chapter 73 Slings
Autologous, Biologic, Synthetic, and Midurethral
Urethral slings are currently the procedure of choice for the surgical correction of female stress urinary incontinence (SUI). A variety of materials (autologous, allograft, and synthetic) and techniques have been pursued for sling placement. The concept of slings for urethral support was first introduced in 1907 by Von Giordano as a gracilis muscle graft wrapping around the urethra (Blaivas, 1994). German surgeons were using slings fashioned from muscle and fascia for slings in children at the turn of the century (Goebell, 1910). The rectus abdominis muscle and fascia were first used by Frangenheim in 1914 and then adapted by Millin for use in recurrent SUI (Frangenheim, 1914; Millin, 1948). It was Aldridge who first understood the abdominal relationship between muscles and urethra and showed that an increase in abdominal pressure caused simultaneous compression of the urethra. He used rectus fascial slings in conjunction with vaginal plastic operations (Aldridge, 1942). Jeffcoate (1953) used the Aldridge technique on 40 women and reported an 86% cure rate. It was McGuire’s use of the pubovaginal sling (PVS) in patients who failed prior retropubic suspension and anterior colporrhaphy, with a cure rate of 91% that reintroduced this procedure (McGuire, 1978). This is the modern description of the PVS, with placement at the bladder neck, in an effort to correct urethral hypermobility and modify the pressure transmission invoked by intra-abdominal pressure changes (Blaivas and Olsson, 1988).
Pubovaginal Sling
In addition to the correction of urethral hypermobility, the PVS (a sling at the bladder neck as distinct from a midurethral sling) is also indicated for intrinsic sphincter deficiency (ISD) associated with urethral hypermobility, SUI presenting with concomitant cystoceles (Cross et al, 1997; Serels et al, 1999), and SUI associated with urethral diverticulum or urethral defects (e.g., urethrovaginal fistula) in which urethral reconstruction is required and in women with SUI and associated neurogenic conditions (Austin et al, 2001). In neurogenic patients, such as those with myelodysplasia, a PVS is indicated for SUI that occurs between clean intermittent catheterizations (once a thorough urodynamic study [UDS] of compliance and bladder capacity is completed). A PVS is used during reconstruction of proximal urethral loss secondary to trauma, or erosion (synthetic material or prolonged Foley catheter), or for interposition in repairs of urethrovaginal fistulae or urethral diverticula (Gormley et al, 1994; McGuire and O’Connell, 1995; Blaivas and Heritz, 1996; Leng and McGuire, 1998). A PVS is also indicated for recurrent SUI, especially after failed retropubic suspensions or prior midurethral sling placement (Beck et al, 1988; Petrou and Frank, 2001). The definition of ISD has evolved from a strict urodynamic criterion to a clinical diagnosis. In understanding surgical treatment it is essential to appreciate that all women with SUI have some component of ISD. The PVS is the gold standard for management of all forms of SUI. The PVS has less morbidity than older retropubic suspensions and a better long-term outcome than needle suspensions and urethral collagen injections. Using female Medicare beneficiary records, Anger and colleagues (2009) identified patterns in the surgical treatment of women with SUI in the United States from 1992 to 2001. By 2001, the PVS became the dominant anti-incontinence procedure, corresponding with a steady decline in needle suspensions and anterior urethropexies (Anger et al, 2009).
Assessment of Intrinsic Sphincter Deficiency
The immobile urethra with poor intrinsic function has been classified as type III incontinence (Blaivas and Olsson, 1988) but is more commonly referred to as ISD. ISD is a complex condition with an imprecise definition and a large variability in measurement. The earliest measurements were by radiographic or endoscopic procedures, confirming an open proximal urethra and urethrovesical junction at rest (Blaivas and Olsson, 1988). Earlier methods for diagnosing ISD included the use of the supine stress test (empty and at capacity) (Lobel and Sand, 1996; Hsu et al, 1999), easy removal of inflated 8-Fr pediatric Foley balloon catheters as a positive office screening tool (Arya et al, 2001), color translabial sagittal ultrasonography for urethral vascularization measurement (Siracusano et al, 2002), ultrasound assessment of bladder neck funneling during straining (Huang and Yang, 2003), and translabial ultrasonography for measurement of the diameter of the urethra (Oliveira et al, 2006). In 2008, Oliveira and colleagues retrospectively reported lower opening detrusor pressure in stress incontinent women with ISD. Smith and Appell (2008) tried to determine whether voiding flow rates in women with symptomatic vaginal prolapse could predict ISD. Flow rates did not predict ISD (Hosker, 2009).
ISD continues to be defined imprecisely by a low-pressure urethra based on urethral closure pressures and abdominal leak point pressures. A maximum urethral closure pressure (MUCP) of 20 cm H2O or less has been a determinant of ISD since McGuire’s (1981) research on failed SUI procedures. Although patients who experienced treatment failure were more likely to have an MUCP of 20 cm H2O or less, McGuire was unable to determine whether closure pressure was the cause or effect of the failures. Therefore, Sand and associates (1987) performed UDS on 86 women undergoing modified Burch colposuspensions. The women were divided into two groups: one with an MUCP of 20 cm H2O or less and one with an MUCP of more than 20 cm H2O. The group with lower urethral closure pressures had a 54% failure rate, compared with 18% in the group with higher urethral closure pressures. Sand and associates concluded that a modified Burch colposuspension was not appropriate in patients younger than age 50 years with an MUCP of 20 cm H2O or less. Also other authors have not used an MUCP of less than or equal to 20 cm H2O to define ISD (Clemons and La Sala, 2007; Fritel et al, 2008). Abdominal leak point pressure (ALPP) less than or equal to 60 cm H2O has been used as a determinant of ISD since videourodynamic analysis by McGuire and colleagues (1993). They found that women who leaked with low abdominal pressures (5 to 60 cm H2O) were most seriously incontinent and 75% had ISD on videourodynamics (proximal urethral pressure <10 cm H2O at 0.5 cm from the bladder neck or a nonfunctioning open sphincter with leakage). There was no correlation between ALPP and MUCP (Hosker, 2009). ISD is clinically relevant, but until the imprecision of diagnosis is addressed it cannot be utilized completely.
Anatomy and Mechanics
Female SUI is due to a combination of urethral hypermobility and ISD. The female urethra lies under the pubic symphysis, and the pubourethral ligaments suspend the anterior urethral wall to the pubic arch. During Valsalva or other stress maneuvers the posterior wall of the urethra slides away from the anterior urethral wall and causes the bladder neck to open in cases of urethral hypermobility. The uneven pressure transmission combined with opening of the bladder neck (funneling) causes a loss of urine with stress maneuvers. ISD arises from defects within the urethra proper, so that the urethral sphincter is unable to coapt and generate enough resting urethral closing pressure to store urine in the bladder. The female urethra is composed of four separate tissue layers that assist in keeping it closed. It is compression from the middle muscular coat that helps to maintain the resting urethral closure mechanism and the outer seromuscular layer that augments this closing pressure. In normal circumstances the resting urethral closing pressure of the internal sphincter exceeds the resting or Valsalva pressure exerted by the bladder. Additionally, the fast-twitch fibers of the external sphincter allow for the sudden voluntary guarding reflex and the slow-twitch fibers provide continuous passive control by the involuntary guarding reflex. In addition to these structures the integrity of the pelvic diaphragm is dependent on the levator ani for continence control. The urethropelvic ligament and pubocervical fascia also provide needed support to the bladder neck and undersurface of the bladder, respectively, to prevent SUI (Vasavada and Rackley, 2011).
The PVS is positioned at the bladder neck to provide urethral compression without obstruction during times of increased intra-abdominal pressure. The ultimate goal is to provide adequate urethral coaptation to increase urethral responsiveness to abdominal pressure. This must be balanced with the risks of ischemia, retention, and erosion from unnecessary tension. Aldridge’s (1942) earliest anatomic descriptions had fascia attached at the medial aspect of the rectus limiting sling mobility and provided no method for avoiding excess tension, resulting in outlet obstruction. The sling was often too short to pass under the urethra. McGuire (1978) modified this technique with a strip of rectus fascia 1 × 12 cm in length, which remained attached laterally on one side. In this technique there was also no way to adjust the tension with one side attached. The modifications by Blaivas and Jacobs (1991) with a free graft of rectus fascia whose tension could be adjusted (by pulling up and tying the sutures at each end of the free graft) are the present concept of the PVS. This allows for a shorter fascial sling. The key to the length of the fascial sling is its method of providing support. It is the incorporation of the sling into the endopelvic fascia and subsequent fibrosis, and not entry into the retropubic space, that prevents SUI. The width (2 to 3 cm) of the PVS ensures that there is sufficient support to provide the needed urethral compression and a cross-sectional area adequate to avoid the formation of a narrow constricting band. The choice of material in PVS requires material longevity and durability to allow for a strong sling scaffolding. The material should be incorporated and remain intact, with limited tissue reaction.
Sling Materials
Various substances have been utilized for construction of a PVS—autologous, allograft, xenograft, or synthetic materials. The ideal material provides long-lasting suburethral support with minimal complications. Ideally, implanted material should be incorporated into the host with minimal tissue reaction. In reality, most materials promote organized fibrosis, reinforcing the sphincteric mechanism through improved suburethral support. Theoretically, a greater degree of fibrosis leads to better clinical results (Bidmead and Cardozo, 2000; Woodruff et al, 2008). Yet, inflammatory infiltration leads to rapid sling material degradation and possible tissue destruction with erosion (Bidmead and Cardozo, 2000). Although there is complete biocompatibility of the autologous sling and negligible urethral erosion, biologic and synthetic graft materials have been increasingly used to decrease operative time, morbidity, pain, and hospital stay (Niknejad et al, 2002). Outcomes of these materials are discussed later.
Autologous Materials
The most commonly used autologous materials include rectus fascia and fascia lata. Rectus fascia is harvested through a suprapubic Pfannenstiel incision. FitzGerald and associates (2000) reported that after sling placement, rectus fascial grafts undergo extensive remodeling with increased fibroblasts and connective tissue on biopsy specimens. Yet, histologic comparison of PVS grafts noted the greatest degree of host fibroblast infiltration and neovascularization in autologous materials with minimal inflammatory or foreign body reaction. The fascial graft changes were consistently intact with a small amount of sling degradation at explantation up to 65 months after placement (Woodruff et al, 2008). The rectus fascia harvest site may be scarred and thickened after prior operations, but this does not compromise its utility for PVS placement. The benefits of autologous tissue include the lack of tissue reaction and negligible urethral erosion (Webster and Gerridzen, 2003). Disadvantages include increased operative time and hospital stay, relative increase in postoperative pain, and suprapubic tissue seroma formation and hernia formation in a rectus fascial PVS (Gomelsky et al, 2003).
Fascia lata is the other commonly utilized autologous material for a PVS. It is harvested from the thigh and has similar properties to rectus fascia (Beck et al, 1988; Latini et al, 2004). Methods for harvest are discussed in the section on operative procedure. Like rectus fascia, fascia lata is completely biocompatibility and is associated with minimal tissue reaction. The recovery time may be less, and there is no risk of future abdominal hernia formation, unlike rectus fascia. Yet, fascia lata requires repositioning of the patient, increased operative time, and operating in an area unfamiliar to urologists (Govier et al, 1997). Wheatcroft and colleagues (1997) reported 67% of their patients had pain on walking for 1 week after surgery. Latini and coworkers (2004) only reported 7% of patients with pain at incision site 1 week postoperatively after using a Crawford fascial stripper. Debility from a thigh hernia has also been reported.
Another autologous material, vaginal wall, has also been used. Raz and associates (1989) described use of in-situ vaginal wall for autologous sling material. This tissue may lack sufficient tensile strength, and there is a risk of epithelial inclusion cyst formation and possible vaginal shortening. Lack of retropubic space dissection may militate against overall efficacy of this procedural variety (Raz et al, 1989; Ghoniem and Hassouna, 1998; Loughlin, 1998; Appell, 2000).
Allograft Materials
Biologic and synthetic graft materials have been increasingly used to decrease operative time, morbidity, pain, and hospital stay. Cadaveric allografts have been used in many nonurologic surgical arenas (e.g., orthopedics, neurosurgery) and eventually adopted for SUI. Allograft slings are currently derived from either cadaveric fascia lata or acellular human dermis. After harvest the allografts are processed by solvent dehydration or by lyophilization (freeze-drying) to remove genetic material and to prevent the transmission of infectious agents. Secondary sterilization may also be achieved by gamma radiation (Gomelsky et al, 2003). Histologic analysis reveals cadaveric dermis to have little host fibroblast infiltration and little neovascularity, particularly in central aspects of the graft. Additionally, inconsistencies were found within the materials grossly, with specimens exhibiting significant thinning and degradation of the graft, disrupting the sling scaffold (Woodruff et al, 2008). No specific allograft has shown a clinical advantage in use. Acellular dermis rehydrates in 0.9% saline quicker than does cadaveric fascia lata (5 minutes vs. 15 to 30 minutes), but each type is pliable, easy to use, and available in a variety of sizes (Gomelsky et al, 2003).
Biomechanical studies have shown that solvent-dehydrated cadaveric fascia lata and acellular dermis have a higher maximal load failure compared with freeze-dried cadaveric fascia lata (Hinton et al, 1992; Lemer et al, 1999). Lemer and associates (1999) prospectively studied the maximum load failure and stiffness of rectus fascia versus freeze-dried fascia versus solvent-dehydrated fascia and cadaveric dermal grafts. The mean values for maximum load to failure, maximum load-graft width, and stiffness were all significantly lower for the freeze-dried fascia lata group compared with the autologous, solvent-dehydrated, and dermal graft groups. The authors believed that ice crystal formation characteristics of tissue freezing may disrupt the collagen matrices, yielding poor strength properties. Dermal grafts differ from fascial allografts because they are derived from skin that is processed to eliminate the epidermis and all immunogenic cellular elements. Dermal grafts provide a protein matrix that serves as a collagen scaffold for the host’s own cellular matrix (Lemer et al, 1999).
Although biomaterials were thought to be a good choice for increased biocompatibility, lower risk of erosion, and lack of response to hormonal stimuli, allografts raise the concern of potential transfer of disease, including human immunodeficiency virus (HIV), hepatitis, and Creutzfeldt-Jacob prion infection. There has been one documented case of HIV transmission from a tissue transplant since the onset of screening in 1985. The estimated risk of acquiring tissue from a properly screened donor infected with HIV is 1 in 1,667,600 (Gallantine and Cespedes, 2002). A few cases of Creutzfeld-Jakob disease (CJD) have been reported after transplantation of cadaveric dura or corneas; however, skin obtained from animals infected with prions has demonstrated no detectable infectious particles. Currently, the theoretical risk of developing CJD from a non-neural allograft is 1 in 3.5 million. No cases of hepatitis or CJD have ever been attributed to the use of processed cadaveric fascia or dermis (Amundsen et al, 2000b; Gallantine and Cespedes, 2002). Within the musculoskeletal tissue transplantation literature, two cases of hepatitis transmission have been reported. The first refers to one tissue donor (cancellous chips) who transmitted HIV, hepatitis B virus, and human T-lymphotrophic virus. These transmissions all occurred before the implementation of extensive donor screening for viruses and bacteria or the availability of validate serologic tests (or both) (Shutkin, 1954). In June 2002, the Centers for Disease Control and Prevention (CDC) reported a case of hepatitis C transmission from minimally processed, cryopreserved patellar tendon allograft. Donor screening was performed during the window period for hepatitis C virus (HCV). Retest of the donor sample with HCV RNA testing confirmed the donor as the source once the recipient reported HCV infection 1 year after transplantation (CDC, 2003; Vangsness, 2006). Despite the low risk of disease transmission, human DNA has been detected in various allograft materials (Choe and Bell, 2001; Hathaway and Choe, 2002). The clinical significance of this is unknown. The theoretical risk of developing hepatitis from allograft graft material is unknown.
Xenograft Materials
Xenografts have been utilized since the 1980s (Descurtins and Buchmann, 1982; Iosif, 1987) owing to their immediate accessibility and use without morbidity. Porcine and bovine xenografts have been used as sling material with decreasing popularity in recent years. The forms of xenograft available for use are porcine dermis or small intestinal submucosa (SIS) and bovine pericardium. Modern processing techniques using diisocyanate to remove genetic material have made porcine safer and more pliable, yet there is significant loss of tensile strength after implantation in a 12-week rabbit model (Dora et al, 2004). Histopathologic analysis has shown porcine SIS to contain growth factors that may reduce significant host-graft immunologic reaction and result in less tissue scarring (Wiedemann and Otto, 2004). Although a majority of data support SIS as nonimmunogenic, animal studies by Thiel and colleagues (2005) suggest that an intense inflammatory reaction 30 to 90 days after subcutaneous implantation occurs. In a report by Kalota (2004), 6 of 18 (33%) patients experienced postoperative inflammation after a PVS procedure. Konig and coworkers (2004) reported a single case of postoperative inflammation with abscess formation. Ho and colleagues (2004) reported a similar reaction in 6 of 10 patients. All of the patients presented with pain and erythema at the abdominal incision, and 2 developed abscesses. Five of the 6 patients with inflammatory responses are currently dry. All were treated conservatively, except 1 patient who required abscess drainage. The etiology is unknown but is likely due to a foreign body reaction from the multilayered (8-ply) SIS material, a reactive manufacturing ingredient, or a tendency for suprapubic fat to produce an inflammatory reaction (John et al, 2008). Kubricht and colleagues (2001) have shown that porcine SIS has less tensile strength than cadaveric fascia lata (Kubricht et al, 2001). Bovine pericardium is available in a preparation either cross-linked with glutaraldehyde or as a non–cross-linked acellular matrix (Gomelsky et al, 2003). Histopathologic comparison of sling materials revealed xenograft (porcine dermis) to have no host fibroblast infiltration, no inflammatory reaction, and no foreign body reaction. Xenograft had the highest propensity to encapsulate. A capsule formed around the porcine dermis, isolating the graft from the periurethral tissue. The graft was described as appearing similar to its original appearance at time of implantation (Woodruff et al, 2008).
Synthetic Materials
In 1959, Francis Usher introduced the first synthetic biomaterial—polyethylene mesh—for use in hernia surgery. In the decades since there has been a transition to polypropylene and the introduction of additional synthetic materials (Amid, 1997). The first synthetic sling, made of nylon, was introduced in 1953 (Kraatz, 1953). The addition of synthetic material for use in PVS surgery brought obvious advantages: unlimited supply of artificial graft material in endless sizes and shapes, consistency in quality, and elimination of harvest site and decreased associated operative time. As compared with absorbable biomaterials, synthetic materials are more uniform, more consistent, and more durable. Additionally, artificial graft materials are sterile, biocompatible, noncarcinogenic, and free of biomaterials (Niknejad et al, 2002). On histopathologic comparison, synthetic materials demonstrated the greatest amount of fibroblast ingrowth and tissue ingrowth into the specimen. There is no degradation or disruption of the graft, and the mesh is completely infiltrated by the viable host tissue. Microscopically, the synthetic material has large amounts of fibroblasts and foreign body reaction characterized by giant cells and occasional microcalcification. This foreign body reaction is not visible grossly by graft disruption, and the graft was completely infiltrated by host tissue (Woodruff et al, 2008).
Artificial graft materials do have potential drawbacks, including graft infection, genitourinary erosion, or vaginal extrusion. The chemical and physical properties of each artificial material and patient characteristics determine how the sling is incorporated into the surrounding tissue and its susceptibility to infection, rejection, erosion, or extrusion. The susceptibility to infection in multifilament fibers is proportional to the porosity and the pore size of the materials (Amid, 1997; Niknejad et al, 2002). Tightly woven mesh provides a safe harbor for small bacteria, excluding macrophages and polymorphonuclear leukocytes. Loosely woven mesh allows tissue ingrowth and neovascularization, without limiting cellular access. Tissue bonding to the mesh strengthens and supports the repair. A tightly woven and large-diameter filament mesh will tend to exhibit increased stiffness or decreased pliability, contributing to migration, extrusion, or erosion. The classification by Amid (1997) used for synthetic materials in hernia surgery may be practically applied to urology as well (Table 73-1). The most frequently used materials are grouped into four types. Type I are totally macroporous prostheses (Atrium, Trelex, Marlex, Prolene) containing pores larger than 75 µm, which is the pore size for admission of macrophages, fibroblasts, blood vessels, and collagen fibers (White et al, 1981; Bobyn et al, 1982; White, 1988). Type II includes totally microporous prostheses (polytetrafluoroethylene [PTFE]: GORE-TEX, Surgical Membrane, and Dualmesh) containing pores less than 10 µm in at least one of their dimensions. Type III includes a macroporous prosthesis with multifilamentous or microporous components (PTFE: Teflon; braided Dacron mesh: Mersilene; braided polypropylene mesh: Surgipro; and perforate PTFE patch: MycroMesh). Lastly, type IV includes biomaterials with submicronic pore size (Silastic, Cellgard (polypropylene sheeting). Type IV is not appropriate for hernia surgery unless used in combination with type I (Amid, 1992). The most commonly utilized synthetic material for a PVS is polypropylene mesh (Table 73–2). It is composed of loosely woven strands of synthetic material, with a pore size greater than 80 µm, permitting passage of macrophages that may allow better host tissue ingrowth compared with the smoother, more tightly woven counterparts (Kobashi et al, 2005). This represents type I among the Amid classification. In fact, Amid (1997) concluded that the risk of infection and seroma formation was avoided by utilization of type I prostheses.
Table 73–1 Amid Classification for Synthetic Materials
| TYPE | DESCRIPTION | BRANDS |
|---|---|---|
| I | Pores >75 µm; macroporous | Atrium, Trelex, Marlex, Prolene |
| II | Pores <10 µm; microporous | PTFE: GORE-TEX, Surgical Membrane, Dualmesh |
| III | Macroporous with multifilamentous or microporous components | PTFE: Teflon, braided Dacron mesh (Mersilene), braided polypropylene mesh (Surgipro), perforated PTFE patch (MicroMesh) |
| IV | Submicronic pore size | Silastic, Cellcard (polypropylene sheeting) |
PTFE, polytetrafluoroethylene.
Adapted from Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997;1:15–21.
Table 73–2 Synthetic Sling Materials
| TRADE NAME | COMPOSITION | DETAILS |
|---|---|---|
| Mersilene | Polyethylene terephthalatae | Multifilament fibers Very porous, becomes firmly embedded in native tissues |
| Teflon | Polytetrafluoroethylene (PTFE) | Multifilament |
| GORE-TEX | Expanded PTFE | Very flexible |
| Silastic | Silicone plus woven polyethylene terephthalate | Minimal tissue reaction, which facilitates removal or revision if necessary |
| ProteGen | Synthetic mesh impregnated with collagen matrix | Removed from market secondary to high rate of vaginal extrusion |
| Marlex, Prolene | Polypropylene | Monofilament with open-weave pattern |
Adapted from Niknejad K, Plzak LS, Staskin DR, Loughlin KR. Autologous and synthetic urethral slings for female incontinence. Urol Clin North Am 2002;29:597–611.
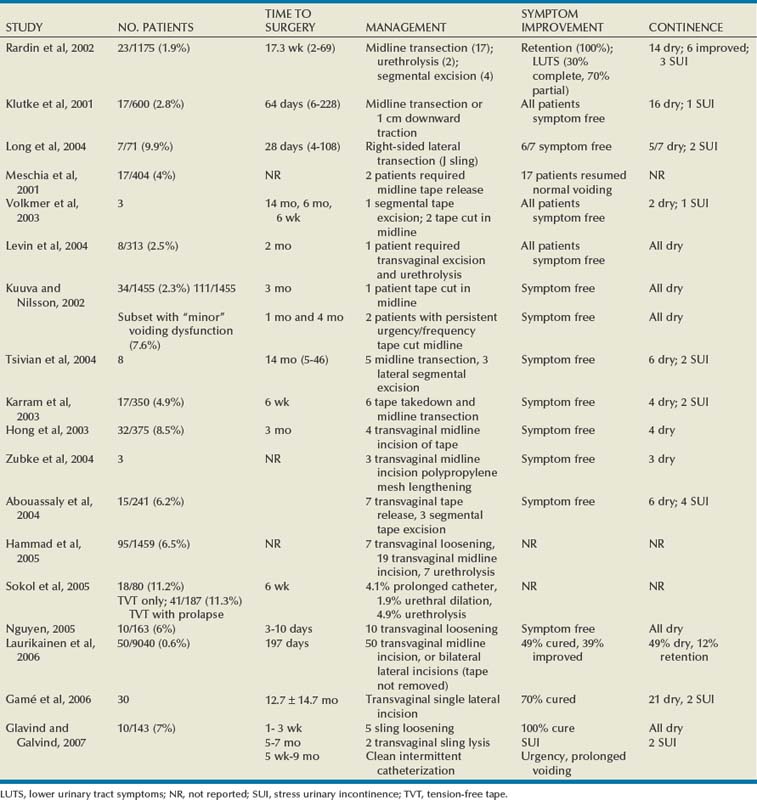
Historically, sling techniques have changed to limit the associated morbid complications. Synthetic material is no longer utilized in a PVS graft to pull the bladder neck into a high retropubic position owing to high erosion rates (see section on complications). Instead, newer approaches position a sling at the midurethra (see later) (Niknejad et al, 2002).
