chapter 64 Evaluation of Patients with Urinary Incontinence and Pelvic Prolapse
Definition and Impact of Pelvic Floor Disorders
Pelvic floor disorders (PFDs), which include urinary incontinence, fecal incontinence, and pelvic organ prolapse (POP), pose a prevalent worldwide health concern. (For the purposes of this chapter, “incontinence” will refer to urinary leakage unless otherwise specified.) On the basis of 2001-2004 data from the National Health and Nutrition Examination Survey (NHANES), an overall prevalence of incontinence of 49.6% in 4229 women older than age 20 was reported. Of those who reported incontinence, 49.8%, 15.9%, and 34.3% reported pure stress urinary incontinence (SUI), pure urgency incontinence (UI), and mixed incontinence, respectively (Dooley et al, 2008). Interestingly, there appears to be a racial disparity with regard to pure SUI and pure UI with the odds of pure SUI being at least 2.5-fold higher in white and Mexican-American women than black women (Dooley et al, 2008; Fenner et al, 2008). The NHANES data from 2004-2005 showed that 23.7% of 1961 women described at least one PFD, and the proportion of women who reported a PFD increased with increasing age and body mass index (BMI) (Nygaard et al, 2008).
The overall prevalence of incontinence in community-dwelling men is 17% and varies with age, race, and socioeconomic status (Abrams et al, 2005; Anger et al, 2006). NHANES data revealed an 11% prevalence in those aged 60 to 64 and 31% in men 85 years or older. Daily incontinence occurs in 42% and weekly incontinence in 24%, with only 22% of those who leaked weekly seeking medical care for the problem (Harris et al, 2007). Unlike in women, there is a prevalence of urgency incontinence (40% to 80%) as compared with mixed incontinence (10% to 30%) or pure SUI (<10%) in men. Overall prevalence is higher in African-American men (21%) than in white men (16%).
Risk factors for development of POP include advanced age, increased parity (vaginal childbirth), genetic predisposition (Twiss et al, 2007), estrogen deficiency, compromised pelvic floor muscle strength, and smoking (Miedel et al, 2009; Samuelsson et al, 2000). Interestingly, recent studies indicate that current or past use of exogenous hormones in the form of oral contraception or hormone replacement therapy may paradoxically serve as a risk factor for the development of urinary incontinence (Samuelsson et al, 2000; Townsend et al, 2009). In men, comorbidities, poor general health, cognitive impairment, stroke, radiation, urinary tract infections, prostate diseases, and diabetes are all associated with incontinence (Shamliyan et al, 2009).
One questionnaire study of 4103 women, conducted in a large managed health care plan, found the prevalence of POP to be 6% (Lawrence et al, 2008). This and other studies have evaluated and found a high co-occurrence of PFDs. Lawrence and colleagues (2008) reported a co-occurrence of at least one additional PFD in approximately 80% of women with SUI or overactive bladder (OAB), 69% of women with POP, and 48% of women with anal incontinence. On the basis of the 2005-2006 NHANES data, Nygaard and colleagues (2008) found that the weighted prevalence of at least one PFD was 23.7%, increasing incrementally with age and parity. Although the prevalence of at least one PFD was 9.7% and 49.7% in women between ages 20 and 39 and those aged 80 or older, respectively, the prevalence was 12.8%, 18.4%, 24.6%, and 32.4% for those who had zero, one, two, and three or more deliveries, respectively. De Boer and colleagues (2010) reported a higher prevalence of OAB in patients with POP than in those without POP, although there was no relationship noted to exist between the grade and stage of POP and the presence of OAB symptoms. Wu and colleagues (2009) used U.S. Census Bureau population projections to forecast the change in PFD prevalence in women between 2010 and 2050. The current estimate of 28.1 million women with at least one PFD in 2010 is projected to increase substantially to 43.8 million in 2050.
The impact of PFDs is far-reaching, carrying a significant potential to affect patient quality of life (QoL), notwithstanding the psychologic burden it produces. Additionally, incontinence creates a tremendous cost to the individual and to society. Hu and colleagues (2004) estimated that the evaluation and management of incontinence and productivity lost due to the condition resulted in a $19.5 billion (year 2000 dollars) cost to society, although sensitivity analysis suggested a potential cost range of between $9.32 and $28 billion. Contemporary numbers might predictably be higher in 2009, although Hu’s most recent analysis demonstrated a 26% cost decrease compared with the 1995 report, which estimated $26.29 billion. The decrease was speculated to be due to various factors including decreased hospital stays and adjusted methods of assessing nursing home stays, routine care product use, and prevalence data. Other reports have demonstrated that medical expenditures for incontinence in the female Medicare population nearly doubled between 1992 and 1998, due primarily to increased outpatient expenditure from 9.1% to 27.3% of total Medicare costs in approximately the same time frame (Thom et al, 2005; Anger et al, 2006).
As a result of increasing awareness of the societal impact of PFDs in addition to the growing emphasis on maximizing QoL in our aging population, tremendous research efforts are under way to improve our understanding of the pathophysiology of these disorders, thereby improving both diagnostic and therapeutic techniques. The importance of evidence-based medicine and meticulous follow-up of patients is driving improvement in the science on which advancements in this subspecialty of urology are being made.
Diagnostic Evaluation
General Considerations
A recent upsurge in research efforts has resulted in the emergence of new diagnostic and therapeutic techniques to address PFDs. As QoL impact has become a focus, many current research efforts include detailed QoL assessment and attempts to quantify and assess the relationship between PFDs and their effects on QoL. Correlating the bother caused by a given PFD with the risk of available therapies is an important consideration.
The type of incontinence affecting an individual must be defined and quantified in order to guide proper treatment planning. Transient or unrelated conditions that can cause leakage should be identified before proceeding with definitive therapy. Table 64–1 contains a mnemonic of transient causes of incontinence (Resnick, 1984). Table 64–2 lists current International Continence Society (ICS) nomenclature and definitions for types of incontinence (Abrams et al, 2002, 2009b). The terminology continues to adjust to reflect the evolving understanding of the condition. The importance of this flexibility has been realized and acknowledged by leaders in the subspecialty of pelvic floor medicine (Chapple, 2009).
Table 64–1 Causes of Transient Incontinence (DIAPPERS)
From Resnick NM. Urinary incontinence in the elderly. Med Grand Rounds 1984;3:281–90.
Table 64–2 Standard International Continence Society Terminology of Lower Urinary Tract Symptoms Pertaining to Continence
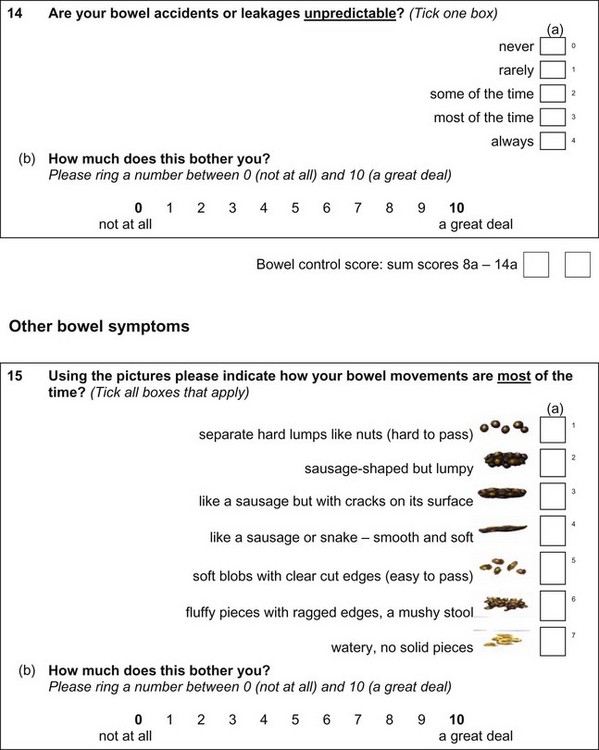
| TERMINOLOGY | DESCRIPTION |
|---|---|
| Urinary incontinence | The complaint of any involuntary leakage of urine |
| Enuresis | Any involuntary loss of urine (should be specified if it is nocturnal) |
| Urgency | The complaint of a sudden compelling desire to pass urine that is difficult to defer |
| Urgency incontinence | The complaint of involuntary leakage accompanied by or immediately preceded by urgency |
| Stress urinary incontinence | The complaint of involuntary leakage on effort or exertion, or on sneezing or coughing |
| Mixed incontinence | The complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing, or coughing |
| Continuous urinary incontinence | The complaint of continuous leakage |
| Other types of urinary incontinence | Situational incontinence (e.g., during sexual intercourse or giggle incontinence) |
| Dependent continence | Situation in which the individual would suffer a recurrence of incontinence if management was withdrawn |
| Contained incontinence | Situation in which incontinence is controlled by being contained in an absorbent product or collecting device (previously “social continence”) |
| Overactive bladder | Urgency with or without urgency incontinence usually with increased daytime frequency and nocturia |
Data from Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn 2002;21:167–78 (reprinted in Urology 2003;61:37–49).
The classification of POP is categorized according to the affected compartment. Simply put, anterior compartment prolapse (cystocele) generally involves descent of the bladder toward the vaginal lumen, posterior prolapse (rectocele) involves the rectum compressing the posterior vaginal wall into the vagina, and apical prolapse is associated with descent of the uterus (uterine procidentia) and/or the bowel (enterocele) at the top of the vagina. Several grading systems exist to quantify the severity of POP and are discussed later and illustrated in Figure 64–1.
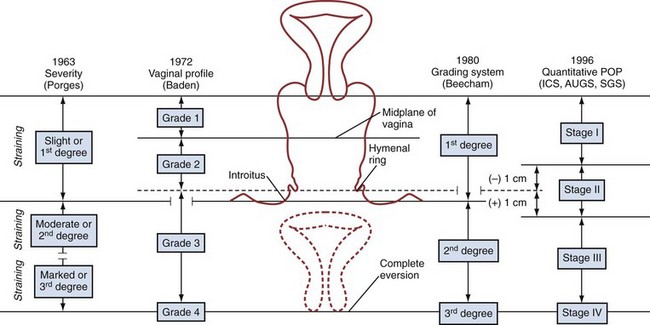
Figure 64–1 Visual comparison of systems used to quantify pelvic organ prolapse
(From Theofrastous JP, Swift SE. The clinical evaluation of pelvic floor dysfunction. Obstet Gynecol Clin North Am 1998;25:783–804.)
Regarding incontinence specifically, the substantiation of a proper diagnosis requires direct observation of urinary leakage by the clinician (Nitti and Blaivas, 2007). It is the belief of many experts that no patient should undergo invasive or irreversible therapies without definitive establishment of the cause of their incontinence. Complete and extensive evaluation can facilitate accurate diagnosis of PFDs to promote optimal treatment planning and counseling of patients.
Key Points: General Considerations
History
A careful history should always be obtained from the patient. However, several studies have indicated that patient history alone is not completely accurate as the sole determinant of incontinence type (Summitt et al, 1992; Jensen et al, 1994). Bates and colleagues (1973) are credited with the dictum, “The bladder is an unreliable witness,” which has been corroborated by many investigators in various forms. Accordingly, all available information including that which is obtained by supplementary examinations should be integrated into the diagnosis.
History of Present Illness
A thorough history is imperative in the evaluation of incontinence. Several queries should be included in a continence and pelvic floor history in order to best portray the patient’s symptoms (Holroyd-Leduc et al, 2008). The incontinence should first be characterized subjectively. Does the leakage occur (1) with physical activity? (2) with a sense of urgency? or (3) without sensory awareness? If the nature of the incontinence is mixed, does one component cause more bother or occur more frequently than the other? Second, the leakage should be quantified if possible. Appraisal of the degree of leakage before therapy can be helpful during postoperative assessment of treatment impact. For the purposes of routine outpatient assessment, this quantification can be achieved on the basis of the number of pads used per day or the frequency of clothing changes due to urinary leakage. In the setting of research or an academic practice, more stringent and objective measures such as pad weight testing are often used (see “Supplemental Evaluation” later). Third, the voiding pattern should be defined. What is the frequency of urination during the day? During the night? Are there any obstructive symptoms? Does the patient feel as though he or she empties the bladder completely? Is the stream strong or does it “trickle”? Does the stream fluctuate during the void? Is it necessary to push or strain or change posture to void or empty the bladder? Fourth, establishment of the duration of symptoms and any inciting events that contributed to the onset of leakage is important. Did the leakage follow a pregnancy or a vaginal delivery? How long ago? Did the leakage start after a strain, a fall, or trauma? Has the patient undergone pelvic or back surgery? In males, has there been any prostate or urethral surgery for benign or malignant disease? Has there been any lower urinary tract instrumentation? Are there any accompanying neurologic symptoms such as numbness or tingling in the extremities, blurry/double vision, balance or coordination changes, or tremor? Finally, it is helpful to determine the impact that the leakage has on the patient’s daily life and activities. Does the incontinence limit the individual’s activity? Has the patient changed his or her lifestyle due to the threat of leakage?
