chapter 22 Surgical Management of Male Infertility
Since the previous edition, the indications for and techniques of surgery for male infertility have been significantly refined, resulting in substantially increased success in the management of male factor infertility. These advances include (1) increasing use of genetic and molecular biologic markers (see Chapters 20 and 21) to better select patients for surgical treatment; (2) improved techniques for microsurgical reconstruction for obstruction; (3) the use of varicocelectomy for enhancement of spermatogenesis in azoospermic or severely oligospermic men (Matthews et al, 1998; Kim et al, 1999); and (4) refined microsurgical techniques for sperm retrieval combined with in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) for men with nonobstructive azoospermia. Even men with nonobstructive azoospermia due to Klinefelter syndrome, once regarded as hopeless cases, can now father biologic offspring with assisted reproductive techniques (Tournaye et al, 1996; Palermo et al, 1998; Ramasamy et al, 2009).
Use of transrectal high-resolution ultrasound, as well as scrotal ultrasound with color flow Doppler, has substantially improved our diagnostic and therapeutic abilities. Transrectal ultrasound of the seminal vesicles provides not only diagnostic information but ultrasound-guided aspiration of the seminal vesicles that allows retrieval of sperm to be used for IVF with ICSI (Jarow, 1996). Power Doppler may allow identification of pockets of sperm production in the testis, which may help guide sperm retrieval in men with nonobstructive azoospermia (Har-Toov et al, 2004; Herwig et al, 2004; Tunc et al, 2005).
IVF with ICSI has expanded our ability to treat even the most severe forms of male-factor infertility such as unreconstructable reproductive tract obstruction and nonobstructive azoospermia. It is, however, a costly procedure and an intense process for the female partner, with associated risks of complications including ovarian hyperstimulation, multiple gestations, and complications of the procedures for oocyte retrieval. Furthermore, as ICSI bypasses all natural biologic barriers, it raises realistic concerns of passing genetic abnormalities to the offspring (Kim et al, 1998; Foresta et al, 2005). Thus proper selection of couples for advanced assisted reproductive technology, along with adequate genetic counseling, is mandatory.
On the other hand, recent analyses clearly indicate that specific treatments for male-factor infertility such as microsurgical reconstruction for obstructive azoospermia and varicocelectomy for impaired testis function, in properly selected patients, remain the safest and most cost-effective ways of managing infertile men (Kolettis and Thomas, 1997; Pavlovich and Schlegel, 1997; Lee et al, 2008). Specific treatment aimed at correcting or enhancing male infertility can upgrade a couple from intensive levels of assisted reproduction to simpler methods or even to naturally conceived pregnancies.
For men with unreconstructable obstruction, as well as men with nonobstructive azoospermia, surgical retrieval of sperm to achieve fertilization, pregnancy, and live birth with IVF and ICSI is a feasible management option. The development and recent refinement of the various techniques of surgical sperm retrieval, from testes, epididymides, or seminal vesicles with percutaneous or open surgical approaches, have expanded the armamentarium of urologists treating infertile men. In particular, the employment of the operating microscope to evaluate and identify individual seminiferous tubules more likely to contain sperm has significantly improved the success of testicular sperm extraction (Schlegel, 1999) while minimizing morbidity significantly (Tsujimura et al, 2002; Ramasamy et al, 2005).
The use of microsurgical techniques has also been extended to varicocelectomy. Varicoceles have long been known to be associated with male infertility and have now clearly been shown to result in progressive, duration-dependent testicular injury (Russell, 1957; Lipshultz and Corriere, 1977; Nagler et al, 1985; Gorelick and Goldstein, 1993; Sigman and Jarow, 1997). Furthermore, microsurgical varicocelectomy, previously reserved only for men with oligospermia, has now been applied to men with nonobstructive azoospermia, resulting in induction of spermatogenesis and successful return of sperm to the ejaculate in many cases (Matthews et al, 1998; Kim et al, 1999; Pasqualotto et al, 2003; Pasqualotto et al, 2006; Ishikawa et al, 2008). Although varicocelectomy has historically been reserved for the treatment of infertile men and varicocele-induced pain, there is an emerging concept of early repair of varicoceles to prevent both future infertility and Leydig cell dysfunction. Substantial evidence has accumulated suggesting that varicocele adversely affects Leydig cell function, resulting in lower serum testosterone levels when compared with age-matched controls without varicocele. Varicocelectomy can halt and even partially reverse this decline (Castro-Magana et al, 1989; Su et al, 1995; Cayan et al, 1999). In selected men, varicocelectomy may be an effective treatment for symptomatic, age-related androgen deficiency (Tanrikut et al, 2011) a condition increasingly referred to as andropause or testosterone deficiency syndrome (TDS). Thus with safer and more effective microsurgical techniques, early varicocelectomy has expanded the urologist’s role from that of salvaging remaining testicular function to that of preventing future infertility and TDS.
When surgery for male infertility is undertaken, only rarely is the life (or death) of the patient at stake. What is at stake when the surgery described in this chapter is undertaken is new life, with the potential for altering not only the quality of a couple’s life but the future of our species. The responsibilities assumed by the surgeon in these circumstances demand the utmost in judgment and skill. Many of the procedures described in this chapter are among the most technically demanding in all of urology. Acquisition of the skills required to perform them demands intensive laboratory training in microsurgery and a thorough knowledge of the anatomy and physiology of the male reproductive system. Attempting such surgery only occasionally and without proper training is a terrible disservice to the patient, the couple, and future humanity.
Surgical Anatomy
The scrotal contents are unique in their accessibility for physical examination, imaging modalities, and surgical intervention. The success of surgery for male infertility and scrotal disorders is predicated on selection of the correct operation and the most appropriate surgical approach. The details of the history and careful physical examination followed by confirmatory, judiciously selected laboratory and imaging procedures are presented in Chapter 21. When surgical intervention for diagnostic or therapeutic purposes is indicated, a thorough understanding of the anatomy (see Chapter 2) and physiology (see Chapter 20) of the male reproductive system is requisite for planning and carrying out a surgical procedure with the highest probability of success and lowest morbidity.
The key points of surgical anatomy follow.
Testicular Blood Supply
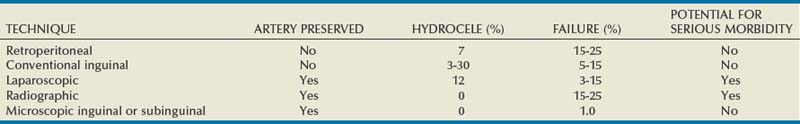
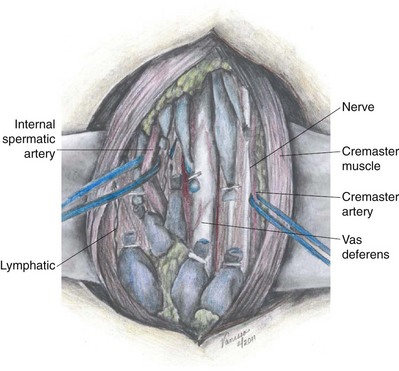
The main blood supply to the testis is from the testicular (internal spermatic) artery arising directly from the aorta (Table 22–1). A second blood supply comes from the artery of the vas deferens (deferential artery), which derives from the hypogastric (internal iliac) artery or the superior vesicle artery (also a branch of the hypogastric). The third blood supply, primarily to the tunica vaginalis but with branches going to the testes, comes from the cremasteric (external spermatic) artery, which derives from the inferior epigastric artery. The testicular artery is the main blood supply to the testes. Its diameter exceeds the diameter of the deferential (vasal) artery plus the cremasteric artery combined (Raman and Goldstein, 2004). Although vasal and cremasteric arteries can provide adequate blood supply to the testes in the event that the testicular artery is ligated, especially in children, atrophy and/or azoospermia has resulted from testicular artery ligation both in adults and children. Experience with the one-stage Fowler-Steven operation for orchiopexy, in which the testicular artery is intentionally ligated, indicates that 20% to 40% of such testes atrophy, although the rate of atrophy is lower in the staged procedure.
Table 22–1 Blood Supply to Testis, Epididymis, and Vas Deferens
| Testis |
| Epididymis |
| Vas Deferens |
Special attention should be paid to men who have undergone vasectomy because the vasal artery has likely been compromised. In these men, maintaining the integrity of the testicular artery in any future operations such as varicocelectomy is critical (Lee JS et al, 2007).
Epididymal Blood Supply
The epididymis has a rich blood supply (see Table 22–1). The superior and medial epididymal arteries derive from the testicular artery. The blood supply to the cauda (inferior pole) of the epididymis derives from the vasal (deferential) artery. The two main blood supplies to the epididymis, running superiorly and inferiorly, form an extensive interconnection such that if the vasal artery is ligated from previous vasectomy, the blood supply to the epididymis from the testicular artery is more than adequate. In addition, in preparation for vasoepididymostomy or vasovasostomy, the epididymis can be intentionally dissected off of the testis and mobilized to the caput (see Technique When Vasal Length Is Severely Compromised) with the inferior and medial epididymal arteries intentionally ligated without adverse consequence. As long as the superior epididymal artery remains intact, the blood supply to the epididymis will be adequate.
Blood Supply of the Vas Deferens
The vas deferens obtains its blood supply from two sources (see Table 22–1). The seminal vesical (abdominal) end of the vas derives its blood supply from the deferential (vasal) artery, a branch of the internal iliac (hypogastric) artery or of its branch, the superior vesicle artery. The testicular end of the vas receives additional blood supply from the inferior epididymal arterial interconnections, which extends onto the vas deferens. The two blood supplies to the vas deferens freely anastomose with each other. After vasectomy, if the vasal vessels are ligated, the testicular end of the vas receives all of its blood supply from branches of the testicular artery and epididymal artery, whereas the seminal vesical (abdominal) end of the vas receives all of its blood supply from the deferential artery. The vas deferens receives no blood supply from the surrounding cremaster muscle or from any blood vessels from the spermatic cord. Therefore if the vas deferens is sectioned or obstructed in two different locations, the intervening segment will fibrose due to lack of blood supply. Therefore two simultaneous vasovasostomies cannot be safely performed on the same vas if the vasal vessels have been interrupted in both locations.
Anatomy of the Excurrent Ducts
Sperm and testicular fluid exit the testes through 7 to 11 tiny efferent ducts. These ducts become convoluted when they exit the testes and form the caput of the epididymis (see Chapter 20). At that level, they freely anastomose with each other. They all coalesce at the distal caput to form a single epididymal tubule from the caput-corpus junction all the way to the vas deferens. Therefore if the epididymis is accidentally injured or ligated distal to the caput, the entire system on that side will be completely obstructed. This is an important consideration when performing epididymal surgery or surgery near the epididymis. Hydrocelectomy is a common surgical procedure that can result in iatrogenic injury to the epididymis. In long-standing large hydroceles, the epididymis is often splayed out and difficult to identify. Generous margins from the epididymis should be allowed when performing hydrocelectomy (see Chapter 37). Orchiopexy for torsion can also result in inadvertent injury to the epididymis. A single stitch through an epididymal tubule in the corpus or cauda will result in complete obstruction of that side. Because there are multiple lobules at the levels of the caput, puncture of a single tubule for sperm aspiration can be safely performed at the most proximal region of the caput without significantly compromising the flow of sperm into the corpus. Multiple punctures of many tubules at the caput, or any puncture distal to the caput, however, can cause obstruction.
Ejaculatory Ducts
The left and right ejaculatory ducts enter the prostatic urethra at the level of the utricle. Obstruction of ejaculatory ducts can lead to azoospermia. Transurethral resection of the ejaculatory ducts (TURED) can relieve the obstruction. TURED should not be considered a benign procedure because it is occasionally associated with significant morbidity (see Transurethral Resection of the Ejaculatory Ducts later). Normally, the ejaculatory ducts contain a valvelike mechanism that prevents reflux of urine into the ejaculatory duct. After transurethral resection of the ejaculatory ducts, a significant percentage of men will develop reflux of urine up the excurrent ductal system (Vazquez-Levin et al, 1994), causing chemical and/or bacterial epididymitis.
Testis Biopsy
Indications
The indications for testis biopsy are detailed in Chapter 21. Briefly, testis biopsy is indicated in azoospermic men with testis of normal size and consistency, palpable vasa deferentia, and normal serum follicle-stimulating hormone (FSH) levels. Under these circumstances, biopsy will distinguish obstructive from nonobstructive azoospermia. In men with congenital absence of vasa and normal serum FSH levels, biopsy always reveals spermatogenesis (Goldstein and Schlossberg, 1988) and biopsy is not necessary before definitive sperm aspiration and IVF with ICSI. Diagnostic biopsy should usually be performed bilaterally irrespective of the size discrepancy of the two testes. Good spermatogenesis is sometimes found in small, firm testes, whereas biopsies of large, healthy testes may reveal maturation arrest.
The ability to achieve pregnancy with only a single testicular sperm has turned biopsy into a potentially therapeutic, as well as diagnostic, procedure. Even men with markedly elevated serum FSH levels and small, soft testes (meaning certain testicular failure) often harbor rare mature sperm in their testes. These sperm can be extracted using techniques described later (see Testicular Sperm Extraction) and employed for IVF with intracytoplasmic injection of testicular sperm.
The recently discovered heterogeneity of the testes of men with nonobstructive azoospermia, coupled with the ability of testicular sperm to acquire motility (Jow et al, 1993), has resulted in changes in the techniques of testis biopsy. Examination of fresh, unfixed tissue for the presence of sperm with tails and possible motility, as well as examination of multiple samples if sperm are not found initially, are now recommended. Furthermore, optimal care requires the availability, at the time of biopsy, of an andrology laboratory capable of processing and cryopreserving any sperm found at the time of biopsy.
Open Testis Biopsy—Microsurgical Technique
Open biopsy remains the gold standard because it provides an optimal amount of tissue both for accurate diagnosis and retrieval of sperm for IVF (Rosenlund et al, 1998; Schlegel, 1999; Dardashti et al, 2000). Open testis biopsy may be performed using either general, spinal, or local anesthetic. Although local anesthesia of just the skin and tunicas without a cord block is uncomfortable, local anesthesia with spermatic cord block can be effective and comfortable. However, there are limitations of the cord block. In animal studies, the incidence of accidental damage to the testicular artery during blind cord block is 5% (Goldstein et al, 1983). In addition, if there has been previous scrotal surgery with scar or adhesions and more extensive dissection and manipulation may be required, the author prefers to use general or spinal anesthetic.
The surgeon’s goals when performing a testis biopsy are to provide an optimal tissue sample, avoid trauma to the specimen, and avoid injury to the epididymis or testicular blood supply. Open biopsy under magnification (preferably with an operating microscope) satisfies these requirements.
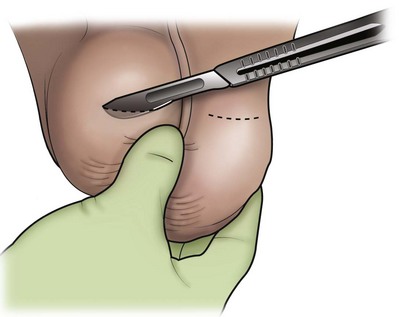
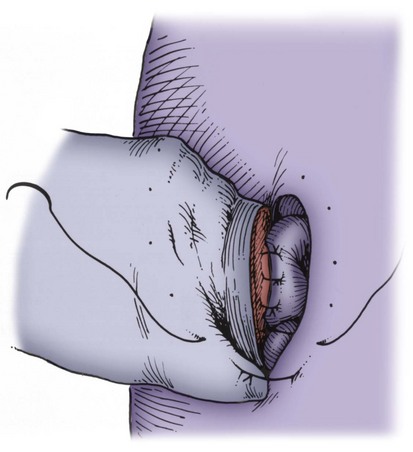
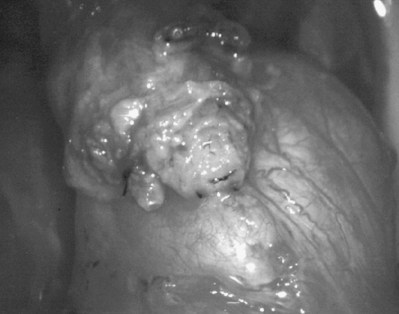
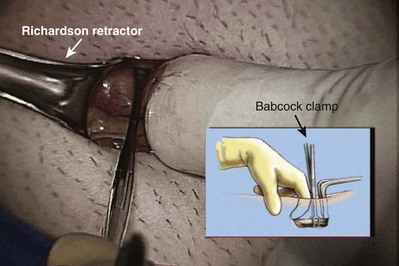
An assistant stretches the scrotal skin tightly over the anterior surface of the testis and confirms that the epididymis is posterior. Bilateral 1-cm transverse scrotal incisions provide good exposure with a minimum of scrotal bleeding (Fig. 22–1). Alternatively, a single vertical incision in the median raphe may be employed. The incision is carried through the skin and dartos muscle, and the tunica vaginalis is opened. If the anatomy is distorted from previous surgery, the epididymis cannot be clearly palpated posteriorly. If the tunica albuginea cannot be clearly identified, the incision should be enlarged and the testis delivered. The edges of the tunica vaginalis are held open with hemostats and any bleeding vessels are cauterized. Use of loupes or, better yet, the operating microscope allows ready identification of a spot on the tunica albuginea relatively free of visible surface vessels. The wound should be dry before incising the tunica albuginea to prevent saturation of the biopsy with blood. A 3- to 4-mm incision is made in the tunica albuginea with a 15-degree microknife (Fig. 22–2A). Small crossing vessels are cauterized with bipolar cautery and divided before excising a pea-sized sample of seminiferous tubules with razor-sharp iris scissors (Fig. 22–2B). When handling testis biopsy material for permanent fixation, avoid tissue handling in any way (including with forceps) because this may traumatize and distort the testicular architecture. The specimen is then deposited directly into Bouin, Zenker, or collidine-buffered glutaraldehyde solution. Formalin fixation results in distortion of testicular histology and should not be used for testis biopsy. A touch-prep is made by blotting the cut surface of the testis several times with a glass slide (Fig. 22–2C) and adding a drop of saline, lactated Ringer, or human tubal fluid with IVF medium and a cover slip. Examination under high power using a light microscope with or without phase contrast will reveal the presence of sperm with tails and allow assessment of motility (Fig. 22-2D). If no sperm are found in the touch prep, a second specimen may be cut for a wet squash prep. In this case the specimen is placed on a slide, a drop of saline is added, and the specimen is crushed under a coverslip (Jow et al, 1993). If no sperm are found, the tunica is closed with two to three interrupted sutures of 5-0 Vicryl (Fig. 22-2E) and another area is biopsied through the same skin incision. As described later in this chapter, use of an operating microscope providing 10× to 25× magnification may allow selective biopsy of larger seminiferous tubules more likely to contain sperm (Schlegel, 1999). If sperm are identified, the slide and additional tissue removed are sent for cryopreservation in the andrology laboratory. The location of the biopsy site where sperm were found is noted, and the tunica albuginea is closed with two to three interrupted sutures of 6-0 nylon. This assists identification of sites of spermatogenesis for future testicular sperm extraction for IVF/ICSI.
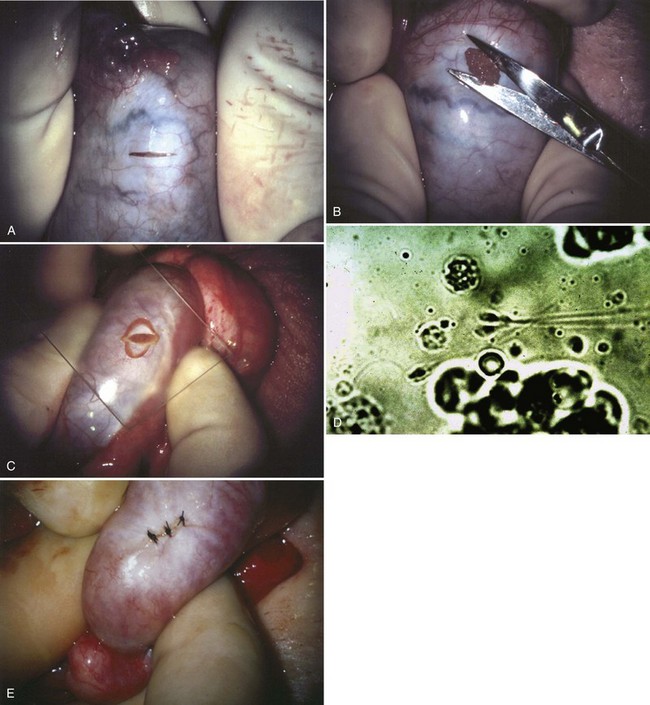
Figure 22–2 A, A 3- to 4-mm incision is made in the tunica albuginea with a 15-degree ultrasharp microknife. B, Small crossing vessels are cauterized with bipolar cautery, and a pea-sized sample of seminiferous tubules are taken with a no-touch technique. C, A touch-prep is made by blotting the cut surface of the testis several times with a glass slide. D, Examination under high power using a light microscope will reveal the presence of sperm and allow assessment of motility. E, The biopsy site is closed with two to three interrupted sutures of 6-0 nylon.
The tunica vaginalis is closed with 5-0 Vicryl suture for hemostasis. Careful closure will assist structural identification in any future scrotal explorations such as epididymal reconstruction, aspiration, or testicular sperm retrieval at the time of IVF with ICSI. The skin is closed with a subcuticular 5-0 Monocryl suture. The wounds are covered with Bacitracin ointment and a fluff-type dressing secured with a snug scrotal support. Antibiotics are unnecessary.
Percutaneous Testis Biopsy
Percutaneous testis biopsy using the same 14-gauge biopsy gun employed for prostatic biopsy is a blind procedure and could result in unintentional injury to either the epididymis or testicular artery. This technique should not be used when previous surgery has resulted in scarring and obliteration of normal anatomy. Diagnostic specimens obtained in this way often contain few tubules with poorly preserved architecture. When performed under local anesthesia, a cord block is necessary to minimize pain. The technique of percutaneous biopsy is described later in Testicular Sperm Extraction. As a therapeutic tool for sperm retrieval, percutaneous biopsy is most useful for fresh sperm retrieval for IVF/ICSI in men with obstructive azoospermia and normal spermatogenesis.
Percutaneous Testicular Aspiration
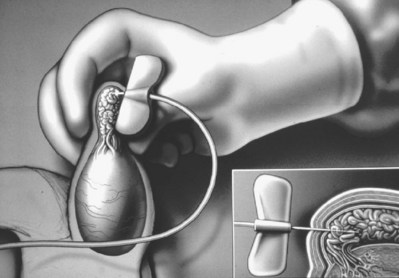
Testicular aspiration performed with a 23-gauge needle or angiocath sheath (Marmar) is probably less invasive and less painful than percutaneous biopsy. Although flow cytometric evaluation of this material can distinguish haploid from diploid cells and therefore confirm the presence or absence of late stages of spermatogenesis (Chan et al, 1984), direct wet examination of the aspirate for sperm and assessment of motility provide the most practical clinical information. Three or four aspirations can be performed until sperm are identified. In cases of obstructive azoospermia, these sperm can be used for IVF with ICSI (Craft et al, 1995) when sperm cannot be retrieved from the epididymis (see Testicular Sperm Extraction).
