Chapter 17 Sleep Medicine
Introduction
Sleep regulation involves the simultaneous operation of two basic highly coupled processes that govern sleep and wakefulness (the “two process” sleep system). The homeostatic process (“Process S”), primarily regulates the length and depth of sleep, and may be related to the accumulation of adenosine and other sleep-promoting chemicals (“somnogens”), such as cytokines, during prolonged periods of wakefulness. This sleep pressure appears to build more quickly in infants and young children, thus limiting the duration of sustained wakefulness during the day and necessitating periods of daytime sleep (i.e., naps). The endogenous circadian rhythm (“Process C”), influences the internal organization of sleep and timing and duration of daily sleep-wake cycles, and govern predictable patterns of alertness throughout the 24 hr day. The “master circadian clock” that controls sleep-wake patterns is located in the suprachiasmatic nucleus (SCN) in the ventral hypothalamus; other “circadian clocks” govern the timing of multiple other physiologic systems in the body (e.g., cardiovascular reactivity, hormone levels, renal and pulmonary functions). Because the human circadian clock is actually slightly longer than 24 hr, intrinsic circadian rhythms must are synchronized or “entrained” to the 24 hr day cycle by environmental cues called zeitgebers. The most powerful of these zeitgebers is the light–dark cycle; light signals are transmitted to the SCN via the circadian photoreceptor system within the retina (functionally and anatomically separate from the visual system), which switch the body’s production of the hormone melatonin off (light) or on (dark) by the pineal gland. Circadian rhythms are also synchronized by other external time cues, such as timing of meals and alarm clocks.
The relative level of sleepiness (sleep propensity) or alertness existing at any given time during a 24 hr period is partially determined by the duration and quality of previous sleep, as well as time awake since the last sleep period (the homeostatic or “sleep drive”). Interacting with this “sleep homeostat” is the 24 hr cyclic pattern or rhythm characterized by clock-dependent periods of maximum sleepiness (“circadian troughs”) and maximum alertness (“circadian nadirs”). There are 2 periods of maximum sleepiness, 1 in the late afternoon (3:00-5:00 PM) and one toward the end of the night (3:00-5:00 AM), and 2 periods of maximum alertness, 1 in mid-morning and 1 in the evening, just prior to sleep onset (the so-called second wind).
Another basic principle of sleep physiology relates to the consequences of the failure to meet basic sleep needs, termed insufficient/inadequate sleep or sleep loss. Adequate sleep is a biologic imperative that appears necessary for sustaining life as well as for optimal functioning. Slow-wave sleep (SWS) appears to be the most “restorative” form of sleep and rapid eye movement (REM) sleep appears not only to be involved in vital cognitive functions, such as the consolidation of memory, but to be an integral component of the growth and development of the central nervous system (CNS). Adequate amounts of both of these sleep stages are necessary for optimal learning. Partial sleep loss (sleep restriction) on a chronic basis accumulates in what is termed a sleep debt and produces deficits equivalent to those seen under conditions of total sleep deprivation. If the sleep debt becomes large enough and is not voluntarily paid back (by obtaining adequate recovery sleep), the body may respond by overriding voluntary control of wakefulness, resulting in periods of decreased alertness, dozing off, and napping, that is excessive daytime sleepiness. The sleep-deprived individual may also experience very brief (several seconds) repeated daytime microsleeps of which he or she may be completely unaware, but which nonetheless may result in significant lapses in attention and vigilance. There is also a relationship between the amount of sleep restriction and performance, with decreased performance correlating with decreased sleep.
Both insufficient quantity and poor quality of sleep in children and adolescents usually result in excessive daytime sleepiness and decreased daytime alertness levels. Sleepiness may be recognizable as drowsiness, yawning, and other classic “sleepy behaviors,” but can also be manifested as mood disturbance, including complaints of moodiness, irritability, emotional lability, depression, and anger; fatigue and daytime lethargy, including increased somatic complaints (headaches, muscle aches); cognitive impairment, including problems with memory, attention, concentration, decision-making, and problem solving; daytime behavior problems, including overactivity, impulsivity, and noncompliance; and academic problems, including chronic tardiness related to insufficient sleep and school failure resulting from chronic daytime sleepiness.
To evaluate sleep problems, it is important to have an understanding of what constitutes “normal” sleep in children and adolescents. Sleep disturbances, as well as many characteristics of sleep itself, have some distinctly different features in children from sleep and sleep disorders in adults. In addition, changes in sleep architecture and the evolution of sleep patterns and behaviors reflect the physiologic/chronobiologic, developmental, and social/environmental changes that are occurring across childhood. These trends may be summarized as the gradual assumption of more adult sleep patterns as children mature:
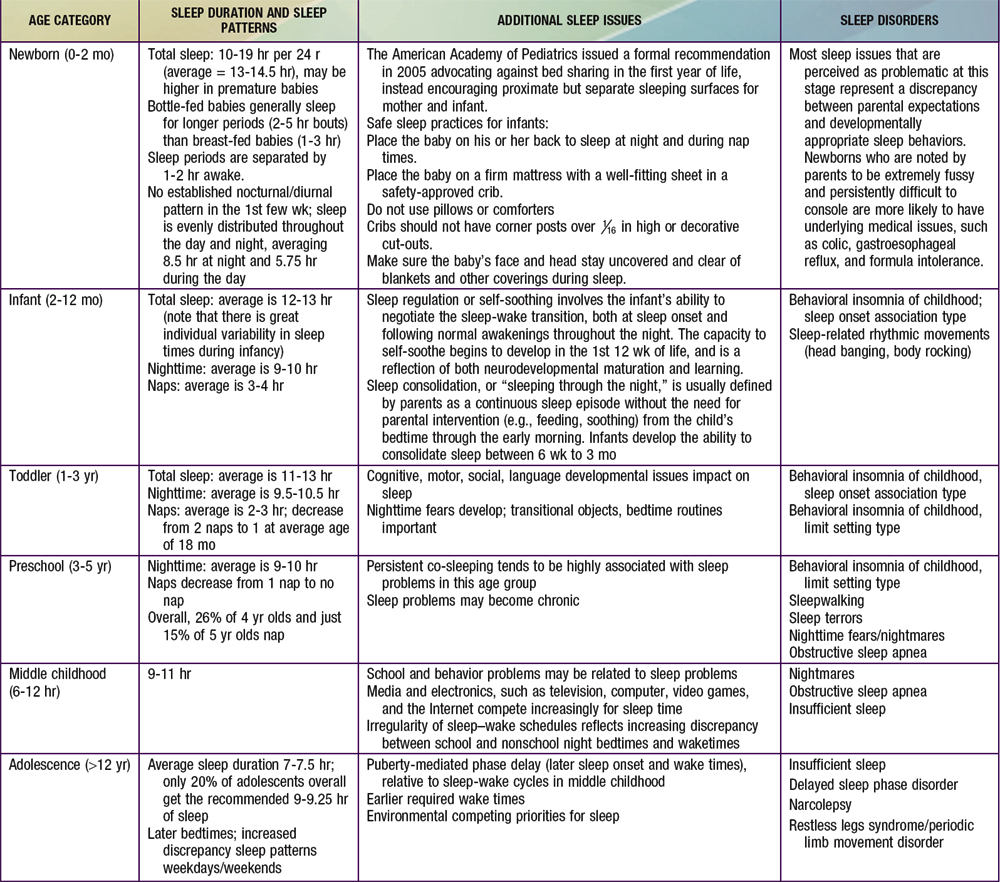
Normal developmental changes in children’s sleep are found in Table 17-1.
