chapter 74 Injection Therapy for Urinary Incontinence
History of Injectable Agents
The quest for injectable agents for stress urinary incontinence (SUI) started at the end of the 19th century when Gersuny (1900) from Vienna suggested periurethral paraffin injection for urethral compression. In 1914, Kelly and Dumm warned about the dangers of embolism after injection and pointed out that this treatment showed only temporary improvement of symptoms. In 1938, Murless first reported the injection of sodium morrhuate, a sclerosing agent synthesized from cod liver oil, into the anterior vaginal wall for treatment of SUI. Of the 20 patients treated, 17 were cured or improved for at least 12 months. Sloughing of a segment of the anterior vaginal wall was seen in 12 patients, and of these 75% were cured. Murless postulated that success was due to contraction of the resulting scar of the anterior vaginal wall. Quackels (1955) reported on the injection of paraffin for incontinence after prostatectomy in 1955; and Sachse (1963), based on previous reports, injected a mineral oil preparation, granugenol oil (Dondren), another sclerosing agent. He reported cures in 12 of 24 men after prostatectomy and 4 of 7 women. However, significant complications of pulmonary emboli and urethral sloughing were seen. With the last case report of distal ureteral stenosis after periurethral injection it was recommended not to use sclerosing agents for incontinence (Bubanz et al, 1980).
Polytetrafluoroethylene (Teflon) paste was first introduced by Berg (1973) and then popularized by Politano and colleagues (1973) in the 1970s. Shortliffe and coworkers (1989) published the first report on glutaraldehyde cross-linked collagen, and Cervigni and colleagues (1994) described the use of autologous fat in woman with SUI. More recently, newer synthetic materials have been described that theoretically should improve efficacy, durability, and safety.
The ideal injectable agent should be easily injectable and conserve its volume over time. It should also be biocompatible, nonantigenic, noncarcinogenic, and nonmigratory and cause little or no inflammatory reaction (Kershen and Atala, 1999) or fibrotic ingrowth (Dmochowski and Appell, 2000). The components of the bulking agent should not separate or dissociate on injection, and if the agent contains microcrystalline or micropolymeric components they should be reasonably uniform spheres of particle sizes above 110 µm that are nonfragile and adhere to host tissue (Dmochowski and Appell, 2000). If the substance used is not successful it should not interfere with subsequent surgical intervention. To date, no substance has met all of these requirements.
Over the past 15 years there has been an evolution of injectable agents and diverse types have been tested. Table 74–1 is a list of the agents that are discussed here. Most of the agents are bulking agents, but recently injection of autologous muscle stem cells for sphincter enhancement and implantable balloons that compress the urethra have been introduced.
Table 74–1 Types of Injectable Agents for Stress Urinary Incontinence
| Biologic |
| BULKING |
| FUNCTIONAL |
| Synthetic |
| Balloons |
Use of Injectable Agents in Female Stress Urinary Incontinence
Pathophysiology of Stress Urinary Incontinence and the Role of Injectable Agents
Please see the online edition of this chapter on the Expert Consult website for a discussion of this topic, along with Figures 74-1 and 74-2.![]()
SUI is thought to be achieved by the interplay of supportive or musculofascial elements and urethral and periurethral functional elements. The anterior vaginal wall supports the urethra by its lateral attachment to the levators (pubococcygeus) and to the endopelvic fascia from the arcus tendineus fasciae pelvis (ATFP) (Fig. 74–1). This has been termed the hammock hypothesis (DeLancey, 1994). In essence it is a “double hammock” that includes not only the vaginal wall but also the posterior aspect of the levator muscles (Fig. 74–2). The lateral vaginal sulci, most clearly found in nulliparous women, at the junction of the lower and middle third of the vaginal wall, represent lateral aspects of the “hammock.” Portions of the pubococcygeus muscle are thought to attach to these sulci within the pelvis and produce elevation during voluntary contraction (Koelbl et al, 2009).
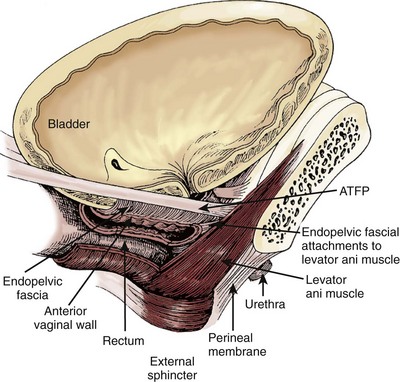
Figure 74–1 The “hammock” hypothesis. The anterior vaginal wall with its attachment to the arcus tendineus fasciae pelvis (ATFP) forms a “hammock” under the urethra and bladder neck.
(From DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 1994;170:1713–23.)
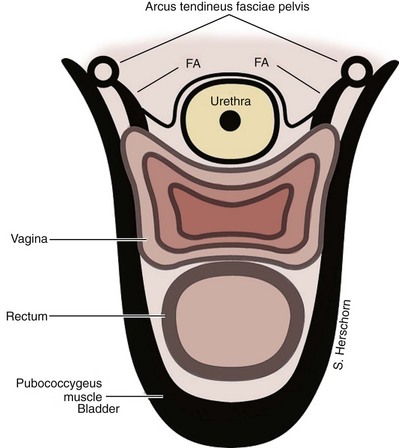
Figure 74–2 Cross section of urethral supports below the bladder neck. The urethra is supported by a “hammock” of anterior vaginal wall suspended to the levators (pubococcygeus muscles) and the fascial attachments (FA) to the tendinous arch of the pelvic fascia. In essence it is a “double hammock.”
The pubourethral ligaments are connective tissue structures that extend from the urethra to the pubic bone (Zacharin, 1963). They are sufficiently condensed to form distinct visible structures on either side of the pubis. Although they form one continuous complex they are divided into anterior and posterior pubourethral ligaments as determined by the location of urethral attachment, either anteriorly or posteriorly (Koelbl et al, 2009). Their role in maintaining continence is discussed later.
The lower third of the vagina is oriented more vertically in the nulliparous woman, and the upper two thirds is horizontal. The uterus and upper vagina are attached to the pelvic side walls by the cardinal and uterosacral ligaments. They rest posteriorly on the levator plate, which is formed by the fusion of the iliococcygeus and the posterior fibers of the pubococcygeus muscles, both part of the pelvic diaphragm. With coughing and straining the pelvic diaphragm muscles contract and the levator hiatus shortens in the anterior direction. This contributes to maintaining a normal position of the urethra and bladder with intact “hammock” support. The ‘hammock hypothesis” suggests that vaginal wall posterior to the urethra provides a backboard against which increasing intra-abdominal forces compress the urethra (DeLancey, 1994). In patients with SUI with hypermobility, restoration of suburethral or bladder neck support can result in symptom improvement or cure. This idea is supported by findings in urethral pressure transmission studies. If the pressure transmission ratio (PTR), defined as the increment in urethral pressure during coughing as a percentage of the simultaneously recorded increment in bladder pressure, is more than 90% it is of value in predicting for the presence of SUI (Cundiff et al, 1997). After successful suspension procedures, patients experience an increase in PTR during coughing (Rosenzweig et al, 1991; Athanassopoulos et al, 1994). However, in stress-continent women the PTR can be more than 100%. This may indicate that in addition to a supported suburethral vaginal wall, active forces such as pelvic floor muscle contraction occur (Constantinou, 2009).
The urethral sphincter mechanism is commonly considered to consist of striated sphincter mass and function, urethral smooth muscle, mucosal and submucosal cushions, and pudendal innervation (Koelbl et al, 2009). The idea that sphincter weakness or intrinsic sphincter deficiency (ISD) could cause incontinence independent of vaginal weakness or urethral mobility was introduced in 1976 by McGuire (Blaivas, 1993). McGuire and colleagues (1993) also characterized SUI due to ISD as associated with abdominal leak point pressures (ALPP) of 15 to 60 cm H2O, continuous leakage, and in 75% no urethral mobility (type III). They concluded that these patients, who would likely fail standard retropubic or needle suspension procedures, would be better treated by pubovaginal slings, artificial sphincters, or injection therapy because these procedures more accurately correct the underlying pathology. They stated that determination of ALPPs with clinical grading of the severity of the incontinence may identify those patients in whom leakage is unrelated to anatomic factors but the result of poor or absent urethral closing function. Furthermore, in the original series of 125 patients, 90% of the women with hypermobility had an ALPP of 60 cm H2O or greater. This separation of the patients on the basis of low ALPP having ISD and high ALPP having hypermobility was reiterated in an accompanying editorial (Blaivas, 1993).
Subsequent studies have not supported the strict division of patients into two categories. In a subsequent publication, McGuire and coworkers (1996) described a middle-pressure group (>60 to 100 cm H2O) with features of both ISD and hypermobility. Kayigil and coworkers (1999) reported that 28% of patients evaluated for SUI had both ISD and hypermobility. Kuo (2003) reported that 39% of subjects with Valsalva leak point pressure (VLPP) less than 60 cm H2O had bladder neck descent on stress testing. Fleischmann and colleagues (2003) reported a lack of relationship between the degree of hypermobility and VLPP or pad weight test in women with SUI. VLPP measures the amount of intra-abdominal or intravesical pressure required to cause incontinence during abdominal straining and is a measure of urethral function during stress maneuvers. There is no consensus about whether it should measured from the supine baseline or the standing resting baseline and about other factors, such as catheter type, placement (vaginal, rectal, intravesical), bladder volume at which measurements are done, patient position, and type of stress maneuver done (Nygaard and Heit, 2004). The variability in results seen in these and other studies as well as the lack of prospective studies predicting outcomes on the basis of VLPP argue against the categorization of patients into separate ISD and hypermobility categories. Despite the lack of its predictability of anatomic information and uncertainties related to standardization of recording methods, low VLPP (without specified values) has been widely accepted as an indicator of ISD (Koelbl et al, 2009).
The concept of separate causes of SUI is now evolving into a continuum with the acknowledgment that many patients with hypermobility also have ISD. There is good evidence that sphincter insufficiency exists in many patients. Perucchini and coworkers (2002) showed in autopsy studies that the number and density of urethral striated muscle fibers at the bladder neck and along the dorsal wall of the urethra decline with aging. Twenty-five years ago Swash and colleagues (1985) reported on pelvic floor and sphincter denervation after childbirth. More recent studies have shown a decrease in the electrophysiologic function of the pudendal nerve (Ismael et al, 2000), the striated urethral sphincter (Takahashi et al, 2000), and the pelvic floor muscles (Gunnarsson and Mattiasson, 1999; Takahashi et al, 2000), as well as prolonged pudendal nerve terminal motor latency (Bakas et al, 2001) in women with SUI. Bladder neck funneling on ultrasonography may be an indication of urethral weakness and ISD. Huang and colleagues (2003) reported funneling at rest in 111 of 320 women with primary SUI and reported that the degree of vaginal relaxation, as well as parameters of intrinsic sphincter function including VLPP and urethral closure pressure, were worse in patients with funneling than without. Similarly, Ghoniem and colleagues (2002) reported varying degrees of funneling in 91 of 100 patients with ISD. Additionally, long-term outcome studies of correction of hypermobility have suggested that there may be more urethral weakness among patients with hypermobility than previously suspected (Koelbl et al, 2009). Recently, Digesu and colleagues (2009) reported that women in whom a modified Burch colposuspension failed had significantly decreased sphincter volume on preoperatively three-dimensional ultrasonography as compared with successfully treated patients. This implies that failures may in part be attributed to concomitant ISD.
The goal of injectable agents is to augment or restore urethral mucosal coaptation and its “hermetic seal effect” contribution to the continence mechanism (Appell and Winters, 2007). It is generally thought that these agents improve intrinsic sphincter function, although the exact mechanism has not been defined (Smith et al, 2009). Bulking agents such as collagen have been reported (McGuire and Appell, 1994; Monga et al, 1995) to augment urethral mucosa and improve coaptation and intrinsic sphincter function, as evidenced by an increase in post-treatment abdominal leak point pressure (ALPP) (Herschorn et al, 1992; Richardson et al, 1995; Winters and Appell, 1995). Bulking agents do not generally obstruct voiding after the initial post-treatment period. Monga and coworkers (1995) showed that successfully treated patients have an increased area and pressure transmission ratio in the first fourth of the urethra. They suggested that placement of the injectable agent at the bladder neck or proximal urethra prevents bladder neck opening under stress, although this is controversial. Proper placement of the injectable agent, possibly just below the bladder neck, rather than actual quantity (Khullar et al, 1997) of the agent improves intrinsic sphincter deficiency (ISD).
Patient Selection, Indications, and Contraindications
Injectable agents are one of the many treatment options for SUI. Although initially it was thought that these agents would be most effective in patients with ISD alone, multiple reports have shown clinical efficacy in patients with hypermobility (Herschorn et al, 1996; Steele et al, 2000; Bent et al, 2001). Injectable agents may provide a rapid response for some patients and are an option for those who do not wish to undergo more invasive procedures. However, these patients must understand that efficacy and duration of these agents are inferior to surgery and follow-up injections may be required. Other possible indications include elderly patients, those with high anesthetic risk, or those willing to accept an improvement rather than cure of their SUI symptoms (Appell et al, 2009).
Detrusor overactivity should be treated before injection because results may be compromised (Herschorn et al, 1996). Severe urethral scarring from radiation or surgery may affect mucosal pliability by preventing bulking and retention of the injectable agent in the urethra.
Contraindications include active urinary tract infection and hypersensitivity to the injectable material.
Patient History
Patients who may be candidates for injectable agents should undergo a diagnostic evaluation to confirm the diagnosis in a similar fashion to other SUI patients. A focused history to characterize the chief complaint including the frequency, severity, and degree of bother should be done. An assessment of other urinary symptoms should be completed, and the desire for treatment should be ascertained. Helpful tools include various validated Symptom and Quality of Life questionnaires and frequency-volume charts (Appell et al, 2009; Abrams et al, 2010).
Physical Examination
The physical examination should provide information about the cause of the lower urinary tract symptoms and suggest additional management options. The general examination should include an abdominal examination to evaluate the skin, surgical incisions, and the presence of any hernias or abdominal masses, including a full bladder.
Pelvic Examination
The patient is placed in the lithotomy position. The external genitalia should be examined for dermatologic lesions and inflammatory conditions. The internal genitalia should be examined for estrogen deficiency, urine or abnormal vaginal discharge, pelvic organ prolapse, and abnormal pelvic masses. The poorly estrogenized vaginal wall has a thinned epithelium with loss of transverse rugae, which are normally present in its lower two thirds (Fantl et al, 1994). The patient should be examined with a comfortably full bladder to assess stress leakage and, if necessary, with an empty bladder to assess other pelvic organ prolapse and masses.
Because incontinence (or pelvic organ prolapse) may not be evident, or its full extent demonstrated, in the dorsal lithotomy position, it has been recommended that the patient be examined in the semi-upright or even upright position (Walters and Karram, 1992). A systematic examination of the vaginal walls and perineum should also be done. (For details, see Chapter 64.)
Urethral mobility can be observed with the patient straining or by the Q-tip test (Crystle et al, 1971). The angles of deflection of the Q-tip at rest and with straining are measured with a goniometer. Hypermobility is defined as a maximum strain axis of more than 30 degrees from the horizontal. Urethral axis testing does not diagnose any form of incontinence because continent women may demonstrate rotational descent of the urethra (Fantl et al, 1986) and incontinent women may have no descent. Although urethral axis testing has been shown to be reproducible (Fantl et al, 1986), it has not been compared with other radiologic methods. However, it may be helpful in assessing the degree of hypermobility.
If the patient has a fixed nonmobile urethra, especially if there has been previous SUI surgery, and leaks with coughing and/or straining, most likely that patient has ISD as the predominant cause of the SUI. However, if hypermobility is present the relative contribution of the causes (ISD vs. anatomic support) cannot be ascertained clinically.
Additional Testing
The initial evaluation of urinary incontinence in women includes a history, physical examination, urinalysis, and measurement of postvoid residual urine (Abrams et al, 2010). The basic evaluation may be satisfactory for proceeding with treatment, including surgery, for patients with straightforward SUI associated with hypermobility with normal postvoid residual volume (Fantl et al, 1996). The indications for additional testing include an inability to make a definitive diagnosis based on symptoms and the initial evaluation, concomitant overactive bladder symptoms, prior lower urinary tract surgery (including failed anti-incontinence procedures), known or suspected neurologic disease affecting the bladder, a negative stress test, abnormal findings of urinalysis such as hematuria or pyuria, abnormal levels of postvoid residual urine, beyond hymen and symptomatic pelvic prolapse, and dysfunctional voiding (Appell et al, 2009). Additional testing can include pad testing and/or use of a voiding diary, urodynamic studies, cystoscopy, and imaging. The evaluation can be tailored to elucidate the patient’s problem.
