chapter 12 Bladder Pain Syndrome (Interstitial Cystitis) and Related Disorders
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a condition diagnosed on a clinical basis and requiring a high index of suspicion on the part of the clinician. Simply put, it should be considered in the differential diagnosis of the patient presenting with chronic pelvic pain that is often exacerbated by bladder filling and associated with urinary frequency. One can argue that it is a symptom-complex because it has a differential diagnosis that should be explored in a timely fashion before or at the time of initiation of empirical therapy (Blaivas, 2007). Once other conditions have been ruled out it can be considered a syndrome that generally responds to one of a variety of therapeutic approaches in the majority of cases. The perception that the original term interstitial cystitis was not at all descriptive of the clinical syndrome or even the pathologic findings in many cases has led to the current effort to reconsider the name of the disorder and even the way it is positioned in the medical spectrum (Hanno, 2008). What was originally considered a bladder disease is now considered a chronic pain syndrome (Janicki, 2003) that may begin as a pathologic process in the bladder in most but not all patients and eventually can develop into a disease that in a small subset of those affected even cystectomy may not benefit (Baskin and Tanagho, 1992). Its relationship to type III chronic pelvic pain syndrome (CPPS)/nonbacterial prostatitis is unclear (Chai, 2002; Hakenberg and Wirth, 2002). Its association with other chronic pain syndromes has taken on more importance recently as a promising clue to unlock the challenging etiologic and therapeutic puzzle of this condition.
BPS/IC encompasses a major portion of the “painful bladder” disease complex. “Painful bladder disorders” comprise a large group of patients with bladder and/or urethral and/or pelvic pain, irritative voiding symptoms (urgency, frequency, nocturia, dysuria), and sterile urine cultures. Painful bladder conditions with well-established causes include radiation cystitis, cystitis caused by microorganisms that are not detected by routine culture methodologies, and systemic disorders that affect the bladder. In addition, many gynecologic disorders can mimic BPS/IC (Kohli et al, 1997; Howard, 2003a, 2003b). BPS/IC has no easily discernible etiology.
The symptoms are allodynic, an exaggeration of normal sensations. There are no pathognomonic findings on pathologic examination, and even the finding of petechial hemorrhages on the bladder mucosa during cystoscopy after bladder hydrodistention under anesthesia is no longer considered the sine qua non of BPS/IC that it had been until a decade ago ( Erickson, 1995; Waxman et al, 1998; Erickson et al, 2005). BPS/IC is truly a diagnosis of exclusion. It may have multiple causes and represent a final common reaction of the bladder to different types of insult.
Definition
“It resembles a constellation of stars; its components are real enough but the pattern is in the eye of the beholder” (Makela and Heliovaara, 1991). This evocative description of fibromyalgia could equally apply to BPS/IC. Indeed, it has been argued, not necessarily convincingly, that each medical specialty has at least one somatic syndrome (irritable bowel syndrome, chronic pelvic pain, fibromyalgia, tension headache, noncardiac chest pain, hyperventilation syndrome) that might be better conceptualized as a part of a general functional somatic syndrome than with the symptom-based classification that we have now that may be more a reflection of professional specialization and access to care (Wessely and White, 2004).
BPS/IC is a clinical diagnosis based primarily on chronic symptoms of pain perceived by the patient to emanate from the bladder and/or pelvis associated with urinary urgency/frequency in the absence of other identified causes for the symptoms. It has been defined and redefined over the past century, and as the problem of definition has become more prominent lately so have the number of definitions and attempts to crystallize just what the diagnosis means (Table 12–1). The International Continence Society (ICS) prefers the term painful bladder syndrome (PBS), defined as “the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology” (Abrams et al, 2002). The ICS reserves the diagnosis of IC to patients with “typical cystoscopic and histological features,” without further specifying these. This definition may miss 36% of patients, primarily because it confines the pain to a suprapubic location and mandates a relationship of pain to bladder filling (Warren et al, 2006).
Table 12–1 Definitions of Bladder Pain Syndrome/Painful Bladder Syndrome/Interstitial Cystitis Syndrome over the Past Century
| YEAR | DEFINITION | SOURCE |
|---|---|---|
| 1887 | Skene: An inflammation that has destroyed the mucous membrane partly or wholly and extended to the muscular parietes | Skene, 1887 |
| 1915 | Hunner: A peculiar form of bladder ulceration whose diagnosis depends ultimately on its resistance to all ordinary forms of treatment in patients with frequency and bladder symptoms (spasms) | Hunner, 1915 |
| 1951 | Bourque: Patients who suffer chronically from their bladder; and we mean the ones who are distressed, not only periodically but constantly, having to urinate at all hours of the day and of the night suffering pains every time they void. | Bourque, 1951 |
| 1978 | Messing and Stamey: Nonspecific and highly subjective symptoms of around-the-clock frequency, urgency, and pain somewhat relieved by voiding when associated with glomerulations upon bladder distention under anesthesia. | Messing and Stamey, 1978 |
| 1990 | Revised NIDDK criteria: Pain associated with the bladder or urinary urgency, and glomerulations or Hunner ulcer on cystoscopy under anesthesia, in patients with 9 months or more of symptoms—at least 8 voids per day, 1 void per night, and cystometric bladder capacity less than 350 mL | Wein et al, 1990 |
| 1997 | NIDDK Interstitial Cystitis Database entry criteria: Unexplained urgency or frequency (7 or more voids per day) or pelvic pain of at least 6 months’ duration in the absence of other definable causes. | Simon et al, 1997 |
| 2008 | European Society for the Study of Interstitial Cystitis: Chronic (>6 months) pelvic pain, pressure, or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom such as persistent urge to void or frequency. Confusable diseases as the cause of the symptoms must be excluded. | Van de Merwe et al, 2008 |
| 2009 | Japanese Urological Association: A disease of the urinary bladder diagnosed by three conditions: (1) lower urinary tract symptoms such as urinary frequency, bladder hypersensitivity and/or bladder pain; (2) bladder pathology proven endoscopically by Hunner ulcer and/or mucosal bleeding after overdistention; and (3) exclusion of confusable diseases such as infection, malignancy, or calculi of the urinary tract. | Homma et al, 2009 |
| 2009 | Society for Urodynamics and Female Urology informal international dialogue consensus meeting: An unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes. | Hanno and Dmochowski, 2009 |
Data from Hanno PM, Lin AT, Nordling J, et al. Bladder pain syndrome. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publication Ltd.; 2009. p. 1459–518.
In the absence of clear criteria for IC, this chapter will refer to BPS/IC and IC interchangeably, because all but recent literature terms the syndrome IC. The definition of the European Society for the Study of Interstitial Cystitis (ESSIC) is a clinically useful one, and changes made since its original iteration have likely made it more sensitive and inclusive (Mouracade et al, 2008). Minor modifications made at a meeting under the auspices of the Society for Urodynamics and Female Urology (SUFU) may be preferred by some clinicians. Perhaps more than for most diseases, how we arrived at this point is instructive and critical to an overall understanding of BPS/IC. The paradigm change that has resulted in morphing what was originally considered a bladder disease (aptly named interstitial cystitis) to a chronic pain syndrome (bladder pain syndrome) also merits discussion.
Historical Perspective
Recent historical reviews confirm that IC was recognized as a pathologic entity during the 19th century (Christmas, 1997; Parsons and Parsons, 2004). Joseph Parrish, a Philadelphia surgeon, described three cases of severe lower urinary tract symptoms in the absence of a bladder stone in an 1836 text (Parrish, 1836) and termed the disorder tic doloureux of the bladder. Teichman and associates (2000) argue that this may represent the first description of IC. Fifty years later Skene (1887) used the term interstitial cystitis to describe an inflammation that has “destroyed the mucous membrane partly or wholly and extended to the muscular parietes.”
Early in the 20th century, at a New England Section meeting of the American Urological Association, Guy Hunner (1915, 1918) reported on eight women with a history of suprapubic pain, frequency, nocturia, and urgency lasting an average of 17 years. He drew attention to the disease, and the red, bleeding areas he described on the bladder wall came to have the pseudonym of Hunner ulcer. As Anthony Walsh (1978) observed, this has proven to be unfortunate. In the early part of the 20th century the very best cystoscopes available gave a poorly defined and ill-lit view of the fundus of the bladder. It is not surprising that when Hunner saw red and bleeding areas high on the bladder wall he thought they were ulcers. For the next 60 years urologists would look for ulcers and fail to make the diagnosis in their absence. The disease was thought to be focal rather than a pancystitis.
Hand (1949) authored the first comprehensive review about the disease, reporting 223 cases. In looking back, his paper was truly a seminal one, years ahead of its time. Many of his epidemiologic findings have held up to this day. His description of the clinical findings bears repeating. “I have frequently observed that what appeared to be a normal mucosa before and during the first bladder distention showed typical interstitial cystitis on subsequent distention.” He notes, “small, discrete, submucosal hemorrhages, showing variations in form … dot-like bleeding points … little or no restriction to bladder capacity.” He portrays three grades of disease, with grade 3 matching the small-capacity, scarred bladder described by Hunner. Sixty-nine percent of patients had grade 1 disease, and only 13% had grade 3 disease.
Walsh (1978) later coined the term glomerulations to describe the petechial hemorrhages that Hand had described. But it was not until Messing and Stamey (1978) discussed the “early diagnosis” of IC that attention turned from looking for an ulcer to make the diagnosis to the concepts that (1) symptoms and glomerulations at the time of bladder distention under anesthesia were the disease hallmarks and (2) the diagnosis was primarily one of exclusion.
Bourque’s Aunt Minnie (she is hard to define, but you know her when you see her) description of IC is over 50 years old and is worth recalling: “We have all met, at one time or another, patients who suffer chronically from their bladder; and we mean the ones who are distressed, not only periodically but constantly, having to urinate often, at all moments of the day and of the night, and suffering pains every time they void. We all know how these miserable patients are unhappy, and how those distressing bladder symptoms get finally to influence their general state of health physically at first, and mentally after a while” (Bourque, 1951).
Although memorable and right on the mark, this description and others like it were not suitable for defining this disease in a manner that would help physicians make the diagnosis and design research studies to learn more about the problem. Physician interest and government participation in research were sparked through the efforts of a group of frustrated patients led by Dr. Vicki Ratner, an orthopedic surgery resident in New York City, who founded the first patient advocacy group, the Interstitial Cystitis Association, in the living room of her small New York City apartment in 1984 (Ratner et al, 1992; Ratner and Slade, 1997). The first step was to develop a working definition of the disease. The modern history of PBS/IC is best viewed through the development of the modern definition.
Evolution of Definition
There are data to suggest that true urinary frequency in women can be defined as regularly having to void at intervals of less than 3 hours and 25% of women older than age 40 years have nocturia at least once (Glenning, 1985; Fitzgerald and Brubaker, 2003). Whereas bladder capacity tends to fall in women by the eighth and ninth decades of life, bladder volume at first desire to void tends to rise as women age (Collas and Malone-Lee, 1996). Based on a 90th percentile cut-off to determine the ranges of normality, the highest “normal” frequency ranges in the 4th decade range from 6 for men to 9 for women (Burgio et al, 1991). Large variation in the degree of bother with varying rates of frequency (Fitzgerald et al, 2002) makes a symptomatic diagnosis of PBS/IC based on an absolute number of voids subject to question, and frequency per volume of intake or even the concept of “perception of frequency” as a problem may be more accurate than an absolute number.
In an effort to define IC so that patients in different geographic areas, under the care of different physicians, could be compared, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) held a workshop in 1987 at which consensus criteria were established for the diagnosis of IC (Gillenwater and Wein, 1988). These criteria were not meant to define the disease but rather to ensure that groups of patients included in basic and clinical research studies would be relatively comparable. After pilot studies testing the criteria were performed, the criteria were revised at another NIDDK workshop a year later (Wein et al, 1990). These criteria are presented in Table 12–2.
Table 12–2 National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diagnostic Criteria for Interstitial Cystitis
From Wein AJ, Hanno PM, Gillenwater JY. Interstitial cystitis: an introduction to the problem. In: Hanno PM, Staskin DR, Krane RJ, Wein AJ, editors. Interstitial cystitis. London: Springer-Verlag; 1990. p. 13–5.
Although meant initially to serve only as a research tool, the NIDDK “research definition” became a de facto definition of this disease, diagnosed by exclusion and colorfully termed a “hole in the air” by Hald (cited in George, 1986). Certain of the exclusion criteria serve mainly to make one wary of a diagnosis of IC but should by no means be used for categorical exclusion of such a diagnosis. However, because of the ambiguity involved, these patients should probably be eliminated from research studies or categorized separately. In particular, exclusion criteria 4, 5, 6, 8, 9, 11, 12, 17, and 18 are only relative. What percentage of patients with idiopathic sensory urgency has IC is unclear (Frazer et al, 1990). The specificity of the finding of bladder glomerulations has come into question (Erickson, 1995; Waxman et al, 1998; Tomaszewski et al, 2001). Similarly, the sensitivity of glomerulations is also unknown, but clearly patients with IC symptoms can demonstrate an absence of glomerulations under anesthesia (Awad et al, 1992; Al Hadithi et al, 2002). Bladder ulceration is extremely rare and exists in less than 5% of patients in my experience and in the experience of others (Sant, 1991). A California series found 20% of patients to have ulceration (Koziol, 1994). Specific pathologic findings represent a glaring omission from the criteria, because there is a lack of consensus as to which pathologic findings, if any, are required for, or even suggestive of, a tissue diagnosis (Hanno et al, 1990, 2005a; Tomaszewski et al, 1999, 2001).
The unexpected use of the NIDDK research criteria by the medical community as a definition of IC led to concerns that many patients suffering from this syndrome might be misdiagnosed. The multicenter Interstitial Cystitis Database (ICDB) study through NIDDK accumulated data on 424 patients with IC, enrolling patients from May 1993 through December 1995. Entry criteria were much more symptom driven than those promulgated for research studies (Simon et al, 1997) and are noted in Table 12–3. In an analysis of the defining criteria (Hanno et al, 1999a, 1999b), it appeared the NIDDK research criteria fulfilled their mission. Fully 90% of expert clinicians agreed that patients diagnosed with IC by those criteria in the ICDB indeed had the disorder. However, 60% of patients deemed to have IC by these experienced clinicians would not have met NIDDK research criteria. Thus, IC remains a clinical syndrome defined by some combination of chronic symptoms of urgency, frequency, and/or pain in the absence of other reasonable causation. Whereas IC symptom and problem indices have been developed and validated (O’Leary et al, 1997; Goin et al, 1998), these are not intended to diagnose or define IC but rather to measure the severity of symptomatology and monitor disease progression or regression (Moldwin and Kushner, 2004).
Table 12–3 Interstitial Cystitis Database (ICDB) Study Eligibility Criteria
From Simon LJ, Landis JR, Erickson DR, Nyberg LM. The Interstitial Cystitis Data Base Study: concepts and preliminary baseline descriptive statistics. Urology 1997;49:64–75.
Nomenclature and Taxonomy
Recent international consultations have essentially agreed that the nomenclature of “interstitial cystitis” should be revised and I will use the terminology of the International Consultation on Incontinence of bladder pain syndrome but keep the term interstitial cystitis to facilitate recognition and understanding. This change implies that it is the symptoms that drive treatment, and the question as to whether interstitial cystitis should refer to a distinct subgroup of the bladder pain syndrome is, as yet, unclear.
The literature over the past 170 years has seen numerous changes in description and nomenclature of the disease. The syndrome has variously been referred to as tic douloureux of the bladder, IC, cystitis parenchymatosa, Hunner ulcer, panmural ulcerative cystitis, urethral syndrome, and painful bladder syndrome (Skene, 1887; Hunner, 1918; Powell and Powell, 1949; Bourque, 1951; Christmas, 1997; Teichman et al, 2000; Dell and Parsons, 2004). The term interstitial cystitis, which Skene is credited with coining and Hunner brought in to common usage, is a misnomer; in many cases not only is there no interstitial inflammation, but also, histopathologically, there may be no inflammation at all (Lynes et al, 1990a; Denson et al, 2000; Tomaszewski et al, 2001; Rosamilia et al, 2003). By literally focusing exclusively on the urinary bladder, the term interstitial cystitis furthermore does not do justice to the condition from both the physician’s and the patient’s perspective. The textual exclusiveness ignores the high comorbidity with various pelvic, extrapelvic, and nonurologic symptoms and associated disorders (Clauw et al, 1997) that frequently precede or develop after the onset of the bladder condition (Wu et al, 2006).
With the formal definition of the term painful bladder syndrome by the ICS in 2002, the terminology discussion became an intense international focal point (Abrams et al, 2002):
One can see in this the beginnings of a new paradigm that might be expected to change the emphasis of both clinical and basic science research and that removes the automatic presumption that the end organ in the name of the disease should necessarily be the sole or primary target of such research.
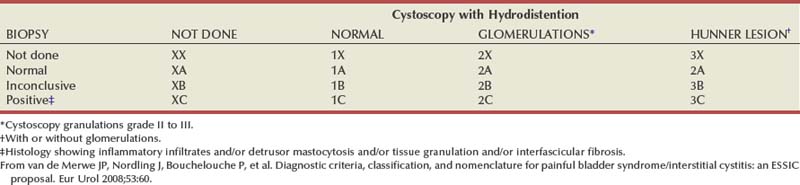
At the major biannual IC research conference in the fall of 2006, held by the NIDDK (Frontiers in Painful Bladder Syndrome/Interstitial Cystitis), the ESSIC group was given a block of time with which to present their thoughts and conclusions. Because (1) the term PBS did not fit into the taxonomy of other pelvic pain syndromes such as urethral or vulvar pain syndromes, (2) as defined by the International Continence Society missed over a third of afflicted patients, and (3) is a term open to different interpretations, the ESSIC suggested that painful bladder syndrome be redesignated as bladder pain syndrome (BPS), followed by a type designation. BPS is indicated by two symbols, the first of which corresponds to cystoscopy with hydrodistention findings (1, 2, or 3 indicating increasing grade of severity) and the second to biopsy findings (A, B, and C indicating increasing grade of pathologic severity) (Table 12–4). Although neither cystoscopy with hydrodistention nor bladder biopsy was prescribed as an essential part of the evaluation, by categorizing patients as to whether either procedure was done and, if so, the results, it is possible to follow patients with similar findings and study each identified cohort to compare natural history, prognosis, and response to therapy (van de Merwe et al, 2008).
As Baranowski and associates (2008) conceived it, BPS is defined as a pain syndrome with a collection of symptoms, the most important of which is pain perceived to be in the bladder. IC is distinguished as an end-organ, visceral-neural pain syndrome, whereas BPS can be considered a pain syndrome that involves the end-organ (bladder) and neurovisceral (myopathic) mechanisms. In IC, one expects an end-organ primary pathologic process. This is not necessarily the case in the broader BPS.
A didactically very demonstrative way to conceptualize the dawning shift in conception of the condition is with the drawing of a target (Fig. 12–1). There may be many causes of chronic pelvic pain. When an etiology cannot be determined, it is characterized as pelvic pain syndrome. To the extent that it can be distinguished as urologic, gynecologic, dermatologic, and the like, it is further categorized by organ system. A urologic pain syndrome can sometimes be further differentiated on the site of perceived pain. Bladder, prostate, testicular, and epididymal pain syndromes follow. Finally, types of BPS can be further defined as IC or simply categorized by ESSIC criteria. Patient groups have expressed their concerns with regard to any nomenclature change that potentially drops the term interstitial cystitis because the U.S. Social Security Administration and private insurances recognize IC but not the term BPS and benefits potentially could be adversely affected. Whether the term interstitial cystitis, as difficult as it is to define and as potentially misleading as it is with regard to etiology and end-organ involvement, should be maintained is a subject of ongoing controversy (Hanno and Dmochowski, 2009).
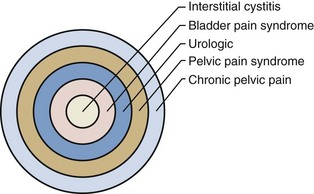
Figure 12–1 Conceptualization of pelvic pain syndrome classification.
(From Hanno P. Interstitial cystitis/painful bladder syndrome/bladder pain syndrome: the evolution of a new paradigm. In: Proceedings of the International Consultation on Interstitial Cystitis. Japan: Comfortable Urology Network; 2008. p. 2–9.)
Key Points
Definition
Urgency is a common complaint of this group of patients. The ICS definition of urgency (Abrams et al, 2002), “the complaint of a sudden compelling desire to pass urine, which is difficult to defer,” could be interpreted as compatible with either detrusor overactivity or BPS/IC depending on the weight one attaches to the word sudden. There are those who see hypersensitivity or sensory urgency as bridging both overactive bladder and BPS/IC (Haylen et al, 2007; Yamaguchi et al, 2007), and the issue has been nicely addressed by Homma (2008). Pain and pressure are more involved in the frequency of BPS/IC, and fear of incontinence seems the reason for the urgency of overactive bladder (Abrams et al, 2005). Thus, urgency is not required to define BPS/IC, because it would tend to obfuscate the borders of overactive bladder and PBS/IC and is unnecessary for definition purposes. Figure 12–2 (Abrams et al, 2005) is a graphic depiction of one view of the relationship between these two, sometimes confused, conditions. The 14% incidence of urodynamic detrusor overactivity in the PBS/IC patients (Nigro et al, 1997) is probably close to what one might expect in the general population if studied urodynamically (Salavatore et al, 2003).
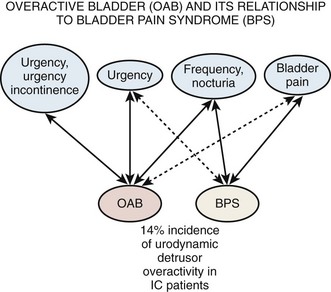
Figure 12–2 Overactive bladder (OAB) and its relationship to bladder pain syndrome (BPS). IC, interstitial cystitis.
