chapter 77 Urinary Tract Fistulae
A fistula represents an extra-anatomic communication between two or more epithelial or mesothelial-lined body cavities or the skin surface. Although most fistulae in the industrialized world are iatrogenic, they may also occur as a result of congenital anomalies, malignancy, inflammation and infection, radiation therapy, iatrogenic (surgical) or external tissue trauma, ischemia, parturition, and a variety of other processes. The potential exists for fistula formation between a portion of the urinary tract (i.e., kidney, ureters, bladder, and urethra) and virtually any other body cavity, including the chest (pleural cavity), gastrointestinal (GI) tract, lymphatics, vascular system, genitalia, skin, and reproductive organs. Classification is generally based on the organ of origin in the urinary tract and the termination point of the fistula (e.g., vagina, skin, GI tract). The presenting symptoms and signs are variable and depend to a large degree on the involved organs, the presence of underlying urinary obstruction or infection, the size of the fistula, and associated medical conditions such as malignancy.
General Considerations
Acquired urinary fistulae in the industrialized world are almost universally unexpected and may result in a great deal of inconvenience, discomfort, and physical disability for the affected individual. They are most often acquired as a result of a medical or surgical intervention for an unrelated problem, and, consequently, considerable emotional and psychologic distress often accompanies the diagnosis and subsequent treatment. This may be expressed as anger, resentment, and disappointment on the part of the patient toward the physician. As a result, not infrequently, the medicolegal aspects of these cases can be very disturbing to the treating health care practitioner, with an increasing proportion of these cases being adjudicated in court (Thomas and Williams, 2000). Only the most naive surgeon would fail to recognize that many of these fascinating reconstructive cases often have significant medicolegal implications, especially in the setting of iatrogenic fistula creation. Nevertheless, minimizing patient discomfort, maintaining a positive and honest patient–physician relationship while providing constant reassurance, and, finally and perhaps most importantly, pursuing expeditious and successful treatment of the fistula will most often result in a satisfactory, non-confrontational, mutually satisfying long-term outcome.
Notably, after the initial diagnosis of a urinary fistula, which results in external urinary leakage, immediate management or control of the urinary leakage is vital. Addressing this quickly will reduce skin breakdown and related complications, as well as alleviate much of the psychologic distress on the part of the affected individual. The judicious use of catheters, pads, and appliances can be very helpful in this regard. Skin care and odor control products are also adjunctive measures in minimizing patient-related distress until definitive therapy and repair of the fistula can be undertaken. The importance of these types of interventions on the patient’s behalf should not be underestimated by the surgeon. These simple measures can often deflect or assuage the anger of an otherwise very disaffected sufferer, thereby reducing the potential for further aggravating an already difficult medical and, possibly, litigious situation.
The principles of repair of urinary fistulae are outlined in Table 77–1 and can be applied to virtually any type of fistula involving the urinary tract (see Table 77–1). Prevention of urinary fistulae is, of course, paramount; however, nutrition, infection, and malignancy are important considerations not only when assessing a patient for the risk of creation of a fistula during any given intervention, but also during an evaluation for the repair of an existing urinary fistula. Although the vast majority of urinary fistulae in the industrialized world occur in healthy, well-nourished individuals, a nutritional assessment may be an important factor in some patients with fistulae, such as those patients with malignancies. Ensuring adequate nutrition is integral to surgical healing in general, but is especially important in the setting of a urinary fistula. Not uncommonly, the catabolic processes contributing to the lack of healing, which may have been a contributing factor in the initial fistula formation, are often ongoing. This is especially relevant in fistulae related to radiation therapy or in debilitated patients.
Table 77–1 Principles of Urinary Fistula Management
Although some types of urinary fistulae will heal with conservative management, surgery often assumes a role in the definitive repair. Repair and reconstruction of urinary fistulae are sometimes complex. These should be approached on a case by case basis, because repair may involve some innovative and even improvisational maneuvers in the operating room. The surgeon should be familiar with a variety of approaches and techniques, because one approach will not be optimal for all patients with a given type of urinary fistulae. Principles of surgical management of urinary fistulae are outlined in Table 77–2. The finding of a persistent fistula after presumably definitive treatment may suggest the existence of other contributing host factors, such as malignancy, nutritional issues, the possibility of an unrecognized foreign body, tissue ischemia, or surgical factors, such as inadequate postoperative urinary drainage, persistent distal urinary obstruction, or technical problems with the surgery.
Table 77–2 Principles of Surgical Repair of Urinary Fistula
Urogynecologic Fistulae
Vesicovaginal Fistulae
Vesicovaginal fistulae (VVF) are the most common acquired fistula of the urinary tract (Gerber and Schoenberg, 1993) and have been known since ancient times (Fig. 77–1). However, it was not until 1663 that Hendrik von Roonhuyse first described surgical repair of VVF by denuding the fistula margins and then reapproximating them with sharpened stiff swan quills (Margolis and Mercer, 1994). Johann Fatio is generally credited with the first successful VVF repair, in 1675, using von Roonhuyse’s technique (Falk and Tancer, 1954). In 1838, using leaden suture, John Peter Mettauer was the first U.S. surgeon to claim a successful VVF closure (Kight, 1967). In 1852, James Marion Sims published his now famous surgical series describing his method of surgical treatment of VVF using silver wire in a transvaginal approach (Sims, 1852). Of note, it was not until his 30th attempt at closure of VVF that he achieved success. However, Sims remains the subject of considerable debate regarding his ethics (Richardson, 1994; Sartin, 2004), because it is unknown whether the patients in his surgical series were willing and consenting participants (all were African-American slaves in pre–Civil War America). He was later to become one of the great figures in the history of operative gynecology. The first successful transabdominal approach to VVF repair was reported by Trendelenburg in 1888, and the concept of an interpositional flap was first proposed and reported in 1928 by Martius, who used a labial fat pad.
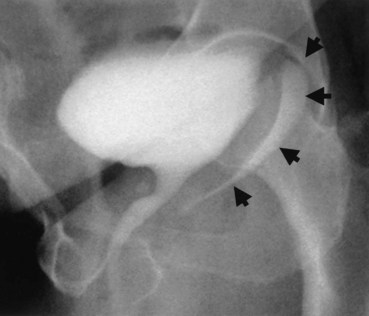
Figure 77–1 Voiding cystourethrogram demonstrates filling of the vagina with voiding due to a post-hysterectomy vesicovaginal fistula.
Etiology and Prevalence
The etiology of VVF differs in various parts of the world. In the industrialized world, the most common cause (>75%) of VVF is injury to the bladder at the time of gynecologic, urologic, or other pelvic surgery (Symmonds, 1984; Lee et al, 1988; Tancer, 1992). Surgical injury to the lower urinary tract most commonly occurs in the setting of hysterectomy (Fig. 77–2), whereas most of the remainder are related to general surgery procedures in the pelvis, anterior colporrhaphy or cystocele repair, anti-incontinence surgery, or other urologic procedures (Armenakas et al, 2004). Of 207 VVF repaired at the University of Caliornia–Los Angeles (UCLA) over a 10-year period ending in 2001, Eilber and colleagues (2003) reported the cause as abdominal hysterectomy in 83%, vaginal hysterectomy in 8%, radiation in 4%, and miscellaneous in 5%. In 1964, Massee and colleagues from the Mayo Clinic reviewed the cause of urogenital fistulae in 262 patients and cited uterine operations as a proximate cause in 73.7%, vaginal wall operations in 6.5%, urinary tract operations in 6.9%, and obstetric operations in 6.5%, with the remainder being due to miscellaneous causes. A later series of greater than 300 fistulae from the same institution cited gynecologic surgery as the cause of VVF in 82%, obstetric procedures in 8%, radiation in 6%, and trauma or fulguration in 4% (Lee et al, 1988). Other causes of VVF in the industrialized world include malignancy, pelvic radiation, and obstetrical trauma (including forceps lacerations and uterine rupture) (Everett and Mattingly, 1956; Gerber and Schoenberg, 1993). The use of vaginal mesh for prolapse repair may also result in VVF (Margulies et al, 2008; Ridgeway et al, 2008). Although fortunately uncommon during labor, approximately 22% of uterine ruptures are associated with a bladder injury (Raghavaiah and Devi, 1975). Prior to 1900, the most common cause of VVF in the United States was obstructed labor (Stothers et al, 1996). However, obstructed labor and obstetric trauma, in general, now account for very few VVF in the United States and other industrialized nations, probably due to the widespread availability of excellent prenatal and perinatal obstetric care.
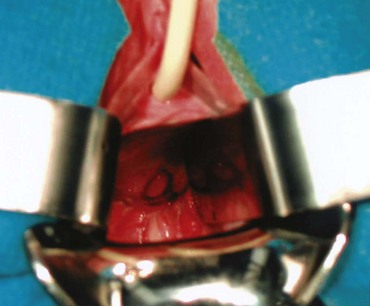
Figure 77–2 Posthysterectomy vesicovaginal fistula (VVF). Retraction with a weighted speculum and Heaney right-angled retractors to provide lateral retraction are needed to visualize this posthysterectomy VVF in a nulliparous woman.
The rate of iatrogenic bladder injury during abdominal hysterectomy is estimated to be between 0.5% and 1.0% (Keettel et al, 1978). Mathevet and colleagues (2001) reported the incidence of bladder injury during vaginal hysterectomy to be 1.7% in 3076 cases, with all injuries being recognized and repaired intraoperatively. Despite the immediate intraoperative repair reported in this series, there were four VVF noted, giving a crude VVF rate during vaginal hysterectomy of 0.13%. The reported incidence of intraoperative bladder injury varies considerably in the literature, depending on whether routine cystoscopy was performed. In series where cystoscopy was not performed, the overall rate of bladder injury was reported to be approximately 2.6 per 1000 cases, whereas in series where cystoscopy was routinely performed, the overall rate of bladder injury was approximately 10.4 per 1000 cases (Gilmour et al, 1999). The incidence of fistula after hysterectomy is estimated to be approximately 0.1% to 0.2% (Harris, 1995). There are approximately 140 to 150 VVF repairs annually in England and Wales (Hilton, 1997).
Posthysterectomy VVF are thought to result most commonly from an incidental unrecognized iatrogenic cystotomy near the vaginal cuff (Kursh et al, 1988). If unrecognized intraoperatively, a pelvic urinoma may develop and ultimately drain out through the vaginal cuff. Ongoing urinary drainage along this tract results in a fistula. Other potential mechanisms for posthysterectomy VVF include tissue necrosis from cautery, a suture placed through both the bladder and vaginal wall during closure of the vaginal cuff, or an attempt to control pelvic bleeding by suture ligature. Tissue ischemia and then necrosis promotes fibrosis and induration, finally resulting in an epithelial or mucosal lining of the tract and the development of a fistula tract. Possibly, factors other than an isolated suture placed through the bladder and vagina are necessary for posthysterectomy VVF formation because, at least, in an animal model, deliberate suture fixation of the bladder to the vagina does not invariably result in VVF in the absence of infection, urinary extravasation, or other complicating factors (Meeks et al, 1997).
In the developing world where routine perinatal obstetrical care may be limited, VVF most commonly occur as a result of prolonged obstructed labor due to cephalopelvic disproportion, with resulting pressure necrosis to the anterior vaginal wall, bladder, bladder neck, and proximal urethra from the baby (Arrowsmith et al, 1996) (Fig. 77–3). The constellation of problems resulting from obstructed labor is not limited to VVF and has been termed the “obstructed labor injury complex” and includes varying degrees of each of the following: urethral loss, stress incontinence, hydroureteronephrosis, renal failure, rectovaginal fistula, rectal atresia, anal sphincter incompetence, cervical destruction, amenorrhea, pelvic inflammatory disease, secondary infertility, vaginal stenosis, osteitis pubis, and foot drop (Arrowsmith et al, 1996). The obstructed labor injury complex occurs largely in developing countries in certain cultures due to several factors, including (1) marriage and conception at a very young age, which results in childbearing in a relatively small and immature pelvis, (2) poor nutrition resulting in stunted skeletal (e.g., pelvic) growth in the mother, and (3) relative absence of qualified prenatal and obstetrical care (Margolis and Mercer, 1994). Patients may suffer with obstructed labor for days in a rural environment, eventually traveling to a distant health care facility only to have a stillborn fetus and a VVF. In direct contradistinction to the epidemiology of VVF in the industrialized world, 96.5% of 932 VVF seen at a single hospital in Nigeria over a 7-year period were temporally associated with labor and delivery (Wall et al, 2004). The incidence of obstetric fistula in developing countries has been estimated at approximately 0.3% to 0.4% of deliveries (Margolis et al, 1994), or between 1 and 4 per 1000 vaginal deliveries (Margolis et al, 1994; Danso et al, 1996). In sub-Saharan Africa the incidence rate has been estimated at 10.3 per 100,000 deliveries (Vangeenderhuysen et al, 2001). An estimate of up to 500,000 new cases of obstetric fistula occur throughout the world annually (Hilton, 2003), although the total morbidity from obstructed maternal labor has been estimated to be in excess of 5 million individuals annually (Kelly, 1991). Risk factors include young or old maternal age, and primigravids (Danso et al, 1996).
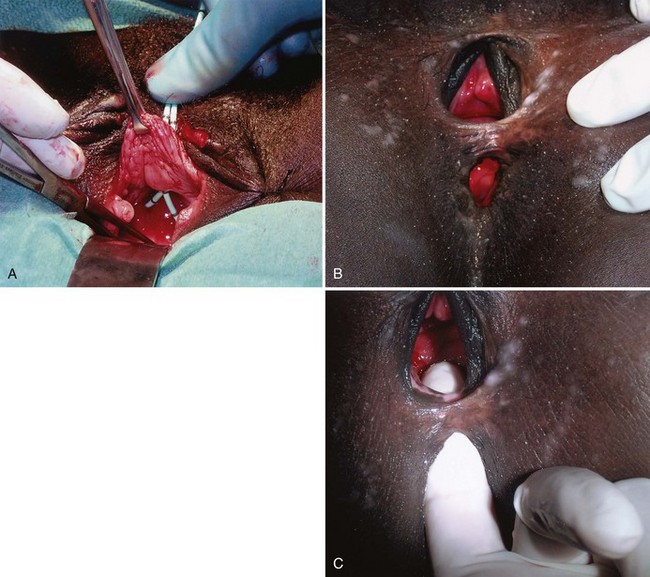
Figure 77–3 Vesicovaginal fistula (VVF) secondary to obstructed labor. A, Note the complete loss of the proximal urethra and bladder neck. The ureteral catheters have been passed through the urethra and delineate the ureteral orifices before repair. B, Large VVF and rectovaginal fistula due to obstructed labor. C, Same patient as in B. Digital examination confirms rectovaginal and vesicovaginal fistula in this patient
(A to C, Courtesy of Mark Morgan, MD, Department of Obstetrics and Gynecology, Hospital of the University of Pennsylvania, Philadelphia, PA.)
Obstetric fistulae tend to be larger, located distally in the vagina, and may involve large portions of the bladder neck and proximal urethra. Even in experienced hands, these are often very difficult to repair due to the extensive soft tissue loss, as well as the ischemia and fibrosis of adjacent tissues (Arrowsmith, 1994; Elkins, 1994). Obstetric fistulae are devastating injuries in the developing world, with significant socioeconomic ramifications (Donnay and Weil, 2004; Wall et al, 2004). The affected individuals, commonly young teens, are often ostracized and shunned from family and friends and become permanent outcasts in society. Only rarely are these individuals able to get adequate care and repair due to the lack of organized health care in many countries in the developing world. Major efforts are underway to improve education, the socioeconomic status of women, and access to health care in these regions (Tahzib, 1983; Tahzib, 1989; Kelly, 1991; Wall, 1996; Donnay and Weil, 2004; Kelly, 2004). With improvements in the delivery of prenatal care in some areas of the developing world, it appears that the incidence of VVF due to obstetric causes may be decreasing as a subset of all VVF, with a proportional increase in the rate of iatrogenic gynecologic fistulae approaching that in industrialized nations (Ayhan et al, 1995; Obi et al, 2008). Another important cause of VVF in some parts of the world is traditional folk treatments such as “gishiri cutting,” which may account for up to 13% of VVF in some series (Tahzib, 1983). This ritual practice involves using a knife to incise the anterior vagina as a treatment for a variety of conditions, including infertility, dyspareunia, dysuria, and back pain.
Other causes of VVF include urologic or gynecologic instrumentation, including percutaneous procedures (Ramsay et al, 1992; Pruthi et al, 2000), retroperitoneal, vascular or pelvic surgery, infectious and inflammatory diseases (Borjas and Rodriguez Diaz 1949; Bland and Gelfand, 1970; Ba-Thike et al, 1992; Monteiro et al, 1995), foreign bodies (including neglected pessaries) (Binstock et al, 1990; Goldstein et al, 1990; Grody et al, 1999), congenital VVF (Rousseau et al, 1996), sexual trauma (Roy et al, 2002), vaginal laser procedures (Colombel et al, 1995), and external violence (see Table 77–3). The three most common locally advanced malignancies that result in VVF include cervical, vaginal, and endometrial carcinoma, which in aggregate account for approximately 3% to 5% of VVF in the industrialized world.
Table 77–3 Etiology of Vesicovaginal Fistula
VVF due to radiation therapy deserve special mention. These VVF may occur several decades after completion of the radiation (Zoubek et al, 1989). The incidence of urinary fistula following radiation therapy varies with the type, dose, and location of the radiation. Both external beam and interstitial (Aristizabal et al, 1983) radiation therapy may result in VVF. An incidence of 1.6% of any type of urinary fistula was noted in one series of greater than 2200 patients treated with a variety of different radiation modalities for cervical carcinoma (Alert et al, 1980). Perez reported a 0.6% to 2.0% incidence of VVF formation in 1456 patients undergoing combined external beam radiotherapy and brachytherapy for stages I to III cervical cancer (Perez et al, 1999). In a series of 2096 patients undergoing therapy for cervical cancer, there was an overall genital fistula rate of 1.8%, including rectovaginal fistulae. All patients diagnosed with fistula had received radiation therapy, and the median interval from completion of radiation to presentation of VVF was 8.7 months (Emmert and Kohler, 1996). Higher radiation doses seem to correlate with a greater risk for overall morbidity, as well as fistula formation (Perez et al, 1984; Perez et al, 1999). The endarteritis as a result of the radiation therapy may involve the surrounding tissues, limiting reconstructive options. An important consideration in any fistula following radiation therapy for malignancy is the possibility that the fistula represents a recurrence of the malignancy. Therefore biopsy of fistula tract should be strongly considered prior to considering definitive repair in these patients.
Intraoperative Risk Factors for Iatrogenic VVF
Clearly, intraoperative injury to the urinary bladder is a primary risk factor for subsequent development of a postoperative VVF. Other risk factors for postoperative VVF formation include prior uterine surgery (cesarean section), endometriosis, infection, diabetes, arteriosclerosis, pelvic inflammatory disease, and prior radiation therapy (Blandy et al, 1991; Likic et al, 2008). In a large series of VVF, Tancer and colleagues (1992) described several factors that increased the risk for VVF following hysterectomy, including prior uterine operation (especially cesarean section), endometriosis, recent cold-knife cervical conization, and prior radiation therapy. Nevertheless, almost 30% of the patients in this series had no identifiable risk factor. The operative approach to hysterectomy is an important factor, because bladder injuries are at least three times more common during abdominal hysterectomy compared with vaginal hysterectomy. Intraoperative recognition and repair of bladder injury is paramount in prevention of VVF; however, despite intraoperative repair, VVF may still result in a substantial number of patients. In 1969, Hutch emphasized five factors in the prevention of VVF during gynecologic surgery: (1) immediate detection of bladder injury using vital dyes if necessary; (2) watertight closure of the bladder; (3) satisfactory extravesical drain placement; (4) avoidance of a vaginal incision, if possible, following recognition of the bladder injury; and (5) prolonged, uninterrupted postoperative bladder drainage (Hutch and Noll, 1970).
