Chapter 646 Vesiculobullous Disorders
Many diseases are characterized by vesiculobullous lesions; they vary considerably in cause, age of onset, and pattern. The morphology of the blister often provides a visual clue to the location of the lesion within the skin. Blisters localized to the epidermal layers are thin-walled, relatively flaccid, and easily ruptured. Subepidermal blisters are tense, thick-walled, and more durable. Biopsies of blisters can be diagnostic because the level of cleavage within the skin and associated findings, such as the nature of the inflammatory infiltrate, are characteristic for a particular disorder. Other diagnostic procedures, such as immunofluorescence and electron microscopy, can often help distinguish vesiculobullous disorders that have nearly identical histopathologic findings (Table 646-1).
Table 646-1 SITES OF BLISTER FORMATION AND DIAGNOSTIC STUDIES FOR THE VESICULOBULLOUS DISORDERS
| DISORDER | BLISTER CLEAVAGE SITE | DIAGNOSTIC STUDIES |
|---|---|---|
| Acrodermatitis enteropathica | IE | Zn level |
| Bullous impetigo | GL | Smear, culture |
| Bullous pemphigoid | SE (junctional) | Direct and indirect immunofluorescence studies |
| Candidosis | SC | KOH preparation, culture |
| Dermatitis herpetiformis | SE | Direct immunofluorescence studies |
| Dermatophytosis | IE | KOH preparation, culture |
| Dyshidrotic eczema | IE | Routine histopathology |
| EB—simplex | IE | Electron microscopy; immunofluorescence mapping |
| EB of the hands and feet | IE | Electron microscopy; immunofluorescence mapping |
| Junctional EB (letalis) | SE (junctional) | Electron microscopy; immunofluorescence mapping |
| Recessive dystrophic EB | SE | Electron microscopy; immunofluorescence mapping |
| Dominant dystrophic EB | SE | Electron microscopy; immunofluorescence mapping |
| Epidermolytic hyperkeratosis | IE | Routine histopathology |
| Erythema multiforme | SE | Routine histopathology |
| Erythema toxicum | SC, IE | Smear for eosinophils |
| Incontinentia pigmenti | IE | |
| Insect bites | IE | Routine histopathology |
| Linear IgA dermatosis | SE | Direct immunofluorescence studies |
| Mastocytosis | SE | Routine histopathology |
| Miliaria crystallina | IC | Routine histopathology |
| Neonatal pustular melanosis | SC, IE | Smear for cells |
| Pemphigus foliaceus | GL | Direct and indirect immunofluorescence studies |
| Tzanck smear | ||
| Pemphigus vulgaris | Suprabasal | Direct and indirect immunofluorescence studies |
| Tzanck smear | ||
| Scabies | IE | Scraping |
| Staphylococcal scalded skin syndrome | GL | Routine histopathology |
| Toxic epidermal necrolysis | SE | Routine histopathology |
| Viral blisters | IE |
EB, epidermolysis bullosa; GL, granular layer; IC, intracorneal; IE, intraepidermal; KOH, potassium hydroxide; SC, subcorneal; SE, subepidermal.
646.1 Erythema Multiforme
Etiology
Among the numerous factors implicated in the etiology of erythema multiforme (EM), infection with herpes simplex virus (HSV) is the most common. HSV labialis and, less commonly, HSV genitalis have been implicated in 60% of episodes of EM and are believed to trigger nearly all episodes of recurrent EM, frequently in association with sun exposure. HSV antigens and DNA are present in skin lesions of EM but are absent in nonlesional skin. Presence of the human leukocyte antigens A33, B62, B35, DQB1*0301, and DR53 is associated with an increased risk of HSV-induced EM, particularly the recurrent form. Most patients experience a single self-limited episode of EM. Lesions of HSV-induced recurrent EM (HA-EM) typically develop 10-14 days after onset of recurrent HSV eruptions, have a similar appearance from episode to episode, but may vary in frequency and duration in a given patient. Not all episodes of recurrent HSV evolve into EM in susceptible patients.
Clinical Manifestations
EM has numerous morphologic manifestations on the skin, varying from erythematous macules, papules, vesicles, bullae, or urticaria-appearing plaques to patches of confluent erythema. The eruption appears most commonly in patients between the ages of 10 and 40 yr and usually is asymptomatic, although a burning sensation or pruritus may be present. The diagnosis of EM is established by finding the classic lesion: doughnut-shaped, target-like (iris or bull’s-eye) papules with an erythematous outer border, an inner pale ring, and a dusky purple to necrotic center (Figs. 646-1 and 646-2).
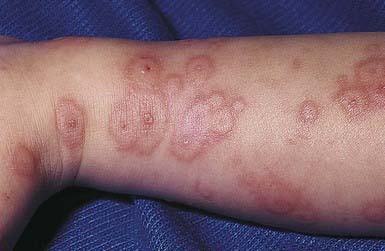
Figure 646-1 Early fixed papules with a central dusky zone on the dorsum of the hand of a child with erythema multiforme due to herpes simplex virus.
(From Weston WL, Lane AT, Morelli J: Color textbook of pediatric dermatology, ed 3, St Louis, 2002, Mosby, p 156.)
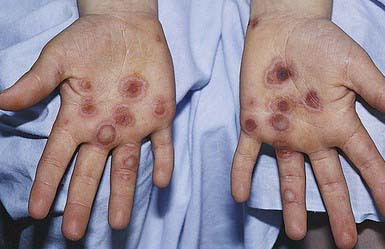
Figure 646-2 “Target” or “iris” lesions with characteristic central dusky zone on palms of a child with erythema multiforme due to herpes simplex virus.