Urodynamic Studies
In the evaluation of the patient with SUI, urodynamic studies before interventional treatment are helpful in specific circumstances. These are to confirm the diagnosis if the symptoms are confusing or complex and other problems are suspected, such as detrusor overactivity, urethral obstruction or voiding dysfunction, low bladder compliance, and/or impaired or absent detrusor contractility (National Institute for Health and Clinical Excellence, 2006; Appell et al, 2009). Urodynamic studies have also been recommended if there has been previous surgery for SUI or anterior compartment prolapse (National Institute for Health and Clinical Excellence, 2006). There is controversy as to whether urodynamic studies are warranted in the straightforward or pure SUI patient with no previous surgery. The National Institute for Health and Clinical Excellence guidelines (2006), similar to the updated American Urological Association guidelines (Appell et al, 2009), did not recommend testing, whereas testing was recommended by the recent 4th International Consultation on Incontinence (Hosker et al, 2009). This was based largely on a report from Agur and coworkers (2009) that showed that only 5.2% of 6276 women in a urodynamic patient database had pure SUI and of those a fourth had other findings that could impact clinical decision making, such as detrusor overactivity, voiding dysfunction, and absence of SUI on testing.
Because injectable agents are indicated for the ISD component of SUI, can urodynamic studies assess ISD? Two measures of urethral function have been used: maximum urethral closure pressure (MUCP) and ALPP. An MUCP of less than or equal to 20 cm H2O has been suggested as indicating clinically significant urethral weakness, but there is controversy regarding the diagnostic and predictive value of urethral pressure profilometry in characterizing ISD (Weber, 2001). Similarly, an ALPP of less than or equal to 60 cm H2O was identified as an indicator of severe ISD (McGuire et al, 1993) but many studies have not confirmed the test’s value in quantifying the degree of ISD (Koelbl et al, 2009). Previously, ALPP measurements of initially less than or equal to 65 cm H2O and then less than or equal to 100 cm H2O were used as indicators of ISD to justify the use of injectable agents (Appell and Winters, 2007). However, because ISD may be present in many patients with SUI with or without urethral hypermobility (Koelbl et al, 2009), the specific value of either the MUCP or ALPP may be of little importance in the clinical decision about the use of injectable agents. As with other patients with SUI who opt for interventional therapy, urodynamic studies are helpful for the reasons mentioned earlier.
Cystoscopy
Routine cystoscopy is not recommended for the evaluation of SUI. However, it is indicated for the evaluation of incontinent patients who have sterile hematuria or pyuria; urgency incontinence to rule out other pathologic processes (e.g., bladder tumor, interstitial cystitis); recurrent or iatrogenic incontinence when surgery is indicated or planned; vesicovaginal fistula or extraurethral incontinence (Tubaro et al, 2009); and when urodynamic studies fail to duplicate symptoms of incontinence (Fantl et al, 1996). Furthermore, preinjection cystoscopy is helpful to make sure that there are no adverse factors or unexpected findings that may prevent or compromise the injection procedure such as extensive urethral scarring from previous surgery, irradiation, or trauma, foreign bodies, or urethral diverticula.
Injection Techniques
The materials can be administered under local anesthesia with cystoscopic control as an outpatient procedure. Both the periurethral and transurethral methods have been done to implant the agent within the urethral wall, preferably into the submucosa or lamina propria. It is thought that the implant should be positioned at the bladder neck or proximal urethra. Different sites can be chosen such as the 3- and 9-o’clock or 4- and 8-o’clock positions. The needle size depends on the viscosity of the agent. Preoperative and postoperative antibiotics are frequently administered. The technique of injection is seen in Figures 74-3 and 74-4. Additional bilateral periurethral infiltration of 2 to 3 mL of 1% or 2% aqueous lidocaine injected lateral to the urethra may improve patient comfort. The goal with current injectable agents is to create mucosal apposition at the end of treatment.
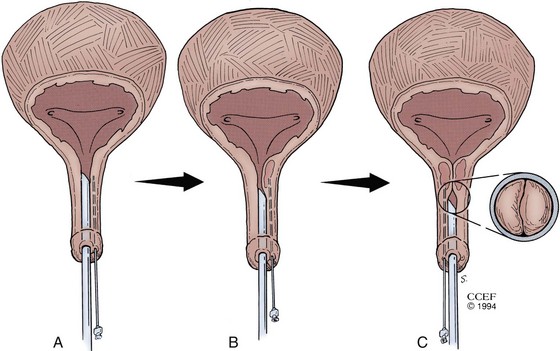
Figure 74–3 Periurethral collagen injection. The 20-Fr cystoscope with a 30-degree lens is positioned in the urethra while the substance is injected into the bladder neck region. A, Appearance of the urethra before treatment. B, Periurethral needle positioned in the proximal urethra below the bladder neck. C, Appearance of the urethra after injection.
(Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1994-2011. All Rights Reserved.)
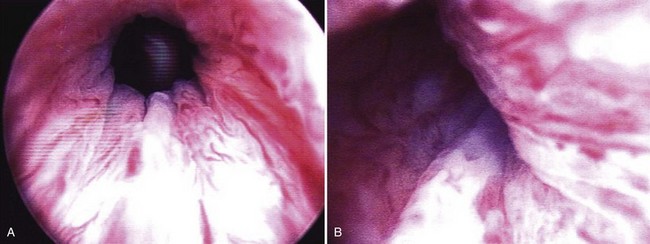
Figure 74–4 A, Cystoscopic view of the open bladder neck region before injection. B, Collagen has been injected via the periurethral route on the patient’s left side. Note the intraluminal bulking effect of the bulking agent.
Periurethral Technique
The patient is placed in the lithotomy position and prepared and draped in the usual sterile fashion. Topical 2% urethral lidocaine jelly is instilled into the meatus. Perimeatal blebs are raised with 1% or 2% aqueous lidocaine at the 3- and 9-o’clock or 4- and 8-o’clock positions 3 to 4 mm lateral to the urethral meatus with a 25-gauge needle. A 20-Fr urethroscope with a 30-degree telescope is inserted into the urethra. The periurethral needle is introduced and advanced parallel to the endoscope sheath until its position can be seen cystoscopically just below the bladder neck within the mucosa. The surgeon can hold the cystoscope in one hand and advance the needle with the other. Care must be taken to prevent the needle from getting too close to or entering the urethral lumen because rupture of the mucosa and extravasation will occur. Rocking the needle will confirm the position of the tip. If penetration of the mucosa occurs, the needle should be removed and repositioned. The substance is injected either unilaterally or bilaterally to create the appearance of “prostatic” lobes (Fig. 74–5). Transvaginal injection with the needle placed through the biopsy port of an ultrasound probe has also been described (Appell, 1996).
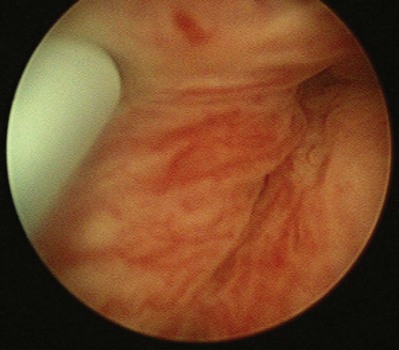
Figure 74–5 Appearance of urethra after injection of collagen with a transurethral needle that is seen on the left. Both sides of the urethra have been injected, giving the appearance of an occlusive prostate.
An 18-gauge “bent tip needle” has been designed for the periurethral approach for placement of carbon-coated zirconium spheres (Durasphere; Boston Scientific, Natick, MA) within the proper plane (Appell and Winters, 2007).
Transurethral Techniques
With Cystoscopic Monitoring
The implant can also be injected transurethrally through the cystoscope with specially designed injection needles or with other devices that do not necessitate cystoscopy. In this approach a 0-, 12-, or 30-degree lens may be used. Various cystoscope sheaths are available, but one with a flat rather than beaked end will prevent the needle from penetrating the urethra proximal to the view from the lens. Endoscopic instrument companies have an array of equipment designed for transurethral injections. The material can be injected through a semi-rigid needle that is advanced through a working element or a flexible needle that is advanced by the surgeon. Both have 22-gauge tips that are about 1 cm long.
The patient is prepared in the same fashion as with the periurethral approach. Topical urethral lidocaine jelly as well as aqueous lidocaine injected periurethrally can be used. The injection needle is inserted into the urethra at a 30- to 45-degree angle and advanced proximally in the submucosal region under the surface of the mucosa. The point of penetration of the urethra has to be at a distance below the bladder neck of more than the length of the needle to prevent extravasation of the substance into the bladder. Injections can be given at the 3-, 6-, and 9-o’clock positions until mucosal apposition is achieved. The cystoscope should not be advanced through the bulked-up urethra and bladder neck to avoid compressing or causing extravasation of the injected material.
Because of its high viscosity, silicone microimplant (Macroplastique; Uroplasty, Inc, Minnetonka, MN) injections require the use of a ratcheted injection gun (Fig. 74–6A). The injection needle is 7 Fr with a 10-mm, 18-gauge needle tip.
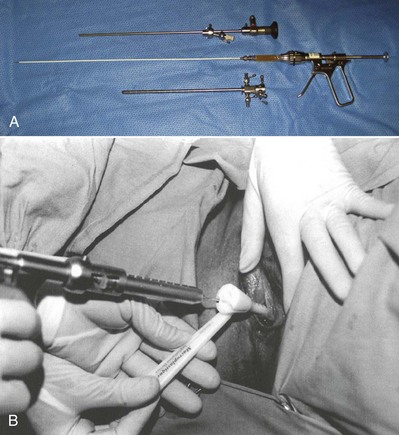
Figure 74–6 A, Silicone macroparticle injection gun. The injection needle is 7 Fr with a 10-mm, 18-gauge needle tip. B, The implantation procedure. The bulking agent is injected through the device using the injection gun and a rigid needle.
(From Tamanini JT, D’Ancona CA, Netto NR Jr. Treatment of intrinsic sphincter deficiency using the Macroplastique Implantation System: two-year follow-up. J Endourol 2004;18[9]:906–11.)
The periurethral and transurethral approaches for collagen were compared first by Faerber and colleagues (1998), who reported no significant difference in success rates and numbers of injections required in 24 patients with transurethral treatment versus 21 with a periurethral approach. However, significantly more collagen was required for the periurethral approach. Schulz and coworkers (2004) reported similar findings in 40 women randomly assigned to either technique. There was no difference in short-term success rate, but the 20 women assigned to the periurethral approach required more collagen than those assigned to the transurethral approach. The transurethral approach is now much more commonly reported than the periurethral approach.
Without Cystoscopic Monitoring
A handheld device that allows the operator to inject Macroplastique transurethrally without cystoscopy was introduced by Henalla and colleagues (2000) in a multicenter trial of 40 patients (see Fig. 74–6B). Device efficacy and acceptability were rated highly by the surgeons at 92.5% and 95%, respectively, and at 3 months 74.3% of patients had a good outcome. Twelve-month outcomes in a cohort of 21 patients who had Macroplastique injections administered with this device were reported by Tamanini and coworkers (2003): 57.1% of patients considered themselves cured, 19% were improved, and 23.8% experienced failure. Outcomes at 2 years showed some deterioration in results, with 47% considered cured, 14.3% improved, and 38.1% failed (Tamanini et al, 2004).
A handheld Implacer device was designed to administer hyaluronic acid dextranomer (Zuidex), but it was withdrawn from the market with failure of the North American multicenter randomized trial to demonstrate noninferiority of Zuidex compared with collagen (Lightner et al, 2009).
Periprocedural Care
Although randomized trials have not been done, prophylactic antibiotics with a fluoroquinolone or trimethoprim-sulfamethoxazole (TMP-SMX) for 24 hours or less can be recommended (Wolf et al, 2008). An additional 2 to 3 days has also been suggested (Appell and Winters, 2007).
After the injection the patient is asked to cough or strain in the supine and then upright positions. If leakage still occurs, more agent may be given. If no leakage is seen the procedure may be terminated. The patient then voids and can be discharged. Urinary retention can be treated by insertion of a fine Foley catheter (12 to 14 Fr or smaller) overnight or intermittent catheterization. Although not specifically reported, an indwelling Foley catheter may cause molding of the agent and lead to early failure, so long-term catheterization should be avoided.
Reinjections
The minimum timing for reinjections varies and depends on the agent. Although collagen can be reinjected within 7 days, most clinicians wait 4 weeks or longer to assess response of the urethra and the need for reinjection (Appell and Winters, 2007). Macroplastique injections can be repeated after 12 weeks. Durasphere can be reinjected after a minimum of 7 days (Lightner et al, 2001) and calcium hydroxyapatite (Coaptite; Bioform Medical, San Mateo, CA) can be reinjected after 1 month or less (Mayer et al, 2007). Reinjection timing with other new agents, if known, is mentioned later in the chapter.
Pitfalls in Reported Study Results of Durability
A number of pitfalls in reporting of injectable studies can lead to inflated success rates. Because injectable agents can be repeated if the patient is not a success or fails, authors should specify whether that time point is after all treatments have been completed or whether it is from baseline. If durability is reported after all injections are administered, then an accurate picture of duration of efficacy can be conveyed. A Kaplan-Meier curve of efficacy has been useful in showing what happens to patients’ continence outcome over time (Herschorn and Radomski, 1997; Lightner et al, 2001). Nevertheless, some studies report duration of results from initial treatment (Richardson et al, 1995) or do not specify it (Monga et al, 1995). This may overestimate success because failures are re-treated and can be counted as successes within the follow-up period. Another pitfall is reporting success rates on cohorts of patients followed for the long term rather than on all patients treated from the start (Stenberg et al, 2003). If the patients in whom the procedure failed or those lost to follow-up are not included in the denominator the success rate is higher.
Outcome Assessment in Bulking Agent Clinical Trials by the U.S. Food and Drug Administration
The Stamey 0 to 3 Grading System (Stamey, 1979) (Table 74–2) for SUI has been recommended as the primary outcome measure by the FDA since the original U.S. collagen trial (McGuire and Appell, 1994). Although the scale is extensively used there is little evidence that it is as valid or reliable as other measures, such as voiding diaries, pad tests, and leak point measurements (Payne et al, 2009).
Table 74–2 Stamey Incontinence Grading System
| Grade 0 | Continent |
| Grade 1 | Patient loses urine with sudden increases in abdominal pressure but not while supine |
| Grade 2 | Patient loses urine with physical stress (walking; changing from a reclining to a standing position; sitting up in bed) |
| Grade 3 | Patient with total incontinence; urine loss unrelated to physical activity and/or position |
Systematic Reviews of Injectable Agents for Women with SUI
The Cochrane Database of Systematic Reviews published an update review of injectable agents in women in 2009 (Keegan et al, 2007). The authors reviewed 12 trials with 1318 women. They concluded that the trials were small and generally of moderate quality. Pending further evidence, injection therapy may represent a useful option for short-term symptomatic relief for women with comorbidity that precludes anesthesia. Two or three injections are likely to be required to achieve a satisfactory result.
Injection therapy was also reviewed at the Fourth International Consultation on Urinary Incontinence (Smith et al, 2009). Evidence for the benefit of injectable agents was also considered to be limited and of short term. Greater symptomatic improvement was observed after surgery, which is of higher risk. There is no evidence for the superiority of any bulking agent over another (although new data on silicone microimplant injections may challenge this [Ghoniem et al, 2009]). There are also no available data comparing bulking agents with nonsurgical or minimal access surgical techniques. It was recommended that, if bulking agents are to be used, women should be made aware that repeat injections are likely to be required, that efficacy diminishes with time, and that this therapy is inferior to that of conventional surgical techniques. Women should also be aware of alternative minimally invasive procedures.
Key Points: Use of Injectable Agents in Female Stress Urinary Incontinence
Currently Used Injectable Agents
Glutaraldehyde Cross-linked Bovine Collagen (Contigen)
Glutaraldehyde cross-linked collagen (Contigen; C.R. Bard, Covington, GA) is a highly purified suspension of bovine collagen in normal saline containing at least 95% type I collagen and 1% to 5% type III collagen (Remacle et al, 1990). This cross-linking makes the collagen resistant to the fibroblast-secreted collagenase. As a result of this, the collagen is only very slightly resorbed. The implant causes little inflammatory reaction or granuloma formation and is colonized by host fibroblasts and blood vessels. It is not known to migrate; however, it does degrade over time with volume loss via absorption of the carrier medium (Kershen and Atala, 1999) and may be replaced by host collagen, to explain its persistence (Keefe et al, 1992). To decrease its antigenicity the collagen is prepared by selective hydrolysis of the nonhelicoidal amino- and carboxy-terminal groups (telopeptides) of the collagen molecule that are the antigenic parts of the molecule (Remacle and Declaye, 1988).
