Chapter 176 Group A Streptococcus
Group A streptococcus (GAS), also known as Streptococcus pyogenes, is a common cause of infections of the upper respiratory tract (pharyngitis) and the skin (impetigo, pyoderma) in children and is a less common cause of perianal cellulitis, vaginitis, septicemia, pneumonia, endocarditis, pericarditis, osteomyelitis, suppurative arthritis, myositis, cellulitis, and omphalitis. These microorganisms also cause distinct clinical entities (scarlet fever and erysipelas), as well as a toxic shock syndrome and necrotizing fasciitis. GAS is also the cause of 2 potentially serious nonsuppurative complications: rheumatic fever (Chapters 176.1 and 432) and acute glomerulonephritis (Chapter 505.1).
Etiology
Group A streptococci are gram-positive coccoid-shaped bacteria that tend to grow in chains. They are broadly classified by their reactions on mammalian red blood cells. The zone of complete hemolysis that surrounds colonies grown on blood agar distinguishes β-hemolytic (complete hemolysis) from α-hemolytic (green or partial hemolysis) and γ (nonhemolytic) species. The β-hemolytic streptococci can be divided into groups by a group-specific polysaccharide (Lancefield carbohydrate C) located in the cell wall. More than 20 serologic groups are identified, designated by the letters A through V. Serologic grouping by the Lancefield method is precise, but group A organisms can be identified more readily by any one of a number of latex agglutination, coagglutination, or enzyme immunoassay procedures. Group A strains can also be distinguished from other groups by differences in sensitivity to bacitracin. A disk containing 0.04 U of bacitracin inhibits the growth of most group A strains, whereas other groups are generally resistant to this antibiotic. GAS can be subdivided into >100 serotypes on the basis of the M protein antigen, which is located on the cell surface and in fimbriae that project from the outer edge of the cell. M typing has traditionally relied primarily on the serologic typing of the surface M protein using available polyclonal sera. However, it is frequently difficult to detect M proteins in this way; a molecular approach to M typing GAS isolates using the polymerase chain reaction is based on sequencing the emm gene of GAS that encodes the M protein. More than 180 distinct M types have been identified using emm typing, and there has been a good correlation between the known serotypes and the emm types.
M serotyping has been valuable for epidemiologic studies; particular GAS diseases tend to be associated with certain M types. Types 1, 12, 28, 4, 3, and 2 (in that order) are the most common causes of uncomplicated streptococcal pharyngitis in the USA. The M types commonly associated with pharyngitis rarely cause skin infections, and the M types commonly associated with skin infections rarely cause pharyngitis. A few of the pharyngeal strains (M type 12) have been associated with glomerulonephritis, but far more of the skin strains (M types 49, 55, 57, and 60) have been considered nephritogenic. A few of the pharyngeal serotypes, but none of the skin strains, have been associated with acute rheumatic fever. Rheumatogenic potential is not solely dependent on the serotype but is a characteristic of specific strains within several serotypes.
Epidemiology
Humans are the natural reservoir for GAS. These bacteria are highly communicable and can cause disease in normal individuals of all ages who do not have type-specific immunity against the particular serotype involved. Disease in neonates is uncommon, probably because of maternally acquired antibody. The incidence of pharyngeal infections is highest in children 5-15 yr of age, especially in young school-aged children. These infections are most common in the northern regions of the USA, especially during winter and early spring. Children with untreated acute pharyngitis spread GAS by airborne salivary droplets and nasal discharge. Transmission is favored by close proximity; therefore, schools, military barracks, and homes are important environments for spread. The incubation period for pharyngitis is usually 2-5 days. GAS has the potential to be an important upper respiratory tract pathogen and to produce outbreaks of disease in the daycare setting. Foods containing GAS occasionally cause explosive outbreaks of pharyngotonsillitis. Children are usually not infectious 24 hr after appropriate antibiotic therapy has been started. Chronic pharyngeal carriers of GAS rarely transmit this organism to others.
Streptococcal pyoderma (impetigo, pyoderma) occurs most frequently during the summer in temperate climates, or year round in warmer climates, when the skin is exposed and abrasions and insect bites are more likely to occur (Chapter 657). Colonization of healthy skin by GAS usually precedes the development of impetigo. Because GAS cannot penetrate intact skin, impetigo usually occurs at the site of open lesions (insect bites, traumatic wounds, burns). Although impetigo serotypes may colonize the throat, spread is usually from skin to skin, not via the respiratory tract. Fingernails and the perianal region can harbor GAS and play a role in disseminating impetigo. Multiple cases of impetigo in the same family are common. Both impetigo and pharyngitis are more likely to occur among children living in crowded homes and in poor hygienic circumstances.
The incidence of severe invasive GAS infections, including bacteremia, streptococcal toxic shock syndrome, and necrotizing fasciitis, has increased in recent years. The incidence appears to be highest in the very young and in older persons. Prior to the routine use of varicella vaccine, varicella was the most commonly identified risk factor in children. Other risk factors include diabetes mellitus, HIV infection, intravenous drug use, and chronic pulmonary or chronic cardiac disease. The portal of entry is unknown in almost 50% of the cases of severe invasive GAS infection; in most cases, it is believed to be skin or mucous membrane. Severe invasive disease rarely follows GAS pharyngitis.
Pathogenesis
Virulence of GAS depends primarily on the M protein, and strains rich in M protein resist phagocytosis in fresh human blood, whereas M-negative strains do not. GAS isolated from chronic pharyngeal carriers contains little or no M protein and are relatively avirulent. The M protein antigen stimulates the production of protective antibodies. These antibodies are type specific. They protect against infection with a homologous M type but confer no immunity against other M types. Therefore, multiple GAS infections attributable to different M types are common during childhood and adolescence. By adult life, individuals are probably immune to many of the common M types in the environment, but because of the large number of serotypes it is doubtful that total immunity is ever achieved.
GAS produces a large variety of enzymes and toxins, including erythrogenic toxins (known as streptococcal pyrogenic exotoxins). Streptococcal pyrogenic exotoxins A, B, and C are responsible for the rash of scarlet fever and are elaborated by streptococci that are infected with a particular bacteriophage. These exotoxins stimulate the formation of specific antitoxin antibodies that provide immunity against the scarlatiniform rash but not against other streptococcal infections. Because GAS can produce 3 different rash-producing pyrogenic exotoxins (A, B, or C), a 2nd attack of scarlet fever may sometimes occur. Streptococcal pyrogenic exotoxins A, B, and C, as well as several newly discovered exotoxins, appear to be involved in the pathogenesis of invasive GAS disease, including the streptococcal toxic shock syndrome.
The roles of most of the other streptococcal toxins and enzymes in human disease have yet to be established. Many of these extracellular substances are antigenic and stimulate antibody production after an infection. However, these antibodies bear no relationship to immunity. Their measurement is useful for evidence of a recent streptococcal infection. The test for antibodies against streptolysin O (antistreptolysin O) is the most commonly used antibody determination. Because the immune response to extracellular antigens varies among individuals as well as with the site of infection, it is sometimes necessary to measure other streptococcal antibodies, such as anti-deoxyribonuclease (anti-DNase).
Clinical Manifestations
The most common infections caused by GAS involve the respiratory tract and the skin and soft tissues.
Respiratory Tract Infections
GAS is an important cause of acute pharyngitis (Chapter 373) and pneumonia (Chapter 392).
Scarlet Fever
Scarlet fever is an upper respiratory tract infection associated with a characteristic rash, which is caused by an infection with pyrogenic exotoxin (erythrogenic toxin)-producing GAS in individuals who do not have antitoxin antibodies. It is now encountered less commonly and is less virulent than in the past, but the incidence is cyclic, depending on the prevalence of toxin-producing strains and the immune status of the population. The modes of transmission, age distribution, and other epidemiologic features are otherwise similar to those for GAS pharyngitis.
The rash appears within 24-48 hours after onset of symptoms, although it may appear with the first signs of illness (Fig. 176-1A). It often begins around the neck and spreads over the trunk and extremities. It is a diffuse, finely papular, erythematous eruption producing a bright red discoloration of the skin, which blanches on pressure. It is often more intense along the creases of the elbows, axillae, and groin. The skin has a goose-pimple appearance and feels rough. The face is usually spared, although the cheeks may be erythematous with pallor around the mouth. After 3-4 days, the rash begins to fade and is followed by desquamation, 1st on the face, progressing downward, and often resembling a mild sunburn. Occasionally, sheetlike desquamation may occur around the free margins of the fingernails, the palms, and the soles. Examination of the pharynx of a patient with scarlet fever reveals essentially the same findings as with GAS pharyngitis. In addition, the tongue is usually coated and the papillae are swollen (Fig. 176-1B). After desquamation, the reddened papillae are prominent, giving the tongue a strawberry appearance (Fig. 176-1C).
