chapter 66 Overactive Bladder
The overactive bladder (OAB) is a prevalent problem, with considerable effects on the quality of life of affected individuals and substantial health economic costs. The condition is symptom based and defined by the International Continence Society (ICS) standardization committee as urgency, with or without urgency incontinence, usually with frequency and nocturia, if there is no proven infection or other obvious pathology (Abrams et al, 2002). A correction had to be put forward when it was realized that the term “urge incontinence” had been used in the original definition (Abrams et al, 2009). OAB is thus a syndrome in which several of the lower urinary tract symptoms (LUTS) relating to the storage of urine coexist, with urinary urgency as the essential parameter. Because urgency will frequently arise at comparatively small bladder volumes, patients tend to describe frequent voids with a low typical voided volume. Nocturia as a symptom is somewhat variable in OAB because the supine position and reduced physical exertion overnight seem to be less provocative in eliciting OAB. A difficulty with the ICS definition is that some patients may void preemptively (defensive voiding), passing urine before urgency arises; they will thus clearly have storage-phase LUTS yet lack the urgency symptom, which is the defining characteristic of OAB.
Urgency with at least one other symptom is essential to diagnose OAB. Thus urgency is the pivotal symptom, defined by the ICS as the complaint of a sudden compelling desire to void that is difficult to defer. However, there remains plenty of room for confusion. For example, a normal “urge to void” is not synonymous with abnormal urgency; the ICS therefore suggested that the term “desire to void” is more appropriate for describing normal filling sensation. Furthermore, there are grounds to consider including “for fear of leakage” alongside the sudden compelling desire to void that is difficult to defer—indeed this was included in earlier ICS definitions. Many OAB patients certainly feel as if they are going to leak, even if they say they never have, commonly expressing anxieties exemplified by, “When I want to go, I have to rush because I think I may wet myself.”
In many OAB patients, urgency incontinence occurs, defined as involuntary leakage of urine, accompanied or immediately preceded by urgency (Abrams et al, 2002). This standardized definition abandoned the requirement that the leakage should be a “social or hygienic problem” to be called incontinence because considerable leakage can occur, which in some individuals may not be a problem to them, particularly in children and the elderly. In a prevalence survey, 69% of women had “any incontinence,” but only 30% found this a “social or hygienic problem” (Swithinbank et al, 1999).
The symptom of “increased daytime frequency” is the complaint by the patient who considers that he or she voids too often by day. There is no minimum number of voids included in the standardized definition. The symptom of nocturia is the complaint that the individual has to wake at night one or more times to void. No agreement has been reached for individuals with differing sleep patterns such as night-shift workers.
Before the introduction of the standardized definitions, the use of several overlapping terms resulted in a confusing situation, hampering many aspects of research and management. The English-speaking world had adopted Patrick Bates’ term unstable bladder to describe involuntary detrusor contractions seen during urodynamic studies as the bladder was filled, while the Scandinavians used the term detrusor hyperreflexia. To resolve the discrepancy, the ICS designated the term unstable bladder to be applied where there was no obvious cause for the contractions and detrusor hyperreflexia for patients whose involuntary contractions had a neurologic cause (Bates et al, 1980); overactive detrusor was used as the generic, overarching term. Nonetheless, the use of different terms in neurologic and non-neurologic patient groups became increasingly difficult. Firstly, several studies showed considerable overlap in the underlying mechanisms. In addition, explaining the terms to the patients and the wider medical profession highlighted the incongruity of the differing terminology. Furthermore, physicians had to be careful not to insult the sensibilities of the patient by inadvertently implying mental instability by using the word “unstable” in relation to bladder symptoms. “Overactive Bladder” was used as the title of a consensus conference, and a formal definition was proposed in 1999 (Abrams and Wein, 1999), culminating in the ICS definition used currently.
Crucially, the current definition of OAB is based on symptoms; in contrast, detrusor overactivity (DO) is a urodynamic observation, characterized by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked (Abrams et al, 2002). OAB and DO are thus not interchangeable terms, and the clinician must be specific in their use. Clinicians need to be clear on this among themselves and when discussing urgency with patients. All measures, for example “warning time” (between first sensation of urgency and eventual voiding), depend on the patients and the clinicians reaching a consensus as to the meaning of urgency. The ICS terminology committee excluded “for fear of leakage” in the new definition of urgency, mainly because many OAB patients do not leak. Perhaps this was not entirely logical because patients can certainly feel as if they are going to leak, even if they have never actually done so. A couple of phrases are often used by patients:
Hence “fear of leakage” is an important concept to patients.
Key Points: OAB Terminology
Pathophysiology and Etiology
The reliance on urgency in defining OAB is a problem for investigation into its scientific basis because it complicates the development of reliable animal models, with which evaluation of subjective symptoms is not possible. Accordingly, research focus has particularly concentrated on abnormalities of afferent signaling and mechanisms underlying DO, the former as the presumed basis of urgency, the latter as it is likely to contribute in a large number of people with OAB. In addition, some hypothetical reasoning has been used to translate scientific observations into clinical reality.
