Chapter 27 Clinical Evaluation and Treatment of Mesenteric Vascular Disease
Evaluation
Clinical evaluation of possible mesenteric ischemia begins with a careful history and physical examination and—above all—an appropriate index of suspicion for the diagnosis. The major etiologies of mesenteric ischemia include mesenteric venous thrombosis (MVT), acute mesenteric ischemia, nonocclusive mesenteric ischemia (NOMI), and chronic mesenteric ischemia (CMI). These differ in their underlying pathologies and the clinical settings in which they occur, but there may be significant overlap in their clinical presentation. The most crucial point is to understand the variety of clinical settings in which intestinal ischemia can occur and to include mesenteric ischemia in the differential diagnosis of patients presenting with abdominal pain. The goal is to achieve a diagnosis prior to the onset of bowel infarction. Without consideration of intestinal ischemia, the appropriate diagnostic evaluation is unlikely to be obtained, resulting in needless additional morbidity and mortality.
Acute Occlusive Mesenteric Ischemia
Signs and symptoms
Acute occlusive mesenteric ischemia is caused by embolism to the superior mesenteric artery (SMA) or acute thrombotic occlusion of an atherosclerotic SMA. Mortality exceeds 60%.1
Thrombotic occlusion of the SMA carries a worse prognosis than embolism to the SMA because with thrombotic occlusion, the SMA occludes proximal to the middle colic artery and interrupts arterial flow to the entire small intestine. Emboli, which usually originate from the heart, typically lodge in the SMA distal to the origin of the middle colic artery, thereby maintaining some perfusion to the small intestine via the middle colic and jejunal artery branches.
Abdominal pain is the most common presenting symptom in patients with occlusive acute mesenteric ischemia, and physical findings can range from nonspecific tenderness to an acute abdomen. Distention, rigidity, and rebound tenderness occur, particularly when the diagnosis of acute mesenteric ischemia is delayed. The classic presentation is sudden onset of acute abdominal pain out of proportion to the physical findings. This reflects profound intestinal ischemia without associated bowel perforation and peritonitis. Vomiting, fever, and diarrhea are present in one third of patients with acute mesenteric ischemia. Patients with embolism tend to present with an acute onset of abdominal pain, whereas patients with thrombosis of a stenotic SMA may have a more delayed presentation.
Laboratory values are typically nonspecific. The majority of patients will have moderate to marked leukocytosis, but about 10% of patients will have a normal white blood cell (WBC) count. Elevated serum amylase and metabolic acidosis may occur in patients with necrotic bowel, but an absence of these findings does not exclude bowel necrosis.
Radiological diagnosis
Ultrasound has a limited role in diagnosing acute occlusive mesenteric ischemia. Duplex ultrasonography in the setting of an acute abdomen is limited by abdominal distention, excessive bowel gas, and patient discomfort.
An abdominal computed tomography (CT) scan is often obtained during evaluation of a patient with abdominal pain. In addition to finding other abdominal pathologies causing abdominal pain, CT scans can detect late findings of acute mesenteric ischemia with a sensitivity of 90%, including bowel luminal dilation, bowel wall thickening, submucosal edema or hemorrhage, pneumatosis intestinalis, and portal venous gas.2 These findings are associated with some degree of intestinal infarction. Emboli tend to lodge distally, and thus the sensitivity of CT scanning in detecting mesenteric arterial embolic occlusion is low and ranges from 37% to 80%3 (Fig. 27-1). In patients with early ischemia, there may be minimal findings on CT scans. These patients benefit most from early diagnosis and definitive mesenteric revascularization before the onset of intestinal infarction. Therefore, a high index of suspicion and thorough physical examination is of the utmost importance in this population of patients.
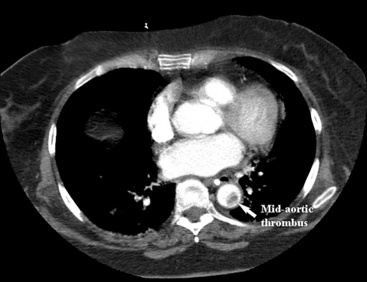
Figure 27-1 Axial computed tomography (CT) scan demonstrating a mid-aortic thrombus that caused embolic occlusion of superior mesenteric artery (SMA).
Mesenteric angiography remains the gold standard for diagnosing vascular lesions associated with acute mesenteric ischemia, but its use is predicated on clinical judgment. In a patient with obvious peritoneal findings and suspected necrotic bowel, as evidenced by hypotension and acidosis, an urgent exploratory laparotomy is required to resect necrotic bowel and perform revascularization. In this emergent situation, preparation and performance of a mesenteric angiogram may delay definitive operative treatment and increase mortality.
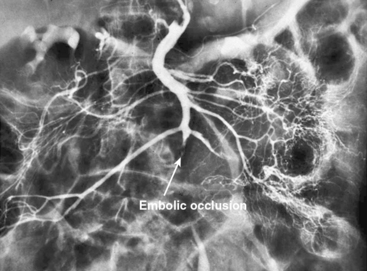
In patients without peritoneal findings but clinically suspected to have mesenteric ischemia, angiography can be performed to make the definitive diagnosis and plan additional therapy. Abrupt occlusion of the SMA distal to the origin of the middle colic artery is typically seen in patients with an embolus (Figs. 27-2 through 27-4), whereas patients with thrombotic occlusion of a chronically stenotic SMA show signs of occlusion beginning at its origin from the aorta.
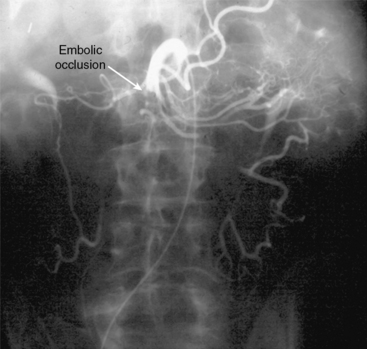
Figure 27-2 Anteroposterior angiogram demonstrating abrupt embolic occlusion of superior mesenteric artery (SMA).
Treatment
Treatment of acute mesenteric occlusive ischemia is aimed at the etiology of the disease. With acute embolic disease, embolectomy can be performed. Thrombotic disease should generally be addressed with a bypass operation. Bypass as an option for acute mesenteric occlusive ischemia treatment is discussed later in the chapter.
Operative Embolectomy
Once angiography has identified embolic disease, the patient is taken to the operating room for abdominal exploration and embolectomy. When performing an SMA embolectomy, a midline incision in the abdomen is made, followed by a thorough examination of the abdominal contents. This may or may not reveal intestinal infarction. The transverse colon is then reflected superiorly, and the SMA is approached at the root of the small-bowel mesentery. The ligament of Treitz is divided and the proximal SMA mobilized. If the embolus is located more distally, the distal SMA may be exposed at the root of the small-bowel mesentery. Embolectomy is performed through a transverse arteriotomy using standard balloon catheters, and the embolus is extracted. The arteriotomy is then closed, and the intestines are again inspected for viability; any nonviable bowel is resected. A Doppler probe can be used to assess the antimesenteric border for intestinal arterial flow. If the bowel viability is equivocal, a “second look” operation can be planned in the following 24 to 48 hours to reassess the bowel and resect if necessary.4
Chronic Mesenteric Ischemia
Signs and symptoms
Patients with CMI are typically women (3:1 female-to-male ratio) between the ages of 40 and 70 years, and a history of recurrent abdominal pain is the most critical factor in the diagnosis. The pain associated with CMI is mid-abdominal or epigastric in origin and is described as colicky or a dull intense ache that may radiate to the back. Pain is postprandial and generally begins 15 to 30 minutes after eating and lasts up to 4 hours. There are no signs of peritonitis, and the degree of pain may reflect the volume of the ingested meal.
