chapter 63 Urinary Incontinence and Pelvic Prolapse
Epidemiology and Pathophysiology
Definition and Classification of Urinary Incontinence
Urinary incontinence (UI) has a considerable social and economic impact. Indeed, it has been estimated that UI in women was the primary cause for more than 1.1 million office visits in 2000 in the United States (Litwin et al, 2005). Hu and colleagues (2004) estimated the total direct and indirect cost of incontinence in the United States in the year 2000 to be approximately $19.5 billion. Incontinence has a larger economic impact than many chronic conditions and diseases.
The lower urinary tract comprises the bladder and urethra and should be considered as a single functioning vesicourethral unit required to store urine and empty efficiently. Most of the bladder’s functional time is spent in storing urine. Dysfunction occurs when there is a breakdown of these fundamental tasks, resulting in storage and/or voiding symptoms, urinary retention, or incontinence. The bladder tends to be an “unreliable witness,” its symptoms often being nonspecific and neither diagnosing the underlying dysfunction nor paralleling the severity of the underlying functional disorder. The customary evaluation of patients with both storage and voiding dysfunction includes an in-depth history and physical examination, as well as appropriate laboratory studies. Endoscopy and radiography provide useful structural information and are used when clinically indicated. Urodynamic studies provide the only objective functional tests of bladder and urethral function and are a valuable adjunct to the investigation of patients with lower urinary tract dysfunction (LUTD). Urodynamics, when properly selected and accurately interpreted, provides health care professionals with an improved diagnostic capability useful in formulating treatment strategies, educating patients, and ultimately improving therapeutic outcome. The results of urodynamics should not be interpreted as a free-standing study but in conjunction with the clinical presentation. Although the interpretation of urodynamics can be complex on occasion, in the majority of cases the indications for urodynamic investigation are evident and its application provides an essential complement to the modern practice of urology, gynecology, and associated specialties.
The “storage” component of lower urinary tract symptoms (LUTS), of which incontinence is unarguably the most troublesome, is a common and important cause of morbidity and impairment of quality of life, in both men and women (Chapple et al, 2008). In order to effectively consider the subject of incontinence, it is important to think in terms of symptoms, signs, urodynamic observations, and conditions, as defined by the International Continence Society (ICS) and summarized in their published standardization report (Abrams, 2002; Abrams et al, 2002).
When a condition cannot be documented by urodynamic observation, it may be “presumed” by clinical documentation.
In this chapter we widely quote the ICS standardization documents, which underpin all clinical and academic evaluation of patients and should form the basis for all therapy. The authors of this chapter want to acknowledge the hard work of all of the authors and contributors to these documents. Signs are observed by the physician by simple means (e.g., observation of the loss of urine with a cough) or by the use of diaries, pad tests, symptoms scores, and validated quality of life instruments. Urodynamic observations are made during urodynamic studies and reflect the definitive pathophysiologic condition that is causing the incontinence (e.g., detrusor overactivity or sphincteric weakness).
Lower Urinary Tract Symptoms
Symptoms are the subjective indicator of a disease or change in condition as perceived by the patient, caregiver, or partner and may lead him or her to seek help from health care professionals. They may be volunteered or described during the patient interview. They are usually qualitative. In general, LUTS cannot be used to make a definitive diagnosis. LUTS can also indicate pathologies other than LUTD such as urinary infection.
Signs Suggestive of Lower Urinary Tract Dysfunction
Signs are observed by the physician including simple means to verify symptoms and quantify them. For example, a classical sign is the observation of leakage on coughing. Observations from frequency volume charts, pad tests, and validated symptom and quality-of-life questionnaires are examples of other instruments that can be used to verify and quantify symptoms.
Urodynamic Observations
Urodynamic observations are observations made during urodynamic studies. For example, an involuntary detrusor contraction (detrusor overactivity) is a urodynamic observation. In general, a urodynamic observation may have a number of possible underlying causes and does not represent a definitive diagnosis of a disease or condition and may occur with a variety of symptoms and signs or in the absence of any symptoms or signs.
Conditions
Conditions are defined by the presence of urodynamic observations associated with characteristic symptoms or signs and/or nonurodynamic evidence of relevant pathologic processes.
LUTS are best considered as three groups of symptoms: storage, voiding, and postmicturition. Storage symptoms will be considered in detail later, and the reader is directed to other chapters for a consideration of the other categories of LUTS.
Storage symptoms are experienced during the storage phase of the bladder and include daytime frequency and nocturia.
Night-time frequency differs from nocturia because it includes voids that occur after the individual has gone to bed but before he or she has gone to sleep and voids that occur in the early morning, which prevent the individual from getting back to sleep as he or she desires.
The International Continence Society (ICS) has defined the symptom of UI as “the complaint of any involuntary loss of urine that is a social or hygienic problem.” When considering incontinence, it is important to particularly consider relevant factors such as type, frequency, severity, precipitating factors, social impact, effect on hygiene and quality of life, the measures used to contain the leakage, and whether or not the individual seeks or desires help because of UI. Urinary leakage may need to be distinguished from sweating or vaginal discharge.
Urgency incontinence can present in different symptomatic forms (e.g., as frequent small losses between micturitions or as a catastrophic leak with complete bladder emptying).
Overactive bladder (OAB), or the urgency frequency symptom syndrome, comprises urgency, with or without urgency incontinence, usually with frequency and nocturia. Urgency is widely held to be a key symptom driving the clinical sequence as demonstrated by Figure 63–1.
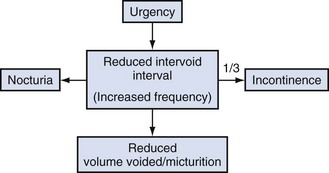
Figure 63–1 The overactive bladder symptom complex.
(From Chapple CR, Artibani W, Cardozo LD, et al. The role of urinary urgency and its measurement in the overactive bladder symptom syndrome: current concepts and future prospects. BJU Int 2005;95:335–40.)
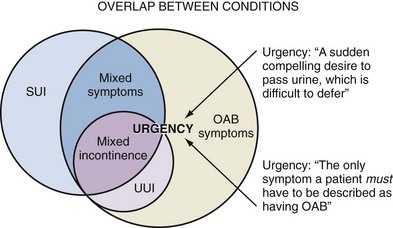
Figure 63–2 Overlap between conditions. OAB, overactive bladder; SUI, stress urinary incontinence; UUI, urgency urinary incontinence.
(From Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol 2006;175:S5–10.)
Coughing may induce a detrusor contraction—hence the sign of stress incontinence is only a reliable indication of urodynamic stress incontinence when leakage occurs synchronously with the first proper cough and stops at the end of that cough.
Definition and Classification of Pelvic Organ Prolapse
More than 40% of women with urethral sphincter incompetence will have a significant cystocele (Cardozo and Stanton, 1980). Conversely, a history of stress incontinence associated with a mild or moderate cystocele is not specific for diagnosing genuine stress incontinence (Summitt et al, 1992). “Occult or latent” incontinence is urethral sphincteric incompetence masked by the presence of pelvic prolapse (Rosenzweig et al, 1992a, 1992b). Not infrequently, incontinent women may note the decrease or disappearance of stress incontinence episodes as the degree of prolapse worsens. The sign of occult stress incontinence is facilitated by the use of a speculum or pessary to reduce the prolapse while a stress maneuver is performed. The method to reduce vaginal prolapse to evaluate latent incontinence is not universally agreed on or standardized at this time.
A large rectocele may cause incomplete bowel evacuation and tenesmus. Sexual function consists of a complex interaction of biologic, psychologic, and social factors that affect both patient and partner. A complex relationship exists between anatomic descent of pelvic organs and physiologic function, and often these components do not correlate well with each other.
Vaginal examination allows the description of observed and palpable anatomic abnormalities and the assessment of pelvic floor muscle function. Segments of the lower reproductive tract should be considered as a substitute for terms as “cystocoele, rectocoele, enterocoele, or urethrovesical junction” because these terms convey an unrealistic certainty relating to the structures associated with the vaginal bulge, especially following previous prolapse surgery. This section draws heavily on the ICS standardization report, which commented on the evaluation of pelvic organ prolapse (Bump et al, 1996) as modified by the ICS standardization report 2002.
Pelvic organ prolapse can occur in association with UI and other lower urinary tract dysfunction and may on occasion mask incontinence.
Pelvic floor muscle function can be qualitatively defined by the tone at rest and the strength of a voluntary or reflex contraction as strong, weak, or absent or by a validated grading system (e.g., Oxford 1 to 5). A pelvic muscle contraction may be assessed by visual inspection, palpation, electromyography, or perineometry. Factors that must be assessed are strength, duration, displacement, and repeatability. Rectal examination allows the description of observed and palpable anatomic abnormalities and is the easiest method of assessing pelvic floor muscle function in children and men. In addition, rectal examination is essential in children with UI to rule out fecal impaction.
Relationship Between Incontinence and Prolapse
Patients with severe prolapse may develop voiding symptoms as a result of urethral kinking, leading to obstruction that is worsened during straining effort (Richardson et al, 1983). For instance, a moderate or severe cystocele may promote urethral compression and kinking, pressure dissipation, and an increase in maximum urethral closure pressures (Bergman et al, 1988; Versi et al, 1998). Clearly, a number of storage urinary symptoms may occur in combination with prolapse. Risk factors contributing to the symptoms of urgency and frequency include age and urogenital atrophy. One study found that women with mild cystoceles had a 20% incidence of detrusor overactivity, and the incidence increased to 52% in those with moderate to severe cystoceles (Enhorning, 1961).
More than 40% of women with urethral sphincter incompetence will have a significant cystocele (Cardozo and Stanton, 1980). A complaint of stress incontinence associated with the appearance of a mild or moderate cystocele, however, is not specific for diagnosing genuine stress incontinence (Walters and Shields, 1988; Summitt et al, 1992).
Occult or latent incontinence is urethral sphincteric incompetence masked by the presence of pelvic prolapse (Rosenzweig et al, 1992). Not infrequently, incontinent women may note the decrease or disappearance of stress incontinence episodes as the degree of prolapse worsens. The office demonstration of the sign of occult stress incontinence is facilitated by the use of a speculum or pessary to reduce the prolapse while a stress maneuver is performed. Its presence may influence management options given to the patient. However, the method to reduce vaginal prolapse to evaluate latent incontinence is not universally agreed on or standardized at this time.
A large rectocele may cause incomplete bowel evacuation and tenesmus, leading some to splint or manually reduce the posterior vaginal segment or perineum to assist in defecation. Patients may describe stool becoming trapped in the rectocele pocket itself. Constipation and straining may worsen the symptoms and lead to left lower quadrant abdominal pain if impaction occurs.
The prevalence of fecal incontinence increases to 17% in populations with pelvic organ prolapse and UI (Jackson et al, 1997) compared with 2% to 3% in the general population (Leigh and Turnberg, 1982; Thomas et al, 1984). The most common mechanisms are an incompetent sphincteric mechanism (secondary to a structural defect or pudendal nerve damage) and overflow incontinence.
Sexual function consists of a complex interaction of biologic, psychologic, and social factors that affect both patient and partner. Various studies have described the presence of sexual problems in patients who present with pelvic organ prolapse and UI (Haase and Skibsted, 1988; Field and Hilton, 1993).
Pathophysiology of Incontinence And Prolapse
Continence
To understand the pathophysiology of urinary incontinence, it is important to be familiar with the micturition cycle and the physiology of normal storage and emptying of urine. The details of the physiology of micturition are provided in Chapter 60. The function of the lower urinary tract is to temporarily store a continuously increasing amount of urine at low pressure and expel it under appropriate circumstances. This is dependent on the coordinated activity of smooth and striated muscles in the bladder, urethra, and pelvic floor. The bladder and urethra constitute a functional unit, which is controlled by a complex interplay between the central and peripheral nervous systems and local regulatory factors (Andersson and Wein, 2004).
Bladder emptying and, conversely, urine storage rely on a complex series of finely tuned and integrated neuromuscular events that involve anatomic and neurologic mechanisms. It is a complex pattern of efferent and afferent signaling in parasympathetic, sympathetic, somatic, and sensory nerves. Malfunction at various levels may result in bladder control disorders, which roughly can be classified as disturbances of filling/storage or disturbances of voiding/emptying. Failure to store urine may lead to various forms of incontinence (mainly urgency and stress incontinence), and failure to empty can lead to urinary retention, which may result in overflow incontinence. A disturbed filling/storage function can, at least theoretically, be improved by agents decreasing detrusor activity, increasing bladder capacity, and/or increasing outlet resistance.
During bladder filling at physiologic rates, detrusor pressure remains nearly constant because of this property of accommodation (Klevmark, 1974), otherwise known as “tonus” or receptive relaxation. Accommodation accounts for the nearly flat cystometric curve that is seen during normal bladder filling. The viscoelastic properties of the bladder, based on its composition of smooth muscle, collagen, and elastin, normally produce a highly compliant structure. When accommodation is impaired, low bladder compliance ensues. This is manifest as a steep rise in detrusor pressure during bladder filling. In addition to the viscoelastic properties of the bladder, the neural control of the lower urinary tract and the anatomy and support of the sphincteric unit are important factors in the maintenance of urinary continence. The micturition reflex is normally under voluntary control and is organized in the rostral brainstem (the pontine micturition center [PMC]). Micturition results from a release from the negative inhibitor effect of the higher centers on the PMC. It requires integration and modulation by the parasympathetic and somatic components of the sacral spinal cord (the sacral micturition center) and the thoracolumbar sympathetic components (Fowler et al, 2008).
The reservoir function of the bladder requires a permanently low intravesical pressure over a wide volume range and a bladder outlet that remains firmly closed, even under conditions of abdominal pressure rises, during the filling phase of the bladder, which represents the majority of its activity cycle. Conversely, during voiding the bladder should be able to develop a sustained contraction of sufficient strength with opening of the bladder outlet offering a low resistance to urinary flow to assure adequate emptying of the bladder. During micturition, the first recorded event is sudden and complete relaxation of the striated sphincteric muscles, characterized by complete electrical silence of the sphincter electromyogram (Tanagho and Miller, 1970). This is followed almost immediately by a rise in detrusor pressure and concomitant fall in urethral pressure as the bladder and proximal urethra become isobaric (Yalla et al, 1980). The bladder neck and urethra open, and voiding ensues. Voluntary interruption of the stream is accomplished by a sudden contraction of the striated periurethral musculature, which, through a reflex mechanism, shuts off the detrusor contraction, aborting micturition. The sphincteric system is also designed to resist physiologic increases in abdominal pressure and thereby prevent the development of incontinence.
Normal storage of urine is therefore dependent on the following:
It is important to identify the underlying bladder abnormality in a reproducible manner. Bladder abnormalities that cause UI include detrusor overactivity and low bladder compliance.
Disordered lower urinary tract function can result from the following:
Patients who have disordered lower urinary tract function in routine clinical practice represent a heterogeneous collection for most of whom there is no identifiable neurologic abnormality. Some of these patients will have a primary neural or muscular disorder (e.g., primary idiopathic detrusor overactivity) in contrast to postobstructive secondary detrusor overactivity, in which the major etiologic factor is likely to be peripheral disruption of local neuromuscular function.
Detrusor overactivity is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. Detrusor overactivity may be phasic, terminal, or both (Abrams et al, 2002). There are certain patterns of detrusor overactivity:
Provocative maneuvers are used during urodynamics in an effort to provoke detrusor overactivity (e.g., rapid filling, use of cooled or acid medium, postural changes, hand washing).
Detrusor overactivity may also be qualified, when possible, according to cause. Examples follow:
In clinical and research practice, the extent of neurologic examination/investigation varies. It is likely that the proportion of neurogenic-to-idiopathic detrusor overactivity will increase if a more complete neurologic assessment is carried out (Table 63–1).
Table 63–1 Causes of Detrusor Overactivity
| Neurogenic Detrusor Overactivity |
| Idiopathic Detrusor Overactivity |
Interpretation of Vesicourethral Dysfunction
Because so many different aspects of vesicourethral function can be impaired in neurologic conditions, interpretation may be difficult. The most important points to clarify in clinical practice are the presence or absence of neurogenic detrusor overactivity and the behavior of the distal urethral sphincter mechanism during voiding.
High-pressure bladders with detrusor–sphincter dyssynergia are prone to develop vesicoureteral reflux, which may ultimately lead to renal impairment. This can be clearly demonstrated by videocystometrography (VCMG). Preservation of renal function is of utmost importance in the management of patients who have chronic neurologic conditions. As a rule of thumb, those patients with a competent bladder outflow and an end filling pressure of 40 cm H2O or higher before leakage occurs—(the detrusor leak point pressure) are at particular risk of developing upper urinary tract problems due to back pressure (McGuire et al, 1996).