Hypotheses of Detrusor Overactivity
Until recently, insight into the pathophysiologic basis of DO has generally focused on cellular mechanisms by which unregulated motor activity could arise. The neurogenic hypothesis states that DO arises from generalized, nerve-mediated excitation of the detrusor muscle (de Groat, 1997). There are several interdependent mechanisms by which this may arise. First, damage to the brain can induce DO by reducing suprapontine inhibition. Second, damage to axonal pathways in the spinal cord allows the expression of primitive spinal bladder reflexes. Third, synaptic plasticity leads to reorganization of sacral activity, with the emergence of new reflexes, which may be triggered by C-fiber bladder afferent neurons. Finally, sensitization of peripheral afferent terminals in the bladder can trigger DO.
The myogenic hypothesis suggests that overactive detrusor contractions result from a combination of an increased likelihood of spontaneous excitation within smooth muscle of the bladder and enhanced propagation of this activity to affect an excessive proportion of the bladder wall (Brading and Turner, 1994; Brading, 1997). Patchy denervation is a common observation in DO, regardless of etiology (German et al, 1995; Charlton et al, 1999; Drake et al, 2000; Mills et al, 2000). A smooth muscle cell deprived of its innervation shows an upregulation of surface membrane receptors and may have altered membrane potential, which increases the likelihood of spontaneous contraction in that cell. DO is also associated with characteristic changes in ultrastructure (Elbadawi et al, 1993; Haferkamp et al, 2003a, 2003b), which could facilitate the propagation of the contraction over a wider proportion of the body of the detrusor than normal. However, the observations in different models of DO and from clinical specimens do not uniformly describe these features.
Afferent Mechanisms in Overactive Bladder and Detrusor Overactivity
The afferent nerve endings are widely distributed in the bladder wall and are particularly dense in the connective tissue underneath the urothelium. In this location, they may be influenced by several cell types. Urothelial cells possess sensory and signaling properties that allow them to respond to their chemical and physical environments and to communicate with subjacent structures (Birder, 2001). Suburothelial interstitial cells lie in close physical proximity to nerve fibers, suggesting a role in sensory transduction or regulation (Wiseman et al, 2003). The number of impulses fired by an afferent ending at any level of distention can be varied physiologically; that is, the gain of the sensory nerve endings can be changed to vary the “afferent sensitivity.” Thus the rate of firing in a particular afferent normally associated with a high vesical volume can occur at lower volumes if the afferent ending has been sensitized. Accordingly, the release of substances from the urothelium, as well as the direct interaction between afferent nerve endings and other cell types, may physiologically influence central reporting of the bladder state and could well contribute pathophysiologically. In theory, OAB may arise if the level of sensory activity is inappropriately high for any given degree of bladder distention, resulting from pathologically sensitized or abnormally numerous afferent nerve endings. The interaction among afferents, urothelium, and interstitial cells is thus interesting, in that modulating their interactions may result in amelioration of symptom severity. The expression of various receptors on urothelium and afferents, particularly members of the transient receptor potential (TRP) family, may thus be relevant in the development of future clinical management options. The sensory information is carried to the spinal cord by myelinated Aδ fibers and unmyelinated C fibers. As a general rule, the Aδ fibers are regarded as responding to passive bladder distention and active detrusor contraction (“in series” mechanoreceptors [Iggo, 1955]), thus conveying information about bladder filling (Janig and Morrison, 1986). C fibers are regarded as responding primarily to chemical irritation of the bladder mucosa (Habler et al, 1990) or to thermal stimulus (Fall et al, 1990). Accordingly, they may be less active in the physiologic state than the Aδ fibers. Nonetheless, there is almost certainly considerable overlap in the sensory information carried by the two types of afferent, and the C fibers may take on a more prominent role in the pathologic state.
