Tumors of the Skeletal System
Osteosarcoma in Dogs
Osteosarcoma (OS) is the most common primary bone tumor in dogs, accounting for up to 85% of malignancies originating in the skeleton.1-5 OS is estimated to occur in more than 10,000 dogs each year in the United States; however, this is probably an underestimation, since not all cases are confirmed or recorded.6,7 The demographics of canine OS have been well reported.1-5,8-19 It is largely a disease of middle-aged to older dogs, with a median age of 7 years. There is a large range in age of onset, with a reported case in a 6-month-old pup20 and a small early peak in age frequency at 18 to 24 months.13 Primary rib OS tends to occur in younger adult dogs with a mean age of 4.5 to 5.4 years.21,22 OS is classically a cancer of large and giant breeds. In a review of 1462 cases of canine OS, dogs weighing more than 40 kg accounted for 29% of all cases and only 5% of their tumors occurred in the axial skeleton. Only 5% of OSs occur in dogs weighing less than 15 kg, but 59% of their tumors originated in the axial skeleton. Increasing weight and, more specifically, height appear to be the most predictive factors for the disease in the dog.23 In the United States, the breeds most at risk for OS are Saint Bernard, Great Dane, Irish setter, Doberman pinscher, Rottweiler, German shepherd, and golden retriever; however, size seems to be a more important predisposing factor than breed.* A hereditary basis for the formation of OS has been suspected, based primarily on the (large) breed prevalence of the disease, as well as the subjective assessment of increased incidence in some related families. Males are reported to be slightly more frequently affected than females (1.1 to 1.5 : 1),† with the exception of the Saint Bernard, Rottweiler, and Great Dane and for dogs with primary OS of the axial skeleton (except rib and spine) in which affected females outnumber males.2,22 However, in 1775 cases of canine OS of all sites treated at Colorado State University between 1978 and 2005, the male-to-female ratio was 1 : 1 (unpublished data, Colorado State University Animal Cancer Center [CSU-ACC] OS Database). Intact males and females were reported to have an increased risk for OS23; however, in the Rottweiler breed, male and female dogs that underwent gonadectomy before 1 year of age had an approximate one in four lifetime risk for bone sarcoma and were significantly more likely to develop bone sarcoma than dogs that were sexually intact.24 There was a highly significant inverse dose-response relationship between duration of lifetime gonadal exposure and incidence rate of bone sarcoma independent of adult height or body weight.
Approximately 75% of OSs occur in the appendicular skeleton, with the remainder occurring in the axial skeleton.2,22 The metaphyseal region of long bones is the most common primary site, with front limbs affected twice as often as rear limbs, and the distal radius and proximal humerus are the two most common locations.11 It is extremely rare for OSs to be located in bones adjacent to the elbow, although there is one report of 12 cases located at the proximal radius or distal humerus.25 There was no prognostic difference in these cases as compared to more common appendicular sites. In the rear limbs, tumors are fairly evenly distributed between the distal femur, distal tibia, and proximal tibia, with the proximal femur a slightly less common site.2 Primary OS distal to the antebrachiocarpal and tarsocrural joints is relatively rare in dogs.26 In 116 cases of canine primary OS in the axial skeleton, it was reported that 27% were located in the mandible, 22% in the maxilla, 15% in the spine, 14% in the cranium, 10% in ribs, 9% in the nasal cavity or paranasal sinuses, and 6% in the pelvis.22 Single reports of OS development in the os penis27 and the patella28 exist for the dog. Clinically documentable multicentric OS at the time of initial diagnosis occurs in less than 10% of all cases.29 OS of extraskeletal sites is rare, but primary OS has been reported in mammary tissue, subcutaneous tissue, spleen, bowel, liver, kidney, testicle, vagina, eye, gastric ligament, synovium, meninges, and adrenal gland.30-35
Etiology
The etiology of canine OS is generally unknown. Some have speculated a viral cause because OS can occur in litter mates and may be experimentally induced by injecting OS cells into canine fetii.36 However, an etiologic virus has not been isolated.
Physical Factors
A simplistic theory based on circumstantial evidence is that because OS tends to occur in major weight-bearing bones adjacent to late-closing physes and heavy dogs are predisposed, multiple minor trauma and subsequent injury to sensitive cells in the physeal region may occur. This may initiate the disease by inducing mitogenic signals, increasing the probability for the development of a mutant lineage. One in vitro study comparing the incidence of microdamage in cadaver radii of small- and large-breed dogs found no difference between the groups.37 OS has been associated with metallic implants used for fracture repair, with chronic osteomyelitis, and with fractures in which no internal repair was used.38-42 OS has also been reported at the site of a bone allograft used for fracture repair 5 years previous.43 Exposure to ionizing radiation can induce OS.35,44-52 In humans exposed to plutonium, 29% and 71% of the OSs were in the appendicular and axial skeleton, respectively, with the spine having the most tumors (36%). An almost identical distribution of plutonium-induced OS was reported for dogs injected with 239Pu as young adults in experimental studies. This distribution of OS is quite different from the distributions of naturally occurring OS for both species and appears to be related to bone volume and turnover. Similar findings were seen for dogs injected with 226R (radium).52 A distribution favoring bone marrow volume was seen for dogs exposed to strontium-90.51 OS is a rare, late complication of radiation therapy (RT) in humans and dogs.44,46,48-50 Three of 87 (3.4%) spontaneous tumor-bearing dogs treated for soft tissue sarcomas developed OS within the field of radiation.44 In another experimental study, 21% of dogs undergoing intraoperative RT (>25 Gy) followed by external-beam RT to the vertebral column developed OS following treatment.48 Secondary OS developed between 1.7 and 5 years after radiation in that study, and the authors speculated that high dose of radiation per fraction or total dose may predispose to this serious late effect of irradiation. OS was reported in 3% of 57 dogs irradiated for acanthomatous epulis of the oral cavity in another study.46 Postirradiation OS in humans comprised approximately 2% to 4% of all OSs reviewed in two large series.53,54
OSs have been concurrently seen in dogs with bone infarcts, but it is not clear whether there is any causal relationship.55-59 Bone infarcts are uncommon, are of unknown etiology, and may be identified as incidental findings by radiography. Bone infarcts are probably not associated with tumor emboli and appear to be more common in smaller breeds. A single report exists of OS occurrence secondary to a bone infarct caused by total hip arthroplasty.60 A case of OS was also reported to be associated with osteochondritis dissecans in the humoral head,61 and another report documents malignant transformation of an aneurysmal bone cyst.62
Genetic Factors
Ample evidence exists implicating the involvement of genetic and heritable factors for the development of OS in dogs. Currently, the most thoroughly described gene mutation that contributes to OS formation and/or progression in dogs is p53.63-70 Initial studies performed in immortalized canine OS cell lines demonstrated that the functionality of the p53 gene was defective based on the incapacity of p53 to regulate appropriately the transcriptional expression of downstream target genes including p21 and mdm2 following genotoxic insult.65 Furthermore, p53 mRNA and protein were overexpressed in 60% of cell lines and correlated with the presence of missense point mutations within the DNA-binding domain.65 Corroborating in vitro cell line studies, mutations in p53 have also been demonstrated in dogs with spontaneously-arising OS. Several studies using either single-strand conformational polymorphism, polymerase chain reaction, or southern blotting, followed by nucleotide sequence analysis have identified missense mutations involving exons 4 to 8 of p53 in 24% to 47% of all spontaneously arising OS samples.63,67,69,70 In addition to exons 4 to 8, the entire gene sequence of p53 has also been assessed by polymerase chain reaction (PCR) and single-strand conformational polymorphism from 59 spontaneously arising appendicular and axial OS samples.64 In 41% of tumors, p53 mutations were identified, with the majority of abnormalities being point mutations (74%), which resulted in an amino acid substitution, with a lesser percentage of mutations (26%) being deletions.64 Finally, through the implementation of targeted microarray-based comparative genomic hybridization analysis of 38 canine OS cases, similar recurrent cytogenetic aberrations classically present in human OS samples were also identified in OS specimens collected from dogs, including loss of heterozygosity (LOH) of the p53 gene in 18% of tumors.71
Substantiation for the presence of p53 mutations in sporadic canine OS has also been documented by immunohistochemical studies because a hallmark of many p53 mutations is enhanced protein stability of this normally labile protein, enabling detection of protein with methodologies such as immunohistochemistry (IHC).72 In one study evaluating p53 protein expressions in 106 osteogenic tumors, a greater percentage of appendicular (84%) OSs overexpressed p53 protein in comparison with OSs arising from the axial skeleton (56%) and other non-OS bone tumors (20%).68 Finally, loss of p53 gene function in 167 osseous tumors has been characterized by p53 nuclear staining frequency and intensity expressed as a p53 index. Of 103 OS samples, 67% stained positively for p53 protein, and the p53 index was significantly greater in OS derived from the appendicular (n = 84) versus axial (n = 38) skeleton.66 Interestingly, the p53 index of appendicular OS derived from Rottweilers was significantly higher than in Great Danes or other commonly affected breeds, supporting the notion that p53 gene mutations may be associated with breed susceptibilities to OS development.24
Another tumor suppressor gene likely to be permissive for OS development is the RB gene. Based on investigations using five tumorigenic immortalized canine OS cell lines, the RB gene signaling pathway was found to be dysregulated with the persistence of hyperphosphorylated RB protein in the absence of mitogen stimulation. Despite apparent aberrant RB gene signaling, reduction in RB protein was identified in only one of five cell lines.65 Corroborating these in vitro findings, the evaluation of 21 spontaneously arising OSs failed to identify gross RB gene alterations by Southern blotting, and protein expressions of RB were identified in all OS samples evaluated.67
Despite normal protein expression of RB in canine OS samples, the observed translational normalcy does not exclude the possibility for allelic deletion of the RB gene because prior studies in human OS samples have demonstrated that LOH at the RB gene locus does not absolutely correlate with inactivation of RB at the protein level.73 Substantiating the possibility that RB gene may have allelic deletion in spontaneously arising canine OS, analysis of 38 OS samples with comparative genomic hybridization techniques identified copy number loss in 11/38 cases (29%), resulting in a correlative reduction or absence of RB protein expression in 62% of OS samples tested.71 Based on these recent findings, it is probable that aberrations in the RB gene indeed participate in sporadic OS formation and/or progression in dogs.
In addition to p53 and RB gene abnormalities, the phosphatase and tensin homolog (PTEN) tumor suppressor gene is suspected to participate in the genetic pathogenesis of canine OS. Original in vitro studies conducted with canine OS cell lines demonstrated that the majority of cell lines (60%) harbored mutations in PTEN, resulting in the absence of gene transcription and protein translation. Corroborating the cell line findings, expression of PTEN was either absent (n = 6) or variable (n = 4) in 15 spontaneously arising OS samples.74 Further support for the loss of PTEN gene in canine OS pathogenesis has been the identification of specific recurrent chromosome copy number aberrations (CNAs) through targeted microarray-based comparative genomic hybridization studies.71,75 In one study utilizing 38 OS samples derived from Rottweilers and golden retrievers, deletion of a chromosomal region (CFA 26q25), which encompasses the PTEN tumor suppressor gene locus, occurred in 42% of OS samples.71 In a subsequent study analyzing 123 OS samples predominantly derived from Rottweilers, greyhounds, golden retrievers, and great Pyrenees, high-resolution comparative genomic hybridization studies similarly identified high recurrent copy number loss encompassing the PTEN gene in 30% of samples.75 In addition to the PTEN gene, other genes identified by high-resolution comparative genome hybridization studies potentially involved in the genetic pathogenesis of OS included overexpression of RHOC and RUNX2 and underexpression of TUSC3.75
A growing body of evidence in dogs supports breed-associated inheritance of OS, especially in Scottish deerhounds, Rottweilers, greyhounds, Great Danes, Saint Bernards, and Irish wolfhounds.24,30,76-79 Many domestic dog breeds have narrow genetic diversity as a consequence of selective breeding practices; this has provided the opportunity to more clearly elucidate the heritability of OS in dogs. For Scottish deerhounds in particular, the reported incidence of OS formation is 15%,76,77 and the narrow heritability in this breed was 0.69, indicating that 69% of the cause for OS development in Scottish deerhounds is due to heritable trait, likely a Mendelian major gene with dominant expression.77 Further studies in Scottish deerhounds using a whole genome linkage approach have mapped a novel locus (OSA1) for OS formation in this breed to CFA34 and provide the opportunity to pinpoint specific candidate genes directly involved in OS etiology for this specific dog breed.
Molecular Factors
Because of the heterogeneous and chaotic nature of OS, it has been difficult to definitively ascribe specific molecular derangements responsible for the etiopathogenesis of OS.80 Nonetheless, substantive progress has been achieved through experimental, preclinical, and comparative investigations to identify dysregulated intracellular signaling and cell survival pathways likely to participate in the pathogenesis of OS. Significantly, the identification and tumorigenic consequences of several putative pathways have been recently characterized not only in immortalized OS cell lines but also in spontaneously arising OS samples.
The MET proto-oncogene encodes a tyrosine kinase receptor that on ligation with hepatocyte growth factor (HGF) mediates multiple cellular functions, including cell scattering, motility, and proliferation. Given its biologic activities, excessive or dysregulated MET signaling in canine OS has been demonstrated to promote tumorigenic phenotypes in cell lines.81-83 Furthermore, in a small pilot study with spontaneously arising OS samples, the expression of MET proto-oncogene was identified in the majority (5/7, 71%) of tumor specimens by northern blot analysis.84 Additionally, a novel germline mutation that results in constitutive receptor phosphorylation and aberrant MET signaling has been identified primarily in the Rottweiler breed.85 In a larger study of 59 primary OS samples, mRNA expressions for MET and HGF were detected by real-time PCR (RT-PCR) in all specimens and suggested the existence of a putative MET/HGF autocrine or paracrine feedback loop.86 In a subset of OS samples, proteolytically activated HGF was identified in homogenized tumor lysates (n = 6) by western blot, and MET protein was expressed by 100% of tumors analyzed (n = 16) by IHC. Increased expression of MET mRNA correlated with regional lymph node metastases, supporting the participation of MET signaling for metastases and cell invasion.
The cellular effects of growth hormone (GH) are mediated through the hepatic production of insulin-like growth factor-1 (IGF-1). In osteoblasts, IGF-1 induces cell mitogenesis and protection from apoptosis, as well as promoting angiogenesis. Derived from experimental and preclinical investigations, aberrant or excessive IGF-1 signaling likely participates in OS pathogenesis. In three canine OS cell lines, the expression and functionality of the IGF-1/IGF-1 receptor (IGF-1R) signaling cascade has been reported.87 In all cell lines, IGF-1R expression was confirmed by northern blot analysis and radioligand binding studies; however, despite uniform receptor expression, IGF-1–mediated promotion of anchorage independent growth and invasion appeared to be cell-line specific.87 Furthermore, to validate IGF-1/IGF-1R as a molecular target, dogs treated with definitive surgery and systemic chemotherapy received a long-acting somatostatin analog (OncoLAR) in an adjuvant setting to attenuate the protumorigenic effects of IGF-1. Despite significant reductions in circulating IGF-1 concentrations, no clinical benefit was detected in dogs treated with OncoLAR compared to placebo.88
The proto-oncogene erbB-2 encodes human epidermal growth factor receptor-2 (HER-2), which is a tyrosine kinase receptor capable of promoting cell transformation and growth. In both dogs and humans, the overexpression of HER2 protein as a result of gene amplification is a negative prognostic factor in mammary carcinoma; however, less clarity exists for the role of HER2 overexpression in OS.89,90 To better characterize HER2 expressions in canine OS, one study evaluated HER2 mRNA transcript and protein expressions in seven cell lines and 10 OS tumor specimens.91 Based on RT-PCR, the majority of OS cell lines (6/7) and 40% of primary OS tumor samples overexpressed HER2. Similarly, the overexpression of HER2 protein was confirmed by IHC. Although not adequately powered, the results of the study suggested the possibility of HER2 overexpression as a negative prognostic factor for survival.
The mammalian target of rapamycin (mTOR) is an evolutionary conserved protein kinase downstream of Akt, which acts as a central hub for the integration of cellular signals induced by growth factors, nutrients, energy, and stress for the purposes of regulating cell cycle progression and growth. As such, aberrant signaling through the mTOR pathway contributes to growth, survival, and chemotherapy resistance in multiple tumor types. To characterize the functionality of the mTOR pathway in canine OS, one study using three canine OS cell lines investigated the expressions of mTOR and p70S6K, a downstream effector protein of mTOR.92 Study results indicated that the mTOR pathway was active in canine OS cells, and phosphorylation of mTOR and p70S6K could be inhibited by rapamycin, resulting in apoptosis and reduced growth of OS cells in vitro. In a complementary phase I dose-escalation study to assess the feasibility of mTOR inhibition as a treatment strategy, the pharmacokinetics and pharmacodynamics of rapamycin were investigated in dogs with primary OS.93 Results from the phase I trial demonstrated that biologically effective concentrations of rapamycin were safely obtainable in dogs and that modulation of mTOR target proteins by rapamycin was achievable within the bone tumor microenvironment.
The tropomyosin-related kinase (Trk) proto-oncogenes encode for high-affinity receptor tyrosine kinases (RTKs), including TrkA, B, and C. Specifically in osteoblasts, TrkA receptor binds nerve growth factor (NGF), resulting in cellular mitogenesis and the inhibition of apoptosis. To characterize the possible involvement of TrkA signaling in OS pathogenesis, one study investigated the expression of TrkA and the functional consequences of TrkA receptor ligation in OS cell lines and tumor samples.94 All canine OS cell lines (n = 2) and the majority of primary OS tumors (10/15) and pulmonary metastatic lesions (9/12) expressed TrkA receptors. Additionally, although TrkA signaling did not induce OS cell mitogenesis, blockade of basal TrkA signaling with either a small molecule inhibitor or blocking antibody increased serum starvation-induced apoptosis. The inhibition studies suggest that TrkA signaling participates in OS cell survival and may serve as a novel druggable target.
Telomeres are highly conserved nucleoprotein complexes at the ends of linear chromosomes that safeguard against harmful recombination events. The maintenance of telomere length and thus prevention of cellular senescence is through the activity of telomerase, a ribonucleoprotein complex that catalyzes the addition of telomeric sequences to the 3′ ends of chromosomes. Telomerase endows cells with infinite replicative capacity, and given its protumorigenic potential, telomerase has been investigated in canine OS. In an exploratory study, telomerase activity was identified in 100% of OS cell lines (5/5) and in the majority of primary tumors (5/6, 83%).95 In a larger corroborative study using 67 OS tumors, telomerase activity was identified in 73% of OS samples and supported the hypothesis that the majority of OSs possess the enzymatic machinery required for infinite replicative capacity.96
In addition to various growth and survival pathways that potentially contribute to OS pathogenesis, the ability of OS cells to interact with their immediate microenvironment found in bone and lung tissues likely influences OS progression and metastases. Tissue invasion and focal osteolysis are hallmark characteristics of OS, and local disease progression is promoted by several OS-associated proteins, including matrix metalloproteinases (MMPs), receptor activator of NF-κB ligand (RANKL), and lysosomal cathepsin K.97-100 Similar to the ability of OS cells to invade local tissues, specific proteins have been identified to participate in the progression of canine OS metastases, including ezrin,101 a cytoskeletal linker protein, as well as CXCR4,102 a chemokine receptor.