Neurogenic detrusor overactivity can be defined as abnormal function of the bladder and urethra due to lesions affecting their innervation, either within the central nervous system or in the peripheral nerves of the lower urinary tract.
Urodynamic investigations are essential to characterize the nature of the detrusor and sphincteric abnormality to do the following:
The interpretation of urodynamics in such complex cases is often difficult and is best performed in specialist centers using VCMG.
Neurologic conditions can alter vesicourethral function by impairing the following:
These alterations may occur in isolation or in combination. Identical abnormalities may also occur without clinical evidence of a neurologic deficit.
Classification of Neuropathic Bladder Dysfunction
Neuropathic bladder dysfunction may be classified into supraspinal, suprasacral, and infrasacral, according to the level of the lesion in relation to the pontine and sacral micturition centers:
Neurologic lesions generally affect bladder and urethral function in a relatively consistent fashion, depending on the area affected and the completeness of the lesion. A basic understanding of the bladder dysfunction associated with each level of injury is necessary to interpret the results of urodynamics properly.
Supraspinal Lesions
Patients who have supraspinal lesions generally empty their bladders efficiently unless they have associated bladder outlet obstruction. Preexisting bladder dysfunction is not uncommon, and urodynamics plays a major role in defining the abnormality and formulating treatment strategies.
Suprasacral Lesions
Patients who have cord lesions above the sacral micturition center initially go through a period of spinal shock in which there is loss of neurologic activity below the level of the injury. Detrusor areflexia (the inability to generate a detrusor contraction due to neurologic causes) and maintenance of some residual sphincteric competence usually results in urinary retention requiring either indwelling or intermittent catheterization.
Recovery is characterized by the gradual return of reflex bladder activity or neurogenic detrusor overactivity mediated through the sacral micturition center, which is intact but separated from higher centers. It usually occurs within 2 to 3 months, but in a small number of patients it may take up to 2 years for reflex activity to return. As reflex bladder activity increases during the recovery phase, the bladder may empty well at the expense of incontinence or high detrusor voiding pressures may develop, resulting in hydronephrosis.
Infrasacral Lesions
Detrusor activity may be absent or diminished if there are lesions of the cell bodies or parasympathetic efferents to the bladder from the sacral roots (S2 to S4). This may be due to trauma to the spinal cord or pelvic nerves or destruction of the cord segment by lesions such as a plaque of multiple sclerosis.
Injury to the conus or sacral roots results in the following:
The weakened urethral sphincter mechanism and paralyzed pelvic floor make patients prone to stress incontinence, especially women.
Some patients empty their bladders by abdominal straining or suprapubic compression (Credé’s maneuver), but the majority need clean intermittent self-catheterization to empty efficiently. For poorly understood reasons, a minority of patients are at risk of developing poorly compliant high-pressure bladders that can lead to renal damage.
Partial infrasacral lesions may result in a mixed pattern of weak or absent detrusor activity, poor compliance, and considerable reflex activity in the pelvic floor muscles.
Idiopathic Detrusor Overactivity
Idiopathic detrusor overactivity can be associated with a variety of non-neurogenic clinical conditions such as bladder outlet obstruction, dysfunctional voiding, and inflammatory reactions (Hebjorn et al, 1976; Awad and McGinnis, 1983; Blaivas, 1988; Resnick et al, 1989; Fantl et al, 1990; Brading and Turner, 1994; Carlson et al, 2001). Elbadawi and colleagues (1993a, 1993b, 1993c) have proposed a possible explanation for age-related detrusor overactivity based on detailed ultrastructural studies of the bladder. They showed a characteristic structural pattern in bladder biopsy specimens obtained from elderly patients with detrusor overactivity. Electron microscopic changes (abundant, distinctive protrusion junctions and abutments) in patients with detrusor overactivity are thought to result in a diminished electrical resistance between detrusor muscle cells and, hence, a hyperexcitable state that results in involuntary detrusor contractions.
Bladder outlet obstruction causes increased voiding pressures and a prolonged duration of voiding. It has been hypothesized that this results in ischemic damage to the intramural ganglia and consequently to a partial denervation of the detrusor muscle (Brading and Turner, 1994). Experiments have shown that denervation of smooth muscle cells may result in supersensitivity to agonists, increased excitability, and increased electrical coupling. These changes may make the detrusor muscle susceptible to the development of involuntary contractions. The detrusor muscle hypertrophy on obstruction does not necessarily play a causative role (Brading and Turner, 1994). A somewhat contrasting and more evidence-based explanation comes from the observation of increased levels of nerve growth factor in the bladders of obstructed rats and humans and increased dimensions of afferent and efferent rat neurons (Steers et al, 1991).
In many cases the etiology is unclear—the cause of idiopathic detrusor overactivity is likely to be neurogenic rather than myogenic, particularly in view of the association between detrusor overactivity and aging. An increased number of putative afferent nerves in the bladder wall of female patients has been reported (Smet et al, 1997). Although no effect of intravesical capsaicin on idiopathic detrusor overactivity was found (Fowler, 2002), efficacy was found in 12 patients treated with the more potent analog resiniferatoxin (Cruz, 2002). This suggests the involvement of the afferent C fibers. The role of non-neurogenic factors cannot be excluded because abnormal cell-to-cell communications have been reported in the bladders of patients with idiopathic overactivity (Elbadawi et al, 1993b).
Bladder Compliance during Filling Cystometry
Bladder compliance describes the relationship between change in bladder volume and change in detrusor pressure. The observation of reduced bladder compliance during conventional filling cystometry is often related to relatively fast bladder filling: the incidence of reduced compliance is markedly lower if the bladder is filled at physiologic rates, as in ambulatory urodynamics.
The viscoelastic properties and neurologic innervation of the bladder under normal conditions allow it to store increasing volumes of urine at low pressure. Thus with normal compliance there is little or no pressure change as the bladder fills. Compliance (Δvolume/Δpressure) is expressed as mL/cm H2O.
Although it is well established that high storage pressures can lead to incontinence and upper tract deterioration (McGuire et al, 1981; Comiter et al, 1997), at the present time there are no standardized or international agreed values to define normal, high, and low compliance. The calculated value of compliance is probably less important than the actual bladder pressure during filling. This is because the compliance value can change depending on the volume over which it is calculated. For this reason, numeric values of compliance are rarely reported. For example, sustained detrusor pressures of 35 to 40 cm H2O or greater during storage, regardless of the bladder volume, can lead to upper tract damage, but similar damage can also occur at lower pressures (McGuire et al, 1981; Churchill et al, 1987). When the detrusor pressure exceeds the pressure capacity of the sphincter to resist that pressure, incontinence results.
Low bladder compliance denotes an abnormal volume-pressure relationship in which there is a high incremental rise in detrusor pressure during bladder filling. Low bladder compliance may be caused by changes in the elastic and viscoelastic properties of the bladder, changes in detrusor muscle tone, or combinations of the two. Clinically, low bladder compliance is most commonly seen in a variety of neurologic conditions, especially lower motor neuron lesions such as spina bifida and cauda equina syndrome. Obstructive uropathy, because of its effect on bladder ultrastructure, is known to cause low bladder compliance and has been identified as a urodynamic risk factor. Leng and McGuire (2003) showed that relief of obstruction by transurethral incision or resection of the prostate can improve compliance.
The clinical causes of low bladder compliance are listed in Table 63–2.
Table 63–2 Causes of Low Bladder Compliance
| Neurogenic |
| Non-Neurogenic (Increased Collagen) |
Sphincteric Mechanisms
The urethra has two main functions:
The innermost mucosal layer in both sexes is organized in longitudinal folds, and during the storage phase, when the urethra is “closed,” this appears in a stellate configuration on cross section. Such a configuration allows significant distensibility, which is necessary during urethral “opening.”
The submucosal layer contains a vascular plexus, which may be involved in improving the seal of a “closed urethra” by transmitting the tension of the urethral muscle to the mucosal folds.
Apart from the obvious anatomic differences (the longer urethra and presence of a prostate gland in men), there are important differences in the configuration of the sphincter mechanisms between males and females. There are two powerful sphincter mechanisms in the male compared with the single weaker intrinsic sphincter mechanism with a weaker bladder neck and also a shorter urethra in the female.
Traditionally, the urethral sphincter is considered to be composed of two components: the internal sphincter, which represents a direct continuation of the detrusor smooth muscle, and the striated external sphincter (Tanagho, 1992). From a clinical standpoint, the bladder neck–proximal urethra normally functions as a sphincter in both sexes. Anatomically there is a unique mixture of smooth and striated muscle, intracellular matrix, and mucosal components contributing to the functional sphincter.
Male Sphincteric Mechanisms
In the male there are two important sphincteric mechanisms: (Hadley et al, 1986).
The proximal sphincter in the male bladder neck provides a powerful mechanism in both maintaining urinary continence and also prevents retrograde ejaculation of semen during sexual activity. In patients with a damaged distal urethral sphincter (e.g., a pelvic fracture-associated urethral disruption), continence can be maintained solely by the proximal bladder neck mechanism. Ultrastructurally, it consists of a powerful inner layer of muscle bundles arranged in a circular orientation.
The distal sphincteric mechanism is also extremely important, as evidenced by its ability to also maintain continence even when the proximal bladder neck mechanism has been rendered totally incompetent by surgical bladder neck incision or a prostatectomy. It is confined to the 3- to 5-mm thickness of the wall of the membranous urethra from the level of the verumontanum down to the distal aspect of the membranous urethra. It is composed mainly of extrinsic striated muscle, which is capable of the sustained contraction necessary for continence and to a lesser degree by intrinsic smooth muscle.
Unlike that in the female, where urethral support can be compromised as a result of childbirth and aging, in the male, compromise to the rhabdosphincter usually occurs after trauma or surgery (e.g., prostatectomy).
Female Sphincteric Mechanisms
Females are much more likely to suffer from UI due to sphincteric deficiency than males because of the much less powerful sphincteric mechanisms. The bladder neck is a far weaker structure than the male bladder neck and is often incompetent, even in nulliparous young women (Chapple et al, 1989). The bladder neck is poorly defined with the muscle fibers having a mainly longitudinal orientation.
Urinary continence usually relies on the integrity of the urethral sphincteric mechanism in females (analogous to the distal sphincter in men). Like the male distal mechanism, it is composed of a longitudinal intrinsic urethral smooth muscle and a larger extrinsic striated muscle component. This sphincter extends throughout the proximal two thirds of the urethra, being most developed in the middle one third of the urethra. Therefore in women the majority of the urethra should be considered to be sphincter active. Damage to the innervation of the urethral sphincter (in particular the pudendal nerve) by obstetric trauma reduces the effectiveness of this mechanism and predisposes to stress UI (Table 63–3).
Table 63–3 Comparison of Male and Female Sphincteric Mechanisms*
| MALE | FEMALE | |
|---|---|---|
| Proximal bladder neck mechanism | Powerful | Weak |
| Distal urethral mechanism | Powerful | Extending along the majority of the length of the female urethra and prone to the effect of exogenous influences such as pelvic floor weakness and damage or denervation consequent on childbirth |
| Prostate | Further increases bladder outlet resistance | Not present |
| Urethra | Long | Short (≈3.5 cm) |
* Indicates why females are much more likely to develop an incompetent urethral mechanism and are prone to urinary incontinence from intrinsic sphincter deficiency.
The principles underlying normal sphincteric function are integrated interactions among a number of factors:
Conditions Causing Sphincter Abnormalities
The causes of sphincteric dysfunction are different in men and women.
In men, sphincter abnormalities are most commonly caused by anatomic disruption after prostate surgery or, less commonly, by trauma or neurologic abnormalities.
In women with stress incontinence it is likely that there is a degree of sphincter weakness in all cases. The sphincter abnormality will lie on a spectrum from a mild degree where the predominant pathophysiologic abnormality is urethral hypermobility (urethral support defect), to those where irrespective of the urethral support defect there is severe intrinsic sphincteric insufficiency (ISD). Urethral hypermobility and support defects in women are most commonly associated with pregnancy and vaginal delivery, pelvic surgery, and chronic abdominal straining (e.g., chronic constipation). In some cases, loss of support many be secondary to neurologic injury. In such cases, nerve injury (e.g., pudendal nerve) can lead to muscle atrophy and subsequent failure of the support mechanism.
Sphincter Abnormalities in Women
Labor and delivery have long been thought to be risk factors for stress incontinence, urogenital prolapse, and anal incontinence. Vaginal delivery may be associated with direct injury to the pelvic soft tissues, as well as partial denervation of the pelvic floor and thus may play a role in the etiology of stress urinary incontinence (SUI).
There are four major mechanisms by which vaginal delivery might lead to sphincteric damage and incontinence:
Vaginal delivery can cause partial denervation with consequent reinnervation in most first deliveries (Allen et al, 1990). A growing body of evidence indicates that multiparity; forceps delivery; increased duration of the second stage of labor, partially related to epidural anesthesia; third-degree perineal tear; and high birth weight (>4000 g) are important factors leading to pudendal nerve injury (Snooks et al, 1986; Handa et al, 1996; Brown and Lumley, 1998; Rortveit et al, 2003). Snooks and colleagues (1984) studied the postpartum innervation of the external anal sphincter and found that the external anal sphincter and its innervation might be damaged by vaginal delivery but not by cesarean section. The abnormalities found were most marked in multiparas and correlated most strongly with a prolonged second stage of labor and forceps delivery.
Several conditions are associated with increased risk for ISD in women:
Sphincter Abnormalities in Men
Sphincter abnormalities in men are caused by trauma or neurologic injury. With prostatectomy, whether for benign disease or with radical prostatectomy, the distal urethral sphincter (particularly the rhabdosphincter) can be damaged by direct injury or injury to the nerve supply or supporting structures (Nitti, 2002). In some cases, there may be preexisting damage to the sphincter that cannot be accurately diagnosed preoperatively (Nitti, 2002). As in women (as noted earlier), radiation and neurologic lesions can cause sphincteric dysfunction. Finally, pelvic trauma or instrumentation that results in trauma to the distal urethral sphincter can result in incontinence, particularly when the PUS is absent or deficient.
Postprostatectomy incontinence, like any urinary incontinence, may be caused by bladder dysfunction, sphincter dysfunction, or a combination of both and may be associated with other lower urinary tract symptoms. Urodynamic investigations are helpful to rule out bladder outlet obstruction or significant bladder dysfunction. Urodynamic studies have demonstrated that the sphincter incompetence occurs as the sole cause in more than two thirds of patients. Isolated bladder dysfunction (detrusor overactivity, poor compliance, detrusor underactivity during voiding) is uncommon, occurring in less than 10% (Ficazzola and Nitti, 1998; Groutz et al, 2000). However, sphincter and bladder dysfunction can coexist in at least one third of incontinent patients.
Bladder dysfunction may occur de novo after prostatectomy, perhaps induced by bladder denervation; may be caused by outlet obstruction; or may be related to preexisting factors such as age. Impaired detrusor contractility and poor compliance resolved in the majority of patients within 8 months (Giannantoni et al, 2004). Decreased sphincter resistance may be due to tissue scarring in some cases and is reflected by a low urethral compliance; however, this parameter is difficult to measure (Groutz et al, 2000). Scarring may lead to an anastomotic stricture and is clinically suspected when both incontinence and decreased force of stream coexist. The preoperative length of the membranous urethra determined on magnetic resonance imaging (MRI) has been shown to be significantly related to time to postoperative continence. When urethral length was greater than 12 mm, 89% of the patients were continent at 1 year, versus 77% with 12 mm or less than this length (Coakley et al, 2002). Urodynamic studies revealed that a reduced functional urethral length was a predictive parameter of incontinence (Hammerer and Huland, 1997; Van Kampen et al, 1998; Wei et al, 2000). Different components of the urethra may also be involved. The urethral intrinsic component responsible for passive continence, as well as the extrinsic component responsible for active continence, may be involved as has been demonstrated in a study using urodynamic evaluation and alpha blockade (Pfister et al, 2002). This may explain passive incontinence despite a high voluntary urethral pressure or that measured during an active squeeze by the patient. Postoperative disruption of the innervation of the posterior urethra may also be involved and can affect both motor and sensory function (John et al, 2000; Bader et al, 2001).
Risk factors for incontinence after transurethral resection of the prostate (TURP) have not been clearly defined, probably because the incidence is so low. However, previous brachytherapy does predispose to post-TURP incontinence, as does significant bladder overactivity and preoperative urinary incontinence. Preoperative lower urinary tract symptoms including urgency incontinence and “overflow incontinence” may improve with de-obstruction secondary to extirpative surgery. Whenever a functional abnormality of the bladder or sphincter abnormalities is suspected, preoperative urodynamic assessment is advised before TURP.