(From Abrams PH, Hanno PM, Wein AJ. Overactive bladder and painful bladder syndrome: there need not be confusion. Neurourol Urodyn 2005;24:149–50.)
Still, there remains some ambiguity, and further research is necessary with regard to urgency. Studies are hampered by the fact that patients tend to use words to describe lower urinary tract symptoms but attribute meanings to the words different from physicians and researchers (Digesu et al, 2008). An analysis of urgency by the University of Maryland group reported that 65% of patients with BPS experienced an urge to urinate to relieve pain, with 46% agreeing that they had an urge to relieve pain and not to prevent incontinence. Still, 21% reported that urgency was for fear of impending incontinence and that this sensation was not present before the onset of BPS symptoms (Diggs et al, 2007). In some patients the term connotes an intensification of the normal urge to void, and in others it is a different sensation (Blaivas et al, 2009).
New efforts to phenotype the chronic urologic pain syndromes (BPS and chronic nonbacterial prostatitis/CPPS in men) are being explored (Nyberg et al, 2009; Shoskes et al, 2009a, 2009b). The hope is that looking at psychological, physical, and organ-specific parameters of afflicted patients, and specifically focusing on associated disorders, will aid in proper selection of therapeutic agents that may have selective specificity for different symptom constellations and also may improve productivity and results of research on etiology, prognosis, and new therapeutic agents.
Epidemiology
Prevalence
Epidemiology studies of PBS/IC are hampered by many problems (Bernardini et al, 1999). The lack of an accepted definition, the absence of a validated diagnostic marker, and questions regarding etiology and pathophysiology make much of the literature difficult to interpret. This is most apparent when one looks at the variation in prevalence reports in the United States and around the world (Table 12–5). These range from 1.2 per 100,000 population and 4.5 per 100,000 females in Japan (Ito et al, 2000), to a questionnaire-based study that suggests a figure in American women of 20,000 per 100,000 (Parsons and Tatsis, 2004)!
Table 12–5 Prevalence of BPS/IC per 100,000 Women
| STUDY | PREVALENCE |
|---|---|
| Oravisto, 1975 (Finland) | 18 |
| Jones, 1989 (USA) | 500 |
| Held et al, 1990 (USA) | 30 |
| Bade et al, 1995 (Netherlands) | 12 |
| Curhan et al, 1999 (USA) | 60 |
| Ito et al, 2000 (Japan) | 4.5 |
| Roberts et al, 2003 (USA) | 1.6 |
| Leppilahti et al, 2005 (Finland) | 300 |
| Clemens, 2007b (USA) | 197 |
| Temml et al, 2007 (Austria) | 306 |
| Song et al, 2009 (China) | 100 |
| Berry et al, 2009 (USA) | 2600 |
It has been estimated that the prevalence of chronic pain due to benign causes in the population is at least 10% (Verhaak et al, 1998). Numerous case series have, until recently, formed the basis of epidemiologic information regarding PBS/IC. Farkas and associates (1977) discussed IC in adolescent girls. Hanash and Pool (1969) reviewed their experience with IC in men. Geist and Antolak (1970) reviewed and added to reports of disease occurring in childhood. A childhood presentation is extremely rare and must be differentiated from the much more common and benign-behaving extraordinary urinary frequency syndrome of childhood, a self-limited condition of unknown etiology (Koff and Byard, 1988; Robson and Leung, 1993). Nevertheless there is a small cohort of children with chronic symptoms of bladder pain, urinary frequency, and sensory urgency in the absence of infection who have been evaluated with urodynamics, cystoscopy, and bladder distention and have findings consistent with the diagnosis of PBS/IC. In Close and colleagues’ review of 20 such children (1996) the median age at onset was younger than 5 years and the vast majority of patients had long-term remissions with bladder distention.
A study conducted at the Scripps Research Institute (Koziol et al, 1993) included 374 patients at Scripps as well as some members of the Interstitial Cystitis Association, the large patient support organization. A more recent, but similar study in England (Tincello and Walker, 2005) concurred with the Scripps findings of urgency, frequency, and pain in the vast majority of these patients, devastating effects on quality of life, and often unsuccessful attempts at therapy with a variety of treatments. Although such reviews provide some information, they would seem to be necessarily biased by virtue of their design.
Several population-based studies have been reported in the literature (Fig. 12–3), and these studies tend to support the reviews of selected patients or from individual clinics and the comprehensive follow-up case-control study by Koziol (1994). The first population-based study (Oravisto, 1975) included “almost all the patients with interstitial cystitis in the city of Helsinki.” This superb, brief report from Finland surveyed all diagnosed cases in a population approaching 1 million. The prevalence of the disease in women was 18.1 per 100,000. The joint prevalence in both sexes was 10.6 cases per 100,000. The annual incidence of new female cases was 1.2 per 100,000. Severe cases accounted for about 10% of the total. Ten percent of cases were in men. The disease onset was generally subacute rather than insidious, and full development of the classic symptom-complex occurred over a relatively short time. IC does not progress continuously but usually reaches its final stage rapidly (within 5 years) in the Koziol study (1993) and then continues without significant change in symptomatology. Subsequent major deterioration was found by Oravisto to be unusual. The duration of symptoms before diagnosis was 3 to 5 years in the Finnish study. Analogous figures in a classic American paper a quarter of a century earlier were 7 to 12 years (Hand, 1949).
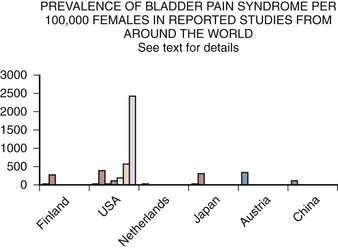
Figure 12–3 Prevalence of bladder pain syndrome per 100,000 females in reported studies from around the world. See text for details.
Another early population study, this one in the United States, first demonstrated the potential extent of what had been considered a very rare disease (Held et al, 1990). The following population groups were surveyed: (1) random survey of 127 board-certified urologists, (2) 64 IC patients selected by the surveyed urologists and divided between the last patient with IC seen and the last patient with IC diagnosed, (3) 904 female patients belonging to the Interstitial Cystitis Association, and (4) random phone survey of 119 persons from the U.S. population. This 1987 study reached the following conclusions:
Other population studies followed. Jones and Nyberg (1997) obtained their data from self-report of a previous diagnosis of IC in the 1989 National Household Interview Survey. The survey estimated that 0.5% of the population, or more than 1 million people in the United States, reported having a diagnosis of IC. There was no verification of this self-report by medical records. Bade and colleagues (1995) did a physician questionnaire-based survey in the Netherlands yielding an overall prevalence of 8 to 16/100,000 females, with diagnosis heavily dependent on pathology and presence of mast cells. This prevalence in females compares to 4.5/100,000 in Japan (Ito et al, 2000).
The Nurses Health Study I and II (Curhan et al, 1999) showed a prevalence of IC between 52 and 67/100,000 in the United States, which was twice the prevalence in the study by Held and associates (1990) and threefold greater than the study by Bade and colleagues (1995) in The Netherlands. It improved on previous studies by using a large sample derived from a general population and careful ascertainment of the diagnosis. If the 6.4% confirmation rate of their study were applied to the Jones and colleagues’ National Health Interview Survey data, the prevalence estimates of the two studies would be nearly identical.
Leppilahti and coworkers (2002, 2005) used the O’Leary-Sant IC symptom and problem index (never validated for making a diagnosis per se) to select women with IC symptoms from the Finnish population register. Of 1331 respondents, 32 had moderate or severe symptoms involving a suspicion of BPS/IC (symptom score 7 or higher). Of 21 who consented to clinical evaluation, 7 had probable or possible BPS/IC. Corrected estimates yielded a prevalence of 300/100,000 women. Similar studies without clinical confirmation suggested prevalence in Austrian women of 306/100,000 (Temml et al, 2007) and in Japanese women of 265/100,000 (Inoue et al, 2009). Using the Bristol Female Lower Urinary Tract Symptoms questionnaire, a 100/100,000 prevalence of BPS symptoms was reported in Fuzhou Chinese women (Song et al, 2009).
Roberts and coworkers (2003), using a physician diagnosis as the arbiter of IC, found annual incidence in Olmsted County, Minnesota, of 1.6 per 100,000 in women and 0.6 per 100,000 in men, a figure remarkably similar to that of Oravisto in Helsinki. The cumulative prevalence by age older than 80 years in the Minnesota study was 114 per 100,000, a figure comparable to that in the Nurses Health Study if one takes into account the younger age group in the Curhan data. Clemens and colleagues (2007b) calculated a prevalence of diagnosed disease in a managed care population of 197 per 100,000 women and 41 per 100,000 men, but when the diagnosis was tested by eliminating those who had not been evaluated with endoscopy or in whom exclusionary conditions existed, the numbers dropped considerably. The Boston Area Community Health Survey estimated a prevalence of painful bladder syndrome symptoms of 1% to 2% of the population depending on the definition used.
Looking at office visits to practices with an interest in urologic problems, 2.8% of patients in Canadian urologist offices had BPS/IC (Nickel et al, 2005) and probable BPS/IC was found in 0.57% in a primary care office in Michigan (Rosenberg and Hazzard, 2005).
An estimation of the prevalence at this time, recognizing that a consistent definition of the condition has not been used in epidemiologic studies, appears to be about 300/100,000 females and a male prevalence of 10% to 20% of the female estimate. A National Institutes of Health (NIH)-sponsored 5-year Rand Corporation epidemiology study is due to be completed in 2010 and may yield the most reliable data yet (Bogart et al, 2007). Preliminary results reported at the annual American Urological Association meeting revealed that 2.5% to 2.7% of the population of U.S. women meet a high specificity symptom definition suggesting BPS/IC. With the use of an epidemiologic definition with higher sensitivity, the prevalence was 6.5%. This translates to a prevalence in women older than 18 years of age in the United States who have symptoms by self-report compatible with BPS/IC of 3.4 to 7.9 million, significantly higher than most previous prevalence estimates (Berry et al, 2009).
Whether the considerable variability in prevalence in studies within the United States and around the world is related to methodology or true differences in incidence is an important question yet to be answered. It is clear that the prevalence of BPS/IC symptoms is much greater than the prevalence of a physician diagnosis of the disease (Clemens et al, 2007a). Familial occurrence of PBS/IC has been reported (Dimitrakov, 2001). A hereditary aspect to incidence has been suggested by Warren and associates (2001, 2004) in a pioneering study who found that adult female first-degree relatives of patients with IC may have a prevalence of IC 17 times that found in the general population. This, together with previously reported evidence showing a greater concordance of IC among monozygotic than dizygotic twins suggests, but does not prove, a genetic susceptibility to IC that could partially explain the discord in prevalence rates in different populations.
Key Point
Prevalence
Characteristics and Natural History
Most studies show a female-to-male preponderance of 5 : 1 or greater (Clemens et al, 2005; Hanno et al, 2005a). In the absence of a validated marker it is often difficult to distinguish PBS/IC from CPPS (nonbacterial prostatitis, prostatodynia) that affects males (Forrest and Schmidt, 2004), and the percentage of men with PBS/IC may actually be higher (Miller et al, 1995, 1997; Novicki et al, 1998). Men tend to be diagnosed at an older age and have a higher percentage of Hunner ulcer in the case series reported (Novicki et al, 1998; Roberts et al, 2003). Costs of the disorder are not insignificant and can range from 4 to 7 thousand dollars a year not including lost wages, costs preceding diagnosis, alternative therapies, and costs attributable to misdiagnosis (Clemens et al, 2008c, 2009).
The Interstitial Cystitis Database Cohort (ICDB) of patients has been carefully studied, and the findings seem to bear out those of other epidemiologic surveys (Propert et al, 2000). Patterns of change in symptoms with time suggest regression to the mean and an intervention effect associated with the increased follow-up and care of cohort participants. Although all symptoms fluctuated there was no evidence of significant long-term change in overall disease severity. The data suggest that PBS/IC is a chronic disease, and no current treatments have a significant impact on symptoms over time in the majority of patients. Quality of life studies suggest that PBS/IC patients are six times more likely than individuals in the general population to cut down on work time owing to health problems but only half as likely to do so as patients with arthritis (Shea-O’Malley and Sant, 1999). There is an associated high incidence of comorbidity, including depression, chronic pain, and anxiety and overall mental health issues (Michael et al, 2000; Rothrock et al, 2002; Hanno et al, 2005a). There seems to be no effect on pregnancy outcomes (Onwude and Selo-Ojeme, 2003).
Female patients with BPS/IC seem to report significant dyspareunia and other manifestations of sexual dysfunction. All domains of female sexual function including sexually related distress, desire, and orgasm frequency can be affected (Ottem et al, 2007; Peters et al, 2007b). Sexual function is an important predictor of physical quality of life and was the only strong predictor of mental quality of life in one recent study of patients with severe BPS/IC (Nickel et al, 2007).
The Boston Area Community Health Survey (BACH) (Link et al, 2008) showed an overall prevalence of symptoms suggestive of BPS of 2% with twice as many women as men affected. It was most common among middle-aged respondents with an earlier peak in women. It also was most common among minorities and those of lower socioeconomic status, and socioeconomic status seemed to overcome any effect due to race or ethnicity. Emotional, sexual, and physical abuse was shown to be a risk factor in BACH (Link and Lutfey, 2007), and this has been borne out in other studies. A Michigan study compared a control group of 464 women with 215 BPS/IC patients and found 22% of the control group having experienced abuse versus 37% of the patient group (Peters and Kalinowski, 2007). Those with a history of sexual abuse may present with more pain and fewer voiding symptoms (Seth and Teichman, 2008). How reliable these data are is not clear, and it would be wrong to jump to any conclusions about abuse in an individual patient. However, practitioners need to have sensitivity for the possibility of an abusive relationship history in all pain patients and in BPS patients in particular. When patients are found to have multiple diagnoses, the rate of previous abuse also increases, and these patients may need referral for further counseling at a traumatic stress center (Fenton et al, 2008).
Key Points
Natural History
Associated Disorders
Knowledge of associated diseases is relevant for the clues it engenders with regard to etiology and possible treatment of this enigmatic pain syndrome. It is well known that patients with chronic pain syndromes including chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder share key symptoms and can often develop overlapping conditions, including chronic pelvic pain (Aaron and Buchwald 2001; Aaron et al, 2001). In a case-control study Erickson found that patients with IC had higher scores than controls for pelvic discomfort, backache, dizziness, chest pain, aches in joints, abdominal cramps, nausea, palpitations, and headache (Erickson et al, 2001). Buffington theorizes that a common stress response pattern of increased sympathetic nervous system function in the absence of comparable activation of the hypothalamic-pituitary-adrenal axis may account for some of these related symptoms (Buffington, 2004). It has been suggested that panic disorder, a diagnosis associated with some BPS/IC patients (Clemens et al, 2008a), may sometimes be a part of a familial syndrome that includes IC, thyroid disorders, and other disorders of possible autonomic or neuromuscular control (Weissman et al, 2004). Depression has been associated with BPS/IC in both men and women (Hall et al, 2008; Clemens et al, 2008a), but whether this is an association or effect of the disorder is uncertain (Fitzgerald et al, 2007).
Newly diagnosed patients are most concerned with the possibility that BPS/IC could be a forerunner of bladder carcinoma. No reports have ever documented a relationship to suggest that IC is a premalignant lesion. Utz and Zincke (1973) discovered bladder cancer in 12 of 53 men treated for IC at the Mayo Clinic. Three of 224 women were eventually diagnosed with bladder cancer. Four years later additional cases were reported (Lamm and Gittes, 1977). Tissot and coworkers (2004) reported 1% of 600 patients previously diagnosed as having IC were found to have transitional cell carcinoma as the cause of symptoms. Somewhat ominously, 2 of these patients had no hematuria. In all patients, irritative symptoms resolved after treatment of the malignancy. From this experience has come the dictum that all patients with presumed IC should undergo cystoscopy, urine cytology, and bladder biopsy of any suspicious lesion to be sure that a bladder carcinoma is not masquerading as BPS/IC. It would seem that in the absence of microhematuria, and with a negative cytology, the risk of missing a cancer is negligible but not zero. There is no evidence that BPS/IC itself is associated with a higher risk of bladder cancer or transitions to cancer over time (Murphy et al, 1982).
A large-scale survey of 6783 individuals diagnosed by their physicians as having IC studied the incidence of associated disease in this population (Alagiri et al, 1997). Data from the 2405 responders was validated by comparison with 277 nonresponders (Fig. 12–4). Allergies were the most common association, with over 40% affected. Allergy was also the primary association in Hand’s study (Hand, 1949). Thirty percent of patients had a diagnosis of irritable bowel syndrome, a finding confirming that of Koziol (1994). Altered visceral sensation has been implicated in irritable bowel syndrome in that these patients experience intestinal pain at intestinal gas volumes that are lower than those that cause pain in healthy persons (Lynn and Friedman, 1993), strikingly similar to the pain on bladder distention in IC.
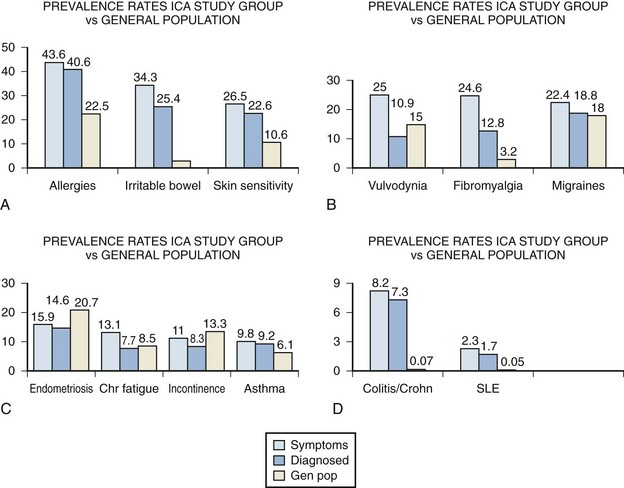
Figure 12–4 Comparison of disease prevalence rates between the Interstitial Cystitis Association (ICA) study group patients who report symptoms of a disorder, those who have been diagnosed with a disorder, and the general population. SLE, systemic lupus erythematosus.
(From Alagiri M, Chottiner S, Ratner V, et al. Interstitial cystitis: unexplained associates with other chronic pain syndromes. Urology 1997;49[Suppl 5A]:52–7.)
Fibromyalgia, another disorder frequently considered functional because no specific structural or biochemical cause has been found, is also overrepresented in the BPS/IC population. This is a painful, nonarticular condition predominantly involving muscles; it is the most common cause of chronic, widespread musculoskeletal pain. It is typically associated with persistent fatigue, nonrefreshing sleep, and generalized stiffness. As in BPS/IC, women are affected at least 10 times more often than men (Consensus document on fibromyalgia, 1993). The association is intriguing because both conditions have nearly identical demographic features, modulating factors, associated symptoms, and response to tricyclic antidepressants (Clauw et al, 1997).
Diagnosed vulvodynia, migraine headaches, endometriosis, chronic fatigue syndrome, incontinence, and asthma had similar prevalence as in the general population. Several publications have noted an association between BPS/IC and systemic lupus erythematosus (SLE) (Fister, 1938; Boye et al, 1979; De la Serna and Alarçon-Segovia, 1981; Weisman et al, 1981; Meulders et al, 1992). The question has always been as to whether the bladder symptoms represent an association of these two disease processes or rather are a manifestation of lupus involvement of the bladder (Yukawa et al, 2008) or even a myelopathy with involvement of the sacral cord in a small group of these patients (Sakakibara et al, 2003). The beneficial response of the cystitis of systemic lupus erythematosus to corticosteroids (Meulders et al, 1992) tends to support the latter view. No association with discoid lupus has been demonstrated (Jokinen et al, 1972b). Although the actual numbers are small, the Alagiri study demonstrated a 30 to 50 times greater incidence of systemic lupus erythematosus in the IC group compared with the general population. Overall, the incidence of collagen vascular disease in the IC population is low. Parsons (1990) found only 2 of 225 consecutive IC patients to have a history of an autoimmune disorder.
Inflammatory bowel disease was found in over 7% of the IC population Alagiri studied, a figure 100 times higher than in the general population. Although unexplained at this time, abnormal leukocyte activity has been implicated in both conditions (Bhone et al, 1962; Kontras et al, 1971).
The University of Maryland group sought antecedent nonbladder syndromes in 313 patients with incident BPS/IC and compared them to 313 matched controls (Warren et al, 2009). They found 11 antecedent syndromes were more often diagnosed in those with BPS/IC, and most syndromes appeared in clusters. The most prominent cluster (45%) comprised fibromyalgia—chronic widespread pain, chronic fatigue syndrome, sicca syndrome, and/or irritable bowel syndrome. Most of the other syndromes and identified clusters were associated with it. They found probable chronic fatigue syndrome in 20% of BPS/IC patients, probable fibromyalgia in 22%, and probable irritable bowel in 27% of the BPS patients. Far fewer had physician reported diagnoses of these syndromes, and odds ratios (OR) for BPS/IC versus controls were 2.5 to 2.9. Study of a managed care database in Portland Oregon revealed that patients coded for gastritis (OR = 12.2), child abuse (OR = 9.3), fibromyalgia (OR = 3.0), anxiety disorder (OR = 2.8), headache (OR = 2.5), and depression (OR = 2.0) were commonly diagnosed with BPS/IC (Clemens et al, 2008b).
A mysterious disorder that has been associated with IC is focal vulvitis. Vulvar vestibulitis syndrome is a constellation of symptoms and findings involving and limited to the vulvar vestibule consisting of (1) severe pain on vestibular touch to attempted vaginal entry, (2) tenderness to pressure localized within the vulvar vestibule, and (3) physical findings confined to vulvar erythema of various degrees (Marinoff and Turner, 1991). McCormack (1990) reported on 36 patients with focal vulvitis, 11 of whom also had IC. Fitzpatrick and coworkers (1993) has added three more cases. The concordance of these noninfectious inflammatory syndromes involving the tissues derived from the embryonic urogenital sinus and the similarity of the demographics argue for a common etiology.
