al. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41:291–296.
92. Gordon, IK, Ye, F, Kent, MS. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am J Vet Res. 2008;69:1079–1084.
93. Paoloni, MC, Mazcko, C, Fox, E, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5:e11013.
94. Fan, TM, Barger, AM, Sprandel, IT, et al. Investigating TrkA expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:1181–1188.
95. Kow, K, Bailey, SM, Williams, ES, et al. Telomerase activity in canine osteosarcoma. Vet Comp Oncol. 2006;4:184–187.
96. Kow, K, Thamm, DH, Terry, J, et al. Impact of telomerase status on canine osteosarcoma patients. J Vet Intern Med. 2008;22:1366–1372.
97. Loukopoulos, P, O’Brien, T, Ghoddusi, M, et al. Characterisation of three novel canine osteosarcoma cell lines producing high levels of matrix metalloproteinases. Res Vet Sci. 2004;77:131–141.
98. Barger, AM, Fan, TM, de Lorimier, LP, et al. Expression of receptor activator of nuclear factor kappa-B ligand (RANKL) in neoplasms of dogs and cats. J Vet Intern Med. 2007;21:133–140.
99. Lana, SE, Ogilvie, GK, Hansen, RA, et al. Identification of matrix metalloproteinases in canine neoplastic tissue. Am J Vet Res. 2000;61:111–114.
100. Schmit, JM, Pondenis, HC, Barger, AM, et al. Cathepsin k expression and activity in canine osteosarcoma. J Vet Intern Med. 2012;26:126–134.
101. Khanna, C, Wan, X, Bose, S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186.
102. Fan, TM, Barger, AM, Fredrickson, RL, et al. Investigating CXCR4 expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:602–608.
103. Barger, A, Graca, R, Bailey, K, et al. Use of alkaline phosphatase staining to differentiate canine osteosarcoma from other vimentin-positive tumors. Vet Pathol. 2005;42:161–165.
104. Neihaus, SA, Locke, JE, Barger, AM, et al. A novel method of core aspirate cytology compared to fine-needle aspiration for diagnosing canine osteosarcoma. J Am Anim Hosp Assoc. 2011;47:317–323.
105. Britt, T, Clifford, C, Barger, A, et al. Diagnosing appendicular osteosarcoma with ultrasound-guided fine-needle aspiration: 36 cases. J Small Anim Pract. 2007;48:145–150.
106. Kirpensteijn, J, Kik, M, Rutteman, GR, et al. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol. 2002;39:240–246.
107. O’Donoghue, LE, Ptitsyn, AA, Kamstock, DA, et al. Expression profiling in canine osteosarcoma: identification of biomarkers and pathways associated with outcome. BMC Cancer. 2010;10:506.
108. Bhandal, J, Boston, SE. Pathologic fracture in dogs with suspected or confirmed osteosarcoma. Vet Surg. 2011;40:423–430.
109. Kim, MS, Lee, SY, Lee, TR, et al. Prognostic effect of pathologic fracture in localized osteosarcoma: a cohort/case controlled study at a single institute. J Surg Oncol. 2009;100:233–239.
110. Ebeid, W, Amin, S, Abdelmegid, A. Limb salvage management of pathologic fractures of primary malignant bone tumors. Cancer Control. 2005;12:57–61.
111. Bacci, G, Ferrari, S, Longhi, A, et al. Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand. 2003;74:449–454.
112. Kuettner, KE, Pauli, BU, Soble, L. Morphological studies on the resistance of cartilage to invasion by osteosarcoma cells in vitro and in vivo. Cancer Res. 1978;38:277–287.
113. Brem, H, Folkman, J. Inhibition of tumor angiogenesis mediated by cartilage. J Exp Med. 1975;141:427–439.
114. Hillers, KR, Dernell, WS, Lafferty, MH, et al. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986-2003). J Am Vet Med Assoc. 2005;226:1364–1367.
115. Boston, SE, Ehrhart, NP, Dernell, WS, et al. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985-2004). J Am Vet Med Assoc. 2006;228:1905–1908.
116. Giuliano, AE, Feig, S, Eilber, FR. Changing metastatic patterns of osteosarcoma. Cancer. 1984;54:2160–2164.
117. Bacci, G, Avella, M, Picci, P, et al. Metastatic patterns in osteosarcoma. Tumori. 1988;74:421–427.
118. Kaya, M, Wada, T, Nagoya, S, et al. Concomitant tumour resistance in patients with osteosarcoma: a clue to a new therapeutic strategy. J Bone Joint Surg Br. 2004;86:143–147.
119. Tsunemi, T, Nagoya, S, Kaya, M, et al. Postoperative progression of pulmonary metastasis in osteosarcoma. Clin Orthop. 2003;407:159–166.
120. Moore, GE, Mathey, WS, Eggers, JS, et al. Osteosarcoma in adjacent lumbar vertebrae in a dog. J Am Vet Med Assoc. 2000;217:1038–1040. [1008].
121. Sajadi, KR, Heck, RK, Neel, MD, et al. The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res. 2004:92–96.
122. Straw, R, Powers, BE, Klausner, J, et al. Canine mandibular osteosarcoma: 51 cases (1980-1992). J Am Anim Hosp Assoc. 1996;32:257–262.
123. Dickerson, ME, Page, RL, LaDue, TA, et al. Retrospective analysis of axial skeleton osteosarcoma in 22 large-breed dogs. J Vet Intern Med. 2001;15:120–124.
124. Mehl, ML, Withrow, SJ, Seguin, B, et al. Spontaneous regression of osteosarcoma in four dogs. J Am Vet Med Assoc. 2001;219:614–617.
125. Mazzaferro, EM, Hackett, TB, Stein, TP, et al. Metabolic alterations in dogs with osteosarcoma. Am J Vet Res. 2001;62:1234–1239.
126. Kazmierski, KJ, Ogilvie, GK, Fettman, MJ, et al. Serum zinc, chromium, and iron concentrations in dogs with lymphoma and osteosarcoma. J Vet Intern Med. 2001;15:585–588.
