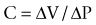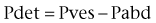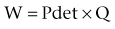chapter 60 Physiology and Pharmacology of the Bladder and Urethra
The medical, social, and economical impact of urinary incontinence, overactive bladder, and voiding dysfunction is staggering (Yoshimura and Chancellor, 2002). In the United States, there are an estimated 34 million community-dwelling men and women with an overactive bladder (Hu et al, 2004). It costs an estimated $19.5 billion and $12.6 billion a year in the United States to manage urinary incontinence and the overactive bladder, respectively (Hu et al, 2003; Hu et al, 2004). With the continued aging of the populations in all developed countries, the problems associated with bladder control will certainly continue to increase. In this chapter, we review the neuromuscular physiology and pathophysiology of the bladder and urethra. We will discuss what is new in pharmacology that may better help our patients with voiding dysfunction. Finally, we will speculate about future treatment methods to conquer urinary incontinence.
The micturition process can be visualized as a complex of neural circuits in the brain and spinal cord that coordinate the activity of smooth muscle in the bladder and urethra (Torrens and Morrison, 1987; de Groat et al, 1993a; Yoshimura and de Groat, 1997). These circuits act as on-off switches to alternate the lower urinary tract between two modes of operation: storage and elimination.
Injuries or diseases of the nervous system in adults can disrupt the voluntary control of micturition, causing the reemergence of reflex micturition and resulting in detrusor overactivity and urgency incontinence (Fig. 60–1) (Torrens and Morrison, 1987; Wein, 1992; de Groat et al, 1993a; Yoshimura and de Groat, 1997). Because of the complexity of the central nervous control of the lower urinary tract, urgency incontinence can result from a variety of neurologic disorders. In addition, urgency incontinence may be due to intrinsic detrusor myogenic abnormalities, resulting in detrusor overactivity (Brading, 1997b). The morphology and function of the detrusor wall are reviewed, including new insights into the urothelium.
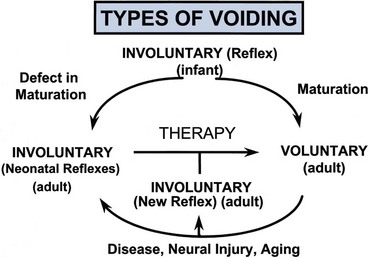
Figure 60–1 Bladder’s “circle of life” by influence of maturation, pathologic processes, and aging. In infants, voiding is initiated and coordinated by reflex circuits. After maturation of central neural pathways, voiding is controlled voluntarily by neural circuitry in higher centers in the brain. A defect in neural maturation allows involuntary voiding to persist in adults. Aging, neural injury, or diseases, such as benign prostatic hyperplasia, can disrupt the central voluntary micturition neural pathways. Pathologic processes can lead to the formation of new reflex circuitry by reemergence of primitive reflex mechanisms that were present in the infant or that appear as the result of synaptic remodeling. The goal of therapy is to reverse the pathologic process and to reestablish normal voluntary control of voiding.
Urethral dysfunction can also be an important cause of urinary incontinence in both women and men. Many health care professionals who are not trained in urology erroneously believe that urethral dysfunction can cause only genuine stress urinary incontinence. This is not true. Urethral dysfunction can also cause urgency incontinence. Increased bladder outlet urethral resistance in men with benign prostatic hyperplasia and in younger men and women with detrusor-sphincter dyssynergia (e.g., spinal cord injury, multiple sclerosis) causes secondary bladder changes. Increased bladder outlet resistance causes secondary bladder remodeling and can result in urgency incontinence. In addition, it can be speculated that in patients with mixed stress and urgency incontinence, stress incontinence can cause urgency incontinence. Leakage of urine into the urethra (stress incontinence) may stimulate urethral afferents that induce an involuntary voiding reflex (urgency incontinence).
This chapter reviews studies in humans and animals that provide insights into
Speculation about what the future holds in terms of research and treatment of lower urinary tract dysfunction ends the chapter. With the mapping of the human genome now completed, we believe that we are on the verge of a paradigm shift in clinical medicine. The future will bring molecular medicine into the hands of the practicing urologists.
Relevant Anatomy and Biomechanics
General Concepts
The bladder can be divided into two parts: a body lying above the ureteral orifices and a base consisting of the trigone and bladder neck, because the two areas are different but homogeneous within themselves regarding neuromorphology and neuropharmacology (Elbadawi and Schenk, 1966). Histologic examination of the bladder body reveals that myofibrils are arranged into fascicles in random directions (Donker et al, 1982). This architecture differs from the discrete circular and longitudinal smooth muscle layers in the ureter or gastrointestinal tract.
The bladder outlet is composed of the bladder base, urethra, and external urethral sphincter (Fig. 60–2). The bladder base has a laminar architecture with a superficial longitudinal layer lying beneath the trigone. A muscle layer deep to the superficial layer is continuous with the detrusor (Tanagho, 1982; Dixon and Gosling, 1987; Zderic et al, 1996). The smaller muscle bundles of the deep muscle layer in the bladder base exhibit a predominantly circular orientation.
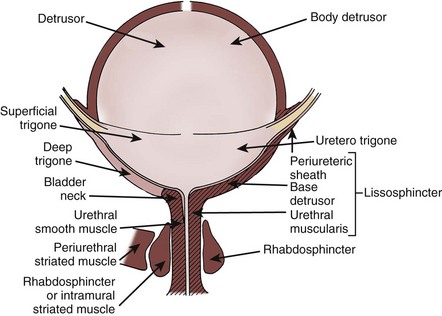
Figure 60–2 Anatomy of the bladder and its outlet, as defined by Gosling and Dixon (left) versus Elbadawi and coworkers (right).
(From Torrens M, Morrison JFB. The physiology of the urinary bladder. Berlin: Springer-Verlag; 1987. p. 1.)
The urethra begins at the internal meatus of the bladder and extends to the external meatus. In the male, four segments are readily identified. The first is the preprostatic portion, or the bladder neck. The prostatic urethra then extends throughout the length of the gland, terminating at its apex. The membranous urethra extends from the prostatic apex through the pelvic floor musculature until it becomes the bulbous and penile urethra (fourth segment) at the base of the penis. In women, the urethra extends throughout the distal third of the anterior vaginal wall from the bladder neck to the meatus. The urethra is composed of tissues that aid continence rather than a single discrete and visible “sphincter.” A network of vascular subepithelial tissue in women contributes to a urethral seal effect.
It is debatable whether the detrusor or trigone muscles project into the proximal urethra (see Fig. 60–2). Embryologic data support the concept of a separate origin for muscles of the bladder and urethra (Dixon and Gosling, 1987; Zderic et al, 1996). Histologic studies show that the longitudinal muscle of the bladder base extends distally into the urethra to form an inner longitudinal layer (Hutch and Rambo, 1967; Tanagho, 1982). Examination of adult and fetal specimens shows that striated and smooth muscles coalesce in the urethra and interdigitate with the fibrous prostatic capsule (Oelrich, 1980). Conversely, a complete and competent ring of smooth muscle at the male bladder neck has been described (Gosling, 1999). No such collar of muscle is identified in the female. The maintenance of continence in men and some women with destruction or opening of the bladder neck argues that the bladder neck may not be the principal site of urinary continence (Chapple et al, 1989). The importance of the bladder neck as the principal zone of maintaining continence remains controversial.
The bladder neck serves an important function in reproduction. In men, closure of the bladder neck facilitates antegrade ejaculation. This is accompanied through a rich noradrenergic innervation by sympathetic nerves that actively contract the bladder neck during ejaculation. However, in women, the density of adrenergic innervation in the bladder neck is reportedly less than that in men (de Groat and Booth, 1993).
Understanding voiding and continence requires some working knowledge of the contractile properties of smooth and striated muscle. The contractile properties of bladder smooth muscle cells are well suited for either urine storage or release. Filling the bladder at a slow physiologic rate maintains an intravesical pressure of less than 10 cm H2O (Klevmark, 1974). Acute denervation of the bladder does not appreciably alter this low filling pressure (Langley and Whiteside, 1951). This concept has been used to support the hypothesis that the intrinsic myogenic or viscoelastic properties of cellular and extracellular components are major contributors to low-pressure bladder filling and compliance (see the section Mechanisms of Detrusor Overactivity). Conversely, neural input is required for the rapid and sustained smooth muscle contraction accompanying voiding.
Bladder Biomechanics
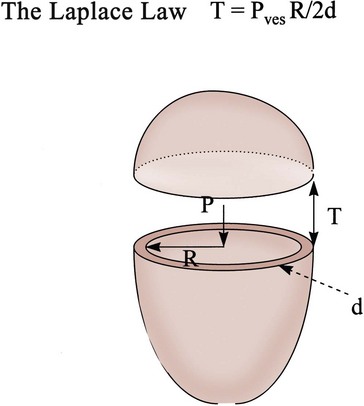
What basic bladder hydrodynamics and biomechanical properties should practicing urologists know, and why should they know them? These are some of the questions we attempt to answer in this section. We lay the foundation of the relationship between bladder shape, size, pressure, and tension as expressed by the Laplace law (Chancellor et al, 1996). Marquis Pierre-Simon de Laplace (1779 to 1827) has been called the Newton of France. Laplace made the keen observation that the tension in the wall of a container necessary to contain a given pressure is directly proportional to the radius of curvature at any point. This is the Laplace law.
It is intuitive to urologists that there is a relationship between intravesical pressure and the size of the bladder and that this affects the tension in the bladder wall. Increased wall tension activates bladder afferent nerves that evoke the sensation of bladder filling and also can evoke involuntary bladder contractions. Large increases in intravesical pressure, especially in a hypertrophic, small-capacity bladder, can dramatically elevate bladder wall tension, producing ischemia, vesicoureteral reflux, and bacterial emptying into the venous or lymphatic systems. Elevated wall tension and intravesical pressure may be the cause of bladder rupture sometimes seen after enterocystoplasty.
The Laplace equation states that there is a direct relationship between wall tension and intravesical pressure and bladder size. In this equation, T is tension, P is intravesical pressure, R is bladder radius, and d is wall thickness. During bladder filling, Pves is relatively constant. With a fully distended bladder, d, because of its relative thinness, is ignored relative to the other parameters unless a hypertrophied wall exists. Thus, T = P • R/2 approximates tension in the full normal bladder (Fig. 60–3).
Filling Mechanics
The viscoelastic behavior of the bladder and urethra depends on both neuromuscular and mechanical properties. Mechanical properties vary with the magnitude of stretch (distention), even in tissue deprived of adenosine triphosphate (ATP) (e.g., postmortem). Mechanical properties are extremely sensitive to tissue structure and composition. In the bladder and urethra, collagen and elastin content have a profound influence on the viscoelastic properties when these tissues are subjected to stress (force per area). Besides smooth muscle, the human bladder is composed of roughly 50% collagen and 2% elastin. With injury, obstruction, or denervation, collagen content increases (Macarak and Howard, 1999). When contractile protein content exceeds collagen, greater distensibility is achieved (compliance). Conversely, when collagen levels increase, compliance falls. Bladder compliance (C) is defined as the change in volume (V) relative to the corresponding change in intravesical pressure (P):
A decrease in compliance or efferent neural input can alter wall tension, cause afferent firing, and thereby change bladder sensations and the volume threshold for micturition. With increasing bladder volume, wall stress or tension (T) increases, as defined by the Laplace equation (see Fig. 60–3).
Bladder Accommodation
The bladder surface undergoes incredible change in size going from empty to full. The percentage change is truly unmatched by any other organ in the body. The change is accommodated by both the urothelium and the bladder wall smooth muscle and connective tissue.
The changes in the thickness of the lamina propria and the detrusor are mechanical requirements for the bladder to accommodate increasing urine volume. During filling, the lamina propria thins at a faster rate than the muscle wall. It has been proposed that bladder wall thinning during filling is the result of a rearrangement of the muscle bundles and also alteration of collagen coil structure (Macarak and Howard, 1999). A combination of muscle and connective tissue spatial changes is required to accommodate urine at low intravesical pressures (Chang et al, 1999).
During filling, the detrusor reorganizes and muscle bundles shift position from a top-to-bottom to a side-to-side configuration. During reorganization, the coiled type III collagen fibers connecting the muscle bundles orthogonally become extended, longer, and taut and assume an orientation such that the fibers become oriented parallel to the lumen.
Voiding Mechanics
Intravesical pressure reflects the combined factors of abdominal (Pabd) and detrusor (Pdet) pressures. Therefore,
Micturition relies on a neurally mediated detrusor contraction, causing Pdet to rise without a significant change in Pabd. To assess the strength of a detrusor contraction, Pdet alone is an insufficient measure. A muscle can use energy either to generate force or to shorten its length. Because the bladder is a hollow viscus, the force developed contributes to Pdet, whereas the velocity of shortening relates to urine flow (Q). There is a trade-off between generating Pdet and urine flow. This has been nicely reviewed by Griffiths (1988). If urethral resistance is low, as in women with sphincter insufficiency and even in normal continent women, Pdet may be almost undetectable; yet, these women with modest Pdet would have normal flow rates. The trade-off between Pdet and Q resembles a curve for constant mechanical power (W) in which
The equation explains why a woman could have normal detrusor contractility and normal detrusor power despite low voiding pressure. During micturition, Pdet reflects outlet resistance. When the urethra opens widely with a high flow (Q), little Pdet is needed to achieve the work necessary to empty the bladder. The key message is that low voiding pressure in a woman does not equate with impaired detrusor contractility; she may simply be able to open her urethra widely. Moreover, pressure-flow nomograms developed for men for diagnosis of obstruction should not be applied to women without validation.
Tissue Biomechanics and Bladder Function
In addition to important molecular and cellular parameters, tissue- and organ-level bladder properties are important to the function of the bladder during filling (Damaser, 1999). Fundamental mechanical properties include the stress-strain relationship, viscoelasticity, and deformation of bladder tissue. Whole-bladder properties include bladder shape, mass, and distention. Organ-level pressure-volume assessment is usually achieved by the cystometrogram. Although it is an essential tool for the urologist, the cystometrogram alone cannot rigorously distinguish between the effects of changes in tissue compliance, and the shape and wall stress distribution of the whole organ. For example, changes in the cystometrogram in spinal cord injury could be a result of alterations in both bladder shape (Ogawa et al, 1988) and tissue properties. Thus to assess intrinsic changes in bladder wall properties, proper assessment of the changes in bladder function requires an understanding of bladder wall biomechanical properties. The biomechanical components contributing to tissue mechanical responses have been studied in isolated detrusor tissue (Wagg and Fry, 1999). However, an ongoing problem is to determine whether clinically observed alterations in detrusor function are due to changes in the contractile apparatus or in the surrounding extracellular matrix.
The complexity of the bladder shape raises similar questions. The bladder can be mechanically idealized as a thin-walled shell. In the theory of thin shells, surface geometry is an integral part of how wall tensions are spatially distributed (Flügge, 1973). That is, a change in surface geometry can alter the stress distributions independent of changes in the mechanical properties. Thus focal points of high curvature on the bladder surface (Fig. 60–4) can potentially induce regions of localized stress concentrations and may be related to bladder deformity, for example, during the spinal cord injury disease process. These phenomena have been underscored by the bladder shape modeling work of Damaser and Lehman (1995), who demonstrated the sensitivity of bladder wall stress distribution on bladder shape.
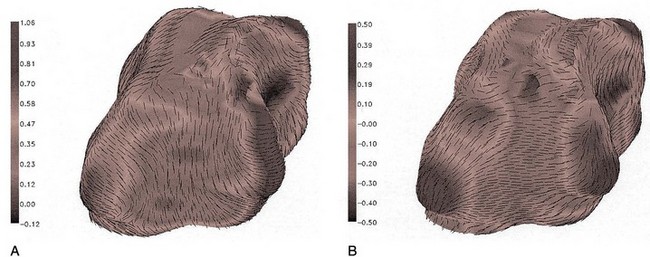
Figure 60–4 Major principal curvature (A) and minor principal curvature (B) for the bladder. Curvature magnitudes are indicated by gray scale and directions by vectors. When the bladder is full, as in this case, wide variations in curvature exist because of contact with the surrounding pelvic structures.
Although it is typically modeled as a spheroid, the bladder is probably one of the more irregularly shaped anatomic structures. Irregularity in bladder shape, especially when it is full, is due to contact with surrounding pelvic structures. Note in particular the complex spatial distribution of surface curvatures, including pointed regions of high curvature and rapid transitions between elliptical (both principal curvatures are positive) and hyperbolic (or saddle-shaped, where one principal curvature component is negative) regions (see Fig. 60–4). By use of the techniques developed by Sacks and colleagues (1993, 1999), this roughness can be removed and the original smooth in-vivo surface recovered (Fig. 60–5).
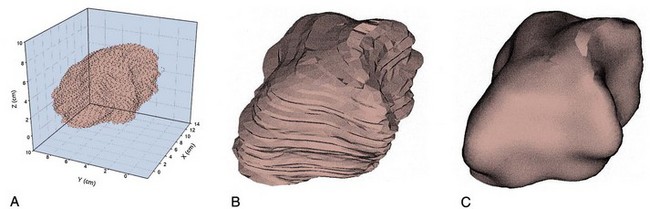
Figure 60–5 Three-dimensional reconstruction of the bladder. A, “Point cloud” of 3146 digitized surface points from a normal human bladder from computed tomographic images. B, The resulting reconstructed bladder surface, revealing a complex, nonspheroidal surface. C, Same surface as in B but with the surface roughness from imaging noise removed.
The Urinary Bladder
The bladder performs several important functions. First, it must store a socially adequate volume of urine. The bladder wall must be able to stretch and rearrange itself to allow an increase in bladder volume without significant rise in pressure. In other words, the bladder wall must be highly compliant. Second, the smooth muscle and intrinsic nerves have to be protected from exposure to urine by the urothelium, which itself must also expand readily during filling. Third, bladder emptying requires synchronous activation of all the smooth muscle of the bladder body, because if only part of the wall contracted, the uncontracted compliant areas would stretch and prevent the increase in pressure necessary for urine to be expelled through the urethra. This is the problem often seen in an elderly man with benign prostatic hyperplasia who develops urinary retention and a bladder diverticulum.
In this section, we review the various components of the bladder, including smooth muscle, stroma, and blood vessels. First, we focus on the detrusor smooth muscle by contrasting the characteristics of smooth and striated muscle, the activation of smooth muscle contractility, the maintenance of bladder tone, and its neural regulation. In addition, we concentrate on the urothelium, because there is now a resurgence of research interest in this thin but complex membrane. Some exciting studies are presented that discuss the barrier, transport, and neurocommunication properties of the urothelium that are just beginning to be understood. Last, we briefly discuss ion channels and how they might contribute to detrusor dysfunction.
Smooth Muscle
There are several universal characteristics of smooth muscle:
Smooth muscle consists of a sheet containing many small, spindle-shaped cells linked together at specific junctions. Smooth muscle cells contain actin and myosin, but these proteins are not arranged in a regular sarcomere pattern. Instead, each smooth muscle cell consists of a more variable matrix of contractile proteins that is attached to the plasma membrane at the junctional complexes between neighboring cells. Smooth muscle maintains a steady level of tension that can be modulated by circulating hormones, by local factors such as nitric oxide, or by activity in the autonomic nerves. Smooth muscle is more adaptable than skeletal muscle and is able to adjust its length over a much wider range than skeletal muscle.
Based on the assumption of a spherical bladder, the circumference of a 400-mL capacity bladder is approximately 30 cm. If the bladder empties to a residual urine volume of 10 mL, the circumference would be only 8 cm. To accomplish this feat, the detrusor would have a change in muscle length of 75%. If the bladder were to be made of skeletal muscle instead, the maximum length change would be only about 30%. The maximum “skeletal muscle bladder” emptying would be only 70% of its contents, leaving a residual urine of 120 mL. Thus the bladder requires the unique property of smooth muscle to accomplish its job. What is assumed to be the stronger skeletal muscle is not up to the job of bladder emptying (Gabella, 1995; Brading et al, 1996; Brading, 1999).
Morphology
The individual smooth muscle cells in the bladder wall are small spindle-shaped cells with a central nucleus; fully relaxed, they are several hundred micrometers long with a 5- to 6-µm maximum diameter. Skeletal muscle fibers are some 20 times wider and thousands of times longer (Smet et al, 1996).
Smooth muscle has a unique range of physiologic properties and consists of sheets containing many small spindle-shaped cells linked together at specific junctions. Each cell has a single nucleus. No cross striations are visible under the microscope, but like skeletal and cardiac muscle, smooth muscle cells contain actin and myosin. In addition, they contain cytoskeletal intermediate filaments that assist in transmission of the force generated during contraction to the neighboring smooth muscle cells and connective tissue. Although there are no Z lines in smooth muscle, they have a functional counterpart in dense bodies that are distributed throughout the cytoplasm and that serve as attachments for both the thin and the intermediate filaments. The thin and thick filaments of smooth muscle fibers are arranged as myofibrils that cross the fibers obliquely in a lattice-like arrangement. The filaments of contractile proteins are attached to the plasma membrane at the junctional complexes between neighboring cells (Fig. 60–6). A comparison of some of the properties of smooth and skeletal muscle is listed in Table 60–1 (Chacko et al, 1999).
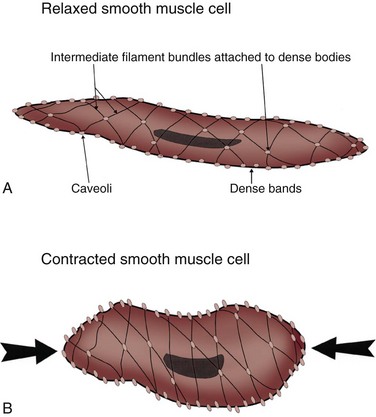
Figure 60–6 The organization of the contractile elements of smooth muscle fibers by a simple model of the contraction of smooth muscle. A, Relaxed smooth muscle cell. B, Contracted smooth muscle cell. Intermediate filaments, dense bodies, and dense bands of smooth muscle fibers harness the pull generated during myosin cross-bridge activity. Intermediate filaments attach to dense bodies scattered throughout the sarcoplasm and occasionally anchor to the dense bands situated between caveolae (invaginations of the sarcolemma). As the obliquely running contractile elements contract, the muscle shortens.
Table 60–1 Comparison of the Properties of Skeletal and Smooth Muscle
| PROPERTY | SKELETAL MUSCLE | SMOOTH MUSCLE |
|---|---|---|
| Cell characteristics | Long cylindrical cells with many nuclei | Spindle-shaped cells with a single nucleus |
| Maximum cell size (length × diameter) | 30 cm × 100 µm | 200 µm × 5 µm |
| Visible striations | Yes | No |
| Ultrastructure | Sarcomere pattern | No sarcomere pattern |
| No immediate filaments | Intermediate filaments | |
| Dense bodies | ||
| Motor innervation | Somatic | Autonomic |
| Type of contracture | Phasic | Mostly tonic, some phasic |
| Contractile activity | Disinhibition of tropomyosin | Active myosin phosphorylation |
| Sliding filaments | ? Sliding filaments | |
| Rapid contraction | Formation of “latch state” | |
| Calcium regulation | Rapid Ca2+ influx via T tubule | Voltage- and receptor-operated Ca2+ channels |
| Release from internal stores | ||
| Basic muscle tone | Neural activity | Intrinsic, extrinsic factors |
| Force of contraction regulated by hormone | No | Yes |
Smooth Muscle Cellular Mechanics
Detrusor smooth muscle contracts and shortens by interaction between thin and thick filaments. The thin actin filaments are anchored at the membranes on the dense bands, or in the cytoplasm on the dense bodies, and interact with the thick filaments through cross bridges formed from the heads of myosin molecules. In both cases, the contractile mechanism is a myosin-activated system. Cross-bridge cycling is initiated when the ATPase activity of the myosin heads is switched on. This is achieved by phosphorylation of two light chains on the cross bridge, through a specific enzyme, myosin light-chain kinase, that is activated by a rise in the intracellular calcium concentration (Hai and Murphy, 1989; Gunst et al, 1993; Andersson and Arner, 2004). Changes in the contractile proteins occur in developing bladders and also during bladder hypertrophy (Wang et al, 1995; Wu et al, 1995; Sjuve et al, 1996).