Key Points: Pubovaginal Sling Materials
Operative Procedure
Preoperative and Preliminary Steps
Before performing a PVS, a thorough history and physical examination is performed. Key points in the history should include preoperative voiding status and symptoms. Specifically, the relationship between stress maneuvers (i.e., cough, sneeze, laugh, weight bearing, exercise) and related incontinence should be elucidated. It is important to identify preoperative storage symptoms, such as frequency, urgency, and urinary urgency incontinence (UUI), because these symptoms may not resolve after a sling procedure. A complete history of all prior vaginal and abdominal operations should include graft material placement, prior urethrolysis, and/or prolapse surgery. A history of prior irradiation is useful because this may compromise the quality of a rectus fascial graft. Physical examination should include an abdominal examination and complete pelvic examination to assess urethral hypermobility, quality of vaginal epithelium, extrusion of previously placed graft material, and prolapse. Cystocele and other forms of prolapse should be assessed, because they may lead to symptomatology and be a source of future obstruction. Special attention should be paid to the anterior vaginal compartment to determine if coexistent prolapse is present. The authors recommend UDS on all women undergoing surgical intervention for SUI, with or without simultaneous prolapse correction. UDS is recommended by the International Consultation on Incontinence (ICI) before undergoing surgical procedures to obtain a more accurate preoperative diagnosis (Griffiths et al, 2005). Knowing the urodynamic abnormalities responsible for urinary manifestations is essential to directing treatment at the underlying pathophysiology. The American Urological Association (AUA) recognizes uroflow, cystometry, leak point pressures, sphincter electromyography, and radiographic visualization as five modalities useful in understanding bladder storage and emptying. Implicitly, diagnosing impaired detrusor contractility or obstruction requires Pdet/uroflow, and cystometry is necessary for identifying detrusor overactivity (DO) (Rutman and Blaivas, 2007). A cough stress test may demonstrate SUI, but it provides no information regarding a patient’s bladder storage and emptying characteristics. This information is essential for tailoring the appropriate treatment plan. “The bladder is an unreliable witness,” because symptoms are not predictive of diagnosis. UDS may aid the clinician in detection of preoperative DO, preoperative abnormal voiding patterns (high postmicturition residual, obstructed flow), and de novo DO (Digesu et al, 2009). Cundiff and colleagues (1997) studied 535 incontinent women and concluded that pure symptoms identified less than half of the patients with urodynamic SUI, recommending UDS for all patients considering surgical intervention. UDS should be performed with and without a pessary if prolapse is present.
This procedure may be performed with the patient under spinal or general anesthesia, based on preference determined between the surgeon and anesthesiologist. A single perioperative dose of cefazolin or fluoroquinolone is given intravenously (as per Centers for Medicare and Medicaid Services recommendations). Bilateral lower extremity sequential compression devices (SCDs) are placed before administration of anesthesia. The patient is placed in the dorsal lithotomy position, and the abdomen (from umbilicus down) and vagina are prepped and draped in sterile fashion. A weighted speculum is placed in the vagina, and an 18-Fr Foley catheter is placed into the urethra and left in place with the bladder left to straight drainage. The patient should be placed in moderate Trendelenburg position, and the surgeon would benefit from a headlight for optimal visualization during vaginal dissection. A vaginal ring retractor is utilized initially for retracting the labia majora for ease with dissection and visualization.
In the case of fascia lata harvesting, the SCDs are placed below the patient’s patella on the harvest side. The knee is elevated and supported with a 1-L bag of intravenous fluid or an appropriate cushion or pad. The involved extremity is internally rotated at the hip and secured to the table using 3-inch tape, below the operative site. The thigh is prepped and draped to expose the anterolateral aspect of the thigh from the greater trochanter to distal to the patella. The greater trochanter and lateral femoral condyle of the femur are identified and marked. These landmarks mark the proximal and distal attachments of the fascia lata (Dwyer and Kreder, 2008).
Graft Harvest
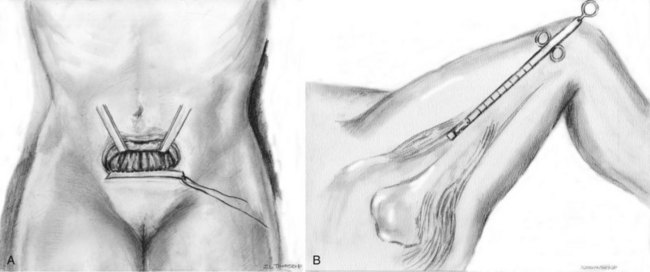
A 6- to 7-cm Pfannenstiel incision is made approximately 2 cm above the pubic symphysis and carried down to the rectus fascia. A 2-cm wide by 8-cm long graft is marked out in the rectus fascia. The premarked graft is harvested out of the rectus fascia. With the use of Allis clamps the edges of the free fascia are freed from the underlying rectus muscle with bovie cautery to aid in a tension-free closure (Fig. 73–1A). The fascia is closed with a running 1 polydioxanone (PDS) suture or other alternative suture. The graft is placed in 0.9% normal saline solution after harvest. On a sterile side table, the overlying fat and perifascial tissue are cleaned off of the graft. A size 1 polydioxanone suture is utilized (perpendicular to sling fibers) at each end of the sling to run across the full width of the sling and tied down. Sutures are left long, and the graft is placed in the 0.9% normal saline until needed.
For fascia lata harvest, a 3-cm longitudinal incision is marked beginning just above the patella over the iliotibial band (see Fig. 73-1B). Dissection is carried down to the level of the fascia lata, where two parallel incisions are made longitudinally in the fascia lata, 2 cm apart. The autologous fascia is bluntly lifted off the underlying muscle and clamped as far distally as possible with a right-angle clamp (3 to 4 cm) and transected, allowing one free end. The free end is secured with a 1-0 polypropylene, polydioxanone, or polyglactin suture, and the proximal fascia lata is lifted off the muscle belly with a thin, malleable retractor. The fascia lata is separated from both the adipose tissue and the muscle fibers by passing the retractor superficial and deep to the fascia lata. With the free distal end under tension, a Crawford fascial stripper is used to extend the fascial incision proximally and divide it before removal. Classically, the fascial strip was 20 × 2 cm in dimension; however, now shorter lengths (8 cm) are used (Karram and Bhatia, 1990). Another 1-0 polypropylene monofilament suture is secured to the other free end of the graft, and the graft is placed in 0.9% normal saline until needed. Immediate compression is applied to the thigh to constrict perforating vessels. The wound is irrigated and closed in three layers without closing the fascia lata. The area is carefully evaluated for bleeding arteries before closure. Once the thigh closure is complete, a compressive wrap is applied to the thigh and the SCD is replaced (to remain in place for the duration of the hospital stay). The compressive bandage should remain in place for 8 hours postoperatively, and the patient should be encouraged to ambulate early postoperatively (Dwyer and Kreder, 2008).
Vaginal Approach
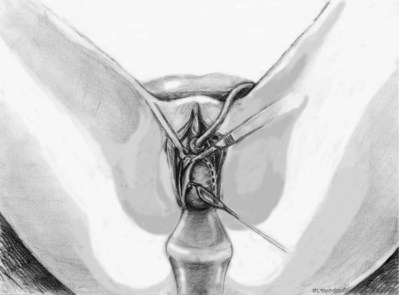
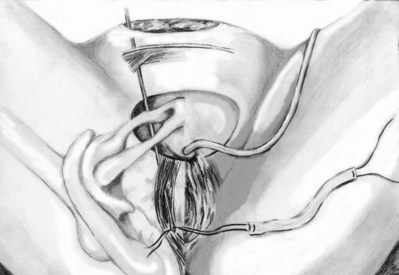
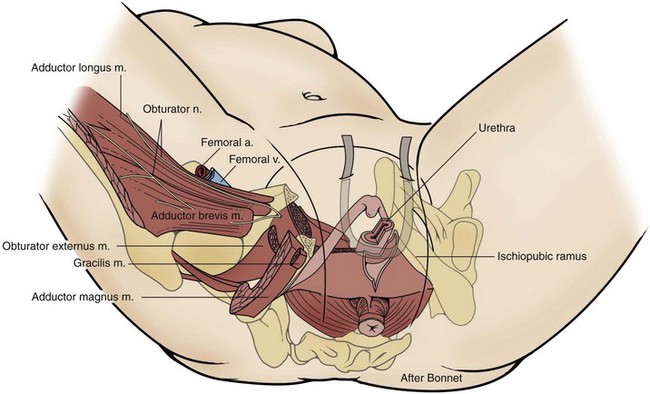
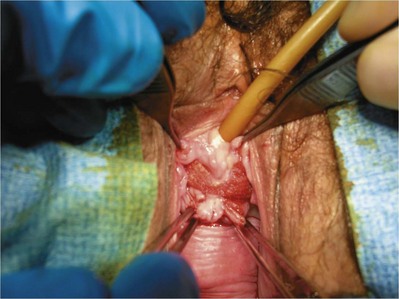
Sterile 0.9% normal saline is injected into the vaginal epithelium, surrounding the urethra to provide hydrodistention and aid in tissue dissection. The authors prefer an inverted-U–shaped incision because it enables adequate exposure to the urethra and bladder and access to the endopelvic fascia and subsequent retropubic space (Fig. 73–2). The top of the incision is approximately 2 cm below the urethral meatus (easily visualized with assistance of an Allis clamp placed immediately below the meatus), and the arms of U should extend to the level of the bladder neck (determined by Foley balloon location). A No. 15 blade knife is used to carry this incision down through the vaginal epithelium, with care to stay above the periurethral and pubocervical fascia (to avoid bleeding and injury to the urethra and bladder). With an Allis clamp and Metzenbaum scissors, thick vaginal epithelial flaps are created. The flaps are retracted with the help of the vaginal ring retractor. Once adequate lateral flaps have been created and the ischiopubic rami are easily palpated, it is appropriate to perforate the endopelvic fascia. It is imperative that the bladder is adequately drained before this maneuver and before later passage of Stamey needles or larger clamps to prevent inadvertent bladder perforation. With the tips of the Metzenbaum scissors pointed upward and to the ipsilateral shoulder, the endopelvic fascia is perforated by remaining directly medial and immediately under the ischiopubic ramus at the superior margin of dissection (Fig. 73–3). Perforation occurs in a superolateral direction, and the dissecting scissors are spread widely to aid in dissection. With the use of blunt finger dissection, the retropubic space is dissected bilaterally (Fig. 73–4). This dissection leads to the connection between the infrapubic and retropubic dissection planes. Simultaneous finger palpation from abdominal and vaginal incisions should be possible, while gently palpating the bladder medially. Aggressive medial mobilization should not be attempted because it may result in bladder injury. Hemostasis should be achieved with bipolar cautery. In the case of women who have undergone prior urethral suspension or sling, more aggressive dissection may be required. The dissection plane into the retropubic space should be immediately adjacent to the periosteum of the pubis, and the dissection should be performed only sharply to minimize risk of injury to the pelvic viscera.
Sling Placement and Fixation
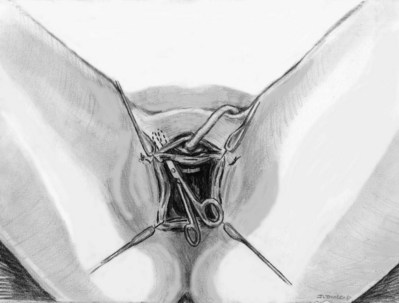
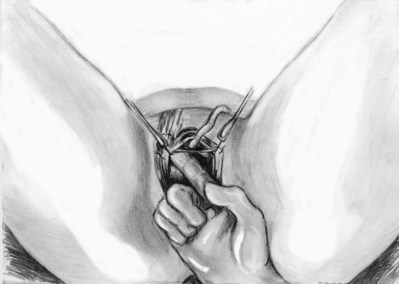
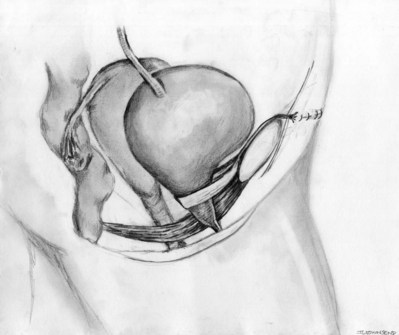
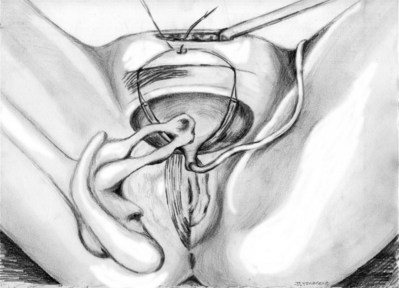
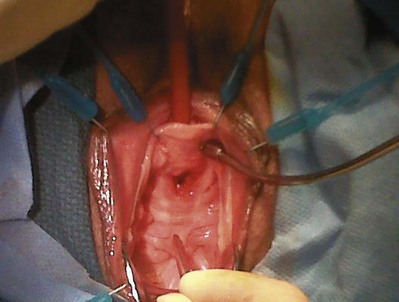
Stamey needles or large clamps are passed from above, through the abdominal incision by careful guidance behind the pubis, and passage is in contact with the pubis throughout until brought out lateral to the bladder and through the vagina (Fig. 73–5). Alternatively, large surgical instruments such as Tonsil clamps may also be used (McGuire, 1978; Blaivas and Olsson, 1988). The bladder must be completely drained before passage of the Stamey needles to avoid inadvertent bladder injury. Cystoscopy should be performed with a 70-degree lens after passage of the needles to confirm the integrity of the bladder, by following the course of the needles (while an assistant moves them). Cystoscopy is not practiced consistently by all urologists after needle passage except when there is visible hematuria or another cause for suspicion (Niknejad et al, 2002; Seung-June et al, 2007). It is the authors’ belief that this step eliminates the morbidity and reoperation required for passage of the sling through the bladder and that cystoscopy is an essential step. In the case of a small bladder injury or inadvertent passage of Stamey needles through the bladder, the needles are removed and passed again and the procedure is completed. Extravesical passage is confirmed. One ampule of indigo carmine is given at this point to confirm ureteral efflux during final cystoscopy for tensioning of sling. The Foley catheter is replaced. The ends of graft suture are passed through the Stamey needle eyelets. After marking the center of the graft with a clamp, the Stamey needles are removed and the ends of the suture are brought out through the abdominal incision and tagged with hemostat clamps (Figs. 73-6 and 73-7). The distal aspect of the graft is sutured to the periurethral tissue with two simple 4-0 polyglactin sutures. The vaginal incision is closed with a watertight, running 2-0 polyglactin suture after complete hemostasis is achieved. Before final tensioning of the sling (see Fig. 73-7) the vagina should be closed and the weighted speculum removed to eliminate factors that can affect the final tension. The polydioxanone sutures are tied down above the rectus fascia (see Fig. 73–7) while cystoscopy with a 30-degree lens is performed to visualize adequate coaptation of the proximal urethra. There is approximately a two-fingerbreadth width between the rectus fascia and the sutures once tied down. The amount of tension may vary owing to the mobility of the urethra or desire to create permanent retention in an individual who will have permanent catheterization. The abdominal incision is closed with a subcuticular 4-0 polyglactin suture. The Foley catheter is left to straight drainage, and a vaginal pack moistened with conjugated estrogens is placed.
Postoperative Care
The vaginal packing is removed on postoperative day 1, and the patient is given a voiding trial once she is ambulating. If the patient voids adequately (after postvoid residual urine confirmation), she is discharged. If she is unable to urinate or has an elevated postvoid residual urine volume, she is discharged with a Foley catheter. She will return within 5 days for a repeat trial of voiding. Patients are instructed to avoid heavy lifting (>5 lb) and sexual intercourse for 6 weeks postoperatively. Sexual intercourse should be resumed only after a physical examination by the surgeon.
Outcomes
Autologous Pubovaginal Sling
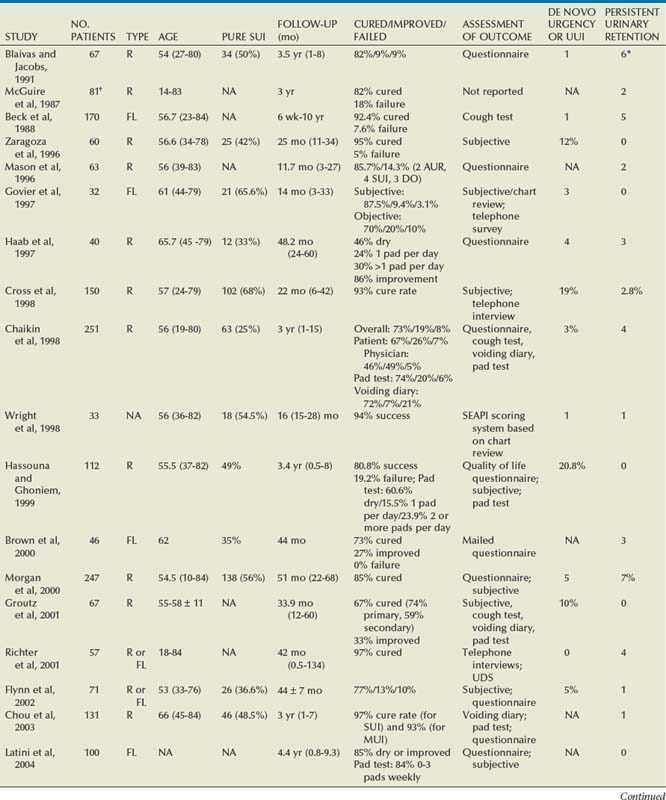
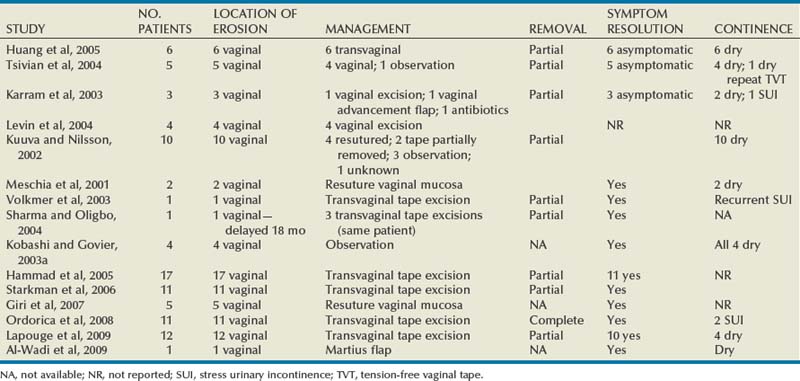
Since McGuire’s reintroduction of the autologous PVS in 1978 with 80% overall success rate, continence rates have been reproducibly satisfactory, even at long-term follow-up (Table 73–3). Earliest series (McGuire, 1987; Blaivas and Jacobs, 1991) with rectus fascial slings include a diverse and complex patient population, with patients having a history of pelvic irradiation, diabetes, spinal cord injury, and pelvic trauma. Poor or no urethral function was associated with 35% of cases (McGuire, 1987). Cure rates among these earliest series were similar (82%). A main cause of failure was urgency and UUI. A majority of the patients who required clean intermittent catheterization postoperatively had a neurogenic bladder and were counseled to expect urinary retention. Harvesting of rectus fascia appears a favored option over fascia lata for autologous material, likely owing to the urologist’s greater comfort with abdominal anatomy. Yet, intermediate and long-term results of fascia lata PVS procedures (Beck et al, 1988; Govier et al, 1997) are comparable to those that use rectus fascia. Beck and colleagues (1988) noted that all three sling failures occurred within the first 22 cases performed and that 10 of the 13 overall failures were due to UUI. Five (3%) of the patients in this study population were extremely bothered by delayed voiding (mean interval of 59.6 days) and underwent a urethrolysis. Govier and associates (1997) administered an independent survey to assess outcomes, yielding worse outcomes than cure rates reported based on their chart review (70% vs. 87.5%, respectively). The vast majority of incontinence reported postoperatively was related to urgency. This patient satisfaction rate was similar to that noted by Brown and associates (2000) with questionnaire-based outcomes. In this group, all of the patients had thigh pain at 1 to 2 weeks and 11% described persistent thigh pain at 6 weeks. Latini and colleagues (2004) used the Crawford fascial stripper to obtain fascia lata. They reported 20% localized numbness at harvest site, 7% with harvest site pain 1 week postoperatively, 5% tendonitis in the harvest site leg, and 1 case of a harvest site hematoma requiring physical therapy. Yet, 83% of respondents indicated that the procedure had a positive effect on their life, 82% would recommend the surgery to a friend, and 83% would undergo the procedure again (Latini et al, 2004).
The rectus fascial autologous sling has been effective up to 7 years (Howden et al, 2006) postoperatively with low morbidity when performed by experienced surgeons. Cure rates have ranged from 46% to 97%, although the measurement of outcomes is varied. Patient-derived cure rates are lower than physician-based or chart review–based outcomes. There is no direct correlation between universally accepted objective and subjective measures of improvement or cure for anti-incontinence procedures (Padmanabhan and Nitti, 2006). The most commonly cited reason for failure relates to urgency symptoms. UUI at follow-up is a common reason for patient dissatisfaction. Reported de novo or UUI rates are 2.0% to 20.8% (Mason and Roach, 1996; Zaragoza, 1996; Haab et al, 1997; Chaikin et al, 1998; Cross et al, 1998; Hassouna an Ghoniem, 1999; Morgan et al, 2000; Groutz et al, 2001; Flynn and Yap, 2002; Albo et al, 2007; Mitsui et al, 2007; Onur et al, 2008). Zaragoza and colleagues (1996) report that their three failures had persistent leakage due to UUI (present preoperatively). One of these patients had previous pelvic irradiation and radiation cystitis, and 1 had additional UDS, confirming bladder instability and lack of urethral obstruction. The short-term complication of postoperative retention that occurred in 60% immediately postoperatively is highlighted. Urinary retention persisted in this group for a median of 6.5 days (1 day to 5 weeks) and resolved in 70% within 10 days. Mason and Roach (1996) confirmed the success of a modified rectus fascial sling (smaller graft, 4 × 2 cm) with intermediate follow-up. Twelve (19%) patients had urgency, and 10 (15.9%) had frank UUI. By 6 months these symptoms had resolved in all but 3 (4.8%), who remained on anticholinergic medication. Haab and associates (1997) provided results of long-term follow-up using a self-administered questionnaire to assess success rates and overall patient satisfaction. Among the 3 patients (7.5%) requiring permanent clean intermittent catheterization, 2 had known sacral arc denervation and were expected to have urinary retention. These investigators cited the main reason for failure as urgency symptoms in 62% of patients at follow-up, with 10% reporting de novo urgency. The low reported dry rate (46%) relates to the large preoperative mixed urinary incontinence (MUI) group (67%) as compared with their cure rate for SUI only (73%).
Cross and colleagues (1998) advocated the use of a PVS in conjunction with anterior colporrhaphy for repair of cystocele to treat both conditions effectively. Thirty-six patients (63%) had a PVS due to a grade III or IV broad-based cystocele and SUI. Thirty-three (92%) of these cystoceles were cured. These authors reported a 28% postoperative urgency/UUI rate with 19% de novo urgency/UUI. Yet only 3% had persistent UUI requiring treatment; 2.8% underwent further urethrolysis to resolve voiding difficulties. Hassouna and colleagues (1999) reported long-term outcomes of a modified rectus fascial PVS. The modified PVS is a modification of the free rectus fascial strip technique popularized by McGuire that includes fixation of the sling to the pubocervical and periurethral ligaments in a four-quadrant manner (Ghoniem, 1991). After the initial reported 95% success rate by Ghoniem, this study confirms a lower overall rate of 80.8%, when including assessment by a patient quality of life questionnaire. All 15 of the patients with documented failures had UUI or severe irritative symptoms. The additional quality of life assessment highlights the impact of UUI, frequency, nocturia, and pain on patient satisfaction.
Chaikin and Blaivas (1998) and Morgan and associates (2000) provide a long-term analysis of the efficacy and quality of life impact of an autologous PVS. Chaikin and Blaivas (1998) reported an SUI cure or improved rate of 92% but also compared instruments for measurement of incontinence (patient assessment, chart review, pad testing, and voiding diary entries). Seventy-five percent of the patients have “complex” SUI, which included “pipe stem urethra,” urethral or vesicovaginal fistula, urethral diverticulum, grade 3 or 4 cystocele, or neurogenic bladder. Concern is raised over the PVS converting an incompetent bladder neck into an obstructing system, with the finding of 23% incidence of DO at 1 year, which increased to 41% at 10 years. The failure rate due to UUI (de novo plus persistent) is similar to that reported by Cross and coworkers (1998) of 22%. Morgan and associates (2000) reported an 85% cure rate at 5 years, yet to achieve this level of cure 14 patients (5.7%) had secondary procedures. This included six collagen injections, three repeat PVS procedures, and five urethrolyses. Eighty percent of these 5 patients had a subsequent return to normal voiding. Chaikin and Blaivas (1998) and Morgan and associates (2000) together demonstrate that when SUI is resolved for more than 1 year after an autologous PVS procedure, the long-term (5 to 10 year) risk of recurrent SUI is low.
Groutz and associates (2001) reported outcomes based on whether the PVS was the primary (57%) or secondary anti-incontinence procedure (43%). The latter group had undergone 1 to 3 prior unsuccessful anti-incontinence procedures for recurrent incontinence. The cure rate was significantly higher in patients with primary incontinence than those with recurrent incontinence (74% vs. 59%, P = .006). Yet there were no surgical failures in either group. All of the patients were either cured or improved based on their outcome score, incorporating 24-hour voiding diary, 24-hour pad test, and patient satisfaction. Richter and associates (2001) assessed long-term effects based on a telephone quality of life survey. This study reports high patient satisfaction, whereas 11.8% of the subjects required regular intermittent self-catheterization; 5.8% had to adopt adaptive positions to facilitate voiding. All of the obstructed patients were satisfied due to freedom from urinary incontinence and did not elect urethrolysis. This may relate to an emphasis of preoperative counseling in conveying the possibility of long-term voiding dysfunction after a PVS procedure. Chou and colleagues (2003) provided long-term outcomes for the effectiveness of an autologous PVS in management of MUI. The population included 48.5% with simple SUI and 51.5% with MUI. On univariate analysis of MUI, the higher number of urgency or UUI episodes preoperatively, the more likely it was that the PVS would fail. Yet, women with SUI and concurrent UUI or DO have a successful PVS outcome at a rate comparable to that in women with simple SUI (97% vs. 93%, respectively, P = .33). DO in 26% of those with MUI did not predict a poor outcome. This is contrary to their earlier observations (Chaikin and Blaivas, 1998).