Regarding pelvic prolapse specifically, important questions focus on whether or not the patient is aware of any prolapse and what, if any, symptomatology and bother the prolapse may be causing. Does the patient feel that anything is falling down out of place in the vagina? Does she need to reduce the prolapse for comfort? Or empty her bladder completely? Or facilitate evacuation of her bowels?
Past medical and surgical histories are vital to the assessment of incontinence insofar as medical conditions and surgeries can affect urinary tract function. Childhood and adult urologic history should be obtained, as should a neurologic history. Neurologic conditions such as Parkinson disease, multiple sclerosis, stroke, spinal cord injury, back surgery, and myelodysplasia can have a considerable impact on lower urinary tract (LUT) function. Medical diagnoses such as diabetes mellitus and dementia can affect continence. Similarly, a history of radiation therapy or neurologic or urologic trauma can affect LUT function, specifically with regard to outlet resistance and/or bladder contractility, stability, and compliance. Although outlet resistance may be compromised by trauma or LUT surgery, urethral strictures related to trauma or neurologic dysfunction that abnormally increase outlet resistance during voiding can cause obstruction and secondary symptoms related to the obstruction.
In women the gynecologic and obstetric history including gravity, parity, and hormonal status is important. Determination of whether the patient is premenopausal, perimenopausal, or postmenopausal, and whether or not she has used any exogenous hormones such as oral contraceptives or local or systemic hormone replacement therapy can be helpful in her overall assessment. As mentioned earlier, although beneficial effects of local hormone replacement therapy are well-established, there have been reports that exogenous hormone therapy can actually increase the risk of SUI (Townsend et al, 2009).
Clearly, previous pelvic surgery can affect LUT function. Anti-incontinence surgery, POP repair, and hysterectomy can contribute to a variety of urinary symptoms in women. Similarly, a history of prostate surgery can give rise to voiding or leakage complaints in men. Abdominoperineal resection can result in neurologic injury that can affect the function of either the bladder or the sphincter (Petrelli et al, 1993), and back surgery can cause a variety of symptoms dependent on the level affected.
Medications
An accurate assessment of medications is critical, particularly in the elderly patient population in whom polypharmacy is common. Many agents can affect urine production, LUT function, and mental status, all of which can have an impact on continence. Special attention should focus on agents that can affect bladder/sphincteric function. Table 64–3 categorizes some commonly used classes of medications by mechanism of action and potential effect on the LUT.
Table 64–3 Pharmacologic Agents That Can Affect the Lower Urinary Tract
| PHARMACOLOGIC EFFECTS | POTENTIAL EFFECTS ON URINARY TRACT |
|---|---|
| Sympathomimetics | Can increase outlet resistance and exacerbate obstructive symptoms/overactive bladder symptoms |
| Can decrease detrusor contractility and precipitate retention | |
| Sympatholytics | Can decrease outlet resistance and exacerbate stress incontinence |
| Anticholinergics | Can contribute to urinary retention, particularly in patients with outlet obstruction |
| Diuretics | Do not affect bladder directly, but because of increased urine production, can aggravate incontinence problems |
Other
As genetics can influence connective tissue integrity, it stands to reason that there may be a potential hereditary role in continence and POP (Twiss et al, 2007). Therefore inquiry about family history of POP may be helpful. Additionally, a thorough review of systems may reveal symptoms that suggest other conditions that could affect pelvic floor function.
Male incontinence, also a prevalent health issue, should be assessed in much the same way as female incontinence, although specific consideration of the impact of the anatomy specific to the male should be considered. Benign prostatic hyperplasia, the evaluation of which is covered in detail in Chapter 92, can cause secondary urgency and urgency incontinence in addition to more “typical” obstructive symptoms such as a decreased force of stream, urinary hesitancy, intermittency, and incomplete bladder emptying. Prostate surgery for benign or malignant disease can contribute to SUI. With this in mind, full assessment of male LUT symptoms (LUTS) should be performed to facilitate proper treatment planning.
Key Points: Evaluation—History
Physical Examination
The general appearance of a patient including details such as age, gait, stature, and fragility can provide important information regarding performance status, neurologic status, and other factors that may direct proper treatment planning. Similarly, an abdominal examination evaluating for incisions, hernias, organomegaly or bladder distention, and habitus is important, particularly if any abdominal surgery may be considered.
Per Medicare coding guidelines (Centers for Medicare/Medicaid, 1997), a female pelvic examination includes at least 7 of the 11 bullet items listed in Table 64–4. The external genitalia should be evaluated with regard to general appearance, estrogen status, lesions, and labial size and adhesions. Estrogen status can be evaluated on the basis of the presence or absence of a urethral caruncle, urethral prolapse, and/or labial adhesions, all of which, if present, may indicate estrogen deficiency. Likewise, attention to the overall tissue appearance and color is important. Hormonally deficient vaginal tissue has a pale, flat, dry appearance with no rugae, as opposed to the healthy, pink rugae of well-estrogenized tissue.
Table 64–4 Components of a Focused Pelvic Examination
|
|
At press time, 7 of 11 bullet points listed above are required to be considered a complete female genitourinary examination. However, other organ systems/body areas not limited to the genitourinary system may be included in a report to accomplish the requirements of various levels of examination.
Data from Centers for Medicare/Medicaid (CMS): Single organ system examination—Genitourinary: 1997 Documentation Guidelines for Evaluation and Management (E/M) Services, jointly approved by the American Medical Association and HCFA with revisions November, 1997.
Urethral position and mobility should be assessed at rest and with straining and coughing. The Q-tip test was developed to objectify the evaluation of urethral mobility (Bergman and Bhatia, 1987; Walters and Diaz, 1987). The discomfort caused to the patient during insertion of the Q-tip can be minimized with the use of intraurethral lidocaine jelly. The Q-tip is inserted into the bladder through the urethra, and the angle that the Q-tip moves from horizontal to its final position with straining is measured. Hypermobility is defined as a Q-tip angle of greater than 30 degrees from horizontal.
Assessment of prolapse should ideally be performed in both the lithotomy and standing positions, the latter facilitated by having the patient stand with one foot elevated on a short stool. Each compartment—the anterior, posterior, and apical (uterus/cervix or vaginal cuff)—should be evaluated methodically and the perineal body assessed for laxity. A complete systematic examination is performed using two posterior blades of a split Grave speculum with and without straining. First, one blade is used to retract the posterior wall to facilitate anterior compartment examination. The blade is then repositioned to retract anteriorly for examination of the posterior compartment. Finally, both blades are inserted simultaneously, one anteriorly and one posteriorly, to isolate the vaginal apex and facilitate examination of the cervical or cuff support. The posterior blade is slowly withdrawn to examine the posterior wall. Next, with the posterior blade in place, the patient is asked to strain. Foreshortening of the posterior wall causes expulsion of the blade and suggests a compromise in the level I support (Delancey, 1992) (cardinal-uterosacral ligament complex) of the vault; if the blade remains in place, this could represent an isolated rectocele or enterocele without vault prolapse. Evaluation for occult SUI should be performed with the anterior wall supported. SUI can be masked if significant prolapse “kinks” the urethra and outlet.
Several classification systems are used to quantify POP, the most widely used of which are the Baden-Walker classification (Baden et al, 1968) and the Pelvic Organ Prolapse Quantification system, known as the “POP-Q” (Bump et al, 1996). The two systems are juxtaposed in Figure 64–1. In the POP-Q system, which was created in an effort to provide objectivity to POP quantification, nine specific points of measurement are obtained in relation to the hymenal ring as illustrated in Figure 64–2A and B. Six vaginal points labeled Aa, Ba, C, D, Ap, and Bp are measured during Valsalva maneuver. Points above the hymen are considered negative and points below the hymen are positive. The genital hiatus (gh) represents the size of the vaginal opening, and the perineal body (pb) represents the distance between the vagina and the anus. The total vaginal length (tvl) is measured by reducing the prolapse and measuring the depth of the vagina. Table 64–5 contains the POP-Q staging criteria, a simplified presentation of the POP-Q system, and Figure 64–3 illustrates an example of the application of the system.
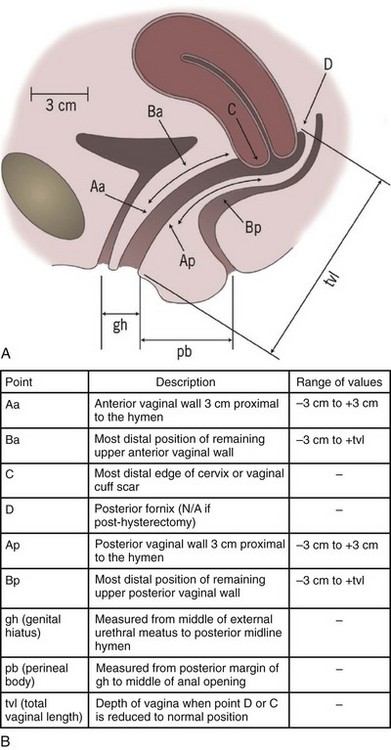
Figure 64–2 A, Landmarks for the POP-Q system. B, POP-Q Points of Reference.
(A, From Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–7.)
Table 64–5 POP-Q Staging Criteria
| STAGE | CRITERIA |
|---|---|
| 0 | Aa, Ap, Ba, Bp at −3 cm and C or D ≤ −(tvl − 2) cm |
| I | Stage 0 criteria not met and leading edge < −1 cm |
| II | Leading edge ≥ −1 cm but ≤ +1 cm |
| III | Leading edge > +1 cm but < +(tvl − 2) cm |
| IV | Leading edge ≥ +(tvl − 2) cm |
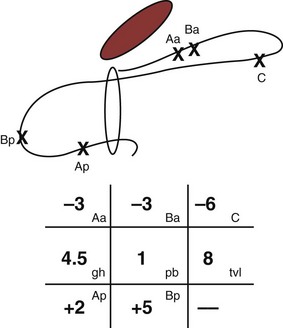
Figure 64–3 Line drawing example of posterior support defect. The anterior compartment is well supported. The leading point of the prolapse is point Bp (+5), which is 5 cm beyond the hymen. Total vaginal length is 8 cm, and point C (−6), the cuff position, has descended 2 cm.
(From Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10–7.)
A neurologic examination is important in any patient with a known or suspected neurologic condition. Attention to the patient’s gait, speech, cognitive status, facial symmetry, sensation in the lower extremities, perineal and perianal regions, lower extremity motor strength, and vaginal and pelvic floor strength can provide helpful information. Mobility and cognitive status can play a role in urinary continence insofar as both can affect a patient’s ability to reach the facilities in a timely fashion. The bulbocavernosus reflex (BCR), which is representative of sacral nerve root levels 2-4 (S2-4), is present in 70% of normal females and 100% of normal males. The BCR is considered positive when squeezing of the glans penis or clitoris results in anal and pelvic floor contraction that can be detected visually or by rectal examination. Alternatively, applying traction to an indwelling Foley catheter to pull the balloon against bladder neck should also precipitate a BCR.
A digital rectal examination (DRE) is important in men to assess the prostate for size, nodularity, or tenderness. In the female, the DRE can facilitate assessment of the rectovaginal septum. Demonstration of a rectocele can be facilitated via anterior pressure applied by a finger placed in the rectum. Anal sphincter tone, which is a reflection of the function at S2-4, is particularly important in neurologic patients with PFD. Patients are asked to voluntarily tighten the pelvic floor as if attempting to stop the flow of urine midstream. Laxity in the rectal sphincter tone may suggest a possible neurologic defect, but it may also be due to patient lack of understanding regarding how to voluntarily control the specific muscle groups necessary for contraction.
