chapter 21 Male Infertility
Introduction: Definition and Demographics of Infertility
Over the past 50 years, we have witnessed dramatic advances in the understanding and treatment of male fertility. The introduction of intracytoplasmic sperm injection (ICSI) in 1992 (Palermo et al, 1992), a technique of in-vitro fertilization using direct insertion of a single sperm into an egg, offered the ability to bypass even some of the most severe etiologies of male subfertility but raised a variety of cost and safety issues. Growing understanding of the genetics of fertility, environmental influences on gonadocytes, and the endocrine basis for germ cell development holds promise to allow more targeted diagnostic and therapeutic interventions. Unlike many other disease states, fertility represents a complex interaction between two individuals involving multiple organ systems. Attempts to isolate pathology to one gender are confounded by the fact that male fertility is not a clearly quantifiable parameter but is dependent on requirements of the individual female reproductive system. Older studies have related 20% of cases of infertility to purely male factor etiology, while an additional 30% to 40% involve both male and female factor pathology (Simmons, 1956). Newer studies have shown little change in this distribution with more than 50% attributable to male factor, despite advances in the diagnosis and management of infertility (Mosher and Pratt, 1991; Thonneau et al, 1991).
Knowledge of the inherent inefficiency of normal human reproduction is critical before we can define infertility. Studies of conception in normal couples reveal that 60% to 75% will conceive within 6 months of unprotected intercourse and 90% by 1 year (Tietze et al, 1950; Spira, 1986). On the basis of this, the classic definition of infertility became the absence of conception after 12 months of regular, unprotected intercourse, a definition supported by the Practice Committee of the American Society for Reproductive Medicine (ASRM). Because a small number of normal couples will conceive between 1 and 2 years, the World Health Organization (WHO) recommends 24 months of unprotected intercourse as the preferred definition of infertility (Rowe, 1993). Geographically diverse population-based studies have reported a remarkably consistent 15% to 20% incidence of infertility (WHO, 1991; Gunnell and Ewings, 1994; Philippov et al, 1998). Although the classic definition of infertility would suggest deferring medical assessment until 12 months of unprotected intercourse, we support performance of a basic, cost-effective evaluation of both partners at the time of presentation for evaluation. Current recommendations by the Practice Committees of the American Urological Association and the American Society for Reproductive Medicine (Male Infertility Best Practice Committee Report, 2006a, 2006b) recommend infertility evaluation before 1 year if (1) male infertility risk factors such as a history of bilateral cryptorchidism are known to be present, (2) female infertility risk factors including advanced female age (older than 35 years) are suspected, or (3) the couple questions the male partner’s fertility potential. A timely but limited evaluation provides early identification and correction of factors that may reduce fertility, as well as reassurance in an emotionally difficult situation for couples. Alleviation of anxiety related to infertility may in itself provide therapeutic value.
Perhaps more than in many other medical assessments, evaluation of infertility requires a methodic approach involving a comprehensive medical history, review of systems, targeted physical examination, and basic laboratory tests. An effective initial approach should be rapid, cost-effective, and noninvasive. Concurrent basic evaluation of the female partner is prudent given the multifactorial etiology of infertility, as well as the potential need for assisted reproductive technologies (ARTs) to treat the male factor. Initial male factor evaluation may suggest the need for more advanced semen, genetic, endocrine, or radiologic tests to arrive at the correct diagnostic and treatment plan.
Whenever possible, treatment should involve correction of a specific problem rather than blanket application of costly assisted reproductive technologies. ART use for male factor infertility in the United States has been estimated to cost almost $18 billion dollars in 1 year alone (Meacham et al, 2007), underscoring the need for addressing the specific cause of male factor infertility. More importantly, ART application without attention to the male factor may mask potentially significant and even life-threatening conditions present in the infertile male, conditions that may occur in up to 1.3% of men and that would only have been diagnosed with a complete medical evaluation (Kolettis and Sabenegh, 2001). However, ART retains an important role in the management of male factor infertility, especially where no etiology for infertility can be identified or in the setting of noncorrectable causes. In addition to assisted reproduction, donor sperm insemination and adoption remain excellent options for the management of noncorrectible infertility.
History and Review of Systems
Successful diagnosis and treatment of infertility requires careful attention to obtaining a thorough history. A diverse variety of specific factors can affect subsequent fertility or sexual function (Table 21–1). Although focus may be on long-term factors that can affect fertility, human spermatogenesis is estimated to involve a 64-day cycle with an additional 5 to 10 days of epididymal transit time on the basis of radioisotope labeling studies (Clermont and Heller, 1963; Franca et al, 2005; Misell et al, 2006). Factors such as fever, illness, or drug use in the several months before semen testing should prompt repeat testing after an additional 3 months to rule out transient detrimental effects.
Table 21–1 Pertinent History in Evaluation of the Infertile Male
Reproductive history is of particular importance in the initial evaluation. Details regarding any prior conceptions the patient may have caused with his present or prior partner, duration of infertility, method of reproductive timing, and prior contraceptive history should be obtained. Primary infertility is defined as the failure to conceive at any time in the past with any prior partner, whereas secondary infertility indicates a prior conception with the current or previous partner. This simple classification can be helpful in narrowing the differential diagnoses because those with secondary infertility are presumed to have normal embryologic development of their reproductive tract and genetic complement.
The timing and frequency of intercourse are an important component of the reproductive history. In recent years, the ease and availability of ovulation predictor kits, which measure midcycle urinary luteinizing hormone (LH) surge as a predictor of impending ovulation, have allowed couples to approach reproductive timing in a more informed and effective fashion. However, many couples are not aware of the viability of spermatozoa within the female reproductive tract with sperm surviving between 2 and 5 days in favorable cervical mucus (Wilcox, 1995). This finding is the basis for the widely offered recommendation of intercourse frequency every 2 days near the time of ovulation, maximizing the chance that viable sperm are available to the oocyte (Tur-Kaspa et al, 1994). Intercourse that is too frequent does not allow replenishment of adequate numbers of spermatozoa within the epididymis, whereas infrequent intercourse may miss the potential window for fertilization. An assessment of erectile and ejaculatory function is also germane to the initial evaluation.
Use of vaginal lubricants is commonplace in reproductive-aged couples with almost one half of couples reporting intermittent use (Oberg et al, 2004). A number of commercially available lubricants that are not marketed as spermicidal agents have been shown to adversely affect sperm motility (Miller, 1994; Kutteh et al, 1996; Anderson et al, 1998) and sperm deoxyribonucleic acid (DNA) integrity (Agarwal et al, 2008). Although some lubricants such as vegetable oil, raw egg white, and Pre-Seed have minimal spermicidal effect (Goldenberg and White, 1975; Edvinsson et al, 1983; Agarwal et al, 2008), it remains optimal to avoid lubricant use if possible and to use minimal concentrations of the least toxic lubricant available, if required.
A variety of pediatric conditions including cryptorchidism, postpubertal mumps orchitis, and testicular torsion or trauma can have significant implications on eventual fertility. Although prepubertal mumps is unlikely to have detrimental effects on fertility, mumps occurring in the postpubertal timeframe is associated with unilateral or bilateral orchitis in up to 40% of children (Werner, 1950) with potentially devastating testicular damage. Testicular torsion or trauma can result in testicular atrophy, as well as the development of antisperm antibodies, which are detrimental to sperm function and motility (Bronson et al, 1984; Puri et al, 1985).
The timing of the onset of puberty may suggest underlying endocrinologic abnormalities. A history of delayed puberty, especially in conjunction with anosmia, is associated with the diagnosis of Kallmann syndrome, or primary hypogonadotropic hypogonadism. On the other hand, precocious puberty may be secondary to congenital adrenal hyperplasia, which may affect future fertility.
Prior scrotal, inguinal, or retroperitoneal surgeries can obstruct the ductal system or interfere with emission or ejaculation of sperm. Classic retroperitoneal lymphadenectomy for testicular cancer frequently results in sympathetic nerve injury leading to anejaculation or retrograde ejaculation (Kedia et al, 1977). Fortunately, with modifications in the surgical template and intentional nerve sparing, ejaculation can be preserved in almost all patients with low-stage disease and in selected patients with more advanced disease (Donohue et al, 1990). Bladder neck surgery and transurethral resection of the prostate can lead to retrograde ejaculation due to bladder neck incompetence. In selected patients, transurethral incision of the prostate can allow preservation of antegrade ejaculation. Vasal injuries from inguinal surgery have seen resurgence with the popularity of polypropylene mesh hernia repairs, which can induce dense fibroblastic reactions leading to vasal obstruction (Shin, 2005).
Systemic diseases in adulthood can affect fertility through a number of different mechanisms. Diabetes mellitus, spinal cord injuries, and multiple sclerosis exert effects through impairment of both ejaculatory and erectile function (Sønksen and Biering-Sørenson, 1992; Sexton and Jarow, 1997). Diseases of the thyroid, both hyper and hypo function, affect both steroid hormone metabolism and sperm quality and have been associated with subfertility (Velazquez and Bellabarba, 1997; Abalovich et al, 1999; Krassas et al, 2002). Subclinical hypothyroidism does not produce significant seminal abnormalities (Trummer et al, 2001).
Neoplasms in general can induce marked impairment of spermatogenesis due to endocrine disturbances, malnutrition, hypermetabolism with associated fever, and immunologic factors (Costabile and Spevak, 1998; Wong et al, 2000). In addition to the global effects of malignancy on reproductive health, specific malignancies such as Hodgkin disease (HD) and testicular germ cell tumors produce significant direct gonadotoxic effects (Petersen et al, 1999; Rueffer et al, 2001). Pretreatment testicular dysfunction associated with HD has been postulated to be due to a variety of mechanisms including genetic abnormalities at the germ cell level, endocrinopathies, systemic release of cytokines injurious to both the seminiferous tubules and the Leydig cells, and negative local effects from intratesticular lymphatic tissue. Testicular tumors impair spermatogenesis by the destruction of surrounding tissue, local secretion of HCG and other paracrine factors, intrascrotal temperature elevation, and alterations in the local blood flow. Cancer treatments including chemotherapy and radiation produce direct toxicity on surviving germ cells, potentially depressing spermatogenic function for many years if recovery occurs at all (Nalesnik et al, 2004; Ståhl et al, 2006).
A detailed history should include a comprehensive assessment of medications, recreational, environmental, and occupational exposures that can impact fertility. Medications can impair fertility by direct toxic effects on gonadocytes, disturbance of the hypothalamic-pituitary-gonadal axis, disruption of ejaculatory or erectile function, and inhibition of libido. Antibiotics including nitrofurantoin, erythromycin, tetracycline, and gentamycin exhibit direct gonadotoxicity or impair sperm function. Androgen production is inhibited by spironolactone, ketoconazole, and cimetidine (Griffin and Wilson, 1991). Treatments for ulcerative colitis such as sulfazalazine are associated with reversible reductions in sperm concentration and motility (Toth, 1979). α Blockers, which are commonly used for treatment of benign prostatic hypertrophy and hypertension, are associated with retrograde ejaculation, an effect that may be more prominent with tamsulosin than with other selective α blockers (Giuliano, 2006). 5-α reductase inhibitors such as finasteride and dutasteride inhibit conversion of testosterone to the metabolically active dihydrotestosterone and are commonly used for treatment of benign prostatic hypertrophy. Use of these agents has been associated with reductions in semen volume, as well as erectile and ejaculatory dysfunction (Giuliano, 2006). Psychotherapeutic medications including the selective serotonin reuptake inhibitors (SSRI), monoamine oxidase inhibitors, phenothiazines, and lithium can suppress the hypothalamic-pituitary-gonadal axis, impair ejaculation and erectile function, and reduce libido (Nudell et al, 2002). Exogenous testosterone and steroid supplementation, whether medically prescribed or used for recreational purposes, can have the most profound detrimental effects on spermatogenesis of the medical agents. Androgenic agents induce hypogonadotropic hypogonadism leading to azoospermia, which can last 6 months or more after cessation of the supplements and, on occasion, may be irreversible (Sigman et al, 2006). Testosterone replacement therapy in hypogonadal men desiring fertility should be avoided, and alternate regimens such as antiestrogens (clomiphene citrate, tamoxiphene) should be considered instead. Recreational drugs have also been implicated as gonadotoxic agents. Marijuana use is associated with gynecomastia, reductions in serum testosterone, decreased sperm counts, and elevated seminal leukocytes (Harmon and Aliapoulios, 1972; Hembree et al, 1979; Close et al, 1990). Abnormal sperm morphology, decreased motility, and low sperm concentrations have been associated with cocaine use (Bracken et al, 1990; Hurd et al, 1992). Although long-term abuse of alcohol is associated with global suppression of the hypothalamic-pituitary gonadal axis and spermatogenesis, moderate intake is not associated with significant deterioration in fertility (Muthusami and Chinnaswammy, 2005).
Although the role of smoking in lung and heart diseases is widely established, the adverse effect of tobacco on male reproductive health is less well known by the general public. Smoking is associated with declines in basic semen parameters such as sperm concentration, viability, forward motility, and morphology (Vine et al, 1996; Künzle et al, 2003), as well as declines in sperm penetration ability and hence fertilization rates (Sofikitis et al, 1995). Defects in these parameters not only affect normal fecundity but also lower assisted reproduction success rates (Joesbury et al, 1998; Zitzmann et al, 2003).
The impacts of environmental and occupational exposures on spermatogenesis are more difficult to prove and quantify. Certain agents such as heavy metals, pesticides such as dibromochloropropane, organic solvents, and heat have been widely associated with gonadotoxicity (Lipshultz and Corriere, 1980; Moreira and Lipshultz, 2008). Industrial lead exposure exerts direct negative effects on both seminiferous tubules and the hypothalamic pituitary axis, resulting in asthenospermia, oligospermia, teratospermia, and ultimately reduced fertility (McGregor and Mason, 1990; Gennart et al, 1992; Shiau et al, 2004).
Inflammatory diseases can have profound effects on the patency of the genital tract and function of the spermatozoa. Infectious diseases such as prostatitis or sexually transmitted infections such as Chlamydia or Neisseria gonorrhea are associated with elevated seminal oxidative stress and leukocytospermia, resulting in abnormal bulk semen parameters, elevated sperm DNA fragmentation, and reduced fertility (Trum, 1998; Pasqualotto, 2000; Aitken et al, 2007). A history of bilateral epididymitis with subsequent azoospermia suggests the possibility of epididymal obstruction. Epididymal granuloma may result from noninfectious diseases such as sarcoidosis (Rao, 2009) or from the sequelae of an active tuberculosis infection. Epididymal sarcoidosis has been associated with azoospermia, which may be reversible with corticosteroid treatments (Svetec, 1998).
Questions regarding a family history of infertility are an underemphasized component of the initial assessment because of a misperception that genetic conditions that cause infertility are inherently nontransmissible. A family history of cystic fibrosis may suggest the diagnosis of congenital bilateral absence of the vas deferens (CBAVD) with its associated vasal, epididymal, and seminal vesicle anomalies. Abnormalities of the androgen receptors should be considered in the setting of a family history of intersex disorders. Today’s widespread use of assisted reproductive technologies such as ICSI allow us to overcome subtle genetic abnormalities that may account for many cases of idiopathic male subfertility. With up to 2% to 4% of European and more than 1% of U.S. children born today (Wright et al, 2007) as a result of these technologies, we would expect the genetic causes of infertility to represent a growing etiology of infertility as these men attempt to conceive in the future, further reinforcing the importance of obtaining a comprehensive family history.
Finally, a complete history should also include an assessment of female factor fertility issues because almost two thirds of infertility can be attributed to the female side, either wholly or in combination with male factors. Failure to incorporate these considerations into the evaluation and management can result in ineffective and unnecessarily expensive treatment courses. Risk factors for female subfertility include but are not limited to advanced age, irregular menstrual cycles, and a history of pelvic pathology including endometriosis and pelvic infections. Fecundity begins to decline sharply after age 35 and is less than 5% by age 40 (Robins and Carson, 2008). Ovulatory dysfunction occurs in 40% of infertile women, accounting for the largest single cause of female infertility (Mosher and Pratt, 1991). A variety of tools are used to assess ovulation; these include basal body temperature charts, midluteal serum progresterone levels, endometrial biopsy, urinary LH prediction kits and transvaginal sonographic detection of ovarian follicles. Tests of ovarian reserve involve an assessment of remaining ovulatory capability and a de facto assessment of ovarian aging. Standard testing includes basal hormone measurements of follicle-stimulating hormone (FSH) and estradiol, as well as dynamic ovarian testing, which involves stimulation of ovulation using clomiphene citrate or gonadotropin (Hofmann et al, 1996). Abnormalities of uterine cavity or tubal anatomy occur in up to 25% of infertile women (Thonneau et al, 1991). Uterine and tubal patency can be assessed with hysterosalpingography (HSG) or laparoscopy with chromotubation. HSG testing involves transcervical injection of contrast material allowing assessment of intrauterine and tubal anatomy. In addition, a number of reports have suggested that the use of oil-based contrast materials may be therapeutic, in addition to the diagnostic value (Al-Fadhli et al, 2006; Luttjeboer et al, 2007). Laparoscopy allows confirmation of HSG findings by direct observation of free spill of contrast (methylene blue or indigo carmine introduced via cervix) and detection of other pathologies such as endometriosis, fibrial phimosis, or peritubular adhesions. At the time of laparoscopy, tubal reconstruction and surgical ablation of endometriosis may be undertaken.
Physical Examination
General Examination
Because fertility issues can be a reflection of the general health, the physical examination should be comprehensive with special attention to the genital examination. Body habitus provides clues to the adequacy of virilization with androgen deficiency suggested by decreased body hair, absence of temporal pattern balding, gynecomastia, and eunuchoid proportions. Abnormalities in these areas suggest possible endocrinopathies to include low serum testosterone, hyperprolactinemia, abnormalities in the estrogen to testosterone ratio, adrenal dysfunction, and genetic syndromes associated with subvirilization to include Klinefelter syndrome (KS). Low androgen levels at the time of puberty may cause disproportionately long extremities due to delayed closure of the epiphyseal plates. Palpation of the thyroid gland will occasionally disclose nodules suggesting hyperfunction or hypofunction, which can affect fertility. Hepatomegaly on abdominal examination raises suspicion for hepatic dysfunction, which may induce altered sex steroid metabolism.
Genital Examination
Genital examination starts with a careful examination of the phallus. Penile curvature, chordee, or hypospadias may interfere with semen deposition in the vaginal vault. A careful examination of the scrotal contents is the most critical part of the examination. The testes should be examined with the patient in both supine and standing positions in a warm room to assist relaxation of the cremasteric muscle. The entire testicular surface should be carefully palpated to assess consistency and rule out masses because infertility has been consistently established as a risk factor for testicular carcinoma (Kolettis and Sabanegh, 2001). Testicular size should be assessed with either an orchidometer, calipers, or sonographic measurement. Normal adult testicular measurements have been established to be at least 4 × 3 cm or 20 mL in volume (Charny, 1960). Because 85% of the testicular volume involves sperm production, decreased testicular size portends impaired spermatogenic potential (Lipshultz and Corriere, 1977). The epididymides should be carefully palpated for enlargement or induration, which can indicate downstream obstruction or inflammatory conditions such as epididymitis. Granulomatous changes of the epididymis have been associated with tuberculosis, bacile Calmette-Guerin (BCG) treatments, and sarcoidosis. Small cystic lesions of the epididymis are common and are usually spermatoceles, which are often nonobstructing. Papillary cystadenomas are less commonly seen and may present in conjunction with von Hippel-Lindau (VHL) disease.
