Chapter 23 Clinical Evaluation of Renal Artery Disease
Data from the National Health and Nutrition Examination Survey (NHANES) 2005 to 2008, extrapolated to 2008, estimated that approximately 76,400,000 adults 20 years of age or older have essential (primary) hypertension.1 Renovascular disease and renal parenchymal disease are the most common secondary causes of hypertension after obesity, excess alcohol ingestion, drug abuse, and oral contraceptive use are excluded.
The presence of anatomical renal artery stenosis (RAS) does not necessarily establish that the hypertension or renal failure is due to RAS. Incidentally discovered RAS is quite common, whereas renovascular hypertension only occurs in 1% to 5% of all patients with hypertension.2,3 Approximately 90% of all renovascular disease is caused by atherosclerosis.4,5 Fibromuscular dysplasia (FMD) is the second most common cause of RAS.6 Patients with atherosclerotic RAS are typically older than age 55 and have the usual risk factors for atherosclerosis, but FMD is more common in younger women. The predominant clinical manifestation of FMD is hypertension; atherosclerotic RAS may present with hypertension, renal failure (ischemic nephropathy), and/or recurrent episodes of congestive heart failure (CHF) and “flash” pulmonary edema.7 Whereas atherosclerotic RAS most often occurs at the ostium or proximal portion of the renal artery, FMD usually occurs in the mid- to distal renal artery and its primary branches (Figs. 23-1 and 23-2).
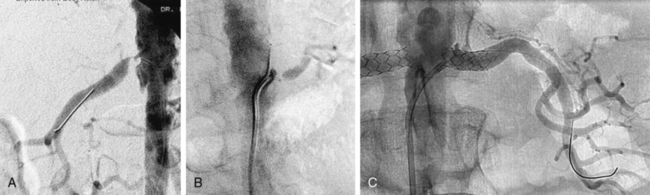
Figure 23-1 A-B, Digital subtraction angiogram (DSA) showing typical features of atherosclerotic renal artery stenosis (RAS). There is severe bilateral ostial RAS. C, Angiogram after stents were placed in right and left renal arteries.
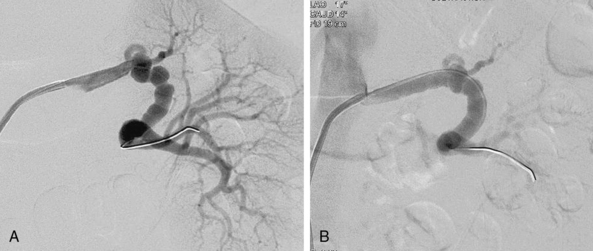
Figure 23-2 A, Digital subtraction angiogram (DSA) demonstrating medial fibroplasia located in mid- to distal part of left renal artery. Note “beading,” with beads larger than normal caliber of artery, typical of medial fibroplasia. B, Angiogram of left renal artery after percutaneous balloon angioplasty. Angiographic appearance is improved, and there was resolution of pressure gradient.
The effects of atherosclerosis on the coronary and carotid arteries are well recognized, but involvement of the renal arteries is frequently overlooked. In addition to the sequelae of RAS (hypertension, renal failure), patients with atherosclerotic RAS succumb prematurely from myocardial infarction (MI) and stroke.8–11 Thus, early diagnosis and treatment is important to avoid the consequences of RAS.
When considering the diagnosis of RAS, it is useful to think in terms of the circumstances in which RAS is likely to occur (Box 23-1).
![]() Box 23-1 Clinical Clues That Suggest Presence of Renal Artery Stenosis
Box 23-1 Clinical Clues That Suggest Presence of Renal Artery Stenosis
Hypertension
Individuals who develop hypertension between the ages of 30 and 55 usually have primary (essential) hypertension. If the initial diagnosis of hypertension is made before the age of 30, it is usually due to FMD if other known secondary causes (obesity, oral contraceptive use, drug abuse, and parenchymal renal disease) have been excluded. Since atherosclerosis occurs in older individuals, it is usually the cause of RAS after the age of 55. In one population-based study of Medicare patients aged 65 or older, the prevalence of atherosclerotic RAS was 6.8%.12 In this cohort, RAS was found in nearly twice as many men as women (9.1% vs. 5.5%; P = 0.053); no significant differences were identified between Caucasians and African American subjects (6.9% vs. 6.7%; P = 0.933).12 Although RAS can be associated with both systolic and diastolic hypertension, the diagnosis of RAS should be seriously considered in individuals who present with new-onset diastolic hypertension after the age of 55, primarily because diastolic blood pressure usually declines after age 55 in normal individuals. It is not uncommon for patients to have primary hypertension for many years, and as they age, develop atherosclerotic RAS. This cohort of patients may have had well-controlled blood pressure that suddenly becomes more difficult to control.
Patients may have anatomically significant RAS and no hypertension at all. Dustan and colleagues reviewed 149 aortograms and found that approximately half of patients with 50% or more RAS did not have hypertension.13 Moreover, in a recent systematic review of 40 studies that evaluated a total of 15,879 patients, the mean prevalence of RAS among patients with suspected renovascular hypertension was 14.1%.11 On further analysis of the patients who were incidentally found to have RAS on imaging studies, 65.5% were hypertensive and 27.5% had renal failure.11 Therefore, the mere presence of RAS and hypertension does not necessarily mean that one is causing the other.
Accelerated or malignant hypertension also has been associated with a very high prevalence of RAS.14Resistant hypertension is defined as failure to normalize blood pressure to less than 140/90 mmHg following an optimal medical regimen consisting of at least three drugs with different mechanisms of action, including a diuretic.15 The diagnosis of renovascular disease should be strongly considered in patients with true drug-resistant hypertension.
Renal Abnormalities
Gifford et al. found that 71% of patients (53 of 75 patients) with an atrophic kidney had severe stenosis or complete occlusion of the renal artery supplying the small kidney.16 Three studies have shown that if there is a discrepancy in size between the two kidneys or if one kidney is atrophic, the contralateral renal artery (normal-sized kidney) is severely stenotic about 60% of the time.2,16,17 Therefore, the presence of an atrophic kidney or a discrepancy in size between the two kidneys demands a thorough investigation for the presence of renovascular disease.
Numerous reports suggest that patients who develop azotemia while receiving angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blocking (ARB) agents have bilateral RAS, RAS to a solitary functioning kidney, or decompensated CHF in the sodium-depleted state.18–22 These clinical scenarios are absolute indications for investigation, since they usually reflect the presence of severe RAS to the entire functioning renal mass, thus placing the patient in jeopardy of renal failure. The mechanisms of acute and chronic renal failure in patients with RAS are discussed in detail in Chapter 22.
