Chapter 111 Contraception
Adolescents bear a disproportionate risk of the adverse consequences of sexual activity, sexually transmitted infections (STIs) (Chapter 114), and early, unintended pregnancy (Chapter 112). Adolescents often do not seek reproductive health care for 6 to 12 mo after initiating sex; many will become pregnant and/or acquire an STI during this interval. Youth who plan sexual initiation are 75% more likely to use contraception at sexual debut. Early adolescents are concrete thinkers, which may limit their ability to plan; most contraception requires some planning. Appropriate educational interventions with adolescents including the health care provider bringing up the topic of prevention can decrease sexual risk behavior.
Epidemiology
Sexual Activity
The median age at 1st intercourse varies greatly across the globe. Among participants ranging in age from 16 to above 65 yr representing all educational levels from 26 middle- and high-income nations, age of sexual debut was 18.9 yr for women and 19.5 yr for men, with an overall range of 23.0 yr in Malaysia to 17.3 yr in Austria (18.0 yr in the USA) (Fig. 111-1). Among developing nations, there is greater diversity; 73% of Liberian women ages 15-19 yr have had intercourse, compared to 53% of Nigerian, 49% of Ugandan, and 32% of Botswanan women. Only 7% of Chinese university students report being sexually experienced. Among U.S. high schools students in 2007, 7% report having initiated sex before age 13 yr, including 16% of African-American, 8% of Hispanic, and 4% of white youth; 48% of high school students are sexually experienced, including 67% African-American, 52% Hispanic, and 44% white.
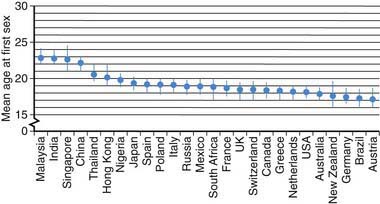
Figure 111-1 Mean age at first sex by country.
(From Durex Network: The face of global sex 2007. First sex: opportunity of a lifetime, Cambridge, UK, 2007, SSL International, p 13. www.durexnetwork.org/en-GB/research/faceofglobalsex/Pages/Home.aspx. Accessed April 22, 2010.)
Factors associated with early sexual activity in nations worldwide include lower expectations for education, poor perception of life options, low school grades, and involvement in other high-risk behaviors. For those who have never had intercourse, being against their religion and morals, avoiding pregnancy or a sexually transmitted infection, and waiting for the right person were the most frequent reasons adolescents report for abstaining.
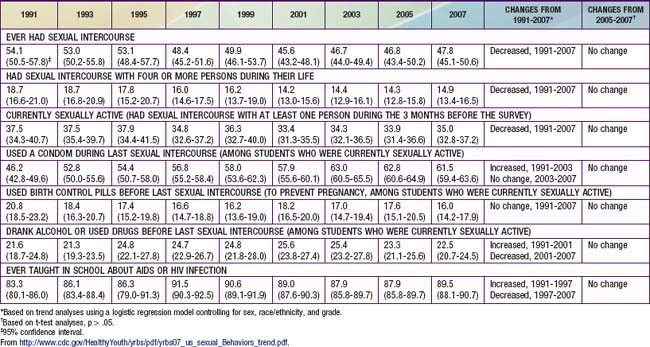
Despite decreases in adverse outcomes of adolescent sexual activity in the USA for the decade at the end of the 20th and beginning of the 21st century, this trend may be reversing. The annual rate of AIDS diagnoses reported among males aged 15-19 yr doubled in the past from 1.3 cases per 100,000 population in 1997 to 2.5 cases in 2006; and rates of gonorrhea and syphilis also increased. Birthrates among adolescents aged 15-19 yr decreased each year from 1991-2005 but increased from 40.5 live births per 1,000 females in 2005 to 42.5 in 2007. The USA has the highest rate of teen pregnancy in the western industrialized world. Of the >750,000 pregnancies among women aged 15-19 yr annually, ~ 80% are unintended (of which 30% are aborted). Consistent with these decreases and subsequent increases in adverse outcomes, the Centers for Disease Control and Prevention “Youth Risk Behavior Survey” reported that among high school students the rate of “ever had intercourse” declined from 54.1% in 1991 to 46.7% in 2005. From 2005 to 2007 there was no further decline; rather, albeit insignificant statistically, all 3 categories of sexual activity (ever had sex, number of partners, currently sexually active) showed increasing trends and both contraception reports (use a condom, use birth control) showed decreasing trends (Table 111-1).