Examination of the spermatic cord in the supine and standing position allows the detection of varicoceles, defined as abnormally dilated scrotal veins. Varicoceles are detected by palpation for assymetry of the spermatic cord, or an impulse, during the Valsalva maneuver. Gentle traction on the testis during this examination can be helpful in more difficult examinations such as patients with high riding testes or exaggerated cremasteric muscle response to Valsalva. Varicoceles are present in 15% of normal males, 19% to 41% in men presenting with primary infertility, and up to 81% of men with secondary infertility (Agarwal et al, 2007). Varicoceles are graded by size with small grade I varicoceles, which are detectable only during the Valsalva maneuver; moderate size grade II varicoceles, which can be palpated without Valsalva; and the large grade III varicoceles, which are visible through the scrotal skin and classically described as feeling like a “bag of worms.” Due to the right-angle insertion of the left gonadal vein into the renal vein with the resulting turbulent flow, varicoceles are more prevalent on the left side with almost 90% presenting on the left side alone. Large unilateral right-side varicoceles and varicoceles that fail to decompress with the supine position suggest the possibility of retroperitoneal or caval pathology such as renal neoplasms and warrant dedicated imaging. A variety of ancillary procedures including ultrasonography with and without Doppler examination, radionucleotide scans such as technetium 99m pyrophosphate, thermography, and venography have been used to corroborate clinical examination findings. In the absence of physical examination findings, varicoceles detected by these procedures alone are considered subclinical and not of clinical significance.
Careful palpation of the vas deferens is also a critical component of the spermatic cord assessment. Inability to palpate the vas deferens is consistent with unilateral or bilateral vasal agenesis and may have genetic or renal implications, which are discussed later in this chapter. Nodularity of the vas is also observed from prior infections such as tuberculosis. Vasal thickening is associated with prior scrotal surgery or downstream obstructions such as inguinal vasal obstruction, potentially from prior surgery or ejaculatory duct obstruction.
Finally, a rectal examination should be performed to evaluate prostatic anatomy for midline cysts such as müllerian duct cysts, which can obstruct the ejaculatory ducts. Prostatic induration or tenderness may be seen in acute or chronic prostatitis. Under normal conditions, the seminal vesicles may not be palpable but may be prominent in the setting of ejaculatory duct obstruction. After obtaining a thorough history and a comprehensive physical examination, the clinician has a number of available tools to further evaluate the infertile male, ranging from the basic semen analysis to testicular biopsy, as well as imaging studies.
Laboratory Evaluation of Male Infertility
Semen Analysis
Semen analysis is one of the most important predictors in determining the fertility potential of a man. Appropriate laboratory testing of semen plays a key role in evaluation of men presenting with infertility. However, semen analysis does not allow for the definitive separation of patients into fertile and sterile, in case of azoospermia. It is important to understand that although the statistical chance of conception decreases as the semen quality declines, it does not reach zero. The basic semen testing is inexpensive and determines the quality and the quantity of the spermatozoa. More advanced testing is available to patients who suffer from idiopathic infertility in order to determine specific causes. The analysis of semen evaluates a variety of parameters including characteristics of spermatozoa, seminal plasma, and non–sperm cells.
Collection and Timing
Physicians should provide patients with standard guidelines for the collection of semen because suboptimal sperm collection remains a frequent cause of error in semen analysis. There should be 2 to 7 days of sexual abstinence before collection. Two separate samples at least 7 days apart should be analyzed (Rowe, 2000; Jeyendran, 2003). The duration of abstinence should be constant, if possible, because each additional day can add as much as 25% in sperm concentration (Carlsen et al, 2004). Lubricants should be avoided because they may interfere with motility results. Coitus interruptus should be discouraged because it often leads to inaccurate results (i.e., the first part of the ejaculate, which contains most of the sperm, may be lost).
Masturbation in a clinical setting is the recommended procedure. Collection is done in a private room in the same facility where the semen will be analyzed. The glans and the penis should be cleaned with a wet paper towel (soap should be avoided). Lubricant use is discouraged but, if necessary, should not be applied to the glans. A clean, sterile container should be used for specimen collection. The container should be provided by the laboratory to avoid contamination or spermicidal effects. The main advantages of this collection method are its simplicity, noninvasiveness, and inexpensiveness (Jeyendran, 2003).
Some men may not be able to achieve adequate erection and ejaculation. Assistance can be provided to them by oral medications such as phosphodiesterase type 5 inhibitors given 30 to 60 minutes before collection. Cavernosal and subcutaneous injections of prostaglandins are less popular but remain possible options for patients who have erectile dysfunction. Seminal pouches that do not contain any spermicides allow the patient to engage in sexual activity if he is incapable of or uncomfortable producing specimens by masturbation. Vacuum erection devices can also be used to obtain erection by creating a vacuum around the penis, generating a pressure differential that fills the corpora with blood. Vibratory stimulation may be used for patients who have suffered spinal cord injury, if the spinal cord lesion is T8 and above (Brown et al, 2006). Rectal probe electro-stimulation induces ejaculation by stimulation of the efferent fibers of the hypogastric plexus. Precautions for autonomic dysreflexia should be taken while doing these procedures because some patients with high spinal cord lesions (T6 and above) can have life-threatening hypertension (Jeyendran, 2003).
In order to allow liquefaction and mixing, semen is placed in a 37° C gently shaking incubator for 30 minutes. The semen sample should be examined within 1 hour of production and receipt in the laboratory. Some of the semen parameters can be affected by a delay in assessment. Motility decreases significantly after 2 hours and progressively diminishes afterwards as free radical activity increases.
The semen analysis characteristics can be classified into two groups: macroscopic and microscopic.
Macroscopic Assessment
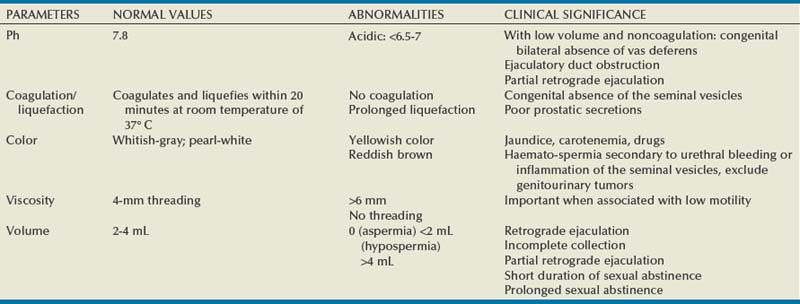
The five macroscopic measurements in a standard sperm analysis have remained fairly constant, with the normal values remaining relatively unchanged since the inception of the semen analysis in the 1950s (Table 21–2). Normal human semen is an off-white to grayish-yellow opalescent fluid. In event of urine contamination, the semen sample has a yellow discoloration. The semen may appear pink in patients with urethral bleeding and yellowish in jaundice patients. During the time of ejaculation, the spermatozoa are suspended in the secretions of prostate, seminal vesicles, bulbo-urethral glands, and other accessory glands that form a coagulum. The specimen usually liquefies within 30 minutes. However, semen obtained from patients with congenital bilateral absence of the vas usually does not form a coagulum and is acidic. Liquefaction is aided by the proteolytic enzyme fibrinolysin, secreted by the prostate. Improper or prolonged liquefaction indicates an ejaculatory duct obstruction or poor prostatic secretion. Viscosity and nonliquefaction are two different phenomena often confused. Viscosity relates to the fluid nature of the sample. It is measured by dropping the semen sample into a container using a pipette and observing the length of the thread formed. Increased viscosity is often associated with infertility because it is known to impair sperm movement. Semen samples that are highly viscous can be treated with enzymes such as trypsin before they are processed for therapeutic purposes. Measurement of pH is a standard component of semen analysis and is largely determined by the secretions from the seminal vesicles and the prostate. The normal range of pH has been defined as 7.2 to 8.0. Because the secretions of seminal vesicles are alkaline, acidic pH indicates congenital absence of the vas with the associated seminal vesicle hypoplasia seen in azoospermic patients (WHO, 1999).
Microscopic Assessment
Sperm Agglutination
The microscopic examination starts with the creation of a wet smear by placing a drop of semen on a slide covered with a cover slip and observing it under 1000× magnification. Sperm agglutination, sperm presence, and subjective motility can be assessed by this method. Sperm adhesion to nonsperm elements (nonspecific agglutination) may indicate accessory gland infection. Sperm-to-sperm agglutination (site-specific agglutination) can be secondary to antisperm antibodies; however, it should be kept in mind that a small degree of agglutination is normal (WHO, 1999). When agglutination is observed, semen cultures and antibody assessment should be performed.
Count and Concentration
Assessment of sperm concentration (number of sperm per milliliter) and sperm count (number of sperm per ejaculate) is conducted after liquefaction. Multiple counting chambers are used for sperm count determination where sperm are counted within a grid pattern. The normal sperm concentration is reported as greater than or equal to 20 million sperm/mL. Attention should be given to collection problems in order to rule out incomplete collection or a short abstinence period before starting evaluation of oligospermia (<20 million sperm/mL). Azoospermia (absence of sperm) may be the result of abnormal spermatogenesis, ejaculatory dysfunction, or obstruction. These specimens should be centrifuged and the pellet examined for the presence of any sperm. Polyspermia (abnormally elevated sperm concentration), although rare, may be caused by a long period of abstinence and is often associated with sperm of poor quality.
When oligospermia is reported, the levels of motility and morphology become especially important. Total motile sperm counts guide decisions on appropriate therapies including the use of ARTs. In cases of azoospermia and severe oligospermia, hormonal evaluation (FSH and testosterone) should be requested. Karyotyping and Y microdeletion may provide valuable information regarding the etiology of the patient’s abnormal semen parameters and important information if in-vitro fertilization (IVF) is being entertained as a treatment option. Foci of microdeletions in the Y chromosome are associated with impaired spermatogenesis and, depending on their location, may predict poor sperm retrieval even with testicular biopsy. Karyotyping may also detect autosomal or X-linked genetic aberrations causing infertility. Knowledge of the chromosome status is important because male offspring conceived with intracytoplasmic sperm insemination (ICSI) or even natural conception most likely will inherit the same microdeletion (Krausz et al, 2000).
Motility
Motility is recognized as the most important predictor of the functional aspect of spermatozoa. Sperm motility is a reflection of the normal development of the axoneme and the maturation that it undergoes within the epididymis. This parameter is subject to significant potential for technical mistakes in the laboratory. The method most commonly employed by laboratories is the simple estimation of the motility of sperm on several fields. This subjective assessment is prone to inaccuracy. Moreover, in-vitro motility of sperm may not reflect the true motility within the female reproductive tract. The sperm motility is graded according to the WHO as follows: A—Rapid forward progress motility; B—Slow or sluggish progressive motility; C—Nonprogressive motility; and D—Immotility. The cutoff value for normal is 50% grade A+B or 25% grade A motility (Rowe, 2000). In addition to organic causes, asthenospermia (sperm motility less than the WHO cutoff levels) can also be artifactual when spermicides, lubricants, or rubber condoms are used. Occasional clumps of agglutinated sperm are of no consequence. However, more than 10% to 15% of clumping of spermatozoa is indicative of antisperm antibodies (ASAs). ASA is known to reduce sperm motility and cause a peculiar shaking pattern that prevents spermatozoa from penetrating through the cervical mucus. ASA testing must be performed to rule out the presence of antibodies. Other potential causes of asthenospermia include prolonged abstinence periods, genital tract infection, partial ductal obstruction, and varicocele. Loss of motility in all spermatozoa or less than 5% to 10% motility can be caused by ultrastructural defects such as absence of axonemal dynein arms or dead sperm (necrospermia) (McLachlan, 2003).
Morphology
Sperm morphology is the most subjective and most difficult-to-standardize semen parameter. Accurate assessment of morphology is critical in the evaluation of an infertile male because it can be a significant predictor of pregnancy. Normal sperm possess an oval head with a well-defined acrosomal region composing 40% to 70% of the head area. The dimensions of the head are 4 to 5.5 µm in length and 2.5 to 3.5 µm in width. The normal sperm are free from head, midpiece, or tail defects. Head defects include microcephalic head (approximately half the size of a normal sperm head), megalocephalic head (one-and-a-half times the size of a normal sperm head), tapered head, round sperm (missing acrosome), and bicephalic or multicephalic head. Neck defects include no tail or improper tail insertions. Midpiece defects comprise elongated, distended, thin, or bent midpieces. Some of the tail defects commonly noted are short, multiple, bent, or broken tails. One common defect includes coiled tail, which is indicative of osmotic stress (McLachlan, 2003).
Sperm morphology is expressed as percentage of abnormal forms present in the semen. The two most common classifications used for the assessment of sperm morphology are the WHO criteria and Kruger’s strict criteria (Table 21–3). When correctable causes of male infertility are not identified, couples with teratozoospermia (<15% normal morphology by WHO method) may be directed to proceed with IVF and ICSI as compared with intrauterine insemination (IUI). Teratozoospermia may occur due to several factors such as fever, varicocele, and stress. Some drugs that affect spermatogenesis are also known to cause morphologic abnormalities. With the advent of ICSI, which requires only one morphologically and functionally normal spermatozoa to fertilize an oocyte, morphologic assessment is losing its significance (Zinaman, 2000).
Table 21–3 Sperm Morphology Classification
| WORLD HEALTH ORGANIZATION 3RD | STRICT WORLD HEALTH ORGANIZATION 4TH (KRUGER) | |
|---|---|---|
| Normal reference range | >30% | >14% |
| Head | ||
| Shape | Oval | Oval, smooth borders |
| Acrosome | 40%-70% of head surface | 40%-70% head surface |
| Size | 4-5.5 mm length 2.5-3.5 mm width Length/width 1.5-1.75 |
3-5 mm length 2-3 mm width |
| Vacuoles | <20% head area | Up to 4 |
| Midpiece | ||
| Shape | Straight regular outlined Axially arched |
Slender, straight, regular outline Axially arched |
| Size | <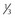 of head area of head area |
<1 mm wide Length 1.5 × head |
| Cytoplasmic droplet | <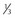 of head area of head area |
<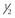 of head area of head area |
| Tail | ||
| Appearance | Slender, uncoiled | Uniform, uncoiled |
| Width | Thinner than midpiece | |
| Length | >45 mm | 10 × head |
Viability
When the motility is reported as less than 5% to 10%, viability testing is recommended because profoundly low motility may indicate dead sperm or necrospermia (McLachlan, 2003). The most common viability assessment involves staining with Eosin Y followed by counter staining with Nigrosin. The viable sperm with its intact cell membrane will not take up the dye and will remain unstained. This test will differentiate necrospermia from immotile sperm secondary to ultrastructural defects such as in Kartagener syndrome and primary cilia dyskinesia.
Hypo-osmotic swelling test (HOST) is an alternative method to assess sperm viability. It is based on the principle that viable sperm have intact cell membranes. Exposure of the sperm to hypo-osmotic fluid will cause water to flow into the viable cells seen as swelling of the cytoplasmic space and curling of the sperm tail. Nonviable sperm with nonfunctional cell membranes will not exhibit this effect because they cannot maintain an osmotic gradient. This reproducible and relatively inexpensive test aids in selection of viable sperm for use in IVF or ICSI, especially when no motile sperm are seen in the cryopreserved specimens (Check, 2002).
Nonsperm Cells
Several nonsperm elements noted on seminal microscopic examination are immature germ cells, epithelial cell, and leukocytes (Branigan et al, 1995; Fedder, 1996). Epithelial cells when present in high numbers are indicative of poor collection. Leukocytes are the most significant nonsperm cellular elements in the semen and are a frequent finding in patients who have unexplained infertility (Branigan et al, 1995). However, in the initial microscopic analysis, the immature spermatozoa may be confused with leukocytes. To confirm the presence of leukocytes, additional testing is therefore required when there are greater than five round cells per high-power field (HPF). Immunocytochemistry is the procedure of choice, but given its expense, it is not widely used in most laboratories. The Endtz test is a reliable alternative because it allows accurate identification of leukocytes that contain enzymes that will react with peroxide and can be visualized with the ortho-toluidine dye (Shekarriz et al, 1995). Initially considered solely as a marker of genital tract infection, contemporary research has shown that leukocytes can be present in the absence of other signs of infection or immune response (Lackner et al, 2006) and that they have intimate links with reactive oxygen species (ROS) (Aitken et al, 1994; Sharma et al, 2001; Saleh et al, 2002; Lackner et al, 2006). The WHO has defined leukocytospermia as levels above 1 × 106 WBC/mL. Studies have shown, however, that ROS levels are elevated even at WBC counts of less than 0.2 × 106/mL, suggesting that much lower levels of leukocytes are pathologic (Sharma et al, 2001; Athayde et al, 2007). In a 12-month follow-up, men who had a negative Endtz test (zero) had a 23.7% chance of initiating pregnancy, whereas leukocytes levels of less than 1 × 106/mL lowered the chances to 15.5% (Athayde et al, 2007). In many andrology laboratories, leukocytospermia determination still has to be requested separately. However, its significance and the ease of determination should place this test among the standard testing that accompanies a basic semen analysis. When leukocytospermia is identified, semen cultures should be performed. Furthermore, red blood cells (RBCs) are also often present in semen. Although small amounts are usually a normal finding, they can be indicative of infection, inflammation, ductal obstruction, or rarely vascular abnormalities.
Computer-Assisted Sperm Analysis
Computer-assisted sperm analysis (CASA) is a semiautomated technique that provides data on sperm density, motility, straight-line and curvilinear velocity, linearity, average path velocity, amplitude of lateral head displacement, flagellar beat frequency, and hyperactivation. It has two distinct advantages over traditional manual analyses: high precision and quantitative assessment of sperm kinematics. Sperm concentration, samplepreparation, and frame rate can, however, affect accuracy of the CASA (Mortimer, 1994). The use of some stains has also affected the accuracy of determining the sperm morphology. Although this technology has theoretic advantages, it has not translated into benefits in clinical practice. This test requires expensive equipment and still requires the active participation of a technician. Therefore at present, these machines are found commonly in andrology laboratories, not in general pathology laboratories, where most of the initial semen analyses are analyzed (Amann and Katz, 2004). Presently, the most important role of CASA is to provide standardized aids in quality control and quality assurance in andrology laboratories, as the emerging use of ICSI has diminished the role of motility assessment in sperm selection (Amann and Katz, 2004).