In men, genitourinary examination as it pertains to voiding function should also include evaluation of the penis for meatal stenosis and, particularly in the post-prostatectomy patient, visible urinary leakage with coughing and straining. Examination for leakage is ideally performed with the patient in the standing position.
Key Points: Evaluation—Physical Examination
Supplemental Evaluation
A variety of measures are available to supplement the history and physical examination in patients with PFDs. Instruments exist to quantify symptoms, their effects on QoL, and the degree of bother experienced by patients with PFDs. Most experts concur that a urinalysis and postvoid residual measurement should be considered in the majority of patients undergoing evaluation for incontinence. However, beyond this, there are no universally agreed-upon guidelines regarding the roles of other studies, such as endoscopy, urodynamics (UDS), and various modalities of radiographic imaging. In circumstances in which further investigation is considered, the value and accuracy (sensitivity and specificity) of the information provided by the given assessment method should be considered in relation to the cost and morbidity of the examination.
Symptom Quantification Instruments
Voiding Diaries
Instruments such as voiding diaries, questionnaires, and pad tests have been developed to aid in the quantification of urinary loss, both symptomatically and volumetrically. Voiding diaries can provide both diagnostic and therapeutic advantages. The use of diaries often helps patients realize their pattern of urination and is more accurate than recall (Larsson et al, 1984; McCormack et al, 1992; Siltberg et al, 1997). Furthermore, the diary can provide patients with insights into those behaviors that can be altered to decrease urinary frequency (Burgio, 2004).
Several studies have demonstrated the adjunctive role that diaries can have in the diagnosis and management of incontinence. Diaries can be helpful over routine subjective history because patient recall is often not as accurate as a formal voiding diary in ascertaining urinary frequency. In a retrospective review of 601 patients who underwent sling surgery and completed bladder diaries, only 47% were accurate about their daytime frequency; 51% overestimated their diurnal frequency, and this overestimation was exaggerated in those who reported voiding more than 10 times a day (Stav et al, 2009). Overestimation rates were similar between patients with and without overactive bladder (OAB) symptoms. Interestingly, 93% of women in this study were accurate about their nighttime frequency. In another study of women with UI, it was noted that the overestimation of incontinence episode frequency occurred more often in those patients who were more bothered by their incontinence. Conversely, Ku and colleagues (2004) found a poor correlation between subjective nocturnal frequency and that noted by 164 patients on their frequency volume charts. Wyman and colleagues (1987) similarly showed a higher correlation of diary-reported frequency in the daytime versus the night.
Martin and colleagues (2006) performed a meta-analysis of 121/6099 papers that compared two or more diagnostic techniques for incontinence and showed that diaries are most cost-effective when used in conjunction with history, particularly in patients undergoing treatment for detrusor overactivity (DO). It should be noted, however, that diaries should not substitute for more formal studies in select patients. One study that retrospectively assessed the Larsson frequency/volume chart as compared with the cystometrogram in 216 patients demonstrated the sensitivity and specificity of the chart with regard to DO to be 52% and 70%, respectively, and with regard to SUI, 66% and 65%, respectively (Tincello and Richmond, 1998). For daily clinical practice, 24-hour diaries should suffice to obtain valuable clinical information regarding LUT function; in academic studies, longer diaries may be requested, but this should be balanced with the well-established knowledge that the more complex a given instrument is (i.e., the more data requested), the lower patient compliance will be in completing it (Groutz et al, 2000).
Questionnaires and Quality of Life Instruments
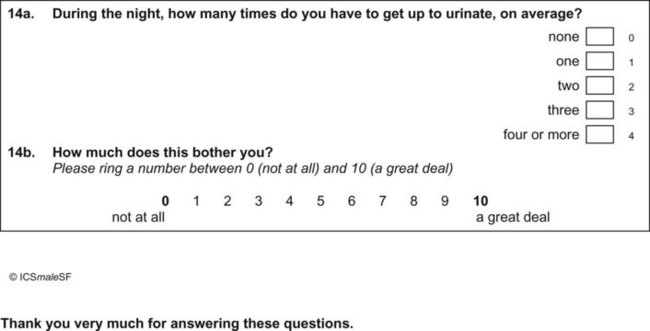
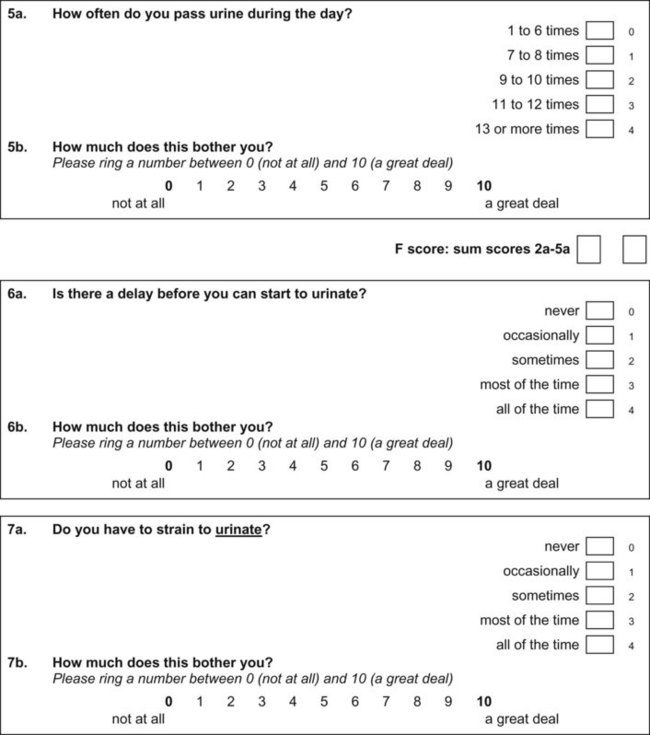
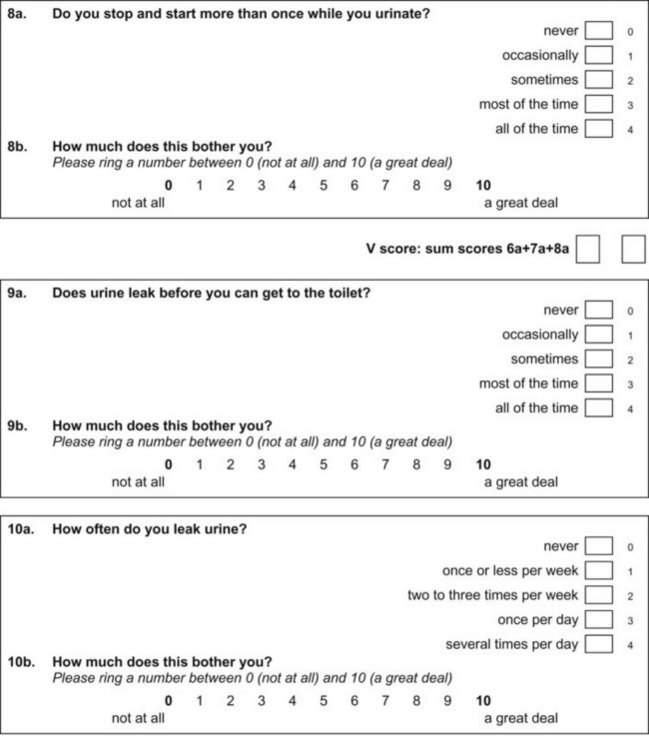
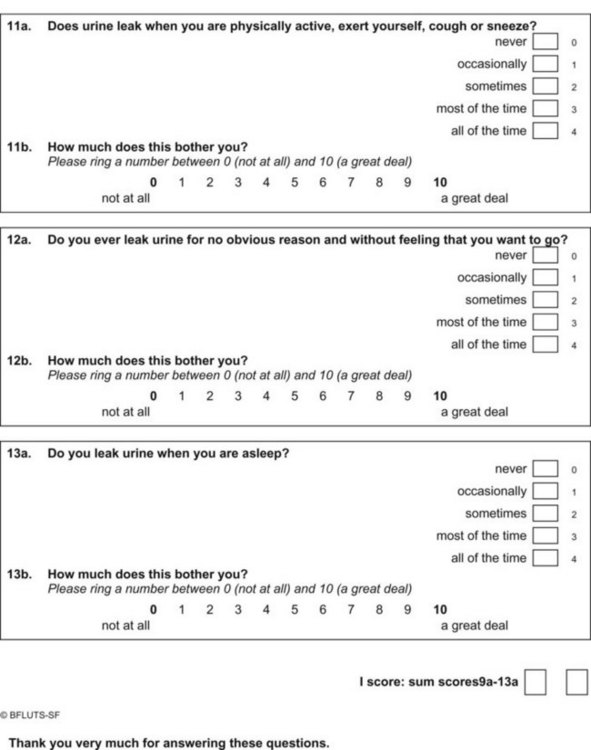
Questionnaires can provide a helpful complement to the patient history and patient-reported outcomes (PROs). A plethora of instruments to evaluate symptoms, degree of bother, and QoL in patients with incontinence and PFDs have been developed in an effort to provide optimal assessment of outcomes and to eliminate the confounding issue of physician bias; many have been validated. Table 64–6 contains validated questionnaires highly recommended by the International Consultation on Incontinence (ICI). One such instrument, the modular ICIQ, was developed by the ICI in an effort to collaboratively develop a universally applicable instrument that could be used internationally to assess pelvic floor function in both clinical practice and research settings (Abrams et al, 2005) and has accordingly been translated in 38 languages. The short form of the ICI questionnaire (ICIQ-SF) has been shown to correlate nicely with both the 1-hour (Franco et al, 2008) and 24-hour (Karantanis et al, 2004) pad test for evaluation of the severity of SUI. Another comparison of the 24-hour pad test, the ICIQ-SF, the International Prostate Symptom Score (IPSS), and the Postoperative Patient Global Impression of Improvement (PGI-I) score in 26 men following perineal sling placement confirmed the construct validity of these instruments (Twiss et al, 2007). There was a strong correlation demonstrated between the ICIQ-SF and PGI-I scores and the percentage reduction in 24-hour pad weight.![]() Appendices 1 to 6 on the Expert Consult website contain the ICIQ-MLUTS (male LUTS), ICIQ-FLUTS (female LUTS), ICIQ-SF (short form), ICIQ-OAB (overactive bladder), ICIQ-VS (vaginal symptoms), and the recently developed ICIQ-B (bowel). At the time of this writing, additional modules covering QoL, sexual function, pediatric LUTS, neurogenic LUTS, nocturia, and bowel function are available or are in development and are or will be available through the ICI (2010).
Appendices 1 to 6 on the Expert Consult website contain the ICIQ-MLUTS (male LUTS), ICIQ-FLUTS (female LUTS), ICIQ-SF (short form), ICIQ-OAB (overactive bladder), ICIQ-VS (vaginal symptoms), and the recently developed ICIQ-B (bowel). At the time of this writing, additional modules covering QoL, sexual function, pediatric LUTS, neurogenic LUTS, nocturia, and bowel function are available or are in development and are or will be available through the ICI (2010).