The bladder muscle has a broad length-tension relationship, allowing tension to be developed over a large range of resting muscle lengths (Uvelius and Gabella, 1980). The tissue shows viscoelasticity that influences muscle tension and is manifested as total bladder wall tension (Venegas, 1991). Isolated detrusor strips show spontaneous mechanical activity to a variable extent. It is more frequently seen in bladders from small mammals (Sibley, 1984) but can also be seen in muscle strips from human detrusor. However, spontaneous fused tetanic contractions, such as those commonly seen in smooth muscles from the gastrointestinal tract and uterus, are almost never seen in normal bladders.
Membrane Electrical Properties and Ion Channels
The smooth muscle of the detrusor is able to support a regenerative action potential from a resting potential of −50 to −60 mV (Montgomery and Fry, 1992; Fry et al, 2002). The action potentials have marked after-hyperpolarizations, and several different K+ channels, such as delayed rectifier and transient outward channels and both small and large calcium-activated K+ channels (SKCa and BKCa, respectively), appear to be involved in determining their shape (Fujii et al, 1990; Brading and Turner, 1996; Heppner et al, 1997; Andersson and Arner, 2004). The upstroke is supported by calcium influx through L-type Ca2+ channels (Rivera, 1998). In addition, a K+ channel opened by reduced intracellular ATP has been demonstrated (Bonev and Nelson, 1993), which, on activation, is profoundly inhibitory to the spontaneous activity (Foster et al, 1989). Several other conductances, which include a nonspecific cation channel linked to the P2X receptor (Inoue and Brading, 1990) and stretch-activated cation channels (Wellner and Isenberg, 1993), have been demonstrated in detrusor smooth muscle.
In response to acetylcholine released from parasympathetic nerve terminals, muscarinic M3 receptors are thought to induce detrusor muscle contractions by calcium entry through nifedipine-sensitive L-type Ca2+ channels (Andersson and Arner, 2004; Andersson and Wein, 2004; Schneider et al, 2004a, 2004b), in addition to increased polyphosphoinositide hydrolysis resulting in inositol 1,4,5-trisphosphate (IP3) production and release of intracellular calcium stores (Iacovou et al, 1990; Eglen et al, 1994; Harriss et al, 1995; Hashitani, Bramich et al, 2000; Fry et al, 2002; Braverman et al, 2006a), although the importance of L-type channel activation in muscarinic M3 receptor–mediated detrusor bladder contractions is still a matter of debate (Fry et al, 2002; Hashitani et al, 2000; Schneider et al, 2004a, 2004b; Braverman et al, 2006a, 2006b; Frazier et al, 2008). The calcium influx through L-type Ca2+ channels also triggers calcium-induced calcium release through ryanodine receptors and activates BKCa channels to generate the membrane after-hyperpolarization (Hashitani and Brading, 2003). The opening of the K+ channel repolarizes the membrane potential by the efflux of positively charged potassium ions from the cell (Mostwin, 1986). In addition, increased polyphosphoinositide hydrolysis after M3 receptor activation can also generate diacylglycerol in the membrane; diacylglycerol can activate protein kinase C, which may be involved in generating the tonic element of the response through modulation of Ca2+ and K+ channels (Zhao et al, 1993).
Calcium also activates a variety of cellular responses when it enters the cytoplasm of a cell by means of transmembrane channels. To be effective as a signal, its concentration must be returned to submicromolar levels, driven by ATP pumps. The Ca2+ pump is a membrane-bound, Ca2+-activated ATPase, similar to the Na+–K+ pump that controls ion balance and membrane potential in all animal cells. These pumps belong to a superfamily of ATPases known as P type, because they depend on the autophosphorylation of a conserved aspartic acid residue using ATP.
Excitation-Contraction Coupling in Smooth Muscle
Like skeletal and cardiac muscle, smooth muscle contracts when intracellular calcium concentration rises. Because smooth muscle does not possess the tubules of the “T system,” the rise in calcium concentration occurs through calcium influx through voltage-gated Ca2+ channels in the plasma membrane or release from the sarcoplasmic reticulum after activation of receptors that increase the formation of IP3. Although calcium serves the same triggering role in all muscle types, the mechanism of activation is different in smooth muscle. The contractile response is slower and longer lasting than that of skeletal and cardiac muscle. This can be explained by the slowness with which smooth muscle is able to hydrolyze ATP during the contractile process. A calcium-binding protein called calmodulin regulates the interaction between actin and myosin. The thin filaments lack troponin and are always ready for contraction. The slow and relatively steady generation of tension enables smooth muscle to generate and to maintain tension with relatively little expenditure of energy (Fig. 60–7 and Table 60–2).
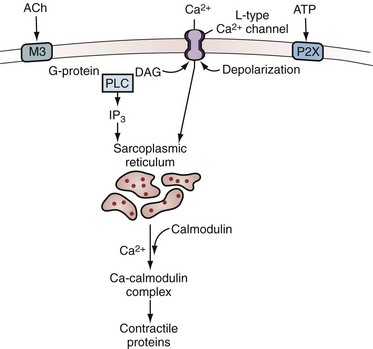
Figure 60–7 Acetylcholine (ACh) and adenosine triphosphate (ATP) pathway in the bladder. Acetylcholine interacts with M3 muscarinic receptors and activates phospholipase C (PLC) through a G protein. Phospholipase activation leads to the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 elicits release of calcium from the sarcoplasmic reticulum through IP3 receptors, and DAG may modulate voltage-sensitive Ca2+ channels in the plasma membrane. ATP acting through P2X purinergic receptors opens nonselective cation channels in the membrane, leading to depolarization that opens voltage-sensitive Ca2+ channels. Both lead to entry of calcium. This triggers release of more calcium from the stores through ryanodine receptors. The rise in intracellular free calcium concentration triggers contraction and may also open calcium-activated channels in the membrane, such as calcium-activated K+ channels, that can modulate the response.
(Redrawn from Brading A. Cellular biology. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth [UK]: Health Publications; 1999. p. 73.)
Table 60–2 Detrusor Smooth Muscle Contraction Sequence
The rise in cytoplasmic calcium concentration brought on by the action potential results in binding of calcium to calmodulin. Calcium-bound calmodulin is then capable of activating myosin light-chain kinase, permitting it to phosphorylate the myosin type II light chain. Phosphorylation of the light chain allows the myosin to interact with actin, leading to force generation (White et al, 1993; Chacko et al, 1994; Andersson and Arner, 2004). The actual geometric arrangement of the actin-myosin complex that permits force generation is unclear. Different isoforms of the myosin heavy chain have been defined by Chacko and associates (1994), as well as the components of the regulatory system linking cytoplasmic calcium levels to contraction (White et al, 1993). The smooth muscle myosin heavy chain is encoded by a single gene, and two heavy-chain variants formed by alternative splicing are identified (SM1 and SM2). Two additional myosin heavy-chain isoforms, SM-A and SM-B, are then formed by alternative splicing in the amino-terminal region, enabling four possible isoforms: SM1-A, SM1-B, SM2-A, and SM2-B (Babu et al, 2000). Because the SM-B isoform is shown to propel actin at a faster velocity (Lauzon et al, 1998), the relatively high expression of SM-B isoform in the bladder shown at the mRNA level (Arafat et al, 2001) would contribute to a comparatively fast smooth muscle type of the bladder (Andersson and Arner, 2004).
Propagation of Electrical Responses
The lack of fused tetanic contractions in normal detrusor smooth muscle strips suggests that there is poor electrical coupling between smooth muscle cells (Uvelius and Mattiasson, 1986). Measurements of tissue impedance support the observation that the detrusor is less well coupled electrically than other smooth muscles (Brading and Mostwin, 1989; Parekh et al, 1990). Poor coupling could be a feature of a normal detrusor that prevents synchronous activation of the smooth muscle cells during bladder filling. Nevertheless, some degree of coupling within a muscle bundle clearly does exist, because it is possible to measure the length constant of a bundle (Seki et al, 1992). There is also evidence for gap-junction coupling between detrusor cells in humans and guinea pigs, detected by whole-cell patch clamp recordings (Wang et al, 2006) and Ca2+ imaging (Neuhaus et al, 2002), respectively. Significant expression of connexins 43 and 45, gap-junction proteins, is found in human detrusor muscles (John et al, 2003; Wang et al, 2006). However, electrical couplings between detrusor cells seem to be reduced during postnatal development because coordinated, large-amplitude, low-frequency contractile activity is seen in the neonate rat bladder declines and is replaced by low-amplitude, high-frequency, more irregular activity in older rats, which appears to depend on the disruption of the intercellular smooth muscle communication (Szell et al, 2003). It has also been suggested that a change in the properties of the cell coupling may underlie the generation of the uninhibited detrusor contractions occurring in overactive and aging bladders (Seki et al, 1992; Brading, 1997b; Brading, 2006).
Smooth Muscle during Filling
As the bladder fills, the myocytes are stretched, leading to activation of nonselective cation channels that permit rapid entry of sodium and of some calcium (Wellner and Isenberg, 1993). Entry of cations depolarizes the smooth muscle membrane potential. If the extent of stretch is mild, there is low activation, and the membrane potential rests at a more depolarized level, predisposing the cell to activation by lower levels of muscarinic agonists. If the extent of the stretch is more significant, the activation of cation channels may be sufficient to depolarize the cell sufficiently for initiation of an action potential. Although individual cells may contract spontaneously, contraction of the bladder as a whole generally requires stimulation by parasympathetic nerves (Andersson, 1993). When the membrane is sufficiently depolarized, L-type Ca2+ channels open and Ca2+ channels in the sarcoplasmic reticulum open, flooding the cell with calcium and resulting in an action potential, thereby leading to spontaneous phasic activity in the bladder (Mostwin, 1986; Kumar et al, 2005).
Between contractions, the sarcoplasmic reticulum accumulates calcium to levels far above those of the cytosol by means of a calcium ATPase (Wall et al, 1990). Calcium stores in the sarcoplasmic reticulum can be released in the absence of action potentials by exposure to caffeine, which renders the Ca2+ channels sensitive to normal ambient cytoplasmic calcium levels.
Interstitial Cells
Recent evidence suggests that the “normal” bladder may be spontaneously active and that exaggerated spontaneous contractions could contribute to the development of detrusor overactivity. In a rat model for detrusor overactivity, local areas of spontaneous contractions are increased and more coordinated in partial outlet-obstructed rat bladders (Drake et al, 2003). However, it is still not clear which cells generate spontaneous activity in the bladder. As mentioned before, detrusor myocytes could be spontaneously active, and electrical coupling through gap junctions could trigger spontaneous contractions (Brading, 1997b, 2006). Alternatively, another population of cells in the bladder known as interstitial cells, or myofibroblasts, has been proposed for a pacemaking role in spontaneous activity of the bladder (Andersson and Arner, 2004; Kumar et al, 2005). Interstitial cells have been identified in the human and guinea pig ureter, urethra, and bladder body (Kumar et al, 2005; Hashitani, 2006; Fry et al, 2007).
Suburothelial Interstitial Cells
In the human bladder, subepithelial interstitial cells, which are also called myofibroblasts, stain for vimentin and α–smooth muscle actin but not for desmin (Fry et al, 2004). These cells are linked by gap junctions consisting of connexin 43 proteins and make close appositions with C-fiber nerve endings in the submucosal layer of the bladder, suggesting that there is a network of functionally connected interstitial cells immediately below the urothelium that may be modulated by other nerve fibers (Fry et al, 2004) (Fig. 60–8). ATP can induce inward currents associated with elevated intracellular Ca2+ in isolated suburothelial interstitial cells (Fry et al, 2007). Immunohistochemical studies show the expression of P2Y receptors, most notably P2Y6 receptors, and M3 muscarinic receptors in suburothelial interstitial cells from guinea pigs (Fry et al, 2007; Grol et al, 2009). In the human bladder, increased expression of muscarinic M2 and M3 receptors in vimentin-stained suburothelial interstitial cells is found and correlates with the urgency score in humans with idiopathic detrusor overactivity (Mukerji et al, 2006). Because ATP or acetyl choline (ACh) is known to be released from the urothelium during bladder stretch, suburothelial interstitial cells are in an ideal position between the urothelium and nerve endings to modify a sensory feedback mechanism. Application of a nitric oxide donor sodium nitroprusside (SNP) also attenuates an increase in intracellular Ca2+ and current responses to ATP in guinea pig interstitial cells, suggesting the cGMP-dependent inhibition of cell activity (Sui et al, 2008).
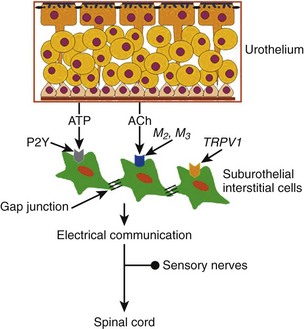
Figure 60–8 Schematic representation of suburothelial interstitial cells (IC), which are also called myofibroblasts. Substances released from the basolateral surface during stretch, such as adenosine triphosphate (ATP) and acetylcholine (ACh), activate afferents in the suburothelial layer through the intermediation of suburothelially located interstitial cells, which express purinergic P2Y receptors, muscarinic M2 and M3 receptors, or capsaicin TRPV1 receptors, and are connected to each other by gap-junction proteins.
Intradetrusor Interstitial Cells
Interstitial cells are also found in the detrusor layer and shown to be spontaneously active (Kumar et al, 2005). These cells are stained for c-Kit and located along both boundaries of muscle bundles in the guinea pig bladder (McCloskey and Gurney, 2002; Hashitani et al, 2004; Hashitani, 2006). These cells can fire Ca2+ waves in response to cholinergic stimulation by M3 muscarinic receptor activation and can be spontaneously active, suggesting that they could act as pacemakers or intermediaries in transmission of nerve signals to smooth muscle cells (McCloskey and Gurney, 2002; Johnston et al, 2008) (Fig. 60–9). However, Hashitani and colleagues (2004) have also suggested that interstitial cells in the detrusor may be more important for modulating the transmission of Ca2+ transients originating from smooth muscle cells rather than being the pacemaker of spontaneous activity because spontaneous Ca2+ transients occur independently in smooth muscles and interstitial cells. It has also been demonstrated that the c-Kit tyrosine kinase inhibitor Glivec decreased the amplitude of spontaneous contractions in the guinea pig bladder (Kubota et al, 2004, 2006) and in muscle strips from the overactive human bladder, in which c-KIT–positive cells were increased compared with normal subjects (Biers et al, 2006), suggesting that targeting these receptors expressed in intradetrusor interstitial cells may provide a new approach for treating overactive bladder. In addition, following application of SNP (a nitric oxide [NO] donor), interstitial cells throughout the bladder, but not detrusor muscle cells, demonstrate cyclic guanosine monophosphate (cGMP) immunoreactivity (Smet et al, 1996; Gillespie et al, 2004). Thus increased levels of cGMP found in interstitial cells by using phosphodiesterase-5 inhibitors, for example, may diminish synchronicity between detrusor muscle bundles (Hashitani, 2006). These cells are also a source of prostaglandin E2 (PGE2) because of their expression of cyclooxygenase and a reduction in spontaneous activity of bladder muscle strips by use of prostaglandin E2 receptor (EP) antagonists in rabbits (Collins et al, 2009).
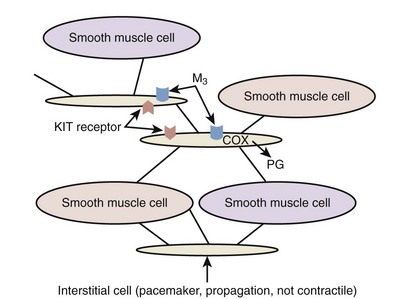
Figure 60–9 Schematic representation of interstitial cells in the detrusor muscle layers. These cells are not contractile but may be pacemakers with spontaneous activity and propagate signals between detrusor muscles. They also express KIT receptors and muscarinic M3 receptors, and can produce prostaglandins (PG), such as PGE2, through activation of cyclooxygenase (COX).
Further research is definitely required to fully understand the role of suburothelial/intradetrusor interstitial cells and their contribution to spontaneous contractions or detrusor overactivity.
Smooth Muscle Tone
The bladder smooth muscle maintains a steady level of contracture and tone. Tone is important in maintaining the capacity of the bladder. Smooth muscle tone depends on many factors, some intrinsic and some extrinsic. Extrinsic factors include activity in the autonomic nerves and circulating hormones; intrinsic factors include the response to stretch, local metabolites, and locally secreted agents such as nitric oxide, and temperature. Consequently, smooth muscle tone does not depend solely on activity in the autonomic nerves or on circulating hormones.
The contraction of smooth muscle is slow, sustained, and resistant to fatigue. Smooth muscle takes 30 times longer to contract and relax than does skeletal muscle and can maintain the same contractile tension for prolonged periods at less than 1% of the energy cost.
Length-Tension Relationship
If a smooth muscle is stretched, there is a corresponding increase in tension immediately after the stretch. This is followed by a progressive relaxation of the tension toward its initial value. This property is unique to smooth muscle and is called stress relaxation (Chancellor et al, 1996).
Compared with skeletal or cardiac muscle, smooth muscle can shorten to a far greater degree. A stretched striated muscle can shorten by perhaps as much as a third of its resting length, whereas a normal resting muscle would shorten by perhaps a fifth. This is perfectly adequate for it to perform its normal physiologic role. In contrast, a smooth muscle may be able to shorten by more than two thirds of its initial length. This unusual property is conferred by the loose arrangement of the thick and thin myofilaments in smooth muscle cells. It is a crucial adaptation because the volume contained by the bladder depends on the cube of the length of the individual muscle fibers. The ability of the detrusor smooth muscle to change its length to such a large degree permits the bladder to adjust to a much wider variation in volume than would be possible for skeletal muscle.
Key Points: Smooth Muscle Mechanics
Stroma
The main constituents of bladder wall stroma are collagen and elastin in a matrix composed of proteoglycans. The main cells are fibroblasts. The passive mechanical properties of the bladder wall depend on the viscoelastic properties of the stroma and of the relaxed detrusor muscle (Cortivo et al, 1981). The stroma has commonly been considered a passive low-metabolic tissue that fills out the space between muscle bundles, vessels, and nerves. In recent years, the important role of the stroma in the adaptation of the bladder to pathophysiologic conditions has been more appreciated (Macarak and Howard, 1999). Bladder hypertrophy is likely to involve an interaction of stroma and smooth muscle. In arteries, disruption of elastin in the stroma can stimulate proliferation of smooth muscle (Li et al, 1998). Although no such mechanisms are yet known in the bladder, it is possible that there could be a more intimate relationship between changes in the composition of the stroma and muscle function and growth than is appreciated at present.
Bladder Wall Collagen
Most of the bladder wall collagen is found in the connective tissue outside the muscle bundles. Changes in the relative amounts of muscle and nonmuscle tissue in the bladder wall would therefore influence collagen concentration. A number of different collagen types have been identified. In the bladder, types I, III, and IV are the most common (Macarak et al, 1995; Andersson and Arner, 2004). Landau and coworkers (1994) developed morphometric and histochemical techniques to determine the percentage volume of connective tissue in the bladder wall and to measure the two major types (I and III) of collagen. These methods quantitate three parameters of bladder ultrastructure: percentage volume of connective tissue, ratio of connective tissue to smooth muscle, and ratio of type III to type I collagen. These parameters have been shown to be abnormally elevated in patients with bladder disease compared with normal patients. They further studied the ultrastructural changes that occur in the wall of dysfunctional bladders to determine the ability of new urodynamic techniques to reliably detect the clinical effect of these histologic changes. The study included 29 consecutive patients undergoing bladder augmentation. Preoperative urodynamic evaluation included measurement of the total bladder capacity, pressure-specific bladder volume, and dynamic analysis of bladder compliance. Full-thickness bladder biopsy specimens were obtained from the dome of the bladders during augmentation. The percentage of connective tissue and the ratio of connective tissue to smooth muscle were determined for all patients. These histologic results were compared with previously established normal values. All 29 patients had decreased bladder compliance, even though 9 had a normal bladder capacity. The ratio of connective tissue to smooth muscle was significantly increased in poorly compliant versus normal bladders. The ratio of type III to type I collagen was also significantly elevated. One can conclude that the poor storage function of poorly compliant bladders is secondary to an alteration in the connective tissue content of the bladder wall, especially increased collagen type III.
In the rat, infravesical obstruction or bladder denervation induces hypertrophy of the detrusor smooth muscle and, in turn, a decrease in the collagen concentration (Uvelius and Mattiasson, 1984, 1986). Aging is associated with a relative decrease in smooth muscle, in both men and women, relative to collagen content (Susset et al, 1978; Lepor et al, 1992). This could perhaps be related to the decreased packing density of submucosal collagen during aging (Levy and Wight, 1990).
Perhaps the most comprehensive work on bladder collagen has been performed by Macarak and Howard (1999), who have speculated that connections must exist between the tension-generating elements (i.e., the smooth muscle cells) and the other components of the bladder. In bladders that become noncompliant (e.g., from spinal cord injury), it is likely that there is some interference with the ability of the collagen fibers to alter their tortuosity. This, predictably, would reduce total bladder capacity. Further studies are required to establish the relationship between compliance changes and the passive mechanical elements of the bladder wall that make up its structural protein matrix.
Bladder Wall Elastin and Matrix
Elastic fibers are amorphous structures composed of elastin and a microfibrillar component located mainly around the periphery of the amorphous component (Rosenbloom et al, 1995). In the mature fiber, the amorphous component composes about 90%. The microfibrils contain a number of glycoproteins. Elastin fibers are sparse in the bladder, compared with collagen, but are found in all layers of the bladder wall (Murakumo et al, 1995). In spinal cord–injured rats, the elastin/collagen ratio first increased and is well correlated with increased bladder compliance due to bladder overdistention up to 6 weeks after injury, including a bladder areflexia phase. Then the ratio is reduced as bladder compliance is decreased due to the emergence of detrusor overactivity 10 weeks after injury, suggesting a potential role for elastin in the modulation of bladder compliance (Nagatomi et al, 2005; Toosi et al, 2008).
The nonfibrillar matrix in the stroma is largely composed of a gel of proteoglycans and water. Proteoglycans are glycoproteins with glycosaminoglycans covalently attached. The arrangement of the proteoglycans in the matrix creates a compartment of tissue water that has a viscous behavior when it is subjected to deformation.
