Chapter 28 Pervasive Developmental Disorders and Childhood Psychosis
The pervasive developmental disorders (PDD) and childhood schizophrenia can be understood as disturbances of brain development with genetic underpinnings. PDD spectrum includes autistic, Asperger’s, childhood disintegrative, Rett’s, and PDD not otherwise specified (NOS) disorders. Children with these disorders all share the inability to attain expected social, communication, emotional, cognitive, and adaptive abilities (Table 28-1).
28.1 Autistic Disorder
Clinical Manifestations
The core features of autistic disorder (AD) include impairments in 3 symptom domains: social interaction; communication; and developmentally appropriate behavior, interests, or activities (Table 28-2). Stereotypical body movements, a marked need for sameness, and a very narrow range of interests are also common.
Table 28-2 DSM-IV-TR DIAGNOSTIC CRITERIA FOR AUTISTIC DISORDER
From American Psychiatric Association: Diagnostic and statistical manual of mental disorders, fourth edition, text revision, Washington, DC, 2000, American Psychiatric Association.
Aberrant development of social skills and impaired ability to engage in reciprocal social interactions are hallmark symptoms of AD. Early social skill deficits can include abnormal eye contact, failure to orient to name, failure to use gestures to point or show, lack of interactive play, failure to smile, lack of sharing, and lack of interest in other children. Some children with AD make no eye contact and seem totally aloof, whereas others show intermittent engagement with their environment and can make inconsistent eye contact, smile, or hug. Most children have some impairment in joint attention, which is the ability to use eye contact and pointing for the purposes of sharing experiences with others. These children show deficits in empathy for what another person might be feeling. They also demonstrate deficits in understanding what another person might be thinking, a lack of a theory of mind.
Children with AD vary in their verbal abilities. They can range from being nonverbal to having some speech (e.g., capable of imitating songs, rhymes, or television commercials). Speech might have an odd prosody or intonation and may be characterized by echolalia (imitative repetition of words), pronoun reversal, nonsense rhyming, and other idiosyncratic language forms. Early abnormal language concerns include absent babbling or gestures by 12 mo, absent single words by 16 mo, absent 2-word purposeful phrases by 24 mo, and any loss of language or social skills at any time.
Play skills in AD are typically aberrant, characterized by little symbolic play, ritualistic rigidity, and preoccupation with parts of objects. The child with AD is often withdrawn and spends hours in solitary play, often with restrictive or repetitive interests and behaviors. Ritualistic behavior prevails, reflecting the child’s need to maintain a consistent, predictable environment. Tantrum-like rages can accompany disruptions of routine.
Intellectual functioning can vary from mental retardation to superior intellectual functioning in select areas (splinter skills, savant behavior). Some children show typical development in certain skills and can even show areas of strength in specific areas, such as puzzles, art, or music.
Visual scanning of hand and finger movements, mouthing of objects, and rubbing of surfaces can indicate a heightened awareness of and sensitivity to some stimuli, whereas diminished responses to pain and lack of startle responses to sudden loud noises reflect lowered sensitivity to other stimuli.
Diagnosis
AD is diagnosed by the clinical examination. The gold standard diagnostic tools are the Autism Diagnostic Interview—Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS), which require referral to a trained professional for administration.
Neuropsychologic and achievement assessment should include intelligence testing to establish overall cognitive function and eligibility for services. Intelligence, as measured by conventional psychologic testing, falls in the functionally retarded range in 30-60% of children with AD. Deficits in language and socialization often make it difficult to obtain an accurate estimate of a child’s intellectual potential. Some children with AD perform adequately in nonverbal tests, and those with developed speech can show adequate intellectual capacity. Separate estimates of verbal and nonverbal (performance) intelligence quotient (IQ) should be obtained. A measure of adaptive functioning such as the Vineland Adaptive Behavior Scales is essential to establish priorities for treatment planning.
Critical elements of the evaluation should include a detailed developmental history with a review of communicative and motor milestones, a medical history including discussion of possible seizures, sensory deficits such as hearing or visual impairment, or other medical conditions associated with AD including fragile-X, Prader-Willi, Smith-Lemli-Opitz, Rett’s, and Angelman’s syndromes, fetal alcohol syndrome, tuberous sclerosis, neurofibromatosis, congenital rubella, or untreated phenylketonuria. The family history should be reviewed for the presence of other developmental disorders. A review of current and past psychotropic medications should include a review of medication dosages and behavioral response, along with adverse effects. The impact of other medications on behavioral status should also be reviewed.