Pathology and Natural Behavior
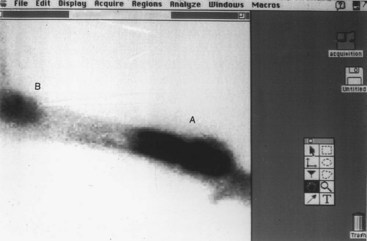
OS is a malignant mesenchymal tumor of primitive bone cells. These cells produce an extracellular matrix of osteoid, and the presence of tumor osteoid is the basis for the histologic diagnosis, differentiating OS from other sarcomas of bone. The histologic pattern may vary between tumors or even within the same tumor. Small biopsy samples of an OS may lead to misdiagnoses such as chondrosarcoma, fibrosarcoma, hemangiosarcoma, or simply reactive bone. These histologic diagnoses from small biopsies must be interpreted with caution. It is important to obtain a histologic analysis of the entire tumor following definitive excision to confirm the diagnosis. There are many histologic subclassifications of OS based on the type and amount of matrix and characteristics of the cells: osteoblastic, chondroblastic, fibroblastic, poorly differentiated, and telangiectatic OS (a vascular subtype). Alkaline phosphatase staining on histopathologic and aspiration cytology specimens has been shown to aid in differentiating OS pathologically from other connective tissue tumors.103-105 In dogs, it has not been well established that there is a difference in the biologic behavior of the different histologic subclassifications; however, histologic grade, based on microscopic features, may be predictive for systemic behavior (metastasis).106 Newer techniques (see previous section) designed to recognize molecular or genetic alterations are being evaluated to determine their potential use in predicting behavior of OS.107 The degree of aneuploidy, as measured by flow cytometry, of primary and metastatic tumors is potentially indicative of biologic behavior.80 OS has very aggressive local effects and causes lysis, production of bone, or both processes, which can occur concurrently (Figure 24-1). The local disease is usually attended by soft tissue swelling. Pathologic fracture of the affected bone can occur. Pathologic fracture at presentation in humans and dogs with OS does not necessarily preclude limb salvage and does not carry a worse prognosis than patients without fracture at presentation.108-111 OS rarely will cross a joint surface. This confinement to the bone may be secondary to collagenase inhibitors limiting progression through synovium.112,113
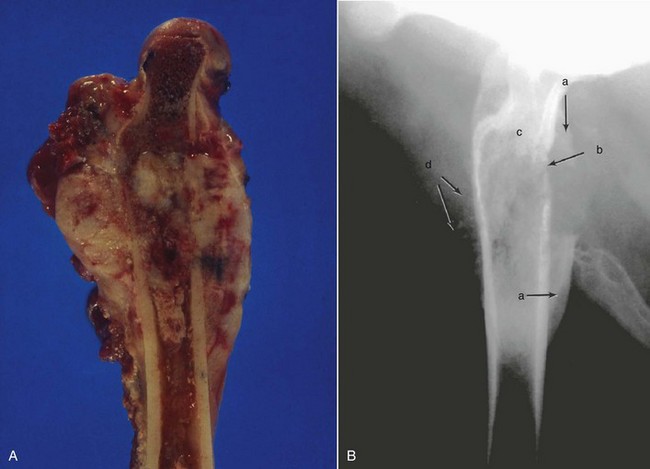
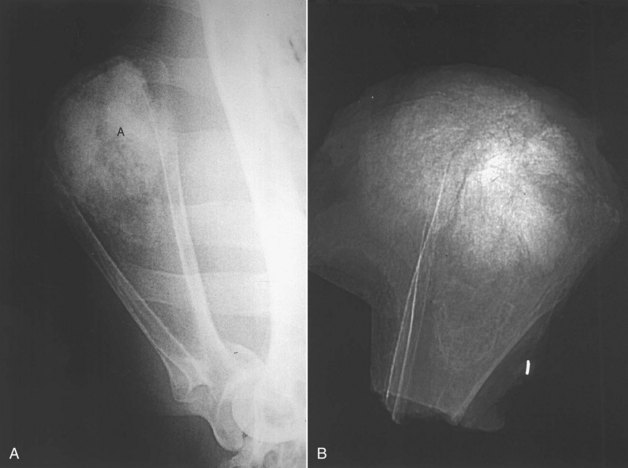
Figure 24-1 A, Gross, longitudinally split specimen of a proximal femoral OS lesion in a dog showing cortical destruction, soft tissue, and osteoid neoplastic components. B, Lateral radiograph of a proximal femoral OS lesion from the case in A. Radiographic features include (a) Codman’s triangle, (b) cortical lysis, (c) loss of trabecular pattern in the metaphyses, and (d) tumor bone extension into the soft tissues in a sunburst pattern.
Metastasis is very common and arises early in the course of the disease, although usually subclinically. Although less than 15% of dogs have radiographically detectable pulmonary or osseous metastasis at presentation, approximately 90% will die within 1 year (median survival time [MST]: 19 weeks) with metastatic disease, usually to the lungs, when amputation is the only treatment.2,15 Metastasis via the hematogenous route is most common; however, on rare occasions, extension to regional lymph nodes may occur.114 Although the lung is the most commonly reported site for metastasis, tumor spread to bones or other soft tissue sites occurs with some frequency.115 An increase in the incidence of bone metastasis following systemic chemotherapy has also been documented in humans and is suspected in dogs.116,117 Possible explanations for this change include a change in the behavior of this cancer independent of treatment; selective killing of metastatic cancer by chemotherapy in certain sites such as the lung, which allows metastasis in other sites to become clinically relevant; lung resection and chemotherapy have improved survival, and bone sites become clinically relevant; more sensitive detection methods, which allow previously undetectable metastases to be seen; or more complete and detailed necropsies compared to those performed previously, which identify asymptomatic metastatic sites. The concept of concomitant tumor resistance has been described in which animals harboring large primary tumors are resistant to the growth of smaller metastatic tumors by systemic angiogenic suppression.118 Development and growth of metastatic lesions can be rapid after the removal of the primary tumor and consequent increase in angiogenic activity in animal OS models.119 Suspected locoregional synchronous regional bone metastases (skip metastases) have also been reported.120 Skip metastases in human patients are a rare (1% to 6%) presentation and have a poor prognosis.121 Whole body magnetic resonance imaging (MRI) or positron emission tomography/computed tomography (PET/CT) imaging can aid in detection of occult skip metastases. Some differences in metastatic behavior have been observed based on the anatomic location of the primary OS site. For example, mandibular OS and, to a degree, other calvarium locations may have a less aggressive metastatic behavior, although contradictory evidence exists (see the later section on Therapy and Prognosis).122,123
There is a report of four cases of histologically confirmed OS that subsequently underwent spontaneous regression without tumor-specific treatment.124 This phenomenon, while extremely rare, has also been reported in humans.
History and Clinical Signs
Dogs with OS of appendicular sites generally present with a lameness and swelling at the primary site. There may be a history of mild trauma just prior to the onset of lameness. This history can often lead to misdiagnosis as another orthopedic or soft tissue injury. The pain is likely due to microfractures or disruption of the periosteum induced by osteolysis of cortical bone with tumor extension from the medullary canal. The lameness worsens and a moderately firm-to-soft, variably painful swelling arises at the primary site. Dogs may present with acute, severe lameness associated with pathologic fractures, although pathologic fractures account for less than 3% of all fractures seen.59 Large- and giant-breed dogs that present with lameness or localized swelling at metaphyseal sites should be evaluated with OS as a likely diagnosis.
The signs associated with axial skeletal OS are site dependent. Signs vary from localized swelling with or without lameness to dysphagia (oral sites), exophthalmos and pain on opening the mouth (caudal mandibular or orbital sites), facial deformity and nasal discharge (sinus and nasal cavity sites), and hyperesthesia with or without neurologic signs (spinal sites). Dogs with tumors arising from ribs usually present because of a palpable, variably painful mass. Respiratory signs are not common even where the lesions have large intrathoracic components, and malignant pleural effusion is quite rare.
Dogs rarely have respiratory signs as the first clinical evidence of pulmonary metastasis; rather, their first signs are usually vague. With radiographically detectable pulmonary metastasis, dogs may remain asymptomatic for many months, but most dogs develop decreased appetites and nonspecific signs such as malaise within 1 month. Hypertrophic osteopathy may develop in dogs with pulmonary metastasis (see Chapter 5).
Systemic Alterations
Alterations in energy expenditure, protein synthesis, urinary nitrogen loss, and carbohydrate flux have been documented in dogs with OS, similar to results documented in humans with neoplasia. Changes were documented in resting energy expenditure and protein and carbohydrate metabolism in dogs with OS. These changes were evident even in dogs that did not have clinical signs of cachexia.125 Systemic, metabolic derangements reported for dogs with OS include lower chromium and zinc levels, lower iron and iron-binding capacity, and increased ferritin levels as compared to normal dogs.126 Hypercalcemia is extremely rare. The impact of these changes on patient treatment, response, or outcome is unknown.
Diagnostic Techniques and Work-Up
Initial evaluation of the primary site involves interpretation of good quality radiographs taken in lateral and craniocaudal projections. Special views may be necessary for lesions occurring in sites other than in the appendicular skeleton. The overall radiographic abnormality of bone varies from mostly bone lysis to almost entirely osteoblastic or osteogenic changes (see Figure 24-1, B). There is an entire spectrum of changes between these two extremes, and the appearance of OS can be quite variable. There are some features, however, that are commonly seen. Cortical lysis is a feature of OS and may be severe enough to leave obvious areas of discontinuity of the cortex leading to pathologic fracture. There is often soft tissue extension with an obvious soft tissue swelling, and new bone (tumor or reactive bone) may form in these areas in a palisading pattern perpendicular or radiating from the axis of the cortex (i.e., “sunburst”). As tumor invades the cortex, the periosteum is elevated and new bone is laid down by the cambium layer providing a triangular-appearing deposition of dense new bone on the cortex at the periphery of the lesion. This periosteal new bone has been called “Codman’s triangle,” but this is not pathognomonic for OS. OS does not directly cross articular cartilage, and primary lesions usually remain monostotic. The tumors may extend into periarticular soft tissues, however, and adjacent bones are at risk because of extension through adjacent soft tissue structures. Other radiographic changes that can attend OS are loss of the fine trabecular pattern in the metaphysis, a vague transition zone at the periphery of the medullary extent of the lesion (rather than a sharp sclerotic margin), or areas of fine punctate lysis. Any one or combinations of these changes may be seen, depending on the size, histologic subtype, location, and duration of the lesion. The radiographic appearance of OS is similar to osteomyelitis, specifically of fungal etiology.127 In cases in which the travel or clinical history might support the possibility of osteomyelitis, a biopsy with submission for histology and culture may be warranted.
Based on signalment, history, physical examination, and radiographic findings, a presumptive diagnosis of OS can be made. Differential diagnoses of lytic, proliferative, or mixed pattern aggressive bone lesions identified on radiographs include other primary bone tumors (chondrosarcoma, fibrosarcoma, hemangiosarcoma); metastatic bone cancer; multiple myeloma or lymphoma of bone; systemic mycosis with bony localization; bacterial osteomyelitis; and, albeit rare, bone cysts.
Other primary bone tumors are far less common but may be suspected, especially in dogs with unusual signalment or tumor location. Metastatic cancer can spread to bone from almost any malignancy. A careful physical examination is important, including a rectal examination, with special attention paid to the genitourinary system to help rule out the presence of a primary cancer. Dogs with a history of cancer in the past should have their original biopsy reviewed and should be restaged for the original disease. Common sites for metastatic bone cancer are lumbar and sacral vertebrae, pelvis, and diaphyses of long bones. There are usually other clues for the diagnosis of multiple myeloma, such as hyperproteinemia, and both multiple myeloma and lymphoma of bone are usually attended by radiographic lesions that are almost entirely lytic. Two classic radiographic appearances of myeloma bone lesions are described: “punched-out” areas of lysis or a generalized osteoporotic thinning of cortices.
Tissue Biopsy
A diagnosis of primary malignant bone tumor may be suggested by signalment, history, physical examination, and radiographic findings. Cytology has not been thought to be definitive for diagnosis; however, it may support the tentative diagnosis, and enough confidence in the diagnosis, combined with clinical features and radiographic appearance, may exist to facilitate a discussion of treatment options. Consistent cytologic criteria of OS has recently been described, and with repeated evaluations, dependent on experience, cytopathologists may be more definitive in making a diagnosis from cytology alone.128,129 Alkaline phosphatase staining of cytologic samples has been shown to differentiate OS from other vimentin-positive tumors.103 However, in most cases, a definitive diagnosis lies in procurement and interpretation of tissue for histopathology. With new treatments such as limb sparing (see subsequent sections), knowledge of the specific tumor type may avoid overextension or inappropriate treatment of bone tumors thought to be OS (e.g., chondrosarcoma, myeloma, or lymphoma). It is crucial to the success of a limb-sparing surgery that the biopsy procedure is planned and performed carefully with close attention to asepsis, hemostasis, and wound closure.130 The skin incision for the biopsy must be small and placed so that it can be completely excised with the tumor at limb sparing without compromising the procedure. Transverse or large incisions must be avoided. It has been recommended that the surgeon who is to perform the definitive surgical procedure (especially if limb sparing) should be the person to perform the preoperative bone biopsy.131
Bone biopsy may be performed as an open incisional, closed needle (Figure 24-2), or trephine biopsy. The advantage of an open technique is that a large sample of tissue is procured, which presumably improves the likelihood of establishing an accurate histologic diagnosis. Unfortunately, this advantage may be outweighed by the disadvantages of an involved operative procedure and risk of postsurgical complications such as hematoma formation, wound breakdown, infection, local seeding of tumor, and pathologic fracture.132,133 Although biopsy with a trephine yields a diagnostic accuracy rate of 93.8%, there is the increased risk of creating pathologic fracture when compared with using a smaller gauge needle.134 This underscores some of the advantages of a closed biopsy using a Jamshidi bone marrow biopsy needle (Jamshidi bone marrow needle, American Pharmaseal Co, Valencia, CA; bone marrow biopsy needle, Sherwood Medical Co, St. Louis, MO) or similar type of needle. Jamshidi needle biopsy has an accuracy rate of 91.9% for detecting tumor versus other disorders and an 82.3% accuracy rate for diagnosis of specific tumor subtype.135 Accuracy of diagnoses from needle core samples can depend on the pathologist’s experience and comfort level with examining small samples. Histology reports indicating the presence of reactive bone should not rule out the presence of a primary bone tumor or other pathology, especially if the radiographic changes suggest tumor. In some cases, it can be very difficult to get the diagnosis by preoperative biopsy (i.e., repeated biopsy attempts yield “reactive bone”) and yet the pathologist has no trouble identifying tumor when the entire specimen is available for histopathologic analysis (e.g., postamputation), This is likely a result of the heterogeneity of the tumor tissue itself and the large amount of reactive bone present within the tumor.
Figure 24-2 A, The Jamshidi bone biopsy needle: cannula and screw-on cap (a), tapered point (b), pointed stylet to advance cannula through soft tissues (c), and probe to expel specimen from cannula (d). B, With the stylet locked in place, the cannula is advanced through the soft tissue until bone is reached. The inset is a close-up view showing stylet against bone cortex. C, The stylet is removed, and the bone cortex penetrated with the cannula. The cannula is withdrawn, and the procedure repeated with redirection of the instrument to obtain multiple core samples. D, The probe is then inserted retrograde into the tip of the cannula to expel the specimen through the base (inset). Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Powers BE, LaRue SM, Withrow SJ, et al: Jamshidi needle biopsy for diagnosis of bone lesions in small animals, J Am Vet Med Assoc 193:206–207, 1988.) J Am Vet Med Assoc
The biopsy site is selected carefully. Radiographs (two views) are reviewed, and the center of the lesion chosen for biopsy. Biopsy at the lesion periphery will often result in sampling the reactive bone surrounding the tumor growth without a resulting diagnosis.135 The skin incision is made so the biopsy tract and any potentially seeded tumor cells can be completely removed at the time of definitive surgery. Care is used to avoid major nerves, vessels, and joint spaces. A 4-inch, 8- or 11-gauge needle is used. With the dog anesthetized, prepared, and draped for surgery, a small stab incision (2 to 3 mm) is made in the skin with a #11 scalpel blade. The bone needle cannula, with the stylet locked in place, is pushed through the soft tissue to the bone cortex. The stylet is removed, and the cannula is advanced through the bone cortex into the medullary cavity using a gentle twisting motion and firm pressure. The opposite cortex is not penetrated. The needle is removed, and the specimen is gently pushed out of the base of the cannula by inserting the probe into the cannula tip. One or two more samples can be obtained by redirecting the needle through the same skin incision so that samples of the transition zone may also be obtained. Ideal specimens should be 1 or 2 cm in length and not fragmented. Biopsy is repeated until solid tissue cores are obtained. Material for culture and cytology may be taken from the samples prior to fixation in 10% neutral buffered formalin. Diagnostic accuracy is improved when samples are evaluated by a pathologist thoroughly familiar with bone cancer. Fluoroscopy or advanced imaging (CT) can assist in obtaining needle-core biopsy samples of suspected bone lesions, especially for axial sites.136
After tumor removal (amputation or limb sparing), histology should be performed on a larger specimen to confirm the preoperative diagnosis. If the clinical and radiographic features are typical for OS, especially when there is little possibility of fungal or bacterial infection, confirmation of histologic diagnosis following surgical treatment of local disease (amputation or limb sparing) can be considered. Few diseases causing advanced destruction of the bone can be effectively treated without removal of the local disease. If the owners are willing to treat aggressively, surgical removal of local disease with biopsy submission following surgery may be acceptable.