Blood Vessels
The bladder has a multilayer vascular plexus, and this fact has morphologic as well as functional implications. When it is carefully examined, the underside of the epithelium is depressed by numerous grooves that are occupied by a dense network of blood capillaries. These vascular grooves allow a large number of capillaries to run at a distance of only a few tenths of a micron from the epithelium (Inoue and Gabella, 1991). The subepithelial capillary plexus may be associated with maintenance of the barrier function of the urothelium, reducing any exposure of the detrusor smooth muscle to substances diffusing from the urine (Hossler and Monson, 1995). It may also play a role in epithelial transport function and be necessary for urothelial metabolism.
Because of the large increase in surface area of the bladder wall during filling, the blood vessels must be able to lengthen considerably. To maintain good blood flow, mechanisms may be needed to ensure that the overall resistance of the vessels, as they lengthen, does not increase sufficiently to reduce the effective perfusion of the tissue. Several groups have investigated the effects of bladder filling on the blood flow. The majority of reports have shown that the blood flow is reduced by distention (Batista et al, 1996; Greenland and Brading, 1996). In patients with a low compliant bladder, there is a marked increase in the intravesicular pressure and a more pronounced decrease in bladder blood flow compared with normal controls (Ohnishi et al, 1994). The principal determinant of blood flow in the bladder wall seems to be intramural tension. During normal filling, the blood flow is able to adapt to the large increase in surface area until the pressure increases in the bladder (Greenland and Brading, 1996).
When the detrusor is deprived of oxygen or a metabolic substrate, as would occur in ischemia, its contractile ability rapidly declines (Levin, 1983; Zhao et al, 1991; Pessina et al, 1997; Levin et al, 2003). It has been suggested that ischemia and reperfusion might lead to damage to intramural neurons and result in the patchy denervation and altered smooth muscle function seen in bladders of patients with detrusor overactivity (Brading, 1997a).
Urothelium
The basic functions of the bladder are storage of urine, maintenance of urine composition, and facilitation of voiding at appropriate time intervals. The urothelium has physiologic functions in relation to all parts of these basic functions and, as such, can no longer be thought of as an inert barrier between urine and plasma.
Structure
The urothelium consists of a variable number of cell layers, depending on the species (Fawcett, 1984). There are generally three distinct layers. A layer of basal cells are germinal in nature and 5 to 10 µm in diameter. Intermediate cells are superficial to these and approximately 20 µm in diameter, and a layer of umbrella cells forms the luminal surface of the urothelium (Lewis, 2000; Apodaca, 2004). These umbrella cells are the largest epithelial cells in the body, measuring 100 to 200 µm in diameter; they are polyhedral, are generally hexagonal, and can flatten and have more surface area with stretching. They may also be multinucleate (Kelly, 1984). The surface of the umbrella cells is covered with a glycosaminoglycan layer (Fig. 60–10).
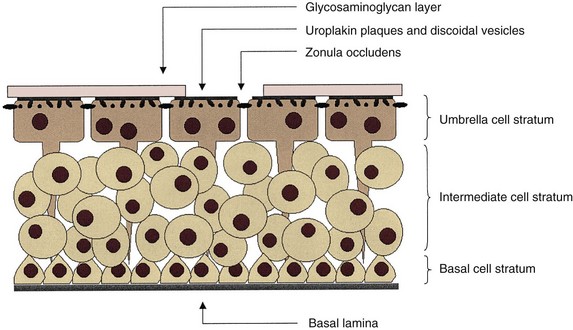
Figure 60–10 Diagrammatic representation of the bladder urothelium. The lumen of the bladder is on top and the lamina propria is below. The glycosaminoglycan layer is shown as discontinuous for illustrative purposes, enabling labeling of deeper structures.
Glycosaminoglycan Layer
The glycosaminoglycan (GAG) layer has been a controversial subject of research into urothelial barrier function. Parsons and associates (Parsons et al, 1990) reported that intravesical treatment of the rabbit bladder with protamine sulfate increased urothelial permeability, to water, urea, and calcium both in vivo and in vitro. This effect was reversed with pentosan polysulfate. They concluded that the protamine sulfate affected the GAG layer and that this was repaired by pentosan polysulfate. However, no microscopic evidence of the anatomic changes was presented in this paper. This study was confirmed by Nickel and coworkers (1998), who compared pentosan polysulfate, heparin, and hyaluronic acid as treatments. The authors concluded that heparin was the best of the three agents in efficacy but pointed out that this may be due to its anti-inflammatory properties. Indeed, the role of the GAG layer may be more in line with an antibacterial adherence function as outlined by Hanno and coworkers (1981). The GAG layer may also be important for the formation and attachment of particulates to the urothelium and stone formation (Hurst, 1994; Grases et al, 1996). However, there are a number of problems with the theory that the GAG layer is the urothelial plasma barrier:
Protamine sulfate increases the apical membrane (luminal surface of umbrella cells) permeability to both monovalent cations and anions. This may be reversed on the basis of the concentration of the protamine, the composition of the bathing solution, and the exposure time of the urothelium to protamine. Prolonged exposure to protamine (>15 minutes) is poorly reversible and is thought to be caused by a decrease in paracellular resistance from cell lysis (Tzan et al, 1993, 1994).
In an indirect method of examining the GAG layer, Madin-Darby canine kidney (MDCK) cells were transfected with MUC-1, the major GAG of the bladder, with different tandem repeat units to vary the length of the glycosylated chain outside the cell. After this treatment, no difference in the transcellular water and urea permeability was found (Lavelle et al, 1997). In summary, the GAG layer may have importance in bacterial antiadherence and in prevention of urothelial damage by large macromolecules. However, there is no definite evidence that the GAG layer acts as the primary epithelial barrier between urine and plasma.
The Umbrella Cells
The large umbrella cells that form the primary urine-plasma barrier are unique in several ways. First, they have the ability to increase and to decrease considerably their surface area primarily at the apical (luminal) surface. Second, they may be multinucleate. Third, they have an unusual apical surface membrane, which is described as an asymmetrical unit membrane, with the outer leaflet consisting of protein plaques and lipid and with a lipid inner leaflet. Fourth, these cells maintain an extremely high gradient between the plasma and the urine in terms of water concentration, urea concentration, potassium concentration, osmolality, and pH.
Umbrella cells are so called for their shape, in that they look like umbrellas over the intermediate cell layer. They may cover several intermediate cells and have a stem that may extend to the lamina propria of the urothelium. They are capable of changing shape and can increase their surface area as the bladder fills and, conversely, decrease their surface area as the bladder empties. How this phenomenon occurs is still under debate. The primary theory is that there are a large number of subapical discoid vesicles with an asymmetrical membrane structure just under the apical membrane. These vesicles decrease in number with bladder stretch and increase the electrical capacitance of the luminal surface of the umbrella cells. This is consistent with the exocytosis of these vesicles to increase the cell surface area (Porter et al, 1967). Conversely, the discoid vesicles may be infoldings of membrane that stretch out and disappear with the requirement for increased surface area (Staehelin et al, 1972). These discoid vesicles are associated with a dense network of filaments (Hicks, 1965), which may be connected to the uroplakin plaques (Minsky and Chlapowski, 1978). The insertion of these vesicles into the apical membrane is a microfilament-dependent system, requiring ATP for insertion but not collapse, (Sarikas and Chlapowski, 1986) that does not require an intact microtubule system (Lewis and de Moura, 1982).
In membrane physiology, the primary determinant of the permeability of the lipid bilayer is the permeability of the individual leaflets. The leaflet that is least permeable will determine the overall permeability of the membrane (Negrete et al, 1996). In the umbrella cell luminal membrane, there is a unique structure where the membrane has most (70% to 90%) of the apical surface covered with protein plaque, with the remaining area consisting of hingelike regions. The composition of the hinge regions is unclear but may be a protein or lipid. The large plaque units are 0.5 µm to 12 nm thick, with subunits approximately 16 nm from center to center (Walz et al, 1995). They are composed of a class of proteins called uroplakins, which are highly conserved across several species (human, monkey, sheep, pig, dog, rabbit, rat, and mouse). There are four uroplakin proteins: UP Ia (27 kD), UP Ib (28 kD), UP II (15 kD), and UP III (47 kD). Antibodies raised against UP I proteins have shown them to be present only in the umbrella cells of the urothelium, and therefore they may act as specific markers of terminally differentiated umbrella cells (Yu et al, 1994; Sun et al, 1999).
The primary function of the uroplakin proteins is hypothesized to be part of the primary plasma-urine barrier. Uroplakins have also been shown to act as the primary attachment site of type 1 piliated uropathogenic Escherichia coli in mice, leading to apoptosis of the umbrella cells that were inhibited by the caspase inhibitor Boc-aspartyl(OMe)-fluoromethylketone (BAF) (Mulvey et al, 1998). Given the highly conserved nature of uroplakins, the same is likely to happen in humans.
Coupled with the theory that the apical asymmetrical membrane of the umbrella cells is the primary barrier of the bladder to urine is the observation that cells are joined by tight junctions consisting of four to six interconnecting strands (Peter, 1978). It is the combination of the umbrella cells and tight junction connections that creates the physical barrier to the movement of substances between urine and blood. Once the umbrella cell layer of the urothelium is breached, there is little morphologic (Peter, 1978) or electrophysiologic (Lewis et al, 1976) diffusive permeability evidence (Negrete et al, 1996; Lavelle et al, 1998, 2000) for a barrier to the movement of water and urea.
Urothelial Permeability
Epithelial permeability, including that of the urothelium, depends on a number of factors. These are passive diffusion, osmotically driven diffusion, active transport, and inertness of the membrane to the solutes to which it is exposed.
Descriptions of finite passage of substances across the urothelium are well known. In 1856, Kaupp reported that the composition and volume of urine were altered with 12-hour voiding patterns instead of hourly voiding. These changes in volume have also been noted in rats during isovolumetric cystometrograms during 3-hour periods (Sugaya et al, 1997), and the rate of water loss has also been estimated by direct measurement of passive water diffusion in vitro in the rabbit (Negrete et al, 1996). There is a passive permeability to most substances in the blood or urine (Hicks, 1975).
The diffusive permeability of the mammalian bladder to water and urea has been directly measured in several species (Lavelle et al, 2000). The permeability of water in the bladder remains remarkably constant, ranging from 4.01 to 5.74 × 10−5 cm/sec, and that of urea, 1.5 to 4.51 × 10−6 cm/sec. Water permeability value in humans is similar at 6.5 × 10−5 cm/sec (Fellows and Marshall, 1972). This value was obtained by estimating the absorption of tritiated water into the plasma after instillation of the tritiated water into the bladder of volunteers. A direct measurement of urothelial diffusive permeability in the human has not yet been made.
Breakdown of the umbrella cells in animal models of cystitis has shown increased water and urea permeability. Presumably, leakage of urine into the detrusor is also responsible for the symptoms of cystitis (Lavelle et al, 1998, 2000). This increase in urothelial permeability with cystitis is increased further by distention of the bladder. The hypothesis is that with distention of the bladder, the weakened urothelium with denuded apical umbrella cells and no real barrier in the intermediate or basal cells is further disrupted, thus allowing further egress of urine constituents into the detrusor. Similar breakdown of the apical cells is thought to occur in most forms of infectious cystitis and also in radiation cystitis.
Direct measurements of the osmotic effect on permeability have not been performed on urothelium. However, the urothelium maintains an osmotic gradient between plasma (300 mOsm/kg approximately) and urine (100 to 1500 mOsm/kg), depending on the level of water balance and diuresis of the individual. In the normal bladder, the osmotic effects of the urine appear to go unnoticed, and the patients have few or no symptoms. However, once the bladder is inflamed, such as in bladder pain syndrome/interstitial cystitis (BPS/IC), the effects of osmotic gradients become important (Gao et al, 1994).
Patients with spinal cord injury or with myelodysplasia tend to have chronic cystitis with bacteriuria and inflamed urothelium. When detrusor activity was increased in the rat by instillation of hyperosmolar compounds, this was accompanied by neurogenic inflammation, including plasma extravasation of Evans blue that could be decreased by pretreatment with the C-fiber afferent neurotoxin capsaicin (Maggi et al, 1990) indicating that hyperosmolar solutions excite afferent nerves. With increased osmolality, detrusor contractions were much stronger and accompanied by blood pressure elevations. These effects were enhanced when the bladder was pretreated with dimethyl sulfoxide to simulate cystitis conditions (Hohlbrugger and Lentsch, 1985; Hohlbrugger, 1987).
Increased permeability of the urothelium in inflammatory conditions has become the basis of the potassium sensitivity test, during which the bladder is distended with 400 mM of potassium chloride solution. Potassium penetrates the urothelium in the inflamed bladder and causes pain by activation of bladder afferent nerves (Parsons, 1996). This does not occur in normal bladders. The validity and accuracy of the intravesical potassium chloride test has not yet been proven clinically by multicenter randomized double-blind studies.
The fact that changes in the osmolality, pH, and ionic composition of the urine can influence the activity of the detrusor is important in the performance of cystometry. The nature of the infusate could alter the outcome of the test, particularly in the neurogenic bladder, where there are usually some changes in the urothelium because of the chronic bacteriuria (Hohlbrugger, 1995).
Ionic Transport
The apical membrane of the urothelium has a high electrical resistance (Lavelle et al, 1998, 2000), whereas the basolateral membrane resistance is approximately 10-fold lower (Clausen et al, 1979). Active sodium transport across the urothelium has been demonstrated (Wickham, 1964; Lewis and Diamond, 1976). Na+ channels that exist on the apical surface of the umbrella cells and in the cytoplasmic vesicles below the apical surface are primarily amiloride sensitive (inhibition) and aldosterone responsive. However, amiloride-insensitive, cation-selective, as well as amiloride-insensitive, unstable cation channels have also been identified. Both of these channels were found to be degradation products of the amiloride-sensitive Na+ channel. The amiloride-sensitive Na+ channel is hydrolyzed by serine proteases such as kallikrein and urokinase and plasmin (normally found in the urine but produced by the kidney) (Lewis et al, 1995).
Sodium that is transported into the cell is removed at the basolateral membrane by a Na+–K+ exchanger. This leaves the cell with a negative intracellular charge. The basolateral membrane contains K+ and Cl− channels, Na+–H+ exchangers, and Cl−–HCO3− exchangers. These channels and exchangers are important in recovery of cell volume during an increase in serosal osmolality (Donaldson and Lewis, 1990). Unfortunately, the precise role of the Na+ channel in the apical membrane of the umbrella cell is unknown. It is possible that the degradation of the channel might follow the filling of the bladder and that the changes in conductance of sodium may be a signaling factor for the bladder and micturition when it reaches capacity. Alternatively, it may be involved in the signaling pathway that allows insertion or removal of apical membrane on expansion of the bladder.
Key Points: Urothelium
The Urethra
The urethra is composed of striated and smooth muscles. Contraction of the longitudinal smooth muscle could play a role in stabilizing the urethra and allowing force generated by the circular muscle elements to occlude the lumen or in aiding in the opening of the bladder neck during micturition. There is controversy about the relative roles of the urethral smooth and striated circular muscles and the lamina propria in generating the urethral pressure profile, but it seems likely that both contribute (Thind, 1995). Blocking striated sphincter activity with nicotinic neuromuscular blocking agents has variable effects and may reduce urethral tone, but rarely by more than 40%, suggesting that the smooth muscles are important. Blocking sympathetic tone with α-adrenoceptor blockers may also reduce urethral pressure by about a third (Torrens and Morrison, 1987). There is little evidence for the involvement of the cholinergic innervation in generating urethral pressure.
Urethral Muscle
The smooth muscle cells in the urethra are gathered into small bundles and linked to each other by many adherens-type junctions but no gap junctions. The smooth muscle bundles in the urethral wall are thinner than in the detrusor and arranged in obvious layers. In humans and larger mammals, there is a relatively thick inner layer that is predominantly longitudinally arranged, and outside this, there is a thinner circular muscle layer. In the lamina propria of the urethra, scattered small bundles of only a few cells are often found.
Striated muscle occurs in the walls of the male and female urethra, where it forms a rhabdosphincter that is separate from the periurethral skeletal muscle of the pelvic floor. In the male, the striated muscle extends from the base of the bladder and the anterior aspect of the prostate to the full length of the membranous urethra. In the female, the striated muscle extends from the proximal urethra distally. The striated sphincter is horseshoe shaped, and the muscle cells are smaller than ordinary skeletal muscle, being 15 to 20 µm in diameter.
Morphology
The urethral stroma has been less extensively studied than the bladder stroma, but it is known to contain primarily longitudinally arranged collagen fibers and elastin fibers (Hickey et al, 1982; Huisman, 1983). The vascular filling of the urethral lamina propria is known to be of importance for urinary continence, although the magnitude of its contribution to continence is still not understood (Rud et al, 1980). Estrogen is known to increase the urethral blood flow, resulting in an increased distention of the lamina propria blood vessels (Brading, 1997a).
Impaired arterial blood supply to the urethra decreases the intraluminal pressure (Rud et al, 1980), but at present, it is not known whether it is the decrease in vascular filling or the urethral hypoxia that mediates the decrease in urethral pressure. It has been suggested that both these mechanisms may be involved because it was shown that the initial drop in urethral pressure was mediated through decreased vascular filling, whereas the later phase was due to a hypoxic effect on the urethral smooth muscle (Greenland and Brading, 1996).
Sphincter Configuration
An issue of continuing confusion is what composes the external sphincter and what the distinction is between the rhabdosphincter and the external striated sphincter proper. In the membranous urethra, a thin smooth muscle layer extends along the entire urethra in the female and throughout the prostate and its capsule in the male (Tanagho, 1982; Dixon and Gosling, 1987). On the anterior surface of the male urethra, an outer layer of circularly oriented striated muscle forms a horseshoe configuration in the adult near the prostatic apex. This striated muscle develops as a complete ring in the fetus and neonate and forms the external urethral sphincter or rhabdosphincter. The periurethral striated muscles of the pelvic floor lie external to the rhabdosphincter (see Fig. 60–2).
Despite the horseshoe configuration with the open end in the posterior direction, urethral pressure recording at the external sphincter during bladder filling increases uniformly along the entire circumference like an iris (Morita, 1989). Norepinephrine or hypogastric nerve stimulation augments this pressure, suggesting a role for adrenergic receptors and sympathetic nerves in the function of the external urethral sphincter (Kakizaki et al, 1991).
The arrangement of muscle forming the distal sphincter of the female differs from that in the male. The female has an attenuated striated sphincter mechanism, as well as additional muscle structures termed the compressor urethrae and urethrovaginal sphincter (DeLancey, 1989). The posterior wall remains rigid if there is adequate pelvic support from muscle and connective tissues. As in the male, the striated components are deficient posteriorly. This relative deficiency of periurethral striated musculature contributes to the difficulty in obtaining reliable external sphincter electromyography results during urodynamic studies in women. This attenuation of distal sphincteric muscles may contribute to urinary incontinence in females after resection of the bladder neck.
In women, urinary continence is maintained during elevations in intra-abdominal pressure by three processes. First, there is passive transmission of abdominal pressure to the proximal urethra. A guarding reflex involving an active contraction of striated muscle of the external urethral sphincter can transiently help continence (Enhorning, 1961; Tanagho, 1982). However, mere transmission of abdominal pressure to proximal urethra does not account for the entire increase in urethral pressure (Constantinou and Govan, 1982). Urethral pressure rises before cough transmission (Fig. 60–11). These findings implicate an active urethral continence (neural) mechanism in women (Constantinou and Govan, 1982). DeLancey proposes the “hammock hypothesis” that abdominal pressure transmitted through the proximal urethra presses the anterior wall against the posterior wall. The posterior wall remains rigid if there is adequate pelvic support from muscle and connective tissues. More distally, based on morphologic data, DeLancey and colleagues (DeLancey 1989, 1997; Sampselle and DeLancey, 1998) have postulated that the urethral attachments to the pubis (pubourethral) and vaginal connections to pelvic muscles and fascia actively change the position of the bladder neck and proximal urethra with voiding. This arrangement compresses the urethra against the pubis during bladder filling and straining. These attachments contain both fascia and smooth muscle (Oelrich, 1983; DeLancey, 1988, 1989). Thus urinary continence results from the combination of active muscle tone and passive anatomic coaptation.
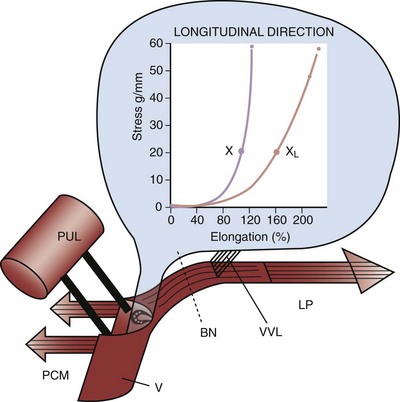
Figure 60–11 Influence of vaginal laxity on muscle force transmission and urinary continence. Inset, Stress extension curve of vagina. BN, bladder neck; LP, levator plate; PCM, pubococcygeus muscle; PUL, pubourethral ligament; V, vaginal hammock; VVL, vaginal attachment to the bladder base; X, normal elasticity; XL, vaginal laxity. The authors propose that an increase in urethral pressure before cough transmission proves that an active continence mechanism is involved in preventing stress urinary incontinence.
(From Petros PE, Ulmsten U. An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol 1993;27[Suppl. 153]:1–93.
Fiber Types of Urethral Striated Muscle
Striated muscles are characterized as slow type and twitch type. Twitch-type myofibrils can be further classified as slow and fast on the basis of functional and metabolic characteristics (Padykula, 1967). Slow-twitch fibers seem ideally suited to maintaining sphincter tone for prolonged periods, whereas fast-twitch fibers may be needed to add to sphincter tone rapidly to maintain continence when intra-abdominal pressure is abruptly increased. Similar to smooth muscle, contraction of striated muscle fibers is governed by intracellular calcium, through interactions with troponin.
The fast-twitch fibers can be recruited rapidly but also fatigue rapidly, and perform predominantly anaerobic metabolism (Markwardt and Isenberg, 1992). Fast-twitch fibers exhibit rapid bursts of contractile force and are rich in myosin ATPase that catalyzes the actin–myosin interaction. The speed of contraction may be correlated with the histochemical reaction of this ATPase and alkaline pH. In addition, fast-twitch muscles are supplied with a fast isoform of the Ca2+-ATPase, which translocates the cytosolic calcium into the abundant sarcoplasmic reticulum to allow rapid relaxation.
In contrast, slow-twitch fibers are found in greater percentage in muscles that require sustained tension, such as the pelvic levators and urethral sphincter. These muscle fibers are recruited and fatigue slowly and can perform high rates of oxidative metabolism because they possess less of the myosin ATPase activity and contain an increased expression of a slow isoform of the Ca2+-ATPase (Markwardt and Isenberg, 1992). These fibers give rise to the background electromyographic activity seen during a urodynamic evaluation.
The external urethral sphincter is composed of two parts. The periurethral striated muscle of the pelvic floor contains both fast-twitch and slow-twitch fibers. The striated muscle of the distal sphincter mechanism contains predominantly slow-twitch fibers (Elbadawi, 1984) and provides more than 50% of the static resistance (Tanagho et al, 1989). Gosling and colleagues (2000) presented histochemical evidence in humans that striated muscle within the distal urethra is composed primarily of slow-twitch myofibrils in contrast to the periurethral striated muscles of the pelvic floor, which contain fast-twitch and slow-twitch fibers. In the male, the rhabdosphincter consists of 35% fast-twitch and 65% slow-twitch fibers (Padykula and Gauthier, 1970). In the female, the ratio of slow-twitch to fast-twitch fibers is 87% slow-twitch and 13% fast-twitch.
