chapter 2 Anatomy of the Lower Urinary Tract and Male Genitalia
This chapter provides a general anatomic framework to guide the pelvic surgeon. The bony, ligamentous, and muscular framework of the pelvis is presented first. Next, the pelvic vessels and nerves and the genital, urinary, and gastrointestinal viscera are discussed. Finally, the perineum and external genitalia are reviewed.
Bony Pelvis
The pelvic bones are the sacrum (the termination of the axial skeleton) and the two innominate bones. The latter are formed by the fusion of the iliac, ischial, and pubic ossification centers at the acetabulum (Fig. 2–1). The ischium and pubis also meet below, in the center of the inferior ramus, to form the obturator foramen. The weight of the upper body is transmitted from the axial skeleton to the innominate bones and lower extremities through the strong sacroiliac (SI) joints. As a whole, the pelvis is divided into a bowl-shaped false pelvis, formed by the iliac fossae and largely in contact with intraperitoneal contents, and the circular true pelvis wherein the urogenital organs lie. At the pelvic inlet, the true and false pelves are separated by the arcuate line, which extends from the sacral promontory to the pectineal line of the pubis. The lumbar lordosis that accompanies erect posture tilts the axis of the pelvic inlet so that it parallels the ground; the pelvic inlet faces anteriorly, and the inferior ischiopubic rami lie horizontal (Fig. 2–2). When approaching the pelvis through a low midline incision, the surgeon gazes directly into the true pelvis.
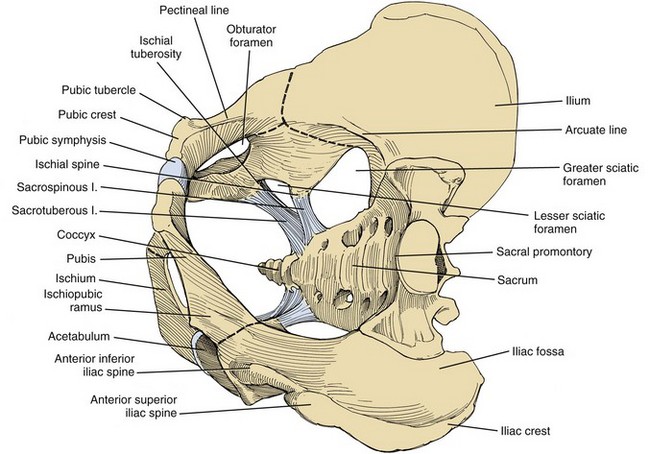
Figure 2–1 The bones and ligaments of the pelvis.
(From Hinman F Jr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993. p. 196.)
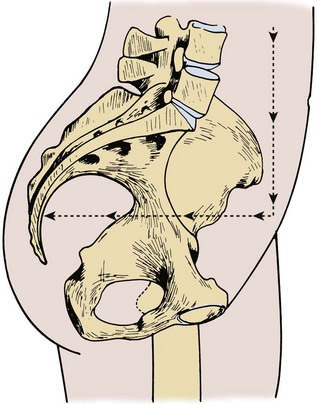
Figure 2–2 Pelvis in standing position. The axis of the pelvic cavity is horizontal because of lumbar lordosis.
(From Zacharin RF. Pelvic floor anatomy and the surgery of pulsion enterocele. New York: Springer-Verlag; 1985. p. 15.)
The anterior and posterior iliac spines, the iliac crests, the pubic tubercles, and the ischial tuberosities are palpable landmarks that orient the pelvic surgeon (see Fig. 2–1). Cooper (pectineal) ligament overlies the pectineal line and offers a sure hold for sutures in hernia repairs and urethral suspension procedures (Fig. 2–3). The ischial spine is palpable transvaginally and attaches to the pelvic diaphragm and the sacrospinous ligament. The sacrospinous ligament separates the greater and lesser sciatic foramina. Together with the sacrotuberous ligament, it stabilizes the SI joint by preventing downward rotation of the sacral promontory. The SI joint, synovial in type, gains additional strength from anterior and posterior ligaments. In pelvic trauma, fractures virtually never involve this joint but occur adjacent to it. The pubes, the thinnest of the pelvic bones, are nearly always fractured, and their fragments may injure the adjacent bladder, urethra, and vagina. Resection or congenital nonunion of the pubes (e.g., bladder exstrophy) does not affect ambulation because of the strength of the SI joint (Waterhouse et al, 1973; Golimbu et al, 1990).
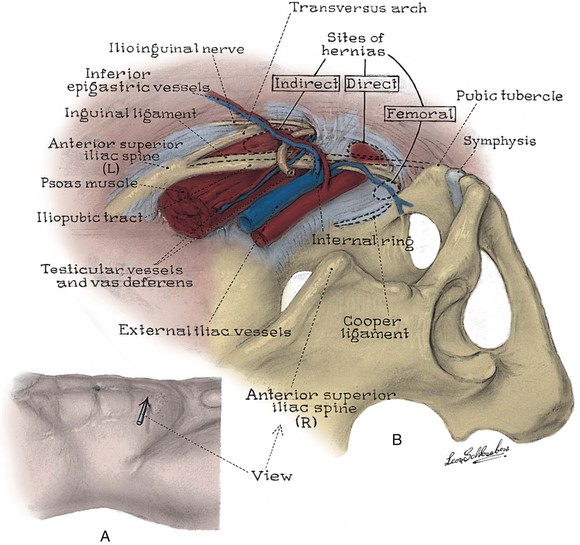
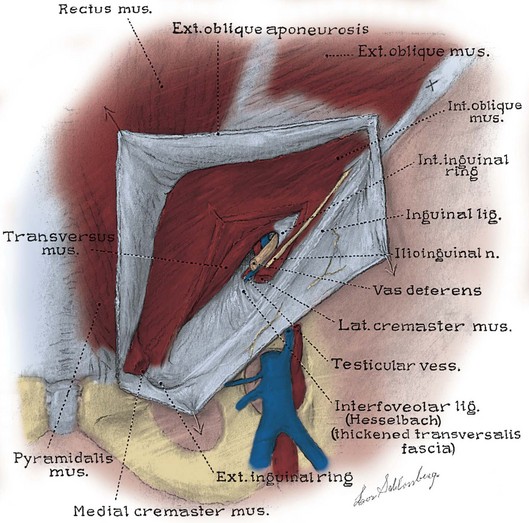
Figure 2–3 Topography (A) and posterior wall (B) of the left inguinal canal, viewed from the preperitoneal space. The location of three types of inguinal hernia is demonstrated.
(From Schlegel PN, Walsh PC. Simultaneous preperitoneal hernia repair during radical pelvic surgery. J Urol 1987;137:1181.)
Anterior Abdominal Wall
Skin and Subcutaneous Fasciae
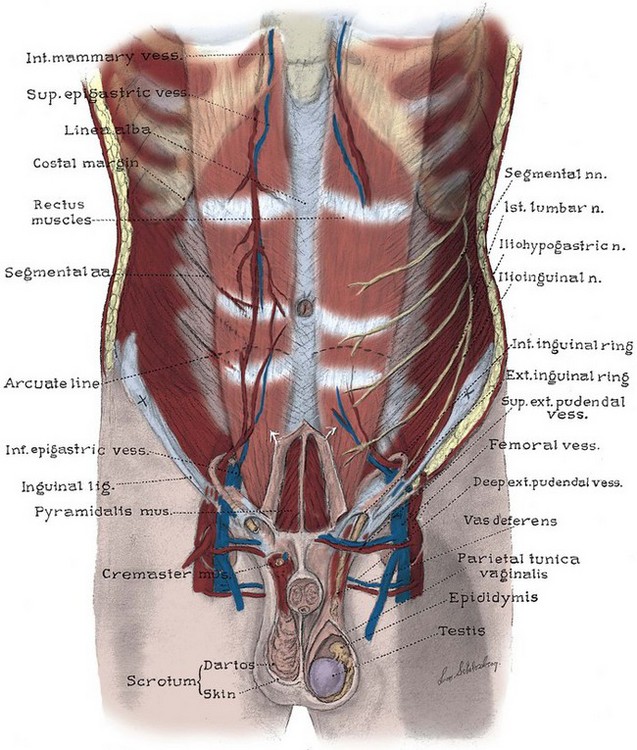
To minimize scarring, incisions of the anterior abdominal wall and flank should follow Langer lines of cleavage. These lines parallel dermal collagen fibers and are oriented along lines of stress. They correspond to the segmental thoracic and lumbar nerves. The skin is backed by Camper fascia, a loose layer of fatty tissue that varies in thickness with the nutritional status of the patient. The superficial circumflex iliac, external pudendal, and superficial inferior epigastric vessels branch from the femoral vessels to run in this layer (Figs. 2-4 and 2-5). The superficial inferior epigastric vessels are encountered during inguinal incisions and can cause troublesome bleeding during placement of pelvic laparoscopic ports.
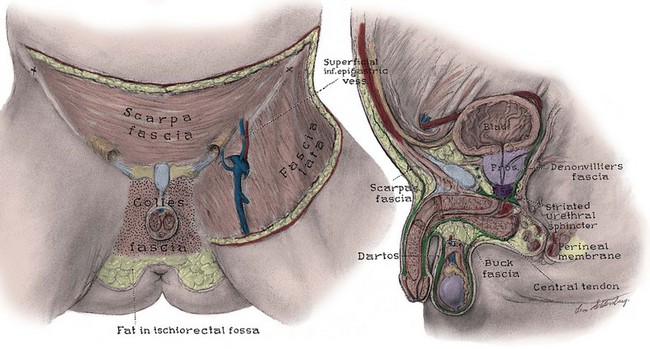
Figure 2–4 Left, Anterior view of the deep fasciae of the abdomen, perineum, and thigh. Note the superficial inferior epigastric artery passing superiorly in Camper fascia. Right, Midline sagittal view of the pelvic fasciae and their attachments.
Scarpa fascia forms a distinct layer deep to Camper fascia, although it may be difficult to discern in older patients. Superiorly and laterally, it blends with Camper fascia. Inferiorly, it fuses with the deep fascia of the thigh 1 cm below the inguinal ligament along a line from the anterior superior iliac spine to the pubic tubercle. Medially, it is continuous with Colles fascia of the perineum (see Fig. 2–4). Colles fascia attaches to the posterior edge of the urogenital diaphragm and the inferior ischiopubic rami. It is continuous with the dartos fascia of the penis and scrotum. These fasciae can limit both the spread of infection in necrotizing fasciitis of the scrotum (Fournier gangrene) and the extent of urinary extravasation in an anterior urethral injury. For instance, blood and urine can accumulate in the scrotum and penis deep to the dartos fascia after an anterior urethral injury. In the perineum, their spread is limited by the fusions of Colles fascia to the ischiopubic rami laterally and to the posterior edge of the perineal membrane; the resulting hematoma is therefore butterfly shaped. Because of these fasciae, bleeding, infection, or urinary extravasation will not extend down the leg or into the buttock but can freely travel up the anterior abdominal wall deep to Scarpa fascia to the clavicles and around the flank to the back.
Abdominal Musculature
The abdominal musculature lies immediately below Scarpa fascia. The origins of the external oblique, internal oblique, and transversus abdominis muscles and the orientation of their fibers are presented in Chapter 1. These muscles terminate on the anterior abdominal wall as broad, tough aponeurotic sheets that fuse in the midline (linea alba) and form the rectus sheath (see Fig. 2–5). The linea alba is avascular and is a convenient point of access to the peritoneal and pelvic cavities. In its upper portion, the anterior rectus sheath is formed by the aponeurosis of the external oblique muscle and a portion of the internal oblique muscle (Fig. 2–6). The posterior sheath is derived from the remaining internal oblique aponeurosis and the transversus abdominis aponeurosis. Two thirds of the distance from the pubis to the umbilicus, the arcuate line is formed, as all aponeurotic layers abruptly pass anterior to the rectus abdominis, leaving this muscle clothed only by transversalis fascia and peritoneum posteriorly.
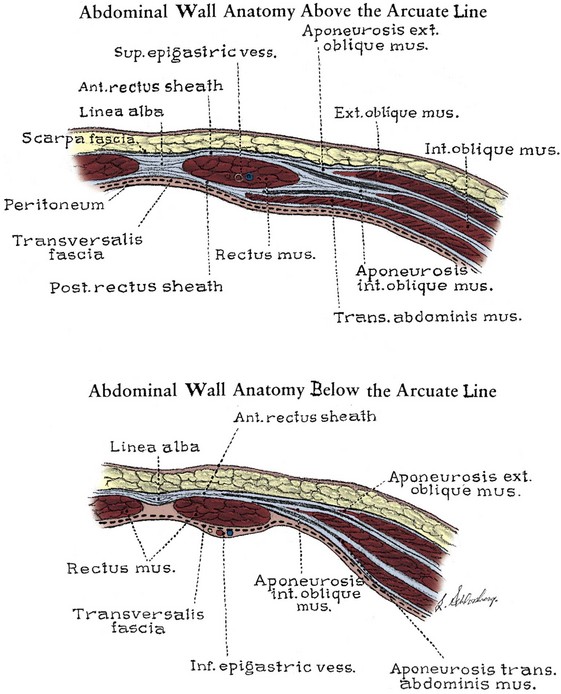
Figure 2–6 Cross section of the rectus sheath. Top, Above the arcuate line, the aponeurosis of the external oblique muscle forms the anterior sheath, and the transversus aponeurosis forms the posterior sheath. The internal oblique muscle splits to contribute to both the anterior and the posterior sheaths. Bottom, Below the arcuate line, all aponeuroses pass anterior to the rectus.
The rectus abdominis arises from the pubis medial to the pubic tubercle and inserts on the xyphoid process and adjacent costal cartilages. The muscle is crossed by three or four tendinous intersections that are firmly attached to the anterior rectus sheath; thus the muscle can be divided transversely without significant retraction. It is supplied by the last six thoracic segmental nerves that enter it laterally. Paramedian incisions lateral to the rectus divide these nerves, cause atrophy of the rectus, and predispose to ventral hernia. Anterior to the rectus and within its sheath, the triangle-shaped pyramidalis muscle arises from the pubic crest and inserts into the linea alba (see Fig. 2–5). It is supplied by the subcostal nerve (T12).
Inguinal Canal
The inguinal canal transmits the spermatic cord in the male, the round ligament in the female, and the ilioinguinal nerve in both sexes (Fig. 2–7; see also Fig. 2–5). Its anterior wall and floor are formed by the external oblique muscle, which folds over at its inferior edge as the inguinal ligament. Above the pubic tubercle, the fibers of the external oblique aponeurosis split to form the lateral edges (crura) of the external inguinal ring. Transverse (intercrural) fibers bridge the crura to form the superior edge of the external ring. By dividing the intracrural fibers, the external oblique can be separated along its fibers to gain access to the cord. The posterior wall of the canal is formed by the transversalis fascia, which lines the inner surface of the abdominal wall. The cord structures pierce this fascia lateral to the inferior epigastric vessels at the internal inguinal ring (see Fig. 2–3). The internal inguinal ring lies midway between the anterior superior iliac spine and the pubic tubercle, above the inguinal ligament, and 4 cm lateral to the external ring. Fibers of the internal oblique and transversus abdominis arise from the iliopsoas fascia and inguinal ligament lateral to the internal ring and arch over the canal to form its roof. They fuse as the conjoint tendon, pass posterior to the cord, and insert into the rectus sheath and pubis. The conjoint tendon reinforces the posterior wall of the inguinal canal at the external ring. With contraction of the internal oblique and transversus muscles, the roof of the canal closes against the floor, preventing herniation of intra-abdominal contents into the canal. Hernias into the canal may occur medial (direct) or lateral (indirect) to the inferior epigastric vessels (see Figs. 2-3 and 2-7).
Internal Surface of the Anterior Abdominal Wall
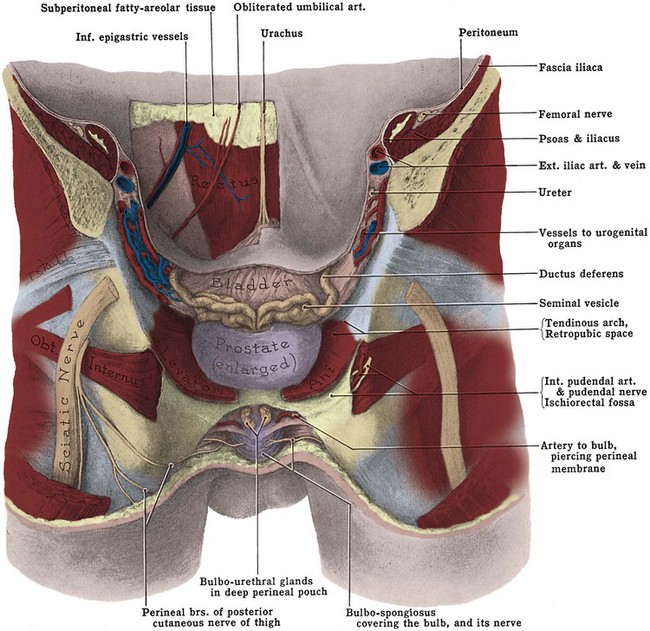
Approached laparoscopically, three elevations of the peritoneum, referred to as the median, medial, and lateral umbilical folds, are visible on the anterior abdominal wall below the umbilicus (Fig. 2–8). The median fold overlies the median umbilical ligament (urachus), a fibrous remnant of the cloaca that attaches the bladder to the anterior abdominal wall. The obliterated umbilical artery in the medial umbilical fold serves as an important landmark for the surgeon. It may be traced to its origin from the internal iliac artery to locate the ureter, which lies on its medial side. During transperitoneal laparoscopic pelvic lymph node dissection, the obturator packet is accessed by incising the peritoneum lateral to the obliterated umbilical artery. In addition, during the performance of transperitoneal laparoscopic or robotic radical prostatectomy, the medial umbilical folds are used as landmarks to guide the dissection of the bladder to expose the space of Retzius. The lateral umbilical fold contains the inferior epigastric vessels as they ascend to supply the rectus abdominis.
Soft Tissues of the Pelvis
Pelvic Musculature
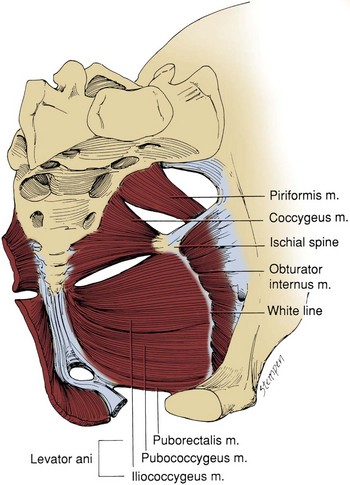
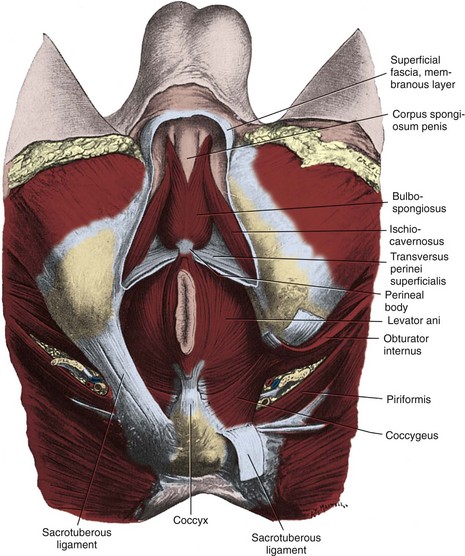
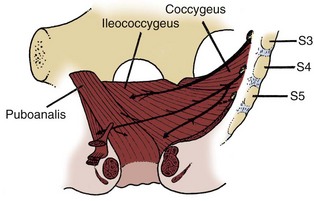
Muscles and fascia line the true pelvis and form its floor. The obturator internus arises from the inner surface of the obturator foramen and the obturator membrane and passes through the lesser sciatic foramen to insert on the femur (see Fig. 2–8). The fascia on the pelvic surface of this muscle is thickened into a tough line extending from the lower half of the pubis to the ischial spine. This tendinous arc of the levator ani serves as the origin of the muscles of the pelvic diaphragm: pubococcygeus and iliococcygeus (Fig. 2–9). These muscles are not truly separable, and they form a diaphragm that closes the pelvic outlet. Anteriorly, a narrow U-shaped hiatus remains, through which the urethra and rectum exit in the male, and the urethra, vagina, and rectum exit in the female (Fig. 2–10). The muscle bordering this hiatus has been referred to as pubovisceral because it provides a sling for (pubourethralis, puborectalis), inserts directly into (pubovaginalis, puboanalis, levator prostatae), or inserts into a structure intimately associated with the pelvic viscera (Lawson, 1974). The pubovisceral group provides strong fixation and support for the pelvic viscera. The coccygeus muscle extends from the sacrospinous ligament to the lateral border of the sacrum and coccyx to complete the pelvic diaphragm. Muscles of the pelvic diaphragm contain type I (slow-twitch) fibers, which provide tonic support to pelvic structures, and type II (fast-twitch) fibers, for sudden increases in intra-abdominal pressure (Gosling et al, 1981).The piriformis muscle arises from the lateral aspect of the sacrum and passes through and fills the greater sciatic foramen to form the posterolateral wall of the pelvis.