127. Wrigley, RH. Malignant versus nonmalignant bone disease. Vet Clin North Am Small Anim Pract. 2000;30:315–347. [vi–vii].
128. Reinhardt, S, Stockhaus, C, Teske, E, et al. Assessment of cytological criteria for diagnosing osteosarcoma in dogs. J Small Anim Pract. 2005;46:65–70.
129. Samii, VF, Nyland, TG, Werner, LL, et al. Ultrasound-guided fine-needle aspiration biopsy of bone lesions: a preliminary report. Vet Radiol Ultrasound. 1999;40:82–86.
130. Ehrhart, N. Principles of tumor biopsy. Clin Tech Small Anim Pract. 1998;13:10–16.
131. Mankin, HJ, Lange, TA, Spanier, SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127.
132. deSantos, LA, Murray, JA, Ayala, AG. The value of percutaneous needle biopsy in the management of primary bone tumors. Cancer. 1979;43:735–744.
133. Simon, MA. Biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1982;64:1253–1257.
134. Wykes, PM, Withrow, SJ, Powers, BE. Closed biopsy for diagnosis of long bone tumors: accuracy and results. J Am Anim Hosp Assoc. 1985;21:489–494.
135. Powers, BE, LaRue, SM, Withrow, SJ, et al. Jamshidi needle biopsy for diagnosis of bone lesions in small animals. J Am Vet Med Assoc. 1988;193:205–210.
136. Vignoli, M, Ohlerth, S, Rossi, F, et al. Computed tomography-guided fine-needle aspiration and tissue-core biopsy of bone lesions in small animals. Vet Radiol Ultrasound. 2004;45:125–130.
137. Barthez, PY, Hornof, WJ, Theon, AP, et al. Receiver operating characteristic curve analysis of the performance of various radiographic protocols when screening dogs for pulmonary metastases. J Am Vet Med Assoc. 1994;204:237–240.
138. Picci, P, Vanel, D, Briccoli, A, et al. Computed tomography of pulmonary metastases from osteosarcoma: the less poor technique: a study of 51 patients with histological correlation. Ann Oncol. 2001;12:1601–1604.
139. Waters, DJ, Coakley, FV, Cohen, MD, et al. The detection of pulmonary metastases by helical CT: a clinicopathologic study in dogs. J Comput Assist Tomogr. 1998;22:235–240.
140. Eberle, N, Fork, M, von Babo, V, et al. Comparison of examination of thoracic radiographs and thoracic computed tomography in dogs with appendicular osteosarcoma. Vet Comp Oncol. 2011;9:131–140.
141. Straw, RC, Cook, NL, LaRue, SM, et al. Radiographic bone surveys. J Am Vet Med Assoc. 1989;195:1458.
142. Berg, J, Lamb, CR, O’Callaghan, MW. Bone scintigraphy in the initial evaluation of dogs with primary bone tumors. J Am Vet Med Assoc. 1990;196:917–920.
143. Hahn, KA, Hurd, C, Cantwell, HD. Single-phase methylene diphosphate bone scintigraphy in the diagnostic evaluation of dogs with osteosarcoma. J Am Vet Med Assoc. 1990;196:1483–1486.
144. Jankowski, MK, Steyn, PF, Lana, SE, et al. Nuclear scanning with 99mTc-HDP for the initial evaluation of osseous metastasis in canine osteosarcoma. Vet Comp Oncol. 2003;1:152–158.
145. Lamb, CR. Bone scintigraphy in small animals. J Am Vet Med Assoc. 1987;191:1616–1622.
146. Parchman, MB, Flanders, JA, Erb, HN, et al. Nuclear medical bone imaging and targeted radiography for evaluation of skeletal neoplasms in 23 dogs. Vet Surg. 1989;18:454–458.
147. Enneking, WF, Spanier, SS, Goodman, MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980:106–120.
148. Lamb, CR, Berg, J, Bengtson, AE. Preoperative measurement of canine primary bone tumors, using radiography and bone scintigraphy. J Am Vet Med Assoc. 1990;196:1474–1478.
149. Leibman, NF, Kuntz, CA, Steyn, PF, et al. Accuracy of radiography, nuclear scintigraphy, and histopathology for determining the proximal extent of distal radius osteosarcoma in dogs. Vet Surg. 2001;30:240–245.
150. Davis, GJ, Kapatkin, AS, Craig, LE, et al. Comparison of radiography, computed tomography, and magnetic resonance imaging for evaluation of appendicular osteosarcoma in dogs. J Am Vet Med Assoc. 2002;220:1171–1176.
151. Wallack, ST, Wisner, ER, Werner, JA, et al. Accuracy of magnetic resonance imaging for estimating intramedullary osteosarcoma extent in pre-operative planning of canine limb-salvage procedures. Vet Radiol Ultrasound. 2002;43:432–441.
152. Bergman, PJ, MacEwen, EG, Kurzman, ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med. 1996;10:76–81.
153. Cho, WH, Song, WS, Jeon, DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17:702–708.
154. Kuntz, CA, Asselin, TL, Dernell, WS, et al. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. 1998;27:417–422.
155. Sottnik, JL, Rao, S, Lafferty, MH, et al. Association of blood monocyte and lymphocyte count and disease-free interval in dogs with osteosarcoma. J Vet Intern Med. 2010;24:1439–1444.
156. Brostrom, LA, Strander, H, Nilsonne, U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982:250–254.
157. Hammer, AS, Weeren, FR, Weisbrode, SE, et al. Prognostic factors in dogs with osteosarcomas of the flat or irregular bones. J Am Anim Hosp Assoc. 1995;31:321–326.
158. Cooley, DM, Waters, DJ. Skeletal neoplasms of small dogs: a retrospective study and literature review. J Am Anim Hosp Assoc. 1997;33:11–23.
159. Wallace, J, Matthiesen, DT, Patnaik, AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg. 1992;21:337–341.
160. Schwarz, PD, Withrow, SJ, Curtis, CR, et al. Partial maxillary resection as a treatment for oral cancer in 61 dogs. J Am Anim Hosp Assoc. 1991;27:616–624.