Common Sleep Disorders
Most sleep problems in children may be broadly conceptualized as resulting from either inadequate duration of sleep for age and sleep needs (insufficient sleep quantity) or disruption and fragmentation of sleep (poor sleep quality) as a result of frequent, repetitive, and brief arousals during sleep. Less common causes of sleep disturbance in childhood involve inappropriate timing of the sleep period (as occurs in circadian rhythm disturbances), or primary disorders of excessive daytime sleepiness (central hypersomnias such as narcolepsy). Insufficient sleep is usually the result of difficulty initiating (delayed sleep onset) and/or maintaining sleep (prolonged night wakings), but, especially in older children and adolescents, may also represent a conscious lifestyle decision to sacrifice sleep in favor of competing priorities, such as homework and social activities. The underlying causes of sleep onset delay/prolonged night wakings or sleep fragmentation may in turn be related to primarily behavioral factors (bedtime resistance resulting in shortened sleep duration) and/or medical causes (obstructive sleep apnea causing frequent, brief arousals).
It should be noted that certain pediatric populations are relatively more vulnerable to acute or chronic sleep problems. These include children with medical problems, including chronic illnesses, such as cystic fibrosis, asthma, and rheumatoid arthritis, and acute illnesses, such as otitis media; children taking medications or ingesting substances with stimulant (e.g., psychostimulants, caffeine), sleep-disrupting (e.g., corticosteroids), or daytime sedating (some anticonvulsants, α-agonists) properties; hospitalized children; and children with a variety of psychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD), depression, bipolar disorder, and anxiety disorders. Children with neurodevelopmental disorders may be more prone to nocturnal seizures, as well as other sleep disruptions, and children with blindness, mental retardation, some chromosomal syndromes (Smith-Magenis, fragile X), and autism spectrum disorders are at increased risk for severe sleep onset difficulty and night wakings, as well as circadian rhythm disturbances.
Insomnia of Childhood
Insomnia may be broadly defined as repeated difficulty initiating and/or maintaining sleep that occurs despite age-appropriate time and opportunity for sleep. These sleep complaints must also result in some degree of impairment in daytime functioning for the child and/or family, which may range from fatigue, irritability, lack of energy, and mild cognitive impairment to effects on mood, school performance, and quality of life. Insomnia complaints may be of a short-term and transient nature (usually related to an acute event), or may be characterized as long-term and chronic. Insomnia is a set of symptoms with a large number of possible etiologies (e.g., pain, medication, medical and psychiatric conditions, learned behaviors) and not as a diagnosis per se. Insomnia, like many behavioral issues in children, is often primarily defined by parental concerns rather than by objective criteria, and therefore should be viewed in the context of family (i.e., maternal depression, stress), child (i.e., temperament, developmental level), and environmental (i.e., cultural practices, sleeping space) considerations.
One of the most common sleep disorders found in infants and toddlers is behavioral insomnia of childhood, sleep onset association type. In this disorder, the child learns to fall asleep only under certain conditions or associations which typically require parental presence, such as being rocked or fed, and does not develop the ability to self-soothe. During the night, when the child experiences the type of brief arousal that normally occurs at the end of a sleep cycle (every 60-90 minutes in infants) or awakens for other reasons, he or she is not able to get back to sleep without those same conditions being present. The infant then “signals” the parent by crying (or coming into the parents’ bedroom, if the child is no longer in a crib) until the necessary associations are provided. The problem is one of prolonged night waking resulting in insufficient sleep (for both child and parent).
Management of night wakings should include establishment of a set sleep schedule and bedtime routine, and implementation of a behavioral program. The treatment approach typically involves a program of rapid withdrawal (extinction) or more gradual withdrawal (graduated extinction) of parental assistance at sleep onset and during the night. Extinction (“cry it out”) involves putting the child to bed at a designated bedtime, “drowsy but awake,” and then systematically ignoring the child until a set time the next morning. Although it has considerable empirical support, extinction is often not an acceptable choice for families. Graduated extinction involves weaning the child from dependence on parental presence with periodic “checks” by the parents at successively longer intervals during the sleep-wake transition; the exact amount of time is determined by the parents’ tolerance for crying and the child’s temperament. The goal is to allow the infant or child to develop skills in self-soothing during the night, as well as at bedtime. In older infants, the introduction of more appropriate sleep associations that will be readily available to the child during the night (transitional objects, such as a blanket or toy), in addition to positive reinforcement (i.e., stickers for remaining in bed), is often beneficial. If the child has become habituated to awaken for nighttime feedings (“learned hunger”), then these feedings should be slowly eliminated. Parents must be consistent in applying behavioral programs to avoid inadvertent, intermittent reinforcement of night wakings; they should also be forewarned that crying behavior often temporarily escalates at the beginning of treatment (“post-extinction burst”).
Bedtime problems, including stalling and refusing to go to bed, are more common in preschool-aged and older children. Sleep disturbances of this type generally fall within the diagnostic category known as behavioral insomnia of childhood, limit setting type, and are often the result of parental difficulties in setting limits and managing behavior, including the inability or unwillingness to set consistent bedtime rules and enforce a regular bedtime, and may be exacerbated by the child’s oppositional behavior. In some cases the child’s resistance at bedtime is due to an underlying problem in falling asleep that is caused by other factors (medical conditions, such as asthma or medication use; a sleep disorder, such as restless legs syndrome; or anxiety) or a mismatch between the child’s intrinsic circadian rhythm (“night owl”) and parental expectations.