Integrative Physiology of Overactive Bladder
The afferent, neurogenic, and myogenic mechanisms alluded to earlier are essentially based on physiology of individual cell types. However, the complexity of lower urinary tract function is substantially increased by the potential interactions between differing cell types and their humoral/paracrine environments. The integrative properties determining the interaction between cell types is manifest in the neural circuits of the central nervous system, as well as the periphery (Fig. 66–1)—the latter working through networks (neural, interstitial cells) and by direct cell communication in the urothelium or muscle cell–to–muscle cell. The presence of interstitial cells in the bladder led to the proposal of a regulatory “myovesical plexus” comprising nerves (excitatory and inhibitory, peripheral and central) and interstitial cells loosely, akin to gut myenteric plexuses. The integrative hypothesis of OAB draws on the various contributory factors to understand the normal micturition cycle, OAB, and DO (Drake et al, 2001). Several observations can be understood by focusing on activity within the bladder wall itself:
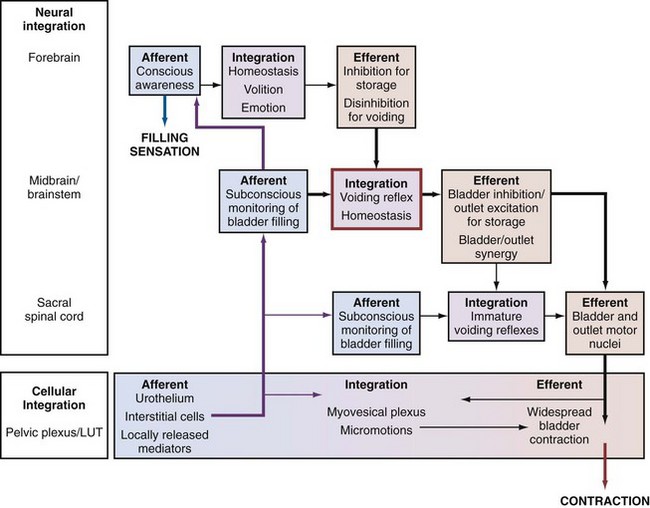
Figure 66–1 The integrative hypothesis of overactive bladder and detrusor overactivity. Neural circuits in the central nervous system and cellular interactions in the periphery integrate afferent input and modulatory influences, in a hierarchy of regulatory structures underpinning normal bladder function. Storage function is characterized by sensations of filling and desire to void at suitable volumes, whereas voiding function is characterized by volitional control and complete emptying. Lesions at various levels have seemingly similar effects because their effects are mediated by final common pathways of sensation and bladder contraction.
The crucial factors in the integrative hypothesis are the level of excitation and degree of propagation in the myovesical plexus structures of the bladder wall. Both will be at low levels during normal bladder filling. Local excitability is high in OAB, whereas propagation alone is high in asymptomatic DO; both are increased where DO is symptomatic. Propagation is high in normal voiding (driven by efferent commands from the central nervous system [CNS]). Increased local excitation with impaired propagation could explain why patients have OAB and a PVR (detrusor hyperactivity with impaired contractility (Resnick and Yalla, 1987).
Central Nervous System
The development of functional brain imaging technology allows estimation of gross activity in specific brain areas and has been used to study bladder filling in normal and symptomatic individuals. Intriguing insights into contributions from various parts of the cerebral cortex such as the insula and the prefrontal cortex have resulted. Alterations in the regional brain activity of symptomatic individuals with OAB have been reported (Griffiths et al, 2007). Understanding brain responses to lower urinary tract activity through autonomic afferent processing networks, as is already under investigation for the gastrointestinal tract, will be crucial in the endeavor to develop improved clinical management options.
Etiology
Neurologic disease is associated with a high prevalence of LUT dysfunction, and this is to be anticipated given the fundamental regulatory influence of innervations on bladder and bladder outlet. Aging is associated with increased LUTS; this may be through the attritional effects on inhibitory innervation and brain function. Bladder outlet obstruction has long been considered an etiologic factor in generation of DO and OAB, but there is now some doubt as to whether this is correct.
Key Points: OAB Pathophysiology
Prevalence
Using the standardized ICS definition of OAB, the EPIC study reported the prevalence of OAB in four European countries and in Canada, reporting an overall OAB prevalence of 11.8%, in the context of a prevalence of 64.3% for at least one LUTS (Irwin et al, 2006). The EPIC study also catalogued multidimensional impact including effects on employment (Coyne et al, 2008; Irwin et al, 2009).
Older studies have to be interpreted according to the definitions they used for LUTS. One prevalence study (Milsom et al, 2001) reported prevalence of OAB symptoms that “occurred singly or in combination,” estimating overall OAB prevalence at 16%. In the studied population, 9.2% had urgency, and this is perhaps closer to the true prevalence of OAB in the community.
The NOBLE study (Stewart et al, 2003) established the prevalence of OAB in more than 5000 community-dwelling individuals in the United States using a validated computer-assisted telephone interview. Men and women had the same prevalence of OAB overall (16.0% and 16.9%, respectively) as defined by the ICS. However, men were shown to have a higher prevalence of “OAB dry” (13.4% as opposed to 7.6% in women) and women had a higher prevalence of “OAB wet” (9.3% as opposed to 2.6% in men). It is assumed that the difference in the prevalence of incontinence is due to the relative weakness of the bladder neck and urethral sphincter mechanism in women, particularly in those who have had children. In women the prevalence of “OAB wet” rose from 2.0% in the youngest group (ages 18 to 24) to 19.1% in those 65 to 74 years of age. Men, on the other hand, did not experience an increase in “OAB wet” until older: 8.22% for those 65 to 74 and 10.2% for those 75 years and older.