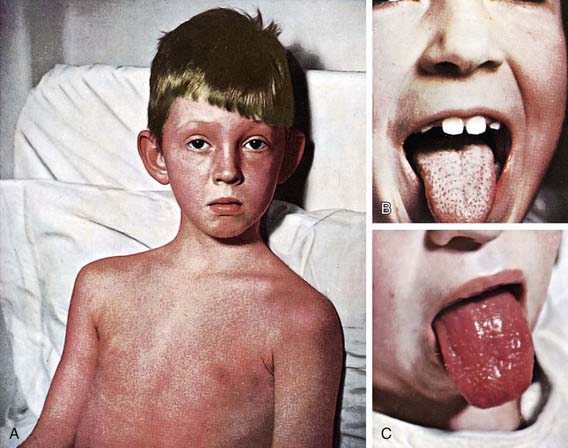
Figure 176-1 Scarlet fever. A, Punctate, erythematous rash (2nd day). B, White strawberry tongue (1st day). C, Red strawberry tongue (3rd day).
(Courtesy Dr. Franklin H. Top, Professor and Head of the Department of Hygiene and Preventive Medicine, State University of Iowa, College of Medicine, Iowa City, IA; and Parke, Davis & Company’s Therapeutic Notes. From Gershon AA, Hotez PJ, Katz SL: Krugman’s infectious diseases of children, ed 11, Philadelphia, 2004, Mosby, plate 53.)
Typical scarlet fever is not difficult to diagnose; the milder form with equivocal pharyngeal findings can be confused with viral exanthems, Kawasaki disease, and drug eruptions. Staphylococcal infections are occasionally associated with a scarlatiniform rash. A history of recent exposure to a GAS infection is helpful. Identification of GAS in the pharynx confirms the diagnosis, if uncertain.
Impetigo
Impetigo (or pyoderma) has traditionally been classified into 2 clinical forms: bullous and nonbullous (Chapter 657). Nonbullous impetigo is the more common form and is a superficial infection of the skin that appears first as a discrete papulovesicular lesion surrounded by a localized area of redness. The vesicles rapidly become purulent and covered with a thick, confluent, amber-colored crust that gives the appearance of having been stuck on the skin. The lesions may occur anywhere but are more common on the face and extremities. If untreated, nonbullous impetigo is a mild but chronic illness, often spreading to other parts of the body, but occasionally is self-limited. Regional lymphadenitis is common. Nonbullous impetigo is generally not accompanied by fever or other systemic signs or symptoms. Impetiginized excoriations around the nares are seen with active GAS infections of the nasopharynx particularly in young children. However, impetigo is not usually associated with an overt streptococcal infection of the upper respiratory tract.
Bullous impetigo is less common and occurs most often in neonates and young infants. It is characterized by flaccid, transparent bullae usually <3 cm in diameter on previously untraumatized skin. The usual distribution involves the face, buttocks, trunk, and perineum. Although Staphylococcus aureus has traditionally been accepted as the sole pathogen responsible for bullous impetigo, there has been confusion about the organisms responsible for nonbullous impetigo. In most episodes of nonbullous impetigo, either GAS or S. aureus, or a combination of these 2 organisms, is isolated. Earlier investigations suggested that GAS was the causative agent in most cases of nonbullous impetigo and that S. aureus was only a secondary invader. However, studies have demonstrated the recent emergence of S. aureus as the causative agent in most cases of nonbullous impetigo. Culture of the lesions is the only way to distinguish nonbullous impetigo caused by S. aureus from that caused by GAS.
Erysipelas
Erysipelas is a relatively rare acute GAS infection involving the deeper layers of the skin and the underlying connective tissue. The skin in the affected area is swollen, red, and very tender. Superficial blebs may be present. The most characteristic finding is the sharply defined, slightly elevated border. At times, reddish streaks of lymphangitis project out from the margins of the lesion. The onset is abrupt, and signs and symptoms of a systemic infection, such as high fever, are often present. Cultures obtained by needle aspirate of the inflamed area often reveal the causative agent.
Perianal Dermatitis
Perianal dermatitis, also called perianal streptococcal disease, is a distinct clinical entity characterized by well-demarcated, perianal erythema associated with anal pruritus, painful defecation, and blood-streaked stools. Physical examination reveals flat, pink to beefy-red perianal erythema with sharp margins extending as far as 2 cm from the anus. Erythema may involve the vulva and vagina. Lesions may be very tender and, particularly when chronic, may fissure and bleed. Systemic symptoms and fever are unusual.
Vaginitis
GAS is a common cause of vaginitis in prepubertal girls (Chapter 543). Patients usually have a serous discharge with marked erythema and irritation of the vulvar area, accompanied by discomfort in walking and in urination.
Severe Invasive Disease
Invasive GAS infection is defined by isolation of GAS from a normally sterile body site and includes 3 overlapping clinical syndromes. The 1st is GAS toxic shock syndrome, which is differentiated from other types of invasive GAS infections by the presence of shock and multiorgan system failure early in the course of the infection (Table 176-1). The second is GAS necrotizing fasciitis characterized by extensive local necrosis of subcutaneous soft tissues and skin. The third is the group of focal and systemic infections that do not meet the criteria for toxic shock syndrome or necrotizing fasciitis and includes bacteremia with no identified focus, meningitis, pneumonia, peritonitis, puerperal sepsis, osteomyelitis, suppurative arthritis, myositis, and surgical wound infections.
Table 176-1 DEFINITION OF STREPTOCOCCAL TOXIC SHOCK SYNDROME
Clinical criteria
Hypotension plus 2 or more of the following:
Renal impairment
Coagulopathy
Hepatic involvement
Adult respiratory distress syndrome
Generalized erythematous macular rash
Soft tissue necrosis
Definite case
Clinical criteria plus group A streptococcus from a normally sterile site
Probable case
Clinical criteria plus group A streptococcus from a nonsterile site
The pathogenic mechanisms responsible for severe, invasive GAS infections, including streptococcal toxic shock syndrome and necrotizing fasciitis, have yet to be defined completely, but an association with streptococcal pyrogenic exotoxins has been suggested. The three original streptococcal pyrogenic exotoxins (A, B, C), the newly discovered streptococcal pyrogenic exotoxins, and potentially other, as yet unidentified toxins produced by GAS act as superantigens, which stimulate an intense activation and proliferation of T lymphocytes and macrophages resulting in the production of large quantities of cytokines. These cytokines are capable of producing shock and tissue injury, and are believed to be responsible for many of the clinical manifestations of severe, invasive GAS infections.
Diagnosis
When attempting to decide whether to perform a microbiologic test on a patient presenting with acute pharyngitis, consideration of the clinical and epidemiologic findings should take place before the test is performed. A history of close contact with a well-documented case of GAS pharyngitis is helpful, as is an awareness of a high prevalence of GAS infections in the community. The signs and symptoms of streptococcal and nonstreptococcal pharyngitis overlap too broadly to allow the requisite diagnostic precision on clinical grounds alone. The clinical diagnosis of GAS pharyngitis cannot be made with certainty even by the most experienced physicians, and bacteriologic confirmation is required.
Culture of a throat swab on a sheep blood agar plate remains the standard for the documentation of the presence of GAS in the upper respiratory tract and for the confirmation of the clinical diagnosis of acute GAS pharyngitis. If performed correctly, a single throat swab cultured on a blood-agar plate has a sensitivity of 90-95% for detecting the presence of GAS in the pharynx.
A disadvantage of culturing a throat swab on a blood-agar plate is the delay (overnight or longer) in obtaining the culture result. Rapid antigen detection tests have been developed for the identification of GAS directly from throat swabs. Although these rapid tests are more expensive than the blood-agar culture, the advantage they offer over the traditional procedure is the speed with which they can provide results. Rapid identification and treatment of patients with streptococcal pharyngitis can reduce the risk for the spread of GAS, allowing the patient to return to school or work sooner, and can reduce the acute morbidity of this illness.