There are no prospective studies evaluating how often atherosclerotic renovascular disease leads to end-stage renal disease (ESRD). Scoble et al. found that atherosclerotic renovascular disease was the cause of ESRD in 14% of patients starting dialysis therapy.23,24 In a retrospective review over a 20-year period in 683 patients, 83 (12%) patients had documented RAS as a cause of ESRD. Since arteriography was only performed in patients with suspected RAS, it is entirely possible that the true incidence of RAS as a cause of ESRD was underestimated. De Mast and Beutler reported that 41% of patients with ESRD had at least one renal artery with more than 50% stenosis.11 Renal artery stenosis must be excluded in every patient starting dialysis if a clear-cut etiology for the ESRD is not known because the mortality in this patient population is extremely high. In the series by Mailloux et al., median survival in patients with ESRD secondary to RAS was 25 months, while 2-, 5-, and 10-year survival was 56%, 18%, and 5%, respectively.9,10
Effects of Renal Artery Stenosis on the Heart
Recurrent CHF and flash pulmonary edema unrelated to ischemic heart disease can result from bilateral RAS (or unilateral RAS to a single functioning kidney). In one renal artery stent series, 39 patients (19% of all patients undergoing renal artery stent implantation from 1991–1997) had recurrent episodes of CHF or flash pulmonary edema as the primary indication for renal artery stenting.25 Nineteen of 39 patients had moderate to severe left ventricular (LV) systolic function. Although not completely understood, the mechanism of CHF may be related in part to the inability to use ACE inhibitors or ARBs to the direct adverse effects of angiotensin II (Ang II) on myocardial function, or to the inability to control volume adequately. If coronary ischemia has been excluded as a cause of CHF, renal revascularization (percutaneous stenting or surgical) is a very effective method of treatment in these individuals.25–27
One retrospective study demonstrated improvement in anginal symptoms in patients undergoing renal artery stent implantation. The mechanism of such improvement is not clearly delineated, but 88% of these patients had improved blood pressure control after stenting. This effect may account at least in part for decreased anginal symptoms.28
Presence of Atherosclerosis in Other Vascular Beds
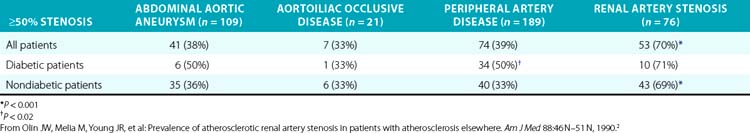
Several series have examined the prevalence of renovascular disease in patients who have atherosclerotic disease elsewhere. To determine the prevalence of atherosclerotic RAS, Olin et al. studied 395 consecutive patients who had undergone arteriography as part of an evaluation for either an abdominal aortic aneurysm, aortoiliac occlusive disease, or peripheral artery disease (PAD)2 (Table 23-1). These patients did not have the usual clinical clues to suggest RAS. High-grade bilateral renal artery disease was present in approximately 13% of patients. In addition, 76 patients had an aortogram performed for suspected RAS, and RAS was present in 70% of these subjects. Other studies have shown that 22% to 59% of patients with PAD have significant RAS.29
It has also been established that RAS is common in patients with coronary artery disease (CAD). Of 7758 patients undergoing cardiac catheterization during a 78-month period of time, 3987 underwent aortography at the time of catheterization to screen for RAS30; 191 (4.8%) had more than 75% RAS, and 0.8% had severe bilateral disease. In a Mayo Clinic series, renal arteries were studied at the time of cardiac catheterization in patients with hypertension.31 Ninety percent of the renal arteries were adequately visualized, and no complications occurred from the aortogram. More than 50% RAS was present in 19.2%, more than 70% stenosis in 7%, and bilateral RAS was present in 3.7% of patients. The likelihood of significant RAS is markedly increased in patients with two or more coronary artery lesions.32 A prospective study of the long-term natural history of patients undergoing cardiac catheterization and renal angiography is needed to determine whether diagnosing RAS in this setting improves patient outcome measures. Renal artery disease is also associated with atherosclerotic disease in the carotid arteries. Louie et al. demonstrated that 46% of patients with more than 60% RAS also had more than 50% carotid stenosis.33 All of these studies support the fact that atherosclerotic RAS is a manifestation of systemic atherosclerosis and reinforce the concept of treating the entire patient, not just the circulatory bed involved at a given point in time.
The presence of RAS even prior to development of ESRD portends a poor prognosis. Patient survival decreases as the severity of RAS increases, with 2-year survival rates of 96% in patients with unilateral RAS, 74% in patients with bilateral RAS, and 47% in patients with stenosis or occlusion to a solitary functioning kidney.34 Dorros et al. demonstrated that as serum creatinine increases, survival decreases in patients with atherosclerotic RAS.35 The 3-year probability of survival was 92 ± 4% for patients with a serum creatinine below 1.4 mg/dL, 74 ± 8% for patients with a serum creatinine of 1.5 to 1.9 mg/dL, and 51 ± 8% for patients with a serum creatinine 2.0 mg/dL or higher.
Long-term survival was investigated in a cohort of 1235 patients who underwent abdominal aortography at the time of cardiac catheterization. The 4-year survival rate of patients without RAS was 88% versus 57% for those with RAS.34
Physical Examination
The physical examination is generally not helpful in the diagnosis of RAS. Evidence of coronary, cerebral, or PAD is associated with a higher likelihood of renal artery disease because of the systemic nature of atherosclerosis. A systolic abdominal bruit is common and nonspecific, but the presence of both a systolic and diastolic bruit auscultated over the epigastrium may point to underlying renal artery disease.36 Presence of a diastolic component to the bruit indicates that the degree of narrowing of the artery is severe, since there is continued flow during diastole.37 An abdominal bruit with a systolic and diastolic component occurs more often in patients with FMD (53%) than in patients who have atherosclerotic disease (12.5%).36 Presence of a bruit is helpful, but absence does not exclude the diagnosis of either atherosclerotic renovascular disease or FMD.
Diagnosis of Renovascular Disease
In the past, indirect methods of assessing the renal arteries were commonly used to diagnose RAS. Intravenous urography is obsolete as a screening tool, owing to its poor sensitivity and specificity.38 Plasma renin activity as a stand-alone screening test is not reliable for diagnosing or excluding renal artery disease. Elevated plasma renin activity may be present in approximately 15% of patients with essential hypertension. In addition, patients with bilateral disease or disease to a solitary functioning kidney may have normal or low plasma renin activity due to extracellular volume expansion, position of the patient during the test, or medication use. The test is less accurate in azotemic patients and in African American patients.39 The captopril test (plasma renin measurement before and after administration of captopril) is not an ideal screening test and is rarely used. Renal vein renin measurement is not a useful test to screen for RAS; in addition, it has little value in determining who will benefit from revascularization. Except under unusual circumstances, this test is rarely used to make clinical decisions.
Captopril Scintigraphy
Radionuclide imaging techniques are a noninvasive and safe way of evaluating renal blood flow and excretory function, but the renal flow scan has unacceptably high false-positive and false-negative rates for diagnosing RAS.40 When an ACE inhibitor such as captopril is added to isotope renography, sensitivity and specificity of the test improve considerably, especially for patients with unilateral RAS. In most instances of unilateral RAS, the glomerular filtration rate (GFR) of the stenotic kidney falls by approximately 30% after captopril administration.41,42 In contrast, the contralateral normal kidney exhibits an increase in GFR, urine flow, and salt excretion despite a reduction in systemic blood pressure. These expected physiological changes within the stenotic and contralateral kidneys are the basis of the asymmetry of renal function following ACE inhibition detected by renal scintigraphy (see Chapter 22).43–45
In patients with normal renal function and unilateral disease, captopril renography has a sensitivity of around 85% to 90% (range 45-94) and specificity around 93% to 98% (range 81-100).46 However, the presence of significant azotemia or bilateral RAS may adversely affect the accuracy of captopril renography. Many investigators have excluded patients with a serum creatinine exceeding 2.5 to 3.0 mg/dL.47 Although the captopril renogram was once the noninvasive diagnostic test of choice for patients with RAS, it is now rarely used because the quality of the images of duplex ultrasound, magnetic resonance angiography (MRA), and computed tomographic angiography (CTA) are excellent, as discussed later.
Imaging Modalities to Detect Renal Artery Stenosis
Although catheter-based renal angiography with pressure gradient measurements is the definitive gold standard of RAS assessment, several noninvasive imaging modalities such as duplex ultrasound, CTA, and MRA, have become more practical first-line tests for the diagnosis of RAS. Imaging has become so sophisticated and accurate, it is seldom necessary to perform catheter-based angiography for the diagnosis of renal artery disease, and it usually is reserved for imaging at the time of percutaneous revascularization. The ideal imaging procedure should48:
1. Identify the main renal arteries as well as accessory or polar vessels.
2. Localize the site of stenosis or disease.
3. Determine the type of disease present (e.g., atherosclerosis, FMD).
4. Provide evidence for the hemodynamic significance of the lesion.
5. Determine the likelihood of a favorable response to revascularization.
6. Identify associated pathology (i.e., abdominal aortic aneurysm, renal mass, etc.) that may have an impact on the treatment of the renal artery disease.
7. Detect restenosis after percutaneous or surgical revascularization.
Duplex ultrasonography, CTA, and MRA do not by themselves fulfill all these criteria. Local expertise and availability, as well as economic costs, often dictate the preferred imaging modality used (Fig. 23-3). Factors that may play a role in determining the optimal screening test include the patient’s renal function, body habitus, and personal preference (e.g., claustrophobia).
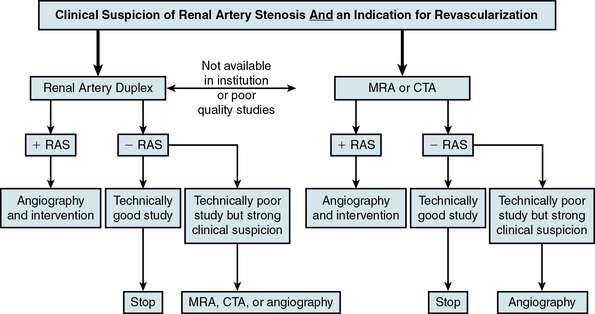
Figure 23-3 Algorithm for diagnosis of renal artery stenosis (RAS).