Clinical Features
Evaluation and Diagnosis
VVF must be distinguished from urinary incontinence due to other causes, including stress (urethral) incontinence, urge (bladder) incontinence, and overflow incontinence.
Presentation
The most common complaint in patients with VVF is constant urinary drainage per vagina. The amount of urinary leakage can vary considerably from patient to patient and may be proportional to the size of the fistula tract. Patients may void a variable amount, depending on the size of the fistula and the volume of urinary leakage. For example, when a large VVF is present, patients may not void at all and simply have continuous leakage of urine into the vagina. Small, pinpoint fistulae may present with intermittent wetness, which is positional in nature. In the supine position, when sleeping, the amount of leakage reported by the patient may be minimal, but upon rising to a seated or standing position, the amount of leakage may increase precipitously. Patients may also complain of recurrent cystitis, perineal skin irritation due to constant wetness, vaginal fungal infections, or rarely pelvic pain. In fact, pain is an uncommon finding in patients with VVF, unless there is considerable skin irritation or the VVF occurred as a result of radiation therapy.
VVF following hysterectomy or other surgical procedures may present upon removal of the urethral catheter, or VVF may present 1 to 3 weeks later with urinary drainage per vagina. It may be possible to identify some patients at high risk for VVF in the immediate postoperative period. Kursh and colleagues (1988) noted that patients who developed VVF following hysterectomy more commonly had postoperative ileus, hematuria, bladder irritability, and elevated white blood cell count compared with a cohort of patients who did not develop VVF. Occasionally, posthysterectomy VVF may go undiagnosed for an extended period of time, because postoperative clear or serosanguinous vaginal discharge is often attributed to the surgery itself.
As noted previously, VVF resulting from radiation therapy may not present for months to years following completion of radiation. These tend to be very challenging reconstructive cases due to the size and complexity of the fistula, the poor quality of the surrounding tissues, and the associated voiding dysfunction due to the radiation effects on the urinary bladder.
Physical Examination
A pelvic exam with a speculum should always be performed in the evaluation of VVF. The bivalved speculum examination usually provides a precise assessment of VVF including the location, size, and number of fistulae. Vaginoscopy has been suggested as an adjunct measure, in some cases, using a modified endoscope to precisely visualize the fistula tract (Redman, 1990). Most commonly, VVF following hysterectomy are located along the anterior vaginal wall at the level of the vaginal cuff (Fig. 77–4). A visual and manual assessment of inflammation surrounding the fistula is necessary, because it may affect timing of the repair. Significant inflammation, infection, or induration around the fistula may mitigate against immediate repair. Relevant vaginal anatomy, including depth, associated prolapse, atrophy, and introital size are carefully recorded, because these may affect the surgical approach to repair. Anatomically, fistulae located high in the vagina, at the level of the hysterectomy cuff in a deep narrow vagina, may be best approached by some surgeons abdominally because a vaginal approach in these patients can be challenging. This may be especially relevant in nulliparous females in whom there is usually very limited pelvic floor laxity or vaginal prolapse. Postmenopausal vaginal atrophy may be treated with preoperative topical estrogen replacement, thereby optimizing the health and vascularity of potential reconstructive flaps. Palpation for masses or other pelvic pathology, which may require attention at the time of fistula repair, is also performed. Notation of prior incisions in the perineum, lower abdomen, and thigh is necessary because these tissues may be required for flap reconstruction when definitive repair is undertaken.
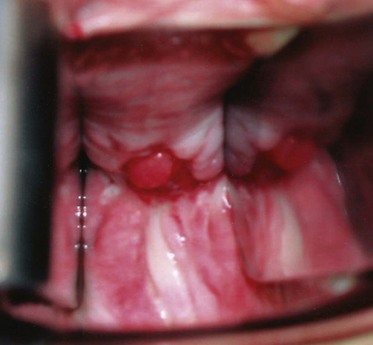
Figure 77–4 Vesicovaginal fistula (VVF) on physical examination. A large VVF is seen at the apex of the vagina after hysterectomy. The VVF in this image is seen as reddish pink bladder mucosa prolapsing into the vagina. Hand-held Heaney right-angled retractors provide lateral retraction in this image.
The presence of a VVF may be confirmed by instilling a vital blue dye into the bladder per urethra and observing whether vaginal drainage is discolored. Small or occult fistulae may be identified in this fashion (Drutz and Mainprize, 1988). A colored solution, such as methylene blue or indigo carmine, is mixed into solution and infused into the bladder. The vagina may be packed with gauze or directly inspected for blue-tinged leakage. If blue-tinged leakage is not apparent and the diagnosis of VVF is in doubt, the sensitivity of this test may be improved by placing a vaginal packing and ambulating the patient for a short period of time. Staining at the introital (distal) end of the packing suggests urinary incontinence or a urethrovaginal fistula, whereas proximal staining suggests a VVF. If the vaginal packing remains dye-free with this maneuver, then the possibility of a ureterovaginal fistula can be investigated with the use of a clean vaginal packing, intravenous indigo carmine (or other vital dye), and a repeat pad test. Blue staining at the proximal end of the pad, following this maneuver, suggests the presence of a ureterovaginal fistula.
A double dye or tampon test may confirm the diagnosis of urinary fistula, as well as suggest the possibility of an associated ureterovaginal or urethrovaginal fistula (Moir, 1973; Raghavaiah, 1974). In one variation of the double dye test, a tampon is placed per vagina. Oral phenazopyridine is administered, and vital blue dye is instilled into the bladder. If the tampon is discolored yellow-orange at the top, it is suggestive of a ureterovaginal fistula. Blue discoloration in the midportion of the tampon suggests VVF, whereas blue staining at the bottom suggests a urethrovaginal fistula.
Clear vaginal discharge following hysterectomy does not invariably represent a urinary fistula or incontinence. Other than normal vaginal secretions, less common causes include a peritoneovaginal fistula (Ginsberg, 1998), lymphatic fistula (Lau and Wong, 1994), vaginitis, and fallopian tube fluid (Leach, 1987). Incontinence following caesarian section, in which a VVF has been excluded, may suggest the possibility of a ureterovaginal or vesicouterine fistula.
Cystoscopy
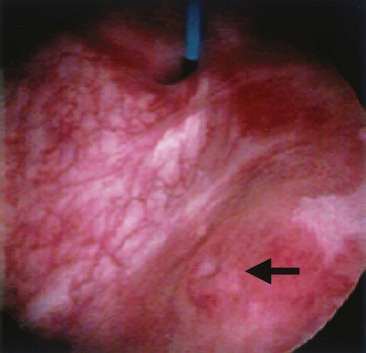
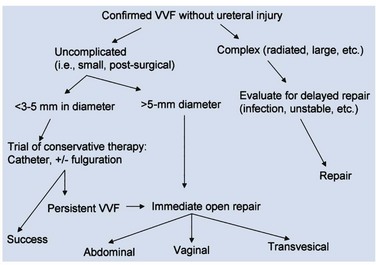
An endoscopic examination should be performed in patients with a suspicion of VVF (Fig. 77–5). Immature fistulae may appear as an area of localized bullous edema without distinct ostia. Mature fistulae may have smooth margins with variably sized ostia. In some cases, multiple pits and cavities along an area of the traumatized posterior bladder wall, in the setting of a small VVF, may make it difficult to identify the exact fistula tract. In these cases, a guidewire or ureteral catheter may be placed through the working channel of the cystoscope and into the fistula tract (Fig. 77–6). Visualization of the wire in the vagina confirms the exact location of the VVF on both the bladder and genital sides. Cystourethroscopy can confirm the presence of the fistula, but also may assess the size of the tract, the presence of collateral fistulae, and the location of the ureteral orifices in relation to the fistula. Small fistulae, usually less than 3 to 4 mm in diameter may be amenable to simple fulguration, which can be performed at the time of cystoscopy (see later discussion) (Stovsky et al, 1994). Importantly, in the setting of a prior history of pelvic malignancy, a biopsy of the fistula is often done to evaluate for the possibility of a recurrent malignancy. Fistulae located near or at the ureteral orifice may require ureteral reimplantation at the time of VVF repair. This type of requirement would usually mitigate against a completely transvaginal attempt at repair.
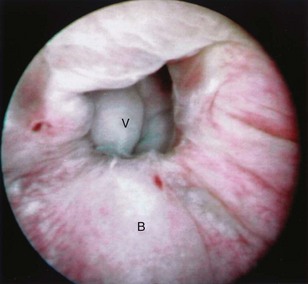
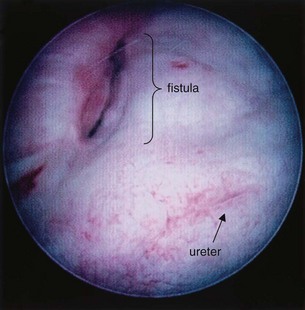
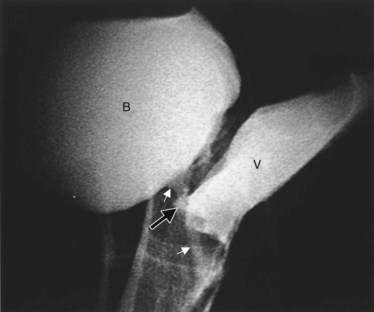
Figure 77–5 Endoscopic view of vesicovaginal fistula (VVF). This is the same patient as in Figure 77–4. The fistula is now seen from the bladder side. This VVF is large enough to see directly into the vagina (V) through the bladder (B).
Imaging
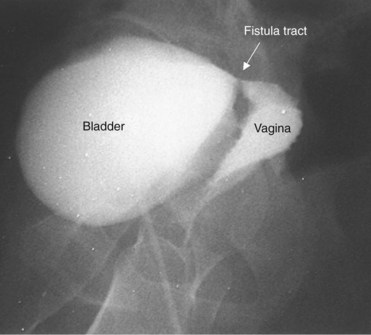
A cystogram and/or voiding cystourethrogram (VCUG) and an upper tract study should be performed in patients being evaluated for a VVF. The cystogram may objectively determine the presence and location of the fistula. Upon filling of the bladder, contrast often begins to opacify the vagina, almost immediately confirming the presence of a VVF. VVF are often best seen in the lateral projection (Fig. 77–7) in which the bladder and vagina are not superimposed. Often, the actual VVF tract may be visible in the lateral projection (Fig. 77–8). However, voiding images may be necessary in some patients with small fistulae, to demonstrate the VVF. The slight increase in intravesical pressure that accompanies micturition is usually adequate to demonstrate even very small fistulae. Importantly, a cystogram that fails to demonstrate a suspected VVF, but lacks voiding images or postvoid images, should be considered nondiagnostic. During voiding, care should be taken to exclude vaginal voiding or reflux of contrast from the introital region cephalad into the vagina, which would produce a falsely positive image. An involuntary bladder contraction can be provoked with rapid filling during cystography, and if the intravesical pressure rises sufficiently, this may also be sufficient to demonstrate a VVF when the filling images of the cystogram failed to demonstrate it. In some instances, a cystogram can also make an assessment of bladder capacity (important in the setting of prior radiotherapy), cystocele, bladder neck competence, and vesicoureteral reflux, any of which may have an impact on operative repair.
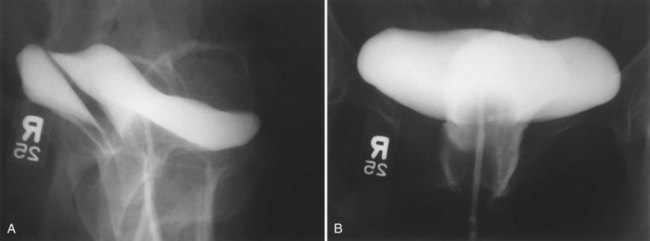
Figure 77–7 Cystogram demonstrating a vesicovaginal fistula (VVF). A, Lateral image demonstrates a posthysterectomy VVF. B, Anteroposterior view. The contrast agent is seen opacifying and outlining the vagina superimposed on the bladder.
Up to 12% of postsurgical VVF have an associated ureteral injury or ureterovaginal fistula (Goodwin and Scardino, 1980); thus, upper urinary tract evaluation is important. Intravenous urography (IVU) (Gerber and Schoenberg, 1993), or, more recently, computed tomography (CT) urography is usually sufficient for this purpose. In one series of 216 consecutive patients with VVF due to obstructed labor, almost 50% of patients were diagnosed with an upper tract abnormality on IVU; caliectasis was found in 71% of those affected; however, almost 10% were found to have a nonfunctioning renal unit (Lagundoye et al, 1976). If there is a suspicion for a ureterovaginal fistula, or if the distal ureter is not well seen IVU, retrograde pyelography may be performed (Blandy et al, 1991) (Fig. 77–9).
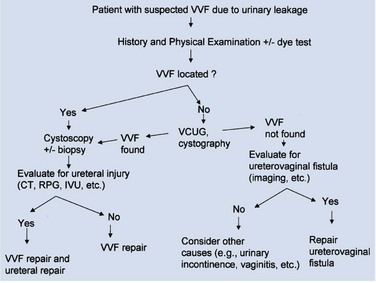
Figure 77–9 Algorithm for the diagnosis of vesicovaginal fistula (VVF). CT, computed tomography; IVU, intravenous urography; RPG, retrograde pyelography; VCUG, voiding cystourethrography.
In addition to contrast cystography and VCUG, CT, ultrasonography, and magnetic resonance imaging (MRI) have been used in the evaluation of VVF (Kuhlman and Fishman, 1990; Outwater and Schiebler, 1993; Yang et al, 1994). Delayed CT visualization of contrast within the vagina is considered highly suspicious for VVF in the majority of cases (Kuhlman and Fishman, 1990) (Fig. 77–10). In cases of suspected VVF, CT should be performed with only intravenous contrast, or, alternatively, a CT cystogram can be performed to isolate the bladder. A vaginal tampon placed per vagina during IVU or CT scan may improve the sensitivity for finding small or occult VVF in patients with an otherwise negative evaluation (Wesolowski and Meaney, 1977). Cross-sectional imaging may also be helpful in assessing for recurrent malignant disease in those with such a history.
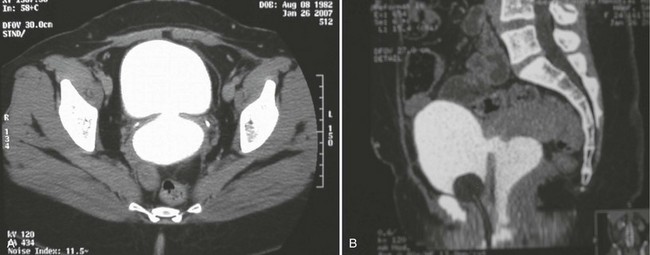
Figure 77–10 CT scan of vesicovaginal fistula (VVF). A, After intravenous administration of the contrast agent, there is high-density material in both the bladder and vagina consistent with a VVF. The fistulous connection between the bladder anteriorly and the vagina posteriorly. B, Sagittal CT reconstruction demonstrating VVF.
Other Studies
Appropriate urine studies including culture, and cytology when indicated, are performed. When infection is detected, appropriate antibiotic coverage is initiated. Urodynamics, including videourodynamics, are generally not necessary in the evaluation of routine posthysterectomy VVF. However, in the setting of a prior history of radiation, radical pelvic surgery (e.g, radical hysterectomy), or in those with preexisting neurogenic vesicourethral dysfunction, a urodynamic evaluation can be used to evaluate for significant detrusor dysfunction, including impaired bladder compliance. In patients with a history of significant symptoms of voiding dysfunction and incontinence preceding the VVF, urodynamic evaluation may help to define these symptoms prior to repair of the VVF (Hilton, 1998), an important element in preoperative counseling regarding potential outcomes and preparation for the optimal operative plan.
Treatment
Conservative and Minimally Invasive Therapy
The goal of treatment of VVF is the rapid cessation of urinary leakage with return of normal and complete urinary and genital function. As noted previously, the physical and psychologic impact of constant urinary incontinence from a VVF can be overwhelming due to the burden of continual wetness, undesirable odor, vaginal and bladder infections and their related discomfort. Bladder catheterization may temporize some of these effects until definitive repair is undertaken but often will not completely eradicate leakage, especially in those with large fistula or those with significant detrusor overactivity. Furthermore, catheterization may provoke additional irritation and pelvic pain and is a constant reminder to the patient of an iatrogenic insult.
Regardless of the aforementioned limitations and drawbacks, a trial of indwelling catheterization and anticholinergic medication for at least 2 to 3 weeks may be warranted in selected patients with newly diagnosed VVF, because spontaneous healing may result (Davits and Miranda, 1991). This is especially applicable in those patients in whom the initial placement of the catheter immediately resolves the vaginal leakage. Tancer (1992) reported three patients with immature VVF tracts who were successfully treated expectantly with indwelling catheterization. All three patients were seen within 3 weeks of the initial surgery and were found to have nonepithelialized VVF tracts on physical examination. Fistulous tracts that remain open 3 or more weeks after adequate Foley drainage are unlikely to resolve without further intervention, especially those which appear completely epithelialized on examination.
Patients with small epithelialized fistulae may benefit from a minimally invasive treatment involving disruption of the epithelial layer of the fistula tract (Fig. 77–11). Catheterization may be combined with minimally invasive electrocoagulation of the fistula tract. In this approach, a small cautery electrode is passed into the fistula tract endoscopically as far as possible. The electrode is slowly withdrawn from the tract with the electrode set on coagulation. The edges of the fistula tract should blanche. Care is taken not to overcoagulate, because this can cause widespread tissue necrosis, sloughing, and enlargement of the fistula. This approach was advocated by O’Conor, as far back as 1938, for small, highly-situated fistulae (O’Conor and Sokol, 1951). Stovsky and colleagues (1994) demonstrated success with endoscopic electrocoagulation and bladder drainage in 11 of 15 cases. All patients had VVF less than 3.5 mm in diameter and were drained for a minimum of 2 weeks post-treatment. It was suggested that disruption of the epithelial component of the VVF tract with subsequent fibrosis, scarring, and closure of the tract is the mechanism by which electrocoagulation exerted its favorable effects. Falk and Orkin (1957) successfully treated eight patients with electrocoagulation and catheter drainage for 10 days. All successfully treated patients had VVF less than 3 mm in diameter; two patients with 6-mm VVF failed this approach. Importantly, in patients with a thin vesicovaginal septum, large VVF, a nonoblique fistula tract, or those with significant inflammation around the fistula tract, fulguration risks failure and the possibility of enlarging the size of the fistula. This approach may also devitalize adjacent tissues, thereby compromising their future utility as flaps. Fibrin sealant has been used as an adjunctive measure to treat VVF (Pettersson et al, 1979; Hedelin et al, 1982). The fibrin sealant may be injected directly into the fistula tract following fulguration as described above. The bladder is then drained for several weeks. Presumably, the gel-like nature of the fibrin sealant plugs the hole until tissue ingrowth occurs from the edges of the fistula. Fibrin sealant has been used successfully in combination with (Morita and Tokue, 1999) and without (Evans et al, 2003b) bovine collagen as an additional “plug.” In general, these conservative measures are useful for small, oblique fistulae (usually less than 2 to 3 mm in diameter), in patients who are agreeable to this course of therapy.
Case reports of other minimally invasive VVF repair techniques have been reported, including laser tissue welding with a Nd-YAG laser (Dogra and Nabi, 2001), as well as transurethral endoscopic suturing (Okamura et al, 1997; McKay, 2001).
Surgical Repair
It has been stated that the best opportunity to achieve successful repair of VVF is with the initial operation (Weed, 1978; Elkins 1994). Previous failed attempts at repair produce scarring, anatomical distortion, and may compromise potential reconstructive flaps. Therefore careful preoperative planning is essential to maximize the chances for a successful result. There is no “best” approach for all patients with VVF.
Timing: Immediate versus Delayed Repair
The timing of VVF repair is somewhat controversial. Repair of VVF should be as expeditious as possible to minimize patient suffering; however, optimal timing for repair should allow consideration of certain medical and surgical factors as well. It is generally accepted that VVF resulting from obstructed labor should be associated with a 3- to 6-month delay prior to definitive repair (Wein et al, 1980a, Arrowsmith, 1994; Waaldijk, 1994) to allow maximum demarcation of ischemic tissue and resolution of the associated edema and inflammatory reaction. Longer periods of time, up to 6 to 12 months, have been advocated for radiation-induced fistulae (Wein et al, 1980a), which are often associated with severe obliterative endarteritis and reduced tissue vascularity.