Successful treatment of limit setting sleep disorder generally involves a combination of parent education regarding appropriate limit setting, decreased parental attention for bedtime-delaying behavior, establishment of bedtime routines, and positive reinforcement (sticker charts) for appropriate behavior at bedtime; other behavioral management strategies that have empirical support include bedtime fading (temporarily setting the bedtime closer to the actual sleep onset time and then gradually advancing the bedtime to an earlier target bedtime). Older children may benefit from being taught relaxation techniques to help themselves fall asleep more readily. Following the principles of sleep hygiene for children is essential (Table 17-2).
Table 17-2 BASIC PRINCIPLES OF SLEEP HYGIENE FOR CHILDREN
When the insomnia is not primarily a result of parent behavior or secondary to another sleep disturbance, or to a psychiatric or medical problem, it is referred to as psychophysiologic or primary insomnia, also sometimes called “learned insomnia.” Primary insomnia usually occurs largely in adolescents and is characterized by a combination of learned sleep-preventing associations and heightened physiologic arousal resulting in a complaint of sleeplessness and decreased daytime functioning. A hallmark of primary insomnia is excessive worry about sleep and an exaggerated concern of the potential daytime consequences. The physiologic arousal can be in the form of cognitive hypervigilance, such as “racing” thoughts; in many individuals with insomnia an increased baseline level of arousal is further intensified by this secondary anxiety about sleeplessness. Treatment usually involves educating the adolescent about the principles of sleep hygiene (Table 17-3), institution of a consistent sleep-wake schedule, avoidance of daytime napping, instructions to use the bed for sleep only and to get out of bed if unable to fall asleep (stimulus control), restricting time in bed to the actual time asleep (sleep restriction), addressing maladaptive cognitions about sleep, and teaching relaxation techniques to reduce anxiety. Hypnotic medications are rarely needed.
Table 17-3 BASIC PRINCIPLES OF SLEEP HYGIENE FOR ADOLESCENTS
Obstructive Sleep Apnea
Sleep-disordered breathing (SDB) in children encompasses a broad spectrum of respiratory disorders that occur exclusively in or are exacerbated by sleep, and includes primary snoring and upper airway resistance syndrome, as well as apnea of prematurity and central apnea. Obstructive sleep apnea (OSA), the most important clinical entity within the SDB spectrum, is a respiratory disorder that is characterized by repeated episodes of prolonged upper airway obstruction during sleep despite continued or increased respiratory effort, resulting in complete (apnea) or partial (hypopnea; ≥50% reduction in airflow) cessation of airflow at the nose and/or mouth, as well as in disrupted sleep. Both intermittent hypoxia and the multiple arousals resulting from these obstructive events likely contribute to significant metabolic, cardiovascular, and neurocognitive/neurobehavioral morbidity.
Primary snoring is defined as snoring without associated ventilatory abnormalities (e.g., apneas or hypopneas, hypoxemia, hypercapnia) or respiratory-related arousals, and is a manifestation of the vibrations of the oropharyngeal soft tissue walls that occur when an individual attempts to breathe against increased upper airway resistance during sleep. Children with primary snoring may still have subtle breathing abnormalities during sleep, including evidence of increased respiratory effort, which in turn may be associated with adverse neurodevelopmental outcomes.
Etiology
In general terms, OSA results from an anatomically or functionally narrowed upper airway; this typically involves some combination of decreased upper airway patency (upper airway obstruction and/or decreased upper airway diameter), increased upper airway collapsibility (reduced pharyngeal muscle tone), and decreased drive to breathe in the face of reduced upper airway patency (reduced central ventilatory drive) (Table 17-4). Upper airway obstruction varies in degree and level (i.e., nose, nasopharynx/oropharynx, hypopharynx) and is most commonly due to adenotonsillar hypertrophy, although tonsillar size does not necessarily correlate with degree of obstruction, especially in older children. Other causes of airway obstruction include allergies associated with chronic rhinitis/nasal obstruction; craniofacial abnormalities, including hypoplasia/displacement of the maxilla and mandible; gastroesophageal reflux with resulting pharyngeal reactive edema; nasal septal deviation; and velopharyngeal flap cleft palate repair. Reduced upper airway tone may result from neuromuscular diseases, including hypotonic cerebral palsy and muscular dystrophies, or hypothyroidism. Reduced central ventilatory drive may be present in some children with Arnold-Chiari malformation and meningomyelocele. In other situations, the etiology is mixed; individuals with Down syndrome, by virtue of their facial anatomy, hypotonia, macroglossia, and central adiposity, as well as the increased incidence of hypothyroidism, are at particularly high risk for OSA, with some estimates of as great as 70% prevalence.
Table 17-4 ANATOMIC FACTORS THAT PREDISPOSE TO OBSTRUCTIVE SLEEP APNEA AND HYPOVENTILATION IN CHILDREN
NOSE
NASOPHARYNGEAL AND OROPHARYNGEAL
CRANIOFACIAL
Although many children with OSA are of normal weight, an increasingly large percentage are overweight or obese, and many of these children are school-aged and younger. There is a significant correlation between weight and SDB (habitual snoring, OSA, central apneas). While adenotonsillar hypertrophy also plays an important etiologic role in overweight/obese children with OSA, mechanical factors related to an increase in the amount of adipose tissue in the throat (pharyngeal fat pads), neck (increased neck circumference), and chest wall and abdomen can create increased upper airway resistance, worsen gas exchange, and increased work of breathing, particularly in the supine position and during REM sleep. There may be a component of blunted central ventilatory drive in response to hypoxia/hypercapnia and hypoventilation as well, particularly in children with morbid or syndrome-based (Prader-Willi) obesity. Overweight and obese children and adolescents are at a particularly high risk for metabolic and cardiovascular complications of SDB, such as insulin resistance and systemic hypertension; morbidly obese children may also be at increased risk for postoperative complications following adenotonsillectomy.
Epidemiology
Overall prevalence of parent-reported snoring in the pediatric population is about 8%; “always” snoring is reported in 1.5-6%, and “often” snoring in 3-15%. When defined by parent-reported symptoms, the prevalence of OSA is 4-11%. The prevalence of pediatric OSA as documented by overnight sleep studies utilizing ventilatory monitoring procedures (e.g., in-lab PSG, home studies) is 1-4% overall, with a reported range of 0.1-13%. Prevalence is also affected by the demographic characteristics, such as age (increased prevalence between 2 and 8 yr), gender (more common in boys, especially after puberty), race/ethnicity (increased prevalence in African-American and Asian children), and family history of OSA.