The medical and genetic evaluation of children with PDD must consider a broad range of disorders (Table 28-3). Approximately 20% of children with AD have macrocephaly, but enlarged head size might not be apparent until after the 2nd yr of life. In the absence of dysmorphic features or focal neurologic signs, additional neuroimaging for investigation of macrocephaly is not usually indicated. Multidisciplinary assessment of AD is optimal in facilitating early diagnosis, treatment, and coordinated multiagency collaboration. Evaluations from various other professionals, including a developmental pediatrician or pediatric neurologist, medical geneticist, child and adolescent psychiatrist, speech-language pathologist, occupational or physical therapist, or medical social worker may be indicated.
Table 28-3 MEDICAL AND GENETIC EVALUATION OF CHILDREN WITH PERVASIVE DEVELOPMENTAL DISORDERS
REQUIRED EVALUATIONS
CONSIDER IF RESULTS OF ABOVE EVALUATIONS ARE NORMAL, AND IN CHILDREN WITH COMORBID MENTAL RETARDATION
METABOLIC TESTING TO CONSIDER BASED ON OTHER CLINICAL FEATURES
OTHER TESTING TO CONSIDER BASED ON CLINICAL FEATURES
ELECTROENCEPHALOGRAPHY IF THE FOLLOWING CLINICAL FEATURES ARE NOTED
From Barbaresi WJ, Katusic SK, Voigt R: Autism: a review of the state of the science for pediatric primary care clinicians, Arch Pediatr Adolesc Med 160:1169, 2006.
Differential Diagnosis
The differential diagnosis includes consideration of the various PDD, mental retardation not associated with PDD (Chapter 33), specific developmental disorders (e.g., of language), early onset psychosis (e.g., schizophrenia), selective mutism, social anxiety (Chapter 23), obsessive-compulsive disorder, stereotypic movement disorder, inhibited-type reactive attachment disorder, and rarely, childhood-onset dementia.
Epidemiology
The incidence of AD has increased steadily over the past 15 yr. There is evidence that the increase in the number of children identified with AD is likely related to changes in the definition of and diagnostic criteria for AD, as well as improvements in the recognition of AD at younger ages. Current estimates of the prevalence rate of all PDD (63.7/10,000) are approximately 1 in 150-160. Disorder-specific prevalence rate estimates includes AD (20.6/10,000), Asperger’s disorder (6/10,000), PDD-NOS (37.1/10,000), Rett’s disorder (0.5-1/10,000 females), and childhood disintegrative disorder (2/100,000). The male : female ratios are estimated to be 4 : 1 for AD and 5 : 1 for Asperger’s disorder.
Pathology
Retrospective analysis of head circumference, in conjunction with MRI studies, has shown differences in the brain structure of children with AD. The head circumference in AD is normal or slightly smaller than normal at birth until 2 mo of age. Afterward children with AD show an abnormally rapid increase in head circumference from 6-14 mo of age, increased brain volume in 2-4 yr olds, increased volume of the cerebellum, cerebrum, and amygdala, and marked abnormal growth in the frontal, temporal, cerebellar, and limbic regions of the brain. Early, accelerated brain growth during the 1st several years of life is followed by abnormally slow or arrested growth, resulting in areas of underdeveloped and abnormal circuitry in parts of the brain. Areas of the brain responsible for higher-order cognitive, language, emotional, and social functions are most affected.
Etiology
The basis for AD is diverse and complex. Multiple genetic regions (chromosomes 16p11.2, 15q24, 11p12-p13) and gene variants (copy number variation, deletions, microdeletions, duplications, inversions, translocations) potentially contribute to abnormal neuronal and axonal growth, synapse formation, and myelination via gene-gene and gene-environment interactions over the course of prenatal and postnatal development. Inheritance patterns of AD demonstrate a 60% concordance rate for monozygotic twins and no concordance in dizygotic twins. A 4 : 1 male : female AD prevalence ratio suggests a sex-linked mechanism in a significant number of cases. An emerging etiologic hypothesis of AD describes spontaneous paternal or maternal genetic mutations that delete or inactivate areas of the genome affecting early brain development.
In utero toxic insults are also thought to carry the potential to produce disruptions in CNS development that can manifest as mental retardation and autistic symptoms. There is no scientifically substantiated association between the administration of the measles-mumps-rubella vaccine and the development of AD. As yet undiscovered environmental factors cannot be ruled out. There may be genetic associations between AD and premature birth as well as childhood-onset schizophrenia, suggesting possible common core neurobiologic processes for subsets of these two heterogeneous clinical groups.
