chapter 37 Surgery of the Scrotum and Seminal Vesicles
Surgical Anatomy of the Scrotum
The scrotum is a unique body component because the superficial anatomic location of the scrotum and scrotal contents facilitates physical examination, imaging, and surgical access. It is crucial to understand the blood supply to the organs within the scrotum when surgical intervention is indicated. This is summarized in Table 37–1.
Table 37–1 Blood Supply to Testis, Epididymis, and Vas Deferens
| TESTIS | EPIDIDYMIS | VAS DEFERENS |
|---|---|---|
The availability of multiple blood supplies to the testis allows continued testicular viability when one or two of the arteries are compromised by injury or ligation.
Scrotal anatomy allows easy access for surgical procedures, including surgery of the scrotal wall, vasectomy, spermatocelectomy, surgery of the epididymis, hydrocelectomy, and surgical treatment of orchitis.
Spread of Scrotal Infections and Postoperative Fluids Based on Scrotal Anatomy
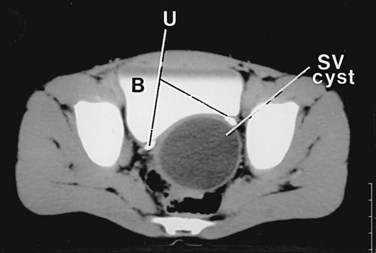
There is a predictable pathway for the spread of scrotal infections and postoperative fluids based on scrotal anatomy. This defined anatomy for the spread of infection is demonstrated in patients with Fournier gangrene (necrotizing fasciitis of the scrotum). Anatomic barriers to the spread of necrotizing fasciitis include the dartos fascia of the penis and scrotum, Colles fascia of the perineum, and Scarpa fascia of the anterior abdominal wall. The testes and epididymes tend to be spared from necrotizing fasciitis of the scrotum (Figs. 37-1 and 37-2) (Gupta et al, 2007).
Figure 37–1 Anatomic barriers to the spread of infection.
(Modified from Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.)
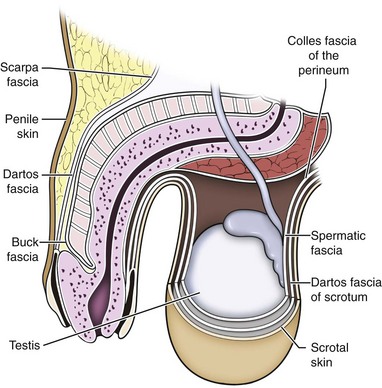
Figure 37–2 Sagittal view of anatomic barriers to the spread of infection.
(Modified from Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.)
Preoperative Preparation
Anesthetic Technique for Scrotal Surgery
Effective anesthetic techniques for scrotal surgery range from local injection with or without sedation to spinal to general anesthesia. The use of a spermatic cord block with local infiltration of 0.5% xylocaine without epinephrine is a simple, cost-effective anesthetic technique that can be implemented by the surgeon for outpatient scrotal surgical procedures. Regional cord block can typically be performed without premedication with satisfactory patient analgesia (Magoha, 1998; Wakefield and Elewa, 1994). Spermatic cord block can be used in patients with large hydroceles for anesthesia by initially percutaneously draining the hydrocele, performing the block, followed by hydrocelectomy (Reale et al, 1998). Outpatient scrotal surgery performed with midazolam sedation and a local block, with sedation reversal at the end of the procedure has a very high patient satisfaction rate (Birch and Miller, 1994).
Preoperative Preparation and the Use of Antibiotics in Scrotal Surgery
The overall infection rate with scrotal surgery is relatively low, ranging from zero to ten percent. There is no difference in the incidence of postoperative wound infections or complications in patients undergoing hydrocelectomy or spermatocelectomy when comparing iodine-based versus chlorhexidine antiseptic preparations. Scrotal cases are considered as class II (clean-contaminated surgeries), which makes it reasonable to use preoperative antibiotics (Kiddoo et al, 2004). The American Urological Association (AUA) best practice policy statement on urologic surgery antimicrobial prophylaxis recommends a single dose of preoperative antibiotics if the patient has risk factors for infection, including advanced age, anatomic anomalies of the urinary tract, poor nutritional status, smoking, chronic corticosteroid use, immunodeficiency, externalized catheters, colonized endogenous/exogenous material, a distant coexistent infection, or prolonged hospitalization. The recommended antibiotic prophylaxis is a dose of a first generation cephalosporin, or clindamycin as an alternative antimicrobial (Wolf et al, 2008). Patients who underwent preoperative clipping for hair removal the morning of surgery had a significantly lower wound infection rate than those who underwent shaving or clipping the night before surgery (Alexander et al, 1983).
Surgery of the Scrotal Wall
Cyst Excision
Patients with multiple scrotal cysts can be managed with surgical excision with excellent cosmetic results and low recurrence rates (Noël et al, 2006).
The classical management of scrotal sebaceous cysts is surgical excision with excellent outcomes and minimal morbidity with good cosmetic results. Less invasive techniques, such as Nd : YAG photocoagulation, have been performed successfully but are not considered standard management (Franco de Castro et al, 2002).
Partial/Total Scrotectomy
Partial scrotectomy is an uncommon procedure. Partial scrotectomy is most commonly performed with infectious processes such as Fournier gangrene. Partial scrotectomy has been advocated after trans-scrotal exploration, orchiectomy, biopsy, or when aspiration has been performed for a scrotal mass and the pathology has revealed a nonseminomatous germ cell tumor of the testis. Prompt and aggressive management has resulted in no local recurrences due to scrotal tumor contamination, even when a tumor was found in the scrotectomy specimen (Johnson and Babaian, 1980; Boileau and Steers, 1984; Leibovitch et al, 1995). In a small group that underwent aggressive local surgical resection and did not receive adjuvant chemotherapy, there was no increase in the local or distant recurrence (Giguere, 1988).
Total scrotectomy is less commonly performed than partial scrotectomy. Total scrotectomy is often necessary when there is extensive involvement of the scrotum with Fournier gangrene. Total scrotectomy has also been described for radical oncologic procedures, concomitantly with cystoprostatectomy, penectomy, or pelvic exenteration with aggressive cases of squamous cell carcinoma of the prostate (Sarma et al, 1991).
Debridement of the Scrotal Wall in Fournier Gangrene
Treatment of Fournier gangrene should include emergent radical surgical debridement and intravenous broad-spectrum antibiotics. When culture results are available the antibiotics can be tailored to the organisms based on sensitivities. Treatment should be performed expeditiously and aggressively, because Fournier gangrene is a life-threatening process. All nonviable and necrotic tissue must be aggressively excised (Fig. 37–3). An empirical broad-spectrum antibiotic regimen for the initial treatment of Fournier gangrene includes a third-generation cephalosporin, an aminoglycoside (if the creatinine clearance is acceptable), and metronidazole. Aggressive fluid resuscitation is required, including the use of blood and blood products when needed. After debridement, adequate nutrition with early enteral feeding, when possible, is crucial for wound healing. Repeat debridement should be performed two days after the initial exploration to excise any remaining nonviable tissue. Multiple resections may be necessary. If the source of the infection is anorectal or the wound is contaminated, a colostomy may need to be performed to divert fecal flow (Ghnnam, 2008). Similarly, patients may require cystostomies for urinary diversion when there is a urinary source exacerbating the necrotizing fasciitis.
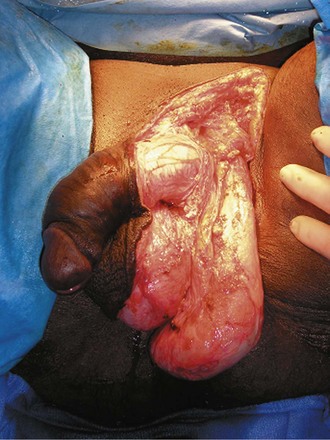
Figure 37–3 Aggressive debridement of Fournier gangrene.
(From Kavoussi PK, Costabile RA. Disorders of scrotal contents: orhcitis, epididymitis, testicular torsion, torsion of the appendages, and Fournier’s gangrene. In: Chapple CR, Steers WD, editors. Practical urology: essential principles and practice. London: Springer-Verlag; 2011.)
Once the patient has been initially treated and resuscitated and all necrotic tissue has been excised, most wounds can be closed secondarily. Large wounds will often require skin grafts for coverage. Fasciocutaneous rotational thigh flaps may be used for coverage with good cosmetic results (Bhatnagar et al, 2008). Wound closure is performed as soon as there is no evidence of infection or remaining necrotic tissue, and there is a viable bed that will allow reapproximation or grafting (Ghnnam, 2008). Patients with less than 50% scrotal skin loss can almost always be closed primarily without major difficulty. The testes may be placed in thigh pouches until the time of definitive reconstruction in cases with major scrotal skin loss (Gudaviciene and Milonas, 2008). Vacuum-assisted closure devices (Wound V.A.C.) have been used to help these complex wounds heal after wide excision and debridement. This technique has been shown to be as effective as conventional wound care in healing wounds. These patients require fewer dressing changes, have less pain, fewer skipped meals, and greater mobility (Ozturk et al, 2009). The use of a small intestinal submucosa graft and fibrin sealant is an option for closure of scrotal defects after excision for Fournier gangrene when standard grafting is not possible (Kavoussi and Bird, 2007).
A severity index has been created and validated to identify prognostic factors in patients suffering from Fournier gangrene. Parameters associated with mortality include abnormalities in heart rate, respiratory rate, serum creatinine, serum bicarbonate, serum lactate, and serum calcium. There is a 46% mortality rate in patients with a severity index score of nine or greater, and a 96% survival rate in patients with a severity index score of less than nine. Necrotizing fasciitis involving the abdominal wall or the lower extremities is associated with increased mortality (Corcoran et al, 2008).
Vasectomy
Vasectomy is a highly effective and safe form of contraception (Schwingl and Guess, 2000). Vasectomy was first described by Sir Ashley Cooper in the United Kingdom when he did experiments to vasectomize dogs (Cooper, 1827). Approximately 526,501 men undergo vasectomy annually in the United States, which makes vasectomy the most commonly performed urologic surgical procedure. 0.01% of men between the ages of 25 and 49 undergo vasectomy annually (Barone et al, 2006). This comprises 11% of all married couples.
Anesthetic Techniques for Vasectomy
Vasectomy can be performed under sedation, spinal, or general anesthesia, although most surgeons perform vasectomy under local anesthesia because the majority of patients tolerate this method well and it minimizes anesthetic complications and morbidity.
The choice of local anesthetic is based on surgeon preference. Options for local anesthesia include the use of 1% or 2% lidocaine without epinephrine or a 50/50 mixture of lidocaine and bupivacaine. The vas deferens is isolated through the scrotal skin and grasped tightly between the thumb and the middle finger of the nondominant hand in a superficial position just beneath the scrotal skin. A 25-gauge needle is used to inject the local anesthetic subcutaneously to raise a small wheel over the vas deferens. Once superficial anesthesia is achieved, the needle is carefully advanced into the vasal sheath, and a small amount of anesthetic is injected. Great care should be taken to use as few punctures as possible and as little needle movement as possible to minimize the risk of hematoma formation.
EMLA (emulsion of lidocaine and prilocaine) cream applied as topical anesthesia on the scrotal skin one hour prior to injection of 1% lidocaine followed by vasectomy does not decrease the pain associated with vasectomy compared with the use of injectable anesthesia alone (Thomas et al, 2008).
The no-needle jet anesthetic technique has been described to eliminate the needle for anesthetic injection. This technique uses the MadaJet medical injector (MADA Medical Products, Carlstadt, NJ) to deliver lidocaine without epinephrine by means of a high-pressure injector through a tiny head to beneath the skin to diffuse a mist of anesthetic around the vas (Weiss and Li, 2005).
Conventional Technique
Vasectomy should be performed in a warm room with warm preparation solution to allow scrotal relaxation, regardless of the technique employed. Shaving should be performed prior to the procedure to minimize the risk of infection. There is no difference in the rate of postvasectomy infection in men randomized to prophylactic antibiotics versus no antibiotics; therefore antibiotic prophylaxis is not recommended (Khan, 1978). In any chosen technique, the use of a single incision or bilateral scrotal incisions is based on surgeon preference. Many surgeons advocate bilateral scrotal incisions to minimize the risk of dividing the same side twice and to allow performing the vasectomy at a position in the midportion of the vas deferens. After induction of adequate local anesthesia, an incision is made over the isolated vas deferens, which is grasped tightly between the thumb and middle fingers. The vasal sheath is sharply divided down to the vas. The vas is delivered through the incision; the deferential artery, nerves, veins, and adjacent tissue are separated from the vas, and the vas is divided. Some surgeons remove a small segment of vas deferens, although most urologists who perform vasectomy reversals prefer not to, which allows easier future reversal. The AUA Policy Statement (American Urological Association, 2007) states that removal of a segment of vas deferens for histologic confirmation is not required or recommended. Most surgeons occlude both the testicular and abdominal ends of the vas with suture ligation, hemoclips, intraluminal fulguration with electrocautery, or fascial interposition. These techniques are further discussed below. The same procedure is repeated on the contralateral vas deferens.
“No-Scalpel” Technique
The no-scalpel vasectomy was initially described in China in 1974 (Li, 1976).
Routine antibiotic use is not needed for vasectomy patients undergoing no-scalpel vasectomy with sterile technique (Seenu and Hafiz, 2005). The no-scalpel technique significantly decreases the rate of hematomas, infections, and pain during the procedure. Patients who undergo the no-scalpel technique also resume sexual activity sooner after surgery and have a shorter operative time than occurs with the conventional technique (Sokal et al, 1999, Cook et al, 2007a).
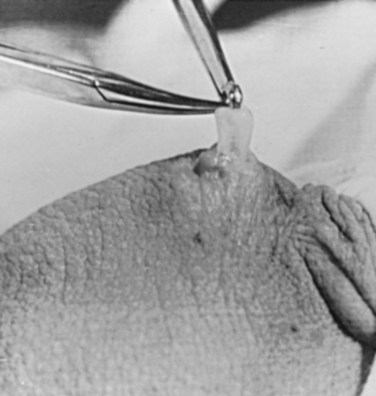
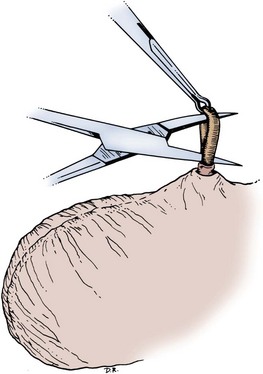
There are several variations to the technique of the no-scalpel vasectomy. One technique involves firmly securing the vas through the skin with a ring-tipped vas deferens fixation clamp (Fig. 37–4) after local anesthesia has been administered as described above (Fig. 37–5). A modified, sharpened tipped curved hemostat (Fig. 37–6) is used to puncture the skin and the vas sheath, and the hemostat is spread to stretch the hole that is made. Then the vas deferens is lifted out, divided, the occlusion technique of choice is employed, inspection is done for hemostasis, and the vas deferens is replaced in the scrotum. The same procedure is performed on the contralateral vas deferens (Huber, 1988). The perforation in the skin can be closed with an absorbable suture, but can also be left open, and will heal well without closure.
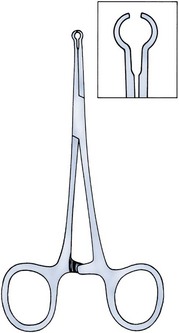
Figure 37–4 Ring-tipped vas deferens fixation clamp. Cantilevered design prevents injury.
(From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.)
