Chapter 55 Chronic Venous Insufficiency
An estimated 25 to 40 million Americans have varicose veins, and more than 2 million have chronic venous insufficiency, including venous edema, skin changes, or venous ulcers.1 The cost of venous ulcer care is greater than $3 billion in the United States; in the United Kingdom, estimates are greater than $1 billion annually.2,3 Patients with advanced venous disease have decreased quality of life and occasionally, severe disease that is limb threatening.4,5
Definition
Chronic venous disease (CVD) includes a series of clinical conditions of varying severity, from varicose veins at one end of the spectrum to venous ulcers at the other. Chronic venous insufficiency (CVI) is the diagnosis given to patients with venous dysfunction causing edema, skin changes, or ulcerations (CEAP class 3-6; Box 55-1). The CEAP classification is used to define, categorize, and grade the severity of all chronic venous disorders.6 This classification provides insight into the clinical presentation (C), etiology (E), anatomy (A), and pathophysiology (P) of the underlying venous disorder.
![]() Box 55-1 The CEAP Classification (Basic)
Box 55-1 The CEAP Classification (Basic)
Adapted from Eklof B, Rutherford RB, Bergan JJ, et al: Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 40:1248–1252, 2004; used with permission.
Clinical Presentation
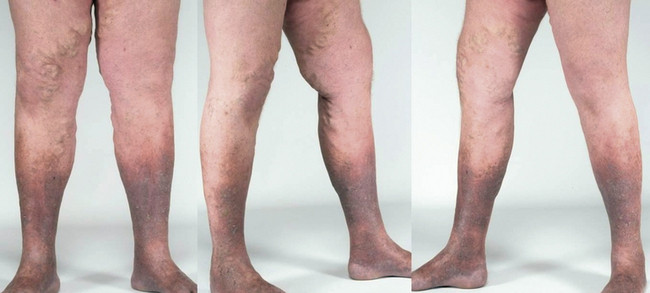
Varicose veins (CEAP class C2) are dilated superficial veins (>3 mm in diameter in the upright position)7 (see Chapter 54). Chronic venous insufficiency includes patients with more advanced disease, with or without varicose veins. These patients have edema, skin changes like dermatosclerosis, pigmentation, corona phlebectatica or eczema, or healed or active venous ulcer8,9 (see Box 55-1).
Increased ambulatory venous hypertension leads to a series of changes in subcutaneous tissue and skin, mostly in the “gaiter” area of the leg, at or above the ankle (Fig. 55-1). These are caused by activation of the endothelial cells (ECs), extravasation of macromolecules and red blood cells, diapedesis of leukocytes, tissue edema, and chronic inflammatory changes.7 These changes are all consequences of either venous valvular incompetence or venous obstruction, or a combination of both.
Etiology
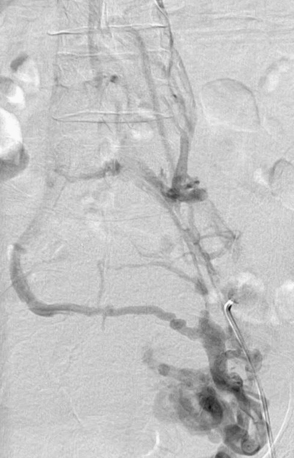
Most cases of venous insufficiency have underlying primary etiology. The most frequent cause is likely intrinsic morphological or biochemical abnormality in the vein wall. The origin of venous reflux in patients with primary varicose veins can be local or multifocal structural weakness of the vein wall, and this can occur together or independently of proximal saphenous vein valvular incompetence.10 Chronic venous insufficiency can also develop as a result of secondary causes such as previous deep venous thrombosis (DVT), deep venous obstruction, superficial thrombophlebitis, or arteriovenous fistula (AVF). May-Thurner’s syndrome (occlusion or stenosis of the left iliac vein due to compression by the overriding right common iliac artery [CIA]) is a cause of CVI that is likely much more frequent than previously thought (Fig. 55-2). Varicose veins may also be congenital and present as venous malformation and eventually lead to CVI. Primary venous insufficiency accounts for approximately 70% of advanced CVI (class 4-6), with the remainder occurring in legs following DVT.11,12
Anatomy
Significant changes have been made to venous terminology over the last decade, and these have been uniformly adopted and promoted by international vascular societies.13,14Superficial veins of the lower limbs are those located between the deep fascia, covering the muscles of the limb, and the skin. The main superficial veins are the great saphenous vein (GSV) and the small saphenous vein (SSV; Fig. 55-3). All previous names used to describe these vessels (greater, long, and lesser) should be abandoned. The GSV originates from the medial superficial veins of the dorsum of the foot and ascends in front of the medial malleolus along the medial border of the tibia, behind the saphenous nerve. There are posterior and anterior accessory saphenous veins in the calf and the thigh. The saphenofemoral junction (SFJ) is the confluence of superficial inguinal veins comprising the GSV and superficial circumflex iliac, superficial epigastric, and external pudendal veins. The GSV in the thigh lies in the saphenous subcompartment of the superficial compartment between the saphenous fascia and deep fascia. The SSV is the primary superficial vein of the posterior aspect of the lower leg and originates posterior to the lateral malleolus and courses posterolaterally in the lower leg to drain into the popliteal vein, behind the knee. The intersaphenous vein (vein of Giacomini) connects the SSV in the posterior thigh with the GSV.15
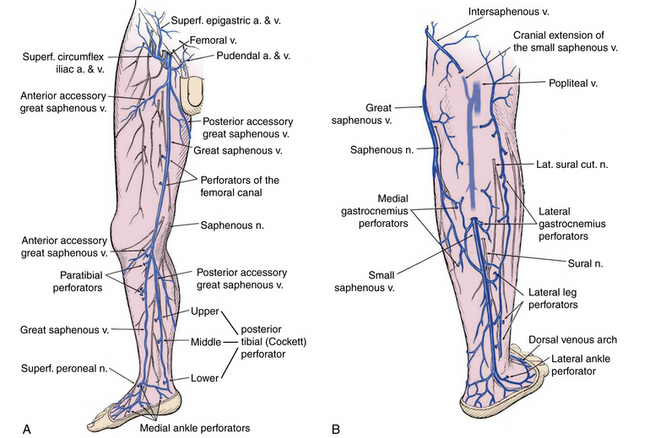
Figure 55-3 A, Medial superficial and perforating veins of the lower limb. B, Posterior superficial and perforating veins of the leg.
(Used with permission of Mayo Foundation for Medical Education and Research.)
Deep veins follow the arterial circulation in the limb and pelvis and are usually paired in the lower leg, accompanying tibial arteries by the same name. The popliteal or the femoral vein can also be paired. The femoral vein runs parallel to the superficial femoral artery (SFA) and replaces the term superficial femoral vein to avoid confusion regarding its importance as a deep vein.14 The pelvic veins include the external, internal, and common iliac veins (CIVs), which drain into the inferior vena cava (IVC). Large gonadal veins drain into the IVC on the right and left renal vein on the left.
Perforator veins traverse the deep muscular fascia and communicate between the superficial and deep venous system at multiple levels in the lower extremity (see Fig. 55-3). These primarily function to drain the superficial system into the deep venous system. The most important leg perforating veins are the medial calf perforators.16 The posterior tibial perforating veins (“Cockett perforators” in the old nomenclature) connect the posterior accessory GSV with the posterior tibial veins and form the lower, middle, and upper groups. They are located just behind the medial malleolus (lower), at 7 to 9 cm (middle) and 10 to 12 cm (upper) from the lower edge of the malleolus. The distance between these perforators and the medial edge of the tibia is 2 to 4 cm.16 Paratibial perforators connect the main GSV trunk with the posterior tibial veins. In the distal thigh, perforators of the femoral canal connect, usually directly, the GSV to the femoral vein.
Bicuspid venous valves are present in all superficial and deep lower-extremity veins and are important in assisting normal unidirectional venous flow. The GSV usually has at least six valves (maximum 14-25),17 with a constant valve present within 2 to 3 cm of the SFJ in 85% of cases (preterminal valve).18 The SSV has a median of 7 to 10 valves (range, 4-13).17 There are valves in the deep veins of the lower limb, but the common femoral or external iliac vein has only one valve in about 63% of cases.17 In 37%, there is no valve in the common iliac vein. The internal iliac vein has a valve in 10%; its tributaries have valves in 9%.19
Although isolated severe incompetence of the superficial system may lead to development of ulcers with high ambulatory pressures, most venous ulcers have underlying multisystem (superficial, deep, perforator) incompetence involving at least two of the three venous systems.20,21 Of 239 patients with venous ulcers evaluated with duplex scanning in three different studies, 144 (60.3%) had incompetent perforating veins, and 141 (59%) had deep vein incompetence or obstruction.22–24
Pathophysiology
Most patients with CVI have primary or secondary venous valvular incompetence. Venous outflow obstruction can be the result of primary venous disease (e.g., May-Thurner’s syndrome),25 or it can be the result of a previous DVT. Occasionally, congenital anomalies like deep vein agenesis or hypoplasia result in venous outflow obstruction.
Venous obstruction results in venous congestion, distal venous hypertension, and venous claudication. Obstruction alone may also result in skin changes and venous ulcerations. Depending on the extent of the collateral circulation, these patients will have pain, swelling, and heaviness of the limb; rarely, the most severe forms of venous obstruction may even interfere with viability of the limb.
Patients with valvular incompetence may have similar symptoms as those with obstruction: varicosity, edema, skin changes, or venous ulcers. Venous claudication or feeling fullness and edema are usually not as severe as with venous obstruction, and relief with limb elevation is immediate.