All patients must undergo a skin test into the volar aspect of the forearm 30 days before treatment. Approximately 3% of patients will have a positive skin test reaction, with 70% showing the reaction within 3 days, indicating a preexisting sensitivity to bovine dermal collagen through dietary exposure. The remaining 30% do not respond until later so a 4-week period is required (Keefe et al, 1992). A negative skin test does not preclude development of a hypersensitivity reaction to subsequent treatment and, although infrequently used, a second skin test has been recommended (Elson, 1989; Stothers and Goldenberg, 1998). Positive responders should be excluded.
Collagen can be injected transurethrally or periurethrally through a 22-gauge needle. It is supplied in syringes that contain about 3 mL of collagen. Ordinarily one to three syringes are used during each treatment.
Collagen is the standard against which all new injectable agents have been compared. The results of these trials are outlined in the following sections. C.R. Bard, Inc., has announced that Contigen will not be available after 2011.
Results
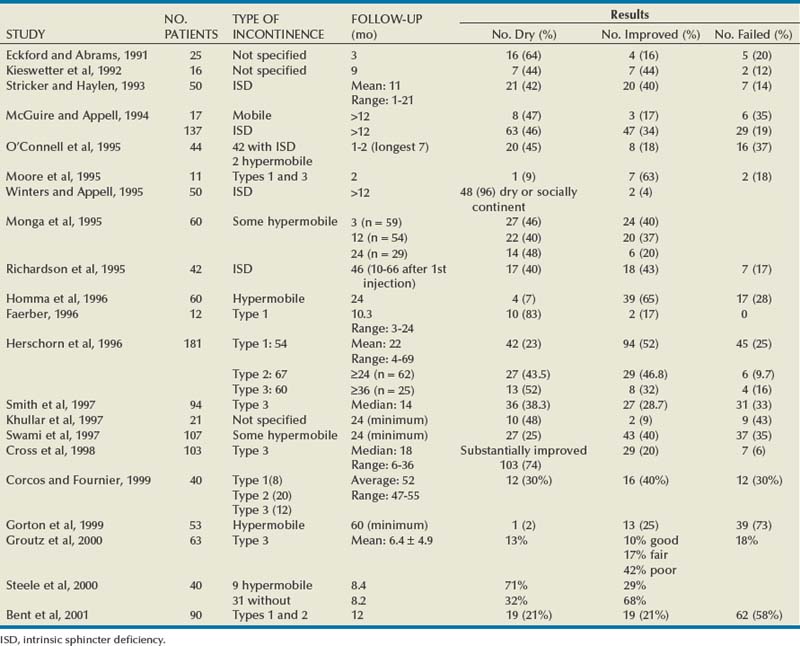
The most extensively reported injectable agent has been collagen (Table 74–3). It was first reported by Shortliffe and colleagues in 1989, and since then numerous reports of its efficacy, safety, ease of administration, and relative lack of morbidity have appeared. The author’s original report, with short-term follow-up of 32 patients for 6 months (Herschorn et al, 1992), showed a cured and improved rate of 90.3%. Longer-term results of more than 1 to 2 years vary from 57%, cure and improved (Khullar et al, 1997), to 94% (Cross et al, 1998). Most patients need one to two treatment sessions with means of 5.6 to 15 mL of collagen. Winters and Appell (1995) reported a 50% rate of complete continence in a multicenter trial after 2 years. Corcos and Fournier (1999) reported 4-year follow-up with 40% improvement and 30% cure. The longest follow-up reported to date is from Gorton and coworkers (1999), who reported on 53 patients with at least 5 years after their last collagen injection: only 14 (26%) had persistent improvement, and of these 1 (2%) was completely dry. Because patients in any series are treated at different times and durations of follow-up vary, a Kaplan-Meier curve is useful to display the persistence of a good result (Herschorn and Radomski, 1997). Figure 74–7 shows that the probability of remaining dry after the last collagen injection was 72% at 1 year, 57% at 2 years, and 45% at 3 years. A similar deterioration over time was reported by Gorton and coworkers (1999).
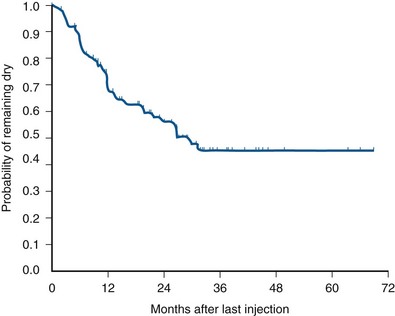
Figure 74–7 Durability: Kaplan-Meier curve showing durability of cure of incontinence after the last collagen injection in 78 patients.
(From Herschorn S, Radomski SB. Collagen injections for genuine stress urinary incontinence: patient selection and durability. Int Urogynecol J Pelvic Floor Dysfunct 1997;8[1]:18–24.)
Multiple factors that may influence outcome have been reported. Previous incontinence surgery was identified by Eckford and Abrams (1991) as a favorable factor but was not supported by others (Herschorn et al, 1996). Preoperative detrusor overactivity may be an adverse factor (Herschorn et al, 1996; Smith et al, 1997). The degree of mucosal coaptation after injection as judged on cystoscopy was not found to correlate with long-term improvement (Kim et al, 1997). Elia and Bergman (1996) used perineal ultrasonography to measure the location of the collagen deposit 3 months after the procedure. They reported that a positive outcome was more likely if collagen was located at a distance of 6 mm or less from the bladder neck. Defreitas and coworkers (2003) correlated a good outcome to circumferential distribution of collagen around the urethra. They did three-dimensional transvaginal ultrasonography in 46 patients at a median follow-up of 14 months and found that the 21 satisfied patients had a higher rate of circumferential distribution compared with the 25 unsatisfied patients. Kuhn and colleagues (2008) compared the site of injection (i.e., bladder neck versus midurethra) in a small randomized study. They found no difference in outcome between the two sites.
Collagen and Hypermobility
The use of collagen for patients with hypermobility has been reported extensively. Moore and colleagues (1995) included patients with hypermobility. Faerber (1996) treated elderly patients with type 1 abnormality. In the report by McGuire and Appell (1994) the results at more than 1 year in women with ISD were similar to those in women with hypermobility, although there were far more women with ISD. Monga and coworkers (1995) included patients with hypermobility and found that cure rates were not reduced for women with up to 2.5 cm of movement. In the author’s series of 181 patients there was no significant difference in outcome in patients with or without hypermobility (Herschorn and Radomski, 1997). Corcos and Fournier (1999) found no difference between patients with and without bladder neck hypermobility in their 4-year follow-up on 40 patients. Steele and colleagues (2000) found that urethral mobility did not significantly affect the success rate. Furthermore, 4 of 6 patients with urethral hypermobility were dry at the 6-month follow-up examination, whereas among the 19 women without hypermobility only a 32% remained dry. Bent and colleagues (2001) reported a 44% cure/improvement rate for 12 months in a cohort of 90 women with SUI and hypermobility. They concluded, as have others, that collagen injection therapy is appropriate for women with hypermobility.
Collagen versus Surgery
Berman and Kreder (1997) found that after an average follow-up of 14.9 months after sling cystourethropexy 71.4% of the patients were continent versus 26.7% of patients with collagen after 21.3 months. They analyzed associated costs and concluded that surgery was more cost effective than collagen. In a multicenter prospective randomized trial, Corcos and colleagues (2005) reported a lower success rate of 53.1% in the collagen-treated patients versus 72.2% in the surgery group. However, general and disease-specific quality of life scores were similar, satisfaction was slightly higher in the surgery group, but complications were less frequent and severe with collagen. Corcos and colleagues concluded that collagen was a reasonable alternative to surgery. The follow-up was relatively short, and the study was done before the era of midurethral slings.
Complications
Treatment-related morbidity has been minimal. Common complications include transient urinary retention, which ranges from 1% to 21% (Herschorn et al, 1992; Winters and Appell, 1995; Appell and Winters, 2007) and can be managed with intermittent catheterization or short-term use of a Foley catheter. Urinary tract infection occurs in 1% to 25% of patients (Herschorn et al, 1992; Winters and Appell, 1995; Appell and Winters, 2007). De novo detrusor overactivity was reported in 11 of 28 elderly women (39%) treated by Khullar and coworkers (1997). Stothers and colleagues (1998) reported de novo urgency with urgency incontinence in 43 of 337 patients (12.6%), 21% of whom did not respond to anticholinergic agents. Hematuria can occur in 2% of patients (Appell and Winters, 2007). Extravasation resolves quickly with flushing away of the dilute collagen suspension and sealing over of the small needle site.
A rare complication is periurethral abscess formation (Sweat and Lightner, 1999). Another is a reaction in the previously negative skin test site after a urethral collagen injection (Stothers and Goldenberg, 1998). This occurred in 3 patients (1.9%) and was associated with arthralgias in 2. This reaction has been reported before in dermatology (Elson, 1989), and two negative pretreatment skin tests have been suggested to prevent it. The potential for hypersensitivity reactions is present because antibody production is stimulated by collagen injection (McClelland and Delustro, 1996).
Vesicovaginal fistula occurring after collagen injections for SUI in 2 women after cystectomy and neobladder was described by Pruthi and colleagues (2000). Carlin and Klutke (2000) reported a urethrovaginal fistula in a woman whose warfarin was not completely reversed. She had a postinjection hematoma that ultimately fistulized to the vagina.
Carbon-Coated Zirconium Beads (Durasphere)
Durasphere consists of nonabsorbable pyrolytic carbon-coated zirconium beads suspended in a water-based polysaccharide carrier gel of 2.8% β-glucan (Lightner et al, 2001). Pyrolytic carbon has been used in medical devices such as heart valves. The bead size ranges from 212 to 500 µm, which is larger than the threshold for particle size migration of 80 µm (Malizia et al, 1984). It is nonantigenic, and no skin testing is required. The agent can be delivered through an 18-gauge needle via the transurethral or periurethral approach with cystoscopic monitoring. It is more viscous than collagen, so greater pressure is required for delivery into the tissues.
As a result of reported difficulty in injecting the agent the beads were modified and made smaller. The bead sizes of Durasphere EXP now range from 90 to 212 µm, still larger than 80 µm threshold for migration.
Results
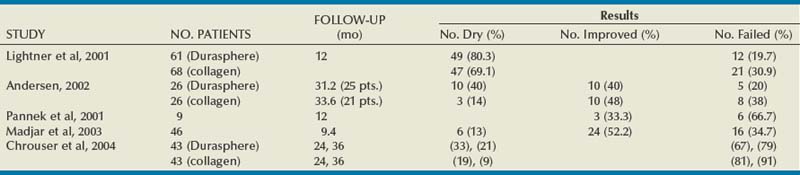
Published results with Durasphere are shown in Table 74–4. In the original multicenter randomized 235-patient clinical trial of Durasphere versus collagen, Lightner and colleagues (2001) reported a 12-month continence grade improvement rate of 76/115 (66.1%) in the Durasphere group versus 79/120 (65.8%) in the collagen group (P = 1.000). They also reported that at 1 year after their last treatment 49 of 61 (80.3%) in the Durasphere group versus 47 of 68 (69.1%) in the collagen group had a continence grade improvement (P = .162). Both injectable agents performed similarly. There was also no difference in number of injections or in results of a pad weight test. However, the injected initial and repeat injection volume of Durasphere was significantly less than those of collagen. It is notable that in the 12-month result after the last injection 45% (106/235) of the study patients were not accounted for.
In another randomized prospective trial of Durasphere versus collagen, Andersen (2002) reported 80% (20/25) of Durasphere patients versus 61.9% (13/21) of collagen patients had a continence grade improvement (P = .205) after a mean of 2.6 and 2.8 years, respectively, after initial treatment. This is similar to the study of Lightner and colleagues (2001).
In a series of 13 women, Pannek and colleagues (2001) reported a decline in success from 76.9% at 6 months to 33% at 12 months. In contrast to the study of Lightner and colleagues (2001) they demonstrated particle migration locally and to distant sites on follow-up plain radiographs. As a result of reported injection difficulties, Madjar and coworkers (2003) modified the technique by injecting local anesthetic into the mucosa to raise a circumferential bleb into which the Durasphere is injected. They reported results on 46/70 (65.7%) of patients. At a mean of 9.4 months, 65.2% of patients considered themselves to be cured or improved.
In a longer-term follow-up matched-cohort study, Chrouser and colleagues (2004) reported initial success of 63% in both groups. With longer follow-up of 24 and 36 months, Durasphere was effective in 33% and 21% whereas collagen was effective in 19% and 9%, respectively. The results were not significantly different. Sokol and coworkers (2008) tried to improve on the results of transurethral collagen by injecting additional periurethral Durasphere. However, they failed to show any benefit. After 6 months there was no difference in cure rates: 33.3% in the combined group versus 29.4% in the collagen alone group.
Complications
In the multicenter randomized trial of Durasphere versus collagen the adverse event profiles were similar (Lightner et al, 2001). However, more women had significantly more post-treatment urgency and acute retention with Durasphere versus collagen: 24.7% and 16.9% versus 11.9% and 3.4%, respectively. Pelvic radiographs taken at 1 and 2 years after injection showed stability of the bulking agents at the injection site. Pannek and associates (2001) reported particle migration. This was attributed to the high pressure necessary to inject the viscous material with large particles, resulting in material displacement into vascular or lymphatic spaces (Appell et al, 2006). Durasphere EXP with its smaller particles may be less likely to lead to this.
Other reported adverse events include urethral mucosal prolapse (Ghoniem and Khater, 2006) and periurethral sterile abscess formation in 2.9% of a series of 135 patients (Madjar et al, 2006).
Silicone Microimplants (Macroplastique)
Silicone microimplants (Harriss et al, 1996) are solid polydimethylsiloxane (silicone rubber) particles suspended in a nonsilicone carrier gel that is absorbed by the reticuloendothelial system and excreted unchanged in the urine. Because 99% of the particles are between 100 µm and 450 µm in diameter, the likelihood of migration is low. Henly and coworkers (1995) demonstrated distant migration of small particles, less than 70 µm, but no migration of particles greater than 100 µm in diameter. Although there was a typical histiocytic and giant cell reaction within the injection site there was no granuloma formation in response to the larger particles. Because the substance is quite viscous it must be injected with an injection gun and a 16-gauge tip transurethral needle. The vials contain 2.5 mL of silicone microimplants.
Results
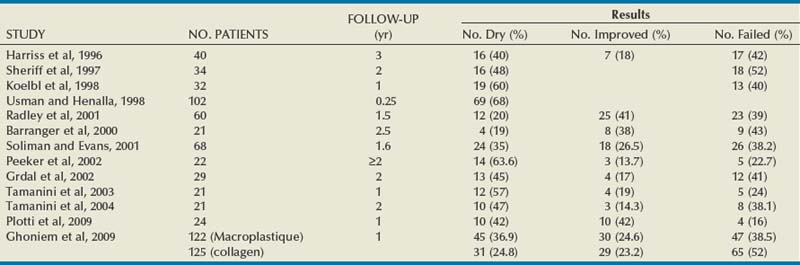
The results for silicone microimplants are shown in Table 74–5. Harriss and colleagues (1996) reported on 40 patients followed for a minimum of 3 years, at which time 16 (40%) were dry, 7 (18%) were improved, and 17 (42%) reported failure of the treatment. Twelve of the 16 required one injection, and 4 needed two injections to become dry. Sheriff and coworkers (1997) reported an overall success of 48% in 34 patients after unsuccessful surgery for SUI, and Koelbl and colleagues (1998) reported a 60% success rate in 32 women after 12 months but noted a time-dependent decrease in success. Radley and coworkers (2001) reported a success rate of 61% (19.6% cured and 41.1% improved) in 60 women after a mean of 19 months. Barranger and coworkers (2000) in a group of 21 patients reported a dry rate of 19%, an improved rate of 38%, and a failure rate of 52% at a median follow-up of 31 months; interestingly, they did not observe a time-dependent decrease in results. Tamanini and coworkers (2003, 2004) reported 1- and 2-year results in 21 patients with the use of a handheld noncystoscopic injector system (see Fig. 74–6B). There was a decrease in continence outcome after 2 years. Plotti and colleagues (2009) reported 84% cure/improvement rates in a series of 24 women with de novo SUI after radical hysterectomy.
Recently Ghoniem and colleagues reported the results of a North American multicenter randomized trial of Macroplastique versus collagen (Ghoniem et al, 2009). After 1 year, 61.5% (75/122) with Macroplastique and 48% (60/125) with collagen had an improvement of at least 1 Stamey grade. This indicated that Macroplastique was noninferior to collagen (P < .001). The proportion of the patients who were dry was higher in the Macroplastique group at 36.9% versus 24.8% (P < .05). However, there were no significant differences in pad weight testing, quality of life scale, or adverse events. The same authors subsequently reported the 2-year results in the Macroplastique group (Ghoniem et al, 2010). Eighty-four percent of those who had a benefit at 12 months maintained that level of cure or improvement to 24 months. That means that of the original 122 patients 51.7% had a good outcome at 2 years.
