Chapter 47 Atheroembolism
Atheroembolism is a rare but serious disorder with significant morbidity from stroke, renal failure, and limb loss. This systemic disorder affects multiple organs and carries a high mortality rate. Atheroembolism can be a single event or recurrent. It can occur spontaneously or following an invasive vascular procedure. It can originate from atherosclerotic or aneurysmal disease and involve single or multiple sites. There is no specific laboratory test that can reliably distinguish cholesterol embolization from other disorders. A definitive diagnosis can only be made with biopsy of involved tissue and histological examination. A high index of clinical suspicion is necessary because atheroembolism may mimic a number of other disorders, leading to potential misdiagnosis. The focus of this chapter will be review of pathophysiology, precipitating factors, clinical syndromes, and management of atheroembolic disease. Prognosis is determined by the extent of systemic involvement and risk of recurrent episodes. As in many vascular disorders, prevention is the best treatment.
Atheroembolism occurs when tiny fragments of an atherosclerotic plaque (in particular, cholesterol crystals) break off from a proximal artery and travel distally in the circulation, ending up in small arteries downstream from its origin. The consequence of this event is microvascular obstruction with tissue ischemia. The abdominal aorta is the most common origin for atheroembolism to the abdominal organs and lower extremities, but any artery with atheromatous disease may be a potential embolic source. End-organ targets include the brain, eye, heart, kidney, gastrointestinal tract, fingers, toes, and skin. The kidneys and skin are the two most common targets in many cases.1 In the setting of an elderly patient who develops sudden onset of pain and ischemia of the distal extremities and unexplained renal failure after an invasive angiographic procedure, atheroembolism should be high on the list of likely diagnoses.2
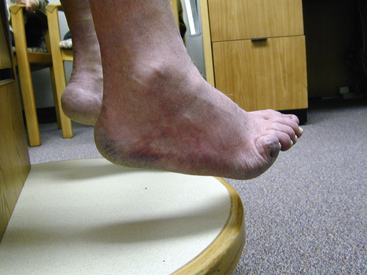
Atheroembolism can present in a number of distinct clinical syndromes (Box 47-1). The blue toe syndrome occurs when arteries to the distal parts of the feet and toes become obstructed by atheromatous embolization causing toe ischemia (Fig. 47-1). Livedo reticularis (localized mottling of the skin) occurs when the atheroembolism involves small cutaneous vessels (Figs. 47-2 and 47-3). When present, this can be an important diagnostic indicator of atheroembolism. Acute and chronic kidney failure can result from aortic or renal artery atheroembolism. Atheroemboli can also travel to the mesenteric arteries, causing intestinal necrosis, or to the splenic, hepatic, or pancreatic arteries, causing localized infarction. Transient ischemic episodes and stroke may result from atheromatous disease of the aortic arch, internal carotid, or vertebral arteries. Atheroembolism to the retinal arteries may present with temporary horizontal monocular visual loss called amaurosis fugax. Funduscopic examination may identify a bright reflection from a cholesterol crystal in a retinal artery known as a Hollenhorst plaque.3 The unifying cause of all atheroembolic syndromes is the presence of atheromatous disease in a proximal artery and ischemic damage to a distal organ or extremity when these fragments embolize and lodge in distal vessels.
![]() Box 47-1 Clinical Syndromes and Manifestations of Atheroembolism
Box 47-1 Clinical Syndromes and Manifestations of Atheroembolism
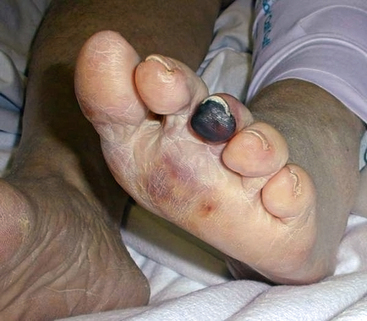
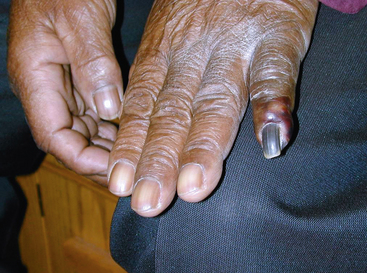
Figure 47-1 Classic blue toe syndrome. Note impending infarction of affected third toe, with livedo of the plantar surface.
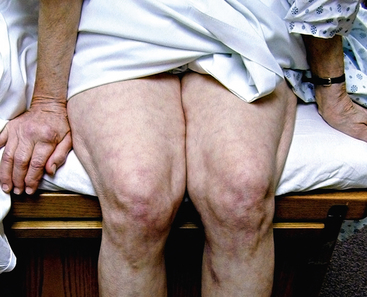
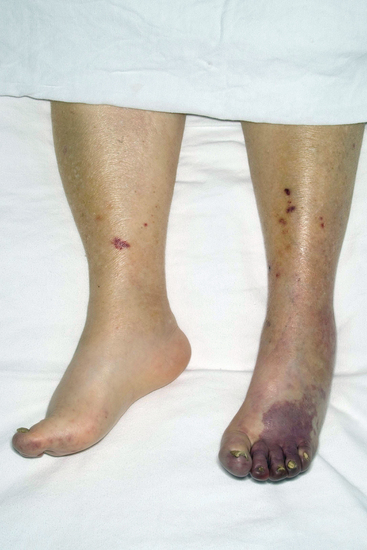
Figure 47-2 Patient with previously undiagnosed abdominal aortic aneurysm (AAA) presenting with atheroembolism to lower extremities. Note typical lacy reticular skin pattern of both thighs.
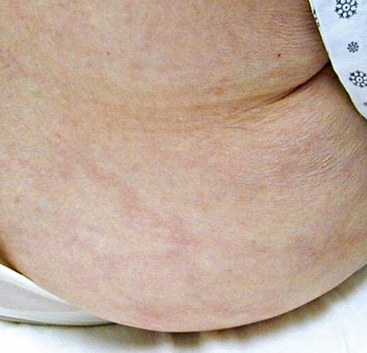
Figure 47-3 Atheroembolism to buttock and flank. Same patient as in Figure 47-2, with renal failure and livedo reticularis as presenting symptoms of previously unknown abdominal aortic aneurysm (AAA).
A number of terms for this syndrome are used interchangeably in the literature, including cholesterol crystal embolization, atheromatous embolization, and atheroembolism. Vascular medicine covers a great deal of internal medicine, and atheroembolism should be in the differential diagnosis of many diseases including vasculitis, infective endocarditis, malignancy, hematological diseases, atypical infections, Raynaud’s syndrome, and acute and chronic renal failure. Atheroembolism has been called “the great masquerader” because it may resemble many other conditions.4 Diagnosis of atheroembolism is usually made on the basis of clinical suspicion, by history and examination, but most importantly by an astute clinician who considers this entity when presented with unusual vascular diseases.
Pathobiology
Atheroembolism originates from atherosclerotic plaque. The pathobiology of atherosclerosis is reviewed in detail in Chapter 8.
Etiology
Atheroembolism may occur spontaneously or be precipitated by angiographic or surgical procedures (iatrogenic). Earlier reports indicated spontaneous episodes of atheroembolism were more common.5,6 Today with increased numbers of vascular procedures, iatrogenic catheter-induced atheroembolism outnumbers spontaneous cases. Currently, over three fourths of atheroembolic renal disease is procedure-related, occurring during or after an angiographic or endovascular procedure7,8 (Figs. 47-4 through 47-6).
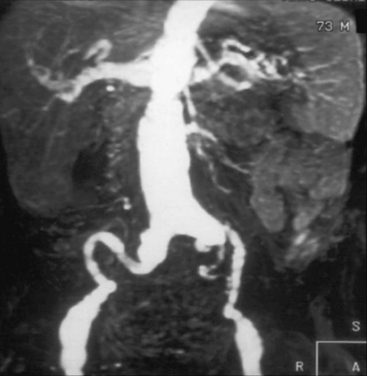
Figure 47-4 Abdominal magnetic resonance imaging (MRI) showing diffuse atherosclerosis of aorta, with “arteriomegaly.” This disease has a high risk of atheroembolism.
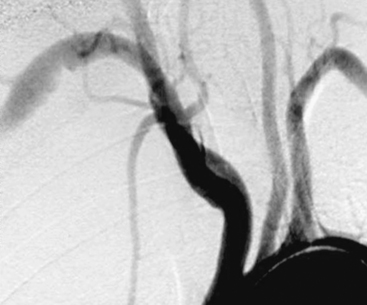
Figure 47-5 Arch aortogram showing right subclavian aneurysm with ulcerated atheromatous disease in nonsmoking young woman with thoracic outlet syndrome, presenting with atheroemboli to fingers.
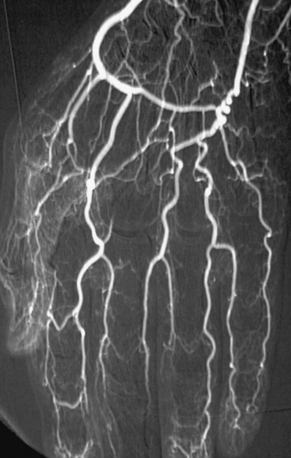
Figure 47-6 Upper-extremity angiogram in patient with hypothenar hammer syndrome, multiple digital artery occlusions resulting in critical ischemia of fingers. Note abnormal ulnar artery tortuosity secondary to local trauma to hand.
Spontaneous Atheroembolism
Who is at risk?
Spontaneous atheroembolism occurs in older patients with advanced atherosclerosis. In Fine’s review of 221 cases of atheroembolism in the English literature, he noted a predominance of patients with underlying atherosclerotic disease including cardiac, carotid, and kidney disease. In particular, many had significant preexisting chronic kidney disease (CKD) evidenced by elevated serum creatinine (Cr) (CKD stage 3 or 4). There was also a high incidence of aortic aneurysms, present in 25% of these patients.5 Many patients with atheroembolic events present with multisystem manifestations. Common presentations of spontaneous atheroembolism included blue toe syndrome, livedo, and progressive renal failure. Stroke/transient ischemic events due to carotid atherosclerosis is one of the best examples of a spontaneous atheroembolic episode. The mechanism of blue toe syndrome has been likened to a brain transient ischemic attack (TIA), but affecting a lower extremity.9 Today, many cases of unexplained progressive renal failure may be due to unsuspected atheroembolism.
In most series, males outnumber females, with mean ages ranging from 63 to 69 years.1,5,10 Skin lesions and renal failure are often the two most common manifestations.1,5 Skin lesions may be the initial clinical sign of atheroembolization (see Fig. 47-3), of which blue toes and livedo reticularis make up 88% of cutaneous findings.1 African Americans are less likely to be diagnosed with atheroembolism, perhaps because the faint cutaneous pattern of livedo is more difficult to see in skin that is more deeply pigmented11 (Fig. 47-7).
How common is spontaneous atheroembolism?