Recent evidence suggests that venous obstruction could be more important than valvular incompetence. Among 504 patients who underwent iliac vein stenting for venous obstruction, symptoms resolved after stenting, despite associated valvular incompetence.26
Diagnostic Evaluation
A thorough history and physical examination should be complemented by duplex scan of the superficial and deep veins to evaluate obstruction and valvular incompetence, as recommended by the recent guidelines of the Society for Vascular Surgery and the American Venous Forum (recommendation grade 1A).27 Subsequent diagnostic testing is carried out based on clinical presentation and examination findings.
History
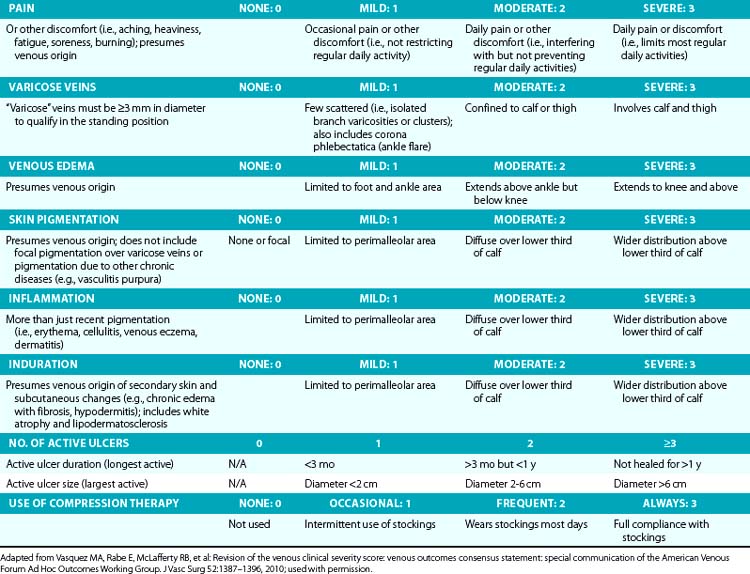
Symptoms related to CVI can include tingling, aching, burning, pain, muscle cramps, swelling, sensation of throbbing or heaviness, itching skin, restless legs, leg tiredness, fatigue, or ulceration in extreme cases.28 These symptoms suggest CVI, particularly if patients notice exacerbation by heat or dependency during the course of the day and relief by resting or leg elevation or use of compression therapy.29 Pain during and after exercise that is relieved with rest and leg elevation (venous claudication) can also be caused by venous outflow obstruction from previous DVT (postthrombotic syndrome) or obstruction of CIVs (May-Thurner’s syndrome).28 Diffuse pain is more frequently associated with axial venous reflux, whereas poor venous circulation in bulging varicose veins usually causes local pain. The Guideline Committee recommends using the revised Venous Clinical Severity Score (VCSS; Table 55-1)30 to grade and document the presenting symptoms of patients with CVD.27
A detailed medical history may establish the diagnosis of primary, secondary, or congenital venous problems. Adequate history should address previous DVT or thrombophlebitis, personal or family history of thrombophilia, medication history (particularly oral contraceptive pills), smoking, obstetric history, and a family history of venous disorders (most patients with varicose veins would be able to relate their parents’ or grandparents’ disease). Premenopausal women with varicose veins should also be questioned for symptoms of pelvic congestion syndrome (pelvic pain, aching, or heaviness; dyspareunia). Advanced age is the most important risk factor for varicose veins and CVI. A positive family history and obesity are risk factors for CVI.31
Physical Examination
Examination should always be performed with the patient in the standing position in a warm room with good light, and should establish the size, location, and distribution of varicose veins and also focus on other signs of venous disease such as edema (partially pitting or nonpitting), skin changes (induration, pigmentation, lipodermatosclerosis, atrophie blanche, eczema, dermatitis, skin discoloration, increased skin temperature), and ulceration (healed or active). Inspection and palpation are essential parts of the examination, and auscultation (for bruits) is particularly helpful in those with vascular malformation and arteriovenous fistula.32 Varicose dilations or venous aneurysms, palpable cord in the vein, tenderness, thrill, bruit, or pulsatility should be recorded. Ankle mobility should also be examined because patients with advanced venous disease frequently have decreased mobility in the ankle joints. Sensory and motor functions of the limb and foot are assessed to help differentiate from diabetic neuropathy or any underlying neurological problem. An abdominal mass or lymphadenopathy can provide a clue to venous compression and outflow obstruction.
Corona phlebectatica (ankle flare or malleolar flare) is a fan-shaped pattern of small intradermal veins located around the ankle or the dorsum of the foot.27 This is considered to be an early sign of advanced venous disease. Inspection of the abdominal wall and perineal and inguinal region should be routinely performed. Perineal, vulvar, or groin varicosities can be seen in iliac vein obstruction or internal iliac vein or gonadal vein incompetence causing pelvic congestion syndrome. Scrotal varicosity may be a sign of gonadal vein incompetence, left renal vein compression between the superior mesenteric artery (SMA) and aorta (nutcracker syndrome), or occasionally even IVC lesions or renal carcinoma.
Classic tourniquet tests for saphenous or perforator incompetence or deep venous occlusion (Trendelenburg test, Ochsner-Mahorner test, Perthes test) are rarely used today. They are mostly of historical interest and should be used in rare instances when duplex scanning or Doppler studies are unavailable.32 Distal palpation and proximal percussion of the saphenous vein, however, are useful tests to suggest valvular incompetence.
Skin lesions other than those already described (e.g., capillary malformations, tumors, onychomycosis, excoriations) should also be noted. An aneurysmal saphenous vein can be misdiagnosed as a femoral hernia or vice versa. Presence of a longer limb, lateral varicosity noted soon after birth, and associated capillary malformations are tipoffs for congenital venous malformation (Klippel-Trénaunay’s syndrome).33 Edema of the dorsum of the foot, squaring of the toes, thick skin, and nonpitting edema are signs of chronic lymphedema. A complete pulse examination should be performed to exclude underlying peripheral arterial disease. The physical examination can be complemented by a handheld Doppler examination, although the latter does not replace evaluation of the venous circulation with color duplex scanning.
The aim of the clinical evaluation is not only to determine the presenting signs and symptoms and type of venous disease (primary, secondary, congenital) but also exclude other etiologies (peripheral artery disease, rheumatoid disease, infection, tumor, or allergies). The physician should also establish the degree of disability caused by the venous disease and its impact on the patient’s quality of life.
Duplex Scanning
Duplex scanning is safe, noninvasive, cost-effective, and reliable and is recommended as the first diagnostic test for all patients with suspected CVD27,34,35 (also see Chapter 12). B-mode imaging permits accurate placement of the pulsed Doppler sample volume, and color flow evaluation makes it easier to establish obstruction, turbulence, and direction of venous and arterial flow,36 increasing diagnostic accuracy in comparison to continuous wave Doppler ultrasonography in the assessment of venous insufficiency.37 Duplex scanning is excellent for evaluation of both infrainguinal venous obstruction and valvular incompetence38 (Fig. 55-4).
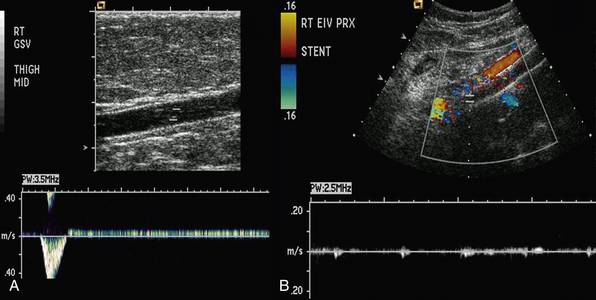
Figure 55-4 A, Duplex of right great saphenous vein (GSV) showing marked incompetence. B, Color duplex of left iliac vein showing absence of flow in stented venous segment, suggesting occlusion.
The appropriate technique of venous duplex scanning has been described in detail by several authors.39 An 8.4- to 9-MHz linear array pulsed-wave Doppler transducer is used most frequently for the deeper veins, with the higher-frequency probe (up to 18 MHz) for detailed assessment of the superficial veins. Evaluation of reflux should be performed with the patient in the upright position, with the leg rotated outward, heel on the ground, and weight taken on the opposite limb.35 The supine position gives both false-positive and false-negative results of reflux.40 All deep veins of the leg are evaluated, followed by evaluation of the superficial veins, including the GSV, SSV, accessory saphenous veins, and perforating veins for a complete examination.
