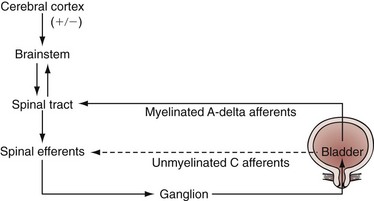
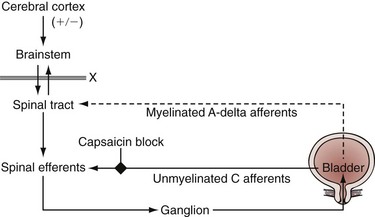
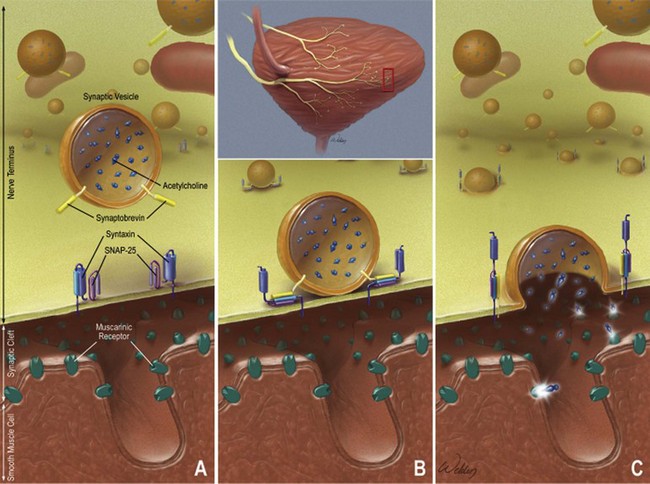
References
Abrams P, Cordozo L, et al. World Health Organization 1st International Consultation on Incontinence, Paris (France): Editions 21 Distributors; 2005.
Aishima M, Tomoda T, et al. Actions of ZD0947, a novel ATP-sensitive K+ channel opener, on membrane currents in human detrusor myocytes. Br J Pharmacol. 2006;149:542-550.
Akasu T, Shinnick-Gallagher P, et al. Adenosine mediates a slow hyperpolarizing synaptic potential in autonomic neurones. Nature. 1984;311(5981):62-65.
Albanese A, Jenner P, et al. Bladder hyperreflexia induced in marmosets by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci Lett. 1988;87(1-2):46-50.
Ali M, Angelo-Khattar M, et al. Urothelial synthesis of prostanoids in the ovine ureter. Urol Res. 1998;26(3):171-174.
Andersson K-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues [Review]. Pharmacol Rev. 1993;45:253-308.
Andersson KE. Clinical pharmacology of potassium channel openers. Pharmacol Toxicol. 1992;70:244-254.
Andersson KE. The overactive bladder:pharmacologic basis of drug treatment. Urology. 1997;50(Suppl. 6A):74-84. discussion 85–89
Andersson KE. The concept of uroselectivity. Eur Urol. 1998;33(Suppl. 2):7-11.
Andersson KE, Appell RC, et al. The pharmacological treatment of urinary incontinence. BJU Int. 1999;84:923-947.
Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84(3):935-986.
Andersson KE, Garcia Pascual A, et al. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992;147:253-259.
Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl. 1995;175:43-53.
Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56(4):581-631.
Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117-128.
Apostolidis A, Fowler CJ. The use of botulinum neurotoxin type A (BoNTA) in urology. J Neural Transm. 2008;115:593-605.
Apostolidis A, Popat R, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977-982.
Apostolidis AP, Dasgupta P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions:a european consensus report. Eur Urol. 2009;55(1):100-120.
Arafat HA, Kim GS, et al. Heterogeneity of bladder myocytes in vitro: modulation of myosin isoform expression. Tissue Cell. 2001;33(3):219-232.
Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J Neurophysiol. 1994;72(6):2903-2910.
Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17(21):8402-8407.
Araki I, Du S, et al. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology. 2004;64:1255-1260.
Atala A. Tissue engineering and regenerative medicine:concepts for clinical application. Rejuvenation Res. 2004;7(1):15-31.
Athwal B, Berkley K, et al. Brain activity associated with the urge to void and bladder fill volume in normal men:preliminary data from a PET study. BJU Int. 1999;84:148-149.
Athwal BS, Berkley KJ, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369-377.
Awad SA, Downie JW, et al. Sympathetic activity in the proximal urethra in patients with urinary obstruction. J Urol. 1976;115(5):545-547.
Awad SA, Downie JW, Kiruluta HG. Alpha-adrenergic agents in urinary disorders of the proximal urethra. Part I. Sphincteric incontinence. Br J Urol. 1978;50:332-335.
Babu J, Warshaw DM, et al. Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microsc Res Tech. 2000;50(6):532-540.
Barinaga M. Secrets of secretion revealed. Science. 1993;260(5107):487-489.
Barrington FJF. The relation of the hind-brain to micturition. Brain. 1921;4:23-53.
Barrington FJF. The component reflexes of micturition in the cat. Parts I and II. Brain. 1931;54:177-188.
Barrington FJF. The component reflexes of micturition in the cat. Part III. Brain. 1941;64:239-243.
Batista JE, Wagner JR, et al. Direct measurement of blood flow in the human bladder. J Urol. 1996;155:630-633.
Batra S, Andersson KE. Oestrogen-induced changes in muscarinic receptor density and contractile responses in the female rabbit urinary bladder. Acta Physiol Scand. 1989;137(1):135-141.
Bazeed MA, Thuroff JW, et al. Histochemical study of urethral striated musculature in the dog. J Urol. 1982;128(2):406-410.
Bean BP, Williams CA, Ceelen PW. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990;10:11-19.
Beckel JM, Kanai A, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103-F110.
Bennett BC, Kruse MN, et al. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004-2009.
Biers SM, Reynard JM, et al. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97(3):612-616.
Birder LA. TRPs in bladder diseases. Biochim Biophys Acta. 2007;1772(8):879-884.
Birder LA, Apodaca G, et al. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275(2 Pt. 2):F226-F229.
Birder LA, Barrick SR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285(3):F423-F429.
Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992;12:4878-4889.
Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265(2 Pt. 2):R326-R333.
Birder LA, de Groat WC. Contribution of C-fiber afferent nerves and autonomic pathways in the urinary bladder to spinal c-fos expression induced by bladder irritation. Somatosens Mot Res. 1998;15(1):5-12.
Birder L, de Groat W, et al. Physiology of the urothelium. In Corcos J, Schick E, editors: Textbook of the neurogenic bladder, 2nd ed, London: Taylor & Francis, 2008.
Birder LA, Kanai AJ, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98(23):13396-13401.
Birder L, Kullmann FA, et al. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther. 2007;323(1):227-235.
Birder LA, Nakamura Y, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856-860.
Birder LA, Ruan HZ, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084-F1091.
Blaivas JG. The neurophysiology of micturition:a clinical study of 550 patients. J Urol. 1982;127(5):958-963.
Blok BF. Central pathways controlling micturition and urinary continence. Urology. 2002;59(5 Suppl. 1):13-17.
Blok BF, de Weerd H, et al. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci Lett. 1997;233(2-3):109-112.
Blok BF, Holstege G. The neuronal control of micturition and its relation to the emotional motor system. Prog Brain Res. 1996;107:113-126.
Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222(3):195-198.
Blok BF, Sturms LM, et al. A PET study on cortical and subcortical control of pelvic floor musculature in women. J Comp Neurol. 1997;389(3):535-544.
Blok BF, Sturms LM, et al. Brain activation during micturition in women. Brain. 1998;121(Pt. 11):2033-2042.
Blok BF, van Maarseveen JT, et al. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neurosci Lett. 1998;249(1):68-70.
Blok BF, Willemsen AT, et al. A PET study on brain control of micturition in humans. Brain. 1997;120(Pt. 1):111-121.
Boggs JW, Wenzel BJ, et al. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688-2697.
Bonev AD, Nelson MT. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am J Physiol. 1993;265:C1723-C1728.
Borda E, Contreras-Ortiz N, et al. In vitro effect of acetylcholine and bethanechol on the contractions of the human detrusor muscle. Influence of prostaglandins. Arch Int Pharmacodyn Ther. 1982;259(1):31-39.
Bosch JL, Groen J. Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol. 1995;154:504-507.
Boselli C, Govoni S, et al. Bladder instability: a re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Auton Pharmacol. 2001;21:219-229.
Brading A, Teramoto T, et al. The relationship between the electrophysiological peroperties of lower urinary tract smooth muscles and their function in vivo. In: Bolton T, editor. Smooth muscle excitation. London: Academic; 1996:403.
Brading A, Turner W. Potassium channels and their modulation in the urogenital tract smooth muscles. In: Evans J, editor. Potassium channels and their modulators:from synthesis to clinical experience. London: Taylor & Francis; 1996:335.
Brading AF. Alterations in the physiological properties of urinary bladder smooth muscle caused by bladder emptying against an obstruction. Scand J Urol Nephrol Suppl. 1997;184:51-58.
Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50(6A Suppl.):57-67. discussion 68–73
Brading AF. The physiology of the mammalian urinary outflow tract. Exp Physiol. 1999;84:215-221.
Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570(Pt. 1):13-22.
Brading AF, Mostwin JL. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br J Pharmacol. 1989;98:1083-1090.
Brading AF, Turner WH. The unstable bladder:towards a common mechanism. Br J Urol. 1994;73(1):3-8.
Brady CM, Apostolidis AN, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93(6):770-776.
Braverman AS, Doumanian LR, et al. M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. II. Denervated rat bladder. J Pharmacol Exp Ther. 2006;316(2):875-880.
Braverman AS, Kohn IJ, et al. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am J Physiol. 1998;274(2 Pt. 2):R517-R523.
Braverman AS, Ruggieri MRSr. Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R701-R708.
Braverman AS, Tibb AS, et al. M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther. 2006;316(2):869-874.
Breyer MD, Hebert RL, Breyer RM. Prostanoid receptors and the urogenital tract. Curr Opin Investig Drugs. 2003;4:1343-1353.
Breyer RM, Bagdassarian CK, et al. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661-690.
Buffington CA, Chew DJ, et al. Feline interstitial cystitis. J Am Vet Med Assoc. 1999;215(5):682-687.
Bultitude MI, Hills NH, Shuttleworth KE. Clinical and experimental studies on the action of prostaglandins and their synthesis inhibitors on detrusor muscle in vitro and in vivo. Br J Urol. 1976;48:631-637.
Burnstock G. P2 purinoceptors: historical perspective and classification. Ciba Found Symp. 1996;198:1-28. discussion 29–34
Burnstock G. Handbook of experimental pharmacology on purinergic and pyrimidinergic signalling. Abbracchio M, Williams W. Springer-Verlag, Berlin, 2000.
Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182-188.
Burnstock GB, Dumsday B, et al. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44(3):451-461.
Bushman W, Steers WD, et al. Voiding dysfunction in patients with spastic paraplegia:urodynamic evaluation and response to continuous intrathecal baclofen. Neurourol Urodyn. 1993;12(2):163-170.
Callahan SM, Creed KE. The effects of oestrogens on spontaneous activity and responses to phenylephrine of the mammalian urethra. J Physiol. 1985;358:35-46.
Candura SM, Messori E, et al. Neural 5-HT4 receptors in the human isolated detrusor muscle: effects of indole, benzimidazolone and substituted benzamide agonists and antagonists. Br J Pharmacol. 1996;118:1965-1970.
Cannon TW, Yoshimura N, Chancellor MB. Innovations in pharmacotherapy for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:367-372.
Cardozo LD, Stanton SL. A comparison between bromocriptine and indomethacin in the treatment of detrusor instability. J Urol. 1980;123(3):399-401.
Caspani O, Heppenstall PA. TRPA1 and cold transduction:an unresolved issue? J Gen Physiol. 2009;133(3):245-249.
Castleden CM, Morgan B. The effect of beta-adrenoceptor agonists on urinary incontinence in the elderly. Br J Clin Pharmacol. 1980;10:619-620.
Castro-Diaz D, Amoros MA. Pharmacotherapy for stress urinary incontinence. Curr Opin Urol. 2005;15(4):227-230.
Chacko S, Chang S, et al. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand J Urol Nephrol Suppl. 2004;215:26-36.
Chacko S, DiSanto M, et al. Contractile protein changes in urinary bladder smooth muscle following outlet obstruction. Adv Exp Med Biol. 1999;462:137-153.
Chacko S, Jacob SS, et al. Myosin I from mammalian smooth muscle is regulated by caldesmon-calmodulin. J Biol Chem. 1994;269(22):15803-15807.
Chai TC, Andersson KE, et al. Altered neural control of micturition in the aged F344 rat. Urol Res. 2000;28(5):348-354.
Chai TC, Gray ML, et al. The incidence of a positive ice water test in bladder outlet obstructed patients:evidence for bladder neural plasticity. J Urol. 1998;160(1):34-38.