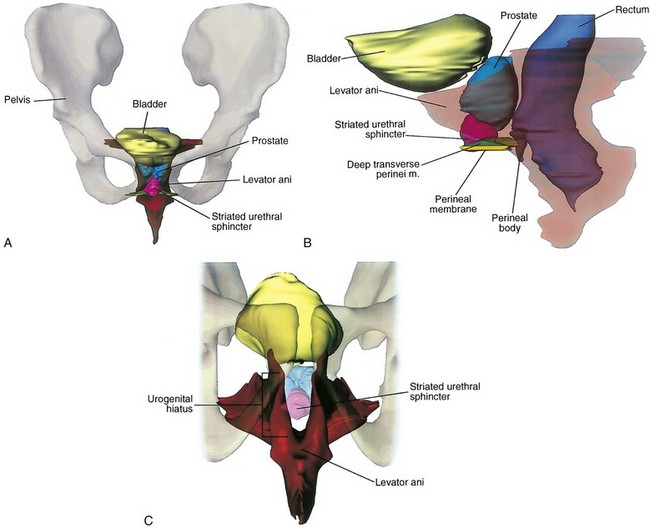
Figure 2–10 Location and contour of the levator ani and pelvic viscera. A, Anterior view demonstrating the near-vertical orientation of the lateral walls of the levator ani and the horizontal wings at its posterior superior aspect. B, Lateral view in which the levator ani has been made transparent. The perineal membrane bridges the urogenital hiatus, and the urethral sphincter fills much of the hiatus. C, View of the levator ani from below showing the urogenital hiatus and the thickened inferior border of the levator ani. The perineal body and related structures are not shown.
(From Brooks JD, Chao W-M, Kerr J. Male pelvic anatomy reconstructed from the visible human data set. J Urol 1998;159:868–72.)
It is important to recognize that the pelvic diaphragm is not flat or bowl shaped, as it is frequently depicted. At the urogenital and anal hiatus, the muscles lie in a near-vertical configuration and are thickened inferiorly (see Fig. 2–10) (Brooks et al, 1998; Myers et al, 1998). Behind the anus, they flatten to form a nearly horizontal diaphragm, referred to as the levator plate. In the female, the levator plate provides critical support to the pelvic viscera, as discussed later.
Pelvic Fasciae
The pelvic fasciae are not merely collagenous; they are also rich in elastic tissue and smooth muscle. This suggests that they are active in the support, and possibly the function, of the pelvic viscera. The pelvic fasciae are continuous with the retroperitoneal fasciae and have been categorized somewhat arbitrarily into outer, intermediate, and inner strata. The outer stratum, or endopelvic fascia, lines the inner surface of the pelvic muscles and is continuous with the transversalis layer of the abdomen. It is fixed to the arcuate line of the pelvis, Cooper ligament, the sacrospinous ligament, the ischial spine, and tendinous arc of the levator ani. The intermediate stratum embeds the pelvic viscera in a fatty, compressible layer that accommodates their filling and emptying. Its tissues are easily swept aside to reveal the retropubic, paravesical, rectogenital, and retrorectal potential spaces. All pelvic vessels and some pelvic nerves travel in this stratum and are subject to injury when these potential spaces are developed at surgery. The intermediate stratum coalesces around vessels and nerves supplying the pelvic organs to form named ligaments (e.g., cardinal, uterosacral, lateral, and posterior vesical) that suspend and tether these organs in the pelvis. This fascia also thickens around the pelvic urogenital organs to form their visceral fascia. These are not true ligaments but rather a meshwork of connective tissue and smooth muscle investing the visceral neurovascular pedicles (DeCaro et al, 1998). The inner stratum lies just beneath the peritoneum and is associated with the entire gastrointestinal tract. In the pelvis, it covers the rectum and dome of the bladder and forms the rectogenital septum (Denonvilliers fascia). This septum is the developmental remains of the rectogenital pouch of peritoneum that extended between the rectum and internal genitalia to the pelvic floor.
The pelvic fasciae have been given a confusing array of appellations by anatomists and surgeons interested in female pelvic organ prolapse. To add to the confusion, the strength of pelvic fasciae can differ significantly between individuals and races and these differences may predispose some individuals to pelvic prolapse (Zacharin, 1985). The pelvic fasciae have three important components: (1) Anteriorly, the puboprostatic ligaments attach to the lower fifth of the pubis, lateral to the symphysis and to the junction of the prostate and external sphincter. They are called the pubourethral ligaments in the female and insert on the proximal third of the urethra (Fig. 2–11 on the Expert Consult website![]() ). (2) Laterally, the arcus tendineus fascia pelvis extends from the puboprostatic (pubourethral) ligament to the ischial spine (see Fig. 2–11). This fascia forms at the junction of the endopelvic and visceral fasciae. It should not be confused with the arcus tendineus levator ani, which lies above its anterior portion (Fig. 2–12). In the male, the arcus tendineus fascia pelvis is found at the base of a sulcus between the pelvic side wall and the prostate and bladder. In the female, it corresponds to the lateral attachment of the anterior bladder wall to the pelvic side wall. Paravaginal suspension procedures for stress urinary incontinence entail lateral reapproximation of the vaginal wall to this tendinous arc (Richardson et al, 1981). The lateral branches of the dorsal venous complex are directly beneath the arcus tendineus fascia pelvis; thus the endopelvic fascia should be opened lateral to this landmark. In the female, the fascia extending medially from this arch carries a variety of names (pubovesical, periurethral, urethropelvic ligament) and provides important support to the urethra and anterior vaginal wall. Damage to this fascia and its attachments has been implicated in urethrocele, cystocele, and stress urinary incontinence. (3) Posterior to the ischial spine, the fascia fans out to either side of the rectum and attaches to the pelvic side wall as the lateral and posterior vesical ligaments. In the female, these are the strong cardinal and uterosacral ligaments. They are not true ligaments; rather, they are condensations of intermediate stratum around visceral neurovascular pedicles. The peritoneum over these ligaments forms discrete folds (rectovesical in the male and rectouterine in the female) that can be appreciated at cystectomy (Fig. 2–13). Taken as a whole, the pelvic fasciae form a Y-shaped scaffolding for the pelvic viscera (see Fig. 2–12).
). (2) Laterally, the arcus tendineus fascia pelvis extends from the puboprostatic (pubourethral) ligament to the ischial spine (see Fig. 2–11). This fascia forms at the junction of the endopelvic and visceral fasciae. It should not be confused with the arcus tendineus levator ani, which lies above its anterior portion (Fig. 2–12). In the male, the arcus tendineus fascia pelvis is found at the base of a sulcus between the pelvic side wall and the prostate and bladder. In the female, it corresponds to the lateral attachment of the anterior bladder wall to the pelvic side wall. Paravaginal suspension procedures for stress urinary incontinence entail lateral reapproximation of the vaginal wall to this tendinous arc (Richardson et al, 1981). The lateral branches of the dorsal venous complex are directly beneath the arcus tendineus fascia pelvis; thus the endopelvic fascia should be opened lateral to this landmark. In the female, the fascia extending medially from this arch carries a variety of names (pubovesical, periurethral, urethropelvic ligament) and provides important support to the urethra and anterior vaginal wall. Damage to this fascia and its attachments has been implicated in urethrocele, cystocele, and stress urinary incontinence. (3) Posterior to the ischial spine, the fascia fans out to either side of the rectum and attaches to the pelvic side wall as the lateral and posterior vesical ligaments. In the female, these are the strong cardinal and uterosacral ligaments. They are not true ligaments; rather, they are condensations of intermediate stratum around visceral neurovascular pedicles. The peritoneum over these ligaments forms discrete folds (rectovesical in the male and rectouterine in the female) that can be appreciated at cystectomy (Fig. 2–13). Taken as a whole, the pelvic fasciae form a Y-shaped scaffolding for the pelvic viscera (see Fig. 2–12).
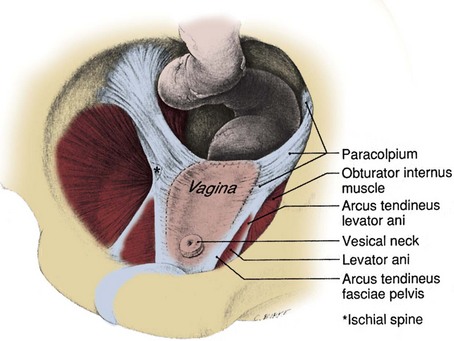
Figure 2–12 Vagina and supportive structures after removal of the bladder and uterus. The arcus tendineus fascia pelvis and the cardinal and uterosacral ligaments (paracolpium) form a continuous structure that supports the pelvic viscera.
(From DeLancey JOL. Structural support of the urethra as it relates to stress urinary continence: the hammock hypothesis. Am J Obstet Gynecol 1994;170:1719.)
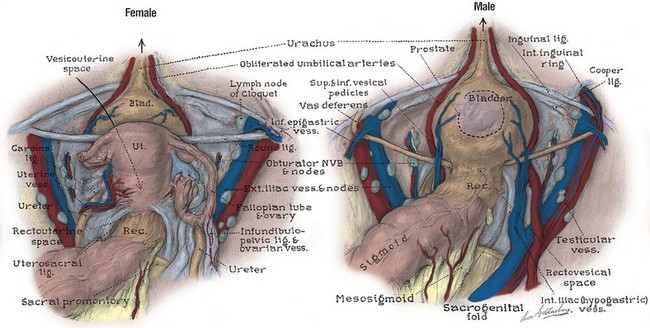
Figure 2–13 Peritoneal surfaces of the female and male pelves. In the female, the ureter passes medial to the ovarian vessels, then deep to the uterine artery within the substance of the cardinal ligament. The sacrogenital and sacrouterine folds represent the posterior portions of pelvic fascial support.
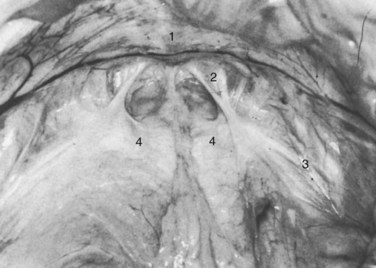
Figure 2–11 Floor of the space of Retzius in a thin, elderly female cadaver. The fat has been removed to show the continuous sheet of endopelvic fascia, and the bladder has been retracted posteriorly. 1, Symphysis pubis; 2, right pubourethral ligament; 3, lateral condensation of endopelvic fascia forming the right arcus tendineus fasciae pelvis; 4, condensation of the endopelvic fascia, which forms a firm, whitish aponeurosis over the proximal urethra and internal vesical orifice.
(From Mostwin JL. Current concepts of female pelvic anatomy and physiology. Urol Clin North Am 1991;18:178.)
Fasciae of the Perineum and the Perineal Body
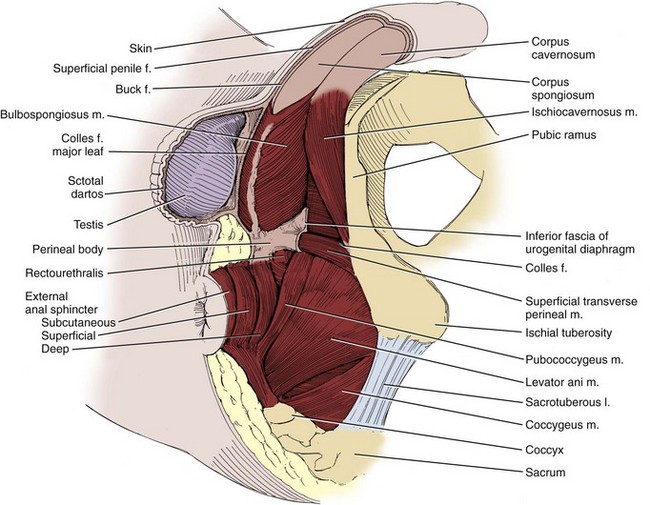
The weakest point in the pelvic floor, the urogenital hiatus, is bridged by the urogenital diaphragm, a structure unique to humans (see Fig. 2–10). The fibrous perineal membrane lies at the center of, and defines, the urogenital diaphragm (see Figs. 2-4, 2-10, and 2-14). It is triangular and spans the inferior ischiopubic rami from the pubis to the ischial tuberosities. Posteriorly, it ends abruptly; the superficial and deep transverse perinei run along its free edge (Fig. 2–15). The external genitalia attach to its inferior surface; superiorly, it supports the urethral sphincter (discussed later). The perineal body represents the point of fusion between the free posterior edge of the urogenital diaphragm and the posterior apex of the urogenital hiatus. This pyramid-shaped structure forms the hub of pelvic support. Virtually every pelvic muscle (superficial and deep transverse perinei, bulbocavernosus, levator ani, rectourethralis, external anal sphincter, striated urethral sphincter) and fascia (perineal membrane, Denonvilliers, Colles, and endopelvic) insert into the perineal body. At its core are abundant elastin and richly innervated smooth muscle, which suggests that it may have a dynamic role in support. Damage to the perineal body during perineal prostatectomy risks postoperative urinary incontinence.
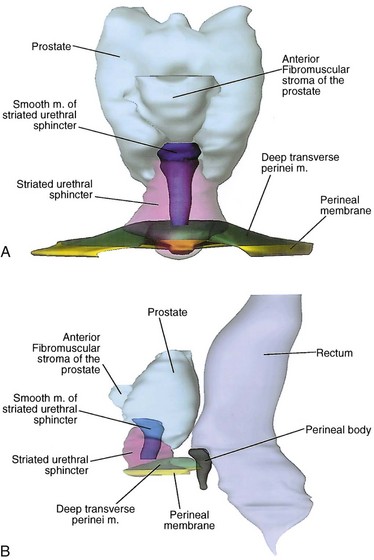
Figure 2–14 Structure of the male striated urethral sphincter. A, Anterior projection shows the cone shape of the sphincter and the smooth muscle of the sphincter. B, Viewed laterally, the anterior wall of the sphincter is nearly twice the length of the posterior wall, although both are of comparable thickness.
(From Brooks JD, Chao W-M, Kerr J. Male pelvic anatomy reconstructed from the visible human data set. J Urol 1998;159:871.)
Pelvic Circulation
Arterial Supply
Major arteries of the pelvis are summarized in Table 2–1. At the bifurcation of the aorta, the middle sacral artery arises posteriorly and travels on the pelvic surface of the sacrum to supply branches to the sacral foramina and the rectum. The common iliac arteries arise at the level of the fourth lumbar vertebra, run anterior and lateral to their accompanying veins, and bifurcate into the external and internal iliac arteries at the SI joint (Fig. 2–16). The external iliac artery follows the medial border of the iliopsoas muscle along the arcuate line and leaves the pelvis beneath the inguinal ligament as the femoral artery (Fig. 2–17). Its inferior epigastric artery is given off proximal to the inguinal ligament and ascends medial to the internal inguinal ring to supply the rectus muscle and overlying skin. Because the rectus is richly collateralized from above and laterally, the inferior epigastric arteries may be ligated with impunity. A rectus myocutaneous flap based on this artery has been used to correct major pelvic and perineal tissue defects. Near its origin, the inferior epigastric artery sends a deep circumflex iliac branch laterally and a pubic branch medially. Both vessels travel on the iliopubic tract and may be injured during inguinal hernia repair. Its cremasteric branch joins the spermatic cord at the internal inguinal ring and forms a distal anastomosis with the testicular artery (Fig. 2–18). In 25% of people, an accessory obturator artery arises from the inferior epigastric artery and runs medial to the femoral vein to reach the obturator canal. This vessel must be avoided during obturator lymph node dissection.
Table 2–1 Arteries of the Pelvis
| ARTERY NAME | ORIGIN | SUPPLIES |
|---|---|---|
| Middle sacral | Aorta | Sacral nerves and sacrum |
| External Iliac Branches | ||
| Inferior epigastric | External iliac | Rectus abdominis muscle and overlying skin and fascia |
| Deep circumflex iliac | Inferior epigastric | Inguinal ligament and surrounding structures laterally |
| Pubic | Inferior epigastric | Inguinal ligament and surrounding structures medially |
| Cremasteric | Inferior epigastric | Vas deferens and testis |
| Internal Iliac Branches | ||
| Superior gluteal | Posterior trunk | Gluteus muscles and overlying skin |
| Ascending lumbar | Posterior trunk | Psoas and quadratus lumborum muscles and adjacent structures |
| Lateral sacral | Posterior trunk | Sacral nerves and sacrum |
| Superior vesical | Anterior trunk | Bladder, ureter, vas deferens, and seminal vesicle |
| Middle rectal | Anterior trunk | Rectum, ureter, and bladder |
| Inferior vesical | Anterior trunk | Bladder, seminal vesicle, prostate, ureter, and the neurovascular bundle |
| Uterine | Anterior trunk | Uterus, bladder, and ureter |
| Internal pudendal | Anterior trunk | Rectum, perineum, and external genitalia |
| Obturator | Anterior trunk | Adductor muscles of the leg and overlying skin |
| Inferior gluteal | Anterior trunk | Gluteus muscles and overlying skin |
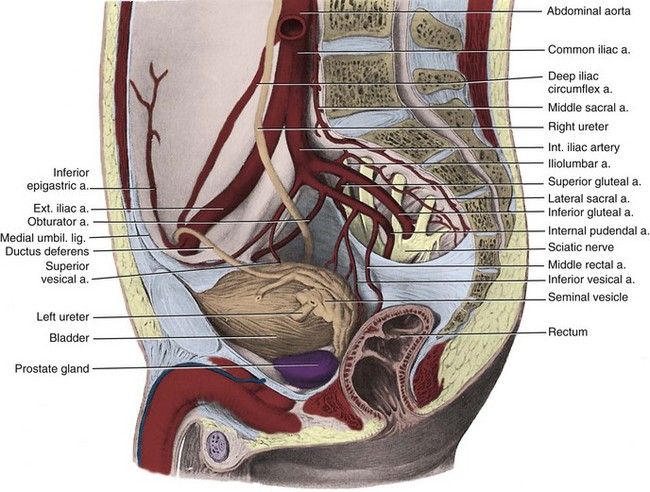
Figure 2–16 Right internal and external iliac arteries. The ureter and vas deferens pass medial to the vessels.
(From Clemente CD. Gray’s anatomy. 30th American ed. Philadelphia: Lea & Febiger; 1985. p. 750.)