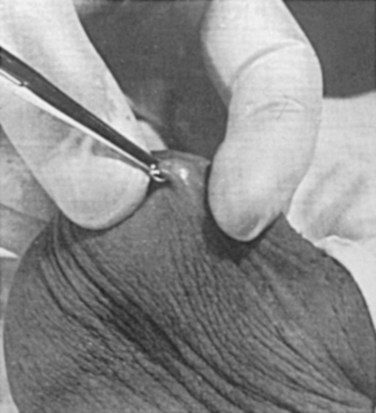
Figure 37–5 Vas fixed in the ring clamp. The scrotal skin is tightly stretched over the most prominent portion of the vas.
(From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.)
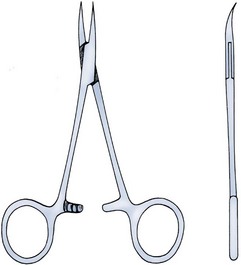
Figure 37–6 Sharp, curved mosquito hemostat.
(From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.)
A variation of this technique employs local anesthesia; fixes the vas through the scrotal skin with the ring-tipped vas deferens fixation clamp; and pierces the scrotal skin, vas sheath, and vas deferens in the midline with a sharpened tipped curved hemostat held at 45 degrees from horizontal (Fig. 37–7). To prepare the vas deferens for division, the clamp is then rotated 180 degrees relative to the pierced vas deferens. The remainder of the procedure is performed in the same manner as described above, and this is done bilaterally (Schlegel and Goldstein, 1992).
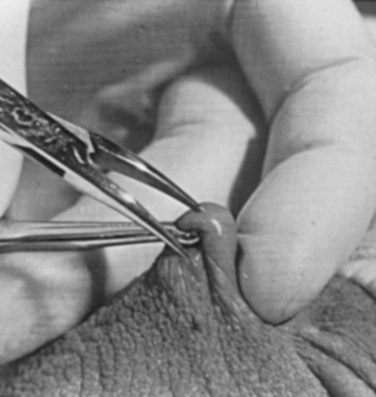
Figure 37–7 Puncture of the skin, vas sheath, and wall into the lumen.
(From Li S, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol 1991;145:341–4.)
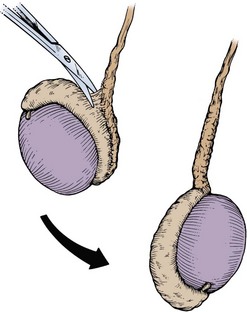
Another technique for performing no-scalpel vasectomy is to isolate the vas deferens after induction of adequate local anesthesia, grasping it tightly between the thumb and middle finger, and puncture the skin overlying the vas deferens with a sharpened tipped curved hemostat. The curved hemostat is used to spread the skin to enlarge the vertical slit in the skin just large enough to allow the ring-tipped vas deferens fixation clamp to fit through to grasp the vas deferens. The vas deferens is grasped and brought up through the puncture, and the vasal fascia and vessels can be spread off the vas deferens to expose the bare vas deferens by spreading the sharp-tipped curved hemostat onto the vasal fascia to open the vasal fascia (Figs. 37-8 and 37-9). The remainder of the vasectomy is performed as described above for bilateral procedure (Li et al, 1991). This modification of making the puncture prior to grasping the vas deferens with the ring-tipped vas deferens fixation clamp was found to significantly decrease the operative time and showed no difference in incision length, postoperative pain, or time to return to work in a randomized prospective evaluation (Chen et al, 2005).
Methods of Vasal Obstruction/Male Sterilization
There are a number of vasectomy occlusion techniques currently employed, including excision and ligation, thermal occlusion with intraluminal electrocautery, mechanical occlusion with hemoclips, fascial interposition, and chemical occlusion with percutaneous techniques.
There have been concerns about the risk of vasal necrosis and sloughing distal to the ligated end when suture ligation is performed, theoretically increasing the risk of recanalization. Low-voltage thermal occlusion with intraluminal electrocautery in the abdominal and testicular ends of the divided vas deferens reduces recanalization rates to less than 0.5% (Schmidt, 1987; Barone et al, 2004). Vasectomy failure rates have been reported to be less than 1% when both the testicular and abdominal ends of the divided vas deferens are occluded with hemoclips (Moss, 1974; Bennett, 1976). Interposition of dartos fascia between the divided ends of the vas deferens is another technique for occlusion. This method has been reported to reduce the recanalization rate even further, nearly to zero (Esho and Cass, 1978; Sokal et al, 2004).
Percutaneous vasectomy has been performed on over 500,000 men in China. This technique employs chemical occlusion by fixing the vas deferens up to the scrotal skin tightly, puncturing the lumen of the vas deferens with a 22-gauge needle, and then cannulating the lumen of the vas deferens with a 24-gauge blunt needle. For confirmation of vas deferens cannulation, Congo red is injected into the lumen of the abdominal end of the right vas deferens, and methylene blue is injected into the lumen of the left vas deferens prior to chemical occlusion by injection of 20 µl of two parts phenol to one part N-butyl-2-cyanoacrylate mixture. Following chemical occlusion the patient should void. If the urine is red, the left side was not cannulated, if it is blue, the right side was not cannulated, and if it is brown, that indicates bilateral successful cannulation (Ban, 1980; Li, 1980). Although these chemicals are not approved for use in the United States by the Food and Drug Administration, they appear to be safe by toxicologic testing and experience in China.
Fascial interposition is the technique that has been found to decrease vasectomy recanalization rates the most significantly. Randomized controlled trials examining the other techniques are not available. Several trials have been performed using irrigation of the abdominal ends of the vas deferens with saline, but there was no difference in time to azoospermia (Cook et al, 2007b).
Open-ended vasectomy, where the testicular portion of the vas deferens remains patent, is another technique that has been evaluated with the aim of decreasing epididymal pressure by performing intraluminal cautery or another method of occlusion on the abdominal end, while leaving the testicular end unoccluded. Ninety-seven percent of patients undergoing open-ended vasectomy develop sperm granulomas. This reduces pressure-induced damage to the epididymis, but increases the vasectomy failure rate to as high as 7% to 50% (Shapiro and Silber, 1979; Goldstein, 1983). There is a significant decrease in the failure rate with open-ended vasectomy when fascial interposition is performed, decreasing the failure rate by approximately 7% (Li et al, 1994).
Performing Vasectomy to Make Microscopic Vasectomy Reversal Easier
Technical aspects of performing vasectomy can affect the ease of microsurgical vasectomy reversal in the future if needed (Mammen et al, 2008). One procedural aspect is that excising a lengthy (>1 cm) segment of vas deferens is associated with the need for a higher scrotal incision, possibly up to the lower inguinal region, with the potential for anastomotic tension with microscopic vasectomy reversal. Vasectomy reversal can be far more difficult when a lengthy portion of the vas deferens has been excised, with concomitant increases in operative time, length of incision, and postoperative pain (Practice Committee of the American Society for Reproductive Medicine, 2006).
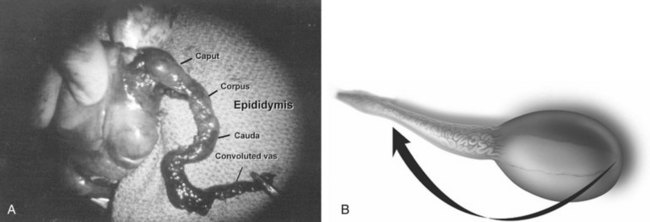
Another procedural aspect is the location along the length of the vas deferens where the vasectomy is performed. Experts in microsurgery agree that the anastomosis is least problematic when the lumen of the vas deferens is largest and most concentric, as opposed to the lumen in the epididymis or the convoluted vas (Mammen et al, 2008). Prospective studies show that the length of the testicular vas deferens present at the time of reversal has a direct correlation with the presence of seminal fluid containing intact sperm at the time of microscopic vasectomy reversal. A testicular length of vas deferens less than 2.7 cm correlates with seminal fluid without intact sperm 85% of the time, and testicular length over 2.7 cm is associated with intact sperm in seminal fluid 94% of the time. For each centimeter increase in testicular remnant length, the probability of whole sperm being present increases fourfold (Witt et al, 1994). Division of the vas deferens should be performed approximately 3 cm distal to the cauda of the epididymis in the straight portion of the vas deferens at the time of vasectomy.
The other technical aspect to consider is the occlusion technique employed. All occlusive modalities for vasectomy carry a similarly high efficacy in terms of postprocedure azoospermia. To date, no specific studies on occlusion technique as a predictor of reversal success have been performed. Simple transection of the vas deferens followed by low-voltage intraluminal cautery occlusion followed by fascial interposition provides successful vasectomy and may result in minimal inflammatory reaction. It would stand to reason that minimizing inflammation near the vas deferens would provide the optimum condition for microscopic vasectomy reversal in the future (Mammen et al, 2008).
Postoperative Care and Follow-up Semen Analysis
Routine postoperative care practices vary, but typically include the use of ice packs on the scrotum intermittently for the first 48 hours, limited heavy or strenuous activity for one week, and the use of nonsteroidal anti-inflammatory drugs, as needed, for pain, if the patient does not have any contraindications to these medications.
There is no vasectomy technique that is 100% effective, and a definitive standard to declare a patient sterile has not been agreed upon. Time to reach azoospermia is variable, although over 80% of patients achieve azoospermia by three months and after twenty ejaculations. Persistent nonmotile sperm are present in 1.4% of postvasectomy patients. This data points to obtaining a semen analysis at three months and twenty ejaculations after vasectomy to reveal azoospermia. If the semen analysis does not show azoospermia, periodic semen analyses can be obtained until azoospermia is achieved. Patients who have small numbers of persistent nonmotile sperm can be advised to cautiously discontinue contraception (Griffin et al, 2005). There is evidence that these men will ultimately reach azoospermia. Vasectomy should be repeated if any motile sperm are found in the ejaculate three months after the initial vasectomy (Aradhya et al, 2005).
An immunodiagnostic test, the SpermCheck Vasectomy (ContraVac, Charlottesville, VA), has been developed to allow patients to test themselves for severe oligospermia or azoospermia at home following vasectomy. This was developed to increase compliance with postvasectomy evaluation of semen parameters. The SpermCheck Vasectomy test was 96% accurate at predicting whether sperm counts were greater or less than a threshold of 250,000 sperm per milliliter (Klotz et al, 2008).
Complications: Local/Postoperative
Local complications of vasectomy include hematoma, infection, Fournier gangrene, chronic scrotal pain, and traumatic fistula/scrotal sinus (Awsare et al, 2005). Surgeon volume and experience is the most important predictor of postoperative complications (Kendrick et al, 1987).
Hematoma is the most common complication of vasectomy. The rate of hematoma formation after vasectomy ranges between 0.09% and 29%, with a mean incidence of 2% (Kendrick et al, 1987). The no-scalpel technique has decreased the hematoma rate to a 0.5% incidence (Pant et al, 2007).
The rate of infection from vasectomy with the conventional technique was reported between 12% and 38%, but decreased to 0.4% with the no-scalpel technique (Appell and Evans, 1980; Pant et al, 2007). Although it is an exceedingly rare complication, Fournier gangrene has been reported as a complication in men undergoing vasectomy (de Diego Rodríguez et al, 2000; Romero Pérez et al, 2004).
Up to 30% of men have short-term scrotal pain lasting a few weeks. However, postvasectomy pain syndrome, or long-term scrotal pain after vasectomy, occurs in approximately one in 1000 vasectomies, although some report the incidence to be as high as 15% (McConaghy et al, 1996; Awsare et al, 2005; Tandon and Sabanegh, 2008). Postvasectomy pain syndrome has no association with immediate postoperative complications such as hematoma or infection. There are several theories about the cause of postvasectomy pain syndrome. One is that dilation of the epididymal duct with obstruction of the testicular end of the vas deferens produces interstitial fibrosis. Another theory is that extravasation of spermatozoa, with epididymal duct rupture forming a sperm granuloma at the site where the vas deferens is transected, results in perineural fibrosis and inflammation, because sperm are highly antigenic (McMahon et al, 1992). This contradicts the previous belief that sperm granulomas are protective against postvasectomy pain syndrome by relieving pressure, although most sperm granulomas are asymptomatic (Tandon and Sebanegh, 2008). Interestingly, there are markedly increased pressures in the epididymis and the testicular end of the vas deferens after vasectomy, but these pressures were not found to be transmitted to the seminiferous tubules in human micropuncture studies (Johnson and Howards, 1975). It is unclear why some patients would develop long-term symptoms and others transient ones. Factors such as age, socioeconomic status, race, environmental, or vasectomy technique have not identified patients at risk for postvasectomy pain syndrome (Tandon and Sebanegh, 2008).
Conservative therapy should be the first line of therapy. This includes scrotal elevation and support, heat or ice (as needed for comfort), and nonsteroidal anti-inflammatory drugs. Empiric antibiotic therapy is not recommended without evidence of infection (Selikowitz and Schned, 1985). Conservative therapy should be employed for at least three months for postvasectomy pain. Spermatic cord blocks should be considered after failure of conservative therapy.
Surgical therapy might be considered if the above methods fail. When pain is clearly localized to a sperm granuloma, excision of the granuloma and intraluminal cautery occlusion of the vas deferens may relieve the pain and prevent recurrence (Schmidt, 1979). Epididymectomy has been performed in patients who had point tenderness to the epididymis, and epididymal dilation after vasectomy and failed conservative therapy. Predictors of poor outcomes with epididymectomy are atypical symptoms, concomitant erectile dysfunction, and a normal-appearing epididymis on scrotal ultrasound (West et al, 2000). Up to 50% of patients who were properly selected for epididymectomy were cured of postvasectomy pain syndrome (Chen and Ball, 1991). The patient must consider that vasectomy reversal will no longer be feasible after epididymectomy. Vasectomy reversal rendered 69% of patients with postvasectomy pain syndrome pain free (Nangia et al, 2000). Although it has only been evaluated in a small number of patients, microscopic denervation of the spermatic cord in patients who had temporary relief with a spermatic cord block resulted in complete pain relief in 76% and improvement in pain in 9% of these men (Levine and Matkov, 2001). The last resort consideration after failure of conservative and more invasive interventions have failed, is orchiectomy for severe intractable pain after vasectomy. In this group, pain relief was reported in 73% of men who underwent inguinal orchiectomy versus 55% in those who underwent scrotal orchiectomy for postvasectomy pain syndrome (Davis et al, 1990).
There is a report of 0.3% rate of scrotal sinus/vasocutaneous fistula after no-scalpel vasectomy (Pant et al, 2007).
Association of Vasectomy with Long-Term Systemic Disease
Previous studies found an increased risk of prostate cancer in men who underwent vasectomy (Giovannucci et al, 1993). Detection bias is thought to be the source of this association of prostate cancer with vasectomy (Millard, 1999). Recent investigations have found that there is no association between vasectomy and prostate cancer (Schuman et al, 1993; Holt et al, 2008). There is no association between vasectomy and prostate cancer in developing countries in which there are low incidences of prostate cancer in the general population as well (Schwingl et al, 2009). Screening recommendations for prostate cancer should be no different in men who have undergone vasectomy than those who have not undergone vasectomy (Healy, 1993).
Vasectomy does not place the patient at an increased long-term risk for cardiovascular disease or atherosclerosis (Coady et al, 2002; Goldacre et al, 2005).