Chalfin SA, Bradley WE. The etiology of detrusor hyperreflexia in patients with infravesical obstruction. J Urol. 1982;127(5):938-942.
Chancellor M, Chartier-Kastler E. Principles of sacral nerve stimulation (SNS) for the treatment of bladder and urethral sphincter dysfunctions. Neuromodulation. 2000;3(1):16-26.
Chancellor MB. Should we be using chili pepper extracts to treat the overactive bladder? J Urol. 1997;158(6):2097.
Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162(1):3-11.
Chancellor MB, Kaplan SA, et al. The cholinergic and purinergic components of detrusor contractility in a whole rabbit bladder model. J Urol. 1992;148(3):906-909.
Chancellor MB, Kaufman J. Case for pharmacotherapy development for underactive bladder. Urology. 2008;72(5):966-967.
Chancellor MB, Lavelle J, et al. Ice-water test in the urodynamic evaluation of spinal cord injured patients. Tech Urol. 1998;4(2):87-91.
Chancellor MB, Rivas DA, et al. Laplace’s law and the risks and prevention of bladder rupture after enterocystoplasty and bladder autoaugmentation. Neurourol Urodyn. 1996;15(3):223-233.
Chancellor MB, Yokoyama T, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19(3):279-287.
Chang HY, Cheng CL, et al. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(1):R224-R234.
Chang HY, Cheng CL, et al. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292(3):F1044-F1053.
Chang SL, Chung JS, et al. Roles of the lamina propria and the detrusor in tension transfer during bladder filling. Scand J Urol Nephrol Suppl. 1999;201:38-45.
Chapple CR, Helm CW, et al. Asymptomatic bladder neck incompetence in nulliparous females. Br J Urol. 1989;64(4):357-359.
Charrua A, Cruz CD, et al. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177(4):1537-1541.
Charrua A, Cruz CD, et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009;181(1):379-386.
Chen Q, Takahashi S, et al. Function of the lower urinary tract in mice lacking alpha1d-adrenoceptor. J Urol. 2005;174(1):370-374.
Chen SY, Wang SD, et al. Glutamate activation of neurons in CV-reactive areas of cat brain stem affects urinary bladder motility. Am J Physiol. 1993;265(4 Pt. 2):F520-F529.
Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40-48.
Cher ML, Kamm KE, et al. Stress generation and myosin phosphorylation in the obstructed bladder. J Urol. 1990;143:355A.
Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22:133-145.
Chess-Williams R. Potential therapeutic targets for the treatment of detrusor overactivity. Expert Opin Ther Targets. 2004;8:95-106.
Chopra B, Barrick SR, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859-871.
Chopra B, Gever J, et al. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol. 2008;294:F821-F829.
Christ GJ, Day NS, et al. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1699-R1709.
Christ GJ, Day NS, et al. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1241-R1248.
Christianson JA, Liang R, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235-243.
Chuang YC, Fraser MO, et al. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975-979.
Chuang YC, Huang CC, et al. Novel action of botulinum toxin on the stromal and epithelial components of the prostate gland. J Urol. 2006;175(3 Pt. 1):1158-1163.
Chuang YC, Yoshimura N, et al. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529-1532.
Chun AL, Wallace LJ, et al. Effect of age on in vivo urinary bladder function in the rat. J Urol. 1988;139:625-627.
Chun AL, Wallace LJ, et al. Effects of age on urinary bladder function in the male rat. J Urol. 1989;141:170-173.
Clapham DE. Some like it hot: spicing up ion channels. Nature. 1997;389(6653):783-784.
Clausen C, Lewis SA, et al. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979;26(2):291-317.
Cockayne DA, Dunn PM, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621-639.
Cockayne DA, Hamilton SG, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407(6807):1011-1015.
Collins C, Klausner AP, et al. Potential for control of detrusor smooth muscle spontaneous rhythmic contraction by cyclooxygenase products released by interstitial cells of Cajal. J Cell Mol Med. 2009;13(9B):3236-3250.
Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol. 1982;127(5):964-969.
Cook SP, McCleskey EW. ATP, pain and a full bladder. Nature. 2000;407(6807):951-952.
Cornelissen LL, Brooks DP, et al. Female, but not male, serotonin reuptake transporter (5-HTT) knockout mice exhibit bladder instability. Auton Neurosci. 2005;122(1-2):107-110.
Cortivo R, Pagano F, et al. Elastin and collagen in the normal and obstructed urinary bladder. Br J Urol. 1981;53(2):134-137.
Craft RM, Cohen SM, et al. Long-lasting desensitization of bladder afferents following intravesical resiniferatoxin and capsaicin in the rat. Pain. 1995;61(2):317-323.
Crowe R, Light K, et al. Vasoactive intestinal polypeptide-, somatostatin- and substance P-immunoreactive nerves in the smooth muscle and striated muscle of the intrinsic external urethral sphincter of patients with spinal cord injury. J Urol. 1986;136:487-491.
Cruz CD, Ferreira D, et al. The activation of the ERK pathway contributes to the spinal c-fos expression observed after noxious bladder stimulation. Somatosens Mot Res. 2007;24(1-2):15-20.
D’Agostino G, Barbieri A, et al. M4 muscarinic autoreceptor-mediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder. J Pharmacol Exp Ther. 1997;283(2):750-756.
D’Agostino G, Bolognesi ML, et al. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor:involvement of the M4 receptor subtype. Br J Pharmacol. 2000;129(3):493-500.
D’Agostino G, Kilbinger H, et al. Presynaptic inhibitory muscarinic receptors modulating [3H] acetylcholine release in the rat urinary bladder. J Pharmacol Exp Ther. 1986;239(2):522-528.
Daly D, Rong W, et al. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583(Pt. 2):663-674.
Damaser MS. Whole bladder mechanics during filling. Scand J Urol Nephrol Suppl. 1999;201:51-58. discussion 76–102
Damaser MS, Lehman SL. The effect of urinary bladder shape on its mechanics during filling. J Biomech. 1995;28(6):725-732.
Dang K, Bielefeldt K, et al. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25(15):3973-3984.
Dang K, Lamb K, et al. Cyclophosphamide-induced bladder inflammation sensitizes and enhances p2x receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99(1):49-59.
Danuser H, Bemis K, et al. Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther. 1995;274(2):820-825.
Danuser H, Thor KB. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the alpha 1 adrenergic receptor antagonist prazosin. J Urol. 1995;153(4):1308-1312.
Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol. 1996;118(1):150-154.
Darblade B, Behr-Roussel D, et al. Piboserod (SB 207266), a selective 5-HT4 receptor antagonist, reduces serotonin potentiation of neurally-mediated contractile responses of human detrusor muscle. World J Urol. 2005;23:147-151.
Das AK, Carlson AM, et al. Improvement in depression and health-related quality of life after sacral nerve stimulation therapy for treatment of voiding dysfunction. Urology. 2004;64:62-68.
DasGupta BR. Structures of botulinum neurotoxin, its functional domains, and perspectives on the crystalline type A toxin. In: Jankovic J, Hallet M, editors. Therapy with botulinum toxin. New York: Marcel Dekker; 1994:15-39.
DasGupta R, Kavia RB, Fowler CJ. Cerebral mechanisms and voiding function. BJU Int. 2007;99:731-734.
Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol. 2005;173(2):635-639.
de Groat WC. Nervous control of the urinary bladder of the cat. Brain Research. 1975;87:201-211.
de Groat WC. Inhibitory mechanisms in the sacral reflex pathways to the urinary bladder. In: Ryall RW, Kelly JS, editors. Iontophoresis and transmitter mechanisms in the mammalian central system. Amsterdam: Elsevier; 1978:366-368.
de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165-187.
de Groat WC. Neuropeptides in pelvic afferent pathways. Experientia Suppl. 1989;56:334-361.
de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59:30-36.
de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64:7-11.
de Groat WC, Booth AM. Synaptic transmission in pelvic ganglia. In: Maggi CA, editor. The autonomic nervous system, vol 1. London: Harwood Academic Publishers; 1993:291-347.
de Groat WC, Booth AM, et al. Neural control of the urinary bladder and large intestine. In: Brooks CM, Koizumi K, Sato A, editors. Integrative functions of the autonomic nervous system. Tokyo: Tokyo University Press; 1979:50-67.
de Groat WC, Booth AM, et al. Parasympathetic preganglionic neurons in the sacral spinal cord. J Auton Nerv Syst. 1982;5:23-43.
de Groat WC, Booth AM, et al. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system, vol 1. London: Harwood Academic Publishers; 1993:227-290.
de Groat WC, Douglas JW, et al. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 1975;94:150-154.
de Groat WC, Fraser MO, et al. Neural control of the urethra. Scand J Urol Nephrol Suppl. 2001;207:35-43.
de Groat WC, Kawatani M, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30(Suppl.):S71-S77.
de Groat WC, Kruse MN, et al. Modification of urinary bladder function after spinal cord injury. Adv Neurol. 1997;72:347-364.
de Groat WC, Nadelhaft I, et al. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135-160.
de Groat WC, Roppolo JR, et al. Neural control of the urinary bladder and colon. In: Tache Y, Wingate D, Burks T, editors. Innervation of the gut: pathophysiological implications. Boca Raton (FL): CRC Press; 1993:167-190.
de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol (London). 1969;200:87-108.
de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol (London). 1976;259:223-237.
de Groat WC, Vizzard MA, et al. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res. 1996;107:97-111.
de Groat WC, Yoshiyama M, et al. Modulation of voiding and storage reflexes by activation of alpha1-adrenoceptors [Review]. Eur Urol. 1999;36(Suppl. 1):68-73.
Delaere KP, Thomas CM, et al. The value of intravesical prostaglandin E2 and F2α in women with abnormalities of bladder emptying. Br J Urol. 1981;53:3069.
DeLancey J. Structural aspects of urethrovesical function in the female. Neurourol Urodyn. 1988;7:509.
DeLancey J. Pubovesical ligaments: a separate structure from the urethral supports (pubourethral ligaments). Neurourol Urodynam. 1989;8:53.
DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15(5):268-274.
Denys P, Chartier-Kastler E, et al. Intrathecal clonide for refractory detrusor hyperreflexia in spinal cord injured patients: a preliminary report. J Urol. 1998;160:2137.
Dickson A, Avelino A, et al. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;141(3):1633-1647.
Dijkema HE, Weil EH, et al. Neuromodulation of sacral nerves for incontinence and voiding dysfunctions. Clinical results and complications. Eur Urol. 1993;24(1):72-76.
Ding YQ, Takada M, et al. Direct projections from the dorsolateral pontine tegmentum to pudendal motoneurons innervating the external urethral sphincter muscle in the rat. J Comp Neurol. 1995;357(2):318-330.
Dinis P, Charrua A, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253-11263.
Diokno AC, Brock BM, et al. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022-1025.
Dixon J, Gosling J. Structure and innervation of human bladder. In: Torrens M, Morrison J, editors. The physiology of the lower urinary tract. Berlin: Springer-Verlag; 1987:3-22.
Dmitrieva N, Burnstock G, et al. ATP and 2-methylthioATP activate bladder reflexes and induce discharge of bladder sensory neurones. Soc Neurosci Abstr. 1998;24:2088.
Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87-97.
Dmitrieva N, Shelton D, et al. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449-459.
Dmitrieva N, Zhang G, et al. Increased alpha1D adrenergic receptor activity and protein expression in the urinary bladder of aged rats. World J Urol. 2008;26(6):649-655.
Doi T, Kamo I, et al. Effects of TAK-637, a tachykinin receptor antagonist, on lower urinary tract function in the guinea pig. Eur J Pharmacol. 1999;383(3):297-303.
Dokita S, Morgan WR, et al. NG-nitro-L-arginine inhibits non-adrenergic, non-cholinergic relaxation in rabbit urethral smooth muscle. Life Sci. 1991;48(25):2429-2436.
Dolber PC, Gu B, et al. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1699-R1706.
Donaldson PJ, Lewis SA. Effect of hyperosmotic challenge on basolateral membrane potential in rabbit urinary bladder. Am J Physiol. 1990;258(2 Pt. 1):C248-C257.
Donker P, Droes JP, et al. Anatomy of the musculature and innovation of the bladder and urethra. In: Chisolm G, Williams D, editors. Scientific foundations of urology. Chicago: Year Book Medical; 1982:404-441.
Downie J. Pharmacological manipulation of central micturition circuitry. Curr Opin Central Periph Nerv Sys. 1999;1:231.
Downie JW, Armour JA. Mechanoreceptor afferent activity compared with receptor field dimensions and pressure changes in feline urinary bladder. Can J Physiol Pharmacol. 1992;70(11):1457-1467.
Downie JW, Bialik GJ. Evidence for a spinal site of action of clonidine on somatic and viscerosomatic reflex activity evoked on the pudendal nerve in cats. J Pharmacol Exp Ther. 1988;246(1):352-358.