(From Weston WL, Lane AT, Morelli J: Color textbook of pediatric dermatology, ed 3, St Louis, 2002, Mosby, p 156.)
EM is characterized by an abrupt, symmetric cutaneous eruption, most commonly on the extensor upper extremities; lesions are relatively sparse on the face, trunk, and legs. The eruption often appears initially as red macules or urticarial plaques that expand centrifugally to form lesions up to 2 cm in diameter with a dusky to necrotic center. Lesions of a particular episode typically appear within 72 hr and remain fixed in place. Oral lesions may occur with a predilection for the vermilion border of the lips and the buccal mucosa, but other mucosal surfaces are spared. Prodromal symptoms are generally absent. Lesions typically resolve without sequelae in about 2 wk; progression to Stevens-Johnson syndrome does not occur. EM may manifest initially as urticarial lesions, but unlike urticaria, a given lesion of EM does not fade within 24 hr.
Pathogenesis
The pathogenesis of EM is unclear, but it may be a host-specific, cell-mediated immune response to an antigenic stimulus, resulting in damage to keratinocytes. HSV Pol1 gene expressed in HA-EM lesions upregulates/activates the transcription factor SP1 and inflammatory cytokines. These cytokines, released by activated mononuclear cells and keratinocytes, may contribute to epidermal cell death and constitutional symptoms.
Pathology
Microscopic findings in EM are variable but may aid in diagnosis. Early lesions typically show slight intercellular edema, rare dyskeratotic keratinocytes, and basal vacuolation in the epidermis and a perivascular lymphohistiocytic infiltrate with edema in the upper dermis. More mature lesions show an accentuation of these characteristics and the development of lymphocytic exocytosis and an intense, perivascular, and interstitial mononuclear infiltrate in the upper third of the dermis. In severe cases, the entire epidermis becomes necrotic.
Differential Diagnosis
The differential diagnosis of EM also includes bullous pemphigoid, pemphigus, linear immunoglobulin (Ig) A dermatosis, graft versus host disease, bullous drug eruption, urticaria, viral infections such as HSV, reactive arthritis syndromes, Kawasaki disease, Behçet disease, allergic vasculitis, erythema annulare centrifugum, and periarteritis nodosa. EM that primarily involves the oral mucosa may be confused with bullous pemphigoid, pemphigus vulgaris, vesiculobullous or erosive lichen planus, Behçet syndrome, recurrent aphthous stomatitis, and primary herpetic gingivostomatitis. Serum sickness-like reaction (SSLR) to cefaclor may also manifest as EM-like lesions; the lesions may develop a dusky to purple center, but in most cases, the eruption of cefaclor-induced SSLR is pruritic, transient, and migratory and is probably urticarial rather than true EM.
Treatment
Treatment of EM is supportive. Topical emollients, systemic antihistamines, and nonsteroidal anti-inflammatory agents do not alter the course of the disease but may provide symptomatic relief. No controlled, prospective studies support the use of corticosteroids in the management of EM. Rather, glucocorticoid therapy may be permissive of HSV replication and make EM episodes more frequent or continuous. Prophylactic oral acyclovir given for 6 mo may be effective in controlling recurrent episodes of HSV-associated EM. On discontinuation of acyclovir, both HSV and EM may recur, although episodes may be less frequent and milder.
Gober MD, Laing JM, Burnett JW, et al. The Herpes simplex virus gene Pol expressed in herpes-associated erythema multiforme lesions upregulates/activates SP1 and inflammatory cytokines. Dermatology. 2007;215:97-106.
Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
Williams PM, Conklin RJ. Erythema multiforme: a review and contrast from Stevens-Johnson syndrome/toxic epidermal necrolysis. Dent Clin North Am. 2005;49:67-76.
646.2 Stevens-Johnson Syndrome
Etiology
Mycoplasma pneumoniae is the most convincingly demonstrated infectious cause of Stevens-Johnson syndrome. Drugs, particularly sulfonamides, nonsteroidal anti-inflammatory agents, antibiotics, and anticonvulsants, are the most common precipitants of Stevens-Johnson syndrome and toxic epidermal necrolysis. HLA-B*1502 and HLA-B*5801 have been implicated in the development of these two disorders in Han Chinese patients receiving carbamazepine and in Japanese patients receiving allopurinol, respectively.
Clinical Manifestations
Cutaneous lesions in Stevens-Johnson syndrome generally consist initially of erythematous macules that rapidly and variably develop central necrosis to form vesicles, bullae, and areas of denudation on the face, trunk, and extremities. The skin lesions are typically more widespread than in EM and are accompanied by involvement of two or more mucosal surfaces, namely the eyes, oral cavity, upper airway or esophagus, gastrointestinal tract, or anogenital mucosa (Fig. 646-3). A burning sensation, edema, and erythema of the lips and buccal mucosa are often the presenting signs, followed by development of bullae, ulceration, and hemorrhagic crusting. Lesions may be preceded by a flu-like upper respiratory illness. Pain from mucosal ulceration is often severe, but skin tenderness is minimal to absent in Stevens-Johnson syndrome, in contrast to pain in toxic epidermal necrolysis. Corneal ulceration, anterior uveitis, panophthalmitis, bronchitis, pneumonitis, myocarditis, hepatitis, enterocolitis, polyarthritis, hematuria, and acute tubular necrosis leading to renal failure may occur. Disseminated cutaneous bullae and erosions may result in increased insensible fluid loss and a high risk of bacterial superinfection and sepsis. New lesions occur in crops, and complete healing may take 4-6 wk; ocular scarring, visual impairment, and strictures of the esophagus, bronchi, vagina, urethra, or anus may remain. Nonspecific laboratory abnormalities in Stevens-Johnson syndrome include leukocytosis, elevated erythrocyte sedimentation rate, and, occasionally, increased liver transaminase levels and decreased serum albumin values. Toxic epidermal necrolysis is the most severe disorder in the clinical spectrum of the disease, involving considerable constitutional toxicity and extensive necrolysis of the mucous membranes and > 30% of the body surface area (Fig. 646-4).