In the classical teaching of VVF, a minimal waiting period of several months is suggested—from the inciting event prior to the definitive repair attempt—to allow reduced tissue edema and inflammation, and optimal pliability of the tissues (Persky and Rabin 1973; Lawson, 1978; O’Conor, 1980; Wein et al, 1980a). O’Conor recommended a waiting period of 3 to 6 months for suprapubic VVF repairs (O’Conor et al, 1973). In this setting, reduced inflammation and edema permit easier identification of tissue planes (and therefore flap development), less bleeding, and less tension on the reapproximated suture lines. However, over the ensuing decades, the enthusiasm for delayed management has waned and, in general, uncomplicated postgynecological urinary fistulae may be repaired as soon as they are identified and confirmed, thereby minimizing patient discomfort and anguish (Collins et al, 1971; Persky et al, 1979; Fourie, 1983; Badenoch et al, 1987a; Cruikshank, 1988; Wang and Hadley 1990; Blandy et al, 1991; Blaivas et al, 1995; Kostakopoulos et al, 1998b). This is especially true when they occur as a result of clean surgical trauma (Wang and Hadley, 1990). Nondelayed closure has also been applied to obstetric fistulae with good results (Waaldijk, 1994; Waaldijk, 2004). Nevertheless, in some cases, the timing of a VVF repair is best tailored to the individual patient (Blaivas et al, 1995). Raz and colleagues (1993) suggested that uncomplicated VVF following abdominal hysterectomy could and should be repaired as expediently as possible transvaginally; however, a 2- to 3-month waiting period may be warranted for some VVF following vaginal hysterectomy. Conversely, if an abdominal approach is being considered following a particularly difficult or complicated abdominal surgery that resulted in the VVF (i.e, complicated by abscess, urinoma), then a delay may be warranted to allow resolution of active inflammation. Another potential reason to delay repair is to treat ongoing infection or inflammation at the level of the vaginal cuff. Periodic reexamination of the vaginal tissues can be performed every 1 to 2 weeks and definitive repair scheduled when suitable pliability is noted (Carr and Webster, 1996).
A vaginal approach can be attempted as soon as 2 to 3 weeks after the initial injury, if conservative therapy fails (i.e., the patient remains wet when a Foley catheter is in place to provide adequate drainage of the bladder) and the patient is in good general health. The vaginal tissues are usually relatively undisturbed from the prior causative surgery, especially if the surgery was transabdominal. Wide healthy vaginal flaps can usually be obtained. In the rare circumstance when a VVF presents within the first 24 to 48 hours postoperatively, an immediate repair can be attempted (Margolis and Mercer, 1994); however, it is possible that these VVF, especially if small in diameter, may heal spontaneously with catheterization over the course of several weeks. It is well documented that similar outcomes can be achieved with early versus delayed abdominal and vaginal repair (Collins et al, 1960; Persky et al, 1979; Zimmern et al, 1985; Badenoch et al, 1987a; Wang and Hadley, 1990; Blandy et al, 1991) with success rates in excess of 90% for uncomplicated VVF.
Approach: Abdominal versus Vaginal
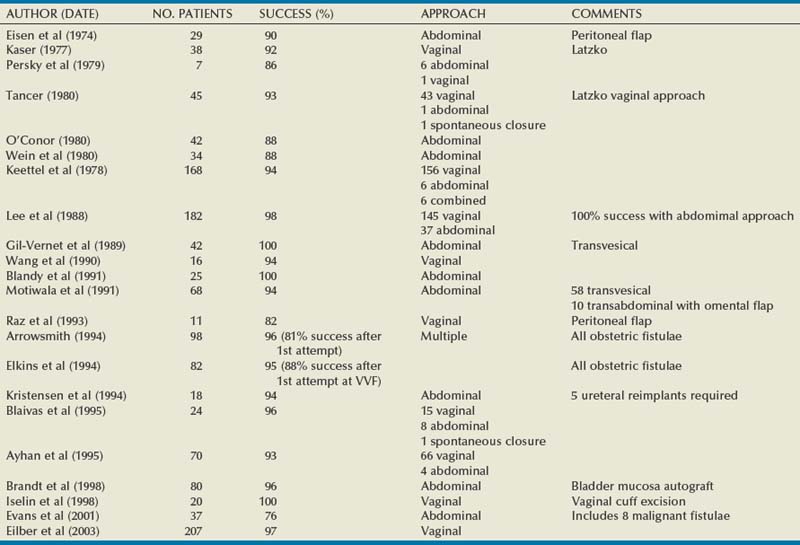
Vesicovaginal fistulae may be repaired through a transvaginal or transabdominal (transvesical) approach. Each approach has merits depending on the particular circumstances of the fistula, and excellent outcomes can be expected with both approaches (Table 77–4). Although factors such as size, location, and the need for adjunctive procedures often influence the choice of approach, the most important factor is the experience of the operating surgeon. Thus there is no preferred approach for all fistulae, and the “optimal” approach to the uncomplicated postgynecological VVF is usually the one that is most successful in the individual surgeon’s hands (Gerber and Schoenberg, 1993; Akman et al, 1999). Although it has been a long-held belief that gynecologists prefer to fix VVF transvaginally, and urologists prefer a transabdominal approach due to their respective training and experience, (Edwards, 1982; Gerber and Schoenberg, 1993), this difference is becoming increasingly blurred as urologists gain more experience and comfort operating transvaginally for a variety of disparate indications.
Table 77–4 Abdominal versus Transvaginal Repair of Vesicovaginal Fistula
| ABDOMINAL | TRANSVAGINAL | |
|---|---|---|
| Incision | Abdominal incision | Vaginal incision(s) can be done immediately in the absence of infection or other complications |
| Timing of repair (elapsed time from fistula creation) | Often delayed 3-6 months | |
| Exposure | Fistula located low on the trigone or near the bladder neck may be difficult to expose transabdominally | Fistula located high at the vaginal cuff may be difficult to expose transvaginally |
| Location of ureters relative to fistula tract | Fistula located near ureteral orifice may necessitate reimplantation | Reimplantation may not be necessary even if fistula tract located near ureteral orifice |
| Sexual function | No change in vaginal depth | Risk of vaginal shortening (e.g., Latzko technique) |
| Use of adjunctive flaps | Omentum, peritoneal flap, rectus abdominus flap | Labial fat pad (Martius fat pad), peritoneal flap, gluteal skin or gracilis myocutaneous flap |
| Relative indications | Large fistulae, location high in a deep narrow vagina, radiation fistulae, failed transvaginal approach, small-capacity bladder requiring augmentation, need for ureteral reimplantation, inability to place patient in the lithotomy position | Uncomplicated fistulae, low fistulae |
The vast majority of VVF in the industrialized world are amenable to a transvaginal repair (Turner-Warwick, 1972; Margolis and Mercer, 1994). The relative advantages of a transvaginal approach compared with an abdominal approach are outlined in Table 77–5 and include shorter operative times, briefer hospital stay, and less blood loss (Goodwin and Scardino, 1979). The principal disadvantages of the transvaginal approach include the relative lack of familiarity of the vaginal cuff anatomy to many urologists; the potential for vaginal shortening, especially with the Latzko approach; and, finally, the difficulty in exposing high or retracted fistulae located near the vaginal cuff, especially in deep, narrow vaginas, or in those without any apical prolapse, such as that found in nulliparous females (see Fig. 77–2). Patients who are unable to get into the high-lithotomy position due to musculoskeletal conditions are not candidates for a transvaginal approach.
Table 77–5 Potential Advantages of a Transvaginal Approach for Posthysterectomy Vesicovaginal Fistula
The abdominal approach to VVF repair is advantageous in several circumstances. If the VVF is associated with other intra-abdominal pathology requiring repair—including an associated ureteral injury (i.e., ureterovaginal fistula), or a complex fistula, including those involving another intra-abdominal organ—then a transabdominal approach is indicated to address the problems simultaneously. If the VVF is located adjacent to the ureteral orifice, some authors have suggested that this is an indication for an abdominal approach (Carr and Webster, 1996), whereas others have not so advocated (Dupont and Raz, 1996). In patients with a small capacity or poorly compliant bladder (often secondary to radiation) requiring augmentation cystoplasty, an abdominal approach is indicated, because both procedures can be performed using the same incision. Complicated fistulae, including those associated with multiple prior failed attempts at repair (Kristensen and Lose, 1994) or those which are quite large (>5 cm), might be best approached abdominally as well. Nevertheless, a prior failed attempt at repair is not necessarily a contraindication to a transvaginal approach because excellent results can be achieved in this setting (Eilber et al, 2003).
Combined transabdominal-transvaginal approaches to VVF have been described (Clark and Holland, 1975; Taylor et al, 1980; Henriksson et al, 1982a). This approach may be applicable selectively in the setting of large, complex, or recurrent VVF after prior attempts at repair.
Handling of Fistula Tract: Excision versus No Excision
A long-held tenet for successful fistula closure, dating back to Sims’ original description in 1852, involves complete excision of the fistulous scar tissue and tract (Fearl and Keizur, 1969; Persky et al, 1979; Wein et al, 1980a; Fourie, 1983). This approach ensures clean, well-vascularized viable edges to be approximated for the initial layer of repair. Simple excision of the scar in an inverted “funnel” shape with careful reapproximation of the defective edges has been shown to be an effective repair of VVF (Iselin et al, 1998; Flynn et al, 2004). However, excision of the fistula tract itself is not always necessary and may even compromise the repair in some patients (Zimmern et al, 1985; Cruikshank, 1988; Tancer, 1992; Raz et al, 1993; Margolis and Mercer, 1994). There are several potential disadvantages of excision of the fistula tract. Firstly, this creates a larger soft tissue defect to be repaired. Excision of the fibrous tract may lead to bleeding, which, if cautery is used, may result in tissue necrosis and impede healing (Eilber et al, 2003). If the VVF is adjacent to the ureter, then excision of the tract may mandate reimplantation of the ureter. Alternatively, if the tract is left in situ, the ureter may be catheterized for the repair and then left undisturbed during a transvaginal operation, obviating the need for reimplantation. In addition, in chronic fistulae, a strong fibrous ring forms outside the epithelialized tract, which maintains some strength through the repair if this layer is incorporated into the closure. This can be an important consideration in those patients with significant detrusor overactivity postoperatively from either the repair itself or the indwelling drainage catheters.
Use of Adjuvant Flaps or Grafts: Type and Application
Prior to embarking on surgical repair, the surgeon should be familiar with a variety of adjuvant flaps and grafts that allow for the interposition of healthy tissue during VVF repair. It is often not possible to predict which patients will require these procedures ahead of time. The indications for tissue interposition are not well defined, but these measures are most commonly used in the setting of radiated tissues, obstetrical fistulae, failed prior repairs, large fistulae, and those with tenuous repairs. The various types of flaps will be discussed in more detail later in the chapter.
Other Considerations
Preoperative estrogen supplementation may be beneficial in the postmenopausal patient with vaginal atrophy and VVF (Massee et al, 1964). There exists little data in the literature, beyond expert opinion, to support their use. However, topical estrogen preparations may improve vascularity (Margolis and Mercer, 1994) and local tissue quality (Carr and Webster, 1996). Therefore a trial of topical estrogens in individuals with postmenopausal vaginal atrophy and a posthysterectomy VVF may be warranted provided that there are no contraindications to their use.
Perioperative intravenous antibiotics are often administered, although their utility in the posthysterectomy VVF repair is questionable in the absence of infection. Whether antibiotics should be administered prophylactically prior to surgical repair is controversial, but at least one study suggests that preoperative antibiotics do not improve outcomes when administered prior to the repair of obstetric fistula (Tomlinson and Thornton, 1998). Treatment of existing infection based on preoperative urine culture is potentially beneficial in preventing bacteremia during surgery. However, prolonged use of broad-spectrum antibiotics postoperatively following repair may result in bacterial resistance and possibly fungal vaginal infections that may compromise suture lines.
Sexual activity should be documented preoperatively. Patients should be specifically queried regarding sexual function and dyspareunia occurring prior to the onset of the event that resulted in the fistula. Although this data is subject to recall bias, it may play an important role in the choice of surgical approach, as well as having medicolegal implications postoperatively. Some types of vaginal procedures for the repair of VVF, including the Latzko partial colpocleisis, may result in vaginal shortening and postoperative dyspareunia. Preoperative documentation of this preexisting condition can be invaluable. Furthermore, adjuvant procedures that may alter vaginal appearance or function, such as the harvesting of a Martius fibrofatty labial flap or an episiotomy, should be carefully discussed with the patient in advance, especially regarding sexual function.
Finally, postoperative drainage following VVF repair can be maintained by single or dual catheters. Some authors suggest that a urethral catheter, alone, provides satisfactory drainage (Collins et al, 1960; Fearl and Keizur, 1968; Tancer, 1980; Leng et al, 1998). Others advocate a suprapubic catheter (Blaivas et al, 1995; Carr and Webster, 1996; Iselin et al, 1998), alone, to minimize bladder spasms and trauma to the surgical repair. Most commonly, both urethral and suprapubic drainage catheters (O’Conor et al, 1973; Wein et al, 1980a; Dupont and Raz, 1996; Eilber et al, 2003) are left postoperatively. The disadvantage to single catheter drainage is principally that the catheter will malfunction, clog, or kink, resulting in bladder filling, eventual overdistention, and disruption of the suture line.
Preoperative Counseling and Indications for Surgery
Surgical repair of a VVF is indicated following confirmation of the diagnosis. As noted previously, a trial of conservative management may be warranted in selected cases, especially in those with a newly diagnosed, small, uncomplicated fistula in which the vaginal leakage significantly improves or resolves with catheter drainage. When conservative measures fail or, if after adequate counseling, the patient requests repair prior to a trial of conservative management, surgical treatment is pursued. Prior to surgery patients should be counseled that most VVF in the industrialized world are repaired on the first attempt in greater than 90% of cases; however, prolonged postoperative urinary catheter drainage is necessary following surgery. Postoperative urinary urgency and frequency are common for a period of time after removal of the catheter but are usually self limited. Finally, the patient should be aware that it may be necessary to alter the surgical plan intraoperatively due to a variety of factors encountered during the operation, and that interpositional flaps or grafts may be used.
Vaginal Techniques
Common vaginal approaches to the repair of uncomplicated VVF are described. The merits of the vaginal approach are reviewed in Tables 77-4 and 77-5.
Vaginal Flap or Flap-Splitting Technique
This approach popularized by Raz and colleagues (Zimmern et al, 1985; Raz et al, 1993; Stothers et al, 1996; Eilber et al, 2003) results in a three-layer closure without the use of an adjuvant flap, and a four-layer closure if a flap is used (Fig. 77–12). It can be performed as an outpatient procedure and is applicable to most simple, uncomplicated VVF.
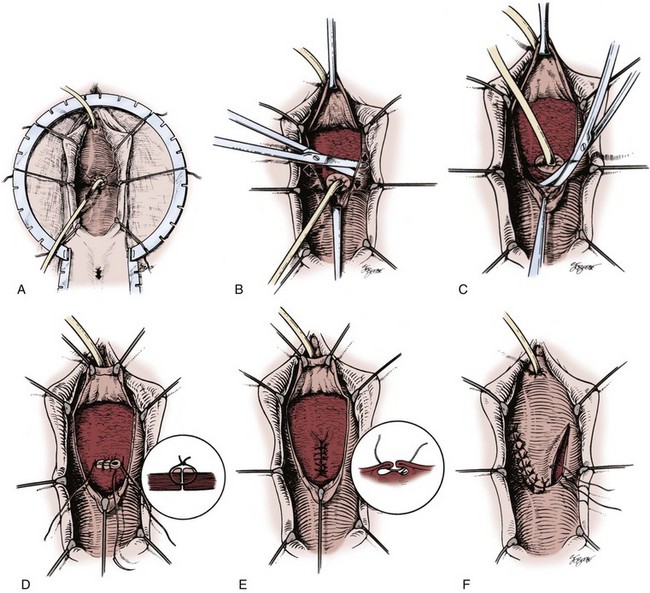
Figure 77–12 Technique of vaginal repair of a posthysterectomy vesicovaginal fistula (VVF). A, Retraction including ring retractor, vaginal speculum, and Foley catheter in the VVF tract. A Foley catheter is seen in the VVF tract providing traction on the vaginal cuff. B, Mobilization of anterior vaginal wall flap. Lateral flaps are developed as well, thereby isolating the VVF tract. C, Mobilization of posterior vaginal wall flap. D, Initial layer of closure is performed without excising the edges of the fistula tract. E, The perivesical fascia is closed with Lembert-type sutures. This line of closure is perpendicular to the initial suture line. F, The vaginal wall flaps are advanced to avoid overlapping suture lines.
(From Ganabathi K, Sirls L, Zimmern P, Leach GE. Vesicovaginal fistulae: reconstructive techniques. In: McAninch J, editor. Traumatic and reconstructive urology. Philadelphia: WB Saunders; 1996. p. 317.)
Step 1 (positioning, preparation, and retraction): The patient is placed in the dorsal lithotomy position, a rectal packing is placed (to aid in identification of the rectum) and the lower abdomen and perineum is prepared with a standard surgical preparation solution. Appropriate exposure is maintained with use of a vaginal weighted speculum, silk labial retraction sutures, and a ring retractor with hooks. A suprapubic tube is placed, cystoscopy with reassessment of the VVF location is performed, and ureteral catheters are placed if the fistula tract is adjacent to or involves the ureteral orifices. A posterolateral episiotomy may be performed to improve exposure in patients with a narrow introitus. A urethral catheter is placed in addition to the suprapubic tube maximizing postoperative urinary drainage (Fig. 77–13A). Any concomitant anti-incontinence or other vaginal surgery that is to be done simultaneous with VVF repair should be done prior to reconstruction to avoid disturbing the repair once completed.
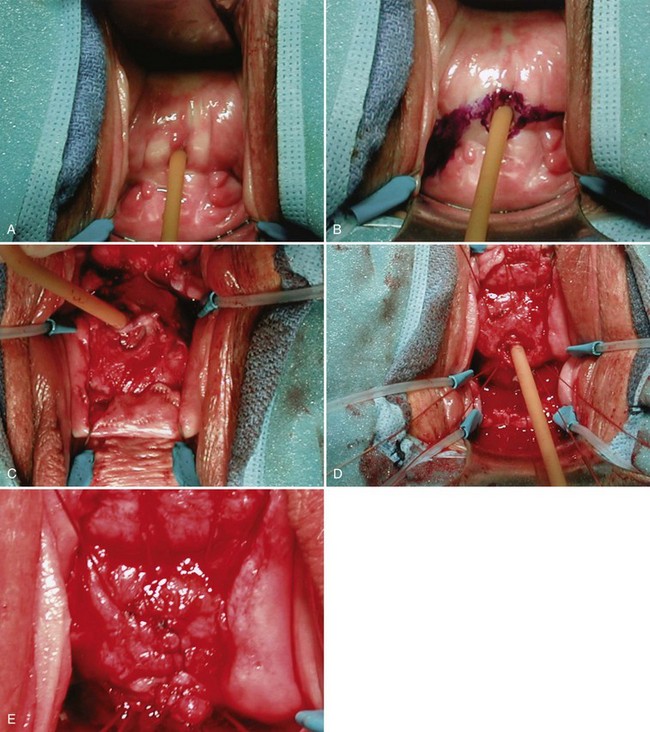
Figure 77–13 Operative repair of vesicovaginal fistula. A, Foley catheter in fistula providing retraction. B, Posterior vaginal flap is marked. C, The vaginal flaps have been developed and are retracted behind the hooks of the ring retractor. Note that the fistula tract is intact surrounding the Foley catheter. D, The first layer of sutures has been placed. The Foley catheter will be removed from the fistula tract and the sutures tied down. E, The second layer of closure is performed by reapproximating the perivesical fascia over the first suture line.