Early Identification
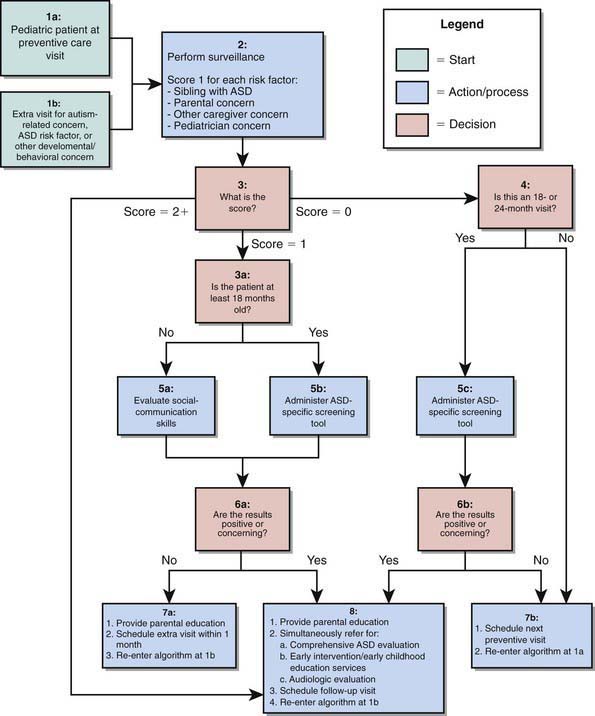
Early identification and intervention of PDD are associated with better outcomes. Several instruments have been developed for screening of PDD in primary care settings including the Checklist for Autism in Toddlers (CHAT), the Modified Checklist for Autism in Toddlers (M-CHAT), and the Pervasive Developmental Disorders Screening Test (PDDST) (Chapter 18) (Fig. 28-1). Failures to meet age-expected language or social milestones are important early red flags for PDD and should prompt an immediate evaluation. Early signs include unusual use of language or loss of language skills, nonfunctional rituals, inability to adapt to new settings, lack of imitation, and absence of imaginary play. Deviations in social and emotional development (such as decreased eye contact, failure to orient to name, and lack of joint attention) can often be detected by 1 yr of age. The absence of expected social, communication, and play behavior often precedes the emergence of odd or stereotypical behaviors or the unusual language usage that is seen in AD in the later years.
Treatment
The primary goals of treatment are to maximize the child’s ultimate functional independence and quality of life by minimizing the core features of the disorder, facilitating development and learning, promoting socialization, reducing maladaptive behaviors, and educating and supporting families. Educational interventions, including behavioral and habilitative (speech, occupational, and physical) therapies, are the cornerstones of treatment for the PDDs. These interventions address communication, social skills, daily-living skills, play and leisure skills, academic achievement, and maladaptive behaviors.
Model early childhood educational programs for children with PDD can be categorized as behavior analytic, developmental, or structured teaching on the basis of the underlying theoretical orientation. Although programs differ in relative emphasis, they share many common goals, including beginning intervention as early as possible; providing intensive intervention (at least 25 hr/wk, 12 mo/yr) in systematically planned educational activities; providing a low student-to-teacher ratio; including parent training; promoting opportunities for interaction with typically developing peers incorporating a high degree of structure through elements such as a predictable routine, visual activity schedules, and clear physical boundaries; implementing strategies to apply learned skills to new environments and situations; and using curricula that address functional spontaneous communication, social skills, functional adaptive skills, reduction of maladaptive behaviors, cognitive skills, and traditional academic skills. Some well-regarded programs that address at least some of these skills include Applied Behavioral Analysis (ABA), Discrete Trial Training (DTT), and Treatment and Education of Autistic and related Communication-handicapped Children (TEACCH). Most educational programs available to young children with PDDs are based in communities in the context of an Individualized Education Program (Chapter 15), and offer an eclectic treatment approach, which may be less effective than standardized protocols.
Parent training and family involvement includes educating parents about PDDs, providing access to needed ongoing supports and services, training and involving them as co-therapists, assisting them in advocating for their child’s needs, and providing emotional support.
Older children and adolescents with relatively higher intelligence, but with poor social skills and psychiatric symptoms, can benefit from more-intensive behavioral or cognitive-behavioral therapy (CBT) and/or supportive psychotherapy. The focus on achieving social communication competence, emotional and behavioral regulation, and functional adaptive skills necessary for independence continues. Every adolescent should be provided with a school-based individualized transition plan, where the focus may shift from academic to vocational services and from remediating deficits to fostering abilities. A vocational assessment can be helpful in this regard.