In addition to simple prevalence, effect on quality of life needs to be evaluated. Both types of OAB have a significant impact on the patients’ quality of life, as measured by generic quality of life instruments. It is not difficult to understand that being “OAB wet” has a profound impact. However, in some studies, the symptom of urgency was shown to have a greater effect on quality of life than incontinence (Coyne et al, 2004, 2008). EpiLUTS was an Internet-based survey of 30,000 people in the United States, United Kingdom, and Sweden documenting symptoms, symptom bother, and health-related quality of life. It derived a substantial amount of information about the impact of LUTS, individually and categorized as storage, voiding, and postmicturition including consequent anxiety and depression. It concluded that storage symptoms were significantly associated with greater impact (Sexton et al, 2009). Many studies in women have shown that urgency incontinence is more bothersome than stress incontinence. In some women, stress incontinence may occur several times a day and urgency incontinence only once or twice a week, but it is for the latter that the patient may demand treatment. This is probably because stress incontinence occurs more predictably and can usually be better contained by pads. OAB is a long-term condition, and ongoing quality of life impairment can be anticipated in many patients (Garnett et al, 2009).
Key Points: OAB Prevalence
Clinical Assessment
Patients with OAB may present to health care workers in various disciplines in either the community or the hospital service. It is vital that the doctor or nurse caring for such individuals is both knowledgeable and sympathetic, taking into account the patient’s psychosocial circumstances. OAB always requires management, and “cure” is not a realistic aspiration; appropriate expectations and honest explanation are therefore crucial. An appropriate initial assessment gives a presumptive diagnosis and thus the basis for empirical treatment. It should also establish the patient’s desire for treatment and which therapies are open to consideration; for example, treatments likely to impair voiding contractility should not be employed if intermittent catheterization is unfeasible or indwelling catheterization will not be tolerated.
History should cover the following:
Focused physical examination requires abdominal and pelvic examination, as well as basic neurologic examination. Assessment of bladder emptying is necessary (most simply by palpating the lower abdomen, if the patient is slim). Urinalysis is important in all patients. The algorithms from the fourth International Consultation on Incontinence 2008 (Abrams et al, 2009) summarize the basic assessment necessary in the evaluation of lower urinary tract dysfunction in men, women, and the frail elderly (Fig. 66–2).
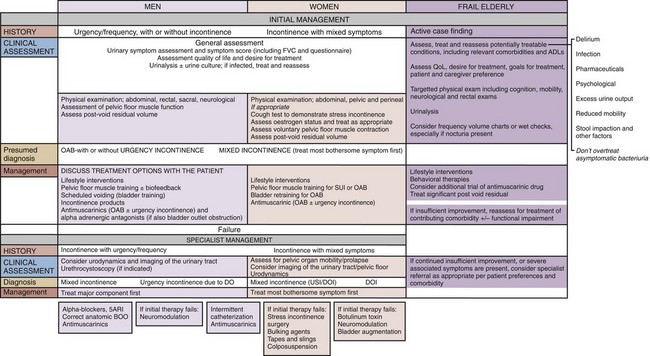
Figure 66–2 Overactive bladder in men, women, and the frail elderly; assessment and management, derived from the respective algorithms of the fourth International Consultation on Incontinence.
(From Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4th ed. Plymouth [MA]: Health Publications; 2009.)
Instruments for Measuring Bladder Sensations and Storage Symptoms
Normal LUT sensations during cystometry are (1) first sensation of filling, (2) normal desire to void, and (3) strong desire to void (Wyndaele and De Wachter, 2002). Whether an individual with OAB also experiences normal sensations is not yet established but seems likely. Commonly, OAB patients have increased daytime frequency, but only a proportion of voids are associated with urgency. Tools for evaluating urgency thus have to deal with the subjective nature of the symptom, the habit of preemptive voiding at low bladder volumes, and the consequent increased frequency with low levels of urgency. A validation process is necessary to ensure tools employed are suitable for clinical or research use (Avery et al, 2004; Abrams et al, 2006).
The urinary sensation scale (Abrams et al, 2005) is as follows:
The urgency percentage scale (Cardozo et al, 2002) has three possible responses:
The Indevus “Urgency Severity Scale” (Bowden et al, 2003), used in trials of trospium, has four responses:
A related strategy used an “urgeometer” (Oliver et al, 2003) in cystometry, asking patients sequentially to press a series of five buttons during bladder filling according to their degree of urgency:
However, the lower three of these scales do not explain to the patients how urgency is defined; thus they are inappropriately named because they actually measure intensity of bladder sensation in differing parts of the sensation spectrum. Other measures such as “warning time” (between first sensation of urgency and eventual voiding) also depend on the patients and clinicians reaching a consensus as to the meaning of urgency (Cardozo and Dixon, 2005).
Storage symptoms questionnaires are now available in a modular format, developed to address recommendations made by the International Consultation on Incontinence (Abrams et al, 2006), known as the ICIQ. The ICIQ has adopted several validated tools and developed new ones where necessary. The King’s Health Questionnaire (Kelleher et al, 1997) and the OAB-q (Coyne et al, 2002) have been adopted as ICIQ modules.
Urgency assessment is a component of more generic LUTS assessment tools and may be of more practical use in clinical practice. For example, the “Patient Perception of Bladder Condition” is straightforward to administer and captures the clinical significance that patients attribute to their OAB (Coyne et al, 2008).