Table 64–6 Instruments Highly Recommended by the International Consultation on Incontinence (Fourth ICI) for Urinary Incontinence, Overactive Bladder (OAB), Lower Urinary Tract Symptoms (LUTS), and Pelvic Organ Prolapse Patient Reported Outcomes (PRO)
| QUESTIONNAIRE | POPULATION | PURPOSE OF INSTRUMENT |
|---|---|---|
| Health-Related Quality of Life (HRQOL) | ||
| BFLUTS (Bristol Female Lower Urinary Tract Symptoms Questionnaire) aka ICIQ-FLUTS (Jackson et al, 1996) | Women, incontinence | To assess female LUTS, particularly incontinence, measure impact on QOL and evaluate treatment outcome |
| DAN-PSS-1 (Danish Prostatic Symptom Score) (Hansen et al, 1995) | Men, BPH | To evaluate males with LUTS suggestive of uncomplicated BPH |
| ICIQ-UI-SF (ICIQ Urinary Incontinence Short Form) (Avery et al, 2004) | Men and women, urinary symptoms | To assess symptoms and impact of urinary incontinence in clinical practice and research |
| ICSmale (ICIQ-MLUTS) (Donovan et al, 1996) | Men with LUTS and possible BPH | To provide evaluation of occurrence and bothersomeness of LUTS and their impact on the lives of men with BPH |
| ICS-QoL (Donovan et al, 1997) | Men with LUTs and possible BPH | To assess impact of LUTS on the lives of men with LUTS |
| IIQ (Incontinence Impact Questionnaire (Wyman et al, 1988) | Women, UI, SUI | To assess impact of incontinence on HRQOL, primarily in patients with SUI |
| IIQ-7 (IIQ-short form) (Uebersax et al, 1995) | Women, UI, SUI | To assess impact of urinary incontinence on HRQOL |
| I-QOL (ICIQ-Uiqol) (Urinary incontinence-specific QoL instrument) (Wagner et al, 1996) | Women, UI | To assess AOL of women with urinary incontinence |
| KHQ (King’s Health Questionnaire) (ICIQ-LUTSqol) (Kelleher et al, 1997) | Men and women, OAB | To assess the impact of LUTS on HRQOL |
| N-QoL (ICIQ-Nqol; Nocturia QOL) (Abraham et al, 2004) | Men and women | To assess the impact of nocturia on QOL |
| OABq-SF (Coyne et al, 2002) | Men and women, OAB | Shortened version of OAB-q to evaluate both continence and incontinence symptoms of OAB and their impact on QOL |
| OAB-q (ICIQ-OABqol) (Coyne et al, 2002) | Men and women with OAB wet and OAB dry | To evaluate both OAB-wet and OAB-dry symptoms and their impact on HRQOL |
| PRAFAB (Protection, Amount Frequency, Adjustment, Body image) (Hendriks et al, 2007) | Women, UI | To evaluate treatment effects for UI in women |
| UISS (Urinary incontinence severity score) (Stach-Lempinen et al, 2001) | Women, UI | To assess symptom severity and impact of urinary incontinence on everyday life |
| Urolife (BPH-QoL9) (Lukacs et al, 1997) | Men, BPH | To assess the impact of BPH and its treatment on the QOL of patients |
| Screeners | ||
| B-SAQ (Bladder-Self-Assessment Questionnaire) (Basra et al, 2007) | Women | Screening tool for the presence of bothersome LUTS in women |
| LUSQ (Leicester Urinary Symptom Questionnaire) (Shaw et al, 2002) | Men and women, LUTS | Condition-specific screener of storage LUTS (urgency, frequency, nocturia, and incontinence) |
| OAB-SS (OAB Symptom Score) (Blaivas et al, 2007) | Men and women, LUTS with and without OAB | 7-item tool to measure overall symptom severity due to the 4 index symptoms of OAB |
| OAB-V8 (OAB Awareness Tool) (Coyne et al, 2005) | Men and women, OAB | 8-item screening tool for use in primary care setting to identify patients who may have OAB |
| QUID (Questionnaire for Urinary Incontinence Diagnosis) (Bradley et al, 2005) | Women, UI and SUI | 6-item tool to diagnose SUI and UI |
| Symptom Bother | ||
| PPBC (Patient Perception of Bladder Condition) (Coyne et al, 2006) | Men and women | To assess patients’ subjective impression of their current urinary problems. Developed as a global assessment of bladder condition |
| UDI-6 (Urogenital Distress Inventory-6/short form) (Uebersax et al, 1995) | Women | To assess LUTS bother, including incontinence, in women |
| Urgency | ||
| IUSS (Indevus Urgency Severity) (Nixon et al, 2005) | OAB with urgency incontinence, men and women | To quantify the level of urgency associated with each toilet void as measured during standard voiding diaries |
| POP Symptoms and QOL | ||
| PFDI (Pelvic Floor Distress Inventory) (Barber et al, 2001) | Women | To quantify the symptoms caused by pelvic prolapse |
| PFIQ (Pelvic Floor Impact Questionnaire) (Barber et al, 2001) | Women | To quantify the effects of pelvic prolapse on quality of life |
BPH, benign prostatic hypertrophy; HRQOL, health-related QOL; ICIQ, International Consultation on Incontinence Questionnaire; ICS, International Continence Society; MLUTS/FLUTS, male/female lower urinary tract symptoms; OAB, overactive bladder; QoL, quality of life; SF, short form; SUI, stress urinary incontinence; UI, urgency incontinence.
Data from Staskin DR. In: Patient-Reported Outcome Assessment. Fourth International Consultation on Incontinence, report of Committee 5, part 5B. 2009. p. 363–412.
A study examining the incontinence- and nonincontinence-related pelvic floor symptoms evaluated by two widely used symptom tools, the UDI-6 and the ICIQ-UI, demonstrated that both symptom questionnaires were sound and correlated with each other and independently with the QoL scores, as measured by the IIQ-7 and IQOL (van de Vaart et al, 2010). This study also uncovered the interesting finding that the delay in time to consultation with a physician was associated with greater bother, emphasizing the importance of heightened awareness of PFDs in the female patient population.
In Martin’s meta-analysis, two studies showed a high sensitivity (0.82 to 0.92) of question 3 in the Urogenital Distress Inventory for SUI; the specificity was 0.51 to 0.69 (Martin et al, 2006). In this study, history alone had a pooled sensitivity of 0.92 (95% CI) and a specificity of 0.56 for the diagnosis of SUI and a sensitivity of 0.61 and specificity or 0.87 for the diagnosis of DO. It should be borne in mind, however, that for higher-risk interventions such as surgery, the most accurate testing available remains multichannel UDS studies.
Pad Tests
Pad tests are generally used for academic purposes. The ICS recommends both a three-day bladder diary and pad weight test as proper measures for symptom quantification in incontinence research (Lose et al, 2001). However, although pad tests can be helpful in quantifying leakage, they are tedious and cumbersome for the patients. Moreover, they do not provide information that is necessary for daily routine clinical practice. The Fourth ICI Committee on initial assessment did not recommend pad tests as part of the initial evaluation in the incontinent patient (Staskin, 2009). From an academic standpoint, however, many investigators advocate for pad tests in clinical trials because pad tests can provide objective, precise information for assessment of actual volume of urine lost over an established period of time.
According to the Third ICI, greater than 1.3 g of urine loss is considered a “positive” 24-hour pad test (Tubaro, 2005), whereas others consider up to 8 g of urine loss in 24 hours to be normal (Lose et al, 1989). Vaginal secretions should also be taken into consideration, although the volume attributable to normal vaginal secretions may be as low as 0.3 g in 24 hours (Karantanis et al, 2003). O’Sullivan et al (2004) evaluated 110 women with incontinence with two 1-hour pad tests and 7 consecutive days of 24-hour pad tests. The severity of the leakage was analyzed in relation to UDS parameters, age, parity, and pelvic floor muscle strength, showing increased severity with increasing age and parity and in those women who demonstrated DO. The authors proposed that 24-hour loss of 1.3 to 20 g, 21 to 74 g, and greater than 75 g signifies “mild,” “moderate,” and “severe” incontinence, respectively. Another study of 144 randomly selected Danish women who underwent 24-hour pad testing revealed a similar loss of urine in the self-reported continent and incontinent groups, or 3.1 and 3.3 g, respectively (Ryhammer et al, 1998).
It is generally agreed that the 24-hour pad test is a clinically more useful tool than the 1-hour pad test (Lose et al, 1989; Matharu et al, 2004); in fact, the test-retest reliability and the predictive value of the 1-hour test in the diagnosis of female incontinence has been shown to be poor (Lose et al, 1986; Lose et al, 1988; Simons et al, 2001; Constantini et al, 2008). Others have advocated the opposite extreme, suggesting that a 20-minute pad test with a standardized bladder volume of 250 mL instilled into the bladder via catheterization had superior sensitivity as compared with the 1-hour test conducted via the ICS standardized method of pad testing (Wu et al, 2006). The ICS method, described in 1988, requires the patient to drink 500 mL of sodium-free liquid in 15 minutes followed by a 30-minute resting period before proceeding with the recommended physical activity (Abrams et al, 1988). One potential concern about this method is the lack of standardization of bladder volume.
Parenthetically, pad use per day (PPD) obtained via patient history is a measure frequently used to quantify urine loss, but one study demonstrated that this is an unreliable measure of incontinence (Dylewski et al, 2007). A retrospective chart review of 145 males and 116 females who underwent artificial urinary sphincter placement and sling surgery, respectively, and who had completed a self-reported pad-use query was performed, and the patients were asked to bring three pads into their clinic visit: one dry “reference” pad and the incontinence pads used for the 24-hour periods preceding and including the day of their visit. The pads were quantified and weighed to determine the grams of urine per pad (GPP). All patients also underwent a 24-hour pad weight test. Only a weak correlation was found between reported pad usage and the 24-hour pad weight, with pad usage measuring only 38% of the variability of incontinence volume. Additionally, although the PPD decreased, the GPP increased with increasing age.
Dye Testing
Dye testing can be helpful to verify that the leakage represents urine versus another fluid such as vaginal discharge or peritoneal fluid and to substantiate the diagnosis of urinary tract fistulae. Oral phenazopyridine 200 mg three times a day colors the urine orange, and this simple test can confirm that the leaking fluid is indeed urine. Diagnosis of a vesicovaginal or urethrovaginal fistula can be supported by blue or orange staining of an intravaginal tampon following intravesical instillation of methylene blue or phenazopyridine hydrochloride (Pyridium) dissolved in sterile water or saline. In the case of a suspected ureterovaginal fistula, intravesical methylene blue with concurrent oral Pyridium can elucidate the fistula location on the basis of the staining pattern on the vaginal tampon. Orange staining suggests a ureteral communication, whereas blue staining connotes a bladder communication (Raghavaiah, 1974). The clinician must keep in mind that simultaneous vesicovaginal and ureterovaginal fistulae can occur.
Key Points: Supplementary Evaluation
Urinalysis
It is generally agreed that urinalysis plays a fundamental role in the evaluation of the incontinent patient or the patient with lower urinary tract symptoms (LUTS) (Abrams et al, 2009a). The urinalysis provides information such as the presence of hematuria, pyuria, glucosuria, or proteinuria that can be indicative of conditions that can cause secondary incontinence. As indicated by the screening dipstick analysis, a microanalysis and/or culture should be performed that may provide guidance regarding further testing or therapy for conditions related to or independent of urinary incontinence.
Postvoid Residual
The volume of urine left in the bladder following routine voiding is termed the postvoid residual (PVR) and should be evaluated in all incontinent patients (Tubaro, 2005; Gormley, 2007). This simple test, which can be performed via in-and-out catheterization or using noninvasive transabdominal ultrasonography, evaluates the bladder’s emptying ability and can be helpful in the diagnosis of overflow incontinence. It is also essential to establish baseline bladder emptying, particularly in stress incontinent patients who may be considered for an anti-incontinence procedure or patients with urinary urgency who may be candidates for therapies aimed at decreasing bladder contractility.
A number of studies have demonstrated that ultrasound is comparable to catheterization in evaluating the PVR, although there are no officially established volumes that define normal or impaired emptying. The Agency for Healthcare Policy and Research (AHCPR) suggests that PVR less than 50 mL represents adequate emptying and PVR greater than 200 mL represents inadequate emptying (U.S. Department of Health and Human Services, 1992). There is no consensus recommendation regarding the significance of PVR between 50 and 200 mL. In one study, Gehrich and colleagues (2007) enrolled 96 healthy women who presented for routine “well-women” checkups. Exclusion criteria included urinary incontinence more than twice a week, urinary retention, neurologic disease, or symptomatic POP. The mean and median PVRs were 19 mL (0 to 145 mL) and 24 ± 29 mL. Fifteen percent had PVR greater than 50 mL, and 95% had PVR less than 100 mL. Another study compared PVR measurements obtained by 3-D bladder scan versus catheterization in 170 women who were undergoing evaluation for SUI but who had never undergone previous pelvic surgery (Tseng et al, 2008). In addition, 35.5% had PVR greater than 50 mL and 15.9% had PVR greater than 100 mL. Ultrasound offered a sensitivity of 64.7% and a specificity of 94.3% in detecting PVR greater than 100 mL. Although several studies support the accuracy of the bladder scan (Al-Shaikh et al, 2009), some suggest that certain sonographic devices may provide more accurate information than others (Ghani et al, 2008).