Patients may ingest some meals without pain early in the course of CMI, so pain may be initially attributed to cholelithiasis, peptic ulcer disease, or malignancy. Patients often undergo extensive evaluation with endoscopy, CT, barium studies, and abdominal ultrasonography prior to reaching a diagnosis of CMI. As the disease progresses, patients begin to experience pain with each meal and may develop a fear of food. Weight loss, the hallmark of CMI, results from limited nutritional intake, not malabsorption; patients with CMI will have normal gastrointestinal absorption test results.
Radiological diagnosis
Mesenteric Angiography
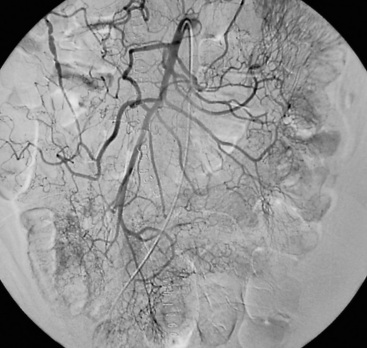
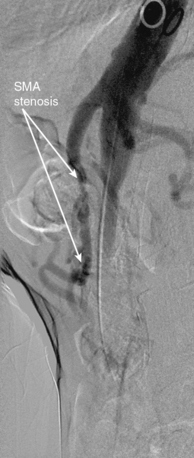
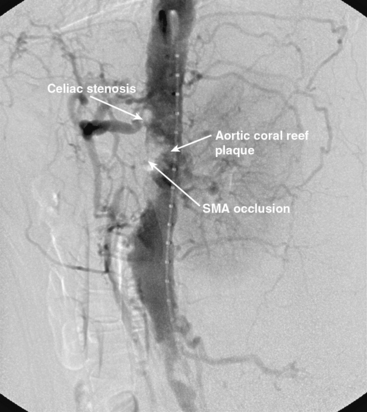
Chronic mesenteric ischemia is a clinical diagnosis. There are no absolute confirmatory tests, but contrast mesenteric angiography is the standard to diagnose arterial lesions associated with CMI. Radiographs are obtained in the lateral and anteroposterior projections. Findings on angiography suggestive of CMI include stenosis or occlusion of the celiac artery (CA) and/or the SMA (Fig. 27-5). When the origins of the CA or SMA are occluded, the more distal vessels are often patent through filling by enlarged and easily visualized pancreaticoduodenal arterial collaterals. Occasionally, one can find mid-SMA stenosis with a normal proximal SMA (Fig. 27-6) or a “coral reef” aorta with associated SMA occlusion (Fig. 27-7). Not all patients with mesenteric artery obstruction, however, have mesenteric ischemia. It is vitally important to differentiate high-grade mesenteric artery stenosis from the clinical entity of CMI.
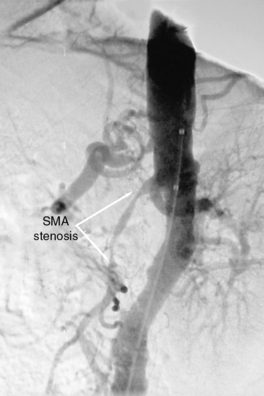
Figure 27-5 Lateral aortogram demonstrating long-segment stenosis of superior mesenteric artery (SMA).
Duplex Ultrasonography
Duplex ultrasonography can serve as a valuable noninvasive screening test for splanchnic artery stenosis and for follow-up in patients with mesenteric artery reconstructions. Despite the accuracy of duplex detection of mesenteric artery stenoses, an appropriate history and physical examination and angiographic confirmation of high-grade stenoses or occlusion of the splanchnic vessels are still required for the diagnosis of CMI. Duplex examination of the mesenteric arteries can be technically difficult and should be performed by vascular technologists with extensive experience in abdominal ultrasound techniques.
In healthy individuals, fasting blood flow velocity waveforms differ between the SMA and the CA. Arterial waveforms reflect end-organ vascular resistance. The liver and spleen have relatively high constant metabolic requirements and are therefore low-resistance organs. As a result, CA waveforms are generally biphasic with a peak systolic component, no reversal of end-systolic flow, and a relatively high end-diastolic velocity (EDV). The normal fasting SMA velocity waveform is triphasic, reflecting the high vascular resistance of the intestinal tract at rest (Figs. 27-8 and 27-9). There is a peak systolic component, often an end-systolic reverse flow component, and a minimal diastolic flow component.
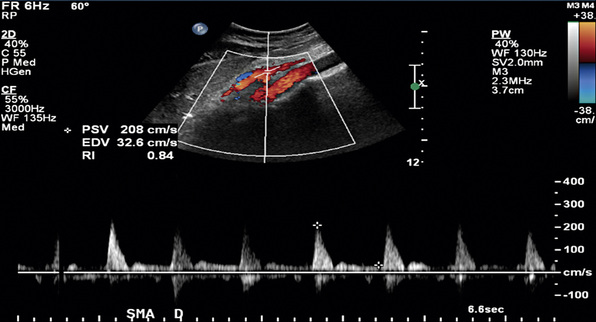
Figure 27-9 Duplex ultrasonography of superior mesenteric artery (SMA), with peak systolic velocity (PSV) of 208 cm/s signifying a normal SMA.
Changes in Doppler-derived arterial waveforms in response to feeding are different in the CA and SMA. Because the liver and spleen have fixed metabolic demands, there is no significant change in CA velocity waveform after eating. Blood flow in the SMA, however, increases markedly after a meal, reflecting a marked decrease in intestinal arterial resistance. Waveform changes in the SMA postprandially include a near doubling of systolic velocity, tripling of the EDV, and loss of end-systolic reversal of blood flow. In addition, there is a small but detectable increase in the diameter of the SMA postprandially. The diameter of the SMA has been shown to be 0.60 ± 0.09 cm in the fasting state and 0.67 ± 0.09 cm after a meal. These changes are maximal at 45 minutes after ingestion of a test meal5 and are dependent on the composition of the meal ingested. Mixed composition meals produce the greatest flow increase in the SMA when compared with equal caloric meals composed solely of fat, glucose, or protein.6
Detection of Splanchnic Arterial Stenosis
Duplex ultrasound can detect hemodynamically significant stenoses in splanchnic vessels. In 1986, investigators at the University of Washington found that flow velocities in stenotic SMAs and CAs were significantly increased when compared with normal SMAs and CAs.7 Quantitative criteria for splanchnic artery stenosis were first developed and validated at Oregon Health & Science University.8
In a blinded prospective study of 100 patients who underwent mesenteric artery duplex scanning and lateral aortography, a peak systolic velocity (PSV) in the SMA of 275 cm/s or more indicated 70% or greater SMA stenosis with a sensitivity of 92%, specificity of 96%, positive predictive value of 80%, negative predictive value of 99%, and accuracy of 96%9 (Fig. 27-10). In the same study, a PSV of 200 cm/s or higher identified 70% or greater angiographic CA stenosis with a sensitivity of 87%, specificity of 80%, positive predictive value of 63%, negative predictive value of 94%, and accuracy of 82% (Fig. 27-11).