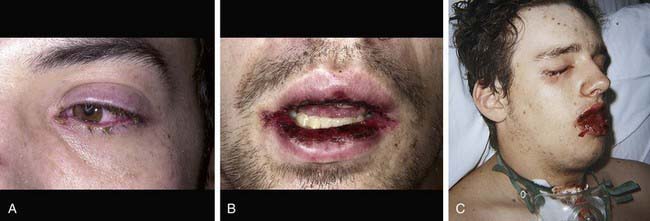
Figure 646-3 Bullae are present on the conjunctivae (A) and in the mouth (B) with Stevens-Johnson syndrome. Sloughing, ulceration, and necrosis in the oral cavity interfere with eating (C). Genital lesions cause dysuria and interfere with voiding.
(From Habif TP, editor: Clinical dermatology, ed 4, Philadelphia, 2004, Mosby, p 631.)
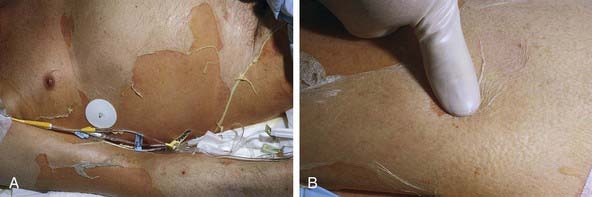
Figure 646-4 A, Large sheets of full-thickness epidermis are shed. B, Toxic epidermal necrolysis begins with diffuse, hot erythema. In hours the skin becomes painful, and with slight thumb pressure, the skin wrinkles, slides laterally, and separates from the dermis (Nikolsky sign).
(From Habif TP, editor: Clinical dermatology, ed 4, Philadelphia, 2004, Mosby, p 633.)
Pathogenesis
Pathogenesis is related to drug-specific CD8+ cytotoxic T cells, with perforin/granzyme B and granulysin triggering keratinocyte apoptosis. This process is followed by expanded enactment of apoptosis involving the interaction of soluble Fas ligand with Fas receptor.
Differential Diagnosis
The differential diagnosis of Stevens-Johnson syndrome includes toxic epidermal necrolysis, urticaria, DRESS (drug rash [or reaction] with eosinophilia and systemic symptoms) syndrome (Chapter 637.2) and other drug eruptions and viral exanthems, including Kawasaki disease.
Treatment
Management of Stevens-Johnson syndrome is supportive and symptomatic. Potentially offending drugs must be discontinued as soon as possible. Ophthalmologic consultation is mandatory because ocular sequelae such as corneal scarring can lead to vision loss. Application of cryopreserved amniotic membrane to the ocular surface during the acute phase of the disease limits the destructive and long-term sequelae. Oral lesions should be managed with mouthwashes and glycerin swabs. Vaginal lesions should be observed closely and treated to prevent vaginal stricture or fusion. Topical anesthetics (diphenhydramine, dyclonine, viscous lidocaine) may provide relief from pain, particularly when applied before eating. Denuded skin lesions can be cleansed with saline or Burrow solution compresses. Antibiotic therapy is appropriate for documented secondary bacterial infection. Treatment may require admission to an intensive care unit; intravenous (IV) fluids; nutritional support; sheepskin or air-fluid bedding; daily saline or Burow solution compresses; paraffin gauze or colloidal gel (Hydrogel) dressing of denuded areas; saline compresses on the eyelids, lips, or nose; analgesics; and urinary catheterization (when needed). A daily examination for infection and ocular lesions, which constitute the major cause of long-term morbidity, is essential. Systemic antibiotics are indicated for documented urinary or cutaneous infections and for suspected bacteremia (due to S. aureus or P. aeruginosa) because infection is the leading cause of death. Prophylactic systemic antibiotics are not necessary. Although corticosteroids are sometimes advocated in early, severe cases of Stevens-Johnson syndrome, no prospective double-blind studies evaluating their efficacy have been reported. Most authorities discourage their use because of reports of increased morbidity and mortality (sepsis) with their administration. IV immunoglobulin (IVIG) (1.5-2.0 g/kg/day × 3 days) should be considered in early disease.
Two severe cases of toxic epidermal necrolysis have been treated successfully with etanercept.
Borchers AT, Lee JL, Naguwa SM, et al. Stevens-Johnson and toxic epidermal necrolysis. Autoimmune Rev. 2008;7:598-605.
Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343-1350.
de Prost N, Ingen-Housz-Oro S, Duong T, et al. Bacteremia in Stevens-Johnson syndrome and toxic epidermal necrolysis. Medicine. 2010;89(1):28-36.
Ferrel PB, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543-1546.
Gregory DG. The ophthalmologic management of acute Stevens-Johnson syndrome. Ocul Surf. 2008;6:87-95.
Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
Hazin R, Ibrahimi OA, Hazin MT, et al. Stevens-Johnson syndrome: pathogenesis, diagnosis and management. Ann Med. 2008;40:129-138.
Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptic and allopurinol related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617-1622.
Williams PM, Conklin RJ. Erythema multiforme: a review and contrast from Stevens-Johnson syndrome/toxic epidermal necrolysis. Dent Clin North Am. 2005;49:67-76.