161. Kosovsky, JK, Matthiesen, DT, Marretta, SM, et al. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet Surg. 1991;20:397–401.
162. Beckwith, K, Eickhoff, J, Dernell, WS, et al. Osteosarcoma of the canine head: a retrospective analysis of 136 cases (1991-2008). Veterinary Cancer Society. 49, 2010. [San Diego, CA].
163. Hendrix, DV, Gelatt, KN. Diagnosis, treatment and outcome of orbital neoplasia in dogs: a retrospective study of 44 cases. J Small Anim Pract. 2000;41:105–108.
164. Duffaud, F, Digue, L, Baciuchka-Palmaro, M, et al. Osteosarcomas of flat bones in adolescents and adults. Cancer. 2000;88:324–332.
165. Pirkey-Ehrhart, N, Withrow, SJ, Straw, RC, et al. Primary rib tumors in 54 dogs. J Am Anim Hosp Assoc. 1995;31:65–69.
166. Baines, SJ, Lewis, S, White, RA. Primary thoracic wall tumours of mesenchymal origin in dogs: a retrospective study of 46 cases. Vet Rec. 2002;150:335–339.
167. Montgomery, RD, Henderson, RA, Powers, RD, et al. Retrospective study of 26 primary thoracic wall tumors in dogs. J Am Anim Hosp Assoc. 1993;29:68–72.
168. Matthiesen, DT, Clark, GN, Orsher, RJ, et al. En bloc resection of primary rib tumors in 40 dogs. Vet Surg. 1992;21:201–204.
169. Trout, NJ, Pavletic, MM, Kraus, KH. Partial scapulectomy for management of sarcomas in three dogs and two cats. J Am Vet Med Assoc. 1995;207:585–587.
170. Norton, C, Drenen, CM, Emms, SG. Subtotal scapulectomy as the treatment for scapular tumour in the dog: a report of six cases. Aust Vet J. 2006;84:364–366.
171. Montinaro, V, Boston, SE, Buracco, P, et al. Evaluation of scapulectomy for primary bone tumors in 42 dogs: a Veterinary Society of Surgical Oncology (VSSO) retrospective study. Vet Surg. 2012. [Accepted for publication].
172. Dernell, WS, Van Vechten, BJ, Straw, RC, et al. Outcome following treatment for vertebral tumors in 20 dogs. J Am Anim Hosp Assoc. 2000;36:245–251.
173. Garzotto, CK, Berg, J, Hoffmann, WE, et al. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med. 2000;14:587–592.
174. Ehrhart, N, Dernell, WS, Hoffmann, WE, et al. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990-1996). J Am Vet Med Assoc. 1998;213:1002–1006.
175. Moore, AS, Dernell, WS, Ogilvie, GK, et al. Doxorubicin and BAY 12-9566 for the treatment of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study. J Vet Intern Med. 2007;21:783–790.
176. McCleese, JK, Bear, MD, Kulp, SK, et al. Met interacts with EGFR and Ron in canine osteosarcoma. Vet Comp Oncol. 2011.
177. Mullins, MN, Lana, SE, Dernell, WS, et al. Cyclooxygenase-2 expression in canine appendicular osteosarcomas. J Vet Intern Med. 2004;18:859–865.
178. Shoeneman, JK, Ehrhart, EJ, 3rd., Eickhoff, JC, et al. Expression and function of survivin in canine osteosarcoma. Cancer Res. 2012;72:249–259.
179. Thamm, DH, O’Brien, MG, Vail, DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet Comp Oncol. 2008;6:126–132.
180. Selvarajah, GT, Kirpensteijn, J, van Wolferen, ME, et al. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. 2009;8:72.
181. Scott, MC, Sarver, AL, Gavin, KJ, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49:356–367.
182. Biller, BJ, Elmslie, RE, Burnett, RC, et al. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. 2007;116:69–78.
183. O’Neill, K, Guth, A, Biller, B, et al. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med. 2009;23:875–881.
184. Rissetto, KC, Rindt, H, Selting, KA, et al. Cloning and expression of canine CD25 for validation of an anti-human CD25 antibody to compare T regulatory lymphocytes in healthy dogs and dogs with osteosarcoma. Vet Immunol Immunopathol. 2010;135:137–145.
185. Biller, BJ, Guth, A, Burton, JH, et al. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med. 2010;24:1118–1123.
186. Liptak, JM, Dernell, WS, Straw, RC, et al. Intercalary bone grafts for joint and limb preservation in 17 dogs with high-grade malignant tumors of the diaphysis. Vet Surg. 2004;33:457–467.
187. Liptak, JM, Dernell, WS, Ehrhart, N, et al. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. 2006;35:518–533.
188. Liptak, JM, Dernell, WS, Lascelles, BD, et al. Intraoperative extracorporeal irradiation for limb sparing in 13 dogs. Vet Surg. 2004;33:446–456.
189. Berg, J, Weinstein, MJ, Schelling, SH, et al. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb-sparing surgery: 22 cases (1987-1990). J Am Vet Med Assoc. 1992;200:2005–2008.
190. LaRue, SM, Withrow, SJ, Powers, BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. 1989;195:1734–1744.
191. Thrall, DE, Withrow, SJ, Powers, BE, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: a dose response assay. Int J Radiat Oncol Biol Phys. 1990;18:1351–1357.
192. Vasseur, P. Limb preservation in dogs with primary bone tumors. Vet Clin North Am Small Anim Pract. 1987;17:889–903.
193. Withrow, SJ, Thrall, DE, Straw, RC, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer. 1993;71:2484–2490.
194. Straw, RC, Withrow, SJ. Limb-sparing surgery versus amputation for dogs with bone tumors. Vet Clin North Am Small Anim Pract. 1996;26:135–143.
195. Pooya, HA, Seguin, B, Mason, DR, et al. Biomechanical comparison of cortical radial graft versus ulnar transposition graft limb-sparing techniques for the distal radial site in dogs. Vet Surg. 2004;33:301–308.
196. Seguin, B, Walsh, PJ, Mason, DR, et al. Use of an ipsilateral vascularized ulnar transposition autograft for limb-sparing surgery of the distal radius in dogs: an anatomic and clinical study. Vet Surg. 2003;32:69–79.