Contraceptive Use
Use of contraception at first sex has increased over the past half-century. Among the residents of 26 middle- and high-income countries listed in Figure 111-2, individuals >65 yr were 8-fold more likely to have used no contraception for their sexual debut, compared to youth currently 16-19 yr of age (among whom 75% used contraception). Females compared to males were 25% more likely to use some form of contraception when they made their sexual debut. Factors increasing the use of contraception at sexual debut include: increasing age among teens up to age 17 yr; spending some time at college; and planning their sexual debut (75% more likely to have used contraception than those who did not plan it). Among U.S. men, data from the National Center for Health Statistics reveals that 48% of those 15-44 yr report using a condom the 1st time they had sex, while 71% of sexually experienced men age 15-19 yr report using a condom the 1st time they have sex. Use of a condom at last episode of intercourse increased from 46% in 1991 to 63% in 2003; in 2007 61.5% reported using a condom; use of birth control pills was 20.8% in 1991 and 16% in 2007 (see Table 111-1). Condoms were the most frequently used method, with dramatic increases from 1995-2002 in non-Hispanic white females (40.8% to 60.8%) and non-Hispanic black males (71% to 86.1%). The type of hormonal method selected also varies by ethnicity, with non-Hispanic white women more likely to select pills (40.7%); black women use pills as the first choice, and use the injectable method twice as much as white women.
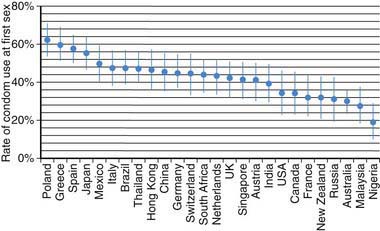
Figure 111-2 Rate of condom use at first sex by country.
(From Durex Network: The face of global sex 2007. First sex: opportunity of a lifetime, Cambridge, UK, 2007, SSL International. www.durexnetwork.org/en-GB/research/faceofglobalsex/Pages/Home.aspx. Accessed April 22, 2010.)
Teens in the USA used medical methods at last intercourse less frequently compared to other teens; 52% in U.S. teens, 56% in Swedish 18-19 yr olds, 67% of French 15-19 yr olds, 72% of British 16-19 yr olds, and 73% of Canadian 15-19 yr olds. A higher likelihood of contraceptive use in women is associated with older age at sexual initiation, aspirations for higher academic achievement, acceptance of one’s own sexuality, and a positive attitude toward contraception.
Contraceptive Counseling
The health screening interview during the adolescent preventive visit offers the opportunity both to support the adolescent who is abstinent to continue to be so and to identify the sexually active adolescent who has unsafe sexual practices (Chapter 106). Adolescents with chronic diseases are particularly vulnerable to having these issues omitted from the health maintenance visit (Chapter 39). There may be particular cautions related to concurrent medication to be noted for these chronically ill teenagers; sexuality and contraceptive issues do need to be addressed. The goals of a counseling intervention with the adolescent are to understand the adolescent’s perceptions and misperceptions about contraceptives, help him or her put the risk of unprotected intercourse in a personal perspective, and educate the adolescent regarding the real risk and contraindications for the various methods available.