Previous studies suggested that vasectomy may be a risk factor for patients with primary progressive aphasia, a dementia syndrome with aphasia as the presenting symptom (Weintraub et al, 2006). There are no longitudinal studies confirming this, and there have been many large, epidemiological studies comparing vasectomized and nonvasectomized men without any increased risk of dementia. There is no evidence that vasectomy adversely affects psychological health status (Thonneau and D’Isle, 1990).
Antisperm Antibodies
There is a disruption of the blood-testis barrier when vasectomy is performed. Of men who undergo vasectomy, 60% to 80% of them have detectable levels of antisperm antibodies in the serum (Fuchs and Alexander, 1983). After vasectomy, 50% to 60% of men develop sperm agglutinating antibodies, and 20% to 30% develop sperm immobilizing antibodies (Kovacs and Frances, 1983). Although some studies suggest that antisperm antibodies persist, others suggest that they diminish in two or more years after vasectomy, but neither immune complex deposition nor circulation are increased in vasectomized men (Witkin et al, 1982).
Key Points: Vasectomy
Spermatocelectomy/Surgery of the Epididymis
Surgical Indications
A spermatocele is a cystic dilation of an epididymal tubule, which is benign in nature. Spermatoceles are very common, occurring incidentally in 30% of men on high-resolution ultrasound, and they are typically asymptomatic and do not cause epididymal obstruction, and therefore rarely require intervention. Men typically seek surgical treatment when the spermatocele has reached the approximate size of the testis and is causing pain with point tenderness (Walsh et al, 2007).
Surgical treatment for chronic epididymitis is poorly studied in clinical trials with no level 1 evidence to support the use of a specific surgical procedure. In one study, ten patients with chronic epididymitis (defined as epididymal pain lasting greater than 3 months), underwent epididymectomy for intractable symptoms. Only one of these patients had significant improvement in pain (Davis et al, 1990). Other authors have reported much higher success rates, such as six out of seven patients (86%) having significant improvement in pain after epididymectomy (Chen and Ball, 1991). Chronic or recurrent epididymitis and persistent epididymalgia with point tenderness to the epididymis may be reasonable indications for epididymectomy (Padmore et al, 1996).
Surgical treatment for chronic epididymitis should only be considered after failure of extensive conservative therapy and after appropriate counseling, with the understanding that the symptoms may not improve after surgery, or may indeed worsen. A retrospective review of men who underwent epididymectomy for chronic epididymitis showed that outcomes were best when the patient had a palpable epididymal abnormality on physical examination. Men in this study without a palpable abnormality, but with sonographic changes, had slightly worse outcomes, and those with neither a palpable abnormality nor a demonstrable ultrasound abnormality did not improve with epididymectomy (Calleary et al, 2009).
Purulent epididymitis diagnosed by a combination of physical examination, ultrasound evaluation, and, occasionally, needle aspiration of the epididymis is an absolute indication for epididymectomy (Arbuliev et al, 2008). This is also the treatment of choice for epididymal abscesses and chronic infectious epididymitis that is unresponsive to antibiotic treatment. Diagnostic epididymal puncture and aspiration should not be performed in men with interest in future fertility, because this will result in epididymal obstruction.
Total epididymectomy may relieve chronic persistent pain localized to the epididymis after vasectomy.
Epididymal malignancies are extremely rare, and 73% of nontransilluminating, solid epididymal masses are benign adenomatoid tumors (Beccia et al, 1976). Surgical extirpation should be considered for adenomatoid tumors, especially if there is any suspicion for malignancy (Alvarez et al, 2009).
Partial/Total Epididymectomy
Any patient undergoing epididymal surgery should be extensively counseled that the surgery may impair his fertility, or even cause sterility if bilateral epididymal surgery is required, because the distal epididymis consists of a single tubule.
Partial/total epididymectomy can be approached scrotally through a median raphe or a unilateral transverse scrotal incision to deliver the testis. The vas deferens is identified, isolated, ligated, and divided. The testicular end of the vas deferens is then followed to the vasoepididymal junction. The tunica vaginalis is opened, and the plane of dissection between the epididymis and the testis is found in order to divide the epididymis from the testis. Great care should be taken to avoid injury to the spermatic cord and testicular artery. The efferent ducts superior to the testicular vasculature are ligated with an absorbable suture to complete the epididymectomy. The edges of the tunica vaginalis where the epididymis was excised are approximated with a running absorbable suture, which helps with hemostasis. The dartos fascia and skin are closed in layers with absorbable suture. In the case of partial epididymectomy, ligations are performed between the testis and epididymis with absorbable suture to excise the affected portion of the epididymis, while leaving the remainder attached to the testis with its vascular supply intact (Figs. 37-10 and 37-11).
Spermatocelectomy/Excision of Epididymal Cysts
Spermatocelectomy can be approached scrotally through a median raphe or a unilateral transverse scrotal incision to deliver the testis. The tunica vaginalis is opened, and the spermatocele is identified and dissected free from the epididymis. The epididymal–spermatocele attachment is ligated and divided to complete the spermatocelectomy. The tunica vaginalis is closed, and the dartos fascia and scrotal skin are reapproximated in layers.
Excision of Epididymal Tumors
As discussed above, the majority of epididymal nontransilluminating masses are benign adenomatoid tumors. Fine needle aspiration of solid epididymal masses has been evaluated and found to be very accurate when compared with surgical pathology (Gupta et al, 2006). When malignancy is suspected, an inguinal approach should be used with clamping of the spermatic cord and delivery of the testis. The addition of testicular hypothermia by adding ice slush to the Chevassu maneuver (of clamping the vessels) was employed and found to salvage three out of five testicles with benign processes after clamping and parenchymal biopsy (Goldstein and Waterhouse, 1983). When malignancy is ruled out, the epididymal mass may be excised as for a spermatocelectomy.
Complications
Complications of epididymectomy, spermatocelectomy, and excision of epididymal masses are rare. Complications include bleeding, infection, damage to the testicular artery with resultant testicular atrophy, and recurrence in cases of spermatocelectomy (Kiddoo et al, 2004; Zahalsky et al, 2004). Complications also include impairment of fertility with obstruction of the epididymal tubules and possible sterility in bilateral cases, necessitating counseling and recommending sperm cryopreservation in men who undergo bilateral procedures and have an interest in fertility. Approximately 17% of patients undergoing spermatocelectomy will have resultant epididymal injury (Zahalsky et al, 2004). The overall complication rate has been reported to be as high as 20%, with the most common complications being persistent scrotal pain and infection. Leaving a scrotal drain did not decrease complication rates (Kiddoo et al, 2004).
Hydrocelectomy
The adult hydrocele is caused by excessive fluid secretion by the visceral tunica albuginea without adequate reabsorption of this fluid by the parietal peritoneum around the testis.
Inguinal Surgical Approach
Men diagnosed with hydroceles, where there is suspicion for concomitant malignancy, should undergo high-resolution scrotal ultrasound. If malignancy is suspected, an inguinal approach should be used to allow control of the spermatic cord in preparation for radical orchiectomy. If this approach is taken and no malignancy is encountered, the testis can be spared and the hydrocele can be repaired by one of the techniques described below.
Scrotal Surgical Approaches
When there is no evidence of malignancy on physical examination and high-resolution ultrasound, hydroceles may be approached scrotally through a median raphe or a transverse unilateral incision. In all techniques, the hydrocele is dissected and delivered intact to allow the easiest dissection. Once it is delivered, an opening in the sac is made away from the testis, epididymis, and cord structures, and the fluid is suctioned out. The hydrocele sac is then opened further to expose the sac for the elected repair. The overall single-treatment success rates for surgical hydrocelectomy range between 90% and 100% (Rodriquez et al, 1981).
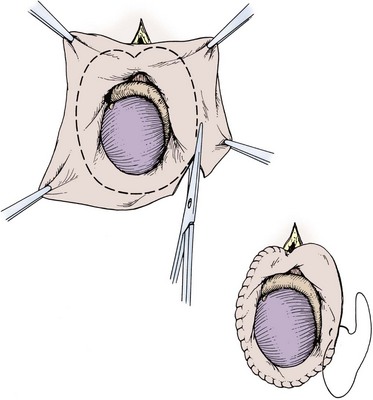
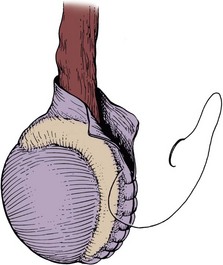
Excisional techniques are the least likely to result in recurrence of a hydrocele. Excising the hydrocele is recommended for long-standing, thick-walled, loculated hydroceles. The hydrocele sac is opened, taking great care not to injure the spermatic cord, and the sac is simply excised, leaving room to oversew the edge of the excised sac without endangering the cord or the epididymis. A 3-0 chromic suture can be used in a baseball stitch manner to oversew the edge of the sac (Fig. 37–12).
The Jaboulay bottleneck technique is a useful method for large, floppy, thin hydrocele sacs. This is performed by excising the sac as described above, but a larger margin of excision should be left between the edge of the sac and the testis and epididymis, to allow for sewing the edges of the sac together behind the cord, without compressing the cord (Fig. 37–13) (Jaboulay, 1902).
If there appears to be a risk for hematoma after excision and oversewing with either technique, a Penrose drain can be left in the dependent portion of the scrotum and fixed in place to the scrotum. The dartos fascia and skin are then closed in layers. Fluffs and a scrotal support are used for the dressing.
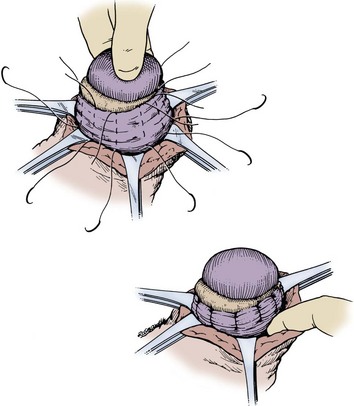
Plication techniques are suitable for thin-walled hydroceles but should not be used in large, long-standing, thick-walled, multiloculated hydroceles because this leaves a large bundle of plicated tissue in the scrotum. The Lord plication technique (1964) is performed by opening the hydrocele as described above, delivering the testis, cauterizing or oversewing the cut edges of the sac, and using interrupted, radially placed chromic sutures to plicate the sac. Closure is then performed as described above. Drains are not necessary with plications (Fig. 37–14).
Sclerotherapy
Sclerotherapy is another treatment option with a single-treatment success rate ranging from 33% to 75% (Levine and Dewolf, 1988). This may be a good choice for patients who cannot tolerate anesthesia or who refuse surgical treatment. The common steps of the procedure include needle aspiration of the hydrocele fluid, followed by injection of local anesthesia, and ultimately instillation of the sclerosing agent. The most commonly used sclerosing agent is tetracycline (500 mg), although 2.5% phenol solutions, 95% alcohol, and ethanolamine oleate have also been used effectively (Nash, 1984; Hellström et al, 1986; Miskowiak and Christensen, 1988). One study showed no statistically significant improvement in patients who underwent aspiration of hydrocele fluid alone versus aspiration plus instillation of tetracycline as a sclerosing agent, with a higher complication rate in the sclerotherapy group (Breda et al, 1992). When sclerotherapy was compared to hydrocelectomy, the success rate was higher in hydrocelectomy, although the hydrocelectomy group had a higher complication rate. Nonetheless, the patients who underwent hydrocelectomy had higher satisfaction rates (Beiko et al, 2003).
Complications
The most common complication after hydrocelectomy is hematoma (Table 37–2). The overall complication rate in hydrocelectomy is approximately 19%, including hematoma, infection, persistent swelling, recurrence, injury to spermatic vessels, and chronic pain. Although the use of a drain in selected patients is recommended, it has not been proven to decrease complication rates thus far (Kiddoo et al, 2004). When repairing large hydroceles, great care must be taken to not injure the epididymis and spermatic vessels, because they may be splayed within the hydrocele layers. Injury to the epididymis or vas deferens can put the patient’s future fertility at risk (Zahalsky et al, 2004).
Table 37–2 Hydrocelectomy Techniques and Risks
| RECURRENCE | HEMATOMA | |
|---|---|---|
| Excision | Decreased | Increased |
| Plication | Increased | Decreased |
Sclerotherapy complications include scrotal pain in 29% to 55% of patients (Rencken et al, 1990; Ovrebø and Vaage, 1991), recurrence, hematoma, and infection; febrile chemical epididymoorchitis has also been reported with sclerotherapy (López Laur, 1989; Beiko et al, 2003). Sclerotherapy can have an adverse effect on fertility and should be avoided in patients with interest in fertility potential (Sigurdsson et al, 1994).
Surgical Treatment of Orchitis and Chronic Scrotal Pain
Orchitis is defined as inflammation of the testicle (Delavierre, 2003). The typical symptoms of orchitis include scrotal pain, swelling, tenderness, and skin fixation over the testicle. The Prehn sign has been described in orchitis and epididymitis when there is relief of pain with elevation of the testicle over the symphysis pubis (Noske et al, 1998). The Prehn sign is nonspecific and nondiagnostic and does not necessarily distinguish epididymo-orchitis from spermatic cord torsion. Orchitis can cause an irreversible affect on spermatogenesis, impacting the quality and number of spermatozoa. Lymphocytic infiltration and seminiferous tubule damage is seen on testicular biopsies of subfertile men with a history of chronic orchitis (Schuppe et al, 2008).
Chronic orchitis is defined as constant or intermittent scrotal pain lasting at least 3 months or longer, and of unclear cause (Costabile et al, 1991). In patients with clinical orchitis, a scrotal ultrasonograph should be obtained because testicular malignancy has been reported to masquerade as orchitis (Vaidyanathan et al, 2008). At least 10% of men with testicular malignancy will initially be incorrectly diagnosed as having an acute inflammatory process or spermatic cord torsion (Cook and Dewbury, 2000). High-frequency transducer sonography (7.5 to 10 MHz) is considered the best modality for evaluation of scrotal pathology including orchitis (Lee and Bhatt, 2008).
Although there is no level 1 evidence for the optimal treatment of chronic orchitis or epididymitis, local supportive therapy, including heat, nerve blocks, analgesics, tricyclic antidepressants, anticonvulsants (such as gabapentin), and anti-inflammatory drugs are common practice and may offer some relief (Davis and Noble, 1992). Other treatment options implemented for chronic epididymitis include phytotherapy, anxiolytics, narcotics, acupuncture, and steroid injection therapy (Nickel et al, 2002). Despite evidence that up to 75% of patients do not have an identifiable bacterial urinary tract infection concomitantly with their clinical epididymitis, antibiotics are routinely given. Empirical antibiotic administration in the absence of positive urine cultures has been steadily increasing, from 75% to 95% between the years of 1965 and 2005. Antibiotic administration does not decrease the length of symptoms or the return to full activity in men without an identifiable bacterial pathogen (Mittemeyer et al, 1966).
Orchiectomy
Surgical treatment for chronic orchitis is poorly studied in clinical trials, with no level 1 evidence to support the use of a specific surgical procedure. Fewer than 250 patients with chronic scrotal pain have been treated with differing surgical therapies in the available literature, despite the common nature of chronic scrotal pain. There is no level 1 evidence that orchiectomy is effective for the treatment of chronic orchalgia. If orchiectomy is recommended, the patient should previously have failed conservative therapy and must be apprised of the risks, benefits, and options of orchiectomy. Because many patients will continue to have pain or have pain recur after orchiectomy, the surgeon should be aware of the medical legal aspects of this action. If orchiectomy is performed, it should be performed through an inguinal incision, because this approach has been shown to have a better outcome than the scrotal approach for orchalgia (Davis and Noble, 1992).
