Chapter 505 Glomerulonephritis Associated with Infections
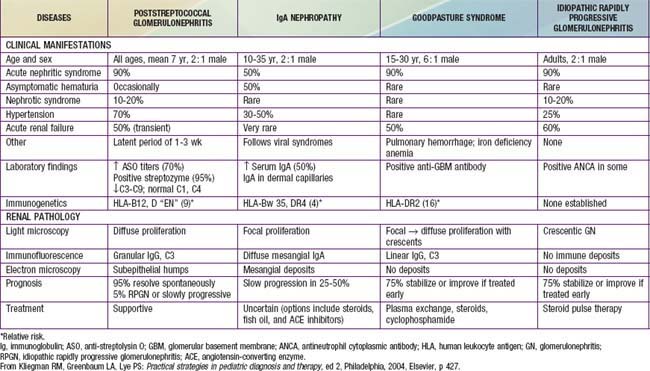
505.1 Acute Poststreptococcal Glomerulonephritis
Group A β-hemolytic streptococcal (GAS) infections are common in children and can lead to the postinfectious complication of acute glomerulonephritis (GN). Acute poststreptococcal glomerulonephritis (APSGN) is a classic example of the acute nephritic syndrome characterized by the sudden onset of gross hematuria, edema, hypertension, and renal insufficiency. It is one of the most common glomerular causes of gross hematuria in children and is a major cause of morbidity in GAS infections.
Etiology and Epidemiology
APSGN follows infection of the throat or skin by certain “nephritogenic” strains of GAS. Epidemics and clusters of household cases occur throughout the world, and 97% of cases occur in less-developed countries. The overall incidence has decreased in industrialized nations, presumably due to improved hygienic conditions. Poststreptococcal GN commonly follows streptococcal pharyngitis during cold-weather months and streptococcal skin infections or pyoderma during warm-weather months. Although epidemics of nephritis have been described in association with throat (serotype 12) and skin (serotype 49) infections, this disease is most commonly sporadic.
Pathology
The kidneys appear symmetrically enlarged. Glomeruli appear enlarged and relatively bloodless and show diffuse mesangial cell proliferation, with an increase in mesangial matrix (Fig. 505-1). Polymorphonuclear leukocyte infiltration is common in glomeruli during the early stage of the disease. Crescents and interstitial inflammation may be seen in severe cases, but these changes are not specific for poststreptococcal GN. Immunofluorescence microscopy reveals a pattern of “lumpy-bumpy” deposits of immunoglobulin and complement on the glomerular basement membrane (GBM) and in the mesangium. On electron microscopy, electron-dense deposits, or “humps,” are observed on the epithelial side of the GBM (Fig. 505-2).
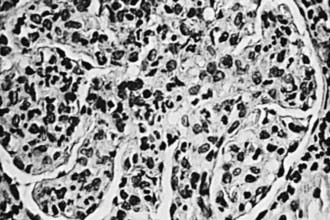
Figure 505-1 Glomerulus from a patient with poststreptococcal glomerulonephritis appears enlarged and relatively bloodless and shows mesangial proliferation and exudation of neutrophils (×400).

Figure 505-2 Electron micrograph in poststreptococcal glomerulonephritis demonstrating electron-dense deposits (D) on the epithelial cell (Ep) side of the glomerular basement membrane. A polymorphonuclear leukocyte (P) is present within the lumen (L) of the capillary. BS, Bowman space; M, mesangium.
Pathogenesis
Morphologic studies and a depression in the serum complement (C3) level provide strong evidence that ASPGN is mediated by immune complexes. Precise mechanisms by which nephritogenic streptococci induce immunologic injury continue to be elucidated. Several mechanisms of immune injury have been supported by experimental models and human studies and include circulating immune complex formation with streptococcal antigens and subsequent glomerular deposition, molecular mimicry whereby circulating antibodies elicited by streptococcal antigens react with normal glomerular antigens, in situ immune complex formation of antistreptococcal antibodies with glomerular deposited antigen, and complement activation by directly deposited streptococcal antigens.