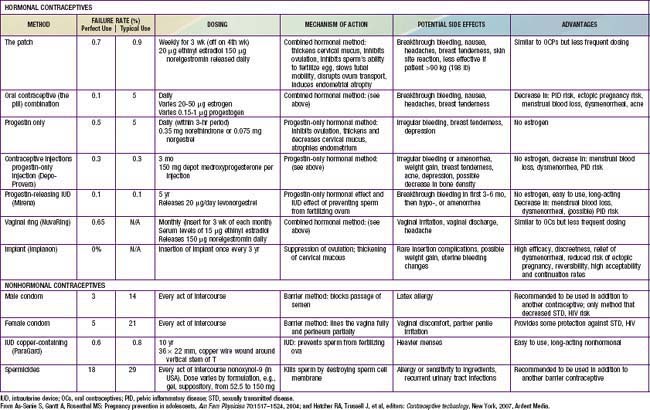
The likelihood that an adolescent will use a contraceptive method depends on such factors as the developmental level of the adolescent, the reproductive history, the involvement in other high-risk behaviors, and the degree of readiness for using contraception. Readiness to use contraception progresses in stages, from (1) precontemplative, not thinking about using contraception; (2) contemplative, giving it some thought, but having no immediate plans; (3) preparative, wanting to try a method in the near future; to (4) active, using contraception. The adolescent should also be made aware of the “perfect” use failure rates versus the “typical” failure rates based on the correct and consistent use of the method (Table 111-2). The pregnancy risk for use of withdrawal as a contraceptive method is probably underestimated in adolescents, and its low efficacy rate should be specifically addressed with young adolescents. Once an adolescent chooses a method, the provider and youth must discuss recognition of the common side effects, with clear plans on management; communication with the provider about the realistic expectation for failure; a contingency plan for that possibility; and strategies for close follow-up (see Table 111-2). A pelvic examination is not required for provision of a contraceptive method. Guidelines from the American College of Obstetrics and Gynecology stipulate that a routine pelvic exam with Pap smear be initiated 3 yr after onset of sexual activity. After this event, annual urine screening for STIs is advised. Confidentiality and consent issues related to contraceptive management are discussed in Chapter 106.
111.1 Barrier Methods
Condoms
This method prevents sperm from being deposited in the vagina. There are no major side effects associated with the use of a condom. The risk of AIDS may have increased the use of condoms among adolescents, with 46.2% of high school students in 1991 reporting using a condom at last sexual intercourse increasing to 61.5% in 2007. Condom use rates vary considerably between countries; condom use at 1st intercourse is higher in many countries compared to the USA (see Fig. 111-2). The main advantages of condoms are their low price, availability without prescription, little need for advance planning, and, most important for this age group, their effectiveness in preventing transmission of STIs, including HIV and human papillomavirus (HPV). Latex condoms are recommended as protection against STIs, to be used along with all nonbarrier medical methods for adolescents. A female condom is available over the counter in single size disposable units. It is a 2nd choice over the male latex condom because of the complexity of properly using the device, its low typical efficacy rate, and the lack of studies in humans demonstrating its effectiveness against STIs. Most adolescents would require intensive education and hands-on practice to use it effectively.
Diaphragm and Cervical Cap
These methods have few side effects but are much less likely to be used by teenagers. Adolescents tend to object to the messiness of the jelly or to the fact that the insertion of a diaphragm may interrupt the spontaneity of sex, or they may express discomfort about touching their genitals.
111.2 Spermicides
A variety of agents containing the spermicide nonoxynol-9 are available as foams, jellies, creams, films, or effervescent vaginal suppositories. They must be placed in the vaginal cavity shortly before intercourse and reinserted before each subsequent ejaculation in order to be effective. Rare side effects consist of contact vaginitis. There has been some concern regarding the vaginal and cervical mucosal damage observed with nonoxynol-9, and the overall impact on HIV transmission is unknown. The finding that nonoxynol-9 is gonococcicidal and spirocheticidal has not been substantiated in randomized clinical trials. Spermicides should be used in combination with condoms.
111.3 Combination Methods
The conjoint use of the condom by the male and spermicidal foam by the female adolescent is extremely effective; the failure rate is 2% (perfect use), without any of the potential side effects and complications associated with the use of other forms of contraception having comparable efficacy. This combination also prevents STIs, including HIV and HPV.