The four essential components of a complete duplex scanning examination for CVD are visibility, compressibility, venous flow, and augmentation. Asymmetry in flow velocity, lack of respiratory variations in venous flow, and waveform patterns at rest and during flow augmentation in the common femoral veins indicate proximal obstruction. Reflux can be elicited in two ways: (1) increased intraabdominal pressure using a Valsalva maneuver for the common femoral vein or SFJ or (2) manual/cuff compression and release of the limb distal to the point of examination. The first is more appropriate for evaluation of reflux in the common femoral vein and at the SFJ, whereas compression and release is the preferred technique more distally on the limb.40 The guidelines recommend the cutoff value for abnormally reversed venous flow (reflux) as 1.0 second in the deep femoropopliteal veins and 500 milliseconds in the superficial and perforator veins.27
Perforating veins are evaluated in patients with advanced disease, usually CEAP class C5-C6, or in those with recurrent varicose veins after previous interventions. The diameter of a clinically relevant “pathological perforator” (e.g., beneath healed or open venous ulcer) can predict valve incompetence. The SVS/AVF Guideline Committee definition of “pathological” perforating veins includes those with outward flow of more than 500 milliseconds, with a diameter of greater than 3.5 mm, located beneath a healed or open venous ulcer (CEAP class C5-C6).27
Plethysmography
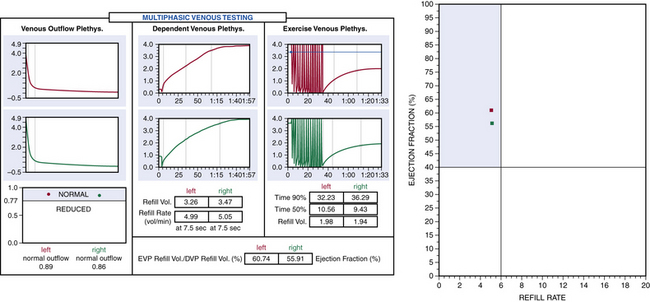
Air or strain-gauge venous plethysmography can provide noninvasive evaluation of calf muscle pump function, global venous reflux, and venous outflow obstruction41–44 (Figs. 55-5 and 55-6). Strain-gauge plethysmography is usually performed with a modified Struckmann protocol, validated by comparison with simultaneously recorded ambulatory venous pressure measurements.45 Strain-gauge or air plethysmography consists of exercise venous plethysmography, measurement of passive refill and drainage, and outflow plethysmography. Plethysmography quantifies venous reflux and obstruction and has been used to monitor venous functional changes and assess physiological outcome of surgical treatments.46,47 Venous plethysmography provides information on venous function in patients with CVI and is a complementary examination to duplex scanning. These studies are especially helpful in patients with suspected outflow obstruction but normal duplex findings, or those suspected of having venous disease due to calf muscle pump dysfunction, but no reflux or obstruction was noted on duplex scanning. Air plethysmography remains one of the few noninvasive techniques that can quantify reflux; other parameters have been reported to be variably useful.44,47 According to the new guidelines, use of air plethysmography is encouraged as best practice in evaluation of patients with CVI (C3-6).27
Intravascular Ultrasound
Recent assessment of patients with iliofemoral venous occlusion suggested that intravascular ultrasound (IVUS) should be used for evaluation of all patients with suspected or confirmed iliac vein obstruction. Intravascular ultrasound can be used in veins with obstruction without occlusion to assess venous wall morphology and mural thickness and identify trabeculations and recanalization, frozen valves, and external compression. Some of these lesions, as emphasized by Raju and Neglen, are not seen with conventional planar venography and provide measurements in assessing the degree of stenosis.48 In addition, in patients who have had an endovenous intervention, IVUS confirms position of the stent in the venous segment and resolution of the stenosis.48
Contrast Venography and Hemodynamic Studies
Ascending or descending (or both) contrast venography for CVI is performed selectively in patients with deep venous obstruction, postthrombotic syndrome, thrombotic or nonthrombotic pelvic vein obstruction (May-Thurner’s syndrome), pelvic congestion syndrome, nutcracker syndrome, vascular malformations, venous trauma, tumors, and if endovenous or open surgical treatment is planned. Descending venography is used to study venous valve incompetence. The Valsalva maneuver is used to grade the severity of reflux (grade 1-4: 1 = to upper thigh, 2 = to distal thigh, 3 = popliteal reflux, 4 = reflux to tibials and perforators).49,50 Since open valve repair is rarely performed today, this test has been less frequently used in recent years. Ascending venography is performed in a standing position to evaluate patency of the superficial and/or deep venous system (Fig. 55-7). It can be used together with direct venous pressure measurements to evaluate patients with varicose veins and associated iliac vein obstruction (May-Thurner’s syndrome). Contrast venography is routinely used in CVD to perform endovenous procedures such as angioplasty or venous stenting or open venous reconstructions. A pressure gradient across iliofemoral obstruction at rest in the supine patient (3 mmHg) is indicative of functional venous obstruction. Arm/foot venous pressure measurements and ambulatory venous pressure (AVP) measurement in a dorsal foot vein are additional tests that can be performed. Detailed descriptions and techniques for these tests are provided in the consensus statement.2
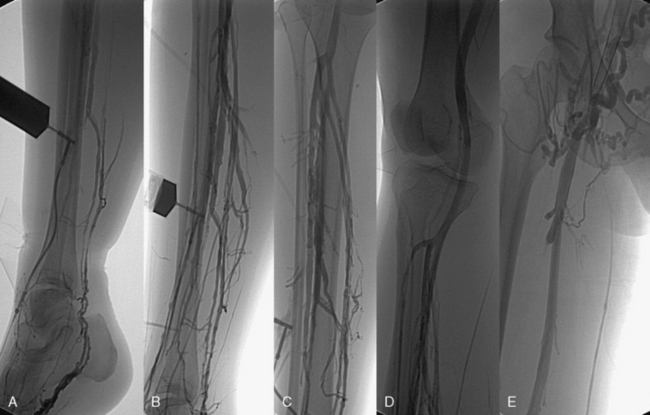
Figure 55-7 A-E, Ascending venogram performed by contrast injection in the pedal veins reveals evidence of chronic thrombotic changes and irregularity in the paired tibial veins (C) and normal popliteal and femoral vein segment (D). Large venous collaterals and absence of contrast in right iliac vein stent suggest obstruction (E).
Computed Tomography and Magnetic Resonance Venography
Early venous disease (C1-2) rarely requires advanced imaging studies other than duplex ultrasonography. Computed tomography (CT) and magnetic resonance imaging (MRI) have progressed tremendously in the last decade and provide excellent three-dimensional (3D) imaging of the venous system. Both modalities are suitable to identify pelvic or iliac venous obstruction in patients with lower-limb varicosity when proximal obstruction or iliac vein compression (May-Thurner’s syndrome) is suspected.48 They are also suitable to establish left renal vein compression (nutcracker syndrome),51 gonadal vein incompetence, and pelvic venous congestion syndrome. Gadolinium-enhanced MRI is especially useful in evaluating patients with vascular malformations, including those with congenital varicose veins.52
Laboratory Evaluation
Based on their history, selected patients with recurrent DVT, thrombosis at a young age, or thrombosis in an unusual site should undergo screening for thrombophilia.27 Laboratory examination is also needed in patients with long-standing recalcitrant venous ulcers, since a small percentage of these patients could have an underlying secondary etiology, including neoplasia, chronic inflammation, and other disorders.53 Patients who undergo general anesthesia for treatment of CVI should undergo appropriate testing to assess suitability for such procedures.
Severity of Venous Disease
The diagnostic evaluation should provide adequate information to quantify and classify the severity of venous disease, using CEAP clinical class and VCSS.6,30 The revised VCSS documents are used to establish disease severity at the first examination and will quantify improvement or deterioration during follow-up. In the basic CEAP classification, only the highest score is used to denote clinical class, and only the main anatomical groups (superficial, perforating, deep) are noted.
The revised format of the classification6 includes two elements in addition to the CEAP findings, the date and diagnostic level of the evaluation:
l. Level 1: history, physical, Doppler examination (handheld).
l. Level 2: noninvasive—duplex scan, plethysmography.
l. Level 3: invasive—venography, venous pressure, IVUS, CT venography, MR venography.
Recording the date and method used to confirm the clinical impression can be added in parentheses after the CEAP recording.
The main purpose of using the CEAP classification in patients with varicose veins is to distinguish primary venous disease causing simple varicose veins from secondary postthrombotic venous insufficiency.7 Evaluation and treatment of the two conditions are distinctly different. Complete CEAP classification and quality-of-life (QOL) evaluation is recommended before and after treatment to help to assess the patient’s perception of the burden of the disease for research purposes. A general QOL instrument such as the SF-36 and one of the disease-specific QOL instruments (e.g., VEINES, CIVIQ 2, Aberdeen, Charing cross) should be used.4,54–56
Treatment of Chronic Venous Insufficiency
Treatment of CVI requires a multimodality approach and has roles for both medical and interventional therapy. A concise flowchart for surgical/interventional treatment decision making is presented in Figure 55-8.
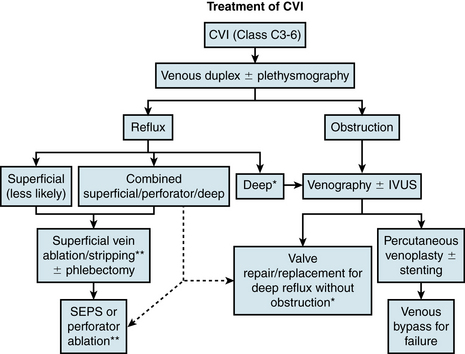
Figure 55-8 Evaluation and management of chronic venous insufficiency (CVI).
Good compression therapy, although not listed, is a must in any treatment protocol. *, Essential to rule out occult underlying obstruction; ** and dotted lines, in selected cases only. IVUS, intravascular ultrasound; SEPS, subfascial endoscopic perforator surgery.
Noninvasive Treatment
Drug treatment
In the United States, no phlebotonic drug is approved by the U.S. Food and Drug Administration (FDA) for treatment of CVI. Elsewhere, multiple phlebotonic drugs are available to treat symptoms of CVI; they have also been used to decrease ankle swelling and accelerate ulcer healing. Many compounds have been tried with varying success, but the most promising drugs include γ-benzopyrones (flavonoids) such as rutoside, diosmin, and hesperidin, micronized purified flavonoid fraction (MPFF), saponins like horse chestnut seed extract (aescin), pentoxifylline, and other plant extracts like French maritime pine bark extract. Synthetic products include calcium dobesilate, naftazone, aminophtone, and chromocarb.57
Precise mechanisms of action of most of these drugs is unknown, but they are postulated to improve venous tone and capillary permeability. Flavonoids appear to affect leukocytes and the endothelium by modifying the degree of inflammation and reducing edema.9 Two different Cochrane reviews found that flavonoids58 and aescin59 appeared to have an effect on edema and restless legs syndrome, but these meta-analyses concluded there is insufficient evidence to support the global use of phlebotonics to treat CVD.