Autopsies studies from the 1940s found an atheroembolic incidence of 3.4% (9 of 267 patients with aortic atherosclerosis).12 A larger and more recent autopsy study involving 2126 elderly patients over a period of 7 years found only 16 cases of spontaneous atheroembolism (incidence <0.1%) despite the high prevalence of severe aortoiliac atheromatous disease and aortic aneurysms.13 Another autopsy study of 372 patients found spontaneous cholesterol embolization occurred in only seven individuals; all were over age 60, and all but one were male, for an incidence of 1.9%. Lesions of different ages were noted, suggesting recurrent episodes to the kidneys and spleen.10 A review of autopsy data at Johns Hopkins from 1973 to 1995 found 0.7% had histological features of atheroembolism.14
The actual incidence of spontaneous atheroembolism is difficult to determine; symptoms may be vague, and clinical features can be subtle or not recognized.15
Procedure-Related Atheroembolism
Cardiovascular surgery
Atheroembolism can occur as a complication of any invasive cardiac or vascular procedure or operation. Atheroembolism has been reported following abdominal aortic aneurysm (AAA) repair, aortoiliac bypass, and aortic and renal arteriography. Atheromatous debris can be dislodged during left heart catheterization, external cardiac message, blunt abdominal trauma, coronary artery bypass surgery, and many endovascular procedures.2,16
The advent of aortography in the 1930s transformed our ability to make accurate vascular diagnosis and expanded treatment options, but brought with it the risk of atheroembolism, especially in patients with atheromatous or aneurysmal disease. A 30% incidence of atheroembolism after abdominal aortography was reported in patients who subsequently died within 6 months of the procedure. The organs most commonly affected were the kidneys and spleen.17
In the 1950s, Thurlbeck and Castleman first reported atheroembolism associated with vascular surgery and attributed this to operative manipulations that included arterial incisions, cannulation, and clamping of major arteries. Postmortem examination of those who died following AAA repair found cholesterol embolization to the kidneys in as many as 77% of patients.18 In a more recent large retrospective series of 1011 patients undergoing angiographic procedures prior to aortic or infrainguinal vascular surgery, 2.9% (29 patients) were identified with atheroembolism. The majority of iatrogenic cases were attributed to aortography as opposed to surgery. The primary sources of embolism in these patients were the abdominal aorta, iliac arteries, and femoropopliteal arteries.19
Massive atheroembolism following aortoiliac stent placement for treatment of aortic aneurysmal disease has been reported but is uncommon.20,21 A study using Doppler ultrasound to identify microembolism found a significantly higher degree of peripheral embolization during endovascular aneurysm repair, compared to conventional surgical aneurysm repair.22
Atheroembolism can also occur after coronary artery bypass grafting (CABG) and valve surgery. Fatal myocardial infarction (MI) due to atheroembolism has been reported during coronary artery bypass operations.23 Procedures that provoke atheroembolism include aortic cannulation, initiation of cardiopulmonary bypass (CPB), and transecting and anastomosis of bypass grafts to the ascending aorta during cardiac surgery. Atheroemboli may originate from the aortic root at the origin of vein grafts or from ruptured plaque in a coronary artery. In one series of 29 patients who died after cardiac surgery, atheroembolism was the causative factor in 22% of all deaths. In this series, atheroembolism to the coronary circulation caused cardiac failure; to the brain caused massive stroke; and to the gastrointestinal tract caused abdominal pain and bleeding.14 Fortunately this is rare. Of 4095 CABG procedures, atheroembolism was found in only nine patients, for an incidence of 0.22%.23 Those undergoing reoperation were found to have a higher incidence of 2.3%.23
Atherosclerosis involving the ascending aorta is a major risk factor for stroke during cardiac surgery. A large autopsy study of 221 patients found severe atherosclerosis of the ascending aorta in patients who died from atheroembolism after cardiac procedures. Atheroembolism occurred in 46 of 123 patients with severe ascending aortic disease, but only 2 of 98 (2%) without ascending aortic disease.24 The incidence of atheroembolism was three times as high after CABG than valve surgery (26.1% vs. 8.9%).24 Older patients with more advanced atheromatous disease of the ascending aorta were at the highest risk. Atheroemboli traveled to the brain in 16%, the spleen in 11%, kidney in 10%, and pancreas in 7%. Two thirds of patients had multiple sites of atheroembolism.24 A 12% stroke risk during CPB was noted in a more recent study if aortic arch atheromas are seen with intraoperative transesophageal echocardiography (TEE).25
Transcranial Doppler (TCD) has documented that the greatest number of microembolic events was found during aortic clamping and initiation of bypass.26 A study where an intraaortic filter was deployed and left in place until the patient was weaned from bypass found that 62% of filters contained atheroma in addition to platelet and fibrin strands.27
Off-pump CABG may reduce cerebral damage due to microembolism.28 A study comparing effects of CABG with and without CPB assessed retinal microembolization by fluorescein angiography and fundus photography. Doppler high-intensity transient signals (HITS) was used to assess emboli to the brain. Five of nine pump patients had retinal microvascular damage, but none was evident in the off-pump patients. Doppler HITS were 20 times more frequent in the CPB patients.28 Off-pump coronary artery bypass may have less risk of atheroembolism by avoiding arterial cannulation and the abrasive effect of CPB on the arterial wall.24,28
Cardiac catheterization
Cholesterol embolization after left heart catheterization is rare but can be devastating when it occurs. Coronary angiography is the most common invasive procedure associated with atheroembolism.8 Clinically detectable cholesterol embolization occurring after cardiac catheterization has resulted in stroke, renal failure, mesenteric ischemia, and lower-extremity tissue loss with gangrene. In some situations it has a high fatality rate.29–33
A retrospective British study by Drost et al. reported 7 cases out of a total of 4587 cardiac catheterization procedures. Most had extensive atherosclerosis and suffered multisystem atheroembolization, with a retinal embolism in one patient, renal failure in five patients, and three requiring toe amputations. Six of the seven had extensive atherosclerosis. Four of these patients died within 4.5 months of this procedure.29
In a large prospective Japanese study, Fukumoto et al. prospectively reviewed 1786 consecutive patients undergoing left heart catheterization in a multicenter study. Diagnostic criteria for atheroembolism included livedo reticularis, blue toe syndrome, digital gangrene, or renal dysfunction. They found an incidence of 1.4 % (25 patients), of which cutaneous findings and renal failure were the two most common clinical findings. If only definite cases were counted, the incidence was lower at 0.75%. Prognosis is poor in some patients, with an in-hospital mortality rate of 16 %.34
Saklayen et al. prospectively evaluated 267 patients undergoing coronary angiography at a Veterans Administration (VA) medical center. A rise in Cr of 0.5 mg/dL or more at 3 weeks after the procedure was the main indicator of atheroembolism. They found an incidence of atheroembolic renal dysfunction of 1.9% (5/263 of patients undergoing coronary angiography).31 Frock identified 17 patients with atheroembolic renal disease out of 14,998 angiographic procedures, an incidence of 0.1%.35 In a prospective study of 1579 patients, Johnson et al. also found the incidence of cholesterol embolization to be very low: a single patient in 1579 coronary angiograms (0.06%).36
Passage of a catheter into the aorta for any endovascular procedure may not only loosen atheromatous plaque but can also scrape off aortic debris into the coronary guiding catheters. During contrast injection, this debris could be injected into the coronary or cerebral artery. In a study of 1000 consecutive coronary interventions, the amount of atheromatous material entering a guiding catheter from passage up the aorta was assessed by allowing blood to passively exit the back of the catheter into a sterile towel. Depending on the catheter shape, aortic debris was recovered in 24% to 65% of interventional cases. Surprisingly, the finding of aortic debris did not correlate with clinically apparent neurological, coronary, or renal ischemic events. Allowing adequate back-bleeding of guiding catheters before injecting contrast was suggested to decrease the risk of atheroembolism found in the guiding catheter from scraping the wall of the aorta during placement.37
Today with more advanced surgical techniques and greater awareness of atheroembolic risk, the incidence of atheroembolism during vascular and cardiac procedures is less common. The promise of distal protection devices to decrease the risk of atheroembolism during a procedure is still being assessed. Atheromatous debris can be recovered from the majority of angioplasty procedures. Development of more flexible catheters and lower-profile devices, along with improved operator technique, should allow for lower incidence of atheroembolic events in the future.38
Intraaortic Balloon Pumps
Karalis et al. addressed the risk of atheroembolism in patients undergoing catheterization when they have a so-called shaggy aorta. In this study, 70 patients were identified with aortic debris found on echocardiography, and 10 had a procedure-related embolic episode. A brachial approach may be a better option in these patients.39
Intraaortic balloon pumps have especially high potential for embolization when placed in an aorta with atherosclerotic debris. In one study, 5 of 10 patients (50%) had an embolic event related to placement of an intraaortic balloon pump.39 If aortic debris is mobile, risk of embolization is especially high.
Spinal cord atheroembolism is a very rare complication of angiography.13,40,41 Spinal cord infarction likely occurs secondary to embolic occlusion of small spinal cord arteries.
Anticoagulation/Thrombolysis Issues
The concern that warfarin could precipitate atheroembolism was first raised in 1961 by Fedar and Auerbach, who reported six patients who had developed painful purple toes after initiation of an oral anticoagulant.42 Since then, anecdotal reports have linked warfarin with spontaneous atheroembolism. Clinical improvement of atheroembolism manifestations, including livedo reticularis, abdominal pain, and even renal function, has been reported after oral anticoagulants were discontinued.43,44 Other anecdotal reports have indicated improvement in skin necrosis and livedo when low-molecular-weight heparin (LMWH) was discontinued.43,45 It is hypothesized that anticoagulation could dissolve or prevent formation of a protective thrombus over an atherosclerotic plaque, leaving it vulnerable to rupture and embolization.
To the contrary, a number of large studies have shown no increased risk of atheroembolism in patients treated with warfarin. Blackshear et al. addressed concerns of warfarin anticoagulation in patients with atrial fibrillation and documented aortic plaque. The SPAF III (Stroke Prevention in Atrial Fibrillation) trial looked at patients with atrial fibrillation and aortic plaque documented by TEE and found that patients assigned to warfarin therapy had an annual cholesterol embolization rate of 0.7 per patient-year, which was lower than those randomized to warfarin plus aspirin. The authors conclude that “elderly patients with AF and aortic plaque can receive adjusted-dose warfarin with a relatively low risk of cholesterol embolism.”46 The French Study of Aortic Plaques in Stroke found no difference in recurrent brain infarction in patients receiving warfarin compared to those on aspirin.47
Many cardiovascular patients are on anticoagulation, and in most case reports, causation is difficult to prove. Elderly patients are most likely to have advanced atherosclerosis and yet have chronic disorders such as atrial fibrillation requiring long-term anticoagulation. Often these patients had undergone other procedures including angiography, which is more likely an explanation for atheroembolic events. Delayed recognition of an acute event or recurrent showers in patients with shaggy aortas may account for the temporal association of atheroembolism with an oral anticoagulant.48
A sensible conclusion is to continue anticoagulation when compelling conditions exist, such as atrial fibrillation and thromboembolism, but consider stopping it if there is a lesser indication.38
Atheroembolism has also been reported to occur after thrombolytic therapy for myocardial infarction.49–52 In some of these case reports, atheroembolism occurred in the absence of any invasive procedure, therefore implicating thrombolysis as a possible culprit. The mechanism of atheroembolism is thought to be dissolution of thrombus overlying atheromatous plaque, exposing ulcerated plaque to the arterial circulation that can embolize distally. Large trials of thrombolysis for acute myocardial infarction (AMI), including GISSI 2 and GUSTO, did not cite atheroembolism as a complication of thrombolytic therapy, so concern of atheroembolization should not be a reason to withhold thrombolysis.53
Blankenship et al. prospectively followed 60 patients with AMI who later underwent CABG. Half of these patients received thrombolytic therapy and half did not. It was concluded the prevalence of cholesterol embolization was not higher in those who received thrombolytic therapy.54
Atheroembolic Syndromes
Livedo and Skin Atheroembolism
Skin findings are the earliest and at times the only clinical finding of atheroembolism. Livedo reticularis is the most common manifestation of skin involvement, noted in 49% of patients.55,56 The incidence of cutaneous manifestations of atheroembolism ranges from 35% to 96%.1,57,58 Livedo has been labeled an underutilized clue to the diagnosis of atheroembolism because it should be considered an important and common indicator of atheroembolism elsewhere—in particular, to the kidneys or mesenteric organs.55,59 For example, the suspicion of renal atheroembolism is markedly strengthened by the finding of livedo reticularis affecting the trunk or abdomen.
Livedo reticularis has a classic appearance as a reddish-blue lacy or netlike color pattern of the skin (Fig. 47-8). It is noted when the cutaneous venous plexus becomes visible owing to desaturated venous blood. In the presence of atheroembolism, small arteries are obstructed, reducing flow into the venous plexus and resulting in stasis of deoxygenated blood.60 Characteristics of livedo reticularis include blanching with local pressure. It is more prominent when the patient is upright and may not be apparent if the patient is examined in the supine position.55 Atheroembolism is less frequently diagnosed in nonwhite individuals because darker skin may disguise visible manifestations.11 Livedo is most commonly seen on the feet and legs, but thighs, buttocks, lower back, abdomen, and upper-extremities can also be affected, depending on the source of the atheroembolism.1
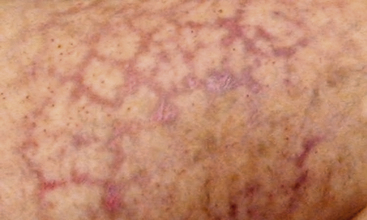
Figure 47-8 Unusual nonblanching livedo reticularis of thigh in patient with suspected livedo vasculitis.