Limitations of Semen Analysis
The true litmus test for male fertility remains the ability to cause pregnancy in vivo. Although the semen analysis is used as a surrogate measure of a man’s fertility potential, it is not a direct measure by any means. Clinical research has shown that normal semen analysis may not reflect defects in sperm function (idiopathic infertility), and men with poor sperm parameters still may cause spontaneous pregnancies. Only 50% of infertile men have recognizable causes detectable by the basic semen analysis (MacLachlan, 2003). The presence of several criteria further reinforces the emerging opinion that the current standards do not reflect the true fertility potential of subjects. The current normal values fail to satisfy clinical and statistical standards (McLachlan, 2003; Nallella et al, 2006) and pose the risk of misclassifying a subject’s true fertility status. In fact, 20% of 18-year-olds would be classified as subfertile using the WHO cutoff of 20 × 106 sperm/mL (Andersen et al, 2000). Studies on semen donors with known fertility status have revealed a significant overlap in the sperm characteristics between fertile and subfertile men (Li et al, 2006; Nallella et al, 2006). Guzick and colleagues (2001) in a study of 1461 men found different cutoff levels in sperm concentration (<13.5 × 106 in subfertile and 48 × 106 in fertile men), percent motility (<32% in subfertile and >63% in fertile men), and normal morphology (<9% in subfertile and >12% in fertile men). Nallella and colleagues in 2006 did a similar study (n = 572) and used the WHO and Tygerberg criteria on subjects with known fertility. They noted that there is low sensitivity (0.48) in detecting subfertile subjects using WHO reference values for sperm concentration and low sensitivity (0.83) using Tygerberg criteria for percentage of normal morphology. Among the variables, motility had the least overlap range and gave the best prediction of the subject’s fertility potential. This is in contrast with the earlier study by Guzick and colleagues, where morphology was reported to provide the highest discriminating power in detecting subfertility among all the semen variables. Clearly, each variable alone is neither a powerful sole discriminator nor a predictor of fertility status, and they must be considered in the context of other parameters and the clinical setting. There remains a need for further studies in larger populations and different demographics before a consensus can be reached on the necessity of resetting current values to increase the predictiveness and utility of the semen analysis (Table 21–4).
Table 21–4 Characteristics of Normal Semen (World Health Organization, 1999)
| Color | White, opalescent |
| Specific gravity | 1.028 |
| pH | 7.35-7.50 |
| Volume | 2-6 mL |
| Count | 2 × 106 spermatozoa/mL or more |
| Motility | ≥50% motile (grades A + B) or 25% with progressive motility (grade A) |
| Morphology | >30% sperm with normal morphology |
| Viability | ≥50% viable sperm |
| Pus cells | <1 × 106/mL of semen |
Sperm Function Assessment
Sperm-Mucus Interaction/Postcoital Test
Cervical mucus is a heterogenous fluid that is composed of 90% water. In order to reach the site of fertilization, the spermatozoon must be able to successfully traverse the cervix and the cervical mucus. In-vitro penetration of spermatozoa through cervical mucus is comparable to in-vivo conditions. The cervical mucus is shown to demonstrate cyclical changes in consistency and to be highly receptive around the time of ovulation. Increase in penetrability is often observed one day before the LH surge. Cervical mucus has been shown to protect the spermatozoa from the hostile environment of the vagina. The penetrability of the spermatozoa through the cervical mucus can be detected by the cervical mucus migration assay. Some methods by which migration can be detected include the postcoital test (PCT). This test can assess cervical environment as a cause of infertility. Accurate timing is crucial because it must be conducted when the cervical mucus is thin and clear just before ovulation. In this test, cervical mucus is examined 2 to 8 hours after normal intercourse. Progressively motile sperm greater than 10 to 20 per HPF is designated as normal. Practical guidelines of the American Society of Reproductive Medicine recommend PCT in the setting of hyperviscous semen, unexplained infertility, or low-volume semen with normal sperm count (Van der Steeg et al, 2004). Medical history and semen analysis can predict PCT results in half of the infertile couples. Poor-quality semen most likely will have poor PCT. Therefore it is not recommended routinely for men who have abnormal semen analyses. Couples who show defective sperm mucus interaction may be advised to proceed with IUI because additional tests are unlikely to affect the management (Guzick et al, 2001). However, abnormal PCT may result from inappropriate timing of the test. Other causes of abnormal PCT include anatomic abnormalities, semen or cervical mucus antisperm antibodies, inappropriately performed intercourse, and abnormal semen. Persistently abnormal PCT in the presence of reasonably good semen parameters should indicate poor cervical mucus quality. The finding of good-quality mucus with nonmotile spermatozoa or immobilized sperm demonstrating shaking motion should lead to the evaluation of both partners for the presence of antisperm antibodies. Although it has fallen out of favor, this test may be useful in patients who are unable or unwilling to produce an ejaculate.
Acrosome Reaction
The Acrosome is a membrane-bound organelle that covers the anterior two thirds of the sperm head. Acrosome reaction is an important prerequisite for successful fertilization. It is an exocytotic event that involves fusion of outer acrosomal membrane and sperm plasma membrane, which enables the exposure of acrosomal contents through the formation of vesicles. The two important acrosomal enzymes that are required to digest the oocyte cumulus cells and zona pellucida include acrosin and hyaluronidase. Acrosome reaction testing is not widely practiced in laboratories and only remains a research interest. However, this test may be recommended in cases of profound abnormalities of head morphology or in the setting of unexplained fertility in patients with poor IVF pregnancy rates. Normal semen samples demonstrate spontaneous acrosome reaction rates of less than 5% and induced acrosome reaction rates of 15% to 40%. Infertile populations have shown high spontaneous rates of acrosome-reacted sperm and low rates of induced acrosome reactions. Although not widely practiced due to its cost and labor, the structure of the acrosome can be studied under transmission electron microscopy. Other techniques such as fluorescence microscopy and beads coated with antiacrosomal antibodies have been developed, but these tests are also not readily available in standard laboratories.
Sperm Penetration Assays/Sperm Zona Binding Tests
The sperm penetration assay (SPA) or the hamster egg penetration assay (HEPT) determines the functional capacity of the spermatozoa necessary to fertilize an oocyte. It is based on the principle that normal spermatozoa can bind and penetrate the oocyte membrane, which is a prerequisite for the fusion of sperm and the oocyte. Zona pellucida is the outermost layer protecting the cytoplasm of the oocyte. It plays an important role in the fertilization process and is shown to be the only physiologic inducer of acrosome reaction. Sperm binds to the species-specific receptor, ZP3, which is found on the zona pellucida of the oocyte. The zona-free hamster oocytes, in which the zona pellucida is stripped, are used to allow cross-species fertilization. Human sperm penetration assay with zona-free hamster eggs determines the ability of sperm to successfully undergo capacitation, acrosome reaction, membrane fusion with oocytes, and chromatin decondensation. The assay is performed by incubating zona-free hamster oocytes in sperm droplets for 1 to 2 hours. The oocytes are examined microscopically for sperm penetration. Penetrations are indicated by swollen sperm heads within the oocyte cytoplasm. Normally, 10% to 30% of ova are penetrated (WHO, 1999). Oligozoospermic and severely teratospermic men have a higher number of defective sperm-zona pellucida interactions, which may account for their low fertility potential in both spontaneous and IVF pregnancies (Liu and Baker, 2004). Despite its low predictive power, SPA is correlated positively with spontaneous pregnancy outcomes (Corson et al, 1988). Sperm capacitation index (SCI) is a variant of the SPA test, assessing the mean number of penetrations per ovum. ICSI has been recommended for couples with an SCI less than 5 instead of standard IVF procedures (Ombelet et al, 1997). Compared with SPA, the zona binding test uses oocytes that failed to fertilize in IVF clinics. The need for human oocyte supply, however, remains a limitation to the use of this test.
Advanced Semen Testing
Antisperm Antibody Testing
The tight Sertoli-cell junctions provide the testis with a barrier that prevents the immune system from coming in contact with the post-meiotic germ cells. However, in certain conditions such as testicular torsion, vasectomy, and testicular trauma, this unique barrier can be violated, resulting in an immune response to sperm, displayed as antisperm antibodies (ASABs). These antisperm antibodies can be several types—sperm agglutinating, sperm immobilizing, or spermotoxic. The sperm agglutinating type causes agglutination of spermatozoa, which reduces the availability of motile spermatozoa penetrating the cervical mucus. Sperm-immobilizing antibodies induce loss in motility of the sperm, which can be identified by the characteristic “shaking” pattern in motility on postcoital test. The spermotoxic type of ASAB causes a complement-dependent loss in viability of spermatozoa.
Approximately 10% of infertile men will present with ASA as compared with 2% of fertile men (Guzick et al, 2001). Sperm parameters are often normal in men with ASA (Munuce et al, 2000). Hence it has been suggested to be tested routinely in all men undergoing infertility work-ups (McLachlan, 2003). Excessive sperm agglutination or an abnormal PCT can suggest the presence of ASA.
The direct ASA test detects sperm-bound immunoglobulins. Indirect testing detects the biologic activity of circulating ASA. False positives can result from nonimmunologic factors (Francavilla et al, 2007). Because only antibodies present on the sperm surface are clinically significant, most investigators prefer direct assays that determine sperm-bound antibodies instead of indirect detection of serum antisperm antibodies. IgG-MAR (mixed antiglobulin reaction) and Sperm MAR are recommended screening tests that are economical and readily available. Immunobead Test (IBT), which measures IgG, IgA, and IgM, may be additionally recommended when either of the previous tests gives a positive result in order to determine if IgA are bound to sperm surface. Acceptable normal values by WHO (1992) standards include less than 10% (IgG MAR) or 20% (IBT) of spermatozoa with adherent particles.
Clinical implications of ASA on male infertility are varied. A weakly positive IgG MAR/IBT in men who have low motile sperm rules out immunologic factors, and no further testing is necessary (Francavilla et al, 2007). ASA are present in 34% to 74% of vasectomized men and persist in 38% to 60% after vasectomy reversal (Broderick et al, 1989; Francavilla et al, 2007). Routine ASA testing is not recommended in this setting because it is of uncertain significance and usually does not affect the decision to do a vasectomy reversal. There are conflicting reports regarding ASA levels after orchidopexy for cryptorchidism (Mirilas et al, 2003). In genitourinary infections, ASA is thought to be a consequence of the inflammatory process rather than cross reactivity to the microorganism (Francavilla et al, 2007).
The decision to proceed with IUI versus ICSI in immunologic infertility can be aided by a zona pellucida (ZP) test. If the sperm exhibit inability for ZP binding, ICSI is the procedure of choice. Presently, flow cytometry techniques are being developed to quantify ASA in individual spermatozoa (Shai et al, 2005). These techniques are also being explored to identify sperm surface antigens for possible immunocontraceptive development.
Electron Microscopy
Spermatozoa may test positive for viability even in the presence of ultrastructural defects. Ultrastructural details of the sperm can only be seen under the electron microscope (EM). Patients who have low sperm motility (<5% to 10%) with high viability (as determined by HOST or Eosin-Nigrosin staining) and density may be appropriate candidates for EM assessment. Subfertile men may demonstrate more serrated and blurred circular sulcus, less intact acrosome membrane, a bigger proportion of the spermatic head, and more droplets attached to the acrosome membrane. Mitochondrial and microtubular defects that are not visible under the usual Papanicolaou smear can be detected.
Biochemical Tests
Acrosin is a serine protease-like enzyme that exhibits a lectin-like carbohydrate binding activity to the zona pellucida glycoproteins. Low acrosin activity has been associated with low sperm density, motility, and poor normal morphology (Xu and Zhan, 2006).
Zinc is necessary for chromatin stability and decondensation, as well as for head–tail detachment during fertilization. It is measured by colorimetric methods with a reference value of 13 mmoL per ejaculate (WHO, 1999). Reports on the effects of zinc in sperm function and semen parameters are quite conflicting. Mankad and colleagues (2006) reported positive correlations between seminal zinc levels, alpha glucosidase, and sperm count; however, there are other reports that showed no significant changes in sperm count and motility with variations in zinc concentration (Abou-Shakra et al, 1989; Lewis-Jones et al, 1996; Sorensen et al, 1999). Zinc levels in seminal plasma are decreased, but spermatozoal zinc levels are increased in asthenozoospermic and oligoasthenozoospermic men (Zhao and Xiong, 2005). A low zinc-to-calcium ratio has been shown to be associated with better motility than high ratio (Sorensen et al, 1999). However, dietary supplementation of zinc did not improve semen variables (Agarwal and Said, 2004).
The seminal vesicles contribute to the bulk of seminal fluid that serves as the transport medium for sperm and contribute to the nutrition in the form of fructose. There is a positive correlation between sperm motility and seminal fructose levels (Lewis-Jones, 1996). Low or absent fructose is seen in ductal obstruction and congenital conditions like CBAVD. Semen fructose testing may be requested when hypo-functioning seminal vesicles are suspected, although morphometric analysis of seminal vesicles using transrectal ultrasound (TRUS) is the recommended test nowadays.
L-carnitine is secreted by the epididymis and is concentrated in the seminal plasma at up to 10 times the serum levels. It has a role in sperm maturation. Low L-carnitine levels are found in oligoasthenozoospermic men (Agarwal and Said, 2004; Sigman et al, 2006). The levels of carnitine can possibly serve as indicators of the level of obstruction in the ductal system. Extremely low concentrations of L-carnitine are found in azoospermic men who have postepididymal obstructions, whereas normal levels are found in azoospermic men who have intratesticular obstructions (Agarwal and Said, 2004). Administration of L-carnitine supplements did not improve sperm density, but contrasting results have been reported for sperm motility changes (Sigman et al, 2006). L-carnitine determinations remain far from becoming mainstream tests in male infertility until significant well-designed studies are conducted.
Alpha glucosidase, tested by fluorimetric methods, has been used to distinguish nonobstructive from obstructive azoospermia. It is used as a specific marker for epididymal function and is believed to play a role in sperm maturation in the epididymis. A cutoff value of 12 mIU/mL distinguishes ductal obstruction from primary testicular failure (Comhaire et al, 2002). The usefulness of this test was questioned by Krause and Bohring (1999), but Comhaire and colleagues (2002), in their review, showed a strong association between α-glucosidase and semen parameters. The cutoff level had 95% specificity in identifying obstructive azoospermia. This suggests that the test can predict IUI response (higher pregnancy rate >78 U per ejaculate) because high levels indicate better zona-binding capacity (Comhaire et al, 2002). The presence of commercial test kits using colorimetric methods promises to make testing accessible and affordable.
Reactive Oxygen Species
Research conducted during the past decade has provided growing support for the concept that excessive production of reactive oxygen species (ROS) is related to abnormal semen parameters and sperm damage. Routine semen analysis remains the backbone of clinical evaluation in male infertility, and determining the levels and sources of excessive ROS generation in semen is currently not included in the routine evaluation of subfertile men. However, the diagnostic and prognostic capabilities of seminal oxidative stress measurement exceed the capabilities of conventional sperm quality tests. An oxidative stress test may accurately discriminate between fertile and infertile men and identify those with a clinical diagnosis of male factor infertility who are likely to initiate a pregnancy if they are followed over a period of time. In addition, such a test can help select subgroups of patients with infertility in which oxidative stress is an important factor and who may benefit from antioxidant supplementation. Although consensus is still required about the type and dosage of antioxidants to be used, rationale and evidence exist supporting their use in infertile men with elevated oxidative stress (Deepinder et al, 2008).
Currently, clinical practice as to the inclusion of ROS measurement is variable, primarily because of the lack of standardization of ROS analytic methods, equipment, and range of normal levels of ROS in semen. The evidence defining high ROS levels as a cause or an effect of abnormal semen parameters and sperm damage is still insufficient on both sides of the question. However, it has been reported that a high level of ROS is an independent marker of male factor infertility in leukocytospermic samples after adjustment for semen characteristics. This finding suggests that ROS may play an important role in the etiology of male factor infertility and encourages the use of ROS measurement as a diagnostic tool in clinical practice, particularly in cases of idiopathic infertility. Although numerous assays for ROS measurement have been introduced, the chemiluminescence assay, determining ROS levels in neat semen, has proven to be an accurate and reliable test for evaluating oxidative stress status. This technique accurately represents an individual’s true in vivo oxidative stress status and overcomes the drawbacks of earlier methods that involve processing semen, a step that may generate ROS by itself. The ROS level for healthy donors with normal standard semen parameters is 1.5 × 104 cpm/20 million sperm/mL. Using this value as a cutoff, infertile men can be classified as either oxidative stress positive (>1.5 × 104 cpm/20 million sperm/mL) or oxidative stress negative (≤1.5 × 104 cpm/20 million sperm/mL), regardless of their clinical diagnosis or standard semen analysis results (Deepinder, et al, 2008).
Sperm DNA Damage
DNA fragmentation was initially described in 1993 and has since been researched as a test to aid fertility prediction in subfertile males. The spermatozoal chromatin is a tightly packed structure because of the disulfide cross linkages between protamines that allow compaction of the nuclear head and protect the DNA fragments from stress and breakage. DNA damage is multifactorial and theories on its etiology include protamine deficiency and mutations that may affect DNA packaging or compaction during spermiogenesis (Agarwal and Said, 2003). Various factors found to be associated with increased sperm DNA damage include tobacco use, chemotherapy, testicular carcinoma, and other systemic cancers (Agarwal and Said, 2003). DNA damage is correlated positively with poor semen parameters, especially low sperm concentration and low sperm motility, leukocytospermia, and oxidative stress (Erenpreiss et al, 2002; Agarwal and Said, 2003; Zini and Libman, 2006). Approximately 8% of subfertile men who have normal semen parameters will have high abnormal DNA (Aitken et al, 1991).
Many tests of sperm DNA damage are now available (Table 21–5). The use of these tests has been driven largely by the growing use of assistive reproductive technologies and awareness that the integrity of the male genome plays an important role in IVF. Sperm DNA damage can be measured directly (fragmentation, oxidation) or indirectly (sperm chromatin compaction). Direct assessment of DNA damage can be obtained by means of single-cell gel electrophoresis assay or “comet” assay (electrophoresis causes DNA fragments to migrate away from the central DNA core, revealing a “comet”), terminal deoxynucleotidyl transferase-mediated dUTP-nick end-labeling or “TUNEL” assay (the ends of fragmented DNA are tagged), and liquid chromatography to measure DNA oxidation levels. DNA damage can also be assessed indirectly by means of sperm chromatin integrity assays and by evaluation of nuclear protein levels. Sperm chromatin integrity assays include slide-based sperm nuclear protein stains (e.g., aniline or toludine blue [detects histones], CMA3 [detects underprotamination]) and DNA stains (e.g., acridine orange [detects denatured or single-stranded DNA]). The sperm chromatin structure assay (SCSA) uses flow cytometry to estimate the percentage of spermatozoa with DNA denaturation (spermatozoa are stained with acridine orange). A cutoff rate of greater than 30% has been shown to be associated with a significant decrease in in-vivo fertilization rates (Evenson and Wixon, 2002). A DNA fragmentation index (DFI) of greater than 30% has a sensitivity of 15% and a specificity of 96%. Meta-analyses by Evenson and Wixon (2002) and Li and colleagues (2006) showed that couples are twice as likely to become pregnant with regular IVF methods if the DFI is less than 30%. Contrasting reports, however, have failed to show significant correlation between DNA damage and idiopathic infertility (Verit et al, 2006). In addition, significant intraindividual variation exists making conclusions using SCSA problematic (Erenpreiss et al, 2006). There is a higher rate of DNA damage in ejaculated or epididymal sperm than in intratesticular spermatozoa. Hence the use of intratesticular spermatozoa from high DFI men is recommended for ICSI (Steele et al, 1999; Greco et al, 2005). ICSI is advised when DFI is above cutoff levels. DNA fragmentation testing can help couples decide on what fertility modality and possible lifestyle modifications they can employ to increase their chances of conception.
