Chapter 391 Parenchymal Disease with Prominent Hypersensitivity, Eosinophilic Infiltration, or Toxin-Mediated Injury
391.1 Hypersensitivity to Inhaled Materials
Extrinsic allergic alveolitis or hypersensitivity pneumonitis (HP) is an immunologically mediated diffuse inflammatory disease of the pulmonary interstitium caused by inhalation of a variety (>200) of different organic antigens. Antigens are typically of animal or vegetable origin and ∼1-5 µm in size and therefore deposit in the alveoli. Reactive antigens, such as a variety of drugs, can occasionally cause HP.
Etiology
Generally an adult disease due to antigen exposure, HP has been reported in children and even infants. There are many types of HP, each related to the offending antigen. The most common forms in the US include farmer’s lung (from moldy hay; antigen is thermophilic actinomycetes), bird fancier or breeder lung (from the feces or urine of a wide variety of bird and animal species), and ventilation or humidifier HP (from contaminated humidifiers or air conditioners; also caused by thermophilic actinomycetes). Exposure to pigeons (pigeon breeder disease) is the most common form in Turkey and Mexico, among other countries. Most case reports of HP in children have been the result of antigens inhaled during avian exposure including family pets and feather filled bedding (pillows, quilts) or exposure to molds. HP may occur after exposure to only one bird.
Pathology and Pathogenesis
HP can present as an acute, subacute, or chronic illness. These forms can be distinguished based on the morphologic features of the pulmonary involvement and the clinical symptoms. In the acute stage, alveolar walls are infiltrated by neutrophils, lymphocytes, plasma cells, and macrophages. The alveolar lumen may contain a proteinaceous exudate mixed with inflammatory cells. Repeated or continuous exposure may result in a subacute presentation characterized by the classic noncaseating granuloma associated with HP. Often, there is an associated terminal and respiratory bronchiolitis; alveoli may have pale foamy macrophages. The chronic form demonstrates further progression of the granulomatous alveolitis, resulting in interstitial fibrosis and honeycombing, primarily affecting the upper lobes. This classification of HP has been questioned as to whether it truly reflects distinct categories of the disease. The distinction between these stages may represent variable responses to the offending antigens and there may be considerable overlap in clinical manifestations.
Exposure to avian serum proteins on feathers or bird droppings produces an inflammatory response. The immune mechanisms involved with inducing these morphologic changes may include immune complex (type III) hypersensitivity (particularly in the acute presentation), delayed cellular (type IV) hypersensitivity, and the alternate complement pathway. Only a small percentage of exposed individuals actually experience clinical symptoms, suggesting an interaction between the nature of the offending antigen, the intensity and duration of exposure, and genetic factors in determining the host response.
Clinical Manifestations
Acute attacks usually occur 4-8 hr after an exposure. Symptoms include fever, chills, cough, dyspnea, myalgia, and malaise that can persist for up to 48 hr. Physical examination usually reveals an ill-appearing, dyspneic child with bibasilar crackles, wheezing, or a normal lung examination. Chest radiographs may also be normal or may demonstrate bilateral ground-glass haziness, often sparing the lung apices and bases, with fine nodulations (Fig. 391-1). These patients may progress to the subacute presentation if exposure continues or recurs. The cough worsens, dyspnea becomes more prominent, and anorexia and weight loss may occur. The chest radiograph at this stage has a more reticulonodular appearance. Long-term exposure can lead to the chronic presentation. Dyspnea and cough are severe; clubbing may be present, as are weight loss, weakness, and hypoxemia. These patients eventually develop chronic alveolitis and fibrosis that can lead to cor pulmonale. Chest radiographs show coarse reticulonodular infiltrates and bronchiectasis primarily in the upper- and mid-lung zones. High-resolution computed tomography (HRCT) scans may be more sensitive in demonstrating bronchiectasis.
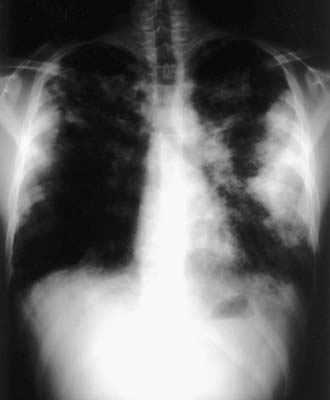
Figure 391-1 Chest radiograph shows bilateral patchy alveolar infiltrate with peripheral consolidation.