Staging and Patient Assessment
Systemic Staging
Examination for evidence of apparent spread of the disease is important. Regional lymph nodes, although rarely involved, should be palpated, and fine needle cytology should be performed on any enlarged node.114 Sites of bone metastasis may be detected by a careful orthopedic examination with palpation of long bones and the accessible axial skeleton. Organomegaly may be detected by abdominal palpation. Usually, pulmonary metastases are undetectable by clinical examination, but careful thoracic auscultation is important to detect intercurrent cardiopulmonary disorders. High-detail thoracic radiographs should be taken during inspiration with the patient awake. Although some controversy exists,137 it is still considered important by most oncologists to include three views: a ventrodorsal or a dorsoventral view and both right and left lateral views. OS pulmonary metastases are generally soft tissue dense and cannot be detected radiographically until the nodules are 6 to 8 mm in diameter. It is uncommon to detect pulmonary metastatic disease at the time of diagnosis (<10% of dogs). Advanced imaging (e.g., CT, MRI, PET/CT) may play a role in patient staging and is used to evaluate for pulmonary metastases and for evaluation of tumor vascularity, soft tissue and medullary involvement, metabolic or functional activity, and response to treatment.138,139 Currently, published treatment recommendations and prognoses are based on the results of plain radiographs. As advanced imaging becomes more commonplace for staging dogs with OS, comparisons to previous protocols will be subject to stage-migration and lead-time bias because earlier diagnosis will result.140
Bone survey radiography has been useful in detecting dogs with second skeletal sites of OS.29 Bone surveys include lateral radiographs of all bones in the body and a ventrodorsal projection of the pelvis using standard radiographic technique appropriate for the region radiographed. In one study, 171 dogs with primary bone tumors underwent radiographic bone surveys and thoracic radiography; at presentation, there was a higher yield in finding other sites of OS with radiographic bone survey (6.4%, 11 of 171 dogs) than with thoracic radiographs (4%, 7 of 171 dogs).141 There are conflicting reports on the usefulness of nuclear scintigraphy (bone scan) (Figure 24-3) for clinical staging of dogs with OS.142-146 Bone scintigraphy was used in one study to identify suspected second bone sites of OS in 14 of 25 dogs with appendicular primaries.143 Seven of these lesions were biopsied and confirmed to be OS. Another study of 70 dogs with appendicular primary bone tumors resulted in only one scintigraphically detectable occult bone lesion.142 In a third report, of 23 dogs with suspected skeletal neoplasia that were evaluated with scintigraphy and radiography, 4 dogs had second skeletal sites suspected to be neoplastic.146 The suspicious site in one of these dogs was found on histologic evaluation to be normal bone. Another study found secondary sites considered highly suspect of bony metastasis in 7.8% of 399 cases; however, most suspected lesions were not subjected to histologic conformation.144 Nuclear bone scan can be a useful tool for the detection and localization of bone metastasis in dogs presenting for vague lameness or signs such as back pain, and, although very sensitive, it is not specific for identifying sites of skeletal tumor. Any region of osteoblastic activity will be identified by this technique, including osteoarthritis and infection. Follow-up with high-detail radiographs of sites found suspicious on scintigraphy will generally help rule out nonneoplastic disease; however, definitive biopsy may be necessary.
Surgical Staging
A surgical staging system for sarcomas of the skeleton has been devised for humans.147 This system is based on the histologic grade (G), the anatomic setting of the primary tumor (T), and regional or distant metastasis (M). There are three stages: stage I, the low-grade (G1) lesions without metastasis; stage II, the high-grade (G2) lesions without metastasis; and stage III, the lesion with regional or distant metastasis regardless of histologic grade. The stages are subdivided by the anatomic setting: A is intracompartmental (T1) and B is extracompartmental (T2). According to this system, most dogs with OS present with stage IIB disease. Scintigraphy can be used to evaluate the degree of bone involvement from a primary bone tumor.148 In one study, scintigraphy overestimated the length of OS disease in limb-sparing patients by 30%, allowing for adequate margin prediction, preoperatively.149 CT may be useful to plan surgery, especially for tumors located in the axial skeleton; however, one study reported that plain radiographs were as accurate as advanced imaging (CT, MRI) in predicting true length of tumor involvement.150 In contrast, MRI was more accurate than plain radiographs or CT in predicting length of tumor involvement for appendicular canine OS in another study.151 This could prove invaluable for limb-sparing patients, and further evaluation is warranted.
Patient Assessment
The patient’s overall health status requires careful assessment. Advancing years do not preclude treatment; however, prolonged anesthesia and chemotherapy may not be tolerated in dogs with organ compromise. Particular attention to the cardiovascular system is important. Coexisting cardiomyopathy or any degree of heart failure may lead to serious complications, particularly during fluid diuresis, anesthesia, or administration of certain chemotherapy agents. Electrocardiogram and echocardiogram should be performed on dogs in which the history or physical findings implicate a cardiac disorder. Renal function must be evaluated prior to administration of cisplatin. A minimum database should include a complete blood count (CBC), platelet count, serum biochemical analysis, and urinalysis.
Known or Suggested Prognostic Factors
Anatomic Location and Signalment
In a multiinstitutional study of 162 dogs with appendicular OS treated with amputation alone, dogs younger than 5 years of age had shorter survival than older dogs.15 Additional studies have related large tumor size13,152,153 and humerus location154,155 to poor outcome. Large tumor size has been reported to be a negative prognostic factor for humans with OS.156 For OS originating from flat bones, small dog size and completeness of excision were positive prognostic indicators.157 Although there are differences in disease distribution and prevalence, documentation of improved survival for small dogs with OS is lacking.158 A negative prognosis can also be predicted by a higher tumor grade and mitotic index, based on the results of one study.106
The biologic behavior for nonappendicular sites of OS appears to be similar (aggressive) with the exception of the mandible and possibly the rest of the calvarium.122,123,159-162 OS affecting the head (mandible, maxilla, and skull) is locally aggressive but has a lower metastatic rate (37%) than appendicular OS.162 Reported median disease-free intervals (DFIs) and survival times for skull OS are 191 days and 204 days, respectively. Dogs with OS of the mandible treated with mandibulectomy alone had a 1-year survival rate of 71% in one study.122 In contrast, maxillary OSs have demonstrated a median survival of 5 months following maxillectomy.159,160 A study evaluating response to treatment for orbital OS reported long-term survival following complete surgical excision.163 Similar behavior is seen for OSs of flat bones in humans.164 Median survival for rib OS lesions is reported to be 3 months for dogs treated with rib resection alone and 8 months for dogs treated with resection and adjuvant chemotherapy.165-168 OS of the canine scapula has been reported to have a poor prognosis when treated with subtotal scapulectomy surgery and chemotherapy.157,169,170 DFI and MST in dogs diagnosed with scapula OS was 210 days (range 118 to 245 days) and 246 days (range 177 to 651 days), respectively.171 Use of adjunctive chemotherapy was prognostic for both increased DFI and survival. Limb function after subtotal scapulectomy is good to excellent.171 Survival of dogs with OS distal to the antebrachiocarpal or tarsocrural joints was somewhat longer (median: 466 days) than survival of dogs with OSs of more common appendicular sites; however, OS in these sites is aggressive, with a high potential for metastasis.26 Vertebral OS is uncommon; however, reported cases indicate aggressive local and systemic behavior.22,172 In 15 dogs treated with a combination of surgery, radiation, and chemotherapy, the median survival was 4 months.172 The biologic behavior of OS in other nonappendicular bone sites (e.g., pelvis) has not been thoroughly evaluated.
Extraskeletal OS is rare and most commonly affects visceral sites (gastrointestinal tract, spleen, liver, kidney, urinary bladder), skin or subcutaneous tissue, or mammary glands. Extraskeletal (soft tissue) OS sites also appear to have aggressive systemic behavior with a high metastatic rate. In one report, extraskeletal OS treated with surgery alone had a median survival of only 1 month, and a median survival of 5 months was obtained for cases treated with surgery and adjuvant chemotherapy.31 In a larger study, soft tissue OSs were separated from mammary gland OSs; median survival of nonmammary gland soft tissue lesions was 1 month and mammary gland lesions 3 months following primarily surgical resection alone.32 The major cause of death was local recurrence (92%) in the soft tissue OS cases and pulmonary metastasis (62.5%) in the mammary gland OS cases.
Dogs presented with stage III disease (measurable metastases) have a very poor prognosis.115 MST of 90 dogs with stage III disease at presentation was 76 days, with a range of 0 to 1583 days. No significant differences in survival times on the basis of age, sex, breed, or primary site were observed. Dogs with bone metastases (132 days) had a longer survival time than dogs with lung (59 days) or lung and other soft tissue (19 days) metastases. Dogs with lymph node metastasis had short survivals with a median of only 57 to 59 days, compared to 318 days for dogs without nodal spread.114,115 Dogs with stage III disease treated palliatively with RT and chemotherapy had a significantly longer survival time (130 days) than dogs in all other treatment groups.
Serum Alkaline Phosphatase
Elevated alkaline phosphatase (AP) has been clearly associated with a poorer prognosis for dogs with appendicular OS in several studies.114,154,173-175 A preoperative elevation of either total (serum) or the bone isoenzyme of AP (>110 U/L or 23 U/L, respectively) is associated with a shorter DFI and survival (Figure 24-4). Likewise, dogs that have elevated preoperative values that do not return to normal within 40 days following surgical removal of the primary lesion also develop earlier metastasis. One study substantiated the predictive nature of elevated preoperative AP levels; however, no association was found for elevated postoperative serum levels.173

Figure 24-4 A, Disease-free interval (DFI) outcome of dogs treated for OS comparing bone alkaline phosphatase levels above and below 23 U/L preoperatively. B, Survival outcome of dogs treated for OS comparing serum alkaline phosphatase levels above and below 110 U/L preoperatively. BALP, Bone alkaline phosphatase; TALP, total alkaline phosphatase. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Erhart N, Dernell WS, Hoffmann WE, et al: Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular OS: 75 cases (1990-1996), J Am Vet Med Assoc 213:1003, 1998.) J Am Vet Med Assoc
Molecular, Genetic, and Immunologic Indices of Prognosis
Although our understanding of the pathogenesis of OS remains incomplete, the availability of canine-specific reagents, as well as sequencing of the canine genome, has permitted the discovery of key molecular, genetic, and immunologic events that might participate in OS progression and metastases. With increased clarity for the critical events involved in directing OS biology, specific tumor- and host-associated characteristics have recently been identified as important factors that influence OS prognosis.
The expression of several molecular proteins, including ezrin, recepteur d’origine nantaise (RON), survivin, vascular endothelial growth factor (VEGF), and cyclooxygenase-2 (COX-2), has been reported to influence DFI and survival times in dogs.101,176-179 Ezrin is a cellular protein belonging to the ERM (ezrin-radixin-moesin) family and serves as a physical and functional anchor site for cytoskeletal F-actin fibers. Because of ezrin’s involvement in cytoskeletal remodeling, it has been demonstrated in murine preclinical models that ezrin is necessary for OS metastases.101 Through the use of a canine tissue microarray with known clinical outcome data (n = 73), it was shown that the presence of high ezrin staining in primary tumors was associated with a significantly shorter median DFI compared to dogs with low primary tumor ezrin staining, 116 versus 188 days, respectively.
HGF receptor (MET) and RON are members of the MET proto-oncogene family of RTKs, and signaling through MET or RON promotes tumorigenesis and the formation of metastases. MET and RON are capable of heterodimerization with one another, resulting in cellular cross-talk that might alter the strength and duration of signal transduction, with resultant protumorigenic effects. Given the role of MET and RON in metastases, their expression in OS has been evaluated in dogs.176 Through the use of a canine OS tissue array with linked outcome data (n = 105), expression of RON but not MET was prognostic for survival. Dogs with high RON expression in their primary tumors lived significantly shorter than dogs with absent, low, or intermediate RON expression.
Survivin is a small protein belonging to the inhibitor of apoptosis (IAP) family and participates in the processes of cell division, as well as apoptosis inhibition. As a dimer, survivin inhibits both caspase-dependent and caspase-independent mediated apoptosis, and its expression can promote tumorigenesis. Given survivin’s antiapoptotic properties, its overexpression might provide a survival advantage to cancer cells and be associated with a negative prognosis. In a recent study, the expression of survivin was characterized in 67 primary OS samples with known outcome data.178 Survivin expression was detected in the majority of tissue samples (65/67), and expression intensity was associated with DFIs. Dogs with primary tumors expressing low survivin immunoreactivity scores achieved significantly longer DFIs than dogs with high survivin immunoreactivity scores within the primary tumor, 331 and 173 days, respectively.
VEGF and the enzymatic activities of COX-2 serve as potent regulators of angiogenesis, and their independent expressions have been associated with a poorer prognosis for a variety of cancers. Angiogenesis is a necessary step for tumor growth and metastases; thus both VEGF and COX-2 have been investigated in dogs with OS.177,179 In one study with 25 dogs treated with definitive surgery and systemic chemotherapy, baseline platelet-corrected serum VEGF concentrations were associated with DFI but not survival time.179 Dogs with VEGF concentration in the lower 50 percentile achieved significantly longer DFIs than dogs with VEGF levels in the upper 50 percentile, 356 and 145 days, respectively. In another study, COX-2 expression was characterized in primary tumors derived from 44 dogs treated with amputation and doxorubicin.177 Immunoreactivity for COX-2 was identified in 88% of primary tumors, although intratumoral COX-2 staining was variable and heterogeneous. A COX-2 immunoreactivity score, a product of stain intensity and percentage of positive cells, was potentially correlated with disease outcome. Dogs with primary tumors demonstrating strong stain intensity (n = 4) had a significantly shorter MST of 86 days in comparison with dogs with tumors staining negative (n = 10, MST: 423 days), poor (n = 19, MST: 399 days), or moderate (n = 11, MST: 370 days) for COX-2. However, given the small sample size of dogs with strong COX-2–staining intensity, the prognostic value of COX-2 expression in OS warrants a more thorough evaluation.
With the near complete sequencing of the canine genome and the commercial availability of canine-specific gene microarrays, it has become possible to characterize and validate specific tumor-associated genetic determinants associated with clinical outcomes and prognosis. In one gene expression profiling study, primary OS tissues were analyzed from two groups of dogs with different clinical outcomes, specifically dogs achieving DFIs either less than 100 days (n = 10) or greater than 300 days (n = 10) following uniform treatment with amputation and systemic chemotherapy.107 Derived from microarray analysis and confirmed by RT-PCR, eight specific gene transcripts were significantly different between poor responders (<100 days) and good responders (>300 days). In dogs categorized as poor responders, six transcripts, including IGF-2 and alcohol dehydrogenase, were downregulated and two transcripts were upregulated in comparison to good responders. To better characterize the molecular pathways associated with the differentially expressed genes identified in microarray analysis, a broader systems approach was used to identify changes in groups of interacting genes or pathways that might contribute to metastatic progression or chemotherapy resistance. In general, pathway expression differences between good and poor responders involved oxidative phosphorylation, bone development, protein kinase A (PKA) signaling, cell adhesion, cytoskeletal remodeling, and immune response.
In a similar expression profiling study, prognostic gene profiles were derived from 32 primary OS tumors derived from two groups of dogs based on survival time.180 Dogs surviving for less than 6 months or greater than 6 months were categorized as either poor or good responders, respectively. Gene profiling identified 51 gene transcripts to be differentially expressed; within the poor responder group, genes uniformly overexpressed were associated with biologic pathways involved in proliferation, drug resistance, and metastases. In addition to identifying differentially expressed genes and associated pathways between dogs categorized as good and poor responders, the findings from the study further substantiated the molecular pathway similarities shared between humans and dogs, including Wnt signaling, integrin signaling, and chemokine/cytokine signaling.
Finally, a highly impactful study was conducted that leveraged the more homogeneous genetic background of dogs diagnosed with OS to detect underlying and conserved gene expression patterns previously undetectable in historic canine and human gene microarray analysis.181 By differential gene expression profiling of early passage immortalized OS cell lines derived from primary tumors, the investigators were able to identify gene signatures associated with G2/M transition and DNA damage checkpoint, as well as microenvironment interactions, which permitted the unbiased segregation of OS samples into distinct molecular subclassification and predicted outcome. Most significantly, the same genetic signatures identified in dogs, also allowed for prognostic molecular classification of human OS—powerfully underscoring the scientific merit derived from comparative oncologic studies.
Perturbations of the immune system are common among cancer patients, and regulatory T-cells (Tregs) and myeloid-derived suppressor cells have the capacity to attenuate effective antitumor immunity responses, with the potential to negatively impact prognosis. Tregs have been characterized in healthy and cancer-bearing dogs,182-184 with some studies demonstrating that dogs with OS have increases in the percentage and absolute counts of circulating Tregs.185 The clinical significance of Tregs on OS prognosis has recently been characterized in a study of 12 dogs treated uniformly with amputation and systemic chemotherapy.185 Dogs with high (above mean) versus low (below mean) percentages of Tregs identified in blood or tumor tissue did not have differences in DFI or survival time. However, a high or low CD8/Treg ratio in the blood was associated with clinical outcomes because dogs with low CD8/Treg ratios (n = 6) were observed to have a significantly shorter survival time than dogs with high CD8/Treg ratios (n = 6).
In addition to Tregs and their potential prognostic value in OS, one study demonstrated that routine hemogram parameters, specifically lymphocyte and monocyte counts, can also predict clinical outcomes in dogs with OS.155 In 69 dogs treated with amputation and systemic chemotherapy, baseline lymphocyte and monocyte counts were associated with DFIs. Shorter DFIs were observed in dogs (n = 69) initially presenting with relative lymphocytosis (≥1000 cells/µL) and relative monocytosis (≥400 cells/µL), and these original conclusions were further substantiated by a second population of OS dogs (n = 21) treated in an identical manner. Mechanistically, it was hypothesized that the association of relative monocytosis and reduced DFI could be the presence of myeloid-derived suppressor cells, a population of cells characterized by their ability to suppress antitumor immune response.
Therapy Directed at the Primary Tumor
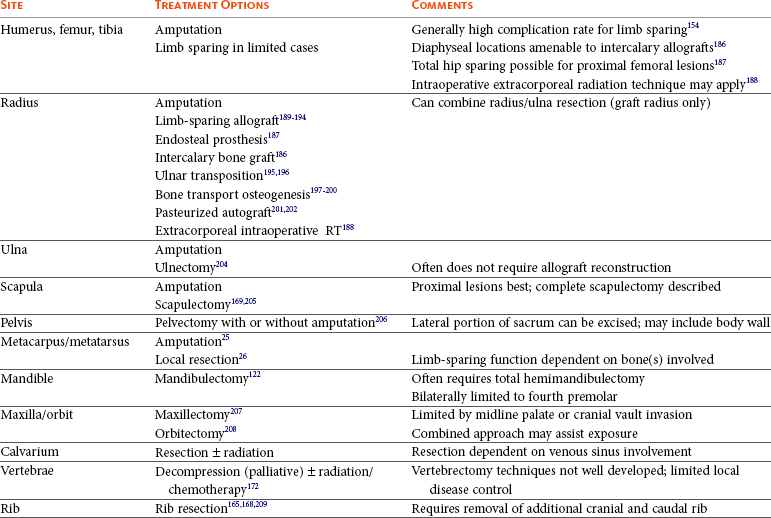
Table 24-1 provides an overview of surgical options for primary bone tumors based on anatomic site.