UI occurring after radical prostatectomy (RP) is a significant problem. Although its rate has lessened in recent years primarily due to a better understanding of pathophysiology and improvements in surgical technique, its prevalence has increased due to the dramatic increase of laparoscopic and robotic-assisted laparoscopic radical prostatectomy as standard treatments for men with prostate cancer in developed countries (Hu et al, 2003, 2004) within the past decade. Based on the body of available data on postoperative continence, it would appear that incontinence rates are similar between open and laparoscopic/robotic approaches. Several studies have compared the techniques either prospectively in nonrandomized fashion (Anastasiadis et al, 2003; Jacobsen et al, 2007) retrospectively (Ahlering et al, 2004) or via limited meta-analysis (Rassweiler et al, 2003; Salomon et al, 2004), and similar continence rates were found. Further prospective comparative studies with open surgery are necessary. Perineal prostatectomy is done by only a limited number of urologists but is still advocated for obese patients. The continence rate was reported as similar to the retropubic route (Weldon et al, 1997; Gray et al, 1999; Harris, 2003).
Data from large multicenter studies and prostate cancer databases suggest that following RP, 1% to 40% of patients complain of persistent urinary incontinence. The incidence of postprostatectomy incontinence (PPI) depends on the definition of UI and the length of follow-up (Olsson et al, 2001; Krupski et al, 2003; Rodriguez et al, 2006). Recent reports of large cohorts use definitions that include “total control/perfect continence,” “occasional leakage but no pad,” and “less than one pad.” Because one third to one half of men who do not wear pads will have occasional leakage of urine, it is important to distinguish among those men who leak enough to require pad use and those who do not. It has been demonstrated that health-related quality of life is strongly correlated with the level of incontinence, and wearing a pad more significantly affects the quality of life than wearing no pad (Litwin et al, 2000). In addition, not all men who leak will elect to have further treatment. Most large cohort studies indicate that between 6% and 9% of patients undergo subsequent surgical treatment for PPI following prostate cancer surgery (Stanford et al, 2000; Begg et al, 2002; Steineck et al, 2002; Penson et al, 2005).
Reported risk factors for incontinence following radical prostatectomy include the following:
Preoperative sphincteric insufficiency (demonstrated by either the preexisting clinical sign of SUI or the urodynamic finding of lower maximal urethral closure pressure) predicts postoperative SUI (Wei et al, 2000; Majoros et al, 2006). Preoperative bladder dysfunction can also contribute to postoperative incontinence. Preexisting abnormalities of detrusor function may predispose to leakage following surgery, especially neurogenic detrusor overactivity due to Parkinson disease, dementia, or spinal cord injury (Khan et al, 1991).
Advancing age as a risk factor is supported by several studies (Leandri et al 1992; Zincke at al, 1994; Eastham et al, 1996; Catalona et al, 1999; Horie et al, 1999; Moore et al, 2007). Others have found advancing age and the number of comorbidities to have a negative impact on the recovering time for continence during the first year after radical prostatectomy (Young et al, 2003). Rogers and colleagues demonstrated that age affected postoperative continence status following laparoscopic RRP. In those younger than 50 years old, 100% achieved 0 to 1 pad per day continence at 1 year, which decreased to 91% and 81% for those 50 to 59 years old and older than 60 years, respectively (P < 0.01) (Rogers et al, 2006). Strasser and colleagues (1998) hypothesized that age-related sphincteric changes may be responsible for the age-related increase in postoperative SUI and successfully demonstrated a progressive reduction in sphincter striated muscle cells with age.
Most large series have found no correlation between the stage of disease and incontinence rates (Eastham et al, 1996; Jonler et al, 1996; Lowe, 1996; Catalona et al, 1999; Pierorazio et al, 2007). However, in certain cases, the stage of disease may affect the surgical technique (i.e., nerve sparing) and incontinence rates may be higher, but this appears to be a reflection on surgical technique and not disease stage (Zincke et al, 1994).
Regarding surgical technique, the many parameters involved in continence have resulted in difficulties in understanding the benefit of certain technical points.
Bladder neck preservation has been reported to improve continence at 3 months (Lowe, 1996), but no difference was evident at 6 and 12 months (Poon et al, 2000; Srougi et al, 2001).
The role of nerve sparing on subsequent continence is debated. Nerve sparing has no significant impact according to Steiner and colleagues (1991), and Lepor and colleagues (2004) recently confirmed this. Others did find benefit (Wei et al, 2000). In particular, Nandipati and colleagues reported that in a cohort of 152 patients followed prospectively, bilateral nerve-sparing surgery was associated with a shorter time to regain continence, as well as improved long-term continence rates compared with non–nerve-sparing surgery. They additionally found that increased age was a risk factor for postprostatectomy incontinence (Nandipati et al, 2007). Burkhard and colleagues similarly demonstrated a positive effect of nerve-sparing surgery on postoperative continence. In a prospective cohort study of 536 patients, PPI developed in 1 of 75 (1.3%), 11 of 322 (3.4%), and 19 of 139 (13.7%) with attempted bilateral, attempted unilateral, and without attempted nerve sparing, respectively. Attempted nerve sparing was, in fact, the only statistically significant factor influencing urinary continence after RRP in this cohort (Burkhard et al, 2006).
Pelvic Floor Support Mechanisms
Two major factors govern the predisposition of the human female to develop either stress UI or prolapse, or both.
Certainly, repeated episodes of vaginal traction can stretch, tear, or weaken urethral muscles and contribute to a chronically weakened urethra characterized by low VLPP or low urethral closure pressures. This is suggested to be typical of intrinsic sphincter deficiency (ISD). Clearly, however, there is no adequate categorization of ISD because most of the measures have limited sensitivity by virtue of their nature and inability to separate out the different components of urethral function. Electrophysiologic studies, MRI, and sonography can be used to quantify the morphologic and functional decline in striated sphincter tone, but analysis of the etiologic factors is difficult because the pathophysiology develops over many years. Successful operations can restore urethral position but probably do not reverse damage to urethral sphincter function, and there are still many question marks about whether this is ever possible.
The complex etiology of stress UI and pelvic organ prolapse and the long time period over which these complications and problems develop strongly supports the need for prospective studies taking account of both nature and nurture. This may allow the investigation of early timely interventions to prevent the further deterioration of their support tissues and urethral closure mechanisms.
From Dichotomy to Continuum between Hypermobility and Intrinsic Sphincter Deficiency
An integrated approach involving anatomy and physiology is essential to an improved understanding of pathophysiology. Clearly a continent bladder outlet in the females depends on multiple factors (resting tone, active contraction, external compression, pressure transmission, integrity of configuration), and the focal point appears to be the midpoint of the urethra. In patients with stress incontinence there is clearly a spectrum between the two components of ISD and urethral hypermobility. Clearly, some patients have primary sphincteric problems, whereas others have an adequately functioning sphincter but significant hypermobility. The majority lie somewhere between these two extremes.
In recent years there has been a gradual change from this dichotomic classification of stress incontinence: hypermobility or ISD. ISD alone is rare, and urethral hypermobility may occur commonly without significant ISD, but usually there is a combination of both in individual patients.
Figure 63–3 demonstrates the potential balance between the relative proportions of ISD and pelvic floor muscle weakness hypermobility seen in any individual patient.
Figure 63–3 The potential balance between the relative proportions of intrinsic sphincter deficiency (ISD) and pelvic floor muscle weakness hypermobility seen in any individual patient.
This evolution in our understanding in part followed the development of the concept of Valsalva leak point pressure (VLPP) introduced by McGuire in 1995 and also from analysis of long-term results of incontinence surgery. Despite lacking standardization of recording methods, as well as lacking consensus on how to deal with any associated prolapse, low VLPP (inferior to 60 cm H2O has been suggested) has been widely considered as an indicator of ISD. Reports in the literature vary considerably regarding the extent of correlation between VLPP and outcome. Similarly, urethral pressure profilometry has been extensively studied in this context but there is a similar lack of correlation both in terms of predictive value and outcome. For instance, a low-pressure urethra may not leak, whereas the high-pressure urethra may. Also, a number of studies have failed to show any correlation between postoperative UPP and outcome. Cystography, although an invasive technique requiring radiologic input, provides a reproducible and easily interpreted technique, which allows the relative contribution of both sphincteric weakness and hypermobility to be assessed (Blaivas and Olsson, 1988).
Consequently, some degree of ISD may exist in many patients where hypermobility is believed to be the only cause of stress incontinence (Kayigil et al, 1999). This understanding supports the increasing use of suburethral slings to treat stress incontinence, whereas formerly this procedure was exclusively suggested for patients with recurrent stress incontinence or severe ISD (Chaikin et al, 1998). Clearly, vaginal support is an important factor in maintaining urinary continence, but in addition one needs to consider the importance of ISD, a discussion hampered by the lack of adequate techniques to evaluate ISD at present.
Urethral Support Mechanisms
The urethral support mechanism comprises all the structures extrinsic to the urethra that offer a supportive backplate on which the proximal urethra and midurethra lie.
The anatomic support is derived from the following:
In recent years the important potential role of connective tissue in the overall physiologic function of the lower urinary tract and pelvic floor has been progressively recognized. The age-related weakening of connective tissues can influence both tissue and organ function, and it is likely that the increasing attention on connective tissue changes will provide a better understanding of pathophysiology, which hopefully will be useful in leading to the more effective management of stress UI and vaginal prolapse.
Urethral hypermobility (increased mobility) is thought to be due to loss of the normal extrinsic support of the urethra by a weakness of both endopelvic fascia and pelvic floor muscles, usually initiated by childbirth but worsened by aging and alterations of hormone level. Stretching, tearing, and avulsion of the levator muscles results in the urogenital hiatus becoming longer and wider. This results in chronic anterior displacement of pelvic organs with loss of any organ support at rest and especially during straining. Stretching or tearing of the cardinal and utero-sacral ligaments may result in anterior displacement of the uterus at rest or during straining. This results in stretching of the vaginal wall and loss of the normal superior vaginal sulcus and vaginal folds.
The consequence of this is a rotational descent of the proximal urethra away from its retropubic position and the eventual development of SUI.
Certainly in lateral cystourethrograms, the main anatomic change is loss of the posterior urethra-vaginal angle with the urethra and trigone falling into the same plane (Jeffcoate and Roberts, 1952; Hodgkinson, 1953; Hodgkinson, 1970).
Consequently, radiographic studies cannot distinguish between lateral or central defects in vaginal wall support because they appear in the same sagittal plane. It is necessary to examine the patient to determine which defect is present and to what extent. Because the proximal urethra rotates out of the focal plane of ultrasonographic probes or MRI, coronal images of vaginal relaxation cannot provide adequate anatomic information during leakage.
Despite the extensive data about anatomic defects, it is difficult to correlate the influence of these defects, the vaginal position, and the urethral closure mechanism. Not all women with stress incontinence have vaginal prolapse; clearly, prolapse repairs do not always cure the stress incontinence and, conversely, women who redevelop stress incontinence after an apparently successful operation do not always have a recurrence of prolapse (Wall et al, 1994).
In conclusion, support and suspension of the pelvic organs is dependent on a healthy pelvic floor striated muscle, intact robust connective tissue, and their attachment to the bony frame of the pelvis. A useful analogy to visualize these factors is to consider a ship lying moored in dock. One can consider the ligaments as the mooring ropes and the pelvic floor musculature as the water on which the ship sits. If the dock is emptied, the influence of the support of the water disappears, leaving all of the tension applied to the mooring robes, which will inevitably weaken and fracture (Fig. 63–4).
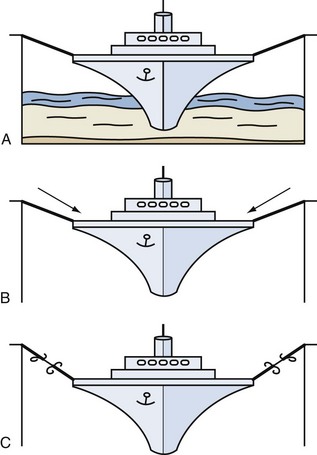
Figure 63–4 Analogy demonstrating the support provided by the A, pelvic floor (water) and ligaments (ropes) to the pelvic organs (ship). B, Consequences of a pelvic floor muscle weakness with increasing strain placed on ligamentous structures. C, Ligamentous damage as a consequence of loss of pelvic floor muscle weakness.
Berglas and Rubin (1953) showed that in the nulliparous patient, the lower one third of the vagina is oriented more vertically, while the upper two thirds deviate horizontally, thereby maintaining the vaginal axis in an almost horizontal position (Fig. 63–5A and B). This configuration is maintained by the posterior attachments of the cervix with the cardinal and utero-sacral ligaments and by the anterior position of the urogenital hiatus. During stressful maneuvers such as coughing or straining, the levator hiatus is shortened anteriorly by contraction of the pubococcygeus muscles (Fig. 63–5C). In the case of genital prolapse when the levator ani support is lost, the vaginal axis becomes more vertical, the urogenital hiatus broadens, and fascial supports are strained (Fig. 63–5D).
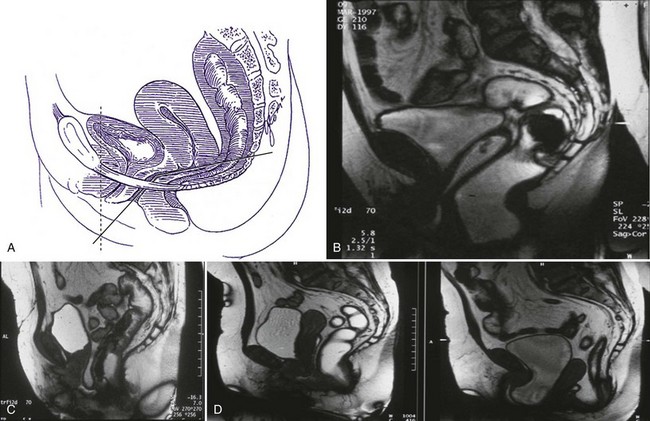
Figure 63–5 A, In the nulliparous patient, the lower one third of the vagina is oriented more vertically, whereas the upper two thirds deviate horizontally, thereby maintaining the vaginal axis in an almost horizontal position. B, In the nulliparous patient, the lower one third of the vagina is oriented more vertically, whereas the upper two thirds deviate horizontally, thereby maintaining the vaginal axis in an almost horizontal position. C, During stressful maneuvers such as coughing or straining, the levator hiatus is shortened anteriorly by contraction of the pubococcygeus muscles. D, In the case of genital prolapse when the levator ani support is lost, the vaginal axis becomes more vertical, the urogenital hiatus broadens, and fascial supports are strained.
Connective Tissue
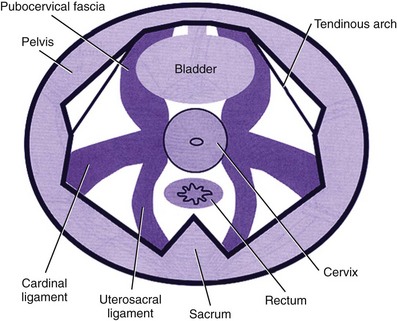
The pubourethral ligament attaches the midurethra to the inferior side of the pubic symphysis and prevents its downward rotational descent (Zacharin, 1963). It works in conjunction with the pubourethralis muscle, a subdivision of the levator ani muscle that forms a sling around the proximal urethra. Together they form the midurethral complex. In particular it has been postulated that an elongation of the posterior pubourethral ligaments may be a significant contributory factor to the loss of urethral support seen in stress incontinence. An important fascial support for the urethra is provided by the urethro-pelvic ligament, which attaches the urethra to the tendineus arc (Rovner et al, 1997).
In addition, the endo-pelvic fascia/pubocervical fascia, extending between the bladder and the vagina, suspends and attaches the vagina and cervix to the pelvic sidewall and to each arcus tendineus fascia pelvis, thereby offering posterior support to the bladder and bladder neck. It has two surfaces: the perivesical fascia on the vaginal side and the endo-pelvic fascia on the abdominal side. Its upper zone supports the bladder above the cervix, the middle zone supports the trigone, and the lower zone supports the bladder neck. Laxity of the fascia in each of the zones will result in uterine prolapse, cystocele, and urethrocele, respectively (DeLancey, 2001).
The arcus tendineus fasciae pelvis are tensile structures located bilaterally on either side of the urethra and vagina that act like the ropes of a suspension bridge and provide the necessary support needed to hang the urethra on the anterior vaginal wall. They originate as fibrous bands from the pubic bone and broaden out as aponeurotic structures moving dorsally to insert into the ischial spine (Fig. 63–6).
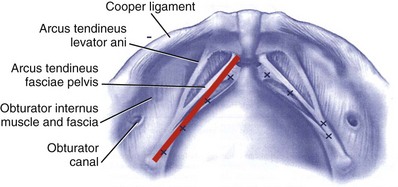
Figure 63–6 The arcus tendineus fasciae pelvis and its relationship to bony structures. The x’s refer to placement of sutures during a paravaginal or vagino–obturator repair.