Step 2 (incision): The fistula tract is cannulated with a small Foley catheter (10 to 12 Fr) and, after inflation of the balloon, gentle downward traction is placed on the Foley catheter, pulling the VVF towards the introitus. Occasionally, a small VVF requires dilation with metal sounds to place the Foley catheter. To facilitate dilation of the fistula tract in these cases, a guidewire may be placed through the fistula tract endoscopically and sequential dilation performed using Goodwin sounds. The gentle traction on the VVF provided by the Foley catheter greatly enhances exposure (see Fig. 77–8). The vaginal flaps are marked (Fig. 77–13B). Saline is then injected into the anterior vaginal wall surrounding the fistula tract and along the lines of the vaginal flaps. The fistula tract is carefully circumscribed. An inverted J-shaped or U-shaped incision that circumscribes the fistula tract is made with the limbs of the J or U extending to the apex of the vagina. The circumscribed fistula is incorporated into the curved portion of the incision. The nature of this incision allows creation of a vaginal wall flap that can be advanced and rotated over the fistula repair. This helps avoid vaginal shortening and overlapping of suture lines during reconstruction. However, some surgeons have recommended that the long end of the incision be extended along the anterior vaginal wall toward the introitus (Wang and Hadley, 1990).
Step 3 (creation of vaginal wall flaps): The vaginal wall flaps are created by dissecting in a proximal, distal, and lateral direction away from the fistula tract (Fig. 77–13C). Initially, dissection may be difficult due to scarring from the insult that resulted in the VVF. It is important to remain in the correct surgical plane while developing the vaginal wall flaps, so not to compromise their vascularity. Adequate mobilization of the vaginal wall distal to the VVF is especially important, because it will be necessary to advance the proximal vaginal wall flap beyond the fistula as the final layer of closure. Each flap is mobilized 2 to 4 cm from the fistula tract, exposing the underlying perivesical fascia. The ring of vaginal wall tissue, where the initial incision circumscribed the fistula opening, is left intact; thus, flap creation is done in healthy tissue, avoiding dissection of the actual fistula tract. This technique facilitates dissection in proper tissue planes, avoids bleeding edges at the resected fistula tract, ensures that closure of the fistula is done with healthy tissue (vaginal wall flaps), and decreases the risk of potential bladder perforation. Wide mobilization of the vagina off the perivesical fascia for a distance of several centimeters bladder allows creation of a tension-free closure.
Step 4 (fistula closure): Closure of the fistulous opening is now done. The catheter in the fistula tract is removed, and the first layer of the repair is performed. Interrupted 3-0 or 4-0 absorbable sutures are placed in a transverse or vertical fashion across the fistula. These sutures incorporate bladder wall and the fistulous tract itself, starting in healthy tissue approximately 0.5 cm away from the margin of the fistula (Fig. 77–13D). Inclusion of the fistula tract in the repair (and not resecting the fistula) provides a strong anchor of supporting tissue for the first layer of the repair. The use of a double-armed suture, with both sides thrown from within the fistula tract outward, facilitates incorporation of good-quality tissue. The Foley catheter can be taken off traction while these sutures are placed to avoid puncturing the balloon. The second layer of the repair is placed with interrupted 2-0 or 3-0 absorbable sutures. These sutures are placed to invert the previous layer by imbricating the perivesical fascia and the deep musculature of the bladder over the first layer/fistula tract (Fig. 77–13E). The sutures should be applied at least 3 to 5 mm from the prior suture line, free of tension, and at a 90-degree angle from the first suture line to minimize overlapping of the two lines of repair. The integrity of the repair is confirmed by filling the bladder with 200 to 300 cc of saline mixed with indigo carmine and observing for vaginal staining. At this point, if desired, an interpositional peritoneal or Martius flap may be mobilized and secured over the existing suture line (Raz et al, 1993; Eilber et al, 2003).
Step 5 (advancement and closure of vaginal wall flap): The final and third layer of closure is done with the vaginal wall flaps that were previously created. The redundant, excess anterior (distal) vaginal flap is excised, and the posterior (proximal) vaginal flap is advanced beyond the fistula closure. This covers the fistula site with fresh, healthy vaginal tissue, which helps avoid overlapping of suture lines.
Step 6: (closure of the vaginal wall): The flap is advanced at least 2 to 3 cm beyond the fistula closure, and the vaginal wall is closed with a running, locking, absorbable 2-0 suture. An antibiotic-impregnated vaginal packing is placed for 24 hours postoperatively. The urethral Foley and suprapubic catheters are left to drain for 10 to 14 days.
Postoperative urinary drainage is essential, and the draining catheter(s) are left in all patients postoperatively until cystography confirms successful repair of the fistula. Generally, imaging is obtained at between 10 and 21 days from the time of repair. If a persistent leak is noted, ongoing catheterization and repeat imaging at a 2- to 3-week interval may demonstrate eventual resolution (Schwab and Rovner, 2003). Anticholinergic agents are given to decrease bladder irritability. A cystogram is done prior to catheter removal to document integrity of the repair. Sexual intercourse is avoided for 2 to 3 months postoperatively.
Complications
Proper surgical technique is critical in avoiding complications. Dissection in the proper surgical planes assures minimal bleeding. When significant bleeding is encountered during the dissection, it is possible that an improper plane of dissection was entered. Careful intraoperative inspection and reevaluation of the surgical planes are warranted. Furthermore, excessive use of cautery may compromise the vascular supply of the tissue flaps used for repair. This may only become evident in the postoperative period with ischemic flaps and recurrence of the fistula. Intraoperative bleeding should be controlled with fine absorbable suture whenever possible. The possibility of ureteral injury is a concern, if the VVF is located adjacent to the insertion of the ureter. If there is doubt regarding a ureteral injury, then cystoscopy should be performed following the intravenous administration of indigo carmine. Blue efflux from the ureteral orifice confirms ureteral patency.
Long-term complications from transvaginal VVF repair include vaginal shortening and stenosis. Careful attention to flap mobilization and reconstruction will minimize this complication. In addition, excessive resection of the vaginal wall should be avoided during reconstruction to avoid vaginal shortening or scarring.
The most important complication of VVF repair is recurrence of the fistula. A repeat transvaginal approach can be attempted with satisfactory success. A careful review of potential factors leading to failure of the initial repair is undertaken, and any remediable factors (i.e., inadequate nutrition, vaginal atrophy, excessive postoperative bladder spasms) are addressed. Strong consideration should be given toward the use of adjuvant flaps in repeat VVF repairs where tissue quality may be suboptimal.
Other Transvaginal Techniques
There are multiple variations to transvaginal fistula repair, including those originally described by Latzko in 1914. The Latzko high-partial colpocleisis is a very popular approach among some reconstructive surgeons, with reported success rates in excess of 90% (Kaser, 1977; Tancer, 1980). This approach may not be as successful as the vaginal flap technique for large obstetric fistulae (Elkins et al, 1988). In this procedure, the fistula tract is isolated, and the tissue surrounding the VVF tract is denuded of vaginal “epithelium” circumferentially for a distance of 1 to 2 cm. Care is taken to avoid deeply “denuding” the vaginal tissues to avoid entry into the bladder or perivesical fascia. The denuded areas are then reapproximated over the fistula tract with a series of interrupted absorbable sutures. Sutures are not placed into the bladder wall or vesical mucosa. The edges of the vaginal wall are then reapproximated as a second layer, creating a partial colpocleisis in some patients. Advantages of the Latzko procedure include minimal blood loss, no need for ureteral reimplantation (even for a fistula adjacent to the ureter, because sutures are not placed through the bladder), and a short convalescence. Potential disadvantages include the possibility for vaginal shortening (Enzelsberger and Gitsch, 1991), as well as the creation of directly overlapping suture lines.
Webster and colleagues reported excellent results with a transvaginal approach to VVF by vaginal cuff excision (Iselin et al, 1998; Flynn et al, 2004). In this approach, the fistula tract is isolated, and the entire epithelialized portion of the tract is excised in the fashion of a wide inverted cone, leaving a funnel-shaped defect from the vesical to the vaginal side of the fistula. The defect is then closed in 3 or 4 layers using absorbable suture. The principal advantages of this technique are that mobilization of vaginal flaps is not required, and vaginal shortening is minimal.
Abdominal Techniques
VVF may be repaired transabdominally, and this is the preferred approach in those cases requiring augmention cystoplasty or ureteral reimplantation. Compared with the vaginal approach, the abdominal approach to VVF repair is associated with a longer recovery time and in-patient hospitalization, greater blood loss, more cosmetic deformity, and, in general, greater morbidity. Abdominal repair of VVF may be performed intraperitoneally or extraperitoneally, as well as transvesically.
Suprapubic Intraperitoneal/Extraperitoneal Approach
The patient is positioned in a low-lithotomy position with access to the vagina in the sterile operative field. Ureteral catheters may be placed preoperatively or intraoperatively to assist in identification of the ureters, especially if the VVF is in close proximity to the ureteral orifices. A lower midline incision is carried out. As classically described by O’Conor and colleagues (O’Conor and Sokol, 1951; O’Conor et al, 1973), the bladder is approached extraperitoneally; however, in some cases, the peritoneum will be entered. The bladder is opened vertically, and the cystotomy is extended down to the opening of the VVF (Fig. 77–14). As the dissection proceeds distally, stay sutures placed on the bladder edges greatly assist in retraction. In addition, a curved sponge stick placed per vagina with gentle upward traction can provide excellent exposure of the VVF. Having bivalved the bladder down to the level of the VVF, the VVF tract is excised, and the dissection is continued beyond the fistula tract to develop the vesicovaginal space (Fig. 77–15A to D). The vagina is carefully dissected and separated from the bladder for a distance of 2 to 3 cm beyond the VVF. The key to the operation is the mobilization of the bladder from the vagina caudal to (beyond) the VVF tract. Following wide mobilization from the bladder, the vagina is closed with a running absorbable suture. At this point, if an interpositional flap of greater omentum is to be used, it is mobilized and then secured 1 to 2 cm distally beyond the excised VVF tract (see later discussion) (Wein et al, 1980a). The bladder is then closed in several layers. A suprapubic tube and urethral catheter are usually left for postoperative drainage. Anticholinergic agents are used liberally in the postoperative period to minimize bladder irritability, which may be problematic.
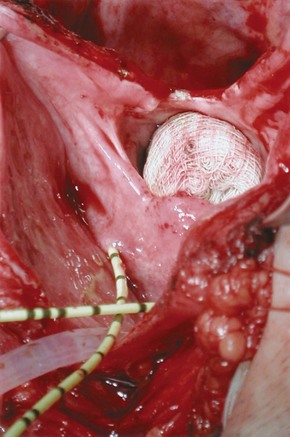
Figure 77–14 Intraoperative photograph from a suprapubic vesicovaginal fistula (VVF) repair. The bladder has been opened anteriorly and bivalved in the midline down to the level of a very large VVF. The gauze packing lies in the vagina. The ureteral catheters were placed to identify the ureters intraoperatively.
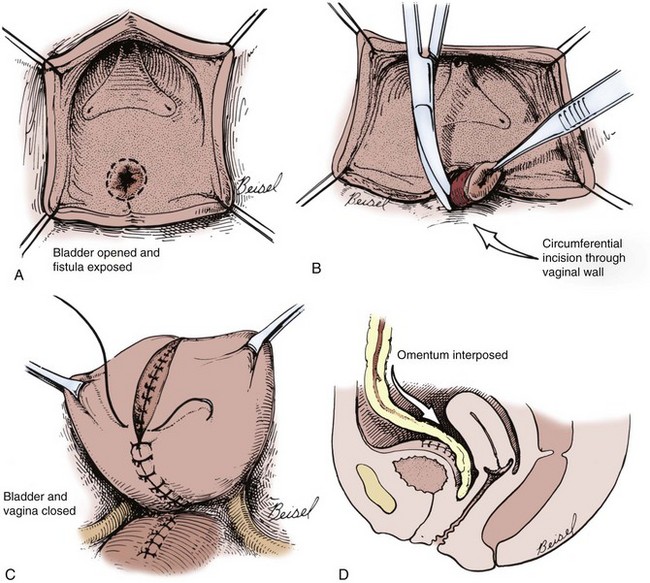
Figure 77–15 Diagrams of suprapubic repair of vesicovaginal fistula (VVF). A, The bladder opened and bivalved down to the level of the VVF. B, The VVF tract is excised. C, After closure of the vagina, the bladder is closed in multiple layers. D, Omentum is interposed between the bladder and vaginal closures.
(From Ganabathi K, Sirls L, Zimmern P, Leach GE. Vesicovaginal fistulae: reconstructive techniques. In: McAninch J, editor. Traumatic and reconstructive urology. Philadelphia: WB Saunders; 1996. p. 315.)
Bladder augmentation or ureteral reimplantation, if necessary, can be incorporated into the suprapubic approach prior to closure of the bladder. Large or small bowel may be used for augmentation cystoplasty, depending on the clinical circumstances.
Transvesical
A suprapubic transvesical approach to VVF repair has also been described (Landes, 1979; Cetin et al, 1988; Gil-Vernet et al, 1989). In this approach, the bladder is opened through a vertical cystotomy but is not bivalved down to the VVF tract. From a transvesical approach, the VVF tract is circumscribed and excised transvesically. The vaginal edges are then carefully mobilized from the bladder. The vagina and bladder are closed sequentially. A V-shaped flap of adjacent posterior bladder wall may be brought down as a flap to close a large gap or to minimize overlapping suture lines (Gil-Vernet et al, 1989). This approach has been successful in both simple and complex fistula (Gil-Vernet et al, 1989; Leng et al, 1998), and modifications have been described incorporating an omental flap (Hellenthal et al, 2007).
Laparoscopic/Robotic Approaches
Laparoscopic VVF repair is an alternative to the classical open approach described previously. Compared with the O’Conor transabdominal approach, laparoscopic repair is reported to be associated with less surgical trauma, shorter convalescence, and lower morbidity (Nezhat et al, 1994; von Theobald et al, 1998; Miklos et al, 1999; Ou et al, 2004). Laparoscopic VVF repair is most useful in the same scenarios as the transabdominal repair, such as in the setting of a high VVF in which a vaginal operation would be anatomically challenging. Dense pelvic adhesions and/or inflammation from prior abdominal surgery can make this approach less desirable in some patients. Furthermore, intracorporeal laparoscopic suturing, a requirement for VVF repair, is an advanced skill many surgeons lack. Because of these limitations, the laparoscopic VVF repair has not been widely adopted.
Successful robotic VVF repair was first reported in 2005 (Melamud et al, 2005), and several small case series have been subsequently reported (Sundaram et al, 2006; Hemal et al, 2008). A five-port technique has been described using a vaginal pack to maintain pneumoperitoneum throughout the case (Sundaram et al, 2006; Hemal et al, 2008). Removal of the vaginal packing without loss of pneumoperitoneum confirms successful closure. Advantages to the robotic technique include three-dimensional visualization, wristed instrumentation reducing the severe angulation required for laparoscopic VVF repair, and technically simpler intracorporeal knot tying.
Although there have been no direct comparisons between the classical transabdominal VVF repair, transvaginal VVF repair, and the minimally invasive robotic/laparoscopic techniques, it is doubtful that a single procedure will emerge as the optimal surgery for all patients with VVF, given the variability in the nature of the condition, the patients in whom it occurs, and the expertise of the individual surgeon.
Adjuvant Procedures in the Repair of VVF: Tissue Interposition
The interposition of a healthy, well-vascularized tissue flap during VVF repair may be beneficial under certain circumstances. Tissue flaps are especially helpful in the setting of a complex fistulae, such as those which have recurred after a prior attempt at repair, those related to previous radiotherapy, ischemic or obstetrical fistulae, large fistulae, and, finally, those associated with a difficult or tenuous closure due to poor tissue quality. For those VVF repaired transvaginally, a labial fat pad (Martius flap) or a peritoneal flap are most commonly used. From a transabdominal approach, omentum or peritoneum is often used as an interpositional flap (Eisen et al, 1974).
Martius Flap
For low or distal fistulae, a Martius fibrofatty labial flap is a reliable source of tissue. The fibrofatty labial flap was first described by Heinrich Martius in 1928. This flap consists of adipose tissue and connective tissue and is the preferential tissue for fistulae involving the trigone, bladder neck, and urethra (Zimmern et al, 1986; Rangnekar et al, 2000a). The blood supply to the flap derives inferiorly from the posterior labial vessels (off the internal pudendal artery), superiorly from the external pudendal artery, and laterally from the obturator artery. The lateral blood supply is sacrificed during mobilization of the flap; the flap may be divided at either its most superior or inferior margin (basing the blood supply on the inferior or superior vascular pedicle, respectively), depending on where the flap will be transferred.
The flap is harvested after the first two layers of closure of the VVF but before advancing the final vaginal wall flap over the repair (see discussion above). To harvest the flap, a vertical incision is made over the labia majora. The borders of dissection include the labiocrural fold laterally, the labia minora and the bulbocavernosus muscle medially, and Colles fascia covering the urogenital diaphragm posteriorly. Flap harvest is accomplished in a lateral to medial fashion. Dissecting down to the adductor muscles laterally before coming around the width of the Martius flap facilitates the harvest of a thick, fatty segment for flap placement. Before final division of the flap inferiorly or superiorly, mobilization may be facilitated by gentle downward traction using a Penrose drain, incorporating the entire thickness of the fibrofatty flap. For a posterior-based flap, the main vascular supply to the flap is located at the base of the labia majora. The anterior segment is clamped and transected anterior to the pubic symphysis (Fig. 77–16).
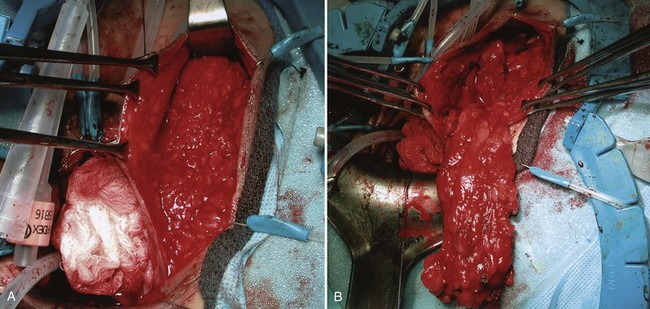
Figure 77–16 Harvesting of a Martius flap. A, The incision is made in the labia. B, A large flap may be obtained.
Having mobilized the flap, a tunnel is created from the labial incision to the site of the fistula repair (Fig. 77–17). A hemostat is used to transfer the fibrofatty flap from the harvest site, through the tunnel, to the level of the fistula repair. The flap is placed over the fistula repair and secured with interrupted absorbable sutures in a tension-free manner. The vaginal wall flap is advanced over the Martius flap and closed as previously described. A small Jackson-Pratt or Penrose drain may be left in the labial incision in the operative bed. The labial incision is closed, and a pressure dressing may be applied to the labial skin incision.
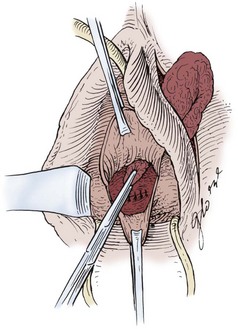
Figure 77–17 Tunneling of the Martius flap.
(From Raz S. Vesicovaginal fistulae. In: Raz S, editor. Atlas of transvaginal surgery. Philadelphia: WB Saunders; 1992. p. 158.)
Rangnekar and colleagues (2000a) reported on the utility of the Martius flap in both urethrovaginal and vesicovaginal fistulae, the majority of which (32 of 46) were due to obstetric trauma. Of the patients undergoing VVF repair, 4 of 21 repairs without a Martius flap failed, compared with none of the 13 patients who underwent an adjuvant Martius flap. Eilber and colleagues (2003) reported that 33 of 34 (97%) of patients undergoing repair of a distal VVF with a Martius flap were cured following the first operation.
For posthysterectomy fistulae, the distance from the labial harvesting site of the Martius flap to the fistula at the apex of the vagina may be considerable. Mobilizing and then tunneling the Martius flap to reach this location may compromise its blood supply and viability. In these cases, a peritoneal flap is preferred (Raz et al, 1993).