The great majority of the rapid antigen detection tests that are currently available have an excellent specificity of >95% when compared with blood-agar plate cultures. False-positive test results are unusual, and, therefore, therapeutic decisions can be made on the basis of a positive test result with confidence. Unfortunately, the sensitivity of most of these tests is 80-90%, possibly lower, when compared with the blood-agar plate culture. Therefore, a negative test does not exclude the presence of GAS, and a confirmatory throat culture should be performed. Newer tests may be more sensitive than other rapid antigen detection tests and perhaps may even be as sensitive as blood-agar plate cultures. However, the definitive studies to determine whether some rapid antigen detection tests are significantly more sensitive than others, and, whether any of these tests are sensitive enough to be used routinely without throat culture confirmation of negative test results, have not been performed. Some experts believe that physicians who use a rapid antigen detection test without culture backup should compare the results with that specific test to those of throat cultures to confirm adequate sensitivity in their practice.
GAS infection can also be diagnosed retrospectively on the basis of an elevated or increasing streptococcal antibody titer. The antistreptolysin O assay is the streptococcal antibody test most commonly used. Because streptolysin O also is produced by group C and G streptococcus, the test is not specific for group A infection. The antistreptolysin O response can be feeble in patients with streptococcal impetigo, and its usefulness for this condition is limited. In contrast, the anti-DNase B responses are present after both skin and throat infections. A significant antibody increase is usually defined as an increase in titer of 2 or more dilution increments between the acute phase and convalescent phase specimens, regardless of the actual height of the antibody titer. Physicians frequently misinterpret streptococcal antibody titers because of a failure to appreciate that the normal levels of these antibodies are higher among school-aged children compared to adults. Both the traditional ASO and anti-DNase B tests are neutralization assays. Newer tests use latex agglutination or nephelometric assays. Unfortunately, these newer tests have not been well-standardized against the traditional neutralization assays. Physicians need to be aware of these potential problems when interpreting the results of streptococcal serologic testing performed on their patients.
A commercially available slide agglutination test for the detection of antibodies to several streptococcal antigens is the Streptozyme test (Wampole Laboratories, Stamford, CT). This test is less well standardized and less reproducible than other antibody tests, and it should not be used as a test for evidence of a preceding GAS infection.
Differential Diagnosis
Viruses are the most common cause of acute pharyngitis in children. Respiratory viruses such as influenza virus, parainfluenza virus, rhinovirus, coronavirus, adenovirus, and respiratory syncytial virus are frequent causes of acute pharyngitis. Other viral causes of acute pharyngitis include enteroviruses and herpes simplex virus (HSV). Epstein-Barr virus (EBV) is a frequent cause of acute pharyngitis that is often accompanied by other clinical findings of infectious mononucleosis (e.g., splenomegaly, generalized lymphadenopathy). Systemic infections with other viral agents including cytomegalovirus, rubella virus, measles virus, and HIV may be associated with acute pharyngitis.
GAS is the most common cause of bacterial pharyngitis, accounting for 15-30% of the cases of acute pharyngitis in children. Groups C and G β-hemolytic streptococcus (Chapter 178) also produce acute pharyngitis in children. Arcanobacterium haemolyticum and Fusobacterium necrophorum are additional less common causes. Neisseria gonorrhoeae can occasionally cause acute pharyngitis in sexually active adolescents. Other bacteria such as Francisella tularensis and Yersinia enterocolitica as well as mixed infections with anaerobic bacteria (Vincent angina) are rare causes of acute pharyngitis. Chlamydia pneumoniae and Mycoplasma pneumoniae have been implicated as causes of acute pharyngitis, particularly in adults. Corynebacterium diphtheriae (Chapter 180) can cause pharyngitis but is rare because of universal immunization. Although other bacteria such as Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae are frequently cultured from the throats of children with acute pharyngitis, their etiologic role in pharyngitis has not been established.
GAS pharyngitis is the only common cause of acute pharyngitis for which antibiotic therapy is definitely indicated. Therefore, when confronted with a patient with acute pharyngitis, the clinical decision that usually needs to be made is whether the pharyngitis is attributable to GAS.
Treatment
Antibiotic therapy for patients with GAS pharyngitis can prevent acute rheumatic fever, shorten the clinical course of the illness, reduce transmission of the infection to others, and prevent suppurative complications. For the patient with classic scarlet fever, antibiotic therapy should be started immediately, but for the vast majority of patients who present with much less distinctive findings, treatment should be withheld until there is some form of bacteriologic confirmation either by throat culture or rapid antigen detection test. Rapid antigen detection tests, because of their high degree of specificity, have made it possible to initiate antibiotic therapy immediately for someone with a positive test result.
GAS is exquisitely sensitive to penicillin, and resistant strains have never been encountered. Penicillin is, therefore, the drug of choice (except in patients who are allergic to penicillin) for pharyngeal infections as well as for suppurative complications. Treatment with oral penicillin V (250 mg/dose bid-tid for ≤60 lb and 500 mg/dose bid-tid for >60 lb PO) is recommended but it must be taken for a full 10 days even though there is symptomatic improvement in 3-4 days. Penicillin V (phenoxyethylpenicillin) is preferred over penicillin G because it may be given without regard to mealtime. The major problem with all forms of oral therapy is the risk that the drug will be discontinued before the 10-day course has been completed. Therefore, when oral treatment is prescribed, the necessity of completing a full course of therapy must be emphasized. If the parents seem unlikely to comply with oral therapy because of family disorganization, difficulties in comprehension, or other reasons, parenteral therapy with a single intramuscular injection of benzathine penicillin G (600,000 IU for ≤60 lb, 1.2 million IU for >60 lb, IM) is the most efficacious and often the most practical method of treatment. Disadvantages include soreness around the site of injection, which may last for several days, and potential for injection into nerves or blood vessels if not administered correctly. The local reaction is diminished when benzathine penicillin G is combined in a single injection with procaine penicillin G, although precautions are necessary to ensure that an adequate amount of benzathine penicillin G is administered.
In several comparative clinical trials, once daily amoxicillin (50 mg/kg, maximum 1,000 mg) for 10 days has been shown to be effective in treating GAS pharyngitis. This somewhat broader-spectrum agent has the advantage of once-daily dosing, which may enhance adherence. In addition, amoxicillin is relatively inexpensive and is considerably more palatable than penicillin V suspension.
A 10-day course of a narrow spectrum oral cephalosporin is recommended for most penicillin-allergic individuals. Several reports indicate that a 10-day course with an oral cephalosporin is superior to 10 days of oral penicillin in eradicating GAS from the pharynx. Analysis of these data suggests that the difference in eradication is due mainly to a higher rate of eradication of carriers included unintentionally in these clinical trials. Some penicillin-allergic persons (up to 10%) are also allergic to cephalosporins, and these agents should not be used in patients with immediate (anaphylactic-type) hypersensitivity to penicillin. Most oral broad-spectrum cephalosporins are considerably more expensive than penicillin or amoxicillin, and the former agents are more likely to select for antibiotic-resistant flora.
Oral clindamycin is an appropriate agent for treating penicillin-allergic patients and resistance to clindamycin among GAS isolates in the USA is currently only about 1%. An oral macrolide (erythromycin or clarithromycin) or azalide (azithromycin) is also an appropriate agent for patients allergic to penicillins. Ten days of therapy is indicated except for azithromycin, which is given for 5 days. Erythromycin is associated with substantially higher rates of gastrointestinal side effects than the other agents. In recent years, macrolide resistance rates among pharyngeal isolates of GAS in most areas of the USA have been around 5-8%. Sulfonamides and the tetracyclines are not indicated for treatment of GAS infections.
Most oral antibiotics must be administered for the conventional 10 days to achieve maximal pharyngeal eradication rates of GAS, but certain newer agents have been reported to achieve comparable bacteriologic and clinical cure rates when given for 5 days or less. However, definitive results from comprehensive studies are not available to allow final evaluation of these proposed shorter courses of oral antibiotic therapy. Therefore, they cannot be recommended at this time. In addition, these antibiotics have a much broader spectrum than penicillin and are generally more expensive, even when administered for short courses.
The majority of patients with GAS pharyngitis respond clinically to antimicrobial therapy, and GAS is eradicated from the pharynx. Post-treatment throat cultures are indicated only in the relatively few patients who remain symptomatic, whose symptoms recur, or who have had rheumatic fever and are, therefore, at unusually high risk for recurrence.