Pharmacotherapy can increase the ability of persons with AD to benefit from educational and other interventions and to remain in less-restrictive environments (Table 28-4). Common targets for pharmacological intervention include associated comorbid conditions and problematic behaviors such as aggression, self-injurious behavior, hyperactivity, inattention, anxiety, mood lability, irritability, compulsive-like behaviors, stereotypic behaviors, and sleep disturbances. After treatable medical causes and modifiable environmental factors have been ruled out, a trial of medication may be considered if the behavioral symptoms cause significant impairment in functioning. Prescribing is best approached with consultation from a practitioner with background and training in developmental disabilities.
Table 28-4 SELECTED POTENTIAL MEDICATION OPTIONS FOR COMMON TARGET SYMPTOMS OR COEXISTING DIAGNOSIS IN CHILDREN WITH AUTISM SPECTRUM DISORDERS
| TARGET SYMPTOM CLUSTERS | POTENTIAL COEXISTING DIAGNOSES | SELECTED MEDICATION CONSIDERATIONS |
|---|---|---|
| Repetitive behavior, behavioral rigidity, obsessive-compulsive symptoms | Obsessive-compulsive disorder, stereotypic movement disorder | |
| Hyperactivity, impulsivity, inattention | Attention-deficit/hyperactivity disorder | |
| Sleep dysfunction | Circadian rhythm sleep disorder, dyssomnia–not otherwise specified | |
| Anxiety | Generalized anxiety disorder, anxiety disorder–not otherwise specified | |
| Depressive phenotype (marked change from baseline including symptoms such as social withdrawal, irritability, sadness or crying spells, decreased energy, anorexia, weight loss, sleep dysfunction) | Major depressive disorder, depressive disorder–not otherwise specified | |
| Bipolar phenotype (behavioral cycling with rages and euphoria, decreased need for sleep, manic-like hyperactivity, irritability, aggression, self-injury, sexual behaviors) | Bipolar I disorder, bipolar disorder–not otherwise specified |
SSRI, selective serotonin reuptake inhibitor.
Modified from Myers SM, Plauche Johnson C, Council on Children with Disabilities: Management of children with autism spectrum disorders, Pediatrics 120:1162-1182, 2007.
Selective serotonin reuptake inhibitors appear to have efficacy for the treatment of co-occurring mood and anxiety symptoms and compulsive-like behaviors among persons with AD. Of the typical antipsychotics, haloperidol has evidence supporting a role in reducing stereotypy and facilitating learning. There has been concern about its use given the high rates of dyskinesias that are incurred. Given a more favorable side-effect profile in this population, atypical neuroleptics have been increasingly used with demonstrated efficacy on the symptoms of agitation, irritability, aggression, self-injury, and severe temper outbursts (Table 28-5; Chapter 19). Risperidone and aripiprazole have been approved by the U.S. Food and Drug Administration (FDA) for treating irritability associated with autism. In moderate doses, stimulants can benefit children with hyperactivity and impulsivity. α-Adrenergic agonists can reduce hyperarousal symptoms including hyperactivity, irritability, impulsivity, and repetitive behavior. The evidence for mood stabilizers in AD is limited.
Table 28-5 DSM-IV-TR DIAGNOSTIC CRITERIA FOR ASPERGER’S DISORDER
From American Psychiatric Association: Diagnostic and statistical manual of mental disorders, fourth edition, text revision, Washington, DC, 2000, American Psychiatric Association.
Prognosis
Most persons with PDD remain within the spectrum as adults, and regardless of their intellectual functioning, they continue to experience problems with independent living, employment, social relationships, and mental health. Some children, especially those with communication abilities, can grow up to live self-sufficient lives in the community with employment. Others remain dependent on their family or require placement in facilities outside the home. Because early, intensive therapy can improve language and social function, delayed diagnosis can lead to a poorer outcome. A better prognosis is associated with higher intelligence, functional speech, and less-bizarre symptoms and behavior. The symptom profile for some children might change as they grow older, and risk of seizures or self-injurious behavior becomes more common.
Allison C, Baron-Cohen S, Wheelwright S, et al. The Q-CHAT (Quantitative Checklist for Autism in Toddlers): a normally distributed quantitative measure of autistic traits at 18-24 months of age: preliminary report. J Autism Dev Disord. 2008;38:1414-1425.
Amaral D, Schuman C, Nordhal C. Neuroanatomy of autism. Trends Neurosci. 2008;31:137-145.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: American Psychiatric Association; 1994.
American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders, J Am Acad Child Adolesc Psychiatry, in press.