111.4 Hormonal Methods
Hormonal methods employ either an estrogenic substance in combination with a progestin or a progestin alone. The major mechanism of action of both the estrogen-progestin combination and the progestin-only methods, is to prevent the surge of luteinizing hormone and, as a result, to inhibit ovulation. Additional effects to the reproductive tract, which may add to contraceptive efficacy, include thickening of the cervical mucus in such a way that prevents sperm penetration.
Combination Oral Contraceptives
Oral contraceptives (OCs) are commonly referred to as “the pill” and contain 35, 30, or 20 µg of estrogenic substance, typically ethinyl estradiol, and a progestin. The pill is one of the most reliable contraceptive methods available; typical-use failure rates in 15-19 yr old women have ranged up to 18.1%. Thrombophlebitis, hepatic adenomas, myocardial infarction, and carbohydrate intolerance are some of the more serious potential complications of exogenous estrogen use. These disorders are exceedingly rare in adolescents. Even though teenage smokers who use OCs have a relative risk of more than 2.0 for myocardial infarction, the likelihood of its occurrence is very small, and thus clinically insignificant, compared to the risk of dying from pregnancy-related complications. Some long-range beneficial effects of estrogen use include decreased risks of benign breast disease, ovarian disease, and anemia.
The short-term adverse effects of OCs, such as nausea and weight gain, often interfere with compliance in adolescent patients. These effects are usually transient and may be overshadowed by the beneficial effects of a shortened menses and the relief of dysmenorrhea. The inhibition of ovulation or the suppressant effect of estrogens on prostaglandin production by the endometrium makes OCs effective in preventing dysmenorrhea (Chapter 110). An initial thought for younger adolescents regarding the potentially unknown effect of estrogens on epiphyseal growth is no longer a concern. Acne may be worsened by some and improved by other OC preparations. The pills with nonandrogenic progestins are particularly effective in reducing acne and hirsutism. Drospirenone, a progestin with antimineralocorticoid activity, has been shown to reduce premenstrual symptomatology, but the potential for hyperkalemia as a side effect eliminates patients with renal, liver, or adrenal diseases and patients on certain medications. A beneficial cardiovascular effect occurs for adolescents taking estrogen-containing OCs; these young women have higher levels of cardioprotective high-density lipoproteins than controls. Although women <35 yr old who smoke are at less risk of cardiovascular complications, adolescents on OCs should be encouraged to stop smoking.
Extended cycling of OCs for adolescents has some anticipated benefits with increased ovarian activity suppression and improved contraceptive efficacy during treatment with drugs that reduce OC efficacy. Seasonale (0.15 mg levonorgestrel/30 µg ethinyl estradiol) was approved by the U.S. Food and Drug Administration (FDA) in September 2003 for extended cycling with 84 active pills and 7 placebo pills, resulting in a cycle of 91 days. The most common side effect is intermenstrual bleeding and/or spotting with the total days of bleeding over the 1st year of treatment being similar for Seasonale subjects and subjects on a 28-day cycle. The unscheduled bleeding pattern diminishes over time. Other advantages include diminished frequency of hormonal withdrawal (premenstrual) effects including headaches and migraines, mood changes, and heavy monthly bleeding.
The first extended-cycle oral contraceptive that supplies continuously throughout the year, Lybrel (90 µg of levonorgestrel and 20 µg of ethinyl estradiol) causes cessation of menstruation for an entire year.
Contraindications to the use of estrogen-containing OCs include hepatocellular disease, migraine headaches, breast disease, any condition in which hypercoagulability may be a problem (replaced cardiac valve, thrombophlebitis, sickle cell anemia) because of the increased levels of factor VIII and decreased production of antithrombin III, and known or suspected pregnancy (Table 111-3). The risks of pregnancy must be balanced against the benefits of reliable contraception in patients with chronic diseases such as diabetes, epilepsy, and sickle cell disease. The initial history taken before prescribing OCs should specifically address these risks. The World Health Organization ranks multiple medical eligibility criteria for safety with the use of hormonal contraception from 4, precluding use, to 1, conditions raising no concerns, and provides a thorough listing for reference purposes.
