Chapter 643 Cutaneous Nevi
Nevus skin lesions are characterized histopathologically by collections of well-differentiated cell types normally found in the skin. Vascular nevi are described in Chapter 642. Melanocytic nevi are subdivided into 2 broad categories: those that appear after birth (acquired nevi) and those that are present at birth (congenital nevi).
Acquired Melanocytic Nevus
Melanocytic nevus is a benign cluster of melanocytic nevus cells that arises as a result of alteration and proliferation of melanocytes at the epidermal-dermal junction.
Epidemiology
The number of acquired melanocytic nevi increases gradually during childhood and more slowly in early adulthood. The number reaches a plateau in the 3rd or 4th decade and then slowly decreases thereafter. The mean number of melanocytic nevi in an adult varies depending on genetics, skin color, and sun exposure. The greater the number of nevi present, the greater is the risk for development of melanoma. Sun exposure during childhood, particularly intermittent, intense exposure of an individual with light skin, and a propensity to burn and freckle rather than tan are important determinants of the number of melanocytic nevi that develop. Red-haired children, despite their light skin and propensity to freckle and sunburn, have fewer nevi than other children. Increased numbers of nevi are also associated with immunosuppression and administration of chemotherapy.
Clinical Manifestations
Nevocellular nevi have a well-defined life history and are classified as junctional, compound, or dermal in accordance with the location of the nevus cells in the skin. In childhood, > 90% of nevi are junctional; melanocyte proliferation occurs at the junction of the epidermis and dermis to form nests of cells. Junctional nevi appear anywhere on the body in various shades of brown; they are relatively small, discrete, flat, and variable in shape. The melanized nevus cells are cuboidal or epithelioid in configuration and occur in nests on the epidermal side of the basement membrane. Although some nevi, particularly those on the palms, soles, and genitalia, remain junctional throughout life, most become compound as melanocytes migrate into the papillary dermis to form nests at both the epidermal-dermal junction and within the dermis. If the junctional melanocytes stop proliferating, nests of melanocytes remain only within the dermis, forming an intradermal nevus. With maturation, compound and intradermal nevi may become raised, dome-shaped, verrucous, or pedunculated. Slightly elevated lesions are usually compound. Distinctly elevated lesions are usually intradermal. With age, the dermal melanocytic nests regress and the nevi gradually disappear.
Prognosis and Treatment
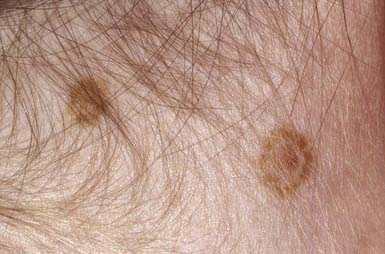
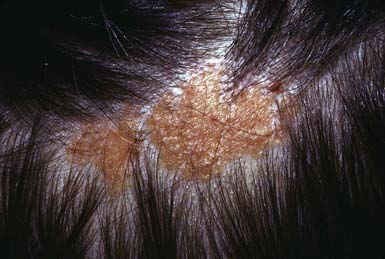
Acquired pigmented nevi are benign, but a very small percentage undergoes malignant transformation. Suspicious changes are indications for excision and histopathologic evaluation; they include rapid increase in size; development of satellite lesions; variegation of color, particularly with shades of red, brown, gray, black, and blue; pigmentary incontinence; notching or irregularity of the borders; changes in texture such as scaling, erosion, ulceration, and induration; and regional lymphadenopathy. Most of these changes are due to irritation, infection, or maturation; darkening and gradual increase in size and elevation normally occur during adolescence and should not be cause for concern. Two common benign changes are clonal nevi (fried-egg moles) and rim nevi. A clonal nevus is light brown with a dark raised center representing a clonal change of a subset of nevus cells within the lesion. Rim nevi are flat and light brown with dark brown rims. They are seen primarily in the scalp (Fig. 643-1). Consideration should be given to the presence of risk factors for development of melanoma and the patient’s parents’ wishes about removal of the nevus. If doubt remains about the benign nature of a nevus, excision is a safe and simple outpatient procedure that may be justified to allay anxiety.