Bandini LG, Anderson SE, Curtin C, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157:259-264.
Barbaresi WJ, Katusic SK, Voigt R. Autism: a review of the state of the science for pediatric primary care clinicians. Arch Pediatr Adolesc Med. 2006;160:1167-1175.
Chen CY, Chen KH, Liu CYL, et al. Increased risk of congenital, neurologic, and endocrine disorders associated with autism in preschool children: cognitive ability differences. J Pediatr. 2009;154:345-350.
Constantino JN, Zhang Y, Frazier T, et al. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349-1356.
Ecker C, Marguand A, Mourao-Miranda J, et al. Describing the brain in autism in five dimensions—magnetic resonance imaging–assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. 2010;30(32):10612-10623.
Giulivi C, Zhang YF, Omanska-Klusek A, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304(21):2389-2396.
Johnson S, Marlow N. Positive screening: results on the modified checklist for autism in toddlers: implications for very preterm populations. J Pediatr. 2009;154:478-480.
Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946-954.
Mandell DS, Wiggins LD, Armstein Carpenter L, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Pub Health. 2009;99:493-498.
Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Human Genetics. 2008;82:1-12.
Morrow EM, Yoo SY, Flavell SW, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218-223.
Myers SM, Plauche Johnson C, Council on Children with Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162-1182.
Ozonoff S, Losif AM, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256-266.
Plauche Johnson C, Myers SM, Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
Rapoport J, Chavez A, Greenstein D, et al. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10-16.
Schendel D, Karapurkar Bhasin T. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121:1155-1164.
Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667-675.
28.2 Asperger’s Disorder
Children with Asperger’s disorder have a qualitative impairment in the development of reciprocal social interaction. They often show repetitive behaviors with restricted, obsessional, and idiosyncratic interests. To meet the DSM-IV-TR diagnostic criteria for Asperger’s disorder, a child must manifest impairments in social interactions and show restrictive, repetitive patterns of behavior, interests, or achievements with other people. These disturbances must cause significant impairments in social or occupational functioning (see Table 28-5).
Unlike children with AD, those with Asperger’s disorder have a history of normal language milestones with single words used by age 2 yr and communicative phrases used by age 3 yr. They have deficits in nonverbal and pragmatic aspects of communication (facial expressions, gestures) but do not have the severe language delays and impairments that characterize AD. Neuropsychologic testing can reveal a pattern consistent with nonverbal learning disability.
Although they are somewhat socially aware, these children appear to others to be peculiar or eccentric. They can be awkward and clumsy and have unusual postures and gait. There are often similar traits in family members. This disorder might represent a form of high-functioning AD (children with autism without cognitive impairment), although this distinction remains controversial. Group social skills training is an effective intervention. CBT has been useful in patients with associated anxiety, and risperidone can improve negative symptoms similar to those seen in schizophrenia. Because children with Asperger’s disorder are at high risk for other psychiatric disorders, particularly mood (Chapter 24) and anxiety disorders (Chapter 23) screening for such problems is an important part of the evaluation.
Children with Asperger’s disorder tend to improve symptomatically and functionally as they mature, with superior IQ conveying an improved prognosis. Thirty percent of children with this disorder develop comorbid psychiatric disorders.
A child who has some symptoms but who does not meet full criteria for AD or Asperger’s disorder is diagnosed as PDD Not Otherwise Specified. This “atypical autism” has a lifelong course with variable outcome and is often associated with comorbid psychiatric disorders.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: American Psychiatric Association; 1994.
American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders, J Am Acad Child Adolesc Psychiatry, in press.
Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946-954.
Myers SM, Plauche Johnson C, Council on Children with Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162-1182.
Plauche Johnson C, Myers SM, Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
Toth K, King B. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry. 2008;165:958-963.
28.3 Childhood Disintegrative Disorder
The essential feature of childhood disintegrative disorder (also termed Heller’s syndrome, dementia infantilis, or disintegrative psychosis) is a marked regression in multiple areas of functioning following a period of at least 2 yr of apparently normal development.
The DSM-IV-TR criteria for this disorder includes apparently normal development for at least the 1st 2 yr after birth as manifested by the presence of age-appropriate verbal and nonverbal communication, social relationships, play, and adaptive behavior along with the clinically significant loss of previously acquired skills (before age 10 yr) in at least two of the following areas: expressive or receptive language, social skills or adaptive behavior, bowel or bladder control, play, and motor skills. The loss of skills usually occurs over a 6- to 9-month period, then plateaus.