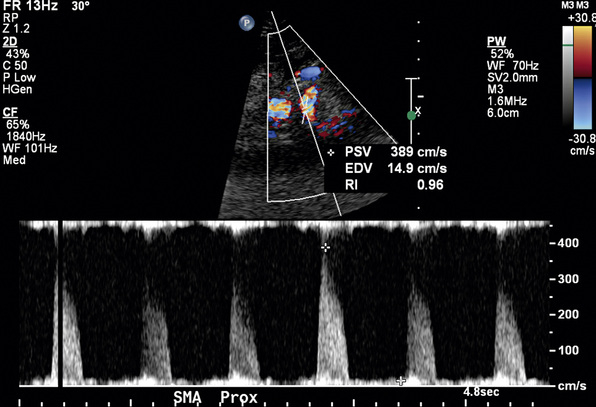
Figure 27-10 Duplex ultrasonography of superior mesenteric artery (SMA), with peak systolic velocity (PSV) of 389 cm/s signifying 70% or greater SMA stenosis.
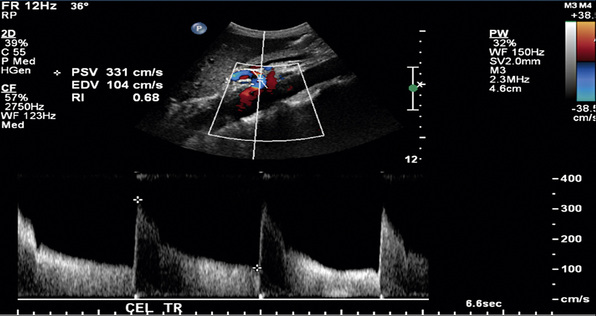
Figure 27-11 Duplex ultrasonography of celiac artery (CA), with peak systolic velocity (PSV) of 331 cm/s signifying 70% or greater CA stenosis.
Other duplex criteria for mesenteric artery stenoses are also in use. An SMA EDV greater than 45 cm/s correlates with 50% or greater SMA stenosis with 92% specificity and 100% sensitivity. A CA EDV of 55 cm/s or higher predicts 50% or greater CA stenosis with 93% sensitivity, 100% specificity, and 95% accuracy.10,11
Postprandial mesenteric duplex scanning has been used as an adjunct to fasting duplex scanning to aide in the diagnosis of mesenteric artery stenoses.12 In patients with less than 70% SMA stenosis, postprandial SMA PSV increases by more than 20% over baseline velocity. The percent increase in SMA PSV is less in patients with 70% or greater SMA stenosis. Specificity for the combination of fasting SMA PSV and postprandial PSV, however, is marginally improved over that provided by a fasting duplex scan alone. Therefore, although theoretically attractive, postprandial duplex scanning offers no significant improvement over fasting mesenteric duplex scanning; its routine use as part of ultrasound assessment of mesenteric artery stenosis is unnecessary. Postprandial examinations are occasionally useful in technically difficult ultrasound studies in that if there is a postprandial response, the insonated vessel can be confirmed as being the SMA.
Duplex ultrasonography is best used as an initial screening study to evaluate for visceral artery stenosis in patients with chronic abdominal pain that may be consistent with CMI. Angiography is required to establish a definitive diagnosis of mesenteric artery stenosis.
Computed Tomography
Standard CT imaging can be of some value in mesenteric artery stenosis evaluation (also see Chapter 14). Currently, however, no studies have compared the accuracy of spiral CT with angiography in assessing visceral artery stenoses. Computed tomography angiography can detect but not precisely quantify proximal stenosis of the CA and SMA, and it is limited by lower resolution and bowel motion artifact in its ability to detect lesions involving more distal branches.13 Computed tomography scans are often obtained to evaluate abdominal pain. Findings suggestive of mesenteric artery stenosis include calcification at the origin of the CA and SMA and lack of contrast enhancement within the vessel lumen (Fig. 27-12).
Magnetic Resonance Imaging
Development of fast breath-hold three-dimensional gadolinium-enhanced magnetic resonance angiography (3D Gd-enhanced MRA) has improved the ability of MRA to detect proximal splanchnic artery lesions (also see Chapter 13). Magnetic resonance angiography is limited in its ability to image more distal visceral branches because of limited spatial resolution, peristaltic and respiratory motion, and chemical shift changes between the vessels and fat. The accuracy of 3D Gd-enhanced MRA in detecting visceral artery stenosis has been well studied. Overall sensitivity and specificity for detecting 75% or greater stenosis or occlusion of the CA, SMA, or inferior mesenteric artery (IMA) were 100% and 95%, respectively.14
In addition to providing anatomical details, magnetic resonance (MR) technology can quantify arterial flow using the phase-contrast magnetic resonance imaging (MRI) technique, which provides information about the presence, magnitude, and direction of blood flow and has been studied in patients with CMI.15 Fasting and postprandial flow in the SMA have been compared in patients with documented atherosclerotic disease and normal volunteers. Mean fasting SMA blood flow has been shown to be higher in atherosclerotic patients than in normal individuals. This difference can be used to evaluate for CMI. The addition of MR oximetry technology, where increased oxygen extraction after a meal is seen in those with significant mesenteric vessel atherosclerosis, is promising with regard to diagnosing CMI.15 Further validation studies correlating the degree of change in mesenteric blood flow and oxygenation with angiographic percentage of stenosis will be necessary before phase-contrast MRI and MR oximetry are universally adopted as routine noninvasive diagnostic methods for patients with CMI.
Treatment
Chronic and Acute Mesenteric Occlusive Disease
The available literature includes no randomized or controlled clinical trials of surgical intervention for treatment of mesenteric ischemia, and none are likely to be performed. Published clinical reports include varied recommendations for treatment, and many do not include descriptions of operative methods. Others describe technically demanding procedures requiring extensive dissections in difficult areas.16,17 Only recently has objective determination of postoperative graft patency been included in clinical series.18,19 For all these reasons, there is no current consensus regarding the surgical details of treatment for intestinal ischemia.
History
In 1936, Dunphy reviewed the medical records of 12 patients dying from intestinal ischemia and discovered that more than half (58%) had evidence of chronic abdominal pain.20 This finding suggested that timely surgical intervention may have prevented progression to intestinal infarction and death. In 1957, Mikkelsen described the arteriographic appearance of typical orificial atherosclerotic lesions affecting the mesenteric arteries. That same year, the first successful surgical procedure (SMA endarterectomy) for treatment of chronic intestinal ischemia was performed by Maynard and Shaw.21
Since then, numerous techniques have been developed to revascularize the mesenteric arteries. One debated issue is the optimal number of vessels to revascularize. Proponents of multiple-vessel or “complete” revascularization have worried that although single-vessel bypass is effective in relieving symptoms initially, there may be a higher incidence of recurrent symptoms secondary to graft failure. With a few exceptions, as noted subsequently, surgery of some sort remains an integral part of the treatment of all etiologies of mesenteric ischemia.
Multiple-vessel revascularization
Multiple-vessel revascularization implies repair or bypass of all diseased or occluded vessels, most often the CA and SMA. Most agree that bypass to the IMA is unnecessary for successful revascularization except in unusual cases. Grafts can be oriented antegrade from the supraceliac aorta or retrograde from the infrarenal aorta or an iliac artery.