The SiSTER trial (Albo et al, 2007), a multicenter, randomized clinical trial, compared the rectus fascial autologous sling to the Burch colposuspension. The two primary outcomes were composite measures of success in terms of overall urinary incontinence measures (no self-reported symptoms of incontinence, increase of less than 15 g in pad weight during a 24-hour pad test, and no medical or surgical treatment for incontinence) and of SUI measures (no self-reported symptoms of SUI and negative stress test), specifically. Whereas success rates were higher for the PVS (47% vs. 38%, P = .01), voiding symptoms were also higher: urinary tract infections (UTIs) (48% vs. 32%), difficulty voiding (14% vs. 2%, P < .001), and postoperative UUI (27% vs. 20%, P = .04). All of the surgical procedures for bladder outlet obstruction (19 of 20) were performed in the sling group.
Mitsui and associates (2007) analyzed the risk factors for postoperative voiding difficulty. Patients with a postvoid residual urine volume greater than 100 mL (P = .05) or 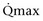 of less than or equal to 20 mL/s (P = .09) in preoperative urodynamics were more likely to require prolonged intermittent self-catheterization. In this group, 28% needed prolonged intermittent self-catheterization (range 4 to 40 months). There have been heterogeneous outcomes in regard to risk factors for postoperative voiding difficulty. This may relate to differences in surgeon skill or surgical method. Although postoperative urgency and UUI are strongly related to failure, there are no preoperative risk factors that consistently predict outcomes after a PVS operation (Mitsui et al, 2007).
of less than or equal to 20 mL/s (P = .09) in preoperative urodynamics were more likely to require prolonged intermittent self-catheterization. In this group, 28% needed prolonged intermittent self-catheterization (range 4 to 40 months). There have been heterogeneous outcomes in regard to risk factors for postoperative voiding difficulty. This may relate to differences in surgeon skill or surgical method. Although postoperative urgency and UUI are strongly related to failure, there are no preoperative risk factors that consistently predict outcomes after a PVS operation (Mitsui et al, 2007).
Petrou and Frank (2001) retrospectively reviewed the safety and efficacy of a repeat PVS for recurrent SUI among 14 women and concluded that repeat PVS is a reasonable treatment option. The original surgeries included autologous PVS (3 patients), cadaveric bone–anchored PVS (3 patients), cadaveric bone–anchored suburethral sling (5 patients), and a vaginal patch sling (3 patients). Postoperative complications included a pelvic abscess related to a prior cadaveric sling and osteomyelitis pubis related to the prior bone anchored sling. One (7%) of the patients developed long-term urinary retention and is on intermittent bladder catheterization; 86% of the patients considered themselves cured or improved.
Value of Autologous Pubovaginal Sling in Urethral Reconstruction
Urethral and bladder neck reconstruction for fistulae and diverticula requires restoration of both anatomy and function. An autologous PVS serves an important role in these reconstructions. Anatomic damage refers to tissue loss and can range from a urethrovaginal fistula to absence of the urethra or vesical neck. The causes of damage include protracted obstetric delivery, anti-incontinence procedures, urethral diverticulectomy or other sequelae of urethral diverticulum, aggressive transurethral resection of the bladder neck, long-term indwelling urethral catheter, pelvic trauma, tumors, and irradiation (Blaivas and Jacobs, 1991). The goals of surgical repair are to restore function and anatomy while fashioning an unobstructed urethra and maintaining continence (Blaivas and Heritz, 1996).
Swiertzewski and McGuire (1993) reviewed the records of 14 women who underwent urethral diverticulectomy during a 3-year period. Only 8 (57%) had symptoms of SUI, confirmed on urodynamics in 7. All 7 women were cured with a diverticulectomy and autologous PVS. One patient demonstrated DO. These investigators concluded that the presence of a urethral diverticulum did not compromise the outcomes of the PVS. Chancellor and coworkers (1994) performed a PVS procedure in 14 women with destroyed urethras secondary to long-term indwelling Foley catheter management of neurogenic bladder dysfunction. Greater tension was applied to the sling suspension to achieve urethral closure. Ten patients had simultaneous intestinal augmentations or diversions, 2 had concurrent suprapubic tube placement, and 2 had adequate bladder capacity and compliance and only had PVS performed. All of the patients achieved continence. The PVS is a simpler addition and avoids the risk of fistula formation associated with bladder neck closure, which has historically been used in this population. Blaivas and Heritz (1996) performed a retrospective study of 49 women who underwent a one-stage urethral reconstruction at up to 11-year follow-up. Forty-one of these women had a concomitant PVS placed for management of preoperative SUI. Only 1 patient experienced obstruction and DO after PVS placement, requiring incision and subsequently is continent. Three of 5 women who had Pereyra repairs for SUI required secondary PVS and are also now continent.
Faerber (1998) reported on 16 women who had simultaneous PVS and diverticulum repairs after UDS. All were either significantly improved (12%) or cured (88%), and only 2 patients developed de novo urgency. The average time for achieving adequate bladder emptying after repair was 5 weeks. For the initial 2 weeks, a Foley catheter is usually in place for urethral healing. Rovner and Wein (2003) reported on the circumferential urethral diverticulum repair among 9 patients who received either end-to-end urethroplasties or dorsal urethroplasties. Based on preoperative SUI status or evidence of open bladder neck on preoperative cystography, 8 patients were recommended to have a concurrent PVS procedure performed. All patients had a rectus fascial PVS sling placed, except 1 who requested a porcine PVS and 1 who refused to have PVS repair. All patients were continent postoperatively, except the patient who refused the PVS procedure and developed de novo SUI. Flisser and Blaivas (2003) evaluated the results of 74 women with urethral pathologic processes that required vaginal flap reconstructions. A majority of the women had required reconstruction for a diverticulum or urethral fistula secondary to iatrogenic causes. Fifty-six of these women had a concomitant PVS placed. Eighty-seven percent (54 women) considered themselves cured postoperatively. Three of 4 patients who had persistent SUI had failed a modified Pereyra procedure but were cured at reoperation. One patient was continent after operative revision of the PVS for obstruction and significant UUI.
Allograft
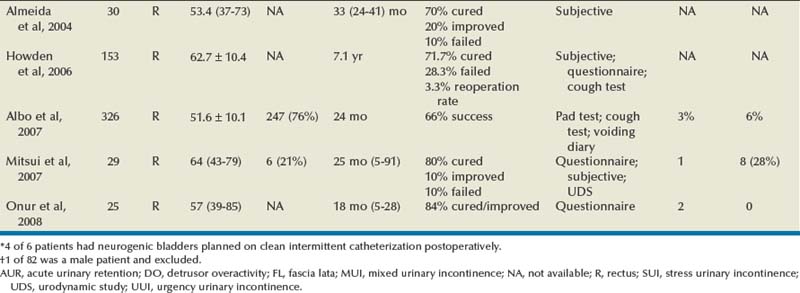
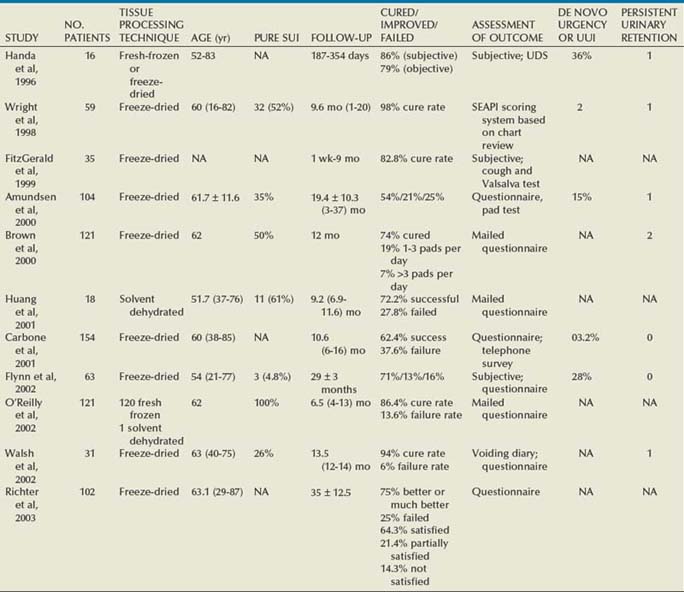
The autologous fascial PVS remains a gold standard with efficacious and durable outcomes, but, owing to the morbidity of graft procurement, cadaveric allograft slings were introduced. This was to provide decreased morbidity, reduce operative time, and minimize pain (Table 73–4). There are limited outcome data owing to questions surrounding efficacy and durability. Early literature used cadaveric frozen or freeze-dried fascia lata with a variety of fixation methods, including suture fixation and bone anchors (Handa et al, 1996; Wright et al, 1998; FitzGerald et al, 1999; Brown and Govier, 2000; Carbone et al, 2001; Flynn and Yap, 2002; O’Reilly and Govier, 2002; Walsh et al, 2002; Amundsen et al, 2003; Richter et al, 2003; Almeida et al, 2004; Howden et al, 2006). Experience has shown that tissue processing techniques can have deleterious effects on cadaveric sling outcome (Nazemi et al, 2008). Lemer and associates (1999) found differences in both tissue integrity and durability between freeze-dried fascia and solvent-dehydrated fascia. They theorized that ice crystal formation characteristics of tissue freezing may disrupt the collagen matrices, yielding poor strength properties. Failure rates for frozen or freeze-dried grafts range from 6.0% to 37.6% (Handa et al, 1996; Wright et al, 1998; FitzGerald et al, 1999; Brown and Govier, 2000; Carbone et al, 2001; Flynn and Yap, 2002; O’Reilly and Govier, 2002; Walsh et al, 2002; Amundsen et al, 2003; Richter et al, 2003; Almeida et al, 2004; Howden et al, 2006).
Handa and associates (1996) reported on some of the earliest short-term outcomes for cadaveric fascia lata, with promising cure rates. Two of their patients (12%) developed abdominal wound infections, which resolved with local care, including drainage. They reported de novo urgency in 36% of the cases, whereas DO resolved in one of four cases. Two of the 3 patients who had SUI recurrence were continent after a synthetic midurethral sling. FitzGerald and associates (1999) reported on 35 patients undergoing cadaveric fascia lata sling placement with a high failure rate of 17%. Symptom recurrence was seen very early (1 week to 5 months). Histopathologic analyses of the retrieved material indicated ongoing processes in the failed graft: disorganized remodeling, areas of graft degeneration, and evidence of immune reaction. These findings led them to conclude that freeze-dried, irradiated donor fascia lata grafts should not be used for urogynecologic procedures due to the high material failure rate.
Three studies (Amundsen et al, 2000b; Walsh et al; 2002; Richter et al, 2003) documented good results without significant adverse outcomes using freeze-dried fascia lata. Amundsen and associates (2000b) utilized questionnaires and pad testing to assess incontinence outcomes in a complex population (80% having had prior pelvic surgery and 49% having had at least 1 prior anti-incontinence procedure). Sixty-five percent of the group had MUI preoperatively, with 41% of them having resolution of UUI; 15% developed de novo UUI, and no erosions were present. In 1 patient who underwent reoperation for a failed sling the fascia lata was found completely intact. The sling was torn from its nonabsorbable suture material, similar to a previously reported case (Chaikin and Blaivas, 1998; Amundsen et al, 2000b). Walsh and colleagues (2002) prospectively evaluated 31 women with promising short-term follow-up. There was complete resolution of SUI in 94% at both 4 months and 1 year. There was also a reduction in the incidence and severity of urgency and UUI after surgery, reflected in the declining use of anticholinergic surgery from before to 4 months and 1 year postoperatively (55% to 32% to 26%). Richter and colleagues (2003) conducted a prospective long-term study using validated questionnaires for a follow-up of 48 months. Difficulty emptying the bladder was described by 58.2% of patients at 12 month follow-up, with 34.2% describing it as slight. By 48 months, 50% of patients continue to have difficulty emptying their bladders. Patients reported a 90.2% satisfaction rate at 12-month follow-up and continued to be satisfied at 48-month follow-up (85.7%).
Two groups documented high failure rates with freeze-dried allograft (Carbone et al, 2001; O’Reilly and Govier, 2002). Carbone and associates (2001) used cadaveric fascia with titanium bone anchors for placement bilaterally in pubic bones. They reported a 38% failure rate and 17% reoperation rate at short-term follow-up of 11 months. Average time to reoperation was 9 months (3 to 15 months). Intraoperative findings at reoperation revealed the titanium anchors to be in position, the polypropylene sutures to be intact, and retropubic fibrosis and scarring of the urethropelvic ligament suggesting appropriate placement of the sling. All of the allogenic fascia appeared to be fragmented, attenuated, or simply absent. These authors have subsequently abandoned the use of cadaveric fascia allografts in all the PVS procedures at their institution. O’Reilly and Govier (2002) reported high intermediate failure rates in 121 women. Eight patients had recurrent SUI at a mean of 6.5 months (4 to 13 months); 7 of the 8 women had previous incontinence surgery and had multiple comorbidities, including neurologic disease, diabetes, previous pelvic irradiation, and previous pelvic surgery. Based on these results and findings by Lemer and associates (1999), this group discontinued use of fresh frozen grafts and switched to solvent dehydrated cadaveric fascia and dermal grafts.
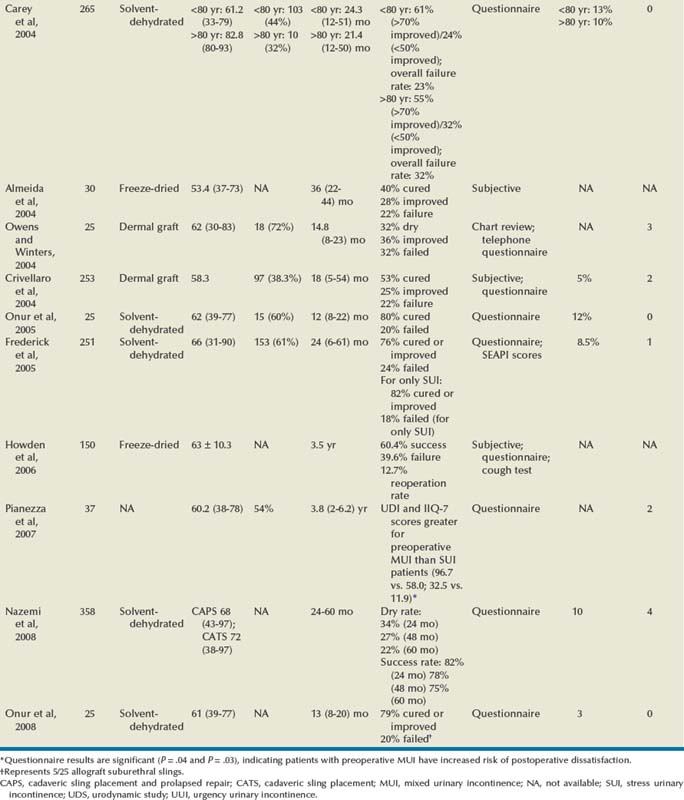
Some studies support the efficacy of solvent-dehydrated fascial slings (Frederick and Leach, 2005; Onur and Singla, 2005; Pianezza et al, 2007; Nazemi et al, 2008), yet previous reported failures coupled with the consistent success and rapid adoption of synthetic midurethral slings has led to abandonment of all types of cadaveric allograft at most centers. Thus there are few data to assess long-term outcomes and define sling outcomes after solvent dehydration techniques (Nazemi et al, 2008).
Huang and colleagues (2001) were the earliest to report unfavorable experiences using solvent-dehydrated allograft in 18 women at short-term follow-up. They reported a 27.8% failure rate with full recurrence of incontinence and subsequently stopped using all allograft as a sling material. On reoperation with an autologous PVS for recurrent SUI the allograft remained only rudimentary and was very friable. Histologic examination of the retrieved allograft revealed wavy collagen fibers with loosely packed fibroblasts and focal areas of degeneration. Carey and Leach (2004) prospectively compared octogenarians and younger women undergoing treatment for incontinence with bone-anchored solvent-dehydrated cadaveric fascia lata. No statistically significant difference in outcome parameters was identified. The rates of de novo urgency were similar between the two groups, yet persistent urgency was greater among the octogenarians. Onur and associates (2005) reported 80% cure rates at 12-month follow-up. In 5 patients (20%) complete recrudescence of symptoms occurred within 6 months of surgery. Frederick and associates (2005) reported on prospective, intermediate-term results for cadaveric prolapse repair with bone-anchored sling (CaPS), with dry/cure rate and improvement rate of 56% and 82%, respectively. There was one case of osteitis pubis related to use of transvaginal bone anchor fixation that resolved with conservative management. Fifty-six percent of the failures occurred after 12 months of follow-up: 80% of these women were satisfied and 77% stated that they would undergo the CaPS procedure again. Using validated questionnaires (i.e., Urogenital Distress Inventory, Short Form [UDI-6]; Incontinent Impact Questionnaire [IIQ-7]), Pianezza and associates (2007) reported on similar levels of long-term patient satisfaction. Patients with preoperative MUI were at the greatest risk for postoperative dissatisfaction. Nazemi and associates (2008) described durable improvements in incontinence episodes, patient satisfaction, and validated quality of life end points when comparing a CaPS and CaTS (cadaveric sling placement alone) cohort. Yet there was a reduction in dry rates with extended follow-up (24 months, 48 months, and 60 months), especially in the CaTS group (23%, 18%, and 9% respectively); 4% of patients presented with sling extrusion and 4% required sling incision and urethrolysis.
Because of early and intermediate graft failure with cadaveric fascia lata, surgeons were prompted to use alternative materials: cadaveric dermal grafts. Crivellaro and associates (2004) presented their prospective series of patients treated with bone-anchored Repliform cadaveric human dermal allograft (LifeCell Corp., The Woodlands, TX) PVS. There was a 22% failure rate, yet based on patient report there was average incontinence improvement at 9 months of 85% and at 18 months of 80%. The 2 patients requiring long-term intermittent catheterization had neurogenic bladders. Owens and Winters (2004) assessed outcome and patient satisfaction with Duraderm allograft (C.R. Bard, Covington, GA). Initial results were promising, with a dry rate of 68% and improved rate of 24%. At intermediate follow-up only 32% of the patients were dry and 36% noted improvement. Two of the 8 patients who experienced graft failure underwent periurethral injections with significant improvement, and 1 is dry after an autologous sling procedure. Surgical reexploration revealed almost complete absence of graft material, without evidence of infection or excessive inflammatory response.
Six studies have compared the outcomes of women undergoing a PVS procedure using either autologous or cadaveric allograft fascia (Wright et al, 1998; Brown and Govier, 2000; Flynn and Yap, 2002; Almeida et al, 2004; Howden et al, 2006; Onur and Singla, 2008). Four groups found the outcomes comparable with equally high success rates and no negligible difference in complications. They concluded that allograft fascia lata may be used as an alternative to autologous fascia for PVS, to reduce operative time and decrease postoperative pain and disability (Wright et al, 1998; Brown and Govier, 2000, Flynn and Yap, 2002; Onur and Singla, 2008). With long-term follow-up, two groups noted superior continence outcomes in the autologous group (Almeida et al, 2004; Howden et al, 2006). Almeida and associates (2004) did not report adverse outcomes in either group. Howden and colleagues (2006) found recurrent symptoms occurred in a higher proportion in the cadaveric group (39.6%) compared with the autologous group (28.3%; P = .04). The reoperation rate was also higher for the allograft group (12.7% vs. 3.3%, P = .003).
Xenograft
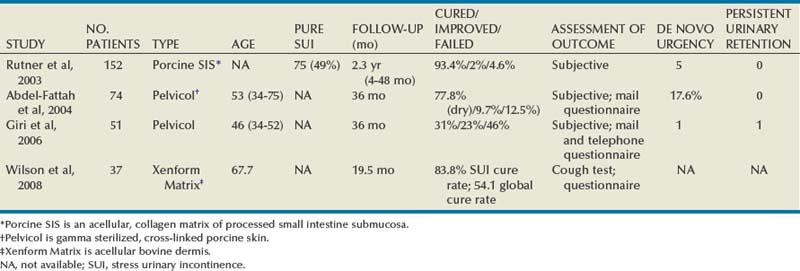
A number of different sling materials are available and have been discussed. Because of the morbidity of autologous fascial harvest, high failure rates of allograft materials, and high erosion rate with synthetic PVS materials, xenografts are an attractive option. They are associated with a low rate of infection, rejection, or extrusion, owing to their incorporation into host tissue (Rutner et al, 2003). Studies present in the literature utilize porcine dermis (Pelvicol), porcine SIS, and bovine dermis as xenografts for use in PVS surgery (Arunkalaivanan and Barrington, 2003; Rutner et al, 2003; Abdel-Fattah et al, 2004;Wiedemann and Otto, 2004; Giri et al, 2006; Wilson et al, 2008) (Table 73–5). Rutner and colleagues (2003) were the first to describe the use of porcine SIS as a bone-anchored PVS in 152 patients with long-term follow-up. They reported cure rates comparable to those with the autologous sling. Among the 7 patients who experienced graft failure (4.6%), 5 had recurrent SUI within 3 months of surgery and the other 2 had recurrences at 9 and 11 months postoperatively. Two of the failures were related to the bone anchors. One patient had repeat porcine SIS PVS, with continued failure to relieve incontinence and 1 patient became dry after carbon bead bulking injections. One patient achieved continence after a urethrolysis 2 years postoperatively. At reoperation, grossly no evidence of the implanted SIS material was found. Biopsy specimens of the periurethral tissue revealed fibrous and muscular tissues with a few remnants of SIS. Wiedemann and Otto (2004) performed the first histopathologic examination of porcine SIS in a series of 15 women with SIS PVS. Three reoperations (20%) were necessary owing to recurrent SUI at a mean of 12.7 months. All 3 patients underwent reoperation with implantation of a polypropylene mesh and achieved immediate continence. Biopsy specimens from the SIS band under the vaginal mucosa revealed only focal residues of SIS without any evidence of tissue reaction. There was also no evidence of a significant immunologic or chronic inflammatory reaction. These authors concluded that the advanced incorporation of the implant would lend to good biocompatibility.
Arunkalaivanan and Barrington (2003) first reported on Pelvicol in a randomized, short-term trial comparing Pelvicol PVS with the tension-free vaginal tape (TVT) procedure. Questionnaire-based cure rates were comparable between the two: 85% in the TVT group and 89% in the Pelvicol implant group. Abdel-Fattah and associates (2004) presented the 3-year follow-up data for this prospective trial. Cure rates remained high and comparable between the two groups: 79.1% for the TVT group and 77.8% for the Pelvicol group. There was no statistical difference in regard to complication rates or postoperative pad score. Giri and colleagues (2006) compared the 3-year efficacy of Pelvicol with autologous rectus fascia in 101 consecutive, nonrandomized patients. Although porcine dermis reduced the associated surgical morbidity, there were significantly inferior long-term cure rates as compared with the autologous PVS. Treatment failure occurred by 9 months in the autologous group and by 24 months after the Pelvicol sling. Repeat UDS indicated SUI as the cause of treatment failure in 18 of 20 (90%) women treated with porcine dermis but only in 3 of 8 (6.5%) women after a rectus fascial sling. These authors concluded that Pelvicol should not be used as a substitute for rectus fascia.
Bovine dermis is the most recently reported material used for a xenograft PVS (Wilson et al, 2008). Women at high risk for sling failure (with advanced age, previous surgical failure, intrinsic sphincter deficiency) underwent either a bovine dermis or autologous rectus fascia PVS procedure with short-term follow-up. Global cure rates and SUI cure rates were not statistically different between the two groups. Four women (8.3%) in the autologous group were reoperated with injections, repeat autologous PVS placement, and anterior colporrhaphy with interposition graft. Two women (5.4%) in the bovine dermis group underwent additional interventions: injections and a repeat autologous PVS placement. Biopsy specimens of the bovine dermis sling material during reoperation (for SUI recurrence at 3 months) revealed the sling was replaced by fibrosis, hemorrhage, and mild chronic inflammatory infiltrate, with no acellular component. Tissue breakdown, represented by intermittent areas of myxoid degeneration, was present and may indicate evidence of early graft failure.