197. Ehrhart, N. Longitudinal bone transport for treatment of primary bone tumors in dogs: technique description and outcome in 9 dogs. Vet Surg. 2005;34:24–34.
198. Tommasini, M, Ehrhart, N, Ferretti, A, et al. Bone transport osteogenesis for limb salvage following resection of primary bone tumors: experience with 6 cases (1991-1996). Vet Comp Orthop Traumatol. 2000;23:43–51.
199. Ehrhart, N, Eurell, JA, Tommasini, M, et al. Effect of cisplatin on bone transport osteogenesis in dogs. Am J Vet Res. 2002;63:703–711.
200. Rovesti, GL, Bascucci, M, Schmidt, K, et al. Limb sparing using a double bone-transport technique for treatment of a distal tibial osteosarcoma in a dog. Vet Surg. 2002;31:70–77.
201. Buracco, P, Morello, E, Martano, M, et al. Pasteurized tumoral autograft as a novel procedure for limb sparing in the dog: A clinical report. Vet Surg. 2002;31:525–532.
202. Morello, E, Vasconi, E, Martano, M, et al. Pasteurized tumoral autograft and adjuvant chemotherapy for the treatment of canine distal radial osteosarcoma: 13 cases. Vet Surg. 2003;32:539–544.
203. Reference deleted in pages.
204. Straw, RC, Withrow, SJ, Powers, BE. Primary osteosarcoma of the ulna in 12 dogs. J Am Anim Hosp Assoc. 1991;27:323–326.
205. Kirpensteijn, J, Straw, RC, Pardo, AD, et al. Partial and total scapulectomy in the dog. J Am Anim Hosp Assoc. 1994;30:313–319.
206. Straw, RC, Withrow, SJ, Powers, BE. Partial or total hemipelvectomy in the management of sarcomas in nine dogs and two cats. Vet Surg. 1992;21:183–188.
207. Lascelles, BD, Thomson, MJ, Dernell, WS, et al. Combined dorsolateral and intraoral approach for the resection of tumors of the maxilla in the dog. J Am Anim Hosp Assoc. 2003;39:294–305.
208. O’Brien, MG, Withrow, SJ, Straw, RC, et al. Total and partial orbitectomy for the treatment of periorbital tumors in 24 dogs and 6 cats: a retrospective study. Vet Surg. 1996;25:471–479.
209. VCOG: Retrospective study of 26 primary tumors of the osseous thoracic wall in dogs. J Am Anim Hosp Assoc. 1993;29:68–72.
210. Withrow, SJ, Hirsch, VM. Owner response to amputation of a pet’s leg. Vet Med Small Anim Clin. 1979;74:332. [334].
211. Carberry, CA, Harvey, HJ. Owner satisfaction with limb amputation in dogs and cats. J Am Anim Hosp Assoc. 1987;23:227–232.
212. O’Brien, MG, Withrow, SJ, Straw, RC. Recent advances in the treatment of canine appendicular osteosarcoma. Comp Cont Ed Pract Vet. 1993;15:613–616.
213. Meyer, MS, Spanier, SS, Moser, M, et al. Evaluating marrow margins for resection of osteosarcoma. A modern approach. Clin Orthop Relat Res. 1999:170–175.
214. Morello, E, Buracco, P, Martano, M, et al. Bone allografts and adjuvant cisplatin for the treatment of canine appendicular osteosarcoma in 18 dogs. J Small Anim Pract. 2001;42:61–66.
215. Tomford, WW, Doppelt, SH, Mankin, HJ, et al. 1983 bone bank procedures. Clin Orthop Relat Res. 1983:15–21.
216. Straw, RC, Powers, BE, Withrow, SJ, et al. The effect of intramedullary polymethylmethacrylate on healing of intercalary cortical allografts in a canine model. J Orthop Res. 1992;10:434–439.
217. Kirpensteijn, J, Steinheimer, D, Park, RD, et al. Comparison of cemented and non-cemented allografts for limb sparing procedures in dogs with osteosarcoma of the distal radius. Vet Comp Orthop Traumatol. 1998;11:178–184.
218. Dernell, WS, Withrow, SJ, Straw, RC, et al. Clinical response to antibiotic impregnated polymethyl methacrylate bead implantation of dogs with severe infections after limb sparing with allograft replacement-18 cases (1994-1996). Vet Comp Orthop Traumatol. 1998;11:94–99.
219. Boston, SE, Duerr, F, Bacon, N, et al. Intraoperative radiation for limb sparing of the distal aspect of the radius without transcarpal plating in five dogs. Vet Surg. 2007;36:314–323.
220. Oya, N, Kokubo, M, Mizowaki, T, et al. Definitive intraoperative very high-dose radiotherapy for localized osteosarcoma in the extremities. Int J Radiat Oncol Biol Phys. 2001;51:87–93.
221. Sakayama, K, Kidani, T, Fujibuchi, T, et al. Definitive intraoperative radiotherapy for musculoskeletal sarcomas and malignant lymphoma in combination with surgical excision. Int J Clin Oncol. 2003;8:174–179.
222. Tsuboyama, T, Toguchida, J, Kotoura, Y, et al. Intra-operative radiation therapy for osteosarcoma in the extremities. Int Orthop. 2000;24:202–207.
223. Farese, JP, Milner, R, Thompson, MS, et al. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc. 2004;225:1567–1572. [1548].
224. Van Ginkel, RJ, Hoekstra, HJ, Meutstege, FJ, et al. Hyperthermic isolated regional perfusion with cisplatin in the local treatment of spontaneous canine osteosarcoma: assessment of short-term effects. J Surg Oncol. 1995;59:169–176.
225. Rossi, CR, Pasquali, S, Mocellin, S, et al. Long-term results of melphalan-based isolated limb perfusion with or without low-dose TNF for in-transit melanoma metastases. Ann Surg Oncol. 2010;17:3000–3007.