Table 21–5 Commonly Used Tests of Sperm DNA Damage
| TEST | MEASURES | CHARACTERISTICS |
|---|---|---|
| Sperm chromatin structure assay | Susceptibility of sperm DNA to denaturation | Objective, flow cytometry–based, indirect assay, complex analysis, used clinically |
| Nuclear protein composition (by protein separation) | Sperm histone and protamine levels | Objective, gel electrophoresis assay, indirect assay, labor intensive |
| Sperm nuclear maturity test (by nuclear staining) | Chromatin compaction, protamine content | Simple, semiquantitative, slide-based, indirect assay |
| Comet assay (by single-cell gel electrophoresis) | Double-stranded DNA breaks (neutral assay) | Objective, quantitative, direct assay, complex image analysis |
| TUNEL assay | Double-stranded DNA breaks | Semiquantitative, direct assay, quantitative if flow cytometry based |
| DNA oxidation | 8-hydroxy-2-deoxyguanosine | Quantitative, direct assay, labor intensive |
Endocrine
Although an uncommon cause of male subfertility, up to 3% of infertile men will have an underlying endocrinopathy (Sigman et al, 1997). Although some authors recommend routine screening of the male hypothalamic-pituitary-gonadal axis in all patients, the consensus opinion favors endocrine evaluation in men with (1) an abnormally low sperm concentration, especially if less than 10 million/mL; (2) impaired sexual function; or (3) other clinical findings suggestive of endocrinopathy such as marked reduction in testicular size or gynecomastia (AUA/ASRM Practice Committee Recommendations, 2006).
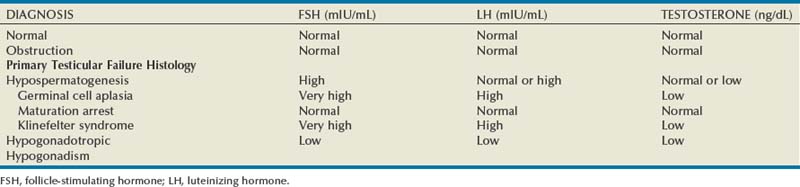
Initial endocrine evaluation in those with indications for testing should include serum follicle-stimulating hormone (FSH) and morning serum testosterone measurements. Gonadotropins and testosterone are secreted in a pulsatile manner, and some advocate pooled specimens drawn at 15 minute intervals to increase accuracy, although most recommend screening with a single morning specimen. Morning specimens are preferred due to a normal physiologic decline in testosterone levels throughout the day. Table 21–6 demonstrates commonly observed endocrine patterns associated with various clinical diagnoses. Under normal conditions, FSH secretion is under negative feedback control via inhibin B, which is produced by the Sertoli cells (Fig. 21–1). Elevations in serum FSH are indicative of disturbances in spermatogenesis such as primary testicular failure (hypergonadotropic hypogonadism), although normal FSH levels do not rule out spermatogenic failure. Obstructive azoospermia is usually associated with normal gonadotropin and testosterone levels. Low serum testosterone levels may indicate hypogonadism of pituitary or hypothalamic origin, as well as primary testicular failure. If initial testing is abnormal, further endocrine testing should be obtained to include a repeated testosterone assay including free and total testosterone levels, serum luteinizing hormone (LH), and serum prolactin levels. Low FSH and LH levels indicate hypogonadotropic hypogonadism such as Kallman syndrome and warrant a complete pituitary hormonal assessment including thyroid stimulating hormone (TSH), adrenal corticotropic hormone (ACTH), and growth hormone assays. Direct measurement of serum inhibin levels may provide a more accurate assessment of spermatocytic health than FSH levels, although most find that the cost of this assay and lack of widespread availability limit its clinical utility (Sussman et al, 2008).
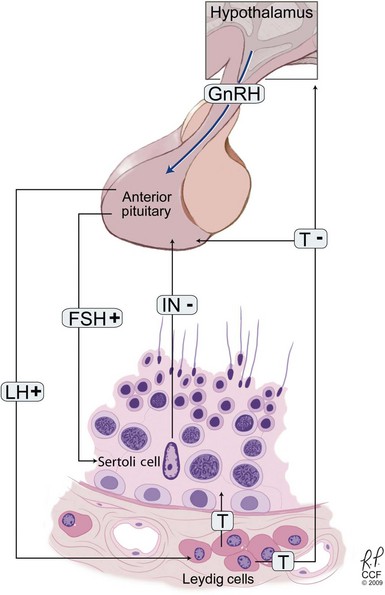
Figure 21–1 The hypothalamic-pituitary-testicular axis. Gonadotropin-releasing hormone (GnRH) is released from the hypothalamus, stimulating luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release. The gonad is stimulated with FSH inducing stimulation of germinal cell epithelium and LH inducing testosterone production by the Leydig cells. Both testosterone (T) and inhibin (IN) downregulate gonadotropin release.
Hyperprolactinemia is usually associated with low serum testosterone often without associated increases in LH levels, suggesting that the hypothalamic-pituitary axis is unresponsive in the setting of elevated serum prolactin levels (Carter et al, 1978). Prolactin tests should be repeated due to marked physiologic variability in serum prolactin levels. Mild serum prolactin elevations (<50 ng/mL) may be seen with medications, stress, and renal insufficiency or may be idiopathic. However, if the prolactin level is persistently elevated, a pituitary tumor such as a prolactinoma should be ruled out with a focused neurologic examination including visual field testing and magnetic resonance imaging of the pituitary fossa.
Estrogen excess may be manifested by gynecomastia, decreased libido, erectile dysfunction, and low serum testosterone levels. Although elevated serum estradiol levels may be from exogenous intake, they are more commonly associated with morbid obesity due to the peripheral aromatization of testosterone to estradiol in adipose cells. Estradiol stimulates sex steroid hormone binding globulin (SHBG) production in the liver, which reduces levels of bioavailable testosterone. SHBG levels are also influenced by a number of other conditions (Table 21–7).
Table 21–7 Factors That Impact Sex Hormone Binding Globulin Levels
| INCREASE | DECREASE |
|---|---|
| Estrogen | Obesity |
| Medications | Medications |
| Anticonvulsants | Progestins |
| Thyroid replacement | Insulin |
| Glucocorticoids | |
| Hyperthyroidism | Hypothyroidism |
| Cirrhosis | Acromegaly |
| Aging | Nephrotic syndrome |
On rare occasions, endocrinopathies involving adrenal or thyroid functions may present with male subfertility. Patients with congenital adrenal hyperplasia (CAH) present with a history of precocious puberty and short stature due to premature closure of the epiphyseal plates. The common variant involving 21-hydroxy deficiency will have elevated serum levels of 17-hydroxyprogesterone and urinary pregnanetriol. Although CAH patients may retain fertility, many will have reduced testicular function due to suppression of gonadotropin levels from direct feedback inhibition of the pituitary from the excessive adrenal androgens.
Thyroid disease, both hyperfunction and hypofunction, may occasionally be associated with male factor infertility, although subclinical hypothyroidism does not impact semen parameters (Trummer et al, 2001). Thyroid function testing of the infertile male is not justified for routine screening but should be reserved for patients with clinical symptoms of thyroid dysfunction.
Genetic Testing
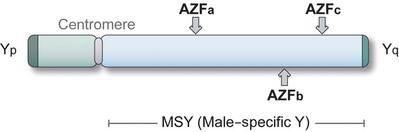
Genetic testing is important for establishment of the etiology of infertility, identification of potential future medical issues for the patient, prediction of therapeutic efficacy from various fertility interventions such as varicocele repair and sperm retrieval, and counseling information to couples regarding transmission risk to offspring. Clinically relevant genetic testing for the infertile male include karyotype and y-linked microdeletion assessment, which are used for evaluation of both nonobstructive azoospermia (NOA) and severe oligospermia, as well as the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is assessed in men with obstructive azoospermia due to CBAVD. Almost 7% of infertile men will have structural or numeric chromosomal abnormalities. The incidence of karyotype anomalies is inversely proportional to the sperm concentration with a prevalence of 10% to 15% in azoospermia, 5% in oligospermia, and less than 1% in patients with normal sperm counts (De Braekeleer and Dao, 1991; Samli et al, 2006). Microdeletions of the Y chromosome have been described in 10% to 15% of patients with severe oligospermia or azoospermia (Pryor et al, 1997). CFTR mutations have been identified in 88% of patients with CBAVD (Ratbi, 2007). Specific genetic syndromes are reviewed later in this chapter.
Other Testing
Imaging Studies
Radiographic evaluation of the infertile male focuses on identification of patients with genital tract obstruction in the vas deferens or ejaculatory duct, as well as ruling out associated pathologies in certain individuals such as testicular masses or renal anomalies. The tests described here are not required in most individuals but should be used judiciously in those with appropriate indications.
Transrectal Ultrasonography
TRUS provides excellent definition of the prostate, seminal vesicles, ampulla of the vas deferens, and the ejaculatory ducts. TRUS is primarily employed to examine patients suspected to have ejaculatory duct obstruction (EDO). These patients usually have low-volume azoospermia (volume <1 mL) with acidic pH and negative semen fructose. TRUS typically employs the 5- to 7-MHz endocavitary probe with scanning in both the longitudinal and transverse planes. Careful examination of verumontanum may identify midline prostatic cysts such as müllerian or wolffian duct cysts or stones obstructing the ejaculatory duct (Fig. 21–2). Often the ejaculatory duct may not be well visualized, but dilation of the seminal vesicles serves as a de facto sign of ejaculatory duct obstruction. Although not always present with ejaculatory duct obstruction, seminal vesicle width in excess of at least 12 to 15 mm or ejaculatory duct diameter greater than 2.3 mm is considered suggestive of obstruction (Carter et al, 1989; Vazquez-Levin et al, 1994; Smith et al, 2008).
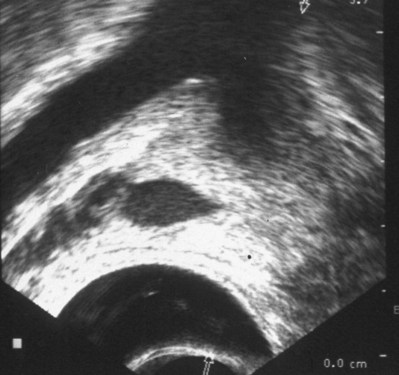
Figure 21–2 Transrectal ultrasound (sagittal image) demonstrating a dilated ejaculatory duct culminating in an ejaculatory duct cyst.
Seminal vesicle aspiration using a 20-gauge needle at the time of TRUS has been used to further increase the specificity of the diagnostic techniques. Significant quantities of sperm are not normally present in the seminal vesicles. Findings of three or more sperm per HPF in the seminal vesicle aspirate support the diagnosis of EDO (Jarow, 1994). Test accuracy is improved by performing aspiration within 24 hours of ejaculation (Jarow, 1996).
Seminovesiculography using transrectal injection of radioopaque contrast (50% renograffin) into the seminal vesicles under TRUS guidance with postinjection radiographs can provide excellent anatomic detail of the seminal vesicles and ejaculatory ducts. Seminal vesicle chromotubation is a variation of seminovesiculography using the injection of dilute indigo carmine or methylene blue (1:5 dilution with saline) into the seminal vesicles via TRUS guidance followed by cystoscopic inspection of the ejaculatory ducts in the prostatic urethra to confirm patency. The dynamic tests of chromotubation and seminal vesiculography offer higher specificity for detection of EDO than static TRUS imaging alone (Purohit et al, 2004). The newest adjunctive technique, ejaculatory duct manometry, involves hydraulic assessment of the ejaculatory ducts at the time of seminal vesicle chromotubation, noting that men with EDO have higher mean ejaculatory duct opening pressures, 116 cm H2O versus 33 cm H2O in fertile controls (Eisenberg, 2008). Despite these advances in diagnostic techniques, criteria for diagnosis of complete EDO remain unclear and those for partial EDO remain controversial.
Scrotal Ultrasonography
Scrotal ultrasound for the infertile male is primarily used to confirm the presence of clinical varicoceles, although it also provides high-quality imaging of scrotal contents that offers the advantage of widespread availability without exposure to ionizing radiation. Although clinical varicoceles do not require confirmation with ultrasound examination, color Doppler ultrasound may be required when the clinical examination is difficult due to body habitus or when the examination is equivocal. Demonstration of reversal of venous blood flow with the Valsalva maneuver or spermatic vein diameters of 3 mm or greater (Fig. 21–3A and B) support the diagnosis of varicocele (Meacham et al, 1994). Scrotal ultrasound is not recommended for screening for subclinical varicoceles because repair of these has not been demonstrated to be of clinical benefit.
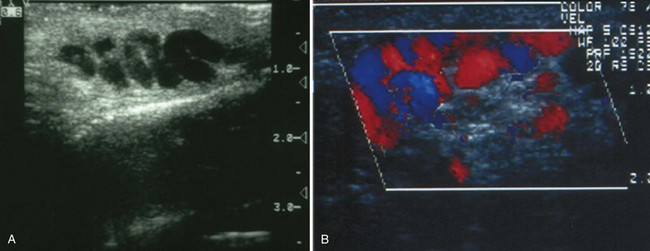
Figure 21–3 Scrotal ultrasound of varicocele. A, Dilated veins in spermatic cord. B, Color Doppler image revealing typical venous reflux with Valsalva maneuver.
In addition, ultrasound examination provides excellent anatomic details of the epididymis and testis, potentially disclosing a number of conditions that may affect fertility. Epididymitis is associated with epididymal enlargement with diffuse hypoechogenicity and is often associated with a reactive hydrocele. Doppler ultrasound will often reveal increased vascularity in the involved epididymis or adjacent testicular region. Epididymal cysts or spermatoceles appear as simple or minimally complex cysts and may cause epididymal outflow obstruction. Testicular germ cell tumors are noted with increased frequency in the subfertile population, and even small, nonpalpable lesions (<0.5 cm) are well visualized with ultrasonography (Fig. 21–4). Testicular microlithiasis are diffuse 1- to 2-mm hyperechogenic nonshadowing foci and have been reported in 3% of subfertile men (Thomas et al, 2000). Although originally thought to represent a radiologic precursor to the development of germ cell tumors, it is now known that testicular microlithiasis is common and does not represent a risk factor for germ cell tumor development or necessitate continued medical surveillance (Costabile and Spevak, 1998).
Abdominal Ultrasonography
Abdominal ultrasound imaging in the infertile male is primarily indicated to rule out associated renal anomalies in patients with vasal agenesis. Vasal agenesis is theorized to result from one of two mechanisms—either mutations in the CFTR gene that do not incur associated renal anomalies or improper morphogenesis of the mesonephric duct before week 7 of gestation, which causes vasal agenesis and unilateral renal agenesis.
The CFTR gene is responsible for regulation of chloride ion transport across epithelial cell membranes. Dysfunction in this transport results in abnormal luminal fluid quality during vasal morphogenesis with resulting vasal agenesis (Oates and Amos, 1993). In patients with proven CFTR gene mutation in association with CBAVD, renal anomalies are unlikely and routine abdominal imaging is not indicated. However, up to 20% of men with CFTR mutation-negative vasal agenesis will have ipsilateral renal anomalies, most commonly renal agenesis, and should have routine abdominal ultrasonography as part of their evaluation.
Vasography
Vasography remains the gold standard test for assessing the patency of the male ductal system. Although procedures such as TRUS, seminal vesicle aspiration, and seminal vesiculography offer minimally invasive imaging to diagnose obstruction, properly performed vasography provides unequalled anatomic detail of the vas deferens, seminal vesicles, and ejaculatory ducts. Vasography is indicated for determination of the site of obstruction in the azoospermic patient with confirmed normal spermatogenesis on testis biopsy. On occasion, it may be used for the severely oligospermic patient in whom there is a high clinical suspicion of unilateral vasal obstruction from iatrogenic injury such as prior inguinal hernia repair (Matsuda, 2000). Vasography may also be used to rule out ejaculatory duct obstruction in the setting of ejaculatory pain. However, it should be emphasized that vasography is not necessary in oligospermic patients who do not have clinical evidence or history (i.e., inguinal surgery) that suggests unilateral obstruction.
Vasography is ideally performed at the time of anticipated reconstruction due to the potential to cause vasal scarring at the vasogram site, although this complication has not been observed in large series (Payne et al, 1985). For antegrade vasography, the straight portion of the vas deferens is isolated in the scrotal region, immediately adjacent to the convoluted vas to allow maximum length of vas deferens if a reconstruction such as a vasoepididymostomy is eventually required. Either a puncture or vasotomy technique may be used to inject contrast into the vas deferens. The puncture technique is preferred if possible because it avoids a full-thickness vasotomy, which necessitates subsequent microsurgical closure, although it is technically more difficult to enter the vasal lumen than with the vasotomy method. With the puncture procedure, a 30-gauge lymphangiogram needle is inserted directly into the proximal vas lumen and contrast is injected in an antegrade fashion (Fig. 21–5A). Alternatively, a microsurgical scalpel may be used to make a hemivasotomy incision through the anterior wall of the vas deferens to expose the vasal lumen and allow placement of a 25-gauge angiocatheter for contrast injection (Fig. 21–5B). This techique allows examination of the intravasal fluid for presence of sperm to confirm epididymal patency. If a hemivasotomy technique is used for the vasogram, the vas will require reconstruction at the end of the procedure with 9-0/10-0 nylon interrupted sutures using standard microsurgical technique. Once the vasal lumen has been intubated, 5 to 10 mL of full- or half-strength contrast (Renografin) is injected in an antegrade fashion. Retrograde injection is not recommended due to the potential for subsequent epididymal scarring or obstruction. Vasography images are obtained using either standard radiographs or fluoroscopy. Methylene blue or indigo carmine may be mixed with contrast (1:10 dilution) to guide the depth of transurethral resection if the vasogram is performed at the time of ejaculatory duct resection. On occasion, contrast will not flow and a 2-0 monofilament suture may be passed in an antegrade fashion to identify the level of obstruction.
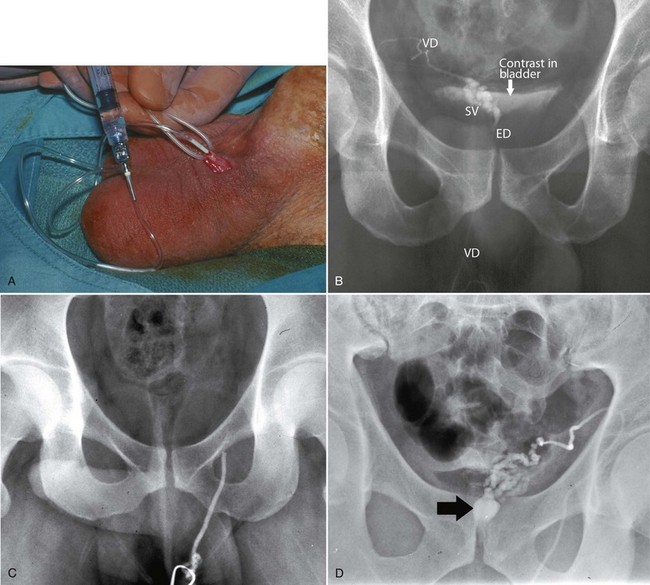
Figure 21–5 Vasography—examples of technique and findings. A, Technique for antegrade injection of contrast into proximal vas deferens. B, Normal vasogram with clearly defined vas deferens (VD), seminal vesicles (SV), ejaculatory duct (ED), and contrast spilling into bladder. C, Obstruction of left inguinal vas deferens due to prior herniorrhaphy. D, Ejaculatory duct obstruction with large cyst (arrow).