646.3 Toxic Epidermal Necrolysis
Epidemiology and Etiology
The pathogenesis of toxic epidermal necrolysis is not proved but may involve a hypersensitivity phenomenon that results in damage primarily to the basal cell layer of the epidermis. Epidermal damage appears to result from keratinocyte apoptosis (Chapter 646.2). This condition is triggered by many of the same factors that are thought to be responsible for Stevens-Johnson syndrome, principally drugs such as the sulfonamides, amoxicillin, phenobarbital, hydantoin, butazones, and allopurinol. Toxic epidermal necrolysis is defined by (1) widespread blister formation and morbilliform or confluent erythema, associated with skin tenderness; (2) absence of target lesions; (3) sudden onset and generalization within 24-48 hr; (4) histologic findings of full-thickness epidermal necrosis and a minimal to absent dermal infiltrate. These criteria categorize toxic epidermal necrolysis as a separate entity from EM.
Clinical Manifestations
The prodrome consists of fever, malaise, localized skin tenderness, and diffuse erythema. Inflammation of the eyelids, conjunctivae, mouth, and genitals may precede skin lesions. Flaccid bullae may develop, although this is not a prominent feature. Characteristically, full-thickness epidermis is lost in large sheets (see Fig. 646-4). Nikolsky sign (denudation of the skin with gentle tangential pressure) is present but only in the areas of erythema (see Fig. 646-4). Healing takes place over 14 or more days. Scarring, particularly of the eyes, may result in corneal opacity. The course may be relentlessly progressive, complicated by severe dehydration, electrolyte imbalance, shock, and secondary localized infection and septicemia. Loss of nails and hair may also occur. Long-term morbidity includes alterations in skin pigmentation, eye problems (lack of tears, conjunctival scarring, loss of lashes), and strictures of mucosal surfaces. The differential diagnosis includes staphylococcal scalded skin syndrome, in which the blister cleavage plane is intraepidermal; graft versus host disease; chemical burns; drug eruptions; toxic shock syndrome; and pemphigus.
Anticonvulsant hypersensitivity syndrome (DRESS syndrome; Chapter 637.2) is a multisystem reaction that appears approximately 4 wk to 3 mo after the start of therapy with phenytoin, carbamazepine, phenobarbitone, primidone, or other drugs, most commonly antibiotics. The mucocutaneous eruption may be identical to that of EM, Stevens-Johnson syndrome, or toxic epidermal necrolysis, but the reaction also typically includes lymphadenopathy as well as fever, hepatic, renal and pulmonary disease, eosinophilia, atypical lymphocytosis, and leukocytosis.
Treatment
Appreciation of the specific etiologic factor is crucial. When the disorder is drug induced, administration of the drug must be discontinued as soon as possible. Management is similar to that for severe burns and may be best accomplished in a burn unit (Chapter 68). It may include strict reverse isolation, meticulous fluid and electrolyte therapy, use of an air-fluid bed, and daily cultures. Systemic antibiotic therapy is indicated when secondary infection is evident or suspected. Skin care consists of cleansing with isotonic saline or Burow solution. Biologic or colloid gel (Hydrogel) dressings alleviate pain and reduce fluid loss. Narcotics are often required for pain relief. Mouth and eye care, as for EM major, may be necessary. Because of an immune mechanism, systemic glucocorticosteroids and IVIG have been used with apparent success. Nonetheless, this treatment remains controversial.
Endorf FW, Cancio LC, Gibran NS. Toxic epidermal necrolysis: clinical guidelines. J Burn Care Res. 2008;29:706-712.
Levi N, Bastuji-Garin S, Mockenhaupt M, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009;123:e297-e304.
646.4 Mechanobullous Disorders
Epidermolysis Bullosa
Diseases categorized under the general term epidermolysis bullosa (EB) are a heterogeneous group of congenital, hereditary blistering disorders. They differ in severity and prognosis, clinical and histologic features, and inheritance patterns, but are all characterized by induction of blisters by trauma and exacerbation of blistering in warm weather. The disorders can be categorized under 3 major headings with multiple subgroupings: epidermolysis bullosa simplex (EBS), junctional epidermolysis bullosa (JEB), and dystrophic epidermolysis bullosa (DEB) (Table 646-2). Kindler syndrome (Kindlin-1 gene), which includes poikiloderma and photosensitivity as well as easy blistering, is also considered a separate form of EB.
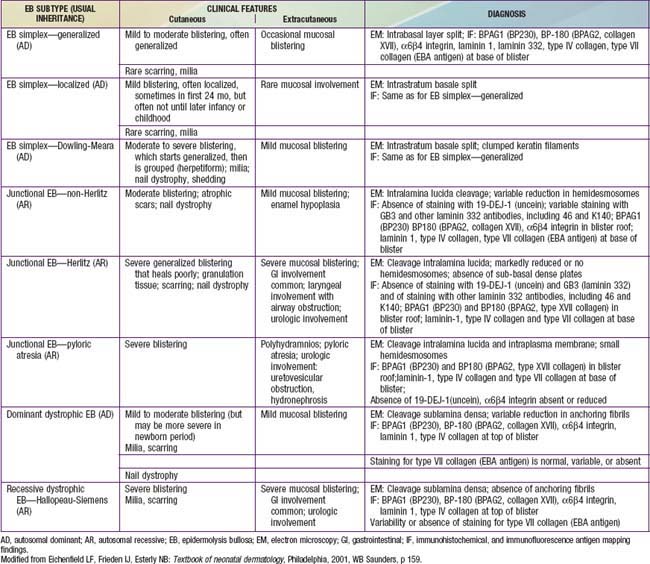
Table 646-2 CLINICAL PRESENTATION AND DIAGNOSIS OF SELECTED EPIDERMOLYSIS BULLOSA SUBTYPES IN THE NEONATAL PERIOD
Epidermolysis Bullosa Simplex
EBS is a nonscarring, autosomal dominant disorder. The defect in most common types of EBS is in keratin 5 or 14, which makes up intermediate filaments of the basal keratinocytes. The intraepidermal bullae result from cytolysis of the basal cells. There are multiple other rare variants with defects that also result in intraepidermal blistering.