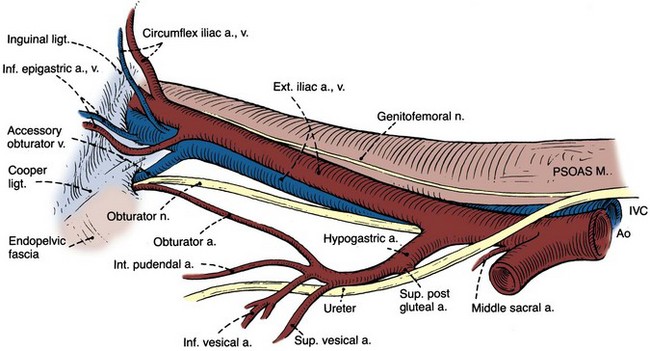
Figure 2–17 Right obturator fossa, showing the iliac vessels and obturator nerve.
(From Skinner DG. Pelvic lymphadenectomy. In: Glenn JF, editor. Urological surgery. 2nd ed. New York: Harper & Row; 1975. p. 591.)
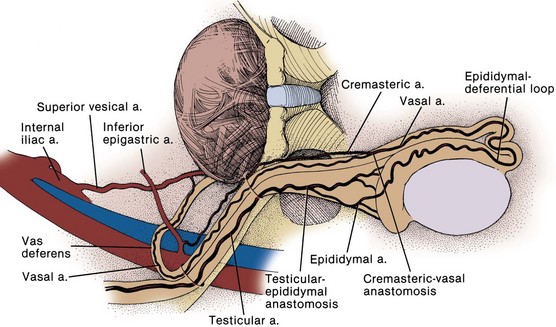
Figure 2–18 Collateral arterial circulation to the testis.
(From Hinman F Jr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993. p. 497.)
The internal iliac (hypogastric) artery descends in front of the SI joint and divides into an anterior and a posterior trunk (see Fig. 2–16). The posterior trunk gives rise to three parietal branches: (1) the superior gluteal, which exits the greater sciatic foramen; (2) the ascending lumbar, which supplies the posterior abdominal wall; and (3) the lateral sacral, which passes medially to join the middle sacral branches at the sciatic foramina.
The anterior trunk gives off seven parietal and visceral branches: (1) The superior vesical artery arises from the proximal portion of the obliterated umbilical artery and gives off a vesiculodeferential branch to the seminal vesicles and vas deferens. The artery of the vas deferens travels the length of the vas to meet the cremasteric and testicular arteries distally (see Fig. 2–18). Because of these anastomoses, the testicular artery may be sacrificed without compromising the viability of the testis. (2) The middle rectal artery gives small branches to the seminal vesicles and prostate and anastomoses with the inferior and superior rectal arteries in the rectal wall. (3) The inferior vesical branches supply the lower ureter, the bladder base, the prostate, and the seminal vesicles. In the female, they supply the ureter, the bladder base, and the vagina. (4) The uterine artery passes above and in front of the ureter (“water flows under the bridge”) to ascend the lateral wall of the uterus and meet the ovarian artery in the lateral portion of the fallopian tube (see Figs. 2-13 and 2-19). The ureter is vulnerable during division of the uterine pedicles. (5) The internal pudendal artery leaves the pelvic cavity through the greater sciatic foramen, passes around the sacrospinous ligament, and enters the lesser sciatic foramen to gain access to the perineum. Its perineal course is discussed later. (6) The obturator artery, variable in origin, travels through the obturator fossa medial and inferior to the obturator nerve and passes through its canal to supply the adductors of the thigh (see Fig. 2–17). (7) The inferior gluteal artery travels through the greater sciatic foramen to supply the buttock and thigh.
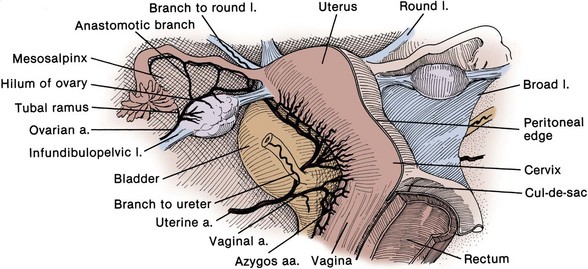
Figure 2–19 Female internal genitalia, from behind. The ureter passes beneath the uterine artery.
(From Hinman F Jr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993. p. 402.)
The internal iliac artery can be ligated to control severe pelvic hemorrhage. Ligation decreases the pulse pressure, allowing hemostasis to occur more readily. Internal iliac blood flow does not stop but reverses its direction because of critical anastomoses (lumbar segmentals to iliolumbar; median sacral to lateral sacral; and superior rectal and middle rectal). Bilateral ligation almost invariably produces vasculogenic impotence.
Venous Supply
The dorsal vein of the penis passes between the inferior pubic arch and the striated urinary sphincter to reach the pelvis, where it trifurcates into a central superficial branch and two lateral plexuses (Reiner and Walsh, 1979) (Fig. 2–20). To minimize blood loss at radical retropubic prostatectomy, the dorsal vein complex is best divided distally, before its ramification. Part of this complex runs within the anterior and lateral wall of the striated sphincter; thus care must be taken not to injure the sphincter when securing hemostasis. The superficial branch pierces the visceral endopelvic fascia between the puboprostatic ligaments and drains the retropubic fat, anterior bladder, and anterior prostate (see Fig. 2–20).
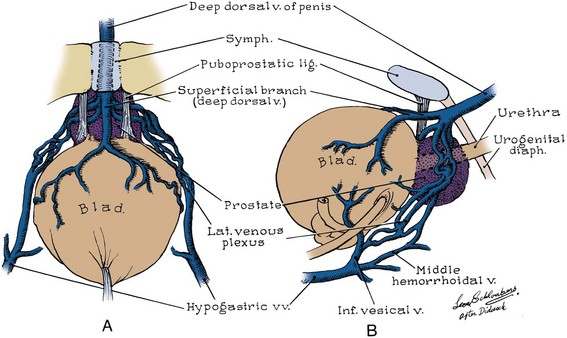
Figure 2–20 Pelvic venous plexus. A, Trifurcation of the dorsal vein of the penis, viewed from the retropubic space. The relationship of the venous branches to the puboprostatic ligaments is shown. B, Lateral view of the pelvic venous plexus after removal of the lateral pelvic fascia. Normally these structures are difficult to see because they are embedded in pelvic fascia.
(From Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol 1979;121:200.)
The lateral plexuses sweep down the sides of the prostate, receiving drainage from it and the rectum, and communicate with the vesical plexuses on the lower part of the bladder. Three to five inferior vesical veins emerge from the vesical plexus laterally and drain into the internal iliac vein. In the female, the dorsal vein of the clitoris bifurcates to empty into the laterally placed vaginal plexuses. These connect with the vesical, uterine, ovarian, and rectal plexuses and drain into the internal iliac veins.
The internal iliac vein is joined by tributaries corresponding to the branches of the internal iliac artery and ascends medial and posterior to the artery. This vein is relatively thin walled and at risk for injury during dissection of the artery or the nearby pelvic ureter. The external iliac vein travels medial and inferior to its artery and joins the internal iliac vein behind the internal iliac artery. In half the patients, one or more accessory obturator veins drain into the underside of the external iliac vein and can be easily torn during lymphadenectomy (see Fig. 2–17).
Pelvic Lymphatics
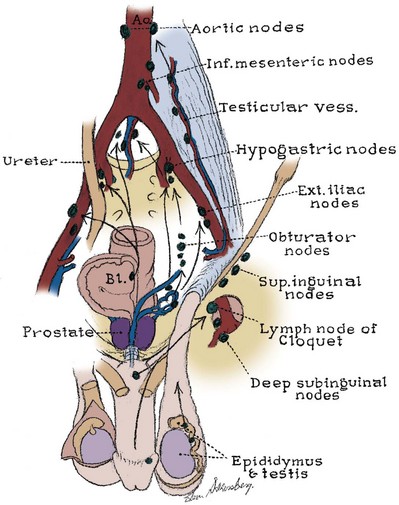
The pelvic lymph nodes can be difficult to appreciate on gross examination because they are embedded in the fatty and fibrous tissue of the intermediate stratum. Three major lymph node groups are associated with the pelvic vessels (Fig. 2–21). A substantial portion of pelvic visceral lymphatic drainage passes through the internal iliac nodes and their tributaries: the presacral, obturator, and internal pudendal nodes. The external iliac nodes lie lateral, anterior, and medial to the vessels and drain the anterior abdominal wall, urachus, bladder, and, in part, internal genitalia. The external genitalia and perineum drain into the superficial and deep inguinal nodes (see later discussion). The inguinal nodes communicate directly with the internal and external iliac chains. The common iliac nodes receive efferent vessels from the external and internal iliac nodes and the pelvic ureter and drain into the lateral aortic nodes.
Pelvic Innervation
Lumbosacral Plexus
The lumbosacral plexus and its rami are well illustrated in Chapter 1; only the pelvic courses of its nerves are reviewed here (see Figs. 2-7 and 2-12 and Table 2–2). The iliohypogastric nerve (L1) travels between, and supplies, the internal oblique and the transversus muscles and pierces the internal and external oblique muscles 3 cm above the external inguinal ring to supply sensation over the lower anterior abdomen and pubis (see Fig. 2–5). The ilioinguinal nerve (L1) passes through the internal oblique muscle to enter the inguinal canal laterally. It travels anterior to the cord and exits the external ring to provide sensation to the mons pubis and anterior scrotum or labia majora (see Figs. 2-5 and 2-7). The genitofemoral nerve (L1, L2) pierces the psoas muscle to reach its anterior surface in the retroperitoneum and then travels to the pelvis and splits into genital and femoral branches. The latter supplies sensation over the anterior thigh below the inguinal ligament. The genital branch follows the cord through the inguinal canal, supplies the cremaster muscle, and supplies sensation to the anterior scrotum.
Table 2–2 Somatic Nerves of the Lower Abdomen and Pelvis
| NERVE NAME | ORIGIN | SUPPLIES |
|---|---|---|
| Iliohypogastric | L1 | Motor supply to internal oblique, transversus muscles, sensation over lower anterior abdominal wall |
| Ilioinguinal | L1 | Sensation over anterior pubis (mons) and anterior scrotum or labia |
| Genitofemoral | L1, L2 | Genital branch: motor supply to cremaster muscle sensation to anterior scrotum Femoral branch: sensation to anterior thigh |
| Femoral | L2, L3, L4 | Motor supply to extensors of the knee sensory to anterior thigh |
| Obturator | L2, L3, L4 | Motor supply to adductors of the thigh, sensation to medial thigh |
| Lumbosacral trunk | L4, L5 | Joins the sacral nerves to form the lumbosacral plexus that supplies motor and sensory innervation to the lower extremities |
| Posterior femoral cutaneous | S2, S3 | Sensation to perineum, posterior scrotum, and posterior thigh |
| Pudendal | S2, S3, S4 | Motor to levator ani, muscles of the urogenital diaphragm, anal and striated urethral sphincter, sensation to the perineum, scrotum, penis |
| Pelvic somatic efferents | S2, S3, S4 | Motor supply to levator ani and striated urethral sphincter |
| Nervi erigentes | S2, S3, S4 | Parasympathetic fibers from the sacral cord supply the pelvic viscera |
For most of its pelvic course, the femoral nerve (L2, L3, L4) travels within the substance of the psoas muscle and then exits its lateral side to pass under the inguinal ligament (Fig. 2–22). It supplies sensation to the anterior thigh and motor innervation to the extensors of the knee. During a psoas hitch, sutures should be placed in the direction of the nerve (and the psoas muscle fibers) to avoid nerve damage or entrapment. Retractor blades must not rest on the psoas muscle because they can produce a femoral nerve palsy, a potentially dangerous setback after pelvic surgery. The lateral femoral cutaneous nerve (L2, L3) may be seen lateral to the psoas in the iliacus fascia.
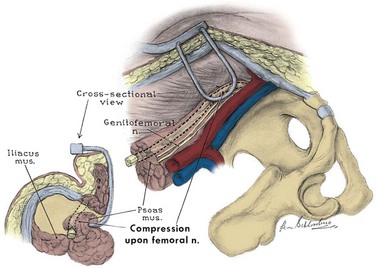
Figure 2–22 Femoral nerve as it relates to the psoas muscle. Retractor blades may compress this nerve to produce a femoral nerve palsy.
(From Burnett AL, Brendler CB. Female neuropathy following major pelvic surgery: etiology and prevention. J Urol 1994;151:163.)
The obturator nerve (L2, L3, L4) emerges in the true pelvis from beneath the psoas muscle, lateral to the internal iliac vessels, and passes through the obturator fossa to the obturator canal. In the fossa, it is lateral and superior to the obturator vessels and surrounded by the obturator and internal iliac lymph nodes. Damage to this nerve during pelvic lymphadenectomy weakens the adductors of the thigh.
The lumbosacral trunk (L4, L5) passes into the true pelvis behind the psoas and unites with the ventral rami of the sacral segmental nerves to form the sacral plexus. This plexus lies on the pelvic surface of the piriformis deep to the endopelvic fascia and posterior to the internal iliac vessels (see Fig. 2–16). It leaves the pelvis through the greater sciatic foramen immediately posterior to the sacrospinous ligament (where it may be injured during sacrospinous culposuspension) and supplies motor and sensory innervation to the posterior thigh and lower leg. An exaggerated lithotomy position may stretch this nerve or place pressure on its peroneal branch at the fibular head to produce foot drop. Pelvic and perineal branches of the sacral plexus include (1) the posterior femoral cutaneous nerve (S2, S3), which, after passing through the greater sciatic foramen, gives an anterior sensory branch to the perineum and posterior scrotum (see Fig. 2–8); (2) the pudendal nerve (S2, S3, S4), which follows the internal pudendal artery to the perineum (to be discussed); (3) the nervi erigentes (S2, S3, S4) to the autonomic plexus; and (4) pelvic somatic efferent nerves from the ventral rami of S2, S3, and S4 (Fig. 2–23). The latter nerves travel on the pelvic surface of the levator ani in close association with the rectum and prostate and are separated from the pelvic autonomic plexus by the endopelvic fascia. They supply the levator ani and extend anteriorly to the striated urethral sphincter (Lawson, 1974; Zvara et al, 1994).
Pelvic Autonomic Plexus
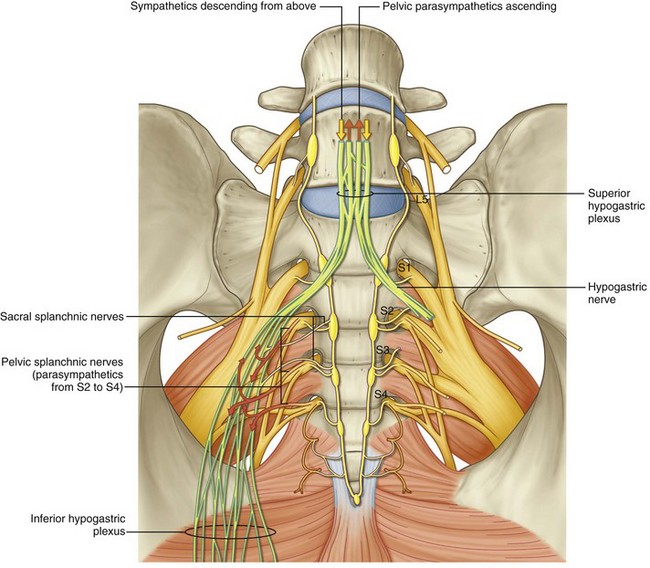
The presynaptic sympathetic cell bodies that project to the pelvic autonomic plexus reside in the lateral column of gray matter in the last three thoracic and first two lumbar segments of the spinal cord. They reach the pelvic plexus by two pathways: (1) The superior hypogastric plexus is formed by sympathetic fibers from the celiac plexus and the first four lumbar splanchnic nerves (Fig. 2–24). Anterior to the bifurcation of the aorta, it divides into two hypogastric nerves that enter the pelvis medial to the internal iliac vessels, anterior to the sacrum, and deep to the endopelvic fascia. (2) The pelvic continuations of the sympathetic trunks pass deep to the common iliac vessels and medial to the sacral foramina and fuse in front of the coccyx at the ganglion impar (see Fig. 2–24). Each chain comprises four to five ganglia that send branches anterolaterally to participate in the formation of the pelvic plexus.
Presynaptic parasympathetic innervation arises from the intermediolateral cell column of the sacral cord. Fibers emerge from the second, third, and fourth sacral spinal nerves as the pelvic splanchnic nerves (nervi erigentes) to join the hypogastric nerves and branches from the sacral sympathetic ganglia to form the inferior hypogastric (pelvic) plexus (see Fig. 2–24). Some pelvic parasympathetic efferent fibers travel up the hypogastric nerves to the inferior mesenteric plexus, where they provide parasympathetic innervation to the descending and sigmoid colon.
The pelvic plexus is rectangular, approximately 4 to 5 cm long, and its midpoint is at the tips of the seminal vesicles (Schlegel and Walsh, 1987). It is oriented in the sagittal plane on either side of the rectum and pierced by the numerous vessels going to and from the rectum, bladder, seminal vesicles, and prostate (Fig. 2–25). Division of these vessels (the so-called lateral pedicles of the bladder and prostate) risks injury to the pelvic plexus with attendant postoperative impotence (Walsh and Donker, 1982; Walsh et al, 1983). The right and left components of the pelvic plexus communicate behind the rectum and anterior and posterior to the vesical neck. Branches of the pelvic plexus follow pelvic blood vessels to reach the pelvic viscera, although nerves to the ureter may join it directly as it passes nearby. Visceral afferent and efferent nerves travel on the vas deferens to reach the testis and epididymis (see later discussion).
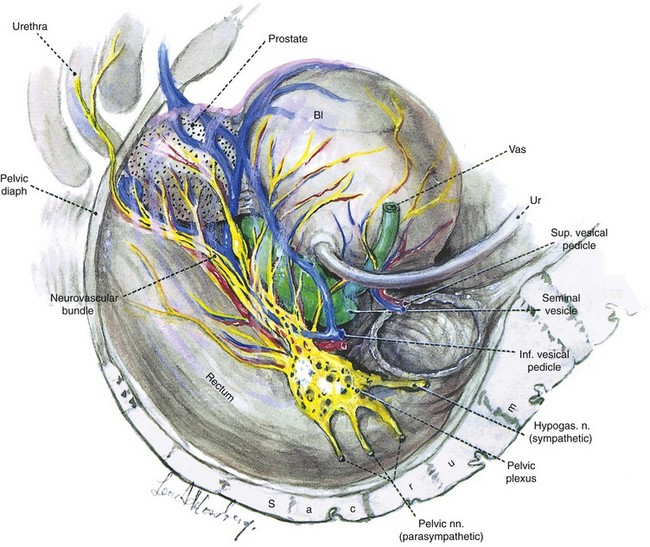
Figure 2–25 Lateral view showing the left pelvic autonomic nervous plexus and its relation to the pelvic viscera.
(From Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol 1987;138:1403.)