Cystoscopy
Endoscopic examination of the bladder is important as a means to evaluate for intravesical or intraurethral pathology that may be contributing to the patient’s symptomatology. Bladder tumors, bladder stones, cystitis, and intravesical or intraurethral foreign bodies such as mesh or suture can contribute to irritative voiding symptoms, recurrent urinary tract infections (UTIs), and incontinence. Patients with a history of previous pelvic floor reconstructive surgery should be evaluated for eroded materials into the LUT. The ureteral orifices should be identified and evaluated for morphology, position, number, and efflux. The bladder mucosa is examined for trabeculation (which can be indicative of bladder outlet obstruction [BOO] and/or overactivity) and estrogen status, and the urethra is assessed for foreign bodies, stricture, diverticulum or fistula, and position.
The role of preoperative cystourethroscopy has been addressed by few authors. Anger and colleagues (2007) analyzed Medicare claims data to assess the effects of preoperative cystoscopy and UDS studies on sling outcomes. The data of a random 5% sample of Medicare beneficiaries during an established 18-month period were used to assess the likelihood of undergoing postoperative studies in those who did and did not undergo the same studies preoperatively. Although patients who underwent preoperative cystoscopy were less likely to undergo postoperative cystoscopy (23.4% vs. 35.2%, P < 0.0001) or UDS (19.3% vs. 34%, P < 0.0001), there were no significant differences in complications or repeat incontinence procedures between the groups. However, Cundiff and Bent (1996) reported that cystoscopy changed the diagnosis and management in 6 of 84 women (7%) who underwent both cystoscopy and UDS for evaluation of LUT dysfunction. Findings included transitional cell carcinoma, cystitis glandularis, intravesical suture, and a urethral diverticulum.
Although the use of routine cystoscopy in the evaluation of the “index” stress urinary incontinence patient is controversial, it should be performed in patients who present with urinary urgency, hematuria, or other irritative symptoms, particularly if they have previously undergone a previous anti-incontinence procedure, pelvic radiation, or pelvic prolapse repair.
Urodynamics
Similar to cystourethroscopy, the routine use of UDS is the subject of much debate; however, one should strongly consider UDS in patients who have failed previous pelvic floor reconstruction or who complain of mixed incontinence, urinary urgency, or obstructive symptoms, and in those who have elevated PVRs or neurologic disease. The benefit of UDS in these situations is to establish baseline bladder capacity, compliance, sensation, stability, and sphincter function before further surgical or therapeutic manipulation. A comprehensive review of UDS is presented in Chapter 62.
LUT function can be simply categorized into storage and emptying. Each of these categories is affected by the bladder (detrusor) and the outlet. Two main questions should be considered in the evaluation of the incontinent patient: (1) Is the problem at hand a storage issue, an emptying issue, or a combination of the two? and (2) Is the etiology of the first question due to a detrusor issue, an outlet issue, or a combination of both?
“Eyeball UDS” is a simple economically favorable alternative to full multichannel UDS that can be helpful in selected patients. The study can determine bladder sensation, compliance, stability, and capacity, as well as outlet competence and PVR. Following voiding, the patient is placed in the lithotomy position and a Foley catheter is placed and the PVR measured. A 60-mL catheter tip syringe with the barrel removed is placed into the end of the catheter. With the syringe held upright, the bladder is filled with sterile fluid through the syringe. The height of the meniscus above the bladder represents the intravesical pressure. The volumes at first sensation, sensation to void, and strong sensation are recorded. During the filling phase, the meniscus in the syringe is observed for a rise and fall that may represent bladder overactivity or a consistent gradual rise that suggests compromised detrusor compliance. The catheter is removed, and a cough stress test is performed by observing the urethra for incontinence during coughing and straining.
Multichannel UDS offers an extensive evaluation of LUT function. This degree of accuracy is important in a variety of circumstances including when conservative treatment methods fail, when the diagnosis is unclear or when previous diagnostic procedures are inconclusive, in patients with clinical pictures complicated by radiation therapy, neurologic disease, or prior failed pelvic floor reconstruction or anti-incontinence surgery, or when patients describe symptoms that cannot be confirmed by the clinician. A complete UDS study evaluates the filling/storage phase by filling cystometrogram and the voiding/emptying phase by uroflowmetry.
Catheters are placed into the bladder and the rectum. The bladder catheter measures the actual pressure within the bladder, termed the vesical pressure (Pves). The rectal catheter measures the abdominal pressure (Pabd). The detrusor pressure (Pdet) is a calculated value (Pves − Pabd) that represents the pressure created by the detrusor independent of the influences of intra-abdominal pressure. During the filling phase, the Pdet is expected to remain low and stable to allow for low-pressure bladder filling. Poorly compliant bladders will show a gradual steady rise in the Pdet as the bladder volume increases. Detrusor overactivity is manifest by intermittent and unpredictable rises in the Pdet. During the voiding phase, the Pdet may rise as the urine flows. Many women normally void with virtually no rise in Pdet. High Pdet with low flow during voiding suggests BOO. Inability to produce a flow with no Pdet, particularly when accompanied by abdominal straining (represented by an undulant Pabd), may indicate an atonic or hypotonic detrusor.
Electromyogram (EMG) and fluoroscopic imaging (“videourodynamics,” VURDS) are helpful adjuncts to the UDS study in selected patients and are covered in detail in Chapter 62. EMG activity is reflective of the activity of the striated muscles of the urethral and anal sphincters and the perineal musculature and can be measured using either surface electrodes or needle electrodes. Although the latter provides more accurate readings, the discomfort caused to the patient and the expertise required for the technique render it far less popular than surface electrodes. The coordination of the EMG and detrusor activity is most helpful during the voiding phase, when failure to relax the pelvic floor can be indicative of pathology. Similarly, recruitment of activity should be noted with BCR and increased abdominal pressure, such as occurs during coughing. VURDS can be useful in the demonstration of anatomy in the upright position, and because the bladder and outlet are visualized “real time,” VURDS can confirm incontinence in patients in whom the diagnosis is difficult and can provide an accurate measure of leak point pressure. It is also helpful to assess the bladder outlet in patients in whom detrusor-sphincter dyssynergia or primary bladder neck obstruction is suspected. Patients can be evaluated for vesicoureteral reflux (VUR). Assessment of detrusor pressures in the face of reflux can provide important information that would be missed without video-imaging.
Recent attention has been placed on scientifically establishing the role and clinical value of UDS. The worth of a test is not solely in its diagnostic accuracy but also in the improvement of the outcomes of subsequent interventions. UDS is frequently used in the evaluation of patients with PFDs. Suboptimal outcomes of anti-incontinence procedures are generally attributed to intrinsic sphincter deficiency (ISD), DO, or baseline dysfunctional voiding. The deliberation regarding the merit of these theories and the ability of UDS to accurately establish these conditions continues. In a study of 655 women with positive stress tests, 10% did not demonstrate urodynamic-SUI (UDS-SUI) (Nager et al, 2007). A meta-analysis based on a Medline search of the literature between 1975 and 1998 suggested that UDS has a positive predictive value (PPV) of only 56% and 79% for pure SUI and SUI with other abnormalities, respectively, while a positive cough test has PPVs for the same of 55% and 91%, respectively (Harvey and Versi, 2001). The Urinary Incontinence Treatment Network (UITN) reported that UDS did not predict postoperative voiding dysfunction or the risk for need of postoperative surgical intervention (Lemack et al, 2008). In another retrospective study in women who underwent retropubic midurethral sling placement for treatment of mixed incontinence, the median opening detrusor pressure was higher in women with postoperative DO than in those with normal postoperative UDS (Panayi et al, 2009).
Much of the literature to date has been limited by the retrospective design and small cohorts of the available studies. The UITN found that the success of anti-incontinence surgery in patients who demonstrated UDS-SUI was nearly twice that of those with non-UDS-SUI (type 0 SUI), although this trend did not reach statistical significance (Nager et al, 2008). Similarly, although the study by Anger and colleagues (2007) did not definitively establish an effect of preoperative UDS specifically on sling outcomes, there was a clear demonstration of a twofold (significant) increased likelihood of undergoing postoperative UDS studies in those patients who did not undergo the study preoperatively as compared with those who did.
With healthcare reform increasingly at the forefront of discussion, cost-effectiveness continues to be a subject of great debate. On the basis of decision-analytic (hypothetical) models, one group deemed UDS not cost-effective relative to the basic office evaluation (Weber and Walters, 2000; Weber et al, 2002). However, one must remain mindful that UDS provides information, not only about the overall diagnosis but also regarding important subtle findings that may direct the clinician in treatment planning and counseling (Summitt et al, 1992; Patel and Chapple, 2008). Although it has been suggested that UDS may not be necessary or useful in straightforward, non-neurologic patients who would be considered for initial conservative management (Colli et al, 2003; NICE, 2006), others have shown that up to 20% of patients with presumed pure SUI may have UDS findings that might alter their treatment or outcomes (Digesu et al, 2009).
Reproducibility of UDS studies, as well as the interpretation of the examination, has been questioned by several studies (Van de Beek et al, 1997; Gupta et al, 2004; Zimmern et al, 2006; Gacci et al, 2007). Others have demonstrated that intraobserver (interpretation repeated by the same individual) interpretation is superior to interobserver (same study read by two different individuals) interpretation for both pressure-flow analyses (Digesu et al, 2003) and filling/voiding studies (Whiteside et al, 2006). Still others have demonstrated no difference in interobserver and intraobserver interpretation, but that live interpretation of a study provides different readings than post hoc interpretation, suggesting that intangible experiential factors may play a role in the interpretation of UDS studies (Smith et al, 2009).
Approximately 40% of patients with POP describe SUI symptoms (Grody, 1998), and UDS-SUI is demonstrated in 70% to 75% of prolapse patients (Roovers and Oelke, 2007). Occult SUI unmasked by reduction of prolapse is reported to be present in 36% to 80% (Richardson et al, 1983; Bergman et al, 1988; Chaikin et al, 2000), and 11% to 50% of clinically continent patients will develop de novo SUI following repair of high-grade prolapse (Bergman et al, 1988; Borstad and Rud, 1989; Gallantine and Cespedes, 2001). Women who demonstrated preoperative UDS-SUI before sacrocolpopexy were more likely to develop postoperative SUI irrespective of whether they received a concomitant Burch colposuspension (Visco et al, 2008). Conversely, following a retrospective review of the records of 76 patients who underwent POP repair, Roovers and colleagues (2007) reported that no UDS parameters predicted postoperative incontinence. In an effort to determine the need for prophylactic sling placement in patients undergoing surgery for high-grade POP, Ballert and colleagues (2009) followed a UDS protocol designed to address the urethra in a standardized fashion. They concluded that in patients who did not subjectively describe or urodynamically demonstrate SUI preoperatively, the risk of intervention due to BOO following sling placement was equivalent to the risk of intervention for SUI in patients who did not receive a sling.
In select patients, particularly those with OAB symptoms and/or known neurogenic bladder, UDS can be helpful in determining the risk of progression to upper tract deterioration. DO, poor compliance, detrusor–external sphincter dyssynergia (DESD), high detrusor storage pressures, VUR, and BOO all represent potential risk factors or the development of upper tract disease, particularly in those patients with sustained Pdet greater than 40 cm H20 (McGuire et al, 1981; Blaivas and Barbalias, 1984; Ghoniem et al, 1989, 1990).
Although multichannel UDS remains the most accurate tool with which to evaluate LUT function, it is clear that multi-institutional randomized prospective studies are of dire importance in addressing many of the unanswered questions regarding the role and contribution of UDS. The accuracy of the study and impact of the findings on treatment outcomes must be established.
Key Points: Evaluation—Urodynamics
Radiographic Imaging
Voiding Cystourethrogram
Standard imaging studies are not necessary in the initial evaluation of women with uncomplicated incontinence (Artibani et al, 2002; Artibani and Cerruto, 2005). However, upper and lower urinary tract imaging in patients in whom renal damage or pelvic pathology are suspected should be performed. Voiding cystourethrogram (VCUG) is optional in patients with recurrent UTIs but can be helpful in the diagnosis of a urethral diverticulum or VUR. VCUG can provide valuable information regarding the contour of the bladder, the presence of VUR, the position of the bladder base with and without straining with the patient in an upright posture, and the position and configuration of the bladder neck during voiding. VCUG can also provide visualization of subtle leakage with coughing or Valsalva maneuver that may be difficult to detect with direct examination.