Recent randomized controlled trials (RCTs) for pentoxifylline have shown some benefit in venous ulcer healing, although all three trials failed to show any statistically significant benefit.60–62 The venous guidelines of the American College of Chest Physicians (ACCP) suggest the use of oral pentoxifylline (400 mg three times daily) in patients with venous ulcers, in addition to local care, a compression garment, or intermittent compression pump (ICP; grade 2B).63 A meta-analysis of five RCTs that included 723 patients with venous ulcers found that at 6 months, the chance of ulcer healing was 32% better in patients treated with adjunctive MPFF than in those managed by conventional therapy alone (relative risk reduction, 32%; 95% confidence interval [CI], 3%-70%).64 For patients with persistent venous ulcers, flavonoids in the form of MPFF given orally or sulodexide administered intramuscularly (IM) and then orally, are suggested in the ACCP guidelines (grade 2B).63
Compression therapy
Compression therapy remains the standard of care for patients with CVI and venous ulcers (class C3-C6). Compression is recommended to decrease ambulatory venous hypertension in patients with CVI, in addition to lifestyle modifications that include weight loss, exercise, and elevation of the legs whenever possible. Ambulatory compression techniques and devices include elastic compression stockings, paste gauze boots (Unna boot), multilayer elastic wraps, dressings, and elastic and nonelastic bandages and nonelastic garments. Pneumatic compression devices (e.g., ICP), applied primarily at night, are also used for patients with refractory edema and venous ulcers.65 The rationale of compression treatment is to compensate for the increased ambulatory venous hypertension. Pressures to compress the superficial veins in supine patients range from 20 to 25 mmHg. In the upright position, pressures around 35 to 40 mmHg have been shown to narrow the superficial veins, and pressures higher than 60 mmHg are needed to occlude them.66
Compression therapy improves calf muscle pump function and decreases reflux in vein segments in patients with CVI.67,68 Although graded compression is effective as primary treatment to aid healing of venous ulceration and as adjuvant therapy to interventions to prevent recurrence of venous ulcers, compliance is important.69 In one study, ulcer healing was 97% in compliant patients and 55% in noncompliant patients (P <0.0001), and recurrence was 16% in compliant patients and 100% in noncompliant patients.69
Systematic reviews of compression treatment for venous ulcers concluded that compression treatment improves ulcer healing compared with no compression, and that high compression is more effective than low compression.70,71 A recent meta-analysis examined data of 692 ulcer patients in eight RCTs and found that ulcer healing was faster by an average of 3 weeks with stockings than with bandages (P = 0.0002), and pain was significantly less (P <0.0001).72 There is no evidence that hydrocolloid or other dressings beneath compression is more effective than compression alone.73
The ESCHAR trial randomized 500 patients with leg ulcers to either compression treatment alone or compression in combination with superficial venous surgery.74,75 Compression consisted of multilayer compression bandaging followed by class 2 (medium compression, 18-24 mmHg) below-knee stockings. Compression treatment alone was as effective as compression with surgery for ulcer healing (65% vs. 65%; hazard ratio, 0.84 [95% CI, 0.77-1.24]), but 12-month ulcer recurrence rates were reduced in the compression with surgery group vs. those with compression alone (12% vs. 28%; hazard ratio, −2.76 [95% CI, −1.78 to −4.27]). This difference in ulcer recurrence rates persisted between the two groups at 4 years.76 Unfortunately, this trial did not have a surgery-only arm; data suggest that saphenous vein disconnection improves venous function and may heal venous ulcers even without compression bandaging in patients with normal deep veins.77
Surgical Treatment
Treatment of superficial vein incompetence
Superficial reflux treatment is the primary treatment modality in patients with CVI. Treatment of superficial reflux leads to decrease in ulcer recurrence, as proved in the ESCHAR trial.74,75 We refer readers to Chapter 54 for details on technique and results of surgical treatment of superficial venous incompetence.
High Ligation, Division, and Stripping
Surgical stripping of the GSV and/or SSV is performed selectively nowadays with the advent of endovenous ablation techniques. Patients who could benefit from open vein stripping include those with a very superficial great or accessory saphenous vein, with large tortuous or aneurysmal saphenous veins, and with partially recanalized veins after thrombophlebitis that cannot be cannulated using the ablation probe. There are limited data for preservation of the GSV using CHIVA78 or ASVAL79 techniques for CVI patients, and these are used selectively in few centers.
Treatment of perforator vein incompetence
An association between incompetent perforating veins and venous ulcers was established more than a century ago by Gay,82 and surgical perforator interruption was recommended by multiple authors to treat venous ulcers.83–86 The importance of incompetent perforators in CVI is supported by the observation that most skin changes and ulcers are seen in the gaiter area, where large incompetent medial perforating veins are located. A correlation between number and size of duplex-based incompetent perforating veins and severity of CVI has been demonstrated.20 Still, the contribution of perforator incompetence to CVI changes is debated, and further randomized studies to establish a firm connection are warranted.
Perforator interruption is reserved for patients with advanced CVI (CEAP classes 5-6) and documented perforator reflux on duplex scanning (see established criteria above). Usually superficial reflux is corrected first. In many cases, superficial reflux correction would lead to resolution of some of the perforator vein incompetence,74,87 although one study showed conflicting results.88 Contraindications to perforator interruption include moderate to severe arterial occlusive disease, infected ulcer, or medically unfit or nonambulatory patient. Relative contraindications include diabetes, renal/liver failure, morbid obesity, autoimmune disorders, recent DVT, and severe lymphedema. Popliteal or proximal deep venous obstruction is also a relative contraindication. A brief description of the anatomy of perforator veins is described in the preceding section and illustrated in Figure 55-1, and details can be found in the article by Mozes et al.16 All identified incompetent perforators are marked on the skin with nonerasable marker during perioperative duplex.
Open Perforator Interruption
Linton’s original open subfascial ligation83 was abandoned because of a wound complication rate of up to 24%.89,90 Many modifications were proposed to this technique, and currently the only potential role for open perforator ligation is in the lateral fascial compartment, owing to limited space in this compartment. Open perforator ligation using duplex guidance and small incisions may limit the extent of surgery. One RCT observed a significantly higher rate of wound complications after open perforator ligation using a modified Linton procedure than after endoscopic perforator ligation (53% vs. 0%; P <0.001).91
Subfascial Endoscopic Perforator Surgery
Hauer’s technique of subfascial endoscopic perforator surgery (SEPS), introduced in 1985,92 uses a single port and is practiced mostly in Europe. O’Donnell was the first to use laparoscopic instrumentation with a two-port technique93; the Mayo Clinic team94 and Conrad95 improved the technique and added carbon dioxide insufflation to the procedure. Between 1992 and 2008, SEPS became the technique of choice for perforator ablation, primarily because of the reduced rate of wound complications.91,96
SEPS is performed under general or epidural anesthesia. Either single or double endoscopic port techniques can be used for dissection and division of medial calf perforators. Most authors use balloon dissection, carbon dioxide insufflation with a pressure of 30 mmHg, and a pneumatic thigh tourniquet inflated to 300 mmHg to avoid any bleeding in the surgical field.97 Division of the fascia of the deep posterior compartment with a paratibial fasciotomy is required to identify all important medial perforating veins. Occlusion of the perforators can be done with endoscopic clips, although most surgeons use an ultrasonic harmonic scalpel for division and transection of the perforators. The wounds are closed, the tourniquet is deflated, and the extremity is wrapped with an elastic bandage. The operation is an outpatient procedure, and patients are encouraged to ambulate 3 hours after the operation.
The North American SEPS registry11 reported on results of SEPS performed in 17 U.S. centers on 155 limbs, 85% with class C5 and C6 disease. Ulcer healing at 1 year was 88%, with the median time to ulcer healing of 54 days. Ulcer recurrence was 16% at 1 year and 28% at 2 years. In this registry, there were 27 patients with class C6 disease who underwent SEPS alone; 78% of the ulcers healed, and the ulcer healing rate was significantly lower (79%) at 2 years than it was in those who had SEPS and superficial ablation (95%; P <0.05). The ulcer recurrence rate (35%) in the SEPS-only group at 2 years was, however, not significantly worse than that in patients who underwent SEPS and superficial ablation alone (25%).
In a systematic review, Tenbrook et al.98 reported results of the SEPS procedure performed with or without superficial ablation on 1140 limbs in one RCT and 19 case series. Ulcers in 88% of limbs healed but recurred in 13% at a mean time of 21 months. Risk factors for nonhealing and recurrence included postoperative incompetent perforating veins, pathophysiological obstruction, previous DVT, and ulcer diameter greater than 2 cm. The authors concluded that surgical treatment including SEPS, with or without saphenous ablation, is recommended for patients with venous ulcers, but RCTs are needed to discern the contributions of compression therapy, superficial venous surgery, and SEPS in patients with advanced CVI. A recent meta-analysis of SEPS by Luebke and Brunkwall99 reviewed data of studies published between 1985 and 2008 and concluded that SEPS used as part of a treatment regimen for severe CVI benefits most patients in the short term, regarding ulcer healing and prevention of ulcer recurrence, but further prospective RCTs are needed to define the long-term benefits of SEPS.
Treatment of deep vein incompetence
The first open venous valve reconstruction was reported by Kistner in 1968.100,101 Since then, many techniques have been developed and modified to treat deep venous insufficiency. Deep valve reconstruction is reserved for advanced CVI patients who have failed other treatment strategies, including compression therapy. Preoperative assessment includes venography in all patients, primarily to rule out underlying proximal venous obstruction causing secondary valvular reflux, but also to grade severity of reflux as described earlier. No specific strategies exist to quantify reflux at a single valve station. Pathological appearance of valves would vary depending on the underlying cause for incompetence, with both primary and secondary (postthrombotic) deep vein reflux contributing to about half the cases.102 In primary valve reflux patients, valves grossly have normal texture with a widened commissure. On the other hand, postthrombotic valves have fibrosis of the vein wall and valve cusp thickening, trabeculation, and sometimes complete destruction of the valve structure. In cases with extensive damage to valve structure, valvuloplasty is not an option; reconstruction is usually carried out using valve or vein transposition.