The frequency volume chart (FVC) (Abrams and Klevmark, 1996) remains the principal method of evaluating frequency and nocturia in an objective way. In OAB, the pattern of voided volumes is characteristically erratic. On the FVC, frequency is defined as the number of voids recorded during waking hours including the last void before sleep and the first void after waking and rising in the morning (Abrams et al, 2002). Nocturia is the number of voids recorded during a night’s sleep: each void is preceded and followed by sleep. The FVC is valuable in highlighting nocturnal urine output because nocturia due to nocturnal polyuria must be considered with other LUTS as likely to mitigate against successful OAB management. The bladder diary additionally collects information on fluid intake and incontinence episodes.
Two symptom scales are undergoing evaluation in nonselected populations. The bladder sensation scale, for use with an FVC, is shown in Table 66–1. The cystometry sensation scale, for use during urodynamic study, is shown in Table 66–2. The approach of using complementary modalities of assessment in conjunction allows a more comprehensive baseline evaluation and is informative for evaluation of treatment response.
Table 66–1 Bladder Sensation Scale
|
1. No sensation of needing to pass urine but passed urine for “social reasons” (e.g., just before going out, unsure where next toilet is); no urgency.
|
Table 66–2 Cystometry Sensation Scale
Mixed Urinary Incontinence and Mixed Incontinence
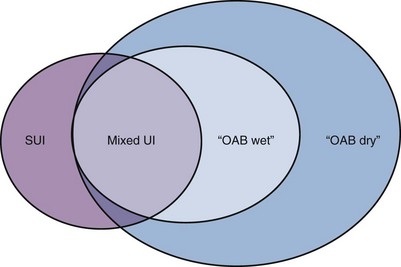
OAB can coexist with stress urinary incontinence (SUI). Mixed urinary incontinence (MUI) is the complaint of involuntary leakage associated with urgency and with exertion, effort, sneezing, or coughing (Abrams et al, 2002). Figure 66–3 describes the relationships among SUI, MUI, and OAB in women. The group illustrated with SUI and “OAB dry” could be described as having “mixed symptoms” because they have key features of SUI and urgency symptoms, but their OAB is not associated with urgency incontinence, so they do not have MUI. In general, MUI is associated with more severe levels of leakage, although it is currently unclear which component, urgency or stress, is responsible: possibly either or both types will prove culpable. Mixed incontinence refers to the coexistence of urinary and fecal incontinence. Clarity of communication is necessary to ensure all parties are aware of the specific situation for each individual under consideration.
Distinguishing Overactive Bladder from Bladder Pain Syndrome
Urgency in the old ICS terminology included a comment as to the reason underpinning the need to pass urine related to “fear of leakage or pain.” Pain aspects now fall into bladder pain syndrome (BPS), which constitutes a symptom complex clearly distinguished from OAB (Abrams et al, 2005; Hanno et al, 2008). BPS is defined as the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology. Figure 66–4 illustrates the relationship between OAB and BPS, and Figure 66–5 indicates the difference in bladder sensation/pain.
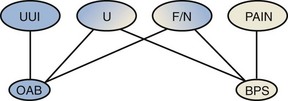
Figure 66–4 Overactive bladder (OAB) and bladder pain syndrome (BPS) both give rise to urgency (U), frequency (F), and nocturia (N); pain, but not urgency urinary incontinence (UUI), is seen in BPS.
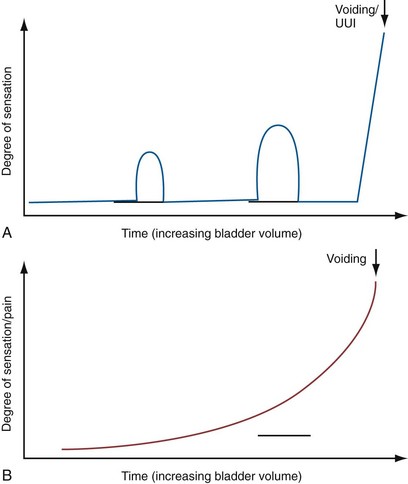
Figure 66–5 The differences in the development of bladder sensation in overactive bladder (OAB) and bladder pain syndrome (BPS). A, In OAB, phasic increases in urgency arise and recede as filling progresses, until severe urgency compels the patient to void or an urgency urinary incontinence (UUI) episode sets in. B, In BPS, pain increases throughout filling, accelerating as filling progresses.
Differences that help to distinguish BPS include the painful nature of symptoms, the steady increase in pain with filling, the more consistent voided volumes compared with OAB, and the ability to defer voiding (albeit at the cost of greater pain). In BPS, suprapubic pain is usual and additional perineal (urethral/vaginal/penile) discomfort/pain can also occur (Fitzgerald et al, 2005); in OAB, urgency is typically felt in the perineum/base of penis or vagina/urethra.