Pathogenesis
The upregulation of inflammatory pathways, as indicated by an increase in peripheral markers of inflammation such as C-reactive protein (CRP), appear to be linked to metabolic dysfunction (e.g., insulin resistance, dyslipidemia) in both obese and non-obese children with OSA. Both systemic inflammation and arousal-mediated increases in sympathetic autonomic nervous system activity with altered vasomotor tone may be key contributors to increased cardiovascular risk in both adults and children with OSA. Mechanical stress on the upper airway induced by chronic snoring may also result in both local mucosal inflammation of adenotonsillar tissues and subsequent upregulation of inflammatory molecules, most notably leukotrienes. Another potential mechanism that may mediate cardiovascular sequelae in both adults and children with OSA is altered endothelial function.
Although yet to be fully elucidated, one of the primary mechanisms by which OSA is believed to exert negative influences on cognitive function appears to involve repeated episodic arousals from sleep leading to sleep fragmentation and resulting sleepiness. An equally important role may be intermittent hypoxia that leads directly to systemic inflammatory vascular changes in the brain. Levels of inflammatory markers such as CRP and cytokine IL-6 are elevated in children with OSA and are also associated with cognitive dysfunction.
Clinical Manifestations
The clinical manifestations of OSA may be divided into sleep-related and daytime symptoms. The most common nocturnal manifestations of OSA in children and adolescents are loud, frequent, and disruptive snoring, breathing pauses, choking or gasping arousals, restless sleep, and nocturnal diaphoresis. Many children who snore do not have OSA, but very few children with OSA do not snore. Most children, like adults, tend to have more frequent and more severe obstructive events in REM sleep and when sleeping in the supine position. Children with OSA may adopt unusual sleeping positions, keeping their necks hyperextended in order to maintain airway patency. Frequent arousals associated with obstruction may result in nocturnal awakenings, but are more likely to cause fragmented sleep.
Daytime symptoms of OSA include mouth breathing and dry mouth, chronic nasal congestion/rhinorrhea, hyponasal speech, morning headaches, difficulty swallowing, and poor appetite. Children with OSA may have secondary enuresis, most likely as a result of the disruption of the normal nocturnal pattern of antidiuretic hormone secretion. Partial arousal parasomnias (sleepwalking and sleep terrors) may occur more frequently in children with OSA, related to the frequent associated arousals and an increased percentage of delta sleep, or SWS.
One of the most important but frequently overlooked sequelae of OSA in children is the effect on mood, behavior, learning, and academic functioning. The neurobehavioral consequences of OSA in children include daytime sleepiness with drowsiness, difficulty in morning waking, and unplanned napping or dozing off during activities, although evidence of frank hypersomnolence tends to be less common in children compared to adults with OSA. Mood changes include increased irritability, mood instability and emotional dysregulation, low frustration tolerance, and depression/anxiety. Behavioral issues include both “internalizing” (i.e., increased somatic complaints and social withdrawal) and “externalizing” behaviors, including aggression, impulsivity, hyperactivity, oppositional behavior, and conduct problems. There is a substantial overlap between the clinical impairments associated with OSA and the diagnostic criteria for ADHD, including inattention, poor concentration, and distractibility (Chapter 30). There also appears to be a selective impact of OSA specifically on “executive functions,” which include cognitive flexibility, task initiation, self-monitoring, planning, organization, and self-regulation of affect and arousal; executive function deficits are also a hallmark of ADHD.
Studies that have looked at changes in behavior and neuropsychologic functioning in children following treatment (usually adenotonsillectomy) for OSA have largely documented significant improvement in outcomes, in both the short and long term, of OSA syndrome post-treatment, including daytime sleepiness, mood, behavior, academics, and quality of life. Many studies have failed to find a dose-dependent relationship between OSA in children and specific neurobehavioral/neurocognitive deficits, suggesting that other factors may influence neurocognitive outcomes, including individual genetic susceptibility, environmental influences such as passive smoking exposure, and co-morbid conditions, such as obesity, shortened sleep duration, and the presence of other sleep disorders.
Diagnosis
The American Academy of Pediatrics clinical practice guidelines provide excellent information for the evaluation and management of uncomplicated childhood OSA (Table 17-5). There are no physical examination findings that are truly pathognomonic for OSA, and most healthy children with OSA appear normal; certain physical examination findings may suggest OSA. Growth parameters may be abnormal (obesity or, less commonly, failure to thrive), and there may be evidence of chronic nasal obstruction (hyponasal speech, mouth breathing, septal deviation, “adenoidal facies”), as well as signs of atopic disease (i.e., “allergic shiners”). Oropharyngeal examination may reveal enlarged tonsils, excess soft tissue in the posterior pharynx, and a narrowed posterior pharyngeal space. Any abnormalities of the facial structure, such as retrognathia and/or micrognathia, midfacial hypoplasia, best appreciated by inspection of the lateral facial profile, increase the likelihood of OSA and should be noted. In very severe cases, there may be evidence of pulmonary hypertension, right-sided heart failure, and cor pulmonale; systemic hypertension, unlike in adults, is relatively uncommon.
Table 17-5 AMERICAN ACADEMY OF PEDIATRICS CLINICAL PRACTICE GUIDELINES FOR OBSTRUCTIVE SLEEP APNEA SYNDROME (APRIL 2002)
All children should be screened for snoring.
Complex high-risk patients should be referred to a specialist.
Patients with cardiorespiratory failure cannot await elective evaluation.
Diagnostic evaluation is useful in discriminating between primary snoring and obstructive sleep apnea syndrome, the gold standard being polysomnography.
Adenotonsillectomy is the first line of treatment for most children, and continuous positive airway pressure (CPAP) is an option for those who are not candidates for surgery or do not respond to surgery.
High-risk patients should be monitored as inpatients postoperatively.
Patients should be re-evaluated postoperatively to determine whether additional treatment is required.
Because no combination of clinical history and physical findings can accurately predict which children with snoring have OSA, the gold standard for diagnosing OSA remains an overnight polysomnogram (PSG).
An overnight PSG is a technician-supervised, monitored study that documents physiologic variables during sleep; sleep staging, arousal measurement, cardiovascular parameters, and body movements (electroencepholography, electrooculography, chin and leg electromyography, ECG, body position sensors, and video recording), and a combination of breathing monitors (oronasal thermal sensor and nasal air pressure transducer for airflow), chest/abdominal monitors (e.g., inductance plethysmography for respiratory effort, pulse oximeter for O2 saturation, end-tidal or transcutaneous CO2 for hypercarbia, snore microphone). The polysomnographic parameter most commonly used in evaluating for sleep disordered breathing is the apnea/hypopnea index (AHI), which indicates the number of apneic and hypopneic events per hr of sleep. It should be noted that currently there are no universally accepted polysomnographic normal reference values and parameters for diagnosing OSA in children, and it is still unclear which parameters best predict morbidity. Normal preschool and early school-aged children may have a total AHI of less than 1.5, and this is the most widely used cutoff value for OSA in children 12 yr and below; in adolescents, the adult cutoff of an AHI ≥5 is generally used. In cases in which the AHI is between 1 and 5 obstructive events per hour, clinical judgment regarding risk factors for SDB, evidence of daytime sequelae, and the technical quality of the overnight sleep study should determine further management.