In EBS—generalized other (formerly Koebner), blisters are usually present at birth or during the neonatal period. Sites of predilection are the hands, feet, elbows, knees, legs, and scalp. Intraoral lesions are minimal, nails rarely become dystrophic and usually regrow even when they are shed, and dentition is normal. Bullae heal with minimal to no scar or milia formation. Secondary infection is the primary complication. The propensity to blister decreases with age, and the long-term prognosis is good. Blisters should be drained by puncturing, but the blister top should be left intact to protect the underlying skin. Erosions may be covered with a semipermeable dressing.
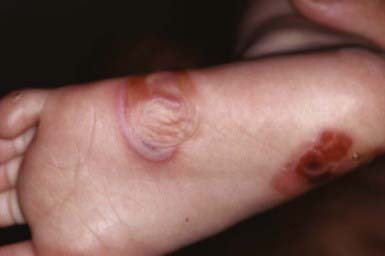
EBS—localized (formerly Weber-Cockayne) predominantly affects the hands and feet and often manifests when a child begins to walk; onset may be delayed until puberty or early adulthood, when heavy shoes are worn or the feet are subjected to increased trauma. Bullae are usually restricted to the hands and feet (Fig. 646-5); rarely, they occur elsewhere, such as the dorsal aspect of the arms and the shins. The disorder ranges from mildly incapacitating to crippling at times of severe exacerbations.
EBS—Dowling-Meara (herpetiformis) is characterized by grouped blisters resembling those of herpes simplex (Fig. 646-6). During infancy, blistering may be severe and extensive, may involve mucous membranes, and may result in shedding of nails, formation of milia, and mild pigmentary changes, without scarring. After the first few months of life, warm temperatures do not appear to exacerbate blistering. Hyperkeratosis and hyperhidrosis of the palms and soles may develop, but generally, the condition improves with age.
Junctional Epidermolysis Bullosa
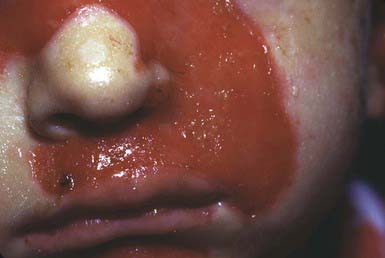
JEB—Herlitz is an autosomal recessive condition that is life threatening. Blisters appear at birth or develop during the neonatal period, particularly on the perioral area, scalp, legs, diaper area, and thorax. Nails eventually become dystrophic and then often permanently lost. Mucous membrane involvement may be severe, and ulceration of the respiratory, gastrointestinal, and genitourinary epithelium has been documented in many affected children, although less frequently than in severe recessive dystrophic epidermolysis bullosa (RDEB). Healing is delayed, and vegetating granulomas may persist for a long time. Large, moist, erosive plaques (Fig. 646-7) may provide a portal of entry for bacteria, and septicemia is a frequent cause of death. Mild atrophy may be seen in areas of recurrent blistering. Defective dentition with early loss of teeth as a result of rampant caries is characteristic. Growth retardation and recalcitrant anemia are almost invariable. In addition to infection, cachexia and circulatory failure are common causes of death. Most patients die within the first 3 yr of life.
JEB—non-Herlitz is a heterogeneous group of disorders. Blistering may be severe in the neonatal period, making differentiation from the Herlitz type difficult. All conditions associated with the Herlitz type may be seen but are usually milder. JEB—non-Herlitz generalized (formerly generalized atrophic benign epidermolysis bullosa) is included as a variant of non-Herlitz JEB. Another variant of non-Herlitz JEB is associated with pyloric atresia.
In all types of JEB, a subepidermal blister is found on light microscopic examination, and electron microscopy demonstrates a cleavage plane in the lamina lucida, between the plasma membranes of the basal cells and the basal lamina. Absence or a great reduction of hemidesmosomes is seen on electron micrographs in JEB—Herlitz and some cases of JEB—non-Herlitz. The defect is in laminin 332 (formerly laminin 5), a glycoprotein associated with anchoring filaments beneath the hemidesmosomes. In JEB—non-Herlitz, defects have also been described in other hemidesmosomal components, such as Col17A1. In JEB—pyloric atresia, the defect is in the α6β4 integrin.
Treatment for junctional epidermolysis bullosa is supportive. The diet should provide adequate calories and supplemental iron. Infections should be treated promptly. Transfusions of packed red blood cells may be required if the patient shows no response to iron and erythropoietin therapy. Tissue-engineered skin grafts (artificial skin derived from human keratinocytes and fibroblasts) may be beneficial.
Dystrophic Epidermolysis Bullosa
All forms of DEB result from mutations in collagen VII, a major component of anchoring fibrils that tether the basement membrane and overlying epidermis to its dermal foundation. The blister is subepidermal in all types of DEB. The type and location of the mutation dictate the severity of the phenotype.