Table 111-3 CONTRAINDICATIONS TO COMBINED HORMONAL CONTRACEPTIVES
ABSOLUTE CONTRAINDICATIONS (CLASS 4 IN THE WHO CLASSIFICATION)
RELATIVE CONTRAINDICATIONS (CLASS 2 OR 3 IN THE WHO CLASSIFICATION)
From Amy JJ, Tripathi V: Contraception for women: an evidence based overview, BMJ 339:563–568, 2009.
Missed Contraceptive Pills
The effectiveness of OC is dependent on compliance, and unfortunately adolescent women may forget to take a pill each day. A pill is considered missed if it is 12 hr late from the designated daily time. If 3 pills are missed, back up contraception is required and if intercourse has occurred, emergency contraception (EC) is indicated (Fig. 111-3). Rules for missed pills are noted in Table 111-4.
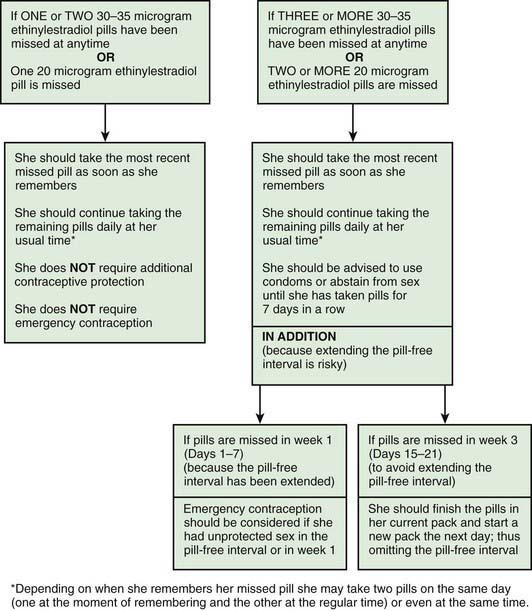
Figure 111-3 Advice for women missing combined oral contraceptives (30-35 µg and 20 µg ethinyl estradiol formulations).
(From Faculty of Family Planning and Reproductive Health Care Effectiveness Unit: FFPRHC Guidance [July 2006]. First prescription of combined oral contraception. The full statement is available at www.ffprhc.org.uk.)
From Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit: Missed pills: new recommendations, April 2005. www.ffprhc.org.uk/admin/uploads/MissedPillRules%20.pdf. Accessed April 23, 2010.
Other Combination Methods
The transdermal patch (Ortho Evra) releases 20 µg ethinyl estradiol and 150 µg norelgestromin daily and is applied to the lower abdomen, buttocks, or upper body. It is worn continuously for 1 wk and changed weekly for a total of 3 wk, then removed to allow menstrual bleeding (see Table 111-2). It should not be applied to the breast. Limited studies in adolescents suggest higher rates of partial or full detachment compared to adults, with high patient satisfaction and 50-83% continuation rates from 3-18 mo of use.
The vaginal contraceptive ring (NuvaRing) is a flexible, transparent, colorless vaginal ring that measures about 2.1 inches in diameter and is inserted into the vagina by the patient. It releases 15 µg ethinyl estradiol and 120 µg etonogestrel per day and remains in place for 3 wk, during which time these hormones are absorbed. If the ring is accidentally expelled, it should be reinserted; however, if it is out of place for more than 3 hr, a back-up method of contraception should be used.
All these methods have contraindications similar to those to oral contraceptives (see Table 111-3).
All-Progestin Contraceptives
Progestin-only oral contraceptives are available for the adolescent in whom the use of estrogen is potentially deleterious: those with liver disease, replaced cardiac valves, or hypercoagulable states. These agents (“mini-pills”) are less reliable in inhibiting ovulation and are associated with a 0.5%/yr pregnancy rate (perfect use). Acceptance by adolescents is limited by the necessity of taking the pill daily, the higher incidence of amenorrhea, and increased breakthrough bleeding.