Group A streptococci possess M proteins, and nephritogenic strains are related to the M protein serotype. The search for the precise nephritogenic antigen suggests that streptococcal pyogenic exotoxin (SPE B) and nephritis-associated streptococcal plasmin receptor (NAPlr) are promising candidates. Both have been identified in glomeruli of affected patients, and in 1 study, circulating antibodies to SPE B were found in all patients. Cross-reactivity of SPE B and other M proteins with various components of the glomerular basement membrane also give evidence for molecular mimicry.
Clinical Manifestations
Poststreptococcal GN is most common in children aged 5-12 yr and uncommon before the age of 3 yr. The typical patient develops an acute nephritic syndrome 1-2 wk after an antecedent streptococcal pharyngitis or 3-6 wk after a streptococcal pyoderma. The history of a specific infection may be absent, because symptoms may have been mild or have resolved without patients receiving specific treatment or seeking the care of a medical provider.
The severity of kidney involvement varies from asymptomatic microscopic hematuria with normal renal function to gross hematuria with acute renal failure. Depending on the severity of renal involvement, patients can develop various degrees of edema, hypertension, and oliguria. Patients are at risk for developing encephalopathy and/or heart failure secondary to hypertension or hypervolemia. Hypertensive encephalopathy must be considered in patients with blurred vision, severe headaches, altered mental status, or new seizures. The effects of acute hypertension not only depend on the severity of hypertension but also the absolute change in comparison to the patient’s baseline blood pressure and the rate at which it has risen. Encephalopathy can also result from the direct toxic effects of streptococcal antigens on the central nervous system. Respiratory distress, orthopnea, and cough may be symptoms of pulmonary edema and heart failure. Peripheral edema typically results from salt and water retention and is common; nephrotic syndrome develops in a minority (<5%) of childhood cases. Nonspecific symptoms such as malaise, lethargy, abdominal pain, or flank pain are common.
The acute phase generally resolves within 6-8 wk. Although urinary protein excretion and hypertension usually normalize by 4-6 wk after onset, persistent microscopic hematuria can persist for 1-2 yr after the initial presentation.
Diagnosis
Urinalysis demonstrates red blood cells (RBCs), often in association with RBC casts, proteinuria, and polymorphonuclear leukocytes. A mild normochromic anemia may be present from hemodilution and low-grade hemolysis. The serum C3 level is significantly reduced in >90% of patients in the acute phase and returns to normal 6-8 wk after onset. Although serum CH50 is commonly depressed, C4 is most often normal in APSGN, or only mildly depressed.
Confirmation of the diagnosis requires clear evidence of a prior streptococcal infection. A positive throat culture report might support the diagnosis or might simply represent the carrier state. On the other hand, a rising antibody titer to streptococcal antigen(s) confirms a recent streptococcal infection. The antistreptolysin O titer is commonly elevated after a pharyngeal infection but rarely increases after streptococcal skin infections. The best single antibody titer to document cutaneous streptococcal infection is the anti-deoxyribonuclease (DNase) B level. If available, a positive streptozyme screen (which measures multiple antibodies to different streptococcal antigens) is a valuable diagnostic tool. Serologic evidence for streptococcal infections is more sensitive than the history of recent infections and far more sensitive than positive bacterial cultures obtained at the time of onset of acute nephritis.
Magnetic resonance imaging of the brain is indicated in patients with severe neurologic symptoms and can demonstrate reversible posterior leukoencephalopathy in the parieto-occipital areas on T2-weighted images. Chest x-ray is indicated in those with signs of heart failure or respiratory distress, or physical exam findings of a heart gallop, decreased breath sounds, rales, or hypoxemia.
The clinical diagnosis of poststreptococcal GN is quite likely in a child presenting with acute nephritic syndrome, evidence of recent streptococcal infection, and a low C3 level. However, it is important to consider other diagnoses such as systemic lupus erythematosus and an acute exacerbation of chronic GN. Renal biopsy should be considered only in the presence of acute renal failure, nephrotic syndrome, absence of evidence of streptococcal infection, or normal complement levels. In addition, renal biopsy is considered when hematuria and proteinuria, diminished renal function, and/or a low C3 level persist more than 2 mo after onset. Persistent hypocomplementemia can indicate a chronic form of postinfectious GN or another disease such as membranoproliferative GN.