Ultrasound
Upper tract imaging is recommended in patients with neurogenic detrusor dysfunction considered to be at high risk for renal damage, chronic urinary retention, suspected extraurethral incontinence, and untreated severe POP. The latter group may develop upper tract obstruction and hydroureteronephrosis related to ureteral “kinking” due to the prolapse. Ultrasonography provides a noninvasive sensitive and specific method of assessing the upper tracts for hydronephrosis. Ultrasonic imaging of the bladder neck for urinary leakage and descent during stress has also been used to diagnose SUI with documented pooled sensitivity and specificity rates in the range of 0.84 to 0.89 and 0.82 to 0.89, respectively (Martin et al, 2006).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has been proposed as an ideal method by which to evaluate the anatomy of the bladder neck and urethra with good correlation with functional studies (Macura, 2006; Macura and Genadry, 2008; Macura et al, 2006). MRI has also been advocated for evaluation of pelvic floor relaxation and POP (Boyadzhyan et al, 2008), particularly in patients undergoing evaluation for complex multicompartmental pelvic floor reconstruction (Macura, 2006; Boyadzhyan et al, 2008), and has been shown to identify changes related to the uterosacral ligaments before and following surgical repair of prolapse (Martin et al, 2006). MRI may also be helpful in identifying those patients in whom a urethral diverticulum may be present. Although urethral diverticula can cause symptoms of urinary leakage, pelvic pain, obstructive voiding symptoms, recurrent urinary tract infections, dyspareunia, and a multitude of nonspecific symptoms, up to 20% of diverticula may be completely asymptomatic (Rovner, 2007). In patients with complaints of SUI who are found to have a urethral diverticulum, a simultaneous anti-incontinence surgery can be offered, in which case proper radiographic imaging such as that which can be obtained with MRI can provide anatomic information germane to optimal surgical planning.
High-resolution MRI allows detailed visualization of the urethra, external sphincter, and supporting structures. Magnetic resonance evaluation focusing specifically on the muscle volume of the sphincter, defects in the sphincteric musculature, funneling of the bladder neck, asymmetry of the pubococcygeus muscle or urethral supporting ligaments, increase in the size of the retropubic space or urethrovesical angle, and abnormal vaginal shape may be helpful in the diagnosis of SUI due to ISD or urethral hypermobility (Macura, 2006). To facilitate assessment of POP using MRI, the HMO (H-line, M-line, organ prolapse) system was developed (Pannu et al, 2000).
Evaluation is performed using rapid half-Fourier T2-weighted images in the midsagittal plane during maximal patient straining. Three fixed points are in the HMO system: A, the inferior margin of the pubic symphysis, B, the posterior levator plate, and C, the junction between the first and second coccygeal segments. Two fixed reference points include the pubococcygeal line (PCL), drawn between points A and C, and point B. Line H, drawn between A and B, represents the anterior-posterior hiatal dimension. Line M, which is the shortest distance between point B and the PCL, represents the degree of pelvic descent. The O component comprises the shortest distance between the H line and the most caudal aspect of the evaluated organ during Valsalva maneuver. Prolapse is graded on the basis of the organ location relative to the H-line in centimeters.
Dynamic MRI can provide integral information in the preoperative assessment of POP, particularly in patients in whom the pelvic examination is difficult and inconclusive. The sensitivity, specificity, and positive predictive value (PPV) of MRI for cystoceles has been reported to be 70% to 100%, 83% to 100%, and 97% to 100%, respectively; 42% to 100%, 54% to 81%, and 33% to 60%, respectively, for vaginal vault prolapse; 87% to 100%, 80% to 83%, and 75% to 91%, respectively, for enteroceles; 83%, 100%, and 100%, respectively, for uterine prolapse, and 87%, 72%, and 66%, respectively, for rectoceles (Gousse et al, 2000; Deval et al, 2003). Other authors have found that dynamic MRI does not correlate well with clinical findings in patients with middle compartment (i.e., apical) prolapse, and much of the literature suggests that the study should be used only as an adjunct to clarify anatomy in complex cases (Cortes et al, 2004) or under investigational circumstances (Tubaro, 2005). Similarly, many authors agree that the posterior compartment is not as easily visualized on dynamic MRI. A study using intrarectal air during MRI did not demonstrate any value of dynamic MRI for evaluation for rectoceles over videoproctography, the latter of which was more sensitive in identifying rectoceles (Matsuoka et al, 2001). For optimal visualization of rectoceles, intrarectal gel is used to provide hyperintensity on T2-weighted images (Macura 2006; Boyadzhyan et al, 2008; Law and Fielding, 2008).
Management
Incontinence Treatment Overview
The approach to treatment of incontinence is contingent on a clear understanding of the etiology and pathophysiology underlying the patient’s symptoms. The clinician must first determine whether the cause of the symptomatology complex is a bladder or an outlet problem or, not uncommonly, a combination of both. Therapeutic options should be considered with the goal of providing an individualized patient-directed treatment plan on the basis of the patient goals and risk-benefit and cost-benefit ratios.
Management of incontinence can be categorized into nonsurgical and surgical options. Underlying causes such as UTI, BOO, bladder stones, foreign body, or bladder tumor should be identified and addressed first. Table 61–3 in Chapter 61 provides an overview of the treatment options available for the management of incontinence; a detailed review of the various therapeutic options is presented in Chapters 68 through 79.
Treatment of incontinence must be tailored to the patient’s needs, goals, and expectations and requires proper counseling on the part of the clinician. Some patients may be satisfied with protective garments and/or urine collection devices such as indwelling or condom catheters or barriers such as urethral plugs or external occlusion devices. Intervention for patients with urgency incontinence may range from behavioral and dietary modification to biofeedback or pharmacotherapy. Sacral neuromodulation, off-label botulinum toxin A detrusor injection, and enteric augmentation of the bladder may be considered in patients with refractory symptoms.
Similarly, patients with SUI may benefit variably from conservative measures using pelvic floor muscle exercises, biofeedback, electrical stimulation, and pharmacotherapy. Urethral bulking injection therapy can provide an intermediate option between nonsurgical and surgical therapies, but surgery remains the mainstay of treatment for SUI. Although needle suspensions remain only as a point of historic discussion, retropubic suspensions have persisted as a reasonable treatment option for SUI. However, slings, using a variety of materials, insertion approaches, and anchoring techniques, have effectively become the standard of care for women with SUI. Injection therapy has not proven a particularly viable option for the treatment of male SUI (which occurs most commonly following prostatectomy for treatment of adenocarcinoma of the prostate), and follow-up of the outcomes with male slings is still early. The artificial urinary sphincter (AUS) remains the prevailing treatment option for postprostatectomy incontinence. The AUS has rarely been used for treatment of SUI in women. In the fortunately rare cases of complete urethral devastation, bladder neck closure or urinary diversion can be considered.
Pelvic Prolapse Treatment Overview
New techniques have been explored to improve on the traditional pelvic floor reconstructive approaches that depend on the inherently compromised tissues of the POP patient. The use of synthetic and biologic graft materials to improve the integrity and durability of POP repairs have become popularized over the past decade. Novel anatomic approaches and “kits” have been developed and have resulted in a dramatic increase in the number of clinicians participating in pelvic floor reconstruction.
The goal of POP repair is to restore the normal anatomy and function of the vagina and the lower urinary and GI tracts. The decision regarding whether to proceed with a transvaginal or a transabdominal approach is dependent on which of the three compartments is affected, the degree of prolapse, and patient and surgeon preference. Apical prolapse involving the uterus typically results in a hysterectomy, although uterine-sparing techniques can be performed. Posthysterectomy apical prolapse can be addressed transvaginally with a uterosacral ligament suspension or a sacrospinous ligament fixation. Several contemporary devices that aim to facilitate high prolapse reduction have been introduced. Nevertheless, the sacrocolpopexy, a transabdominal approach that can be performed either open or minimally invasively using laparoscopy or robotic assistance, remains the gold standard repair for apical prolapse. A Y-shaped mesh typically composed of polypropylene is attached to the apex of the vagina and bridged to the sacrum to return the vagina to its normal axis.
New techniques of pelvic floor reconstruction continue to emerge parallel with increasing efforts to understand pelvic floor anatomy and function. A comprehensive overview of current surgical management of pelvic prolapse is presented in Chapter 72.
Conclusion
The ultimate goal of pelvic floor reconstruction is to restore the normal anatomy and function of the vagina, bladder, and surrounding structures. Proper evaluation of the pelvic floor anatomy and function should theoretically maximize the probability of favorable outcomes. Additionally, with a growing emphasis on QoL-enrichment and a simultaneously increasing cost of health care, economically sound and durable treatments are the aspiration. Accordingly, efforts to develop methods by which to measure symptoms and evaluate outcomes continue. New techniques designed to provide robust, minimally invasive options to achieve this objective have been developed, and as our appreciation of the pelvic floor continues to advance, further approaches will undoubtedly arise.

Appendix 1 ICIQ-MLUTS. (ICIQ modules and associated permissions can be requested through www.ICIQ.net.)

Appendix 2 ICIQ-FLUTS. (ICIQ modules and associated permissions can be requested through www.ICIQ.net.)

Appendix 3 ICIQ-UI Short Form. (ICIQ modules and associated permissions can be requested through www.ICIQ.net.)

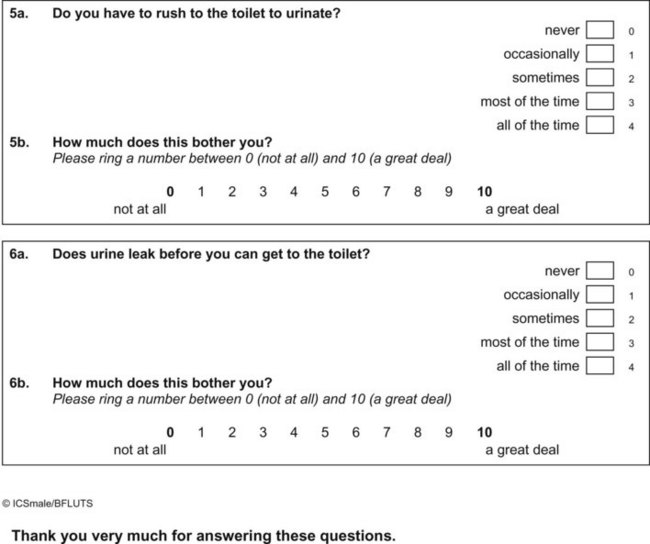
Appendix 4 ICIQ-OAB. (ICIQ modules and associated permissions can be requested through www.ICIQ.net.)


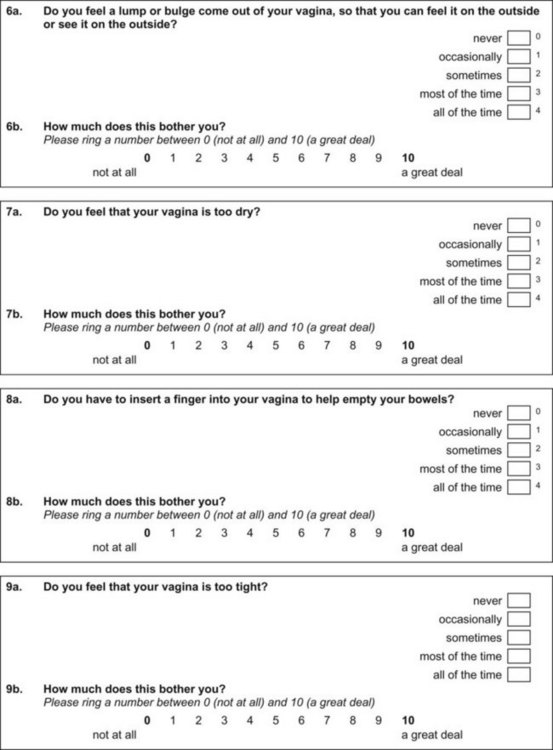


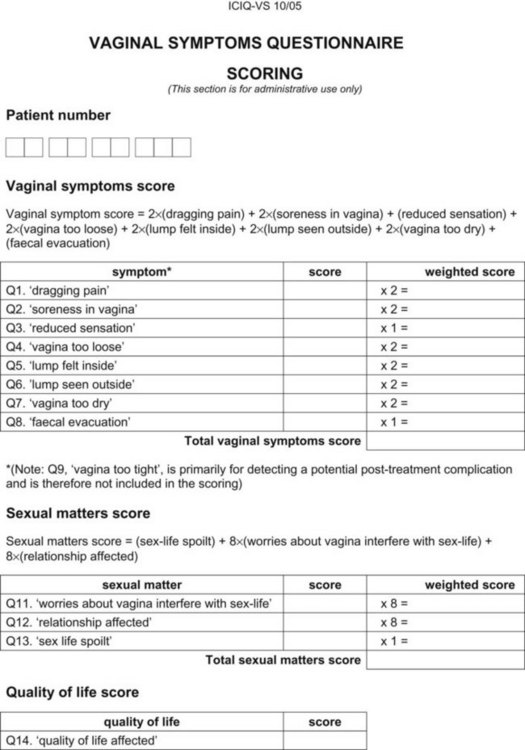
Appendix 5 ICIQ-VS. (ICIQ modules and associated permissions can be requested through www.ICIQ.net.)