There are also abnormalities of functioning in at least two of the following areas: qualitative impairment in social interaction (e.g., impairment in nonverbal behaviors, failure to develop peer relationships, lack of social or emotional reciprocity), qualitative impairments in communication (e.g., delay or lack of spoken language, inability to initiate or sustain a conversation, stereotyped and repetitive use of language, lack of varied make-believe play), and restricted, repetitive, and stereotyped patterns of behavior, interest, and activities, including motor stereotypes and mannerisms.
The exact causes of this disorder are unknown. The loss of language and social skills related to social interaction and self-care portend a poor prognosis. These children face permanent disability and require long-term care. Treatment involves behavior therapy and medications (e.g., antipsychotic medication for severe behavior dyscontrol).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: American Psychiatric Association; 1994.
American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders, J Am Acad Child Adolesc Psychiatry, in press.
Myers SM, Plauche Johnson C, Council on Children with Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162-1182.
Plauche Johnson C, Myers SM, Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
28.4 Childhood Schizophrenia
The signs and symptoms of schizophrenia in children are classified in the DSM-IV-TR into 2 broad domains of positive and negative symptoms (see Table 28-6 on the Nelson Textbook of Pediatrics website at www.expertconsult.com ![]() ). Positive symptoms include hallucinations, delusions, disorganized speech, and/or disorganized or catatonic behavior. Negative symptoms include flattening of affect, social withdrawal, loss of motivation, and cognitive impairments. These latter symptoms are related to poorer premorbid functioning and an increased familial risk of schizophrenia. Children with schizophrenia have more-severe premorbid neurodevelopmental abnormalities, increased cytogenetic anomalies, and stronger family histories of psychotic disorders in comparison to their adult counterparts.
). Positive symptoms include hallucinations, delusions, disorganized speech, and/or disorganized or catatonic behavior. Negative symptoms include flattening of affect, social withdrawal, loss of motivation, and cognitive impairments. These latter symptoms are related to poorer premorbid functioning and an increased familial risk of schizophrenia. Children with schizophrenia have more-severe premorbid neurodevelopmental abnormalities, increased cytogenetic anomalies, and stronger family histories of psychotic disorders in comparison to their adult counterparts.
Table 28-6 DSM-IV-TR DIAGNOSTIC CRITERIA FOR SCHIZOPHRENIA
Note: Only one criterion A symptom is required if delusions are bizarre or hallucinations consist of a voice keeping up a running commentary on the person’s behavior or thoughts or two or more voices are conversing with each other
From American Psychiatric Association: Diagnostic and statistical manual of mental disorders, fourth edition, text revision, Washington, DC, 2000, American Psychiatric Association.
Epidemiology
The average age of onset of schizophrenia is 18 yr in men and 25 yr in women. The prevalence of childhood-onset schizophrenia is 1/10,000; prevalence of adult schizophrenia is 1/100. Men tend to be affected twice as often as women, with males affected approximately 5 yr earlier than females. Less than 20% of patients with schizophrenia diagnosed before age 12 yr were younger than 10 yr, and boys were predominant in most studies. More than half of patients with onset before age 18 yr have moderate to severe impairment in adulthood.
Etiology
Schizophrenia is a disorder of brain development, with susceptibility genes acting in conjunction with developmental and environmental factors to effect brain pathophysiologic processes long before the overt manifestation of clinical symptoms. Although schizophrenia has a significant genetic component, with heritability of about 80%, the genetics are complex and the interpretation of genetic data has proved difficult. Lifetime risk of developing this disorder is 5-20 times higher in first-degree biological relatives of affected persons when compared to the general population. Twin and family studies have revealed increased familial aggregation for childhood-onset schizophrenia and a higher concordance in earlier age of onset in monozygotic compared to dizygotic twins with schizophrenia.
Velocardiofacial syndrome (VCFS or diGeorge Syndrome; Chapter 119) is associated with psychosis in childhood and in adult life. The deletion on chromosome 22q11.2 found in VCFS is 80 times more common in adults with schizophrenia and 240 times more common in childhood-onset schizophrenia. Childhood-onset schizophrenia is preceded by and comorbid with PDD in 30-50% of cases.
Neurotransmitter systems, particularly CNS dopamine circuits, are hypothesized to have a key role in the pathophysiology of schizophrenia. The dopamine hypothesis is derived in part from the identification of D2 receptor blockade as the mechanism for the action of antipsychotic medications. Increased lateral ventricular volume, decreased gray matter volume, increased basal ganglia volume, and abnormal hemispheric asymmetries are noted in childhood-onset schizophrenia.