Exposure and preparation of valves is similar for both repair and transfer techniques. An arm is prepped out to provide access to the axillary/brachial vein in case vein transfer is needed. Usually only one valve is repaired, the first femoral vein valve, despite reflux at multiple stations in the femoral vein. The deep femoral vein (DFV) valve is also repaired in case it is incompetent. Meticulous dissection is carried out to identify the valve attachment lines, which may require subadventitial dissection in some postthrombotic cases. Intact valve lines signify preserved valve apparatus that can be repaired in most cases, in contrast to absence or disruption of valve attachment lines when repair is not possible.102 A strip test is used to confirm valve incompetence.
Valvuloplasty can be performed from within (internal as originally described by Kistner) or from outside (external). The goal for both approaches is to appose the commissure and achieve valve tightening. An obvious advantage of internal valvuloplasty is repair under direct vision, although opening the vein increases the risk of surgical trauma to the vein and valve apparatus. To overcome this disadvantage of external valvuloplasty, we have used an angioscopic external valvuloplasty technique where an angioscope is introduced from a tributary proximal to the repaired valve and helps with visualization during suture placement.103
Valve transfer can be achieved using two methods: either transfer of a segment of vein with a competent valve and using it as an interposition graft (usually an axillary/brachial vein segment from the arm); or transposition of the incompetent vein onto a competent vein segment (usually femoral vein is transposed to the adjacent GSV or DFV). Vein transposition requires an adjacent patent competent vein, which is not always true in many cases where the GSV has already been ablated/stripped and the DFV is also incompetent. Also, long-term competence is a concern, owing to increased chance of reflux with dilation as a result of increased flow.104 Axillary vein transfer is technically demanding, with 40% incidence of incompetent axillary valves in situ, and carries additional morbidity of a second incision.
Other less commonly used techniques include prosthetic sleeve reconstruction, de novo valve reconstruction, cryopreserved allograft, and artificial venous valves. None of these techniques have been widely adopted, and all previous attempts at artificial or cryopreserved valves were met with miserable failure. A new “neovalve” technique described by Maleti and Lugli105 holds tremendous promise, but their excellent results, at least in the short term, have not been duplicated at other centers.
Perioperative complications are rare, with minimal or no mortality in these cases. The incidence of DVT has been reported to be between 0% and 11%, with higher rates in postthrombotic patients.49,106 Long-term outcomes of deep vein incompetence have reported 60% to 80% ulcer healing,102 with better outcomes in primary disease and better outcomes with valvuloplasty compared to valve transfer techniques.49
Prevention of postthrombotic superficial/deep vein incompetence could be a key point of intervention, in view of limited success with valve reconstruction once the valves are damaged in these patients. It has been shown that duration of venous occlusion clearly affects the likelihood of secondary reflux.107 Multilevel disease and recurrent thrombosis are the two most predictive factors for future CVI.108 Early spontaneous lysis leads to preserved valve function and fewer symptoms.109 Therefore, there may be a role for prevention of venous reflux with early thrombus lysis. This is discussed further in Chapter 52. Also, recent data suggest that venous obstruction is more important than valvular incompetence. In a study of 504 patients who underwent iliac vein stenting, stenting was sufficient to control symptoms in the majority of patients with combined outflow obstruction and deep venous reflux, without need to correct underlying reflux.26
Treatment of deep vein obstruction
Open surgical reconstructions have been challenging because of multiple factors affecting graft patency,110 including inadequate graft material, low venous flow, and the thrombophilia that often occurs in these patients. Endovascular stenting is first-line therapy, with excellent midterm and even long-term results in patients with chronic symptomatic venous stenosis and short occlusions,111 but stenting is not possible or successful in all patients with long femoro-iliocaval venous occlusions.112
Femorofemoral Crossover Bypass
Femorofemoral crossover bypass can be performed for unilateral iliofemoral obstruction with suprapubic transposition of the GSV (Palma-Dale procedure; Fig. 55-9). The contralateral GSV is tunneled subcutaneously to the groin incision of the affected limb. An end-to-side anastomosis is performed between the saphenous vein and ipsilateral common femoral vein. This anastomosis can be spatulated onto the ipsilateral GSV if the femoral or deep femoral veins are diseased and the GSV is the predominant outflow in the affected leg. An externally supported 10- to 12-mm diameter polytetrafluoroethylene (PTFE) graft is used if the GSV is inadequate, less than 5 mm in diameter, or of poor quality. An (AVF) is performed between the conduit and SFA in selected cases and marked with a Silastic sheath for easy identification at a later time.
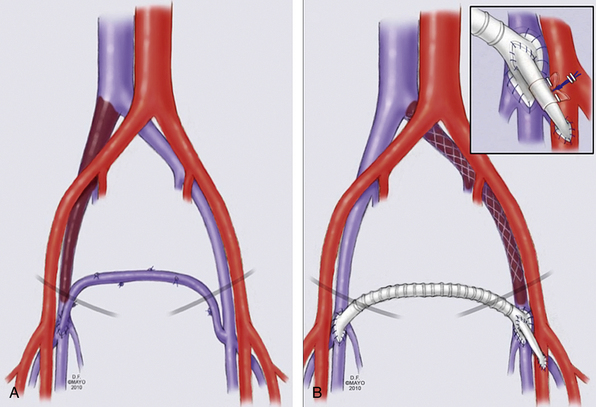
Figure 55-9 Schematic presentation of femorofemoral crossover venous bypass with either the contralateral great saphenous vein (GSV) (A) or prosthetic graft (B).
An arteriovenous fistula (AVF) is shown in inset, with marking Silastic sheath.
(Used with permission of Mayo Foundation for Medical Education and Research.)
Femoroiliac or Iliocaval Bypass
For femoroiliac or iliocaval bypass, the iliac vein is exposed via a flank incision, and the femoral vein is exposed through a standard groin incision. In cases with CIV occlusion, the infrahepatic cava is used as outflow, exposed through a midline incision. These “short” bypasses have a hemodynamic advantage due to the length and high flow. We prefer an externally supported 10- to 14-mm PTFE graft for these bypasses.
Femorocaval or Complex Bypass
Long bypasses from the femoral vein to the IVC have poor results because of the hemodynamics of flow across the femoral vein. Most of these patients also have extensive postphlebitic changes in the femoral and distal veins, making these procedures technically challenging and prone to failure due to poor inflow. Patients with bilateral disease, or those with obstruction of the suprarenal or suprahepatic IVC who have failed endovascular intervention, are evaluated for a complex reconstruction using either a bifurcated graft or tube graft with contralateral jump graft.
Saphenopopliteal Bypass
Saphenopopliteal bypass is popularly known as the May-Husni procedure and is indicated for femoral or proximal popliteal vein obstruction. The GSV, which most commonly is the major outflow from the leg via collaterals in these patients, is exposed above the knee joint, and a direct anastomosis is performed between the GSV and popliteal vein (end to side). Alternatively, a free vein conduit or prosthetic can be used in case the ipsilateral GSV is not suitable.
In a recent report on open surgical reconstruction for chronic iliofemoral occlusion, 50 patients underwent 52 open reconstructive procedures for CVI over a 25-year period.113 Twenty-nine patients underwent a femorofemoral crossover bypass, and 17 underwent a short bypass (femoroiliac or iliocaval). Early graft occlusion occurred in 17%, requiring reoperation, and discharge patency was 96%. There was no mortality or pulmonary embolism (PE). Five-year primary and secondary patency, respectively, for Palma vein grafts was 70% and 78%; for femoroiliac and ilio-infrahepatic IVC bypasses, 63% and 86%; and for femoro-infrahepatic IVC bypasses, 31% and 57%. May-Thurner’s syndrome with associated chronic thrombosis, use of prosthetic grafts, and endoscopic vein harvesting were each noted to be adversely related to long-term graft patency. More than 60% of patients had no venous claudication and no or minimal swelling. In multiple large series, the Palma procedure has shown good to excellent patency rates of 70% to 85% on mid- to long-term follow-up.114
Endovenous Treatment
Treatment of superficial vein incompetence
Endovenous treatment of superficial vein incompetence using radiofrequency (RFA) or laser (endovenous laser treatment [EVLT]) ablation or foam sclerotherapy has made significant advancement over the last decade and has replaced open surgical treatment in most centers. We again refer readers to Chapter 54 for details on technique and results of endovenous treatment of superficial venous incompetence.
Treatment of perforator incompetence
The emergence of ultrasonographically guided thermal ablation and sclerotherapy in recent years has transformed the techniques of perforator ablation.96,115–117
Advantages of percutaneous ablation of perforators (PAPS) include the low risk of a minimally invasive procedure that is easily repeatable and can be performed under local anesthesia in an office setting.118 PAPS is performed under ultrasound guidance, with direct needle puncture of the perforating vein performed under local anesthesia, with the patient in the reverse Trendelenburg position to allow for full venous distention. The tip of the needle should be at or just below the fascia in the vein to minimize deep vessel and nerve injury. After confirmation of probe position, local anesthesia is used to infiltrate surrounding tissues before treatment, and the patient is then placed in the Trendelenburg position.
Radiofrequency ablation of the perforating veins can be accomplished using a new intravascular ablation device (ClosureRFS Stylet, VNUS Medical Technologies, San Jose, Calif.). Intraluminal placement of the RF stylet is confirmed by ultrasonography and also by measuring impedance: values between 150 and 350 ohms indicate the intravascular location of the tip of the probe. Treatment is performed with a target temperature of 85 °C. All four quadrants of the vein wall are treated for 1 minute each. The catheter is then withdrawn 1 to 2 mm, and a second treatment is performed. The treatment is finished with applying compression to the region of the treated perforating vein.