Atypical Melanocytic Nevus
Atypical melanocytic nevi occur both in an autosomal dominant familial melanoma-prone setting (familial mole–melanoma syndrome, dysplastic nevus syndrome, BK mole syndrome) and as a sporadic event. Only 2% of all pediatric melanomas occur in individuals with this familial syndrome; melanoma develops before age 20 yr in 10% of individuals with the syndrome. Malignant melanoma has been reported in children with the dysplastic nevus syndrome as young as 10 yr. Risk for development of melanoma is essentially 100% in individuals with dysplastic nevus syndrome who have 2 family members who have had melanomas. The term atypical mole syndrome describes lesions in those individuals without an autosomal dominant familial history of melanoma but with > 50 nevi, some of which are atypical. The lifetime risk of melanoma associated with dysplastic nevi in this context is estimated to be 5-10%.
Atypical nevi tend to be large (5-15 mm) and round to oval. They have irregular margins and variegated color, and portions of them are elevated. These nevi are most common on the posterior trunk, suggesting that intermittent, intense sun exposure has a role in their genesis. They may also occur in sun-protected areas such as the breasts, buttocks, and scalp. Atypical nevi do not usually develop until puberty, although scalp lesions may be present earlier. Atypical nevi demonstrate disordered proliferation of atypical intraepidermal melanocytes, lymphocytic infiltration, fibroplasia, and angiogenesis. It may be helpful to obtain histopathologic documentation of dysplastic change by biopsy to identify these individuals. It is prudent to excise borderline atypical nevi in immunocompromised children or in those treated with irradiation or chemotherapeutic agents. Although chemotherapy has been associated with the development of a greater number of melanocytic nevi, it has not been directly linked to increased risk for development of melanoma. The threshold for removal of clinically atypical nevi is also lower at sites that are difficult to observe, such as the scalp. Children with atypical nevi should undergo a complete skin examination every 6-12 mo. In these children, photographic mole mapping serves as a useful adjunct in following nevus change. Parents must be counseled about the importance of sun protection and avoidance and should be instructed to look for early signs of melanoma on a regular basis, approximately every 3-4 mo.
Congenital Melanocytic Nevus
Congenital melanocytic nevi are present in ≈1% of newborn infants. These nevi have been categorized by size: giant congenital nevi are >20 cm in diameter (adult size) or >5% of the body surface, small congenital nevi are <2 cm in diameter, and intermediate nevi are in between these dimensions. Congenital nevi are characterized by the presence of nevus cells in the lower reticular dermis; between collagen bundles; surrounding cutaneous appendages, nerves, and vessels in the lower dermis; and occasionally extending to the subcuticular fat. Identification is often uncertain, however, because they may have the histologic features of ordinary junctional, compound, or intradermal nevi. Some nevi that were not present at birth display histopathologic features of congenital nevi; these should not be considered congenital. Furthermore, congenital nevi may be difficult to distinguish clinically from other types of pigmented lesions, adding to the difficulty that parents may have in identifying nevi that were present at birth. The clinical differential diagnosis includes mongolian spots, café-au-lait spots, smooth muscle hamartoma, and dermal melanocytosis (nevi of Ota and Ito).
Sites of predilection for small congenital nevi are the lower trunk, upper back, shoulders, chest, and proximal limbs. The lesions may be flat, elevated, verrucous, or nodular and may be various shades of brown, blue, or black. Given the difficulty in identifying small congenital nevi with certainty, data regarding their malignant potential are controversial and likely overstated. The true incidence of melanoma in congenital nevi, especially small and medium-sized lesions, is unknown. Removal of all small congenital nevi is not warranted because the development of melanoma in a small congenital nevus is an exceedingly rare event before puberty. A number of factors must be weighed in the decision about whether or not to remove a nevus, including its location, the ability to be monitor it clinically, the potential for scarring, the presence of other risk factors for melanoma, and the presence of atypical clinical features.