The majority of the fast-twitch fibers and about a fourth of the slow-twitch fibers in the intramural striated muscle of the human membranous urethral sphincter show positive staining for nitric oxide synthase in the sarcolemma (Ho et al, 1998). Moreover, the striated periurethral muscles of the pelvic floor are adapted for the rapid recruitment of motor units required during increases in abdominal pressure. It has been speculated that the successful treatment of stress incontinence by pelvic floor exercises or electrostimulation is caused by the conversion of fast-twitch to slow-twitch striated muscle fibers (Bazeed et al, 1982).
In addition to striated muscle, the external sphincter appears to contain smooth muscle, which receives noradrenergic innervation. Investigators have shown that stimulation of the hypogastric nerve elicits myogenic potentials in the external urethral sphincter (Kakizaki et al, 1991). Whether this activity is the result of smooth or striated muscle is unclear. Because these potentials persist after α-adrenergic blockade, investigators postulate that it arises from striated muscle.
Lamina Propria and Paraurethral Tissue
Results are divergent regarding the clinical significance of connective tissue outside the urethra. Paraurethral tissue biopsy specimens from premenopausal women with stress incontinence contain 30% more collagen, and the diameter of the fibrils is 30% larger than in controls (Falconer et al, 1998a). Postmenopausal stress-incontinent women, on the other hand, have no difference in collagen concentration compared with their age-matched controls (Falconer et al, 1998b). Others, however, have found a decreased periurethral collagen concentration (Rechberger et al, 1993) and a decreased collagen I to collagen III ratio (Keane et al, 1997) in patients with stress incontinence.
Neural Control of the Lower Urinary Tract
Peripheral Nervous System
The lower urinary tract is innervated by three sets of peripheral nerves involving the parasympathetic, sympathetic, and somatic nervous systems (Fig. 60–12). Pelvic parasympathetic nerves arise at the sacral level of the spinal cord, excite the bladder, and relax the urethra. Lumbar sympathetic nerves inhibit the bladder body and excite the bladder base and urethra. Pudendal nerves excite the external urethral sphincter. These nerves contain afferent (sensory) as well as efferent axons (Wein, 1992; de Groat et al, 1993a; Sugaya et al, 1997; Yoshimura et al, 2008).
Figure 60–12 Diagram showing the sympathetic, parasympathetic, and somatic innervation of the urogenital tract of the male cat. Sympathetic preganglionic pathways emerge from the lumbar spinal cord and pass to the sympathetic chain ganglia (SCG) and then through the inferior splanchnic nerves (ISN) to the inferior mesenteric ganglia (IMG). Preganglionic and postganglionic sympathetic axons then travel in the hypogastric nerve (HGN) to the pelvic plexus and the urogenital organs. Parasympathetic preganglionic axons that originate in the sacral spinal cord pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. Sacral somatic pathways are contained in the pudendal nerve, which provides an innervation to the penis and the ischiocavernosus (IC), bulbocavernosus (BC), and external urethral sphincter (EUS) muscles. The pudendal and pelvic nerves also receive postganglionic axons from the caudal sympathetic chain ganglia. These three sets of nerves contain afferent axons from the lumbosacral dorsal root ganglia. PG, prostate gland; U, ureter; VD, vas deferens.
Parasympathetic Pathways
Parasympathetic preganglionic neurons innervating the lower urinary tract are located in the lateral part of the sacral intermediate gray matter in a region termed the sacral parasympathetic nucleus (Nadelhaft et al, 1980; Morgan et al, 1981; de Groat et al, 1993a; Morgan et al, 1993; de Groat et al, 1996). Parasympathetic preganglionic neurons send axons through the ventral roots to peripheral ganglia, where they release the excitatory transmitter acetylcholine (de Groat and Booth, 1993). Parasympathetic postganglionic neurons in humans are located in the detrusor wall layer as well as in the pelvic plexus. This is an important fact to remember because patients with cauda equina or pelvic plexus injury are neurologically decentralized but may not be completely denervated. Cauda equina injury allows possible afferent and efferent neuron interconnection at the level of the intramural ganglia (de Groat et al, 1993a; de Groat et al, 1996).
Sympathetic Pathways
Sympathetic outflow from the rostral lumbar spinal cord provides a noradrenergic excitatory and inhibitory input to the bladder and urethra (Andersson, 1993). Activation of sympathetic nerves induces relaxation of the bladder body and contraction of the bladder outlet and urethra, which contribute to urine storage in the bladder. The peripheral sympathetic pathways follow a complex route that passes through the sympathetic chain ganglia to the inferior mesenteric ganglia and then through the hypogastric nerves to the pelvic ganglia.
Somatic Pathways
The external urethral sphincter motoneurons are located along the lateral border of the ventral horn, commonly referred to as the Onuf nucleus (Fig. 60–13) (Thor et al, 1989). Sphincter motoneurons also exhibit transversely oriented dendritic bundles that project laterally into the lateral funiculus, dorsally into the intermediate gray matter, and dorsomedially toward the central canal.
Figure 60–13 Cross section of sacral spinal cord; neuroanatomic distribution of primary afferent and efferent components of storage and micturition reflexes. For purposes of clarity, afferent components are shown only on the left, and efferent components are shown only on the right. Both components are, of course, distributed bilaterally and thus overlap extensively. Visceral afferent components represent bladder, urethral, and genital (glans penis or clitoris) afferent fibers contained in the pelvic and pudendal nerves. Cutaneous perineal afferent components represent afferent fibers that innervate the perineal skin contained in the pudendal nerve. Muscle spindle afferent components represent Ia/b afferent fibers contained in the levator ani nerve that innervate muscle spindles in the levator ani muscle. EUS, external urethral sphincter; LCP, lateral collateral projection; MCP, medial collateral projection; SPN, sacral parasympathetic nucleus.
Afferent Pathways
Afferent axons in the pelvic, hypogastric, and pudendal nerves transmit information from the lower urinary tract to the lumbosacral spinal cord (de Groat, 1986; Janig and Morrison, 1986; Yoshimura et al, 2008). The primary afferent neurons of the pelvic and pudendal nerves are contained in sacral dorsal root ganglia (DRG), whereas afferent innervation in the hypogastric nerves arises in the rostral lumbar DRG. The central axons of the DRG neurons carry the sensory information from the lower urinary tract to second-order neurons in the spinal cord (Morgan et al, 1981; de Groat, 1986; Thor et al, 1989; de Groat et al, 1996) (see Fig. 60–13). Visceral afferent fibers of the pelvic (Morgan et al, 1981) and pudendal (Thor et al, 1989) nerves enter the cord and travel rostrocaudally within the Lissauer tract.
Pelvic nerve afferents, which monitor the volume of the bladder and the amplitude of the bladder contraction, consist of myelinated (Aδ) and unmyelinated (C) axons (Table 60–3). During neuropathic conditions and possibly inflammatory conditions, there is recruitment of C fibers that form a new functional afferent pathway that can cause urgency incontinence and possibly bladder pain.
Sensing bladder volume is of particular relevance during urine storage. On the other hand, afferent discharges that occur during a bladder contraction have an important reflex function and appear to reinforce the central drive that maintains the detrusor contraction. Afferent nerves that respond to both distention and contraction, that is, “in-series tension receptors,” have been identified in the pelvic and hypogastric nerves of cats and rats (see Table 60–3) (Iggo, 1955; Floyd et al, 1976; Morrison, 1997). Afferents that respond only to bladder filling have been identified in the rat bladder (Morrison, 1998) and appear to be volume receptors, possibly sensitive to stretch of the mucosa. In the cat bladder, some in-series tension receptors may also respond to bladder stretch (Downie and Armour, 1992). In the rat, there is evidence that many C bladder afferents are volume receptors that do not respond to bladder contractions, a property that distinguishes them from in-series tension receptors (Morrison, 1998).
In the mouse pelvic nerve, four classes of bladder afferents (serosal, muscular, muscular/urothelial, and urothelial) have been identified based on responses to receptive field stimulation with different mechanical stimuli, including probing, stretch, and stroking the urothelium. Both low-threshold, representing 65% to 80% of the total population, and high-threshold stretch-sensitive muscular afferents are present (Daly et al, 2007; Xu and Gebhart, 2008). The muscular afferents can be sensitized by application of a combination of inflammatory mediators (bradykinin, serotonin, prostaglandin, and histamine at pH 6.0) (Xu and Gebhart, 2008).
In the guinea pig bladder, four classes of C-fiber afferents have also been detected (Zagorodnyuk et al, 2006, 2007). The first class, muscle mechanoreceptors, are activated by stretch but not by mucosal stroking with light (0.05 to 0.1 mN) von Frey hairs or by hypertonic saline, α,β-methylene ATP or capsaicin. Removal of the urothelium did not affect their stretch-induced firing. The second class, muscle-mucosal mechanoreceptors, are activated by both stretch and mucosal stroking, by hypertonic solution, α,β-methylene-ATP but not by capsaicin. Stroking- and stretch-induced firing is significantly reduced by removal of the urothelium. The third class of afferents, mucosal high-responding mechanoreceptors, are stretch insensitive but can be activated by mucosal stroking, hypertonic solution, α,β-methylene-ATP, and capsaicin. Stroking-induced activity is reduced by removal of the urothelium. The fourth class of afferents, mucosal low-responding mechanoreceptors, are stretch insensitive but can be weakly activated by mucosal stroking but not by hypertonic solution, α,β-methylene-ATP, or capsaicin. Removal of the urothelium reduces stroking-induced firing. A recent study by Zagorodnyuk and colleagues (2009) also showed that mechanotransduction during stretch or mucosal stroking in the second and third classes of afferents, stretch-sensitive muscle-mucosal mechanoreceptors, and stretch-insensitive, mucosal high-responding afferents, are not dependent upon Ca2+-dependent exocytotic release of mediators or ATP, but are likely to be induced by benzamil-sensitive, stretch-activated ion channels on nerve endings. This is because stretch- or stroking-induced firing of these afferents are not affected by the nonselective P2 purinoreceptor antagonist or Ca2+-free solution, but are inhibited by benzamil, a mechanogated channel blocker. These results suggest that substances released from the urothelium during mechanical stimulation, such as ATP, may not be involved in the modulation of the activity of bladder afferents located in the vicinity of the urothelium, at least in the normal condition, which is against the current theory of the urothelium–afferent interaction by urothelially released substances during bladder stretch.
Species differences, as well as differences of nomenclature, might account for some of the variations in reported properties of bladder afferents. For example, the conduction velocity that differentiates Aδ and C fibers is 2 m/sec in the cat, whereas it is 1.3 m/sec in the rat (Waddell et al, 1989). In the cat, Aδ bladder afferents appear to be low-threshold mechanoreceptors (Häbler et al, 1993), whereas C-bladder afferents (Häbler et al, 1990) are generally mechanoinsensitive (“silent C fibers”) (see Table 60–3). Some of the latter may be nociceptive and have been found to be sensitized by intravesical administration of chemicals (such as high potassium), low pH, high osmolality, and irritants such as capsaicin (Fig. 60–14) (Maggi et al, 1987; McMahon and Abel, 1987; Häbler et al, 1990; Wen et al, 1994; Wen and Morrison, 1994, 1996; Zagorodnyuk et al, 2009). After exposure to these substances, the sensitivity of bladder mechanoreceptors to distention increases, and some “silent” afferents become mechanoreceptive.
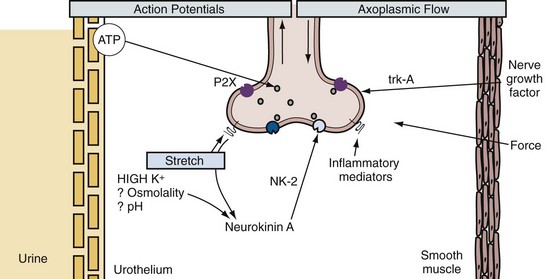
Figure 60–14 Actions of chemical mediators that may sensitize mechanosensory nerve endings in the bladder mucosa. Adenosine triphosphate (ATP) can be released from the urothelium and may sensitize the mechanoreceptors, which respond to stretch of the mucosa during bladder distention. Neuropeptides transported to the sensory ending by axoplasmic transport may be released during distention and chemical stimulation, and neurokinin A can act on NK2 autoreceptors, which sensitize the mechanosensitive endings. This mechanism can be induced by high urinary potassium concentrations and possibly by other sensitizing solutions within the bladder lumen, such as those with high osmolality or low pH; the presence in the tissues of inflammatory mediators may also sensitize the endings. The smooth muscle can generate force that may influence some mucosal endings, and the production of nerve growth factor is another mechanism that can influence the mechanosensitivity of the sensory ending through the TrkA receptor.
In the urethra, afferent nerves are distributed between the muscle fibers, around blood vessels, in the urothelium, and in a dense suburothelial plexus (Crowe et al, 1986; Tainio, 1993; Fahrenkrug and Hannibal, 1998). In some species, afferent nerves extend to the luminal surface of the urothelium. The striated sphincter muscle that surrounds the urethra receives a very sparse afferent innervation that is localized primarily to nerve bundles passing between the muscle bundles. Specialized tension receptors (muscle spindles) that are innervated by large-diameter myelinated group IA afferents and that are prominent in most striated muscles are absent (Gosling et al, 1981) or are present in low density (Lassmann, 1984) in striated sphincter muscles.
Afferent Nerves and Interorgan Cross Sensitization
Axonal tracing studies revealed that a subpopulation (6% to 21%) of afferent neurons in dorsal root ganglia of the rat and mouse can innervate multiple pelvic organs (Keast and de Groat, 1992; Christianson et al, 2007). In the rat, the largest percentage of these neurons is present in the thoracolumbar DRG; whereas in the mouse the larger percentage is in the lumbosacral DRG. Subsequent studies provided evidence that chemical irritation of one organ (either the urinary bladder or the colon) can facilitate/sensitize the activity of the other organ (Pezzone et al, 2005). Electrophysiologic experiments showed that acute colonic irritation can sensitize bladder C-fiber afferents to mechanical and chemical stimuli (Ustinova et al, 2006, 2007) and enhance the firing of lumbosacral spinal interneurons receiving afferent input from the bladder (Qin et al, 2005). Lumbosacral afferent neurons in the L6-S2 DRG innervating both the colon and bladder (“convergent neurons”) exhibited decreased voltage and current thresholds for action potential firing three days after colonic irritation with trinitrobenzene sulfonic acid (TNBS) in rats (Malykhina et al, 2006). The effect persisted for 30 days in the absence of overt colonic inflammation. Colitis also enhanced the responses to capsaicin and increased the peak amplitude of tetrodotoxin-resistant Na+ currents in bladder afferent neurons isolated from the L6-S2 DRG (Malykhina et al, 2004).
Changes in bladder function after colonic inflammation also appear to be mediated by a change in the cholinergic efferent pathway to the bladder (Noronha et al, 2007). During the active colonic inflammation, three days after TNBS instillation into the colon, the bladder was not inflamed, but the contractions of bladder strips induced by electrical field stimulation or carbachol, a muscarinic receptor agonist, were reduced, while the contractions induced by potassium (KCl) were not changed. During and after recovery of the colonic inflammation (15 to 30 days), the contractile responses of the bladder returned to normal. It was suggested that bladder dysfunction was mediated by visceral organ cross talk induced by sensitization of a subpopulation of afferents innervating both the bladder and colon.
Key Points: Afferent Pathways
Reflex Circuitry Controlling Micturition
Multiple reflex pathways organized in the brain and spinal cord mediate coordination between the urinary bladder and the urethra. The central pathways controlling lower urinary tract function are organized as simple on-off switching circuits (Fig. 60–15) that maintain a reciprocal relationship between the urinary bladder and the urethral outlet (de Groat, 1975; de Groat et al, 1993a). The principal reflex components of these switching circuits are listed in Table 60–4 and illustrated in Figure 60–16. Some reflexes promote urine storage, whereas others facilitate voiding. It is also possible that individual reflexes might be linked together in a serial manner to create complex feedback mechanisms. For example, the bladder to external urethral sphincter guarding reflex that triggers sphincter contractions during bladder filling could, in turn, activate sphincter muscle afferents that initiate an inhibition of the parasympathetic excitatory pathway to the bladder. Thus a bladder to sphincter to bladder reflex pathway could, in theory, contribute to the suppression of bladder activity during urine storage. Alterations in these primitive reflex mechanisms may contribute to neurogenic bladder dysfunction. Direct activation of these reflexes by electrical stimulation of the sacral spinal roots very likely contributes to therapeutic effects of sacral nerve root neuromodulation (Dijkema et al, 1993; Chancellor and Chartier-Kastler, 2000).
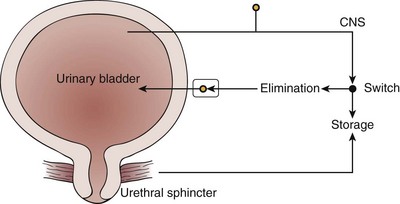
Figure 60–15 Diagram illustrating the anatomy of the lower urinary tract and the “switchlike” function of the micturition reflex pathway. During urine storage, a low level of afferent activity activates efferent input to the urethral sphincter. A high level of afferent activity induced by bladder distention activates the switching circuit in the central nervous system (CNS), producing firing in the efferent pathways to the bladder, inhibition of the efferent outflow to the sphincter, and urine elimination.
Table 60–4 Reflexes to the Lower Urinary Tract
| AFFERENT PATHWAY | EFFERENT PATHWAYS | CENTRAL PATHWAY |
|---|---|---|
| Urine Storage | ||
| Low-level vesical afferent activity (pelvic nerve) | External sphincter contraction (somatic nerves) | Spinal reflexes |
| Internal sphincter contraction (sympathetic nerves) | ||
| Detrusor inhibition (sympathetic nerves) | ||
| Ganglionic inhibition (sympathetic nerves) | ||
| Sacral parasympathetic outflow inactive | ||
| Micturition | ||
| High-level vesical afferent activity (pelvic nerve) | Inhibition of external sphincter activity | Spinobulbospinal reflex |
| Inhibition of sympathetic outflow | ||
| Activation of parasympathetic outflow to the bladder | ||
| Activation of parasympathetic outflow to the urethra | ||
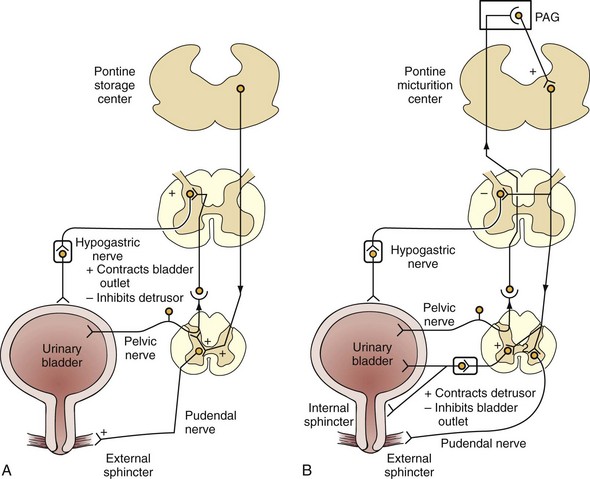
Figure 60–16 Mechanism of storage and voiding reflexes. A, Storage reflexes. During the storage of urine, distention of the bladder produces low-level bladder afferent firing. Afferent firing, in turn, stimulates the sympathetic outflow to the bladder outlet (base and urethra) and pudendal outflow to the external urethral sphincter. These responses occur by spinal reflex pathways and represent “guarding reflexes,” which promote continence. Sympathetic firing also inhibits detrusor muscle and transmission in bladder ganglia. B, Voiding reflexes. At the initiation of micturition, intense vesical afferent activity activates the brainstem micturition center, which inhibits the spinal guarding reflexes (sympathetic and pudendal outflow to the urethra). The pontine micturition center also stimulates the parasympathetic outflow to the bladder and internal sphincter smooth muscle. Maintenance of the voiding reflex is through ascending afferent input from the spinal cord, which may pass through the periaqueductal gray matter (PAG) before reaching the pontine micturition center.
The Storage Phase of the Bladder
Intravesical pressure measurements during bladder filling in both humans and animals reveal low and relatively constant bladder pressures when bladder volume is below the threshold for inducing voiding (Fig. 60–17). The accommodation of the bladder to increasing volumes of urine is primarily a passive phenomenon dependent on the intrinsic properties of the vesical smooth muscle and stroma, as well as the quiescence of the parasympathetic efferent pathway (Torrens and Morrison, 1987; de Groat et al, 1993a; Yoshimura et al, 2008). The bladder to sympathetic reflex also contributes as a negative feedback or urine storage mechanism that promotes closure of the urethral outlet and inhibits neurally mediated contractions of the bladder during bladder filling (de Groat and Theobald, 1976) (see Table 60–4). Reflex activation of the sympathetic outflow to the lower urinary tract can be triggered by afferent activity induced by distention of the urinary bladder (de Groat and Theobald, 1976; de Groat et al, 1993a). This reflex response is organized in the lumbosacral spinal cord and persists after transection of the spinal cord at the thoracic levels (Fig. 60–18). However, this bladder to sympathetic mechanism to suppress bladder contractions during urine storage may be weak in humans, given that bilateral retroperitoneal lymph node dissection, in which the sympathetic chains are destroyed, has no discernible alteration of filling or storage function in humans.
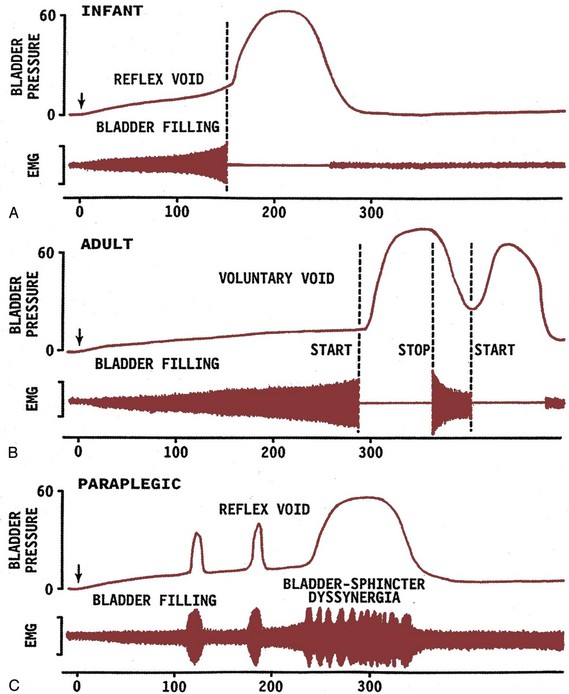
Figure 60–17 Combined cystometrogram and sphincter electromyogram (EMG) comparing reflex voiding responses in an infant (A) and in a paraplegic patient (C) with a voluntary voiding response in an adult (B). The x-axis in all records represents bladder volume in milliliters, and the y-axis represents bladder pressure in centimeters of water and electrical activity of the electromyographic recording. On the left side of each trace, the arrows indicate the start of a slow infusion of fluid into the bladder (bladder filling). Vertical dashed lines indicate the start of sphincter relaxation that precedes by a few seconds the bladder contraction in A and B. In B, note that a voluntary cessation of voiding (stop) is associated with an initial increase in sphincter electromyographic activity followed by a reciprocal relaxation of the bladder. A resumption of voiding is again associated with sphincter relaxation and a delayed increase in bladder pressure. On the other hand, in the paraplegic patient (C), the reciprocal relationship between bladder and sphincter is abolished. During bladder filling, transient uninhibited bladder contractions occur in association with sphincter activity. Further filling leads to more prolonged and simultaneous contractions of the bladder and sphincter (bladder-sphincter dyssynergia). Loss of the reciprocal relationship between bladder and sphincter in paraplegic patients interferes with bladder emptying.