CTA, computed tomographic angiography; MRA, magnetic resonance angiography.
(Adapted from Carman T, Olin JW: Diagnosis of renal artery stenosis: what is the optimal diagnostic test? Curr Interv Cardiol Rep 2:111–118, 2000.)48
Duplex Ultrasonography
Duplex ultrasonography (also see Chapter 12), which is composed of real-time brightness (B-mode/gray scale) imaging and color pulsed-wave Doppler, has the advantages of being noninvasive, the least expensive of the imaging modalities, and provides both anatomical and functional information about the arterial segments being evaluated. Duplex ultrasonography also does not require the use of potentially nephrotoxic agents.
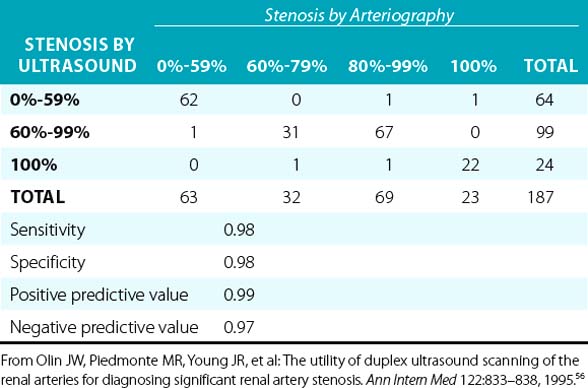
Overall, when compared to angiography, duplex ultrasonography has a sensitivity and specificity of 84% to 98% and 62% to 99%, respectively, when used to diagnose RAS.49–55 In a prospective blinded study, there was a very good correlation between duplex ultrasonography and angiography (Table 23-2). In addition, it was determined that if the end-diastolic velocity (EDV) was 150 cm/s or greater, the degree of stenosis was likely to be 80% or more.56
Renal artery ultrasound should be performed from both an anterior and oblique (or posterior [flank]) approach (Fig. 23-4). In the longitudinal view, the peak systolic flow velocity in the aorta is recorded at the level of the renal arteries. The renal-to-aortic ratio (RAR), which is the ratio of the highest peak systolic value (PSV) in the renal arteries to the PSV in the aorta, can then be calculated to help classify the degree of stenosis (see Table 23-3).54,56
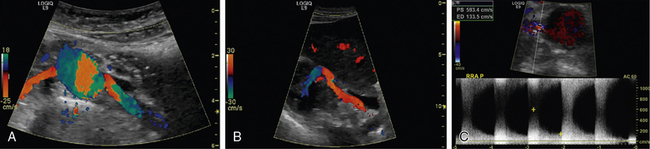
Figure 23-4 A, Color duplex ultrasound of renal arteries from an anterior approach. The right renal artery takes off at approximately 9-10 o’clock and the left renal artery at 3-4 o’clock. B, Color duplex ultrasound from oblique view imaging from kidney to proximal renal artery. Note how entire renal artery is visualized from this approach. C, Duplex ultrasound from anterior view. There is turbulence to flow on color Doppler and markedly increased velocities of blood flow (peak systolic velocity [PSV] 593 cm/sec and end-diastolic velocity [EDV] 134 cm/sec), indicating severe stenosis.
Table 23-3 Duplex Criteria for Diagnosis of Renal Artery Stenosis
| RAR <3.5 and PSV <200 cm/s | 0%-59% |
| RAR ≥3.5 and PSV >200 cm/s | 60%-99% |
| RAR >3.5 and EDV ≥150 cm/s | 80%-99% |
| Absent flow, low-amplitude parenchymal signal | Occluded |
EDV, end-diastolic velocity; PSV, peak systolic velocity; RAR, renal-to-aortic ratio.
The renal arteries are best visualized in a transverse (short-axis) view. Using the B-mode image and a 60-degree angle of insonance, the arteries are interrogated with pulsed wave Doppler. The Doppler should be swept through the artery from its origin to the renal hilum, which will allow the examiner to survey the artery for velocity shifts along the entire course of the renal artery. Velocities should be recorded at the origin, proximal, mid-, and distal arterial segments. From an oblique approach, the renal artery can be visualized at the renal hilum and followed to the aorta. By studying the patient from an anterior and an oblique approach, Doppler velocity measurements are obtained in two views, assuring that a focal stenosis is not missed and that the angle of insonation is correct. Since medial fibroplasia most often occurs in the mid- to distal renal artery, the oblique approach is particularly good for detecting this type of stenosis. It is important to note that segmental Doppler interrogation (spot-checking) of the renal artery velocities is inadequate and often leads to an inaccurate result.54,56,57 When there is a discrepancy in kidney size of 1.5 cm or greater, the ultrasonographer should search very carefully for the presence of RAS or an occluded renal artery.
A three-category classification scheme based on the PSV within the proximal segment of the renal arteries is commonly used: 0% to 59% stenosis; 60% to 99% stenosis, and total occlusion. If the PSV is greater than 200 cm/s and turbulence is present in color Doppler flow, the stenosis would be classified as 60% to 99%. In the presence of a severe stenosis, there may be characteristic spectral broadening of the Doppler arterial waveform or parvus-tardus waveform just distal to the lesion. In addition to the PSV, the RAR is also used to help classify the degree of stenosis (Table 23-2). The caveat is that the RAR is not an accurate representation of the degree of stenosis when the aortic velocity is less than 40 cm/s or greater than 100 cm/s, or when an abdominal aortic aneurysm, or aortic stent graft is present.
Indirect assessment using the acceleration time (AT), acceleration index (AI), and resistance index (RI) have been used by some investigators to diagnose RAS. However, direct measurement of blood flow velocities in the visualized segments of the renal arteries is the most accurate method of determining whether significant RAS is present.
There are two other important advantages of duplex ultrasonography. First, duplex ultrasonography may help identify patients who will have a favorable clinical outcome after surgical or catheter-based renal revascularization.58 The RI is calculated as follows: [1-(end-diastolic velocity/peak systolic velocity)] × 100 (Fig. 23-5). Using a zero-degree angle of insonation, the peak systolic velocity and EDV are measured within the parenchyma of the kidney. Two studies help support use of the RI. A prospective study followed 138 patients with more than 50% RAS who underwent renal artery angioplasty or surgery for blood pressure control or preservation of renal function. A renal RI of 80 or greater identified patients in whom angioplasty or surgery was not associated with improved blood pressure, renal function, or kidney survival. Ninety-seven percent of patients with an increased renal RI demonstrated no improvement in blood pressure, and 80% had no improvement in renal function. The authors suggested that the increased RI identifies structural abnormalities in the small vessels of the kidney. Such small-vessel disease is typical of long-standing hypertension associated with nephrosclerosis or glomerulosclerosis.59 Similar conclusions were drawn from a more recent study that retrospectively evaluated the significance of associating preprocedural RI with postintervention outcomes (endovascular or open surgical repair for RAS treatment). Crutchley et al. found that a preprocedural RI of 0.8 or higher was highly associated with a postprocedural decline in renal function, and that the RI was also highly predictive of all-cause mortality.60 However, not all investigators believe RI is an accurate predictor of response to renal artery revascularization. A prospective study of renal stent placement in 241 patients demonstrated that individuals with an elevated RI (> 80) achieved a favorable blood pressure response and renal functional improvement a year after renal arterial intervention.61,62 Zeller et al. demonstrated that patients with the most abnormal RI values experienced the greatest magnitude of benefit.61 Until more information becomes available, an elevated RI should not be considered a contraindication to performing renal artery revascularization.63
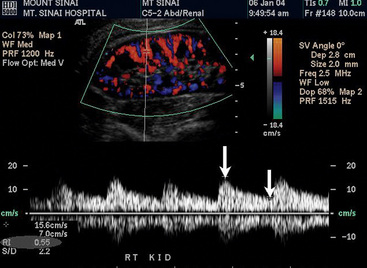
Figure 23-5 Measurement of resistance index (RI) {[1-(end-diastolic velocity/peak systolic velocity)] × 100}. Parenchyma of the kidney is visualized. Note blood flow within kidney. Doppler angle is zero degrees to optimize Doppler waveform. Color velocity scale is set low to optimize color flow. By measuring peak systolic velocity (PSV) (arrow) and end-diastolic velocity (EDV) (arrow), ultrasound machine calculates RI (shown in the gray area at bottom left portion of this image). RI = 0.55 × 100 = 55.