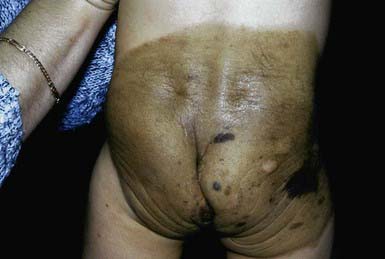
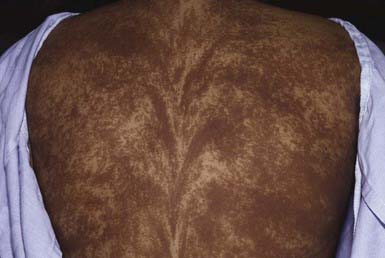
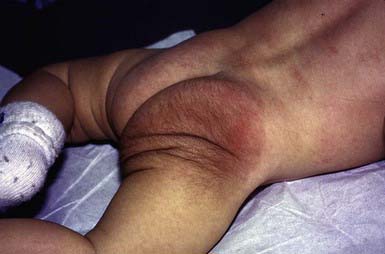
Giant congenital pigmented nevi (<1/20,000 births) occur most commonly on the posterior trunk (Fig. 643-2) but may also appear on the head or extremities. These nevi are of special significance because of their association with leptomeningeal melanocytosis (neurocutaneous melanocytosis) and their predisposition for development of malignant melanoma. Leptomeningeal involvement occurs most often when the nevus is located on the head or midline on the trunk, particularly when associated with multiple “satellite” melanocytic nevi (>20 lesions). Nevus cells within the leptomeninges and brain parenchyma may cause increased intracranial pressure, hydrocephalus, seizures, retardation, and motor deficits and may result in melanoma. Malignancy can be identified by careful cytologic examination of the cerebrospinal fluid for melanin-containing cells. MRI demonstrates asymptomatic leptomeningeal melanosis in ≈ 30% of individuals with giant congenital nevus of the type described above. The overall incidence of malignant melanoma arising in a giant congenital nevus has been estimated to be ≈ 5-10% but is more likely to be approximately 1-2%. The median age at diagnosis of the melanomas that arise within a giant congenital nevus is 7 yr. The mortality rate approaches 100%. The risk of melanoma is greater in patients in whom the predicted adult size of the nevus is > 40 cm. Management of giant congenital nevi remains controversial and should involve the parents, pediatrician, dermatologist, and plastic surgeon. If the nevus lies over the head or spine, MRI may allow detection of neural melanosis, the presence of which makes gross removal of a nevus from the skin a futile effort. In the absence of neural melanosis, early excision and repair aided by tissue expanders or grafting may reduce the burden of nevus cells and thus the potential for development of melanoma, but at the cost of many potentially disfiguring operations. Nevus cells deep within subcutaneous tissues may evade excision. Random biopsies of the nevus are not helpful, but biopsy of newly expanding nodules is indicated. Follow-up every 6 mo for 5 yr and every 12 mo thereafter is recommended. Serial photographs of the nevus may aid in detecting changes.
Melanoma
Malignant melanoma accounts for 1-3% of all pediatric malignancies, and approximately 2% of all melanomas occur before age 20 yr. The incidence of melanoma continues to increase. Melanoma is 7 times more frequent in the 2nd decade of life than in the 1st decade of life. Melanoma develops primarily in white individuals, on the head and trunk in males, and on the extremities in females. Risk factors for development of melanoma include the presence of the familial atypical mole–melanoma syndrome or xeroderma pigmentosum; an increased number of acquired melanocytic nevi, or atypical nevi; fair complexion; excessive sun exposure, especially intermittent exposure to intense sunlight; a personal or family (first-degree relative) history of a previous melanoma, giant congenital nevus, and immunosuppression. In previously well children, ultraviolet (UV) radiation is responsible for most melanomas. Fewer than 5% of childhood melanomas develop within giant congenital nevi or in individuals with the familial atypical mole–melanoma syndrome. Approximately 40-50% of the time, melanoma develops at a site where there was no apparent nevus. The mortality rate from melanoma is related primarily to tumor thickness and the level of invasion into the skin. The overall mortality rate reaches ≈ 40%, regardless of whether the tumor arises in a child or adult.