Drake MJ, Hedlund P, et al. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003;170(1):276-279.
Dray A, Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Pharmacol Exp Ther. 1984;231:254-260.
Dray A, Metsch R. Morphine and the centrally-mediated inhibition of urinary bladder motility in the rat. Brain Res. 1984;297:191-195.
Dressler D, Saberi FA, Barbosa ER. Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr. 2005;63:180-185.
Du S, Araki I, et al. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007;70(4):826-831.
Du S, Araki I, et al. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008;72(2):450-455.
Dupont M, Steers WD, et al. Neural plasticity and alterations in nerve growth factor and norepinephrine in response to bladder inflammation. J Urol. 1994;151:284.
Dykstra DD, Sidi AA. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: a double-blind study. Arch Phys Med Rehabil. 1990;71:24-26.
Dykstra DD, Sidi AA, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol. 1988;139:919-922.
Eglen R, Hedge S, et al. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531.
Eglen RM, Reddy H, et al. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol Sci. 1994;15:114-119.
Ehler FJ, Griffin MT, et al. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther. 2005;313(1):368-378.
Ehren I, Hammarstrom M, et al. Induction of calcium-dependent nitric oxide synthase by sex hormones in the guinea-pig urinary bladder. Acta Physiol Scand. 1995;153(4):393-394.
Ek A. Innervation and receptor functions of the human urethra. Scand J Urol Nephrol Suppl. 1977;45:1-50.
Ek A, Andersson KE, et al. The effects of long-term treatment with norephedrine on stress incontinence and urethral closure pressure profile. Scand J Urol Nephrol. 1978;12(2):105-110.
Ekstrom J, Iosif CS, et al. Effects of long-term treatment with estrogen and progesterone on in vitro muscle responses of the female rabbit urinary bladder and urethra to autonomic drugs and nerve stimulation. J Urol. 1993;150(4):1284-1288.
Elbadawi A. Ultrastructure of vesicourethral innervation. II. Postganglionic axoaxonal synapses in intrinsic innervation of the vesicourethral lissosphincter:a new structural and functional concept in micturition. J Urol. 1984;131(4):781-790.
Elbadawi A, Schenk EA. Dual innervation of the mammalian urinary bladder:a histochemical study of the distribution of cholinergic and adrenergic nerves. Am J Anatomy. 1966;119:405-427.
Elliott RA, Castleden CM, et al. The effect of in vivo oestrogen pretreatment on the contractile response of rat isolated detrusor muscle. Br J Pharmacol. 1992;107(3):766-770.
Enhorning G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta Chir Scand Suppl. 1961;276:1-68.
Espey MJ, Downie JW, et al. Effect of 5-HT receptor and adrenoceptor antagonists on micturition in conscious cats. Eur J Pharmacol. 1992;221(1):167-170.
Espey MJ, Du HJ, et al. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res. 1998;798(1-2):101-108.
Everaerts W, Gevaert T, et al. On the origin of bladder sensing:Tr(i)ps in urology. Neurourol Urodyn. 2008;27(4):264-273.
Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261-1272.
Fajardo O, Meseguer V, et al. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons:pharmacological and genetic evidence. J Neurosci. 2008;28(31):7863-7875.
Falconer C, Blomgren B, et al. Different organization of collagen fibrils in stress-incontinent women of fertile age. Acta Obstet Gynecol Scand. 1998;77(1):87-94.
Falconer C, Ekman-Ordeberg G, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand. 1998;77(1):95-100.
Fall M, Lindstrom S. Electrical stimulation. A physiologic approach to the treatment of urinary incontinence. Urol Clin North Am. 1991;18(2):393-407.
Fall M, Lindström S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol (London). 1990;427:281-300.
Fawcett D. The urinary system. In: Bloom W, editor. Textbook of histology. 12th ed. New York: Chapman & Hall; 1984:728-764.
Fedirchuk B, Hochman S, et al. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Exp Brain Res. 1992;89(3):511-516.
Fellows GJ, Marshall DH. The permeability of human bladder epithelium to water and sodium. Invest Urol. 1972;9(4):339-344.
Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol. 1997;505(Pt. 2):503-511.
Flood HD, Liu JL, et al. Sex differences in the nitric oxide (NO): mediated smooth muscle component and striated muscle component of urethral relaxation in rats. Neurourol Urodyn. 1995;14:517.
Floyd K, Hick VE, Morrison JF. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976;259:457-471.
Flügge W, editor. Stresses in shells, 2nd ed. Springer, New York, 1973, 525.
Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506(6):989-1002.
Foster CD, Speakman MJ, et al. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989;63(3):284-294.
Fowler CJ, Beck RO, et al. Intravesical capsaicin for treatment of detrusor hyperreflexia. J Neurol Neurosurg Psychiatry. 1994;57:169-173.
Fowler CJ, Griffiths D, et al. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453-466.
Fowler CJ, Jewkes D, et al. Intravesical capsaicin for neurogenic bladder dysfunction. Lancet. 1992;339:1239.
Fraser MO, Flood HD, et al. Urethral smooth muscle relaxation is mediated by nitric oxide (NO) released from parasympathetic postganglionic neurons. J Urol. 153, 1995. 461A–461A
Frazier EP, Peters SL, et al. Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4-6):449-462.
Fry CH, Ikeda Y, et al. Control of bladder function by peripheral nerves:avenues for novel drug targets. Urology. 2004;63(3 Suppl. 1):24-31.
Fry CH, Skennerton D, et al. The cellular basis of contraction in human detrusor smooth muscle from patients with stable and unstable bladders. Urology. 2002;59(5 Suppl. 1):3-12.
Fry CH, Sui GP, et al. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26(6 Suppl.):914-919.
Fry CH, Wu C. Initiation of contraction in detrusor smooth muscle. Scand J Urol Nephrol Suppl. 1997;184:7-14.
Fujii K, Foster CD, et al. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990;99:779-785.
Fukuda H, Koga T. Midbrain stimulation inhibits the micturition, defecation and rhythmic straining reflexes elicited by activation of sacral vesical and rectal afferents in the dog. Exp Brain Res. 1991;83(2):303-316.
Furuta A, Asano K, et al. Role of alpha2-adrenoceptors and glutamate mechanisms in the external urethral sphincter continence reflex in rats. J Urol. 2009;181(3):1467-1473.
Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995;24:159-187.
Gabella G, Uvelius B. Urinary bladder of rat:fine structure of normal and hypertrophic musculature. Cell Tissue Res. 1990;262(1):67-79.
Gajewski J, Downie JW, Awad SA. Experimental evidence for a central nervous system site of action in the effect of alpha-adrenergic blockers on the external urinary sphincter. J Urol. 1984;132:403-409.
Galeano C, Jubelin B, et al. Micturition reflexes in chronic spinalized cats: the underactive detrusor and detrusor-sphincter dyssynergia. Neurourol Urodyn. 1986;5:45-63.
Gao X, Buffington CA, et al. Effect of interstitial cystitis on drug absorption from urinary bladder. J Pharmacol Exp Ther. 1994;271(2):818-823.
Geirsson G. Evidence of cold receptors in the human bladder:effect of menthol on the bladder cooling reflex. J Urol. 1993;150(2 Pt. 1):427-430.
Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J Urol. 1995;154:1825-1829.
Geirsson G, Lindstrom S, et al. The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. J Urol. 1999;162(6):1890-1896.
Gevaert T, Vriens J, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453-3462.
Gillespie JI. Phosphodiesterase-linked inhibition of nonmicturition activity in the isolated bladder. BJU Int. 2004;93(9):1325-1332.
Gillespie JI, Markerink-van Ittersum M, et al. cGMP-generating cells in the bladder wall:identification of distinct networks of interstitial cells. BJU Int. 2004;94(7):1114-1124.
Gilpin SA, Gilpin CJ, et al. The effect of age on the autonomic innervation of the urinary bladder. Br J Urol. 1986;58(4):378-381.
Goins WF, Yoshimura N, et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748-1754.
Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629-2646.
Gopalakrishnan M, Shieh CC. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin Ther Targets. 2004;8:437-458.
Gosling J. Gross anatomy of the lower urinary tract. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication; 1999:23-56.
Gosling JA, Dixon JS, Lendon RG. The autonomic innervation of the human male and female bladder neck and proximal urethra. J Urol. 1977;118:302-305.
Gosling JA, Dixon JS, et al. A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol. 1981;53:35-41.
Gosling JA, Kung LS, et al. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000;163(4):1349-1356.
Grases F, Garcia-Ferragut L, Costa-Bauza A. Study of the early stages of renal stone formation: experimental model using urothelium of pig urinary bladder. Urol Res. 1996;24:305-311.
Green SA, Alon A, et al. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J Urol. 2006;176(6 Pt. 1):2535-2540. discussion 2540
Greenland JE, Brading AF. Urinary bladder blood flow changes during the micturition cycle in a conscious pig model. J Urol. 1996;156(5):1858-1861.
Griffiths D. Mechanics of micturition. In: Yalla S, McGuire E, Elbadawai A, Blaivas J, editors. Neurourology and urodynamics: principles and practice. New York: Macmillan; 1988:96-105.
Griffiths D, Derbyshire S, et al. Brain control of normal and overactive bladder. J Urol. 2005;174:1862-1867.
Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27(6):466-474.
Griffiths D, Tadic SD, et al. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37:1-7.
Grol S, Essers PB, et al. M(3) muscarinic receptor expression on suburothelial interstitial cells. BJU Int. 2009;104(3):398-405.
Gu B, Fraser MO, et al. Induction of bladder sphincter dyssynergia by kappa-2 opioid receptor agonists in the female rat. J Urol. 2004;171(1):472-477.
Gu B, Thor KB, et al. Effect of 5-hydroxytryptamine1 serotonin receptor agonists on noxiously stimulated micturition in cats with chronic spinal cord injury. J Urol. 2007;177:2381-2385.
Guarneri L, Ibba M, et al. The effect of mCPP on bladder voiding contractions in rats are mediated by the 5HT2A/5-HT2C receptors. Neurourol Urodyn. 1996;15:316.
Gunst SJ, Wu MF, et al. Contraction history modulates isotonic shortening velocity in smooth muscle. Am J Physiol. 1993;265(2 Pt. 1):C467-C476.
Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol. 1999;39(3):289-296.
Häbler HJ, Janig W, et al. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545-562.
Häbler HJ, Janig W, et al. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449-460.
Haferkamp A, Mundhenk J, et al. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004;46(6):799-805.
Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285-298.
Hald T, Horn T. The human urinary bladder in ageing. Br J Urol. 1998;82(Suppl. 1):59-64.
Hampel C, Dolber PC, et al. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002;167(3):1513-1521.
Hanno PM, Fritz RW, et al. Heparin—examination of its antibacterial adsorption properties. Urology. 1981;18:273-276.
Hanyu S, Iwanaga T, et al. Distribution of serotonin-immunoreactive paraneurons in the lower urinary tract of dogs. Am J Anat. 1987;180(4):349-356.
Harriss DR, Marsh KA, et al. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J Urol. 1995;154:1241-1245.
Hashimoto K, Oyama T, et al. Neuronal excitation in the ventral tegmental area modulates the micturition reflex mediated via the dopamine D1 and D2 receptors in rats. J Pharmacol Sci. 2003 Jun;92(2):143-148.
Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576(Pt. 3):707-714.
Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140(1):159-169.
Hashitani H, Bramich NJ, et al. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524(Pt. 2):565-579.
Hashitani H, Yanai Y, et al. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559(Pt. 2):567-581.
Hawthorn MH, Chapple CR, et al. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129(3):416-419.
Hayashi Y, Takimoto K, et al. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1661-R1670.
Hegde SS, Choppin A, et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120(8):1409-1418.
Hendrix SL, Cochrane BB, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293(8):935-948.
Heppner TJ, Bonev AD, Nelson MT. Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110-C117.
Hergenhahn M, Adolf W, et al. Resiniferatoxin and other esters of novel polyfunctional diterpenes from Euphorbia resinifera and euspina. Tetra Lett. 1975;19:1595.
Hickey DS, Phillips JI, Hukins DW. Arrangements of collagen fibrils and muscle fibres in the female urethra and their implications for the control of micturition. Br J Urol. 1982;54:556-561.
Hicks RM. The fine structure of the transitional epithelium of rat ureter. J Cell Biol. 1965;26(1):25-48.
Hicks RM. The mammalian urinary bladder:an accommodating organ. Biol Rev Camb Philos Soc. 1975;50(2):215-246.
Hirayama A, Fujimoto K, et al. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62(5):909-913.
Hirayama A, Fujimoto K, et al. Nocturia in men with lower urinary tract symptoms is associated with both nocturnal polyuria and detrusor overactivity with positive response to ice water test. Urology. 2005;65:1064-1069.
Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res. 1984;298(1):51-65.