Treatment
There are presently no universally accepted guidelines regarding the indications for treatment of pediatric SDB (i.e., including primary snoring and OSA). Current recommendations largely emphasize weighing what is known about the potential cardiovascular, metabolic, and neurocognitive sequelae of SDB in children in combination with the individual health care professional’s clinical judgment. The decision of whether and how to treat OSA specifically in children is contingent on a number of parameters, including severity (nocturnal symptoms, daytime sequelae, sleep study results), duration of disease, and individual patient variables such as age, co-morbid conditions, and underlying etiologic factors. In the case of moderate to severe disease (AHI >10), the decision to treat is usually straightforward, and most pediatric sleep experts recommend that any child with an apnea index >5 should be treated.
In the majority of cases of pediatric OSA, adenotonsillectomy is the first-line treatment in any child with significant adenotonsillar hypertrophy, even in the presence of additional risk factors such as obesity. Adenotonsillectomy in uncomplicated cases generally (70-90% of children) results in complete resolution of symptoms; regrowth of adenoidal tissue after surgical removal occurs in some cases. Groups considered high-risk include young children (<3 yr old), as well as those with severe OSA documented on polysomnography, significant clinical sequelae of OSA (e.g., failure to thrive), or associated medical conditions, such as craniofacial syndromes, morbid obesity, and hypotonia. All patients should be re-evaluated postoperatively to determine whether additional evaluation and/or treatment are required. If there are significant residual risk factors (e.g., obesity) or continued symptoms of OSA, a follow-up sleep study at least 6 wk post-adenotonsillectomy may be indicated.
Additional treatment measures that may be appropriate include weight loss, positional therapy (attaching a firm object, such as a tennis ball, to the back of a sleep garment to prevent the child from sleeping in the supine position), and aggressive treatment of additional risk factors when present, such as asthma, seasonal allergies, and gastroesophageal reflux; there is some evidence that intranasal corticosteroids and leukotriene inhibitors may be helpful in mild OSA. Other surgical procedures, such as uvulopharyngopalatoplasty, and maxillofacial surgery (mandibular distraction osteogenesis and maxillomandibular advancement), are seldom performed in children but may be indicated in selected cases. Oral appliances, such as mandibular advancing devices and tongue retainers, are typically considered for adolescents in whom facial bone growth is largely complete.
Continuous or bilevel positive airway pressure (nasal CPAP or BiPAP) is the most common treatment for OSA in adults and can be used successfully in children and adolescents. CPAP delivers humidified, warmed air through an interface (mask, nasal pillows) that, under pressure, effectively “splints” the upper airway open. Optimal pressure settings (that abolish or significantly reduce respiratory events without increasing arousals or central apneas) are determined in the sleep lab during a full night CPAP titration. Efficacy studies at the current pressure and retitrations should be conducted periodically with long-term use (every 6 mo in young children and at least yearly or with significant weight changes in older children and adolescents). CPAP may be recommended if removing the adenoids and tonsils is not indicated, if there is residual disease following adenotonsillectomy, or if there are major risk factors that are not amenable to treatment with surgery (obesity, hypotonia).
Parasomnias
Parasomnias are defined as episodic nocturnal behaviors that often involve cognitive disorientation and autonomic and skeletal muscle disturbance. Parasomnias may be further characterized as occurring primarily during NREM sleep (partial arousal parasomnias) or in association with REM sleep, including nightmares, hypnogogic hallucinations, and sleep paralysis; other common parasomnias include sleep-talking. Sleep-related movement disorders, including restless legs syndrome/periodic limb movement disorder (RLS/PLMD) and rhythmic movement disorder (head banging, body rocking), are reviewed in a separate section below.
Etiology
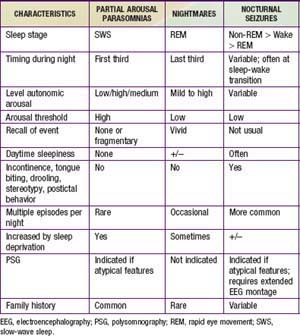
Partial arousal parasomnias, which include sleepwalking, sleep terrors, and confusional arousals are more common in preschool and school-aged children because of the relatively higher percentage of SWS in younger children. Any factor that is associated with an increase in the relative percentage of SWS (certain medications, previous sleep deprivation) may increase the frequency of events in a predisposed child. There appears to be a genetic predisposition for both sleepwalking and night terrors. In contrast, nightmares, which are much more common than the partial arousal parasomnias but are often confused with them, are concentrated in the last third of the night, when REM sleep is most prominent. Partial arousal parasomnias may also be difficult to distinguish from nocturnal seizures. Table 17-6 summarizes similarities and differences among these nocturnal arousal events.
Epidemiology
Many children (15-40%) sleepwalk on at least one occasion; the prevalence of children who regularly sleepwalk is approximately 17%, and 3-4% have frequent episodes. Sleepwalking may persist into adulthood, with the prevalence in adults of about 4%. The prevalence is approximately 10 times greater in children with a family history of sleepwalking. Approximately 1-6% of children experience sleep terrors, primarily during the preschool and elementary school years, and the age of onset is usually between 4 and 12 yr. Because of the common genetic predisposition, the prevalence of sleep terrors in children who sleepwalk is about 10%. Although sleep terrors can occur at any age from infancy through adulthood, most individuals outgrow sleep terrors by adolescence. Confusional arousals commonly co-occur with sleepwalking and sleep terrors; prevalence rates have been estimated to be upwards of 15% in children ages 3-13 yr.
Clinical Manifestations
The partial arousal parasomnias have several features in common. Because they typically occur at the transition out of “deep” or SWS, partial arousal parasomnias have clinical features of both the awake (ambulation, vocalizations) and the sleeping (high arousal threshold, unresponsiveness to the environment) states; there is usually amnesia for the events. The typical timing of partial arousal parasomnias during the first few hours of sleep is related to the predominance of SWS in the first third of the night; the duration is typically a few minutes (sleep terrors) to an hour (confusional arousals). Sleep terrors are sudden in onset and characteristically involve a high degree of autonomic arousal (i.e., tachycardia, dilated pupils), while confusional arousals typically arise more gradually from sleep, may involve thrashing around but usually not displacement from bed, and are often accompanied by slow mentation on arousal from sleep (“sleep inertia”). Sleepwalking may be associated with safety concerns (e.g., falling out of windows, wandering outside). Avoidance of, or increased agitation with, comforting by parents or attempts at awakening are also common features of all partial arousal parasomnias.
