Chapter 201 Bartonella
The spectrum of disease resulting from human infection with Bartonella species includes the association of bacillary angiomatosis and cat-scratch disease (CSD) with Bartonella henselae. Six major Bartonella species are pathogenic for humans: B. henselae, Bartonella quintana, Bartonella bacilliformis, Bartonella elizabethae, Bartonella vinsonii, and Bartonella clarridgeiae (Table 201-1). Several other Bartonella species have been found in animals, particularly rodents and moles.
Members of the genus Bartonella are gram-negative, oxidase-negative, fastidious aerobic rods that ferment no carbohydrates. B. bacilliformis is the only species that is motile, achieving motility by means of polar flagella. Optimal growth is obtained on fresh media containing 5% or more sheep or horse blood in the presence of 5% carbon dioxide. The use of lysis centrifugation for specimens from blood on chocolate agar for extended periods (2-6 wk) enhances recovery.
201.1 Bartonellosis (Bartonella bacilliformis)
The 1st human Bartonella infection described was bartonellosis, a geographically distinct disease caused by B. bacilliformis. There are 2 predominant forms of illness due to B. bacilliformis: Oroya fever, a severe, febrile hemolytic anemia, and verruca peruana (verruga peruana), an eruption of hemangioma-like lesions. B. bacilliformis also causes asymptomatic infection. Bartonellosis is also called Carrión disease in honor of the Peruvian medical student who inoculated himself with blood from a verruca and 21 days later had Oroya fever. He died 39 days after the inoculation, thus proving the unitary etiology of the 2 clinical illnesses.
Etiology
B. bacilliformis is a small, motile, gram-negative organism with a brush of 10 or more unipolar flagella, which appear to be important components for invasiveness. An obligate aerobe, it grows best at 28°C in semisolid nutrient agar containing rabbit serum and hemoglobin.
Epidemiology
Bartonellosis is a zoonosis found only in mountain valleys of the Andes Mountains in Peru, Ecuador, Colombia, Chile, and Bolivia at altitudes and environmental conditions favorable for the vector, which is the sandfly, Lutzomyia verrucarum.
Pathogenesis
After the sandfly bite, Bartonella organisms enter the endothelial cells of blood vessels, where they proliferate. Found throughout the reticuloendothelial system, they then re-enter the bloodstream and parasitize erythrocytes. They bind on the cells, deform the membranes, and then enter intracellular vacuoles. The resultant hemolytic anemia may involve as many as 90% of circulating erythrocytes. Patients who survive this acute phase may or may not experience the cutaneous manifestations, which are nodular hemangiomatous lesions or verrucae ranging in size from a few millimeters to several centimeters.
Clinical Manifestations
The incubation period is 2-14 wk. Patients may be totally asymptomatic or may have nonspecific symptoms such as headache and malaise without anemia.
Oroya fever is characterized by fever with rapid development of anemia. Clouding of the sensorium and delirium are common symptoms and may progress to overt psychosis. Physical examination demonstrates signs of severe hemolytic anemia, including icterus and pallor, sometimes in association with generalized lymphadenopathy.
In the pre-eruptive stage of verruca peruana (Fig. 201-1), patients may complain of arthralgias, myalgias, and paresthesias. Inflammatory reactions such as phlebitis, pleuritis, erythema nodosum, and encephalitis may develop. The appearance of verrucae is pathognomonic of the eruptive phase. Lesions vary greatly in size and number.
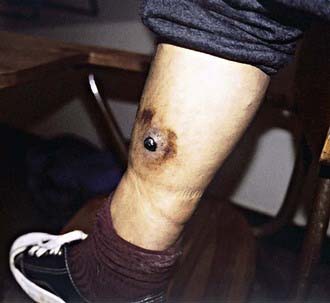
Figure 201-1 A single large lesion of verruca peruana on the leg of an inhabitant of the Peruvian Andes. Such lesions are prone to superficial ulceration, and their vascular nature may lead to copious bleeding. Ecchymosis of the skin surrounding the lesion is also evident.
(Courtesy of Dr. J.M. Crutcher, Oklahoma State Department of Health, Oklahoma City.)