Perforator EVLT is done with a 16-gauge angiocatheter (for 600-μm laser fiber) or a 21-gauge micropuncture needle (for a 400-μm laser fiber).119 Intraluminal placement of the access catheter is confirmed by ultrasonography and aspiration of blood at or just below the fascial level. Various methods of energy application are used, and perforating veins are treated at three locations, each segment receiving between 60 and 120 joules in 1 or 2 treatments.117,119 The rest of the procedure is similar to RFA.
Sclerotherapy of perforating veins is done with a 25-, 27-, or 30-gauge needle. A wire may be placed into the deep system for better control of the access. Ultrasonographically guided sclerotherapy can be performed using different agents. Care should be taken to avoid injection of the agent into the accompanying artery; 0.5 to 1 mL of the sclerosant is injected with the leg elevated to avoid flow into the deep system, and compression is applied over the treated perforators.119
Outcomes of PAPS are largely unknown. Most publications report small numbers of patients with short follow-up, frequently treated for mild disease (class C2-C3). Most data provided are on safety and surrogate endpoints, such as perforating vein occlusions, but less so on clinical and functional endpoints. A systematic review of five recently published cohort studies and seven unpublished case series found a mean occlusion rate of 80%, with a mean follow-up of less than 2 months.96 Ultrasonographically guided sclerotherapy is gaining rapid acceptance because perforating veins can be accessed easily with a small needle, without much pain to the patient. Significant improvement in VCSS was noted after ultrasonographically guided sclerotherapy using morrhuate sodium in 80 limbs with predominantly perforator incompetence, and ulcers rapidly healed in 86.5%, with a mean time to heal of 36 days.116 The ulcer recurrence rate was 32% at a mean of 20 months despite low compliance (15%) with compression hose. New and recurrent perforators were identified in 33% of limbs, and ulcer recurrence was associated with perforator recurrence and the presence of postthrombotic syndrome.
Treatment of deep vein incompetence
Endovascular treatment of deep vein incompetence using stents mounted with native or artificial valves has been fraught with failure. New designs in development are still in infancy, awaiting good clinical data.120
Treatment of deep vein obstruction
Deep vein obstruction, either postthrombotic or nonthrombotic, has recently been shown to be significant contributor to CVD. Adequate tests to quantify the exact role of deep vein obstruction are lacking, so experts now recommend routine use of IVUS for detailing venous assessment.111 In one study, more than one fourth of patients were shown to have more than 50% stenosis based on IVUS, whereas venogram failed to reveal any obstruction.121
Endovenous stenting (Fig. 55-10) is performed under local or general anesthesia in an endovascular suite. Access is achieved under ultrasound guidance, either via the femoral or jugular vein. After venography and IVUS (as needed), obstructed segments are crossed using a hydrophilic wire. Predilation is performed with high-pressure balloons, with which the risk of rupture is low to none. Chronic occlusive lesions usually require serial dilation to a final diameter of 12 to 18 mm. Self-expanding stents are used in the entire diseased segment. The Wallstent (Boston Scientific, USA) is used by most interventionalists because of the external compression due to high radial force in the common iliac vein. Large-diameter nitinol stents are used less frequently. Extension of the stent across the inguinal ligament is less of a concern in the venous segment.122 In cases of IVC obstruction, a large Gianturco (Cook Inc., USA) or Wallstent is used. We prefer to use a large Wallstent, and inside this a Gianturco stent, all the way to the caval bifurcation. Subsequent iliac stenting is performed with a Wallstent or nitinol self-expanding stents.123 Coverage of the renal veins with Gianturco stents has not been of concern in our experience and that of others.124
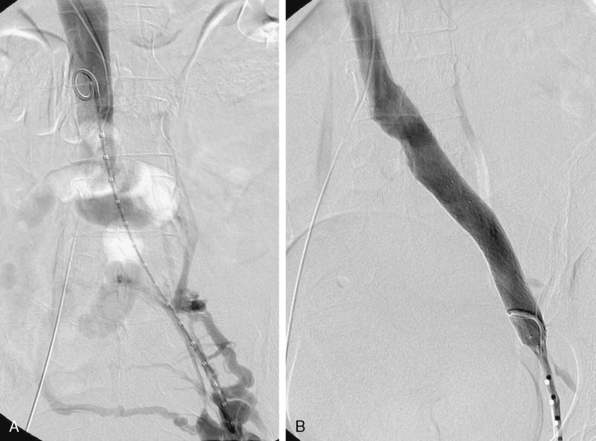
Figure 55-10 Successful recanalization and stenting of left iliac vein in a 43-year-old woman, with excellent luminal result and predominant flow through iliac vein (B) rather than pelvic collaterals (A).
In patients with common femoral vein obstruction and proximal disease, vascular access could be impossible, so hybrid reconstruction may be necessary. Endovenous recanalization and stent placement is combined with femoral vein endophlebectomy and patch angioplasty in these cases. Balloon angioplasty and stenting is performed prior to or at completion of the patch angioplasty. Jugular vein access is obtained for retrograde recanalization as an alternative to or in combination with femoral access. The stent is extended proximally to the healthy vein and across the inguinal ligament distally. With recent experience, we extend the distal end of the stent into the venous patch.113
Technical success for endovascular iliac vein recanalization is reported to be 87% to 100% in multiple series.123 Inferior vena cava occlusion does decrease the ability to cross the occluded segment, and technical success in these cases is reported to be 66% in one series.124 Mid- to long-term secondary patency is very good in successful cases, ranging from 75% at 48 months to 93% at 36 months.123 Patients with nonthrombotic obstruction fare much better than those with thrombotic occlusion.125 Cumulative rate of improvement of pain and swelling at 5 years after treatment of venous outflow obstruction was 78% and 55%, respectively, and reflux parameters did not deteriorate after stenting in one large study.26 In a study from Mayo Clinic, we also observed marked improvement in hemodynamics, venous function, and symptoms after successful treatment of venous outflow obstruction with stenting126 (Fig. 55-11). We noted improvement in both venous outflow and calf muscle pump function, and the residual volume fraction had decreased at the expense of venous reflux, which increased (increase of median venous filling index by 24%).
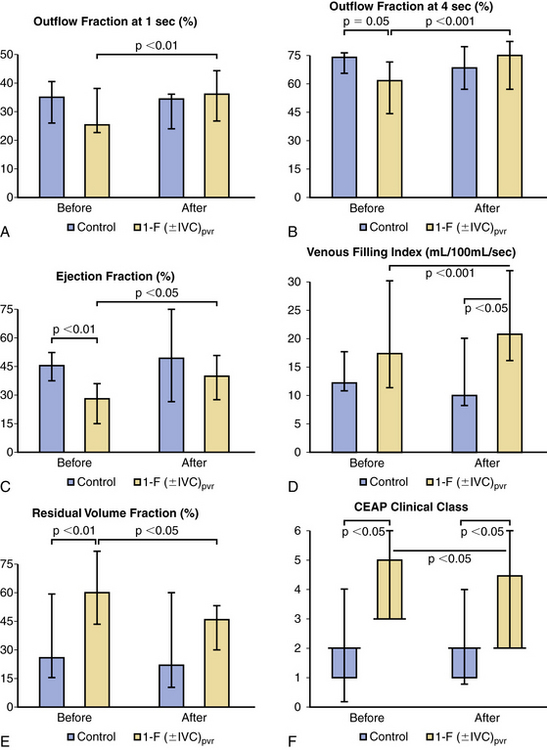
Figure 55-11 A-F, Effects of successful treatment of venous outflow obstruction using venous stents. Venous hemodynamics, including venous outflow (outflow fraction at 1 and 4 seconds; A-B), calf muscle pump function (ejection fraction; C), amount of venous reflux (venous filling index; D), and venous hypertension (residual volume fraction; E), and the CEAP clinical class (F) in 23 limbs with chronic iliofemoral (IF) inferior vena cava (IVC) thrombosis (DVT) and nine control limbs, before (30 days) and after (median, 8.4; interquartile range, 3-11.8 months) successful IF (IVC) venous stenting. Data are median and interquartile range.
(From Delis KT, Bjarnason H, Wennberg PW, et al: Successful iliac vein and inferior vena cava stenting ameliorates venous claudication and improves venous outflow, calf muscle pump function, and clinical status in post-thrombotic syndrome. Ann Surg 245: 130–139, 2007, with permission.)
Stenting improved the CEAP class status of the patients. Incapacitating venous claudication, noted in 62.5% of patients before stenting, was eliminated in all after stenting. The Mayo Clinic study concluded that successful treatment of venous outflow obstruction in patients with CVI ameliorates venous claudication, normalizes outflow, and enhances calf muscle pump function, resulting in significant clinical improvement of CVD. Increase in the amount of venous reflux of the stented limbs indicated that elastic or inelastic compression support of the successfully stented limbs would be pivotal in preventing disease progression. The study also supports the notion that venous obstruction is likely more important than venous valvular incompetence.126
Assessment of Treatment Outcome
Clinical outcome studies evaluate results of procedures on patient-focused outcomes, including symptom improvement, recurrence of varicosities, healing or recurrence of skin ulcers, improvement in the chronic progressive symptoms of CVD, improved QOL, and cosmetic improvement.127 These can be assessed using multiple questionnaires, including QOL measures for symptom relief and CEAP classification, in combination with revised VCSS for disease severity outcomes (reader is referred to previous section on “Severity of Venous Disease” for details). The Recurrent Varicose Veins After Surgery (REVAS) classification,128 a descriptive evaluation of recurrent and residual varicosities based on the physician’s assessment, can be used for cosmetic outcome, although it has some limitations.