Complications
Self-limited side effects of urinary tract infection, hematuria, dysuria, urgency, frequency, voiding difficulty, and urinary retention have been reported (Ghoniem et al, 2009). Rarely erosion of the injectable occurs. The lack of a granulomatous reaction and migration of the large silicone particles may provide some benefit over smaller-particle injectable agents such as polytetrafluoroethylene, although long-term data are not yet available. Despite the laboratory and clinical evidence of safety with the large particles, concerns still exist about the small silicone particle migration and long-term tissue response to the injection (Henly et al, 1995).
Calcium Hydroxyapatite (Coaptite)
Calcium hydroxyapatite (Coaptite; Bioform Medical, San Mateo, CA), which is a normal constituent of bone, can be manufactured into particles of a spherical mean diameter of 100 µm (75 to 125 µm) suspended in a carboxymethylcellulose gel carrier. This exogenous substance has been used in orthopedic and dental applications (Bucholz, 2002), as well as soft tissue augmentation of vocal cords (Belafsky and Postma, 2004), face (Tzikas, 2004), and the ureteral orifice for reflux (Mevorach et al, 2006). Animal experiments have demonstrated the safety and biocompatibility of this substance. It stimulates fibroblast infiltration when placed into soft tissue, which may explain its long-term bulking effect after degradation of the carrier gel (Mayer et al, 2007).
The implant is currently supplied in 1-mL syringes and can be injected via a transurethral approach though a 21-gauge needle.
Results
Mayer and colleagues (2001) reported initial results in 10 women with ISD and limited hypermobility. After 1 year, 7 reported substantial improvement, 2 improved, and 1 had no change. No significant complications were reported. In a randomized prospective trial of calcium hydroxyapatite compared with collagen, Mayer and coworkers (2007) reported results in 231 women. Up to 5 injections were performed in the first 6 months of the trial. At 12 months, 83 (63.4%) of 131 patients who had injections of calcium hydroxyapatite showed improvement of one Stamey grade or more versus 57 (57%) of 100 collagen patients (P = .34). There was no difference in cure (39% calcium hydroxyapatite vs. 37% collagen) and improvement (50% calcium hydroxyapatite vs. 46% collagen) rates. More patients who had had calcium hydroxyapatite injections required only one injection, and the total average injected volume was lower (4.0 mL vs. 6.6 mL, respectively; P < .0001).
Complications
Minor adverse events such as transient retention (41%), urinary tract infection, and urgency incontinence (5.7%) were reported (Mayer et al, 2007). Serious adverse events were vaginal wall erosion of the implant and dissection of the material beneath the trigone. Because this agent has a greater particle density than collagen it is thought to have the potential to cause more local tissue pressure effects.
Autologous Fat
Autologous fat has been used for aesthetic and defect reconstruction since the 1980s (Billings and May, 1989). Although fat is biocompatible and readily available, 50% to 90% of the transferred adipose tissue graft may not survive (Horl et al, 1991). Graft survival depends on minimal handling, low suction pressure during liposuction, and the use of large-bore needles. Smaller grafts survive better than larger ones (Bircoll and Novack, 1987).
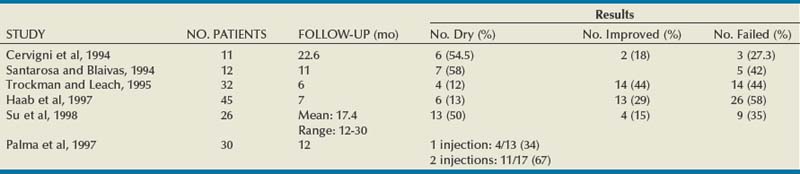
The procedure involves harvesting abdominal wall fat by liposuction either under local (Trockman and Leach, 1995) or general anesthesia (Su et al, 1998). The injection is usually carried out via the periurethral route with a 16- or 18-gauge needle. Postprocedural care may involve intermittent catheterization or even a suprapubic tube (Su et al, 1998).
Results
A number of reports of urethral fat injections have been published and appear in Table 74–6. Most of the series report short-term results with success apparently lower than that of other injectable agents, apart from the study of Su and colleagues (1998) with a follow-up of more than 12 months. Palma and coworkers (1997) showed that repeat injections improved the cure rate from 31% to 64%. Haab and colleagues (1997) reported a comparative study with collagen. After a mean of 7 months, 13% of the women with fat injection were cured versus 24% of the women with collagen injections. The subjective improvement rate was also higher with the collagen. Lee and colleagues (2001) reported a randomized double-blind study of autologous fat versus saline injection. At 3 months, 6 of 27 (22.2%) and 6 of 29 (20.7%) women were cured or improved in the fat and saline groups, respectively. In this study, periurethral fat injection did not appear to be more efficacious than placebo in treating SUI.
Since the publication of the randomized trial of Lee and colleagues (2001) showing that fat was no more efficacious than saline, no further publications have appeared in the literature. Furthermore, the report of a death from fat embolism (Currie et al, 1997) most likely discouraged additional studies. However, experimental work on the use of adipose-derived stem cells to help improve function after urethral injury has progressed in the rat model (Lin et al, 2010).
Complications
Reported complications are similar to other injectable agents with urinary infection, retention, hematuria, and extravasation. Additional problems with donor site, the abdominal wall, such as pain, hematomas, and infection may also be seen. Other noteworthy complications are urethral pseudolipoma (Palma et al, 1996) and fat embolism (Sweat and Lightner, 1999), one of which was fatal (Currie et al, 1997).
Key Points: Currently Used Injectable Agents for Female Stress Urinary Incontinence
New Agents Undergoing Development
Polyacrylamide Hydrogel (Bulkamid)
Polyacrylamide hydrogel (PAHG) is a nontoxic, nonresorbable sterile aqueous gel consisting of 2.5% cross-linked polyacrylamide and 97.5% nonpyrogenic water. It is homogeneous, stable, and nonbiodegradable and has tissue-like viscosity and elasticity. It is being used in plastic surgery (Breiting et al, 2004) and ophthalmology (Lloyd et al, 2001).
Lose and coworkers (2006) reported 12-month results in a study with 25 women. The injection was delivered transurethrally with a 23-gauge needle. Four women were lost to follow-up. Eleven women (44%) underwent a second injection after 3 months because of lack of effect. Overall, 8 (32%) were dry and 9 (36%) experienced improvement. Minor adverse events included urinary tract infections (40%), transient retention (10%), urgency (8%), urgency incontinence (8%), and nocturia (4%). Multicenter randomized trials are underway in North America and Europe.
Porcine Dermal Collagen
Porcine dermal collagen implants have been used in hernia repairs and pelvic floor reconstruction (Harper, 2001; Dench et al, 2006). This substance is maintained in its original three-dimensional form and is close to human dermis in architecture (Meyer et al, 1978). It is biocompatible, and patients do not have to be skin tested as for bovine collagen.
Bano and colleagues (2005) reported early results of a randomized trial of porcine dermal collagen versus Macroplastique. There were 25 patients in each arm. The porcine collagen was injected periurethrally in 21 patients. At 6 months, 15 (60%) of 25 in the collagen group were dry and 10 were unchanged or worse (40%). In the Macroplastique group, 9 (36%) were dry, 1 was improved (4%), and 14 (56%) were unchanged or worse. There were no significant differences between the groups. Minor complications of transient retention and urgency incontinence were seen in both groups and were similar.
Autologous Chondrocytes
A bulking agent composed of autologous chondrocytes has been used to treat children with vesicoureteral reflux (Diamond and Caldamone, 1999). Animal studies of the implant demonstrated stability and lack of migration over time (Atala et al, 1994; Cozzolino et al, 1999). The injectable material consists of autologous chondrocytes in a calcium alginate gel administered endoscopically through a 22-gauge needle. The chondrocytes obtained from biopsy of the external pinna of the patient’s ear are expanded in tissue culture and combined with a carrier gel that degrades after injection.
Bent and coworkers (2001) reported 12-month results in 32 women after a single outpatient injection in a multicenter trial. Incontinence grading indicated 16 patients dry and 10 improved, for a total of 26 (81.3%). Side effects were minimal.
Implantable Balloons
To obviate the degradation and movement of injectable materials Yoo and coworkers (1997) developed a self-detachable implantable balloon system. The balloon is a silicone elastomer with a check valve that prevents escape of the solution that is injected at the time of implant. The filling solution is a biocompatible cross-linked hydrogel that maintains its volume within the silicone shell. The balloons are inserted into the submucosal area, usually periurethrally, with cystoscopic control.
Pycha and coworkers (1998) reported that 8 (42%) of 19 women were dry and 7 (36.8%) were improved after a mean of 14.4 months. The patients with hypermobility had a poor outcome. Mazouni and colleagues (2004) reported results in 45 women who were observed for a mean of 3 months. The overall success rate was 65%, with 24 dry and 2 improved. Complications included detrusor overactivity, balloon rupture on insertion, and balloon extrusion.
Adjustable Continence Therapy (ACT)
The Adjustable Continence Therapy (ACT) device (Uromedica, Plymouth, MN) consists of two inflatable silicone balloons attached to silicone tubing with a titanium and silicone port (Fig. 74–8). The procedure can be done under local, regional, or general anesthesia. The balloons are placed into the periurethral space at the bladder neck with introducer devices inserted through two 1-cm incisions in the labial sulci at the level of the vaginal introitus. The procedure is carried out under fluoroscopic guidance with a contrast-filled Foley balloon positioned at the bladder neck. After the correct position is ascertained the device balloons are inflated with 1.0 to 1.5 mL of an isotonic solution (sterile water and contrast material).The aim is to increase urethral resistance and support the bladder neck with the inflated balloons (Stecco et al, 2006). The ports are buried in the subcutaneous tissue of the labia to enable postoperative reinjection of the balloons if necessary (Kocjancic et al, 2008). Beginning at 6 weeks, additional fluid, usually with volumes of 2 mL, can be added to the balloons by injecting percutaneously through the port sites, if necessary. The technology was developed as an alternative to bulking agents that would not migrate (Kocjancic et al, 2008).
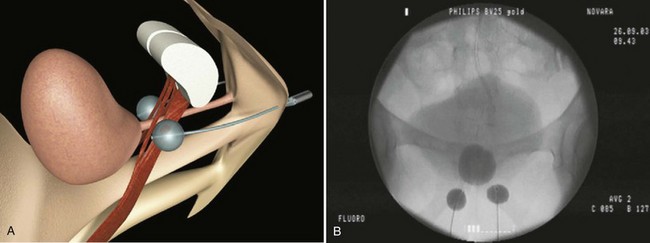
Figure 74–8 Illustration of Adjustable Continence Therapy balloon device for women. A, Schematic diagram showing percutaneous placement of the balloons on both sides of the bladder neck. B, Fluoroscopic picture showing contrast agent in the bladder, Foley catheter, and balloons.
(From Aboseif SR, Franke EI, Nash SD, et al. The adjustable continence therapy system for recurrent female stress urinary incontinence: 1-year results of the North America Clinical Study Group. J Urol 2009;181[5]:2187–91.)
Kocjancic and coworkers (2008) implanted 49 patients, of whom 38 were observed for more than 1 year. Of these, 26 of 38 (68%) were dry, 6 of 38 (16%) were improved, and 6 of 38 (16%) experienced failure. In 62%, one to five fluid additions were necessary throughout the follow-up period. Wachter and coworkers (2008) reported a series of 41 women: the dry rate was 44%, marked improvement was 15%, and 41% had slight improvement or no change; device adjustment was need in 70% of patients. Aboseif and coworkers (2009) reported a North American study consisting of 162 patients of whom 84% had undergone at least one unsuccessful surgical procedure for SUI. Of those observed for 1 year or more, 107 of 140 (76.4%) improved by one or more Stamey grades, 67 of 130 (52%) had less than 2 g of urine on pad weight testing, and 102 of 126 (81%) had a greater than 50% reduction in provocative pad weight. Within 9 months of implantation a mean of 2.3 balloon readjustments were required. Because volume adjustments were done during the follow-up periods in all series there were no data available on continence outcome long after cessation of fluid addition.
Complications
Complications have occurred in 24% (Aboseif et al, 2009) to 39% (Wachter et al, 2008) of patients, with most classified as mild to moderate. Common ones include port or balloon erosion (13%), balloon or port migration (8%), urinary retention (8%), and balloon failure (4%). Kocjancic and coworkers (2008) removed the device in 11 (22%) and then reimplanted it in all of them. Aboseif and coworkers (2009) reimplanted the device in half of the 28 (20% of the total) patients who required its removal, and Wachter and coworkers (2008) explanted 6 devices (15%) owing to nonresponse.
Overall, the procedure may be more suited to patients after surgical failures. Long-term durability still has to be established.
Autologous Myoblasts
Myoblasts, the mononuclear precursor cells of skeletal muscle, can differentiate into multinucleated muscle fibers capable of muscle contraction. They fuse to form myotubes, which stop dividing with maturation and can express protein for a prolonged period (Yokoyama et al, 2000). These cells can be harvested from skeletal muscle outside the urinary tract. The aim is to improve urinary sphincter function with urethral sphincter injections of these cells.
In a short-term feasibility animal study, Chancellor and coworkers (2000) injected mouse myoblasts into rat urethras and bladders. After 3 to 4 days the harvested organs showed viable cells with fusion of injected cells to form myotubes. The same group subsequently used adult rat muscle–derived allografts containing satellite cells and myoblasts and injected it into autologous animals and compared it with injected collagen. After 30 days there was almost no collagen but there was a large cell mass bulging into the urethral lumen (Yokoyama et al, 2001). Mitterberger and coworkers (2007) injected allograft myoblasts in a porcine model and demonstrated a 300% increase in urethral pressure values and survival of the injected cells with formation of myofibers.
Mitterberger and coworkers (2008) reported on 20 women with SUI who underwent transurethral ultrasound-guided injection of autologous fibroblast injection into the urethral submucosal and myoblast injection into the rhabdosphincter. The cells were cultured from skeletal muscle forearm biopsy specimens. Two years after therapy, 16 were cured, 2 were improved, and 2 were lost to follow-up. Apart from 1 patient with transient retention, no complications were encountered. Strasser and associates (2007), from the same group in Innsbruck, Austria, published results of a randomized trial of autologous myoblast versus collagen injection, but the article was subsequently retracted by the Lancet (Kleinert and Horton, 2008).
Carr and colleagues (2008) reported results in 8 women followed for a mean of 16 months after transurethral or periurethral injection of autologous skeletal thigh muscle–derived stem cells. Five of 8 improved with one reporting being dry. No serious adverse events were reported.
This technology looks promising and may provide advantages over bulking agents. No long-term data are available at present, and further studies are underway.
Agents That Are No Longer Used
The reader is referred to the electronic version of this edition on the Expert Consult website. ![]()
Polytetrafluoroethylene Paste (PTFE, Teflon, Urethrin)
Polytetrafluoroethylene (Teflon) paste is composed of equal parts Teflon paste and glycerin with polysorbate 20 (Cole, 1993). Teflon is a resin polymer with a very high molecular weight and high viscosity and is composed of small (40 µm in diameter) particles. It is inert, is stable, and does not induce an allergic response. However, it does cause a local inflammatory response with histiocytes phagocytizing the particles and coalescing to form foreign body giant cells and a granuloma. There is also fibrous tissue ingrowth that adds to the bulk formed by the Teflon. Because of the small particle size, Malizia and colleagues (1984) also showed distant migration of Teflon particles to pelvic nodes, lung, brain, and kidneys of experimental animals.
Teflon paste has been used to treat urinary incontinence since 1964, but it was not reported until 1973 by Berg. Since that time, numerous reports relating to its use in treating incontinence have appeared in the literature.
It may be injected via the periurethral route, and volumes of up to 10 to 20 mL are reported. The procedure is done under local or spinal anesthesia, and injections may be repeated after 6 months. The author has modified the procedure by injecting small amounts (2.5 mL) via the periurethral approach under local anesthetic (Herschorn and Glazer, 2000).
Results
There are wide-ranging outcomes with longer-term series showing poorer results (33% to 76% cure and improved) than those of short-term series (57% to 86%) (Politano, 1982; Lim et al, 1983; Schulman et al, 1984; Deane et al, 1985; Vesey et al, 1988; Beckingham et al, 1992; Harrison et al, 1993; Lopez et al, 1993; Lotenfoe et al, 1993).