Less common cutaneous findings in atheroembolism include splinter hemorrhages, petechiae, purpura, and erythematous nodules. Cholesterol embolism to the skin has been called a pseudovasculitic syndrome.61 Livedo due to atheroembolism has been mistaken for small-vessel vasculitis in 16% of patients.62 The diagnosis of atheroembolism can be confirmed by skin biopsy, and is positive in 92% of cases.57,62
In young women, livedo reticularis may be a common normal finding due to cold-induced vasospasm of skin vessels, and classically disappears with warming. It can also be seen in a number of diseases including collagen vascular disorders, antiphospholipid antibody syndrome, disseminated intravascular coagulation (DIC), vasculitides such as systemic lupus erythematosus (SLE) and polyarteritis nodosa, infective endocarditis, cryoglobulinemia, and hyperviscosity disorders.38
Atheroembolic Renal Disease
About half of all reported cases of atheroembolism involve the kidney.7 The kidney receives a major percentage of the cardiac output and is the most common site for atheroembolism, followed by skin and gastrointestinal tract.1,63 In clinical practice, it has been estimated that up to 10% of all cases of acute renal failure may be due to atheroembolism.64
Atheroembolic renal disease is defined as a syndrome of renal failure secondary to obstruction of small kidney arteries, arterioles, and glomerular capillaries by cholesterol crystal atheroembolism dislodged from the aorta or proximal renal arteries.65 Renal atheroembolism may occur spontaneously in patients who have advanced atheromatous disease of the abdominal aorta, but more frequently it occurs as a complication of an angiographic or endovascular procedure. As noted earlier, passage of a catheter or guidewire through the aorta or renal arteries may dislodge atheromatous plaque fragments that travel to the kidneys, where they remain in small vessels. Today, approximately three fourths of renal atheroembolization cases are iatrogenic secondary to invasive procedures, in particular angiography. Coronary angiography is the most common angiographic procedure.7,8
The exact incidence of spontaneous atheroembolic renal disease is difficult to determine because most studies are retrospective. However, approximately 20% of atheroembolic episodes to the kidneys are thought to be unprovoked spontaneous episodes.65 In addition, many atheroembolic episodes are subclinical, difficult to diagnose, and may be missed unless specifically looked for.
In renal biopsy studies, the prevalence of renal atheroembolism in all patients and age groups is quite low, ranging from 0.31% to 2.4%.8,10 Moolenaar and Lamers reviewed the Netherlands experience of 842 cases of cholesterol crystal embolization in the Dutch National Pathology Information System and found an incidence of 6.0 cases per million population. Among autopsy reports, they also found a low incidence of 0.31%.63 In other renal biopsy series, Greenberg et al. found 24/500 had atheroembolic findings.66 In another large series of 755 renal biopsies, atheroemboli were discovered in 8 patients (1.1%).67 Selection bias may account for the low prevalence; these patients were selected for biopsy because of unexplained recent worsening of renal function.
Atheroembolism to the kidneys can also occur during any vascular surgical procedure, in particular AAA resection, renal revascularization, and aortoiliac or aortofemoral bypass. In those who died after aortic surgery or an angiographic procedure, the finding of atheroembolism at autopsy ranged from 12% to 77%.8 Atheromatous emboli to the kidney was documented in 77% of patients following surgical repair of abdominal aortic aneurysms.2
Atheroembolism to the kidneys also occurs as complication of endovascular procedures (Box 47-2). Modi and Rao reports 85% of patients presenting with atheroembolic renal disease underwent an invasive vascular procedure within the prior 3 months (abdominal aorta, coronary, or carotid angiography).65 Olin has stated that atheroembolism likely occurs in every patient undergoing an endovascular procedure (renal artery angioplasty and stent) for atherosclerotic renal disease.68 A study by Kawarada et al. used intrarenal duplex ultrasound to detect microembolic signals and found that emboli occurred in all 13 patients undergoing renal artery stent implantation, in particular post dilation of the stent.69 Underappreciation of this frequent occurrence is because clinicians attribute acute renal failure after a procedure to another cause such as contrast-induced nephropathy.
A prospective study at a VA medical center looked at renal failure after cardiac catheterization. Atheroembolism was suspected on the basis of a 0.5 mg/dL or more rise in Cr 3 weeks after a coronary angiogram. Although the incidence of renal impairment was low in this group, two of the five died of renal failure. Of note, none of the five had skin signs of livedo, and the diagnosis of atheroembolism would have been missed on examination.31
Atheroembolism to the kidneys is underrecognized as a cause of acute and chronic renal disease. In one review of 259 patients who underwent renal biopsy for acute renal failure, cholesterol emboli were found in 6.9% of cases. Of note, 15 of 18 of these patients were clinically unsuspected to have atheroembolism as a cause of renal failure. Another study found 7.5% of patients with acute renal failure had documented atheroembolism on renal biopsy.70
In one review, Mayo and Swartz estimated that 4% of all inpatient nephrology consults were due to atheroembolism.64 This may be a conservative estimate because older patients with multiple risk factors accounted for a higher proportion of in-hospital nephrology consults. Of those consults seen with acute renal failure, 5% to 10% were felt to be due to atheroembolic renal disease.64
Atheromatous emboli and cholesterol crystals tend to lodge in arcuate and interlobar arteries which are 150 to 200 microns in diameter.8,65 Cholesterol crystal emboli not only cause mechanical vessel obstruction but also set up an endothelial inflammatory reaction that some have labeled microcrystalline angiitis.8 This is characterized by polymorphonuclear leukocyte and eosinophil infiltration around the vessel, and subsequently mononuclear cells with giant cell formation in the perivascular tissues. Endothelial distortion, intimal proliferation, perivascular fibrosis, and sometimes extraluminalization of crystals can be seen.67 With thrombus formation, there is further microvascular occlusion of renal vessels. Over 2 to 4 weeks, there is a progressive gradual decline in renal function following an acute atheroembolic episode. Renal infarction or necrosis is rare because the process is patchy and does not obstruct the larger feeding arteries to the kidney.
Atheroembolic renal disease presents as acute or subacute renal dysfunction in older patients, rarely younger than age 50, usually affecting those with preexisting renal insufficiency.5,35,65,71 Patients with atheroembolism have multiple risk factors including smoking, diabetes, hypertension, and hyperlipidemia. A review of 52 cases of atheroembolic renal failure at the Massachusetts General Hospital from 1981 to 1990 found these patients were more likely to have significant hypertension and coronary and peripheral artery disease (PAD).72
The clinical course may be variable. In contrast-induced nephropathy, renal failure occurs immediately after dye infusion, with a peak in Cr within several days and resolving in less than 2 weeks.8 Unlike contrast-induced nephropathy, atheroembolic renal failure may slowly worsen over a period of weeks to months, likely because of recurrent spontaneous showers of emboli. The kidney responds to ischemic damage with inflammatory changes that lead to glomerular sclerosis, tubular atrophy, and interstitial fibrosis.38,65 Sometimes, features of focal segmental necrotizing glomerulonephritis and crescentic glomerulonephritis are seen in renal biopsy specimens.65,66,73
Atheroembolism to kidneys may be subclinical. Subacute presentation is more common with progressive renal failure over several weeks. In one report, the average time interval between an angiographic procedure and diagnosis of atheroembolic renal disease was 5 weeks.35 Renal function declined over 3 to 8 weeks.72
A chronic form of renal failure may be mild and can be clinically silent. Atheroembolism may present acutely, with onset within 1 week, or be subacute with delayed onset of renal impairment 2 to 6 weeks after an inciting procedure. A step-and-plateau drop in renal function has been described, perhaps owing to intermittent recurrent showers of cholesterol crystals over a period of time. One to two thirds of patients with atheroembolic renal disease will need dialysis. Lye et al. reviewed the English literature in 1993, noting that 40% of patients required dialysis.6 Only 20% to 30% will recover sufficient renal function to stop dialysis.6,8,74
Clinical features are often absent but may include flank pain and gross hematuria due to renal infarction. Abdominal pain, nausea, vomiting, and blood loss can result from embolization to the gastrointestinal tract. In approximately half of these patients, there may be livedo reticularis or purple toe discoloration due to cholesterol embolization to the skin. Fever, malaise, and weight loss may be accompanying systemic symptoms.65
Severe or resistant hypertension has been noted in up to half of patients with atheroembolic renal disease.6,15 Hypertension may result from activation of renin angiotensin system, or be volume mediated secondary to inability of the kidney to excrete excess fluid. Malignant hypertension can occur with atheromatous embolization to the kidneys.75
Laboratory testing
Laboratory testing generally shows nonspecific findings. Although elevated creatinine, proteinuria, and eosinophilia have been reported in up to 80% of patients in the acute stage, these findings are inconsistently found.15,76 Anemia, leukocytosis, thrombocytopenia, and elevated inflammatory markers including sedimentation rate and C-reactive protein (CRP) are occasionally noted.1
The finding of eosinophils in the urine has been considered to be a very important diagnostic feature of renal atheroembolism. In a report by Wilson et al., urine eosinophils were found in 8 of 24 patients who had biopsy-proven atheroembolic disease. Six of the eight patients had more than 5% of urinary white cells as eosinophils.77 Hansel’s stain may increase the identification of urinary eosinophils. Urinary eosinophils, however, can be seen in other kidney disorders such as acute interstitial nephritis and other allergic disorders. Urinalysis may show hyaline or granular casts. Proteinuria may be present but is rarely severe enough to cause nephrotic syndrome.78 Urine sediment is usually inactive and unremarkable.78,79
Blood tests may show eosinophilia ranging from 14% to 80%, but again this is not a consistent finding.8,72 Eosinophilia is thought to be due to inflammatory changes in the kidney with immune activation. Kasinath et al. reviewed the literature and observed that 29 of 36 reports noted eosinophilia in association with renal atheroembolism. In this patient series, they found eosinophil counts ranging from 540 to 2000 cells/mm3.76 Modi and Rao found that 60% of patients had eosinophilia,65 and Lye et al. reported an incidence of 71%.6 Eosinophilia may be transient and seen only in the acute phase. Despite not always being present, if the eosinophil count is greater than 500 cells per μL, many clinicians feel this is a contributing finding, helpful in establishing a possible diagnosis of atheroembolism.7
The definitive diagnosis of atheroembolic kidney disease is confirmed histologically by the demonstration of cholesterol crystals in arcuate and interlobular arteries of the kidney. The sensitivity of a single renal biopsy may be only 75% owing to the patchy distribution of atheroembolism; however, with two biopsies, 94% are positive.65
Renal biopsies are not done in every patient with renal insufficiency, but a high degree of suspicion of atheroembolism in the appropriate setting (e.g., after an invasive vascular procedure) is necessary to make the correct diagnosis. It is important to be aware of many potential causes of renal failure in vascular patients, including contrast nephropathy, volume depletion from diuretics, renal artery or vein thrombosis, renal artery stenosis, nephrotoxic agents (e.g., antiinflammatory agents, angiotensin inhibitors), drug-induced interstitial nephritis, and glomerulonephritis. Atheroembolic renal disease can also mimic vasculitis. In many cases, renal failure after an angiographic procedure is incorrectly attributed to contrast-induced acute tubular necrosis. Clinical differentiation between the two is important.
Prognosis and treatment
The prognosis for atheroembolic renal disease is generally poor.1 Some 30% to 40% of patients with recurrent atheroembolic events have irreversible end-stage kidney failure requiring long-term hemodialysis.6,80,81 Dialysis dependency is also an indicator of poor prognosis. In one study, those who progressed to end-stage renal failure had a mortality rate of 75%, compared to 17% in those who recovered renal function.35 Scolari et al. reported poor outcomes for 354 subjects followed for 2 years, of whom 116 required dialysis and 102 died. These patients were elderly with advanced cardiovascular disease and comorbidities including heart failure and renal disease.7,82 Another series also reported an overall mortality rate of 58% over 15 months. Most of these patients died of cardiac failure.1 The overall outcome and prognosis is influenced by severity of atheroembolism and presence of preexisting kidney and cardiovascular comorbidities.