Treatment
Management of partial arousal parasomnias involves some combination of parental education and reassurance, good sleep hygiene, and avoidance of exacerbating factors such as sleep deprivation and caffeine. Particularly in the case of sleepwalking, it is important to institute safety precautions such as use of gates in doorways and at the top of staircases, locking of outside doors and windows, and installation of parent notification systems such as bedroom door alarms. Scheduled awakenings, a behavioral intervention that involve having the parent wake the child approximately 15 to 30 min before the time of night that the first parasomnia episode is most likely to be successful in situations in which partial arousal episodes occur on a nightly basis. Pharmacotherapy is rarely necessary, but may be indicated in cases of frequent or severe episodes, high risk of injury, violent behavior, or serious disruption to the family; the primary pharmacologic agents used are potent SWS suppressants, primarily benzodiazepines and tricyclic antidepressants.
Sleep-Related Movement Disorders: Restless Legs Syndrome/Periodic Limb Movement Disorder and Rhythmic Movements
Restless legs syndrome (RLS) is a neurologic, primarily sensory disorder, characterized by uncomfortable sensations in the lower extremities that are accompanied by an almost irresistible urge to move the legs. The sensations are usually at least partially relieved by movement, including walking, stretching, and rubbing, but only as long as the motion continues. RLS is a clinical diagnosis that is based on the presence of these key symptoms. Periodic limb movement disorder (PLMD) is characterized by periodic, repetitive, brief (0.5-10 sec), and highly stereotyped limb jerks typically occurring at 20 to 40 sec intervals. These movements occur primarily during sleep, most commonly occur in the legs, and frequently consist of rhythmic extension of the big toe and dorsiflexion at the ankle. The diagnosis of periodic limb movements (PLMs) requires overnight polysomnography to document the characteristic limb movements with anterior tibialis EMG leads.
Etiology
“Early-onset” RLS (i.e., onset of symptoms before 35-40 yr of age), often termed “primary” RLS, appears to have a particularly strong genetic component. Variants of the PTPRD gene are associated with RLS. Low serum iron levels in both adults and children may be an important etiologic factor for the presence and severity of both RLS symptoms and PLMs. As a marker of decreased iron stores, serum ferritin levels in both children and adults with RLS are frequently low. The underlying mechanism that has been postulated is related to the role of iron as a cofactor of tyrosine hydroxylase in a rate-limiting step of the synthesis of dopamine; in turn, dopaminergic dysfunction has been implicated as playing a key role particularly in the genesis of the sensory component of RLS, as well as in PLMD. Certain medical conditions, including diabetes mellitus, end-stage renal disease, cancer, rheumatoid arthritis, hypothyroidism, and pregnancy, may also be associated with RLS/PLMD, as are specific medications (i.e., antihistamines such as diphenhydramine, antidepressants, and H-2 blockers such as cimetidine) and substances (e.g., caffeine).
Epidemiology
Previous studies have found prevalence rates of RLS in the pediatric population ranging from 1-6%; the percent of 8-17 yr olds meeting criteria for “definite” RLS is approximately 2%. Prevalence rates of PLMs greater than 5 per hour in clinical populations of children referred for sleep studies range from 5-27%; in survey studies of PLM symptoms, rates are 8-12%. Several studies in referral populations have found that PLMs occur in as much as one fourth of children diagnosed with ADHD.
Clinical Manifestations
In addition to the sensory component and the urge to move the legs, most RLS episodes begin or are exacerbated by rest or inactivity, such as lying in bed to fall asleep or riding in a car for prolonged periods. A unique feature of RLS is that the timing of symptoms also appears to have a circadian component, in that they often peak in the evening hours. Some children may complain of “growing pains,” although this is considered a nonspecific feature. Because RLS symptoms are usually worse in the evening, bedtime struggles and difficulty falling asleep are 2 of the most common presenting complaints. In contrast to patients with RLS, individuals with PLMs are usually unaware of these movements; these movements may result in arousals during sleep and consequent significant sleep disruption. Parents of children with RLS/PLMD may complain that their child is a restless sleeper, moves around or even falls out of bed during the night.
Treatment
The decision of whether and how to treat RLS depends on the level of severity (intensity, frequency, and periodicity) of sensory symptoms, the degree of interference with sleep, and the impact of daytime sequelae in a particular child or adolescent. With PLMs, for an index (PLMs per hr) less than 5, usually no treatment is recommended; for an index over 5, the decision to specifically treat PLMs should be based on the presence or absence of nocturnal symptoms (restless or nonrestorative sleep) and daytime clinical sequelae. A reasonable initial approach would be to promote good sleep hygiene (including restricting caffeine) and instituting iron supplements in children if serum ferritin levels are low (<50); the recommended dose of ferrous sulfate is typically in the range of 4-6 mg/kg/day for a duration of 3-6 mo. Medications that increase dopamine levels in the CNS, such as ropinirole and pramipexole, have been found to be effective in relieving RLS/PLMD symptoms in adults; data in children are extremely limited.
Sleep-related rhythmic movements, including head banging, body rocking, and head rolling, are characterized by repetitive, stereotyped, and rhythmic movements or behaviors that involve large muscle groups. These behaviors typically occur with the transition at sleep at bedtime, but also at nap times and following nighttime arousals. Children typically engage in these behaviors as a means of soothing themselves to (or back to) sleep; they are much more common in the 1st yr of life and usually disappear by 4 yr of age. In most instances, rhythmic movement behaviors are benign, because sleep is not significantly disrupted as a result of these movements and associated significant injury is rare. These behaviors typically occur in normally developing children, and in the vast majority of cases their presence does not indicate that there is some underlying neurological or psychological problem. Usually, the most important aspect in management of sleep-related rhythmic movements is reassurance to the family that this behavior is normal, common, benign, and self-limited.
Narcolepsy
Hypersomnia is a clinical term that is used to describe a group of disorders characterized by recurrent episodes of excessive daytime sleepiness (EDS), reduced baseline alertness, and/or prolonged nighttime sleep periods that interfere with normal daily functioning. It is important to recognize that there are many potential causes of EDS, which may be broadly grouped as “extrinsic” (e.g., secondary to insufficient and/or fragmented sleep) or “intrinsic” (e.g., resulting from an increased need for sleep). Narcolepsy is a chronic lifelong CNS disorder, typically presenting in adolescence and early adulthood, that is characterized by profound daytime sleepiness and resultant significant functional impairment. Other symptoms frequently associated with narcolepsy, cataplexy, hypnogogic/hypnopompic hallucinations, and sleep paralysis, may be conceptualized as representing the “intrusion” of REM sleep features into the waking state.