Key Points: Pubovaginal Sling Outcomes and Urethral Reconstruction
Complications
Erosion and Extrusion
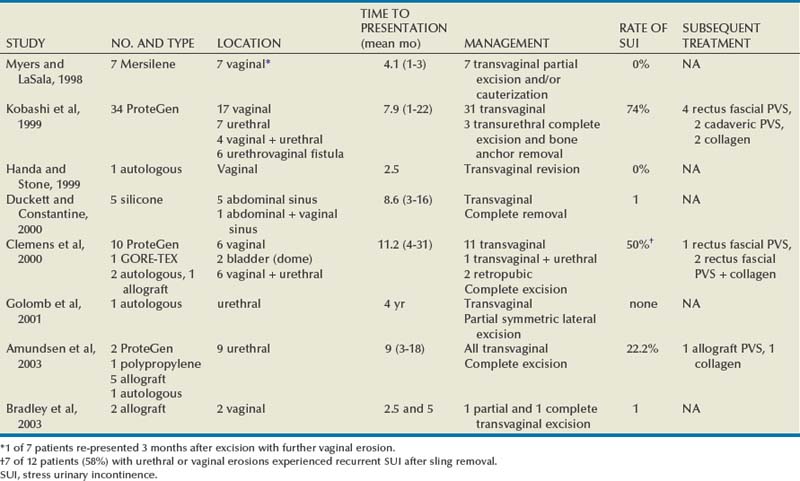
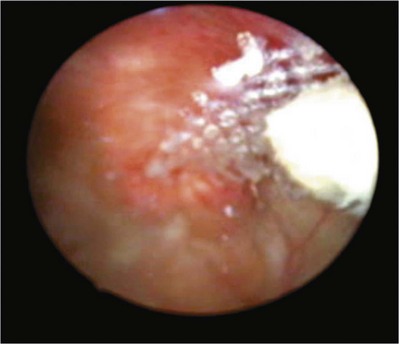
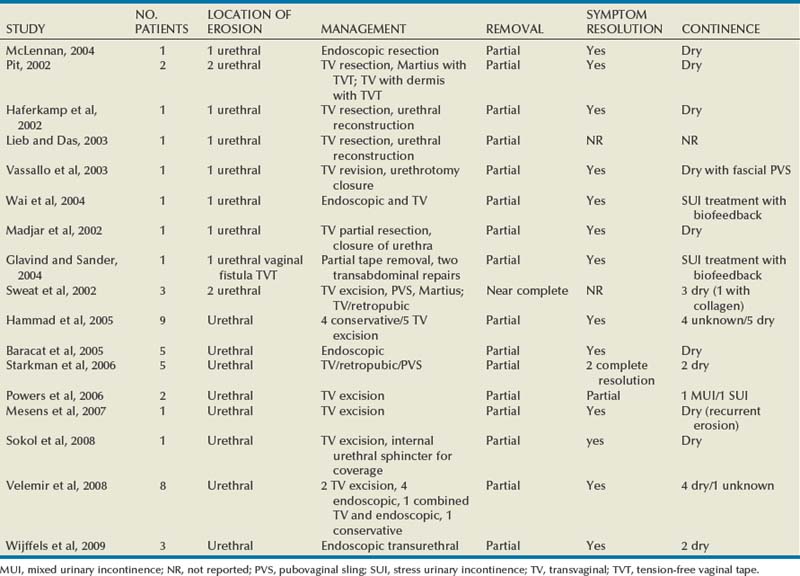
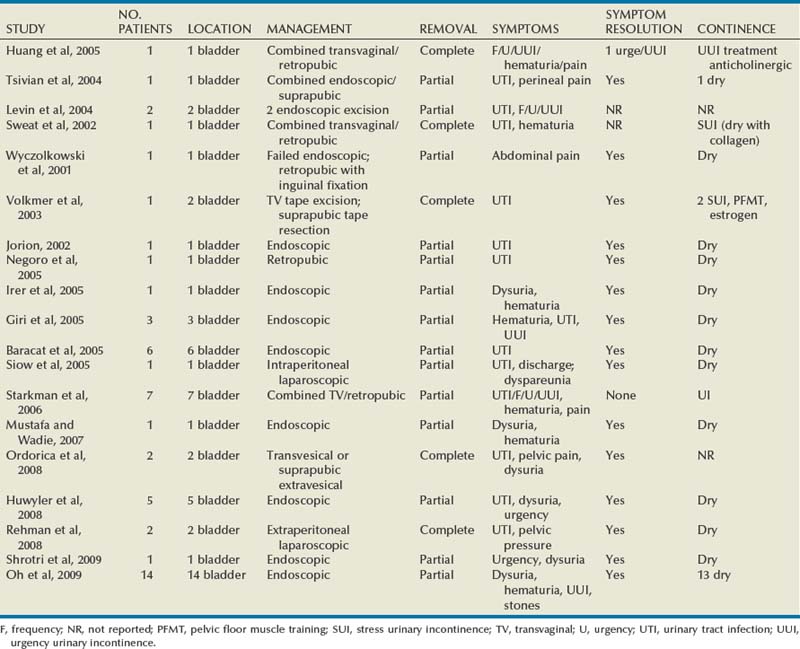
The incidence of sling erosion is dependent on the composition of sling material. Synthetic slings erode 15 times more often into the urethra and extrude 14 times more often into the vagina than autologous, allograft and xenograft slings (Blaivas and Sandhu, 2004). This is based on a meta-analysis of peer-reviewed literature in 1997, citing a urethral erosion incidence of 0.02% and vaginal extrusion of 0.007% in 1515 patients who underwent synthetic slings. This is compared with a urethral erosion incidence of 0.003% and vaginal extrusion of 0.0001% in 1715 patients undergoing autologous and allograft slings (Leach et al, 1997). In subsequent studies, most erosions have been associated with synthetic slings, particularly woven polyester slings (Summit et al, 1992; Bent et al, 1993; Weinberger and Ostergard, 1995; Myers and LaSala, 1998; Kobashi et al, 1999; Duckett and Constantine, 2000; Clemens et al, 2000; Amundsen et al, 2003; Chen et al, 2010), but recently there are a few reported cases of autologous and allograft slings (Handa and Stone, 1999; Golomb et al, 2001; Amundsen et al, 2003; Bradley et al, 2003; Blaivas and Sandhu, 2004) (Table 73–6).
Most urethral erosions are diagnosed 1 to 18 months after the original surgery, with a mean presentation of approximately 9 months. Presenting symptoms often include urinary retention, UUI, and MUI. Synthetic sling erosions are often also associated with vaginal discharge, vaginal pain/pressure, suprapubic pain, and recurrent UTIs. The etiology is multifactorial, including local tissue factors (i.e., postsurgical scarring, urethral atrophy, estrogen deficiency, and radiation-induced ischemia) or surgical techniques (i.e., excessive tension, dissection too near urethra, or perforation of urethra or bladder) (Blaivas and Sandhu, 2004). Golomb and colleagues (2001) reported a rare case of autologous sling erosion into the midurethra after traumatic urethral catheterization for prolonged urinary retention.
Erosion of an autologous PVS is rare. There are only four cases of erosions documented in peer-reviewed literature. Handa and Stone (1999) and Golomb and colleagues (2001) reported on an individual case of an autologous sling eroding through the midurethra. Possible causative factors include misplacement or incorrect technique in sling passage or positioning, excessive tension, or traumatic urethral instrumentation after placement of a PVS (i.e., for retention, hematuria clot evacuation, surveillance cystoscopy). In both cases the eroded portion of the sling was excised and the urethra was closed (Handa and Stone, 1999; Golomb et al, 2001). Clemens and colleagues (2000) described two cases of bladder dome erosion by an autologous rectus fascial sling that presented as recurrent UTI, dysuria, and UUI. One of these patients presented with a bladder calculus on sling material that was visible on cystoscopy. The other woman presented with edema and suture at the dome. In both cases adequate cystoscopy with 30- and 70-degree lenses after Stamey needle passage may have avoided this. Both cases were successfully managed with endoscopic removal of stitches and treatment of the bladder stone. No further treatments were necessary (Clemens et al, 2000).
Eight cases of allograft erosion and extrusion were found within the literature between three groups (Clemens et al, 2000; Amundsen et al, 2003; Bradley et al, 2003). Among the three cases of vaginal extrusion (Clemens et al, 2000; Bradley et al, 2003) of an allograft PVS, patients commonly presented with vaginal bleeding, persistent SUI, and irritative voiding symptoms. All three cases were managed successfully with transvaginal exploration, partial sling removal, and closure of the vagina. The patients did not require reoperation or further treatment. Management of autologous and allograft sling urethral erosion is usually done with incision or excision of that part of the sling that has eroded and simple closure of the urethra (Blaivas and Sandhu, 2004). Rarely are additional coverage measures (i.e., Martius flap) required. No cases of xenograft erosion or extrusion are present in the literature.
Erosions and extrusion of a synthetic PVS were very common and associated with significant morbidity. Synthetic material is no longer utilized for bladder neck slings. In fact, the ProteGen sling (Boston Scientific, Natick, MA) was recalled in January 1999 because of the high erosion rates (Clemens et al, 2000). The other 60 cases of erosion and extrusion found within the literature are cases of synthetic PVS: ProteGen, Mersilene, silicone, GORE-TEX or polypropylene (Myers and LaSala, 1998; Kobashi et al, 1999; Clemens et al, 2000; Duckett and Constantine, 2000; Amundsen et al, 2003). Rates of rejection or erosion, usually requiring removal of the synthetic material historically, vary from 2.7% to 22% (Summit et al, 1992; Bent et al, 1993; Weinberger and Ostergard, 1995). Most authors agree that erosions from synthetic slings require complete removal of the sling and all other foreign materials (sutures, bone anchors, screws) whenever present (Blaivas and Sandhu, 2004). Myers and LaSala (1998) reported on 7 patients with vaginal extrusion among 116 women with bladder neck Mersilene mesh slings. All of the cases presented within 4 to 12 weeks of initial operation and were managed with trimming of visible mesh, excision of granulation tissue, and re-covering of erosion. One of these 7 women presented with recurrent extrusion (Myers and LaSala, 1998). Of note, whereas follow-up of these 7 women was up to 37 months, many of the other 109 patients were likely also to have had subsequent erosions or extrusions. Kobashi and colleagues (1999) published the multi-institutional results of ProteGen sling (bovine collagen with bone anchors) erosions and extrusions in 34 women. The most common presenting complaints were delayed vaginal discharge in 21 (32%), vaginal pain or pressure in 21 (62%), suprapubic pain in 11 (32%), and recurrent UTI in 5 (15%). The synthetic sling was entirely removed. Attempt was additionally made to remove all bone anchors. One patient with residual pain after sling removal had a “hot area” at the bone anchor site revealed on bone scintigraphy (Kobashi et al, 1999). Duckett and Constantine (2000) came to a similar conclusion about silicone as sling material as other groups reported with the ProteGen sling. Among the initial 7 women who had a silicone PVS placed, 5 experienced silicone erosion and sinus formation. The study was terminated due to the high complication rates. Clemens and colleagues (2000) reported on complications associated with 10 cases of ProteGen and 1 case of GORE-TEX. This included four ProteGen extrusions, six ProteGen erosions with extrusions, and one GORE-TEX extrusion. All of the cases were managed with transvaginal removal of sling sutures and bone anchors wherever possible. Five of these 14 women had recurrent SUI, including 1 who opted to have an autologous sling placed at the time of ProteGen sling removal owing to persistent SUI after the initial surgery. Other symptoms resolved among all of the patients (Clemens et al, 2000). Amundsen and colleagues (2003) performed complete sling removal, urethral repair, and additional placement of a Martius flap in patients with two of the three synthetic erosions that occurred. They noted an intense inflammatory reaction with superimposed infection, leading to extensive urethral loss. This was thought to contribute to the significant recurrent SUI (ISD), requiring a PVS and collagen (Amundsen et al, 2003). The incidence of recurrent SUI in contemporary series of urethral erosions after synthetic PVS placement is 74% to 100%, often necessitating a secondary PVS (Kobashi et al, 1999; Clemens et al, 2000).
There are few studies concerning treatment of urethral erosions after a PVS procedure, success rates, and rates of continence. Urethral reconstruction is successful a majority of the time, yet continence is achieved in only 17% to 56% of patients (Clemens et al, 2000; Amundsen et al, 2003) who had erosions secondary to a synthetic sling. When erosions were managed with concurrent placement of a new PVS, Flisser and Blaivas (2003) reported an 87% continence rate. Secondary procedures such as bulking agents and PVS surgery are successful in a majority of patients (Blaivas and Sandhu, 2004). However, if the bladder neck is involved with the erosion the authors have noted much lower overall continence rates, even with the use of concomitant autologous slings at the time of reconstruction.
Key Points: Erosion and Extrusion
Voiding Dysfunction Secondary to Bladder Outlet Obstruction
The voiding dysfunction that develops from iatrogenic outlet obstruction by a PVS is related to obstruction, DO, or impaired detrusor contractility. The incidence of voiding dysfunction after continence surgery varies widely in the literature, from 2.5% to 35% (Foster and McGuire, 1993; Carr and Webster, 1997; Cross et al, 1998; Chaliha and Stanton, 1999). The traditional PVS is known to have higher rates of voiding dysfunction than the Burch colposuspension (Stanton et al, 1983). A multi-institutional, randomized clinical trial (SiSTER) compared the autologous rectus fascia PVS procedure and the Burch colposuspension. Although success rates were higher for women who underwent the sling procedure there was significantly greater voiding dysfunction (63% vs. 47%, P < .001): UTIs, difficulty voiding, and postoperative UUI (Albo et al, 2007). A meta-analysis by the AUA Stress Urinary Incontinence Clinical Guidelines Panel reported that the incidence of urinary retention more than 4 weeks after PVS placement was 8% and the risk of permanent retention “generally does not exceed 5%” (Leach et al, 1997). In a series of 252 women at 4-year follow-up, Morgan and colleagues (2000) reported a prolonged urinary retention rate of only 2.4%. Patients may present with complete urinary retention or obstructive voiding symptoms and less obvious irritative symptoms, including UUI (Carr and Webster, 1997). Obstruction may present as recurrent UTIs, prolonged suprapubic pain, and painful voiding, even if emptying is completed. Nitti and colleagues (2002) found that 16% of patients who required PVS lysis did not have obstructive symptoms or retention. Some studies report persistent postoperative UUI and urgency as presenting more commonly (8% to 25%) than frank retention after a PVS (Cross et al, 1998). The risk of iatrogenic obstruction usually relates to technical factors (i.e., placement and tension of sutures or sling material). When there is an inadequately supported bladder neck/proximal urethra there is potential for continued SUI. When the sling is tied too tightly there is excessive elevation of the bladder neck toward the pubic bone, causing “hypersuspension” or overcorrection of the urethrovesical angle.
Preoperative voiding dysfunction affects a patient’s ability to empty after anti-incontinence surgery. Detrusor hypocontractility, a condition that is present preoperatively, may manifest symptomatically with even a “relative obstruction” when urethral resistance is increased by an anti-incontinence surgery. During preoperative counseling these patients should be warned of the possibility of postoperative voiding dysfunction. Dysfunctional voiding or failure of relaxation of the external (striated) urethral sphincter may affect emptying after surgery (FitzGerald and Brubaker, 2001). A patient who habitually voids by abdominal straining may have difficulty emptying after incontinence surgery. Because of the variability of presenting symptoms after a PVS procedure it is important to ascertain the predominant symptom through a focused history. The temporal relationship between anti-incontinence surgery and onset of persistent voiding symptoms is key. For example, de novo urgency may actually exist as a result of urethral obstruction. On physical examination an abnormal urethral angulation, a foreshortened nonpliable vagina, or a nonmobile urethra may be noted. Hypersuspension is usually not evident on physical examination. The postvoid residual urine volume is very important in the evaluation of voiding dysfunction, although no clear cut-off values exist (Siddighi and Karram, 2007). Cystoscopy is useful to rule out a bladder pathologic process, sling erosion, and a hypersuspended urethra. Videourodynamics are useful in selected cases at the physician’s discretion. Most important remain the temporal relationship between the symptoms and the surgical procedure and the main criterion for a sling incision or urethrolysis.
There are no well-established risk factors for patients who are likely to experience obstruction after anti-incontinence surgery. UDS has been used to investigate multiple factors to determine which may be predictive. Miller and associates (2003) noted that women undergoing an allograft PVS who voided with no or minimal detrusor pressure (19%) had a significantly increased risk of postoperative retention. In contrast, no patient with a detrusor contraction developed retention postoperatively. Weinberger and Ostergard (1995) studied 108 women undergoing synthetic PVS procedures and found that the absence of detrusor contractions predicted delayed returns to normal voiding. Valsalva voiding had no affect on the incidence of postoperative retention or voiding dysfunction in either of these studies. The association between low voiding detrusor pressures and Valsalva voiding with subsequent voiding dysfunction was not seen by others (McLennan et al, 1998). UDS is useful in understanding voiding dynamics of incontinent women, yet low detrusor pressure or Valsalva voiding preoperatively should not exclude patients from having an anti-incontinence procedure. Lemack and colleagues (2008) examined the preoperative and postoperative urodynamic data for patients enrolled in the SiSTER trial (prospective, randomized clinical trial comparing the PVS procedure with Burch colposuspension) to predict voiding dysfunction after surgery. UDS findings did not predict postoperative voiding dysfunction or the risk of surgical revision in the PVS group. Recently, there have been anecdotal data on correlation between preoperative postvoid residual urine volume and urodynamic  with postoperative retention (Mitsui et al, 2007). No study to date has shown a definitive relationship between preoperative urodynamic findings and risk of postoperative retention.
with postoperative retention (Mitsui et al, 2007). No study to date has shown a definitive relationship between preoperative urodynamic findings and risk of postoperative retention.
A key factor in assessing voiding dysfunction is prolapse that was either uncorrected at the time of surgery or occurred postoperatively. Prolapse of sufficient size may kink or angulate and externally compress the urethra. Apical, anterior, and posterior prolapse must be ruled out as a cause of the urethral obstruction. Kobashi and colleagues reported on a technique of combined cystocele repair and PVS using a single piece of cadaveric fascia. Although no patients required surgery for obstruction the follow-up was only 6 months and long-term data are lacking (Kobashi et al, 2000). There is a paucity of literature on the affects of concurrent PVS and prolapse repair on postoperative emptying and voiding symptoms. Barnes and associates (2002) reported on 38 women with grade 3 to 4 pelvic prolapse and occult SUI who underwent concurrent PVS with prolapse repair. No patient developed permanent urinary retention. Two (9.5%) of the women developed de novo UUI. Existing UUI resolved in 45%. These investigators concluded that concurrent surgery had little negative effect on postoperative bladder emptying. Data are lacking on risk factors for voiding dysfunction after an anti-incontinence procedure performed with surgery for pelvic organ prolapse.
Whereas transient urinary retention is common, most patients return to spontaneous voiding within the first 10 days (Zaragoza, 1996; Cross et al, 1998). Obstruction after a PVS procedure may improve or resolve with time, which is the reason most physicians historically prefer waiting 3 months before considering surgical intervention. Persistent voiding dysfunction is initially treated conservatively. This includes temporary catheter drainage, clean intermittent catheterization, timed voiding, double voiding, biofeedback, pelvic floor muscle training, and antimuscarinic agents, which are extremely effective in specific cases. Anecdotal evidence shows improvement in urethral obstruction with urethral dilation and downward traction. These results may vary from temporary relief to worsening of the urethral rigidity secondary to periurethral fibrosis (Zimmern et al, 1987; Beck et al, 1988). This, like resection or incision of the bladder neck, is likely to fail because the sling is extraluminal. Additionally, these procedures may be associated with damage to the sphincter or bladder neck, leading to worsened incontinence, in addition to postoperative fibrosis and bladder neck contracture (Ghoniem and Elgamasy, 1995). It is not the authors’ practice to perform urethral dilation. When conservative measures fail, surgical intervention is indicated.
Surgical management of bladder outlet obstruction after a PVS procedure traditionally involves complete urethrolysis by a retropubic, transvaginal, or suprameatal approach, with success rates of 65% to 93% (Foster and McGuire, 1993; Carr and Webster, 1997; Cross et al, 1998; Goldman, 1999; Petrou et al, 1999). Most of these series include patients with obstruction after many different anti-incontinence procedures. Only two groups have stratified their results specifically for the PVS (Foster and McGuire, 1993; Petrou et al, 1999). Foster and McGuire (1993) reported that transvaginal urethrolysis was successful in 50% of PVS obstructions, which was less than both needle suspensions (75%) or retropubic urethropexy (63%). Their conclusion was that transvaginal lateral dissection is insufficient in relieving the direct suburethral compressive force of the sling. Petrou and coworkers (1999) reported on the suprameatal approach as superior to the transvaginal approach, allowing access and division of the lateral wings of the sling. Eight of 12 patients had successful results. In a series of 12 women, Petrou and Young (2002) reported resolution of obstruction in 10 patients with new-onset SUI (18% of women) after retropubic urethrolysis. Carr and Webster (1997) reported complete or significant resolution of symptoms in 86% of patients with retropubic urethrolysis. Recurrent SUI after formal urethrolysis is reported as 0% to 19% (Table 73–7).
Ghoniem and Elgamasy (1995) were the first to report on the successful use of sling incision and interposition of a free graft of vaginal wall for obstruction. Sling incision has comparable success rates (84% to 100%) without the longer operative time and potential morbidity of a formal urethrolysis (McLennan and Bent, 1997; Defreitas and Herschorn, 2000; Shenassa et al, 2000; Amundsen et al, 2000a; Kusuda, 2001; Nitti et al, 2002; Goldman, 2003; Thiel et al, 2005). Theoretically the interposition of autologous graft material between cut ends of the sling was to prevent recurrent incontinence. Others have found that this technique did not live up to this expectation. Shenassa and associates (2000) and McLennan and Bent (1997) used vaginal wall interposition in 12 and 4 women, respectively. The success rate was 92% and 100%, but SUI recurred in 25% in each series. Several small series have reported on successful sling incision without graft interposition. Kusuda and colleagues (2001) reported successful outcomes for 5 patients who underwent lateral sling incision. Defreitas and Herschorn (2000) had a 94% success rate in 16 women after lateral sling incision, with a 34% rate of recurrent SUI. Lateral incision is beneficial to avoid urethral injury in cases in which the sling is identified but the dissection plane between the urethra and sling is difficult. Amundsen and associates (2000a) used midline incision of a PVS in 10 of 32 patients, in whom the sling was easily identified. In the rest, a formal urethrolysis with entrance into the retropubic space was performed. The overall success rate was 84%, but the results were not stratified between sling incision and formal urethrolysis. In 9 of 12 of the obstructing autologous slings in this series the sling material could not be identified and was replaced by dense fibrosis. Nitti and colleagues (2002) reported on 19 women who underwent a PVS lysis for obstruction. The success rate was 84% and the recurrent SUI rate was 17%. Two of the 3 women who experienced failure of their procedures underwent subsequent successful retropubic urethrolysis. This allowed for complete release of all retropubic space scarring, which likely contributed to the failure of the suburethral sling release. Goldman (2003) performed simple sling incision in 14 women with iatrogenic urethral obstruction. This included 3 patients with a midurethral polypropylene mesh sling. Thirteen of 14 (93%) patients had complete or significant improvement of presenting complaints, and 1 (7%) required subsequent urethrolysis. Twenty-one percent (3 patients) developed recurrent SUI, with only 1 patient requiring treatment (repeat mesh midurethral sling). Long-term results after simple sling incision were reported on 13 women with catheter-dependent urinary retention after PVS surgery by Thiel and colleagues (2005). At 5-year follow-up, patients reported 45% cure and 45% improvement; 7.7% of the women noted recurrent SUI but chose to not pursue further therapy.
There are no preoperative or urodynamic parameters that predict success or failure of urethrolysis. Foster and McGuire (1993) found that patients with DO had a higher rate of failure but later studies contradicted this. Carr and Webster (1997) found the only parameter predictive of success was no prior urethrolysis. Nitti and Raz (1994) found that as the postvoid residual urine volume increased so did the failure. This has not been confirmed by others. Four of these women who failed to generate a contraction during UDS testing had successful urethrolysis. They also reported that UDS findings in patients whose procedures were considered failures after transvaginal urethrolysis failed to elucidate the reason for their continued voiding dysfunction. Because of the limitation of UDS in evaluation of these patients the temporal relationship of urethrolysis to the onset of symptoms is relied on as an indicator of obstruction. If a patient fails to resume voiding or improve significantly, then continued obstruction is suspected.
Failure of urethrolysis may be due to persistent or recurrent obstruction, DO, impaired detrusor contractility, or learned voiding dysfunction. Recurrent obstruction may result from periurethral fibrosis and scarring or intrinsic damage to the urethra that has occurred from the prior urethrolysis surgery. The most common reason is likely due to insufficient dissection and lysis of the urethra. Scarpero and colleagues (2003) reported on the value of repeat urethrolysis after failed urethrolysis in 24 women. Both transvaginal and retropubic approaches were chosen depending on the clinical situation. Obstruction was cured in 92%, but storage symptoms only resolved in 12%. Sixty-nine percent were improved and continued to require antimuscarinic therapy. SUI recurred in 18% of the women. This supports the use of repeat urethrolysis in the presence of initial failure or in cases in which the aggressiveness of the initial dissection is unknown. After an aggressive transvaginal urethrolysis, a retropubic urethrolysis may be considered (Scarpero et al, 2003).
Refractory storage symptoms after a urethrolysis are a challenging problem to treat. Overactive bladder symptoms are refractory in more than 50%, affecting patient satisfaction and quality of life. There are no predictors of outcome after urethrolysis, yet DO preoperatively may suggest an increased likelihood of refractory overactive bladder (Starkman et al, 2008b). There are no established guidelines for management. Twenty-five women with UUI after urogynecologic surgery (19 PVS, 3 retropubic suspension, and 3 transperitoneal vesicovaginal fistula repair; 4 required further urethrolysis) were retrospectively analyzed after sacral neuromodulation stimulation (SNS) therapy (Starkman et al, 2007). SNS was effective (80% reported > 50% improvement and 6 were continent), and there were no significant differences in response based on age, duration of symptoms, type of surgery, or urodynamic parameters. Starkman and associates (2007, 2008b) evaluated SNS in the management of 8 women who had at least one urethrolysis. Six patients had a favorable response during test stimulation and underwent implantation of a pulse generator. All 6 of the patients significantly improved, and the 2 who failed test stimulation perceived no change (Starkman et al, 2008b). In addition to antimuscarinic agents, SNS should be considered as an option for de novo or refractory urgency and UUI after urethrolysis.
Key Points: Voiding Dysfunction after a Pubovaginal Sling Procedure
Mixed Urinary Incontinence
The treatment of patients with mixed urgency and SUI is complicated and often involves a combination of anticholinergic therapy and surgery. Medical therapy for MUI is associated with significant resolution of the urgency component in only two thirds of patients (Nordling et al, 1979; Stephenson and Mundy, 1994). This therapy does not address the bladder outlet and would not be expected to achieve complete dryness. Anti-incontinence surgery may cure or aggravate the urgency symptoms and lead to de novo urgency. This aspect of anti-incontinence surgery is unpredictable and a major cause of patient dissatisfaction. Osman and associates (2003) evaluated outcomes of patients with stress and sensory UUI (no DO on UDS) compared with a control group of patients with pure SUI. Patients were divided between a Burch colposuspension study arm (Valsalva leak point pressure [VLPP] >90 cm H2O) with 24 patients and a PVS study arm (VLPP < 90 cm H2O) with 26 patients. Twelve PVS procedures and 8 Burch colposuspensions were performed among the SUI-only control subjects, using the same leak point parameters. The incidence of persistent UUI was 12% among the PVS patients with MUI. Twenty percent of controls with PVS had de novo UUI. The incidence of residual urgency was not significantly higher than that of de novo urgency in those with genuine SUI. Chou and colleagues (2003) used a validated questionnaire, voiding diary, and pad testing to report outcomes of the PVS for MUI, compared with pure SUI. Among 98 patients, 46 (48.5%) had pure SUI and 52 (51.5%) had MUI (26% with DO). The cure/improved rates were 97% among the SUI group and 93% in the MUI cases (P = .33). Increasing episodes of urgency and UUI on preoperative voiding diary correlated directly with surgical failure, whereas voiding frequency was associated with cure. This frequent voiding may have been adapted by patients preoperatively to avoid incontinence. Stress-induced DO is unique and difficult to treat. It is thought that traction on the pelvic nerves when increased abdominal pressure is applied to weakened pelvic supportive tissue causes this form of DO (Serels et al, 2000). Records of 36 patients with Valsalva-induced DO were reviewed by Serels and associates. Seventy-five percent of patients had resolution of UUI, and 92% achieved cure. Leak point pressures did not correlate with outcome. The presence of residual urgency is similar to de novo urgency after a PVS procedure. Increasing episodes of urgency and UUI may correlate with surgical failure. The PVS is an effective treatment option for stress-induced DO with cure rates similar to those of simple SUI.