Ho KM, McMurray G, et al. Nitric oxide synthase in the heterogeneous population of intramural striated muscle fibres of the human membranous urethral sphincter. J Urol. 1998;159(3):1091-1096.
Hohlbrugger G. Changes of hypo- and hypertonic sodium chloride induced by the rat urinary bladder at various filling stages. Evidence for an increased transurothelial access of urine to detrusor nerve and muscle cells with distension. Eur Urol. 1987;13:83-89.
Hohlbrugger G. The vesical blood-urine barrier:a relevant and dynamic interface between renal function and nervous bladder control. J Urol. 1995;154(1):6-15.
Hohlbrugger G, Lentsch P. Intravesical ions, osmolality and pH influence the volume pressure response in the normal rat bladder, and this is more pronounced after DMSO exposure. Eur Urol. 1985;11:127-130.
Holstege G. Micturition and the soul. J Comp Neurol. 2005;493:15-20.
Holstege G, Mouton LJ. Central nervous system control of micturition. Int Rev Neurobiol. 2003;56:123-145.
Holstege JC, Van Dijken H, et al. Distribution of dopamine immunoreactivity in the rat, cat and monkey spinal cord. J Comp Neurol. 1996;376:631-652.
Homma Y, Imajo C, et al. Urinary symptoms and urodynamics in a normal elderly population. Scand J Urol Nephrol Suppl. 1994;157:27-30.
Homolya L, Steinberg TH, et al. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150(6):1349-1360.
Horby-Petersen J, Schmidt PF, et al. The effects of a new serotonin receptor antagonist (ketanserin) on lower urinary tract function in patients with prostatism. J Urol. 1985;133(6):1094-1098.
Hossler FE, Monson FC. Microvasculature of the rabbit urinary bladder. Anat Rec. 1995;243(4):438-448.
Hotta H, Morrison JF, et al. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995;45(5):823-836.
Hu P, Meyers S, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200-F1207.
Hu S, Kim HS. Modulation of ATP-sensitive and large-conductance Ca++-activated K+ channels by Zeneca ZD6169 in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther. 1997;280(1):38-45.
Hu TW, Wagner TH, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61(6):1123-1128.
Hu TW, Wagner TH, et al. Costs of urinary incontinence and overactive bladder in the United States:a comparative study. Urology. 2004;63(3):461-465.
Hu VY, Zvara P, et al. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173(3):1016-1021.
Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contrib Gynecol Obstet. 1983;10:1-31.
Hurst RE. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J Urol. 1994;12:3-10.
Hutch JA, Rambo ONJr. A new theory of the anatomy of the internal urinary sphincter and the physiology of micturition. 3. Anatomy of the urethra. J Urol. 1967;97(4):696-704.
Iacovou JW, Hill SJ, Birmingham AT. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol. 1990;144:775-779.
Igawa Y, Mattiasson A, et al. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J Urol. 1993;150(2 Pt. 1):537-542.
Igawa Y, Yamazaki Y, et al. Functional and molecular biological evidence for a possible beta3-adrenoceptor in the human detrusor muscle. Br J Pharmacol. 1999;126:819-825.
Igawa Y, Zhang X, et al. Cystometric findings in mice lacking muscarinic M2 or M3 receptors. J Urol. 2004;172(6 Pt. 1):2460-2464.
Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593-607.
Ikeda K, Kobayashi S, et al. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol. 2002;366(2):97-103.
Imamura M, Negoro H, et al. Basic fibroblast growth factor causes urinary bladder overactivity through gap junction generation in the smooth muscle. Am J Physiol Renal Physiol. 2009;297:F46-F54.
Inoue R, Brading AF. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990;100(3):619-625.
Inoue T, Gabella G. A vascular network closely linked to the epithelium of the urinary bladder of the rat. Cell Tissue Res. 1991;263(1):137-143.
Iosif CS, Batra S, et al. Estrogen receptors in the human female lower uninary tract. Am J Obstet Gynecol. 1981;141(7):817-820.
Ishiura Y, Yoshiyama M, et al. Central muscarinic mechanisms regulating voiding in rats. J Pharmacol Exp Ther. 2001;297(3):933-939.
Ishizuka O, Alm P, et al. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66(4):1009-1014.
Ishizuka O, Gu BJ, et al. Functional role of central muscarinic receptors for micturition in normal conscious rats. J Urol. 2002;168:2258-2262.
Ishizuka O, Igawa Y, et al. Role of intrathecal tachykinins for micturition in unanaesthetized rats with and without bladder outlet obstruction. Br J Pharmacol. 1994;113:111-116.
Ishizuka O, Mattiasson A, et al. Effects of neurokinin receptor antagonists on L-dopa induced bladder hyperactivity in normal conscious rats. J Urol. 1995;154(4):1548-1551.
Ishizuka O, Mattiasson A, et al. Role of spinal and peripheral alpha 2 adrenoceptors in micturition in normal conscious rats. J Urol. 1996;156(5):1853-1857.
Ishizuka O, Persson K, et al. Micturition in conscious rats with and without bladder outlet obstruction:role of spinal alpha 1-adrenoceptors. Br J Pharmacol. 1996;117(5):962-966.
Ito K, Iwami A, et al. Therapeutic effects of the putative P2X3/P2X2/3 antagonist A-317491 on cyclophosphamide-induced cystitis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4-6):483-490.
Jaggar SI, Scott HC, et al. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83(3):442-448.
Jallat-Daloz I, Cognard JL, et al. Neural-epithelial cell interplay:in vitro evidence that vagal mediators increase PGE2 production by human nasal epithelial cells. Allergy Asthma Proc. 2001;22(1):17-23.
Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87-114.
Jeremy JY, Tsang V, et al. Eicosanoid synthesis by human urinary bladder mucosa:pathological implications. Br J Urol. 1987;59(1):36-39.
Johansson S, Fall M. The pathology of interstitial cystitis. In: Sant GR, editor. Interstitial cystitis. Philadelphia: Lippincott-Raven; 1997:143-151.
John H, Wang X, et al. Evidence of gap junctions in the stable nonobstructed human bladder. J Urol. 2003;169(2):745-749.
Johnston L, Carson C, et al. Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am J Physiol Renal Physiol. 2008;294(3):F645-F655.
Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Horm Behav. 1997;32:1-10.
Jung SY, Fraser MO, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162(1):204-212.
Kaiho Y, Kamo I, et al. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292(2):F639-F646.
Kaiho Y, Nishiguchi J, et al. The effects of a type 4 phosphodiesterase inhibitor and the muscarinic cholinergic antagonist tolterodine tartrate on detrusor overactivity in female rats with bladder outlet obstruction. BJU Int. 2008;101:615-620.
Kakizaki H, de Groat WC. Role of spinal nitric oxide in the facilitation of the micturition reflex by bladder irritation. J Urol. 1996;155:355-360.
Kakizaki H, Fraser MO, et al. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am J Physiol. 1997;272:R1647.
Kakizaki H, Koyanagi T, Kato M. [Urethral response to sympathetic nerve stimulation in the cat.]. Nippon Hinyokika Gakkai Zasshi. 1991;82:1664-1670. [in Japanese]
Kakizaki H, Yoshiyama M, de Groat WC. Role of NMDA and AMPA glutamatergic transmission in spinal c-fos expression after urinary tract irritation. Am J Physiol. 1996;270:R990-R996.
Kakizaki H, Yoshiyama M, et al. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1407-R1413.
Kamo I, Chancellor MB, et al. Differential effects of activation of peripheral and spinal tachykinin neurokinin(3) receptors on the micturition reflex in rats. J Urol. 2005;174(2):776-781.
Kanie S, Yokoyama O, et al. GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1230-R1238.
Kaplan SA, Chancellor MB, et al. Bladder and sphincter behavior in patients with spinal cord lesions. J Urol. 1991;146(1):113-117.
Kawatani M, Nagel J, de Groat WC. Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1986;249:117-132.
Kawatani M, Rutigliano M, de Groat WC. Vasoactive intestinal polypeptide produces ganglionic depolarization and facilitates muscarinic excitatory mechanisms in a sympathetic ganglion. Science. 1985;229:879-881.
Kawatani M, Suzuki T, de Groat WC. Corticotropin releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Syst. 1996;61:218-226.
Keane DP, Sims TJ, et al. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104(9):994-998.
Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615-623.
Keast JR, Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience. 1998;85(2):543-556.
Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424(4):577-587.
Kelly S. The urinary system. In: Kelley DE, Wood RL, Enders AC, editors. Bailey’s textbook of microscopic anatomy. Baltimore: Williams & Wilkins; 1984:645-686.
Khan MA, Dashwood MR, et al. Up-regulation of endothelin (ET(A) and ET(B)) receptors and down-regulation of nitric oxide synthase in the detrusor of a rabbit model of partial bladder outlet obstruction. Urol Res. 1999;27:445-453.
Khawaja AM, Rogers DF. Tachykinins:receptor to effector. Int J Biochem Cell Biol. 1996;28(7):721-738.
Khera M, Somogyi GT, et al. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987-993.
Kitta T, Kakizaki H, et al. Brain activation during detrusor overactivity in patients with Parkinson’s disease:a positron emission tomography study. J Urol. 2006;175(3 Pt. 1):994-998.
Klarskov P, Horby-Petersen J. Influence of serotonin on lower urinary tract smooth muscle in vitro. Br J Urol. 1986;58(5):507-513.
Klevmark B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol Scand. 1974;90(3):565-577.
Kondo S, Morita T, et al. Muscarinic cholinergic receptor subtypes in human detrusor muscle studied by labeled and nonlabeled pirenzepine, AFDX-116 and 4DAMP. Urol Int. 1995;54(3):150-153.
Kontani H, Inoue T, Sakai T. Dopamine receptor subtypes that induce hyperactive urinary bladder response in anesthetized rats. Jpn J Pharmacol. 1990;54:482-486.
Kontani H, Maruyama I, et al. Involvement of alpha 2-adrenoceptors in the sacral micturition reflex in rats. Jpn J Pharmacol. 1992;60(4):363-368.
Krause JE, Chenard BL, et al. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Curr Opin Investig Drugs. 2005;6(1):48-57.
Krenz NR, Meakin SO, et al. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci. 1999;19(17):7405-7414.
Kruse MN, Bray LA, et al. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54(3):215-224.
Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neurosci Lett. 1993;152(1-2):141-144.
Kruse MN, Noto H, et al. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182-190.
Kubota Y, Biers SM, et al. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006;25:205-210.
Kubota Y, Hashitani H, et al. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008;27:330-340.
Kubota Y, Kajioka S, et al. Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci. 2004;115:64-73.
Kuhtz-Buschbeck JP, Gilster R, et al. Control of bladder sensations: an fMRI study of brain activity and effective connectivity. Neuroimage. 2009;47:18-27.
Kuhtz-Buschbeck JP, van der Horst C, et al. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477-1481.
Kullmann FA, Artim D, et al. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol. 2008;294:F971-F981.
Kullmann FA, Artim DE, et al. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977-1987.
Kumar V, Cross RL, et al. Recent advances in basic science for overactive bladder. Curr Opin Urol. 2005;15(4):222-226.
Lagos P, Ballejo G. Role of spinal nitric oxide synthase-dependent processes in the initiation of the micturition hyperreflexia associated with cyclophosphamide-induced cystitis. Neuroscience. 2004;125:663-670.
Lai FM, Cobuzzi A, Spinelli W. Characterization of muscarinic receptors mediating the contraction of the urinary detrusor muscle in cynomolgus monkeys and guinea pigs. Life Sci. 1998;62:1179-1186.
Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150-156.
Landau EH, Jayanthi VR, et al. Loss of elasticity in dysfunctional bladders:urodynamic and histochemical correlation. J Urol. 1994;152(2 Pt. 2):702-705.
Langley LL, Whiteside JA. Mechanism of accommodation and tone of urinary bladder. J Neurophysiol. 1951;14(2):147-152.
Lashinger ES, Steiginga MS, et al. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008;295:F803-F810.
Lassmann G. [Muscle spindles and sensory nerve endings in the urethral sphincter.]. Acta Neuropathol. 1984;63(4):344-346.
Latifpour J, Kondo S, et al. Autonomic receptors in urinary tract:sex and age differences. J Pharmacol Exp Ther. 1990;253(2):661-667.
Lauzon AM, Tyska MJ, et al. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil. 1998;19(8):825-837.
Lavelle JP, Apodaca G, et al. Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. Am J Physiol. 1998;274:F205-F214.
Lavelle JP, Meyers SA, et al. Urothelial pathophysiological changes in feline interstitial cystitis:a human model. Am J Physiol Renal Physiol. 2000;278(4):F540-F553.
Lavelle JP, Negrete HO, et al. Low permeabilities of MDCK cell monolayers:a model barrier epithelium. Am J Physiol. 1997;273(1 Pt. 2):F67-F75.
Lazzeri M, Beneforti P, et al. Intravesical capsaicin for treatment of severe bladder pain: a randomized placebo controlled study. J Urol. 1996;156:947-952.