An early report from the Mayo Clinic first suggested that “complete” revascularization resulted in decreased symptomatic recurrence.22 A subsequent report including these patients and others indicated that graft patency and survival in patients with three-vessel revascularization were improved compared to single-vessel revascularization.23 The authors speculated that this difference in outcome was a result of complete revascularization, which theoretically provides an additional measure of safety. These two studies, however, were limited to patients with chronic intestinal ischemia and did not use objective methods to determine postoperative graft patency. In the latter study, McAfee et al.23 noted that symptoms of recurrent ischemia were an unreliable measure of graft patency because two of their three early occlusions were asymptomatic. Lack of symptoms may have resulted from the presence of additional patent grafts. Although these retrospective studies suggest that complete revascularization resulted in fewer recurrences and deaths, the results were not statistically significant.
Some believe that antegrade orientation provides better inflow than retrograde orientation because prograde flow is less turbulent, there may be less graft kinking, and the supraceliac aorta is usually less diseased than the infrarenal aorta or an iliac artery. In the Mayo Clinic series published in 1981, the symptomatic recurrence rate was 26%; none of these grafts were antegrade.22 In more current studies in which the majority of grafts are positioned antegrade, the recurrence rate is lower.24 Clearly, the reduction in recurrence is multifactorial and cannot be attributed solely to graft orientation.
More recent data suggest the rate of symptomatic recurrence is unaffected by the number of vessels revascularized or graft orientation. In a study of 91 patients treated for CMI with a bypass procedure, there were patients with both single- and multiple-vessel reconstructions and with grafts in either orientation. Survival was unaffected by number of vessels revascularized. Patients with retrograde grafts had decreased survival, but these patients were older than those with antegrade grafts.24
Single-vessel revascularization
Proponents of single-vessel revascularization have reported long-term results similar to multiple-vessel revascularizations. Series from France have shown SMA reconstruction alone to be a durable form of treatment for intestinal ischemia. Kieny et al.25 performed 60 direct or indirect (using a short prosthetic segment) reimplantations of the SMA (10% of patients had additional vessels reconstructed) in patients with atherosclerotic lesions of the visceral arteries. Mean follow-up was 8.5 years; five patients (8.3%) developed recurrences, and one patient died as a result. The 5-year actuarial survival was 69.6%.
Favorable results for single-vessel revascularization have also been reported in the United States.26,27 Stanton et al.26 performed 20 reconstructions in 17 patients, and at 60.9 months they found no symptomatic recurrences. One method of mesenteric revascularization is transaortic endarterectomy (TAE), with antegrade aortoceliac bypass reserved for older or poor-risk patients.28 Transaortic endarterectomy usually involves revascularization of both the celiac axis and the SMA. Similar recurrence rates have been observed between the two techniques, with 86% of patients in both groups being asymptomatic at 5 years. Durable relief of symptoms did not appear to correlate with number of visceral arteries repaired.
At Oregon Health & Science University, the surgical approach to managing acute and CMI has changed in the last 2 decades. In 1994, Gentile et al.27 reported 26 patients who had 29 isolated bypasses to the SMA for intestinal ischemia (23 chronic, 5 acute, 1 asymptomatic). Perioperative mortality was 10%. Mean follow-up was 40 months, and the life table–determined 4-year primary graft patency rate and survival rate were 89% and 82%, respectively. This compared favorably with contemporary reports in the literature. Based on this experience, revascularization of the SMA alone is recommended for most cases of intestinal ischemia.
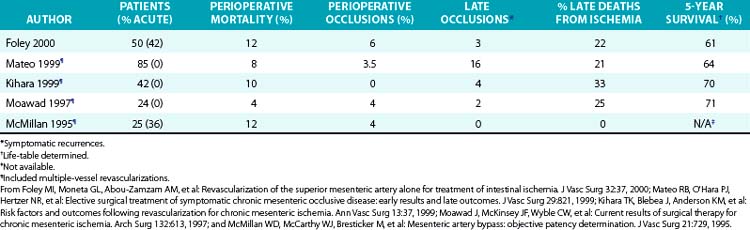
Foley et al. recently reported a series of 50 SMA revascularizations, employing objective means to determine graft postoperative patency.19 This series differed from others with respect to the larger number of patients with previous attempts at revascularization (24%), higher percentage of patients presenting with acute ischemia (42%), and higher percentage of patients requiring simultaneous bowel resection (28%). Overall perioperative mortality (12%), however, was comparable to other recent series. Perioperative mortality was 3% for patients operated on electively. The incidence of perioperative graft occlusions (6%) was similar to other recent series, only one of which contains a significant number of patients presenting with acute intestinal ischemia. Three graft occlusions occurred during long-term follow-up and resulted in death in two patients, accounting for 22% of late deaths. In this series, the number of symptomatic late graft occlusions, number of deaths attributable to recurrent ischemia, and life table–determined survival were comparable to other recent series employing more complete visceral revascularizations (Table 27-1).
Although acute mesenteric ischemia is accompanied by a higher perioperative mortality rate, McMillan et al.29 found no differences in long-term patency of bypass grafts between patients with acute or chronic ischemia. Two of the three late occlusions in this series occurred in patients whose initial graft was placed for CMI, but one of these occluded in the perioperative period and was replaced. Revascularization of the SMA alone continues to compare favorably with more complete mesenteric revascularizations. Several authors have noted that symptoms are an insensitive measure of graft failure.23,29 With improvements in duplex scanning, several studies have objective data for long-term graft patency.29–31
Indications for operation
Revascularization is clearly indicated for symptomatic intestinal ischemia. Revascularization for asymptomatic high-grade SMA obstruction is recommended only in patients undergoing otherwise indicated aortic surgery for aneurysmal or occlusive disease. In this group of patients, acute intestinal ischemia following aortic surgery has been well documented, and SMA reconstruction seems prudent.32
Techniques of superior mesenteric artery bypass
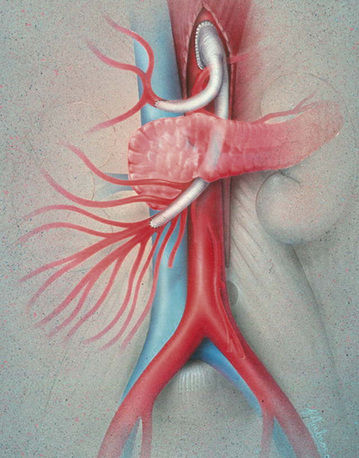
Retrograde Bypass
The distal infrarenal aorta as an origin for an SMA bypass graft has advantages and disadvantages. This exposure is familiar, and risks of dissection and clamping are less than with more proximal aortic exposures. In addition, the procedure can be readily combined with other intraabdominal vascular procedures. The primary disadvantage is that the infrarenal aorta and iliac arteries are frequently calcified, increasing the technical difficulty of the proximal anastomosis.