Initial Treatment
Basic evaluation allows diagnosis of OAB and baseline severity assessment, at which point initial treatment can be instigated:
In addition to the patient’s perceptions regarding response, repeat measures using symptom assessment tools and the frequency volume chart are valuable in providing objective markers because subjective perceptions and recollections of LUTS are notoriously unreliable. Should these initial therapies fail both subjectively and objectively, and invasive treatment be contemplated by the patient and clinician, then urodynamic evaluation is usually desirable.
Urodynamic Evaluation
It is common practice to employ conservative management and oral pharmacotherapy without a urodynamic diagnosis. Where the initial treatment is deemed inadequate and specialist referral is made, it is incumbent on the specialist clinician to ensure that conservative measures have been followed fully. Before urodynamic tests are requested, the reasons for “failure” of drug therapy should be explored:
For each circumstance, altered drug dose or agent may achieve sufficient improvement to obviate the need to consider more invasive investigation and treatment. Only when considerable efforts have been made should conservative therapy and antimuscarinic medications be considered unsuccessful.
Urodynamic evaluation should be considered where conservative and drug therapy fails adequately to manage OAB in a patient who is sufficiently healthy and is considering more invasive therapeutic interventions due to the impact of the symptoms on his or her quality of life. Whether to discontinue antimuscarinic drugs before the test can be argued either way; stopping the drugs gives the best chance of observing DO if present, while continuing them allows evaluation of the mechanism underlying residual refractory symptoms.
The primary aim of urodynamic studies is to reproduce the patient’s symptoms and identify additional factors likely to influence management decisions. The two main urodynamic diagnoses associated with OAB are DO and increased filling sensation. Figure 66–6 shows the relationship between the symptom-based diagnosis of OAB and the urodynamic-based diagnosis of DO. It is worth emphasizing that DO may not be present in some patients with OAB, especially women (Hashim and Abrams, 2006), and that some patients with DO are asymptomatic (i.e., do not have OAB). OAB without DO could be a consequence of bladder wall micromotions, causing distortion that leads to sensations, but without affecting a sufficient proportion of the bladder wall to cause pressure change (Drake et al, 2005). In some patients considered to have OAB on the basis of their symptoms, the ultimate diagnosis turns out to be urodynamic stress incontinence (USI), perhaps because contact of urine with urethral receptors can trigger a reinforcement reflex. Additional diagnoses relevant to management include bladder outlet obstruction and inefficient bladder emptying because treatment measures aiming to improve bladder reservoir function may cause collateral problems with voiding, perhaps necessitating intermittent self-catheterization.
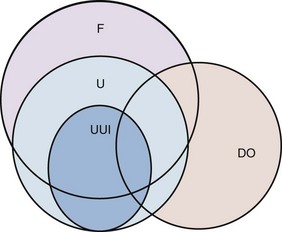
Figure 66–6 Correlation between the symptom-based diagnosis of overactive bladder (F, frequency; U, urgency; UUI, urgency urinary incontinence) and the urodynamic diagnosis of detrusor overactivity (DO). The correlation is best for urgency incontinence and least good for frequency (day and/or night).
Ideally, the patient should be seated or standing for filling cystometry because OAB symptoms are usually experienced when upright. However, where there is severe DO at low volumes, it may be difficult to ascertain if urinary stress incontinence (USI) is also present. In these patients, a second filling cycle in the supine position generally makes DO less overt (Al-Hayek et al, 2008), allowing more fluid to be instilled for determining whether USI is an additional diagnosis.
The urodynamic report must clearly state whether the patients’ symptoms were reproduced completely, reproduced in part, or not reproduced to ensure that due caution is exercised in major treatment decisions where there is diagnostic uncertainty.
Subclassification of Detrusor Overactivity and Technical Aspects of Urodynamics
The presence of a relevant neurologic condition distinguishes neurogenic DO from idiopathic DO, when there is no defined cause. Some clinicians feel that DO can arise as a consequence of BOO, but prevalence of DO is 60% in men who have had transurethral resection of the prostate previously (Thomas et al, 2005), despite the fact that they remain unobstructed. Accordingly, relief of BOO does not lead to permanent disappearance of DO. Although symptomatic improvement is seen in many of these men, it is probably less reliable than in men without DO.
DO has a variety of patterns on urodynamic traces. The ICS report describes two types:
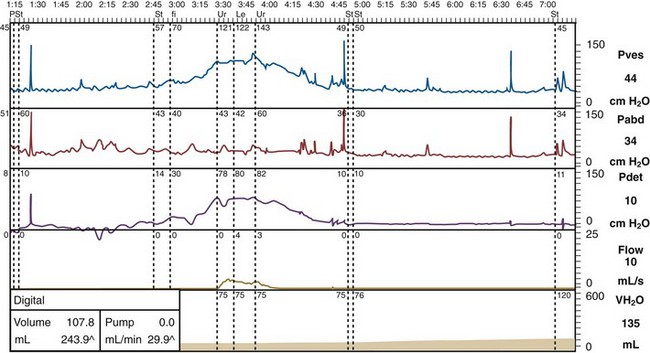
Figure 66–7 Detrusor overactivity trace. Bladder pressure is plotted in blue, rectal pressure in red, detrusor pressure in purple, flow in orange, and filling in light brown. This is a filling cystometry, so the presence of flow signifies incontinence. Incontinence in this individual is seen as a consequence of a phasic rise in detrusor pressure, at a comparatively low bladder volume.