Lazzeri M, Beneforti P, et al. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000;164:676-679.
Lecci A, Giuliani S, et al. Evidence for a role of tachykinins as sensory transmitters in the activation of micturition reflex. Neuroscience. 1993;54:827-837.
Lecci A, Giuliani S, et al. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J Pharmacol Exp Ther. 1992;262:181-189.
Lecci A, Giuliani S, et al. Involvement of spinal tachykinin NK1 and NK2 receptors in detrusor hyperreflexia during chemical cystitis in anaesthetized rats. Eur J Pharmacol. 1994;259:129-135.
Lecci A, Giuliani S, et al. MEN 11,420, a peptide tachykinin NK2 receptor antagonist, reduces motor responses induced by the intravesical administration of capsaicin in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1997;356(2):182-188.
Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Pept. 2001;101:1-18.
Lee HY, Bardini M, et al. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163(6):2002-2007.
Lee SJ, Nakamura Y, et al. Effect of (+/-)-epibatidine, a nicotinic agonist, on the central pathways controlling voiding function in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R84-R90.
Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11-18.
Lepor H, Sunaryadi I, et al. Quantitative morphometry of the adult human bladder. J Urol. 1992;148:414-417.
Levin R, Brendler K, Van Arsdalen KN, Wein AJ. Functional response of the rabbit urinary bladder to anoxia and ischaemia. Neurourol Urodyn. 1983;42:54.
Levin R, Wein A. Neurophysiology and neuropharmacology. In: Fitzpatrick JM, Krane RJ, editors. Bladder. New York: Churchill Livingstone; 1995:47-70.
Levin RM, Kitada S, et al. Experimental hyperreflexia:effect of intravesical administration of various agents. Pharmacology. 1991;42(1):54-60.
Levin RM, O’Connor LJ, et al. Focal hypoxia of the obstructed rabbit bladder wall correlates with intermediate decompensation. Neurourol Urodyn. 2003;22:156-163.
Levin RM, Ruggieri MR, et al. Beta adrenergic stimulation of cyclic AMP production in the rabbit urinary bladder. Neurourol Urodyn. 1986;5:227.
Levin RM, Shofer FS, et al. Estrogen-induced alterations in the autonomic responses of the rabbit urinary bladder. J Pharmacol Exp Ther. 1980;215(3):614-618.
Levin RM, Wein AJ. Neurophysiology and neuropharmacology. In: Fitzpatrick J, Krane R, editors. Bladder. New York: Churchill Livingstone; 1995:47-70.
Levy BJ, Wight TN. Structural changes in the aging submucosa:new morphologic criteria for the evaluation of the unstable human bladder. J Urol. 1990;144(4):1044-1055.
Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867-F874.
Lewis SA, Berg JR, et al. Modulation of epithelial permeability by extracellular macromolecules. Physiol Rev. 1995;75(3):561-589.
Lewis SA, de Moura JL. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature. 1982;297(5868):685-688.
Lewis SA, Diamond JM. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol. 1976;28(1):1-40.
Lewis SA, Eaton DC, et al. The mechanism of Na+ transport by rabbit urinary bladder. J Membr Biol. 1976;28(1):41-70.
Li DY, Brooke B, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276-280.
Li L, Jiang C, et al. Changes of gap junctional cell-cell communication in overactive detrusor in rats. Am J Physiol Cell Physiol. 2007;293:C1627-C1635.
Lieu PK, Sa′adu A, et al. The influence of age on isometric and isotonic rat detrusor contractions. J Gerontol A Biol Sci Med Sci. 1997;52:M94-M96.
Lin AT, Yang CH, et al. Correlation of contractile function and passive properties of rabbit urinary bladder subjected to outlet obstruction—an in vitro whole bladder study. J Urol. 1992;148(3):944-948.
Lin AT, Yang CH, Chang LS. Impact of aging on rat urinary bladder fatigue. J Urol. 1997;157:1990-1994.
Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75(4):1503-1514.
Liu Y, Allen GV, et al. Parabrachial nucleus influences the control of normal urinary bladder function and the response to bladder irritation in rats. Neuroscience. 2007;144(2):731-742.
Liu Z, Sakakibara R, et al. Micturition-related neuronal firing in the periaqueductal gray area in cats. Neuroscience. 2004;126(4):1075-1082.
Longhurst PA, Briscoe JA, et al. The role of cyclic nucleotides in guinea-pig bladder contractility. Br J Pharmacol. 1997;121:1665-1672.
Longhurst PA, Eika B, et al. Comparison of urinary bladder function in 6 and 24 month male and female rats. J Urol. 1992;148:1615-1620.
Lowe EM, Anand P, et al. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79(4):572-577.
Lumb BM, Morrison JF. An excitatory influence of dorsolateral pontine structures on urinary bladder motility in the rat. Brain Res. 1987;435(1-2):363-366.
Macarak EJ, Ewalt D, et al. The collagens and their urologic implications. Adv Exp Med Biol. 1995;385:173-177.
Macarak EJ, Howard PS. The role of collagen in bladder filling. Adv Exp Med Biol. 1999;462:215-223. discussion 225–233
Madersbacher S, Pycha A, et al. The aging lower urinary tract:a comparative urodynamic study of men and women. Urology. 1998;51(2):206-212.
Madersbacher S, Pycha A, et al. Interrelationships of bladder compliance with age, detrusor instability, and obstruction in elderly men with lower urinary tract symptoms. Neurourol Urodyn. 1999;18(1):3-15.
Maggi CA. The dual, sensory and efferent function of the capsaicin-sensitive primary sensory nerves in the bladder and urethra. In: Maggi CA, editor. Nervous control of the urogenital system, vol. 1. London: Harwood Academic Publishers; 1993:383-422.
Maggi CA, Abelli L, et al. Motor and inflammatory effect of hyperosmolar solutions on the rat urinary bladder in relation to capsaicin-sensitive sensory nerves. Gen Pharmacol. 1990;21(1):97-103.
Maggi CA, Borsini F, et al. Effect of acute or chronic administration of imipramine on spinal and supraspinal micturition reflexes in rats. J Pharmacol Exp Ther. 1989;248(1):278-285.
Maggi CA, Giuliani S, et al. Further studies on the mechanisms of the tachykinin-induced activation of micturition reflex in rats: evidence for the involvement of the capsaicin-sensitive bladder mechanoreceptors. Eur J Pharmacol. 1987;136:189-205.
Mallory BS, Roppolo JR, et al. Pharmacological modulation of the pontine micturition center. Brain Research. 1991;546:310-320.
Malloy BJ, Price DT, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol. 1998;160(3 Pt. 1):937-943.
Malykhina AP, Qin C, et al. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601-2605.
Malykhina AP, Qin C, et al. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936-948.
Mansfield KJ, Liu L, et al. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol. 2005;144:1089-1099.
Marcelli M, Shao TC, et al. Induction of apoptosis in BPH stromal cells by adenoviral-mediated overexpression of caspase-7. J Urol. 2000;164(2):518-525.
Markwardt F, Isenberg G. Gating of maxi K+ channels studied by Ca2+ concentration jumps in excised inside-out multi-channel patches (myocytes from guinea pig urinary bladder). J Gen Physiol. 1992;99(6):841-862.
Martin SW, Radley SC, et al. Relaxant effects of potassium-channel openers on normal and hyper-reflexic detrusor muscle. Br J Urol. 1997;80:405-413.
Masaki T. Historical review. Endothelin Trends Pharmacol Sci. 2004;25:219-224.
Masuda H, Chancellor MB, et al. Effects of cholinesterase inhibition in supraspinal and spinal neural pathways on the micturition reflex in rats. BJU Int. 2009;104:1163-1169.
Masuda H, Kim JH, et al. Inhibitory roles of peripheral nitrergic mechanisms in capsaicin-induced detrusor overactivity in the rat. BJU Int. 2007;100(4):912-918.
Matsui M, Motomura D, et al. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97(17):9579-9584.
Matsui M, Motomura D, et al. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22(24):10627-10632.
Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997;44:539-547.
Matsumoto G, Hisamitsu T, de Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci Lett. 1995;183:58-61.
Matsumoto G, Hisamitsu T, de Groat WC. Non-NMDA glutamatergic excitatory transmission in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Brain Res. 1995;693:246-250.
Matsuura S, Downie JW, et al. Micturition evoked by glutamate microinjection in the ventrolateral periaqueductal gray is mediated through Barrington’s nucleus in the rat. Neuroscience. 2000;101(4):1053-1061.
Matsuura S, Kakizaki H, et al. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035-2039.
Mattiasson A, Andersson KE, Sjogren C. Adrenoceptors and cholinoceptors controlling noradrenaline release from adrenergic nerves in the urethra of rabbit and man. J Urol. 1984;131:1190-1195.
Mbaki Y, Ramage AG. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol. 2008;155(3):343-356.
McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168(2):832-836.
McGuire E. Experimental observations on the integration of bladder and urethral function. Trans Am Assoc Genitourin Surg. 1977;68:38.
McGuire E, Morrissey S, et al. Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol. 1983;129(1):197-199.
McGuire EJ. Experimental observations on the integration of bladder and urethral function. Invest Urol. 1978;15(4):303-307.
McMahon SB, Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109-127.
McMahon SB, Morrison JF. Spinal neurones with long projections activated from the abdominal viscera of the cat. J Physiol. 1982;322:1-20.
McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res. 1982;234:237-249.
McMurray G, Dass N, et al. Purinergic mechanisms in primate urinary bladder. Br J Urol. 1997;80:182.
Meredith AL, Thorneloe KS, et al. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746-36752.
Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl. 2):S88-S119.
Mills IW, Greenland JE, et al. Studies of the pathophysiology of idiopathic detrusor instability:the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol. 2000;163(2):646-651.
Minsky BD, Chlapowski FJ. Morphometric analysis of the translocation of lumenal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J Cell Biol. 1978;77(3):685-697.
Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2001;895:223-232.
Miyazato M, Kaiho Y, et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2008;295(1):F264-F271.
Miyazato M, Sasatomi K, et al. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R336-R342.
Miyazato M, Sugaya K, et al. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp Neurol. 2003;183:232-240.
Miyazato M, Sugaya K, et al. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J Urol. 2005;173(1):314-317.
Miyazato M, Sugaya K, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009;16(5):660-668.
Mochizuki T, Sokabe T, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284(32):21257-21264.
Mohammed H, Hannibal J, et al. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter:effects of age. Urol Res. 2002;30(4):248-255.
Mohammed HA, Santer RM. Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur J Morphol. 2002;40(5):293-301.
Montgomery BS, Fry CH. The action potential and net membrane currents in isolated human detrusor smooth muscle cells. J Urol. 1992;147:176-184.
Morgan C, Nadelhaft I, et al. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415-440.
Morgan CW, de Groat WC, et al. Intracellular injection of neurobiotin or horseradish peroxidase reveals separate types of preganglionic neurons in the sacral parasympathetic nucleus of the cat. J Comp Neurol. 1993;331:161-182.
Mori K, Noguchi M, et al. Decreased cellular membrane expression of gap junctional protein, connexin 43, in rat detrusor muscle with chronic partial bladder outlet obstruction. Urology. 2005;65:1254-1258.
Morita T, Ando M, et al. Species differences in cAMP production and contractile response induced by beta-adrenoceptor subtypes in urinary bladder smooth muscle. Neurourol Urodyn. 1993;12:185-190.
Morita T, Tsuchida S. Sterographic urethral pressure profile. Urol Int. 1989;39:199.
Morrison J, Birder L, Craggs M. Neural control. In: Abrams P, Cardozo L, et al, editors. Incontinence. Plymouth (UK): Health Publications; 2005:363-422.
Morrison J, Namasivayam S, Eardley I. 1998. ATP may be a natural modulator of the sensitivity of bladder mechanoreceptors during slow distensions. Presented at 1st International Consultation on Incontinence. Monaco; June 28-July 1, 1998. p. 84.
Morrison JF. The physiological mechanisms involved in bladder emptying. Scand J Urol Nephrol Suppl. 1997;184:15-18.
Morrison JF, Sato A, et al. The influence of afferent inputs from skin and viscera on the activity of the bladder and the skeletal muscle surrounding the urethra in the rat. Neurosci Res. 1995;23:195-205.
Mostwin JL. The action potential of guinea pig bladder smooth muscle. J Urol. 1986;135(6):1299-1303.
Mukerji G, Yiangou Y, et al. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol. 2006;176(1):367-373.
Mulvey MA, Lopez-Boado YS, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494-1497.
Murakami S, Yoshida M, et al. Change in acetylcholine release from rat bladder with partial outlet obstruction. BJU Int. 2008;101(5):633-639.
Murakumo M, Ushiki T, et al. Three-dimensional arrangement of collagen and elastin fibers in the human urinary bladder: a scanning electron microscopic study. J Urol. 1995;154:251-256.