Antibiotic therapy for a patient with nonbullous impetigo can prevent local extension of the lesions, spread to distant infectious foci, and transmission of the infection to others. However, the ability of antibiotic therapy to prevent poststreptococcal glomerulonephritis has not been demonstrated. Patients with a few superficial, isolated lesions and no systemic signs can be treated with topical antibiotics. Mupirocin is a safe and effective agent that has become the topical treatment of choice. If there are widespread lesions or systemic signs, oral therapy with coverage for both GAS and S. aureus is needed. With the rapid emergence of methicillin-resistant S. aureus in many communities, consideration should be given to using clindamycin alone or a combination of trimethoprim-sulfamethoxazole and amoxicillin as first-line therapy. Oral cefuroxime is an effective treatment of perianal streptococcal disease.
Theoretical considerations and experimental data suggest that intravenous clindamycin is a more effective agent for the treatment of severe, invasive GAS infections than intravenous penicillin. However, because a small proportion of the GAS isolates in the USA are resistant to clindamycin, clindamycin should be used in combination with penicillin for these infections until susceptibility to clindamycin has been established. If necrotizing fasciitis is suspected, immediate surgical exploration or biopsy is required to identify a deep soft tissue infection that should be debrided immediately. Patients with streptococcal toxic shock syndrome require rapid and aggressive fluid replacement, management of respiratory or cardiac failure, if present, and anticipatory management of multiorgan system failure. Limited data suggest that intravenous gamma globulin may be effective in the management of streptococcal toxic shock syndrome. Intravenous immunoglobulin should be reserved for those patients who do not respond to other therapeutic measures.
Complications
Suppurative complications from the spread of GAS to adjacent structures were common before antibiotics became available. Cervical lymphadenitis, peritonsillar abscess, retropharyngeal abscess, otitis media, mastoiditis, and sinusitis still occur in children in whom the primary illness has gone unnoticed or in whom treatment of the pharyngitis has been inadequate. GAS pneumonia can also occur.
Acute rheumatic fever (Chapter 176.1) and acute poststreptococcal glomerulonephritis (Chapter 505.1) are both nonsuppurative sequelae of infections with GAS that occur after an asymptomatic latent period. They are both characterized by lesions remote from the site of the GAS infection. Acute rheumatic fever and acute glomerulonephritis differ in their clinical manifestations, epidemiology, and potential morbidity. In addition, acute glomerulonephritis can occur after a GAS infection of either the upper respiratory tract or the skin, but acute rheumatic fever can occur only after an infection of the upper respiratory tract.
Poststreptococcal Reactive Arthritis
Poststreptococcal reactive arthritis has been used to describe a syndrome characterized by the onset of acute arthritis following an episode of GAS pharyngitis in a patient whose illness does not otherwise fulfill the Jones criteria for the diagnosis of acute rheumatic fever. There is still considerable debate about whether this entity represents a distinct syndrome or is a manifestation of acute rheumatic fever. Although poststreptococcal reactive arthritis usually involves the large joints, in contrast to the arthritis of acute rheumatic fever, it may involve small peripheral joints as well as the axial skeleton and is typically nonmigratory. The latent period between the antecedent episode of GAS pharyngitis and the poststreptococcal reactive arthritis may be considerably shorter (usually <10 days) than that typically seen with acute rheumatic fever. In contrast to the arthritis of acute rheumatic fever, poststreptococcal reactive arthritis does not respond dramatically to therapy with aspirin or other nonsteroidal antiinflammatory agents. In addition, the reactive arthritis is usually not migratory, and fewer patients have a fever >38°C. Even though no more than half of the patients with poststreptococcal reactive arthritis who have a throat culture performed have GAS isolated, all have serologic evidence of a recent GAS infection. Because a small proportion of patients with poststreptococcal reactive arthritis have been reported to subsequently develop valvular heart disease, these patients should be carefully observed for several months for clinical evidence of carditis. Some experts recommend that these patients receive secondary prophylaxis for up to 1 yr; however, the effectiveness of this approach is not well established. If clinical evidence of carditis is not observed, the prophylaxis can then be discontinued. If valvular disease is detected, the patient should be classified as having had acute rheumatic fever and should continue to receive secondary prophylaxis.
Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcus pyogenes (PANDAS)
PANDAS describes a group of neuropsychiatric disorders (particularly obsessive-compulsive disorders, tic disorders, and Tourette syndrome) for which a possible relationship with GAS infections has been suggested (Chapter 22). It has been demonstrated that patients with Sydenham chorea (a manifestation of acute rheumatic fever) frequently have obsessive-compulsive symptoms and that a subset of patients with obsessive-compulsive and tic disorders will have chorea as well as acute exacerbations following GAS infections. Therefore, it has been proposed that this subset of patients with obsessive-compulsive and tic disorders produce autoimmune antibodies in response to a GAS infection that cross react with brain tissue similar to the autoimmune response believed to be responsible for the manifestations of Sydenham chorea. It has also been suggested that secondary prophylaxis that prevents recurrences of Sydenham chorea might also be effective in preventing recurrences of obsessive-compulsive and tic disorders in these patients. Because of the proposed autoimmune mechanism, it has also been suggested that these patients may benefit from immunoregulatory therapy such as plasma exchange or intravenous immunoglobulin therapy. The possibility that PANDAS could represent an extension of the spectrum of acute rheumatic fever is intriguing, but it should be considered only as a yet-unproven hypothesis. Until carefully designed and well-controlled studies have established a causal relationship between PANDAS and GAS infections, routine laboratory testing for GAS to diagnose, long-term antistreptococcal prophylaxis to prevent, or immunoregulatory therapy (e.g., intravenous immunoglobulin, plasma exchange) to treat exacerbations of this disorder are not recommended (Chapter 22).
Prognosis
The prognosis for appropriately treated GAS pharyngitis is excellent, and complete recovery is the rule. When therapy is provided within 9 days of onset, acute rheumatic fever is prevented. There is no evidence that acute poststreptococcal glomerulonephritis can be prevented once pharyngitis or pyoderma with a nephritogenic strain of GAS has occurred. In rare instances, particularly in neonates or in children whose response to infection is compromised, fulminant pneumonia, septicemia, and death may occur despite usually adequate therapy.
Prevention
The only specific indication for long-term use of antibiotics to prevent GAS infections is for patients with a history of acute rheumatic fever or rheumatic heart disease. Mass prophylaxis is generally not feasible except to reduce the number of infections during epidemics of impetigo and to control epidemics of pharyngitis in military populations and in schools. Because the ability of antimicrobial agents to prevent GAS infections is limited, a streptococcal vaccine offers the possibility of a more effective approach.
Results of phase 1 and phase 2 studies in adults of a 26-valent plus streptococcal protective antigen (Spa) M protein-based recombinant vaccine demonstrated that the vaccine was well tolerated with no evidence of induction of human tissue-reactive antibodies. The vaccine was also immunogenic and produced opsonizing bactericidal antibodies. Published data suggest that immunization with this candidate vaccine could produce protection against approximately 85% of the isolates causing pharyngitis, 93% of the isolates associated with acute rheumatic fever, and 88% of the isolates associated with invasive GAS disease in the USA and could have significant impact on the overall burden of GAS disease. However, matching a multivalent M type specific vaccine to current circulating strains is likely to be less complete in Asia and other developing areas of the world. In addition, emergence of new emm types and the possibility that nonvaccine serotypes or genotypes of clinical importance may replace those contained in the vaccine is a concern. Other current candidate GAS vaccines such as a C5a peptidase, cysteine protease, fibronectin-binding protein, and group A carbohydrate vaccine are based on common protective antigens and would result in increased strain coverage and reduced concerns for serotype replacement.
Barash J, Mashiach E, Navon-Elkan P, et al. Differentiation of post-streptococcal reactive arthritis from acute rheumatic fever. J Pediatr. 2008;153:696-699.
Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240-245.
Chan KH, Kraai TL, Richter GT, et al. Toxic shock syndrome and rhinosinusitis in children. Arch Otolaryngol Head Neck Surg. 2009;135:538-542.
Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79:383-390.
Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53-63.
Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young. Circulation. 2009;119:1541-1551.
Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17:571-580.