Diagnosis
The diagnosis is established on clinical grounds in conjunction with a blood smear demonstrating organisms or with blood culture. The anemia is macrocytic and hypochromic, with reticulocyte counts as high as 50%. B. bacilliformis may be seen on Giemsa stain preparation as red-violet rods in the erythrocytes. In the recovery phase, organisms change to a more coccoid form and disappear from the blood. In the absence of anemia, the diagnosis depends on blood cultures. In the eruptive phase, the typical verruca confirms the diagnosis. Antibody testing has been used to document infection.
Treatment
B. bacilliformis is sensitive to many antibiotics, including rifampin, tetracycline, and chloramphenicol. Treatment is very effective in rapidly diminishing fever and eradicating the organism from the blood. Chloramphenicol (50-75 mg/kg/day) has been considered the drug of choice, because it is also useful in the treatment of concomitant infections such as Salmonella. Fluoroquinolones have been used successfully as well. Blood transfusions and supportive care are critical in patients with severe anemia. Antimicrobial treatment for verruca peruana is considered when there are >10 cutaneous lesions, if the lesions are erythematous or violaceous, or if the onset of the lesions was <1 mo before presentation. Oral rifampin is effective in the healing of lesions. Surgical excision may be needed for lesions that are large and disfiguring or that interfere with function.
Prevention
Prevention depends on avoidance of the vector, particularly at night, by the use of protective clothing and insect repellents (Chapter 168).
201.2 Cat-Scratch Disease (Bartonella henselae)
The most common presentation of Bartonella infection is CSD, which is a subacute, regional lymphadenitis caused by B. henselae. It is the most common cause of chronic lymphadenitis that persists for >3 wk.
Etiology
B. henselae can be cultured from the blood of healthy cats. B. henselae organisms are the small pleomorphic gram-negative bacilli visualized with Warthin-Starry stain in affected lymph nodes from patients with CSD. Development of serologic tests that showed prevalence of antibodies in 84-100% of cases of CSD, culturing of B. henselae from CSD nodes, and detection of B. henselae by polymerase chain reaction (PCR) in the majority of lymph node samples and pus from patients with CSD confirmed the organism as the cause of CSD. Occasional cases of CSD may be caused by other organisms; 1 report described a veterinarian with CSD caused by B. clarridgeiae.
Epidemiology
CSD is common, with more than 24,000 estimated cases per year in the USA. It is transmitted by cutaneous inoculation. Most (87-99%) patients have had contact with cats, many of which are kittens <6 mo of age, and >50% have a definite history of a cat scratch or bite. Cats have high-level Bartonella bacteremia for months without any clinical symptoms; kittens are more frequently bacteremic than adult cats. Transmission between cats occurs via the cat flea, Ctenocephalides felis. In temperate zones, the majority of cases occur between September and March, perhaps in relation to the seasonal breeding of domestic cats or to the close proximity of family pets in the fall and winter. In tropical zones, there is no seasonal prevalence. Distribution is worldwide, and infection occurs in all races.
Cat scratches appear to be more common among children, and boys are affected more often than girls. CSD is a sporadic illness; usually only 1 family member is affected, even though many siblings play with the same kitten. However, clusters do occur, with family cases within weeks of one another. Anecdotal reports have implicated other sources, such as dog scratches, wood splinters, fishhooks, cactus spines, and porcupine quills.
Pathogenesis
The pathologic findings in the primary inoculation papule and affected lymph nodes are similar. Both show a central avascular necrotic area with surrounding lymphocytes, giant cells, and histiocytes. Three stages of involvement occur in affected nodes, sometimes simultaneously in the same node. The first stage consists of generalized enlargement with thickening of the cortex and hypertrophy of the germinal center and with a predominance of lymphocytes. Epithelioid granulomas with Langhans giant cells are scattered throughout the node. The middle stage is characterized by granulomas that increase in density, fuse, and become infiltrated with polymorphonuclear leukocytes, with beginning central necrosis. In the final stage, necrosis progresses with formation of large pus-filled sinuses. This purulent material may rupture into surrounding tissue. Similar granulomas have been found in the liver, spleen, and osteolytic lesions of bone when those organs are involved.
Clinical Manifestations
After an incubation period of 7-12 days (range 3-30 days), 1 or more 3- to 5-mm red papules develop at the site of cutaneous inoculation, often reflecting a linear cat scratch. These lesions are often overlooked because of their small size but are found in at least 65% of patients when careful examination is performed (Fig. 201-2). Lymphadenopathy is generally evident within a period of 1-4 wk (Fig. 201-3). Chronic regional lymphadenitis is the hallmark, affecting the 1st or 2nd set of nodes draining the entry site. Affected lymph nodes in order of frequency include the axillary, cervical, submandibular, preauricular, epitrochlear, femoral, and inguinal nodes. Involvement of more than 1 group of nodes occurs in 10-20% of patients, although at a given site, half the cases involve several nodes.