An injectable progestin, medroxyprogesterone acetate (Depo-Provera, DMPA), is highly effective as birth control in a dose of 150 mg as a deep intramuscular injection, with failure rates typically at 0.3-0.4% (see Table 111-2). DMPA is particularly attractive for adolescents who have difficulty with compliance, are intellectually impaired, and are chronically ill or have a relative contraindication to estrogen use. Although concern has been directed toward the potential for loss in bone mineral density in adolescents, thereby potentially increasing their risk for osteoporosis later in life, recent studies have found that bone density is recovered after discontinuation of the method. Health care providers may want to consider a contraceptive containing estrogen in teens who are already at high risk for low bone density, such as those who have chronic renal disease, who are wheelchair bound, or who have eating disorders or chronic amenorrhea (Chapter 698).
The long-acting progestational agent levonorgestrel (Norplant) is not available in the USA. A 3-yr implant with a single rod containing etonogestrel (Implanon) releasing 60 µg/day received FDA approval in the USA in 2006. Like the progestin-only injectable contraceptive, the implant conveys a high degree of contraceptive efficacy and its main mechanism of action is suppression of ovulation. Also like the injectable contraceptive, the implant does not require daily or even weekly compliance. One potential unique complication of this method relates to infection and other serious side effects after implantation; however, these events are rare, occurring in less than 1% of patients. Minor side effects, such as bruising or skin irritation are more common but tend to resolve without treatment. Implant removal, through a minor surgical procedure, occurs at the end of 3 years.
American Society for Reproductive Medicine Practice Committee. Hormonal contraception: recent advances and controversies. Fertil Steril. 2008;90:S103-S113.
Cromer BA, Scholes D, Berenson A, et al. Depot medroxyprogesterone acetate and bone mineral density in adolescents—the Black Box Warning: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 2006;39:296-301.
Dunn N. Oral contraceptives and venous thromboembolism. BMJ. 2009;339:521-524.
FDA Talk Paper. Black box warning added concerning long-term use of Depo-Provera contraceptive injection. November 17, 2004 www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html
Gupta N, Corrado S, Goldstein M. Hormonal contraception in the adolescent. Pediatr Rev. 2008;29:386-396.
Mansour D, Fraser IS. Missed contraceptive pills and the critical pill-free interval. Lancet. 2005;365:1670-1671.
Peterson HB, Curtis KM. Long-acting methods of contraception. N Engl J Med. 2005;353:2169-2175.
Practice Committee of the American Society for Reproductive Medicine. Hormonal contraception: recent advances and controversies. Fertil Steril. 2008;90:S103-S113.
Zhang L, Chen J, Wang Y, et al. Pregnancy outcome after levonorgestrel-only emergency contraception failure: a prospective cohort study. Hum Reprod. 2009;24:1605-1611.
111.5 Emergency Contraception
Unprotected intercourse at mid-cycle carries a pregnancy risk of 20-30%. At other times during the cycle, the risk is 2-4%. The risk may be reduced or eliminated by intervention as soon as possible after unprotected intercourse with a “window” up to 120 hr. Indications for use of emergency contraception are listed in Table 111-5. In 2006, the FDA approved the emergency contraceptive drug Plan B as an over-the-counter option for women aged 18 yr and older. Experience in adolescent women demonstrates more effective use of EC with advance provision and is not associated with more frequent unprotected intercourse or less condom or pill use.
Table 111-5 POTENTIAL INDICATIONS FOR USE OF EMERGENCY CONTRACEPTION
* The usual interval for use of depot medroxyprogesterone acetate as contraception is every 12 wk.
Adapted from Allen RH, Goldberg AB: Emergency contraception: a clinical review, Clin Obstet Gynecol 50:927–936, 2007.