Complications of Testis Biopsy
Carefully performed, testis biopsy is associated with few complications (Schlegel and Su, 1997; Dardashti et al, 2000). The most serious complication associated with testis biopsy is inadvertent biopsy of the epididymis. If histologic evaluation of the biopsy material reveals epididymis with sperm within the epididymal tubule, obstruction of the epididymis at the site of the biopsy is certain. If, however, there are no sperm within the epididymal tubules, the patient is either obstructed above the level of the epididymal biopsy or has primary seminiferous tubular failure and no harm has been done.
The most common complication of testis biopsy is hematoma. Hematomas can be quite large and may require drainage. Use of magnification to avoid vessels and a bipolar cautery for hemostasis will help prevent this complication. Proper closure of the well-vascularized tunica vaginalis with a continuous suture of 5-0 Vicryl will minimize bleeding and adhesions.
With the rich blood supply of the scrotum and its contents, wound infection is rare in the absence of hematoma and antibiotics are unnecessary.
Vasography
Indications
The absolute indications for vasography are as follows:
Relative indications for vasography are as follows:
Vasography should answer the questions:
If the testis biopsy reveals many sperm, consider the following:
Vasography with radiographic contrast media and intraoperative radiograph are rarely indicated. There is no need to perform vasography at the time of testis biopsy for azoospermia unless immediate reconstruction is planned and the touch/wet prep biopsy reveals mature sperm with tails. If not meticulously performed, vasography can cause stricture or even obstruction at the vasography site, which can complicate subsequent reconstruction (Howards et al, 1975a; Poore et al, 1997). In addition, vasography is of no value in making the diagnosis of epididymal obstruction and the majority of nonvasectomy-related obstructions are epididymal.
If testis biopsy reveals normal spermatogenesis and the vasa are palpable, vasography, if necessary, should be performed only at the time of definitive repair of obstruction. General anesthesia provides the most flexibility for scrotal exploration, vasography, and repair of obstruction. Although local anesthesia can provide adequate analgesia, patients are often unable to lie still through several hours of microsurgery. Long-acting hypobaric spinal or continuous epidural anesthesia can be satisfactory alternatives.
Technique of Vasography and Interpretation of Findings
Inguinal hernia repair, particularly when performed in childhood, is known to be associated with vasal injury leading to obstruction. If there is no previous inguinal incision and the side of obstruction is unknown, the testis is delivered through a high vertical scrotal incision (see Scrotal later). The vas deferens is identified and isolated at the junction of the straight and convoluted portions of the vas deferens. Using an operating microscope and 10-power magnification, the vasal sheath is longitudinally incised and the vasal vessels carefully preserved (Fig. 22–3A).
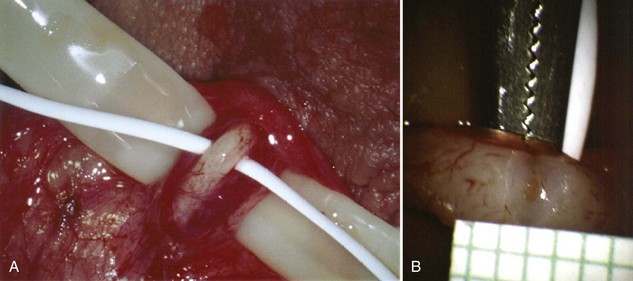
Figure 22–3 A, Using an operating microscope and 10× magnification, the vasal sheath is longitudinally incised and vasal vessels carefully preserved. B, Under 25× magnification, a 15-degree microknife is used to hemitransect the vas until the lumen is revealed.
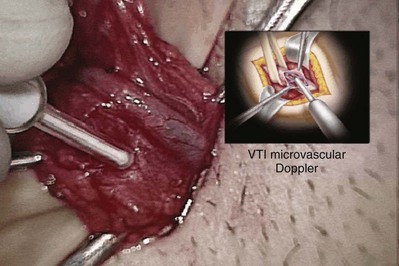
A clean segment of bare vas is delivered and surrounded with a vessel loop. A straight clamp is placed beneath the vas to act as a platform. Under 25-power magnification, a 15-degree microknife is used to hemitransect the vas until the lumen is revealed (Fig. 22–3B). Any fluid exuding from the lumen is placed on a slide, mixed with a drop of saline, and sealed with a coverslip for microscopic examination. If the vasal fluid is devoid of sperm with repeated sampling after milking the epididymis and convoluted vas, epididymal obstruction is present. The end of the vas toward the seminal vesicles is then cannulated with a 24-gauge angiocatheter sheath and injected with 1 mL of lactated Ringer solution with a 1-mL tuberculin syringe to confirm its patency (Fig. 22–4). If the Ringer passes easily, formal vasography is not necessary. If further proof of patency of the vas deferens is desired, 1 mL of 50% dilute indigo carmine may be injected and the bladder catheterized. The presence of blue/green dye in the urine confirms patency of the vas. Indigo carmine diluted 50/50 with lactated Ringer solution is preferred instead of methylene blue because, even at low concentrations, methylene blue kills sperm and renders them useless for cryopreservation or for immediate IVF/ICSI (Chang et al, 1998; Sheynkin et al, 1999b; Wood et al, 2003). If motile sperm are found in the vas, the testicular end should be gently barbotaged with 0.2 mL of human tubal fluid media and the fluid processed by the andrology laboratory for sperm cryopreservation for potential future use for IVF/ICSI. This should be done before injection with indigo carmine or x-ray contrast material (Sheynkin et al, 1999a).
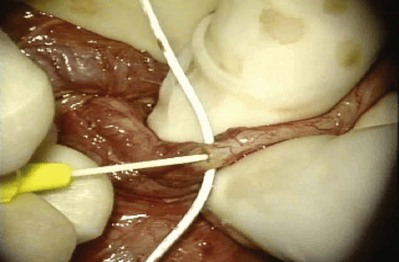
Figure 22–4 The end of the vas toward the seminal vesicles is cannulated with a 24-gauge angiocatheter and then injected with 1 mL of lactated Ringer solution with a 1-mL tuberculin syringe to confirm its patency.
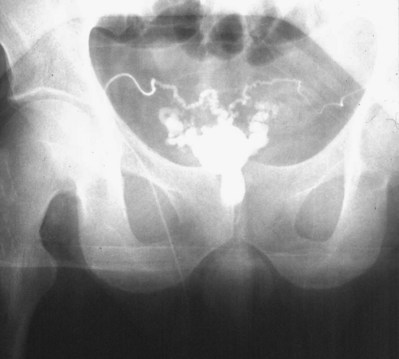
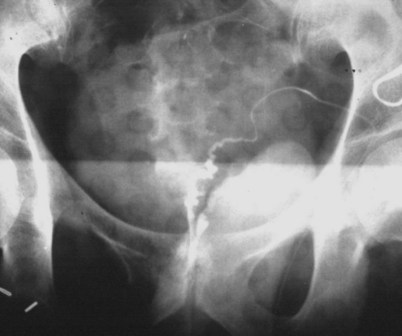
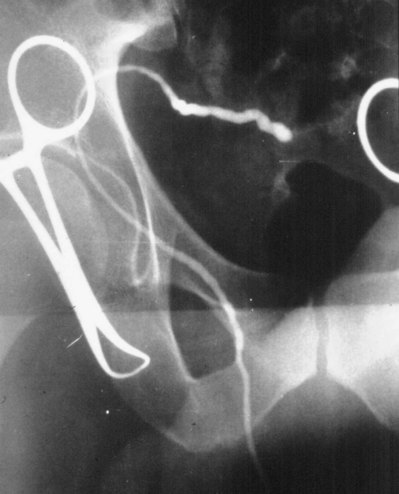
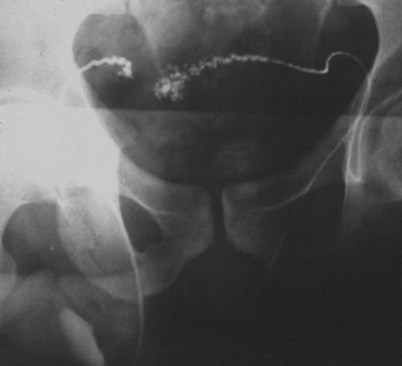
If a large amount of fluid is found in the vasal lumen and microscopic examination reveals the presence of sperm, the obstruction is toward the seminal vesicle end of the vas. In these cases the vas is usually markedly dilated. A 2-0 Proline suture can be passed toward the seminal vesicle end of the vas and a clamp placed on the Proline when the suture passes no farther. This is particularly useful for delineating the site of inguinal obstruction from prior groin surgery. If the obstruction is proximal to the inguinal scar, formal vasography is performed by passing a No. 3 whistle-tip ureteral catheter toward the seminal vesicle end of the vas. A 16-Fr Foley catheter is placed in the bladder, and the balloon is filled with 5 mL of air. Placing the balloon on gentle traction before vasography prevents reflux of contrast into the bladder, which can obscure detail (Fig. 22–5). The air-filled balloon also identifies the location of the bladder neck relative to any obstruction. After the vasa have been cannulated, vasograms are performed with the injection of 0.5 mL of water-soluble contrast media (Fig. 22–6). If vasography reveals obstruction at the site of the ejaculatory ducts (Fig. 22–7), indigo carmine is injected in both vasa to assist a transurethral resection (TUR) of the ejaculatory ducts (see Diagnosis later). If both vasa are visualized after injection of contrast into only one vas (Fig. 22–8), it means both vasa empty into a single cavity, usually a midline ejaculatory duct cyst.
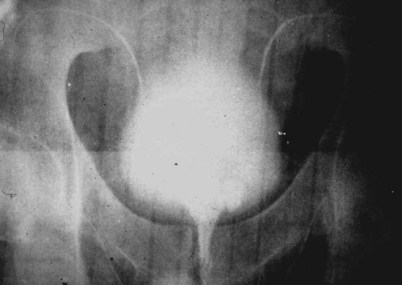
Figure 22–5 Placing the balloon on gentle traction before vasography prevents reflux of contrast into the bladder, which can obscure detail.
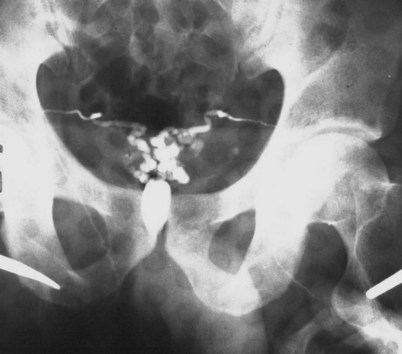
Figure 22–8 Both vasa are visualized after injection of contrast into one vas only, revealing distal obstruction.
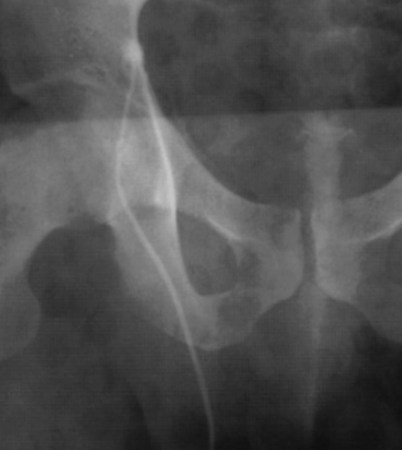
Vasography may reveal the vas deferens ending blindly, far from the ejaculatory ducts (Fig. 22–9). This finding indicates congenital partial absence of the vas deferens, and these patients should be tested for cystic fibrosis mutations (see Chapter 21). If this is found bilaterally (Fig. 22–10), reconstruction is impossible but vasal or epididymal sperm can be aspirated into standard laboratory pipettes (see Microsurgical Epididymal Sperm Aspiration Techniques later) and cryopreserved for future IVF with ICSI. If vasography reveals obstruction in the inguinal region (Fig. 22–11), either inguinal vasovasostomy or crossed transeptal vasovasostomy, using the contralateral unobstructed vas (see Crossed Vasovasostomy later), may be performed. The hemitransected vasography sites are carefully closed microsurgically using two or three interrupted 10-0 monofilament nylon for the mucosa and 9-0 for the muscularis and adventitia (see Microsurgical Multilayer Microdot Method later).
If the vasal fluid reveals no sperm and vasography confirms patency of the seminal vesicle end of the vas, the vas is completely transected and the seminal vesicle end is prepared for vasoepididymostomy (see Vasoepididymostomy later). If the vasal fluid reveals many sperm and vasography is normal, either retrograde ejaculation, lack of emission, or aperistalsis of the vas (Tiffany and Goldstein, 1985; Tillem and Mellinger, 1999) is the cause of the azoospermia.
Fine-Needle Vasography
Exposure of the vas in its straight portion may allow vasography to be performed with a fine needle, obviating the need for hemitransection of the vas. Dewire and Thomas (1995) employed a 30-gauge lymphangiogram needle attached to Silastic tubing. When the sensation of puncture of the lumen is detected, 50% water-soluble contrast is injected to confirm patency radiographically. This has proven to be a difficult technique for even experienced microsurgeons to master. Accurate evaluation of vasal fluid for sperm is difficult because it is so scant. If barbotage with saline or lactated Ringer solution reveals the presence of sperm, then epididymal obstruction has been excluded and contrast can be injected. Collection of vasal sperm for cryopreservation is difficult with this technique. Percutaneous vasography through the scrotal skin has been successfully performed in China (Li) using the same ringed percutaneous fixation clamp employed for the no-scalpel vasectomy. After fixation of the vas beneath the scrotal skin, the vas lumen is punctured with a 22-gauge sharp needle and cannulated with a 24-gauge blunt needle through which vasography is performed. This technique is even more difficult than the direct-vision technique with a fine needle.
Complications of Vasography
Stricture
Multiple attempts at percutaneous vasography using sharp needles can result in stricture or obstruction at the vasography site. Imprecise closure of a vasotomy can also result in stricture and obstruction (Howards et al, 1975b; Poore et al, 1997). Non-water-soluble contrast agents may also result in stricture and should not be employed for vasography.
Injury to the Vasal Blood Supply
If the vasal blood supply is injured at the site of vasography, vasovasostomy proximal to the vasography site may result in ischemia, necrosis, and obstruction of the intervening segment of vas.
Transrectal Vasography and Seminal Vesiculography
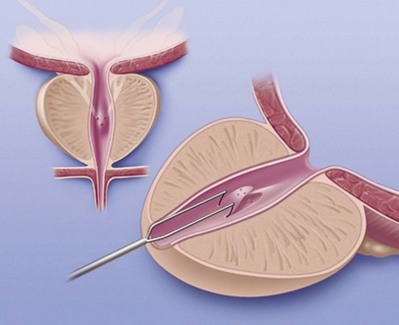
If transrectal ultrasound reveals markedly dilated seminal vesicles and/or a midline müllerian duct cyst in a man with obstructive azoospermia, transrectal aspiration followed by instillation of indigo carmine mixed with radiographic contrast is a useful diagnostic maneuver (Jarow, 1994; Katz et al, 1994; Riedenklau et al, 1995).
The same bowel prep and antibiotic coverage used for transrectal prostate biopsy is employed. The fine-needle aspirate is examined for sperm. If sperm are present it means at least one vas and epididymis are patent. One-half mL of indigo carmine is diluted with 1.5 mL of 50% water-soluble contrast and instilled. If a flat plate reveals a potentially resectable lesion, a TUR of the ejaculatory ducts is performed (see Transurethral Resection of the Ejaculatory Ducts later). Visualization of blue dye effluxing from the ejaculatory ducts or an unroofed cyst aids in determining the adequacy of the resection (Cornel et al, 1999).
This technique obviates the need for formal open scrotal vasography in men with transrectally accessible lesions. If sperm are found in the aspirate, a TUR may immediately be undertaken without violating the scrotum. Sperm-laden aspirates may be frozen for future IVF with ICSI if surgery fails.
If no sperm are found in the aspirated fluid, it suggests that secondary epididymal obstruction exists. Simultaneous TUR of the ejaculatory ducts and vasoepididymostomy is rarely successful. In the face of both ejaculatory duct obstruction and bilateral epididymal obstruction, the best option would be epididymal sperm aspiration for cryopreservation for future IVF/ICSI.
Vasovasostomy
Introduction
The number of American men who undergo vasectomy has remained stable at about 500,000 per year, as has the divorce rate of 50%. Surveys suggest that 2% to 6% of vasectomized men will ultimately seek reversal. Furthermore, obstructive azoospermia can be the result of iatrogenic injuries to the vas deferens, usually from hernia repair, in 6% of azoospermic men (Sheynkin et al, 1998a; Shin et al, 2005).
Preoperative Evaluation
Before attempted surgical reconstruction of the reproductive tract, adequate spermatogenesis should be documented. A prior history of natural fertility prevasectomy is usually adequate. In other cases a testicular biopsy may be indicated to confirm the presence of spermatogenesis.
Laboratory Tests
Anesthesia
General anesthesia is preferred. Slight movements are greatly magnified by the operating microscope and disturb performance of the anastomosis. In cooperative patients regional or even local anesthesia with sedation can be employed if the vasal ends are easily palpable, a sperm granuloma is present, and/or the time interval since vasectomy is short, decreasing the likelihood of secondary epididymal obstruction. When large vasal gaps are present, extensions of the incisions high into the inguinal canal may be necessary. Furthermore, if vasoepididymostomy is necessary, the operating time could exceed 4 or 5 hours. Local anesthesia limits the options available to the surgeon. Hypobaric spinal anesthesia with long-acting agents such as Marcaine can provide 4 to 5 hours of anesthesia time and has the advantage of eliminating lower body motion. Epidural anesthesia with an indwelling catheter can be equally effective.
Surgical Approaches
Scrotal
Bilateral high vertical scrotal incisions provide the most direct access to the obstructed site in cases of vasectomy reversal. Length is usually a problem on the abdominal end but not on the testicular end. Mark the location of the external inguinal ring (Fig. 22–12). If the vasal gap is large or the vasectomy site is high, this incision can easily be extended toward the external ring. If the vasectomy site is low, it is easy to pull up the testicular end. This incision should be made at least 1 cm lateral to the base of the penis. The testis should be delivered with the tunica vaginalis left intact. This provides excellent exposure of the entire scrotal vas deferens and, if necessary, the epididymis.
Infrapubic Incision
An infrapubic incision provides excellent exposure when the abdominal end of the vas is short but awkward when vasoepididymostomy is necessary. In addition, surgeons should be cautious that, when vasal gaps are large, tension on the anastomosis may not be apparent during the surgery until an attempt is made to place the testis back into the scrotum. Overall this incision has no advantage over inguinal extension of a high scrotal incision.
Inguinal Incision
An inguinal incision is the preferred approach in men when obstruction of the inguinal vas deferens from prior herniorrhaphy or orchiopexy is strongly suspected. Incision through the previous scar usually leads directly to the site of obstruction. If the obstruction turns out to be scrotal or epididymal, it is a simple matter to deliver the testis through the inguinal incision or through a separate scrotal incision to perform the anastomosis.
Preparation of the Vasa
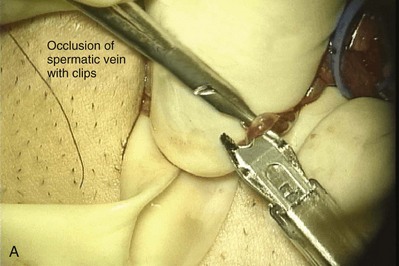
The vas is grasped above and below the site of obstruction with two Babcock clamps. Penrose drains replace the Babcock clamps and assist the dissection. The vasal vessels and periadventitial sheath are included. Transillumination of the periadventitial sheath, by properly adjusting the operating light, allows clear visualization of the blood vessels, which facilitates dissection of the periadventitial sheath and prevents damage to the vasal vessels. The vas is mobilized enough to allow a tension-free anastomosis. In order to preserve good blood supply the vas should not be stripped of its sheath. The obstructed segment and, if present, sperm granuloma at the vasectomy site should be dissected out and excised. By staying right on the vas and/or sperm granuloma during this dissection, the risk of injuring the testicular artery is reduced. Injury to adjacent cord structures, especially the testicular artery, is likely to result in testicular atrophy because the vasal artery has usually been interrupted at the vasectomy site.
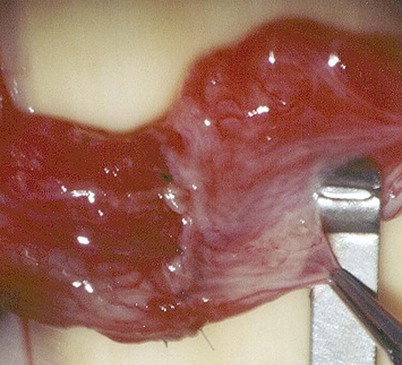
When large vasal gaps are present, a gauze-wrapped index finger is used to bluntly separate the cord structures from the vas. Blunt finger dissection through the external ring will free the vas to the internal inguinal ring if additional abdominal side length is necessary. These maneuvers will leave all the vasal vessels intact. When the vasal gap is extremely large, additional length can be achieved by dissecting the entire convoluted vas free of its attachments to the epididymal tunica (Fig. 22–13), allowing the testis to drop upside down. These maneuvers can provide an additional 4 to 6 cm of length. In order to maintain the integrity of the vasal vessels, this dissection is best performed using magnifying loupes or the operating microscope under low power. If the amount of vas removed is so large that even these measures fail to allow a tension-free anastomosis, the incision can be extended to the internal inguinal ring, the floor of the inguinal canal cut, and the vas rerouted under the floor, as in a difficult orchiopexy. An additional 4 to 6 cm of length can be obtained by dissecting the epididymis off of the testis from the vasoepididymal junction to the caput epididymis (Fig. 22–14A and B). The superior epididymal vessels are left intact and provide adequate blood supply to the testicular end of the vas. With this combination of maneuvers, up to 10 cm gaps can be bridged.
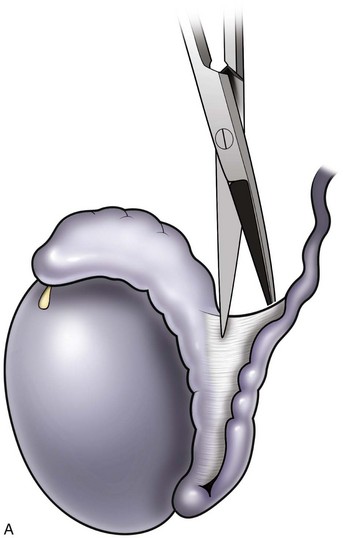
Figure 22–13 A and B, An additional 4 to 6 cm of length can be obtained by dissecting the epididymis off of the testis from the vasoepididymal junction to the caput epididymis.