Surrogate outcome measures are commonly used in reporting outcomes of treatment, although caution should be used. Surrogate outcomes may include patency of the ablated saphenous or perforating vein, patency of a venous bypass or stent, or hemodynamic results after interventions.
Conclusions
Treatment of CVI has progressed markedly over the last decade. This can be attributed to better understanding of the pathophysiology of venous disease, increased awareness of complications among both patients and care providers, and the advent of endovascular techniques and minimally invasive technology. Prevention of skin complications with compression therapy and early treatment of CVI with endovenous thermal ablations are essential. Endovenous ablation therapy has replaced open treatment of superficial reflux and is likely to replace open surgical treatment of perforator incompetence as well. Venous stenting is now the first line of treatment for deep venous outflow obstruction; open surgical reconstruction is indicated only in patients who fail or are not candidates for stenting. Further research is still needed to develop more effective treatment of deep vein valvular incompetence.
1 Kaplan R.M., Criqui M.H., Denenberg J.O., et al. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37(5):1047–1053.
2 Nicolaides A.N. Investigation of chronic venous insufficiency: a consensus statement (France, March 5-9, 1997). Circulation. 2000;102(20):E126–E163.
3 Gillespie D.L., Kistner B., Glass C., et al. Venous ulcer diagnosis, treatment, and prevention of recurrences. J Vasc Surg. 2010;52(Suppl 5):8S–14S.
4 Smith J.J., Guest M.G., Greenhalgh R.M., et al. Measuring the quality of life in patients with venous ulcers. J Vasc Surg. 2000;31(4):642–649.
5 Korn P., Patel S.T., Heller J.A., et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg. 2002;35(5):950–957.
6 Eklof B., Rutherford R.B., Bergan J.J., et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248–1252.
7 Kistner R., Eklof B. Classification and etiology of chronic venous disease. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;37–46.
8 Raju S., Neglen P. Clinical practice. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360(22):2319–2327.
9 Eberhardt R.T., Raffetto J.D. Chronic venous insufficiency. Circulation. 2005;111(18):2398–2409.
10 Labropoulos N., Giannoukas A.D., Delis K., et al. Where does venous reflux start? J Vasc Surg. 1997;26(5):736–742.
11 Gloviczki P., Bergan J.J., Rhodes J.M., et al. Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American subfascial endoscopic perforator surgery registry. The North American Study Group. J Vasc Surg. 1999;29(3):489–502.
12 Kalra M., Gloviczki P., Noel A.A., et al. Subfascial endoscopic perforator vein surgery in patients with post-thrombotic venous insufficiency–is it justified? Vasc Endovascular Surg. 2002;36(1):41–50.
13 Caggiati A., Bergan J.J., Gloviczki P., et al. Nomenclature of the veins of the lower limb: extensions, refinements, and clinical application. J Vasc Surg. 2005;41(4):719–724.
14 Caggiati A., Bergan J.J., Gloviczki P., et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36(2):416–422.
15 Delis K.T., Knaggs A.L., Khodabakhsh P. Prevalence, anatomic patterns, valvular competence, and clinical significance of the Giacomini vein. J Vasc Surg. 2004;40(6):1174–1183.
16 Mozes G., Gloviczki P., Menawat S.S., et al. Surgical anatomy for endoscopic subfascial division of perforating veins. J Vasc Surg. 1996;24(5):800–808.
17 Gloviczki P., Mozes G. Development and anatomy of the venous system. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;12–24.
18 Pang A.S. Location of valves and competence of the great saphenous vein above the knee. Ann Acad Med Singapore. 1991;20(2):248–250.
19 LePage P.A., Villavicencio J.L., Gomez E.R., et al. The valvular anatomy of the iliac venous system and its clinical implications. J Vasc Surg. 1991;14(5):678–683.
20 Labropoulos N., Delis K., Nicolaides A.N., et al. The role of the distribution and anatomic extent of reflux in the development of signs and symptoms in chronic venous insufficiency. J Vasc Surg. 1996;23(3):504–510.
21 van Rij A.M., Solomon C., Christie R. Anatomic and physiologic characteristics of venous ulceration. J Vasc Surg. 1994;20(5):759–764.
22 Labropoulos N., Giannoukas A.D., Nicolaides A.N., et al. The role of venous reflux and calf muscle pump function in nonthrombotic chronic venous insufficiency. Correlation with severity of signs and symptoms. Arch Surg. 1996;131(4):403–406.
23 Labropoulos N., Leon M., Geroulakos G., et al. Venous hemodynamic abnormalities in patients with leg ulceration. Am J Surg. 1995;169(6):572–574.
24 Hanrahan L.M., Araki C.T., Rodriguez A.A., et al. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg. 1991;13(6):805–811. discussion 811–802
25 May R., Thurner J. A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left-sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45(23–24):912–922.
26 Raju S., Darcey R., Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51(2):401–408. discussion 408
27 Gloviczki P., Comerota A., Dalsing M., et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 Suppl):2S–48S.
28 Langer R.D., Ho E., Denenberg J.O., et al. Relationships between symptoms and venous disease: the San Diego population study. Arch Intern Med. 2005;165(12):1420–1424.
29 Eklof B., Perrin M., Delis K.T., et al. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(2):498–501.
30 Vasquez M.A., Rabe E., McLafferty R.B., et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52(5):1387–1396.
31 Rabe E., Pannier F. Epidemiology of chronic venous disorders. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;105–112.
32 Bradbury A., Ruckley C.V. Clinical presentation and assessment of patients with venous disease. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;331–341.
33 Gloviczki P., Driscoll D.J. Klippel-Trenaunay syndrome: current management. Phlebology. 2007;22(6):291–298.
34 Cavezzi A., Labropoulos N., Partsch H., et al. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs–UIP consensus document. Part II. Anatomy. Eur J Vasc Endovasc Surg. 2006;31(3):288–299.
35 Coleridge-Smith P., Labropoulos N., Partsch H., et al. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs–UIP consensus document. Part I. Basic principles. Eur J Vasc Endovasc Surg. 2006;31(1):83–92.
36 Meissner M.H., Moneta G., Burnand K., et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl S):4S–24S.
37 McMullin G.M., Coleridge Smith P.D. An evaluation of Doppler ultrasound and photoplethysmography in the investigation of venous insufficiency. Aust N Z J Surg. 1992;62(4):270–275.
38 Labropoulos N., Tiongson J., Pryor L., et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38(4):793–798.
39 Abai B., Labropoulos N. Duplex scanning for chronic venous obstruction and valvular incompetence. Gloviczki P., ed. Handbook of venous disorders, ed 3, 1. London: Hodder Arnold, 2009;142–155.
40 Markel A., Meissner M.H., Manzo R.A., et al. A comparison of the cuff deflation method with Valsalva’s maneuver and limb compression in detecting venous valvular reflux. Arch Surg. 1994;129(7):701–705.
41 Struckmann J. Venous investigations: the current position. Angiology. 1994;45(6 Pt 2):505–511.
42 Struckmann J.R. Assessment of the venous muscle pump function by ambulatory strain gauge plethysmography. Methodological and clinical aspects. Dan Med Bull. 1993;40(4):460–477.
43 Rooke T.W., Heser J.L., Osmundson P.J. Exercise strain-gauge venous plethysmography: evaluation of a “new” device for assessing lower limb venous incompetence. Angiology. 1992;43(3 Pt 1):219–228.
44 Criado E., Farber M.A., Marston W.A., et al. The role of air plethysmography in the diagnosis of chronic venous insufficiency. J Vasc Surg. 1998;27(4):660–670.
45 Lurie F., Rooke T. Evaluation of venous function by indirect noninvasive tests (plethysmography). Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;156–159.
46 Rhodes J.M., Gloviczki P., Canton L., et al. Endoscopic perforator vein division with ablation of superficial reflux improves venous hemodynamics. J Vasc Surg. 1998;28(5):839–847.
47 Park U.J., Yun W.S., Lee K.B., et al. Analysis of the postoperative hemodynamic changes in varicose vein surgery using air plethysmography. J Vasc Surg. 2010;51(3):634–638.
48 Neglen P., Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35(4):694–700.
49 Masuda E.M., Kistner R.L. Long-term results of venous valve reconstruction: a four- to twenty-one-year follow-up. J Vasc Surg. 1994;19(3):391–403.
50 Kistner R.L., Ferris E.B., Randhawa G., et al. A method of performing descending venography. J Vasc Surg. 1986;4(5):464–468.
51 Reed N.R., Kalra M., Bower T.C., et al. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009;49(2):386–393. discussion 393–384
52 Lee B.B., Bergan J., Gloviczki P., et al. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2009;28(6):434–451.
53 Labropoulos N., Manalo D., Patel N.P., et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45(3):568–573.
54 Kahn S.R., M’Lan C.E., Lamping D.L., et al. Relationship between clinical classification of chronic venous disease and patient-reported quality of life: results from an international cohort study. J Vasc Surg. 2004;39(4):823–828.
55 Launois R., Reboul-Marty J., Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5(6):539–554.
56 Lamping D.L., Schroter S., Kurz X., et al. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37(2):410–419.
57 Coleridge Smith P. Drug treatment of varicose veins, venous edema and ulcers. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;359–365.
58 Martinez M.J., Bonfill X., Moreno R.M., et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. (3):2005. CD003229
59 Pittler M.H., Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. (1):2006. CD003230
60 Nelson E.A., Prescott R.J., Harper D.R., et al. A factorial, randomized trial of pentoxifylline or placebo, four-layer or single-layer compression, and knitted viscose or hydrocolloid dressings for venous ulcers. J Vasc Surg. 2007;45(1):134–141.
61 Dale J.J., Ruckley C.V., Harper D.R., et al. Randomised, double blind placebo controlled trial of pentoxifylline in the treatment of venous leg ulcers. BMJ. 1999;319(7214):875–878.