The second major advantage of duplex ultrasonography is its ability to detect restenosis after percutaneous therapy or surgical bypass64–66 (Fig. 23-6). Unlike MRA (which may be affected by artifact or scatter produced by the stent), ultrasound transmission through the stent is not a problem. Computed tomographic angiography has not been adequately studied in this respect. Hudspeth et al. compared angiography to duplex ultrasound for follow-up of RAS after angioplasty and demonstrated a sensitivity and specificity of 69% and 98%, respectively, for detecting stenosis greater than 60%.65 In a more contemporary series, Bakker et al. showed that duplex ultrasonography was an excellent technique to detect restenosis after stent implantation. In 33 consecutive patients using threshold values of 226 cm/s for peak systolic velocity and 2.7 for RAR, sensitivities and specificities were 100% and 90%, and 100% and 84%, respectively.66 In a series of 134 patients with renal artery stents, velocity-derived criteria were developed. All patients with a PSV of less than 241 cm/s were free of in-stent restenosis, while all patients with a PSV of 300 cm/s or greater had in-stent restenosis as confirmed by CTA or catheter-based angiogram. If the PSV was between 241 and 299 cm/s, a judgment was required assessing the degree of turbulence and appearance on gray scale and color Doppler. Using these criteria, the sensitivity was 91%, specificity was 97%, positive predictive value 91%, negative predictive value 96%, and accuracy 95%.67 All patients who have undergone percutaneous intervention should be placed in a surveillance program in an attempt to identify restenosis and treat it before the artery occludes. Following PTA and stent implantation, a renal artery duplex should be obtained at the first office visit, 6 months, 12 months, and yearly thereafter.7,68
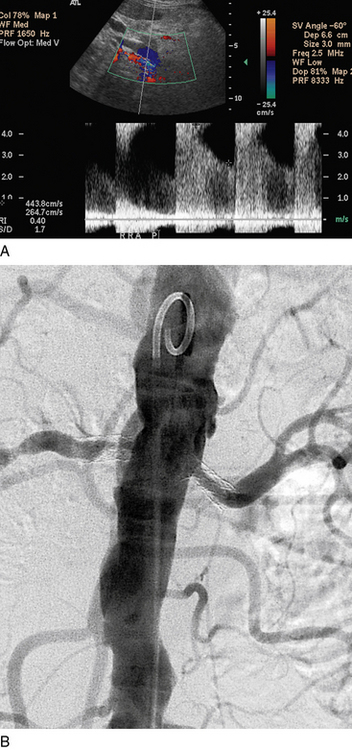
Figure 23-6 A, Duplex ultrasound demonstrating severe stenosis on first surveillance ultrasound 6 months after bilateral renal artery stent implantation. There is turbulence on color image. Peak systolic velocity (PSV) in right renal artery is 444 cm/s, and end-diastolic velocity (EDV) is 265 cm/s, with a renal-to-aortic ratio of 7.4. This is consistent with an 80%-99% stenosis. B, Digital subtraction angiogram (DSA) of same patient demonstrating severe bilateral in-stent restenosis, right more severe than left.
There are several limitations of duplex ultrasonography. It is technically demanding, there is a steep learning curve, and it is particularly challenging in the obese individual. The sensitivity of identifying accessory renal arteries is only about 67%.49 In addition, approximately 5% of renal artery ultrasound studies are of suboptimal quality because of the presence of too much bowel gas. It is highly recommended that these patients return to be studied at a later date after having not eaten in the previous 12 hours.
Magnetic Resonance Angiography
Magnetic resonance angiography (also see Chapter 13) of the renal arteries can be performed rapidly with excellent image quality, does not involve ionizing radiation, and allows for direct visualization of the aorta and renal arteries. Furthermore, MRA can provide functional assessment of blood flow via absolute blood flow rate and GFR measurements.69 There is recent evidence that functional renal perfusion can be assessed by MRA.70,71
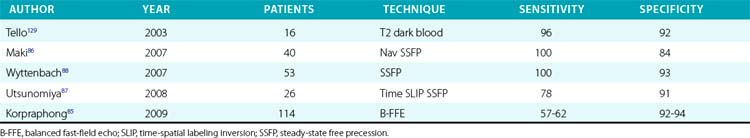
Compared to conventional catheter angiography as the reference standard, three-dimensional (3D) contrast-enhanced gadolinium MRA has a mean sensitivity of 96% and mean specificity of 93%72–83 (Table 23-4). Magnetic resonance angiography techniques that do not use gadolinium contrast agents have improved significantly and can be comparable to contrast-enhanced MRA in diagnostic quality.84 These gadolinium-free techniques, such as 3D time-of-flight (TOF) and inversion-recovery steady-state free precession (SSFP), have similar sensitivities (≈︀ 92%) and specificities (94%) for detecting RAS84–88 (Table 23-5 and Fig. 23-7).
Table 23-4 Accuracy of Three-Dimensional Gadolinium Magnetic Resonance Angiography for Renal Artery Stenosis
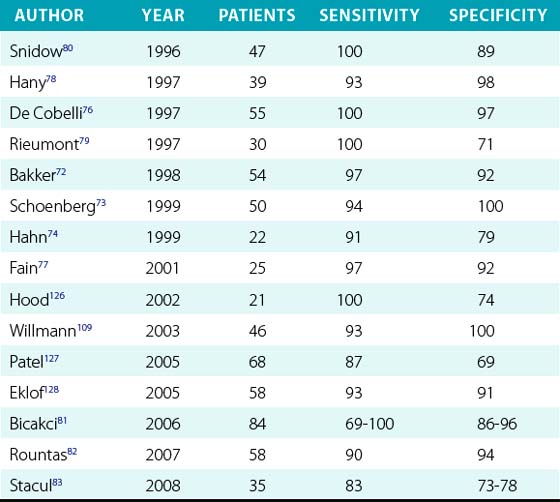
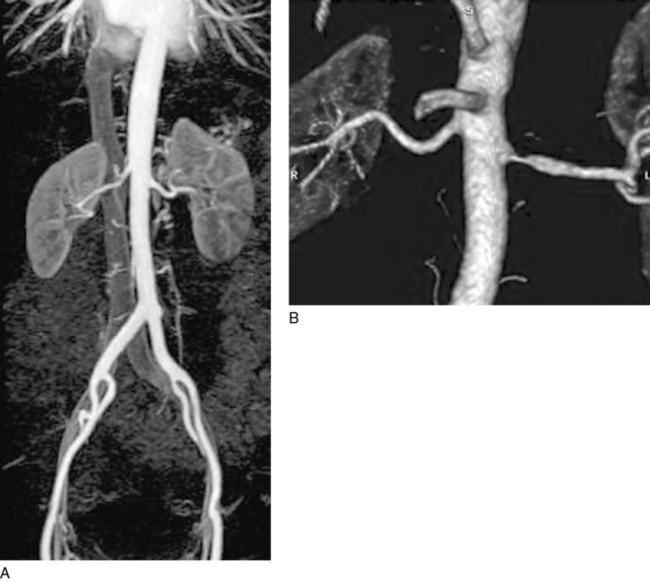
Figure 23-7 A, Three-dimensional (3D) gadolinium-enhanced magnetic resonance angiogram (MRA) demonstrating normal renal arteries bilaterally. There is an excellent view of aorta from diaphragm to inguinal ligament. By imaging a large field of view, one can be certain not to miss an accessory renal artery. Kidneys are also well seen with this technology. Inferior vena cava can be seen in background. B, Severe atherosclerotic renal artery stenosis (RAS) of left renal artery. Right renal artery was normal.
Contrast-enhanced 3D MRA has become a commonly used modality for renal artery imaging because of its ability to produce 3D angiographic images with excellent image quality and improved speed of acquisition.57,70,89,90 Contrast-enhanced 3D MRA exploits the T1-shortening effects of gadolinium-based contrast agents. Blood appears bright, and stationary tissues have a dark appearance. Use of gadolinium shortens image acquisition times, significantly limiting artifact due to patient movement and respiration.91 Because signal intensity with gadolinium is concentration dependent and not flow based, low-flow related artifacts are reduced, and visualization of small vessels is improved compared to other MRI techniques.92 Contrast-enhanced MRA is performed using fast 3D gradient echo pulse sequences. These pulse sequences are available primarily at higher magnetic field strengths (1.0 and 1.5 Tesla). Because hundreds of images are acquired, 3D image processing is subsequently performed to project vessels in views of high diagnostic interest.