(From de Groat WC. Basic neurophysiology and neuropharmacology. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth [UK]: Health Publications; 1999. p. 112.)
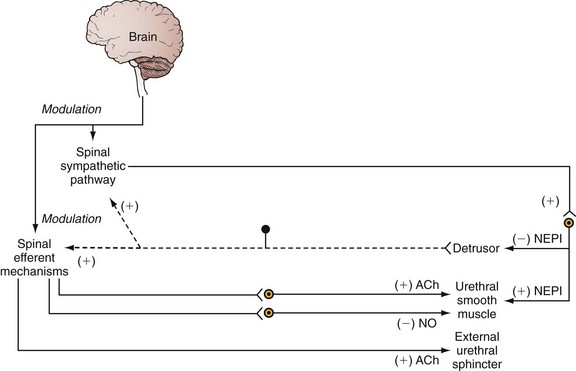
Figure 60–18 Diagram showing bladder to urethra reflex pathways. Afferent pathway (dashed line) from the detrusor activates spinal reflex mechanisms that induce firing in somatic cholinergic nerves to the external urethral sphincter, sympathetic adrenergic nerves to the urethral smooth muscle, and cholinergic and nitrergic nerves to the urethral smooth muscle. Bulbospinal pathways from the brain can modulate these spinal reflex mechanisms. ACh, acetylcholine; NEPI, norepinephrine; NO, nitric oxide; excitatory (+) and inhibitory (−) mechanisms.
During bladder filling, the activity of the sphincter electromyogram also increases (see Fig. 60–17), reflecting an increase in efferent firing in the pudendal nerve and an increase in outlet resistance that contributes to the maintenance of urinary continence. Pudendal motoneurons are activated by bladder afferent input (the guarding reflex) (Park et al, 1997), whereas during micturition the motoneurons are reciprocally inhibited (de Groat et al, 1993a). External urethral sphincter motoneurons are also activated by urethral or perineal afferents in the pudendal nerve (Fedirchuk et al, 1992). This reflex may represent, in part, a continence mechanism that is activated by proprioceptive afferent input from the urethra or pelvic floor and that induces closure of the urethral outlet. These excitatory sphincter reflexes are organized in the spinal cord. Inhibition of external urethral sphincter reflex activity during micturition is dependent, in part, on supraspinal mechanisms, because it is weak or absent in chronic spinal animals and humans, resulting in simultaneous contractions of bladder and sphincter (i.e., detrusor-sphincter dyssynergia) (Rossier and Ott, 1976; Blaivas, 1982).
Sphincter to Bladder Reflexes
It is well known that stimulation of somatic afferent pathways projecting in the pudendal nerve to the caudal lumbosacral spinal cord can inhibit voiding function. The inhibition can be induced by activation of afferent input from various sites, including the penis, vagina, rectum, perineum, urethral sphincter, and anal sphincter (de Groat et al, 1979, 1993a, 2001). Electrophysiologic studies in cats showed that the inhibition was mediated by suppression of interneuronal pathways in the sacral spinal cord and also by direct inhibitory input to the parasympathetic preganglionic neurons (de Groat et al, 1982).
On the basis of experiments in our laboratory and the review of medical literature, we believe that contractions of the external urethral sphincter, and possibly other pelvic floor striated muscles, stimulate firing in muscle proprioceptive afferents, which then activate central inhibitory mechanisms to suppress the micturition reflex (Fig. 60–19). A similar inhibitory mechanism has been identified in monkeys by directly stimulating the anal sphincter muscle (McGuire et al, 1983). In monkeys, at least part of the inhibitory mechanism must be localized in the spinal cord, because it persisted in T4 chronic paraplegic animals.
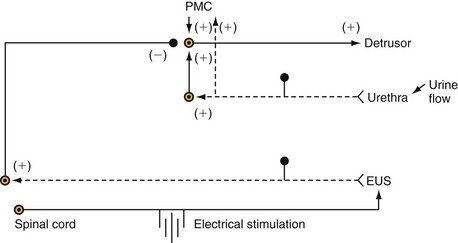
Figure 60–19 Urethra to bladder reflexes. Activity in afferent nerves (dashed lines) from the urethra can facilitate parasympathetic efferent outflow to the detrusor by means of a supraspinal pathway passing through the pontine micturition center (PMC), as well as a spinal reflex pathway. Afferent input from the external urethral sphincter (EUS) can inhibit parasympathetic outflow to the detrusor through a spinal reflex circuit. Electrical stimulation of motor axons in the S1 ventral root elicits an EUS contraction and EUS afferent firing that, in turn, inhibits reflex bladder activity, excitatory (+) and inhibitory (−) mechanisms.
The Emptying Phase of the Bladder
The storage phase of the bladder can be switched to the voiding phase either involuntarily (reflexively) or voluntarily. The former is readily demonstrated in the human infant or in patients with neuropathic bladder when the bladder wall tension due to increased volume of urine exceeds the micturition threshold. At this point, increased afferent firing from tension receptors in the bladder reverses the pattern of efferent outflow, producing firing in the sacral parasympathetic pathways and inhibition of sympathetic and somatic pathways. The expulsion phase consists of an initial relaxation of the urethral sphincter (see Fig. 60–17) followed in a few seconds by a contraction of the bladder, an increase in bladder pressure, and the flow of urine. Relaxation of the urethral smooth muscle during micturition is mediated by activation of a parasympathetic pathway to the urethra that triggers the release of nitric oxide, an inhibitory transmitter (Andersson, 1993; Bennett et al, 1995), and by removal of excitatory inputs to the urethra. Secondary reflexes elicited by flow of urine through the urethra facilitate bladder emptying (Torrens and Morrison, 1987; de Groat et al, 1993a; Jung et al, 1999). These reflexes require the integrative action of neuronal populations at various levels of the neuraxis (see Fig. 60–16A). The parasympathetic outflow to the detrusor and urethra has a more complicated central organization involving spinal and spinobulbospinal pathways passing through a micturition center in the pons (pontine micturition center) (see Fig. 60–16B).
Urethra to Bladder Reflexes
A landmark in the historical progress of neurourology is the contribution of Barrington. Using his keen observational skills, Barrington (1931, 1941) reported that urine flow or mechanical stimulation of the urethra with a catheter could excite afferent nerves that, in turn, facilitated reflex bladder contractions in the anesthetized cat (see Fig. 60–19). He proposed that this facilitatory urethra to bladder reflex could promote complete bladder emptying. Barrington identified two components of this reflex. One component was activated by a somatic afferent pathway in the pudendal nerve and produced facilitation by a supraspinal mechanism involving the pontine micturition center (Barrington, 1931) (see Fig. 60–19). Studies have confirmed the existence of this type of reflex by the pudendal nerve because low-frequency electrical stimulation of afferent axons in the pudendal nerve in humans, or the deep perineal nerve (a caudal branch of the pudendal nerve) in cats, can initiate reflex bladder contractions and voiding (Shefchyk and Buss, 1998; Boggs et al, 2005). The other component was activated by a visceral afferent pathway in the pelvic nerve and produced facilitation by a spinal reflex mechanism (Barrington, 1941).
Studies (Jung et al, 1999) in the anesthetized rat have also provided additional support for Barrington’s findings (Dokita et al, 1991). Measurements of reflex bladder contractions, under isovolumetric conditions during continuous urethral perfusion (0.075 mL/min), revealed that the frequency of micturition reflexes was significantly reduced when urethral perfusion was stopped or after infusion of lidocaine (1%) into the urethra. Intraurethral infusion of nitric oxide donors (S-nitroso-N-acetylpenicillamine [SNAP], or nitroprusside, 1 to 2 mM) markedly decreased urethral perfusion pressure (approximately 30%) and decreased the frequency of reflex bladder contractions (45% to 75%) but did not change the amplitude of bladder contractions (Fig. 60–20). Desensitization of the urethral afferent with intraurethral capsaicin also dramatically altered the micturition reflex (Fig. 60–21). It was concluded that activation of urethral afferents during urethral perfusion could modulate the micturition reflex in the rat. This may be an explanation of why stress incontinence and urgency incontinence often occur together in women. Jung and colleagues (1999) speculated that in women with mixed incontinence, leakage of urine into the urethra can stimulate afferents and induce or increase detrusor overactivity. The theory is that stress incontinence can induce urgency incontinence (Lavelle et al, 2000). Surgical cure of the stress incontinence of women with mixed incontinence has resolved the urgency incontinence in up to half of the patients.
Figure 60–20 Effects of intraurethral S-nitroso-N-acetylpenicillamine (SNAP) on the bladder pressure (Pves) and urethral pressure (Pura) in normal female rats. A, Before treatment. B, After intraurethral administration of SNAP (2 mM). Urethral perfusion pressure immediately decreased. In addition, bladder contraction frequency was significantly decreased. The duration of reflex urethral relaxation was increased.
(From Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex: implication for the relationship between stress incontinence and detrusor instability. J Urol 1999;162:204–12.)
Figure 60–21 Effects of intraurethral capsaicin on the bladder pressure (Pves) and urethral pressure (Pura) in normal female rats. A, Before treatment. B, After intraurethral administration of capsaicin (100 µM). Initially, intraurethral capsaicin instillation increased the bladder contraction frequency, but 30 minutes after continuous infusion, the activity was blocked.
(From Jung SY, Fraser MO, Ozawa H, et al: Urethral afferent nerve activity affects the micturition reflex: implication for the relationship between stress incontinence and detrusor instability. J Urol 1999;162:204–12.)
Spinal and Supraspinal Pathways Involved in the Micturition Reflex
Spinal Cord
In the spinal cord, afferent pathways terminate on second-order interneurons that relay information to the brain or to other regions of the spinal cord, including the preganglionic and motor nuclei. Because disynaptic or polysynaptic pathways, but not monosynaptic pathways, mediate bladder, urethral, and sphincter reflex, interneuronal mechanisms must play an essential role in the regulation of lower urinary tract function. Electrophysiologic (de Groat et al, 1981; Araki and de Groat, 1997) and neuroanatomic (Birder and de Groat, 1993; Vizzard et al, 1995; Nadelhaft and Vera, 1996; Sugaya et al, 1997) techniques have identified lower urinary tract interneurons in the same regions of the cord that receive afferent input from the bladder.
As shown in Figure 60–13, horseradish peroxidase labeling techniques in the cat revealed that afferent projections from the external urethral sphincter and levator ani muscles (i.e., pelvic floor) project into different regions of the sacral spinal cord. The external urethral sphincter afferent terminals are located in the superficial layers of the dorsal horn and at the base of the dorsal horn (laminae V to VII and lamina X), whereas the levator ani afferents project into a region just lateral to the central canal and extend into the medial ventral horn. The external urethral sphincter afferents overlap closely with the central projections of visceral afferents in pelvic nerve that innervate the bladder and urethra (Morgan et al, 1981). Intracellular labeling experiments also showed that the dendritic patterns of external urethral sphincter motoneurons (Sasaki, 1994) and parasympathetic preganglionic neurons (Morgan et al, 1993) are similar. Pharmacologic experiments revealed that glutamic acid is the excitatory transmitter in these pathways. In addition, approximately 15% of interneurons medial to the sacral parasympathetic nucleus in laminae V through VII make inhibitory synaptic connections with the preganglionic neurons (de Groat et al, 1996). These inhibitory neurons release γ-aminobutyric acid (GABA) and glycine. Reflex pathways that control the external sphincter muscles also use glutamatergic excitatory and GABAergic and glycinergic inhibitory interneuronal mechanisms.
Tracing with neurotropic viruses, such as pseudorabies virus, has been particularly useful. Pseudorabies virus can be injected into a target organ and then move intra-axonally from the periphery to the central nervous system. In the nervous system, the virus can replicate and then pass retrogradely across synapses to infect second- and third-order neurons in the neural pathways (Vizzard et al, 1995; Nadelhaft and Vera, 1996; Sugaya et al, 1997). Because pseudorabies virus can be transported across many synapses, it could sequentially infect all the neurons that connect directly or indirectly to the lower urinary tract. Interneurons identified by retrograde transport of pseudorabies virus injected into the urinary bladder are located in the region of the sacral parasympathetic nucleus, the dorsal commissure, and the superficial laminae of the dorsal horn (de Groat et al, 1996; Nadelhaft and Vera, 1996; Sugaya et al, 1997). A similar distribution of labeled interneurons has been noted after injection of virus into the urethra (Vizzard et al, 1995) or the external urethral sphincter (Nadelhaft and Vera, 1996), indicating a prominent overlap of the interneuronal pathways controlling the various target organs of the lower urinary tract.
The micturition reflex can be modulated at the level of the spinal cord by interneuronal mechanisms activated by afferent input from cutaneous and striated muscle targets. Micturition reflex can also be modulated by inputs from visceral organs (de Groat et al, 1975; McGuire, 1977; de Groat, 1978; de Groat et al, 1981; McMahon and Morrison, 1982; Torrens and Morrison, 1987; de Groat et al, 1993a; Morrison et al, 1995; Yoshimura et al, 2008). Stimulation of afferent fibers from various regions (anus, colon-rectum, vagina, uterine cervix, penis, perineum, pudendal nerve) can inhibit the firing of sacral interneurons evoked by bladder distention (de Groat et al, 1981). This inhibition may be a result of presynaptic inhibition at primary afferent terminals or be due to direct postsynaptic inhibition of the second-order neurons. Direct postsynaptic inhibition of bladder preganglionic neurons can also be elicited by stimulation of somatic afferent axons in the pudendal nerve or visceral afferents from the distal bowel (de Groat and Ryall, 1969; de Groat, 1978). Suppression of detrusor overactivity in patients by sacral root stimulation may reflect, in part, activation of the afferent limb of these visceral-bladder and somatic-bladder inhibitory reflexes (Wheeler et al, 1992; Bosch and Groen, 1995; Chancellor and Chartier-Kastler, 2000).
Pontine Micturition Center and Brainstem Modulatory Mechanisms
Various studies indicate that the micturition reflex is normally mediated by a spinobulbospinal reflex pathway passing through relay centers in the brain (see Fig. 60–16B) (de Groat, 1975; Torrens and Morrison, 1987; de Groat et al, 1993a; Yoshimura et al, 2008). Studies in animals by use of brain-lesioning techniques revealed that neurons in the brainstem at the level of the inferior colliculus have an essential role in the control of the parasympathetic component of micturition (Torrens and Morrison, 1987; de Groat et al, 1993a; Yoshimura and de Groat, 1997; Yoshimura et al, 2008). Removal of areas of brain above the colliculus by intercollicular decerebration usually facilitates micturition by elimination of inhibitory inputs from more rostral centers. However, transections at any point below the colliculi abolish micturition.
The dorsal pontine tegmentum has been firmly established as an essential control center for micturition in normal subjects. First described by Barrington (1921), it has subsequently been called the Barrington nucleus, the pontine micturition center (Blok and Holstege, 1997), or the M region (Blok and Holstege, 1996; Holstege et al, 1996) because of its medial location.
In addition to providing axonal inputs to the locus ceruleus and the sacral spinal cord (Ding et al, 1995; Otake and Nakamura, 1996; Valentino et al, 1996), neurons in the pontine micturition center (PMC) also send axon collaterals to the paraventricular thalamic nucleus, which is thought to be involved in the limbic system modulation of visceral behavior (Otake and Nakamura, 1996). Some neurons in the PMC also project to the periaqueductal gray region (PAG) (Blok et al, 1998), which regulates many visceral activities as well as pain pathways (Valentino et al, 1995). Thus neurons in the PMC communicate with multiple supraspinal neuronal populations that may coordinate micturition with other functions of the organism. Although the circuitry in humans is uncertain, brain imaging studies have revealed increases in blood flow in this region of the pons during micturition (Blok et al, 1997b). This change presumably reflects increases in neuronal activity. Thus the PMC appears critical for micturition across species.
Neurons in the PMC provide direct synaptic inputs to sacral preganglionic neurons (Blok and Holstege, 1997) as well as to GABAergic neurons in the sacral dorsal commissure region (Blok et al, 1997a). The former neurons carry the excitatory outflow to the bladder, whereas the latter neurons are thought to be important in mediating an inhibitory influence on external urethral sphincter motoneurons (Blok et al, 1998). Because of these reciprocal connections, the PMC can promote bladder–sphincter synergy. Studies in rats indicate that activation of bladder preganglionic neurons by input from the PMC can be blocked by inotropic glutamate receptor antagonists, suggesting that neurons in the PMC use glutamate as a neurotransmitter (Matsumoto et al, 1995a).
Besides the PMC region, transneuronal virus tracing methods have identified many populations of neurons in the brainstem that are involved in the control of bladder, urethra, and the urethral sphincter, including medullary raphe nuclei, which contain serotonergic neurons; the locus coeruleus, which contains noradrenergic neurons; PAG; and the A5 noradrenergic cell group (Nadelhaft and Vera, 1992; Vizzard et al, 1995; Sugaya et al, 1997). Other anatomical studies in which anterograde tracer substances were injected into brain areas and then identified in terminals in the spinal cord are consistent with the virus tracing data. Projections from neurons in the lateral pons (L-region or nucleus locus subcoeruleus) terminate rather selectively in the sphincter motor nucleus to enhance the contraction of pelvic floor muscle and increased urethral pressure during the storage phase in cats, providing evidence that this area in the lateral pons functions as “the pontine continence center” (Blok, 2002; Sugaya et al, 2003). The parabrachial nuclei (PBN) at the dorsolateral pons, which send projections to the sacral spinal cord neurons, are also known to be involved in the control of micturition (Lumb and Morrison, 1987; de Groat et al, 1993b). A recent study in rats has shown that firing of PBN neurons corresponds to bladder contraction during cystometry and that the medial subnuclei of PBN plays an important role in the mediation of normal bladder contractions (Liu et al, 2007).
In the midbrain, there is increasing evidence that the mesencephalic PAG, which directly receives information of bladder fullness by ascending spinal tract neurons and sends projections to the PMC, seems to play an important role in the integration of the micturition reflex (Blok, 2002; Holstege, 2005). Stimulation of the pelvic nerve results in short latency potentials in the caudal periaqueductal gray (Noto et al, 1991). Electrical and chemical stimulation of the ventrolateral PAG induces micturition in rats and cats (Matsuura et al, 2000; Taniguchi et al, 2002), and this PAG-mediated micturition reflex seems to be induced by subsequent activation of the PMC following PAG stimulation (Matsuura et al, 2000). In cats, there are different patterns of neuronal firing in response to storage/micturition cycles in the PAG, and stimulation of the rostral PAG can inhibit the micturition reflex (Liu et al, 2004; Numata et al, 2008). Thus the PAG appears to function as a relay center between the PMC and spinal cord neurons that are critically involved in the excitatory and inhibitory control of the micturition reflex.
Central Pathways That Modulate the Micturition Reflex
Transneuronal virus tracing methods have identified virus-infected cells in several regions of the hypothalamus and the cerebral cortex after injection of pseudorabies virus into the lower urinary tract in animals (Nadelhaft and Vera, 1992; Vizzard et al, 1995; Sugaya et al, 1997). Neurons in the cortex were located primarily in the medial frontal cortex. Tracers injected into the paraventricular nucleus of the hypothalamus labeled terminals in the sacral parasympathetic nucleus as well as the sphincter motor nucleus (Holstege and Mouton, 2003). The influence of the cortex on voiding function could be mediated by a number of pathways, including direct cortical projections from the prefrontal cortex and insular cortex to the PMC or projections through the hypothalamus and the extrapyramidal system (de Groat et al, 1993a). Studies in humans indicate that voluntary control of voiding is dependent on connections between the frontal cortex and the septal-preoptic region of the hypothalamus, as well as on connections between the paracentral lobule and the brainstem. Lesions to these areas of cortex appear to directly increase bladder activity by removing cortical inhibitory control (de Groat et al, 1993a).
Human Brain Imaging Studies
In the last decade, the areas of the brain involved in the control of micturition have been examined in human brain imaging studies using single photon emission–computed tomography (SPECT), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI) (DasGupta et al, 2007; Griffiths and Tadic, 2008) (Fig. 60–22). Some studies evaluated the brain areas responsible for the perception of bladder fullness and the sensation of the desire to void during bladder filling, whereas others examined brain activity during micturition, voluntary contractions of the pelvic floor during urine withholding, or during cold stimulation of the bladder. PET scan studies in normal men and women revealed that during voiding two cortical areas (the dorsolateral prefrontal cortex and anterior cingulate gyrus) were active (i.e., exhibited increased blood flow). The hypothalamus, including the preoptic area, as well as the pons and the PAG, also showed activity in concert with voluntary micturition (Blok et al, 1997c, 1998). Another PET study during voiding also confirmed that micturition was associated with increased activity in the pons, inferior frontal gyrus, hypothalamus, and PAG, while also showing activity in several other cortical areas (postcentral gyrus, superior frontal gyrus, thalamus, insula, and globus pallidus) and the cerebellar vermis (Nour et al, 2000).
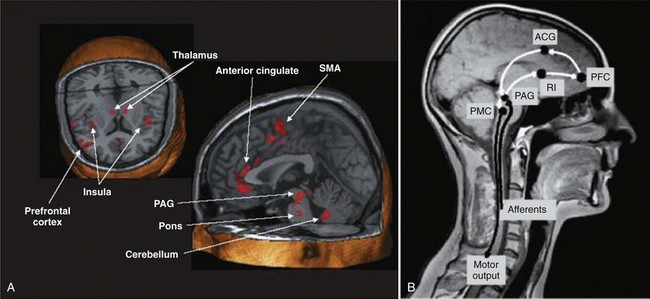
Figure 60–22 A, Areas of brain activation during the urinary storage phase based on human imaging studies using PET and fMRI. SMA, supplementary motor area. PAG, periaqueductal gray. B, Simplified model of supraspinal control system. Ascending afferent fibers (afferents) carrying the information from the bladder synapse in the PAG and are relayed to the right insula (RI), forming the substrate for sensation. The anterior cingulate gyrus (ACG) is responsible for monitoring, arousal, and efferent output to the PAG and pontine micturition center (PMC). The prefrontal cortex (PFC) is involved in voluntary decision about voiding and generates efferent signals to control ACG and ultimately PMC. The PMC then provides motor output to cause voiding.
(A, Modified from Fowler A, Griffiths CJD, et al. The neural control of micturition. Nat Rev Neurosci 2008;9:453–66; B, modified from Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn 2008; 27:466–74.)