Complications
Because relatively large volumes of Teflon have been injected with the patient under general anesthesia, the incidence of urinary retention at 25% (Politano, 1982) is higher than that of collagen. Storage symptoms have also been seen transiently in 20% (Schulman et al, 1984). Urinary infection is rare at 2% (Lim et al, 1983). Perineal discomfort may occur in 5% (Politano, 1982), and transient fever may occur in 5% of patients. Perforation and extravasation can occur and, if recognized at the time of injection, the Teflon should be removed. Although Teflon particles can migrate (Malizia et al, 1984), only one case of migration of clinical significance has been reported in the literature in humans. Claes and colleagues (1989) described a woman previously treated with large volumes of periurethral Teflon for urinary incontinence who later presented with lymphocytic alveolitis and fever. Light microscopy showed Teflon particles in the lungs. She was treated successfully with corticosteroids. Mittleman and Marraccini (1983) reported an incidental postmortem finding of interstitial pulmonary granulomas in a previously asymptomatic man who had received Teflon. Kiilholma and coworkers (1993) reported three complications in 22 women—a sterile periurethral abscess, a urethral diverticulum, and a urethral granuloma—that all required surgical intervention. Although neoplastic transformation was hypothesized (Malizia et al, 1984) no clinical occurrence has been reported. Furthermore, in a long-term rat study, Dewan and associates (1995) demonstrated no increase in tumor risk and no tumors found at the injection site.
Despite the potential for complications with Teflon the actual rate of reported problems is low. However, Teflon is not used as an injectable now.
Hyaluronic Acid Dextranomer (Deflux, Zuidex)
Non–animal-stabilized hyaluronic acid dextranomer (Deflux, Zuidex) copolymer comprises dextranomer microspheres (80 to 250 µm) in a carrier gel of non–animal-stabilized hyaluronic acid. The gel is a biocompatible, biodegradable material free of animal products, has no immunogenic properties, and has been shown not to migrate to different organs after submucosal injection (Stenberg et al, 1999). Stenberg and colleagues first reported the use of this substance in 20 women. They injected it transurethrally through a cystoscope. After 6 months 9 were cured, 7 were improved, and in 3 the procedure failed. Between 6 and 7 years later 5 remained dry and 4 were still improved. Overall, 9 of the original 20 women had a long-term response.
Subsequently the procedure was modified by injecting the copolymer through a system consisting of a handle (Implacer) through which four needles with attached syringes are mounted. A plastic protector is pushed forward to sheathe the needles and then is inserted into the urethra and positioned below the bladder neck by measuring the distance from the meatus. The barrel is slid backward to unsheathe the four needles and a total of 2.8 mL of copolymer is injected blindly into the urethra, 0.7 mL into each quadrant. Van Kerrebroeck and colleagues (2004) reported early results in 42 women. Thirty-two (76%) had improvement in leakage at 3 and 12 months. Chapple and colleagues (2005) reported the results of 142 patients treated in a European multicenter trial. The protocol consisted of an initial injection followed by another at 8 weeks, if required. A total of 61 patients (43%) underwent the second injection. At month 12, 77% of the patients demonstrated a positive response that was a greater than or equal to 50% decrease in leakage on provocative testing.
Lightner and colleagues (2009) reported 12-month outcomes of a North American prospective 2 : 1 randomized trial of Zuidex-Implacer versus collagen injected cystoscopically in 344 women. The study failed to demonstrate that Zuidex was noninferior to collagen. The proportion of women who achieved a 50% reduction in urinary leakage on provocation testing, the primary outcome, was achieved in 84% of collagen-treated women versus 65% of Zuidex-treated women. This negative trial prompted the company to withdraw the Zuidex product. A similar product Deflux, used mainly for vesicoureteral reflux without the Implacer, is still available.
Complications
The reported side effects were similar to those of other injectable agents apart from injection site infections and pseudocyst formation requiring drainage or excision (Chapple et al, 2005; Petrou et al, 2006; Abdelwahab and Ghoniem, 2007). Urethrovaginal fistula after sterile abscess has also been reported (Hilton, 2009).
Ethylene Vinyl Alcohol (EVOH, Tegress)
Ethylene vinyl alcohol copolymer was an intraurethral bulking agent dispensed in a dimethyl sulfoxide carrier as a clear odorless solution that diffuses into the tissue after injection. The EVOH copolymer becomes a soft, spongy, hydrophilic substance that bulks the tissue.
In an unpublished North American clinical trial of 237 women randomized two to one to EVOH versus collagen, at 12 months 18.4% of women treated with EVOH and 16.5% treated with collagen were dry according to Stamey grade (Appell and Winters, 2007). Dry pad weights were reported with 37.8% of the EVOH patients versus 32.1% of those treated with collagen. Pad weight improvement was significantly better in the EVOH group. In a study from the United Kingdom with 33 women observed for a minimum of 35 months, Kuhn and colleagues (2008) reported that 15 (45%) considered themselves as completely continent and 23 (69%) were satisfied. However, Hurtado and coworkers (2007) reported that after a mean of 4.5 months only 2 of 19 patients (10.5%) had at least a 50% improvement in their symptoms.
Complications
In the North American clinical trial the most common complications were urinary tract infection, delayed voiding, dysuria, exposed material, urinary urgency and frequency, hematuria, and genitourinary tenderness (Hurtado et al, 2007). An erosion rate of 36.8% was reported in the series of 19 patients from Hurtado and coworkers (2008). In a series of 18 men treated off-label with EVOH and followed for an average of 4.2 months, the complication rate was 58.8%, with erosions occurring in 41.1% (Hurtado et al, 2008). This compound is no longer available as an injectable agent owing to reports from clinicians, and EVOH was voluntarily withdrawn by C. R. Bard, Inc.
Pathophysiology of Incontinence after Prostatectomy and Use of Injectable Agents for Male Stress Urinary Incontinence
Incontinence after prostatectomy may be caused by bladder dysfunction, sphincter dysfunction, or a combination of the two. Urodynamic investigations are helpful to rule out bladder outlet obstruction or significant bladder dysfunction. In addition to incontinence symptoms, storage and voiding symptoms may be associated (Gray et al, 1999; Hollenbeck et al, 2002). Urodynamic studies have demonstrated that sphincter incompetence occurs as the sole cause in more than two thirds of patients whereas isolated bladder dysfunction (detrusor overactivity, poor compliance, detrusor underactivity during voiding) is uncommon, occurring in less than 10% of patients (Ficazzola and Nitti, 1998; Groutz et al, 2000). However, sphincter and bladder dysfunction can coexist in at least one third of incontinent patients. Decreased sphincter resistance may be due to tissue scarring in some cases and is reflected by a low urethral compliance; however, this parameter is difficult to measure (Groutz et al, 2000). Scarring may lead to an anastomotic stricture evidenced by endoscopy or urethrography and is clinically suspected when incontinence and decreased force of stream coexist.
The preoperative length of the membranous urethra determined on magnetic resonance imaging has been shown to be related to time to postoperative continence (Coakley et al, 2002). Urodynamic studies have revealed that a reduced functional urethral length was predictive of incontinence (Hammerer and Huland, 1997; Van Kampen et al, 1998; Wei et al, 2000). The state of a patient’s pelvic floor may also influence continence or return to continence after radical prostatectomy. Physiotherapy and pelvic floor rehabilitation have been shown to improve or enhance continence (decreased time to final continence level) in the postoperative period in two randomized studies, but only if such measures are instituted before or immediately after catheter removal (Van Kampen et al, 2000; Parekh et al, 2003). Maximum difference between physiotherapy and no treatment is achieved at 3 months, with almost no difference at 12 months. A randomized study in which randomization occurred 6 weeks after surgery showed no difference in continence at 6 months (Wille et al, 2003).
Bulking agents are the most minimally invasive treatment for incontinence after prostatectomy after conservative measures have been tried. They work theoretically by adding bulk and increasing coaptation at the level of the bladder neck and proximal urethra. Several agents have been used, including bovine collagen (Contigen) and silicone microimplants (Macroplastique). All agents share the similar problems, including the need for multiple injections and treatment sessions, deterioration of effect over time, and low cure rates. It remains to be seen if improvements in outcomes can be achieved with alternative agents or if the concept of urethral bulking has achieved its maximum benefit with currently available agents (Herschorn et al, 2010). An alternative technology with implanted adjustable volume balloons (ProACT) will be discussed.
Workup
Basic evaluation includes history, physical examination, urinalysis, and measurement of postvoid residual urine. A frequency-volume chart (Griffiths et al, 1993) or bladder diary is also helpful. A pad test quantifies the severity of incontinence. The 24-hour home test is the most accurate pad test for quantification and diagnosis and the most reproducible (Mouritsen et al, 1989). The 1-hour pad test is widely used because it is more easily done and standardized. The amount of postvoid residual urine is a good estimation of voiding efficiency (Diokno et al, 1988; Starer and Libow, 1988).
Blood tests (blood urea nitrogen, creatinine, glucose) are recommended if compromised renal function is suspected or documented (Fantl et al, 1996).
Uroflowmetry is also helpful to assess voiding function.
Cystourethroscopy is done to verify integrity of the urethral wall, bladder neck, and the status of the bladder (e.g., trabeculation, stones, diverticula). Injectable agents are not effective with scarred supramembranous urethras because the tissues cannot expand with the agent. The status of the urethra should be assessed preoperatively.
Invasive urodynamic studies are useful to assess bladder and urethral function before interventional therapy (Herschorn et al, 2010). Detrusor overactivity or decreased bladder compliance demonstrated on multichannel testing may be contributing to symptoms and may merit alternative treatment. Sphincter weakness can be documented by the Valsalva (McGuire et al, 1993) or cough (Schick, 1985) abdominal leak point pressure, but its reproducibility has been studied in women and not men. Although ALPP may be helpful, it has been shown to be a poor predictor of incontinence severity after radical prostatectomy (Twiss et al, 2005). Furthermore, in male patients, ALPP measurement may be better via a rectal catheter because a urethral catheter may occlude a scarred urethra (Flood et al, 1996). Although definite evidence supporting the predictive value of urodynamic studies is lacking, they are still recommended before interventional treatment (Hosker et al, 2009).
Injection Techniques
Retrograde Injection
The patient is placed in the lithotomy position, and the surgical field is prepared in the usual sterile fashion (Fig. 74–9A). The procedure can be performed with local, regional, or general anesthesia. If local anesthesia is employed, 2% topical urethral lidocaine jelly can be inserted 10 minutes before instrumentation. In some patients, preoperative sedation may also be of benefit. A 20- or 22-Fr cystoscopic sheath is employed using a 0- or 30-degree lens. Collagen is provided in a 3.0-mL Luer-Lok syringe containing 2.5 mL of injectable material. The syringe attaches to a 5-Fr injection catheter containing a 1.5-cm 20-gauge needle at the tip. Most injections are done transurethrally under cystoscopic vision.
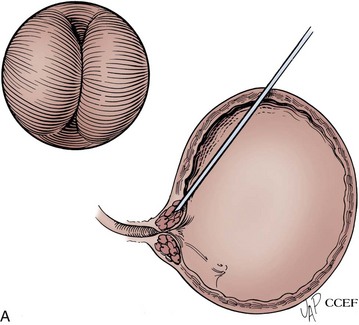
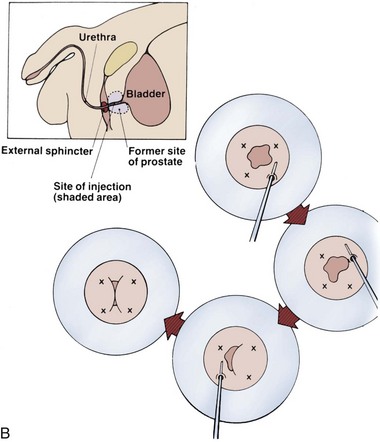
Figure 74–9 A, Retrograde urethral injection technique in men. Schematic representation of transurethral circumferential injection in a male after prostatectomy. B, Antegrade urethral technique injection in the male after radical prostatectomy.
(A, Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1994-2011. All Rights Reserved.)
After a prostatectomy the urethra is frequently scarred and not very pliable; thus, several needle insertions are frequently needed to deposit sufficient material to produce urethral coaptation. The injection is completed in up to four quadrants after localization of the appropriate level in the proximal urethra. The needle is advanced under the urethral mucosa with the beveled portion of the needle facing the urethral lumen to allow for layering of the material. The injectable material is then delivered, creating a bleb under the urethral mucosa that protrudes into the urethral lumen. This is performed in a circumferential manner in four quadrants, creating a bleb in each quadrant. After completion, the urethral mucosa should be completely coapted and creates the appearance of an obstructed urethra. Extrusion of the injectable agent into the urethral lumen as the needle is withdrawn may occur. This may be prevented in most cases by leaving the needle in place for at least 30 seconds after the injection is completed or by flushing the material with saline. The loss of additional material is diminished by preventing advancement of the cystoscope proximal to the injection sites. If material extravasation occurs in all quadrants during injection, the procedure should be terminated and rescheduled (Appell and Winters, 2007).
Antegrade Injection
Because of less than optimal results an alternative method of injecting collagen in men was introduced using a suprapubic antegrade approach. The antegrade approach has the advantage of direct visualization of the bladder neck and the injection of material into more supple, less scarred urethra (see Fig. 74–9B). The patient is placed in the lithotomy position, and flexible cystoscopy is carried out, simultaneously filling the bladder. A percutaneous suprapubic puncture allows placement of two guide wires into the bladder. The site is dilated to 16 Fr with Amplatz dilators. A sheath may be used, but commonly the cystoscope (flexible or rigid pediatric) can be advanced over one of the guide wires into the bladder. A small red rubber catheter or the flexible cystoscope can be used to assist in localization of the bladder neck. The needle is placed just under the urethral mucosa at the level of the bladder neck and advanced proximally, meaning it is pulled back toward the bladder. The bladder neck closes off completely as the material is injected. Injection stops after the bladder neck closes off, the two sides meeting in apposition near the midline. A small suprapubic tube can be placed to avoid the possible necessity of urethral catheterization.
Periprocedural Care
Prophylactic antibiotics with a fluoroquinolone or trimethoprim-sulfamethoxazole for 24 hours or less can be recommended (Wolf et al, 2008). Therapy for an additional 2 to 3 days also has been suggested (Appell and Winters, 2007).
The patient then voids and can be discharged. Urinary retention can be treated by insertion of a fine Foley catheter (12 to 14 Fr or smaller) overnight or intermittent catheterization. Although not specifically reported, an indwelling Foley catheter may cause molding of the agent and lead to early failure; thus long-term catheterization should be avoided.
Currently Used Injectable Agents
Glutaraldehyde Cross-linked Bovine Collagen (Contigen)
Collagen has been injected transurethrally with cystoscopic monitoring into the supramembranous urethra. It has also been injected percutaneously through a suprapubic approach in an antegrade manner. The published results for both techniques are in Tables 74-7 and 74-8.
Table 74–7 Results of Retrograde Transurethral Collagen Injection Therapy for Incontinence after Prostatectomy
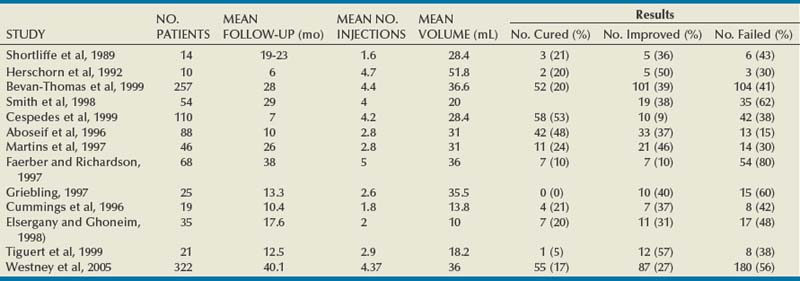
Table 74–8 Results of Antegrade Injection Collagen Injection Therapy for Incontinence after Prostatectomy

For collagen, “success rates” for incontinence after prostatectomy range from 36% to 69%, with 4% to 20% of patients reporting being dry (McGuire and Appell, 1994; Aboseif et al, 1996; Cummings et al, 1996; Sanchez-Ortiz et al, 1997; Smith et al, 1998; Cespedes et al, 1999; Klutke et al, 1999; Tiguert et al, 1999). Unfortunately, the end points in most of these studies are subjectively based, making comparisons difficult; however, it is clear that cure rates (total dryness) are low, and multiple injections are required to achieve modest rates of subjective improvement. There is no advantage of delivery technique (retrograde vs. antegrade). Several authors have identified factors that negatively affect results, including extensive scarring or stricture formation, previous irradiation, and high-grade SUI and low ALPP (Aboseif et al, 1996; Sanchez-Ortiz et al, 1997; Smith et al, 1998; Cespedes et al, 1999). One study reported more favorable results for collagen in treating incontinence after transurethral prostatectomy as opposed to radical prostatectomy (35.2% “social continence” vs. 62.5%) (Smith et al, 1998). Westney and coworkers (2005) reported long-term results in 322 men observed for a mean of 40.1 months. Overall, 55 patients (17%) were dry for a mean of 11.1 months and required 3.8 injections with a total volume of 29.3 mL. In those who experienced any improvement the mean duration of response was 6.3 months. They concluded that collagen was of some benefit but that the duration of response was limited.