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178. reprinted in Urology 2003;61:37–49
Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol. 2009;19:380-394.
Bump RC Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
CMS 97 guidelines for focused female pelvic examination. Documentation Guidelines for Evaluation and Management (E/M) Services, jointly approved by the American Medical Association and HCFA with revisions November, 1997.
DeLancey JO. Anatomical aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6Pt1):1717-1724.
Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(111):1311-1316.
Abraham L, Hareendran A, Mills IW, et al. Development and validation of a quality-of-life measure for men with nocturia. Urology. 2004;63(3):481-486.
Abrams P, Avery K, Gardener N, Donovan J. The International Consultation on Incontinence modular questionnaire: www-iciq.net, on behalf of the ICIQ Advisory Board. J Urol, 2005;175, 1063-1066.
Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function: the International Continence Society Committee on Standardisation of Terminology. Scand J Urol Nephrol Suppl. 1988;11:5-19.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol and Urodyn. 2002;21:167-178. reprinted in Urology 2003;61:37–49
Abrams P, Cardozo L, Khoury S, Wein A. Incontinence. Proceedings from the 3rd International Consultation on Incontinence. Paris: Health Publications; 2005.
Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181(4):1779-1787.
Abrams P, Ribani W, Cardozo L, et al. Reviewing the ICS 2002 Terminology report: the ongoing debate. Neurourol Urodyn. 2009;28:287.
Al-Shaikh G, Larochelle A, Campbell CE, et al. Accuracy of bladder scanning in the assessment of postvoid residual volume. J Obstet Gynaecol Can. 2009;31(6):526-532.
Anger JT, Rodriguez LV, Wang Q, et al. The role of preoperative testing on outcomes after sling surgery for stress urinary incontinence. J Urol. 2007;178:1364-1369.
Anger JT, Saigal CS, Madison R, et althe Urologic Diseases of America Project. Increasing costs of urinary incontinence among female Medicare beneficiaries. J Urol. 2006;176:247-251.
Anger JT, Saigal CS, Stothers L, et alUrologic Diseases of America Project. The prevalence of urinary incontinence among community dwelling men: results from the National Health and Nutrition Examination survey. J Urol. 2006;176(2):2103-2108.
Artibani W, Andersen JT, Gajewski JB. Imaging and other investigations. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth (MA): Plymbridge Distributors; 2002:425-478.
Artibani W, Cerruto MA. The role of imaging in urinary incontinence. BJU Int. 2005;95(5):699-703.
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurorol Urodyn. 2004;23(4):322-330.
Baden WF, Walker TA, Lindsay HJ. The vaginal profile. Tex Med J. 1968;64:56-58.
Ballert KN, Biggs GY, Isenalumhe A, et al. Managing the urethra at transvaginal pelvic organ prolapse repair: a urodynamic approach. J Urol. 2009;181:679-684.
Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388-1395.
Basra R, Artibani W, Cardozo L, et al. Design and validation of a new screening instrument for lower urinary tract dysfunction: the bladder control self-assessment questionnaire (B-SAQ). Eur Urol. 2007;52(1):230-237.
Bates CP, Loose H, Stanton SLR. The objective study for incontinence after repair operations. Surg Gynaecol Obstet. 1973;136:12-22.
Bergman A, Bhatia NN. Urodynamic appraisal of the Marshal-Marchetti test in women with stress urinary incontinence. Urology. 1987;29(4):458-462.
Bergman A, Koonings PP, Ballard CA. Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol. 1988;158(5):1171-1175.
Blaivas JG, Barbalias GA. Detrusor-external sphincter dyssynergia in men with multiple sclerosis: an ominous urologic condition. N Urol. 1984;131(1):91-94.
Blaivas JG, Panagopoulos G, Weiss JP, Somaroo C. Validation of the overactive bladder symptom score. J Urol. 2007;178(2):543-547.
Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continence women: a clinical and urodynamic follow-up study. Acta Obstet Gynecol Scand. 1989;68:545-549.
Boyadzhyan L, Raman SS, Raz S. Role of static and dynamic MR imaging in surgical pelvic floor dysfunction. Radiographics. 2008;28:949-967.
Bradley CS, Rovner ES, Morgan MA, et al. A new questionnaire for urinary incontinence diagnosis in women: development and testing. Am J Obstet Gynecol. 2005;192(1):66-73.
Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
Burgio KL. Current perspectives on management of urgency using bladder and behavioral training. J Am Acad Nurs Pract. 2004;16(10 Suppl.):4-7.
Centers for Medicare/Medicaid (CMS). Single organ system exam—Genitourinary: 1997 Documentation Guidelines for Evaluation and Management (E/M) Services, jointly approved by the American Medical Association and HCFA with revisions. November 1997.
Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
Chapple C. Old words revisited—the importance of standardisation. Neurourol Urodyn. 2009;28:267.
Colli E, Artibani W, Goka J, et al. Are urodynamic tests useful tools for the initial conservative management of non-neurogenic urinary incontinence? A review of the literature. Eur Urol. 2003;43(1):63-69.
Constantini E, Lazzeri M, Bini V, et al. Sensitivity and specificity of one-hour pad test as a predictive value for female urinary incontinence. Urol Int. 2008;81(2):153-159.
Cortes E, Reid WMN, Singh K, Berger L. Clinical examination and dynamic magnetic resonance imaging in vaginal vault prolapse. Obstet Gynecol. 2004;103:41-46.
Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006;49(6):1079-1086.
Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563-574.
Coyne K, Zyczynski T, Margolis MK, et al. Validation of overactive bladder awareness tool for use in primary care settings. Adv Ther. 2005;22(4):381-394.
Cundiff GE, Bent AE. The contribution of urethrocystoscopy to evaluation of lower urinary tract dysfunction in women. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7(6):307-311.
De Boer TA, Salvatore S, Cardozo L, et al. Pelvic organ prolapse and overactive bladder. Neurourol Urodyn. 2010;29(1):30-39.
DeLancey JO. Anatomical aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6Pt1):1717-1724.
Deval B, Vulierme MP, Poilpot S, et al. Imaging of pelvic floor prolapse. J Gynecol Obstet Biol Reprod (Paris). 2003;32(1):22-29.
Digesu GA, Hendricken C, Fernando R, Khullar V. Do women with pure stress urinary incontinence need urodynamics? Urology. 2009;74:278-282.
Digesu GA, Hutchings A, Salvatore S, et al. Reproducibility and reliability of pressure flow parameters in women. BJOG. 2003;110:774-776.
Donovan JL, Abrams P, Peters TJ, et al. The ICS-”BPH” Study: the psychometric validity and reliability of the ICS male questionnaire. Br J Urol. 1996;77(4):554-562.
Donovan JL, Kay HE, Peters TH, et al. Using the ICSQoL to measure the impact of lower urinary tract symptoms on quality of life: evidence from the ICS-”BPH” Study. International Continence Society—Benign Prostatic Hyperplasia. Br J Urol. 1997;80(5):712-721.
Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656-661.
Dylewski DA, Jamison MG, Borawski KM, et al. A statistical comparison of pad numbers versus pad weights in the quantification of urinary incontinence. Neurourol and Urodyn. 2007;26:3-7.
Fenner DR, Trowbridge ER, Patel DA, et al. Establishing the prevalence of incontinence study: racial differences in women’s patterns of urinary incontinence. J Urol. 2008;179(4):1455-1460.
Franco AV, Lee F, Fynes MM. Is there an alternative to pad tests? Correlation of subjective variables of severity of urinary loss to the 1-h pad test in women with stress urinary incontinence. BJU Int. 2008;102(5):586-590.
Gacci M, Del Popolo G, Artibani W, et al. Visual assessment of uroflowmetry curves: description and interpretation by urodynamicists. World J Urol. 2007;25:333-337.
Gallantine ML, Cespedes RD. Occult stress urinary incontinence and the effect of vaginal vault prolapse on abdominal leak point pressures. Urology. 2001;57:40-44.
Gehrich A, Stany MP, Fischer JR, et al. Establishing a mean postvoid residual volume in asymptomatic perimenopausal and postmenopausal women. Obstet and Gynecol. 2007;110(4):827-832.
Ghani KR, Pilcher J, Rowland D, et al. Portable ultrasonography and bladder volume accuracy—a comparative study using three-dimensional ultrasonography. Urology. 2008;72(1):24-28.
Ghoniem GM, Bloom DA, McGuire EJ, Stewart KL. Bladder compliance in meningomyelocele children. J Urol. 1989;141(6):1404-1406.
Ghoniem GM, Roach MB, Lewis VH, Harmon EP. The value of leak pressure and bladder compliance in the urodynamic evaluation of meningomyelocele patients. J Urol. 1990;144(6):1440-1442.
Gormley EA. Evaluation of the patient with incontinence. Can J Urol. 2007;14(Suppl. 1):58-62.
Gousse AE, Barbaric ZL, Safir MH, et al. Dynamic half-Fourier acquisition, single shot turbo spin echo magnetic resonance imaging for evaluating the female pelvis. J Urol. 2000;164(5):1606-1613.
Grody MHT. Urinary incontinence and concomitant prolapse. Clin Obstet Gynecol. 1998;41:777-785.
Groutz A, Blaivas JG, Chaikin DC, et al. Noninvasive outcome measures o urinary incontinence and lower urinary tract symptoms: a multicenter study of micturition diary and pad tests. J Urol. 2000;164(3 Pt 1):698-701.
Gupta A, Defreitas GA, Lemack GE. The reproducibility of urodynamic findings in a healthy female volunteers: results of repeated studies in the same setting and at short-term follow-up. Neurourol Urodyn. 2004;23:311-316.
Hansen BJ, Flyger H, Brasso K, et al. Validation of the self-administered Danish Prostatic Symptoms Score (DAN-PSS-1). Clinical assessment of indications and outcomes of transurethral prostatectomy for uncomplicated benign prostatic hyperplasia. Br J Urol. 1995;76(4):451-458.
Harris SS, Link LL, Tennstedt SL, et al. Care seeking and treatment for urinary incontinence in a diverse population. J Urol. 2007;177(2):680-684.
Harvey MA, Versi E. Predictive value of clinical evaluation of stress urinary incontinence: a summary of the published literature. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(1):31-37.
Hendriks EJ, Bernards At, Berghmans BC, de Bie RA. The psychometric properties of the PRAFAB-questionnaire: a brief assessment questionnaire to evaluate severity of urinary incontinence in women. Neurourol Urodyn. 2007;26(7):512-518.
Holroyd-Leduc JM, Tannenbaum C, Thorpe KE, Straus SE. What type of urinary incontinence does this woman have? JAMA. 2008;299(12):1446-1456.
Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
International Consultation on Incontinence. ICI modular questionnaires. www.iciq.net/index.html. accessed 27.03.11
Jackson S, Donovan J, Brookers S, et al. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77(6):805-812.
Jensen JK, Nielsen FRJr, Ostergard DR. The role of patient history in the diagnosis of urinary incontinence. Obstet Gynecol. 1994;83(5 Pt. 2):904-910.
Karantanis E, Fynes M, Moore KH, Stanton SL. Comparison of the ICIQ-SF and 24-hour pad test with other measures for evaluating the severity of urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(2):111-116.