62 Falanga V., Fujitani R.M., Diaz C., et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1999;7(4):208–213.
63 Hirsh J., Guyatt G., Albers G.W., et al. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(Suppl 6):71S–109S.
64 Coleridge-Smith P., Lok C., Ramelet A.A. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30(2):198–208.
65 Moneta G., Partsch B. Compression therapy for venous ulceration. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;348–358.
66 Partsch B., Partsch H. Calf compression pressure required to achieve venous closure from supine to standing positions. J Vasc Surg. 2005;42(4):734–738.
67 Ibegbuna V., Delis K.T., Nicolaides A.N., et al. Effect of elastic compression stockings on venous hemodynamics during walking. J Vasc Surg. 2003;37(2):420–425.
68 Zajkowski P.J., Proctor M.C., Wakefield T.W., et al. Compression stockings and venous function. Arch Surg. 2002;137(9):1064–1068.
69 Mayberry J.C., Moneta G.L., Taylor L.M.Jr, et al. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109(5):575–581.
70 Fletcher A., Cullum N., Sheldon T.A. A systematic review of compression treatment for venous leg ulcers. BMJ. 1997;315(7108):576–580.
71 Partsch H., Flour M., Smith P.C. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27(3):193–219.
72 Amsler F., Willenberg T., Blattler W. In search of optimal compression therapy for venous leg ulcers: a meta-analysis of studies comparing diverse [corrected] bandages with specifically designed stockings. J Vasc Surg. 2009;50(3):668–674.
73 Palfreyman S., Nelson E.A., Michaels J.A. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ. 2007;335(7613):244.
74 Barwell J.R., Davies C.E., Deacon J., et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363(9424):1854–1859.
75 Gohel M.S., Barwell J.R., Taylor M., et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335(7610):83.
76 Geerts W.H., Bergqvist D., Pineo G.F., et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(Suppl 6):381S–453S.
77 Scriven J.M., Hartshorne T., Thrush A.J., et al. Role of saphenous vein surgery in the treatment of venous ulceration. Br J Surg. 1998;85(6):781–784.
78 Criado E., Lujan S., Izquierdo L., et al. Conservative hemodynamic surgery for varicose veins. Semin Vasc Surg. 2002;15(1):27–33.
79 Pittaluga P., Chastanet S., Rea B., et al. Midterm results of the surgical treatment of varices by phlebectomy with conservation of a refluxing saphenous vein. J Vasc Surg. 2009;50(1):107–118.
80 Passman M. Transilluminated powered phlebectomy in the treatment of varicose veins. Vascular. 2007;15(5):262–268.
81 Bergan J.J. Varicose veins: hooks, clamps, and suction. Application of new techniques to enhance varicose vein surgery. Semin Vasc Surg. 2002;15(1):21–26.
82 Gay J. On varicose disease of the lower extremities and its allied disorders: skin discoloration, induration, and ulcer: being the Lettsomian Lectures delivered before the Medical Society of London in 1867. London: John Churchill and Sons; 1868.
83 Linton R.R. The communicating veins of the lower leg and the operative technic for their ligation. Ann Surg. 1938;107(4):582–593.
84 Cockett F.B. The pathology and treatment of venous ulcers of the leg. Br J Surg. 1955;43(179):260–278.
85 Dodd H. The diagnosis and ligation of incompetent ankle perforating veins. Ann R Coll Surg Engl. 1964;34:186–196.
86 Homans J. The operative treatment of varicose veins and ulcers, based open a classification of these lesions. Surg Gynecol Obstet. 1916;22:143–158.
87 Mendes R.R., Marston W.A., Farber M.A., et al. Treatment of superficial and perforator venous incompetence without deep venous insufficiency: is routine perforator ligation necessary? J Vasc Surg. 2003;38(5):891–895.
88 Stuart W.P., Adam D.J., Allan P.L., et al. Saphenous surgery does not correct perforator incompetence in the presence of deep venous reflux. J Vasc Surg. 1998;28(5):834–838.
89 Wilkinson G.E.Jr, Maclaren I.F. Long term review of procedures for venous perforator insufficiency. Surg Gynecol Obstet. 1986;163(2):117–120.
90 Negus D., Friedgood A. The effective management of venous ulceration. Br J Surg. 1983;70(10):623–627.
91 Pierik E.G., van Urk H., Hop W.C., et al. Endoscopic versus open subfascial division of incompetent perforating veins in the treatment of venous leg ulceration: a randomized trial. J Vasc Surg. 1997;26(6):1049–1054.
92 Hauer G. Endoscopic subfascial discussion of perforating veins–preliminary report. Vasa. 1985;14(1):59–61.
93 O’Donnell T.J.Jr. Surgical treatment of incompetent perforating veins. In: Bergan J.J., Kistner R.L. Atlas of venous surgery. Philadelphia: W. B. Saunders Company; 1992:111–124.
94 Gloviczki P., Cambria R.A., Rhee R.Y., et al. Surgical technique and preliminary results of endoscopic subfascial division of perforating veins. J Vasc Surg. 1996;23(3):517–523.
95 Conrad P. Endoscopic exploration of the subfascial space of the lower leg with perforator vein interruption using laparoscopic equipment: a preliminary report. Phlebology. 1994;9(4):154–157.
96 O’Donnell T.F. The role of perforators in chronic venous insufficiency. Phlebology. 2010;25(1):3–10.
97 Gloviczki P., Bergan J.J. Atlas of endoscopic perforator vein surgery. London: Springer, 1998.
98 Tenbrook J.A.Jr, Iafrati M.D., O’Donnell T.F.Jr, et al. Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg. 2004;39(3):583–589.
99 Luebke T., Brunkwall J. Meta-analysis of subfascial endoscopic perforator vein surgery (SEPS) for chronic venous insufficiency. Phlebology. 2009;24(1):8–16.
100 Kistner R.L. Surgical repair of a venous valve. Straub Clin Proc. 1968;34:41–43.
101 Kistner R.L. Surgical repair of the incompetent femoral vein valve. Arch Surg. 1975;110(11):1336–1342.
102 Raju S. Surgical repair of deep vein valve incompetence. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;472–482.
103 Gloviczki P., Merrell S.W., Bower T.C. Femoral vein valve repair under direct vision without venotomy: a modified technique with use of angioscopy. J Vasc Surg. 1991;14(5):645–648.
104 Raju S., Fountain T., Neglen P., et al. Axial transformation of the profunda femoris vein. J Vasc Surg. 1998;27(4):651–659.
105 Lugli M., Guerzoni S., Garofalo M., et al. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49(1):156–162. 162 e151–152, discussion 162
106 Raju S., Fredericks R. Valve reconstruction procedures for nonobstructive venous insufficiency: rationale, techniques, and results in 107 procedures with two- to eight-year follow-up. J Vasc Surg. 1988;7(2):301–310.
107 Meissner M.H., Manzo R.A., Bergelin R.O., et al. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18(4):596–605. discussion 606–598
108 Ziegler S., Schillinger M., Maca T.H., et al. Post-thrombotic syndrome after primary event of deep venous thrombosis 10 to 20 years ago. Thromb Res. 2001;101(2):23–33.
109 O’Shaughnessy A.M., Fitzgerald D.E. Natural history of proximal deep vein thrombosis assessed by duplex ultrasound. Int Angiol. 1997;16(1):45–49.
110 Gloviczki P., Hollier L.H., Dewanjee M.K., et al. Experimental replacement of the inferior vena cava: factors affecting patency. Surgery. 1984;95(6):657–666.
111 Neglen P. Endovascular reconstruction for chronic iliofemoral vein obstruction. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;491–502.
112 Raju S., Neglen P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50(2):360–368.
113 Garg N., Gloviczki P., Karimi K.M., et al. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. J Vasc Surg. 2011;53(2):383–393.
114 Jost C.J., Gloviczki P., Cherry K.J.Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33(2):320–327. discussion 327–328
115 Hingorani A.P., Ascher E., Marks N., et al. Predictive factors of success following radio-frequency stylet (RFS) ablation of incompetent perforating veins (IPV). J Vasc Surg. 2009;50(4):844–848.
116 Masuda E.M., Kessler D.M., Lurie F., et al. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43(3):551–556. discussion 556–557
117 Proebstle T.M., Herdemann S. Early results and feasibility of incompetent perforator vein ablation by endovenous laser treatment. Dermatol Surg. 2007;33(2):162–168.
118 Marks N., Hingorani A., Ascher E. New office-based vascular interventions. Perspect Vasc Surg Endovasc Ther. 2008;20(4):340–345.
119 Elias S. Percutaneous ablation of perforating veins. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;536–544.
120 Dalsing M. Artificial venous valves. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;483–490.
121 Neglen P., Berry M.A., Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20(6):560–571.
122 Neglen P., Tackett T.P.Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48(5):1255–1261.
123 Bjarnason H. Endovascular reconstruction of complex iliocaval venous occlusions. Gloviczki P., ed. Handbook of venous disorders, ed 3, vol 1. London: Hodder Arnold, 2009;503–513.
124 Raju S., Hollis K., Neglen P. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44(4):820–827.
125 Neglen P., Hollis K.C., Olivier J., et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979–990.
126 Delis K.T., Bjarnason H., Wennberg P.W., et al. Successful iliac vein and inferior vena cava stenting ameliorates venous claudication and improves venous outflow, calf muscle pump function, and clinical status in post-thrombotic syndrome. Ann Surg. 2007;245(1):130–139.
127 Kundu S., Lurie F., Millward S.F., et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: joint statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Surg. 2007;46(3):582–589.
128 Perrin M., Allaert F.A. Intra- and inter-observer reproducibility of the Recurrent Varicose Veins after Surgery (REVAS) classification. Eur J Vasc Endovasc Surg. 2006;32(3):326–332.