Kidneys, adrenal glands, and surrounding soft tissues are evaluated by T1- and T2-weighted image acquisition. Time-of-flight (high-velocity jet within stenosis appears black owing to signal loss), phase contrast (gadolinium injection allows phase shift difference detection and rendering of renal arterial blood flow), and maximal intensity projection are the most widely applied MRA imaging techniques. After 20 minutes of source image acquisition, additional time is required for reformatting. As with CTA, software allows for both two dimensional (2D) and 3D reconstruction, which increases diagnostic yield. Proper equipment, software, and technical expertise are critical for optimal renal MRA and account for significant variability of study quality between institutions.
Despite recent advances, MRA is still limited by several factors, including high cost and imaging artifacts, such as those attributed to patient movement, and difficulty resolving highly tortuous vessels and the smaller accessory renal arteries. Magnetic resonance angiography acquisition times are longer than those for CTA, and patients must therefore be able to remain motionless for minutes at a time. Moreover, MRA may not be possible for patients with claustrophobia and those with metal clips, pacemakers, or other metallic devices. For these reasons, MRA is most useful in patients after inconclusive preliminary workup for RAS or in those with a high clinical suspicion for renovascular hypertension or with contraindications to other imaging modalities. Magnetic resonance angiography is also not useful for monitoring patients after renal artery angioplasty and stenting because of artifact produced by the stent. It also has a tendency to overestimate stenosis severity and may miss accessory renal arteries if the field of view is too narrow.
Exposure to gadolinium-based contrast agents in the setting of renal failure has been associated with nephrogenic systemic fibrosis (NSF), an exceedingly rare condition that involves fibrosis of the skin, joints, eyes, and internal organs.93–95 Current recommendations advise against administering gadolinium contrast to individuals with a GFR below 30 mL/min/1.73m2 or those with acute renal failure or acute deterioration of chronic renal failure.95
Computed Tomographic Angiography
Computed tomographic angiography (also see Chapter 14) can be performed rapidly and safely for assessment of renal artery disease. Multidetector-row CTA provides excellent image quality with higher resolution than could be obtained previously with single-detector-row technology. Most clinical imaging centers currently use 64- to 256-multidetector-row scanners, with 320-multidetector-row scanners currently reserved mostly for research applications or for studying the coronary arteries and bypass grafts.96,97 Advantages of CTA over catheter-based angiography are98–103: volumetric acquisition, demonstrating better visualization of the anatomy from multiple angles and in multiple planes after a single acquisition; improved visualization of soft tissues and other adjacent anatomical structures; less invasive and thus fewer complications; and lower cost.
Computed tomographic angiography has several advantages over MRA, such as higher spatial and temporal resolution, absence of flow-related phenomena that may distort MRA images, and capability to visualize calcification and metallic implants such as endovascular stents or stent grafts. Computed tomographic angiography also involves markedly decreased total examination time, with most 64-multidetector scanners currently performing a complete vascular examination of the abdominal aorta, mesenteric, renal, and iliac arteries in 5 to 10 seconds with submillimeter spatial resolution. When exposure to ionizing radiation is a concern (e.g., in younger patients), MRA may be the preferred imaging modality.
The increased speed of acquisitions coupled with subsecond gantry rotations obtained with multidetector-row CTA allows for greater longitudinal coverage for a given scan duration and greater spatial resolution.104 This may not be of as much importance for assessing renal artery disease, but it has great advantages when assessing the thoracoabdominal, aortoiliac, and lower-extremity inflow and runoff, which may require up to 1400 mm of coverage.105 Rapid acquisition of images allows for reduction in the amount of iodinated contrast material needed while maintaining excellent and uniform vascular enhancement.98,101–103,106
Thin beam collimation (< 1 mm), rotational speed of the tube, and rate of table feed are key parameters in determining imaging protocols. The first set of images produced are sequential or overlapping axial images, which should be interpreted with full attention to all nonvascular structures including bones, bowel, visceral organs, and lung. To create angiographic representations, post-processing of the volumetric data is necessary. The best post-processed images are created from overlapping submillimeter reconstructed images (Fig. 23-8). In the absence of overlap, the angiographic images may have a marked stair-step appearance.
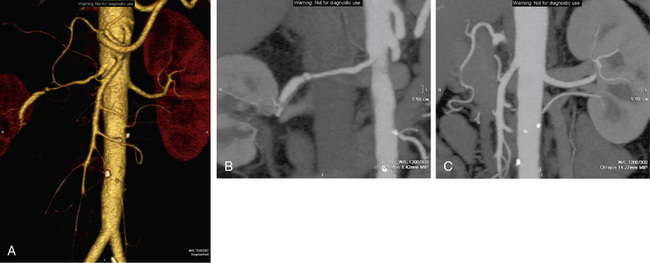
Figure 23-8 Three-dimensional (3D) computed tomographic angiogram (CTA) of renal arteries with 250 multidetector CT scanner.
Volume rendering (A) and maximal intensity projection (MIP) (B-C) demonstrating a dissection and severe focal stenosis of right renal artery and a normal left renal artery.
Over the past several years, more complex post-processing algorithms have been formulated to display volumetric data, including maximum intensity projection (MIP), shaded surface display (SSD), and volumetric rendering (VR).106–108 These techniques allow manipulation of raw data so as to optimize visualization of relevant lesions or disease processes. An important common pitfall is selective visualization of the maximally opacified vascular lumen. Both automated and manual creation of post-processed images risk inadvertent rejection of critical vascular and nonvascular information. Post-processed images alone should never be used for interpretation of CT angiography.105
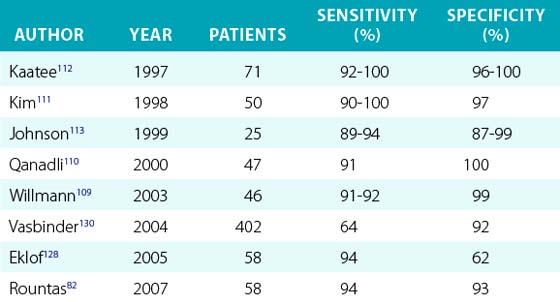
The sensitivity of CTA for RAS ranges from 89% to 100% and specificity from 82% to 100%77,103,105,109–113 (Table 23-6). The area of acquisition should include the area from just proximal to the celiac artery to and including the iliac arteries. This will ensure that accessory renal arteries are detected and associated aortic and visceral artery pathology is not overlooked.
Table 23-6 Accuracy of Computed Tomographic Angiography for Assessment of Visceral and Renal Artery Stenosis
Results obtained using duplex ultrasound, MRA, or CTA are not nearly as good for assessing RAS secondary to FMD; catheter-based angiography remains the imaging modality of choice if FMD is suspected.6,114
Catheter-Based Angiography
Although duplex ultrasonography, MRA, and CTA have replaced catheter-based angiography for the diagnosis of RAS in most circumstances, catheter-based angiography remains the gold standard. It is the most accurate test to diagnose RAS secondary to both atherosclerosis and FMD. It can clearly visualize branch vessels and cortical blood flow and is excellent for identifying accessory renal arteries.
Digital subtraction angiography (DSA) has replaced screen-film angiography in the majority of institutions for vascular applications. The resolution of DSA is less than that of screen film but can approach three to four line pairs per millimeter with current equipment (see Fig. 23-1). The standard imaging matrix is now 1024 × 1024, with image intensifiers that range up to 16 inches in diameter. Flat-panel image intensifiers will soon become available. It is important to recognize that the renal arteries often come off of the aorta posteriorly, and therefore oblique views of the aorta may be needed to adequately visualize the origin of the renal arteries. Pressure gradients should also be obtained to confirm the physiological significance of a given lesion.