Amputation
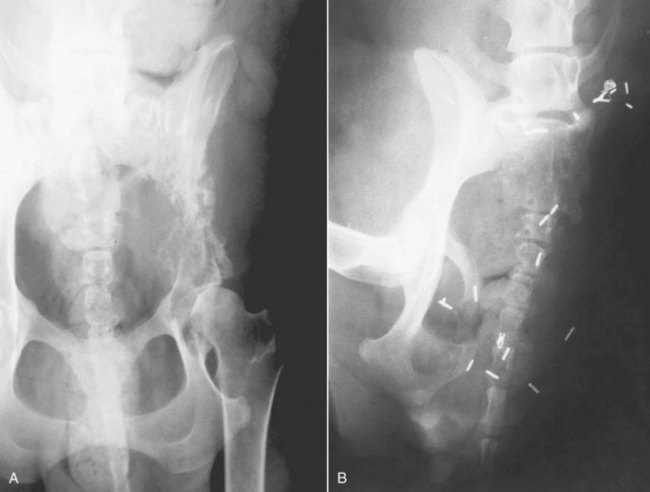
Amputation of the affected limb is the standard local treatment for canine appendicular OS. Even large- and giant-breed dogs usually function well after limb amputation, and most owners are pleased with their pets’ mobility and quality of life after surgery.210,211 Even moderate preexisting degenerative joint disease at the level found in most older, large-breed dogs is rarely a contraindication for amputation. Most dogs will readily compensate, and although the osteoarthritis may progress more rapidly in the three-legged dog, this rarely results in a clinical problem. Severe preexisting orthopedic or neurologic conditions may cause poor results in some cases, and careful preoperative examination is important. A complete forequarter amputation for forelimb lesions is generally recommended, as is a coxofemoral disarticulation amputation for hind leg lesions. This level of amputation ensures complete local disease removal and also results in a more cosmetic and functional outcome. For proximal femoral lesions, a complete amputation and en bloc acetabulectomy is recommended to obtain proximal soft tissue margins (Figure 24-5). Surgery alone must be considered palliative for OS because microscopic metastatic disease is present in the vast majority of cases at diagnosis, and amputation does not address these.
Limb-Sparing Surgery
Although most dogs function well with amputation, in some dogs limb sparing would be preferred, such as dogs with severe preexisting orthopedic or neurologic disease or dogs with owners who absolutely will not permit amputation. Until recently, only a few reports of limb sparing in dogs, with limited follow-up, have appeared in the literature.189-193 To date, more than 600 limb-sparing procedures have been performed at Colorado State University Animal Cancer Center (CSU-ACC). Limb function has generally been good to excellent in most dogs, and survival has not been adversely affected by removing the primary tumor with marginal resection.194
Suitable candidates for limb sparing include dogs with OS clinically and radiographically confined to the leg, dogs in which the primary tumor affects less than 50% of the bone (as determined radiographically), and dogs that are in otherwise good general health. Other criteria for consideration include absence of pathologic fracture, less than 360-degree involvement of soft tissues, and a firm/definable soft tissue mass versus an edematous lesion. Early in the development of limb-sparing procedures, many dogs treated at CSU-ACC received some form of preoperative treatment (i.e., primary or neoadjuvant intraarterial [IA] cisplatin, intravenous [IV] cisplatin, RT to the tumor bone, or a combination of radiotherapy with IV or IA cisplatin). Results from 21 dogs treated with RT alone given in large doses per fraction prior to limb sparing were unsatisfactory for preservation of life or limb.191 Many of the dogs treated with two preoperative IA cisplatin doses 21 days apart, with the last treatment 21 days prior to surgery, showed marked decrease in the degree of vascularization of the tumor. This represented a high degree of induced tumor necrosis in the resected specimen, especially when combined with RT, and facilitated limb sparing.193,212 Currently at CSU-ACC, case selection predetermines the use of local chemotherapy or preoperative downstaging for limb-sparing cases, and most dogs receive systemic carboplatin, doxorubicin, or combination therapy after surgery (see the later section on Systemic Adjuvant Therapy for Dogs with Osteosarcoma).
The most suitable patients for limb sparing are dogs with tumors in the distal radius or ulna because function following limb sparing and carpal arthrodesis is good. Arthrodesis of the scapulohumeral, coxofemoral, stifle, or tarsal joints following limb sparing generally results in only fair to poor function.154 Resulting poor function, combined with a high complication rate, has generally led surgeons away from recommending limb sparing near these joints. Limb sparing is a complicated process and requires a coordinated team effort between surgical and medical oncologists, radiologists, pathologists, and technical staff. Several methods of limb sparing have been described, each with unique advantages and limitations. The choice of limb-sparing method depends on several factors, including owner choice, patient personality, surgeon experience, and individual risk factors. At the CSU-ACC, owners are given a choice of limb-sparing procedures and informed about the risks and benefits of each method compared with amputation. A brief description of the surgical options for a distal radial location (most common) follows. In all cases, cephalosporin antibiotics are administered via IV immediately preoperatively, intraoperatively, and for 24 hours postoperatively. Meticulous aseptic technique is essential.
Allograft Limb Sparing: For a distal radial site, the dog is placed in lateral or dorsal recumbency, with the affected limb uppermost. A skin incision is made on the dorsolateral aspect of the antebrachium from a point just distal to the elbow, to just proximal to the metacarpophalangeal joint. Any biopsy tracts are excised en bloc. Soft tissue is dissected to the level of the tumor pseudocapsule. Care is taken not to enter the tumor. The bone is cut with an oscillating bone saw 3 to 5 cm proximal to the proximal radiographic (or scintigraphic) margin of the tumor. Extensor muscles attached to the tumor pseudocapsule are transected at a level to maintain 2 to 3 cm soft tissue margins. The joint capsule is incised, keeping close to the proximal row of carpal bones. For tumors of the middiaphysis, tumor resection follows similar guidelines with the exception that an attempt to spare extensor and flexor muscle groups is undertaken so the joint (above and below) may be spared.186 The ulna is sectioned sagittally with an osteotome, and the medial ulnar cortex adjacent to the tumor is removed en bloc with the radius. For tumors that have extension to the ulna (rare), the ulna is also cut with a bone saw, and the distal one-third or more is removed with the tumor. Care is taken to preserve as much vasculature as possible, especially on the palmar surface.
Large vessels associated with the tumor are ligated and divided. Surgical hemostatic staples (Surgiclip, United States Surgical Corp, New York) are very useful. The specimen is radiographed, then submitted for histologic evaluation, including assessment of completeness of surgical margins and percentage of tumor necrosis. In addition, a sample of bone marrow proximal to the resection level (radius) is obtained for histologic evaluation of marrow involvement. Intraoperative frozen section histology is used in humans to assess the adequacy of surgical resection of primary bone tumors during limb sparing.213 Although this technique is being used in veterinary medicine, it is still considered somewhat unreliable for bone specimens.
A fresh-frozen cortical allograft is thawed in 1 L of an antibiotic and saline solution (Neomycin 1 g, polymyxin B 500,000 U, potassium penicillin 5,000,000 U), the articular cartilage is removed, the graft is cut to fit, and the medullary cavity reamed to remove fat and cellular debris.214,215 The articular cartilage of the proximal carpal bones is removed, and the allograft is stabilized in compression using Association for the Study of Internal Fixation (ASIF/AO) principles. A dynamic compression plate with a minimum of three screws proximal and four screws distal to the graft is used; 3.5-mm broad locking plates of up to 22 hole size or a custom-designed limb salvage plate are appropriate in most cases, but for very large dogs, 4.5-mm narrow or broad plates are selected. The plate is fastened in the patient to the allograft with two or three screws, removed from the surgery site, and the medullary canal of the allograft is filled with polymethyl methacrylate (Palacos radiopaque bone cement, Smith & Nephew Richards, Inc., Memphis, TN) bone cement containing amikacin (1 g amikacin to 40 g of polymer powder). This provides support for the screws during revascularization of the graft and acts as a reservoir for antibiotics. The healing of the allograft is not significantly impeded by the presence of the cement and has been shown to significantly decrease the incidence of orthopedic failure, including allograft fracture and screw pullout.216,217 The plate extends proximally in the host radius and distally to a level just proximal to the metacarpophalangeal joint (Figure 24-6). For intercalary limb spares, the plate extends proximally and distally to meet or exceed ASIF standards with the intent to spare joint motion.
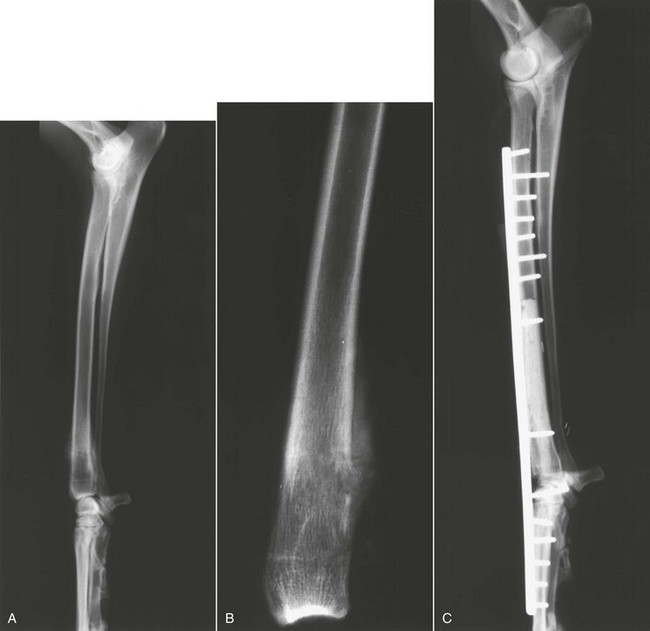
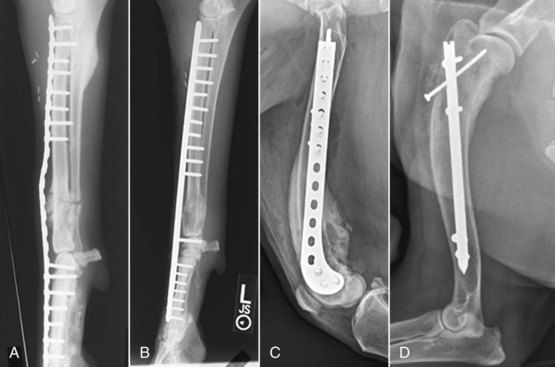
Figure 24-6 Limb sparing. A, Preoperative lateral radiograph of a distal radial OS lesion in a dog. B, Craniocaudal specimen radiograph following tumor resection of the case in A. C, Lateral postoperative radiograph following allograft placement and plate fixation of the case in A and B.
The wound is thoroughly lavaged with saline, and it is at this point that local (polymer) chemotherapy may be implanted. A closed suction drain is inserted adjacent to the allograft, and the wound is closed. The leg is supported in a padded bandage. The drain is removed the day after surgery in most cases. It is most important to prevent self-mutilation (licking) after surgery, and Elizabethan collars should be used as necessary. No external coaptation is used and most dogs use the limb fairly well by 10 days after surgery. Postoperative foot swelling can be considerable but usually resolves by 2 weeks. Although decreased exercise is recommended for the first 4 to 6 weeks to allow soft tissues to heal, no exercise restriction need apply after this time. In fact, it is important that limb use is encouraged, even in early postoperative times, so that flexure contracture of the digits does not occur. Early weight bearing will often decrease the occurrence and incidence of postoperative swelling.
The advantages to allograft limb sparing include the absence of external fixation, and little owner involvement is required in the postoperative period aside from bandage changes in the first 2 weeks. The disadvantages are the high infection rate, potential for local recurrence, and the need for permanent internal hardware. Canine limb spare patients have an infection rate of approximately 40% and 50%. Once an infection occurs, it may be controlled with long-term antibiotic therapy but is rarely, if ever, resolved.218 Infection may result in soft tissue defects from draining tracts, exposure of the plate or allograft, and hardware loosening. Revision surgeries, either for hardware complications or soft tissue reconstruction, are not uncommon. Additionally, amputation for catastrophic implant failure, local recurrence, or unmanageable infection is sometimes required.
Metal Endoprosthesis Limb Sparing.: The metal endoprosthesis limb-sparing technique utilizes a commercially available metal endoprosthesis with a modified bone plate (Figure 24-7). The surgery is nearly identical to the procedure described earlier; however, instead of reconstruction with an allograft, the endoprosthesis is used to span the radial defect. A prospective comparison of complications between allograft limb sparing and metal endoprosthesis limb sparing was published in 2006.187 No significant differences in the number of complications were noted between metal implants and allografts in the patient population studied. An endoprosthesis is an attractive alternative to cortical allografts for limb salvage of the distal aspect of the radius in dogs because surgical and oncologic outcomes are similar, but the endoprosthesis is an immediately available off-the-shelf implant that is not complicated by the bone harvesting and banking requirements associated with cortical allografts.

Figure 24-7 Lateral radiographic projection of a limb-sparing technique using a commercially available metal endoprosthesis with a modified bone plate in a dog with distal radial and ulnar OS. A limb-sparing plate spans host radius and metacarpus, connecting to the implant, which abuts the host radius proximally and the radial carpal bone distally. A negative suction drain has also been placed at the surgical site to decrease postoperative fluid accumulation.
Pasteurized Tumoral Autograft.: Two reports exist of a limb-sparing technique that involves removal of the segment of bone with the tumor and pasteurizing the bone segment at 65° C for 40 minutes, followed by reimplantation.201,202 Limb function was good in 12 of 13 dogs with a 15% local recurrence, 31% infection, and 23% implant failure rate. The advantages of this method are that there is no need for an allograft, and anatomic apposition is excellent. The disadvantages are similar to the allograft technique in terms of complications; however, overall survival and disease-free progression were similar to other studies.
Longitudinal Bone Transport Osteogenesis.: The longitudinal bone transport osteogenesis (BTO) technique for limb sparing has been reported in veterinary patients (Figure 24-8).197,198 This method utilizes Ilizarov (circular) fixators and the principles of distraction osteogenesis to create bone in the defect following tumor resection. Prior to surgery, a five- to six-ring circular fixator is constructed to allow one central ring (termed a transport ring) to move independently from the rest of the fixator. Following the same procedure for removal of the tumor and preparation of the radiocarpal bone described earlier, the circular fixator is placed on the limb and attached to the remaining radius using tensioned 1.6-mm diameter wires. A longitudinal section of normal bone (termed the transport segment) from the radius immediately proximal to the defect is osteotomized and attached to the transport ring with wires. Following a 3- to 7-day delay period, the osteotomized bone segment is slowly transported into the defect at a rate of 1 mm per day. Distraction osteogenesis occurs in the trailing distraction pathway. New bone continues to form longitudinally within the defect proximal to the transport segment for as long as the steady, slow distraction continues. When the transport segment reaches the radiocarpal bone (docking), the transport segment is compressed to the radiocarpal bone and heals to create an arthrodesis. The circular fixator remains on the limb while the newly formed bone remodels and the arthrodesis occurs. This technique is compatible with cisplatin, carboplatin, and combination chemotherapy.197,199
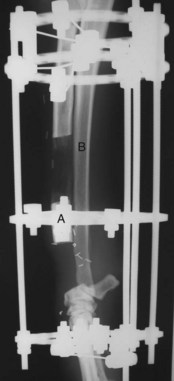
Figure 24-8 Lateral radiographic projection of a limb-sparing technique known as longitudinal bone transport osteogenesis (BTO). In this case, a distal radial OS was removed and BTO was accomplished using circular fixators and the principles of distraction osteogenesis to create bone in the defect remaining following tumor resection. Briefly, a longitudinal section of normal bone (A), termed the transport segment, from the radius is osteotomized and attached to the transport ring, and the osteotomized bone segment is slowly transported into the defect at a rate of 1 mm per day. Distraction osteogenesis occurs in the trailing distraction pathway (B).
The advantages to BTO limb sparing are the lack of internal hardware; the low risk of infection due to the autologous, vascularized nature of the replacement bone; and the ability of the new bone tissue to remodel over time. Patients are typically weight bearing within the first 48 hours and once the incision is healed do not require exercise restriction. The disadvantage of the BTO procedure is the extensive client involvement needed to perform the daily distractions on the fixator and the extended amount of time the fixator remains on the limb. Double level longitudinal transport and translational transport of the ulna can significantly diminish the time required for distraction and have been used successfully in a case of limb salvage for a distal tibial OS.200
Ulna Transposition Limb Sparing.: The vascularized ulna transposition technique uses the ipsilateral distal ulna as an autograft to reconstruct the distal radial defect by rotating the graft into position while preserving the caudal interosseous artery and vein.195,196 Following excision of the tumor as described previously, two transverse osteotomies of the ulna are made. The distal osteotomy is performed at the level of the isthmus proximal to the facet that articulates with the radius. The proximal ulna osteotomy is performed 1 to 2 mm distal to the level of the radial osteotomy. Direct visualization of the caudal interosseous artery and vein allows these structures to be preserved during dissection of the autograft. The ulna graft is “rolled over” into the radial defect and fixed using a bone plate that extends from the proximal radius to the distal one-third of metacarpal IV (i.e., carpal arthrodesis).
Advantages to the ulna transposition technique are that there is no distant donor site morbidity; the replacement bone is autologous; and the graft is vascularized, making it less likely to get infected, and possibly speeding healing. The disadvantages to this technique are that the ulna transposition technique may be more prone to biomechanical complications in the postoperative period due to its smaller size relative to the radius and the need for permanent internal hardware.195
Nonsurgical Alternative Methods of Limb Salvage
Intraoperative Radiation Therapy and Extracorporeal Intraoperative Radiation Therapy
The extracorporeal intraoperative RT (IORT) technique for limb sparing has been utilized in a small number of canine OS patients,188,219 as well as in human patients with extremity bone tumors.220-222 This limb-salvage technique involves a surgical approach to the bone with osteotomy above or below the affected site (depending on the anatomic location of the tumor) and reflection of normal soft tissues from the tumor-affected bone. The neurovascular bundle, muscle, and skin are held away from the affected bone, and the tumor is pivoted from the site on the intact joint tissue. The patient is then transported to the radiation suite and a single dose of 70 Gy radiation is then directed to the tumor, taking care to spare the distracted neurovascular bundle. The irradiated bone is then anatomically replaced and surgical fixation of the osteotomy applied with dynamic compression plating, an interlocking nail system or a combination. One advantage of IORT for limb salvage over surgical limb salvage is that it can be used to preserve limb function in anatomic sites that are not amenable to reliable surgical limb salvage (e.g., proximal humerus).154 Patients treated with IORT had good limb function in the immediate postoperative period; however, complications related to surgery or radiation led to implant revisions in 69% of cases within 5 to 9 months of initial surgery, including four amputations. Pathologic fracture of the irradiated bone was the most common complication. Additionally, local tumor recurrence occurred in four patients and infection in four patients. A modification of this technique includes complete temporary removal of diaphyseal tumors, performing extracorporeal radiation, and then reimplantation and stabilization. In situ radiation of distal femur and any tibial tumors can be performed without osteotomy. The disease-free and overall success rates for limb and joint salvage for extracorporeal IORT were 46% and 54%, respectively. The MST for dogs with appendicular sarcoma treated with limb- and joint-sparing extracorporeal IORT was 298 days (range: 116 to 1775 days).