A properly performed vasogram will opacify the scrotal and inguinal portions of the vas deferens with subsequent filling of the ipsilateral seminal vesicle and the bladder (Fig. 21–5C). Failure to opacify the bladder represents either insufficient injection of contrast or evidence of obstruction (Fig. 21–5D).
Venography
Venography of the internal spermatic veins has been used to diagnose and treat varicoceles. As a diagnostic test, venography is arguably the most sensitive imaging modality but specificity remains its limitation. Although nearly 100% of clinical varicocele patients will demonstrate reflux on venographic examination, left internal spermatic vein reflux has been reported in up to 70% of patients without a palpable varicocele (Ahlberg et al, 1966; Narayan et al, 1980). False-positive studies may be due to examination technique factors such as high-pressure contrast instillation or placement of the catheter tip through a valve in the proximal portion of the internal spermatic vein. Because of the high false-positive rate and the invasive nature of the test, venography is not indicated for routine screening in the subfertile male. It does have utility in patients with presumed postvaricocelectomy recurrence both for confirmation of the diagnosis and embolization of persistent vessels (Fig. 21–6A and B).
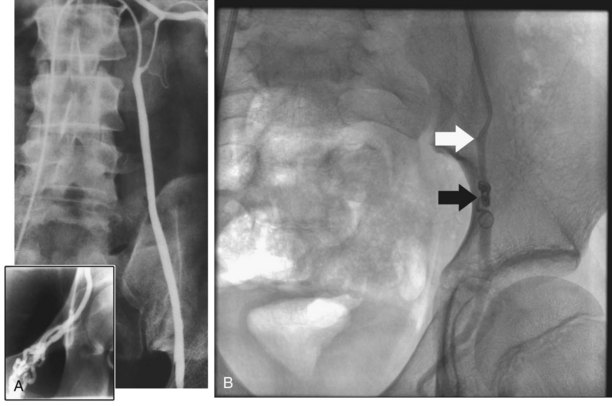
Figure 21–6 Venography for evaluation and treatment of varicoceles. A, Venogram demonstrates reflux through incompetent venous valves of the left internal spermatic vein consistent with left varicocele. Inset reveals inguinal plexis of gonadal veins. B, Venous embolization procedure with catheter tip (white arrow) and deployed coils (black arrow).
Percutaneous embolization of varicoceles has been described using deployed coils, balloons, and sclerotherapy. Although it may be used for initial treatment, higher recurrence rates than surgical repair and unsuccessful procedure rates support embolization as a second-line therapy (Khera and Lipshultz, 2008). In addition, rare safety issues such as balloon migration, femoral vein injury, and anaphylactic reactions to contrast material further reinforce the recommendation that embolization is not the initial procedure of choice (Matthews et al, 1992; Zini et al, 1997). However, embolization does have a role in the management of patients with a persistent or recurrent varicocele after varicocele ligation. Antegrade scrotal sclerotherapy has also been described as first-line varicocele therapy with success rates as high as 95%, although this technique awaits validation with larger series and with long-term follow-up (Tauber and Johnson, 1994; Ficarra et al, 2002).
Testicular Biopsy
Testicular biopsy has two roles in the management of male infertility: diagnostic for the differentiation of obstruction from nonobstructive testicular pathology and therapeutic for sperm harvest with the intention of use for ICSI. Sperm retrieval techniques are discussed later in the chapter and reviewed in detail in Chapter 22.
Diagnostic testicular biopsy is primarily indicated for evaluation of the azoospermic patient presenting with a clinical picture suggestive of obstruction to include normal testicular size and consistency and normal serum FSH levels. Although some have suggested testicular biopsy for moderate oligospermia (<5 to 10 million/mL), most clinicians do not find that this provides useful prognostic or therapeutic information. On occasion, a diagnostic biopsy may be performed in a patient with clinical evidence of testicular failure (small-volume testes, high serum FSH level) to assess ability to perform sperm harvest for ICSI in the future. However, in this setting, a diagnostic biopsy should be coupled with sperm retrieval and cryopreservation to mitigate the need for a repeat biopsy in the future. Men with known obstructive etiologies such as prior vasectomy or vasal agenesis do not require routine testicular biopsy. Unilateral testicular biopsy is usually sufficient to assess the azoospermic patient for obstruction. Bilateral biopsy may be performed if there is suggestion of asymmetric pathology such as unilateral testicular failure from a situation such as cryptorchidism and contralateral obstruction such as from prior inguinal surgery with vasal injury. Although testicular biopsy may be performed concurrently with planned reconstruction, reconstruction is often performed at a later surgery to allow permanent section analysis of the tissue by pathologists. Frozen sections of the testis or touch preparations of testicular tissue have less accuracy for identification of normal spermatogenesis than permanent sections. Testicular biopsy specimens should be placed in specific solutions such as Bouin’s, Zenker’s, or buffered glutaraldehyde because the normal formalin tissue preservative will introduce distortion artifacts into the specimen, making histologic analysis less accurate.
Interpretation of testicular biopsies requires an experienced pathologist because analysis is descriptive rather than quantitative in nature. Electron microscopy of the germinal epithelium has not been shown to provide clinical benefit over standard light microscopy. A variety of objective histologic scoring methods have been described in an effort to quantify spermatogenesis and predict outcomes from surgical reconstruction for obstruction, but these have not gained widespread acceptance due to the time-consuming nature of the analysis and the lack of validated studies (Johnsen, 1970; Silber and Rodriguez-Rigau, 1981). DNA flow cytometry has also been employed to quantify spermatogenesis, but this has not gained popularity for similar reasons (Kaufman and Nagler, 1987; Hellstrom et al, 1990). At the present time, the most commonly used system for analysis of testicular biopsy remains the histologic classification across a spectrum of standard patterns ranging from normal to germ cell aplasia with hypospermatogenesis and maturation arrest in between. Although single patterns may be seen throughout the biopsy, multiple patterns may often be identified.
Normal
More than 85% of testicular volume consists of seminiferous tubules made up of progressively maturing germ cells and their supporting Sertoli cells. Blood vessels and Leydig cells in the interstitial areas provide the rest of the cellular complement. Spermatogenesis proceeds in an orderly fashion from spermatogonia along the basement membrane to spermatocytes and finally mature spermatozoa adjacent to the tubular lumen (Fig. 21–7A). Type A spermatogonia provide the stem cell complement and have a diploid complement of chromosomes (2N). These cells undergo a mitotic division to replenish the stem cell complement and provide type B spermatogonia (2N). Type B spermatogonia undergo DNA synthesis, resulting in spermatocytes that have a tetraploid complement of chromosomes (4N). Two subsequent meiotic divisions result in spermatids with the final haploid number of chromosomes (1N). Further maturation results in a progression from round to elongated spermatids and then to the final product, mature spermatozoa. Human testes exhibit multiple stages of spermatogenesis in a single tubular section (patchwork pattern), unlike other mammalian testes, which produce waves of spermatogenesis progressing along a tubule.
Figure 21–7 Diagnostic findings on testicular biopsy. A, Normal. B, Hypospermatogenesis. C, Maturation arrest. D, Germinal cell aplasia (Sertoli cell–only pattern).
In the setting of azoospermia, a normal testicular biopsy is considered pathognomonic of ductal obstruction. Although focal areas of hypospermatogenesis and maturation arrest have been observed in the presence of obstruction, particularly in the setting of prior vasectomy, these are not classic findings for obstruction (Joshi et al, 1972; Perera, 1978). Dilated tubules with intraluminal debris are also commonly noted in patients exhibiting obstruction.
Hypospermatogenesis
Hypospermatogenesis is associated with reduced numbers of all germ cells, but all stages of spermatogenesis remain present in the histologic section (Fig. 21–7B). The degree of reduction determines whether the patient is oligospermic or azoospermic. A critical level of sperm production is required before sperm can be detected in the ejaculate, accounting for the common observation of hypospermatogenesis in the azoospermic patient.
Maturation Arrest
As the name suggests, maturation arrest involves a block of sperm maturation at a specific stage anywhere along the path of spermatogenesis (Fig. 21–7C). Maturation arrest most commonly occurs at the primary spermatocyte or late spermatid stages. Late maturation arrest may be difficult to distinguish from a normal biopsy, but the presence of mature sperm on testicular touch prep supports the diagnosis of normal spermatogenesis. Complete maturation arrest will produce azoospermia, whereas patients with partial maturation arrest may present with severe oligospermia. The remaining testicular architecture including the Sertoli and Leydig cells, as well as the basement membranes, are usually normal. Interestingly, maturation arrest is often associated with normal endocrine profiles such as FSH and inhibin levels due to an intact hypothalamic-pituitary-gonadal negative feedback system. Thus the clinical presentation of maturation arrest and ductal obstruction may be similar because both are associated with normal testicular volume and similar endocrine profiles.
Germinal Aplasia
Germinal aplasia, also called Sertoli cell-only syndrome, has small seminiferous tubules that are completely devoid of germ cells (Fig. 21–7D). The interstitial component, as well as the Sertoli cells and basement membranes, are normal. The diagnosis of germinal aplasia is suggested in an azoospermic patient with small-volume testes and elevated levels of FSH consistent with primary testicular failure. Although there are no current treatments to repopulate the testis with germ cells, low levels of spermatogenesis may be seen in other areas of the testis, which forms the basis for the use of microsurgical dissection testis biopsy (micro-TESE) to retrieve sperm in some of these patients for eventual ICSI use.
End-Stage Testes
Thickened basement membranes, tubular and peritubular sclerosis, and absence of both germ cells and Sertoli cells are characteristic histologic findings for end-stage testes. These patients will have azoospermia with profoundly small, soft testes (2- to 3-mL volume). Although this is characteristic of KS, some patients may still have small foci of retained spermatogenesis, which may be identified by micro-TESE. This histology may also be seen with cryptorchid testes.
Diagnostic and Treatment Categories of Male Infertility
Upon completion of a focused physical and laboratory examination, patients may be grouped into common patterns on the basis of seminal parameters (Table 21–8) and etiologic categories (Table 21–9). In the following sections, we review diagnostic criteria and appropriate therapy for specific diseases.
Table 21–8 Distribution of Seminal Parameters in Patients Presenting for Infertility Evaluation
| TYPE | INCIDENCE (%) |
|---|---|
| Abnormal semen parameter | 37 |
| Motility | 26 |
| Asthenospermia | 24 |
| Oligospermia | 8 |
| Agglutination | 2 |
| Volume | 2 |
| Morphology | 1 |
| Azoospermia | 8 |
| All parameters normal | 55 |
From Lipshultz L. Subfertility. In: Kaufman JJ, editor. Current urologic therapy. Philadelphia: WB Saunders; 1980.
Table 21–9 Distribution of Etiology of Male Infertility
| CATEGORY | NUMBER | PERCENT (%) |
|---|---|---|
| Varicocele | 603 | 42.2 |
| Idiopathic | 324 | 22.7 |
| Obstruction | 205 | 14.3 |
| Normal/female factor | 119 | 7.9 |
| Cryptorchidism | 49 | 3.4 |
| Immunologic | 37 | 2.6 |
| Ejaculatory dysfunction | 18 | 1.3 |
| Testicular failure | 18 | 1.3 |
| Drug/radiation | 16 | 1.1 |
| Endocrinopathy | 16 | 1.1 |
| Others (all <1%) | 31 | 2.1 |
Data from Nagler HM, Martinis FG. Varicocele. In: Lipshultz LI, Howards S, editors. Infertility in the male. St. Louis: Mosby Year Book; 1997. p. 336–59.
The goal of the infertility evaluation is to cause a successful pregnancy in the safest, most natural, expeditious, and cost-effective manner possible. Although advanced ART technologies such as ICSI are helpful tools in the armentarium for the management of infertility, all attempts should be made to correct underlying male factor etiologies before proceeding with these treatments. The high cost of these technologies, the shifting of treatment burden of male factor to the female partner, and intrinsic safety issues with ICSI mandate a complete exploration of correctable male factor issues before proceeding with ICSI.
Semen Parameter Categories
Azoospermia
Azoospermia is defined as the absence of sperm in the ejaculate and is identified in 10% to 15% of infertile males (Jarow et al, 1989). Before proceeding with further diagnostic procedures, the diagnosis of azoospermia should be confirmed with at least two centrifuged semen specimens to rule out severe oligospermia. The finding of even small quantities of sperm in the centrifuged specimen rules out complete ductal obstruction such as CBAVD and also offers the potential for immediate sperm cryopreservation for ICSI cycles.
Although there is a myriad of causes of azoospermia, etiologies fall into three general categories: pretesticular, testicular, and post-testicular. Causes can usually be differentiated on the basis of semen volume (low vs. normal), physical exam findings of testicular volume and presence of vas deferens as well as serum FSH level (Fig. 21–8).
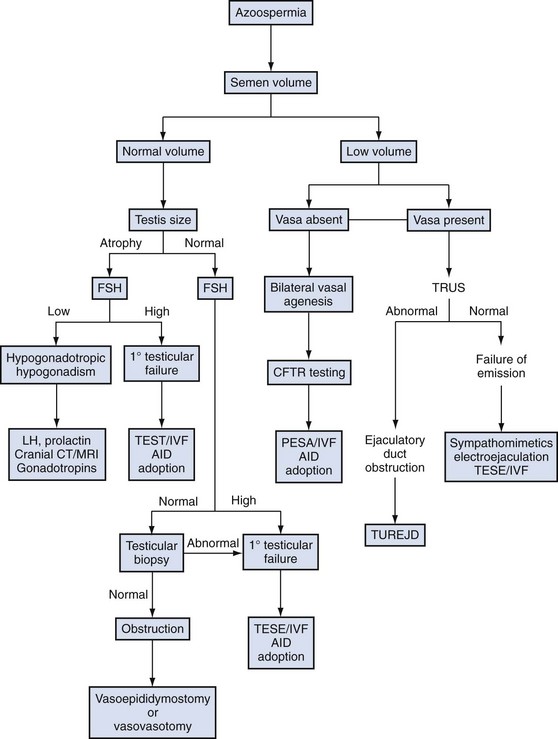
Figure 21–8 Algorithm for evaluation and treatment of azoospermia. AID, artificial insemination by donor semen; CFTR, cystic fibrosis transmembrane conductance regulator; CT/MRI, computed tomography/magnetic resonance imaging; FSH, follicle-stimulating hormone; IVF, in-vitro fertilization; LH, leuteinizing hormone; PESA, percutaneous epididymal sperm aspiration; TESE, testicular sperm extraction; TRUS, transrectal ultrasound; TUREJD, transurethral resection of the ejaculatory ducts.
Pretesticular causes, also called secondary testicular failure, are usually endocrine in nature and relate to either congenital (Kallman syndrome) or acquired hypogonadotropic hypogonadism, which are addressed in detail later in this chapter.
Testicular etiologies, broadly termed as primary testicular failure, are intrinsic disorders of spermatogenesis. Direct testicular pathology may derive from genetic abnormalities such as Y-chromosome microdeletions or chromosomal abnormalities, varicocele-induced testicular damage, gonadotoxic effects from medications or environmental exposures, and idiopathic infertility, which constitute the majority.
Ejaculatory dysfunction or obstruction of the genital tract account for the post-testicular pathologies, which constitute 40% of cases of azoospermia (Best Practice Committees of American Urological Association and American Society for Reproductive Medicine, 2006). Pretesticular and post-testicular causes are often amenable to treatment, which may restore fertility, whereas the success rates for intervention in testicular pathology are much more modest.
Although both primary and secondary testicular failure will be associated with marked reduction in testicular volume, these entities can be distinguished by serum endocrine testing to include FSH, LH, testosterone, and prolactin levels. High serum FSH levels, typically greater than two times normal, are indicative of primary testicular failure, and diagnostic testicular biopsy is not required to rule out obstructive etiologies. Primary testicular failure in conjunction with azoospermia, commonly termed nonobstructive azoospermia (NOA), is best managed with testicular sperm harvest for eventual ICSI. The absolute degree of serum FSH elevation has not proven predictive of success rates for sperm retrieval (Vernaeve et al, 2004; Hibi et al, 2005; Harris and Sandlow, 2008). Azoospermic patients with normal testicular size, palpable vas deferens, and normal serum FSH levels require a diagnostic testicular biopsy to differentiate genital tract obstruction from disorders of spermatogenesis such as maturation arrest.
Obstructive azoospermia accounts for 40% of cases of azoospermia (Practice Committee, American Society of Reproductive Medicine, 2008). A normal testicular biopsy is pathognomonic for genital tract obstruction. If these patients desire restoration of patency, they will require scrotal exploration and vasography at the time of planned reconstruction to identify the site of obstruction. Genital tract obstruction may occur anywhere along the sperm transport system to include the rete testis, efferent ductules, epididymis, vas deferens, or ejaculatory ducts. Obstruction at the level of the epididymis or vas deferens may be successfully treated with microsurgical reconstruction, either vasoepididymostomy for epididymal obstruction or vasovasostomy for vasal obstruction. Elective vasectomy remains the leading cause of obstructive azoospermia and subsequent infertility. In the absence of prior injury to the vas either intentionally from vasectomy or iatrogenic injury of inguinal vas from hernia repair, most men with idiopathic ductal obstruction will be obstructed at the level of the epididymis. Epididymal obstruction may be due to prior trauma, infection, or the result of downstream vasal obstruction from an elective vasectomy. Although vasectomy usually results in a single obstructive site, the development of high intraluminal pressures after a vasectomy can result in rupture of the delicate epididymal tubule with secondary obstruction in the epididymis. Epididymal obstruction rarely occurs within 4 years of a vasectomy but is present in more than 60% of patients on one or both sides after 15 years of vasal obstruction (Fuchs and Burt, 2002). The absence of proximal vasal fluid or thick, viscous, pastelike fluid is indicative of a concurrent epididymal obstruction and such patients will require a vasoepididymostomy for their reconstruction. The outcome of reconstruction in vasectomized men depends on a number of factors including the obstructive interval, the quality of the fluid from the proximal vas at the time of surgery, and the surgeon’s microsurgical experience (Sabanegh, 2009).
Azoospermia in conjunction with low semen volumes and palpable vas deferens is most likely caused by ejaculatory duct obstruction (EDO), although rarely, ejaculatory dysfunction can cause a similar presentation. Disorders of ejaculation such as retrograde ejaculation will more frequently cause oligospermia in conjunction with low semen volume and are readily diagnosed by the presence of sperm in a postejaculation urine specimen. The diagnosis of EDO is suggested by low semen volume with acidic pH and absent semen fructose. TRUS will often reveal midline prostatic cysts, dilated ejaculatory ducts, or dilated seminal vesicles (>1.5 cm in width), and seminal vesicle aspiration may produce large numbers of sperm. Ultimately, EDO may be confirmed by vasography at the time of planned transurethral resection of the ejaculatory ducts (TUREJD) to relieve the obstruction.
Oligospermia
Oligospermia is defined as a sperm density of less than 20 million/mL. Unlike the situation with azoospermia, the number of potential diagnoses causing oligospermia can be quite vast and the etiology is often idiopathic. Oligospermia is rarely seen as an isolated seminal abnormality but is usually associated with disturbances in motility and morphology. Endocrinopathies are rarely observed in patients with concentrations higher than 10 million/mL, so routine endocrine screening with serum testosterone and FSH levels are reserved for counts lower than 10 million (Sigman and Jarow, 1997). Testis biopsy is not indicated in the setting of mild to moderate oligospermia, although it can be considered in patients with sperm concentrations under 1 million/mL in whom ductal obstruction is considered on the basis of history or physical examination findings.