Fulford and colleagues (1999) were the first group to utilize videourodynamics in the assessment of how a PVS affects the urgency syndrome. Ninety-seven percent (83 of 85 women) were symptomatically cured of SUI but only 66 (78%) were satisfied with the surgical result, owing to persistence of urgency symptoms in 27. Among these 27 women, 9 (41%) had an open bladder neck at rest compared with 4 of 50 (8%) women without UUI (P < .01) postoperatively. The storage symptoms resolved in 69% (32 patients), almost all of whom had a closed bladder neck at rest. Yet the postoperative urgency symptoms were not significantly associated with any preoperative clinical or urodynamic variables. Schrepferman and associates (2000) attempted to predict urinary urgency resolution after a PVS procedure with preoperative videourodynamics. Seventy women with MUI were divided into two groups of sensory urgency (one or more episodes of subjective symptoms without DO) and motor urgency (one or more episodes of subjective urgency symptoms correlating with DO). DO was documented in 41 patients. They concluded that there was a significantly greater resolution of urinary urgency symptoms in those with low-pressure motor urgency than high-pressure motor urgency or sensory urgency. Overall, urgency resolution rate in patients with MUI was 51% in this study (Schrepferman et al, 2000). Stoffel and colleagues (2008) found no difference in preoperative videourodynamics between women with MUI and urodynamic DO and those without DO. MUI patients with DO had less improvement in UDI-6 scores than MUI patients without DO, despite similar reduction in pad use/day. The presence of preoperative DO on UDS may relate to decreased quality of life and decreased urgency resolution rates after a PVS procedure. There are no data on consistent videourodynamic parameters that relate to outcomes of MUI after a PVS.
Key Points: Pubovaginal Sling for Mixed Urinary Incontinence
Nonurologic Complications
Beyond discussion of voiding dysfunction and erosions after PVS placement, the AUA Female Stress Urinary Incontinence Clinical Guidelines Panel (1997) reported on surgical management of female SUI, which included summary data of published outcomes data on complications (Table 73-8). Although an extensive literature review was performed there was insufficient information to estimate the following complications: large bowel injury, peripheral nerve injury, and vascular injury. The literature available for UTI had variable definitions and bias. Due to death being a rare complication, the panel estimated a death rate of approximately 5 per 10,000 procedures, combining all SUI procedures (retropubic suspension, transvaginal suspensions, anterior repairs, and PVS surgeries).
Table 73–8 American Urological Association Female Stress Urinary Incontinence Clinical Guidelines Panel Complication Summary
| COMPLICATION | % |
|---|---|
| Postoperative urgency | |
| For patients with urgency and DO preoperatively | 46 (24-68) |
| For patients with urgency and no DO preoperatively | 34 (13-61) |
| For patients with no urgency but with DO preoperatively | 20 (5-45) |
| For patients with no urgency and no DO preoperatively | 7 (3-11) |
| Retention | |
| Longer than 4 weeks | 8 (6-11) |
| Permanent | <5 |
| Transfusion | 4 (2-7) |
| General medical complications (significant/nonsignificant) | 4 (2-5)/6 (3-10) |
| Subjective complications | 6 (2-13) |
| Complications requiring surgery | 3 (2-5) |
DO, detrusor overactivity.
Anger and associates (2007a) analyzed Medicare claims data and determined short-term complications after sling surgery among female beneficiaries aged 65 years and older. A total of 1356 sling procedures were performed between 1999 and 2001. In 3 months after the procedure, 12.5% women developed surgical or urologic complications and 33.6% were diagnosed with UTIs. At 1 year after the operation the following nonurologic complications were reported: bowel injury or obstruction (6.6%), cardiac (9.1%), thromboembolic (2.6%), pulmonary (15.3%), and other (22.1%). Multivariate analysis revealed that race, age, and comorbidity did significantly impact on both urologic and nonurologic complications. Nonwhite subjects were more likely to experience urologic and nonurologic postoperative complications. Women 65 to 69 years were significantly less likely to experience nonurologic complications or undergo treatment for outlet obstruction or re-treatment for incontinence than women older than 75 years.
Midurethral Slings
Since the inception of the PVS approximately a century ago, experimentation has continued with multiple types of slings composed of different materials using different suspension techniques. Materials used as sling constituents have included autografts, allografts, xenografts, and, more recently, synthetic variants. Methods of anchoring these slings have also undergone development and advancement; however, the ideal method of suspension remains to be completely defined. Sling placement was classically described at the level of the bladder neck in an effort to correct urethral hypermobility and enhance pressure transmission invoked by intra-abdominal straining.
However, competing theories arose initially as espoused by Zaccharin in the 1960s and DeLancey in the 1990s (Zaccharin, 1968; DeLancey, 1994). In these theories the importance of the pubourethral ligaments and their function in maintaining the integrity of urinary control furthered the concept of the importance of the midurethral mechanism for preservation of urinary incontinence under stress circumstances. These findings further demonstrate the fact that hypermobility is a secondary finding noted in association with incontinence but not causative of the condition of effort-related urinary loss (SUI). Studies performed by Asmussen and Ulmsten (1983) demonstrated the importance of the distal components of the urethra for preserving urethral closure during stress events. They demonstrated that MUCP occurs at the level of the midurethra and this closure mechanism is associated with intrinsic urethral function in continent women. Ingelman-Sundberg (1953) noted that the pubococcygeal muscles also played a role in the midurethral mechanism, inserting at the level of the midurethra just outside the vaginal epithelial wall, and propounded this anatomic finding as being important when considering methods to correct urinary incontinence. Westby and colleagues (1982) demonstrated that in continent women, midurethral closure is associated with the area of MUCPs on pressure evaluation of the urethra and considered this phenomenon to be due to the confluence of anatomic structures in that area.
Petros and Ulmsten (1993), using these theories, proposed a unifying concept now known as the midurethra theory (previously the integral theory). They postulated that injury arising from surgery, parturition, aging, or hormonal deprivation led to weakening or damage of the pubourethral ligaments, impairing midurethral function and anterior urethral wall support, thus resulting in urinary incontinence. They theorized that this damage was not only a ligamentous injury but also a representation of weakening of the pubococcygeal muscles at the level of the midurethra. It has been shown that weakness of soft tissue in this area and specifically connective tissue can contribute to urinary incontinence (Ulmsten et al, 1987). Ultrasound techniques provide greater understanding of the vesicourethral dynamics by giving a detailed view of the exact anatomic location of the midurethral sling. Lo and associates (2003) conducted early ultrasound studies to describe dynamic urethral kinking (or rotational urethral descent) as the morphologic feature (92% of the time) contributing to continence after a TVT procedure. Preoperative real-time imaging demonstrates the motion of anterior and posterior urethral walls in the same axis and vector, regardless of the urethral descent. Midurethral tape binding impedes the movement of the posterior urethra above the tape, directing its motion away from an inferior course in an anteroinferior or anterior direction. Inward movement of the posterior urethral wall results in urethral narrowing. Width, position, and appearance of slings on ultrasonography are similar: a V at rest, round angulation on Valsalva maneuver, and closed angulation at maximum retaining. It is closer angulation during a Valsalva maneuver that may be associated with postoperative voiding dysfunction (Dietz and Wilson, 2004; Masata et al, 2006; Chene et al, 2008; Kociszewski et al, 2008).
The Tension-Free Vaginal Tape Procedure
The TVT procedure was developed using the concepts of the integral theory. The provisions of this operation include a minimally invasive approach, which would supplement the diminished midurethral mechanism and produce the ingrowth of new host tissues after implantation for purposes of further supplementing support introduced by the procedure. As initially described, the procedure was performed with the patient under local anesthesia to allow ambulatory delivery of the intervention and enable intraoperative testing of the urethral compression.
Initially, several types of material were evaluated until the final material was chosen, a synthetic polypropylene monofilament mesh with pore size under 150 µm. Also, this material allowed optimal migration of host inflammatory components (leukocytes and macrophages) into the mesh for purposes of infectious surveillance and host wound healing (imbibition and inosculation). It was found that this material was optimal for inciting fibrous tissue ingrowth. This type of mesh is known as a type 1 mesh and has previously been described in the general surgical literature as being favorable from the standpoints of its mechanical properties (stretch and elasticity) (Dietz et al, 2001, 2003). Efforts have been made to standardize the procedure by incorporating certain technical approaches and safety features for purposes of avoiding injury to surrounding structures. To date, more than 400,000 of these procedures have been performed worldwide.
The Procedure
The device consists of two specially curved 5-mm-diameter insertion needles that are attached to a 40-cm segment of polypropylene tape that is 11 mm wide. The tape is covered with a clear plastic sheath, which protects the tape from contamination and allows easy passage through host tissues. A rigid catheter guide is placed in the bladder with an 18-Fr Foley catheter to help deflect the bladder away from the locale of needle path insertion. An ergonomic handle is attached to the insertion needles for actual placement of the needles during the procedure. This handle is reusable and sterilizable.
The midurethra sling procedure (transvaginal tape) is performed with the patient in the dorsal lithotomy position with a significant degree of flexion (70 degrees or more) of the thighs. The patient has received parenteral sedation and then local anesthetics are placed in the region of insertion of the device (vaginal wall and retropubic space). Approximately 5 mL of local anesthetic is injected into the vaginal area as well as into the planned suprapubic insertion skin sites. For placement of retropubic local anesthesia, another 20 mL of local anesthetic agent is injected into the area along the posterior aspect of the pubic bone to the level of the urogenital diaphragm. Additional vaginal infiltration includes approximately 10 mL injected on either side of the urethra to the level of the urogenital diaphragm.
After appropriate anesthesia, two small suprapubic stab incisions are created just above the level of the symphysis pubis, approximately 2 cm lateral to the midline. A third midline vaginal incision approximately 1.5 cm wide is created approximately 1.5 cm from the external meatus of the urethra, between that structure and the bladder neck. After the vaginal incision is created, minimal dissection is performed using Metzenbaum scissors under the vaginal flaps on either side to elevate the vaginal epithelium from the underlying periurethral tissue to the level of the pubocervical fascia, which is not perforated. The TVT needle is then placed in the dissection tunnel immediately beneath the vaginal epithelium on one side of the urethra with the needle tip situated in close proximity to the lower rim of the pubic ramus. With the use of controlled pressure, the needle is elevated through the endopelvic fascia, into the space of Retzius, through the rectus muscles, and through the previously created suprapubic skin incision. During this maneuver the needle is kept in close contact with the intrapelvic surface of the pubic bone to avoid perforation of the lower urinary tract and also to avoid intraperitoneal entry. Tactile contact ensures direct apposition of metal to bone, as does slow graded pressure during needle advancement.
Simultaneous deflection of the lower urinary tract is accomplished during insertion using the catheter guide and catheter with the pelvic viscera deflected away from the side of needle insertion. The same maneuver is performed contralaterally so that each needle exits through the appropriate skin incision. Cystoscopy is performed to exclude needle penetration of the lower urinary tract. The use of a 70-degree lens is essential, as is complete distention of the bladder with irrigant to exclude subtle tangential injury. If perforation is noted, the needle is withdrawn and passed once more in the same area in an effort to avoid further perforation. Once cystoscopy has demonstrated no evidence of bladder injury, the tape is brought through the incisions and tension adjustment of the tape is performed. Tension adjustment is most commonly performed by inserting either a surgical instrument (clamp) or a metallic sound between the tape and urethra while the covering plastic sheath is removed from the field. The tape is set to tension such that with the bladder full to 300 mL of saline and the patient now aroused and asked to cough, no incontinence occurs during the stress maneuver. Redundant tape is then excised at the level of the suprapubic skin incisions, and all incisions are closed (Fig. 73–8).
The procedure may also be performed with the use of regional or general anesthesia according to the surgeon’s preference. Outcome data support the incorporation of midurethral sling techniques with concomitant vaginal prolapse repairs.
Results
In reviewing the extensive results reporting for the midurethral sling, several caveats must be entertained. Outcomes are reported in varying fashions using different tools, lengths of follow-up, and overall definitions of success and failure. These factors should be kept in mind when attempting to cross-compare different groups and procedural nuances.
Initial results with the midurethral sling technique approximated 80% (author-defined) success rates (Ulmsten et al, 1996). A subsequent prospective multicenter trial that included 130 women with genuine SUI who were observed for 1 year revealed success rates of 91% (Ulmsten et al, 1998). Seven percent were considered improved, and only 2% were deemed failures. Complication rates were low, including one bladder perforation and one wound infection. Voiding dysfunction was also relatively low, with 1 patient experiencing retention for 12 days, which resolved spontaneously, and 3 patients with less than 3 days of voiding dysfunction (regarding catheterization), which the authors defined as a short-term voiding problem.
On the basis of these findings, further studies were then embarked upon. Nilsson and Kuuva (2001) evaluated 161 consecutive TVT operations in cases of which 28% had failed prior incontinence surgery, 11% had ISD, and 37% had MUI. At 16 months mean follow-up, the overall objective cure rate was 87%, with 7% significantly improved and another 5% considered failures. Bladder injury rate at the time of insertion was 3.7%, and 4.3% of women experienced short-term de novo voiding dysfunction. Urgency symptoms arising after surgery occurred in 3% of women, yet 80% of the women who had preoperative urgency symptoms had relief of those symptoms at their 16-month visit. No serious complications were noted.
Long-term results mirror the short-term experience with this procedure. Success rates ranging from 81% to 90% have been reported at more than 3 years. Ulmsten and associates (1999) reported an 86% success rate in 50 women at 3 years. Olsson and Kroon (1999) reported 90% success in 51 women at 3 years. Doo and colleagues (2006) evaluated long-term efficacy and safety of this procedure among 134 Korean women. The overall 5-year success rate was 94.9%, with an 86.6% patient satisfaction rate. Although success rates between 1 and 5 years were similar (97.7% and 94.9%), the cure rate decreased from 90.1% to 76.9%. Nilsson and coworkers reported success rates of 84.7% at 5 years (Nilsson et al, 2001) and 81.3% at 7 years (Nilsson et al, 2004) in a consistent cohort of 90 women. Liapis and colleagues (2008) prospectively assessed the efficacy of the TVT in 65 women: at 5-year follow-up the objective cure rate was 53% and failure rate was 9.4%, whereas at 7-year follow-up the objective cure rate was 80% and the failure rate 13.5%. Song and associates (2009) reported on the second longest follow-up (≥7 years) in 306 women with a cure rate of 84.6%; they reported on 6 patients who had complications from mesh exposure. As a continuation of their earlier work, Nilsson and coworkers (2008) provide the longest (11 years) prospective observational cohort study of 90 women with primary SUI. Ninety percent of these women were objectively cured, and 77% reported subjective cure. No late onset adverse effects or cases of tape erosion were seen. The Austrian Urogynecology Working Group published their data compiled within the Austrian central registry beginning in 1998 (Table 73–9). There were no serious complications and no mortality within the registry; 363 (45%) of those in the registry had a TVT in combination with other procedures (Tamussino et al, 2001). These long-term studies have all attempted to evaluate risk factors for declining effectiveness. There does appear to be a tendency for higher failure rates to be associated with advancing age at the time of procedure and also with diminished urethral function (ISD).
Twelve randomized clinical trials have compared the TVT with traditional incontinence procedures in published peer-reviewed journals. This includes five that compared TVT with open colposuspension (Ward and Hilton, 2002, 2004, 2008; Bai et al, 2005; El-Barky et al, 2005), four comparing TVT with laparoscopic colposuspension (Persson et al, 2002; Ustun et al, 2003; Paraiso et al, 2004; Valpas et al, 2004), two comparing TVT with a fascial sling (Bai et al, 2005; Wadie et al, 2005), and one that compared TVT with no treatment (Campeau et al, 2007) (Table 73–10).
Table 73–10 Comparison of Cure Rates between the Tension-Free Vaginal Tape (TVT) and Traditional Incontinence Management
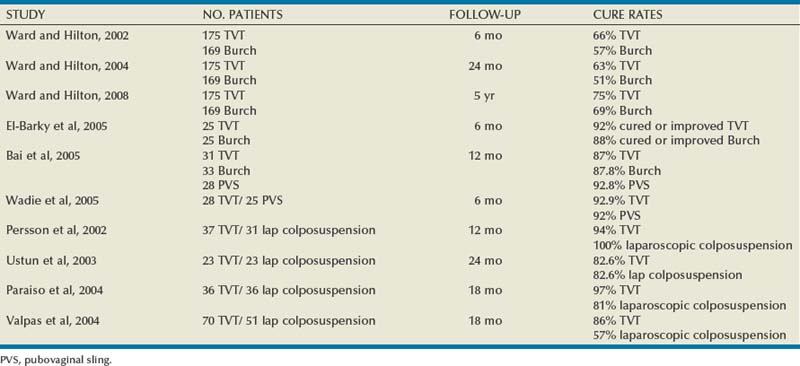
Among the TVT versus Burch comparisons, the trials by Ward and Hilton (2002, 2004, 2008) and Valpas and associates (2004) enrolled the greatest number of patients. The United Kingdom/Ireland cooperative group compared TVT with open colposuspension published data at short (Ward and Hilton, 2002), intermediate (Ward and Hilton, 2004), and long-term (Ward and Hilton, 2008) follow-up. At 6-month follow-up, 344 women were randomized to TVT and Burch colposuspension study arms, with no significant difference in cure rates. Surgery with TVT was associated with more operative complications (i.e., bladder injury), yet colposuspension was associated with more postoperative complications and longer recovery (Ward and Hilton, 2002). At 2-year follow-up, 175 and 169 women were randomized to TVT and Burch colposuspension, respectively. A variety of outcome measures, including subjective and objective measures, were utilized. Overall cure rates noted in the study were lower than other reported cure rates, with 63% of TVT patients and 51% of colposuspension patients being cured. This is likely due to their strict definition of cure (1-hour pad test with change in weight <1 g) and failures including both dropouts and those lost to follow-up. There were significantly more patients in the colposuspension group needing intermittent self-catheterization (<0.0045) and surgery for pelvic organ prolapse (<0.0042) than in the TVT group (Ward and Hilton, 2004). At long-term follow-up (5 years) there were no difference in cure rates. Consistent with earlier studies, prolapse was seen more commonly in the Burch colposuspension group. Two tape extrusions (only one symptomatic) were found in the TVT group. Unlike reports of 27% de novo urgency and UUI in the literature (Jarvis, 1994) after colposuspension, this group reported less than 2% after TVT and less than 5% after colposuspension (Ward and Hilton, 2008). Ward and Hilton (2002) and El-Barky and colleagues (2005) found operation time, hospital stay, and time until return to normal activity significantly shorter in the TVT group. El-Barky and colleagues (2005) reported two bladder perforations in the TVT group (<0.05), whereas wound infections (<0.05) were more common among the Burch colposuspension patients. Bai and associates (2005) compared TVT with open colposuspension and fascial sling: at 3- and 6-month follow-up there were no differences in cure rates between the operations, but at 12 months the patients with a PVS had significantly higher cure rates (92.8%) than those who had the colposuspension (87.8%) or the TVT procedure (87.0%). Wadie and associates (2005) found PVS and TVT procedures to be equally effective. The TVT procedure is cost effective and superior in terms of impact on health care spending compared with open colposuspension (Manca et al, 2003).
Four randomized trials (Persson et al, 2002; Ustun et al, 2003; Paraiso et al, 2004; Valpas et al, 2004) have been performed comparing TVT with laparoscopic colposuspension. Valpas and associates (2004) and Persson and coworkers (2002) reported results at 12 months, and Paraiso and colleagues (2004) reported outcomes at 18 months. The results of the laparoscopic comparator trials revealed TVT cure rates in the range of 86% to 97%, with colposuspension rates ranging from 57% to 100%, depending on the reporting method. There was no apparent difference between procedures in the Persson and coworkers’ trial, but Paraiso and colleagues and Valpas and associates noted significant differences in success in the two study groups. These trials noted no other apparent differences between techniques other than that the TVT group recovered more rapidly and had a lower need for subsequent urogenital prolapse procedures than the colposuspension group. Dean and colleagues (2006) reviewed seven randomized trials comparing TVT and laparoscopic colposuspension and found no statistically significant difference in reported subjective cure rates within 18 months. Yet the overall objective cure rate was significantly higher for TVT. Novara and colleagues (2008) performed a meta-analysis of 33 randomized controlled trials comparing tension-free midurethral slings with other anti-incontinence procedures. Complications were similar between TVT and Burch colposuspension, with the exception of bladder perforation (higher in TVT group) and reoperation rate (higher in the Burch colposuspension study arm). TVT and PVS procedures were found to be equally effective, with better cure rates than the Burch colposuspension (Novara et al, 2008).
Because of the success of the TVT operation, several modifications to the retropubic procedure and use of different tape materials arose. These retropubic modifications have not been evaluated extensively. Only seven randomized studies exist comparing the TVT with different retropubic slings. Three trials compared the TVT with the suprapubic arc sling (SPARC), a polypropylene tape material approaching the midurethra from the abdominal incision (Tseng et al, 2005; Andonian et al, 2005; Lord et al, 2006). Tseng and associates (2005) found the TVT and SPARC to be equally effective. The SPARC group had a greater (12.9%) number of bladder perforations than the TVT group (0%). This was not statistically significant, but the authors believed it was clinically significant. Andonian and associates (2005) randomized 84 patients to either study arm and found no statistically significant difference between SPARC and TVT, in terms of objective cure rates at 12-month follow-up. Tape erosion, infected pelvic hematoma, and UTI were only found postoperatively in the SPARC group, yet there were no differences in other perioperative complications (bladder perforation, blood loss, hospital stay, urinary retention, postoperative analgesia). Lord and colleagues (2006) found the TVT group to have a lower rate of vaginal erosion (4.8% vs. 10.5%) and a statistically significantly higher subjective cure rate (87.1% vs. 76.5%) than the SPARC group. The SPARC was found to be more difficult to adjust correctly, and a statistically significant number of patients required loosening of the tape (P =.002). Campeau and colleagues (2007) compared elderly women who either had a TVT or had to wait 6 months for surgery, using quality of life questionnaires. The women who had the TVT procedure had a significantly higher quality of life (P < .0001), yet 22.6% experienced bladder perforation and 12.9% had urinary retention.
Arunkalaivanan and Barrington (2003) compared the TVT procedure with the use of allogenic acellular porcine collagen (Pelvicol) in a questionnaire-based study. They reported on both 12-month and 36-month results and found no difference in subjective cure rates. TVT was compared in three studies to the intravaginal sling (IVS), a multifilament, miniporous polypropylene tape material produced by Tyco that is passed similar to a SPARC. Rechberger and associates (2009) reported no differences in cure rates at 13-month follow-up of 50 patients per group. Complications were similar with the exception of postoperative acute urinary retention occurring significantly more commonly among the patients having the TVT procedure. Meschia and colleagues (2006) compared the TVT and IVS with intermediate follow-up and reported subjective cure rates of 80% (TVT) and 78% (IVS) and objective cure rates of 85% (TVT) and 72% (IVS). Eight (9%) of the IVS patients experienced vaginal erosion, with none found in the TVT group. The TVT was compared with the IVS and SPARC by Lim and associates (2005) in the SUSPEND trial. There was no significant difference between the cure rates: 87.9% (TVT) versus 81.5% (IVS) and 71.4% (SPARC). There was a significantly greater rate of tape extrusion in the SPARC group. Balakrishnan and coworkers (2007) followed a subgroup of IVS patients from the Lim and associates’ (2005) group up to 30 months and found 13% with sling erosions, requiring removal of the sling. Of the 29 patients (47%) from this initial IVS group seen 12 to 34 months postoperatively, 24% experienced sling erosion with associated sinus formation, requiring sling removal. In Novara and colleagues’ (2007) meta-analysis the TVT was found to be more efficacious than the IVS and SPARC.
Special Groups of Patients
Elderly
Although there is a large body of literature that demonstrates the efficacy and low morbidity associated with midurethral slings for SUI there are few data evaluating the effect of advanced age on outcomes. Aging affects the lower urinary tract both anatomically and functionally. The aging lower urinary tract has a higher rate of DO, UUI, and ISD. Emptying abnormalities related to impaired contractility are also more common in elderly persons. Older women are more likely to have had prior procedures for incontinence and therefore may have higher rates of urethral fixation. More severe vaginal atrophy related to long-standing lack of salubrious estrogen support of the vaginal tissues in the older woman could pose a greater risk of poor healing and erosion after vaginal incontinence surgery. Older patients are generally considered poorer surgical candidates because of medical comorbidities that could complicate the surgery, the surgical outcome, or the postoperative course. Prior studies and prevailing attitudes maintain that retropubic suspensions and vaginal procedures for SUI in elderly patients have variable rates of success (Gillon and Stanton, 1984; Schmidbauer et al, 1986; Couillard et al, 1994; Chilaka et al, 2002).
Few studies of relatively small numbers of patients examine the safety and efficacy of midurethral slings in older women. In all these studies, “old” is defined as 70 years of age and older. The most robust of the studies is a prospective comparison of 460 consecutive women who underwent TVT surgery (Gordon et al, 2005); 157 (34%) were elderly, and all women underwent UDS preoperatively and 3 months postoperatively. Outcome measures were compared between elderly and younger patients with follow-up of at least 12 months, and mean follow-up was 26 months. Preoperatively, a statistically significant greater prevalence of MUI was noted in the older (31%) versus younger (23%) patients. Concomitant pelvic organ prolapse surgery was undertaken in 84% of older patients and 67% of younger patients. The main outcome measures evaluated were perioperative morbidity, postoperative SUI, persistent or de novo UUI, and voiding dysfunction. Intraoperative complications were infrequent. The rates of blood loss were equal, and there were significantly fewer bladder perforations in the elderly patients. The incidence of postoperative fever, UTI, wound infection, and hematoma formation was similar in the two groups. Older patients did experience some age-related morbidities such as pulmonary embolism (2 patients), cardiac arrhythmia (2 patients), deep vein thrombosis (1 patient), and pneumonia (1 patient), whereas of the younger patients only 1 had a cardiac arrhythmia. Older women experienced no increased risk of urethral erosion or vaginal extrusion. The rates of postoperative voiding dysfunction necessitating catheterization for more than 1 week were low and similar between groups. Only 1 patient, an older woman, required urethrolysis. Rates of persistent SUI were uniformly low and no higher in the older women. Rates of UUI were similar between groups, but the rate of de novo UUI was greater to a statistically significant degree in the older patients (18% vs. 4%).