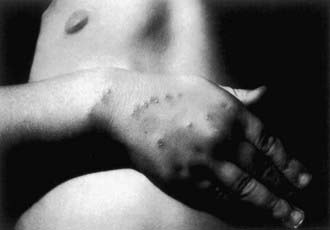
Figure 201-2 A child with typical cat-scratch disease demonstrating the original scratch injuries and the primary papule that soon thereafter developed proximal to the middle finger.
(Courtesy of Dr. V.H. San Joaquin, University of Oklahoma Health Sciences Center, Oklahoma City.)
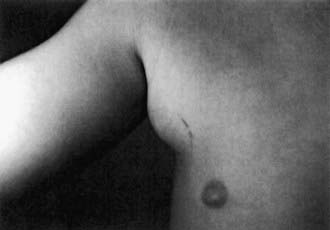
Figure 201-3 Right axillary lymphadenopathy followed the scratches and development of a primary papule in this child with typical cat-scratch disease.
(From Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, ed 6, vol 2, Philadelphia, 2006, Elsevier, p 2737.)
Nodes involved are usually tender and have overlying erythema but without cellulitis. They usually range between 1 and 5 cm in size, although they can become much larger. Between 10% and 40% eventually suppurate. The duration of enlargement is usually 1-2 mo, with persistence up to 1 yr in rare cases. Fever occurs in about 30% of patients, usually 38-39°C. Other nonspecific symptoms, including malaise, anorexia, fatigue, and headache, affect less than a third of patients. Transient rashes, which may occur in about 5% of patients, are mainly truncal maculopapular rashes. Erythema nodosum, erythema multiforme, and erythema annulare are also reported.
CSD is usually a self-limited infection that spontaneous resolves within a few weeks to months. The most common atypical presentation is Parinaud oculoglandular syndrome, which is unilateral conjunctivitis followed by preauricular lymphadenopathy and occurs in 2-17% of patients with CSD (Fig. 201-4). Direct eye inoculation as a result of rubbing with the hands after cat contact is the presumed mode of spread. A conjunctival granuloma may be found at the inoculation site. The involved eye is usually not painful and has little or no discharge but may be quite red and swollen. Submandibular or cervical lymphadenopathy may also occur.
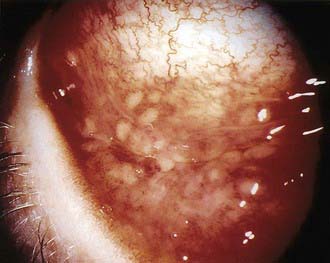
Figure 201-4 The granulomatous conjunctivitis of Parinaud oculoglandular syndrome is associated with ipsilateral local lymphadenopathy, usually preauricular and less commonly submandibular.
(From Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, ed 6, vol 2, Philadelphia, 2006, Elsevier, p 2739.)
More severe, disseminated illness occurs in a small percentage of patients and is characterized by presentation with high fever, often persisting for several weeks. Other prominent symptoms include significant abdominal pain and weight loss. Hepatosplenomegaly may occur, although hepatic dysfunction is rare (Fig. 201-5). Granulomatous changes may be seen in the liver and spleen. Another common site of dissemination is bone, with the development of granulomatous osteolytic lesions, associated with localized pain but without erythema, tenderness, or swelling. Other uncommon manifestations are neuroretinitis with papilledema and stellate macular exudates, encephalitis, fever of unknown origin, and atypical pneumonia.
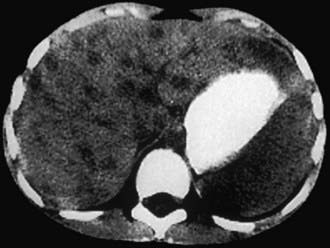
Figure 201-5 In this CT scan of a patient with hepatic involvement of cat-scratch disease, the absence of enhancement of the multiple lesions after contrast infusion is consistent with the granulomatous inflammation of this entity. Treated empirically with various antibiotics without improvement before establishment of this diagnosis, the patient subsequently recovered fully with no further antimicrobial therapy.