Microsurgical Denervation of the Spermatic Cord
Some surgeons have attempted microsurgical denervation of the spermatic cord for symptomatic relief of chronic scrotal pain. Microsurgical denervation of the spermatic cord was performed in 79 men on 95 testicular units for chronic orchalgia over a mean duration of 62 months. There was complete relief of pain in 71% of the patients, partial relief in 17%, and there was no change from the preoperative status in 12%, with no patients experiencing worsened postoperative pain. The mean follow-up was 20.3 months (Strom and Levine, 2008). Microscopic denervation should only be considered if the patient has temporary relief of orchalgia with a spermatic cord block with 10 ml of 0.5% bupivacaine (Levine et al, 1996).
To mobilize the spermatic cord, microsurgical denervation is performed by the same approach as microscopic subinguinal ligation of the spermatic veins for varicocele repair. Microscopic transection of all branches of the genitofemoral nerve along the spermatic cord is performed while preserving the testicular artery, the vas deferens, the vasal vessels, and some of the lymphatics (Fig. 37–15). This procedure can also be performed laparoscopically (Cadeddu et al, 1999). If fertility is not an issue of concern, it is recommended to divide the vas deferens as well to eliminate the sympathetic innervation, which may contribute to orchalgia by a sympathetic dystrophy component (Levine et al, 1996).
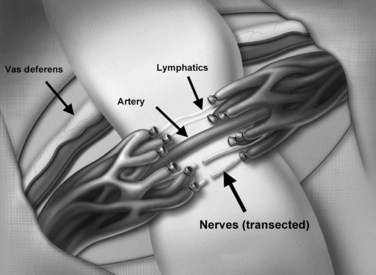
Figure 37–15 Microsurgical denervation. The goal is to transect all branches of the genitofemoral nerve while preserving the vas deferens, vasal vessels, testicular artery, and lymphatics.
Large, clinically palpable varicoceles can cause orchalgia. The pain typically improves with the patient in the supine position, as the varicocele decompresses. In a small series of men who underwent microscopic varicocelectomy for clinically palpable varicoceles with concomitant orchalgia, just over 50% had resolution of pain and 90% had improvement (Chawla et al, 2005). Higher success rates were reported in a previous, larger-powered, retrospective study (Peterson et al, 1998). Smaller, nonclinical varicoceles are unlikely to cause orchalgia and should be managed conservatively.
Retractile testes are another source of scrotal pain in the adult male. A careful history must be elicited to make this diagnosis, as the patient may not demonstrate a retractile testis on examination. The history is consistent with orchalgia only when the testis retracts up toward the external inguinal ring. This is typically seen in younger men with hyperactive cremasteric reflexes. Orchiopexy can be performed in these patients as it would in patients with intermittent torsion (Forte et al, 2003). When orchiopexy is offered, it should be performed by a dartos pouch technique to prevent retraction, as is recommended for testicular torsion (Redman and Barthold, 1995).
The other surgical treatment for orchalgia secondary to the retractile testis is to perform microscopic release of the cremasteric muscle. This is performed in a similar manner to microscopic subinguinal varicocelectomy. The spermatic cord is mobilized and isolated and the cremasteric muscle is divided circumferentially while preserving the vasculature of the spermatic cord and the vas deferens. This effectively releases the spermatic cord, thus not allowing retraction of the testis with hypercontraction of the cremasteric muscles.
Key Points: Surgery of the Scrotal Contents
Surgery of the Seminal Vesicles
In 1561, Gabriele Fallopius, a renowned Italian anatomist and physician, first described the seminal vesicles as paired male organs. He was considered an authority in the field of sexuality and advocated the use of condoms to decrease the transmission of syphilis. There was a great deal of interest in the seminal vesicles in the late 19th century due to their discovered involvement with inflammatory diseases (Brewster, 1985).
Seminal vesicle secretions contribute 50% to 80% of the volume of the ejaculate. The pH of the secreted fluid is neutral to slightly alkaline, and the mean volume is approximately 2.5 mL. The secreted fluid contains fructose and other carbohydrates necessary for sperm motility. It also contains a coagulation factor and prostaglandins A, B, E, and F (Tauber et al, 1975).
Primary disease processes of the seminal vesicles are very rare, although secondary processes are seen more commonly. Diagnosis of such entities has improved over the years with advanced imaging. Surgical access and management of seminal vesicle pathology can be difficult for the urologist due to the anatomic location.
Anatomy
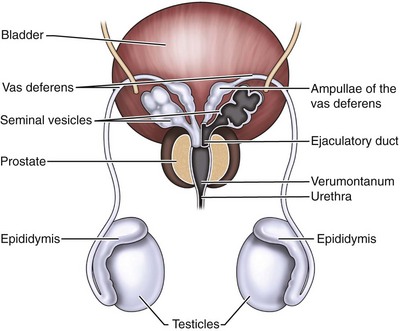
The seminal vesicles are paired male organs with no equivalent in the female. It is useful to understand the developmental anatomy of the seminal vesicles to gain a full understanding of the anatomy in the adult patient. The seminal vesicles begin as bilateral dorsolateral bulbous dilations of the distal mesonephric ducts between 12 and 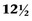 weeks of gestation. By 13 weeks, these dilations have enlarged and the ejaculatory ducts are beginning to form in the developing prostate (Brewster, 1985). The seminal vesicle and the ampulla of the vas deferens join posterior and superior to the prostate to form the ejaculatory duct (Nguyen et al, 1996). By the early portion of the seventh month, the seminal vesicle has multiple outpouchings and a widened main central lumen (Fig. 37–16) (Brewster, 1985).
weeks of gestation. By 13 weeks, these dilations have enlarged and the ejaculatory ducts are beginning to form in the developing prostate (Brewster, 1985). The seminal vesicle and the ampulla of the vas deferens join posterior and superior to the prostate to form the ejaculatory duct (Nguyen et al, 1996). By the early portion of the seventh month, the seminal vesicle has multiple outpouchings and a widened main central lumen (Fig. 37–16) (Brewster, 1985).
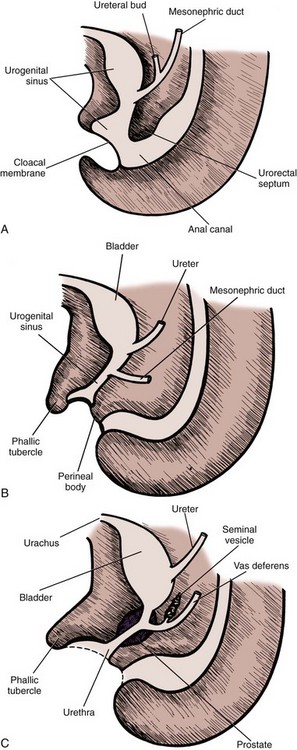
Figure 37–16 Intrauterine (fetal) development of the seminal vesicles. A, Fifth week. B, Eighth week. C, Thirteenth week.
(Redrawn from Langman J. Medical embryology. 4th ed. Baltimore: Williams & Wilkins; 1981. p. 242–3.)
The adult seminal vesicle measures 5 to 6 cm in length and 3 to 5 cm in diameter with a volume capacity of 13 cm, although they decrease in size as men age (Redman, 1987). At the terminal portion of the vas deferens within the prostate, the major lumen of the seminal vesicle empties into the ejaculatory duct. The ejaculatory duct is in continuity with the seminal vesicle but does not share the thick muscular wall of the seminal vesicle (Fig. 37–17) (Nguyen et al, 1996). The arterial supply to the seminal vesicle is from the vesiculodeferential artery, branching off from the umbilical artery (Braithwaite, 1952). Venous drainage of the seminal vesicle follows the arterial supply draining through the vesiculodeferential veins and the inferior vesicle plexus. Innervation of the seminal vesicles is by the hypogastric nerve (adrenergic and cholinergic) and the pelvic nerve. Lymphatic drainage of the seminal vesicles is through the internal iliac nodes (Mawhinney and Tarry, 1991).
Congenital Anatomic Anomalies of the Seminal Vesicles
The incidence of unilateral seminal vesicle agenesis is 0.6% to 1%. Unilateral seminal vesicle agenesis may be associated with ipsilateral renal anomalies and unilateral absence of the vas deferens. This is thought to be secondary to an embryological insult at the seventh week of gestation prior to the separation of the ureteral bud from the mesonephric duct. If this insult occurs after the seventh week, it is unlikely that renal agenesis would be associated with the seminal vesicle agenesis (Hall and Oates, 1993). It is common to find bilateral absence of the seminal vesicles along with congenital bilateral absence of the vas deferens, which is typically associated with a cystic fibrosis transmembrane receptor mutation. Of these men, 70% to 80% carry the genetic mutation associated with cystic fibrosis (Anguiano et al, 1994; Chillon et al, 1995). Treatment is only necessary when fertility becomes an issue.
Infectious Processes of the Seminal Vesicles
Infection of the seminal vesicles is seen in underdeveloped countries more frequently than in the United States. The causative agents are Mycobacterium tuberculosis and Schistosoma haematobium. The diagnosis of bacterial seminal vesiculitis can be made by transrectal or perineal needle aspiration. Surgery is not usually indicated, and culture-specific systemic antibiotics are the treatment of choice (Gutierrez et al, 1994). Very rarely, seminal vesiculectomy is necessary to prevent recurrent bacteremia or to eliminate persistent symptoms (Indudhara et al, 1991). Seminal vesicle abscesses are rare, but have been associated with diabetes mellitus, chronic indwelling catheters, and endoscopic instrumentation (Gutierrez et al, 1994). Management of seminal vesicle abscesses is discussed below along with management of seminal vesicle cysts. Infection, obstruction, or the combination of the two can result in calculi formation in the seminal vesicles. Patients with seminal vesicle calculi present with hematospermia, perineal pain, painful ejaculation, and infertility. These stones can be managed through an open or laparoscopic vesiculectomy, or the stone can be retrieved endoscopically using a small-caliber ureteroscope (Ozgök et al, 2005; Cuda et al, 2006; Han et al, 2008).
Evaluation of Abnormalities of the Seminal Vesicles
Normal seminal vesicles are not palpable on digital rectal examination. When a seminal vesicle cyst is present, the area immediately above the prostate may be compressible on digital rectal examination. This same area may feel firm or solid when a seminal vesicle tumor is present.
Semen analysis revealing a low seminal volume and a lack of liquefaction and fructose may indicate ejaculatory duct obstruction or absence of seminal vesicles (Goldstein and Schlossberg, 1988).
High-resolution transrectal ultrasonography (TRUS) has become the mainstay of imaging for the diagnostic evaluation of seminal vesicle pathology, because it is a reliable and inexpensive imaging modality. On TRUS, the seminal vesicles can be found just superior to the prostate, between the bladder and the rectum, and can be well visualized in the anteroposterior and sagittal views. The normal seminal vesicles should appear as flat, elongated, paired structures in the above described positions (Fig. 37–18). Along with the seminal vesicles, the ampullae of the vas deferens, the ejaculatory ducts within the prostate, and the verumontanum can be imaged and evaluated by TRUS. Abnormalities such as seminal vesicle obstruction, aplasia, atrophy, and cyst formation can be identified by TRUS (Carter et al, 1989).
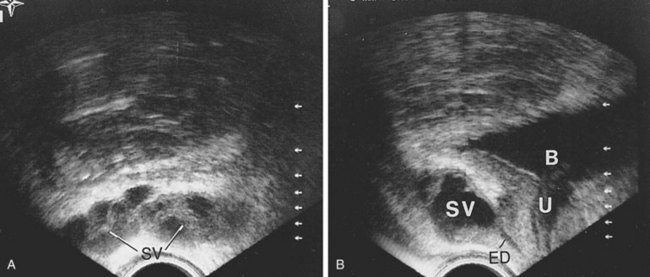
Figure 37–18 Transrectal ultrasound examination of normal seminal vesicles. A, Transverse view. B, Sagittal view. B, bladder; ED, ejaculatory duct; SV, seminal vesicle; U, urethra.
Seminal vesicle obstruction may result in seminal vesicle dilation identifiable by TRUS with the following characteristics: anteroposterior diameter of greater than 15 mm, length greater than 35 mm, and large anechoic areas, which contain sperm when aspirated (Jarow, 1996; Colpi et al, 1997). Asymptomatic cystic dilation of the seminal vesicle was incidentally found in 5% of men undergoing TRUS for prostate cancer screening (Wessels et al, 1992).
TRUS revealing hyperechoic solid masses in the seminal vesicles (isoechoic to the prostate) are concerning for tumor. If there is a unilateral solid mass in the seminal vesicle, it is more likely to be a primary tumor, whereas if it is present in bilateral seminal vesicles, it is more likely to be a secondary tumor from a primary prostate, rectal, or bladder malignancy. TRUS-guided biopsy or aspiration is necessary to assist in the diagnosis.
Computed tomography (CT) has also been used to evaluate the seminal vesicles. The normal appearance of the seminal vesicles on CT is that of paired structures just below the bladder with medium contrast similar to muscle (Goldstein and Schlossberg, 1988). A seminal vesicle cyst imaged by CT scan appears as a well-defined retrovesicular fluid density with the attenuation of water, from 0 to 10 Hounsfield units, cephalad to the prostate gland (Fig. 37–19). CT accurately images seminal vesicle anomalies, and is a good modality to allow for imaging of the ipsilateral kidney concomitantly (Arora et al, 2007).
In cases of primary seminal vesicle malignancy, CT scan may be useful to further characterize the lesion prior to intervention. A tumor within the seminal vesicle on CT has a higher attenuation than the normal seminal vesicle, but the tumor may appear cystic secondary to tumor necrosis (King et al, 1989). It is not possible to differentiate malignant tumors from benign ones on CT scan alone, although secondary tumors from the bladder, rectum, or prostate may have a more contiguous appearance (Sussman et al, 1986). The CT findings of a leiomyosarcoma of the seminal vesicle are described as an irregular mass, resulting in enlargement of the seminal vesicle with displacement of the prostate gland (Upreti et al, 2003). CT, as well as magnetic resonance imaging (MRI), allows metastatic evaluation when seminal vesicle neoplasms are further characterized (Dahms et al, 1999).
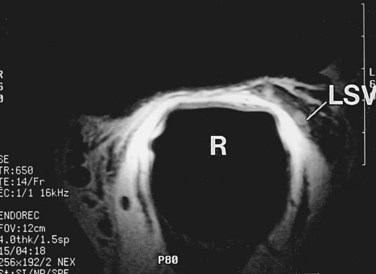
MRI is another useful imaging modality for the seminal vesicles, which gives more anatomic detail than CT. On T2-weighted images, the ampulla of the vas deferens is visible approximately 71% of the time and exhibits low signal intensity. The seminal vesicles exhibit high signal intensity 79% of the time, low signal intensity 19% of the time, and a heterogeneous signal intensity 2% of the time on T2-weighted images (Roy et al, 1993). On T2-weighted images, the seminal vesicles generally have similar or higher intensity than fat in patients less than 70 years of age, and typically have signal intensity lower than that of fat in patients greater than 70 years of age. The convolutions of the seminal vesicles can be seen on T1-weighted imaging with contrast (Fig. 37–20) (Secaf et al, 1991).
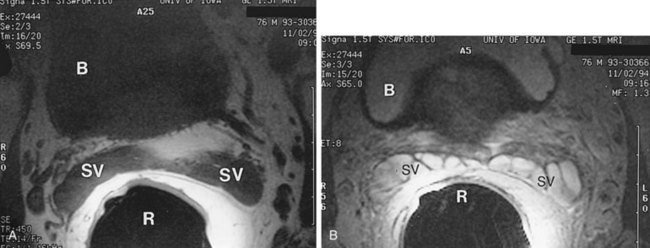
Figure 37–20 Transaxial MRI of normal seminal vesicles (SV) with endorectal coli. A, T1-weighted image. B, T2-weighted image. B, bladder; R, rectum.