Given the lack of effective therapy for melanoma, prevention and early detection are the most effective measures. Emphasis should be given to avoidance of intense midday sun exposure between 10 AM and 3 PM; wearing of protective clothing such as a hat, long sleeves, and pants; and use of sunscreen. Early detection includes frequent clinical and photographic examinations of patients at risk (dysplastic nevus syndrome) and prompt response to rapid changes in nevi (size, shape, color, inflammation, bleeding or crusting, and sensation). The ABCD rule (asymmetry, border irregularities, color variability, diameter >6 mm), which is a useful screening tool for adults, may not be as effective for children.
Halo Nevus
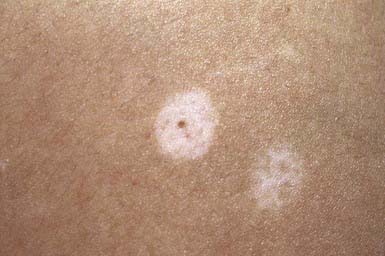
Halo nevi occur primarily in children and young adults, most commonly on the back (Fig. 643-3). Development of the lesion may coincide with puberty or pregnancy. Several pigmented nevi frequently develop halos simultaneously. Subsequent disappearance of the central nevus over several months is the usual outcome, and the depigmented area usually repigments. Excision and histopathologic examination of the lesion is indicated only when the nature of the central lesion is in question. An acquired melanocytic nevus occasionally develops a peripheral zone of depigmentation over a period of days to weeks. There is a dense inflammatory infiltrate of lymphocytes and histiocytes in addition to the nevus cells. The pale halo reflects disappearance of the melanocytes. This phenomenon is associated with congenital nevi, blue nevi, Spitz nevi, dysplastic nevi, neurofibromas, and primary and secondary malignant melanoma, and occasionally with poliosis, Vogt-Koyanagi-Harada syndrome, and pernicious anemia. Patients with vitiligo have an increased incidence of halo nevi. Individuals with halo nevi have circulating antibodies against the cytoplasm of melanocytes and nevus cells.
Spitz Nevus (Spindle and Epithelioid Cell Nevus)
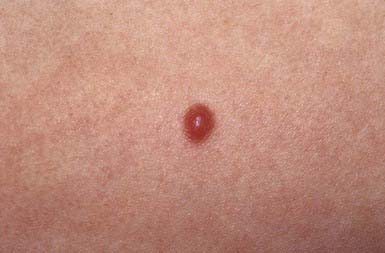
Spitz nevus manifests most commonly in the 1st 2 decades of life as a pink to red, smooth, dome-shaped, firm, hairless papule on the face, shoulder, or upper limb (Fig. 643-4). Most are <1 cm in diameter, but they can achieve a size of 3 cm. Rarely, they occur as numerous grouped lesions. Visually similar lesions include pyogenic granuloma, hemangioma, nevocellular nevus, juvenile xanthogranuloma, and basal cell carcinoma, but these entities are histologically distinguishable. Spitz nevus may be difficult to distinguish histopathologically from malignant melanoma because nuclear atypia is a common feature, particularly after local recurrence of the nevus. Difficulty arises in the fact that many other clinical types of melanocytic nevi have a similar histologic appearance. Local recurrence after excision may occur up to 5% of the time. If a nevus arouses clinical suspicion that it may be a melanoma, an excisional biopsy of the entire lesion is recommended. If the margins of excision of a Spitz nevus are positive, re-excision of the site is prudent to avoid difficulties in histopathologic interpretation of the lesion in the future.
Zosteriform Lentiginous Nevus (Agminated Lentigine)
Zosteriform lentiginous nevus is a unilateral, linear, bandlike collection of numerous 2- to 10-mm brown or black macules on the face, trunk, or limbs. The nevus may be present at birth or may develop during childhood. There are higher numbers of melanocytes in elongated rete ridges of the epidermis.