Treatment of renal atheroembolism is preventive (to avoid further episodes of atheroembolism) and supportive.8 General measures include avoidance of nephrotoxic agents, including angiographic contrast and antiinflammatory drugs. Hypertension and congestive heart failure (CHF) should be managed with appropriate medications such as vasodilators and diuretics. Aggressive management of hypertension may decrease proteinuria.79 Dialysis may be needed for uremia, volume excess, and electrolyte imbalances.
Belenfant et al. showed positive outcomes with aggressive supportive care in 67 patients admitted to a renal intensive care unit (ICU) for management of acute renal failure in the setting of multisystem cholesterol embolization. Clinical features included pulmonary edema, gastrointestinal ischemia, cutaneous ischemia, and retinal embolism. Treatment consisted of anticoagulation withdrawal, avoidance of invasive procedures, management of CHF with angiotensin-converting enzyme (ACE) inhibitors, and loop diuretics. Ultrafiltration or hemodialysis was used when needed for renal support. Enteral or parenteral nutrition supplementation was provided when needed. Some patients were treated empirically with steroids. Improved outcomes in multiorgan cholesterol embolism were reported, although the in-hospital mortality rate was 16%. Of the 56 patients who survived initial hospitalization and were ultimately discharged, there was a 77% 1-year and 52% 4-year survival; 32% remained on maintenance hemodialysis for irreversible renal failure.80
Pharmacological treatment for renal atheroembolism is empirical because there are no prospective clinical trials, and no definitive treatment has been established. Anecdotal reports suggest the use of steroids to reduce the inflammatory response associated with atheroembolism to the kidney.83,84 Cholesterol crystals act like foreign bodies setting up an inflammatory reaction with a cascade of systemic mediators of inflammation. Steroids reduce the inflammatory reaction and may have a favorable response.85,86 Belenfant et al. treated those with new cholesterol embolization, as well as those with declining clinical status, with corticosteroids and noted improved constitutional symptoms and nutrition.80 Scolari et al. found the risk of dialysis and death was lower in those patients on steroids.7
In an anecdotal report, simvastatin (40 mg daily) was associated with improved renal function in some patients with renal biopsy–documented atheroembolism.87 Corticosteroids and plasma exchange for treatment of atheroembolic renal disease has been tried for management.88 Treatment of cyanotic toes with lovastatin was noted in a case report of a man with diffuse atheromatous disease and gangrenous toes.89
Prevention is the best management, in particular avoiding additional invasive angiographic procedures in high-risk patients with extensive atheromatous disease of the aorta. If procedures are necessary, distal embolic protection devices may improve outcomes for arterial interventions.90
Gastrointestinal Tract Atheroembolism
The gastrointestinal tract is one of the common sites for aortic atheroembolism and ranks as the third most frequently affected organ system after skin and renal involvement.63 Cholesterol embolization should be in the differential diagnosis of all patients with atherosclerosis presenting with gastrointestinal symptoms after a vascular interventional procedure. Symptoms may be nonspecific and difficult to diagnose but include abdominal pain, fever, and diarrhea. Gastrointestinal bleeding due to mucosal infarcts and ulceration caused by bowel ischemia may occur.57,91,92 In severe cases, intestinal infarction may require urgent surgery for necrotic bowel or bowel perforation. The colon is most commonly involved. Multiple emboli over time may result in stricture formation, bowel obstruction, or polypoid lesions.93–95 Rarely, atheroembolism to the gut can mimic colon cancer.96 Sometimes symptoms may be misdiagnosed for months until a catastrophic event such as small-bowel perforation with peritonitis or bleeding occurs.97 The liver, gallbladder, and pancreas are also uncommon sites for atheroembolism, but there are case reports of acute pancreatitis and acute acalculous cholecystitis from aortic atheroembolism.98–103 Cholesterol embolization has been reported after protracted vomiting.104 The stomach is rarely involved. An endoscopic biopsy should include submucosa to detect cholesterol clefts in small arterioles.91 Most patients with atheroembolism to the gastrointestinal tract have advanced atherosclerosis, and atheroembolism affects multiple organs.92 When atheroembolism involves the gastrointestinal tract, cholesterol embolization can also be seen as livedo reticularis or associated blue toe syndrome.97 At least half of these cases are precipitated by an angiographic procedure and have a high mortality rate.91
Lower Extremities and Blue Toe Syndrome
In 1961, Feder and Auerbach described six patients who presented with painful purple toes and noted findings of dark-tinged discoloration of the plantar surfaces and sides of the first and second toes bilaterally. He noted the toes were painful and tender to touch, and that the blue discoloration of the skin blanched with local pressure, which he felt differentiated this entity from localized hematoma or purpura. These patients were older, with ages ranging from 53 to 69, and had atherosclerotic cardiovascular diseases including diabetes, stroke, or congestive heart failure. Most of these patients had intact peripheral pulses.42 Feder and Auerbach associated these skin changes with initiation of warfarin anticoagulation but recognized these features were not due to warfarin-induced skin necrosis.
Fifteen years later in 1976, Karmody et al. established the term blue toe syndrome, recognizing that the sudden onset of pain and cyanosis was the result of a microembolic event to the digital arteries. Angiography in a number of these patients localized the source of embolism to the femoral, popliteal, or aortoiliac arteries.9
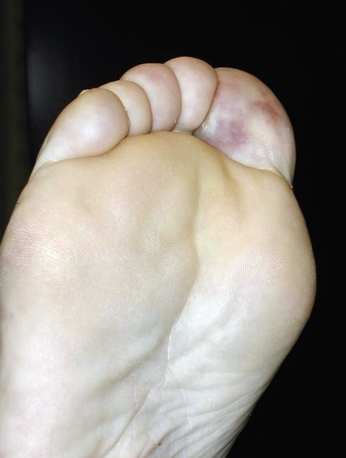
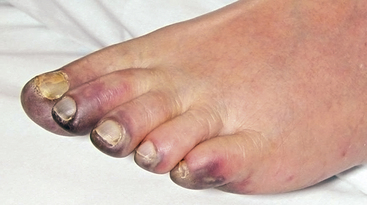
The typical patient with blue toe syndrome presents with sudden onset of painful cyanotic skin lesions that may involve one or many toes. Cyanosis results from decreased arterial inflow along with impaired venous outflow, leading to stasis of desaturated blood. Initially, the cyanosis blanches with pressure, but with worsening ischemia and tissue damage, the blue discoloration may become nonblanchable. The affected toe is dark blue in color, painful due to ischemia, and exquisitely tender to touch. Digital ischemia can progress to cause skin necrosis, ulceration, and black gangrene. Livedo reticularis of the foot may also be present involving the base of the affected toe, forefoot, plantar surface, or heel (Figs. 47-9 through 47-13). Myalgias due to muscle atheroembolism may occur, with clinical features of local muscle tenderness, sometimes with actual myonecrosis.105
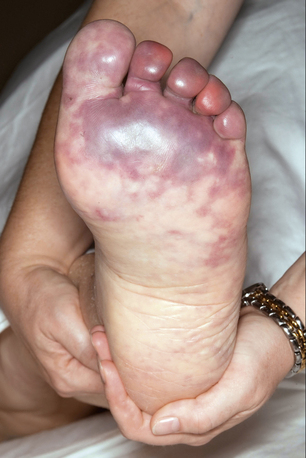
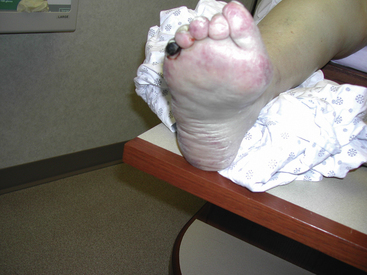
Figure 47-12 Plantar surface of same patient as in Figure 47-11, showing purple toes and severe atheroembolism to forefoot.
Clinical examination should be the initial step to determine the source of atheroembolism. In 78% of patients, peripheral pulses including pedal pulses are intact.1 Although distal pulses are palpable in the classic case of blue toe syndrome, occlusive arterial disease is present in up to half of patients, based on ankle- brachial indices (ABIs) less than 0.9.106 Careful pulse examination with auscultation for bruits may suggest proximal arterial disease. A widened expansile common femoral or popliteal pulse may suggest an aneurysm. Palpation of the abdomen may reveal an aortic aneurysm. The location of livedo, such as the foot, thigh, or abdomen, suggests a more proximal site.
Lower-extremity atheromatous emboli can originate from focal or diffuse atherosclerosis, from stenotic or aneurysmal disease, and from disease above or below the inguinal ligament.106 If both lower extremities are involved, this suggests the origin of the atheroembolism is proximal to the aortic bifurcation. In unilateral blue toe syndrome, the culprit site is likely at or distal to the iliac artery. Common sites of atherosclerosis are the common or external iliac artery (EIA), the superficial femoral artery (SFA) at the adductor hiatus (due to stenotic disease), and the popliteal artery (arising from a local aneurysm). The aortoiliac segment is the most common origin for atheroembolism, accounting for two thirds of cases.106–109 One third of cases are found to arise from the femoropopliteal arteries.106
When no embolic source is readily identifiable, imaging of the thoracic aorta is important and may reveal a coral reef (see later discussion) or mobile plaque as the source of distal atheroemboli. Sometimes a solitary embolic source cannot be isolated, owing to the diffuse nature of atherosclerosis. For example, in one study, arteriograms showed diffuse disease at both aortoiliac and femoropopliteal levels in 40% of patients, making it difficult to discern the likely source of atheroembolism.
Only a few years ago, angiography was the gold standard to search for a culprit lesion, but now with advances in noninvasive imaging, computed tomographic angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasound, and TEE are all first-line well-established methods to image the aorta.110,111
Blue toes due to atheroembolism should be differentiated from other skin and systemic disorders (Fig. 47-14). Blue toes can also be caused by benign cold-induced reversible vasospasm, similar to Raynaud’s phenomenon of the fingers. Other vascular diseases that can present with a blue toe include pernio, thromboangiitis obliterans (TAO) (Buerger’s disease), and digital artery thrombosis. Paraproteinemia (e.g., myeloma) or myeloproliferative disorders (e.g., polycythemia vera, essential thrombocytosis) may cause small-vessel thrombosis. Cryofibrinogenemia results from complexes of fibrinogen, fibrin, and proteins that precipitate with cold. Secondary forms are associated with cancers, rheumatological diseases, and infections. Cryoglobulinemia results from immunoglobulins (Igs) that precipitate in the cold. There are three types: type 1 cryoglobulinemia occurs in association with lymphoproliferative disease (e.g., chronic lymphocytic lymphoma); types 2 and 3 may be associated with viral hepatitis infections. Hirschmann and Raugi defines the blue toe syndrome as a “blue or violaceous discoloration of one or more toes in the absence of trauma (fracture or strain), cold-induced injury (pernio or frostbite), or disorders that produce systemic cyanosis (methemoglobinemia or hypoxia)”.60
The short- and long-term outcome after an atheroembolic episode is variable depending on the extent of atheroembolism and resulting tissue damage. For many patients, the prognosis for atheroembolism is poor, sometimes requiring limb amputation. Improvement may occur but may take several weeks for pain to slowly subside, and longer for skin color changes to improve. In more severe cases, the affected toe(s) may progress to necrosis with black gangrene. If carefully managed, the gangrene may stay dry and demarcate from healthy tissue, allowing future autoamputation of the distal or entire toe. Wet gangrene may lead to infection and may require surgical amputation. A toe amputation may heal satisfactorily at a demarcation line if large-vessel arterial perfusion is intact.