New developments in hardware and software have led to greater diagnostic accuracy and better safety. Bolus chasing, rapid image acquisition, vessel diameter analysis, regional pixel shifting, image stacking, 3D reconstructions from rotational angiograms, and angioscopic representations of DSA data are now routinely available from manufacturers.105,115–119
Carbon dioxide (CO2) angiography provides an alternative to conventional angiography or DSA using iodinated contrast agents. This may be particularly useful in patients with renal insufficiency in whom contrast exposure may accelerate the decline of renal function. When compared to conventional angiography, CO2 angiography has a sensitivity of 83% and a specificity of 99%.120,121
Advantages of DSA are the high resolution compared to current cross-sectional imaging techniques, ability to selectively evaluate individual vessels, access direct physiological information such as pressure gradients, and utilization as a platform for intervention. Disadvantages are exposure to ionizing radiation, use of iodinated contrast agents (contrast-induced nephropathy), and risks related to vascular access (pseudoaneurysm, hematoma, retroperitoneal bleed) and catheterization (atheromatous embolization). Nevertheless, until an alternative platform is developed for intervention or completely MR-compatible devices become available, DSA will continue to have a central role in the management of patients with vascular disease.
Renal Angiography at the Time of Cardiac Catheterization
This controversial subject has led to numerous debates over the most appropriate management strategy for patients with CAD and possible RAS. It has been demonstrated that patients with CAD have a higher prevalence of RAS than the general population. In addition, patients with RAS have a markedly increased mortality from cardiovascular disease. Conlon et al. reported that the 4-year survival for patients with no RAS detected at the time of cardiac catheterization was 90% compared to survival rates of 70% for 50% to 75% stenosis, 68% for 75% to 95% stenosis, and 48% for more than 95% stenosis.30,122 Proponents of angiography at the time of catheterization state that the procedure can be performed accurately with no added risk and provide the cardiologist with knowledge that the patient has RAS so that the patient can then be followed serially and treated with optimal secondary preventive measures.31 Those against routine angiography claim that knowing that the patient has RAS adds nothing to the patient’s overall management other than to tempt the angiographer to stent the stenotic lesion in the absence of accepted clinical indications.46,123 This has been termed the renal oculosten(t)otic reflex.124
It is appropriate to perform renal angiography at the time of cardiac catheterization if acceptable indications for renal artery intervention are present.125 Further prospective natural history studies in this asymptomatic population are needed, however, to answer the question of whether routine screening should be performed at the time of cardiac catheterization.
1 Pleis J.R., Lucas J.W., Ward B.W. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat. 2009;10(242):1–157.
2 Olin J.W., Melia M., Young J.R., et al. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88(1N):46N–51N.
3 Harding M.B., Smith L.R., Himmelstein S.I., et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992;2(11):1608–1616.
4 Safian R.D., Textor S.C. Renal-artery stenosis. N Engl J Med. 2001;344(6):431–442.
5 Dworkin L.D., Cooper C.J. Clinical practice. Renal-artery stenosis. N Engl J Med. 2009;361(20):1972–1978.
6 Olin J.W., Sealove B.A. Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg. 2011;53(3):826–836.
7 Olin J.W. Atherosclerotic renal artery disease. Cardiol Clin. 2002;20(4):547–562.
8 Connolly J.O., Higgins R.M., Walters H.L., et al. Presentation, clinical features and outcome in different patterns of atherosclerotic renovascular disease. QJM. 1994;87(7):413–421.
9 Mailloux L.U., Napolitano B., Bellucci A.G., et al. Renal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: a 20-year clinical experience. Am J Kidney Dis. 1994;24(4):622–629.
10 Mailloux L.U., Bellucci A.G., Napolitano B., et al. Survival estimates for 683 patients starting dialysis from 1970 through 1989: identification of risk factors for survival. Clin Nephrol. 1994;42(2):127–135.
11 de Mast Q., Beutler J.J. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27(7):1333–1340.
12 Hansen K.J., Edwards M.S., Craven T.E., et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36(3):443–451.
13 Dustan H.P., Humphries A.W., DeWolfe V.G. Normal arterial pressure in patients with renal arterial stenosis. JAMA. 1964;187:1028–1029.
14 Davis B.A., Crook J.E., Vestas R.E., et al. Prevalence of renovascular hypertension in patients with grade III or IV hypertensive retinopathy. N Engl J Med. 1979;301:1273–1276.
15 Chobanian A.V., Bakris G.L., Black H.R., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289(19):2560–2571.
16 Gifford R.W.Jr., McCormack L.J., Poutasse E.F. The atrophic kidney: its role in hypertension. Mayo Clin Proc. 1965;40(834):852.
17 Lawrie G.M., Morris G.C.Jr., Glaeser D.H., et al. Renovascular reconstruction: factors affecting long-term prognosis in 919 patients followed up to 31 years. Am J Cardiol. 1989;63(15):1085–1092.
18 Textor S.C., Tarazi R.C., Novick A.C., et al. Regulation of renal hemodynamics and glomerular filtration in patients with renovascular hypertension during converting enzyme inhibition with captopril. Am J Med. 1984;76(5B):29–37.
19 Textor S.C., Novick A.C., Steinmuller D.R., et al. Renal failure limiting antihypertensive therapy as an indication for renal revascularization. A case report. Arch Intern Med. 1983;143(11):2208–2211.
20 Silas J.H., Klenka Z., Solomon S.A., et al. Captopril induced reversible renal failure: a marker of renal artery stenosis affecting a solitary kidney. BMJ. 1983;286(6379):1702–1703.
21 Packer M., Lee W.H., Medina N., et al. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med. 1987;106:346–354.
22 Textor S.C. Renal failure related to ACE inhibitors. Semin Nephrol. 1997;17:67–76.
23 Scoble J.E., Maher E.R., Hamilton G. Atherosclerotic renovascular disease causing renal impairment–a case for treatment. Clin Nephrol. 1989;31:119–122.
24 Scoble J.E. Renal artery stenosis as a cause of renal impairment: implications for treatment of hypertension and congestive heart failure. J R Soc Med. 1999;92(10):505–510.
25 Gray B.H., Olin J.W., Childs M.B., et al. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med. 2002;7(4):275–279.
26 Pickering T.G., Herman L., Devereux R.B., et al. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet. 1988;2(8610):551–552.
27 Diamond J.R. Flash pulmonary edema and the diagnostic suspicion of occult renal artery stenosis. Am J Kidney Dis. 1993;21(3):328–330.
28 Khosla S., Kunjummen B., Manda R., et al. Prevalence of renal artery stenosis requiring revascularization in patients initially referred for coronary angiography. Catheter Cardiovasc Interv. 2003;58(3):400–403.
29 Scobel J.E. The epidemiology and clinical presentation of atherosclerotic renal artery disease. In: Novick A.C., Scoble J.E., Halmilton G. Renal vascular disease. London: WB Saunders Co, Ltd; 1996:303–314.
30 Conlon P.J., Little M.A., Pieper K., et al. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60(4):1490–1497.
31 Rihal C.S., Textor S.C., Breen J.F., et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc. 2002;77(4):309–316.
32 Weber-Mzell D., Kotanko P., Schumacher M., et al. Coronary anatomy predicts presence or absence of renal artery stenosis. A prospective study in patients undergoing cardiac catheterization for suspected coronary artery disease. Eur Heart J. 2002;23(21):1684–1691.
33 Louie J., Isaacson J.A., Zierler R.E., et al. Prevalence of carotid and lower extremity arterial disease in patients with renal artery stenosis. Am J Hypertens. 1994;7(5):436–439.
34 Conlon P., O’Riordan E., Kalra P. New insights into the epidemiologic and clinical manifestations of atherosclerotic renovascular disease. Am J Kidney Dis. 2000;35(4):573–587.
35 Dorros G., Jaff M., Mathiak L., et al. Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98(7):642–647.
36 Eipper D.F., Gifford R.W.Jr., Stewart B., et al. Abdominal bruits in renovascular hypertension. Am J Cardiol. 1976;37:48–52.
37 Olin J.W. Evaluation of the peripheral circulation. In: Izzo J.L., Sicca D.A., Black H.R. Hypertension primer. ed 4. Dallas: American Heart Association; 2007:374–378.
38 Canzanello V.J., Textor S.C. Noninvasive diagnosis of renovascular disease. Mayo Clin Proc. 1994;69(12):1172–1181.
39 Emovon O.E., Klotman P.E., Dunnick N.R., et al. Renovascular hypertension in blacks. Am J Hypertens. 1996;9(1):18–23.
40 Maxwell M.H., Lupu A.N., Taplin G.V. Radioisotope renogram in renal arterial hypertension. J Urol. 1968;100(4):376–383.
41 Ploth D.W. Angiotensin-dependent renal mechanisms in two-kidney, one-clip renal vascular hypertension. Am J Physiol. 1983;245(2):F131–F141.