Peritoneal Flap
The use of a peritoneal flap during transvaginal repair of a complex VVF is a simple procedure that does not require extravaginal harvesting of the flap. This technique is primarily used in conjunction with repair of a high-lying posthysterectomy VVF (Raz et al, 1993; Eilber et al, 2003). Notably, peritoneal flaps may also be used as an adjunctive measure during transabdominal repair of VVF, although the approach and technique are vastly different (Eisen et al, 1974).
Following a two-layer closure as described previously, the peritoneum is identified posteriorly. The peritoneum and preperitoneal fat is identified, isolated, and mobilized from the caudal origin of the vaginal wall flap using sharp dissection. Usually, dissection just beyond the posterior wall of the bladder will expose the edge of the peritoneum in the anterior cul-de-sac (Raz et al, 1993). The peritoneum is identified as a distinct layer from the bladder. The peritoneum is not opened but is mobilized and then advanced over the fistula repair and secured with interrupted absorbable sutures in a tension-free manner (Fig. 77–18). If a peritoneotomy is made during dissection or mobilization, the peritoneal defect can be closed as the flap is secured to the perivesical fascia over the fistula repair. The vaginal flap is then advanced and closed as previously described.
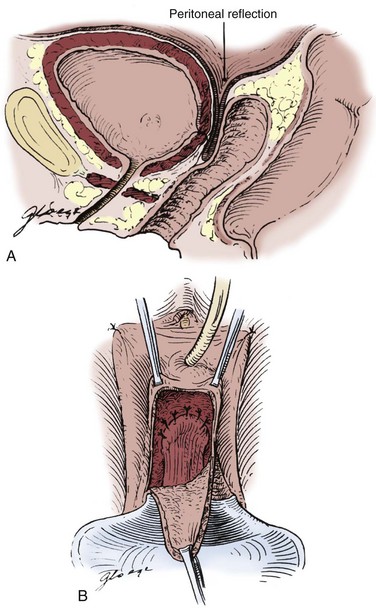
Figure 77–18 Peritoneal flap. A, Diagrammatic representation of the location of the peritoneal flap during vesicovaginal fistula repair. B, Diagram of peritoneal flap advanced over fistula repair.
(A and B, From Raz S. Fistulae: transvaginal repair of vesicovaginal and urethrovaginal fistulae. In: Raz S, editor. Atlas of transvaginal surgery. 2nd ed. Philadelphia, WB Saunders; 2002. p. 242.)
Raz initially reported success in 9 of 11 patients with high VVF undergoing peritoneal flap placement (Raz et al, 1993). A later study from the same institution reported on the use of peritoneal flaps in 83 patients, of whom 80 were cured following the first operation (Eilber et al, 2003). Two of the 3 failed patients were successfully repaired with a repeat transvaginal repair and peritoneal flap, whereas the 1 patient required a transabdominal repair with omental interposition.
Greater Omentum
The omentum is a particularly useful structure in the repair of VVF. Although most commonly used as an adjunct during transabdominal VVF repair (an interpositional layer between the bladder and vagina), it has occasional utility in transvaginal VVF repair, if it had been brought down into the pelvis during a prior surgical procedure. Favorable properties of the omentum include its ability to be mobilized on a well-vascularized pedicle into the deep pelvis without tension, its inherent lymphatic properties, its ability to contribute to healing even in the presence of infection, and the ease with which epithelialization occurs on its surface (Turner-Warwick, 1976; Wein et al, 1980b).
The blood supply of the greater omentum derives principally from the right and left gastroepiploic arteries, as well as the distal branches of the gastroduodenal and splenic arteries, respectively. The right and left gastroepiploic arteries join along the greater curvature of the stomach to form the gastroepiploic arch. The arterial anatomy within the greater omentum is variable but usually consists of a right and left omental artery, and occasionally a middle omental artery, all of which run perpendicular to their origin off the gastroepiploic arch. The caliber of the right gastroepiploic artery is usually larger than the left one, which generally favors a pedicle based on this artery; however, in practice, a pedicle based on either artery may be used (Kiricuta and Goldstein, 1972; Bissada and Bissada, 1992). In addition, anatomically, the origin of the right gastroepiploic artery is somewhat caudal compared with the left one, allowing a slight advantage in reaching into the deep pelvis. In some cases, the free distal end of the greater omentum is long enough to reach into the deep pelvis tension-free without any further mobilization. In either situation, the omental flap is secured with absorbable suture to healthy tissue at a location distal to and beyond the closed VVF tract, between the vagina and bladder. Securing the omental flap beyond and between the suture lines of the closed viscera prevents overlying or apposed suture lines. Evans and colleagues (2001) reported retrospectively on the utility of an omental interpositional flap in 37 patients undergoing transabdominal VVF. Of the 29 patients with a benign etiology of VVF, all 10 patients in whom an omental flap was used were cured, compared with only 12 of 19 (63%) in whom an omental flap was not used. Orford and Theron (1985) reported a 93% cure rate with the use of an omental pedicle graft in 52 patients undergoing VVF repair. In addition to its utility in routine posthysterectomy VVF, the omentum is reported to be a useful adjunct in complicated or complex cases, such as those associated with large VVF (Kiricuta and Goldstein, 1972; Bissada and McDonald, 1983), obstetric VVF (Baines et al, 1976; Sharma et al, 1980), and those associated with radiation therapy (Bissada and Bissada, 1992; Evans et al, 2001).
Other Flap and Graft Techniques
A variety of flaps including gracilis muscle flaps (Izes et al, 1992), labial myocutaneous flaps (Symmonds and Hill, 1978), seromuscular intestinal flaps (Mraz and Sutory, 1994), and rectus abdominis flaps (Menchaca et al, 1990; Viennas et al, 1995; Reynolds et al, 2008) have been used as adjunctive measures in the repair of complex VVF. Obstetric fistulae associated with significant urethral loss may be repaired, in part, with the use of anterior or posterior bladder flaps (Hanash and Sieck, 1983; Elkins et al, 1992; Khanna, 1992). The gracilis muscle in the medial thigh is a convenient adjunct to repair large soft tissue defects, especially those associated with radiation therapy (Obrink and Bunne, 1978; Heckler, 1980). The gracilis muscle is in close proximity to the vagina and has a reliable blood supply. The muscle is mobilized through a thigh incision from its distal attachment on the tibial condyle, with care to preserve its blood supply. It is tunneled cephalad into the vagina subcutaneously and secured over the fistula. Bilateral gracilis muscle flaps can be used for total vaginal reconstruction.
Bladder mucosa as a free graft has been used for repair of VVF (Brandt et al, 1998; Ostad et al, 1998; Sharifi-Aghdas et al, 2002). The bladder is approached extraperitoneally and a small cystotomy is performed. The fistula tract is identified and denuded of mucosa circumferentially for approximately 1 cm. A free graft of bladder mucosa is harvested from the edge of the cystotomy and placed over the denuded VVF tract and secured in place with absorbable suture. Brandt and colleagues (1998) reported a 96.3% success rate in 80 patients. Ostad and colleagues (1998) reported on 6 patients with complex, high, radiated, large or recurrent VVF, all of whom were cured by this technique.
Outcomes of VVF Repair
The success rate reported for a simple VVF repair in the modern era, whether through an abdominal or vaginal approach, is in excess of 90% (Table 77–6). Long-term follow-up suggests that the majority of patients are greatly improved, although minimally bothersome symptoms may persist in a minority of patients over the long term, causing little impact on quality of life (Dolan et al, 2008). Complicated VVF, including those resulting from obstetric causes (Elkins et al, 1988; Arrowsmith, 1994), larger fistulae, as well as those associated with radiation therapy, generally have lower success rates (Massee et al, 1964; Hedlund and Lindstedt, 1987; Langkilde et al, 1999; Ockrim et al, 2009). Massee and colleagues (1964) reported a 93.8% cure rate of urinary vaginal fistulae due to surgical trauma, but only a 66.7% cure rate with those due to radiation therapy. Some authors have suggested that urinary diversion should be strongly considered as primary therapy (Murray et al, 2002) for radiation-induced fistulae, because the results with surgical repair in this group is less than optimal (Langkilde et al, 1999). In patients with obstetric fistulae associated with loss of the bladder neck and proximal urethra, relatively high rates of persistent severe sphincteric incontinence are noted despite successful repair of the VVF (Murray et al, 2002; Browning, 2004), and this type of fistula is involved in the majority of treatment failures (Kelly, 1992). Thus despite a technically successful VVF repair, functionally, from the patient’s perspective, some of these individuals may not be significantly improved following the repair due to severe stress urinary incontinence. Pubovaginal slings and periurethral injectable agents are often beneficial in these patients, once the VVF has been surgically closed (Arrowsmith, 1994). In the developing world, access to synthetic midurethral slings is limited. Futhermore, the safety of using such materials in the setting of extensive reconstruction, such as that after repair of an obstetric fistula, is not established.
VVF and Urinary Diversion
In some patients, repair of VVF is not possible or multiple surgical attempts have failed. This is probably most commonly associated with existing pelvic malignancy, severe radiation damage, and/or large soft tissue loss, especially in the setting of obstetric fistula. However, some patients may simply not be candidates for repair due to coexistent medical morbidities, making them a prohibitive surgical risk. In the former group, urinary diversion, either in the form of a urinary conduit (Kisner and Kesner, 1987), or a continent reservoir can be considered. Fistulae in patients who are not candidates for surgical intervention may be managed by percutaneous ureteral occlusion and permanent nephrostomy (Kinn et al, 1986; Stern et al, 1987; Hubner et al, 1992; Farrell et al, 1997; Amsellem-Ouazana et al, 2006; Natarajan et al, 2007; Shindel et al, 2007).
In the developing world, where catheters and ostomy appliances are either too expensive or completely unavailable, continent urinary diversion or incontinent urostomies are often not practical, which presents ethical issues with the alternative treatments (Wall et al, 2008). In these situations, internal urinary diversion with ureterosigmoidostomy has some application in patients with unreconstructable lower urinary tracts (Attah and Ozumba, 1993). It should be recognized that this is clearly a last-resort operation due to its significant metabolic and neoplastic potential.
Key Points: Vesicovaginal Fistulae
Ureterovaginal Fistulae
Ureteral fistulae to the genital tract in the female most often connect with the vagina but have also been reported to connect with other genital structures, including the fallopian tube (Crochet et al, 2008) or uterus (Billmeyer et al, 2001). Risk factors for the development of ureterovaginal fistulae include endometriosis, obesity, pelvic inflammatory disease (Symmonds, 1976), as well as radiation therapy and pelvic malignancy. Nevertheless, Symmonds has noted that the patient with a ureteral injury following gynecologic surgery is typically one who had an uncomplicated, technically easy hysterectomy for minimal disease (Symmonds, 1976). Thus, except for those oncologic cases where a segment of ureter is deliberately excised, many ureteral injuries are likely due to technical or iatrogenic factors.
Etiology and Presentation
The most common etiology for ureterovaginal fistulae is surgical injury to the distal ureter, with gynecologic procedures being by far the most common cause (Symmonds, 1976; Dowling et al, 1986; Badenoch et al, 1987b; Lee et al, 1988; Blandy et al, 1991). The vast majority of ureterovaginal fistulae occur during procedures for benign rather than malignant indications (Mandal et al, 1990), including hysterectomy most commonly, but also caesarean section, cystocele repair, and other pelvic surgery such as infertility procedures (von Eye et al, 2008; Mongiu et al, 2009) (Table 77–7). Regarding the approach for hysterectomy, the risk of ureteral injury appears to be greatest during laparoscopic hysterectomy, followed by abdominal and then vaginal hysterectomy (Harkki-Siren et al, 1998). The incidence of iatrogenic ureteral injury during major gynecologic surgery is estimated to be about 0.5% to 2.5% (Symmonds, 1976; Payne, 1996; Gilmour et al, 1999).
Table 77–7 Etiology of Ureterovaginal Fistula
| Gynecologic Surgery |
| Other Pelvic Surgical Procedures |
| Other |
The mechanism of injury resulting in iatrogenic postoperative ureterovaginal fistulae includes ureteral laceration or transection, blunt avulsion, crush injury, partial or complete suture ligation, and, finally, ischemia due to operative devitalization of the ureteral vascular supply and/or cautery injury. Overall, the ureter is most commonly injured during gynecologic surgery in the distal one third or pelvic portion, which is, accordingly, the only location at which a ureteral injury may result in a ureterovaginal fistula. Not uncommonly this occurs inadvertently during an attempt by the surgeon to control active bleeding using clamps or suture ligation of large tissue segments in the deep pelvis.
The pelvic ureter is intimately related to the female genital tract throughout its course. In the deep pelvis, the ureter passes at the lateral edge of the uterosacral ligament and ventral to the uterine artery, and then passes just lateral to the cervix and fornix of the vagina. In close apposition to these structures, the ureter must be carefully avoided during any gynecologic procedure in the deep pelvis. Any injury to the ureter that exposes the ureteral lumen (i.e., laceration), or results in delayed necrosis of a portion of the ureter (i.e., suture ligation) and subsequent urinary extravasation, may lead to a fistula. A ureterovaginal fistula may result from a sequence of events, including urinary extravasation from the ureteral injury, urinoma formation, subsequent extension along nonanatomic planes created during surgery, and eventual drainage through the vaginal incision or an ischemic area of the vaginal cuff. Infection, prior radiation therapy, or other factors that may impede healing probably promote the development of ureterovaginal fistulae under these circumstances.
The most common presenting symptom is the onset of constant urinary incontinence 1 to 4 weeks following surgery (Mandal et al, 1990). This may have been preceded by several days of flank or abdominal pain, nausea, and low-grade fever, presumably as a result of urinoma and/or obstruction of the kidney (Lee et al, 1988). Flank pain will often be masked in the postoperative period due to the use of postoperative narcotic analgesics. Importantly, and in direct contrast to VVF, in the setting of continuous urine leakage from a ureterovaginal fistula, patients will continue to report normal voiding habits as bladder filling is maintained from the contralateral, presumably undamaged, upper urinary tract.
Diagnosis and Management
Diagnosis and confirmation of a ureterovaginal fistula can usually be accomplished with a combination of a relevant history, physical examination, and appropriate radiologic studies, including cross-sectional imaging (such as a CT urogram), intravenous urography (IVU), and retrograde pyelography (Mandal et al, 1990) (Fig. 77–19). A double dye test may be of some value in differentiating between a ureterovaginal and vesicovaginal fistula as a cause of ongoing urinary leakage (Raghavaiah, 1974). Suspicion of a ureterovaginal fistula should prompt upper tract imaging (Badenoch et al, 1987a). The IVU or CT urogram most commonly will demonstrate some degree of ureteral obstruction and associated caliectasis or ureteral dilation (Selzman et al, 1995). These findings in the presence of constant vaginal drainage strongly suggest a ureterovaginal fistula. Alternatively, if the fistula is mature and large, the upper urinary tract may appear completely unremarkable; however, urine will be seen opacifying the vagina prior to the postvoid image (Fig. 77–20). Antegrade pyelography following nephrostomy tube decompression of a partially obstructed ureter may be associated with similar findings (Fig. 77–21). A high oblique or lateral film may be necessary to differentiate the contrast in the bladder from that in the vagina. A retrograde pyelogram may show the ureter and fistula well, or may demonstrate an abrupt termination of the ureter 2 to 4 cm from the ureteral orifice. Retrograde pyelography may be the single best test to diagnose a ureteral injury (Payne, 1996). If retrograde pyelography demonstrates the fistula, as well as ureteral continuity, then an attempt at stenting is warranted. Cystography is performed primarily to exclude a coexistent VVF. A cystogram will not demonstrate the ureterovaginal fistula unless there is preexisting vesicoureteral reflux. Ureterovaginal fistulae may be seen on CT urography (Fig. 77–22); however, an additional role for such cross-sectional imaging is to evaluate for an associated pelvic abscess or undrained urinoma.
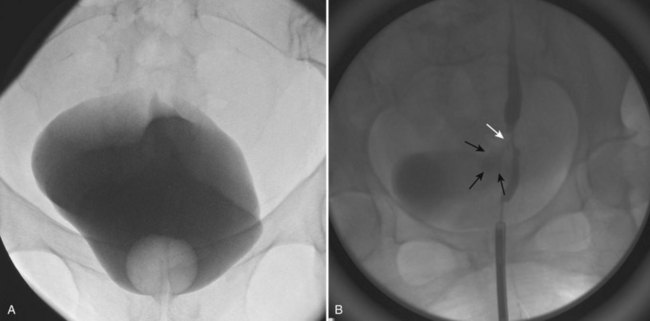
Figure 77–19 Diagnosis of ureterovaginal fistula. A, Normal cystogram in a woman complaining of constant urinary drainage since undergoing an abdominal hysterectomy. B, Retrograde pyelogram demonstrates a distal ureteric stricture with a small fistula tract (white arrow) with contrast in the vagina (black arrows) superimposed on contrast in the bladder from the contralateral retrograde ureterogram.
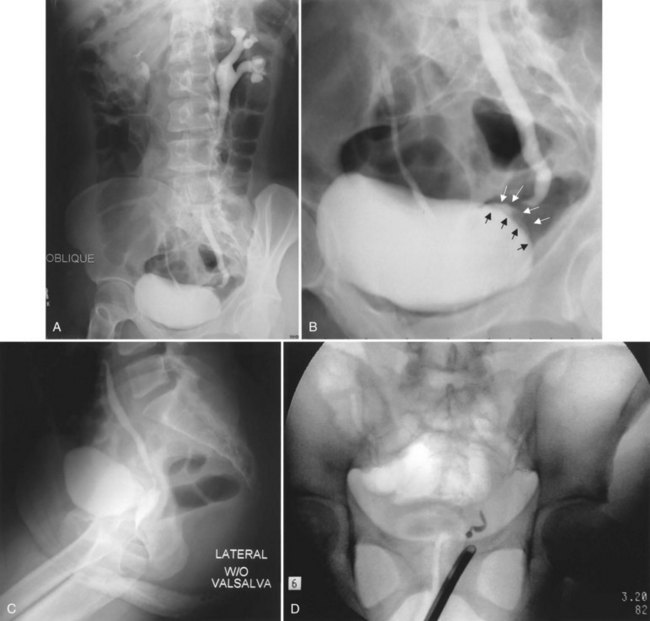
Figure 77–20 Ureterovaginal fistula. A, Oblique view on intravenous urography shows mild left-sided hydroureteronephrosis associated with a distal tapering of the ureter. B, Faint and subtle opacification of the vagina (white arrows) is somewhat obscured by bladder filling (bladder edge indicated by black arrows) on this oblique image. C, Lateral view demonstrates the ureter clearly entering the vagina. D, Retrograde pyelogram demonstrates abrupt termination of the distal ureter.
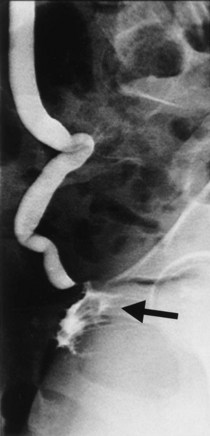
Figure 77–21 Ureterovaginal fistula. Antegrade nephrostogram demonstrates mild ureterectasis with opacification of the vagina (arrow).
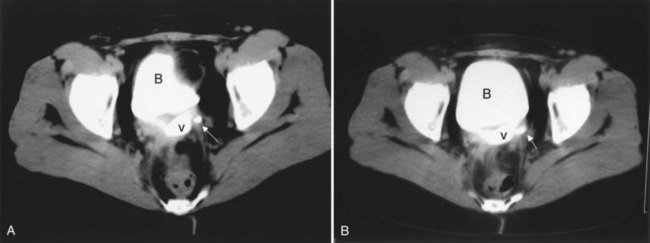
Figure 77–22 CT scan of ureterovaginal fistula. A, CT scan demonstrates contrast material within the bladder (B) and vagina (v). The ureter is seen (arrow) adjacent to the vagina. B, This image shows the ureter (arrow) entering the vagina.