Although some individuals recover after a single episode of atheroembolism, a recurrent episode can cause further irreparable damage resulting in extensive tissue damage and necrosis. Spontaneous embolic episodes tend to be recurrent. With very extensive atheroembolism, skin necrosis may occur, affecting much of the foot; this has been referred to as trash foot19 (Fig. 47-15). This is potentially limb threatening and is associated with a high mortality rate, owing to coexistent multisystem disease. In one study where trash foot occurred in 19 of 29 patients (7 bilateral) following abdominal aorta or lower-extremity revascularization, 8 patients underwent major amputations, and 5 minor amputations.19 The risk of major amputation after extensive atheroembolism varies from 10% to 27% depending on the reported series.9,106,112 Mortality is also significant in multisystem atheroembolism, as in one recent study where 31% of patients died during a follow-up period of 15 months.113
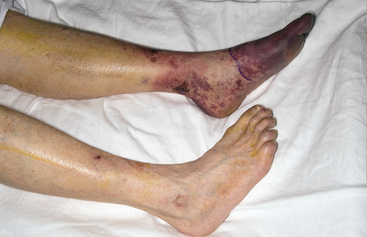
Figure 47-15 Postangiographic atheroembolism to foot, resulting in critical irreversible foot ischemia requiring below-knee amputation.
Operative management
Once lower-extremity atheroembolism occurs, management principles are to prevent recurrent embolic episodes and provide local care for the affected extremity. General treatment measures are discussed later under the section on treatment.
Surgical or endovascular intervention has been advocated because of the high likelihood of recurrent atheroembolic events leading to worsening irreversible tissue ischemia with risk of limb amputation.9,112 Embolic recurrence may be as high as 50% to 80%, with a 40% to 60% risk of tissue loss.106,107 The goal of surgical intervention is to remove or exclude the source of embolization and prevent recurrent episodes leading to organ and extremity loss. Treatment choice is determined by location and severity of disease. The optimal surgical intervention depends on the individual patient. Arterial bypass, endarterectomy, and angioplasty with stent placement have been reported to be effective in selected patients by preventing recurrent embolization.107,114
A retrospective study at Washington University Medical Center found 62 patients with renal or cutaneous manifestations of atheroembolism. Angiography was done in almost all patients. The aorta and iliac arteries (80%) were felt to be the most common source of atheroembolism, followed by femoral (13%), popliteal (3%), and subclavian (3%) arteries.108 Bypass grafting procedures were performed on 42 patients to exclude the native diseased artery. Other patients underwent a combination of endarterectomy and bypass grafts. Limb salvage was accomplished in 98%, although 31% had minor amputations. No further episodes of atheroembolism occurred over a mean follow-up period of 20 months.108
Keen et al. reviewed his experience of surgical management of atheroembolism in 100 patients with lower-extremity, visceral, and upper-extremity atheroembolism who were followed for 12 years.109 Aortoiliac occlusive disease was present in 47 patients, and aortic aneurysm was present in 20 patients (average size, 3.5 cm). Operations to exclude the embolic source included aortic bypass or aortoiliac endarterectomy, femoral and popliteal endarterectomy, or bypass graft. Several patients underwent extra-anatomical reconstruction.109 Despite surgery, 6 of 97 had recurrent events, and 9 required major leg amputations with 10 toe amputations.
Friedman and Krishnasastry reviewed a small group of high-risk surgical patients presenting with rest pain, ulceration, or gangrene due to atheroemboli to both lower extremities. These patients, who were not candidates for direct aortic reconstruction because of preexisting medical comorbidities, underwent ligation of the EIA with axillary-bifemoral bypass. Initial limb salvage was accomplished in all, with no limb loss over the next 52 months.115 Kaufman et al. also reported a small group of high-risk patients in whom limb salvage and healing of foot ulcers was accomplished in the majority with an axillobifemoral bypass with exclusion of the external iliacs.116
Hollier et al. reviewed 88 patients with shaggy aorta syndrome who suffered atheromatous embolization (Fig. 47-16). Surgical correction was performed in 27 patients, including endarterectomy, external iliac ligation, and graft replacement. The best outcome (lowest morbidity and mortality) for those with lower-extremity atheroembolism was with ligation of the distal EIA and extra-anatomical bypass.117 The author noted that surgery was not always successful in preventing visceral infarction or renal failure.117
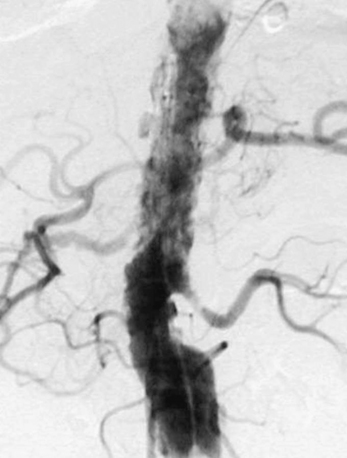
Figure 47-16 Aortogram demonstrating diffuse atheromatous disease, so-called shaggy aorta, with high atheroembolic potential.
Primary angioplasty for iliac or superficial femoral lesions can also be successful for focal high-grade stenotic lesions of the iliac or superficial femoral artery.114,118 Although there may be concern of provoking further embolization, some studies show good results. In one series of 10 patients treated with primary angioplasty, none had embolization at the time of the procedure, and 8 of the 10 had no recurrent atheroembolic episodes. The patients in this series were more likely to have single focal high-grade stenotic lesions of an iliac or SFA amenable to angioplasty, as opposed to more diffuse proximal atherosclerotic plaque.112,114
A self-expanding stent has been used to treat atheroembolism arising from an isolated segment of the iliac artery.107 For more complex plaques, a covered stent has been successfully deployed and offers the advantage of excluding the diseased segment by trapping the atheroma and thrombus under the covered stent.107,119 In one case report of three patients with iliac artery disease treated with a self-expanding stent covered with Dacron, no recurrences of microembolism were noted after 16 months, and the toe lesions healed.107
Aortic stent grafting is now commonly used in the management of abdominal aortic aneurysms. In a retrospective study of 19 patients with symptomatic lower-extremity atheroembolism presenting with ischemic ulcers or toe gangrene and an abdominal aneurysm, an aortic stent graft was deployed to exclude an abdominal aortic aneurysm. At 1-year follow-up, eight of nine patients had resolution of ischemic toe symptoms.113 The authors note that although stent graft repair of AAA may prevent future embolization, it is important to not miss coexisting thoracic aortic disease.
Arterial filters have also been employed in the superficial femoral artery, carotid, renal, and many other vessels. As filter development continues to advance, this may become an adjunctive procedure in the future.120
Upper-Extremity Atheroembolism
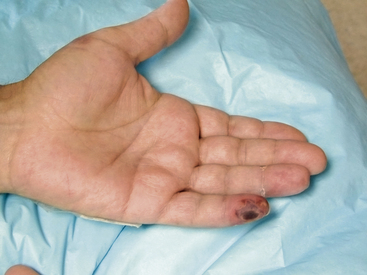
In the upper extremity, atheroembolism is said to be uncommon.108 Atherosclerosis may involve the aortic arch and branch vessels. Two sites in particular are important. The subclavian artery is a common site for atherosclerosis. Unequal upper-extremity blood pressures should raise suspicion of disease at or proximal to the subclavian artery level. Most individuals with subclavian atherosclerotic disease are asymptomatic, but this can be a source of atheroembolism to the arm and the fingers (Fig. 47-17). Thoracic outlet syndrome causes extrinsic compression of the subclavian artery as it passes under the clavicle and over the first rib. This is a site for aneurysm formation and subsequent atheroembolism to the hand and fingers. Finally, at the wrist level, repetitive pounding injury to the hypothenar side of the palm, as occurs in carpenters and car mechanics, can result in ulnar artery aneurysm with atheroembolism to the hand and fingers.
Coral Reef Plaque
A coral reef plaque is an exophytic calcified discrete mass that is prone to distal embolization.121 These are more commonly located in the posterior wall of the suprarenal aorta.122 Although unusual, they occur in patients with generalized atherosclerosis. They can be treated by surgical thromboendarterectomy or bypass.123,124 Endovascular stent placement has also been successful in patients symptomatic with claudication from aortic lumen compromise by a coral reef.125
Ascending Aorta/Arch Atheroma and Stroke
Sixty years ago, cerebral vasospasm was speculated to cause stroke. Today, carotid artery disease and atrial fibrillation are known to be major causes of nonhemorrhagic stroke. With the advent of TEE, aortic arch plaque has been identified as an important potential source of cerebral embolic stroke.126,127 Case-controlled prospective studies have shown a clear association between atherosclerotic disease of the ascending aorta/aortic arch and risk of ischemic stroke.128 Autopsy studies found ulcerated plaque in the aortic arch to be present in 26% of 239 patients with cerebrovascular disease (both stroke and brain hemorrhage) but only 5% of patients with another neurological diagnosis.128,129 Another large study using TEE to detect aortic atheroma compared 215 consecutive patients with first stroke or TIA to 202 control subjects and confirmed that atheroma in the ascending aorta and arch are a significant risk factor for stroke.130
Characterization of aortic plaque morphology (hypoechoic plaque, ulceration, calcification, sessile or mobile thrombus) is important in prediction of stroke.131 In particular, the thickness of plaque in the ascending aorta and arch correlates with risk of stroke. When patients with acute stroke were compared to consecutive controls, 28% had plaques of 4 mm or more in thickness in those with unexplained stroke, compared to 8% of 172 patients who had a known or suspected cause of brain infarct.128 In the French Study of Aortic Plaques in Stroke, TEE was done to quantify aortic arch atheromatous disease in 331 patients aged 60 or older admitted to hospital with an acute stroke. The incidence of recurrent brain infarction was 11.9% per 100 person-years in those with aortic plaque thickness greater than 4 mm, compared to 2.8% for those with minimal plaque (<1 mm). The presence of atherosclerotic arch plaque thickness of 4 mm or greater was found to be an independent predictor of recurrent brain infarction and cardiovascular events.132 Even moderate atheromatous disease (defined as intimal thickness of the ascending aorta and arch of > 2 mm) is a significant risk factor for stroke after cardiac surgery.133
Mobile Atheroma
Atheromatous plaque that is protruding and pedunculated has been correlated with an increased risk of stroke. The presence of mobile components, however, denotes the highest risk for stroke.109,132,134–141 In a study of 118 elderly patients studied with intraoperative TEE, 3 of 12 patients (25%) with a mobile aortic arch atheroma had suffered a perioperative stroke, compared to 2 of 118 (2%) patients without a mobile atheroma.134 A mobile mass overlying an atheromatous plaque is likely adherent thrombus and therefore is more likely to respond to anticoagulation than the underlying plaque itself. Whether to use anticoagulation for mobile thrombus in the aorta, however, remains controversial.
Warfarin anticoagulation has been advocated for the management of mobile aortic atheroma. A study by Dressler et al. documented effectiveness of warfarin in preventing recurrent embolic events in those with symptomatic thoracic aorta mobile atheroma compared to those not receiving anticoagulation.142 He reviewed 31 patients with mobile aortic atheroma who presented with a systemic embolic event. At follow-up, those patients not receiving warfarin had a much higher incidence of vascular events (45% vs. 5%). Recurrent strokes occurred in 38% of patients. There were no strokes in those on warfarin.142 Of note, those with small mobile atheroma were not treated with warfarin, and recurrent strokes occurred in 37% of these patients.142
Warfarin was noted to be more effective than antiplatelet medications in another study of patients with severe ascending aortic atheromatous disease.140 Further randomized controlled trials would be needed to address anticoagulation vs. antiplatelet therapy for this important issue.