Collagen injection does not adversely affect outcomes of artificial sphincter implantation and does not increase the complication rate (Gomes et al, 2000), nor does collagen injection adversely affect the outcome of the bone-anchored male sling (Comiter, 2002; Onur and Singla, 2006). The cost efficacy of injections remains to be determined.
Complications
In addition to those reported in the section in females, worsening of incontinence symptoms may occur in 1.5% of patients (Westney et al, 2005).
Silicone Microimplants (Macroplastique)
Initial success has been demonstrated with silicone microimplants, but results deteriorate over time. Bugel and coworkers (1999) treated 15 patients. They noted rapid deterioration after initial improvements with success rates of 40%, 71%, 33%, and 26% at 1, 3, 6, and 12 months, respectively. They also noted that a urethral closure pressure of at least 30 cm H2O was essential for success. Kylmala and colleagues (2003) studied 50 patients with mild to moderate SUI (average 48 mL on 1-hour pad test), with 12% achieving short-term continence after one injection and an additional 20%, 18%, and 10% achieving continence with two, three, and four injections, respectively. Follow-up, however, was limited to 3 months. In a series of 14 men with spinal injuries and SUI, Hamid and associates (2003) reported that 5 (36%) were dry, 3 (21%) improved, and 6 (43%) experienced failure after a mean follow-up of 35 months.
In a randomized trial of the Artificial Urinary Sphincter (AUS) versus Macroplastique injection in patients with minimal SUI (the vast majority had SUI after surgery for benign prostatic hyperplasia, with greater than a third of the cohort suffering from SUI after radical retropubic prostatectomy), Imamoglu and colleagues (2005) demonstrated no difference in success with AUS versus Macroplastique in men with mild incontinence. However, in patients with more severe incontinence, the AUS was superior and minimal improvement was seen after transurethral implantation of Macroplastique.
Adjustable Balloons (ProACT)
The adjustable balloon procedure is based on the concept of passive compression utilizing balloons located on either side of the urethra. Balloons may be progressively inflated until there is optimal coaptation, thereby achieving continence. The biomaterial name ACT (Adjustable Continence Therapy) was originally developed for female SUI and subsequently was applied to male incontinence. The male ProACT device (Uromedica, Plymouth, MN) was first reported in 2000 (Hubner, 2000).
The device consists of a silicone elastomer balloon attached to an injectable titanium port with a silicone tube (Fig. 74–10). A balloon is implanted on either side of the urethra, either under the bladder neck for post–radical prostatectomy incontinence or under the verumontanum for incontinence after transurethral resection of the prostate. The ports are positioned subcutaneously in the scrotum, allowing simple access for percutaneous adjustment of the balloon volume. The implantation is performed under general or spinal anesthesia through a short perineal incision. A trocar covered with a U-shaped sheath is inserted up to the site of implantation and then the balloon is pushed along inside the sheath. Fluoroscopic and urethroscopic guidance is used for the procedure. Transrectal ultrasound-guided implantation (Gregori et al, 2006, 2010) is a possible option. The balloons are filled with 2 mL of isotonic sterile water and contrast medium during the initial procedure. After approximately 1 month, the balloons are refilled with 1 mL of this solution at each period (maximum filling is 8 mL) until continence is achieved. The adjustments of the filling are volume limited and are carried out step by step to obtain a pseudocapsule surrounding the balloons to minimize the risk of urethral erosion or migration.
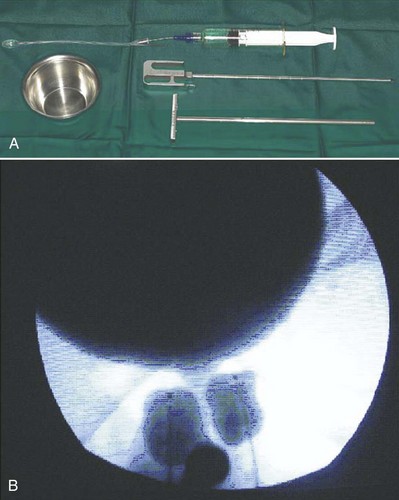
Figure 74–10 Illustration of Adjustable Continence Therapy balloon device for men. A, Balloon, trocar, and U-shaped insertion device are shown. Syringe with contrast agent is connected to balloon port. B, Fluoroscopic picture showing contrast agent in the bladder and urethra with balloons near the bladder neck.
(From Trigo-Rocha F, Gomes CM, Pompeo AC, et al. Prospective study evaluating efficacy and safety of Adjustable Continence Therapy [ProACT] for post radical prostatectomy urinary incontinence. Urology 2006;67[5]:965–69.)
Results from seven studies are shown in Table 74–9. Of 170 patients reported by Hubner and Schlarp (2005) and Lebret and colleagues (2008), one third became pad free. In other studies 70% of patients utilized no pad or just 1 pad daily (Hubner and Schlarp, 2005; Trigo-Rocha et al, 2006; Cansino Alcaide et al, 2007; Lebret et al, 2008). Mean procedure time of 35 minutes was reported. Along with this improvement in pad use there were parallel improvements in Incontinence-Quality of Life questionnaire (I-QOL) score (Hubner and Schlarp, 2005; Trigo-Rocha et al, 2006; Lebret et al, 2008). The mean number of postoperative adjustments of the balloon was 3 to 5, with some patients requiring up to 15.
Table 74–9 Results and Complications of Adjustable Balloons (ProACT) in Urinary Incontinence after Prostatectomy
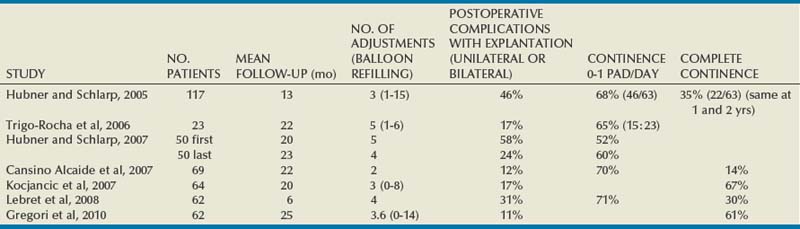
In a simultaneously treated cohort study from two centers, Crivellaro and colleagues (2008) reported no difference in outcome of the adjustable balloons versus the bone-anchored male sling. At a mean follow-up of 19 months after use of adjustable balloons, 30 of 44 men (68%) were dry and 7 (16%) were improved versus 23 of 36 (64%) and 8 (22%) after a bone-anchored male sling procedure, respectively, after a mean of 33 months (P > .05).
Complications
The most common perioperative complication is urethral or bladder perforation, necessitating termination of the implant on the perforated side. However, contralateral implantation was not adversely affected, and repeat ipsilateral implantation was invariably achieved after healing of the urethral or bladder wall. Lebret and colleagues (2008) reported a perforation rate of 10% and Hubner and Schlarp (2007) reported a rate of 18% early in their series but a lower urethral perforation rate in more recent cases, illustrating a relatively short learning curve for optimal balloon placement near the urethral/bladder wall. Temporary urinary retention was reported at 5% (Hubner and Schlarp, 2007). Voiding was restored by removing fluid from the balloon.
Device explantation is related to balloon failure, infection, erosion, or migration. The explantation rate ranged from 11% to 58% (Hubner and Schlarp, 2005, 2007; Trigo-Rocha et al, 2006; Cansino Alcaide et al, 2007; Lebret et al, 2008) but decreased with experience (Hubner and Schlarp, 2007). Device removal is straightforward, because a deflated balloon can be explanted transperineally. Reported risk factors for failure and complications were prior external beam radiation therapy (Lebret et al, 2008; Gregori et al, 2010) and severe preoperative incontinence (Gregori et al, 2010). Kocjancic and colleagues (2007) reported a continence rate of 67% in nonirradiated patients compared with 36% in irradiated patients.
Conclusion
The adjustable balloon (ProACT) technique appears to be a feasible procedure to improve continence in the short and medium term. Appropriate candidates include those with mild to moderate leakage and no previous radiation. The benefit of an adjustable system should be weighed against the need for multiple sessions to refill the balloons and with reported rate of perioperative and postoperative complications necessitating removal.
The technology appears to have better results than injectable agents in men. Longer follow-up is needed before definitive comparison to male sling or artificial sphincter can be made (Herschorn et al, 2010). The device is not commercially available in the United States.
Key Points: Use of Injectable Agents in Male Stress Urinary Incontinence
Use of Injectable Agents for Incontinence after Urinary Diversion
There are very few reports in the literature about the use of injectable agents after urinary diversion. Izes and colleagues (1997) demonstrated in a canine study that collagen injected circumferentially into the lumen of the ileocecal valve can increase the leak point pressure for up to 1 month after injection. Subsequently, Smith and colleagues (1998) reported results of collagen in four women and two men with leaking Indiana pouches. They injected a mean of 16 mL circumferentially into the ileocecal junction to obtain visual closure of the bowel lumen. After a mean follow-up of 26 months 5 of the 6 patients remained dry.
Use of injectable agents has been reported in women after construction of a neobladder. Tchetgen and colleagues (2001) reported on 3 patients with SUI after cystectomy and orthotopic neobladder. An average of two injections was given with a cure in 1, improvement in 1, and no change in 1. Wilson and colleagues (2004) administered transurethral collagen to 11 patients and pyrolytic carbon-coated zirconium beads to 1 patient with SUI after cystectomy and construction of a neobladder. After a mean of 22.5 months 2 patients (16.7%) were dry although continued to have urinary frequency. Four patients (33.3%) were improved, and 6 were unchanged. These researchers concluded that the treatment response was not optimal nor durable.
New-onset vesicovaginal fistula has been reported in 2 patients after collagen injection for SUI after cystectomy and construction of a neobladder (Pruthi et al, 2000). Both patients underwent transvaginal fistula repair with pubovaginal sling procedures.
To date, the success of injectable agents for these clinical circumstances is limited.
Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181(1):204-210.
Herschorn S, Bruschini H, Comiter C, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29(1):179-190.
Herschorn S, Steele DJ, Radomski SB. Followup of intraurethral collagen for female stress urinary incontinence. J Urol. 1996;156(4):1305-1309.
Koelbl H, Nitti VW, Baessler K, et al. Pathophysiology of urinary incontinence, faecal incontinence, and pelvic organ prolapse. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:255-330.
Lightner D, Rovner E, Corcos J, et al. Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology. 2009;74(4):771-775.
Smith AR, Dmochowski RR, Hilton P, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:1191-1272.
Abdelwahab HA, Ghoniem GM. Obstructive suburethral mass after transurethral injection of dextranomer/hyaluronic acid copolymer. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1379-1380.
Aboseif SR, Franke EI, Nash SD, et al. The adjustable continence therapy system for recurrent female stress urinary incontinence: 1-year results of the North America Clinical Study Group. J Urol. 2009;181(5):2187-2191.
Aboseif SR, O’Connell HE, Usui A, et al. Collagen injection for intrinsic sphincteric deficiency in men. J Urol. 1996;155(1):10-13.
Abrams P, Andersson KE, Birder L, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29(1):213-240.
Agur W, Housami F, Drake M, et al. Could the National Institute for Health and Clinical Excellence guidelines on urodynamics in urinary incontinence put some women at risk of a bad outcome from stress incontinence surgery? BJU Int. 2009;103(5):635-639.
Andersen RC. Long-term follow-up comparison of Durasphere and Contigen in the treatment of stress urinary incontinence. J Low Genit Tract Dis. 2002;6(4):239-243.
Appell R. Collagen injections. In: Raz S, editor. Female urology. Philadelphia: WB Saunders; 1996:399-405.
Appell R, Dmochowski RR, Blaivas JG, et al. Guideline for the surgical management of female stress urinary incontinence: 2009 update. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/stress2009/chapter1.pdf, 2009.
Appell RA, Dmochowski RR, Herschorn S. Urethral injections for female stress incontinence. BJU Int. 2006;98(Suppl. 1):27-30. discussion 31
Appell RA, Vasavada SP, Rackley RR, et al. Percutaneous antegrade collagen injection therapy for urinary incontinence following radical prostatectomy. Urology. 1996;48(5):769-772.
Appell RA, Winters JC. Injection therapy for urinary incontinence. In: Wein A, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh urology. Philadelphia: Saunders Elsevier; 2007:2272-2287.
Atala A, Kim W, Paige KT, et al. Endoscopic treatment of vesicoureteral reflux with a chondrocyte-alginate suspension. J Urol. 1994;152(2 Pt. 2):641-643. discussion 644
Athanassopoulos A, Melekos MD, Speakman M, et al. Stamey endoscopic vesical neck suspension in female urinary stress incontinence: results and changes in various urodynamic parameters. Int Urol Nephrol. 1994;26:293-299.
Bakas P, Liapis A, Karandreas A, Creatsas G. Pudendal nerve terminal motor latency in women with genuine stress incontinence and prolapse. Gynecol Obstet Invest. 2001;51:187-190.
Bano F, Barrington JW, Dyer R. Comparison between porcine dermal implant (Permacol) and silicone injection (Macroplastique) for urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(2):147-150. discussion 150
Barranger E, Fritel X, Kadoch O, et al. Results of transurethral injection of silicone micro-implants for females with intrinsic sphincter deficiency. J Urol. 2000;164(5):1619-1622.
Beckingham IJ, Wemyss-Holden G, Lawrence WT. Long-term follow-up of women treated with perurethral Teflon injections for stress incontinence. Br J Urol. 1992;69:580-583.
Belafsky PC, Postma GN. Vocal fold augmentation with calcium hydroxylapatite. Otolaryngol Head Neck Surg. 2004;131(4):351-354.
Bent AE, Foote J, Siegel S, et al. Collagen implant for treating stress urinary incontinence in women with urethral hypermobility. J Urol. 2001;166(4):1354-1357.
Bent AE, Tutrone RT, McLennan MT, et al. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20(2):157-165.
Berg S. Polytef augmentation urethroplasty: correction of surgically incurable urinary incontinence by injection technique. Arch Surg. 1973;107(3):379-381.
Berman CJ, Kreder KJ. Comparative cost analysis of collagen injection and fascia lata sling cystourethropexy for the treatment of type III incontinence in women [see comments]. J Urol. 1997;157(1):122-124.
Bevan-Thomas R, Wesley OL, Cespedes RD, et al. Long-term follow-up of periurethral collagen injections for male intrinsic deficiency. J Urol. 1999;161:257. [abstract]
Billings EJr, May JWJr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83(2):368-381.
Bircoll M, Novack BH. Autologous fat transplantation employing liposuction techniques. Ann Plast Surg. 1987;18(4):327-329.
Blaivas JG. Incontinence. J Urol. 1993;150:1455.
Breiting V, Aasted A, Jorgensen A, et al. A study on patients treated with polyacrylamide hydrogel injection for facial corrections. Aesthetic Plast Surg. 2004;28(1):45-53.
Bubanz H, Truss F, Zimmermann A. [Ureterstenosis after periurethral injection of granugenol oil (author’s transl)]. Urologe A. 1980;19(3):143-144.
Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395:44-52.
Bugel H, Pfister C, Sibert L, et al. [Intraurethral macroplastic injections in the treatment of urinary incontinence after prostatic surgery]. Prog Urol. 1999;9(6):1068-1076.
Cansino Alcaide JR, Alvarez Maestro M, Martin Hernandez M, et al. [Paraurethral ballon implantation in the treatment of male urinary incontinence. La Paz University Hospital experience]. Arch Esp Urol. 2007;60(6):647-655.
Carlin BI, Klutke CG. Development of urethrovaginal fistula following periurethral collagen injection. J Urol. 2000;164(1):124.
Carr LK, Steele D, Steele S, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):881-883.
Cervigni M, Tomiselli G, Perricone C, et al. [Endoscopic treatment of sphincter insufficiency with autologous fat injection]. Arch Ital Urol Androl. 1994;66(4 Suppl):219-224.
Cespedes RD, Leng WW, McGuire EJ. Collagen injection therapy for postprostatectomy incontinence. Urology. 1999;54(4):597-602.
Chancellor MB, Yokoyama T, Tirney S, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19(3):279-287.
Chapple CR, Haab F, Cervigni M, et al. An open, multicentre study of NASHA/Dx Gel (Zuidex) for the treatment of stress urinary incontinence. Eur Urol. 2005;48(3):488-494.
Chrouser KL, Fick F, Goel A, et al. Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended followup. J Urol. 2004;171(3):1152-1155.