Jeng A, Beheshti M, Li J, et al. The role of β-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;89(4):217-226.
Kaul R, McGeer A, Norby-Teglund A, et al. Intravenous immunoglobulin therapy in streptococcal toxic shock syndrome—a comparative observational study. Clin Infect Dis. 1999;28:800-807.
Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883-886.
Lennon DR, Farrell E, Martin DR, et al. Once-daily amoxicillin versus twice-daily penicillin V in group A β-haemolytic streptococcal pharyngitis. Arch Dis Child. 2008;93:474-478.
Malhotra-Kumar S, Lammens C, Coenen S, et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci on healthy volunteers: a randomized, double-blind, placebo-controlled study. Lancet. 2007;369:482-490.
The Medical Letter. Extended release amoxicillin for strep throat. Med Lett. 2009;51:17.
Navarini S, Erb T, Schaad UB, et al. Randomized, comparative efficacy trial of oral penicillin versus cefuroxime for perinanal streptococcal dermatitis in children. J Pediatr. 2008;153:799-802.
Shulman ST, Tanz RR, Dale JB, et al. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49:78-84.
Stevens DL. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med. 2000;51:271-288.
176.1 Rheumatic Fever
Etiology
There is considerable evidence to support the link between GAS upper pharyngitis tract infections and acute rheumatic fever and rheumatic heart disease. As many as 66% of the patients with an acute episode of rheumatic fever have a history of an upper respiratory tract infection several weeks before, and the peak age and seasonal incidence of acute rheumatic fever closely parallel those of GAS infections. Patients with acute rheumatic fever almost always have serologic evidence of a recent GAS infection. Their antibody titers are usually considerably higher than those seen in patients with GAS infections without acute rheumatic fever. Outbreaks of GAS pharyngitis in closed communities, such as boarding schools or military bases, may be followed by outbreaks of acute rheumatic fever. Antimicrobial therapy that eliminates GAS from the pharynx also prevents initial episodes of acute rheumatic fever, and long-term, continuous prophylaxis that prevents GAS pharyngitis also prevents recurrences of acute rheumatic fever.
Not all of the serotypes of GAS can cause rheumatic fever. When some strains (M type 4) were present in a very susceptible rheumatic population, no recurrences of rheumatic fever occurred. In contrast, episodes of pharyngitis with other serotypes prevalent in the same population were associated with frequent recurrences. The concept of rheumatogenicity is further supported by the observation that although serotypes of GAS frequently associated with skin infection are often isolated from the upper respiratory tract, they rarely cause recurrences of rheumatic fever in individuals with a previous history of rheumatic fever. In addition, certain serotypes of GAS (M types 1, 3, 5, 6, 18, 24) are more frequently isolated from patients with acute rheumatic fever than are other serotypes.
Epidemiology
The incidence of acute rheumatic fever in some developing countries exceeds 50 per 100,000 children. Worldwide, rheumatic heart disease remains the most common form of acquired heart disease in all age groups, accounting for as much as 50% of all cardiovascular disease and as much as 50% of all cardiac admissions in many developing countries. Striking differences are evident in the incidence of acute rheumatic fever and rheumatic heart disease among different ethnic groups within the same country; many, but not all, of these differences appear to be related to differences in socioeconomic status.
In the USA, at the beginning of the 20th century, acute rheumatic fever was a leading cause of death among children and adolescents, with annual incidence rates of 100-200/100,000 population. In addition, rheumatic heart disease was a leading cause of heart disease among adults <40 yr of age. At that time, as many as 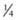 of the hospital beds in the USA were occupied by patients with acute rheumatic fever or its complications. By the 1940s, the annual incidence of acute rheumatic fever had decreased to 50/100,000, and over the next 4 decades, the decline in incidence accelerated rapidly. By the early 1980s, the annual incidence in some areas of the USA was as low as 0.5/100,000 population. This sharp decline in the incidence of acute rheumatic fever has been observed in other industrialized countries as well.
of the hospital beds in the USA were occupied by patients with acute rheumatic fever or its complications. By the 1940s, the annual incidence of acute rheumatic fever had decreased to 50/100,000, and over the next 4 decades, the decline in incidence accelerated rapidly. By the early 1980s, the annual incidence in some areas of the USA was as low as 0.5/100,000 population. This sharp decline in the incidence of acute rheumatic fever has been observed in other industrialized countries as well.
The explanation for this dramatic decline in the incidence of acute rheumatic fever and rheumatic heart disease in the USA and other industrialized countries is not clear. Historically, acute rheumatic fever has been associated with poverty, particularly in urban areas. Much of the decline in the incidence of acute rheumatic fever in industrialized countries during the preantibiotic era can probably be attributed to improvements in living conditions. A number of studies have suggested that, of the various manifestations of poverty, crowding, which contributes to the spread of GAS infections, is the one most closely associated with the incidence of acute rheumatic fever. The decline in incidence of acute rheumatic fever in industrialized countries over the past 4 decades has also been attributable in large measure to the greater availability of medical care and to the widespread use of antibiotics. Antibiotic therapy of GAS pharyngitis has been important in preventing initial attacks and, particularly, recurrences of the disease. In addition, the decline can be attributed, at least in part, to a shift in the prevalent strains of GAS from rheumatogenic to nonrheumatogenic strains.
A dramatic outbreak of acute rheumatic fever in the Salt Lake City area began in early 1985, and 198 cases were reported by the end of 1989. Other outbreaks were reported between 1984 and 1988 in Columbus and Akron, OH; Pittsburgh, PA; Nashville and Memphis, TN; New York, NY; Kansas City, MO; Dallas, TX; and among recruits at the San Diego Naval Training Center in California and at the Fort Leonard Wood Army Training Base in Missouri. Evidence suggests that this resurgence of acute rheumatic fever was focal and not nationwide.
Certain rheumatogenic serotypes (types 1, 3, 5, 6, and 18) that were isolated infrequently during the 1970s and early 1980s dramatically reappeared during these focal outbreaks. The appearance of these rheumatogenic strains in selected communities was probably a major factor in these outbreaks of acute rheumatic fever. Another property of GAS that has been associated with rheumatogenicity is the formation of highly mucoid colonies. Mucoid strains of GAS had only rarely been isolated from throat cultures in recent years. However, during these focal outbreaks of acute rheumatic fever, mucoid strains of GAS were commonly isolated from patients, family members, and members of the surrounding community.
In addition to the specific characteristics of the infecting GAS, the risk of a particular person developing acute rheumatic fever is also dependent on various host factors. The incidence of both initial attacks and recurrences of acute rheumatic fever peaks in children 5-15 yr of age, the age of greatest risk for GAS pharyngitis. Patients who have had one attack of acute rheumatic fever tend to have recurrences, and the clinical features of the recurrences tend to mimic those of the initial attack. In addition, there appears to be a genetic predisposition to acute rheumatic fever. Studies in twins have shown a higher concordance rate of acute rheumatic fever in monozygotic than in dizygotic twin pairs. Some investigators have also demonstrated an association between the presence of both specific human leukocyte antigen (HLA) markers and a specific B-cell alloantigen (D8/17) and susceptibility to acute rheumatic fever; others have been unable to confirm these associations.
Pathogenesis
The pathogenic link between a GAS infection of the upper respiratory tract and an attack of acute rheumatic fever, characterized by organ and tissue involvement far removed from the pharynx, is still not clear. One of the major obstacles to understanding the pathogenesis of acute rheumatic fever and rheumatic heart disease has been the inability to establish an animal model. Several theories of the pathogenesis of acute rheumatic fever and rheumatic heart disease have been proposed, but only 2 are seriously considered: the cytotoxicity theory and the immunologic theory.
The cytotoxicity theory suggests that a GAS toxin may be involved in the pathogenesis of acute rheumatic fever and rheumatic heart disease. GAS produces several enzymes that are cytotoxic for mammalian cardiac cells, such as streptolysin O, which has a direct cytotoxic effect on mammalian cells in tissue culture. Most of the proponents of the cytotoxicity theory have focused on this enzyme. However, one of the major problems with the cytotoxicity hypothesis is its inability to explain the latent period between GAS pharyngitis and the onset of acute rheumatic fever.