In case examples, peripheral embolization from plaque-related mobile thrombus in the thoracic aorta has been successfully treated with warfarin. In one case report, a 71-year-old man had atheroembolism to the toes after vomiting. A large mobile mass in the descending thoracic aorta was identified by TEE. After 3 months of warfarin anticoagulation, there was virtual resolution of the aortic mass, suggesting it was thrombus covering atheromatous plaque. The toe pain and discoloration from atheroembolism also resolved completely.104
Stroke due to atheroembolism can occur during carotid surgery. Microemboli occurs during carotid endarterectomy and can be detected by TCD ultrasound. Ackerstaff et al. found a positive correlation with the occurrence of microembolism during carotid endarterectomy and perioperative TIA and stroke. Magnetic resonance imaging also documented new ischemic brain lesions in these patients.143
Carotid sinus massage may reproduce syncope in patients with carotid sinus hypersensitivity but also cause inadvertent atheroembolic stroke in patients with carotid atherosclerosis.144
Embolic stroke should be suspected in the proper clinical setting. In young patients with unexplained embolism, TEE has identified unsuspected mobile aortic arch thrombus on atherosclerotic plaque.127 Brain imaging characteristically shows multiple small ischemic lesions. Brain pathology has also documented multiple cholesterol emboli.145 Lacunar infarcts are generally thought to be due to small brain infarcts from hypertension. It is possible some lacunar infarcts may be due to cholesterol emboli.146
General Treatment Measures for Atheroembolic Disease
Preventive
It has been said many times that the best treatment for many diseases is prevention.147 Angiography is the most common iatrogenic cause of atheroembolism, responsible for up to 80% of cases, so safer angiographic techniques and newer technologies may decrease the incidence of catheter-induced atheroembolism.8 For example a “no touch technique” has been advocated to reduce potential for intimal disruption during renal artery stenting. A second guide is placed within the guide catheter to minimize contact between the guide catheter and aorta.148 Arterial filters have also been successfully employed in the superficial artery.120 Distal protection devices are available for renal and cerebral angioplasty.149–151 Cerebral protection devices may reduce stroke during carotid artery stenting, but device-related complications can occur.147,152
Reduction of atherosclerotic burden and plaque stabilization has been a major focus for preventing coronary events and should also be a focus for preventing atheroembolism. Reducing lipid content in the plaque core, decreasing inflammation and inflammatory cells, and reducing vasa vasorum neovascularization are future strategies.
Control of lifestyle-related risk factors includes cessation of cigarette smoking and avoidance of all tobacco products, avoiding obesity, adult-onset diabetes, and elevated triglycerides, with recognition and management of the metabolic syndrome and decreasing salt in the diet. Avoiding physical inactivity by pursuing an aerobic exercise program is an important step to preventing progression of atheromatous disease and therefore lessening the risk of atheroembolism.153
Pharmacological therapy consists of statin medications, antiplatelet agents, ACE inhibitors, often in combination, as well as medications to control hyperglycemia and hypertension.
HMG-CoA reductase inhibitors, or statins, are well-established medications for lowering cholesterol and reducing cardiovascular mortality. Statin medications likely have multiple effects that include antiinflammatory properties, improvement in endothelial function, and reducing blood thrombogenicity. They may also have immunomodulatory effects, decreasing recruitment of monocytes and T cells into the arterial wall and stabilizing arterial plaque, thus decreasing the risk of plaque rupture.154
Many large trials have confirmed the benefits of statin agents in secondary prevention of cardiovascular events.153,155 Slowing atherosclerosis progression is a laudable goal. Recently, regression of atheroma volume (of almost 7%) has been documented in the ASTEROID trial, which achieved an average low-density lipoprotein (LDL) decline from 130.4 to 60.8 mg/dL and increase in high-density lipoprotein (HDL) by 14.7%.156 Cholesterol education program guidelines for lipid management suggest an initial treatment goal of LDL less than 100 mg/dL, except in those at highest risk with established atheromatous disease, who should have an LDL goal of less than 70 mg/dL.153
There are no large randomized trials of statin medications in atheroembolism, but there are a number of observational case reports. Tunick et al. looked at the rate of cholesterol embolism in 519 patients with complex aortic plaque seen during TEE in a retrospective study in which atheroembolism occurred in 21%. Although treatment was not randomized, multivariate analysis showed a benefit of statin medication, with absolute risk reduction of 17%. No protective effect was found for warfarin or antiplatelet drugs.157
Antiplatelet agents prevent platelet activation and thrombosis. Commonly used oral antiplatelet agents include aspirin (75-365 mg/day), dipyridamole plus aspirin, and clopidogrel 75 mg daily. Many clinical trials have documented the effectiveness of combined antiplatelet therapy in coronary disease. Aspirin blocks the enzyme cyclooxygenase (COX)-1 to prevent synthesis of thromboxane A2 (TxA2), thus inhibiting platelet aggregation. Thienopyridines act by blocking adenosine diphosphate (ADP)-mediated platelet aggregation via the glycoprotein (GP)-IIb/IIIa receptor. Dipyridamole is a phosphodiesterase (PDE) inhibitor that reduces the breakdown of cyclic adenosine and guanine monophosphate (cAMP and cGMP) and increases activity of prostacyclin(PGI2), which inhibits platelet aggregation.158 It is not known whether antiplatelet agents prevent recurrent atheroembolism.
Cilostazol has antiplatelet and vasodilatory effects. One small study of five patients reported healing of distal limb ulcers and improved large- and small-vessel perfusion, but there was no control group and therefore no compelling evidence that ulcer healing was related to this drug.159 Iloprost therapy has been used for cholesterol emboli syndrome.160 Anecdotal treatment of blue toe syndrome has included nifedipine and pentoxifylline.161 With fixed microvascular disease, there is no clear benefit of vasodilator therapy in the majority of patients with atheroembolism.
Although anticoagulation may be of benefit for mobile thrombus overlying atheromatous plaque, it is not routinely used in asymptomatic patients with nonmobile atheroma.162
Supportive
Organ failure has to be treated. Kidney disease has to be monitored, with correction of electrolyte abnormalities, volume excess, and uremia. Renal replacement therapy with dialysis may be temporary or permanent. Heart failure and hypertension should be managed with diuretics, renin-angiotensin-aldosterone system (RAAS) antagonists, β-blockers, and vasodilators. The HOPE trial showed benefit for ACE inhibitors in patients with vascular disease or diabetes, with reduced rates of stroke, myocardial infarction, and overall death.163 The efficacy of renin-angiotensin system inhibition in preventing recurrent atheroembolism is not known.
Wound care may be needed for management of lower-extremity skin necrosis, using surgical wound débridement and application of topical agents and dressings. Limited amputation of toes or forefoot is necessary in some patients, and limb amputations may be necessary for patients with irreversible ischemia with gangrene. Antibiotics may be necessary for infection. A fluffy, soft vascular boot can protect the ischemic foot from trauma. Pneumatic arterial pumps can improve arterial perfusion in some patients.
Effective pain control is very important; ischemic pain can be severe and persist for weeks after lower-extremity atheroembolism.164 The degree of pain from blue toe syndrome may seem to be out of proportion to the extent of tissue involvement but reflects microvascular necrosis. Besides narcotic and neurotransmitter pharmaceutical agents, several other modalities have been used for pain associated with lower-extremity ischemia.
Lumbar sympathectomy was popular 50 years ago when surgical options for PAD were more limited. There has always been controversy regarding actual benefit in patients with atherosclerotic disease.165–168 Lumbar sympathectomy historically has been done for critical leg ischemia with gangrene, ischemic ulcerations, and rest pain. A 60% improvement rate has been reported, although not all respond. In one retrospective review of 45 patients (50 limbs) with toe gangrene treated with lumbar sympathectomy, amputation rate remained high at 40%.169,170 More recently, some surgeons continue to advocate lumbar sympathectomy for selected patients in situations of threatened tissue loss.162 The hope is that this may improve collateral blood flow and open arteriovenous connections by relieving smooth muscle constriction.162 Some studies do show improved tissue perfusion and enhanced healing in addition to surgical reconstruction.
Today, a lumbar sympathetic block may be done by local injection, with the goal of improving skin warmth and pain. A spinal cord stimulator has been of benefit in a few selected patients.164
Other treatment options may include hyperbaric oxygen or pneumatic leg compression to improve distal perfusion in selected cases of extremity atheroembolism.171
Conclusions
Atheroembolism is a rare but serious disorder that can occur spontaneously or be a complication of invasive cardiac and vascular procedures. Angiography is the most common iatrogenic cause, responsible for up to 80% of cases.8 Be aware that atheroembolic skin changes may mimic other disorders.
A presumptive diagnosis is often based on clinical features. The diagnosis requires a high index of suspicion in an appropriate clinical setting, such as exposure to a precipitating factor, unexplained renal failure, and cutaneous signs of atheroembolization.2
A definitive diagnosis requires histological confirmation of cholesterol crystals in a biopsy of muscle, skin, or affected organ. There is no single definitive laboratory test except biopsy of involved tissue to confirm the diagnosis of atheroembolic disease.
It is important to determine the most likely embolic source. When atheroembolism involves the lower extremities, atherosclerotic or aneurysmal disease of the aortoiliac segment accounts for two thirds of cases.107,108
Atheroembolism can be recurrent, and in those patients there is a 40% to 60% risk of tissue loss.106,107 The goal of surgical and endovascular treatment is to exclude the embolic source and prevent recurrent episodes. A covered stent or extra-anatomical bypass may be an option in high-risk patients.
Management of mobile atheroma is controversial, but warfarin seems to be effective in preventing symptomatic thromboembolism in some patients.142 Anticoagulation is not routine therapy in patients with diffuse atherosclerosis.
The best treatment of atheroembolism is prevention. Ultimately, prevention of atherosclerosis will prevent atheroembolism.
1 Jucgla A., Moreso F., Muniesa C., et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786–793.
2 Kiechle F.L., McLaughlin J.H., Yang S.S., et al. Atheromatous embolization. Am J Emerg Med. 1983;1:299–301.
3 Hollenhorst R.W. Significance of bright plaques in the retinal arterioles. Trans Am Ophthalmol Soc. 1961;59:252–273.
4 Lie J.T. Cholesterol atheromatous embolism. The great masquerader revisited. Pathol Annu. 1992;27(Pt 2):17–50.
5 Fine M.J., Kapoor W., Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology. 1987;38:769–784.
6 Lye W.C., Cheah J.S., Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol. 1993;13:489–493.
7 Scolari F., Ravani P., Gaggi R., et al. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116:298–304.
8 Scolari F., Ravani P. Atheroembolic renal disease. Lancet. 2010;375:1650–1660.
9 Karmody A.M., Powers S.R., Monaco V.J., et al. “Blue toe” syndrome. An indication for limb salvage surgery. Arch Surg. 1976;111:1263–1268.
10 Cross S.S. How common is cholesterol embolism? J Clin Pathol. 1991;44:859–861.
11 Saklayen M.G. Atheroembolic renal disease: preferential occurrence in whites only. Am J Nephrol. 1989;9:87–88.
12 Flory C. Arterial occlusions produced by emboli from eroded aortic atheromatous plaques. Am J Pathol. 1945;21:549–565.
13 Kealy W.F. Atheroembolism. J Clin Pathol. 1978;31:984–989.
14 Doty J.R., Wilentz R.E., Salazar J.D., et al. Atheroembolism in cardiac surgery. Ann Thorac Surg. 2003;75:1221–1226.
15 Rosman H.S., Davis T.P., Reddy D., et al. Cholesterol embolization: clinical findings and implications. J Am Coll Cardiol. 1990;15:1296–1299.
16 Hertzer N.R. Peripheral atheromatous embolization following blunt abdominal trauma. Surgery. 1977;82:244–247.
17 Ramirez G., O’Neill W.M.Jr, Lambert R., et al. Cholesterol embolization: a complication of angiography. Arch Internal Med. 1978;138:1430–1432.
18 Thurlbeck W.M., Castleman B. Atheromatous emboli to the kidneys after aortic surgery. N Engl J Med. 1957;257:442–447.
19 Sharma P.V., Babu S.C., Shah P.M., et al. Changing patterns of atheroembolism. Cardiovasc Surg. 1996;4:573–579.
20 Lindholt J.S., Sandermann J., Bruun-Petersen J., et al. Fatal late multiple emboli after endovascular treatment of abdominal aortic aneurysm. Case report. Int Angiol. 1998;17:241–243.
21 Zempo N., Sakano H., Ikenaga S., et al. Fatal diffuse atheromatous embolization following endovascular grafting for an abdominal aortic aneurysm: report of a case. Surg Today. 2001;31:269–273.
22 Thompson M.M., Smith J., Naylor A.R., et al. Microembolization during endovascular and conventional aneurysm repair. J Vasc Surg. 1997;25:179–186.
23 Keon W.J., Heggtveit H.A., Leduc J. Perioperative myocardial infarction caused by atheroembolism. J Thorac Cardiovasc Surg. 1982;84:849–855.