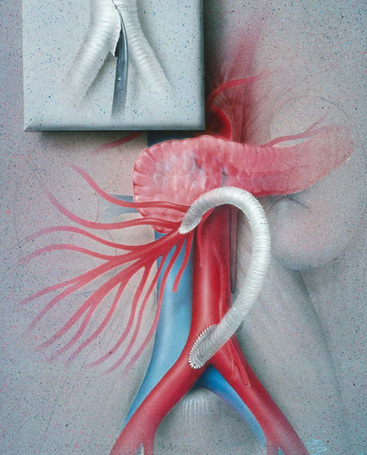
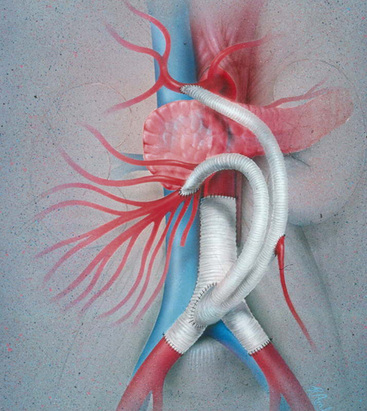
Prosthetic grafts are used most often in cases of mesenteric revascularization. Exceptions are cases complicated by bowel necrosis. For these patients, vein grafts are preferred to minimize the possibility of graft infection. Special attention to graft configuration must be paid to avoid graft kinking when the graft is placed in a retrograde configuration. A preference for the origin of the graft is from the area of the junction of the aorta and right common iliac artery (CIA), although any suitable site on the infrarenal aorta or either CIA is satisfactory. A single limb is cut from a bifurcation graft in the manner described by Wylie et al.; this provides a “flange” for sewing and prevents anastomotic stricture (Fig. 27-13). The ligament of Treitz is dissected. The proximal (inflow) anastomosis is completed first. The graft is then arranged first cephalad, then turning anteriorly and inferiorly a full 180 degrees to terminate in an antegrade anastomosis to the anterior wall of the SMA—just beyond the inferior border of the pancreas (Fig. 27-14). The graft is excluded from the peritoneal cavity by closing the mesenteric peritoneum, reapproximating the ligament of Treitz, and closing the posterior parietal peritoneum.
Antegrade Bypass
Antegrade bypasses originate from the anterior surface of the aorta proximal to the CA. The proximal aorta is exposed through the upper midline (Fig. 27-15) or, when the intraabdominal supraceliac aorta is calcified, using a low thoracoabdominal incision. Antegrade bypass provides prograde flow to the mesenteric vessels and is clearly the preferred approach in patients with contraindications to use of the infrarenal aorta or an iliac artery as a bypass origin. Visceral bypass grafts can be constructed to many supraceliac aortas with partial-occlusion clamping of the aorta, although in most cases the “partial” occlusion is near-total occlusion. Transient hepatic and renal ischemia is usually well tolerated but is a potential disadvantage to the antegrade approach. To minimize the risk associated with supraceliac aortic surgery, the procedure should be reserved for patients in whom this arterial segment is angiographically normal. Significantly diseased supraceliac aortas are dangerous origins for a visceral artery bypass.
Antegrade grafts to the SMA are normally tunneled behind the pancreas and anastomosed to the anterior wall of the SMA in end-to-side fashion (Fig. 27-16). A disadvantage of antegrade bypass is that the retropancreatic space is limited, and great care is necessary when tunneling the graft. Some surgeons advocate prepancreatic tunneling to avoid compression of the graft within the tunnel. A prepancreatic tunnel, however, places the graft in opposition to the posterior wall of the stomach and theoretically increases the possibility of graft infection. Occasionally, in the setting of very focal SMA origin disease and an easily mobilized pancreas, the antegrade bypass can be constructed entirely superior to the pancreas, obviating the need for a retropancreatic tunnel.
Postoperative Monitoring of Graft Patency
The authors use sterilized Doppler probes to confirm normal flow signals in visceral artery bypass grafts and in the native mesenteric arteries distal to the anastomotic sites after graft completion. Arterial Doppler signals should be easily detected on the antimesenteric border of the revascularized bowel as well. It is important to repeat Doppler insonation after all packs and retractors have been removed and after the viscera have been returned to the peritoneum. This approach helps minimize technical failures from graft kinking.
In contrast to the situation with other vascular repairs, continuous monitoring of the patency of visceral artery repairs is impossible in the postoperative period. Postoperative graft thrombosis may be asymptomatic or confused with other causes of postoperative pain. When symptoms do occur with resumption of oral intake, reoperation may be difficult or impossible because of postoperative inflammatory scarring. Thus, routine imaging of the reconstruction 5 to 7 days postoperatively to confirm visceral revascularization patency is prudent (Figs. 27-17 and 27-18). If the graft is occluded or otherwise unsatisfactory, reoperation is mandatory.
Postoperative Care
Patients with chronic visceral ischemia often have significant ischemic bowel injury that requires time for recovery. “Food fear” due to preoperative postprandial pain may persist at least temporarily. Prolonged periods of inability to achieve adequate oral nutrition are frequent following visceral revascularization. For this reason, total parenteral nutrition is used liberally. Some patients with severe preoperative ischemia develop postoperative revascularization syndrome, which consists of abdominal pain, tachycardia, leukocytosis, and intestinal edema. It has been attributed to intestinal vasospasm after revascularization.33 Any departure from a normal postoperative course should prompt arteriography, reexploration, or both. Delayed diagnosis of graft occlusion or intestinal necrosis is usually fatal.
Endovascular therapy for mesenteric occlusive disease
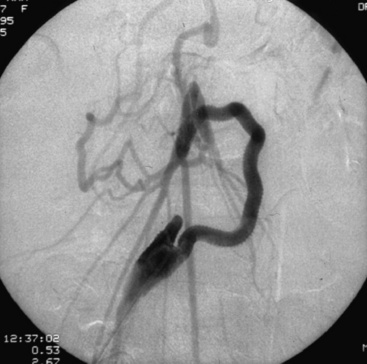
Catheter-based therapy has become an accepted method for the treatment of mesenteric occlusive disease, especially in patients who are frail and unable to tolerate an operation, and those who have short-segment SMA disease. Whereas long-segment stenoses, heavily calcified arteries, or irregular plaques are generally more amenable to operative revascularization, short-segment SMA disease allows for angioplasty and stenting (Figs. 27-19 and 27-20) with good short-term and reasonable long-term results. Initial published series reported immediate technical success with endovascular therapy, but long-term success rates were disappointing early on and inferior to open surgery. Mortality and complication rates in these studies range from 0% to 6% and 0% to 32%, respectively.
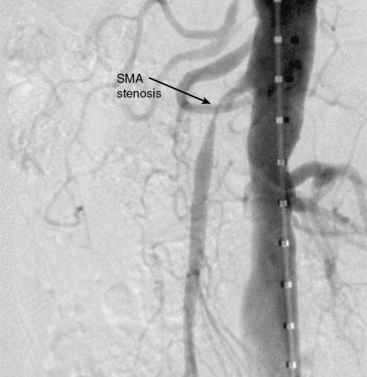
Figure 27-19 Lateral aortogram demonstrating stenosis of superior mesenteric artery (SMA) just prior to stent placement.
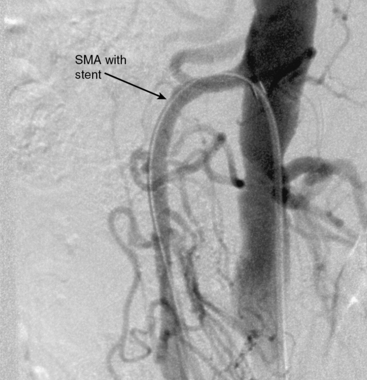
Figure 27-20 Lateral aortogram demonstrating patency of superior mesenteric artery (SMA) just after stent placement.