A small case series of five distal radius IORT cases described joint sparing of the radiocarpal joint by avoiding transcarpal plating.219 Radiocarpal joint function could not be preserved long term in any dog due to complications related to implant failure, deep tissue infection, and pathologic fracture that was treated by amputation or pancarpal plating. The IORT technique for limb salvage cannot be recommended currently due to the high complication rate associated with orthopedic implants and infection in irradiated bone.
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) offers the ability to deliver high-dose RT to the tumor volume with relative sparing of the surrounding normal tissues by use of image guidance and a sharp drop off in dose intensity (Figure 24-9). In this respect, it is a refinement of the IORT technique that avoids the need for surgical exposure. A nonsurgical limb-salvage technique using SRS was developed at the University of Florida, and initial results were reported in 11 dogs.223 Adjuvant carboplatin chemotherapy was used in six dogs immediately prior to radiation treatment for its potential radiosensitization action in addition to its conventional cytotoxic qualities, and five dogs received radiosurgery alone. Five dogs developed pathologic fractures, and one dog developed infection. Acute effects to the skin were mild to moderate in most dogs. Limb use in the dogs that received SRS and chemotherapy was excellent. The reported overall median survival was 363 days in this series; however, the population size was small. Advantages of this technique include limb preservation for anatomic sites not amenable to reliable surgical limb salvage, the normal tissue-sparing effects of stereotactic RT (SRT) compared to conventional RT, no surgical procedures, and good-to-excellent limb function. Disadvantages of the technique include access to equipment that is not typically available to veterinarians and the high complication rate with postirradiation pathologic fracture. These initial results suggested that SRS could provide a viable nonsurgical limb-sparing alternative. Since the publication of this first report, other veterinary centers with linear accelerators capable of delivery of SRT have adapted the SRT limb-salvage technique.
Figure 24-9 Dose color wash map of radiation distribution for SRT of distal radius OS lesion showing steep dose-gradient drop-off between tumor volume and normal tissues.
At CSU, an SRT protocol using a Varian Trilogy linear accelerator was developed for treatment of canine extremity OS. The current SRT protocol utilizes a 3 × 12 Gy (total dose 36 Gy) SRT protocol with fractions delivered on consecutive days. Carboplatin chemotherapy is administered within 2 hours before or after the first or second SRT fraction. Standard adjuvant chemotherapy with either single-agent carboplatin or doxorubicin or combination chemotherapy is continued after SRT at 3 weeks interval for 4 to 6 cycles. We have seen two suspected cases of “radiation recall” skin effects associated with the use of doxorubicin, so care is recommended with use of this chemotherapeutic agent when used in conjunction with SRT. We recommend administration of pamidronate (a bisphosphonate) as soon as possible after diagnosis and ideally 24 to 72 hours before SRT. We do not recommend continued bisphosphonate therapy after SRT because there is no ongoing tumor-associated osteolysis or bone remodeling processes in the irradiated field based on histopathologic examination. Local tumor control has been excellent as assessed by static radiographic findings on serial evaluation, repeat scintigraphic imaging in a few cases, and decreases in urine N-telopeptide (NTx) levels. A mean percentage of tumor necrosis of greater than 90% has been observed on 12 retrieved limb specimens collected at a variety of time points after SRT. Pathologic fracture has been the most frequent complication after SRT. This is due to the amount of preexisting tumor-associated osteolysis and postirradiation bone necrosis and the loss of dynamic bone remodeling and healing capacity after SRT. Smaller, more blastic lesions are better candidates for SRT limb salvage than larger, more lytic lesions. Pathologic fracture has been treated by internal fixation, external coaptation, or amputation. In more recent cases, preemptive stabilization has been used for more lytic tumors immediately after the third fraction of SRT to help prevent fracture (Figure 24-10). Using data in which surgical stabilization is employed when needed, the overall limb survival rate is 83%. The MST for the first 50 dogs treated with this SRT and adjuvant chemotherapy limb-salvage technique is 275 days, which is similar to reported survival times for amputation or surgical limb salvage and adjuvant chemotherapy. Further research into the potential beneficial roles for total radiation dose reduction and adjuvant therapies such as radioprotectant and other bisphosphonates and parathyroid hormone is indicated.
Isolation of Limb Circulation and Perfusion
Isolated limb perfusion with chemotherapy has been used in humans and dogs with sarcomas and melanomas as a sole treatment or to downstage local disease and allow limb sparing.224-226 Isolated limb perfusion (ILP) allows delivery of high concentrations of chemotherapy, as well as delivery of compounds that are poorly tolerated systemically. Varying degrees of local toxicity are reportedly dependent on the drugs used. Successful use of ILP in canine OS has been reported.224 One study determined that appendicular bone tumors have significantly higher interstitial fluid pressure and lower blood flow than do adjacent, unaffected soft tissues.227 ILP may be a method to facilitate delivery of therapeutic drug concentrations to primary tumors for preoperative downstaging prior to limb salvage.
Systemic administration of 153samarium ethylenediamine-tetramethylene phosphonate (153Sm-EDTMP) is limited by systemic myelotoxicity (see Radioisotopes section later). In a study at CSU, 153Sm-EDTMP (37 MBq/kg) was administered via ILP through the isolated limb circulation for 1 hour in nine dogs with primary OS of the appendicular skeleton 3 weeks prior to amputation to evaluate the potential for decreased systemic toxicity and to induce a clinically meaningful percentage of tumor necrosis prior to primary tumor removal. No systemic toxicity was observed. Despite good dosimetry to the lesion, the mean percent tumor necrosis was 27.6% (Std Dev ± 17.6%; range 3.4% to 56.4%), which was similar to mean percent tumor necrosis of 26.8% in untreated OS cases.228 This low tumor necrosis percentage may be due to incomplete perfusion of the 153Sm-EDTMP, the heterogeneous nature of OS, and the inability of the beta particles to exert a cytotoxic effect on the noncalcified regions of the tumor due to their short track length (3 mm). Future experiments should evaluate dose escalation in the perfused limb now that systemic safety has been demonstrated with the ILP technique.
Summary of Outcome Following Limb Salvage for Dogs with Osteosarcoma
There is no significant difference in survival rates for dogs treated with amputation and cisplatin compared to dogs treated with limb sparing and cisplatin.194 Overall, limb function has been satisfactory, with approximately 80% of dogs experiencing good-to-excellent limb function.16 Limb-sparing surgery is usually combined with some form of adjuvant therapy, and complications can arise in any or all phases of treatment (chemotherapy, radiation, or surgery). High-dose external-beam RT may complicate wound and bone healing and potentiate infection.191 Moderate-dose external-beam radiation in combination with chemotherapy may, however, be useful for control of local disease, as indicated by percentage of tumor necrosis data.193,228 The major complications related to limb-sparing surgery are recurrent local disease, allograft infection, and implant complications. In a review of 220 limb-sparing surgeries performed at CSU-ACC, the 1-year local recurrence-free rate determined by Kaplan-Meier life table analysis is over 76% with 60% alive at 1 year.194
In two case series, 40% and 47.5% of dogs, respectively, developed allograft infections.194,229 The majority had their infections adequately controlled with systemic antibiotics with or without local antibiotics (antibiotic-impregnated polymethyl methacrylate beads).218 Many of these dogs continued to have evidence of infection; however, their function was not severely affected. In severe and uncontrolled infections, allografts had to be removed and a small number of dogs required amputation. An unexpected finding has been that dogs with allograft infections experienced a statistically significant prolongation of overall survival times compared to dogs with limb sparing without infected allografts.230 This finding has also been reported in humans with deep infections after limb-salvage surgery for OS.231 A mouse model of OS examined the effects of infection on tumor angiogenesis and innate immunity and demonstrated that chronic localized bacterial infection could elicit significant systemic antitumor activity, depending on natural killer (NK) cells and macrophages.232
Surgery for Nonappendicular and Less Common Appendicular Sites of OS
Certain primary bone tumors of the pelvis can be removed by hemipelvectomy and, although these surgeries are difficult, function and cosmetic outcome have been excellent (see Figure 24-5).206,233 Kramer et al provide an excellent review of the technique for hemipelvectomy and the surgical and oncologic factors to be considered with this surgery.233 SRT has been used for long-term palliation of primary bone tumors arising from the pelvis. The total radiation dose that can be delivered to tumors in this location is limited by the dose tolerance of the colon, rectum, nerves, and perineum within the treatment field. A 5-fraction SRT protocol is used at CSU in preference to the 3-fraction protocol used for extremity OS to help spare these normal tissues. Concurrent administration of carboplatin during SRT for intrapelvic or pelvic tumors is avoided due to the increased frequency of complications observed.
Vertebral OS sites are the most difficult sites to adequately treat local disease. Techniques of complete vertebrectomy are not well established in veterinary medicine,234 and surgery often is an attempt to decompress dogs with neurologic deficits or intractable pain and to obtain a diagnosis.172 Present recommendations are to perform surgery in cases that require decompression (with or without stabilization) and institute RT (discussed later) and chemotherapy. SRT is an emerging treatment option for vertebral body tumors. RT likely plays a role in the treatment of OS of the vertebrae. In a series of 14 dogs with vertebral OS treated between 1986 and 1995, 12 had surgery to decompress the spinal cord, 7 were treated with OPLA-Pt implanted in a distant intramuscular site, and 11 were given IV cisplatin. Nine dogs were treated with fractionated external-beam RT. All dogs had surgery, RT, or both, and no dog was treated with chemotherapy alone. Four dogs improved neurologically, four dogs worsened, and six dogs remained the same. The median survival of 135 days after treatment was relatively short.172 Local disease recurrence rather than metastasis was the usual cause of death.
A combination allograft and custom total joint arthroplasty has been described for successful limb salvage of a proximal femur OS.235 An intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage has been described in a case series of four dogs for distal radius and distal tibia OS lesions.236 A case report using an ITAP prosthesis for a traumatic distal tibia injury has also been reported.237 The success of an ITAP prosthesis requires a biologic seal to be formed between the skin and the prosthesis to prevent the possibility of deep infection.
Bone tumors originating in proximal sites of the scapula can be successfully removed by partial scapulectomy (Figure 24-11).169,171,205 Dogs function well with partial scapulectomy but take 1 to 3 months to improve. Seroma formation is common in the immediate postoperative period. Intensive postoperative physical therapy is important in these cases to regain normal function. Significant gait abnormalities may occur after complete scapulectomy by disarticulation at the scapulohumeral joint.171 Small primary tumors of the ulna can be removed by partial ulnectomy, and reconstruction is rarely needed.204 Tumors located in the metatarsal and metacarpal bones can be treated with local resections or partial foot amputation.237a Experience at our institution supports removal of a single bone or the central two bones in most dogs, with good functional outcome. In small dogs, removal of the medial or lateral two bones can result in normal function. Resection to this extent has not been attempted in larger dogs. Mandibulectomy and maxillectomy are appropriate surgeries for primary bone tumors of oral sites.160,161,238,238a,239 In a survey of owners of dogs undergoing partial mandibulectomy or maxillectomy, 85% were satisfied with the outcome despite 44% citing difficulty in eating as a complication.236 Tumors of periorbital sites can be removed by orbitectomy.208 Rib tumors can be removed by thoracic wall resection, and the defect can be reconstructed with polypropylene mesh (Marlex mesh, CR Bard Inc, Billerica, MD) with plastic plates (Lubra plates, Lubra Co, Fort Collins, CO) for large defects or by muscle flap techniques.165,167,168 Diaphragmatic advancement can be used for caudally located defects.
Radiation
At present, the role of RT for curative-intent local tumor control is still evolving in veterinary medicine. Currently, the most common role of RT in dogs with appendicular OS is for palliation of bone pain (see later section on Palliative Treatment: Primary and Metastatic Bone Cancer Pain). RT at relatively high total doses can cause considerable necrosis of primary OS in dogs and humans, either before limb salvage to downstage the primary tumor to improve the success of local disease control following removal or as a primary therapy for unresectable tumors.191,193,240-242 As a primary therapy, an MST of 209 days was reported in 14 dogs with appendicular OS treated with fractionated high-dose radiation (median dose of 57 Gy) to the primary tumor and systemic chemotherapy for micrometastasis.243 Similar results are seen in humans with extremity OS treated with high-dose radiation, with and without surgical stabilization.220 The introduction of SRT has allowed delivery of high doses of radiation to the tumor volume with excellent local tumor control and relative sparing of the surrounding normal tissues.
Radioisotopes
The bone-seeking, beta emitter radioisotope, 153Sm-EDTMP, has been used to treat primary OS and metastatic bone neoplasia in dogs and humans via systemic IV administration (see later section on Palliative Treatment: Primary and Metastatic Bone Pain). Radium-223 chloride (Alpharadin, Algeta ASA, Oslo), a high linear energy transfer (LET) alpha-emitting radioisotope, has been recently approved by the Food and Drug Administration (FDA) and European regulatory authorities for treatment of multifocal metastatic bone cancer in men affected by hormone refractory prostate cancer.244,245 Radium-223 chloride is a natural bone-seeking radioisotope that has been shown to prolong survival in patients with advanced prostate cancer in phase II and III clinical trials.246 In acute and long-term repeat dose toxicity studies in normal beagles, the main side effect was temporary and reversible myelosuppression (leukocytopenia and thrombocytopenia). Radium-223 chloride was tolerated well when administered at 50 KBq/kg IV once per month for 6 consecutive months and at single doses up to 450 KBq/kg.
Systemic Adjuvant Therapy for Dogs with Osteosarcoma
For the most effective management of canine OS, multimodality therapy is required to address both local and metastatic disease. Although amputation and limb-sparing surgeries, as well as nonsurgical techniques such as SRT, have proved highly effective for local OS management, the ability to control the progression of OS metastases remains an urgent clinical challenge, and substantive improvements in DFIs and survival times await advances in systemic antimetastatic treatment options.
Systemic chemotherapy remains the backbone for the management of OS metastases, and it is improbable that the discovery of new chemotherapeutic agents or dose-intensification with existing agents will dramatically improve current clinical outcomes. The future of OS management will likely depend on combining conventional cytotoxic agents with targeted molecular therapeutics or immunomodulatory agents. As such, considerable research focus has been committed to discovering and validating new combination therapies for improving the long-term prognosis of canine OS. Current adjuvant chemotherapies, used singly or in combination with other cytotoxic agents, are discussed in the following section.
Chemotherapy
Table 24-2 provides an abbreviated summary of conventional chemotherapeutic agents used in the adjuvant setting, evaluated as single agents or in combination in at least 20 dogs. More detailed information regarding the studies highlighted in Table 24-2 is provided in the following section.
Table 24-2
Abbreviated Summary of Historic and Current Adjuvant Chemotherapy Protocols Derived from Studies with at least 20 Dogs*
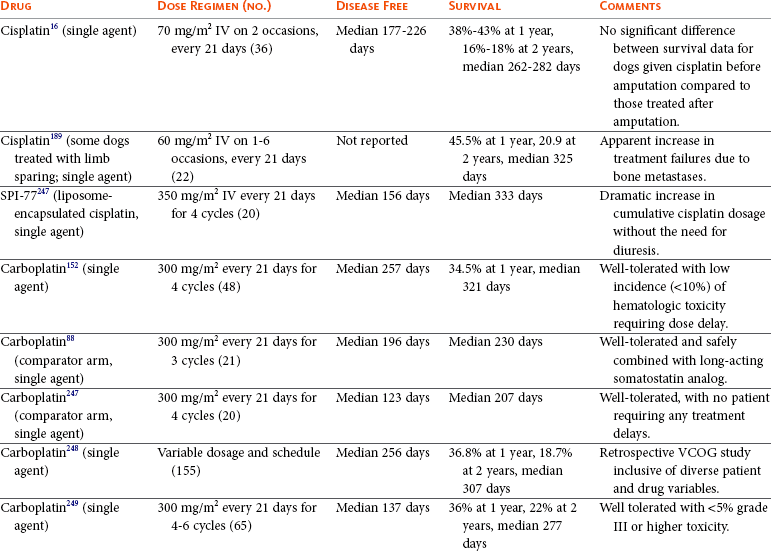
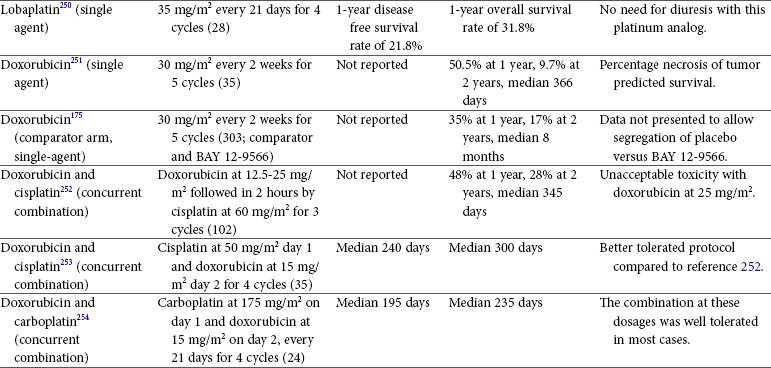
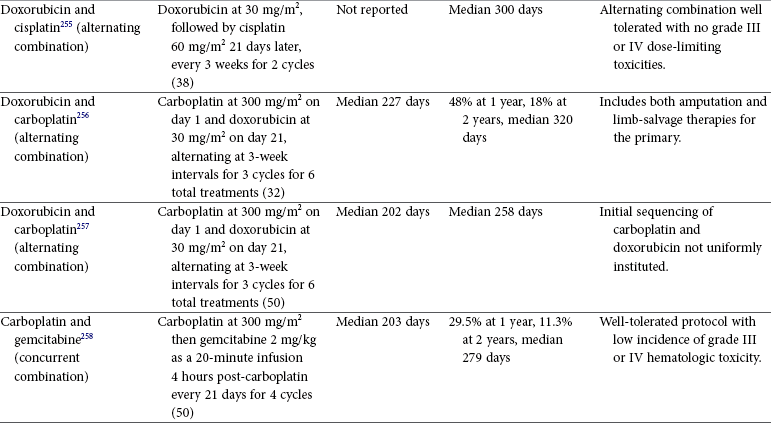
VCOG, Veterinary Cooperative Oncology Group.