24 Blauth C.I., Cosgrove D.M., Webb B.W., et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac surgery. J Thorac Cardiovasc Surg. 1992;;103:1104–1111. discussion 1111–1102
25 Tunick P.A., Krinsky G.A., Lee V.S., et al. Diagnostic imaging of thoracic aortic atherosclerosis. AJR Am J Roentgenol. 2000;174:1119–1125.
26 Baker A.J., Naser B., Benaroia M., et al. Cerebral microemboli during coronary artery bypass using different cardioplegia techniques. Ann Thorac Surg. 1995;59:1187–1191.
27 Harringer W. Capture of particulate emboli during cardiac procedures in which aortic cross-clamp is used. International Council of Emboli Management Study Group. Ann Thorac Surg. 2000;70:1119–1123.
28 Ascione R., Ghosh A., Reeves B.C., et al. Retinal and cerebral microembolization during coronary artery bypass surgery: a randomized, controlled trial. Circulation. 2005;112:3833–3838.
29 Drost H., Buis B., Haan D., et al. Cholesterol embolism as a complication of left heart catheterisation. Report of seven cases. Br Heart J. 1984;52:339–342.
30 Colt H.G., Begg R.J., Saporito J.J., et al. Cholesterol emboli after cardiac catheterization. Eight cases and a review of the literature. Medicine. 1988;67:389–400.
31 Saklayen M.G., Gupta S., Suryaprasad A., et al. Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology. 1997;48:609–613.
32 Gaines P.A., Cumberland D.C., Kennedy A., et al. Cholesterol embolisation: a lethal complication of vascular catheterisation. Lancet. 1988;1:168–170.
33 Gjesdal K., Orning O.M., Smith E. Fatal atheromatous emboli to the kidneys after left-heart catheterisation. Lancet. 1977;2:405.
34 Fukumoto Y., Tsutsui H., Tsuchihashi M., et al. Cholesterol Embolism Study I. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211–216.
35 Frock J., Bierman M., Hammeke M., et al. Atheroembolic renal disease: experience with 22 patients. Nebr Med J. 1994;79:317–321.
36 Johnson L.W., Esente P., Giambartolomei A., et al. Peripheral vascular complications of coronary angioplasty by the femoral and brachial techniques. Cathet Cardiovasc Diagn. 1994;31:165–172.
37 Keeley E.C., Grines C.L. Scraping of aortic debris by coronary guiding catheters: a prospective evaluation of 1,000 cases. J Am Coll Cardiol. 1998;32:1861–1865.
38 Liew Y.P., Bartholomew J.R. Atheromatous embolization. Vasc Med. 2005;10:309–326.
39 Karalis D.G., Quinn V., Victor M.F., et al. Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J. 1996;131:1149–1155.
40 Blankenship J.C., Mickel S. Spinal cord infarction resulting from cardiac catheterization. Am J Med. 1989;87:239–240.
41 Harrington D., Amplatz K. Cholesterol embolization and spinal infarction following aortic catheterization. Am J Roentgenol Radium Ther Nucl Med. 1972;115:171–174.
42 Feder W., Auerbach R. “Purple toes”: an uncommon sequela of oral coumarin drug therapy. Ann Intern Med. 1961;55:911–917.
43 Bruns F.J., Segel D.P., Adler S. Control of cholesterol embolization by discontinuation of anticoagulant therapy. Am J Med Sci. 1978;275:105–108.
44 Hyman B.T., Landas S.K., Ashman R.F., et al. Warfarin-related purple toes syndrome and cholesterol microembolization. Am J Med. 1987;82:1233–1237.
45 Carron P.L., Florea A., Ducloux D., et al. Atheroembolic disease associated with the use of low-molecular-weight heparin during haemodialysis. Nephrol Dial Transplant. 1999;14:520–521.
46. Blackshear JL, Zabalgoitia M, Pennock G, et al: Warfarin safety and efficacy in patients with thoracic aortic plaque and atrial fibrillation. SPAF TEE Investigators. Stroke Prevention and Atrial Fibrillation. Transesophageal echocardiography, Am J Cardiol 83:453–455.
47 Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334:1216–1221.
48 Bols A., Nevelsteen A., Verhaeghe R. Atheromatous embolization precipitated by oral anticoagulants. Int Angiol. 1994;13:271–274.
49 Boudes P. Cholesterol emboli and streptokinase therapy. JAMA. 1988;259:1180.
50 Gupta B.K., Spinowitz B.S., Charytan C., et al. Cholesterol crystal embolization-associated renal failure after therapy with recombinant tissue-type plasminogen activator. Am J Kidney Dis. 1993;21:659–662.
51 Wong F.K., Chan S.K., Ing T.S., et al. Acute renal failure after streptokinase therapy in a patient with acute myocardial infarction. Am J Kidney Dis. 1995;26:508–510.
52 Arora R.R., Magun A.M., Grossman M., et al. Cholesterol embolization syndrome after intravenous tissue plasminogen activator for acute myocardial infarction. Am Heart J. 1993;126:225–228.
53 GUSTO. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682.
54 Blankenship J.C., Butler M., Garbes A. Prospective assessment of cholesterol embolization in patients with acute myocardial infarction treated with thrombolytic vs. conservative therapy. Chest. 1995;107:662–668.
55 Chaudhary K., Wall B.M., Rasberry R.D. Livedo reticularis: an underutilized diagnostic clue in cholesterol embolization syndrome. Am J Med Sci. 2001;321:348–351.
56 Faraggiana T., Muda A.O. Atheromatous embolism. Contrib Nephrol. 1996;119:67–73.
57 Ben-Horin S., Bardan E., Barshack I., et al. Cholesterol crystal embolization to the digestive system: characterization of a common, yet overlooked presentation of atheroembolism. Am J Gastroenterol. 2003;98:1471–1479.
58 Scolari F., Tardanico R., Zani R., et al. Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089–1109.
59 Chesney T.M. Atheromatous embolization to the skin. Am J Dermatopathol. 1982;4:271–273.
60 Hirschmann J.V., Raugi G.J. Blue (or purple) toe syndrome. J Am Acad Dermatol. 2009;60:1–20. quiz 21–22
61 Cappiello R.A., Espinoza L.R., Adelman H., et al. Cholesterol embolism: a pseudovasculitic syndrome. Semin Arthritis Rheum. 1989;18:240–246.
62 Falanga V., Fine M.J., Kapoor W.N. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194–1198.
63 Moolenaar W., Lamers C.B. Cholesterol crystal embolization in the Netherlands. Arch Internal Med. 1996;156:653–657.
64 Mayo R.R., Swartz R.D. Redefining the incidence of clinically detectable atheroembolism. Am J Med. 1996;100:524–529.
65 Modi K.S., Rao V.K. Atheroembolic renal disease. J Am Soc Nephrol. 2001;12:1781–1787.
66 Greenberg A., Bastacky S.I., Iqbal A., et al. Focal segmental glomerulosclerosis associated with nephrotic syndrome in cholesterol atheroembolism: clinicopathological correlations. Am J Kidney Dis. 1997;29:334–344.
67 Jones D.B., Iannaccone P.M. Atheromatous emboli in renal biopsies. An ultrastructural study. Am J Pathol. 1975;78:261–276.
68 Olin J.W. Atheroembolic renal disease: underdiagnosed and misunderstood. Catheter Cardiovasc Interv. 2007;70:789–790.
69 Kawarada O., Yokoi Y., Takemoto K. The characteristics of dissemination of embolic materials during renal artery stenting. Catheter Cardiovasc Interv. 2007;70:784–788.
70 Haas M., Spargo B.H., Wit E.J., et al. Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35:433–447.
71 Hara S., Asada Y., Fujimoto S., et al. Atheroembolic renal disease: clinical findings of 11 cases. J Atheroscler Thromb. 2002;9:288–291.
72 Thadhani R.I., Camargo C.A.Jr, Xavier R.J., et al. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine. 1995;74:350–358.
73 Goldman M., Thoua Y., Dhaene M., et al. Necrotising glomerulonephritis associated with cholesterol microemboli. Br Med J (Clin Res Ed). 1985;290:205–206.
74 Theriault J., Agharazzi M., Dumont M., et al. Atheroembolic renal failure requiring dialysis: potential for renal recovery? A review of 43 cases. Nephron. 2003;94:c11–c18.
75 Dalakos T.G., Streeten D.H., Jones D., et al. “Malignant” hypertension resulting from atheromatous embolization predominantly of one kidney. Am J Med. 1974;57:135–138.
76 Kasinath B.S., Corwin H.L., Bidani A.K., et al. Eosinophilia in the diagnosis of atheroembolic renal disease. Am J Nephrol. 1987;7:173–177.
77 Wilson D.M., Salazer T.L., Farkouh M.E. Eosinophiluria in atheroembolic renal disease. Am J Med. 1991;91:186–189.
78 Haqqie S.S., Urizar R.E., Singh J. Nephrotic-range proteinuria in renal atheroembolic disease: report of four cases. Am J Kidney Dis. 1996;28:493–501.
79 Williams H.H., Wall B.M., Cooke C.R. Reversible nephrotic range proteinuria and renal failure in atheroembolic renal disease. Am J Med Sci. 1990;299:58–61.
80 Belenfant X., Meyrier A., Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. Am J Kidney Dis. 1999;33:840–850.
81 Herzog A.L., Wanner C. Case report: atheroembolic renal disease in a 72-year-old patient through coronary intervention after myocardial infarction. Hemodial Int. 2008;12:406–411.
82 Scolari F., Ravani P., Pola A., et al. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. J Am Soc Nephrol. 2003;14:1584–1590.
83 Fabbian F., Catalano C., Lambertini D., et al. A possible role of corticosteroids in cholesterol crystal embolization. Nephron. 1999;83:189–190.
84 Nakayama M., Nagata M., Hirano T., et al. Low-dose prednisolone ameliorates acute renal failure caused by cholesterol crystal embolism. Clin Nephrol. 2006;66:232–239.
85 Graziani G., Santostasi S., Angelini C., et al. Corticosteroids in cholesterol emboli syndrome. Nephron. 2001;87:371–373.
86 Hasegawa M., Sugiyama S. Apheresis in the treatment of cholesterol embolic disease. Ther Apher Dial. 2003;7:435–438.
87 Woolfson R.G., Lachmann H. Improvement in renal cholesterol emboli syndrome after simvastatin. Lancet. 1998;351:1331–1332.
88 Hasegawa M., Kawashima S., Shikano M., et al. The evaluation of corticosteroid therapy in conjunction with plasma exchange in the treatment of renal cholesterol embolic disease. A report of 5 cases. Am J Nephrol. 2000;20:263–267.
89 Cabili S., Hochman I., Goor Y. Reversal of gangrenous lesions in the blue toe syndrome with lovastatin–a case report. Angiology. 1993;44:821–825.
90 Dubel G.J., Murphy T.P. Distal embolic protection for renal arterial interventions. Cardiovasc Intervent Radiol. 2008;31:14–22.
91 Moolenaar W., Lamers C.B. Gastrointestinal blood loss due to cholesterol crystal embolization. J Clin Gastroenterol. 1995;21:220–223.
92 Paraf F., Jacquot C., Bloch F., et al. Cholesterol crystal embolization demonstrated on GI biopsy. Am J Gastroenterol. 2001;96:3301–3304.
93 Blundell J.W. Small bowel stricture secondary to multiple cholesterol emboli. Histopathology. 1988;13:459–462.
94 Mulliken J.B., Bartlett M.K. Small bowel obstruction secondary to atheromatous embolism. A case report and review of the literature. Ann Surg. 1971;174:145–150.
95 Gramlich T.L., Hunter S.B. Focal polypoid ischemia of the colon: atheroemboli presenting as a colonic polyp. Arch Pathol Lab Med. 1994;118:308–309.
96 Chan T., Levine M.S., Park Y. Cholesterol embolization as a cause of cecal infarct mimicking carcinoma. AJR Am J Roentgenol. 1988;150:1315–1316.
97 Fujiyama A., Mori Y., Yamamoto S., et al. Multiple spontaneous small bowel perforations due to systemic cholesterol atheromatous embolism. Intern Med. 1999;38:580–584.