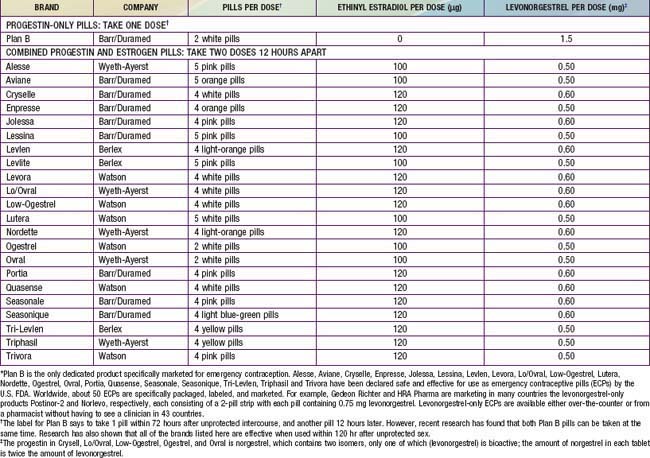
The Yuzpe method is commonly used in the USA, consisting of combination pills totaling 200 µg ethinyl estradiol and 2.0 mg norgestrel or 1.0 mg levonorgestrel. Pills that can be utilized for this method are shown in Table 111-6. The high-dose combination OCs disrupt the luteal phase hormone pattern, creating an unstable and unsuitable uterine lining for implantation. If used mid-cycle, when ovulation is about to occur, the high-dose estrogen and progestin blunt the luteinizing hormone surge and impair ovulation. This method is effective in reducing the risk of pregnancy by 75%. The most common side effects are nausea (50%) and vomiting (20%), prompting some clinicians to prescribe or recommend antiemetics along with the OCs. A urine pregnancy test is usually required prior to dispensing the pills to rule out an existing pregnancy. There is some controversy about the need to do this, since there is no evidence to suggest that OCs used in this manner affect early fetal development and the dose as prescribed would not disrupt a previously undetected pregnancy. The EC kit prepackaged for this method (Preven) was withdrawn from the market in 2004. A progestin-only EC kit was FDA approved in 1999 and contains 2 tablets, each with 0.75 mg levonorgestrel. Nausea and vomiting are uncommon side effects, and in a recent comparison, levonorgestrel proved more effective at preventing pregnancy than the Yuzpe method.
Table 111-6 TWENTY-THREE ORAL CONTRACEPTIVES THAT CAN BE USED FOR EMERGENCY CONTRACEPTION IN THE UNITED STATES*
Mifepristone (RU-486) is an antiprogestin agent that blocks the binding of progestin to its receptor. It prevents ovulation or interferes with the luteal phase of the menstrual cycle and is as effective as Plan B for emergency contraception.
Teens can access EC information through a hotline at 1-888-NOT-2-LATE. A 2-wk follow-up appointment is recommended following any of the methods to determine the effectiveness of treatment and to diagnose a possible early pregnancy. The visit also provides an opportunity to counsel the adolescent, explore the situation leading up to the unprotected intercourse, test for STDs, and initiate continuing contraception when appropriate. Pap smear screening is not initiated until 21 yr of age.
Allen RH, Goldberg AB. Emergency contraception: a clinical review. Clin Obstet Gynecol. 2007;50:927-936.
American Academy of Pediatrics Committee on Adolescence. Emergency contraception. Pediatrics. 2005;116:1026-1035.
American Academy of Pediatrics Committee on Adolescence. Emergency contraception. Pediatrics. 2005;116:1038-1047.
American College of Obstetricians and Gynecologists. Clinical management guidelines—emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
Prine L. Emergency contraception, myths and facts. Obstet Gynecol Clin North Am. 2007;34:127-136.