*Few of these protocols include sufficient numbers for adequate statistical power and fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably among protocols presented. Therefore evaluations of efficacy among protocols are subject to bias, making direct comparisons difficult and indeed precarious.
Cisplatin: Single Agent
Similar to its activity in an adjuvant or neoadjuvant setting for the treatment of pediatric OS, cisplatin has been demonstrated to improve survival in dogs with OS after amputation in several studies with small-to-modest patient population sizes.16,189 In one report, 36 dogs received 2 treatments of cisplatin every 21 days at a dosage of 70 mg/m2.16 In approximately half of the dogs (group 1, n = 17), cisplatin was administered postoperative; for the remaining dogs (group 2, n=19), cisplatin was administered at diagnosis (preamputation) and again 21 days later immediately following amputation (perioperative). The median survival for group 1 (262 days; 1- and 2-year survival rates of 38% and 18%, respectively) was not significantly different from that of group 2 dogs (282 days; 1- and 2-year survival rates of 43% and 16%, respectively); suggesting that the administration of cisplatin in a neoadjuvant setting did not improve clinical outcomes. In a second study, 22 dogs with appendicular OS were treated with definitive surgery (amputation or limb-sparing), and 1 week after surgical recovery began treatment with cisplatin at 60 mg/m2 every 21 days for 1 to 6 treatment cycles.189 The MST achieved by dogs receiving cisplatin was estimated to be 46.4 weeks, and 1- and 2-year survival rates were estimated to be 45.5% and 20.9%, respectively.
Despite the fact that some studies have documented cisplatin’s antimetastatic effects at relatively low-dose intensities (50 mg/m2 every 4 weeks), the recommended dose for cisplatin is 70 mg/m2 body surface area, given intravenously every 3 weeks. Although cisplatin is relatively well tolerated, it is highly emetogenic and preemptive treatment with antiemetics such as maropitant should be recommended.259,260 In addition to its emetogenic potential, a more serious side effect of cisplatin is the induction of nephrotoxicity. Therefore normal baseline renal function is essential prior to administration, and diuresis protocols must be performed concurrent with treatment. The diuresis protocol used most commonly involves a 4-hour pretreatment diuresis with 18.3 mL/kg/hr of 0.9% NaCl, followed by a 20-minute infusion of cisplatin diluted in a volume of 0.9% NaCl that will allow the same (18.3 mL/kg/hr) fluid infusion rate as the rate before diuresis. The cisplatin infusion is then followed by an additional 2 hours of diuresis with 18.3 mL/kg/hr of 0.9% NaCl.261
Carboplatin: Single Agent
Carboplatin is a second-generation platinum compound that is less nephrotoxic than cisplatin with comparable antitumor activity. Given its ease of administration, carboplatin has largely supplanted the use of cisplatin in the postoperative setting. In the first study reporting the tolerability and activity of carboplatin, 48 dogs with OS were treated with amputation and intent-to-treat with 4 doses of carboplatin (300 mg/m2 every 21 days).152 Carboplatin was well tolerated, with neutropenia being identified as the dose-limiting toxicity. For the entire study population (n = 48), the median DFI and survival time achieved was 257 and 321 days, respectively.
Despite the initial report of carboplatin’s comparable activity to cisplatin,152 two prospective randomized studies using single-agent carboplatin as a comparator arm demonstrated less impressive antimetastatic effects.88,247 In one study, dogs were treated with a single neoadjuvant dose of carboplatin and amputation 7 days later, then received 2 additional treatments with carboplatin (300 mg/m2) every 21 days. On completion of carboplatin, dogs were then randomized to receive either placebo (n = 21) or a long-acting somatostatin analog (n = 23).88 For dogs treated with carboplatin and placebo, the median DFI and survival time achieved was 196 and 230 days, respectively. In a second study comparing the activity of a liposome-encapsulated cisplatin formulation (SPI-77) versus single-agent carboplatin, 40 dogs were treated with a single neoadjuvant dose of either SPI-77 (350 mg/m2) or carboplatin (300 mg/m2) 1 week prior to amputation, then received an additional 3 treatments of SPI-77 or carboplatin every 21 days.247 No difference was identified between treatment groups, with dogs receiving single-agent carboplatin achieving median DFI and survival time of 123 and 207 days, respectively.
Two relatively large, retrospective studies have been conducted to substantiate the activity of carboplatin for managing pulmonary micrometastases.248,249 In a study initiated by the Veterinary Cooperative Oncology Group (VCOG), 155 dogs treated with amputation and carboplatin (variable dosage and schedule) achieved median DFI and survival times of 256 and 307 days, respectively.248 In a second retrospective investigation, 65 dogs undergoing amputation and subsequent carboplatin at a dosage of 300 mg/m2 every 21 days for 4 to 6 treatment cycles achieved median DFI and survival time of 137 and 277 days, respectively.249
Lobaplatin: Single Agent
Lobaplatin, a third-generation platinum compound, has been investigated in the adjuvant setting for the treatment of canine OS. In a study of 28 dogs, lobaplatin at 35 mg/m2 was administered as an IV bolus every 21 days for 4 treatments.250 Lobaplatin was well tolerated, with a low incidence of vomiting. Transient and reversible hematologic toxicity manifested as neutropenia and thrombocytopenia were observed 7 to 10 days following lobaplatin administration. The 1-year survival percentage was reported to be 32%, which is comparable to what is achieved with cisplatin or carboplatin.
Doxorubicin: Single Agent
Doxorubicin is considered effective for delaying the development and progression of micrometastatic disease in dogs with OS. However, doxorubicin’s antimetastatic effects are more definitively substantiated when administered every 2 weeks, rather than every 3 weeks. In one study, doxorubicin was given at a dosage of 30 mg/m2 every 2 weeks for 5 treatments to 35 dogs with appendicular OSs in a neoadjuvant setting. Dogs were treated with 2 or 3 dosages of doxorubicin prior to amputation and continued to receive doxorubicin postsurgery for a total of 5 treatments.251 The 1- and 2-year survival rates were 50.5% and 9.7%, respectively. In a second study evaluating the activity of an MMP inhibitor (BAY 12-9566), 303 dogs were treated with amputation and doxorubicin (30 mg/m2 every 2 weeks for a total of 5 treatment cycles) and then randomized to receive daily oral placebo or BAY 12-9566.175 No difference in survival time was identified between dogs receiving placebo or BAY 12-9566, and the MST for all 303 dogs was 8 months.
Doxorubicin/Cisplatin: Concurrent Combination
With the establishment of single-agent activity of doxorubicin and cisplatin for the management of canine OS in the postoperative setting, two studies were conducted to evaluate the tolerability and activity of doxorubicin/cisplatin combinations producing summation dose intensities of greater than one.252,253,262 In one study, 102 dogs treated with combination doxorubicin/cisplatin either 2 or 10 days postamputation were evaluated.252 Doxorubicin was administered at a dosage of 12.5-25 mg/m2 during saline diuresis and before cisplatin administration. The dosage of cisplatin was 60 mg/m2 and was given with 6-hour saline diuresis. Dogs received combination doxorubicin/cisplatin every 21 days for a total of 3 treatments. Unacceptable toxicity was associated with doxorubicin administered at 25 mg/m2 in conjunction with cisplatin at 60 mg/m2, which resulted in approximately 10% treatment-associated mortality. With the exclusion of deaths associated with therapy, dogs capable of tolerating the protocol achieved MST of 11 to 11.5 months, with 1-year, 2-year, and 3-year survival rates of 48%, 28%, and 18%, respectively. In a second, less dose-intense combination study, 35 dogs with appendicular OS underwent amputation and chemotherapy with cisplatin and doxorubicin every 21 days for up to 4 cycles.253 Cisplatin was administered at a dosage of 50 mg/m2 in conjunction with 6-hour saline diuresis, and doxorubicin was administered at a dosage of 15 mg/m2 24 hours later. Of the original 35 dogs, only 16 patients completed all four treatment cycles. For all dogs, the median DFI and survival time achieved was 240 and 300 days, respectively.
Doxorubicin/Carboplatin: Concurrent Combination
Given the modest-to-severe toxicity associated with doxorubicin/cisplatin combination therapy, one study investigated if combination tolerability could be improved by replacing cisplatin with carboplatin. The rationale to use carboplatin was based on its comparable anticancer activities and improved side effect profile relative to cisplatin, which excludes the need for saline diuresis and minimizes the likelihood of severe emesis. Twenty-four dogs were treated with definitive surgery, followed by combination chemotherapy consisting of carboplatin (175 mg/m2) administered on day one and followed by doxorubicin (15 mg/m2) on day two.254 Combination doxorubicin/carboplatin was administered every 21 days for a maximum of 4 treatment cycles. Nineteen dogs completed four treatment cycles, and the tolerability of the combination was good with mild gastrointestinal toxicity reported in approximately 50% of patients and rare frequency of grade III hematologic toxicity or greater. The median DFI and survival time achieved was 195 and 235 days, respectively, and not considered superior to historic single-agent studies.
Doxorubicin/Cisplatin: Alternating Combination
To minimize toxicosis associated with concurrent combination protocols with summation dose intensities greater than 1.0 but to still exploit differing mechanisms of action of two compounds, alternating two drug protocols has been investigated for canine OS. In one of the first alternating two drug protocols described, 38 dogs were treated with definitive surgery alone (n = 19) or with adjuvant chemotherapy (n = 19) alternating doxorubicin and cisplatin.255 Doxorubicin (30 mg/m2) was the first drug to be administered 14 days after surgery, and cisplatin (60 mg/m2) was given 21 days later; treatment was repeated for a total of 4 treatments (2 doxorubicin and 2 cisplatin). For dogs treated with amputation alone, the MST was 175 days; whereas for dogs treated with amputation (n = 17) or complete resection (n = 2; rib OS) followed by alternating doxorubicin and cisplatin therapy, the MST was 300 days. The alternating combination of full-dose doxorubicin and cisplatin did not result in any grade III or IV dose-limiting toxicities and significantly improved survival time in comparison to amputation alone.
Doxorubicin/Carboplatin: Alternating Combination
The tolerability and activity of full-dose, alternating combinations with doxorubicin and carboplatin have been recently conducted. In one study, 32 dogs were treated with amputation or limb-sparing surgery, then subsequently treated with carboplatin (300 mg/m2, or 10 mg/kg if <15 kg) and 21 days later received doxorubicin (30 mg/m2, or 1.0 mg/kg if < 15 kg).256 Dogs received up to 3 treatment cycles (3 carboplatin and 3 doxorubicin). Alternating carboplatin and doxorubicin therapy was well tolerated, and out of 88 doses of carboplatin and 82 doses of doxorubicin administered, only one grade III neutropenia, one grade III thrombocytopenia, and one grade III vomiting were recorded. The median DFI and survival times were 227 and 320 days, respectively; with 1- and 2-year survival rates being 48% and 18%, respectively.
In a second confirmatory study, 50 dogs were treated with amputation and 10 to 14 days postoperative received alternating combination chemotherapy with carboplatin (300 mg/m2) and doxorubicin (30 mg/m2) every 21 days for 3 cycles (3 carboplatin and 3 doxorubicin).257 However, dogs were not standardized to receive carboplatin first and doxorubicin second; and of the 50 dogs, 30 received carboplatin first, and the remaining 20 dogs received doxorubicin as their initial treatment. Adverse toxicities, including grade III or IV hematologic toxicity in 18% of dogs, and grade III or IV gastrointestinal toxicity in 12% of dogs were recorded. The median DFI and survival time were 202 and 258 days, respectively.
Carboplatin/Gemcitabine: Concurrent Combination
Based on in vitro data demonstrating strong synergism between carboplatin and gemcitabine in canine OS cell lines at pharmacokinetically achievable concentrations and durations of exposure,263 a prospective study was conducted to assess if combination chemotherapy with carboplatin and gemcitabine could improve clinical outcomes in dogs with OS.258 Fifty dogs were treated with amputation, and 14 days postsurgery were treated first with carboplatin (300 mg/m2) and then gemcitabine was administered as a 20-minute IV infusion 4 hours postcarboplatin administration. Dogs were treated every 21 days for up to 4 dosages of carboplatin and gemcitabine. The combination of carboplatin and gemcitabine was well-tolerated with 74% of patients receiving all 4 treatment cycles, and only 5 episodes of grade III or IV hematologic toxicity was recorded. The median DFI and survival time were 203 and 279 days, respectively; with 1- and 2-year survival rates being 29.5% and 11.3%, respectively.
Preliminary Agents
Satraplatin, by virtue of its lipophilicity, is a platinum drug formulation with high oral bioavailability. Given the advantages of novel oral drug formulations, the pharmacokinetics and tolerability of oral satraplatin was determined in tumor-bearing dogs in a phase I dose-escalation study.264 Oral satraplatin was administered daily for 5 consecutive days every 3 to 4 weeks for up to a total of 4 treatment cycles. Dose-limiting toxicity was myelosuppression, with grade III and IV neutropenia and/or thrombocytopenia being documented with satraplatin doses equal to or greater than 35 mg/m2/day for 5 consecutive days. Out of the 23 dogs enrolled in the phase I study, 12 dogs had a diagnosis of appendicular OS (6 micrometastatic and 6 gross macroscopic). In the six dogs receiving satraplatin in the adjuvant setting, the median DFI and survival time were 456 and 659 days, respectively. Based on these early findings derived from a small subset of dogs with OS, satraplatin should be investigated more formally for appendicular OS.
Immunotherapy
Harnessing and directing the immune system for controlling micrometastatic disease remains a highly desirable anticancer strategy. Despite the various forms of immunotherapies such as monoclonal antibodies and dendritic cell vaccines currently instituted for the treatment of metastatic tumor histologies in humans, only a few immunotherapy studies in dogs have been conducted as randomized, double-blind trials. The best documented and clinically effective immunotherapy trials for dogs with OS have evaluated the anticancer immune effects associated with the administration of liposome-encapsulated muramyl tripeptide-phosphatidylethanolamine (L-MTP-PE). Being a lipophilic derivative of muramyl dipeptide, a synthetic analog of a Mycobacterium cell wall component, L-MTP-PE has been demonstrated to augment canine alveolar macrophage tumoricidal properties as supported by enhanced cytotoxicity against OS cells in vitro.265
In an initial clinical study of 27 dogs, the single-agent activity of intravenously administered L-MTP-PE was assessed immediately following amputation. Dogs were treated twice weekly with either L-MTP-PE (n = 14) or empty liposomes (n = 13) for a duration of 8 weeks. Dogs receiving L-MTP-PE achieved a significant prolongation in median DFI compared to dogs treated with empty liposomes (168 versus 58 days). Similarly, the MST was significantly longer for dogs receiving L-MTP-PE than dogs treated with empty liposomes (222 versus 77 days).266 Based on these comparisons, it was concluded that L-MTP-PE induced beneficial immunobiologic effects capable of delaying the progression of pulmonary micrometastatic disease.
After establishing the anticancer activity of single-agent L-MTP-PE when used in the adjuvant setting, two subsequent randomized, double-blind studies were conducted to determine the effectiveness of combining L-MTP-PE with cisplatin.267 In trial 1, dogs underwent limb amputation and received cisplatin every 4 weeks for 4 treatments. On completion of cisplatin therapy, dogs (n = 25) without overt evidence of pulmonary metastases were randomized to receive either L-MTP-PE (n = 11) or empty liposomes (n = 14) twice a week for 8 consecutive weeks. Dogs receiving L-MTP-PE had a significantly longer MST in comparison to dogs treated with empty liposomes, 14.4 versus 9.8 months.
Unlike trial 1 in which the cisplatin and L-MTP-PE were administered serially, the study design of trial 2 sought to characterize the anticancer activities of concurrently administered cisplatin and L-MTP-PE. All dogs (n = 64) were treated with limb amputation and cisplatin every 3 weeks for 4 treatments. Within 24 hours following the first cisplatin treatment, dogs were randomized to concurrently receive either L-MTP-PE twice a week (n = 21), L-MTP-PE once a week (n = 21), or empty liposomes once a week (n = 22) for a duration of 8 weeks. Disappointingly, dogs receiving concurrent L-MTP-PE (twice or once weekly) and cisplatin failed to demonstrate any improvement in DFI or survival time when compared to dogs treated with concurrent empty liposome (once weekly) and cisplatin. The reported MSTs for L-MTP-PE and empty liposome groups were 10.3, 10.5, and 7.6 months, respectively.
Molecular-Targeted Therapies for Dogs with Osteosarcoma
Micrometastatic Adjuvant Setting
The molecular pathogenesis for OS remains incomplete; however, a growing body of scientific evidence supports the participatory role of several cell signaling pathways that promote OS growth and survival. One particular growth factor signaling cascade more thoroughly investigated in OS has been GH and IGF-1. The putative roles of GH and IGF-1 in OS pathogenesis is supported by several clinical observations in both humans and dogs. For pediatric OS, the peak incidence of OS development occurs during the adolescent growth spurt,268 which coincides with the greatest circulating concentrations of GH and IGF-1. Similarly in dogs, large to giant skeletal size is a strong positive determinant for appendicular OS development. Given the central roles of GH and IGF-1 in skeletal growth and homeostasis, as well as their role in cell survival, it has been hypothesized that aberrant or excessive GH and IGF-1 signaling is likely involved in OS pathogenesis.269 To investigate the biologic consequences of attenuating GH and IGF-1 autocrine and/or paracrine signaling in OS, a randomized clinical trial in 44 dogs with OS was conducted in which circulating IGF-1 concentrations were suppressed with the administration of a long-acting analog of somatostatin (OncoLAR).88 All dogs were treated with amputation and carboplatin in combination with either OncoLAR (n = 23) or vehicle (n = 21). The administration of OncoLAR resulted in a 43% reduction in circulating IGF-1 concentrations in comparison to baseline values; however, disappointingly, the suppression in IGF-1 did not translate result in improved DFIs or overall survival times in comparison to dogs receiving vehicle.
Molecular therapies with the capacity to delay or inhibit the development of pulmonary metastases would dramatically improve current treatment outcomes. As such, novel anticancer agents have been designed to selectively inhibit obligate steps necessary for successful tumor cell invasion and metastasis. One specific strategy has been the inhibition of MMPs, which are proteolytic enzymes involved in local tissue invasion and metastases. Based on documented gelatinolytic activities of MMP-2 and MMP-9 in canine cell lines and OS samples,97,99 it has been rationalized that specific inhibitors of MMP activity might have the potential to increase the metastasis-free period after amputation and systemic chemotherapy. As such, a prospective, double-blind, randomized, placebo-controlled clinical trial evaluating the adjuvant activity of a MMP-2 and -9 inhibitor (BAY 12-9566), was evaluated in dogs with OS.175 Following amputation and doxorubicin therapy, dogs without radiographic evidence of pulmonary metastatic disease (n = 223) were randomized and treated with either BAY 12-9566 (10 mg/kg) or placebo control daily until clinical failure. The addition of BAY 12-9566 did not improve DFI or survival time in comparison to placebo control. Correlating with the absence of biologic effect, serum MMP-2 and -9 activities were not different between dogs receiving BAY 12-9566 or placebo.