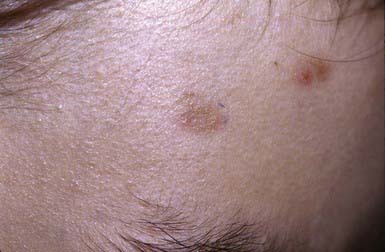
Dominant dystrophic epidermolysis bullosa (DDEB) is the most common type of DEB. The spectrum of DDEB is varied. Blisters may be manifest at birth and are often limited and characteristically form over acral bony prominences. The lesions heal promptly, with the formation of soft, wrinkled scars, milia, and alterations in pigmentation (Fig. 646-8). Abnormal nails and nail loss are common. In many cases, the blistering process is mild, causing little restriction of activity and not impairing growth and development. Mucous membrane involvement tends to be minimal.
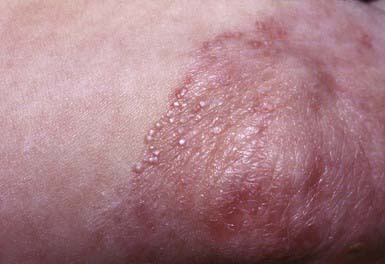
Figure 646-8 Scarring with milia formation over the knee in dominant dystrophic epidermolysis bullosa.
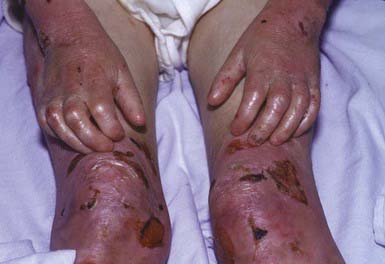
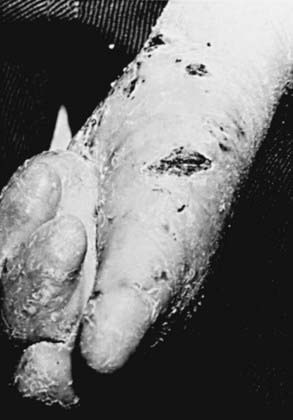
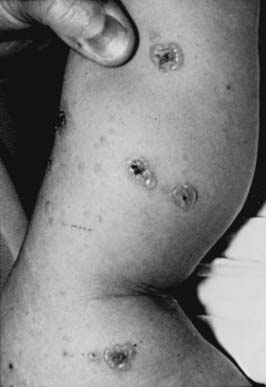
RDEB—severe generalized (formerly RDEB—Hallopeau-Siemens) is the most incapacitating form of epidermolysis bullosa, although the clinical spectrum is wide. Some patients have blisters, scarring, and milia formation primarily on the hands, feet, elbows, and knees (Fig. 646-9). Others have extensive erosions and blister formation at birth that seriously impedes their care and feeding. Mucous membrane lesions are common and may cause severe nutritional deprivation, even in older children, whose growth may be retarded. During childhood, esophageal erosions and strictures, scarring of the buccal mucosa, flexion contractures of joints secondary to scarring of the integument, development of cutaneous carcinomas, and the development of digital fusion may significantly limit the quality of life (Fig. 646-10).
Although the skin becomes less sensitive to trauma with aging in patients with RDEB, the progressive and permanent deformities complicate management, and the overall prognosis is poor. Foods that traumatize the buccal or esophageal mucosa should be avoided. If esophageal scarring develops, a semiliquid diet and esophageal dilatations may be required. Stricture excision or colonic interposition may be needed to relieve esophageal obstruction. In infants, severe oropharyngeal involvement may necessitate the use of special feeding devices such as a gastrostomy tube. Iron therapy for anemia, intermittent antibiotic therapy for secondary infections, which are a common cause of death, and periodic surgery for release of digits may reduce morbidity. Tissue-engineered skin grafts containing keratinocytes and fibroblasts are of some benefit. Allogeneic bone marrow transplantation may also be beneficial.
Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
Fine JD, Hall M, Weiner M, et al. The risk of cardiomyopathy in inherited epidermolysis bullosa. Br J Dermatol. 2008;159:677-682.
Fine JD, Johnson LB, Weiner M, et al. Cause-specific risks of childhood death in inherited epidermolysis bullosa. J Pediatr. 2008;152:276-280.
Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363(7):629-638.
646.5 Pemphigus
Pemphigus Vulgaris
Etiology/Pathogenesis
Pemphigus vulgaris (PV) is caused by circulating antibodies to desmoglein III that result in suprabasal cleaving with consequent blister formation. Desmoglein III is a 30-kd glycoprotein that is complexed with plakoglobin, a plaque protein of desmosomes. The desmogleins are a subfamily of the cadherin family of cell adhesion molecules.
Clinical Manifestations
PV usually first appears as painful oral ulcers, which may be the only evidence of the disease for weeks or months. Subsequently, large, flaccid bullae emerge on nonerythematous skin, most commonly on the face, trunk, pressure points, groin, and axillae. Nikolsky sign is present. The lesions rupture and enlarge peripherally, producing painful, raw, denuded areas that have little tendency to heal. When healing occurs, it is without scarring, but hyperpigmentation is common. Malodorous, verrucous, and granulomatous lesions may develop at sites of ruptured bullae, particularly in the skinfolds; as this pattern becomes more pronounced, the condition may be more properly referred to as pemphigus vegetans. Because the course may rapidly lead to debility, malnutrition, and death, prompt diagnosis is essential. Neonatal PV develops in utero as a result of placental transfer of maternal antidesmoglein antibodies from women who have active PV, although it may occur when the mother is in remission. High antepartum maternal titers of PV antibodies and increased maternal disease activity correlate with a poor fetal outcome, including demise.