The goal of therapy is the expeditious resolution of urinary leakage, avoidance of urosepsis, and preservation of renal function. Once the diagnosis is made, prompt drainage of the affected upper urinary tract is essential (Gerber and Schoenberg, 1993), because partial ureteral obstruction is often present. An attempt at ureteral stenting or percutaneous nephrostomy tube decompression is warranted as soon as possible (Dowling et al, 1986; Kostakopoulos et al, 1998a) if direct open surgical repair is not immediately considered. Occasionally, conservative, nonoperative management, alone, will result in fistula closure in patients with ureteral continuity and a normal-appearing ureter beyond the fistula (Alonso et al, 1986), although this is unusual. Endoscopic management, including ureteral stenting, may be sufficient to promote closure of the fistula in some cases. Dowling and colleagues (1986) reported that 11 of 23 patients with ureteral injuries recognized postoperatively were successfully managed with nephrostomy tube drainage and/or ureteral stenting. Selzman and coworkers (1995) reported successful management of 7 of 20 ureterovaginal fistulae with internal ureteral stenting alone. None of the 7 patients in whom a ureteral stent was successfully placed required open surgery. In general, if ureteral continuity can be demonstrated on imaging, retrograde placement of a stent is often possible. In some cases, an antegrade stent placement will be successful where a retrograde attempt had failed.
If ureteral stenting is unsuccessful due to complete ureteral occlusion, or prolonged leakage persists despite stenting, then formal surgical repair is indicated (Fig. 77–23). Timing of the repair of ureterovaginal fistulae is controversial. Some authors advocate early repair (Talbert et al, 1965; Flynn et al, 1979; Badenoch et al, 1987b; Blandy et al, 1991; Selzman et al, 1995), while others recommend a delay of 4 to 8 weeks (Hulse et al, 1968; Lee and Symmonds, 1971). More recent literature suggests that early repair is preferred and is not associated with an increase in morbidity or higher failure rates (Payne, 1996).
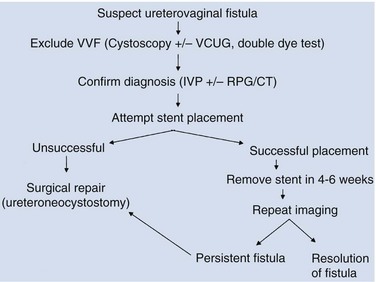
Figure 77–23 Algorithm for diagnosis and management of ureterovaginal fistula. CT, computed tomography; IVP, intravenous pyelography; RPG, retrograde pyelography; VCUG, voiding cystourethrography; VVF, vesicovaginal fistula.
As noted previously, ureterovaginal fistulae most commonly result from injury to the distal one third of the ureter below the level of the iliac vessels. The usual site of the injury in the very distal ureter and the surrounding fibrosis and inflammation usually precludes primary repair of the fistula. Therefore open surgical repair most commonly involves ureteroneocystostomy. The ureter is located and dissected as distally as possible in the pelvis. Care is taken to preserve the periureteral adventitial layer to prevent ureteral ischemia. The ureter is divided distally, and a ureteroneocystostomy is performed with or without a psoas hitch. Occasionally, a Boari flap may be necessary due to extensive ureteral injury. Robotic and laparoscopic repairs have been reported using the same principles as open repair (Modi et al, 2005; Laungani et al, 2008; Modi et al, 2008; Patil et al, 2008), with the potential benefits of shorter hospitalization and quicker convalescence. Rarely, transureteroureterostomy, ileal substitution of the ureter, or renal autotransplantation are required. In patients with a normal contralateral kidney in whom there is extensive renal damage due to obstruction or infection, nephrectomy may be the most expeditious method of management.
Successful repair of ureterovaginal fistulae is expected in greater than 90% of cases. Blandy and colleagues reported on early repair of iatrogenic injury to the ureter in 43 cases including 30 ureterovaginal fistulae. All patients were cured using a combination of techniques including the Boari flap (Blandy et al, 1991). Others have reported similar results (Lee and Symmonds, 1971; Flynn et al, 1979; Mandal et al, 1990).
Ureteral fistulae to other gynecologic organs have been reported. Ureterouterine fistulae may occur as a result of caesarean section, uterine malignancy and elective abortion (Keegan and Forkowitz, 1982; Lazarevski and Badiev, 1996; Wang and Hung, 1997; Sheen et al, 1998). Ureterofallopian tube fistulae have also been reported as a consequence of laparoscopic fulguration of endometriosis (Steckel et al, 1993).
Vesicouterine Fistulae
Etiology and Presentation
Vesicouterine fistulae are among the least common urogynecologic fistulae. Less than 100 cases were reported in the world literature between 1908 and 1986, with no series having greater than four patients (Tancer, 1986). However, the incidence of this condition is increasing in parallel with the rising numbers of low-segment caesarean sections being done worldwide (Porcaro et al, 2002). Caesarean section is by far the most common cause of this unusual fistula (Tancer, 1986; Miklos et al, 1995; Vu et al, 1995). Tancer related that of the 74 cases of vesicouterine fistulae reported from 1947 to 1986, 57 followed low-segment caesarean section, 7 followed vaginal operative delivery, while the remaining cases were related to a variety of disparate scenarios, including induced abortion, hysterectomy, and dilation and curettage (D&C) (Tancer, 1986). Jozwik and colleagues (1997) reported that 21 of 24 vesicouterine fistulae treated over a 12-year period occurred following caesarean section, the majority of which were repeat caesarean sections. Those undergoing vaginal birth after a prior caesarean section (VBAC) are also at risk for vesicouterine fistula (Gil and Sultana, 2001). Vesicouterine fistulae may occur spontaneously as a result of a ruptured uterus during obstructed labor. In these cases, the posterior bladder wall may tear along the uterine rupture line creating the potential for a fistula. Bladder wall invasion by chorionic villi penetrating beyond the uterine serosa, placenta percreta, may also create a vesicouterine fistula (Krysiewicz et al, 1988). A foreign body such as an intrauterine device (IUD) (Schwartzwald et al, 1986), uterine artery embolization (Sultana et al, 2002), brachytherapy (Memon et al, 1998), and traumatic bladder catheterization (Futter and Baker, 1995) have all been reported to cause vesicouterine fistula. In most cases, simultaneous injury to the bladder and uterus is the inciting event. An unrecognized and unrepaired (occult) bladder injury, or incorporation of a portion of the bladder during closure of the uterus following any number of operations, may result in a vesicouterine fistula. Anatomically, the most common location of the fistula is along the posterior bladder wall in the midline, or from the genital side, just cephalad to the internal cervical os.
Unlike other types of urogynecologic fistulae, vesicouterine fistulae may or may not present with constant urinary incontinence due to the sphincter-like activity of the cervix; the exception is in the setting of an incompetent cervix where urinary leakage is constant. In this clinical setting, which typically occurs following vaginal delivery, urine flows from the bladder through the fistula into the uterine cavity and then into the vagina through an incompetent cervical os. Tancer (1986) described 12 such patients, with urinary incontinence as the presenting symptom, out of 15 patients with vesicouterine fistulae following vaginal operative delivery. However, in the setting of a relevant clinical history, vesicouterine fistulae will also present with menouria and cyclical hematuria in the setting of urinary continence. Youssef syndrome describes the presenting symptom complex of vesicouterine fistula: menouria, cyclic hematuria with associated apparent amenorrhea, infertility, and urinary continence (Youssef, 1957; Tancer, 1986) in a patient who has undergone prior low-segment caesarean section. Endometriosis of the bladder, in which cyclical hematuria may be present, must be differentiated from this condition.
Diagnosis and Management
Diagnosis of vesicouterine fistula can be made by a combination of cystoscopy and radiographic studies, although a high degree of suspicion is necessary to pursue the diagnosis if initial radiographic studies prove negative (Smayra et al, 2005). Cystoscopy may demonstrate a midline lesion along the posterior bladder wall (Fig. 77–24). Urine cytology may reveal endothelial cells. Instillation of contrast material into the bladder (cystogram) will outline the uterine cavity (Fig. 77–25), whereas a hysterosalpingogram will demonstrate filling of the bladder. MRI, CT (Fig. 77–26), and ultrasonography have been used in the diagnosis and evaluation of vesicouterine fistulae as well (Mercader et al, 1995; Huang and Yao, 1996; Murphy et al, 1999). An IVU or contrast-enhanced CT scan can be used to exclude concomitant ureteral injury.
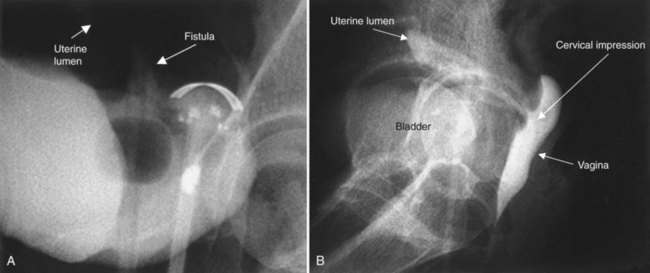
Figure 77–25 Cystogram demonstrating vesicouterine fistula in a postpartum woman. A, Filling of the bladder demonstrates a small amount of contrast material cephalad to the tip of the Foley catheter. The uterine cavity is faintly seen. B, Postvoid image demonstrates filling of the uterine cavity and cervical canal. The bladder is not well seen. This patient is immediately postpartum. Contrast material in the vagina outlines the incompetent cervical canal and os.
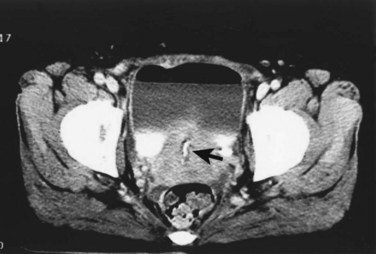
Figure 77–26 CT scan with contrast enhancement demonstrating a vesicouterine fistula. Contrast material is seen layering in the partially filled urinary bladder; contrast opacification of the uterine canal (arrow) suggests a vesicouterine fistula.
Several different approaches have been advocated for the treatment of vesicouterine fistulae. Spontaneous resolution may occur, and Graziotti and colleagues (1978) noted that only five such cases, including theirs, had previously been reported in the literature. Therefore these authors recommend against a “precocious” surgical repair of these lesions as time may allow some fistulae to resolve without surgery. Prolonged indwelling bladder catheterization or fulguration of the fistula tract followed by bladder drainage may be successful in select cases, especially those with small, immature fistulae (Graziotti et al, 1978; Molina et al, 1989; Ravi et al, 2003; Novi et al, 2004). Hormonal induction of menopause will induce involution of the puerperal uterus, and this principle has been used with some success in treating this condition as well (Hemal et al, 1994; Jozwik and Jozwik, 1999; Ravi et al, 2003). Jozwik and colleagues (1999) reported successful treatment in 8 of 9 patients using hormonal manipulation.
Surgical therapy for vesicouterine fistulae is often contingent on the specific reproductive wishes of the patient (Smayra et al, 2005), as well as other surgical factors, but is considered definitive therapy (DiMarco et al, 2006; Rao et al, 2006). If there is no further desire for childbearing, then transabdominal hysterectomy and bladder closure should be considered. Ureteral stents can be placed to facilitate identification of the ureters intraoperatively. Following performance of the hysterectomy, the fistula tract on the posterior bladder wall is excised and the bladder is closed primarily. An omental flap can be placed into the deep pelvis to buttress the bladder closure and separate the bladder closure from the vaginal closure to reduce the possibility of a postoperative vesicovaginal fistula. For the patient who desires preservation of fertility, uterine-sparing surgery can be considered. In a manner similar to an O’Conor transabdominal VVF repair, the bladder is opened and bivalved down to the fistula tract. Careful dissection allows separation of the bladder from the uterus beyond the fistula tract. The fistula tract is excised from both structures, the uterus and bladder are closed individually, and an interpositional flap, usually omentum, is secured between the two organs. Fertility is possible following repair of vesicouterine fistula. Lotocki and colleagues (1996) reported five pregnancies with four full-term deliveries in a cohort of 16 patients who had undergone uterine-sparing surgery for vesicouterine fistulae. Minimally invasive laparoscopic and robotic surgical approaches to the repair of vesicouterine fistulae have been reported (Miklos, 1999; Tarhan et al, 2007; Ramalingam et al, 2008).
Key Points: Vesicouterine Fistulae
Urethrovaginal Fistulae
Etiology and Presentation
In the industrialized world, urethrovaginal fistulae most commonly occur as a result of vaginal surgery, including anti-incontinence surgery, anterior vaginal wall prolapse surgery, and urethral diverticulectomy (Henriksson et al, 1982b; Webster et al, 1984; Blaivas, 1989; Glavind and Larsen, 2001). Importantly, with the rising popularity of midurethral synthetic slings for the treatment of stress urinary incontinence, there is the potential for increasing numbers of urethrovaginal fistulae due to iatrogenic urethral injury at the time of sling placement (Reisenauer et al, 2007; Morton and Hilton, 2009) (Fig. 77–27). In the developing world, similar to the etiology of VVF, obstructed labor is the most common cause of urethrovaginal fistulae (Elkins, 1994) (Fig. 77–28). In the setting of obstructed labor, this condition occurs due to extensive ischemic necrosis and often extends beyond the bladder neck, resulting in a combined urethrovesicovaginal fistula. These are extraordinarily challenging cases to repair due to the size of the fistula, ischemia of the surrounding tissues, and implications for continence in those individuals with destruction of the bladder neck and proximal urethra. Fortunately, due to modern obstetrical care in the industrialized world, these are rarely seen. Other causes of urethrovaginal fistulae include radiation therapy for pelvic malignancy, trauma (including pelvic fracture), and vaginal neoplasms. Another important cause in the long-term–care setting is urethral catheter erosion (Trop and Bennett, 1992; Andrews and Shah, 1998). In patients with poor sensation, especially the cognitively or otherwise neurologically impaired patient, pressure necrosis from a chronically indwelling catheter may result in a traumatic hypospadius and urethrovaginal fistula. If not recognized, over time the catheter may erode to the level of the bladder neck and beyond, effectively creating a bivalved urethra and a urethrovesicovaginal fistula (Fig. 77–29).
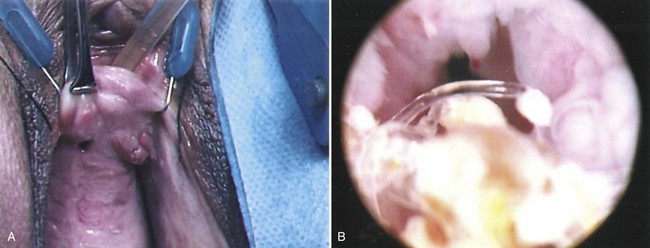
Figure 77–27 Urethrovaginal fistula following midurethral synthetic sling. A, Intraoperative view demonstrating a urethrovaginal fistula at the bladder neck following a midurethral sling. B, Cystoscopy demonstrates intraurethral sling material with calcified material.
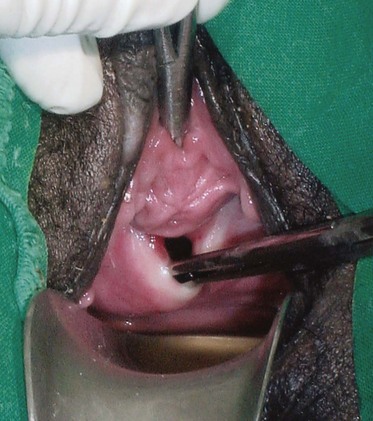
Figure 77–28 Urethrovaginal fistula at the bladder neck from obstructed labor. A hemostat is seen entering the urethral meatus.
(Courtesy of Mark Morgan, MD, Department of Obstetrics and Gynecology, Hospital of the University of Pennsylvania, Philadelphia, PA.)
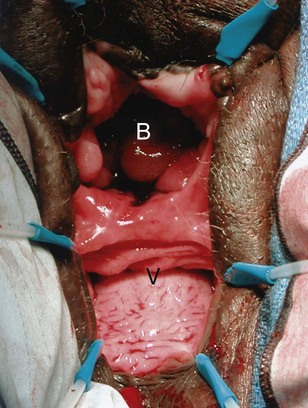
Figure 77–29 Severe complete erosion of the urethra secondary to chronic indwelling urinary catheter. This patient with advanced multiple sclerosis underwent surgical bladder neck closure and ileovesicostomy (ileal chimney). B, bladder; V, vagina.
Symptoms of urethrovaginal fistulae are largely dependent on the size and location of the fistula along the urethral lumen. A small fistula may produce only minimal leakage, whereas a large urethrovaginal fistula can present with continuous urine drainage. Proximal fistulae can be associated with stress incontinence, or, if they are located at the bladder neck, continuous incontinence may result, similar to that associated with vesicovaginal fistulae. Distal fistulae beyond the sphincteric mechanism may be completely asymptomatic or may be associated with a splayed urinary stream. Occasionally, distal fistulae are associated with vaginal voiding and pseudoincontinence, so called because the patient only complains of urinary incontinence when rising from a seated position after voiding. This occurs as a result of urine accumulation in the vagina during voiding, with emptying occurring upon standing—so-called vaginal voiding (Fig. 77–30).
Diagnosis and Management
The diagnosis of urethrovaginal fistula can often be made on physical examination and cystourethroscopy; however, voiding cystourethrography is most useful (Fig. 77–31), especially in complex cases. Small fistulae may be very difficult to locate on physical examination, even with a speculum, due to the surrounding vaginal rugation (Fig. 77–32). It is important to note that an associated vesicovaginal fistula will be found in up to 20% of cases, and therefore a thorough evaluation of the entire lower urinary tract is warranted (Lee et al, 1988).
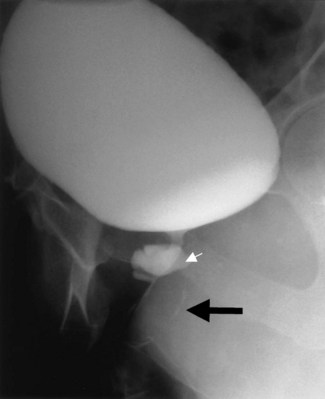
Figure 77–31 This patient was referred for the evaluation of persistent incontinence after urethral diverticulectomy. A voiding cystourethrogram demonstrates incomplete resection of the urethral diverticulum (white arrow) with a pinpoint postoperative urethrovaginal fistula (black arrow shows a small amount of contrast material faintly outlining the anterior vaginal wall). Resection of the residual diverticulum with repair of the fistula by a Martius flap was curative.
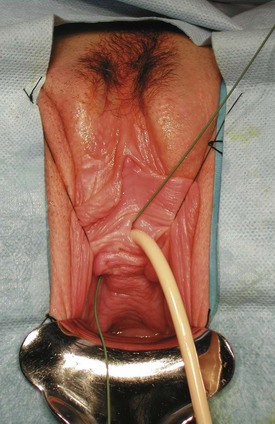
Figure 77–32 Urethrovaginal fistula. Identification of the exact fistula tract can be difficult in redo cases. Passage of a flexible wire endoscopically from the urethral lumen into the vagina can aid in locating the fistula tract.
Endoscopic examination of the urethra should be performed. However, due to its short length, the female urethra may be difficult to fully examine with a standard rigid cystoscope, because the irrigation fluid is discharged 1 to 2 cm proximal to the lens. A flexible cystoscope or a specially designed female cystoscope with a short beak is very helpful in visualizing the entire urethral lumen, because the irrigation fluid is discharged next to the lens, distending the adjacent urethral lumen (Fig. 77–33). Once the diagnosis is made on cystoscopy, the bladder is examined for additional fistulae. Videourodynamics may accurately characterize any associated incontinence, including that associated with detrusor dysfunction. This study also assesses the anatomic relationship of the fistula to the bladder neck and urethra, as well as examines associated VVF.
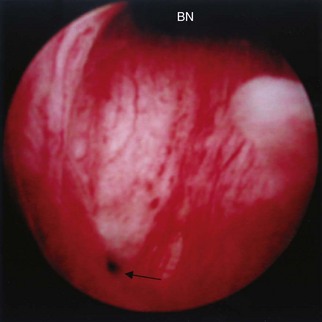
Figure 77–33 Endoscopic view of a urethrovaginal fistula (arrow). This patient complained of ongoing urinary incontinence for 10 years after an endoscopic bladder neck suspension. BN, bladder neck.