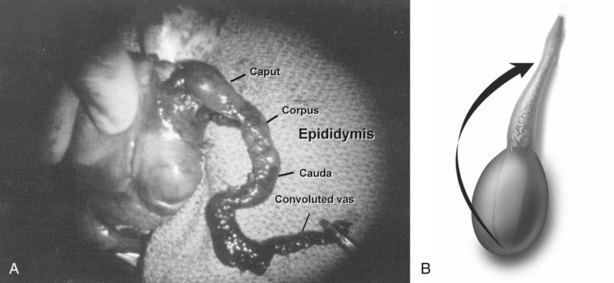
Figure 22–14 A and B, An additional 4 to 6 cm of length can be obtained by dissecting the epididymis off of the testis from the vasoepididymal junction to the caput epididymis.
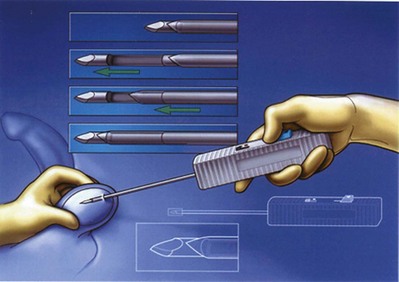
After the vasa have been freed, the testicular end of the vas is cut transversely. An ultrasharp knife drawn through a slotted 2-, 2- to 5-, or 3-mm diameter nerve holding clamp (Accurate Surgical and Scientific Instrument Corp., Westbury, NY) yields a perfect 90-degree cut (Fig. 22–15). The cut surface of the testicular end of the vas deferens is inspected using 15- to 25-power magnification. A healthy white mucosal ring that springs back immediately after gentle dilation should be seen. The muscularis should appear smooth and soft. A gritty-looking muscularis layer indicates the presence of scar/fibrotic tissues. The cut surface should look like a bull’s-eye with the three vasal layers distinctly visible. Healthy bleeding should be noted from both the cut edge of the mucosa and the surface of the muscularis. If the blood supply is poor or the muscularis is gritty, the vas is recut until healthy tissue is found. The vasal artery and vein are then clamped and ligated with 6-0 nylon. Small bleeders are controlled with a microbipolar forcep set at low power. Once a patent lumen has been established on the testicular end, the vas is milked and a clean glass slide is touched to its surface. The vasal fluid is immediately mixed with a drop or two of saline or lactated Ringer solution and preserved under a cover slip for microscope examination. The abdominal end of the vas deferens is prepared in a similar manner, and the lumen gently dilated with a microvessel dilator and cannulated with a 24-gauge angiocatheter sheath. Injection of saline or lactated Ringer solution confirms its patency. After the Ringer injection and a test dilation, the vas is recut to obtain a fresh surface. A minimum of instrumentation of the mucosa should be performed.
Figure 22–15 An ultrasharp knife drawn through a slotted 2.5- or 3-mm diameter nerve-holding clamp (Accurate Surgical and Scientific Instrument Corp., Westbury, NY) yields a perfect 90-degree cut.
After preparation, the ends of the vasa are stabilized with a Microspike approximating clamp (Goldstein, 1985) to remove all tension before performing the anastomosis. Isolating the field through a slit in a rubber dam prevents microsutures from sticking to the surrounding tissue. A sterile tongue blade covered with a large Penrose drain is placed beneath the ends of the vasa to provide a platform on which to perform the anastomosis.
When to Perform Vasoepididymostomy
The gross appearance of fluid expressed from the testicular end of the vas is usually predictive of findings on microscopic examination (Table 22–2). If microscopic examination of the vasal fluid reveals the presence of sperm with tails, vasovasostomy is performed. If no fluid is found, a 24-gauge angiocatheter sheath is inserted into the lumen of the testicular end of the vas and barbotaged with 0.1 mL of saline while the convoluted vas is vigorously milked. The barbotage fluid is expressed onto a slide and examined. Men with large sperm granulomas often have virtually no dilation of the testicular end of the vas and little or no fluid initially; however, with barbotage and vigorous milking, sperm can invariably be found in this scant fluid. If there is no sperm granuloma and the vas is absolutely dry and spermless after multiple samples are examined, vasoepididymostomy is indicated. If the fluid expressed from the vas is found to be thick, white, water insoluble, and like toothpaste in quality, microscopic examination rarely reveals sperm. Under these circumstances, the tunica vaginalis is opened and the epididymis inspected. If clear evidence of obstruction is found (i.e., an epididymal sperm granuloma with dilated tubules above and collapsed tubules below), vasoepididymostomy is performed. When in doubt or if not experienced with vasoepididymostomy, vasovasostomy should be performed. However, only 15% of men with bilateral absence of sperm in the vasal fluid after barbotage and an intensive search will have sperm return to the ejaculate after vasovasostomy (Sheynkin et al, 2000).
Table 22–2 Relationship between Gross Appearance of Vasal Fluid and Microscopic Findings
| VASAL FLUID APPEARANCE | MOST COMMON FINDINGS ON MICROSCOPIC EXAMINATION | SURGICAL PROCEDURE INDICATED |
|---|---|---|
| Copious, crystal clear, watery | No sperm in fluid | Vasovasostomy |
| Copious, cloudy thin, water soluble | Usually sperm with tails | Vasovasostomy |
| Copious, creamy yellow, water soluble | Usually many sperm heads; occasionally sperm have short tails | Vasovasostomy |
| Copious, thick, white toothpaste-like, water insoluble | No sperm | Vasoepididymostomy |
| Scant white thin fluid | No sperm | Vasoepididymostomy |
| Dry spermless vas—no granuloma at vasectomy site | No sperm | Vasoepididymostomy |
| Scant fluid, granuloma present at vasectomy site | Barbotage fluid reveals sperm | Vasovasostomy |
When copious, crystal-clear, water-like fluid squirts out from the vas and no sperm are found in this fluid, a vasovasostomy is performed because the likelihood is that sperm will return to the ejaculate after vasovasostomy is performed.
Multiple Vasal Obstructions
If saline injection reveals that the abdominal end of the vas deferens is not patent, a 2-0 nylon or polypropylene suture is gently threaded into the vas lumen to determine the site of obstruction. If the obstruction is within 5 cm of the original vasectomy site, the abdominal end of the vas deferens may be dissected to this site and excised. The incision should then be extended inguinally to free the vas up extensively toward the internal inguinal ring. The testicular end then should also be freed up to the vasoepididymal junction. If the site of the second obstruction is so far from the vasectomy site that two vasovasostomies are necessary, a single crossed vasovasostomy should be performed to yield one good system (see Crossed Vasovasostomy earlier). If this is not possible, vasal or epididymal sperm is aspirated into micropipets and cryopreserved for future IVF with ICSI (see Sperm Retrieval Techniques later). Simultaneous vasovasostomies at two separate sites may lead to devascularization of the intervening segment with fibrosis and necrosis.
Varicocelectomy and Vasovasostomy
When men presenting for vasovasostomy or vasoepididymostomy are found to have significant varicoceles on physical examination, it is tempting to repair the varicoceles at the same time. When varicocelectomy is properly performed, all spermatic veins are ligated and the only remaining avenues for testicular venous return are the vasal veins. In men who have had vasectomy and are presenting for reversal, the vasal veins are likely to be compromised from either the original vasectomy or the reversal itself. Furthermore, the integrity of the vasal artery in those men is also likely to be compromised. Varicocelectomy in such men requires preservation of the testicular artery as the primary remaining testicular blood supply, as well as preservation of some avenue for venous return.
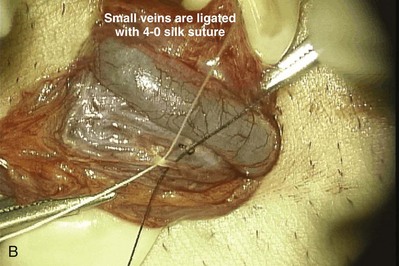
Microscopic varicocelectomy can assure preservation of the testicular artery in most cases. Deliberate preservation of the cremasteric veins provides venous return. In one series of 570 men presenting for vasectomy reversal, 19 had large varicoceles (20 left, 7 bilateral). Microsurgical varicocelectomy was performed at the same time as vasovasostomy. The cremasteric veins and the fine network of veins adherent to the testicular artery were left intact for venous return and to minimize the chances of injury to the testicular artery. Postoperatively 5 of 26 varicoceles recurred (22%) (Goldstein, 1995). This compares with a recurrence rate of less than 1% in 2700 varicocelectomies performed by the author in nonvasectomized men in whom the vasal vessels were intact and the cremasteric veins and periarterial venous network were ligated. However, Mullhall and colleagues performed a series of simultaneous microsurgical vasovasostomies and varicocelectomies without intentionally preserving the cremasteric and periarterial network. They reported a low recurrence rate and no cases of atrophy (Mulhall et al, 1997). Interestingly, the increase in recurrences when the cremasteric veins and periarterial venous network were left intact suggests that these veins contribute to a significant proportion of varicocele recurrences.
If varicocelectomy is performed at the same time as vasovasostomy or vasoepididymostomy, it is important that a microscope be used and the testicular artery preserved. A safer approach is to do the vasovasostomy or vasoepididymostomy first. The semen quality is then assessed postoperatively. If necessary, varicocelectomy can be safely performed 6 months or more later when venous and arterial channels have formed across the anastomotic line. This two-stage delayed approach has been completed a dozen times with no atrophy or recurrence.
Anastomotic Techniques: Keys to Success
All successful vasovasostomy techniques depend on adherence to surgical principles that are universally applicable to anastomoses of all tubular structures.
In human vasovasostomy, the lumen on the testicular side is usually dilated, often to diameters two to five times that of the abdominal side. Techniques that work well with lumina of equal diameters may be less successful when applied to lumina of markedly discrepant diameters.
Sperm are highly antigenic and provoke an inflammatory reaction when they escape from the normally intact lining of the excurrent ducts of the male reproductive tract. Extravasated sperm adversely influence the success of vasovasostomy (Hagan and Coffey, 1977). Unlike blood vessel anastomoses, in which platelets and clotting factors seal the gaps between sutures, vasal and epididymal fluid contain no platelets or clotting factors, so the water-tightness of the anastomosis is entirely dependent on the mucosal sutures.
When an anastomosis is performed under tension, sperm may appear in the ejaculate for several months after surgery. Ultimately, sperm counts and motility will decrease and azoospermia may ensue. At re-exploration only a thin fibrotic band is found at the anastomotic site. This can be prevented by adequately freeing up the vasa and placement of re-enforcing sutures in the sheath of the vas.
If the cut vas exhibits poor blood supply, it should be recut until healthy bleeding is encountered. If extensive resection is necessary, additional length should be obtained using the techniques previously described.
If the mucosa or cut surface of the vas exhibits poor distensibility after dilation, peels away from the underlying muscularis, or shreds easily, then the vas should be cut back until healthy mucosa is found. Surgeons should be aware that if a needle electrocautery was used in vasectomy, the area of damage to the mucosa and muscularis by the electric current may extend far beyond the tip of the needle cautery. If the muscularis is found to be fibrotic or gritty, the vas must be recut until healthy tissue is found.
Setup
An operating microscope providing variable magnification from 6 to 32 power is employed. A diploscope providing identical fields for both surgeon and assistant is preferred. Foot pedal controls for a motorized zoom and focus leave the surgeon’s hands free.
Both surgeon and assistant should be comfortably seated on microsurgical chairs that stabilize the chest and arms. This dramatically improves stability and accuracy. An inexpensive alternative is a simple rolling stool with a round bean bag (meditation pillow) taped on top for padding. Two arm boards, placed on either side of the surgeon and built up to the appropriate height with folded blankets taped to the board, provide excellent arm support. A right-handed surgeon should sit on the patient’s right side so that his or her forehand stitch is always on the smaller, more difficult abdominal-side lumen.
Microsurgical Multilayer Microdot Method
This method of vasovasostomy can handle lumina of markedly discrepant diameters in the straight or convoluted vas. The microdot technique ensures precise suture placement by exact mapping of each planned suture. The microdot method separates the planning from the placement (Goldstein et al, 1998). This allows focus on only one task at a time and results in substantially improved accuracy.
A microtip marking pen (Devon Skin Marker Extra Fine No. 151) is used to map out planned needle exit points. Exactly six mucosal sutures are used for every anastomosis because it is easy to map out and always results in a leakproof closure even when the lumen diameters are markedly discrepant.
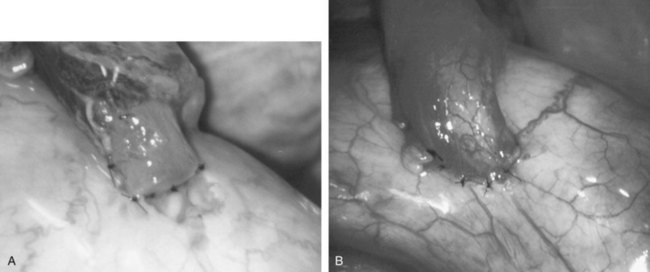
Immediately after drying the cut surface of the testicular end of vas with a Weck cell, a dot is made at 3 o’clock halfway between the mucosal ring and the outer edge of the muscle layer. A line is extended out from this dot to serve as a reference point. The second dot is made at 9 o’clock, and a line is extended from this dot as well. Additional dots are placed at 11, 1, 5, and 7 o’clock for a total of six. The abdominal end of the vas is marked in the same way to exactly match the testicular end (Fig. 22–16). Monofilament 10-0 nylon sutures, double-armed with 70-µm diameter taper-point needles bent into a fish hook configuration (available from Sharpoint and Ethicon), are used. Double-armed sutures allow inside-out placement (Fig. 22–17), eliminating the need for manipulation of the mucosa and the possibility of back-walling. If the mucosal rings are not sharply defined, stain the cut surfaces of the vasal ends with indigo carmine to highlight the mucosa (Sheynkin et al, 1999a). The anastomosis is begun with the placement of three 10-0 mucosal sutures anteriorly (Fig. 22–18). On the small abdominal side lumen, gently and momentarily dilate the lumen with a microvessel dilator just before placement of the sutures. For accurate mucosal approximation, include only a small amount of mucosa but one-third to one-half the muscle wall thickness. Include exactly the same amount of tissue in the bites on each side. The needle should exit through the center of each dot. After placement, tie the three mucosal sutures. Place two 9-0 monofilament nylon deep muscularis sutures exactly in between the previously placed mucosal sutures, just above, but not through the mucosa (Fig. 22–19) and then tie them. These sutures seal the gaps between the mucosal sutures without trauma to the mucosa from the larger 100-µm diameter cutting needle required to penetrate the tough vas muscularis and adventitia. Rotate the vas 180 degrees (Fig. 22–20) and place three additional 10-0 sutures through each microdot and then tie them to complete the mucosal portion of the anastomosis (Fig. 22–21). Just before tying the last mucosal suture, irrigate the lumen with heparinized Ringer solution to prevent the formation of clot in the lumen. After completion of the mucosal layer (Fig. 22–22), place four more 9-0 deep muscularis sutures exactly in between each mucosal suture, just above but not penetrating the mucosa. Place four to six 9-0 nylon interrupted sutures, between each muscular suture. This is a purely adventitial layer that covers the underlying mucosal suture. Finish the anastomosis by approximating the vasal sheath with six interrupted sutures of 8-0 nylon completely covering the anastomosis and relieving it of all tension (Fig. 22–23).
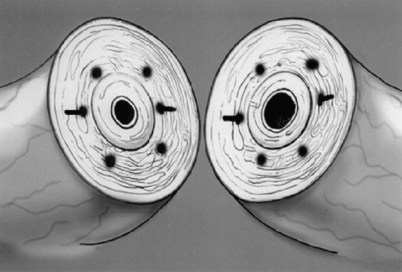
Figure 22–16 The abdominal end of the vas is marked in the same way to exactly match the testicular end.
Figure 22–19 Place two 9-0 monofilament nylon deep muscularis sutures exactly in between the previously placed mucosal sutures, just above, but not through, the mucosa.
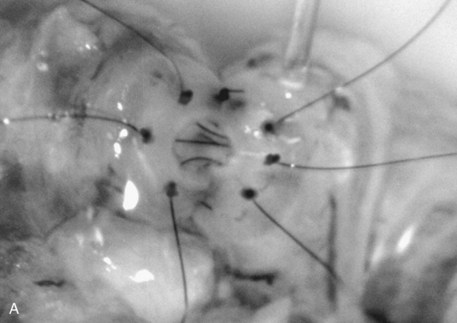
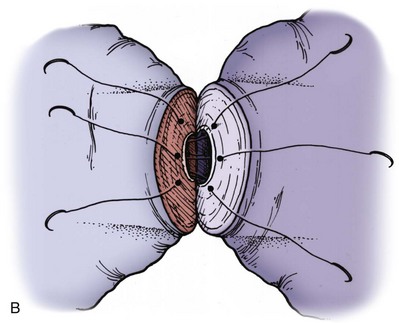
Figure 22–20 A and B, Rotate the vas 180 degrees and then place three more 10-0 sutures through the remaining microdots.
Anastomosis in the Convoluted Vas
Vasovasostomy performed in the convoluted portion of the vas deferens is technically more demanding than anastomoses in the straight portion. Fear of cutting back into the convoluted vas in order to obtain healthy tissue may lead surgeons to complete an anastomosis in the straight portion when the testicular end of the vas has poor blood supply, unhealthy or friable mucosa, or gritty fibrotic muscularis. Adherence to the following principles will enable anastomosis in the convoluted vas to succeed as often as those in the straight portion.
Crossed Vasovasostomy
This is a useful procedure that often provides an easy solution for otherwise difficult problems (Lizza et al, 1985; Hamidinia, 1988; Sheynkin et al, 1998a). Crossover is indicated in the following circumstances:
It is preferable to perform one anastomosis with a high probability of success (vasovasostomy) rather than two operations with a much lower chance of success (i.e., unilateral vasovasoepididymostomy and contralateral transurethral resection of the ejaculatory ducts).
Technique
Transect the vas attached to the atrophic testis at the junction of its straight and convoluted portion and confirm its patency with a Ringer or indigo carmine vasogram (Fig. 22–25). Dissect the contralateral vas with the normal testis toward the inguinal obstruction. Clamp and transect it as high up as possible with a right-angle clamp. Cross the testicular end of the vas through a capacious opening made in the scrotal septum and proceed with vasovasostomy as described earlier. This procedure is much easier than inguinal vasovasostomy, which requires finding both ends of the vas within the dense scar of a previous inguinal operation.
Transposition of the Testis
Occasionally when vasal length is critically short, a tension-free crossed anastomosis can best be accomplished by testicular transposition (Fig. 22–26). The spermatic cord is always longer than the vas. The testes will comfortably cross through a generous opening in the septum and sit nicely in the contralateral scrotal compartment.
Wound Closure
If the vasal dissection was extensive, Penrose drains are brought out the dependent portion of the right and left hemiscrota and fixed in place with sutures and safety pins preferably before beginning the anastomosis. Placement of drains at the end of the procedure may potentially disturb the anastomosis. The dartos layer is approximated with interrupted 4-0 absorbable sutures and the skin with subcuticular sutures of 5-0 Monocryl. The wound heals with a fine scar. The use of through-and-through skin closures, which give an unacceptable railroad-track-looking scar, should be avoided. Virtually all of our procedures are performed on an ambulatory basis. If drains were placed, the patients are given detailed instructions (with explicit drawings) on how to remove the drains the next morning.
Postoperative Management
Sterile fluff gauze dressings are held in place with a snug-fitting scrotal supporter. Only perioperative antibiotics are used. Patients are discharged with a prescription for acetaminophen with codeine. They shower 48 hours after surgery. They wear a scrotal supporter at all times (except in the shower), even when sleeping, for 6 weeks postoperatively. Thereafter a scrotal supporter is worn during athletic activity until pregnancy is achieved. Desk work is resumed in 3 days. No heavy work or sports are allowed for 3 weeks. No intercourse or ejaculation is allowed for 4 weeks postoperatively. Semen analyses are obtained at 1, 3, and 6 months postoperatively and every 6 months thereafter. If azoospermia persists at 6 months, a redo vasovasostomy or vasoepididymostomy will be necessary.
Postoperative Complications
The most common complication is hematoma. In 2500 operations seven small hematomas occurred. None required surgical drainage. Most were walnut sized and perivasal. They take 6 to 12 weeks to resolve. Wound infection has not occurred. Late complications include sperm granuloma at the anastomotic site (≈5%). This is usually a harbinger of eventual obstruction. Late stricture and obstruction are disappointingly common (see later). Progressive loss of motility followed by decreasing counts indicates stricture. Our recent change from Proline to nylon sutures (Sheynkin et al, 1999a), use of the microdot system to prevent leaks, extensive dissection of the vas until healthy mucosa and muscularis are identified, constant attention to the preservation of good blood supply, as well as generous use of scrotal support until pregnancy is established, has reduced the incidence of late obstruction from 12% (Matthews et al, 1995) to 5% (Kolettis and Thomas, 1997) at 18 months after surgery. Because of the risk of late stricture and obstruction, we strongly encourage cryopreservation of semen specimens as soon as motile sperm appear in the ejaculate.
Long-Term Follow-Up Evaluation after Vasovasostomy
When sperm are found in the vasal fluid on at least one side at the time of surgery, the anastomotic technique described results in appearance of sperm in the ejaculate in 99.5% of men (Goldstein et al, 1998). Pregnancy has occurred in 52% of couples followed for at least 2 years and 63% when female factors are excluded with outcomes dependent on the time since vasectomy and female partner age (Kolettis et al, 2003; Boorjian et al, 2004; Kolettis et al, 2005; Gerrard et al, 2007).
Surgery of the Epididymis
Introduction
Detailed knowledge of epididymal anatomy and physiology (presented in Chapters 2 and 20) is essential before undertaking surgery of this delicate but important structure. Sperm motility and fertilizing capacity progressively increase during passage through the 200-µm diameter, 12- to 15-foot-long, tightly coiled single tubule. When the epididymis is obstructed and functionally shortened after vasoepididymostomy, even short lengths of epididymis are able to adapt and allow some sperm to acquire motility and fertilizing capacity (Silber, 1989a, 1989b; Jow et al, 1993). Adaptation may gradually continue up to 2 years after surgical reconstruction, with progressive improvement in the fertility and motility of sperm. Nevertheless, preservation of the greatest possible length of functional epididymis is most likely to result in the best sperm quality after vasoepididymostomy (Schoysman and Bedford, 1986; Schlegel and Goldstein, 1993). Furthermore, because the wall of the epididymis is thinnest in the caput region and gradually thickens, due to the increasing numbers of smooth muscle cells in its more distal (inferior) end, anastomoses are technically easier to perform and more likely to succeed in distal regions. Because the corpus and cauda epididymis is a single tubule with a small diameter, injury or occlusion of a tubule anywhere along its length will lead to total obstruction of outflow at that level. For these reasons, magnification, with loupes for macrodissection and with the operating microscope for anastomosis, is essential for performing all epididymal surgery.
Fortunately, the epididymis is blessed with a rich blood supply derived from the testicular vessels superiorly and the deferential vessels inferiorly (see Testicular Blood Supply earlier and Chapter 2). Because of the extensive interconnections between these branches, either the testicular or deferential branches (but not both) to the epididymis may be divided without compromising epididymal viability.