Seminal Quality Abnormalities
Isolated defects may occur in motility or morphology, known as asthenospermia or teratospermia, respectively, while global sperm quality abnormalities are described with the term oligoasthenoteratospermia (OAT). Asthenospermia may be iatrogenic from delayed processing in the laboratory or may be due to a prolonged abstinence period. Persistent asthenospermia in a properly processed specimen, while often idiopathic, may be seen in association with varicoceles, genital tract infections, ultrastructural cilia abnormalities such as immotile cilia syndrome, and immunologic infertility in association with antisperm antibodies.
Teratospermia is a common finding, especially with use of the strict morphologic criteria (Tygerberg or Kruger) applied by many andrology laboratories. Bizarre morphologies of the sperm head such as pinhead sperm, multiheaded sperm, or round-headed sperm, which suggest acrosome deficiency, undoubtedly have clinical significance, although there is growing controversy regarding the predictive utility of abnormal morphology in general, largely due to the subjective nature of the test making it difficult to standardize (Agarwal et al, 2008). Abnormal sperm morphology has not been correlated with recurrent spontaneous miscarriages or abnormalities in offspring (Rosenbusch et al, 1992; Hill et al, 1994).
Low ejaculate volume may also be a contributing factor to subfertility. Reduction in ejaculate volume is most commonly due to improper specimen collection but may be associated with CBAVD with associated hypoplasia of the seminal vesicles, hypoandrogenism, retrograde ejaculation, or obstruction of the ejaculatory duct. Relative acidity of the seminal pH and low semen fructose suggest absence of seminal vesicle contribution to the semen, pointing to either CBAVD or EDO as lead possible causes of subfertility.
Normal Bulk Semen Parameters
Up to 50% of men presenting for an infertility evaluation will have normal bulk semen parameters, representing a particularly difficult population to be assigned an etiology for subfertility and reinforcing the inherent inability of standard semen analyses to assess sperm function. In these couples, particular attention should be given to identifying occult female factor fertility issues, as well as assessing coital frequency to optimize reproductive timing for conception. On occasion, antisperm antibodies in the cervical mucus may inhibit sperm motility in vivo and prevent fertilization. This situation can be quantified with indirect antisperm antibody testing of the cervical mucus, although an in-vivo assessment of compatibility of sperm with the cervical mucus can be provided with the postcoital test (PCT). Couples with an abnormal PCT may benefit from intrauterine insemination, which bypasses the hostile cervical factors.
Varicocele
Varicoceles represent the most common attributable cause of primary and secondary infertility in the male (see Table 21–9). Although there is a large body of research on varicoceles dating back to Tulloch’s first report of varicocele ligation for male infertility in 1955, much of the subsequent research has suffered from methodologic flaws, lack of standardized definitions for varicoceles, and failure to control confounding issues such as concurrent female factor infertility. Despite these limitations, varicocele treatment remains the most commonly performed surgery for the correction of male factor subfertility.
Varicoceles have been attributed to turbulent venous flow related to the right angle insertion of the left testicular vein into the left renal vein, an explanation supported by the left-sided predominance of these lesions. The insertion of the right testicular vein directly into the inferior vena cava is believed to provide less flow turbulence and back pressure, which translates into a lower incidence of venous dilation in the right spermatic cord. In addition, incompetent or absent venous valves in the gonadal veins allow retrograde reflux of blood into the scrotum with the standing position (Braedel et al, 1994). On rare occasions, a “nutcracker phenomenon” has been described where the left renal vein may be compressed between the superior mesenteric artery and the aorta producing elevated pressure in the left gonadal vein and resulting in a varicocele.
The mechanism of varicocele-induced impairment of spermatogenesis remains the subject of much debate. The lead theory postulates that testicular cellular processes are exquisitely temperature dependent. Venous pooling from a varicocele produces elevated intrascrotal temperature resulting in reductions in testosterone synthesis by Leydig cells, injury to germinal cell membranes, altered protein metabolism, and reduced Sertoli cell function (Khera and Lipshultz, 2008). Varicocele ligation has been demonstrated to be associated with reductions in intrascrotal temperature (Wright et al, 1997). It has also been suggested that free reflux of renal and adrenal metabolites from the left renal vein are directly gonadotoxic. Spermatic cord blood catecholamine and prostaglandin levels appear elevated in varicocele patients, although the direct gonadotoxic effects of these compounds remain to be established in humans (Comhaire et al, 1974; Ito et al, 1982). Other proposed mechanisms for varicocele-induced subfertility include impaired venous drainage with resulting hypoxia, poor clearance of gonadotoxins, and elevated levels of oxidative stress. The level of seminal oxidative stress correlates with varicocele grade and improves with treatment of the varicocele (Allamaneni et al, 2004; Khera et al, 2007). Although conflicting evidence exists for each theory, it appears that the mechanism of varicocele damage is multifactorial.
Treatment of varicoceles in the subfertile patient has produced variable results on the basis of the definition of the varicocele (subclinical vs. clinical) and the method of intervention used. The various methods of varicocele treatment all involve ligation or occlusion of dilated gonadal veins. Surgical ligation has been approached through retroperitoneal, inguinal, and subinguinal dissection, whereas embolization is a radiologic procedure. These techniques are discussed in depth in a later chapter. The most current guidelines in 2008 by the Best Practice Committee of the American Society for Reproductive Medicine recommend treatment of a varicocele in the infertile patient when all of the following conditions are met: (1) varicocele is palpable on physical examination; (2) the couple has known infertility; (3) the female partner has normal fertility or a potentially treatable cause of infertility; and (4) the male partner has abnormal semen parameters or abnormal results from sperm function tests. Patients with subclinical varicoceles are not candidates for varicocele treatment due to a lack of demonstrated efficacy in this population (Yamamoto et al, 1996). On occasion, large varicoceles will produce clinical symptoms such as dull hemiscrotal discomfort or sense of heaviness and these patients will benefit from varicocele treatment.
Adolescent males with unilateral or bilateral clinical varicoceles and ipsilateral testicular hypotrophy are also candidates for varicocele repair. Although there remains controversy with the threshold for testis size reduction to support varicocele intervention in the adolescent, authors have suggested 2-mL volume or 20% volume decrement from the contralateral testis as evidence of significant varicocele-induced damage (Gargollo and Diamond, 2009). Further support can be provided by pretreatment semen analysis, although this can be challenging to obtain in the adolescent patient. Recovery of testicular volume, so called “catch-up growth,” has been reported to occur in up to 80% of boys with grade II or III varicoceles (Kass and Belman, 1987; Gershbein et al, 1999), although this has been challenged by a more recent study that suggests testicular growth will occur spontaneously in conservatively managed patients with varicoceles (Kolon et al, 2008). In the absence of testicular growth retardation, adolescents with varicoceles should be followed with annual testicular size assessments, as well as semen analysis if possible to assist early identification and intervention of varicocele-induced testicular damage.
Outcomes of varicocele treatment remain the subject of some controversy, although substantial evidence exists to support intervention in patients with clinical varicoceles and abnormal semen quality. The bulk of studies (Table 21–10) has shown improvements in seminal parameters with varicocele repair (Schlesinger et al, 1994) and specific functional testing to include sperm penetration assay (Ohl et al, 2007), sperm DNA fragmentation levels (Zini et al, 2005), and oxidative stress levels (Mostafa et al, 2001). A recent meta-analysis incorporating studies using current clinical guidelines for varicocele repair reported mean increases in sperm density of 9.7 million/mL, motility increases of 9.9%, and WHO sperm morphology improvement by 3% (Agarwal et al, 2007). Semen quality has been reported to improve in 51% to 78% of infertile men after varicocele treatment (Turek, 2005). The impact of varicocele grade on post-treatment semen quality remains unclear, although the bulk of studies suggests that larger varicoceles produce more significant impairment in semen parameters and the repair of bilateral varicoceles produced increased benefit over the repair of unilateral varicoceles (Richardson et al, 2008). In the setting of underlying genetic abnormalities associated with the subfertility, varicocele treatment does not appear to produce significant improvement in outcomes (Cayan et al, 2001). Azoospermic patients with varicoceles have shown some potential for return of sperm to their ejaculate after varicocele treatment (Table 21–11), although studies have been relatively small with modest conception results. The vast majority of azoospermic patients with return of sperm postvaricocele treatment will still require advanced ART such as in-vitro fertilization to obtain conception.
Table 21–10 Outcomes of Varicocele Treatment in Nonazoospermia Men (Controlled Trials) for Clinical Varicoceles
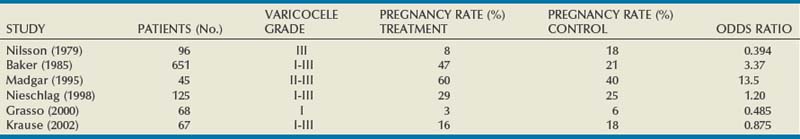
Spontaneous pregnancy rates after varicocele treatment have been reported to average between 30% and 50% in the larger series (Abdulmaaboud et al, 1998; Segenreich et al, 1998; Perimenis et al, 2001) with pregnancies occurring at an average of 8 months after treatment (Pryor, 1987). A recent meta-analysis after surgical varicocelectomy noted that the odds of spontaneous pregnancy were 2.87 times higher over the nontreatment group (Marmar et al, 2007). Cost-benefit analyses have generally been supportive of varicocele treatment over ART techniques (Schlegel, 1997). In addition, 30% to 50% of couples who are felt to require ART due to low semen quality may be able to avoid this after varicocele treatment (Cayan et al, 2002) or have improved efficacy with post-treatment intrauterine insemination (Daitch et al, 2001).
Cryptorchidism
Cryptorchidism is a relatively common condition noted in 2.7% of newborns and up to 0.8% of 1 year olds (Score, 1964). It is a well-known etiology for subfertility and has been associated with reduced testicular size and sperm concentration, as well as reductions in serum inhibin and elevations in serum FSH levels (de Gouveia Brazao et al, 2003; Caroppo et al, 2005). It is important to distinguish cryptorchid testes from retractile testes, a condition involving hyperactive cremasteric muscles causing the testis to periodically reside in the inguinal canal or high scrotum. Although retractile testes have been associated with depressed spermatogenesis, more severe reductions in semen quality have been identified in cryptorchid patients (Caroppo et al, 2005). Suggested mechanisms for cryptorchidism-induced subfertility include testicular dysgenesis, impaired endocrine axis, immunologic damage, and obstruction. Controversies remain as to the exact effect on fertility from unilateral versus bilateral cryptorchidism and the protective effect and timing of orchidopexy. Early studies reported significant detrimental effects on semen parameters with even unilateral cryptorchidism (Kogan, 1985; Cendron, 1989), but newer work suggests that unilateral cryptorchidism may be associated with modest or no significant impact on fertility (Lee, 2005; Murphy, 2007). The level of the cryptorchid testis is predictive of spermatogenic impairment with germinal cell aplasia found in 20% to 40% of inguinal testes versus 90% of intra-abdominal testes (Hadziselimovic, 1984). One epidemiologic study comparing fertility in men with either unilateral or bilateral cryptorchidism versus age-matched controls reported paternity rates of 89% in unilateral cryptorchidism, 93% in age-matched controls, and 65% in patients with a history of bilateral cryptorchidism (Lee, 2005). Some studies have suggested that orchiopexy at younger ages, typically younger than 4 years of age, is associated with improved fertility outcomes (Coughlin et al, 1999; Lee, 2002), although the question still awaits large longitudinal studies.
Endocrinopathies
Endocrine-derived causes of infertility may occur at the hypothalamic, pituitary, or gonadal levels. Originally believed to be a rare cause of infertility, growing evidence suggests that hypogonadism, as defined by the U.S. Food and Drug Association (FDA), established normal testosterone level of 300 ng/dL may be highly prevalent in infertile males, occurring in 45%, 43%, and 35% of men with nonobstructive azoospermia, oligospermia, and normal semen parameters, respectively (Sussman et al, 2008).
Primary hypogonadism, also called primary testicular failure or hypergonadotrophic hypogonadism, is defined as low serum testosterone and elevated gonadotropin level consistent with organ function failure at the level of the testes.
Secondary hypogonadism, also called hypogonadotropic hypogonadism, has low testosterone levels in conjunction with low gonadotropin levels.
The time of onset of hypogonadism induces variable clinical presentations. Failure of testosterone surge at the expected time of puberty produces delayed or absent puberty, growth retardation, and small, soft testis. Patients with onset of hypogonadism after puberty will have normal secondary sexual characteristics and body habitus but small firm testis consistent with possible fibrosis after normal testicular development.
Hypogonadotropic Hypogonadism
Isolated gonadotropin deficiency or hypogonadotropic hypogonadism (HH) is a relatively rare cause of subfertility accounting for less than 1% of cases of male infertility and may be congenital or acquired (Sigman and Jarow, 1997).
Acquired causes of hypogonadotropic hypogonadism include pituitary disease, which may result from prior surgery, infarction, tumors, or infection; metabolic disorders; and a variety of other medical conditions (Table 21–12).
Table 21–12 Acquired Causes of Hypogonadotropic Hypogonadism
Although variants of congenital hypogonadotropic hypogonadism have been described (Table 21–13), the anosmic form or Kallman syndrome is the most commonly reported, occurring in between 1:10,000 and 1:60,000 births (Oates, 1997). In addition to the anosmia and azoospermia noted with Kallman syndrome, other clinical features may include midline facial defects such as cleft palate; gynecomastia; neurologic abnormalities (mental retardation, oculomotor defects, deafness, and synkinesia); unilateral renal agenesis; cryptorchidism; micropenis; and pes cavus (Sussman et al, 2008). Kallman syndrome results from failure to secrete gonadotropin-releasing hormone (GnRH) by the hypothalamus, leading to low gonadotropin levels and ultimately, failure to transition the prepubertal testis to postpubertal level of function. The GnRH deficiency noted with Kallman syndrome is the result of failed embryonic migration of neuroendocrine GnRH cells from the olfactory epithelium to the forebrain. Absence or hypoplasia of the olfactory bulbs accounts for the concurrent anosmia. Kallman syndrome represents a genetically diverse group of diseases with both X-chromosome linked inheritance via mutations in the KAL1 gene, as well as autosomal dominant inheritance via mutations in the fibroblast growth factor receptors 1 and 8 (FGFR1, FGFR8), the prokineticin receptor-2 (PROKR2), and the prokineticin-2 (PROK2) genes. Mutations in these five genes are found in less than 30% of Kallman syndrome patients reinforcing the need for further genetic study on these complex patients (Dode, 2009).
Table 21–13 Variants of Congenital Hypogonadotropic Hypogonadism
From Sussman EM, Chudnovsky A, Niederberger CS. Hormonal evaluation of the infertile male: has it evolved? Urol Clin North Am 2008;35(2):147–55.
Prader-Willi syndrome (PWS), another form of congenital hypogonadotropic hypogonadism, has features that include infantile hypotonia, obesity, cryptorchidism, short stature, and mental retardation. It is caused by microdeletions or mutations on the paternal chromosome 15 at the q11 or q13 location (Smeets et al, 1992). Because of concurrent medical problems in these patients, most do not seek treatment for infertility.
Hypogonadotropic hypogonadism usually presents in the late teenage years when the patient fails to undergo the usual pubertal changes with secondary virilization. It is of critical importance to distinguish HH from constitutional delay of growth and puberty (CGDP) because they share many clinical and hormonal features and yet require markedly different treatments. Usually, CGDP patients will attain spontaneous puberty by age 18 or may require transient androgen replacement for puberty induction but then will progress through normal development and fertility. HH patients will require lifelong androgen replacement for maintenance of virilization and episodes of exogenous gonadotropin treatment off androgen therapy for fertility induction as described later. Because differentiation is not always possible on the basis of serum testosterone and gonadotropin levels, a number of physiologic and stimulatory tests have been described to discriminate these conditions to include serial serum LH measurements, prolactin response to TRH, and GnRH and human chorionic gonadotropins (HCG) stimulation tests (Segal, 2009). Patients with CGDP often demonstrate LH pulses with serial overnight blood draws and gonadotropin surge in response to GnRH stimulation. HH patients have minimal variability in LH levels and do not demonstrate significant increases in gonadotropins levels with administration of GnRH.
In general, treatment of HH syndromes is directed to two ultimate goals: (1) induction of normal serum androgen levels to allow appropriate virilization and bone growth and (2) the induction of spermatogenesis and, ultimately, fertility. During adolescence, puberty and virilization are usually initiated with exogenous androgen treatment. Various effective methods of testosterone replacement therapy exist including intramuscular testosterone enanthate or cypionate (200 mg every 2 weeks), transdermal patches (5 to 10 mg/day), testosterone gel, or buccal tablets. Adequacy of replacement can be assessed by serum testosterone levels, which should be within the midrange of normal, and observation of the desired phenotypic response to therapy. Exogenous testosterone is effective in induction of virilization but will inhibit spermatogenesis, so alternate therapies will be required when the patient desires fertility.
Induction of spermatogenesis in the HH patient requires the maintenance of intratesticular testosterone levels that are exponentially higher than that obtainable with conventional androgen replacement therapy. To obtain such levels, the usual first-line therapy for HH involves treatment with human chorionic gonadotropin (hCG), which is biologically similar to LH, inducing Leydig cell production of testosterone. hCG is administered subcutaneously at a dose of 1500 to 2000 IU two to three times per week for 4 to 6 months. When testicular volume and serum testosterone levels are stable without significant further improvement, FSH stimulation is added to the treatment regimen to induce spermatogenesis. FSH may be administered in the form of human menopausal gonadotropin (hMG), a compound that contains both FSH and LH in equal doses or with recombinant human FSH formulations. Human menopausal gonadotropin is administered at a dose of 75 IU two to three times per week, and recombinant FSH usually uses doses of 37.5 to 75 IU three times per week. FSH therapy is continued until attainment of sperm concentrations of at least 5 million per mL in the ejaculate or pregnancy is obtained. The vast majority of patients will be able to conceive after gonadotropin therapy, although 71% of the patients with subsequent fertility have sperm concentrations considerably lower than normal, suggesting that these patients are often able to conceive with low counts (Burris et al, 1988). Interestingly, 10% of patients may maintain normal serum testosterone levels after cessation of all endocrine therapy, implying that HH may be reversible in some patients (Raivio et al, 2007). Prior treatment of HH patients with testosterone does not affect success with future gonadotropin therapy (Ley and Leonard, 1985; Hamman and Berg, 1990).
Hypogonadotropic hypogonadism patients with intact pituitary function can also be effectively treated with pulsatile GnRH therapy supplied via subcutaneous infusion from portable pump or intranasal administration (Klingmuller, 1985; Aulitzky, 1988). Although direct GnRH therapy is effective, inconvenience of administration and expense do not justify this choice over traditional gonadotropins therapy at the present time (Liu, 1988). In addition, selected patients with HH presenting after puberty with intact pituitary function may respond to clomiphene citrate, an antiestrogen therapy (Whitten et al, 2006).