The differential diagnosis of poststreptococcal GN includes many of the causes of hematuria listed in Tables 503-2 and 505-1 and an algorithm to help with diagnosis is presented in Figure 505-3. Acute postinfectious GN can also follow other infections with coagulase-positive and coagulase-negative staphylococci, Streptococcus pneumoniae, and gram-negative bacteria. Bacterial endocarditis can produce a hypocomplementemic GN with renal failure. Acute GN can occur after certain fungal, rickettsial, and viral diseases, particularly influenza.
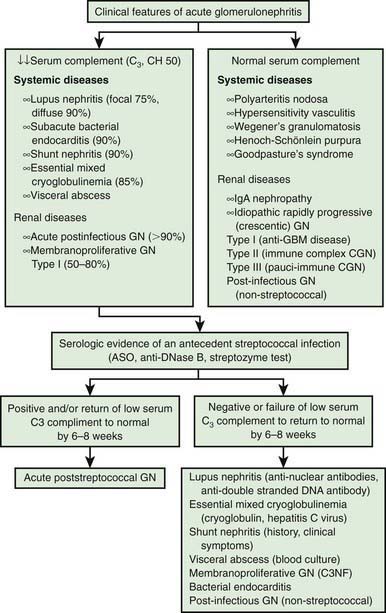
Figure 505-3 Differential diagnosis of acute glomerulonephritis (GN). ASO, anti-streptolysin O; GBM, glomerular basement membrane.
(Adapted from Sulyok E: Acute proliferative glomerulonephritis. In Avner ED, Harmon WE, Niaudet P, editors: Pediatric nephrology, ed 5, Philadelphia, 2004, Lippincott Williams & Wilkins, pp 601–613.)
Complications
Acute complications result from hypertension and acute renal dysfunction. Hypertension is seen in 60% of patients and is associated with hypertensive encephalopathy in 10% of cases. Although the neurologic sequelae are often reversible with appropriate management, severe prolonged hypertension can lead to intracranial bleeding. Other potential complications include heart failure, hyperkalemia, hyperphosphatemia, hypocalcemia, acidosis, seizures, and uremia. Acute renal failure can require treatment with dialysis.
Prevention
Early systemic antibiotic therapy for streptococcal throat and skin infections does not eliminate the risk of GN. Family members of patients with acute GN, especially young children, should be considered at risk and be cultured for group A β-hemolytic streptococci and treated if appropriate. Family pets, particularly dogs, have also been reported as carriers.
Treatment
Management is directed at treating the acute effects of renal insufficiency and hypertension (Chapter 529.1). Although a 10-day course of systemic antibiotic therapy with penicillin is recommended to limit the spread of the nephritogenic organisms, antibiotic therapy does not affect the natural history of GN. Sodium restriction, diuresis usually with intravenous furosemide, and pharmacotherapy with calcium channel antagonists, vasodilators, or angiotensin-converting enzyme inhibitors are standard therapies used to treat hypertension.
Prognosis
Complete recovery occurs in >95% of children with APSGN. Recurrences are extremely rare. Mortality in the acute stage can be avoided by appropriate management of acute renal failure, cardiac failure, and hypertension. Infrequently, the acute phase is severe and leads to glomerulosclerosis and chronic renal disease in <2% of affected children. The true incidence of chronic renal disease that emerges later in adulthood as a result of childhood APSGN and reduction of functioning nephron number remains unknown.
Ahn SY, Ingulli E. Acute poststreptococcal glomerulonephritis: an update. Curr Opin Pediatr. 2008;20:157-162.
Clark G, White RHR, Glasgow EF, et al. Poststreptococcal glomerulonephritis in children: clinicopathological correlations and long-term prognosis. Pediatr Nephrol. 1988;2:381-388.