Pathology
Biopsy of a fresh small blister reveals a suprabasal (intraepidermal) blister containing loose, acantholytic epidermal cells that have lost their intercellular bridges and thus their contact with one another. Immunofluorescence staining (IF) with an IgG antibody produces a characteristic pattern on direct immunofluorescence preparations of both involved and uninvolved skin of essentially all patients. Serum IgG antibody titers to desmoglein correlate with the clinical course in many patients; thus, serial determinations may have predictive value.
Differential Diagnosis
PV must be differentiated from EM, bullous pemphigoid, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
Treatment
The disease is best treated initially with systemic methylprednisolone 1-2 mg/kg/day. Azathioprine, cyclophosphamide, and methotrexate therapy all have been useful in maintenance regimens. IVIG given in cycles may be beneficial to patients whose disease does not respond to steroids. Rituximab with IVIG replacement has been effective in the management of severe pemphigus. Excellent control of the disease may be obtained, but relapse is common.
Pemphigus Foliaceus
Etiology/Pathogenesis
Pemphigus foliaceus is caused by circulating antibodies to a 50-kd portion of the 160-kd desmosomal glycoprotein desmoglein I, which result in subcorneal cleavage leading to superficial erosions. This extremely rare disorder is characterized by subcorneal blistering; the site of cleavage is high in the epidermis rather than suprabasal as in PV.
Clinical Manifestations
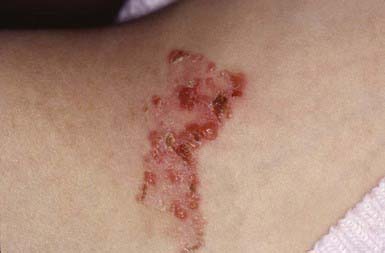
The superficial blisters rupture quickly, leaving erosions surrounded by erythema that heal with crusting and scaling (Fig. 646-11). Nikolsky sign is present. Focal lesions are usually localized to the scalp, face, neck, and upper trunk. Mucous membrane lesions are minimal or absent. Pruritus, pain, and a burning sensation are frequent complaints. The clinical course varies but is generally more benign than that of PV. Fogo selvagem, which is endemic in certain areas of Brazil, is identical clinically, histopathologically, and immunologically to pemphigus foliaceus.
Pathology
An intraepidermal acantholytic bulla high in the epidermis is diagnostic. It is imperative to select an early lesion for biopsy. Immunofluorescent staining with an IgG antibody reveals a characteristic intercellular staining pattern similar to that of PV but higher in the epidermis.
Differential Diagnosis
When generalized, the eruption may resemble exfoliative dermatitis or any of the chronic blistering disorders; localized erythematous plaques simulate seborrheic dermatitis, psoriasis, impetigo, eczema, and systemic lupus erythematosus.
For localized disease, superpotent topical steroids used twice a day may be all that is needed for control until remission. For more generalized disease, long-term remission is usual after suppression of the disease by systemic methylprednisolone (1 mg/kg/day) therapy. Dapsone (25-100 mg/day) also may be used.
Bullous Pemphigoid
Etiology/Pathogenesis
Bullous pemphigoid (BP) is caused by circulating antigens to either the 180-kd or 230-kd BP antigen that result in a subepidermal blister. The 230-kd protein is part of the hemidesmosome, whereas the 180-kd antigen localizes to both the hemidesmosome and the upper lamina lucida and is a transmembrane collagenous protein.
Clinical Manifestations
The blisters of BP typically arise in crops on a normal, erythematous, eczematous, or urticarial base. Bullae appear predominantly on the flexural aspects of the extremities, in the axillae, and on the groin and central abdomen. Infants have involvement of the palms, soles, and face more frequently than older children. Individual lesions vary greatly in size, are tense, and are filled with serous fluid that may become hemorrhagic or turbid. Oral lesions occur less frequently and are less severe than in PV. Pruritus, a burning sensation, and subcutaneous edema may accompany the eruption, but constitutional symptoms are not prominent.
Pathology
Biopsy material should be taken from an early bulla arising on an erythematous base. A subepidermal bulla and a dermal inflammatory infiltrate, predominantly of eosinophils, can be identified histopathologically. In sections of a blister or perilesional skin, a band of immunoglobulin (usually IgG) and C3 can be demonstrated in the basement membrane zone by direct immunofluorescence. Indirect immunofluorescence studies of serum have positive results in ≈ 70% of cases for IgG antibodies to the basement membrane zone; the titers, however, do not correlate well with the clinical course.
Diagnosis and Differential Diagnoses
BP rarely occurs in children but must be considered in the differential diagnosis of any chronic blistering disorder. The differential diagnosis includes bullous erythema multiforme, pemphigus, linear IgA dermatosis, bullous drug eruption, dermatitis herpetiformis, herpes simplex infection, and bullous impetigo, which can be differentiated by histologic examination, immunofluorescence studies, and cultures. The large, tense bullae of BP can generally be distinguished from the smaller, flaccid bullae of PV.
Treatment
Localized bullous pemphigoid can be successfully suppressed with superpotent topical twice a day. Generalized disease usually requires systemic methylprednisolone (1 mg/kg/day) therapy. Rarely are other immunosuppressive treatments necessary. Ultimately, the condition usually remits permanently.
Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60:595-603.
Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2009;357:545-552.
Razzaque Ahmed A, Spigelman Z, Cavacini LA, et al. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355:1772-1778.
Yazganoglu KD, Baykal C, Kucukoglu R. Childhood pemphigus vulgaris: five cases in 16 years. J Dermatol. 2006;33:846-849.