42 Nally J.V., Barton D.P. Contemporary approach to diagnosis and evaluation of renovascular hypertension. Urol Clin North Am. 2001;28(4):781–791.
43 Black H.R., Bourgoignie J.J., Pickering T., et al. Report of the Working Party Group for Patient Selection and Preparation. Am J Hypertens. 1991;4(12 Pt 2):745S–746S.
44 Setaro J.F., Chen C.C., Hoffer P.B., et al. Captopril renography in the diagnosis of renal artery stenosis and the prediction of improvement with revascularization. The Yale Vascular Center experience. Am J Hypertens. 1991;4(12 Pt 2):698S–705S.
45 Setaro J.F., Saddler M.C., Chen C.C., et al. Simplified captopril renography in diagnosis and treatment of renal artery stenosis. Hypertension. 1991;18(3):289–298.
46 Olin J.W., Begelman S.M. Renal artery disease. In: Topol E., ed. Textbook of Cardiovascular medicine. ed 2. Philadelphia: Lippincott Raven; 2002:2139–2159.
47 Fommei E., Ghione S., Hilson A.J., et al. Captopril radionuclide test in renovascular hypertension: a European multicentre study. European Multicentre Study Group. Eur J Nucl Med. 1993;20(7):617–623.
48 Carman T.L., Olin J.W. Diagnosis of renal artery stenosis: what is the optimal diagnostic test? Curr Interv Cardiol Rep. 2000;2(2):111–118.
49 Hansen K.J., Tribble R.W., Reavis S.W., et al. Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12(3):227–236.
50 Hoffmann U., Edwards J.M., Carter S., et al. Role of duplex scanning for the detection of atherosclerotic renal artery disease. Kidney Int. 1991;39(6):1232–1239.
51 Kohler T.R., Zierler R.E., Martin R.L., et al. Noninvasive diagnosis of renal artery stenosis by ultrasonic duplex scanning. J Vasc Surg. 1986;4(5):450–456.
52 Malatino L.S., Polizzi G., Garozzo M., et al. Diagnosis of renovascular disease by extra- and intrarenal Doppler parameters. Angiology. 1998;49(9):707–721.
53 Miralles M., Cairols M., Cotillas J., et al. Value of Doppler parameters in the diagnosis of renal artery stenosis. J Vasc Surg. 1996;23(3):428–435.
54 Olin J.W. Role of duplex ultrasonography in screening for significant renal artery disease. Urol Clin North Am. 1994;21(2):215–226.
55 Williams G.J., Macaskill P., Chan S.F., et al. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. AJR Am J Roentgenol. 2007;188(3):798–811.
56 Olin J.W., Piedmonte M.R., Young J.R., et al. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122(11):833–838.
57 Carman T.L., Olin J.W., Czum J. Noninvasive imaging of the renal arteries. Urol Clin North Am. 2001;28(4):815–826.
58 Radermacher J., Chavan A., Bleck J., et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344(6):410–417.
59 Soulez G., Therasse E., Qanadli S.D., et al. Prediction of clinical response after renal angioplasty: respective value of renal Doppler sonography and scintigraphy. AJR Am J Roentgenol. 2003;181(4):1029–1035.
60 Crutchley T.A., Pearce J.D., Craven T.E., et al. Clinical utility of the resistive index in atherosclerotic renovascular disease. J Vasc Surg. 2009;49(1):148–155.
61 Zeller T., Muller C., Frank U., et al. Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv. 2003;58(4):510–515.
62 Zeller T., Frank U., Muller C., et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation. 2003;108(18):2244–2249.
63 Hirsch A.T., Haskal Z.J., Hertzer N.R., et al: ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation, J Am Coll Cardiol 47(6):1239–1312, 2006.
64 Taylor D.C., Moneta G.L., Strandness D.E.Jr. Follow-up of renal artery stenosis by duplex ultrasound. J Vasc Surg. 1989;9(3):410–415.
65 Hudspeth D.A., Hansen K.J., Reavis S.W., et al. Renal duplex sonography after treatment of renovascular disease. J Vasc Surg. 1993;18(3):381–388.
66 Bakker J., Beutler J.J., Elgersma O.E., et al. Duplex ultrasonography in assessing restenosis of renal artery stents. Cardiovasc Intervent Radiol. 1999;22:475–480.
67 Galin I., Trost B., Kang K., et al. Validation of renal duplex ultrasound in detecting renal artery stenosis post stenting. J Am Coll Cardiol. 2008;51(Suppl I 10):A317.
68 White C.J., Olin J.W. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med. 2009;6(3):176–190.
69 Soulez G., Oliva V.L., Turpin S., et al. Imaging of renovascular hypertension: respective values of renal scintigraphy, renal Doppler US, and MR angiography. Radiographics. 2000;20(5):1355–1368.
70 Schoenberg S.O., Knopp M.V., Londy F., et al. Morphologic and functional magnetic resonance imaging of renal artery stenosis: a multireader tricenter study. J Am Soc Nephrol. 2002;13(1):158–169.
71 Aumann S., Schoenberg S.O., Just A., et al. Quantification of renal perfusion using an intravascular contrast agent (part 1): results in a canine model. Magn Reson Med. 2003;49(2):276–287.
72 Bakker J., Beek F.J., Beutler J.J., et al. Renal artery stenosis and accessory renal arteries: accuracy of detection and visualization with gadolinium-enhanced breath-hold MR angiography. Radiology. 1998;207(2):497–504.
73 Schoenberg S.O., Essig M., Bock M., et al. Comprehensive MR evaluation of renovascular disease in five breath holds. J Magn Reson Imaging. 1999;10(3):347–356.
74 Hahn U., Miller S., Nagele T., et al. Renal MR angiography at 1.0 T: three-dimensional (3D) phase-contrast techniques versus gadolinium-enhanced 3D fast low-angle shot breath-hold imaging. AJR Am J Roentgenol. 1999;172(6):1501–1508.
75 Tan K.T., van Beek E.J., Brown P.W., et al. Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin Radiol. 2002;57(7):617–624.
76 De Cobelli F., Vanzulli A., Sironi S., et al. Renal artery stenosis: evaluation with breath-hold, three-dimensional, dynamic, gadolinium-enhanced versus three-dimensional, phase-contrast MR angiography. Radiology. 1997;205(3):689–695.
77 Fain S.B., King B.F., Breen J.F., et al. High-spatial-resolution contrast-enhanced MR angiography of the renal arteries: a prospective comparison with digital subtraction angiography. Radiology. 2001;218(2):481–490.
78 Hany T.F., Debatin J.F., Leung D.A., et al. Evaluation of the aortoiliac and renal arteries: comparison of breath-hold, contrast-enhanced, three-dimensional MR angiography with conventional catheter angiography. Radiology. 1997;204(2):357–362.
79 Rieumont M.J., Kaufman J.A., Geller S.C., et al. Evaluation of renal artery stenosis with dynamic gadolinium-enhanced MR angiography. AJR Am J Roentgenol. 1997;169(1):39–44.
80 Snidow J.J., Johnson M.S., Harris V.J., et al. Three-dimensional gadolinium-enhanced MR angiography for aortoiliac inflow assessment plus renal artery screening in a single breath hold. Radiology. 1996;198(3):725–732.
81 Bicakci K., Soker G., Binokay F., et al. Estimation of the ratio of renal artery stenosis with magnetic resonance angiography using parallel imaging technique in suspected renovascular hypertension. Nephron Clin Pract. 2006;104(4):c169–c175.
82 Rountas C., Vlychou M., Vassiou K., et al. Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD-enhanced MR angiography, and digital subtraction angiography. Ren Fail. 2007;29(3):295–302.
83 Stacul F., Gava S., Belgrano M., et al. Renal artery stenosis: comparative evaluation of gadolinium-enhanced MRA and DSA. Radiol Med. 2008;113(4):529–546.
84 Glockner J.F., Takahashi N., Kawashima A., et al. Non-contrast renal artery MRA using an inflow inversion recovery steady state free precession technique (Inhance): comparison with 3D contrast-enhanced MRA. J Magn Reson Imaging. 2010;31(6):1411–1418.
85 Korpraphong P., Tovanabutra P., Muangsomboon K. Renal artery stenosis: diagnostic performance of balanced fast field gradient echo MRA. J Med Assoc Thai. 2009;92(8):1077–1083.