Androgen Excess
Excess systemic levels of androgen paradoxically inhibit spermatogenesis due to direct feedback inhibition of gonadotropin secretion at the level of the hypothalamus and pituitary. This ultimately results in low intratesticular levels of testosterone, which are inadequate for the maintenance of spermatogenesis, an observation that provides the impetus for growing research examining the potential contraceptive role of exogenous testosterone.
Excess states may result from either endogenous production or exogenous administration of androgens. Elevations in endogenous production of androgens may be caused by congenital abnormalities of steroid metabolism such as congenital adrenal hyperplasia or androgen-producing tumors of the testis or adrenal gland. Congenital adrenal hyperplasia (CAH), the most common endogenous etiology of androgen excess, encompasses a variety of specific enzyme defects in cortisol and aldosterone synthesis. Inadequate systemic cortisol levels result in excessive pituitary release of ACTH leading to hyperstimulation of the adrenal gland with subsequent release of adrenal androgens and suppression of pituitary gonadotropin release. Deficiency in the 21-hydroxylase enzyme accounts for 90% of cases of CAH. Although severe deficiency in 21-hydroxylase activity can present in the neonatal period with salt wasting, milder deficiencies present more commonly in childhood with phallic enlargement, precocious puberty, and advanced skeletal maturation. Elevated levels of serum 17-hydroxyprogesterone (often >1000 ng/dL) and urinary pregnanetriol support the diagnosis of 21-hydroxylase deficiency. The impact on subsequent fertility is variable, depending on the degree of androgen excess. Treatment for CAH consists of glucocorticoid replacement, which reduces ACTH levels and adrenal androgen production. In one small series of adult patients with CAH, 60% of men were able to cause pregnancies either with treatment or even in the absence of CAH treatment (Urban, 1978).
Anabolic steroids represent a growing etiology of male subfertility with a similar mechanism of spermatogenic disruption to that observed with CAH. It remains problematic to accurately predict the impact of these agents because of the variety of performance-enhancing substances used, as well as the variation in doses employed. Older studies of semen quality in athletes using high doses of anabolic steroids revealed severe impairment of sperm concentration, motility, and morphology (Knuth et al, 1989). Although cessation of use allowed normalization of seminal parameters in an average of 4 months, cases of persistent azoospermia have been reported occurring more than 1 year later (Jarow, 1990). To avoid the fertility-related side effects, anabolic steroid users have employed modified regimens that add hCG with steroid to protect spermatogenesis. These combinations, although causing transient impairment in semen quality, seem to allow preservation of spermatogenesis in some patients (Karila et al, 2004). In patients with persistent azoospermia or severe oligospermia after steroid abuse, hCG alone or in combination with hMG has been successfully used to restore spermatogenesis (Pundir, 2008).
Hyperprolactinemia
Because of its low yield, screening serum prolactin measurements in the infertile male is not indicated unless associated with erectile dysfunction, low serum testosterone levels, or decreased libido. Although often idiopathic, hyperprolactinemia can be associated with medications, physiologic stress, and pituitary tumors. Because of the marked physiologic variability noted with serum prolactin levels, elevated levels should be confirmed by at least one additional measurement. If elevated prolactin levels are confirmed (>18 ng/dL), anatomic imaging of the sella turcica is required, usually with magnetic resonance imaging with gadolinium contrast. Although macroadenomas may require surgical excision, microadenomas are usually responsive to dopamine agonist therapy with either bromocriptine or cabergoline. Cabergoline offers advantages over bromocriptine to include longer half-life, allowing less frequent dosing and an improved side effect profile without compromising therapeutic efficacy. Despite limited data on the therapeutic efficacy of dopamine agonist therapy for the infertile male with hyperprolactinemia, several small studies have reported improvements in sperm concentration and motility, as well as normalization of serum prolactin levels with treatment (Laufer et al, 1981; Mancini et al, 1984).
Estrogen Excess
Regardless of the source of the estrogen excess, estrogens inhibit GnRH secretion by the hypothalamus and gonadotropin release by the pituitary. In addition to the effects on the hypothalamic-pituitary-gonadal axis, elevations in serum estrogen levels produce direct deleterious effects on spermatogenesis (Hammoud et al, 2008). Hormonal evaluation in the infertile patient will reveal low levels of serum FSH, LH, and testosterone with elevations in serum estrone and estradiol (E2).
In the male, hyperestrogenemia may result from hepatic disease, estrogen-producing tumors, or, most commonly, obesity. Sertoli or Leydig cell testicular tumors and adrenal cortical tumors may rarely produce elevated estrogen levels. In the obese patient, elevations in serum estrogen derive from elevated peripheral aromatization of androgen to estrogen occurring in adipose tissue via an aromatase enzyme. The level of serum androgen inversely correlates with the degree of obesity (Giagulli et al, 1994; Tchernof et al, 1995). This provides the scientific basis for the use of selective medical therapy with aromatase inhibitors in men with reduced testosterone-to-estrogen ratios, which are discussed later in this chapter. In the obese patient, weight loss and bariatric surgery have been associated with improvements in endocrine profiles (Kaukua et al, 2003; Globerman et al, 2005), although the ultimate effect on fertility remains subject to further study.
Genetic Syndromes
Disorders of Sex Chromosome Number and Structure
Klinefelter Syndrome (47,XXY)
KS, or 47,XXY male syndrome, is the most common genetic cause of nonobstructive azoospermia (NOA), accounting for up to 10% of these cases (Oates, 2003; Visootsak and Graham, 2006). It has been reported to occur in between 1:500 and 1:1000 live male births (Nielsen and Wohlert, 1991; Simpson et al, 2003). The majority of KS patients have a pure 47,XXY karyotype due to nondisjunction during the meiotic phase of the parental gametes, although 10% of patients are mosaic if the nondisjunction occurs during the mitotic cell division of embryogenesis (Therman et al, 1993). The extra X chromosome may be of maternal or paternal origin, but advancing paternal age is a risk factor for sperm with an XY complement and subsequent KS offspring. The frequency of XY sperm was reported to be 10%, 31%, and 160% higher in men in their 30s, 40s, and 50s, respectively, as compared with men in their 20s (Lowe et al, 2001; Eskenazi et al, 2002).
Although the exact mechanism of the subfertility induced by the supernumerary X chromosome remains to be defined, this karyotype results in severe spermatogenic and androgenic failure. In 1942 Klinefelter originally described a triad of gynecomastia, hypergonadotropic hypogonadism, and infertility (Klinefelter et al, 1942). Since the original report, KS has been identified with a wide spectrum of clinical presentations. The most severe form presents with delayed or absent puberty, incomplete virilization, and eunuchoid appearance. These patients tend to have tall, slender statures with long legs, narrow shoulders, and proportionately shorter torsos. Often they present for medical attention during adolescence and may be placed on testosterone replacement to induce puberty. On the other end of the developmental spectrum are patients who have adequate androgen levels to induce puberty but are noted to have KS during a fertility evaluation in their adulthood. Both extremes of presentation share common features of small testicular size (<8 to 10 mL testis volume), elevated serum gonadotropin levels, and azoospermia, although mosaic patients may occasionally have rare sperm identified in semen.
In addition to the detrimental effects on testicular function, KS is associated with significant developmental, neoplastic, and metabolic implications. Early studies suggested severe cognitive and behavioral abnormalities in KS patients, yet more recent reports have identified only mild cognitive impairment with speech and language deficiencies, as well as fine motor developmental delays (Youings et al, 2000; Fales et al, 2003). KS patients have been described to have as much as a 50-fold increased risk of breast cancer development reinforcing the need for monthly breast self-examination and screening in this population (Swerdlow et al, 2005). They have also been noted to have an increased risk of non-Hodgkin lymphoma and extragonadal mediastinal germ cell tumors (Aguirre et al, 2006; Oates, 2008). The underlying hypogonadism in KS patients predisposes them to the development of metabolic syndrome with the associated findings of abdominal obesity, hyperlipidemia, diabetes mellitus, and cardiovascular disease with an associated increase in risk of mortality from diabetic or cardiovascular complications (Lanfranco et al, 2004; Ishikawa, 2008).
Although most KS patients will present with azoospermia, rare cases of unassisted paternity in these patients exist, reinforcing the need for careful semen analysis of centrifuged specimens to assess for severe oligospermia, or cryptospermia. The predominant testicular histology in these patients is germinal cell aplasia with seminiferous tubular sclerosis, but there can be small areas of residual complete spermatogenesis. Advances in testicular biopsy techniques including microsurgical dissection have allowed successful sperm retrieval in up to 69% of KS patients (Denschlag et al, 2004; Gonsalves et al, 2005; Schiff et al, 2005). These techniques are covered in detail in Chapter 22. Sperm retrieved from KS patients is associated with high fertilization rates with ICSI, although there have been isolated reports of KS genotypes in offspring (Ron-El et al, 2000; Komori et al, 2004; Okada et al, 2005). This reinforces the need for all KS patients to be offered genetic counseling before proceeding with surgical sperm retrieval, both for their own health issues and the potential implications for offspring.
46,XX Male Syndrome
Also called the sex reversal syndrome, 46,XX male syndrome has an incidence of 1:20,000 male babies (de la Chapelle, 1972). Although sharing some similarities in presentation with KS including small testicular size, gynecomastia, and azoospermia, these patients have shorter stature than average men, an increased incidence of hypospadias, and normal levels of cognitive function (Vorona et al, 2007). In 90% of these patients, the testis-determining (SRY) gene including a small distal portion of the short arm of the Y chromosome (Yp) has been translocated to either one of the X chromosomes or to an autosome allowing differentiation of the bipotential gonads into testes (Schiebel et al, 1997). In the remaining 46,XX men, the SRY gene is not identified and it is presumed that other Y-chromosome genes have translocated to an autosome (Rajender et al, 2006). Unlike KS patients, these men are missing the AZFa, AZFb, and AZFc genetic regions, resulting in a complete absence of spermatogenesis. At the current time, there are no reports of successful sperm harvest and testicular biopsy is not recommended for prognostic or therapeutic purposes.
47,XYY Syndrome
Found in 0.1% of male births, the 47,XYY karyotype is characterized by a normal male phenotype and endocrine profile (Oates, 2002). This syndrome has been associated with reduced intelligence and antisocial behavior, although its mechanism has been controversial (Oates, 1997). These patients typically retain some degree of spermatogenesis, although they may have severe oligospermia or complete azoospermia. Fortunately, only a small percentage of spermatogonia have an abnormal genetic complement (0.35% 24,XY; 0.43% 24,YY) suggesting a low risk of genetic abnormalities in spontaneous or ICSI-derived offspring of these patients (Egozcue et al, 2000). It is still recommended that these patients receive genetic counseling before any testicular sperm harvest or ART procedure.
Noonan Syndrome
Noonan syndrome (NS) is commonly referred to as male Turner syndrome because both syndromes share numerous clinical features. Unlike the female Turner syndrome (45X0), the inheritance pattern for NS is autosomal dominant with a normal 46,XY karyotype. Although the exact chromosomal locus remains to be determined, studies have implicated chromosome 12 in some cases (Jamieson et al, 1994; Robin et al, 1995). The syndrome is characterized by short stature, webbing of the neck, cubitus valgus, pulmonary stenosis, hypertrophic cardiomyopathy, low-set ears, and ptosis. Fertility may be normal, but 77% of these patients will have cryptorchidism with resulting spermatogenic failure and elevated gonadotropin levels (Sharland et al, 1992).
Y-Linked Microdeletions
Testing for Y chromosome microdeletion is indicated in men with presumed nonobstructive etiology of azoospermia and severe oligospermia (concentration < 5 million/mL) to assess the prognosis for surgical sperm retrieval and to assist genetic counseling for affected couples. Normal molecular anatomy of the Y chromosome is critical both for gonadal development and function. The Y chromosome is an acrocentric chromosome with an off-center centromere creating asymmetric short (Yp) and long (Yq) arms incorporating 60 million base pairs (Morton, 1991). The Y chromosome has two important regions—the euchromatic zone, which includes Yp, the centromere, and the proximal portion of Yq, and the heterochromatic zone, which comprises the distal Yq. The heterochromatic region does not appear to have transcriptional function, while the euchromatic zone has a number of important genetic loci that are critical to the development of normal spermatogenesis (Fig. 21–9). Of particular importance is the sex-determining region Y (SRY) gene located on Yp, which is critical in initiating the cascade that converts the embryonic bipotential gonad to the eventual testis.
Deletions or mutations of critical areas of the Y chromosome may cause profound disruption of spermatogenesis. Early cytogenetic analysis of infertile men suggested the presence of a specific region on Yp termed the azoospermia factor (AZF), which is of paramount importance for normal spermatogenesis (Tiepolo and Zuffardi, 1976). Subsequent advances in chromosomal molecular mapping allowed the identification of three regions of Y-chromosome microdeletion: AZFa, AZFb, and AZFc (Vogt, 1998). Deletions in the AZFa region have been reported in 1% of men with nonobstructive azoospermia. This region contains two genes, DDX3Y (also known as DBY) and USP9Y, which are felt to be important for normal spermatogenesis (Kamp et al, 2000). AZFa microdeletion is associated with germinal cell aplasia histology of the testis, and current literature suggests that an attempt at testicular sperm retrieval (TESE) is not indicated because the success rate is poor (Blagosklonova et al, 2000; Hopps et al, 2003; Oates, 2008).
Like AZFa, microdeletions in the AZFb region are uncommon and are associated with poor surgical sperm retrieval success rates, especially if associated with concurrent microdeletion in other loci. The critical spermatogenic gene in the AZFb region is the RNA-binding motif (RBM) gene, which produces an RNA-binding protein localized to germ cell nuclei (Ma et al, 1993). Microdeletion in the AZFc region is the most most commonly noted, in up to 13% and 6% of azoospermic and severely oligospermic men, respectively (Reijo et al, 1996). The AZFc region is the location for the Deleted in Azoospermia (DAZ) gene, which encodes an RNA-binding protein noted primarily in spermatogonia (Saxena et al, 2000; Collier et al, 2005). Isolated AZFc microdeletion portends a better prognosis because more than 50% of azoospermic men will have successful sperm retrieval (Silber et al, 1998; Oates 2002).
Considering the fact that Y chromosome microdeletions do not have direct health implications to patients other than in the fertility arena, couples should receive genetic counseling before obtaining and using surgically retrieved sperm for ICSI. The male offspring of these patients will be phenotypically normal but will harbor the father’s microdeletion and thus are expected to have similar fertility challenges in their adulthood (Silber and Repping, 2002).
Disorders of Internal Ductal Development
Congenital Bilateral Absence of the Vas Deferens
CBAVD represents the mildest phenotypic presentation of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction with cystic fibrosis at the severe end of the spectrum (Oates, 2008). The cystic fibrosis (CF) gene is located on chromosome 7 and encodes for CFTR, a membrane-spanning protein that assists transmembrane chloride ion transport, which is critical for the maintenance of epithelial-lined lumen. If only one CF allele is mutated, the patient may present with CBAVD without the pulmonary and pancreatic manifestations described in classic cystic fibrosis. However, if both alleles manifest mutations and critically reduce the CFTR reserve, patients may exhibit full-blown CF, although the degree of dysfunction depends on the nature of the CF gene mutations involved.
CBAVD accounts for 6% of cases of obstructive azoospermia. The descriptive name incorporates only a portion of the associated anomalies because these patients will also have a prominent caput with absence of the distal two thirds of the epididymis, atrophy, or hypoplasia of the seminal vesicles in addition to the characteristic vasal agenesis. In the absence of seminal vesicle contributions, the ejaculate will be azoospermic with low volume (typically <0.5 mL) and acidic pH (6.5) due to unbuffered scant prostatic secretions. Although TRUS can be used to confirm the seminal vesicle anomalies, the diagnosis of CBAVD can usually be made on the basis of clinical and semen findings. Spermatogenesis is normal and biopsy is not required unless undertaken for sperm retrieval for eventual ICSI. Renal anatomy is usually normal except in cases of non-CF mutation CBAVD, as discussed later.
Development of vasal agenesis is secondary to one of two genetic transmission patterns, either CF gene mutation or aberrant development of the early mesonephric duct. Almost 80% of men with CBAVD will have at least one detectable CF mutation with more than 500 types identified to date (Claustres, 2005). The most common mutation observed in CBAVD patients is a three-base pair deletion called delta-508, which accounts for almost 70% of CFTR mutations (Kerem et al, 1989). In CBAVD men without identifiable CF mutation, the syndrome is probably secondary to yet unidentified CF mutations or to improper morphogenesis of the mesonephric duct. These patients should be screened with a renal imaging study because up to 40% will have renal agenesis or fusion anomalies (Oates, 2002). Renal screening is not necessary for CBAVD patients testing positive for CF mutations. Clearly, all patients with CBAVD should have thorough genetic counseling before proceeding with fertility treatment.
Because spermatogenesis is usually normal in these patients, sperm retrieval techniques via a percutaneous or open surgical route from either the caput epididymis or testis are usually successful in extracting excellent quality spermatozoa for ICSI (Phillipson, 2000). Cryopreserved sperm from these patients produce equally high pregnancy rates with ICSI similar to those observed with fresh specimens (Oates, 1996).
Ultrastructural Sperm Anomalies
Primary Ciliary Dyskinesia
Primary ciliary dyskinesia (PCD), also known as the immotile cilia syndrome, encompasses a spectrum of disorders involving ultrastructural abnormalities of the flagellum affecting all ciliated cells including the lining of the respiratory and sinus tracts, as well as sperm motility. Normal ciliary axonemal structure involves a 9 + 2 microtubular arrangement with 9 pairs surrounding a central doublet with axonemal bridges that connect the outer doublet to the central pair. Movement generation requires intact ATPase function that localizes to the dynein complex located in the peripheral doublets. Electron microscopy studies have demonstrated a variety of abnormalities in PCD including absence of outer and inner dynein arms, radial spokes, and central doublet. Of these, the lack of both dynein arms is the most commonly observed deficiency (Rott, 1979).
Typically, affected patients may rarely present with infertility as their initial complaint. They will rather initially present in childhood with chronic bronchiectasis and sinusitis due to dysfunctional movement of mucus through the upper airways and sinuses.
Kartagener syndrome is a variant of PCD with three components including chronic sinusitis and bronchiectasis, situs inversus, and infertility as manifested by low or absent sperm motility. The prevalence of PCD and Kartagener syndrome are 1 in 20,000 and 1 in 40,000, respectively (McClure, 1997). Ciliary dyskinesia has also been described in association with retinitis pigmentosa, presumably due to the axonemal support requirements of retinal cells (van Dorp et al, 1992). The genetic basis for PCD has been elusive due to the heterogeneous nature of the syndrome and the multitude of substructures required for ciliary function. Most cases appear to be inherited in an autosomal recessive pattern, although there have been isolated reports of autosomal dominant or X-linked transmission (Yokota et al, 1993; Narayan et al, 1994; Blouin et al, 2000). Diagnosis can be confirmed by electron microscopy examination of ciliary axonemal structure. Although there is no cure for the ultrastructural defects, sperm have been used successfully for ICSI with resulting normal births (Kay and Irvine, 2000). Genetic counseling is highly recommended before pursuing ICSI in these patients.