An immune-mediated pathogenesis for acute rheumatic fever and rheumatic heart disease has been suggested by the clinical similarity of acute rheumatic fever to other illnesses produced by immunopathogenic processes and by the latent period between the GAS infection and acute rheumatic fever. The antigenicity of a large variety of GAS products and constituents and the immunologic cross reactivity between GAS components and mammalian tissues also lends support to this hypothesis. Common antigenic determinants are shared between certain components of GAS (M protein, protoplast membrane, cell wall group A carbohydrate, capsular hyaluronate) and specific mammalian tissues (e.g., heart, brain, joint). For example, certain M proteins (M1, M5, M6, and M19) share epitopes with human tropomyosin and myosin. Additionally, the involvement of GAS superantigens such as pyrogenic exotoxins in the pathogenesis of acute rheumatic fever has been proposed.
Clinical Manifestations and Diagnosis
Because no clinical or laboratory finding is pathognomonic for acute rheumatic fever, T. Duckett Jones in 1944 proposed guidelines to aid in diagnosis and to limit overdiagnosis. The Jones criteria, as revised in 1992 by the American Heart Association (AHA) (Table 176-2) are intended only for the diagnosis of the initial attack of acute rheumatic fever and not for recurrences. There are 5 major and 4 minor criteria and an absolute requirement for evidence (microbiologic or serologic) of recent GAS infection. The diagnosis of acute rheumatic fever can be established by the Jones criteria when a patient fulfills 2 major criteria or 1 major and 2 minor criteria and meets the absolute requirement. Even with strict application of the Jones criteria, overdiagnosis as well as underdiagnosis of acute rheumatic fever may occur. There are 3 circumstances in which the diagnosis of acute rheumatic fever can be made without strict adherence to the Jones criteria. Chorea may occur as the only manifestation of acute rheumatic fever. Similarly, indolent carditis may be the only manifestation in patients who 1st come to medical attention months after the onset of acute rheumatic fever. Finally, although most patients with recurrences of acute rheumatic fever fulfill the Jones criteria, some may not.
Table 176-2 GUIDELINES FOR THE DIAGNOSIS OF INITIAL ATTACK OF RHEUMATIC FEVER (JONES CRITERIA, UPDATED 1992)
| MAJOR MANIFESTATIONS* | MINOR MANIFESTATIONS | SUPPORTING EVIDENCE OF ANTECEDENT GROUP A STREPTOCOCCAL INFECTION |
|---|---|---|
| Carditis Polyarthritis Erythema marginatum Subcutaneous nodules Chorea |
Clinical features: Arthralgia Fever |
Positive throat culture or rapid streptococcal antigen test Elevated or increasing streptococcal antibody titer |
| Laboratory features: Elevated acute phase reactants: |
||
| Erythrocyte sedimentation rate | ||
| C-reactive protein | ||
| Prolonged PR interval |
* The presence of 2 major or of 1 major and 2 minor manifestations indicates a high probability of acute rheumatic fever if supported by evidence of preceding group A streptococcal infection.
From Jones criteria, updated 1992. JAMA 268:2069–2073, 1992. Copyright American Medical Association.
Major Manifestations
Migratory Polyarthritis
Arthritis occurs in about 75% of patients with acute rheumatic fever and typically involves larger joints, particularly the knees, ankles, wrists, and elbows. Involvement of the spine, small joints of the hands and feet, or hips is uncommon. Rheumatic joints are generally hot, red, swollen, and exquisitely tender; even the friction of bedclothes is uncomfortable. The pain can precede and can appear to be disproportionate to the other findings. The joint involvement is characteristically migratory in nature; a severely inflamed joint can become normal within 1-3 days without treatment, as 1 or more other large joints become involved. Severe arthritis can persist for several weeks in untreated patients. Monoarticular arthritis is unusual unless antiinflammatory therapy is initiated prematurely, aborting the progression of the migratory polyarthritis. If a child with fever and arthritis is suspected of having acute rheumatic fever, it frequently is useful to withhold salicylates and observe for migratory progression. A dramatic response to even small doses of salicylates is another characteristic feature of the arthritis, and the absence of such a response should suggest an alternative diagnosis. Rheumatic arthritis is typically not deforming. Synovial fluid in acute rheumatic fever usually has 10,000-100,000 white blood cells/mm3 with a predominance of neutrophils, a protein level of about 4 g/dL, a normal glucose level, and forms a good mucin clot. Frequently, arthritis is the earliest manifestation of acute rheumatic fever and may correlate temporally with peak antistreptococcal antibody titers. There is often an inverse relationship between the severity of arthritis and the severity of cardiac involvement.
Carditis
Carditis and resultant chronic rheumatic heart disease are the most serious manifestations of acute rheumatic fever and account for essentially all of the associated morbidity and mortality. Rheumatic carditis is characterized by pancarditis, with active inflammation of myocardium, pericardium, and endocardium (Chapter 432). Cardiac involvement during acute rheumatic fever varies in severity from fulminant, potentially fatal exudative pancarditis to mild, transient cardiac involvement. Endocarditis (valvulitis) is a universal finding in rheumatic carditis, whereas the presence of pericarditis or myocarditis is variable. Myocarditis and/or pericarditis without evidence of endocarditis is rarely due to rheumatic heart disease. Most cases consist of either isolated mitral valvular disease or combined aortic and mitral valvular disease. Isolated aortic or right-sided valvular involvement is uncommon. Serious and long-term illness is related entirely to valvular heart disease as a consequence of a single attack or recurrent attacks of acute rheumatic fever. Valvular insufficiency is characteristic of both acute and convalescent stages of acute rheumatic fever, whereas valvular stenosis usually appears several years or even decades after the acute illness. However, in developing countries where acute rheumatic fever often occurs at a younger age, mitral stenosis and aortic stenosis may develop sooner after acute rheumatic fever than in developed countries and can occur in young children.
Acute rheumatic carditis usually presents as tachycardia and cardiac murmurs, with or without evidence of myocardial or pericardial involvement. Moderate to severe rheumatic carditis can result in cardiomegaly and congestive heart failure with hepatomegaly and peripheral and pulmonary edema. Echocardiographic findings include pericardial effusion, decreased ventricular contractility, and aortic and/or mitral regurgitation. Mitral regurgitation is characterized by a high-pitched apical holosystolic murmur radiating to the axilla. In patients with significant mitral regurgitation, this may be associated with an apical mid-diastolic murmur of relative mitral stenosis. Aortic insufficiency is characterized by a high-pitched decrescendo diastolic murmur at the upper left sternal border.
Clinically, rheumatic carditis is almost always associated with a murmur of valvulitis. Several investigators and advisory groups have suggested that subclinical valvular regurgitation be accepted as evidence of rheumatic carditis. Subclinical valvular regurgitation is echocardiographically identified pathological mitral or aortic regurgitation inaudible to skilled auscultation. Although controversial, subclinical valvular regurgitation is not currently accepted as either a major or minor Jones criterion by the AHA in the guidelines for the diagnosis of acute rheumatic fever (Chapter 432).
Carditis occurs in about 50-60% of all cases of acute rheumatic fever. Recurrent attacks of acute rheumatic fever in patients who had carditis with the initial attack are associated with high rates of carditis. The major consequence of acute rheumatic carditis is chronic, progressive valvular disease, particularly valvular stenosis, which can require valve replacement.
Chorea
Sydenham chorea occurs in about 10-15% of patients with acute rheumatic fever and usually presents as an isolated, frequently subtle, neurologic behavior disorder. Emotional lability, incoordination, poor school performance, uncontrollable movements, and facial grimacing, exacerbated by stress and disappearing with sleep, are characteristic. Chorea occasionally is unilateral. The latent period from acute GAS infection to chorea is usually longer than for arthritis or carditis and can be months. Onset can be insidious, with symptoms being present for several months before recognition. Clinical maneuvers to elicit features of chorea include (1) demonstration of milkmaid’s grip (irregular contractions of the muscles of the hands while squeezing the examiner’s fingers), (2) spooning and pronation of the hands when the patient’s arms are extended, (3) wormian darting movements of the tongue upon protrusion, and (4) examination of handwriting to evaluate fine motor movements. Diagnosis is based on clinical findings with supportive evidence of GAS antibodies. However, in patients with a long latent period from the inciting streptococcal infection, antibody levels may have declined to normal. Although the acute illness is distressing, chorea rarely, if ever, leads to permanent neurologic sequelae.