The cardinal ligaments and the more medially placed utero-sacral ligaments support the uterus and cervix; their relaxation results in uterine prolapse (Fig. 63–7).
Pelvic Floor Musculature
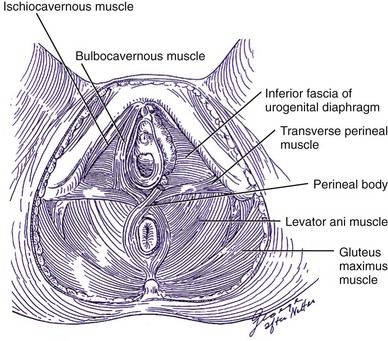
The pelvic floor musculature, represented by the levator ani muscles, carries the weight of the pelvic contents and prevents the abdominal pressure from stretching the ligamentous support structures. The levator ani includes the puborectalis muscle, which surrounds the rectum connecting the pubic bones anteriorly in a U-shaped configuration, the pubococcygeus muscle, which crosses from the pubis to the coccyx, and the iliococcygeus muscle. The last one arises laterally from the arcus tendineus levator ani and forms a horizontal sheet that spans the posterior opening of the pelvis, providing a shelf on which the pelvic organs lie. The urethra and vagina pass through an aperture in the levator musculature, the urogenital hiatus. The levator ani contains not only type I fibers providing resting tone but also type II (fast-twitch) fibers that, under stress, maintain the urethral closure and prevent stretching of the pelvic ligaments.
The constant muscle tone (Critchley et al, 1980) maintained by predominantly type I (slow-twitch) striated muscle fibers compresses the vagina and urethra anteriorly toward the pubic bone and keeps the hiatus closed (DeLancey and Hurd, 1998).
The pudendal nerve provides somatic innervation to the striated muscle of levator ani, as well as to the striated muscle within the external anal and urethral sphincters. The intrapelvic somatic fibers travel along the anterior vaginal wall from the sacral segments S2 to S4 to provide asomatic nerve supply to the pelvic floor (Borirakchanyavat et al, 1997). Neuromuscular injuries have been proposed as an important factor in predisposing to pelvic floor dysfunction. Childbirth and age are the two major factors predisposing to pelvic floor denervation (Smith et al, 1989). Other factors include pelvic surgery (rectal and vaginal surgery with extensive pelvic dissection), radiotherapy, and congenital neurologic conditions such as spina bifida and muscular dystrophy (Fig. 63–8).
Current Theories Relating to the Maintenance of Continence
The traditional view has been that urethral support is critical to the maintenance of continence during increases in abdominal pressure. It was thought that loss of structural urethral support resulted in varying degrees of descent of the bladder neck and urethra, and it was thought that the resultant urethral hypermobility is the principal cause of SUI (Hodgkinson, 1953; Green, 1968; McGuire et al, 1976; Hodgkinson, 1978; Blaivas, 1988; Walters and Jackson, 1990). It was also thought that the normally supported bladder neck and proximal urethra sit in a high retropubic position and that increased intra-abdominal pressure is transmitted equally to both bladder and urethra. In cases of stress-induced urethral descent, the urethra attains a low, dependent position. Increased intra-abdominal pressure is then transmitted unequally to the urethra and bladder, and when bladder pressure exceeds urethral pressure, SUI ensues (McGuire and Herlihy, 1977; Constantinou and Govan, 1982; Westby et al, 1982; Constantinou, 1985; Bump et al, 1988; Rosenzweig et al, 1991). However, the fact that many women are totally continent despite clinical evidence of urethral hypermobility somewhat refutes that theory. This implies that the normal urethra remains closed even during stress-induced descent (Walters and Diaz, 1987).
The most commonly held view of the pathophysiology of stress incontinence secondary to urethral hypermobility is based on DeLancey’s theory of urethral support, the so-called hammock theory (DeLancey, 1994). In anatomic specimens in which increases in abdominal pressure were simulated, he found that the urethra lies in a position where it can be compressed against a hammock-like musculofascial layer on which the bladder and urethra rest (DeLancey, 1994). In this model, it is the stability of this supporting layer rather than the position of the urethra that determines stress continence. Urethral support is supplied by connective tissue and muscle arranged to resist the downward pressure created by increases in abdominal pressure. The urethra is intimately connected to the anterior vaginal wall, and the connections of these two structures to the levator ani muscle complex and arcus tendineus fascia pelvis (ATFP) determine the stability of the urethra. The ATFP is a fibrous fascial band that stretches from the pubic bone anteriorly to the ischial spine. The fascial covering of the levator ani consists of two leaves: the endopelvic fascia (abdominal side) and the pubocervical fascia (vaginal side). The two leaves fuse laterally to attach on to the ATFP, creating a hammock of support under the urethra and bladder neck. Normally, with rises in intra-abdominal pressure, the urethra is compressed against the supporting structures, which act like a backboard and prevent loss of urine (DeLancey, 1997). When the supporting structures fail, there can be rotational descent of the bladder neck and proximal urethra during increases in abdominal pressure. If the urethra opens concomitantly, SUI ensues. If this supporting layer establishes its stability, although at a lower level, continence may still be preserved (DeLancey, 1996). These findings have important clinical implications that support the contention that the primary goal of surgery for sphincteric incontinence in women is to provide a backboard against which the bladder neck and proximal urethra can be compressed during increases in abdominal pressure. In fact, some of the most popular procedures for the treatment of stress incontinence do not change the position of the urethra or even correct mobility (Lo et al, 2001; Sarlos et al, 2003; Minaglia et al, 2005). An additional observation by DeLancey (1990) is that the medial portion of the levator ani muscle has a direct connection to the endopelvic fascia and the vaginal wall, and contraction of the levators contributes to stabilization of the urethra during sudden increases in abdominal pressure (e.g., a cough).
In a series of elegant MRI studies of the pelvic floor, stress incontinence was found to occur when there was unequal movement of the anterior and posterior walls of the bladder neck and proximal urethra during stress. With good anterior wall support and a lax posterior wall, the urethral lumen is literally pulled open as the posterior wall moves away from the anterior wall, resulting in incontinence (Yang et al, 1991; Yang et al, 1993; Mostwin et al, 1995). Conversely, one would expect less incontinence if there is similar laxity of the anterior wall.
Furthermore, ultrasound imaging of the bladder neck and proximal urethra, in both normal and stress-incontinent patients, demonstrated that opening (funneling) of the bladder neck was the common denominator underlying stress incontinence, not urethral hypermobility. Anterior support of the urethra is provided by the pubourethral ligaments, attaching the midurethra to the inferior aspect of the pubic bone. These work in conjunction with the pubourethralis muscle of the levator ani complex, which forms a sling around the proximal urethra and prevents rotational descent (Plzak and Staskin, 2002). Together, the two structures form the midurethral complex, which is now thought to play a significant role in the maintenance of stress continence along with the suburethral hammock described earlier. According to Mostwin and colleagues (1995): “It is the relative, not the absolute, position of the urethra with respect to the pubis that may contribute to urethral opening in stress incontinence. The length and strength of the suburethral complex may vary in patients. In those with shorter, stronger anterior fascial support, only a small loss of suburethral support may be sufficient to permit distraction (of the anterior and posterior urethral walls) during rotational descent.” In contrast to the explanation that proposes a pure transmitted pressure effect, the mechanism of shear force suggests that transmitted energy mechanically opens the proximal and middle urethra (Plzak and Staskin, 2002).
The exact point of continence in the urethra, if such a point exists, has been debated. For years it was thought to be the bladder neck; however, the work of Constantinou and Govan (1981) and Petros and Ulmstem (1990, 1998) suggested that the midurethra supported anteriorly by the pubourethral ligaments is the most important zone of continence. Constantinou and Govan (1981) showed that pressure change ratios in the urethra/bladder are highest at the midurethra in continent control patients and in postsurgical patients after endoscopic bladder neck suspension, whereas the high midurethral pressure is lost in women with stress incontinence. In 1990 Petros and Ulmstem published the integral theory of stress and urge incontinence. According to the theory, female stress incontinence and urgency incontinence have a common etiology. They proposed that support of the anterior vaginal wall is provided by three separate but synergistic mechanisms. The anterior pubococcygeus muscle lifts the anterior vaginal wall to compress the urethra; the bladder neck is closed by traction of the underlying vaginal wall in a backward and downward fashion; and the pelvic floor musculature, under voluntary control, draws the hammock upward, closing the bladder neck. Overall laxity of the anterior vaginal wall causes dissipation of all of these forces, resulting in stress incontinence. They further suggested that laxity of the anterior vaginal wall causes activation of stretch receptors in the bladder neck and proximal urethra that can trigger an inappropriate micturition reflex, resulting in detrusor overactivity. These theories are not mutually exclusive, and there is probably a least some truth in each. The fact that both bladder neck and midurethral slings have been shown to be successful in treating stress incontinence supports all these theories (Ulmsten et al, 1998; Olsson and Kroon, 1999; Nilsson and Kuuva, 2001; Rezapour and Ulmsten, 2001; Nilsson et al, 2004). Further reports of successful treatment of mixed incontinence with slings lend credence to the integral theory.
In 1980 McGuire and colleagues first introduced the concept of intrinsic sphincteric deficiency (ISD) when, in a retrospective analysis of the results of anti-incontinence surgery, they noted that some women who had undergone multiple retropubic operations had a deficient sphincteric mechanism characterized by an open bladder neck and proximal urethra at rest with minimal or no urethral descent during stress. They stated that ISD denotes an intrinsic malfunction of the urethral sphincter, regardless of its anatomic position. Contemporary theories suggest that all patients with sphincteric incontinence have some degree of ISD. Considerations that support this theory include the fact that the normal urethra is intended to remain closed no matter what the degree of stress or rotational descent. Furthermore, many women with urethral hypermobility remain continent (Versi et al, 1986). Finally, urethral hypermobility and ISD may (and often do) coexist in the same patient. Because urethral hypermobility and ISD often coexist, we believe that they do not necessarily define discrete classes of patients (Nitti and Combs, 1996; Fleischmann et al, 2003). Thus these parameters may be used to characterize incontinence but not necessarily classify patients. Classification systems have been devised to characterize stress incontinence by the presence or absence of urethral mobility, the degree of mobility, and the presence of rotational descent (Green, 1962; McGuire et al, 1976; Blaivas and Olsson, 1988). However, it is recommended that rather than “classifying” stress incontinence, it makes more sense to simply characterize it by two parameters—the degree of urethral mobility and sphincter strength.
Epidemiology of Urinary Incontinence and Prolapse
The authors would like to acknowledge the major contribution made by the International Consultation on Incontinence Committee 1 on Epidemiology of Urinary and Faecal Incontinence and Pelvic Organ Prolapse (Milsom et al, 2009), on which the following section is based.
Background Terminology
Epidemiology is the scientific study of the distribution and determinants of disease in people. The epidemiology and natural history of UI vary greatly between the genders. Causes and risk factors are different. Therefore it is most practical to discuss the epidemiology of incontinence in men and women separately.
Prevalence is defined as the probability of experiencing a symptom or having a condition or a disease within a defined population and at a defined time point. The concept is important for establishing the distribution of the condition in the population and for projecting the need for health and medical services.
Incidence is defined as the probability of developing the condition under study during a defined time period. Incidence is usually reported for a 1-, 2-, or 5-year time interval.
When discussing the epidemiology and impact of incontinence, it is important to distinguish between its prevalence and incidence.
The relative risk (RR) estimates the magnitude of an association between exposure and a condition and indicates the likelihood of having the condition in the exposed group relative to those who are not exposed (e.g., do not have the risk factor). An RR of 1.0 indicates that the rates in the exposed and nonexposed groups are identical and thus that there is no association between the exposure and the condition in that specific dataset. A value greater than 1.0 indicates a positive association or an increased risk. An RR of 2.5 for UI indicates that there is a 2.5 times increased risk or that the persons in question are 250% more likely to have incontinence than those without the risk factor.
The odds ratio (OR) is the odds for having a risk factor in persons with a condition divided by the odds among those without the condition. An OR of 2.5 for UI may be interpreted as meaning that in this sample the odds in favor of having incontinence are 2.5 times higher among those with the risk factor than among those without.
For a condition with high prevalence, like UI or pelvic organ prolapse, OR and RR will not be identical, but in practice the results can be interpreted similarly. Results should always be given with a 95% confidence interval (CI).
Incontinence
UI is a common symptom that may affect women at all ages, and there is a wide range of severity and nature of symptoms. UI is not a life-threatening disease, but the symptoms may seriously influence the physical, psychologic, and social well-being of the affected individuals.
General Comments
Virtually all epidemiologic studies rely on participant self-reporting of UI. Multiple studies have examined the association between self-reported incontinence and clinically demonstrable incontinence and by type of incontinence based on self-reported compared with type based on clinical diagnosis. Although self-reporting and clinical diagnosis are highly correlated, they are inherently different. Sandvik and colleagues (1995) validated diagnostic questions used in a survey against a final diagnosis made by a gynecologist after urodynamic evaluation. The percentage of stress-only incontinence increased from 51% to 77%, and mixed incontinence was reduced from 39% to 11% while the proportion with urgency-only UI remained virtually the same (10% vs. 12%). For public health purposes, self-report has the advantage of reflecting the woman’s experience and allows further characterization of the incontinence by frequency and quantity of urine loss. Despite the uncertainty in determining the type of incontinence, the differences in the epidemiology of urgency and stress incontinence are potentially important given the presumed differences in underlying pathophysiology. Differences in the association of stress and urgency UI (based on self-report) with respect to age, race/ethnicity, and risk factors suggest that questionnaire-based determination of UI type is useful and should be included in all epidemiologic studies of UI (Thom, 1998).
Usually incontinence is defined by the episode frequency (e.g., one or more episodes in the past year or past month or typically leaking at least once a week or daily). UI can also be defined by severity (a combination of frequency and quantity). Probably the most widely used measure of severity is the Sandvik Severity index (Sandvik et al, 1993, 2000), which is calculated by multiplying the reported frequency (four levels) by the amount of leakage (three levels). Incontinence can also be characterized by impact, which is usually assessed either in terms of bothersomeness or by the degree to which it restricts a woman’s activity (e.g., the Incontinence Impact Questionnaire) (Shumaker et al, 1994).
The vast majority of epidemiologic studies of female UI have been conducted in the United States, Canada, Europe, Australia, or Japan. A few additional studies have been reported from other Asian countries. Thus we know little about the prevalence, incidence, and risk factors for UI in Africa or other parts of the developing world.
Prevalence
Estimates of UI prevalence differ for a variety of reasons other than variation in true prevalence:
Prevalence in General Population of Adult Women
A review of 36 general population studies from 17 countries in the 2004 Third ICI edition (Hunskaar, 2005) found that the prevalence estimates for the most inclusive definitions of UI (“ever,” “any,” or “at least once in the past 12 months”) ranged from 5% (Van Oyen and Van Oyen, 2002) to 69% (Swithinbank et al, 1999), with most studies reporting a prevalence of any UI in the range of 25% to 45%. For daily incontinence, prevalence estimates typically range between 5% and 15% for middle-aged and older women (Thomas et al, 1980; Yarnell et al, 1981; Holst and Wilson, 1988; Rekers et al, 1992; Lara and Nacey, 1994; Hannestad et al, 2000). Studies reported since 2004 have added important information on prevalence of incontinence in women younger than 30 and older than 80 years of age, particularly for prevalence of incontinence by type. These studies are consistent with previous studies reporting that older women are more likely to have mixed and urgency incontinence (Diokno et al, 1986; Molander et al, 1990; Roberts et al, 1998; Nuotio et al, 2003), whereas young and middle-aged women generally report stress incontinence (Hording et al, 1986; Holst and Wilson, 1988; Burgio et al, 1991; Samuelsson et al, 1997; Hannestad et al, 2000). Overall, approximately half of all incontinent women are classified as stress incontinent. A smaller proportion are classified as mixed incontinent, and the smallest fraction as urgency incontinent. A recent study that included the entire adult age range by Hannestad and colleagues (2000) demonstrated a fairly regular increase in prevalence of mixed incontinence across the age range and a decrease in prevalence of stress incontinence from the 40- to 49-year-old age group through the 60- to 69-year-old group.
Prevalence in Long-Term Care Facilities
Several studies have documented a higher prevalence of UI in women residing in long-term care facilities compared with community-dwelling women (Ouslander et al, 1982; Sgadari et al, 1997; Hunskaar et al, 1998; Aggazzotti et al, 2000; Saxer et al, 2008). Because UI is associated with dementia, limited mobility, and other comorbid conditions, prevalence of UI is higher in facilities with residents requiring a higher level of care. In addition to being more frequent, UI in long-term care residents tends to be more severe, costly, and more burdensome on caregivers compared with UI in the community (Ouslander and Schnelle, 1995).