86 Maki J.H., Wilson G.J., Eubank W.B., et al. Navigator-gated MR angiography of the renal arteries: a potential screening tool for renal artery stenosis. AJR Am J Roentgenol. 2007;188(6):W540–W546.
87 Utsunomiya D., Miyazaki M., Nomitsu Y., et al. Clinical role of non-contrast magnetic resonance angiography for evaluation of renal artery stenosis. Circ J. 2008;72(10):1627–1630.
88 Wyttenbach R., Braghetti A., Wyss M., et al. Renal artery assessment with nonenhanced steady-state free precession versus contrast-enhanced MR angiography. Radiology. 2007;245(1):186–195.
89 Prince M.R., Chenevert T.L., Foo T.K., et al. Contrast-enhanced abdominal MR angiography: optimization of imaging delay time by automating the detection of contrast material arrival in the aorta. Radiology. 1997;203(1):109–114.
90 Zhang J., Pedrosa I., Rofsky N.M. MR techniques for renal imaging. Radiol Clin North Am. 2003;41(5):877–907.
91 Saloner D. Determinants of image appearance in contrast-enhanced magnetic resonance angiography: a review. Invest Radiol. 1998;33:488–495.
92 Thornton J., O’Callaghan J., Walshe J., et al. Comparison of digital subtraction angiography with gadolinium-enhanced magnetic resonance angiography in the diagnosis of renal artery stenosis. Eur Radiol. 1999;9(5):930–934.
93 Kribben A., Witzke O., Hillen U., et al. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol. 2009;53(18):1621–1628.
94 Perazella M.A. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens. 2009;18(6):519–525.
95 Prince M.R., Zhang H.L., Roditi G.H., et al. Risk factors for NSF: a literature review. J Magn Reson Imaging. 2009;30(6):1298–1308.
96 de Graaf F.R., van Velzen J.E., Witkowska A.J., et al. Diagnostic performance of 320-slice multidetector computed tomography coronary angiography in patients after coronary artery bypass grafting. Eur Radiol. 2011;21(11):2285–2296.
97 van Velzen J.E., de Graaf F.R., Kroft L.J., et al. Performance and efficacy of 320-row computed tomography coronary angiography in patients presenting with acute chest pain: results from a clinical registry. Int J Cardiovasc Imaging. 2011. May 26. [Epub ahead of print] DOI 10.1007/s10554-011-9889-z
98 Rubin G.D. Three-dimensional helical CT angiography. Radiographics. 1994;14(4):905–912.
99 Rubin G.D. MDCT imaging of the aorta and peripheral vessels. Eur J Radiol. 2003;45(Suppl 1):S42–S49.
100 Rubin G.D. 3-D imaging with MDCT. Eur J Radiol. 2003;45(Suppl 1):S37–S41.
101 Rubin G.D. Techniques for performing multidetector-row computed tomographic angiography. Tech Vasc Interv Radiol. 2001;4(1):2–14.
102 Bluemke D.A., Chambers T.P. Spiral CT angiography: an alternative to conventional angiography. Radiology. 1995;195(2):317–319.
103 Liu P.S., Platt J.F. CT angiography of the renal circulation. Radiol Clin North Am. 2010;48(2):347–365.
104 Bluemke D.A., Soyer P.A., Chan B.W., et al. Spiral CT during arterial portography: technique and applications. Radiographics. 1995;15(3):623–637.
105 Olin J.W., Kaufman J.A., Bluemke D.A., et al. Atherosclerotic Vascular Disease Conference. American Heart Association, Imaging, Writing Group IV. Circulation. 2004;109:2626–2633.
106 Zeman R.K., Silverman P.M., Vieco P.T., et al. CT angiography. AJR Am J Roentgenol. 1995;165(5):1079–1088.
107 Ibukuro K., Charnsangavej C., Chasen M.H., et al. Helical CT angiography with multiplanar reformation: techniques and clinical applications. Radiographics. 1995;15(3):671–682.
108 Addis K.A., Hopper K.D., Iyriboz T.A., et al. CT angiography: in vitro comparison of five reconstruction methods. AJR Am J Roentgenol. 2001;177(5):1171–1176.
109 Willmann J.K., Wildermuth S., Pfammatter T., et al. Aortoiliac and renal arteries: prospective intraindividual comparison of contrast-enhanced three-dimensional MR angiography and multi-detector row CT angiography. Radiology. 2003;226(3):798–811.
110 Qanadli S.D., Mesurolle B., Coggia M., et al. Abdominal aortic aneurysm: pretherapy assessment with dual-slice helical CT angiography. AJR Am J Roentgenol. 2000;174(1):181–187.
111 Kim T.S., Chung J.W., Park J.H., et al. Renal artery evaluation: comparison of spiral CT angiography to intra-arterial DSA. J Vasc Interv Radiol. 1998;9(4):553–559.
112 Kaatee R., Beek F.J., de Lange E.E., et al. Renal artery stenosis: detection and quantification with spiral CT angiography versus optimized digital subtraction angiography. Radiology. 1997;205(1):121–127.
113 Johnson P.T., Halpern E.J., Kuszyk B.S., et al. Renal artery stenosis: CT angiography–comparison of real-time volume-rendering and maximum intensity projection algorithms. Radiology. 1999;211(2):337–343.
114 Slovut D.P., Olin J.W. Fibromuscular dysplasia. N Engl J Med. 2004;350(18):1862–1871.
115 Bosanac Z., Miller R.J., Jain M. Rotational digital subtraction carotid angiography: technique and comparison with static digital subtraction angiography. Clin Radiol. 1998;53(9):682–687.
116 Seymour H.R., Matson M.B., Belli A.M., et al. Rotational digital subtraction angiography of the renal arteries: technique and evaluation in the study of native and transplant renal arteries. Br J Radiol. 2001;74(878):134–141.
117 Meijering E.H., Niessen W.J., Bakker J., et al. Reduction of patient motion artifacts in digital subtraction angiography: evaluation of a fast and fully automatic technique. Radiology. 2001;219(1):288–293.
118 Meijering E.H., Niesssen W.J., Viergever M.A. Retrospective motion correction in digital subtraction angiography: a review. IEEE Trans Med Imaging. 1999;18(1):2–21.
119 Ashleigh R.J., Hufton A.P., Razzaq R., et al. A comparison of bolus chasing and static digital subtraction arteriography in peripheral vascular disease. Br J Radiol. 2000;73(872):819–824.
120 Schreier D.Z., Weaver F.A., Frankhouse J., et al. A prospective study of carbon dioxide-digital subtraction vs standard contrast arteriography in the evaluation of the renal arteries. Arch Surg. 1996;131(5):503–507.
121 Hawkins I.F.Jr., Wilcox C.S., Kerns S.R., et al. CO2 digital angiography: a safer contrast agent for renal vascular imaging? Am J Kidney Dis. 1994;24(4):685–694.
122 Conlon P.J., Athirakul K., Kovalik E., et al. Survival in renal vascular disease. J Am Soc Nephrol. 1998;9(2):252–256.
123 Textor S.C. Progressive hypertension in a patient with “incidental” renal artery stenosis. Hypertension. 2002;40(5):595–600.
124 White C.J. The renal oculosten(t)otic reflex. Cathet Cardiovasc Diagn. 1996;37(3):251.
125 White C.J., Jaff M.R., Haskal Z.J., et al. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation. 2006;114(17):1892–1895.
126 Hood M.N., Ho V.B., Corse W.R. Three-dimensional phase-contrast magnetic resonance angiography: a useful clinical adjunct to gadolinium-enhanced three-dimensional renal magnetic resonance angiography? Mil Med. 2002;167(4):343–349.
127 Patel S.T., Mills J.L.Sr., Tynan-Cuisinier G., et al. The limitations of magnetic resonance angiography in the diagnosis of renal artery stenosis: comparative analysis with conventional arteriography. J Vasc Surg. 2005;41(3):462–468.
128 Eklof H., Ahlstrom H., Bostrom A., et al. Renal artery stenosis evaluated with 3D-Gd-magnetic resonance angiography using transstenotic pressure gradient as the standard of reference. A multireader study. Acta Radiol. 2005;46(8):802–809.
129 Tello R., Mitchell P.J., Witte D.J., et al. T2 dark blood MRA for renal artery stenosis detection: preliminary observations. Comput Med Imaging Graph. 2003;27(1):11–16.
130 Vasbinder G.B., Nelemans P.J., Kessels A.G., et al. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med. 2004;141(9):674–682.