(Courtesy of Dr. V.H. San Joaquin, University of Oklahoma Health Sciences Center, Oklahoma City.)
Diagnosis
In most cases, the diagnosis can be strongly suspected on clinical grounds in a patient with history of exposure to a cat. The U.S. Centers for Disease Control and Prevention (CDC) has developed an indirect immunofluorescent assay (IFA) that has shown good correlation with disease. Other IFA and enzyme-linked immunoassay tests are commercially available, although little comparative data are available. Most patients have elevated antibody titers at presentation; however, the timing of immunoglobulin G (IgG) and IgM response to B. henselae can be quite variable. There is cross reactivity among Bartonella species, particularly B. henselae and B. quintana.
If tissue specimens are obtained, bacilli may be visualized with Warthin-Starry and Brown-Hopps tissue stains. Bartonella DNA can be identified through PCR analysis of tissue specimens. Culturing of the organism is not generally practical for clinical diagnosis.
Differential Diagnosis
The differential diagnosis of CSD includes virtually all causes of lymphadenopathy (Chapter 490). The more common entities include pyogenic lymphadenitis, primarily from staphylococcal or streptococcal infections, atypical mycobacterial infections, and malignancy. Less common entities are tularemia, brucellosis, and sporotrichosis. Epstein-Barr virus, cytomegalovirus, and Toxoplasma gondii infections usually cause more generalized lymphadenopathy.
Laboratory Findings
Routine laboratory tests are not helpful. The erythrocyte sedimentation rate is often elevated. The white blood cell count may be normal or mildly elevated. Hepatic transaminases may be elevated in systemic disease. Ultrasonography or CT may reveal many granulomatous nodules in the liver and spleen; the nodules appear as hypodense round irregular lesions.
Treatment
Antibiotic treatment of CSD is not always needed and is not clearly beneficial. For most patients, treatment consists of conservative symptomatic care and observation. Studies show a significant discordance between in vitro activity of antibiotics and clinical effectiveness. For many patients, diagnosis is considered in the context of failure to respond to β-lactam antibiotic treatment of presumed staphylococcal lymphadenitis.
A small prospective study of oral azithromycin (500 mg on day 1, and then 250 mg on days 2-5; for smaller children, 10 mg/kg/24hr on day 1 and 5 mg/kg/24hr on days 2-5) showed a decrease in initial lymph node volume in 50% of patients during the 1st 30 days, but after 30 days there was no difference in lymph node volume. No other clinical benefit was found. For the majority of patients, CSD is self-limited, and resolution occurs over weeks to months without antibiotic treatment. Azithromycin, clarithromycin, trimethoprim-sulfamethoxazole, rifampin, ciprofloxacin, and gentamicin appear to be the best agents if treatment is considered.
Suppurative lymph nodes that become tense and extremely painful should be drained by needle aspiration, which may need to be repeated. Incision and drainage of nonsuppurative nodes should be avoided because chronic draining sinuses may result. Surgical excision of the node is rarely necessary.
Children with hepatosplenic CSD appear to respond well to rifampin at a dose of 20 mg/kg for 14 days, either alone or in combination with trimethoprim-sulfamethoxazole.
Complications
Encephalopathy, which can occur in as many as 5% of patients with CSD, typically manifests 1-3 wk after the onset of lymphadenitis as the sudden onset of neurologic symptoms, which often include seizures, combative or bizarre behavior, and altered level of consciousness. Imaging studies are generally normal. The cerebrospinal fluid is normal or shows minimal pleocytosis and protein elevation. Recovery occurs without sequelae in nearly all patients but may take place slowly over many months.
Other neurologic manifestations include peripheral facial nerve paralysis, myelitis, radiculitis, compression neuropathy, and cerebellar ataxia. One patient has been reported to have encephalopathy with persistent cognitive impairment and memory loss.
Stellate macular retinopathy has been associated with several infections, including CSD. Children and young adults present with unilateral or rarely bilateral loss of vision with central scotoma, optic disc swelling, and macular star formation from exudates radiating out from the macula. The findings usually resolve completely, with recovery of vision, generally within 2-3 mo. The optimal treatment for the neuroretinitis is unknown, although treatment of adults with doxycycline and rifampin for 4-6 wk has had good results.