Conversely, because the epididymal branches of the testicular artery are medial to and separate from the main testicular artery and veins, surgical procedures may be performed on the epididymis without compromise to testicular blood supply.
Vasoepididymostomy
Introduction
Before the development of microsurgical techniques, accurate approximation of the vasal lumen to that of a specific epididymal tubule was not possible. Vasoepididymostomy was performed by aligning the vas deferens adjacent to a slash made in multiple epididymal tubules and hoping a fistula would form. Results with this primitive technique were poor. Microsurgical approaches allow accurate approximation of the vasal mucosa to that of a single epididymal tubule (Silber, 1978), resulting in marked improvement in the patency and pregnancy rates (Schlegel and Goldstein, 1993; Chan et al, 2005). Microsurgical vasoepididymostomy, however, is the most technically demanding procedure in all of microsurgery. In virtually no other operation are results so dependent on technical perfection. Microsurgical vasoepididymostomy should only be attempted by experienced microsurgeons who perform the procedure frequently.
Indications
The indications for vasoepididymostomy at the time of vasectomy reversal are reviewed in Vasovasostomy earlier. For obstructive azoospermia not due to vasectomy, vasoepididymostomy is indicated when the testis biopsy reveals complete spermatogenesis and scrotal exploration reveals the absence of sperm in the vasal lumen with no vasal or ejaculatory duct obstruction. The preoperative evaluation is identical to that described in the previous section on vasovasostomy.
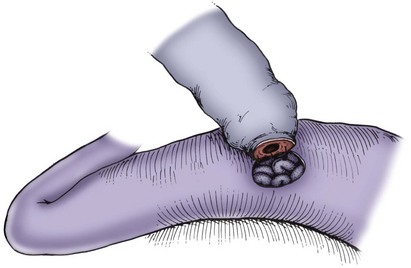
Microsurgical End-to-Side Vasoepididymostomy
End-to-side techniques of vasoepididymostomy have the advantage of being minimally traumatic to the epididymis and relatively bloodless (Table 22–3) (Wagenknecht et al, 1980; Krylov and Borovikov, 1984; Fogdestam et al, 1986; Thomas, 1987). The end-to-side technique does not disturb the epididymal blood supply. When the level of epididymal obstruction is clearly demarcated by the presence of markedly dilated tubules proximally and collapsed tubules distally, the site at which the anastomosis should be performed is readily apparent. The end-to-side approach has the advantage of allowing accurate approximation of the muscularis and adventitia of the vas deferens to a precisely tailored opening in the tunica of the epididymis. This is the preferred technique when vasoepididymostomy is performed simultaneously with inguinal vasovasostomy because it is possible to preserve the vasal blood supply deriving from epididymal branches of the testicular artery (Fig. 22–27). This provides blood supply to the segment of vas intervening between the two anastomoses. Maintenance of the deferential artery’s contribution to the testicular blood supply is also important in situations where the integrity of the testicular artery is in doubt due to prior surgery such as orchiopexy, nonmicroscopic varicocelectomy, or hernia repair.
Table 22–3 Comparison of Three Common Techniques for Vasoepididymostomy
| TECHNIQUES | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Intussusception | 2-3 sutures placed in the epididymal tubule provide 4 and 6 points of fixation. Virtually bloodless anastomosis. |
Cannot assess tubular fluid for sperm before anastomosis setup. |
| End-Side | Virtually bloodless anastomosis. Epididymal fluid can be examined before anastomosis. |
Difficult suture placement to a collapsed tubule. |
| End-End | Epididymal fluid can be examined before anastomosis. Easy and rapid identification of the level of obstruction in the epididymis. Allows upward mobilization of epididymis to bridge a large vasal gap. |
Difficult hemostasis on transected epididymis. Difficult to identify the proper tubule for anastomosis. Difficult outer-layer closure. Vasal blood supply from inferior epididymal artery is sacrificed. |
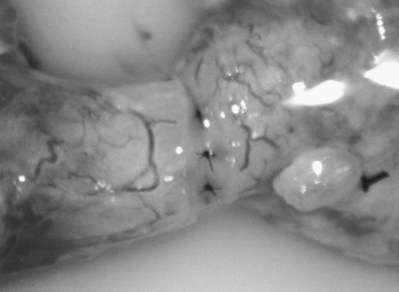
The testis is delivered through a 3- to 4-cm-high vertical scrotal incision. The vas deferens is identified, isolated with a Babcock clamp, and then surrounded with a Penrose drain at the junction of the straight and convoluted portions of the vas deferens. Using 8- to 15-power magnification provided by the operating microscope, the vasal sheath is longitudinally incised with a microknife and a bare segment of vas stripped of its carefully preserved vessels is delivered. The vas is hemitransected with the ultrasharp knife until the lumen is entered (Fig. 22–28). The vasal fluid is sampled. If microscopic examination of this fluid reveals the absence of sperm, the diagnosis of epididymal obstruction is confirmed. Patency of the vas and ejaculatory ducts is confirmed by cannulating the abdominal end of the vas with a 24-gauge angiocatheter sheath and gently injecting lactated Ringer solution with a 1-mL tuberculin syringe (see Fig. 22–4). Further confirmation of patency may be obtained by injecting indigo carmine, catheterizing the bladder, and observing blue-tinged urine. The vas is then completely transected using a 2.5-mm slotted nerve clamp (see Fig. 22–28), and the vas is prepared as for vasovasostomy as described earlier (see Preparation of the Vasa).
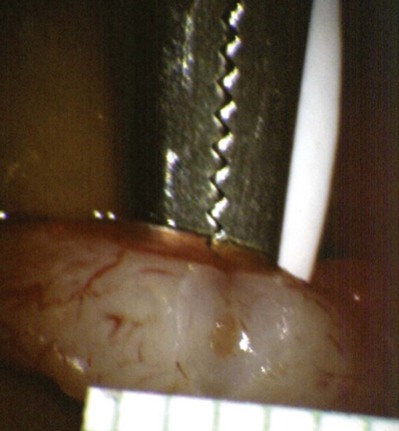
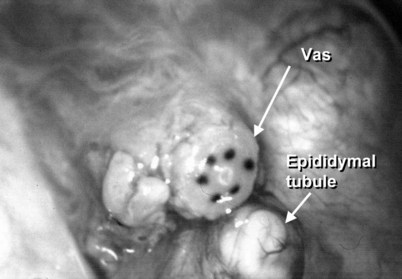
After opening the tunica vaginalis, the epididymis is inspected under the operating microscope. An anastomotic site is selected above the area of suspected obstruction, proximal to any visible sperm granulomas, where dilated epididymal tubules are clearly seen beneath the epididymal tunica (Fig. 22–29). A relatively avascular area is grasped with sharp jeweler’s forceps and the epididymal tunica tented upward. A 3- to 4-mm buttonhole is made in the tunica with microscissors to create a round opening that matches the outer diameter of the previously prepared vas deferens. The epididymal tubules are then gently dissected with a combination of sharp and blunt dissection until dilated loops of tubule are clearly exposed (Fig. 22–30). If the level of obstruction is not clearly delineated after the buttonhole opening is made in the tunic, a 70-µm diameter tapered needle from the 10-0 nylon microsuture is used to puncture the epididymal tubule beginning as distal as possible and fluid is sampled from the puncture site. When sperm are found, the puncture sites are sealed with microbipolar forceps, a new buttonhole is made in the epididymal tunic just proximally, and the tubule is prepared as described previously.
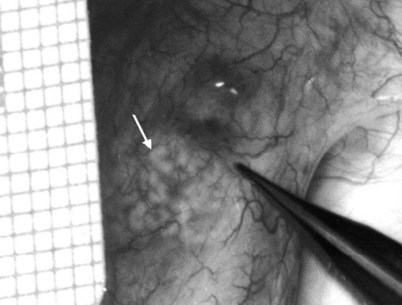
Figure 22–29 An anastomotic site is selected above the area of suspected obstruction, proximal to any visible sperm granulomas, where dilated epididymal tubules are clearly seen beneath the epididymal tunica, as marked by an arrow.
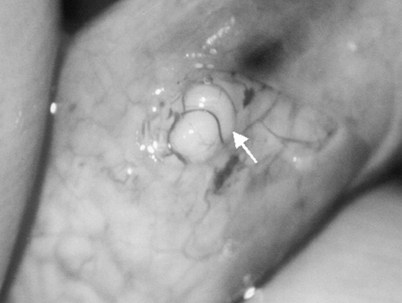
Figure 22–30 The epididymal tubules are then gently dissected with a combination of sharp and blunt dissection until dilated loops of tubule are clearly exposed.
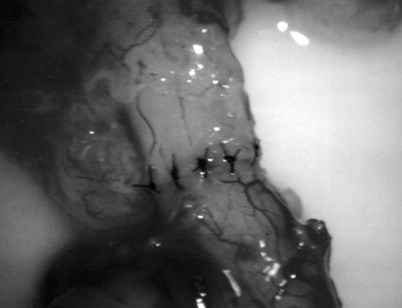
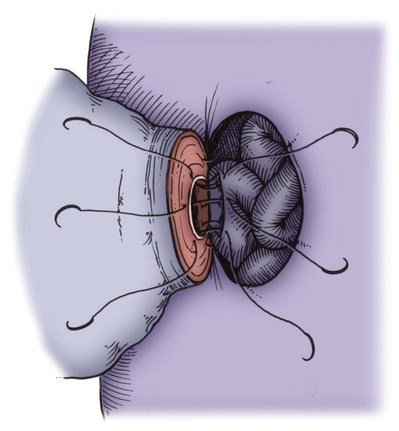
The vas deferens is drawn through an opening in the tunica vaginalis and secured in proximity to the anastomotic site with two to four interrupted sutures of 6-0 polypropylene placed through the vasal adventitia and the tunica vaginalis. The vasal lumen should reach the opening in the epididymal tunica easily, with length to spare. The posterior edge of the epididymal tunica is then approximated to the posterior edge of the vas muscularis and adventitia with two to three interrupted sutures of double-armed 9-0 nylon (Fig. 22–31). This is done in such a way as to bring the vasal lumen in close approximation to the epididymal tubule selected for anastomosis.
Classic End-to-Side Technique
Under 25× to 32× magnification, using small curved microscissors or a 15-degree microknife, an opening about 0.3 to 0.5 mm in diameter is made in the selected tubule. Epididymal fluid is touched to a slide, diluted with saline or lactated Ringer solution, and inspected under the microsope for sperm. If no sperm are found, the opening in the tubule is closed with 10-0 sutures, the vas is detached, and the tunica incision is closed with 9-0 nylon. The procedure is then repeated more proximally in the epididymis.
Once sperm are identified, they are aspirated into glass capillary tubes and flushed into media for cryopreservation (Fig. 22–32) (see Open Tubule Technique later) (Matthews et al, 1995). Indigo carmine solution is dripped on the cut tubule to outline the mucosa. Methylene blue kills sperm instantly even when diluted, rendering the sperm useless for cryopreservation (Sheynkin et al, 1999b). Indigo carmine, diluted 50%, is safe for sperm. The posterior mucosal edge of the epididymal tubule is approximated to the posterior edge of the vasal mucosa with two interrupted sutures of 10-0 monofilament nylon sutures double armed with fish-hook 70-µm diameter tapered needles (Fig. 22–33). The lumen is irrigated with lactated Ringer solution just before placement of each suture to keep the epididymal lumen open. The lumen is irrigated with heparinized saline just before tying the last mucosal suture to prevent clots from obstructing the lumen. Unlike blood vessels, there are no platelets and fibrinogen to seal a leaky anastomosis and no clot lysis factor to dissolve clots. After these mucosal sutures are tied, the anterior mucosal anastomosis is completed with two to four additional 10-0 sutures. The outer muscularis and adventitia of the vas are then approximated to the cut edge of the epididymal tunica with 6 to 10 additional interrupted sutures of 9-0 nylon double armed with 100-µm diameter needles (Fig. 22–34A and B). The vasal sheath is secured to the epididymal tunica with three to five sutures of 9-0 nylon. The testis and epididymis are gently returned to the tunica vaginalis, which is closed with 5-0 Vicryl. Penrose drains are usually not necessary. The scrotum is closed as previously described for vasovasostomy.
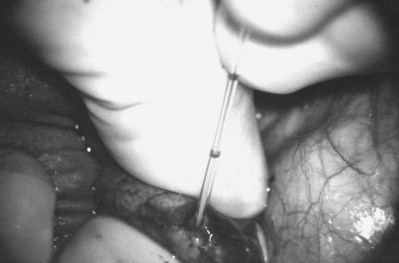
Figure 22–32 Once sperm are identified, they are aspirated into glass capillary tubes and flushed into media for cryopreservation.
End-to-Side Intussusception Techniques
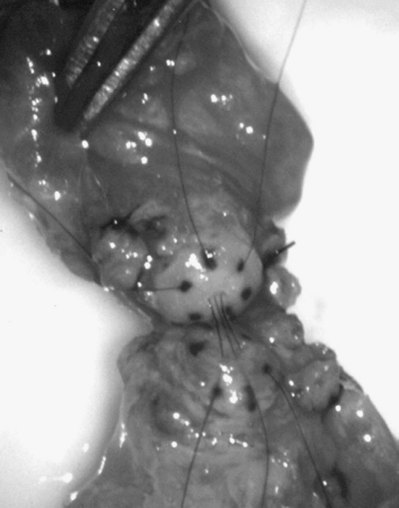
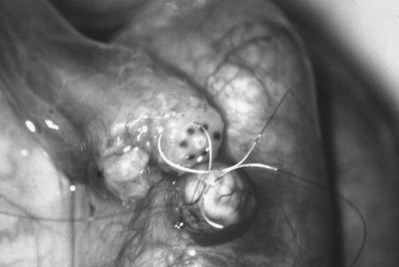
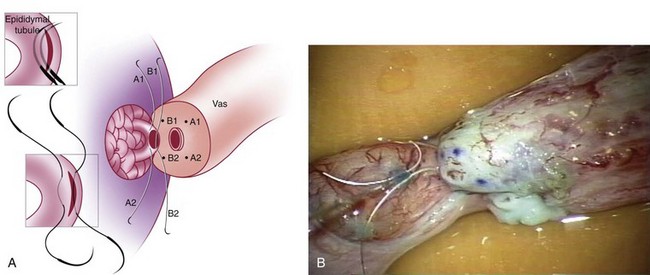
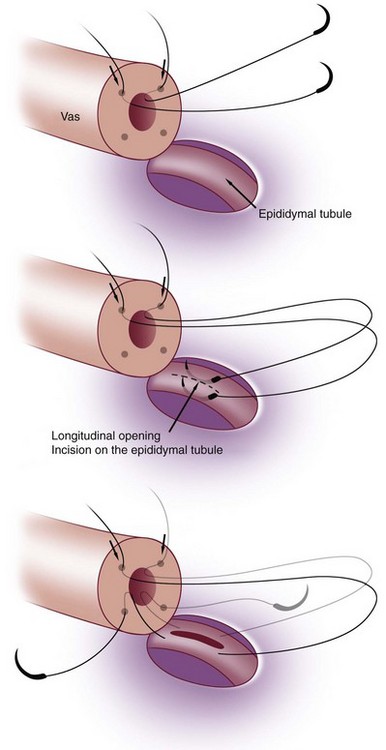
This method, originally described as the triangulation technique, was introduced by Berger (1998). There are several advantages of the methods over previous techniques (see Table 22–3) (Goldstein, 1999). The setup is identical to that for the classical end-to-side vasoepididymostomy. After the vas is fixed to the opening in the epididymal tunica, six microdots are placed on the cut surface of the vas in an identical fashion to that described for vasovasostomy. The epididymal tubule selected is dissected with blunt microscissors and the micro needle holder until it is free of surrounding tissue and prominent. The tubule is then stained with indigo carmine. Using 10-0 monofilament nylon sutures approximately 2 inches in length, double armed with 70-µm diameter fish hook–shaped tapered needles, three sutures are placed in the epididymal tubule in a triangulation fashion. The apex of the triangle faces the inferior edge of the vasal mucosa. The needles are not pulled through but left in situ, creating a triangle of needles (Fig. 22–35). Using a 15-degree microknife with the blade pointing upward, a generous opening is made in the epididymal tubule in the center of the triangle created by the three needles (Fig. 22–36).
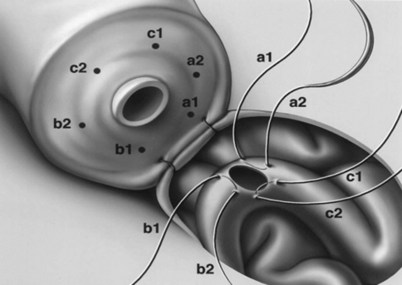
Figure 22–36 Using a 15-degree microknife with the blade pointing upward, a generous opening is made in the epididymal tubule in the center of the triangle created by the three needles.
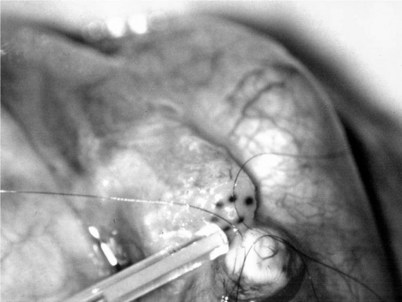
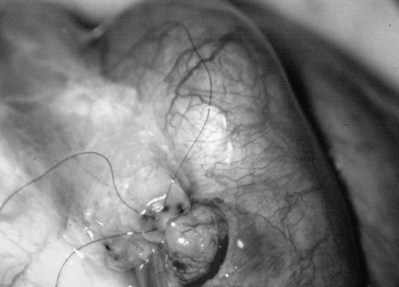
The three needles are then pulled through. The six needles are now laid out so as to avoid a spaghetti-like tangle. A glass slide is touched to the fluid exuding from the opening in the epididymal tubule and mixed with human tubal fluid media, covered with a coverslip, and examined by the surgeon using the separate bench microscope under 400-power magnification. If sperm are present (whether motile or not), the decision is made to proceed with the anastomosis. Sperm are aspirated into micropipets first (Fig. 22–37) and expressed into human tubal fluid media and sent for cryopreservation if motility is observed. Sperm that initially appear immotile, when mixed with human tubule fluid, often regain motility adequate for successful cryopreservation. Even immotile sperm should be placed in the media and evaluated for potential cryopreservation. If the needles are pulled through before placing the microsurgical sutures or before making an opening in the epididymal tubule, epididymal fluid and sperm would immediately leak through the suture hole, causing the tubule to collapse. This makes placement of subsequent sutures and creation of the opening in the tubule considerably more difficult. Leaving the needles in the epididymal tubule before making the opening also prevents accidental cutting of the sutures when making the opening in the center of the triangle. After abundant sperm have been aspirated into micropipets and cryopreserved, the six needles are passed inside out the vas deferens exiting through the six previously placed microdots in the order indicated (Fig. 22–38). Each pair of sutures is then sequentially tied beginning with suture a1 and a2, then b1 and b2, and finally c1 and c2. Tying of these sutures intussuscepts the epididymal tubule into the vas lumen (Fig. 22–39). This creates a water-tight closure (Fig. 22–40). In addition, the flow of epididymal fluid from the epididymal tubule into the vas deferens tends to plaster the edges of the epididymal tubule against the mucosal walls of the vas deferens, further helping create a leakproof closure. The second layer of the anastomosis is completed in an identical fashion to that described for the classical end-to-side operation described earlier (Fig. 22–41A and B).
Two-Stitch Variation of the Intussusception Technique (Preferred Technique)
For anastomoses to small epididymal tubules such as those found in the caput or to the efferent ductules (Chan et al, 2005), the three-stitch triangulation technique may be impossible. We now employ a two-stitch longitudinal intussusception technique for all vasoepididymostomies. It is much easier to perform and is even more successful. With this method, four microdots are marked on the cut surface of the vas deferens and two parallel sutures are placed in the distended epididymal tubule longitudinally, but not pulled through (Fig. 22–42A and B). Marmar suggests mounting two needles in the needle holder and placing them simultaneously transversely in the tubule. However, if the needles are not pulled through to avoid leakage of fluid and tubular collapse, they can be placed one at a time with greater control and accuracy (Chan et al, 2005; Schiff et al, 2005). Using a 15-degree microknife, an opening is made exactly between and parallel to the two previously placed sutures. Of note, we have also developed a single-arm technique of vasoepididymostomy, which is almost as effective as the double-arm technique (Fig. 22–43) (Monoski et al, 2007). This technique is valuable when double-armed sutures are not available.
Technique When Vasal Length Is Severely Compromised
When there is inadequate length of the vas deferens to reach the dilated epididymal tubule without tension, the epididymis can be dissected down to the vasoepididymal junction and then dissected off the testes as in the older end-to-end operation.
After the vas has been prepared, the tunica vaginalis is opened and the testis is delivered. Inspection of the epididymis under the operating microscope may reveal a clearly delineated site of obstruction. Often, a discrete yellow sperm granuloma is noted, above which the epididymis is indurated and the tubules are dilated and below which the epididymis is soft and the tubules are collapsed. If the level of obstruction is not clearly delineated, a 70-µm tapered needle from the 10-0 nylon microsuture is used to puncture the epididymal tubule beginning as distal as possible and fluid is sampled from the puncture site until sperm are found. At that level the puncture is sealed with microbipolar forceps, and the epididymis is ligated just proximal to the puncture site with a 6-0 nylon. The epididymis is then dissected off the testis and flipped up to obtain additional length (Fig. 22–44). To do this, the epididymis is encircled with a small Penrose drain at the level of obstruction and, using 2.5-power loupe magnification, dissected off of the testis for 3 to 5 cm, yielding sufficient length to perform the anastomosis. Usually a nice plane can be found between the epididymis and testis, and injury to the epididymal blood supply can be avoided by staying right on the tunica albuginea of the testis. The inferior and, if necessary, middle epididymal branches of the testicular artery are ligated and divided to free up an adequate length of epididymis. The superior-epididymal branches entering the epididymis at the caput are always preserved and can provide adequate blood supply to the entire epididymis. The tunica vaginalis in then closed over the testis with 5-0 Vicryl. This prevents drying of the testis and thrombosis of the surface testicular vessels during the anastomosis. The dissected epididymis remains outside the tunica vaginalis.
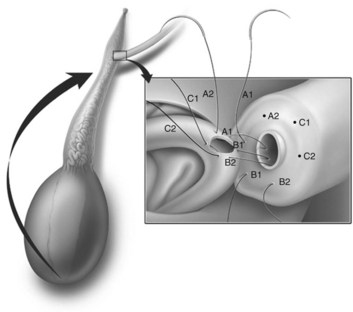
Figure 22–44 In addition, if vasal length is compromised, the epididymis can be dissected to the caput by ligating the inferior and medial epididymal vessels and flipping the epididymis up, providing additional length.
