Chapter 393 Bronchiectasis
Bronchiectasis, a disease characterized by irreversible abnormal dilatation and anatomic distortion of the bronchial tree, likely represents a common end stage of a number of nonspecific and unrelated antecedent events. Its incidence has been decreasing overall in developed countries, but it persists as a problem in developing countries and among some ethnic groups in industrialized nations. In at least 1 series of children with bronchiectasis (not due to cystic fibrosis), the male : female ratio was 2 : 1.
Pathophysiology and Pathogenesis
In the developed world, cystic fibrosis (Chapter 395) is the most common cause of clinically significant bronchiectasis. Other conditions associated with bronchiectasis include primary ciliary dyskinesia, foreign body aspiration, aspiration of gastric contents, immune deficiency syndromes (especially humoral immunity), and infection, especially pertussis, measles, and tuberculosis. Bronchiectasis can also be congenital, as in Williams-Campbell syndrome, in which there is an absence of annular bronchial cartilage, and Marnier-Kuhn syndrome (congenital tracheobronchomegaly), in which there is a connective tissue disorder. Other disease entities associated with bronchiectasis are right middle lobe syndrome (chronic extrinsic compression of right middle lobe bronchus by hilar lymph nodes) and yellow nail syndrome (pleural effusion, lymphedema, discolored nails).
Three basic mechanisms are involved in the pathogenesis of bronchiectasis. Obstruction can occur because of tumor, foreign body, impacted mucus due to poor mucociliary clearance, external compression, bronchial webs, and atresia. Infections due to Bordetella pertussis, measles, rubella, togavirus, respiratory syncytial virus, adenovirus, and Mycobacterium tuberculosis induce chronic inflammation, progressive bronchial wall damage, and dilatation. Chronic inflammation similarly contributes to the mechanism by which obstruction leads to bronchiectasis. Inflammatory mediators such as neutrophil elastase, interleukin-6, interleukin-8, and tumor necrosis factor-α (TNF-α) have been found to be elevated in the airways of patients with bronchiectasis. The mechanism by which bronchiectasis occurs in congenital forms is likely related to abnormal cartilage formation. The common thread in the pathogenesis of bronchiectasis consists of difficulty clearing secretions and recurrent infections with a “vicious circle” of infection and inflammation resulting in airway injury and remodeling.
Bronchiectasis can manifest in any combination of three pathologic forms, best defined by high-resolution CT (HRCT) scan. In cylindrical bronchiectasis, the bronchial outlines are regular, but there is diffuse dilatation of the bronchial unit. The bronchial lumen ends abruptly because of mucous plugging. In varicose bronchiectasis, the degree of dilatation is greater, and local constrictions cause an irregularity of outline resembling that of varicose veins. There may also be small sacculations. In saccular (cystic) bronchiectasis, bronchial dilatation progresses and results in ballooning of bronchi that end in fluid- or mucus-filled sacs. This is the most severe form of bronchiectasis. Bronchiectasis lies within a disease spectrum of chronic pediatric suppurative lung disease. The following definitions have been proposed: prebronchiectasis (chronic or recurrent endobronchial infection with nonspecific HRCT changes—may be reversible); HRCT bronchiectasis (clinical symptoms with HRCT evidence of bronchial dilation—may persist, progress, or improve and resolve); established bronchiectasis (like the previous but with no resolution within 2 yr). Therefore, early diagnosis and aggressive therapy are important to prevent the development of established bronchiectasis.
Clinical Manifestations
The most common complaints in patients with bronchiectasis are cough and production of copious purulent sputum. Younger children may swallow the sputum. Hemoptysis is seen with some frequency. Fever can occur with infectious exacerbations. Anorexia and poor weight gain may occur as time passes. Physical examination typically reveals crackles localized to the affected area, but wheezing as well as digital clubbing may also occur. In severe cases, dyspnea and hypoxemia can occur. Pulmonary function studies may demonstrate an obstructive, restrictive, or mixed pattern. Typically, impaired diffusion capacity is a late finding.
Diagnosis
Conditions that can be associated with bronchiectasis should be ruled out by appropriate investigations (e.g., sweat test, immunologic work-up). Chest radiographs of patients with bronchiectasis tend to be nonspecific. Typical findings can include increase in size and loss of definition of bronchovascular markings, crowding of bronchi, and loss of lung volume. In more severe forms, cystic spaces, occasionally with air-fluid levels and honeycombing, may occur. Compensatory overinflation of unaffected lung may be seen. Thin-section HRCT scanning is the gold standard, because it has excellent sensitivity and specificity. CT provides further information on disease location, presence of mediastinal lesions, and the extent of segmental involvement. The addition of radiolabeled aerosol inhalation to CT scanning can provide even more information. The CT findings in patients with bronchiectasis typically include cylindrical (“tram lines,” “signet ring appearance”), varicose (bronchi with “beaded contour”), cystic (cysts in “strings and clusters”), or mixed forms (Fig. 393-1). The lower lobes are most commonly affected.