Treating Gross Metastatic Disease
Resection of pulmonary metastasis from OSs or other solid tumors has been reported in humans.270 One report of 36 dogs treated with pulmonary metastasectomy for OS exists.271 Lesions located subpleurally were gently lifted from the lung parenchyma by thumb forceps and a single pursestring of 2-0 or 3-0 polygalactin 910 (Vicryl, Ethicon, Sommerville, NJ) suture was tied around the base of normal tissue. Larger lesions located deeper in the lung parenchyma were removed by complete or partial lobectomy using surgical staples (TA30 or TA55, United States Surgical Corp, New York). No chemotherapy was given after these surgeries. Although the initial treatments varied between dogs, the MST of the entire group was 487 days. The median survival after pulmonary metastasectomy was 176 days (range 20 to 1495 days). The criteria established for case selection for pulmonary metastasectomy in order to maximize the probability of long survival periods are (1) primary tumor in complete remission, preferably for a long relapse-free interval (>300 days); (2) one or two nodules visible on plain thoracic radiographs; (3) cancer only found in the lung (negative bone scan); and perhaps (4) long doubling time (>30 days) with no new visible lesions within this time. Pulmonary metastasectomy can also be performed for palliative relief in dogs with hypertrophic osteopathy.272 Thorascopic lung lobectomy has been described for removal of primary and metastatic lung tumors.273
Surgical intervention to treat metastatic skeletal lesions with limb-sparing surgery alone or combined with stereotactic RT have been used in a few selected cases at the authors’ institution, but this is not routinely recommended. A whole body technetium bone scan or PET/CT scan with F-18 is strongly recommended to identify the complete extent of metastatic skeletal disease prior to any surgical intervention. A recent report describes the feasibility of aggressive cancer treatment consisting of fracture fixation and postoperative chemotherapy as a treatment option for dogs with fractures associated with primary OS.108 Dogs with stage III OS with bone metastases without pulmonary metastases are reported to have longer survival times than dogs with pulmonary metastases.115 With the increased availability of locking plates, intraoperative imaging and minimally invasive osteosynthetic techniques, stabilization of metastatic bone lesions or fractures is a palliative treatment option that may provide increased quality of life for selected patients and owners.
Chemotherapy and Receptor Tyrosine Kinase Inhibitors for Gross (Macroscopic) Osteosarcoma
The treatment of gross measurable OSs with conventional cytotoxic agents or RTK inhibitors remains unsatisfactory. In part, the ineffectiveness of systemic therapies to cytoreduce gross measurable OS lesions is due to the development of drug-resistant clones, in conjunction with unfavorable and altered drug biodistribution within large tumor microenvironments. Because formidable biologic barriers are operative within macroscopic tumors that favor cancer cell survival, the majority of studies demonstrate only marginal effectiveness of conventional cytotoxic agents or RTK inhibitors for the management of gross, measurable OS.
In one study, 45 dogs that had either developed gross metastatic OS following standard-of-care therapy (amputation and systemic chemotherapy) or diagnosed at presentation with metastatic OS were treated with single-agent chemotherapies, including cisplatin (n = 31), doxorubicin (n = 11), or mitoxantrone (n = 3).274 Out of the 45 dogs treated, only 1 dog achieved a short-lived (21-day) partial response, findings which suggest that conventional cytotoxic agents that are effective in the adjuvant setting are not efficacious for managing macroscopic metastatic OS. In a second study evaluating the tolerability and anticancer activities of paclitaxel in dogs with measurable tumor burdens, two out of nine dogs with macroscopic pulmonary metastatic OS achieved a partial response, suggesting that inhibitors of microtubule depolymerization might have modest activity for the management of gross measurable OS lesions.275
In addition to cytotoxic agents, the RTK inhibitor, toceranib phosphate (Palladia), has recently demonstrated preliminary anticancer activity across a broad range of tumor histologies, including metastatic pulmonary OS. Targets of toceranib include several members of the split-kinase family such as VEGF receptor, platelet-derived growth factor (PDGF) receptor, and c-kit.276 Based on toceranib’s ability to inhibit multiple signaling pathways, its potential activity was evaluated in 23 dogs with measurable pulmonary OS metastases.277 Toceranib was orally administered at a median dose 2.7 mg/kg (every other day or Monday/Wednesday/Friday) and resulted in a partial response rate of 4.3% (1/23 dogs) and stable disease rate of 43.5% (10/23 dogs).
Strategies to enhance the susceptibility of metastatic cancers to conventional chemotherapeutic agents have also been recently conducted. By virtue of their chromatin remodeling effects, histone deacetylase inhibitors (HDACi) used in combination with cytotoxic agents might enhance the nuclear accumulation of cytotoxic agents and improve therapeutic outcomes. Based on this premise, a phase I dose-escalation study combining valproic acid and doxorubicin was conducted in dogs with various spontaneous tumors to determine the tolerability and activity of this HDACi/chemotherapy combination.278 Three dogs with macroscopic OS pulmonary metastases were treated with valproic acid and doxorubicin, with one dog achieving durable disease stabilization.
Investigational Therapies for Gross Metastatic Disease
Because the pulmonary parenchyma has a large absorptive surface area and high blood flow, the administration of therapeutic agents directly to it remains a potential tractable and noninvasive method for localized and systemic drug delivery. Although the majority of clinical indications for aerosol drug delivery are the management of inflammatory airway pathologies (i.e., bronchitis), the ability of inhalation therapy to achieve high drug concentrations directly to the lungs with the minimization of systemic toxicities provides justification to test this method of drug delivery for the management of macroscopic pulmonary OS metastases in dogs.
Two studies have been conducted to evaluate the feasibility, tolerability, and anticancer activities of aerosolized cytotoxic therapies in dogs diagnosed with macroscopic pulmonary OS metastases.279,280 In one study, six dogs with macroscopic pulmonary OS metastases were treated every 14 days for a total of 6 treatment cycles with inhalation doxorubicin, paclitaxel, or both.279 Aerosolization therapy was well tolerated, with no dose-limiting hematologic or biochemical toxicity associated with inhalation therapy. However, in dogs treated with aerosolized doxorubicin, pulmonary histologic changes were identified at necropsy in some patients consisting of toxin-induced pneumonitis, multifocal interstitial fibrosis, alveolar histiocytosis, and type II pneumocyte proliferation. Measurable anticancer activity was documented in two dogs (partial remission) treated with inhalation doxorubicin and one dog (complete remission) treated with inhalation paclitaxel. The duration of complete remission in the one responder dog treated with inhalation paclitaxel was durable, lasting greater than 325 days.
In addition to doxorubicin and paclitaxel, aerosolized gemcitabine has also been evaluated in dogs with metastatic OSs. Although categorized as a pyrimidine antimetabolite that belongs to the nucleoside analog family, it was demonstrated in preclinical mouse OS xenograft models that the anticancer activity of aerosolized gemcitabine was mediated though the upregulation of Fas receptor expression on the surface of pulmonary metastatic OS cells.281 Because lung epithelium basally expresses Fas ligand, the restoration of Fas receptor expression by OS cells would consequently render them susceptible to Fas receptor-/Fas ligand-mediated apoptosis. Based on this preclinical information, a comparative study with aerosolized gemcitabine was conducted in 20 dogs with macroscopic pulmonary OS metastases.280 Dogs were treated twice weekly with inhalation gemcitabine and monitored for toxicity and anticancer activity. Aerosolized gemcitabine was well tolerated with no dose-limiting hematologic or biochemical toxicity reported and minimal histologic lung pathology following inhalation therapy. Mechanistic anticancer activities of aerosolized gemcitabine were supported by the identification of increases in percentage of necrosis, Fas receptor expression, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positivity in macroscopic pulmonary OS metastatic lesions; however, clinically relevant tumor reductions, either partial or complete, were not achieved in any of the dogs treated.
Augmentation of Antitumor Immunity
Cytokines are cellular peptides, some of which actively aid or stimulate the immune system to recognize and attack cancer cells. Although numerous cytokines participate in shaping the strength, specificity, and longevity of antitumor immune responses, interleukin-2 (IL-2) is a critical cytokine necessary for stimulating the growth, differentiation, and survival of antigen-specific cytotoxic T-cells. Additional immune effects orchestrated by IL-2 include the facilitation of immunoglobulin production by B-cells, as well as the differentiation and proliferation of NK cells. Despite the pleiotropic and desirable antitumor immune activities of IL-2, its systemic administration has been clinically limited due to severe toxicities. As such, alternative delivery strategies have been investigated to attenuate IL-2–associated toxicities, yet maximize its potent immunomodulatory effects.
For the treatment of pulmonary metastases, the localized deposition of IL-2 or the preferential gene expression of IL-2 within the lung parenchyma has been investigated as a novel and effective treatment option in dogs with macroscopic OS metastases. Initial studies evaluated the antitumor activities of liposomal IL-2 when delivered directly to the pulmonary parenchyma in the form of inhalation therapy.282 Dogs were nebulized with liposomal IL-2 daily for 30 days, and immunomodulatory effects of IL-2 were confirmed by increases in bronchoalveolar lavage effector cell numbers and lytic activities, with resultant complete regression of macroscopic pulmonary OS metastases in two of four dogs. The duration of complete regression was durable in responder dogs, lasting between 12 and 20 months. Alternatively to inhaled liposomal IL-2 delivery strategies, IV gene therapy as liposome-DNA complexes encoding IL-2 has also been investigated.283 Based on its preferential accumulation and subsequent transgene expression within the lung parenchyma, the tolerability, immunomodulatory effects, and antitumor activity of IV liposome DNA complexes encoding IL-2 were evaluated in 20 dogs with chemotherapy-resistant macroscopic OS metastases. Following administration, the immunomodulatory effects of liposome DNA complexes were substantiated by the induction of fever, leukogram changes, monocyte activation, and increased NK cell activities. On completion of 12 consecutive weekly IV treatments with liposome DNA complexes, measurable responses were achieved in 3 out of 20 dogs, with one complete remission and two partial remissions.
Additional to IL-2 therapy, other investigations have evaluated alternative strategies for activating the immune system including the IV administration of a genetically-modified and attenuated bacterial species, Salmonella typhimurium (VNP20009).284 Based on the premise that anaerobic bacteria have potential as novel immunomodulatory cancer therapeutics, a phase I dose escalation study was conducted with VNP20009 in 41 dogs with spontaneous cancers. Dose-limiting toxicity associated with VNP20009 administration included fever and vomiting, symptoms associated with systemic immune activation. Importantly, preferential tumor tissue tropism of VNP20009 was confirmed by gene transcription and bacterial culture techniques in a substantial proportion of tumor samples. In a subset of four dogs with macroscopic OS pulmonary metastases, one partial remission was achieved for a duration of 68 days.
Palliative Treatment: Primary and Metastatic Bone Cancer Pain
Although pain is an evolutionarily conserved protective mechanism, its categorization can be differentiated based on temporal aspects (acute, chronic, or intermittent), intensity (mild, moderate, severe, or excruciating), and anatomic origin (somatic, visceral, or neuropathic). The sensation of pain is mediated by specialized afferent nerve endings, called nociceptors, that initiate sodium channel opening and subsequent neuronal depolarization events within small, myelinated Aδ and unmyelinated C fibers. Generated neuronal impulses are propagated through the dorsal horn of the spinal cord via the dorsal root ganglia, where they synapse with second-order neurons of the gray matter with subsequent modulation of impulse intensity. The resultant nociceptive information is carried to the brain via the spinothalamic tracts, where it can be integrated, processed, and recognized in multiple areas of the brain.285
The greatest density of afferent nociceptors responsible for pain-impulse generation is found at the periosteal surface and medullary cavity, specifically in bone. As such, malignant perturbations affecting these bone anatomic compartments are associated with intense pain.286,287 In dogs with OS, the generation of bone cancer pain is attributed to two specific host responses. First, the invasive growth of malignant osteoblasts in the bone microenvironment results in the release of chemical mediators by nonneoplastic stromal cells, which in turn stimulate nociceptors and lead to the generation of painful sensations. Second, the genesis, maintenance, and exacerbation of bone cancer pain are directly attributed to dysregulated and pathologic osteoclastic bone resorption.286,287 Based on these mechanisms of bone cancer pain generation, the most effective management of malignant osteolytic pain would combine the eradication of malignant osteoblasts in bone matrix and the inhibition of tumor-induced osteoclastic bone resorption.
Palliative Radiation Therapy
RT is considered the most effective treatment modality for the management of osteolytic bone pain in human cancer patients and likewise has been investigated and extensively applied to alleviating bone cancer pain in dogs diagnosed with OS. Mechanistically, the analgesic effects of ionizing radiation may be attributed to the induction of apoptosis in both malignant osteoblasts and resorbing osteoclasts,288 and in dogs, these effects have been supported by percent tumor necrosis assessment.193,228,289 As such, ionizing radiation reduces overall tumor burden and attenuates the degree of osteoclastic resorption within the focal OS microenvironment.
Multiple palliative radiation protocols have been evaluated and reported in the veterinary literature, with the majority of dosing schemes utilizing 2 to 4 individual treatments of 6 to 10 Gy fractions. Although variable and subjectively reported in these studies, the alleviation of bone cancer pain was achieved in the majority of OS dogs treated and ranged from 74% to 93%. Although the majority of dogs symptomatically improved following palliative RT, the median time interval of subjective pain alleviation was not durable and ranged from 53 to 130 days.290-295 Because most conventional palliative radiation protocols only utilize 2 to 4 treatment fractions, the total cumulative radiation dose administered is relatively low (<32 Gy); therefore acute and late radiation toxicity is not a limiting factor for the majority of patients treated. This also may allow repetition of palliative radiation protocols in the same patient subsequent to return of pain as the tumor ultimately advances. Although megavoltage palliative RT appears effective when used as a single-agent treatment option for short-term pain management, some investigations suggest that the concurrent administration of IV systemic chemotherapy along with palliative radiation might enhance analgesic response rates and durations.293 Systemic chemotherapy is indicated for the delay of micrometastatic disease development in dogs diagnosed with appendicular OS, thus the adjuvant institution of chemotherapy combined with palliative RT might have the dual benefit of improving local pain control and improving overall survival time through the delay of micrometastatic disease development.
Radiopharmaceuticals
153Samarium is a radioisotope that undergoes gamma and beta decay, allowing for concurrent biodistribution tracking studies, as well as therapeutic ionizing radiation delivery within a 2- to 3-mm deposition radius. When 153Samarium is conjugated to EDTMP, which is a bisphosphonate, the resultant compound 153Sm-EDTMP preferentially concentrates in areas of increased osteoblastic activity and binds to exposed hydroxyapatite crystals.296 By virtue of its osteotropism and defined radius of ionizing radiation deposition, 153Sm-EDTMP is currently used as a radiopharmaceutical for the palliative treatment of multifocal, skeletal metastases in humans afflicted with breast or prostate carcinoma.297
Similar to humans diagnosed with skeletal malignant osteolysis, the use of 153Sm-EDTMP has been investigated and reported for alleviating bone cancer pain in dogs with appendicular and axial OS.298-301 In dogs treated with 153Sm-EDTMP, the predicted radiation dose equivalent achieved within the immediate bone tumor microenvironment has been estimated to approximate 20 Gy,298 although its intratumoral biodistribution is expected to be nonhomogeneous based on regional differences in reparative osteoblastic activities. Following IV 153Sm-EDTMP administration, the majority (63% to 83%) of dogs with OS demonstrate improved lameness scores and activity levels, suggesting the achievement of pain palliation.298-301 Despite clinical improvement in most dogs treated, the duration of pain alleviation has not been extensively documented but appears to approximate similar durations of pain control achieved with megavoltage teletherapy. In a small fraction (<5%) of treated dogs, 153Sm-EDTMP has resulted in the complete involution of malignant skeletal lesions, resulting in durable pain alleviation and prolonged survival times. Overall, 153Sm-EDTMP is well-tolerated; however, side effects associated with treatment include transient decreases in platelet and white blood cell counts as a consequence of beta energy deposition within the proximity of pluripotent marrow stem cells.300
Aminobisphosphonates
Aminobisphosphonates (NBPs) are synthetic analogs of inorganic pyrophosphate that were initially utilized for diagnostic purposes in bone scanning, based on their ability to preferentially adsorb to sites of active bone mineral remodeling. The pharmaceutical use of NBPs has gained wide acceptance in the therapy of human nonneoplastic bone resorptive disorders, such as osteoporosis and Paget’s disease.302 In addition to the management of these metabolic disorders, NBPs are considered first-line treatment for malignant skeletal osteolysis, including paraneoplastic hypercalcemia, multiple myeloma, and metastatic bone diseases in human cancer patients.303,304
The effective treatment of bone disorders by NBPs is attributed to their differential effect on bone resorption and bone mineralization. At concentrations safely obtainable in vivo, NBPs inhibit bone resorption without inhibiting the process of bone mineralization. Mechanistically, the bone protective effects exerted by NBPs are through the induction of osteoclast apoptosis, which results in the net attenuation of pathologic bone resorption.305,306 Specifically, NBPs interfere with posttranslational prenylation of small guanosine triphosphate (GTP)-binding proteins, including Ras, Rho, and Rac,307 and the disruption of these small GTP-binding proteins results in the failure of normal intracellular signaling and interaction with the extracellular matrix, thereby triggering osteoclast apoptosis.
Although NBPs are commercially available in different formulations, the effective management of tumor-induced hypercalcemia, osteolytic bone metastases of breast cancer, and osteolytic lesions of multiple myeloma appear to require the administration of IV NBP and at relatively high doses. Historically, pamidronate and zoledronate have been the two most commonly utilized IV NBP formulations in human oncology for their ability to decrease bone pain, improve quality of life, delay progression of the bone lesions, and decrease the frequency of malignant skeletal events.
Because OS is characterized by focal and aggressive malignant osteolysis, the investigation of NBPs has been a focus of clinical interest, and several studies have demonstrated the cytotoxic effects of NBPs against canine OS cell lines in vitro. In three independent studies, it was concluded that various NBPs, including alendronate, pamidronate, and zoledronate exerted dose- and time-dependent cytotoxicity in immortalized canine OS cell lines.308-310 Although the in vitro studies were in agreement with the cytotoxic effects of NBPs on canine OS cell viability, the inhibitory concentration 50% (IC50) derived from the experiments varied among the studies. Nonetheless, the documented direct cytotoxic effects of NBPs on canine OS cell lines served as a springboard to rationally investigate the bone pain–alleviating effects of NBPs in dogs with OS.