An association has been reported between IC and Sjögren syndrome, an autoimmune exocrinopathy with a female preponderance manifested by dry eyes, dry mouth, and arthritis but that can also include fever, dryness, and gastrointestinal and lung problems. Van de Merwe and colleagues (1993) investigated 10 IC patients for the presence of Sjögren syndrome. Two patients had both the keratoconjunctivitis sicca and focal lymphocytic sialoadenitis allowing a primary diagnosis of Sjögren syndrome. Only 2 patients had neither finding. These researchers later (2003) reported an incidence of 28% of Sjögren syndrome in patients with IC. The incidence of symptoms of PBS/IC in patients with Sjögren syndrome has been estimated to be up to 5% (Leppilahti et al, 2003).
A negative correlation with diabetes has been noted (Parsons, 1990; Koziol, 1994; Warren et al, 2009).
Further epidemiologic studies are warranted because the epidemiology of this disorder may ultimately yield as many clues to etiology and treatment as other avenues of research. The heterogeneity of causes and symptoms of CPPS suggests that proper clinical phenotyping could foster the development of better treatments for individual phenotypes and more successful treatments for all afflicted patients (Baranowski et al, 2008; Shoskes et al, 2009b).
Etiology
It is likely that PBS/IC has a multifactorial etiology that may act predominantly through one or more pathways resulting in the typical symptom-complex (Holm-Bentzen et al, 1990; Mulholland and Byrne, 1994; Erickson 1999; Levander, 2003; Keay et al, 2004b) (Fig. 12–5). There are numerous theories regarding its pathogenesis, but confirmatory evidence gleaned from clinical practice has proven sparse. Among numerous proposals that shall be further explored in this section are “leaky epithelium,” mast cell activation, and neurogenic inflammation, or some combination of these and other factors leading to a self-perpetuating process resulting in chronic bladder pain and voiding dysfunction (Elbadawi, 1997). Irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, and various other chronic pain disorders may precede or follow the development of BPS/IC in some patients, but development of associated syndromes is not inevitable by any means, and their relationship to etiology is currently unknown (Warren et al, 2009; Nickel et al, 2010).
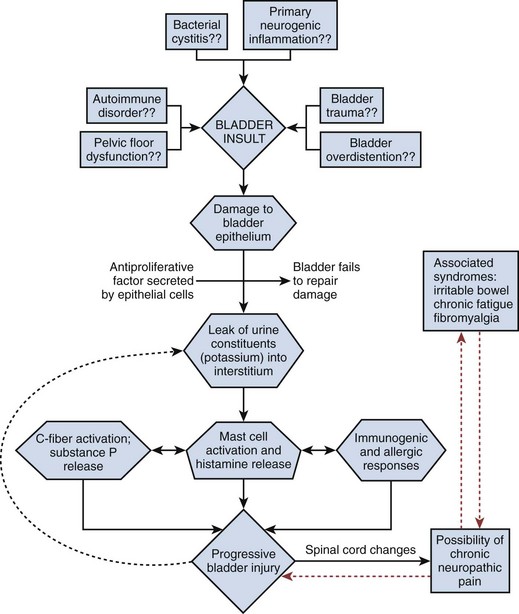
Figure 12–5 Hypothesis of etiologic cascade of bladder pain syndrome.
(From Hanno P, Lin AT, Nordling J, et al. Bladder pain syndrome. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publication Ltd.; 2009. p. 1459–518.)
Animal Models
The problem with animal models is how accurately they mirror the human situation. A section on animal models appears in the electronic edition along with Figure 12–6 (see the Expert Consult website![]() ).
).
Until recently, lacking an easily available animal model of the naturally occurring disease, researchers have had to devise animal models to study isolated symptoms of BPS/IC, hoping to uncover the root causes of the symptomatology (Ruggieri et al, 1990). Bullock and associates (1992) reported a mouse model in which bladder inflammation could be induced by the injection of syngeneic bladder antigen. The model demonstrated that a component in the Balb/cAN mouse is capable of inducing a bladder-specific, adoptively transferable, cell-mediated autoimmune response that exhibits many characteristics of clinical IC but was difficult to reproduce (Klutke et al, 1997).
A guinea pig model in which bladder inflammation was induced by the instillation of a solution containing a protein to which the animal had been previously immunized resulted in bladder inflammation (Christensen et al, 1990; Kim et al, 1992), as did a rat model of allergic cystitis using a local challenge of ovalbumin in previously sensitized rates (Ahluwalia et al, 1998). Changes in the rat model were dependent on mast cell degranulation and activation of sensory C fibers.
Ghoniem and coworkers (1995) studied 4 female African green monkeys challenged with intravesical acetone. Not surprisingly, they exhibited symptoms of BPS. Rivas and associates (1997) performed similar experiments using dilute hydrochloric acid in a rat model. A rat model for neurogenic cystitis using pseudorabies virus demonstrated that inflammatory changes in the spinal cord can result in dramatic, neurogenically mediated changes in the bladder (Doggweiler et al, 1998).
The problem with all of these animal models relates to whether they mirror the human disease to any great extent. Buffington has described what appears to be a naturally occurring animal model of BPS/IC (Fig. 12–6). Two thirds of cats with lower urinary tract disease have sterile urine and no evidence of other urinary tract disorders (Kruger et al, 1991). A portion of these cats experience frequency and urgency of urination, pain, and bladder inflammation (Houpt, 1991). Glomerulations have been observed in the bladders of these animals. GP-51, a glycosaminoglycan (GAG) commonly found in the surface mucin covering the mucosa of the normal human bladder and decreased in IC, shows a decreased expression in cats with this symptom-complex (Press et al, 1995), originally termed feline urologic syndrome. Bladder Aδ afferents in these cats are more sensitive to pressure changes than are afferents in normal cats (Roppolo et al, 2005). They also demonstrate an increase in baseline nitric oxide production in smooth muscle and mucosal strips when compared with healthy cats with evidence of altered mucosal barrier function (Birder et al, 2005).
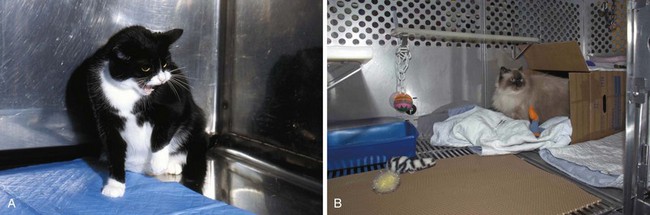
Figure 12–6 A, Photograph of a cat with feline interstitial cystitis (FIC). Note posture of defensive aggression in response to reflection in the stainless steel cage wall. B, Photograph of a cat with FIC in an enriched cage. Environmental modification intended to reduce perception of external threat has been found to be an effective approach to treatment of cats with FIC in both laboratory and clinical studies.
(A and B, Courtesy of Tony Buffington.)
This is now referred to as “feline interstitial cystitis” (Buffington et al, 1999). It is associated with urinary urgency, frequency, and pain with sterile urine, bladder mastocytosis, increased histamine excretion, increased bladder permeability, decreased urinary GAG excretion (Buffington et al, 1996), and increased plasma norepinephrine concentrations (Buffington and Pacak, 2001b).
Although animal models can yield clues to etiology, all theories must ultimately be tested in humans with the disease.
Infection
Often, a diagnosis of BPS/IC is made only after a patient has been seen by a number of physicians and treated with antibiotics for presumed urinary tract infection without resolution of symptoms (Held et al, 1990). The symptom-complex looks to the patient and physician like an infectious process (Porru et al, 2004). The epidemiology of urinary tract infection and its predominance in women mirror the BPS/IC data (Warren, 1994). The acute to subacute onset in many patients has fascinated clinicians who often associate an insidious onset with a chronic condition such as PBS/IC.
Reverse logic led some to suspect that antibiotics may be instrumental in causing IC (Holm-Bentzen et al, 1990). Most patients have been treated with antibiotics once or several times before the diagnosis is made. Numerous antibiotics, primarily in the penicillin family, can induce a cystitis (Bracis et al, 1977; Moller, 1978; Chudwin et al, 1979; Cook et al, 1979; Marx and Alpert, 1984), but no evidence has ever been documented that these antibiotics or the supposedly “surface active” nitrofurantoins or tetracyclines have any involvement in pathogenesis of IC (Ruggieri et al, 1987; Levin et al, 1988).
To determine whether there is an infectious cause of BPS certain procedures are necessary (Warren, 1994). Not just urine but bladder epithelium as well must be cultured for appropriate microorganisms, including bacteria, viruses, and fungi. Because some organisms might be culturable yet fastidious, special culture techniques should be used. Because some organisms in urine or tissue might be viable but nonculturable, specific nonculture techniques for discovery and identification should be employed. Most important, the same procedures must be carried out in a control population.
Attempts to show an infectious etiology go back to the dawn of the disease, but the case has never been a strong one (Duncan and Schaeffer, 1997). Hunner (1915) originally proposed that IC resulted from chronic bacterial infection of the bladder wall secondary to hematogenous dissemination. Harn and associates (1973) proposed a relationship between IC and streptococcal and post-streptococcal inflammation. They produced a progressive chronic inflammation in rabbit bladders by injecting small numbers of Streptococcus pyogenes in the bladder wall. The discovery that Helicobacter pylori is related to the pathogenesis of chronic atrophic gastritis and peptic ulcer disease and that antibiotic treatment can heal ulceration (Parsonnet et al, 1991; National Institutes of Health, Consensus Development Panel, 1994; Sung et al, 1995) has continued to focus attention of researchers in IC on the possibility that an infectious etiology is not only reasonable but will ultimately be found. Studies of H. pylori itself have failed to demonstrate an association with IC (English et al, 1998; Agarwal and Dixon 2003; Atug et al, 2004; Haq et al, 2004). Wilkins and colleagues (1989) found bacteria in catheterized urine specimens and/or bladder biopsy specimens in 12 of 20 patients with IC. However, 8 of the isolates were fastidious bacteria, Gardnerella vaginalis, and Lactobacillus species and no controls were included in the study. Polyomaviruses have been reported to cause a BPS/IC-like syndrome that responds to cidofovir treatment (Eisen et al, 2009). These viruses are excreted intermittently in the urine of healthy, asymptomatic adults, making diagnosis of a true infection problematic.
Negative studies far outnumber the positive ones. Hanash and Pool (1970) performed viral, bacterial, and fungal studies on 30 IC patients and failed to substantiate an infectious cause. Hedelin and coworkers (1983) found only 3 of 19 IC patients to have urine cultures positive for Ureaplasma urealyticum and indirect hemagglutination antibodies to Mycoplasma hominis to be no greater than in controls. Potts and colleagues (2000) cultured U. urealyticum in 22 of 48 patients with “chronic urinary symptoms” and had great success in these patients (none of whom had established IC) with short courses of commonly prescribed antibiotics. Given the history of empirical use of antibiotics in the vast majority of IC patients, it is doubtful if this group represents even a small percentage of the IC diagnosed population. Nevertheless it illustrates that BPS/IC is a diagnosis of exclusion and that the urine culture is critical whereas an empirical short course of antibiotics is certainly reasonable if the patient has not already been treated for presumed infection. Empirical use of doxycycline has been successful in this situation (Burkhard et al, 2004).
The development of highly sensitive, rapid, and specific molecular methods of identifying infectious agents by the direct detection of DNA or RNA sequences unique to a particular organism (Naber, 1994) resulted in a flurry of activity into the search for a responsible virus or microorganism. Hampson and associates (1993) could find no evidence of mycobacterial involvement in 8 cases of BPS/IC using DNA probes. Haarala and colleagues (1996) confirmed an absence of bacterial DNA in the bladder of 11 BPS/IC patients with no documented history of urinary tract infection. Hukkanen and associates (1996) reported an absence of adenovirus and BK virus genomes in urinary bladder biopsy specimens of IC patients. Domingue and coworkers’ (1995) provocative finding of the presence of bacterial 16S rRNA genes in bladder biopsy specimens from 29% of IC patients but not from control bladders, and his discovery of 0.22-µm filterable forms in culture of biopsy tissue from 14 of 14 IC patients and none of 15 controls has never been confirmed or repeated.
A preliminary study found a statistically significant increase in urine polymerase chain reaction studies to Chlamydia pneumoniae major outer membrane protein gene in patients with BPS/IC as compared with controls (Franke et al, 1999). Other studies have shown that similar percentages of both IC and control patient populations have nonculturable bacteria in their bladder on the basis of polymerase chain reaction studies of bladder biopsy specimens (Heritz et al, 1997; Keay et al, 1998a). The spirochete Borrelia burgdorferi has been found in the bladder biopsy specimens and urine of patients with Lyme disease and can cause frequency, urgency, and nocturia. DNA studies have failed to show a role for Borrelia in IC (Haarala et al, 2000).
The role of infection in the pathogenesis of BPS/IC remains a mystery. At this time there are little data to support the role of an infectious etiology but investigators keep returning to an infectious theory. New insights into the mechanisms by which bacteria adhere, grow, and persist in association with host tissue and form intracellular pods capable of subverting host defense mechanisms and allowing replication within epithelial cells lay the foundation for a possible role of infection in initiating the BPS/IC pathologic cascade (Kau et al, 2005). The University of Maryland group proposes a model of BPS in which bladder epithelial damage such as that caused by bacterial cystitis may be the first step leading to a low-level inflammatory response we call BPS/IC (Keay and Warren, 1998). Domingue writes that “It is logical to suggest that even if organisms are not causative agents, their presence may lead to immune and host-cell responses that could initiate or exacerbate an inflammatory state” (Domingue and Ghoniem, 1997).
If infection does play a role it would be predicted that appropriate treatments to minimize microbial presence in the tissue would significantly improve the morbidity associated with BPS/IC. Durier’s incredible series (1992) purporting to cure 27 of 27 IC patients with the use of up to five sequential antibiotics covering the anaerobic spectrum has never been duplicated. Warren and associates’ (2000) prospective, double-blind, placebo-controlled, randomized trial of 50 patients may well prove the end of long-term empirical antibiotic treatment in established BPS/IC. Either a placebo or antibiotics (sequential doxycycline, erythromycin, metronidazole, clindamycin, amoxicillin, and ciprofloxacin for 3 weeks each) were administered for 18 weeks. Most patients guessed the arm to which they were assigned. Of the 25 patients in the active arm, 80% had new nonurinary symptoms perceived as side effects. There was minimal improvement in some patients associated with the active arm of the study, but the conclusion that intensive antibiotics do not represent a major advance in therapy for IC seems well justified.
Although the concept that a urinary tract infection may trigger BPS in some patients is appealing (Elgavish et al, 1995; Elbadawi, 1997), it is unlikely that active infection is involved in the ongoing pathologic process or that antibiotics have a role to play in treatment.
Autoimmunity/Inflammation
Immune/neuroimmune mechanisms may have an important role in the pathogenesis of BPS/IC. Excessive release of sensory nerve neurotransmitters and mast cell inflammatory mediators is thought by some to be responsible for the development and propagation of symptoms (Luo, 2005). Inflammation results in altered nerve growth factor content of the bladder and in morphologic changes in sensory and motor neurons innervating the bladder. Such neuroplasticity may be a possible explanation for the association of bladder inflammation with long-term symptoms and pain after inflammation subsides (Dupont et al, 2001). Up to one third of BPS/IC patients may have an acute urinary tract infection that immediately precedes the onset of chronic symptoms (Warren et al, 2008). However, abnormal differentiation in the urothelium with a loss of barrier function markers and altered differentiation markers may be independent and occur independently of inflammation (Hauser et al, 2008).
The role of inflammation may stem from inflammation originating in organs other than the bladder. Pain syndromes such as irritable bowel syndrome and BPS, which are associated with visceral hyperalgesia, are often comorbid with endometriosis and chronic pelvic pain. One of the possible explanations for this phenomenon is viscerovisceral cross-sensitization, in which increased nociceptive input from an inflamed pelvic organ sensitizes neurons that receive convergent input to the same dorsal root ganglion (DRG) from an unaffected visceral organ. Visceral sensory integration in the dorsal root ganglia has been demonstrated in a rodent model and may underlie the observed comorbidity of female pelvic pain syndromes (Li et al, 2008).
For many years the possibility that BPS may represent some type of autoimmune disorder has been considered. Narrowly defined, autoimmune diseases are clinical syndromes caused by the activation of T cells or B cells, or both, in the absence of an ongoing infection or other discernible cause (Davidson and Diamond, 2001). To establish a disease as autoimmune, three types of evidence can be considered: (1) direct evidence from transfer of pathogenic antibody or pathogenic T cells; (2) indirect evidence based on reproduction of the autoimmune disease in experimental animals; and (3) circumstantial evidence from clinical clues (Rose and Bona, 1993). Circumstantial evidence would include (1) association with other autoimmune diseases in the same individual or same family; (2) lymphocytic infiltration of target organ; (3) statistical association with a particular major histocompatibility complex haplotype; and (4) favorable response to immunosuppression.
Circumstantial evidence by itself cannot define an autoimmune disease, and at this point the case for autoimmunity in BPS/IC is far from clear. Three different possibilities exist: (1) BPS is caused by a direct autoimmune attack on the bladder, (2) some of the autoimmune symptoms and pathology of BPS arise indirectly as a result of tissue destruction and inflammation from other causes, and (3) autoimmune phenomena in BPS patients are coincident and unrelated to the disease (Ochs, 1997).
Silk (1970) found bladder antibodies in 9 of 20 IC patients and none in 35 pathologic or normal control patients. Gordon and associates (1973) found antibladder antibodies present in biopsy specimens from 6 of 8 IC patients and in 3 of 5 control patients. No control patient demonstrated antibodies in the muscle, whereas 3 of 5 IC patients with muscle in the biopsy did so. Jokinen and colleagues (1972a) looked at sera from 33 IC patients and found 28 with an antinuclear antibody titer of 1 : 10 or greater, but no bladder-specific antibodies were detected with immunofluorescence. There was poor correlation between antinuclear antibody titers and symptom severity. These researchers noted in a later study (Jokinen et al, 1973) that elevated antibody titers against cell nuclei and crude kidney homogenate decreased within 12 months after cystectomy in 3 IC patients. All of this provided hints that BPS/IC could fall into the autoimmune group of diseases.
Oravisto (1980) summarized the world literature on this idea in 1980, concluding that the chronic course of disease, the absence of infection, the pathologic findings, the occurrence of antinuclear antibodies, and the reported responses to corticosteroids at that time provided strong circumstantial evidence of autoimmunity. He discounted the paucity of activated lymphocytes that speak against an autoimmune process. Studies on autoantibodies in BPS/IC have shown that these mainly consist of antinuclear antibodies (Jokinen et al, 1972a) similar to the autoantibody profiles in some systemic diseases such as Sjögren syndrome, which is well known to be of autoimmune origin (Tan, 1989; Leppilahti et al, 2003). Mattila (1982) presented evidence of immune deposits in the bladder vascular walls in 33 of 47 BPS/IC patients. Studying sera from 41 patients with IC, he concluded that the classical pathway activation of the complement system was involved, supporting the possibility that a chronic local immunologic process was indeed occurring (Mattila et al, 1983). The autoantibodies tested were found to be directed against cytoskeletal intermediate filaments. Because the autoantibodies have to gain access to intracellular structures to cause in-vivo deposits, primary tissue injury of unknown etiology has to be postulated (Mattila and Linder, 1984).
Anderson and colleagues (1989) studied 26 patients with BPS/IC and compared them to a control group of similar age and sex with other urologic complaints. They performed a standard autoimmune profile and looked for specific antibodies to normal human bladder in the serum. Sixty-five percent of IC patients and 36% of controls demonstrated non–organ-specific antibodies; 40% of IC patients had antinuclear antibodies; 75% of IC patients and 40% of controls had antibladder antibodies present in the serum. There was no increase in immunoglobulin deposition in the bladder epithelium in IC patients versus controls. Although IC patients demonstrated a nonspecific increase in antibody formation, this was not significantly different from a similar group of other urologic patients. The lack of specificity indicates the immunologic findings are likely secondary to inflammation rather than a primary etiology.
In a study looking for active immune cellular deposition in BPS/IC patients, no statistically significant difference between controls and nonulcerative IC patients was identified (Harrington et al, 1990). In contrast, the ulcerative BPS group had focal sheets of plasma cells, aggregates of T cells, B-cell nodules, a decreased or normal helper-to-suppressor cell ratio, and suppressor cytotoxic cells in germinal centers. Flow cytometry analysis of peripheral blood lymphocyte subsets showed increased numbers of secretory-Ig–positive B cells and activated lymphocytes in the nonulcerative group and increased numbers of secretory-Ig–positive cells and activated lymphocytes in the ulcerative group. These results may suggest a partial role for an immune mechanism in IC. Erickson and coworkers (1997a) have also noted a major difference in inflammatory cell types as well as clinical features in BPS/IC patients with severe inflammation, suggesting two different patient groups with two different underlying pathophysiologies.
Hanno and colleagues (1990) found CD4 cell predominance in all layers of the bladder in BPS/IC patients. Christmas (1994) reported increased numbers of CD4+ and CD8+ T cells in bladder biopsy specimens from patients with IC and bacterial cystitis as compared with controls. These T cells were present in the urothelium and submucosa but not in the detrusor. Control bladder tissue demonstrated only CD8 cells in the urothelium and both CD4+ and CD8+ cells in the submucosa. The number of plasma cells was significantly greater in IC patients than in normal controls and controls with bacterial cystitis.
MacDermott and colleagues (1991b) found a normal distribution of peripheral blood lymphocytes in IC patients, a finding not supportive of an autoimmune mechanism in the disease. The lamina propria showed a predominance of CD4 (helper T cells) lymphocytes over CD8 cells in both IC and other cystitis patients. The same pattern was seen in the epithelium of patients with bacterial or mechanical cystitis, but patients with IC had a predominance of CD8 lymphocytes in the urothelium, which was identical to that found in control subjects. The findings suggest that the urothelium is not involved in the inflammatory reaction, as is the lamina propria, making the urothelium an unlikely source for the initiating factor.