Murray E, Malley SE, et al. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172(6 Pt. 1):2434-2439.
Musselman DM, Ford AP, et al. A randomized crossover study to evaluate Ro 115-1240, a selective alpha1A/1L-adrenoceptor partial agonist in women with stress urinary incontinence. BJU Int. 2004;93(1):78-83.
Nadelhaft I, Degroat WC, et al. Location and morphology of parasympathetic preganglionic neurons in the sacral spinal cord of the cat revealed by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1980;193(1):265-281.
Nadelhaft I, Vera PL. Reduced urinary bladder afferent conduction velocities in streptozotocin-diabetic rats. Neurosci Lett. 1992;135:276-278.
Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375(3):502-517.
Nagasaka Y, Yokoyama O, et al. Effects of opioid subtypes on detrusor overactivity in rats with cerebral infarction. Int J Urol. 2007;14:226-231.
Nagata K, Duggan A, et al. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052-4061.
Nagatomi J, DeMiguel F, et al. Early molecular-level changes in rat bladder wall tissue following spinal cord injury. Biochem Biophys Res Commun. 2005;334:1159-1164.
Naglo AS, Nergardh A, Boreus LO. Influence of atropine and isoprenaline on detrusor hyperactivity in children with neurogenic bladder. Scand J Urol Nephrol. 1981;15:97-102.
Nakamura Y, Ishiura Y, et al. Role of protein kinase C in central muscarinic inhibitory mechanisms regulating voiding in rats. Neuroscience. 2003;116:477-484.
Namasivayam S, Eardley I, et al. Purinergic sensory neurotransmission in the urinary bladder:an in vitro study in the rat. BJU Int. 1999;84(7):854-860.
Naughton MJ, Wyman JF. Quality of life in geriatric patients with lower urinary tract dysfunction. Am J Med Sci. 1997;314:219-227.
Negrete HO, Lavelle JP, et al. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271(4 Pt. 2):F886-F894.
Negrete HO, Rivers RL, et al. Individual leaflets of a membrane bilayer can independently regulate permeability. J Biol Chem. 1996;271(20):11627-11630.
Nergardh A, Boreus LO. Autonomic receptor function in the lower urinary tract of man and cat. Scand J Urol Nephrol. 1972;6:32-36.
Neuhaus J, Pfeiffer F, et al. Alterations in connexin expression in the bladder of patients with urge symptoms. BJU Int. 2005;96:670-676.
Neuhaus J, Wolburg H, et al. Detrusor smooth muscle cells of the guinea-pig are functionally coupled via gap junctions in situ and in cell culture. Cell Tissue Res. 2002;309(2):301-311.
Ng YK, de Groat WC, et al. Smooth muscle and neural mechanisms contributing to the downregulation of neonatal rat spontaneous bladder contractions during postnatal development. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R2100-R2112.
Nickel JC, Downey J, et al. Relative efficacy of various exogenous glycosaminoglycans in providing a bladder surface permeability barrier. J Urol. 1998;160:612-614.
Niku SD, Stein PC, et al. A new method for cytodestruction of bladder epithelium using protamine sulfate and urea. J Urol. 1994;152(3):1025-1028.
Nilvebrant L, Andersson KE, et al. Tolterodine—a new bladder-selective antimuscarinic agent. Eur J Pharmacol. 1997;327(2-3):195-207.
Nishiguchi J, Hayashi Y, et al. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology. 2005;66(6):1332-1337.
Nishimoto T, Latifpour J, et al. Age-dependent alterations in beta-adrenergic responsiveness of rat detrusor smooth muscle. J Urol. 1995;153(5):1701-1705.
Nishimura T, Tokimasa T. Purinergic cation channels in neurons of rabbit vesical parasympathetic ganglia. Neurosci Lett. 1996;212(3):215-217.
Nishino Y, Masue T, et al. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006;97(4):747-751. discussion 751
Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003;170(2 Pt. 1):649-653.
Nordling J. Influence of the sympathetic nervous system on lower urinary tract in man. Neurourol Urodynam. 1983;2:3.
Noronha R, Akbarali H, et al. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293:F1461-F1467.
Noto H, Roppolo JR, et al. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int. 1991;47(Suppl. 1):19-22.
Nour S, Svarer C, et al. Cerebral activation during micturition in normal men. Brain. 2000;123(Pt. 4):781-789.
Numata A, Iwata T, et al. Micturition-suppressing region in the periaqueductal gray of the mesencephalon of the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1996-R2000.
Nuotio M, Jylha M, et al. Urgency, urge incontinence and voiding symptoms in men and women aged 70 years and over. BJU Int. 2002;89:350-355.
O’Donnell PD. Central actions of bethanechol on the urinary bladder in dogs. J Urol. 1990;143(3):634-637.
Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980;158(2):229-246.
Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223-232.
Ogawa T, Kamo I, et al. Differential roles of peripheral and spinal endothelin receptors in the micturition reflex in rats. J Urol. 2004;172:1533-1537.
Ogawa T, Sasatomi K, et al. Therapeutic effects of endothelin-A receptor antagonist on bladder overactivity in rats with chronic spinal cord injury. Urology. 2008;71(2):341-345.
Ogawa T, Seki S, et al. Dopaminergic mechanisms controlling urethral function in rats. Neurourol Urodyn. 2006;25:480-489.
Ogawa T, Yoshida T, et al. [Bladder deformity in traumatic spinal cord injury patients.]. Hinyokika Kiyo. 1988;34(7):1173-1178.
Ohnishi N, Kishima Y, et al. [A new method of measurement of the urinary bladder blood flow in patients with low compliant bladder.]. Hinyokika Kiyo. 1994;40(8):663-667.
Ohtake A, Ukai M, et al. In vitro and in vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats. Eur J Pharmacol. 2004;492(2-3):243-250.
Okragly AJ, Niles AL, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161(2):438-442.
Olah ME, Ren H, et al. Adenosine receptors: protein and gene structure. Arch Int Pharmacodyn Ther. 1995;329(1):135-150.
O’Reilly BA, Kosaka AH, et al. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001;87(7):617-622.
O’Reilly BA, Kosaka AH, et al. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001;165(5):1730-1734.
Otake K, Nakamura Y. Single neurons in Barrington’s nucleus projecting to both the paraventricular thalamic nucleus and the spinal cord by way of axon collaterals: a double labeling study in the rat. Neurosci Lett. 1996;209(2):97-100.
Ozawa H, Chancellor MB, et al. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162(6):2211-2216.
Padykula H, Gauthier GF. Morphological and cytochemical characteristics of fiber types in mammalian skeletal muscle. Explanatory concepts in neuromuscular dystrophy and related disorders, Amsterdam: Excerpta Medica International Congress Series, No. 147, 1967. p. 117–31
Padykula HA, Gauthier GF. The ultrastructure of the neuromuscular junctions of mammalian red, white, and intermediate skeletal muscle fibers. J Cell Biol. 1970;46(1):27-41.
Palea S, Artibani W, et al. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;150(6):2007-2012.
Palea S, Lluel P, et al. Involvement of 5-hydroxytryptamine (HT)7 receptors in the 5-HT excitatory effects on the rat urinary bladder. BJU Int. 2004;94(7):1125-1131.
Pandita RK, Andersson KE. Effects of intravesical administration of the K+ channel opener, ZD6169, in conscious rats with and without bladder outflow obstruction. J Urol. 1999;162:943-948.
Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168(3):1230-1234.
Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545-550.
Pandita RK, Persson K, et al. Capsaicin-induced bladder overactivity and nociceptive behaviour in conscious rats: involvement of spinal nitric oxide. J Auton Nerv Syst. 1997;67(3):184-191.
Parekh AB, Brading AF, Tomita T. Studies of longitudinal tissue impedance in various smooth muscles. Prog Clin Biol Res. 1990;327:375-378.
Park JM, Bloom DA, et al. The guarding reflex revisited. Br J Urol. 1997;80(6):940-945.
Parsons CL. Potassium sensitivity test. Tech Urol. 1996;2(3):171-173.
Parsons CL, Boychuk D, et al. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol. 1990;143(1):139-142.
Payne CK, Mosbaugh PG, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol. 2005;173:1590-1594.
Persson K, Pandita R, Andersson K. Bladder hyperactivity in spontaneously hypertensive rat is decreased by alpha adrenoceptor blockade. J Urol. 1997;157:255.
Pessina F, McMurray G, et al. The effect of anoxia and glucose-free solutions on the contractile response of guinea-pig detrusor strips to intrinsic nerve stimulation and the application of excitatory agonists. J Urol. 1997;157:2375-2380.
Peter S. The junctional connections between the cells of the urinary bladder in the rat. Cell Tissue Res. 1978;187(3):439-448.
Peters KM, Feber KM, et al. Sacral versus pudendal nerve stimulation for voiding dysfunction:a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24(7):643-647.
Petit H, Wiart L, et al. Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal Cord. 1998;36:91-94.
Pezzone MA, Fraser MO, et al. The discovery of the pacemaker cells of the urinary tract. J Urol. 1999;161:41.
Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953-1964.
Pfisterer MH, Griffiths DJ, et al. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54(3):405-412.
Phelan WF, Yoshimura N, et al. Nerve growth factor (NGF) gene therapy improves rat diabetic cystopathy but does not increase pain sensation. J Urol. 2000;163:245.
Pontari MA, Braverman AS, et al. The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol. 2004;286(5):R874-R880.
Pool JL. Role of the sympathetic nervous system in hypertension and benign prostatic hyperplasia. Br J Clin Pract Suppl. 1994;74:13-17.
Porter KR, Kenyon K, et al. Specializations of the unit membrane. Protoplasma. 1967;63(1):262-274.
Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454:200-211.
Qiao LY, Vizzard MA. Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis. J Comp Neurol. 2004;469:262-274.
Qin C, Malykhina AP, et al. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967-1978.
Ramage AG, Wyllie MG. A comparison of the effects of doxazosin and terazosin on the spontaneous sympathetic drive to the bladder and related organs in anaesthetized cats. Eur J Pharmacol. 1995;294(2-3):645-650.
Ranson RN, Dodds AL, et al. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003;972(1-2):149-158.
Rechberger T, Donica H, et al. Female urinary stress incontinence in terms of connective tissue biochemistry. Eur J Obstet Gynecol Reprod Biol. 1993;49(3):187-191.
Resnick NM. Urinary incontinence. Lancet. 1995;346:94-99.
Rice A. Topical spinal administration of a nitric oxide synthase inhibitor prevents the hyperreflexia associated with a rat model of persistent visceral pain. Neurosci Lett. 1995;187:111.
Rivera L, McMurray G, Brading AF. The role of calcium stores in the action of muscarinic agonists on mammalian urinary bladder smooth muscle. J Physiol. 1998;507P:21P.
Roppolo JR, Tai C, et al. Bladder Adelta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol. 2005;173(3):1011-1015.
Rosenbloom J, Koo H, et al. Elastic fibers and their role in bladder extracellular matrix. Adv Exp Med Biol. 1995;385:161-172. discussion 179–184
Rossier AB, Ott R. Bladder and urethral recordings in acute and chronic spinal cord injury patients. Urol Int. 1976;31(1-2):49-59.
Rubanyi GM, Polokoff MA. Endothelins:molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46(3):325-415.
Rud T, Andersson KE, et al. Factors maintaining the intraurethral pressure in women. Invest Urol. 1980;17(4):343-347.
Saban R, Undem BJ, et al. Differential release of prostaglandins and leukotrienes by sensitized guinea pig urinary bladder layers upon antigen challenge. J Urol. 1994;152(2 Pt. 1):544-549.
Sacks MS, Chuong CJ, et al. In vivo 3-D reconstruction and geometric characterization of the right ventricular free wall. Ann Biomed Eng. 1993;21:263-275.
Sacks MS, Vorp DA, et al. In vivo three-dimensional surface geometry of abdominal aortic aneurysms. Ann Biomed Eng. 1999;27:469-479.
Saenz de Tejada I, Mueller JD, et al. Endothelin in the urinary bladder. I. Synthesis of endothelin-1 by epithelia, muscle and fibroblasts suggests autocrine and paracrine cellular regulation. J Urol. 1992;148(4):1290-1298.
Saito M, Gotoh M, et al. Influence of aging on the rat urinary bladder function. Urol Int. 1991;47(Suppl. 1):39-42.
Saito M, Kondo A, et al. Age-related changes in the response of the rat urinary bladder to neurotransmitters. Neurourol Urodyn. 1993;12:191-200.
Sakakibara R, Nakazawa K, et al. Micturition-related electrophysiological properties in the substantia nigra pars compacta and the ventral tegmental area in cats. Auton Neurosci. 2002;102(1-2):30-38.
Sampselle CM, DeLancey JO. Anatomy of female continence. J Wound Ostomy Continence Nurs. 1998;25(2):63-70. 72–74
Santer RM, Dering MA, et al. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002;96:73-81.