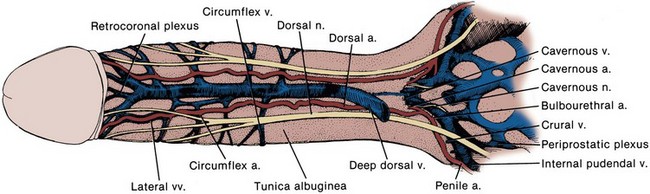
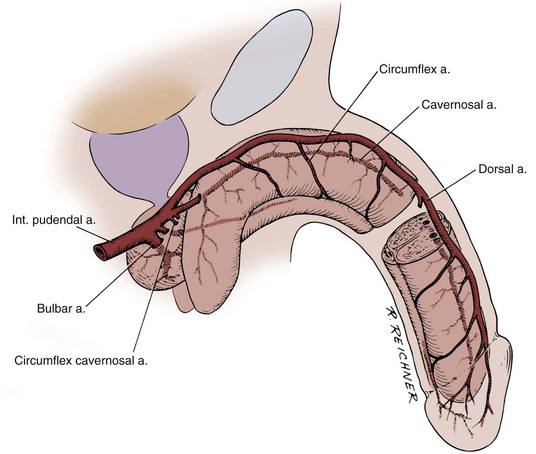
The most caudal portion of the pelvic plexus gives rise to the innervation of the prostate and the important cavernosal nerves (Walsh and Donker, 1982). After passing the tips of the seminal vesicles, these nerves lie within leaves of the lateral endopelvic fascia near its juncture with, but outside, Denonvilliers fascia (Lepor et al, 1985). They travel at the posterolateral border of the prostate on the surface of the rectum and are lateral to the prostatic capsular arteries and veins (see Fig. 2–25). Because the nerves are composed of multiple fibers not visible on gross inspection, these vessels serve as a surgical landmark for the course of these nerves (the neurovascular bundle of Walsh). During radical prostatectomy, the nerves are most vulnerable at the apex of the prostate, where they closely approach the prostatic capsule at the 5- and 7-o’clock positions. On reaching the membranous urethra, the nerves divide into superficial branches, which travel on the lateral surface of the striated urethral sphincter at 3- and 9-o’clock positions, and deep fibers, which penetrate the substance of this muscle and send twigs to the bulbourethral glands. As the nerves reach the hilum of the penis, they join to form one to three discrete bundles, related to the urethra at 1- and 11-o’clock positions, superficial to the cavernous veins and dorsomedial to the cavernous arteries (Fig. 2–26) (Lue et al, 1984; Breza et al, 1989). With the arteries, they pierce the corpora cavernosa to supply the erectile tissue (see later discussion). Small fibers also join the dorsal nerves of the penis as they course distally. In the female, the nerves to vestibular bodies and corpora cavernosa of the clitoris travel between the anterior vaginal wall and the bladder in association with the lateral venous plexuses.
Pelvic Viscera
Rectum
The rectum begins with the disappearance of the sigmoid mesentery opposite the third sacral vertebra. Peritoneum continues anteriorly over the upper two thirds of the rectum as the rectovesical pouch in males and as the rectouterine pouch (of Douglas) in females (Fig. 2–27; see also Fig. 2–11). Incision of the anterior wall of this peritoneal pouch exposes the seminal vesicles behind the bladder. Inferior to this pouch, the anterior rectum is related to its fascial continuation (the rectogenital or Denonvilliers fascia) down to the level of the striated urethral sphincter (see Figs. 2-4, 2-27, and 2-28). The rectum describes a gentle curve on the sacrum, coccyx, and levator plate (see Fig. 2–24) and receives innervation from the laterally placed pelvic autonomic plexus and blood supply from the superior (from inferior mesenteric), middle (from internal iliac), and inferior (from internal pudendal) rectal arteries.
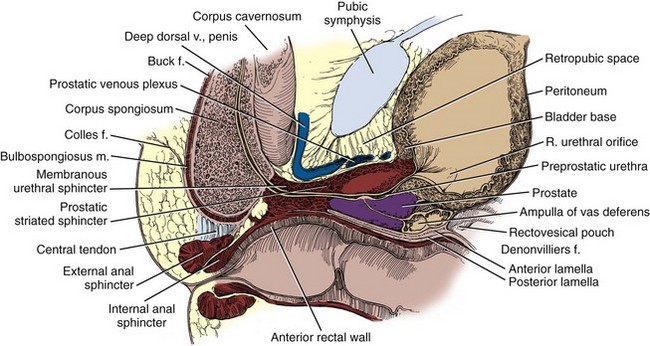
Figure 2–27 Sagittal section through the prostatic and membranous urethra, demonstrating the midline relations of the pelvic structures.
(From Hinman F Jr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993. p. 356.)
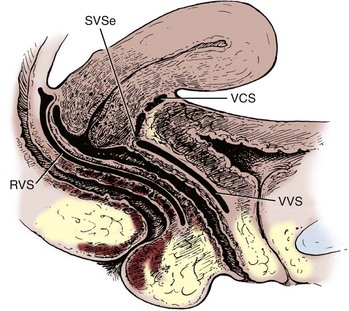
Figure 2–28 Median sagittal section of the female pelvis, showing the potential spaces between the pelvic organs. The posterior two thirds of the vagina lies nearly horizontal and rests with the uterine cervix on the rectum, which is in turn supported by the posterior portion of levator ani (the levator plate, not shown). RVS, rectovaginal space, the anterior wall is formed by the rectovaginal (Denonvilliers) fascia; SVSe, supravaginal septum, the fusion between the bladder and cervix; VCS, vesicocervical space; VVS, vesicovaginal space.
(From Nichols DH, Randall CL. Vaginal surgery. 3rd ed. Baltimore: Williams & Wilkins; 1989. p. 34.)
The rectal wall is composed of an inner layer of circular smooth muscle and a virtually continuous sheet of outer longitudinal smooth muscle derived from the tenia of the colon. In its lowest part, the rectum dilates to form the rectal ampulla. At the most inferior portion of the ampulla, anterior fibers of the longitudinal muscle leave the rectum to join Denonvilliers fascia and the posterior striated urethral sphincter in the apex of the perineal body (Brooks et al, 2002). During perineal prostatectomy, these fibers, the rectourethralis muscle, are 2 to 10 mm thick and must be divided to gain access to the prostate (Fig. 2–29). The apices of the prostate and rectal ampulla are in close proximity, and rectal injuries during radical prostatectomy commonly occur at this location. As the rectourethralis is given off, the rectum makes a right-angle turn posteroinferiorly to exit the pelvis at the anal canal (see Fig. 2–10). The anatomy of the anal canal is considered with the perineum.
Pelvic Ureter
The ureter is divided into abdominal and pelvic portions by the common iliac artery. The structure of the ureter and its abdominal course are reviewed in Chapter 1. Intraoperatively, the ureter is identified by its peristaltic waves and is readily found anterior to the bifurcation of the common iliac artery. At ureteroscopy, pulsations of this artery can be seen in the posterior ureteral wall. The ovarian vessels (infundibulopelvic ligament) cross the iliac vessels anterior and lateral to the ureter, and dissection of the ovarian vessels at the pelvic brim is a common cause of ureteral injury (see Fig. 2–13) (Daly and Higgins, 1988). Pyeloureterography discloses a narrowing of the ureter at the iliac vessels, and ureteral calculi frequently become lodged at this location. Because the ureter and iliac vessels rest on the arcuate line, the ureter is subject to compression and obstruction by the gravid uterus and by masses within the true pelvis.
The ureters come within 5 cm of each other as they cross the iliac vessels. On entering the pelvis, they diverge widely along the pelvic side walls toward the ischial spines. The ureter travels on the anterior surface of the internal iliac vessels and is related laterally to the branches of the anterior trunk. Near the ischial spine, the ureter turns anteriorly and medially to reach the bladder. In men, the anteromedial surface of the ureter is covered by peritoneum, and the ureter is embedded in retroperitoneal connective tissue, which varies in thickness (see Fig. 2–13). As the ureter courses medially, it is crossed anteriorly by the vas deferens and runs with the inferior vesical arteries, veins, and nerves in the lateral vesical ligaments. Viewed from the peritoneal side, the ureter is just lateral and deep to the rectogenital fold. In women, the ureter first runs posterior to the ovary and then turns medially to run deep to the base of the broad ligament before entering a loose connective tissue tunnel through the substance of the cardinal ligament (see Fig. 2–13). As in the male, the ureter can be found slightly lateral and deep to the rectouterine folds of peritoneum. It is crossed anteriorly by the uterine artery and can be injured during hysterectomy. As it passes in front of the vagina, it crosses 1.5 cm anterior and lateral to the uterine cervix. The ureter can be injured at this level during hysterectomy, resulting in a ureterovaginal fistula. The ureter courses 1 to 4 cm on the anterior vaginal wall to reach the bladder. Occasionally, a stone lodged in the distal ureter can be palpated through the anterior vaginal wall. The intramural ureter is discussed with the bladder.
The pelvic ureter receives abundant blood supply from the common iliac artery and most branches of the internal iliac artery. The inferior vesical and uterine arteries usually supply the ureter with its largest pelvic branches. Blood supply to the pelvic ureter enters laterally; thus the pelvic peritoneum should be incised only medial to the ureter. Intramural vessels of the ureter run within the adventitia and generally follow one of two patterns. In approximately 75% of specimens, longitudinal vessels run the length of the ureter and are formed by anastomoses of segmental ureteral vessels. In the remaining ureters, the vessels form a fine interconnecting mesh (plexiform) with less collateral flow (Shafik, 1972). Therefore primary repair of injuries to the pelvic ureter fare poorly and are more prone to stricture formation (Hinman, 1993). Lymphatic drainage of the pelvic ureter is to the external, internal, and common iliac nodes. Pathologic enlargement of the common and internal iliac nodes can encroach on and obstruct the ureter.
The pelvic ureter has rich adrenergic and cholinergic autonomic innervation derived from the pelvic plexus. The functional significance of this innervation is unclear, inasmuch as the ureter continues to contract peristaltically after denervation. Afferent neural fibers travel through the pelvic plexus and account for the visceral quality of referred pain from ureteral irritation or acute obstruction.
Bladder
Relationships
When filled, the bladder has a capacity of approximately 500 mL and assumes an ovoid shape. The empty bladder is tetrahedral and is described as having a superior surface with an apex at the urachus, two inferolateral surfaces, and a posteroinferior surface or base with the bladder neck at the lowest point (see Fig. 2–27).
The urachus anchors the bladder to the anterior abdominal wall (see Fig. 2–8). There is a relative paucity of bladder wall muscle at the point of attachment of the urachus, predisposing to diverticula formation. The urachus is composed of longitudinal smooth muscle bundles derived from the bladder wall. Near the umbilicus, it becomes more fibrous and usually fuses with one of the obliterated umbilical arteries. Urachal vessels run longitudinally, and the ends of the urachus must be ligated when it is divided. An epithelium-lined lumen usually persists throughout life and uncommonly gives rise to aggressive urachal adenocarcinomas (Begg, 1930). In rare instances, luminal continuity with the bladder serves as a bacterial reservoir or results in an umbilical urinary fistula.
The superior surface of the bladder is covered by peritoneum. Anteriorly, the peritoneum sweeps gently onto the anterior abdominal wall (see Fig. 2–13). With distention, the bladder rises out of the true pelvis and separates the peritoneum from the anterior abdominal wall. It is therefore possible to perform a suprapubic cystostomy without risking entry into the peritoneal cavity. Posteriorly, the peritoneum passes to the level of the seminal vesicles and meets the peritoneum on the anterior rectum to form the rectovesical space.
Anteroinferiorly and laterally, the bladder is cushioned from the pelvic side wall by retropubic and perivesical fat and loose connective tissue. This potential space (of Retzius) may be entered anteriorly by dividing the transversalis fascia and provides access to the pelvic viscera as far posteriorly as the iliac vessels and ureters (see Fig. 2–11). The bladder base is related to the seminal vesicles, ampullae of the vas deferentia, and terminal ureter. The bladder neck, located at the internal urethral meatus, rests 3 to 4 cm behind the midpoint of the symphysis pubis. It is firmly fixed by the pelvic fasciae (see earlier discussion) and by its continuity with the prostate; its position changes little with varying conditions of the bladder and rectum.
In the female, the peritoneum on the superior surface of the bladder is reflected over the uterus to form the vesicouterine pouch and then continues posteriorly over the uterus as the rectouterine pouch (see Fig. 2–13). The vagina and uterus intervene between the bladder and the rectum so that the base of the bladder and urethra rest on the anterior vaginal wall. Because the anterior vaginal wall is firmly attached laterally to the levator ani, contraction of the pelvic diaphragm (e.g., during increases in intra-abdominal pressure) elevates the bladder neck and draws it anteriorly. In many women with stress incontinence, the bladder neck drops below the pubic symphysis. In infants, the true pelvis is shallow and the bladder neck is level with the upper border of the symphysis. The bladder is a true intra-abdominal organ that can project above the umbilicus when full. By puberty, the bladder has migrated to the confines of the deepened true pelvis.
Structure
The internal surface of the bladder is lined with transitional epithelium, which appears smooth when the bladder is full but contracts into numerous folds when the bladder empties. This urothelium is usually six cells thick and rests on a thin basement membrane. Deep to this, the lamina propria forms a relatively thick layer of fibroelastic connective tissue that allows considerable distention. This layer is traversed by numerous blood vessels and contains smooth muscle fibers collected into a poorly defined muscularis mucosa. Beneath this layer lies the smooth muscle of the bladder wall. The relatively large muscle fibers form branching, interlacing bundles loosely arranged into inner longitudinal, middle circular, and outer longitudinal layers (Fig. 2–30). However, in the upper aspect of the bladder, these layers are clearly not separable, and any one fiber can travel between each of the layers, change orientation, and branch into longitudinal and circular fibers. This meshwork of detrusor muscle is ideally suited for emptying the spherical bladder.
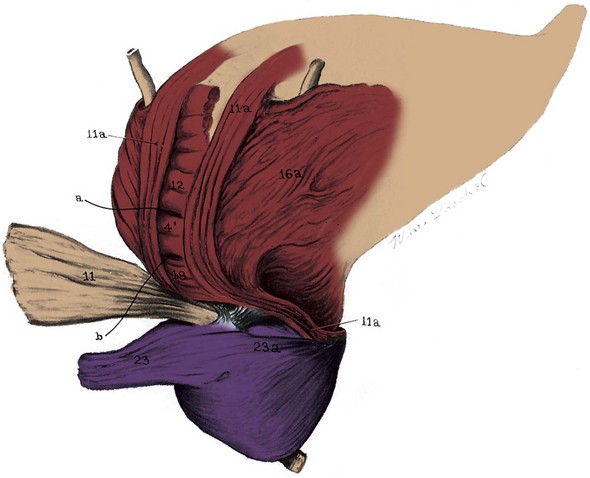
Figure 2–30 Dissection of the male bladder. 11, Posterior outer longitudinal detrusor, which forms the backing of the ureters (folded back); 11a, posterolateral portion of the outer longitudinal muscle forming a loop around the anterior bladder neck; 4′, 12, and 18, middle circular layer backing the trigone; 23 and 23a, lateral pedicle of the prostate.
(From Uhlenhuth E. Problems in the anatomy of the pelvis. Philadelphia: JB Lippincott; 1953. p. 187.)
Near the bladder neck, the detrusor muscle is clearly separable into the three layers described earlier. Here, the smooth muscle is morphologically and pharmacologically distinct from the remainder of the bladder because the large-diameter muscle fascicles are replaced by much finer fibers. The structure of the bladder neck appears to differ between men and women. In men, radially oriented inner longitudinal fibers pass through the internal meatus to become continuous with the inner longitudinal layer of smooth muscle in the urethra.
The middle layer forms a circular preprostatic sphincter that is responsible for continence at the level of the bladder neck (Fig. 2–31 on the Expert Consult website![]() ). The bladder wall posterior to the internal urethral meatus and the anterior fibromuscular stroma of the prostate form a continuous ringlike structure at the bladder neck (Brooks et al, 1998). The fact that perfect continence can be maintained in men in whom the striated urethral sphincter is destroyed attests to the efficacy of this sphincter (Waterhouse et al, 1973). This muscle is richly innervated by adrenergic fibers, which, when stimulated, produce closure of the bladder neck (Uhlenhuth, 1953). Damage to the sympathetic nerves to the bladder, as a result of diabetes mellitus or retroperitoneal lymph node dissection for testis cancer, can cause retrograde ejaculation.
). The bladder wall posterior to the internal urethral meatus and the anterior fibromuscular stroma of the prostate form a continuous ringlike structure at the bladder neck (Brooks et al, 1998). The fact that perfect continence can be maintained in men in whom the striated urethral sphincter is destroyed attests to the efficacy of this sphincter (Waterhouse et al, 1973). This muscle is richly innervated by adrenergic fibers, which, when stimulated, produce closure of the bladder neck (Uhlenhuth, 1953). Damage to the sympathetic nerves to the bladder, as a result of diabetes mellitus or retroperitoneal lymph node dissection for testis cancer, can cause retrograde ejaculation.
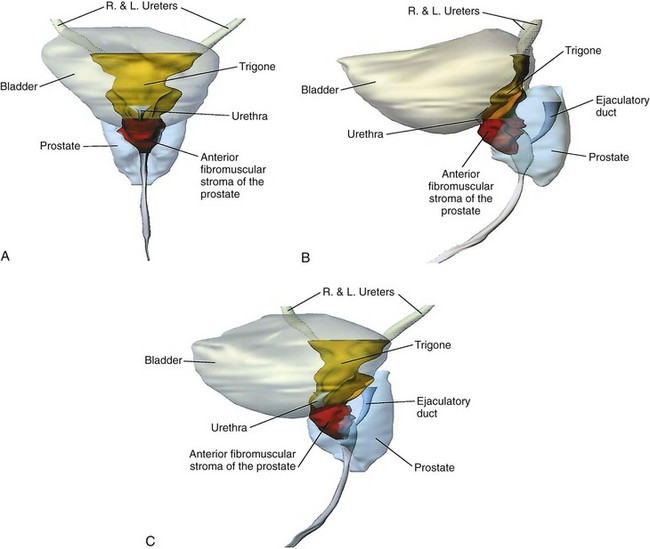
Figure 2–31 Structure of the male bladder neck and trigone. A, Anterior view reveals that the trigone narrows below the ureteral orifices and then widens at the bladder neck to become continuous with the anterior fibromuscular stroma of the prostate. B, Lateral projection shows that the trigone and anterior fibromuscular stroma are in continuity. The trigone thickens near the bladder neck as it meets the anterior fibromuscular stroma. C, Oblique view shows this structure at the bladder neck, where it forms the internal urethral sphincter.
(From Brooks JD, Chao WM, Kerr J. Male pelvic anatomy reconstructed from the visible human data set. J Urol 1998;159:870.)
The outer longitudinal fibers are thickest posteriorly at the bladder base. In the midline, they insert into the apex of the trigone and intermix with the smooth muscle of the prostate to provide a strong trigonal backing. Laterally, the fibers from this posterior sheet pass anteriorly and fuse to form a loop around the bladder neck (see Fig. 2–30). This loop is thought to participate in continence at the bladder neck. On the lateral and anterior surfaces of the bladder, the longitudinal fibers are not as well developed. Some anterior fibers course forward to join the puboprostatic ligaments in men and the pubourethral ligaments in women. These fibers contribute smooth muscle to these supports and are speculated to contribute to bladder neck opening during micturition (DeLancey, 1989).
At the female bladder neck, the inner longitudinal fibers converge radially to pass downward as the inner longitudinal layer of the urethra, as described earlier. The middle circular layer does not appear to be as robust as that of the male, and several authors have denied its existence altogether (Gosling, 1979, 1985; Williams et al, 1989). Although several other investigators have noted an anterior loop of external longitudinal muscle (Fig. 2–32), the authors just cited deny the existence of this structure as well. They maintain instead that the external fibers pass obliquely and longitudinally down the urethra to participate in forming the inner longitudinal layer of smooth muscle. Regardless, the female bladder neck differs strikingly from the male in possessing little adrenergic innervation. In addition, its sphincteric function is limited; in 50% of continent women, urine enters the proximal urethra during a cough (Versi et al, 1986).