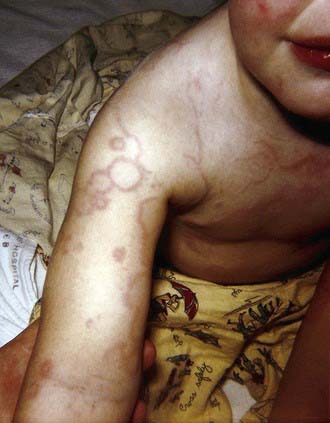
Erythema Marginatum
Erythema marginatum is a rare (<3% of patients with acute rheumatic fever) but characteristic rash of acute rheumatic fever. It consists of erythematous, serpiginous, macular lesions with pale centers that are not pruritic (Fig. 176-2). It occurs primarily on the trunk and extremities, but not on the face, and it can be accentuated by warming the skin.
Subcutaneous Nodules
Subcutaneous nodules are a rare (≤1% of patients with acute rheumatic fever) finding and consist of firm nodules approximately 1 cm in diameter along the extensor surfaces of tendons near bony prominences. There is a correlation between the presence of these nodules and significant rheumatic heart disease.
Minor Manifestations
The 2 clinical minor manifestations are arthralgia (in the absence of polyarthritis as a major criterion) and fever (typically temperature ≥102°F and occurring early in the course of illness). The 2 laboratory minor manifestations are elevated acute-phase reactants (e.g., C-reactive protein, erythrocyte sedimentation rate) and prolonged PR interval on electrocardiogram (1st degree heart block). However, a prolonged P-R interval alone does not constitute evidence of carditis or predict long-term cardiac sequelae.
Recent Group A Streptococcus Infection
An absolute requirement for the diagnosis of acute rheumatic fever is supporting evidence of a recent GAS infection. Acute rheumatic fever typically develops 2-4 wk after an acute episode of GAS pharyngitis at a time when clinical findings of pharyngitis are no longer present and when only 10-20% of the throat culture or rapid streptococcal antigen test results are positive. One third of patients with acute rheumatic fever have no history of an antecedent pharyngitis. Therefore, evidence of an antecedent GAS infection is usually based on elevated or increasing serum antistreptococcal antibody titers. A slide agglutination test (Streptozyme) has been introduced, and it is purported to detect antibodies against 5 different GAS antigens. Although this test is rapid, relatively simple to perform, and widely available, it is less standardized and less reproducible than other tests and should not be used as a diagnostic test for evidence of an antecedent GAS infection. If only a single antibody is measured (usually antistreptolysin O), only 80-85% of patients with acute rheumatic fever have an elevated titer; however, 95-100% have an elevation if 3 different antibodies (antistreptolysin O, anti-DNase B, antihyaluronidase) are measured. Therefore, when acute rheumatic fever is suspected clinically, multiple antibody tests should be performed. Except for patients with chorea, clinical findings of acute rheumatic fever generally coincide with peak antistreptococcal antibody responses. Most patients with chorea have elevation of antibodies to 1 or more GAS antigens. However, in patients in whom there is a long latent period from the inciting GAS infection, antibody levels may have declined to within the normal range. The diagnosis of acute rheumatic fever should not be made in patients with elevated or increasing streptococcal antibody titers who do not fulfill the Jones criteria because such titer changes may be coincidental. This is most often true in younger, school-aged children, many of whom have GAS pyoderma in the summer or unrelated GAS pharyngitis during the winter and spring months.
Differential Diagnosis
The differential diagnoses of rheumatic fever include many infectious as well as noninfectious illnesses (Table 176-3). When children present with arthritis, a collagen vascular disease must be considered. Rheumatoid arthritis in particular must be distinguished from acute rheumatic fever. Children with rheumatoid arthritis tend to be younger and usually have less joint pain relative to their other clinical findings than those with acute rheumatic fever. Spiking fevers, lymphadenopathy, and splenomegaly are more suggestive of rheumatoid arthritis than acute rheumatic fever. The response to salicylate therapy is also much less dramatic with rheumatoid arthritis than with acute rheumatic fever. Systemic lupus erythematosus can usually be distinguished from acute rheumatic fever on the basis of the presence of antinuclear antibodies with systemic lupus erythematosus. Other causes of arthritis such as gonococcal arthritis, malignancies, serum sickness, Lyme disease, sickle cell disease, and reactive arthritis related to gastrointestinal infections (e.g., Shigella, Salmonella, Yersinia) should also be considered.
Table 176-3 DIFFERENTIAL DIAGNOSIS OF ACUTE RHEUMATIC FEVER
| ARTHRITIS | CARDITIS | CHOREA |
|---|---|---|
| Rheumatoid arthritis | Viral myocarditis | Huntington chorea |
| Reactive arthritis (e.g., Shigella, Salmonella, Yersinia) | Viral pericarditis | Wilson disease |
| Serum sickness | Infective endocarditis | Systemic lupus erythematosus |
| Sickle cell disease | Kawasaki disease | Cerebral palsy |
| Malignancy | Congenital heart disease | Tics |
| Systemic lupus erythematosus | Mitral valve prolapse | Hyperactivity |
| Lyme disease (Borrelia burgdorferi) | Innocent murmurs | |
| Gonococcal infection (Neisseria gonorrhoeae) |
When carditis is the sole major manifestation of suspected acute rheumatic fever, viral myocarditis, viral pericarditis, Kawasaki disease, and infective endocarditis should also be considered. Patients with infective endocarditis may present with both joint and cardiac manifestations. These patients can usually be distinguished from patients with acute rheumatic fever by blood cultures and the presence of associated findings (e.g., hematuria, splenomegaly, splinter hemorrhages). When chorea is the sole major manifestation of suspected acute rheumatic fever, Huntington chorea, Wilson disease, systemic lupus erythematosus, and various encephalitides should also be considered. These other diseases are usually identified by the history, laboratory studies, and clinical findings.
Treatment
All patients with acute rheumatic fever should be placed on bed rest and monitored closely for evidence of carditis. They can be allowed to ambulate as soon as the signs of acute inflammation have subsided. However, patients with carditis require longer periods of bed rest.
Antibiotic Therapy
Once the diagnosis of acute rheumatic fever has been established and regardless of the throat culture results, the patient should receive 10 days of orally administered penicillin or erythromycin or a single intramuscular injection of benzathine penicillin to eradicate GAS from the upper respiratory tract. After this initial course of antibiotic therapy, the patient should be started on long-term antibiotic prophylaxis.
Anti-inflammatory Therapy
Anti-inflammatory agents (e.g., salicylates, corticosteroids) should be withheld if arthralgia or atypical arthritis is the only clinical manifestation of presumed acute rheumatic fever. Premature treatment with one of these agents may interfere with the development of the characteristic migratory polyarthritis and thus obscure the diagnosis of acute rheumatic fever. Agents such as acetaminophen can be used to control pain and fever while the patient is being observed for more definite signs of acute rheumatic fever or for evidence of another disease.
Patients with typical migratory polyarthritis and those with carditis without cardiomegaly or congestive heart failure should be treated with oral salicylates. The usual dose of aspirin is 100 mg/kg/day in 4 divided doses PO for 3-5 days, followed by 75 mg/kg/day in 4 divided doses PO for 4 wk. Determination of the serum salicylate level is not necessary unless the arthritis does not respond or signs of salicylate toxicity (tinnitus, hyperventilation) develop. There is no evidence that nonsteroidal antiinflammatory agents are any more effective than salicylates.
Patients with carditis and cardiomegaly or congestive heart failure should receive corticosteroids. The usual dose of prednisone is 2 mg/kg/day in 4 divided doses for 2-3 wk followed by a tapering of the dose that reduces the dose by 5 mg/24 hr every 2-3 days. At the beginning of the tapering of the prednisone dose, aspirin should be started at 75 mg/kg/day in 4 divided doses for 6 wk. Supportive therapies for patients with moderate to severe carditis include digoxin, fluid and salt restriction, diuretics, and oxygen. The cardiac toxicity of digoxin is enhanced with myocarditis.
Termination of the antiinflammatory therapy may be followed by the reappearance of clinical manifestations or of laboratory abnormalities. These “rebounds” are best left untreated unless the clinical manifestations are severe; salicylates or steroids should be reinstated in such cases.