On MRI, seminal vesicle agenesis is best exemplified on T1-weighted axial images (Fig. 37–21). Care must be taken to not mistake the vesicoprostatic venous plexus for small glands. Arteriovenous malformations appear as large ectatic vessels adjacent to the lateral edge of the seminal vesicle. After androgen ablation, seminal vesicles demonstrate low signal intensity on T2-weighted images and appear small in size (Secaf et al, 1991). The appearance of seminal vesicles after pelvic radiation reveals a decrease in size in a third of the patients. The seminal vesicles have a normal MRI appearance in 63% of the patients after pelvic radiation, 21% had normal signal intensity but had fewer tubules, 8% had diffuse loss of signal intensity appearing hypointense to fat, and 8% were hypointense to fat on T2-weighted images (Chan and Kressel, 1991). A seminal vesicle cyst may have variable signal intensities on T1-weighted images but typically demonstrates fluid signal intensities on T2-weighted images and does not enhance with the administration of intravenous gadolinium. Increased T1-weighted intensity represents increased proteinaceous concentration within the cyst or hemorrhage (Arora et al, 2007). Hemorrhagic seminal vesicle cysts have high signal intensity on T1- and T2-weighted images (Sue et al, 1989). A benign primary mass of the seminal vesicle appears as a sharply marginated mass arising from the seminal vesicle. The most common form of malignancy affecting the seminal vesicle is invasion of prostate cancer directly into the seminal vesicle. This can make the seminal vesicle appear large but does not always do so, and the seminal vesicle has low signal intensity on T2-weighted images (Secaf et al, 1991). MRI is not necessary to make the diagnosis in cases with a seminal vesicle abnormality. If there is a palpable seminal vesicle abnormality, TRUS should be performed with biopsy if there is suspicion for malignancy. MRI is helpful with hemorrhagic lesions, and body imaging is useful for staging.
Surgical Approaches to the Seminal Vesicles
The surgical approach to the seminal vesicles is mainly dependent on the expertise and comfort of the surgeon, although the characteristics of the lesion may impact the decision on the approach.
Preoperative preparation for seminal vesicle surgery should include a bowel preparation the evening before surgery, in case of the uncommon occurrence of a bowel injury; GoLYTELY is recommended. A prophylactic systemic antibiotic is administered preoperatively, and two doses are given postoperatively. The use of intermittent compression stockings to prevent deep vein thrombosis during surgery is recommended.
Anterior Surgical Approaches to the Seminal Vesicles
The anterior surgical approach to the seminal vesicles has been well established and is a good open approach for patients with large benign masses or cysts, and for patients with an ectopic ureter draining into a seminal vesicle cyst, so the kidney, ureter, and seminal vesicle can all be approached concomitantly.
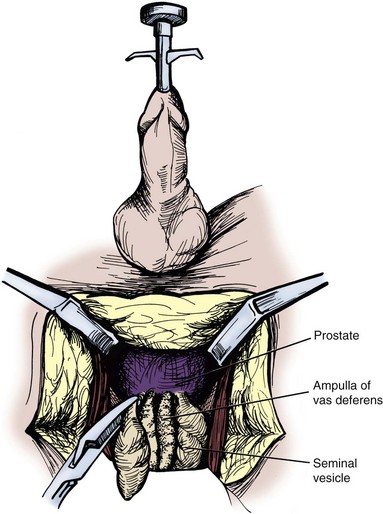
A popular open anterior approach is the transvesicle approach, which has been well described (Walker and Bowles, 1968; Politano et al, 1975). A lower midline infraumbilical incision is made sharply, and the rectus muscles are divided in the midline. The space of Retzius is developed. The anterior bladder is exposed, and a self-retaining retractor is placed. A longitudinal incision, about 7 to 10 cm long, is made in the anterior bladder wall, taking care to stay at least 3 to 4 cm proximal to the bladder neck. Moist sponges are placed in the bladder dome, and a bladder blade for the retractor is used to gently offer exposure. The ureteral orifices should be identified, and 8-Fr feeding tubes can be passed gently up the ureters to help with identification of the intramural ureters. A 5-cm longitudinal incision is made in the midline of the trigone with electrocautery on cutting current. Once the incision goes through the posterior bladder muscle, the ampullae of the vas deferens should be visible just below the bladder neck. The seminal vesicles should be identified just lateral to the ampullae of the vas deferens on the prostatic base. The seminal vesicles should be dissected free completely. Metal clips are placed on the vascular pedicle. A clip is placed on the proximal end of the vas deferens. A 2-0 chromic tie is used to ligate the end of the seminal vesicle at the prostate. The seminal vesicle is resected and removed. Care must be taken not dissect too deep through Denonvilliers fascia posteriorly, to not endanger the rectum. The posterior bladder wall is closed in two layers with a 2-0 absorbable suture in the muscle and a 4-0 absorbable suture in the mucosa. The sponges and ureteral catheters are removed, a 20-Fr urethral catheter is placed, and the anterior bladder wall is closed in the same fashion as the posterior bladder wall. A suction drain is placed in the perivesicle space, not overlying the suture line, and is brought out through a separate stab incision (Fig. 37–22). This drain can be removed when the output is less than 50 ml per day, typically in 2 to 3 days. The urethral catheter can be removed in 5 to 7 days. This approach has a lower rectal injury rate, although it places the ureters at a higher risk for injury and is more prone to blood loss.
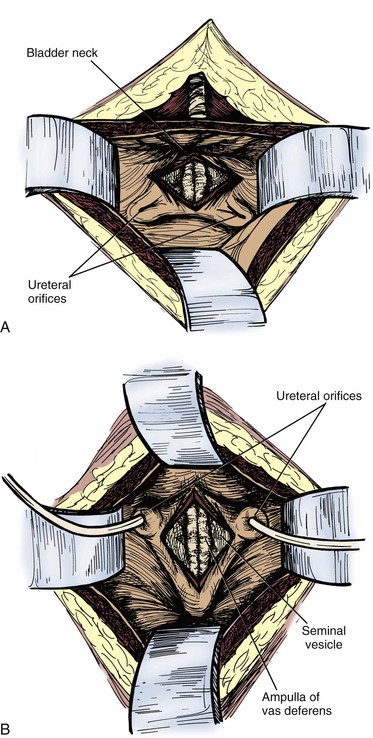
Figure 37–22 Transvesical approach to seminal vesiculectomy. A, Vertical incision between the ureteral orifices. B, Transverse incision 2 cm superior to the bladder neck below the ureteral orifices.
(Redrawn from Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989. p. 382.)
The perivesicle approach is useful in pediatric patients with a large seminal vesicle cyst, so that nephroureterectomy can be performed along with seminal vesiculectomy. A midline or Pfannenstiel incision will offer adequate exposure for this approach. Finger dissection is used to dissect the bladder from the lateral pelvic sidewall on the side with the cyst. The seminal vesicle cyst should be readily identifiable, the seminal vesicle should be dissected free in its entirety, and a 1-0 chromic can be placed through the cyst as a traction suture to assist with dissection. The ureter must be identified crossing the vas deferens to prevent ureteral injury. The superior, and possibly the inferior, vesicle arteries may be sacrificed to offer exposure to the base of the seminal vesicle. The cyst should be dissected away from the bladder, and any seminal vesicle vessels can be clipped and divided. When the base of the seminal vesicle is accessed at the prostate–seminal vesicle junction, it can be ligated with a 2-0 absorbable suture. Dissection of the proximal portion of the seminal vesicle must be done carefully by hugging the cyst, so not to injure the neurovascular bundle, which is directly lateral to the seminal vesicle. A clip is then placed just distal to the previously placed suture, and the seminal vesicle is resected. A suction drain is placed adjacent to the seminal vesicle bed and is brought out through a separate stab incision. The drain and urethral catheter may both be removed in 24 hours, as long as there is not excessive drainage through the drain.
A third anterior approach to the seminal vesicles is the retrovesicle approach and is useful for bilateral seminal vesiculectomies for bilateral small cysts or benign masses (de Assis, 1952). A urethral catheter should be placed, a midline incision is made, and the peritoneum is entered. A transverse incision is made in the peritoneal reflection over the rectum at the posterior bladder wall, taking great care to not enter the rectum. The posterior bladder is sharply dissected off the anterior rectal wall until the ampullae of the vas deferens and the tips of the seminal vesicles are visible. The seminal vesicles are dissected free to the base at the prostate–seminal vesicle junctions and are ligated and resected. A suction drain is placed at the posterior bladder and brought out through a separate stab incision. The incision is closed (Fig. 37–23).
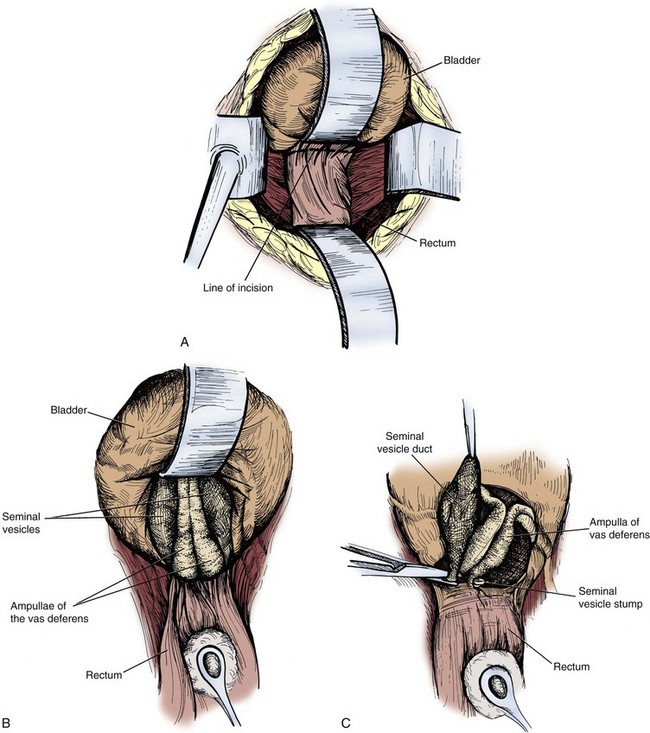
Figure 37–23 Retrovesical approach to seminal vesiculectomy. A, Incision line between base of bladder and peritoneal reflection over the rectum. B, Caudal dissection reveals the ampullae of the vas deferens on the midline and seminal vesicles immediately lateral to them. C, The duct of the seminal vesicle is ligated and transected.
(Redrawn from Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989. p. 379–80.)
Transperineal Surgical Approach to the Seminal Vesicles
The patient should be positioned as they would be for perineal prostatectomy. The same incision is made as would be for perineal prostatectomy, but the rectum needs to be dissected free high on the base of the prostate. A transverse incision is then made in Denonvilliers fascia above the base of the prostate–seminal vesicle junctions. This incision can be made longitudinally to allow better preservation of the neurovascular bundles for potency (Weldon and Tavel, 1988). A Lowsley retractor can be placed through the urethra into the bladder to elevate the prostate to provide traction to allow for easier dissection of the base of the seminal vesicle. Just below Denonvilliers fascia and above the prostate, the ampulla of the vas deferens can be dissected. If the surgery is being done for benign pathology, dissection can be carried laterally to the seminal vesicle. If it is being done for malignancy or recurrent infection, the ampulla of the vas deferens should be included in the specimen with a wider resection. The seminal vesicle should be dissected completely free, and the base should be ligated at the prostate–seminal vesicle junction with a 2-0 suture. A second tie or clip should be placed on the distal seminal vesicle, and the seminal vesicle should be divided. The divided end of the seminal vesicle is then grasped with an Allis clamp, and the Metzenbaum scissors are used in a spreading motion to free the rest of the seminal vesicle from surrounding tissue while using the Allis for traction. Metal clips are placed on the vascular pedicle and it is divided, typically within 1 cm of the distal tip of the seminal vesicle. The seminal vesicle is removed, a Penrose drain is left in the seminal vesicle bed, and the incision is closed in layers. The drain can be removed in 24 hours if there is minimal drainage. The transperineal approach offers minimal blood loss, minimal pain, and early ambulation (Fig. 37–24).
Posterior Surgical Approach to the Seminal Vesicles
Although the transcoccygeal approach is the least familiar to most urologists, it may be useful for patients who have had previous suprapubic or perineal surgeries. The patient is positioned in a prone, jackknife position (Kreager and Jordan, 1965). An L-shaped hockey stick incision is made from midway on the sacrum, 10 cm from the tip of the coccyx, and angled at the tip of the coccyx down the gluteal cleft stopping 3 cm from the anus. The lateral side of the coccyx is carefully divided free from the rectum and removed. The layers of the gluteus maximus are swept aside until the rectosigmoid is reached, and then it is carefully dissected from the underside of the sacrum. The lateral rectal wall is divided free medially from the levator ani muscle until the prostate is encountered on the side of the seminal vesicle pathology. Dissection is carried superior to the base of the prostate in the midline until the ampulla of the vas deferens is identified with the seminal vesicle just lateral to the ampulla. The seminal vesicle should be dissected and resected as previously described in the above approaches. A Penrose drain should be placed at the bed of the seminal vesicle and brought out through a separate stab incision from the closure. The drain can be removed in two to three days if there is no drainage (Fig. 37–25).
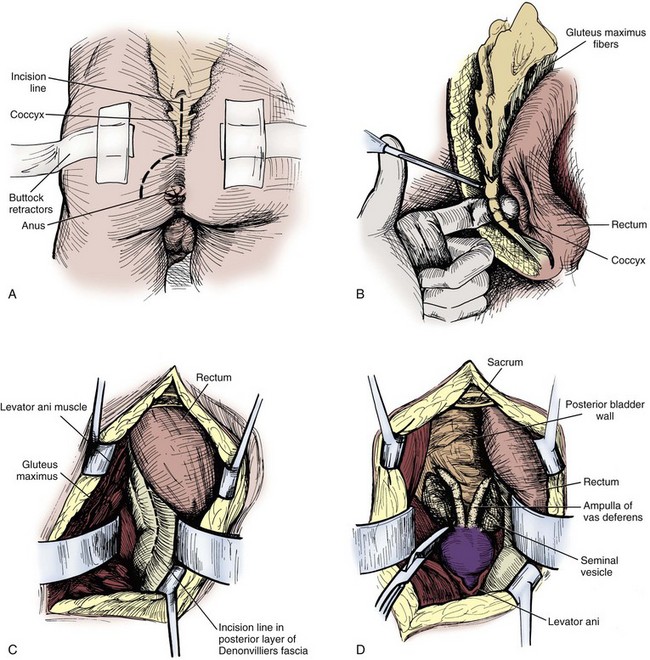
Figure 37–25 Transcoccygeal seminal vesiculectomy. A, Incision line over the lower sacrum on coccyx surrounding the anus. B, Dissection of the coccyx. C, Incision of Denonvilliers fascia after the rectum has been displaced. D, Exposure of the prostate and seminal vesicles.
(Redrawn from Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989. p. 381.)