226. Deroose, JP, van Geel, AN, Burger, JW, et al. Isolated limb perfusion with TNF-alpha and melphalan for distal parts of the limb in soft tissue sarcoma patients. J Surg Oncol. 2011.
227. Zachos, TA, Aiken, SW, DiResta, GR, et al. Interstitial fluid pressure and blood flow in canine osteosarcoma and other tumors. Clin Orthop Relat Res. 2001:230–236.
228. Powers, BE, Withrow, SJ, Thrall, DE, et al. Percent tumor necrosis as a predictor of treatment response in canine osteosarcoma. Cancer. 1991;67:126–134.
229. Withrow, SJ, Liptak, JM, Straw, RC, et al. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol. 2004;11:705–713.
230. Lascelles, BD, Dernell, WS, Correa, MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. 2005;12:1073–1083.
231. Jeys, LM, Grimer, RJ, Carter, SR, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14:2887–2895.
232. Sottnik, JL, U’Ren, LW, Thamm, DH, et al. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. 2010;59:367–378.
233. Kramer, A, Walsh, PJ, Seguin, B. Hemipelvectomy in dogs and cats: technique overview, variations, and description. Vet Surg. 2008;37:413–419.
234. Chauvet, AE, Hogge, GS, Sandin, JA, et al. Vertebrectomy, bone allograft fusion, and antitumor vaccination for the treatment of vertebral fibrosarcoma in a dog. Vet Surg. 1999;28:480–488.
235. Liptak, JM, Pluhar, GE, Dernell, WS, et al. Limb-sparing surgery in a dog with osteosarcoma of the proximal femur. Vet Surg. 2005;34:71–77.
236. Fitzpatrick, N, Smith, TJ, Pendegrass, CJ, et al. Intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage in 4 dogs. Vet Surg. 2011;40:909–925.
237. Drygas, KA, Taylor, R, Sidebotham, CG, et al. Transcutaneous tibial implants: a surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg. 2008;37:322–327.
237a. Liptak, JM, Dernell, WS, Rizzo, SA, et al. Partial foot amputation in 11 dogs. J Am Anim Hosp Assoc. 2005;41:47–55.
238. Fox, LE, Geoghegan, SL, Davis, LH, et al. Owner satisfaction with partial mandibulectomy or maxillectomy for treatment of oral tumors in 27 dogs. J Am Anim Hosp Assoc. 1997;33:25–31.
238a. White, RAS. Mandibulectomy and maxillectomy in the dog: long-term survival in 100 cases. J Small Anim Pract. 1991;32:69–74.
239. Withrow, SJ, Holmberg, DL. Mandibulectomy in the treatment of oral cancer. J Am Anim Hosp Assoc. 1983;19:273–286.
240. Gaitan-Yanguas, M. A study of the response of osteogenic sarcoma and adjacent normal tissues to radiation. Int J Radiat Oncol Biol Phys. 1981;7:593–595.
241. Caceres, E, Zaharia, M, Valdivia, S, et al. Local control of osteogenic sarcoma by radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1984;10:35–39.
242. Machak, GN, Tkachev, SI, Solovyev, YN, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clinic Proc. 2003;78:147–155.
243. Walter, CU, Dernell, WS, LaRue, SM, et al. Curative-intent radiation therapy as a treatment modality for appendicular and axial osteosarcoma: a preliminary retrospective evaluation of 14 dogs with the disease. Vet Comp Oncol. 2005;3:1–7.
244. Liepe, K. Alpharadin, a 223Ra-based alpha-particle-emitting pharmaceutical for the treatment of bone metastases in patients with cancer. Curr Opin Investig Drugs. 2009;10:1346–1358.
245. Nilsson, S, Strang, P, Ginman, C, et al. Palliation of bone pain in prostate cancer using chemotherapy and strontium-89: a randomized phase II study. J Pain Symptom Manage. 2005;29:352–357.
246. Nilsson, S, Franzen, L, Parker, C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594.
247. Vail, DM, Kurzman, ID, Glawe, PC, et al. STEALTH liposome-encapsulated cisplatin (SPI-77) versus carboplatin as adjuvant therapy for spontaneously arising osteosarcoma (OSA) in the dog: a randomized multicenter clinical trial. Cancer Chemother Pharmacol. 2002;50:131–136.
248. Phillips, B, Powers, BE, Dernell, WS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc. 2009;45:33–38.
249. Saam, DE, Liptak, JM, Stalker, MJ, et al. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996-2006). J Am Vet Med Assoc. 2011;238:195–206.
250. Kirpensteijn, J, Teske, E, Kik, M, et al. Lobaplatin as an adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a phase II evaluation. Anticancer Res. 2002;22:2765–2770.
251. Berg, J, Weinstein, MJ, Springfield, DS, et al. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206:1555–1560.
252. Berg, J, Gebhardt, MC, Rand, WM. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer. 1997;79:1343–1350.
253. Chun, R, Garrett, LD, Henry, C, et al. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc. 2005;41:382–387.
254. Bailey, D, Erb, H, Williams, L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 2003;17:199–205.
255. Mauldin, GN, Matus, RE, Withrow, SJ, et al. Canine osteosarcoma: treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med. 1988;2:177–180.
256. Kent, MS, Strom, A, London, CA, et al. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med. 2004;18:540–544.
257. Bacon, NJ, Ehrhart, NP, Dernell, WS, et al. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999-2006). J Am Vet Med Assoc. 2008;232:1504–1510.
258. McMahon, M, Mathie, T, Stingle, N, et al. Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med. 2011;25:511–517.
259. de la Puente-Redondo, VA, Tilt, N, Rowan, TG, et al. Efficacy of maropitant for treatment and prevention of emesis caused by intravenous infusion of cisplatin in dogs. Am J Vet Res. 2007;68:48–56.
260. Vail, DM, Rodabaugh, HS, Conder, GA, et al. Efficacy of injectable maropitant (Cerenia) in a randomized clinical trial for prevention and treatment of cisplatin-induced emesis in dogs presented as veterinary patients. Vet Comp Oncol. 2007;5:38–46.