Globozoospermia
Globozoospermia, also called the round-headed sperm syndrome, is an unusual condition noted in less than 0.1% of infertile males (Dam et al, 2007a). Although sperm concentration and motility are normal, spermatozoa have a characteristic circular head due to the absence of the acrosomal cap and enzyme package including acrosin. This deficiency prevents the sperm from penetrating the zona pellucida and outer investment of the oocyte and from obtaining fertilization. Morphologic abnormalities have also been described in the nuclear chromatin, midpiece, and mitochondrial sheath. Familial studies strongly argue for genetic transmission with some evidence suggesting a mutation in the SPATA16 gene, which is a spermatogenesis-specific locus, although the exact mode remains to be described (Carrell et al, 1999; Dam et al, 2007b). Standard ICSI has been successfully used for these patients, although some authors have suggested that it be combined with calcium ionophore oocyte activation to improve fertilization rates (Rybouchin et al, 1997; Kilani et al, 1998; Dirican et al, 2007).
Fibrous Sheath Dysplasia
Fibrous sheath dysplasia (FSD), also known as the Stump-tail syndrome, describes a malformation of the fibrous sheath investing the sperm tail, resulting in structural and functional impairment (Rawe et al, 2001). Spermatozoa have a distinctive morphologic appearance with coiled or markedly shortened tails and are immotile. Although the genetic basis of FSD has not yet been established, there are troubling reports that the sperm have higher rates of diploidy and sex chromosomal disomy, suggesting the need for genetic counseling before proceeding with fertility treatment (Baccetti et al, 2005; Moretti and Collodel, 2006). Despite these concerns, ICSI has been used successfully for patients with FSD (Olmedo et al, 2000).
Ejaculatory Dysfunction
Although an uncommon cause of male infertility, ejaculatory dysfunction is responsive to a variety of therapies allowing restoration of fertility in most patients. Ejaculation is a complex process requiring coordinated inputs from both the central and peripheral neural systems to produce expulsion of semen from the urethra. There are three distinct phases of semen production: emission, closure of the bladder neck, and ejaculation. In response to visual or sensory erotic stimulation to the cerebral cortex, signals are conducted down the thoracolumbar sympathetic nerves, resulting in contraction of prostatic smooth muscle, seminal vesicles, and the vas deferens and allowing deposition of pre-ejaculatory fluid into the posterior urethra, a process called emission. Bladder neck closure occurs concurrently with emission in response to sympathetic innervations. Finally, semen is propelled in an antegrade fashion from the posterior urethra in response to rhythmic contractions of the periurethral and pelvic floor muscles.
Various classifications of ejaculatory dysfunction have been described, but three broad categories account for most of the disturbances observed in the infertile male: functional, neurogenic, and retrograde.
Functional Ejaculatory Dysfunction
Functional disorders include premature and delayed ejaculation. Premature ejaculation (PE) is a common form of male sexual dysfunction, occurring in more than 30% of men between the ages of 18 and 60 (Laumann et al, 1999). PE may cause significant emotional distress in couples, yet the vast majority of patients will still have intravaginal ejaculation minimizing the infertility implications of this condition. If ejaculation is consistently occurring before intromission, semen may be collected by the couple and used for intravaginal insemination at home.
Delayed ejaculation is defined as persistent difficulty or inability to ejaculate despite the presence of sexual desire and erection. No clear neurologic basis for this condition has yet been established and it is widely believed to represent a psychogenic issue (Ohl et al, 2008). Some authors have described an association with strict religious upbringing and frequent use of masturbation (Stewart and Ohl, 1989; Perelman, 2006). Some men may benefit from sex therapy or vibratory stimulation to induce ejaculation, but many will require electroejaculation or surgical sperm retrieval for eventual fertility.
Neurogenic Anejaculation
Neurogenic anejaculation is usually the result of a spinal cord injury (SCI). In addition to the ejaculatory dysfunction in these patients, many will also have erectile dysfunction and deficiencies in spermatogenesis related to thermal dysregulation of the testes, stasis of spermatozoa, and chronic genital tract infections. Oral therapies are rarely of value in these patients and most require penile vibratory stimulation (PVS), electroejaculation (EEJ), or surgical sperm retrieval to circumvent the lack of ejaculation.
Penile vibratory stimulation involves direct vibratory stimulation of the penile frenular region using specially designed equipment. Specific vibratory parameters including a vibration amplitude of 2.5 at 100 Hz appear optimal for ejaculation induction in SCI men (Sønksen, 1994). The best candidates for PVS are patients with complete SCI who have an intact ejaculatory reflex system: Specifically, patients with complete upper motor neuron lesions above T10 with preservation of sacral efferents, thoracolumbar sympathetic outflow, and intact communication between sacral and thoracolumbar segments will exhibit the best response to PVS because approximately 70% of these patients will have ejaculation in response to treatment (Ohl et al, 1996; Bird et al, 2001). Men with incomplete spinal cord lesions may have poor response to PVS because of cortical inhibition of the reflex arc preventing ejaculation. In addition, patients with lower spinal cord lesions or peripheral neural root injuries are unlikely to respond to PVS. The most serious adverse event associated with both PVS and EEJ is autonomic dysreflexia, a massive sympathetic discharge generally associated with SCI above the T6 level. Blood pressure monitoring is essential during any procedure on SCI patients. Men who are prone to autonomic dysreflexia may be given sublingual nifedipine, 10 to 20 mg 10 minutes before the procedure to minimize the blood pressure elevation (Steinberger et al, 1990). In general, PVS is well tolerated and can even be performed by the patient at home in the absence of autonomic dysreflexia. Sønksen reviewed the efficacy of PVS in SCI men reporting 102 pregnancies in 619 couples using home vaginal insemination or clinic intrauterine insemination (Sønksen and Biering-Sørenson, 1992). PVS remains the first-line therapy for SCI patients, producing better ejaculate quality than noted with EEJ procedures (Brackett et al, 1997; Ohl et al, 1997).
Electroejaculation may be used for SCI men who have failed a trial of PVS and is usually effective for any level of spinal cord injury, as well as in men with anejaculation from a variety of other mechanisms including retroperitoneal nerve injury from prior surgery such as retroperitoneal lymphadenectomy, diabetic neuropathy, multiple sclerosis, spina bifida, and psychogenic anejaculation. In the EEJ procedure, a rectal probe is inserted and pulsed direct electrical stimulation is applied to induce ejaculation. Although SCI patients often do not require an anesthetic during the procedure, men who retain normal pelvic and perirectal sensation will need general or spinal anesthesia. Because retrograde ejaculation often results, patients should have bladder catheterization before the procedure to minimize urine contact with the ejaculate. In addition, instillation of a sperm-friendly buffered medium into the bladder, systemic alkalinization with sodium bicarbonate, and hydration before the procedure will improve sperm survival in the bladder. Antegrade ejaculate is collected and the patient is again catheterized at the end of the procedure to recover any retrograde ejaculate. Sigmoidoscopy is also performed before and after the procedure to evaluate for preexisting rectal pathology that could interfere with the EEJ procedure, as well as identify any injuries resulting from the electrical stimulation of the rectal wall. Rectal injuries are rare, occurring in less than 0.1% of EEJ procedures, but they require prompt identification and treatment to minimize morbidity (Ohl and Sønksen, 1997). Electroejaculation procedures have been reported to produce ejaculates adequate for intrauterine insemination in 71% of men with SCI and 87% of patients with anejaculation postretroperitoneal lymph node resection (Ohl, 1989, 1995).
Regardless of the etiology of anejaculation, surgical sperm retrieval via either percutaneous or open biopsy of the epididymis or testis remains an effective option for management. One study conducted a cost-benefit analysis of EEJ coupled with intrauterine insemination versus ICSI using surgical sperm retrieval in SCI patients, noting that EEJ was the cost-effective approach if the procedure could be performed without anesthesia. However, in those patients requiring anesthesia for the EEJ, the cost-benefit analysis favored surgical sperm retrieval via local anesthetic followed by ICSI (Ohl, 2001).
Retrograde Ejaculation
Retrograde ejaculation occurs when seminal fluid preferentially flows into the bladder, instead of the normal antegrade direction due to failure of the bladder neck to close. The diagnosis is confirmed by identification of 10 to 15 sperm per HPF in a centrifuged specimen of postejaculation urine. The causes of retrograde ejaculation include incompetence of the bladder neck usually from prior surgery such as transurethral resection of the prostate gland, medications including α blockers and antidepressants, and diseases causing neurologic pathology such as diabetes and multiple sclerosis. Before initiating therapy, potential reversible etiologies such as medications should be removed if possible. In the absence of correctible etiologies, a trial of sympathomimetic medication therapy may be useful in increasing sympathetic tone of the bladder neck and vas deferens, especially in patients with slowly progressive disease such as diabetic neuropathy or in those with failure of emission due to disruption of retroperitoneal sympathetic innervation from prior surgery. Table 21–14 lists some of the standard medication regimens described. Medical therapy can produce a spectrum of results including conversion of retrograde to antegrade ejaculation, increase in ejaculatory volume, and development of retrograde ejaculation in a previously anejaculatory patient (Brooks et al, 1980; Kamischke and Nieschlag, 2002). These regimens must be used judiciously because side effects include tachycardia and hypertension, which may be particularly concerning in the diabetic patient at risk for occult cardiovascular disease.
Table 21–14 Medication Regimens for Treatment of Retrograde Ejaculation
| MEDICATION | DOSE |
|---|---|
| Phenylpropanolamine | 75 mg po bid |
| Ephedrine | 25-50 mg po qid |
| Pseudoephedrine | 60 mg po qid or 120 mg po bid |
| Imipramine | 25 mg po bid |
Patients with retrograde ejaculation who do not respond to medical therapy may have sperm recovered from the bladder for use in intrauterine insemination as detailed earlier. With proper preparation of the bladder to retain sperm viability and laboratory sperm processing, this technique is an effective and economical method of treatment (Van der Linden, 1992).
Immunologic Infertility
Immunologic mechanisms of infertility remain poorly understood and controversial, both in cause and treatment. The development of antisperm antibodies (ASA) results from a variety of conditions that cause disruption of the blood-testis immune barrier, resulting in exposure of sperm antigens to the systemic immune system. Elevated levels of ASA have been associated with gonadal trauma, testicular torsion, cryptorchidism, varicocele, genital infections, and prior testicular biopsy, although it has long been recognized that many patients will have no clear inciting event (Ansbacher and Gangai, 1975; Koskimies and Hovatta, 1982; Witkin and Toth, 1983; Golomb et al, 1986). Prior vasectomy is a leading cause of clinically significant immunologic infertility with ASA development reported in 34% to 74% of vasectomized men and persisting in 38% to 60% after vasectomy reversal (Broderick et al, 1989; Francavilla et al, 2007). The potential detrimental effects of ASA depend on the location of binding to the sperm and include impaired sperm motility, reduced binding and penetration of the zona pellucida, inhibition of the acrosome reaction, and reduced sperm survival in the female reproductive tract. The degree of fertility impairment is related to the amount of antibody binding, with one series noting IVF fertilization rates of 27% in men with 80% or more of sperm bound with IgG or IgA versus 78% in patients with less than 80% binding (Clarke et al, 1985).
A critical assessment of effective treatment options for immunologic infertility is hampered by a lack of controlled, prospective studies and the absence of a standard definition of the disease. Current treatment strategies use two main approaches: immunosuppressive therapy or assisted reproduction using laboratory techniques to lower antibody levels. Immunosuppression using corticosteroid therapy remains the most commonly used therapeutic approach with reported pregnancy rates ranging from 6% to 50% despite consistent reductions in antibody titers (Turek and Lipshultz, 1994). Many different regimens have been employed in uncontrolled studies, making it difficult to critically compare results. Hendry conducted a 6-month randomized trial using high-dose prednisolone given on days 1 through 10 of the female partner’s cycle followed by a rapid taper over 2 days (Hendry et al, 1990). Pregnancy rates were 31% in the treatment group compared with 9% in the untreated men. A more recent double-blind, placebo-controlled study using methylprednisolone treatment for three cycles reported a significant reduction in sperm-associated IgG titers but no significant improvement in pregnancy rates in the treated group (Haas and Manganiello, 1997). Although therapy may be helpful in some patients, this therapeutic benefit must be weighed against the potential risks of steroid treatment including fluid retention, bone loss, gastrointestinal bleeding, and aseptic necrosis of the femoral head (Naz, 2004).
Assisted reproduction strategies for immunologic infertility have centered on using semen processing techniques to attempt removal of ASA for IUI and IVF. Simple sperm wash and percoll gradient methods of sperm preparation do not reliably remove ASA attached to the sperm surface (Haas and D’Cruz, 1988; Windt et al, 1989; Almagor et al, 1992). Despite the persistent presence of ASA after sperm processing, studies have suggested that IUI may be an effective and economical treatment for ASA-mediated infertility in some couples. One series reported a pregnancy rate of 64% after three cycles of IUI in men with ASA who produced ejaculate into a sterile medium (Ombelet et al, 1997). These results were considerably better than the conception rates per cycle of 3% to 10% reported in ASA-positive men using untreated sperm (Haas,1991; Francavilla et al, 1992). Intracytoplasmic sperm injection appears to be an effective treatment for ASA-mediated infertility when IUI fails, although there have been some concerning reports regarding postfertilization development. Fertilization and cleavage rates with ICSI treatment appear to be similar in ASA-positive and ASA-negative men (Lahteenmaki et al, 1995). However, ASA may have direct negative effects on developing embryos as manifested by higher embryonic degeneration and miscarriage rates than those observed in ASA-negative men (Naz, 2004).
Idiopathic Infertility
Despite the diagnostic advances in the field of male infertility, more than 30% of patients will still have no discernible cause for abnormal semen analyses (Nieschlag, 1997). Although it is anticipated that future developments will allow identification of the etiology for subfertility in these patients, at present they are considered idiopathic disorders that defy specific treatment recommendations. In the absence of obvious causality, therapy involves the employment of empiric medical therapy or assisted reproductive techniques to be discussed later. Empiric pharmacologic treatments have usually involved endocrine agents, with a variety of different tested therapies (Table 21–15). Although small series provide support for some empiric therapies, large placebo-controlled trials are lacking in this area, further confounding decision making in this difficult population, especially in the context of a 26% background pregnancy rate for untreated couples with abnormal semen parameters (Collins et al, 1983; Siddiq and Sigman, 2002).
Table 21–15 Empiric Pharmacologic Therapy
Empiric Medical Therapy
Gonadotropin-Releasing Hormone Agonists
The justification for use of gonadotropin-releasing hormone agonists (GnRHs) involves the observation that these agents are effective for treatment of hypogonadotropic hypogonadism, implying that stimulation of FSH elevation may be beneficial even in the absence of known deficiency. Although multiple studies of empiric GnRH therapy have yielded conflicting results, two small controlled studies failed to show significant improvements in semen parameters (Badenoch et al, 1988; Crottaz et al, 1992). In addition, most studies have not shown significant increases in pregnancy rates, further reinforcing the conclusion that the expense of the therapy is not justified by improvements in fertility outcomes.
Gonadotropins
Multiple studies employing various formulations to include hCG, hMG and recombinant forms of gonadotropins have yielded conflicting results. A Cochrane meta-analysis suggested a modest 9% increase in pregnancy rates in the 3-month GnRH treatment group over controls, although even this analysis was underpowered to allow definitive assessment of impact due to lack of studies meeting selection criteria (Attia et al, 2006). Studies of GnRH treatment in men before ICSI have not shown significant improvements in pregnancy rates (Ashkenazi et al, 1999; Baccetti et al, 2004).
Antiestrogens
Antiestrogens remain the most commonly employed medical therapy for idiopathic male infertility. The two main formulations, clomiphene citrate and tamoxifen citrate, are nonsteroidal selective estrogen receptor modulators. By blocking the estrogen receptors at the hypothalamus and pituitary levels, this class of agents minimizes the estrogenic-mediated inhibition of gonadotropin release, resulting in elevation of gonadotropin levels. Numerous clinical trials with both agents have been conducted with conflicting results. Although some controlled studies with clomiphene citrate have shown improvement in semen parameters (Ronnberg, 1980; Wang et al, 1983; Micic and Dotlic, 1985), others have failed to show significant changes (Abel et al, 1982; Sokol et al, 1988; WHO, 1992). A meta-analysis of available studies did not reveal a significant improvement in pregnancy rates with clomiphene therapy (Liu, 2003). Similar randomized controlled trials using tamoxifen therapy have not conclusively supported efficacy in either improved semen parameters or pregnancy rates (Torok, 1985; Krause et al, 1992). A Cochrane meta-analysis combining 10 clomiphene and tamoxifen randomized controlled studies failed to show improvements in pregnancy rates with therapy (Vandekerckhove et al, 2000). A more recent study examined combination therapy with tamoxifen (20 mg/day) and testosterone replacement (120 mg/day testosterone undecanoate) for patients with idiopathic oligoteratospermia, noting improvements in semen parameters and pregnancy rates (Adamopoulos et al, 2003). These results remain to be confirmed in other large controlled studies. Other investigators used clomiphene therapy for men with nonobstructive azoospermia, titrating the dose to elevate serum testosterone levels to between 600 and 800 ng/dL (Hussein et al, 2005). In this noncontrolled trial, 64% had spontaneous return of sperm to the ejaculate with mean density of 3.8 million/mL. In those with persistent azoospermia, testicular biopsy was successful in retrieving sperm for ICSI in all cases.
Aromatase Inhibitors
Aromatase inhibitors suppress the activity of aromatase, a cytochrome P-450 enzyme concentrated in the testes, liver, brain, and adipose tissue. Aromatase is responsible for the conversion of testosterone to estradiol, so blockade produces functional effects similar to the antiestrogen class of medications. Aromatase inhibitors have been postulated to have incremental benefit over antiestrogens in patients with lower serum testosterone to estradiol ratios (<10) and in obese patients. Testolactone and anastrozole are the two main agents that have been used for the treatment of idiopathic infertility. Although one study using testolactone noted improvement in semen parameters in men with severe idiopathic oligospermia (Pavlovich et al, 2001), a controlled crossover trial failed to identify treatment benefit over placebo (Clark and Sherins, 1989). A newer study by Raman examined subgroups of infertile men with abnormal testosterone to estradiol (T/E2) ratios treated with either testolactone or anastrozole (Raman and Schlegel, 2002). Men in both treatment groups had significant improvements in their T/E2 ratios, sperm concentration, morphology, and motility, although pregnancy rates were not reported. Although these reports are intriguing, the expense of these agents and the absence of large controlled studies will limit use until further study.
Antioxidant Therapy
With growing awareness of the role of oxidative stress in idiopathic male infertility, antioxidant supplementation has become a common form of empiric therapy. The antioxidant vitamins α-tocopherol (vitamin E), ascorbic acid (vitamin C), and the retinoids (vitamin A), as well as L-carnitine, an amino acid important for mitochondrial metabolism, have all been postulated to have therapeutic benefit for male subfertility. Various studies of antioxidant vitamin supplementation have yielded conflicting conclusions on both semen parameter and pregnancy rate results (Suleiman et al, 1996; Rolf et al, 1999; Keskes-Ammar et al, 2003). More recent studies in infertile men with elevated levels of sperm DNA fragmentation revealed improvements in fragmentation rates and increased rates of ICSI-derived pregnancy following vitamin C and E supplementation (Greco et al, 2005a-c). Randomized double-blind studies examining the effect of L-carnitine administration in men with idiopathic asthenospermia have yielded contrasting results on post-treatment semen parameters, preventing definitive conclusions on the efficacy of supplementation (Balercia et al, 2005; Sigman et al, 2006).