Miller and coworkers (1992) investigated the function of peripheral blood lymphocytes from nonulcerative IC patients, testing the proliferative response and cytokine production of T cells to nonspecific mitogenic stimulation and the proliferative response of T cells to urine components. Proliferation and cytokine production after mitogen stimulation were the same for controls and BPS/IC patients. Moreover, no immunologic response to IC urine by autologous peripheral blood lymphocytes in in-vitro assays was observed. Their findings cast doubt on theories suggesting that IC is an autoimmune disease.
Numerous inflammatory mediators have been studied with regard to their relation to BPS (Elgebaly et al, 1992; Felsen et al, 1994; Lotz et al, 1994; Steinert et al, 1994; Zuraw et al, 1994). Patients with BPS/IC exhibit varying degrees of inflammation that can separate them into clusters (Tomaszewski et al, 2001; Green et al, 2004). Bladder inflammation in IC is categorized by elevated urinary interleukin-6 (Erickson et al, 1997a) and activation of the kallikrein-kinin system (Rosamilia et al, 1999b). The absence of urinary interleukin-1β in IC argues against an immunologic or autoimmune etiology of the disorder (Martins et al, 1994). Neurogenic inflammation may play a role in the etiology, because long-term exposure of afferent nerve terminals to inflammatory mediators can alter ion channels and result in bladder hyperalgesia (Buffington and Wolfe, 1998; Yoshimura and de Groat, 1999). Substance P itself does not seem to be the single initiator of inflammation in the bladder, and its blockade does not protect the bladder in animal models from inflammatory responses (Luber-Narod et al, 1997). Urinary nitric oxide synthase (NOS) activity is known to be elevated in patients with urinary infection and thought to play a role in the bladder’s response to infection and in the inflammatory process that follows infection. The finding that urinary NOS activity is decreased in BPS/IC patients has puzzled researchers but could explain the reduction in functional bladder capacity associated with the disorder (Smith et al, 1996; Foster et al, 1997).
Urothelial cell activation in IC may result in aberrant immune responses and immune activation within the bladder wall (Liebert et al, 1993) that could relate to pathogenesis of the disease but might not reflect initiating etiology (Ochs et al, 1994). It has been proposed that inflammatory and/or immune responses in IC could be exacerbated by persistent activation of the nuclear factor-kappa B (NF-κB) (Abdel-Mageed and Ghoniem, 1998; Abdel-Mageed, 2003). Angiogenic factors such as platelet-derived endothelial cell growth factor/thymidine phosphorylase and transforming growth factor-β may be involved in the inflammatory process to induce painful symptoms in patients with IC or bladder carcinoma (Ueda et al, 2002).
The exact role of autoimmunity in IC remains controversial (Ochs, 1997). Suplatast tosylate, a new immunoregulator, has shown efficacy in a small, uncontrolled IC study in which improvements in symptoms and bladder capacity were correlated with changes in autoimmune parameters (Ueda et al, 2000). Although the immune system remains a target for therapy, no clear indication of a primary role for autoimmunity as the cause of IC has been observed (Liebert, 1997).
Key Points
Autoimmunity and Inflammation
Mast Cell Involvement
Although mast cells are thought of primarily in the context of allergic disorders, and certain acute inflammatory responses, these cells have also been implicated in biologic responses as diverse as angiogenesis and wound healing, bone remodeling, peptic ulcer disease, atherosclerosis, and reactions to neoplasms (Galli, 1993). Mast cells remain one of the most enigmatic cells in the body. They secrete significant amounts of numerous proinflammatory mediators that contribute to a number of chronic inflammatory conditions, including stress-induced intestinal ulceration, rheumatoid arthritis, scleroderma, and Crohn disease. They have been described even among the lowest order of animals, having been discovered in the frog mesentery over 100 years ago. Their raison d’etre may be for initiating and coordinating the host’s inflammatory and immune responses against microbial pathogens (Abraham and Malaviya, 1997). Recently they have been implicated in a range of neuroinflammatory diseases, especially those worsened by stress (Theoharides, 2004; Theoharides and Cochrane, 2004). These include multiple sclerosis, migraines, inflammatory arthritis, atopic dermatitis, coronary inflammation, irritable bowel syndrome, and BPS/IC. They may be activated through their Fc receptors by immunoglobulins other than IgE, as well as by anaphylatoxins, neuropeptides, and cytokines to secrete mediators selectively without overt degranulation.
Mast cells have frequently been reported to be associated with IC, both as a pathogenetic mechanism and as a pathognomonic marker (Simmons, 1961; Bhone et al, 1962; Smith and Dehner, 1972; Larsen et al, 1982; Hofmeister et al, 1997). The association of bladder mastocytosis, IC, and irritable bowel syndrome (Pang et al, 1996) and chronic urticaria (Sant et al, 1997) is intriguing. Evidence of their importance is mounting, suggesting that they may serve as the final common pathway through which the symptomatic condition is expressed. Mast cells produce, among other compounds, histamine. Histamine release in tissue causes pain, hyperemia, and fibrosis, all notable features of IC.
Simmons (1961) was the first to suggest mast cells as a cause of IC. Contribution of mast cells to the cellular infiltrate in IC (Fig. 12–7) has been shown to vary from about 20% in nonulcer IC patients to 65% in patients with ulceration (Sant et al, 1988; Enerback et al, 1989). Mast cells participate in allergic reactions (hypersensitivity type I) during which IgE antibody is synthesized in response to specific antigens. IgE binds to mast cell receptors, and antigen binds to the IgE, leading to degranulation (Lagunoff et al, 1983). Other triggers of mast cell secretion include acetylcholine, anaphylatoxins, cationic peptides such as substance P, chemicals, contrast agents, cytokines, opioids, antihistamines, exercise, hormones, viruses, and bacterial toxins (Sant and Theoharides, 1994). Mast cells promote infiltration of neutrophils, T and B lymphocytes, monocytes, and eosinophils. T lymphocytes secrete substances capable of activating mast cells, thus perpetuating the cycle of inflammation (Kaplan et al, 1985).
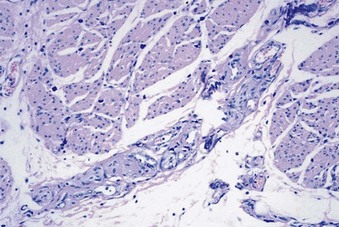
Figure 12–7 Giemsa stain shows detrusor mastocytosis and nerve hypertrophy in bladder pain syndrome (original magnification ×400).
(Courtesy of John Tomaszewski, Hospital of the University of Pennsylvania, Department of Pathology.)
Since the presence of mast cells within the bladder wall was first recognized (Simmons and Bunce, 1958), numerous investigators have tried to determine whether there is an increase in the number of mast cells in the bladder of patients with IC or differences in their location or functional state (Larsen et al, 1982; Kastrup et al, 1983; Fall et al, 1987; Feltis et al, 1987; Lynes et al, 1987; Christmas and Rode, 1991). An increase in urothelial mast cells appears to be part of the generalized inflammatory cell reaction regardless of etiology, and not a specific feature of IC, whereas the presence of increased numbers of mast cells in the detrusor is more specific for IC. However, one study did report detrusor mastocytosis in 64% of IC patients and 80% of a control group with other urologic disease, with no statistically significant difference between the mean number of detrusor mast cells in the two groups (Hanno et al, 1990).
Aldenborg and associates (1986) reported that mast cells are found predominantly in the detrusor muscle in patients with classic IC, but there is also a secondary population of mast cells in the lamina propria and the bladder epithelium, with staining characteristics distinct from those in the detrusor. None of these epithelial mast cells was found in controls. These findings were interpreted to indicate a transepithelial migration of mast cells in patients with IC. This second population of mast cells does not appear to be involved in the nonulcer type of IC (Aldenborg et al, 1989). This mucosal population of mast cells can also differ from the mast cells found in deeper tissues in physiologic responses and release of secretory products (Sant, 1991). The “mucosal mast cells” are susceptible to aldehyde fixation and require special fixation and staining techniques for proper demonstration. Detrusor mast cells are not susceptible to fixation techniques. Recent studies have shown that although all human mast cells contain the proteinase tryptase there is a population of mast cells that also contain the proteinase chymase. The mast cell expansion in IC involves both types (Yamada et al, 2000). Mast cell activation is far more pronounced in the ulcerative form, which in addition displays prominent inflammation, in contrast to nonulcer IC in which it is sparse. Thus the basic pathologic processes may differ (Peeker et al, 2000b). Because activated mast cells lose their histologically identifiable granules once degranulation occurs, estimates of mast cell density using standard histologic techniques may underestimate mast cell numbers (Sant and Theoharides, 1994).
Electron microscopy has confirmed that mast cells in IC are more likely to be degranulated or activated than those found in other conditions (Larsen et al, 1982; Theoharides and Sant, 1991; Theoharides et al, 1995). In at least a subpopulation of IC patients this may be explained by increased stimulation of mast cells by stem cell factor (Pang et al, 1998). A chronic exposure of detrusor muscle to histamine in IC patients is suggested by the finding that there is an impairment of the direct contractile response to histamine in detrusor muscle affected by IC in comparison to control detrusor, suggesting a receptor desensitization (Palea et al, 1993). The clinical relationship between an increased number of mast cells and symptoms of IC has not been definitively established. Some studies have found no correlation (Holm-Bentzen et al, 1987b; Lynes et al, 1987; Dondore et al, 1996). Although mast cell infiltration in intestinal segments used for augmentation has been associated with pain and failure of the procedure (Kisman et al, 1991), others have shown that mast cell infiltration in intestine used in the urinary tract is the norm and not pathologic (MacDermott et al, 1990).
Many of the substances that have been shown to induce mast cell secretion are released from neurons that innervate the organ containing the mast cells (Christmas et al, 1990). The capsaicin-sensitive sensory neurons that innervate the bladder are thought to have a dual “sensory-efferent” function, in which an axon reflex-induced release of neuropeptides results in local inflammation (Foreman, 1987; Barbanti et al, 1993). Hand (1949) reported an increase in the submucosal nerve density in IC, a phenomenon confirmed by Christmas and associates (1990), who showed an increase in nerve fiber proliferation in IC but not in patients with bacterial or lupus cystitis. Increased innervation by nerves releasing substances affecting mast cells could lead to increased mast cell secretion. Among these substances is acetylcholine. Mast cells can be stimulated by cholinergic agonists to secrete serotonin (Theoharides and Sant, 1991). Substance P–containing fibers have been found to be increased in bladders from IC patients and are found adjacent to mast cells (Pang et al, 1995b). In mice, mast cells modulate the inflammatory response of the bladder to substance P and to Escherichia coli lipopolysaccharide (Bjorling et al, 1999).
An increase in adrenergic but not cholinergic nerves in IC patients as compared with controls has been reported (Hohenfellner et al, 1992). These researchers also found increased numbers of neurons staining for vasoactive intestinal polypeptide and neuropeptide Y (NPY), both of which are associated with sympathetic nerves. Studies in rats have revealed that psychological stress can activate bladder mast cells through the action of sensory neuropeptides (Spanos et al, 1997; Alexacos et al, 1999). Diurnal cortical variations have been associated with symptom levels in PBS/IC (Lutgendorf et al, 2002), and the mast cell may represent a pathway for stress to be reflected in bladder symptomatology.
Mast cells can alter their environment by regulating tissue gene expression (Saban et al, 2001). The finding of increased synthesis of urinary leukotriene E4 in patients with IC and detrusor mastocytosis when compared with healthy controls suggests that cysteinyl containing leukotrienes are involved in the inflammatory reaction observed in the urinary bladder of patients with IC and may be produced from tissue mast cells in the bladder wall or macrophages (Bouchelouche et al, 2001a).
Could mast cell products be useful in diagnosing BPS/IC? They are not specific for BPS/IC and are increased in bladder carcinoma (Serel et al, 2004). Elevated histamine levels have been found in bladder biopsy specimens of IC patients (Kastrup et al, 1983; Lynes et al, 1987; Enerback et al, 1989) as well as from bladder washings (Lundeberg et al, 1993). Holm-Bentzen and colleagues (1987c) reported a significantly elevated urinary excretion of 1,4-methylimidazole acetic acid, the major metabolite of histamine. Others have found no differences between IC and controls in random spot tests of urinary histamine (Yun et al, 1992). Levels were elevated after hydrodistention in IC patients but not in control subjects, which is a possible consequence of hydrodistention and resultant mast cell degranulation. El Mansoury and coworkers (1994) found increased methylhistamine, a histamine metabolite, in spot and 24-hour urine samples from IC patients as compared with controls. Although such an increase could still be interpreted as indicating a systemic rather than a bladder syndrome, subsequent findings of elevated mast cell tryptase in the urine of IC patients could come only from the bladder (Boucher et al, 1995). Erickson and colleagues (2004) reported that urine methylhistamine is not useful as an objective marker of response to bladder distention or as a predictor of response to distention or as a substitute for bladder biopsy to determine mast cell counts.
The realization that mast cells are associated with the syndrome of BPS by no means diminishes the other multiple theories of causation. The poor clinical results with antihistamine therapy would argue against their being a primary factor. Their very presence could be related to injury from any of the proposed theories of etiology, and degranulation could likewise reflect a final common pathway resulting in pain and frequency from multiple causes. Rickard and Lagunoff (1995) proposed, based on results with mast cell granules and epithelial cells in tissue culture, that mast cells could contribute to failure of epithelialization of the bladder surface after injury by two potential mechanisms: (1) inhibition of epithelial cell replication and (2) interference with epithelial cell spreading, thus resulting in the “leaky epithelium” found in some patients. Mast cells may actually be the mediator through which female hormones play a role, accounting for the 10 : 1 female-to-male preponderance of the disease (Vliagoftis et al, 1992; Pang et al, 1995a; Patra et al, 1995; Bjorling and Wang, 2001). Estradiol augments the secretion of mast cell histamine in response to substance P. It has been proposed that the symptoms of BPS/IC may depend on an imbalance of the relative number of estrogen receptors to progesterone receptors on bladder mast cells (Letourneau et al, 1996).
To summarize, much important IC research has centered on the mast cell. These cells are strategically localized in the urinary bladder close to blood vessels, lymphatics, nerves, and detrusor smooth muscle (Saban et al, 1997). Studies to date strongly suggest that BPS is a syndrome with neural, immune, and endocrine components in which activated mast cells play a central, although not primary, pathogenetic role in many patients (Elbadawi and Light, 1996; Filippou et al, 1999). Studies in a mast cell–deficient mouse model demonstrate that mast cells promote cystitis pain and bladder pathophysiology through the separable actions of histamine and tumor necrosis factor, respectively, suggesting that pain is independent of pathology and inflammation and that histamine receptors may represent direct therapeutic targets for pain in BPS/IC (Rudick et al, 2008).
Bladder Glycosaminoglycan Layer and Epithelial Permeability
Until the early 1970s most investigators thought that the major barrier to free flow of urinary constituents was at the level of the epithelial cells. Tight junctions between urothelial cells, specialized “umbrella cells” lining the surface, and direct bactericidal activity of the vesical mucosa were thought capable of defense of the internal milieu from bacteria, molecules, and ions in the urine (Ratliff et al, 1994). Staehelin and colleagues (1972) proposed that lipid and other hydrophobically bonded materials were important in any barrier to permeability in the luminal membrane because permeants leaked through the interplaque regions if the particles alone limited transport. It has been shown that inflammation of the underlying muscle and lamina propria can disrupt the bladder permeability barrier by damaging tight junctions and apical membranes and causing sloughing of epithelial cells. Leakage of urinary constituents through the damaged epithelium may then exacerbate the inflammation in the underlying tissues (Lavelle et al, 1998, 2000).
It was Parsons and Hurst (1990) who hypothesized and popularized the concept that IC in a subset of patients is the result of some defect in the epithelial permeability barrier of the bladder surface GAGs. The major classes of GAGs include hyaluronic acid, heparin sulfate, heparin, chondroitin 4-sulfate and chondroitin 6-sulfate, dermatan sulfate, and keratan sulfate. These carbohydrate chains, coupled to protein cores, produce a diverse class macromolecules, the proteoglycans (Trelstad, 1985). GAGs exist as a continuous layer on the bladder urothelium (Dixon et al, 1986; Cornish et al, 1990). Except heparin, all the other types of GAGs have been found on the bladder surface (Ruoslahti, 1988). The GAG layer functions as a permeability and antiadherence barrier. When impaired, its functions can be duplicated by exogenous GAG (Hanno et al, 1978). In the absence of this protective layer in the urinary bladder its susceptibility to infection would increase, and the production of nitric oxide in the urothelial cells, and of substance P in the intraepithelial afferent C-fiber terminals, increases. Consequently, the permeability of both the urothelium and the blood vessels in the mucous membrane increases and the blood flow slows owing to vasodilatation (Hohlbrugger, 1999).
Parsons and Hurst (1990) reported a lower excretion of urinary uronic acid and GAGs in IC patients than in normal volunteers and hypothesized that a leaky transitional epithelium might be absorbing these substances to its surface. The data are interesting in that one might expect urinary GAG to increase with injury to the bladder and decrease with resolution (Uehling et al, 1988). The San Diego group (Lilly and Parsons, 1990; Parsons et al, 1990) went on to show experimentally that one can damage the GAG layer with protamine sulfate with resultant back-diffusion of urea through the bladder lumen, and that this urea loss can be prevented with a bladder instillation of exogenous GAG (heparin). By placing a solution of concentrated urea into the bladder of IC patients and measuring absorption versus controls, Parsons and associates (1991) found support for his theory in patients with IC. The rationale of the epithelial permeability school is nicely summarized in four publications (Parsons 1993, 1994; Hurst et al, 1997; Hohlbrugger and Riedl, 2000) and provides a comprehensive, if somewhat imperfect, theory of the disorder.
Support for an epithelial abnormality from a different perspective has come from Bushman and coworkers (1994), who found aneuploid DNA profiles on barbotage specimens from IC patients that may signal an underlying abnormality of the epithelial cell population in some patients with IC. Wilson and colleagues (1995) identified a loss of type IV collagen in the urothelial basement membrane in 5 of 11 IC patients. Hurst’s group (1996) studied bladder biopsy specimens of IC patients and control subjects and concluded that there is a deficit of bladder luminal and basal proteoglycans associated with the disorder. The basal abnormality may reflect an altered urothelial differentiation program. In a later study, IC bladder biopsy specimens showed abnormalities in 24 of 27 patients when examined by immunohistochemical assessment of E-cadherin, ZO-1, uroplakin, and chondroitin sulfate (Slobodov et al, 2004). Erickson and coworkers (1996) measured a glycoprotein (epitectin) in the urine of IC patients and found a decrease compared with a control population, although a significant overlap was detected. Buffington and Woodworth (1997) gave 6 IC patients and 6 control subjects oral fluorescein dye. IC patients had higher levels of fluorescein in their plasma and lower urinary excretion of the dye, suggesting altered membrane permeability and increased fluorescein reabsorption in the bladder wall of IC patients. Erickson and colleagues (1998) compared urinary levels of hyaluronic acid in IC patients and control subjects, reporting higher urinary hyaluronic acid in the patient group, which possibly was accounted for by leakage of this GAG across the epithelium.
In feline IC, the only naturally occurring animal model of the disorder, the urothelium has been shown to have decreased transepithelial resistance and increased water and urea permeability compared with controls in response to hydrodistention (Lavelle et al, 2000). This indicates that barrier function is compromised, which could lead to the sensitization of sensory nerves by irritants from urine crossing into the muscle layer. Bladder strip studies have shown that bladders of cats with feline IC have significantly more spontaneous Ca2+ transients in the mucosal layer than control bladders. Coupled with increased sensitivity of muscarinic receptors in the mucosal layer, these bladders may manifest enhanced smooth muscle spontaneous contractions. This finding could play a contribution in the symptoms of BPS/IC (Ikeda et al, 2009). In fact, human cell culture studies comparing BPS/IC and normal bladder urothelial cells have shown greater sensitivity of IC bladder urothelial cells to carbachol, suggesting that BPS/IC pathobiology may also include alterations in muscarinic signaling (Gupta et al, 2009).
Further data for an abnormal surface mucin came from Moskowitz and colleagues (1994) who studied biopsy specimens from 23 IC patients with regard to the presence of a glycoprotein component of the surface mucin referred to as GP1 and compared the results to those in 11 normal controls. Qualitative GP1 changes in a majority of IC patients were identified. GP1 reactivity was noted in all controls but was absent in 35% of IC patients and diminished in 61%. This study may provide evidence of an abnormal bladder urothelium, but the effects of bladder distention in the IC group is unknown and may have contributed to the results. There were no pathologic controls used, and no attempt was made to correlate GP1 reactivity with IC symptoms (Messing, 1994). Castration in female rabbits is associated with bladder mucosal changes resulting in increased mucosal permeability (Parekh et al, 2004). Birder and colleagues have shown that feline IC results in increased baseline production of nitric oxide due to inducible nitric oxide synthase (iNOS) (Birder et al, 2005). These changes in transmitter release may have a role in altering mucosal barrier properties.
Vascular endothelial growth factor (VEGF) plays a key role in bladder inflammation and is closely associated with the vascular alterations observed in patients with BPS/IC. VEGF and coreceptors (neuropilins [NRPs]) are strongly expressed in human bladder urothelium. NRP2 and VEGF-R1 are significantly downregulated in IC when compared with control subjects (Saban et al, 2008). GAG modification of NRPs plays a critical role in modulating VEGF/NRP signaling (Shintani et al, 2006), suggesting another possible mechanism in which a GAG deficiency could act by interfering with the functionality of this signaling system.