Prevalence during Pregnancy and Postpartum
Studies of UI during pregnancy have reported period prevalences of 32% to 64% for all UI and 40% to 59% for stress (including mixed) UI. Period prevalence is higher in parous than in nulliparous women (Dimpfl et al, 1992; Marshall et al, 1998; Morkved and Bo, 1999; Hvidman et al, 2002), whereas new-onset UI (cumulative incidence) during pregnancy is higher in primigravida women (Marshall et al, 1998; Morkved and Bo, 1999; Hvidman et al, 2002). Point prevalence of UI is low in the first trimester, rises rapidly in the second trimester, and increases further, though less rapidly, in the third trimester (Marshall et al, 1998; Morkved and Bo, 1999). Severity of incontinence appears to increase during pregnancy as well (Burgio et al, 1996). Data recently reported from the Norwegian Mother and Child Cohort Study, a large (N = 43,279) population-based study, confirms these observations (Wesnes et al, 2007). In this study the prevalence of stress incontinence from before to during pregnancy rose from 9% to 31% in nulliparous women and from 24% to 42% in parous women. In contrast, mixed incontinence showed a similar rise in both groups (from 6% to 16% and from 8% to 20%, respectively). Urgency incontinence remained virtually unchanged in both groups at less than 5%.
Estimating the postpartum prevalence of UI is complex because in addition to differences in study design, method of ascertainment, and choices of definition of incontinence, prevalence may also depend on the number of previous births, type of delivery, and history of previous incontinence. Nearly all postpartum UI is stress related. Most studies did not provide information on the frequency of leakage or degree of bother. Overall, it appears that any incontinence is reported by 15% to 30% of women during the postpartum year, weekly or greater incontinence is reported by 5% to 10%, and daily UI is reported by less than 5% of women. Excluding women with UI before pregnancy reduced the prevalence of postpartum incontinence by 3% to 4% in the one study reporting separate prevalences for each group (Glazener et al, 2006). Studies that included women delivered by caesarean section and delivered vaginally consistently found a lower prevalence in those delivered by caesarean section. Studies reporting prevalence in women delivered by elective caesarean section and by caesarean section after initiation of labor showed a substantially lower prevalence of postpartum UI in the elective caesarean section group.
Prevalence in Different Racial and Ethnic Groups
Although most epidemiologic studies of UI have been conducted on Caucasian populations in the United States, Canada, and Europe, there are now a substantial number of studies in Asian countries (Herzog and Fultz, 1990; Dimpfl et al, 1992; Burgio et al, 1996; Marshall et al, 1998; Morkved and Bo, 1999; Hvidman et al, 2002; Jumadilova et al, 2005; Anger et al, 2006b; Glazener et al, 2006; Waetjen et al, 2007; Wesnes et al, 2007). Studies have also been conducted on racial/ethnic groups in the United States (Burgio et al, 1991; Fultz et al, 1999; Sampselle et al, 2002; Grodstein et al, 2003; Nygaard et al, 2003; Jackson et al, 2004; Anger et al, 2006a; Danforth et al, 2006; Thom et al, 2006; Waetjen et al, 2007; Dooley et al, 2008; Fenner et al, 2008; Markland et al, 2008, 2009). Because of the substantial variation in prevalence of incontinence between studies, it is best to examine differences by race and ethnicity in the same study. Most striking is the lower prevalence of stress UI in black and Asian compared with white women. In most studies, blacks have half to a quarter the prevalence of stress UI compared with white women but the same or slightly higher prevalence of urgency UI and a slightly lower prevalence of mixed UI. Thus the difference in prevalence of UI between white and black women appears to be almost entirely the result of a markedly lower prevalence of stress UI symptoms in black women. In contrast, the lower prevalence of UI seen in Asian women compared with white women reflects a lower prevalence of both stress and urgency UI. The comparison of prevalences between Hispanic and non-Hispanic white women is complex in that some studies have found a lower prevalence of UI in Hispanic women while others have reported the prevalence to be higher in this group. This heterogeneity may be explained, at least in part, by differences in prevalence among a subpopulation of Hispanic women. Studies such as the SWAN study (Sampselle et al, 2002) that include Hispanic women who are primarily of Caribbean origin have reported lower UI prevalence, whereas those enrolling primarily Mexican-American women have reported higher UI prevalences (Thom et al, 2006; Daneshgari et al, 2008; Dooley et al, 2008). This relationship is further supported by the NHANES data reported by Anger and colleagues, which found prevalences of any UI to be 36% in Mexican-American women and 30% in other Hispanic women (Anger et al, 2006a; Daneshgari et al, 2008).
Key Points: Prevalence of Urinary Incontinence
Potential Risk Factors
Many epidemiologic studies have investigated potential risk factors for UI. The design of most of these studies has been cross sectional using prevalent UI. In cross-sectional studies, the temporal relationship between some potential risk factors such as diet, physical activity, medications, or body weight is ambiguous because the development of UI can lead to changes in behavior. Since 2004, several prospective studies have examined risk factors for incident UI that have allowed for evaluation of the temporal relationship. Associations in all observational studies are subject to potential confounding, necessitating multivariate analysis to estimate the independent association between risk factors and UI. The risk factors of estrogen use and body mass (obesity) have been examined in randomized, controlled trials. With the exception of age, the following risk factors are considered to be at least potentially modifiable.
Key Points: Conditions Predisposing to Urinary Incontinence (Milsom et al, 2009)
Impact and Consequences
The impact of UI is typically assessed by degree of bother or by scales that measure the impact of incontinence on functional status and quality of life. A single question asking patients to report the degree of bother they experience from their incontinence provides an important dimension to characterizing UI in addition to frequency and amount of urine lost. Typically, incontinence that is more severe, of longer duration, and is urgent or mixed is also more bothersome (van der Vaart et al, 2002). Validated scales measure the impact of UI. One of the first scales, the Incontinence Impact Questionnaire (Shumaker et al, 1994), uses 30 items asking whether incontinence limits activities in four domains. Several other similar scales exist, some specific for urgency or stress incontinence or for overactive bladder (Shumaker et al, 1994; Uebersax et al, 1995; Jackson et al, 1996; Wagner et al, 1996; Kelleher et al, 1997; Brown et al, 1999; DuBeau et al, 1999). In addition to severity of UI, interference with sexual activities and incontinence at night is associated with greater reported impact on quality of life (Huang et al, 2006).
Incontinence has been identified as a potential risk factor for falls and nursing home admissions. In a prospective study of more than 6000 older women, women with weekly urgency, but not stress, incontinence were more likely to fall during the 3-year follow-up, adjusting for multiple other factors (adjusted OR = 1.3, 95% CI = 1.1 to 1.4) (Brown et al, 2000). Similarly, in a study of 472 long-term care residents in Germany, UI at baseline predicted falls over the next 12 months after adjusting for a variety of patient factors and comorbid conditions (Kron et al, 2003). UI was the second strongest predictor of falls (a history of falls being first). However, it is possible that both falls and UI are markers for frailty that cannot completely be adjusted for. Whether treatment of UI would reduce the number of falls has not been established.
UI has also been reported as a risk factor for nursing home admissions even after adjusting for multiple comorbid conditions (Thom et al, 1997a, 1997b). However, another study that also adjusted for additional measures of frailty including activities of daily living found that the association became weaker and nonsignificant (Holroyd-Leduc et al, 2004).
UI has substantial, well-documented economic consequences as well. Annual costs of UI are estimated to be more than $20 billion in direct costs (primarily costs of routine care including out-of-pocket costs) (Wilson et al, 2001), which is greater than the annual costs for breast, cervical, uterine, and ovarian cancer combined (Subak et al, 2006). There are also substantial indirect costs from work loss and missed volunteer work (Thom et al, 2005).
Treatment Seeking and Treatment
The proportion of women with any UI in the past 12 months who report having sought medical treatment ranges from 12% (Sampselle et al, 2002) to 53% (Mardon et al, 2006) with about half the studies showing 25% to 30% (Rekers et al, 1992; Hannestad et al, 2002; Hagglund et al, 2003; Hsieh et al, 2008). All studies that have examined treatment seeking by type of incontinence have found that women with urgency or mixed incontinence are more likely to seek treatment than women with stress incontinence (Hannestad et al, 2002; Danforth et al, 2006; Zhu et al, 2008). As expected, the proportion of women seeking treatment is strongly associated with frequency, severity, and bothersomeness, with several studies reporting proportions of 50% to 80% of women with daily, moderate to severe, or bothersome incontinence (Hannestad et al, 2002; Kinchen et al, 2003; Diokno et al, 2004; Gasquet et al, 2006).
Reasons given by people for not seeking help include not regarding incontinence as abnormal or serious, embarrassment, considering incontinence to be a normal part of aging, having low expectations of treatment, and thinking they should cope on their own. Other characteristics such as age, general health, or attitude toward health care may also play a role.
Key Points: Consequences of Urinary Incontinence
Urinary Incontinence in Men
General Comments
Key Points: Urinary Incontinence in Men
Prevalence
A systematic review of 21 studies reported a prevalence of UI in older men ranging from 11% to 34% (median = 17, pooled mean = 22%). In the same review, the prevalence of daily UI in men ranged from 2% to 11% (median = 4%, pooled mean = 5%) (Thom, 1998). A wide definition of UI, older age, inclusion of institutionalized men, and the use of self-reporting methods tend to result in higher prevalence rates (Koyama et al, 1998; Thom, 1998). The wide span of results may be explained by the variation in the population studied, the definition of incontinence used, and the methods used in the surveys.
For any definition of UI, there is a steady increase in prevalence with increasing age.
Incontinence Subtypes
Due to differences in pathologic anatomy and pathophysiology of UI in men and women, there is a different distribution in incontinence subtypes. Recent studies confirmed our previous reports of the predominance of urgency incontinence (40% to 80%), followed by mixed forms of UI (10% to 30%) and stress incontinence (<10%) (Herzog and Fultz, 1990).
The higher percentages of the urgency and mixed types of incontinence are more significant in studies involving older people. In fact, the increasing prevalence of any UI by age in men is largely due to the contribution of urgency incontinence rather than stress incontinence. One study demonstrated an increasing rate of urgency UI from 0.7% between ages 50 and 59 to 2.7% between 60 and 69 to 3.4% for respondents 70 years and older. Stress UI was steady at 0.5%, 0.5%, and 0.1% for these groups, respectively (Ueda et al, 2000). On the other hand, Maral and colleagues (2001) reported increasing prevalence of SUI with age: 0.9% between ages 35 and 44, 1.2% between ages 45 and 54, 3.8% between ages 55 and 64, and 4.9% at age 65 and older.
Most studies report a significant fraction of other/unclassified type of urinary incontinence. One study reported that a majority of men with UI had overflow and functional types of incontinence (Bortolotti et al, 2000), whereas another found constant dribbling in 7% of their respondents (Damian et al, 1998). Terminal dribbling or postvoid dribbling is another type of leakage in men that is difficult to assign to the conventional subtypes of UI. In an Australian survey, 12% of respondents reported frequent terminal dribbling (Sladden et al, 2000).
Severity
When it comes to severity, the distribution in men follows that of the women. Estimates for severe UI in older women tend to be about twice as high as for older men (Herzog and Fultz, 1990).
Race/Ethnicity
Few studies have looked at the impact of race or ethnicity on the prevalence of UI among men. A four-country study presented lower prevalences of reported UI among men from Korea (4%) and France (7%) than in men from Britain (14%) and Denmark (16%) (Boyle et al, 2003). On the other hand, unpublished data from the MESA study did not indicate differences in prevalence among white male respondents compared with African-American respondents.
Incidence and Remission
Literature on the incidence of male UI is scarce. The MESA study (Herzog and Fultz, 1990) found a 1-year incidence rate for men older than 60 years of 9% to 10%. In a population-based survey in the UK among men at least 40 years of age, the 1-year incidence of UI was noted to be 3.8% (McGrother et al, 2004). A review of a health organization database of males at least 65 years old revealed a UI incidence of 23.8 per 1000 person years. Malmsten (1997) analyzed the age of onset of UI for each age cohort. Mean starting age for all men was 63 years. The mean duration was about 8 to 10 years in the cohorts. Substantial remission rates for UI in males were noted by the MESA study, higher among men (27% to 32%) than women (11% to 13%) (Herzog and Fultz, 1990). A similarly high 1-year remission rate of 39.6% was noted among British males (McGrother et al, 2004). One possible explanation for the difference in the published incidence and remission rates in men compared with women is the predominance of urgency type incontinence among men and its close relation to overactive bladder with and without incontinence. Another factor is the close association among urgency UI and prostate gland disease, infections, or bowel dysfunction, all of which are relatively amenable to treatment or may improve even without treatment.
Potential Risk Factors for Urinary Incontinence
There is relatively little research concerning conditions and factors that may be associated with UI in men, and clear risk factors are poorly documented. However, a few available studies have identified potential risk factors, which are described next.
Age
As in women, increasing age is correlated with increasing prevalence of UI. Multivariate analysis in several studies has shown that age is an independent risk factor for incontinence (Muscatello et al, 2001; Boyle et al, 2003; Landi et al, 2003; Nelson and Furner, 2005; Diokno et al, 2007). Compared with women, however, there seems to be a steadier increase in prevalence in men with increasing age.
Lower Urinary Tract Symptoms and Infections
LUTS, like urgency, nocturia, a feeling of incomplete voiding, and reduced flow, are typically associated with UI (Diokno et al, 1986; Hunskaar, 1992; Schmidbauer et al, 2001). In one study, UI was reported by 15% of men without voiding symptoms, frequency, or urgency and by 34% of those with such symptoms (Diokno et al, 1986).
Studies have also reported that urinary tract infections and cystitis are strongly associated with male UI (Damian et al, 1998; Ueda et al, 2000), with an OR of 3.7 for UI in men reporting cystitis (Ueda et al, 2000) and an OR of 12.5 among men with recurrent infections (Bortolotti et al, 2000). It should be noted that reports indicating a positive association between UTI and incontinence involved men older than 60 years.
Functional and Cognitive Impairment, Physical Activity
Mobility problems such as use of a wheelchair or aids to walking, as well as diagnosed arthritis or rheumatism or having a fall during the past year, were significantly greater among incontinent than continent men (Diokno et al, 1990; Damian et al, 1998).
Neurologic Disorders
Many specific neurologic diseases may lead to UI (Resnick and Yalla, 1987). Neurogenic detrusor overactivity is seen commonly in meningo-myelocele patients and in spinal injuries, Parkinson disease, and multiple sclerosis. Underactive bladder dysfunction due to a cauda equina lesion or diabetes might cause overflow or a paralyzed pelvic floor and hence stress incontinence. Men who have suffered a stroke were at increased risk for incontinence with an OR of 7.1 (Ueda et al, 2000). A case control study of age-matched, long-term stroke male survivors with controls showed a higher prevalence of UI among stroke survivors compared with controls (17% vs. 9%). In addition, among UI sufferers, the stroke survivors were found to have higher frequency and more leakage than controls. In a study of 235 stroke patients, the occurrence of UI correlated with motor weakness (OR 5.4), visual defects (OR 4.8), and dysphagia (OR 4.0).
Prostatectomy
A well-known iatrogenic cause of male incontinence is prostatectomy, but the attributable risk for this factor in the population of men with UI is unknown. In a Norwegian survey of elderly men with UI, almost a third had undergone prostatectomy (Hunskaar, 1992).
In a cross-sectional study among men in Vienna, Schmidbauer and colleagues identified previous prostatectomy to be associated with UI (Schmidbauer et al, 2001).
TURP seems to be followed by an incidence of stress incontinence of about 1%. A randomized controlled trial comparing TURP, laser prostatectomy, and evaporization of the prostate for benign disease showed comparable incontinence rates immediately and up to 12 months postoperatively (van Melick et al, 2003).
Radical prostatectomy seems to induce UI at a much higher rate than TURP. The overall prevalence of postradical prostatectomy incontinence ranges from 2% to nearly 60%. This wide range may be explained by many factors including differences in study characteristics, population characteristics, study site, definition used, and timing of assessment of continence in relation to the surgery. The era of development of the procedure has also been found to be associated with the prevalence rates (Hu et al, 2003), as well as the various procedural modifications of the surgery (see later). Postprostatectomy incontinence rates elicited from symptoms reported by patients are generally two to three times higher than those from physicians’ observations. Studies that have performed both assessments in the same population confirm this observation that doctors underestimate postprostatectomy incontinence by as much as 75% (Ojdeby et al, 1996; Donnellan et al, 1997; McKammon et al, 1997; Olsson et al, 2001).
Incontinence rates after prostatectomy seem to decline steadily with time and plateau 1 to 2 years after surgery (Lowe, 1996; Weldon et al, 1997; Goluboff et al, 1998; Horie et al, 1999; Walsh et al, 2000; Moinzadeh et al, 2003). This emphasizes the need for a long follow-up period to establish continence status postprostatectomy. An actuarial study among 647 postprostatectomy men estimated UI rate of 13% at 1 year and 7% at 2 years postsurgery (Saranchuk et al, 2005).