In a study by Sharafuddin et al., 25 patients underwent angioplasty and stenting of the SMA or CA.34 Primary patency determined by ultrasound was 92% at 6 months. Early series established that endovascular treatment of mesenteric artery stenosis is technically feasible, but no data were available with respect to long-term durability. More recently, Lee et al. showed a primary patency rate of 69% at 7 years, but freedom from recurrent symptoms was only 56%. Although endoluminal therapy for CMI carries low morbidity and mortality, long-term therapeutic benefit is not as reliably achieved; catheter-based treatment should be reserved for patients without a good surgical option.35
To date, there have been several studies, mostly retrospective, comparing the durability of endovascular revascularization for CMI with operative bypass. A comparison was made by Kasirajan et al.36 where 28 patients were treated with percutaneous angioplasty (PTA) with and without stenting and compared to a previously reported series of 85 patients treated with a variety of operative procedures for CMI. Early complication rates and mortality rates were similar, but the rate of recurrent symptoms was higher in the PTA/stent group.36 A second group, Sivamurthy et al.,37 evaluated 60 patients treated with either operative reconstruction or angioplasty and stenting for CMI. Perioperative and 3-year mortality rates were similar between the groups, and overall patency rates were not statistically different. The major difference between these groups was the number of patients free from recurrent symptoms, which was worse in the angioplasty and stent group than in the operative group (46% vs. 71%, respectively).37
A recent paper by Davies et al.38 confirms prior conclusions. Their group performed a retrospective review of 27 patients with 56 diseased vessels; 17 operative revascularizations were performed (38 vessels), and 15 endovascular reconstructions were performed (28 vessels). Both groups were similar in comorbidities and anatomy of disease. The primary patencies of the operative revascularizations and endovascular reconstructions groups was 83% and 54%, respectively. More patients in the operative revascularization group were free from symptoms at 1 and 2 years than in the endovascular repair group (100% vs. 73%, P = 0.014).38 In another recent publication, Schermerhorn et al. looked at the Nationwide Inpatient Sample to compare mortality and complication rates between patients treated with PTA and stenting and patients treated with open surgery for mesenteric ischemia. This study included over 6000 patients undergoing PTA/stent and over 16,000 patients undergoing open surgery. Overall mortality rates were lower for those patients undergoing PTA/stent than after open surgery for both acute mesenteric ischemia (16% vs. 28%) and CMI (3.7% vs. 13%).39
Given these data, endovascular reconstruction for mesenteric ischemia is feasible and can be a good option, although long-term symptom recurrence is worse with angioplasty and stenting. Endovascular options, however, could be considered in patients requiring more time to improve nutritional status prior to undergoing elective bypass operations, or perhaps in those patients who have an expected short-term lifespan.
Summary
Symptomatic CMI remains uncommon. Recognition and treatment of CMI may avoid progression to acute ischemia, alleviate symptoms, and provide durable long-term relief. This may be accomplished by a number of techniques. Single-vessel bypass to the SMA compares favorably in terms of graft patency, death from recurrent ischemia, and survival to recent reports of intestinal revascularizations employing bypasses to multiple arteries.
With the small numbers of patients in previously published series, as well as differences in patient selection, it has been difficult to demonstrate a significant benefit of one technique over others. The technical issues involved in mesenteric revascularization are basic vascular surgical principles: choice of proximal anastomosis, distal target, and conduit. It is largely accepted that prosthetic grafts are effective for mesenteric revascularization. However, there has been considerable debate surrounding the choice of inflow vessel, number of vessels revascularized, and orientation of the graft. Surgeons should choose a revascularization procedure for CMI that fits the patient. It is not necessary to rigidly adhere to a single approach. If the operation is well planned and technically well performed, excellent results can be expected.
Acute Nonocclusive Mesenteric Ischemia
Signs and symptoms
Acute NOMI occurs as a result of severe and prolonged mesenteric arterial vasospasm without evidence of arterial or venous obstruction and has been well documented. Twenty-five percent of patients with acute mesenteric ischemia have NOMI, and mortality rates between 30% and 90% have been reported.40 Patients with NOMI are often critically ill with decreased cardiac output and episodes of hypotension. They also tend to have significant comorbidities that can result in decreased intestinal perfusion. Although a hypoperfusion state is present in most patients with NOMI, some have NOMI due to visceral vasoconstriction alone, as is the case with cocaine or ergot intoxication.41,42 Early definitive diagnosis and treatment are essential for patient survival.
Recognition of factors associated with NOMI is critical to its prompt diagnosis. These include acute myocardial infarction (AMI), congestive heart failure (CHF), valvular heart disease, aortic dissection, cardiopulmonary bypass (CPB), renal failure requiring hemodialysis, sepsis, and the use of pharmacological agents such as vasopressors and digitalis.43–45
Findings on physical examination are varied and do not confirm or exclude the diagnosis of NOMI. Abdominal pain may be present and can vary widely in character, location, and intensity but is absent in 20% to 25% of patients with NOMI.44 Abdominal distention with occult or frank gastrointestinal bleeding may be present. As in occlusive acute mesenteric ischemia, laboratory values are nonspecific.
Radiological diagnosis
Radiological evaluation of patients with NOMI is similar to that of patients with occlusive acute mesenteric ischemia. Plain abdominal films are obtained to rule out a perforated viscus. If technically feasible, duplex ultrasonography may detect persistent flow in the mesenteric arteries and exclude occlusive disease.
Patients suspected to have NOMI should undergo urgent mesenteric angiography to confirm the diagnosis. Significant mortality is associated with a delayed diagnosis. Images in the anteroposterior and lateral planes are obtained. Findings of NOMI include patent mesenteric arterial trunks, with tapered or spastic narrowing of visceral artery branches and impaired filling of intramural vessels.44
Treatment
The primary treatment of NOMI is correction of the systemic condition leading to generalized hypoperfusion. The largest percentage of patients with NOMI have severe cardiac failure.46–53 Etiology of the cardiac failure is not as important as optimization of blood pressure and cardiac output with as little dependence as possible on agents that result in peripheral vasoconstriction.
In the past, digitalis was frequently used to treat congestive heart failure. Although digitalis is used much less frequently in modern practice, many patients remain on this drug or one of its derivatives. Patients treated with digitalis preparations are at risk for NOMI in the setting of worsening congestive heart failure.50 Animal experiments indicate that baseline intestinal arterial resistance is not altered by digitalis, but compared with controls, arterial resistance in animals treated with digitalis does increase in response to intestinal venous hypertension.54 Thus, patients who are treated with digitalis and have increases in portal pressure, such as occur with worsening heart failure, may be more susceptible to development of NOMI as a result of arterial mesenteric vasoconstriction. In patients with possible NOMI, digitalis preparations must be withdrawn and alternative medications used to treat underlying cardiac abnormalities.
In patients with peritonitis, an operation is required to adequately evaluate bowel viability. For this reason, catheter-based therapy alone is insufficient in patients with peritoneal findings. At operation, the bowel is inspected for viability and necrotic intestine removed. A handheld Doppler instrument is used to assess the mesenteric vessels proximally and distally.55 Intravenous (IV) fluorescein is also used to evaluate areas of possible ischemia; absent, perivascular, or patchy fluorescein patterns represent areas of ischemia.56 A “second look” procedure within 24 to 48 hours allows for reassessment of bowel viability, and additional bowel resection can be performed if necessary.