261. Ogilvie, GK, Krawiec, DR, Gelberg, HB, et al. Evaluation of a short-term saline diuresis protocol for the administration of cisplatin. Am J Vet Res. 1988;49:1076–1078.
262. DeRegis, CJ, Moore, AS, Rand, WM, et al. Cisplatin and doxorubicin toxicosis in dogs with osteosarcoma. J Vet Intern Med. 2003;17:668–673.
263. McMahon, MB, Bear, MD, Kulp, SK, et al. Biological activity of gemcitabine against canine osteosarcoma cell lines in vitro. Am J Vet Res. 2010;71:799–808.
264. Selting, KA, Wang, X, Gustafson, DL, et al. Evaluation of satraplatin in dogs with spontaneously occurring malignant tumors. J Vet Intern Med. 2011;25:909–915.
265. Kurzman, ID, Shi, F, Vail, DM, et al. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm. 1999;14:121–128.
266. MacEwen, EG, Kurzman, ID, Rosenthal, RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81:935–938.
267. Kurzman, ID, MacEwen, EG, Rosenthal, RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1:1595–1601.
268. Savage, SA, Mirabello, L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011;2011:548151.
269. Polednak, AP. Human biology and epidemiology of childhood bone cancers: a review. Hum Biol. 1985;57:1–26.
270. Downey, RJ. Surgical treatment of pulmonary metastases. Surg Oncol Clin N Am. 1999;8:341.
271. O’Brien, MG, Straw, RC, Withrow, SJ, et al. Resection of pulmonary metastases in canine osteosarcoma: 36 cases (1983-1992). Vet Surg. 1993;22:105–109.
272. Liptak, JM, Monnet, E, Dernell, WS, et al. Pulmonary metastasectomy in the management of four dogs with hypertrophic osteopathy. Vet Comp Oncol. 2004;2:1–12.
273. Lansdowne, JL, Monnet, E, Twedt, DC, et al. Thoracoscopic lung lobectomy for treatment of lung tumors in dogs. Vet Surg. 2005;34:530–535.
274. Ogilvie, GK, Straw, RC, Jameson, VJ, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987-1991). J Am Vet Med Assoc. 1993;202:304–306.
275. Poirier, VJ, Hershey, AE, Burgess, KE, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222.
276. London, CA, Hannah, AL, Zadovoskaya, R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768.
277. London, C, Mathie, T, Stingle, N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia) in solid tumours. Vet Comp Oncol. 2011.
278. Wittenburg, LA, Gustafson, DL, Thamm, DH. Phase I pharmacokinetic and pharmacodynamic evaluation of combined valproic acid/doxorubicin treatment in dogs with spontaneous cancer. Clin Cancer Res. 2010;16:4832–4842.
279. Hershey, AE, Kurzman, ID, Forrest, LJ, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999;5:2653–2659.
280. Rodriguez, CO, Jr., Crabbs, TA, Wilson, DW, et al. Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv. 2010;23:197–206.
281. Koshkina, NV, Kleinerman, ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer. 2005;116:458–463.
282. Khanna, C, Anderson, PM, Hasz, DE, et al. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer. 1997;79:1409–1421.
283. Dow, S, Elmslie, R, Kurzman, I, et al. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther. 2005;16:937–946.
284. Thamm, DH, Kurzman, ID, King, I, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. 2005;11:4827–4834.
285. Portenoy, RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247.
286. Clohisy, DR, Mantyh, PW. Bone cancer pain. Clin Orthop Relat Res. 2003:S279–S288.
287. Goblirsch, MJ, Zwolak, PP, Clohisy, DR. Biology of bone cancer pain. Clin Cancer Res. 2006;12:6231s–6235s.
288. Goblirsch, M, Mathews, W, Lynch, C, et al. Radiation treatment decreases bone cancer pain, osteolysis and tumor size. Radiat Res. 2004;161:228–234.
289. Withrow, SJ, Powers, BE, Straw, RC, et al. Tumor necrosis following radiation therapy and/or chemotherapy for canine osteosarcoma. Chir Organi Mov. 1990;75:29–31.
290. Green, EM, Adams, WM, Forrest, LJ. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc. 2002;38:445–451.
291. Knapp-Hoch, HM, Fidel, JL, Sellon, RK, et al. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 2009;45:24–32.
292. Mueller, F, Poirier, V, Melzer, K, et al. Palliative radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. In Vivo. 2005;19:713–716.
293. Ramirez, O, III., Dodge, RK, Page, RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 1999;40:517–522.
294. Bateman, KE, Catton, PA, Pennock, PW, et al. 0-7-21 radiation therapy for the palliation of advanced cancer in dogs. J Vet Intern Med. 1994;8:394–399.
295. McEntee, MC, Page, RL, Novotney, CA, et al. Palliative radiotherapy for canine appendicular osteosarcoma. Vet Radiol Ultrasound. 1993;34:367–370.
296. Holmes, RA. [153Sm]EDTMP: a potential therapy for bone cancer pain. Semin Nucl Med. 1992;22:41–45.
297. Serafini, AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer. 2000;88:2934–2939.
298. Aas, M, Moe, L, Gamlem, H, et al. Internal radionuclide therapy of primary osteosarcoma in dogs, using 153Sm-ethylene-diamino-tetramethylene-phosphonate (EDTMP). Clin Cancer Res. 1999;5:3148s–3152s.
299. Barnard, SM, Zuber, RM, Moore, AS. Samarium Sm 153 lexidronam for the palliative treatment of dogs with primary bone tumors: 35 cases (1999-2005). J Am Vet Med Assoc. 2007;230:1877–1881.
300. Lattimer, JC, Corwin, LA, Jr., Stapleton, J, et al. Clinical and clinicopathologic response of canine bone tumor patients to treatment with samarium-153-EDTMP. J Nucl Med. 1990;31:1316–1325.
301. Milner, RJ, Dormehl, I, Louw, WK, et al. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J S Afr Vet Assoc. 1998;69:12–17.