(From Wubbel C, Fulmer D, Sherman J: Chronic eosinophilic pneumonia: a case report and national survey, Chest 123:1763–1766, 2003.)
When removed from the antigenic environment (occupational exposure), the patient will improve (on weekends or days off) only to have recurrent symptoms on reexposure.
Diagnosis
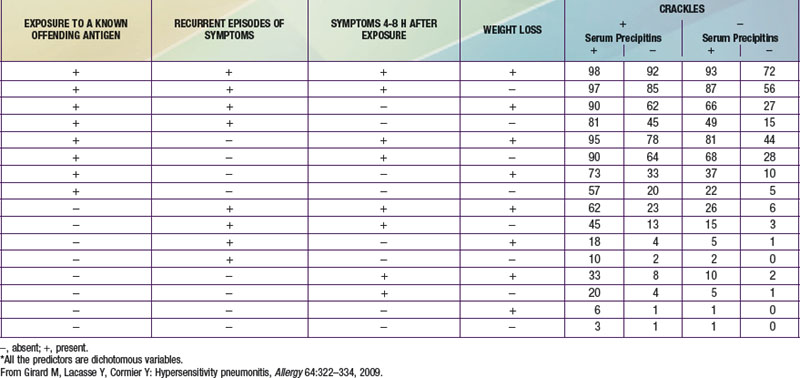
The diagnosis of HP is based primarily on the clinical presentation in association with a suspicious exposure. Because the clinical presentation is nonspecific, a high index of suspicion is crucial. Children with HP will meet most of the basic diagnostic criteria that have been proposed for adults. A variety of diagnostic criteria recommendations have been proposed. Major criteria include symptoms compatible with HP, evidence of exposure (antibody studies), compatible chest x-ray or HRCT findings, bronchoalveolar lavage (BAL) lymphocytosis, histologic changes compatible with HP, and a positive antigen provocation challenge. Minor criteria include bibasilar crackles, decreased diffusion capacity, and hypoxemia. The presence of 4 major and 2 minor criteria are very suggestive of HP, especially when other diseases with similar presentations have been excluded. A number of laboratory tests may also be helpful in confirming a strong clinical suspicion. HP patients often demonstrate a modest leukocytosis with neutrophilia with a left shift, and modest elevation of the erythrocyte sedimentation rate. Serum levels of immunoglobulins (IgG, IgM, and IgA) are often elevated. Skin testing to particular antigens lacks sensitivity and specificity, as do serum precipitins to specific antigens. Both these tests may indicate exposure but are often positive in individuals without the clinical disease. In adults, BAL fluid typically demonstrates a marked lymphocytosis (often up to 70%) particularly of the CD8+ suppressor T cells, although in children the CD4 : CD8 ratio is not increased. BAL fluid may also contain higher levels of immunoglobulins. Pulmonary function tests classically demonstrate a restrictive pattern with impaired gas exchange (diffusion capacity). The presence of a mild obstructive pattern during the acute stage is a poor prognostic indicator. Some have advocated an inhalational challenge either in the laboratory or by re-exposure to the environment. Challenge testing can be dangerous and therefore should be undertaken only in appropriately equipped diagnostic centers. A diagnostic algorithm is proposed in Figure 391-2. Table 391-1 provides criteria for estimating the probability of HP; an individual living on a farm presenting with recurrent episodes of respiratory symptoms, inspiratory crackles and testing positive for the corresponding precipitating antibodies, would have an 81% chance of having HP, while an individual with progressive dyspnea and inspiratory crackles only would have a probability of HP of less than 1%.
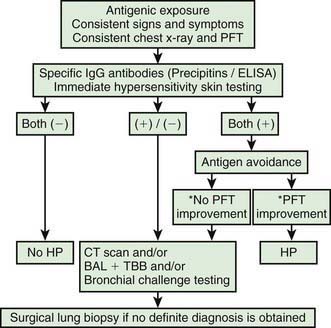
Figure 391-2 Diagnostic algorithm for hypersensitivity pneumonitis. *PFT improvement: ≥ 20% increase of FVC, FEV 1, and/or DLCO in PFTs. BAL, bronchoalveolar lavage; CT, computed tomography; DLCO, carbon monoxide diffusing capacity; ELISA, enzyme-linked immunosorbent assay; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HP, hypersensitivity pneumonitis; PFT, pulmonary function test; TBB, transbroncheal biopsy.