Other PET studies that examined the changes in brain activity during filling of the bladder revealed that increased activity occurred in the PAG, the midline pons, the anterior and midcingulate gyrus, anterior insula, and bilaterally in the frontal lobes (Athwal et al, 1999; Athwal et al, 2001; Matsuura et al, 2002). The results were consistent with the notion that the PAG receives information about bladder fullness and then relays this information to other brain areas involved in the control of bladder storage. In fMRI studies in men and women, activation during urinary storage is found in the supplementary motor area, midcingulate cortex, insula, and right prefrontal cortex, and the right anterior insula and midbrain PAG were more active at higher than at lower bladder volumes (Kuhtz-Buschbeck et al, 2005, 2009). Other fMRI studies also addressed the effect of pelvic floor contraction with a full bladder, and reported activation of the parietal cortex, cerebellum, putamen and supplementary motor area (Zhang et al, 2005), or the frontal cortex, basal ganglia, and cerebellum (Seseke et al, 2006).
Brain imaging studies have also been performed to identify changes in cerebral perception of detrusor overactivity. A PET study in patients with Parkinson disease reported activation of the PAG, supplementary motor area, basal ganglia, and cerebellum during detrusor overactivity, (Kitta et al, 2006). When comparing healthy control subjects and those with confirmed overactivity using fMRI, infusion into the bladder is associated with activity in the PAG, thalamus, insula, and anterior cingulated gyrus; however, weak responses or deactivation in the prefrontal cortex or the limbic system, as well as exaggerated responses in the anterior cingulate gyrus at large bladder volumes, are observed in urgency-incontinent patients (Griffiths et al, 2005, 2007). Based on the results in human imaging studies, Griffiths proposes that (1) during urine storage in healthy subjects, bladder and urethral afferents received in the PAG and mapped in the insula form normal sensations of desire to void, which is monitored by the anterior cingulate gyrus, and the voiding reflex is continuously inhibited until the decision to void is made in the prefrontal cortex; and (2) urgency-incontinent patients have weak responses or deactivation observed in the prefrontal cortex or the limbic system, leading to a defect of supraspinal bladder control, which may cause urgency incontinence, and enhanced responses in the anterior cingulate gyrus, which may be due to abnormal bladder afferents or related to a loss of control in other brain areas, and could be correlated with the urgency sensation (Griffiths and Tadic, 2008) (see Fig. 60–22). Radiographic imaging of the central nervous system (CNS), with control and dysregulation of the lower urinary tract, is an exciting area of research, but the results are preliminary. Subjects are conscious and thinking about their bladder during the test and may have an indwelling catheter that may alter what parts of the brain are activated naturally or artificially.
Hypothesis of Mechanism of Action of Sacral Neuromodulation
Neuromodulation of the sacral nerves and, more recently, pudendal nerves is now commonly used for the treatment of refractory overactive bladder and urinary retention relating to pelvic floor dysfunction (Das et al, 2004; Leng and Chancellor, 2005). Some have criticized the inconsistency that electrical stimulation of the sacral nerve can paradoxically inhibit incontinence in the overactive bladder (OAB) group of patients and yet aid the retentive patients to void. But neuromodulation has worked for both these groups of patients. The effects of sacral neuromodulation may depend on electrical stimulation of afferent axons in the spinal roots that, in turn, modulate voiding and continence reflex pathways in the central nervous system. The afferent system is the most likely target, because beneficial effects can be elicited at intensities of stimulation that do not activate movements of striated muscles (Vadusek et al, 1986; Thon et al, 1991; de Groat et al, 1997). Sacral neuromodulation activates somatic afferent axons that modulate sensory processing and micturition reflex pathways in the spinal cord. Urinary retention and dysfunctional voiding can be resolved by inhibition of the guarding reflexes. Detrusor overactivity can be suppressed by direct inhibition of bladder preganglionic neurons. Inhibition of interneuronal transmission in the afferent limb of the micturition reflex can also block detrusor overactivity. Thus the principle behind sacral neuromodulation can be summarized as somatic afferent inhibition of sensory processing in the spinal cord.
Rational for Neuromodulation to Facilitate Voiding
In adults, brain pathways are necessary to turn off sphincter and urethral guarding reflexes to allow efficient bladder emptying. Thus spinal cord injury produces bladder-sphincter dyssynergia and inefficient bladder emptying by eliminating the brain mechanisms involved (Fig. 60–23). This may also occur after more subtle neurologic lesions in patients with idiopathic urinary retention, such as after a bout of prostatitis or urinary tract infection. Before the development of brain control of micturition, at least in animals, the stimulation of somatic afferent pathways passing through the pudendal nerve from the perineum can initiate efficient voiding by activating bladder efferent pathways and turning off the excitatory pathways to the urethral outlet (de Groat et al, 1993a; Kruse and de Groat, 1993). Tactile stimulation of the perineum in the cat also inhibits the bladder–sympathetic reflex component of the guarding reflex mechanism. The sacral nerve stimulation may elicit similar responses in patients with urinary retention, and it may turn off excitatory outflow to the urethral outlet and promote bladder emptying. Because sphincter activity can generate afferent input to the spinal cord that can, in turn, inhibit reflex bladder activity, an indirect benefit of suppressing sphincter reflexes would be a facilitation of bladder activity.
Figure 60–23 When there is a sudden increase in intravesical pressure, such as during a cough, the urinary sphincter contracts by means of the spinal guarding reflex to prevent urinary incontinence (guarding reflex). The spinal guarding reflexes can be turned off by the brain for urination. In cases of neurologic diseases, the brain cannot turn off the guarding reflex and retention can occur. The sacral nerve stimulation (SNS) restores voluntary micturition in cases of voiding dysfunction and urinary retention but inhibits the guarding reflex.
Rationale for Neuromodulation to Inhibit the Overactive Bladder
Several reflex mechanisms may be involved in the sacral neuromodulation suppression of detrusor overactivity. Afferent pathways projecting to the sacral cord can inhibit bladder reflexes in animals and humans. The source of afferent input may be from sphincter muscles, distal colon, rectum, anal canal, vagina, uterine cervix, and cutaneous afferents from the perineum (Fig. 60–24). As mentioned previously, two mechanisms have been identified in animals for somatic and visceral afferent inhibition of bladder reflexes. The most common mechanism is suppression of interneuronal transmission in the bladder reflex pathway (de Groat and Theobald, 1976; Kruse et al, 1990; Kruse and de Groat, 1993). It is assumed that this inhibition occurs, in part, on the ascending limb of the micturition reflex and therefore blocks the transfer of information from the bladder to the PMC. This action would prevent involuntary (reflex) micturition but not necessarily suppress voluntary voiding that would be mediated by descending excitatory efferent pathways from the brain to the sacral parasympathetic preganglionic neurons. A second inhibitory mechanism is mediated by a direct inhibitory input to the bladder preganglionic neurons. This can be induced by electrical stimulation of the pudendal nerve or by mechanical stimulation of the anal canal and distal bowel. It is not elicited by tactile stimulation of penile or perineal afferents; this mechanism would be much more effective in turning off bladder reflexes, because it would directly suppress firing in the motor outflow from the spinal cord.
Figure 60–24 The voiding reflex involves afferent neurons from the bladder that project on spinal tract neurons that ascend to the brain. Descending pathways connect to parasympathetic efferent nerves to contract the bladder (bladder-bladder reflex). A spinal bladder-urethra reflex is activated by a similar bladder afferent innervation. In cases of supraspinal dysfunction, overactive micturition reflexes occur. The sacral nerve stimulation (SNS) inhibits urinary urgency, frequency, and urge incontinence by inhibiting the bladder-bladder and bladder-urethra reflexes.
Pudendal Nerve Stimulation
The pudendal nerve is a peripheral branch of the sacral nerve roots, and stimulating the pudendal allows afferent stimulation to all three of the sacral nerve roots (S2, S3, S4), and that may raise the stimulation threshold needed for micturition and inhibit detrusor activity. Because this is a more peripheral nerve, it is less likely that stimulation of the sciatic and sural nerves will occur, thus decreasing the potential risk for discomfort in the thighs, calves, and feet as seen on occasion with sacral stimulation at the S3 nerve root. The pudendal nerve arises from the sacral plexus within the pelvis; it must go around the pelvic floor to reach the ischioanal fossa. In the pelvis, it runs on the piriformis and then passes laterally through the greater sciatic foramen to enter the gluteal region. Here it lies inferior to the piriformis as does the sciatic nerve, the inferior gluteal neurovascular bundle, and the nerve to the quadratus femoris. The pudendal nerve curls around the spine of the ischium, lying superficial to the sacrospinous ligament, and then passes into the lesser sciatic notch to enter the ischioanal fossa. The nerve then divides into the inferior rectal, the perineal, and the dorsal nerve of the penis or clitoris.
Afferent pudendal nerve stimulation has been demonstrated to inhibit the micturition reflex, abolish uninhibited detrusor contractions, and increase bladder capacity in animals and humans (Fall and Lindstrom, 1991). Peters and colleagues (2005) compared the effectiveness of sacral and pudendal nerve stimulation for voiding dysfunction in a prospective, single-blinded, randomized crossover trial of 30 subjects (22 with urgency/frequency, 5 with urgency incontinence, and 3 with urinary retention) scheduled for sacral implantation a tined quadripolar lead consented to the placement of a second pudendal lead. Twenty-four of the 30 subjects demonstrated a significant clinical response and had an implantable pulse generator placed. Sacral nerve stimulation resulted in 46% improvement in symptoms, while pudendal nerve stimulation demonstrated 63% improvement in symptoms. Urgency-incontinence episodes were reduced by approximately 47%; however, this did not reach statistical significance because of small sample size (n = 5).
Inhibitory and Excitatory Stimulation Frequencies of the Pudendal-Bladder Reflexes
The exact mechanism of action of neuromodulation is unknown. In addition, there are no studies involving neuromodulation that look at programming parameters (pulse width, intensity, or frequency) and their impact on voiding function. The pudendal nerve may have a dual mechanism depending on the frequency and continuity of stimulation. A recent study by Tai et al (2007) in anesthetized spinal cord–injured cats demonstrated that at 3 Hz, stimulation of the pudendal nerve inhibited bladder function and decreased bladder pressures, whereas intermittent stimulation at 20 Hz improved the efficiency of the bladder to empty (Tai et al, 2007). Furthermore, the clinical outcomes of continuous (which potentially can fatigue the urethral sphincter and accommodate the nerve) and intermittent stimulation have not been explored. It would directly suppress firing in the motor outflow from the spinal cord.
Developmental Changes of Bladder Reflexes
The neonatal bladder is a conduit of urine more than a storage organ. Without cerebral control, the bladder will reflexively empty into a diaper when it reaches functional capacity. Therefore by definition, a normal toddler has detrusor overactivity. Neonatal animal bladder strips have repetitive spontaneous contraction, which can be initiated by pacemaker cells within the bladder and propagated by gap junctions between cells (see Fig. 60–1) (Pezzone et al, 1999; Szell et al, 2003) and/or directly induced by enhanced smooth muscle activity due to the low level expression of Ca2+-activated K+ channels (Ng et al, 2007).
The neonatal pathways responsible for bladder overactivity do not disappear with growth and development; rather, increasing cerebral maturation actively inhibits them. Inhibition rather than resolution of neonatal reflexes may be difficult to grasp initially, but this concept holds true across most concepts of neuroscience. With disease processes such as neurologic diseases and aging, the neonatal reflexes are released and involuntary detrusor contractions recur (de Groat, 1975).
One of the most interesting clinical demonstrations of the reemergence of the primitive neonatal micturition reflex is the bladder ice-water test. The bladder ice-water cooling test is performed by quickly instilling up to 100 mL of 4° C sterile saline solution. The normal adult will sense cold but maintain a stable bladder. However, infants and neuropathic patients will develop involuntary detrusor contractions with this test (Chancellor and de Groat, 1999).
The bladder cooling response is triggered by activation of cold receptors within the bladder wall. The receptors are supplied by unmyelinated C-fiber afferent neurons (Chancellor and de Groat, 1999). The cooling response represents a neonatal reflex that may be unmasked by central neuropathologic processes, analogous to the appearance of the Babinski reflex in pyramidal tract lesions. The cooling response is present (development of involuntary detrusor contraction) in neurologically normal infants and children until about the age of 4 years. The reflex becomes absent with further maturation of the nervous system, but the response may be unmasked by pathologic processes that disturb the descending neuronal control of normal voiding (Geirsson et al, 1999). It has also been suggested that men with prostatic bladder outlet obstruction also have an ice-water test response. Chai and colleagues (1998) reported an ice-water test response in 12 of 17 patients (71%) with bladder outlet obstruction, but their method of ice-water infusion has been criticized (Geirsson et al, 1999). Hirayama and colleagues (2003) have also reported that an ice-water test response was found in 35 (27%) of 127 patients with benign prostatic hyperplasia and that the patients with the ice-water test response had detrusor overactivity and higher bladder outlet obstruction index than the nonresponders on the pressure flow study.
Pharmacology
Peripheral Pharmacology
Traditional teaching in uropharmacology is that if a drug works on the bladder or urethra, it is working on the postjunctional receptors. In this section, we present evidence for prejunctional as well as postjunctional actions of drugs.
Muscarinic Mechanisms
Detrusor strips from normal human bladders are contracted by cholinergic muscarinic receptor agonists and by electrical stimulation of intrinsic cholinergic nerves. Contractile responses can be completely abolished by atropine (Sibley, 1984). There are at least five receptor subtypes based on molecular cloning and four different receptor subtypes based on pharmacology (M1 to M5) (Somogyi et al, 1994; Wang et al, 1995; Eglen et al, 1996; Yamaguchi, 1996; Hegde et al, 1997).
Pharmacologically, M1, M2, and M3 receptor subtypes have been found in the human bladder by receptor binding assays (Kondo et al, 1995); all M1 to M5 receptor mRNAs are detected by reverse transcription–polymerase chain reaction assays (Andersson and Wein, 2004; Mansfield et al, 2005). Although ligand receptor binding studies revealed that M2 receptors predominate, M3 receptors mediate cholinergic contractions (Eglen et al, 1994; Harriss et al, 1995; Yamaguchi, 1996; Hegde et al, 1997; Lai et al, 1998). Stimulation of M3 receptors by acetylcholine leads to IP3 hydrolysis due to phospholipase C activation and then to the release of intracellular calcium and a smooth muscle contraction (Harriss et al, 1995; Fry et al, 2002) (Fig. 60–25). The involvement of transmembrane flux of calcium ions through nifedipine-sensitive L-type Ca2+ channels has also been indicated in M3 receptor–mediated detrusor muscle contractions because the L-type Ca2+ channel inhibitor nifedipine strongly suppressed carbachol-induced detrusor contractions, whereas the phospholipase C inhibitor or the store-operated Ca2+ channel inhibitor caused little inhibition in rats and humans (Andersson and Arner, 2004; Andersson and Wein 2004; Schneider et al, 2004a, 2004b; Frazier et al, 2008) (see Fig. 60–25). However, other studies have indicated the major contribution of the phospholipase C–mediated mechanism to M3 receptor–induced detrusor contractions, because phospholipase C inhibitors significantly suppressed carbachol-induced detrusor contractions in rats (Braverman et al, 2006a, 2006b), and intracellular calcium elevation after carbachol application was observed without membrane depolarization in human bladders, which is required for the opening of L-type Ca2+ channels (Fry et al, 2002). Hashitani and colleagues (2000) have reported that the stimulation of muscarinic receptors activates both calcium influx through L-type Ca2+ channels and calcium release from intracellular calcium stores in guinea pig bladders.
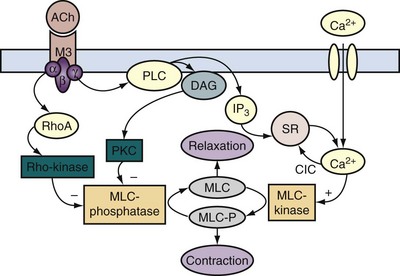
Figure 60–25 Transmitter signal pathways involved in activation of detrusor contractions through muscarinic M3 receptors. There seem to be differences between species in the contribution of the different pathways in contractile activation. ACh, acetylcholine; CIC, calcium-induced calcium release; DAG, diacylglycerol; IP3, inositol 1.4.5-trisphosphate; MLC, myosin light chain; PKC, protein kinase C; PLC, phospholipase C; SR, sarcoplasmic reticulum.
(From Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 2004;56:581–631.)
It has also been proposed (Hegde et al, 1997; Ehler et al, 2005) that coactivation of M2 receptors could enhance the response to M3 stimulation by (1) inhibition of adenylate cyclase, thereby suppressing sympathetically mediated depression of detrusor muscle; (2) inactivation of K+ channels; or (3) activation of nonspecific cation channels. In addition, because the specific Rho-kinase inhibitor Y-27632 reportedly suppresses carbachol-induced detrusor contractions in rats and humans, muscarinic receptor activation in detrusor smooth muscles is likely to stimulate the Rho-kinase pathway, leading to a direct inhibition of myosin phosphatase that induces calcium sensitization to enhance the ability of the muscle to generate the same contractile force with lower levels of intracellular calcium (Andersson and Wein, 2004; Schneider et al, 2004a, 2004b; Frazier et al, 2008). Although the involvement of M3 receptors for the Rho-kinase activation has been suggested (Andersson and Arner, 2004; Andersson and Wein, 2004; Schneider et al, 2004a) (see Fig. 60–25), a study has also suggested the participation of M2 receptors in this mechanism because Y-27632 not only suppressed carbachol-induced muscle contractions but also increased the affinity of darifenacin, an M3 receptor antagonist with approximately a 30-fold selectivity for M3 over M2 receptors, for inhibiting carbachol-induced contractions of rat bladders (Braverman et al, 2006a, 2006b). It has also been reported that the muscarinic receptor subtype–mediated detrusor contractions shift from M3 to M2 receptor subtype in certain pathologic conditions, such as obstructed or denervated hypertrophied bladders in rats (Braverman and Ruggieri, 2003; Braverman et al, 2006a, 2006b), as well as in bladder muscle specimens from patients with neurogenic bladder dysfunction (Pontari et al, 2004).
Studies using constructed mutant mice lacking the M3 receptor or the M2 and M3 receptors have demonstrated that this subtype plays key roles in salivary secretion, pupillary constriction, and detrusor contractions (Matsui et al, 2000, 2002; Igawa et al, 2004). However, M3-mediated signals in digestive and reproductive organs are dispensable, probably because of redundant mechanisms through other muscarinic acetylcholine receptor subtypes or other mediators (Matsui et al, 2000). In addition, it has also been found that male M3 knockout mice had a distended bladder and larger bladder capacity compared with females, indicating a considerable sex difference in the micturition mechanism (Matsui et al, 2002; Igawa et al, 2004). Thus M3 or M2 and M3 double-knockout mice should provide a useful animal model for the detrusor overactivity pathophysiology and pharmacology.
Muscarinic receptor antagonists tolterodine and oxybutynin (Table 60–5) are the most widely prescribed drugs for urinary incontinence. Oxybutynin is a nonspecific muscarinic antagonist with additional smooth muscle relaxant properties. The smooth muscle relaxation properties of oxybutynin may be clinically relevant only with intravesical instillation of the drug. Because new antimuscarinic drugs are such a hot topic for pharmaceutical development, all urologists should be aware of the existence of muscarinic receptor subtypes and their distribution in the lower urinary tract and other organs.
Table 60–5 Drugs with Bladder Action
| CLASSIFICATION | EXAMPLES | PHARMACOLOGIC ACTION |
|---|---|---|
| Anticholinergic agents | Atropine | Inhibit muscarinic receptors, thus reducing the response to cholinergic stimulation; used to reduce pressure during bladder filling and for the treatment of unstable bladder contractions |
| Glycopyrrolate | ||
| Oxybutynin | ||
| Propantheline | ||
| Tolterodine | ||
| Smooth muscle relaxants | Dicyclomine | Direct smooth muscle relaxation reduces intravesical pressure during filling and reduces severity and presence of unstable bladder contractions; most of these agents have some degree of anticholinergic action |
| Flavoxate | ||
| Calcium antagonists | Diltiazem | Used in the treatment of unstable bladder contractions to reduce the magnitude of the spikes by reducing the entrance of calcium during an action potential |
| Nifedipine | ||
| Verapamil | ||
| Potassium channel openers | Cromakalim | Act to increase the membrane potential and thus reduce the myogenic initiation of unstable bladder contractions |
| Pinacidil | ||
| Prostaglandin synthesis inhibitors | Flurbiprofen | Prostaglandins have been implicated in increased smooth muscle tone and in the induction of spontaneous activity. Inhibition of prostaglandin synthesis could promote relaxation of the bladder during filling and decrease spontaneous activity of the bladder. |
| β-Adrenergic agonists | Isoproterenol | Stimulation of β receptors induces relaxation of the bladder body, resulting in a decrease in intravesical pressure during filling. |
| Terbutaline | ||
| Tricyclic antidepressants | Amitriptyline | These agents have anticholinergic, direct smooth muscle relaxant, and norepinephrine reuptake inhibition properties. |
| Imipramine | ||
| α-Adrenergic agonists | Ephedrine | Increase urethral tone and closure pressure by direct stimulation of α-adrenergic receptors |
| Phenylpropanolamine | ||
| Midodrine | ||
| Pseudoephedrine | ||
| Afferent nerve inhibitors | DMSO | Reduce the sensory input from bladder and thereby increase bladder capacity and reduce detrusor overactivity |
| Capsaicin | ||
| Resiniferatoxin | ||
| Estrogen | Estradiol | Direct application to the vagina or oral therapy may increase the thickness of the urothelial mucosa, making a better seal and reducing the incidence of incontinence. Other actions may include increasing adrenergic effects on the urethra and increasing blood flow. |
DMSO, dimethyl sulfoxide.
We will briefly present two additional issues regarding the effect of antimuscarinic drugs on the bladder and salivary glands that have clinical relevancy. First, antimuscarinic drugs are metabolized, and their metabolites have pharmacologic effects. It has been shown that oxybutynin has less of a dry mouth effect than does its metabolite desethyoxybutynin (Gupta and Sathyan, 1999). Therefore the controlled-release formulation of oxybutynin maintains the efficacy of immediate-release oxybutynin but with significantly fewer side effects. Tolterodine and solifenacin have been shown in cats and rats, respectively, to have less activity on the salivary gland muscarinic receptors than on the bladder muscarinic receptors (Nilvebrant et al, 1997; Ohtake et al, 2004). Second, the site and speed of antimuscarinic metabolism appear to have profound effects in terms of clinical efficacy and side effects.
Muscarinic receptors are also located prejunctionally on cholinergic nerve terminals in the bladder (D’Agostino et al, 1986; Somogyi and de Groat, 1992; Somogyi et al, 1996; D’Agostino et al, 1997; Braverman et al, 1998; D’Agostino et al, 2000). Activation of M1 prejunctional receptors facilitates acetylcholine release (Somogyi and de Groat, 1992; Somogyi et al, 1996), whereas activation of M2 to M4 receptors inhibits the release (D’Agostino et al, 1997; Braverman et al, 1998). It has been proposed that inhibitory M2 to M4 receptors are preferentially activated by autofeedback mechanisms during short periods of low-frequency nerve activity and thereby suppress cholinergic transmission during urine storage (Somogyi and de Groat, 1992). Conversely, M1 receptors are activated during more prolonged, high-frequency nerve firing that would occur during voiding and thus participate in an amplification mechanism to promote complete bladder emptying. M1-mediated facilitation of transmitter release involves the activation of a phospholipase C–protein kinase C signaling cascade that appears to facilitate the opening of L-type Ca2+ channels that are necessary for prejunctional facilitation of acetylcholine release from parasympathetic nerve terminals (Somogyi et al, 1996, 1997). Inhibitory and facilitatory muscarinic receptors are also present in the central nervous system (de Groat et al, 1993a; Yoshimura and de Groat, 1997; Ishiura et al, 2001) and in bladder parasympathetic ganglia, where they modulate nicotinic transmission (de Groat and Booth, 1993). The ability to modulate neurotransmission in the bladder through the selective activation and inhibition of specific presynaptic receptors may lead, in the future, to novel forms of pharmacologic therapy for specific forms of lower urinary tract dysfunction.