A similar rate of postoperative de novo urgency in elderly women undergoing TVT was documented in a smaller study of 76 women (Sevestre et al, 2003). One strength of this study is that a description of the degree of urethral hypermobility is given. Of these women, 53% had urethral hypermobility defined by a Q-tip test greater than 30 degrees, 28.9% had undergone prior incontinence procedures, and 4 (5.2%) showed UDS evidence of DO. At a mean follow-up of 24.6 months, 67% of the patients were cured, as determined by questionnaire and examination. Ten (13.7%) had persistent SUI, and all of these had negative Q-tip tests preoperatively; and 14 (18.4%) had UUI. Satisfaction with the procedure was 82%, and rates of dissatisfaction were higher in those with de novo UUI. For the older women with a negative Q-tip test, the cure rate was 71%, compared with 100% in women with a positive test. The rate of immediate urinary retention was 26.3%, but only 1 patient had urinary retention for more than 1 week. One case of bladder perforation and one case of vaginal erosion were reported. Liapis and associates (2006) similarly correlated the degree of urethral hypermobility and outcomes after a TVT procedure among women aged 65 to 85 years. An overall cure rate of 76% was reported, with positive correlation to bladder neck mobility. Of patients in whom the angle of displacement on the Q-tip test was less than 30 degrees, 42% became continent, whereas 90% were continent among those with an angle greater than 30 degrees. Among those in whom the angle was less than 10 degrees, 80% remained incontinent.
The success rates and complications after colposuspension and TVT procedure were compared between women older than age 70 years and younger women (Pugsley et al, 2005). The cure rates were similar between the age groups. There was an increased incidence of UTI among the elderly women with Burch colposuspensions and the TVT patients. After the Burch colposuspension, women older than age 70 required more long-term self-catheterization. Among the elderly group with the TVT procedure, postoperative UDS was required more often for voiding dysfunction and eventual need for sling lysis was higher. Touloupidis and coworkers (2007) reported a cure rate of 96% among women with mean age 72.3 years; however, these authors were concerned by the rate of bladder perforation (5.8%) and de novo urgency (9.8%).
Overall, cure rates at least in older women with urethral hypermobility are comparable to those in younger women. Complication rates vary, with some studies citing a higher rate of age-related morbidities but no apparent increase in intraoperative complications. Jha and colleagues (2009) evaluated factors influencing outcome with the TVT procedure and found that age did not impact significantly on outcomes for SUI reduction or improvement in quality of life. Postoperative voiding dysfunction or increased de novo urgency does seem to be a complication of greater incidence and significant impact in older women. Postoperative urgency symptoms in as many as 60% of women older than 70 years have been reported (Allahdin et al, 2004), and 44% developed the symptom de novo. Bafghi and associates (2005) noted cure rates among patients younger than 70 years of 97.5% versus 78.5% among patients older than 70 years (P = .001). The satisfaction among the younger group was also higher, 92.6% and 66.7%, respectively. This difference was attributed to higher rates of de novo and persistent UUI in those older than age 75 years.
A sensation of impaired emptying postoperatively has also been reported (Sevestre et al, 2003). Furthermore, one study documented a 4-fold increase in the need for repeated UDS in older women after TVT surgery and an approximately 30-fold higher risk of further surgery to divide the tape.
Another important measure of the success of a TVT procedure in elderly patients is quality of life assessments. The outcome of TVT surgery was assessed prospectively in older women at a mean follow-up of 22 months by a validated health-related quality of life instrument, the King’s Health Questionnaire (Walsh et al, 2004). The improvement in SUI was greater in the age group younger than 70, which is not clearly attributed to any preoperative factor. Rates of preoperative DO were 24% versus 9% in older and younger women, respectively. Older women had a history of prior surgery more often than younger women (67% vs. 28%) and lower leak point pressures, but no specific comment on their physical examination and degree of urethral hypermobility was made. Although the outcome for the TVT procedure was successful, the hospital course may be longer, as found by Walsh and colleagues (2004). The mean hospital stay in their series was 6 days, indicating that the postoperative morbidity in this age group was significant. The reasons for the longer hospital stays were not given in this study.
Hellberg and colleagues (2007) used a mailed questionnaire for 970 consecutive TVT procedures performed between 1995 and 2001 to compare outcomes between women older than 75 years and younger women at a mean follow-up of 5.7 years. The older women were significantly more often on hormone replacement therapy, had lower education, and had history of recurrent UTIs, previous vaginal repair, and previous incontinence surgery. The elderly patients more commonly had MUI and required longer hospitalization postoperative care. The unfavorable cure rates among the older women (55.7% vs. 79.7%, P = .0001) were due to higher failure rate for SUI, rather than for urgency symptoms.
Most recently, a multicenter, prospective randomized clinical trial was performed to compare TVT surgery versus no treatment in elderly women with SUI. Campeau and colleagues (2007) studied 69 women older than the age of 70 years who consented to be randomized to undergo immediate TVT surgery or wait for 6 months before submitting to the same surgery (control group). The main outcomes measured included the Incontinence-Quality of Life (I-QOL) Questionnaire, the Patient Satisfaction Questionnaire, and the Urinary Problems Self-Assessment Questionnaire. At 6 months after randomization the group of elderly women who underwent immediate TVT surgery had significant improvement in quality of life and patient satisfaction and fewer urinary complaints compared with the group of women waiting for the same surgery. Importantly, no age-related morbidity was observed in the immediate surgery group.
Obesity
Whether obesity affects surgical outcome with the TVT procedure is controversial. A few small studies have examined the safety and efficacy of TVT surgery in this population. In a prospective study of 242 women with genuine SUI, women were stratified into three groups on the basis of body mass index (BMI) (Mukherjee and Constantine, 2001). The cure rate in obese women was 90% versus 95% in women with a BMI of 25 to 29 and 85% in those with a BMI less than 25. No women experienced a wound infection, and there was no higher rate of retropubic hematoma in obese women. In addition, obese patients undergoing TVT surgery did not demonstrate a statistically significant difference in the incidence of voiding dysfunction: de novo urgency symptoms, UUI, or voiding disorders. Although few in number, studies with 6- to 12-month follow-up support equal efficacy and no difference in intraoperative or postoperative complication rate. No relation between obesity and the occurrence of bladder injury has been found (Lovatsis et al, 2003; Rafii et al, 2003). Skriapas and associates (2006) matched 31 women with BMI greater than 40 with 52 women with BMI less than 30 and reported on their 18.5 month follow-up after a TVT procedure. The continence rates among the morbidly obese group were not significantly different from those of the control group, 87% and 92% (P = .103), respectively. The early postoperative complications were significantly higher among the morbidly obese patients. Killingsworth and coworkers (2009) reported on 127 overweight to obese women and concluded that success rates, patient satisfaction, and complications were not significantly different with increasing BMI. Meschia and colleagues (2007) had similar findings to those of Mukherjee and Constantine (2001) and Killingsworth and coworkers (2009) with no differences in BMI of successes and failure, suggesting that the TVT procedure could be confidently used in overweight women. Muller and associates (2007) reviewed randomized and retrospective clinical trials in the English TVT surgical literature from 1998 to 2006. Neither age older than 70 years nor morbid obesity was a risk factor for failure of the TVT procedure; however, there was an increase of de novo urgency among the elderly women and those with a BMI greater than 35.
Hellberg and colleagues (2007) conducted a questionnaire-based study, including 970 consecutive TVT procedures performed between 1995 and 2001 to compare outcomes between women with a BMI greater than 35 (n = 61) and those who had normal weight at a mean follow-up of 5.7 years. There was a sharp decline in long-term cure rates between women with a BMI less than 25 and those with a BMI greater than or equal to 35: 81.2% and 52.1%, respectively (P = .0005). The obese women were significantly older, had a higher parity, were less educated, and more often had diabetes and chronic bronchitis. The women with normal weight had higher mean postvoid residual urine volumes after surgery and required more adjustments of tape. The increased failure rate among the obese population was due to low cure rates of both stress and urgency components.
Despite the success of the TVT procedure in obese women there is a reported case of necrotizing surgical site infection after TVT placement in a 53-year-old obese woman (Connolly, 2004). Morbidly obese patients have a 44% increased risk of postoperative infectious morbidity (Forse et al, 1989). Obesity poses a greater risk for necrotizing fasciitis than diabetes in obstetric and gynecologic procedures (Gallup et al, 2002). Standard antibiotic prophylaxis and the time of surgery may need to be altered for the morbidly obese to provide adequate tissue levels, and infected tapes require complete removal. Obese women, like all women undergoing midurethral sling, should be counseled about the risk of infection.
Concomitant Pelvic Organ Prolapse
A large proportion of women with SUI have associated pelvic organ prolapse that requires concurrent treatment. Many studies have explored the outcomes of TVT with concomitant surgery. The advantage of using a synthetic sling with concomitant surgery is that operative time is reduced sizably and blood loss from the TVT portion is usually minimal compared with autologous slings or retropubic suspensions. Some of the theoretical risks of midurethral slings with concomitant transvaginal surgery are that the increased dissection will increase exposure of the graft, leading to greater rates of infection, erosion, or vaginal extrusion, or that increased blood loss or anatomic distortion from the concomitant procedures could increase the rate of sling migration and postoperative voiding dysfunction or obstruction. These studies tend to be small and have short follow-up, but results suggest that the TVT procedure can be added to prolapse surgery with minimal morbidity. Success rates in combined TVT and prolapse repair vary from 88% to 93% (Jomaa, 2001; Huang et al, 2003; Meltomaa et al, 2004). Even in questionnaire-based assessments 3 years after surgery the data do not show a statistical difference in the cure rate of SUI and incidence of urgency symptoms after TVT alone or in combination with other vaginal surgery (Meltomaa et al, 2004). Transient urinary retention occurred more often in patients undergoing concomitant vaginal surgery, but urethrolysis rates were low and not statistically different between groups. Interpretations of rates of postoperative urinary retention are limited by variation in the definition of retention, and therefore caution must be exercised when reviewing outcomes.
Gordon and colleagues (2005) examined TVT surgery as a prophylactic procedure for SUI in prolapse repairs. None of the patients had undergone prior incontinence surgery. With a mean follow-up of 14 months, no patient developed symptomatic SUI but 3 had a positive stress test urodynamically. Six of 9 patients with preoperative urgency had persistent symptoms, and 4 (13.3%) developed de novo urgency without evidence of obstruction. No woman had urinary retention lasting more than 2 weeks. Subsequent 5-year data from a prospective analysis of a large group of women undergoing TVT for occult SUI combined with transvaginal repair of second- or third-degree prolapse showed a low incidence of complications (Groutz et al, 2004). The authors reported that one case of bladder perforation was managed conservatively without consequence. Two patients experienced extended voiding difficulty requiring catheterization for more than 7 days, but urethrolysis was not necessary in any case. Three cases of vaginal erosion were documented. Two patients developed recurrent symptomatic SUI, and another 15 patients were found to have asymptomatic urodynamically confirmed SUI. Eight patients developed de novo UUI, and 72% of the 18 patients with preoperative UUI had postoperative persistent incontinence. Yuan and associates (2008) retrospectively reviewed results of 12 patients who underwent concurrent TVT and Prolift repair of prolapse. Thirty percent of the patients initially presenting with SUI had moderate to severe anterior prolapse. All patients had satisfactory results. The Cochrane Incontinence Group reviewed 22 randomized trials of surgical prolapse repair that included 2368 women. It was concluded that the addition of a TVT procedure to endopelvic fascial plication, Burch colposuspension, and abdominal sacrocolpopexy may reduce the incidence of postoperative SUI, but issues of cost and associated adverse effects were unclear (Maher et al, 2008).
Although concurrent surgery does not appear to alter success of a TVT procedure, whether concurrent surgery alters the time to efficient voiding or incidence of urinary retention has been examined separately in a retrospective study of 267 women (66% having concurrent prolapse repair) (Sokol et al, 2005). Without standard definitions of urinary retention, results are not comparable between studies. Sokol and associates (2005) noted that there was no significant difference in median days to voiding and rate of urinary retention based on prolapse repair status. Yet, increasing age, decreasing BMI, and postoperative UTI were independent predictors of time to adequate voiding. Only previous history of incontinence surgery was an independent predictor of urinary retention. No statistically significant difference in the rate of urethrolysis between TVT surgery alone and TVT with prolapse repair was found.
Unlike most other authors, Partoll (2002) claimed that urinary retention is far more common after combined procedures than with a TVT procedure alone. Results showed a 94% cure rate at 11 months and an alarming 43% rate of urinary retention after concurrent anterior or posterior repair. However, in her study, “urinary retention” was defined as not meeting the criteria for catheter removal on postoperative day 2. The expectation of voiding efficiently within 48 hours of surgery, rather than the more lenient expectations in other studies, probably accounts for the higher rate of retention found in her study. Using Medicare claims data on a 5% national random sample of female beneficiaries who underwent sling procedures, Anger reviewed 1356 cases. Of 1356 sling cases, 467 (34.4%) had concomitant prolapse repairs. Women who underwent prolapse repair at the time of the sling surgery were significantly more likely to be diagnosed with postoperative outlet obstruction (9.4% vs. 5.5%, P < .007) but less likely to undergo a repeat procedure for SUI or reoperation for prolapse within 1 year after sling surgery (Anger et al, 2008).
When TVT procedures are placed for only urodynamic or occult SUI at the time of prolapse repair, the risk of intervention due to obstruction is equal to the risk of intervention due to SUI when no clinical, urodynamic, or occult SUI is present and no TVT was placed (8.5% and 8.3%, respectively) (Ballert et al, 2009). Ballert and colleagues (2009) did report that the risk of intervention for SUI in patients with clinical SUI but no urodynamic or occult SUI and no midurethral sling was 30%.
TVT surgery performed with either transvaginal or laparoscopic-assisted vaginal hysterectomy and anterior/posterior colporrhaphy has been shown to have success rates similar to those in published series of a TVT procedure alone (Huang et al, 2005). Complication rates were also in accordance with other TVT surgical series, with 2% bladder perforation, 11% postoperative urgency, and 11% postoperative voiding difficulty. A study specifically looking at the complications and cure rates of TVT procedures performed with or without vaginal hysterectomy found that there was no overall difference (Darai et al, 2002). The TVT-hysterectomy group did have a trend toward more bladder perforation and lower postoperative urinary flow rates, but it was not statistically significant. Objective and subjective cures for this group were 92.5% and 75%, respectively, which was not significantly different from the TVT-alone group.
Anger and associates (2007b) evaluated the relationship between provider specialty and outcomes of sling surgery specifically related to concurrent prolapse management. Utilizing 1999 to 2000 Medicare data, they reported on 1063 slings performed by urologists and 246 by gynecologists. Urologists performed concomitant prolapse repairs in 29.1% of cases versus 55.7% among gynecologists (P < .0001). After a year, urologists were more likely to perform a repeat incontinence procedure (9.3% vs. 4.9%, P = .024) and prolapse repair (26.0% vs. 12.2%, P < .0001). Their findings suggest that urologists should identify and manage prolapse at the time of evaluation of urinary incontinence, to avoid the morbidity and cost of repeat surgery.
The design and methodology for the OPUS trial is presently being finalized. This will attempt to determine whether the use of a concomitant prophylactic anti-incontinence procedure may prevent development of SUI symptoms in women undergoing prolapse surgery and to evaluate the cost-effectiveness of this prophylactic approach. Ethical issues may arise because the protocol includes a sham incision in the control arm and patients may be reluctant to accept random assignment for invasive randomization (Wei et al, 2009).
Mixed Incontinence
Evidence suggests that the TVT procedure has successful outcomes for women with MUI symptoms. However, interpretation of these studies should be judicious because many outcomes were reported on the basis of symptoms only, with no UDS substantiation. There are presently no randomized control trials evaluating the TVT placement for MUI symptoms.
In a retrospective analysis of 112 consecutive women with genuine SUI and MUI the objective cure rate as measured by clinical and urodynamic examination was 89.3% at a mean follow-up of 25 months. Objective cure was defined as no evidence of SUI, a negative stress provocation test, and any urinary retention or residual volume greater than 150 mL. No difference was found in the objective cure rate between patients with genuine SUI and those with MUI. The overall subjective cure rate determined by the Contilife questionnaire was 66%. Subjective cure was lower than objective cure in both patients with genuine SUI and patients with MUI, 69.3% and 54.2%, respectively. The type of incontinence did not alter the incidence of postoperative voiding difficulty. Ten of the 24 patients with MUI had persistence of the urgency component (Jeffry et al, 2002).
Holmgren and colleagues (2005) evaluated the outcome of TVT surgery in women with SUI and MUI with mailed questionnaires 2 to 8 years postoperatively. This was a large cohort of 970 women, and the 78% response rate was remarkable. Five hundred eighty women with SUI and 112 women with UUI were eligible for analysis. However, UDS was not performed in these women preoperatively or postoperatively, and therefore categorization of their incontinence was based only on a positive stress test and a history of leakage immediately preceded by urgency. The questionnaire itself is not fully described in this article; therefore, it is not clear whether a validated instrument was used. Specific questions regarding SUI and UUI were posed, and respondents selected options including worsened, unchanged, improved, almost cured, and cured. For analysis, the women were grouped into cohorts by the number of years since they had undergone TVT surgery. The mean age of women with MUI was greater than the age of women with SUI (67 vs. 61.2), and this was a statistically significant difference. Adjustment for age was made in the analysis. In addition, women with MUI had a statistically significant higher BMI, rate of cesarean delivery, and prevalence of urinary frequency than those with SUI. More women with MUI had a history of irradiation for gynecologic malignancy. Although the numbers were small, irradiation may have had repercussions for bladder function that cannot be fully elucidated by questionnaire and could skew results. Sixty percent of women with MUI were cured up to 3 years after surgery, but outcome declined steadily thereafter. By 6 to 8 years postoperatively the cure rate in women with MUI was only 30%. Urgency and episodes of UUI increased with time after the TVT procedure. In the final analysis the lower rate of cure in the patients with MUI may be due to the other variables in this population, such as age, cesarean section, irradiation, and higher BMI. These differences in the populations confound the ability to assess outcome and highlight the limitations of this study design. Overall, despite the population variables, early outcomes of TVT surgery are good and equal in women with SUI and those with UUI. The diminishment of results over time needs to be confirmed by a prospective study (Holmgren et al, 2005).
In a similar Finnish study, 191 women who underwent TVT procedures were evaluated by examination or telephone interview for outcome at a mean follow-up of 17 months. Sixty-four (34%) of these women had preoperative stress-predominant MUI. None of the women had preoperative UDS; instead, the preoperative diagnosis was based on symptoms. Cure after the TVT surgery was judged as self-report of completely dry in any stress situation. At latest follow-up, 164 of the 187 patients were completely cured, for a cure rate of 87.7%. The cure rate in women with MUI was 69% compared with a cure rate of 97% in the women with pure SUI. This outcome difference was statistically significant. No difference in cure rate was found between women who had concomitant surgery or TVT surgery alone. Sixty percent of the women with MUI considered themselves improved from an UUI perspective as well. The lower cure rate in the MUI patients is not fully known. No description of the preoperative physical examination is given, and it is not known how many of them were among the 149 (78%) who had urethral hypermobility. The authors suggested that, on the basis of these results, preoperative UDS should be performed in women with MUI before anti-incontinence surgery (Laurikainen and Killholma, 2003).
Segal and colleagues (2004) evaluated 98 women after TVT placement explicitly to answer the question of what happens to the UUI. The outcome of TVT surgery in women with mixed incontinence or significant SUI with associated frequency and urgency was assessed retrospectively by a variety of methods: subjectively by patients’ symptoms, by the rate of anticholinergic use before and after TVT, and by before and after quality of life questionnaires. One strength of this study is that patients with concomitant surgery were excluded to minimize the confounding effect of other causes on UUI or frequency and urgency. Sixty-five women were identified as having UUI, and follow-up occurred at 3 months and 1 year. Several preoperative factors were looked at for risk of postoperative frequency, urgency, or UUI requiring anticholinergic agents. On the basis of preoperative subjective symptoms, the urgency component was found to be resolved in 63.1% after the TVT procedure. Two patients with complaints of UUI only but SUI identified on UDS had persistent UUI requiring anticholinergic drug treatment. Seventy-five patients had preoperative urinary frequency and urgency, which resolved in 57.3% after TVT surgery. Thirty (57.7%) of the 52 patients requiring anticholinergic agents preoperatively no longer needed medication after surgery. Only 4 (8.7%) patients needed anticholinergic agents for the first time after TVT surgery. Of all the variables assessed as possible risk factors for postoperative OAB requiring an anticholinergic agent, only a history of prior anti-incontinence surgery was statistically significant. Patients with prior surgery were eight times more likely to have postoperative OAB requiring anticholinergic therapy. Overall, the resolution of preoperative UUI was 63% and the resolution of preoperative urinary frequency and urgency was 57.3%. Resolution based on no longer needing anticholinergic medication postoperatively was 57.7%. TVT surgery resulted in statistically significant improvement in quality of life scores postoperatively in women with stress-predominant MUI and with SUI with urinary frequency and urgency (Segal et al, 2004).
Rezapour and Ulmsten (2001) reported their 5-year data on the efficacy and safety of TVT in women with MUI. In all of these women the urgency component was sensory, and no woman had urodynamic evidence of DO. Eighty women were evaluated, and results were reported in a retrospective fashion. All had undergone UDS preoperatively, and all were found to have SUI as well as motor detrusor contractions during filling. At follow-up, 85% were reported as cured and an additional 4% had improved symptoms on the basis of pad testing and symptom questionnaire. These investigators concluded that UDS was essential before surgery to analyze presenting symptoms. Only 1 patient had prolonged retention (6 weeks), but 8% were found to have small hematomas and 1 patient required exploration for bleeding.
Basu and Duckett (2007) reported on two case reports of women with DO and clinical SUI but no evidence of urodynamic SUI. In both cases the SUI and DO resolved with TVT placement and postoperative anticholinergic treatment. Yet, both women continued to have persistent urgency requiring prolonged anticholinergic therapy.
The short-term outcomes of TVT surgery and use of outside-in transobturator tape (TOT) and SPARC in women with MUI were reported by Paick and colleagues (2008). There was no significant difference in cure rates for SUI. Preoperative DO was an independent risk factor for treatment failure of the UUI component. Athanasiou and associates (2009) compared the efficacy of the TVT procedure and inside-out transobturator tape (TVT-O) for treatment of urinary incontinence in women with MUI and idiopathic DO. There were no subjective differences in outcome, yet women undergoing the TVT procedure were less likely to have persistent DO postoperatively.
Kulseng-Hanssen and colleagues (2008) evaluated results of TVT placement in 1113 women with MUI based on the patient’s predominant bother, that is, SUI, UUI, or SUI and UUI equally. There were no differences in cure rates between the three groups. Patients with predominant SUI bother had significantly better results at 7 and 38 months than the other two groups, especially those complaining mainly of UUI. Eleven percent of women had an increase in UUI 38 months after the TVT procedure. The authors concluded that patients with predominant UUI have poorer results than those with predominant SUI. In a retrospective evaluation of long-term effects of TVT surgery on OAB and urodynamic SUI, Leron and colleagues (2009) noted 88.7% cure and 9% improvement with 62.2% improvement in OAB symptoms. There was no change in severity of symptoms in a third of the patients. De novo OAB symptoms developed in 6.2%. Using the AUA Symptom Index for measurement of outcomes, Ballert and coworkers (2008) found no difference in storage, voiding, or total score between patients with SUI and MUI or those undergoing TVT versus TVT-O placement. Sinha and associates (2008) used the Medical Epidemiology and Social Aspects of Ageing (MESA) and other outcome measures used by the British Society of Urogynaecology (BSUG) database for short-term evaluation of TVT in women with MUI. SUI and UUI were either cured or improved in 78% and 75% of women, respectively. The postoperative global impression of outcome noted great or moderate improvement in 75% of cases and reduction in mean MESA scores of 69% (P < .001).
Duckett and associates (2008) used perineal ultrasonography in 77 women with DO and urodynamic SUI to determine whether the position of a TVT has any effect on the resolution of irritative symptoms. They found that placement of the TVT on any part of the urethra is not more likely to resolve irritative bladder symptoms. Three studies have attempted to correlate preoperative variables with outcomes of TVT in women with MUI (Basu and Duckett, 2007; Gamble et al, 2008; Panayi et al, 2009). Duckett and Basu (2007) reported on correlation between preoperative pressure-flow studies and resolution of DO and OAB symptoms after a TVT procedure. Pressure-flow studies were compared before and after TVT surgery. Women whose  decreased significantly after the TVT placement were more likely to have persistent OAB symptoms. The flow rate was significantly higher in women before the TVT placement, with an objective cure of DO after TVT placement than in those with persistent DO. Basu and Duckett believed that this supported an obstructive cause in women with persistent DO. Gamble and associates (2008) found that age, nocturia, maximum capacity, and choice of sling procedure (lowest rate of DO with TVT-O, followed by TVT, SPARC, and then bladder neck sling) impacted on the persistence of DO and UUI. Panayi and coworkers (2009) found higher median preoperative opening detrusor pressure in women with DO postoperatively (33 cm H2O vs. 26 cm H2O, P < .05).
decreased significantly after the TVT placement were more likely to have persistent OAB symptoms. The flow rate was significantly higher in women before the TVT placement, with an objective cure of DO after TVT placement than in those with persistent DO. Basu and Duckett believed that this supported an obstructive cause in women with persistent DO. Gamble and associates (2008) found that age, nocturia, maximum capacity, and choice of sling procedure (lowest rate of DO with TVT-O, followed by TVT, SPARC, and then bladder neck sling) impacted on the persistence of DO and UUI. Panayi and coworkers (2009) found higher median preoperative opening detrusor pressure in women with DO postoperatively (33 cm H2O vs. 26 cm H2O, P < .05).