The surgical repair of urethrovaginal fistulae is challenging and can often be more difficult than repair of VVF. This is due to several factors, including extensive soft tissue defects as well as the lack of local viable tissue for a multilayer repair (Keettel et al, 1978). Repair of urethrovaginal fistulae usually involves the use of rotational vaginal wall flaps, but anterior- (Elkins et al, 1992) and posterior-based (Khanna, 1992) bladder flap tubes have been used as well.
A number of factors should be considered prior to repair. If the fistula is due to a foreign body, such as a synthetic sling (Reisenauer et al, 2007), the foreign material should be excised as widely as possible from the margins of the fistula, debridement performed on the associated devitalized or inflamed tissue and then the fistula closed with healthy tissues and flaps, if necessary. Small fistulae may be managed by a multilayered closure, usually with an interpositional graft such as a Martius flap (Webster et al, 1984; Leach, 1991). Larger fistulae, including those resulting from obstructed labor, may require extensive surgery, including urethral reconstruction (Tehan et al, 1980; Elkins et al, 1992; Wang and Hadley, 1993). Distal fistulae without associated voiding symptoms or incontinence may be observed or, alternatively, can be managed with an extended meatotomy (Lamensdorf et al, 1977). The quality of the vaginal tissues should be optimized prior to operative repair, which may include the use of antibiotics to treat associated infection, or topical estrogen treatment in those patients with significant atrophic vaginitis. Similar to VVF, timing of repair is controversial. Some authors have suggested that a waiting time of 2 to 6 months is advisable in most patients, with a waiting period of up to 1 year in those with radiation fistulae (Webster et al, 1984; Zimmern et al, 1986). Other authors have advocated immediate repair as soon as the vaginal tissues are free of infection and inflammation (Blaivas, 1989; Blaivas et al, 1995).
Operative Technique
The repair of urethrovaginal fistulae is conceptually very similar to the vaginal flap repair of VVF described previously. The patient is positioned in the dorsal lithotomy position, and urethral and suprapubic catheters are placed. A ring retractor is helpful for exposure. Allis clamps are used to reflect the vaginal wall cephalad exposing the fistula. In some patients, an episiotomy may facilitate exposure to the fistula improving visualization for repair (Webster et al, 1984). However, due to the distal (with respect to the vagina) location of these lesions, this is not usually necessary.
After infiltrating the fistula margins with injectable saline, the fistula tract is circumscribed through the vaginal wall (Fig. 77–34). The fistula tract and immediate surrounding epithelium is not typically excised, because this will create a larger defect in the urethra that will be more difficult to close without undue tension. However, the vaginal wall is dissected several millimeters from the edge of the circumscribed fistula tract in a radial orientation. An inverted U- or J-shaped incision is marked along the anterior vaginal wall, with the base of the U or J at the margin of the circumscribed fistula tract. This is infiltrated with injectable saline and the anterior vaginal wall flap is developed exposing the underlying periurethral fascia to the level of the bladder neck or beyond, depending on the size and location of the fistula. If a concomitant anti-incontinence procedure is planned, dissection may be carried laterally from the edges of the flap at the level of the bladder neck and the retropubic space entered.
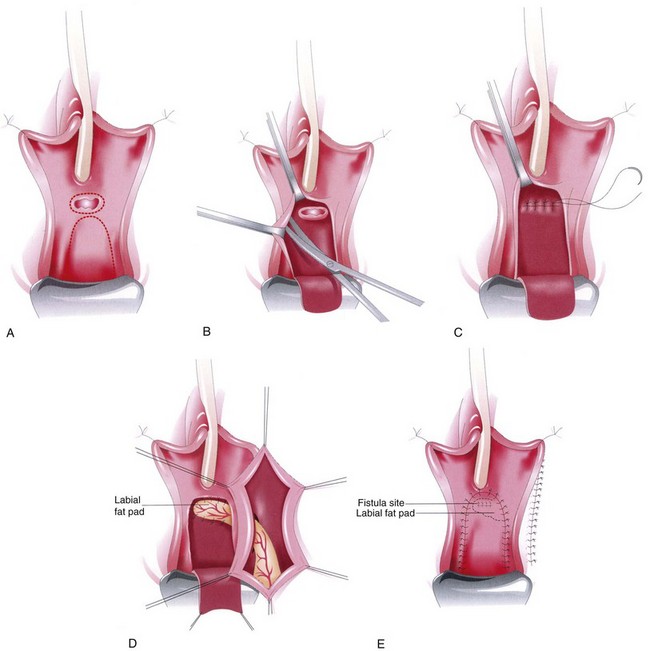
Figure 77–34 Operative diagram of urethrovaginal fistula repair. A, Inverted U incision is made in the anterior vaginal wall with the base of the U at the proximal margin of the fistula. The fistula is circumscribed. B, The anterior vaginal wall flap is mobilized, exposing the periurethral fascia. Dissection is also carried out laterally and distally from the margins of the fistula. The edges of the fistula tract are not excised. C, The epithelialized margins of the fistula tract are reapproximated with absorbable suture for the initial layer of closure. The periurethral fascia may be closed as a second layer, imbricating the initial layer of closure (not shown). D, A Martius flap may be harvested from the labia majora and tunneled as an additional layer of closure. E, The anterior vaginal wall flap is advanced over the closure and secured with absorbable suture.
(From Leach GE, Kobashi KC. Urethral diverticulum and fistula. In: Cardozo L, Staskin D, editors. Textbook of female urology and urogynecology. London: Isis Medical Media; 2001. p. 721–45.)
The edges of the circumscribed tract are reflected over the fistula and apposed with absorbable suture to create the first layer of closure. The periurethral fascia is then reapproximated in a perpendicular suture to the first layer of closure, thereby minimizing overlap. At this point, a Martius flap or other adjuvant flap can be secured over the periurethral fascia. Finally, the anterior vaginal wall flap is brought over the repair, including a Martius flap if used, and secured beyond the original fistula tract.
Adjuvant Flaps and Procedures in the Repair of Urethrovaginal Fistula
Various types of soft tissue flaps are often an important component of a successful urethrovaginal fistula repair because fistula excision and vaginal flap advancement has been historically associated with a high rate of failure (Birkhoff et al, 1977; Keettel et al, 1978; Davis et al, 1980; Patil et al, 1980; Leach, 1991; Fall, 1995; Bruce et al, 2000; Rangnekar et al, 2000b). A variety of adjuvant procedures have been used in the repair of urethrovaginal fistulae, including, most commonly, a Martius labial fat flap, but also gracilis and rectus abdominis muscle, myocutaneous flaps, vaginal wall flaps, fibrin glue, and free labial skin grafts (Keettel et al, 1978; McKinney, 1979; Tolle et al, 1981; Webster et al, 1984; Krogh et al, 1989; Leach, 1991; Izes et al, 1992; Candiani et al, 1993; Fall, 1995; Rangnekar et al, 2000b).
Webster and colleagues (1984) reported on 11 patients with urethrovaginal fistulae, of which 10 underwent surgical repair. Three of the 10 had recurrence of the fistula, all of whom were salvaged with a subsequent repair combined with a Martius labial fat pad interposition. Two patients had primary repair with a Martius flap (see prior discussion under VVF for a more complete discussion of the Martius flap), and both were cured. These authors recommended a Martius flap for all patients undergoing urethrovaginal fistula repair. Bruce and colleagues (2000) reported on the use of a pedicled rectus abdominis muscle flap based on the inferior epigastic artery for complex and refractory urethrovaginal fistulae in 6 patients. All patients had failed at least one attempt at repair with a Martius flap. Six of 6 patients remained successfully closed at a mean follow-up of 23 months. One of 6 patients had persistent urge incontinence postoperatively. The authors concluded that the rectus abdominis flap is a useful adjunct in the repair of complex or refractory urethrovaginal fistulae.
Suprapubic, as opposed to urethral catheterization, has been suggested postoperatively for at least 14 days to allow adequate healing (Tehan et al, 1980; Webster et al, 1984), although the use of only a single drainage catheter postoperatively is not universally agreed upon; other authors use both a suprapubic and urethral catheter (Leach, 1991). Anticholinergics are administered liberally to reduce bladder irritability. Usually, a VCUG with contrast administered through the suprapubic tube is obtained to document resolution of the fistula.
Stress incontinence (SUI) may persist following repair of urethrovaginal fistulae. Whether repair of SUI should be done concomitantly with the fistula surgery, or should be deferred until after repair of the fistula, is controversial. Blaivas and colleagues (1989) argued that sphincteric incontinence should be repaired at the time of fistula surgery with a Martius flap interposed between the fistula repair and a pubovaginal fascial sling. Webster and colleagues (1984) suggested that stress incontinence (SUI) associated with a proximal or mid-urethral urethrovaginal fistula should not be corrected until the fistula is closed and the patient reassessed for persistent incontinence. These authors suggest, however, that SUI associated with distal urethrovaginal fistula can be repaired concomitantly.
Overall, the success rate of urethrovaginal fistula repair is variable but is not generally considered to be as high as that for VVF repair (Gerber and Schoenberg, 1993). Not uncommonly, two or more procedures may be necessary to gain a satisfactory result (Webster et al, 1984).
Uroenteric Fistulae
Vesicoenteric Fistulae
Etiology and Presentation
Vesicoenteric fistulae commonly occur in the setting of bowel disease, such as diverticulitis, colorectal carcinoma, and Crohn disease. Less common causes include radiation, infection, and trauma—external penetrating trauma, as well as that due to iatrogenic surgical trauma. Diverticulitis is the most common cause of colovesical fistulae in most series, accounting for approximately 70% of cases (Mileski et al, 1987; Pollard et al, 1987; Walker et al, 2002; Najjar et al, 2004) (Table 77–8). The second most common cause of vesicoenteric fistulae is cancer, followed by Crohn disease. The peak incidence of vesicoenteric fisula is between 55 and 65 years of age, although fistulae due to Crohn disease present much earlier (Badlani et al, 1980). Approximately 2% of patients with diverticulitis may experience a colovesical fistula as a complication of their disease (Hafner et al, 1962). In a multi-institutional retrospective review of 400 patients with Crohn disease over a 10-year period, 8 patients (2%) were found to have enterovesical fistulae (Gruner et al, 2002). The underlying GI tract disease strongly influences the type of fistula; ileovesical fistulae are more common in Crohn disease than in cancer, and colovesical fistulae are usually due to diverticulitis.
Table 77–8 Causes of Vesicoenteric Fistulae*
| Diverticulitis | 65%-75% |
| Malignancy | 10%-15% |
| Crohn disease | 5%-6% |
| Other (trauma, appendiceal abscess, foreign body) | <5% |
* See Morse and Dretler, 1974; Amendola et al, 1984; Pollard et al, 1987; Schofield, 1988; Walker et al, 2002.
Symptoms of vesicoenteric fistulae may originate from the urinary or gastrointestinal tract; however, in general, lower urinary tract symptoms are more common at presentation (Morse and Dretler, 1974; Ray et al, 1976). In the early stages, symptoms are nonspecific and relate to lower urinary tract dysfunction. Lower urinary tract symptoms include pneumaturia, frequency, urgency, suprapubic pain, recurrent urinary tract infections (UTIs), and hematuria (Table 77–9). Pneumaturia is considered the most common presenting symptom noted in 50% to 70% of cases (Morse and Dretler, 1974; Pontari et al, 1992; Jarrett and Vaughan, 1995; Solem et al, 2002). GI symptoms may include fecaluria and tenesmus. The classic presentation of vesicoenteric fistula is described as Gouverneur syndrome and consists of suprapubic pain, urinary frequency, dysuria, and tenesmus (Sans et al, 1986). Recurrent UTIs or cystitis refractory to antibiotic therapy may suggest a colovesical fistula (Rao et al, 1987). Although it is rare for enterovesical fistulae to present with sepsis (Woods et al, 1988), patients presenting with such signs are found to have urinary obstruction in approximately 70% of cases (Mileski et al, 1987).
Table 77–9 Presenting Symptoms of Vesicoenteric Fistula*
| Pneumaturia | 52%-77% |
| Fecaluria | 36%-51% |
| Urinary tract infection symptoms (frequency, urgency, dysuria) | 44%-45% |
| Fever and chills | 41% |
| Abdominal pain | 25% |
| Nonspecific gastrointestinal symptoms | 25% |
| Hematuria | 5%-22% |
| Orchitis | 10% |
| Urine per rectum | 5% |
* Morse and Dretler, 1974; Pontari et al, 1992; Najjar et al, 2004.
Diagnosis
Many studies exist for the diagnosis of enterovesical fistulae; however, there are significant problems with both false negatives and false positives among the diagnostic modalities, and thus the diagnosis is often made on clinical grounds. Cystoscopy has the highest yield in identifying a potential lesion, with some type of abnormality noted on endoscopic exam in greater than 90% of cases (Morse and Dretler, 1974) (Fig. 77–35). However, the findings on cystoscopy are often nonspecific and include localized erythema, papillary, or bullous change; a definitive diagnosis using cystoscopy can be made in only 35% to 46% of cases (Woods et al, 1988; Pontari et al, 1992). Cystoscopy and biopsy of abnormal-appearing tissue, or an established fistula tract in the setting of a history of malignancy is indicated to evaluate for the possibility of a malignant fistula.
Figure 77–35 Cystoscopic view of a chronic long-standing colovesical fistula. Unlike the majority of these fistulae, there is minimal inflammation around the fistula tract.
Cross-sectional imaging, especially CT scanning has become the imaging modality of choice (Goldman et al, 1984; Goldman et al, 1985; Jarrett and Vaughan, 1995; Gruner et al, 2002). CT or MRI (Haggett et al, 1995) may localize the fistula tract, as well as the involved segment of bowel. The triad of findings on CT that are suspicious for colovesical fistulae consist of (1) bladder wall thickening adjacent to a loop of thickened colon, (2) air in the bladder (in the absence of previous lower urinary manipulation), and (3) the presence of colonic diverticula (Labs et al, 1988). Labs and colleagues (1988) correctly diagnosed colovesical fistulae on CT in 11 of 12 patients with surgically confirmed lesions. CT is now generally considered to be the most sensitive and specific modality for the diagnosis of colovesical fistulae in suspect patients (Najjar et al, 2004), with diagnostic accuracy as high as 90% to 100% (Goldman et al, 1985; Jarrett and Vaughan, 1995). The high diagnostic accuracy of CT in detecting enterovesical fistulae relates to its ability to detect even a very small amount of air in the bladder (Fig. 77–36). Although air in the bladder on diagnostic imaging is very suggestive of colovesical fistulae, false positives may be created by recent instrumentation (catheterization, cystoscopy) or an active UTI with a gas-forming organism. CT scanning should be done after the administration of oral contrast but prior to the administration of intravenous contrast to permit detection of barium within the bladder. Ultrasonagraphy has also been reported to be useful in the diagnosis of colovesical fistulae. A characteristic “beak” sign may be noted; however, this study is not usually performed in the routine evaluation of the patient with a suspected enterovesical fistula (Chen et al, 1990).
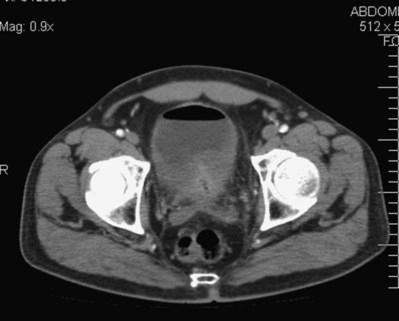
Figure 77–36 CT scan of colovesical fistula. Note the air in the bladder with the thickened posterior bladder wall.
Although commonly used, cystography and transrectal contrast studies (e.g., barium enema) are generally less likely to demonstrate the fistula. Rao and colleagues (1987) reported that 14 of 24 barium enemas either demonstrated or were suspicious for colovesical fistulae in a series of surgically treated patients. Barium enemas and/or colonoscopy may be valuable adjunctive studies in evaluating for colonic disease, such as malignancy, as a cause of the fistula; the preoperative knowledge of such a condition can considerably alter management decisions. Nevertheless, barium enemas have limited utility in the diagnosis of enterovesical fistulae due to low sensitivity (Amendola et al, 1984). The Bourne test, however, can be a useful adjunctive study in the evaluation of colovesical fistulae (Bourne, 1964). As described, the Bourne test is performed following a nondiagnostic barium enema. The first voided urine following the barium enema is immediately centrifuged and then examined radiographically. Radiodense particles in the urine are considered a positive test and evidence for a vesicoenteric fistula. Amendola and colleagues (1984) reported a positive Bourne test in 9 of 10 patients with colovesical fistulae; the Bourne test was the only evidence for a colovesical fistula in 7 such patients who had an otherwise negative evaluation with a combination of other diagnostic studies.
The diagnosis of vesicoenteric fistulae may be confirmed by the oral administration of activated charcoal which, in the setting of a fistula, will appear in the urine as black particles (Geier et al, 1972). This test provides no anatomic information regarding the location of the fistula but is useful in confirming the diagnosis in suspect cases. Intrarectal administration of vital dyes has been advocated for the diagnosis of occult colovesical fistula; however, the dye may be absorbed and then excreted in the urine, giving a false positive test (Deshmukh et al, 1977).
Management
Nonoperative management is a viable option in selected patients with vesicoenteric fistula. Amin and colleagues (1984) reported on 4 of 30 patients with colovesical fistulae secondary to diverticulitis who were observed without active intervention for 3 to 14 years. There were no significant long-term sequelae in this select patient population. In nontoxic, minimally symptomatic patients with nonmalignant causes of enterovesical fistulae, a trial of medical therapy including intravenous total parenteral nutrition (Dudrick et al, 1999), bowel rest, and antibiotics may be warranted. This may be the preferred initial approach, especially in patients with Crohn disease, in whom the notion of immediate exploratory laparotomy and bowel resection is often discouraged due to the chronic relapsing nature of the disease (Evans et al, 2003a). There is also the potential for successful medical management of other fistulae resulting from inflammatory bowel disease (Mahadevan et al, 2003; Parsi et al, 2004).
The goal of operative management is to separate and close the involved organs with minimal anatomic disruption and normal long-term function of both systems. Unfortunately, enterovesical fistulae may be complicated by intense pelvic inflammation, pelvic abscess, and phlegmon formation (in some cases), requiring complex staged reconstructions (Shackley et al, 2000). Bowel resection and/or partial cystectomy may be necessary to obtain viable tissue margins to ensure adequate, watertight closure of the involved viscera. An interpositional flap of greater omentum is often placed between the repaired bowel and urinary bladder to prevent overlapping suture lines and provide a well-vascularized surface for healing.
Both single and multistage procedures have been advocated, depending on the clinical circumstances (Morse and Dretler, 1974; Castro, 1975; Ray et al, 1976; Morrison and Addison, 1983; Mileski et al, 1987; Pollard et al, 1987; Rao et al, 1987; Shackley et al, 2000; Walker et al, 2002; Najjar et al, 2004). A one-stage procedure involves removal of the fistula, closure of the involved organs, and primary reanastomosis of the bowel following resection of the involved bowel segment. A two-stage approach advocates removal of the fistula, closure of the involved organs, and creation of a temporary proximal diverting colostomy, with a later return to the operating room for colostomy takedown once the fistula tract is demonstrated to be closed. The choice of whether to proceed with a one-stage or two-stage repair is influenced by the location and cause of the fistula, the patient’s general condition, the presence of a pelvic abscess, and the presence of colonic obstruction (McConnell et al, 1980). Patients with an inflammatory cause of the fistula, but without gross contamination, can be treated with a one-stage procedure, whereas those with an unprepared bowel, gross contamination, or abscess may require a multistage procedure (Mileski et al, 1987; Shackley et al, 2000). As noted previously, most patients with colovesical fistulae present electively with lower urinary tract symptoms, not emergently in extremis with sepsis (Mileski et al, 1987). Therefore adequate preoperative support, including bowel preparation, nutritional supplementation, and appropriate antibiotics can be used in the majority of cases allowing an elective one-stage approach.