302. Lipton, A. New therapeutic agents for the treatment of bone diseases. Expert Opin Biol Ther. 2005;5:817–832.
303. Coleman, RE. Therapeutic use of bisphosphonates in oncology. BMJ. 1994;309:1233.
304. Coleman, RE, McCloskey, EV. Bisphosphonates in oncology. Bone. 2011;49:71–76.
305. Carano, A, Teitelbaum, SL, Konsek, JD, et al. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990;85:456–461.
306. Hughes, DE, Wright, KR, Uy, HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487.
307. Luckman, SP, Hughes, DE, Coxon, FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589.
308. Ashton, JA, Farese, JP, Milner, RJ, et al. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005;66:885–891.
309. Farese, JP, Ashton, J, Milner, R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim. 2004;40:113–117.
310. Poirier, VJ, Huelsmeyer, MK, Kurzman, ID, et al. The bisphosphonates alendronate and zoledronate are inhibitors of canine and human osteosarcoma cell growth in vitro. Vet Comp Oncol. 2003;1:207–215.
311. Tomlin, JL, Sturgeon, C, Pead, MJ, et al. Use of the bisphosphonate drug alendronate for palliative management of osteosarcoma in two dogs. Vet Rec. 2000;147:129–132.
312. Fan, TM, de Lorimier, LP, Charney, SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74–80.
313. Fan, TM, de Lorimier, LP, O’Dell-Anderson, K, et al. Single-agent pamidronate for palliative therapy of canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2007;21:431–439.
314. Ryan, SD, Timbie, JW, Haussler, KK, et al. Randomized, placebo-controlled, clinical trial of radiation therapy with or without pamidronate for palliative treatment of canine appendicular osteosarcoma. Vet Comp Oncol. 2011;9:e1–e49.
315. Fan, TM, Charney, SC, de Lorimier, LP, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2009;23:152–160.
316. Spugnini, EP, Vincenzi, B, Caruso, G, et al. Zoledronic acid for the treatment of appendicular osteosarcoma in a dog. J Small Anim Pract. 2009;50:44–46.
317. Fan, TM, de Lorimier, LP, Garrett, LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med. 2008;22:380–387.
318. Vail, DM, MacEwen, EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792.
319. Rowell, JL, McCarthy, DO, Alvarez, CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388.
320. Withrow, SJ, Wilkins, RM. Cross talk from pets to people: translational osteosarcoma treatments. ILAR J. 2010;51:208–213.
321. Mankin, HJ, Hornicek, FJ, Rosenberg, AE, et al. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004:286–291.
322. Wilkins, RM, Cullen, JW, Camozzi, AB, et al. Improved survival in primary nonmetastatic pediatric osteosarcoma of the extremity. Clin Orthop Relat Res. 2005;438:128–136.
323. Bacci, G, Ferrari, S, Bertoni, F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli, according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–4027.
324. Bacci, G, Ruggieri, P, Bertoni, F, et al. Local and systemic control for osteosarcoma of the extremity treated with neoadjuvant chemotherapy and limb salvage surgery: the Rizzoli experience. Oncol Rep. 2000;7:1129–1133.
325. Okada, K, Unni, KK, Swee, RG, et al. High grade surface osteosarcoma: a clinicopathologic study of 46 cases. Cancer. 1999;85:1044–1054.
326. Banks, WC. Parosteal osteosarcoma in a dog and a cat. J Am Vet Med Assoc. 1971;158:1412–1415.
327. Withrow, SJ, Doige, CE. En block resection of a juxtacortical and three intra-osseous osteosarcomas of the zygomatic arch in dogs. J Am Anim Hosp Assoc. 1980;16:867–872.
328. Moores, AP, Beck, AL, Baker, JF. High-grade surface osteosarcoma in a dog. J Small Anim Pract. 2003;44:218–220.
329. Brodey, RS, Misdorp, W, Riser, WH, et al. Canine skeletal chondrosarcoma: a clinicopathologic study of 35 cases. J Am Vet Med Assoc. 1974;165:68–78.
330. Sylvestre, AM, Brash, ML, Atilola, MAO, et al. A case series of 25 dogs with chondrosarcoma. Vet Comp Orthop Traumatol. 1992;5:13–17.
331. Doige, CE. Multiple cartilaginous exostoses in dogs. Vet Pathol. 1987;24:276–278.
332. Gee, BR, Doige, CE. Multiple cartilaginous exostoses in a litter of dogs. J Am Vet Med Assoc. 1970;156:53–59.
333. Doige, CE, Pharr, JW, Withrow, SJ. Chondrosarcoma arising in multiple cartilaginous exostosis in a dog. J Am Anim Hosp Assoc. 1978;14:605–611.
334. Popovitch, CA, Weinstein, MJ, Goldschmidt, MH, et al. Chondrosarcoma: a retrospective study of 97 dogs (1987-1990). J Am Anim Hosp Assoc. 1994;30:81–85.
335. Anderson, WI, Carberry, CA, King, JM, et al. Primary aortic chondrosarcoma in a dog. Vet Pathol. 1988;25:180–181.
336. Flanders, JA, Castleman, W, Carberry, CA, et al. Laryngeal chondrosarcoma in a dog. J Am Vet Med Assoc. 1987;190:68–70.
337. Greenlee, PG, Liu, SK. Chondrosarcoma of the mitral leaflet in a dog. Vet Pathol. 1984;21:540–542.
338. Southerland, EM, Miller, RT, Jones, CL. Primary right atrial chondrosarcoma in a dog. J Am Vet Med Assoc. 1993;203:1697–1698.
339. Weller, RE, Dagle, GE, Perry, RL, et al. Primary pulmonary chondrosarcoma in a dog. Cornell Vet. 1992;82:447–452.
340. Patnaik, AK, Mattiesen, DT, Zawie, DA. Two cases of canine penile neoplasm: squamous cell carcinoma and mesenchymal chondrosarcoma. J Am Anim Hosp Assoc. 1988;24:403–406.
341. Aron, DN, DeVreis, R, Short, CE. Primary tracheal chondrosarcoma in a dog: a case report with description of surgical and anesthetic techniques. J Am Anim Hosp Assoc. 1980;16:31–37.