Karantanis E, O’Sullivan R, Moore KH. The 24-hour pad test in continence women and men: normal values and cyclical alternations. BJOG. 2003;110(6):567-571.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinence women. Br J Obstet Gynaecol. 1997;104(12):1374-1379.
Ku JH, Hong SK, Kim HH, et al. Is questionnaire enough to assess number of nocturic episodes? Prospective comparative study between data from questionnaire and frequency-volume chart. Urology. 2004;64:966-969.
Larsson B, Andersson KE, Batra S, et al. Effects of estradiol on norepinephrine-induced contraction, alpha-adrenoreceptor number, and norephinephrine content in the female rabbit urethra. J Pharmacol Exp Ther. 1984;229(2):557-563.
Law YM, Fieldling JR. MRI of pelvic floor dysfunction: Review. AJR. 2008;191:45-53.
Lawrence JM, Lukacz ES, Nager CW, et al. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet and Gynecol. 2008;111(3):678-685.
Lemack GE, Kraus S, Litman H, et al. Normal preoperative urodynamic testing does not predict voiding dysfunction after Burch colposuspension versus pubovaginal sling, for the Urinary Incontinence Treatment Network. J Urol, 2008;180, 2076-2080.
Lose F, Fantl JA, Victor A, et al. Outcome measures for research in adult women with symptoms of lower urinary tract dysfunction. Standardization Committee of the International Continence Society. Acta Obstet Gynecol Scand. 2001;80:981-985.
Lose F, Gammelgaard J, Jorgensen TJ. The on-hour pad-weighing test: reproducibility and the correlation between the test result, the start volume in the bladder and the diuresis. Neurourol Urodyn. 1986;5:17-21.
Lose G, Jørgensen L, Thunedborg P. 24-hour pad weighing test versus 1-hour ward test in the assessment of mild stress incontinence. Acta Obstet Gynecol Scand. 1989;68(3):211-215.
Lose G, Rosenkilde P, Gammelgaard J, Schroeder T. Pad-weighing test performed with standardized bladder volume. Urology. 1988;23(1):78-80.
Lukacs B, Comet D, Grange JC, Thibault P. Construction and validation of a short-form benign prostatic hypertrophy health-related quality-of-life questionnaire. BPH Group in General Practice. Br J Urol. 1997;80(5):722-730.
Macura KJ. Magnetic resonance imaging of pelvic floor defects in women. Magn Reson Imaging. 2006;17:417-426.
Macura KJ, Genadry RR. Female urinary incontinence: pathophysiology, methods of evaluation and role of MR imaging. Abdom Imaging. 2008;33(3):371-380.
Macura KJ, Genadry RR, Bluemke DA. MR imaging of the female urethra and supporting ligaments in assessment of urinary incontinence: spectrum of abnormalities. Radiographics. 2006;26:1135-1149.
Martin DR, Salman K, Wilmot CC, Galloway NT. MR imaging evaluation of the pelvic floor for the assessment of vaginal prolapse and urinary incontinence. Magn Reson Imaging Clin N Am. 2006;14(4):523-535.
Martin JL, Williams KS, Sutton AJ, et al. Systemic review and meta-analysis of methods of diagnostic assessment for urinary incontinence. Neurourol Urodyn. 2006;25(7):674-683.
Matharu GS, Assassa RP, Williams KS, et al. Objective assessment of urinary incontinence in women: comparison of the one-hour and 24 hour pad tests. Eur Urol. 2004;45(2):208-212.
Matsuoka H, Wexner SD, Desai MB, et al. A comparison between magnetic resonance imaging and videoproctography in patients with constipation. Dis Colon Rectum. 2001;44(4):571-576.
McCormack M, Infante-Rivard C, Schick E. Agreement between clinical methods of measurement of urinary frequency and functional bladder capacity. Br J Urol. 1992;69:17.
McGuire EJ, Woodside JR, Borden A, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126(2):205-209.
Miedel A, Tegerstedt G, Maehle-Schmidt M, et al. Nonobstetric risk factors for symptomatic pelvic prolapse. Obstet Gynecol. 2009;113(5):1089-1097.
Nager CW, Albo ME, FitzGerald MP, et alUrinary Incontinence Treatment Network. Reference urodynamic values for stress incontinent women. Neurourol Urodyn. 2007;26:333-340.
Nager CW, FitzGerald MP, Kraus SR, et al. Urodynamic measures do not predict stress continence outcomes after surgery for stress urinary incontinence in selected women, for the Urinary Incontinence Treatment Network. J Urol, 2008;179, 1470-1474.
Nitti VW, Blaivas JG. Urinary incontinence: epidemiology, pathophysiology, evaluation, and management overview. In: Wein AJ, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders Elsevier; 2007:2059.
Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174(2):604-607.
Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in U.S. women. JAMA. 2008;300(111):1311-1316.
O’Sullivan R, Karantanis E, Stevermuer TL, et al. Definition of mild, moderate and severe incontinence on the 24-hour pad test. BJOG. 2004;111(8):859-862.
Panayi DC, Duckett J, Digesu GA, et al. Pre-operative opening detrusor pressure is predictive of detrusor overactivity following TVT in patients with pre-operative mixed urinary incontinence. Neurourol Urodyn. 2009;28(1):82-85.
Pannu HK, Kaufman JS, Cundiff GW, et al. Dynamic MR imaging of pelvic organ prolapse: spectrum of abnormalities. Radiographics. 2000;20:1567-1582.
Patel AK, Chapple CR. Urodynamics in the management of female stress incontinence–which test and when? Curr Opin Urol. 2008;18:359-364.
Petrelli NJ, Nagel S, Rodruiguez-Bigas M, et al. Morbidity and mortality following abdominoperineal resection for rectal adenocarcinoma. Am Surg. 1993;59(7):400-404.
Raghavaiah NV. Double-dye test to diagnose various types of vaginal fistulas. J Urol. 1974;112(6):811-812.
Resnick NM. Urinary incontinence in the elderly. Med Grand Rounds. 1984;3:281-290.
Richardson DA, Bent AE, Ostergard DR. The effect of uterovaginal prolapse on urethrovesical pressure dynamics. Am J Obstet Gynecol. 1983;146(8):901-905.
Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):455-460.
Roovers JP, van Laar JO, Loffeld C, et al. Does urodynamic investigation improve outcome in patients undergoing prolapse surgery? Neurourol Urodyn. 2007;26:170-175.
Rovner E. Bladder and urethral diverticula. In: Wein AJ, Kavoussi LR, Novick AC, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders Elsevier; 2007:2361-2390.
Ryhammer AM, Laurberg S, Djurhuus JC, Hermann AP. No relationship between subjective assessment of urinary incontinence and pad test weight gain in a random population sample of menopausal women. J Urol. 1998;159(3):800-803.
Samuelsson E, Victor A, Svärdsudd K. Determinants of urinary incontinence in a population of young and middle-aged women. Acta Obstet Gynecol Scand. 2000;79(3):208-215.
Shamliyan TA, Wyman JF, Pink R, et al. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11(3):145-165.
Shaw C, Matthews RJ, Perry SI, et al. Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptoms Questionnaire. BU Int. 2002;90(3):205-215.
Siltberg J, Larsson G, Victor A. Frequency/volume chart: the basic tool for investigating urinary symptoms. Acta Obstet Gynecol Scand Suppl. 1997;166:24-27.
Simons AM, Yoong WC, Buckland S, Moore KH. Inadequate repeatability of the one-hour pad test: the need for a new incontinence outcome measure. BJOG. 2001;108(3):315-319.
Smith PP, Hurtado EA, Appell RA. Post hoc interpretation of urodynamic evaluation is qualitatively different than interpretation at the time of urodynamic study. Neurourol Urodyn. 2009. Epub ahead of print
Stach-Lempinen B, Kujansuu E, Laippala P, Metsanoja R. Visual analogue scale, urinary incontinence severity score and 15 D—psychometric testing of three different health-related quality-of-life instruments for urinary incontinence women. Scand J Urol Nephrol. 2001;35(6):476-483.
Staskin DR. In Patient-Reported Outcome Assessment. Fourth International Consultation on Incontinence, report of Committee 5, part 5B, p. 363–412, 2009.
Stav K, Dwyer PL, Rosamilia A. Women overestimate daytime urinary frequency: the importance of the bladder diary. J Urol. 2009;181(5):2176-2180.
Summitt RLJr, Stovall TG, Bent AE, Ostergard DR. Urinary incontinence: correlation of history and brief office evaluation with multichannel urodynamic testing. Am J Obstet Gynecol. 1992;166(6Pt1):1835-1840.
The National Institute for Health and Clinical Excellence. The management of urinary incontinence in women. London, RCOG press http://www.nice.org.uk/guidance/CG40/guidnance/pdf/English/download.dspx accessed 2006
Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: urinary incontinence in women—national trends in hospitalizations, office visits, treatment and economic impact. J Urol. 2005;173:1295-1301.
Tincello DG, Richmond DH. The Larsson frequency/volume chart is not a substitute for cystometry in the investigation of women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9(6):391-396.
Townsend MK, Curhan GC, Resnick NM, Grodstein F. Oral contraceptive use and incident urinary incontinence in premenopausal women. J Urol. 2009;181(5):2170-2175.
Tseng LH, Liang CC, Chang YL, et al. Postvoid residual urine in women with stress incontinence. Neurourol Urodyn. 2008;27(1):48-51.
Tubaro A, Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Third International Consultation on Incontinence. Plymouth (MA): Health Publications, Ltd., 2005.
Twiss C, Triaca V, Rodriguez LV. Familial transmission of urogenital prolapse and incontinence. Curr Opin Obstet Gynecol. 2007:464-468.
Twiss CO, Fischer MC, Nitti NW. Comparison between reduction in 24-hour pad weight, International Consultation on Incontinence-Short Form (ICIQ-SF) score, International Prostate Symptom Score (IPSS), and post-operative Patient Global Impression of Improvement (PGI-I) score in patient evaluation after male perineal sling. Neurourol Urodyn. 2007;26:8-13.
Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory, Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131-140.
US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. Clinical practice guidelines: urinary incontinence in adults. Washington (DC): U.S. Department of Health and Human Services; March 1992.
Van de Beek C, Stoevelaar HJ, McDonnell J, et al. Interpretation of uroflowmetry curves by urologists. J Urol. 1997;157:164-168.
van de Vaart J, Falconer C, Quail D, et al. Patient reported outcomes tools in an observational study of female stress urinary incontinence. Neururol Urodyn. 2010;29(3):348-353.
Visco AG, Brubaker L, Nygaard I, et alPelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607-614.
Wagner TH, Patrick DL, Bavendam TG, et al. Quality of life of persons with urinary incontinence: development of a new measure. Urology. 1996;47(1):67-71.
Walters MD, Diaz K. Q-tip test: a study of continent and incontinent women. Obstet Gynecol. 1987;70(2):208-211.
Weber AM, Taylor RJ, Wei JT, et al. The cost-effectiveness of preoperative testing (basic office assessment vs. urodynamics) for stress urinary incontinence in women. BJU Int. 2002;89(4):356-363.
Weber AM, Walters MD. Cost-effectiveness of urodynamic testing before surgery for women with pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol. 2000;183(6):1338-1346.
Whiteside JL, Hijaz A, Imrey PB, et al. Reliability and agreement of urodynamics interpretations in a female pelvic medicine center. Obstet Gynecol. 2006;108:315-323.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. women. Obstet Gynecol. 2009;114(6):1278-1283.
Wu WY, Sheu BC, Lin HH. Comparison of 20-minute pad test versus one-hour pad test in women with stress urinary incontinence. Urology. 2006;68:764-768.
Wyman JF, Choi SC, Harkins SW, et al. The urinary diary in evaluation of incontinent women: a test-retest analysis. Obstet Gynecol. 1988;71(6 Pt. 1):812-817.
Wyman JF, Harkins SW, Choi SC, et al. Psychosocial impact of urinary incontinence in women. Obstet Gynecol. 1987;70(3 Pt. 1):278-281.
Zimmern P, Nager CW, Albo M, et alUrinary Incontinence Treatment Network. Inter-rater reliability of filling cystometrogram interpretation in a multicenter study. J Urol. 2006;175:2174-2177.