Some have cited the “potassium sensitivity test” as providing strong evidence for a population with mucosal leak (Parsons et al, 1994b). Parsons and colleagues placed water or 0.4 M potassium chloride (KCl) intravesically into normal volunteers and IC patients. Water did not provoke pain in either group, but KCl provoked the symptom in 4.5% of normal subjects and 70% of IC patients. Symptomatic responses were reduced in patients on heparinoid therapy. Similar findings occur in patients with radiation cystitis (100%) (Parsons et al, 1994c), urinary infection (100%) (Parsons et al, 1998), detrusor instability (25%) (Parsons et al, 1998), “urethral syndrome” (55%) (Parsons et al, 2001b), and more than 80% of women with endometriosis, vulvodynia, and pelvic pain (Parsons et al, 2001a, 2002b). Up to 33% of unselected Turkish women may test positive (Sahinkanat et al, 2008). Eighty-four percent of men with prostatitis also have a positive test (Parsons and Albo, 2002). The poor specificity of the potassium test suggests that it does not provide unequivocal evidence of a permeability dysfunction. Because it is known that the normal bladder epithelium can never be absolutely tight, and there is always some leak, however small (Hohlbrugger, 1997), it is conceivable that the findings of pain with KCl are related to a hypersensitivity of the sensory nerves in this condition, rather than to pathologic epithelial permeability, at least in some patients. In fact, KCl administered intravesically to cats with feline IC seems to inhibit afferent firing of peripheral Aδ fibers. Heightened sensitivity of afferent nerve fibers can explain KCl results without necessarily evoking increased permeability (Lutgendorf and Kreder, 2005; Roppolo et al, 2005). Intravesical administration of KCl has since been proposed as a diagnostic test for IC (Parsons et al, 1998) (see later).
How central abnormal epithelial permeability is to IC is, however, by no means clear. Tamm-Horsfall protein (THP), a high-molecular-weight glycoprotein synthesized exclusively by the ascending loop of Henle and the distal tubule of the kidney, has been studied as a potential marker of urothelial permeability. Fowler and associates (1988) provided graphic data that the urothelium might be leaky in IC. With immunohistochemical techniques this group assayed the bladder biopsy specimens of 14 IC patients and 10 normal control subjects for intraurothelial THP to assess indirectly the in-vivo permeability of the urothelium. Eight pathologic controls were also assessed. Ten of 14 IC patients versus 1 of 18 controls demonstrated intraurothelial THP. Serum THP autoantibody is higher in BPS/IC patients versus controls (Neal et al, 1991). It is known that excretion rates of THP vary widely, even in repeat samples taken from the same individual (Reinhart et al, 1990). Subsequent studies in IC have failed to show differences in the presence of intraurothelial THP in the IC population versus controls, nor in antibody reactivity to THP (Stone et al, 1992; Stein et al, 1993). Parsons and associates (2007) reported that the THP protein is qualitatively different in patients with IC than in controls, even if the urinary concentration of the protein is the same. Bade and coworkers (1996) failed to find THP in the bladder tissue from 10 IC patients. Others have suggested that when THP is seen in bladder tissue it is an incidental finding of no clinical significance. The finding of intraurothelial THP has not been shown to be a harbinger of IC or any other bladder disorder (Truong et al, 1994).
Finally, we must look at a body of literature that has failed to find GAG abnormality or hyperpermeability. Ultrastructural, biochemical, and functional studies of bladder GAGs have not supported this theory (Collan et al, 1976; Dixon et al, 1986; Johansson and Fall, 1990; Ruggieri et al, 1991). Nickel’s group (Nickel et al, 1993) reported sophisticated electron micrography using a specific antimucus, antisera stabilization technique to study the ultrastructural morphologic appearance of the GAG layer. No significant difference in the morphologic appearance of the mucus or GAG layer was noted in IC versus control subjects. Urinary chondroitin sulfates, heparan sulfate, and total sulfated GAGs normalized to creatinine are not altered in IC (Erickson et al, 1997b). Although an increased ratio of total GAGs to sulfated GAGs in IC may indicate an altered GAG layer, whether it reflects a cause or is a result of the primary pathologic process(s) is unknown (Wei et al, 2000). That leaves one to postulate an as yet unknown functional abnormality, rather than GAG deficiency, to account for any increase in permeability.
Chelsky and coworkers (1994) measured bladder permeability in IC using direct measurement by transvesical absorption of technetium-99m–labeled diethylenetriaminepentaacetic acid (99mTc-DTPA). Whereas some IC patients had a more permeable bladder than others, the same was true for normal volunteers. Increased permeability in the IC group could not be demonstrated. However, three IC patients had marked absorption of 99mTc-DTPA and may represent a subpopulation of patients with increased epithelial permeability. Intravesical instillation of 10% and 20% ethanol in rabbits was reported to be a reliable quantitative measure of bladder hyperpermeability by the San Diego group (Monga et al, 2001) and subsequently failed to demonstrate bladder permeability in humans with IC (Gordon et al, 2003).
Overall, it does seem that there is a population of BPS/IC patients with increased epithelial permeability but the issue is far from closed. Increased mucosal permeability is nonspecific and a consequence of bladder inflammation and also occurs with cyclophosphamide-induced bladder injury, bacterial infection, and cystitis after intravesical challenge with antigen after sensitization (Engelmann et al, 1982; Kim et al, 1992). It may also be a consequence of aging itself (Jacob et al, 1978). Whether this represents a primary cause of IC or merely reflects the result of an as yet unidentified source of inflammation is unclear. Treatments that tend to damage the GAG layer, including transurethral resection and laser of ulcerated areas, bladder distention, and the administration of silver nitrate, oxychlorosene (Clorpactin), or the organic solvent dimethyl sulfoxide (DMSO) have all been used with varying results to treat IC.
One does not need increased permeability of the mucosa as a foundation of the many potential causes or contributing factors to BPS/IC. Enhanced adenosine triphosphate (ATP) release from the urothelium of patients with BPS/IC has been described (Kumar et al, 2007). This nonneuronal ATP may act as a sensory neurotransmitter. Increased purinergic activity may thus lead to a condition in which the bladder is oversensitive to distention.
Increased permeability and epithelial dysfunction must be only a part of the story.
Inhibition of Uroepithelial Cell Proliferation: Antiproliferative Factor
The finding that cells from the bladder lining of normal controls grow significantly more rapidly in culture than cells from BPS/IC patients led Keay and associates (1996) at the University of Maryland to the discovery of an antiproliferative factor (APF) produced by the urothelium of IC patients. Normal bladder cells were cultured in the presence of urine from patients with IC, asymptomatic controls, bacterial cystitis, and vulvovaginitis. Only urine from IC patients inhibited bladder cell proliferation (Keay et al, 1998b). The presence of APF was found to be a sensitive and specific biomarker for IC (Keay et al, 2001) (Table 12–6). It was found in bladder urine but not in renal pelvic urine of IC patients, indicating production by the bladder urothelial cells (Keay et al, 1999). Subsequent studies indicated that APF is associated with decreased production of heparin-binding epidermal growth factor–like growth factor (HB-EGF) (Keay et al, 2000, 2003b). APF activity was related to increased production of epidermal growth factor (EGF), insulin-like growth factor-1, and insulin-like growth factor binding protein-3 by the bladder cells from IC patients but not by the cells from healthy bladders. Studies of IC patients and asymptomatic controls showed urine levels of APF, HB-EGF, and EGF to reliably separate out IC from controls (Keay et al, 2001; Erickson et al, 2002).
Table 12–6 Prevalence of Urine Antiproliferative Factor Activity in Interstitial Cystitis Patients and Control Groups
| GROUPS | NO. OF PATIENTS POSITIVE/TOTAL | % POSITIVE |
|---|---|---|
| Patients | ||
| Interstitial cystitis | 206/219 | 94 |
| Controls | ||
| Asymptomatic | 10/113 | 9 |
| Overactive bladder | 2/32 | 6 |
| Bacterial cystitis | 7/58 | 12 |
| Microscopic hematuria | 2/19 | 10 |
| Stress incontinence | 1/10 | 10 |
| Neurogenic bladder | 0/11 | 0 |
| Benign prostatic hyperplasia | 1/14 | 7 |
| Nonbacterial prostatitis | 1/16 | 6 |
| Vulvovaginitis | 0/12 | 0 |
| Miscellaneous | 1/16 | 6 |
From Keay SK, Zhang CO, Shoenfelt J, et al. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor–like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology 2001;57:9.
APF levels in the urine were found to discriminate between men with IC versus those with CPPS or nonbacterial prostatitis (Keay et al, 2004a). APF activity dropped significantly in IC patients within 2 hours after hydrodistention (Chai et al, 2000b) and after 5 days of sacral neuromodulation (Chai et al, 2000a). Cell culture studies showed that APF actually caused decrease in HB-EGF and increase in EGF, mirroring the differences in urine levels of these growth factors between IC patients and controls and suggesting that APF is the primary abnormality (Keay et al, 2003b).
Whereas APF may prove to be a useful marker for BPS/IC, it may also unlock the etiology of the syndrome. It has been hypothesized by Keay and colleagues (2003a) that BPS/IC may result from an inhibition of bladder epithelial cell proliferation caused by the APF, which is mediated by its regulation of growth factor production from bladder cells. Conceivably, any of a variety of injuries to the bladder (infection, trauma, and overdistention) in a susceptible individual may result in BPS/IC if APF is present and suppresses production of HB-EGF (Keay and Warren, 1998). Theoretically, if production of APF could be “turned off” by genetic techniques, or its effects were nullified by exogenous HB-EGF, the clinical syndrome might be prevented. HB-EGF functionally antagonizes APF activity via a mitogen-activated protein kinase pathway activation (Kim et al, 2009a).
APF has been purified (Fig. 12–8) and proved to be a frizzled 8 protein that belongs to a newly discovered family of proteins that seem to be important in the development of nerve tissues, skin, and the lining of organs (Keay et al, 2004b). The frizzled family of receptors is critically involved in embryogenesis, and there is substantial evidence that members of this family also regulate tissue homeostasis in many different organs in the adult (Schulte and Bryja, 2007).
Figure 12–8 Composition and structure of antiproliferative factor.
(From Keay SK, Szekely Z, Conrads TP, et al: An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci U S A 2004;101:11803–8.)
It appears that the cell cycle regulatory protein TP53 is an important mediator of APF-induced effects on bladder epithelial cells (Kim et al, 2007). APF treatment suppresses cell proliferation by cell cycle arrest of human bladder urothelial cells. Evidence shows that TP53 levels increase significantly after APF stimulation; TP53 downregulation enhances the suppressive effect of APF on cell growth; and overexpression of TP53 induces cell cycle arrest in the absence of APF. It is possible that targeting of TP53 could be a means of abrogating the pathologic effects of urinary APF and lead to new options for clinical therapy.
Studies are ongoing to confirm the research by Keay and colleagues and expand on its significance in diagnosis and development of a rational treatment approach (Rashid et al, 2004). The development of animal models with specific signaling abnormalities found in BPS/IC epithelial, endothelial, smooth muscle, neuronal, or immune cells to determine whether these animals develop a disease similar to the human disorder would be an extremely useful and potentially very productive pathway to learning more about potential therapeutic pathways (Keay, 2008).
Neurobiology
Neuropeptides present in primary afferents and the dorsal horn of the spinal cord have an important role in the mediation of nociceptive input under normal conditions. Under pathologic conditions, such as chronic inflammation or after peripheral nerve injury, the production of peptides and peptide receptors is dramatically altered, leading to a number of functional consequences (Wiesenfeld-Hallin and Xu, 2001). Inflammatory painful stimuli, especially if repeated, can chronically alter innervation, central pain-processing mechanisms, and tissue responses (Steers and Tuttle, 1997). It has been known for some time that the sensory nervous system can generate some of the manifestations of inflammation (Foreman, 1987; Dimitriadou et al, 1991, 1992). Activation of capsaicin-sensitive afferent neurons locally and centrally may be involved in stress-related pathologic changes in the rat bladder (Ercan et al, 2001). Activation of sensory nerves, specifically pain fibers, is known to trigger neurogenic inflammation through release of neuropeptides such as substance P, neurokinin A, and calcitonin gene–related protein, and subsequent increase in vascular permeability, with leukocyte adhesion and tissue edema. The neuropeptide mediators have been shown to also cause degranulation of mast cells with release of additional potent mediators of inflammation and to lead to injury and increased permeability of epithelial surfaces (Elbadawi and Light, 1996). An increase in nerve fibers within the suburothelium and detrusor muscle in ulcerative IC has been noted (Lundeberg et al, 1993). A correlation was found between the number of nerve fibers and numbers of mast cells as well as between the number of nerve fibers and the amount of histamine. Consolidating the leaky urothelium theory and mast cell activation, neurogenic inflammation is an attractive proposal for etiology and can readily accommodate infectious, immunologic, and autoimmunologic mechanisms as factors (Elbadawi and Light, 1996).
Harrison and colleagues (1990) proposed that small-diameter sensory nerves in the bladder wall may have a role in the transmission of the sensation of pain and in the triggering of inflammatory reactions rather than forming the afferent limb of the micturition reflex. Abelli and associates (1991) demonstrated in the rat urethra that mechanical irritation alone can cause neuropeptide release from peripheral capsaicin-sensitive primary afferent neurons, resulting in neurogenic inflammation. Extracellular ATP can act through the purinergic receptor subtype P2X3 to transmit a pain signal to the central nervous system. These subunits expressed by cultured IC bladder urothelial cells are upregulated during in-vitro stretch and may phenotypically mimic sensory neurons (Sun and Chai, 2004).
Several pieces of additional information support a theory of neurogenic inflammation. Levels of nerve growth factor are elevated in bladder biopsy specimens of IC patients (Lowe et al, 1997). Studies in rats using pseudorabies virus clearly show that bladder inflammation can be induced from a somatic structure through a neural mechanism and that central nervous system dysfunction can bring about a peripheral inflammation (Doggweiler et al, 1998; Jasmin et al, 1998). Pelvic nerve stimulation in the rat increases urothelial permeability that is antagonized by capsaicin, indicating both an efferent effect of afferent nerves and afferent-mediated neuroepithelial interaction (Lavelle et al, 1999).
Numerous studies indicate increased sympathetic activity in IC. Hohenfellner and colleagues (1992) suggested that IC is associated with increased sympathetic outflow into the bladder and altered metabolism of vasoactive intestinal polypeptide and neuropeptide Y. Neuropeptide Y (NPY) inhibits bladder afferents and therefore may be involved in autonomic disturbances affecting the bladder. Elevation of urinary catecholamines in IC patients and plasma catecholamines in cats with feline IC has been observed (Stein et al, 1999; Buffington and Pacak, 2001a), as has an increased density and number of nerve fibers immunoreactive for tyrosine hydroxylase in IC patients (Peeker et al, 2000a). Whether these changes reflect a cause of IC or are merely the result of long-standing intense pain and a severely pathologic voiding pattern is unknown.
Galloway and associates (1991) proposed that the changes in IC may be explained by an increase in sympathetic discharge, analogous to that seen in reflex sympathetic dystrophy (RSD) of limbs. The pathology in RSD is the development of abnormal synaptic activity between sensory afferent and sympathetic efferent neurons. Nerve cells in the spinal cord become hypersensitive to sensory input, and this sustains abnormal sympathetic outflow and corresponding vasomotor dysregulation. The excess sympathetic outflow leads to constriction of blood vessels and tissue ischemia, setting up further sensory changes and perpetuating the cycle. In RSD there is usually a trigger event leading to these changes. With the acute phase of RSD, regional signs of inflammation are evident in the affected extremity. One school of thought believes an inflammatory response to an injury initiates RSD. Increased capillary permeability is a direct result (Goris and Jan, 1998). Perhaps a urinary infection could trigger such a pathologic cycle in some IC patients?
Herbst and colleagues (1937) produced bladder lesions in a dog resembling the ulceration of IC by ligating the blood vessels to the posterior bladder wall and infecting the area with Streptococcus viridans. Studies using laser Doppler flowmetry have shown that when the bladder is distended under anesthesia the blood flow increases in control patients to a statistically significant degree as compared with IC patients (Irwin and Galloway, 1993; Pontari et al, 1999). Another study has purported to show that topical heparin therapy can normalize urothelial permeability and vesical blood flow in IC (Hohlbrugger et al, 1998). Decreased microvascular density has been described in the suburothelium but not in the deeper mucosa in bladder biopsy specimens of women with IC (Rosamilia et al, 1999a). Hyperbaric oxygen has been suggested to have a limited effectiveness for the treatment of BPS/IC (van Ophoven et al, 2004b, 2006; Tanaka et al, 2007) as well as radiation-induced cystitis (Weiss and Neville, 1989).
If lumbar sympathetic blocks can decrease the pain of IC, a role of the sympathetic nervous system in IC pathogenesis is a reasonable supposition (Irwin et al, 1993; Doi et al, 2001). An increase in sympathetic activity has been shown in cats with feline IC (Buffington and Pacak, 2001b; Buffington et al, 2002). Similar findings have been reported in a small study of IC patients (Dimitrakov et al, 2001), and sympathetic activity may be an underlying common denominator in many disorders associated with PBS/IC (Buffington, 2004).
Nevertheless, no studies performed to date can confirm any case of IC is related to the syndrome of reflex sympathetic dystrophy (Ratliff et al, 1994). No single test can be used to exclude sympathetically maintained pain, and there are no clear symptoms that predict sympathetically mediated pain (Baron, 2000). In the animal model, bladder ischemia is associated with detrusor overactivity or impaired detrusor contraction, not sensory urgency (Azadzoi et al, 1999). Those patients with RSD who have voiding symptoms rarely have a picture that would be confused with IC (Chancellor et al, 1996).
Before leaving the neurogenic theory of etiology it is important to note that the nervous system itself almost surely contributes to the chronic nature of this pain syndrome, regardless of initiating cause (Vrinten et al, 2001). Repetitious stimulation of a peripheral nerve, at sufficient intensity to activate C fibers, results in a progressive buildup of the magnitude of the electrical response recorded in the second-order dorsal horn neurons. This “windup” phenomenon is central to the concept of chronic pain. Biochemically it is dependent on activation of N-methyl-D aspartate (NMDA) receptors in the spinal cord (Bennett, 1999). With persistent NMDA receptor activation, spinal cord cells undergo trophic changes and the pain resulting from subsequent stimulation becomes exaggerated and prolonged. This “pain memory” in the spinal cord may be what causes IC patients to become refractory to different therapies (Brookoff, 1997). NMDA-receptor–driven formation of new connections in the spinal cord may account for the expansion of the pain field.
Upregulation of the CNS and augmented sensory processing has been referred to as non-nociceptive pain (NNP) (Bennett, 1999). The four characteristic features of NNP would seem to apply very well to the clinical syndrome of IC (Table 12–7). Chronic neuropathic pain may continue after the resolution of tissue damage and persist on the basis of a maladaptive mechanism (Urban et al, 2002).
Table 12–7 Non-Nociceptive Pain: Characteristic Clinical Features
|
1. The description of the pain seems inappropriate in comparison with the degree of tissue pathology, or no tissue pathology may be discernible.
|
From Bennett RM. Emerging concepts in the neurobiology of chronic pain: evidence of abnormal sensory processing in fibromyalgia. Mayo Clin Proc 1999;74:385–98.
Burnstock’s observation (1999) that ATP has a role in mechanosensory transduction by the epithelial lining of hollow viscus organs such as bladder has been followed up by Sun and colleagues (2001). Stretched epithelial cells lining hollow organs release ATP, which acts on purinergic nociceptive receptors on subepithelial sensory nerve terminals. ATP levels were significantly elevated in the urine of PBS/IC patients, and the stretch-activated release of ATP was augmented in IC urothelium.
Neurogenic inflammation may be the cause of some cases of BPS or may be the result of other initiating etiologic events. It is not incompatible with the central role of the mast cell or with the leaky epithelium theory. It conceivably could result in the appearance of autoimmune phenomena or result from an episode of infection. The central nervous system may also be implicated in dysregulation of the pelvic floor, resulting in chronic pelvic pain and contributing to IC (Zermann et al, 1999) and perhaps in the rare cases of IC that chronologically seem to relate to trauma or pelvic surgery (Zermann et al, 1998). It is an etiologic theory that provides fertile ground for new treatment possibilities.
Pelvic Organ Cross-Sensitization
Clinical observations of viscerovisceral referred pain in patients with gastrointestinal and genitourinary disorders suggest an overlap of neurohumoral mechanisms underlying both bowel and urinary bladder dysfunctions. Close proximity of visceral organs within the abdominal cavity complicates identification of the exact source of chronic pelvic pain, where it originates, and how it relocates with time. Cross-sensitization among pelvic structures may contribute to chronic pelvic pain of unknown etiology and involves convergent neural pathways of noxious stimulus transmission from two or more organs. Inflammation, nerve injury, ischemia, peripheral hyperalgesia, metabolic disorders, and other pathologic conditions can dramatically alter the function of directly affected pelvic structures as well as organs located next to a damaged domain (Malykhina, 2007). It has been demonstrated in a rat model that acute colitis can sensitize lumbosacral spinal neurons receiving input from the urinary bladder (Qin et al, 2008). Acute colitis and acute cystitis in the rat model can each cross-sensitize the bladder and colon, respectively (Pezzone et al, 2005).
It is thought that chronic widespread pain in chronic fatigue syndrome and fibromyalgia (disorders associated with BPS/IC in many patients) can be a consequence of central sensitization. Central sensitization is an increased central neuronal responsiveness and causes hyperalgesia, allodynia, and referred pain and hyperalgesia across multiple spinal segments, leading to chronic widespread pain. Triggers include windup or temporal summation, dysregulated descending inhibitory pathways, and upregulated facilitatory modulation. Windup or temporal summation is the result of repetitive noxious stimuli, leading to an increase in electrical discharges in the dorsal horn. Inhibitory modulation can be impaired by abnormalities in the central nervous system, and the facilitatory pain pathways can be stimulated by certain behavioral and cognitive factors (Meeus and Nijs, 2007).