Nonphasic changes in detrusor pressure before micturition should be regarded as changes in bladder compliance rather than as DO. Technical artifacts are easily introduced by moving a patient during urodynamics. Sometimes this change in pressure occurs if the patient is moved to the standing or sitting position, having had the bladder filled while supine.
If DO is not seen during filling, the investigator should attempt to use any provocations that the patient says lead to OAB symptoms such as the sound of running water. In some individuals, DO can be elicited by asking the patient to cough, but this must not be confused with stress urinary incontinence. Filling rate needs to be considered carefully; a short period of rapid filling can provoke the emergence of DO. However, fast filling can mask DO and cause low compliance, particularly in neurogenic DO.
There is no minimum value of amplitude below which a phasic detrusor contraction during filling does not constitute DO (Abrams et al, 2002). Earlier ICS reports (Bates et al, 1980) stated that, in order to diagnose “detrusor instability,” the contraction should be at least 15 cm H2O. However, it was later recognized that involuntary detrusor contractions of lower amplitude can cause significant symptoms, so the 1988 ICS report (Abrams et al, 1988) was altered accordingly. Measurement of the amplitude has been used as a way of describing the severity of DO (Abrams, 1984) (Fig. 66–8).
Ambulatory Urodynamics
The advent of ambulatory urodynamics has complicated the discussion because DO may be apparent in up to 60% of asymptomatic women (Heslington and Hilton, 1996). Does DO in these circumstances have any clinical relevance? It is not clear from the papers whether DO during ambulatory urodynamics in normal individuals is symptomatic or asymptomatic. If it were symptomatic, but this was a symptom the individual did not experience in everyday life, then the conclusion would be that the DO was artifactual, perhaps due to the presence of the pressure-measuring catheter “irritating” the bladder wall. Thus the requirements of good urodynamic practice to document the reproduction of a patient’s symptoms are particularly pertinent in this context.
Management
The fourth International Consultation of Incontinence has produced a comprehensive review of the spectrum of incontinence management, using the International Consultation on Urological Diseases’ adaptation of the AHCPR/ Oxford System of levels of evidence and grades of recommendation to evaluate the evidence from the scientific literature. The initial and specialized management algorithms for women show the care pathways for OAB and DO (Abrams et al, 2009) (see Fig. 66–2). Management of OAB is covered in detail elsewhere in this book. In brief, therapy for OAB can be divided into four classes of treatment:
For intractable OAB, options are appliances, catheters (urethral or suprapubic), urethral closure, and urinary diversion.
Key Points: OAB Treatment
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178.
Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence, 4th ed, Plymouth: Health Publications Ltd, 2009.
Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994 Jan;73(1):3-8.
de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50(6A Suppl.):36-52. discussion 3–6
Drake MJ, Mills IW, Gillespie JI. Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet. 2001 Aug 4;358(9279):401-403.
Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007 Aug 1;37(1):1-7.
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306-1314. discussion 14–15
Abrams P. Bladder instability: concept, clinical associations and treatment. Scand J Urol Nephrol Suppl. 1984;87:7-12.
Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28(4):287.
Abrams P, Avery K, Gardener N, Donovan J. The International Consultation on Incontinence Modular Questionnaire: www.iciq.net. J Urol. 2006;175(3 Pt. 1):1063-1066. discussion 6
Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. The International Continence Society Committee on Standardisation of Terminology. Scand J Urol Nephrol Suppl. 1988;114:5-19.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178.
Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence, 4th ed, Plymouth (MA): Health Publications Ltd, 2009.
Abrams P, Guan Z, Wang J, Hussain I. Gender analysis of data from two 12-week controlled trials; tolterodine reduces overactive bladder related nocturnal frequency in patients with overactive bladder and nocturia. Eur Urol Suppl. 2005;4:61.
Abrams P, Hanno P, Wein A. Overactive bladder and painful bladder syndrome: there need not be confusion. Neurourol Urodyn. 2005;24(2):149-150.
Abrams P, Klevmark B. Frequency volume charts: an indispensable part of lower urinary tract assessment. Scand J Urol Nephrol Suppl. 1996;179:47-53.
Abrams P, Wein A. The overactive bladder and incontinence: definitions and a plea for discussion. Neurourol Urodyn. 1999;18:413-416.
Al-Hayek S, Belal M, Abrams P. Does the patient’s position influence the detection of detrusor overactivity? Neurourol Urodyn. 2008;27(4):279-286.
Avery K, Donovan J, Peters TJ, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322-330.
Bates P, Bradley WE, Glen E, et al. [Function of the lower urinary tract. 3rd Report on standardization of terminology (author’s transl)]. Urologe A. 1980;19(5):315-317.