Laparoscopic/Robot-Assisted Surgical Approach to the Seminal Vesicles
Laparoscopic surgery on the seminal vesicles is most commonly performed concomitantly with prostate surgery. The technique for laparoscopic approach to the seminal vesicles was first described in 1993 (Kavoussi et al, 1993). Laparoscopy has also been applied to seminal vesiculectomy without prostatectomy and has been reported in a case of amyloid of the seminal vesicle (Vandwalle et al, 2007). Robot-assisted laparoscopy has also been used to excise seminal vesicle cysts (Moore et al, 2007; Selli et al, 2008). Patients are positioned in the supine position with careful padding of all pressure points, and the arms are tucked and padded. A split-leg table is necessary for the robot-assisted technique. Wide cloth tape is used across the chest and hips to secure the patient to the table, and the table is placed in steep Trendelenburg position. Prior to gaining access, a urethral catheter and an orogastric tube should be placed to decompress the bladder and stomach for subsequent trochar placement. To gain access, the Varess needle is placed periumbilically and a pneumoperitoneum is achieved, not exceeding pressures of 15 mm Hg. After an adequate pneumoperitoneum is achieved, the laparoscopic ports can be placed, placing the first one with an optical trocar for the camera port, and the following ones under direct laparoscopic visualization. The ports can be placed in either a horseshoe or a diamond arrangement for pure laparoscopy and can be placed in the same position as would be for prostatectomy for robot assistance (Fig. 37–26) (Menon et al, 2003; Lee et al, 2004). The peritoneum is then incised between the two obliterated umbilical ligaments just anterior to the rectum in the pouch of Douglas. The seminal vesicles can then be visualized and should be dissected carefully to avoid injury to the neurovascular bundles or the surrounding viscera. Monopolar energy should not be used in order to minimize injury to surrounding structures, and much of this dissection can safely be performed sharply. The seminal vesicle arterial pedicle can be managed with a clip or with bipolar cautery. The seminal vesicle should be dissected toward its junction with the ampulla of the vas deferens, and both can be clipped together at the base. The specimen can then be placed in an extraction bag and can be removed through one of the laparoscopic ports. The ports are then closed under visualization.
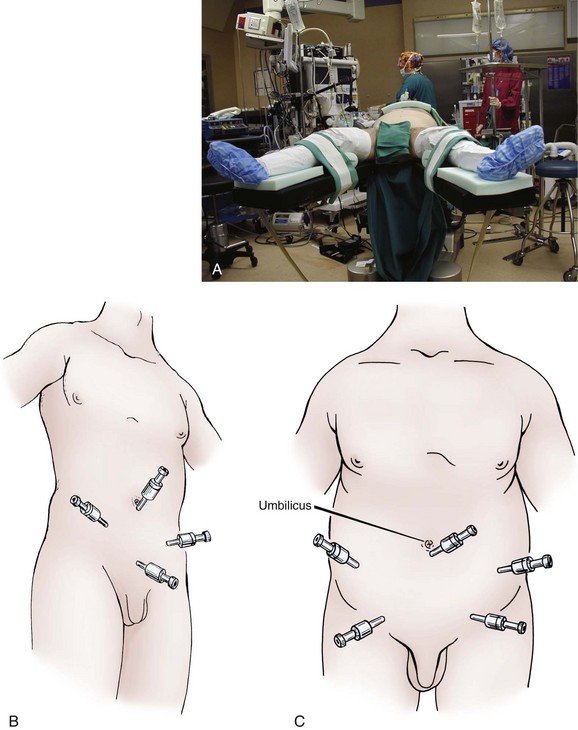
Figure 37–26 A, Positioning of the patient for laparoscopic seminal vesicle dissection. B, Diamond configuration of laparoscopic ports. C, Inverted U-shaped configuration of laparoscopic ports in obese patients.
(From Winifield HN. Laparoscopic pelvic lymph node dissection for urological pelvic malignancies. Atlas Urol Clin North Am 1993;1:33–47.)
The extraperitoneal laparoscopic approach to the seminal vesicles was first described in 1997, and was performed concomitantly with radical prostatectomy (Raboy et al, 1997). In the following years, this approach gained more popularity (Bollens et al, 2001; Stolzenburg et al, 2003). A 1.5-cm periumbilical incision is made, and the preperitoneal space is entered. A balloon trocar is introduced into the preperitoneal space, and insufflation is performed under direct vision. The details of the technique are further described in detail in Chapter 103.
Surgical Treatment of Seminal Vesicle Cysts
Seminal vesicle cysts are thought to be secondary to ejaculatory duct obstruction and can be congenital or acquired (Heaney et al, 1987; King et al, 1991; Conn et al, 1992). Seminal vesicle cysts are associated with ipsilateral renal agenesis or dysplasia in two thirds of patients; the cysts are secondary to maldevelopment of the distal mesonephric duct and are an error in ureteral budding (Beeby, 1974). Seminal vesicle cysts have also been associated with polycystic kidney disease. In one report, seminal vesicle cysts were identified in 60% of patients with polycystic kidney disease, and some authors recommend that all patients with seminal vesicle cysts undergo renal imaging (Alpern et al, 1991; Hihara et al, 1993; Danaci et al, 1998). Seminal vesicle cysts should only be treated if they are symptomatic or result in ejaculatory duct obstruction and affect fertility (Surya et al, 1988).
Seminal vesicle cysts can be drained by a number of techniques. TRUS-guided aspirations or transperineal aspirations can be performed on small seminal vesicle cysts that are symptomatic or obstructing the ejaculatory ducts. If the cyst reaccumulates fluid, resulting in recurrent symptoms or obstruction, it may be aspirated again with the injection of a sclerosing agent such as tetracycline. A small abscess in the seminal vesicle can be managed similarly with drainage (Frye and Loughlin, 1988; Shabsigh et al, 1989; Gutierrez et al, 1994). If the seminal vesicle cyst is proximal, adjacent to the prostate, transurethral resection can be performed to unroof the cyst at the 5-o’clock and 7-o’clock positions just distal to the bladder neck (Frye and Loughlin, 1988; de Lichtenberg and Hvidt, 1989). The same outcome has been reported by incising the seminal vesicle cyst to drain it cystoscopically with the use of a Collin knife (Gonzalez and Dalton, 1998). Seminal vesicle abscesses can be managed in a similar fashion. Some groups have reported using semirigid ureteroscopes to treat seminal vesicle cysts and abscesses (Razvi and Denstedt, 1995; Shimada and Yoshida, 1996; Okubo et al, 1998). If the above techniques for drainage of seminal vesicle cysts are unsuccessful, open or laparoscopic excision can be performed (Moudouni et al, 2006). Seminal vesiculectomy, along with nephroureterectomy should be performed in cases with an ectopic ureter. If the above techniques for seminal vesicle abscess fail, open drainage is required (Kore et al, 1994).
Surgical Treatment of Tumors of the Seminal Vesicles
Benign tumors of the seminal vesicle that occur more commonly than malignant ones include fibromas, leiomyomas, cystadenomas, schwannomas, and papillary adenomas (Mostofi and Price, 1973; Lundhus et al, 1984; Narayana, 1985; Mazur et al, 1987; Bullock, 1988; Gentile et al, 1994; Latchamsetty et al, 2002; Lee et al, 2006). Primary papillary adenomas and cystadenomas of the seminal vesicle typically occur in middle-aged men and are almost never bilateral, and they appear as simple cysts on imaging; therefore, the diagnosis is typically one made on final pathology after excision (Mazur et al, 1987). Amyloid localized to the seminal vesicles has also been reported (Jun et al, 2003). Twenty percent of men over the age of 76 have subepithelial deposits of amyloid in the seminal vesicles, and the reported incidence in male autopsies is 4% to 17% (Pitkanen et al, 1983; Ramchandani et al, 1993). Patients should only be treated if they are symptomatic and the diagnosis of amyloid of the seminal vesicle is made. Hydatid cysts of the seminal vesicle have also been reported (Kuyumcuoglu et al, 1991; Papathanasiou et al, 2006).
Seminal vesicle malignancies are extremely rare and are difficult to diagnose, because patients are typically asymptomatic until late in the course of the disease process. Primary malignancies of the seminal vesicles are extremely rare, and serum PSA and tissue biopsies can help differentiate primary malignancies from extension/metastasis of lymphoma, prostate, bladder, or rectal cancer. It is thought that the seminal vesicles low proliferative activity accounts for the low incidence of primary malignancies of the seminal vesicle (Meyer et al, 1982). Primary adenocarcinoma of the seminal vesicle occurs in patients greater than 50 years old. Serum PSA is normal and serum carcinoembryonic antigen is elevated (Mostofi and Price, 1973; Benson et al, 1984; Tanaka et al, 1987; Chinoy and Kulkarni, 1993; Thiel and Effert, 2002). Primary sarcoma of the seminal vesicle is an extremely rare malignancy, which is diagnosed by biopsy, and is usually discovered late in the course of the disease process (Benson et al, 1984; Chiou et al, 1985; Schned et al, 1986; Tanaka et al, 1987; Davis et al, 1988; Kawahara et al, 1988). All sarcoma types of the seminal vesicle, including leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, and müllerian adenosarcoma-like tumor, behave very aggressively, and radical extirpation has varying outcomes (Amirkhan et al, 1994; Lamont et al, 1991; Laurila et al, 1992; Berger et al, 2002). Cystosarcoma phylloides and seminoma have also been reported as primary malignancies of the seminal vesicles (Fain et al, 1993; Adachi et al, 1991). Primary squamous cell carcinoma of the seminal vesicle has been reported and treated with surgical extirpation followed by adjuvant radiation therapy with success with short-term follow-up (Tabata et al, 2002).
Solid seminal vesicle masses that are benign on biopsy and show no evidence of local spread should be treated with seminal vesiculectomy only if they are symptomatic, which is a very rare occurrence. Solid masses of the seminal vesicles that are proven to be malignant by biopsy or with a high suspicion for malignancy should be surgically treated, although the optimal treatment is still debated because there have been so few primary seminal vesicle malignancies treated at any institution. Large primary malignancies of the seminal vesicles have been treated with radical pelvic surgery: cystoprostatectomy with pelvic lymphadenectomy or pelvic exenteration. Adjuvant therapy has not proven to be effective, although the only survivors in the literature underwent radical surgery followed by pelvic radiation and/or androgen ablation.
Complications of Seminal Vesicle Surgery
Complications of seminal vesicle surgery are minimized by the surgeon selecting the approach that he or she is most comfortable and facile performing. The surgeon must be aware of the following seminal vesiculectomy specific complications. Rectal injury can occur with the perineal approach to the seminal vesicles. If a preoperative bowel preparation was administered and there is no gross fecal contamination, a two-layer closure of the rectum may be performed closing the mucosal layer with a running 3-0 absorbable suture, and the submucosal layer with interrupted 4-0 silks. The anus should also be dilated prior to the end of the case. A temporary colostomy should be considered with a large rectal injury or gross fecal contamination. The bladder may also be injured during the perineal approach. The bladder should be closed in two layers, leaving a urethral catheter in place for 7 to 10 days postoperatively. During the perineal approach, the ureter may be injured. If this occurs, the injury should be repaired, or a double-J ureteral stent may be placed and left in place for 10 to 14 days postoperatively. Hemorrhage is least likely in the perineal approach, and the transvesicle approach is more prone to blood loss.
Complications of the laparoscopic approach include general laparoscopic complications, such as trocar injury to the bowel or great vessels, extraperitoneal insufflation, abdominal wall bleeding, and gas embolism. Bladder and rectal injuries may be repaired laparoscopically, as long as there is not gross fecal contamination with rectal injuries. The distal ureter is close to the tip of the seminal vesicle, and, if the ureter is injured it may be repaired laparoscopically, although a ureteral reimplant may be necessary, and performed open or laparoscopically, depending on the experience of the surgeon. It is recommended that a ureteral stent and a pelvic drain be left in such cases. The neurovascular bundle runs just lateral to the tips of the seminal vesicles; therefore, injury to the neurovascular bundle can result in erectile dysfunction, regardless of the surgical approach. Potential complications of endoscopic management of the seminal vesicles include postvoid dribbling due to urinary reflux and infection (Goluboff et al, 1995).
Key Points: Surgery of the Seminal Vesicles
Brewster SF. The development and differentiation of human seminal vesicles. J Anat. 1985;143:45-55.
Carter SS, Shinohara K, Lipshultz LI. Transrectal ultrasonography in disorders of the seminal vesicles and ejaculatory ducts. Urol Clin North Am. 1989;16:773-790.
Corcoran AT, Smaldone MC, Gibbons EP, et al. Validation of the Fournier’s gangrene severity index in a large contemporary series. J Urol. 2008;180(3):944-948.
Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol. 1990;143:936-939.
Goldstein M, Schlossberg S. Men with congenital absence of the vas deferens often have seminal vesicles. J Urol. 1988;140(1):85-86.
Holt SK, Salinas CA, Stanford JL. Vasectomy and the risk of prostate cancer. J Urol. 2008;180(6):2565-2567. discussion 2567–8
Kavoussi LR, Schuessler WW, Vancaillie TG, et al. Laparoscopic approach to the seminal vesicles. J Urol. 1993;150:417-419.
Kiddoo DA, Wollin TA, Mador DR. A population based assessment of complications following outpatient hydrocelectomy and spermatocelectomy. J Urol. 2004;171(2 Pt. 1):746-748.
Mittemeyer BT, Lennox KW, Borski AA. Epididymitis: a review of 610 cases. J Urol. 1966;95:390-392.
Selikowitz S, Schned A. A late post-vasectomy syndrome. J Urol. 1985;134:494.
Sokal D, Irsula B, Hays M, et al. Vasectomy by ligation and excision, with or without fascial interposition: a randomized controlled trial, Investigator Study Group. BMC Med, 2004;2, 6.
Sokal D, McMullen S, Gates D, Dominik R, the Male Sterilization Investigator Team. A comparative study of the no scalpel and standard incision approaches to vasectomy in 5 countries. J Urol. 1999;162:1621-1625.
Adachi Y, Rokujyo M, Kojma H, et al. Primary seminoma of the seminal vesicle: report of a case. J Urol. 1991;146:857-859.
Alexander JW, Fischer JE, Boyajian M, et al. The influence of hair-removal methods on wound infections. Arch Surg. 1983;118:347-352.
Alpern MB, Dorfman RE, Gross BH, et al. Seminal vesicle cysts: association with adult polycystic kidney disease. Radiology. 1991;180:79-80.
Alvarez Maestro M, Tur Gonzalez R, Alonso Dorrego JM, et al. Adenomatoid tumors of the epididymis and testicle: report of 9 cases and bibliographic review. Arch Esp Urol. 2009;62(2):137-411.
American Urological Association. Policy statement on vasectomy and prostate cancer. www.auanet.org/content/guidelines-and-quality-care/policy-statements/v/vasectomy-and-prostate-cancer.cfm, 2007. [accessed 23.05.11]
Amirkhan RH, Molberg KH, Wiley EL, et al. Primary leiomyosarcoma of the seminal vesicle. Urology. 1994;44:132-135.
Anguiano A, Oates RD, Amos JA, et al. Congenital bilateral absence of the vas deferens: A primarily genital form of cystic fibrosis. JAMA. 1994;267:1794-1797.
Appell R, Evans P. Vasectomy: etiology of infectious complications. Fertil Steril. 1980;33:52-53.
Aradhya KW, Best K, Sokal DC. Recent developments in vasectomy. BMJ. 2005;330:296-299.
Arbuliev MG, Arbuliev KM, Gadzhiev DP, Abunimekh BKH. [Diagnosis and treatment of acute epididymoorchitis. Urologiia. 2008;May–Jun(3):49-52.
Arora SS, Breiman RS, Webb EM, et al. CT and MRI of congenital anomalies of the seminal vesicles. AJR Am J Roentgenol.. 2007;189(1):130-135.