Intrinsic Sphincteric Deficiency
In most evidence sources, ISD is defined urodynamically as a leak point pressure less than 60 cm H2O or MUCPs less than 20 cm H2O. However, several of these articles fail to describe the patients’ physical examination or reveal whether any urethral hypermobility existed. What is clear from the current literature is that success of midurethral slings is less in the patient with a fixed urethra and that low leak point pressures seem to portend a poorer outcome, but this may be due to lack of urethral mobility in the same patient. Rezapour and associates (2001) published a prospective 4-year follow-up study of 49 women with ISD who underwent TVT surgery. Forty-one of these women had hypermobility of the urethra; the other 8 had immobile urethras. Postoperatively, 36 (74%) of patients were cured, 12% were improved, and 7 patients (14%) experienced failure of the procedure. None of the women with fixed urethras was cured: 3 improved and in 5 the procedure failed. Other studies support lower cure rates of TVT surgery in women with low leak point pressures when rates of urethral hypermobility are not statistically different (Paick et al, 2004). Urethral mobility before midurethral sling procedures has been shown to be predictive of success. The more the proximal urethra moves during Valsalva maneuvers, the better the cure rate for incontinence (Fritel et al, 2002). Leak point pressures alone have not been shown to predict outcome after a midurethral sling (Gutierrez Banos et al, 2004; Rodriguez et al, 2004); therefore, low leak point pressures are not necessarily a contraindication to TVT surgery. Leak point pressures in the era of the midurethral sling should be correlated with the patient’s physical examination and used to counsel patients about their risk for a reduced chance of success.
A deficient sphincter mechanism is an important risk factor for failure of conventional anti-incontinence procedures. It is difficult to evaluate the efficacy of surgical treatment for ISD because no universally accepted definition presently exists. A few studies have been performed to assess the clinical effectiveness of the TVT procedure in women with ISD (Ghezzi et al, 2006; O’Connor et al, 2006; Sang et al, 2007; Rechberger et al, 2009). In all of these studies, patients were included with urodynamic SUI from ISD, based on a VLPP less than 60 cm H2O. Ghezzi and coworkers (2006) prospectively studied 35 patients and noted a 93.7% cure rate at 12.5 month follow-up. Two of the 3 patients who failed the procedure were noted to have a fixed urethra preoperatively. In a retrospective study, Song and colleagues (2009) compared the treatment outcome of the TVT procedure in patients with ISD (31 patients) and non-ISD (80 patients) SUI after 1-year follow-up. Whereas at the 1-month follow-up there was significant difference in cure rates between the ISD (87%) and non-ISD patients (100%), by 1 year after surgery there was no significant difference. Rechberger and associates (2009) conducted a prospective, randomized trial with a follow-up of 18 months comparing the clinical effectiveness of retropubic and transobturator midurethral slings. Although the efficacy of both techniques was comparable, the retropubic route was more efficient in the ISD group.
Mode of Anesthesia
In the original description of the midurethral sling, anesthesia was local so that the patient would be able to perform a cough as a method of adjusting sling tension intraoperatively. It was believed that this would minimize the incidence of obstruction; however there is conflicting information in the literature regarding whether the mode of anesthesia affects the incidence of intraoperative and postoperative complication. Two studies have failed to show a difference in the efficacy or safety of the TVT procedure performed with the use of local or spinal anesthesia (Wang and Chen, 2001; Adamiak et al, 2002). In a study of 103 women who underwent TVT procedures, a comparison of local and spinal anesthesia was made; 67 women underwent the procedure with local anesthesia and 36 underwent TVT with spinal anesthesia (Adamiak et al, 2002). In the postoperative evaluation there was no difference in the success rate of the TVT surgery performed; there was also no difference in the rate of complications between the groups. There was a difference in the patients’ ability to perform a cough test effectively during the procedure because of spinal anesthesia, but this did not result in an increase in the rate of postoperative obstruction. It appears that tensioning the sling by cough stress test is not necessary. Lo and colleagues (2002b) demonstrated that vigorous manual pressure against the abdominal wall and intraoperative introital ultrasonography to set tension resulted in no obstruction in 45 patients at 1 year. No comparisons of tensioning techniques have been performed to the authors’ knowledge, and many different methods are used. Outcomes in women who undergo TVT surgery in combination with a prolapse repair under general or spinal anesthesia are similar to those in women who undergo TVT surgery alone.
Key Points: Tension-Free Vaginal Tape Surgical Results in Special Populations
Complications
Complications associated with the TVT procedure appear to be within the acceptable range for incontinence procedures. Quality of life is affected not only by the outcome of the incontinence surgery from the standpoint of cure or improvement but also by the appearance of voiding difficulties, UTIs, and other adverse consequences of the surgical procedure itself. The evidence appears to suggest that a minimally invasive procedure that is standardized can decrease rates of complications. The Finnish TVT registry demonstrates a pronounced learning curve for surgeons adopting this new technology (Kuuva and Nilsson, 2003). This registry is unique in that all TVT procedures are recorded in this database, and therefore the data are reflective of an entire national experience with a new procedure. A second registry is currently maintained in Austria and includes more than 3000 cases but it does not involve all surgeons in the country (Tamussino et al, 2001). Overall, the rate of complications associated with the TVT procedure is relatively low. Bladder injury in the two national registries ranges from 2.7% to 3.8%. Voiding dysfunction is reported as approximately 7.6%, and wound healing problems are less than 1%. Other studies have also reported complication rates in smaller but still significant groups of patients (Table 73–11).
Table 73–11 Complications as Reported by Recent Large Series (Two of Which Represent National Registries)*
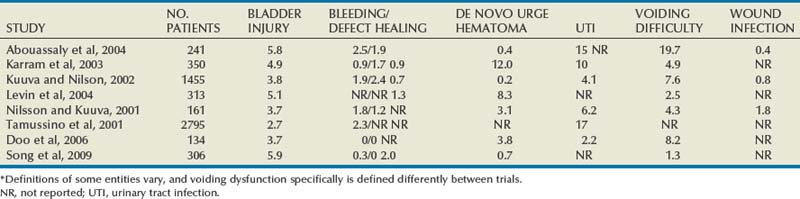
The definition of voiding dysfunction in these studies varies; however, the common definition is the need for catheterization, with short-term catheterization usually being defined as 2 days. The highest voiding dysfunction rate (19.7%) was reported by Abouassaly and colleagues (2004) at 48 hours. The highest UTI rates were reported in the Austrian National Registry (17%) (Tamussino et al, 2001). However, in this registry the infection rate may be reflective of the operative tendency to leave indwelling catheters in the majority of cases. Bodelsson and associates (2002) found that the risk of bladder perforation was three times higher in patients undergoing regional versus local anesthesia.
When UUI and urgency symptoms are evaluated, patients with MUI experienced cure rates of approximately 80% with resolution in patients with urgency in 50% to 80% of cases. The appearance of de novo urgency occurs in 0.2% to 15% of patients without an apparent escalation of risk over time from the initial procedure. Long-term follow-up suggests approximately 6% of patients with de novo urgency postoperatively (Nilsson et al, 2001).
Intraoperative and postoperative bleeding are also variably defined, often by estimation of volume of blood loss. Hemorrhage, however, is relatively rare. Hemorrhage, defined as greater than 200 mL or postoperative hematoma, occurs in approximately 2% of patients and can usually be managed by observation or local compression. Flock and colleagues (2004) reported a rate of 4.1% of 249 patients experiencing hemorrhage of 300 mL or more and requiring surgical intervention.
Although rare, vaginal, urethral, and intravesical erosion of the midurethral sling polypropylene mesh is a particularly feared complication of the technique, largely because of advances in materials science. Tape erosion has been reported in the literature with an incidence of 0.3% to 23%, and currently there is no clear consensus on the management of this particular complication. Given the extensive experience with the TVT device, the majority of reported complications have been associated with this device; however, given the similarity between the various devices, it is reasonable to assume that these results will be noted with other types of midurethral slings (Clemens et al, 2000; Volkmer et al, 2003).
The incidence of TVT erosion in the reported literature is extremely low. Many attribute this phenomenon to the macroporous characteristics of the polypropylene mesh. These characteristics allow excellent incorporation of fibroblasts, macrophages, white blood cells, collagen, and neovascular tissue within the interstices of the tape. The excellent tissue ingrowth facilitates integration of the mesh with the surrounding host tissues and decreases tape encapsulation, all of which minimize the risk of an erosion complication. The erosion rate reported in the literature for polypropylene mesh is 0.5% to 1.3% (Meschia et al, 2001; Kuuva and Nilsson, 2002; Karram et al, 2003; Levin et al, 2004).
Voiding dysfunction after genitourinary erosion of synthetic midurethral slings has been reported. Starkman and associates retrospectively reviewed the voiding dysfunction in 19 patients with genitourinary erosion of polypropylene mesh slings after mesh excision. They reported on a total of 11 vaginal, 7 intravesical, and 5 urethral erosions. After surgery, only 4 patients (21%) achieved complete resolution of symptoms. Persistent voiding dysfunction was present in 15 patients (79%), including persistent or de novo pelvic pain, storage symptoms, and SUI. Ten patients were incontinent after surgery (4 had SUI, 2 had UUI, and 4 had MUI) (Starkman et al, 2006).
The Austrian Urogynecology Working Group reported no serious complications and no mortality within their registry (Tamussino et al, 2001) (see Table 73–9). Two percent were noted to have intraoperative bleeding, and 4% had bladder perforation. Among those reported to have bleeding, most were treated conservatively and only 0.8% required conversion or reoperation (Kolle et al, 2005).
Terminology
The term erosion implies that the mesh has either entered the lower genitourinary tract (urethra or bladder) or has penetrated through the vaginal epithelium. The latter circumstance may better be termed exposure or extrusion (exposure may be preferable because the underlying causative mechanism may not be identifiable) and may arise from either mechanical or wound-related variables. The actual mechanism of erosion is still poorly understood. There are a number of theoretical possibilities, including subclinical infection, poor tissue ingrowth into the sling, disturbed wound healing, rolling or twisting of the tape, and excessive friction between host tissue and the tape. Also, iatrogenic injury and surgeon technical error should be considered as factors in the etiology of erosive complications (Kobashi and Govier, 2003a, 2003b; Domingo et al, 2005). Biomechanical properties of the sling material play a major role in the incidence of complications related to tape erosion. Although various materials have been historically utilized for sling implants there has been a trend in the contemporary literature toward the use of macroporous polypropylene slings. The increased pore size allows excellent tissue ingrowth, promotes integration with the surrounding host tissues, and decreases encapsulation and infection (Dietz et al, 2001, 2003; Slack et al, 2005). Adherence to meticulous surgical technique and utilization of polypropylene tapes with favorable biomechanical properties may help the surgeon minimize this particular complication.
Vaginal Erosion
Vaginal tape erosion or extrusion is a rare complication after the TVT procedure (Fig. 73–9). In three large studies the incidence was reported to be 1.1%, 1.3%, and 0.5% (Meschia et al, 2001; Levin et al, 2004; Huang et al, 2005). Most cases present within a few weeks to a few months after the midurethral sling procedure. One case of delayed vaginal erosion at 18 months has been reported (Sharma and Oligbo, 2004). Symptoms of vaginal erosion include vaginal discharge (with variable constituents and different amounts of blood and inflammatory components), a palpable rough surface in the vagina, sexual discomfort (including partner related), pelvic pain, inguinal discomfort, and lower urinary tract symptoms (LUTS) (urgency, frequency, persistent incontinence, hematuria). Symptoms are often nonspecific, and therefore a high index of suspicion is required. Careful vaginal examination usually identifies an area on the anterior vaginal wall with separated epithelial edges and exposed mesh. The management of this complication is not standardized, and there are various reports claiming successful outcomes with observation, partial tape excision, complete tape excision, and reapproximation of the vaginal mucosa over the exposed tape (Table 73–12).
Historically, most authors have advocated removal of the exposed tape when a vaginal erosion occurs. Reports of conservative management in the literature are sparse. Kobashi and Govier (2003a) reported their experience managing 4 patients (3 SPARC and 1 TVT) with vaginal erosion in a conservative fashion. Two patients presented with persistent vaginal discharge and 2 were completely asymptomatic. All patients were observed with serial physical examinations, and all patients had spontaneous reepithelialization of the mesh at 3 months, which they attributed to the macroporous characteristics of the polypropylene mesh facilitating excellent tissue ingrowth. This is less likely with other synthetic materials such as GORE-TEX, polyester, and silicone, because epithelialization is not likely to occur (Leach et al, 1997; Kobashi et al, 1999).
In a review by Huang and associates (2005), six vaginal erosions and one bladder erosion after creation of polypropylene synthetic slings were initially expectantly managed. In 4 patients with vaginal mesh erosion of less than 1 cm, conservative management was initiated for a 3-month period. One of these patients was observed for 24 months without adverse sequelae. In contrast to the results seen by Kobashi and Govier (2003b), none of the patients in their series had vaginal epithelialization over the area of erosion. Therefore, all 6 patients underwent TVT excision in conjunction with excision of all fibrotic vaginal tissues. Symptoms resolved in all patients, and all patients were continent at their last follow-up. Although all patients ultimately required surgical intervention, the authors believed that a trial of conservative management in appropriately selected patients (i.e., erosion <1 cm) was reasonable; and if no epithelialization occurs at 3 months of follow-up the tape should be surgically removed. In a review of tape-related complications in a series of 200 patients, Tsivian and coworkers (2004) observed five vaginal tape erosions. Four patients required surgical excision of the exposed tape, and 1 asymptomatic patient was being observed conservatively without adverse outcomes. Four of the 5 patients were dry, and 1 with recurrent SUI was dry with repeated TVT surgery. Symptoms resolved in all patients after partial tape excision and judicious vaginal debridement. Furthermore, in a large series of 350 TVT procedures reported by Karram and colleagues (2003), three vaginal erosions were noted with 1 patient managed with local estrogen cream and antibiotics. This patient had done well without recurrence of symptoms or additional morbidity at last follow-up. In the same series, another patient underwent careful excision of the vaginal tissues with coverage of the tape with a vaginal advancement flap with satisfactory results. The third patient required partial excision of the exposed tape and redeveloped SUI. In all patients, symptoms related to the tape erosion resolved after management.
In the Finish nationwide review of 1455 TVT procedures, 10 patients were identified with vaginal polypropylene tape exposure. Three of these patients were managed without surgical intervention with good results and maintenance of continence (Kuuva and Nilsson, 2002). Four patients had the vaginal mucosa resutured over the exposed tape, and 2 patients required partial tape excision. One patient was lost to follow-up, and management was unknown at the time of their report. According to this national registry, continence was maintained in all patients, regardless of the management. Australasian data on erosions was presented by Hammad and associates (2005) that included 17 vaginal erosions. Thirty-five percent of the erosions were asymptomatic and identified by vaginal examination. Symptomatic patients complained of palpable tape and vaginal discharge, local pain, UTI, and dyspareunia. All vaginal erosions were managed with either partial or complete sling removal. Five (29%) of these patient with erosions had simultaneous anti-incontinence procedures. Data are unavailable on the other 12 patients.
Among 166 patients in whom TVT procedures performed, 5 (3%) developed vaginal extrusion between 4 and 40 months postoperatively (Giri et al, 2007). Patients complained of vaginal discharge, pain, bleeding, and dyspareunia. The eroded margin of vaginal mucosa was trimmed and closed over the tape. All of the patients were subsequently symptom free at 12-month follow-up. Ordorica and colleagues (2008) reported on 11 vaginal erosions after nonautologous sling (33 retropubic, 2 bone anchors, 3 TOT) placement. Involved sling and suture were excised. The bone anchors were unable to be removed. An autologous sling was placed in the same setting if the patient complained of SUI at presentation. Two patients had recurrent or persistent SUI, 2 had de novo urgency/frequency, and 1 developed osteitis pubis. Lapouge and colleagues (2009) described their management of 12 patients with vaginal erosions. Initially all patients were managed with partial transvaginal excision. Eight (67%) had no complaints after excision, and 50% of these were continent. Al-Wadi and associates (2009) presented a case report of a women with vaginal extrusion of mesh after a TVT procedure that was managed without excision of the exposed sling. After failure of conservative treatment, a Martius graft was used for coverage, with preservation of the exposed mesh and closure of the vagina. These authors believed that removal of sling material increased the patient’s chance of recurrent incontinence. At 5-month follow-up the woman is asymptomatic and dry.
Chen and associates (2008) analyzed risk factors associated with vaginal erosion (6/239, 2.5%) after synthetic sling placement. Women with diabetes were 8.3 time more at risk of developing vaginal erosion after synthetic sling placement. The vaginal erosion-free rate during the 24-month follow-up decreased significantly in women with diabetes. There was also a 10.7% more vaginal erosion rate associated with type III multifilamentous polypropylene sling (intravaginal slingplasty) than with type I monofilament polypropylene sling (TVT and TVT-O, P = .054). These authors encourage counseling women with diabetes of the risk of erosion.
The sexual function of women after correction of erosion from a midurethral sling was determined using a validated questionnaire (Female Sexual Function Index [FSFI]) by Kuhn and coworkers (2009a, 2009b). Among 21 erosions, 3 healed with topical estrogen; and 18 larger defects required operative intervention. Initially, vaginal closure was attempted, but 2 patients had recurrent erosion. One patient had repeat vaginal closure, and the other had partial sling excision and vaginal closure. The results for the domains of desire, arousal, lubrication, satisfaction, and pain improved significantly. The results pertaining to orgasm remained unchanged.
Key Points: Tension-Free Vaginal Tape Procedure—Vaginal Erosion
Urethral Erosion
Urethral erosion is defined as presence of sling material within the urethral lumen (Fig. 73–10). A review of the published literature by the AUA Stress Urinary Incontinence Clinical Guidelines Panel found 5 cases of urethral erosion after 1715 autologous fascial slings (0.003%) and 27 cases of urethral erosion after 1515 synthetic PVS procedures (0.02%) (Leach et al, 1997). The exact incidence of urethral erosion after the newer tension-free midurethral sling procedures is, as yet, undetermined. Factors thought to contribute to urethral erosion include compromised urethral blood supply (i.e., radiation therapy or estrogen deficiency), excessive sling tension, extensive dissection too close to the urethra with subsequent urethral devascularization, iatrogenic urethral injury (at time of device insertion), and traumatic catheterization/dilation. Furthermore, twisting or rolling of the tape can create a ridge that leads to pressure necrosis and erosion through the urethra (Dell and O’Kelley, 2005). Management of this complication is extremely challenging with the possibility of significant morbidity, because access to the tape is traditionally gained by incising the urethra, although endoscopic management has been attempted.
Presenting symptoms are varied. In almost all published cases, voiding dysfunction is predominant, with typical symptoms including urgency, UUI, obstructive voiding, urinary retention, history of self-catheterization, urethral dilations, recurrent UTI, and persistent urinary incontinence (Haferkamp et al, 2002; Madjar et al, 2002; Pit, 2002; Sweat et al, 2002; Lieb and Das, 2003; Vassallo et al, 2003; Glavind and Sander, 2004; McLennan, 2004; Tsivian et al, 2004; Wai et al, 2004). Diagnosis is often delayed in these patients for an extended period of time. In a review by Amundsen and associates (2003), the average time from placement of the initial PVS to diagnosis of urethral erosion was 9 months.
Diagnosis is made by confirming the presence of the tape within the urethral lumen during cystoscopy. Voiding cystourethrography has also been useful adjunctively by documenting a dilated proximal urethra related to high-grade obstruction caused by the eroded tape (Lieb and Das, 2003).
Management of urethral tape erosion has typically involved transvaginal urethrotomy and excision of the exposed tape. In selected cases an autologous fascial sling and Martius labial fat pad graft can be utilized at the discretion of the surgeon. Pit (2002) described two cases of urethral erosion. The urethra was incised, and the tape was cut at the level of the mucosa. The tape was then dissected on its medial edge toward the inferior ischiopubic ramus and cut bilaterally, which allowed the tape to be removed from the periurethral fascia. A Martius graft was placed over the urethra in one case, and a cadaveric fascia lata graft was used in the second case. A second TVT was then placed over the tissue bolsters without complication. In a review by Sweat and colleagues (2002), transvaginal tape excision was accomplished by identifying and dividing the tape in the midline, followed by sharp lateral dissection along the medial border of the tape, freeing the mesh from the urethra and bladder neck. The endopelvic fascia was perforated and the tape cut in the retropubic space. A Martius fat pad graft was interposed over the urethral closure in one case and an autologous fascial sling placed over the graft in both cases. Both patients improved symptomatically and were continent during postoperative follow-up. Glavind and coworkers (2004) reported a urethrovaginal fistula caused by urethral erosion of the polypropylene tape. Transvaginal tape excision failed to resolve the fistula, and the patient required two transabdominal fistula repairs until closure was achieved and symptom resolution occurred. Persistent SUI was managed by conservative means with satisfactory results. Sokol and Urban (2008) reported use of the internal urinary sphincter for covering the defect after sling resection. They believe this may reduce the risk of fistula formation and be a less morbid option than a Martius flap. Four other additional case reports reported excellent results utilizing a midline transvaginal approach with partial tape excision and closure of the urethra (Haferkamp et al, 2002; Madjar et al, 2002; Lieb and Das, 2003; Wai et al, 2004). The postoperative results reported by each of these investigators were excellent, with all patients achieving symptom resolution after surgical intervention. In 2 patients mild recurrent SUI was successfully managed with biofeedback (Glavind and Sander, 2004; Wai et al, 2004). In 2 patients continence was achieved by intraoperative fascial sling, and in 1 patient a postoperative fascial sling resulted in satisfactory continence (Vassallo et al, 2003; Wai et al, 2004).
Three cases without ideal outcomes utilizing transvaginal excision have been reported. Mesens and associates (2007) described a patient who had recurrence of her erosion paraurethrally 3 months after the initial transvaginal excision. Powers and colleagues (2006) describe two women who presented 1 and 3 years after TVT surgery with urethral erosion. Both patients had UUI and pain at presentation and underwent transvaginal excision. Both women had recurrence of SUI and 1 had persistence of her UUI.
In 2004, McLennan successfully managed a TVT urethral erosion endoscopically. Hysteroscopic scissors were used to transect the tape flush with the urethral mucosa. Catheter drainage was continued postoperatively for 72 hours. The patient remained symptom free and continent at 10 months of follow-up. In a case report by Wai and associates (2004), a combined endoscopic and transvaginal approach was used successfully to manage a difficult urethral erosion. Initially, the tape was cut in the midline transurethrally. Transvaginal periurethral dissection was performed, and the tape was identified and removed from the dense fibrous attachments. An accidental urethrotomy was noted and closed without difficulty. The theoretical advantage of this technique is that, with endoscopic transection of the tape, entry into the urethral lumen is unnecessary. Wijffels and coworkers (2009) utilized an endoscopic transurethral approach successfully in three cases of urethral erosion. The visible tape is grasped with forceps and cut while on traction with scissors. This is performed on both sides of the urethra. One patient had recurrent SUI and another had a TVT placed. Otherwise there were no complaints. A similar approach was used by Baracat and coworkers (2005) successfully for five urethral erosions.
Nine urethral erosions (0.6%) were reported in the Australasian data presented by Hammad and associates (2005). Thirty percent of these erosions presented more than 1 year after surgery, and 89% were symptomatic. Presenting symptoms included urinary retention, bleeding, and local pain. One case was discovered incidentally on cystoscopy. Unlike the reported vaginal erosions, 33% of the urethral erosions occurred when the polypropylene sling followed another anti-incontinence procedure. Four (44%) were managed conservatively (2 patients were too frail to undergo operative intervention). Five patients (56%) had transvaginal excision: all were cured and dry. Velemir and colleagues (2008) presented a retrospective analysis of eight cases of urethral erosion that were managed with a combination of transvaginal and endoscopic approaches. One patient refused intervention. Two had transvaginal excision of mesh, with 1 developing recurrent SUI, although continent with Zuidex bulking injections followed by laparoscopic Burch colposuspension. One underwent a combined endoscopic and transvaginal excision of mesh but required later endoscopic removal of residual mesh; the patient was dry and asymptomatic following her second endoscopic mesh excision. Four patients were managed endoscopically. Three of the 4patients required repeat endoscopic attempts at mesh removal and urethral stone removal. Two patients had recurrent SUI and were treated with Zuidex bulking injections and a TVT-O procedure.
Key Points: Tension-Free Vaginal Tape Procedure—Urethral Erosion
Intravesical Tape Erosion
The finding of synthetic mesh within the lumen of the urinary bladder is another particularly distressing complication (Fig. 73-11). This complication is also exceedingly rare after the TVT procedure with only case reports in the published literature. Bladder injury recognized at the time of cystoscopy is much more common, occurring with a frequency between 2% and 11% of cases (Nilsson et al, 2001). Recognition of this complication allows the surgeon to remove and reintroduce the trocar in a controlled fashion, with placement of the tape in the correct anatomic plane. The vast majority of intravesical tape erosions are most likely due to unrecognized cystotomy and placement of the tape within the urinary bladder at the time of surgery. True erosion of the tape across the seromuscular wall of the bladder into the lumen is much less likely. Thus, performing complete and thorough cystoscopic examination of the bladder with adequate hydrodistention is critical to minimize this complication. Occasionally the insertion trocar may telescope the vesical wall during insertion and may obscure intravesical entry.
Patients typically present after their TVT procedure with a variety of symptoms. A review of seven reported cases in the literature was included in a case report by Negoro and colleagues (2005). Typical symptoms can include lower abdominal pain, intermittent gross hematuria, recurrent UTI, urgency, frequency, dysuria, and urinary incontinence.