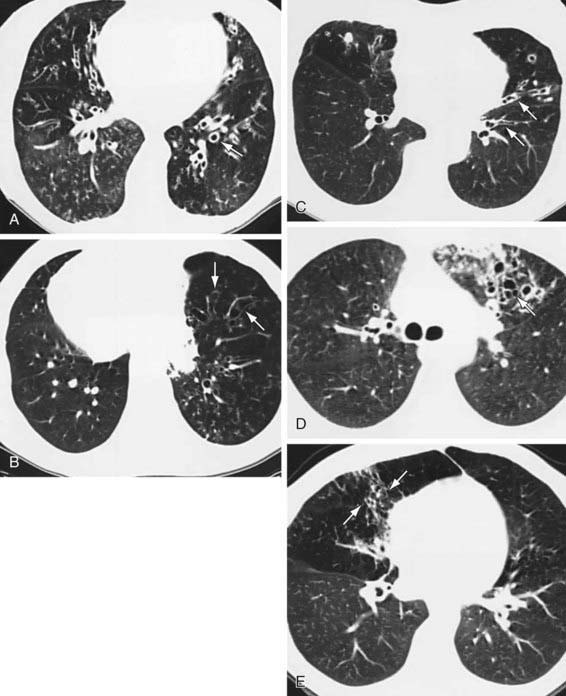
Figure 393-1 High-resolution CT scans of lungs with bronchiectasis. A, Dilated and thickened airways (arrow). B, Airways do not taper (arrows) toward the periphery in a patient with Kartagener syndrome. C, Varicose changes (dilated and beaded airways [arrows]). D, Clustered cysts or saccules (arrow) as well as a peripheral infiltrate. E, Middle lobe bronchiectasis (arrows) in a patient with Mycobacterium avium complex infection.
(From Barker AF: Bronchiectasis, N Engl J Med 346:1383, 2002.)
Treatment
The initial therapy for patients with bronchiectasis is medical and aims at decreasing airway obstruction and controlling infection. Chest physiotherapy (postural drainage), antibiotics, and bronchodilators are essential. Two to four weeks of parenteral antibiotics are often necessary to manage acute exacerbations adequately. Antibiotic choice is dictated by the identification and sensitivity of organisms found on deep throat, sputum (induced or spontaneous), or bronchoalveolar lavage fluid cultures. Long-term prophylactic oral (macrolide) or nebulized antibiotics may be beneficial. Any underlying disorder (immunodeficiency, aspiration) that may be contributing must be addressed. When localized bronchiectasis becomes more severe or resistant to medical management, segmental or lobar resection may be warranted. Lung transplantation can also be performed in patients with bronchiectasis. A review of randomized trials among adult patients with bronchiectasis did not find evidence to support the routine use of inhaled corticosteroids.
Prognosis
Children with bronchiectasis often suffer from recurrent pulmonary illnesses, resulting in missed school days, stunted growth, osteopenia, and osteoporosis. Overall, however, the prognosis for patients with bronchiectasis has improved considerably in the past few decades. Earlier recognition or prevention of predisposing conditions, more powerful and broad-spectrum antibiotics, and improved surgical outcomes are likely reasons.
Barker AF. Clinical manifestations and diagnosis of bronchiectasis. UpToDate, last revised December 30, 2008, http://www.uptodate.com.
Bilton D. Update on non-cystic fibrosis bronchiectasis. Curr Opin Pulmon Med. 2008;14:595-599.
Boren EJ, Teuber SS, Gershwin ME. A review of non-cystic fibrosis pediatric bronchiectasis. Clin Rev Allerg Immunol. 2008;34:260-273.
Evans DJ, Bara AI, Greenstone M: Prolonged antibiotics for purulent bronchiectasis in children and adults, Cochrane Database Syst Rev (2):CD001392, 2007.
Fall A, Spenser D. Paediatric bronchiectasis in Europe: what now and where next? Paediatr Respir Rev. 2006;7:268-274.
Guran T, Turan S, Karadag B, et al. Bone mineral density in children with non-cystic fibrosis bronchiectasis. Respiration. 2008;75:432-436.
Kapur N, Bell S, Kolbe J, et al: Inhaled steroids for bronchiectasis, Cochrane Database Syst Rev (1):CD000996, 2009.
Rama M, Yousef E. Bronchiectasis and chronic asthma: how common in pediatrics? Allergy Asthma Proc. 2006;27:354-358.
Santamaria F, Montella S, Camera L, et al. Lung structure abnormalities, but normal lung function in pediatric bronchiectasis. Chest. 2006;130:480-486.
Sirmali M, Karasu S, Turut H, et al. Surgical management of bronchiectasis in childhood. Eur J Cardio-Thorac Surg. 2007;31:120-123.
Stafler P, Carr SB. Non-cystic fibrosis bronchiectasis: its diagnosis and management. Arch Dis Child Educ Pract Ed. 2010;95:73-82.