Nevus Spilus (Speckled Lentiginous Nevus)
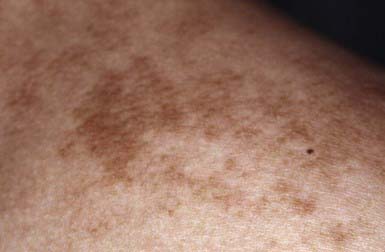
Nevus spilus is a flat brown patch within which are darker flat or raised brown melanocytic elements (Fig. 643-5). It varies considerably in size and can occur anywhere on the body. The color of the macular component may vary from light to dark brown, and the number of darker lesions may be low or high. Nevus spilus is rare at birth and is commonly acquired in late infancy or early childhood. Dark elements within the nevus are usually present initially and tend to increase in number gradually over time. The darker macules represent nevus cells in a junctional or dermal location; the patch has increased numbers of melanocytes in a lentiginous epidermal pattern. The malignant potential of these nevi is uncertain; nevus spilus is found more commonly in individuals with melanoma than in matched control subjects. The nevi need not be excised, unless atypical features or recent clinical changes are noted.
Nevus of Ota and Nevus of Ito
Nevus of Ota is more common among females, Asian, and black patients. This nevus consists of a permanent patch composed of partially confluent blue, black, and brown macules. Enlargement and darkening may occur with time. Occasionally, some areas of the nevus are raised. The macular nevi resemble mongolian spots in color and occur unilaterally in the areas supplied by the 1st and 2nd divisions of the trigeminal nerve. Nevus of Ota differs from a mongolian spot, not only by its distribution but also by having a speckled rather than a uniform appearance. Both are forms of mid-dermal melanocytosis. Nevus of Ota also has a greater concentration of elongated, dendritic dermal melanocytes located in the upper rather than the lower portion of the dermis. This nevus is sometimes present at birth; in other cases, it may arise during the 1st or 2nd decade of life. Patchy involvement of the conjunctiva, hard palate, pharynx, nasal mucosa, buccal mucosa, or tympanic membrane occurs in some patients. Malignant change is exceedingly rare. Laser therapy may effectively decrease the pigmentation.
Nevus of Ito is localized to the supraclavicular, scapular, and deltoid regions. This nevus tends to be more diffuse in its distribution and less mottled than nevus of Ota. It is also a form of mid-dermal melanocytosis. The only available treatments are masking with cosmetics and laser therapy.
Blue Nevi
The common blue nevus is a solitary, asymptomatic, smooth, dome-shaped, blue to blue-gray papule <10 mm in diameter on the dorsal aspect of the hands and feet. Rarely, common blue nevi form large plaques. Blue nevus is nearly always acquired, often during childhood and more commonly in females. Microscopically, it is characterized by groups of intensely pigmented spindle-shaped melanocytes in the dermis. This nevus is benign.
The cellular blue nevus is typically 1-3 cm in diameter and occurs most frequently on the buttocks and in the sacrococcygeal area. In addition to collections of deeply pigmented dermal dendritic melanocytes, cellular islands composed of large spindle-shaped cells are noted in the dermis and may extend into the subcutaneous fat. A histologic continuum may be seen from blue nevi to cellular blue nevi. A combined nevus is the association of a blue nevus with an overlying melanocytic nevus.
The blue-gray that is characteristic of these nevi is an optical effect caused by dermal melanin. Longer wavelengths of visible light penetrate to the deep dermis and are absorbed there by melanin; shorter-wavelength blue light cannot penetrate deeply but instead is reflected back to the observer.
Nevus Depigmentosus (Achromic Nevus)
Nevi depigmentosi are usually present at birth; they are localized macular hypopigmented patches or streaks, often with bizarre, irregular borders (Fig. 643-6). They can resemble hypomelanosis of Ito clinically, except that they are more localized and often unilateral. Small lesions may also resemble the ash leaf macules of tuberous sclerosis. Nevi depigmentosi appear to represent a focal defect in transfer of melanosomes to keratinocytes.
Epidermal Nevi
Epidermal nevi may be visible at birth or may develop in the first months or years of life. They affect both sexes equally and usually occur sporadically. Epidermal nevi are hamartomatous lesions characterized by hyperplasia of the epidermis and/or adnexal structures in a focal area of the skin.