It is possible that a combination of central sensitization and pelvic cross talk may account for the association of BPS/IC with bowel symptoms in some patients and the use of dietary alterations for managing the severity of BPS symptoms. Relatively minor gut stimuli, which otherwise cause no symptoms, could exacerbate established, bladder-driven pelvic pain, because even slight increases of inputs from a second site like the gut might lead to a sum of inputs that is considerably elevated above a threshold necessary to induce pain (Rudick et al, 2007; Klumpp and Rudick, 2008). Central pain amplification might also account for the increased startle responses described in female BPS/IC patients in the context of a threat of abdominal pain (Twiss et al, 2009).
Key Points
Sensitization of Neural Pathways
Nitric Oxide Metabolism
Regulation of urinary NOS activity has been proposed to be of importance for immunologic responses in BPS. The oral administration of L-arginine, the substrate for nitric oxide production (Moncada and Higgs, 1993) has been shown to increase nitric oxide–related enzymes and metabolites in the urine of patients with BPS (Smith et al, 1996). It has been reported that differences in nitric oxide evaporation between ulcerative and nonulcerative BPS allows for subtyping of cases meeting the NIDDK criteria. This could potentially replace cystoscopy as a tool for identifying ulcerative BPS/IC (Logadottir et al, 2004). Increased levels of endogenously formed nitric oxide correspond to increased iNOS in mRNA expression and protein levels in BPS patients. iNOS has been found to be localized to the urothelium and has also been found in macrophages in the bladder mucosa. Whether high levels of endogenously formed nitric oxide are a part of the pathogenesis in BPS/IC and whether it has a protective or damaging role remain to be elucidated (Koskela et al, 2008).
Urine Abnormalities
Current theories of pathogenesis generally involve access of a component of urine to the interstices of the bladder wall, resulting in an inflammatory response induced by toxic, allergic, or immunologic means. The substance in the urine may be a naturally occurring one—a substance that acts as an initiator only in particularly susceptible individuals—or may act like a true toxin, gaining access to the urine by a variety of mechanisms or metabolic pathways (Wein and Broderick, 1994). Clemmensen and colleagues (1988) noted that 8 of 11 IC patients had positive skin reactions to patch tests with their own urine. Immediate reactions were not observed, and the histology suggested a toxic rather than an allergic reaction. Lynes and associates (1990b) were unable to find a urinary myotropic substance unique to IC patients. The San Diego group found IC urine to result in higher cell death of cultured transitional cells than normal urine, suggesting a toxic compound in the urine of some IC patients (Parsons and Stein, 1990). They identified heat-labile, cationic components of low molecular weight that bind to heparin and that when separated from the bulk of urinary wastes are cytotoxic to urothelial cells as well as underlying smooth muscle cells (Parsons et al, 2000). They reported a 12% increase in 72-kD stress protein in cells treated with urine from IC patients compared with controls (Ito et al, 1998).
Others have not been able to demonstrate in-vitro cytotoxicity (Beier-Holgersen et al, 1994) or immunohistochemical changes in the nociceptive centers in the spinal cord or bladder wall when IC urine was compared with control urine (Baykara et al, 2003). Efforts to induce an IC-like picture in the rabbit bladder from exposure to urine of IC patients have failed to demonstrate conclusive changes (Perzin et al, 1991; Ruggieri et al, 1993; Kohn et al, 1998). Increased levels of soluble mediators associated with activation of sensory neurons and/or mast cells have been found in the urine of both IC and bladder cancer patients (Okragly et al, 1999).
Circumstantial evidence for the toxicity of IC urine is suggested by the failure of substitution cystoplasty and continent diversions in some of these patients because of the development of pain or contraction of the bowel segment over time (Nielsen et al, 1990; Baskin and Tanagho, 1992; Trinka et al, 1993; Lotenfoe et al, 1995) and the histologic findings similar to IC found to occur in bowel used to augment the small-capacity IC bladder (Singh and Thomas, 1996; McGuire et al, 1973). Intestinal mucosa in contact with urine undergoes progressive changes as long as 3 years after surgery, and the significance of the histologic IC-like changes has been questioned (MacDermott et al, 1990; Davidsson et al, 1996).
The Role of Genetics in BPS
Warren and colleagues (2001) report findings from a small cohort of twins that demonstrated a greater concordance of BPS among monozygotic than among dizygotic twins. This finding suggested that there could be a genetic susceptibility to BPS. A later study by the same research group (Warren et al, 2004) suggested that adult female first-degree relatives of patients with BPS may have a prevalence of IC 17 times that found in the general population. This coupled with the previously reported twin data suggests, but does not prove, a genetic component adds to the susceptibility for BPS.
The report by Weissman and associates (2004) of the increased frequency of BPS in patients and their first-degree relatives with panic disorder and other seemingly disparate disorders has suggested that there is a familial syndrome consisting of BPS and other disorders of possible autonomic or neuromuscular dysfunction. More recent studies by the same group (Talati et al, 2008) from a case-control study suggested that this syndrome might include other anxiety disorders as well and that families with and without this collection of symptoms were genetically distinguishable on chromosome 13.
Gene expression profiles in cultured IC cells have been investigated and compared with controls (Erickson et al, 2008a). Few differences were appreciated, indicating that rather than being genetically based the abnormal urothelium in BPS/IC may be due to post-translational changes and/or to the bladder environment. It has been suggested that epigenetic reprogramming mechanisms in the bladder may provide an explanation for uroepithelial, mast cell, and nerve cell abnormalities in BPS/IC, as well as propagation of this altered state in the absence of the signal that may have triggered it (Elgavish, 2009). Viewing medically unexplained symptoms from the perspective of underlying developmental influences involving epigenetic modulation of gene expression that affect function of a variety of organs based on familial predispositions rather than from the traditional viewpoint of isolated organ-originating diseases may open up new areas of investigation for this class of “functional” disorders (Buffington, 2009).
Other Potential Causes
Various other etiologic theories have been proposed (Ratliff et al, 1994), but none has received much scientific support. Voiding almost hourly, always having to be aware of how far the nearest restroom facilities are, and suffering constant pain would be expected to lead to psychological stress. However, could there be individual differences in the propensity to develop IC that result from a dysregulation of anxiety and mood (Nesse, 1999; Bodden-Heidrich, 2004)? There are no data currently to suggest that stress initiates the chronic syndrome of BPS/IC, although it certainly can increase symptom severity (Lutgendorf et al, 2000). Cats restricted to indoor living are five times more likely to have urinary problems as cats allowed outdoors (Buffington, 2002). Female patients with PBS/IC have been shown to have increased heart rate at baseline and throughout a laboratory mental stress challenge but did not demonstrate greater autonomic reactivity to stress (Lutgendorf et al, 2004). Until stress can be shown to produce PBS/IC de novo in humans, it is just as reasonable to speculate that the stress is a result of the syndrome as a primary cause of it.
Speculation that abnormalities in or obstruction of lymphatics or vascular structures is causative has never been borne out. The fact that some of these patients have had a hysterectomy probably relates more to the attempt of the physician to treat chronic pelvic pain than a postsurgical change causing the IC syndrome (Chung, 2004).
The knowledge that there is at least a 5 : 1 female-to-male preponderance immediately makes the role of the hormonal milieu potentially important (Bjorling and Wang, 2001). Paradoxically, it is known that estrogens can control hematuria in hemorrhagic cystitis, perhaps by decreasing the fragility of the mucosal microvasculature of the bladder (Liu et al, 1990). Estradiol augments while the estrogen receptor blocker tamoxifen inhibits mast cell secretion (Vliagoftis et al, 1992). Bladder mast cells express high-affinity estrogen receptors, and there is a higher number of such cells present in patients with IC compared with controls. Although this may help explain why IC is so common in women, the hormonal role can only account for the propensity of IC to occur in females and not be the ultimate etiology.
Pelvic floor dysfunction has been associated with PBS/IC for many years (Schmidt and Vapnek, 1991), and uncontrolled trials suggest that treatment of this disorder can be effective in ameliorating symptoms (Lilius et al, 1973; Doggweiler-Wiygul et al, 2001, 2002; Holzberg et al, 2001; Weiss, 2001; Oyama et al, 2004). Speculation that abnormalities of the pelvic floor muscular function may contribute to the cause of some cases of CPPS in men are well accepted (Segura et al, 1979; Schmidt and Vapnek, 1991; Zermann et al, 1999) and a similar case might be made for patients with PBS/IC, although scientific support for a direct etiologic relationship is lacking.
One can understand the complexity of BPS/IC by looking at the myriad theories of etiology and the associated disorders that have been described. Complex functional disorders with patterns of comorbidity, where there is overlap of what had once been regarded as independent syndromes, appear to fall outside the biomedical framework. It has been said that they “fully escape the profession’s divided and divisive grip. By their mere existence, they challenge the medical community’s view of the body, their understanding of what it is to be human and question whether present approaches to obtaining knowledge are adequate for helping people who suffer” (Kirkengen and Ulvestad, 2007).
Key Points
Etiology
Pathology
Please see the Expert Consult website![]() for more discussion of this topic.
for more discussion of this topic.
Pathology can be consistent with the diagnosis of BPS, but there is no histology that is pathognomonic of this syndrome. The role of histopathology in the diagnosis of BPS is primarily one of excluding other possible diagnoses. One must rule out carcinoma and carcinoma in-situ, eosinophilic cystitis, tuberculous cystitis, as well as any other entities with a specific tissue diagnosis (Hellstrom et al, 1979; Johansson and Fall, 1990; Tsiriopoulos et al, 2006).
Although earlier reports described a chronic, edematous pancystitis with mast cell infiltration, submucosal ulcerations, and involvement of the bladder wall and chronic lymphocytic infiltrate (Smith and Dehner, 1972; Jacobo et al, 1974), these were cases culled from patients with severe disease and not representative of the majority of cases currently diagnosed. The pathologic findings in BPS are not consistent. There has been a great variation in the reported histologic appearance of biopsy specimens from BPS patients and even variation among specimens taken from the same patients over time (Gillenwater and Wein, 1988).
Lepinard and colleagues (1984) reported a pancystitis affecting the three layers of bladder wall. In nonulcerative disease the vesical wall was never normal, with epithelium being thinned and muscle being affected. Johansson and Fall (1990) looked at 64 patients with ulcerative disease and 44 with nonulcerative IC. The former group had mucosal ulceration and hemorrhage, granulation tissue, intense inflammatory infiltrate, elevated mast cell counts, and perineural infiltrates. The nonulcerative group, despite the same severe symptoms, had a relatively unaltered mucosa with a sparse inflammatory response, the main feature being multiple, small, mucosal ruptures and suburothelial hemorrhages that were noted in a high proportion of patients. Because these specimens were almost all taken immediately after hydrodistention, how much of the admittedly minimal findings in the nonulcer group were purely iatrogenic is a matter of speculation.
Completely normal biopsy specimens are not uncommon in the nonulcerative BPS group (Johansson and Fall, 1994). Transition from nonulcerative to ulcerative BPS is a rare event (Fall et al, 1987), and pathologically the two types of IC may be completely separate entities. Although mast cells are more commonly seen in the detrusor in ulcerative BPS (Holm-Bentzen et al, 1987b), they are also common in patients with idiopathic bladder instability (Moore et al, 1992). Mastocytosis in BPS is best documented by tryptase immunocytochemical staining (Theoharides et al, 2001). Larsen and colleagues (2008) recommend taking biopsy specimens from the detrusor of patients with suspected BPS and examining them with tryptase-stained 3-µm thick sections, with every seventh section used for quantification. They consider 27 mast cells/mm2 indicative of mastocytosis. Despite attempts to develop a diagnostic algorithm based on the detrusor to mucosa mast cell ratio and nerve fiber proliferation (Hofmeister et al, 1997), mast cell counts per se have no place in the differential diagnosis of this clinical syndrome.
Mast cells could be valuable in clinical phenotyping, but as yet that is unproven. Mast cells trigger inflammation that is associated with local pain, but the mechanisms mediating pain are unclear. In a murine model of neurogenic cystitis, Rudick and colleagues (2008) demonstrated that mast cells promote cystitis pain and bladder pathophysiology through the separable actions of histamine and tumor necrosis factor, respectively. Therefore, pain is independent of pathology and inflammation and histamine receptors may represent direct therapeutic targets for the pain of BPS and other chronic pain conditions.
Lynes and coworkers (1990a) concluded that biopsy specimens are often not helpful in confirming the diagnosis. Although BPS patients in their study had a higher incidence and degree of denuded epithelium, ulceration, and submucosal inflammation, none of these findings was pathognomonic. In addition, these “typical” findings occurred only in BPS patients with pyuria or small bladder capacity. Epithelial and basement membrane thickness, submucosal edema, vascular ectasia, fibrosis, and detrusor muscle inflammation and fibrosis were not significantly different in the BPS and control patients.
Attempts to definitively diagnose BPS by electron microscopy have also been unsuccessful. Collan’s group (1976), in the first such study, wrote that the similarity of the ultrastructure of epithelial cells in control subjects and IC patients makes it improbable that the disease process originates in the epithelium. Other investigators found no differences in the morphologic appearances of the glycocalyx and of urothelial cells in patients with IC when compared with control subjects (Dixon et al, 1986). Anderstrom and colleagues (1989) saw no surface characteristics specific for IC but believed that the mucin layer covering the urothelial cells seemed reduced in patients with IC compared with control subjects, a fact disputed by Nickel et al in a very elegant paper (1993). Elbadawi and Light (1996) observed ultrastructural changes sufficiently distinctive to be diagnostic in specimens submitted for pathologic confirmation of nonulcerative IC. Marked edema of various tissue elements and cells appeared to be a common denominator of many observed changes. The wide-ranging discussion by Elbadawi (1997) of the etiology of IC is fascinating, but the pathologic findings are potentially marred by the methodology, in that specimens were obtained after diagnostic hydrodistention.
So what is the place of pathologic examination of tissue in BPS? Attempts to classify the painful bladder by the pathoanatomic criteria described by Holm-Bentzen (1989) are of questionable value. There is a group of patients with what she describes as “nonobstructive detrusor myopathy” (Holm-Bentzen et al, 1985). In her series these patients with degenerative changes in the detrusor muscle often had residual urine, a history of urinary retention, and an absence of sensory urgency on cystometry with bladder capacities over 400 mL. Most would not be clinically confused with BPS. A similar English series (Christmas et al, 1996b), however, included patients who met NIDDK research criteria and associated detrusor myopathy with diminished detrusor compliance and ultimate bladder contracture.
The Interstitial Cystitis Database (ICDB) study worked backward from symptoms to pathology and concluded that certain symptoms are predictive of specific pathologic findings (Tomaszewski et al, 1999, 2001). Denson and associates (2000) analyzed forceps biopsies from 65 females and 4 males with BPS. Ten percent of specimens showed vasodilatation or submucosal edema. Inflammation was absent in 30% of patients, and mild in another 41%. Cystoscopic changes did not correlate with degree of inflammation. Hanus and colleagues (2001) studied 84 biopsy specimens from 112 BPS patients and reported a linear relationship between the mean bladder capacity under anesthesia and severity of glomerulations. They did not find a correlation between severity of symptoms and histopathologic changes observed by light or electron microscopy.
Rosamilia reviewed the pathology literature pertaining to BPS and presented her own data (Rosamilia et al, 2003; Hanno et al, 2005a). She compared forceps biopsy specimens obtained with the use of anesthesia from 35 control subjects and 34 PBS/IC patients, 6 with bladder capacities less than 400 mL. Epithelial denudation, submucosal edema, congestion and ectasia, and inflammatory infiltrate were increased in the BPS group. Submucosal hemorrhage did not differentiate the groups, but denuded epithelium was unique to the BPS group and more common in those with severe disease. The most remarkable finding in her study was that histologic parameters were normal and indistinguishable from control subjects in 55% of BPS subjects. Method of biopsy can be important in interpreting findings, because transurethral resection biopsy specimens tend to show mucosal ruptures, submucosal hemorrhage, and mild inflammation (Johansson and Fall, 1990) whereas histology is normal approximately half the time with cold-cup forceps biopsies (Mattila, 1982; Lynes et al, 1990a; Rosamilia et al, 2003).
Histopathology plays a supportive diagnostic role at best (Johansson et al, 1997). Major reconstructive procedures appear to have better outcomes in patients with pathology consistent with Hunner lesions (Rossberger et al, 2007). Inflammatory features can be seen in 24% to 76% of patients without a visible Hunner lesion (Erickson et al, 2008b). Although recent studies suggest that a severely abnormal pathologic process may be associated with poor prognosis (Mcdougald and Landon, 2003; Nordling et al, 2003), this is not necessarily the case (MacDermott et al, 1991a). At this time, excluding other diseases that are pathologically identifiable is the primary clinical utility of bladder biopsy in this group of patients.
Diagnosis
BPS/IC can be considered a functional pain disorder (Mayer and Bushnell, 2009) and one of the chronic visceral pain syndromes, affecting the urogenital and rectal area, many of which are well described but poorly understood (Wesselmann et al, 1997; Wesselmann, 2001). These include vulvodynia, orchialgia, penile pain, perineal pain, and rectal pain. In men, many of the entities have now been included in the rubric of CPPS and can be difficult to distinguish from BPS/IC (Hakenberg and Wirth, 2002; Forrest and Schmidt, 2004). The diagnosis of BPS/IC is by its very nature based on the definition. In the past this was by default the symptom criteria enumerated by the NIDDK (Hanno et al, 1999a; Hanno et al, 1999b) (see Table 12–2). It has now morphed largely into a diagnosis of chronic pain, pressure, or discomfort associated with the bladder, usually accompanied by urinary frequency in the absence of any identifiable cause (Hanno et al, 2005a, 2005b). Diagnostic approaches vary widely, and general agreement on a diagnostic algorithm remains a future goal (Chai, 2002; Nordling, 2004; Nordling et al, 2004). A current algorithm is illustrated in Figure 12–9. The disorder can be very difficult to diagnose until symptoms become well established, unless one has a high level of suspicion (Porru et al, 2004). Frequency and pelvic pain of long duration perceived to be related to the bladder unrelated to other known causes establishes a working diagnosis. It is often difficult for patients to distinguish between sensations of pain, pressure, discomfort, and urgency. Ask a patient why he or she voids hourly, and it is usually because of discomfort rather than convenience. Heavy reliance on other aspects of the NIDDK research criteria will result in underdiagnosing more than half of the cases (Hanno et al, 1999b). IC symptom scales (O’Leary et al, 1997; Goin et al, 1998; Moldwin and Kushner, 2004), such as the American Urological Association’s symptom score for BPH, are designed to evaluate the severity of symptomatology and monitor disease progression or regression with or without treatment. They have not been validated as diagnostic criteria.
One must rule out infection and less common conditions including but not limited to carcinoma (Utz and Zincke, 1974; Tissot et al, 2004), eosinophilic cystitis (Hellstrom et al, 1979; Sidh et al, 1980; Littleton et al, 1982; Aubert et al, 1983; Abramov et al, 2004), malakoplakia, schistosomiasis, scleroderma (Batra and Hanno, 1997), and detrusor endometriosis (Sircus et al, 1988; Price et al, 1996). In men younger than the age of 50, video-urodynamics are useful to rule out voiding dysfunction resulting from vesical neck obstruction, “pseudo” dyssynergia, or impaired contractility (Kaplan et al, 1996). Musculoskeletal dysfunction may also play a role in causation or increasing symptom severity and should be looked for in the diagnostic phase of evaluation (Prendergast and Weiss, 2003). Reports of successful treatment of IC symptoms by laparoscopic adhesiolysis (Chen et al, 1997) or urethral diverticulum excision (Daneshgari et al, 1999) give credence to the fact that IC is a diagnosis of exclusion. Many drugs including cyclophosphamide, aspirin, nonsteroidal anti-inflammatory agents, and allopurinol have caused a nonbacterial cystitis that resolves with drug withdrawal (Bramble and Morley 1997; Gheyi et al, 1999).
Various gynecologic problems can mimic the pain of IC (Kohli et al,1997). The pelvic congestion syndrome, a condition of the reproductive years and equally prevalent among parous and nulliparous women, is manifest by shifting location of pain, deep dyspareunia and postcoital pain, and exacerbation of pain after prolonged standing (Stones, 2003). Similar symptoms can be seen in BPS/IC. Other gynecologic disorders can include pelvic tumors, vaginal atrophy, vulvodynia, vestibulitis, pelvic relaxation, pelvic adhesive disease, levator ani myalgia, and undiagnosed chronic pelvic pain (Myers and Aguilar, 2002). Pelvic surgery is more common in women with a diagnosis of BPS/IC than in a control population as studied by Ingber and associates (2008). In this series the diagnosis of BPS/IC occurred 1 to 5 years after hysterectomy in most patients, suggesting that pelvic surgery may be performed for pain related to undiagnosed BPS.
Endometriosis can be a cause of pelvic pain (Evans et al, 2007), an idea largely based on findings of two randomized, placebo-controlled studies of laser laparoscopy (Sutton et al, 1995, 1997; Abbott et al, 2004). Nevertheless, it is disconcerting that any claim linking endometriosis with pain fails to account for the common experience that identical lesions can be found in symptomatic and asymptomatic women (Vercellini, 1997). Between 2% and 43% of asymptomatic women are found to have endometriosis (Moen and Stokstad, 2002). Furthermore, there does not appear to be any risk for patients with asymptomatic mild endometriosis to develop symptoms even after more than 10 years (Moen and Stokstad, 2002). Although 70% to 90% of women with chronic pelvic pain have endometriosis, this does not definitively establish causation (Gambone et al, 2002). For these reasons, laparoscopy, which is not considered essential before initiating hormonal treatment of endometriosis (Ling, 1999; Howard, 2003b), should not be considered a part of any routine evaluation of BPS/IC unless an experienced practitioner believes it is likely to benefit the patient.
A presumptive diagnosis can be made merely by ruling out known causes of frequency and pain/urgency in a patient with compatible chronic symptoms (Table 12–8). Often this will involve a complete history, physical examination, appropriate cultures, and local cystoscopy. In the absence of microhematuria the value of cytology is questionable (Duldulao et al, 1997) but something we still consider important, especially if bladder carcinoma in-situ is a serious possibility, as in patients older than 40 and those with a smoking history. The report of a large series of BPS/IC patients indicating that 1% actually had transitional cell carcinoma and that four of the six cancer patients did not have microhematuria, provides evidence for the justification of local cystoscopic examination (Tissot et al, 2004).