Treatment
Children with schizophrenia and their families require an array of mental health services to address their psychologic, social, educational, and cultural needs. Given the insidious onset and chronic course of the disorder, the patient must be followed longitudinally, with periodic reassessment to hone diagnostic accuracy and tailor services to meet the patient’s and family’s needs. Integrated psychopharmacologic, psychotherapeutic, psychoeducational, and case-management services are often necessary. Psychoeducation about the illness with an assessment of the potential role of stigma in treatment participation is critical for improving adherence with treatment recommendations. Assessing a child’s strengths and vulnerabilities as well as available environmental resources is critical in devising an effective treatment plan. School and community liaison work to develop and maintain a day-to-day schedule for the patient is important. Specialized educational programs should be considered within the school system. Effective and collaborative communication among the family, the primary care clinician, a child psychiatrist, and other mental health providers increases the potential for the patient’s optimal functioning.
First-generation (typical) and second-generation (atypical) antipsychotic medications have been shown to be effective in reducing psychotic symptoms (Chapter 19 and Table 19-5). In general, the atypical medications have become the first line of treatment in this disorder. Although clozapine has been shown to be effective in treating both positive and negative symptoms, its increased risk for agranulocytosis and seizures has limited its use until after multiple trials of other antipsychotic medications have failed.
These medications all have significant side effects including extrapyramidal symptoms (EPS) (e.g., restlessness and dyskinesias), weight gain, metabolic syndrome, hyperprolactinemia, diabetes, hematologic adverse effects (e.g., leukopenia or neutropenia), seizures, hepatotoxicity, neuroleptic malignant syndrome, and cardiovascular effects. For atypical antipsychotics, body mass index, blood pressure, fasting blood glucose, fasting lipid profiles, and abnormal movements should be closely monitored. Significant weight gain occurs early in treatment with these medications. Regular physical activity and nutritional balance should be part of a comprehensive treatment plan. Abnormal movements can be monitored using the AIMS (Abnormal Involuntary Movement Scale). If there is a family or personal history suggesting cardiac disease, electrocardiograms should also be monitored.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: American Psychiatric Association; 1994.
American Academy of Child and Adolescent Psychiatry. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(Suppl):4S-23S.
Fazel S, Langstrom N, Hjern A, et al. Schizophrenia, substance abuse, and violent crime. JAMA. 2009;301:2016-2023.
Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomized clinical trial. Lancet. 2009;371:1085-1096.
Kumra S, Schulz SC. Editorial: research progress in early-onset schizophrenia. Schizophrenia Bulletin. 2008;34:15-17.
Nicodemus KK, Law AJ, Radulesca E, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67(10):991-1001.
Rapoport J, Chavez A, Greenstein D, et al. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10-16.
Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225-234.
Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330-350.
Wicks S, Hjern A, Dalman C. Social risk or genetic liability for psychosis? A study of children born in Sweden and reared by adoptive parents. Am J Psychiatry. 2010;167:1240-1246.
Xu B, Roos JL, Levy S, et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880-885.
28.5 Psychosis Associated with Epilepsy
Psychosis associated with epilepsy has been reported in children and adults and may be more common than expected. Also called schizophrenic-like psychosis of epilepsy, the disorder can manifest delusions, hallucinations, and poor insight. The characterization is complicated by the facts that anticonvulsant drugs can precipitate psychosis and antipsychotic drugs can lower the seizure threshold, producing seizures. In addition, epilepsy may be a risk factor for schizophrenia.
Ictal-induced psychosis is a form of nonconvulsive status epilepticus, usually complex partial status that can last for hours to days and is associated with periods of impaired consciousness. Brief interictal psychosis can last days to weeks and is associated with paranoia, delusions, and auditory hallucinations. Chronic interictal psychosis resembles schizophrenia and manifests with paranoia, visual hallucinations, and catatonia.
Postictal psychosis is the most common type; it lasts up to 1 wk and then spontaneously remits. It is characterized by delusions, mania, and catatonia.
The diagnosis requires a strong index of suspicion and EEG monitoring.
Treatment requires appropriate anticonvulsant drugs and, if the psychosis persists, initiating low-dose antipsychotic agents.
Devinsky O. Postictal psychosis: common, dangerous, and treatable. Epilepsy Curr. 2008;8:31-34.
Elliott B, Joyce E, Shorvon S. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res. 2009;85(2–3):172-186.
Farooq S, Sherin A: Interventions for psychotic symptoms concomitant with epilepsy (review), Cochrane Database Syst Rev (4):CD006118, 2008.