If the epididymis is indurated and dilated throughout its length, it is dissected to the vasoepididymal (VE) junction. This dissection is often assisted by first dissecting the convoluted vas to the VE junction from below, and then, after encircling the epididymis with a Penrose drain, dissecting the epididymis to the VE junction from above. In this way the entire VE junction can be freed up. This will allow preservation of maximal epididymal length in cases of distal obstruction near the VE junction. After the epididymis is dissected off of the testis and flipped up, a three-stitch end-to-side intussusception anastomosis is performed as described earlier.
Long-Term Follow-Up—Evaluation and Results
Microsurgical vasoepididymostomy in the hands of experienced and skilled microsurgeons will result in the appearance of sperm in the ejaculate in 50% to 85% of men. Patency rates with the intussusception technique can exceed 80% (Berger, 1998; Brandell, 1999; Marmar, 2000). With the classic end-to-side or older end-to-end method, the patency rate is about 70% and 43% of men with sperm will impregnate their wives after a minimum follow-up of 2 years (Schlegel and Goldstein, 1993; Pasqualotto et al, 1999). With the intussusception techniques, patency rates are 70% to 90% (Kolettis and Thomas, 1997; Chan et al, 2005; Schiff et al, 2005). Regardless of technique, pregnancy rates are higher the more distal the anastomosis is performed (Silber, 1989a). With the older end-to-end or end-to-side methods, at 14 months after surgery 25% of initially patent anastomoses have shut down (Matthews et al, 1995). With intussusception technique, the late shut-down rates appear to be less than 10% but long-term follow-up with these techniques has not been reported. Nevertheless, we recommend banking sperm both intraoperatively (Matthews and Goldstein, 1996) and as soon as they appear in the ejaculate postoperatively after vasoepididymostomy, regardless of technique employed. In men with low counts or poor sperm quality postoperatively and men who remain azoospermic, the sperm intraoperatively cryopreserved can be used for IVF with intracytoplasmic sperm injection. Persistently azoospermic men without cryopreserved sperm can opt for either a redo vasoepididymostomy and/or microscopic epididymal sperm aspiration combined with IVF and intracytoplasmic sperm injection (see Microsurgical Epididymal Sperm Aspiration Techniques).
Transurethral Resection of the Ejaculatory Ducts
Ejaculatory duct obstruction is usually a congenital anomaly that represents the opposite end of the spectrum of excurrent ductal system anomalies that begin with congenital complete absence of the vas deferens and most of the epididymis. When the aplastic segment occurs at the terminal end of the vas, where the ejaculatory duct enters the urethra, it is potentially correctable by transurethral resection (Paick et al, 2000; Schroeder-Printzen et al, 2000; Kadioglu et al, 2001; Ozgok et al, 2001; Yurdakul et al, 2008). Occasionally ejaculatory duct obstruction results from chronic prostatitis or extrinsic compression of the ejaculatory ducts by prostate or seminal vesical duct cysts (Cornel et al, 1999; Paick et al, 2000; Kadioglu et al, 2001). Higher ejaculatory duct pressures have been directly measured in men with ejaculatory duct obstruction (Eisenberg et al, 2008).
Diagnosis
The workup leading to the diagnosis of probable ejaculatory duct obstruction is covered in Chapter 21. Briefly, ejaculatory duct obstruction is suspected in azoospermic or severely oligospermic and/or asthenospermic men with at least one palpable vas deferens, a low semen volume, acid semen pH and negative, equivocal, or low semen fructose levels. If these men have normal serum levels of FSH and testis biopsy reveals normal spermatogenesis, the diagnosis of ejaculatory duct obstruction is entertained.
Digital rectal examination may reveal a midline cystic structure. Transrectal sonography is key for the diagnosis and treatment of ejaculatory duct obstruction. A midline cystic lesion or dilated ejaculatory ducts and seminal vesicles can be visualized sonographically. As described in Transrectal Vasography and Seminal Vesiculography earlier in this chapter, transrectal ultrasound-guided aspiration of the cystic or dilated ejaculatory ducts or seminal vesicles is performed (Jarow, 1994). The aspirate is examined microscopically and if motile sperm are found, they are cryopreserved and 2 to 3 mL indigo carmine diluted with water-soluble radiographic contrast are instilled. If a radiograph confirms a potentially resectable lesion, TUR of the ejaculatory ducts is performed without the need for prior vasography because the presence of sperm in the seminal vesicles indicates that at least one epididymis is patent and that the cyst or dilated ejaculatory duct communicates with a nonobstructed vas. The instillation of indigo carmine assists in localizing the opening of the ejaculatory duct and confirms when resection has successfully opened the obstructed system. Transrectal sonography with aspiration should be performed immediately before anticipated surgery and employs the same bowel prep and antibiotic prophylaxis used for transrectal prostate biopsy.
If no sperm are found in the aspirate, vasography, as described earlier in Technique of Vasography and Interpretation of Findings, is necessary. If no sperm are found in either vas when the vasotomy is made and vasography reveals ejaculatory duct obstruction, it is best to abandon attempts at reconstruction and simply perform microsurgical epididymal sperm aspiration and cryopreservation for future IVF/ICSI. Simultaneous vasoepididymostomy and TUR of the ejaculatory ducts rarely works. If ejaculatory duct obstruction is confirmed by vasography employing a 50% water-soluble contrast medium and sperm are present in the vasa, the 3-Fr whistle-tip ureteral vasography stents are left in place so that a dilute indigo carmine solution can be injected by the assistant to aid resection.
Technique
Cold knife incision alone almost always leads to reobstruction. The resectoscope, with the 24-Fr cutting loop, is engaged with a finger placed in the rectum providing anterior displacement of the posterior lobe of the prostate. The ejaculatory ducts course between the bladder neck and the verumontanum and exit at the level of and along the lateral aspect of the verumontanum (Fig. 22–45). Resection of the veru will often reveal the dilated ejaculatory duct orifice or cyst cavity. Resection should be carried out in this region with great care in order to preserve the bladder neck proximally, the striated sphincter distally and the rectal mucosa posteriorly. Efflux of indigo carmine from dilated orifices confirms adequate resection. Avoid excessive coagulation. If formal vasography was performed, the hemivasotomies are carefully closed employing microsurgical technique. A Foley catheter is left overnight and the patient receives an additional 7 days of oral antibiotics.
Complications
Reflux
Reflux of urine into the ejaculatory ducts, vas, and seminal vesicles occurs after a majority of resections. This can be documented by voiding cystourethrography or measuring semen creatinine levels (Malkevich et al, 1994). Contamination of semen by urine impairs sperm quality.
Epididymitis
Reflux can lead to acute and chronic epididymitis. Recurrent epididymitis often results in epididymal obstruction. The incidence of epididymitis after TUR is probably underestimated. Symptomatic chemical epididymitis may occur from refluxing urine. Chronic low-dose antibacterial suppression such as that employed for vesicoureteral reflux may be necessary until pregnancy is achieved. If epididymitis is chronic and recurrent, vasectomy or even epididymectomy may be necessary.
Retrograde Ejaculation
Even when care has been taken to spare the bladder neck, retrograde ejaculation is common after TUR. Pseudoephedrine 120 mg orally, 90 minutes before ejaculation, or Ornade Spansules (chlorpheniramine and phenylpropanolamine) twice a day for a week may prevent this. If this is not successful, sperm can be retrieved from alkalinized urine and used for either intrauterine insemination or IVF with ICSI.
Results
TUR of the ejaculatory ducts results in increased semen volume about two thirds of the time and appearance of sperm in the ejaculate in about 50% of previously azoospermic men. Pregnancy rates are based on case reports and small series (Goldwasser et al, 1985; Paick et al, 2000; Ozgok et al, 2001; Fuse et al, 2003; Yurdakul et al, 2008). If viable sperm appear in the ejaculate but the quality is poor, IVF with ICSI is recommended. This approach currently yields delivery rates of up to 38.5% per attempt. Because of the potential for serious complications, TUR should only be performed in azoospermic men or in severely oligoasthenospermic men and only after the couple has stated they are unwilling to do IVF and have been fully apprised of the risks of TUR.
Electroejaculation
Men with neurologic impairments in sympathetic outflow such as seen in traumatic spinal cord injury, demyelinating neuropathies (multiple sclerosis), and diabetes following retroperitoneal lymph node dissection frequently have abnormalities in or absence of seminal emission. Ejaculation can be induced in most of these men, especially those with high spinal cord injury, with vibratory stimulation (Schellan, 1968; Brindley, 1981; Bennett et al, 1987; Brackett et al, 1997; Ohl et al, 1997). For those men that do not respond to vibratory stimulation, electroejaculation has proven to be a safe and effective means of obtaining motile sperm suitable for assisted reproduction techniques (IUI, IVF/ICSI).
The procedure is performed under general anesthesia except for men with a complete spinal cord injury, who do not require anesthesia. In men with a high thoracic spinal cord lesion (above T6) or in those men with prior history of autonomic dysreflexia, pretreatment, 15 minutes before the procedure, with 20 mg of sublingual nifedipine is employed. These men should have intravenous access and their blood pressure and pulse should be monitored every 2 minutes before, during, and for 20 minutes following electroejaculation. In the event of a sympathetic outflow (autonomic dysreflexia), termination of the procedure should be sufficient to break the response. However, intravenous access allows for delivery of sympatheticolytic agents should they become necessary.
Before placing the patient in the lateral decubitus position, the bladder is catheterized and emptied. A 12-Fr or 14-Fr Silastic catheter lubricated with a small amount of mineral oil is used because commonly employed lubricants are spermicidal. Ten mL of buffer (HEPES-BSA) is instilled into the bladder. Before the electroejaculation sequence, a digital rectal examination and anoscopy are performed. A rectal probe with three large horizontal stripes is well lubricated, inserted with the electrodes facing anteriorly and applied against the posterior aspect of the prostate and seminal vesicles. The probe is connected to a variable-output power source that simultaneously records probe temperature through a thermistor in the rectal probe. Electrostimulation is started at 3 to 5 volts and increased in 1-volt increments with each stimulation (Ohl et al, 2001). An assistant records probe temperatures and number of stimulations to full erection and ejaculation and collects the ejaculate in a sterile wide mouth plastic container. The number of stimulations and maximum voltage required are variable and the ejaculate may be retrograde. If probe temperature rises rapidly or above 40° C, stimulation is suspended until the temperature falls below 38° C or probes are changed. At the completion of stimulation, anoscopy is again performed to check for rectal injury. The bladder is recatheterized to obtain any retrograde-ejaculated sperm. The specimens are then delivered to the laboratory for processing. A second electroejaculation sequence can be immediately performed under the same anesthetic to obtain additional sperm.
Employing this technique, sperm can be recovered in more than 90% of men. Overall pregnancy rates of up to 40% can be achieved after multiple cycles with intrauterine insemination. Use of IVF with ICSI will yield 50% live delivery rates for a single (albeit costly) procedure if motile sperm are obtained.
Sperm Retrieval Techniques
Men with congenital absence or bilateral partial aplasia of vas deferens, or those with failed or surgically unreconstructable obstructions, can now be treated by using sperm retrieval techniques in conjunction with in vitro fertilization (Table 22–4) (Temple-Smith et al, 1985; Silber et al, 1990; Schlegel et al, 1994; Craft et al, 1995; Sheynkin et al, 1998b; Janzen et al, 2000; Levine et al, 2003; Qiu et al, 2003; Anger et al, 2004). These techniques are also useful for intraoperative retrieval of sperm during reconstructive procedures such as vasoepididymostomy, which have significant failure rates. The intraoperatively retrieved sperm may be used immediately if the wife has been prepared for IVF or may be cryopreserved for a future IVF with ICSI cycle in the event the reconstructive surgery is unsuccessful. Sperm obtained from chronically obstructed systems usually have poor motility and decreased fertilizing capacity. The use of intracytoplasmic sperm injection (ICSI) combined with IVF is essential to achieve optimal results.
Table 22–4 Surgical Techniques for Sperm Retrieval
| ADVANTAGES | DISADVANTAGES | |
|---|---|---|
| MESA (microsurgical epididymal sperm aspiration) | Microsurgical procedure allows lower complication rate. Epididymal sperm has better motility than testicular sperm. Large number of sperm can be harvested for cryopreservation of multiple vials in a single procedure. |
Requires anesthesia and microsurgical skills. Not indicated for nonobstructive azoospermia. |
| PESA (percutaneous epididymal sperm aspiration) | No microsurgical skill required. Local anesthesia. Epididymal sperm has better motility than testicular sperm. |
Complications include hematoma, pain, and vascular injury to testes and epididymis. Variable success in obtaining sperm. Small quantity of sperm obtained than with MESA. Not indicated in nonobstructive azoospermia. |
| TESA (testicular sperm aspiration) | No microsurgical skill required. Local anesthesia. Can be used for obstructive azoospermia. |
Immature or immotile testicular sperm. Small quantity of sperm obtained. Poor results in nonobstructive azoospermia. Complications include hematoma, pain, and vascular injury to testes and epididymis. |
| TESE (testicular sperm extraction) | Low complication rate if performed microsurgically. Preferred technique for nonobstructive azoospermia. |
Requires anesthesia and microsurgical skills. |
Microsurgical Epididymal Sperm Aspiration Techniques
Open Tubule Technique
The technique described here can be employed for either intraoperative sperm retrieval at the time of vasoepididymostomy or as an isolated procedure in men with congenital absence of the vas or unreconstructable obstructions (Matthews and Goldstein, 1996; Nudell et al, 1998). A median raphe approach through two small transverse scrotal incisions within the scrotal skin folds is used. After delivery of the testis, the tunica vaginalis is opened and the epididymis inspected under 16× to 25× magnification using the operating microscope. The epididymal tunica is incised over a dilated tubule as described previously for vasoepididymostomy. Meticulous hemostasis is obtained using the bipolar cautery. A dilated tubule is isolated and incised with a 15-degree microknife. The fluid is touched to a slide, a drop of human tubal fluid media is added, a coverslip is placed, and the fluid examined. If no sperm are obtained, the epididymal tubule and tunica are closed with 10-0 and 9-0 monofilament nylon sutures, respectively, and an incision is made more proximally in the epididymis or even at the level of the efferent ductules until motile sperm are obtained.
As soon as motile sperm are found, a dry micropipet (5 µL; Drummond Scientific Co., Broomall, PA) is placed adjacent to the effluxing epididymal tubule (Fig. 22–46). A standard hematocrit pipette is less satisfactory but can be used if micropipets are not available. Sperm are drawn into the micropipet by simple capillary action. Negative pressure, as is generated by action of an in-line syringe, should not be applied during sperm recovery because this may disrupt the delicate epididymal mucosa. Two micropipets may be employed simultaneously to increase the speed of sperm recovery.
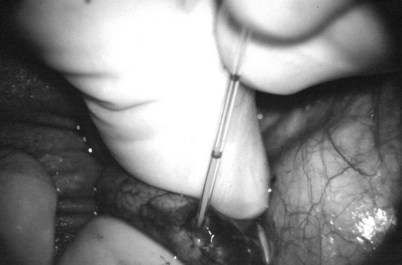
Figure 22–46 As soon as motile sperm are found, a dry micropipet (5 µL; Drummond Scientific Co., Broomall, PA) is placed adjacent to the effluxing epididymal tubule.
The highest rate of flow is observed immediately following incision of the tubule. Progressively better-quality sperm are often found following the initial washout. Gentle compression of the testis and epididymis enhances flow from the incised tubule. With patience, 10 to 20 µL of epididymal fluid can be recovered.
The micropipet is connected to a short (3 to 5 cm) segment of medical-grade silicone tubing (American Scientific Products; McGaw Park, IL). Alternatively, the tubing attached to a 22-gauge butterfly needle may be employed. A 20-gauge needle fitted to a Luer-tip syringe is then placed in line. The fluid is flushed with IVF media (0.5 to 1.0 mL) into a sterile container. Once a micropipet has been used, it is discarded. Residual fluid in the pipette will disrupt capillary action. A typical procedure requires 4 to 12 micropipets. The sperm bank should be instructed to cryopreserve the aspirate in multiple vials (aliquots) so that several IVF cycles may be attempted if required (Janzen et al, 2000; Anger et al, 2004).
Experience with the technique has revealed that, paradoxically, in obstructed systems, sperm motility is better more proximally in the epididymis with the most motile sperm often found in the efferent ductules (Fig. 22–47). Motility immediately after aspiration and, consequently, fertilization rates are highest in men who have the longest length of epididymal tubule available. Even when packed with debris distally, the epididymal tubule may be capable of secreting substances that can diffuse proximally and benefit sperm motility and fertilizing capacity.
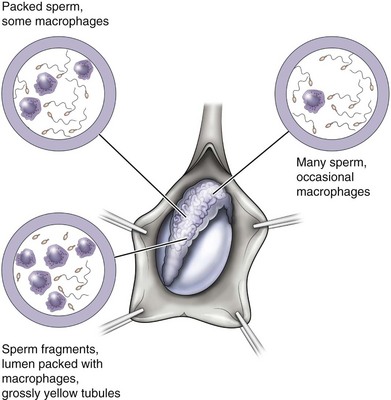
Figure 22–47 Experience with the technique has revealed that, paradoxically, in obstructed systems, sperm motility is better more proximally in the epididymis with the most motile sperm often found in the efferent ductules.
Employing ICSI, ongoing pregnancy or delivery rates exceeding 60% have been achieved with this technique using either fresh or cryopreserved epididymal sperm (Schlegel et al, 1995; Nudell et al, 1998). Epididymal sperm aspiration can be done electively, with the cryopreserved sperm used for multiple future IVF cycles (Janzen et al, 2000; Anger et al, 2004).
Percutaneous Epididymal Sperm Aspiration
Percutaneous puncture of the epididymis with a fine needle (Fig. 22–48) has been successfully employed to obtain sperm and achieve pregnancies (Shrivastav et al, 1994; Craft and Tsirigotis, 1995; Levine et al, 2003; Qiu et al, 2003; Lin et al, 2004). The technique is less reliable than the open retrieval, and the small quantities of sperm obtained are sometimes inadequate for cryopreservation. Reported pregnancy rates are half those achieved with open techniques (Sheynkin et al, 1998b). In view of the enormous costs and effort involved in IVF, epididymal sperm retrieval under direct vision is the preferred technique.
Testicular Sperm Extraction
The indications for testicular sperm extraction (TESE) are:
Testicular sperm has been retrieved employing one of three techniques:
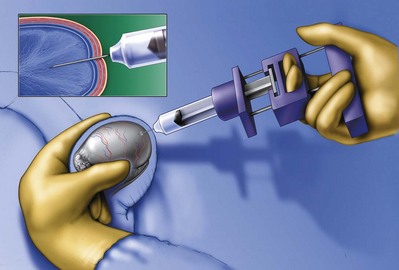
Figure 22–50 Percutaneous aspiration (testicular sperm aspiration) with a high-suction glass syringe and a 23-gauge needle. This is the least invasive but requires 10 to 20 passes to obtain an adequate yield.
The percutaneous methods are most appropriate in men with normal spermatogenesis and obstructive azoospermia in whom adequate numbers of sperm can be retrieved in a small amount of tissue (Craft et al, 1995). The pros and cons of these three methods are discussed earlier in Testis Biopsy.
Microsurgical Testicular Sperm Extraction
The use of an operating microscope for standard open diagnostic testes biopsy allows identification of an area in the tunica albuginea free of blood vessels (Fig. 22–51), minimizing the risk of injury to testicular blood supply and allowing a relatively blood-free biopsy specimen (Dardashti et al, 2000). Employing the microscope for testis biopsy, Schlegel (1999) discovered that in men with nonobstructive azoospermia, some of the tubules were larger than others. The larger tubules are more likely to yield sperm. Previous studies revealed that testicular biopsy in men with nonobstructive azoospermia display considerable heterogeneity. Examination of permanently fixed biopsy specimens that display heterogeneity reveal that tubules with spermatogenesis are of considerably larger diameter than tubules composed of Sertoli cells only. This difference can be readably observed under the operating microscope (Fig. 22–52).
Figure 22–51 The use of an operating microscope for standard open diagnostic testes biopsy allows identification of an area in the tunica albuginea free of blood vessels.
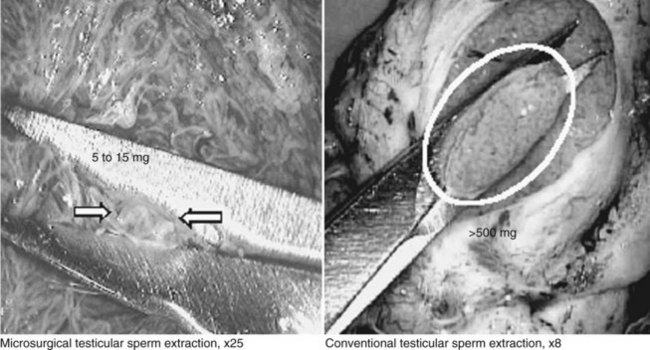
Figure 22–52 Tubules with spermatogenesis are of considerably larger diameter than tubules that are Sertoli-cell only. This difference can be readily observed under the operating microscope.
Technique
With the patient under general or regional anesthetic, the testes are exposed through either a single midline median raphe incision or two transverse incisions within the skin lines and between the scrotal blood vessels. The testes are delivered into the wound. The tunica vaginalis is opened, and the operating microscope is brought into the field. Using 10-power magnification, an avascular plane is identified on the anterior surface of the tunica albuginea. Using a 15-degree microknife, a generous transverse incision is made between the blood vessels through the tunica albuginea. Small blood vessels that are seen coursing across the incision are coagulated with the microbipolar cautery prior to incising them. This yields a blood-free field. The seminiferous tubules are observed. Sertoli-cell-only tubules tend to be thin, white, and stringy. Tubules with active spermatogenesis are larger, plumper, and somewhat yellow. Using a micro needle holder, the seminiferous tubules are dissected in an attempt to identify larger tubules. If such a tubule is found, a sharp curved iris scissor is used to selectively excise these tubules. The sample is placed in human tubal fluid media, microdissected, and immediately examined by an andrology laboratory technician present in the operating room. After sperm have been found, hemostasis is obtained with the microbipolar cautery. The incision in the tunica albuginea is closed with a 6-0 nylon suture. The testis is returned to the tunica vaginalis, which is closed with a continuous suture of 5-0 Vicryl. If necessary, the opposite testis is explored.
Results
Using the microdissection technique, sperm have been identified in 50% of men explored. In those men in whom sperm are found, a pregnancy rate of 50% has been achieved at Cornell using IVF/ICSI. The spontaneous abortion rate is 19%. The high rate of spontaneous abortion is probably owing to the increased incidence of chromosomal abnormalities and DNA damage in the sperm of men with nonobstructive azoospermia (Rucker et al, 1998). Even in severe cases of congenital or acquired testicular failure, as in Sertoli-cell-only syndrome (Su et al, 1999), postchemotherapy azoospermia (Chan et al, 2001) and nonmosaic (47XXY) Klinefelter syndrome (Palermo et al, 1998; Ramasamy et al, 2009) sperm have been found and pregnancy and live births achieved (Table 22–5).