Mesenteric Venous Thrombosis
Signs and symptoms
Patients with MVT present with a wide range of symptoms ranging from asymptomatic state to an acute abdomen with peritoneal signs on physical exam. Acute mesenteric ischemia occurs in approximately 25% of patients as a result of MVT.57,58 Abdominal pain is the most common symptom and is present in approximately 80% of patients with documented MVT.58 Typically, patients present with prolonged abdominal discomfort associated with abdominal distention related to increasing intestinal edema. With transmural bowel infarction, peritoneal findings may be present in addition to other symptoms such as nausea, vomiting, and/or gastrointestinal bleeding, which can be present in 20% to 30% of patients. Leukocytosis and metabolic acidosis may accompany MVT that has resulted in bowel infarction; these patients generally have reduced intravascular volume as a result of fluid third-spacing. In addition to urgent anticoagulation, they often need aggressive fluid resuscitation. Ileus is also present in these patients, and bowel rest and decompression with nasogastric suction is required.
Mesenteric venous thrombosis can be classified into primary or secondary thrombosis. Primary MVT is associated with hereditary or acquired hypercoagulation disorders including factor V Leiden and deficiencies of protein C, protein S, and antithrombin III. Secondary MVT can result from malignancy or inflammatory disorders, and is also associated with trauma, cirrhosis, portal hypertension, or oral contraceptives.
Radiological diagnosis
Plain abdominal radiographs are usually obtained in patients with abdominal pain. Free air suggestive of a perforated viscus should be ruled out. However, in most patients with MVT, plain abdominal radiographs show a nonspecific bowel gas pattern and are generally nondiagnostic.
In patients who have minimal abdominal pain or are asymptomatic, duplex ultrasonography may be used to evaluate patency of the mesenteric veins. The examination is performed after a period of fasting, and blood flow velocities within the aorta, inferior vena cava (IVC), hepatic veins, portal vein, hepatic artery, splenic vein, and superior mesenteric vein are evaluated. Additional information that can be obtained from duplex ultrasonography include the presence or absence of ascites, recanalized umbilical vein, and/or liver mass. Hepatopetal (toward the liver) or hepatofugal (away from the liver) flow within the portal vein can also be determined.
Duplex ultrasonography is limited in the evaluation of the mesenteric veins when there is severe ascites, recent surgery or liver biopsy, and obesity. Occasionally the liver is located high in the right upper quadrant and is obscured by ribs. In patients with peritoneal findings, duplex ultrasonography is difficult to perform because of patient discomfort and significant amounts of bowel gas.
Currently, contrast-enhanced abdominal CT scanning is the diagnostic study of choice in patients suspected of having MVT. In addition to MVT, CT scanning can accurately detect portal and ovarian vein thrombosis. Other suggestive findings include bowel-wall thickening, pneumatosis intestinalis, or mesenteric edema. In one series, contrast-enhanced abdominal CT scanning was diagnostic for MVT in 90% of patients.58
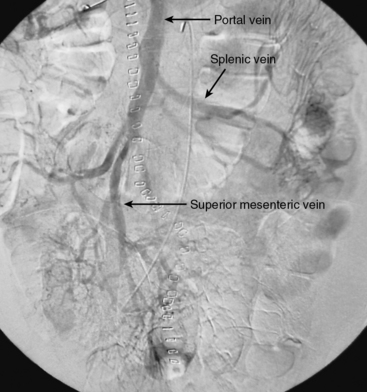
Arterioportography is indicated when associated arterial ischemia is suspected or when findings on abdominal CT scanning are equivocal. The mesenteric venous system cannot be directly punctured, but is visualized indirectly through catheter-directed contrast injections into the SMA and CA, followed by delayed filming (Fig. 27-21). Mesenteric venous thrombosis is demonstrated by a filling defect within the mesenteric veins.
Treatment
Urgent laparotomy is undertaken in patients with peritoneal findings. This is a minority of patients with MVT. Perioperative broad-spectrum antibiotics are administered. Findings at laparotomy consist of edema and cyanotic discoloration of the mesentery and bowel wall with thrombus involving the distal mesenteric veins. Complete thrombosis of the superior mesenteric vein is rare, occurring in only 12% of patients undergoing laparotomy for suspected MVT.59 The arterial supply to the involved bowel is usually intact. Nonviable bowel is resected and primary anastomosis performed. If viability of the remaining bowel is in question, a repeat “second look” operation is performed in 24 to 48 hours. Thrombolytic therapy or surgical thrombectomy of mesenteric veins is not required; these interventions are not usually technically successful.
In patients without peritoneal findings, anticoagulation with IV unfractionated heparin (UFH) is promptly initiated, and the patient is observed with serial abdominal examinations while maintaining bowel rest. Ileus may be prolonged, so hyperalimentation should be considered early. Once the patient’s clinical status improves, oral intake can be cautiously introduced. A search for a predisposing primary or secondary hypercoagulable condition is required. In the interim, the patient is transitioned to oral anticoagulation over 3 to 4 days, once intestinal function has returned. Lifelong anticoagulation is usually maintained, especially in cases of idiopathic MVT or when an uncorrectable hypercoagulable state has been identified.
1 Char D.J., Cuadra S.A., Hines G.L., et al. Surgical intervention for acute intestinal ischemia: experience in a community teaching hospital. Vasc Endovascular Surg. 2003;37:245.
2 Sreenarasimhaiah J. Diagnosis and management of intestinal ischemic disorders. BMJ. 2003;326:1372.
3 Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256:93–101.
4 Lin P.H., Bush R.L., Lumsden A.B. Treatment of acute visceral artery occlusive disease. In: Zelenock G.B., Huber T.S., Messina L.M., et al. Mastery of vascular and endovascular surgery. Philadelphia: Lippincott, Williams & Wilkins; 2006:295.
5 Jager K., Bollinger A., Valli C., et al. Measurement of mesenteric blood flow by duplex scanning. J Vasc Surg. 1986;3:462.
6 Moneta G.L., Taylor D.C., Helton W.S., et al. Duplex ultrasound measurement of postprandial intestinal blood flow: effect of meal composition. Gastroenterology. 1988;95:1294.
7 Nicholls S.C., Kohler T.R., Martin R.L., et al. Use of hemodynamic parameters in the diagnosis of mesenteric insufficiency. J Vasc Surg. 1986;3:507.
8 Moneta G.L., Yeager R.A., Dalman R., et al. Duplex ultrasound criteria for diagnosis of splanchnic artery stenosis or occlusion. J Vasc Surg. 1991;14:511.
9 Moneta G.L., Lee R.W., Yeager R.A., et al. Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg. 1993;17:79.
10 Bowersox J.C., Zwolak R.M., Walsh D.B., et al. Duplex ultrasonography in the diagnosis of celiac and mesenteric artery occlusive disease. J Vasc Surg. 1991;14:780.
11 Zwolak R.M., Fillinger M.F., Walsh D.B., et al. Mesenteric and celiac duplex scanning: a validation study. J Vasc Surg. 1998;27:1078.
12 Gentile A.T., Moneta G.L., Lee R.W., et al. Usefulness of fasting and postprandial duplex ultrasound examinations for predicting high-grade superior mesenteric artery stenosis. Am J Surg. 1995;169:476.
13 Aschoff A.J., Stuber G., Becker B.W., et al. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging. 2008;34:345–357.
14 Shih M.C., Hagspiel K.D. CTA and MRA in mesenteric ischemia: part 1, role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188:452–461.
15 Chow L.C., Chan F.P., Li K.C. A comprehensive approach to MR imaging of mesenteric ischemia. Abdom Imaging. 2002;27:507–516.