Sarikas SN, Chlapowski FJ. Effect of ATP inhibitors on the translocation of luminal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycle of the rat urinary bladder. Cell Tissue Res. 1986;246(1):109-117.
Sasaki K, Chancellor MB, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723-2730.
Sasaki M. Morphological analysis of external urethral and external anal sphincter motoneurones of cat. J Comp Neurol. 1994;349(2):269-287.
Schiavo G, Rossetto O, et al. Botulinum neurotoxins are zinc proteins. J Biol Chem. 1992;267(33):23479-23483.
Schiavo G, Santucc A, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99-103.
Schneider T, Fetscher C, et al. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther. 2004;309(3):1148-1153.
Schneider T, Hein P, et al. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J Pharmacol Exp Ther. 2004;308(1):47-53.
Schroder A, Tajimi M, et al. Protective effect of an oral endothelin converting enzyme inhibitor on rat detrusor function after outlet obstruction. J Urol. 2004;172(3):1171-1174.
Schurch B, Hauri D, et al. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol. 1996;155:1023-1029.
Schurch B, Stohrer M, et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692-697.
Schussler B. Comparison of mode of action of prostaglandin E2 and sulprostone, a PGE2 derivative on the lower urinary tract in healthy women. Urol Res. 1990;18:349.
Schwinn DA, Roehrborn CG. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol. 2008;15(3):193-199.
Scott FB, Quesada EM, Cardus D. Studies on the dynamics of micturition: observations on healthy men. J Urol. 1964;92:455-463.
Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in DRG neurons of the adult rat. J Neurophysiol. 2003;90:21-31.
Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205(1):92-100.
Sculptoreanu A, de Groat WC, et al. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett. 2005;381(1-2):42-46.
Sculptoreanu A, Kullmann FA, et al. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci. 2008;27(12):3171-3181.
Seki N, Karim OM, et al. Changes in action potential kinetics following experimental bladder outflow obstruction in the guinea pig. Urol Res. 1992;20(6):387-392.
Seki S, Igawa Y, et al. Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats. Neurourol Urodyn. 2001;20(1):105-113.
Seki S, Sasaki K, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269-2274.
Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72(5):2420-2430.
Seseke S, Baudewig J, et al. Voluntary pelvic floor muscle control—an fMRI study. Neuroimage. 2006;31:1399-1407.
Shapiro E. Effect of estrogens on the weight and muscarinic cholinergic receptor density of the rabbit bladder and urethra. J Urol. 1986;135:1084.
Sharma A, Goldberg MJ, et al. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40(2):161-167.
Shefchyk S, Buss R. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137-140.
Shibata T, Watanabe M, et al. Different expressions of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and N-methyl-D-aspartate receptor subunit mRNAs between visceromotor and somatomotor neurons of the rat lumbosacral spinal cord. J Comp Neurol. 1999;404:172-182.
Sibley GN. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984;354:431-443.
Sillen U, Rubenson A, et al. Central cholinergic mechanisms in L-DOPA induced hyperactive urinary bladder of the rat. Urol Res. 1982;10(5):239-243.
Silva C, Ribeiro MJ, et al. The effect of intravesical resiniferatoxin in patients with idiopathic detrusor instability suggests that involuntary detrusor contractions are triggered by C-fiber input. J Urol. 2002;168(2):575-579.
Sjuve R, Haase H, et al. Contraction kinetics and myosin isoform composition in smooth muscle from hypertrophied rat urinary bladder. J Cell Biochem. 1996;63:86-93.
Smet PJ, Jonavicius J, et al. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337-348.
Smith CP, Chancellor MB. Emerging role of botulinum toxin in the management of voiding dysfunction. J Urol. 2004;171:2128-2137.
Smith CP, Franks ME, et al. Effect of botulinum toxin A on the autonomic nervous system of the rat lower urinary tract. J Urol. 2003;169:1896-1900.
Smith CP, Gangitano DA, et al. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int. 2008;52:1068-1075.
Smith CP, Vemulakonda VM, et al. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47:291-297.
Smith PR, Mackler SA, et al. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am J Physiol. 1998;274:F91-F96.
Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J Auton Nerv Syst. 1992;37:S89-S97.
Somogyi GT, Tanowitz M, de Groat WC. M-1 muscarinic receptor mediated facilitation of acetylcholine release in the rat urinary bladder but not in the heart. J Physiol. 1994;480:81-89.
Somogyi GT, Tanowitz M, et al. M1 muscarinic receptor facilitation of ACh and noradrenaline release in the rat urinary bladder is mediated by protein kinase C. J Physiol. 1996;496:245-254.
Somogyi GT, Zernova GV, et al. Role of L- and N-type Ca2+ channels in muscarinic receptor-mediated facilitation of ACh and noradrenaline release in the rat urinary bladder. J Physiol. 1997;499(Pt. 3):645-654.
Sosnowski M, Stevens CW, et al. Assessment of the role of A1/A2 adenosine receptors mediating the purine antinociception, motor and autonomic function in the rat spinal cord. J Pharmacol Exp Ther. 1989;250(3):915-922.
Souslova V, Cesare P, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407(6807):1015-1017.
Speakman MJ, Brading AF, et al. Bladder outflow obstruction—a cause of denervation supersensitivity. J Urol. 1987;138(6):1461-1466.
Staehelin LA, Chlapowski FJ, et al. Lumenal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images. J Cell Biol. 1972;53(1):73-91.
Steers WD. Darifenacin: Pharmacology and clinical usage. Urol Clin North Am. 2006;33(4):475-482. viii
Steers WD, Broder SR, et al. Mechanical stretch increases secretion of parathyroid hormone-related protein by cultured bladder smooth muscle cells. J Urol. 1998;160(3 Pt. 1):908-912.
Steers WD, Ciambotti J, et al. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci. 1990;10:1943-1951.
Steers WD, Ciambotti J, et al. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991;310(3):401-410.
Steers WD, Clemow DB, et al. Observations from the spontaneously hypertensive rat. Insight into NGF regulation and noradrenergic hyper-innervation in the lower urinary tract. Adv Exp Med Biol. 1999;462:283-292. discussion 311–320
Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379-385.
Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988;140:864-871.
Steers WD, de Groat WC. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am J Physiol. 1989;257:R1441-R1449.
Steers WD, Tuttle JB. Neurogenic inflammation and nerve growth factor: possible roles in interstitial cystitis. In: Sant GR, editor. Interstitial cystitis. Philadelphia: Lippincott-Raven; 1997:67-75.
Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3(2):101-110.
Stein RJ, Santos S, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004;172:1175-1178.
Streng T, Axelsson HE, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391-399.
Sugaya K, Kadekawa K, et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int J Urol. 2003;10(11):569-574. discussion 575
Sugaya K, Matsuyama K, et al. Electrical and chemical stimulations of the pontine micturition center. Neurosci Lett. 1987;80:197-201.
Sugaya K, Roppolo JR, et al. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett. 1997;223(3):197-200.
Sui GP, Wu C, et al. Modulation of bladder myofibroblast activity:implications for bladder function. Am J Physiol Renal Physiol. 2008;295(3):F688-F697.
Sun TT, Liang FX, et al. Uroplakins as markers of urothelial differentiation. Adv Exp Med Biol. 1999;462:7-18. discussion 103–14
Susset JG, Servot-Viguier D, et al. Collagen in 155 human bladders. Invest Urol. 1978;16:204-206.
Szallasi A, Blumberg PM. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990;47(16):1399-1408.
Szallasi A, Fowler CJ. After a decade of intravesical vanilloid therapy: still more questions than answers. Lancet Neurol. 2002;1:167-172.
Szell EA, Somogyi GT, et al. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2003;285:R809-R816.
Szolcsanyi J. Effect of capsaicin, resiniferatoxin and piperine on ethanol-induced gastric ulcer of the rat. Acta Physiol Hung. 1990;75(Suppl.):267-268.
Tai C, Wang J, et al. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26(4):570-577.
Tainio H. Neuropeptidergic innervation of the human male distal urethra and intrinsic external urethral sphincter. Acta Histochem. 1993;94:197-201.
Takeda M, Mizusawa T, et al. Adrenergic B1-,B2-, and B3- receptor subtypes in the detrusor of human urinary bladder—evaluation by mRNA expression and isometric contraction. Neurourol Urodyn. 1997;16:365.
Taki N, Taniguchi T, et al. Evidence for predominant mediation of alpha1-adrenoceptor in the tonus of entire urethra of women. J Urol. 1999;162(5):1829-1832.
Tammela T, Kontturi M, et al. Intravesical prostaglandin F2 for promoting bladder emptying after surgery for female stress incontinence. Br J Urol. 1987;60:43-46.
Tanagho EA. The ureterovesical junction: anatomy and physiology. In: Chishold G, Williams D, editors. Scientific foundations of urology. Chicago: Year Book Medical; 1982:295-404.
Tanagho EA, Schmidt RA, et al. Neural stimulation for control of voiding dysfunction:a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol. 1989;142(2 Pt. 1):340-345.
Tanaka H, Kakizaki H, et al. Effects of a selective metabotropic glutamate receptor agonist on the micturition reflex pathway in urethane-anesthetized rats. Neurourol Urodyn. 2003;22(6):611-616.
Taniguchi N, Miyata M, et al. A study of micturition inducing sites in the periaqueductal gray of the mesencephalon. J Urol. 2002;168(4 Pt. 1):1626-1631.
Tempest HV, Dixon AK, et al. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344-1348.
Testa R, Guarneri L, et al. Characterization of alpha 1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur J Pharmacol. 1993;249(3):307-315.
Testa R, Guarneri L, et al. Effect of several 5-hydroxytryptamine(1A) receptor ligands on the micturition reflex in rats: comparison with WAY 100635. J Pharmacol Exp Ther. 1999;290:1258-1269.
Theobald RJJr, de Groat WD. The effects of purine nucleotides on transmission in vesical parasympathetic ganglia of the cat. J Auton Pharmacol. 1989;9(3):167-181.
Thind P. The significance of smooth and striated muscles in the sphincter function of the urethra in healthy women. Neurourol Urodyn. 1995;14(6):585-618.
Thiruchelvam N, Wu C, et al. Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1296-R1305.
Thon W, Baskin L, et al. Surgical principles of sacral foramen electrode implantation. World J Urol. 1991;9:133.
Thor KB, Donatucci C. Central nervous system control of the lower urinary tract:new pharmacological approaches to stress urinary incontinence in women. J Urol. 2004;172(1):27-33.
Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274(2):1014-1024.
Thor KB, Katofiasc MA, et al. The role of 5-HT(1A) receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290-297.
Thor KB, Morgan C, et al. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288(2):263-279.
Thorneloe KS, Sulpizio AC, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J Pharmacol Exp Ther. 2008;326:432-442.
Toosi KK, Nagatomi J, et al. The effects of long-term spinal cord injury on mechanical properties of the rat urinary bladder. Ann Biomed Eng. 2008;36:1470-1480.
Torrens M. Urethral sphincteric responses to stimulation of the sacral nerves in the human female. Urol Int. 1978;33:22.
Torrens M, Morrison JFB. The physiology of the lower urinary tract. London, New York: Springer-Verlag; 1987.
Trivedi S, Potter-Lee L, et al. Calcium dependent K-channels in guinea pig and human urinary bladder. Biochem Biophys Res Commun. 1995;213:404-409.
Truss MC, Becker AJ, et al. Selective pharmacological manipulation of the smooth muscle tissue of the genitourinary tract: a glimpse into the future. BJU Int. 1999;83(Suppl. 2):36-41.
Truss MC, Stief C, et al. Initial clinical experience with the selective phosphodiesterase (PDE)-a isoenzyme inhibitor vinpocetine in the treatment of urge-incontinence and low complicance bladder. J Urol. 1997;157:727A.
Truss MC, Stief CG, et al. Phosphodiesterase 1 inhibition in the treatment of lower urinary tract dysfunction:from bench to bedside. World J Urol. 2001;19(5):344-350.
Truss MC, Uckert S, et al. Effects of various phosphodiesterase-inhibitors, forskolin, and sodium nitroprusside on porcine detrusor smooth muscle tonic responses to muscarinergic stimulation and cyclic nucleotide levels in vitro. Neurourol Urodyn. 1996;15:59-70.
Tsukimi Y, Mizuyachi K, et al. Cold response of the bladder in guinea pig:involvement of transient receptor potential channel, TRPM8. Urology. 2005;65(2):406-410.
Turner WH, Brading AF. Smooth muscle of the bladder in the normal and the diseased state:pathophysiology, diagnosis and treatment. Pharmacol Ther. 1997;75(2):77-110.
Tyagi P, Thomas CA, et al. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 2009;35:76-83.
Tzan CJ, Berg J, Lewis SA. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J Membr Biol. 1993;133:227-242.