The technique of radical prostatectomy affects UI rates. Modifications associated with lower UI rates include the perineal approach (Bishoff et al, 1998; Gray et al, 1999) and preservation of neurovascular bundle (Eastham et al, 1996; Van Kampen et al, 1998; Wei et al, 2000). Bladder neck preservation affords earlier return to continence compared with bladder neck resection, but with similar UI rates after 1 year (Poon et al, 2000; Srougi et al, 2001). One study showed earlier recovery of UI after tennis racquet reconstruction and bladder neck preservation compared with bladder neck resection with puboprostatic ligament preservation, but with similar UI rates at 1 year (Lowe, 1996; Noh et al, 2003). However, continence status assessed more than 12 months after surgery showed even lower rates of UI after bladder neck preservation (11.6%) compared with resection (4.9%) in one study (Lowe, 1996). UI after laparoscopic procedures seems to be higher initially but approximates that of open techniques by 1 year (Jacobsen et al, 2007).
Older age at time of surgery has been found to be associated with a higher prevalence of postprostatectomy UI (Eastham et al, 1996; Goluboff et al, 1998; Van Kampen et al, 1998; Gray et al, 1999; Wei et al, 2000; Kundu et al, 2004). One study showed a doubled risk for every 10 years of age beginning at age 40 (Catalona et al, 1999). Another study suggested that rather than absolutely affecting final continence prevalence, elderly men need a longer time to achieve continence after surgery (Horie et al, 1999). Two studies found no relation between age at surgery and UI (Donnellan et al, 1997; Kao et al, 2000).
Other factors have been found to be associated with a higher prevalence of postprostatectomy UI, although not consistently. Such factors include prior TURP, preoperative lower urinary tract symptoms, obesity, clinical stage, prostate-specific antigen (PSA), prostate volume, and Gleason score (Eastham et al, 1996; Moul et al, 1998; Van Kampen et al, 1998; Kundu et al, 2004; Lee et al, 2006). A retrospective analysis of 156 patients who had undergone preoperative MRI of the prostate and were followed up postprostatectomy showed that time to return to continence was associated with the variation in the shape of the prostatic apex. The prostatic apex that does not overlap with the membranous urethra was found to be significantly associated with an early return of continence (Lee et al, 2006). The 5-year cohort study of the Prostate Cancer Outcomes Study found that among prostatectomy patients, race and ethnic differences were related to urinary incontinence, with African-Americans having better recovery compared with non-Hispanic whites and Hispanics (Johnson et al, 2004).
Adjuvant radiotherapy has not been found to affect postprostatectomy incontinence rates when assessed beyond 1 year (Formenti et al, 2000; Hofmann et al, 2003; Kundu et al, 2004).
Factors of Unclear Association with Urinary Incontinence in Men
A 9-year study of Janssen (2007) showed increasing rates of UI with increasing body mass index (BMI) among the older men and women. However, multivariate analysis failed to show increased BMI (overweight and obese levels) as an independent risk factor for the development of UI. In a study including a younger population in Australia, obesity was noted to be associated with UI with an OR of 3.2 (1.2 to 9.0) (Muscatello et al, 2001). In this study, however, being merely overweight was not associated with UI. Several studies in the older persons have shown an association between physical activity and UI among women that is not seen among men (Boyle et al, 2003; Kikuchi et al, 2007).
Key Points: Epidemiology of Male Incontinence
Epidemiology of Overactive Bladder
The studies reported in the literature have used different populations and ways of selecting samples, and the definition of OAB has changed slightly after its initial description, although this was standardized after the 2002 ICS standardization report.
The prevalence of OAB in adult males varies from 10.2% to 17.4% and in females from 7.7% to 31.3%. Clearly different definitions, the different age groups studied and maybe cultural differences affect the rates (level 2 evidence). OABwet was less prevalent than OABdry except for one implausible-looking result. The response rate was quite low in many of these studies, and sometimes it was impossible to know the response rate (e.g., when quota sampling was used).
A common finding was that the rates increased with age, but this was not universal (level 2 evidence). Some studies found that after adjusting for state of health, the relationship with age went away, and some found that it only applied to OABwet. Usually OAB was more prevalent in females, and this was much more pronounced for OABwet, which sometimes meant that OABdry was the same for males and females. OAB was related to general health problems and in particular diabetes. It is likely to be related to urinary problems in childhood.
Epidemiology of Pelvic Organ Prolapse
General Comments and Definitions
Pelvic organ prolapse (POP) refers to loss of support for uterus, bladder, colon, or rectum leading to prolapse of one or more of these organs into the vagina. The ICS Pelvic Organ Prolapse Quantification (POPQ) examination defines prolapse by measuring the descent of specific segments of the reproductive tract during Valsalva strain relative to a fixed point, the hymen. As noted earlier, the POPQ system describes the anatomic findings of pelvic organ prolapse without consideration for symptoms and bother perceived by the woman. Validation of this system has shown it to be highly reliable (Hall et al, 1996). The stages of prolapse severity are arbitrarily defined, and there is no clear differentiation between normal anatomic variation and mild POP. For research purposes there is consensus for use of the POPQ system until further evidence might clarify the distinction between normal variation and mild prolapse (Weber et al, 2001).
Determining POP on the basis of self-reported symptoms is difficult because of the lack of specificity and sensitivity of most symptoms attributed to pelvic organ prolapse (Ellerkmann et al, 2001) and the fact that prolapse above the level of the hymenal ring is usually asymptomatic (Lukacz et al, 2005). The only exception appears to be a sensation of bulging into the vagina, which is most strongly associated with prolapse at or below the hymeneal ring (Swift, 2000; Hendrix et al, 2002). A recent study of 110 women found that a question asking about a feeling of something bulging in or dropping out of their vagina had a sensitivity of 84% and a specificity of 94% for POP at or beyond the hymeneal ring on examination (Lukacz et al, 2005). Seeing prolapse would presumably be even more specific but is too uncommon to be useful as a definition.
Prevalence of PELVIC ORGAN PROLAPSE
The prevalence of POP based on a sensation of a mass bulging into the vagina was remarkably consistent, ranging between 5% and 10%. The study by Eva and colleagues (2003) reported a substantially higher prevalence included in the definition of POP, pelvic heaviness, or digital pressure on the perineum or in the vagina to aid with defecation. Reports from the Women’s Health Initiative (WHI) Oestrogen Plus Progestin Trial and randomized controlled trial (Handa et al, 2004; Nygaard et al, 2004; Bradley et al, 2007), although not actually population based, recruited the women in the trial from the community rather than from women seeking gynecologic care and provided important information on the prevalence of POP based on pelvic examination. The prevalence of observed prolapse in women enrolled in the WHI trial is similar to the prevalence found in the population-based study that also used pelvic examination (Samuelsson et al, 1999), although the prevalence of each type of prolapse was higher in the WHI study (Hendrix et al, 2002). In both studies, prolapse occurs most frequently in the anterior compartment, next most frequently in the posterior compartment, and least in the apical compartment.
Two studies that examined prolapse by race found that black women had the lowest prevalence and Hispanic women the highest after controlling for multiple other factors in multivariate analysis (Hendrix et al, 2002; Rortveit et al, 2007). The study reported by Rortveit and colleagues (2007) based on symptoms found adjusted ORs of 0.4 (95% CI = 0.2 to 0.8) for black and 1.3 (95% CI = 0.8 to 2.2) for Hispanic women, with white women as the referent group. Hendrix and colleagues (2002) reported adjusted ORs of 0.6 (95% CI = 0.5 to 0.8) for black and 1.2 (95% CI = 1.0 to 1.5) for Hispanic women compared with white women for POP based on genital examination.
Incidence
Only two studies that reported the incidence of new POP could be located. Both studies were done on subgroups of women enrolled in the WHI Oestrogen Plus Progestin Trial. The first study of 412 women enrolled at the University of California, Davis site used a standardized pelvic examination repeated every 2 years over 8 years (Handa et al, 2004). The incidence of new cystocele, rectocele, and uterine prolapse was 9%, 6%, and 2%, respectively. Annual rates of remission from grade 1 (prolapse to above introitus) were relatively common for each type of POP (24%, 22%, and 48%, respectively) but less common from grade 2 or 3 (prolapse to or beyond introitus) (9%, 3%, and 0%, respectively). In a second study of 259 women, postmenopausal women with a uterus were examined using the POP-Q at baseline and annually for 3 years. POP was defined as prolapse to or beyond the hymeneal ring. The incidence of new POP was 26% at 1 year and 40% at 3 years, with remission rates of 21% at 1 year and 19% at 3 years (Bradley et al, 2007).
Several studies have reported the annual incidence of surgery for POP in the United States and at least one in the United Kingdom. A longitudinal study of more than 17,000 women in the United Kingdom, age 25 to 39 at baseline, reported an annual rate of prolapse surgery of 0.16% (Mant et al, 1997). This rate is consistent with the rate of approximately 0.2% per year reported in the United States (Brown et al, 2002; Boyles et al, 2003). One U.S. study reported an annual incidence rising with age from 0.05% in women age 30 to 39 to 0.5% in women age 70 to 79 with an estimated lifetime cumulative risk of surgery from prolapse of 7% to 11% (Olsen et al, 1997). A recent U.S. study reported similar surgical rates: 0.07% for women age 18 to 39, 0.24% for women age 40 to 59, and 0.31% for women age 60 to 79 (Shah et al, 2008). Surgical rates drop substantially after age 80 (Olsen et al, 1997; Shah et al, 2008). Estimating rates of prolapse surgery has the advantage of use of hospital discharge data on procedures, which is highly accurate for the procedure performed but less accurate for the indications for the procedures, particularly when a procedure may have more than one indication.
Key Points: Epidemiology of Pelvic Organ Prolapse
Abrams P, Artibani W, Cardozo L, et alInternational Continence Society. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28(4):287.
Abrams P, Cardozo L, Fall M, et alStandardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178.
. Incontinence 4th International Consultation on Incontinence. Abrams P, Cardozo L, Khoury S, Wein A. Health Publication Ltd, United Kingdom, 2009. ISBN 0-9546956-8-2
Chapple CR, MacDiarmid SA, Patel A. Urodynamics made easy, 3rd ed. Oxford (UK): Churchill: Livingstone Elsevier; 2009.
Chapple CR, Wein AJ, Abrams P, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol. 2008 Sep;54(3):563-569.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713-1720.
DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15:268-274.
International Continence Society. Documents and forums. icsoffice.org/documents/documents.aspx?FolderID=3, 2011. [accessed 05.06.11]
Petros PP, Ulmsten U. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7-31.
Schafer W, Abrams P, Limin L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261-274.
Turner Warwick R, Whiteside G. Symposium on clinical urodynamics. Urol Clin North Am. 1979;6:1-293.
Wein AJ. Classification of neurogenic voiding dysfunction. J Urol. 1981;125:605-609.
Abrams P. ICS standardization documents. http://www.icsoffice.org/ASPNET_Membership/Membership/Documents/Documents.aspx. accessed 2002
Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28:287.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Aggazzotti G, Pesce F, Grassi D, et al. Prevalence of urinary incontinence among institutionalized patients: a cross-sectional epidemiologic study in a midsized city in northern Italy. Urology. 2000;56:245-249.
Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology. 2004;63(5):819-822.
Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770-779.
Anastasiadis AG, Salomon L, Katz R, et al. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003;62(2):292-297.
Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581-631.
Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: results from the National Health and Nutrition Examination Survey. J Urol. 2006;175:601-604.
Anger JT, Saigal CS, Pace J, et al. True prevalence of urinary incontinence among female nursing home residents. Urology. 2006;67:281-287.
Awad SA, Mcginnis RH. Factors that influence the incidence of detrusor instability in women. J Urol. 1983;130:114-115.
Bader P, Hugonnet CL, Burkhard FC, Studer UE. Inefficient urethral milking secondary to urethral dysfunction as an additional risk factor for incontinence after radical prostatectomy. J Urol. 2001;166(6):2247-2252.
Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144.
Berglas B, Rubin I.C. Study of the supportive structures of the uterus by levator myography. Surg Gynecol Obstet. 1953;97:677-692.
Bergman A, Koonings PP, Ballard CA. Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol. 1988;158:1171-1175.
Bishoff JT, Motley G, Optenberg SA, et al. Incidence of fecal and urinary incontinence following radical perineal and retropubic prostatectomy in a national population. J Urol. 1998;160:454-458.
Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958-963.
Blaivas JG. Techniques of evaluation. In: Yalla SV, editor. Neurourology and urodynamics. New York: Macmillan, 1988.
Blaivas JG, Barbalias GA. Characteristics of neural injury after abdominoperineal resection. J Urol. 1983;129:84-87.
Blaivas JG, Olsson CA. Stress incontinence: classification and surgical approach. J Urol. 1988;139:727-731.
Borirakchanyavat S, Aboseif SR, Carroll PR, et al. Continence mechanism of the isolated female urethra: an anatomical study of the intrapelvic somatic nerves. J Urol. 1997;158:822-826.
Bortolotti A, Bernardini B, Colli E, et al. Prevalence and risk factors for urinary incontinence in Italy. Eur Urol. 2000;37:30-35.
Boyle P, Robertson C, Mazzetta C, et al. The prevalence of male urinary incontinence in four centres: the UREPIK study. BJU Int. 2003;92:943-947.
Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol. 2003;188:108-115.
Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994;73:3-8.
Bradley CS, Zimmerman MB, Qi Y, Nygaard I.E. Natural history of pelvic organ prolapse in postmenopausal women. Obstet Gynecol. 2007;109:848-854.
Brown JS, Posner SF, Stewart AL. Urge incontinence: new health-related quality of life measures. J Am Geriatr Soc. 1999;47:980-988.
Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
Brown JS, Waetjen LE, Subak LL, et al. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;186:712-716.
Brown S, Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol. 1998;105:156-161.
Bump RC, Fantl JA, Hurt WG. Dynamic urethral pressure profilometry pressure transmission ratio determinations after continence surgery: understanding the mechanism of success, failure, and complications. Obstet Gynecol. 1988;72:870-874.
Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
Burgio KL, Locher JL, Zyczynski H, et al. Urinary incontinence during pregnancy in a racially mixed sample: characteristics and predisposing factors. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:69-73.
Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. J Urol. 1991;146:1255-1259.
Burkhard FC, Kessler TM, Fleischmann A, et al. Nerve sparing open radical retropubic prostatectomy—does it have an impact on urinary continence? J Urol. 2006;176(1):189-195.
Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. J Urol. 1998;160:1301-1306.
Cardozo LD, Stanton SL. Genuine stress incontinence and detrusor instability—a review of 200 patients. Br J Obstet Gynaecol. 1980;87:184-190.
Carlson KV, Rome S, Nitti VW. Dysfunctional voiding in women. J Urol. 2001;165:143-147. discussion 147–8
Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433-438.
Chaikin DC, Rosenthal J, Blaivas JG. Pubovaginal fascial sling for all types of stress urinary incontinence: long-term analysis. J Urol. 1998;160:1312-1316.
Chang PL, Fan HA. Urodynamic studies before and/or after abdominoperineal resection of the rectum for carcinoma. J Urol. 1983;130:948-951.
Chapple CR, Helm CW, Blease S, et al. Asymptomatic bladder neck incompetence in nulliparous females. Br J Urol. 1989;64:357-359.
Chapple CR, Wein AJ, Abrams P, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol. 2008;54:563-569.
Churchill BM, Gilmour RF, Williot P. Urodynamics. Pediatr Clin North Am. 1987;34:1133-1157.
Coakley FV, Eberhardt S, Kattan MW, et al. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002;168(3):1032-1035.
Comiter CV, Sullivan MP, Schacterle RS, et al. Urodynamic risk factors for renal dysfunction in men with obstructive and nonobstructive voiding dysfunction. J Urol. 1997;158:181-185.
Constantinou CE. Resting and stress urethral pressures as a clinical guide to the mechanism of continence in the female patient. Urol Clin North Am. 1985;12:247-258.
Constantinou CE, Govan DE. Contribution and timing of transmitted and generated pressure components in the female urethra. Prog Clin Biol Res. 1981;78:113-120.
Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol. 1982;127:964-969.
Critchley HO, Dixon JS, Gosling JA. Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int. 1980;35:226-232.
Cruz F. Vanilloid receptor and detrusor instability. Urology. 2002;59:51-60.
Damian J, Martin-Moreno JM, Lobo F, et al. Prevalence of urinary incontinence among Spanish older people living at home. Eur Urol. 1998;34:333-338.
Daneshgari F, Imrey PB, Risendal B, et al. Differences in urinary incontinence between Hispanic and non-Hispanic white women: a population-based study. BJU Int. 2008;101:575-579.
Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339-345.
DeLancey JO. Anatomy and physiology of urinary continence. Clin Obstet Gynecol. 1990;33:298-307.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713-1720. discussion 1720–3
DeLancey JO. Stress urinary incontinence: where are we now, where should we go? Am J Obstet Gynecol. 1996;175:311-319.
DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15:268-274.
DeLancey JO. Anatomy. In: Cardozo L, Staskin D, editors. Textbook of female urology and urogynaecology. London Florence (KY): Isis Medical Media, Distributed in the USA by Fulfilment Center, Taylor & Francis, 2001.
DeLancey JO, Hurd WW. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet Gynecol. 1998;91:364-368.
Dimpfl T, Hesse U, Schussler B. Incidence and cause of postpartum urinary stress incontinence. Eur J Obstet Gynecol Reprod Biol. 1992;43:29-33.
Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022-1025.
Diokno AC, Brock BM, Herzog AR, Bromberg J. Medical correlates of urinary incontinence in the elderly. Urology. 1990;36:129-138.
Diokno AC, Burgio K, Fultz NH, et al. Medical and self-care practices reported by women with urinary incontinence. Am J Manag Care. 2004;10:69-78.
Diokno AC, Estanol MV, Ibrahim IA, Balasubramaniam M. Prevalence of urinary incontinence in community dwelling men: a cross sectional nationwide epidemiological survey. Int Urol Nephrol. 2007;39:129-136.
Donnellan SM, Duncan HJ, Macgregor RJ, Russell JM. Prospective assessment of incontinence after radical retropubic prostatectomy: objective and subjective analysis. Urology. 1997;49:225-230.
Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: results from the National Health and Nutrition Examination Survey. J Urol. 2008;179:656-661.
DuBeau CE, Kiely DK, Resnick NM. Quality of life impact of urge incontinence in older persons: a new measure and conceptual structure. J Am Geriatr Soc. 1999;47:989-994.
Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707-1713.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal versus impaired contractility. J Urol. 1993;150:1657-1667.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J Urol. 1993;150:1668-1680.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. IV. Bladder outlet obstruction. J Urol. 1993;150:1681-1695.
Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337. discussion 1337–8
Enhorning G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta Chir Scand Suppl. 1961;Suppl 276:1-68.
Eva UF, Gun W, Preben K. Prevalence of urinary and fecal incontinence and symptoms of genital prolapse in women. Acta Obstet Gynecol Scand. 2003;82:280-286.
Fantl JA, Wyman JF, Mcclish DK, Bump RC. Urinary incontinence in community-dwelling women: clinical, urodynamic, and severity characteristics. Am J Obstet Gynecol. 1990;162:946-951. discussion 951–2
Fenner DE, Trowbridge ER, Patel DA, et al. Establishing the prevalence of incontinence study: racial differences in women’s patterns of urinary incontinence. J Urol. 2008;179:1455-1460.
Ficazzola MA, Nitti VW. The etiology of post-radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol. 1998;160(4):1317-1320.
Field SM, Hilton P. The prevalence of sexual problems in women attending for urodynamic investigation. Int Urogynecol J. 1993;4:212-215.
Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;169:999-1002.
Formenti SC, Lieskovsky G, Skinner D, et al. Update on impact of moderate dose of adjuvant radiation on urinary continence and sexual potency in prostate cancer patients treated with nerve-sparing prostatectomy. Urology. 2000;56:453-458.
Fowler CJ. Bladder afferents and their role in the overactive bladder. Urology. 2002;59:37-42.
Fowler CJ, Griffiths D, De Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453-466.
Fultz NH, Herzog AR. Measuring urinary incontinence in surveys. Gerontologist. 1993;33:708-713.
Fultz NH, Herzog AR. Prevalence of urinary incontinence in middle-aged and older women: a survey-based methodological experiment. J Aging Health. 2000;12:459-469.
Fultz NH, Herzog AR, Raghunathan TE, et al. Prevalence and severity of urinary incontinence in older African American and Caucasian women. J Gerontol A Biol Sci Med Sci. 1999;54:M299-M303.
Gasquet I, Tcherny-Lessenot S, Gaudebout P, et al. Influence of the severity of stress urinary incontinence on quality of life, health care seeking, and treatment: a national cross-sectional survey. Eur Urol. 2006;50:818-825.
Gerstenberg TC, Nielsen ML, Clausen S, et al. Bladder function after abdominoperineal resection of the rectum for anorectal cancer. Urodynamic investigation before and after operative in a consecutive series. Ann Surg. 1980;191:81-86.
Giannantoni A, Mearini E, Di Stasi SM, et al. Assessment of bladder and urethral sphincter function before and after radical retropubic prostatectomy. J Urol. 2004;171(4):1563-1566.
Glazener CM, Herbison GP, Macarthur C, et al. New postnatal urinary incontinence: obstetric and other risk factors in primiparae. BJOG. 2006;113:208-217.
Goluboff ET, Saidi JA, Mazer S, et al. Urinary continence after radical prostatectomy: the Columbia experience. J Urol. 1998;159:1276-1280.
Gosling JA, Dixon JS, Critchley HO, Thompson SA. A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol. 1981;53:35-41.
Gray M, Petroni GR, Theodorescu D. Urinary function after radical prostatectomy: a comparison of the retropubic and perineal approaches. Urology. 1999;53:881-890. discussion 890–1
Green THJr. Development of a plan for the diagnosis and treatment of urinary stress incontinence. Am J Obstet Gynecol. 1962;83:632-648.
Green THJr. The problem of urinary stress incontinence in the female: an appraisal of its current status. Obstet Gynecol Surv. 1968;23:603-634.
Grodstein F, Fretts R, Lifford K, et al. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428-434.
Groutz A, Blaivas JG, Chaikin DC, et al. The pathophysiology of post-radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol. 2000;163(6):1767-1770.
Haab F, Zimmern PE, Leach GE. Female stress urinary incontinence due to intrinsic sphincteric deficiency: recognition and management. J Urol. 1996;156:3-17.
Haase P, Skibsted L. Influence of operations for stress incontinence and/or genital descensus on sexual life. Acta Obstet Gynecol Scand. 1988;67:659-661.
Hadley HR, Zimmern PE, Raz S. The treatment of male urinary incontinence. In Campbell MF, Walsh PC, editors: Campbell’s urology, 5th ed, Philadelphia: Saunders, 1986.
Hagglund D, Walker-Engstrom ML, Larsson G, Leppert J. Reasons why women with long-term urinary incontinence do not seek professional help: a cross-sectional population-based cohort study. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:296-304. discussion 304
Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. Am J Obstet Gynecol. 1996;175:1467-1470. discussion 1470–1
Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997;157(1):233-236.
Handa VL, Garrett E, Hendrix S, et al. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27-32.
Handa VL, Harris TA, Ostergard DR. Protecting the pelvic floor: obstetric management to prevent incontinence and pelvic organ prolapse. Obstet Gynecol. 1996;88:470-478.
Hannestad YS, Rortveit G, Hunskaar S. Help-seeking and associated factors in female urinary incontinence. The Norwegian EPINCONT Study. Epidemiology of Incontinence in the County of Nord-Trondelag. Scand J Prim Health Care. 2002;20:102-107.
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150-1157.
Harris MJ. Radical perineal prostatectomy: cost efficient, outcome effective, minimally invasive prostate cancer management. Eur Urol. 2003;44(3):303-308.
Hebjorn S, Andersen JT, Walter S, Mouritzen Dam A. Detrusor hyperreflexia. A survey on its etiology and treatment. Scand J Urol Nephrol. 1976;10:103-109.
Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160-1166.
Herzog AR, Fultz NH. Epidemiology of urinary incontinence: prevalence, incidence and correlates in community populations. Urology Suppl. 36, 1990.
Herzog AR, Fultz NH. Prevalence and incidence of urinary incontinence in community-dwelling populations. J Am Geriatr Soc. 1990;38:273-281.
Hodgkinson CP. Relationships of the female urethra and bladder in urinary stress incontinence. Am J Obstet Gynecol. 1953;65:560-573.
Hodgkinson CP. Stress urinary incontinence—1970. Am J Obstet Gynecol. 1970;108:1141-1168.
Hodgkinson CP. Metallic bead-chain urethrocystographyin preoperative and postoperative evaluation of gynecologic urologic problems. Clin Obstet Gynecol. 1978;21:725-735.
Hofmann T, Gaensheimer S, Buchner A, et al. An unrandomized prospective comparison of urinary continence, bowel symptoms and the need for further procedures in patients with and with no adjuvant radiation after radical prostatectomy. BJU Int. 2003;92:360-364.
Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. J Am Geriatr Soc. 2004;52:712-718.
Holst K, Wilson PD. The prevalence of female urinary incontinence and reasons for not seeking treatment. N Z Med J. 1988;101:756-758.
Hording U, Pedersen KH, Sidenius K, Hedegaard L. Urinary incontinence in 45-year-old women. An epidemiological survey. Scand J Urol Nephrol. 1986;20:183-186.
Horie S, Tobisu KI, Fujimoto H, et al. Urinary incontinence after non-nerve-sparing radical prostatectomy with neoadjuvant androgen deprivation. Urology. 1999;53:561-567.
Hsieh CH, Su TH, Chang ST, et al. Prevalence of and attitude toward urinary incontinence in postmenopausal women. Int J Gynaecol Obstet. 2008;100:171-174.
Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CaPSURE. J Urol. Feb 2004;171(2 Pt. 1):703-707.
Hu JC, Gold KF, Pashos CL, et al. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169:1443-1448.
Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
Huang AJ, Brown JS, Kanaya AM, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med. 2006;166:2000-2006.
Hunskaar S. One hundred and fifty men with urinary incontinence. I. Demography and medical history. Scand J Prim Health Care. 1992;10:21-25.
Hunskaar S. Committee 1 on Epidemiology of Urinary and Faecal Incontinence and Pelvic Organ Prolapse. In Abrams P, editor: Incontinence (edition 2005) 4th International Consultation on Incontinence, 4th ed, Health Publications Ltd, 2005.
Hunskaar S, Ostbye T, Borrie MJ. Prevalence of urinary incontinence in elderly Canadians with special emphasis on the association with dementia, ambulatory function, and institutionalization. Norwegian J Epidemiol. 1998;8:177.
Hvidman L, Foldspang A, Mommsen S, Bugge Nielsen J. Correlates of urinary incontinence in pregnancy. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:278-283.
Jackson RA, Vittinghoff E, Kanaya AM, et al. Urinary incontinence in elderly women: findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004;104:301-307.
Jackson S, Donovan J, Brookes S, et al. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77:805-812.
Jackson SL, Weber AM, Hull TL, et al. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89:423-427.
Jacobsen NE, Moore KN, Estey E, Voaklander D. Open versus laparoscopic radical prostatectomy: a prospective comparison of postoperative urinary incontinence rates. J Urol. 2007;177:615-619.
Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring). 2007;15:1827-1840.
Jeffcoate TN, Roberts H. Observations on stress incontinence of urine. Am J Obstet Gynecol. 1952;64:721-738.
John H, Sullivan MP, Bangerter U, et al. Effect of radical prostatectomy on sensory threshold and pressure transmission. J Urol. 2000;163(6):1761-1766.
Johnson TK, Gilliland FD, Hoffman RM, et al. Racial/Ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. J Clin Oncol. 2004;22:4193-4201.
Jonler M, Madsen FA, Rhodes PR, et al. A prospective study of quantification of urinary incontinence and quality of life in patients undergoing radical retropubic prostatectomy. Urology. 1996;48(3):433-440.
Jumadilova Z, Zyczynski T, Paul B, Narayanan S. Urinary incontinence in the nursing home: resident characteristics and prevalence of drug treatment. Am J Manag Care. 2005;11:S112-S120.
Kao TC, Cruess DF, Garner D, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol. 2000;163:858-864.
Kayigil O, Iftekhar Ahmed S, Metin A. The coexistence of intrinsic sphincter deficiency with type II stress incontinence. J Urol. 1999;162:1365-1366.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104:1374-1379.
Khan Z, Mieza M, Starer P, Singh VK. Post-prostatectomy incontinence. A urodynamic and fluoroscopic point of view. Urology. 1991;38(5):483-488.
Kikuchi A, Niu K, Ikeda Y, et al. Association between physical activity and urinary incontinence in a community-based elderly population aged 70 years and over. Eur Urol. 2007;52:868-874.
Kinchen KS, Burgio K, Diokno AC, et al. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt). 2003;12:687-698.
Klevmark B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol Scand. 1974;90:565-577.
Koyama W, Koyanagi A, Mihara S, et al. Prevalence and conditions of urinary incontinence among the elderly. Methods Inf Med. 1998;37:151-155.
Krahn HP, Morales PA. The effect of pudendal nerve anesthesia on urinary continence after prostatectomy. J Urol. 1965;94:282-285.
Kron M, Loy S, Sturm E, et al. Risk indicators for falls in institutionalized frail elderly. Am J Epidemiol. 2003;158:645-653.
Krupski TL, Saigal CS, Litwin MS. Variation in continence and potency by definition. J Urol. 2003;170(4 Pt. 1):1291-1294.
Kundu SD, Roehl KA, Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227-2231.
Landi F, Cesari M, Russo A, et al. Potentially reversible risk factors and urinary incontinence in frail older people living in community. Age Ageing. 2003;32:194-199.
Lapides J, Sweet RB, Lewis LW. Role of striated muscle in urination. J Urol. 1957;77:247-250.
Lara C, Nacey J. Ethnic differences between Maori, Pacific Island and European New Zealand women in prevalence and attitudes to urinary incontinence. N Z Med J. 1994;107:374-376.
Leandri P, Rossignol G, Gautier JR, Ramon J. Radical retropubic prostatectomy: morbidity and quality of life. Experience with 620 consecutive cases. J Urol. 1992;147(3 Pt. 2):883-887.
Lee SE, Byun SS, Lee HJ, et al. Impact of variations in prostatic apex shape on early recovery of urinary continence after radical retropubic prostatectomy. Urology. 2006;68:137-141.
Leigh RJ, Turnberg LA. Faecal incontinence: the unvoiced symptom. Lancet. 1982;1:1349-1351.
Leng WW, McGuire EJ. Obstructive uropathy induced bladder dysfunction can be reversible: bladder compliance measures before and after treatment. J Urol. 2003;169:563-566.
Lepor H, Kaci L, Xue X. Continence following radical retropubic prostatectomy using self-reporting instruments. J Urol. 2004;171(3):1212-1215.
Litwin MS, Pasta DJ, Yu J, et al. Urinary function and bother after radical prostatectomy or radiation for prostate cancer: a longitudinal, multivariate quality of life analysis from the Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2000;164(6):1973-1977.
Litwin MS, Saigal CS, Yano EM, et al. Urologic diseases in America Project: analytical methods and principal findings. J Urol. 2005;173:933-937.
Lo TS, Wang AC, Horng SG, et al. Ultrasonographic and urodynamic evaluation after tension free vagina tape procedure (TVT). Acta Obstet Gynecol Scand. 2001;80:65-70.
Lowe BA. Comparison of bladder neck preservation to bladder neck resection in maintaining postrostatectomy urinary continence. Urology. 1996;48:889-893.
Lukacz ES, Lawrence JM, Buckwalter JG, et al. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:272-284.
Majoros A, Bach D, Keszthelyi A, et al. Urinary incontinence and voiding dysfunction after radical retropubic prostatectomy (prospective urodynamic study). Neurourol Urodyn. 2006;25(1):2-7.
Malmsten UG, Milsom I, Molander U, Norlen LJ. Urinary incontinence and lower urinary tract symptoms: an epidemiological study of men aged 45 to 99 years. J Urol. 1997;158:1733-1737.
Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104:579-585.
Maral I, Ozkardes H, Peskircioglu L, Bumin MA. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128-1133.
Markland AD, Gerety MB, Goode PS, et al. Urinary incontinence in community-dwelling older Mexican American and European American women. Arch Gerontol Geriatr. 2009;48:232-237.
Markland AD, Goode PS, Burgio KL, et al. Correlates of urinary, fecal, and dual incontinence in older African-American and white men and women. J Am Geriatr Soc. 2008;56:285-290.
Marshall K, Thompson KA, Walsh DM, Baxter GD. Incidence of urinary incontinence and constipation during pregnancy and postpartum: survey of current findings at the Rotunda Lying-In Hospital. Br J Obstet Gynaecol. 1998;105:400-402.
McGrother CW, Donaldson MM, Shaw C, et al. Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int. 2004;93:763-769.
McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am. 1996;23:253-262.
McGuire EJ, Herlihy E. The influence of urethral position on urinary continence. Invest Urol. 1977;15:205-207.
McGuire EJ, Lytton B, Kohorn EI, Pepe V. The value of urodynamic testing in stress urinary incontinence. J Urol. 1980;124:256-258.