Bates P, Bradley WE, Glen E, et al. [Function of the lower urinary tract. 3. Report on terminology standardization: studies on urination analysis, pressure-flow functions and residual urine]. Z Urol Nephrol. 1980;73(10):768-772.
Birder LA. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc West Pharmacol Soc. 2001;44:85-86.
Bowden A, Colman S, Sabounjian L, et al. Psychometric validation of an urgency severity scale (IUSS) for patients with overactive bladder (abstract). Neurourol Urodyn. 2003;22:531.
Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50(6A Suppl.):57-67.
Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994 Jan;73(1):3-8.
Cardozo L, Dixon A. Increased warning time with darifenacin: a new concept in the management of urinary urgency. J Urol. 2005;173(4):1214-1218.
Cardozo L, Prescott K, Serdarevic D, Skillern L. Validation of the urgency perception scale. Value Health. 2002;5:277.
Charlton RG, Morley AR, Chambers P, Gillespie JI. Focal changes in nerve, muscle and connective tissue in normal and unstable human bladder. BJU Int. 1999;84(9):953-960.
Coyne KS, Elinoff V, Gordon DA, et al. Relationships between improvements in symptoms and patient assessments of bladder condition, symptom bother and health-related quality of life in patients with overactive bladder treated with tolterodine. Int J Clin Pract. 2008;62:925-931.
Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7(4):455-463.
Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563-574.
Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388-1395.
de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50(6A Suppl.):36-52. discussion 3–6
Drake MJ. The integrative physiology of the bladder. Ann R Coll Surg Engl. 2007;89(6):580-585.
Drake MJ, Harvey IJ, Gillespie JI. Autonomous activity in the isolated guinea pig bladder. Exp Physiol. 2003;88(1):19-30.
Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 2005;95(7):1002-1005.
Drake MJ, Hedlund P, Harvey IJ, et al. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003;170(1):276-279.
Drake MJ, Hedlund P, Mills IW, et al. Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest. 2000;80(10):1491-1499.
Drake MJ, Mills IW, Gillespie JI. Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet. 2001;358(9279):401-403.
Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J Urol. 1993;150(5 Pt. 2):1668-1680.
Fall M, Lindstrom S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281-300.
Fitzgerald MP, Kenton KS, Brubaker L. Localization of the urge to void in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24:633-637.
Garnett S, Swithinbank L, Ellis-Jones J, Abrams P. The long-term natural history of overactive bladder symptoms due to idiopathic detrusor overactivity in women. BJU Int. 2009 Apr 15.
German K, Bedwani J, Davies J, et al. Physiological and morphometric studies into the pathophysiology of detrusor hyperreflexia in neuropathic patients. J Urol. 1995;153(5):1678-1683.
Gillespie JI, Harvey IJ, Drake MJ. Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol. 2003;88(3):343-357.
Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37(1):1-7.
Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545-562.
Haferkamp A, Dorsam J, Elbadawi A. Ultrastructural diagnosis of neuropathic detrusor overactivity: validation of a common myogenic mechanism. Adv Exp Med Biol. 2003;539(Pt. A):281-291.
Haferkamp A, Dorsam J, Resnick NM, et al. Structural basis of neurogenic bladder dysfunction. III. Intrinsic detrusor innervation. J Urol. 2003;169(2):555-562.
Hanno P, Nordling J, van Ophoven A. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 2008;18(4):353-358.
Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? J Urol. 2006;175(1):191-194. discussion 4–5
Heslington K, Hilton P. Ambulatory monitoring and conventional cystometry in asymptomatic female volunteers. Br J Obstet Gynaecol. 1996;103(5):434-441.
Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128(3):593-607.
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306-1314. discussion 14–15
Irwin DE, Milsom I, Kopp Z, et al. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol. 2009;56:14-20.
Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87-114.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104(12):1374-1379.
Mills IW, Greenland JE, McMurray G, et al. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol. 2000;163(2):646-651.
Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760-766.
Oliver S, Fowler C, Mundy A, Craggs M. Measuring the sensations of urge and bladder filling during cystometry in urge incontinence and the effects of neuromodulation. Neurourol Urodyn. 2003;22(1):7-16.
Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257(22):3076-3081.
Sexton CC, Coyne KS, Kopp Z, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int. 2009;103(Suppl. 3):12-23.
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327-336.
Swithinbank LV, Donovan JL, du Heaume JC, et al. Urinary symptoms and incontinence in women: relationships between occurrence, age, and perceived impact. Br J Gen Pract. 1999;49(448):897-900.
Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic followup of transurethral resection of prostate for bladder outlet obstruction. J Urol. 2005;174(5):1887-1891.
Van Os-Bossagh P, Kosterman LM, Hop WC, et al. Micromotions of bladder wall in chronic pelvic pain (CPP): a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(2):89-96.
Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91(1):89-93.
Wyndaele JJ, De Wachter S. Cystometrical sensory data from a normal population: comparison of two groups of young healthy volunteers examined with 5 years interval. Eur Urol. 2002;42(1):34-38.