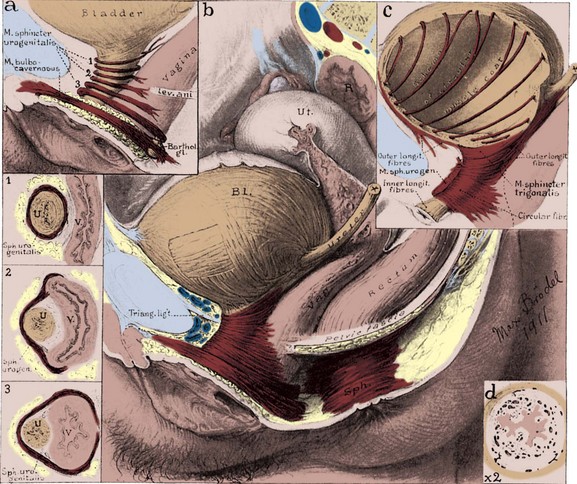
Figure 2–32 Female bladder and striated urethral sphincter. a, Diagram of striated urethral sphincter showing disposition of the muscle fibers. 1, The proximal third of the sphincter encircles the urethra entirely. 2, The middle bundles surround the urethra in front and pass off the lateral sides to blend with the vaginal wall (compressor urethrae). 3, The distal portion surrounds the urethra and vagina together and has been called the urethrovaginal sphincter. The bulbocavernosus also acts as a sphincter around the vaginal vestibule. b, Urethral sphincter in its entirety. The relationship of the pelvic viscera is shown. Interlacing detrusor fibers are also demonstrated. c, Posterolateral outer longitudinal detrusor muscle, looping anterior to the bladder neck. Inner longitudinal smooth muscle fibers run the length of the urethra, deep to the striated sphincter. d, Cross section of the urethra, showing thick, highly vascularized lamina propria and folded mucosa, which act as a urethral seal. Longitudinal smooth muscle surrounds the lamina propria.
(Original art in the Max Brödel Archives, Department of Art as Applied to Medicine, The Johns Hopkins University School of Medicine.)
Ureterovesical Junction and the Trigone
As the ureter approaches the bladder, its spirally oriented mural smooth muscle fibers become longitudinal. Two to 3 cm from the bladder, a fibromuscular sheath (of Waldeyer) extends longitudinally over the ureter and follows it to the trigone (Tanagho, 1992). The ureter pierces the bladder wall obliquely, travels 1.5 to 2 cm, and terminates at the ureteral orifice (Fig. 2–33). As it passes through a hiatus in the detrusor (intramural ureter), it is compressed and narrows considerably. This is a common site in which ureteral stones become impacted. The intravesical portion of the ureter lies immediately beneath the bladder urothelium and therefore is quite pliant; it is backed by a strong plate of detrusor muscle. With bladder filling, this arrangement is thought to result in passive occlusion of the ureter, like a flap valve. Indeed, reflux does not occur in fresh cadavers when the bladder is filled (Thomson et al, 1994). Vesicoureteral reflux is thought to result from insufficient submucosal ureteral length and poor detrusor backing. Chronic increases in intravesical pressure resulting from bladder outlet obstruction can cause herniation of the bladder mucosa through the weakest point of the hiatus above the ureter and produce a “Hutch diverticulum” and reflux (Hutch et al, 1961).
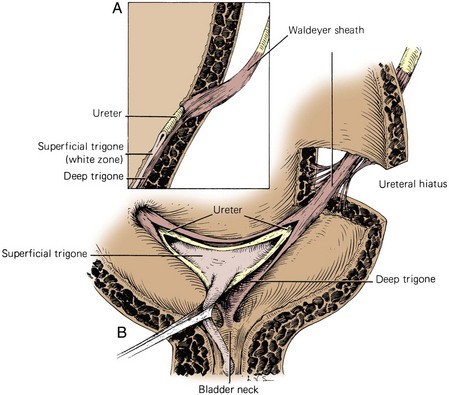
Figure 2–33 Normal ureterovesical junction and trigone. A, Section of the bladder wall perpendicular to the ureteral hiatus shows the oblique passage of the ureter through the detrusor and also shows the submucosal ureter with its detrusor backing. Waldeyer sheath surrounds the prevesical ureter and extends inward to become the deep trigone. B, Waldeyer sheath continues in the bladder as the deep trigone, which is fixed at the bladder neck. Smooth muscle of the ureter forms the superficial trigone and is anchored at the verumontanum.
(From Tanagho EA, Pugh RCB. The anatomy and function of the ureterovesical junction. Br J Urol 1963;35:151.)
The triangle of smooth urothelium between the two ureteral orifices and the internal urethral meatus is referred to as the trigone of the bladder (see Fig. 2–33). The fine longitudinal smooth muscle fibers from each ureter fan out over the base of the bladder to form a triangular sheet of muscle that extends from the two ureteral orifices to the internal urethral meatus. The edges of this muscular sheet can be thickened between the ureteral orifices (the interureteric crest or Mercier bar) and between the ureters and the internal urethral meatus (Bell muscle).
The muscle of trigone forms three distinct layers: (1) a superficial layer, derived from the longitudinal muscle of the ureter, which extends down the urethra to insert at the verumontanum; (2) a deep layer, which continues from Waldeyer sheath and inserts at the bladder neck; and (3) a detrusor layer, formed by the outer longitudinal and middle circular smooth muscle layers of the bladder wall. Through its continuity with the ureter, the superficial trigonal muscle anchors the ureter to the bladder. During ureteral reimplantation, this muscle is tented up and divided in order to gain access to the space between Waldeyer sheath and the ureter. In this space, only loose fibrous and muscular connections are found. This anatomic arrangement helps prevent reflux during bladder filling by fixing and applying tension to the ureteral orifice. As the bladder fills, its lateral wall telescopes outward on the ureter, thereby increasing intravesical ureteral length (Hutch et al, 1961).
The urothelium overlying the muscular trigone is usually only three cells thick and adheres strongly to the underlying muscle by a dense lamina propria. During filling and emptying of the bladder, this mucosal surface remains smooth.
Bladder Circulation
In addition to the vesical branches, the bladder may be supplied by any adjacent artery arising from the internal iliac artery. For convenience, surgeons refer to the vesical blood supply as the lateral and posterior pedicles, which, when the bladder is approached from the rectovesical space, are lateral and posteromedial to the ureters, respectively. These pedicles are the lateral and posterior vesical ligaments in the male and part of cardinal and uterosacral ligaments in the female (see Fig. 2–13). The veins of the bladder coalesce into the vesicle plexus and drain into the internal iliac vein. Lymphatics from the lamina propria and muscularis drain to channels on the bladder surface, which run with the superficial vessels within the thin visceral fascia. Small paravesical lymph nodes can be found along the superficial channels. The bulk of the lymphatic drainage passes to the external iliac lymph nodes (see Fig. 2–21). Some anterior and lateral drainage may go through the obturator and internal iliac nodes, whereas portions of the bladder base and trigone may drain into the internal and common iliac groups.
Bladder Innervation
Autonomic efferent fibers from the anterior portion of the pelvic plexus (the vesical plexus) pass up the lateral and posterior ligaments to innervate the bladder. The bladder wall is richly supplied with parasympathetic cholinergic nerve endings and has abundant postganglionic cell bodies. Sparse sympathetic innervation of the bladder has been proposed to mediate detrusor relaxation but probably lacks functional significance. A separate nonadrenergic, noncholinergic (NANC) component of the autonomic nervous system participates in activating the detrusor, although the neurotransmitter has not been identified (Burnett, 1995). As mentioned, the male bladder neck receives abundant sympathetic innervation and expresses α1-adrenergic receptors. The female bladder neck has little adrenergic innervation. Nitric oxide synthase–containing neurons have been identified in the detrusor, particularly at the bladder neck, where they assist relaxation during micturition. The trigonal muscle is innervated by adrenergic and nitric oxide synthase–containing neurons. Like the bladder neck, it relaxes during micturition. Afferent innervation from the bladder travels with both sympathetic (via the hypogastric nerves) and parasympathetic nerves to reach cell bodies in the dorsal root ganglia located at thoracolumbar and sacral levels. As a consequence, presacral neurectomy (division of the hypogastric nerves) is ineffective in relieving bladder pain.
Prostate
Relationships
The normal prostate weighs 18 g; measures 3 cm in length, 4 cm in width, and 2 cm in depth; and is traversed by the prostatic urethra (see Fig. 2–27). Although ovoid, the prostate is referred to as having anterior, posterior, and lateral surfaces, with a narrowed apex inferiorly and a broad base superiorly that is contiguous with the base of the bladder. It is enclosed by a capsule composed of collagen, elastin, and abundant smooth muscle. Posteriorly and laterally, this capsule has an average thickness of 0.5 mm, although it may be partially transgressed by normal glands. Microscopic bands of smooth muscle extend from the posterior surface of the capsule to fuse with Denonvilliers fascia. Loose areolar tissue defines a thin plane between Denonvilliers fascia and the rectum. On the anterior and anterolateral surfaces of the prostate, the capsule blends with the visceral continuation of endopelvic fascia. Toward the apex, the puboprostatic ligaments extend anteriorly to fix the prostate to the pubic bone. The superficial branch of the dorsal vein lies outside this fascia in the retropubic fat and pierces it to drain into the dorsal vein complex.
Laterally, the prostate is cradled by the pubococcygeal portion of levator ani and is directly related to its overlying endopelvic fascia (see Figs. 2-8 and 2-10). Below the juncture of the parietal and visceral endopelvic fascia (arcus tendineus fascia pelvis), the pelvic fascia and prostate capsule separate and the space between them is filled by fatty areolar tissue and the lateral divisions of the dorsal vein complex. During a radical retropubic prostatectomy, the endopelvic fascia should be divided lateral to the arcus tendineus fascia pelvis to avoid injury to the venous complex. In the process, the endopelvic fascia overlying the levator ani is actually peeled off the muscle and displaced medially with the prostate. Although this is truly a parietal endopelvic fascia, it is commonly referred to as the “lateral prostatic fascia” (Myers, 1994). As mentioned earlier, the cavernosal nerves run posterolateral to the prostate in the substance of the parietal pelvic fascia (lateral prostatic fascia). Thus to preserve these nerves, this fascia must be incised lateral to the prostate and anterior to the neurovascular bundle (Walsh et al, 1983). Therefore an understanding of the fascial layers that overlie the prostate is crucial in the performance of an accurate nerve-sparing radical prostatectomy (Fig 2–34).
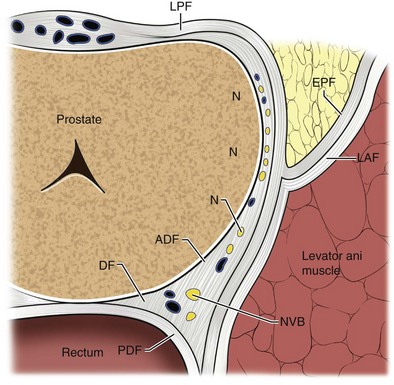
Figure 2–34 Cross section of prostate with prostatic fascial layers outlined including the lateral prostatic fascia (LPF), endopelvic fascia (EPF), levator ani fascia (LAF), Denonvilliers fascia (DF), anterior lamina of Denonvilliers fascia (ADF), posterior lamina of Denonvilliers fascia, neurovascular bundle (NVB), and lateral nerves (N).
(From Walz J, Graefen M, Huland H. Basic principles of anatomy for optimal surgical treatment of prostate cancer. World J Urol 2007;25:31–8.)
Recently, with the adoption of robotic and laparoscopic radical prostatectomy techniques, there has been a renewed interest in the description of these fascial layers with respect to the neurovascular bundle because these layers may be better visualized with the magnification afforded by these techniques (Tewari et al, 2006). Better definition of the “lateral prostatic fascia” at the level of the prostatic pedicles has become necessary in preserving the cavernosal nerves because the dissection is often performed antegrade in these techniques. Anatomic studies have revealed nerve bundles that course along the prostate laterally and anteriorly to the nerves, commonly referred to as the neurovascular bundle (Eichelberg et al, 2007; Raychaudhuri and Cahill, 2008). Whether these nerves contribute to erectile function remains controversial.
The apex of the prostate is continuous with the striated urethral sphincter (see Fig. 2–14). Histologically normal prostatic glands can be found to extend into the striated muscle with no intervening fibromuscular stroma or “capsule.” At the base of the prostate, outer longitudinal fibers of the detrusor fuse and blend with the fibromuscular tissue of the capsule. As mentioned, the middle circular and inner longitudinal muscles extend down the prostatic urethra as a preprostatic sphincter. As with the apex, no true capsule separates the prostate from the bladder. In surgically resected prostate carcinomas, this peculiar anatomic arrangement can make interpretation of these margins difficult and has led some pathologists to propose that the prostate does not possess a true capsule (Epstein, 1989).
Structure
The prostate is composed of approximately 70% glandular elements and 30% fibromuscular stroma. The stroma is continuous with capsule and is composed of collagen and abundant smooth muscle. It encircles and invests the glands of the prostate and contracts during ejaculation to express prostatic secretions into the urethra.
The urethra runs the length of the prostate and is usually closest to its anterior surface. It is lined by transitional epithelium, which may extend into the prostatic ducts. The urothelium is surrounded by an inner longitudinal and an outer circular layer of smooth muscle. A urethral crest projects inward from the posterior midline, runs the length of the prostatic urethra, and disappears at the striated sphincter (Fig. 2–35). To either side of this crest, a groove is formed (prostatic sinuses) into which all glandular elements drain (McNeal, 1972). At its midpoint, the urethra turns approximately 35 degrees anteriorly, but this angulation can vary from 0 to 90 degrees (see Figs. 2-27, 2-31, 2-36, and 2-37). This angle divides the prostatic urethra into proximal (preprostatic) and distal (prostatic) segments that are functionally and anatomically discrete (McNeal, 1972, 1988). In the proximal segment, the circular smooth muscle is thickened to form the involuntary internal urethral (preprostatic) sphincter described earlier. Small periurethral glands, lacking periglandular smooth muscle, extend between the fibers of the longitudinal smooth muscle to be enclosed by the preprostatic sphincter. Although these glands constitute less than 1% of the secretory elements of the prostate, they can contribute significantly to prostatic volume in older men as one of the sites of origin of benign prostatic hypertrophy.
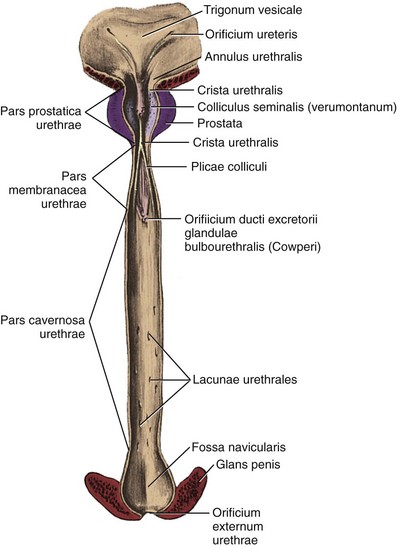
Figure 2–35 Posterior wall of the male urethra.
(From Anson BJ, McVay CB. Surgical anatomy. 6th ed. Philadelphia: WB Saunders; 1984. p. 833.)
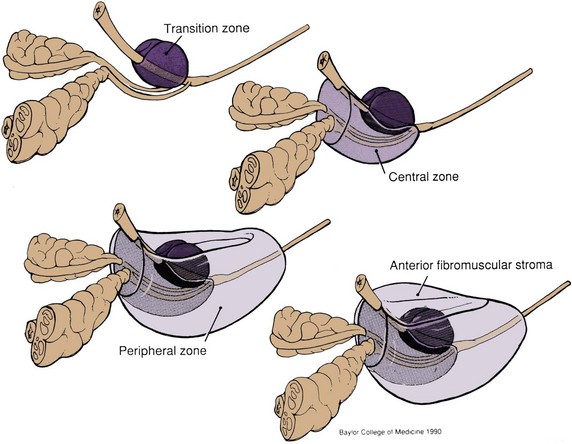
Figure 2–36 Zonal anatomy of the prostate as described by J.E. McNeal (Normal histology of the prostate. Am J Surg Pathol 1988;12:619–33). The transition zone surrounds the urethra proximal to the ejaculatory ducts. The central zone surrounds the ejaculatory ducts and projects under the bladder base. The peripheral zone constitutes the bulk of the apical, posterior, and lateral aspects of the prostate. The anterior fibromuscular stroma extends from the bladder neck to the striated urethral sphincter.
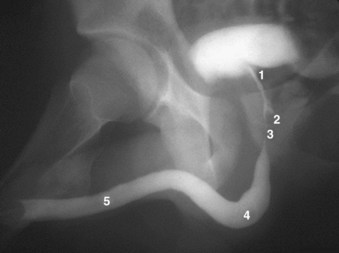
Figure 2–37 Retrograde urethrogram of the male urethra demonstrating urethral anatomy. 1, prostatic urethra; 2, verumontanum, into which enter the ejaculatory ducts; 3, membranous urethra, note physiologic narrowing of urethral luminal diameter due to external striated sphincter; 4, bulbar urethra; 5, pendulous urethra.
Beyond to the urethral angle, all major glandular elements of the prostate open into the prostatic urethra. The urethral crest widens and protrudes from the posterior wall as the verumontanum (see Figs. 2-35, 2-37, and 2-38). The small slitlike orifice of the prostatic utricle is found at the apex of the verumontanum and may be visualized cystoscopically. The utricle is a 6-mm müllerian remnant in the form of a small sac that projects upward and backward into the substance of the prostate. In males with ambiguous genitalia, it may form a large diverticulum that protrudes from the posterior side of the prostate. To either side of the utricular orifice, the two small openings of the ejaculatory ducts may be found. The ejaculatory ducts form at the juncture of the vas deferens and seminal vesicles and enter the prostate base, where it fuses with the bladder. They course nearly 2 cm through the prostate in line with the distal prostatic urethra and are surrounded by circular smooth muscle (see Fig. 2–36; see also Figs. 2-27 and 2-31).
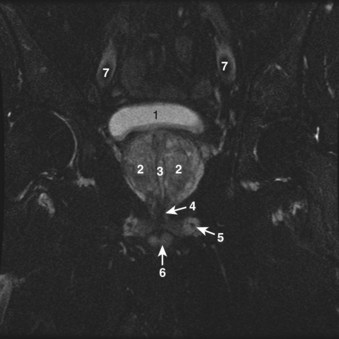
Figure 2–38 Axial T2-weighted magnetic resonance image of the male pelvis through the prostate gland and adjacent structures. 1, urinary bladder; 2, lateral lobes of prostate; 3, verumontanum; 4, striated urethral sphincter; 5, inferior pubic ramus; 6, corpus spongiosum in cross section; 7, external iliac artery.
In general, the glands of the prostate are tubuloalveolar with relatively simple branching and are lined with simple cuboidal or columnar epithelium. Scattered neuroendocrine cells, of unknown function, are found between the secretory cells. Beneath the epithelial cells, flattened basal cells line each acinus. Each acinus is surrounded by a thin layer of stromal smooth muscle and connective tissue.
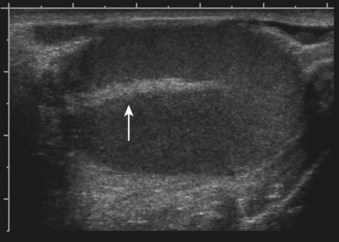
The glandular elements of the prostate have been divided into discrete zones, distinguished by the location of their ducts in the urethra, by their differing pathologic lesions, and, in some cases, by their embryologic origin (see Fig. 2–36). These zones can be demonstrated clearly with transrectal ultrasonography (Fig 2–39). At the angle dividing the preprostatic and prostatic urethra, the ducts of the transition zone arise and pass beneath the preprostatic sphincter to travel on its lateral and posterior sides. Normally, the transition zone accounts for 5% to 10% of the glandular tissue of the prostate. A discrete fibromuscular band of tissue separates the transition zone from the remaining glandular compartments and may be visualized at transrectal ultrasonography of the prostate. The transition zone commonly gives rise to benign prostatic hypertrophy, which expands to compress the fibromuscular band into a surgical capsule seen at enucleation of an adenoma. It is estimated that 20% of adenocarcinomas of the prostate originate in this zone.