(Modified from Morell F, Roger A, Reyes L, et al: Bird fancier’s lung: a series of 86 patients, Medicine 87:110–130, 2008.)
The differential diagnosis includes asthma, sarcoidosis, idiopathic pulmonary fibrosis and other causes of interstitial lung disease.
Treatment
The most effective therapeutic approach is complete avoidance of the suspected antigen. Prednisone (1-2 mg/kg/24 hr) is of some benefit, but an extended course, perhaps up to 6-12 mo, is required. In general, prognosis is quite good, but if exposure persists, pulmonary fibrosis may occur and patients may not completely recover their baseline lung function.
Ceviz N, Kaynar H, Olgun H, et al. Pigeon breeder’s lung in childhood: is family screening necessary? Pediatr Pulmonol. 2006;41:279-282.
Engelhart S, Rietschel E, Exner M, et al. Childhood hypersensitivity pneumonitis associated with fungal contamination of indoor hydroponics. Int J Hyg Environ Health. 2009;212:18-20.
Girard M, Lacasse Y, Cormier Y. Hypersensitivity pneumonitis. Allergy. 2009;64:322-334.
Hanak V, Globin JM, Ryu JH. Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc. 2007;82:812-816.
Kurup VP, Zacharisen MC, Fink JN. Hypersensitivity pneumonitis. Indian J Chest Dis Allied Sci. 2006;48:115-128.
Lacasse Y, Selman M, Costabel U, et al. Classification of hypersensitivity pneumonitis. Int Arch Allergy Immunol. 2009;149:161-166.
Morell F, Roger A, Reyes L, et al. Bird fancier’s lung: a series of 86 patients. Medicine. 2008;87:110-130.
Natarjan A, Sutton P, Spencer DA, et al. Pigeon fancier’s lung in childhood. Respiratory Medicine Extra. 2006;2:71-73.
Stauffer Ettlin M, Pache JC, Renevey F, et al. Bird breeder’s disease: a rare diagnosis in young children. Eur J Pediatr. 2006;165:55-61.
Venkatesh P, Wild L. Hypersensitivity pneumonitis in children. Pediatr Drugs. 2005;7:235-244.
391.2 Silo Filler Disease
Silo filler disease (also referred as silage gas poisoning or silo filler pneumoconiosis) is typically caused by nitrogen dioxide toxicity. Nitrogen dioxide is produced in silos (particularly corn silos) within a few hr of filling and reaches a maximum concentration within about 2 days. Dangerous concentrations of gas can remain in a closed silo for as long as 2 wk. After entering a silo within this time frame without proper protection, a person may experience various degrees of silo filler disease.
Pathogenesis
The degree of lung injury depends on the duration of exposure and the concentration of nitrogen dioxide. Shortly after inhalation, nitrogen dioxide is hydrolyzed to nitrous and nitric acid, causing chemical pneumonitis and pulmonary edema, primarily affecting type I pneumocytes and ciliated cells lining the airways. Biopsy or autopsy findings include diffuse pulmonary edema, diffuse alveolar damage, hyperplastic airway epithelium, widened intralobular septa, and bronchiolitis obliterans. With severe exposures, methemoglobinemia can occur. Alteration of macrophage activity and immune function can also occur, resulting in increased risk of infection.
Clinical Manifestations and Diagnosis
Most exposures result in mild symptoms that are self-limited. Cough and dyspnea typically occur immediately on entering the silo and are often associated with wheezing, nausea, a choking sensation, ocular irritation, and fatigue. Rarely, symptoms are delayed days or even weeks after exposure. Radiologic manifestations include pulmonary edema in the perihilar areas and bases. Some patients recover and the pulmonary edema clears rapidly. In others, this initial phase is followed by a period of remission for 2-3 wk, which then gives way to a 2nd phase characterized by fever, progressive dyspnea, cyanosis, and cough associated with widespread, scattered miliary opacities that can become confluent and take on a more patchy and nodular appearance. These patients may suffer from methemoglobinemia. Complications include secondary infection and bronchiolitis obliterans. Diagnosis necessitates a careful medical and occupational history, as specific laboratory confirmation of silo filler disease is unavailable. Cows can also be affected.