Tzan CJ, Berg JR, Lewis SA. Mammalian urinary bladder permeability is altered by cationic proteins: modulation by divalent cations. Am J Physiol. 1994;267:C1013-C1026.
Ukai M, Yuyama H, et al. Participation of endogenous endothelin and ETA receptor in premicturition contractions in rats with bladder outlet obstruction. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(3):197-203.
Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478-F1487.
Ustinova EE, Gutkin DW, et al. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol. 2007;292(1):F123-F130.
Uvelius B, Arner A, et al. Contractile and cytoskeletal proteins in detrusor muscle from obstructed rat and human bladder. Neurourol Urodyn. 1989;3:396.
Uvelius B, Gabella G. Relation between cell length and force production in urinary bladder smooth muscle. Acta Physiol Scand. 1980;110(4):357-365.
Uvelius B, Mattiasson A. Collagen content in the rat urinary bladder subjected to infravesical outflow obstruction. J Urol. 1984;132:587-590.
Uvelius B, Mattiasson A. Detrusor collagen content in the denervated rat urinary bladder. J Urol. 1986;136(5):1110-1112.
Vadusek D, Light J, et al. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986;5:381.
Vadyanaathan S, Rao MS, et al. Enhancement of detrusor reflex activity by naloxone in patients with chronic neurogenic bladder dysfunction. J Urol. 1981;126:500.
Valentino RJ, Chen S, et al. Evidence for divergent projections to the brain noradrenergic system and the spinal parasympathetic system from Barrington’s nucleus. Brain Res. 1996;732:1-15.
Valentino RJ, Pavcovich LA, et al. Evidence for corticotropin-releasing hormone projections from Barrington’s nucleus to the periaqueductal gray and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363(3):402-422.
Valera S, Talabot F, et al. Characterization and chromosomal localization of a human P2X receptor from the urinary bladder. Receptors Channels. 1995;3(4):283-289.
van Waalwijk van Doorn ES, Remmers A, Janknegt RA. Extramural ambulatory urodynamic monitoring during natural filling and normal daily activities: evaluation of 100 patients. J Urol. 1991;146:124-131.
Venegas JG. Viscoelastic properties of the contracting detrusor. I. Theoretical basis. Am J Physiol. 1991;261(2 Pt. 1):C355-C363.
Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273-284.
Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21(2):125-138.
Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006;152:97-115.
Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844:174-187.
Vizzard MA, Erdman SL, et al. Differential distribution of nitric oxide synthase in neural pathways to urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res. 1994;646:279-291.
Vizzard MA, Erdman SL, et al. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370(2):191-202.
Vizzard MA, Erickson VL, et al. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629-640.
Vlaskovska M, Kasakov L, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21(15):5670-5677.
Waddell PJ, Lawson SN, et al. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience. 1989;30(3):577-584.
Wagg A, Fry CH. Visco-elastic properties of isolated detrusor smooth muscle. Scand J Urol Nephrol Suppl. 1999;201:12-18.
Wagner G, Husslein P, et al. Is prostaglandin E2 really of therapeutic value for postoperative urinary retention? Results of a prospectively randomized double-blind study. Am J Obstet Gynecol. 1985;151(3):375-379.
Wall SM, Sands JM, et al. Net acid transport by isolated perfused inner medullary collecting ducts. Am J Physiol. 1990;258(1 Pt. 2):F75-F84.
Walter S, Wolf H, et al. Urinary incontinence in post-menopausal women treated with oestrogens: a double blind clinical trial. Urol Int. 1978;33:135.
Walz T, Haner M, et al. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed twisted ribbon structure. J Mol Biol. 1995;248(5):887-900.
Wang EC, Lee JM, et al. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol. 2003;285:F651-F663.
Wang ECY, Lee J-M, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412-2422.
Wang HZ, Brink PR, et al. Gap junction channel activity in short-term cultured human detrusor myocyte cell pairs:gating and unitary conductances. Am J Physiol Cell Physiol. 2006;291(6):C1366-C1376.
Wang ZE, Gopalakurup SK, et al. Expression of smooth muscle myosin isoforms in urinary bladder smooth muscle during hypertrophy and regression. Lab Invest. 1995;73:244-251.
Wang ZY, Wang P, et al. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139(1):158-167.
Wein A. Neuromuscular dysfunction of the lower urinary tract. In Walsh P, Retik A, Stamey T, Vaughan E, editors: Campbell’s urology, 6th ed, Philadelphia: WB Saunders, 1992.
Wein AJ. Pharmacologic options for the overactive bladder. Urology. 1998;51:43-47.
Wellner MC, Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993;466:213-227.
Wen J, Kawatani M, et al. The effects of urinary pH on pelvic nerve mechanoreceptors and silent afferents in the rat bladder. J Physiol. 1994;481:24P.
Wen J, Morrison J. The effects of changing urinary composition on silent afferents in the rat bladder. J Physiol. 1994;476:45P.
Wen J, Morrison J. Sensitization of pelvic afferent neurones from the rat bladder. Auton Nerv Sys. 1996;58:187.
Wheeler JSJr, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100-103.
White S, Martin AF, et al. Identification of a novel smooth muscle myosin heavy chain cDNA:isoform diversity in the S1 head region. Am J Physiol. 1993;264(5 Pt. 1):C1252-C1258.
Wickham JE. Active transport of sodium ion by the mammalian bladder epithelium. Invest Urol. 1964;2:145-153.
Willette RN, Sauermelch CF, Hieble JP. Role of alpha-1 and alpha-2 adrenoceptors in the sympathetic control of the proximal urethra. J Pharmacol Exp Ther. 1990;252:706-710.
Wilson PD, Faragher B, et al. Treatment with oral piperazine oestrone sulphate for genuine stress incontinence in postmenopausal women. Br J Obstet Gynaecol. 1987;94(6):568-574.
Wu C, Kentish JC, Fry CH. Effect of pH on myofilament Ca(2+)-sensitivity in alpha-toxin permeabilized guinea pig detrusor muscle. J Urol. 1995;154:1921-1924.
Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99(1):244-253.
Xu X, Gordon E, et al. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin). 2009;3:156-160.
Yalla SV, Rossier AB, et al. Functional contribution of autonomic innervation to urethral striated sphincter: studies with parasympathomimetic, parasympatholytic and alpha adrenergic blocking agents in spinal cord injury and control male subjects. J Urol. 1977;117:494.
Yamada T, Ugawa S, et al. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem. 2009;57(3):277-287.
Yamaguchi O. Beta3-adrenoceptors in human detrusor muscle. Urology. 2002;59:25-29.
Yamaguchi O, Shishido K, et al. Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. J Urol. 1996;156(3):1208-1213.
Yamamoto M, Harm SC, et al. Parathyroid hormone-related protein in the rat urinary bladder:a smooth muscle relaxant produced locally in response to mechanical stretch. Proc Natl Acad Sci U S A. 1992;89(12):5326-5330.
Yamamoto T, Hanioka N, et al. Contribution of tachykinin receptor subtypes to micturition reflex in guinea pigs. Eur J Pharmacol. 2003;477:253-259.
Yamamoto T, Honbo T, et al. General pharmacology of the new antimuscarinic compound vamicamide. Arzneimittelforschung. 1995;45(12):1274-1284.
Yamamoto T, Sakakibara R, et al. Striatal dopamine level increases in the urinary storage phase in cats:an in vivo microdialysis study. Neuroscience. 2005;135(1):299-303.
Yanagisawa M, Kurihara H, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411-415.
Yokoyama H, Sasaki K, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2008;20(1):63-71.
Yokoyama O, Ootsuka N, et al. Forebrain muscarinic control of micturition reflex in rats. Neuropharmacology. 2001;41:629-638.
Yokoyama O, Yoshiyama M, et al. Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol Regul Integr Comp Physiol. 1999;276:R935-R942.
Yono M, Foster HEJr, et al. Doxazosin-induced up-regulation of alpha 1A-adrenoceptor mRNA in the rat lower urinary tract. Can J Physiol Pharmacol. 2004;82(10):872-878.
Yono M, Foster HEJr, et al. Age related changes in the functional, biochemical and molecular properties of alpha1-adrenoceptors in the rat genitourinary tract. J Urol. 2006;176(3):1214-1219.
Yoshida M, Inadome A, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67(2):425-430.
Yoshimura N. Bladder afferent pathway and spinal cord injury:possible mechanisms inducing hyperreflexia of the urinary bladder. Progress in Neurobiology. 1999;57:583-606.
Yoshimura N, Bennett NE, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26(42):10847-10855.
Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002;168(5):1897-1913.
Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra, 9th ed. Wein AJ, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh urology, vol 3. WB Saunders, Philadelphia, 2007, 1922-1972.
Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol. 1997;4:111-125.
Yoshimura N, Kaiho Y, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4-6):437-448.
Yoshimura N, Kuno S, et al. Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br J Pharmacol. 2003;139:1425-1432.
Yoshimura N, Mizuta E, et al. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Neuropharmacology. 1993;32:315-321.
Yoshimura N, Mizuta E, et al. Therapeutic effects of dopamine D1/D2 receptor agonists on detrusor hyperreflexia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsonian cynomolgus monkeys. J Pharmacol Exp Ther. 1998;286:228-233.
Yoshimura N, Sasa M, et al. Contraction of urinary bladder by central norepinephrine originating in the locus coeruleus. J Urol. 1988;139:423-427.
Yoshimura N, Sasa M, et al. Alpha-1 adrenergic receptor-mediated excitation from the locus coeruleus of the sacral parasympathetic preganglionic neuron. Life Sci. 1990;47:789-797.
Yoshimura N, Sasa M, et al. Dopamine D-1 receptor mediated inhibition of micturition reflex by central dopamine from the substantia nigra. Neurourol Urodyn. 1992;11:535.
Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304-311.
Yoshiyama M, de Groat WC. Supraspinal AMPA and NMDA glutamatergic transmission in the micturition reflex in the rat. Soc Neurosci Abstr. 1996;22:93.
Yoshiyama M, de Groat WC. Supraspinal and spinal alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132:1017-1026.
Yoshiyama M, de Groat WC. Role of spinal metabotropic glutamate receptors in regulation of lower urinary tract function in the decerebrate unanesthetized rat. Neurosci Lett. 2007;420(1):18-22.
Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide on lower urinary tract functions in rats with intact or transected spinal cords. Exp Neurol. 2008;211:449-455.
Yoshiyama M, Roppolo JR, et al. Alterations by urethane of glutamatergic control of micturition. Eur J Pharmacol. 1994;264:417-425.
Yoshiyama M, Roppolo JR, et al. Effects of LY215490, a competitive AMPA receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther. 1997;280:894-904.
Yu HJ, Wein AJ, Levin RM. Age-related differential susceptibility to calcium channel blocker and low calcium medium in rat detrusor muscle: response to field stimulation. Neurourol Urodyn. 1996;15:563-576.
Yu J, Lin JH, et al. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125(1):171-182.
Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294(5):F1146-F1156.
Yunoki T, Zhu HL, et al. Comparative studies of ZD0947, a novel ATP-sensitive K(+) channel opener, on guinea pig detrusor and aortic smooth muscles. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:309-319.
Zagorodnyuk VP, Brookes SJ, et al. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J Physiol. 2009;587(Pt. 14):3523-3538.
Zagorodnyuk VP, Costa M, et al. Major classes of sensory neurons to the urinary bladder. Auton Neurosci. 2006;126:390-397.
Zagorodnyuk VP, Gibbins IL, et al. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147-163.
Zderic S, Levin R, et al. Voiding function and dysfunction: relevant anatomy, physiology, pharmacology and molecular biology. In: Gillenwater J, Grayhack J, Howards S, Duckett J, editors. Adult and pediatric urology. Chicago: Mosby Year Book; 1996:1159-1219.
Zhang H, Reitz A, et al. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174-180.
Zhang X, Igawa Y, et al. Effects of resiniferatoxin desensitization of capsaicin-sensitive afferents on detrusor over-activity induced by intravesical capsaicin, acetic acid or ATP in conscious rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(5):473-479.
Zhao Y, Wein AJ, et al. Effect of anoxia on in vitro bladder function. Pharmacology. 1991;43:337-344.
Zhao Y, Wein AJ, et al. Role of calcium in mediating the biphasic contraction of the rabbit urinary bladder. Gen Pharmacol. 1993;24(3):727-731.
Zhong Y, Banning AS, et al. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120(3):667-675.
Zhong Y, Dunn PM, et al. Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglion neurons. Br J Pharmacol. 1998;125(4):771-781.
Zhong Y, Dunn PM, et al. Multiple P2X receptors on guinea-pig pelvic ganglion neurons exhibit novel pharmacological properties. Br J Pharmacol. 2001;132(1):221-233.
Zvara P, Braas KM, et al. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI). Ann N Y Acad Sci. 2006;1070:622-628.
Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9.
Zvarova K, Dunleavy JD, et al. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192(1):46-59.