Muscarinic Selectivity
Pharmacologically defined subtype-selective drugs have been developed. Darifenacin and vamicamide have been demonstrated to be relatively selective for the M3 subtype (Yamamoto et al, 1995; Andersson, 1997; Steers, 2006). However, they are not necessarily tissue selective, because salivary glands and other tissues also contain M3 muscarinic receptors. Currently, several drugs are being tested for their tissue selectivity. Tolterodine appears to be a muscarinic antagonist that has selectivity for the bladder compared with the salivary gland, even though it may not be an M3 subtype–selective antagonist (Nilvebrant et al, 1997; Andersson, 1998). More recently, solifenacin has also shown selectivity to the bladder over the salivary gland; the receptor selectivity of solifenacin to M3 receptors over M2 receptors (10-fold) is similar to that of oxybutynin (Ikeda et al, 2002; Ohtake et al, 2004). Thus, therapeutically, it is more important to be tissue selective than subtype selective (Nilvebrant, et al. 1997; Andersson, 1998). A truly bladder-selective antimuscarinic drug with no side effects is the “holy grail” of overactive bladder drug therapy.
Key Points: Muscarinic Mechanisms
Purinergic Mechanisms
Purinergic contribution to parasympathetic stimulation in vivo, or field stimulation in vitro, has been proved to exist in a variety of species including rat, rabbit, and guinea pig (Burnstock et al, 1972; Chancellor et al, 1992; Burnstock, 1996). However, there is less evidence that purinergic neurotransmission exists in humans, at least regarding normal responses to stimulation, but it may play a role in pathologic conditions such as detrusor overactivity or bladder outlet obstruction (Palea et al, 1993; Burnstock, 2000; O’Reilly et al, 2001a).
ATP acts on two families of purinergic receptors: an ion channel family (P2X) and a G protein–coupled receptor family (P2Y) (Inoue and Brading, 1990; Inoue and Gabella, 1991; McMurray et al, 1997). Seven P2X subtypes and eight P2Y subtypes have been identified. Analysis of the structure-activity relationships of a series of excitatory purinergic agonists on the guinea pig bladder revealed an order of potency consistent with P2X1 or P2X2 receptors (Burnstock, 2001; Zhong et al, 2001). In other species (rabbit, cat, and rat), various studies suggested that multiple purinergic excitatory receptors are present in the bladder (Burnstock, 2000). Immunohistochemical experiments with specific antibodies for different P2X receptors showed that P2X1 receptors are the dominant subtype in membranes of rat detrusor muscle and vascular smooth muscle in the bladder (Lee et al, 2000). Clusters of P2X1 receptors were detected on rat bladder smooth muscle cells, some of which were closely related to nerve varicosities. Northern blotting and in-situ hybridization revealed the presence of P2X1 and P2X4 mRNA in the bladder (Valera et al, 1995). The predominant expression of P2X1 receptors has also been confirmed in the human bladder (O’Reilly et al, 2001a, 2001b). Investigators also found that the amount of P2X1 receptors was increased in the obstructed bladder compared with the control bladder, suggesting upregulated purinergic mechanisms in the overactive bladder due to bladder outlet obstruction (O’Reilly et al, 2001a). In addition, ATP also seems to act through P2Y receptors in the smooth muscle to suppress cholinergic and purinergic contractions (Lee et al, 2000; Burnstock, 2001).
Purinergic nerves are likely to have other functions in the lower urinary tract, because excitatory receptors for ATP are present in parasympathetic ganglia (Theobald and de Groat, 1989; Nishimura and Tokimasa, 1996; Zhong et al, 1998, 2001), afferent nerve terminals (Dmitrieva et al, 1998; Namasivayam et al, 1999; Lee et al, 2000; Burnstock, 2001), and urothelial cells (Buffington et al, 1999; Vlaskovska et al, 2001). Excitatory purinergic receptors in pelvic ganglia have been demonstrated in the cat (Theobald and de Groat, 1989), rabbit (Nishimura and Tokimasa, 1996), and rat (Zhong et al, 1998, 2001).
P2X3 receptors that have been identified in small-diameter afferent neurons in DRG have also been detected immunohistochemically in the wall of the bladder and ureter in a suburothelial plexus of afferent nerves (Lee et al, 2000). Studies using patch clamp recordings in rats have also demonstrated that the majority (90%) of bladder afferent neurons projecting by pelvic nerves responded to ATP and α,β-methylene ATP with persistent currents, suggesting that bladder afferent pathways in the pelvic nerve express predominantly P2X2/3 heteromeric receptors rather than P2X3 homomeric receptors (Zhong et al, 2003; Dang et al, 2005). Intravesical or intra-arterial administration of ATP- or 2-methylthio-ATP–activated bladder afferent fibers and enhanced reflex bladder activity (Dmitrieva et al, 1998; Namasivayam et al, 1999; Pandita and Andersson, 2002; Zhang et al, 2003; Nishiguchi et al, 2005). On the other hand, desensitization of purinergic receptors by intravesical administration of α,β-methylene ATP, a purinergic receptor agonist, or administration of a receptor antagonist, suramin, depressed bladder afferent activity (Dmitrieva et al, 1998; Namasivayam et al, 1999). In P2X3 knockout mice, afferent activity induced by bladder distention was significantly reduced (Burnstock, 2000; Cockayne et al, 2000; Cook and McCleskey, 2000; Souslova et al, 2000). Furthermore, deletion of the gene encoding P2X2 and loss of heteromeric P2X2/3 receptors also result in a marked urinary bladder hyporeflexia, as evidenced by increased thresholds for bladder contractions during bladder filling (Cockayne et al, 2005). These data indicate that purinergic receptors are involved in mechanosensory as well as nociceptive signaling in the bladder.
ATP is also released by afferent nerves and might act in an autofeedback manner to regulate afferent excitability. Two studies (Dmitrieva et al, 1998; Morrison, 1998) presented evidence for purinergic sensitization of bladder afferent nerve endings. Intra-arterial injection of ATP can activate bladder afferent nerves (Dmitrieva et al, 1998), whereas suramin, an antagonist of certain types of ATP receptors (P2X purinergic receptors), given intravesically reduced by 50% the firing of bladder mechanoreceptors induced by bladder distention (Morrison, 1998). Intravesical administration of α,β-methylene ATP also reduced the firing of mechanosensitive afferents, presumably by desensitizing purinergic receptors on the afferent terminals.
In addition, adenosine, which can be formed by the metabolism of ATP, can depress parasympathetic nerve-evoked bladder contractions by activating P1 inhibitory receptors in parasympathetic ganglia (Akasu et al, 1984), in postganglionic nerve terminals, and in the bladder muscle (Cockayne et al, 2000; Burnstock, 2001). Adenosine P1 receptors have been further classified into a number of subtypes (i.e., A1, A2A, A2B, and A3) (Olah et al, 1995). A study has demonstrated that adenosine reduces the force of nerve-mediated contractions by acting predominantly at presynaptic sites at the nerve-muscle junction through a subtype of an adenosine receptor (the A1 receptor in guinea pigs), although these actions of adenosine are less evident in human detrusor muscles (Fry et al, 2004).
Adrenergic Mechanisms
β-Adrenergic
Stimulation of β2- and β3-adrenergic receptors that exist in the human detrusor results in the direct relaxation of the detrusor smooth muscle (Andersson, 1993; Morita et al, 1993; Nishimoto et al, 1995; Levin and Wein, 1995). In addition, β-adrenergic–stimulated relaxation is mediated through the stimulation of adenylate cyclase and the accumulation of cyclic AMP (cAMP) (Levin et al, 1986; Andersson, 1993; Andersson and Arner, 2004). Because β-adrenoceptor–mediated relaxation of the human detrusor was not blocked by selective β1− or β2-adrenoceptor antagonists, such as dobutamine and procaterol, but was blocked by selective β3-adrenoceptor antagonists, the relaxation induced by adrenergic stimulation of the human detrusor is mediated mainly through β3-adrenoceptor activation (Igawa et al, 1999; Yamaguchi, 2002; Andersson and Arner, 2004). A quantitative analysis by reverse transcription–polymerase chain reaction has also confirmed that the β3-adrenergic receptor is the most highly expressed subtype among α− and β-adrenoceptor subtypes at the mRNA level in human bladders (Nomiya and Yamaguchi, 2003). Therefore β3-receptor agonists have been proposed to be effective for treatment of detrusor overactivity, and this is an exciting area of research for overactive bladder (Andersson and Arner, 2004; Tyagi et al, 2009). β-Adrenergic blockers have also been advocated for urinary incontinence due to inappropriate reflex urethral relaxation, because propranolol prevents the reduction in urethral pressure after sacral root stimulation (McGuire, 1978). However, β-adrenergic antagonists are not particularly useful in treating bladder or urethral disorders (Castleden and Morgan, 1980; Naglo et al, 1981; Takeda et al, 1997).
A second pharmacologic method of increasing cAMP levels is with phosphodiesterase (PDE) inhibitors. PDE is the enzyme that catalyzes the degradation of cAMP to AMP and thus limits the action of cAMP (Andersson, 1997; Longhurst, et al, 1997). There are several classes of PDEs that have individual substrate affinities, specific species and tissue distributions, and pharmacologic selectivities (Truss et al, 1996; Longhurst et al, 1997). Currently, there is considerable research trying to identify the specific isoform of PDE present in the bladder as opposed to that in the penis (Truss et al, 1996). Selective inhibition of bladder PDE would result in both an increase in the basal levels of cAMP (and possibly relaxation of the detrusor) and enhancement of the sensitivity and efficacy of β-adrenergic agonists. In the isolated guinea pig bladder, the frequency of agonist-induced phasic activity is slowed by cAMP, and degradation of intracellular cAMP in the cells responsible for phasic activity appears to involve primarily PDE4 (Gillespie 2004). PDE4 inhibitors are shown to suppress detrusor overactivity in a rat model of bladder outlet obstruction induced by partial urethral ligation (Kaiho et al, 2008). Thus a selective β agonist and a selective PDE inhibitor might be an effective combination for the relaxation of the detrusor in the future.
α-Adrenergic
Although α-adrenergic stimulation is not prominent in the normal bladder, recent evidence indicates that under pathologic conditions, such as detrusor overactivity associated with bladder outlet obstruction, the α-adrenergic receptor density, especially the α1D-receptor subtype, can increase to such an extent that the norepinephrine-induced responses in the bladder are converted from relaxation to contraction (Andersson and Arner, 2004). In rats with outflow obstruction, the proportion of α1D-receptor subtype in the total α1-receptor mRNA in the bladder is increased to 70% from 25% in normal rat bladders (Hampel et al, 2002), and urinary frequency is suppressed by an inhibition of α1D and α1A receptors by tamsulosin, whereas α1A-receptor suppression by 5-methyl-urapidil has no effect. Moreover, α1D-receptor knockout mice have larger bladder capacity and voided volumes than do their wild-type controls, which supports an important role of α1D receptors in the control of bladder function (Chen et al, 2005). However, in humans, there is the predominant expression of α1D receptors already in the normal bladder (Malloy et al, 1998), and the level of expression of α-adrenoceptor mRNA, which is considerably low compared with β3 adrenoceptors in normal bladders, was not increased in the bladder with outflow obstruction (Nomiya and Yamaguchi, 2003). Thus the contribution of α1D receptors to detrusor overactivity observed in a variety of pathologic conditions, including obstructive uropathy and incontinence, still needs to be established (Andersson and Arner, 2004).
α-Adrenergic mechanisms are more important in urethral function. Substantial pharmacologic and physiologic evidence indicates that urethral tone and intraurethral pressure are influenced by α-adrenergic receptors. The presence of α1 and α2 adrenoceptors has been shown in the urethra of various species including humans. Among α1 adrenoceptors, the α1A adrenoceptor is the major subtype expressed in urethral smooth muscle at the mRNA and protein levels (Yono et al, 2004; Michel and Vrydag, 2006). Isolated human urethral smooth muscle contracts in response to α-adrenergic agonists (Yalla et al, 1977; Awad et al, 1978; Nordling, 1983; Mattiasson et al, 1984). It is also reported in the rabbit that the urethral contraction is mediated by the α1A-adrenoceptor subtype (Testa et al, 1993; Michel and Vrydag, 2006). Likewise, hypogastric nerve stimulation and α-adrenergic agonists raise intraurethral pressure, which is blocked by α1-adrenergic antagonists (Awad et al, 1976; Yalla et al, 1977). These findings provide the rationale for use of α-adrenergic agonists to promote urine storage by increasing urethral resistance.
Conversely, α-adrenergic receptor antagonists facilitate urine release in conditions of functionally increased urethral resistance, such as benign prostatic hyperplasia. Although the α1A adrenoceptor is the major subtype in the prostate and urethra, highly selective α1A- adrenoceptor antagonists (e.g., RS-17053) do not alter lower urinary tract symptom (LUTS) scores in men with BPH, but these agents are effective at relaxing prostate smooth muscle and increasing urine flow in men (Schwinn and Roehrborn, 2008). In contrast, α1-adrenoceptor antagonists that contain α1D-adrenoceptor blocking activity improve bladder-based symptoms in humans (Nishino et al, 2006), suggesting the important role of the α1D adrenoceptors for storage symptoms associated with bladder outlet obstruction, receptors potentially located at the bladder or the spinal cord (Schwinn and Roehrborn, 2008).
α2-Adrenergic antagonists increase the release of norepinephrine from urethral tissues through a presynaptic mechanism, but this does not affect the contractility of urethral smooth muscle in vitro (Mattiasson et al, 1984; Willette et al, 1990; Michel and Vrydag, 2006). The human urethra lacks postjunctional α2-adrenergic receptors, although in-vitro prejunctional activation of these receptors produces a feedback inhibition of norepinephrine release. Pharmacologic and electrophysiologic data suggest that adrenergic nerves influence excitatory cholinergic transmission in pelvic ganglia. de Groat and Booth (1993) have shown, in the cat, that hypogastric nerves inhibit excitatory cholinergic transmission in vesical ganglia by activation of α2-adrenergic receptors (Fig. 60–26). Conversely, β-adrenergic agonists facilitate transmission in vesical ganglia.
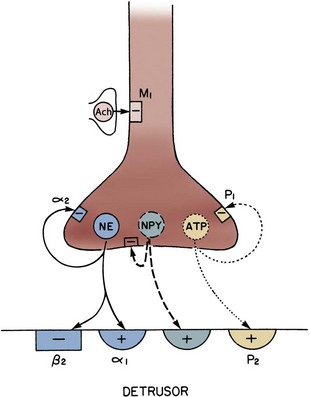
Figure 60–26 Diagram of possible transmitters in an adrenergic terminal supplying the bladder or urethra. Norepinephrine (NE) release can activate α1-adrenergic receptors and produce contraction (+) or β receptors and cause relaxation (−) of the detrusor. Feedback inhibition of NE release through α2 receptors can also occur. Neuropeptide Y (NPY) can produce smooth muscle contraction (+) or inhibit acetylcholine (ACh) release (not shown), or feedback can inhibit NE release. Adenosine triphosphate (ATP) can activate P2 receptors in the detrusor, which elicit contraction (+) or inhibit (−) further ATP release through P1 prejunctional receptors. ACh release from terminals in synaptic contact with an adrenergic varicosity can inhibit firing of adrenergic axons by activation of M1 receptors.
Urethral Tone in Women
Taki and associates (1999) separated the entire length of the human female urethra into several parts and studied regional contractile effect to norepinephrine, clonidine, acetylcholine, and potassium chloride. Their findings suggest that sympathetic innervation helps maintain resting urethral tonus, mainly through α1 adrenoceptors. With the recent identification of at least three distinct subtypes of α1 adrenoceptors with distinct pharmacologic profiles, it may be possible to develop urethra-specific adrenergic agonists for the treatment of stress urinary incontinence. A small-scale, placebo-controlled clinical study has demonstrated that activation of the α1A/1L-adrenoceptor subtype, which is a pharmacologic isoform of the α1A-adrenoceptor gene product, was effective in reducing the number of incontinence episodes in women with mild to moderate stress urinary incontinence (Musselman et al, 2004), suggesting an important role of α1 adrenoceptors in the urethral continence mechanism, although the data are still preliminary.
Key Points: Adrenergic Mechanisms
Nitric Oxide
Nitric oxide (NO) has been identified as a major inhibitory transmitter mediating relaxation of the urethral smooth muscle during micturition (Andersson et al, 1992; Andersson, 1993; Andersson and Persson, 1995; Bennett et al, 1995). In 1931, Barrington hypothesized that stimulation of the pelvic nerve, in addition to evoking a bladder contraction, produces urethral relaxation. Indeed, urodynamic studies document a reduction in urethral pressure just before a bladder contraction (Scott et al, 1964; van Waalwijk van Doorn et al, 1991). In support of the contention that parasympathetic nerves mediate this urethral relaxation, sacral root stimulation reduces urethral pressure (McGuire, 1978; Torrens, 1978). Cholinergic innervation to the urethra has been supported by histochemical identification of acetylcholinesterase-staining fibers and ultrastructural data showing varicosities containing small clear vesicles in the human urethra (Ek, 1977; Gosling et al, 1977). Exogenous cholinergic agonists do not relax but rather contract urethral smooth muscle in vivo (Nergardh and Boreus, 1972; Ek et al, 1978) and in vitro (Ek et al, 1978). Therefore parasympathetic pathways mediating urethral relaxation must rely on a noncholinergic transmitter.
In the rat, NO is released by postganglionic nerves arising from neurons in the major pelvic ganglia (Fraser et al, 1995) (see Fig. 60–18). These neurons contain nitric oxide synthase (NOS), the enzyme that synthesizes NO, as well as nicotinamide adenine dinucleotide phosphate diaphorase, a marker for NOS (Vizzard et al, 1994). Electrophysiologic studies in female rats showed that electrical stimulation of the lumbosacral (L6-S1) spinal roots elicits simultaneous bladder contractions and urethral relaxation (Fraser et al, 1995). The urethral relaxation was inhibited by NOS inhibitors, which did not alter the bladder responses. The inhibition was reversed by administration of L-arginine, a precursor of NO. The electrically evoked urethral relaxation was abolished by ganglionic blocking agents, indicating that it was mediated by stimulation of preganglionic parasympathetic axons in the lumbosacral roots.
NO is also involved in controlling bladder afferent nerve activity. Inhibitors of NOS, given systemically or intrathecally, do not affect normal micturition in conscious or anesthetized rats. However, detrusor overactivity that accompanies irritation with turpentine, acetic acid, or cyclophosphamide is ameliorated by spinal application of NOS inhibitors (Rice, 1995; Kakizaki and de Groat, 1996; Lagos and Ballejo, 2004). However, intravesically administered capsaicin induces detrusor overactivity that is not influenced by an intrathecally applied NOS inhibitor, although the behavioral effects of the irritation are reduced (Pandita et al, 1997). It is believed that NO is involved in mediating N-methyl-D-aspartate (NMDA) receptor–dependent effects but not those involving neurokinin 2 (NK2) receptors. Overall, the NO mechanism at the spinal level has an excitatory effect on the micturition reflex.
However, NO seems to have an inhibitory effect in the bladder. Intravesical application of NO can suppress bladder overactivity due to cyclophosphamide-induced bladder irritation in rats (Ozawa et al, 1999). Intravesical oxyhemoglobin, an NO scavenger, also induces bladder overactivity as evidenced by reductions in bladder capacity and micturition volume, which is prevented by L-arginine or enhanced by the guanylate cyclase inhibitor in rats (Pandita et al, 2000). Masuda and colleagues (2007) also showed that bladder overactivity induced by intravesical capsaicin instillation was enhanced by a NOS inhibitor (L-NAME) administered intravenously or intravesically, and that these L-NAME–induced excitatory effects were significantly suppressed by desensitization of C-fiber afferent pathways by capsaicin pretreatment. Thus NO released locally in the bladder can mediate inhibitory effects by modulation of bladder afferent activity (Yoshimura et al, 2001).
Possible Gender Difference in Cholinergic and Nitrergic Innervation in the Urethra
A parasympathetic cholinergic excitatory input to the urethra (see Fig. 60–18) has been identified in male but not in female rats (Flood et al, 1995; Kakizaki et al, 1997). This was demonstrated by measuring intraurethral pressure during voiding after blockade of striated external urethral sphincter activity with a neuromuscular blocking agent. Under these conditions, urethral pressure increased during micturition in male rats. This urethral reflex was blocked by hexamethonium (a ganglionic blocking agent), markedly reduced by atropine, and increased by an NOS inhibitor. However, it was not changed by transection of sympathetic nerves or administration of an α1-adrenergic blocking agent (prazosin). These results indicate that in male rats, parasympathetic nerve activity induces a predominant cholinergic muscarinic contraction of the urethra and a subordinate NO-mediated relaxation. These studies implicate possible gender differences in parasympathetic and especially nitrergic pathways in the human urethra.
Smooth Muscle Tone and Nitric Oxide
NO-mediated smooth muscle relaxation is due to increased production of intracellular cyclic guanosine monophosphate (cGMP). The second messengers cAMP and cGMP are synthesized from the corresponding nucleoside triphosphates by their respective membrane-bound or soluble adenylate or guanylate cyclases. cAMP and cGMP are inactivated by PDEs by hydrolytic cleavage of the 3′-ribose phosphate bond. Therefore the level of intracellular second messengers can be regulated by PDE isoenzymes (Truss et al, 1999, 2001).
Because of their central role in regulating smooth muscle tone and the considerable variation of PDE isoenzymes among species and tissues, PDEs have become an attractive target for drug development. So far, 7 PDE families, 16 genes, and 33 individual enzyme proteins have been defined. The hope is that bladder-specific PDEs can be developed.
Studies have reported that PDEs 1 to 5 exist in human detrusor smooth muscle (Truss et al, 1996). The PDE1-selective inhibitor vinpocetine has been tested, with encouraging but preliminary results, in patients with detrusor overactivity who did not respond to conventional anticholinergic drugs (Truss et al, 1997, 2001).
Afferent Neuropeptides
Afferent neurons innervating the lower urinary tract exhibit immunoreactivity for various neuropeptides, such as substance P (SP), calcitonin gene–related peptide (CGRP), pituitary adenylate cyclase–activating polypeptide (PACAP), leucine enkephalin, corticotropin-releasing factor, and vasoactive intestinal polypeptide (VIP) (de Groat, 1986; de Groat, 1989; Keast and de Groat, 1992; Maggi, 1993; Vizzard, 2001, 2006), as well as growth-associated protein-43 (GAP43), nitric oxide synthase (NOS) (Vizzard et al, 1996), glutamic acid, and aspartic acid (Keast and Stephensen, 2000). These substances have been identified in many species and at one or more locations in the afferent pathways including (1) afferent neurons in lumbosacral dorsal root ganglia, (2) afferent nerves in the peripheral organs, and (3) afferent axons and terminals in the lumbosacral spinal cord (Kawatani et al, 1985, 1986, 1996; Morrison et al, 2005). The majority (>70%) of bladder DRG neurons in rats appear to contain multiple neuropeptides—CGRP, substance P, or PACAP being the most common. In cats, VIP is also contained in a large percentage of bladder DRG neurons (de Groat, 1989).