The first reported description in the veterinary literature was the use of oral alendronate for the palliative management of two dogs with OS.311 Based on the unexpectedly long survival times reported in this anecdotal study, the authors suggested that NBP therapy might have a role in managing canine malignant bone disorders. Given that IV NBPs have been historically used for the management of malignant osteolysis in humans, a prospective study principally evaluating the safety of IV pamidronate was conducted in 33 dogs diagnosed with primary and secondary skeletal tumors.312 IV pamidronate (1.0 mg/kg diluted with 0.9% sodium chloride to a total volume of 250 ml) as a 2-hour constant rate infusion (CRI) every 28 days was well tolerated, and in a subset of dogs, the bone biologic and clinically relevant therapeutic effects of IV pamidronate were documented as significant reductions in urine NTx concentrations, increases in relative primary tumor bone mineral density (rBMD) as assessed by dual-energy x-ray absorptiometry (DEXA), and subjective pain alleviation.
Following the established safety of IV pamidronate in dogs with skeletal tumors, a second study of 43 dogs treated with IV pamidronate (comparing 1.0 mg/kg versus 2.0 mg/kg) was conducted to further characterize the biologic activity of pamidronate specifically for the management of appendicular OS-associated bone pain and pathologic bone resorption.313 Overall, 12/43 (28%) OS-bearing dogs treated with single-agent IV pamidronate achieved pain alleviation for greater than 4 months. In addition to the subjective analgesic effects of pamidronate reported by pet owners, changes in urine NTx concentrations and DEXA-assessed rBMD correlated with therapeutic response.
Although original studies have focused on the palliative effects of pamidronate when used as a single-agent, a recent study has documented the synergistic activity of pamidronate when coupled with palliative RT in dogs with appendicular OS through the use of subjective and objective surrogate endpoints. In a prospective, double-blind, randomized, placebo-controlled clinical trial, dogs with appendicular OS were to receive palliative RT (8 Gy, days 1 and 2) plus 0.9% saline infusion or pamidronate once every 4 weeks for 3 treatments (12-week study).314 Prior to initial palliative RT and subsequent to each IV treatment with either 0.9% saline or pamidronate, all dogs were evaluated by force plate gait analysis, urine NTx concentrations, numeric lameness evaluation, and owner quality-of-life questionnaires. Out of 17 dogs, 8 received 0.9% saline and 9 received IV pamidronate (1.0 mg/kg). The saline placebo group dogs experienced a significant increase in numeric lameness score between weeks 0 and 12, and pamidronate significantly lowered the lameness scores on week 12 compared to saline. In addition, dogs receiving pamidronate had a significantly greater vertical impulse and total stance time on weeks 4 through 12 compared to saline placebo-treated dogs. Based on these findings, the addition of pamidronate to palliative therapy appeared to improve limb function compared to palliative RT alone.
In another study evaluating the benefit of combining pamidronate with conventional therapies for managing bone cancer pain, a double-blind, placebo-controlled study of 50 dogs with OS receiving palliative radiation, doxorubicin, and either saline placebo or pamidronate was conducted.315 The median pain-free interval for dogs receiving adjuvant pamidronate or saline was 76 days and 75 days, respectively. Despite the apparent lack of pet owner–perceived analgesia, dogs that received adjuvant pamidronate did demonstrate improved quality-of-life scores and more importantly, superior bone biologic effects representative of decreased malignant bone resorption at the level of the primary tumor. Collectively, the findings from this clinical trial suggest that adjuvant pamidronate may not subjectively improve analgesia when dogs are already receiving treatment with megavoltage radiation and doxorubicin but still exert beneficial bone biologic effects within the bone tumor microenvironment in dogs with OS.
Although the majority of palliative studies have documented the effects of pamidronate, other more potent IV NBPs for managing malignant bone pain have also been evaluated in dogs with skeletal tumors. Zoledronate possesses 100-fold greater antiresorptive potency in comparison with pamidronate and has the advantage of being safely administered over a shorter period of time than other NBPs. In one case report, the use of IV zoledronate administered every 28 days was effective for the long-term pain management of a dog diagnosed with OS affecting the distal radius.316 In a larger study, the bone biologic effects of IV zoledronate were evaluated in dogs diagnosed with primary and secondary skeletal tumors.317 In this study, zoledronate was administered at a dose of 0.25 mg/kg as a 15-minute CRI every 28 days and was well tolerated with no overt biochemical evidence of renal toxicity in patients receiving multiple monthly infusions. In 10 dogs with appendicular OS, 50% of dogs treated achieved pain alleviation for greater than 4 months and also demonstrated significant increases in rBMD. The observation for increased rBMD in conjunction with pain alleviation suggests that zoledronate inhibits local malignant osteolysis and the generation of pain within the immediate bone-tumor microenvironment.
Comparative Aspects
Animal models for the study of human diseases are important to our understanding of the mechanism and etiology of disease and for the development and refinement of therapeutic strategies. Spontaneously developing diseases in animal populations are particularly useful for study.318-320 Canine OS has many similarities to human OS in terms of genetic similarities, clinical presentation, biologic behavior, and metastatic progression and has been shown through many studies to be a valuable comparative model for study (Table 24-3).7,320 OS is more common in dogs than in humans; therefore case accrual is more rapid. Because disease progression is more rapid in dogs than in humans, results of novel treatment protocols can be reported earlier than those of similar trials in humans. Research costs for clinical trials in dogs are less compared to those in human clinical trials, and, from an animal welfare standpoint, no disease is induced and dogs with cancer can potentially be helped through the course of the research.
Table 24-3
Comparison of Canine and Human Osteosarcoma Characteristics
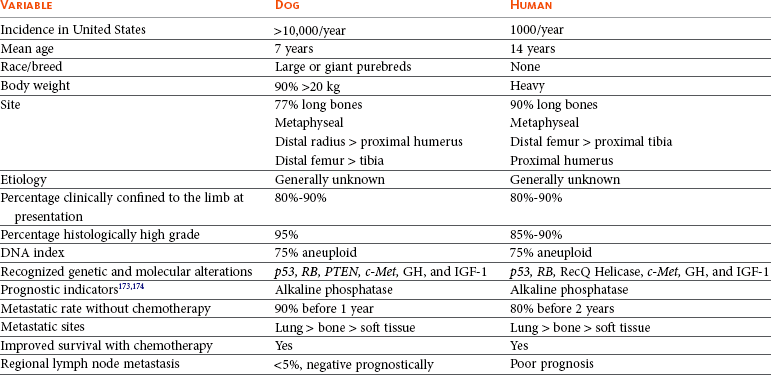
GH, Growth hormone; IGF-1, insulin-like growth factor-1.
Modified with permission from Withrow SJ, Powers BE, Straw RC, et al: Comparative aspects of osteosarcoma: dog versus man, Clin Orthop Relat Res 270:159–167, 1991.
OS is an uncommon cancer of humans affecting mainly children in their second decade of life and remains a very serious, aggressive solid tumor. Fortunately, there has been a great improvement in survival rates with the use of established multidrug adjuvant protocols. The long-term survival rate for human OS is presently 60%, which contrasts to the 20% expected 5-year survival rates of the early 1980s. A retrospective study of 648 human OS patients reported a mean survival of 3 years; however, this represented all cases at one institution since the 1970s and therefore improvements over time were not presented.321 Factors that negatively affected outcome included older age, advanced local or systemic stage, axial location, larger size, and a lower percentage of necrosis following neoadjuvant treatment. The type of surgery (amputation or limb sparing) did not impact outcome, supporting the need for advancement of systemic medical therapy to impact survival. Limb-sparing programs are becoming more common, and many survivors of OS retain functional, pain-free limbs. In two reports, one from the United States322 and one from Italy,323,324 aggressive neoadjuvant chemotherapy resulted in limb-sparing success of 93.5% and 83%, respectively, and a projected 10-year survival of 93%.
Bone Surface Osteosarcoma
OS usually originates from elements within the medullary canal of bones (intraosseous OS); however, there are forms of this cancer that originate from the outside surface of bones. Periosteal OS is a high-grade form of surface OS and seems to arise from the periosteal surface but has invasive characteristics seen radiographically.325 There is cortical lysis with extension of the tumor into the bone and surrounding soft tissues. These tumors are histologically similar to intraosseous OS and have similar aggressive biologic behavior.
In contrast, parosteal OS, or juxtacortical OS, arises from the periosteal surface of bones but appears less aggressive than periosteal OS both radiographically and in terms of biologic behavior. Parosteal OS is relatively uncommon and has a moderately well-circumscribed radiographic appearance. The tumors grow out from the periosteal side of a cortex and cortical lysis is usually very mild on radiographs. Histologically, these tumors look more benign compared to intraosseous or periosteal OS. These tumors contain well-differentiated cartilage, fibrous tissue, and bone with sparse regions of sarcoma cells adjacent to tumor osteoid. Histologic specimens must be evaluated carefully because it is often easy to miss the areas of tumor cells and misdiagnose the lesion as osteoma, chondroma, or reactive bone. These tumors generally do not invade the medullary canal and tend to grow out from the bone on broad pedicles. Diagnosis is based on typical histologic and radiographic findings.
Parosteal OS is usually slow growing but can induce pain at the local site. Metastases can occur, but the prognosis for long-term survival is much better than for intraosseous OS.326,327 Control of parosteal OS can be achieved by en bloc resection of the tumor with the adjacent cortical bone. This has been reported for tumors of the zygomatic arch (Figure 24-12).327 If full-thickness cortex needs to be removed for tumors on long bones, reconstruction may be performed using autogenous corticocancellous bone, such as a rib, ileal crest, or allogeneic cortical bone. A report described a surface OS without cortical destruction (similar to parosteal) that had an aggressive histology and biologic behavior.328
Other Primary Bone Tumors of Dogs
Primary bone tumors other than OS make up somewhere between 5% and 10% of bone malignancies in dogs. These tumors are chondrosarcomas, hemangiosarcomas, fibrosarcomas, lymphomas (see Chapter 32, Section A), and plasma cell tumors (see Chapter 32, Section D).
It can be difficult to distinguish chondroblastic OS from chondrosarcoma, fibroblastic OS from fibrosarcoma, and telangiectatic OS from hemangiosarcoma when only small amounts of biopsy tissue are evaluated.135 This makes interpretation of older reports difficult in terms of trying to establish the true incidence of the different types of primary bone tumors. This also underscores the importance of evaluating the entire excised specimen to validate the preoperative biopsy. All too often a bone malignancy thought to be relatively low grade from preoperative biopsy is upgraded to a true OS once the histology of the surgical specimen is reviewed. This may change the prognosis and postsurgical treatment plan.
Chondrosarcoma
Chondrosarcoma is the second most common primary tumor of bone in humans and dogs and accounts for approximately 5% to 10% of all canine primary bone tumors.2-5,329 Chondrosarcomas are characterized histologically by anaplastic cartilage cells that elaborate a cartilaginous matrix. There is a spectrum of degree of differentiation and maturation of the cells within and between each tumor. Histologic grading systems have been devised.330 The etiology is generally unknown, although chondrosarcoma can arise in dogs with preexisting multiple cartilaginous exostosis.331-333 In a clinicopathologic study of 97 dogs with chondrosarcoma, the mean age was 8.7 years (ranging from 1 to 15 years) and golden retrievers were at a higher risk of developing chondrosarcoma than any other breed.334 There was no sex predilection, and 61% of the tumors occurred on flat bones. Chondrosarcoma can originate in the nasal cavity, ribs, long bones, pelvis, extraskeletal sites (such as the mammary gland, heart valves, aorta, larynx, trachea, lung, and omentum), vertebrae, facial bones, digits, and os penis.35,330,334-341 The nasal cavity is the most common site for canine chondrosarcoma.330,334
Chondrosarcoma is generally considered to be slow to metastasize. Tumor location rather than histologic grade was prognostic in one study.330 The reported median survival of dogs with nasal chondrosarcoma ranges from 210 days to 580 days with various treatments (RT, rhinotomy and RT, and rhinotomy alone; see Chapter 23, Section B).334,342 Metastatic disease is not a reported feature of nasal chondrosarcoma in dogs. The reported median survival for dogs with rib chondrosarcoma varies widely.21,167,330,343 Reports prior to 1992 contained few cases that were treated with intent to cure, but 15 dogs with rib chondrosarcoma treated with en bloc resection in a more recent study had a median survival of 1080 days.165 The median survival for dogs with chondrosarcoma was 540 days in a study of five dogs treated with amputation alone.334 Death was usually associated with metastatic disease. A reliable adjuvant chemotherapeutic agent is not known for canine chondrosarcoma. In humans, chondrosarcoma is considered a local disease, with a moderate rate of metastasis, which can be predicted by histologic grade. Aggressive surgical resection often results in long-term tumor control.344 Although this tumor is generally considered resistant to standard RT, the authors have noted objective responses to coarse-fraction radiation protocols in a handful of cases in which surgery was not an option.
Hemangiosarcoma
Primary hemangiosarcoma of bone is rare and accounts for less than 5% of all bone tumors (see Chapter 33, Section A). This disease generally affects middle-aged to older dogs and can occur in dogs of any size. This is a highly metastatic tumor, and virtually all dogs affected will develop measurable metastatic disease within 6 months of diagnosis. Metastases can be widely spread throughout various organs such as lungs, liver, spleen, heart, skeletal muscles, kidney, brain, and bones. Dogs can present with multiple lesions, making it difficult to determine the site of primary disease. Histologically, hemangiosarcoma is composed of highly anaplastic mesenchymal cells, which are precursors to vascular endothelium. The cells are arranged in chords separated by a collagenous background and may appear to be forming vascular channels or sinuses. Cellular pleomorphism and numerous mitotic figures are features of this highly malignant disease. There is profound bone lysis, and the malignant cells aggressively invade adjacent normal structures. The lesion, however, may be confused with telangiectatic OS, especially if the diagnosis is based on small tissue samples. Often the dominant radiographic feature is lysis; however, hemangiosarcoma does not have an unequivocally unique radiographic appearance, and diagnosis is based on histology.
If hemangiosarcoma is diagnosed, the dog must be thoroughly staged with thoracic and abdominal films, bone survey radiography or bone scintigraphy, and ultrasonographic evaluation, particularly of the heart and abdominal organs. Right atrial hemangiosarcoma may be present without clinical or radiographic signs of pericardial effusion. The prognosis is poor, and even dogs with hemangiosarcoma clinically confined to one bony site have less than a 10% probability of surviving 1 year following complete excision. Cyclophosphamide, vincristine, and doxorubicin have been used in combination as an adjuvant protocol, and the reported median survival of dogs with nonskeletal hemangiosarcoma is 172 days.345 In a patient population represented by a variety of primary tumor sites, doxorubicin as a single-agent adjuvant seemed to be as effective as the combination of drugs, with an MST of 172 days in patients in which all gross disease is surgically resected.346 In a group of dogs with splenic hemangiosarcoma, L-MTP-PE resulted in a median survival of 277 days, compared to 143 days for dogs receiving empty liposomes.347
Fibrosarcoma
Primary fibrosarcoma is also a rare tumor of dogs and accounts for less than 5% of all primary bone tumors.4 Unfortunately, the difficulty in distinguishing fibrosarcoma from fibroblastic OS histologically (especially from small tissue samples) renders study of this tumor difficult. In one report, 11 dogs thought to have fibrosarcoma were reevaluated after complete resection and the histologic diagnosis was changed to OS in 6 dogs.348 Histologic characteristics of fibrosarcoma have been described as interwoven bundles of fibroblasts within a collagen matrix permeating cancellous and cortical bone but not associated with osteoid produced by the tumor cells. Host-derived new bone can be seen, however, especially at the periphery of the tumor.
Complete surgical resection of the primary lesion is recommended for dogs with fibrosarcoma clinically confined to the primary site. This treatment may be curative, although metastatic potential may be considerable. There is no good evidence that adjuvant chemotherapy is of any benefit in preventing metastatic disease. It has been postulated that primary fibrosarcoma of bone has a propensity to metastasize to such sites as the heart, pericardium, skin, and bones rather than lung.348
Multilobular Osteochondrosarcoma
Multilobular osteochondrosarcoma (MLO) is an uncommon tumor that generally arises from the skull of dogs.349-351 Many names have been used to describe this disease, including chondroma rodens and multilobular osteoma. These tumors have a characteristic appearance on radiographs, CT, and MRI: generally the borders of the tumor are sharply demarcated with limited lysis of adjacent bone, and there is a coarse granular mineral density throughout (Figure 24-13).352,353 However, there is one report of an MLO of the vertebra that did not have radiographic abnormalities.354 Histologically, these tumors are composed of multiple lobules, each centered on a core of cartilaginous or bony matrix that is surrounded by a thin layer of spindle cells. A histologic grading system has been described.350,351 These tumors have the potential to recur locally following incomplete resection, and metastasis can occur. In one report of 39 dogs, the median age of affected dogs was 8 years, the median weight was 29 kg, and there was no breed or sex predilection.351 Slightly less than 50% of dogs had local tumor recurrence following resection at a median time of approximately 800 days. A little over half the dogs developed metastases after treatment; however, time to metastasis was prolonged with a median of 542 days. The MST was 800 days. Local tumor recurrence and metastasis after treatment appears to be predicted by histologic grade and the ability to obtain histologically complete resection. Local tumor excision with histologically complete surgical margins appears to offer a good opportunity for long-term tumor control, especially for low-grade lesions. When metastatic lesions are identified by thoracic radiography, dogs may remain asymptomatic for their lung disease for up to 1 year or more. The role of chemotherapy and RT in the management of MLO is not well defined.
Metastatic Tumors of Bone
Almost any malignant tumor can metastasize to bone via the hematogenous route. The lumbar vertebrae, femur, humerus, rib, and pelvis are common sites for cancer spread, possibly because these are predilection sites for bone metastasis from the common urinogenital malignancies, such as prostate, bladder, urethral, and mammary cancer.355,356 Metastatic lesions in long bones frequently affect the diaphysis, likely because of the proximity to a nutrient foramen. Nuclear scintigraphy is a sensitive technique to detect bone metastasis. A whole-skeleton bone scan is recommended when metastatic bone cancer is suspected because it is common for multiple sites of metastasis to be present, even if the patient is symptomatic for only one bone.
Benign Tumors of Bone
Osteomas are benign tumors of bone.357 Radiographically, these are well-circumscribed, dense bony projections, which are usually not painful to palpation. Histologically, they are composed of tissue nearly indistinguishable from reactive bone. The diagnosis is made after considering the history and physical examination, as well as radiographic and histologic findings. The most important differential diagnosis is MLO when the lesion occurs on the skull. Treatment for osteoma is simple surgical excision, which is usually curative.