342. Lana, SE, Dernell, WS, LaRue, SM, et al. Slow release cisplatin combined with radiation for the treatment of canine nasal tumors. Vet Radiol Ultrasound. 1997;38:474–478.
343. Yamaguchi, T, Toguchida, J, Yamamuro, T, et al. Allelotype analysis in osteosarcomas: Frequent allele loss on 3q, 13q, 17p, and 18q. Cancer Res. 1992;52:2419–2423.
344. Bjornsson, J, McLeod, RA, Unni, KK, et al. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–2119.
345. Hammer, AS, Couto, CG, Filppi, J, et al. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:160–166.
346. Ogilvie, GK, Powers, BE, Mallinckrodt, CH, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;10:379–384.
347. Vail, DM, MacEwen, EG, Kurzman, ID, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. 1995;1:1165–1170.
348. Wesselhoeft-Ablin, LA, Berg, RJ, Schelling, SH. Fibrosarcoma in the canine appendicular skeleton. J Am Anim Hosp Assoc. 1991;27:303–309.
349. Banks, TA, Straw, RC. Multilobular osteochondrosarcoma of the hard palate in a dog. Aust Vet J. 2004;82:409–412.
350. Straw, RC, LeCouteur, RA, Powers, BE, et al. Multilobular osteochondrosarcoma of the canine skull: 16 cases (1978-1988). J Am Vet Med Assoc. 1989;195:1764–1769.
351. Dernell, WS, Straw, RC, Cooper, MF, et al. Multilobular osteochondrosarcoma in 39 dogs: 1979-1993. J Am Anim Hosp Assoc. 1998;34:11–18.
352. Hathcock, JT, Newton, JC. Computed tomographic characteristics of multilobular tumor of bone involving the cranium in 7 dogs and zygomatic arch in 2 dogs. Vet Radiol Ultrasound. 2000;41:214–217.
353. Lipsitz, D, Levitski, RE, Berry, WL. Magnetic resonance imaging features of multilobular osteochondrosarcoma in 3 dogs. Vet Radiol Ultrasound. 2001;42:14–19.
354. Stoll, MR, Roush, JK, Moisan, PG. Multilobular tumour of bone with no abnormalities on plain radiography in a dog. J Small Anim Pract. 2001;42:453–455.
355. Cooley, DM, Waters, DJ. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J Vet Intern Med. 1998;12:288–293.
356. Cornell, KK, Bostwick, DG, Cooley, DM, et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate. 2000;45:173–183.
357. Johnson, KA, Cooley, AJ, Darien, DL. Zygomatic osteoma with atypical heterogeneity in a dog. J Comp Pathol. 1996;114:199–203.
358. Chester, DK. Multiple cartilaginous exostoses in two generations of dogs. J Am Vet Med Assoc. 1971;159:895–897.
359. Hunt, GB, Malik, R, Johnson, KA. What is your diagnosis? Benign bone cyst in the distal portion of the left femur. J Am Vet Med Assoc. 1991;199:1071–1072.
360. Pernell, RT, Dunstan, RW, DeCamp, CE. Aneurysmal bone cyst in a six-month-old dog. J Am Vet Med Assoc. 1992;201:1897–1899.
361. Basher, AWP, Doige, CE, Presnell, KR. Subchondral bone cysts in a dog with osteochondrosis. J Am Anim Hosp Assoc. 1988;24:321–326.
362. Schrader, SC, Burk, RL, Liu, SK. Bone cysts in two dogs and a review of similar cystic bone lesions in the dog. J Am Vet Med Assoc. 1983;182:490–495.
363. Biery, DN, Goldschmidt, M, Riser, WH, et al. Bone cysts in the dog. Vet Am Vet Radiol. 1976;17:202–212.
364. Grabias, S, Mankin, H. Chondrosarcoma arising in histologically proved unicameral bone cyst. J Bone Joint Surg. 1973;56:1501–1503.
365. Dorn, CR, Taylor, DO, Schneider, R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318.
366. Bitetto, WV, Patnaik, AK, Schrader, SC, et al. Osteosarcoma in cats: 22 cases (1974-1984). J Am Vet Med Assoc. 1987;190:91–93.
367. Heldmann, E, Anderson, MA, Wagner-Mann, C. Feline osteosarcoma: 145 cases (1990-1995). J Am Anim Hosp Assoc. 2000;36:518–521.
368. Turrel, JM, Pool, RR. Primary bone-tumors in the cat: a retrospective study of 15 cats and a literature-review. Vet Radiol. 1982;23:152–166.
369. Spugnini, EP, Ruslander, D, Bartolazzi, A. Extraskeletal osteosarcoma in a cat. J Am Vet Med Assoc. 2001;219:60–62. [49].
370. Stimson, EL, Cook, WT, Smith, MM, et al. Extraskeletal osteosarcoma in the duodenum of a cat. J Am Anim Hosp Assoc. 2000;36:332–336.
371. Dhaliwal, RS, Johnson, TO, Kitchell, BE. Primary extraskeletal hepatic osteosarcoma in a cat. J Am Vet Med Assoc. 2003;222:340–342. [316].
372. Dimopoulou, M, Kirpensteijn, J, Moens, H, et al. Histologic prognosticators in feline osteosarcoma: a comparison with phenotypically similar canine osteosarcoma. Vet Surg. 2008;37:466–471.
373. Pool, RR, Carrig, CB. Multiple cartilaginous exostoses in a cat. Vet Pathol. 1972;9:350–359.
374. Carpenter, J, Andrews, L, Holzworth, J. Tumors and tumor-like lesions. In: Holzworth J, ed. Diseases of the cat: medicine and surgery. Philadelphia: WB Saunders, 1987.
375. Liu, SK, Dorfman, HD, Patnaik, AK. Primary and secondary bone tumours in the cat. J Small Anim Pract. 1974;15:141–156.
376. Quigley, PJ, Leedale, AH. Tumors involving bone in the domestic cat: a review of 58 cases. Vet Pathol. 1983;20:670–686.
377. Riddle, WE, Jr., Leighton, RL. Osteochondromatosis in a cat. J Am Vet Med Assoc. 1970;156:1428–1430.