Diagnosis is made with a high index of suspicion by the clinician and cystoscopic examination confirming the presence of the tape within the lumen of the urinary bladder. Imaging including computed tomography and cystography may help in difficult cases but is not a substitute for cystoscopic examination. A review of intravesical polypropylene tape erosions is presented in Tables 73-13 and 73-14.
Management goals are aimed at removing the portion of polypropylene tape from the urinary tract and reconstructing the lower urinary tract. Observational treatment is not recommended because the tape often becomes encrusted, leading to stone formation, persistent lower urinary tract storage/voiding symptoms, recurrent UTI, and intermittent gross hematuria. Different techniques and surgical approaches have been advocated with varying levels of invasiveness, complexity, and success.
In a report by Negoro and colleagues (2005) a retropubic approach was used to resect the intravesical portion of the tape. The bladder was closed with absorbable suture, and catheter drainage was maintained postoperatively. The patient was symptom free and continent at 10-month follow-up. Volkmer and coworkers (2003), Sweat and associates (2002), and Huang and colleagues (2003) used a combined transvaginal and abdominal approach to remove the tape in its entirety. One patient had residual urgency and frequency treated with anticholinergic agents; the other patients had resolution of symptoms but recurrent SUI managed with collagen in one patient and pelvic floor muscle training and estrogen in the remaining two patients.
Ordorica and associates (2008) reported two vesical erosions after nonautologous sling placement. These were managed with either transvesical removal of mesh or a suprapubic extravesical approach. Lapouge and colleagues (2009) retrospectively reviewed five bladder erosions after TVT placement that were initially managed endoscopically. Two patients went on to need open transvesical removal of residual sling material, owing to insufficient removal on endoscopy or recurrent symptoms. All 5 patients were dry after the last treatment. Oh and Ryu (2009) evaluated the efficacy of transurethral resection in 14 patients with intravesical mesh. The intravesical mesh was resected deep into the perivesical fat. The patients presented with dysuria, hematuria, pelvic pain, and urgency. Six patients presented with stone encasing the mesh. Thirteen (92.9%) had mesh completely removed during a mean follow-up of 18 months. One patient had recurrent bladder stones. One patient had mild SUI and UUI, which was controlled with anticholinergic agents; no further treatment for SUI was performed.
Jorion (2002) excised the tape endoscopically using an offset nephroscope transurethrally and a 5-mm laparoscopic trocar placed suprapubically. Laparoscopic grasping forceps were used to grasp the tape, and endoscopic shears excised the mesh flush with the bladder mucosa, allowing the tape to be easily removed. Cystoscopy at 1 month revealed healed mucosa, and the patient was continent and symptom free. In a separate report by Tsivian and coworkers (2004), the tape was cut endoscopically. Because of dense adhesions and adherent calculus the tape could not be extracted endoscopically. Therefore, a suprapubic approach was required to remove the stone and intravesical tape. Wyczolkowski and associates (2001) attempted to cut the tape endoscopically without success. Transabdominal exploration allowed the tape to be excised, and the cut ends were secured to the inguinal ligaments bilaterally. Holmum:yttrium-aluminum-garnet (YAG) laser excision of encrusted intravesical TVT has been successful in 15 cases (Baracat et al, 2005; Giri et al, 2005; Huwyler et al, 2008; Shrotri et al, 2010). Holmium laser destruction (vaporization) of visible tape has also been performed successfully in two cases (Hodroff et al, 2004; Lane et al, 2005). In two cases, patients presented with bladder stones that had formed on intravesical portions of TVT. Endoscopic lithotripsy of the calculi and transurethral resection of the TVT material was performed. Both patients demonstrated complete mucosal healing and remain asymptomatic and dry (Irer et al, 2005; Mahmoud and Wadie, 2007). There are three reported cases of successful laparoscopic removal of intravesical mesh after retropubic sling placement. In one of these cases a three-port intraperitoneal approach was used (Siow et al, 2005), and in the other two a three-port extraperitoneal approach was used (Rehman et al, 2008). All of the patients were free of symptoms and dry after the mesh removal.
It is unclear from the literature whether complete or partial tape excision is preferred. The authors routinely use the open cystotomy approach for complete tape excision. This approach is necessitated by a large surface area of tape present in the vesical lumen or in the case of tape collocation near the bladder neck, trigone, or ureteral orifices. Only patients with complete tape removal had recurrent SUI; the patients with partial excision maintained continence postoperatively. The significance of this, given the small number of cases, is unclear, but it may imply that suburethral support is maintained when the midurethral portion of tape is left in situ. The endoscopic approach is intriguing because it is minimally invasive and with the addition of the laparoscopic trocar achieved a satisfactory outcome. This should be duplicated by others before it is universally adopted to treat patients with this particular complication.
Voiding Dysfunction
Voiding dysfunction is a well-recognized complication of suburethral sling procedures for SUI. Although it is difficult to quantify objectively, a review by the AUA guidelines panel concluded that the incidence of permanent urinary retention after suburethral slings does not exceed 5% (Leach et al, 1997). Anti-incontinence surgery, regardless of the technique, has some effect on outlet resistance. As a result, clinical symptoms of urethral obstruction have not been eliminated by the TVT procedure with an incidence in the literature of 1.9% to 9.9% (Klutke et al, 2001; Meschia et al, 2001; Kuuva and Nilsson, 2002, 2003; Volkmer et al, 2003; Levin et al, 2004; Long et al, 2004).
Despite the fact that TVT is described as a tension-free procedure there is controversy about whether tension or resistance is directed at the bladder outlet. In one study of 404 TVT procedures, UDS before and after surgery showed an increase in voiding time but no difference in flow rate, urethral closure pressure, or urethral functional length (Meschia et al, 2001). A study by Lo and colleagues (2001) showed no statistical difference in several UDS parameters (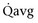 ,
, 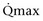 , postvoid residual urine volume, MUCP, and functional urethral length) in 82 patients before and after TVT. In another study, Wang and Chen (2001) looked at filling cystometry, uroflowmetry, and urethral pressure profile data before and after TVT and found the only statistically significant difference to be in resting MUCP. Several other studies have also shown no statistical difference in UDS pressure flow variables after the TVT procedure (Wang, 2000; Wang and Chen, 2003a, 2003b; Lin et al, 2004).
, postvoid residual urine volume, MUCP, and functional urethral length) in 82 patients before and after TVT. In another study, Wang and Chen (2001) looked at filling cystometry, uroflowmetry, and urethral pressure profile data before and after TVT and found the only statistically significant difference to be in resting MUCP. Several other studies have also shown no statistical difference in UDS pressure flow variables after the TVT procedure (Wang, 2000; Wang and Chen, 2003a, 2003b; Lin et al, 2004).
In contrast, Gateau and associates (2003) analyzed UDS findings before and after TVT surgery in 112 patients and reported consistent decreases in 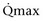 , increased mean Pdet
, increased mean Pdet 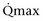 , increased mean urethral resistance, and elevated postvoid residual urine volume. They concluded from their data that TVT surgery leads to obstructive changes in the bladder outlet. Sander and colleagues (2002) evaluated the voiding phase before and 1 year after the TVT procedure. They found both subjective and objective changes in the voiding phase, with 78% of patients experiencing more difficult voiding and significant decreases in
, increased mean urethral resistance, and elevated postvoid residual urine volume. They concluded from their data that TVT surgery leads to obstructive changes in the bladder outlet. Sander and colleagues (2002) evaluated the voiding phase before and 1 year after the TVT procedure. They found both subjective and objective changes in the voiding phase, with 78% of patients experiencing more difficult voiding and significant decreases in 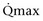 , corrected
, corrected 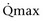 , and
, and  . Postvoid residual urine volume was also significantly increased, although not greater than 25% capacity. Furthermore, in another study comparing the TVT procedure and Burch colposuspension, a significant decrease in the flow rate was observed after surgery (Atherton and Staton, 2000).
. Postvoid residual urine volume was also significantly increased, although not greater than 25% capacity. Furthermore, in another study comparing the TVT procedure and Burch colposuspension, a significant decrease in the flow rate was observed after surgery (Atherton and Staton, 2000).
There are contemporary UDS data both supporting and refuting bladder outlet obstructive changes after the TVT procedure. The physician must therefore consider these issues when counseling patients with SUI before surgical intervention with TVT. It would be most helpful when selecting patients for the TVT procedure to identify preoperative factors predictive of voiding dysfunction and urinary retention after surgery. One study assessed differences in surgical outcome after TVT by subgrouping patients into two groups, those with dysfunctional voiding and those with normal voiding (Wang and Chen, 2003b). Dysfunctional voiding in this study was defined as having both a free 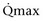 of less than 12 mL/sec and a Pdet
of less than 12 mL/sec and a Pdet 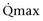 of greater than 20 cm H2O as defined by Blaivas and Groutz (2000). Statistically significant differences in pressure-flow variables of free
of greater than 20 cm H2O as defined by Blaivas and Groutz (2000). Statistically significant differences in pressure-flow variables of free 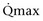 and Pdet
and Pdet 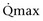 between the two groups were observed, as well as a higher objective cure rate in the normal voiding group. In a separate study by Hong and colleagues (2003), 375 patients were analyzed to see which factors predicted urinary retention after the TVT procedure. Urinary retention, defined as the need to catheterize for 72 hours or longer after surgery, was identified in 32 patients. Twenty-eight patients resumed normal voiding within 3 months, and 4 patients required a TVT release procedure. Patients’ age, parity, peak flow rate, and history of hysterectomy predicted urinary retention on univariate analysis, and only peak flow rate predicted urinary retention on multivariate analysis. Finally, a small study of 14 patients showed that low Pdet
between the two groups were observed, as well as a higher objective cure rate in the normal voiding group. In a separate study by Hong and colleagues (2003), 375 patients were analyzed to see which factors predicted urinary retention after the TVT procedure. Urinary retention, defined as the need to catheterize for 72 hours or longer after surgery, was identified in 32 patients. Twenty-eight patients resumed normal voiding within 3 months, and 4 patients required a TVT release procedure. Patients’ age, parity, peak flow rate, and history of hysterectomy predicted urinary retention on univariate analysis, and only peak flow rate predicted urinary retention on multivariate analysis. Finally, a small study of 14 patients showed that low Pdet 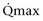 on the preoperative pressure-flow study correlated with elevated postvoid residual urine volume in 3 patients after a TVT procedure (Kawashima et al, 2004).
on the preoperative pressure-flow study correlated with elevated postvoid residual urine volume in 3 patients after a TVT procedure (Kawashima et al, 2004).
Urethral obstruction after suburethral sling surgery can arise in variety of ways. Patients may complain of straining to void, incomplete emptying, urgency and frequency, hesitancy, UUI, elevated postvoid residual urine volume, recurrent UTI, and total urinary retention. These symptoms usually cause patients bother and prompt evaluation and treatment after the initial surgical procedure.
The optimal evaluation for patients with postoperative voiding dysfunction is poorly defined in the literature. In a study by Carr and Webster (1997) there were no parameters (urodynamic, previous surgery, time from suspension to urethrolysis, and surgical approach) that predicted which patients would benefit from urethrolysis. The decision to perform urethrolysis was based on a clear temporal relationship between onset of symptoms and the surgical procedure. In a study by Petrou and coworkers (1999) there was no difference in urethrolysis outcomes with respect to patients with urodynamic obstruction versus those who had surgery based on clinical criteria. Under ideal circumstances, videourodynamics would differentiate patients with high-pressure, low-flow voiding consistent with obstruction and patients with detrusor hypocontractility. At this time there does not appear to be enough evidence correlating urodynamic parameters with surgical outcomes. Therefore, UDS can be useful in selected cases at the physician’s discretion; however, it appears that the temporal relationship correlating symptoms with an antecedent surgical procedure should be the primary criterion in selecting patients for urethrolysis and tape release procedures. Cystoscopy is useful to rule out bladder pathology, urethral tape erosion, and a hypersuspended bladder neck.
In most cases, persistent postoperative voiding dysfunction is initially treated conservatively. Temporary catheter drainage, clean intermittent catheterization, timed voiding, biofeedback, pelvic floor muscle training, and selective medical therapy have all been successful to some degree in managing postoperative voiding dysfunction. Several reports have shown some benefit with urethral dilation (Hong et al, 2003; Mishra et al, 2005). However, when these conservative measures fail, surgical intervention is usually indicated. The urethrolysis literature is more mature and robust in the management of urethral obstruction secondary to traditional PVS surgery. A discussion of the results of surgical management of urethral obstruction after a TVT procedure follows (Table 73–15).
In a large review of 1175 TVT procedures, Rardin and colleagues (2002) found 23 (1.9%) women with persistent voiding dysfunction. Twenty patients had urinary retention or incomplete emptying, 3 patients had symptoms of refractory urgency and UUI, and 7 had both. Symptoms of incomplete bladder emptying usually arose in the immediate postoperative period, and LUTS arose in delayed fashion some weeks later. All patients were refractory to conservative regimens and underwent TVT release at a mean time of 17.3 weeks after the TVT procedure. Tape release was performed in the majority (17 patients), with mild urethrolysis in 2 patients and segmental tape excision (2 to 11 mm) in 4 patients. Relief of impaired bladder emptying was complete in 100%, and relief of urgency/UUI was complete in 30% and partial in 70%. Fourteen patients (61%) maintained their baseline continence, 6 (26%) were improved over baseline, and 3 (13%) had recurrent SUI.
Kuuva and Nilsson (2002, 2003) reviewed complications associated with the TVT procedure from the nationwide database in Finland. In their analysis of 1455 TVT procedures, 34 cases (2.3%) of urinary retention were reported and duration was recorded for 23 patients. A normal voiding pattern was resumed in 14 patients (2 days to 2 weeks), 2 patients (5 to 6 weeks), and 6 patients (within 24 hours) after surgery. One patient required that the tape be released by cutting it in the midline, and normal voiding resumed. In 111 cases the authors noted “minor” postoperative voiding difficulty (e.g., urgency, frequency) that resolved without intervention in the majority of patients. Thirteen cases were identified that lasted up to 4 months, and 2 of these patients required surgical transection of the tape to achieve a normal voiding pattern.
In another large review by Klutke and colleagues (2001), 17 of 600 patients (2.8%) required reoperation secondary to urinary retention and persistent obstructive symptoms. In their series, tape release was performed at a mean of 64 days after the TVT procedure. The tape was identified and either released with downward traction for 1 cm or cut in the midline. There was one urethral injury, which was repaired without sequelae. Symptoms resolved in all patients after tape release, all patients voided to completion, and 16 patients remained continent. In a review by Long and associates (2004), 7 of 71 patients (9.9%) underwent lateral tape excision to treat patients with evidence of urethral obstruction. All patients had irritative symptoms or elevated amounts of residual urine and 6 patients voided with significant straining. Six of the patients had urodynamic obstruction (Pdet > 20 cm H2O and 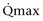 < 12 mL/sec) and 1 patient had low-pressure, low-flow voiding. Long and associates described a technique transecting the tape lateral to the midline on the right side of the periurethral fascia, leaving the tape in the shape of a J underneath the urethra. Symptoms resolved in 6 patients, and 5 patients were continent. Of note, in the 2 patients who had recurrent SUI after transection of the tape, the tape was cut on postoperative day 4 in both cases. In the other cases the tape was cut after a minimum of 14 days after TVT. Therefore, on the basis of their experience, the authors recommended a minimum waiting period of 2 weeks before surgical intervention.
< 12 mL/sec) and 1 patient had low-pressure, low-flow voiding. Long and associates described a technique transecting the tape lateral to the midline on the right side of the periurethral fascia, leaving the tape in the shape of a J underneath the urethra. Symptoms resolved in 6 patients, and 5 patients were continent. Of note, in the 2 patients who had recurrent SUI after transection of the tape, the tape was cut on postoperative day 4 in both cases. In the other cases the tape was cut after a minimum of 14 days after TVT. Therefore, on the basis of their experience, the authors recommended a minimum waiting period of 2 weeks before surgical intervention.
In a study reviewing outcomes and complications of 404 TVT procedures, Meschia and coworkers (2001) found 17 patients (4%) who had voiding difficulties defined as residual urine greater than 100 mL. At 1-month follow-up, 15 patients resumed normal voiding patterns and the remaining 2 patients required the tape to be cut in the midline to release tension; normal voiding was observed thereafter. A review by Volkmer and coworkers (2003) found 3 patients with permanent urinary retention after the TVT procedure. UDS revealed high-grade obstruction in each patient utilizing the Abrams-Griffiths nomogram. Two patients had the tape cut in the midline transvaginally, and 1 patient had segmental excision of the entire suburethral portion of the tape. All 3 patients were able to resume normal voiding without significant residual urine, and 2 of the 3 patients remained continent. In the patient with recurrent SUI the suburethral portion of the tape was excised and she underwent repeated TVT surgery to restore continence.
Levin and colleagues (2004) reviewed a series of 313 patients who underwent the TVT procedure. They identified 8 patients (2.5%) who had urinary retention with incomplete bladder emptying. Seven patients required temporary catheterization for a short period of time before resumption of normal voiding at a follow-up of 1 month. The remaining patient had persistent urinary retention 2 months after TVT surgery, and UDS showed obstruction on the pressure-flow study. Transvaginal urethrolysis with tape excision was performed, and the patient thereafter resumed complete bladder emptying with complete continence. Tsivian and associates (2004) found 8 of 12 patients who required repeated surgery after a TVT procedure to have urethral obstruction, with complaints ranging from severe urgency symptoms to straining to void. All patients had incomplete bladder emptying, and 5 patients had objective evidence of obstruction on pressure-flow UDS. All 8 women underwent corrective surgery with resolution of symptoms, and only 2 women suffered from recurrent SUI symptoms.
Karram and coworkers (2003) reviewed their series of TVT procedures looking at the incidence of postoperative complications. Seventeen women (4.9%) were performing clean intermittent self-catheterization beyond 7 days. Another 42 women with irritative LUTS were taking anticholinergic medications more than 6 weeks after surgery. Twenty-eight patients underwent urethral dilation, which provided symptom relief in 23; and 6 patients with persistent urinary retention had transvaginal takedown and incision of the tape. Normal voiding resumed in all 6 of these patients, although 2 experienced recurrent SUI.
Zubke and colleagues (2004) managed 3 patients with urethral obstruction after TVT with a novel surgical technique. They cut the tape in the midline with a transvaginal approach and sutured the edges of the tape to a Prolene mesh, thus lengthening the tape. All 3 patients were continent and resumed normal voiding after intervention. Mishra and colleagues performed urethral dilation on 3 of 52 cases who had retention 3 months after TVT. Two of the three patients voided effectively after urethral dilation. In this series the postoperative retention rate is significantly higher (23%) than published data. Although many practitioners report anecdotal success, no randomized trials exist in peer-reviewed literature. It is the authors’ opinion that urethral dilation is of limited utility and if used too aggressively may be detrimental. There are concerns about the potentially traumatic nature of dilation, which could induce scarring of the urethra. Hammad and associates (2005) reported on their incidence and management of urinary retention in an Australasian cohort after 1459 midurethral slings (993 TVT, 466 SPARC). Postoperative retention occurred in 95 patients, but surgical intervention was only required in 33 patients. This included sling loosening, lysis, and urethrolysis. This study does not provide information on time to presentation of obstruction or of results after treatment of obstruction.
Sokol and coworkers (2005) described the management of obstruction after a TVT procedure with and without concurrent prolapsed repair. The median days to voiding (8 vs. 5 days) and rate of urinary retention were similar between patients with and without prolapse repair. Increasing age, decreasing BMI, and postoperative UTI were independent predictors of time to adequate voiding, whereas previous history of incontinence surgery was the only independent variable predictive of urinary retention. Sling loosening was reported by Nguyen (2005) within approximately 1 week after TVT placement. All patients had a suprapubic tube placed at time of initial TVT, which was removed once the patient was able to void at least 75% of total bladder volume on two consecutive occasions. All patients were continent after mobilization, and quality of life scores of the nonvoiders did not differ from those of voiders 1 year after surgery.
A nationwide analysis of obstruction after TVT surgery was performed by Finnish authors (Laurikainen and Killholma, 2006). A retrospective review of 9040 patients who received a TVT procedure was reviewed. Approximately 50% were cured with sling lysis and remained continent. Four (12%) continued to have retention after lysis. There was no difference in continence rate among patients based on interval between a TVT procedure and sling lysis. Repeat sling lysis and urethrolysis were options used in managing the refractory retention.
Gamé and colleagues (2006) presented results of 30 women requiring sling lysis with a single lateral incision over a 4-year period. Seventy percent were continent after intervention, and 2 women developed recurrent SUI. Glavind and Glavind (2007) evaluated different treatment options for managing prolonged voiding dysfunction after TVT placement (clean intermittent catheterization, sling loosening, and lysis). Seven percent of their initial cohort developed prolonged voiding difficulties. Five patients experienced loosening at between 1 and 3 weeks; 2 underwent sling lysis between 5 and 7 months, and 3 patients chose to perform extended clean intermittent catheterization. The sling loosening study arm was the only group who was subsequently continent. The authors concluded that voiding issues should be followed closely for the first 1 to 2 weeks and intervention should not be delayed if symptoms persist. The value of this study is limited by the large variability in time between presentation of symptoms after TVT and intervention. It clearly supports timely evaluation and intervention for obstruction or related symptoms.
Three studies attempted to relate preoperative or intraoperative variables to postoperative voiding dysfunction. Takacs and colleagues (2007) found that a poor quality intraoperative cough test predicted immediate postoperative retention. The quality of the cough test was determined by whether every cough produced urinary incontinence. This study is limited by subjective measurements of the cough test. The cough test is no longer commonly performed intraoperatively for setting sling tension. Shukla and colleagues (2007) found no significant predictive factors but described a trend toward long-term voiding difficulty after concurrent posterior vaginal repairs and in women with low preoperative flow rates. Vervest and colleagues (2007) performed a prospective cohorts study in 703 women with a TVT procedure and reported the need for postoperative catheterization for more than 24 hours in 11% and sling lysis in 1.3% of patients. Using validated questionnaires, these authors found that the negative impact of abnormal voiding on TVT outcomes is greater than in those without voiding dysfunction. Yet, this impact decreased significantly after a TVT procedure, implying a considerable improvement in quality of life.
Key Points: Tension-Free Vaginal Tape Procedure—Voiding Dysfunction
Other Complications
A diverse group of complications have been reported with the midurethral sling, aside from those mentioned previously. These include vascular injury, bowel perforation, dyspareunia, pain (inguinal, suprapubic, pelvic), and infection-related complications. Death has also occurred after sling implant and been directly attributable to the procedure.
Wound-related complications include minor superficial cutaneous infections, pelvic abscesses, and UTI. A case of necrotizing fasciitis was reported in an obese, diabetic patient. This resolved after intensive resuscitation (Connolly, 2004). Interestingly, a review of necrotizing fasciitis in gynecologic surgery found that obesity (88%), hypertension (65%), and diabetes (47%) were all strong factors in the development of this infection after surgery (Gallup et al, 2002).
Serious complications such as vascular perforation or intestinal perforation remain relatively low. The rate of serious vascular complications in the Finnish registry was 0.07% (Kuuva and Nilson, 2002), and the Austrian registry reported a bowel perforation rate of 0.04% (Tamussino et al, 2001). In a systemic evaluation of the occurrence of postoperative retropubic hematoma formation, Flock and colleagues (2004) reported a rate of 4.1% among 249 consecutive cases, with only 4 patients with hematomas exceeding 300 mL and requiring surgical intervention.
Groin and suprapubic pain are potential problems after TVT placement. Thigh pain is usually associated with the transobturator approach (see later). A randomized controlled study from Finland (Laurikainen and Killhoma, 2006) reveals 16% of women in the transobturator (inside-to-outside) group had groin pain compared with only 1.5% of those in the TVT study arm. This groin pain persisted longer after the TVT-O than after the TVT (Daneshgari et al, 2008). Long and associates (2009) and Wang and colleagues (2009) both also found the TVT-O group to have significantly more postoperative groin and thigh pain. Duckett and Jain (2005) reviewed different strategies for managing groin pain after a TVT procedure or other similar midurethral slings in five women (1%). Initial conservative management was successful in most patients. Those with persistent or severe pain (four women) were given combination of corticosteroid and local anesthetic injections. Two women developed recurrent pain and had the TVT excised, with significant pain relief. Doo and associates (2006) noted persistent suprapubic pain in three (2.2%) women at 5-year follow-up after a TVT procedure.
Varying degrees of sexual impairment after TVT insertion (0% to 15%) have been associated with dyspareunia (Kuhn et al, 2009a). Among 20% prospectively evaluated who reported sexual impairment after TVT placement, 14.5% related this to dyspareunia (none preoperatively vs. eight postoperatively, P < 0.01) (Mazouni et al, 2004). Marszalek and colleagues (2007) performed a cross-sectional analysis and noted that the 14.3% deterioration in sexual function was significantly associated with de novo urgency, dyspareunia, and a sensation of incomplete emptying. The sexual satisfaction of the TVT and Burch colposuspension were compared (Demirkesen, 2008). Fifty-four percent expressed a negative change, of which the majority suffered from dyspareunia. The TVT procedure appeared to more adversely affect sexual satisfaction, but the difference was not significant. Kuhn and coworkers (2009a) reviewed the impact of sling removal for postoperative female de novo dyspareunia and found that pain improved significantly.
Review of the U.S. Food and Drug Administration safety database for complications reported to that agency is noted in Table 73–16. Catastrophic complications are rare but do occur and may result in mortality: 11 reported deaths exist in the database from 1998 to July 2009.
Table 73–16 Summary of All Significant Complications Reported to the U.S. Food and Drug Administration with the Midurethral Sling TVT: 1998-2009
| Bladder erosion | 50 |
| Urethral erosion | 51 |
| Vaginal erosion | 239 |
| Bowel perforation | 48 |
| Major vascular | 26 |
| Blood loss >200 mL | 36 |
| Plastic sheath malfunction | 51 |
| Leg pain | 44 |
| Needle broken from mesh | 154 |
This analysis is limited by reporting inaccuracies and the incomplete nature of adverse event reporting in common practice.
Key Points: Tension-Free Vaginal Tape Procedure—Voiding Dysfunction