Laparoscopic management of colovesical fistulae has been reported, albeit with a relatively high rate of conversion to open repair (Joo et al, 1997).
Ureteroenteric Fistulae
Fistulae between the ureter and the bowel are most likely to occur in the setting of inflammatory bowel disease such as Crohn disease. The segment of bowel most likely to be involved is the terminal ileum (Banner, 1987), and thus the vast majority cases of ureteroenteric fistulae are unilateral and right sided (Sigel et al, 1977). Rarely, diverticulitis (Ney et al, 1986; Cirocco et al, 1994; Maeda et al, 1998) or ulcerative colitis will lead to a left- sided ureteroenteric fistula (Sigel et al, 1977). Involvement of the ureter is usually at the level of the sacral promontory (Sigel et al, 1977). Other causes of ureteroenteric fistulae include calculous disease, tuberculosis (TB), external and iatrogenic (surgical) trauma, radiation therapy, and transitional cell carcinoma (Javadpour et al, 1973; Sankaran et al, 1974; McElwee et al, 1983; Sumiya et al, 1985; Flood et al, 1992; Goetz et al, 1992; Toporoff et al, 1992; Oh et al, 2002). Unlike vesicoenteric fistulae, ureteroenteric fistulae are more likely to present with bowel rather than urinary symptoms. Pain may also be reported in the hip, flank, or anterior thigh.
The diagnosis is most often made by retrograde pyelography, although CT and IVU are useful as well. Barium contrast studies of the small bowel will often show a diseased segment of bowel but will only rarely demonstrate the fistula. Treatment involves ureterolysis and possible bowel resection. Ureteral resection is not necessary if it can be separated from the involved bowel segment and stented. Unfortunately, in many of these cases, significant renal damage has occurred prior to the definitive diagnosis, and thus nephrectomy may be necessary for definitive management (Sigel et al, 1977).
Pyeloenteric Fistulae
Pyelointestinal fistulae represent an epithelialized connection between the renal pelvis or collecting system of the kidney and the GI tract. Chronic inflammatory disease, such as xanthogranulomatous pyelonephritis or other infectious diseases involving the kidney or bowel, historically has been the most common cause of this condition (Schwartz et al, 1970; Greene et al, 1975; Bhargava et al, 1982; Cheatle et al, 1985; Desmond et al, 1989; Yildiz et al, 1993; Majeed et al, 1997). However, iatrogenic surgical trauma, especially that related to percutaneous renal surgery and percutaneous nephrolithotomy (PCNL), has been associated with an increasing number of such fistulae (LeRoy et al, 1985; Culkin et al, 1990) (Fig. 77–37). Penetrating external trauma, malignancy, ulcer disease, ingested foreign bodies, and complex calculous disease may also result in pyeloenteric fistulae (Brust and Morgan 1974; Mooreville et al, 1988; Blatstein and Ginsberg, 1996; Ginsberg et al, 1996; Chen et al, 2002). Right-sided pyeloenteric fistulae most often involve the duodenum due to their close anatomical relationship, whereas left-sided pyeloenteric fistulae most commonly involve the descending colon.
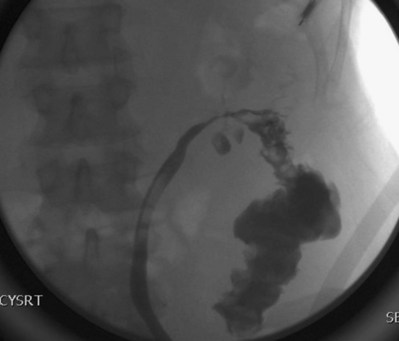
Figure 77–37 Pyelocolonic fistula following cryosurgical ablation of a renal tumor. Retrograde pyeloureterogram demonstrates contrast extravasation into the colon.
The majority of patients present with nonspecific symptoms, including malaise, nonspecific GI symptoms, urinary frequency, flank mass, or tenderness. Approximately 60% to 70% of patients present with flank pain, fever, and pyuria (Desmond et al, 1989). Iatrogenic fistulae as a result of endourologic procedures may present with minimal symptoms. In some cases, they are only noted incidentally on postoperative nephostogram (Culkin et al, 1990). Diagnosis can be made with a combination of urography, retrograde pyelography, and nephrostogram. The GI tract may be studied with a barium swallow or enema.
Historically, pyeloenteric fistulae were treated by nephrectomy and closure of the GI tract (Greene et al, 1975); however, given the changing nature of the cause of these lesions, in many cases the fistula may be initially treated conservatively, especially if associated with a normally functioning kidney. A large nephrostomy tube, enteric suction or bowel rest, antibiotics, and removal of any foreign body (e.g., a stone) may be attempted. Internal stenting of the urinary tract may be pursued for maximal drainage (Desmond et al, 1989). Fistulae associated with a poorly functioning kidney are best treated by primary closure of the bowel and nephrectomy.
Urethrorectal (Rectourethral or Prostatorectal) Fistulae
Acquired rectourethral fistulae (RUF) may occur in the male under a variety of clinical circumstances, including that related to prostatectomy for benign or malignant disease, cryotherapy, pelvic radiotherapy, anorectal surgery, external penetrating trauma (Bukowski et al, 1995; al-Ali and Kashmoula, 1997), urethral instrumentation (Thompson and Marx, 1990), locally advanced prostatic or rectal malignancy, infection (e.g., TB) (Okaneya et al, 1988), ruptured prostatic abscess, or inflammatory disease (e.g., Crohn disease) (Stamler et al, 1985; Fazio et al, 1987). Congenital rectourethral fistula associated with imperforate anus is covered in Chapter 128.
Etiology and Presentation
The incidence of rectourethral fistulae following radical retropubic prostatectomy (RRP) is low, but due to the frequency with which the operation is performed, it is the most common etiology of RUF in most modern series (Stephenson and Middleton, 1996; Nyam and Pemberton 1999; Renschler and Middleton, 2003). Rectal injury during radical prostatectomy occurs in less than 1% to 2% of patients (Igel et al, 1987; Borland and Walsh, 1992; McLaren et al, 1993; Guillonneau et al, 2003). In a Mayo RRP series, there were 27 documented rectal injuries in 2212 patients (McLaren et al, 1993). In this series, 26 of 27 injuries were recognized intraoperatively and repaired. Six patients underwent temporary colostomy and 4 patients developed RUF. In a community-based practice, Harpster and colleagues (1995) reported rectal injuries in 7 of 516 patients undergoing RRP (1.4%) and in 1 of 17 patients undergoing radical perineal prostatectomy (Harpster et al, 1995). Five rectal injuries were recognized and repaired intraoperatively. There were three RUF reported in this series; none closed with conservative management and all required formal repair. In 2003, Rassweiler and colleagues reported one RUF in a series of 219 patients undergoing RRP but seven RUF in 538 patients undergoing laparoscopic radical prostatectomy. All operations were performed by the same surgical team. Guillonneau and colleagues (2003) reported a 1.3% incidence of rectal injury in 1000 consecutive patients undergoing laparoscopic radical prostatectomy. Eleven of 13 injuries were recognized and repaired intraoperatively with a two-layer closure. One of these patients subsequently underwent temporary colostomy for complications related to the injury. Both patients with rectal injuries that were recognized postoperatively underwent colostomy. One of the 2 patients presenting late with a rectal injury developed a RUF requiring a primary surgical closure.
In the setting of radical prostatectomy, a prior history of pelvic radiation therapy, rectal surgery, or TURP is associated with an increased risk of RUF (Thompson and Marx, 1990; McLaren et al, 1993). RUF that occur following radical prostatectomy are usually seen at the vesicourethral anastomosis and are often due to an unrecognized rectal injury at the time of surgery. However, when a rectal injury is recognized and repaired intraoperatively, RUF is extremely uncommon. Borland and Walsh reported 10 rectal injuries in 1000 nonirradiated patients undergoing radical prostatectomy at Johns Hopkins Hospital; however, no patient developed a rectourethral fistula (Borland and Walsh, 1992). Nine of 10 patients had a two-layer closure performed with an omental interpositional flap at the time of injury. One patient underwent a temporary diverting colostomy as the rectal injury was diagnosed and repaired postoperative day 2. Anal sphincter dilation performed on all patients, and they received 7 to 14 days of postoperative antibiotics.
The reported incidence of RUF following cryosurgical ablation as primary therapy for localized carcinoma of the prostate is between 0.5% and 2% (Zippe, 1996; Long et al, 2001), whereas the rate of RUF following cryotherapy as salvage therapy for prostate cancer is somewhat higher at approximately 3.3% (Chin et al, 2001). The incidence of RUF following brachytherapy for prostate cancer, reported by Theodorescu and colleagues (2000), is 0.4%. Rectourethral fistulae have also been reported following high-intensity focused ultrasound treatment (HIFU) (Uchida et al, 2002; Blana et al, 2004), HIFU combined with external beam radiotherapy (6% incidence) (Gelet et al, 2004), laparoscopic radical prostatectomy (Guillonneau et al, 2003; Katz et al, 2003; Rassweiler et al, 2003; Dafnis et al, 2004), and transurethral thermotherapy for benign prostatic hyperplasia (BPH) (Norby and Frimodt-Moller, 2000). The incidence of RUF in Crohn disease is estimated to be approximately 0.3% (Stamler et al, 1985) (Fig. 77–38). Fistulae due to Crohn disease are complex, and management should be individualized (Stamler et al, 1985; Fazio et al, 1987; Santoro et al, 1995; Cools et al, 1996; Rius et al, 2000).
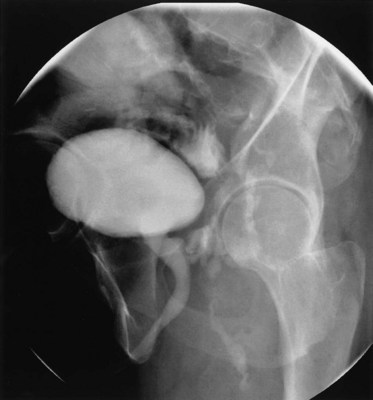
Figure 77–38 Rectourethral fistula due to Crohn disease. Contrast material is seen posterior to the bladder on this voiding image from voiding cystourethrography.
(Courtesy of Nancy Curry, MD, Department of Radiology, Medical University of South Carolina, Charleston, SC.)
The presentation of rectourethral fistula is variable. Symptoms may include fecaluria, hematuria, UTI, nausea, vomiting, and fever. Peritonitis and sepsis may occur as well. Digital rectal examination often permits palpation of the fistula tract along the anterior rectal wall. Cystoscopy and sigmoidoscopy (Shin et al, 2000) visualize the fistula tract in the vast majority of cases and provide a mechanism for biopsy. In patients with a history of pelvic malignancy, biopsy of the fistula is suggested to evaluate for a local recurrence of the tumor (Shin et al, 2000). Voiding cystourethrography (VCUG) or retrograde urethrography (RUG) usually provide a definitive diagnosis of RUF (Fig. 77–39). The exact anatomic location and size of the fistula is also usually well delineated on VCUG or RUG, providing important information in surgical planning. Lateral projections may be necessary to visualize small fistulae, because contrast in the rectum or urethra can sometimes obscure extremely thin fistulous tracts. Upper tract imaging should be performed in patients with rectourethral fistulae to exclude a related ureteral injury. It is important to make an assessment of continence and sphincteric function in patients with RUF following radical prostatectomy. Given the location of most RUF at or near the vesicourethral anastomosis and the membranous urethra, there is a risk for persistent severe stress incontinence postoperatively following RUF repair (Ghoniem et al, 2008). In patients with RUF and severe stress incontinence, closure of the RUF may not be sufficient to bring about continence in many patients. Additional procedures may be needed to bring about a satisfactory result in these patients; this is an important issue to discuss in preoperative patient counseling.
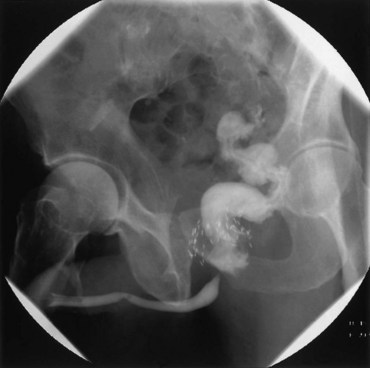
Figure 77–39 Rectourethral fistula after brachytherapy for carcinoma of the prostate. A retrograde urethrogram demonstrates filling of the rectum in this patient who presented several years after brachytherapy with fecaluria. The bladder is not opacified. The brachytherapy seeds are seen in the area of the prostate.
Management
Most RUF will require surgical repair (Bukowski et al, 1995; Stephenson and Middleton, 1996) although it is clear that some will close with conservative management. RUF following open or laparoscopic prostatectomy may heal spontaneously with catheter drainage, bowel rest, and intravenous hyperalimentation. In some cases, fecal diversion is necessary. Rassweiler and colleagues (2003) reported that 6 of 8 RUF patients were treated successfully in such a manner. Two patients required a temporary colostomy. Noldus and colleagues (1999) reported closure with conservative management in 7 of 13 patients with RUF following radical prostatectomy or cystoprostatectomy. Six patients eventually were successfully closed with a transanal Latzko procedure (see below). Successful minimally invasive management has been reported, as well, using endoscopic suturing, fulguration of the fistula tract, and the application of fibrin glue (Wilbert et al, 1996).
Surgical repair of RUF can be challenging, and the basic tenets of operative fistula repair are especially relevant in these cases (see Table 77–2). Several surgical approaches have been advocated and are outlined below. Single and staged repairs have been described for the repair of RUF. The controversy surrounding the staged repair centers on the issue of whether or not to perform fecal diversion at all, or to perform it prior to or at the time of repair of the urinary tract. Some authors have advocated fecal diversion and staged repair of all RUF (Shin et al, 2000). This is considered the standard conservative approach and, in combination with an indwelling urethral catheter, permits a trial of spontaneous healing of the fistula without open manipulation of the urinary tract. In support of the single-stage repair, a successful one-stage approach limits the potential morbidity and cost of multiple procedures that, by design, accompanies the staged repair. Suggested guidelines for cases in which a one-stage approach might be appropriate include surgically induced, small RUF not associated with infection, an abscess, or a poor bowel preparation (Wood and Middleton, 1990; Nunoo-Mensah et al, 2008). Staged repairs might be considered in cases of large fistulae, those associated with radiation therapy, uncontrolled local or systemic infection, immunocompromised states, or inadequate bowel preparation at the time of definitive repair (Stephenson and Middleton, 1996; Nunoo-Mensah et al, 2008).
Transrectal approaches with and without division of the anal sphincter have been described for the operative repair of RUF. The York-Mason procedure is a transrectal, transsphincteric approach that has been found to be effective and with low morbidity (Henderson et al, 1981; Prasad et al, 1983; Wood and Middleton, 1990; Stephenson and Middleton, 1996; Fengler and Abcarian, 1997; Renschler and Middleton, 2003). Classically, this is a staged repair with fecal diversion performed prior to repair of the RUF. However, in patients with small, nonirradiated fistulae, a single-stage approach can be used, provided that a vigorous bowel prepapration and broad-spectrum antibiotics are used (Renschler and Middleton, 2003). For repair of the urinary tract, the patient is placed prone on the operating room table in the jack-knife position. A full-thickness incision through the posterior anus and dorsal rectal wall is performed and deepened down to the level of the coccyx through the external anal sphincter (Fig. 77–40). Care is taken to mark or tag each layer of the anal sphincter as it is divided. Later in the procedure during closure, careful anatomical reapproximation of the layers of the external anal sphincter is necessary to avoid the devastating complication of anal incontinence postoperatively. The anorectal incision as described provides excellent exposure of the fistula in the anterior rectal wall. The fistula tract is excised, and the anterior rectal wall is mobilized circumferentially around the fistula margins. The urethra is closed, and then the anterior rectal wall is closed. Finally, the rectal mucosa is reapproximated, providing a three-layer closure. Alternatively, following excision of the RUF, an anterior rectal wall flap can be developed and advanced over the fistula (al-Ali and Kashmoula, 1997), similar to the transvaginal repair of VVF using the vaginal wall (see previous discussion under VVF repair). Closure of the incision is performed by reapproximating the posterior rectal wall and then sequentially closing the layers of the anal sphincter in an anatomic fashion. Results with the York-Mason procedure have been excellent. In the largest series of patients undergoing the York-Mason procedure, Renschler and Middleton (2003) reported a successful repair in 22 of 24 patients. One of the two failures was subsequently repaired with another York-Mason procedure. No serious complications were reported, and no patient developed anal incontinence or anal stenosis. Similar excellent results have been noted by other authors (Prasad et al, 1983; Bukowski et al, 1995; Fengler and Abcarian, 1997).
Figure 77–40 Diagram of York-Mason approach to the repair of rectourethral fistula.
(From Middleton RG. Rectourethral fistula repair. In: Krane RJ, Siroky MB, Fitzpatrick JM, editors. Operative urology. Philadelphia: Churchill Livingstone; 2000. p. 286.)
In contrast to the transrectal, transsphincteric approach, the transanal approach to RUF repair does not involve division of the anal sphincter. Exposure of the fistula is provided by dilation of the anus and fixed retraction. The Latzko procedure is one such approach (Noldus et al, 1999; Hata et al, 2002). Similar to the Latzko method described previously for transvaginal VVF repair (see prior discussion), the fistula tract and surrounding rectal mucosa is denuded in all four quadrants. The fistula is then closed in three layers. The major disadvantage to this approach is the relatively poor exposure and lack of maneuverability within the operative field. Rectal advancement flaps have also been used to successfully treat RUF through a transanal approach (Fazio et al, 1987; Dreznik et al, 2003; Garofalo et al, 2003).
A perineal approach to RUF has been advocated by some authors in selected cases of RUF (Bukowski et al, 1995; Nyam and Pemberton, 1999; Youssef et al, 1999; Zmora et al, 2003). Anatomically, this is a familiar approach for many urologists and has the added advantage of local access to a variety of potential interpositional flaps. Excellent results have been obtained with the perineal approach in combination with an interpositional flap, including gracilis muscle (Ryan et al, 1979; Rius et al, 2000; Zmora et al, 2003; Ghoniem et al, 2008; Gupta et al, 2008), pedicled Dartos muscle (Venable, 1989; Youssef et al, 1999; Yamazaki et al, 2001; Varma et al, 2007), penile skin (Morgan, 1975), levator muscle (Goodwin et al, 1958), and bladder (Kokotas and Kontogeorgos, 1983). Transabdominal approaches to RUF have been described with limited success (Bukowski et al, 1995; Nyam and Pemberton, 1999; Shin et al, 2000). The principal advantage of this technique is the availability of greater omentum for an interpositional flap. Potential disadvantages include the morbidity and prolonged postoperative convalescence associated with a laparotomy incision, poor exposure of the operative field (with limited maneuverability in the deep pelvis), and the risk of urinary and fecal incontinence.
The repair of rectourethral fistulae following external beam radiotherapy, (Chrouser et al, 2005), combined radiotherapy (Marguet et al, 2007), brachytherapy (Lane et al, 2006), or cryosurgical ablation of the prostate can be especially difficult (Izawa et al, 2000a; Elliott et al, 2006). These fistulae may be large and are associated with considerable induration, fibrosis, and ischemia for a variable distance around the fistula limiting reconstructive options. Urinary reconstruction may not be possible in some of these cases, necessitating urinary diversion. Moreira and colleagues (2004) reported on 11 cases of RUF following brachytherapy for the treatment of prostate cancer, all of whom underwent diverting colostomy. Three patients with satisfactory baseline continence underwent primary repair by a York-Mason approach with a gracilis flap; 7 patients underwent urinary diversion combined with radical pelvic surgery (6 cystoprostatectomy, 1 prostatectomy); and 1 refused repair. Izawa and colleagues (2000b) reported on the management of severe complications of cryosurgical ablation of the prostate, including two cases of prostatopubic fistulae and one rectourethral fistula. Two of these patients underwent cystoprostatectomy, and one had a bladder neck closure and continent reconstruction.