Etiology
There is a specific deficit in the hypothalamic orexin/hypocretin neurotransmitter system in the genesis of narcolepsy with cataplexy. The underlying pathogenesis of narcolepsy involves selective loss of cells that secrete hypocretin/orexin in the lateral hypothalamus; it has been postulated that autoimmune mechanisms, possibly triggered by viral infections, in combination with a genetic predisposition and environmental factors, may be involved. Human leukocyte antigen testing also shows a strong association with narcolepsy; however, the vast majority of individuals with this antigen do not have narcolepsy. Although the majority of cases of narcolepsy are considered idiopathic, “secondary” narcolepsy with cataplexy may also result from CNS insults.
Epidemiology
The prevalence of narcolepsy is reported to be between 3 and 16 per 10,000, with the prevalence of narcolepsy with cataplexy approximately 0.2-0.5/10,000. The risk of developing narcolepsy with cataplexy in a first-degree relative of a narcoleptic patient is estimated at 1-2%; this represents an increase of 10- to 40-fold compared to the general population.
Clinical Manifestations and Diagnosis
The typical onset of symptoms of narcolepsy is in adolescence and early adulthood, although symptoms may initially present in school-aged and even younger children. The early manifestations of narcolepsy are often ignored, misinterpreted, or misdiagnosed as other medical, neurologic, and psychiatric conditions, and the appropriate diagnosis is frequently delayed for a number of years.
The most prominent clinical manifestation of narcolepsy is profound daytime sleepiness, characterized by both an increased baseline level of daytime drowsiness and by the repeated occurrence of sudden and unpredictable sleep episodes. These “sleep attacks” are often described as “irresistible” in that the child or adolescent is unable to stay awake despite considerable effort, and they occur even in the context of normally stimulating activities (e.g., during meals, in the middle of a conversation). Very brief (several seconds) sleep attacks may also occur in which the individual may “stare off,” appear unresponsive, or continue to engage in an ongoing activity (automatic behavior). Cateplexy is considered pathognomonic for narcolepsy. Cataplexy is rarely the first symptom of narcolepsy, but it often develops within the 1st year of the onset of EDS. It is described as an abrupt, bilateral, partial or complete loss of muscle tone, classically triggered by an intense positive emotion (e.g., laughter, surprise). The cataplectic attacks are typically brief (seconds to minutes), and fully reversible, with complete recovery of normal tone when the episode ends. Hypnogogic/hypnopompic hallucinations involve vivid visual, auditory, and sometimes tactile sensory experiences occurring during transitions between sleep and wakefulness, primarily at sleep onset (hypnogogic) and sleep offset (hypnopompic). Sleep paralysis is the inability to move or speak for a few seconds or minutes at sleep onset or offset, and often accompanies the hallucinations. Other symptoms associated with narcolepsy include disrupted nocturnal sleep, inattention, and behavioral and mood issues.
Overnight polysomnography followed by a multiple sleep latency test (MSLT) are strongly recommended components of the evaluation of a patient with profound unexplained daytime sleepiness or suspected narcolepsy. The purpose of the overnight PSG is to evaluate for primary sleep disorders, such as OSA that may cause EDS. The MSLT involves a series of 5 opportunities to nap (20 min long), during which narcoleptics demonstrate a pathologically shortened sleep onset latency as well as periods of REM sleep occurring immediately after sleep onset.
Treatment
An individualized narcolepsy treatment plan usually involves education, good sleep hygiene, behavioral changes, and medication. Scheduled naps may be helpful. Medications such as psychostimulants and modafinil are often prescribed to control the EDS. The goal should be to allow the fullest possible return of normal functioning in school, at home, and in social situations. Medications such as tricyclic antidepressants and serotonin reuptake inhibitors may also be used to control the REM-associated phenomena, such as cataplexy, hypnogogic hallucinations, and sleep paralysis.
Delayed Sleep Phase Disorder
Delayed sleep phase disorder (DSPD), a circadian rhythm disorder, involves a significant, persistent, and intractable phase shift in sleep-wake schedule (later sleep onset and wake time) that conflicts with the individual’s normal school, work, and/or lifestyle demands. DSPD may occur at any age, but is most common in adolescents and young adults.
Etiology
Individuals with DSPD often start out as night owls; that is, they have an underlying predisposition or circadian preference for staying up late at night and sleeping late in the morning, especially on weekends, holidays, and summer vacations. The underlying pathophysiology of DSPD is still unknown, although some authors have theorized that it involves an intrinsic abnormality in the circadian oscillators that govern the timing of the sleep period.
Clinical Manifestations
The most common clinical presentation is sleep initiation insomnia when the individual attempts to fall asleep at a “socially acceptable” desired bedtime, accompanied by extreme difficulty getting up in the morning even for desired activities, and daytime sleepiness. Sleep maintenance is generally not problematic, and no sleep onset insomnia is experienced if bedtime coincides with the preferred sleep onset time (e.g., on weekends, school vacations). School tardiness and frequent absenteeism are often present.
Treatment
The goal in the treatment of DSPD is basically 2-fold: first, shifting the sleep-wake schedule to an earlier time, and second, maintaining the new schedule. Gradual advancement of bedtime in the evening and rise time in the morning typically involves shifting bedtime/wake time earlier by 15-30 min increments; more significant phase delays (difference between current sleep onset and desired bedtime) may require “chronotherapy,” which involves delaying bedtime and wake time by 2-3 hr daily to every other day. Exposure to light in the morning (either natural light or a “light box”) and avoidance of evening light exposure are often beneficial. Exogenous oral melatonin supplementation may also be used; larger doses (i.e., 5 mg) are typically given at bedtime, but some studies have suggested that physiologic doses of oral melatonin (0.3-0.5 mg) administered in the afternoon or early evening (i.e., 5-7 hr before the habitual sleep onset time) seem to be most effective in advancing the sleep phase.