Table 22–5 Testicular Sperm Extraction Outcomes by Diagnosis from Schlegel
| CONDITION | RETRIEVAL |
|---|---|
| Klinefelter syndrome | 68% |
| AZFc deletions | 70% |
| Sertoli cell only | 37% |
| Postchemotherapy | 53% |
| Cryptorchidism (postorchiopexy) | 74% |
| Maturation arrest | 40% |
| AZFa, AZFb deletions | 0% |
From Chan et al, 2001; Hopps et al, 2003b; Raman and Schlegel, 2003; Hung et al, 2007; Ramasamy and Schlegel, 2007; and Ramasamy et al, 2009.
Postmortem Sperm Retrieval
Postmortem sperm retrieval and cryopreservation (but no pregnancies) were initially reported by Rothman in 1980 and employed removal and mincing of the epididymis. The retrieved sperm can be frozen and subsequently used to achieve pregnancy. Pregnancy has now been achieved with sperm retrieved postmortem using IVF with ICSI (Tash et al, 2003; Dostal et al, 2005; reviewed in Benshushan and Schenker, 1998).
Retrieval of sperm from the vas can be performed using the technique described previously for vasography. Once the vas has been delivered, a hemivasotomy is made with a 15-degree microknife (as described earlier in Technique of Vasography and Interpretation of Findings). The testicular end of the vas is cannulated with a 22-gauge angiocatheter and the vas irrigated with a 0.2-mL volume of human tubal fluid medium while the convoluted vas and epididymis are massaged.
Twenty-four million sperm with 70% grade 1 motile sperm were retrieved 13 hours after death with this technique (Schlegel, personal communication). The ethical appropriateness of such retrieval is the most important issue surrounding its use.
Varicocelectomy
Introduction
Varicocele is by far the most commonly performed operation for the treatment of male infertility. Varicocele is found in approximately 15% of the general population, 35% of men with primary infertility, and 75% to 81% of men with secondary infertility. Animal and human studies have demonstrated that varicocele is associated with a progressive and duration-dependent decline in testicular function (Russell, 1957; Lipshultz and Corriere, 1977; Nagler et al, 1985; Harrison et al, 1986; Kass and Belman, 1987; Hadziselimovic et al, 1989; Chehval and Purcell, 1992; Gorelick and Goldstein, 1993; Witt and Lipshultz, 1993).
Repair of varicocele will halt any further damage to testicular function (Kass and Belman, 1987; Gorelick and Goldstein, 1993) and, in a large percentage of men, result in improved spermatogenesis (Dubin and Amelar, 1977; Schlegel and Goldstein, 1992), as well as enhanced Leydig cell function (Su et al, 1995). The potentially important role of urologists in preventing future infertility underscores the importance of using a varicocelectomy technique that minimizes the risk of complications and recurrence. Table 22–6 summarizes the pros and cons of various methods of varicocele repair.
Scrotal Operations
Various surgical approaches have been advocated for varicocelectomy. The earliest recorded attempts at repair of varicocele date to antiquity and involved external clamping of the scrotal skin including the enlarged veins. In the early 1900s an open scrotal approach was employed, involving the mass ligation and excision of the varicosed plexus of veins. At the level of the scrotum, however, the pampiniform plexus of veins are intimately entwined with the coiled testicular artery. Therefore scrotal operations are to be avoided because damage to the arterial supply of the testis frequently results in testicular atrophy and further impairment of spermatogenesis and fertility.
Retroperitoneal Operations
Retroperitoneal repair of varicocele involves incision at the level of the internal inguinal ring (Fig. 22–53), splitting of the external and internal oblique muscles, and exposure of the internal spermatic artery and vein retroperitoneally near the ureter. This approach has the advantage of isolating the internal spermatic veins proximally, near the point of drainage into the left renal vein. At this level, only one or two large veins are present and, in addition, the testicular artery has not yet branched and is often distinctly separate from the internal spermatic veins. Retroperitoneal approaches involve ligation of the fewest number of veins. This approach is still a commonly employed method for the repair of varicocele, especially in children.
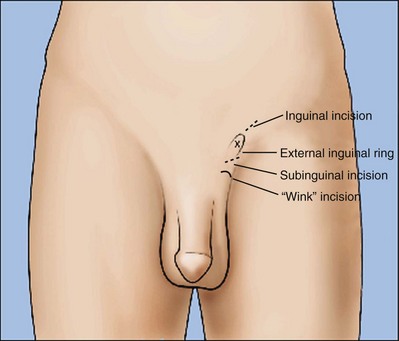
Figure 22–53 Retroperitoneal repair of varicocele involves incision at the level of the internal inguinal ring.
A disadvantage of a retroperitoneal approach is the high incidence of varicocele recurrence, especially in children and adolescents, when the testicular artery is intentionally preserved. Recurrence rates after retroperitoneal varicocelectomy are in the range of 15% (Homonnai et al, 1980; Rothman et al, 1981; Watanabe et al, 2005). Failure is usually due to preservation of the periarterial plexus of fine veins (venae comitantes) along with the artery. These veins have been shown to communicate with larger internal spermatic veins. If left intact, they may dilate and cause recurrence. Less commonly, failure is due to the presence of parallel inguinal or retroperitoneal collaterals, which may exit the testis and bypass the ligated retroperitoneal veins, rejoining the internal spermatic vein proximal to the site of ligation (Sayfan, 1981; Murray et al, 1986). Dilated cremasteric veins, another cause of varicocele recurrence (Sayfan et al, 1980), cannot be identified with a retroperitoneal approach. Positive identification and preservation of the 1.0- to 1.5-mm testicular artery via the retroperitoneal approach is difficult, especially in children where the artery is small. The operation involves working in a deep hole. Because at this level the internal spermatic vessels cannot be delivered into the wound, they must be dissected and ligated in situ in the retroperitoneum. In addition, the difficulty in positively identifying and preserving lymphatics using this approach results in postoperative hydrocele formation after 7% to 33% of retroperitoneal operations (Szabo and Kessler, 1984). The incidence of recurrence appears to be higher in children, with rates reported between 15% and 45% in adolescents (Gorenstein et al, 1986; Levitt et al, 1987; Reitelman et al, 1987). Kass (1992) reports that recurrence can be markedly reduced in children and adolescents by intentional ligation of the testicular artery (Kass and Marcol, 1992). This assures ligation of the periarterial network of fine veins. Although reversal of testicular growth failure has been documented with intentional testicular artery ligation at the time of retroperitoneal repair in children, the effect of artery ligation on subsequent spermatogenesis is uncertain. In adults bilateral artery ligation has been documented to occasionally cause azoospermia and testicular atrophy. At least it is inarguable that testicular artery ligation will not enhance testicular function.
Laparoscopic Varicocelectomy
Laparoscopic repair is in essence a retroperitoneal approach and many of the advantages and disadvantages are similar to those of the open retroperitoneal approach (Donovan and Winfield, 1992; Hagood et al, 1992; Enquist et al, 1994; Hirsch et al, 1998; Riccabona et al, 2003; Watanabe et al, 2005).
Using the laparoscope, the internal spermatic vessels and vas deferens can be clearly visualized through the laparoscope as they course through the internal inguinal ring. The magnification provided by the laparoscope allows visualization of the testicular artery. With experience, the lymphatics may be visualized and preserved as well (Glassberg et al, 2008). With laparoscopic varicocelectomy the internal spermatic veins are ligated at the same level as the retroperitoneal (Palomo) approach described earlier in Retroperitoneal Operations. Laparoscopic varicocelectomy should allow preservation of the testicular artery in a majority of cases, as well as preservation of lymphatics. The incidence of varicocele recurrence would be expected to be similar to that associated with the open retroperitoneal operations. These recurrences would be due to collaterals joining the internal spermatic vein near its entrance to the renal vein, or entering the renal vein separately.
Currently reported series of laparoscopic varicocelectomy report a recurrence rate of 2.9% to 4.5% in most recent series (May et al, 2006; Glassberg et al, 2008; Barroso et al, 2009), but up to 17% in some (Al-Said et al, 2008). An artery ligation but lymphatic-sparing laparoscopic technique has markedly reduced the incidence of postoperative hydrocele formation in children (Glassberg et al, 2008). The potential complications of laparoscopic varicocelectomy (injury to bowel, vessels or viscera, air embolism, peritonitis) are significantly more serious than those associated with the open techniques. Furthermore, laparoscopic varicocelectomy requires a general anesthetic. The microsurgical techniques described next can be performed using local or regional anesthesia and employ an incision of 2.5 to 3 cm for unilateral repair. This is equal to or less than the sum of incisions employed for a laparoscopic approach. Postoperative pain and recovery from the laparoscopic technique are the same as those associated with subinguinal varicocelectomy (Hirsch et al, 1998). Finally, laparoscopic varicocelectomy is less cost-effective than open varicocelectomy. In the hands of an experienced laparoscopist, the approach is a reasonable alternative for the repair of bilateral varicoceles (Donovan and Winfield, 1992; Diamond et al, 2009; Mendez-Gallart et al, 2009; Tong et al, 2009).
Microsurgical Inguinal and Subinguinal Operations: Preferred Approaches
Introduction
Inguinal and subinguinal varicocelectomy are currently the most popular approaches. They have the advantage of allowing the spermatic cord structures to be pulled up and out of the wound so that the testicular artery, lymphatics, and small periarterial veins may be more easily identified. In addition, an inguinal or subinguinal approach allows access to external spermatic and even gubernacular veins, which may bypass the spermatic cord and result in recurrence if not ligated. Lastly, an inguinal or subinguinal approach allows access to the testis for biopsy or examination of the epididymis for obstruction.
Traditional approaches to inguinal varicocelectomy involve a 5- to 7-cm incision made over the inguinal canal, opening of the external oblique aponeurosis, and encirclement and delivery of the spermatic cord. The cord is then dissected, and all the internal spermatic veins are ligated (Dubin and Amelar, 1977). The vas deferens and its vessels are preserved. An attempt is made to identify and preserve the testicular artery and, if possible, the lymphatics. In addition, the cord is elevated and any external spermatic veins that are running parallel to the spermatic cord or perforating the floor of the inguinal canal are identified and ligated. Compared with retroperitoneal operations, conventional nonmagnified inguinal approaches lower the incidence of varicocele recurrence but do not alter the incidence of either hydrocele formation or testicular artery injury. Conventional inguinal operations are associated with an incidence of postoperative hydrocele formation varying from 3% to 15% with an average incidence of 7% (Szabo and Kessler, 1984). Analysis of the hydrocele fluid has clearly indicated that hydrocele formation following varicocelectomy is due to ligation of the lymphatics (Szabo and Kessler, 1984). The incidence of testicular artery injury during nonmagnified inguinal varicocelectomy is unknown. Case reports, however, suggest that this complication may be more common than realized. It can result in testicular atrophy and, if the operation is performed bilaterally, azoospermia may ensue in a previously oligospermic man. Furthermore, Starzl and his transplant group reported a 14% incidence of testicular atrophy and 70% incidence of hydrocele formation when the spermatic cord was divided and only the vas and vasal vessels were preserved (Penn et al, 1972).
The introduction of microsurgical technique to varicocelectomy has resulted in a substantial reduction in the incidence of hydrocele formation (Goldstein et al, 1992; Marmar and Kim, 1994; Matthews et al, 1998; Cayan et al, 2000). This is because the lymphatics can be more easily identified and preserved. Furthermore, the use of magnification enhances the ability to identify and preserve the 0.5- to 1.5-mm testicular artery, thus avoiding the complications of atrophy or azoospermia.
Advocates of nonmicrosurgical techniques contend that the deferential (vasal) artery and, if preserved, the cremasteric artery, will provide adequate blood supply to the testes to prevent atrophy. However, anatomic studies have known that the diameter of the testicular artery is greater than the diameter of the deferential artery and cremasteric artery combined (Raman and Goldstein, 2004). The testicular artery is the main blood supply to the testis. Experience with the one-stage Fowler and Steven orchiopexy, in which the testicular artery is intentionally ligated, reveals that a substantial percentage of such procedures results in an atrophic testis. Also, animal models indicate that artery preservation varicocelectomy results in improved testicular ultrastructure, whereas artery ligation resulted in further deterioration of ultrastructure (Zheng et al, 2008). At the very least, it is inarguable that ligation of the testicular artery is unlikely to enhance testicular function.
Anesthesia
If the testis is delivered, as described later, regional or light general anesthesia is preferred. If only the cord is delivered, local anesthesia with a 50/50 combination of 0.25% bupivacaine and 1% lidocaine is satisfactory with adjunctive intravenous heavy sedation. After infiltration of the skin and subcutaneous tissues, the cord is infiltrated before delivery. Blind cord block carries with it a small risk of inadvertant testicular artery injury (Goldstein, 1983). A 30-gauge needle should therefore be employed for cord block to minimize the risk of injury and hematoma.
Inguinal and Subinguinal Approaches
The introduction of the subinguinal approach, just below the external inguinal ring (Marmar et al, 1985) obviates the need for opening any fascial layer and is associated with less pain and a rapid recovery comparable with laparoscopic procedures. At the subinguinal level, however, significantly more veins are encountered, the artery is more often surrounded by a network of tiny veins that must be ligated, and the testicular artery has often divided into two or three branches, making arterial identification and preservation more difficult (Hopps et al, 2003a).
Subinguinally, the arterial pulsations are often dampened by compression on the edge of the external ring, making its identification somewhat more difficult than when the external oblique is opened. Table 22–7 summarizes the criteria for performing the operation inguinally (external oblique opened) versus subinguinally (fascia intact). In general, it is best to use a subinguinal approach in men with a history of any prior inguinal surgery. Under these circumstances the cord is usually stuck to the undersurface of the external oblique and opening the fascia risks injury to the cord. A subinguinal approach is easier in obese men in whom opening and closing the fascia is difficult through a small incision. A subinguinal approach is easier in men with high, lax, capacious external rings and in men with long cords and low-lying testes. In these men the level of the external ring is fairly proximal to the testis and opening the fascia will not result in a significant diminution in the number of veins to be ligated or in the branching of the testicular artery.
Table 22–7 Indications for Inguinal (External Oblique Opened) versus Subinguinal (Fascia Intact) Varicocelectomy
| INGUINAL | SUBINGUINAL |
|---|---|
| Children or prepubertal adolescents | Prior inguinal surgery |
| Solitary testis | Obesity |
| Tight, low external ring | Lax, capacious external ring High external ring |
| Short cord, high-lying testis | Long cord with low-lying testis |
| Less experienced with microsurgical repair | Very experienced with microsurgical repair |
Always open the external oblique in children or prepubertal adolescents without prior inguinal surgery. In children the testicular artery is very small and systemic blood pressure is low, making identification of the artery difficult in a subinguinal approach. The fascia should also be opened in men with a solitary testis in whom preservation of the artery is critical. Exposure of the cord more proximally (at the inguinal level) allows identification of the artery before it has branched, where clear pulsations are more readily observed.
Open the fascia in thin men with tight low external rings, high-riding testes, and short cords. The microdissection will be quicker and easier and it is easy to open and close the fascia in thin men. A subinguinal operation is significantly more difficult than a high inguinal operation and should only be used by surgeons who perform the operation frequently. An inguinal operation is employed when simultaneous ipsilateral hernia repair is performed.
Before making the incision, the location of the external inguinal ring is determined by invagination of the scrotal skin and marked. The size of the incision is determined by the size of the testis when delivery of the testis (see later) is planned. Atrophic testes can be delivered through a 2- to 2.5-cm incision. Large testes require a 3-cm incision. The incision is made within Langer lines to minimize scarring.
If the decision is made to perform an inguinal operation and thus to open the fascia, the incision is begun at the external ring and extended laterally 2 to 3.5 cm along Langer lines (Fig. 22–54). If the operation is to be performed subinguinally, the incision is placed in the skin lines just below the external ring (Fig. 22–55).
Figure 22–54 If the decision is made to perform an inguinal operation, and thus to open the fascia, the incision is begun at the external ring and extended laterally 2 to 3/5-cm along Langer lines.
Figure 22–55 If the operation is to be performed subinguinally, the incision is placed in the skin lines just below the external ring.
Camper fascia and Scarpa fascia are divided with the electrocautery between the blades of a Crile clamp. The superficial epigastric artery and vein, if encountered, are retracted or, alternately, clamped, divided, and ligated.
If an inguinal approach is selected, the external oblique aponeurosis is cleaned and opened the length of the incision to the external inguinal ring in the direction of its fibers. A 3-0 absorbable suture placed at the apex of the external oblique incision assists later closure.
The spermatic cord is grasped with a Babcock clamp and delivered through the wound. The ilioinguinal and genital branches of the genitofemoral nerve are excluded from the cord, which is then surrounded with a large Penrose drain. If a subinguinal incision was made, Camper and Scarpa fascia are incised as described earlier. An index finger is introduced into the wound and along the cord into the scrotum. The index finger is then hooked under the external inguinal ring, retracting it cephalad. A small Richardson retractor is slid along the back of the index finger and retracted caudad over the cord toward the scrotum (Fig. 22–56). The spermatic cord will be revealed between the index finger and retractor. The assistant grasps the cord with a Babcock clamp and delivers it through the wound. The cord is surrounded with a large Penrose drain.
Dissection of the Cord
The operating microscope is then brought into the field. Under 10-power magnification, the external and internal spermatic fascias are opened (Fig. 22–57A and B). The magnification is increased to 10 to 25 power and, after irrigation with 1% papaverine solution, the cord is inspected for the presence of pulsations revealing the location of the testicular artery. A micro-Doppler is extremely useful in identifying arteries (Fig. 22–58). Once the testicular artery is identified, it is dissected free of all surrounding tissue, tiny veins, and lymphatics using a fine-tipped nonlocking micro needle holder and microforceps. The artery is encircled with a vessel loop for positive identification and gentle retraction (Fig. 22–59). The suspected artery is tested by elevating the artery with the tips of the micro needle holder until it is completely occluded and then slowly lowering it until a pulsating blush of blood appears just over the needle holder. If the artery is not immediately identified, the cord is carefully dissected beginning with the largest veins. The veins are stripped clean of adherent lymphatics (Fig. 22–60) and the underside of the largest veins inspected for an adherent artery. In approximately 50% of cases the testicular artery is adherent to the undersurface of a large vein (Beck et al, 1992). All veins within the cord, with the exception of the vasal veins, are doubly ligated with either hemoclips (Fig. 22–61A) or by passing two 4-0 silk ligatures, one black and one white, beneath the vein (Fig. 22–61B). These are then tied, and the vein is divided. Medium hemoclips are used for veins 5 mm or larger, small automatic hemoclips for veins 1 to 5 mm, and 4-0 silk for veins smaller than 2 mm. The use of an automatic clip applier (Ligaclip small size, Ethicon, Somerville, NJ) significantly reduces operating time. The bipolar cautery can be used for veins smaller than 0.5 mm. The vasal veins are preserved providing venous return. If the vas deferens is accompanied by dilated veins greater than 3 mm in diameter, they are dissected free of the vasal artery and ligated. The vas deferens is always accompanied by two sets of vessels. As long as at least one set of vasal veins remains intact, venous return will be adequate. At the completion of the dissection, the cord is run over the index finger and inspected to verify that all veins have been identified and ligated. Small veins adherent to the testicular artery are dissected free and ligated or, if smaller than 1 mm, cauterized using a bipolar unit with a jeweler’s forcep tip and divided. Cremasteric arteries are found (usually between and adherent of two cremasteric veins) and preserved in at least 90% of cases. Recent studies employing power-Doppler in men with nonobstructive azoospermia undergoing testicular sperm extraction have found that tubules containing sperm are most likely to be found in areas of the testis with the greatest blood supply. Therefore logic would dictate that preservation of maximum testicular blood supply including both testicular and cremasteric arteries would be beneficial to testicular function. At the completion of the dissection, only the testicular arteries, cremasteric arteries, cremaster muscle fibers, nerves, lymphatics, and vas deferens with its vessels remain (Fig 22–62). After adequate hemostasis is achieved, the cord is returned to its bed.
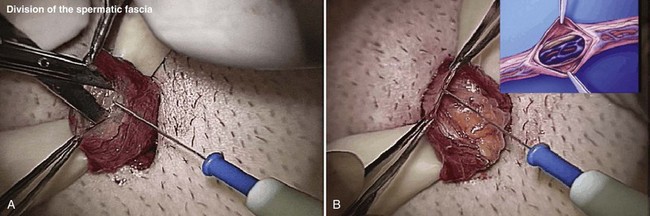
Figure 22–57 A and B, The operating microscope is then brought into the field. Under 4- to 6-power magnification, the external and internal spermatic fascias are opened.
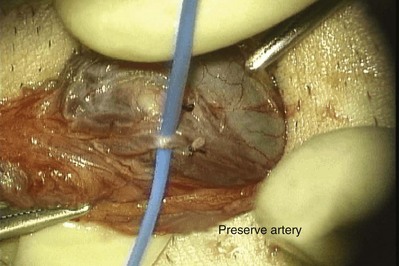
Figure 22–59 The artery is encircled with a vessel loop for positive identification and gentle retraction.
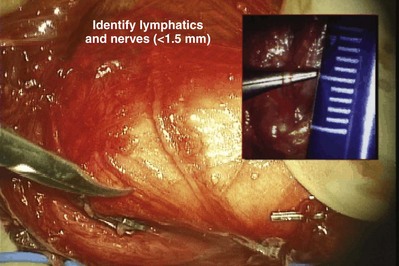
Figure 22–60 If the artery is not immediately identified, the cord is carefully dissected, beginning with the largest veins. The veins are stripped clean of adherent lymphatics.
Delivery of the Testis
Delivery of the testis through a small inguinal or subinguinal incision guarantees direct visual access to all possible avenues of testicular venous drainage. Delivery of only the cord allows access to most external spermatic collaterals but may miss those close to the testis and will not allow access to scrotal or gubernacular collaterals, which have been demonstrated radiographically to be the cause of 10% of recurrent varicoceles (Kaufman et al, 1983). With gentle upward traction on the cord and upward pressure on the testis through the invaginated scrotum, the testis is easily delivered through the wound. All external spermatic veins are identified and doubly ligated with hemoclips and divided (Fig. 22–63). The gubernaculum is inspected for the presence for veins exiting from the tunica vaginalis. These are either cauterized or doubly clipped and divided. When this step is completed, all testicular venous return must be within the Penrose-surrounded cord.
Figure 22–63 All external spermatic veins are identified and doubly ligated with hemoclips and divided.
Hydroceles are found in 15% of testes associated with varicoceles. As little as 3 mL of hydrocele fluid can significantly alter testicular temperature regulation (Wysock et al, 2009). If a hydrocele is noted when the testis is delivered, it is repaired. Small ones may be treated with excision of a segment of the hydrocele sac and cauterization of the edges. Larger hydroceles are treated with either a bottleneck or excision technique. The temporary high-venous pressure immediately after varicocelectomy can make good hemostasis difficult to achieve after excisional hydrocelectomy. Therefore there should be no hesitation to employ a scrotal Penrose drain placed in the dependant portion of the scrotal for 24 hours after combined varicocelectomy and excisional hydrocelectomy. The testis is then returned to the scrotum, and the Penrose drain is left beneath the cord structures.