Epidermal nevi are classified into a number of variants, depending on the morphology and extent of the individual nevus and the predominant epidermal structure. An epidermal nevus may appear initially as a discolored, slightly scaly patch that, with maturation, becomes more linear, thickened, verrucous, and hyperpigmented. Systematized refers to a diffuse or extensive distribution of lesions, and ichthyosis hystrix indicates that the distribution is extensive and bilateral (Fig. 643-7). Morphologic types include pigmented papillomas, often in a linear distribution; unilateral hyperkeratotic streaks involving a limb and perhaps a portion of the trunk; velvety hyperpigmented plaques; and whorled or marbled hyperkeratotic lesions in localized plaques or over extensive areas of the body along Blaschko lines. An inflammatory linear verrucous variant is markedly pruritic and tends to become erythematous, scaling, and crusted.
The histologic pattern evolves as an epidermal nevus matures, but epidermal hyperplasia of some degree is apparent in all stages of development. One or another dermal appendage may predominate in a particular lesion. These nevi must be distinguished from lichen striatus, lymphangioma circumscriptum, shagreen patch of tuberous sclerosis, congenital hairy nevi, linear porokeratosis, linear lichen planus, linear psoriasis, the verrucous stage of incontinentia pigmenti, and nevus sebaceus (Jadassohn). Keratolytic agents such as retinoic acid and salicylic acid may be moderately effective in reducing scaling and controlling pruritus, but definitive treatment requires full-thickness excision; recurrence is usual if more superficial removal is attempted. Alternatively, the nevus may be left intact. Epidermal nevi are occasionally associated with other abnormalities of the skin and soft tissues, eyes, and nervous, cardiovascular, musculoskeletal, and urogenital systems. In these instances, the disorder is referred to as epidermal nevus syndrome, and a mosaic phenotype is expressed. This syndrome, however, is not a distinct clinical entity. The well-established syndromes that involve a type of epidermal nevus and distinct birth defects include the proteus and CHILD (congenital hemidysplasia with ichthyosiform erythroderma and limb defects) syndromes.
Nevus Sebaceus (Jadassohn)
A relatively small, sharply demarcated, oval or linear, elevated yellow-orange plaque that is usually devoid of hair, nevus sebaceus occurs on the head and neck of infants (Fig. 643-8). It may occur occasionally on the trunk. Although the lesion is characterized histopathologically by an abundance of sebaceous glands, all elements of the skin are represented. It is frequently flat and inconspicuous in early childhood. With maturity, usually during adolescence, the lesions become verrucous and studded with large rubbery nodules. The changing clinical appearance reflects the histologic pattern, which is characterized by a variable degree of hyperkeratosis, hyperplasia of the epidermis, malformed hair follicles, and often a profusion of sebaceous glands and the presence of ectopic apocrine glands. It is believed that these nevi form from pluripotential primary epithelial germ cells, which can dedifferentiate into various epithelial tumors. Consequently, during adulthood, these nevi are frequently complicated by secondary malignancies and benign adnexal tumors, most commonly basal cell carcinoma or syringocystadenoma papilliferum. Deletions in the PTCH gene, the putative gene defect in basal cell carcinoma, have been found in sebaceus nevi. The treatment of choice is total excision before adolescence. Sebaceus nevi associated with central nervous system, skeletal, and ocular defects represent a variant of the epidermal nevus syndrome.
Becker Nevus (Becker Melanosis)
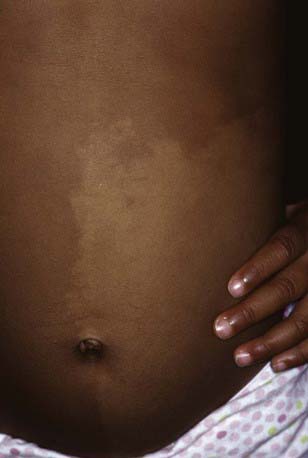
Becker nevus develops predominantly in males, during childhood or adolescence, initially as a hyperpigmented patch. The lesion commonly develops hypertrichosis, limited to the area of hyperpigmentation, and evolves into a unilateral, slightly thickened, irregular, hyperpigmented plaque. The most common sites are the upper torso and upper arm (Fig. 643-9). The nevus shows an increased number of basal melanocytes and variable epidermal hyperplasia. Becker melanosis is commonly associated with a smooth muscle hamartoma, which may appear as slight perifollicular papular elevations or slight induration. Stroking of such a lesion may induce smooth muscle contraction and make the hairs stand up. The nevus is benign, has no risk for malignant change, and is rarely associated with other anomalies.