Sydenham Chorea
Because chorea often occurs as an isolated manifestation after the resolution of the acute phase of the disease, anti-inflammatory agents are usually not indicated. Sedatives may be helpful early in the course of chorea; phenobarbital (16-32 mg every 6-8 hr PO) is the drug of choice. If phenobarbital is ineffective, then haloperidol (0.01-0.03 mg/kg/24 hr divided bid PO) or chlorpromazine (0.5 mg/kg every 4-6 hr PO) should be initiated.
Complications
The arthritis and chorea of acute rheumatic fever resolve completely without sequelae. Therefore, the long-term sequelae of rheumatic fever are usually limited to the heart (Chapter 432).
The AHA has published updated recommendations regarding the use of prophylactic antibiotics to prevent infective endocarditis (Chapter 431). The AHA recommendations do not suggest routine prophylaxis any longer for patients with rheumatic heart disease. However, the maintenance of optimal oral health care remains an important component of an overall health care program. For the relatively few patients with rheumatic heart disease in whom IE prophylaxis remains recommended, such as those with prosthetic valves or prosthetic material used in valve repair, the current AHA recommendations should be followed (Chapter 431). These recommendations advise using an agent other than a penicillin to prevent IE in those receiving penicillin prophylaxis for rheumatic fever because oral α-hemolytic streptococci are likely to have developed resistance to penicillin.
Prognosis
The prognosis for patients with acute rheumatic fever depends on the clinical manifestations present at the time of the initial episode, the severity of the initial episode, and the presence of recurrences. Approximately 70% of patients with carditis during the initial episode of acute rheumatic fever recover with no residual heart disease; the more severe the initial cardiac involvement, the greater the risk is for residual heart disease. Patients without carditis during the initial episode are unlikely to have carditis with recurrences. In contrast, patients with carditis during the initial episode are likely to have carditis with recurrences, and the risk for permanent heart damage increases with each recurrence. Patients who have had acute rheumatic fever are susceptible to recurrent attacks following reinfection of the upper respiratory tract with GAS. Therefore, these patients require long-term continuous chemoprophylaxis.
Before antibiotic prophylaxis was available, 75% of patients who had an initial episode of acute rheumatic fever had one or more recurrences during their lifetimes. These recurrences were a major source of morbidity and mortality. The risk of recurrence is highest immediately after the initial episode and decreases with time.
Approximately 20% of patients who present with “pure” chorea who are not given secondary prophylaxis develop rheumatic heart disease within 20 yr. Therefore, patients with chorea, even in the absence of other manifestations of rheumatic fever, require long-term antibiotic prophylaxis.
Prevention
Prevention of both initial and recurrent episodes of acute rheumatic fever depends on controlling GAS infections of the upper respiratory tract. Prevention of initial attacks (primary prevention) depends on identification and eradication of the GAS that produces episodes of acute pharyngitis. Individuals who have already suffered an attack of acute rheumatic fever are particularly susceptible to recurrences of rheumatic fever with any subsequent GAS upper respiratory tract infection, whether or not they are symptomatic. Therefore, these patients should receive continuous antibiotic prophylaxis to prevent recurrences (secondary prevention).
Primary Prevention
Appropriate antibiotic therapy instituted before the 9th day of symptoms of acute GAS pharyngitis is highly effective in preventing 1st attacks of acute rheumatic fever from that episode. However, about 30% of patients with acute rheumatic fever do not recall a preceding episode of pharyngitis.
Secondary Prevention
Secondary prevention is directed at preventing acute GAS pharyngitis in patients at substantial risk of recurrent acute rheumatic fever. Secondary prevention requires continuous antibiotic prophylaxis, which should begin as soon as the diagnosis of acute rheumatic fever has been made and immediately after a full course of antibiotic therapy has been completed. Because patients who have had carditis with their initial episode of acute rheumatic fever are at a relatively high risk for having carditis with recurrences and for sustaining additional cardiac damage, they should receive long-term antibiotic prophylaxis well into adulthood and perhaps for life.
Patients who did not have carditis with their initial episode of acute rheumatic fever have a relatively low risk for carditis with recurrences. Antibiotic prophylaxis should continue in these patients until the patient reaches 21 yr of age or until 5 yr have elapsed since the last rheumatic fever attack, whichever is longer. The decision to discontinue prophylactic antibiotics should be made only after careful consideration of potential risks and benefits and of epidemiologic factors such as the risk for exposure to GAS infections.
The regimen of choice for secondary prevention is a single intramuscular injection of benzathine penicillin G (600,000 IU for children ≤60 lb and 1.2 million IU for those >60 lb) every 4 wk (Table 176-4). In certain high-risk patients, and in certain areas of the world where the incidence of rheumatic fever is particularly high, use of benzathine penicillin G every 3 wk may be necessary because levels of penicillin may decrease to marginally effective amounts after 3 wk. In the USA, the administration of benzathine penicillin G every 3 wk is recommended only for those who have recurrent acute rheumatic fever despite adherence to a 4-wk regimen. In compliant patients, continuous oral antimicrobial prophylaxis can be used. Penicillin V given twice daily and sulfadiazine given once daily are equally effective when used in such patients. For the exceptional patient who is allergic to both penicillin and sulfonamides, a macrolide (erythromycin or clarithromycin) or azalide (azithromycin) may be used. The duration of secondary prophylaxis is noted in Table 176-5.
Table 176-4 CHEMOPROPHYLAXIS FOR RECURRENCES OF ACUTE RHEUMATIC FEVER
| DRUG | DOSE | ROUTE |
|---|---|---|
| Penicillin G benzathine | 600,000 U for children, ≤60 lb 1.2 million U for children >60 lb, every 4 wk* |
Intramuscular |
| OR | ||
| Penicillin V | 250 mg, twice a day | Oral |
| OR | ||
| Sulfadiazine or sulfisoxazole | 0.5 g, once a day for patients ≤60 lb | Oral |
| 1.0 g, once a day for patients >60 lb | ||
| FOR PEOPLE WHO ARE ALLERGIC TO PENICILLIN AND SULFONAMIDE DRUGS | ||
| Macrolide or azalide | Variable | Oral |
* In high-risk situations, administration every 3 weeks is recommended.
From Gerber MA, Baltimore RS, Eaton CB, et al: Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Circulation 119:1541–1551, 2009.
Table 176-5 DURATION OF PROPHYLAXIS FOR PEOPLE WHO HAVE HAD ACUTE RHEUMATIC FEVER: RECOMMENDATIONS OF THE AMERICAN HEART ASSOCIATION
| CATEGORY | DURATION |
|---|---|
| Rheumatic fever without carditis | 5 yr or until 21 yr of age, whichever is longer |
| Rheumatic fever with carditis but without residual heart disease (no valvular disease*) | 10 yr or until 21 yr of age, whichever is longer |
| Rheumatic fever with carditis and residual heart disease (persistent valvular disease*) | 10 yr or until 40 yr of age, whichever is longer, sometimes lifelong prophylaxis |
* Clinical or echocardiographic evidence.
From Gerber MA, Baltimore RS, Eaton CB, et al: Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Circulation 119:1541–1551, 2009.
Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155-168.
Dajani AS, Ayoub E, Bierman FZ, et al. Guidelines for the diagnosis of rheumatic fever: Jones criteria, 1992 update. JAMA. 1992;268:2069-2073.
Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young. Circulation. 2009;119:1541-1551.
Lennon D. Acute rheumatic fever in children. Paediatr Drugs. 2004;6:363-373.
Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470-476.
Martin JM, Barbadora KA. Continued high caseload of rheumatic fever in western Pennsylvania: possible rheumatogenic EMM types of Streptococcus pyogenes. J Pediatr. 2006;149:58-63.
Miyake CY, Gauvreau K, Tani LY, et al. Characteristics of children discharged from hospitals in the United States in 2000 with the diagnosis of acute rheumatic fever. Pediatrics. 2007;120:503-508.
Shulman ST, Stollerman G, Beall B, et al. Temporal changes in streptococcal M protein types and the near-disappearance of acute rheumatic fever in the United States. Clin Infect Dis. 2006;42:441-447.
Tani LY, Veasy LG, Minich LL, et al. Rheumatic fever in children younger than 5 years: is the presentation different? Pediatrics. 2003;112:1065-1068.
Walker AR, Tani LY, Thompson JA, et al. Rheumatic chorea: relationship to systemic manifestations and response to corticosteroids. J Pediatr. 2007;151:679-683.
Zomorrodi A, Wald ER. Sydenham’s chorea in Western Pennsylvania. Pediatrics. 2006;117:e675-e679.