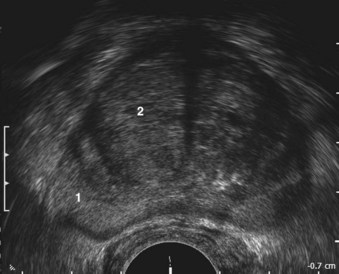
Figure 2–39 Transrectal ultrasound of the prostate demonstrating the 1, peripheral zone and 2, transition zone.
The ducts of the central zone arise circumferentially around the openings of the ejaculatory ducts. This zone constitutes 25% of the glandular tissue of the prostate and expands in a cone shape around the ejaculatory ducts to the base of the bladder. The glands are structurally and immunohistochemically distinct from the remaining prostatic glands (which branch directly from the urogenital sinus), which has led to the suggestion that they are of wolffian origin (McNeal, 1988). In keeping with this suggestion, only 1% to 5% of adenocarcinomas arise in the central zone, although it may be infiltrated by cancers from adjacent zones.
The peripheral zone makes up the bulk of the prostatic glandular tissue (70%) and covers the posterior and lateral aspects of the gland. Its ducts drain into the prostatic sinus along the entire length of the (postsphincteric) prostatic urethra. Seventy percent of prostatic cancers arise in this zone, and it is the zone most commonly affected by chronic prostatitis.
Up to one third of the prostatic mass may be attributed to the nonglandular anterior fibromuscular stroma. This region normally extends from the bladder neck to the striated sphincter, although considerable portions of it may be replaced by glandular tissue in adenomatous enlargement of the prostate. It is directly continuous with the prostatic capsule, anterior visceral fascia, and anterior portion of the preprostatic sphincter and is composed of elastin, collagen, and smooth and striated muscle. It is rarely invaded by carcinoma.
Clinically, the prostate is often spoken of as having two lateral lobes, separated by a central sulcus that is palpable on rectal examination, and a middle lobe, which may project into the bladder in older men. These lobes do not correspond to histologically defined structures in the normal prostate but are usually related to pathologic enlargement of the transition zone laterally and the periurethral glands centrally.
Vascular Supply
Most commonly, the arterial supply to the prostate arises from the inferior vesical artery. As it approaches the gland, the artery (often several) divides into two main branches (Fig. 2–40). The urethral arteries penetrate the prostatovesical junction posterolaterally and travel inward, perpendicular to the urethra. They approach the bladder neck in the 1- to 5-o’clock and 7- to 11-o’clock positions, with the largest branches located posteriorly. They then turn caudally, parallel to the urethra, to supply it, the periurethral glands, and the transition zone. Thus in benign prostatic hypertrophy, these arteries provide the principal blood supply of the adenoma (Flocks, 1937). When these glands are resected or enucleated, the most significant bleeding is commonly encountered at the bladder neck, particularly at the 4- and 8-o’clock positions.
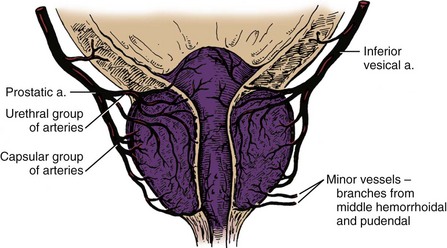
Figure 2–40 Arterial supply of the prostate.
(Adapted from Flocks RH. The arterial distribution within the prostate gland: its role in transurethral prostatic resection. J Urol 1937;37:527.)
The capsular artery is the second main branch of the prostatic artery. This artery gives off a few small branches that pass anteriorly to ramify on the prostatic capsule. The bulk of this artery runs posterolateral to the prostate with the cavernous nerves (neurovascular bundles) and ends at the pelvic diaphragm. The capsular branches pierce the prostate at right angles and follow the reticular bands of stroma to supply the glandular tissues. Venous drainage of the prostate is abundant through the periprostatic plexus (see Fig. 2–20).
Lymphatic drainage is primarily to the obturator and internal iliac nodes (see Fig. 2–21). A small portion of drainage may initially pass through the presacral group or, less commonly, the external iliac nodes.
Nerve Supply
Sympathetic and parasympathetic innervation from the pelvic plexus travels to the prostate through the cavernous nerves. Nerves follow branches of the capsular artery to ramify in the glandular and stromal elements. Parasympathetic nerves end at the acini and promote secretion; sympathetic fibers cause contraction of the smooth muscle of the capsule and stroma. α1-Adrenergic blockade diminishes prostate stromal and preprostatic sphincter tone and improves urinary flow rates in men affected with benign prostatic hypertrophy; this emphasizes that this disease affects both the stroma and the epithelium. Peptidergic and nitric oxide synthase–containing neurons also have been found in the prostate and may affect smooth muscle relaxation (Burnett, 1995). Afferent neurons from the prostate travel through the pelvic plexuses to pelvic and thoracolumbar spinal centers. A prostatic block may be achieved by instilling local anesthetic into the pelvic plexuses.
Membranous Urethra
In its course from the apex of the prostate to the perineal membrane, the membranous urethra spans on average 2 to 2.5 cm (range 1.2 to 5 cm) (Myers, 1991) (see Fig 2–37). It is surrounded by the striated (external) urethral sphincter, which is often incorrectly depicted as a flat sheet of muscle sandwiched between two layers of fascia. The striated sphincter is actually signet ring shaped, broad at its base, and narrowing as it passes through the urogenital hiatus of the levator ani to meet the apex of the prostate (see Fig. 2–14; see also Figs. 2-10, 2-27, and 2-38). In utero, this muscle forms a vertically oriented tube that extends from the perineal membrane to the bladder neck (Oelrich, 1980). As the prostate grows, posterior and lateral portions of this muscle atrophy, although transverse fibers persist on the entire anterior prostate through adulthood. At the apex of the prostate, circular fibers surround the urethra and thin posteriorly to insert into a fibrous raphe. Distally, the fibers do not meet posteriorly; rather, they acquire an Ω shape as they fan out laterally over the perineal membrane. Throughout its length, the posterior portion of the striated sphincter inserts into the perineal body. When the sphincter contracts, the walls of the urethra are pulled posteriorly toward the perineal body (Strasser et al, 1998). In contrast to the levator ani, the sphincter consists only of fine, type I (slow-twitch) fibers, rich in acid-stable myosin ATPase, which appear designed for tonic contraction. The myofibrils are surrounded by abundant connective tissue that blends with adjacent supporting structures.
The striated sphincter is related anteriorly to the dorsal vein complex (which may invade its anterior portion with age) and laterally to the levator ani. Connective tissue from deep within the lateral and anterior walls inserts into the puboprostatic ligaments posteriorly and into the suspensory ligament of the penis anteriorly to form a sling of fibrous tissue that suspends the urethra from the pubis (Steiner, 1994). A similar suspensory mechanism is found in the female urethra (see later discussion and Fig. 2–41). Two bulbourethral glands lie superior to the perineal membrane and are invested in the broad base of sphincter muscle. During sexual excitement, these glands secrete clear mucus into the bulbous urethra.
Figure 2–41 Urethral suspensory mechanism. The pubourethral ligament (P.U.L.) is composed of an anterior portion (suspensory ligament of the clitoris), a posterior portion (pubourethral ligament of endopelvic fascia), and an intermediate portion that bridges the other two.
(From Milley PS, Nichols DH. The relationship between the pubo-urethral ligaments and the urogenital diaphragm in the human female. Anat Rec 1971;170:283.)
The striated sphincter corresponds to the location of peak urethral closing pressure and is responsible for continence after prostatectomy. Components involved in generating this closing pressure are (1) the pseudostratified columnar epithelium, which contracts into radial folds as it meets to occlude the lumen; (2) the submucosa, which is rich with blood vessels and soft connective tissue and contributes to urethral sealing (Raz et al, 1972); (3) the longitudinal and circular urethral smooth muscle (intrinsic component of the external sphincter); (4) the striated sphincter; and (5) the pubourethral component of the levator ani.
Gross dissection and retrograde axonal tracing techniques have confirmed that the striated sphincter is supplied by the pudendal nerve (Tanagho et al, 1982). However, urologists have long been puzzled as to why pudendal nerve sectioning does not ablate sphincter activity. Lawson (1974) and Zvara and colleagues (1994) identified a second source of somatic innervation to the sphincter: a branch of the sacral plexus that runs on the pelvic surface of the levator ani (see Fig. 2–23). Injury to this nerve at radical prostatectomy may contribute to postoperative urinary incontinence (Hollabaugh et al, 1997). Autonomic innervation to the intrinsic smooth muscle of the membranous urethra is likely given by the cavernous nerves as they pass nearby, although dividing these nerves does not appear to affect urinary continence significantly (Steiner et al, 1991). Afferent fibers from the striated sphincter have not been defined but are sure to have interesting and important functional roles because this muscle lacks proprioceptive muscle spindles (Gosling et al, 1981).
Vas Deferens and Seminal Vesicle
As it arises from the tail of the epididymis, the vas (ductus) deferens is somewhat tortuous for 2 to 3 cm (Fig. 2–42). It runs posterior to the vessels of the cord and through the inguinal canal and emerges in the pelvis lateral to the inferior epigastric vessels (see Fig. 2–3). At the internal ring, it diverges from the testicular vessels and passes medial to all structures of the pelvic side wall to reach the base of the prostate posteriorly (see Figs. 2-3, 2-13, and 2-16). The terminal vas is dilated and tortuous (ampulla) and is capable of storing spermatozoa. The vas has a thick wall of outer longitudinal and inner circular smooth muscle and is lined by pseudostratified columnar epithelium with nonmotile stereocilia.
Figure 2–42 Testis and epididymis. A, One to three seminiferous tubules fill each compartment and drain into the rete testis in the mediastinum. Twelve to 20 efferent ductules become convoluted in the head of the epididymis and drain into a single coiled duct of the epididymis. The vas is convoluted in its first portion. B, Cross section of the testis, showing the mediastinum and septations continuous with the tunica albuginea. The parietal and visceral tunica vaginalis are confluent where the vessels and nerves enter the posterior aspect of the testis.
The seminal vesicle is a lateral outpouching of the vas, approximately 5 cm long, with a capacity of 3 to 4 mL (see Figs. 2-8, 2-36, and 2-43). Despite its name, it does not store sperm but contributes the largest portion of fluid to the ejaculate. The seminal vesicle comprises a single coiled tube with several outpouchings that is lined by columnar epithelium with goblet cells. The tube is encased in a thin layer of smooth muscle and is held in its coiled configuration by a loose adventitia.
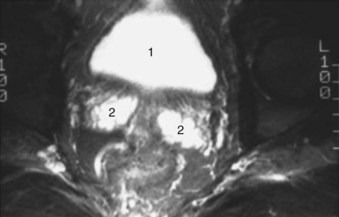
Figure 2–43 Axial T2-weighted magnetic resonance image of the male pelvis illustrating the 1, bladder and 2, seminal vesicles.
The seminal vesicle and ampulla of the vas lie posterior to the bladder. The ureter enters the bladder medial to the tip of the seminal vesicle. As the seminal vesicle and vas join to form the ejaculatory duct, their smooth muscle coats fuse with the prostatic capsule at its base. Denonvilliers fascia or, occasionally, the rectovesical pouch of peritoneum separates these structures from the rectum (see Fig. 2–27). Unless involved by a pathologic process, these structures are not palpable on rectal examination.
The blood supply for the seminal vesicles and vasa comes from the vesiculodeferential artery, a branch of the superior vesical artery. This artery supplies the vas throughout its length and then passes onto the anterior surface of the seminal vesicle near its tip. During radical prostatectomy the artery is invariably found between the vas and seminal vesicle and must be isolated and controlled. Additional arterial supply may come from the inferior vesical artery. The pelvic vas and seminal vesicle drain into the pelvic venous plexus. Lymphatic drainage passes to the external and internal iliac nodes (see Fig. 2–21). Innervation arises from the pelvic plexus, with major excitatory efferents contributed by the (sympathetic) hypogastric nerves (Kolbeck and Steers, 1993).
Female Pelvic Viscera
The uterus measures 8 × 6 × 4 cm in a normal woman and is composed largely of dense smooth muscle (see Figs. 2-19 and 2-44). It has a narrowed neck, the cervix, which opens through the anterior vaginal wall and a broad corpus that is capped by the rounded fundus. As discussed earlier, it lies in front of the rectum and over the dome of bladder; its impression can be observed cystoscopically (see Figs. 2-28 and 2-32). The fallopian tubes extend laterally from the junction of the corpus and fundus and are draped by leaves of peritoneum called the broad ligaments (see Figs. 2-13 and 2-19). As they extend to the pelvic side walls, the fallopian tubes angle up and backward to open posteromedially. The tubes are divided into four segments—uterine, isthmus, ampulla, and infundibulum—and are crowned by the fimbriae. The ovary rests posterior to the elbow of the tube and is supported by its own peritoneal fold, the mesovarium. The ureter may be found directly posterior to the ovary, covered by pelvic peritoneum. The infundibulopelvic ligament, mentioned earlier, suspends the ovary and lateral fallopian tube from the pelvic side wall and transmits the ovarian vessels to both structures. The round ligament of the ovary passes medially through the broad ligament to fix the ovary to the lateral wall of the uterus. Beneath its point of attachment, the round ligament of the uterus passes laterally, in the leaves of the broad ligament, to exit through to the inguinal canal and attach to the labial fat pad (see Figs. 2-13 and 2-19).
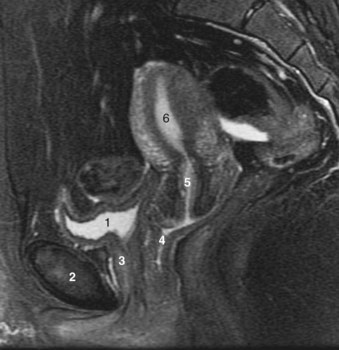
Figure 2–44 Key anatomic relationships in the female pelvis illustrated on sagittal midline T2-weighted magnetic resonance image. 1, urinary bladder; 2, pubic symphysis; 3, urethra; 4, vagina—note that the vagina is retroflexed posteriorly over the levator plate. The levator plate provides critical female pelvic support; 5, cervix; 6, uterus.
The uterine artery crosses in front of the ureter and runs in the broad and cardinal ligaments to supply the proximal vagina, uterus, and medial two thirds of the fallopian tube (see Fig. 2–19). It is joined by a rich plexus of uterine veins that freely connect with the ovarian veins. Nerves from the pelvic plexus travel to the female pelvic viscera through the cardinal and uterosacral ligaments in the company of the vessels; therefore hysterectomy can cause a flaccid neurogenic bladder.
The vagina extends inward from the vestibule at a 45-degree angle and then turns horizontal over the levator plate (see Figs. 2-28 and 2-44). It is lined by rugated nonkeratinized squamous epithelium backed by a thick, well-vascularized lamina propria. It is surrounded by a smooth muscle coat of inner circular and stronger external longitudinal layers. In cross section, the vagina is H shaped (see Fig. 2–41) as a result of firm attachments of its anterior wall to the levator ani at the arcus tendineus fascia pelvis, and of its posterior wall to the rectovaginal septum. The anterior vaginal wall is pierced by the cervix proximally. The shallow fossae around the cervix are referred to as the anterior, lateral, and posterior fornices. Because the apex of the vagina is covered with the peritoneum of the rectouterine pouch, the peritoneal cavity may be accessed through the posterior fornix (see Fig. 2–28).
Immediately in front of the cervix, the base of the bladder rests on the vaginal wall. Smooth muscle fibers tether the posterior bladder wall and base to the uterine cervix and vagina (see Figs. 2-28 and 2-44). Division of these fibers yields posterior access to the vesicovaginal space. This space extends distally to the proximal third of the urethra (where the urethra and vagina fuse) and is limited to each side by the lateral ligaments of the bladder. It may be accessed transvaginally through incision of the anterior vaginal wall in front of the cervix. Incision of the anterior vaginal wall to either side of the urethra leads into the retropubic space (see Fig. 2–12). The tough leaves of the visceral endopelvic fascia are felt medially and should be included in all transvaginal urethral suspension procedures (Mostwin, 1991).
The vagina is separated from the rectum by the rectovaginal septum (see Fig. 2–28), and rectoceles result from a loss of integrity of this septum. Deep to this septum lies a second potential space, the rectovaginal space. The bowel may herniate into this space to form an enterocele. On its lateral surfaces, the vagina is related to the levator ani. Near the vestibule, fibers of the levator ani blend and fuse with the vaginal muscularis. The vaginal vessels and nerves lie on the anterolateral surface of the vagina deep to the arcus tendineus fascia pelvis.
Female Urethra
On average, the female urethra traverses 4 cm from the bladder neck to the vaginal vestibule (see Fig. 2–44). Its lining changes gradually from transitional to nonkeratinized stratified squamous epithelium. Many small mucous glands open into the urethra and can give rise to urethral diverticula. Distally, these glands group together on either side of the urethra (Skene glands) and empty through two small ducts to either side of the external urethral meatus. A thick, richly vascular submucosa supports the urethral epithelium and glands (see Fig. 2–32). Together, the mucosa and submucosa form a cushion that contributes significantly to urethral closure pressure (Raz et al, 1972). These layers are estrogen dependent; at menopause they may atrophy, resulting in stress incontinence. A relatively thick layer of inner longitudinal smooth muscle continues from the bladder to the external meatus to insert into periurethral fatty and fibrous tissue. In contrast to the male proximal urethra, no circular smooth muscle sphincter can be identified. A rather thin layer of circular smooth muscle envelops the longitudinal fibers throughout the length of the urethra. It is thought that the longitudinal smooth muscle of the urethra contracts coordinately with the detrusor during micturition to shorten and widen the urethra (Gosling, 1979).
The striated urethral sphincter invests the distal two thirds of the female urethra (Oelrich, 1983). It is composed exclusively of delicate type I (slow-twitch) fibers surrounded by abundant collagen. Proximally, it forms a complete ring around the urethra that corresponds to the zone of highest urethral closure pressure (see Fig. 2–32). Farther down the urethra, the fibers do not meet posteriorly but continue off the lateral sides of the urethra onto the anterior and lateral wall of the vagina. Contraction of these fibers (the compressor urethrae) closes the urethra against the fixed anterior vaginal wall. Near the vestibule, the fibers completely surround the urethra and vagina to form a urethrovaginal sphincter. Contraction of this muscle group, along with bulbospongiosus, tightens the urogenital hiatus.
The suspensory ligament of the clitoris (anterior urethral ligament) and the pubourethral ligaments (posterior urethral ligaments) form a sling that suspends the urethra beneath the pubis (see Figs. 2-12 and 2-41) (Zacharin, 1963). The striated urethral sphincter receives dual somatic innervation, like that in the male, from the pudendal and pelvic somatic nerves (Borirakchanyavat et al, 1997). Little sympathetic innervation is found in the female urethra. Parasympathetic cholinergic fibers are found throughout the smooth muscle. Somatic and autonomic nerves to the urethra travel on the lateral walls of the vagina near the urethra. During transvaginal incontinence surgery, the anterior vaginal wall should be incised laterally to avoid these nerves and prevent type III urinary incontinence (Ball et al, 1997).