Pais PJ, Kump T, Greenbaum LA. Delay in diagnosis in poststreptococcal glomerulonephritis. J Pediatr. 2008;153:560-564.
Rodriquez-Iturbe B, Mezzano S. Acute postinfectious glomerulonephritis. In: Avner ED, Harmon WE, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelburg, Germany: Springer-Verlag; 2009:743-756.
White AV, Hoy WE, McCredie DA. Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Med J Aust. 2001;10:492-496.
505.2 Other Chronic Infections
GN is a recognized complication of various chronic infections. Classic examples include bacterial endocarditis caused by viridans Streptococcus and other organisms, and ventriculoatrial shunts infected with Staphylococcus epidermidis. Other infections, observed less commonly in children than adults, include hepatitis B virus, hepatitis C virus, syphilis, and candidiasis. Parasitic infections associated with glomerular disease include malaria, schistosomiasis, leishmaniasis, filariasis, hydatid disease, trypanosomiasis, and toxoplasmosis. In each condition, the infecting organism has low virulence and the host is chronically infected with foreign antigen. In the presence of high levels of circulating antigen, the host’s antibody response leads to formation of immune complexes that deposit in the kidneys and initiate glomerular inflammation. Foreign antigens can also stimulate an autoimmune response through the production of antibodies that cross react with such antigens incorrectly “recognized” as glomerular structural components.
The renal histopathology can resemble poststreptococcal GN, membranous GN, or membranoproliferative GN. The clinical manifestations are generally those of an acute nephritic or nephrotic syndrome. The serum C3 and CH50 complement levels are often decreased.
It has been demonstrated in HIV-associated nephropathy (HIVAN) that direct viral infection of nephrons can occur because renal cells express a variety of lymphocyte chemokine receptors that are essential for and facilitate viral invasion. The renal expression of HIV infection is quite variable and includes an immune complex injury and a direct cytopathic effect. The classic histopathologic lesion of HIVAN is focal segmental glomerulosclerosis.
Prompt eradication of any infection before severe glomerular injury occurs usually results in resolution of the GN. Progression to end-stage renal failure has been described but is uncommon. Spontaneous resolution of hepatitis B infection is common in children (30-50%) and results in remission of the glomerulopathy. Specific antivirals, interferon therapy, plasmapheresis, and immunosuppressive treatment have all been used successfully in adults with hepatitis C disease, but no controlled trials with any of these agents have been performed in pediatric patients.
Chesney RW, O’Regan S, Guyda HJ, et al. Candida endocrinopathy syndrome with membranoproliferative glomerulonephritis: demonstration of glomerular Candida antigen. Clin Nephrol. 1976;5:232-238.
Connor FL, Rosenberg AR, Kennedy SE, et al. HBV associated nephrotic syndrome: resolution with oral lamivudine. Arch Dis Child. 2003;88:446-449.
Hendrickse RG, Adeniyi A. Quartan malarial nephrotic syndrome in children. Kidney Int. 1979;16:64-74.
Hunte W, Al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3:1351-1355.
Meyers CM, Seeff LB, Stehman-Breen CO, et al. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631-657.
Naicker S, Fabian J, Naidoo S, et al. Infection and glomerulonephritis. Semin Immunopathol. 2007;29:397-414.
Nasr SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in the modern era. Medicine. 2008;87:21-32.
Neugarten J, Baldwin DS. Glomerulonephritis in bacterial endocarditis. Am J Med. 1984;77:297-304.
Ozdamar SO, Gucer S, Tinaztepe K. Hepatitis-B virus associated nephropathies: a clinicopathological study in 14 children. Pediatr Nephrol. 2003;181:23-28.
Ray PE, Xu L, Rakusan T, et al. A 20-year history of childhood HIV-associated nephropathy. Pediatr Nephrol. 2004;10:1075-1092.
Vella J, Carmody M, Campbell E, et al. Glomerulonephritis after ventriculo-atrial shunt. Q J Med. 1995;88:911-918.