111.6 Intrauterine Devices
Intrauterine devices (IUDs) are small, flexible, plastic objects introduced into the uterine cavity through the cervix. They differ in size, shape, and the presence or absence of pharmacologically active substances (copper or progesterone). The mechanism of action of the TCu380A IUD is uncertain, although they render the endometrium unsuitable for implantation by inducing a local polymorphonuclear leukocyte response and production of prostaglandin. The levonorgestrel IUD may also have various contraceptive actions, from thickening of cervical mucus and inhibiting sperm survival to suppressing the endometrium. Both types of IUDs are effective in preventing pregnancy in 97-99% of women. Although early studies suggested an increased risk for upper genital tract infection due theoretically to the presence of a foreign body in the cervix, more recent work has refuted these earlier concerns. Because of this clinicians have been encouraged to reconsider use of IUDs in adolescents despite relatively high prevalence rates of STIs.
American Academy of Pediatrics Committee on Adolescence. Contraception and adolescents. Pediatrics. 2007;120:1135-1148.
Amy JJ, Tripathi V. Contraception for women: an evidence based overview. BMJ. 2009;339:563-568.
Benagiano G, von Hertzen H. Towards more effective emergency contraception? Lancet. 2010;375:527-528.
2011 Centers for Disease Control and Prevention. Contraceptive methods available to patients of office-based physicians and title X clinics—United States, 2009–2010. MMWR. 2011;60(1):104.
Centers for Disease Control and Prevention. U.S medical eligibility criteria for contraceptive use, 2010. MMWR. 59(No. RR-4), 2010.
Centers for Disease Control and Prevention. Sexual and reproductive health of persons aged 10-24 years—United States, 2002-2007. MMWR Morb Mortal Wkly Rep. 2009;58:1-60.
Centers for Disease Control and Prevention. Trends in HIV- and STD-related risk behaviors among high school students—United States, 1991–2007. MMWR Morb Mortal Wkly Rep. 2008;57:817-821.
Darroch JE, Singh SS, Frost JJ, et al. Differences in teenage pregnancy rates among five developed countries: the roles of sexual activity and contraceptive use. Fam Plann Perspect. 2001;33:244-250.
De Irala J, Alonso A. Changes in sexual behaviours to prevent HIV. Lancet. 2006;368:1749-1750.
Duffy K, Wimberly Y, Brooks C. Adolescent contraceptive care for the practicing pediatrician. Adolesc Med State Art Rev. 2009;20:168-187.
Durex Network. The face of global sex 2007. First sex: opportunity of a lifetime. Cambridge, UK Accessed April 23, 2010 www.durexnetwork.org/en-GB/research/faceofglobalsex/Pages/Home.aspx, 2007. SSL International
Grossman D, Holt K, Pena M, et al. Self-induction of abortion among women in the United States. Reprod Health Matters. 2010;18(36):136-146.
Kirby D. Reducing pregnancy and risky behaviour in teenagers. BMJ. 2009;339:116-117.
Manlove J, Ikramullah E, Mincieli L, et al. Trends in sexual experience, contraceptive use, and teenage childbearing: 1992–2002. J Adolesc Health. 2009;44:413-423.
2011 The Medical Letter. Ella: a new emergency contraceptive. Med Lett. 2011;53(1355):3-4.
Monasterio E, Hwang LY, Shafer MA. Adolescent sexual health. Curr Probl Pediatr Adolesc Health Care. 2007;37:302-325.
Paranjothy S, Broughton H, Adappa R, et al. Teenage pregnancy: who suffers? Arch Dis Child. 2009;94:239-245.
Raymond EG, Halpern V, Lopez LM. Pericoital oral contraception with levonorgestrel. Obstet Gynecol. 2011;117(3):673-681.
Santelli JS, Lindberg LD, Finer LB, et al. Explaining recent declines in adolescent pregnancy in the United States: the contribution of abstinence and improved contraceptive use. Am J Public Health. 2007;97:1541-1548.
Shelton JD. Why multiple sexual partners? Lancet. 2009;374:367-368.
Stammers T. Sexual health in adolescents. BMJ. 2007;334:103-104.
Tripp J, Viner R. Sexual health, contraception, and teenage pregnancy. Br Med J. 2005;330:590-593.
World Health Organization. Medical eligibility criteria for contraceptive use. ed 3 www.who.int/reproductive-health/publications/MEC_3/, 2004. Accessed April 23, 2005