646.6 Dermatitis Herpetiformis
Etiology/Pathogenesis
In dermatitis herpetiformis (DH), IgA antibodies are directed at epidermal transglutaminase. Gluten-sensitive enteropathy is found in all patients with DH, although the majority are asymptomatic or have minimal gastrointestinal symptoms (Chapter 330.2). The severity of the skin disease and the responsiveness to gluten restriction do not correlate with the severity of the intestinal inflammation. An antibody to smooth muscle endomysium is found in 70-90% of patients with DH. Ninety percent of patients with the disease express HLA DQ2. HLA DQ2–negative patients with DH usually express HLA DQ8.
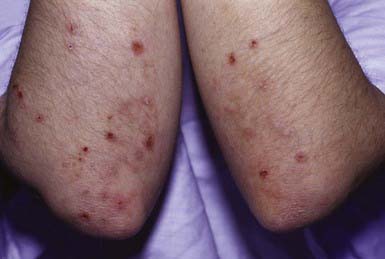
Clinical Manifestations
DH is characterized by symmetric, grouped, small, tense, erythematous, stinging, intensely pruritic papules and vesicles. The eruption is pleomorphic, including erythematous, urticarial, papular, vesicular, and bullous lesions. Sites of predilection are the knees, elbows, shoulders, buttocks, and scalp; mucous membranes are usually spared. Hemorrhagic lesions may develop on the palms and soles. When pruritus is severe, excoriations may be the only visible sign (Fig. 646-12).
Pathology
Subepidermal blisters composed predominantly of neutrophils are found in dermal papillae. The presence of granular IgA in the dermal papillary tips is diagnostic.
Differential Diagnosis
DH may mimic other chronic blistering diseases and may also resemble scabies, papular urticaria, insect bites, contact dermatitis, and papular eczema.
Treatment
Patients with DH show response within weeks to months to a gluten-free diet. Alternatively, oral administration of dapsone (0.5-2.0 mg/kg/day divided qd or bid) provides immediate relief from the intense pruritus but must be used with caution because of possible serious side effects (methemoglobinemia, hemolysis, and hypersensitivity syndrome [sulfone syndrome]). Local antipruritic measures may also be useful. Jejunal biopsy is indicated to diagnose gluten-sensitive enteropathy, because cutaneous manifestations may precede malabsorption. The disease is chronic and either a gluten-free diet or dapsone must be continued indefinitely to prevent relapse.
Alonso-Llamazares J, Gibson LE, Rogers RS. Clinical, pathologic and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007;46:910-919.
Templet JT, Welsh JP, Cusack CA. Childhood dermatitis herpetiformis: a case report and review of the literature. Cutis. 2007;80:473-476.
Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91.
646.7 Linear IgA Dermatosis (Chronic Bullous Dermatosis of Childhood)
Etiology/Pathogenesis
Linear IgA dermatosis is a heterogeneous autoimmune disorder with antibodies targeting multiple antigens. It is caused by circulating IgA antibodies, most commonly to LABD97 and LAD-1, which are degradation proteins of BP180 (type 17 collagen). Linear IgA dermatosis may also be seen as a drug eruption. Most cases of drug-induced linear IgA dermatosis are related to vancomycin, although anticonvulsants, ampicillin, cyclosporine, and captopril have been implicated.
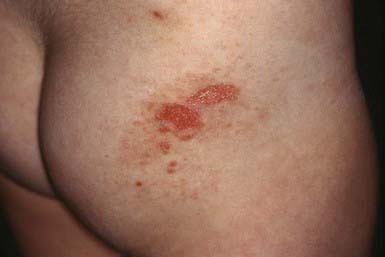
Clinical Manifestations
This rare dermatosis is most common in the 1st decade of life, with a peak incidence during the preschool years. The eruption consists of many large, tense bullae filled with clear or hemorrhagic fluid that develop on a normal or erythematous, urticarial base. Areas of predilection are the genitals and buttocks (Fig. 646-13), the perioral region, and the scalp. Sausage-shaped bullae may be arranged in an annular or rosette-like fashion around a central crust (Fig. 646-14). Erythematous plaques with gyrate margins bordered by intact bullae may develop over larger areas. Pruritus may be absent or very intense, and systemic signs or symptoms are absent.
Pathology
The subepidermal bullae are infiltrated with a mixture of inflammatory cells. Neutrophilic abscesses may be noted in the dermal papillary tips, indistinguishable from those of dermatitis herpetiformis. The infiltrate may also be largely eosinophilic, resembling that in BP. Therefore, direct immunofluorescence studies are required for a definitive diagnosis of linear IgA dermatosis; perilesional skin demonstrates linear deposition of IgA and sometimes IgG and C3 at the dermal-epidermal junction. Immunoelectron microscopy has localized the immunoreactants to the sublamina densa, although a combined sublamina densa and lamina lucida pattern has also been seen.
Differential Diagnosis
The eruption can be distinguished by histopathologic and immunofluorescence studies from pemphigus, BP, DH, and EM. Gram stain and culture preclude the diagnosis of bullous impetigo.
Treatment
Many cases of linear IgA dermatosis respond favorably to oral dapsone (see treatment of DH). Children who show no response to dapsone may benefit from oral therapy with a methylprednisolone (1 mg/kg/day) or a combination of these drugs. The usual course is 2-4 yr, although some children have persistent or recurrent disease; there are no long-term sequelae.
Ho JC, NG PL, Tan SH, et al. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. 2007;24:E40-E43.
Nanda A, Dvorak R, Al-Sabah H, et al. Linear IgA disease of childhood: an experience from Kuwait. Pediatric Dermatol. 2006;23:443-447.
Onodera H, Mohm MCJr, Yoshinda A, et al. Drug-induced linear IgA bullous dermatosis. J Dermatol. 2005;32:759-764.