Health Supervision
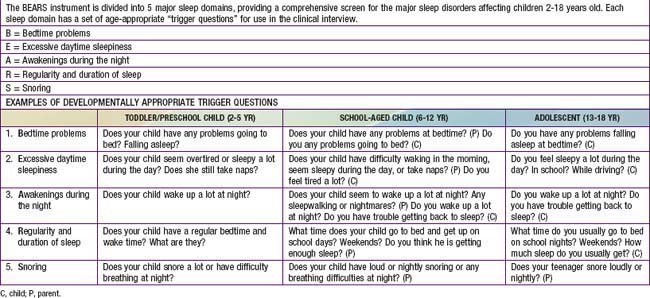
It is especially important for pediatricians to both screen for and recognize sleep disorders in children and adolescents during health encounters. The well child visit is an opportunity to educate parents about normal sleep in children and to teach strategies to prevent sleep problems from developing (primary prevention) or from becoming chronic, if problems already exist (secondary prevention). Developmentally appropriate screening for sleep disturbances should take place in the context of every well child visit and should include a range of potential sleep problems; one simple sleep screening algorithm, the “BEARS,” is outlined in Table 17-7. Because parents may not always be aware of sleep problems, especially in older children and adolescents, it is also important to question the child directly about sleep concerns. The recognition and evaluation of sleep problems in children requires both an understanding of the association between sleep disturbances and daytime consequences, such as irritability, inattention, and poor impulse control, and familiarity with the developmentally appropriate differential diagnoses of common presenting sleep complaints (difficulty initiating and maintaining sleep, episodic nocturnal events). An assessment of sleep patterns and possible sleep problems should be part of the initial evaluation of every child presenting with behavioral and/or academic problems, especially ADHD.
Effective preventive measures include educating parents of newborns about normal sleep amounts and patterns. The ability to regulate sleep, or control internal states of arousal to fall asleep at bedtime and to fall back asleep during the night, begins to develop in the first 12 wk of life. Thus, it is important to recommend that parents put their 2-4 mo old infants to bed “drowsy but awake” to avoid dependence on parental presence at sleep onset and to foster the infants’ ability to self-soothe. Other important sleep issues include discussing the importance of regular bedtimes, bedtime routines, and transitional objects for toddlers, and providing parents and children with basic information about good “sleep hygiene” and adequate sleep amounts.
The cultural and family context within which sleep problems in children occur should be considered. Co-sleeping of infants and parents is a common and accepted practice in many ethnic groups, including African-Americans, Hispanics, and Southeast Asians. The goal of independent self-soothing in young infants may not be shared by these families. On the other hand, the institution of co-sleeping by parents as an attempt to address a child’s underlying sleep problem, rather than as a lifestyle choice, is likely to yield only a temporary respite from the problem and may set the stage for more significant sleep issues.
Evaluation of Pediatric Sleep Problems
The clinical evaluation of a child presenting with a sleep problem involves obtaining a careful medical history to assess for potential medical causes of sleep disturbances, such as allergies, concomitant medications, and acute or chronic pain conditions. A developmental history is important because of the aforementioned frequent association of sleep problems with developmental delays and autism spectrum disorders. Assessment of the child’s current level of functioning (school, home) is a key part of evaluating possible mood, behavioral, and neurocognitive sequelae of sleep problems. Current sleep patterns, including the usual sleep duration and sleep-wake schedule, are often best assessed with a sleep diary, in which parents record daily sleep behaviors for an extended period. A review of sleep habits, such as bedtime routines, daily caffeine intake, and the sleeping environment (e.g., temperature, noise level) may reveal environmental factors that contribute to the sleep problems. Nocturnal symptoms that may be indicative of a medically based sleep disorder, such as OSA (loud snoring, choking or gasping, sweating) or PLMs (restless sleep, repetitive kicking movements), should be elicited. An overnight sleep study is seldom warranted in the evaluation of a child with sleep problems unless there are symptoms suggestive of OSA or periodic leg movements, unusual features of episodic nocturnal events, or daytime sleepiness that is unexplained.
Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82-90.
Capdevila OS, Kheirandish-Gozal L, Dayyat E, et al. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274-282.
Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505-509.
Guilleminault C, Quo S, Huynh NT, et al. Orthodontic expansion treatment and adenotonsillectomy in the treatment of obstructive sleep apnea in prepubertal children. Sleep. 2008;31:953-957.
Horsley T, Clifford T, Barrowman N, et al. Benefits and harms associated with the practice of bed sharing: a systematic review. Arch Pediatr Adolesc Med. 2007;161:237-245.
Ivanenko A, editor. Sleep and psychiatric disorders in children and adolescents. New York: Informa Healthcare, 2008.
Jenni OG, Carskadon MA. Sleep behavior and sleep regulation from infancy through adolescence: normative aspects. Sleep Med Clin. 2007;2:321-329.
Khatwa U, Kothare SV. Restless legs syndrome and periodic limb movements disorder in the pediatric population. Curr Opin Pulm Med. 2010;16:559-567.
Kothare S, Kaleyias J. Narcolepsy and other hypersomnias in children. Curr Opin Pediatr. 2008;20:666-675.
Loughlin GM. Primary snoring in children—no longer benign. J Pediatr. 2009;155:306-307.
Mayer G, Wilde-Frenz J, Kurella B. Sleep related rhythmic movement disorder revisited. J Sleep Res. 2007;16:110-116.
Mindell J, Owens J. A clinical guide to pediatric sleep: diagnosis and management of sleep problems in children and adolescents. Philadelphia: Lippincott Williams & Wilkins; 2009.
Morgenthaler TI, Owens J, Alessi C, et al. Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children: an American Academy of Sleep Medicine report. Sleep. 2006;29:1277-1281.
Owens J. Socio-cultural considerations and sleep practices in the pediatric population. Sleep and disorders of sleep in women. Driver H, editor. Sleep Medicine Clinics, 3, 2008;97-107.
Petit D, Touchette E, Tremblay RE, et al. Dyssomnias and parasomnias in early childhood. Pediatrics. 2007;119:e1016-1025.
Picchietti D, Allen RP, Walters AS, et al. Restless legs syndrome: prevalence and impact in children and adolescents—the Peds REST study. Pediatrics. 2007;120:253-266.
Picchietti MA, Picchietti DL. Advances in pediatric restless legs syndrome: iron, genetics, diagnosis and treatment. Sleep Med. 2010;11:643-651.
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea. Pediatrics. 2002;109:704-712.
Sheldon SH, Ferber R, Kryger MH, editors. Principles and practices of pediatric sleep medicine. Philadelphia: Elsevier, 2005.
Simard V, Nielsen TA, Tremblay RE, et al. Longitudinal study of preschool sleep disturbance. Arch Pediatr Adolesc Med. 2008;162:360-367.
Stores G. Aspects of parasomnias in children and adolescence. Arch Dis Child. 2009;94:63-69.
Touchette E, Mongrain V, Petit D, et al. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008;162:343-349.
Wiggs L. Behavioural aspects of children’s sleep. Arch Dis Child. 2009;94:59-62.
Yang Q, Li L, Yang R, et al. Family-based and population-based association studies validate PTPRD as a risk factor for restless legs syndrome. Movement Disorders. 24 Jan 2011. DOI: 10.1002/mds.23459