Treatment
Prevention of silo filler disease by minimizing exposure during the initial 2 wk after filling is key. Treatment is primarily supportive. Although no controlled trial has been conducted, high-dose prednisone (up to 30 mg/kg/day) is of some benefit in reducing the severity and duration of the symptoms. Bronchodilators may also be of some limited benefit. Methemoglobinemia is treated with methylene blue in severe cases. Nitric oxide therapy has been reported to improve oxygenation in patients with silo filler disease. Silo filler disease can be fatal, result in complete recovery, or leave patients with chronic lung disease associated with fibrosis and emphysema.
Meulenbelt J. Nitrogen and nitrogen oxides. Medicine. 2007;35:638.
Rasmussen MD, Bascom R. Silo filler’s disease. www.emedicine.com.
Tanaka N, Emoto T, Matsumoto T, et al. Inhalational lung injury due to nitrogen dioxide: high resolution computed tomography findings in 3 patients. JCAT. 2007;31:808-811.
391.3 Paraquat Lung
Paraquat is the most toxic dipyridilium herbicide. Concentrated solutions (12-20%) tend to be more dangerous than dilute solutions. Its toxic effects result from the production of superoxides and other highly reactive free radicals that cause the peroxidation of cell membranes and selective mitochondrial damage, resulting in cell death. Paraquat selectively concentrates in the lungs because of an amine uptake process that exists in alveolar epithelial cells. Additionally, paraquat-induced injury is significantly increased in the presence of high concentrations of oxygen. Although its use is banned or restricted in some countries, paraquat is still used extensively, particularly in many developing and transitional countries including tourist destinations. Most cases of paraquat intoxication are self-inflicted (suicide attempts). There have been case reports of fetal poisoning after maternal ingestion of paraquat (readily crosses placenta) with poor prognosis for the fetus.
Pathophysiologically, there is direct injury to the alveolar-capillary membrane and surfactant loss, acute respiratory distress syndrome, progressive intra-alveolar pulmonary fibrosis, and respiratory failure.
Clinical Manifestations
There are 3 degrees of paraquat intoxication that are dose related. Patients with mild poisoning after ingestion of <20 mg of paraquat ion/kg body weight are usually asymptomatic or may develop vomiting, diarrhea, and mild impairment of gas exchange. Moderate to severe poisoning follows ingestion of 20-50 mg of paraquat ion/kg. These patients typically suffer from vomiting, diarrhea, and caustic injury to the oral or esophageal mucosa and may develop renal failure, hepatic dysfunction, and progressive pulmonary fibrosis, resulting in respiratory failure and death. Acute fulminant poisoning follows ingestion of >50 mg of paraquat ion/kg. Marked ulceration of the oropharynx and esophagus occurs, with multiorgan failure and 100% mortality within hours of ingestion. Measuring the plasma paraquat concentration may be the best marker of exposure and severity (paraquat nomograms) and for predicting prognosis, although the urine dithionite test (turns urine blue if paraquat is present) has been used, as well.
Treatment
Treatment is generally supportive because no antidote exists. Gastric lavage, activated charcoal, hemoperfusion, and hemodialysis are of limited value in significant poisoning. There have been rare individual case reports of successful treatment of paraquat poisoning with antioxidant therapy consisting of deferoxamine infusion and acetylcysteine. Others have reported some success with combination immunosuppressive therapy consisting of intravenous corticosteroids and cyclophosphamide or prolonged repeated pulse corticosteroid therapy. Results after lung transplantation are poor, likely related to long-term storage of paraquat in muscle and eventual reaccumulation in the transplanted lungs.
Overall prognosis is poor. Survivors generally suffer from a persistent restrictive defect with impaired diffusion capacity on tests of pulmonary function.
Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11:387-391.
Chomchai C, Tiawilai A. Fetal poisoning after maternal paraquat ingestion during third trimester of pregnancy: case report and literature review. J Med Toxicol. 2007;3:182-186.