Female Pelvic Support
The pelvic muscles and fasciae cooperate to prevent prolapse of the urogenital organs through the hiatus. Three functional supportive elements are recognized: (1) the pubovisceral and perineal muscles, which form a sphincter around the urogenital hiatus (see Fig. 2–32); (2) the levator plate, which acts as a horizontal shelf beneath the bladder, uterine cervix, posterior vagina, and rectum (see Fig. 2–28); and (3) the cardinal and uterosacral ligaments, which anchor the pelvic viscera over the levator plate (Zacharin, 1985; Mostwin, 1991; DeLancey, 1993). The pelvic muscles contract tonically to counteract gravitational forces. In response to stress, the levator ani contracts, closing the urogenital hiatus and increasing the anteroposterior length of the levator plate. Increased intra-abdominal pressure forces the pelvic viscera downward against a fixed levator plate, closing the vagina like a flap valve.
Pelvic and perineal muscles play the greatest role in pelvic support. Damage to the perineal body during parturition destroys the urogenital sphincter, enlarges the urogenital hiatus, and erodes the levator plate. Aging and birth trauma partially denervate and weaken the levator ani (Snooks et al, 1985). With loss of muscular support, intra-abdominal forces impinge directly on the pelvic fasciae; over time, these either tear or stretch. Procedures to correct pelvic prolapse or urinary incontinence that rely solely on these fasciae may be successful initially but do not fare well over time (Trockman et al, 1995). Repair of a single pelvic defect—a cystocele for instance—may unmask another (enterocele, rectocele); therefore successful repair of pelvic prolapse must address all components of anatomic support (Zacharin, 1985; DeLancey, 1993).
Perineum
The perineum lies among the pubis, thighs, and buttocks and is limited superiorly by the levator ani. Viewed from below, the symphysis pubis, ischial tuberosities, and coccyx outline the diamond shape of the perineum; the inferior ischiopubic rami and sacrotuberous ligaments form its bony and ligamentous walls (Fig. 2–45; see also Figs. 2-29 and 2-46). A line drawn through the ischial tuberosities divides the perineum into an anal and a urogenital triangle.
(From Anson BJ, McVay CB. Surgical anatomy. 6th ed. Philadelphia: WB Saunders; 1984. p. 893.)
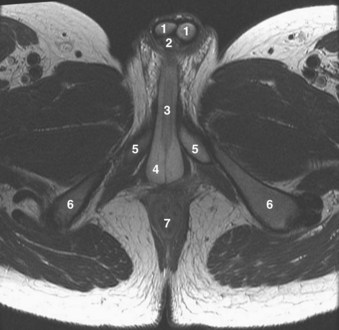
Figure 2–46 Axial T1-weighted magnetic resonance image of male pelvis. 1, corpora cavernosa in cross section; 2, corpus spongiosum in cross section; 3, corpus spongiosum in perineum; 4, bulbospongiosus muscle, which assists urine or seminal fluid expulsion from bulbar urethra; 5, crura of corpora cavernosa—note divergence of crura as they insert on pubic bone; 6, ischial tuberosities; 7, rectum.
Anal Triangle
At the apex of the prostate, the rectum turns approximately 90 degrees posteriorly and inferiorly to become the anus (see Figs. 2-10 and 2-14). It traverses 4 cm to reach the skin near the center of the anal triangle. The subcutaneous fat that surrounds the anus is continuous with that of the urogenital triangle, buttocks, and medial thigh. Laterally, the fat fills the ischiorectal fossa, a space bounded by the levator ani medially, and obturator internus, and the sacrotuberous ligament laterally (see Fig. 2–15). Anteriorly, this space extends into a recess above the urogenital diaphragm; posteriorly, it is continuous with the intermediate stratum of the pelvis through the sciatic foramina. Through this continuity, infections may travel between the perineum and the pelvic cavity.
The anal sphincter is divided into internal and external components. The internal sphincter represents a thickening of the inner circular smooth muscle layer of the rectum. The outer longitudinal smooth muscle thins beyond the rectourethralis and blends with the external sphincter, although a few fibers insert in the skin around the anus (corrugator cutis ani) to give it a puckered appearance. The external sphincter surrounds the internal sphincter and is divided into subcutaneous, superficial, and deep portions. The subcutaneous part attaches to the perineal body by collagenous and muscular fibers that are thickest superficially and referred to as the central tendon of the perineum. The superficial sphincter attaches to the perineal body and coccyx. At the posterior inflection of the rectum, the deep sphincter blends with the puborectalis sling of levator ani. At this level, a firm band may be felt on rectal examination and corresponds to the internal and external sphincter. Division of this muscular band results in fecal incontinence. The prostate may be accessed anterior to the sphincter, by dividing the central tendon and sphincteric attachments to the perineum (Young procedure) or by following the anterior rectal wall beneath the external anal sphincter (Belt procedure).
Male Urogenital Triangle
The entire urogenital triangle is bridged by the urogenital diaphragm. The scrotum hangs from the anterior aspect of the urogenital triangle; in the posterior aspect, skin and subcutaneous fat overlie Colles fascia. The perineal membrane and the posterior and lateral attachments of Colles fascia limit a potential space known as the superficial pouch (see Figs. 2-4, 2-15, and 2-29). In this space, the three erectile bodies of the penis have their bony and fascial attachments (the root of the penis). The paired corpora cavernosa attach to the inferior ischiopubic rami and perineal membrane and are surrounded by the ischiocavernosus muscles. The corpus spongiosum dilates as the bulb of the penis and is fixed to the center of the perineal membrane. It is encompassed by the bulbospongiosus muscles (see Fig. 2–46) that arise from the perineal body and from a central tendinous raphe and pass around the bulb to attach to the perineal membrane and dorsum of the penis. Contraction of the ischiocavernosus and bulbospongiosus muscles compresses the erectile bodies and potentiates penile erection. The transversus perinei muscles (superficial and deep) run along the posterior edge of the perineal membrane and are thought to stabilize the perineal body. Deep to the perineal membrane rests the striated urethral sphincter (discussed earlier).
Blood supply to the anal and urogenital triangles is derived largely from the internal pudendal vessels (Fig. 2–47). After entering the perineum through the lesser sciatic foramen, the artery runs in a fascial sheath on the medial aspect of obturator internus, the pudendal canal (of Alcock). Early in its course, it gives off three or four inferior rectal branches to the anus. Its perineal branch pierces Colles fascia to supply the muscles of the superficial pouch and continues anteriorly to supply the back of the scrotum. The internal pudendal terminates as the common penile artery (to be discussed).
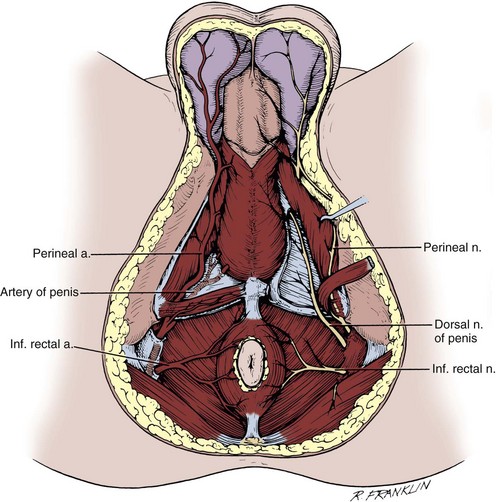
Figure 2–47 Male perineum, illustrating the internal pudendal artery and its branches on the left and the pudendal nerve and its branches on the right.
The internal pudendal veins communicate freely with the dorsal vein complex by piercing the levator ani. These communicating vessels enter the pelvic venous plexus on the lateral surface of the prostate and are a common, often unexpected, source of bleeding during apical dissection of the prostate. The inferior rectal veins anastomose with the middle and superior rectal veins and produce an important connection between the portal and systemic circulation. Obstruction of the portal or systemic venous system may cause shunting of collateral venous drainage through the portal system, manifested by hemorrhoids.
The pudendal nerve follows the vessels in their course through the perineum (see Fig. 2–47). Its first branch, the dorsal nerve of the penis, travels ventral to the main pudendal trunk in Alcock canal. Several inferior rectal branches supply the external sphincter muscle and provide sensation to perianal skin. The perineal branches follow the perineal artery into the superficial pouch to supply the ischiocavernosus, bulbospongiosus, and transversus perinei muscles. A few of these branches continue anteriorly to supply sensation to the posterior scrotum. Additional perineal branches pass deep to the perineal membrane to supply the levator ani and striated urethral sphincter.
Penis
As discussed, the root of the penis is fixed to the perineum within the superficial pouch. The corpora cavernosa join beneath the pubis (penile hilum) to form the major portion of the body of the penis. They are separated by a septum that becomes pectiniform distally, so their vascular spaces freely communicate. They are enclosed by the tough tunica albuginea, which is predominantly collagenous (Fig. 2–48). Its outer longitudinal and inner circular fibers form an undulating meshwork when the penis is flaccid and appear tightly stretched with erection (Goldstein et al, 1982). Smooth muscle bundles traverse the erectile bodies to form the endothelium-lined cavernous sinuses. These sinuses give the erectile tissue a spongy appearance on gross examination.
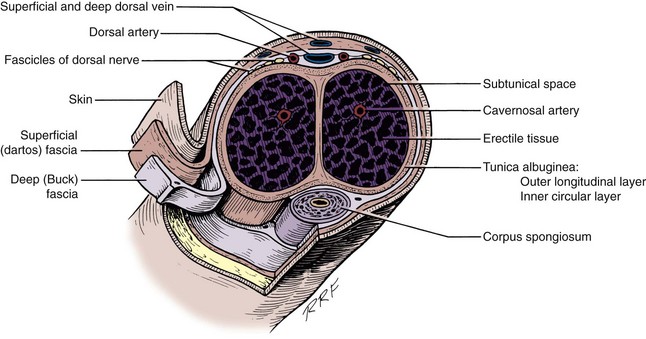
Figure 2–48 Cross section of the penis, demonstrating the relationship between the corporal bodies, penile fascia, vessels, and nerves.
(From Devine Jr CJ, Angermeier KW. Anatomy of the penis and mate perineum. AUA Update Series 1994;13:10–23.)
Distal to the bulb, the corpus spongiosum tapers and runs on the underside (ventrum) of the corpora cavernosa (see Fig. 2–46) and then expands to cap them as the glans penis. The corona separates the base of the glans from the shaft of the penis. The spongiosum is traversed throughout its length by the anterior urethra, which begins at the perineal membrane (see Figs. 2-35 and 2-37). The anterior urethra is dilated in its bulbar and glanular segments (fossa navicularis) and narrowest at the external meatus. Proximally, it is lined by stratified and pseudostratified columnar epithelium, distally by stratified squamous epithelium. The mucus-secreting glands (of Littre) may be seen as small outpouchings of the mucosa.
Buck fascia surrounds both cavernosal bodies dorsally and splits to surround the spongiosum ventrally (see Fig. 2–48). Elastic and collagenous fibers from the rectus sheath blend with and surround Buck fascia as the fundiform ligament of the penis. Deeper fibers from the pubis form the suspensory ligament of the penis. In the perineum, Buck fascia fuses with the tunica albuginea deep to the muscles of the erectile bodies (Uhlenhuth et al, 1949). Distally, it fuses with the base of the glans at the corona. Bleeding from a tear in the corporal bodies (e.g., penile fracture) is usually contained within Buck fascia, and ecchymosis is limited to the penile shaft.
The skin of the penile shaft is highly elastic and without appendages (hair or glandular elements), except for the smegma-producing glands at the base of the corona. It is devoid of fat and quite mobile because of the loose attachment of its dartos backing to Buck fascia. Distally, it folds over the glans as the foreskin and attaches firmly below the corona. Its blood supply is independent of the erectile bodies and is derived from the external pudendal branches of the femoral vessels (see Fig. 2–5). These vessels enter the base of the penis to run longitudinally in the dartos fascia as a richly anastomotic network. Thus penile skin may be mobilized on a vascular pedicle as the ideal tissue for urethral reconstruction. The skin of the glans is immobile as a result of its direct attachment to the underlying, thin tunica albuginea.
The common penile artery continues in Alcock canal, above the perineal membrane, and terminates in three branches to supply the erectile bodies (Fig. 2–49). The bulbourethral artery penetrates the perineal membrane to enter the spongiosum from above at its posterolateral border. This large, short artery can be difficult to isolate and control during urethrectomy. It supplies the urethra, spongiosum, and glans. The cavernosal artery pierces the corporal body in the penile hilum to near the center of its erectile tissue. It gives off straight and helicine arteries that ramify to supply the cavernous sinuses. The dorsal artery of the penis passes between the crus penis and the pubis to reach the dorsal surface of the corporal bodies. It runs between the dorsal vein and the dorsal penile nerve and with them attaches to the underside of Buck fascia (see Fig. 2–26). As it courses to the glans, it gives off cavernous branches and circumferential branches to the spongiosum and urethra. The rich blood supply to the spongiosum allows safe division of the urethra during stricture repair (Devine and Angermeier, 1994).
The surgeon contemplating penile revascularization must be aware that the penile arteries are highly variable in their branching, courses, and anastomoses (Bare et al, 1994). It is not uncommon for a single cavernosal artery to supply both corporal bodies or to be absent altogether. Alternatively, an accessory pudendal artery may supplement or completely replace branches of the common penile artery. This artery usually arises from the obturator or inferior vesical arteries and runs anterolateral to or within the prostate to reach the penis in the company of the dorsal vein. This artery has been identified in 7 of 10 cadaveric specimens (Breza et al, 1989) and noted at 4% of radical prostatectomies (Polascik and Walsh, 1995); its resection at prostatectomy may adversely affect postoperative potency (Droupy et al, 1999).
At the base of the glans, several venous channels coalesce to form the dorsal vein of the penis, which runs in a groove between the corporal bodies and drains into the preprostatic plexus (see Fig. 2–26). The circumflex veins originate in the spongiosum and pass around the cavernosa to meet the deep dorsal vein perpendicularly. They are present only in the distal two thirds of the penile shaft and number 3 to 10. Intermediary venules form from the cavernous sinuses to drain into a subtunical capillary plexus. These plexuses give rise to emissary veins, which commonly follow an oblique path between the layers of the tunica and drain into the circumflex veins dorsolaterally. Emissary veins in the proximal third of the penis join on the dorsomedial surface of the cavernous bodies to form two to five cavernous veins. At the hilum of the penis, these vessels pass between the crura and the bulb, receiving branches from each, and join the internal pudendal veins. Valves are found in the emissary, cavernosal, and deep dorsal veins and may thwart attempts to revascularize the penis by arteriovenous anastomosis (Sohn, 1994).
The dorsal nerves provide sensory innervation to the penis. These nerves follow the course of the dorsal arteries and richly supply the glans (see Fig. 2–26). Small branches from the perineal nerve supply the ventrum of the penis near the urethra as far as the glans distally (Uchio et al, 1999). These nerves must be anesthetized when performing a penile block to numb the ventrum of the penis. The route of the cavernous nerves has been described. After piercing the corporal bodies, they ramify in the erectile tissue to supply sympathetic and parasympathetic innervation from the pelvic plexus. Tonic sympathetic tone inhibits erection. Parasympathetic nerves release acetylcholine, nitric oxide, and vasoactive intestinal polypeptide, which cause the cavernosal smooth muscle and arterial relaxation necessary for erection (Burnett, 1995). It is thought that during erection, the subtunical venules are occluded by being compressed against the nondistensible tunica albuginea. Insufficient venous occlusion, particularly in vessels draining into the deep dorsal and cavernosal veins, is thought to cause vasculogenic impotence.
Scrotum
The scrotal skin is pigmented, hair bearing, devoid of fat, and rich in sebaceous and sweat glands. It varies from loose and shiny to highly folded with transverse rugae, depending on the tone of the dartos smooth muscle. A midline raphe runs from the urethral meatus to the anus and represents the line of fusion of the genital tubercles. Deep to this raphe, the scrotum is separated into two compartments by a septum.
The dartos layer of smooth muscle is continuous with Colles, Scarpa, and the dartos fascia of the penis (see Figs. 2-4 and 2-29). The testes are suspended by their cords in the scrotal compartments. As the testes descend, they acquire coverings from the layers of the abdominal wall, known as the spermatic fascia, that form part of the scrotal wall (Fig. 2–50). The external spermatic fascia derives from the external oblique fascia and remains firmly attached to the borders of the external ring. The cremasteric muscle and fascia arise from the internal oblique muscle and attach laterally to the inguinal ligament and iliopsoas fascia and medially to the pubic tubercle. The internal spermatic fascia is a continuation of the transversalis fascia. The parietal and visceral tunica vaginalis surround the testis with a mesothelium-lined pouch and are derived from the peritoneum. They are continuous at the posterolateral border of the testis at its mesentery, where it is fixed to the scrotal wall. The testis is also fixed at its lower pole by the gubernaculum. Occasionally, the mesentery and gubernaculum may be deficient, leaving the testis unfixed (bell-clapper deformity) and predisposing to torsion of the cord.
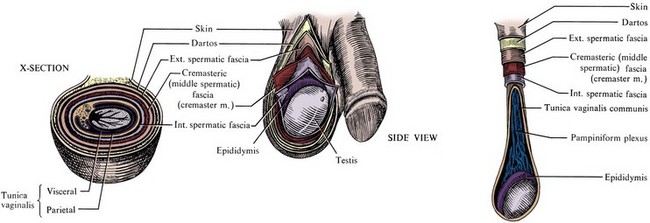
Figure 2–50 Scrotum and its layers.
(From Pansky B. Review of gross anatomy. 6th ed. New York: McGraw-Hill; 1987. p. 483.)
The anterior wall of the scrotum is supplied by the external pudendal vessels and the ilioinguinal and genitofemoral nerves (see Fig. 2–5). The anterior vessels and nerves typically run parallel to the rugae and do not cross the raphe; thus transverse or midline raphe scrotal incisions are most hemostatic. The back of the scrotum is supplied by the posterior scrotal branches of the perineal vessels and nerves (see Fig. 2–47). In addition, the posterior femoral cutaneous nerve (S3) gives a perineal branch to supply the scrotum and perineum (see Fig. 2–8). In accordance with their origin, the spermatic fasciae have a blood supply (cremasteric, vasal, testicular) separate from that of the scrotal wall. Fournier gangrene usually does not involve these structures, and they may be spared during débridement.
Perineal Lymphatics
The penis, scrotum, and perineum drain into the inguinal lymph nodes. These nodes may be divided into superficial and deep groups, which are separated by the deep fascia of the thigh (fascia lata). In relation to the external pudendal, superficial inferior epigastric, and superficial circumflex iliac vessels, the superficial nodes lie at the saphenofemoral junction. At the saphenous opening (fossa ovalis) in the fascia lata, the greater saphenous vein joins the femoral vein and the superficial nodes communicate with the deep group. Most of the deep inguinal nodes lie medial to the femoral vein and send their efferents through the femoral ring (beneath the inguinal ligament) to the external iliac and obturator nodes. Just outside the femoral ring, a large node (Cloquet or Rosenmuller node) is consistently present.
The scrotal lymphatics do not cross the median raphe and drain into the ipsilateral superficial inguinal lymph nodes. Lymphatics from the shaft of the penis converge on the dorsum and then ramify to both sides of the groin. Those of the glans pass deep to Buck fascia dorsally and drain to superficial and deep groups in both sides of the groin. Direct lymphatic channels from the glans to the pelvic nodes, which bypass the inguinal nodes, have been proposed by anatomists; however, clinical studies have not confirmed their existence. Other studies have suggested that all penile lymphatic drainage passes through “sentinel nodes,” which lie medial to the superficial inferior epigastric veins. Clinical studies have also called this speculation into question (Catalona, 1988). The perineal skin and fasciae drain into superficial inguinal nodes; the structures of the superficial pouch likely drain into the superficial and deep inguinal node groups.