Many of these peptides, which are contained in capsaicin-sensitive, C-fiber bladder afferents, are released in the bladder by noxious stimulation and contribute to inflammatory responses by triggering plasma extravasation, vasodilation, and alterations in bladder smooth muscle activity (Maggi, 1993; Ishizuka et al, 1994, 1995b). These agents also function as transmitters at afferent terminals in the spinal cord.
Tachykinins
The tachykinins are a family of small peptides sharing a common C-terminal sequence, Phe-Xaa-Gly-Leu-Met-NH2, whose main members are substance P, neurokinin A, and neurokinin B. Tachykinins are found in both central and peripheral nervous systems. In the peripheral nerves, tachykinins are predominantly located in the terminals of nonmyelinated, sensory C fibers. The diverse biologic effects of the tachykinins are mediated through three receptors, designated NK1, NK2, and NK3, which belong to the superfamily of seven transmembrane-spanning G protein–coupled receptors (Khawaja and Rogers, 1996). Substance P is the most potent tachykinin for the NK1 receptor, whereas neurokinin A exhibits the highest affinity for the tachykinin NK2 receptor, and neurokinin B for the tachykinin NK3 receptor (Table 60–6). All receptor subtypes have been identified in the bladder of humans and animals such as rats, mice, and dogs (Lecci and Maggi, 2001; Andersson and Arner, 2004).
Table 60–6 Tachykinins and Tachykinin Receptors
| TACHYKININ | RECEPTOR |
|---|---|
| Substance P | NK1 |
| Neurokinin A | NK2 |
| Neurokinin B | NK3 |
Tachykinins released from capsaicin-sensitive sensory C fibers in response to irritation in the bladder can act on (1) NK1 receptors in blood vessels to induce plasma extravasation and vasodilation, (2) NK2 receptors to stimulate bladder contractions, and (3) NK2 receptors on primary afferent terminals to increase the excitability during bladder filling or during bladder inflammation (de Groat, 1989; Andersson, 1993; Morrison et al, 1995; Lecci and Maggi, 2001). A study by Kamo and associates (2005) also demonstrated that activation of NK3 receptors on capsaicin-sensitive C-fiber afferents in the rat bladder can increase the excitability during bladder filling.
Intrathecal administration of NK1 antagonists (RP 67580 and CP 96345) or systemic application of centrally acting NK1 antagonists (GR 205171 and CP 99994) increased bladder capacity in normal rats and guinea pigs, respectively, without changing voiding pressure, whereas NK2, NK3, or peripherally acting NK1 antagonists were ineffective (Lecci et al, 1993; Yamamoto et al, 2003). Detrusor overactivity in rats induced by chemical cystitis, intravesical administration of capsaicin, or intravenous injection of L-dopa was also suppressed by intrathecal injection of NK1 antagonists (Ishizuka et al, 1994, 1995b; Lecci et al, 1994). Detrusor overactivity induced by capsaicin was reduced by an NK2 antagonist (SR 48965) that did not influence normal voiding (Lecci et al, 1997). In the anesthetized guinea pigs, TAK-637, an NK1 receptor antagonist, administered orally or intravenously, also increased the volume threshold for inducing micturition and inhibited the micturition reflex induced by capsaicin applied topically to the bladder (Doi et al, 1999). In a clinical study, an NK1 receptor antagonist, aprepitant, is also shown to effectively decrease the average daily number of micturitions and urgency episodes compared with placebo at 8 weeks in women with idiopathic overactive bladder (Green et al, 2006). These results indicate that sensory input to the spinal cord from non-nociceptive bladder afferents is mediated by tachykinins acting on NK1 receptors, whereas input from nociceptive afferents in the bladder can be mediated by NK1, NK2, and NK3 receptors. In addition, tachykinin NK3 receptor activation in the spinal cord can inhibit the micturition reflex through an activation of the spinal opioid mechanism (Kamo et al, 2005).
Autofeedback mechanisms may also be important at afferent nerve terminals (see Fig. 60–14). As mentioned earlier, some stimuli are known to release neuropeptides from afferent nerves, and these neuropeptides may, in turn, sensitize the afferents. NK2 agonists were found (Wen and Morrison, 1996) to sensitize bladder mechanoreceptors by acting on NK2 autoreceptors in the sensory endings in the bladder mucosa to produce the combination of effects found previously for other sensitizing agents (Morrison, 1998). The NK2 receptor blocker SR 48968 decreases the sensitivity of bladder mechanoreceptors and also blocks the sensitization produced by NK2 agonists and high urinary potassium levels. This suggests that the sensitization produced by intravesical chemical stimuli may be due to a mechanism using the NK2 receptor. On the basis of these findings, it could be hypothesized that high urinary potassium concentration or higher levels of bladder distention release neurokinin A from sensory endings, and that the sensitization is due to the action of the peptide on local NK2 autoreceptors on the sensory endings. It has also been shown that sensory neurons obtained from rat DRG can be excited by NK2 agonists and inhibited by NK3 agonists through modulation of Ca2+ channel activity mediated by protein kinase C activation (Sculptoreanu and de Groat, 2003). NK2 receptor activation also leads to PKC-induced phosphorylation of TRPV1 channels, resulting in an increase in capsaicin-evoked currents in rat DRG neurons (Sculptoreanu and de Groat, 2007; Sculptoreanu et al, 2008).
Other Neuropeptides
Other afferent neuropeptides have effects on the peripheral organs or the central reflex pathways controlling the lower urinary tract. However, the effects can vary in different species and at different sites in the lower urinary tract. CGRP applied exogenously or released from primary afferents relaxes smooth muscle and produces vasodilation. The effect of CGRP on bladder is prominent in the guinea pig and dog but is absent in the rat and human bladder (Andersson 1993). VIP, which is contained in C-fiber afferents, as well as in postganglionic neurons (de Groat and Booth, 1993), inhibits spontaneous contractile activity in isolated bladder muscle from several species, including humans. However, VIP usually has little effect on bladder contractions induced by muscarinic receptor agonists or by nerve stimulation (Andersson, 1993). In-vivo studies in the cat revealed that VIP facilitates muscarinic, but not nicotinic transmission, in bladder parasympathetic ganglia and also depresses neurally evoked contractions of the bladder (de Groat and Booth, 1993).
In the spinal cord, VIP-containing afferent pathways have been implicated in the recovery of bladder reflexes after spinal injury. In cats with chronic spinal injury, VIP immunoreactivity, which is a marker for C-fiber afferent terminals, is distributed over a wider area of the lateral dorsal horn, suggestive of afferent axonal sprouting after spinal injury (Yoshimura and de Groat, 1997; Doi et al, 1999). In addition, the effects of intrathecal administration of VIP are changed. In normal cats, VIP inhibits the micturition reflex; whereas in paraplegic cats, VIP facilitates the micturition reflex. These findings suggest that the action of a putative C-fiber afferent transmitter may underlie the emergence of C-fiber bladder reflexes in the paraplegic cat. In the normal rat, VIP and PACAP, another member of the secretin-glucagon-VIP peptide family, also facilitate the micturition reflex by actions on the spinal cord (Ishizuka et al, 1995a; Yoshiyama and de Groat, 2008). In rats with spinal cord injury, an increase in expression of PACAP-immunoreactivity in bladder DRG neurons and expansion of PACAP-IR afferent axons in the lumbosacral spinal cord are observed, and intrathecal administration of PACAP6-38, a PAC1 PACAP–receptor antagonist, reduces premicturition contractions during bladder filling and reduces maximal voiding pressure, suggesting that activation of PAC1 receptors by endogenous PACAP contributes to the micturition reflex and bladder overactivity in spinalized rats (Zvarova et al, 2005; Zvara et al, 2006).
Patch clamp studies in neonatal rat spinal-slice preparations revealed that PACAP has a direct excitatory action on parasympathetic preganglionic neurons due in part to blockade of K+ channels (Miura et al, 2001). In addition, PACAP has an indirect action by activating excitatory interneurons.
Prostanoids, Endothelins, and Hormones
Prostanoids
Prostanoids (prostaglandins and thromboxanes), which comprise a family of oxygenated metabolites of arachidonic acid, by the enzymatic activity of cyclooxygenases 1 and 2, are manufactured throughout the lower urinary tract and have been implicated in bladder contractility, inflammatory responses, and neurotransmission. Biopsy specimens of human bladder mucosa contain prostaglandin (PG) I2, PGE2, PGE2α, and thromboxane A. In decreasing order of potency, PGF2α, PGE, and PGE2 contract the human detrusor (Andersson, 1993; Andersson and Arner, 2004). The actions of prostanoids are mediated by specific receptors on cell membranes. The receptors include the DP, EP, FP, IP, and TP receptors that preferentially respond to PGD2, PGE2, PGF2α, PGI2, and thromboxane A2, respectively. Furthermore, EP is subdivided into four subtypes: EP1, EP2, EP3, and EP4 (Breyer et al, 2001, 2003). The slow onset of action for these substances suggests a modulatory role for prostaglandins. Some prostaglandins may affect neural release of transmitters, whereas others inhibit acetylcholinesterase activity. These actions provide mechanisms whereby prostaglandins could potentially augment the amplitude of cholinergic-induced detrusor contractions (Borda et al, 1982).
Attempts to use prostaglandins to facilitate voiding have had mixed results. Intravesical PGE2 has been shown to enhance bladder emptying in women with urinary retention and patients with neurogenic voiding dysfunction (Bultitude et al, 1976; Vadyanaathan et al, 1981; Tammela et al, 1987). Others have failed to find PGE2 useful to facilitate complete evacuation of the bladder ((Delaere et al, 1981; Wagner et al, 1985). Intravesical PGE2 does produce urgency and involuntary bladder contractions (Schussler, 1990). Consistent with this finding, inhibition of prostaglandin synthesis with indomethacin reduces detrusor overactivity (Cardozo and Stanton, 1980).
Endothelins
Endothelins (ETs), a family of 21–amino acid peptides (originally isolated from bovine aortic endothelial cells), include ET-1, ET-2, and ET-3, which are encoded by separate genes and mediate a variety of biologic actions through two distinct G protein–coupled receptor subtypes, the endothelin-A (ETA) and the endothelin-B (ETB) receptor (Yanagisawa et al, 1988; Masaki, 2004). The ETA receptor subtype has a higher affinity for ET-1 and ET-2 than for ET-3; the ETB receptor subtype binds all ETs with equal affinity (Rubanyi and Polokoff, 1994). ET-1, which is known to be primarily produced by human endothelial cells, can induce prolonged contractile responses in isolated urinary bladder muscle strips in various species (Maggi et al, 1990; Khan et al, 1999). In humans and rabbits, ET-like immunoreactivity is identified in almost all cell types in the bladder, including bladder epithelium, vascular endothelium, detrusor, and vascular smooth muscles, and fibroblasts; it plays a role in control of bladder smooth muscle tone, regulation of local blood flow, and bladder wall remodeling in pathologic conditions (Saenz de Tejada et al, 1992). In a rabbit model of bladder outlet obstruction, ET-1 and ETA receptor binding sites in detrusor smooth muscle and urothelium, as well as ETB receptor binding sites in detrusor smooth muscle, were significantly increased (Khan et al, 1999). In addition, the endothelin-converting enzyme inhibitor WO-03028719, which suppresses ET-1 production, can improve voiding efficiency and suppress detrusor overactivity in a rat model of bladder outlet obstruction (Schroder et al, 2004). YM598, a selective ETA receptor antagonist, also reduces detrusor overactivity in urethral obstructed rats (Ukai et al, 2006). These results suggest that the increase in ET-1 expression and ET receptors could be involved in detrusor hyperplasia and overactivity seen in patients with bladder outlet obstruction resulting from benign prostatic hyperplasia.
There is also evidence that ETs have a role in modulation of sensory function in the peripheral and central nervous system. The activation of ETA receptors in capsaicin-sensitive C-fiber afferents in the bladder induces detrusor overactivity, whereas ETA receptor activation in the spinal cord can inhibit the micturition reflex through activation of a spinal opioid mechanism in rats (Ogawa et al, 2004). In spinal cord–injured rats, the bladder ET-1 level was increased, and the application of ABT-627, an ETA antagonist, suppresses C-fiber–mediated detrusor overactivity. Taken together, modulation of ETA receptor activity in bladder afferent pathways or the spinal cord could be effective in treating bladder overactivity or painful conditions (Ogawa et al, 2008).
Parathyroid Hormone–Related Peptide
Some locally released substances cause detrusor relaxation. Parathyroid hormone–related peptide is manufactured by bladder smooth muscle. Stretch in vivo (Yamamoto et al, 1992) and in vitro (Steers et al, 1998) increases parathyroid hormone–related peptide. Slow or gradual distention could release local relaxants, thereby maintaining low filling pressures.
Sex Steroids
Differences in responses of human and animal bladders to the effect of drugs suggest that sex steroids play a role in detrusor contractility. It is not unusual for women to note changes in voiding, bladder pain, or continence at different times of their menstrual cycle. Sex steroids do not directly affect bladder contractility, but they modulate receptors and influence growth of bladder tissues. Estrogen receptors are expressed by the trigone in women (Iosif et al, 1981). Levin and associates (1980) noted that bladder body muscle from young female rabbits treated with estrogens exhibits increased responsiveness to α-adrenergic, cholinergic, and purinergic agonists. Others have seen a decreased density of adrenergic and muscarinic receptors in the bladder after estrogen administration (Shapiro, 1986; Batra and Andersson, 1989). In contrast to the study by Levin and coworkers (1980), Elliot and associates (1992) showed that bladder smooth muscle from estrogen-treated rats exhibited decreased contractions.
Estrogens also increase adrenergic receptors in the urethra (Callahan and Creed, 1985). Ekstrom and associates (1993) reported that estrogen administration to ovariectomized rabbits unmasked contractile responses to α-adrenergic agonists, whereas contracted and normal rabbit bladders demonstrated no response to these agents. Some clinicians have combined these agents to elevate urethral pressure in patients with stress incontinence (Wilson et al, 1987). However, the clinical efficacy of the combined use of estrogen with α agonists has been questioned (Walter et al, 1978). The effect of estrogens on urinary continence in females probably reflects the multiple actions of this hormone on adrenergic receptors, vasculature, and urethral morphology. In addition, progesterone increases electrical and cholinergic contractions of the bladder. Exogenous estrogens and progesterones also induce NOS activity in bladders of female guinea pigs (Ehren et al, 1995). This effect is postulated to contribute to relief of detrusor overactivity with hormonal treatment. However, the use of estrogens alone to treat either stress urinary incontinence or urgency incontinence has given disappointing results (Abrams et al, 2005), and studies have suggested that estrogen may be associated with an increase in urinary incontinence in postmenopausal women (Hendrix et al, 2005).
Androgen treatment in the male rat has been reported to have similar effects on synaptic connections, as well as effects on motoneuronal somatic and dendritic size in the androgen-sensitive motoneurons innervating the bulbocavernosus and levator ani muscles of the rat (Jordan, 1997; Matsumoto, 1997). Testosterone treatment can also influence the size of postganglionic neurons in the major pelvic ganglion of the male rat (Keast and Saunders, 1998). Thus further studies are needed to evaluate the influence of changes in hormonal environment on the neural pathways controlling the lower urinary tract.
“Transducer” Function of the Urothelium
Whereas the urothelium has historically been viewed primarily as a “barrier,” there is increasing evidence that urothelial cells display a number of properties similar to sensory neurons (nociceptors and mechanoreceptors) and that both types of cells use diverse signal-transduction mechanisms to detect physiologic stimuli. Examples of “sensor molecules” (i.e., receptors and ion channels) associated with neurons that have been identified in urothelium include receptors for bradykinin (Chopra et al, 2005), neurotrophins (TrkA and p75) (Murray et al, 2004), purines (P2X and P2Y) (Lee et al, 2000; Hu et al, 2002; Birder et al, 2004; Tempest et al, 2004; Chopra et al, 2008), norepinephrine (α and β) (Birder et al, 1998; Birder et al, 2002), acetylcholine (nicotinic and muscarinic) (Chess-Williams, 2002; Beckel et al, 2006; Kullmann et al, 2008a), protease-activated receptors, amiloride-/mechanosensitive Na+ channels such as ENaC (Smith et al, 1998; Wang et al, 2003; Araki et al, 2004), and a number of TRP channels (TRPV1, TRPV2, TRPV4, TRPM8) (Birder and de Groat, 1998; Birder et al, 2001, 2002, 2007; Stein et al, 2004; Gevaert et al, 2007).
When urothelial cells are activated through these receptors and ion channels in response to mechanical as well as chemical stimuli, they can, in turn, release chemical mediators such as NO, ATP, acetylcholine, and substance P (Ferguson et al, 1997; Birder et al, 1998; Burnstock, 2001; Birder et al, 2003; Chess-Williams, 2004). These agents are known to have excitatory and inhibitory actions on afferent nerves that are close to or in the urothelium (Bean et al, 1990; Dmitrieva et al, 1998; Birder et al, 2001; Yoshimura et al, 2008). Chemicals released from urothelial cells may act directly on afferent nerves or indirectly through an action on suburothelial interstitial cells (also referred to as myofibroblasts) that lie in close proximity to afferent nerves. Myofibroblasts are extensively linked by gap junctions and can respond to chemicals that in turn modulate afferent nerves (Fowler et al, 2008). Thus it is believed that urothelial cells and myofibroblasts can participate in sensory mechanisms in the urinary tract by chemical coupling to the adjacent sensory nerves.
NO can be released by the urothelium, particularly during inflammation (Birder et al, 1998). The release of NO may be evoked by the calcium ionophore A-23187, norepinephrine, and capsaicin. Substance P also acts on receptors on urothelial cells to release NO. The adrenergic release of NO from bladder strips was reduced by 85% after removal of the urothelium. Denervation of the bladder did not completely block the release of capsaicin-induced NO production, suggesting other sites of production. This is consistent with the observations that capsaicin released NO from cultured rat, cat, rabbit, and human urothelial cells and that the TRPV1 capsaicin receptor is expressed in cultured urothelial cells. NOS expression in afferent neurons is also increased in chronic bladder inflammation. Given that NO does not have much effect on the detrusor muscle but does inhibit Ca2+ channels in rat bladder afferent neurons (Yoshimura et al, 2001), the role of NO in the urothelium has still to be clarified. However, NO released locally in the bladder appears to have an inhibitory effect on afferent activity in the bladder because suppression of endogenous NO by intravesical oxyhemoglobin, an NO scavenger, or L-NAME, a NOS inhibitor, enhances bladder activity in rats (Pandita et al, 2000; Masuda et al, 2007).
ATP released from urothelial cells during stretch can activate a population of suburothelial bladder afferents expressing P2X3 receptors, signaling changes in bladder fullness and pain (Ferguson et al, 1997; Burnstock, 2001). Accordingly, P2X3 null mice exhibit a urinary bladder hyporeflexia, suggesting that this receptor, as well as neural–epithelial interactions, are essential for normal bladder function (Cockayne et al, 2000). This type of regulation may be similar to epithelium-dependent secretion of mediators in airway epithelial cells, which are thought to modulate submucosal nerves and bronchial smooth muscle tone and may play an important role in inflammation (Homolya et al, 2000; Jallat-Daloz et al, 2001). Thus it is possible that activation of bladder nerves and urothelial cells can modulate bladder function directly or indirectly by the release of chemical factors in the urothelial layer. ATP released from the urothelium or surrounding tissues may also play a role in the regulation of membrane trafficking. This is supported by studies in the urinary bladder in which urothelium-derived ATP release purportedly acts as a trigger for exocytosis, in part by autocrine activation of urothelial purinergic (P2X, P2Y) receptors (Wang et al, 2005). These findings suggest a mechanism whereby urothelial cells sense or respond to ATP and thereby translate extracellular stimuli into functional processes.
Prostaglandins are also released from the urothelium. These are assigned two possible functions: regulation of detrusor muscle activity and cytoprotection of the urothelium, based on effective treatment of hemorrhagic cystitis by prostaglandins (Jeremy et al, 1987). The predominant forms found in human urothelium from biopsy specimens are 6-oxo-PGF2α > PFE2 > PGF2α > thromboxane B2. PGI2 (prostacyclin) is also produced. These findings were confirmed and further developed in the guinea pig, in which it was found that the major production of prostaglandins occurred in the urothelium. The production of prostaglandins also increased greatly with inflammation (Saban et al, 1994). Prostaglandin synthesis also occurs in the ureter, where it is speculated to be important in the regulation of ureteral peristalsis and also in reducing the development of blood clots in the lumen of the ureter (Ali et al, 1998).
Evidence also suggests that the involvement of the muscarinic receptor in bladder function extends beyond detrusor contractility and into afferent sensory functioning. Muscarinic receptors are found on the urothelium at high density (Hawthorn et al, 2000), and there is a basal release of acetylcholine from the urothelium that is increased by stretch and aging (Yoshida et al, 2006). Thus activation of the muscarinic receptors in the urothelium releases substances that modulate afferent nerves and smooth muscle activity (Hawthorn et al, 2000; de Groat, 2004; Kullmann et al, 2008b).
The urothelium also releases substances called urothelium-derived inhibitory factors, which decrease the force of detrusor muscle contraction in response to muscarinic stimulation (Hawthorn et al, 2000; Kumar et al, 2005). The molecular identity of this factor is not known; however, pharmacologic studies suggest that it is not NO, a prostaglandin, prostacyclin, adenosine, catecholamine, GABA, or one that acts through apamine-sensitive, small-conductance K+ channels. It has been shown that an inhibitory response through this factor is attenuated in a fetal model of bladder outlet obstruction (Thiruchelvam et al, 2003). Further studies are required to clarify the identity of this substance and its role in bladder function.
Serotonin
Serotonin (5-HT) has been found in neuroendocrine cells along the urethra and in the prostate ((Hanyu et al, 1987). In animals, 5-HT2 and, possibly, 5-HT3 agonists contract the urethra. If inflammatory conditions promote release of serotonin from paraurethral cells, irritative symptoms, such as the urethral syndrome, may arise because of serotonergic mechanisms. The 5-HT2 antagonist ketanserin has been shown to reduce urethral pressure in humans (Horby-Petersen et al, 1985). However, the reduction in urethral pressure after ketanserin administration can also be the result of blockade of α-adrenergic receptors (Thor and Katofiasc, 1995).
5-HT also has several pharmacologic effects on mammalian urinary bladders, both in vitro and in vivo. Human and pig isolated detrusor muscles are known to contract in a concentration-dependent manner in response to 5-HT (Klarskov and Horby-Petersen, 1986). In human isolated urinary bladder, there was potentiation of the contractions induced by electrical field stimulation, mediated by the 5-HT4 receptor subtype (Candura et al, 1996; Darblade et al, 2005). A similar response is present on guinea pig detrusor muscle through 5-HT2A and 5-HT4 receptors, whereas in the rabbit and the rat, the receptors involved are the 5-HT3 and 5-HT7 subtypes, respectively (Palea et al, 2004).