McGuire EJ, Lytton B, Pepe V, Kohorn EI. Stress urinary incontinence. Obstet Gynecol. 1976;47:255-264.
McGuire EJ, Woodside JR, Border TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplasic patients. J Urol. 1981;126:205-209.
McGuire EJ, Yalla SV, Elbadawi A. Abnormalities of vesicourethral function following radical pelvic extirpative surgery. In: Yalla SV, editor. Neurourology and urodynamics. New York: Macmillan, 1988.
McKammon KA, Kolm P, Main B, Schellhammer PF. Comparative quality of life analysis after radical retropubic prostatectomy: objective and subjective analysis. Urology. 1997;49:225.
Milsom I, Altman D, Lapitan MC, et al. Committee 1 on epidemiology of urinary and faecal incontinence and pelvic organ prolapse. In: Abrams P, editor. Incontinence: 4th International Consultation on Incontinence. Paris, July 5-8, 2008. Health Publications Ltd, 2009.
Minaglia S, Ozel B, Hurtado E, et al. Effect of transobturator tape procedure on proximal urethral mobility. Urology. 2005;65:55-59.
Moinzadeh A, Shunaigat AN, Libertino JA. Urinary incontinence after radical retropubic prostatectomy: the outcome of a surgical technique. BJU Int. 2003;92:355-359.
Molander U, Milsom I, Ekelund P, Mellstrom D. An epidemiological study of urinary incontinence and related urogenital symptoms in elderly women. Maturitas. 1990;12:51-60.
Moore KN, Truong V, Estey E, Voaklander DC. Urinary incontinence after radical prostatectomy: can men at risk be identified preoperatively? J Wound Ostomy Continence Nurs. 2007;34(3):270-279.
Morkved S, Bo K. Prevalence of urinary incontinence during pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:394-398.
. Incontinence: 3rd International Consultation on Incontinence. Morrison J, Birder L, Craggs M, Al E. Health Publications, Paris, 2005.
Mostwin JL, Yang A, Sanders R, Genadry R. Radiography, sonography, and magnetic resonance imaging for stress incontinence. Contributions, uses, and limitations. Urol Clin North Am. 1995;22:539-549.
Moul JW, Mooneyhan RM, Kao TC, et al. Preoperative and operative factors to predict incontinence, impotence and stricture after radical prostatectomy. Prostate Cancer Prostatic Dis. 1998;1:242-249.
Muscatello DJ, Rissel C, Szonyi G. Urinary symptoms and incontinence in an urban community: prevalence and associated factors in older men and women. Intern Med J. 2001;31:151-160.
Nandipati KC, Raina R, Agarwal A, Zippe CD. Nerve sparing surgery significantly affects long-term continence after radical prostatectomy. Urology. 2007;70(6):1127-1130.
Nelson RL, Furner SE. Risk factors for the development of fecal and urinary incontinence in Wisconsin nursing home residents. Maturitas. 2005;52:26-31.
Nilsson CG, Falconer C, Rezapour M. Seven-year follow-up of the tension-free vaginal tape procedure for treatment of urinary incontinence. Obstet Gynecol. 2004;104:1259-1262.
Nilsson CG, Kuuva N. The tension-free vaginal tape procedure is successful in the majority of women with indications for surgical treatment of urinary stress incontinence. BJOG. 2001;108:414-419.
Nitti V. Post-prostatectomy incontinence. In Walsh PC, editor: Campbell’s urology, 8th ed, Philadelphia: Saunders, 2002.
Nitti VW, Combs AJ. Correlation of Valsalva leak point pressure with subjective degree of stress urinary incontinence in women. J Urol. 1996;155:281-285.
Noh C, Kshirsagar A, Mohler JL. Outcomes after radical retropubic prostatectomy. Urology. 2003;61:412-416.
Norton C. Personal communication.
Nuotio M, Jylha M, Luukkaala T, Tammela TL. Urinary incontinence in a Finnish population aged 70 and over. Prevalence of types, associated factors and self-reported treatments. Scand J Prim Health Care. 2003;21:182-187.
Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104:489-497.
Nygaard I, Turvey C, Burns TL, et al. Urinary incontinence and depression in middle-aged United States women. Obstet Gynecol. 2003;101:149-156.
Ojdeby G, Claezon A, Brekkan E, et al. Urinary incontinence and sexual impotence after radical prostatectomy. Scand J Urol Nephrol. 1996;30:473-477.
Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
Olsson I, Kroon U. A three-year postoperative evaluation of tension-free vaginal tape. Gynecol Obstet Invest. 1999;48:267-269.
Olsson LE, Salomon L, Nadu A, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology. 2001;58:570-572.
Ouslander JG, Kane RL, Abrass IB. Urinary incontinence in elderly nursing home patients. JAMA. 1982;248:1194-1198.
Ouslander JG, Schnelle JF. Incontinence in the nursing home. Ann Intern Med. 1995;122:438-449.
Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173(5):1701-1705.
Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7-31.
Petros PP, Ulmsten U. An anatomical classification—a new paradigm for management of female lower urinary tract dysfunction. Eur J Obstet Gynecol Reprod Biol. 1998;80:87-94.
Pfister C, Cappele O, Dunet F, et al. Assessment of the intrinsic urethral sphincter component function in postprostatectomy urinary incontinence. Neurourol Urodyn. 2002;21(3):194-197.
Pierorazio PM, Spencer BA, McCann TR, et al. Preoperative risk stratification predicts likelihood of concurrent PSA-free survival, continence, and potency (the trifecta analysis) after radical retropubic prostatectomy. Urology. 2007;70(4):717-722.
Plzak L3rd, Staskin D. Genuine stress incontinence theories of etiology and surgical correction. Urol Clin North Am. 2002;29:527-535.
Poon M, Ruckle H, Bamshad BR, et al. Radical retropubic prostatectomy: bladder neck preservation versus reconstruction. J Urol. 2000;163:194-198.
Rassweiler J, Seemann O, Schulze M, et al. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol. 2003;169(5):1689-1693.
Raz S, Caine M, Zeigler M. The vascular component in the production of intraurethral pressure. J Urol. 1972;108:93-96.
Rekers H, Drogendijk AC, Valkenburg H, Riphagen F. Urinary incontinence in women from 35 to 79 years of age: prevalence and consequences. Eur J Obstet Gynecol Reprod Biol. 1992;43:229-234.
Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257:3076-3081.
Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N Engl J Med. 1989;320:1-7.
Rezapour M, Ulmsten U. Tension-free vaginal tape (TVT) in women with recurrent stress urinary incontinence—a long-term follow up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S9-11.
Richardson DA, Bent AE, Ostergard DR. The effect of uterovaginal prolapse on urethrovesical pressure dynamics. Am J Obstet Gynecol. 1983;146:901-905.
Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc. 1998;46:467-472.
Rodriguez EJr, Skarecky DW, Ahlering TE. Post-robotic prostatectomy urinary continence: characterization of perfect continence versus occasional dribbling in padfree men. Urology. 2006;67(4):785-788.
Rogers CG, Su LM, Link RE, et al. Age stratified functional outcomes after laparoscopic radical prostatectomy. J Urol. 2006;176(6 Pt. 1):2448-2452.
Rortveit G, Brown JS, Thom DH, et al. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109:1396-1403.
Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol. 2003;189:1268-1274.
Rosenzweig BA, Bhatia NN, Nelson AL. Dynamic urethral pressure profilometry pressure transmission ratio: what do the numbers really mean? Obstet Gynecol. 1991;77:586-590.
Rosenzweig BA, Pushkin S, Blumenfeld D, Bhatia NN. Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol. 1992;79:539-542.
Rosenzweig BA, Soffici AR, Thomas S, Bhatia NN. Urodynamic evaluation of voiding in women with cystocele. J Reprod Med. 1992;37:162-166.
Rovner ES, Ginsberg DA, Raz S. The UCLA surgical approach to sphincteric incontinence in women. World J Urol. 1997;15:280-294.
Salomon L, Sebe P, De La Taille A, et al. Open versus laparoscopic radical prostatectomy: part II. BJU Int. 2004;94(2):244-250.
Sampselle CM, Harlow SD, Skurnick J, et al. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100:1230-1238.
Samuelsson E, Victor A, Tibblin G. A population study of urinary incontinence and nocturia among women aged 20-59 years. Prevalence, well-being and wish for treatment. Acta Obstet Gynecol Scand. 1997;76:74-80.
Samuelsson EC, Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299-305.
Sandvik H, Hunskaar S, Seim A, et al. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497-499.
Sandvik H, Hunskaar S, Vanvik A, et al. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48:339-343.
Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137-145.
Saranchuk JW, Kattan MW, Elkin E, et al. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol. 2005;23:4146-4151.
Sarlos D, Kuronen M, Schaer GN. How does tension-free vaginal tape correct stress incontinence? Investigation by perineal ultrasound. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:395-398.
Saxer S, Halfens RJ, De Bie RA, Dassen T. Prevalence and incidence of urinary incontinence of Swiss nursing home residents at admission and after six, 12 and 24 months. J Clin Nurs. 2008;17:2490-2496.
Schmidbauer J, Temml C, Schatzl G, et al. Risk factors for urinary incontinence in both sexes. Analysis of a health screening project. Eur Urol. 2001;39:565-570.
Sgadari A, Topinkova E, Bjornson J, Bernabei R. Urinary incontinence in nursing home residents: a cross-national comparison. Age Ageing. 1997;26(Suppl 2):49-54.
Shah AD, Kohli N, Rajan SS, Hoyte L. The age distribution, rates, and types of surgery for pelvic organ prolapse in the USA. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:421-428.
Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res. 1994;3:291-306.
Sladden MJ, Hughes AM, Hirst GH, Ward JE. A community study of lower urinary tract symptoms in older men in Sydney, Australia. Aust N Z J Surg. 2000;70:322-328.
Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37-49.
Smith AR, Hosker GL, Warrell DW. The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. Br J Obstet Gynaecol. 1989;96:24-28.
Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet. 1984;2:546-550.
Snooks SJ, Swash M, Henry MM, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1:20-24.
Srougi M, Nesrallah LJ, Kauffmann JR, et al. Urinary continence and pathological outcome after bladder neck preservation during radical retropubic prostatectomy: a randomized prospective trial. J Urol. 2001;165:815-818.
Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354-360.
Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991;88:1709-1715.
Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790-796.
Steiner MS. Anatomic basis for the continence-preserving radical retropubic prostatectomy. Semin Urol Oncol. 2000;18:9-18.
Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol. 1991;145(3):512-514. discussion 514–15
Strasser H, Frauscher F, Helweg G, et al. Transurethral ultrasound: evaluation of anatomy and function of the rhabdosphincter of the male urethra. J Urol. 1998;159(1):100-104.
Subak LL, Brown JS, Kraus SR, et al. The “costs” of urinary incontinence for women. Obstet Gynecol. 2006;107:908-916.
Summitt RLJr, Stovall TG, Bent AE, Ostergard DR. Urinary incontinence: correlation of history and brief office evaluation with multichannel urodynamic testing. Am J Obstet Gynecol. 1992;166:1835-1840. discussion 1840–4
Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183:277-285.
Swithinbank LV, Donovan JL, Du Heaume JC, et al. Urinary symptoms and incontinence in women: relationships between occurrence, age, and perceived impact. Br J Gen Pract. 1999;49:897-900.
Tanagho EA. Anatomy of the lower urinary tract and mechanical interpretation of storage and voiding. Curr Opin Urol. 1992;2:245-247.
Tanagho EA, Miller ER. Initiation of voiding. Br J Urol. 1970;42:175-183.
Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998;46:473-480.
Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing. 1997;26:367-374.
Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: urinary incontinence in women-national trends in hospitalizations, office visits, treatment and economic impact. J Urol. 2005;173:1295-1301.
Thom DH, Van Den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983-989.
Thom DH, Van Den Eeden SK, Ragins AI, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259-264.
Thomas TM, Egan M, Walgrove A, Meade TW. The prevalence of faecal and double incontinence. Community Med. 1984;6:216-220.
Thomas TM, Plymat KR, Blannin J, Meade TW. Prevalence of urinary incontinence. Br Med J. 1980;281:1243-1245.
Tulloch AG. The vascular contribution to intraurethral pressure. Br J Urol. 1974;46:659-664.
Turner-Warwick RW. The sphincter mechanisms: their relation to prostatic enlargement and its treatment. In: Hinman F, Boyarsky S, editors. Benign prostatic hypertrophy. New York: Springer, 1983.
Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14:131-139.
Ueda T, Tamaki M, Kageyama S, et al. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol. 2000;7:95-103.
Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
Van Der Vaart CH, De Leeuw JR, Roovers JP, Heintz AP. The effect of urinary incontinence and overactive bladder symptoms on quality of life in young women. BJU Int. 2002;90:544-549.
Van Kampen M, De Weerdt W, Van Poppel H, et al. Prediction of urinary continence following radical prostatectomy. Urol Int. 1998;60:80-84.
Van Melick HH, Van Venrooij GE, Eckhardt MD, Boon TA. A randomized controlled trial comparing transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia: analysis of subjective changes, morbidity and mortality. J Urol. 2003;169:1411-1416.
Van Oyen H, Van Oyen P. Urinary incontinence in Belgium; prevalence, correlates and psychosocial consequences. Acta Clin Belg. 2002;57:207-218.
Versi E, Cardozo LD, Studd JW, et al. Internal urinary sphincter in maintenance of female continence. Br Med J (Clin Res Ed). 1986;292:166-167.
Versi E, Lyell DJ, Griffiths DJ. Videourodynamic diagnosis of occult genuine stress incontinence in patients with anterior vaginal wall relaxation. J Soc Gynecol Investig. 1998;5:327-330.
Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol. 2007;165:309-318.
Wagner TH, Patrick DL, Bavendam TG, et al. Quality of life of persons with urinary incontinence: development of a new measure. Urology. 1996;47:67-71. discussion 71–2
Wall LL, Helms M, Peattie AB, et al. Bladder neck mobility and the outcome of surgery for genuine stress urinary incontinence. A logistic regression analysis of lateral bead-chain cystourethrograms. J Reprod Med. 1994;39:429-435.
Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58-61.
Walters MD, Diaz K. Q-tip test: a study of continent and incontinent women. Obstet Gynecol. 1987;70:208-211.
Walters MD, Jackson GM. Urethral mobility and its relationship to stress incontinence in women. J Reprod Med. 1990;35:777-784.
Walters MD, Shields LE. The diagnostic value of history, physical examination, and the Q-tip cotton swab test in women with urinary incontinence. Am J Obstet Gynecol. 1988;159:145-149.
Weber AM, Abrams P, Brubaker L, et al. The standardization of terminology for researchers in female pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:178-186.
Wei JT, Dunn RL, Marcovich R, et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164:744-748.
Weldon VE, Tavel FR, Neuwirth H. Continence, potency and morbidity after radical perineal prostatectomy. J Urol. 1997;158:1470-1475.
Wesnes SL, Rortveit G, Bo K, Hunskaar S. Urinary incontinence during pregnancy. Obstet Gynecol. 2007;109:922-928.
Westby M, Asmussen M, Ulmsten U. Location of maximum intraurethral pressure related to urogenital diaphragm in the female subject as studied by simultaneous urethrocystometry and voiding urethrocystography. Am J Obstet Gynecol. 1982;144:408-412.
Wheeler JSJr, Culkin DJ, O’Hara RJ, Canning JR. Bladder dysfunction and neurosyphilis. J Urol. 1986;136:903-905.
Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398-406.
Yalla SV, Sharma GV, Barsamian EM. Micturitional static urethral pressure profile: a method of recording urethral pressure profile during voiding and the implications. J Urol. 1980;124:649-656.
Yang A, Mostwin JL, Genadry R, Sanders R. Patterns of prolapse demonstrated with dynamic fast scan MRI: reassessment of conventional concepts of pelvic floor weakness. Neurourol Urodyn. 1993;12:310-311.
Yang A, Mostwin JL, Rosenshein NB, Zerhouni EA. Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology. 1991;179:25-33.
Yarnell JW, Voyle GJ, Richards CJ, Stephenson TP. The prevalence and severity of urinary incontinence in women. J Epidemiol Community Health. 1981;35:71-74.
Young MD, Weizer AZ, Silverstein AD, et al. Urinary continence and quality of life in the first year after radical perineal prostatectomy. J Urol. 2003;170(6 Pt. 1):2374-2378.
Zacharin RF. The suspensory mechanism of the female urethra. J Anat. 1963;97:423-427.
Zhu L, Lang J, Wang H, et al. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China. Menopause. 2008;15:566-569.
Zincke H, Oesterling JE, Blute ML, et al. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152(5 Pt. 2):1850-1857.
Zinner NR, Sterling AM, Ritter RC. Role of inner urethral softness in urinary continence. Urology. 1980;16:115-117.