Table 12–8 International Consultation on Incontinence 2009: Diagnosis of Bladder Pain Syndrome
| History |
| A general thorough medical history should emphasize: |
| Physical Examination |
| The physical examination should emphasize: |
| Laboratory Testing |
| Symptom Evaluation |
| Urodynamics (optional) |
| Cystoscopy with or without hydrodistention under anesthesia (optional) |
| Bladder biopsy (optional) |
From Hanno P, Lin AT, Nordling J, et al. Bladder pain syndrome. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Paris: Health Publication Ltd.; 2009. p. 1459–518.
It must be recognized that one may sacrifice a certain level of confidence in the diagnosis without the supporting evidence that can be furnished by additional studies. In a long-term illness such as IC, many patients and physicians ultimately want to base a diagnosis and treatment plan on the most complete data set possible (Rovner and Wein, 1999). A more thorough evaluation for IC would also include a urodynamic evaluation and cystoscopy under anesthesia with hydrodistention of the bladder (Hanno et al, 1990; Hanno, 1994b). Bladder biopsy is indicated only if necessary to rule out other disorders that might be suggested by the cystoscopic appearance. Cystoscopy under anesthesia with bladder distention has been important in the identification of a Hunner lesion. Experimental data suggest that measurement of increased nitric oxide levels in the bladder can also accurately identify those with ulcerative disease (Logadottir et al, 2004). The diagnosis is generally subject to more rigorous testing in Europe (Fall et al, 2008) than in North America, where symptoms in the absence of other obvious causes seems to be the gold standard (Nordling 2004; Nordling et al, 2004; Hanno et al, 2005a). Japanese guidelines are described in Table 12–9.
Table 12–9 Recommended Tests for Diagnosis of Interstitial Cystitis
| MANDATORY | RECOMMENDED | OPTIONAL |
|---|---|---|
| Clinical history | Urine culture | Ultrasonography |
| Physical examination | Urine cytology | Urodynamic study |
| Urinalysis | Symptom scoresQuality of life scores | Radiographic examination |
| Frequency-volume chart | Potassium testBiopsy | |
| Residual urine measurement | ||
| Prostate specific antigen | ||
| Cystoscopy | ||
| Hydrodistention |
From Homma Y, Ueda T, Ito T, et al. Japanese guideline for diagnosis and treatment of interstitial cystitis. Int J Urol 2009; 16:4–16. ©Japanese Urological Association.
Although sensations reported during cystometric bladder filling are subjective, they have a normal pattern and may be helpful in distinguishing a bladder pathologic process (Wyndaele, 1998). Many dispute the need for urodynamic study (Cameron and Gajewski, 2009), but Siroky (1994) and Kim and colleagues (2009b) argue that not only can it help to assess bladder compliance and sensation and reproduce the patient’s symptoms during bladder filling but also it can help to rule out detrusor overactivity. Women with pain on filling can be indistinguishable from those with detrusor overactivity in their perception of bladder fullness (Creighton et al, 1991). One should be wary of diagnosing IC in patients with discrete, involuntary bladder contractions whose symptoms respond to antimuscarinic therapy. The two problems coexist in 15% to 19% of patients (Gajewski and Awad, 1997; Kirkemo et al, 1997), but the pathophysiology is possibly very different. Patients who respond to anticholinergic medication tend not to respond to standard therapy for IC (Perez-Marrero et al, 1987). If involuntary contractions are noted and the patient’s symptoms of frequency and pain continue despite treatment for overactive bladder, one is on firmer ground in considering a diagnosis of IC. Complex cases may benefit from full video-urodynamic studies (Carlson et al, 2001).
Cystometry in conscious IC patients generally demonstrates normal function, the exception being decreased bladder capacity and hypersensitivity. Pain on bladder filling that reproduces the patient’s symptoms is very suggestive of IC. Bladder compliance in patients with IC is normal, because hypersensitivity would prevent the bladder from filling to the point of noncompliance (Siroky, 1994; Rovner and Wein, 1999). The possible addition of including a second cystometrogram after instillation of intravesical lidocaine to help to determine if pain is bladder related is a provocative one worth further study (Teichman et al, 1997). It is not uncommon to find evidence of outlet obstruction in BPS/IC, presumably related to associated pelvic floor dysfunction (Cameron and Gajewski, 2009).
Long before it was considered a diagnostic tool, cystoscopy with hydrodistention of the bladder was used as a therapeutic modality for IC (Bumpus, 1930). When done under anesthesia this procedure allows for sufficient distention of the bladder to afford visualization of either glomerulations or Hunner ulcers (Figs. 12-10 and 12-11). After filling to 80 cm H2O for 1 to 2 minutes, the bladder is drained and refilled. The terminal portion of the effluent is often tinged with blood. Reinspection will reveal the pinpoint petechial hemorrhages that develop throughout the bladder after distention and are not usually seen after examination without anesthesia (Nigro and Wein, 1997).
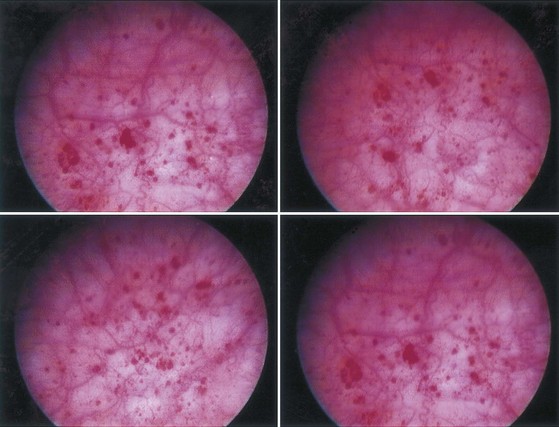
Figure 12–10 Typical appearance of glomerulations after bladder distention in a patient with nonulcerative bladder pain syndrome.
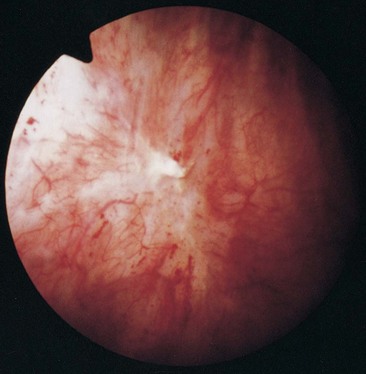
Figure 12–11 Typical appearance of a Hunner lesion in a patient with bladder pain syndrome before bladder distention.
Glomerulations are not specific for IC (Erickson, 1995; Waxman et al, 1998), and only when seen in conjunction with the clinical criteria of pain and frequency can the finding of glomerulations be viewed as potentially significant. Glomerulations can be seen after radiation therapy, in patients with carcinoma, after exposure to toxic chemicals or chemotherapeutic agents, and often in patients on dialysis or after urinary diversion when the bladder has not filled for prolonged periods. They have been reported in the majority of men with prostate pain syndromes, begging the question as to whether CPPS in men is closely linked with IC (Berger et al, 1998). They are observed in 20% of men undergoing transurethral prostatectomy for lower urinary tract symptoms (Furuya et al, 2007). We have speculated that they may simply reflect the response of the bladder to distention after a prolonged period of chronic underfilling because of sensory urgency rather than result from a primary pathologic process. Although the presence of a Hunner ulcer has been associated with pain and urinary urgency, neither the findings of bloody irrigating fluid nor glomerulations are strongly associated with any particular symptom in patients in the IC database (Messing et al, 1997).
Further confusion arises when the patient demonstrates the symptoms of IC but the cystoscopic findings under anesthesia are completely normal. This occurred in 8.7% of patients undergoing cystoscopy with hydrodistention of the bladder entered into the IC database (Messing et al, 1997). Awad and colleagues recognized this entity soon after the NIDDK research criteria had been described. They reported on a series of patients in whom the symptomatology, urodynamic evaluation, histology, and response to therapy were identical to that of IC and in whom findings on cystoscopy with hydrodistention of the bladder were normal. It was termed idiopathic reduced bladder storage (Awad et al, 1992). Clinical, urodynamic, and cystoscopic data strongly suggest that the presence of glomerulations is not selecting out a meaningful difference in patients with symptoms of BPS/IC (Al Hadithi et al, 2002). The presence of glomerulations on cystoscopy under anesthesia meeting the NIDDK criteria may identify a group of patients with worse daytime frequency and nocturia and lower bladder capacity under anesthesia but does not have any relationship to biopsy findings, bladder pain, or urgency (Erickson et al, 2005).
The Search for a Marker
What is the value of a “diagnostic test” in what is essentially a clinical syndrome defined by a symptom-complex? If a patient has chronic pain associated with the bladder usually accompanied by urinary frequency with no discernible cause, BPS/IC is diagnosed. In essence, once well-characterized pathologic entities are ruled out, the patient makes the diagnosis by relating symptoms; much like a patient with impotence makes that diagnosis. Testing for impotence may give clues as to etiology but impotence cannot be ruled out in a patient who cannot function sexually by doing a test!
This is not to say that establishment of a valid diagnostic marker would not be a major advance in our understanding of IC. Just as with phenotyping, it will be important largely to the extent that it can predict prognosis in a given group of patients, predict response to therapy in a given group of patients, and/or distinguish between BPS/IC and another possible cause of the symptom-complex that has been diagnosed. Ultimately, marker identification may enable us to stratify patients with the symptom-complex in such a way that treatments will be specific to the specific etiology (disease) the patient has. As various causes are identifiable, the diagnosis of BPS may itself become a rarity, much as what has happened to “acute urethral syndrome” (Stamm et al, 1980).
In just such an effort, numerous investigators have looked at the mast cell as a possible diagnostic marker for IC. The current standard involves detrusor muscle biopsy specimens examined with tryptase staining of 3-µm thick sections, with every seventh section used for quantification (Larsen et al, 2008). The finding of 27 mast cells/mm3 is considered indicative of mastocytosis. The results in the past have been very contradictory; and at this time, in terms of the use of mast cell criteria in diagnosis, the issue remains moot (Kastrup et al, 1983; Feltis et al, 1987; Holm-Bentzen et al, 1987c; Lynes et al, 1987; Hanno et al, 1990; Christmas and Rode, 1991; Moore et al, 1992; Dundore et al, 1996; Hofmeister et al, 1997). Methylhistamine, a histamine metabolite found in the urine and thought to reflect mast cell activation was not associated with symptom scores, response to bladder distention, cystoscopic findings, or bladder biopsy features, including mast cell determination by tryptase staining (Erickson et al, 2004).
Attempts have been made to look at other markers (Erickson, 2001), including eosinophil cationic protein (Lose et al, 1987), GAG excretion (Hurst et al, 1993), and urinary histamine and methylhistamine (El Mansoury et al, 1994). Proposals for measuring smooth muscle isoactin expression (Rivas et al,1997) and urinary levels of neurotrophin-3, nerve growth factor, glial cell line–derived neurotrophic factor, and tryptase (Okragly et al, 1999) have been suggested. Low levels of GP51, a urinary glycoprotein with a molecular weight of 5 kD have been documented in IC patients compared with normal controls and patients with other urinary tract diseases (Byrne et al, 1999). Cell cultures (Elgavish et al, 1997) have been proposed as a screening technique.
The measurement of elevated nitric oxide levels in air instilled and incubated in the bladder has been proposed for office screening (Lundberg et al, 1996; Ehren et al, 1999). Increased levels of endogenously formed nitric oxide in patients with IC correspond to increased iNOS mRNA expression and protein levels in these patients. Furthermore, iNOS was found to be localized to the urothelium but it was also found in macrophages in the bladder mucosa (Koskela et al, 2008). The simple technique allows for discrimination of ulcer from nonulcer disease (Logadottir et al, 2004) and may provide an objective measure of treatment response (Hosseini et al, 2004).
The urine APF identified by Keay and colleagues (see earlier) may prove to be an accurate marker of BPS/IC if it can be confirmed by other centers and become a biochemical rather than biologic assay. It appears to have the highest sensitivity and specificity of the variety of possible markers tested and fits nicely into an etiologic schema (Keay et al, 2001; Erickson et al, 2002). It has also been shown to differentiate men with PBS/IC symptoms from controls and differentiate men with bladder-associated pain and irritative voiding symptoms from those with pelvic/perineal pain alone and other nonspecific findings compatible with the CPPS in men (CPPS III), previously referred to as “nonbacterial prostatitis” (Keay et al, 2004a). This question as to whether CPPS and BPS/IC are two different disorders will doubtless be the subject of future research and is an integral question that the NIDDK is hoping to answer with current research (see www.mappnetwork.org).
Much work in markers is ongoing. Uroplakin III-delta 4 is a potential marker for identifying nonulcerative IC (Zeng et al, 2007). Most recently, the feasibility of diagnosing IC in humans and domestic cats from the spectra of dried serum films (DSFs) using infrared microspectroscopy has been reported (Rubio-Diaz et al, 2009).
Potassium Chloride Test
Parsons has championed an intravesical KCl challenge, comparing the sensory nerve provocative ability of sodium versus potassium using a 0.4-M KCl solution. Pain and provocation of symptoms constitutes a positive test. Whether the results indicate abnormal epithelial permeability in the subgroup of positive patients or hypersensitivity of the sensory nerves is unclear. Normal bladder epithelium can never be absolutely tight, and there is always some leak, however small (Hohlbrugger, 1997). The concentration of potassium used is 400 mEq/L, far exceeding the physiologic urinary concentrations of 20 to 80 mEq/L depending on dietary intake (Vander, 1995). Healthy controls can distinguish KCl from sodium chloride, although they do not experience severe pain (Roberto et al, 1997). The hope is that this test may stratify patients into those who will respond to certain treatments (perhaps those designed to fortify the GAG layer), but to date this information is lacking (Teichman and Nielsen-Omeis, 1999).
Used as a diagnostic test for IC, the KCl test is not valid (Chambers et al, 1999). The gold standard in defining BPS/IC for research purposes has been the NIDDK criteria. These criteria are recognized to constitute a set of patients that virtually all researchers can agree have PBS/IC, although they are far too restrictive to be used in clinical practice (Hanno et al, 1999b). Thus, this group of patients should virtually all be positive if the KCl test is to have the sensitivity needed to aid in diagnosis. Up to 25% of patients meeting the NIDDK criteria will have a negative KCl test (Parsons et al, 1998). In the group it should perform the best in it is lacking in sensitivity.
When we look at the specificity side of the equation, in the universe of unselected persons, recent studies reported a 36% false-positive rate in asymptomatic men (Yilmaz et al, 2004) and a 33% positive rate in a fixed population of Turkish textile workers (Sahinkanat et al, 2008). In the patient population with confounding conditions for which we would want help in sorting out BPS/IC from other disorders, 25% of patients with overactive bladder test positive and virtually all patients with irritative symptoms from radiation cystitis and urinary tract infection test positive (Parsons et al, 1994b, 1998). The results with CP/CPPS in men are variable, but 50% to 84% of men have been reported to test positive (Parsons and Albo, 2002; Yilmaz et al, 2004; Parsons et al, 2005). In women with pelvic pain the results are similar (Parsons et al, 2002b); and based on these findings, Parsons and colleagues (2002a) have expressed the view that BPS/IC may affect over 20% of the female population of the United States. Another way to interpret the findings would be that the KCl test is very nonspecific, missing a significant number of BPS/IC patients and overdiagnosing much of the population.
Prospective and retrospective studies looking at the KCl test for diagnosis in patients presenting with symptoms of BPS/IC have found no benefit of the test in comparison with standard techniques of diagnosis (Chambers et al, 1999; Gregoire et al, 2002; Kuo, 2003), nor is it useful in monitoring results of treatment (Sairanen et al, 2007). The development of a painless modification of the KCl test (Daha et al, 2003) using cystometric capacity and a 0.2-M solution may improve acceptability among patients, but further research is needed to determine what place, if any, this test will have in the diagnostic or treatment algorithm.
Confusable Diseases (Differential Diagnosis)
The diagnosis of BPS can be made on the basis of exclusion of confusable diseases and confirmed by the recognition of the presence of the specific combination of symptoms and signs of BPS. If the main urinary symptoms are not explained by a single diagnosis, the presence of a second diagnosis is possible. One must remember that BPS may occur together with confusable diseases such as chronic or remitting urinary infections or endometriosis. Table 12–10 summarizes confusable diseases related to BPS and their mode of exclusion based on aforementioned diagnostic proposals and procedures as outlined by the ESSIC group (van de Merwe et al, 2008).
Table 12–10 Confusable Diseases for Bladder Pain Syndrome
| CONFUSABLE DISEASE | EXCLUDED OR DIAGNOSED BY |
|---|---|
| Carcinoma and carcinoma in situ | Cystoscopy and biopsy |
| Infection with: | |
| Common intestinal bacteria | Routine bacterial culture |
| Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Mycoplasma genitalium, Corynebacterium urealyticum, Candida species, Mycobacterium tuberculosis | Special cultures Dipstick; if “sterile” pyuria culture for M. tuberculosis |
| Herpes simplex and human papillomavirus | Physical examination |
| Radiation | Medical history |
| Chemotherapy, including immunotherapy with cyclophosphamide | Medical history |
| Anti-inflammatory therapy with tiaprofenic acid | Medical history |
| Bladder neck obstruction and neurogenic outlet obstruction | Uroflowmetry and ultrasonography |
| Bladder stone | Imaging or cystoscopy |
| Lower ureteral stone | Medical history and/or hematuria; upper urinary tract imaging (CT or IVP) |
| Urethral diverticulum | Medical history and physical examination |
| Urogenital prolapse | Medical history and physical examination |
| Endometriosis | Medical history and physical examination |
| Vaginal candidiasis | Medical history and physical examination |
| Cervical, uterine, and ovarian cancer | Physical examination |
| Incomplete bladder emptying (retention) | Postvoid residual urine volume measured by ultrasound evaluation |
| Overactive bladder | Medical history and urodynamics |
| Prostate cancer | Physical examination and PSA test |
| Benign prostatic obstruction | Uroflowmetry and pressure-flow studies |
| Chronic bacterial prostatitis | Medical history, physical examination, culture |
| Chronic nonbacterial prostatitis | Medical history, physical examination, culture |
| Pudendal nerve entrapment | Medical history, physical examination; nerve block may prove diagnosis |
| Pelvic floor muscle–related pain | Medical history, physical examination |
CT, computed tomography; IVP, intravenous pyelogram; PSA, prostate-specific antigen.
From van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 2008;53:60–7.
Key Points
Diagnosis
Classification
IC was originally described as bladder disease with severe inflammation of the bladder wall described by Hunner (1915) as an ulcer. The lesion is, however, not an ulcer but a vulnus (weakness, vulnerability) that can ulcerate on distention, and the name of the bladder lesion has consequently been changed to Hunner lesion (van de Merwe et al, 2008). The finding of a Hunner lesion could therefore originally be regarded as a diagnostic criterion for IC. Messing and Stamey introduced glomerulations as another typical finding for IC, and this was included in the NIDDK criteria (Wein et al, 1990). Fall and associates (1987) proposed that patients with Hunner lesion (classic IC) and patients with glomerulations (nonulcerative type) represented two different subtypes with different clinical pictures, different outcomes, and different responses to treatment (Peeker and Fall, 2002), meaning that patients fulfilling the NIDDK criteria represent at least two different patient populations. Moreover, up to 60% of patients clinically believed to have BPS by experienced clinicians do not fulfill the NIDDK criteria (Hanno et al, 1999b) and whether these patients are comparable to the patients fulfilling the NIDDK criteria is unknown. Finally, Japanese urologists consider that “interstitial cystitis” should be preserved as a disease name reserved for patients with urinary symptoms and cystoscopic findings of glomerulations or Hunner lesion as outlined in the NIDDK criteria (Homma, 2008).
In an attempt to unite these different philosophies into a coherent schema, the ESSIC proposed a classification of BPS based on findings during cystoscopy with hydrodistention and morphologic findings in bladder biopsy specimens (van de Merwe et al, 2008) (see Table 12–4). The classification includes groups not having had cystoscopy with hydrodistention (group X) as well as groups not having had morphologic investigation of bladder biopsy specimens (group XX). By using this classification future research will be able to identify if findings of glomerulations and/or Hunner lesion as well as morphologic changes in bladder biopsy specimens do have significant importance for disease prognosis and/or treatment outcome.
Treatment
Conservative Therapies
Once the diagnosis has been made one must decide whether to institute therapy or employ a policy of conservative “watchful waiting.” If the patient has not had an empirical course of antibiotics for his or her symptoms by the time the PBS/IC diagnosis is made, such a trial is reasonable. Doxycycline has been reported efficacious in a Swiss study (Burkhard et al, 2004). Further attempts to alleviate symptoms with antibiotics are unlikely to be worthwhile and are not recommended in the absence of positive cultures. Stress reduction, exercise, warm tub baths, and efforts by the patient to maintain a normal lifestyle all contribute to overall quality of life (Whitmore, 1994). In a controlled study of 45 PBS/IC patients and 31 healthy controls, higher levels of stress were related to greater pain and urgency in patients with IC but not in the control group (Rothrock et al, 2001). Maladaptive strategies for coping with stress may impact adversely on symptoms (Rothrock et al, 2003). Biofeedback, soft tissue massage, and other physical therapies may aid in muscle relaxation of the pelvic floor (Mendelowitz and Moldwin, 1997; Meadows, 1999; Holzberg et al, 2001; Lukban et al, 2001; Markwell, 2001). This is a reasonable intervention given the association of pelvic floor dysfunction and BPS/IC (Peters et al, 2007a), and an NIDDK trial is now in its final phases and should provide further information on efficacy of physical therapy. A preliminary NIDDK trial demonstrated the feasibility of such a study and strongly suggested the efficacy of physical therapy when compared with global therapeutic massage (National Institutes of Health, 2008a, 2008b; FitzGerald et al, 2009).