98 Funabiki K., Masuoka H., Shimizu H., et al. Cholesterol crystal embolization (CCE) after cardiac catheterization: a case report and a review of 36 cases in the Japanese literature. Japan Heart J. 2003;44:767–774.
99 Orvar K., Johlin F.C. Atheromatous embolization resulting in acute pancreatitis after cardiac catheterization and angiographic studies. Arch Intern Med. 1994;154:1755–1761.
100 Moolenaar W., Kreuning J., Eulderink F., et al. Ischemic colitis and acalculous necrotizing cholecystitis as rare manifestations of cholesterol emboli in the same patient. Am J Gastroenterol. 1989;84:1421–1422.
101 Moolenaar W., Lamers C.B. Cholesterol crystal embolization and the digestive system. Scand J Gastroenterol. 1991;188:69–72.
102 Harvey R.L., Doberneck R.C., Black W.C.3rd. Infarction of the stomach following atheromatous embolization. Report of a case and literature review. Gastroenterology. 1972;62:469–472.
103 Bourdages R., Prentice R.S., Beck I.T., et al. Atheromatous embolization to the stomach: an unusual cause of gastrointestinal bleeding. Am J Dig Dis. 1976;21:889–894.
104 Blackshear J.L., Jahangir A., Oldenburg W.A., et al. Digital embolization from plaque-related thrombus in the thoracic aorta: identification with transesophageal echocardiography and resolution with warfarin therapy. Mayo Clin Proc. 1993;68:268–272.
105 Donohue K.G., Saap L., Falanga V. Cholesterol crystal embolization: an atherosclerotic disease with frequent and varied cutaneous manifestations. J Eur Acad Dermatol Venereol. 2003;17:504–511.
106 Wingo J.P., Nix M.L., Greenfield L.J., et al. The blue toe syndrome: hemodynamics and therapeutic correlates of outcome. J Vasc Surg. 1986;3:475–480.
107 Kumins N.H., Owens E.L., Oglevie S.B., et al. Early experience using the wall graft in the management of distal microembolism from common iliac artery pathology. Ann Vasc Surg. 2002;16:181–186.
108 Baumann D.S., McGraw D., Rubin B.G., et al. An institutional experience with arterial atheroembolism. Ann Vasc Surg. 1994;8:258–265.
109 Keen R.R., McCarthy W.J., Shireman P.K., et al. Surgical management of atheroembolization. J Vasc Surg. 1995;;21:773–780. discussion 780–771
110 Applebaum R.M., Kronzon I. Evaluation and management of cholesterol embolization and the blue toe syndrome. Curr Opin Cardiol. 1996;11:533–542.
111 Spittell P.C., Seward J.B., Hallett J.W.Jr. Mobile thrombi in the abdominal aorta in cases of lower extremity embolic arterial occlusion: value of extended transthoracic echocardiography. Am Heart J. 2000;139:241–244.
112 Matchett W.J., McFarland D.R., Eidt J.F., et al. Blue toe syndrome: treatment with intra-arterial stents and review of therapies. J Vasc Interv Radiol. 2000;11:585–592.
113 Carroccio A., Olin J.W., Ellozy S.H., et al. The role of aortic stent grafting in the treatment of atheromatous embolization syndrome: results after a mean of 15 months follow-up. J Vasc Surg. 2004;40:424–429.
114 Kumpe D.A., Zwerdlinger S., Griffin D.J. Blue digit syndrome: treatment with percutaneous transluminal angioplasty. Radiology. 1988;166:37–44.
115 Friedman S.G., Krishnasastry K.V. External iliac ligation and axillary-bifemoral bypass for blue toe syndrome. Surgery. 1994;115:27–30.
116 Kaufman J.L., Saifi J., Chang B.B., et al. The role of extraanatomic exclusion bypass in the treatment of disseminated atheroembolism syndrome. Ann Vasc Surg. 1990;4:260–263.
117 Hollier L.H., Kazmier F.J., Ochsner J., et al. “Shaggy” aorta syndrome with atheromatous embolization to visceral vessels. Ann Vasc Surg. 1991;5:439–444.
118 Brewer M.L., Kinnison M.L., Perler B.A., et al. Blue toe syndrome: treatment with anticoagulants and delayed percutaneous transluminal angioplasty. Radiology. 1988;166:31–36.
119 Dougherty M.J., Calligaro K.D. Endovascular treatment of embolization of aortic plaque with covered stents. J Vasc Surg. 2002;36:727–731.
120 Van Thielen J, Hendriks JMH, Hertoghs M, et al: Filters placed in the superficial femoral arteries for limb salvage in a high-surgical-risk patient with atheroembolism: results at 2 years, J Endovasc Ther 17:399–401.
121 Rosenberg G.D., Killewich L.A. Blue toe syndrome from a “coral reef” aorta. Ann Vasc Surg. 1995;9:561–564.
122 Schlieper G., Grotemeyer D., Aretz A., et al. Analysis of calcifications in patients with coral reef aorta. Ann Vasc Surg. 2010;24:408–414.
123 Schulte K.M., Reiher L., Grabitz L., et al. Coral reef aorta: a long-term study of 21 patients. Ann Vasc Surg. 2000;14:626–633.
124 Teebken O.E., Pichlmaier M.A., Kuhn C., et al. Severe obstructive calcifications affecting the descending and suprarenal abdominal aorta without coexisting peripheral atherosclerotic disease–coral reef aorta. Vasa. 2006;35:206–208.
125 Holfeld J., Gottardi R., Zimpfer D., et al. Treatment of symptomatic coral reef aorta by endovascular stent-graft placement. Ann Thorac Surg. 2008;85:1817–1819.
126 Bernard Y. Value of transoesophageal echocardiography for the diagnosis of embolic lesions from the thoracic aorta. J Neuroradiol. 2005;32:266–272.
127 Laperche T., Laurian C., Roudaut R., et al. Mobile thromboses of the aortic arch without aortic debris. A transesophageal echocardiographic finding associated with unexplained arterial embolism. The Filiale Echocardiographie de la Société Française de Cardiologie. Circulation. 1997;96:288–294.
128 Amarenco P., Cohen A., Tzourio C., et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479.
129 Amarenco P., Duyckaerts C., Tzourio C., et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225.
130 Jones E.F., Kalman J.M., Calafiore P., et al. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218–224.
131 Cohen A., Tzourio C., Bertrand B., et al. Aortic plaque morphology and vascular events: a follow-up study in patients with ischemic stroke. FAPS Investigators. French Study of Aortic Plaques in Stroke. Circulation. 1997;96:3838–3841.
132 Di Tullio M.R., Homma S., Jin Z., et al. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008;52:855–861.
133 Djaiani G., Fedorko L., Borger M., et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356–e358.
134 Katz E.S., Tunick P.A., Rusinek H., et al. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70–77.
135 Karalis D.G., Chandrasekaran K., Victor M.F., et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73–78.
136 Tunick P.A., Kronzon I. Protruding atherosclerotic plaque in the aortic arch of patients with systemic embolization: a new finding seen by transesophageal echocardiography. Am Heart J. 1990;120:658–660.
137 Tunick P.A., Perez J.L., Kronzon I. Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427.
138 Tunick P.A., Rosenzweig B.P., Katz E.S., et al. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23:1085–1090.
139 Tunick P.A., Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545–554.
140 Ferrari E., Vidal R., Chevallier T., et al. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol. 1999;33:1317–1322.
141 Naghavi M., Libby P., Falk E., et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672.
142 Dressler F.A., Craig W.R., Castello R., et al. Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke. J Am Coll Cardiol. 1998;31:134–138.
143 Ackerstaff R.G., Jansen C., Moll F.L., et al. The significance of microemboli detection by means of transcranial Doppler ultrasonography monitoring in carotid endarterectomy. J Vasc Surg. 1995;21:963–969.
144 Beal M.F., Park T.S., Fisher C.M. Cerebral atheromatous embolism following carotid sinus pressure. Arch Neurol. 1981;38:310–312.
145 Ezzeddine M.A., Primavera J.M., Rosand J., et al. Clinical characteristics of pathologically proved cholesterol emboli to the brain. Neurology. 2000;54:1681–1683.
146 Laloux P., Brucher J.M. Lacunar infarctions due to cholesterol emboli. Stroke. 1991;22:1440–1444.
147 Cremonesi A., Manetti R., Setacci F., et al. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34:1936–1941.
148 Feldman R.L., Wargovich T.J., Bittl J.A. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter Cardiovasc Interv. 1999;46:245–248.
149 Henry M., Henry I., Klonaris C., Polydorou, et al. Renal angioplasty and stenting under protection: the way for the future? Catheter Cardiovasc Interv. 2003;60:299–312.
150 Henry M., Henry I., Klonaris C., et al. Benefits of cerebral protection during carotid stenting with the PercuSurge GuardWire system: midterm results. J Endovasc Ther. 2002;9:1–13.
151 Kawarada O., Yokoi Y., Takemoto K., et al. Double-wire technique in balloon-protected carotid artery stenting. J Interv Cardiol. 2007;20:55–62.
152 Kastrup A., Groschel K., Krapf H., et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34:813–819.
153 Grundy S.M., Cleeman J.I., Merz C.N.B., et al. Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732.
154 Blanco-Colio L.M., Tunon J., Martin-Ventura J.L., et al. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23.
155 Cannon C.P., Steinberg B.A., Murphy S.A., et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445.
156 Nissen S.E., Nicholls S.J., Sipahi I., et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565.
157 Tunick P.A., Nayar A.C., Goodkin G.M., et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320–1325.
158 Ling G., Ovbiagele B. Oral antiplatelet therapy in the secondary prevention of atherothrombotic events. Am J Cardiovasc Drugs. 2009;9:197–209.
159 Dean S.M., Vaccaro P.S. Successful pharmacologic treatment of lower-extremity ulcerations in 5 patients with chronic critical limb ischemia. J Am Board Fam Pract. 2002;15:55–62.
160 Elinav E., Chajek-Shaul T., Stern M. Improvement in cholesterol emboli syndrome after iloprost therapy. BMJ. 2002;324:268–269.
161 Carr M.E.Jr, Sanders K., Todd W.M. Pain relief and clinical improvement temporally related to the use of pentoxifylline in a patient with documented cholesterol emboli–a case report. Angiology. 1994;45:65–69.
162 Jennings W.C., Corder C.N., Jarolim D.R., et al. Atheromatous embolism: varied clinical presentation and prognosis. South Med J. 1989;82:849–852.
163 Yusuf S., Sleight P., Pogue J., et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators, [Erratum appears in N Engl J Med 2000 Mar 9;342(10):748.] [Erratum appears in 2000 May 4;342(18):1376.]. N Engl J Med. 2000;342:145–153.
164 Ghilardi G., Massaro F., Gobatti D., et al. Temporary spinal cord stimulation for peripheral cholesterol embolism. J Cardiovasc Surg (Torino). 2002;43:255–258.
165 Haimovici H., Steinman C., Karson I.H. Evaluation of lumbar sympathectomy. Advanced occlusive arterial disease. Arch Surg. 1964;89:1089–1095.
166 Fulton R.L., Blakeley W.R. Lumbar sympathectomy: a procedure of questionable value in the treatment of arteriosclerosis obliterans of the legs. Am J Surg. 1968;116:735–744.
167 Berardi R.S., Siroospour D. Lumbar sympathectomy in the treatment of peripheral vascular occlusive disease. Ten-year study. Am J Surg. 1975;130:309–314.
168 Berry R.E., Flotte C.T., Coller F.A. A critical evaluation of lumbar sympathectomy for peripheral arteriosclerotic vascular disease. Surgery. 1955;37:115–129.
169 Kim G.E., Ibrahim I.M., Imparato A.M. Lumbar sympathectomy in end stage arterial occlusive disease. Ann Surg. 1976;183:157–160.
170 Lee B.Y., Madden J.L., Thoden W.R., et al. Lumbar sympathectomy for toe gangrene. Long-term follow-up. Am J Surg. 1983;145:398–401.
171 Vella A., Carlson L.A., Blier B., et al. Circulator boot therapy alters the natural history of ischemic limb ulceration, [Erratum appears in Vasc Med 2000;5(2):128.]. Vasc Med. 2000;5:21–25.