The external oblique aponeurosis, if opened, is reapproximated with continuous suturing using the previously placed 3-0 suture. Scarpa fascia and Camper fascia are reapproximated with a single or continuous 3-0 plain catgut suture, and the skin is approximated with a 5-0 monofilament absorbable subcuticular suture reinforced by two to three sterile adhesive strips (Steri-Strips) (Fig. 22–64). A scrotal supporter is applied and stuffed with fluff-type dressings. The patient is discharged on the day of surgery with a prescription for Tylenol with codeine. Light work may be resumed in 2 or 3 days.
Radiographic Occlusion Techniques
Intraoperative venography has been employed to visualize the venous collaterals, which if left unligated, may result in varicocele recurrence (Sayfan, 1981; Belgrano et al, 1984; Levitt et al, 1987; Zaontz and Firlit, 1987). Intraoperative venography does reduce the incidence of varicocele recurrence, but the two-dimensional view afforded often does not enable the surgeon to identify the location of all collaterals.
Radiographic balloon or coil occlusion of the internal spermatic veins has been successfully employed for varicoceles (Lima et al, 1978; Walsh and White, 1981; Weissbach et al, 1981). These techniques are performed under a local anesthetic through a small cut-down incision over the femoral vein. The recurrence rate after balloon occlusion was originally 11% and more recently is reportedly as low as 4% (Kaufman et al, 1983; Mitchell et al, 1985; Murray et al, 1986; Matthews et al, 1992). Failure to successfully cannulate small collaterals and external spermatic veins results in recurrence. Venographic placement of a balloon or coil in the internal spermatic vein is successfully accomplished in 75% to 90% of attempts (White et al, 1981; Morag et al, 1984; Winkelbauer et al, 1994; Sivanathan and Abernethy, 2003). Therefore a significant number of men undergoing attempted radiographic occlusion will ultimately require a surgical approach. In addition, the radiographic techniques take between 1 and 3 hours to perform compared with 25 to 45 minutes required for surgical repair. Although rare, serious complications of radiographic balloon or coil occlusion have included migration of the balloon or coil into the renal vein, resulting in loss of a kidney, pulmonary embolization of the coil or balloon (Matthews et al, 1992), femoral vein perforation or thrombosis, and anaphylactic reaction to radiographic contrast medium. Antegrade scrotal sclerotherapy via cannulation of a scrotal vein has been employed in Europe (Tauber and Johnsen, 1994; Ficarra et al, 2002; Minucci et al, 2004). The recurrence rate is similar to balloon or coil techniques. Long-term follow-up is not available, and the consequence of escape of the sclerosing agent into the renal vein and vena cava is unknown. In addition, the larger the varicocele, the higher the failure and recurrence rate with this technique. We have seen many men referred with late (2 to 5 years) recurrence after radiographic occlusion. They typically present as slow-filling veins that become prominent at the end of the day. Initial cursory physical examination can miss these recurrences. We believe these recurrences are likely due to recanalization through the coils because, unlike surgical repair, the veins are not ligated and divided.
Complications of Varicocelectomy
Hydrocele
Hydrocele formation is the most common complication reported after nonmicroscopic varicocelectomy. The incidence of this complication varies from 3% to 33%, with an average incidence of about 7%. Analysis of the protein concentration of hydrocele fluid indicates that hydrocele formation after varicocelectomy is due to lymphatic obstruction (Szabo and Kessler, 1984). At least half of postvaricocelectomy hydroceles grow to a size large enough to warrant surgical excision due to the discomfort and growth of the hydrocele to a large size. The effect of hydrocele formation on sperm function and fertility is uncertain. It is known that men with varicocele have significantly elevated intratesticular temperatures (Zorgniotti et al, 1979; Goldstein and Eid, 1989), and this appears to be an important pathophysiologic phenomenon mediating the adverse effects of varicocele on fertility (Saypol et al, 1981). The development of a large hydrocele creates an abnormal insulating layer that surrounds the testis. This may impair the efficiency of the counter-current heat exchange mechanism and therefore obviate some of the benefits of varicocelectomy (Wysock et al, 2009).
Use of magnification to identify and preserve lymphatics can virtually eliminate the risk of hydrocele formation after varicocelectomy (Goldstein et al, 1992; Marmar and Kim, 1994; Glassberg et al, 2008). The management of postvaricocelectomy hydrocele is identical to that for other forms of hydrocele (see Chapter 37).
Testicular Artery Injury
The diameter of the testicular artery in humans is 1.0 to 1.5 mm. The testicular artery supplies two thirds of the testicular blood supply, and the vasal and cremasteric arteries supply the remaining one third (Raman and Goldstein, 2004). Microdissections of the human spermatic cord have revealed that the testicular artery is closely adherent to a large internal spermatic vein in 40% of men. In another 20% of men the testicular artery is surrounded by a network of tiny veins (Beck et al, 1992). During the course of cord dissection for varicocelectomy, the artery may go into spasm and even in its unconstricted state is often difficult to positively identify and preserve. Injury or ligation of the testicular artery carries with it the risk of testicular atrophy and/or impaired spermatogenesis. Starzl’s transplant group (Penn et al, 1972) reported a 14% incidence of frank testicular atrophy when the testicular artery was purposely ligated. The actual incidence of testicular artery ligation during varicocelectomy is unknown, but some studies suggest it is common (Wosnitzer and Roth, 1983). Animal studies indicate that the risk of testicular atrophy after testicular artery ligation varies from 20% to 100% (MacMahon et al, 1976; Goldstein, 1983). In humans, atrophy after artery ligation is probably less likely due to the contribution of the cremasteric and vasal arterial supply (Raman and Goldstein, 2004). In children the potential for neovascularization and compensatory hypertrophy of the vasal and cremasteric vessels is probably greater than in adults, making atrophy after testicular artery ligation less likely. Use of magnifying loupes, or preferably an operating microscope and/or a fine-tipped Doppler probe, assists identification and preservation of the testicular artery and therefore minimizes the risk of testicular injury. Radiographic balloon or coil occlusion techniques also eliminate this risk.
Varicocele Recurrence
The incidence of varicocele recurrence following surgical repair varies from 0.6% to 45% (Barbalias et al, 1998; Lemack et al, 1998; Cayan et al, 2000; Al-Kandari et al, 2007). Recurrence is more common after repair of pediatric varicoceles. Radiographic studies of recurrent varicoceles visualize periarterial, parallel inguinal or midretroperitoneal collaterals, or, more rarely, transcrotal collaterals (Kaufman et al, 1983). Retroperitoneal operations miss parallel inguinal collaterals. Nonmagnified inguinal operations have a lower incidence of varicocele recurrence but fail to address the issue of scrotal collaterals or small veins surrounding the testicular artery. The microsurgical approach with delivery of the testis lowers the incidence of varicocele recurrence to less than 1% compared with 9% using conventional inguinal techniques (Goldstein et al, 1992; Marmar and Kim, 1994).
Results
Varicocelectomy results in significant improvement in semen analysis in 60% to 80% of men. Reported pregnancy rates after varicocelectomy vary from 20% to 60% (Marmar et al, 2007). A randomized controlled trial of surgery versus no surgery in infertile men with varicoceles revealed a pregnancy rate of 44% at 1 year in the surgery group versus 10% in the control group (Abdel-Meguid, 2010). In our series of 1500 microsurgical operations 43% of couples were pregnant at 1 year (Goldstein and Tanrikut, 2006) and 69% at 2 years when couples with female factors were excluded. Microsurgical varicocelectomy results in return of sperm to the ejaculate in up to 60% of azoospermic men with palpable varicoceles (Matthews et al, 1998; Kim et al, 1999; Pasqualotto et al, 2006; Lee et al, 2007; Ishikawa et al, 2008).
The results of varicocelectomy are also related to the size of the varicocele. Repair of large varicoceles results in a significantly greater improvement in semen quality than repair of small varicoceles (Steckel et al, 1993; Jarow et al, 1996). In addition, large varicoceles are associated with greater preoperative impairment in semen quality than small varicoceles, and consequently overall pregnancy rates are similar regardless of varicocele size. Some evidence suggests that the younger the patient is at the time of varicocele repair, the greater the improvement after repair and the more likely the testis is to recover from varicocele-induced injury (Kass et al, 1987). Varicocele recurrence, testicular artery ligation, or postvaricocelectomy hydrocele formation are often associated with poor postoperative results. In infertile men with low serum testosterone levels, microsurgical varicocelectomy alone results in substantial improvement in serum testosterone levels (Su et al, 1995; Cayan et al, 1999; Younes, 2003; Rosoff et al, 2009).
Summary
Varicocele is an extremely common entity, present in 15% of the male population. Varicoceles are found in approximately 35% of men with primary infertility but 75% to 81% of men with secondary infertility. Mounting evidence clearly demonstrates that varicocele causes progressive duration-dependent injury to the testis. Larger varicoceles appear to cause more damage than small varicoceles and, conversely, repair of large varicoceles results in greater improvement of semen quality. Varicocelectomy can halt the progressive duration-dependent decline in semen quality found in men with varicoceles. The earlier the age at which varicocele is repaired, the more likely is recovery of spermatogenic function. Variococelectomy can also improve Leydig cell function, resulting in increased testosterone levels (Su et al, 1995; Cayan et al, 1999; Younes, 2003).
The most common complications after varicocelectomy are hydrocele formation, testicular artery injury, and varicocele persistence or recurrence. The incidence of these complications can be reduced by employing microsurgical techniques, inguinal or subinguinal operations, and exposure of the external spermatic and scrotal veins. Employment of these advanced techniques of varicocelectomy provide a safe, effective approach to elimination of varicocele, preservation of testicular function, and, in a substantial number of men, an increase in semen quality and likelihood of pregnancy (Abdel-Meguid, 2010).
Orchiopexy in Adults
It is well known that cryptorchidism is associated with a high incidence of infertility even when unilateral. Long hot baths and saunas in humans, on a regular basis, have been shown to impair spermatogenesis. Elevated testicular temperature is also thought to be the primary pathophysiology feature of varicocele (Zorgniotti, 1980; Saypol et al, 1981; Goldstein and Eid, 1989; Wright et al, 1997). Spermatogenesis is exquisitely temperature sensitive. Both animal and human studies have shown that artificial elevation of testicular temperature results in impaired spermatogenesis (Shin et al, 1997; Perez-Crespo et al, 2008; Shiraishi et al, 2009). It will also preserve testicular hormonal function. The technique of orchiopexy in adults is identical to that employed for children. Even with a normal contralateral testis, orchiopexy is worthwhile to bring down a unilateral undescended testis to, if possible, a scrotal location where it can be examined. Leydig cell function in undescended testis can be retained. Orchiopexy in adults with bilateral undescended testes can induce spermatogenesis and allow pregnancy (Shin et al, 1997). Even a solitary cryptorchid testis, when properly placed in scrotum, can provide enough testosterone to obviate the need for hormone replacement. When orchiopexy is performed in adults, regular self-examination and yearly sonography are mandatory.
Retractile Testes in Adults
Retractile testes in boys are usually not surgically repaired if the testes can be manually manipulated to stay down into the scrotum either in the office or under anesthesia. The fate of persistently retractile testis in adults is unknown. A subset of infertile men have retractile testis (Caucci et al, 1997). The semen analyses of these men often demonstrate a typical stressed pattern similar to those of men with varicoceles. These men, however, do not have palpable varicoceles. They all have at least one and frequently both testes that retract out of the scrotum and into the abdomen and remain there for an hour or more a day. In some men these testes remain in the abdomen virtually all the time, except when in a warm shower or under anesthesia. It is likely that these testes will suffer from impaired temperature regulation and impaired spermatogenesis. Scrotal orchiopexy can improve the semen quality and fertility of some of these men.
When scrotal orchiopexy is performed for retractile testis, a dartos pouch operation should be performed. Simple suture orchiopexy of the tunica albuginea of the testis to the dartos, such as is performed sometimes to prevent torsion, will not prevent retraction of these testes into the groin. Creation of a dartos pouch will keep the testis well down into the scrotum and permanently prevent retraction. This is also the most reliable and safest technique for the prevention of testicular torsion (Redman and Barthold, 1995).
A 3- to 4-cm transverse incision is made in the low scrotal skin folds overlying the testis. The incision is kept superficial, just through the dermis and not into the dartos. A large pouch must be created to accommodate the adult testis. The place of dissection is above the dartos and just below the skin, which is kept thin.
After a capacious pouch is created, the dartos and underlying tunica vaginalis are vertically incised and the testis delivered. The cremasteric fibers overlying the spermatic cord are divided and ligated to minimize the tendency of the testis to retract. The opening in the dartos is closed around the cord (but not too tightly) to prevent the testis from falling out of the pouch. The cut edge of the everted tunica is approximated to the opening in the dartos with interrupted synthetic monofilament absorbable sutures. This allows placement of the testis in the pouch without the need for fixation sutures in the tunica albuginea (Redman and Barthold, 1995). The skin is closed over the testis with interrupted sutures of 4-0 chromic catgut. This technique obviates the risk of inadvertent injury to and bleeding from the testicular artery, which courses just under the tunica albuginea (Jarow, 1990).
Acknowledgments
I thank Vanessa L. Dudley and Philip Shihua Li, MD, for their immeasurable assistance in the preparation of this manuscript.
Special thanks to Wayland Hsiao, MD, who critically reviewed this manuscript and provided invaluable suggestions resulting in substantial improvement in its content.
Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59(3):455-461.
Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96(4):598-601.
Chehval MG, Purcell MG. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992;57(1):174-177.
Esteves SC, Oliveira FV, Bertolla RP. Clinical outcome of intracytoplasmic sperm injection in infertile men with treated and untreated clinical varicocele. J Urol. 2010;184(4):1442-1446.
Goldstein M, Gilbert BR, Dicker AP, et al. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148(6):1808-1811.
Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159(1):188-190.
Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol. 2006;3:381-391.
Kim ED, Leibman BB, Grinblat DM, Lipshultz LI. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162(3 Pt. 1):737-740.
Kolettis PN, Thomas AJJr. Vasoepididymostomy for vasectomy reversal: a critical assessment in the era of intracytoplasmic sperm injection. J Urol. 1997;158(2):467-470.
Lee R, Goldstein M, Ullery BW, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol. 2009;181(1):264-269.
Marmar JL, Agarwal A, Prabakaran S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88(3):639-648.
Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe aligoasthenospermia. Fertil Steril. 1998;70(1):71-75.
Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol. 2007;177(4):1447-1449.
Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14(1):131-135.
Steckel J, Dicker AP, Goldstein M. Relationship between varicocele size and response to varicocelectomy. J Urol. 1993;149(4):769-771.
Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 2011. [Epub ahead of print]
Abdel-Meguid T, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59(3:455-461.
Al-Kandari AM, Shabaan H, et al. Comparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trial. Urology. 2007;69(3):417-420.
Al-Said S, Al-Naimi A, et al. Varicocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approaches. J Urol. 2008;180(1):266-270.
Anger JT, Wang GJ, et al. Sperm cryopreservation and in vitro fertilization/intracytoplasmic sperm injection in men with congenital bilateral absence of the vas deferens: a success story. Fertil Steril. 2004;82(5):1452-1454.
Barbalias GA, Liatsikos EN, et al. Treatment of varicocele for male infertility: a comparative study evaluating currently used approaches. Eur Urol. 1998;34(5):393-398.
Barroso UJr, Andrade DM, et al. Surgical treatment of varicocele in children with open and laparoscopic Palomo technique: a systematic review of the literature. J Urol. 2009;181(6):2724-2728.
Beck EM, Schlegel PN, et al. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 1992;148(4):1190-1194.
Belgrano E, Puppo P, et al. The role of venography and sclerotherapy in the management of varicocele. Eur Urol. 1984;10(2):124-129.
Bennett CJ, Ayers JW, et al. Electroejaculation of paraplegic males followed by pregnancies. Fertil Steril. 1987;48(6):1070-1072.
Benshushan A, Schenker JG. The right to an heir in the era of assisted reproduction. Hum Reprod. 1998;13(5):1407-1410.
Berger RE. Triangulation end-to-side vasoepididymostomy. J Urol. 1998;159(6):1951-1953.
Boorjian S, Lipkin M, et al. The impact of obstructive interval and sperm granuloma on outcome of vasectomy reversal. J Urol. 2004;171(1):304-306.
Brackett NL, Padron OF, et al. Semen quality of spinal cord injured men is better when obtained by vibratory stimulation versus electroejaculation. J Urol. 1997;157(1):151-157.
Brandell RA, Goldstein M. Reconstruction of the male reproductive tract using the microsurgical triangulation technique for vasoepididymostomy. J Urol. 1999;161(Suppl.):350.
Brindley GS. Reflex ejaculation under vibratory stimulation in paraplegic men. Paraplegia. 1981;19(5):299-302.
Carpi A, Menchini Fabris FG, et al. Fine-needle and large-needle percutaneous aspiration biopsy of testicles in men with nonobstructive azoospermia: safety and diagnostic performance. Fertil Steril. 2005;83(4):1029-1033.
Castro-Magana M, Angulo MA, et al. Improvement of Leydig cell function in male adolescents after varicocelectomy. J Pediatr. 1989;115(5 Pt. 1):809-812.
Caucci M, Barbatelli G, et al. The retractile testis can be a cause of adult infertility. Fertil Steril. 1997;68(6):1051-1058.
Cayan S, Kadioglu A, et al. The effect of microsurgical varicocelectomy on serum follicle stimulating hormone, testosterone and free testosterone levels in infertile men with varicocele. BJU Int. 1999;84(9):1046-1049.
Cayan S, Kadioglu TC, et al. Comparison of results and complications of high ligation surgery and microsurgical high inguinal varicocelectomy in the treatment of varicocele. Urology. 2000;55(5):750-754.
Chan PT, Brandell RA, et al. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96(4):598-601.
Chan PT, Palermo GD, et al. Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy. Cancer. 2001;92(6):1632-1637.
Chan SL, Lipshultz LI, et al. Deoxyribonucleic acid (DNA) flow cytometry: a new modality for quantitative analysis of testicular biopsies. Fertil Steril. 1984;41(3):485-487.
Chang HC, Chen SC, et al. The effect of biological dyes and contrast media on the vas deferens in Long Evans rats. Int J Androl. 1998;21(5):308-312.
Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992;57(1):174-177.
Cornel EB, Dohle GR, et al. Transurethral deroofing of midline prostatic cyst for subfertile men. Hum Reprod. 1999;14(9):2297-2300.
Craft I, Tsirigotis M. Simplified recovery, preparation and cryopreservation of testicular spermatozoa. Hum Reprod. 1995;10(7):1623-1626.
Craft I, Tsirigotis M, et al. Percutaneous epididymal sperm aspiration and intracytoplasmic sperm injection in the management of infertility due to obstructive azoospermia. Fertil Steril. 1995;63(5):1038-1042.
Dardashti K, Williams RH, et al. Microsurgical testis biopsy: a novel technique for retrieval of testicular tissue. J Urol. 2000;163(4):1206-1207.
Dewire DM, Thomas AV. Microsurgical end-to-side vasoepididymostomy. In: Goldstein M, editor. Surgery of male infertility. Philadelphia: WB Saunders; 1995:128-134.
Diamond DA, Xuewu J, et al. Varicocele surgery: a decade’s experience at a children’s hospital. BJU Int. 2009;104(2):246-249.
Donovan JF, Winfield HN. Laparoscopic varix ligation. J Urol. 1992;147(1):77-81.
Dostal J, Utrata R, et al. Post-mortem sperm retrieval in new European Union countries: case report. Hum Reprod. 2005;20(8):2359-2361.
Dubin L, Amelar RD. Varicocelectomy: 986 cases in a twelve-year study. Urology. 1977;10(5):446-449.
Eisenberg ML, Walsh TJ, et al. Ejaculatory duct manometry in normal men and in patients with ejaculatory duct obstruction. J Urol. 2008;180(1):255-260. discussion 260
Enquist E, Stein BS, et al. Laparoscopic versus subinguinal varicocelectomy: a comparative study. Fertil Steril. 1994;61(6):1092-1096.
Ficarra V, Porcaro AB, et al. Antegrade scrotal sclerotherapy in the treatment of varicocele: a prospective study. BJU Int. 2002;89(3):264-268.
Fogdestam I, Fall M, et al. Microsurgical epididymovasostomy in the treatment of occlusive azoospermia. Fertil Steril. 1986;46(5):925-929.
Foresta C, Garolla A, et al. Genetic abnormalities among severely oligospermic men who are candidates for intracytoplasmic sperm injection. J Clin Endocrinol Metab. 2005;90(1):152-156.
Friedler S, Raziel A, et al. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Hum Reprod. 1997;12(7):1488-1493.
Fuse H, Mizuno I, et al. Transurethral treatment of ejaculatory duct obstruction in infertile men. Arch Androl. 2003;49(6):429-431.
Gerrard ERJr, Sandlow JI, et al. Effect of female partner age on pregnancy rates after vasectomy reversal. Fertil Steril. 2007;87(6):1340-1344.
Glassberg KI, Poon SA, et al. Laparoscopic lymphatic sparing varicocelectomy in adolescents. J Urol. 2008;180(1):326-330. discussion 330–1
Goldstein M. Microspike approximator for vasovasostomy. J Urol. 1985;134(1):74.
Goldstein M. Simultaneous microsurgical vasovasostomy and varicocelectomy: caveat emptor. American Society of Reproductive Medicine; 1995.
Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142(3):743-745.
Goldstein M, Gilbert BR, et al. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148(6):1808-1811.
Goldstein M, Li PS, et al. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159(1):188-190.
Goldstein M, McCallum S, Li PS. Microsurgical vasoepididymostomy: end-to-side triangulation technique. J Urol. 1999;161(Suppl.):93.
Goldstein M, Schlossberg S. Men with congenital absence of the vas deferens often have seminal vesicles. J Urol. 1988;140(1):85-86.
Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol. 2006;3(7):381-391.
Goldstein M, Young GPH, Einer-Jensen N. Testicular artery damage due to infiltration with a fine gauge needle: experimental evidence suggesting that blind cord block should be abandoned. Surg Forum. 1983;24:653-656.
Goldwasser BZ, Weinerth JL, et al. Ejaculatory duct obstruction: the case for aggressive diagnosis and treatment. J Urol. 1985;134(5):964-966.
Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59(3):613-616.
Gorenstein A, Katz S, et al. Varicocele in children: to treat or not to treat—venographic and manometric studies. J Pediatr Surg. 1986;21(12):1046-1050.
Hadziselimovic F, Herzog B, et al. Testicular and vascular changes in children and adults with varicocele. J Urol. 1989;142(2 Pt. 2):583-585. discussion 603–5
Hagan KF, Coffey DS. The adverse effects of sperm during vasovasostomy. J Urol. 1977;118(2):269-273.
Hagood PG, Mehan DJ, et al. Laparoscopic varicocelectomy: preliminary report of a new technique. J Urol. 1992;147(1):73-76.