Testes
The testes are 4 to 5 cm long, 3 cm wide, and 2.5 cm deep and have a volume of 30 mL. They are enclosed in a tough capsule comprising (1) the visceral tunica vaginalis; (2) tunica albuginea, with collagenous and smooth muscle elements; and (3) the tunica vasculosa. The epididymis attaches to the posterolateral aspect of the testis. Beneath it, the tunica albuginea projects inward to form the mediastinum testis, the point at which vessels and ducts traverse the testicular capsule (see Fig. 2–42). Septa radiate from the mediastinum to attach to the inner surface of the tunica albuginea to form 200 to 300 cone-shaped lobules, each of which contains one or more convoluted seminiferous tubules. Each tubule is U shaped and has a stretched length of nearly 1 meter. Interstitial (Leydig) cells lie in the loose tissue surrounding the tubules and are responsible for testosterone production. Toward the apices of the lobules, the seminiferous tubules become straight (tubuli recti) and enter the mediastinum testis to form an anastomosing network of tubules lined by flattened epithelium. This network, known as the rete testis (Fig. 2–51), forms 12 to 20 efferent ductules and passes into the largest portion of the epididymis, the caput. Here, the efferent ductules enlarge, become more convoluted, and form conical lobules. The duct from each lobule drains into a single epididymal duct, which winds approximately 6 m within the fibrous sheath of the epididymis to form its body and tail. As the duct approaches the tail, it thickens and straightens to become the vas deferens.
The spermatic cord is composed of the vas deferens, testicular vessels, and spermatic fasciae. As discussed in Chapter 1, the testicular arteries arise from the aorta and travel in the intermediate stratum of the retroperitoneum to reach the internal inguinal ring. Lateral to the internal inguinal ring, the attachments of the intermediate stratum form the lateral spermatic fascia. These attachments can be taken down at orchidopexy to gain cord length. At the internal ring, the vessels are joined by the genital branch of the genitofemoral nerve, ilioinguinal nerve, cremasteric artery, and vas deferens and its artery.
In its course to the testis, the testicular artery branches into an internal artery and an inferior testicular artery and into a capital artery to the head of the epididymis (see Fig. 2–18). The level of this branching varies and has been noted to occur within the inguinal canal in 31% to 88% of cases (Beck et al, 1992; Jarow et al, 1992). When performing an inguinal varicocelectomy, the surgeon must remember that there may be two or three arterial branches at this level (Hopps et al, 2003). A rich arterial anastomosis occurs at the head of the epididymis, between the testicular and the capital arteries, and at the tail between the testicular, epididymal, cremasteric, and vasal arteries (see Fig. 2–18). The testicular arteries enter the mediastinum and ramify in the tunica vasculosa, principally in the anterior, medial, and lateral portions of the lower pole and the anterior segment of the upper pole (Fig. 2–52 on the Expert Consult website![]() and Fig. 2–53). Thus placement of a traction suture through the lower pole tunica albuginea risks damaging these important superficial vessels and devascularizing the testis (Jarow, 1991). Testicular biopsy should be carried out in the medial or lateral surface of the upper pole, where the risk of vascular injury is minimal.
and Fig. 2–53). Thus placement of a traction suture through the lower pole tunica albuginea risks damaging these important superficial vessels and devascularizing the testis (Jarow, 1991). Testicular biopsy should be carried out in the medial or lateral surface of the upper pole, where the risk of vascular injury is minimal.
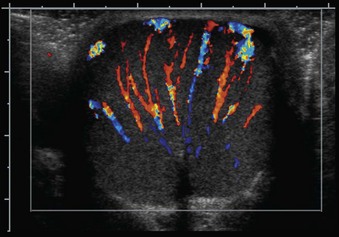
Figure 2–53 Doppler ultrasound of testis demonstrating spokelike radiation of testicular vessels originating from the mediastinum testis.
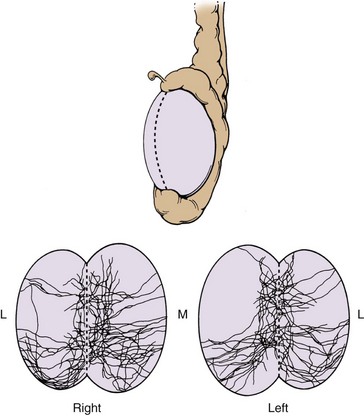
Figure 2–52 Distribution of subtunical testicular arteries compiled from 27 right and 26 left vascular casts. The highest density of subtunical arteries is found at the anterior upper pole and the entire lower pole. Lateral and medial sides of the upper pole are relatively free of arterial branches.
(From Jarow JP. Clinical significance of intratesticular arterial anatomy. J Urol 1991;145:777.)
The testicular veins form several highly anastomotic channels that surround the testicular artery as the pampiniform plexus. This arrangement allows countercurrent heat exchange, which cools the blood in the testicular artery. At the level of the inguinal canal, the veins join to form two or three channels and then a single vein that drains into the inferior vena cava on the right and the renal vein on the left. The testicular veins may anastomose with the external pudendal, cremasteric, and vasal veins (Fig. 2–54). These connections can allow varicoceles to recur after ablative procedures. Testicular lymphatic vessels drain to the para-aortic and interaortocaval nodes as detailed in Chapter 1.
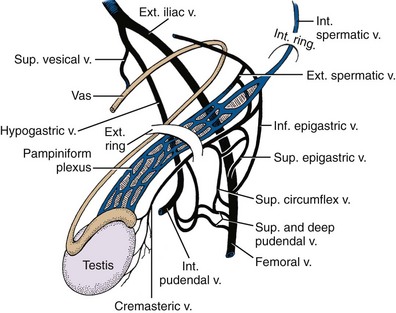
Figure 2–54 Venous drainage of the testis and epididymis. Note connections between the pampiniform plexus and the saphenous, internal iliac, and external iliac veins.
Visceral innervation to the testis and epididymis travels by two routes. A portion arises in the renal and aortic plexuses and travels with the gonadal vessels. Additional gonadal afferent and efferent nerves course from the pelvic plexus in association with the vas deferens (Rauchenwald et al, 1995). Intractable orchialgia may respond to anesthesia of the pelvic plexus (Zorn et al, 1994). Intriguingly, some afferent and efferent nerves cross over to the contralateral pelvic plexus (Taguchi et al, 1999). This neural cross-communication might explain how pathologic processes in one testis (e.g., tumor or varicocele) may affect the function of the contralateral testis. The genital branch of the genitofemoral nerve supplies sensation to the parietal and visceral tunica vaginalis and the overlying scrotum.
Female Urogenital Triangle
The vestibule of the vagina runs vertically throughout the length of the urogenital triangle. The labia majora form its lateral sides and fuse anteriorly as the hood of the clitoris. The subcutaneous fat pad of the mons pubis continues posteriorly in the labia majora to frame the vestibule. The labial fat pads receive blood supply from the external pudendal vessels and may be raised on these vessels as a rotational flap for repair of vesicovaginal or urethrovaginal fistulas (Fig. 2–55). The urethra enters the vestibule between the clitoris and the vagina.
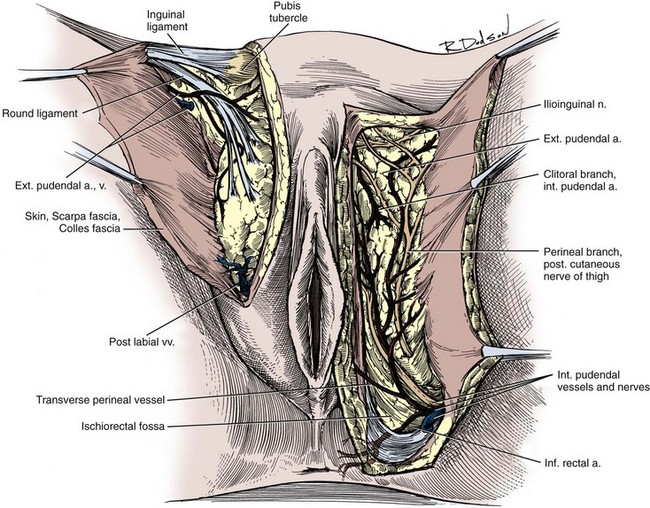
Figure 2–55 Arteries and nerves of the female perineum.
(From Doherty MG. Clinical anatomy of the pelvis. In: Copeland LJ, editor. Textbook of gynecology. Philadelphia: WB Saunders; 1993. p. 51.)
The structure of the superficial pouch is similar to that of the male (Fig. 2–56). The crura of the clitoris attach to the inferior ischiopubic rami, surrounded by the ischiocavernosus muscles, and converge to form the body of the clitoris. The vestibular bulbs lie to either side of the vaginal vestibule, covered by the bulbospongiosus muscles. As homologues of the penile bulb, they are composed of erectile tissue and meet anteriorly to form the glans of the clitoris. The vestibular glands are deep to the vestibular bulbs but, unlike the bulbourethral glands in the male, are superficial to the perineal membrane. Their ducts travel 2 cm to open in the vaginal vestibule on the posteromedial sides of the labia minora. The perineal membrane, pierced in its center by the vagina, is less well developed than that of the male. The innervation, blood supply, and lymphatic drainage of the external genitalia and superficial pouch are similar to those described in the male.
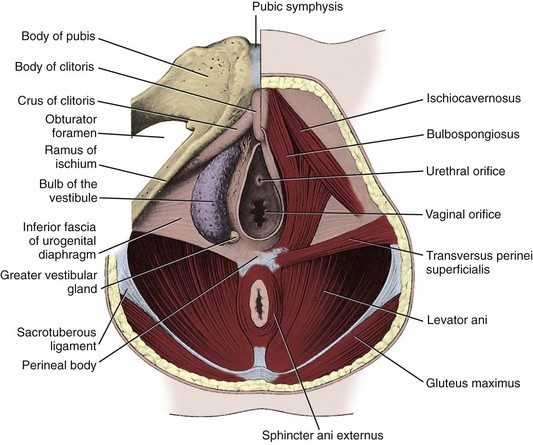
Figure 2–56 Female superficial perineal pouch. On the left side, the muscles have been removed to show the vestibular bulb and Bartholin gland.
(From Williams PL, Warwick R. Gray’s anatomy. 35th British ed. Philadelphia: WB Saunders; 1973. p. 1364.)
Key Points
DeLancey JOL. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897-909.
Hinman FJr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993.
Myers RP. Radical prostatectomy: pertinent surgical anatomy. Atlas Urol Clin North Am. 1994;2:1-18.
Uhlenhuth E. Problems in the anatomy of the pelvis. Philadelphia: JB Lippincott; 1953.
Williams PL, Warwick R, Dyson M, Bannister LH. Gray’s anatomy, 37th ed. New York: Churchill Livingstone; 1989.
Ball TP, Teichman JMH, Sharkey FE, et al. Terminal nerve distribution to the urethra and bladder neck: considerations in the management of stress urinary incontinence. J Urol. 1997;158:827-829.
Bare RL, DeFranzo A, Jarow JP. Intraoperative arteriography facilitates penile revascularization. J Urol. 1994;151:1019-1021.
Beck EM, Schlegel PN, Goldstein M. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 1992;148:1190-1194.
Begg RC. The urachus: its anatomy, histology and development. J Anat. 1930;64:170-183.
Borirakchanyavat S, Aboseif SR, Carroll PR, et al. Continence mechanism of the isolated female urethra: an anatomical study of the intrapelvic somatic nerves. J Urol. 1997;158:822-826.
Breza J, Aboseif SR, Ovis BR, et al. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437-443.
Brooks JD, Chao W-M, Kerr J. Male pelvic anatomy reconstructed from the visible human data set. J Urol. 1998;159:868-872.
Brooks JD, Eggener SE, Chow W-M. Anatomy of the male rectourethralis muscle. Eur Urol. 2002;41:94-100.
Burnett AL. Nitric oxide control of lower genitourinary tract functions: a review. Urology. 1995;45:1071-1083.
Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of the saphenous veins: technique and preliminary results. J Urol. 1988;140:306-310.
Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
DeCaro R, Aragona F, Herms A, et al. Morphometric analysis of the fibroadipose tissue of the female pelvis. J Urol. 1998;160:707-713.
DeLancey JOL. The pubovesical ligament: a separate structure from the urethral supports (“pubo-urethral ligaments”). Neurourol Urodyn. 1989;8:53-62.
DeLancey JOL. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897-909.
Devine CJJr, Angermeier KW. Anatomy of the penis and male perineum. AUA Update Series. 1994;13:10-23.
Droupy S, Hessel A, Benoît G, et al. Assessment of the functional role of accessory pudendal arteries in erection by transrectal color Doppler ultrasound. J Urol. 1999;162:1987-1991.
Eichelberg C, Erbersdobler A, Michl U, et al. Nerve distribution along the prostatic capsule. Eur Urol. 2007;51:105-111.
Epstein JI. The prostate and seminal vesicles. In: Sternberg SS, editor. Diagnostic surgical pathology. 1st ed. New York: Raven; 1989:1393-1432.
Flocks RH. The arterial distribution within the prostate gland: its role in transurethral prostatic resection. J Urol. 1937;37:524-548.
Goldstein AMB, Meehan JP, Zakhary R, et al. New observations on microarchitecture of corpora cavernosa in man and possible relationship to mechanism of erection. Urology. 1982;20:259-266.
Golimbu M, Al-Askari S, Morales P. Transpubic approach for lower urinary tract surgery: a 15 year experience. J Urol. 1990;143:72-76.
Gosling JA. The structure of the bladder and urethra in relation to function. Urol Clin North Am. 1979;6:31-38.
Gosling JA. The structure of the female lower urinary tract and pelvic floor. Urol Clin North Am. 1985;12:207-214.
Gosling JA, Dixon JS, Critchley HOD, Thompson SA. A comparative study of human external sphincter and periurethral levator ani muscles. Br J Urol. 1981;53:35-41.
Hinman FJr. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993.
Hollabaugh RSJr, Dmochowski RR, Steiner MS. Neuroanatomy of the male rhabdosphincter. Urology. 1997;49:426-434.
Hopps CV, Lemer ML, Schlegel PN, et al. Intraoperative varicocele anatomy: a microscopic study of the inguinal versus the subinguinal approach. J Urol. 2003;170:2366-2370.
Hutch JA, Ayers RD, Loquvam GS. The bladder musculature with special reference to the ureterovesical junction. J Urol. 1961;85:531-539.
Jarow JP. Clinical significance of intratesticular anatomy. J Urol. 1991;145:777-779.
Jarow JP, Ogle A, Kaspar J, Hopkins M. Testicular artery ramification within the inguinal canal. J Urol. 1992;147:1290-1292.
Kolbeck SC, Steers WD. Origin of neurons supplying the vas deferens of the rat. J Urol. 1993;149:918-921.
Lawson JON. Pelvic anatomy: Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244-252.
Lepor H, Gregerman M, Crosby R, et al. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207-212.
Lue TF, Zeineh SJ, Schmidt RA, Tanagho EA. Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol. 1984;131:273-280.
McNeal JE. The prostate and prostatic urethra: a morphologic synthesis. J Urol. 1972;107:1008-1016.
McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619-633.
Mostwin JL. Current concepts of female pelvic anatomy and physiology. Urol Clin North Am. 1991;18:175-195.
Myers RP. Male urethral sphincteric anatomy and radical prostatectomy. Urol Clin North Am. 1991;18:211-227.
Myers RP. Radical prostatectomy: pertinent surgical anatomy. Atlas Urol Clin North Am. 1994;2:1-18.
Myers RP, Cahill DR, Devine RM, et al. Anatomy of radical prostatectomy as defined by magnetic resonance imaging. J Urol. 1998;159:2148-2158.
Oelrich TM. The urethral sphincter in the male. Am J Anat. 1980;158:229-246.
Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223-232.
Polascik TJ, Walsh PC. Radical retropubic prostatectomy: the influence of accessory pudendal arteries on the recovery of sexual function. J Urol. 1995;153:150-152.
Rauchenwald M, Steers WD, Desjardins C. Efferent innervation of the rat testis. Biol Reprod. 1995;52:1136-1143.
Raychaudhuri B, Cahill D. Pelvic fasciae in urology. Ann R Coll Surg Engl. 2008;90:633-637.
Raz S, Caine M, Zeigler M. The vascular component in the production of intraurethral pressure. J Urol. 1972;108:93-96.
Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;121:198-200.
Richardson CA, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357-362.
Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987;138:1402-1406.
Shafik A. A study of the arterial pattern of the normal ureter. J Urol. 1972;107:720-722.
Snooks SJ, Badenoch DF, Tiptaft RC, Swash M. Perineal nerve damage in genuine stress urinary incontinence: an electrophysiological study. Br J Urol. 1985;57:422-426.
Sohn MHH. Current status of penile revascularization for the treatment of erectile dysfunction. J Androl. 1994;15:183-186.
Steiner MS. The puboprostatic ligament and the male urethral suspensory mechanism: an anatomic study. Urology. 1994;44:530-534.
Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol. 1991;145:512-514.
Strasser H, Frauscher F, Helweg G, et al. Transurethral ultrasound: evaluation of anatomy and function of the rhabdosphincter of the male urethra. J Urol. 1998;159:100-105.
Taguchi K, Tsukamoto T, Murakami G. Anatomical studies of the autonomic nervous system in the human pelvis by the whole-mount staining method: left-right communicating nerves between bilateral pelvic plexuses. J Urol. 1999;161:320-325.
Tanagho EA. Anatomy of the lower urinary tract. In: Walsh PC, Retik AB, Stamey TA, Vaughan ED, editors. Campbell’s urology. 6th ed. Philadelphia: WB Saunders; 1992:40-69.
Tanagho EA, Schmidt RA, Gomes De Araujo C. Urinary striated sphincter: what is its nerve supply? Urology. 1982;20:415-417.
Tewari A, Takenaka A, Mtui E, et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006;98:314-323.
Thomson AS, Dabhoiwala NF, Verbeek FJ, Lamers WH. The functional anatomy of the ureterovesical junction. Br J Urol. 1994;73:284-291.
Trockman BA, Leach GE, Hamilton J, et al. Modified Pereyra bladder neck suspension: 10-year mean followup using outcomes analysis in 125 patients. J Urol. 1995;154:1841-1847.
Uchio EM, Yang CC, Kromm BG, Bradley WE. Cortical evoked responses from the perineal nerve. J Urol. 1999;162:1983-1986.
Uhlenhuth E. Problems in the anatomy of the pelvis. Philadelphia: JB Lippincott; 1953.
Uhlenhuth E, Smith RD, Day EC, Middleton EB. A re-investigation of Colles’ and Buck’s fasciae in the male. J Urol. 1949;62:542-563.
Versi E, Cardozo LD, Studd JWW, et al. Internal urinary sphincter in maintenance of female continence. BMJ. 1986;292:166-173.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492-497.
Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473-485.
Waterhouse K, Abrahams JI, Gruber H, et al. The transpubic approach to the urinary tract. J Urol. 1973;109:486-490.
Williams PL, Warwick R, Dyson M, Bannister LH. Gray’s anatomy, 37th ed. New York: Churchill Livingstone; 1989.
Zacharin RF. The suspensory mechanism of the female urethra. J Anat (Lond). 1963;97:423-427.
Zacharin RF. Pelvic floor anatomy and the surgery of pulsion enterocele. New York: Springer-Verlag; 1985.
Zorn BH, Watson LR, Steers WD. Nerves from the pelvic plexus contribute to chronic orchialgia. Lancet. 1994;343:1161.
Zvara P, Carrier S, Kour N-W, Tanagho EA. The detailed neuroanatomy of the human striated urethral sphincter. Br J Urol. 1994;74:182-187.