Awsare NS, Krishnan J, Boustead GB, et al. Complications of vasectomy. Ann R Coll Surg Engl. 2005;87(6):406-410.
Ban SL. Sterility by vas injection method. Hu Nan Med J. 1980;5:49-50.
Barone MA, Irsula B, Chen-Mok M, Sokal DC, Investigator Study Group. effectiveness of vasectomy using cautery. BMC Urol. 2004;4:10.
Barone MA, Hutchinson PL, Johnson CH, et al. Vasectomy in the United States, 2002. J Urol. 2006;176(1):232-236.
Beccia DJ, Krane RJ, Olsson CA. Clinical management of non-testicular intrascrotal tumors. J Urol. 1976;116(4):476-479.
Beeby DI. Seminal vesicle cyst associated with ipsilateral renal agenesis: case report and review of literature. J Urol. 1974;112:120-122.
Beiko DT, Kim D, Morales A. Aspiration and sclerotherapy versus hydrocelectomy for treatment of hydroceles. Urology. 2003;61(4):708-712.
Bennett AH. Vasectomy without complication. Urology. 1976;7:184-185.
Benson RCJr, Clark WR, Farrow GM. Carcinoma of the seminal vesicle. J Urol. 1984;132:483-485.
Berger AP, Bartsch G, Horninger W. Primary rhabdomyosarcoma of the seminal vesicle. J Urol. 2002;168(2):643.
Bhatnagar AM, Mohite PN, Suthar M. Fournier’s gangrene: a review of 110 cases for aetiology, predisposing conditions, microorganisms, and modalities for coverage of necrosed scrotum with bare testes. N Z Med J. 2008;121(1275):46-56.
Birch BR, Miller RA. Walk-in, walk-out day case genito-scrotal surgery with sedation reversal. A survey of patient attitudes and morbidity. Br J Urol. 1994;74(5):658-664.
Boileau MA, Steers WD. Testis tumors: the clinical significance of the tumor-contaminated scrotum. J Urol. 1984;132(1):51-54.
Bollens R, Vanden Bossche M, Roumeguere T, et al. Extraperitoneal laparoscopic radical prostatectomy. Results after 50 cases. Eur Urol. 2001;40:65-69.
Braithwaite JL. The arterial supply of the male urinary bladder. Br J Urol. 1952;24:64-71.
Breda G, Giunta A, Gherardi L, et al. Treatment of hydrocele: randomised prospective study of simple aspiration and sclerotherapy with tetracycline. Br J Urol. 1992;70(1):76-77.
Brewster SF. The development and differentiation of human seminal vesicles. J Anat. 1985;143:45-55.
Bullock KN. Cystadenoma of the seminal vesicle. J R Soc Med. 1988;81:294-295.
Cadeddu JA, Bishoff JT, Chan DY, et al. Laparoscopic testicular denervation for chronic orchalgia. J Urol. 1999;162:733-735.
Calleary JG, Masood J, Hill JT. Chronic epididymitis: is epididymectomy a valid surgical treatment? Int J Androl. 2009;32:468-472.
Carter SS, Shinohara K, Lipshultz LI. Transrectal ultrasonography in disorders of the seminal vesicles and ejaculatory ducts. Urol Clin North Am. 1989;16:773-790.
Chan TW, Kressel HY. Prostate and seminal vesicles after irradiation: MR appearance. J Magn Reson Imaging. 1991;1(5):503-511.
Chawla A, Kulkarni G, Kamal K, Zini A. Microsurgical varicocelectomy for recurrent or persistent varicoceles associated with orchalgia. Urology. 2005;66(5):1072-1074.
Chen TF, Ball RY. Epididymectomy for post vasectomy pain: histological review. Br J Urol. 1991;68:407-413.
Chen KC, Peng CC, Hsieh HM, Chiang HS. Simply modified no-scalpel vasectomy (percutaneous vasectomy)—a comparative study against the standard no-scalpel vasectomy. Contraception. 2005;71(2):153-156.
Chillon M, Casals T, Mercier B, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475-1480.
Chinoy RF, Kulkarni JN. Primary papillary adenocarcinoma of the seminal vesicle. Indian J Cancer. 1993;30:82-84.
Chiou RK, Limas C, Lange PH. Hemangiosarcoma of the seminal vesicle: case report and literature review. J Urol. 1985;134:371-373.
Coady SA, Sharrett AR, Zheng ZJ, et al. Vasectomy, inflammation, atherosclerosis and long-term followup for cardiovascular diseases: no associations in the atherosclerosis risk in communities study. J Urol. 2002;167(1):204-207.
Colpi GM, Negri L, Nappi RE, et al. Is transrectal ultrasonography a reliable diagnostic approach in ejaculatory duct sub-obstruction? Hum Reprod. 1997;12:2186-2191.
Conn IG, Peeling WB, Clements R. Complete resolution of a large seminal vesicle cyst: evidence for an obstructive aetiology. Br J Urol. 1992;69:636-639.
Cook JL, Dewbury K. The changes seen on high-resolution ultrasound in orchitis. Clin Radiol. 2000;55:13-18.
Cook LA, Pun A, van Vliet H, et al. Scalpel versus no-scalpel incision for vasectomy. Cochrane Database Syst Rev 2007a;(2):CD004112.
Cook LA, van Vliet H, Lopez LM, et al. Vasectomy occlusion techniques for male sterilization. Cochrane Database Syst Rev 2007b;(2):CD003991.
Cooper A. The anatomy and surgical treatment of abdominal hernia. London: Longman; 1827.
Corcoran AT, Smaldone MC, Gibbons EP, et al. Validation of the Fournier’s gangrene severity index in a large contemporary series. J Urol. 2008;180(3):944-948.
Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol. 1991;146:1571-1574.
Cuda SP, Brand TC, Thibault GP, Stack RS. Case report: endoscopic laser lithotripsy of seminal-vesicle stones. J Endourol. 2006;20(11):916-918.
Dahms SE, Hohenfellner M, Linn JF, et al. Retrovesical mass in men: pitfalls of differential diagnosis. J Urol. 1999;161(4):1244-1248.
Danaci M, Akpolat T, Bastemir M, et al. The prevalence of seminal vesicle cysts in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1998;13:2825-2828.
Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol. 1990;143:936-939.
Davis NS, Merguerian PA, DiMarco PL, et al. Primary adenocarcinoma of seminal vesicle presenting as bladder tumor. Urology. 1988;32:466-468.
Davis BE, Noble MJ. Analysis and management of chronic orchalgia. AUA Update Series. 1992;11:lesson 2.
de Assis JS. Seminal vesiculectomy. J Urol. 1952;68:747-753.
de Diego Rodríguez E, Correas Gómez MA, Martín García B, et al. Fournier’s gangrene after vasectomy. Arch Esp Urol. 2000;53(3):275-278.
de Lichtenberg MH, Hvidt V. Transurethral, transprostatic incision of a seminal vesicle cyst. Scand J Urol Nephrol. 1989;23:303-305.
Delavierre D. Orchi-epididymitis. Ann Urol (Paris). 2003;37(6):322-338.
Esho JO, Cass AS. Recanalization rate following methods of vasectomy using interposition of fascial sheath of vas deferens. J Urol. 1978;120:178-179.
Fain JS, Cosnow I, King BF, et al. Cystosarcoma phyllodes of the seminal vesicle. Cancer. 1993;71:2055-2061.
Forte F, Bitelli M, Sorrenti S, et al. Testicular fixation in adult retractile testis: technical notes. Chir Ital. 2003;55(1):145-147.
Franco de Castro A, Truhán D, Carretero González P, Alcover García J. [Nd-YAG laser photocoagulation of scrotal sebaceous cysts]. Actas Urol Esp. 2002;26(2):121-123.
Frye K, Loughlin K. Successful transurethral drainage of bilateral seminal vesicle abscesses. J Urol. 1988;139:1323-1324.
Fuchs EF, Alexander N. Immunologic considerations before and after vasovasostomy. Fertil Steril. 1983;40:497-499.
Gentile AT, Moseley HS, Quinn SF, et al. Leiomyoma of the seminal vesicle [review]. J Urol. 1994;151(4):1027-1029.
Ghnnam WM. Fournier’s gangrene in Mansoura Egypt: a review of 74 cases. J Postgrad Med. 2008;54(2):106-109.
Giguere JK, Stablein DM, Spaulding JT, et al. The clinical significance of unconventional orchiectomy approaches in testicular cancer: a report from the Testicular Cancer Intergroup Study. J Urol. 1988;139(6):1225-1228.
Giovannucci E, Ascherio A, Rimm EB, et al. A prospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269:873-877.
Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancer and cardiovascular disease after vasectomy: an epidemiological database study. Fertil Steril. 2005;84(5):1438-1443.
Goldstein M. Vasectomy failure using an open-ended technique. Fertil Steril. 1983;40:699-700.
Goldstein M, Schlossberg S. Men with congenital absence of the vas deferens often have seminal vesicles. J Urol. 1988;140(1):85-86.
Goldstein M, Waterhouse K. When to use the Chevassu maneuver during exploration of intrascrotal masses. J Urol. 1983;130:1199-1200.
Goluboff ET, Kaplan SA, Fisch H. Seminal vesicle urinary reflux as a complication of transurethral resection of the ejaculatory ducts. J Urol. 1995;153:1234-1235.
Gonzalez CM, Dalton DP. Endoscopic incision of a seminal vesicle cyst. Urology. 1998;51:831.
Griffin T, Tooher R, Nowakowski K, et al. How little is enough? The evidence for post-vasectomy testing. J Urol. 2005;174(1):29-36.
Gudaviciene D, Milonas D. Scrotal reconstruction using thigh pedicle flaps after scrotal skin avulsion. Urol Int. 2008;81(1):122-124.
Gupta A, Dalela D, Sankhwar SN, et al. Bilateral testicular gangrene: does it occur in Fournier’s gangrene? Int Urol Nephrol. 2007;39(3):913-915.
Gupta N, Rajwanshi A, Srinivasan R, Nijhawan R. Fine needle aspiration of epididymal nodules in Chandigarh, north India: an audit of 228 cases. Cytopathology. 2006;17(4):195-198.
Gutierrez R, Carrere W, Llopis J, et al. Seminal vesicle abscess: two case reports and a review of the literature. Urol Int. 1994;52:45-47.
Hall S, Oates RD. Unilateral absence of the scrotal vas deferens associated with contralateral mesonephric duct anomalies resulting in infertility: laboratory, physical and radiographic findings, and therapeutic alternatives. J Urol. 1993;150:1161-1164.
Han P, Yang YR, Zhang XY, Wei Q. Laparoscopic treatment of a calcium fluorophosphate stone within a seminal vesicle cyst. Asian J Androl. 2008;10(2):337-340.
Healy B. From the National Institutes of Health. Does vasectomy cause prostate cancer? JAMA. 1993;269:2620.
Heaney JA, Pfister RC, Meares EMJr. Giant cyst of the seminal vesicle with renal agenesis. Am J Radiol. 1987;149:139-140.
Hellström P, Malinen L, Kontturi M. Sclerotherapy for hydroceles and epididymal cysts with ethanolamine oleate. Ann Chir Gynaecol. 1986;75(1):51-54.
Hihara T, Ohnishi H, Muraishi O, et al. MR imaging of seminal vesicle cysts associated with adult polycystic kidney disease. Radiat Med. 1993;11:24-26.
Holt SK, Salinas CA, Stanford JL. Vasectomy and the risk of prostate cancer. J Urol. 2008;180(6):2565-2567. discussion 2567–8
Huber DH. The no-scalpel vasectomy: a new technique. AVSC News. 1988;26(1):1-2.
Indudhara R, Das K, Sharma M, et al. Seminal vesiculitis due to Mycobacterium gastri leading to male infertility. Urol Int. 1991;46:99-100.
Jaboulay M. Chirurgie des centers nerveux, des visceres et des member, vol II. Storck, Lyon, France, 1902. p. 192
Jarow JP. Transrectal ultrasonography in the diagnosis and management of ejaculatory duct obstruction. J Androl. 1996;17:467-472.
Johnson AL, Howards SS. Intratubular hydrostatic pressure in testis and epididymis before and after vasectomy. Am J Physiol. 1975;228:556-564.
Johnson DE, Babaian RJ. The case for conservative surgical management of the ilioinguinal region after inadequate orchiectomy. J Urol. 1980;123(1):44-46.
Jun SY, Kim KR, Cho KS, Ro JY. Localized amyloidosis of seminal vesicle and vas deferens: report of two cases [review]. J Korean Med Sci. 2003;18(3):447-451.
Kavoussi PK, Bird ET. Repair of a scrotal wound defect after Fournier’s gangrene: a novel approach. Issues Urol. 2007;19(2):75-77.
Kavoussi LR, Schuessler WW, Vancaillie TG, et al. Laparoscopic approach to the seminal vesicles. J Urol. 1993;150:417-419.
Kawahara M, Matsuhashi M, Tajima M, et al. Primary carcinoma of seminal vesicle. Urology. 1988;32:269-272.
Kendrick J, Gonzales B, Huber D, et al. Complications of vasectomies in the United States. J Fam Pract. 1987;25:245-248.
Khan AR. Preliminary report of a comparative study of vasectomy with and without prophylactic antibiotic. FRP Technical Report No 15. 1978. p. 11.
Kiddoo DA, Wollin TA, Mador DR. A population based assessment of complications following outpatient hydrocelectomy and spermatocelectomy. J Urol. 2004;171(2 Pt. 1):746-748.
King BF, Hattery RR, Lieber MM, et al. Seminal vesicle imaging. Radiographics. 1989;9:653-676.
King BF, Hattery RR, Lieber MM, et al. Congenital cystic disease of the seminal vesicle. Radiology. 1991;178:207-211.
Klotz KL, Coppola MA, Labrecque M, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180(6):2569-2576.
Kore RN, McLoughlin J, Kabala J, et al. Seminal vesicle abscess. Br J Urol. 1994;73:331-332.
Kovacs GT, Frances M. Vasectomy. What are the long-term risks? Med J Aust. 1983;2(11):564-567.
Kreager JAJr, Jordan WPJr. Transcoccygeal approach to the seminal vesicles. Am Surg. 1965;31:126-127.
Kuyumcuoglu U, Erol D, Germiyanoglu C, et al. Hydatid cyst of the seminal vesicle. Int Urol Nephrol. 1991;23:479-483.
Lamont JS, Hesketh PJ, de las Morenas A, Babayan RK. Primary angiosarcoma of the seminal vesicle. J Urol. 1991;146:165-167.
Latchamsetty KC, Elterman L, Coogan CL. Schwannoma of a seminal vesicle. Urology. 2002;60(3):515.
Laurila P, Leivo I, Makisalo H, et al. Müllerian adenosarcomalike tumor of the seminal vesicle. Arch Pathol Lab Med. 1992;116:1072-1076.
Leibovitch I, Baniel J, Foster RS, Donohue JP. The clinical implications of procedural deviations during orchiectomy for nonseminomatous testis cancer. J Urol. 1995;154(3):935-939.
Lee CB, Choi HJ, Cho DH, Ha US. Cystadenoma of the seminal vesicle. Int J Urol. 2006;13(8):1138-1140.
Lee DI, Eichel L, Skarecky DW, et al. Robotic laparoscopic radical prostatectomy with a single assistant. Urology. 2004;63:1172-1175.
Lee JC, Bhatt S, Dogra VS. Imaging of the epididymis. Ultrasound Q. 2008;24(1):3-16.