Joshi CN, Booth FA, Sigurdson ES, et al. Postictal psychosis in a child. Pediatr Neurol. 2006;34:388-391.
Kanner AM, Dunn DW. Diagnosis and management of depression and psychosis in children and adolescents with epilepsy. J Child Neurol. 2004;19:S65-S72.
28.6 Acute Phobic Hallucinations of Childhood
Among adults, hallucinations are viewed as synonymous with psychosis and as harbingers of serious psychopathology. In children, hallucinations can be part of normal development or can be associated with nonpsychotic psychopathology, psychosocial stressors, drug intoxication, or physical illness. The first clinical task in evaluating children and adolescents who report hallucinations is to sort out those that are associated with severe mental illness from those that derive from other causes (Fig. 28-2).

Figure 28-2 Evaluation of hallucinations.
(From Kliegman RM, Greenbaum LA, Lye PS: Practical strategies in pediatric diagnosis and therapy, ed 2, Philadelphia, 2004, Elsevier/Saunders, p 601.)
Clinical Manifestations
Hallucinations are perceptions (typically auditory, visual, tactile, or olfactory) that occur in the absence of identifiable external stimuli. Hallucinations can be further categorized as nondiagnostic (such as hearing footsteps, knocking, or one’s name) and diagnostic (such as hearing one or more voices saying words other than one’s own name).
In children with nonpsychotic hallucinations, the symptoms of psychosis are absent. Nonpsychotic hallucinations commonly occur in the context of severe traumatic stress, developmental difficulties, social and emotional deprivation, parents whose own psychopathology promotes a breakdown in the child’s sense of reality, cultural beliefs in mysticism, and unresolved mourning. Auditory hallucinations of voices telling the child to do bad things may be more often associated with disruptive behavior disorders than with psychotic diagnoses. Hearing a voice invoking suicide is often associated with depression. Trauma-related auditory hallucinations are commonly associated with post-traumatic stress disorder or a brief psychotic disorder with marked stressors. Thus, the content of the hallucinations may be relevant in understanding the underlying psychopathology and/or developmental issues.
Diagnosis and Differential Diagnosis
The differential diagnosis of hallucinations comprises a broad range of psychiatric disorders, including diagnoses in which hallucinations are not the hallmark feature, but may be viewed as associated symptoms (e.g., post-traumatic stress disorder, nonpsychotic mood disorders, and disruptive behavior disorders); diagnoses that are defined by psychotic features (e.g., brief psychotic disorder, schizophrenia, major depression with psychotic features, bipolar disorder with psychotic features); and at-risk clinical states (poor reality testing). In addition, nonpsychiatric disorders can manifest with hallucinations, including drug intoxications (cannabis, LSD, cocaine, amphetamines, barbiturates), medication side effects (steroids, anticholinergic medications, stimulant medications), and physical illnesses (thyroid, parathyroid, adrenal, and Wilson’s disease; electrolyte imbalance; infections; migraines; seizures; and brain tumors).
Acute phobic hallucinations are benign and common and occur in previously healthy preschool children. The hallucinations are often visual or tactile, last 10-60 min, and occur at any time but most often at night. The child is quite frightened and might complain that bugs or snakes are crawling over him or her and attempt to remove them. The cause is unknown. The differential diagnosis includes drug overdose or poisoning, high fever, encephalitis, and psychosis. The child’s fear is not alleviated by reassurance by the parents or physician, and the child is not amenable to reason. Findings on physical and mental status examinations are otherwise normal. Symptoms can persist for 1-3 days, slowly abating over 1-2 wk. Treatment with benzodiazepines may be beneficial.
Treatment
The evaluation of the underlying condition directs the type of treatment needed. Nonpsychotic hallucinations suggest the need for disorder-specific psychotherapy (e.g., trauma-focused CBT for post-traumatic stress disorder) and perhaps adjunctive medication (e.g., an antidepressant for depression or anxiety, or a brief trial of antipsychotic medication). CBT focused on helping the youth understand the origin of the hallucinations and develop coping strategies for stressful situations also may be helpful for older children and adolescents. Psychotic hallucinations suggest the need for antipsychotic medication.
McGee R, Williams S, Poulton R. Hallucinations in nonpsychotic children. J Am Acad Child Adolesc Psychiatry. 2000;39:12-13.
Owen MJ, Craddock N. Diagnosis of functional psychoses: time to face the future. Lancet. 2009;373:190-191.
Schreier A, Wolke D, Thomas K, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66:527-536.
Sosland M, Edelsohn G. Hallucinations in children. Curr Psychiatry Rep. 2005;7:180-188.