Claes H, Stroobants D, Van Meerbeek J, et al. Pulmonary migration following periurethral polytetrafluoroethylene injection for urinary incontinence. J Urol. 1989;142:821-822.
Coakley FV, Eberhardt S, Kattan MW, et al. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002;168(3):1032-1035.
Cole HM. Diagnostic and therapeutic technology assessment. Use of Teflon preparations for urinary incontinence and vesicoureteral reflux. JAMA. 1993;269:2975-2980.
Comiter CV. The male sling for stress urinary incontinence: a prospective study. J Urol. 2002;167(2 Pt 1):597-601.
Constantinou CE. Dynamics of female pelvic floor function using urodynamics, ultrasound and magnetic resonance imaging (MRI). Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl. 1):S159-S165.
Corcos J, Collet JP, Shapiro S, et al. Multicenter randomized clinical trial comparing surgery and collagen injections for treatment of female stress urinary incontinence. Urology. 2005;65(5):898-904.
Corcos J, Fournier C. Periurethral collagen injection for the treatment of female stress urinary incontinence: 4-year follow-up results. Urology. 1999;54(5):815-818.
Cozzolino DJ, Cendron M, DeVore DP, et al. The biological behavior of autologous collagen-based extracellular matrix injected into the rabbit bladder wall. Neurourol Urodyn. 1999;18(5):487-495.
Crivellaro S, Singla A, Aggarwal N, et al. Adjustable continence therapy (ProACT) and bone anchored male sling: comparison of two new treatments of post prostatectomy incontinence. Int J Urol. 2008;15(10):910-914.
Cross CA, English SF, Cespedes RD, et al. A followup on transurethral collagen injection therapy for urinary incontinence. J Urol. 1998;159(1):106-108.
Crystle CD, Charme LS, Copeland WE. Q-tip test in stress urinary incontinence. Obstet Gynecol. 1971;38(2):313-315.
Cummings JM, Boullier JA, Parra RO. Transurethral collagen injections in the therapy of post–radical prostatectomy stress incontinence. J Urol. 1996;155(3):1011-1013.
Cundiff GW, Harris RL, Theofrastous JP, Bump RC. Pressure transmission ratio reproducibility in stress continent and stress incontinent women. Neurourol Urodyn. 1997;16:161-166.
Currie I, Drutz HP, Deck J, et al. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: case report and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(6):377-380.
Deane AM, English P, Hehir M, et al. Teflon injection in stress incontinence. Br J Urol. 1985;57:78-80.
Defreitas GA, Wilson TS, Zimmern PE, et al. Three-dimensional ultrasonography: an objective outcome tool to assess collagen distribution in women with stress urinary incontinence. Urology. 2003;62(2):232-236.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713-1720.
Dench JE, Scott SM, Lunniss PJ, et al. External pelvic rectal suspension (the express procedure) for internal rectal prolapse, with or without concomitant rectocele repair: a video demonstration. Dis Colon Rectum. 2006;49(12):1922-1926.
Dewan PA, Owen AJ, Byard RW. Long-term histological response to subcutaneously injected Polytef and Bioplastique in a rat model. Br J Urol. 1995;76:161-164.
Diamond DA, Caldamone AA. Endoscopic correction of vesicoureteral reflux in children using autologous chondrocytes: preliminary results. J Urol. 1999;162(3 Pt 2):1185-1188.
Digesu GA, Robinson D, Cardozo L, Khullar V. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2009;28:90-94.
Diokno AC, Brown MB, Brock BM, et al. Clinical and cystometric characteristics of continent and incontinent noninstitutionalized elderly. J Urol. 1988;140(3):567-571.
Dmochowski RR, Appell RA. Injectable agents in the treatment of stress urinary incontinence in women: where are we now? Urology. 2000;56(6 Suppl. 1):32-40.
Eckford SD, Abrams P. Para-urethral collagen implantation for female stress incontinence. Br J Urol. 1991;68(6):586-589.
Elia G, Bergman A. Periurethral collagen implant: ultrasound assessment and prediction of outcome. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7(6):335-338.
Elsergany R, Ghoniem GM. Collagen injection for intrinsic sphincteric deficiency in men: a reasonable option in selected patients. J Urol. 1998;159(5):1504-1506.
Elson ML. The role of skin testing in the use of collagen injectable materials. J Dermatol Surg Oncol. 1989;15(3):301-303.
Faerber GJ. Endoscopic collagen injection therapy in elderly women with type I stress urinary incontinence. J Urol. 1996;155(2):512-514.
Faerber GJ, Belville WD, Ohl DA, et al. Comparison of transurethral versus periurethral collagen injection in women with intrinsic sphincter deficiency. Tech Urol. 1998;4(3):124-127.
Faerber GJ, Richardson TD. Long-term results of transurethral collagen injection in men with intrinsic sphincter deficiency. J Endourol. 1997;11(4):273-277.
Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1994;83(1):12-18.
Fantl JA, Hurt WG, Bump RC, et al. Urethral axis and sphincteric function. Am J Obstet Gynecol. 1986;155(3):554-558.
Fantl JA, Newman DK, Colling J, et al. Urinary incontinence in adults: acute and chronic management. Clinical practice guideline no. 2 update. Rockville, MD, U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, March 1996.
Ficazzola MA, Nitti VW. The etiology of post–radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol. 1998;160(4):1317-1320.
Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;169:999-1002.
Flood HD, Alevizatos C, Liu JL. Sex differences in the determination of abdominal leak point pressure in patients with intrinsic sphincter deficiency. J Urol. 1996;156(5):1737-1740.
Gersuny R. Uber eine subcutane Prosthese. Z Heilkunde Wien Leipzig. 1900;21:199.
Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181(1):204-210.
Ghoniem G, Corcos J, Comiter C, et al. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol. 2010;183(4):1444-1449.
Ghoniem G, Elgamasy AN, Elsergany R, Kapoor DS. Grades of intrinsic sphincteric deficiency (ISD) associated with female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:99-105.
Ghoniem G, Khater U. Urethral prolapse after durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(3):297-298.
Gomes CM, Broderick GA, Sanchez-Ortiz RF, et al. Artificial urinary sphincter for post-prostatectomy incontinence: impact of prior collagen injection on cost and clinical outcome. J Urol. 2000;163(1):87-90.
Gorton E, Stanton S, Monga A, et al. Periurethral collagen injection: a long-term follow-up study. BJU Int. 1999;84(9):966-971.
Gray M, Petroni GR, Theodorescu D. Urinary function after radical prostatectomy: a comparison of the retropubic and perineal approaches. Urology. 1999;53(5):881-890. discussion 890-81
Grdal M, Tekin A, Erdoan K, et al. Endoscopic silicone injection for female stress urinary incontinence due to intrinsic sphincter deficiency: impact of coexisting urethral mobility on treatment outcome. Urology. 2002;60(6):1016-1019.
Gregori A, Romano AL, Scieri F, et al. Transrectal ultrasound-guided implantation of Adjustable Continence Therapy (ProACT): surgical technique and clinical results after a mean follow-up of 2 years. Eur Urol. 2010;57(3):430-436.
Gregori A, Simonato A, Lissiani A, et al. Transrectal ultrasound guided implantation of the ProACT adjustable continence therapy system in patients with post–radical prostatectomy stress urinary incontinence: a pilot study. J Urol. 2006;176(5):2109-2113. discussion 2113
Griebling TL, Kreder KJJr, Williams RD. Transurethral collagen injection for treatment of postprostatectomy urinary incontinence in men. Urology. 1997;49(6):907-912.
Griffiths DJ, McCracken PN, Harrison GM, et al. Relationship of fluid intake to voluntary micturition and urinary incontinence in geriatric patients. Neurourol Urodyn. 1993;12(1):1-7.
Groutz A, Blaivas JG, Chaikin DC, et al. The pathophysiology of post–radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol. 2000;163(6):1767-1770.
Groutz A, Blaivas JG, Kesler SS, et al. Outcome results of transurethral collagen injection for female stress incontinence: assessment by urinary incontinence score. J Urol. 2000;164(6):2006-2009.
Gunnarsson M, Mattiasson A. Female stress, urge, and mixed urinary incontinence are associated with a chronic and progressive pelvic floor/vaginal neuromuscular disorder: An investigation of 317 healthy and incontinent women using vaginal surface electromyography. Neurourol Urodyn. 1999;18:613-621.
Haab F, Zimmern PE, Leach GE. Urinary stress incontinence due to intrinsic sphincteric deficiency: experience with fat and collagen periurethral injections. J Urol. 1997;157(4):1283-1286.
Hamid R, Arya M, Khastgir J, et al. The treatment of male stress urinary incontinence with polydimethylsiloxane in compliant bladders following spinal cord injury. Spinal Cord. 2003;41(5):286-289.
Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997;157(1):233-236.
Harper C. Permacol: clinical experience with a new biomaterial. Hosp Med. 2001;62(2):90-95.
Harrison SC, Brown C, O’Boyle PJ. Periurethral Teflon for stress urinary incontinence: medium-term results. Br J Urol. 1993;71:25-27.
Harriss DR, Iacovou JW, Lemberger RJ. Peri-urethral silicone microimplants (Macroplastique) for the treatment of genuine stress incontinence. Br J Urol. 1996;78(5):722-725. discussion 726–8
Henalla SM, Hall V, Duckett JR, et al. A multicentre evaluation of a new surgical technique for urethral bulking in the treatment of genuine stress incontinence. Br J Obstet Gynaecol. 2000;107(8):1035-1039.
Henly DR, Barrett DM, Weiland TL, et al. Particulate silicone for use in periurethral injections: local tissue effects and search for migration. J Urol. 1995;153(6):2039-2043.
Herschorn S, Bruschini H, Comiter C, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29(1):179-190.
Herschorn S, Glazer AA. Early experience with small volume periurethral polytetrafluoroethylene for female stress urinary incontinence. J Urol. 2000;163:1838-1842.
Herschorn S, Radomski SB. Collagen injections for genuine stress urinary incontinence: patient selection and durability. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(1):18-24.
Herschorn S, Radomski SB, Steele DJ. Early experience with intraurethral collagen injections for urinary incontinence. J Urol. 1992;148(6):1797-1800.
Herschorn S, Steele DJ, Radomski SB. Followup of intraurethral collagen for female stress urinary incontinence. J Urol. 1996;156(4):1305-1309.
Hilton P. Urethrovaginal fistula associated with “sterile abscess” formation following periurethral injection of dextranomer/hyaluronic acid co-polymer (Zuidex) for the treatment of stress urinary incontinence—a case report. Br J Obstet Gynaecol. 2009;116(11):1527-1530.
Hollenbeck BK, Lipp ER, Hayward RA, et al. Concurrent assessment of obstructive/irritative urinary symptoms and incontinence after radical prostatectomy. Urology. 2002;59(3):389-393.
Homma Y, Kawabe K, Kageyama S, et al. Injection of glutaraldehyde cross-linked collagen for urinary incontinence: two-year efficacy by self-assessment. Int J Urol. 1996;3(2):124-127.
Horl HW, Feller AM, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26(3):248-258.
Hosker G, Rosier P, Gajewski J, et al. Dynamic testing. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:413-522.
Huang WC, Yang JM. Bladder neck funneling on ultrasound cystourethrography in primary stress urinary incontinence: a sign associated with urethral hypermobility and intrinsic sphincter deficiency. Urology. 2003;61:936-941.
Hubner W. Adjustable Continence Therapy (ACT) for male post prostatectomy stress incontinence. Rio de Janeiro, Brazil: Brazilian Congress of Urology; 2000.
Hubner WA, Schlarp OM. Treatment of incontinence after prostatectomy using a new minimally invasive device: adjustable continence therapy. BJU Int. 2005;96(4):587-594.
Hubner WA, Schlarp OM. Adjustable continence therapy (ProACT): evolution of the surgical technique and comparison of the original 50 patients with the most recent 50 patients at a single centre. Eur Urol. 2007;52(3):680-686.
Hurtado E, McCrery R, Appell R. The safety and efficacy of ethylene vinyl alcohol copolymer as an intra-urethral bulking agent in women with intrinsic urethral deficiency. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:869-873.
Hurtado E, McCrery R, Appell R. Complications of ethylene vinyl alcohol copolymer as an intraurethral bulking agent in men with stress urinary incontinence. Urology. 2008;71(4):662-665.
Imamoglu MA, Tuygun C, Bakirtas H, et al. The comparison of artificial urinary sphincter implantation and endourethral Macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol. 2005;47(2):209-213.
Ismael SS, Amarenco G, Bayle B, Kerdraon J. Postpartum lumbosacral plexopathy limited to autonomic and perineal manifestations: clinical and electrophysiological study of 19 patients. J Neurol Neurosurg Psychiatry. 2000;68:771-773.
Izes JK, Bihrle W3rd, Bihrle R. Ileocecal valve resistance augmentation using glutaraldehyde cross-linked collagen: a canine model for endoscopic salvage of the leaking Indiana reservoir. J Urol. 1997;158(4):1369-1371.
Kayigil O, Iftekhar Ahmed S, Metin A. The coexistence of intrinsic sphincter deficiency with type II stress incontinence. J Urol. 1999;162:1365-1366.
Keefe J, Wauk L, Chu S, et al. Clinical use of injectable bovine collagen: a decade of experience. Clin Mater. 1992;9(3–4):155-162.
Keegan PE, Atiemo K, Cody JD, et al. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2007;(3):10.1002/14651858.
Kelly HA, Dumm WM. Urinary incontinence in women without manifest injury to the bladder. Surg Gynecol Obstet. 1914;8:444-450.
Kershen RT, Atala A. New advances in injectable therapies for the treatment of incontinence and vesicoureteral reflux. Urol Clin North Am. 1999;26(1):81-94. viii
Khullar V, Cardozo LD, Abbott D, et al. GAX collagen in the treatment of urinary incontinence in elderly women: a two year follow up. Br J Obstet Gynaecol. 1997;104(1):96-99.
Kieswetter H, Fischer M, Wober L, et al. Endoscopic implantation of collagen (GAX) for the treatment of urinary incontinence. Br J Urol. 1992;69(1):22-25.
Kiilholma PJ, Chancellor MB, Makinen J, et al. Complications of Teflon injection for stress urinary incontinence. Neurourol Urodyn. 1993;12:131-137.
Kim YH, Kattan MW, Boone TB. Correlation of urodynamic results and urethral coaptation with success after transurethral collagen injection. Urology. 1997;50(6):941-948.
Kleinert S, Horton R. Retraction—autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: a [corrected] randomised controlled trial. Lancet. 2008;372(9641):789-790.
Klutke JJ, Subir C, Andriole G, et al. Long-term results after antegrade collagen injection for stress urinary incontinence following radical retropubic prostatectomy. Urology. 1999;53(5):974-977.
Kocjancic E, Crivellaro S, Ranzoni S, et al. Adjustable continence therapy for the treatment of male stress urinary incontinence: a single-centre study. Scand J Urol Nephrol. 2007;41(4):324-328.
Kocjancic E, Crivellaro S, Smith JJ3rd, et al. Adjustable continence therapy for treatment of recurrent female urinary incontinence. J Endourol. 2008;22(7):1403-1407.
Koelbl H, Nitti VW, Baessler K, et al. Pathophysiology of urinary incontinence, faecal incontinence, and pelvic organ prolapse. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:255-330.
Koelbl H, Saz V, Doerfler D, et al. Transurethral injection of silicone microimplants for intrinsic urethral sphincter deficiency. Obstet Gynecol. 1998;92(3):332-336.
Kuhn A, Stadlmayr W, Lengsfeld D, et al. Where should bulking agents for female urodynamic stress incontinence be injected? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):817-821.
Kuo HC. Videourodynamic analysis of the relationship of Valsalva and cough leak point pressures in women with stress urinary incontinence. Urology. 2003;61:544-548.
Kylmala T, Tainio H, Raitanen M, et al. Treatment of postoperative male urinary incontinence using transurethral Macroplastique injections. J Endourol. 2003;17(2):113-115.
Lebret T, Cour F, Benchetrit J, et al. Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology. 2008;71(2):256-260.
Lee PE, Kung RC, Drutz HP. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol. 2001;165(1):153-158.
Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of Durasphere. Urology. 2001;58(1):12-15.
Lightner D, Rovner E, Corcos J, et al. Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology. 2009;74(4):771-775.
Lim KB, Ball AJ, Feneley RC. Periurethral Teflon injection: a simple treatment for urinary incontinence. Br J Urol. 1983;55:208-210.
Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12(1):88-95.
Lloyd AW, Faragher RG, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22(8):769-785.
Lopez AE, Padron OF, Patsias G, Politano VA. Transurethral polytetrafluoroethylene injection in female patients with urinary continence. J Urol. 1993;150:856-858.