Hematologic manifestations include hemolytic anemia, thrombocytopenic purpura, nonthrombocytopenic purpura, and eosinophilia. Leukocytoclastic vasculitis similar to Henoch-Schönlein purpura has been reported in association with CSD in 1 child. A systemic presentation of CSD with pleurisy, arthralgia or arthritis, mediastinal masses, enlarged nodes at the head of the pancreas, and atypical pneumonia also has been reported.
Prognosis
The prognosis for CSD in a normal host is generally excellent, with resolution of clinical findings over weeks to months. Recovery is occasionally slower and may take as long as a year.
Prevention
Person-to-person spread of Bartonella infections is not known. Isolation of the affected patient is not necessary. Prevention would require elimination of cats from households, which is not practical or necessarily desirable. Awareness of the risk of cat (and particularly kitten) scratches should be emphasized to parents.
Arisoy ES, Correa AG, Wagner ML, et al. Hepatosplenic cat-scratch disease in children: selected clinical features and treatment. Clin Infect Dis. 1999;28:778-784.
Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452.
Batts S, Demers DM. Spectrum and treatment of cat-scratch disease. Pediatr Infect Dis J. 2004;23:1161-1162.
Carithers HA, Margileth AM. Cat scratch disease: acute encephalopathy and other neurologic manifestations. Am J Dis Child. 1991;145:98-101.
Fournier PE, Lelievre H, Ekyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine. 2001;80:245-251.
Jacobs R, Schutze G. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998;26:80-84.
Metzkor-Cotter E, Kletter Y, Avidor B, et al. Long-term serological analysis and clinical follow-up of patients with cat scratch disease. Clin Infect Dis. 2003;37:1149-1154.
Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol. 1999;10:209-216.
201.3 Trench Fever (Bartonella quintana)
Etiology
The causative agent of trench fever was first designated Rickettsia quintana, was then assigned to the genus Rochalimaea, and now has been reassigned as B. quintana.
Epidemiology
Trench fever was first recognized as a distinct clinical entity during World War I, when more than a million troops in the trenches were infected. The disease became quiescent until World War II, when it again was epidemic. It is extremely rare in the USA.
Humans are the only known reservoir. No other animal is naturally infected, and usual laboratory animals are not susceptible. The human body louse, Pediculus humanus var. corporis, is the vector and is capable of transmission to a new host 5-6 days after feeding on an infected person. Lice excrete the organism for life; transovarian passage does not occur. Humans may have prolonged asymptomatic bacteremia for years.
Clinical Manifestations
The incubation period for trench fever averages about 22 days (range 4-35 days). The clinical presentation is highly variable. Symptoms can be very mild and brief. About half of infected persons have a single febrile illness with abrupt onset lasting 3-6 days. In other patients, prolonged, sustained fever may occur. More commonly, patients have periodic febrile illness with 3 to 8 episodes lasting 4-5 days each, sometimes occurring over a period of a year or more. This form is reminiscent of malaria or relapsing fever (Borrelia recurrentis). Afebrile bacteremia can occur.
Clinical findings usually consist of fever (typically with a temperature of 38.5-40°C), malaise, chills, sweats, anorexia, and severe headache. Common findings include marked conjunctival injection, tachycardia, myalgias, arthralgias, and severe pain in the neck, back, and legs. Crops of erythematous macules or papules may occur on the trunk in as many as 80% of patients. Splenomegaly and mild liver enlargement may be noted.
Diagnosis
In non-epidemic situations, it is impossible to establish a diagnosis of trench fever on clinical grounds, because the findings are not distinctive. A history of body louse infection or having been in an area of epidemic disease should heighten suspicions. B. quintana can be cultured from the blood with modification to include culture on epithelial cells. Serologic tests for B. quintana are available, but there is cross reaction with B. henselae.
201.4 Bacillary Angiomatosis and Bacillary Peliosis Hepatis (Bartonella henselae and Bartonella quintana)
Both B. henselae and B. quintana cause vascular proliferative disease called bacillary angiomatosis (BA) and bacillary peliosis in severely immunocompromised persons, primarily adult patients with AIDS or cancer and organ transplant recipients. Subcutaneous and lytic bone lesions are strongly associated with B. quintana, whereas peliosis hepatis is associated exclusively with B. henselae.