Levine LA, Dewolf WC. Aspiration and tetracycline sclerotherapy solution for hydrocele. J Urol. 1988;139:959-960.
Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchialgia. J Urol. 2001;165:1927-1929.
Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol. 1996;155:1005-1007.
Li S. Ligation of vas deferens by clamping method under direct vision. Chin Med J. 1976;4:213-214.
Li S. Percutaneous injection of vas deferens. Chin J Urol. 1980;1:193-198.
Li SQ, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol. 1991;145(2):341-344.
Li SQ, Xu B, Hou YH, et al. Relationship between vas occlusion techniques and recanalization. Adv Contracept Deliv Syst. 1994;10(3–4):153-159.
López Laur JD, Parisi J. Sclerotherapy of hydroceles and spermatoceles with oxytetracyclines. Actas Urol Esp. 1989;13(6):439-440.
Lord PH. A bloodless operation for the radical cure of idiopathic hydrocele. Br J Surg. 1964;51:914.
Lundhus E, Bundgaard N, Sorensen FB. Cystadenoma of the seminal vesicle. Scand J Urol Nephrol. 1984;18:341-342.
Magoha GA. Local infiltration and spermatic cord block for inguinal, scrotal and testicular surgery. East Afr Med J. 1998;75(10):579-581.
Mammen T, Hwang K, Costabile R. Performing vasectomy to ensure future vasovasostomy success. Urol Times. 2008;36:24-25.
Mawhinney MG, Tarry WF. Male accessory sex organs and androgen action. In: Lipshultz LI, Howards SS, editors. Infertility of the male. New York: Churchill Livingstone; 1991:129-137.
Mazur MT, Myers JL, Maddox WA. Cystic epithelial-stromal tumor of the seminal vesicle. Am J Surg Pathol. 1987;11:210-217.
McConaghy P, Paxton LD, Loughlin V. Chronic testicular pain following vasectomy. Br J Urol. 1996;77:328.
McMahon AJ, Buckley J, Taylor A, et al. Chronic testicular pain following vasectomy. Br J Urol. 1992;69:188-191.
Menon M, Tewari A, Peabody J, the VIP Team. Vattikuti Institute prostatectomy: technique. J Urol. 2003;169:2289-2292.
Meyer JS, Sufrin G, Martin SA. Proliferative activity of benign human prostate, prostatic adenocarcinoma and seminal vesicle evaluated by thymidine labeling. J Urol. 1982;128:1353-1356.
Millard PS. Review: bias may contribute to association of vasectomy with prostate cancer. West J Med. 1999;171(2):91.
Miskowiak J, Christensen AB. Treatment of hydrocele testis by injection of tetracycline. Eur Urol. 1988;14(6):440-441.
Mittemeyer BT, Lennox KW, Borski AA. Epididymitis: a review of 610 cases. J Urol. 1966;95:390-392.
Moore CD, Erhard MJ, Dahm P. Robot-assisted excision of seminal vesicle cyst associated with ipsilateral renal agenesis [review]. J Endourol. 2007;21(7):776-779.
Moss WM. Sutureless vasectomy, an improved technique; 1300 cases performed without failure. Fertil Steril. 1974;27:1040-1045.
Mostofi FK, Price EB. Tumors of the seminal vesicle. In: Mostofi FK, Price EB, editors. Tumors of the male genital system. Washington, DC: Armed Forces Institute of Pathology; 1973:259.
Moudouni SM, Tligui M, Doublet JD, et al. Laparoscopic excision of seminal vesicle cyst revealed by obstruction urinary symptoms. Int J Urol. 2006;13(3):311-314.
Nangia AK, Myles JL, Thomas AJr. Vasectomy reversal for the postvasectomy pain syndrome: a clinical and histological evaluation. J Urol. 2000;164:1939-1942.
Narayana A. Tumors of the epididymis, seminal vesicles, and vas deferens (spermatic cord). In: Culp DA, Loening SA, editors. Genitourinary oncology. Philadelphia: Lea & Febiger; 1985:385-398.
Nash JR. Sclerotherapy for hydrocele and epididymal cysts: a five-year study. Br Med J (Clin Res Ed). 1984;288(6431):1652.
Nguyen HT, Etzell J, Turek PJ. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol. 1996;155:1639-1642.
Nickel JC, Siemens DR, Nickel KR, Downey J. The patient with chronic epididymitis: characterization of an enigmatic syndrome. J Urol. 2002;167(4):1701-1704.
Noël B, Bron C, Künzle N, et al. Multiple nodules of the scrotum: histopathological findings and surgical procedure. A study of five cases. J Eur Acad Dermatol Venereol. 2006;20(6):707-710.
Noske HD, Kraus SW, Altinkilic BM, Weidner W. Historical milestones regarding torsion of the scrotal organs. J Urol. 1998;159:13-26.
Okubo K, Maekawa S, Aoki Y, et al. In vivo endoscopy of the seminal vesicle. J Urol. 1998;159:2069-2070.
Ovrebø KK, Vaage S. [Tetracycline sclerotherapy of hydroceles and spermatoceles. Tidsskr Nor Laegeforen. 1991;111(26):3171-3173.
Ozgök Y, Kilciler M, Eydur E, et al. Endoscopic seminal vesicle stone removal. Urology. 2005;65(3):591.
Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier’s gangrene. Am J Surg. 2009;197:660-665. discussion 665
Padmore D, Norman RW, Millard OH. Analyses of indications for and outcomes of epididymectomy. J Urol. 1996;156:95.
Pant PR, Sharma J, Subba S. Scrotal haematoma: the most common complication of no-scalpel vasectomy. Kathmandu Univ Med J (KUMJ). 2007;5(2):279-280.
Papathanasiou A, Voulgaris S, Salpiggidis G, et al. Hydatid cyst of the seminal vesicle. Int J Urol. 2006;13(3):308-310.
Peterson AC, Lance RS, Ruiz HE. Outcomes of varicocele ligation done for pain. J Urol. 1998;159:1565-1567.
Pitkanen P, Westermark P, Cornwell GG3rd, et al. Amyloid of the seminal vesicles. A distinctive and common localized form of senile amyloidosis. Am J Pathol. 1983;110:64-69.
Politano VA, Lankford RW, Susaeta R. A transvesical approach to total seminal vesiculectomy: a case report. J Urol. 1975;113:385-388.
Practice Committee of the American Society for Reproductive Medicine. Vasectomy reversal. Fertil Steril. 2006;86(Suppl. 5):S268-S271.
Raboy A, Ferzli G, Albert P. Initial experience with extraperitoneal endoscopic radical retropubic prostatectomy. Urology. 1997;50:849-853.
Ramchandani P, Schnall MD, LiVolsi VA, et al. Senile amyloidosis of the seminal vesicles mimicking metastatic spread of prostatic carcinoma on MR images. AJR Am J Roentgenol. 1993;161:99-100.
Razvi HA, Denstedt JD. Endourologic management of seminal vesicle cyst. J Endourol. 1995;8:429-431.
Reale C, Corinti R, Galullo B, et al. Anesthetic infiltration of the spermatic cord in surgery for voluminous hydrocele. Arch Ital Urol Androl. 1998;70(Suppl. 3):43-46.
Redman JF. Anatomy of the genitourinary system. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW, editors. Adult and pediatric urology. 2nd ed. St Louis: Mosby-Year Book; 1987:3-62.
Redman JF, Barthold JS. A technique for atraumatic scrotal pouch orchiopexy in the management of testicular torsion. J Urol. 1995;154:1511-1512.
Rencken RK, Bornman MS, Reif S, Olivier I. Sclerotherapy for hydroceles. J Urol. 1990;143(5):940-943.
Rodriquez WC, Rodriquez DD, Fortuno RF. The operative treatment of hydrocele: a comparison of 4 basic techniques. J Urol. 1981;125:804-805.
Romero Pérez P, Merenciano Cortina FJ, Rafie Mazketli W, et al. Vasectomy: study of 300 interventions. Review of the national literature and of its complications. Actas Urol Esp. 2004;28(3):175-214.
Roy C, Morel M, Saussine C, et al. Magnetic resonance imaging of seminal vesicles. Normal and pathological aspects. J Radiol. 1993;74(3):171-178.
Sarma DP, Weilbaecher TG, Moon TD. Squamous cell carcinoma of prostate. Urology. 1991;37(3):260-262.
Schlegel PN, Goldstein M. No-scalpel vasectomy. Semin Urol. 1992;10(4):252-256.
Schmidt SS. Spermatic granuloma: an often painful lesion. Fertil Steril. 1979;31:178-181.
Schmidt SS. Vasectomy. Urol Clin North Am. 1987;14:149-154.
Schned AR, Ledbetter JS, Selikowitz SM. Primary leiomyosarcoma of the seminal vesicle. Cancer. 1986;57:2202-2206.
Schuman LM, Coulson AH, Mandel JS, et al. Health status of American men—a study of post-vasectomy sequelae. J Clin Epidemiol. 1993;46:697-958.
Schuppe HC, Meinhardt A, Allam JP, et al. Chronic orchitis: a neglected cause of male infertility? Andrologia. 2008;40(2):84-91.
Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923-936.
Schwingl PJ, Meirik O, Kapp N, Farley TM, HRP Multicenter Study of Prostate Cancer and Vasectomy. Prostate cancer and vasectomy: a hospital-based case-control study in China, Nepal and the Republic of Korea. Contraception. 2009;79(5):363-368.
Secaf E, Nuruddin RN, Hricak H, et al. MR imaging of the seminal vesicles. AJR Am J Roentgenol. 1991;156(5):989-994.
Seenu V, Hafiz A. Routine antibiotic prophylaxis is not necessary for no scalpel vasectomy. Int Urol Nephrol. 2005;37(4):763-765.
Selikowitz S, Schned A. A late post-vasectomy syndrome. J Urol. 1985;134:494.
Selli C, Cavalleri S, De Maria M, et al. Robot-assisted removal of a large seminal vesicle cyst with ipsilateral renal agenesis associated with an ectopic ureter and a müllerian cyst of the vas deferens. Urology. 2008;71(6):1226.e5-1226.e7.
Shabsigh R, Lerner S, Fishman IJ, Kadmon D. The role of transrectal ultrasonography in the diagnosis and management of prostatic and seminal vesicle cysts. J Urol. 1989;141:1206-1209.
Shapiro EI, Silber SJ. Open-ended vasectomy, sperm granuloma, and post-vasectomy orchialgia. Fertil Steril. 1979;32:546-550.
Shimada M, Yoshida H. Ex vivo ultrathin endoscopy of the seminal vesicles. J Urol. 1996;156:1338.
Sigurdsson T, Johansson JE, Jahnson S, et al. Polidocanol sclerotherapy for hydroceles and epididymal cysts. J Urol. 1994;151:898-901.
Sokal D, McMullen S, Gates D, Dominik R. the Male Sterilization Investigator Team. A comparative study of the no scalpel and standard incision approaches to vasectomy in 5 countries. J Urol. 1999;162:1621-1625.
Sokal D, Irsula B, Hays M, et al. Vasectomy by ligation and excision, with or without fascial interposition: a randomized controlled trial [ISRCTN77781689]. BMC Med. 2004;2:6.
Stolzenburg J, Do M, Rabenalt R, et al. Endoscopic extraperitoneal radical prostatectomy: initial experience after 70 procedures. J Urol. 2003;169:2066-2071.
Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180(3):949-953.
Sue DE, Chicola C, Brant-Zawadzki MN, et al. MR imaging in seminal vesiculitis. J Comput Assist Tomogr. 1989;13:662-664.
Surya BV, Washecka R, Glasser J, Johanson KE. Cysts of the seminal vesicles: diagnosis and management. Br J Urol. 1988;62:491-493.
Sussman SK, Dunnick NR, Silverman PM, Cohan RH. Carcinoma of the seminal vesicle: CT appearance. J Comput Assist Tomogr. 1986;10:519-520.
Tabata K, Irie A, Ishii D, et al. Primary squamous cell carcinoma of the seminal vesicle. Urology. 2002;59(3):445.
Tanaka T, Takeuchi T, Oguchi K, et al. Primary adenocarcinoma of the seminal vesicle. Hum Pathol. 1987;18:200-202.
Tandon S, Sabanegh EJr. Chronic pain after vasectomy: a diagnostic and treatment dilemma. BJU Int. 2008;102(2):166-169.
Tauber PF, Zaneveld LJ, Propping D, Schumacher GF. Components of human split ejaculate. J Reprod Fertil. 1975;43:249-267.
Thiel R, Effert P. Primary adenocarcinoma of the seminal vesicles [review]. J Urol. 2002;168(5):1891-1896.
Thomas AA, Nguyen CT, Dhar NB, et al. Topical anesthesia with EMLA does not decrease pain during vasectomy. J Urol. 2008;180(1):271-273.
Thonneau P, D’Isle B. Does vasectomy have long-term effects on somatic and psychological health status? Int J Androl. 1990;13(6):419-432.
Upreti L, Bhargava SK, Kumar A. Imaging of primary leiomyosarcoma of the seminal vesicle. Australas Radiol. 2003;47(1):70-72.
Vaidyanathan S, Hughes PL, Mansour P, Soni BM. Seminoma of testis masquerading as orchitis in an adult with paraplegia: proposed measures to avoid delay in diagnosing testicular tumours in spinal cord injury patients. Sci World J. 2008;8:149-156.
Vandwalle J, Dugardin F, Petit T, et al. Haemospermia due to seminal vesicle amyloidosis. Treatment by laparoscopic vesiculectomy. A case report. Prog Urol. 2007;17(7):1382-1384.
Wakefield SE, Elewa AA. Spermatic cord block: a safe technique for intrascrotal surgery. Ann R Coll Surg Engl. 1994;76(6):401-402.
Walker WC, Bowles WT. Transvesical seminal vesiculotomy in treatment of congenital obstruction of seminal vesicles: case report. J Urol. 1968;90:324-326.
Walsh TJ, Seeger KT, Turek PJ. Spermatoceles in adults: when does size matter? Arch Androl. 2007;53(6):345-348.
Weintraub S, Fahey C, Johnson N, et al. Vasectomy in men with primary progressive aphasia. Cogn Behav Neurol. 2006;19(4):190-193.
Weiss RS, Li PS. No-needle jet anesthetic technique for no-scalpel vasectomy. J Urol. 2005;173(5):1677-1680.
Weldon VE, Tavel FR. Potency-sparing radical perineal prostatectomy: anatomy, surgical technique and initial results. J Urol. 1988;140:559-562.
Wessels EC, Ohori M, Granthyre JE, et al. The prevalence of cystic dilatation of the ejaculatory ducts detected by transrectal ultrasound (TRUS) in a self-referred (screening) group of men. J Urol. 1992;147:456.
West AF, Leung HY, Powell PH. Epididymectomy is an effective treatment for scrotal pain after vasectomy. BJU Int. 2000;85:1097-1099.
Witkin SS, Zelikovsky G, Bongiovanni AM. Sperm-related antigens, antibodies, and circulating immune complexes in sera of recently vasectomized men. J Clin Invest. 1982;70:33-40.
Witt MA, Heron S, Lipshultz LI. The post-vasectomy length of the testicular vasal remnant: a predictor of surgical outcome in microscopic vasectomy reversal. J Urol. 1994;151(4):892-894.
Wolf JS, Bennett CJ, Dmochowski RR, et al. American Urological Association best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
Zahalsky MP, Berman AJ, Nagler HM. Evaluating the risk of epididymal injury during hydrocelectomy and spermatocelectomy. J Urol. 2004;171:2291-2292.