Lose G, Mouritsen L, Nielsen J. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006;98:100-104.
Lotenfoe R, O’Kelly JK, Helal M, Lockhart JL. Periurethral polytetrafluoroethylene paste injection in incontinent female subjects: surgical indications and improved surgical technique. J Urol. 1993;149:279-282.
Madjar S, Covington-Nichols C, Secrest CL. New periurethral bulking agent for stress urinary incontinence: modified technique and early results. J Urol. 2003;170(6 Pt 1):2327-2329.
Madjar S, Sharma AK, Waltzer WC, et al. Periurethral mass formations following bulking agent injection for the treatment of urinary incontinence. J Urol. 2006;175(4):1408-1410.
Malizia AAJr, Reiman HM, Myers RP, et al. Migration and granulomatous reaction after periurethral injection of polytef (Teflon). JAMA. 1984;251(24):3277-3281.
Martins FE, Bennett CJ, Dunn M, et al. Adverse prognostic features of collagen injection therapy for urinary incontinence following radical retropubic prostatectomy. J Urol. 1997;158(5):1745-1749.
Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69(5):876-880.
Mayer RD, Lightfoot M, Jung I. Preliminary evaluation of calcium hydroxylapatite as a transurethral bulking agent for stress urinary incontinence. Urology. 2001;57(3):434-438.
Mazouni C, Bladou F, Karsenty G, et al. Minimally invasive surgery for female urinary incontinence: experience with periurethral microballoon implantation. J Endourol. 2004;18(9):901-905. discussion 905
McClelland M, Delustro F. Evaluation of antibody class in response to bovine collagen treatment in patients with urinary incontinence. J Urol. 1996;155(6):2068-2073.
McGuire EJ, Appell RA. Transurethral collagen injection for urinary incontinence. Urology. 1994;43(4):413-415.
McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am. 1996;23:253-262.
McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol. 1993;150(5 Pt 1):1452-1454.
Mevorach RA, Hulbert WC, Rabinowitz R, et al. Results of a 2-year multicenter trial of endoscopic treatment of vesicoureteral reflux with synthetic calcium hydroxyapatite. J Urol. 2006;175(1):288-291.
Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39-52.
Mitterberger M, Marksteiner R, Margreiter E, et al. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol. 2008;179(1):226-231.
Mitterberger M, Pinggera GM, Marksteiner R, et al. Functional and histological changes after myoblast injections in the porcine rhabdosphincter. Eur Urol. 2007;52(6):1736-1743.
Mittleman RE, Marraccini JV. Pulmonary Teflon granulomas following periurethral Teflon injection for urinary incontinence. Arch Pathol Lab Med. 1983;107:611-612.
Monga AK, Robinson D, Stanton SL. Periurethral collagen injections for genuine stress incontinence: a 2-year follow-up. Br J Urol. 1995;76(2):156-160.
Moore KN, Chetner MP, Metcalfe JB, et al. Periurethral implantation of glutaraldehyde cross-linked collagen (Contigen) in women with type I or III stress incontinence: quantitative outcome measures. Br J Urol. 1995;75(3):359-363.
Mouritsen L, Berild G, Hertz J. Comparison of different methods for quantification of urinary leakage in incontinent women. Neurourol Urodyn. 1989;8:579-587.
Murless BC. The injection treatment of stress incontinence. J Obstet Gynaecol Br Emp. 1938;45:67-73.
National Institute for Health and Clinical Excellence. Urinary incontinence: the management of urinary incontinence in women. http://www.nice.org.uk/nicemedia/pdf/CG40fullguideline.pdf. accessed 15.10.09
Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607-620.
O’Connell HE, McGuire EJ, Aboseif S, et al. Transurethral collagen therapy in women. J Urol. 1995;154(4):1463-1465.
Onur R, Singla A. Comparison of bone-anchored male sling and collagen implant for the treatment of male incontinence. Int J Urol. 2006;13(9):1207-1211.
Palma PC, Riccetto CL, Herrmann V, et al. Repeated lipoinjections for stress urinary incontinence. J Endourol. 1997;11(1):67-70.
Palma PC, Riccetto CL, Netto Junior NR. Urethral pseudolipoma: a complication of periurethral lipo-injection for stress urinary incontinence in a woman. J Urol. 1996;155(2):646.
Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;166(4):1350-1353.
Parekh AR, Feng MI, Kirages D, et al. The role of pelvic floor exercises on post-prostatectomy incontinence. J Urol. 2003;170(1):130-133.
Payne C, Brown J, Castro D, et al. Research. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:1713-1766.
Peeker R, Edlund C, Wennberg AL, et al. The treatment of sphincter incontinence with periurethral silicone implants (Macroplastique). Scand J Urol Nephrol. 2002;36(3):194-198.
Perucchini D, DeLancey JO, Ashton-Miller JA, et al. Age effects on urethral striated muscle. II. Anatomic location of muscle loss. Am J Obstet Gynecol. 2002;186:356-360.
Petrou SP, Pak RW, Lightner DJ. Simple aspiration technique to address voiding dysfunction associated with transurethral injection of dextranomer/hyaluronic acid copolymer. Urology. 2006;68(1):186-188.
Plotti F, Zullo MA, Sansone M, et al. Post–radical hysterectomy urinary incontinence: a prospective study of transurethral bulking agents injection. Gynecol Oncol. 2009;112(1):90-94.
Politano VA. Periurethral polytetrafluoroethylene injection for urinary incontinence. J Urol. 1982;127:439-442.
Politano VA, Small MP, Harper JM, et al. Periurethral Teflon injection for urinary incontinence. Trans Am Assoc Genitourin Surg. 1973;65:54-57.
Pruthi RS, Petrus CD, Bundrick WJ. New onset vesicovaginal fistula after transurethral collagen injection in women who underwent cystectomy and orthotopic neobladder creation: presentation and definitive treatment. J Urol. 2000;164(5):1638-1639.
Pycha A, Klingler CH, Haitel A, et al. Implantable microballoons: an attractive alternative in the management of intrinsic sphincter deficiency. Eur Urol. 1998;33(5):469-475.
Quackels R. Deux incontinences après adénectomie guéries par injection de paraffine dans la périnée. Acta Urol Belg. 1955;23:259-261.
Radley SC, Chapple CR, Mitsogiannis IC, et al. Transurethral implantation of Macroplastique for the treatment of female stress urinary incontinence secondary to urethral sphincter deficiency. Eur Urol. 2001;39(4):383-389.
Remacle MJ, Bertrand B, Eloy P, et al. The use of injectable collagen to correct velopharyngeal insufficiency. Laryngoscope. 1990;100(3):269-274.
Remacle MJ, Declaye XJ. Gax-collagen injection to correct an enlarged tracheoesophageal fistula for a vocal prosthesis. Laryngoscope. 1988;98(12):1350-1352.
Richardson TD, Kennelly MJ, Faerber GJ. Endoscopic injection of glutaraldehyde cross-linked collagen for the treatment of intrinsic sphincter deficiency in women. Urology. 1995;46(3):378-381.
Rosenzweig BA, Bhatia NN, Nelson AL. Dynamic urethral pressure profilometry pressure transmission ratio: what do the numbers really mean? Obstet Gynecol. 1991;77:586-590.
Sachse H. [Treatment of urinary incontinence with sclerosing solutions: indications, results, complications]. Urol Int. 1963;15:225-244.
Sanchez-Ortiz RF, Broderick GA, Chaikin DC, et al. Collagen injection therapy for post–radical retropubic prostatectomy incontinence: role of Valsalva leak point pressure. J Urol. 1997;158(6):2132-2136.
Santarosa RP, Blaivas JG. Periurethral injection of autologous fat for the treatment of sphincteric incontinence. J Urol. 1994;151(3):607-611.
Schick E. Objective assessment of resistance of female urethra to stress: a scale to establish degree of urethral incompetence. Urology. 1985;26(5):518-526.
Schulman CC, Simon J, Wespes E, Germeau F. Endoscopic injections of Teflon to treat urinary incontinence in women. Br Med J (Clin Res Ed). 1984;288:192.
Schulz JA, Nager CW, Stanton SL, et al. Bulking agents for stress urinary incontinence: short-term results and complications in a randomized comparison of periurethral and transurethral injections. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(4):261-265.
Sheriff MK, Foley S, McFarlane J, et al. Endoscopic correction of intractable stress incontinence with silicone micro-implants. Eur Urol. 1997;32(3):284-288.
Shortliffe LM, Freiha FS, Kessler R, et al. Treatment of urinary incontinence by the periurethral implantation of glutaraldehyde cross-linked collagen. J Urol. 1989;141(3):538-541.
Smith AR, Dmochowski RR, Hilton P, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:1191-1272.
Smith DN, Appell RA, Rackley RR, et al. Collagen injection therapy for post-prostatectomy incontinence. J Urol. 1998;160(2):364-367.
Smith DN, Appell RA, Winters JC, et al. Collagen injection therapy for female intrinsic sphincteric deficiency. J Urol. 1997;157(4):1275-1278.
Smith JJ3rd, Swierzewski SJ3rd, Bihrle W3rd, et al. Endoscopic injection of glutaraldehyde cross-linked collagen for efferent limb incompetence in the Indiana reservoir. J Urol. 1998;159(3):804-805.
Sokol ER, Aguilar VC, Sung VW, et al. Combined trans- and periurethral injections of bulking agents for the treatment of intrinsic sphincter deficiency. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):643-647.
Soliman S, Evans C. Endoscopic Macroplastique™ injection for the treatment of female stress incontinence: role and efficacy. Afr J Urol. 2001;7(2):45-50.
Stamey TA. Urinary incontinence in the female. In: Harrison JH, Gittes RF, Stamey TA, et al, editors. Campbell’s urology. Philadelphia: WB Saunders; 1979:2272-2293.
Starer P, Libow LS. The measurement of residual urine in the evaluation of incontinent nursing home residents. Arch Gerontol Geriatr. 1988;7(1):75-81.
Stecco A, Saponaro A, Crivellaro S, et al. Can MRI predict which patients are most likely to benefit from percutaneous positioning of volume-adjustable balloon devices? Urol Int. 2006;76(3):240-246.
Steele AC, Kohli N, Karram MM. Periurethral collagen injection for stress incontinence with and without urethral hypermobility. Obstet Gynecol. 2000;95(3):327-331.
Stenberg A, Larsson E, Lindholm A, et al. Injectable dextranomer-based implant: histopathology, volume changes and DNA-analysis. Scand J Urol Nephrol. 1999;33(6):355-361.
Stenberg A, Larsson G, Johnson P. Urethral injection for stress urinary incontinence: long-term results with dextranomer/hyaluronic acid copolymer. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(5):335-338. discussion 338
Stothers L, Goldenberg SL. Delayed hypersensitivity and systemic arthralgia following transurethral collagen injection for stress urinary incontinence. J Urol. 1998;159(5):1507-1509.
Stothers L, Goldenberg SL, Leone EF. Complications of periurethral collagen injection for stress urinary incontinence. J Urol. 1998;159(3):806-807.
Strasser H, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet. 2007;369:2179-2186.
Stricker P, Haylen B. Injectable collagen for type 3 female stress incontinence: the first 50 Australian patients. Med J Aust. 1993;158(2):89-91.
Su TH, Wang KG, Hsu CY, et al. Periurethral fat injection in the treatment of recurrent genuine stress incontinence. J Urol. 1998;159(2):411-414.
Swami S, Batista JE, Abrams P. Collagen for female genuine stress incontinence after a minimum 2-year follow-up. Br J Urol. 1997;80(5):757-761.
Swash M, Snooks SJ, Henry MM. Unifying concept of pelvic floor disorders and incontinence. J R Soc Med. 1985;78:906-911.
Sweat SD, Lightner DJ. Complications of sterile abscess formation and pulmonary embolism following periurethral bulking agents. J Urol. 1999;161(1):93-96.
Takahashi S, Homma Y, Fujishiro T, et al. Electromyographic study of the striated urethral sphincter in type 3 stress incontinence: evidence of myogenic-dominant damages. Urology. 2000;56:946-950.
Tamanini JT, D’Ancona CA, Netto NRJr. Treatment of intrinsic sphincter deficiency using the Macroplastique implantation system: two-year follow-up. J Endourol. 2004;18(9):906-911.
Tamanini JT, D’Ancona CA, Tadini V, et al. Macroplastique implantation system for the treatment of female stress urinary incontinence. J Urol. 2003;169(6):2229-2233.
Tchetgen MB, Sanda MG, Montie JE, et al. Collagen injection for the treatment of incontinence after cystectomy and orthotopic neobladder reconstruction in women. J Urol. 2001;163:212. [abstract]
Tiguert R, Gheiler EL, Gudziak MR. Collagen injection in the management of post–radical prostatectomy intrinsic sphincteric deficiency. Neurourol Urodyn. 1999;18(6):653-658.
Trigo-Rocha F, Gomes CM, Pompeo AC, et al. Prospective study evaluating efficacy and safety of Adjustable Continence Therapy (ProACT) for post–radical prostatectomy urinary incontinence. Urology. 2006;67(5):965-969.
Trockman BA, Leach GE. Surgical treatment of intrinsic urethral dysfunction: injectables (fat). Urol Clin North Am. 1995;22(3):665-671.
Tubaro A, Artibani W, Bartram CI, et al. Imaging and other investigations. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence—4th international consultation. Paris: Health Publications; 2009:541-630.
Twiss C, Fleischmann N, Nitti VW. Correlation of abdominal leak point pressure with objective incontinence severity in men with post–radical prostatectomy stress incontinence. Neurourol Urodyn. 2005;24(3):207-210.
Tzikas TL. Evaluation of the Radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg. 2004;6(4):234-239.
Uroplasty, Inc. (Minnetonka, MN). Macroplastique® implants (MPQ-1.5 & MPQ-2.5): instructions for use. http://www.uroplasty.com/Uroplasty/view/files/pdf/4171.pdf.
Usman F, Henalla S. A single transurethral Macroplastique injection as primary treatment for stress incontinence in women. J Obstet Gynaecol. 1998;18(1):56-60.
Van Kampen M, De Weerdt W, Van Poppel H, et al. Prediction of urinary continence following radical prostatectomy. Urol Int. 1998;60(2):80-84.
Van Kampen M, De Weerdt W, Van Poppel H, et al. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355(9198):98-102.
van Kerrebroeck P, ter Meulen F, Larsson G, et al. Treatment of stress urinary incontinence using a copolymer system: impact on quality of life. BJU Int. 2004;94(7):1040-1043.
Vesey SG, Rivett A, O’Boyle PJ. Teflon injection in female stress incontinence. Effect on urethral pressure profile and flow rate. Br J Urol. 1988;62:39-41.
Wachter J, Henning A, Roehlich M, et al. Adjustable continence therapy for female urinary incontinence: a minimally invasive option for difficult cases. Urol Int. 2008;81(2):160-166.
Wainstein MA, Klutke CG. Antegrade techniques of collagen injection for post-prostatectomy stress urinary incontinence: the Washington University experience. World J Urol. 1997;15:310-315.
Walters MD, Karram MM. Enterocele. American Urogynecologic Society Quarterly Report, 1992, 10.
Weber AM. Is urethral pressure profilometry a useful diagnostic test for stress urinary incontinence? Obstet Gynecol Surv. 2001;56(11):720-735.
Wei JT, Dunn RL, Marcovich R, et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164(3 Pt 1):744-748.
Westney OL, Bevan-Thomas R, Palmer JL, et al. Transurethral collagen injections for male intrinsic sphincter deficiency: the University of Texas-Houston experience. J Urol. 2005;174(3):994-997.
Wille S, Sobottka A, Heidenreich A, et al. Pelvic floor exercises, electrical stimulation and biofeedback after radical prostatectomy: results of a prospective randomized trial. J Urol. 2003;170(2 Pt 1):490-493.
Wilson S, Quek ML, Ginsberg DA. Transurethral injection of bulking agents for stress urinary incontinence following orthotopic neobladder reconstruction in women. J Urol. 2004;172(1):244-246.
Winters JC, Appell R. Periurethral injection of collagen in the treatment of intrinsic sphincteric deficiency in the female patient. Urol Clin North Am. 1995;22(3):673-678.
Wolf JS, Bennett CJ, Dmochowski R, et al. Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/antimicroprop08.pdf, 2008.
Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000;18(1):56-61.
Yokoyama T, Yoshimura N, Dhir R, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165(1):271-276.
Yoo JJ, Magliochetti M, Atala A. Detachable self-sealing membrane system for the endoscopic treatment of incontinence. J Urol. 1997;158(3 Pt 2):1045-1048.
Zacharin RF. The suspensory mechanism of the female urethra. J Anat. 1963;97:423-427.