Mendelowitz and Moldwin (1997) had a 69% success rate in 16 patients treated with electromyographic biofeedback, but treatment response did not correlate to changes in muscle identification and the placebo effect may have been considerable. Acupuncture has been used for PBS/IC and many other chronic pain syndromes. There is limited evidence that it is more effective than nontreatment for chronic pain, and inconclusive evidence that acupuncture is more effective than placebo, sham acupuncture, or standard care (Ezzo et al, 2000). In general, results with acupuncture for PBS/IC have been disappointing (Geirsson et al, 1993).
Diet
Elaborate dietary restrictions are unsupported by any literature, but many patients do find their symptoms are adversely affected by specific foods and would do well to avoid them (Koziol et al, 1993; Koziol, 1994). Often this includes caffeine, alcohol, artificial sweeteners, hot pepper, and beverages that might acidify the urine such as cranberry juice (Shorter et al, 2007). Anecdotal association of IC with many foods has spawned the recommendation of various “IC diets” with little in the way of objective, scientific basis (Table 12–11). The only placebo-controlled dietary study, although small, failed to demonstrate a relationship between diet and symptoms (Fisher et al, 1993). Bade and colleagues found that IC patients tend to have a healthier diet than the general population but could discern no rationale for dietary or fluid intake change other than decreasing caffeine intake (Bade et al, 1997b). Nguan and coworkers (2005) performed a prospective, double-blind, crossover study consisting of crossover instillations of urine at physiologic pH (5.0) and neutral buffered pH (7.5). There was no statistically significant difference in subjective pain scores, suggesting that adjusting urine pH with diet or dietary supplements may have little influence on symptomatology. Orange and grapefruit juices, rich in potassium and citrate, tend to increase urinary pH (Wabner and Pak, 1993) but are avoided by many IC patients based on “IC diet” recommendations. Urinary alkalinization may be worth trying, but supporting studies are lacking. Some patients have had benefit with calcium glycerophosphate, an over-the-counter food acid–reducing agent (Hill et al, 2008), but supporting controlled trials are lacking.
Table 12–11 Interstitial Cystitis Association Recommendations of Foods to Avoid
Modified from Interstitial Cystitis Association. IC/PBS food list. <www.ichelp.org/Document.Doc?id=7>; 2009 [accessed 15.05.11].
A large NIH study in which patients with newly diagnosed BPS/IC were treated with a focus on four targeted areas: (1) how to control or manage their symptoms, (2) controlling fluid intake, (3) changing diet which may improve symptoms, and (4) bladder training/urge suppression. A behavioral approach to stress and pain management was also used to help patients learn skills to reduce stress in their lives. Forty-five percent of 135 patients randomized to this approach without additional medication were moderately or markedly improved at the 12-week end point (Foster et al, 2010).
Unfortunately, education and self-help are often not sufficient, and most patients will require one or more of a variety of therapies.
Oral Therapies
Some oral medications that have been used for treatment of BPS are listed in Table 12–12 and discussed in the following sections.
Table 12–12 Some Oral Medications That Have Been Used for Treatment of BPS: Oxford System Recommendations
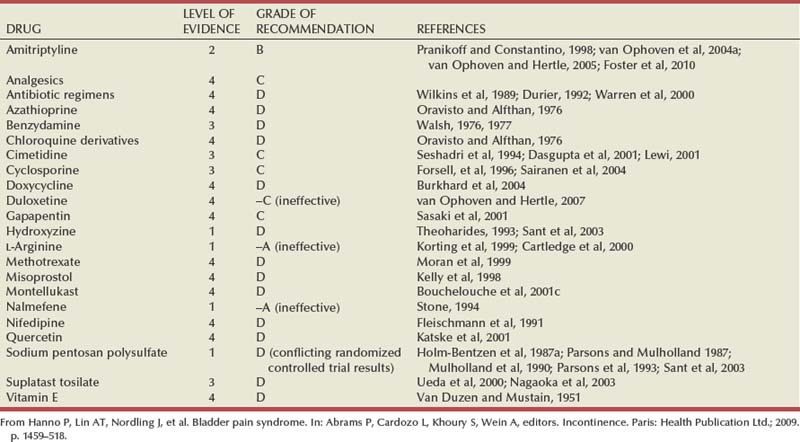
Amitriptyline
Amitriptyline, a tricyclic antidepressant, has become a staple of oral treatment for BPS/IC. The tricyclic antidepressants possess varying degrees of at least three major pharmacologic actions: (1) they have central and peripheral anticholinergic actions at some but not all sites, (2) they block the active transport system in the presynaptic nerve ending that is responsible for the reuptake of the released amine neurotransmitters serotonin and norepinephrine, and (3) they are sedatives, an action that occurs presumably on a central basis but perhaps is related to their antihistaminic properties. Amitriptyline, in fact, is one of the most potent tricyclic antidepressants in terms of blocking H1-histaminergic receptors (Baldessarini, 1985). There is also evidence that they desensitize α2-adrenergic receptors on central noradrenergic neurons. Paradoxically they also have been shown to block α-adrenergic receptors and serotonin receptors. Theoretically tricyclic agents have actions that might tend to stimulate predominantly β-adrenergic receptors in bladder body smooth musculature, an action that would further facilitate urine storage by decreasing the excitability of smooth muscle in that area (Barrett and Wein, 1987).
Hanno and Wein (1987) first reported a therapeutic response in IC after noting a “serendipitous” response to amitriptyline in one of their patients concurrently being treated for depression. The following year a similar report appeared relating a response to desipramine hydrochloride (Renshaw, 1988). Reasoning that a drug used successfully at relatively low dosages for many types of chronic pain syndromes, which would also have anticholinergic properties, β-adrenergic bladder effects, sedative characteristics, and strong H1-antihistaminic activity would seem to be ideal for IC, Hanno and associates (1989) performed the first clinical trial with promising results. A subsequent follow-up study (Hanno, 1994a) reported that in 28 of 43 patients who could tolerate therapy for at least a 3-week trial at a dosage of 25 mg at bedtime gradually increasing to 75 mg at bedtime over 2 weeks, 18 had total remission of symptoms with a mean follow-up of 14.4 months, 5 dropped out because of side effects, and 5 derived no clinical benefit. Benefits were apparent within 4 weeks. All patients had failed hydrodistention and intravesical DMSO therapy. Sedation was the main side effect. Kirkemo and colleagues (1990) treated 30 patients and had a 90% subjective improvement rate at 8 weeks. Both studies noted that patients with bladder capacities over 450 to 600 mL under anesthesia seemed to have the best results. Another uncontrolled study of 11 patients with urinary frequency and pelvic pain (Pranikoff and Constantino, 1998) related success in 9 of the patients, with 5 reporting complete resolution of symptoms and 4 significant relief. Two patients could not tolerate the medication. In a 4-month intent-to-treat placebo-controlled double-blind trial of 50 patients, 63% on amitriptyline at doses of 25 to 75 mg (dose as tolerated) at bedtime reported good or excellent satisfaction versus 4% on placebo (van Ophoven et al, 2004a). At 19-month follow-up there was little tachyphylaxis and good response rates were observed in the entire spectrum of BPS/IC symptoms (van Ophoven and Hertle, 2005).
The large, double-blind, randomized controlled trial by the NIDDK comparing education and behavioral modification with and without oral amitriptyline showed a 55% response to the study arm that included both medication and conservative therapy compared with a 45% response to education and behavioral therapy alone (Foster et al, 2010). The difference was not statistically significant. However, if only patients who could tolerate 25 mg or more of medication or placebo are included the success compared with conservative therapy alone was 73% compared with 53% at 12 weeks. Frequently, O’Leary-Sant symptom and problem scores also showed significant improvement. Thus, on an intent-to-treat basis there was not significant benefit from amitriptyline, but in the 62% of patients who could tolerate the relatively low doses of drug the benefits appear substantial. Patients should be cautioned about fatigue, constipation, dry mouth, increased appetite, and dizziness. Slowly titrating the dose on a weekly basis, beginning at 10 mg at bedtime and increasing by 10 mg weekly to a maximum tolerated dose of 50 mg at bedtime seems to minimize side effects.
Amitriptyline has proven analgesic efficacy with a median preferred dose of 50 mg in a range of 25 to 150 mg/day. This range is lower than traditional doses for depression of 150 to 300 mg. The speed of onset of effect is much faster (1 to 7 days) than reported in depression, and the analgesic effect is distinct from any effect on mood (McQuay and Moore, 1997). Tricyclic antidepressants are contraindicated in patients with long QT syndrome or significant conduction system disease (bifascicular or trifascicular block) after recent myocardial infarction (within 6 months), unstable angina, congestive heart failure, frequent premature ventricular contractions, or a history of sustained ventricular arrhythmias. They should be used with caution in patients with orthostatic hypotension (Low and Dotson, 1998). Doses greater than 100 mg are associated with increased relative risk of sudden cardiac death (Ray et al, 2004).
Other Antidepressants
Other tricyclic antidepressants that have been used for BPS. One trial employed the combination of doxepin and piroxicam, a cyclooxygenase-2 inhibitor. Twenty-six of 32 patients (81%) experienced remission of symptoms (Wammack et al, 2002). Another study reported satisfactory outcome with desipramine (Renshaw, 1988). The safety and efficacy of duloxetine, a serotonin and norepinephrine reuptake inhibitor, for BPS/IC was assessed in an observational study with 48 women (van Ophoven and Hertle, 2007). Patients were prospectively treated for 2 months following an uptitration protocol to the target dose of 40 mg duloxetine twice daily. Five patients were identified as responders, and 17 patients dropped out due to side effects, including nausea in all 17 patients. No severe adverse events were reported. In the 5 responders, the 40-mg twice-daily dose was required to see efficacy. Overall, duloxetine did not result in clinically meaningful improvement of symptoms.
Antihistamines
The use of antihistamines goes back to the late 1950s and stems from work by Simmons (1961), who postulated that the local release of histamine may be responsible for, or accompany, the development of, IC. He reported on 6 patients treated with pyribenzamine. The results were far from dramatic, with only half of the patients showing some response. The therapy is notable for this disease in that it was very logically conceived. It has been Theoharides who has spearheaded mast cell research in this field and been a major modern proponent of antihistamine therapy (Theoharides, 1994). He has used the unique piperazine H1-receptor antagonist hydroxyzine, a first-generation antihistamine (Simons 2004), which can block neuronal activation of mast cells (Minogiannis et al, 1998). In 40 patients treated with 25 mg at bedtime increasing over 2 weeks (if sedation was not a problem) to 50 mg at night and 25 mg in the morning, virtually every symptom evaluated improved by 30%. Only 3 patients had absolutely no response. As with many IC drug reports, these responses were evaluated subjectively and without being blinded or placebo controlled. A subsequent study suggested improved efficacy in patients with documented allergies and/or evidence of bladder mast cell activation (Theoharides and Sant 1997a, 1997b). No significant response to hydroxyzine was found in an NIDDK placebo-controlled trial (Sant et al, 2003).
Why an H2 antagonist would be effective is unclear, but uncontrolled studies show improvement of symptoms in two thirds of patients taking cimetidine in divided doses totaling 600 mg (Seshadri et al, 1994; Lewi, 1996). It proved effective in a double-blind, placebo-controlled trial (Thilagarajah et al, 2001), but histologic studies show the bladder mucosa to be unchanged before and after treatment and the mechanism of any efficacy remains unexplained (Dasgupta et al, 2001). Cimetidine is a common treatment in the United Kingdom, where over a third of patients reported having used it (Tincello and Walker, 2005).
Pentosan Polysulfate
Parson’s suggestion that a defect in the epithelial permeability barrier, the GAG layer, contributes to the pathogenesis of IC has led to an attempt to correct such a defect with the synthetic sulfated polysaccharide sodium pentosan polysulfate (PPS), a heparin analogue available in an oral formulation, 3% to 6% of which is excreted into the urine (Barrington and Stephenson, 1997). It is sold under the trade name Elmiron. Studies have been contradictory.
Fritjofsson and coworkers (1987) treated 87 patients in an open multicenter trial in Sweden and Finland. Bladder volume with and without anesthesia was unchanged. Relief of pain was complete in 35% and partial in 23% of patients. Daytime frequency decreased from 16.5 to 13, and nocturia decreased from 4.5 to 3.5. Mean voided volumes increased by almost a tablespoon in the nonulcer group. Holm-Bentzen and associates (1987a) studied 115 patients in a double-blind, placebo-controlled trial. Symptoms, urodynamic parameters, cystoscopic appearance, and mast cell counts were unchanged after 4 months. Bladder capacity under anesthesia increased significantly in the group with mastocytosis, but this had no bearing on symptoms or awake capacity.
Parsons and colleagues (1983) had a more encouraging initial experience, and subsequently the results of two placebo-controlled multicenter trials in the United States were published (Mulholland et al, 1990; Parsons et al, 1993). In the initial study, overall improvement of greater than 25% was reported by 28% of the PPS-treated group versus 13% in the placebo group. In the later study the respective figures were 32% on drug versus 16% on placebo. Average voided volume on PPS increased by 20 mL. No other objective improvements were documented. An NIDDK 2 × 2 factorial study to evaluate PPS and hydroxyzine looked at each drug used alone and in combination and compared results with a placebo group (Sant et al, 2003). Patients were treated for 6 months. No statistically significant response to either medication was documented. No significant trend was seen in the PPS treatment groups (34%) compared with non-PPS groups (18%). Of the 29 patients on PPS alone, 28% had global response (the primary end point) of moderately or markedly improved versus 13% on placebo, a number remarkably similar to the results in the 3-month pivotal trials, although not reaching statistical significance in the 6-month study. A subsequent industry-sponsored trial showed no dose-related efficacy response in the range of 300 to 900 mg/day, but adverse events were dose related (Nickel et al, 2001). Another 6-month trial that compared PPS with cyclosporine yielded a 19% response rate for PPS compared with 75% global response to cyclosporine (Sairanen et al, 2005a).
Long-term experience with PPS is consistent with efficacy in a subset of patients that may drop below 30% of those initially treated (Jepsen et al, 1998). Tachyphylaxis seems to be uncommon in responders. Adverse events with PPS occurred in less than 4% of patients at the dose of 100 mg three times daily (Hanno, 1997) and include reversible alopecia, diarrhea, nausea, and rash. Rare bleeding problems have been reported (Rice et al, 1998). It promotes cellular proliferation in vitro in the MCF-7 breast cancer cell line, and caution has been suggested in prescribing it in groups at high-risk for breast cancer and premenopausal females (Zaslau et al, 2004). A 3- to 6-month treatment trial is generally required to see symptom improvement. In a small trial, PPS has shown efficacy when administered intravesically (Bade et al, 1997a). It may be of value in the management of radiation cystitis (Parsons, 1986; Hampson and Woodhouse, 1994) and cyclophosphamide cystitis (Toren and Norman, 2005), but its value in the treatment of BPS/IC seems marginal.
Immunosuppressant Drugs
Cyclosporine
Cyclosporine, a widely used immunosuppressive drug in organ transplantation, was the subject of a novel BPS trial (Forsell et al, 1996). Eleven patients received cyclosporine for 3 to 6 months at an initial dose of 2.5 to 5 mg/kg/day and a maintenance dose of 1.5 to 3 mg/kg/day. Micturition frequency decreased, and mean and maximum voided volumes increased significantly. Bladder pain decreased or disappeared in 10 patients. After cessation of treatment, symptoms recurred in the majority of patients.
In a longer-term follow-up study, 20 of 23 refractory IC patients on cyclosporine therapy followed for a mean of 60.8 months became free of bladder pain. Bladder capacity more than doubled. Eleven patients subsequently stopped therapy; and in 9 the symptoms recurred within months but responded to reinitiating cyclosporine (Sairanen et al, 2004). Sairanen and colleagues (2005b) further found that cyclosporine was far superior to sodium PPS in all clinical outcome parameters measured at 6 months. Patients who responded to cyclosporine had a significant reduction of urinary levels of EGF (Sairanen et al, 2008).
Suplatast Tosylate
Suplatast tosylate is an immunoregulator that selectively suppresses IgE production and eosinophilia via suppression of helper T cells that produce interleukin-4 and interleukin-5. It is used in Japan to treat allergic disorders, including asthma, atopic dermatitis, and rhinitis. Ueda and colleagues (2000) reported a small study in 14 women with IC. Treatment for 1 year resulted in a significantly increased bladder capacity and decreased urinary urgency, frequency, and lower abdominal pain in 10 women. Concomitant changes occurred in blood and urine markers, suggesting an immune system response. Larger, multicenter, randomized controlled trials in the United States and Japan have not led to the governmental approval of the BPS/IC indication or the introduction of the drug into the United States.
Azathioprine and Chloroquine Derivatives
In a single report in 1976, Oravisto and Alfthan (1976) used azathioprine or chloroquine derivatives for BPS patients not responding to other treatments. About 50% patients responded.
Mycophenolate Mofetil
In an aborted multicenter randomized placebo-controlled NIDDK trial, mycophenolate mofetil (Cellcept 1 to 2 g/day in divided doses) failed to show efficacy in the treatment of symptoms of refractory BPS/IC (National Institutes of Health, 2007). The trial, which included 59 patients randomized 2 : 1 to the active arm, was halted when the U.S Food and Drug Administration issued a new black box warning for the drug (miscarriage and congenital malformations have been associated with its use), and an interim analysis showed no benefit.
Miscellaneous Agents
L-Arginine
Foster and colleagues (1997) were the original proponents of L-arginine in the therapy of IC. Eight patients with IC were given 500 mg of L-arginine three times daily. After 1 month, urinary NOS activity increased eightfold and 7 of the 8 patients noticed improvement in symptoms. An open-label study of 11 patients showed improvement in all 10 of the patients who remained on L-arginine for 6 months (Smith et al, 1997).
An open-label study of 9 women in Sweden failed to find any change in symptom scores or in nitric oxide production in the bladder (Ehren et al, 1998). A placebo-controlled randomized trial of 53 BPS/IC patients could find no difference on an intent-to-treat analysis between drug and placebo-treated patients (Korting et al, 1999). A smaller randomized placebo-controlled crossover trial of 16 BPS patients found no clinically significant improvement with L-arginine and concluded that it could not be recommended for IC treatment (Cartledge et al, 2000).
The body of evidence does not support the use of L-arginine for the relief of symptoms of IC.
Quercitin
Quercetin, a bioflavonoid available in many over-the-counter products, may have the anti-inflammatory effects of other members of this class of compounds found in fruits, vegetables, and some spices. Katske and coworkers (2001) administered 500 mg twice daily to 22 BPS patients for 4 weeks. All but 1 patient had some improvement in the O’Leary-Sant symptom and problem scores as well as in a global assessment score. Further studies are necessary to determine efficacy.
Antibiotics
Warren and associates (2000) randomized 50 patients to receive 18 weeks of placebo or antibiotics including rifampin plus a sequence of doxycycline, erythromycin, metronidazole, clindamycin, amoxicillin, and ciprofloxacin for 3 weeks each. Intent-to-treat analysis demonstrated that 12 of 25 patients in the antibiotic and 6 of 25 patients in the placebo group reported overall improvement while 10 and 5, respectively, noticed improvement in pain and urgency. The study was complicated by the fact that 16 of the patients in the antibiotic group underwent new BPS therapy during the study as did 13 of the placebo patients. There was no statistical significance reached. What was statistically significant were adverse events in 80% of participants who received antibiotic compared with 40% in the placebo group. Nausea and/or vomiting and diarrhea were the predominant side effects. Most patients on antibiotics correctly guessed what treatment arm they were in, and those who guessed correctly were significantly more likely to note improvement after the study. No duration in improvement after completion of the trial of antibiotics was reported.
Burkhard and colleagues (2004) recorded a 71% success in 103 women presenting with a history of urinary urgency and frequency and chronic urethral and/or pelvic pain often associated with dyspareunia and/or a history of recurrent urinary tract infection. This was a large, inclusive group and one that is probably broader than the BPS we are focusing on. Nevertheless, they recommended empirical doxycycline in this group. The overwhelming majority of BPS patients have been treated with empirical antibiotics before diagnosis.
At this time there is no evidence to suggest that antibiotics have a place in the therapy for BPS in the absence of a culture-documented infection (Maskell, 1995). Nevertheless, it would not be unreasonable to treat patients with one empirical course of antibiotic if they have never been on an antibiotic for their urinary symptoms.
Methotrexate
Low-dose oral methotrexate significantly improved bladder pain in 4 of 9 women with BPS but did not change urinary frequency, maximum voided volume, or mean voided volume (Moran et al, 1999). No placebo-controlled randomized trial has been done with this agent.
Montelukast
Mast cell triggering releases two types of proinflammatory mediators, including granule stored preformed types such as heparin and histamine and newly synthesized prostaglandins and leukotriene B4 and C4. Classic antagonists, such as montelukast, zafirlukast, and pranlukast, block cysteinyl leukotriene-1 receptors. In a pilot study (Bouchelouche et al, 2001b), 10 women with IC and detrusor mastocytosis received 10 mg of montelukast daily for 3 months. Frequency, nocturia, and pain improved dramatically in 8 of the patients. Further study would seem to be warranted, especially in patients with detrusor mastocytosis, which is defined as more than 28/mm2.
Nifedipine
The calcium channel antagonist nifedipine inhibits smooth muscle contraction and cell-mediated immunity. In a pilot study (Fleischmann, 1994), 30 mg of an extended-release preparation was administered to 10 female patients and titrated to 60 mg daily in 4 of the patients who did not get symptom relief. Within 4 months 5 patients showed at least a 50% decrease in symptom scores and 3 of the 5 were asymptomatic. No further studies have been reported.
Misoprostol
The oral prostaglandin analogue misoprostol was studied in 25 patients at a dose of 600 µg/day (Kelly et al, 1998). At 3 months 14 patients were significantly improved, and at 6 months 12 patients still had a response. A cytoprotective action in the urinary bladder was postulated.