Dinis-Oliveira RJ, Sarmento A, Reis P, et al. Acute paraquat poisoning: report of a survival case following intake of a potential lethal dose. Pediatr Emerg Care. 2006;22:537-540.
Gil HW, Kang MS, Yang JO, et al. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clinl Toxicol. 2008;46:513-518.
Hong KH, Jung JH, Eo EK. A case of moderate paraquat intoxication with pulse therapy in the subacute stage of pulmonary fibrosis. J Korean Soc Clin Toxicol. 2008;6:130-133.
Lin JL, Lin-Tan DT, Chen KH, et al. Repeated pulse of methylprednisone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34:368-373.
Pinto Pereira LM, Boysielal K, Siung-Chang A. Pesticide regulation, utilization, and retailers’ selling practices in Trinidad and Tobago, West Indies: current situation and needed changes. Rev Panam Salud Publica. 2007;22:83-90.
Senarathna L, Eddleston M, Wilks MF, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251-259.
391.4 Eosinophilic Lung Disease
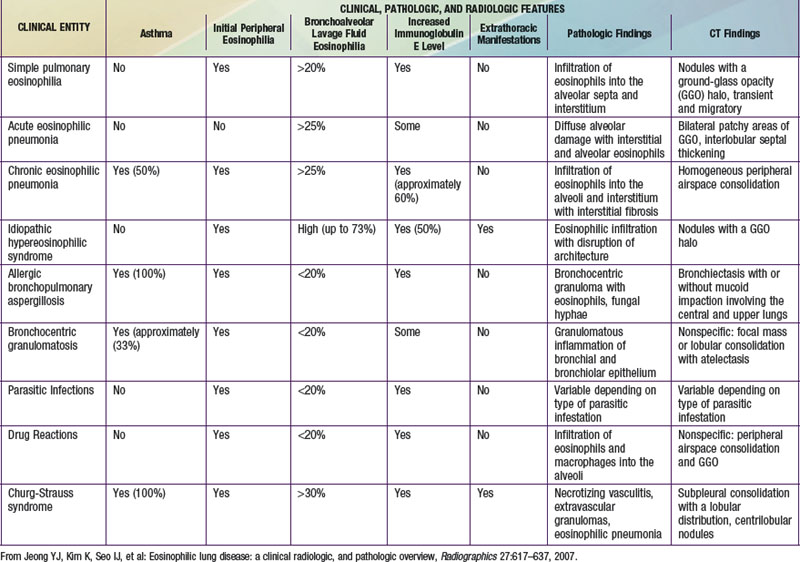
The findings of pulmonary infiltrates and circulating or tissue eosinophilia describe the heterogenous group of disorders referred to as eosinophilic lung diseases or pulmonary infiltrates with eosinophilia (PIE) (see Table 391-2 the Nelson Textbook of Pediatrics website at www.expertconsult.com). There are numerous classification schemes for these types of lung disease. PIE syndromes can be divided into primary (idiopathic) and secondary eosinophilic lung diseases. Primary eosinophilic lung diseases include simple pulmonary eosinophilia (Löffler syndrome), acute eosinophilic pneumonia, chronic eosinophilic pneumonia, and idiopathic hypereosinophilic syndrome. Secondary eosinophilic lung diseases include tropical pulmonary eosinophilia, pulmonary eosinophilia with asthma, polyarteritis nodosa, Churg-Strauss syndrome, allergic bronchopulmonary aspergillosis (ABPA), and drug-induced eosinophilic lung disease. Additional lung diseases such as idiopathic pulmonary fibrosis, Langerhans cell granuloma, and other interstitial lung diseases may have associated eosinophilia but are better classified elsewhere.
Löffler syndrome, the most common PIE syndrome reported in children, is characterized by migrating pulmonary infiltrates accompanied by peripheral blood eosinophilia but minimal respiratory symptoms. This term is rarely used today, and it is likely that most patients with this diagnosis have allergic bronchopulmonary helminthiasis (parasites), medication reactions, or ABPA.