Hamidinia A. Transvasovasostomy—an alternative operation for obstructive azoospermia. J Urol. 1988;140(6):1545-1548.
Har-Toov J, Eytan O, et al. A new power Doppler ultrasound guiding technique for improved testicular sperm extraction. Fertil Steril. 2004;81(2):430-434.
Harrington TG, Schauer D, et al. Percutaneous testis biopsy: an alternative to open testicular biopsy in the evaluation of the subfertile man. J Urol. 1996;156(5):1647-1651.
Harrison RM, Lewis RW, et al. Pathophysiology of varicocele in nonhuman primates: long-term seminal and testicular changes. Fertil Steril. 1986;46(3):500-510.
Herwig R, Tosun K, et al. Tissue perfusion essential for spermatogenesis and outcome of testicular sperm extraction (TESE) for assisted reproduction. J Assist Reprod Genet. 2004;21(5):175-180.
Hirsch IH, Abdel-Meguid TA, et al. Postsurgical outcomes assessment following varicocele ligation: laparoscopic versus subinguinal approach. Urology. 1998;51(5):810-815.
Homonnai ZT, Fainman N, et al. Varicocelectomy and male fertility: comparison of semen quality and recurrence of varicocele following varicocelectomy by two techniques. Int J Androl. 1980;3(4):447-458.
Hopps CV, Goldstein M. Microsurgical reconstruction of iatrogenic injuries to the epididymis from hydrocelectomy. J Urol. 2006;176(5):2077-2079. discussion 2080
Hopps CV, Lemer ML, et al. Intraoperative varicocele anatomy: a microscopic study of the inguinal versus subinguinal approach. J Urol. 2003;170(6 Pt. 1):2366-2370.
Hopps CV, Mielnik A, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660-1665.
Howards SS, Jessee S, et al. Micropuncture and microanalytic studies of the effect of vasectomy on the rat testis and epididymis. Fertil Steril. 1975;26(1):20-27.
Howards SS, Johnson A, et al. Micropuncture and microanalytic studies of the rat testis and epididymis. Fertil Steril. 1975;26(1):13-19.
Hung AJ, King P, et al. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178(2):608-612. discussion 612
Ishikawa T, Kondo Y, et al. Effect of varicocelectomy on patients with unobstructive azoospermia and severe oligospermia. BJU Int. 2008;101(2):216-218.
Janzen N, Goldstein M, et al. Use of electively cryopreserved microsurgically aspirated epididymal sperm with IVF and intracytoplasmic sperm injection for obstructive azoospermia. Fertil Steril. 2000;74(4):696-701.
Jarow JP. Intratesticular arterial anatomy. J Androl. 1990;11(3):255-259.
Jarow JP. Seminal vesicle aspiration in the management of patients with ejaculatory duct obstruction. J Urol. 1994;152(3):899-901.
Jarow JP. Seminal vesicle aspiration of fertile men. J Urol. 1996;156(3):1005-1007.
Jarow JP, Ogle SR, et al. Seminal improvement following repair of ultrasound detected subclinical varicoceles. J Urol. 1996;155(4):1287-1290.
Jow WW, Steckel J, et al. Motile sperm in human testis biopsy specimens. J Androl. 1993;14(3):194-198.
Kadioglu A, Cayan S, et al. Does response to treatment of ejaculatory duct obstruction in infertile men vary with pathology? Fertil Steril. 2001;76(1):138-142.
Kass EJ, Belman AB. Reversal of testicular growth failure by varicocele ligation. J Urol. 1987;137(3):475-476.
Kass EJ, Chandra RS, et al. Testicular histology in the adolescent with a varicocele. Pediatrics. 1987;79(6):996-998.
Kass EJ, Marcol B. Results of varicocele surgery in adolescents: a comparison of techniques. J Urol. 1992;148(2 Pt. 2):694-696.
Katz D, Mieza. M, Nagler HM. Ultrasound guided transrectal seminal vesiculography; a new approach to the diagnosis of male reproductive tract abnormalities. American Urological Association Annual Meeting, San Francisco, 1994.
Kaufman SL, Kadir S, et al. Mechanisms of recurrent varicocele after balloon occlusion or surgical ligation of the internal spermatic vein. Radiology. 1983;147(2):435-440.
Kim ED, Bischoff FZ, et al. Genetic concerns for the subfertile male in the era of ICSI. Prenat Diagn. 1998;18(13):1349-1365.
Kim ED, Leibman BB, et al. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162(3 Pt. 1):737-740.
Kolettis PN, Fretz P, et al. Secondary azoospermia after vasovasostomy. Urology. 2005;65(5):968-971.
Kolettis PN, Sabanegh ES, et al. Pregnancy outcomes after vasectomy reversal for female partners 35 years old or older. J Urol. 2003;169(6):2250-2252.
Kolettis PN, Thomas AJJr. Vasoepididymostomy for vasectomy reversal: a critical assessment in the era of intracytoplasmic sperm injection. J Urol. 1997;158(2):467-470.
Krylov VS, Borovikov AM. Microsurgical method of reuniting ductus epididymis. Fertil Steril. 1984;41(3):418-423.
Lee JS, Park HJ, et al. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology. 2007;69(2):352-355.
Lee R, Goldstein M, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol. 2009;181(1):264-269.
Lee R, Li PS, et al. A decision analysis of treatments for obstructive azoospermia. Hum Reprod. 2008;23(9):2043-2049.
Lee RK, Li PS, et al. Simultaneous vasectomy and varicocelectomy: indications and technique. Urology. 2007;70(2):362-365.
Lemack GE, Goldstein M. Presence of sperm in the pre-vasectomy reversal semen analysis: incidence and implications. J Urol. 1996;155(1):167-169.
Lemack GE, Uzzo RG, et al. Microsurgical repair of the adolescent varicocele. J Urol. 1998;160(1):179-181.
Levine LA, Dimitriou RJ, et al. Testicular and epididymal percutaneous sperm aspiration in men with either obstructive or nonobstructive azoospermia. Urology. 2003;62(2):328-332.
Levitt S, Gill B, et al. Routine intraoperative post-ligation venography in the treatment of the pediatric varicocele. J Urol. 1987;137(4):716-718.
Lima SS, Castro MP, et al. A new method for the treatment of varicocele. Andrologia. 1978;10(2):103-106.
Lin YH, Huang LW, et al. Intentional cryopreservation of epididymal spermatozoa from percutaneous aspiration for dissociated intracytoplasmic sperm injection cycles. Acta Obstet Gynecol Scand. 2004;83(8):745-750.
Lipshultz LI, Corriere JNJr. Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117(2):175-176.
Lizza EF, Marmar JL, et al. Transseptal crossed vasovasostomy. J Urol. 1985;134(6):1131-1132.
MacMahon RA, O’Brien MC, et al. The use of microsurgery in the treatment of the undescended testis. J Pediatr Surg. 1976;11(4):521-526.
Malkevich DA, Mieza M, Nagler HM. Patency of ejaculatory ducts after transurethral resection of ejaculatory ducts verified by reflux seen on voiding cystourethrogram. J Urol. 1994;151:301A.
Marmar JL. Modified vasoepididymostomy with simultaneous double needle placement, tubulotomy and tubular invagination. J Urol. 2000;163(2):483-486.
Marmar JL, Agarwal A, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88(3):639-648.
Marmar JL, DeBenedictis TJ, et al. The management of varicoceles by microdissection of the spermatic cord at the external inguinal ring. Fertil Steril. 1985;43(4):583-588.
Marmar JL, Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data. J Urol. 1994;152(4):1127-1132.
Matthews GJ, Goldstein M. A simplified method of epididymal sperm aspiration. Urology. 1996;47(1):123-125.
Matthews GJ, Matthews ED, et al. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril. 1998;70(1):71-75.
Matthews GJ, Schlegel PN, et al. Patency following microsurgical vasoepididymostomy and vasovasostomy: temporal considerations. J Urol. 1995;154(6):2070-2073.
Matthews RD, Roberts J, et al. Migration of intravascular balloon after percutaneous embolotherapy of varicocele. Urology. 1992;39(4):373-375.
May M, Johannsen M, et al. Laparoscopic surgery versus antegrade scrotal sclerotherapy: retrospective comparison of two different approaches for varicocele treatment. Eur Urol. 2006;49(2):384-387.
Mendez-Gallart R, Bautista-Casasnovas A, et al. Laparoscopic Palomo varicocele surgery: lessons learned after 10 years’ follow up of 156 consecutive pediatric patients. J Pediatr Urol. 2009;5(2):126-131.
Mercan R, Urman B, et al. Outcome of testicular sperm retrieval procedures in non-obstructive azoospermia: percutaneous aspiration versus open biopsy. Hum Reprod. 2000;15(7):1548-1551.
Minucci S, Mazzoni G, et al. Evolution in varicocele sclerosing treatment: the ante/retrograde (A/R) approach. Arch Ital Urol Androl. 2004;76(1):29-33.
Mitchell SE, Shuman LS, et al. Biliary catheter drainage complicated by hemobilia: treatment by balloon embolotherapy. Radiology. 1985;157(3):645-652.
Monoski MA, Schiff J, et al. Innovative single-armed suture technique for microsurgical vasoepididymostomy. Urology. 2007;69(4):800-804.
Morag B, Rubinstein ZJ, et al. Percutaneous venography and occlusion in the management of spermatic varicoceles. AJR Am J Roentgenol. 1984;143(3):635-640.
Mulhall JP, Stokes S, et al. Simultaneous microsurgical vasal reconstruction and varicocele ligation: safety profile and outcomes. Urology. 1997;50(3):438-442.
Murray RRJr, Mitchell SE, et al. Comparison of recurrent varicocele anatomy following surgery and percutaneous balloon occlusion. J Urol. 1986;135(2):286-289.
Nagler HM, Li XZ, et al. Varicocele: temporal considerations. J Urol. 1985;134(2):411-413.
Nudell DM, Conaghan J, et al. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod. 1998;13(5):1260-1265.
Ohl DA, Sonksen J, et al. Electroejaculation versus vibratory stimulation in spinal cord injured men: sperm quality and patient preference. J Urol. 1997;157(6):2147-2149.
Ohl DA, Wolf LJ, et al. Electroejaculation and assisted reproductive technologies in the treatment of anejaculatory infertility. Fertil Steril. 2001;76(6):1249-1255.
Ostad M, Liotta D, et al. Testicular sperm extraction for nonobstructive azoospermia: results of a multibiopsy approach with optimized tissue dispersion. Urology. 1998;52(4):692-696.
Ozgok Y, Tan MO, et al. Diagnosis and treatment of ejaculatory duct obstruction in male infertility. Eur Urol. 2001;39(1):24-29.
Paick J, Kim SH, et al. Ejaculatory duct obstruction in infertile men. BJU Int. 2000;85(6):720-724.
Palermo GD, Schlegel PN, et al. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N Engl J Med. 1998;338(9):588-590.
Pasqualotto FF, Agarwal A, et al. Fertility outcome after repeat vasoepididymostomy. J Urol. 1999;162(5):1626-1628.
Pasqualotto FF, Lucon AM, et al. Induction of spermatogenesis in azoospermic men after varicocele repair. Hum Reprod. 2003;18(1):108-112.
Pasqualotto FF, Sobreiro BP, et al. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85(3):635-639.
Pavlovich CP, Schlegel PN. Fertility options after vasectomy: a cost-effectiveness analysis. Fertil Steril. 1997;67(1):133-141.
Penn I, Mackie G, et al. Testicular complications following renal transplantation. Ann Surg. 1972;176(6):697-699.
Perez-Crespo M, Pintado B, et al. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75(1):40-47.
Poore RE, Schneider A, et al. Comparison of puncture versus vasotomy techniques for vasography in an animal model. J Urol. 1997;158(2):464-466.
Qiu Y, Yang DT, et al. Successful pregnancy and birth after intrauterine insemination using caput epididymal sperm by percutaneous aspiration. Asian J Androl. 2003;5(1):73-75.
Rajfer J, Binder S. Use of biopsy gun for transcutaneous testicular biopsies. J Urol. 1989;142(4):1021-1022.
Raman JD, Goldstein M. Intraoperative characterization of arterial vasculature in spermatic cord. Urology. 2004;64(3):561-564.
Raman JD, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J Urol. 2003;170(4 Pt. 1):1287-1290.
Ramasamy R, Ricci JA, et al. Successful fertility treatment for Klinefelter’s syndrome. J Urol. 2009;182(3):1108-1113.
Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol. 2007;177(4):1447-1449.
Ramasamy R, Yagan N, et al. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65(6):1190-1194.
Redman JF, Barthold JS. A technique for atraumatic scrotal pouch orchiopexy in the management of testicular torsion. J Urol. 1995;154(4):1511-1512.
Reitelman C, Burbige KA, et al. Diagnosis and surgical correction of the pediatric varicocele. J Urol. 1987;138(4 Pt. 2):1038-1040.
Riccabona M, Oswald J, et al. Optimizing the operative treatment of boys with varicocele: sequential comparison of 4 techniques. J Urol. 2003;169(2):666-668.
Riedenklau E, Buch JP, et al. Diagnosis of vasal obstruction with seminal vesiculography: an alternative to vasography in select patients. Fertil Steril. 1995;64(6):1224-1227.
Rosenlund B, Kvist U, et al. A comparison between open and percutaneous needle biopsies in men with azoospermia. Hum Reprod. 1998;13(5):1266-1271.
Rosoff JS, Tanrikut C, Goldstein M, et al. Prognostic nomogram for the estimation of serum testosterone level following varicocele ligation. American Urological Asssociation Annual Meeting, Chicago, IL, 2009.
Rothman CM, Newmark H3rd, et al. The recurrent varicocele—a poorly recognized problem. Fertil Steril. 1981;35(5):552-556.
Rucker GB, Mielnik A, et al. Preoperative screening for genetic abnormalities in men with nonobstructive azoospermia before testicular sperm extraction. J Urol. 1998;160(6 Pt. 1):2068-2071.
Russell JK. Varicocele, age, and fertility. Lancet. 1957;273(6988):222.
Sayfan J, Adam YG, et al. A new entity in varicocele subfertility: the cremasteric reflux. Fertil Steril. 1980;33(1):88-90.
Sayfan J, Adam YG, Soffer Y. A natural venous bypass causing postoperative recurrence of a varicocele. J Androl. 1981;2:108-110.
Saypol DC, Howards SS, et al. Influence of surgically induced varicocele on testicular blood flow, temperature, and histology in adult rats and dogs. J Clin Invest. 1981;68(1):39-45.
Schellan T. Induction of ejaculation by electrovibration. Fertil Steril. 1968;19:566.
Schiff J, Chan P, et al. Outcome and late failures compared in 4 techniques of microsurgical vasoepididymostomy in 153 consecutive men. J Urol. 2005;174(2):651-655. quiz 801
Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14(1):131-135.
Schlegel PN, Berkeley AS, et al. Epididymal micropuncture with in vitro fertilization and oocyte micromanipulation for the treatment of unreconstructable obstructive azoospermia. Fertil Steril. 1994;61(5):895-901.
Schlegel PN, Goldstein M. Anatomical approach to varicocelectomy. Semin Urol. 1992;10(4):242-247.
Schlegel PN, Goldstein M. Microsurgical vasoepididymostomy: refinements and results. J Urol. 1993;150(4):1165-1168.
Schlegel PN, Palermo GD, et al. Micropuncture retrieval of epididymal sperm with in vitro fertilization: importance of in vitro micromanipulation techniques. Urology. 1995;46(2):238-241.
Schlegel PN, Palermo GD, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49(3):435-440.
Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12(8):1688-1692.
Schoysman RJ, Bedford JM. The role of the human epididymis in sperm maturation and sperm storage as reflected in the consequences of epididymovasostomy. Fertil Steril. 1986;46(2):293-299.
Schroeder-Printzen I, Ludwig M, et al. Surgical therapy in infertile men with ejaculatory duct obstruction: technique and outcome of a standardized surgical approach. Hum Reprod. 2000;15(6):1364-1368.
Sheynkin YR, Chen ME, et al. Intravasal azoospermia: a surgical dilemma. BJU Int. 2000;85(9):1089-1092.
Sheynkin YR, Hendin BN, et al. Microsurgical repair of iatrogenic injury to the vas deferens. J Urol. 1998;159(1):139-141.
Sheynkin YR, Li PS, et al. Comparison of absorbable and nonabsorbable sutures for microsurgical vasovasostomy in rats. Urology. 1999;53(6):1235-1238.
Sheynkin YR, Starr C, et al. Effect of methylene blue, indigo carmine, and Renografin on human sperm motility. Urology. 1999;53(1):214-217.
Sheynkin YR, Ye Z, et al. Controlled comparison of percutaneous and microsurgical sperm retrieval in men with obstructive azoospermia. Hum Reprod. 1998;13(11):3086-3089.
Shin D, Lemack GE, et al. Induction of spermatogenesis and pregnancy after adult orchiopexy. J Urol. 1997;158(6):2242.
Shin D, Lipshultz LI, et al. Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg. 2005;241(4):553-558.
Shiraishi K, Takihara H, et al. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol. 2009;28:359-364.
Shrivastav P, Nadkarni P, et al. Percutaneous epididymal sperm aspiration for obstructive azoospermia. Hum Reprod. 1994;9(11):2058-2061.
Sigman M, Jarow JP. Ipsilateral testicular hypotrophy is associated with decreased sperm counts in infertile men with varicoceles. J Urol. 1997;158(2):605-607.
Silber SJ. Microscopic vasoepididymostomy: specific microanastomosis to the epididymal tubule. Fertil Steril. 1978;30(5):565-571.
Silber SJ. Results of microsurgical vasoepididymostomy: role of epididymis in sperm maturation. Hum Reprod. 1989;4(3):298-303.
Silber SJ. Role of epididymis in sperm maturation. Urology. 1989;33(1):47-51.
Silber SJ, Ord T, et al. Congenital absence of the vas deferens. The fertilizing capacity of human epididymal sperm. N Engl J Med. 1990;323(26):1788-1792.
Sivanathan C, Abernethy LJ. Retrograde embolisation of varicocele in the paediatric age group: a review of 10 years’ practice. Ann R Coll Surg Engl. 2003;85(1):50-51.
Steckel J, Dicker AP, et al. Relationship between varicocele size and response to varicocelectomy. J Urol. 1993;149(4):769-771.
Su LM, Goldstein M, et al. The effect of varicocelectomy on serum testosterone levels in infertile men with varicoceles. J Urol. 1995;154(5):1752-1755.
Su LM, Palermo GD, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol. 1999;161(1):112-116.
Szabo R, Kessler R. Hydrocele following internal spermatic vein ligation: a retrospective study and review of the literature. J Urol. 1984;132(5):924-925.
Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 2011. [Epub ahead of print]
Tash JA, Applegarth LD, et al. Postmortem sperm retrieval: the effect of instituting guidelines. J Urol. 2003;170(5):1922-1925.
Tauber R, Johnsen N. Antegrade scrotal sclerotherapy for the treatment of varicocele: technique and late results. J Urol. 1994;151(2):386-390.
Temple-Smith PD, Southwick GJ, et al. Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J In Vitro Fert Embryo Transf. 1985;2(3):119-122.
Thomas AJJr. Vasoepididymostomy. Urol Clin North Am. 1987;14(3):527-538.
Tiffany P, Goldstein M. Aperistalsis of the vas deferens corrected with administration of ephedrine. J Urol. 1985;133(6):1060-1061.
Tillem SM, Mellinger BC. Azoospermia due to aperistalsis of the vas deferens: successful treatment with pseudoephedrine. Urology. 1999;53(2):417-419.
Tong Q, Zheng L, et al. Lymphatic sparing laparoscopic Palomo varicocelectomy for varicoceles in children: intermediate results. J Pediatr Surg. 2009;44(8):1509-1513.
Tournaye H, Staessen C, et al. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum Reprod. 1996;11(8):1644-1649.
Tsujimura A, Matsumiya K, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17(11):2924-2929.
Tunc L, Alkibay T, et al. Power Doppler ultrasound mapping in nonobstructive azoospermic patients prior to testicular sperm extraction. Arch Androl. 2005;51(4):277-283.
Vazquez-Levin MH, Dressler KP, et al. Urine contamination of seminal fluid after transurethral resection of the ejaculatory ducts. J Urol. 1994;152(6 Pt. 1):2049-2052.
Wagenknecht LV, Klosterhalfen H, et al. Microsurgery in andrologic urology. I. Refertilization. J Microsurg. 1980;1(5):370-376.
Walsh PC, White RIJr. Balloon occlusion of the internal spermatic vein for the treatment of varicoceles. JAMA. 1981;246(15):1701-1702.
Watanabe M, Nagai A, et al. Minimal invasiveness and effectivity of subinguinal microscopic varicocelectomy: a comparative study with retroperitoneal high and laparoscopic approaches. Int J Urol. 2005;12(10):892-898.
Weissbach L, Thelen M, et al. Treatment of idiopathic varicoceles by transfemoral testicular vein occlusion. J Urol. 1981;126(3):354-356.
White RIJr, Kaufman SL, et al. Occlusion of varicoceles with detachable balloons. Radiology. 1981;139(2):327-334.
Winkelbauer FW, Ammann ME, et al. Doppler sonography of varicocele: long-term follow-up after venography and transcatheter sclerotherapy. J Ultrasound Med. 1994;13(12):953-958.
Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology. 1993;42(5):541-543.
Wood BL, Doncel GF, et al. Effect of diltiazem and methylene blue on human sperm motility, viability and cervical mucus penetration: potential use as vas irrigants at the time of vasectomy. Contraception. 2003;67(3):241-245.
Wosnitzer M, Roth JA. Optical magnification and Doppler ultrasound probe for varicocelectomy. Urology. 1983;22(1):24-26.
Wright EJ, Young GP, et al. Reduction in testicular temperature after varicocelectomy in infertile men. Urology. 1997;50(2):257-259.
Wysock JS, Schwartz M, Goldstein M. Hydroceles associated with varicoceles: incidence and mathematical model of their insulating effects. American Urological Association Annual Meeting, Chicago, 2009.
Younes AK. Improvement of sexual activity, pregnancy rate, and low plasma testosterone after bilateral varicocelectomy in impotence and male infertility patients. Arch Androl. 2003;49(3):219-228.
Yurdakul T, Gokce G, et al. Transurethral resection of ejaculatory ducts in the treatment of complete ejaculatory duct obstruction. Int Urol Nephrol. 2008;40(2):369-372.
Zaontz MR, Firlit CF. Use of venography as an aid in varicocelectomy. J Urol. 1987;138(4 Pt. 2):1041-1042.
Zheng YQ, Zhang XB, et al. The effects of artery-ligating and artery-preserving varicocelectomy on the ipsilateral testes in rats. Urology. 2008;72(5):1179-1184.
Zorgniotti AW. Testis temperature, infertility, and the varicocele paradox. Urology. 1980;16(1):7-10.
Zorgniotti AW, Toth A, et al. Infrared thermometry for testicular temperature determinations. Fertil Steril. 1979;32(3):347-348.