16 Zeller T., Rastan A., Sixt S. Chronic atherosclerotic mesenteric ischemia. Vasc Med. 2010;15:333–338.
17 Beebe H.G., MacFarlane S., Raker E.J. Supraceliac aortomesenteric bypass for intestinal ischemia. J Vasc Surg. 1987;5:749.
18 Kruger A.F., Walker P.J., Foster W.J., et al. Open surgery for atherosclerotic chronic mesenteric ischemia. J Vasc Surg. 2007;46:941–945.
19 Foley M.I., Moneta G.L., Abou-Zamzam A.M., et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32:37.
20 Dunphy J.E. Abdominal pain of vascular origin. Am J Med Sci. 1936;192:109.
21 Shaw R.S., Maynard E.P.III. Acute and chronic thrombosis of the mesenteric arteries associated with malabsorption: a report of two cases successfully treated with thromboembolectomy. N Engl J Med. 1958;258:874.
22 Hollier L.H., Bernatz P.E., Pairolero P.C., et al. Surgical management of chronic intestinal ischemia: a reappraisal. Surgery. 1991;90:940.
23 McAfee M.K., Cherry K.J., Naessens J.M., et al. Influence of complete revascularization on chronic mesenteric ischemia. Am J Surg. 1992;164:220.
24 Park W.M., Cherry K.J.Jr, Chua H.K., et al. Current results of open revascularization for chronic mesenteric ischemia: a standard for comparison. J Vasc Surg. 2002;35:853.
25 Kieny R., Batellier J., Kretz J. Aortic reimplantation of the superior mesenteric artery for atherosclerotic lesions of the visceral arteries: sixty cases. Ann Vasc Surg. 1990;4:122.
26 White C.J. Chronic mesenteric ischemia: diagnosis and management. Prog Cardiovasc Dis. 2011;54:36–40.
27 Gentile A.T., Moneta G.L., Taylor L.M.Jr, et al. Isolated bypass to the superior mesenteric artery for intestinal ischemia. Arch Surg. 1994;129:926.
28 Rapp J.H., Reilly L.M., Qvarfordt P.G., et al. Durability of endarterectomy and antegrade grafts in the treatment of chronic visceral ischemia. J Vasc Surg. 1986;3:799.
29 McMillan W.D., McCarthy W.J., Bresticker M., et al. Mesenteric artery bypass: objective patency determination. J Vasc Surg. 1995;21:729.
30 Moneta G.L. Screening for mesenteric vascular insufficiency and follow-up of mesenteric bypass procedures. Semin Vasc Surg. 2001;14:186.
31 Oderich G.S., Malgor R.D., Ricotta J.J. Open and endovascular revascularization for chronic mesenteric ischemia: tabular review of the literature. Ann Vasc Surg. 2009;23(5):700–712.
32 Connolly J.E., Stemmer E.A. Intestinal gangrene as the result of mesenteric arterial steal. Am J Surg. 1973;126:197.
33 Gewertz B.L., Zarins C.K. Postoperative vasospasm after antegrade mesenteric revascularization: a report of three cases. J Vasc Surg. 1991;14:382.
34 Sharafuddin M.J., Olson C.H., Sun S., et al. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. 2003;38:692.
35 Lee R.W., Bakken A.M., Palchik E., et al. Long-term outcomes of endoluminal therapy for chronic atherosclerotic occlusive mesenteric disease. Ann Vasc Surg. 2008;22:541–546.
36 Kasirajan K., O’Hara P.J., Gray B.H., et al. Chronic mesenteric ischemia: open surgery versus percutaneous angioplasty and stenting. J Vasc Surg. 2001;33:63–71.
37 Sivamurthy N., Rhodes J.M., Lee D., et al. Endovascular versus open mesenteric revascularization: immediate benefits do not equate with short-term functional outcomes. J Am Coll Surg. 2006;202:859–867.
38 Davies R., Wall M.L., Silverman S.H., et al. Surgical versus endovascular reconstruction for chronic mesenteric ischemia: a contemporary UK series. Vasc Endovascular Surg. 2009;43(2):157–164.
39 Schermerhorn M.L., Giles K.A., Hamdan A.D., et al. Mesenteric revascularization: management and outcomes in the United States, 1988–2006. J Vasc Surg. 2009;50:341–348.
40 Klotz S., Vestring T., Rotker J., et al. Diagnosis and treatment of nonocclusive mesenteric ischemia after open heart surgery. Ann Thorac Surg. 2001;72:1583.
41 Green F.L., Ariyan S., Stausel H.C.Jr. Mesenteric and peripheral vascular ischemia secondary to ergotism. Surgery. 1977;81:311.
42 Myers S.I., Clagett G.P., Valentine R.J., et al. Chronic intestinal ischemia caused by intravenous cocaine use: report of two cases and review of the literature. J Vasc Surg. 1996;23:724.
43 Diamond S., Emmett M., Henrich W. Bowel infarction as a cause of death in dialysis patients. JAMA. 1986;256:2545.
44 Bassiouny H. Nonocclusive mesenteric ischemia. Surg Clin North Am. 1997;77:319.
45 Valentine R., Whelan T., Meyers H. Non-occlusive mesenteric ischemia in renal patients: recognition and prevention of intestinal gangrene. Am J Kidney Dis. 1990;15:598.
46 Zeier M., Weisel M., Ritz E. Non-occlusive mesenteric infarction in dialysis patients: risk factors, diagnosis, intervention and outcome. Int J Artif Organs. 1992;15:387.
47 John A., Tuerff S., Kerstein M. Nonocclusive mesenteric infarction in hemodialysis patients. J Am Coll Surg. 2000;190:84.
48 Aldrete J.S., Hansy S.Y., Laws H.L., et al. Intestinal infarction complicating low cardiac output states. Surg Gynecol Obstet. 1977;144:371.
49 Williams L.F., Anastasia L.F., Hasiotis C.A. Nonocclusive mesenteric infarction. Am J Surg. 1967;114:376.
50 Britt L.G., Cheek R.C. Nonocclusive mesenteric vascular disease: clinical and experimental observations. Ann Surg. 1969;169:704.
51 Garofalo M., Borioni R., Nardi P., et al. Early diagnosis of acute mesenteric ischemia after cardiopulmonary bypass. J Card Surg. 2002;43:455–459.
52 Venkateswaran R.V., Charman S.C., Goddard M., et al. Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication? Eur J Cardiothorac Surg. 2002;22:534–538.
53 Landreueau R.J., Fry W.J. The right colon as a target organ of nonocclusive mesenteric ischemia. Arch Surg. 1990;125:591.
54 Kim E.H., Gewertz B.L. Chronic digitalis administration alters mesenteric vascular reactivity. J Vasc Surg. 1987;5:382.
55 Hobson R.W.II, Wright C.B., Rich N.M., et al. Assessment of colonic ischemia during aortic surgery by Doppler ultrasound. J Surg Res. 1976;20:231.
56 Gloviczki P., Bergman R.T., Stanson A.W., et al. The role of intravenous fluorescein in the detection of colon ischemia during aortic reconstruction. Int Angiol. 1992;11:281.
57 Rhee R.Y., Gloviczki P., Jost C., et al. Acute mesenteric venous thrombosis. In: Gloviczki P., Yao JST. Handbook of venous disorders. New York: Arnold; 2001:321.
58 Morasch M.D., Ebaugh J.L., Chiou A.C., et al. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg. 2001;34:680.
59 Rhee R.Y., Gloviczki P., Mendonca C.T., et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20:688.