Bacillary Angiomatosis
Lesions of cutaneous BA, also known as epithelioid angiomatosis, are the most easily identified and recognized form of Bartonella infection in immunocompromised hosts. They are found primarily in patients with AIDS who have very low CD4 counts. The clinical appearance can be quite diverse. The vasoproliferative lesions of BA may be cutaneous or subcutaneous and may resemble the vascular lesions (verruca peruana) of B. bacilliformis in immunocompetent persons, characterized by erythematous papules on an erythematous base with a collarette of scale. They may enlarge to form large pedunculated lesions and may ulcerate. Trauma may result in profuse bleeding.
BA may be clinically indistinguishable from Kaposi sarcoma. Other considerations in the differential diagnosis are pyogenic granuloma and verruca peruana (B. bacilliformis). Deep soft tissue masses caused by BA may mimic a malignancy.
Osseous BA lesions commonly involve the long bones. These lytic lesions are very painful and highly vascular and are occasionally associated with an overlying erythematous plaque. The high degree of vascularity produces a very positive result on a technetium-Tc 99m methylene diphosphonate bone scan, resembling that of a malignant lesion.
Lesions can be found in virtually any organ, producing similar vascular proliferative lesions. They may appear raised, nodular, or ulcerative when seen on endoscopy or bronchoscopy. They may be associated with enlarged lymph nodes with or without an obvious local cutaneous lesion. Brain parenchymal lesions have been described.
Bacillary Peliosis
Bacillary peliosis affects the reticuloendothelial system, primarily the liver (peliosis hepatis) and less frequently the spleen and lymph nodes. It is a vasoproliferative disorder characterized by random proliferation of venous lakes surrounded by fibromyxoid stroma harboring numerous bacillary organisms. Clinical findings include fever and abdominal pain in association with abnormal results of liver function tests, particularly a markedly increased alkaline phosphatase level. Cutaneous BA or splenomegaly may be associated, with or without thrombocytopenia or pancytopenia. The vascular proliferative lesions in the liver and spleen appear on CT scan as hypodense lesions scattered throughout the parenchyma. The differential diagnosis includes hepatic Kaposi sarcoma, lymphoma, and disseminated infection with Pneumocystis carinii or Mycobacterium avium complex.
Bacteremia and Endocarditis
B. henselae, B. quintana, B. vinsonii, and B. elizabethae all have been reported to cause bacteremia or endocarditis. They are associated with symptoms such as prolonged fevers, night sweats, and profound weight loss. A cluster of cases in Seattle in 1993 occurred in a homeless population with chronic alcoholism. These patients with high fever or hypothermia were thought to represent “urban trench fever,” but no body louse infestation was associated. Some cases of culture-negative endocarditis may represent Bartonella endocarditis. One report described central nervous system involvement with B. quintana infection in 2 children.
Diagnosis
Diagnosis of BA is made initially by biopsy. The characteristic small vessel proliferation with mixed inflammatory response and the staining of bacilli by Warthin-Starry silver staining distinguish BA from pyogenic granuloma or Kaposi sarcoma (Chapter 254). Travel history can usually preclude verruca peruana.
Culture is impractical for CSD but is the diagnostic procedure for suspected bacteremia or endocarditis. Use of the lysis centrifugation technique or fresh chocolate or heart infusion agar with 5% rabbit blood with prolonged incubation may increase the yield of culture. PCR can also be a useful tool.
Treatment
Bartonella infections in immunocompromised hosts caused by both B. henselae and B. quintana have been treated successfully with antimicrobial agents. BA responds rapidly to erythromycin, azithromycin, and clarithromycin, which are the drugs of choice. Alternative choices are doxycycline or tetracycline. Severely ill patients with peliosis hepatis, endocarditis, or osteomyelitis may be treated initially with intravenous erythromycin or doxycycline and the addition of rifampin or gentamicin. The use of an aminoglycoside for a minimum of 2 wk is associated with improved prognosis in endocarditis. A Jarisch-Herxheimer reaction may occur. Relapses may follow, and prolonged treatment for several months may be necessary.
Prevention
Immunocompromised persons should consider the potential risks of cat ownership because of the risks for Bartonella infections as well as toxoplasmosis and enteric infections. Those who elect to obtain a cat should adopt or purchase a cat >1 yr of age and in good health. Prompt washing of any wounds from cat bites or scratches is essential.
Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008;121:e1413-e1425.
Inoue K, Maruyama S, Kabeta H, et al. Exotic small mammals as potential reservoirs of zoonotic Bartonella spp. Emerg Infect Dis. 2009;15:526-532.
Rolain JM, Brouqui P, Koehler JE, et al. Recommendations for the treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921-1933.