Nevus Comedonicus
An uncommon organoid nevus of epithelial origin, nevus comedonicus consists of linear plaques of plugged follicles that simulate comedones; they may be present at birth or may appear during childhood. The horny plugs represent keratinous debris within dilated, malformed pilosebaceous follicles. The lesions are most often unilateral and may develop at any site. Rarely, they are associated with other congenital malformations, including skeletal defects, cerebral anomalies, and cataracts. Although these lesions are often asymptomatic, some affected individuals experience recurrent inflammation, resulting in cyst formation, fistulas, and scarring. There is no effective treatment except full-thickness excision; palliation of larger lesions may be achieved by regular applications of a retinoic acid preparation.
Connective Tissue Nevus
Connective tissue nevus is a hamartoma of collagen, elastin, and/or glycosaminoglycans of the dermal extracellular matrix. It may occur as a solitary defect or as a manifestation of an associated disorder. These nevi may occur at any site but are most common on the back, buttocks, arms, and thighs. They are skin-colored, ivory, or yellow plaques, 2-15 cm in diameter, composed of many tiny papules or grouped nodules that are frequently difficult to appreciate visually because of the subtle color changes. The plaques have a rubbery or cobblestone consistency on palpation. Biopsy findings are variable and include increased amounts and/or degeneration or fragmentation of dermal collagen, elastic tissue, or ground substance. Similar lesions occurring with tuberous sclerosis are called shagreen patches; however, shagreen patches consist only of excessive amounts of collagen. The association of many small papular connective tissue nevi with osteopoikilosis is called dermatofibrosis lenticularis disseminata (Buschke-Ollendorf syndrome).
Smooth Muscle Hamartoma
Smooth muscle hamartoma is a developmental anomaly resulting from hyperplasia of the smooth muscle (arrector pili) associated with hair follicles. It is usually evident at birth or shortly thereafter as a flesh-colored or lightly pigmented plaque with overlying hypertrichosis on the trunk or limbs (Fig. 643-10). Transient elevation or a rippling movement of the lesion, caused by contraction of the muscle bundles, can sometimes be elicited by stroking of the surface. Smooth muscle hamartoma can be mistaken for congenital pigmented nevus, but the distinction is important because the former has no risk for malignant melanoma and need not be removed.
Altaykan A, Ersov-Evans S, Erkin G, et al. Basal carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
Crane LA, Mokrohisky ST, Dellavalle RP, et al. Melanocytic nevus development in Colorado children born in 1998. Arch Dermatol. 2009;145:1-9.
Guidbakke KK, Khachemoune A, Deng A, et al. Naevus comedonicus: a spectrum of body involvement. Clin Exp Dermatol. 2007;32:488-492.
Gupta M, Berk DR, Gray C, et al. Morphologic features and natural history of scalp nevi in children. Arch Dermatol. 2010;146(5):506-511.
Huynh PM, Grant-Kels JM, Grin CM. Childhood melanoma: update and treatment. Int J Dermatol. 2005;44:715-723.
Kazakov DV, Calonie E, Zelger B, et al. Sebaceous carcinoma arising in nevus sebaceous of Jadassohn: a clinicopathological study of five cases. Am J Dermatopathol. 2007;29:242-248.
Kinsler VA, Birley J, Atherton DJ. Great Ormond Street Hospital for Children registry for congenital melanocytic naevi: prospective study 1988–2007. Part 1: epidemiology, phenotype and outcome. Br J Dermatol. 2009;160:143-150.
Krengel S, Hauschild A, Schafer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol. 2006;155:1-8.
Schaffer JV. Pigmented lesions in children: when to worry. Curr Opin Pediatr. 2007;19:430-440.
Sulit DJ, Guardiano RA, Krvida S. Classic and atypical Spitz nevi: review of the literature. Cutis. 2007;79:141-146.
Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
Zaal LH, Mooi WJ, Klip H, et al. Risk of malignant transformation of congenital melanocytic nevi: a retrospective nationwide study from the Netherlands. Plast Reconstr Surg. 2005;116:1902-1909.