Epidemiology
PIE syndromes appear to be much less common in the pediatric population than in adults. In one series, only 6% of cases occurred in persons <20 yr of age. There have been scattered case reports of acute eosinophilic pneumonia and chronic eosinophilic pneumonia in children. Children previously classified as having Löffler syndrome make up the majority of pediatric cases. Most pediatric case reports suggest an equal number of males and females being affected; there may be geographic differences because 1 group reports a female preponderance in children, similar to adult patients with PIE syndromes. Age at presentation ranges from infancy to adolescence.
Pathology and Pathogenesis
In the pediatric population, the most common etiology of PIE syndromes includes parasite infections and drug reactions. The prevalence of individual parasite infections varies geographically. The most common parasite causing PIE syndromes in the USA is Ascaris lumbricoides (Chapter 283). The eggs are ingested. After the larvae hatch, they pass through the intestinal wall and migrate to the lungs, causing an intense inflammatory reaction. Alveolar macrophages, lymphocytes, neutrophils, and eosinophils are the most striking inflammatory cells. Other common parasites include Strongyloides species, Toxocara canis (dog roundworm, visceral larva migrans), and Ancylostoma braziliense (“creeping eruption”). In Africa, South America, and Southeast Asia, the filarial worms Wuchereria bancrofti and Brugia malayi cause tropical pulmonary eosinophilia.
Several drugs have also been reported to cause PIE syndromes. Sulfasalazine, penicillin, ampicillin, ibuprofen, and cromolyn are a few of these drugs that are of common use in pediatric patients. Immunologic mechanisms may be the means by which these drugs result in an intense pulmonary inflammatory reaction.
Clinical Manifestations
Patients with PIE syndromes caused by parasites or drugs present with malaise, chronic cough, intermittent fevers, dyspnea, wheezing, and, occasionally, abdominal pain, rash, and weight loss. Symptom duration varies. In acute eosinophilic pneumonia, symptoms are usually present for less than a month and typically do not recur once treated. In chronic eosinophilic pneumonia, symptoms are present for more than 6 mo and often recur despite successful treatment. Results of physical examination vary but often show tachypnea, crackles, and wheezing.
Diagnosis
The diagnosis of a PIE syndrome is made by clinical manifestations with associated blood eosinophilia and chest radiographic findings. Radiologically, these patients present with nonspecific interstitial, alveolar, or mixed infiltrates. The infiltrates tend to be bilateral and diffuse. Patients with chronic eosinophilic pneumonia demonstrate peripheral infiltrates with central sparing (“photographic negative of pulmonary edema”). Patients with acute eosinophilic pneumonia may not have peripheral (blood) eosinophilia. In addition, eosinophilic lung diseases can be diagnosed by the presence of pulmonary infiltrates and eosinophilia on bronchoalveolar lavage or the presence of parasitic larvae in bronchoscopic or gastric lavage. Lung biopsy can be used to make the diagnosis.
Treatment
Therapy varies with the type of PIE syndrome. Parasite and drug-induced PIE syndromes have a good prognosis and resolve spontaneously with supportive care and removal of exposure. Rarely, medications to eradicate the parasites may be warranted. Patients with other forms of eosinophilic lung diseases including acute eosinophilic pneumonia and chronic eosinophilic pneumonia usually require a course of corticosteroids. Prognosis for patients with most forms of eosinophilic lung disease is good. Acute eosinophilic pneumonia, however, can be life threatening if left untreated.
Jeong YJ, Kim K, Seo IJ, et al. Eosinophilic lung disease: a clinical radiologic, and pathologic overview. Radiographics. 2007;27:617-639.
Mann B. Eosinophilic lung disease. Clinical Medicine: Circulatory, Respiratory and Pulmonary Medicine. 2008;2:99-108.
Nathan N, Guillemot N, Aubertin G, et al. Chronic eosinophilic pneumonia in a 13-year-old child. Eur J Pediatr. 2008;167:1203-1207.
Pigakis K, Meletis G, Ferdoutsis M, et al. Idiopathic eosinophilic lung diseases: a new approach to pathogenesis and treatment. Pneumon. 2008;21:156-166.
Vijayan VK. Tropical pulmonary eosinophilia: pathogenesis, diagnosis and management. Curr Opin Pulm Med. 2007;13:428-433.
Wechsler ME. Pulmonary eosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:477-492.